Abstract
Asthma has long attracted extensive attention because of its recurring symptoms of reversible airflow obstruction, airway hyperresponsiveness (AHR) and airway inflammation. Although accumulating evidence has enabled gradual increases in understanding of the pathogenesis of asthma, many questions regarding the mechanisms underlying asthma onset and progression remain unanswered. Recent advances delineating the potential functions of endoplasmic reticulum (ER) stress in meeting the need for an airway hypersensitivity response have revealed critical roles of unfolded protein response (UPR) pathways in asthma. In this review, we highlight the roles of ER stress and UPR activation in the etiology, pathogenesis and treatment of asthma and discuss whether the related mechanisms could be targets for therapeutic strategies.
Keywords: Asthma, endoplasmic reticulum stress, unfolded protein response, therapeutic strategies
Background
Asthma is a chronic airway inflammatory disease that can be subdivided into several phenotypes on the basis of clinical, physiological and inflammatory markers [1]. The main manifestations of asthma are shortness of breath, wheezing, and chest tightness, which are caused by variable airflow restriction. The symptoms of asthma attacks are reversible: they can resolve within a short time after treatment, or even spontaneously, and only a few of them are persistent [2]. Asthma attacks are often induced by certain factors, such as house dust mites (HDMs), tobacco smoke, chemical irritants, air pollution and viral infections [3]. Many patients have attacks with obvious biological rules that occur or worsen at 2~6 am every morning and generally occur in spring or winter [4]. Asthma is a major global disease that with considerable public health consequences, including high morbidity and very high rates of mortality in severe cases. The disease is reported to kill nearly 250,000 people worldwide each year [5].
The endoplasmic reticulum (ER), which is distributed throughout the cytosol, is a specialized organelle in eukaryotic cells. The ER forms an extensive network that has many connections with other organelles in the cell [6]. Ample connections between the ER and endocytic organelles are observed in many cell types, highlighting the prominent physiological roles of the ER. This organelle is mainly responsible for folding and modifying proteins synthesized in ribosomes and for transporting these proteins to Golgi bodies via vesicles. In addition, the ER also exhibits other functions; for example, it regulates lipid biosynthesis, calcium homeostasis and cellular stress [7]. ER stress involves accumulation of unfolded or misfolded proteins due to disruption of homeostasis in the ER. ER stress is caused by many factors, such as excessive protein processing loads, insufficient nutrition supply, viral infection, calcium imbalance, and reduction/oxidation (REDOX) imbalance. ER stress can trigger calcium ion imbalance, ER overloading, apoptotic pathway activation and other adverse reactions. When ER suffers from such imbalance, various pathways are activated to restore the normal functioning of the organelle; however, these pathways also elicit side effects. The activity of these collective pathways is known as the unfolded protein response (UPR) [8]. ER stress is involved in the pathogeneses of various diseases; therefore, in recent years, it has been studied extensively in the context of diseases such as obesity, diabetes, neurodegenerative diseases, asthma and pulmonary fibrosis [9-12]. Although the contributions of ER stress to many diseases have been studied, the role of ER stress in asthma remains unclear. Therefore, in this review, we discuss the influence of ER stress on asthma from the perspectives of disease triggers, pathophysiological characteristics and treatment. Our aim is to deepen and expand the current understanding of the relationship between asthma and ER stress so that better therapeutic strategies can be developed in clinical settings to combat this widespread disease.
Asthma initiation and progression
The pathogenesis of asthma is complex and multifaceted on the cell, tissue and organ levels, involving an intricate regulatory network under the combined action of genetic and environmental factors (Figure 1). Asthma is a disease with genetic predisposition and strong family trends, as has been confirmed by numerous genome-wide association studies (GWASs) [13]. The major genetic determinants include IL-1RL1, ST2, IL-33R, IL-33, SMAD3, IL-2RB, GSDML and ORMDL3 [14,15]. Among them, ORMDL3, which is specifically associated with the risk for childhood-onset asthma, is correlated with the degree of ER stress [16]. Asthma attacks are also caused by a variety of environmental factors, including smoking, allergies and fungal infections. Asthma is defined as a chronic airway inflammatory disease, and on the cellular level, it can be roughly divided into Th2 inflammation-related and non-Th2 inflammation-related asthma. The inflammation associated with Th2-type asthma is mainly eosinophilic inflammation, which can be further classified as allergic or nonallergic inflammation. Th2-mediated allergic eosinophilic inflammation is the classic inflammation type in asthma [17]. When exogenous allergens enter the body and are phagocytized by antigen-presenting cells, Th2 cells are activated to produce related Th2 cytokines (such as IL-4, IL-5 and IL-13). These cytokines can activate B cells to synthesize and secrete IgE, which can bind to mast cells, and induce the release of various active mediators that cause asthma-related symptoms. Th2 cells can also directly activate eosinophils, mast cells, and alveolar macrophages to secrete such mediators [18,19]. In addition, Th2-mediated eosinophilic inflammation can result from the activation of type 2 innate lymphoid cells (ILC2 cells) by IL-25, IL-33 and thymic stromal lymphopoietin (TSLP) in a T cell-independent manner [20]. Non-Th2 inflammation is mediated predominantly by Th1 and Th17 pathways. Th1/Th17 cells can secrete IL-17, ILC3 and other cytokines to activate alveolar macrophages and neutrophils, thus causing neutrophil inflammation. Suppression of Th2-type inflammation can upregulate Th17 immunity and increase the levels of Th1/Th17 cytokines; therefore, some neutrophilic asthma may be iatrogenic, occurring as a consequence of Th2-suppressing asthma therapies such as corticosteroids [21]. In addition to inflammation, there are two other important features of asthma: airway hyperresponsiveness (AHR) and airway remodeling. In AHR, the airway become highly sensitive to various stimuli, exhibiting an overly strong or premature contraction response when exposed to these stimuli. AHR is mainly caused by chronic airway inflammation and is significantly affected by genetic factors. Airway remodeling is an important pathological feature of asthma that mainly manifests as mucous metaplasia of airway epithelial cells, hyperplasia and hypertrophy of smooth muscle, and distant subepithelial deposition of collagen [22,23].
Figure 1.
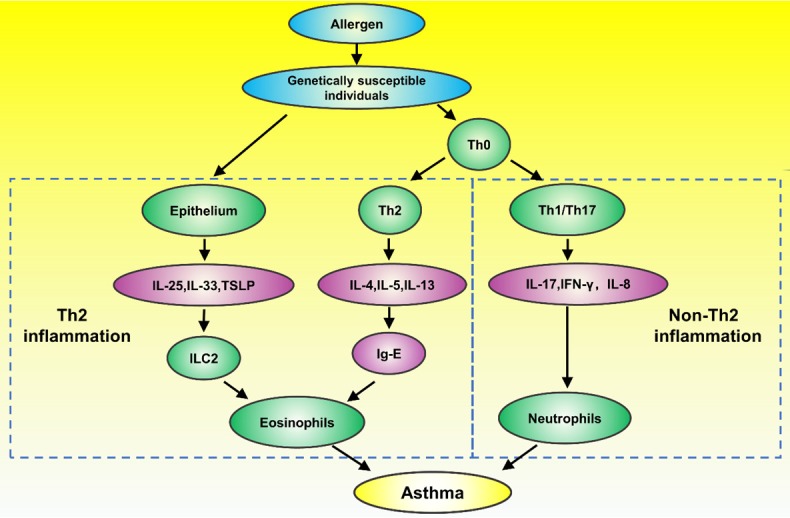
Overview of the pathogenesis of asthma. The combined influence of genetic and environmental factors mediates Th2 and non-Th2 types of inflammation, leading to the production of various cytokines by eosinophils, neutrophils, mast cells, etc. and ultimately causing AHR, mucus hypersecretion and airway remodeling. ILC2: type 2 innate lymphoid cells; TSLP: thymic stromal lymphopoietin; IFN-γ: interferon-γ.
ER stress and the UPR
Adaptive UPR
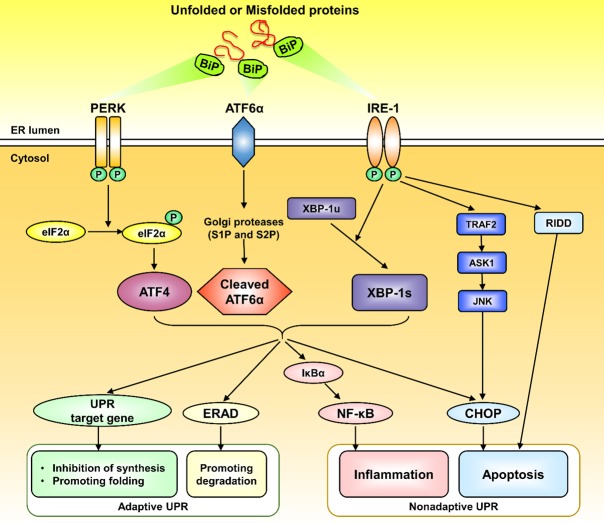
As mentioned above, pathophysiological states that increase the demand for protein folding or that disrupt normal folding processes result in accumulation of misfolded proteins in the ER, which leads to ER stress and activation of the UPR [7]. Three transmembrane proteins that mediate three major UPR pathways exist in the ER lumen: activating transcription factor 6α (ATF6α), protein kinase RNA-like ER kinase (PERK) and inositol-requiring protein 1α (IRE1α). ATF6α contains a leucine zipper domain that is activated by proteases, and PERK and IRE1α contain similar cytoplasmic Ser/Thr kinase domains that are activated by autophosphorylation [24]. These three transmembrane proteins act as sensors and inhibit ER stress through physical interaction with immunoglobulin--binding protein (BiP)/GRP78, a chaperone of the heat shock protein family. Once ER stress is induced, BiP combines with misfolded proteins and disassociates from the three transmembrane proteins, and UPR pathways are triggered to restore protein folding homeostasis [25-27]. The first pathway is mediated by ATF6α. Under stress conditions, ATF6α is activated and transported into the Golgi, where its N-terminal cytosolic domain is cleaved by the proteases S1P and S2P. Then, cleaved ATF6α enters the nucleus to induce the transcription of several UPR-related genes to promote protein folding. The second pathway is mediated by PERK; when PERK is activated, it phosphorylates eukaryotic translation initiation factor 2α (eIF2α), which can then upregulate the translation of ATF4 mRNA to reduce initiation codon (AUG) recognition and translation, thereby reducing protein synthesis and the burden of ER damage. The third pathway is mediated by IRE1α; activated IRE1α elicits the activity of an endoribonuclease that selectively cleaves a 26-nucleotide segment from X-box-binding protein 1 (XBP-1) mRNA to create transcriptionally active XBP-1s, which then enters the nucleus to activate UPR-associated genes and ER-associated degradation (ERAD) [25,28-31].
Nonadaptive UPR
The initial purpose of UPR pathway activation is to prevent excessive accumulation of misfolded proteins, maintain ER homeostasis, and promote ER functional recovery (through the adaptive UPR). However, sustained or prolonged activation of the UPR pathway can lead to toxicity and side effects, causing cell inflammatory states and even apoptosis (through the nonadaptive UPR) [32]. Nuclear factor-κB (NF-κB) is a transcription factor that induces the expression of inflammatory response-related genes that encode cytokines, chemokines, and adhesion receptors [33]. According to previous studies, NF-κB is closely associated with ER stress, and UPR pathway activation can activate NF-κB in a variety of ways, thus causing relevant inflammatory responses [34,35]. C/EBP homologous protein (CHOP), a major transcription factor regulating cell death under ER stress, can be activated by all three UPR pathways [36]. CHOP can upregulate the proapoptotic gene Bax/Bak and downregulate the antiapoptotic gene Bcl-2, resulting in mitochondrial-related dysfunction, caspase-12 and caspase-9 activation, caspase cascade reaction initiation, and ultimately apoptosis [37,38]. In addition, some other pathways can induce apoptosis. For instance, long-term ER stress causes continuous interaction between IRE1α and tumor necrosis factor-related factor 2 (TRAF2), which activates the downstream apoptotic signal regulation kinase 1 (ASK1)-JNK signaling pathway, triggering apoptosis [39]. Furthermore, activation of IRE1α can mediate mRNA breakdown through regulated IRE1-dependent decay (RIDD) [40]. The three UPR pathways are intricately interconnected with many intersections, and they are often activated together (Figure 2).
Figure 2.
Classic UPR signaling pathways. Under ER stress, Bip dissociates from three ER stress sensors (ATF6, PERK, and IREα) and binds to unfolded or misfolded proteins. The UPR pathway includes adaptive UPR and nonadaptive UPR. Adaptive UPR can reduce ER stress and restore homeostasis. Persistent ER stress can lead to nonadaptive UPR, causing stress-related damage such as inflammation and apoptosis. BiP: binding protein; ATF6α: activating transcription factor 6α; PERK: protein kinase RNA-like ER kinase; IRE1α: inositol-requiring protein 1α; eIF2α: eukaryotic translation initiation factor 2α; ATF4: activating transcription factor 4; XBP-1: X-box-binding protein 1; ERAD: ER-associated degradation; NF-κB: nuclear factor-κB; CHOP: C/EBP homologous protein; TRAF2: tumor necrosis factor-related factor 2; ASK1: apoptotic signal regulation kinase 1; RIDD: regulated IRE1 dependent decay.
ER stress and its role in the etiology of asthma
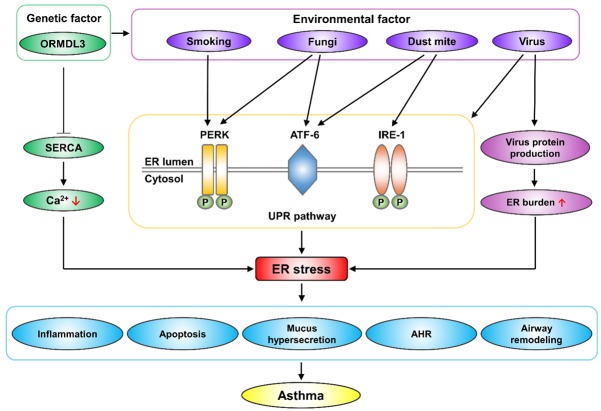
Asthma is a disease with complex characteristics and a polygenic inheritance tendency. The etiology of the disease is closely related to heredity and the environment. In individuals with certain genetic susceptibility, asthma can be triggered by allergens inhalations, smoking, and viral or fungal infection.
Genetic factors related to ER stress in asthma: ORMDL3
ORMDL3 is an ER transmembrane protein that contains 153 amino acids and is encoded by a gene on chromosome 17q21. The discovery of ORMDL3 has been important in the context of a variety of inflammatory diseases including asthma [41]. Studies have shown that genetic variation affecting the expression of the ORMDL3 protein is an important determinant of asthma susceptibility [42-44]. Mechanistically, abnormal ORMDL3 expression can lead to calcium outflow from the ER by inhibiting the sarco-ER calcium pump (SERCA); decreased calcium content in the ER is one of the major causes of the UPR, mainly through the PERK-eIF2α pathway [45]. Pesticide exposure is often associated with asthma attacks; for example, benomyl, a common pesticide, can increase intracellular Ca2+ levels and trigger asthma. However, ORMDL3 knockout alleviates the effects of benomyl on intracellular Ca2+ and proinflammatory cytokines associated with the pathogenesis of asthma [46]. Genes play regulatory roles; rather than acting independently, genetic factors usually act in combination with environmental factors. Notably, ORMDL3 also mediates host-pathogen interactions. Increased ORMDL3 expression can regulate rhinovirus-induced ER stress and IFN-γ production [47], while ORMDL3 silencing can significantly reduce the expression of the rhinovirus receptor ICAM1. In addition, ORMDL3 silencing can reduce ER stress after IL-1β stimulation and reduce the release of inflammatory factors such as IL-6 and IL-8 [16]. In an ER stress model induced by Alternaria, ORMDL3 has been found to drive the ATF6-mediated UPR pathway arm and to further activate the ERAD pathway [48]. However, some studies have revealed inconsistent results; for example, it has been suggested that changes in ORMDL3 in airway epithelial cells do not play important roles in regulating immune responses and UPRs in the lungs [49]. Furthermore, Debeuf et al. suggested that wild-type, ORMDL3-deficient (Ormdl3-/-) and ORMDL3-overexpressing (Ormdl3Tg/wt) mice showed no differences in asthma characteristics after HDM sensitization [50]. In general, the effect of ORMDL3 on asthma has been widely recognized, but whether ORMDL3 plays a role through ER stress or other pathways needs to be further studied.
Asthma-associated environmental factors and ER stress
Dust mite
Many microorganisms can cause asthma attacks, including HDMs, fungi, virus, and others. Among these, HDMs, which is ubiquitous in the air, is one of the most important causes of asthma [51]. Studies have reported that allergic reactions to HDMs in early childhood are significant determinants of the subsequent development of asthma [52]. HDM stimulation can significantly upregulate ER stress-related markers in bronchial epithelial cells, mainly through the ATF-6 and IRE-1 pathways. The ATF-6 pathway can further activate the downstream CHOP pathway, inducing caspase-3 activation and apoptosis. In addition, the ER chaperones GRP78, GRP94, and ER-resident protein 57 (ERP57) are also markedly upregulated by HDMs [53]. ERP57 is an endoplasmic REDOX chaperone protein involved in the folding and secretion of glycoproteins, and is significantly upregulated in epithelial cells of asthmatic patients. However, in a mouse model of allergic asthma, inflammatory cell counts and airway resistance induced by HDM stimulation have been found to be significantly reduced in ERp57 knockout mice compared with wild-type mice [54]. In contrast to HDMs, Dermatophagoides farinae (Der f) mainly induce ER stress by activating IL-25; IL-25 activates the downstream CHOP pathway through PERK-eIF2α arm, which further upregulates the expression of caspase3 and downregulates the expression of Bcl-2 to mediate apoptosis [55]. Some targeted treatment strategies have been developed for HDM-induced ER stress and asthma attacks. For example, tauroursodeoxycholic acid (TUDCA), a taurine-coupled form of ursodeoxycholic acid mainly used in the treatment of cholestatic liver disease, effectively inhibits apoptosis partly by modulating the PERK-eIF2α ER stress pathway and the Akt pathway [56,57]. In the context of asthma, TUDCA markedly reduces HDM-mediated airway inflammation, mucus secretion, AHR, and airway remodeling by inhibiting ER stress [58]. In addition, given its inhibitory effect on ER stress, the effects of TUDCA on many other conditions, such as diabetes, retinopathy and neurological diseases, have also been studied [59,60]. Platycodi Radix extract (PRE) has been identified as another possible drug for the treatment or prevention of HDM-related allergic airway inflammation that acts by inhibiting ER stress and its related reactive oxygen species (ROS) signaling pathway [61].
Fungi
Fungi are also present as allergens in indoor air, especially in dark, damp and poorly ventilated areas. Recently, studies have suggested that ER stress and associated molecules, including phosphoinositide 3-kinase-δ (PI3K-δ), may be vital for the development of fungal infection associated asthma [63]. Among fungi, Alternaria spp. and Aspergillus fumigatus (Af) have been identified as the most important risk factors for asthma mediated by ORMDL3 [64]. Alternaria spp. potently induce cellular stress and the UPR by activating ATF6-XBP1 signaling [48]. Studies have shown that PI3K-δ inhibitors can effectively inhibit ER stress and the inflammatory response in an Afinduced cortisol-resistant mouse model [65]. The overall effects of PI3K-δ inhibition on ER stress induced by fungi are achieved through reductions in inflammation-associated intra-ER hyperoxidation, disruption of protein disulfide isomerase (PDI) chaperone activity and stabilization of ER membrane fluidity and permeability [66]. In addition, PI3K-δ inhibition can improve Af-induced allergic inflammation by regulating the production of mitochondrial ROS (mtROS) and thereby modulating the NLRP3 inflammasome [67]. However, ER stress affects not only the human body but also the AbHacA gene of the fungus itself, which encodes the major UPR transcription regulator in Alternaria spp. Deletion of the AbHacA gene prevents induction of the UPR, resulting in a complete loss of virulence associated with cell wall defects [68].
Viruses
A growing number of reports have linked acute asthma attacks to respiratory viral infections. For example, the influenza virus, one of the most common airway pathogens, can cause airway inflammation and asthma attacks by mediating ER stress and subsequent UPRs [69]. Mechanistically, eosinophils have emerged as important links between airway virus infection and allergic asthma exacerbation. When the airway is infected by a virus, eosinophils are activated to clear the virus from the respiratory tract. At the same time, endoplasmic reticulum stress occurs, causing the secretion of activated mediators that can induce asthma-related symptoms. However, this process requires the presence of prolyl isomerase. The eosinophils of prolyl isomerase knockout mice cannot activate the ER stress-induced UPR and fail to activate the intrinsic immune response, thus failing to clear viruses [70].
Smoking
In addition to microbial factors, cigarette smoke is an important trigger for asthma, especially in children whose parents smoke. ER stress plays a significant role in smoking-induced inflammation, apoptosis and autophagy. According to a gene set variation analysis of the bronchial epithelial cell transcriptome, current-smokers show enrichment of ER stress-associated genes compared with ex-smokers and nonsmokers [71]. In vitro, cigarette smoke extract (CSE) can significantly upregulate various ER stress markers (IER1α, PERK, GRP78, eIF2α, ATF4, CHOP) and induce related inflammatory responses, leading to upregulation of inflammatory markers (IL-6, IL-8, NF-κB) [72,73]. In addition, CSE can induce autophagy and apoptosis in alveolar epithelial cells through ER stress pathways. Smoking-mediated downregulation of PERK, GRP78, and eIF2α in the UPR pathway upregulates CHOP and ATF4, promotes epithelial cell apoptosis and inhibits autophagy. However, there is a delicate balance among ER stress, apoptosis and autophagy induced by smoking, and different UPR pathways have disparate regulatory effects on apoptosis and autophagy. In addition, apoptosis and autophagy are regulated by mutual inhibition [73]. However, CSE can also increase the expression of HRD1, which, when overexpressed, can mediate ERAD to reduce ER stress-induced apoptosis as an adaptive protective measure [74]. Given that smoking can induce apoptosis through ER stress pathways, protective drugs targeting ER stress have become the focus of attention. Progranulin, a glycoprotein, has been reported to play a protective role in the context of smoking-induced apoptosis. CSE-induced alveolar epithelial cell apoptosis is significantly decreased in progranulin-overexpressing cells, and the activation levels of ER stress-associated markers are correlated with progranulin expression levels [75]. Another study has shown that H2S inhibits lung tissue injury by reducing CSE-induced pulmonary ER stress in vivo and attenuates nicotine-induced ER stress-mediated bronchial epithelial cell apoptosis in vitro [76]. However, considering the toxicity of H2S itself, it is difficult to translate its use into clinical application.
In general, asthma is associated with a variety of environmental factors that are closely related to ER stress. Targeting ER stress may provide a new direction for the future treatment of asthma attacks induced by such factors.
ER stress and its role in the pathophysiological characteristics of asthma
The pathophysiological characteristics of asthma include chronic airway inflammation, epithelial apoptosis, AHR and excessive mucus secretion, which eventually cause airway remodeling. During this process, ER stress plays an important role in regulating epithelial cell inflammation and apoptosis and affects mucus secretion, collagen deposition and smooth muscle hyperplasia.
ER stress-mediated airway inflammation and epithelial apoptosis
Chronic airway inflammation is the first major feature of asthma. The newly discovered function of the ER as a regulator of inflammation suggests potential strategies for the treatment of various inflammation-related diseases. NF-κB, which is a transcription factor, regulates the expression of many genes involved in inflammation [77]. In the context of ER stress, various UPR pathways can reduce the expression of IκBα, an inhibitory protein of NF-κB, in different ways, thereby upregulating NF-κB to mediate the inflammatory response [78,79]. First, phosphorylated IRE1 can bind with TRAF2 to activate the JNK/AKT pathway, leading to phosphorylation of IκB kinase (IKK). IKK activation leads to the cleavage of IκBα, which eventually induces the activation of NF-κB [80]. Second, PERK activation by autophosphorylation results in eIF2α phosphorylation and ATF4 activation, which can inhibit the translation of various proteins, including IκBα, thereby reducing IκBα production and inducing NF-κB transcription [81,82]. Third, ATF6 is cleaved into active ATF6α and ATF6β in the Golgi, which induces the phosphorylation of Akt in a specific way and then inhibits the expression of IκBα, leading to the activation of NF-κB [83]. In addition to the three classic UPR pathways, BIP, a chaperone protein that mediates the initiation of the UPR, can directly leak into the cytoplasm to induce the activation of IKK and then downregulate IκBα, leading to the activation of NF-κB [84]. In addition, the sigma-1 receptor, an ER-resident protein, can restrict cytokine expression and the endonuclease activity of the ER stress sensor IRE-1 but does not inhibit the classic inflammatory signaling pathways [85]. ER stress plays an important role in regulating inflammation through NF-κB and this phenomenon has also been verified to be involved in asthma. The results of one study in which samples obtained from airway epithelial brushing of individuals with asthma and normal subjects were analyzed by RNA sequencing showed that the expression of type 2 markers, IFN-stimulated genes (ISGs) and ER stress-related genes was significantly higher in the asthma group than in the normal group; in addition ER stress was obviously correlated with the type 2 inflammatory response and ISGs [86]. Researchers have also confirmed that the expression levels of ER stress markers (p-PERK, ATF4 and CHOP) are elevated in lung tissue in ovalbumin (OVA)-lipopolysaccharide (LPS)-OVA mouse models and that tunicamycin, used to induce ER stress, can further increase the expression levels of inflammatory cytokines, suggesting that PERK-ATF4-CHOP signaling is associated with airway inflammation in the context of neutrophilic asthma [87]. In addition, the ER stress blocker 4-PBA can significantly reduce the expression of NF-κB, resulting in downregulation of the expression of Th2 cytokines (IL-4, IL-5, IL-13) and airway inflammatory response factors (IL-1β, TNF-α, IFN-γ) as well as reductions in the populations of neutrophils and eosinophils [88].
CHOP, an ER stress marker and transcription factor, plays a core role in ER stress-induced apoptosis. CHOP can be activated by three classic UPR pathways: the PERK-eIF2α-ATF4, ATF6-Golgi-cleaved ATF6, and IRE1-XBP1 pathways. In addition, IRE1 also activates the ASK1-JNK pathway through TRAF2 to mediate the activation of CHOP [89,90]. After CHOP is activated, it can promote apoptosis through multiple other pathways. Activated CHOP can upregulate the expression of Bim and downregulate the expression of Bcl2, thereby inducing apoptosis by affecting the Bax/Bak-mediated permeability of the mitochondrial outer membrane. Increased permeability of the mitochondrial outer membrane can then lead to a caspase cascade to mediate apoptosis [91,92]. Another major pathway of apoptosis mediated by CHOP is the ERO1α-IP3R-calcium-CaMKII pathway. In this pathway, CHOP activates IP3R (a calciumrelease channel in the ER) via ERO1α and then induces the release of calcium from the ER into the cytoplasm. The resulting increase in intracellular calcium ion content activates the calcium-sensing kinase CaMKII, inducing calcium-dependent apoptosis [93,94]. CHOP can also induce apoptosis in other ways, such as through the DR4-DR5-caspase8 pathway [95].
During the past few years, our laboratory has focused on the role of CHOP in pulmonary diseases. We obtained the first evidence that pulmonary fibrosis alters CHOP expression and ER stress both in patients with idiopathic pulmonary fibrosis (IPF) and in animals with bleomycin-induced pulmonary fibrosis. Consistent with these observations, mice deficient in Chop are protected from bleomycin-induced lung injury and fibrosis. Specifically, loss of Chop significantly attenuates TGF-β production and M2 macrophage infiltration in the lungs following bleomycin induction. Mechanistic studies have revealed that Chop deficiency suppresses the M2 program in macrophages, which subsequently attenuates TGF-β secretion. Loss of Chop enhances the expression of SOCS1 and SOCS3, thereby inhibiting STAT6/PPARγ signaling, which is essential for the macrophage M2 program [96]. Similarly, a study on an OVA-induced allergic airway inflammatory model has revealed that Chop regulates STAT6 phosphorylation, thereby enhancing the expression of mouse transcription factor EC (Tfec), which then transcribes IL-4 receptor α (IL-4Rα) to promote the M2 program in macrophages [11]. Taken together, these data provide novel insights into the role of ER stress in modulating M2 macrophage polarization, which contributes to the pathogeneses of fibrotic and asthmatic diseases.
ER stress-mediated hypersecretion of mucus
Increased airway mucus secretion, mucus retention, and mucus plug formation are other major characteristics of asthma. In mammals, 5 mucin-related genes mediate mucus formation; among them, MUC5AC and MUC5B are significantly highly expressed in airways and regulate mucus secretion in the respiratory system [97]. A genetic analysis of small airway epithelial cells brushed through fiberoptic bronchoscopy revealed a list of 73 MUC5AC-associated core genes with 9 categories, one of which included ER stress-related genes [98]. Recently, ER stress has begun to be regarded as a target for inhibition of mucus secretion. Researchers have explored the abilities of numerous molecules involved in ER stress to affect mucus secretion. For example, the expression of MUC5AC in an OVA-LPS-OVA-induced mouse asthma model has been found to be significantly downregulated by 4-PBA, an ER stress inhibitor [99]; astragalus polysaccharide (APS) has been found to significantly reduce ER stress marker levels and reduce mucus production [100]; kaempferol has been found to alleviate mucus hypersecretion by blocking bronchial epithelial ER stress through inhibition of IRE1α-TRAF2-JNK pathway activation, thus reducing the expression of MUC5AC [101]; and knockout of anterior gradient homolog 2 (AGR2), a gene associated with airway and intestinal epithelial mucin production, has been found to reduce MUC5AC and MUC5B expression and mucus secretion in an OVA-LPS-OVA-induced mouse asthma model [102]. IRE1β is a component of the UPR pathway; but unlike IRE1α, it is expressed only in the gut and respiratory system. IRE1β can upregulate the expression of AGR2 by mediating XBP-1 splicing, thereby increasing mucus secretion [103]. In addition to affecting mucus secretion through AGR2, XBP-1s can be activated by IRE-1β and directly bind to the proximal region of the MUC5B promoter variant rs35705950, thereby inducing mucus hypersecretion [104]. In addition to the IRE1 pathway, the NF-κB, ATF6, and CHOP signaling pathways also involved in the regulation of mucus secretion. Inhibition of ER stress by Lyn kinase leads to blockade of NF-κB, thereby downregulating MUC5AC expression [105], and siRNA-mediated knockdown of XBP-1, CHOP, and ATF6 can decrease the mRNA expression and protein levels of MUC5AC and MUC5B [106]. Interestingly, ER stress can regulate the expression of MUC5AC, and MUC5AC can in turn exert a regulatory effect on ER stress. MUC5AC knockout in cells attenuates the increases in ER stress markers caused by LPS stimulation [107].
ER stress-mediated AHR and airway remodeling
In AHR an airway is highly sensitive to various stimulating factors, and airway smooth muscle contraction is hyperactive. The main causes of AHR are chronic inflammation, airway epithelial injury, apoptosis, and abnormal airway smooth muscle contraction [108]. As mentioned above, ER stress is involved in the development of airway inflammation and airway epithelial cell apoptosis. However, the contraction of airway smooth muscle is also regulated by ER stress [109]. Contraction of smooth muscle depends on two key factors: intracellular calcium concentrations and smooth muscle calcium sensitivity. ER stress can induce intracytoplasmic calcium imbalance by activating CHOP; this is one of the mechanisms by which ER stress mediates abnormal smooth muscle contraction [110]. Acetaldehyde stimulation significantly increases the levels of ER stress markers in airway smooth muscle cells, resulting in upregulation of the expression of NF-κB, a key molecule regulating inflammation [111]. In addition, while PI3Kδ is closely associated with fungal infection-mediated asthma attacks, as we have mentioned, it can also induce airway inflammation and AHR by activating NF-κB signaling through ER-associated ROS and RIDD-RIG-I activation [112]. In general, ER stress participates in AHR by regulating the inflammatory response, apoptosis, and smooth muscle sensitivity.
The combined action of the above pathological features ultimately causes airway remodeling, or airway structural changes. The main characteristics of airway remodeling are subepithelial collagen deposition and fibrotic proliferation after epithelial injury. Studies have confirmed the close relationship between ER stress and pulmonary fibrosis. ER stress can induce pulmonary fibrosis by mediating apoptosis and the epithelial-mesenchymal transition (EMT) in epithelial cells, the polarization of macrophages to M2-type cells and the activation of fibroblasts [24,113]. The IRE1-XBP1 pathway can promote the EMT by mediating snail expression, thereby causing fiber proliferation [114]. N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP) can reduce type II alveolar epithelial cell apoptosis and inhibit collagen deposition by suppressing the PERK/eIF2 α/CHOP pathway [115]. In conclusion, ER stress can mediate inflammation, apoptosis, mucus hypersecretion, smooth muscle contraction and collagen deposition through multiple pathways that together constitute the pathological mechanism of asthma.
Role of ER stress in the treatment of asthma
Traditional treatments for asthma include glucocorticoids combined with beta agonists or leukotriene modulators. However, a subset of asthma cases are steroid-resistant, especially Th2-mediated nonallergic eosinophilic asthma and non-Th2 asthma cases [116]. ER stress participates closely in the pathogenesis of steroid-resistant asthma by mediating the activation of the PI3K, MAPK and NF-κB pathways [117]. The molecular mechanisms of ER stress and steroid-resistant asthma overlap, suggesting a therapeutic strategy for severe asthma [118]. Thus far, many compounds have been studied or used for ER stress-based asthma treatment. Suhuang antitussive capsules, which are adjuvant drugs commonly used in the field of pneumology, disrupt NLRP3 inflammasome activation by inhibiting ER stress in the context of asthma [119]. In addition, conjugated bile acids (CBAs) have been reported to be able to inhibit allergen-induced UPRs and airway allergic disease in mice by specifically binding to ATF6 α [120]. 4μ8c, an inhibitor of IRE1α RNase, can reduce the secretion of IL-4, IL-5, and IL-13 proteins by activated CD4+ splenocytes in naive mice and reduce the secretion of IL-5 by established TH2 cells [121]. In addition, trimethylamine-N-oxide (TMAO) and 4-phenylbutyric acid can reduce UPR marker levels, airway inflammation, and remodeling in a dose-dependent manner [122]; gubenfangxiao decoction can significantly attenuate persistent airway inflammation in a respiratory syncytial virus (RSV)-OVA-induced asthma mouse model at least partially through inhibition of ER stress [123]; Ghrelin can inhibit ER stress by stimulating the Akt signaling pathway, thereby reducing inflammatory responses and ameliorating asthma in mice [124]; and sevoflurane can restrain ER stress to recover the protein processing of aquaporins, thus relieving asthma [125]. Given the increasing evidence of the relationship between ER stress and asthma, we believe that targeting ER stress may be a new approach for steroid-resistant asthma treatment.
Conclusion
We reviewed recent studies on ER stress mainly from the perspectives of the etiology and pathogenesis of asthma. Both genetic and environmental factors mediate ER stress, which in turn regulates airway inflammation, apoptosis, mucus secretion, AHR and airway remodeling in different ways to ultimately cause asthma (Figure 3). The reviewed studies thoroughly demonstrate the close relationship between ER stress and asthma. However, additional in-depth mechanisms are worth elucidating, and uncertainties remain, such as how to best translate the existing mechanistic research into clinical applications. In the future, targeting ER stress may be a new strategy for asthma treatment, especially in the context of steroid-resistant asthma.
Figure 3.
The relationship between ER stress and asthma. Under a genetic background dominated by high ORMDL3 expression, various environmental factors participate in inducing ER stress. ER stress further mediates inflammation, apoptosis, airway mucus hypersecretion, AHR and airway remodeling through different mechanisms. ER stress can be targeted as a new way to treat asthma. ER: endoplasmic reticulum; SERCA: Sarco-ER calcium pump; ATF6α: activating transcription factor 6α; PERK: protein kinase RNA-like ER kinase; IRE1α: inositol-requiring protein 1α; AHR: airway hyperresponsiveness.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81800068).
Disclosure of conflict of interest
None.
References
- 1.Ray A, Oriss TB, Wenzel SE. Emerging molecular phenotypes of asthma. Am J Physiol Lung Cell Mol Physiol. 2015;308:L130–140. doi: 10.1152/ajplung.00070.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mikalsen IB, Dalen I, Karlstad O, Eide GE, Magnus M, Nystad W, Oymar K. Airway symptoms and atopy in young children prescribed asthma medications: a large-scale cohort study. Pediatr Pulmonol. 2019;54:1557–1566. doi: 10.1002/ppul.24437. [DOI] [PubMed] [Google Scholar]
- 3.Kim H, Mazza J. Asthma. Allergy Asthma Clin Immunol. 2011;7(Suppl 1):S2. doi: 10.1186/1710-1492-7-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Acevedo N, Zakzuk J, Caraballo L. House dust mite allergy under changing environments. Allergy Asthma Immunol Res. 2019;11:450–469. doi: 10.4168/aair.2019.11.4.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dharmage SC, Perret JL, Custovic A. Epidemiology of asthma in children and adults. Front Pediatr. 2019;7:246. doi: 10.3389/fped.2019.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Mattia T, Tomasetto C, Alpy F. Faraway, so close! Functions of endoplasmic reticulum-endosome contacts. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865:158490. doi: 10.1016/j.bbalip.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 7.Shakeri A, Zirak MR, Wallace Hayes A, Reiter R, Karimi G. Curcumin and its analogues protect from endoplasmic reticulum stress: mechanisms and pathways. Pharmacol Res. 2019;146:104335. doi: 10.1016/j.phrs.2019.104335. [DOI] [PubMed] [Google Scholar]
- 8.Moore KA, Hollien J. The unfolded protein response in secretory cell function. Annu Rev Genet. 2012;46:165–183. doi: 10.1146/annurev-genet-110711-155644. [DOI] [PubMed] [Google Scholar]
- 9.Villalobos-Labra R, Subiabre M, Toledo F, Pardo F, Sobrevia L. Endoplasmic reticulum stress and development of insulin resistance in adipose, skeletal, liver, and foetoplacental tissue in diabesity. Mol Aspects Med. 2019;66:49–61. doi: 10.1016/j.mam.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Martinez G, Khatiwada S, Costa-Mattioli M, Hetz C. ER proteostasis control of neuronal physiology and synaptic function. Trends Neurosci. 2018;41:610–624. doi: 10.1016/j.tins.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Zhu J, Zhang L, Zhang Z, He L, Mou Y, Deng Y, Cao Y, Yang P, Su Y, Zhao J, Zhang S, Yu Q, Hu J, Chen Z, Ning Q, Xiang X, Xu Y, Wang CY, Xiong W. Role of C/EBP homologous protein and endoplasmic reticulum stress in asthma exacerbation by regulating the IL-4/signal transducer and activator of transcription 6/transcription factor EC/IL-4 receptor alpha positive feedback loop in M2 macrophages. J Allergy Clin Immunol. 2017;140:1550–1561. e1558. doi: 10.1016/j.jaci.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 12.Bueno M, Lai YC, Romero Y, Brands J, St Croix CM, Kamga C, Corey C, Herazo-Maya JD, Sembrat J, Lee JS, Duncan SR, Rojas M, Shiva S, Chu CT, Mora AL. PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. J Clin Invest. 2015;125:521–538. doi: 10.1172/JCI74942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, von Mutius E, Farrall M, Lathrop M, Cookson W. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363:1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramasamy A, Kuokkanen M, Vedantam S, Gajdos ZK, Couto Alves A, Lyon HN, Ferreira MAR, Strachan DP, Zhao JH, Abramson MJ, Brown MA, Coin L, Dharmage SC, Duffy DL, Haahtela T, Heath AC, Janson C, Kähönen M, Khaw KT, Laitinen J, Le Souef P, Lehtimäki T Australian Asthma Genetics Consortium collaborators. Madden PAF, Marks GB, Martin NG, Matheson MC, Palmer CD, Palotie A, Pouta A, Robertson CF, Viikari J, Widen E, Wjst M, Jarvis DL, Montgomery GW, Thompson PJ, Wareham N, Eriksson J, Jousilahti P, Laitinen T, Pekkanen J, Raitakari OT, O’Connor GT, Salomaa V, Jarvelin MR, Hirschhorn JN. Correction: genome-wide association studies of asthma in population-based cohorts confirm known and suggested loci and identify an additional association near HLA. PLoS One. 2013;8 doi: 10.1371/annotation/9630862b-4676-4b82-9869-8d8fbb2a2e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ober C, Yao TC. The genetics of asthma and allergic disease: a 21st century perspective. Immunol Rev. 2011;242:10–30. doi: 10.1111/j.1600-065X.2011.01029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Willis-Owen SAG, Spiegel S, Lloyd CM, Moffatt MF, Cookson WOCM. The ORMDL3 asthma gene regulates ICAM1 and has multiple effects on cellular inflammation. Am J Respir Crit Care Med. 2019;199:478–488. doi: 10.1164/rccm.201803-0438OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16:45–56. doi: 10.1038/ni.3049. [DOI] [PubMed] [Google Scholar]
- 18.Eltboli O, Brightling CE. Eosinophils as diagnostic tools in chronic lung disease. Expert Rev Respir Med. 2013;7:33–42. doi: 10.1586/ers.12.81. [DOI] [PubMed] [Google Scholar]
- 19.Kulkarni NS, Hollins F, Sutcliffe A, Saunders R, Shah S, Siddiqui S, Gupta S, Haldar P, Green R, Pavord I, Wardlaw A, Brightling CE. Eosinophil protein in airway macrophages: a novel biomarker of eosinophilic inflammation in patients with asthma. J Allergy Clin Immunol. 2010;126:61–69. e63. doi: 10.1016/j.jaci.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doherty TA, Khorram N, Lund S, Mehta AK, Croft M, Broide DH. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J Allergy Clin Immunol. 2013;132:205–213. doi: 10.1016/j.jaci.2013.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choy DF, Hart KM, Borthwick LA, Shikotra A, Nagarkar DR, Siddiqui S, Jia G, Ohri CM, Doran E, Vannella KM, Butler CA, Hargadon B, Sciurba JC, Gieseck RL, Thompson RW, White S, Abbas AR, Jackman J, Wu LC, Egen JG, Heaney LG, Ramalingam TR, Arron JR, Wynn TA, Bradding P. TH2 and TH17 inflammatory pathways are reciprocally regulated in asthma. Sci Transl Med. 2015;7:301ra129. doi: 10.1126/scitranslmed.aab3142. [DOI] [PubMed] [Google Scholar]
- 22.Boulet LP. Airway remodeling in asthma: update on mechanisms and therapeutic approaches. Curr Opin Pulm Med. 2018;24:56–62. doi: 10.1097/MCP.0000000000000441. [DOI] [PubMed] [Google Scholar]
- 23.Fehrenbach H, Wagner C, Wegmann M. Airway remodeling in asthma: what really matters. Cell Tissue Res. 2017;367:551–569. doi: 10.1007/s00441-016-2566-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, Wang Y, Pandupuspitasari NS, Wu G, Xiang X, Gong Q, Xiong W, Wang CY, Yang P, Ren B. Endoplasmic reticulum stress, a new wrestler, in the pathogenesis of idiopathic pulmonary fibrosis. Am J Transl Res. 2017;9:722–735. [PMC free article] [PubMed] [Google Scholar]
- 25.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 26.Saibil H. Chaperone machines for protein folding, unfolding and disaggregation. Nat Rev Mol Cell Biol. 2013;14:630–642. doi: 10.1038/nrm3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 28.Janssens S, Pulendran B, Lambrecht BN. Emerging functions of the unfolded protein response in immunity. Nat Immunol. 2014;15:910–919. doi: 10.1038/ni.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, Lavail MM, Walter P. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korennykh A, Walter P. Structural basis of the unfolded protein response. Annu Rev Cell Dev Biol. 2012;28:251–277. doi: 10.1146/annurev-cellbio-101011-155826. [DOI] [PubMed] [Google Scholar]
- 31.Jensen MB, Jasper H. Mitochondrial proteostasis in the control of aging and longevity. Cell Metab. 2014;20:214–225. doi: 10.1016/j.cmet.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang M, Kaufman RJ. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature. 2016;529:326–335. doi: 10.1038/nature17041. [DOI] [PubMed] [Google Scholar]
- 33.Taniguchi K, Karin M. NF-kappaB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18:309–324. doi: 10.1038/nri.2017.142. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tam AB, Mercado EL, Hoffmann A, Niwa M. ER stress activates NF-kappaB by integrating functions of basal IKK activity, IRE1 and PERK. PLoS One. 2012;7:e45078. doi: 10.1371/journal.pone.0045078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Badiola N, Penas C, Minano-Molina A, Barneda-Zahonero B, Fado R, Sanchez-Opazo G, Comella JX, Sabria J, Zhu C, Blomgren K, Casas C, Rodriguez-Alvarez J. Induction of ER stress in response to oxygen-glucose deprivation of cortical cultures involves the activation of the PERK and IRE-1 pathways and of caspase-12. Cell Death Dis. 2011;2:e149. doi: 10.1038/cddis.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woo CW, Kutzler L, Kimball SR, Tabas I. Toll-like receptor activation suppresses ER stress factor CHOP and translation inhibition through activation of eIF2B. Nat Cell Biol. 2012;14:192–200. doi: 10.1038/ncb2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Senkal CE, Ponnusamy S, Bielawski J, Hannun YA, Ogretmen B. Antiapoptotic roles of ceramide-synthase-6-generated C16-ceramide via selective regulation of the ATF6/CHOP arm of ER-stress-response pathways. FASEB J. 2010;24:296–308. doi: 10.1096/fj.09-135087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang J, Liang Y, Lin Y, Liu Y, YouYou , Yin W. IRE1alpha-TRAF2-ASK1 pathway is involved in CSTMP-induced apoptosis and ER stress in human non-small cell lung cancer A549 cells. Biomed Pharmacother. 2016;82:281–289. doi: 10.1016/j.biopha.2016.04.050. [DOI] [PubMed] [Google Scholar]
- 40.Coelho DS, Domingos PM. Physiological roles of regulated Ire1 dependent decay. Front Genet. 2014;5:76. doi: 10.3389/fgene.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma X, Long F, Yun Y, Dang J, Wei S, Zhang Q, Li J, Zhang H, Zhang W, Wang Z, Liu Q, Zou C. ORMDL3 and its implication in inflammatory disorders. Int J Rheum Dis. 2018;21:1154–1162. doi: 10.1111/1756-185X.13324. [DOI] [PubMed] [Google Scholar]
- 42.Moffatt MF, Kabesch M, Liang L, Dixon AL, Strachan D, Heath S, Depner M, von Berg A, Bufe A, Rietschel E, Heinzmann A, Simma B, Frischer T, Willis-Owen SA, Wong KC, Illig T, Vogelberg C, Weiland SK, von Mutius E, Abecasis GR, Farrall M, Gut IG, Lathrop GM, Cookson WO. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature. 2007;448:470–473. doi: 10.1038/nature06014. [DOI] [PubMed] [Google Scholar]
- 43.Fang Q, Zhao H, Wang A, Gong Y, Liu Q. Association of genetic variants in chromosome 17q21 and adult-onset asthma in a Chinese Han population. BMC Med Genet. 2011;12:133. doi: 10.1186/1471-2350-12-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.James B, Milstien S, Spiegel S. ORMDL3 and allergic asthma: from physiology to pathology. J Allergy Clin Immunol. 2019;144:634–640. doi: 10.1016/j.jaci.2019.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cantero-Recasens G, Fandos C, Rubio-Moscardo F, Valverde MA, Vicente R. The asthma-associated ORMDL3 gene product regulates endoplasmic reticulum-mediated calcium signaling and cellular stress. Hum Mol Genet. 2010;19:111–121. doi: 10.1093/hmg/ddp471. [DOI] [PubMed] [Google Scholar]
- 46.Jang Y, Lee AY, Kim JE, Jeong SH, Kim JS, Cho MH. Benomyl-induced effects of ORMDL3 overexpression via oxidative stress in human bronchial epithelial cells. Food Chem Toxicol. 2016;98:100–106. doi: 10.1016/j.fct.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 47.Liu YP, Rajamanikham V, Baron M, Patel S, Mathur SK, Schwantes EA, Ober C, Jackson DJ, Gern JE, Lemanske RF Jr, Smith JA. Association of ORMDL3 with rhinovirus-induced endoplasmic reticulum stress and type I Interferon responses in human leucocytes. Clin Exp Allergy. 2017;47:371–382. doi: 10.1111/cea.12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loser S, Gregory LG, Zhang Y, Schaefer K, Walker SA, Buckley J, Denney L, Dean CH, Cookson WOC, Moffatt MF, Lloyd CM. Pulmonary ORMDL3 is critical for induction of Alternaria-induced allergic airways disease. J Allergy Clin Immunol. 2017;139:1496–1507. e1493. doi: 10.1016/j.jaci.2016.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hsu KJ, Turvey SE. Functional analysis of the impact of ORMDL3 expression on inflammation and activation of the unfolded protein response in human airway epithelial cells. Allergy Asthma Clin Immunol. 2013;9:4. doi: 10.1186/1710-1492-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Debeuf N, Zhakupova A, Steiner R, Van Gassen S, Deswarte K, Fayazpour F, Van Moorleghem J, Vergote K, Pavie B, Lemeire K, Hammad H, Hornemann T, Janssens S, Lambrecht BN. The ORMDL3 asthma susceptibility gene regulates systemic ceramide levels without altering key asthma features in mice. J Allergy Clin Immunol. 2019;144:1648–1659. e9. doi: 10.1016/j.jaci.2019.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gregory LG, Lloyd CM. Orchestrating house dust mite-associated allergy in the lung. Trends Immunol. 2011;32:402–411. doi: 10.1016/j.it.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sporik R, Holgate ST, Platts-Mills TA, Cogswell JJ. Exposure to house-dust mite allergen (Der p I) and the development of asthma in childhood. A prospective study. N Engl J Med. 1990;323:502–507. doi: 10.1056/NEJM199008233230802. [DOI] [PubMed] [Google Scholar]
- 53.Hoffman SM, Tully JE, Nolin JD, Lahue KG, Goldman DH, Daphtary N, Aliyeva M, Irvin CG, Dixon AE, Poynter ME, Anathy V. Endoplasmic reticulum stress mediates house dust mite-induced airway epithelial apoptosis and fibrosis. Respir Res. 2013;14:141. doi: 10.1186/1465-9921-14-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoffman SM, Chapman DG, Lahue KG, Cahoon JM, Rattu GK, Daphtary N, Aliyeva M, Fortner KA, Erzurum SC, Comhair SA, Woodruff PG, Bhakta N, Dixon AE, Irvin CG, Janssen-Heininger YM, Poynter ME, Anathy V. Protein disulfide isomerase-endoplasmic reticulum resident protein 57 regulates allergen-induced airways inflammation, fibrosis, and hyperresponsiveness. J Allergy Clin Immunol. 2016;137:822–832. e827. doi: 10.1016/j.jaci.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuan X, Wang J, Li Y, He X, Niu B, Wu D, Lan N, Wang X, Zhang Y, Dai X, Wang X, Liu Z, Li G. Allergy immunotherapy restores airway epithelial barrier dysfunction through suppressing IL-25 -induced endoplasmic reticulum stress in asthma. Sci Rep. 2018;8:7950. doi: 10.1038/s41598-018-26221-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li P, Fu D, Sheng Q, Yu S, Bao X, Lv Z. TUDCA attenuates intestinal injury and inhibits endoplasmic reticulum stress-mediated intestinal cell apoptosis in necrotizing enterocolitis. Int Immunopharmacol. 2019;74:105665. doi: 10.1016/j.intimp.2019.05.050. [DOI] [PubMed] [Google Scholar]
- 57.Rani S, Sreenivasaiah PK, Kim JO, Lee MY, Kang WS, Kim YS, Ahn Y, Park WJ, Cho C, Kim DH. Tauroursodeoxycholic acid (TUDCA) attenuates pressure overload-induced cardiac remodeling by reducing endoplasmic reticulum stress. PLoS One. 2017;12:e0176071. doi: 10.1371/journal.pone.0176071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Siddesha JM, Nakada EM, Mihavics BR, Hoffman SM, Rattu GK, Chamberlain N, Cahoon JM, Lahue KG, Daphtary N, Aliyeva M, Chapman DG, Desai DH, Poynter ME, Anathy V. Effect of a chemical chaperone, tauroursodeoxycholic acid, on HDM-induced allergic airway disease. Am J Physiol Lung Cell Mol Physiol. 2016;310:L1243–1259. doi: 10.1152/ajplung.00396.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vang S, Longley K, Steer CJ, Low WC. The unexpected uses of urso- and tauroursodeoxycholic acid in the treatment of non-liver diseases. Glob Adv Health Med. 2014;3:58–69. doi: 10.7453/gahmj.2014.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gronbeck KR, Rodrigues CM, Mahmoudi J, Bershad EM, Ling G, Bachour SP, Divani AA. Application of tauroursodeoxycholic acid for treatment of neurological and non-neurological diseases: is there a potential for treating traumatic brain injury? Neurocrit Care. 2016;25:153–166. doi: 10.1007/s12028-015-0225-7. [DOI] [PubMed] [Google Scholar]
- 61.Lee HY, Lee GH, Kim HK, Chae HJ. Platycodi Radix and its active compounds ameliorate against house dust mite-induced allergic airway inflammation and ER stress and ROS by enhancing anti-oxidation. Food Chem Toxicol. 2019;123:412–423. doi: 10.1016/j.fct.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 62.Yang L, Hirose S, Suzuki K, Hiroi T, Takaiwa F. Expression of hypoallergenic Der f 2 derivatives with altered intramolecular disulphide bonds induces the formation of novel ER-derived protein bodies in transgenic rice seeds. J Exp Bot. 2012;63:2947–2959. doi: 10.1093/jxb/ers006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jeong JS, Kim SR, Lee YC. Can controlling endoplasmic reticulum dysfunction treat allergic inflammation in severe asthma with fungal sensitization? Allergy Asthma Immunol Res. 2018;10:106–120. doi: 10.4168/aair.2018.10.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Snelgrove RJ, Gregory LG, Peiro T, Akthar S, Campbell GA, Walker SA, Lloyd CM. Alternaria-derived serine protease activity drives IL-33-mediated asthma exacerbations. J Allergy Clin Immunol. 2014;134:583–592. e586. doi: 10.1016/j.jaci.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee KS, Jeong JS, Kim SR, Cho SH, Kolliputi N, Ko YH, Lee KB, Park SC, Park HJ, Lee YC. Phosphoinositide 3-kinase-delta regulates fungus-induced allergic lung inflammation through endoplasmic reticulum stress. Thorax. 2016;71:52–63. doi: 10.1136/thoraxjnl-2015-207096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee HY, Lee GH, Kim HR, Lee YC, Chae HJ. PI3K-delta controls endoplasmic reticulum membrane fluidity and permeability in fungus-induced allergic inflammation. Br J Pharmacol. 2020;177:1556–1567. doi: 10.1111/bph.14917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jeong JS, Lee KB, Kim SR, Kim DI, Park HJ, Lee HK, Kim HJ, Cho SH, Kolliputi N, Kim SH, Lee YC. Airway epithelial phosphoinositide 3-kinase-delta contributes to the modulation of fungi-induced innate immune response. Thorax. 2018;73:758–768. doi: 10.1136/thoraxjnl-2017-210326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Joubert A, Simoneau P, Campion C, Bataille-Simoneau N, Iacomi-Vasilescu B, Poupard P, Francois JM, Georgeault S, Sellier E, Guillemette T. Impact of the unfolded protein response on the pathogenicity of the necrotrophic fungus Alternaria brassicicola. Mol Microbiol. 2011;79:1305–1324. doi: 10.1111/j.1365-2958.2010.07522.x. [DOI] [PubMed] [Google Scholar]
- 69.Yeganeh B, Rezaei Moghadam A, Tran AT, Rahim MN, Ande SR, Hashemi M, Coombs KM, Ghavami S. Asthma and influenza virus infection: focusing on cell death and stress pathways in influenza virus replication. Iran J Allergy Asthma Immunol. 2013;12:1–17. [PubMed] [Google Scholar]
- 70.Shen ZJ, Hu J, Kashi V, Bochkov YA, Gern JE, Malter JS. TLR-7 stress signaling in differentiating and mature eosinophils is mediated by the prolyl isomerase Pin1. J Immunol. 2018;201:3503–3513. doi: 10.4049/jimmunol.1800881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takahashi K, Pavlidis S, Ng Kee Kwong F, Hoda U, Rossios C, Sun K, Loza M, Baribaud F, Chanez P, Fowler SJ, Horvath I, Montuschi P, Singer F, Musial J, Dahlen B, Dahlen SE, Krug N, Sandstrom T, Shaw DE, Lutter R, Bakke P, Fleming LJ, Howarth PH, Caruso M, Sousa AR, Corfield J, Auffray C, De Meulder B, Lefaudeux D, Djukanovic R, Sterk PJ, Guo Y, Adcock IM, Chung KF on behalf of the U-BIOPRED study group. Sputum proteomics and airway cell transcripts of current and ex-smokers with severe asthma in U-BIOPRED: an exploratory analysis. Eur Respir J. 2018;51 doi: 10.1183/13993003.02173-2017. [DOI] [PubMed] [Google Scholar]
- 72.Wang Y, Wu ZZ, Wang W. Inhibition of endoplasmic reticulum stress alleviates cigarette smoke-induced airway inflammation and emphysema. Oncotarget. 2017;8:77685–77695. doi: 10.18632/oncotarget.20768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.He B, Chen Q, Zhou D, Wang L, Liu Z. Role of reciprocal interaction between autophagy and endoplasmic reticulum stress in apoptosis of human bronchial epithelial cells induced by cigarette smoke extract. IUBMB Life. 2019;71:66–80. doi: 10.1002/iub.1937. [DOI] [PubMed] [Google Scholar]
- 74.Tan SX, Jiang DX, Hu RC, Dai AG, Gan GX, Fu DY, Kong CC, Chen YR, Wang LL, Li J. Endoplasmic reticulum stress induces HRD1 to protect alveolar type II epithelial cells from apoptosis induced by cigarette smoke extract. Cell Physiol Biochem. 2017;43:1337–1345. doi: 10.1159/000481845. [DOI] [PubMed] [Google Scholar]
- 75.Lee KY, Park SY, Park S, Hong GH, Moon KA, Kim YS, Oh YM, Kwon HS, Kim TB, Moon HB, Cho YS. Progranulin protects lung epithelial cells from cigarette smoking-induced apoptosis. Respirology. 2017;22:1140–1148. doi: 10.1111/resp.13023. [DOI] [PubMed] [Google Scholar]
- 76.Lin F, Liao C, Sun Y, Zhang J, Lu W, Bai Y, Liao Y, Li M, Ni X, Hou Y, Qi Y, Chen Y. Hydrogen sulfide inhibits cigarette smoke-induced endoplasmic reticulum stress and apoptosis in bronchial epithelial cells. Front Pharmacol. 2017;8:675. doi: 10.3389/fphar.2017.00675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Giuliani C, Napolitano G, Bucci I, Montani V, Monaco F. Nf-kB transcription factor: role in the pathogenesis of inflammatory, autoimmune, and neoplastic diseases and therapy implications. Clin Ter. 2001;152:249–253. [PubMed] [Google Scholar]
- 78.Hasnain SZ, Lourie R, Das I, Chen AC, McGuckin MA. The interplay between endoplasmic reticulum stress and inflammation. Immunol Cell Biol. 2012;90:260–270. doi: 10.1038/icb.2011.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cao SS, Luo KL, Shi L. Endoplasmic reticulum stress interacts with Inflammation in Human Diseases. J Cell Physiol. 2016;231:288–294. doi: 10.1002/jcp.25098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kaneko M, Niinuma Y, Nomura Y. Activation signal of nuclear factor-kappa B in response to endoplasmic reticulum stress is transduced via IRE1 and tumor necrosis factor receptor-associated factor 2. Biol Pharm Bull. 2003;26:931–935. doi: 10.1248/bpb.26.931. [DOI] [PubMed] [Google Scholar]
- 81.Jiang HY, Wek SA, McGrath BC, Scheuner D, Kaufman RJ, Cavener DR, Wek RC. Phosphorylation of the alpha subunit of eukaryotic initiation factor 2 is required for activation of NF-kappaB in response to diverse cellular stresses. Mol Cell Biol. 2003;23:5651–5663. doi: 10.1128/MCB.23.16.5651-5663.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Deng J, Lu PD, Zhang Y, Scheuner D, Kaufman RJ, Sonenberg N, Harding HP, Ron D. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol Cell Biol. 2004;24:10161–10168. doi: 10.1128/MCB.24.23.10161-10168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yamazaki H, Hiramatsu N, Hayakawa K, Tagawa Y, Okamura M, Ogata R, Huang T, Nakajima S, Yao J, Paton AW, Paton JC, Kitamura M. Activation of the Akt-NF-kappaB pathway by subtilase cytotoxin through the ATF6 branch of the unfolded protein response. J Immunol. 2009;183:1480–1487. doi: 10.4049/jimmunol.0900017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shkoda A, Ruiz PA, Daniel H, Kim SC, Rogler G, Sartor RB, Haller D. Interleukin-10 blocked endoplasmic reticulum stress in intestinal epithelial cells: impact on chronic inflammation. Gastroenterology. 2007;132:190–207. doi: 10.1053/j.gastro.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 85.Rosen DA, Seki SM, Fernandez-Castaneda A, Beiter RM, Eccles JD, Woodfolk JA, Gaultier A. Modulation of the sigma-1 receptor-IRE1 pathway is beneficial in preclinical models of inflammation and sepsis. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aau5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bhakta NR, Christenson SA, Nerella S, Solberg OD, Nguyen CP, Choy DF, Jung KL, Garudadri S, Bonser LR, Pollack JL, Zlock LT, Erle DJ, Langelier C, Derisi JL, Arron JR, Fahy JV, Woodruff PG. IFN-stimulated gene expression, type 2 inflammation, and endoplasmic reticulum stress in asthma. Am J Respir Crit Care Med. 2018;197:313–324. doi: 10.1164/rccm.201706-1070OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guo Q, Li H, Liu J, Xu L, Yang L, Sun Z, Zhou B. Tunicamycin aggravates endoplasmic reticulum stress and airway inflammation via PERK-ATF4-CHOP signaling in a murine model of neutrophilic asthma. J Asthma. 2017;54:125–133. doi: 10.1080/02770903.2016.1205085. [DOI] [PubMed] [Google Scholar]
- 88.Kim SR, Kim DI, Kang MR, Lee KS, Park SY, Jeong JS, Lee YC. Endoplasmic reticulum stress influences bronchial asthma pathogenesis by modulating nuclear factor kappaB activation. J Allergy Clin Immunol. 2013;132:1397–1408. doi: 10.1016/j.jaci.2013.08.041. [DOI] [PubMed] [Google Scholar]
- 89.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hu H, Tian M, Ding C, Yu S. The C/EBP homologous protein (CHOP) transcription factor functions in endoplasmic reticulum stress-induced apoptosis and microbial infection. Front Immunol. 2018;9:3083. doi: 10.3389/fimmu.2018.03083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ma R, Yang L, Niu F, Buch S. HIV Tat-mediated induction of human brain microvascular endothelial cell apoptosis involves endoplasmic reticulum stress and mitochondrial dysfunction. Mol Neurobiol. 2016;53:132–142. doi: 10.1007/s12035-014-8991-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shah A, Vaidya NK, Bhat HK, Kumar A. HIV-1 gp120 induces type-1 programmed cell death through ER stress employing IRE1alpha, JNK and AP-1 pathway. Sci Rep. 2016;6:18929. doi: 10.1038/srep18929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Timmins JM, Ozcan L, Seimon TA, Li G, Malagelada C, Backs J, Backs T, Bassel-Duby R, Olson EN, Anderson ME, Tabas I. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J Clin Invest. 2009;119:2925–2941. doi: 10.1172/JCI38857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lu M, Lawrence DA, Marsters S, Acosta-Alvear D, Kimmig P, Mendez AS, Paton AW, Paton JC, Walter P, Ashkenazi A. Opposing unfolded-protein-response signals converge on death receptor 5 to control apoptosis. Science. 2014;345:98–101. doi: 10.1126/science.1254312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yao Y, Wang Y, Zhang Z, He L, Zhu J, Zhang M, He X, Cheng Z, Ao Q, Cao Y, Yang P, Su Y, Zhao J, Zhang S, Yu Q, Ning Q, Xiang X, Xiong W, Wang CY, Xu Y. Chop deficiency protects mice against bleomycin-induced pulmonary fibrosis by attenuating M2 macrophage production. Mol Ther. 2016;24:915–925. doi: 10.1038/mt.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Evans CM, Kim K, Tuvim MJ, Dickey BF. Mucus hypersecretion in asthma: causes and effects. Curr Opin Pulm Med. 2009;15:4–11. doi: 10.1097/MCP.0b013e32831da8d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang G, Xu Z, Wang R, Al-Hijji M, Salit J, Strulovici-Barel Y, Tilley AE, Mezey JG, Crystal RG. Genes associated with MUC5AC expression in small airway epithelium of human smokers and non-smokers. BMC Med Genomics. 2012;5:21. doi: 10.1186/1755-8794-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ran Q, Zhang L, Qiu YH, Wing X, Li GP. Effects of 3-methyladenine on airway inflammation, airway hyperresponsiveness and mucus secretion in asthmatic mice. Zhonghua Jie He He Hu Xi Za Zhi. 2019;42:185–192. doi: 10.3760/cma.j.issn.1001-0939.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 100.Lu Y, Xing QQ, Xu JY, Ding D, Zhao X. Astragalus polysaccharide modulates ER stress response in an OVA-LPS induced murine model of severe asthma. Int J Biol Macromol. 2016;93:995–1006. doi: 10.1016/j.ijbiomac.2016.09.058. [DOI] [PubMed] [Google Scholar]
- 101.Park SH, Gong JH, Choi YJ, Kang MK, Kim YH, Kang YH. Kaempferol inhibits endoplasmic reticulum stress-associated mucus hypersecretion in airway epithelial cells and ovalbumin-sensitized mice. PLoS One. 2015;10:e0143526. doi: 10.1371/journal.pone.0143526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schroeder BW, Verhaeghe C, Park SW, Nguyenvu LT, Huang X, Zhen G, Erle DJ. AGR2 is induced in asthma and promotes allergen-induced mucin overproduction. Am J Respir Cell Mol Biol. 2012;47:178–185. doi: 10.1165/rcmb.2011-0421OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Martino MB, Jones L, Brighton B, Ehre C, Abdulah L, Davis CW, Ron D, O’Neal WK, Ribeiro CM. The ER stress transducer IRE1beta is required for airway epithelial mucin production. Mucosal Immunol. 2013;6:639–654. doi: 10.1038/mi.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen G, Ribeiro CMP, Sun L, Okuda K, Kato T, Gilmore RC, Martino MB, Dang H, Abzhanova A, Lin JM, Hull-Ryde EA, Volmer AS, Randell SH, Livraghi-Butrico A, Deng Y, Scherer PE, Stripp BR, O’Neal WK, Boucher RC. XBP1S regulates MUC5B in a promoter variant-dependent pathway in idiopathic pulmonary fibrosis airway epithelia. Am J Respir Crit Care Med. 2019;200:220–234. doi: 10.1164/rccm.201810-1972OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang X, Yang X, Li Y, Wang X, Zhang Y, Dai X, Niu B, Wu J, Yuan X, Xiong A, Liu Z, Zhong N, Wu M, Li G. Lyn kinase represses mucus hypersecretion by regulating IL-13-induced endoplasmic reticulum stress in asthma. EBioMedicine. 2017;15:137–149. doi: 10.1016/j.ebiom.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim MH, Bae CH, Choi YS, Na HG, Song SY, Kim YD. Endoplasmic reticulum stress induces MUC5AC and MUC5B expression in human nasal airway epithelial cells. Clin Exp Otorhinolaryngol. 2019;12:181–189. doi: 10.21053/ceo.2018.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shang Y, Wang F, Bai C, Huang Y, Zhao LJ, Yao XP, Li Q, Sun SH. Dexamethasone protects airway epithelial cell line NCI-H292 against lipopolysaccharide induced endoplasmic reticulum stress and apoptosis. Chin Med J (Engl) 2011;124:38–44. [PubMed] [Google Scholar]
- 108.Cockcroft DW, Davis BE. Mechanisms of airway hyperresponsiveness. J Allergy Clin Immunol. 2006;118:551–559. doi: 10.1016/j.jaci.2006.07.012. quiz 560-551. [DOI] [PubMed] [Google Scholar]
- 109.Delmotte P, Sieck GC. Interaction between endoplasmic/sarcoplasmic reticulum stress (ER/SR stress), mitochondrial signaling and Ca(2+) regulation in airway smooth muscle (ASM) Can J Physiol Pharmacol. 2015;93:97–110. doi: 10.1139/cjpp-2014-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Que CX, Yu YJ, Chen H, Shi C, Xue AM. Research progress and forensic application on the pathogenesis of coronary artery spasm. Fa Yi Xue Za Zhi. 2018;34:60–66. doi: 10.3969/j.issn.1004-5619.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 111.Kaphalia L, Kalita M, Kaphalia BS, Calhoun WJ. Effects of acute ethanol exposure on cytokine production by primary airway smooth muscle cells. Toxicol Appl Pharmacol. 2016;292:85–93. doi: 10.1016/j.taap.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 112.Kim HK, Lee GH, Bhattarai KR, Junjappa RP, Lee HY, Handigund M, Marahatta A, Bhandary B, Baek IH, Pyo JS, Kim HK, Chai OH, Kim HR, Lee YC, Chae HJ. PI3Kdelta contributes to ER stress-associated asthma through ER-redox disturbances: the involvement of the RIDD-RIG-I-NF-kappaB axis. Exp Mol Med. 2018;50:e444. doi: 10.1038/emm.2017.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Burman A, Tanjore H, Blackwell TS. Endoplasmic reticulum stress in pulmonary fibrosis. Matrix Biol. 2018;68-69:355–365. doi: 10.1016/j.matbio.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mo XT, Zhou WC, Cui WH, Li DL, Li LC, Xu L, Zhao P, Gao J. Inositol-requiring protein 1-X-box-binding protein 1 pathway promotes epithelial-mesenchymal transition via mediating snail expression in pulmonary fibrosis. Int J Biochem Cell Biol. 2015;65:230–238. doi: 10.1016/j.biocel.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 115.Zhang L, Xu D, Li Q, Yang Y, Xu H, Wei Z, Wang R, Zhang W, Liu Y, Geng Y, Li S, Gao X, Yang F. N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP) attenuates silicotic fibrosis by suppressing apoptosis of alveolar type II epithelial cells via mediation of endoplasmic reticulum stress. Toxicol Appl Pharmacol. 2018;350:1–10. doi: 10.1016/j.taap.2018.04.025. [DOI] [PubMed] [Google Scholar]
- 116.Hansbro PM, Kaiko GE, Foster PS. Cytokine/anti-cytokine therapy - novel treatments for asthma? Br J Pharmacol. 2011;163:81–95. doi: 10.1111/j.1476-5381.2011.01219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim SR, Lee YC. Endoplasmic reticulum stress and the related signaling networks in severe asthma. Allergy Asthma Immunol Res. 2015;7:106–117. doi: 10.4168/aair.2015.7.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jeong JS, Kim SR, Cho SH, Lee YC. A novel insight on endotyping heterogeneous severe asthma based on endoplasmic reticulum stress: beyond the “type 2/non-type 2 dichotomy”. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20030713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Qin W, Wu X, Jia Y, Tong X, Guo C, Chen D, Wang Z, Tan N. Suhuang antitussive capsule inhibits NLRP3 inflammasome activation and ameliorates pulmonary dysfunction via suppression of endoplasmic reticulum stress in cough variant asthma. Biomed Pharmacother. 2019;118:109188. doi: 10.1016/j.biopha.2019.109188. [DOI] [PubMed] [Google Scholar]
- 120.Nakada EM, Bhakta NR, Korwin-Mihavics BR, Kumar A, Chamberlain N, Bruno SR, Chapman DG, Hoffman SM, Daphtary N, Aliyeva M, Irvin CG, Dixon AE, Woodruff PG, Amin S, Poynter ME, Desai DH, Anathy V. Conjugated bile acids attenuate allergen-induced airway inflammation and hyperresponsiveness by inhibiting UPR transducers. JCI Insight. 2019;4 doi: 10.1172/jci.insight.98101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Poe C, Youngblood C, Hodge K, Kemp K. Treatment of established TH2 cells with 4mu8c, an inhibitor of IRE1alpha, blocks IL-5 but not IL-4 secretion. BMC Immunol. 2019;20:3. doi: 10.1186/s12865-018-0283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Makhija L, Krishnan V, Rehman R, Chakraborty S, Maity S, Mabalirajan U, Chakraborty K, Ghosh B, Agrawal A. Chemical chaperones mitigate experimental asthma by attenuating endoplasmic reticulum stress. Am J Respir Cell Mol Biol. 2014;50:923–931. doi: 10.1165/rcmb.2013-0320OC. [DOI] [PubMed] [Google Scholar]
- 123.Lu Y, Xu JY, Zhang XH, Zhao X. Gu-Ben-Fang-Xiao decoction attenuates sustained airway inflammation by suppressing ER stress response in a murine asthma remission model of respiratory syncytial virus infection. J Ethnopharmacol. 2016;192:496–509. doi: 10.1016/j.jep.2016.09.039. [DOI] [PubMed] [Google Scholar]
- 124.Fu T, Wang L, Zeng Q, Zhang Y, Sheng B, Han L. Ghrelin ameliorates asthma by inhibiting endoplasmic reticulum stress. Am J Med Sci. 2017;354:617–625. doi: 10.1016/j.amjms.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 125.Lv CM, Wu HM, Wu L, Xu GH, Yang ZL, Shen QY. Sevoflurane modulates AQPs (1,5) expression and endoplasmic reticulum stress in mice lung with allergic airway inflammation. Biosci Rep. 2019;39 doi: 10.1042/BSR20193282. [DOI] [PMC free article] [PubMed] [Google Scholar]