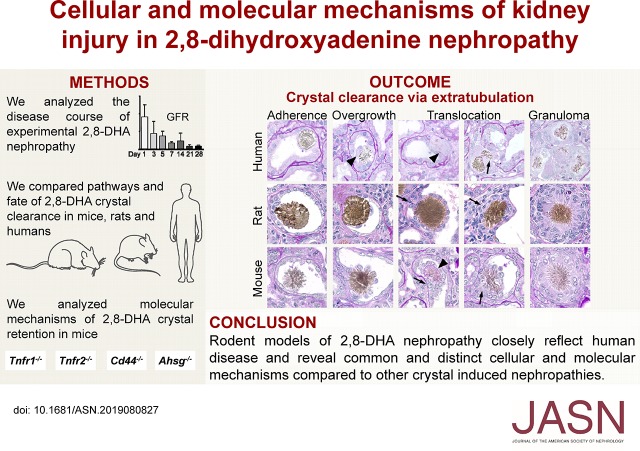
Significance Statement
Lack of well characterized experimental models of 2,8-dihydroxyadenine nephropathy—resulting from formation of 2,8-dihydroxyadenine crystals within renal tubules due to a rare hereditary deficiency of adenine phosphoribosyltransferase in humans (or excessive adenine load in animals)—has hindered achieving a better understanding of underlying disease mechanisms. The authors demonstrate that crystal formation, deposition, and clearance, as well as the resulting renal tubular injury, inflammation, fibrosis, and loss of kidney function, are virtually identical in experimental rodent models induced by an adenine-enriched diet and in patients with adenine phosphoribosyltransferase deficiency. These models are thus suitable to study cellular mechanisms, such as crystal clearance (via a reparative process the authors call extratubulation), or to identify relevant molecular pathways, such as TNF receptor 1–dependent crystal retention, that might inform the development of novel treatments.
Keywords: tubular injury, extratubulation, tubulointerstitial inflammation, renal fibrosis, TNFR, adenine associated nephropathy
Visual Abstract
Abstract
Background
Hereditary deficiency of adenine phosphoribosyltransferase causes 2,8-dihydroxyadenine (2,8-DHA) nephropathy, a rare condition characterized by formation of 2,8-DHA crystals within renal tubules. Clinical relevance of rodent models of 2,8-DHA crystal nephropathy induced by excessive adenine intake is unknown.
Methods
Using animal models and patient kidney biopsies, we assessed the pathogenic sequelae of 2,8-DHA crystal-induced kidney damage. We also used knockout mice to investigate the role of TNF receptors 1 and 2 (TNFR1 and TNFR2), CD44, or alpha2-HS glycoprotein (AHSG), all of which are involved in the pathogenesis of other types of crystal-induced nephropathies.
Results
Adenine-enriched diet in mice induced 2,8-DHA nephropathy, leading to progressive kidney disease, characterized by crystal deposits, tubular injury, inflammation, and fibrosis. Kidney injury depended on crystal size. The smallest crystals were endocytosed by tubular epithelial cells. Crystals of variable size were excreted in urine. Large crystals obstructed whole tubules. Medium-sized crystals induced a particular reparative process that we term extratubulation. In this process, tubular cells, in coordination with macrophages, overgrew and translocated crystals into the interstitium, restoring the tubular luminal patency; this was followed by degradation of interstitial crystals by granulomatous inflammation. Patients with adenine phosphoribosyltransferase deficiency showed similar histopathological findings regarding crystal morphology, crystal clearance, and renal injury. In mice, deletion of Tnfr1 significantly reduced tubular CD44 and annexin two expression, as well as inflammation, thereby ameliorating the disease course. In contrast, genetic deletion of Tnfr2, Cd44, or Ahsg had no effect on the manifestations of 2,8-DHA nephropathy.
Conclusions
Rodent models of the cellular and molecular mechanisms of 2,8-DHA nephropathy and crystal clearance have clinical relevance and offer insight into potential future targets for therapeutic interventions.
A broad range of clinically diverse disorders is caused by tissue deposition of crystals causing acute or chronic organ injury, commonly referred to as crystallopathies.1 Although such crystals are composed of a variety of substances and originate from different intrinsic or extrinsic ions or metabolites, previous data suggested common pathophysiologic pathways driving organ injury, potentially uncovering targets for therapeutic interventions in a wide range of human diseases with crystal deposits.2–5 Several types of crystals are formed and deposited within the kidney, causing renal damage and leading to various crystal-induced nephropathies.4,6 Calcium phosphate–, calcium oxalate–, and uric acid–containing crystals are the most common to cause such nephropathies, followed by several rare types of crystals, including 2,8-dihydroxyadenine (2,8-DHA) precipitates.6–8 In healthy kidneys, the tubular epithelial cells have a crystal-repulsive surface and urinary anions coating the crystal surface prevent cellular adhesion.9 It was proposed that an initiating and essential step in calcium crystal–induced kidney injury is a switch of tubular epithelial cells toward a “crystal-binding” phenotype, characterized by de novo expression of various molecules, including CD44 or TNF receptors (TNFR).10,11 After cellular adhesion, crystals induce injury and a proinflammatory phenotype of tubular cells, involving intrarenal mononuclear phagocytes expressing the nucleotide-binding domain, leucine-rich repeat family, pyrin domain–containing 3 (NLRP3) inflammasome, toll-like receptors (TLRs), high-mobility group protein B1 (HMGB1), and NF κ-light-chain-enhancer of activated B cells (NF-κB).5,12–15
2,8-DHA crystal nephropathy can be induced by increased ingestion or intravenous administration of adenine in the course of blood transfusion,16 albeit the clinical relevance of this is unknown and likely remains clinically insignificant. Another cause of 2,8-DHA nephropathy is adenine phosphoribosyltransferase (APRT) deficiency, a rare autosomal recessive genetic disorder characterized by perturbation of the adenine salvage pathway, resulting in the conversion of adenine to 2,8-DHA by xanthine oxidoreductase.17,18 These patients develop nephrolithiasis and progressive CKD with recurrent episodes of AKI, which is independent of the stone.18 The estimated prevalence of this disorder is 1:50,000–1:100,000, but some reports suggest that these numbers might be an underestimation.19–21 2,8-DHA nephropathy can also recur in transplanted kidneys and frequently results in allograft failure in undertreated individuals.22–24 The standard therapy consists of a xanthine oxidoreductase inhibitor, allopurinol or febuxostat,21,25 which can prevent or ameliorate the progressive course of the disease if initiated early.26 However, even treated patients develop episodes of AKI, usually due to inadequate xanthine oxidoreductase inhibitor dosing, and occasionally patients do not tolerate the available drugs or long-term treatment. Little is known about the mechanisms driving renal injury in 2,8-DHA nephropathy and whether the pathophysiologic processes might be similar to the more common calcium crystal–induced nephropathies.
Given the need to better understand the pathogenesis of rare diseases,27 we comprehensively analyzed the fate, distribution, and consequences of renal 2,8-DHA crystal deposition both in rodent models of 2,8-DHA nephropathy and in kidney specimens from patients with APRT deficiency. Our aims were to compare experimental 2,8-DHA nephropathy in rodents with the human disease and to explore molecular pathways in 2,8-DHA nephropathy to identify mechanisms and targets for clinical interventions.
Methods
Study Design and Ethics
Patients
Formalin-fixed paraffin-embedded kidney biopsy specimens and autopsy kidney specimens obtained from patients with APRT deficiency were collected from Landspitali–The National University Hospital of Iceland and the University Hospital Hamburg Eppendorf. In total, we analyzed human kidney specimens from seven patients with APRT deficiency. The patients’ clinical data were also collected in Landspitali–The National University Hospital of Iceland. The studies were approved by the National Bioethics Committee of Iceland (NBC 09–072) and the Icelandic Data Protection Authority. All living patients or their legal guardians provided written, informed consent for participation in the studies.
Animal Experiments
Animals were housed under standard conditions (specific pathogen free) in a light-, temperature-, and humidity-controlled environment with free access to tap water and standard or adenine-enriched rodent chow. All animal protocols were approved by the local government authorities (Landesamt für Umwelt und Verbraucherschutz Nordrhein Westfalen or Regierung von Oberbayern).
Mouse Model of 2,8-DHA Nephropathy
2,8-DHA nephropathy was induced in 10–12-week-old male mice, or 60-week-old male and female mice, by administration of a 0.2% adenine-containing chow (Altromin, Lage, Germany). Body weight was measured weekly. Before euthanasia, kidney function was evaluated by noninvasive measurement of BP via tail cuff (CODA; Kent Scientific Corporation, Torrington, CT); measurement of serum creatinine, serum urea, serum calcium, and serum phosphate using an autoanalyzer; and measurement of urinary protein and creatinine after collection of 12–16-hour urine samples in metabolic cages. Upon euthanasia, mice were perfused via the heart using physiologic saline during ketamine (100 mg/kg body wt) and xylazine (10 mg/kg body wt) anesthesia administered intraperitoneally. The kidneys were removed and immediately processed for histologic, ultrastructural, biochemical, or molecular analyses.
For a comprehensive analysis of the progressive mouse model, a time course study was performed. Male C57BL/6N mice (Charles River, Erkrath, Germany) were fed with a 0.2% adenine diet and the kidneys were removed for analysis on days 1, 3, 5, 7, 14, 21, and 28 (n=4 in each group).
To analyze the kidney disease progression in elderly mice, 60-week-old male and female mice (n=6) with a mixed C57BL/6–129/Sv background were fed with a 0.2% adenine diet for 21 days. Littermates (n=4) served as healthy age-matched controls.
Cd44−/− mice (n=9) were originally obtained from the Jackson Laboratory (Stock 005085, Bar Harbor, ME) and bred on a mixed background (C57BL/6-SV129 mice). Hemizygous Cd44+/− littermates were used as controls (n=6). Both groups were fed with a 0.2% adenine diet for 7 days. Ahsg−/− mice (cloning and targeted deletion of the mouse Ahsg [α2-Heremans-Schmid-glycoprotein] gene [fetuin-A]28) were maintained on a C57BL/6N background (n=5) and were analyzed after 14 days of adenine-enriched diet in comparison with wild-type mice (n=4). Tnfr1−/− (n=5) and Tnfr2−/− (n=7) mice were originally obtained from the Jackson Laboratory (Stock 002818 and 002620, Bar Harbor, ME) and were also bred on a C57BL/6J background.11 Both strains were analyzed after 14 days of adenine-enriched diet and compared with C57BL/6J wild-type mice (n=5).
To demonstrate tubular obstruction by 2,8-DHA crystals, wild-type mice were i.v. injected with 10 mg rhodamine-labeled 10-kDa dextrane in 100 µl saline (Sigma, Steinheim, Germany) on day 26 of the adenine-enriched diet. Animals were euthanized 60 minutes after injection and the kidneys were removed, embedded in O.C.T., and snap frozen.
Rat Model of 2,8-DHA Nephropathy
2,8-DHA nephropathy was induced in nine male Fisher344 (F344) rats, weighing 200–220 g, by administration of an adenine-enriched diet (supplemented with 0.75% adenine) (Altromin, Lage, Germany).29 Kidney function was assessed every week by measurement of serum urea, serum creatinine, serum calcium, and serum phosphate using an autoanalyzer, and proteinuria which was monitored by 24-hour urine samples for protein and creatinine measurement collected from animals housed in metabolic cages. BP was assessed with noninvasive measurements via tail cuff (CODA). Rats were euthanized by an overdose of isoflurane after 2, 3, and 4 weeks and the kidneys were harvested for histologic and biochemical analyses.
Human Kidney Tissue Specimens
Formalin-fixed paraffin-embedded kidney biopsy specimens and autopsy kidney specimens obtained from patients with APRT deficiency were collected from Landspitali–The National University Hospital of Iceland and the University Hospital Hamburg Eppendorf. In total, we analyzed human kidney specimens from seven patients with APRT deficiency. The patients’ clinical data are shown in Table 1.
Table 1.
Clinical data from patients with APRT deficiency
| Patient No. | Yr | Kidney Specimen | Sex | Age (yr) | Serum Creatinine (µmol/l) | eGFR (ml/min per 1.73 m2) |
|---|---|---|---|---|---|---|
| 1 | 1967 | Autopsy | Male | 37 | 1856 | 3 |
| 2 | 1993 | Kidney biopsy | Female | 44 | 446 | 10 |
| 3 | 1995 | Kidney biopsy | Female | 55 | 1038 | 3 |
| 4 | 1999 | Nephrectomy | Female | 1 | 25 | 129 |
| 5 | 2011 | Kidney biopsy | Male | 42 | 935 | 5 |
| 6 | 2015 | Resection of kidney tumors | Female | 68 | 143 | 33 |
Data were collected at the time of tissue sampling. eGFR was calculated from serum creatinine, using the CKD Epidemiology Collaboration creatinine equation in adults and the modified Schwartz equation in children.30,31 Nonstandardized serum creatinine values were reduced by 5% before eGFR was calculated, as previously described.32
Kidney specimens from six patients in a cohort of 33 individuals in Iceland were available for histologic examination (Table 1). Patient no. 1 was a man with a history of nephrolithiasis who at the age of 35 years was found to have CKD of unknown cause that progressed to end-stage kidney failure 2 years later. He died of uremic complications in 1967, before dialysis became available in Iceland. APRT deficiency had not yet been described at the time of his death, but after his brother was diagnosed with the disorder a review of the autopsy findings showed renal features consistent with 2,8-dihydroxyadeninuria. Patient no. 2 was a 44-year-old woman with a past history of recurrent kidney stones, presumed to be composed of uric acid, and stage 4 CKD who presented with AKI. Two years before, she had undergone removal of the right kidney due to damage caused by stone disease. Patient no. 3 was a 55-year-old woman who presented with severe AKI requiring transient hemodialysis. She had no past history of stone disease. Patient no. 4 presented at the age of 9 months with a kidney stone resulting in the diagnosis of APRT deficiency. At the age of 14 months, obstructive AKI due to bilateral stone impaction occurred, eventually resulting in unilateral nephrectomy 5 months later. Patient no. 5 was a 42-year-old man, with a history of recurrent kidney stones since childhood and CKD of unknown cause, who presented with severe AKI. Patient no. 6 was a 68-year-old woman with a history of APRT deficiency diagnosed in 2004 when she presented with AKI, culminating in stage 3b CKD after institution of treatment with allopurinol. She underwent partial nephrectomy due to a small mass in the left kidney that was found to be an oncocytoma.
Urinary DHA excretion values from the time when kidney tissue specimens were obtained are not available because the measurement technique had not been developed. All patients except patient no. 1 (deceased) have been shown to have elevated levels of urine 2,8-DHA since the ultra-performance liquid chromatography–tandem mass spectrometry (UPLC-MS/MS) assay became available in 2016.
Histologic findings in kidney specimens from patients no. 1, 3, and 4 have been reported previously.33
Renal Morphology and Immunohistochemistry
Murine and rat renal tissues for histopathologic analyses were fixed in methyl Carnoy’s solution and embedded in paraffin. Sections of 1 µm were stained with periodic acid–Schiff (PAS) and counterstained with hematoxylin for overall assessment of kidney damage and with hematoxylin and eosin (HE) for quantification of intrarenal crystals. Some sections were also stained with acid fuchsin orange G to visualize fibrosis, methenamine-silver to visualize basement membranes, or van Kossa to stain calcium phosphate.
Tubulointerstitial injury, defined as tubular damage and atrophy, interstitial inflammation, and fibrosis, was scored semiquantitatively in PAS-stained sections, depending on the extent of the whole renal cortex affected, as follows: no damage, 0%–1%; 1, 2%–25%; 2, 26%–50%; 3, 51%–75%; 4, 76%–100%. HE stainings were photographed in polarized light using a digital bright-field microscope (BZ-9000; Keyence, Japan). The total area of bright crystals on dark tissue background was quantified in renal cortices using ImageJ (National Institutes of Health, Bethesda, MD). The size of the crystals was measured semiautomatically using Keyence BZII Analyzer software (Keyence, Japan).
Immunohistochemistry and immunofluorescence analyses were performed as previously described29,34 and the primary antibodies used are listed in Table 2. Negative controls remained negative (data not shown). Human FFPE samples for clinical diagnostics stained with PAS and HE were reanalyzed. Additional histology samples were stained with van Kossa or silver stain, or immunohistochemistry against keratin, CD68, Ki-67, or TNFR1.
Table 2.
Primary antibodies for immunohistochemistry or immunofluorescence staining
| Target Protein | Antibody | Company |
|---|---|---|
| α-SMA | Mouse-anti-human SMA (clone 1A4) | Dako/Agilent, M085101–2 (US) |
| Aquaporin-2 | Rabbit-anti-AQ2 (polyclonal) | Abcam, ab15116 (UK) |
| CD3 | Rat-anti-human CD3 (cloneCD3–12) | Bio-Rad, MCA1477 (GER) |
| CD11b | Rat-anti-mouse CD11b (clone M1/70) PE-labeled | eBioscience/Thermo Fisher Scientific, 12–0112–82 (US) |
| CD11c | Hamster-anti-mouse CD11c (clone N418) APC-labeled | eBioscience/Thermo Fisher Scientific, 17–0114–82 (US) |
| CD13 | Rabbit-anti-CD13 (clone EPR4058) | Abcam, ab108310 (UK) |
| CD44 | Rat-anti-mouse CD44 (clone IM7) | BD Pharmingen, 553131 (US) |
| CD68 | Mouse-anti-rat CD68 (clone ED1) | Bio-Rad, MCA341R (GER) |
| CD68 | Mouse-anti-human CD68 (clone KP1) | Dako/Agilent, M081401 (US) |
| Collagen III | Goat-anti-type III collagen (antiserum) | Southern Biotech, 1330–01 (US) |
| Murine monocytes/macrophages (ER-HR3) | Rat-anti-mouse monocyte antigen (clone ER-HR3) | BMA Biomedicals, T-2012 (CHE) |
| F4/80 | Rat-anti-mouse F4/80 (clone CI:A3–1) | Bio-Rad, MCA497G (GER) |
| Fibronectin | Rabbit-anti-rat fibronectin (polyclonal) | Millipore/Merck AB1954 (GER) |
| KIM-1 | Goat-anti-mouse TIM-1/KIM-1/HAVCR (polyclonal) | R&D Systems, AF1817 (US) |
| Lipocalin-2 (NGAL) | Rat-anti-human lipocalin-2/NGAL (clone 220310) | R&D Systems, MAB1757 (US) |
| Lipocalin-2 (NGAL) (for double staining) |
Rabbit-anti-NGAL (polyclonal) | Thermo Fisher Scientific, PA5–79590 (US) |
| Cytokeratin (Pan CK) | Mouse-anti-pan Cytokeratin (clone Lu5) | Abcam, ab17155 (UK) |
| Proliferation (Ki-67, MIB1) | Mouse-anti-human (clone KI-67P) | Dianova, DIA-505 (GER) |
| Proliferation (PCNA) | Mouse-anti-PCNA (clone PC10) | Calbiochem/Merck, NA03 (GER) |
| Sm22α/Transgelin | Rabbit-anti-Transgelin (polyclonal) | Abcam, ab14106 (UK) |
| Tamm–Horsfall protein | Rabbit-anti-human THP (polyclonal) | SantaCruz, sc-20631 (GER) |
| TNFR1 | Rabbit-anti-TNFR1 (polyclonal) | Abcam, ab19139 (UK) |
| TNFR2 | Rabbit-anti-TNFR2 (clone EPR1653) | Abcam, ab15116 (UK) |
| Vimentin | Mouse-anti-vimentin (clone V9) | Dako/Agilent, M0725 (US) |
α-SMA, α–skeletal muscle actin; AQ-2, aquaporin-2; CD, cluster of differentiation; GER, Germany; CHE, Switzerland; F4/80, EGF-like module–containing mucin-like hormone receptor–like 1; TIM-1, T-cell immunoglobulin and mucin domain 1; HAVCR, Hepatitis A virus cellular receptor; NGAL, neutrophil gelatinase–associated lipocalin, lipocalin-2; CK, cytokeratin; PCNA, proliferating cell nuclear antigen; SM22α, Smooth muscle protein 22-α/Transgelin; THP, Tamm–Horsfall protein, uromodulin; TNFR, tumor necrosis factor receptor.
Immunofluorescence was visualized using a digital fluorescence microscope (BZ-9000). Using a whole-slide scanner (NanoZoomer HT; Hamamatsu Photonics, Hamamatsu, Japan), fully digitalized images of immunohistochemically stained slides were further processed and analyzed using the viewing software NDP.view (Hamamatsu Photonics, Hamamatsu, Japan) and ImageJ. The percentage of positively stained area was analyzed in whole cortices at ×20 magnification as described previously.34,35 All analyses were performed in a blinded manner.
Electron Microscopy
The renal cortex of the kidney specimens was cut into small pieces (approximately 1×1×1 mm3) and fixed in a solution containing 0.1 M sodium cacodylate, 2% paraformaldehyde, and 2.5% glutaraldehyde for several days at 4°C. According to standard protocols, the tissue was washed in 0.1 M Soerensen’s phosphate buffer and dehydrated, followed by critical point drying in liquid CO2 and coating with a 10-nm gold/palladium layer (Sputter Coater EM SCD500; Leica, Wetzlar, Germany). Samples were viewed in a high-vacuum environment with 10-kV acceleration voltage using an environmental scanning electron microscope (ESEM XL30 FEG; FEI, Eindhoven, NL).
Determination of Urine 2,8-DHA
Urine 2,8-DHA was measured using the UPLC-MS/MS assay as previously described.36 Briefly, a standard curve and quality control samples were prepared using urine from healthy C57BL/6 mice (purchased from BioIV, West Sussex, UK). Mouse urine samples were diluted 1:15 (v/v) in 10 mM NH4OH and 50 µl of the diluted samples pipetted into a 96-well plate, followed by the addition of 100 µl of 10 mM NH4OH and 50 µl of internal standard working solution. The samples were subsequently mixed in the plate for 3 minutes and centrifuged at 3100 rpm for 10 minutes at 20°C before injection (volume 5 µl) into the UPLC-MS/MS system. We used a Waters ACQUITY UPLC device coupled to a Quattro PremierTM XE triple quadrupole mass spectrometer (Waters Corporation, Milford, MA) using electrospray ionization in positive ionization mode for the analysis of 2,8-DHA in mouse urine samples. The analytic column used was an ACQUITY HSS T3 (1.8 µm, 100×2.1 mm2; Waters Corporation, Wexford, Ireland), and mobile phase A consisted of 2 mM ammonium acetate and mobile phase B of acetonitrile.
Reverse Transcription–Quantitative PCR
Total RNA was isolated from renal cortex tissue using the RNeasy Mini Kit (Qiagen, Hilden, Germany). RNA purity determination, cDNA synthesis, and the RT-PCR were performed as described.29,34,35 Reverse transcription–quantitative PCR was carried out using an ABI Prism 7300 sequence detector (Applied Biosystems, Weiterstadt, Germany). In each reaction, 0.75 µl of cDNA was amplified in a 25-µl volume using the qPCR Core Kit for SYBR Green I (Eurogentec, Seraing, Belgium). The PCR conditions consisted of 50°C for 2 minutes followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Primers were designed from sequences in the GenBank database using Primer Express software (Applied Biosystems). Primer sequences are listed in Table 3. GAPDH cDNA amplification was used as an internal standard.
Table 3.
Primers for reverse transcription–quantitative PCR
| Gene | Forward Primer 5′–3′ | Reverse Primer 5′–3′ |
|---|---|---|
| Anxa2 | GCACATTGCTGCGGTTTGTCAG | CACCAACTTCGATGCTGAGAGG |
| Ccl5 | AGTGCTCCAATCTTGCAGTCG | CACTTCTTCTCTGGGTTGGCA |
| Cd44 | TCCGAATTAGCTGGACACTC | CCACACCTTCTCCTACTATTGAC |
| Gapdh | GGCAAATTCAACGGCACAGT | AGATGGTGATGGGCTTCCC |
| Havcr1 | GCAAGGATTCCACTTTCCGTT | GCACCCTGCAGTCATTCAGA |
| Lcn2 | GGCCTCAAGGACGACAACA | TCACCACCCATTCAGTTGTCA |
| Tnfr1 | GCAACAGCACCGCAGTAGCTGA | GTGCGTCCCTTGCAGCCACT |
| Tnfr2 | CTGGGTCGCGCTGGTCTTGC | CAAGACAACCTGGGCGGGCA |
Anxa2, Annexin A2; Ccl5, Chemokine (C-C motif) ligand 5, Rantes; CD44, cluster of differentiation 44; Gapdh, Glyceraldehyde 3-phosphate dehydrogenase; Havcr1, Hepatitis A virus cellular receptor 1, TIM-1, KIM-1; Lcn2, Lipocalin-2, neutrophil gelatinase-associated lipocalin; Tnfr, tumor necrosis factor receptor.
Statistical Analyses
All values are presented as mean±SD. Statistical significance was determined using t test when comparing two groups or one-way ANOVA with Tukey correction when comparing more than two groups. Statistical significance was defined as P≤0.05.
Results
2,8-DHA Nephropathy Leads to Progressive Kidney Disease, Characterized by Crystal Deposits, Tubular Injury, Inflammation, and Fibrosis
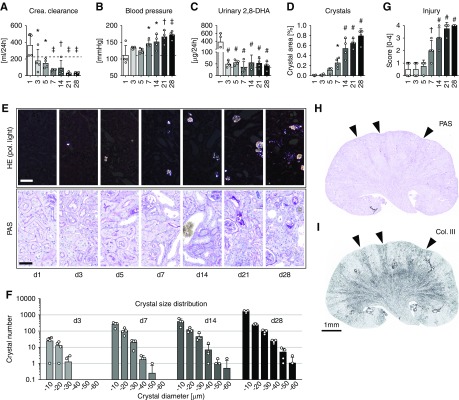
We first induced 2,8-DHA nephropathy in mice and rats by adenine-enriched diet and compared them with healthy animals on a regular diet. We analyzed the time course of the disease in C57BL/6N mice on days 1, 3, 5, 7, 14, 21, and 28 after starting the diet, representing early (days 1–5), established (days 7–14), and advanced (days 21–28) disease stages. Kidney function progressively declined during the disease course with a 52% reduction in creatinine clearance already present on day 3 and a reduction of up to 90% on days 21 and 28 (Figure 1A). Serum urea and phosphate levels progressively increased in parallel (Supplemental Table 1). All mice developed significant and progressive hypertension from day 7 onwards (Figure 1B), but no proteinuria was observed and the blood cell counts remained normal (Supplemental Table 1). Urinary 2,8-DHA concentration, a biomarker of APRT deficiency,36 increased 19-fold in the mice with 2,8-DHA nephropathy on day 1, followed by a constant ten-fold increase in urinary 2,8-DHA excretion thereafter (Figure 1C, Supplemental Table 1).
Figure 1.
Adenine overload leads to 2,8-DHA–induced kidney damage and reduced kidney function in mice. Male C57BL/6N mice fed with 0.2% adenine chow were euthanized on days 1, 3, 5, 7, 14, 21, and 28 (n=4/group). Kidney function progressively decreased during the time course of the adenine-enriched diet shown by (A) significant decline in creatinine clearance and (B) increase in systolic BP. Dashed lines indicate values from healthy mice. The (C) urinary 2,8-DHA excretion and the (D) amount of intrarenal 2,8-DHA crystals significantly increased during 4 weeks of adenine-enriched diet. Crystals were quantified in HE-stained tissue sections using bright-field and polarizing light microscopy. (E) Representative pictures from HE- and PAS-stained tissue sections. (F) Detailed analyses of crystal number and size distribution. (G) PAS-stained sections were scored for tubulointerstitial damage using a semiquantitative score (0–4). (H and I) Whole PAS- and collagen type III–stained kidneys from mice fed with 0.2% adenine diet for 21 days showed a cortico-medullary striped pattern of injury and fibrosis (arrowheads). Scale bars, 50 µm. *P<0.05, †P<0.01, ‡P<0.001, #P<0.0001 versus day 1. Col., collagen; Crea., creatinine; d, day; Pol., polarizing.
Macroscopically, starting from day 14 of 2,8-DHA nephropathy, the kidneys appeared shrunken with an irregular surface (Supplemental Figure 1A). The number and size of crystal spicules exponentially increased throughout the time course (Figure 1, D–F). From day 3 onward, histology showed brownish spheres consisting of spicule-shaped 2,8-DHA crystals, which were birefringent in polarized light microscopy (Supplemental Figure 1, B–D). We could not detect crystals in any other organs (data not shown). Tubular damage was the leading pathologic finding, with mild and focal injury on days 1, 3, and 5, followed by progressive damage from day 7 onwards, finally leading to diffuse injury involving the entire renal cortex and medulla at day 28 (Figure 1, E and G). Glomeruli remained unaffected (Supplemental Figure 1E).
Tubular injury was accompanied by inflammation, characterized by increased expression of the chemokine Ccl5 and mononuclear cell infiltrates, mainly composed of F4/80+ macrophages and resident renal dendritic cells37 and ErHr3+ monocytes and monocyte-derived macrophages representing a subset of F4/80+ cells,38 with up to a 5000-fold increase in positively stained cortical area on day 28 (Supplemental Figure 2, A–E). Macrophages accumulated around injured cortical and medullary tubules and frequently formed interstitial granulomas around crystals. The cells forming the granulomas and multinucleated giant cells were CD44+ (which also stained injured tubules), CD11b+, CD11c+, partly F4/80+, but ErHr3− (Supplemental Figure 2, F and G). Tubular injury and inflammation were followed by interstitial fibrosis, starting with a phenotype switch of cortical interstitial fibroblasts toward α-SMA+ myofibroblasts (Supplemental Figure 3A), associated with pathologic deposition of extracellular matrix, including collagen type III, in late stages (Supplemental Figure 3B). The fibrotic niches were always found in proximity to damaged tubules, which in most cases contained crystals. Whole-kidney cross-sections demonstrated that tubular damage, atrophy, and fibrosis developed in a striped cortico-medullary pattern (Figure 1, H and I). In these atrophic and fibrotic areas glomeruli were located in close proximity, suggesting prominent early loss of groups of nephrons, and only at later stages did all nephrons become affected.
We next investigated whether there is an age-, sex-, or species-dependent disease susceptibility. Mice aged 60 weeks exposed to adenine also developed a similar extent of renal injury and loss of kidney function compared with young mice with 2,8-DHA nephropathy (Supplemental Table 2). Male 60-week-old mice showed significantly more crystals and more extensive tubular injury compared with female littermates, but this did not translate into significant differences in kidney function or renal interstitial fibrosis (Supplemental Figure 4, A–J). The adenine-enriched diet induced 2,8-DHA nephropathy in Fisher344 (Supplemental Figure 5, Supplemental Table 3) and Wistar (data not shown) rats, with identical pathologic features as in the mouse models.
Collectively, we showed that renal 2,8-DHA crystals induce progressive kidney disease, which is independent of species, age, and also, at least in terms of kidney function, sex.
2,8-DHA crystals exhibit four distinct pathways of clearance from tubules in rodent models and patients with APRT deficiency.
Degeneration of adjacent nephron groups was suggestive of injury primarily to collecting ducts draining several nephrons. In the kidney cortex, medulla, and papilla, most 2,8-DHA crystals localized to CD13+ proximal tubules and to Tamm–Horsfall protein (THP1)+ thick ascending limbs of the loops of Henle, but not to Aquaporin-2+ collecting ducts (Supplemental Figure 6, A–C). Occasionally, small crystals were observed in the renal pelvis (Supplemental Figure 6D), but large crystal aggregates, i.e., calculi, were never observed. Apart from being a tubular segment marker, THP1 was proposed to be protective in crystal nephropathies by limiting the extent of crystallization.39 In line with this finding, some of the crystals located in the thick ascending limbs were coated with THP1, whereas adjacent apical tubular cell THP1 positivity was lost (Supplemental Figure 6B).
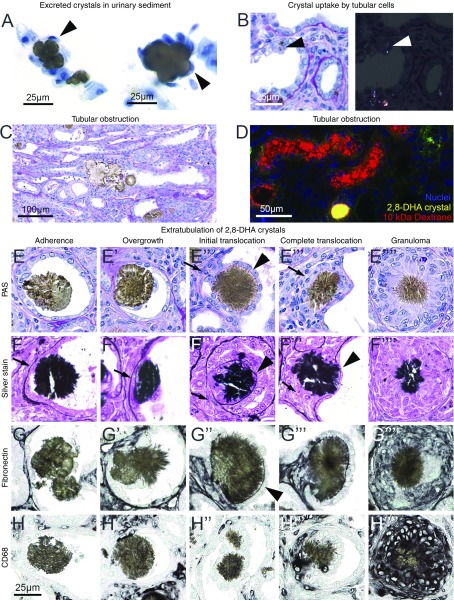
Several mechanisms of calcium oxalate crystal clearance from renal tubules have been proposed.8,40 In this study, we found that, first, 2,8-DHA-crystals were excreted as evidenced by crystalluria in both rats and mice. Some of the urine crystals were overgrown by cells (Figure 2A). Second, very small crystals were internalized and likely degraded by tubular cells (Figure 2B). Third, larger crystals or crystal aggregates led to tubular obstruction (Figure 2C), confirmed by the injection of rhodamine-labeled 10-kDa dextrane. This dye is rapidly excreted in the urine; however, it remained in the lumen in the case of obstructed tubules (Figure 2D). Fourth, it was mainly the medium-sized 2,8-DHA crystals that translocated from the tubular lumen to the interstitium (Figure 2, E–H, Supplemental Figure 7, A–C), here referred to as “crystal extratubulation.” Detailed analyses revealed several distinct steps of extratubulation (Figure 2, E–H), initiated by crystal adhesion to the injured tubular epithelial cells. The tubular cells beneath the crystals showed prominent signs of injury and were eventually lost. At this stage, macrophages accumulated in adjacent interstitium and started to degrade the basement membrane. In parallel, tubular cells adjacent to the crystal began to proliferate and migrate onto the crystals, finally completely overgrowing the crystal (Figure 2, E’–H’ and E’’–H’’). The cells overgrowing the crystals started to form a new basement membrane on top of the crystal, paralleled by complete degradation of the original basement membrane below the crystal (Figure 2, E’’’–H’’’). Ultimately, this process restored the tubular lumen and the crystals were surrounded and cleared by macrophages, commonly forming granulomas (Figure 2, E’’’’–H’’’’).
Figure 2.
The mechanisms of 2,8-DHA renal tubular clearance in mice and rats. (A) In urine sediments of rats, crystals were found overgrown with cells. Cells (black arrowheads) were stained with trypan blue. (B) PAS-stained section examined by bright-field and polarized light microscopy, showing 2,8-DHA particles that have been phagocytosed by tubular epithelial cells (arrowheads). (C) A big crystal aggregate obstructing an entire tubule, seen in a rat kidney section. (D) A cryosection of a kidney on day 26 from a mouse receiving the 0.2% adenine diet. The mouse was injected intravenously with 10 mg of rhodamine-labeled 10-kDa dextrane 60 minutes before euthanasia. Red fluorescent dextrane is filtered into the urine but is retained upstream of an obstructive 2,8-DHA crystal. (E–H) The translocation process of 2,8-DHA crystals is shown in kidney sections from a rat fed a 0.75% adenine diet, stained by PAS (E–E’’’’) and methenamine-silver (F–F’’’’) and immunochemically for fibronectin (G–G’’’’) and CD68 (H–H’’’’). In the left panel the first step of 2,8-DHA crystal adhesion to flattened epithelium is illustrated. In the next stage of extratubulation, tubular epithelial cells started to overgrow the crystal. The first “climber” cells were in direct contact with the crystal and migrated on top of it. These cells then started to form a new basement membrane (arrowheads). The original basement membrane below the crystal at the side of the adhesion was degraded or interrupted (arrows). CD68+ macrophages got into contact with crystals and started to degrade them. The degradation process often proceeded in parallel to formation of CD68+ granulomas (right panel), which surrounded a big crystal in the center and were partially formed by multinucleated cells. Scale bar, 25, 50, or 100 µm, respectively.
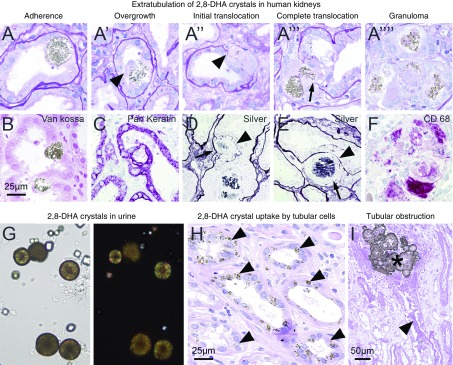
Compared with animal models of 2,8-DHA nephropathy, patients with APRT deficiency showed similar histopathologic findings with respect to crystal morphology, crystal clearance, and renal injury (Figure 3; clinical data of patients are given in Table 1). All steps of extratubulation were also observed in all seven cases of analyzed patient kidney specimens, e.g., van Kossa–negative crystals adherent to tubules were overgrown by keratin-expressing tubular cells (Figure 3, A and A’, B and C), and as soon as the original basement membrane was disrupted (Figure 3, A’’ and A’’’, D and E), the crystals fully translocated to the interstitium and were degraded by CD68+ macrophages (Figure 3, A’’’’ and F). 2,8-DHA crystals were also present in patient urine samples (Figure 3G), whereas frequent uptake of small crystals by tubular cells (Figure 3H) and obstruction of tubules by large crystal aggregates was also observed (Figure 3I). Glomeruli were not affected (Supplemental Figure 8). Taken together, experimental animals fed with adenine-enriched diet and patients with APRT deficiency show highly comparable features of 2,8-DHA nephropathy, supporting the relevance of the animal model for the human disease.
Figure 3.
Crystal clearance mechanisms in patients with 2,8-DHA nephropathy. (A) Extratubulation of 2,8-DHA crystals in PAS-stained human kidney biopsy specimens. The pathologic process was similar to the animal models. (B) 2,8-DHA crystals were negative in sections stained with van Kossa. (C) Keratin+ tubular epithelial cells overgrew adherent crystals and formed a new basement membrane (arrowheads), whereas the original membrane was degraded (arrows) as shown by silver staining (D and E). (F) CD68+ macrophages degraded crystals in the setting of granulomatous reactions. (G) 2,8-DHA crystals in urine sediments from patients with APRT deficiency. (H) Small crystals were taken up by tubular epithelial cells (arrowheads) and big 2,8-DHA crystal aggregates obstructed whole tubules which subsequently became necrotic (arrowhead). Scale bars, 25 or 50 µm, respectively.
2,8-DHA Nephropathy Gives Rise to Distinct Phenotypes of Tubular Cells
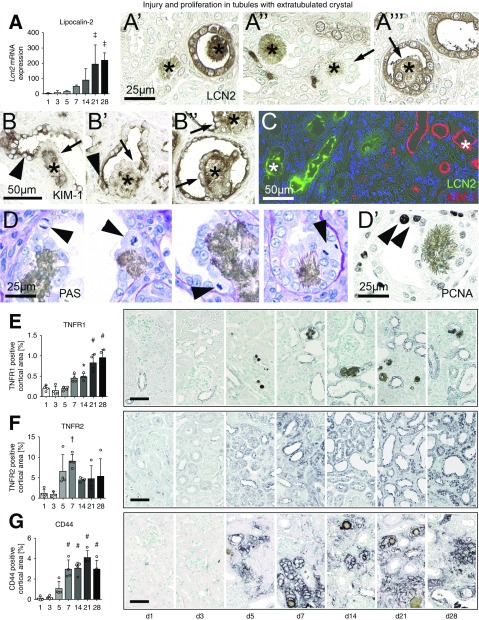
In mice, the expression of the tubular injury marker lipocalin-2 (Lcn2) followed the progressive course of tubular injury with >200-fold upregulation on days 21 and 28 (Figure 4A). LCN2 was expressed in some but not all tubules containing crystals and was particularly strong in the neutrophils located beneath the tubules with extratubulating crystals (Figure 4, A’–A’’’). This is in line with our observation that crystals often adhered to CD13+ proximal tubules, whereas LCN2 expression is known to be localized to the distal parts of the nephron.41 Expression of kidney injury molecule–1 (KIM-1, Havcr1), as a marker of proximal tubular injury,42 followed a similar course (Supplemental Figure 9A), but was localized to a more specific population of cells neighboring the crystals and only very rarely localized to cells overgrowing the crystals (Figure 4, B–B’’). Double staining of LCN2 and KIM-1 showed that both markers never colocalized in the same tubules (Figure 4C), confirming adherence and extratubulation of 2,8-DHA crystals in different parts of the nephron. Because KIM-1 is also a marker of regenerating tubular cells,43 we further analyzed the phenotype of these two distinct tubular cell populations, i.e., the overgrowing and the neighboring tubular cells. In line with KIM-1 staining, mitotic figures and the proliferation markers PCNA and Ki-67 were only found in the neighboring cells and not in the overgrowing cells. This showed that cells adjacent to the crystals give rise to the cells migrating and overgrowing the crystals, supporting that crystal extratubulation involves cellular regeneration (Figure 4, D and D’, rat; Supplemental Figure 9B, human).
Figure 4.
Characterization of tubular cell phenotypes and injury in rodents with 2,8-DHA nephropathy. (A) The injury marker Lcn2 (lipocalin-2) was progressively upregulated during the murine nephropathy time course on the mRNA level. (A’) Crystals (stars) were adherent and extratubulated in (A’’) LCN2− as well as (A’’’) LCN2+ tubules (pictures from mouse). (B) Tubules with extratubulating crystals were KIM-1+ (arrowheads). Cells that migrated on the crystal (stars), however, were KIM-1− in most (first two examples, arrows) but not all cases (third example, arrow, images from rats). (C) Immunofluorescence double staining showed that tubules with crystals were either LCN2+ or KIM-1+. (D) At sites of extratubulation, we only observed proliferation of tubular epithelial cells visualized by mitotic figures in PAS sections or by PCNA immunohistochemistry directly next to the 2,8-DHA crystal, but never in cells that overgrew the crystal (images from rats). Immunohistochemistry quantification and representative images showed increased expression of (E) TNFR1, (F) TNFR2, and (G) CD44 during the time course of the 2,8-DHA nephropathy in mice. Scale bars, 50 or 25 µm. *P<0.05, †P<0.01, ‡P<0.001, #P<0.0001 versus day 1. d, day.
TNFR1, TNFR2, and CD44 have been implicated in the pathogenesis of calcium oxalate nephropathy,10,11 but their role in 2,8-DHA nephropathy is unknown. In healthy kidneys, TNFR1 was expressed in principal cells of collecting ducts in both cortex and medulla (Supplemental Figure 9, C and C’). In experimental 2,8-DHA nephropathy, the number of TNFR1+ tubular cells progressively increased during the disease course and they were already detectable at the earliest time point, in particular in dilated tubules (Figure 4E). The basolateral or apical expression of TNFR1 changed to a circumferential staining pattern during the disease. Crystals were mostly adherent and extratubulated in TNFR1+ tubules (Supplemental Figure 9, C’’ and C’’’), but less often in TNFR− tubules (Supplemental Figure 9C’’’’). The number of TNFR2+ tubular cells increased during the course of the kidney disease, peaking on days 5 and 7 (Figure 4F), and showed de novo and mainly cytoplasmic expression (Supplemental Figure 9, D and D’’). 2,8-DHA crystals were adherent to TNFR2+ tubules and overgrown by TNFR2+ cells (Supplemental Figure 9, D’’’ and D’’’’). During the course of the disease, de novo tubular expression of CD44 was detected on day 5, increased until day 7, and remained constantly upregulated thereafter (Figure 4G, Supplemental Figure 9E). CD44 expression showed a basolateral or circumferential membranous pattern and appeared in most tubules with adherent crystals. Similar to TNFR1, a few crystals also extratubulated in CD44− tubules (Supplemental Figure 9E’). CD44 was expressed by the urothelium of the renal pelvis which occasionally also bound crystals or crystal aggregates (Supplemental Figure 6 D’’ and D’’’, Supplemental Figure 9E’’’’).
In summary, crystal extratubulation in human and experimental 2,8-DHA nephropathy involves distinct populations of tubular epithelial cells that express several molecules proposed to be involved in calcium oxalate nephropathy.
Tnfr1−/− but not Tnfr2−/−, Cd44−/−, or Ahsg−/− mice were protected from 2,8-DHA nephropathy.
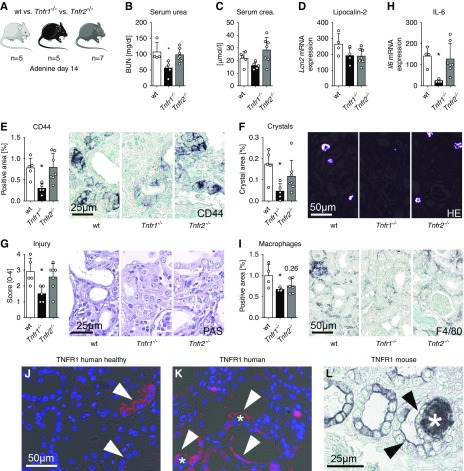
We have previously shown that both TNFR1 and TNFR2 are important in calcium oxalate nephropathy.11 Compared with wild-type mice, Tnfr1−/− but not Tnfr2−/− mice challenged with adenine-enriched diet had less severe renal failure (Figure 5, A–C). Lcn2 mRNA expression was slightly reduced in both knockout lines (Figure 5D), but only Tnfr1−/− mice significantly downregulated expression of CD44 and annexin 2 (Figure 5E, Supplemental Figure 10A). The number of cortical 2,8-DHA crystals was significantly reduced in Tnfr1−/− mice, but not in Tnfr2−/− mice (Figure 5F). Similarly, the degree of tubular injury was significantly decreased by 50% only in Tnfr1−/− (Figure 5G). Although expression of TNFR ligand Tnfa was similar in all groups (Supplemental Figure 10B), Tnfr1−/− mice showed significantly reduced Il6 expression and less infiltration of F4/80+ macrophages (Figure 5, H and I). We found no difference in α-SMA expression but significantly reduced collagen III accumulation in Tnfr1−/− mice (Supplemental Figure 10, C and D). Because deletion of Tnfr1 showed protective effects in mice, we analyzed the expression in kidney tissue from patients with APRT deficiency. In healthy kidneys, TNFR1 was only expressed in a cytoplasmic or granular pattern by a small population of tubular cells (Figure 5J). In patient kidneys with 2,8-DHA nephropathy, TNFR1 was upregulated and was predominantly expressed in injured dilated tubules and tubules containing extratubulating crystals, as well as by some interstitial cells (Figure 5K). This pattern resembles TNFR1 expression in murine kidneys (Figure 5L, Supplemental Figure 9, C–C’’’’).
Figure 5.
Deletion of Tnfr1 but not Tnfr2 ameliorated 2,8-DHA nephropathy. (A) Experimental design. Compared with wild-type mice, Tnfr1−/− but not Tnfr2−/− had (B) significantly reduced serum urea concentration and (C) slightly lowered serum creatinine. (D) Lcn2 mRNA expression tended to be reduced in both mouse lines. (E) CD44 expression, (F) the number of intrarenal crystals in kidney sections stained with HE and viewed by polarized light, as well as (G) tubulointerstitial injury scores of PAS sections were significantly reduced in Tnfr1−/−. Reduced inflammation was detected by (H) significantly decreased Il6 mRNA expression and (I) significantly decreased infiltration of F4/80+ cells. Representative TNFR1 immunofluorescence images in kidneys from (J) healthy individuals and from (K) patients with APRT deficiency, as well as (L) immunohistochemistry of a kidney from a mouse fed a 0.2% adenine diet. In healthy human kidneys, TNFR1 was only expressed by a few tubular epithelial cells in a luminal, cytoplasmic, or granular pattern (arrowheads). In human and murine kidneys with 2,8-DHA crystal nephropathy, TNFR1 was upregulated and expressed by dilated tubules (arrowheads) and tubules with extratubulating crystals (stars). Scale bars, 25 or 50 µm, respectively. wt, wild-type. *P<0.05 versus wt.
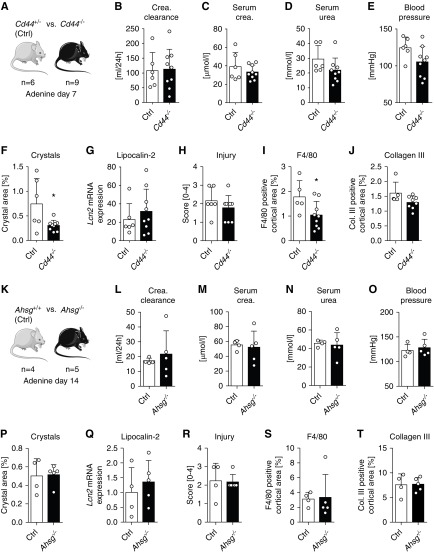
CD44 was described to be expressed de novo by injured or regenerating tubular cells that bind calcium oxalate crystals in rats and humans.10,44 We compared Cd44−/− mice and Cd44+/− littermates with 2,8-DHA nephropathy (Figure 6A), because CD44 expression during the disease was comparable between wild-type and heterozygous Cd44+/− mice, whereas no expression was found in Cd44−/− mice (Figure 4F, Supplemental Figure 11A). Compared with heterozygous Cd44+/− mice, Cd44−/− mice showed no significant change in kidney function and systolic BP (Figure 6, B–E), despite significantly fewer cortical crystals in the Cd44−/− animals (Figure 6F). This did not translate into mitigation of tubular damage assessed by mRNA expression of Lcn2, Havcr1, and Anxa2, or histologic assessment of tubulointerstitial injury (Figure 6, G and H, Supplemental Figure 11, B–E), despite significantly reduced numbers of F4/80+ immune cells in the Cd44−/− mice (P=0.043; Figure 6I, Supplemental Figure 11F). The reduced immune cell influx is most likely due to a known defect of leukocyte adhesion and tissue influx in Cd44−/− mice.45 The extent of fibrosis was similar in both groups (Figure 6J, Supplemental Figure 11, G and H).
Figure 6.
Neither deletion of Cd44 nor of Ahsg was protective against 2,8-DHA nephropathy. (A) Experimental design of studies in Cd44 mice. No differences between Cd44−/− and Cd44+/− mice after 7 days of 0.2% adenine diet were detected for the kidney function parameters (B) creatinine clearance, (C) serum creatinine, (D) serum urea, or (E) systolic BP. Furthermore, there were no differences in the amount of (F) intrarenal crystals in HE-stained kidney sections, (G) mRNA expression of Lcn2, or (H) tubulointerstitial injury scored in PAS sections. (I) Expression of F4/80 was significantly reduced in Cd44−/− mice, whereas there was (J) no difference in collagen III accumulation. (K) Experimental design in Ahsg mice. No differences could be detected between Ahsg−/− and wild-type (Ahsg+/+) mice in terms of (L) creatinine clearance, (M) serum creatinine, (N) serum urea, (O) systolic BP, (P) amount of intrarenal 2,8-DHA crystals, (Q) Lcn2 expression, (R) extent of tubulointerstitial injury, (S) F4/80 expression, and (T) collagen type III expression after 14 days of adenine-enriched diet. Col. collagen; Crea., creatinine; Ctrl, control (i.e., wild-type or hemizygous, respectively). *P<0.05 versus Ctrl.
Finally, we analyzed the role of α2-HS glycoprotein (Ahsg/fetuin-A), a hepatic protein involved in calcified matrix metabolism.46 Luminal fetuin-A was found to work as a natural inhibitor against calcification by megalin-dependent tubular reuptake of urinary calcium in the proximal tubules, protecting the kidney from nephrocalcinosis in rats.47 In addition, AHSG was also shown to be involved in inflammation.48 We did not find any differences between Ahsg−/− mice and wild-type mice with 2,8-DHA nephropathy. Both groups showed similar kidney function, numbers of renal crystals, and levels of tubular damage, inflammation, and fibrosis (Figure 6, K–T, Supplemental Figure 11, I–O).
In summary, compared with calcium oxalate nephropathy, only TNFR1 was involved in the pathogenesis of experimental 2,8-DHA nephropathy.
Discussion
2,8-DHA nephropathy is a rare disease exclusively affecting the kidneys and leading to progressive loss of kidney function. It occurs in patients with APRT deficiency, a rare genetic disease for which the diagnosis is often missed.24,49 So far, the lack of well characterized preclinical models hindered a better understanding of disease mechanisms what could lead to novel therapeutic approaches for established 2,8-DHA crystal-induced nephropathy. In the present comprehensive analyses, we demonstrated that the intrarenal crystal formation, deposition, and clearance, as well as the resulting renal tubular injury, inflammation, fibrosis, and loss of kidney function, are virtually identical in experimental rodent models of 2,8-DHA nephropathy and patients with APRT deficiency. These important data confirmed the translational relevance of using a rodent model to study a human disease. We used a model of 2,8-DHA nephropathy induced by feeding the animals an adenine-enriched diet, a simple and easily reproducible approach. The kidney disease manifests rapidly; the initial stages can be analyzed within 7 days and the advanced stages at days 14 and 21 after the disease induction.
We also found that the model is robust and leads to similar renal manifestations independent of species, i.e., mice or rats, confirming earlier data obtained in dogs,50 which are known to suffer from APRT deficiency.51,52 Importantly, the 2,8-DHA nephropathy model was also independent of mouse or rat strain or the age of the animals. This observation renders the dietary 2,8-DHA nephropathy model easily applicable to most transgenic animals. This will strongly facilitate our understanding of the key molecular and cellular mechanisms in the pathogenesis of 2,8-DHA nephropathy, but also of more general processes, such as crystal clearance as shown in this study or common mechanisms promoting AKI, tubular atrophy or fibrosis, and ECM turnover.29,53–57 In addition, this model can be easily modified by variation of the adenine concentration in the chow or by pausing the diet, thereby modulating the disease course and severity.53,58 Another model to study human APRT deficiency uses Aprt−/− mice, which also develop 2,8-DHA nephropathy.59,60 However, this model is only available in mice and crossbreeding them with other genetically modified mice requires significant time. Moreover, the phenotype cannot be controlled as easily as in the dietary model, and because the high crystal burden was only seen at an age of 120–240 days, the knockout mouse model would be less suitable for effective and high-throughput preclinical testing.
We have previously proposed that common pathologic mechanisms, such as the NLRP3 inflammasome component or necroptosis, are responsible for crystal-induced kidney diseases.1,4 Most of the functional in vivo experiments were performed using calcium oxalate nephropathy models, but some were also confirmed in 2,8-DHA nephropathy models, for example, in case of the inflammasome.54 The NLRP3 inflammasome was shown to mediate a proinflammatory immune response of renal dendritic cells by inducing IL-1β production. Nlrp3 deletion or NLRP3 inhibition by CP-456,773 or BHB (β-hydroxybutyrate) was protective in murine models of oxalate and 2,8-DHA nephropathy by reducing inflammation and renal fibrosis.54,61,62 To further address this question of common crystal-induced pathomechanisms, we here analyzed the involvement of four putative pathogenic mediators in 2,8-DHA nephropathy, namely TNFR1, TNFR2, CD44, and AHSG (all data are summarized in Supplemental Table 4). All of these molecules were previously shown to be implicated in the pathogenesis of calcium oxalate nephropathy, particularly in the initiating step of crystal adhesion to tubular cells.10,11,47 We found upregulation of TNFR1 and 2 and CD44 in experimental 2,8-DHA nephropathy, similar to previous reports in calcium oxalate nephropathy.10,11,44 Nevertheless, only TNFR1−/− mice were protected from 2,8-DHA nephropathy, which is in contrast to calcium oxalate nephropathy.11 For oxalate crystals we have previously shown that, unlike the proinflammatory NLRP3 inflammasome, TNFR was involved in crystal-to-tubular adhesion that might be directly affected by the type of crystal. In this study we found that only deletion on Tnfr1 resulted in reduced expression of the adhesion molecules annexin 2 and CD44, which might be a reason for reduced amounts of crystals and less renal damage. Fetuin-A was previously shown to limit nephrocalcinosis47 and have anti-inflammatory effects.48 In our study we could not detect any effects of Ahsg deletion on 2,8-DHA nephropathy severity, neither crystal accumulation nor inflammation, suggesting that Ahsg function may only be relevant for calcium phosphate handling.46 Overall, these data suggest that although some pathologic processes observed in crystal-induced diseases are common to all types of crystals, specific molecular mechanisms that depend on the type of crystal also exist. Our findings suggest TNFR1 as a potential therapeutic target in 2,8-DHA nephropathy, which might have direct clinical implications because various inhibitors of TNF/TNFR already exist or are being developed, such as infliximab, adalimumab, golimumab, certolizumab, or etanercept. We have previously shown that a small TNFR inhibitor, R-7050, was renoprotective in calcium oxalate nephropathy.11 Compared with antibodies or larger fusion proteins, such small-molecule inhibitors exhibit higher glomerular excretion into the tubular lumen and might therefore be better suited for targeting tubular cells.
Although differences between the underlying molecular mechanisms in various crystal nephropathies might exist, 2,8-DHA nephropathy can be used to analyze common mechanisms, including crystal clearance. We have shown here that the clearance of 2,8-DHA crystals in both the animal models and patients with APRT deficiency is similar to the clearance processes previously described for calcium oxalate crystals,4,40 and proposed that crystal size is an important determinant of future crystal fate. We particularly focused on and comprehensively described an intriguing mechanism of crystal clearance in which tubular cells actively overgrow and translocate larger crystals into the interstitium, thereby restoring tubular patency. This regenerative process was characterized by the appearance of phenotypically distinct tubular cells overgrowing the crystals, which could be termed “climber cells,” and neighboring cells giving rise to the climber cells, which could be termed “base camp cells.” This process also involved macrophages, which accumulated at sites of crystal translocation to subsequently degrade the crystals in a granulomatous inflammatory process. We found that the granuloma-forming macrophages were CD68+, CD11b+, CD11c+, and F4/80+ but ErHr3−, indicating that they might originate from resident renal macrophages rather than from infiltrating monocytes.38 The process was previously described for calcium oxalate monohydrate in rats63 and humans,64 and was termed “exotubulosis.” We instead propose the term “crystal extratubulation,” given its resemblance to the previously described “embolus extravasation,” as an alternative mechanism of microvascular recanalization.65 Our data showed reduced renal 2,8-DHA crystal retention in both Cd44−/− and Tnfr1−/− mice, which in Cd44−/− did not translate into renoprotective effects. Like Tnfr1−/−, Cd44−/− mice also showed a significant reduction of F4/80+ macrophages, a well described phenotype in Cd44−/− mice.66 This alone did not lead to significant improvement of kidney function. We believe that there might be three potential explanations for this finding. First, compared with calcium oxalate, it is possible that CD44 is only partly involved in the binding of 2,8-DHA crystals. Second, Cd44 deficiency was shown to have either anti-45,66 or proinflammatory67,68 effects, which might lead to a shift in macrophage subtypes and affect the outcome. Third, we have analyzed the Cd44−/− animals at an early disease time point, i.e., day 7, when inflammation, fibrosis, and decrease in kidney function were only mild. We chose this time point to better rule out the known effects of CD44 on macrophages and inflammation, which could have obscured the effects of CD44 on crystal retention.
Male mice with Aprt deficiency were reported to experience greater crystal burden and more pronounced renal fibrosis compared with female mice.60,69 Others also reported sex differences in the dietary adenine–associated nephropathy model in rats, with male rats exhibiting a significantly greater decline in kidney function than females, whereas both developed similar degrees of renal fibrosis.70 This is comparable to our results, showing a greater number of crystals and a stronger lipocalin-2 expression in male animals, but with only a trend toward higher serum urea concentration and no significant difference in histologic parameters of kidney injury compared with female littermates. These data suggest sex differences in the susceptibility to kidney injury, albeit these are not reflected in significant differences in clinical parameters and are not reported for patients with APRT deficiency.
In conclusion, we showed that rodent models of 2,8-DHA nephropathy closely reflect the human disease seen in patients with APRT deficiency and are highly suitable preclinical models for studies of mechanisms, including crystal extratubulation; for identification of crystal-specific molecular disease pathways, such as TNFR1 antagonism; and for testing the efficacy of therapeutic interventions.
Disclosures
Dr. Anders reports consultancy fees from GlaxoSmithKline, Inositec, Secarna, Previpharma, and Janssen. All remaining authors have nothing to disclose.
Funding
This study was financially supported by the German Research Foundation (Deutsche Forschungsgemeinschaft (DFG): SFB/TRR57, SFB/TRR219, BO3755/3-1, BO3755/6-1, AN372/16-2, and 24-1), the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung (BMBF): STOP-FSGS-01GM1901A), the RWTH Interdisciplinary Centre for Clinical Research (Interdisziplinäres Zentrum für Klinische Forschung (IZKF): O3-2), and the Medical Faculty of the RWTH Aachen University (START 09/15). Dr. Edvardsson and Dr. Palsson are supported by the Rare Kidney Stone Consortium (U54DK083908), a part of the National Center for Advancing Translational Sciences (NCATS) Rare Diseases Clinical Research Network. The Rare Kidney Stone Consortium is funded through collaboration between NCATS and the National Institute of Diabetes and Digestive and Kidney Diseases.
Data and Materials Availability
All data associated with this study are present in the paper or Supplemental Materials.
Supplementary Material
Acknowledgments
The authors thank Simon Otten, Marie Cherelle Timm, Claudia Gavranic, and Esther Stüttgen for excellent technical assistance and Mareike Hoss and Stephan Rütten for help with scanning electron microscopy.
Dr. Klinkhammer and Prof. Boor were responsible for the overall study design. Dr. Klinkhammer, Dr. Djudjaj, Dr. Kunter, Dr. Foresto-Neto, and Dr. Mulay performed the experiments. Dr. Thorsteinsdottir conducted the mass spectrometry. Dr. Edvardsson, Dr. Palsson, Dr. Hardarson, and Dr. Wiech selected and contributed patient samples. Dr. Palsson and Dr. Edvardsson collected and interpreted the clinical data from patients with adenine phosphoribosyltransferase deficiency and the results of urine dihydroxyadenine measurements. Dr. Klinkhammer and Dr. Mulay analyzed the data. Dr. Klinkhammer and Prof. Boor contributed to the writing of the manuscript. All authors discussed and critically reviewed the manuscript.
The study sponsors had no role in study design, collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019080827/-/DCSupplemental.
Supplemental Table 1. Blood pressure and laboratory blood and urine parameters of mice with 2,8-DHA nephropathy.
Supplemental Table 2. Comparison of young and aged mice with 2,8-DHA nephropathy.
Supplemental Table 3. Kidney function in rats with 2,8-DHA nephropathy.
Supplemental Table 4. Summary of performed mouse experiments.
Supplemental Figure 1. Basic characteristics of the rodent 2,8-DHA nephropathy model.
Supplemental Figure 2. 2,8-DHA nephropathy is characterized by massive inflammation in mice and rats.
Supplemental Figure 3. 2,8-DHA nephropathy is characterized by progressive renal fibrosis in mice.
Supplemental Figure 4. 2,8-DHA nephropathy in aging mice.
Supplemental Figure 5. 2,8-DHA nephropathy in rats.
Supplemental Figure 6. Location of intrarenal 2,8-DHA crystals in rats and mice.
Supplemental Figure 7. Extratubulation in mouse kidneys.
Supplemental Figure 8. In patients with APRT deficiency, renal glomeruli are not directly affected by the crystal nephropathy.
Supplemental Figure 9. Characterization of tubular phenotype in murine and human 2,8-DHA nephropathy.
Supplemental Figure 10. 2,8-DHA nephropathy in Tnfr1−/− and Tnfr2−/− mice.
Supplemental Figure 11. 2,8-DHA nephropathy in Cd44−/− and Ahsg−/− mice.
References
- 1.Mulay SR, Anders HJ: Crystallopathies. N Engl J Med 375: e29, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J: Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440: 237–241, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Mulay SR, Desai J, Kumar SV, Eberhard JN, Thomasova D, Romoli S, et al.: Cytotoxicity of crystals involves RIPK3-MLKL-mediated necroptosis. Nat Commun 7: 10274, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mulay SR, Anders HJ: Crystal nephropathies: Mechanisms of crystal-induced kidney injury. Nat Rev Nephrol 13: 226–240, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al.: NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464: 1357–1361, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herlitz LC, D’Agati VD, Markowitz GS: Crystalline nephropathies. Arch Pathol Lab Med 136: 713–720, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Sakhaee K: Recent advances in the pathophysiology of nephrolithiasis. Kidney Int 75: 585–595, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vervaet BA, Verhulst A, D’Haese PC, De Broe ME: Nephrocalcinosis: New insights into mechanisms and consequences. Nephrol Dial Transplant 24: 2030–2035, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Lieske JC, Deganello S, Toback FG: Cell-crystal interactions and kidney stone formation. Nephron 81[Suppl 1]: 8–17, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Asselman M, Verhulst A, De Broe ME, Verkoelen CF: Calcium oxalate crystal adherence to hyaluronan-, osteopontin-, and CD44-expressing injured/regenerating tubular epithelial cells in rat kidneys. J Am Soc Nephrol 14: 3155–3166, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Mulay SR, Eberhard JN, Desai J, Marschner JA, Kumar SV, Weidenbusch M, et al.: Hyperoxaluria requires TNF receptors to initiate crystal adhesion and kidney stone disease. J Am Soc Nephrol 28: 761–768, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinon F: Mechanisms of uric acid crystal-mediated autoinflammation. Immunol Rev 233: 218–232, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Correa-Costa M, Braga TT, Semedo P, Hayashida CY, Bechara LR, Elias RM, et al.: Pivotal role of Toll-like receptors 2 and 4, its adaptor molecule MyD88, and inflammasome complex in experimental tubule-interstitial nephritis. PLoS One 6: e29004, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okabe C, Borges RL, de Almeida DC, Fanelli C, Barlette GP, Machado FG, et al.: NF-κB activation mediates crystal translocation and interstitial inflammation in adenine overload nephropathy. Am J Physiol Renal Physiol 305: F155–F163, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Oyama Y, Hashiguchi T, Taniguchi N, Tancharoen S, Uchimura T, Biswas KK, et al.: High-mobility group box-1 protein promotes granulomatous nephritis in adenine-induced nephropathy. Lab Invest 90: 853–866, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warner WL: Toxicology and pharmacology of adenine in animals and man. Transfusion 17: 326–332, 1977 [DOI] [PubMed] [Google Scholar]
- 17.Bollée G, Dollinger C, Boutaud L, Guillemot D, Bensman A, Harambat J, et al.: Phenotype and genotype characterization of adenine phosphoribosyltransferase deficiency. J Am Soc Nephrol 21: 679–688, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Runolfsdottir HL, Palsson R, Agustsdottir IM, Indridason OS, Edvardsson VO: Kidney disease in adenine phosphoribosyltransferase deficiency. Am J Kidney Dis 67: 431–438, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verma R, Niraimathi M, Prasad P, Agrawal V: Dihydroxyadenine crystal-induced nephropathy presenting with rapidly progressive renal failure. Kidney Res Clin Pract 37: 287–291, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cochran B, Kovačíková T, Hodaňová K, Živná M, Hnízda A, Niehaus AG, et al.: Chronic tubulointerstitial kidney disease in untreated adenine phosphoribosyl transferase (APRT) deficiency: A case report. Clin Nephrol 90: 296–301, 2018 [DOI] [PubMed] [Google Scholar]
- 21.Mac-Way F, Desmeules S, Latulippe E, de Cotret PR, Agharazii M: 2,8-Dihydroxyadeninuria-induced progressive renal failure. NDT Plus 1: 437–439, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaidan M, Palsson R, Merieau E, Cornec-Le Gall E, Garstka A, Maggiore U, et al.: Recurrent 2,8-dihydroxyadenine nephropathy: A rare but preventable cause of renal allograft failure. Am J Transplant 14: 2623–2632, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.George SA, Al-Rushaidan S, Francis I, Soonowala D, Nampoory MRN: 2,8-Dihydroxyadenine nephropathy identified as cause of end-stage renal disease after renal transplant. Exp Clin Transplant 15: 574–577, 2017 [DOI] [PubMed] [Google Scholar]
- 24.Bollée G, Cochat P, Daudon M: Recurrence of crystalline nephropathy after kidney transplantation in APRT deficiency and primary hyperoxaluria. Can J Kidney Health Dis 2: 31, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bollée G, Harambat J, Bensman A, Knebelmann B, Daudon M, Ceballos-Picot I: Adenine phosphoribosyltransferase deficiency. Clin J Am Soc Nephrol 7: 1521–1527, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Runolfsdottir HL, Palsson R, Agustsdottir IM, Indridason OS, Edvardsson VO: Long-term renal outcomes of APRT deficiency presenting in childhood. Pediatr Nephrol 34: 435–442, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boycott KM, Hartley T, Biesecker LG, Gibbs RA, Innes AM, Riess O, et al.: A diagnosis for all rare genetic diseases: The horizon and the next frontiers. Cell 177: 32–37, 2019 [DOI] [PubMed] [Google Scholar]
- 28.Jahnen-Dechent W, Schinke T, Trindl A, Müller-Esterl W, Sablitzky F, Kaiser S, et al.: Cloning and targeted deletion of the mouse fetuin gene. J Biol Chem 272: 31496–31503, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Klinkhammer BM, Kramann R, Mallau M, Makowska A, van Roeyen CR, Rong S, et al.: Mesenchymal stem cells from rats with chronic kidney disease exhibit premature senescence and loss of regenerative potential. PLoS One 9: e92115, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al.: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skali H, Uno H, Levey AS, Inker LA, Pfeffer MA, Solomon SD: Prognostic assessment of estimated glomerular filtration rate by the new Chronic Kidney Disease Epidemiology Collaboration equation in comparison with the Modification of Diet in Renal Disease Study equation. Am Heart J 162: 548–554, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Edvardsson V, Palsson R, Olafsson I, Hjaltadottir G, Laxdal T: Clinical features and genotype of adenine phosphoribosyltransferase deficiency in Iceland. Am J Kidney Dis 38: 473–480, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Djudjaj S, Lue H, Rong S, Papasotiriou M, Klinkhammer BM, Zok S, et al.: Macrophage migration inhibitory factor mediates proliferative GN via CD74. J Am Soc Nephrol 27: 1650–1664, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buhl EM, Djudjaj S, Babickova J, Klinkhammer BM, Folestad E, Borkham-Kamphorst E, et al.: The role of PDGF-D in healthy and fibrotic kidneys. Kidney Int 89: 848–861, 2016 [DOI] [PubMed] [Google Scholar]
- 36.Thorsteinsdottir M, Thorsteinsdottir UA, Eiriksson FF, Runolfsdottir HL, Agustsdottir IM, Oddsdottir S, et al.: Quantitative UPLC-MS/MS assay of urinary 2,8-dihydroxyadenine for diagnosis and management of adenine phosphoribosyltransferase deficiency. J Chromatogr B Analyt Technol Biomed Life Sci 1036-1037: 170–177, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rogers NM, Ferenbach DA, Isenberg JS, Thomson AW, Hughes J: Dendritic cells and macrophages in the kidney: A spectrum of good and evil. Nat Rev Nephrol 10: 625–643, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Jong JP, Voerman JS, van der Sluijs-Gelling AJ, Willemsen R, Ploemacher RE: A monoclonal antibody (ER-HR3) against murine macrophages. I. Ontogeny, distribution and enzyme histochemical characterization of ER-HR3-positive cells. Cell Tissue Res 275: 567–576, 1994 [DOI] [PubMed] [Google Scholar]
- 39.Mo L, Huang HY, Zhu XH, Shapiro E, Hasty DL, Wu XR: Tamm–Horsfall protein is a critical renal defense factor protecting against calcium oxalate crystal formation. Kidney Int 66: 1159–1166, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Vervaet BA, Verhulst A, Dauwe SE, De Broe ME, D’Haese PC: An active renal crystal clearance mechanism in rat and man. Kidney Int 75: 41–51, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Schmidt-Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P, et al.: Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol 18: 407–413, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Waanders F, van Timmeren MM, Stegeman CA, Bakker SJ, van Goor H: Kidney injury molecule-1 in renal disease. J Pathol 220: 7–16, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Lim AI, Tang SC, Lai KN, Leung JC: Kidney injury molecule-1: More than just an injury marker of tubular epithelial cells? J Cell Physiol 228: 917–924, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Verhulst A, Asselman M, Persy VP, Schepers MS, Helbert MF, Verkoelen CF, et al.: Crystal retention capacity of cells in the human nephron: Involvement of CD44 and its ligands hyaluronic acid and osteopontin in the transition of a crystal binding- into a nonadherent epithelium. J Am Soc Nephrol 14: 107–115, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Rouschop KM, Roelofs JJ, Claessen N, da Costa Martins P, Zwaginga JJ, Pals ST, et al.: Protection against renal ischemia reperfusion injury by CD44 disruption. J Am Soc Nephrol 16: 2034–2043, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Jahnen-Dechent W, Heiss A, Schäfer C, Ketteler M: Fetuin-A regulation of calcified matrix metabolism. Circ Res 108: 1494–1509, 2011 [DOI] [PubMed] [Google Scholar]
- 47.Matsui I, Hamano T, Mikami S, Inoue K, Shimomura A, Nagasawa Y, et al.: Retention of fetuin-A in renal tubular lumen protects the kidney from nephrocalcinosis in rats. Am J Physiol Renal Physiol 304: F751–F760, 2013 [DOI] [PubMed] [Google Scholar]
- 48.Wang H, Sama AE: Anti-inflammatory role of fetuin-A in injury and infection. Curr Mol Med 12: 625–633, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ceballos-Picot I, Perignon JL, Hamet M, Daudon M, Kamoun P: 2,8-Dihydroxyadenine urolithiasis, an underdiagnosed disease. Lancet 339: 1050–1051, 1992 [PubMed] [Google Scholar]
- 50.Minkowsky O: Untersuchungen zur Physiologie und Pathologie der Harnsäure bei Säugethieren. Arch Exper Path u Pharmacol 41: 375–420, 1898 [Google Scholar]
- 51.Furrow E, Pfeifer RJ, Osborne CA, Lulich JP: An APRT mutation is strongly associated with and likely causative for 2,8-dihydroxyadenine urolithiasis in dogs. Mol Genet Metab 111: 399–403, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Houston DM, Moore AE, Mendonca SZ, Taylor JA: 2,8-Dihydroxyadenine uroliths in a dog. J Am Vet Med Assoc 241: 1348–1352, 2012 [DOI] [PubMed] [Google Scholar]
- 53.Morishita Y, Ohnishi A, Watanabe M, Ishibashi K, Kusano E: Establishment of acute kidney injury mouse model by 0.75% adenine ingestion. Ren Fail 33: 1013–1018, 2011 [DOI] [PubMed] [Google Scholar]
- 54.Ludwig-Portugall I, Bartok E, Dhana E, Evers BD, Primiano MJ, Hall JP, et al.: An NLRP3-specific inflammasome inhibitor attenuates crystal-induced kidney fibrosis in mice. Kidney Int 90: 525–539, 2016 [DOI] [PubMed] [Google Scholar]
- 55.Diwan V, Brown L, Gobe GC: Adenine-induced chronic kidney disease in rats. Nephrology (Carlton) 23: 5–11, 2018 [DOI] [PubMed] [Google Scholar]
- 56.Tamura M, Aizawa R, Hori M, Ozaki H: Progressive renal dysfunction and macrophage infiltration in interstitial fibrosis in an adenine-induced tubulointerstitial nephritis mouse model. Histochem Cell Biol 131: 483–490, 2009 [DOI] [PubMed] [Google Scholar]
- 57.Rahman A, Yamazaki D, Sufiun A, Kitada K, Hitomi H, Nakano D, et al.: A novel approach to adenine-induced chronic kidney disease associated anemia in rodents. PLoS One 13: e0192531, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Diwan V, Mistry A, Gobe G, Brown L: Adenine-induced chronic kidney and cardiovascular damage in rats. J Pharmacol Toxicol Methods 68: 197–207, 2013 [DOI] [PubMed] [Google Scholar]
- 59.Engle SJ, Stockelman MG, Chen J, Boivin G, Yum MN, Davies PM, et al.: Adenine phosphoribosyltransferase-deficient mice develop 2,8-dihydroxyadenine nephrolithiasis. Proc Natl Acad Sci U S A 93: 5307–5312, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Evan AP, Bledsoe SB, Connors BA, Deng L, Liang L, Shao C, et al.: Sequential analysis of kidney stone formation in the Aprt knockout mouse. Kidney Int 60: 910–923, 2001 [DOI] [PubMed] [Google Scholar]
- 61.Mulay SR, Kulkarni OP, Rupanagudi KV, Migliorini A, Darisipudi MN, Vilaysane A, et al.: Calcium oxalate crystals induce renal inflammation by NLRP3-mediated IL-1β secretion. J Clin Invest 123: 236–246, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Anders HJ, Suarez-Alvarez B, Grigorescu M, Foresto-Neto O, Steiger S, Desai J, et al.: The macrophage phenotype and inflammasome component NLRP3 contributes to nephrocalcinosis-related chronic kidney disease independent from IL-1-mediated tissue injury. Kidney Int 93: 656–669, 2018 [DOI] [PubMed] [Google Scholar]
- 63.de Bruijn WC, Boevé ER, van Run PR, van Miert PP, Romijn JC, Verkoelen CF, et al.: Etiology of experimental calcium oxalate monohydrate nephrolithiasis in rats. Scanning Microsc 8: 541–549; discussion 549–550, 1994 [PubMed] [Google Scholar]
- 64.Wiech T, Hopfer H, Gaspert A, Banyai-Falger S, Hausberg M, Schröder J, et al.: Histopathological patterns of nephrocalcinosis: A phosphate type can be distinguished from a calcium type. Nephrol Dial Transplant 27: 1122–1131, 2012 [DOI] [PubMed] [Google Scholar]
- 65.Lam CK, Yoo T, Hiner B, Liu Z, Grutzendler J: Embolus extravasation is an alternative mechanism for cerebral microvascular recanalization. Nature 465: 478–482, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rampanelli E, Dessing MC, Claessen N, Teske GJ, Joosten SP, Pals ST, et al.: CD44-deficiency attenuates the immunologic responses to LPS and delays the onset of endotoxic shock-induced renal inflammation and dysfunction. PLoS One 8: e84479, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van der Windt GJ, Florquin S, de Vos AF, van’t Veer C, Queiroz KC, Liang J, et al.: CD44 deficiency is associated with increased bacterial clearance but enhanced lung inflammation during Gram-negative pneumonia. Am J Pathol 177: 2483–2494, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van der Windt GJ, van ’t Veer C, Florquin S, van der Poll T: CD44 deficiency is associated with enhanced Escherichia coli-induced proinflammatory cytokine and chemokine release by peritoneal macrophages. Infect Immun 78: 115–124, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stockelman MG, Lorenz JN, Smith FN, Boivin GP, Sahota A, Tischfield JA, et al.: Chronic renal failure in a mouse model of human adenine phosphoribosyltransferase deficiency. Am J Physiol 275: F154–F163, 1998 [DOI] [PubMed] [Google Scholar]
- 70.Diwan V, Small D, Kauter K, Gobe GC, Brown L: Gender differences in adenine-induced chronic kidney disease and cardiovascular complications in rats. Am J Physiol Renal Physiol 307: F1169–F1178, 2014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.