Abstract
To describe the outcomes of autografts and synthetics in anterior cruciate ligament (ACL) and posterior cruciate ligament (PCL) reconstruction with respect to instrumented laxity measurements, patient‐reported outcome scores, complications, and graft failure risk. We searched PubMed, Cochrane Library, and EMBASE for published randomized controlled trials (RCT) and case controlled trials (CCTs) to compare the outcomes of the autografts versus synthetics after cruciate ligament reconstruction. Data analyses were performed using Cochrane Collaboration RevMan 5.0. Nine studies were identified from the literature review. Of these studies, three studies compared the results of bone–patellar tendon–bone (BPTB) and ligament augmentation and reconstruction system (LARS), while six studies compared the results of four‐strand hamstring tendon graft (4SHG) and LARS. The comparative study showed no difference in Lysholm score and failure risk between autografts and synthetics. The combined results of the meta‐analysis indicated that there was a significantly lower rate of side‐to‐side difference > 3 mm (Odds Ratio [OR] 2.46, 95% confidence intervals [CI] 1.44–4.22, P = 0.001), overall IKDC (OR 0.40, 95% CI 0.19–0.83, P = 0.01), complications (OR 2.54, 95% CI 1.26–5.14, P = 0.009), and Tegner score (OR −0.31, 95% CI −0.52–0.10, P = 0.004) in the synthetics group than in the autografts group. This systematic review comparing long‐term outcomes after cruciate ligament reconstruction with either autograft or synthetics suggests no significant differences in failure risk. Autografts were inferior to synthetics with respect to restoring knee joint stability and patient‐reported outcome scores, and were also associated with more postoperative complications.
Keywords: Cruciate ligament, LARS, Meta‐analysis, Systematic review
Introduction
Arthroscopically assisted anterior cruciate ligament (ACL) or posterior cruciate ligament (PCL) reconstruction has been widely used for patients with cruciate ligament lesions, and advances in arthroscopic surgery have yielded good clinical results. However, controversy continues over the choice of graft tissue, including autografts, allografts, and synthetic ligaments. Two of the most common autografts used are bone–patellar tendon–bone (BPTB) and four‐strand hamstring tendon (4SHT)1. Arguably, autograft cruciate reconstruction is the gold standard, providing reliable long‐term results. Regardless of its type, autograft harvest can result in a degree of morbidity, which may negatively affect recovery after ACL reconstruction2. The use of allografts has increased in recent years because they offer less donor‐site morbidity, shorter surgical and anesthesia times, fewer postoperative complications, faster postoperative recovery, lower incidence of postoperative arthrofibrosis, less postoperative pain, and an unlimited graft source in the setting of multi‐ligament and revision reconstructions3, 4. However, allografts are associated with a higher expense, a risk of disease transmission, delayed healing, ligamentization, and an increased risk of graft rupture in the younger, more active population4, 5.
There have been numerous systematic reviews comparing the results of autograft hamstring versus bone–patellar tendon–bone grafts6, 7, 8, 9, 10. Data from this analysis suggested that there was insufficient evidence to recommend one graft choice over the other in major clinical results between graft types. Compared with 4SHT autografts, ACL reconstruction with BPTB autografts may more effectively restore knee joint stability and allow patients to return to higher levels of activity, but it may also result in an increase in long‐term anterior knee pain, kneeling pain, and higher rates of osteoarthritis.
There have also been many studies comparing autograft with allograft5, 11, 12. Prodromos et al.11 showed that the overall stability rate was 72% for all autografts compared with 59% for all allografts (P < 0.001), which did not account for the effect of irradiation on the allograft tissue. Christopher et al.5 and Mariscalco et al.12 compared exclusively nonirradiated allograft tissue with autograft in a systematic review and concluded that there was no significant difference between the two graft sources in outcomes.
The purpose of this systematic review was to conduct a meta‐analysis to compare the effectiveness of cruciate ligament reconstruction using either autografts or synthetics. We hypothesize that autografts or synthetics show no difference in: (i) instrumented laxity measurements; (ii) patient‐reported outcome scores; (iii) complications; or (iv) graft failure risk after cruciate ligament reconstruction.
Materials and Methods
Literature Search and Study Selection
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) guidelines were followed from the inception of the study. A review of the literature was performed by two authors (L Li and XM Cao) using PubMed, Cochrane Library, and EMBASE from inception through January 2018. Key search terms included (“ACL,” OR “anterior cruciate ligament,” OR “PCL,” OR “posterior cruciate ligament,”) and [(“The Ligament Augmentation and Reconstruction System,” OR “LARS artificial ligament,” OR “artificial ligament”). No language limitations were imposed. The reference lists of the selected articles were reviewed to identify additional studies not found in the original search.
Inclusion and Exclusion Criteria
Two researchers reviewed the generated list of unique articles for studies that met the following inclusion criteria: (i) Participants: the autografts and synthetics in the treatment of cruciate ligament lesions and minimum 2‐year follow‐up; (ii) Interventions and comparisons: at least two study groups in ACL or PCL reconstruction with either autograft or synthetics, the type of graft fixation was not limited; (iii) Outcome measure: the outcome assessments included instrumented laxity measurements, patient‐reported outcome scores, complications, and graft failure risk; (iv) Study design: CCTs, both randomized controlled trial (RCTs) and case controlled trials (CCTs). The following studies were excluded: (i) case‐based reports or reviews without original data; (ii) studies with imprecise experimental design; and (iii) studies with incomplete data or incorrect data.
Data Extraction and Quality Assessment
Two investigators independently extracted data that met our inclusion and exclusion criteria. Disagreement between two reviewers was resolved by discussion or consultation with a third reviewer when necessary. The following information was extracted: details on study designs; patient demographic characteristics; length of clinical follow‐up, percentage lost to follow‐up, description of surgical technique, and clinical variables including follow‐up time, complications, knee laxity measurements with KT‐1000/KT‐2000 arthrometer, patient‐reported quantitative outcome measures (International Documentation Knee Documentation Committee [IKDC] grade, Lysholm score, Tegner activity scale). If a study did not specifically state whether it was retrospective or prospective in nature, it was assumed to be retrospective. Failure was defined either as complete or partial rupture of the ligament or as a documented “failure” as defined by each included study. The methodological quality of RCTs was evaluated independently by two of the authors (K Li and L Guo) using the tool for assessing risk of bias described in Chapter 8 of the Cochrane Handbook Systematic Reviews of Interventions (Version 5.1.0). This tool is comprised of a description and judgment for each entry in a “risk of bias” table, where each entry addresses a specific feature of the study. The judgment for each entry involves answering a question, with answers “Yes” indicating low risk of bias, “No” indicating high risk of bias, and “Unclear” indicating either lack of information or uncertainty over the potential for a bias. Quality assessment of non‐RCTs was performed according to the Methodological Index for Non‐Randomized studies (MINORs)13, which scores range from 0 to 24. Disagreement was resolved by consulting a third reviewer.
Statistical Analysis
Data analysis was performed using RevMan 5.3 software. Two authors (JG Lu and ZZ Duan) checked the data during entry to ensure that there were no errors. Dichotomous outcomes were expressed in odds ratio (OR) and the mean difference (MD) was used for continuous outcomes, both 95% confidence intervals (CI). A χ2 test was used to assess heterogeneity between different studies, inter‐study heterogeneity was assumed in cases in which I2 > 50% or P value <0.114, and ORs were pooled according to random‐effects models. Alternatively, fixed‐effects models were used. A P value less than 0.05 was considered statistically significant. A sensitivity analysis was conducted by excluding one study in each round and investigating the influence of a single study on the overall meta‐analysis estimate.
Results
Search Results
A total of 56 potentially relevant articles were identified, with a review of article reference lists revealing a further two publications. After screening the titles and abstracts, 48 studies were excluded. After reading the full‐text of the remained 11 studies, we enrolled nine studies on 521 patients that met all inclusion criteria2, 15, 16, 17, 18, 19, 20, 21, 22, including seven English and two Chinese articles. The literature search process is presented in Fig. 1.
Figure 1.
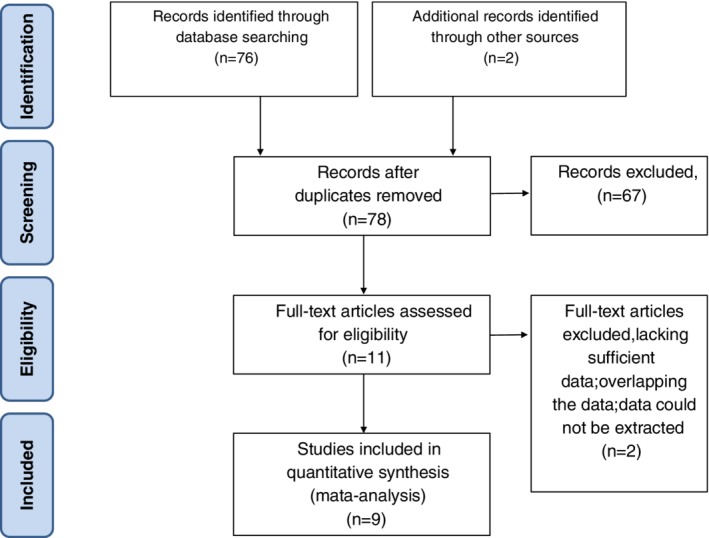
Flow chart of study selection process for the meta‐analysis.
Study Characteristics
The nine studies were published between 2002 to 2017 and comprised a total of 521 patients with an age range from 17 to 56 years. All studies’ reported groups were matched in terms of age, gender, severity, and course of disease. The mean length of follow‐up was 48.8 months (range, 24 to 122 months) in the allograft group and 48 months (range, 18 to 120 months) in the synthetics group. The allograft group contained 79.4% male and 20.6% female patients, and the synthetics group contained 76.5% male and 23.5% female patients. Of the nine studies included in this review, there were three randomized controlled trial, the other six studies were relatively high‐quality retrospective study (Table 1). One study used LARS as an augmentation to short or thin hamstring autograft rather than a sole constituent of the graft2.
Table 1.
Overview of included studies*
| Authors | Year | Journal | Procedure Date Range | Ligament | Patients enrolled (A/S) | Study Design | Country | Mean Length of Follow up (Range) months | % Follow‐up | Male/Female | Age,y, Mean (Range) (A/S) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nau15 | 2002 | JBJS | 1996–1998 | ACL | 27/26 | RCT | USA | 24 | 96.23 | 36/17 | 30.87 ± 8.66/31.03 ± 8.98 |
| Fan16 | 2008 | CJRRS | 2002–2005 | ACL | 27/15 | Cohort | China | 26.3(2243)/24.1(18–40) | 100 | 34/8 | 20–52/17–40 |
| Li17 | 2009 | SICOT | 2002–2006 | PCL | 15/21 | RCT | China | 28.8/26.4 | 100 | 30/6 | 20–43/18–47 |
| Liu18 | 2010 | SICOT | 2003–2004 | ACL | 32/28 | Cohort | China | 49 (48–52) | 100 | 45/15 | 32 (20–56)/36 (18–54) |
| Li 19 | 2012 | EJOST | 2007–2011 | ACL | 26/24 | RCT | China | 30 | 100 | 38/12 | 30.6(18–50)/32.6(18–50) |
| Pan20 | 2013 | AOTS | 2004–2006 | ACL | 30/32 | Cohort | China | >48 | 100 | 44/18 | 33.93 ± 6.34/35.88 ± 11.30 |
| Xu21 | 2014 | AOTS | 2006–2008 | PCL | 16/19 | Cohort | China | 51(46–57) | 100 | 17/18 | 29.1 ± 5.7/ 28.6 ± 6.8 |
| Hamido2 | 2015 | OTSR | 2007–2008 | ACL | 45/27 | Cohort | Kuwait | 59(58–62) | 100 | 71/1 | 20(18–31)/24(21–35) |
| Chen22 | 2017 | AJSM | 2004–2007 | ACL | 73/38 | Cohort | China | 122.9 ± 11.8/120.8 ± 26.9 | 83 | 92/19 | 28.6 ± 8.8(17–52) /27.6 ± 9.3 (17–54) |
A/S, Autografts/Synthetics; ACL, anterior cruciate ligament; AJSM, The American Journal of Sports Medicine; AOTS, Arch Orthop Trauma Surg; CJRRS, Chinese Journal of Reparative and Reconstructive Surgery; EJOST, Eur J Orthop Surg Traumatol; JBJS, The journal of bone and joint surgery; OTSR, Orthopedics & Traumatology: Surgery & Research; PCL, posterior cruciate ligament; SICOT, International Orthopedics.
Assessment of Risk of Bias
Three RCTs and six high‐quality retrospective studies included in this meta‐analysis have gone through a strict quality assessment. The Cochrane Handbook for Systematic Review of Interventions was consulted to assess the quality of RCTs (Fig. 2) For the three RCTs, all studies have definite selection criteria and are described as “randomized,” and two studies describe the methods for random sequence generation. One study describes allocation concealment or blinding methods15. All studies had a low risk of incomplete outcome data and selectively reporting results. The MINORS scale was applied for six non‐RCTs and the total score is 19.
Figure 2.
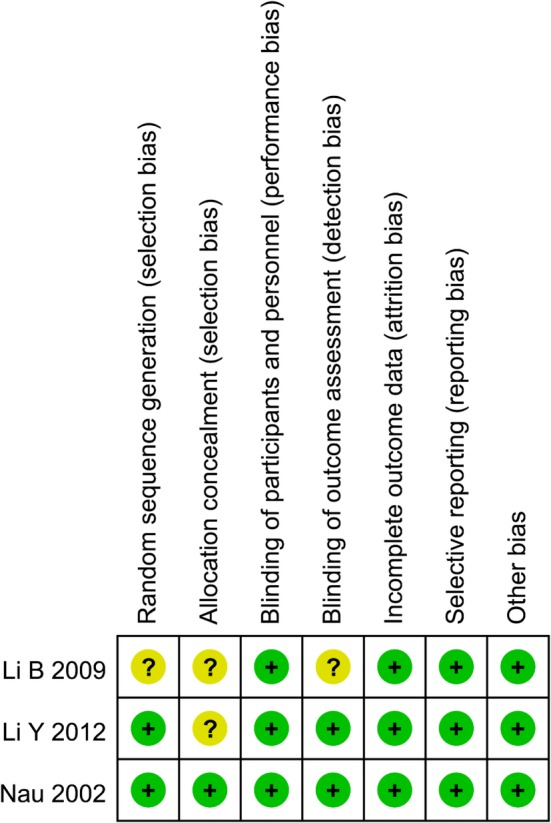
Quality of methodology of the RCTs. +, low risk of bias?, unclear risk of bias.
Surgical Technique
All surgeries were performed with the arthroscopic technique by the senior surgeon. Table 2 details the surgical techniques used for each report. For autograft procedures, three studies used BPTB and six studies used 4SHT (Fig. 3 A,B). For synthetic procedures, all studies used LARS (Fig. 3C). All studies used interference screw fixation for both tibial and femoral bone plugs in synthetic group, but most did not delineate whether these were metal or biocomposite, and only one study used bioabsorbable screw. Autograft fixation was slightly more variable, with femoral tunnels relying on interference screws in six studies15, 17, 18, 19, 20, 21, titanium button in two studies16, 22, bioabsorbable screw2 and crosspins in one study22. The tibial fixation of autografts included interference screws in six studies15, 17, 18, 19, 20, 21, bioabsorbable screw in one study2, steel plate in one study16, and screw and/or spiked washer in one study22.
Table 2.
Overview of surgical details for included studies*
| Authors | No. of Autografts (%) | No. of Synthetics (%) | Autografts | Synthetic | Autografts | Synthetics | ||
|---|---|---|---|---|---|---|---|---|
| Femoral Fixation | Tibial Fixation | Femoral Fixation | Tibial Fixation | |||||
| Nau15 | 27 | 26 | BPTB | LARS | inteinterference screw | interference screw | interference screw | interference screw |
| Fan16 | 27 | 15 | 4SHG | LARS | titanium button | steel plate | interference screw | interference screw |
| Li17 | 15 | 21 | 4SHG | LARS | inteinterference screw | interference screw | interference screw | interference screw |
| Liu18 | 32 | 28 | 4SHG | LARS | inteinterference screw | interference screw | interference screw | interference screw |
| Li19 | 26 | 24 | BPTB | LARS | inteinterference screw | interference screw | interference screw | interference screw |
| Pan20 | 30 | 32 | BPTB | LARS | inteinterference screw | interference screw | interference screw | interference screw |
| Xu21 | 16 | 19 | 4SHG | LARS | inteinterference screw | interference screw | interference screw | interference screw |
| Hamido2 | 45 | 27 | 4SHG | LARS | bioabsorbable screw | bioabsorbable screw | bioabsorbable screw | bioabsorbable screw |
| Chen22 | 73 | 38 | 4SHG | LARS | titanium button or; cross‐pin system | Screw and/or spiked washer | interference screw | interference screw |
BPTB, bone–patellar tendon–bone; LARS, ligament augmentation and reconstruction system; 4SHG, four‐strand hamstring tendon graft.
Figure 3.
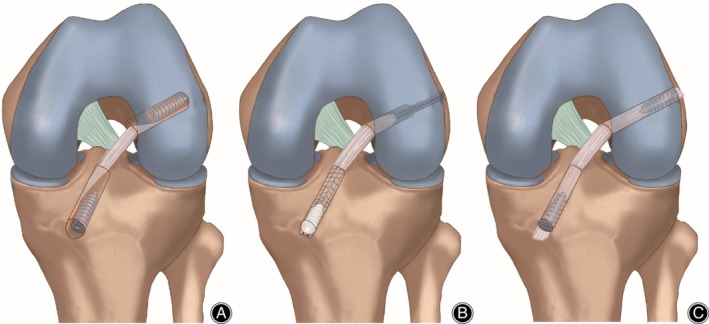
Schematic illustration of ACL reconstruction with BPTB(A), 4SHT(B) and LARS (C). BPTB, bone‐patellar tendon‐bone; 4SHG, four‐strand hamstring tendon graft; LARS, ligament augmentation and reconstruction system.
Instrumented Laxity Across All Studies
Instrumented laxity testing was reported in eight studies2, 16, 18, 19, 20, 21, 22 as mean side‐to‐side difference at maximum follow‐up using maximum manual tension with either the KT‐1000 or KT‐2000 arthrometer (Table 3). The stability results of five studies showed that there was no significant difference between the two groups, four studies showed that the LARS group had significantly less anterior displacement than the autografts group. The pooled risk of instrumented anteroposterior laxity greater than 3 mm was 4.4% (95% CI, 0.6% to 11.6%) in the autografts group and 4.9% (95% CI, 2.8% to 7.4%) in the synthetics group (Fig. 4).
Table 3.
Instrumented laxity*
| Authors | Side‐to‐Side Difference Autografts, mm | Side‐to‐Side Difference Synthetics, mm | Significance |
|---|---|---|---|
| Nau15 | 2.38 ± 1.80 | 4.86 ± 3.80 | ns |
| Fan16 | 5 (19%) > 3 mm | 3 (20%) > 3 mm | ns |
| Li17 | NR | NR | P < 0.05 |
| Liu18 | 2.4 ± 0.5 | 1.2 ± 0.3 | P = 0.013 |
| Li19 | 8. 9 ± 4.2 | 5.3 ± 4.1 | P = 0.004 |
| Pan20 | 2.62 ± 2.12 | 2.29 ± 2.03 | P > 0.5 |
| Xu21 | 3.28 ± 1.95 | 3.27 ± 2.13 | P > 0.05 |
| Hamido2 | 2.5 ± 0.5 | 1.1 ± 0.3 | P = 0.027 |
| Chen22 | 2.4 ± 2.1 | 1.5 ± 1.5 | P = 0.131 |
Results are reported as mean ± SD. NR, not reported; ns, not significant.
Figure 4.
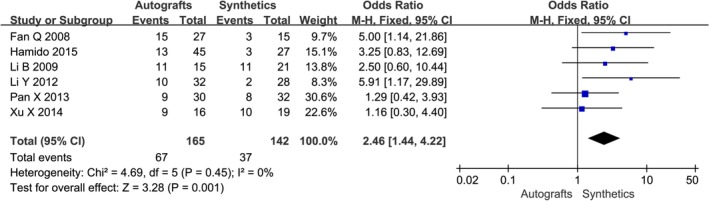
The forest plot of instrumented laxity autografts and synthetics after cruciate ligament reconstruction. In this and subsequent figures, CI, confidence interval; df, degrees of freedom.
Clinical Outcomes
A combination of IKDC scores, patient‐reported Lysholm scores, and/or Tegner activity scores were reported in all studies; the related information is detailed in Table 4. Six studies used the objective IKDC scoring system to describe the results of the ACL reconstruction as normal (A), nearly normal (B), abnormal (C), and severely abnormal (D). The IKDC scores of the six trials were treated as dichotomous variables: normal and nearly normal versus abnormal and severely abnormal. The IKDC scores were normal and nearly normal in 136/164 patients in the autografts group and 140/151 patients in the synthetics group, respectively. Mean Lysholm scores at follow‐up ranged from 85 to 98 in the autografts group and from 87 to 98 in the synthetics group. Mean Tegner scores at follow‐up ranged from 4.93 to 8.31 in the autografts group and from 5.03 to 8.57 in the synthetics group. The meta‐analysis showed no difference in Lysholm score after cruciate ligament reconstruction with autografts compared with synthetics (OR −0.75, 95% CI −2.25–0.75, P = 0.41) (Fig. 5). The combined results of the meta‐analysis indicated there was a significantly lower rate of overall IKDC (OR 0.40, 95% CI 0.19–0.83, P = 0.01) (Fig. 6) and Tegner score (OR −0.31, 95% CI −0.52–0.09, P = 0.005) (Fig. 7) in the autografts group than in the synthetics group.
Table 4.
Clinical outcomes*
| Authors | Overall IKDC | Lysholm | Tegner | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Autografts | Synthetics | Significance | Autografts | Synthetics | Significance | Autografts | Synthetics | Significance | |
| Nau15 | NR | NR | ns | NR | NR | NR | NR | NR | ns |
| Fan16 | NR | NR | NR | 87.80 ± 3.41 | 88.90 ± 3.30 | ns | 4.93 (3–6) | 5.03(3–7) | P > 0.05 |
| Li17 | 8A,3B,3C,1D | 14A,5B,2C,0D | P = 0.285 | 85 (33–100) | 93 (43–100) | P < 0.05 | 6 (1–9) | 7 (2–10) | P < 0.05 |
| Liu18 | 22A,6B,4C,0D | 21A,5B,2C,0D | P > 0.05 | 92.1 ± 7.9 | 94.6 ± 9.2 | P = 0.259 | 6.2 ± 1.6 | 6.6 ± 1.8 | P = 0.387 |
| Li19 | 22A,2B,2C,0D | 22A,2B,0C,0D | P = 0.448 | 98.14 ± 0 0.43 | 98.32 ± 0.13 | P = 0.055 | 8.31 ± 0.63 | 8.57 ± 0.46 | P = 0.105 |
| Pan20 | 14A,12B,4C,0D | 19A,9B,4C,0D | P > 0.1 | 93.13 ± 9.03 | 94.09 ± 6.75 | P > 0.5 | 5.83 ± 1.18 | 6.16 ± 1.17 | P > 0.05 |
| Xu21 | 9A,6B,1C,0D | 10A,7B,2C,0D | P > 0.05 | 87.9 ± 7.7 | 87.0 ± 6.8 | P > 0.05 | 6.31 ± 0.79 | 6.42 ± 0.84 | P > 0.05 |
| Hamido2 | 26A,6B,11C,2D | 20A,6B,1C,0D | P = 0.05 | 90.1 ± 6.9 | 95.3 ± 7.3 | P = 0.239 | 6.7 ± 1.5 | 7.4 ± 1.8 | P = 0.368 |
| Chen22 | 91.6 ± 5.1 | 89.8 ± 5.3 | P = 0.124 | 92.5 ± 5.0 | 91.5 ± 4.8 | P = 0.251 | 5.5 ± 1.7 | 6.0 ± 1.8 | P = 0.286 |
IKDC, International Knee Documentation Committee; NR, not reported; ns, not significant.
Figure 5.
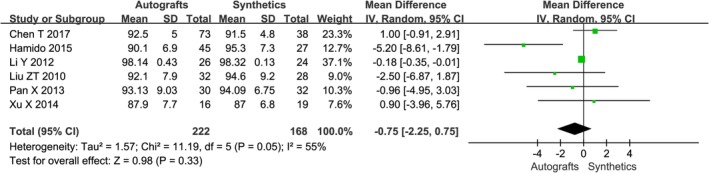
The forest plot of Lysholm score between autografts and synthetics after cruciate ligament reconstruction.
Figure 6.
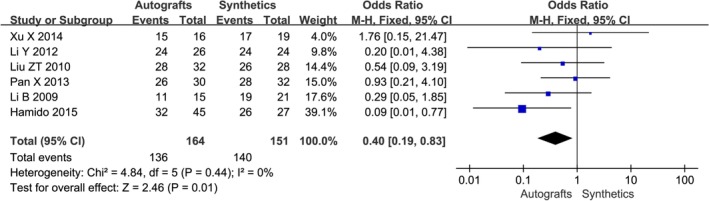
The forest plot of overall IKDC between autografts and synthetics after cruciate ligament reconstruction.
Figure 7.
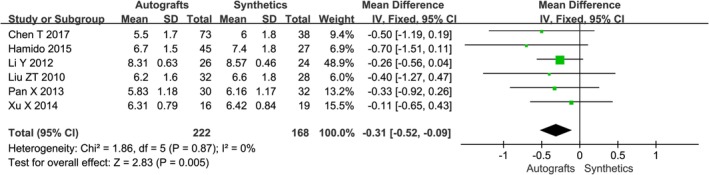
The forest plot of Tegner score between autografts and synthetics after cruciate ligament reconstruction.
Complications
Table 5 details the complications encountered in each group. The complications results of eight studies showed that there was no significant difference between the two groups and just one study16 showed that the synthetics group had significantly less complications than the autografts group. Table 5 details the complications encountered in each group at the time of most recent follow‐up. The meta‐analysis results are shown in Fig. 8. There was a statistical difference among the postoperative complications between the two groups (OR 2.54, 95% CI 1.26–5.14, P = 0.009), indicating that complications of synthetics were less than autografts.
Table 5.
Complications*
| Authors | Autografts | Synthetics | |||||
|---|---|---|---|---|---|---|---|
| No. of Patients at Follow‐up | Complications,% (n) | Description of Complications | No. of Patients at Follow‐up | Complications,% (n) | Description of Complications | Significance | |
| Nau15 | 26 | 7.7 (2) | 1 Superficial infection; 1 Screw‐related problem | 25 | 4 (1) | 1 Screw‐related problem | ns |
| Fan16 | 27 | 25.9 (7) | 2 loss of last 5° of extension; 5 loss of 5–10° of full flexion | 15 | 0 (0) | NR | P < 0.05 |
| Li17 | 15 | 26.7 (4) | 1 anterior knee Pain; 2 paraesthesia; 1 arthrofibrosis | 21 | 4.8 (1) | 1 anterior knee pain | NR |
| Liu18 | 32 | 9.4 (3) | 2 loss of 5° of full flexion; 1 arthrofibrosis | 28 | 3.6 (1) | 1 Screw‐related problem | NR |
| Li19 | 26 | 11.5 (3) | 1 Screw‐related problem 2 anterior knee | 24 | 8.3 (2) | 1 Screw‐related problem; 1 anterior knee Pain | NR |
| Pan20 | 30 | 0 (0) | NO | 32 | 0 (0) | NO | NR |
| Xu21 | 16 | 31.3 (5) | 2 anteromedial knee pain; 3 paraesthesia | 19 | 5.3 (1) | 1 synovitis | NR |
| Hamido2 | 45 | 4.5 (2) | 1 paraesthesia; 1 arthrofibrosis and loss of; last 5–10° of extension | 27 | 0(0) | NR | NR |
| Chen22 | 73 | 8.2 (6) | 2 Screw‐related problem; 3 Donor site morbidity, 1Superficial infection | 38 | 10.5 (4) | 3 Screw‐related problem; 1Synovitis | NR |
NR, not reported; ns, not significant.
Figure 8.
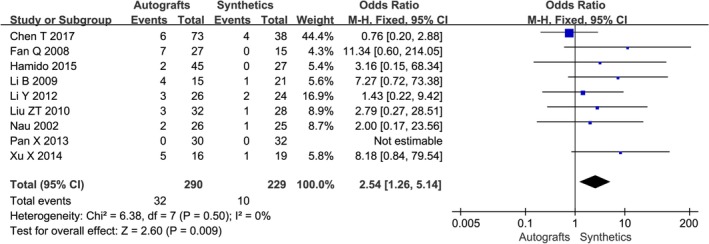
The forest plot of complications between autografts and synthetics after cruciate ligament reconstruction.
Failure Risk Across All Studies
Graft rupture was labeled as failure, with other reasons for reoperation categorized as “complications.” Nau et al.15 reported two patients were lost to follow‐up (one in each group) and each was assumed to be a failure of reconstruction. Chen et al.22 evaluated primary ACL reconstruction using either synthetics with remnant preservation or hamstring autografts and observed cumulative failure in three patients (3/38, 7.9%) and six patients (6/73, 8.2%), respectively, for patients with synthetics and hamstring autografts at 10 years post‐operation. Seven studies reported zero failures. No studies showed any statistically significant difference in the rates of graft failure between the two groups.
Discussion
A substantial body of literature has examined the factors influencing outcome after ACL reconstruction, including comparisons of tunnel placement, fixation technique, and graft choice. Although many studies argue in favor of one form of autograft over another, confounding variables of such a complex surgery are often difficult to control, and may influence results9. Meta‐analysis allows us to quantitatively analyze multiple prospective comparative studies with similar study objectives to increase sample size and improve statistical power. Thus, we performed this up‐to‐date meta‐analysis of comparative studies to assess the safety and efficacy of cruciate ligament reconstruction that use either autografts or synthetics in order to provide a reference for the selection of autograft. Our study had the advantage of long‐term follow‐up of more than 2 years and is the first study to our knowledge comparing these two grafts.
Admittedly, autograft cruciate reconstruction is the gold standard, providing reliable long‐term results, while this method somewhat reminds us of robbing “Peter to pay Paul.” Logically, an off‐the‐shelf graft without self‐tissue sacrifice would be an ideal choice. Shorter surgical and anesthesia time, fewer postoperative complications, reduced morbidity at the harvest site, faster postoperative recovery and lower incidence of postoperative arthrofibrosis, and less postoperative pain are considered to be the main advantages of allograft usage for ACL replacement3. On the other hand, allograft usage may be associated with higher rates of re‐rupture, limited availability, a delayed healing and ligamentization process in comparison to autografts, risk of disease transmission, and expensiveness23. Since the 1970s, synthetic devices have been available for use in the management of the cruciate‐injured knee. These devices have the intended benefits of avoiding donor‐site morbidity, providing a strong stabilizing construct, and allowing aggressive rehabilitation and a relatively rapid return to sporting activity without the risks of disease transmission and rejection. LARS is a third generation of such synthetic ligament, designed to overcome the issues of graft failure and synovitis which led previous generations of synthetic ligaments to fall out of favor. It consists of two distinct segments: an intraosseous and an intra‐articular segment. Its intraosseous segment is composed of longitudinal fibers bound together by a transverse knitted structure while the intra‐articular segment consists of multiple parallel longitudinal fibers twisted at 90° angles24. The intra‐articular multifilament part of the prosthesis is implanted in a twisted fashion to imitate the natural cruciate ligament. This avoids shearing forces between the fibers and interferes during combined tension, torsion, and flexion. Additionally, the intra‐articular segment of the graft acts as a scaffold inducing fibroblastic ingrowth between the fibers of the ligament due to the porosity of the material25, 26, 27. Ingrown soft tissue between the ligament fibers acts as a viscoelastic element and protects the ligament against friction at the opening of the bony canal as well as between the artificial fibers themselves15.
Our meta‐analysis included nine studies. The pooled ORs of these studies indicated that autografts were inferior to synthetics for restoring knee joint stability, patient‐reported outcome scores and were associated with more postoperative complications, but there was no significant difference between two groups with regard to graft failure in any of the studies. Another drawback of autografts is that graft strength decreases during the long period of revascularization. This may lead to graft laxity or rupture during early rehabilitation28. Currently, the BPTB and hamstring autografts are the most commonly used grafts. The autologous and allogeneic bone grafts in the bone tunnel must undergo tendon “religamentization.” However, the phenomenon of ligamentization occurs in the successfully reconstructed human cruciate ligament between 6 month to 1 year after operation, being slowly revascularized and presenting most histologic and functional properties28, 29. In this process, necrosis and replacement of bone graft occurs alternatively, which is prone to collapse and loosening during this course. The tunnel wall is reconstructed by fiber tissue and the enlargement of the tunnel is likely to occur during this period, but the artificial ligament in the bone tunnel did not undergo necrosis or religamentization30. The synthetic materials can provide immediate strength and stability for the knee after reconstruction, allowing for early function recovery18, 30. Four studies evaluated patient‐reported outcomes postoperatively at different times of follow‐up, which suggested that symptom relief and restoration of function of those with synthetics were statistically higher at 6 months or 1 year postoperatively, demonstrating that patients with synthetics could return to sports earlier15, 17, 19, 22. Krupa et al. also indicated a significant progress from preoperative to short‐term postoperative result in reducing anterior translation and anterolateral rotational instability of the tibia relative to the femur in patients who had undergone ACL reconstruction with a synthetic LARS graft4.
The strengths of this review are its well‐conducted clinical trials and relatively long‐term follow‐up of the papers included in the analysis. To our knowledge, no previously published systematic review of the influence of autograft choice on outcome after cruciate ligament reconstruction surgery has had such rigid inclusion and exclusion criteria. The included studies are all high‐level data with at least two‐year minimum follow‐up and thus provide a high level evidence for clinical decisions. A review by Newman and Atkinson indicated the support of the current literature for the use of LARS in the short to medium term in patients who have undergone ACL reconstruction. However, the authors highlighted the need for high‐quality studies with long‐term follow‐up to determine whether the use of LARS is preferable to autologous grafts31. Batty et al. wrote systematic review was to assess the safety and efficacy of different synthetic devices in cruciate ligament surgery, a limitation of this review is the paucity of well‐conducted clinical trials included and the exclusion of no‐English‐language studies (n = 8)32. Another two systematic reviews assess the effectiveness of LARS as a surgical option for ACL and PCL, but they were limited by the paucity of high‐level, high‐quality evidence33, 34.
Be that as it may, there are several limitations in this study. Firstly, although nine studies were added in this study since the latest systematic review, just three of them were RCT, the other six studies were relatively high‐quality retrospective studies, and thus usually has more potential sources of bias and confounding factors. This might weaken the strength of the findings, and more high‐quality randomized controlled trials with long‐term follow‐up are necessary to make a firm conclusion. Secondly, all the articles included ACL and PCL reconstruction. Thirdly, the majority of studies were limited in their statistical power by small sample size. Further standardization of rehabilitation, utilization of a blinded clinical examiner, and use of additional validated patient‐reported outcome measure would improve the strength of conclusions and meta‐analysis.
Our study is the first meta‐analysis to compare the results of the autografts and synthetic ligaments. The combined results of this meta‐analysis indicate a statistical difference between autografts and synthetics in terms of negative outcomes of instrumented laxity, IKDC scores, patient‐reported Lysholm scores, and Tegner activity scores. Thus, our study concludes that cruciate ligament reconstruction with LARS achieved better postoperative effects in terms of restoring knee joint function and stability than autografts and was associated with less postoperative complications. The main advantages of the use of a synthetic ligament in ACL reconstruction are: the immediate recovery of stability and the timely rehabilitation and avoidance of sacrifice of autologous structures. We consider LARS artificial ligament to be an alternative graft for cruciate ligament reconstruction, especially (i) when early rehabilitation is imperative, (ii) in the presence of multiple ligament injuries, and (iii) in revision surgeries in which the availability of autologous tissue for reconstruction is limited.
Acknowledgements
The project was supported by National Natural Science Foundation of China 81772415 and 81972075, Doctoral Fund of the Second Hospital of Shanxi Medical University 201801‐1.This work was also supported by Research Project Supported by Shanxi Scholarship Council of China HGKY2019099.
Declarations of Interest: None.
Contributor Information
Chuan Xiang, Email: xcml7275@163.com.
Lei Wei, Email: leiwei43@hotmail.com.
References
- 1. Gifstad T, Sole A, Strand T, Uppheim G, Grontvedt T, Drogset JO. Long‐term follow‐up of patellar tendon grafts or hamstring tendon grafts in endoscopic ACL reconstructions. Knee Surg Sports Traumatol Arthrosc, 2013, 21: 576–583. [DOI] [PubMed] [Google Scholar]
- 2. 2. Hamido F, Al Harran H, Al Misfer AR, et al Augmented short undersized hamstring tendon graft with LARS® artificial ligament versus four‐strand hamstring tendon in anterior cruciate ligament reconstruction: preliminary results. Orthop Traumatol Surg Res, 2015, 101: 535–538. [DOI] [PubMed] [Google Scholar]
- 3. Zeng C, Gao SG, Li H, et al Autograft versus allograft in anterior cruciate ligament reconstruction: a meta‐analysis of randomized controlled trials and systematic review of overlapping systematic reviews. Arthroscopy, 2016, 32: 153–163.e18. [DOI] [PubMed] [Google Scholar]
- 4. Krupa S, Krolikowska A, Reichert P. Postoperative knee joint stability following anterior cruciate ligament reconstruction using the ligament advanced reinforcement system. Polim Med, 2016, 46: 155–161. [DOI] [PubMed] [Google Scholar]
- 5. Joyce CD, Randall KL, Mariscalco MW, Magnussen RA, Flanigan DC. Bone‐patellar tendon‐bone versus soft‐tissue allograft for anterior cruciate ligament reconstruction: a systematic review. Art Ther, 2016, 32: 394–402. [DOI] [PubMed] [Google Scholar]
- 6. Magnussen RA, Carey JL, Spindler KP. Does autograft choice determine intermediate‐term outcome of ACL reconstruction? Knee Surg Sports Traumatol Arthrosc, 2011, 19: 462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li S, Chen Y, Lin Z, Cui W, Zhao J, Su W. A systematic review of randomized controlled clinical trials comparing hamstring autografts versus bone‐patellar tendon‐bone autografts for the reconstruction of the anterior cruciate ligament. Arch Orthop Trauma Surg, 2012, 132: 1287–1297. [DOI] [PubMed] [Google Scholar]
- 8. Xie X, Liu X, Chen Z, Yu Y, Peng S, Li Q. A meta‐analysis of bone‐patellar tendon‐bone autograft versus four‐strand hamstring tendon autograft for anterior cruciate ligament reconstruction. Knee, 2015, 22: 100–110. [DOI] [PubMed] [Google Scholar]
- 9. Poehling‐Monaghan KL, Salem H, Ross KE, et al Long‐term outcomes in anterior cruciate ligament reconstruction: a systematic review of patellar tendon versus hamstring autografts. Orthop J Sports Med, 2017, 5: 2325967117709735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mohtadi NG, Chan DS, Dainty KN, Whelan DB. Patellar tendon versus hamstring tendon autograft for anterior cruciate ligament rupture in adults. Cochrane Database Syst Rev, 2011: CD005960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Prodromos C, Joyce B, Shi K. A meta‐analysis of stability of autografts compared to allografts after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc, 2007, 15: 851–856. [DOI] [PubMed] [Google Scholar]
- 12. Mariscalco MW, Magnussen RA, Mehta D, Hewett TE, Flanigan DC, Kaeding CC. Autograft versus nonirradiated allograft tissue for anterior cruciate ligament reconstruction: a systematic review. Am J Sports Med, 2014, 42: 492–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non‐randomized studies (minors): development and validation of a new instrument. ANZ J Surg, 2003, 73: 712–716. [DOI] [PubMed] [Google Scholar]
- 14. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials, 1986, 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 15. Nau T, Lavoie P, Duval N. A new generation of artificial ligaments in reconstruction of the anterior cruciate ligament. Two‐year follow‐up of a randomised trial. J Bone Joint Surg Br, 2002, 84: 356–360. [DOI] [PubMed] [Google Scholar]
- 16. Fan Q, Fan J. Comparison between four‐strand semitendinosus tendon autograft and ligament advanced reinforcement system for anterior cruciate ligament reconstruction by arthroscopy. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi, 2008, 22: 676–679. [PubMed] [Google Scholar]
- 17. Li B, Wen Y, Wu H, Qian Q, Wu Y, Lin X. Arthroscopic single‐bundle posterior cruciate ligament reconstruction: retrospective review of hamstring tendon graft versus LARS artificial ligament. Int Orthop, 2009, 33: 991–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu ZT, Zhang XL, Jiang Y, Zeng BF. Four‐strand hamstring tendon autograft versus LARS artificial ligament for anterior cruciate ligament reconstruction. Int Orthop, 2010, 34: 45–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li Y, Zhang W, Wu Y, et al A comparison of effectiveness between ligament advanced reinforcement system and bone‐patellar tendon‐bone autograft for anterior cruciate ligament reconstruction. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi, 2012, 26: 1045–1050. [PubMed] [Google Scholar]
- 20. Pan X, Wen H, Wang L, Ge T. Bone‐patellar tendon‐bone autograft versus LARS artificial ligament for anterior cruciate ligament reconstruction. Eur J Orthop Surg Traumatol, 2013, 23: 819–823. [DOI] [PubMed] [Google Scholar]
- 21. Xu X, Huang T, Liu Z, et al Hamstring tendon autograft versus LARS artificial ligament for arthroscopic posterior cruciate ligament reconstruction in a long‐term follow‐up. Arch Orthop Trauma Surg, 2014, 134: 1753–1759. [DOI] [PubMed] [Google Scholar]
- 22. Chen T, Zhang P, Chen J, Hua Y, Chen S. Long‐term outcomes of anterior cruciate ligament reconstruction using either synthetics with remnant preservation or hamstring autografts: a 10‐year longitudinal study. Am J Sports Med, 2017, 45: 2739–2750. [DOI] [PubMed] [Google Scholar]
- 23. Satora W, Krolikowska A, Czamara A, Reichert P. Synthetic grafts in the treatment of ruptured anterior cruciate ligament of the knee joint. Polim Med, 2017, 47: 55–59. [DOI] [PubMed] [Google Scholar]
- 24. Huang JM, Wang Q, Shen F, Wang ZM, Kang YF. Cruciate ligament reconstruction using LARS artificial ligament under arthroscopy: 81 cases report. Chin Med J, 2010, 123: 160–164. [PubMed] [Google Scholar]
- 25. Trieb K, Blahovec H, Brand G, Sabeti M, Dominkus M, Kotz R. In vivo and in vitro cellular ingrowth into a new generation of artificial ligaments. Eur Surg Res, 2004, 36: 148–151. [DOI] [PubMed] [Google Scholar]
- 26. Wang CL, Hsiao CK, Hsu AT, Dung CZ, Chang CH. Biocompatibility and mechanical property of lars artificial ligament with tissue ingrowth. J Mech Med Biol, 2012, 12: 1250012. [Google Scholar]
- 27. Yu SB, Yang RH, Zuo ZN, Dong QR. Histological characteristics and ultrastructure of polyethylene terephthalate LARS ligament after the reconstruction of anterior cruciate ligament in rabbits. Int J Clin Exp Med, 2014, 7: 2511–2518. [PMC free article] [PubMed] [Google Scholar]
- 28. Marumo K, Saito M, Yamagishi T, Fujii K. The "ligamentization" process in human anterior cruciate ligament reconstruction with autogenous patellar and hamstring tendons: a biochemical study. Am J Sports Med, 2005, 33: 1166–1173. [DOI] [PubMed] [Google Scholar]
- 29. Meller R, Brandes G, Drogemuller C, et al Graft remodeling during growth following anterior cruciate ligament reconstruction in skeletally immature sheep. Arch Orthop Trauma Surg, 2009, 129: 1037–1046. [DOI] [PubMed] [Google Scholar]
- 30. Huang JM, Liu HY, Chen FR, et al Characteristics of bone tunnel changes after anterior cruciate ligament reconstruction using ligament advanced reinforcement system artificial ligament. Chin Med J, 2012, 125: 3961–3965. [PubMed] [Google Scholar]
- 31. Newman SD, Atkinson HD, Willis‐Owen CA. Anterior cruciate ligament reconstruction with the ligament augmentation and reconstruction system: a systematic review. Int Orthop, 2013, 37: 321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Batty LM, Norsworthy CJ, Lash NJ, Wasiak J, Richmond AK, Feller JA. Synthetic devices for reconstructive surgery of the cruciate ligaments: a systematic review. Art Ther, 2015, 31: 957–968. [DOI] [PubMed] [Google Scholar]
- 33. Machotka Z, Scarborough I, Duncan W, Kumar S, Perraton L. Anterior cruciate ligament repair with LARS (ligament advanced reinforcement system): a systematic review. Sports Med Arthrosc Rehabil Ther Technol, 2010, 2: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smith C, Ajuied A, Wong F, Norris M, Back D, Davies A. The use of the ligament augmentation and reconstruction system (LARS) for posterior cruciate reconstruction. Art Ther, 2014, 30: 111–120. [DOI] [PubMed] [Google Scholar]


