Abstract
We report the first total synthesis of (+)-granatumine A, a limonoid alkaloid with PTP1B inhibitory activity, in ten steps. Over the course of this study, two key methodological advances were made: a cost-effective procedure for ketone α,β-dehydrogenation using allyl-Pd catalysis, and a Pd-catalyzed protocol to convert epoxyketones to 1,3-diketones. The central tetrasubstituted pyridine is formed by a convergent Knoevenagel condensation and carbonyl-selective electrocyclization cascade, which was followed by a direct transformation of a 2H-pyran to a pyridine. These studies have led to the structural revision of two members of this family.
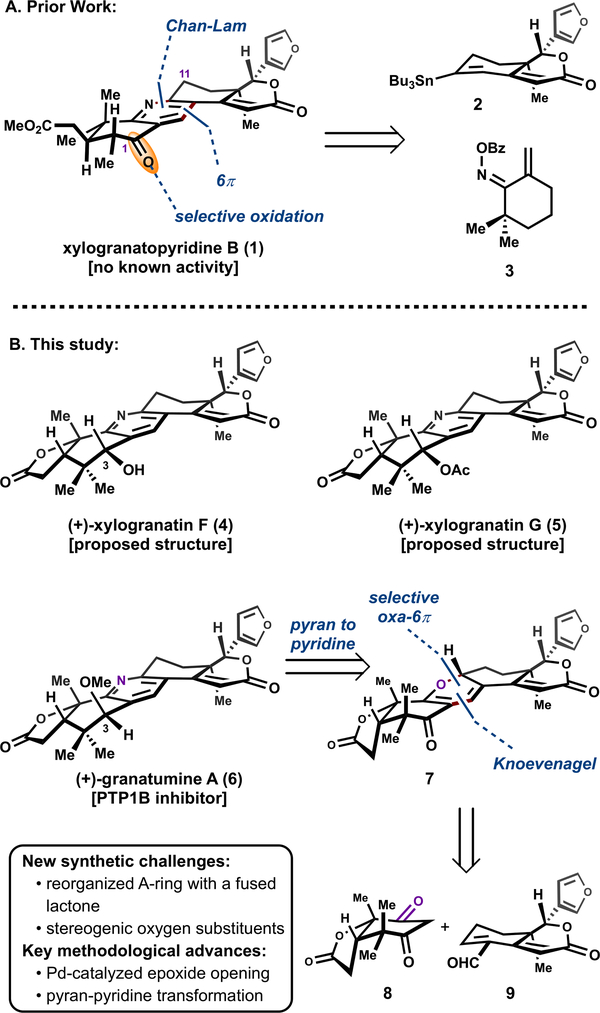
Protein tyrosine phosphatase 1B (PTP1B) has emerged as an exciting target for the treatment of many ailments, such as diabetes, cancer, and neurodegenerative diseases.1 Granatumine A (6), a bislactone limonoid alkaloid isolated from the Chinese mangrove (Xylocarpus granatum), has shown moderate inhibitory activity against PTP1B, while the related limonoid alkaloid xylogranatopyridine B (1) was found to be inactive.2 This increased potency may arise due to the synthetically demanding structural differences, namely the presence of an acid-labile C3 benzylic ether substituent and a reorganized A-ring with a fused lactone.
The C3 substituent present in the bislactone limonoid alkaloids is a point of diversity in this class of natural products (4−6), as depicted in Figure 1B.3 The differing reported stereochemistries at C3 across this series of limonoid natural products prompted the question of whether some of these members had been structurally misassigned.3 In order to resolve the stereochemical assignment and provide a means to modulate structurally the pharmacologically relevant compounds, we undertook the total synthesis of the bislactone limonoid alkaloids.4
Figure 1.
(A) Previous work on the limonoid alkaloids. (B) Retrosynthetic analysis toward bislactone limonoid alkaloids.
Previously, our laboratory disclosed a strategy to assemble the tetrasubstituted pyridine ring present in xylogranatopyridine B (1) by employing a stannyl-Liebeskind pyridine synthesis followed by a late-stage selective benzylic oxidation at C1 (Figure 1A).5 In the context of the more structurally complex bislactone alkaloids, this approach was unsuccessful, which suggested the introduction of the necessary oxidation state at an earlier stage and subsequent functional group interchange to access the variance at C3 of the bislactone limonoid alkaloids. The obstructing pyridine was retrosynthetically reduced to a 2H-pyran (7), which would serve as a cyclic equivalent to a Knoevenagel condensation product via a carbonyl-selective oxa-6π electrocyclization.6,7 This tactical combination would simultaneously enable a convergent approach using fragments 8 and 9, and differentiate the two ketones present in compound 8.
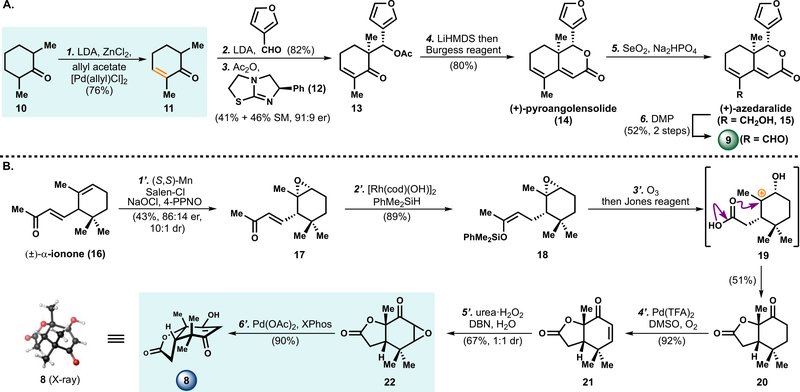
The synthesis of aldehyde 9 began with the dehydrogenation of ketone 10 (Scheme 1A). Attempts to reproduce known literature procedures for the production of 2,6-dimethylcyclohex-2-en-1-one (11), such as Birch reduction8a or bromination/elimination protocols,8b resulted in variable results and purification challenges on large scale. Utilizing our laboratory’s palladium-catalyzed ketone dehydrogenation methodology,9 ketone 10 was converted to the corresponding enone (11) in 76% yield. Importantly, the conditions were modified to be more cost-effective by employing inexpensive allyl acetate as oxidant, LDA as base, and 1 mol % palladium loading; and thus might be broadly applied in the scalable synthesis of synthetic building blocks.
Scheme 1. (A) Total Synthesis of Pyroangolensolide (14) and Azedaralide (15); (B) Synthesis of A-Ring Diketone (8)a.
a Reagents and conditions: (1) LDA (1.5 equiv), ZnCl2 (2.0 equiv), −40 °C, THF, 0.5 h, then allyl acetate (1.2 equiv), 1 mol % [Pd(allyl)Cl]2, 60 °C, 5 h, 76%; (2) LDA (1.2 equiv), 3-furaldehyde (1.2 equiv), −78 °C, THF, 0.5 h, 82%; (3) 10 mol % (+)-tetramisole, Ac2O (0.5 equiv), PhMe, 0 °C, 10 h, 41%, 91:9 er, 46% SM; (4) LiHMDS (4.0 equiv), −78 to 23 °C, 4 h, then Burgess reagent (4.0 equiv), 60 °C, 3 h, 80%; (5) SeO2 (2.5 equiv), Na2HPO4 (5.0 equiv), 1,4-dioxane, 100 °C, 14 h; (6) Dess–Martin periodinane (1.5 equiv), CH2Cl2, 23 °C, 1 h, 52% over 2 steps; (1′) 5 mol % (S,S)-(+)-N,N′-Bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminomanganese(III) chloride, 4-phenylpyridine-N-oxide (5 mol %), aq. NaOCl (1.0 equiv), CH2Cl2, 0 to 23 °C, 4 h, 43%, 86:14 er, 10:1 dr; (2′) 1 mol % [Rh(cod)(OH)]2, PhMe2SiH (1.3 equiv), THF, 23 to 60 °C, 4 h, 89%; (3′) O3, acetone, −78 °C, 0.5 h, then Jones reagent (2.0 equiv), 0 to 23 °C, 2 h, 51%; (4′) O2 balloon, Pd(TFA)2 (5 mol %), DMSO (10 mol %), AcOH, 80 °C, 12 h, 92%; (5′) urea·H2O2 (3.0 equiv), DBN (3.0 equiv), H2O (9.0 equiv), THF, 0 to 23 °C, 5 h, 67%, 1:1 dr; (6′) Pd(OAc)2 (5 mol %), XPhos (5 mol %), toluene, 120 °C, 15 h, 90%.
A diastereoselective aldol reaction was performed with 3-furaldehyde and enone 11 to afford the alcohol product in 82% yield.10 An acylative kinetic resolution with (+)-tetramisole (12)11 was conducted on the resulting alcohol to form acetate (+)-13 in 41% yield and 91:9 er with 46% recovered starting material. The unreacted enantiomer could be recycled to 11 by a retro-aldol reaction in the presence of K2CO3 and MeOH in 85% yield, and a total yield of 57% of (+)-13 could be obtained after one round of recycling (see Supporting Information). An intramolecular aldol reaction occurred upon treatment of (+)-13 with LiHMDS, and dehydration of the resulting alkoxide with Burgess reagent formed the degraded limonoid (+)-pyroangolensolide (14) in 80% yield.10 This marks the first enantioselective synthesis of (+)-14 and the absolute configuration of (+)-14 was confirmed by X-ray diffraction.
The interconversion of (+)-pyroangolensolide (14) to (+)-azedaralide (15) by site-selective allylic oxidation of the methyl group has been a previously described synthetic challenge.12 After examining a wide range of allylic oxidation conditions, we discovered that (+)-azedaralide and aldehyde 9 were formed smoothly upon treatment of 14 with SeO2 and Na2HPO4. Treatment of the resulting alcohol with Dess–Martin periodinane (DMP) furnished 9 in 52% yield over two steps, and 14 could be recovered in 27% yield.
For the synthetic route to the 1,3-diketone 8, we identified (±)-α-ionone (16), a widely available terpene building block, as a starting material goal because they are a near structural match. Modification of 16 would require oxidative cleavage, lactone formation, and installation of the carbonyl oxidation state at C3. Although there are many stepwise approaches to synthesize enantioenriched α-ionone,13 we viewed a direct resolution of the commercially available material as ideal for an efficient synthesis of 17. In fact, the previously reported synthesis of (−)-epoxy-α-ionone (17) via lipase-resolution proceeded via a low-yielding five-step sequence.14
The direct kinetic resolution of (±)-α-ionone (16) was realized using Jacobsen’s commercially available (S,S)-Mn-salen epoxidation catalyst,15 4-PPNO, and buffered bleach as the terminal oxidant to provide (−)-17 in 43% yield, 86:14 er, and an inconsequential mixture of diastereomers (10:1 dr), Scheme 1B. Hydrosilylation of the enone functionality with [Rh(cod)(OH)]2 and phenyldimethylsilane16 furnished enoxysilane 18 in an excellent isolated yield on decagram scale (89%). Subjection of enoxysilane 18 to ozonolytic oxidative cleavage followed by addition of Jones reagent provided ketone 20 in 51% yield. This presumably occurs via the intermediacy of carboxylic acid (19), which cyclizes onto the tertiary carbocation formed by acid-mediated epoxide opening. The resulting secondary alcohol would then undergo additional oxidation to form ketone 20.
Employing Stahl’s ketone α,β-dehydrogenation conditions,17 20 was selectively converted to enone 21. Utilizing alternative ketone dehydrogenation conditions, including our laboratory’s allyl-Pd-mediated method,9 resulted in unselective product formation or incomplete conversion of 20. Likewise, other methods were found to be ineffective. Nucleophilic epoxidation of enone 21 resulted in epoxide 22 in 67% yield and an inconsequential 1:1 mixture of diastereomers.
Treatment of the mixture of diastereomeric epoxides (22) with the previously described Pd-mediated α,β-epoxyketone opening conditions18 did not result in the formation of diketone 8. A broad examination of phosphine ligands, including those developed by Buchwald and co-workers,19 revealed that XPhos could effectively promote the transformation of 22 to 8 in 90% yield. These newly discovered conditions for synthesis of 1,3-diketones from α,β-epoxyketones may find use in other challenging contexts in which traditional epoxide opening conditions are unsuccessful. More generally, this approach for 1,3-diketone synthesis may be a useful tactic in other settings, as we have found here.
With a scalable route to 8 and 9, the convergent fragment coupling through a Knoevenagel condensation was investigated (Scheme 2A). Treatment of 8 and aldehyde 9 with ethylenediammonium diacetate (EDDA)20,21 formed presumed enedione intermediate 23, which under thermal conditions spontaneously underwent an oxa-6π electrocyclization to give 2H-pyran 7 as the only product on a gram scale in 47% yield from 9. The oxa-6π electrocyclization allowed for a regioselective C1 ketone protection in the presence of the C3 ketone.7e
Scheme 2. (A) Total Synthesis of (+)-Granatumine A (6); (B) Structural Revision of (+)-Xylogranatin F (29) and (+)-Xylogranantin G (28)a.
aReagents and conditions: (7) ethylenediammonium diacetate (0.7 equiv), 9 (1.5 equiv), (CH2 Cl)2, 65 °C, 4 h, 47%; (8) LiBH4 (4.5 equiv), CeCl3·7H2O (4.5 equiv), CF3CH2OH/THF (1:1), 0 to 23 °C, 3 h, 61%; (9) HO-NH2·HCl (5.0 equiv), LiOAc·H2O (6.0 equiv), MeOH, 80 °C, 12 h, 34% 4 + 35% 26; (10a) SOCl2 (7.0 equiv), CH2Cl2, 40 °C, 12 h, then NaOMe (10.0 equiv), MeOH, 70 °C, 5 h, 82%, 1:1 dr; (10b) Zn (4.0 equiv), CeCl3·7H2O (2.0 equiv), MeOH, 23 °C, 6 h, 67%; (10c) SOCl2 (7.0 equiv), CH2Cl2, 40 °C, 12 h, then Zn(OAc)2 (10.0 equiv), AcOH, 100 °C, 12 h, 74%, 1:1 dr; (11) K2CO3 (7.0 equiv), MeOH, 60 °C, 5 h, 87%.
Intrigued by this selective pyran formation, we conducted computational investigations to determine the origins of regioselectivity, and these studies suggest that the observed product is both kinetically and thermodynamically favored.22 At the ωB97x-D/6–311+G(2d,p)//ωB97x-D/6–31+G(d,p) level of theory,23 the transition states for the two 6π electrocyclizations differ by only 1.0 kcal/mol, slightly favoring the pathway leading to the observed pyran product 7 (Figure 2A). Additionally, the ground state energy of pyran 7 is significantly lower in energy than its regioisomer regio-7 (2.8 kcal/mol). In order to obtain experimental evidence for the thermodynamic preference for the observed pyran 7, regio-7 was subjected to the reaction conditions used for condensation and electrocyclization, which resulted in full conversion to the pyran 7 in 69% isolated yield (Figure 2B). Interestingly, treatment of the pyran 7 with TiCl4 resulted in formation of regio-7 in 30% yield, and pyran 7 was recovered in 60% yield.21 A related curiosity is that similar regioisomeric limonoid natural products have been isolated recently.24 For both the synthetically relevant electrocyclization and for experiments conducted as part of the mechanistic investigations, high torquoselectivity was observed—in each instance, a single diastereomer was observed by 1H NMR (see Supporting Information for computational evaluation of the torquoselectivity).
Figure 2.
(A) Computational investigations toward the regioselective pyran formation. Free energies (in kcal/mol) and structures were determined using ωB97x-D/6–311+G(2d,p)//ωB97x-D/6–31+G(d,p). (B) Interconversion investigations between pyran regioisomers.
The selective differentiation of the ketone functionalities through pyran formation allowed for installation of the necessary C3 functionality present in the bislactone limonoids. Luche reduction of the C3 ketone resulted in allylic alcohol 24 in 61% yield as a single diastereomer. The use of CF3CH2OH as solvent prevented decomposition pathways when more nucleophilic solvents were employed, such as MeOH. Treatment of alcohol (24) with hydroxylamine in refluxing methanol resulted in the formation of the compound with the originally proposed structure of (+)-xylogranatin F (4) in 34% yield and the pyridine N-oxide 26 in 35% yield.6,7 These products presumably arise via elimination of water from the intermediate 25 by two different pathways as shown in Scheme 2A. Intermediate 25 in turn likely arises via a retro-6π electrocyclization,25 followed by oxime formation, and 6π electrocyclization.26,27 The structure of 4 was confirmed by X-ray diffraction, conclusively demonstrating that the structure of (+)-xylogranatin F was misassigned.
In order to complete the synthesis of (+)-granatumine A, numerous derivatives of 4 were synthesized, including a tosylate, mesylate and phosphate, and an SN2 displacement with methoxide was attempted. However, the undesired stereochemical outcome was obtained, namely the retention of configuration, which we attributed to an SN1 solvolysis pathway. Fortunately, chlorination of the benzylic alcohol (4) with thionyl chloride, and in situ methanolysis resulted in formation of (+)-granatumine A (6) as a separable 1:1 mixture of diastereomers. The structure of (+)-granatumine A (6) had previously been reported by X-ray crystallography.2
The other major product of the pyridine synthesis cascade, the pyridine N-oxide (26), was reduced to the corresponding pyridine using Zn and CeCl328 to form 3-deoxy-xylogranatin F (27), which may itself be a natural product that has not yet been isolated. To demonstrate the failure of our previously described synthetic strategy for limonoid alkaloid synthesis for the bislactone limonoid alkaloids, conversion of 27 to (+)-xylogranatin F by benzylic oxidation was attempted without success. This result is consistent with our expectation that the bislactone alkaloids are more challenging substrates for benzylic oxidation due to the increased steric hindrance and heightened deactivation by inductive effects.
In the view that NMR structure prediction calculations may not define the correct structure of xylogranatin F, we planned to use chemical synthesis to resolve this ambiguity. Given the putative biosynthesis, we hypothesized that the correct structure of xylogranatin F is the C3 epimer 29.29 Hence, chlorination of alcohol 4 and displacement of the resulting chloride with Zn(OAc)2 resulted in the formation of (+)-xylogranatin G (28) and C3-epi-28 with a 1:1 dr. Hydrolysis of the acetate 28 provided 29, whose spectral data matched those reported for (+)-xylogranatin F; hydrolysis of C3-epi-28 provided 4. As an alternative approach to directly access (+)-xylogranatin F, reduction of ketone 7 to C3-epi-24 was attempted, but unsuccessful.
In summary, we have demonstrated the first asymmetric synthesis of (+)-granatumine A in 10 steps from commercially available 2,6-dimethylcyclohexanone and α-ionone, and revised the structures of (+)-xylogranatin F and (+)-xylogranatin G by the reassignment of the C3 stereocenter. Over the course of this work, we discovered new Pd-catalyzed ketone α,β-dehydrogenation and α,β-epoxy ketone opening conditions. The convergent synthesis employing a key pyran to pyridine conversion provided efficient access to bislactone limonoid alkaloids and may facilitate the biological study of these and related compounds.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge Dr. Brandon Mercado for X-ray crystallography of compounds 4, 8, and 14. Financial support for this work was provided by Yale University, the Sloan Foundation, Amgen, Bristol-Myers Squibb (Graduate Fellowship to A.W.S.), the National Science Foundation (GRFP to A.W.S) and the NIH (GM118614). We gratefully acknowledge the National Science Foundation for financial support in the establishment of the Yale University High Performance Computing (HPC) Center (CNS 08-21132).
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.9b04508.
Experimental procedures, X-ray diffraction, spectroscopic data for all new compounds including 1H- and 13C NMR spectra (PDF)
Crystallographic data for C26H27NO6 (CIF)
Crystallographic data for C11H14O4, 0.5(CHCl3), 0.5(H2O) (CIF)
Crystallographic data for C15H16O3 (CIF)
REFERENCES
- (1).Zhang S; Zhang Z-Y PTP1B as a drug target: recent developments in PTP1B inhibitor discovery. Drug Discovery Today 2007, 12, 373–381. [DOI] [PubMed] [Google Scholar]
- (2).For isolation of granatumine A, also known as xylogranatopyridine A, and xylogranatopyridine B, see:Li J; Guo Y; Zhou Z; Liu H; Gao L Chinese Patent CN 103880854 A, June 25, 2014.Zhou Z-F; Liu H-L; Zhang W; Kurtán T; Mándi A; Bényei A; Li J; Taglialatela-Scafati O; Guo Y-W Bioactive rearranged limonoids from the Chinese mangrove Xylocarpus granatum Koenig. Tetrahedron 2014, 70, 6444–6449.
- (3).For recent isolation of bislactone limonoid alkaloids, see:Cui J; Ouyang J; Deng Z; Lin W Structure elucidation of an unprecedented alkaloid and a new limonoid from Xylocarpus granatum. Magn. Reson. Chem. 2008, 46, 894–897.Wu J; Zhang S; Bruhn T; Xiao Q; Ding H; Bringmann G Xylogranatins F−R: Antifeedants from the Chinese Mangrove, Xylocarpus granatum, A New Biogenetic Pathway to Tetranortriterpenoids. Chem. - Eur. J. 2008, 14, 1129–1144.Pan J-Y; Chen SL; Li, M-Y; Li J; Yang M-H; Wu J Limonoids from the Seeds of a Hainan Mangrove, Xylocarpus granatum. J. Nat. Prod. 2010, 73, 1672–1679.
- (4).For recent limonoid total syntheses, see:Behenna DC; Corey EJ Simple Enantioselective Approach to Synthetic Limonoids. J. Am. Chem. Soc. 2008, 130, 6720–6721.Faber JM; Eger WA; Williams CM Enantioselective Total Synthesis of the Mexicanolides: Khayasin, Proceranolide, and Mexicanolide. J. Org. Chem. 2012, 77, 8913–8921.Yamashita S; Naruko A; Nakazawa Y; Zhao L; Hayashi Y; Hirama M Total Synthesis of Limonin. Angew. Chem., Int. Ed. 2015, 54, 8538–8541.Lv C; Yan X; Tu Q; Di Y; Yuan C; Fang X; Ben-David Y; Xia L; Gong J; Shen Y; Yang Z; Hao X Isolation and Asymmetric Total Synthesis of Perforanoid A. Angew. Chem., Int. Ed. 2016, 55, 7539–7543.Schuppe AW; Newhouse TR Assembly of the Limonoid Architecture by a Divergent Approach: Total Synthesis of (±)-Andirolide N via (±)-8α-Hydroxycarapin. J. Am. Chem. Soc. 2017, 139, 631–634.Pinkerton DM; Chow S; Eisa NH; Kainth K; Vanden Berg TJ; Burns JM; Guddat LW; Savage GP; Chadli A; Williams CM Synthesis of the seco-Limonoid BCD Ring System Identifies a Hsp90 Chaperon Machinery (p23) Inhibitor. Chem. - Eur. J. 2019, 25, 1451–1455.
- (5).Schuppe AW; Huang D; Chen Y; Newhouse TR Total Synthesis of (−)-Xylogranatopyridine B via a Palladium-Catalyzed Oxidative Stannylation of Enones. J. Am. Chem. Soc. 2018, 140, 2062–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).For a review on electrocyclization reactions in biomimetic synthesis, see: Beaudry CM; Malerich JP; Trauner D Biosynthetic and Biomimetic Electrocyclizations. Chem. Rev 2005, 105, 4757–4778. [DOI] [PubMed] [Google Scholar]
- (7).For select recent examples of 6π-electrocyclization sequences in natural product synthesis, see:Zhang W; Ready JM A Concise Total Synthesis of Dictyodendrins F, H, and I Using Aryl Ynol Ethers as Key Building Blocks. J. Am. Chem. Soc. 2016, 138, 10684–10692.Li H; Chen Q; Lu Z; Li A Total Syntheses of Aflavazole and 14-Hydroxyaflavinine. J. Am. Chem. Soc. 2016, 138, 15555–15558.Feng J; Lei X; Bao R; Li Y; Xiao C; Hu L; Tang Y Enantioselective and Collective Total Syntheses of Xanthanolides. Angew. Chem., Int. Ed. 2017, 56, 16323–16327.Murray LA; McKinnie SMK; Pepper HP; Erni R; Miles ZD; Cruickshank MC; López-Pérez B; Moore BS; George JH Total Synthesis Establishes the Biosynthetic Pathway to the Naphterpin and Marinone Natural Products. Angew. Chem., Int. Ed. 2018, 57, 11009–11014.Bellavance G; Barriault L Modular Total Syntheses of Hyperforin, Papuaforins A, B, and C via Gold(I)-Catalyzed Carbocyclization. J. Org. Chem. 2018, 83, 7215–7230.Palani V; Hugelshofer CL; Kevlishvili I; Liu P; Sarpong R A Short Synthesis of Delavatine A Unveils New Insights into SiteSelective Cross-Coupling of 3,5-Dibromo-2-pyrone. J. Am. Chem. Soc. 2019, 141, 2652–2660.
- (8).Safaryn JE; Chiarello J; Chen K-M; Joullie MM A Convenient Synthesis of (±) Ascochlorin. Tetrahedron 1986, 42, 2635–2642.Kende AS; Fludzinski P; Hill JH Chloroacetylenesas Michael Acceptors. 3. Mechanism and Synthetic Utility of Enolate Reactions with Halogenated Olefins and Chloroacetylenes. J. Am. Chem. Soc. 1984, 106, 3551–3562.
- (9).For ketone α,β-dehydrogenation, see:Chen Y; Huang D; Zhao Y; Newhouse TR Allyl-Palladium-Catalyzed Ketone Dehydrogenation Enables Telescoping with Enone α,β-Vicinal Difunctionalization. Angew. Chem., Int. Ed. 2017, 56, 8258–8262.Huang D; Zhao Y; Newhouse TR Synthesis of Cyclic Enones by Allyl-Palladium-Catalyzed α,β-Dehydrogenation. Org. Lett 2018, 20, 684–687.
- (10).For previous syntheses of (±)-pyroangolensolide, see:Fukuyama Y; Tokoroyama T; Kubota T Total Synthesis of Pyroangolensolide. Tetrahedron Lett 1973, 14, 4869–4872.Drewes SE; Grieco PA; Huffman JC A Short Synthesis of dl-epi-Pyroangolensolide and dl-Pyroangolensolide: Confirmation of the Structures of Pyroangolensolide and Calodendrolide. J. Org. Chem. 1985, 50, 1309−1311.Fernández-Mateos A; De la Fuente Blanco, J. A. Synthesis of Limonoid Model Insect Antifeedants through Stereoselective Aldol Addition Reactions. J. Org. Chem. 1991, 56, 7084–7092.Fernández-Mateos A; Grande Benito M; Pascual Coca G; Rubio González R; Tapia Hernández C Synthesiś of dl-Pyroangolensolide. Tetrahedron 1995, 51, 7521–7526.
- (11).Birman VB; Li X Benzotetramisole: A Remarkably Enantioselective Acyl Transfer Catalyst. Org. Lett. 2006, 8, 1351–1354. [DOI] [PubMed] [Google Scholar]
- (12).Baker LA; Williams CM; Bernhardt PV; Yanik GW Azedaralide: total synthesis, relative and absolute stereochemical assignment. Tetrahedron 2006, 62, 7355–7360. [Google Scholar]
- (13).(a) Fehr C; Guntern O Efficient Synthesis of Enantiomerically Pure α-Ionone from (R)- and (S)-α-Damascone. Helv. Chim. Acta 1992, 75, 1023–1028. [Google Scholar]; (b) Soorukram D; Knochel P Enantioselective Synthesis of α-Ionone Derivatives Using an Anti SN2’ Substitution of Functionalized Zinc Organometallics. Org. Lett. 2004, 6, 2409–2411. [DOI] [PubMed] [Google Scholar]; (c) Bovolenta M; Castronovo F; Vadalà A; Zanoni G; Vidari G A Simple and Efficient Highly Enantioselective Synthesis of α-Ionone and α-Damascone. J. Org. Chem. 2004, 69, 8959–8962. [DOI] [PubMed] [Google Scholar]
- (14).Aleu J; Brenna E; Fuganti C; Serra S Lipase-mediated synthesis of the enantiomeric forms of 4,5-epoxy-4,5-dihydro-α-ionone and 5,6-epoxy-5,6-dihydro-α-ionone. A new direct access to enantiopure (R)- and (S)-α-ionone. J. Chem. Soc., Perkin Trans 1 1999, 271–278. [Google Scholar]
- (15).For examples of kinetic resolutions using Mn-Salen complexes, see:Jacobsen EN; Zhang W; Muci AR; Ecker JR; Deng L Highly enantioselective epoxidation catalysts derived from 1,2-diaminocyclohexane. J. Am. Chem. Soc. 1991, 113, 7063–7064.Vander Velde SL; Jacobsen EN Kinetic Resolution of Racemic Chromenes via Asymmetric Epoxidation: Synthesis of (+)-Teretifolione B. J. Org. Chem. 1995, 60, 5380–5381.Hamada T; Irie R; Katsuki T How Does Chiral Oxo(salen)manganese(V) Complex Discriminate the Enantioface of Simple Olefins? Scope and Limitation of Salen-Catalyzed Epoxidation. Synlett 1994, 479–481.Noguchi Y; Irie R; Fukuda T; Katsuki T Mn-salen catalyzed asymmetric epoxidation of (±)-3-alkylindene: Reagent-dependent stereoselectivity. Tetrahedron Lett. 1996, 37, 4533–4536.
- (16).Mori A; Kato T [Rh(OH)(cod)]2 (cod = 1,5-Cyclooctadiene): A Highly Efficient Catalyst for 1,4-Hydrosilylation of α,β-Unsaturated Carbonyl Compounds. Synlett 2002, 1167–1169. [Google Scholar]
- (17).Diao T; Stahl SS Synthesis of Cyclic Enones via Direct Palladium-Catalyzed Aerobic Dehydrogenation of Ketones. J. Am. Chem. Soc. 2011, 133, 14566–14569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).(a) Suzuki M; Watanabe A; Noyori R Palladium(0)-catalyzed reaction of α,β-epoxy ketones leading to β-diketones. J. Am. Chem. Soc. 1980, 102, 2095–2096. [Google Scholar]; (b) Inouye Y; Kojima T; Owada J; Kakisawa H Preparation of Bicyclo[3.3.1]nonane-2,4-dione Derivatives. Bull. Chem. Soc. Jpn. 1987, 60, 4369–4375. [Google Scholar]; (c) Suzuki M; Watanabe A; Noyori R Palladium(0)-catalyzed isomerization of α,β-epoxy ketones to β-diketones. Recl. Trav. Chim. Pays-Bas 1988, 107, 230–236. [Google Scholar]; (d) Ragan JA; Makowski TW; am Ende DJ; Clifford PJ; Young GR; Conrad AK; Eisenbeis SA A Practical Synthesis of Cycloheptane-1,3-dione. Org. Process Res. Dev. 1998, 2, 379–381. [Google Scholar]; (e) Sharp MJ; Fang FG Efficient Construction of 6-Azasteroids: Dual Inhibitors of Steroidal 5α-Reductase. Bioorg. Med. Chem. Lett. 1998, 8, 3291–3294. [DOI] [PubMed] [Google Scholar]; (f) Lehmann TE; Kuhn O; Krüger J Process Development and Pilot Plant Scale Synthesis of Spiro[3.5]nonane-6,8-dione. Org. Process Res. Dev. 2003, 7, 913–916. For a review, see: [Google Scholar]; (g) Muzart J Pd-Mediated Reactions of Epoxides. Eur. J. Org. Chem. 2011, 4717–4741. [Google Scholar]
- (19).Surry DS; Buchwald SL Dialkylbiaryl phosphines in Pd-catalyzed amination: a user’s guide. Chem. Sci. 2011, 2, 27–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Riveira MJ; Quiroga GN; Mata EG; Gandon V; Mischne MP Cycloisomerization of Conjugated Trienones and Isomeric 2H-Pyrans: Unified Strategy toward Cyclopenta[b]furans. J. Org. Chem. 2015, 80, 6515–6519. [DOI] [PubMed] [Google Scholar]
- (21).Malerich JP; Maimone TJ; Elliott GI; Trauner D Biomimetic Synthesis of Antimalarial Naphthoquinones. J. Am. Chem. Soc. 2005, 127, 6276–6283. [DOI] [PubMed] [Google Scholar]
- (22).For experimental evidence of the reversibility of oxa-6π electrocyclizations, see: Marvell EN; Chadwick T; Caple G; Gosink T; Zimmer G Rates of Electrocyclic Reactions. Conversion of α-Pyrans to Cis Dienones. J. Org. Chem. 1972, 37, 2992–2997. [Google Scholar]
- (23).Phillips EM; Mesganaw T; Patel A; Duttwyler S; Mercado BQ; Houk KN; Ellman JA Synthesis of ent-Ketorfanol via a C–H Alkenylation/Torquoselective 6π Electrocyclization Cascade. Angew. Chem., Int. Ed. 2015, 54, 12044–12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Li W-S; Mándi A; Liu J-J; Shen L; Kurtán T; Wu J Xylomolones A-D from the Thai Mangrove Xylocarpus moluccensis: Assignment of Absolute Stereostructures and Unveiling a Convergent Strategy for Limonoid Biosynthesis. J. Org. Chem. 2019, 84, 2596–2606. [DOI] [PubMed] [Google Scholar]
- (25).For select examples of retro-oxa-6π electrocyclizations, see:Cerfontain H; Geenevasen JA; van Noort PCM Photochemistry of dienones. Part 7. On the photosensitized isomerization of (E)-β-ionone and its isomeric α-pyran. Evidence for exciplex formation between the α-pyran and fluoren-9-one. J. Chem. Soc., Perkin Trans 2 1980, 1057–1062.Sastry MNV; Claessens S; Habonimana P; De Kimpe N Synthesis of the Natural Products 3-Hydroxymollugin and 3-Methoxymollugin. J. Org. Chem. 2010, 75, 2274–2280.Hall AJ; Roche SP; West LM Synthesis of Briarane Diterpenoids: Biomimetic Transannular Oxa-6π electrocyclization Induced by a UVA/UVC Photoswitch. Org. Lett. 2017, 19, 576–579.
- (26).For an example of 2H-pyran to pyridine conversion with hydrazine, see: Micale N; Zappala M; Grasso S Synthesis and̀ antitumor activity of 1,3-benzodioxole derivatives. Farmaco 2002, 57, 853–859. [DOI] [PubMed] [Google Scholar]
- (27).Vargas DF; Larghi EL; Kaufman TS The 6π-azaelectrocyclization of azatrienes. Synthetic applications in natural products, bioactive heterocycles, and related fields. Nat. Prod. Rep. 2019, 36, 354–401. [DOI] [PubMed] [Google Scholar]
- (28).Yoo BW; Jung H II; Kim SH; Ahn YS; Choi JY Selective and Efficient Deoxygenation of Amine-N-Oxides with CeCl3·7H2O/Zinc System. Bull. Korean Chem. Soc. 2013, 34, 359–360. [Google Scholar]
- (29).For a recent example of stereochemical reassignment based on putative biosynthesis and NMR calculations, see: Kutateladze AG; Krenske EH; Williams CM Reassignments and Corroborations of Oxo-Bridged Natural Products Directed by OSE and DU8+NMR Computation. Angew. Chem., Int. Ed. 2019, 58, 7107–7112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.