Lyme disease, which is epidemic in certain communities, primarily in the northeastern United States, is caused by the tick-borne spirochete Borrelia burgdorferi (also called Borreliella burgdorferi). When untreated, the disease usually occurs in stages with different manifestations at each stage (1). In the northeastern United States, the infection usually begins with a slowly expanding skin lesion, erythema migrans (stage 1), often accompanied by nonspecific symptoms, including headache, myalgias, arthralgias, fever, malaise, and fatigue. Within weeks (stage 2), neurologic or cardiac abnormalities may develop. Months later (stage 3), usually following a latent period, intermittent or persistent monoarticular or oligoarticular arthritis commonly develops, lasting for several years, accompanied by minimal, if any, systemic symptoms. Rarely, patients have late neurologic involvement, characterized by a subtle encephalopathy or sensory polyneuropathy. Thus, in most patients, the natural history of Lyme disease, without treatment, is one of persistent infection for several years, with latent periods and changing system involvement.
Treatment and ongoing disease manifestations
Depending on the disease manifestation, the infection can usually be treated successfully with 2–4 weeks of oral antibiotic therapy. However, arthritis, a late disease manifestation, can be more difficult to treat. We start with oral antibiotic therapy, usually doxycycline, for 30 days (2). If patients have minimal or no response, we treat with IV antibiotics, usually ceftriaxone, for another month, and in most patients, the arthritis improves. One theory to explain this finding is that tendons, a relatively avascular niche in and around affected joints, may be infected, and better tissue penetration with IV antibiotics is necessary for successful treatment (3).
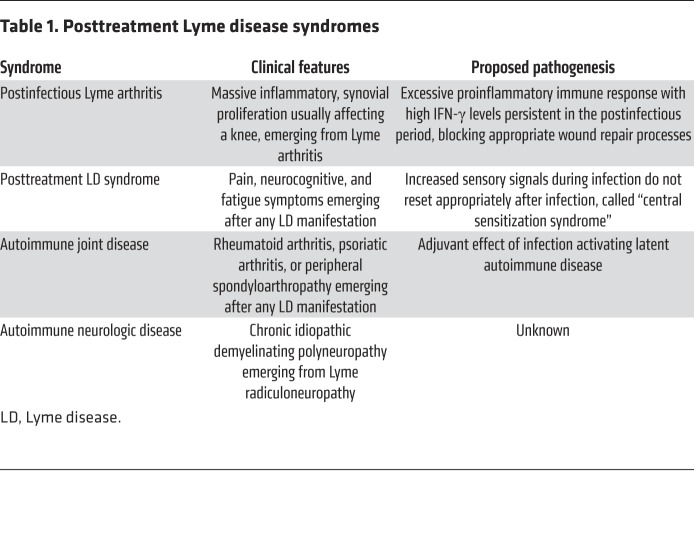
Lyme disease may be complicated by the emergence of disabling postantibiotic syndromes. Importantly, there is more than one posttreatment syndrome, each with a different pathogenesis (Table 1). Physicians are often in a quandary regarding whether these patients still have active infection or postinfectious phenomena. Should patients be treated with more antibiotic therapy, or should they be treated in other ways?
Table 1. Posttreatment Lyme disease syndromes.
We have focused our studies on postantibiotic (also called postinfectious or antibiotic-refractory) Lyme arthritis (LA). In these patients, the arthritis seems to change after antibiotic treatment. Joint effusions are not as large, but massive synovial proliferation develops, usually in one or both knees, that may even worsen in the postantibiotic period, lasting months to several years. The synovial lesion in these patients is similar to that seen in other forms of chronic inflammatory arthritis, including rheumatoid arthritis, though evidence of vascular damage, including obliterative microvascular lesions, is a feature of postinfectious LA synovia (4).
Causes and consequences of postantibiotic LA
The basic pathogenetic feature of postinfectious LA is the development of an excessive, dysregulated proinflammatory immune response during the infection, characterized by exceptionally high IFN-γ levels, which persists in the postinfectious period (4). Patients with a polymorphism in the TLR-1 gene who are infected with B. burgdorferi outer surface protein C (OspC) type A (RST1) strains are at risk for excessively high IFN-γ levels (5). Similarly, presentation of an epitope of B. burgdorferi OspA (OspA164–175) by certain HLA-DR alleles, such as DRB1:0401, leads to high IFN-γ levels (6). Furthermore, in patients with postinfectious LA, a high percentage of CD4+CD25+ T cells, which are ordinarily regulatory T cells, become effector cells that secrete large amounts of IFN-γ, leading to an imbalance in Th1 effector–T regulatory cell function (7).
The consequences of this excessive proinflammatory response in Lyme synovia include vascular damage, autoimmune and cytotoxic processes, and fibroblast proliferation and fibrosis (4). An important driver of innate immune responses may be persistence of B. burgdorferi peptidoglycan in synovial fluid, which may be especially difficult to clear (8). In addition, patients with postinfectious LA often have T and B cell responses to Lyme disease–associated autoantibodies, endothelial cell growth factor, apolipoprotein B-100, or matrix metalloproteinase-10 (9). These responses may reflect damage to blood vessels and the extracellular matrix, leading to presentation of autoantigens by synovial fibroblasts and endothelial cells, which become unconventional antigen-presenting cells that secrete proinflammatory cytokines (10).
In a transcriptomic analysis of postinfectious LA synovial tissue, a heightened IFN-γ signature correlated inversely with the expression of genes involved in the repair of damaged tissue (4). Thus, high numbers of IFN-γ–producing cells in synovia may prevent repair of tissue damaged by the infection, blocking the return to tissue homeostasis even after spirochetal killing. Analysis of miRNAs supports this paradigm (11). During active infection, miRNA expression in synovial fluid reflects the type of immune response associated with bacterial killing. In postinfectious LA, miRNA expression in synovia reflects chronic inflammation, synovial proliferation, and breakdown of wound repair processes.
After oral and IV antibiotics, culture and PCR results for B. burgdorferi have been uniformly negative in postinfectious LA synovial tissue (12). We treat such patients with disease-modifying antirheumatic drugs, the standard of care for other forms of autoimmune, chronic inflammatory arthritis, and we have not observed reemergence of infection in these patients. Thus, we do not think that this LA complication requires persistent infection for disease expression.
Posttreatment Lyme disease syndrome
The clinical features and pathogenesis of what is often called posttreatment Lyme disease syndrome (PTLDS) are quite different. After any disease manifestation, but perhaps more commonly following neurologic involvement, patients may experience pain, neurocognitive, or fatigue symptoms, emerging during or within several months after antibiotic treatment of the infection, lasting months or years (13). These patients may have severe pain around joints (tender points), headache, brain fog, sleep disorder, and incapacitating fatigue, which have a major impact on the quality of life. Such symptoms may also follow certain other infections or physical or emotional trauma, or the precipitating events may not be apparent. PTLDS or “chronic Lyme disease” has become a diagnosis for this type of syndrome not only in patients with recent Lyme disease but also in those who have little or no evidence of previous Lyme disease.
The pathogenesis of PTLDS is not well understood. Although elevated levels of IL-23 or CCL19 have been reported (14, 15), PTLDS does not seem to be a syndrome characterized primarily by excessive immune responses, as in postinfectious LA. Rather, with infection, the brain is sensitized to pain signals, an alarm signal, and fatigue necessitates rest. One hypothesis is that in patients with PTLDS the enhanced sensory signals during the infection do not reset after infection, and the increased sensitivity to pain, brain fog, and marked fatigue persist, called a “central sensitization syndrome” (16).
These symptoms are often difficult to treat effectively; symptomatic treatments and stress reduction are generally recommended (13). However, many patients with chronic Lyme disease feel that antibiotic therapy is the only treatment that has helped them, suggesting that they still have active spirochetal infection. This has led some physicians to treat with multiple antibiotic regimens for months or years, and advocacy groups have formed that promote this approach.
The case for and against long-term antibiotic treatment
Many studies have been done to address the persistent infection hypothesis. In bacterial culture systems, some B. burgdorferi organisms may persist despite exposure to doxycycline or ceftriaxone (17). In culture, daptomycin plus doxycycline and cefoperazone eradicated the most resistant, aggregated microcolony form of B. burgdorferi persisters (18). However, bacterial persisters in culture may be an in vitro phenomenon commonly observed with many bacterial species, which may be explained by the kinetics of antibiotic killing of bacteria not exposed to a host immune system (19).
There is limited evidence for spirochetal persistence after antibiotic treatment in certain animal models of B. burgdorferi infection. When C3H/HeN mice, an inbred strain susceptible to severe B. burgdorferi–induced arthritis, were infected with stationary-phase, aggregated forms of B. burgdorferi, the combination of daptomycin plus doxycycline and cefoperazone for 30 days eradicated the spirochetes, as assessed by culture of ear punch biopsies, whereas doxycycline or ceftriaxone alone did not (20). In macaque monkeys, 24 animals had positive skin biopsy cultures during the first 4 weeks of infection, prior to antibiotic therapy (21). At postmortem, after treatment with 4 days of ceftriaxone and 28 days of doxycycline, only 1 of 12 monkeys in each of the treated and untreated groups had positive cultures. In a second experiment in which xenodiagnosis was used to assess infection 7 or 11 months after infection, rare spirochetes of unproven viability were visualized in the ticks that fed on 2 of 3 treated monkeys. On postmortem examination of the monkeys, a few spirochetes grew slowly in cultures of organ tissues, but subculture was unsuccessful. Building upon these studies, xenodiagnosis experiments were carried out in human patients with PTLDS (22). B. burgdorferi DNA, which may persist after active infection, was detected by PCR in ticks that fed on 1 of 17 patients, but all cultures of the ticks were negative. Additionally, skin biopsies were performed at the site of tick feeding in 11 patients, and all cultures and PCR results of skin samples were negative.
The major problem with the persistent infection hypothesis is that 4 double-blind, placebo-controlled trials have not shown a sustained difference between case and control patients (23–26). In the largest trial, 129 patients were randomized to receive IV ceftriaxone for 1 month followed by oral doxycycline for 2 months or placebo IV and oral preparations for the same duration (23). Before treatment, culture and PCR tests for B. burgdorferi on blood or cerebrospinal fluid were uniformly negative. After 6 months, the percentage of patients whose symptoms had improved, remained the same, or worsened was equivalent in antibiotic-treated and placebo-treated patients. Are there other antibiotic regimens, such as the 3-antibiotic regimen used to kill spirochetal persisters in culture, that may be more effective in human patients than doxycycline or IV ceftriaxone? To answer that question, it would be necessary to do a double-blind, placebo-controlled trial of the 3-antibiotic regimen in patients with PTLDS, accompanied by appropriate culture and PCR testing, as done in the IV ceftriaxone and doxycycline trial.
Both doxycycline and ceftriaxone have the potential for beneficial effects other than bacterial killing, including antiinflammatory or neuroprotective effects. However, if these mechanisms accounted for improvement in symptoms with antibiotics, the antibiotic-treated group would still be expected to do better than the placebo-treated group, which was not the case. Moreover, prolonged use of antibiotics has the potential for significant adverse effects, including untoward reactions during treatment, and antibiotic-induced gut dysbiosis is a risk factor for the later development of certain autoimmune diseases or malignancies (27). With the current lack of evidence of persistent infection or antibiotic efficacy in human patients with PTLDS, the Infectious Diseases Society of America recommends against treatment of such patients with long-term antibiotics (28).
Systemic autoimmune diseases following Lyme disease
Finally, systemic autoimmune or autoinflammatory joint diseases, including rheumatoid arthritis, psoriatic arthritis, or peripheral spondyloarthropathy, may develop weeks to months after Lyme disease, most commonly following antibiotic-treated erythema migrans (29). These complications may be due to the chance occurrence of two diseases, or alternatively, the infection may serve as an adjuvant activating latent autoimmune disease. In addition, we have observed several cases of chronic idiopathic demyelinating polyneuropathy, an autoimmune neurologic disease, emerging from Lyme radiculoneuropathy. Importantly, these autoimmune diseases have required immunosuppressive or antiinflammatory therapy for successful treatment, not further antibiotic therapy.
Conclusions
Lyme disease can usually be treated successfully with 2–4 weeks of oral antibiotic therapy, or if necessary, with 4 additional weeks of IV antibiotics. However, disabling posttreatment syndromes may still develop, which appear to result primarily from disadvantageous or maladaptive host responses to the infection that persist after spirochetal killing with antibiotics.
Acknowledgments
ACS’s work is supported by the NIH (AI R01-101175 and AI R01-144365), the Mathers Foundation, and the Eshe Fund.
Version 1. 04/13/2020
Electronic publication
Version 2. 05/01/2020
Print issue publication
Footnotes
Conflict of interest: The author has declared that no conflict of interest exists.
Copyright: © 2020, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2020;130(5):2148–2151. https://doi.org/10.1172/JCI138062.
References
- 1.Steere AC. Lyme disease. N Engl J Med. 2001;345(2):115–125. doi: 10.1056/NEJM200107123450207. [DOI] [PubMed] [Google Scholar]
- 2.Steere AC, Angelis SM. Therapy for Lyme arthritis: strategies for the treatment of antibiotic-refractory arthritis. Arthritis Rheum. 2006;54(10):3079–3086. doi: 10.1002/art.22131. [DOI] [PubMed] [Google Scholar]
- 3. Arvikar S KM, Oza A, Steere AC. Ultrasonographic examinations show highly prevalent abnormalities of hamstring tendons in Lyme arthritis patients. Paper presented at: American College of Rheumatology/Association of Rheumatology Health Professionals Annual Meeting; October 19–24, 2018; Chicago, Illinois, USA. http://acrabstracts.org/abstract/ultrasonographic-examinations-show-highly-prevalent-abnormalities-of-hamstring-tendons-in-lyme-arthritis-patients. Accessed March 11, 2020.
- 4.Lochhead RB, Arvikar SL, Aversa JM, Sadreyev RI, Strle K, Steere AC. Robust interferon signature and suppressed tissue repair gene expression in synovial tissue from patients with postinfectious, Borrelia burgdorferi-induced Lyme arthritis. Cell Microbiol. 2019;21(2):e12954. doi: 10.1111/cmi.12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strle K, Shin JJ, Glickstein LJ, Steere AC. Association of a Toll-like receptor 1 polymorphism with heightened Th1 inflammatory responses and antibiotic-refractory Lyme arthritis. Arthritis Rheum. 2012;64(5):1497–1507. doi: 10.1002/art.34383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iliopoulou BP, Guerau-de-Arellano M, Huber BT. HLA-DR alleles determine responsiveness to Borrelia burgdorferi antigens in a mouse model of self-perpetuating arthritis. Arthritis Rheum. 2009;60(12):3831–3840. doi: 10.1002/art.25005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vudattu NK, Strle K, Steere AC, Drouin EE. Dysregulation of CD4+CD25(high) T cells in the synovial fluid of patients with antibiotic-refractory Lyme arthritis. Arthritis Rheum. 2013;65(6):1643–1653. doi: 10.1002/art.37910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jutras BL, et al. Borrelia burgdorferi peptidoglycan is a persistent antigen in patients with Lyme arthritis. Proc Natl Acad Sci U S A. 2019;116(27):13498–13507. doi: 10.1073/pnas.1904170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crowley JT, et al. Matrix metalloproteinase-10 is a target of T and B cell responses that correlate with synovial pathology in patients with antibiotic-refractory Lyme arthritis. J Autoimmun. 2016;69:24–37. doi: 10.1016/j.jaut.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lochhead RB, et al. Interferon-gamma production in Lyme arthritis synovial tissue promotes differentiation of fibroblast-like synoviocytes into immune effector cells. Cell Microbiol. 2019;21(2):e12992. doi: 10.1111/cmi.12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lochhead RB, et al. MicroRNA expression shows inflammatory dysregulation and tumor-like proliferative responses in joints of patients with postinfectious Lyme arthritis. Arthritis Rheumatol. 2017;69(5):1100–1110. doi: 10.1002/art.40039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, McHugh GA, Damle N, Sikand VK, Glickstein L, Steere AC. Burden and viability of Borrelia burgdorferi in skin and joints of patients with erythema migrans or Lyme arthritis. Arthritis Rheum. 2011;63(8):2238–2247. doi: 10.1002/art.30384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aucott JN. Posttreatment Lyme disease syndrome. Infect Dis Clin North Am. 2015;29(2):309–323. doi: 10.1016/j.idc.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Strle K, Stupica D, Drouin EE, Steere AC, Strle F. Elevated levels of IL-23 in a subset of patients with post–Lyme disease symptoms following erythema migrans. Clin Infect Dis. 2014;58(3):372–380. doi: 10.1093/cid/cit735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aucott JN, et al. CCL19 as a chemokine risk factor for posttreatment Lyme disease syndrome: a prospective clinical cohort study. Clin Vaccine Immunol. 2016;23(9):757–766. doi: 10.1128/CVI.00071-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Batheja S, Nields JA, Landa A, Fallon BA. Post-treatment Lyme syndrome and central sensitization. J Neuropsychiatry Clin Neurosci. 2013;25(3):176–186. doi: 10.1176/appi.neuropsych.12090223. [DOI] [PubMed] [Google Scholar]
- 17.Sharma B, Brown AV, Matluck NE, Hu LT, Lewis K. Borrelia burgdorferi, the causative agent of Lyme disease, forms drug-tolerant persister cells. Antimicrob Agents Chemother. 2015;59(8):4616–4624. doi: 10.1128/AAC.00864-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng J, Auwaerter PG, Zhang Y. Drug combinations against Borrelia burgdorferi persisters in vitro: eradication achieved by using daptomycin, cefoperazone and doxycycline. PLoS ONE. 2015;10(3):e0117207. doi: 10.1371/journal.pone.0117207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. doi: 10.1016/j.amjmed.2019.12.007. Baker PJ. A review of antibiotic-tolerant persisters and their relevance to posttreatment Lyme disease symptoms [published online January 9, 2020]. Am J Med . [DOI] [PubMed]
- 20.Feng J, et al. Stationary phase persister/biofilm microcolony of Borrelia burgdorferi causes more severe disease in a mouse model of Lyme arthritis: implications for understanding persistence, Post-treatment Lyme Disease Syndrome (PTLDS), and treatment failure. Discov Med. 2019;27(148):125–138. [PubMed] [Google Scholar]
- 21.Embers ME, et al. Persistence of Borrelia burgdorferi in rhesus macaques following antibiotic treatment of disseminated infection. PLoS ONE. 2012;7(1):e29914. doi: 10.1371/journal.pone.0029914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marques A, et al. Xenodiagnosis to detect Borrelia burgdorferi infection: a first-in-human study. Clin Infect Dis. 2014;58(7):937–945. doi: 10.1093/cid/cit939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klempner MS, et al. Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. N Engl J Med. 2001;345(2):85–92. doi: 10.1056/NEJM200107123450202. [DOI] [PubMed] [Google Scholar]
- 24.Krupp LB, et al. Study and treatment of post Lyme disease (STOP-LD): a randomized double masked clinical trial. Neurology. 2003;60(12):1923–1930. doi: 10.1212/01.WNL.0000071227.23769.9E. [DOI] [PubMed] [Google Scholar]
- 25.Fallon BA, et al. A randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathy. Neurology. 2008;70(13):992–1003. doi: 10.1212/01.WNL.0000284604.61160.2d. [DOI] [PubMed] [Google Scholar]
- 26.Berende A, et al. Randomized trial of longer-term therapy for symptoms attributed to Lyme disease. N Engl J Med. 2016;374(13):1209–1220. doi: 10.1056/NEJMoa1505425. [DOI] [PubMed] [Google Scholar]
- 27.Cao Y, et al. Long-term use of antibiotics and risk of colorectal adenoma. Gut. 2018;67(4):672–678. doi: 10.1136/gutjnl-2016-313413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wormser GP, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43(9):1089–1134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 29.Arvikar SL, Crowley JT, Sulka KB, Steere AC. Autoimmune arthritides, rheumatoid arthritis, psoriatic arthritis, or peripheral spondyloarthritis following Lyme disease. Arthritis Rheumatol. 2017;69(1):194–202. doi: 10.1002/art.39866. [DOI] [PMC free article] [PubMed] [Google Scholar]