Abstract
Background
Minimal residual disease (MRD) is a standard measurement for response assessment in multiple myeloma (MM). Despite new treatments, high-risk MM patients continue to have poor prognosis. We evaluated the impact of MRD- in high vs standard- risk patients.
Methods
We retrospectively evaluated all consecutive MM patients who had routine MRD testing by 1-tube 8-color advanced flow cytometry with 2,000,000 events and sensitivity level 10−5 at our center from 2015–2018 after initial therapy. Kaplan-Meier and log-rank test were used to assess survival estimates and differences between study groups.
Results
136 patients with MRD testing after initial therapy/autologous transplant (ASCT) were identified. At a median follow-up of 14 months (1–36 months), Progression-free survival (PFS) and overall survival (OS) were significantly worse in high-risk vs standard-risk patients. During the study period, 50% of high-risk group had progressed (relapse and/or death) vs 20% in standard-risk group (p=0.0006). No patients with standard-risk died, but 4 (14%) in the high-risk group did (p=0.0007). Regardless of MRD status, high-risk patients had statistically significant worse PFS than standard-risk; at median follow-up 10% standard-risk/MRD-; 20% standard-risk/MRD+; 40% high-risk/MRD-; 45% high-risk/MRD+ had either relapsed or died (p=0.0041). MRD status did not impact significantly OS in either group (p=0.0914), however longer follow up is needed to assess survival.
Conclusion
Genetic abnormalities (FISH/GEP) remain a powerful prognostic indicator for myeloma regardless of MRD status. For newly diagnosed myeloma patients treated with novel triple initial therapy and frontline ASCT, achieving MRD negative status didn’t mitigate poor prognosis outcomes of high-risk MM patients.
Keywords: multiple myeloma, newly diagnosed myeloma, minimal residual disease, high-risk multiple myeloma
Overall, patients with multiple myeloma who achieve deep remissions including minimal residual disease negativity have better clinical outcomes when compared to those do not. In this study, we aimed to describe the impact of testing for MRD negativity in a cohort of myeloma patients outside of clinical trial. We found that for most patients MRD negativity does support improved clinical outcomes. However, in our cohort of patients with high-risk myeloma, MRD negativity does not seem to impact the overall poor prognosis.
MRD negativity is associated with improved PFS and OS in multiple myeloma in patients treated on clinical trials. There is less information on MRD testing in patients outside of clinical trials and whether this may be useful clinically. In this study, we describe the clinical outcomes of 136 myeloma patients who had MRD tested outside of clinical trials as per current IMWG response criteria guidelines. In our cohort, patients with low-risk myeloma had improved outcomes if they reached MRD/flow negativity whereas patients with high-risk myeloma had overall poor outcomes regardless of MRD/flow status. MRD status can predict prognosis in patients with multiple myeloma and should be routinely tested, when available, in the clinic, as part of a routine response assessment.
INTRODUCTION
Although multiple myeloma (MM) remains, mostly, an incurable plasma cell disorder, overall survival (OS) and progression free survival (PFS) have improved considerably due to emerging new treatments (1–4). Decades ago, only a fraction of myeloma patients responded to initial therapy. Now, nearly all patients with newly diagnosed myeloma are able to achieve a first remission to treatment (1, 2, 4). In parallel to this progress, response monitoring has also been updated over time from complete remission (CR), to stringent CR (sCR) and now with CR with minimal residual disease negativity (5, 6).
Over the past decade, two meta-analysis showed that both transplant eligible or ineligible MM patients who achieved a CR had longer PFS and OS when compared to patients who did not achieve a CR (7, 8). Despite this, the majority of these patients who had achieved a CR, still relapsed later on. Similarly to other hematologic malignancies such as chronic myeloid leukemia, acute promyelocytic leukemia and acute lymphoblastic leukemia, patients with MM who are in CR and MRD negative had prolonged PFS and OS when compared to those who were in CR MRD positive (9, 10). Several studies have shown that MM patients who achieve a CR MRD negative have significantly better PFS and OS compared to those who don’t achieve MRD negativity. These patients in CR MRD positive had, in fact, clinical outcomes that were similar to those patients who only achieved a partial response (PR) or very good partial response (VGPR) (11–25).
Deep MRD monitoring in the bone marrow compartment can be successfully achieved through advanced flow cytometry and next-generation sequencing (NGS). Additionally, extramedullary disease and bone marrow focal lesions can be detected effectively with whole body positron emission tomography/ computed tomography (WB PET/CT) or magnetic resonance imaging (MRI). Although conventional multicolor flow cytometry (MFC) could evaluate and discriminate long-term survival between MRD positive and negative MM patients with high applicability as 90 – 95%, its sensitivity of 10−4 to 10−5 remained lower than that of molecular methods (Allele-specific oligonucleotide polymerase chain reaction (ASO-PCR) and NGS) (11, 20–22). Advanced flow cytometry with the appropriate antibody and fluorochrome selections can improve the sensitivity to make it comparable to that of NGS methods (26–29). Importantly, advanced flow cytometry can easily identify hemodilution, is cheaper and has rapid turn-around time. Unfortunately, it does require a fresh specimen, within 24-hour which is actually impractical, and expert dependent (28). Both the International Myeloma Working Group (IMWG) 2016 and the International Clinical Cytometry Society (ICCS) 2016 consensus guidelines recommended that, at a minimum advanced flow cytometry should evaluate at least 2 −5 × 106 total events per sample to detect aberrant plasma cells at a sensitivity of 10−5 (6, 30).
The European Myeloma Network has developed a standardized, non-expert-dependent advanced flow cytometry technique called EuroFlow (31, 32). Comparison of Euroflow and conventional 8-color MFC or advanced flow cytometry showed that advanced flow cytometry has higher sensitivity than conventional MFC and was comparable to NGS (26). Memorial Sloan Kettering Cancer Center (NYC, NY) has also developed a 10-color one tube advanced flow cytometry test that has been validated with EuroFlow showing comparable sensitivity (28, 33). MD Anderson Cancer Center (Houston, TX) has been using MFC to measure MRD in myeloma since 2014 (at a sensitivity of 10−4) and advanced flow cytometry following guidelines from the IMWG and ICCS since 2015 (at a sensitivity of 10−5) for the routine care of patients with MM. More recently, randomized clinical trials suggest that the depth of MRD negativity also correlates with improved clinical outcomes. Perrot et al evaluated MRD sensitivity in transplant eligible newly diagnosed MM patients treated with bortezomib, lenalidomide and dexamethasone. In this study, they demonstrated that an MRD level of 10−6 was predictive of superior PFS when compared to 10−5 or 10−4 (34). In this study, we set out to describe the clinical outcomes and practice patterns of all consecutive newly diagnosed myeloma (NDMM) patients who had MRD evaluation according to current MRD consensus guidelines at MD Anderson Cancer Center. Additionally, we wanted to describe the implications of reaching MRD negativity in high risk versus standard risk myeloma.
METHODS
Patients and treatment
We retrospectively reviewed electronic medical records of all consecutive patients who received standard of care MRD testing from January 2015 to April 2018. The aim of the study was to describe the treatment and practice patterns both in high risk and standard risk NDMM who had MRD testing done as part of standard of care prognosis and disease monitoring. All patients were tested by advanced flow cytometry including at least 2 × 106 total events (sensitivity 10−5) and had MRD testing done at least once either after initial therapy (if patient elected not to proceed with upfront autologous stem cell transplant (ASCT)) or within 6 months after ASCT to evaluate the impact of MRD status in high- versus standard- risk patients. High risk myeloma patients were defined as having positive FISH or cytogenetics for amplification 1q ≥ 4 copies and/or t(4;14) and/or t(14;16) and/or deletion 17p and/or high risk gene expression profiling (GEP70, Quest Diagnostics, Secaucus, NJ).
For all patients, initial therapy after diagnosis was either received at MD Anderson Cancer Center or in community centers. Treatment regimens varied according to preference at each center. This study was approved by the MD Anderson Cancer Center Institutional Research Ethics committee.
Myeloma response assessment
Our center routinely evaluates serum protein electrophoresis, immunofixation, serum free light chains and ratio, and serum immunoglobulin levels at diagnosis at every cycle during initial therapy, at 3 months after ASCT, and monthly during the maintenance phase. Response and MRD were assessed by IMWG 2016 consensus criteria (6). NDMM patients who achieved at least a PR after initial therapy for myeloma (if no transplant was done) or within 6 months after ASCT (if upfront transplant was done) and before maintenance therapy was initiated were eligible. In our center, some patients with less than a CR are tested for MRD. These are patients whose bone marrow biopsies are requested after a pre-set amount of treatment cycles, who are responding to therapy and who are believed to be close to VPGR or CR status. Given discomfort and cost of bone marrow biopsy procedures, our center errs on the side of caution and orders MRD testing in responding patients as repeat bone marrow biopsy for the sole purpose of checking MRD status may not be feasible. Patients with PR or VGPR who had a negative MRD test are labeled as “flow negative at MRD level of detection” and are not thought of as being in CR.
MRD assessment
MRD by advanced flow cytometry was initiated at MD Anderson in 2015 according to consensus guideline practices (35, 36). Bone marrow specimens were prepared with a pre-lysis technique within 24 hours after bone marrow aspiration collection. Routinely, we use an 8-color 1 tube panel, consisting of CD138, CD38, and CD45 to identify the total number of plasma cells, combined with CD19, CD27, CD81, CD59, and CD117 to identify abnormal plasma cells. Abnormal plasma cells are identified when they express two or more aberrant markers. A second tube which comprises of CD138, CD38, CD45, CD56, polyclonal cytoplasmic kappa light chain and polyclonal cytoplasmic lambda light chain is applied when the first tube cannot obtain adequate data. The total number of events included was at least 2,000,000 events to detect 20 aberrant cells with a lower limit of detection is 1 × 10−5.
Statistical analysis
Descriptive statistics including mean, standard deviation, median, and range for continuous variables such as number of aberrant cells, and frequency counts and percentages for categorical variables such as MRD status and level of risk were done. Kaplan-Meier method was used to estimate the time-to-event endpoints including PFS and OS. PFS was calculated from the date of MRD testing until disease progression or death. OS was calculated from the date of MRD testing to the date of death. The log-rank test was used to evaluate the difference in the time-to-event endpoints between/among patient groups. Statistical software SAS 9.4 (SAS, Cary, NC) and S-Plus 8.2 (TIBCO Software Inc., Palo Alto, CA) were used for the analyses. Multivariate analysis was not performed due to the limited number of events. Cox proportional hazards model was fitted for covariate R-ISS.
RESULTS
Patients
A total 960 MRD flow cases were done at MD Anderson for MM patients from January 2015 until April 2018. Of these, only 370 cases tested a minimum of 2 million total events. From these 370 MRD cases, 152 cases were excluded because they did not meet eligibility criteria (Figure 1). From the remaining 218 cases, 170 cases were tested in NDMM, 2 cases were tested in patients when they were NDMM and then again later on as they relapsed from initial treatment (RRMM) and 46 cases were tested in RRMM only. From these 172 cases, only 136 were tested in patients who were NDMM and had at least a PR after initial therapy (if patient elected not to proceed with upfront ASCT) or within 6 months after ASCT.
Figure 1:
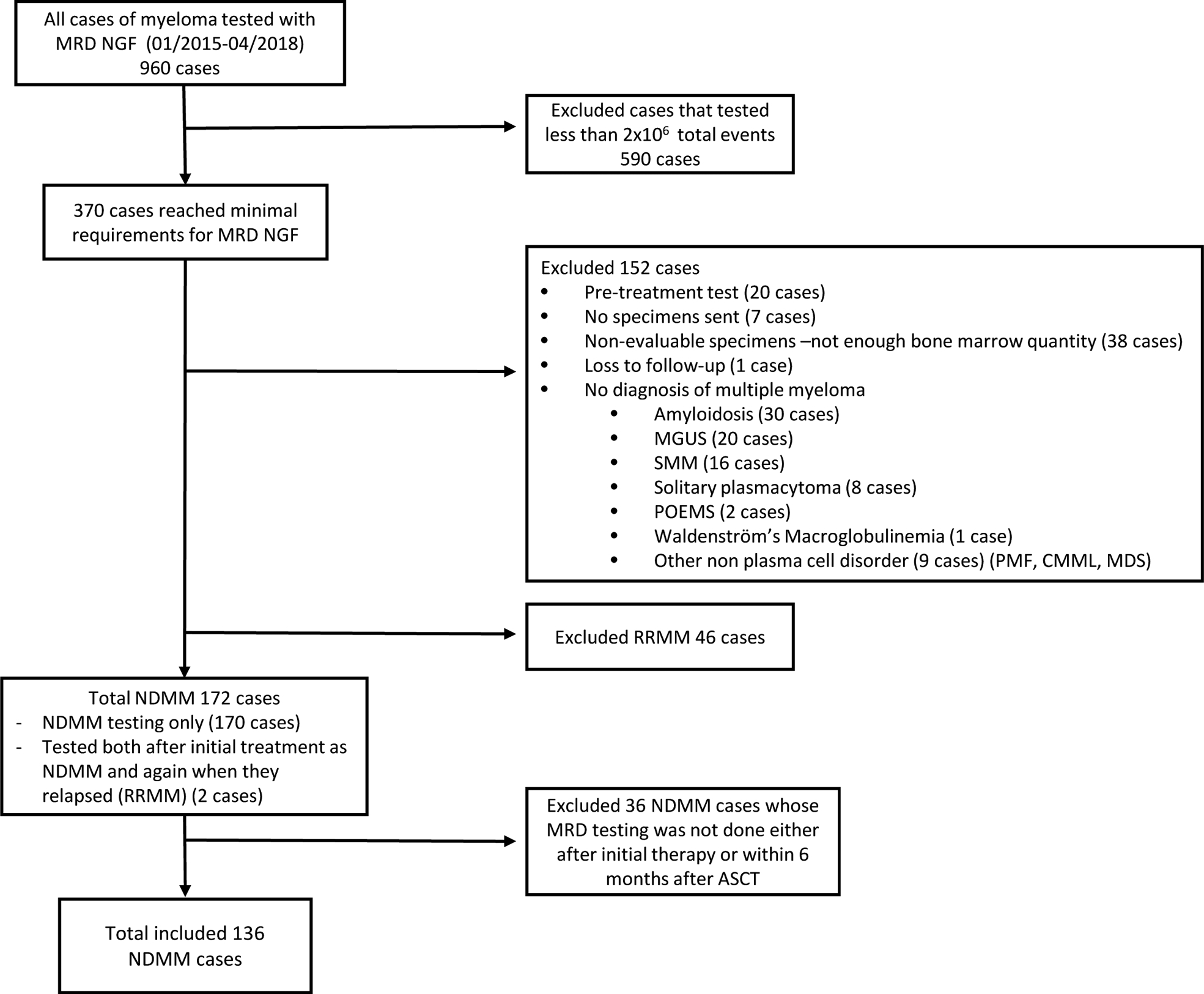
Trial flow.
Patients’ baseline characteristics and treatments are described in Table 1. Median age for all patients was 61 years old (range, 28–83) and 33% of patients were older than 65 year-old. 127 patients (93%) received initial therapy with at least 3 agents and 133 patients (98%) were treated with novel agents. The most frequently used initial therapy regiments were carfilzomib, lenalidomide or cyclophosphamide and dexamethasone (n=63, 46.3%) and bortezomib, lenalidomide/thalidomide or cyclophosphamide and dexamethasone (n=60, 44%). 29 patients (22%) had high-risk myeloma. Ninety percent of patients had an upfront ASCT. All patients for whom maintenance information was available (n=107, 78%) received continued therapy after initial treatment or ASCT and the majority of them received lenalidomide maintenance (n=62, 58%). Twenty out of 29 high-risk patients had maintenance information. Seventy-five percent of patients (15/20 patients) received three-drug combination (five with carfilzomib/ lenalidomide/dexamethasone, five with elotuzumab/lenalidomide/dexamethasone, three with ixazomib/ lenalidomide/dexamethasone, one with carfilzomib/pomalidomide/dexamethasone, and one with bortezomib/ lenalidomide/dexamethasone) as maintenance therapy and the other five patients received either lenalidomide alone (n=4) or pomalidomide/dexamethasone (n=1). The majority of patients in our center underwent upfront ASCT (n=123, 90%) with the oldest patient having an upfront ASCT at 83 years of age. The median age of upfront ASCT versus non-(or delayed) ASCT was 61 (range, 28 – 83) vs 58 (range, 32 – 75), respectively (p= 0.185).
Table 1:
Baseline characteristics, transplant status and treatments received.
| All patients n=136/100% | MRD negative or flow negative at MRD level post initial therapy or ASCT; n=72 /53% | MRD positive post initial therapy or ASCT; n=64 /47% | p-value (comparing MRDpos and MRDneg) | |
|---|---|---|---|---|
| Age, median (range) | 61 (28, 83) | 62 (32, 83) | 59 (28, 79) | 0.233 |
| -Female | 54 (39.71) | 27 (37.5) | 27 (42.2) | |
| -Plasma cell leukemia | 2 (1.5) | 1 (1.4) | 1 (1.6) | |
| -IgG kappa and IgA kappa | 1 (0.7) | 1 (1.4) | 0 | |
| High risk GEP70 (n=5) | 4 (80) | 2 (100) | 2 (66.7) | 0.414 |
| -3 | 27 (23.7) | 14 (24.1) | 14 (23.2) | |
| - 3 | 16 (14.7) | 9 (15.5) | 7 (13.7) | |
| -mCBAD | 2 (1.5) | 1 (1.4) | 1 (1.6) | |
| ASCT | 123 (90.4) | 65 (90.3) | 58 (90.6) | 0.9452 |
| -Thalidomide/D | 1 (0.9) | 0 | 1 (1.9) |
Abbreviations: Amp, amplification; D, dexamethasone; FRD, panobinostat/lenalidomide/dexamethasone; GEP70, gene expression profiling done at baseline (Quest Diagnostics, Secaucus, NJ; Ig, immunoglobulin; KCD, carfilzomib/cyclophosphamide/dexamethasone; KRD, carfilzomib/lenalidomide/dexamethasone; KPD, carfilzomib/pomalidomide/dexamethasone; Len, lenalidomide; mCBAD, modified Cyclophosphamide, Bortezomib, Doxorubicin, and Dexamethasone; MGUS, monoclonal gammopathy undetermined significance; MM, multiple myeloma; MPT, melphalan/prednisolone/thalidomide; n, number; RD, lenalidomide/dexamethasone; R-ISS, Revised Multiple Myeloma International Staging System; SMM, Smoldering multiple myeloma; VCD, bortezomib/cyclophosphamide/dexamethasone; VD, bortezomib/dexamethasone ; VRD, bortezomib/lenalidomide/dexamethasone; VTD, bortezomib/thalidomide/dexamethasone.
High risk genetics were defined as presence of at least one of the following abnormalities: t(4,14), t(14,16), deletion 17p, amplification 1q ≥ 4 copies or high risk GEP70.
Risk stratification data were available for 129 patients for deletion 17p and t(4;14), 117 patients for amplification of 1q and 5 patients for GEP70.
Maintenance information was available for 107 patients.
Minimal residual disease status
A total of 72 patients (53%) achieved MRD negativity or were flow negative at MRD level after initial therapy with or without upfront ASCT. In particular, 31 out of 69 (44.93%) NDMM patients achieved MRD negativity post initial treatment without upfront ASCT; whereas, 52 out of 82 (63.4%) NDMM patients achieved MRD negativity within 6 months post upfront ASCT. 13 patients had MRD testing done after initial therapy/before upfront ASCT and after ASCT. Among these 13 patients, 4 patients had persistent MRD positivity, 6 patients had MRD turn negative from positive and 3 patients had persistent MRD negativity after upfront ASCT when compared to measurements before ASCT. In our study, only 8% of patients who were in CR (sCR+CR) were MRD positive, whereas 83% of patients who had a PR were flow positive at MRD level of detection (p<0.001) (Table 2). In the high-risk patient group, 50% of patients in CR where MRD negative (n=7/15) whereas only 38% of those not in CR (PR/VGPR) were flow negative at MRD level (n=4/12) (Table S1).
Table 2.
MRD status by IMWG response assessment.
| MRD negative (n=72) | MRD positive (n=61) | p-value | |
|---|---|---|---|
| PR* (n=30), n(%) | 5 (17) | 25 (83) |
Patients with either VGPR or PR are considered to be flow bone marrow aspirate negative at MRD level of detection rather than MRD negative.
Minimal residual disease status and genetic risk
Genetic data was available for 129 patients, 100 (78%) of whom were standard risk and 29 (22%) were high risk MM. 54 (54%, n=54/100) patients with standard risk myeloma and 12 (41%, n=12/29 patients) with high risk myeloma were MRD negative after initial therapy with or without ASCT (p=0.233). In detail, 8 out of 14 patients (57.1%), 4 out of 11 patients (36.4%), 1 out of 2 patients (50%), and 2 out of 4 patients (50%) with monosomy 17, t(4;14), amplification 1q ≥ 4 copies, and high GEP70 score reached MRD negativity, respectively. The rate at which standard or high-risk myeloma achieved MRD negativity or were bone marrow flow negative at MRD level of detection was not statistically different (p=0.23) in these series of 29 high risk myeloma patients (Table 1).
Survival outcomes
For all patients (n=136), at a median follow-up of 14 months (1–36 months), median PFS and OS were not reached (Figure 2 and 3). One- and two-year PFS were 85.3% (95% CI= 79.4% – 92.7%) and 73.3% (95% CI=59.7% – 90%) (Figure 2). Of the 136 patients, 19 patients relapsed. Of these 19 patients, 10 out of 29 (35.5%) were high risk patients and 9 out of 100 (9%) patients were low risk, p=0.001.
Figure 2:
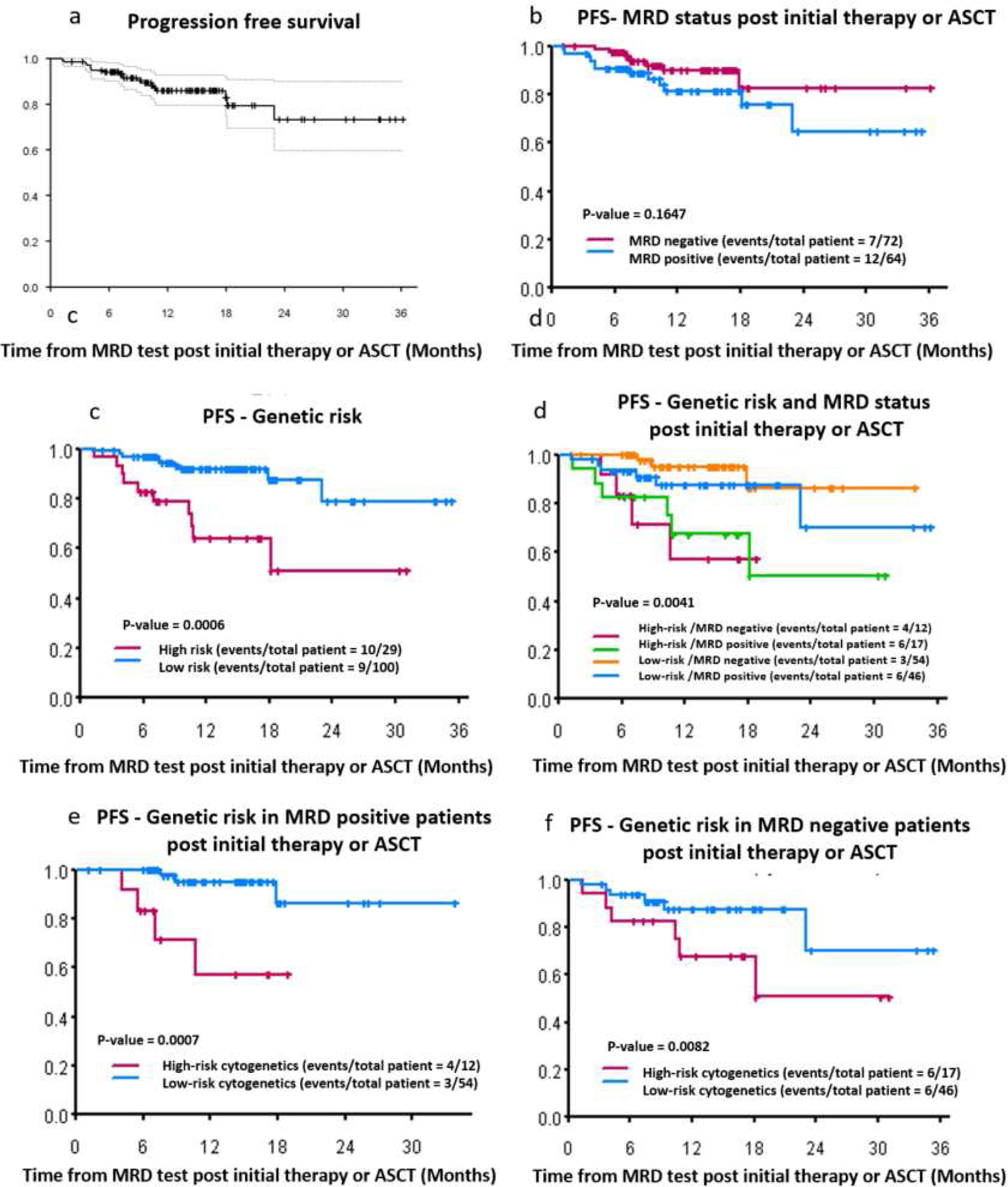
Clinical outcomes (all patients n=136). (a) Progression free survival for all patients (n=136), (b) PFS by MRD status post initial therapy or ASCT, (c) PFS by high versus low/standard risk myeloma patients, (d) PFS according to genetic risk and MRD status, (e) PFS according to genetic risk in MRD positive patients, (f) PFS according to genetic risk in MRD negative patients
Figure 3:
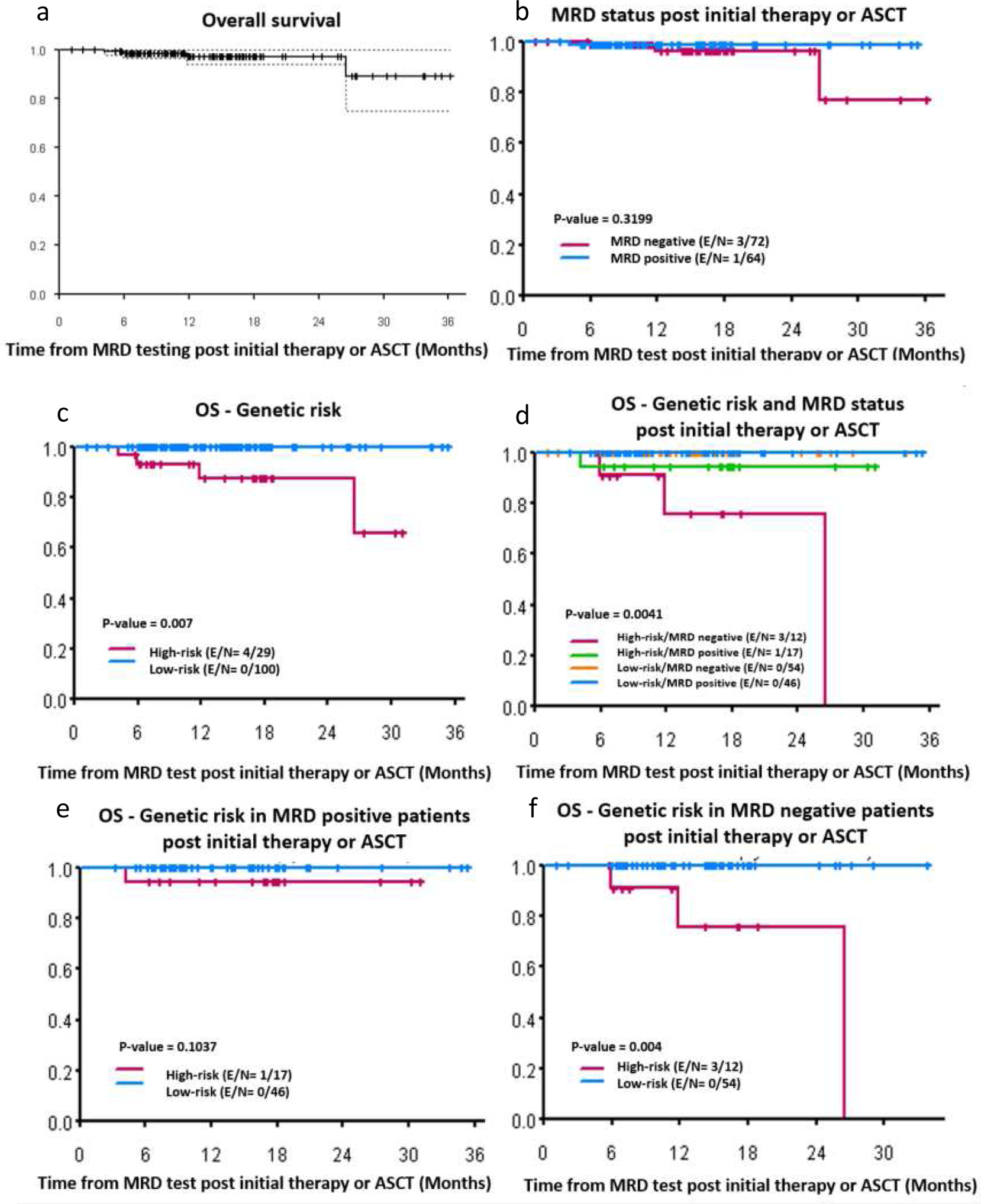
Clinical outcomes. (a) Overall survival for all patients (n=136), (b) OS by MRD status post initial therapy or ASCT, (c) OS by high versus low/standard risk myeloma patients, (d) OS according to genetic risk and MRD status, (e) OS according to genetic risk in MRD positive patients, (f) OS according to genetic risk in MRD negative patients
According to MRD status, 7 out of 72 MRD negative patients and 12 out of 64 MRD/flowpositive patients experienced disease relapse. Patients who were MRD/flow negativeand MRD/flow positive had 1- and 2- year PFS at 90% (95% CI= 82% – 98%) versus 81% (95% CI= 71% - 93%) and 83% (95% CI= 69% – 99%) versus 65% (95% CI= 45% - 93%), respectively (p=0.1647). Low risk patients had significantly better PFS than high risk patients. 92% (95% CI= 86% – 98%) versus 64% (95% CI= 47% – 87%) and 79% (95% CI= 62% – 100%) versus 51% (95% CI= 30% – 87%) of standard- and high-risk patients survived without disease progression or death at 1 and 2 year, respectively (p=0.0006). Interestingly, at a sensitivity of 10−5, high risk patients showed significantly worse PFS and OS, regardless of MRD status (Figure 2 and 3). At the median follow up of 14 months, 10% standard-risk/MRD negative; 20% standard-risk/MRD positive; 40% high-risk/MRD negative 45% high-risk/MRD positive had either relapsed or died (p=0.0041). Median OS for high-risk patients (either those who were CR/MRD negative or non-CR/flow negative) was not reached (p=0.175). Median PFS for high-risk patients in CR and MRD negative was not reached, however the median PFS for high-risk patients who were not in CR and flow negative was 23 months (p=0.495). (Figure S1 and S2). No statistical significant differences were observed in PFS or OS in patients with high-risk myeloma by MRD/flow or CR status (Figures S3–S8).
In our cohort, 4 patients died during this follow-up time and all of them had high-risk MM. Three of them had t(4;14) and R-ISS stage III and one of them had deletion17p (Figure 3). The hazard ratio for progression or death in 107 patients with available data for R-ISS stage was: 12.65 for R-ISS 2 versus 1 (95% CI: 1.57 ~ 101.85) and 32.25 (95% CI: 3.54 ~ 293.49) for R-ISS 3 versus 1 (p=0.007).
DISCUSSION
In this single center, retrospective study we aimed to evaluate the relationship between MRD status after initial therapy in NDMM with clinical outcomes in consecutively treated patients outside of a clinical trial. We had previously reported our experience using MFC at a sensitivity of 10−4 in a total of 95 patients to report MRD outcomes (37). Here, we report on a larger series of patients using advanced flow cytometry with a higher sensitivity as established by IMWG and ICCS MRD guidelines.
The MRD rate in this cohort of patients was 53% after either initial therapy or ASCT. Most of our patients received initial therapy with either VRD (n=43, 32%) or KRd (n=60, 44%). The Intergroupe Francophone du Myelome (IFM) 2009 study comparing VRD with delayed versus upfront ASCT in NDMM patients reported MRD negative rates of 65% versus 79%, respectively (38). Lower MRD negative rates in our patient cohort can be explained by a shorter duration of initial therapy (especially in the cohort of patients that had MRD tested after initial therapy which is usually given for 2–4 months in our center and without undergoing transplantation). In addition, almost a half of this cohort used KRd as first-line treatment. Some of us, had reported a higher MRD negative rate of 92% in 45 KRd-treated NDMM patients after 8 months of therapy (24). However, cross comparisons are difficult since most of our patients receive 2–4 months of initial KRd therapy instead of 8 months. In the future, to increase the rates of MRD negativity in our patient population and, perhaps to improve clinical outcomes, one could consider prolonging the time of initial therapy before transplant to 4–6 months or adding a fourth agent (i.e. CD38 monoclonal antibody). This is in line with other reports suggesting that patients with NDMM treated with KRd take, on average, 6 months to reach MRD negativity (39). Gu et al using advanced flow cytometry at 10−5 sensitivity in 104 NDMM patients showed an MRD negativity rate of 36.5% which is a little lower than the one reported in this study (17).
The median PFS was not reached both for MRD negative and positive patients. The 1-and 3- year PFS tended to be better in MRD negative patients, 90% versus 81% and 83% versus 65% (p=0.1647). This difference in PFS between MRD negative and positive patients may be non-significant because of the short follow-up time, the low incidence of relapsed disease or the depth of MRD detection level used in this study of 10−5 instead of the deeper 10−6 level sensitivity level. Other studies have shown that MRD status does impact PFS (9, 10, 17, 24, 40). In contrast, the impact of MRD status on survival in high-risk NDMM patients remains more controversial. Our study continues to show that patients with high risk myeloma have a much higher rate of early relapse or death than low risk myeloma, even when treated with initial novel triple therapy, including KRd, as 35% (10/29 patients) of high risk versus 9% (9/100 patients) (p=0.001) of low risk patients relapsed or died during follow up. This was true even for patients who had high risk myeloma and were MRD negative. The 1-year PFS rate was 90%, 80%, 60%, and 55% in standard-risk/MRD negative, standard-risk/MRD positive, high-risk/MRD negative, and high-risk/MRD positive, respectively (p=0.0041). Detailed analysis performed on our cohort of high-risk patients by either CR or MRD/flow status did not show any statistically significant differences in PFS or OS between CR/non-CR patients or MRD/flow negative or positive patients. This may be due to the small number of patients in the high-risk cohort (n=29), short follow up and low number of progression/death events. Larger cohorts, longer follow up and higher levels of sensitivity of MRD test (i.e. 10−6) may be needed to show a difference in PFS/OS in high-risk myeloma patients who are in CR/non-CR and reach MRD/flow negativity status.
Deletion (17p), t(4; 14) and t(14; 16) are genetic abnormalities that are well known to correlate with worse outcomes in myeloma and are widely regarded as high risk according to the IMWG classification. Our study also included amplification of 1q in 4 or more copies and high risk score GEP70 because they are linked to poor outcomes (41–44). Our rates of MRD negativity in high versus low risk myeloma- 41.4% vs 54%, respectively (p=0.233)- are similar to those reported by others (23, 25, 45). Similar to our findings, others have reported that despite reaching MRD negativity, high-risk myeloma continues to be an independent factor for poor prognosis (14, 46). In contrast, four other studies using advanced flow cytometry at 10−5 (47) or NGS with a sensitivity of 10−6 (23, 25, 45) have shown that high risk myeloma patients who achieve MRD negativity have similar PFS and OS to those in the low risk group. More studies are needed in particular in high risk myeloma to evaluate the impact of MRD on risk status including the sensitivity threshold for MRD testing in this particular group. It has been reported that only around 10% of patients with deletion 17p could become MRD negative (23, 25, 45) which was different from the results on our study were deletion 17p patients could achieve MRD negativity in 57% of patients. This difference might be because half of our patients received more intense initial regimens such as KRd.
Some of our limitations are the nature of this study being retrospective with the possibility of the introduction of bias and short median follow up of only 14 months counted from the date that MRD testing was performed. Moreover, outcomes were evaluated based on a one-time MRD assessment. Our study is valuable in reporting clinical outcomes in patients with myeloma using advanced flow cytometry MRD for prognosis and monitoring as part of a standard of care response assessment tool. It provides further evidence that, in low risk myeloma, MRD is a good predictor of outcomes, however, in high risk myeloma more studies are needed to evaluate the role of MRD status in prognosis. In the future, serial monitoring of MRD status during initial therapy, ASCT and maintenance may be more informative to predict prognosis to make treatment decisions (17).
MRD status after initial therapy determines clinical outcomes in myeloma. However, high-risk genetics and GEP remain factors that predict prognosis independently of MRD status. Deeper sensitivity, serial MRD monitoring, evaluation of myeloma biology and the use of MRD guided treatment may be helpful in the future to improve survival for all patients with multiple myeloma.
Supplementary Material
Acknowledgments
This work was supposed by a grant of the International Myeloma Foundation Black Swan Initiative, without which this work would not have been implemented. This work was also supported in part by The MD Anderson Cancer Center Support Grant (P30 CA016672), the Leukemia and Lymphoma Society Specialized Center of Research (LLS SCOR), the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, the Multiple Myeloma Research Foundation, the Perelman Family Foundation and the University of Texas MD Anderson Moon Shot Program. RZO, the Florence Maude Thomas Cancer Research Professor, would like to acknowledge support from the National Cancer Institute (R01s CA184464 and 194264, and U10 CA032102), the Leukemia & Lymphoma Society (SCOR-12206-17), the Adelson Medical Research Foundation, the Brock Family Myeloma Research Fund, and the Jean Clarke High-Risk Myeloma Research Fund. We would like to thank participating patients and their families.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This manuscript is being submitted in conjunction with presentation at the 55th Annual Meeting of the American Society of Clinical Oncology, Chicago, USA.
Declaration of Interests
C. Kunacheewa reports no conflicts of interest.
E. Manasanch has received research support from Sanofi, Quest Diagnostics, Novartis, JW Pharma, Merck; consultant fees from Takeda, Celgene, Sanofi and Adaptive Biotechnologies.
H. Lee has received consulting fees from Adaptive Biotechnologies, Celgene, Pimera and Takeda and research support from Amgen, Daiichi Sankyo, Janssen and Takeda.
D. Weber reports no conflicts of interest
S. Thomas has received consulting fees from Amgen and research support from Acerta Pharma, Amgen, Array BioPharma, Bristol-Myers-Squibb, Celgene and Idera.
B. Amini reports no conflicts of interest.
S. Srour reports no conflicts of interest.
Q. Bashir reports no conflicts of interest.
M. H. Qazilbash reports no conflicts of interest.
L. Feng reports no conflicts of interest.
P. Lin reports no conflicts of interest.
R Orlowski has received consulting fees from Amgen, Bristol-Myers-Squibb, Celgene, GSK Biologicals, Ionis Phamraceuticals, Janssen, Juno Therapeutics, Kite Pharma, Legend Biotech, Molecular Partners, Sanofi, Servier, and Takeda and research support from BioTheryX.
Reference
- 1.Corso A, Nozza A, Lazzarino M, Klersy C, Zappasodi P, Arcaini L, et al. Plateau phase in multiple myeloma: an end-point of conventional-dose chemotherapy. Haematologica. 1999;84(4):336–41. [PubMed] [Google Scholar]
- 2.Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111(5):2516–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benboubker L, Dimopoulos MA, Dispenzieri A, Catalano J, Belch AR, Cavo M, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. The New England journal of medicine. 2014;371(10):906–17. [DOI] [PubMed] [Google Scholar]
- 4.Rajan AM, Rajkumar SV. Treatment of newly diagnosed myeloma: Bortezomib-based triplet. Seminars in oncology. 2016;43(6):700–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9):1467–73. [DOI] [PubMed] [Google Scholar]
- 6.Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. The Lancet Oncology. 2016;17(8):e328–e46. [DOI] [PubMed] [Google Scholar]
- 7.van de Velde HJ, Liu X, Chen G, Cakana A, Deraedt W, Bayssas M. Complete response correlates with long-term survival and progression-free survival in high-dose therapy in multiple myeloma. Haematologica. 2007;92(10):1399–406. [DOI] [PubMed] [Google Scholar]
- 8.Gay F, Larocca A, Wijermans P, Cavallo F, Rossi D, Schaafsma R, et al. Complete response correlates with long-term progression-free and overall survival in elderly myeloma treated with novel agents: analysis of 1175 patients. Blood. 2011;117(11):3025–31. [DOI] [PubMed] [Google Scholar]
- 9.Munshi NC, Avet-Loiseau H, Rawstron AC, Owen RG, Child JA, Thakurta A, et al. Association of Minimal Residual Disease With Superior Survival Outcomes in Patients With Multiple Myeloma: A Metaanalysis. JAMA Oncol. 2017;3(1):28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landgren O, Devlin S, Boulad M, Mailankody S. Role of MRD status in relation to clinical outcomes in newly diagnosed multiple myeloma patients: a meta-analysis. Bone marrow transplantation. 2016;51(12):1565–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarasquete ME, Garcia-Sanz R, Gonzalez D, Martinez J, Mateo G, Martinez P, et al. Minimal residual disease monitoring in multiple myeloma: a comparison between allelic-specific oligonucleotide real-time quantitative polymerase chain reaction and flow cytometry. Haematologica. 2005;90(10):1365–72. [PubMed] [Google Scholar]
- 12.Paiva B, Vidriales M-B, Cerveró J, Mateo G, Pérez JJ, Montalbán MA, et al. Multiparameter flow cytometric remission is the most relevant prognostic factor for multiple myeloma patients who undergo autologous stem cell transplantation. Blood. 2008;112(10):4017–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paiva B, Gutiérrez NC, Rosiñol L, Vídriales M-B, Montalbán M-Á, Martínez-López J, et al. High-risk cytogenetics and persistent minimal residual disease by multiparameter flow cytometry predict unsustained complete response after autologous stem cell transplantation in multiple myeloma. Blood. 2012;119(3):687–91. [DOI] [PubMed] [Google Scholar]
- 14.Rawstron AC, Child JA, de Tute RM, Davies FE, Gregory WM, Bell SE, et al. Minimal residual disease assessed by multiparameter flow cytometry in multiple myeloma: impact on outcome in the Medical Research Council Myeloma IX Study. J Clin Oncol. 2013;31(20):2540–7. [DOI] [PubMed] [Google Scholar]
- 15.Lahuerta JJ, Paiva B, Vidriales MB, Cordon L, Cedena MT, Puig N, et al. Depth of Response in Multiple Myeloma: A Pooled Analysis of Three PETHEMA/GEM Clinical Trials. J Clin Oncol. 2017;35(25):2900–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu B, Thall P, Milton DR, Sasaki K, Bashir Q, Shah N, et al. High-risk myeloma and minimal residual disease postautologous-HSCT predict worse outcomes. Leuk Lymphoma. 2018:1–11. [DOI] [PubMed] [Google Scholar]
- 17.Gu J, Liu J, Chen M, Huang B, Li J. Longitudinal Flow Cytometry Identified “Minimal Residual Disease” (MRD) Evolution Patterns for Predicting the Prognosis of Patients with Transplant-Eligible Multiple Myeloma. Biol Blood Marrow Transplant. 2018;24(12):2568–74. [DOI] [PubMed] [Google Scholar]
- 18.Gupta R, Kumar L, Dahiya M, Mathur N, Harish P, Sharma A, et al. Minimal residual disease evaluation in autologous stem cell transplantation recipients with multiple myeloma. Leuk Lymphoma. 2017;58(5):1234–7. [DOI] [PubMed] [Google Scholar]
- 19.Paiva B, Chandia M, Puig N, Vidriales MB, Perez JJ, Lopez-Corral L, et al. The prognostic value of multiparameter flow cytometry minimal residual disease assessment in relapsed multiple myeloma. Haematologica. 2015;100(2):e53–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silvennoinen R, Lundan T, Kairisto V, Pelliniemi TT, Putkonen M, Anttila P, et al. Comparative analysis of minimal residual disease detection by multiparameter flow cytometry and enhanced ASO RQ-PCR in multiple myeloma. Blood cancer journal. 2014;4:e250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puig N, Sarasquete ME, Balanzategui A, Martinez J, Paiva B, Garcia H, et al. Critical evaluation of ASO RQ-PCR for minimal residual disease evaluation in multiple myeloma. A comparative analysis with flow cytometry. Leukemia. 2014;28(2):391–7. [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Lopez J, Lahuerta JJ, Pepin F, Gonzalez M, Barrio S, Ayala R, et al. Prognostic value of deep sequencing method for minimal residual disease detection in multiple myeloma. Blood. 2014;123(20):3073–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takamatsu H, Takezako N, Zheng J, Moorhead M, Carlton VEH, Kong KA, et al. Prognostic value of sequencing-based minimal residual disease detection in patients with multiple myeloma who underwent autologous stem-cell transplantation. Annals of oncology : official journal of the European Society for Medical Oncology. 2017;28(10):2503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korde N, Roschewski M, Zingone A, Kwok M, Manasanch EE, Bhutani M, et al. Treatment With Carfilzomib-Lenalidomide-Dexamethasone With Lenalidomide Extension in Patients With Smoldering or Newly Diagnosed Multiple Myeloma. JAMA oncology. 2015;1(6):746–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Avet-Loiseau H, Corre J, Lauwers-Cances V, Chretien M-L, Robillard N, Leleu X, et al. Evaluation of Minimal Residual Disease (MRD) By Next Generation Sequencing (NGS) Is Highly Predictive of Progression Free Survival in the IFM/DFCI 2009 Trial. Blood. 2015;126(23):191. [Google Scholar]
- 26.Flores-Montero J, Sanoja-Flores L, Paiva B, Puig N, Garcia-Sanchez O, Bottcher S, et al. Next Generation Flow for highly sensitive and standardized detection of minimal residual disease in multiple myeloma. Leukemia. 2017;31(10):2094–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roshal M Minimal Residual Disease Detection by Flow Cytometry in Multiple Myeloma: Why and How? Seminars in hematology. 2018;55(1):4–12. [DOI] [PubMed] [Google Scholar]
- 28.Royston DJ, Gao Q, Nguyen N, Maslak P, Dogan A, Roshal M. Single-Tube 10-Fluorochrome Analysis for Efficient Flow Cytometric Evaluation of Minimal Residual Disease in Plasma Cell Myeloma. Am J Clin Pathol. 2016;146(1):41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takamatsu H, Yoroidaka T, Fujisawa M, Kobori K, Hanawa M, Yamashita T, et al. Comparison of minimal residual disease detection in multiple myeloma by SRL 8-color single-tube and EuroFlow 8-color 2-tube multiparameter flow cytometry. Int J Hematol. 2019. [DOI] [PubMed] [Google Scholar]
- 30.Soh KT, Tario JD Jr, Wallace PK. Diagnosis of Plasma Cell Dyscrasias and Monitoring of Minimal Residual Disease by Multiparametric Flow Cytometry. Clin Lab Med. 2017;37(4):821–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rawstron AC, Orfao A, Beksac M, Bezdickova L, Brooimans RA, Bumbea H, et al. Report of the European Myeloma Network on multiparametric flow cytometry in multiple myeloma and related disorders. Haematologica. 2008;93(3):431–8. [DOI] [PubMed] [Google Scholar]
- 32.Flores-Montero J, de Tute R, Paiva B, Perez JJ, Bottcher S, Wind H, et al. Immunophenotype of normal vs. myeloma plasma cells: Toward antibody panel specifications for MRD detection in multiple myeloma. Cytometry B Clin Cytom. 2016;90(1):61–72. [DOI] [PubMed] [Google Scholar]
- 33.Roshal M, Flores-Montero JA, Gao Q, Koeber M, Wardrope J, Durie BGM, et al. MRD detection in multiple myeloma: comparison between MSKCC 10-color single-tube and EuroFlow 8-color 2-tube methods. Blood advances. 2017;1(12):728–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perrot A, Lauwers-Cances V, Corre J, Robillard N, Hulin C, Chretien ML, et al. Minimal residual disease negativity using deep sequencing is a major prognostic factor in multiple myeloma. Blood. 2018;132(23):2456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arroz M, Came N, Lin P, Chen W, Yuan C, Lagoo A, et al. Consensus guidelines on plasma cell myeloma minimal residual disease analysis and reporting. Cytometry B Clin Cytom. 2016;90(1):31–9. [DOI] [PubMed] [Google Scholar]
- 36.Stetler-Stevenson M, Paiva B, Stoolman L, Lin P, Jorgensen JL, Orfao A, et al. Consensus guidelines for myeloma minimal residual disease sample staining and data acquisition. Cytometry B Clin Cytom. 2016;90(1):26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu B, Thall P, Milton DR, Sasaki K, Bashir Q, Shah N, et al. High-risk myeloma and minimal residual disease postautologous-HSCT predict worse outcomes. Leuk Lymphoma. 2019;60(2):442–52. [DOI] [PubMed] [Google Scholar]
- 38.Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. The New England journal of medicine. 2017;376(14):1311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tageja N, Korde N, Kazandjian D, Panch S, Manasanch E, Bhutani M, et al. Combination therapy with carfilzomib, lenalidomide and dexamethasone (KRd) results in an unprecedented purity of the stem cell graft in newly diagnosed patients with myeloma. Bone Marrow Transplant. 2018;53(11):1445–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Avet-Loiseau H, Lauwers-Cances V, Corre J, Moreau P, Attal M, Munshi N. Minimal Residual Disease in Multiple Myeloma: Final Analysis of the IFM2009 Trial. Blood. 2017;130(Suppl 1):435. [Google Scholar]
- 41.Fonseca R, Van Wier SA, Chng WJ, Ketterling R, Lacy MQ, Dispenzieri A, et al. Prognostic value of chromosome 1q21 gain by fluorescent in situ hybridization and increase CKS1B expression in myeloma. Leukemia. 2006;20(11):2034–40. [DOI] [PubMed] [Google Scholar]
- 42.Yu W, Guo R, Qu X, Qiu H, Li J, Zhang R, et al. The amplification of 1q21 is an adverse prognostic factor in patients with multiple myeloma in a Chinese population. Onco Targets Ther. 2016;9:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sawyer JR, Tian E, Heuck CJ, Johann DJ, Epstein J, Swanson CM, et al. Evidence of an epigenetic origin for high-risk 1q21 copy number aberrations in multiple myeloma. Blood. 2015;125(24):3756–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Claussen CM, Lee H, Shah JJ, Richards T, Shah N, Patel K, et al. Gene Expression Profiling Predicts Clinical Outcomes in Newly Diagnosed Multiple Myeloma Patients in a Standard of Care Setting. Blood. 2016;128(22):5628. [Google Scholar]
- 45.Perrot A, Lauwers-Cances V, Corre J, Robillard N, Hulin C, Chretien ML, et al. Minimal residual disease negativity using deep sequencing is a major prognostic factor in multiple myeloma. Blood. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oliva S, Gambella M, Gilestro M, Muccio VE, Gay F, Drandi D, et al. Minimal residual disease after transplantation or lenalidomide-based consolidation in myeloma patients: a prospective analysis. Oncotarget. 2017;8(4):5924–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paiva B, Cedena MT, Puig N, Arana P, Vidriales MB, Cordon L, et al. Minimal residual disease monitoring and immune profiling in multiple myeloma in elderly patients. Blood. 2016;127(25):3165–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


