Abstract
Evidence for dosing, efficacy, and safety of most medications used to treat neonates is sparse. Thus, dosing is usually derived by extrapolation from adult and pediatric pharmacologic data with scaling by bodyweight or body surface area. This may lead to drug dosing that is unsafe or ineffective. However, new strategies are being developed and studied to dose medications in critically ill neonates. Mass spectroscopy technology capable of quickly analyzing drug levels is readily available. Software that integrates population pharmacokinetics and pharmacodynamics with data from sparse samples from neonates allows for timely adjustments of dosing to achieve the desired effect while minimizing adverse outcomes. Some genetic polymorphisms that affect drug response in neonates have also been reported. This review highlights aspects of drug response and how it is impacted by prematurity, assesses pharmacogenomic studies in neonates, and offers suggestions for innovative pharmacokinetic/pharmacodynamic model-based approaches which combine population or physiology-based pharmacology data, Bayesian analysis, and electronic decision support tools for precision dosing in neonates while illustrating examples where this approach can be used to optimize medical therapy in neonates. Barriers to implementing precision dosing in neonates and how to overcome them are also discussed.
Keywords: Neonate, Pharmacodynamics, Pharmacokinetics, Pharmacology, Precision Dosing, Population pharmacokinetics
Introduction
Several federal agencies in the United States, including the National Institute of Health, the US Food and Drug Administration and the Office of National Coordinator for Health Information Technology have been charged with the implementation of individualized medicine in the clarion call for Precision Medicine Initiative1,2. In neonates, however, the task is daunting because of the lack of data despite the Best Pharmaceuticals for Children Act (BPCA) and Pediatric Research Equity Act (PREA) legislation, European Medicines Agency, and International Neonatal Consortium encouraging more research into the safe and effective use of medications in children. Dynamic postnatal physiologic changes in neonates, together with an incomplete understanding of the ontogeny of important enzyme systems and pharmacogenetics/genomics that are responsible for interindividual variability in drug disposition and response are not accounted for with current dosing regimens. Dosing recommendations for most medications prescribed to neonates are not validated and do not address unique characteristics in this population. Thus, dosing schemes are either suboptimal or are fraught with avoidable adverse effects. Technological advances in bedside pharmacologic testing and electronic health systems provide an opportunity for evidence-based dosing of medications in neonates. Individualized dosing can best be achieved by integration of pharmacokinetic/pharmacodynamic (PK/PD) principles with developmental pharmacology, therapeutic drug monitoring, and model-based decision support using Bayesian adaptive control3–6. This strategy allows the realization for individualized dosing in order to achieve the desired drug exposure for efficacy while minimizing the risk of adverse effects.
In this review, we highlight aspects of drug response and how they are impacted by prematurity, assess pharmacogenomic studies in neonates, and provide suggestions for innovative PK/PD model-based approaches which combine population or physiology-based PK and PD data, Bayesian analysis and readily available decision support tools for precision dosing in neonates. Where relevant, we summarized some successful use of this model in neonates.
Why Neonatal Pharmacology is Unique
Pharmacology data are sparse in neonates because of the inherent difficulty in conducting conventional pharmacokinetic studies in vulnerable populations and the relatively small population of preterm infants from which to sample. Dosing of medications in neonates often relies on extrapolation from adult or older children data through scaling based on body weight or body surface area. Yet, successful application of adult or older children pharmacology data to neonates is hampered due to unique developmental physiological processes, changing body composition, and a non-linear relationship between growth and development in neonates which contribute to poor correlation between drug dosage and serum concentrations 1,7–11. These developmental changes, and to a lesser extent, genetic variation, are important determinants of drug response. The absorption, distribution, metabolism, and elimination of medications are often quantified with PK parameters such as bioavailability, clearance, volume of distribution, and half-life. These determine PK measures such as peak and trough concentrations and area under the concentration versus time curve. These PK parameters and measures are usually stated as a mean with a standard deviation and are used to create dosing guidelines for an average patient. However, they typically fail to account for the often large inter-individual variability in PK and PD. The neonate’s dynamic physiology impacts drug disposition and response, suggesting they could benefit tremendously from precision dosing. It also implies that utilizing data from other populations, such as adults or from older children, may not be appropriate for neonates. The need for neonates-specific data is also underscored by the heterogeneous population in which the bodyweight may vary more than 10-fold, gestational age could span between 24 and 42 weeks, postnatal age ranges from 0 to 30 days, and their size is affected by in utero environment that could result in growth restriction or small-for-gestational age infants in contrast to appropriate- or large-for-gestational age infants with implications for drug, particularly in weight-based dosing. For example, should a 30-day old former 24-week infant weighing 1 kg be dosed the same as a 1-day old 28-week infant who also weighs 1 kg? These are important questions that are not addressed by the current dosing schemes in neonates.
Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
Neonatal pharmacology is challenged by the fact that for a given drug dose there is a range of clinical responses related to interindividual PK and PD variability resulting in limited predictability. PK variability leads to differences in drug exposure between patients for a given dose and can be related to body composition, protein binding capacity, organ function maturity, cardiac output, or ontogeny of transporters and drug metabolizing enzymes 3,12–15. Factors contributing to PD variability, which could explain the relationship of drug exposure to drug effect, are less well understood but examples include bacterial antibiotic resistance, severity of illness, or interindividual differences in receptor binding affinity16. The quest then is to take into account this PK/PD variability in selecting the appropriate drug dosing regimen. PK/PD modeling attempts to describe and predict the dose-exposure-response relationship for a given drug in a given patient. While a number of modeling techniques have been developed to account for this task, two of the most commonly-used approaches are the combination of either population PK or physiology-based PK models with the PD response17.
Introduced in the 1980s, population PK uses descriptive equations to explain the relationship between physiology and PK, the interindividual variability in these relationships, and their residual intraindividual variability18. Population PK studies simultaneously use data from multiple subjects in a population who have drug concentrations collected at different time points. This allows for random sparse sampling which is desirable in the Neonatal Intensive Care Unit (NICU) population and results in a robust analysis describing the PK of the drug. By applying population PK through non-linear mixed effects modeling, covariates can be identified to determine PK parameters and their variability. Covariates include factors such as age, bodyweight, biomarkers of liver or renal function, or, to a lesser extent, pharmacogenetic markers are utilized to partially explain PK variability in this population. Once PK parameters, typically clearance and volume of distribution, are estimated then an initial dosing regimen can be designed. This is the first advantage of this patient-tailored dosing technique, where evidence-based guidelines can be used to account for interindividual variability by incorporating the identified covariates in order to implement the most appropriate dosing regimen19. As an example, this model-based approach has been demonstrated to be useful in dosing vancomycin in neonates, safely increasing the rate of target concentration attainment from 41% to 72%20. The better-informed dosing scheme is then modified when the remaining variability is adjusted for through therapeutic drug monitoring combined with Bayesian analysis techniques described below.
Physiology-based PK (PBPK) models are mechanistic models combining anatomy and physiology data of the patient population with the biochemical characteristics of the drug that describe the absorption, distribution, metabolism, and excretion of the drug21. In this model a patient is represented by a large number of compartments corresponding to different organs and tissues which are connected by flow rates paralleling blood flow22. Typically, adult models are constructed first and medication parameters determined. Then patient-specific parameters can be modified for the neonate23. However, this may be challenging in neonates, and especially in preterm newborns, due to rapidly evolving enzyme systems and changing physiology24,25. As more is learned about the ontogeny of these enzyme systems, PBPK modeling can better predict drug exposure. Combined with PK-PD data, PBPK modeling can then facilitate precision dosing of drugs.
While PK models predict the time course of drug exposure for a given dose, PD modeling relates exposure to the effects of the drug on the body. Exposure can be measured in a number of ways including peak or trough concentration of a medication, area under the plasma drug concentration-time curve for a specific time period, or determining the duration of time a drug concentration is within a predetermined target exposure range. Characterizing the PD of drugs in neonates is significantly hampered by the lack of defined biomarkers or PD outcomes measures for neonatal disease processes and adverse effects. For instance, differing definitions of bronchopulmonary dysplasia26 and delayed diagnosis of neurodevelopmental impairment27 hinder the ability to associate drug exposure with changes in disease severity or outcomes. The impact of development on PD measures of drugs also still needs to be further investigated. For example, how does the exposure goal for caffeine in the treatment of apnea of prematurity change as a neonate develops? Is the goal the same at 26 weeks as it is at 32 weeks postmenstrual age? Answers to these important questions will help in advance precision dosing in neonates.
Effects of Pharmacogenomics/Pharmacogenetics on PK/PD
No review of personalized drug dosing is complete without a discussion about pharmacogenomics. The Human Genome Project has enabled technologies to probe diseases in order to refine clinical care for risk stratification, prevention, diagnosis, and treatment. Pharmacogenomics, the study of variability in drug response due to genetic factors, includes the prediction of a patient’s response to a specific therapy, and susceptibility to toxicity and adverse events7. Pharmacogenetics deals with individual genetic variations that may explain some of the drug dose-exposure-effect relationship and its interindividual variability. These genetic variations may impact all aspects of drug response by altering drug distribution in the tissues, drug elimination from the body, or the PD of the drug28,29. Polymorphisms in genes encoding proteins that are important in drug transport and metabolism or in drug receptors may be important covariates to consider with respect to drug dosing. Although a single polymorphism by itself may not be sufficient to explain the variability in drug exposure or response, a combination of variants from multiple genes may act synergistically to impact drug response. Poignant illustrations include genetic polymorphisms in the principal morphine metabolizing enzyme, UGT2B7, and the mu opioid receptor. UGT2B7 −900G>A polymorphism results in increased drug metabolism thereby contributing to lower morphine exposure after a single dose of intravenous morphine administration30. Similarly, a polymorphism in the gene encoding the mu opioid receptor (OPRM1 118A>G) combined with a polymorphism in catechol-O-methyltransferase (COMT 472G>A) has been associated with an increased need for a rescue dose of morphine in neonates requiring mechanical ventilation31.
While some neonatal pharmacogenomic studies have confirmed several associations reported in adult or pediatric populations, some phenotype-genotype associations are anticipated to be unique to the newborn period29. Hence, pharmacogenomic studies being advocated through the Precision Medicine Initiatives by the US government2 and the 100,000 Genome Project in the United Kingdom must be broadened to include neonatal clinical syndromes28,29. Unique neonatal conditions like neonatal abstinence syndrome can only be studied in the newborn population where outcomes have shown to be dependent on the genotype of the mother and the infant32. In addition, there is a dynamic temporal interplay between developmental and pharmacogenomic factors. Determining the age when genetic variations become the predominant factor for differences in drug response is crucial to evidence-based dosing algorithms in neonates. A recent study showed that morphine exhibits age-dependent extraction as a result of developmental increases in OCT1 and UGT2B7 protein expression/activity and hepatic bloodflow33. This temporal versus pharmacogenomics contribution to drug response in neonates is further demonstrated by CYP2C8*3, CYP2C9*2, and CYP2C9*3 polymorphisms that show lower ibuprofen clearance in adults34. It might be assumed genetic polymorphisms that reduce drug clearance would increase ibuprofen effectiveness but these polymorphisms have no effect in the ductus arteriosus response to ibuprofen in preterm infants35. The decreased enzyme activity of CYP2C8 and CYP2C9 in early life likely explains this35,36. Incorporating developmental and genetic data about drug metabolizing enzymes, transporter, and receptor systems along with age-related physiological changes in neonates into predictive PK/PD models is warranted to allow for more precise dosing.
Bayesian Analysis
While population and physiology-based PK/PD modeling and pharmacogenetics can provide improved dosing regimens in neonates, Bayesian analysis allows for truly individualized precision dosing. Bayesian analysis uses previously collected information (“a priori”), such as a PBPK model-based approach or population PK parameters (e.g., clearance and volume of distribution) of a drug for a defined population, subsequent patient-specific information (“a posteriori”), and biomarker data such as one or more measured drug concentrations at known time points37. With the knowledge of the dosing history, a time-exposure curve can then be constructed and graphically displayed for the clinician. Furthermore, this analysis then allows the clinician to simulate dosing regimens to predict drug concentrations (Bayesian forecasting) or to determine a dosing strategy to best achieve the target concentration (Bayesian adaptive control)38. Infants have been included in some Bayesian adaptive control research to optimize drug treatment39–41; however, the use of this method has been understudied in neonates. Conventional guidelines for monitoring medications used commonly in neonates, such as gentamicin and vancomycin, recommend waiting to assure a steady state after multiple doses before checking a drug level. One major advantage of utilizing PK modeling coupled with Bayesian analysis is that the behavior of the drug in an individual patient can be modeled starting with the first dose and with obtaining just one drug level. In addition, this process obviates the need to wait for attainment of steady-state 42–44. Thus, Bayesian analysis allows clinicians to achieve the optimal dose more quickly.
Therapeutic Drug Management with Decision Support Tools
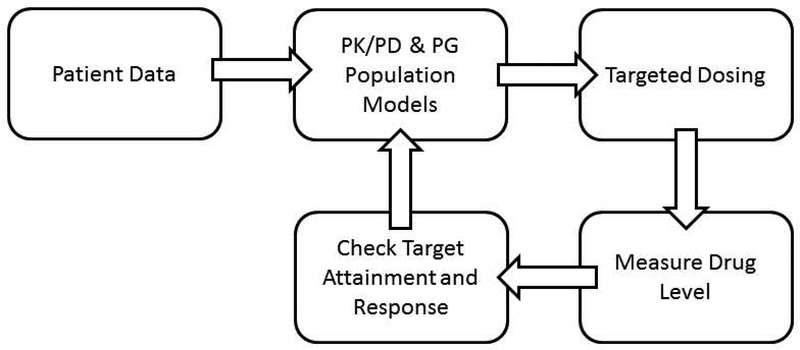
For many drugs, dose is a poor predictor of serum concentration and outcome in neonates. There are no simple clinical or patient-related factors that reliably inform drug response and adverse effects. Replacing the current trial and error paradigm used in clinical practice with a model that tailors dosing to each individual neonate’s profile is possible through the use of bedside drug concentration testing coupled with Bayesian modeling and integrated electronic decision support tool45. Tandem mass spectrometry has become widely available 46. Its use in the analysis of minute amounts of specimens collected on dried blood spots (DBS) for accurate and reliable drug concentration analysis has been reported47,48. Advances in paper spray mass spectrometry technology have the enormous potential to enable rapid quantification of drug levels 49. Assessing drug exposure in real time will allow feedback for appropriate dose adjustments. While PK/PD modeling can be used for initial drug dosing50,51, to further refine exposure, Bayesian adaptive modeling techniques can be utilized to predict and control drug exposure at a target level (See Figure 1)38. However, exposure does not always equal response. Once a drug concentration has been quantified, these data can be compared to target dosing ranges and correlated to clinical response in order to determine the dosing adjustment needed to achieve desired response. Systems pharmacology platforms using Bayesian estimation are then utilized to determine dosing adjustment recommendations, as has been reported in neonates52. User-friendly software for PK/PD model-based precision dosing is available53.
Fig. 1.
Schematic diagram of PK/PD modeling with Bayesian adaptive support tools using fluconazole as an example in a two week old infant born at 24 weeks gestation. Patient data (gestational age of 24 weeks, post-natal age of 2 weeks, and not on ECMO) are incorporated into the PK/PD model to target drug exposure based on the exposure-response relationship. When drug plasma level is outside the target range or clinical response is suboptimal, the drug concentration is input into the model to further refine the dosing regimen. This can be repeated if the desired outcome is not reached or the patient’s clinical status changes
Clinical Examples: Optimizing Neonatal Fluconazole and Acetaminophen Dosing Using Model-Informed Strategies
Population PK modeling has been used to optimize the dosing of fluconazole in neonates54. From 55 infants, born between 23 and 40 weeks gestational age and less than 120 days old, fluconazole clearance and volume of distribution were determined via population PK analysis. 55. Using liquid chromatography-tandem mass spectrometry analysis (LC-MS/MS) fluconazole serum concentration measurements were made. The ensuing new model suggested that weight-based fluconazole dosing should be based on both gestational age at birth and postnatal age. Thus, an eight-week old infant born at 24 weeks gestation may require a different dosing regimen from a one-day old infant born at 32 weeks despite a similar postmenstrual age of 32 weeks. Subsequent Monte Carlo simulations revealed higher fluconazole doses were required in infants than is typically recommended56. Further PK and safety trial work provided recommendations for an appropriate loading dose of 25 mg/kg to achieve target concentrations more quickly in infants less than 60 days old57. Dosing in special circumstances, such as extracorporeal membrane oxygenation, was also explored. This revealed an increased volume of distribution thus requiring a further increase in the treatment loading dose to 35 mg/kg58. Despite these improvements, 10 – 20% of infants still do not reach a sufficient AUC target for treatment with fluconazole56. Implementing these recommendations clinically is challenging because it can be cumbersome for clinicians to appropriately adjust fluconazole dosing based on gestational age at birth, post-natal age, and other clinical factors. The decision support tool can automatically account for these variables and further adjust the dosing regimen so more infants are in the desired target range for fluconazole exposure. This work underscores the utility of the data in the decision support tool.
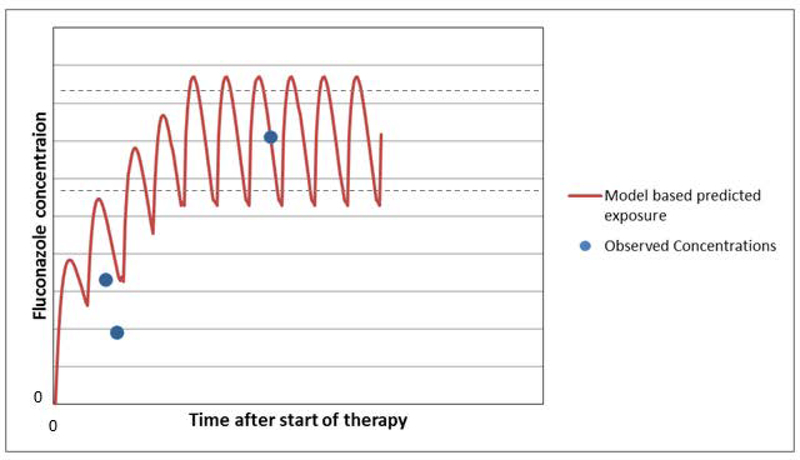
As shown in Figure 2, fluconazole dose is initiated based on the neonate’s gestational and postnatal ages. So for a 28-week infant who is 2 weeks old, a loading dose of 25 mg/kg is administered. Because clinical response is difficult to assess early on in fungal infections a drug level must be obtained. If the fluconazole level is suboptimal per the PK/PD model then a new dosing regimen can be determined and the level checked again. If there is a change in clinical status, such as impaired renal function, concurrent administration of medication that might impact PK or PD, or during critical illness, follow-up samples can be obtained and the Bayesian dose optimization process repeated (learn and confirm). This strategy can also be used to study the exposure-effect relationship to better define the target exposure range thereby maximizing the likelihood of response while minimizing the risk of adverse effects59.
Fig. 2.
Using fluconazole treatment as an example in a two week infant born at 24 weeks gestation who is not on ECMO and has normal renal function the initial dosing regimen would suggest a loading dose of 25 mg/kg followed by a maintenance dose of 12 mg/kg every 12 hours. After fluconazole has been started a plasma drug level is obtained. Using the dosing information and drug level the 24 hour AUC can be calculated using the population PK model and compared to the goal (> 400 mg*hr/L). In this case the 24 hour AUC is too low. The decision support tool using Bayesian optimization then recommends a new dosing regimen so that the target AUC is obtained. The new dosing scheme with a higher maintenance dose every 12 hours after giving a one time loading dose is interrogated by obtaining another fluconazole level. The updated dosing regimen is subsequently simulated in the population PK model to determine the new 24 hour AUC. This time it is appropriate, thus the right dose has been determined for this patient
Using a PBPK model-based approach as the “a priori” information to describe the dose-exposure relationship in neonates is also possible4,60. Rather than using the population PK data to determine the initial dosing regimen, the PBPK model would inform the targeted dosing regimen. The prescribed dosing regimen would be adjusted in a similar fashion as described above to attain the desired exposure target or response. For acetaminophen which is used in the NICU for pain control and ductus arteriosus closure, a published neonatal PBPK model exists61. This model could be used to minimize the risk of liver toxicity to optimize therapy to close the ductus arteriosus when used with Bayesian adaptive modeling techniques.
Clinical Example: Using Bayesian Methods to Optimize Morphine Dosing
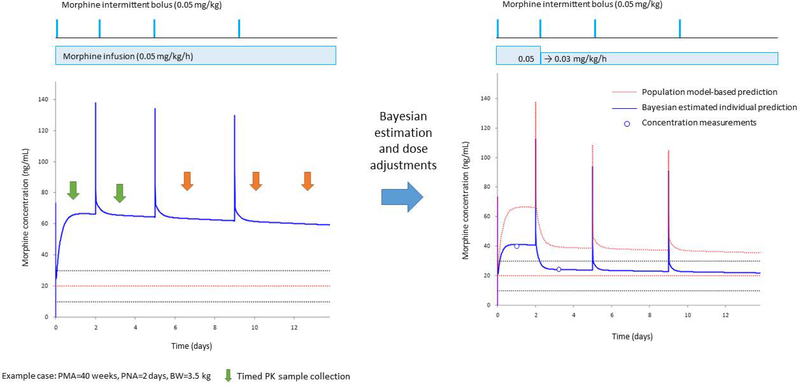
At Cincinnati Children’s Hospital Medical Center, we have developed an electronic health record-linked decision support platform for precision dosing of morphine in neonates(Alexander A. Vinks. Cincinnati Children’s Medical Center. Electronic Health Record (EHR)-embedded Decision Support Platform for Individualized Precision Drug Treatment in Neonates. Gerber Foundation. http://www.gerberfoundation.org/pediatric-health.). This population PK/PD model-informed precision dosing platform using Bayesian estimation allows the clinician to adjust the dosing regimen to optimize the drug therapy in real time. The example in Figure 3 shows the predicted morphine PK profile (blue line on the left panel and dashed red line in the right panel) based on the infant’s weight and gestational age and dosing regimen administered (continuous infusion plus bolus doses as needed, based on the pain scores). Two measured morphine levels (open circles in the right panel) revealed the concentration to be less than the expected value. Based on the feedback from these measured morphine concentrations, a new individually-predicted PK profile is developed (blue line in the right panel) using Bayesian analysis. The dotted lines represent the potential target range of 10–30 ng/mL (mean 20 ng/mL in red) as suggested by Anderson and van den Anker59. This example was reported retrospectively to develop this technology but it illustrates its power to tailor morphine therapy in neonates in real time. The lower than expected observed concentrations suggest this study subject has a higher clearance than the “average” neonate and the dosing regimen could have been adjusted accordingly to safely provide analgesia (blue line in the right panel). Could the number of painful experiences for the neonate be decreased using this tool? Could the tool be used to better control pain without putting the infant at increased risk for adverse effects from morphine? Is the target morphine concentration range correct and how does it change over time? How does the clinician incorporate this information to make clinical decisions? The platform is undergoing prospective evaluation as a decision support tool to answer these questions.
Fig. 3.
Bayesian estimation integrated within the electronic health record allows for precision dosing in neonates. The figure represents model-based PK simulations for a standard neonate (postmenstrual age: 40 weeks, postnatal age: 2 days and body weight: 3.5 kg) as an example. The left panel shows the population PK model-based morphine PK profile (solid blue line) for a standard morphine initial continuous infusion and “as needed” bolus doses. The Upper panel represents the morphine doses for intermittent bolus (horizontal lines) and continuous infusion (box). The dotted lines represent the potential target concentration range of 10–30 ng/mL (mean 20 ng/mL in red). Vertical arrows represent the time of PK sample collection by timed (green) or opportunistic sampling (orange). The right panel shows the adjusted dose and resulting individual PK profile based on the Bayesian estimation. The observed concentrations (open circles) were less than the expected concentration based on the population PK profile (dotted red line) suggesting this neonate had a higher clearance when compared to the average neonate. Therefore, the continuous infusion dose was decreased by 40% to target to the individual predicted concentration (blue line) fitted within the suggested target range (horizontal dotted lines). (Figure courtesy of Dr. Tomoyuki Mizuno, Cincinnati Children’s Hospital Medical Center)
Overcoming Limitations to the Implementation of Therapeutic Drug Management
The clinical utility of population models is not intuitive and before Bayesian analysis techniques are fully adopted a number of impediments must be overcome. Lack of knowledgeable and well trained staff in modeling and simulation makes this technology difficult to use at the bedside. This can be overcome by utilizing new and future decision support tools to automate the Bayesian adaptive control process without the involvement of clinical pharmacologists. However, this may present another possible barrier because the generated dosing recommendations are potentially boundless. An assay error or incorrectly recorded drug dosing or timing of sample could result in the algorithm recommending a harmful or ineffective dosing plan. The clinician must be aware of this and warning messages could be built into the system to alert care providers to doses that are outside of an accepted range. Thus, this technology has the potential to reduce the risk of medication errors. Also, the best use of Bayesian analysis requires real time measurement of medication concentrations but not all facilities are equipped to perform this function. DBS samples have the advantage that they could be mailed overnight to regional centers for LC-MS/MS analysis and results returned by electronic mail so dosing adjustments can be undertaken. This still requires the clinician to have access to a decision support system integrated into the electronic health record. Web-based platforms offer a solution that can make this possible for optimizing dosing. Furthermore, using plasma as the source for drug levels may not reflect the drug exposure at the receptor site. Medications need to be studied individually to test this assumption. Assay variability can also complicate results and this difference needs to be solved. Finally, perhaps the most significant barrier is the lack of information concerning exposure-response relationships for many drugs used in the NICU. The model assumes a known target concentration exists but often this is not the case59. The development of PD biomarkers to assess desired outcomes and side effects must be undertaken to better guide dosing62. Using Bayesian analysis to decrease some of the PK variability by controlling drug exposure will aid in these PD studies.
Conclusion
Precision dosing is possible in neonates through model-informed Bayesian analysis methods that take advantage of PK/PD models, bedside pharmacologic testing, and electronic decision support tools. The application of this learn-confirm and apply approach allows neonates to fully realize the benefits of PK/PD model-based individualized dosing. It may seem axiomatic that this approach will improve outcomes but hypothesis-driven research is warranted in order to move this science forward.
In conclusion, despite rapidly changing physiology and incomplete knowledge of developmental pharmacology and pharmacogenomics in neonates, improved efficacy and better safety can be realized through PK/PD modeling and Bayesian analysis. It is further anticipated that the Precision Medicine Initiative that aims to capitalize on advances in genome biology, next-generation sequencing and digital health coupled with ongoing PK/PD studies will complement the gains that have been realized through BPCA and PREA legislations that mandate or encourage inclusion of infants in drug clinical studies.
Acknowledgements
Joshua Euteneuer was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development under award number 5T32HD069054 (Cincinnati Training Program in Pediatric Clinical & Developmental Pharmacology). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest
Joshua C. Euteneuer, Suyog Kamatkar, Tsuyoshi Fukuda, Alexander A. Vinks, and Henry T. Akinbi declare that they have no conflict of interest.
Fellows of the American College of Clinical Pharmacology
Alexander A. Vinks is a Fellow of the American College of Clinical Pharmacology
References
- 1.Wiles JR, Vinks AA, Akinbi H. Federal legislation and the advancement of neonatal drug studies. J Pediatr. 2013;162(1):12–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372(9):793–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology--drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–1167. [DOI] [PubMed] [Google Scholar]
- 4.Vinks AA, Emoto C, Fukuda T. Modeling and simulation in pediatric drug therapy: Application of pharmacometrics to define the right dose for children. Clin Pharmacol Ther. 2015;98(3):298–308. [DOI] [PubMed] [Google Scholar]
- 5.Admiraal R, van Kesteren C, Boelens JJ, Bredius RG, Tibboel D, Knibbe CA. Towards evidence-based dosing regimens in children on the basis of population pharmacokinetic pharmacodynamic modelling. Arch Dis Child. 2014;99(3):267–272. [DOI] [PubMed] [Google Scholar]
- 6.Darwich AS, Ogungbenro K, Vinks AA, et al. Why has model-informed precision dosing not yet become common clinical reality? lessons from the past and a roadmap for the future. Clin Pharmacol Ther. 2017;101(5):646–656. [DOI] [PubMed] [Google Scholar]
- 7.Allegaert K, van de Velde M, van den Anker J. Neonatal clinical pharmacology. Paediatric anaesthesia. 2014;24(1):30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahmood I Prediction of drug clearance in children from adults: a comparison of several allometric methods. British journal of clinical pharmacology. 2006;61(5):545–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pauwels S, Allegaert K. Therapeutic drug monitoring in neonates. Arch Dis Child. 2016;101(4):377–381. [DOI] [PubMed] [Google Scholar]
- 10.Emoto C, Fukuda T, Mizuno T, et al. Age-dependent changes in sirolimus metabolite formation in patients with neurofibromatosis type 1. Ther Drug Monit. 2015;37(3):395–399. [DOI] [PubMed] [Google Scholar]
- 11.Brussee JM, Calvier EA, Krekels EH, et al. Children in clinical trials: towards evidence-based pediatric pharmacotherapy using pharmacokinetic-pharmacodynamic modeling. Expert Rev Clin Pharmacol. 2016;9(9):1235–1244. [DOI] [PubMed] [Google Scholar]
- 12.Friis-Hansen B Water distribution in the foetus and newborn infant. Acta Paediatr Scand Suppl. 1983;305:7–11. [DOI] [PubMed] [Google Scholar]
- 13.Tayman C, Rayyan M, Allegaert K. Neonatal pharmacology: extensive interindividual variability despite limited size. J Pediatr Pharmacol Ther. 2011;16(3):170–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brouwer KL, Aleksunes LM, Brandys B, et al. Human Ontogeny of Drug Transporters: Review and Recommendations of the Pediatric Transporter Working Group. Clin Pharmacol Ther. 2015;98(3):266–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hines RN. Developmental expression of drug metabolizing enzymes: impact on disposition in neonates and young children. Int J Pharm. 2013;452(1–2):3–7. [DOI] [PubMed] [Google Scholar]
- 16.Lin JH. Pharmacokinetic and pharmacodynamic variability: a daunting challenge in drug therapy. Curr Drug Metab. 2007;8(2):109–136. [DOI] [PubMed] [Google Scholar]
- 17.Downes KJ, Hahn A, Wiles J, Courter JD, Vinks AA. Dose optimisation of antibiotics in children: application of pharmacokinetics/pharmacodynamics in paediatrics. Int J Antimicrob Agents. 2014;43(3):223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheiner LB. The population approach to pharmacokinetic data analysis: rationale and standard data analysis methods. Drug Metab Rev. 1984;15(1–2):153–171. [DOI] [PubMed] [Google Scholar]
- 19.Zeilmaker GA, Pokorna P, Mian P, et al. Pharmacokinetic considerations for pediatric patients receiving analgesia in the intensive care unit; targeting postoperative, ECMO and hypothermia patients. Expert Opin Drug Metab Toxicol. 2018;14(4):417–428. [DOI] [PubMed] [Google Scholar]
- 20.Leroux S, Jacqz-Aigrain E, Biran V, et al. Clinical Utility and Safety of a Model-Based Patient-Tailored Dose of Vancomycin in Neonates. Antimicrob Agents Chemother. 2016;60(4):2039–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nestorov I Whole-body physiologically based pharmacokinetic models. Expert Opin Drug Metab Toxicol. 2007;3(2):235–249. [DOI] [PubMed] [Google Scholar]
- 22.Jones H, Rowland-Yeo K. Basic concepts in physiologically based pharmacokinetic modeling in drug discovery and development. CPT Pharmacometrics Syst Pharmacol. 2013;2:e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maharaj AR, Edginton AN. Physiologically based pharmacokinetic modeling and simulation in pediatric drug development. CPT Pharmacometrics Syst Pharmacol. 2014;3:e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edginton AN, Schmitt W, Willmann S. Development and evaluation of a generic physiologically based pharmacokinetic model for children. Clinical pharmacokinetics. 2006;45(10):1013–1034. [DOI] [PubMed] [Google Scholar]
- 25.Khalil F, Laer S. Physiologically based pharmacokinetic models in the prediction of oral drug exposure over the entire pediatric age range-sotalol as a model drug. AAPS J. 2014;16(2):226–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poindexter BB, Feng R, Schmidt B, et al. Comparisons and Limitations of Current Definitions of Bronchopulmonary Dysplasia for the Prematurity and Respiratory Outcomes Program. Ann Am Thorac Soc. 2015;12(12):1822–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Natalucci G, Latal B, Koller B, et al. Effect of Early Prophylactic High-Dose Recombinant Human Erythropoietin in Very Preterm Infants on Neurodevelopmental Outcome at 2 Years: A Randomized Clinical Trial. JAMA. 2016;315(19):2079–2085. [DOI] [PubMed] [Google Scholar]
- 28.Leeder JS, Kearns GL, Spielberg SP, van den Anker J. Understanding the relative roles of pharmacogenetics and ontogeny in pediatric drug development and regulatory science. J Clin Pharmacol. 2010;50(12):1377–1387. [DOI] [PubMed] [Google Scholar]
- 29.Leeder JS, Kearns GL. Interpreting pharmacogenetic data in the developing neonate: the challenge of hitting a moving target. Clin Pharmacol Ther. 2012;92(4):434–436. [DOI] [PubMed] [Google Scholar]
- 30.Matic M, Norman E, Rane A, et al. Effect of UGT2B7 −900G>A (−842G>A; rs7438135) on morphine glucuronidation in preterm newborns: results from a pilot cohort. Pharmacogenomics. 2014;15(12):1589–1597. [DOI] [PubMed] [Google Scholar]
- 31.Matic M, Simons SH, van Lingen RA, et al. Rescue morphine in mechanically ventilated newborns associated with combined OPRM1 and COMT genotype. Pharmacogenomics. 2014;15(10):1287–1295. [DOI] [PubMed] [Google Scholar]
- 32.Wachman EM, Hayes MJ, Sherva R, et al. Association of maternal and infant variants in PNOC and COMT genes with neonatal abstinence syndrome severity. Am J Addict. 2017;26(1):42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Emoto C, Johnson TN, Neuhoff S, Hahn D, Vinks AA, Fukuda T. PBPK Model of Morphine Incorporating Developmental Changes in Hepatic OCT1 and UGT2B7 Proteins to Explain the Variability in Clearances in Neonates and Small Infants. CPT Pharmacometrics Syst Pharmacol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia-Martin E, Martinez C, Tabares B, Frias J, Agundez JA. Interindividual variability in ibuprofen pharmacokinetics is related to interaction of cytochrome P450 2C8 and 2C9 amino acid polymorphisms. Clin Pharmacol Ther. 2004;76(2):119–127. [DOI] [PubMed] [Google Scholar]
- 35.Durrmeyer X, Hovhannisyan S, Medard Y, et al. Are cytochrome P450 CYP2C8 and CYP2C9 polymorphisms associated with ibuprofen response in very preterm infants? PLoS One. 2010;5(8):e12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johansson M, Strahm E, Rane A, Ekstrom L. CYP2C8 and CYP2C9 mRNA expression profile in the human fetus. Front Genet. 2014;5:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jelliffe R, Bayard D, Milman M, Van Guilder M, Schumitzky A. Achieving target goals most precisely using nonparametric compartmental models and “multiple model” design of dosage regimens. Ther Drug Monit. 2000;22(3):346–353. [DOI] [PubMed] [Google Scholar]
- 38.Neely M, Jelliffe R. Practical, individualized dosing: 21st century therapeutics and the clinical pharmacometrician. J Clin Pharmacol. 2010;50(7):842–847. [DOI] [PubMed] [Google Scholar]
- 39.Zhao W, Cella M, Della Pasqua O, Burger D, Jacqz-Aigrain E, Pediatric European Network for Treatment of Asg. Population pharmacokinetics and maximum a posteriori probability Bayesian estimator of abacavir: application of individualized therapy in HIV-infected infants and toddlers. Br J Clin Pharmacol. 2012;73(4):641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCune JS, Bemer MJ, Barrett JS, Scott Baker K, Gamis AS, Holford NH. Busulfan in infant to adult hematopoietic cell transplant recipients: a population pharmacokinetic model for initial and Bayesian dose personalization. Clin Cancer Res. 2014;20(3):754–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mizuno T, Emoto C, Fukuda T, Hammill AM, Adams DM, Vinks AA. Model-based precision dosing of sirolimus in pediatric patients with vascular anomalies. Eur J Pharm Sci. 2017. [DOI] [PubMed] [Google Scholar]
- 42.Rybak MJ, Lomaestro BM, Rotschafer JC, et al. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis. 2009;49(3):325–327. [DOI] [PubMed] [Google Scholar]
- 43.Neofax TEY. Twentyfourth Edition, Montvale, NJ, USA: Thomson Reuters; 2011. [Google Scholar]
- 44.Pickering LK, Baker CJ, Kimberlin DW. Red Book, (2012). AAP Books. 2012. [Google Scholar]
- 45.Mould DR, D’Haens G, Upton RN. Clinical Decision Support Tools: The Evolution of a Revolution. Clin Pharmacol Ther. 2016;99(4):405–418. [DOI] [PubMed] [Google Scholar]
- 46.Grebe SK, Singh RJ. LC-MS/MS in the Clinical Laboratory - Where to From Here? Clin Biochem Rev. 2011;32(1):5–31. [PMC free article] [PubMed] [Google Scholar]
- 47.Verplaetse R, Henion J. Quantitative determination of opioids in whole blood using fully automated dried blood spot desorption coupled to on-line SPE-LC-MS/MS. Drug Test Anal. 2016;8(1):30–38. [DOI] [PubMed] [Google Scholar]
- 48.Li W, Doherty J, Moench P, Flarakos J, Tse FL. LC-MS/MS bioanalysis of loratadine (Claritin) in dried blood spot (DBS) samples collected by subjects in a clinical research study. J Chromatogr B Analyt Technol Biomed Life Sci. 2015;983–984:117–124. [DOI] [PubMed] [Google Scholar]
- 49.Manicke NE, Yang Q, Wang H, Oradu S, Ouyang Z, Cooks RG. Assessment of paper spray ionization for quantitation of pharmaceuticals in blood spots. International Journal of Mass Spectrometry. 2011;300(2):123–129. [Google Scholar]
- 50.Ku LC, Smith PB. Dosing in neonates: special considerations in physiology and trial design. Pediatr Res. 2015;77(1–1):2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krekels EH, Tibboel D, de Wildt SN, et al. Evidence-based morphine dosing for postoperative neonates and infants. Clin Pharmacokinet. 2014;53(6):553–563. [DOI] [PubMed] [Google Scholar]
- 52.Wang J, Edginton AN, Avant D, Burckart GJ. Predicting neonatal pharmacokinetics from prior data using population pharmacokinetic modeling. J Clin Pharmacol. 2015;55(10):1175–1183. [DOI] [PubMed] [Google Scholar]
- 53.Fuchs A, Csajka C, Thoma Y, Buclin T, Widmer N. Benchmarking therapeutic drug monitoring software: a review of available computer tools. Clinical pharmacokinetics. 2013;52(1):9–22. [DOI] [PubMed] [Google Scholar]
- 54.Autmizguine J, Guptill JT, Cohen-Wolkowiez M, Benjamin DK, Jr., Capparelli EV. Pharmacokinetics and pharmacodynamics of antifungals in children: clinical implications. Drugs. 2014;74(8):891–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wade KC, Wu D, Kaufman DA, et al. Population pharmacokinetics of fluconazole in young infants. Antimicrob Agents Chemother. 2008;52(11):4043–4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wade KC, Benjamin DK Jr., Kaufman DA, et al. Fluconazole dosing for the prevention or treatment of invasive candidiasis in young infants. Pediatr Infect Dis J. 2009;28(8):717–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Piper L, Smith PB, Hornik CP, et al. Fluconazole loading dose pharmacokinetics and safety in infants. Pediatr Infect Dis J. 2011;30(5):375–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watt KM, Benjamin DK Jr., Cheifetz IM, et al. Pharmacokinetics and safety of fluconazole in young infants supported with extracorporeal membrane oxygenation. Pediatr Infect Dis J. 2012;31(10):1042–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anderson BJ, van den Anker J. Why is there no morphine concentration-response curve for acute pain? Paediatr Anaesth. 2014;24(3):233–238. [DOI] [PubMed] [Google Scholar]
- 60.Mahmood I, Ahmad T, Mansoor N, Sharib SM. Prediction of Clearance in Neonates and Infants (</= 3 Months of Age) for Drugs That Are Glucuronidated: A Comparative Study Between Allometric Scaling and Physiologically Based Pharmacokinetic Modeling. J Clin Pharmacol. 2017;57(4):476–483. [DOI] [PubMed] [Google Scholar]
- 61.Jiang XL, Zhao P, Barrett JS, Lesko LJ, Schmidt S. Application of physiologically based pharmacokinetic modeling to predict acetaminophen metabolism and pharmacokinetics in children. CPT Pharmacometrics Syst Pharmacol. 2013;2:e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Valitalo PA, Krekels EH, van Dijk M, Simons S, Tibboel D, Knibbe CA. Morphine Pharmacodynamics in Mechanically Ventilated Preterm Neonates Undergoing Endotracheal Suctioning. CPT Pharmacometrics Syst Pharmacol. 2017;6(4):239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]