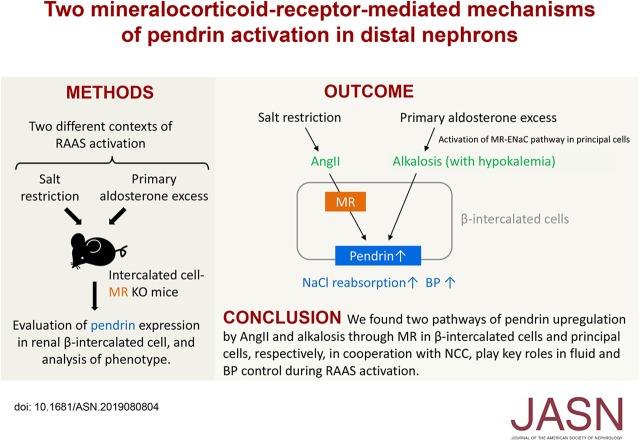
Significance Statement
Pendrin expressed in renal β-intercalated cells is involved in sodium chloride reabsorption in distal nephron, and plays an essential role in fluid homeostasis and BP control in conjunction with sodium chloride cotransporter in distal convoluted tubules. Using intercalated cell–specific mineralocorticoid receptor knockout mice, the authors found two distinct pathways of pendrin activation: by angiotensin II elevation, mediated by mineralocorticoid receptor in intercalated cells, and by hypokalemic alkalosis, mediated by mineralocorticoid receptor in principal cells. Moreover, they demonstrated that pendrin activation, in cooperation with sodium chloride cotransporter, contributes to the maintenance of fluid homeostasis during dietary salt restriction and to the development of salt-sensitive hypertension during aldosterone excess. They also clarified that activation of mineralocorticoid receptor at the two nephron sites plays a key role in thiazide-resistant hypertension.
Keywords: mineralocorticoid receptor, aldosterone, angiotensin II, pendrin, sodium chloride cotransporter, blood pressure
Visual Abstract
Abstract
Background
Regulation of sodium chloride transport in the aldosterone-sensitive distal nephron is essential for fluid homeostasis and BP control. The chloride-bicarbonate exchanger pendrin in β-intercalated cells, along with sodium chloride cotransporter (NCC) in distal convoluted tubules, complementarily regulate sodium chloride handling, which is controlled by the renin-angiotensin-aldosterone system.
Methods
Using mice with mineralocorticoid receptor deletion in intercalated cells, we examined the mechanism and roles of pendrin upregulation via mineralocorticoid receptor in two different models of renin-angiotensin-aldosterone system activation. We also used aldosterone-treated NCC knockout mice to examine the role of pendrin regulation in salt-sensitive hypertension.
Results
Deletion of mineralocorticoid receptor in intercalated cells suppressed the increase in renal pendrin expression induced by either exogenous angiotensin II infusion or endogenous angiotensin II upregulation via salt restriction. When fed a low-salt diet, intercalated cell–specific mineralocorticoid receptor knockout mice with suppression of pendrin upregulation showed BP reduction that was attenuated by compensatory activation of NCC. In contrast, upregulation of pendrin induced by aldosterone excess combined with a high-salt diet was scarcely affected by deletion of mineralocorticoid receptor in intercalated cells, but depended instead on hypokalemic alkalosis through the activated mineralocorticoid receptor–epithelial sodium channel cascade in principal cells. In aldosterone-treated NCC knockout mice showing upregulation of pendrin, potassium supplementation corrected alkalosis and inhibited the pendrin upregulation, thereby lowering BP.
Conclusions
In conjunction with NCC, the two pathways of pendrin upregulation, induced by angiotensin II through mineralocorticoid receptor activation in intercalated cells and by alkalosis through mineralocorticoid receptor activation in principal cells, play important roles in fluid homeostasis during salt depletion and salt-sensitive hypertension mediated by aldosterone excess.
The renin-angiotensin-aldosterone system (RAAS) plays a critical part in the regulation of sodium chloride reabsorption in the distal nephron. Traditionally, aldosterone causes activation of epithelial sodium channel (ENaC) via the mineralocorticoid receptor (MR) in principal cells (PCs) of the connecting tubule and collecting duct.1,2 In primary and secondary aldosteronism, ENaC and thiazide-sensitive sodium chloride cotransporter (NCC) in the distal convoluted tubules (DCTs) perform an important function in the fluid and electrolyte homeostasis and the control of BP. During volume depletion, activation of NCC owing to elevated angiotensin II (AngII) levels increases sodium chloride reabsorption to maintain normal BP.3,4 In turn, the decreased distal flow reduces sodium reabsorption by ENaC despite aldosterone excess, thus resulting in the inhibition of potassium excretion via renal medullary potassium channel.5 Conversely, during a high-potassium diet, increased plasma potassium concentration decreases NCC activity,6 and in turn, the increased distal flow augments sodium reabsorption via aldosterone-activated ENaC and promotes urinary potassium excretion.7 This phenomenon is called the “aldosterone paradox.” Moreover, hypokalemia induced by ENaC/renal medullary potassium channel activation during a mineralocorticoid excess activates NCC,8 which contributes to salt-sensitive hypertension.9 Thus, NCC plays a key role in sodium and potassium homeostasis under the control of the RAAS.
In addition to NCC, pendrin, a chloride/bicarbonate exchanger in β-intercalated cells (ICs) in the connecting tubule and cortical collecting duct, participates in the regulation of sodium chloride reabsorption10,11 in coordination with apical sodium-dependent chloride/bicarbonate exchanger.12 Accordingly, pendrin is involved in fluid homeostasis10 and in the development of salt-sensitive hypertension.13 Of note, pendrin and NCC are activated by elevated AngII levels during salt depletion14,15 and hypokalemia induced by aldosterone excess.16 Moreover, pendrin serves as a complement to NCC in fluid homeostasis and BP control during volume depletion.17 It is noteworthy that both AngII and hypokalemia induced by aldosterone excess induces dephosphorylation of MR in ICs, thereby resulting in an increase in the ligand affinity of its receptor.18 This finding led us to the hypothesis that pendrin activation depends on IC-MR, in contrast to the absence of DCT-MR involvement in NCC activation.8 Given the lack of evidence indicating MR dependence of pendrin activation, we aimed to create mice with a conditional MR knockout (KO) in ICs to test whether the AngII- and hypokalemia-induced pendrin activation is mediated by IC-MR. We demonstrated that AngII-induced pendrin activation depends on IC-MR, whereas the hypokalemia-induced pendrin activation is barely mediated by IC-MR and rather depends on hypokalemic metabolic alkalosis that is mediated by PC-MR activation. Moreover, we found that the two mechanisms of pendrin activation, in cooperation with NCC, contributes to the maintenance of normal BP during salt restriction and to the development of salt-sensitive hypertension by aldosterone excess.
Methods
Mice
All animal procedures were approved by the Animal Care Committee of The University of Tokyo. In MR-flox mice, the loxP site was inserted as previously described,19 to delete the entire exon 2 of the Nr3c2 gene (encoding MR). Crossing MR-flox mice in the C57BL/6 background with Atp6v1b1-cre mice (Atp6v1b1-cre) in the 129/Sv background that express Cre recombinase under the control of the Atp6v1b1 (encoding vacuolar H+-ATPase B1 subunit) promoter,20 we obtained MRflox/flox;Atp6v1b1-cre+/− (MRB1-KO) mice and littermate MRflox/flox (MRFlox) mice, in expected Mendelian ratios. They were maintained as a mixed inbred strain. Crossing Pax8-rtTA mice21 in the C57BL/6 background (B6.Cg-Tg[Pax8-rtTA2S*M2]1Koes/J; stock number 007176; Jackson Laboratory) with tetO-cre mice22 in the C57BL/6 background (B6.Cg-Tg[tetO-cre]1Jaw/J; stock number 006234; Jackson Laboratory), we obtained Pax8-rtTA+/−;tetO-cre+/− mice. These were then crossed with MRflox/flox mice in the C57BL/6 background to generate MRflox/flox;Pax8-rtTA+/−;tetO-cre+/− mice. To induce recombination, the mice were fed a doxycycline-containing diet (0.1% in diet AIN-76A; Research Diets) for 4 weeks, followed by 2 weeks of a wash-out period, where they were fed a normal diet (MRPax8-KO mice). MRflox/flox;Pax8-rtTA+/−;tetO-cre+/− littermates that were fed a diet without doxycycline, or MRflox/flox that were fed a doxycycline-containing diet (one mouse in the high-salt diet group) served as controls (MRControl). NCC KO mice23 were obtained from Mutant Mouse Resource and Research Centers (B6.Cg-Slc12a3tm1Ges/Mmnc, strain 16148). The genetically modified mice used in the experiments were males between 12 and 24 weeks of age. C57BL/6J mice aged 9–10 weeks were also used in some experiments.
AngII Treatment
Mice that were fed a normal diet were treated with AngII (1000 ng/kg per minute, administered subcutaneously; FUJIFILM Wako) for 1 week using a miniosmotic pump (model 2002; Alzet).
Salt Depletion and Add-On Hydrochlorothiazide Treatment
Mice were fed a high-salt diet (8% NaCl) for 1 week, and then their nutrition was either switched to a low-salt diet (0.03% NaCl) or kept unchanged. After 2 weeks, the mice that were fed the low-salt diet received either hydrochlorothiazide (HCTZ) treatment (300 mg/L in drinking water, 80 mg/kg per day; Sigma-Aldrich) for 6 days or no treatment. HCTZ was dissolved in drinking water with mild heating. The bottles of drinking water containing HCTZ were exchanged with new ones every other day.
Metabolic Cage Study
Mice were fed with a low-salt diet (0.03% NaCl) and moved to metabolic cages (Natsume) on the 11th day. After 3 days of acclimation, the mice that were fed the low-salt diet received massage of the lower abdomen to empty the bladder, followed by intraperitoneal injection of HCTZ (20 mg/kg). After the HCTZ injection, urine was collected under light mineral oil for 4 hours. The mice received abdominal massage again at the end of urine collection. Urine Na+ content was determined using a sodium ion meter (LAQUAtwin Na-11; HORIBA). In another experiment, the mice that were fed with low-salt diet and acclimated in metabolic cages received HCTZ treatment (300 mg/L in drinking water, 80 mg/kg per day) for 6 days. Urine was collected under light mineral oil every 24 hours.
Aldosterone Treatment Combined with High-Salt Diet
Mice were fed a high-salt diet (8% NaCl) for 1 week and then treated with aldosterone (200 μg/kg per day, administered subcutaneously; Sigma-Aldrich) for 2 weeks by means of the miniosmotic pump. On these mice, we started pharmacologic and dietary manipulations including amiloride (20 mg/L in drinking water, 10 mg/kg per day; Sigma-Aldrich) and potassium supplementation (8% KCl, 8% NaCl), just after implantation of the aldosterone pump. In an experiment with potassium restriction, mice were fed a potassium-normal and high-salt diet (1% K, 8% NaCl) for 1 week, and then, at the start of aldosterone infusion, the nutrition was either switched to a potassium-depleted high-salt diet (0% K, 8% NaCl) or kept unchanged. Treatment with either acetazolamide (300 mg/L in drinking water sweetened by 1% sucrose, 250 mg/kg per day; Tokyo Chemical Industry) or vehicle was begun at the start of aldosterone infusion. Because treatment of NCC KO mice with a full dose of aldosterone (200 μg/kg per day, administered subcutaneously) and the high-salt diet (8% NaCl) unexpectedly caused massive polyuria and hydronephrosis, we reduced the dose (aldosterone to 100 μg/kg per day, administered subcutaneously, and NaCl to 2% in chow, respectively). This dose was well tolerated regardless of the presence of potassium as a dietary supplement (8% KCl, 2% NaCl).
Bicarbonate Loading and Potassium Depletion in Adrenalectomized Mice
Bilateral adrenalectomy was performed via a dorsal incision under isoflurane anesthesia, as described previously.15 The mice received dexamethasone (60 μg/kg per day, administered subcutaneously; FUJIFILM Wako) every other day as a glucocorticoid replacement, and were fed the high-salt potassium-normal diet (8% NaCl, 1% K) to compensate for the natriuresis after adrenalectomy. After 7 days of recovery from the surgical procedure, the mice either were treated with oral bicarbonate (0.28 M NaHCO3 in drinking water) for 3 days or received no treatment. After that, they were fed either a high-salt low-potassium diet (8% NaCl, 0% K) or the high-salt potassium-normal diet for 4 days. Successful removal of adrenal glands was confirmed by undetectable levels of plasma aldosterone.
Measurement of BP
In some experiments, BP was measured using a radiotelemetry system as previously described.15 Mice were anesthetized with isoflurane (FUJIFILM Wako), and the left carotid artery was isolated. The tip of the catheter of the PA-C10 transmitter (Data Sciences International) was inserted into the carotid artery and advanced into the aortic arch, with the telemeter body positioned in the subcutaneous pocket of the left flank. The experiments were started after more than 7 days of recovery from the surgical procedure. Arterial BP in a conscious mouse was directly monitored by radiotelemetry by means of a RPC-1 receiver, APR-1 ambient-pressure monitor, and a Data-Quest-ART-Silver 4.2 acquisition system (Data Sciences International). Mean arterial pressure (MAP) was recorded in 10-second intervals every 15 minutes, and hourly MAP levels were calculated using the average of four sequential MAP records. We also measured systolic BP with a tail-cuff system (BP-98A; Softron). Measurements were performed during the day, and average systolic BP was calculated from five consecutive measurements in a calm state.
Blood Collection and Analysis
We collected blood into a heparin lithium–coated syringe after cutting down the right femoral artery of mice under anesthesia. Immediately, 80 μl of whole blood was loaded into an EC8+ cartridge for blood analysis on an i-STAT 1 analyzer (Abbott). The remaining heparinized blood was centrifuged at 2400×g for 15 minutes at 4°C to obtain the plasma fraction.
Quantitative RT-PCR
Total RNA was extracted with the RNeasy Mini Kit (Qiagen) and converted into complementary DNA via the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Quantitative PCR was performed using the Power SYBR Green PCR Master Mix (Applied Biosystems) on a StepOnePlus Real-Time PCR System (Applied Biosystems). The primers used are listed in Supplemental Table 1.
Immunoblot Analysis
We performed immunoblot analysis as described previously,15 with some modifications. Kidney samples were homogenized in a buffer consisting of 10 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1% of IGEPAL-CA630, 50 mM NaF, 10 mM sodium pyrophosphate, 1 mM sodium orthovanadate, and a protease inhibitor cocktail (cOmplete, Roche) to prepare protein lysates. In some experiments, the cortex and medulla were excised from kidney slices under a stereoscopic microscope. Equal amounts of protein were mixed with Laemmli sample buffer supplemented with 2-mercaptoethanol and were incubated at 20°C for 30 minutes. Each sample was resolved by SDS-PAGE and transferred to polyvinylidene difluoride membranes. The membranes were incubated with primary antibodies overnight at 4°C, followed by incubation with the corresponding HRP-conjugated secondary antibodies. Signals were detected and scanned with the ECL Prime Western Blotting Detection Reagent (GE Healthcare) and ImageQaunt LAS 4000 mini (GE Healthcare), respectively. We used antibodies against pendrin (a gift from Prof. Aronson, Yale University),24 against α-ENaC and pT53-NCC (gifts from Prof. Loffing, University of Zurich),25 against NCC (AB3553; Millipore), and against GAPDH (sc-32233; Santa Cruz Biotechnology).
Immunofluorescence Staining
We performed immunofluorescence staining as described elsewhere,15 with some modifications. Cryosections (6 μm thick) fixed with 4% paraformaldehyde were boiled in citrate buffer (pH 6.0) for antigen retrieval and then blocked with 5% normal donkey serum. The sections were then incubated with a primary antibody followed by incubation with the corresponding secondary donkey antibody conjugated with a fluorescent dye such as Alexa Fluor 488, 555, or 633 (Invitrogen). We used the antibodies against pendrin (a gift from Prof. Aronson, Yale University),24 β-ENaC (SPC-404D; StressMarq), vacuolar H+-ATPase B1 subunit (sc-21206; Santa Cruz Biotechnology), NCC (AB3553), anion exchanger 1 (AE11-A; Alpha Diagnostic), and Calbindin1 (sc-7692; Santa Cruz Biotechnology). Double or triple immunostaining was performed by repeating the process of blocking and subsequent incubation with primary and secondary antibodies. DAPI (catalog number D523; Dojindo) was used for counterstaining of nuclei. Immunofluorescence staining of the MR in mouse kidney sections with a mouse monoclonal anti-MR antibody (clone 1D5, a gift from Prof. C.E. Gomez-Sanchez, University of Mississippi Health Center)26 was performed using a mouse-on-mouse immunodetection kit (M.O.M. ImmPRESS HRP Polymer Kit; Vector Laboratories) in combination with the cyanine 3 tyramide signal amplification kit (Opal 3-Plex Kit; PerkinElmer). Images were captured using a TCS SP5 confocal laser scanning microscope with the Leica Application Suite Advanced Fluorescence software (Leica) and were processed for figure preparation in the ImageJ software (National Institutes of Health). The pixel intensity of pendrin labeling on lines drawn from apical surface into the cytosolic area were obtained using ImageJ software.
Statistical Analyses
All statistical analyses were performed in the JMP software (version Pro 13; SAS Institute Inc.). Data are expressed as mean±SEM, unless specified otherwise. For multiple comparisons of continuous variables, we performed ANOVA with the Tukey–Kramer post hoc test. We conducted the unpaired t test for comparisons of continuous variables between two groups, and in some experiments that involved several time points, Bonferroni correction was applied after the unpaired t test. Wilcoxon rank-sum test was carried out for comparisons of either ordinal variables or continuous but not normally distributed variables between two groups. Data with a P value <0.05 were considered statistically significant. Statistical parameters can be found within the figure and table legends. In all mouse experiments, the n value represents the number of mice receiving a given treatment.
Results
MR Deletion in ICs Suppresses AngII-Induced Upregulation of Pendrin in Kidneys
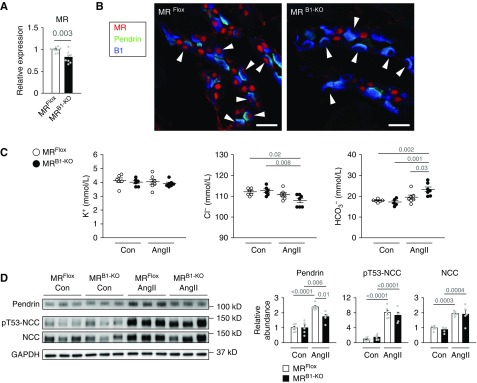
We generated mice with genetically deleted MR in ICs by crossing MR-floxed mice19 with Atp6v1b1-cre mice.20 As expected, MRflox/flox;Atp6v1b1-cre+/− (MRB1-KO) mice manifested a small but significant reduction in the renal expression of the MR gene as compared with MRflox/flox (MRFlox) littermate controls (Figure 1A). They showed MR ablation in around 90% of ICs, including pendrin-positive β-ICs (Figure 1B). MRB1-KO mice had comparable body weight and systolic BP relative to MRFlox mice in the steady state (Supplemental Figure 1, A and B).
Figure 1.
MR deletion in ICs suppressed the AngII-induced increase in pendrin amounts. (A) Quantitative analysis of MR gene expression in the kidneys of MRflox/flox (MRFlox) mice and MRflox/flox;Atp6v1b1-cre+/− (MRB1-KO) mice (n=8–9 per group). The expression was normalized to a reference gene (Rps29) and reported relative to the MRFlox group. (B) Immunofluorescence staining of MR (red) with counterstaining of pendrin (green) for β-ICs and vacuolar H+-ATPase B1 subunit (B1, blue) for all ICs in the kidneys. MR staining yielded negative results in ICs including pendrin-positive β-ICs (arrowheads). (C and D) MRFlox mice and MRB1-KO mice either were treated with AngII (1000 ng/kg per minute, administered subcutaneously) or received no treatment (Con) for 7 days. (C) Plasma concentration of electrolytes (n=5–7 per group). ANOVA, P=0.006 in Cl−, and P<0.001 in HCO3−. (D) Representative immunoblots and quantities of pendrin, phosphorylated NCC (pT53-NCC, as an index of activation), and NCC in the kidney lysates (n=5–6 per group). ANOVA, P<0.001 in all proteins. The expression was normalized to a loading control (GAPDH) and reported relative to the MRFlox–Con group. Data are expressed as mean±SEM. Statistical significance was analyzed by the unpaired t test in (A) and ANOVA followed by the Tukey–Kramer post hoc test in (C and D); significant differences are indicated by horizontal bars with P values. Scale bars, 20 μm.
First, we administered AngII to MRB1-KO mice and MRFlox mice. In MRFlox mice, AngII did not affect plasma K+, Cl−, or HCO3− concentration (Figure 1C), but significantly increased the pendrin amount in the kidneys along with raising the amounts of total and phosphorylated NCC, which is indicative of NCC activation (Figure 1D). The MR deletion in ICs significantly suppressed the AngII-induced upregulation of pendrin (Figure 1D). Notably, metabolic alkalosis was noted in the AngII-treated MRB1-KO mice (Figure 1C), possibly owing to the suppression of pendrin-driven chloride/bicarbonate exchange. In contrast, MR deletion in ICs did not alter AngII-induced activation of NCC (Figure 1D).
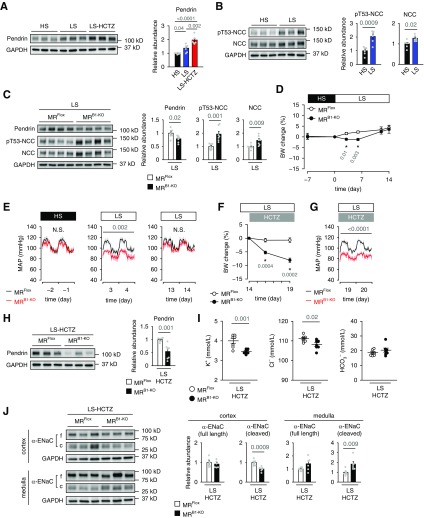
Next, we examined whether IC-MR is involved in the increased pendrin expression induced by elevated endogenous AngII concentration owing to dietary salt restriction. The salt restriction increased pendrin expression in the kidneys along with NCC activation in wild-type mice, resultantly keeping the body weight and electrolytes unchanged (Figure 2, A and B, Supplemental Figure 2, A–C). In addition, administration of NCC blocker HCTZ to mice fed a low-salt diet, which might enhance the activation of endogenous RAAS, further increased the pendrin expression without change in body weight and electrolytes (Figure 2A, Supplemental Figure 2, A–C), suggesting that pendrin is involved in the compensation for NCC inhibition to maintain volume balance. During dietary salt restriction, pendrin expression was lower in MRB1-KO mice than in MRFlox mice (Figure 2C). The reduced expression of pendrin was associated with transient body weight loss and hypotension (Figure 2, D and E, Supplemental Figure 2D) in salt-depleted MRB1-KO mice, but their body weight and BP gradually recovered and reached the same levels as the BP and body weight at the end of the low-salt diet period in salt-depleted MRFlox mice (Figure 2, D and E, Supplemental Figure 2D), possibly through the compensatory activation of NCC (Figure 2C). The compensatory NCC activation in salt-depleted MRB1-KO mice is supported by augmented urinary sodium excretion in response to acute administration of HCTZ (Supplemental Figure 3A). Resultantly, chronic (6-day) administration of NCC blocker HCTZ to salt-depleted MRB1-KO mice caused progressive body weight loss (Figure 2F), increased sodium excretion in the urine (Supplemental Figure 3B), and significant hypotension (Figure 2G, Supplemental Figure 2E), along with suppression of pendrin expression (Figure 2H). Notably, after HCTZ treatment, plasma concentration of K+ was lower in MRB1-KO mice than in MRFlox mice (Figure 2I), possibly owing to increased medullary ENaC activity (Figure 2J). Moreover, the HCTZ-treated MRB1-KO mice had a lower plasma Cl− concentration and showed a tendency for an increase in plasma HCO3− concentration as compared with the HCTZ-treated MRFlox mice (Figure 2I), suggesting that the AngII-induced increase in pendrin activity was inhibited by the IC-MR deletion. These results reveal that the upregulation of pendrin mediated by MR in ICs plays a complementary part in conjunction with NCC activation in the maintenance of normal BP during salt restriction.
Figure 2.
Mice with MR deletion in ICs showed reduced expression of pendrin, a volume loss, and hypotension after salt depletion and NCC blockade. (A and B) C57BL/6J mice were fed a high-salt diet (HS; 8% NaCl) for 1 week and then were switched or not switched to a low-salt diet (LS; 0.03% NaCl). After 2 weeks, the LS-fed mice were treated or not treated with HCTZ for 6 days. Immunoblots of renal pendrin (A), pT53-NCC, and NCC (B) (n=6 per group). ANOVA P<0.001 in (A). (C–J) MRFlox and MRB1−KO mice were fed HS for 1 week, followed by LS for 2 weeks, and then treated with HCTZ for 6 days. (C) Immunoblots of pendrin, pT53-NCC, and NCC after 1 week of LS (n=7 per group). (D) Body weight change before and after the salt depletion (n=7 per group). (E) Telemetric monitoring of MAP during HS and LS (n=6 per group). (F) Body weight change after HCTZ treatment (n=7 per group). (G) Telemetric monitoring of MAP after HCTZ treatment (n=6 per group). Immunoblots of pendrin (H) and plasma concentration of electrolytes (I) after 6 days of HCTZ treatment (n=7 per group). (J) Immunoblots of full-length (f) and cleaved forms (c, as an index of activation) of α-ENaC in the renal cortex or medulla (n=6–7 per group). Data are expressed as mean±SEM. Statistical significance was analyzed by ANOVA followed by the Tukey–Kramer post hoc test in (A), the unpaired t test in (B, C, E, and G–J), and unpaired t test with Bonferroni correction in (D and F). The average values of hourly MAP during two consecutive days in each mouse were analyzed in (E and G). Significant differences are indicated by horizontal bars or asterisks with P values.
Upregulation of Pendrin during Aldosterone Excess Is Driven by Hypokalemic Metabolic Alkalosis
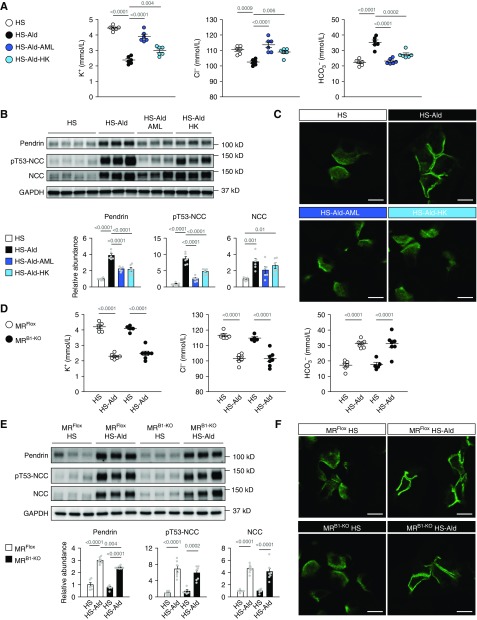
Next, we investigated the mechanism of pendrin regulation during mineralocorticoid excess that also causes MR activation in the distal nephron. Wild-type mice that were fed a high-salt diet with the continuous infusion of aldosterone developed marked hypokalemia and metabolic alkalosis with hypochloremia (Figure 3A). These mice manifested a significant increase in the pendrin amount along with NCC activation (Figure 3B). In addition to the change in total amount of pendrin, previous reports demonstrated mineralocorticoid-induced change in subcellular distribution of pendrin; the immune-gold labels of pendrin changed from the apical cytosolic vesicles to the apical plasma membrane after mineralocorticoid treatment.27,28 In accordance with this report, immunofluorescence staining showed the flat distribution of pendrin labeling from the apical membrane to the subapical cytosolic region in mice fed a high-salt diet, but with the additional treatment of aldosterone, the labeling was sharply concentrated in the region of apical plasma membrane (Figure 3C, Supplemental Figure 4, A–C). Moreover, treatments with either ENaC inhibitor amiloride or dietary supplementation with potassium attenuated hypochloremic metabolic alkalosis (Figure 3A), and resulted in the reversal of the aldosterone-induced upregulation of pendrin (Figure 3B) as previously reported,16 which is associated with remarkable change in pendrin distribution in the apical membrane region to the subapical cytosolic region (Figure 3C, Supplemental Figure 4C). The aldosterone-induced NCC activation was also attenuated by both treatments, thus attenuating the aldosterone-induced BP elevation (Supplemental Table 2). These data suggest that the aldosterone-induced upregulation of pendrin and NCC is attributable to hypokalemic metabolic alkalosis.
Figure 3.
Upregulation of pendrin during aldosterone excess is driven by hypokalemic metabolic alkalosis, independently of MR in ICs. (A–C) C57BL/6J mice fed the high-salt diet (HS; 8% NaCl) either were treated with aldosterone (Ald; 200 μg/kg per day, administered subcutaneously) or received no treatment for 14 days. Ald treatment was combined with either amiloride (AML) or dietary potassium supplementation (high potassium; HK) or was administered alone. (A) Plasma concentration of electrolytes (n=6 per group). ANOVA, P<0.001 in all electrolytes. (B) Representative immunoblots and quantities of pendrin, pT53-NCC, and NCC in kidney lysates (n=6 per group). ANOVA, P<0.001 in pendrin and pT53-NCC, and P=0.002 in NCC. (C) Representative immunofluorescence staining of pendrin (green) in the kidneys. (D–F) MRFlox and MRB1-KO mice fed the HS diet (8% NaCl) either were treated with Ald (200 μg/kg per day, administered subcutaneously) or received no treatment for 14 days. (D) Plasma concentrations of electrolytes (n=5–7 per group). ANOVA, P<0.001 in all electrolytes. (E) Representative immunoblots and quantities of pendrin, pT53-NCC, and NCC in the kidney lysates (n=5–7 per group). ANOVA, P<0.001 in all proteins. (F) Representative immunofluorescence staining of pendrin (green) in the kidneys. Data are expressed as mean±SEM. Statistical significance was analyzed by ANOVA followed by the Tukey–Kramer post hoc test; significant differences are indicated by horizontal bars with P values. Scale bars, 10 μm.
To test the involvement of IC-MR in pendrin regulation induced by mineralocorticoid excess, we administered aldosterone to MRB1-KO mice. Aldosterone treatment decreased plasma potassium concentration associated with hypochloremic alkalosis equally in MRB1-KO mice and MRFlox mice (Figure 3D). Contrary to our expectations, the aldosterone-induced change in subcellular localization of pendrin was unchanged by the IC-MR deletion (lower versus upper in the right panels of Figure 3F), despite slightly lower expression of pendrin in aldosterone-treated MRB1-KO mice than in aldosterone-treated MRFlox mice (Figure 3E). This finding suggested that aldosterone-induced regulation of pendrin was not affected by IC-MR deletion. Consistently, plasma Cl− and HCO3− levels, which are modified by pendrin function, were not affected by the IC-MR deletion in the aldosterone-treated mice (Figure 3D), and this result revealed that pendrin was regulated by aldosterone regardless of the presence of IC-MR. NCC activation did not differ between aldosterone-treated MRB1-KO mice and MRFlox mice (Figure 3E), which had similar levels of plasma potassium (Figure 3D). Consistent with the little change in the regulation of pendrin and NCC, BP elevation was comparable between aldosterone-infused MRB1-KO and MRFlox mice (Supplemental Table 3).
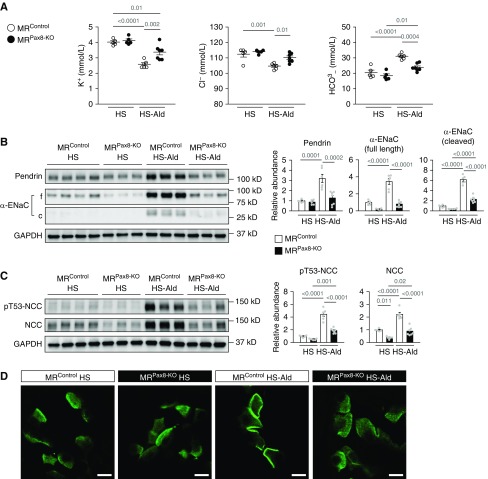
Upon ENaC activation–induced hypokalemic metabolic alkalosis during mineralocorticoid excess via the activation of PC-MR, aldosterone-induced hypokalemia and hypochloremic metabolic alkalosis were suppressed in the mice with genetically deleted MR in entire nephron (MRPax8-KO) (Figure 4, A and B, Supplemental Figure 5, A–D). In accordance with the suppression of hypokalemic metabolic alkalosis, aldosterone-induced upregulation of pendrin with concentrated distribution of pendrin label in the region of apical membrane disappeared in MRPax8-KO mice (Figure 4, B and D), along with attenuation of the NCC activation (Figure 4C). As a result, aldosterone-induced salt-sensitive hypertension was ameliorated in MRPax8-KO mice (Supplemental Table 4). The present findings that aldosterone-induced upregulation of pendrin with the change in subcellular localization were not affected by MR deletion in ICs but attenuated by the correction of hypokalemic alkalosis by each treatment of MR deletion in entire nephron, ENaC inhibitor, and potassium supplementation led us to the plausible hypothesis that hypokalemic alkalosis by the MR–ENaC activation in renal PCs mainly contributes to the regulation of pendrin during mineralocorticoid excess.
Figure 4.
Effects of MR deletion in the entire nephron on pendrin upregulation induced by aldosterone. (A–D) Mice with MR deletion in entire nephron (MRPax8-KO) and control littermates (MRControl) that were fed the high-salt diet (HS; 8% NaCl) either were treated with aldosterone (Ald; 200 μg/kg per day, administered subcutaneously) or received no treatment for 14 days. (A) Plasma concentrations of electrolytes (n=5–7 per group). ANOVA, P<0.001 in K+, P<0.001 in Cl−, and P<0.001 in HCO3−. (B and C) Representative immunoblots and quantities of pendrin, full-length (f) and cleaved form (c, as an index of activation) of α-ENaC (B), pT53-NCC, and NCC (C) in the kidney lysates (n=5–7 per group). ANOVA, P<0.001 in all proteins. (D) Representative immunofluorescence staining of pendrin (green) in the kidneys. Data are expressed as mean±SEM. Statistical significance was analyzed using ANOVA followed by the Tukey–Kramer post hoc test; significant differences are indicated by horizontal bars with P values. Scale bars, 10 μm.
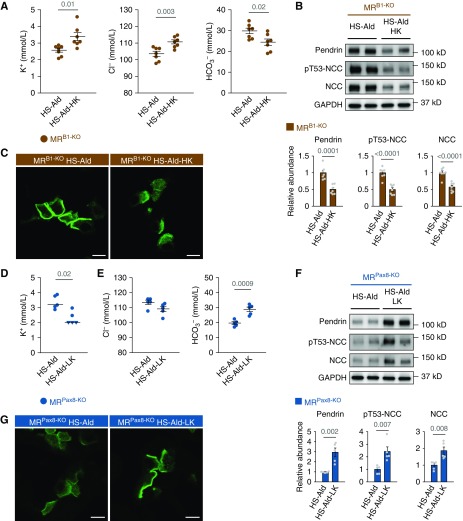
Next, we tested whether the correction and induction of hypokalemia can reverse the upregulation and downregulation of pendrin observed in aldosterone-treated MRB1-KO mice and MRPax8-KO mice, respectively. Potassium supplementation attenuated hypokalemia and hypochloremic metabolic alkalosis in aldosterone-treated MRB1-KO mice (Figure 5A), and reversed the aldosterone-induced upregulation of pendrin with attenuation of the concentrated pendrin distribution in the region of apical membrane (Figure 5, B and C), along with suppression of NCC activation (Figure 5B), resulting in a significant reduction of systolic BP in aldosterone-treated MRB1-KO mice (Supplemental Table 5). Conversely, dietary potassium depletion induced hypokalemia and metabolic alkalosis in aldosterone-treated MRPax8-KO mice (Figure 5, D and E), and significantly increased pendrin abundance with induction of concentrated distribution of pendrin label in the apical membrane region (Figure 5, F and G) that accompanies NCC activation (Figure 5F). Thus, pendrin regulation owing to aldosterone excess is a result of hypokalemia and alkalosis that is independent of IC-MR, but depends on PC-MR.
Figure 5.
The influence of dietary potassium manipulations on pendrin expression in aldosterone-treated mice with MR deletion in the ICs and entire nephron. (A–C) MRB1-KO mice that were fed the high-salt diet (HS; 8% NaCl) were treated with aldosterone (Ald; 200 μg/kg per day, administered subcutaneously) for 14 days, with or without dietary potassium supplementation (high potassium; HK). (A) Plasma concentrations of electrolytes (n=7 per group). (B) Representative immunoblots and quantities of pendrin, pT53-NCC, and NCC in the kidney lysates (n=7 per group). (C) Representative immunofluorescence staining of pendrin (green) in the kidneys. (D–G) MRPax8-KO mice that were fed a HS (8% NaCl) were treated with Ald (200 μg/kg per day, administered subcutaneously) for 14 days, with or without dietary potassium depletion (low potassium; LK). (D) Plasma concentration of potassium (n=5 per group). Some values below the limit of detection (2 mmol/L) are presented as triangular plots. The horizontal bars indicate the median values in each group. (E) Plasma concentration of chloride and bicarbonate (n=5 per group). (F) Representative immunoblots and quantities of pendrin, pT53-NCC, and NCC in the kidney lysates (n=5 per group). (G) Representative immunofluorescence staining of pendrin (green) in the kidneys. Data are expressed as mean±SEM in (A, B, E, and F). Statistical significance was analyzed with the unpaired t test in (A, B, E, and F) and by Wilcoxon rank-sum test in (D); significant differences are indicated by horizontal bars with P values. Scale bars, 10 μm.
Metabolic Alkalosis Rather Than Hypokalemia Is Involved in Pendrin Regulation Induced by Aldosterone Treatment
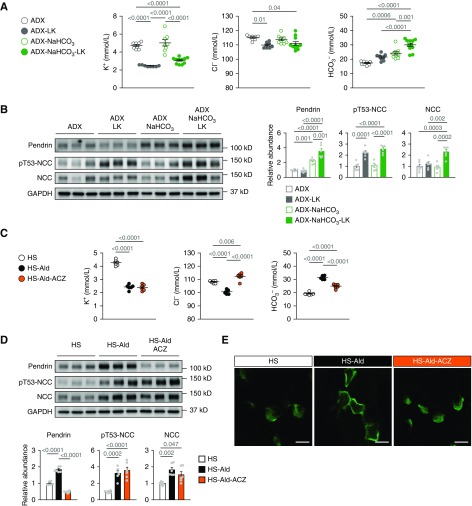
To determine whether the pendrin regulation induced by hypokalemic alkalosis is mediated by hypokalemia or alkalosis, we examined the individual effects of hypokalemia and metabolic alkalosis on pendrin expression. Given that either hypokalemia29 or metabolic alkalosis30 can suppress aldosterone secretion from adrenal glands, we used wild-type mice that had undergone adrenalectomy to eliminate corticosteroids secretion from the adrenal glands. Notably, the low-potassium diet induced hypokalemia without alkalosis (Figure 6A), but did not affect pendrin expression (Figure 6B). In contrast, bicarbonate loading induced mild metabolic alkalosis (Figure 6A) without a change in serum potassium concentration, and definitely increased the pendrin amount (Figure 6B). These results are in line with those of other studies on mice that had not undergone adrenalectomy,31,32 confirming that alkalosis-induced upregulation of pendrin is independent of the aldosterone–MR pathway. Moreover, the combination of dietary potassium depletion with bicarbonate loading caused severe metabolic alkalosis (Figure 6A) and further increased the pendrin amount (Figure 6B) along with induction of concentrated distribution of pendrin label in the region of apical membrane (Supplemental Figure 6), as observed in aldosterone-treated mice. To sum up, metabolic alkalosis rather than hypokalemia upregulated pendrin, but the combination of hypokalemia and alkalosis was needed for the full regulation of pendrin.
Figure 6.
Metabolic alkalosis rather than hypokalemia is involved in pendrin activation induced by aldosterone infusion. (A and B) Adrenalectomized (ADX) C57BL/6J mice either were pretreated with NaHCO3 loading (0.28 M in drinking water) for 3 days or received no pretreatment and then were fed either a low-potassium diet (LK) or normal diet for 4 days. (A) Plasma concentrations of electrolytes (n=6 per group). ANOVA, P<0.001 in K+, P=0.005 in Cl−, and P<0.001 in HCO3−. (B) Representative immunoblots and quantities of pendrin, pT53-NCC, and NCC in the kidney lysates (n=6 per group). ANOVA, P<0.001 in all proteins. (C–E) C57BL/6J mice that were fed the high-salt diet (HS; 8% NaCl) either were treated with aldosterone (Ald; 200 μg/kg per day, administered subcutaneously) for 14 days or received no treatment. Ald treatment was either combined with acetazolamide (ACZ) or administered alone. (C) Plasma concentration of electrolytes (n=6 per group). ANOVA, P<0.001 in all electrolytes. (D) Representative immunoblots and quantities of pendrin, pT53-NCC, and NCC in the kidney lysates (n=6 per group). ANOVA, P<0.001 in pendrin and pT53-NCC, and P=0.003 in NCC. (E) Representative immunofluorescence staining of pendrin (green) in the kidneys. Data are expressed as mean±SEM. Statistical significance was analyzed using ANOVA followed by the Tukey–Kramer post hoc test; significant differences are indicated by horizontal bars with P values. Scale bars, 10 μm.
To confirm the indispensability of metabolic alkalosis for the pendrin regulation induced by mineralocorticoid excess, we corrected metabolic alkalosis in aldosterone-treated mice. Treatment with a carbonic anhydrase inhibitor, acetazolamide significantly reversed metabolic alkalosis without changing serum potassium (Figure 6C), and thus eliminated the aldosterone-induced upregulation of pendrin with concentrated distribution in the region of apical membrane (Figure 6, D and E). In contrast, aldosterone-induced activation of NCC was unaffected by acetazolamide, through sustained hypokalemia (Figure 6D). Thus, aldosterone-induced regulation of pendrin was owing to alkalosis (not to hypokalemia), in contrast to NCC activation by hypokalemia.
The Important Role of Pendrin Regulation in Salt-Sensitive Hypertension in Aldosterone-Treated NCC KO Mice
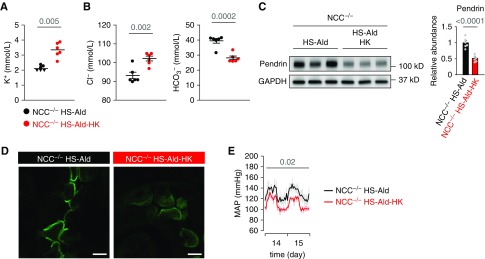
Pendrin regulation was induced by alkalosis in mineralocorticoid excess; however, its contribution to BP elevation was unclear. Indeed, in aldosterone-treated MRB1-KO mice, dietary supplementation with potassium decreased BP (Supplemental Table 5) in conjunction with the suppression of pendrin but with concurrent inhibition of NCC (Figure 5B). To dissect the roles of pendrin and NCC in salt-induced BP elevation upon treatment with aldosterone, we administered aldosterone to salt-loaded NCC KO mice. Aldosterone-treated NCC KO mice with hypokalemic alkalosis (Supplemental Figure 7, A and B) showed sustained BP elevation (Supplemental Figure 7E), possibly through upregulation of pendrin with concentrated distribution of pendrin label in the region of apical membrane (Supplemental Figure 7, C and D). However, the reversal of hypokalemic alkalosis by potassium supplementation (Figure 7, A and B) significantly decreased BP (Figure 7E) through a significant reduction in pendrin expression with the attenuation of the concentrated distribution in the apical membrane region (Figure 7, C and D). These results revealed that upregulation of pendrin by hypokalemic alkalosis, in conjunction with NCC, contributed to aldosterone-induced salt-sensitive hypertension.
Figure 7.
The important role of pendrin activation in salt-sensitive hypertension in aldosterone-treated NCC KO mice. (A–E) NCC KO mice (NCC−/−) that were fed a high-salt diet (HS; 2% NaCl) were treated with aldosterone (Ald; 100 μg/kg per day, administered subcutaneously) for 2 weeks with or without dietary potassium supplementation (high potassium; HK). (A) Plasma concentration of potassium (n=6 per group). Some values below the limit of detection (2 mmol/L) are presented as triangular plots. The horizontal bars indicate the median values in each group. (B) Plasma concentration of chloride and bicarbonate (n=6 per group). (C) Representative immunoblots and quantities of pendrin in the kidney lysates (n=6 per group). (D) Representative immunofluorescence staining of pendrin (green) in the kidneys. (E) Telemetric monitoring of MAP after 2 weeks of aldosterone infusion (n=6 per group). Data are expressed as mean±SEM in (B, C, and E). Statistical significance was analyzed using Wilcoxon rank-sum test in (A) and the unpaired t test in (B and C). The average value of hourly MAP during two consecutive days was calculated for each mouse, and statistical significance between the two groups was analyzed with the unpaired t test in (E); significant differences are indicated by horizontal bars with P values. Scale bars, 10 μm.
Discussion
We demonstrated two distinct mechanisms regulating renal pendrin expression during RAAS activation. AngII-induced upregulation of pendrin mediated by IC-MR is involved in the maintenance of fluid and BP during volume depletion, whereas the mineralocorticoid excess–induced regulation of pendrin is driven by hypokalemic metabolic alkalosis through activation of the MR–ENaC pathway in PCs, thereby leading to the development of salt-sensitive hypertension.
A previous report showed that MR in ICs is phosphorylated at a site in ligand binding domain in steady state, and the phosphorylated MR shows the less ability of ligand binding.18 AngII decreased phosphorylation of MR at the site in ICs, increasing the ligand affinity, and the dephosphorylation was associated with upregulation of pendrin.18 Supporting the involvement of MR dephosphorylation in the regulation of pendrin, AngII-induced pendrin upregulation was inhibited by either treatment of the MR antagonist or removal of mineralocorticoid by adrenalectomy.15 Moreover, the exogenous AngII-induced increase in the pendrin amount was significantly reversed by the MR deletion in ICs, thereby causing hypochloremic metabolic alkalosis (Figure 1C), and this result revealed the involvement of IC-MR in AngII-induced upregulation of pendrin with increased activity. Given that chloride/bicarbonate exchange is the fundamental function of pendrin,33 the phenotype of hypochloremic alkalosis observed in AngII-treated MRB1-KO mice is similar to that of pendrin KO mice with AngII upregulation.10 Moreover, increased endogenous AngII concentration owing to dietary salt restriction increases the expression of NCC and pendrin to maintain normal BP. BP remained normal through compensatory NCC activation, even with reduced pendrin expression by IC-MR deletion. Notably, the additional treatment with HCTZ markedly decreased BP and body weight in salt-restricted MRB1-KO mice. The decreased BP and body weight were associated with the suppression of pendrin expression, and the phenotype was similar to that of the pendrin and NCC double-KO mice.17 These results mean that the MR in ICs mediates AngII-driven upregulation of pendrin during volume depletion, and this mechanism cooperates with NCC to salvage fluid and maintain normal BP. According to another important finding—that IC-MR deletion induces hypokalemia in HCTZ-treated mice fed a low-salt diet—double inhibition of NCC and pendrin may increase distal flow, resulting in increased ENaC-dependent potassium secretion in the medulla. This assumption is on the basis of our finding of increased expression levels of cleaved α-ENaC, indicating ENaC activation in the renal medulla of HCTZ-treated MRB1-KO mice (Figure 2J). Of note, the dissociated expression of ENaC (upregulated in the medulla and downregulated in the cortex) has also been observed in the IC proton pump–deficient mice in association with pendrin downregulation.34 Moreover, the inhibitory influence of pendrin on ENaC-dependent potassium secretion is supported by other studies showing that the deletion of the pendrin gene attenuates the hyperkalemia observed in mice with a pseudohypoaldosteronism type II–causing mutation in WNK4 that mimics AngII excess in the distal nephron, by increasing ENaC-dependent potassium secretion.35 These observations indicate that AngII-driven upregulation of pendrin during volume depletion, which is mediated by MR in ICs, complements the NCC to maintain normal BP and potassium homeostasis.
During mineralocorticoid excess, we observed a prominent increase of pendrin and concentrated distribution of pendrin label in the region of apical membrane as reported previously.28 Nonetheless, MR deletion in ICs, without changes in plasma Cl− and HCO3− concentrations in aldosterone-treated mice, did not affect the concentrated distribution of pendrin label in the region of apical membrane, thus implying negligible changes in pendrin function by IC-MR deletion. This independence of MR from pendrin regulation was supported by the present finding of the bicarbonate-induced pendrin upregulation in the adrenalectomized mice. On the basis of a rather small expression of 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2), which converts corticosterone (cortisol in humans) into the inactive form, in ICs,36 IC-MR is already occupied by corticosterone and hence less sensitive to the change in aldosterone.37 In contrast, the MR in PCs, which sufficiently express 11β-HSD2,36 is activated by aldosterone, which in turn activates MR–ENaC signaling causing hypokalemic alkalosis, resulting in upregulation of pendrin with change in subapical distribution. Indeed, the correction of hypokalemic alkalosis by treatment with either the ENaC inhibitor amiloride or potassium supplementation reversed the aldosterone-induced upregulation of pendrin, in parallel with the decreased activation of NCC. Moreover, upon treatment with acetazolamide, the reversal of metabolic alkalosis occurred without changes in serum potassium concentration, and the reversal of pendrin upregulation took place without changes in NCC activation. Accordingly, bicarbonate loading increased pendrin expression via alkalosis without changing serum potassium concentration or NCC activity, whereas the low-potassium diet without changes in serum bicarbonate did not change pendrin expression, but rather activated NCC through hypokalemia. These results clearly mean that metabolic alkalosis rather than hypokalemia is the major driver of pendrin regulation during mineralocorticoid excess, although hypokalemia rather than alkalosis induces NCC activation.
Several investigators have demonstrated that pendrin is involved in the development of salt-sensitive hypertension because IC-specific overexpression of pendrin causes salt-sensitive hypertension,13 and BP elevation during mineralocorticoid excess is suppressed in pendrin KO mice.28 However, the parallel activation of NCC induced by hypokalemia also contributes to mineralocorticoid-induced salt-sensitive hypertension.9 In this study, pendrin was upregulated by hypokalemic alkalosis in aldosterone-treated NCC-KO mice to sustain BP elevation. The reversal of hypokalemic metabolic alkalosis by potassium supplementation inhibited the aldosterone-induced upregulation of pendrin with change in subcellular distribution, leading to a significant reduction in BP. These results indicate that hypokalemic alkalosis–induced regulation of pendrin, in cooperation with NCC, contributes to salt-sensitive hypertension during mineralocorticoid excess.
In clinical practice, thiazide diuretics are used as a first-line treatment of hypertension. Nevertheless, long-term treatment with HCTZ is often associated with thiazide resistance, possibly because of RAAS activation and the resultant hypokalemic alkalosis. In this study, we found that pendrin regulation induced by elevated AngII and hypokalemic alkalosis contributes to the maintenance of normal BP during dietary salt restriction and the development of salt-sensitive hypertension during mineralocorticoid excess, respectively. This finding prompted us to hypothesize that pendrin is a therapeutic target in thiazide-resistant hypertension. Spironolactone is effective at lowering systolic BP in patients with resistant hypertension, which is defined as BP that remains above the target level despite treatment with at least three antihypertensive drugs, including a diuretic.38 Thus, an MR antagonist may be a preferable antihypertensive drug for the treatment of thiazide-resistant hypertension, given that it can result in either the reversal of hypokalemic metabolic alkalosis by the blockade of the MR–ENaC pathway in PCs or the inhibition of AngII-induced upregulation of pendrin via the blockade of the MR in ICs (Supplemental Figure 8).
Of note, some studies using the Atp6v1b1-cre line reported unexpected gene deletion in some population of PC-like cells in connecting tubules.39,40 This observation was supported by open data (https://cello.shinyapps.io/kidneycellexplorer/) of a recent study by single-cell analysis of gene expression in the mice kidneys,41 which indicated that Atp6v1b1 gene is expressed not only in ICs but also among the population of PC-like cells in connecting tubules and DCT cells, despite the smaller expression than in ICs. In accordance with these studies, we observed MR deletion in around 40% of β-ENaC–positive cells in connecting tubules, in <5% of β-ENaC–positive cells in both cortical and medullary collecting ducts, and in around 10% of NCC-positive cells, in the MRB1-KO mice (Supplemental Figure 9, A and B). Notably, the MR deletion was observed in >90% of both α-ICs and β-ICs. Thus, some possibilities remain that results of the experiments using MRB1-KO mice are not only attributable solely to the deletion of MR in β-ICs but influenced by MR deletion in these non–β-IC cells. Indeed, it is expected that the partial MR deletion in PC-like cells in connecting tubules might cause hyperkalemic acidosis, through the inhibition of MR–ENaC pathway. However, gene deletion of α-ENaC using Atp6b1v1-cre reportedly showed no phenotype in salt deletion.40 In addition, in our study, MRB1-KO mice showed neither elevation of plasma potassium nor decrease in bicarbonate (Figure 1C), with comparable hypokalemic alkalosis induced by aldosterone to MRflox (Figure 3D), suggesting little effect of MR deletion in PC-like cells in connecting tubules on the pendrin regulation. According to the concern about the effect of acidosis induced by suppression of H+-ATPase in α-ICs, moreover, it should be noted that the pendrin expression is suppressed in AngII-treated MRB1-KO despite the presence of metabolic alkalosis as compared with the AngII-treated MRFlox mice (Figure 1C), and this result implies that the pendrin regulation by AngII might not be attributed to the alteration in acid-base status induced by the MR-deletion in non–β-IC cells, but instead depend on MR activation in β-ICs. However, the suppression of AngII-induced pendrin upregulation by MRB1-KO was not complete despite >90% deletion of MR in β-IC (Figure 1D), which might be counteracted by alkalosis-induced upregulation of pendrin (Figure 1C). Otherwise, the presence of the other unknown IC-MR–independent pathway remains. Further study is needed to clarify the precise mechanism of AngII-induced pendrin upregulation.
In conclusion, we uncovered two distinct mechanisms of pendrin regulation by the MR in ICs and PCs; these mechanisms are active during AngII concentration elevation and mineralocorticoid excess. Both mechanisms contribute to the regulation of the fluid balance and BP (along with the parallel activation of NCC) and may be therapeutic targets in thiazide-resistant hypertension.
Disclosures
Dr. Ayuzawa reports other from Asahi Group Holdings, other from Astellas Pharma, other from Chugai Pharmaceutical, other from EA Pharma, other from Mitsubishi Tanabe Pharma Corporation, other from Mochida Pharmaceutical, other from MSD K.K., other from Nippon Boehringer Ingelheim, other from Omron Healthcare, other from Shionogi & Co., other from Toray Industries, outside the submitted work. Dr. Fujita reports other from Asahi Group Holdings, other from Astellas Pharma, other from Chugai Pharmaceutical, other from EA Pharma, other from Mitsubishi Tanabe Pharma Corporation, other from Mochida Pharmaceutical, other from MSD K.K., other from Nippon Boehringer Ingelheim, other from Omron Healthcare, other from Shionogi & Co., other from Toray Industries, outside the submitted work. Dr. Hirohama reports other from Asahi Group Holdings, other from Astellas Pharma, other from Chugai Pharmaceutical, other from EA Pharma, other from Mitsubishi Tanabe Pharma Corporation, other from Mochida Pharmaceutical, other from MSD K.K., other from Nippon Boehringer Ingelheim, other from Omron Healthcare, other from Shionogi & Co., other from Toray Industries, outside the submitted work. Dr. Marumo reports other from Asahi Group Holdings, other from Astellas Pharma, other from Chugai Pharmaceutical, other from EA Pharma, other from Mitsubishi Tanabe Pharma Corporation, other from Mochida Pharmaceutical, other from MSD K.K., other from Nippon Boehringer Ingelheim, other from Omron Healthcare, other from Shionogi & Co., other from Toray Industries, outside the submitted work. Dr. Nishimoto reports other from Asahi Group Holdings, other from Astellas Pharma, other from Chugai Pharmaceutical, other from EA Pharma, other from Mitsubishi Tanabe Pharma Corporation, other from Mochida Pharmaceutical, other from MSD K.K., other from Nippon Boehringer Ingelheim, other from Omron Healthcare, other from Shionogi & Co., other from Toray Industries, outside the submitted work. Dr. Ueda reports other from Asahi Group Holdings, other from Astellas Pharma, other from Chugai Pharmaceutical, other from EA Pharma, other from Mitsubishi Tanabe Pharma Corporation, other from Mochida Pharmaceutical, other from MSD K.K., other from Nippon Boehringer Ingelheim, other from Omron Healthcare, other from Shionogi & Co., other from Toray Industries, outside the submitted work.
Dr. Kawarazaki reports other from Asahi Group Holdings, other from Astellas Pharma, other from Chugai Pharmaceutical, other from Mitsubishi Tanabe Pharma Corporation, other from Mochida Pharmaceutical, other from Nippon Boehringer Ingelheim, other from Omron Healthcare, other from Shionogi & Co., other from Toray Industries, outside the submitted work.
Funding
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (grant numbers JP15H05788, JP26860546, JP16K15494, and JP18K15969), the AMED-CREST from the Japan Agency for Medical Research and Development (AMED), and the Japan Foundation for Applied Enzymology.
Supplementary Material
Acknowledgments
We thank Prof. Thomas M. Coffman (Duke University) for providing the Atp6v1b1-cre mice, Prof. Günther Schütz (German Cancer Research Center) for the MR-floxed mice, Prof. Celso E. Gomez-Sanchez (University of Mississippi Medical Center) for the anti-MR antibody, Prof. Peter S. Aronson (Yale University) for the anti-pendrin antibody, and Prof. Johannes Loffing (University of Zurich) for the anti–pT53-NCC and anti–α-ENaC antibodies.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019080804/-/DCSupplemental.
Supplemental Figure 1. Baseline characteristics of MRB1-KO mice.
Supplemental Figure 2. Characteristics of normal and MRB1-KO mice after salt depletion and NCC blockade.
Supplemental Figure 3. Urinary analysis of MRB1-KO mice after salt depletion and NCC blockade.
Supplemental Figure 4. Aldosterone-induced change in subcellular distribution of pendrin.
Supplemental Figure 5. Creation of mice with MR deletion in the entire nephron.
Supplemental Figure 6. Potassium depletion upon alkali loading induces concentrated distribution of pendrin label in the region of apical membrane.
Supplemental Figure 7. Pendrin regulation and salt-sensitive hypertension induced by aldosterone in NCC KO mice.
Supplemental Figure 8. The possible mechanism of thiazide-resistant hypertension.
Supplemental Figure 9. MR deletion in each cell types of aldosterone-sensitive distal nephron in MRB1-KO mice.
Supplemental Table 1. Primer pairs used for quantitative RT-PCR.
Supplemental Table 2. Effects of amiloride and dietary potassium supplementation on systolic BP in C57BL/6J mice that were fed a high-salt diet and treated with aldosterone.
Supplemental Table 3. Systolic BP in MRFlox mice and MRB1-KO mice that were fed a high-salt diet and received either aldosterone treatment or no treatment.
Supplemental Table 4. Systolic BP in MRControl and MRPax8-KO mice that were fed a high-salt diet and received either aldosterone treatment or no treatment.
Supplemental Table 5. Systolic BP in MRB1-KO mice fed a high-salt diet and treated with aldosterone either with or without dietary potassium supplementation.
References
- 1.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA: Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest 104: R19–R23, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loffing J, Zecevic M, Féraille E, Kaissling B, Asher C, Rossier BC, et al.: Aldosterone induces rapid apical translocation of ENaC in early portion of renal collecting system: Possible role of SGK. Am J Physiol Renal Physiol 280: F675–F682, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Castañeda-Bueno M, Cervantes-Pérez LG, Vázquez N, Uribe N, Kantesaria S, Morla L, et al.: Activation of the renal Na+:Cl- cotransporter by angiotensin II is a WNK4-dependent process. Proc Natl Acad Sci U S A 109: 7929–7934, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schultheis PJ, Lorenz JN, Meneton P, Nieman ML, Riddle TM, Flagella M, et al.: Phenotype resembling Gitelman’s syndrome in mice lacking the apical Na+-Cl- cotransporter of the distal convoluted tubule. J Biol Chem 273: 29150–29155, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Hoover RS: Angiotensin II: A candidate for an aldosterone-independent mediator of potassium preservation during volume depletion. Kidney Int 79: 377–379, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Rengarajan S, Lee DH, Oh YT, Delpire E, Youn JH, McDonough AA: Increasing plasma [K+] by intravenous potassium infusion reduces NCC phosphorylation and drives kaliuresis and natriuresis. Am J Physiol Renal Physiol 306: F1059–F1068, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arroyo JP, Ronzaud C, Lagnaz D, Staub O, Gamba G: Aldosterone paradox: Differential regulation of ion transport in distal nephron. Physiology (Bethesda) 26: 115–123, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Terker AS, Yarbrough B, Ferdaus MZ, Lazelle RA, Erspamer KJ, Meermeier NP, et al.: Direct and indirect mineralocorticoid effects determine distal salt transport. J Am Soc Nephrol 27: 2436–2445, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ueda K, Nishimoto M, Hirohama D, Ayuzawa N, Kawarazaki W, Watanabe A, et al.: Renal dysfunction induced by kidney-specific gene deletion of Hsd11b2 as a primary cause of salt-dependent hypertension. Hypertension 70: 111–118, 2017. [DOI] [PubMed] [Google Scholar]
- 10.Wall SM, Kim YH, Stanley L, Glapion DM, Everett LA, Green ED, et al.: NaCl restriction upregulates renal Slc26a4 through subcellular redistribution: Role in Cl- conservation. Hypertension 44: 982–987, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Pech V, Wall SM, Nanami M, Bao HF, Kim YH, Lazo-Fernandez Y, et al.: Pendrin gene ablation alters ENaC subcellular distribution and open probability. Am J Physiol Renal Physiol 309: F154–F163, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leviel F, Hübner CA, Houillier P, Morla L, El Moghrabi S, Brideau G, et al.: The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest 120: 1627–1635, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacques T, Picard N, Miller RL, Riemondy KA, Houillier P, Sohet F, et al.: Overexpression of pendrin in intercalated cells produces chloride-sensitive hypertension. J Am Soc Nephrol 24: 1104–1113, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verlander JW, Hong S, Pech V, Bailey JL, Agazatian D, Matthews SW, et al.: Angiotensin II acts through the angiotensin 1a receptor to upregulate pendrin. Am J Physiol Renal Physiol 301: F1314–F1325, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirohama D, Ayuzawa N, Ueda K, Nishimoto M, Kawarazaki W, Watanabe A, et al.: Aldosterone is essential for angiotensin II-induced upregulation of pendrin. J Am Soc Nephrol 29: 57–68, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu N, Hirohama D, Ishizawa K, Chang WX, Shimosawa T, Fujita T, et al.: Hypokalemia and pendrin induction by aldosterone. Hypertension 69: 855–862, 2017. [DOI] [PubMed] [Google Scholar]
- 17.Soleimani M, Barone S, Xu J, Shull GE, Siddiqui F, Zahedi K, et al.: Double knockout of pendrin and Na-Cl cotransporter (NCC) causes severe salt wasting, volume depletion, and renal failure. Proc Natl Acad Sci U S A 109: 13368–13373, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shibata S, Rinehart J, Zhang J, Moeckel G, Castañeda-Bueno M, Stiegler AL, et al.: Mineralocorticoid receptor phosphorylation regulates ligand binding and renal response to volume depletion and hyperkalemia. Cell Metab 18: 660–671, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berger S, Wolfer DP, Selbach O, Alter H, Erdmann G, Reichardt HM, et al.: Loss of the limbic mineralocorticoid receptor impairs behavioral plasticity. Proc Natl Acad Sci U S A 103: 195–200, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller RL, Lucero OM, Riemondy KA, Baumgartner BK, Brown D, Breton S, et al.: The V-ATPase B1-subunit promoter drives expression of Cre recombinase in intercalated cells of the kidney. Kidney Int 75: 435–439, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Traykova-Brauch M, Schönig K, Greiner O, Miloud T, Jauch A, Bode M, et al.: An efficient and versatile system for acute and chronic modulation of renal tubular function in transgenic mice. Nat Med 14: 979–984, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA: Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci U S A 99: 10482–10487, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris RG, Hoorn EJ, Knepper MA: Hypokalemia in a mouse model of Gitelman’s syndrome. Am J Physiol Renal Physiol 290: F1416–F1420, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Knauf F, Yang CL, Thomson RB, Mentone SA, Giebisch G, Aronson PS: Identification of a chloride-formate exchanger expressed on the brush border membrane of renal proximal tubule cells. Proc Natl Acad Sci U S A 98: 9425–9430, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, et al.: Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int 83: 811–824, 2013. [DOI] [PubMed] [Google Scholar]
- 26.Gomez-Sanchez CE, de Rodriguez AF, Romero DG, Estess J, Warden MP, Gomez-Sanchez MT, et al.: Development of a panel of monoclonal antibodies against the mineralocorticoid receptor. Endocrinology 147: 1343–1348, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Wall SM, Hassell KA, Royaux IE, Green ED, Chang JY, Shipley GL, et al.: Localization of pendrin in mouse kidney. Am J Physiol Renal Physiol 284: F229–F241, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Verlander JW, Hassell KA, Royaux IE, Glapion DM, Wang ME, Everett LA, et al.: Deoxycorticosterone upregulates PDS (Slc26a4) in mouse kidney: Role of pendrin in mineralocorticoid-induced hypertension. Hypertension 42: 356–362, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Cannon PJ, Ames RP, Laragh JH: Relation between potassium balance and aldosterone secretion in normal subjects and in patients with hypertensive or renal tubular disease. J Clin Invest 45: 865–879, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kassirer JP, Appleton FM, Chazan JA, Schwartz WB: Aldosterone in metabolic alkalosis. J Clin Invest 46: 1558–1571, 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner CA, Finberg KE, Stehberger PA, Lifton RP, Giebisch GH, Aronson PS, et al.: Regulation of the expression of the Cl-/anion exchanger pendrin in mouse kidney by acid-base status. Kidney Int 62: 2109–2117, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Frische S, Kwon TH, Frøkiaer J, Madsen KM, Nielsen S: Regulated expression of pendrin in rat kidney in response to chronic NH4Cl or NaHCO3 loading. Am J Physiol Renal Physiol 284: F584–F593, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA, et al.: Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci U S A 98: 4221–4226, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gueutin V, Vallet M, Jayat M, Peti-Peterdi J, Cornière N, Leviel F, et al.: Renal β-intercalated cells maintain body fluid and electrolyte balance. J Clin Invest 123: 4219–4231, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.López-Cayuqueo KI, Chavez-Canales M, Pillot A, Houillier P, Jayat M, Baraka-Vidot J, et al.: A mouse model of pseudohypoaldosteronism type II reveals a novel mechanism of renal tubular acidosis. Kidney Int 94: 514–523, 2018. [DOI] [PubMed] [Google Scholar]
- 36.Kyossev Z, Walker PD, Reeves WB: Immunolocalization of NAD-dependent 11 beta-hydroxysteroid dehydrogenase in human kidney and colon. Kidney Int 49: 271–281, 1996. [DOI] [PubMed] [Google Scholar]
- 37.Funder JW, Pearce PT, Smith R, Smith AI: Mineralocorticoid action: Target tissue specificity is enzyme, not receptor, mediated. Science 242: 583–585, 1988. [DOI] [PubMed] [Google Scholar]
- 38.Václavík J, Sedlák R, Plachy M, Navrátil K, Plásek J, Jarkovsky J, et al.: Addition of spironolactone in patients with resistant arterial hypertension (ASPIRANT): A randomized, double-blind, placebo-controlled trial. Hypertension 57: 1069–1075, 2011. [DOI] [PubMed] [Google Scholar]
- 39.Kortenoeven ML, Pedersen NB, Miller RL, Rojek A, Fenton RA: Genetic ablation of aquaporin-2 in the mouse connecting tubules results in defective renal water handling. J Physiol 591: 2205–2219, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poulsen SB, Praetorius J, Damkier HH, Miller L, Nelson RD, Hummler E, et al.: Reducing αENaC expression in the kidney connecting tubule induces pseudohypoaldosteronism type 1 symptoms during K+ loading. Am J Physiol Renal Physiol 310: F300–F310, 2016. [DOI] [PubMed] [Google Scholar]
- 41.Ransick A, Lindström NO, Liu J, Zhu Q, Guo JJ, Alvarado GF, et al.: Single-cell profiling reveals sex, lineage, and regional diversity in the mouse kidney. Dev Cell 51: 399–413.e7, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.