Key Points
Question
What is the effect of PCSK9 inhibitor evolocumab on different types and sizes of myocardial infarction among patients with stable atherosclerosis receiving statin therapy?
Findings
In a prespecified analysis of the Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER) trial, a total of 1288 myocardial infarctions occurred in 1107 patients. Most of the myocardial infarctions prevented by evolocumab were type 1, and consistent reductions were seen for type 1 and type 4, ST-segment elevation, and non–ST-segment elevation myocardial infarction, and those with higher biomarker levels.
Meaning
Results of this study suggest that low-density lipoprotein cholesterol–lowering therapy with evolocumab resulted in benefit across multiple myocardial infarction subtypes.
Abstract
Importance
The PCSK9 inhibitor evolocumab reduced major vascular events in the Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER) trial, yet the types and sizes of myocardial outcomes in FOURIER have not been previously explored.
Objective
To assess the types and sizes of myocardial infarction (MI) and the effect of evolocumab on MI by subtype.
Design, Setting, and Participants
A prespecified analysis of a multicenter double-blind randomized clinical trial. Patients were randomized to evolocumab or placebo and followed up for a median of 2.2 years. The study included 27 564 patients with stable atherosclerotic disease receiving statin therapy. Clinical end points were evaluated by the Thrombolysis in Myocardial Infarction clinical events committee. Rates presented are 3-year Kaplan-Meier estimates. Data were collected from 2013 to 2016 and analyzed from June 2017 to December 2019.
Main Outcomes and Measures
Myocardial infarction was defined based on the third universal MI definition, and further classified according to MI type (universal MI subclass, ST-segment elevation myocardial infarction [STEMI] vs non–STEMI) and by MI size (determined by peak troponin level).
Results
A total of 27 564 patients were randomized, with a mean (SD) age of 62.5 (9.0) years, and 20 795 (75%) were male. Of these, 1107 patients experienced a total of 1288 MIs. Most MIs (68%) were atherothrombotic (type 1), with 15% from myocardial oxygen supply-demand mismatch (type 2) and 15% percutaneous coronary intervention–related (type 4). Sudden death (type 3) and coronary artery bypass grafting–related (type 5) accounted for a total of 21 MIs (<2%). Evolocumab significantly reduced the risk of first MI by 27% (4.4% vs 6.3%; hazard ratio [HR], 0.73; 95% CI, 0.65-0.82; P < .001), type 1 by 32% (2.9% vs 4.5%; HR, 0.68; 95% CI, 0.59-0.79; P < .001), and type 4 by 35% (0.8% vs 1.1%; HR, 0.65; 95% CI, 0.48-0.87; P = .004), with no effect on type 2 (0.9% vs 0.8%; HR, 1.09; 95% CI, 0.82-1.45; P = .56). Most MIs (688 [59.8%]) had troponin levels greater than or equal to 10 times the upper limit of normal. The benefit was highly significant and consistent regardless of the size of MI with a 34% reduction in MIs with troponin level greater than or equal to 10 times the upper limit of normal (2.6% vs 3.7%; HR, 0.66; 95% CI, 0.56-0.77; P < .001) and a 36% reduction in the risk of STEMI (1.0% vs 1.5%; HR, 0.64; 95% CI, 0.49-0.84; P < .001).
Conclusions and Relevance
Low-density lipoprotein cholesterol lowering with evolocumab was highly effective in reducing the risk of MI. This reduction with evolocumab included benefit across multiple subtypes of MI related to plaque rupture, smaller and larger MIs, and both STEMI and non–STEMI. These data are consistent with the known benefit of low-density lipoprotein cholesterol lowering and underscore the reduction in clinically meaningful events.
Trial Registration
ClinicalTrials.gov Identifier: NCT01764633
This prespecified analysis of a randomized clinical trial evaluates the effect of evolocumab on type and size of subsequent myocardial infarction.
Introduction
Lipid-lowering treatment with statins has been reported to reduce cardiovascular events including myocardial infarction (MI), with greater benefit for more intensive lipid lowering.1 Major coronary events are consistently and robustly reduced by lowering low-density lipoprotein cholesterol [LDL-C] levels with statin therapy.1 These benefits extend to nonstatin LDL-C lowering with ezetimibe,2 proprotein convertase subtilisin-kexin type 9 (PCSK9) inhibitors,3 and in one trial of a cholesterol ester transfer protein inhibitor.4 This event reduction has been observed in patients at varying levels of risk. As such, lipid-lowering therapy is a cornerstone of primary and secondary prevention of MI.5
The Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER) trial was the first large-scale trial to demonstrate a reduction in cardiovascular (CV) outcomes with PCSK9 inhibition in patients with cardiovascular disease.3 Overall, evolocumab reduced the primary composite end point of cardiovascular death, MI, stroke, hospitalization for unstable angina, or coronary revascularization events by 15% and the key secondary end point of CV death, MI, or stroke by 20% over a median of 2.2 years. A robust 27% reduction in MI contributed to the effect on the primary end point.3
Myocardial infarction is characterized by the death of cardiac myocytes, generally diagnosed by elevation of cardiac biomarkers along with clinical evidence of ischemia.6 Biomarker assays and definitions of MI have evolved over time and a recognition that the prognosis associated with different clinical circumstances (spontaneous or procedural), subtypes (ST-elevation or non-ST elevation) or sizes of MI is not uniform.7,8 We therefore sought to examine the MI outcomes in FOURIER to better understand the events prevented.
Methods
Study Population
The FOURIER trial was a randomized, double-blind, placebo-controlled trial of evolocumab (either 140 mg every 2 weeks or 420 mg monthly, per patient preference) on a background of statin therapy that enrolled 27 564 patients aged 40 to 85 years with clinically evident cardiovascular disease (prior MI, prior nonhemorrhagic stroke, or symptomatic peripheral arterial disease). The details of the design and implementation have been previously published.9 The trial protocol is available in Supplement 1. Exclusions particularly relevant to the current analysis included MI within 4 weeks of randomization and planned or expected cardiac surgery or revascularization within 3 months after randomization. Ethics committee approvals for the FOURIER trial were obtained from all relevant organizations locally or through a central institutional review board within the country. Each patient provided written informed consent, which included use of data by the TIMI (Thrombolysis in Myocardial Infarction) Study Group.
Outcomes
The primary end point of FOURIER was time to first occurrence of the composite of cardiovascular death, MI, stroke, coronary revascularization, or hospitalization for unstable angina. A central clinical events committee led by the TIMI Study Group, whose members were unaware of treatment assignment and lipid levels, adjudicated all efficacy end points. Trial-based definitions of the end points have been published previously, and MI definitions were based on the third universal definition of MI.6,9
This study focuses on a prespecified descriptive analysis of MI end points. The TIMI clinical events committee adjudicated the events based on the third universal MI definitions.6,9 When events were confirmed to be MI, the clinical events committee further classified the events. All confirmed MI events were categorized by the clinical events committee into universal MI subtypes, subtypes related to electrocardiogram (ST-segment elevation myocardial infarction [STEMI] or non-STEMI), and by peak cardiac biomarkers. Acute and chronic myocardial injury not associated with ischemia were classified as no MI. Most events were type 1 (spontaneous atherothrombotic), type 2 (myocardial oxygen supply-demand mismatch), and type 4 (percutaneous coronary intervention [PCI]–related). A total of 21 events (<2% of the total) were either type 3 (presenting with typical symptoms and dying before cardiac biomarkers could be drawn) or type 5 (associated with coronary artery bypass grafting) and owing to a low proportion of events are not included in subtype analyses. Percutaneous coronary intervention–related MIs (type 4) were tabulated by subtype (related to PCI, stent thrombosis, or coronary artery bypass grafting) but then combined for the purpose of additional analyses (Figure 1). For description, MI was classified by size according to the peak cardiac troponin level elevation compared with the site-reported MI limit and categorized by multiples of upper limit of normal elevations for cardiac biomarkers. When comparing evolocumab to placebo, because of small numbers of events in each category, groups are expressed as 7 nonexclusive cumulative bins (≥1, ≥3, ≥5, ≥10, ≥25, ≥50, and ≥100 times elevation of upper limit of normal). To assess the timing of benefit, we performed serial landmark analyses of MI from 0 to 6 months, 6 to 12 months, 12 to 18 months, and greater than 18 months. Patients alive at the beginning of the landmark period were included regardless of MI in a prior period.
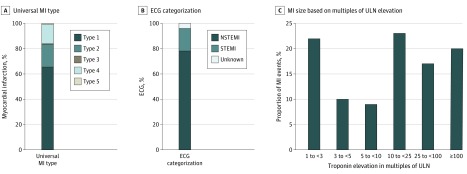
Figure 1. MI Type, ECG Categorization, and MI Size.
ECG indicates electrocardiogram; MI, myocardial infarction; STEMI, ST-segment elevation MI; NSTEMI, non-STEMI; ULN, upper limit of normal. Panel C values were rounded to the nearest whole number.
Statistical Analyses
All efficacy analyses of evolocumab vs placebo were conducted using randomized groups and on an intention-to-treat basis. Kaplan-Meier event rates were calculated through 3 years, and P values for time-to-event analyses are from log-rank tests. Patients could experience more than 1 MI during the study period, so analyses evaluating subtypes of MI included the first MI of that subtype. Hazard ratios (HRs) and 95% CIs for the effect of evolocumab vs placebo were generated using a Cox proportional hazards regression model with adjustment for the stratification factors of geographic region and baseline LDL-C level. To assess the risk of death associated with MIs of different size, we examined the proportion of patients who died during the follow-up period and after an MI that occurred during the trial compared with the survival of those without MI. If a patient had more than 1 MI, they were categorized according to the largest MI they experienced. To account for differences in baseline characteristics, an extended Cox proportional hazards regression analysis was performed using MI as a time-dependent covariate. Additional features in the Cox model included age, sex, body mass index, race/ethnicity, region, prior MI at baseline, history of stroke, peripheral artery disease, hypertension, diabetes, congestive heart failure, PCI, coronary artery bypass grafting and treatment group. Adjusted HRs are presented. All analyses were conducted using Stata/IC, version 14.2 (StataCorp LP) or SAS, version 9.4 (SAS Institute Inc). Two-sided P values <.05 were considered significant without correction for multiple comparisons. Data were collected from 2013 to 2016 and analyzed from June 2017 to December 2019.
Results
Population and MI Types
A total of 27 564 patients were randomized, with a mean (SD) age of 62.5 (9.0) years, and 20 795 (75%) were male. Of these, 1107 patients experienced a total of 1288 MIs. The number of participants randomized and constituting follow-up are shown in eFigure 1 in Supplement 2.3 Baseline characteristics of patients with MI compared with those without MI during follow-up are shown in the eTable in Supplement 2. Patients with MI during follow-up were more likely to be older, to have diabetes, an MI, or prior coronary revascularization prior to enrollment, and residual multivessel coronary disease.
Most MIs (68%) were atherothrombotic (type 1), with 15% from myocardial oxygen supply-demand mismatch (type 2), and 15% from PCI-related (type 4) (Figure 1). Less than 2% of the total MIs were either type 3 or type 5. When categorized by electrocardiogram type, 78% of MIs were non-STEMI, 18% were STEMI, and in 3.5% a designation based on electrocardiogram could not be determined (Figure 1). Myocardial infarction size by troponin level elevation could be determined in 1150 of 1288 adjudicated events (89%). Of those events, 59.8% had troponin level elevation greater than or equal to 10 times, including 23% with troponin level elevation 10 to less than 25 times the upper limit of normal, 17% with troponin level elevation 25 to less than 100 times the upper limit of normal, and 20% with troponin level elevation 100 or more times the upper limit of normal (Figure 1).
Larger MI size was associated with a higher risk of coronary heart disease death (Table). Compared with patients with no MI, patients with MI between 1 and 10 times the upper limit of normal had an approximately 7 times higher rate of subsequent cardiovascular death (HR, 6.47; 95% CI, 3.85-10.89; P < .001); those with biomarkers between 10 and 100 times the upper limit of normal had more than 10 times the rate of CV death (HR, 10.79; 95% CI, 6.63-17.56; P < .001), and those greater than or equal to 100 times the upper limit of normal had more than a 20 times increase in CV death (HR, 21.41; 95% CI, 13.63-33.62; P < .001).
Table. Risk of Coronary Death Following Largest MI, by MI Size .
| Subgroup | Total No. | No. (%) | Adjusted HR (95% CI) | P value |
|---|---|---|---|---|
| Coronary death | ||||
| No myocardial infarction | 26 457 | 261 (1.0) | 1 [Reference] | NA |
| 1 to <10 × ULN | 365 | 16 (4.4) | 6.47 (3.85-10.89) | <.001 |
| 10 to <100 × ULN | 326 | 18 (5.5) | 10.79 (6.63-17.56) | <.001 |
| ≥100 × ULN | 222 | 22 (9.9) | 21.41 (13.63-33.62) | <.001 |
Abbreviations: HR, hazard ratio; MI, myocardial infarction; NA, not applicable; ULN, upper limit of normal.
Evolocumab and MI
MI Type
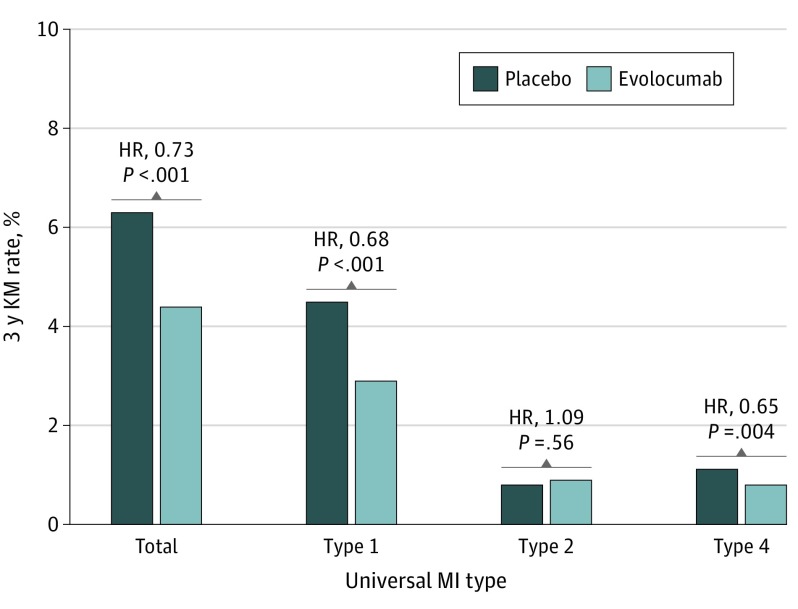
Evolocumab significantly reduced the risk of first MI by 27% (4.4% vs 6.3%; HR, 0.73; 95% CI, 0.65-0.82; P < .001). There was a significant benefit for both spontaneous and procedural MIs. Type 1 MI was reduced by 32% (2.9% vs 4.5%; HR, 0.68; 95% CI, 0.59-0.79; P < .001). Type 4 MI was reduced by 35% (0.8% vs 1.1%; HR, 0.65; 95% CI, 0.48-0.87; P = .004), with no effect on type 2 MI (0.9% vs 0.8%; HR, 1.09; 95% CI, 0.82-1.45; P = .56) (Figure 2).
Figure 2. Effect of Evolocumab by Universal MI Type.
Because of the small numbers of events, types 3 and 5 are not presented individually. HR indicates hazard ratio; KM, Kaplan-Meier; MI, myocardial infarction.
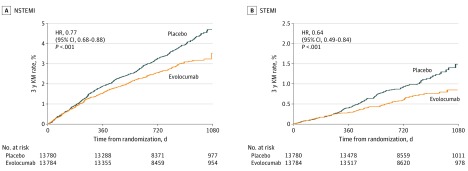
The benefit of evolocumab was also consistent regardless of the electrocardiogram categorization of MI. Specifically, evolocumab significantly reduced the risk of non–STEMI by 23% (3.5% vs 4.7%; HR, 0.77; 95% CI, 0.68-0.88; P < .001) and STEMI by 36% (1.0% vs 1.5%; HR, 0.64; 95% CI, 0.49-0.84; P < .001). The pattern and timing of benefit for STEMI and non–STEMI are similar with an apparent separation of event curves beginning at approximately 6 months for each and continuing to separate throughout follow-up (Figure 3). This pattern is also seen for both type 1 and type 4 MI (eFigure 2 in Supplement 2).
Figure 3. Effect of Evolocumab by MI Type.
HR indicates hazard ratio; KM, Kaplan-Meier; MI, myocardial infarction; NSTEMI, non–ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction.
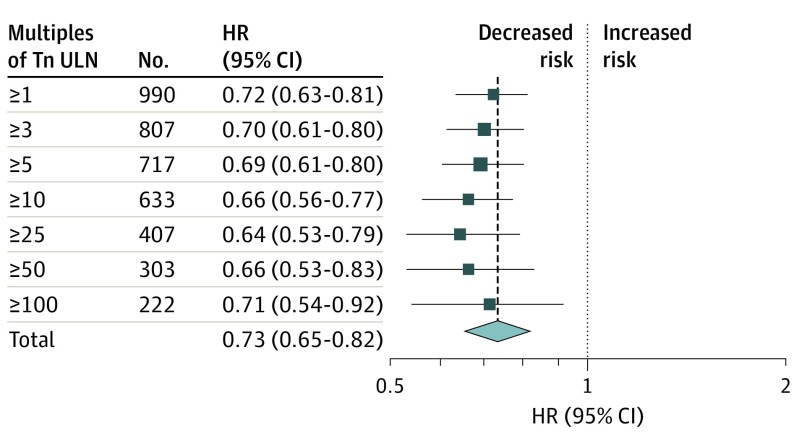
Troponin level values were available for 1150 MIs. Using multiples of upper limit of normal elevation of troponin level, most MIs (688 [59.8%]) had troponin levels greater than or equal to 10 times the upper limit of normal. The benefit was highly significant and consistent regardless of the size of MI with a 34% reduction in MIs with troponin level greater than or equal to 10 times the upper limit of normal (2.6% vs 3.7%; HR, 0.66; 95% CI, 0.56-0.77; P < .001). Figure 4 shows HRs and 95% CIs of all MIs categorized by elevation of troponin level. There was a consistent benefit with HRs ranging from 0.64 to 0.72 across the differing troponin level thresholds (Figure 4).
Figure 4. Effect of Evolocumab by MI Size Based on Multiples of Troponin ULN Elevation.
HR indicates hazard ratio; Tn, troponin; ULN, upper limit of normal.
MI Timing
As with the overall primary composite outcome in FOURIER,3 both a greater proportional and absolute reduction in MIs were seen as patients were treated longer with evolocumab. For the first year of treatment, there was a 20% reduction in MI (1.9% vs 2.4%; HR, 0.80; 95% CI, 0.68-0.94; P = .006), with a greater reduction beyond the first year, with a 35% reduction (2.7% vs 4.2%; HR, 0.65; 95% CI, 0.55-0.77; P < .001). When examined with greater granularity, there was a nonsignificant difference in the first 6 months (1.06% vs 1.14%; HR, 0.92; 95% CI, 0.74-1.16; P = .49) (eFigure 3 in Supplement 2). However, after the first 6 months, in each of the periods 6 to 12 months, 12 to 18 months, and greater than 18 months, we observed at least a 31% (HR, 0.69; 95% CI, 0.55-0.87) relative risk reduction for MI, reaching a 35% (HR, 0.65; 95% CI, 0.52-0.80) reduction in the risk of MI after 18 months of treatment.
Discussion
The key observations from this analysis are related to the natural history of MI in this population and the effects of evolocumab. In this population of patients with stable atherosclerosis followed up for a median of 2.2 years, we observed that MIs were most frequently spontaneous type 1 MIs, non–STEMI, and with cardiac troponin level values greater than 10 times the upper limit of normal. Myocardial infarctions of increasing size were associated with a stepwise increase in the risk of death. Evolocumab reduced the risk of spontaneous and procedural MI, but had no effect on type 2 (myocardial oxygen supply-demand mismatch) events. The benefit for evolocumab was consistent for MIs generally considered more severe, including STEMI and those with larger elevations of cardiac biomarkers. In addition, the benefit for reduction of MI appeared to have a time dependence with a lesser reduction over the first 6 months and larger reductions thereafter.
We expected that the benefit of lipid-lowering therapy would be greatest for spontaneous MI. The relative proportions of different types of incident MI are dependent on the characteristics of the study population. Because FOURIER included a stable population without planned cardiac procedures, most were spontaneous. A clear reduction in spontaneous events was observed, as would be expected from prior lipid-lowering therapy studies. Previous studies have demonstrated an effect of statins on reducing MI, but the data are less clear on the effect of PCI-related MI10,11 with inconsistent results among trials. In FOURIER, because of the exclusion of planned PCI, patients were in general treated with intensive lipid-lowering therapy with evolocumab for long periods prior to PCI, possibly allowing for plaque stabilization. Nevertheless, apparent benefits of evolocumab compared with placebo on PCI-related MI emerged within approximately 8 months of treatment initiation. We did not observe a benefit for evolocumab in universal type 2 MI (myocardial oxygen supply-demand mismatch). There are few data regarding prevention of such events with other treatments known to be effective for reducing CV events. These events tend to be observed in patients who are older and with more comorbidities than spontaneous MI, can be more frequently misclassified from nonischemic injury, and are less clearly associated with CV death than type 1 MI.12 These features and the observations that type 2 MIs are not affected by intensive lipid-lowering therapy suggest that it may be more appropriate for future trial end points to focus on type 1 MIs.
We observed consistent reductions in the most severe MI events in FOURIER with significant reductions of MI with higher troponin level elevations (including more than 100 times the upper limit of normal) and patients with STEMI. In addition, we observed an increasing benefit for the reduction of MI events with longer-term follow-up. We also observed that the benefit in MI extended during the full duration of follow-up after a lag period of approximately 6 months, with subsequent time periods showing greater benefit. Although FOURIER did not demonstrate a reduction of CV death during the trial,3 the reduction of larger and severe MIs raises the possibility that over a longer duration of follow-up, such a benefit may have been possible. This hypothesis is supported by the clear and graded association of increasing MI size with increased risk, suggesting that a robust reduction of larger MIs would lead over time to a mortality reduction. In another trial of a PCSK9 that had longer follow-up, a nominal reduction in mortality was achieved.13
Limitations
This study has limitations. The FOURIER trial was not designed or powered to examine MI as an isolated outcome, although significant reductions were seen. The trial relied on spontaneous reporting of events to trigger events for clinical events committee review, so it is possible that smaller MIs or procedural MIs may have been underreported, but event definitions were applied uniformly by the clinical events committee and there is no reason to believe that there would be any systematic bias related to the evolocumab comparisons. This analysis does not allow for a clear examination of the mechanism of benefits seen.
Conclusions
In this study, the lowering of LDL-C levels with evolocumab was highly effective in reducing the risk of MI. This reduction included benefit across multiple subtypes of MI related to spontaneous and procedure-related plaque rupture, smaller and larger MIs, and both STEMI and non–STEMI. These data are consistent with the known benefits of LDL-C lowering and underscore the reduction in clinically meaningful events.
Trial Protocol
eTable: Baseline Characteristics by MI During Follow Up
eFigure 1. CONSORT Diagram
eFigure 2. KM Rates of MI Stratified by Universal MI Types
eFigure 3. Effect of Evolocumab by Month of Treatment
References
- 1.Baigent C, Blackwell L, Emberson J, et al. ; Cholesterol Treatment Trialists’ (CTT) Collaboration . Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681. doi: 10.1016/S0140-6736(10)61350-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cannon CP, Blazing MA, Giugliano RP, et al. ; IMPROVE-IT Investigators . Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387-2397. doi: 10.1056/NEJMoa1410489 [DOI] [PubMed] [Google Scholar]
- 3.Sabatine MS, Giugliano RP, Keech AC, et al. ; FOURIER Steering Committee and Investigators . Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713-1722. doi: 10.1056/NEJMoa1615664 [DOI] [PubMed] [Google Scholar]
- 4.Bowman L, Hopewell JC, Chen F, et al. ; HPS3/TIMI55–REVEAL Collaborative Group . Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med. 2017;377(13):1217-1227. doi: 10.1056/NEJMoa1706444 [DOI] [PubMed] [Google Scholar]
- 5.Stone NJ, Robinson JG, Lichtenstein AH, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25)(suppl 2):S1-S45. doi: 10.1161/01.cir.0000437738.63853.7a [DOI] [PubMed] [Google Scholar]
- 6.Thygesen K, Alpert JS, Jaffe AS, et al. ; Writing Group on the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction; ESC Committee for Practice Guidelines (CPG) . Third universal definition of myocardial infarction. Eur Heart J. 2012;33(20):2551-2567. doi: 10.1093/eurheartj/ehs184 [DOI] [PubMed] [Google Scholar]
- 7.Bonaca M, Scirica B, Sabatine M, et al. Prospective evaluation of the prognostic implications of improved assay performance with a sensitive assay for cardiac troponin I. J Am Coll Cardiol. 2010;55(19):2118-2124. doi: 10.1016/j.jacc.2010.01.044 [DOI] [PubMed] [Google Scholar]
- 8.Bonello L, De Labriolle A, Lemesle G, et al. Prognostic value of procedure-related myocardial infarction according to the universal definition of myocardial infarction in saphenous vein graft interventions. Am Heart J. 2009;157(5):894-898. doi: 10.1016/j.ahj.2008.12.020 [DOI] [PubMed] [Google Scholar]
- 9.Sabatine MS, Giugliano RP, Keech A, et al. Rationale and design of the Further cardiovascular OUtcomes Research with PCSK9 Inhibition in subjects with Elevated Risk trial. Am Heart J. 2016;173:94-101. doi: 10.1016/j.ahj.2015.11.015 [DOI] [PubMed] [Google Scholar]
- 10.Berwanger O, Santucci EV, de Barros E Silva PGM, et al. ; SECURE-PCI Investigators . Effect of Loading Dose of Atorvastatin Prior to Planned Percutaneous Coronary Intervention on Major Adverse Cardiovascular Events in Acute Coronary Syndrome: The SECURE-PCI Randomized Clinical Trial. [published correction appears in JAMA. 2018 Sep 4;320(9):938]. JAMA. 2018;319(13):1331-1340. doi: 10.1001/jama.2018.2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz GG, Olsson AG, Ezekowitz MD, et al. ; Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) Study Investigators . Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA. 2001;285(13):1711-1718. doi: 10.1001/jama.285.13.1711 [DOI] [PubMed] [Google Scholar]
- 12.Arora S, Strassle PD, Qamar A, et al. Impact of type 2 myocardial infarction (MI) on hospital-level MI outcomes: implications for quality and public reporting. J Am Heart Assoc. 2018;7(7):e008661. doi: 10.1161/JAHA.118.008661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz GG, Steg PG, Szarek M, et al. ; ODYSSEY OUTCOMES Committees and Investigators . Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097-2107. doi: 10.1056/NEJMoa1801174 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable: Baseline Characteristics by MI During Follow Up
eFigure 1. CONSORT Diagram
eFigure 2. KM Rates of MI Stratified by Universal MI Types
eFigure 3. Effect of Evolocumab by Month of Treatment