Abstract
Timothy J Key and colleagues describe the evidence linking diet and nutrition to cancer risk, concluding that obesity and alcohol are the most important factors
Scientists have suspected for decades that nutrition has an important influence on the risk of developing cancer. Epidemiological studies as early as the 1960s showed that cancer rates varied widely between populations and that cancer rates in migrants moving from low to high risk countries could rise to equal or sometimes exceed the rates in the host population.1 2 These observations implied the existence of important environmental causes of cancer, and other studies showed strong correlations between many types of cancer and dietary factors; for example, countries with high intakes of meat had high rates of colorectal cancer.3 Furthermore, experiments in animals showed that cancer rates could be altered by manipulating diet, with compelling evidence that restricting energy intake causes a general reduction in cancer development.4 5
Cancer is predicted to be the leading cause of death in every country of the world by the end of this century.6 Although dietary factors are thought to be important in determining the risk of developing cancer, establishing the exact effects of diet on cancer risk has proved challenging. Here we describe the relatively few dietary factors that clearly influence risk of cancers along the digestive tract (from top to bottom) and of other common types of cancer,7 8 as well as challenges for future research.
Cancers of the oral cavity and pharynx
Nasopharyngeal cancer is common in a few populations around the globe, such as the Cantonese population in southern China and some indigenous populations of South East Asia, the Arctic, north Africa, and the Middle East.9 Consumption of foods preserved with salt has been linked with this cancer, and the mechanism might be through nitrosamine formation or reactivation of the Epstein-Barr virus.10 Based on case-control studies, Chinese style salted fish has been classified as a carcinogen by the International Agency for Research on Cancer (IARC), part of the World Health Organization.10
For oral and pharyngeal cancers overall, eating more fruits, vegetables, and related micronutrients such as vitamin C and folate is associated with lower cancer risk (boxes 1 and 2). These associations, however, might be influenced by residual confounding by smoking (a major non-dietary risk factor7 14) and alcohol consumption, so the evidence is only suggestive of a protective effect.8 14
Box 1. Are fruit and vegetables important determinants of cancer risk—and what about vegetarians?
Early case-control studies indicated that higher intakes of fruit and vegetables were associated with a lower risk of several types of cancer.11 But subsequent prospective studies, which are not affected by recall or selection bias, produced much weaker findings. In the 2018 World Cancer Research Fund report neither fruits nor vegetables were considered to be convincingly or probably associated with the risk of any cancer.8 There was suggestive evidence for protection of some cancers, and risk might increase at very low intakes. Specific components of certain fruits and vegetables might have a protective action.
Vegetarians eat no meat or fish and usually eat more fruit and vegetables than comparable non-vegetarians. The risk of all cancer sites combined might be slightly lower in vegetarians and vegans than in non-vegetarians, but findings for individual cancers are inconclusive. 12
Box 2. Do vitamins and minerals reduce cancer risk?
By definition, deficiencies of vitamins and essential minerals cause ill health; this might include increased susceptibility to some types of cancer, but establishing the details of any such effects has proved difficult. High dose vitamin or mineral supplements have not reduced cancer risk in well nourished populations and might increase risk; for example, high dose β carotene might increase the risk of lung cancer.13 Vitamin and mineral supplements should not be used for cancer prevention, although they can be important for other aspects of health, such as folic acid supplements for women before conception.
Oesophageal cancer
There are two types of oesophageal cancer: squamous cell carcinoma and adenocarcinoma. The squamous form predominates in most of the world, whereas adenocarcinoma is relatively common only in Western countries, where rates have recently increased. Obesity is an established risk factor for adenocarcinoma, probably partly owing to reflux of stomach contents into the oesophagus.15 16 Alcohol increases the risk of squamous cell carcinoma but not of adenocarcinoma.17 Smoking increases the risk of both types, with a larger effect for squamous cell carcinoma.17
Oesophageal cancer incidence rates are very high in parts of eastern and southern Africa, Linzhou (China), and Golestan (Iran).6 17 People in high risk populations have often consumed a restricted diet, low in fruit, vegetables, and animal products, so deficiencies of micronutrients have been postulated to explain the high risk (boxes 1 and 2). Despite several observational studies and some randomised trials, however, the relative roles of various micronutrients are not yet clear.17 18 19 20 In Western countries early case-control studies indicated a protective role for fruit and vegetables,21 22 but more recently published prospective studies show weaker associations, which might be due to residual confounding from smoking and alcohol consumption.16
Consumption of drinks such as tea and mate when scalding hot is associated with an increased risk of oesophageal cancer,23 24 25 and drinking beverages above 65°C is classified by IARC as probably carcinogenic to humans.26
Stomach cancer
Stomach cancer is the fifth most common cancer worldwide, with the highest rates in eastern Asia.6 Eating large amounts of salted foods, such as salt preserved fish, is associated with an increased risk27; this might be caused by the salt itself or by carcinogens derived from the nitrites in many preserved foods. Salted food might increase the risk of Helicobacter pylori infection (an established cause of stomach cancer)28 and act synergistically to promote development of the disease.29 Some evidence indicates that eating large amounts of pickled vegetables increases the risk of stomach cancer because of the production of N-nitroso compounds by mould or fungi, which are sometimes present in these foods.30 31
The risk of stomach cancer might be decreased by diets high in fruit and vegetables and for people with high plasma concentrations of vitamin C (boxes 1 and 2).32 A trial in Linzhou, China, showed that supplementation with β carotene, selenium, and α tocopherol resulted in a significant reduction in stomach cancer mortality,18 and other trials have indicated enhanced regression of precancerous lesions with the use of supplements of vitamin C, β carotene, or both.33 34 Prospective studies in Japan have also shown an inverse association between stomach cancer risk and green tea consumption in women (the majority of whom are non-smokers), perhaps related to polyphenols.35 These studies indicate a protective role of antioxidant micronutrients or other antioxidant compounds, but these associations need clarification.
Colorectal cancer
Colorectal cancer is the third most common cancer in the world.6 Overweight and obesity increase risk,8 36 37 as do alcohol and smoking.7
Ecological analyses show striking positive correlations between eating meat and colorectal cancer rates.3 38 In 2015 IARC classified processed meat as carcinogenic to humans and unprocessed red meat as probably carcinogenic,39 40 partly based on a meta-analysis reporting an increase in risk of 17% for each daily 50 g increment in consumption of processed meat and 18% for each 100 g increment in consumption of red meat.41 More recent systematic reviews have reported smaller increases in risk for unprocessed red meat.8 42
The chemicals used to preserve processed meat, such as nitrates and nitrites, might increase exposure of the gut to mutagenic N-nitroso compounds.40 Both processed and unprocessed red meat also contain haem iron, which might have a cytotoxic effect in the gut and increase formation of N-nitroso compounds. Cooking meat at high temperatures can generate mutagenic heterocyclic amines and polycyclic aromatic hydrocarbons.40 Whether any of these putative mechanisms explain the association between eating red and processed meat and risk for colorectal cancer is unclear.39 40
Higher consumptions of milk and calcium are associated with a moderate reduction in risk of colorectal cancer.8 43 44 45 Calcium might be protective by forming complexes with secondary bile acids and haem in the intestinal lumen. Higher circulating concentrations of vitamin D are associated with a lower risk,46 but this might be confounded by other factors such as physical activity. Mendelian randomisation studies of genetically determined vitamin D have not supported a causal relation.47 48
In the 1970s Burkitt suggested that the low rates of colorectal cancer in parts of Africa were caused by the high consumption of dietary fibre.49 Prospective studies have shown that consuming 10 g more total dietary fibre a day is associated with an average 10% reduction in risk of colorectal cancer; further analyses suggest that cereal fibre and wholegrain cereals are protective, but not fibre from fruit or vegetables.50 51
High dietary folate intake has been associated with reduced risk of colorectal cancer, and adequate folate status maintains genomic stability,8 but high folate status might promote the growth of colorectal tumours.52 Whether folate or folic acid have any material impact on the risk of colorectal cancer is uncertain. Most randomised trials of folic acid supplementation have found no effect,53 54 and although studies of the gene for methylenetetrahydrofolate reductase have indicated that lower circulating folate is associated with a slightly lower risk, the interpretation of these genetic data is not straightforward. 55
Liver cancer
Alcohol is the main diet related risk factor for liver cancer, probably through the development of cirrhosis and alcoholic hepatitis.7 Overweight and obesity also increase risk.8 Aflatoxin, a mutagenic compound produced by the fungus Aspergillus in foods such as grains, nuts, and dried fruit when stored in hot and humid conditions, is classified as a carcinogen by IARC and is an important risk factor in some low income countries (for people with active hepatitis virus infection).56 The major non-dietary risk factor is chronic infection with hepatitis B or C viruses.
Some studies indicate an inverse association between coffee drinking and risk of liver cancer.8 Coffee might have a true protective effect because it contains many bioactive compounds,57 58 but the association might be influenced by residual confounding, as well as by reverse causation if subclinical liver disease reduces appetite for coffee.
Pancreatic cancer
Obesity increases risk of pancreatic cancer by about 20%.8 Diabetes is also associated with increased risk, and a mendelian randomisation analysis indicates that this is due to raised insulin rather than diabetes itself.59 Studies of dietary components and risk have been inconclusive.8
Lung cancer
Lung cancer is the most common cancer in the world, and heavy smoking increases risk around 40-fold.6 7 Prospective studies have indicated that diets higher in fruits and vegetables are associated with a slightly lower risk of lung cancer in smokers, but not in never smokers.60 61 The weak inverse association of fruit and vegetables with lung cancer risk in smokers might perhaps indicate some true protective effect, but it might simply be due to residual confounding by smoking (box 1). Trials that tested supplements of β carotene (and retinol in one trial) to prevent lung cancer showed an unexpected higher risk of lung cancer in participants in the intervention group.13 62
Breast cancer
Breast cancer is the second most common cancer in the world.6 Reproductive and hormonal factors are key determinants of risk.63 Obesity increases breast cancer risk in postmenopausal women, probably by increasing circulating oestrogens, which are produced by aromatase in adipose tissue.64 Most studies have shown that obesity in premenopausal women is associated with a reduction in risk, perhaps due to lower hormone levels related to an increased frequency of anovulation. 65 Alcohol increases risk by about 10% for each drink consumed daily8 66; the mechanism might involve increased oestrogens.
Much controversy has surrounded the hypothesis that a high fat intake in adulthood increases breast cancer risk. Early case-control studies supported this hypothesis, but prospective observational studies have overall been null,8 and two randomised controlled trials of a reduced fat diet were also null.67 68
Studies of other dietary factors including meat, dairy products, and fruit are generally inconclusive.8 Some recent studies have indicated an inverse association between vegetable intake and risk of oestrogen receptor negative breast cancer8 69 70 and between dietary fibre and overall risk.8 71 Isoflavones, largely from soya, have been associated with a lower risk of breast cancer in Asian populations.72 These associations are potentially important and should be investigated for causality.
Prostate cancer
Prostate cancer is the fourth most common cancer in the world.6 The only well established risk factors are age, family history, black ethnicity, and genetic factors.73 Obesity probably increases the risk for more aggressive forms of prostate cancer.8
Lycopene, primarily from tomatoes, has been associated with a reduced risk, but the data are not conclusive.8 Some studies have indicated that risk might be reduced with higher levels of other micronutrients including β carotene, vitamin D, vitamin E, and selenium, but the findings from trials and mendelian randomisation analyses are overall null or inconclusive.47 74 75 76
Isoflavones, largely from soya foods, have been associated with a reduced risk for prostate cancer in Asian men,77 and plasma concentrations of the isoflavone equol might be inversely associated with prostate cancer risk in men in Japan.78
Substantial evidence shows that prostate cancer risk is increased by high levels of the hormone insulin-like growth factor 1, which stimulates cell division, and further research is needed to determine whether dietary factors, such as animal protein, might influence prostate cancer risk by affecting production of this hormone.79
Evaluations by expert groups
Given the huge variation in diets around the world and the large number of cancers that diets can influence, how do we know which foods or diets should be avoided and which should be recommended? The World Cancer Research Fund (WCRF) and IARC have reviewed the carcinogenic risk of foods and nutrients using systematic reviews of the evidence and evaluation by expert panels. As with much nutritional research the topic is complex, but the WCRF and IARC have identified nutritional factors with convincing evidence or probable evidence of cancer risk.
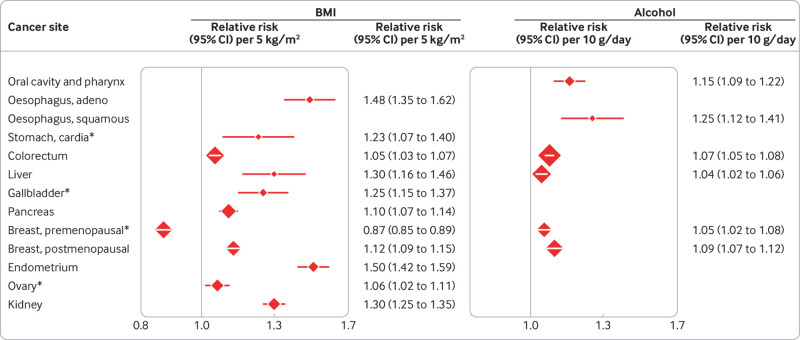
WCRF and IARC concluded that obesity and alcohol cause cancer at several sites (fig 1). For overweight and obesity, increases in risk for every 5 kg/m2 rise in body mass index (BMI) vary from 5% for colorectal cancer to 50% for cancer of the endometrium (IARC also considered the evidence to be sufficient for meningioma, thyroid cancer, and multiple myeloma).80 For alcohol, risk increases for each 10 g rise in consumption a day vary from 4% for liver cancer to 25% for squamous cell carcinoma of the oesophagus.
Fig 1.
Body mass index (BMI), alcohol, and cancer risk. Convincing associations according to the World Cancer Research Fund8 or the International Agency for Research on Cancer (marked by asterisks), or both,10 80 with relative risks from meta-analyses.8 We also consider the association between BMI and risk of breast cancer in premenopausal women to be convincing.65 RR, relative risk (plotted with squares proportional to amount of statistical information); CI, confidence interval
Processed meat was judged to be a convincing cause of cancer by both WCRF and IARC; in the most recent WCRF report the relative risk for colorectal cancer was 1.16 (1.08 to 1.26) for each 50 g/day increment.8 IARC judged Chinese-style salted fish to be a carcinogen (with a relative risk of nasopharyngeal cancer of 1.31 (1.16 to 1.47) for each additional serving per week), 8 10 as well as foods contaminated with aflatoxin.56 Neither expert body judged any dietary factor to be convincingly protective against cancer.
Uncertainty remains
WCRF and IARC judged some associations between nutritional factors and cancer risk to be “probably” causal or protective (table 1). Some researchers might think that the criteria for “probable” are not stringent enough. Further evidence might change the conclusions, and this should be kept in mind when using the reports to estimate the likely effects of diet or to make dietary recommendations. Notably, WCRF also categorised adult and young adulthood body fatness as probably protective for premenopausal breast cancer; with new evidence65 we consider this convincing, so the association is shown in figure 1 rather than table 1.
Table 1.
Still uncertain: dietary and nutritional factors that expert groups have classified as “probable” causal or protective factors for cancer
| Cancer | Probably increase risk | Probably decrease risk |
|---|---|---|
| Oral cavity and pharynx | Obesity | |
| Oesophagus | Drinking very hot beverages Mate (for squamous cell carcinoma) |
|
| Stomach | Food preserved by salting Alcohol |
|
| Colorectum | Red meat | Foods containing dietary fibre Wholegrains Dairy products Calcium supplements |
| Liver | Coffee* | |
| Prostate, aggressive disease | Obesity | |
| Endometrium | Glycaemic load | Coffee* |
| Kidney | Alcohol |
Obesity probably increases the risk of cancers of the oral cavity and pharynx and of aggressive prostate cancer. Alcohol probably increases the risk of stomach cancer but is inversely associated with the risk of kidney cancer, which might indicate a true biological effect or reflect residual confounding or bias.81 Very hot drinks probably increase the risk of cancer of the oesophagus, foods preserved by salting probably increase the risk of stomach cancer, and several dietary factors probably reduce the risk of colorectal cancer. The expert panels also concluded that the risk of endometrial cancer is probably increased by a diet with a high glycaemic load. Coffee was judged to probably be protective for liver and endometrial cancer, but some of the current authors think that this conclusion is too strong and that the data on coffee and endometrial cancer might be affected by selective publication of only part of the evidence.82
Independently from overweight and obesity, greater adult height is associated with the risk of several cancers (box 3).
Box 3. Why do taller people have a higher risk of cancer?
The risk for most types of cancer increases with height. A WCRF systematic review showed that increases in risk for each 5 cm increment in height ranged from 4% for prostate cancer to 12% for malignant melanoma.83 The mechanism is uncertain but might be related to taller people having more stem cells at risk of cancer or a factor such as insulin-like growth factor 1 having effects on both height and cancer risk.84 Undernutrition causes restricted growth, and some aspects of adequate nutrition during childhood and adolescence, such as an ample intake of energy and protein, might lead to relatively greater height and a higher overall cancer risk.83 It is not clear, however, whether better understanding of this pathway could lead to strategies for reducing cancer risk.
Acrylamide, a chemical produced during high temperature cooking and in the manufacture of many types of carbohydrate-rich foods (such as potato chips, cereal crispbreads, and coffee), is classified by IARC as probably carcinogenic to humans.85 This conclusion was based largely on studies in experimental animals; epidemiological studies have been mostly null or inconclusive86 but are limited by the difficulty of estimating long term exposure and by confounding owing to smoking. Recent research on possible mutational signatures of this chemical indicate that it might contribute to risk.87
How important is diet as a preventable cause of cancer?
Figure 2 shows recent estimates of the proportions of cancer cases in the UK attributable to modifiable risk factors, including dietary factors classified by WCRF or IARC as convincing causes of cancer. 88 Overweight and obesity is the second largest attributable cause, responsible for 6.3% of cancers in the UK, and is the largest cause in non-smokers. Alcohol (3.3%), dietary fibre (3.3%), and processed meat (1.5%) are also among the top 10 causes (although dietary fibre is currently classed by WCRF as only “probable”). Analyses from some other countries have produced broadly similar estimates; recent estimates for Brazil were 4.9% for overweight and obesity, 3.8% for alcohol, 0.8% for dietary fibre, and 0.6% for processed meat. 89 In Japan, however, where the prevalence of obesity is lower, estimates were 1.1% for overweight and obesity and 6.3% for alcohol (and 1.6% for salt). 90
Fig 2.
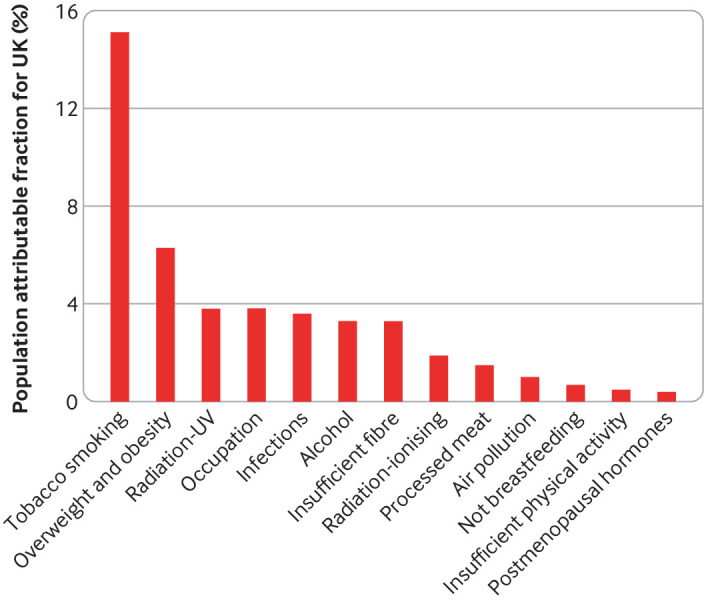
Percentages of cancer cases in the UK attributable to different exposures.88
The way forward
Research into the effects of nutrition on health is difficult. 91 We have summarised here the relatively few well established clear links between nutrition and cancer, but future research might show further important risk factors—perhaps for specific food components or for broader dietary patterns, such as so called plant based diets. To move forward, the new generation of studies needs to improve estimates of long term exposure with, for example, repeated dietary records, which are now feasible using web based questionnaires.92 Biomarkers of dietary intake and nutritional status can be used more extensively, and new biomarkers might be found through metabolomics, for example, but they will need to be validated and interpreted in the light of possible confounding and reverse causation. For some exposures, both for intake and nutritional status, mendelian randomisation will help to clarify causality,93 and randomised trials will be needed to test specific hypotheses. It will also be important to attempt to coordinate systematic analyses of all the data available worldwide, to reduce the risk of publication bias.94 For public health and policy, the top priority should be tackling the known major diet related risk factors for cancer, particularly obesity and alcohol.
Key messages.
Obesity and alcohol increase the risk of several types of cancer; these are the most important nutritional factors contributing to the total burden of cancer worldwide
For colorectal cancer, processed meat increases risk and red meat probably increases risk; dietary fibre, dairy products, and calcium probably reduce risk
Foods containing mutagens can cause cancer; certain types of salted fish cause nasopharyngeal cancer, and foods contaminated with aflatoxin cause liver cancer
Fruits and vegetables are not clearly linked to cancer risk, although very low intakes might increase the risk for aerodigestive and some other cancers
Other nutritional factors might contribute to the risk of cancer, but the evidence is currently not strong enough to be sure
Contributors and sources: All authors contributed to the first draft of the manuscript and provided critical revisions. All authors gave intellectual input to improve the manuscript and have read and approved the final version. TJK is the guarantor. The authors all have experience in nutritional epidemiology, with particular expertise in cancers of the gastrointestinal tract (KEB, RS, ST), breast cancer (TJK), prostate cancer (TJK, APC), cancer in Asia (RS, ST), and mendelian randomisation (KKT). Sources of information for this article included published systematic reviews and primary research articles based on prospective observational studies and randomised controlled trials.
Competing interests: We have read and understood BMJ policy on declaration of interests and have no relevant interests to declare.
This research was partly supported by Cancer Research UK (C8221/A19170) and the Wellcome Trust Our Planet Our Health (Livestock, Environment and People, LEAP 205212/Z/16/Z). KEB is supported by the Girdlers’ New Zealand Health Research Council Fellowship. KKT is supported by WCRF (2014/1180).
References
- 1. Haenszel W, Kurihara M. Studies of Japanese migrants. I. Mortality from cancer and other diseases among Japanese in the United States. J Natl Cancer Inst 1968;40:43-68. [PubMed] [Google Scholar]
- 2. Shimizu H, Mack TM, Ross RK, Henderson BE. Cancer of the gastrointestinal tract among Japanese and white immigrants in Los Angeles County. J Natl Cancer Inst 1987;78:223-8. [PubMed] [Google Scholar]
- 3. Armstrong B, Doll R. Environmental factors and cancer incidence and mortality in different countries, with special reference to dietary practices. Int J Cancer 1975;15:617-31. 10.1002/ijc.2910150411 [DOI] [PubMed] [Google Scholar]
- 4. Moreschi C. Beziehungen zwischen Ernährung und Tumorwachstum. Ztschr f Immunitätsforsch. 1909;2:651. [Google Scholar]
- 5. McCay CM, Pope F, Lunsford W. Experimental prolongation of the life span. Bull N Y Acad Med 1956;32:91-101. [PMC free article] [PubMed] [Google Scholar]
- 6. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 7. Swerdlow AJ, Doll RS, Peto R. Epidemiology of cancer. In: Warrell DA, Cox TM, Firth JD, eds. Oxford Textbook of Medicine. 5th ed Oxford University Press, 2010: 193-218 10.1093/med/9780199204854.003.0601 . [DOI] [Google Scholar]
- 8.World Cancer Research Fund, American Institute for Cancer Research. Diet, nutrition, physical activity and cancer: a global perspective. Continuous update project expert report 2018. https://www.wcrf.org/dietandcancer.
- 9.Chang ET, Adami HO. Nasopharyngeal cancer. In: Adami H-O, Hunter DJ, Lagiou P, Mucci L. Textbook of Cancer Epidemiology, Third edition. Eds, Oxford, UK: Oxford University Press, 2018:159-181. [Google Scholar]
- 10. Secretan B, Straif K, Baan R, et al. WHO International Agency for Research on Cancer Monograph Working Group A review of human carcinogens—Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol 2009;10:1033-4. 10.1016/S1470-2045(09)70326-2 [DOI] [PubMed] [Google Scholar]
- 11. World Cancer Research Fund/American Institute for Cancer Research Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. AICR, 2007. [Google Scholar]
- 12. Appleby PN, Key TJ. The long-term health of vegetarians and vegans. Proc Nutr Soc 2016;75:287-93. . 10.1017/S0029665115004334 [DOI] [PubMed] [Google Scholar]
- 13. Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med 1994;330:1029-35. 10.1056/NEJM199404143301501 [DOI] [PubMed] [Google Scholar]
- 14.Rider JR, Brennan P, Lagiou P. Oral and pharyngeal cancer. In: Adami H-O, Hunter DJ, Lagiou P, Mucci L, eds. Textbook of Cancer Epidemiology, 3rd ed. Oxford, UK: Oxford University Press, 2018:137-157. [Google Scholar]
- 15. Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D, Million Women Study Collaboration Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ 2007;335:1134. 10.1136/bmj.39367.495995.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vingeliene S, Chan DSM, Vieira AR, et al. An update of the WCRF/AICR systematic literature review and meta-analysis on dietary and anthropometric factors and esophageal cancer risk. Ann Oncol 2017;28:2409-19. 10.1093/annonc/mdx338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abnet CC, Nyrén O, Adami HO. Esophageal cancer. In: Adami H-O, Hunter DJ, Lagiou P, Mucci L, eds. Textbook of Cancer Epidemiology, 3rd ed. Oxford, UK: Oxford University Press, 2018:183-211. [Google Scholar]
- 18. Blot WJ, Li JY, Taylor PR, et al. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst 1993;85:1483-92. 10.1093/jnci/85.18.1483 [DOI] [PubMed] [Google Scholar]
- 19. Hashemian M, Poustchi H, Abnet CC, et al. Dietary intake of minerals and risk of esophageal squamous cell carcinoma: results from the Golestan Cohort Study. Am J Clin Nutr 2015;102:102-8. 10.3945/ajcn.115.107847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hashemian M, Murphy G, Etemadi A, et al. Toenail mineral concentration and risk of esophageal squamous cell carcinoma, results from the Golestan Cohort Study. Cancer Med 2017;6:3052-9. 10.1002/cam4.1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cheng KK, Sharp L, McKinney PA, et al. A case-control study of oesophageal adenocarcinoma in women: a preventable disease. Br J Cancer 2000;83:127-32. 10.1054/bjoc.2000.1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Terry P, Lagergren J, Hansen H, Wolk A, Nyrén O. Fruit and vegetable consumption in the prevention of oesophageal and cardia cancers. Eur J Cancer Prev 2001;10:365-9. 10.1097/00008469-200108000-00010 [DOI] [PubMed] [Google Scholar]
- 23. Lubin JH, De Stefani E, Abnet CC, et al. Maté drinking and esophageal squamous cell carcinoma in South America: pooled results from two large multicenter case-control studies. Cancer Epidemiol Biomarkers Prev 2014;23:107-16. 10.1158/1055-9965.EPI-13-0796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen Y, Tong Y, Yang C, et al. Consumption of hot beverages and foods and the risk of esophageal cancer: a meta-analysis of observational studies. BMC Cancer 2015;15:449. 10.1186/s12885-015-1185-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yu C, Tang H, Guo Y, et al. China Kadoorie Biobank Collaborative Group Hot tea consumption and its interactions with alcohol and tobacco use on the risk for esophageal cancer: A population-based cohort study. Ann Intern Med 2018;168:489-97. 10.7326/M17-2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Loomis D, Guyton KZ, Grosse Y, et al. International Agency for Research on Cancer Monograph Working Group Carcinogenicity of drinking coffee, mate, and very hot beverages. Lancet Oncol 2016;17:877-8. 10.1016/S1470-2045(16)30239-X [DOI] [PubMed] [Google Scholar]
- 27. Takachi R, Inoue M, Shimazu T, et al. Japan Public Health Center-based Prospective Study Group Consumption of sodium and salted foods in relation to cancer and cardiovascular disease: the Japan Public Health Center-based Prospective Study. Am J Clin Nutr 2010;91:456-64. 10.3945/ajcn.2009.28587 [DOI] [PubMed] [Google Scholar]
- 28. Fox JG, Dangler CA, Taylor NS, King A, Koh TJ, Wang TC. High-salt diet induces gastric epithelial hyperplasia and parietal cell loss, and enhances Helicobacter pylori colonization in C57BL/6 mice. Cancer Res 1999;59:4823-8. [PubMed] [Google Scholar]
- 29. Nozaki K, Shimizu N, Inada K, et al. Synergistic promoting effects of Helicobacter pylori infection and high-salt diet on gastric carcinogenesis in Mongolian gerbils. Jpn J Cancer Res 2002;93:1083-9. 10.1111/j.1349-7006.2002.tb01209.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tsugane S. Salt, salted food intake, and risk of gastric cancer: epidemiologic evidence. Cancer Sci 2005;96:1-6. 10.1111/j.1349-7006.2005.00006.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye W, Nyrén O, Adami HO. Stomach cancer. In: Adami H-O, Hunter DJ, Lagiou P, Mucci L, eds. Textbook of Cancer Epidemiology, 3rd ed. Oxford, UK: Oxford University Press, 2018:213-241. [Google Scholar]
- 32. Lam TK, Freedman ND, Fan JH, et al. Prediagnostic plasma vitamin C and risk of gastric adenocarcinoma and esophageal squamous cell carcinoma in a Chinese population. Am J Clin Nutr 2013;98:1289-97. 10.3945/ajcn.113.061267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Correa P, Fontham ET, Bravo JC, et al. Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-helicobacter pylori therapy. J Natl Cancer Inst 2000;92:1881-8. 10.1093/jnci/92.23.1881 [DOI] [PubMed] [Google Scholar]
- 34. Plummer M, Vivas J, Lopez G, et al. Chemoprevention of precancerous gastric lesions with antioxidant vitamin supplementation: a randomized trial in a high-risk population. J Natl Cancer Inst 2007;99:137-46. 10.1093/jnci/djk017 [DOI] [PubMed] [Google Scholar]
- 35. Inoue M, Sasazuki S, Wakai K, et al. Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan Green tea consumption and gastric cancer in Japanese: a pooled analysis of six cohort studies. Gut 2009;58:1323-32. 10.1136/gut.2008.166710 [DOI] [PubMed] [Google Scholar]
- 36. Pischon T, Lahmann PH, Boeing H, et al. Body size and risk of colon and rectal cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC). J Natl Cancer Inst 2006;98:920-31. 10.1093/jnci/djj246 [DOI] [PubMed] [Google Scholar]
- 37. Kyrgiou M, Kalliala I, Markozannes G, et al. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ 2017;356:j477. 10.1136/bmj.j477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kono S. Secular trend of colon cancer incidence and mortality in relation to fat and meat intake in Japan. Eur J Cancer Prev 2004;13:127-32. 10.1097/00008469-200404000-00006 [DOI] [PubMed] [Google Scholar]
- 39. Bouvard V, Loomis D, Guyton KZ, et al. International Agency for Research on Cancer Monograph Working Group Carcinogenicity of consumption of red and processed meat. Lancet Oncol 2015;16:1599-600. 10.1016/S1470-2045(15)00444-1 [DOI] [PubMed] [Google Scholar]
- 40. International Agency for Research on Cancer Red meat and processed meat. IARC Monographs on the Evaluation of Carcinogenic risks to Humans. Vol 114 IARC, 2018. [Google Scholar]
- 41. Chan DS, Lau R, Aune D, et al. Red and processed meat and colorectal cancer incidence: meta-analysis of prospective studies. PLoS One 2011;6:e20456. 10.1371/journal.pone.0020456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schwingshackl L, Schwedhelm C, Hoffmann G, et al. Food groups and risk of colorectal cancer. Int J Cancer 2018;142:1748-58. 10.1002/ijc.31198 [DOI] [PubMed] [Google Scholar]
- 43. Cho E, Smith-Warner SA, Spiegelman D, et al. Dairy foods, calcium, and colorectal cancer: a pooled analysis of 10 cohort studies. J Natl Cancer Inst 2004;96:1015-22. 10.1093/jnci/djh185 [DOI] [PubMed] [Google Scholar]
- 44. Murphy N, Norat T, Ferrari P, et al. Consumption of dairy products and colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC). PLoS One 2013;8:e72715. 10.1371/journal.pone.0072715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang X, Keum N, Wu K, et al. Calcium intake and colorectal cancer risk: Results from the nurses’ health study and health professionals follow-up study. Int J Cancer 2016;139:2232-42. 10.1002/ijc.30293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McCullough ML, Zoltick ES, Weinstein SJ, et al. Circulating vitamin D and colorectal cancer risk: an international pooling project of 17 cohorts. J Natl Cancer Inst 2018. 10.1093/jnci/djy087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dimitrakopoulou VI, Tsilidis KK, Haycock PC, et al. GECCO Consortium. PRACTICAL Consortium. GAME-ON Network (CORECT, DRIVE, ELLIPSE, FOCI-OCAC, TRICL-ILCCO) Circulating vitamin D concentration and risk of seven cancers: Mendelian randomisation study. BMJ 2017;359:j4761. 10.1136/bmj.j4761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. He Y, Timofeeva M, Farrington SM, et al. SUNLIGHT consortium Exploring causality in the association between circulating 25-hydroxyvitamin D and colorectal cancer risk: a large Mendelian randomisation study. BMC Med 2018;16:142. 10.1186/s12916-018-1119-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Burkitt DP. Editorial: Large-bowel cancer: an epidemiologic jigsaw puzzle. J Natl Cancer Inst 1975;54:3-6. 10.1093/jnci/54.1.3 [DOI] [PubMed] [Google Scholar]
- 50. Aune D, Chan DS, Lau R, et al. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ 2011;343:d6617. 10.1136/bmj.d6617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Reynolds A, Mann J, Cummings J, Winter N, Mete E, Te Morenga L. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet 2019;393:434-45. 10.1016/S0140-6736(18)31809-9 [DOI] [PubMed] [Google Scholar]
- 52. Kim YI. Folate: a magic bullet or a double edged sword for colorectal cancer prevention? Gut 2006;55:1387-9. 10.1136/gut.2006.095463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vollset SE, Clarke R, Lewington S, et al. B-Vitamin Treatment Trialists’ Collaboration Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet 2013;381:1029-36. 10.1016/S0140-6736(12)62001-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Oliai Araghi S, Kiefte-de Jong JC, Dijk SCV, et al. Folic acid and vitamin-B12 supplementation and the risk of cancer: long-term follow-up of the B-vitamins for the Prevention Of Osteoporotic Fractures (B-PROOF) trial. Cancer Epidemiol Biomarkers Prev 2018. 10.1158/1055-9965.EPI-17-1198. [DOI] [PubMed] [Google Scholar]
- 55. Kennedy DA, Stern SJ, Matok I, et al. Folate intake, MTHFR polymorphisms, and the risk of colorectal cancer: a systematic review and meta-analysis. J Cancer Epidemiol 2012;2012:952508. 10.1155/2012/952508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. International Agency for Research on Cancer Aflatoxins. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol 100F. IARC, 2012. [Google Scholar]
- 57. Aleksandrova K, Bamia C, Drogan D, et al. The association of coffee intake with liver cancer risk is mediated by biomarkers of inflammation and hepatocellular injury: data from the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr 2015;102:1498-508. 10.3945/ajcn.115.116095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bamia C, Stuver S, Mucci L. Cancer of the liver and biliary tract. In: Adami H-O, Hunter DJ, Lagiou P, Mucci L, eds. Textbook of Cancer Epidemiology, 3rd ed. Oxford, UK: Oxford University Press, 2018:277-307. [Google Scholar]
- 59. Carreras-Torres R, Johansson M, Gaborieau V, et al. The role of obesity, type 2 diabetes, and metabolic factors in pancreatic cancer: a mendelian randomization study. J Natl Cancer Inst 2017;109. 10.1093/jnci/djx012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vieira AR, Abar L, Vingeliene S, et al. Fruits, vegetables and lung cancer risk: a systematic review and meta-analysis. Ann Oncol 2016;27:81-96. 10.1093/annonc/mdv381 [DOI] [PubMed] [Google Scholar]
- 61. Pirie K, Peto R, Green J, Reeves GK, Beral V, Million Women Study Collaborators Lung cancer in never smokers in the UK Million Women Study. Int J Cancer 2016;139:347-54. 10.1002/ijc.30084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med 1996;334:1150-5. 10.1056/NEJM199605023341802 [DOI] [PubMed] [Google Scholar]
- 63. Collaborative Group on Hormonal Factors in Breast Cancer Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet 2002;360:187-95. 10.1016/S0140-6736(02)09454-0 [DOI] [PubMed] [Google Scholar]
- 64. Grodin JM, Siiteri PK, MacDonald PC. Source of estrogen production in postmenopausal women. J Clin Endocrinol Metab 1973;36:207-14. 10.1210/jcem-36-2-207 [DOI] [PubMed] [Google Scholar]
- 65. Schoemaker MJ, Nichols HB, Wright LB, et al. Premenopausal Breast Cancer Collaborative Group Association of body mass index and age with subsequent breast cancer risk in premenopausal women. JAMA Oncol 2018;4:e181771. 10.1001/jamaoncol.2018.1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Allen NE, Beral V, Casabonne D, et al. Million Women Study Collaborators Moderate alcohol intake and cancer incidence in women. J Natl Cancer Inst 2009;101:296-305. 10.1093/jnci/djn514 [DOI] [PubMed] [Google Scholar]
- 67. Prentice RL, Caan B, Chlebowski RT, et al. Low-fat dietary pattern and risk of invasive breast cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 2006;295:629-42. 10.1001/jama.295.6.629 [DOI] [PubMed] [Google Scholar]
- 68. Martin LJ, Li Q, Melnichouk O, et al. A randomized trial of dietary intervention for breast cancer prevention. Cancer Res 2011;71:123-33. 10.1158/0008-5472.CAN-10-1436 [DOI] [PubMed] [Google Scholar]
- 69. Farvid MS, Chen WY, Rosner BA, et al. Fruit and vegetable consumption and breast cancer incidence: Repeated measures over 30 years of follow-up. Int J Cancer 2018. 10.1002/ijc.31653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jung S, Spiegelman D, Baglietto L, et al. Fruit and vegetable intake and risk of breast cancer by hormone receptor status. J Natl Cancer Inst 2013;105:219-36. 10.1093/jnci/djs635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Key TJ, Balkwill A, Bradbury KE, et al. Foods, macronutrients and breast cancer risk in postmenopausal women: a large UK cohort. Int J Epidemiol 2019;48:489-500. 10.1093/ije/dyy238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dong JY, Qin LQ. Soy isoflavones consumption and risk of breast cancer incidence or recurrence: a meta-analysis of prospective studies. Breast Cancer Res Treat 2011;125:315-23. . 10.1007/s10549-010-1270-8 [DOI] [PubMed] [Google Scholar]
- 73. Price AJ, Key TJ. Epidemiology of prostate cancer. In: Hamdy FC, Eardley I, eds. Oxford Textbook of Urological Surgery. 1st ed Oxford University Press, 2017. [Google Scholar]
- 74. Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009;301:39-51. 10.1001/jama.2008.864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gaziano JM, Glynn RJ, Christen WG, et al. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA 2009;301:52-62. 10.1001/jama.2008.862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Heinonen OP, Albanes D, Virtamo J, et al. Prostate cancer and supplementation with alpha-tocopherol and beta-carotene: incidence and mortality in a controlled trial. J Natl Cancer Inst 1998;90:440-6. 10.1093/jnci/90.6.440 [DOI] [PubMed] [Google Scholar]
- 77. Yan L, Spitznagel EL. Soy consumption and prostate cancer risk in men: a revisit of a meta-analysis. Am J Clin Nutr 2009;89:1155-63. 10.3945/ajcn.2008.27029 [DOI] [PubMed] [Google Scholar]
- 78. Perez-Cornago A, Appleby PN, Boeing H, et al. Circulating isoflavone and lignan concentrations and prostate cancer risk: a meta-analysis of individual participant data from seven prospective studies including 2,828 cases and 5,593 controls. Int J Cancer 2018;143:2677-86. 10.1002/ijc.31640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Travis RC, Appleby PN, Martin RM, et al. A meta-analysis of individual participant data reveals an association between circulating levels of IGF-I and prostate cancer risk. Cancer Res 2016;76:2288-300. 10.1158/0008-5472.CAN-15-1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, International Agency for Research on Cancer Handbook Working Group Body fatness and cancer–viewpoint of the IARC Working Group. N Engl J Med 2016;375:794-8. 10.1056/NEJMsr1606602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bagnardi V, Rota M, Botteri E, et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer 2015;112:580-93. 10.1038/bjc.2014.579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yang TO, Crowe F, Cairns BJ, Reeves GK, Beral V. Tea and coffee and risk of endometrial cancer: cohort study and meta-analysis. Am J Clin Nutr 2015;101:570-8. 10.3945/ajcn.113.081836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.World Cancer Research Fund/American Institute for Cancer Research. Height and birthweight and the risk of cancer. Available at: https://www.wcrf.org/sites/default/files/Height-and-birthweight_0.pdf. 2018.
- 84. Giovannucci E. A growing link-what is the role of height in cancer risk? Br J Cancer 2019;120:575-6. 10.1038/s41416-018-0370-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. International Agency for Research on Cancer Acrylamide. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. IARC, 1994. [Google Scholar]
- 86. Pelucchi C, Bosetti C, Galeone C, La Vecchia C. Dietary acrylamide and cancer risk: an updated meta-analysis. Int J Cancer 2015;136:2912-22. 10.1002/ijc.29339 [DOI] [PubMed] [Google Scholar]
- 87. Zhivagui M, Ng AWT, Ardin M, et al. Experimental and pan-cancer genome analyses reveal widespread contribution of acrylamide exposure to carcinogenesis in humans. Genome Res 2019;29:521-31. 10.1101/gr.242453.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Brown KF, Rumgay H, Dunlop C, et al. The fraction of cancer attributable to modifiable risk factors in England, Wales, Scotland, Northern Ireland, and the United Kingdom in 2015. Br J Cancer 2018;118:1130-41. 10.1038/s41416-018-0029-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Rezende LFM, Lee DH, Louzada MLDC, Song M, Giovannucci E, Eluf-Neto J. Proportion of cancer cases and deaths attributable to lifestyle risk factors in Brazil. Cancer Epidemiol 2019;59:148-57. 10.1016/j.canep.2019.01.021 [DOI] [PubMed] [Google Scholar]
- 90. Inoue M, Sawada N, Matsuda T, et al. Attributable causes of cancer in Japan in 2005—systematic assessment to estimate current burden of cancer attributable to known preventable risk factors in Japan. Ann Oncol 2012;23:1362-9. 10.1093/annonc/mdr437 [DOI] [PubMed] [Google Scholar]
- 91. Mozaffarian D, Rosenberg I, Uauy R. History of modern nutrition science—implications for current research, dietary guidelines, and food policy. BMJ 2018;361:k2392. 10.1136/bmj.k2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Liu B, Young H, Crowe FL, et al. Development and evaluation of the Oxford WebQ, a low-cost, web-based method for assessment of previous 24 h dietary intakes in large-scale prospective studies. Public Health Nutr 2011;14:1998-2005. 10.1017/S1368980011000942 [DOI] [PubMed] [Google Scholar]
- 93. Pierce BL, Kraft P, Zhang C. Mendelian randomization studies of cancer risk: a literature review. Curr Epidemiol Rep 2018;5:184-96. 10.1007/s40471-018-0144-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ioannidis JPA. The challenge of reforming nutritional epidemiologic research. JAMA 2018;320:969-70. 10.1001/jama.2018.11025 [DOI] [PubMed] [Google Scholar]