Abstract
Despite replicated evidence for working memory deficits in youth with ADHD, no study has comprehensively assessed all three primary ‘working’ subcomponents of the working memory system in these children. Children ages 8–13 with (n=45) and without (n=41) ADHD (40% female; Mage=10.5; 65% Caucasian/Non-Hispanic) completed a counterbalanced battery of nine tasks (three per construct) assessing working memory reordering (maintaining and rearranging information in mind), updating (active monitoring of incoming information and replacing outdated with relevant information), and dual-processing (maintaining information in mind while performing a secondary task). Detailed analytic plans were preregistered. Bayesian t-tests indicated that, at the group level, children with ADHD exhibited significant impairments in working memory reordering (BF10=4.64 x 105; d=1.34) and updating (BF10=9.49; d=0.64), but not dual-processing (BF01=1.33; d=0.37). Overall, 67%–71% of youth with ADHD exhibited impairment in at least one central executive working memory domain. Reordering showed the most ADHD-related impairment, with 75% classified as below average or impaired, and none demonstrating strengths. The majority of children with ADHD (52%–57%) demonstrated average or better abilities in the remaining two domains, with a notable minority demonstrating strengths in updating (8%) and dual-processing (20%). Notably, impairments in domain-general central executive working memory, rather than individual subcomponents, predicted ADHD severity, suggesting that common rather than specific working memory mechanisms may be central to understanding ADHD symptoms. These impairment estimates extend prior work by providing initial evidence that children with ADHD not only exhibit heterogeneous profiles across cognitive domains but also exhibit significant heterogeneity within subcomponents of key cognitive processes.
Keywords: working memory, central executive, heterogeneity, ADHD, preregistration
Though several cognitive processes have been consistently implicated in attention-deficit/hyperactivity disorder (ADHD), working memory has garnered particular attention in recent years. Working memory performance has been linked to ADHD symptoms (e.g., Kofler et al., 2010; Rapport et al., 2009; Sarver, Rapport, Kofler, Raiker, & Friedman, 2015) and associated impairments (Kofler et al., 2017; McQuade, Murray-Close, Shoulberg, & Hoza, 2013; Simone, Marks, Bedard, & Halperin, 2018), as well as the natural course of symptom change (Karalunas et al., 2017) and stimulant treatment response (Hawk et al., 2018). As a group, children with ADHD demonstrate large-magnitude differences in working memory task performance when compared with typically-developing peers (Kasper, Alderson, & Hudec, 2012; Martinussen, Hayden, Hogg-Johnson, & Tannock, 2005). However, not all children with ADHD experience working memory deficits (Kasper et al., 2012; Nigg, Willcutt, Doyle, & Sonuga-Barke, 2005), and there is increasing interest in documenting the heterogeneity of cognitive function in this population (Feczko et al., 2019). Focusing on working memory specifically, there is a fairly wide range of estimates in the percentage of children with ADHD identified as impaired. At least two studies suggest that working memory impairments are present in only approximately 30% of youth with ADHD (Coghill, Seth, & Matthews, 2014; Wahlstedt, Thorell, & Bohlin, 2009). In contrast, through a meta-regression approach, Kasper et al. (2012) estimated that 98% of children with ADHD are expected to score below the mean of a typically-developing group, with 81%–84% demonstrating clinically-significant impairment. Similar to these meta-regression estimates, Karalunas and colleagues (2017) showed that approximately 85% of children with ADHD exhibited impaired working memory, with the majority of these cases (55%) demonstrating stable impairment across time, and Kofler and colleagues (2019) showed that 62% of children with ADHD were impaired in working memory.
Taken together, the literature clearly shows that working memory is central for understanding ADHD. Yet, many different paradigms and tasks have been used to assess working memory, and an underappreciation of the multi-component nature of the construct has resulted in little consideration of the aspects of working memory that are being evaluated, leading to wide heterogeneity in effect size and impairment estimates (Kasper et al., 2012). Including all memory-related tasks in a general ‘working memory’ category limits our ability to move beyond the notion of a general working memory deficit in ADHD to characterize the more nuanced nature of these deficits. The issue is further complicated by construct validity concerns with several widely-used ‘working memory’ tests (e.g., backward digit span; Wells et al., 2018) and the common practice of using a single clinically-oriented assessment tool as an indicator of working memory functioning. While this approach is understandable when these tools are the only available measures, clinical assessments have been criticized when the goal is to understand variation across a spectrum ranging from normal to abnormal functioning (Snyder, Miyake, & Hankin, 2015).
Working Memory
Working memory refers to a multicomponent system involving domain-specific storage (i.e., phonological versus visuospatial), as well as a domain-general central executive. The central executive is the ‘working’ component of working memory and is responsible for numerous complex functions that involve coordinating and acting upon information held within the domain-specific short-term memory stores as described below (Baddeley, 2003; 2012). Internally-stored information often must be manipulated or modulated in some way, and meta-analytic work suggests that ADHD/Control group differences are more pronounced on tasks with higher central executive demands, as opposed to tasks that primarily require the storage of information (Kasper et al., 2012).
Despite consistent evidence that central executive functioning is the most impaired component of working memory in youth with ADHD, surprisingly little is known regarding which specific central executive functions are impaired in youth with the disorder. The present study utilized the 3-domain model of central executive processes (Wager & Smith, 2003), which differentiates functionally and neuroanatomically among processes involving serial/temporal reordering of stimuli, continuous updating of relevant information in working memory, and the ability to maintain relevant information in mind when competing stimuli are introduced. Meta-analytic neuroimaging evidence shows that there are core frontal and parietal regions that support central executive processes broadly; at the same time, tasks that require different central executive abilities demonstrate distinct cortical activation patterns (Wager & Smith, 2003; see also Rottschy et al., 2012).
Reordering requires an individual to hold a series of information in mind and rearrange that information. As an example, imagine that you are at the grocery store when your partner calls and asks you to pick up milk, bread, coffee, ice cream, and lunchmeat. To save time, you decide to rearrange that list based on the items’ locations in the store (e.g., non-refrigerated items first, and ice cream last so it does not melt before you get home). You would have to temporarily hold the original list in mind while reordering it based on your ruleset and ultimately replacing the original mental list with the new list. Cognitive tasks that assess reordering usually require participants to rearrange a series of letters, numbers, or locations according to some maintained rule provided by the experimenter (e.g., smallest to largest). Reordering requires active manipulation of material stored within short-term memory, whereas the other two central executive processes do not require manipulation.
Updating involves continuous monitoring of incoming information and the deletion and replacement of irrelevant information with updated, relevant information (Miyake et al., 2000). For example, consider again your grocery list described above. As you pick each item off the shelf, you update your mental list by deleting those items while continuing to rehearse the remaining list. Then, imagine that your partner calls back and tells you that on second thought you do not need milk (turns out there is a full gallon that was hiding in the back of the fridge) but asks you to get orange juice instead. You then update your mental list by deleting the no-longer-relevant item (milk) and replacing it with the now-relevant item (juice). Updating tasks require participants to monitor a stream of incoming information; based on each piece of new information, participants must then make judgments about whether the information currently held in working memory should be replaced by the new information (i.e., if the new information is more relevant than past information).
Finally, when individuals are engaged in dual-processing, they must hold information in mind while also performing another cognitively demanding task. Returning to your grocery list, as you continue to rehearse the list mentally, you have to engage in secondary processing tasks to select the individual products. For example, you stand in the bread aisle reading packaging and evaluating your options: Do you want whole grain? Reduced calorie? Perhaps you are comparing carbohydrate counts or prices across the many options. These verbally-mediated processes (comparing different bread choices) compete for cognitive resources with your attempt to remember your grocery list because both processes rely on the same phonological system. The better your dual-processing abilities are, the more likely you will be able to actively consider different product features without forgetting the other items on your list that you still need to purchase. In a laboratory setting, dual-processing tasks interleave the presentation of to-be-remembered target stimuli with a demanding, secondary processing task. Thus, participants are required to engage in a secondary task of the same modality as the primary task, which yields interference effects that increase demands on controlled attention and the central executive (Conway et al., 2005).
Current Study
The present study comprehensively examined the three primary subcomponents of central executive working memory in ADHD with the goal of clarifying how the type of central executive demand impacts estimates of diagnostic group differences and impairment rates. We utilized tasks developed from the cognitive science literature to evaluate 1) diagnostic group differences across the domains of reordering, updating, and dual-processing and 2) the percentage of children with ADHD with impairment in each domain.
Our preregistered hypotheses were as follows: (1) we predicted that the ADHD group would perform significantly worse than the control group across all three components of central executive functioning; (2) based on previous studies that have evaluated these domains among separate groups of children (e.g., Friedman et al., 2017; Hutchinson, Bavin, Efron, & Sciberras, 2012; Kofler et al., 2019), we predicted the largest magnitude between-group effect size for reordering, followed by updating, with the smallest effect size expected for dual-processing; and (3) we expected approximately 60%–85% of children in the ADHD group to exhibit impairment in at least one central executive working memory component based on recent prevalence estimates of overall working memory impairment in ADHD (Karalunas et al., 2017; Kofler et al., 2019). Due to the lack of research across these three components, no specific predictions were made regarding the percentage of children with impairment in each domain.
Method
Preregistration and Open Data
Detailed data analytic plans were preregistered at https://osf.io/gcq26. There were no departures from the preregistered plan, with one exception. Consistent with a deficit-focused perspective, we originally defined impairment as scores at/below the 10th percentile of the non-ADHD group, with all other scores lumped into an unimpaired category. While examining performance patterns, we discovered that a sizable minority of children with ADHD demonstrated average or better performance in at least one domain. We therefore elected to expand our categorical descriptive ranges to better understand these cases by adopting qualitative descriptive categories derived from common standardized cognitive tests (e.g., Wechsler, 2014). Therefore, findings regarding ADHD-related impairments remain hypothesis-confirming, while conclusions regarding ADHD-related strengths in each central executive component should be considered hypothesis-generating given that we did not preregister the latter definition. The de-identified dataset (.jasp) and annotated results output (including test statistics) are available for peer review: https://osf.io/wj37y/.
Participants
The sample included 45 children with and 41 children without ADHD (8–13 years old; see Table 1 for participant characteristics). Both groups were recruited through community resources (e.g., pediatricians, schools, community mental health clinics) to participate in a research study at a university laboratory. All families received no-cost psychoeducational evaluations for participation, which included evaluation of a child’s intellectual abilities and academic achievement, as well as their current behavioral and emotional symptoms and functioning.
Table 1.
Participant characteristics
| ADHD (N=45) M (SD) | Non-ADHD (N=41) M (SD) | BF10 | BF01 | Cohen’s d | |
|---|---|---|---|---|---|
| Sex (male/female) | 28/17 | 24/17 | 3.50 | ||
| Ethnicity (C/AA/A/H/M) | 32/6/0/4/3 | 24/5/3/7/2 | 2.48 | ||
| Age | 10.27 (1.49) | 10.79 (1.56) | 1.53 | 0.34 | |
| SES Total Score | 46.00 (12.19) | 51.45(11.08) | 1.73 | 0.47 | |
| IQ | 102.02 (15.44) | 109.71 (10.41) | 4.88 | 0.58 | |
| BASC Attention Problems | |||||
| Parent | 65.82 (8.60) | 59.34 (10.44) | 15.15 | 0.68 | |
| Teacher | 62.51 (8.65) | 55.61 (11.20) | 17.77 | 0.69 | |
| BASC Hyperactivity | |||||
| Parent | 67.89(12.71) | 56.41 (12.71) | 301.22 | 0.90 | |
| Teacher | 60.84 (14.91) | 54.63 (13.09) | 1.37 | 0.44 | |
| Working Memory Component Z-Scores | |||||
| Reordering | −0.53 (0.89) | 0.58 (0.76) | 4.64 x 105 | 1.34 | |
| Updating | −0.29 (1.02) | 0.32 (0.89) | 9.49 | 0.64 | |
| Dual-Processing | −0.17 (1.15) | 0.19 (0.77) | 1.33 | 0.37 | |
Note. BASC = Behavior Assessment System for Children. BF = Bayes Factor; BF01 represents support for the null hypothesis over the alternative, and BF10 indicates support for the alternative hypothesis; BF10 is the inverse of BF01 (i.e., BF10 = 1/BF01); BF values >3 are considered “significant” support.
A comprehensive evaluation was conducted for all interested participants. The evaluation included semi-structured clinical interviews (Kiddie Schedule for Affective Disorders and Schizophrenia; K-SADS Kaufman et al., 1997), parent and teacher ratings of children’s ADHD symptoms (DuPaul ADHD Rating Scale-4/5; DuPaul et al., 2016), behavioral and emotional functioning (Behavior Assessment System for Children; BASC-2/3; Reynolds & Kamphaus, 2004; 2015), and children’s intellectual functioning (Wechsler Intelligence Scale for Children-IV Short Form; Sattler, Dumont, & Coalson, 2016) and achievement (Kaufman Test of Educational Achievement-3; Kaufman & Kaufman, 2014). Children in the ADHD group (1) met criteria for a DSM-5 diagnosis of ADHD Combined (n=35), Inattentive (n=9), or Hyperactive/Impulsive Presentation (n=1) by the directing clinical psychologist based on the K-SADS, (2) exhibited borderline/clinical elevations on at least one parent and one teacher ADHD subscale, and (3) exhibited current impairment based on parent report.
Any participants currently taking stimulant medication (n=23) had medication withheld at least 24 hours before testing. Given the high rate of comorbidity between ADHD and other mental health disorders, children with comorbidity were included (Larson, Russ, Kahn, & Halfon, 2011; Wilens et al., 2002). In the current sample, comorbid conditions included anxiety (n=10), depression (n=2), autism spectrum (n=4), and oppositional defiant (n=5) disorders.
Children in the Non-ADHD group included both typically-developing (TD) healthy controls (n=21) and clinical controls (n=20). Typically-developing children had normal developmental histories based on parent report and did not meet criteria for any behavioral or emotional disorder based on the assessment measures described above. Children who were diagnosed with clinical disorders other than ADHD were also included in the Non-ADHD group to control for comorbidities in the ADHD group to maximize the likelihood that ADHD/Non-ADHD between-group differences could be attributable to ADHD specifically rather than psychopathology generally. Comorbidities reflect clinical consensus best estimates (Kosten & Rounsaville, 1992), and included anxiety (n=9), depression (n=3), autism spectrum (n=5), and oppositional defiant (n=1) disorders. Importantly, there was no difference in the frequency of comorbid conditions across ADHD and Non-ADHD groups (BF01= 0.69–3.20).
Children in both groups were excluded for gross neurological, sensory, or motor impairment, history of seizure disorder, psychosis, intellectual disability, and use of non-stimulant medications that could not be withheld for testing.
Procedure
This study was approved by the Institutional Review Board at Florida State University, and caregivers and children gave consent/assent before study enrollment. Cognitive testing occurred during a larger battery of two sessions that lasted approximately three hours each. All tests were counterbalanced within and across sessions to minimize order/fatigue effects. Children received brief breaks after each task, and preset longer breaks every 2–3 tasks.
Measures
Working memory reordering1.
The working memory reordering tasks developed by Rapport et al. (2008) were used for the current study, along with the Kofler et al. (2018) episodic buffer working memory task (see Supplemental Figure 1 for visual depictions of all tasks). All three tasks involve serial reordering of characters presented (numbers, black dot locations), and reordering of a target stimulus (letter, red dot location) into the final serial position recalled. Stimuli were presented at a rate of 1 per second (800 ms presentation, 200 ms ISI). After five practice trials (80% correct required), trials were presented in two, 12-trial blocks of mixed set sizes, with 6 unique trials of each memory load (set sizes 3–6 per block). Short breaks were provided between each block (approximately 1 minute). Task duration was approximately 3.4 (phonological), 2.8 (visuospatial), and 3.0 (episodic buffer) minutes per block. Partial-credit unit scoring was used at each set size for each task, as recommended (Conway et al., 2005).
Phonological working memory task (PHWM).
Children were presented a series of numbers and a letter that never appeared first or last and were instructed to recall the numbers in order from least to greatest, and to say the letter last (e.g., 4-H-6-2 is correctly recalled as 2-4-6-H). Two trained research assistants, shielded from the participant’s view, recorded oral responses independently (interrater reliability in our prior studies has consistently been greater than 97% agreement).
Visuospatial working memory task (VSWM).
Children were shown nine squares arranged in three offset vertical columns on a computer monitor. A series of 2.5 cm diameter dots (3, 4, 5, or 6) were presented sequentially. No two dots appeared in the same square on a given trial. All dots were black except for one red dot that never appeared first or last. Children were instructed to re-order the dot sequence by keying the spatial locations on a modified keyboard, with the black dot locations in the serial order presented, followed by the red dot’s location last.
Episodic buffer working memory (EBWM).
The episodic buffer working memory task combined the phonological and visuospatial tasks. Children were presented a series of numbers and a letter that appeared in the visuospatial squares described above. Children were instructed to remember the spatial location of each number/letter, reorder the numbers in ascending order and put the letter last (e.g., 4-H-6-2 is correctly recalled as 2-4-6-H), and respond by keying the corresponding squares in the position in which they appeared on the screen on a modified keyboard. Thus, successful performance on the task required children to bind the phonological (numbers and letter) with the visuospatial (location each number/letter appeared) information.
Working memory updating.
Letter updating.
The Miyake et al. (2000) letter memory test was adapted for use with children. Letters were presented on the screen one at a time, and children were instructed to keep track of the last three letters presented. To ensure the task required continuous updating, children were instructed to rehearse out loud the last three letters by mentally adding the most recent letter, dropping the fourth letter back and then saying the new string of three letters out loud (Miyake et al., 2000). The number of letters presented (4–8 stimuli presented/trial, 1200 ms presentation, 2400 ms ISI) was varied randomly across trials to ensure that successful performance required continuous updating until the end of each trial. After a practice block (three correct trials required), four blocks of three trials each were administered (12 trials total). Children responded via mouse click.
N-back task.
The N-back task is arguably the most commonly used continuous updating test (Schmiedek, Lövdén, & Lindenberger, 2014). The high-density, double-letter (1-back) N-back task described by Denney (Denny, Rapport, & Chung, 2005) was used in the current study. A practice block of 30 stimuli (10 targets) was included (80% correct required). The N-back task included 180 trials, during which capital letters (3.5 cm height and width) were displayed one at a time (200 ms presentation, 800 ms ISI). Children were instructed to press a mouse button each time a target letter was identical to the letter immediately preceding it (i.e., 1 back in the sequence). One-back targets comprised 60 (33.3%) of the 180 stimuli (Denney et al., 2005); task duration was three minutes.
Keep track.
The Miyake et al. (2000) keep track test was adapted for use with children. In this computerized task, exemplars from 2–5 categories (animals, vehicles, clothing, shapes, body parts) were presented on the screen one at a time, and children were instructed to keep track of the last exemplar from each category. Each category included three stimuli (e.g., the animal category included pictures of a dog, cat, and fish) that were presented randomly. Children were instructed to rehearse out loud the current exemplar from each category after each stimulus was presented (Miyake et al., 2000). Stimuli were presented randomly with the caveat that at least one exemplar per category was presented before the recall phase. The number of stimuli presented (9–13 stimuli presented/trial, 1200 ms presentation, 2400 ms ISI) was varied randomly across trials. The task included a practice block (two correct trials required) and four test blocks of three trials each that were administered in ascending set size order (2–5 categories presented; 12 trials total).
Dual-processing working memory.
The counting span and animal/animal context span tasks described below were developed based on the counting span and reading span working memory tasks described by Conway et al. (2005), adapted for use with children. The secondary processing task was either experimenter-paced (counting span) or self-paced (animal span, animal context span), or included stimuli with low information processing demands (animal span) or high information processing demands (animal context span).
Counting span.
Children were sequentially shown screens containing a random number of black dots and between 1 and 9 red dots (all 2.5 cm diameter). Children were instructed to verbally report the number of red dots as each screen was presented, ignoring the black dots. After a predetermined number of screens (set sizes 3, 4, 5, and 6), children were asked to indicate via mouse click the number of red dots on each screen in serial order. Each screen was displayed for 500 ms per red dot (e.g., screens with 6 red dots remained visible for 3000 ms; ISI = 500 ms). Sixteen total trials (4 per set size, presented randomly) were completed following a practice round that terminated after two correct trials.
Animal span.
Stimuli included exemplars of six different animals (dogs, spiders, birds, fish, lions, walruses). Children were sequentially shown screens containing a picture of a single animal at the top of the screen and six response boxes on the bottom of the screen and were instructed to click the response box that matched the picture (e.g., clicking ‘dog’ when viewing a picture of a dog). After each animal, children silently read and responded to a true/false sentence by clicking the corresponding button on the screen (e.g., “Fish fly in the sky. True or False”). After a predetermined number of animal-sentence pairs (memory set sizes 3–6), children were asked to recall via mouse click the animals in serial order. The sentences were presented last in each animal-sentence pair to ensure interference effects prior to recall (Unsworth & Engle, 2007). The number of trial pairs before each recall phase was unpredictable to maximize working memory demands, and children completed a total of eight trials. Following Engle et al. (1999), children received performance feedback during both the primary and secondary task components. All task components were self-paced.
Animal context span.
This task was identical to the animal span task, except that children had to infer which animal was “hidden” in each picture based on the context. Each animal context stimulus featured a scene that included a ‘hidden animal’ (depicted as a white circle with a black question mark) that could be inferred based on the rest of the picture.
ADHD symptom severity.
T-scores from the parent- and teacher-reported Attention Problems and Hyperactivity scales of the BASC-2/3 were used to measure severity of ADHD symptoms.
Data Reduction
The primary outcome variables were stimuli correct per trial at each block/set size (4 variables per task) for all working memory tasks except the n-back, which provided two outcome variables (total omission and total commission errors). Rather than using observed variables, we reduced task performance data by computing Bartlett maximum likelihood weighted averages. This approach isolates reliable variance across indicators of each central executive domain, providing common variance thought to represent the underlying process (DiStefano, Zhu, & Mindrila, 2009). Working memory task data were represented as formative (mean-based scores) rather than reflective indicators (confirmatory factors) as recommended (Willoughby et al., 2016). A component score was generated for each central executive domain and used in all analyses reported below. The task variables were reduced using an a priori specified 3-component principal components analysis with varimax rotation (49.8% of variance explained; see Supplementary Table 1 for component loadings). Using the Bartlett weighted averages approach, each task variable potentially contributes to each component, with the amount of variance contributed weighted according to that indicator’s loading on the component. An orthogonal rotation was pre-specified to maximize distinction between working memory domains given the primary research questions; we also present results of a direct oblimin oblique rotation in the sensitivity analyses section for comparison given prior evidence for the separability and interrelatedness of the various components.
Working memory impairment was defined as a score at or below the 10th percentile of the non-ADHD group. Specifically, all component scores were standardized relative to the non-ADHD group (i.e., the mean and SD of the non-ADHD group’s Bartlett weighted scores were used to compute standardized scores for all participants), and z-scores of −1.28 (i.e., the 10th percentile) or below were considered impaired. This approach is advantageous over defining impairment as scores at or below the nth lowest non-ADHD case because it accounts for any potential non-normality in the non-ADHD group (see Figures 1 and 2). Although the 10th percentile is an arbitrary cutoff, it is based on precedence in the ADHD neurocognitive heterogeneity literature (Coghill et al., 2014), and previous research suggests that this approach produces results that are highly consistent with objective methods of identifying impairment, such as the Jacobson and Truax (1991) Reliable Change Index (Kofler et al., 2019). Throughout the manuscript, reference to an impaired or non-impaired group refers to working memory impairment (as opposed to functional impairment related to ADHD symptoms).
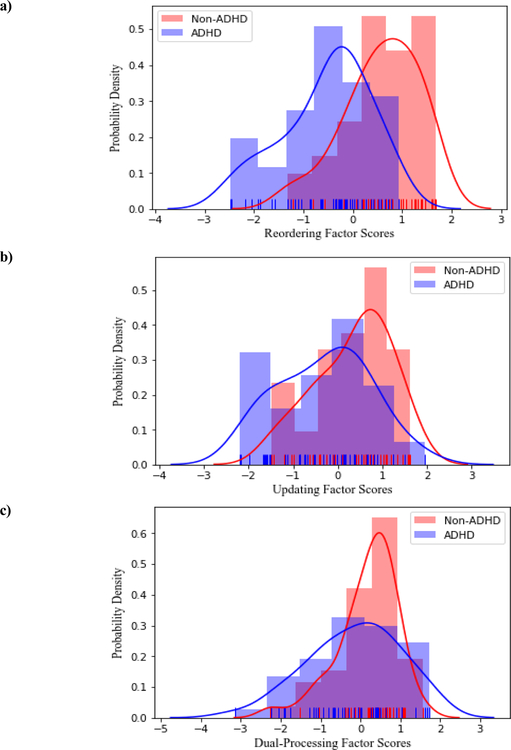
Figure 1.
Distributions of standardized scores for working memory a) reordering, b) updating, and c) dual-processing. The rugs (tick marks) represent performance for each individual child.
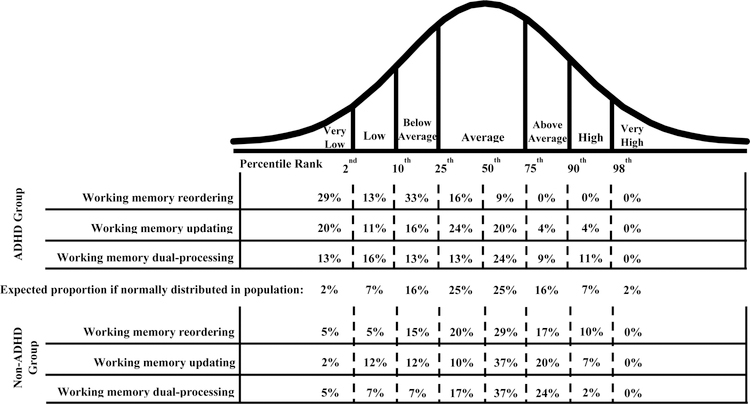
Figure 2.
Descriptive figure of the category in which children with and without ADHD performed based on qualitative descriptions from standardized cognitive assessments. Participants performing at or below the 10th percentile (corresponding to the Very Low and Low categories) were considered Impaired in that particular working memory domain. Note that percentages do not always add to 100 due to rounding.
Data Analytic Plan
Task variables were examined for outliers (> 3 SDs) prior to factor score reduction (described above), but none were observed. The current study utilized Bayesian analyses to test study hypotheses. Bayesian methods can provide estimates of the magnitude of support for both the alternative and null hypotheses simultaneously (Rouder & Morey, 2012; Wagenmakers et al., 2016), making it advantageous for evaluating equivalence across groups, in addition to the typical evaluation of group differences. Instead of a p-value, these analyses provide BF10, which is the Bayes Factor of the alternative hypothesis (H1) against the null hypothesis (H0). BF10 is an odds ratio, where values above 3.0 are considered moderate evidence supporting the alternative hypothesis (i.e., statistically significant evidence for the alternative hypothesis). BF10 values above 10.0 are considered strong (>30 = very strong, >100 = decisive/extreme support; Wagenmakers et al., 2016). Conversely, BF01 is the Bayes Factor of the null hypothesis (H0) against the alternative hypothesis (H1). BF01 is the inverse of BF10 (i.e., BF01 = 1/BF10), and is reported when the evidence indicates a lack of an effect (i.e., favors the null hypothesis; Rouder & Morey, 2012). BF01 values are interpreted identically to BF10. Inferential tests are supplemented with Cohen’s d effect sizes.
Diagnostic group differences for each working memory subcomponent were evaluated with three (one for each domain) Bayesian t-tests conducted in JASP 0.9.2. Bayesian chi-square tests then evaluated whether children in the ADHD group were more likely to be classified as impaired than children in the Non-ADHD group. Sensitivity analyses were conducted using a direct oblimin oblique rotation, which permits working memory components to covary, as well as specifying a 1-component solution to explore the extent to which model specification impacted results.
Follow-up analyses compared impaired and non-impaired groups on demographic variables and ADHD symptom severity. Impaired and non-impaired group differences in ADHD symptom severity (based on the BASC) were evaluated using a series of 2 WM Impairment (present versus absent) x 2 Informant (parent versus teacher) x 2 Symptom Domain (inattention versus hyperactivity/impulsivity) Bayesian mixed-model ANOVAs. Main effects were corrected for multiple testing by fixing to 0.5 the prior probability that the null hypothesis holds across all comparisons (JASP Team 2017; Westfall, Johnson, & Utts, 1997). Separate models were run for each of the three central executive components.
Results
Preliminary Analyses
There were no differences between the ADHD and Non-ADHD groups in terms of age (BF01= 1.58), socioeconomic status (BF10= 1.73), and ethnicity (BF01= 2.48), and the groups were equivalent in terms of sex (BF01= 3.50; Table 1). Children in the Non-ADHD group had slightly higher IQ than those in the ADHD group (BF10= 4.88); IQ was not included as a covariate based on compelling statistical, methodological, and conceptual rationale against covarying IQ when investigating cognitive processes in ADHD (Dennis et al., 2009). Task data from subsets of the current battery have been reported for a subset of the current sample to examine conceptually distinct hypotheses (Kofler et al., 2017; 2018; 2019). Data for the study’s primary outcomes (composite, multi-task estimates of working memory reordering, updating, and dual-processing) have not been reported previously.
As shown in Supplemental Table 1, most but not all of the working memory task variables loaded primarily on their hypothesized components. Interestingly, the keep track task appears to primarily require working memory reordering and dual-processing, whereas the counting span task showed a stronger association with updating than dual-processing, and the n-back task cross-loaded with all three working memory components. Overall, each component was comprised primarily of their pre-specified tasks (Miyake et al., 2000; Wager & Smith, 2003); therefore, we retained the reordering, updating, and dual-processing terminology throughout the results below.
Diagnostic Group Differences
Consistent with hypotheses and as shown in Figures 1 and 2, as a group, children with ADHD demonstrated significant impairments in working memory reordering (BF10= 4.64 x 105; d= 1.34) and working memory updating (BF10= 9.49; d= 0.64). There was no evidence to suggest that children with ADHD had deficits in the dual-processing working memory domain (BF01= 1.33; d= 0.37)2.
Central Executive Impairments in ADHD
Across the three working memory subcomponents, 67% (n=30) of youth with ADHD demonstrated impairments in at least one domain, with 36% (n=16) impaired in a single domain, 27% (n=12) impaired in two domains, and two participants (4%) impaired in all three working memory domains. Children with ADHD were significantly more likely to exhibit impairment in working memory reordering than children without ADHD (BF10= 108.40). Evidence for diagnostic group differences in impairment rates for working memory updating (BF10= 1.51) and dual-processing (BF01= 1.85) was inconclusive. Interestingly, 27% of children with ADHD demonstrated a strength in one or more working memory domains, defined as above average or better performance relative to the Non-ADHD distribution (see Figures 1 and 2). Patterns of strengths and impairments for each domain are summarized below.
Working memory reordering.
As shown in Figure 2, 42% of children with ADHD exhibited impairment in working memory reordering, and an additional 33% fell within the below average range. The remaining 25% demonstrated average working memory reordering. None of the children with ADHD showed above average or better working memory reordering relative to the Non-ADHD comparison group. The distribution of reordering abilities was significantly different between children with and without ADHD (BF10= 9.62 x 103), such that children with ADHD were significantly more likely to perform in the very low range (BF10= 27.48), and significantly less likely than their Non-ADHD peers to perform in the average (BF10= 4.12) or better ranges (BF10= 32.49 and 4.40 for above average and high, respectively).
Working memory updating.
As shown in Figure 2, 31% of children with ADHD were impaired on working memory updating, with an additional 16% falling within the below average range. The remaining 52% showed average (44%) or better (8%) working memory updating. The distribution of updating abilities did not significantly differ between children with versus without ADHD (BF10 = 2.05).
Working memory dual-processing.
For working memory dual-processing, 29% of children with ADHD were impaired, and an additional 13% fell within the below average range. The remaining 57% showed average (37%) or better (20%) dual-processing abilities. There were no detectable differences in the distribution of dual-processing abilities for children with vs. without ADHD (BF10 = 1.47).
Profiles of Impaired and Non-Impaired Children
Generally, there was no evidence that impaired and non-impaired groups differed with respect to sex, medication status, age, IQ, or socioeconomic status (all BF10 < 3), with two exceptions. Girls were more likely than boys to be classified as impaired on tasks of working memory reordering (BF10 = 15.87), and children with impaired updating performance were younger than children with intact updating performance (BF10 = 7.63).
Results of the 2 Impairment (yes/no) x 2 Informant (parent versus teacher) x 2 Symptom Domain (inattention versus hyperactivity/impulsivity) Bayesian mixed-model ANOVAs revealed that parents’ symptom ratings were significantly higher/more severe than teacher ratings (BF10 = 375.00 – 384.60), and that inattention and hyperactivity/impulsivity ratings were equivalent (BF01 = 6.00 – 6.25) across all three models (i.e., a separate model was run for each working memory domain). There was no evidence that impaired versus unimpaired groups differed in ADHD symptom severity for working memory reordering (BF01 = 1.78) or updating (BF01 = 1.64). On dual-processing tasks, there was significant evidence against symptom severity differences between the impaired and non-impaired groups (BF01 = 3.27). Given the incongruence between these results and those obtained in previous studies, we conducted exploratory analyses using the 1-factor component score as we have done previously (Kofler et al., 2019). Consistent with previous reports, results indicated significantly higher ADHD symptoms in the Impaired versus Non-impaired group (BF10= 3.65). In other words, ADHD symptom severity appears to covary as a function of domain-general (shared) central executive impairment rather than as a function of process-specific impairments.
Sensitivity Analyses
Sensitivity analyses first evaluated the extent to which our decision to specify an orthogonal rotation impacted results. Overall, the pattern of results with a 3-component oblique solution (which permits covariation among the working memory components) were highly consistent with those obtained when specifying an orthogonal rotation, though the oblique solution suggested overall greater working memory difficulties in youth with ADHD. Specifically, the between-group effect sizes were larger by approximately d=0.30 for reordering and dual-processing when process overlap was not minimized (Cohen’s ds = 1.67, 0.62, and 0.69 for reordering, updating, and dual-processing, respectively). Among youth with ADHD, 71% demonstrated impaired working memory, with 18% (n=8) impaired in one domain, 38% (n=17) impaired in two domains, and 16% (n=7) impaired in all three domains.
Finally, we tested a 1-component solution. Results were highly consistent with the 3-component solutions. A large between-group effect size was observed (d = 1.67), and 62% (n=28) of youth with ADHD were considered impaired in working memory abilities.
Discussion
Although our understanding of neurocognitive deficits in youth with ADHD has improved markedly in recent decades, substantial questions persist regarding the nature of these deficits and their contribution to core areas of functioning in youth with the disorder. Demonstrable differences in working memory abilities are consistently observed in youth with ADHD (Kasper et al., 2012; Martinussen et al., 2005). However, failure to appreciate the well-documented heterogeneity of cognitive dysfunction (Fair et al., 2012; Nigg et al., 2005), as well as the extensive use of tasks designed to detect global neuropsychological dysfunction rather than characterize the full range of children’s abilities in working memory and its component processes (Snyder et al., 2015), has hindered efforts to comprehensively characterize cognition in this population (Coghill et al., 2014). The present study is the first to address these limitations by comprehensively examining central executive working memory functioning in youth with ADHD by evaluating the extent to which the magnitude of diagnostic group differences and prevalence of impairment differed across the central executive subprocesses of reordering, updating, and dual-processing.
Similar to previous studies evaluating heterogeneity across different cognitive functions (Kofler et al., 2019; Nigg et al., 2005), we found that, among ADHD youth with any working memory impairment, the majority (36%) were impaired in one central executive subprocess, with fewer demonstrating impairment in two (27%) or three (4%) domains. These impairment estimates provide evidence that children with ADHD not only exhibit heterogeneity across cognitive processes but also exhibit heterogeneity within subcomponents of key cognitive processes. For working memory specifically, these results suggest that use of the broad term ‘working memory’ when describing any short-term memory task, regardless of its storage and central executive demands, may lead to wide variability in estimates of diagnostic group differences and impairment rates (Kasper et al., 2012). To improve clarity moving forward, we urge researchers to use multiple tasks per working memory component and state specifically which component(s) of working memory their tests are intended to assess.
Overall, 67%–71% of youth with ADHD were impaired in at least one of the three central executive domains. This overall estimate falls in between the 62% (Kofler et al., 2019) and 85% (Karalunas et al., 2017) estimates reported in recent studies using latent/component methodologies, but below the 81%–84% estimates via meta-regression using group-level performance differences (Kasper et al., 2012). Taken together, there appears to be convergence among more recent studies suggesting that working memory deficits in children with ADHD are significantly more prevalent than previously estimated, with cross-study estimates increasing from approximately one-third in earlier studies (Coghill et al., 2014; Wahlstedt et al., 2009) to between two-thirds and three-fourths in recent studies (current study; Karalunas et al., 2017; Kofler et al., 2019). A key difference between studies reporting lower vs. higher impairment estimates is the use of observed variables in studies with lower impairment rates and the use of latent or component scores in studies with higher impairment estimates. This pattern may suggest that including non-construct-related variance by using observed indicators may create noise that obfuscates the degree of functioning in the central process of interest (DiStefano et al., 2009). Interestingly, the current estimates are somewhat lower than the 81%–84% (Kasper et al., 2012) to 85% (Karalunas et al., 2017) of children identified as impaired in working memory in previous meta-analytic and longitudinal studies. An important consideration in comparing the current results with both previous studies is participant age range. That is, although 85% of children with ADHD were classified as impaired at age 7 by Karalunas and colleagues (2017), 30% of these children showed an upward trajectory over time and no longer demonstrated impaired working memory by age 13; the current study’s participants were slightly older (8–13 years old), and our estimates of impairment likely would have been higher had we included a younger age group or oversampled the younger end of our age distribution. This hypothesis is consistent with meta-regression findings, which included age as a significant moderator that increased effect sizes, and thus population non-overlap estimates used to estimate impairment rates (Kasper et al., 2012).
While our finding that more than two-thirds of youth with ADHD demonstrate impairment in at least one central executive subcomponent was perhaps unsurprising, of critical interest in the current study was the extent to which specific domains of central executive impairment are implicated in the disorder and the extent to which this finding adds to our growing understanding of heterogeneity in neurocognitive dysfunction in ADHD. Across central executive domains, impairments in central executive processes that support the ability to rearrange information within short-term memory (i.e., reordering) were the most robust, with 75% of youth with ADHD exhibiting below average or impaired serial/temporal reordering abilities. In contrast, over half (52%–57%) of children with ADHD demonstrated average or better performance in updating and dual-processing. This pattern is also evident from the distributions of scores in the ADHD group (see Figure 2). Children in the ADHD group were more likely to exhibit reordering performance that fell at the low end of the distribution, whereas the non-ADHD group’s reordering scores were concentrated in the average and above average ranges. In contrast, the distributions of updating and dual-processing abilities for the ADHD and non-ADHD groups overlapped to a greater degree. Assuming that a researcher’s goal is not to evaluate updating or dual-processing specifically, these results suggest that employing tasks that place heavy demands on reordering may be particularly important for evaluation of ADHD-related impairments in working memory. However, inclusion of a multi-component battery is likely to remain ideal for comprehensive evaluation of working memory abilities in this population, particularly given the significantly higher estimates of impairment obtained across all central executive domains (67%) relative to our reordering-specific estimates (42%).
Despite the nearly exclusive focus on cognitive deficits in the literature, there is an increasing interest in identifying resilience factors that may mitigate the impact of ADHD on functioning (Dvorsky & Langberg, 2016). To date, few person-level characteristics have been investigated as potential resilience or protective factors (see Dvorsky & Langberg, 2016 for a review). Given the established association between working memory and functional outcomes in ADHD (e.g., Kofler et al., 2017; Simone et al., 2018), it seems reasonable to hypothesize that intact abilities in at least some working memory processes may be a plausible child-level protective factor that may promote resiliency, even in the context of an ADHD diagnosis. In the current study, over half of youth with ADHD exhibited average or better performance on at least one central executive working memory subprocess, and some even showed strengths in updating (8%) and dual-processing (20%). Though these results warrant replication with larger sample sizes that capture the full range of performance from weaknesses to strengths, Karalunas et al. (2017) also identified a small group of children with ADHD who had better working memory performance than their typically-developing peers (~15%), suggesting preliminary evidence that this finding replicates across studies and may therefore be theoretically important. An important avenue for future work will be to evaluate whether exhibiting average or above average neurocognitive abilities does in fact attenuate the risk for poor outcomes. A more thorough understanding of equifinality, as well as risk and protective factors, will require evaluation of how deficits in some areas and strengths in others may interact to impact the expression of the disorder, persistence and remission, and/or clinical course in key domains of functioning. Finally, current treatments designed to improve ADHD outcomes via working memory training have been largely unsuccessful (Melby-Lervag & Hulme, 2013; Rapport et al., 2013). The sizable minority of ADHD youth with average or better abilities in at least one central executive process indicates that the training may be targeting a non-impaired process for a subset of children, which might obfuscate or attenuate treatment effects for children with greater working memory impairments.
Given the increasing recognition of heterogeneity as a meaningful clinical phenomenon, identifying demographic and clinical characteristics that are associated with impairment will further refine our understanding of within- and between-group heterogeneity. To this end, we found that impaired and non-impaired groups of central executive functioning did not differ across key demographic variables (e.g., medication status, age, medication status, or socioeconomic status), with two exceptions. Girls were more likely than boys to exhibit impaired reordering abilities, and, not surprisingly, younger children were at greater risk of being classified as impaired in updating. While the sample size in this study prevented further examination of these relationships, studies examining neurocognitive functioning in females with ADHD are scarce (Miller & Hinshaw, 2010), highlighting the need for substantial work to better understand demographic factors impacting our understanding of cognitive functioning in ADHD.
Results from our preregistered analytic plan showed that impaired and non-impaired children in each central executive component did not differ in ADHD symptom severity. Interestingly, however, our exploratory analyses showed strong evidence linking variation in ADHD symptom severity with overall central executive working memory impairments (i.e., impairment on the one-factor component). These later findings are consistent with previous work linking working memory abilities and ADHD symptoms (Halperin et al., 2008; Karalunas et al., 2017; Kofler et al., 2019) and extend this line of inquiry by honing in on the specific aspects of working memory that portend inattentive and hyperactive/impulsive behavior. That is, the current findings suggest that children’s ADHD symptoms are linked specifically with domain-general (shared) central executive impairment rather than process-specific impairments. These findings provide an important avenue for future research and suggest that underdevelopment and/or hypoactivity of frontal/parietal regions commonly activated by all three central executive components, rather than process-specific cortical activation, may drive children’s inattentive and hyperactive/impulsive behavioral symptoms (Shaw et al., 2008; Wager & Smith, 2003).
This pattern of results could also represent equifinality; that is, behavioral symptoms may arise from dysfunction in different central executive subcomponents for different children, so associations between working memory and symptoms may be obfuscated when each domain is tested separately. Finally, the current study utilized the three-domain central executive framework of Wager and Smith (2003), as it was best-suited for the current study’s aims of evaluating central executive heterogeneity. Alternate conceptualizations of working memory, particularly the maintenance component of working memory, have been proposed (e.g., D’Esposito & Postle, 2015; Nyberg & Eriksson, 2016), and adopting one of these alternative frameworks in future investigations may help isolate links between ADHD symptoms and specific aspects of working memory. For practical purposes, these findings highlight the need for working memory assessment batteries that use multiple tasks to assess multiple central executive subcomponents of working memory and dimension reduction methods that extract domain-general and domain-specific variance to further evaluate how heterogeneity in central executive functioning relates to individual differences in key functional domains implicated in ADHD.
Limitations
Given the novelty of these data, we look forward to replication studies that address limitations of the current study. The use of an arbitrary cut-point to define impairment is not ideal, even though at least one previous study found no meaningful differences in impairment estimates through the use of an arbitrary cut-point versus objectively-defined impairment (Kofler et al., 2019). Relatedly, children with ADHD were classified as “impaired” based on their performance relative to a group without ADHD, but this provides no information regarding the extent to which central executive impairments impact functioning in their daily lives. Being able to link impairment domain with real-world functioning will provide key insights into the types of accommodations a child may need to reduce the functional impairment associated with working memory difficulties. The use of a non-ADHD control group comprised of both healthy and clinical controls was ideal for the current study’s goal of isolating ADHD-specific working memory impairments, but it precludes any inferences regarding the presence of working memory deficits (or strengths) in disorders other than ADHD. Though the healthy control and clinical control groups did not differ in working memory abilities in the present study, future studies that include larger samples of both control groups will be well-suited to evaluate heterogeneity in working memory abilities in other disorders for which neurocognitive impairment is thought to play a central role.
Task impurity and construct overlap is a well-documented issue in the measurement of cognitive processes (Conway et al., 2005; Snyder et al., 2015). Although the ADHD/working memory literature frequently refers to individual tasks as measuring a specific central executive domain, we found that some of the tasks loaded on multiple factors, resulting in slightly reduced clarity when describing performance on tasks involving reordering, updating, or dual-processing. This pattern highlights the need for broad assessment of a cognitive domain with multiple indicators per domain. For example, inhibitory control can be fractionated into subprocesses involving action restraint, action cancellation, and action postponing (Bari & Robbins, 2013). Future work that incorporates indicators of these three subcomponents will clarify ADHD-related inhibitory control difficulties by evaluating the extent to which performance and impairment are similar across these subcomponents of inhibitory control.
Research and Clinical Implications
The current study provides a critical, nuanced examination of central executive working memory and its component processes in youth with ADHD. Overall, the findings indicate that approximately 67%–71% of children with ADHD have impaired working memory in at least one domain. Impairments in working memory reordering were particularly pronounced (75% demonstrating below average or lower abilities; d=1.34–1.67), whereas most children with ADHD demonstrated average or better updating and dual-processing abilities. At the same time, it was domain-general central executive abilities that predicted ADHD symptom severity, suggesting that mechanisms shared across central executive subcomponents may be particularly important for understanding working memory’s relation to behavior and functioning. Future studies are needed to probe the extent to which each working memory subdomain is linked with ADHD-related impairments in academic, peer, family, and other important areas of functioning (Pelham, Fabiano, & Massetti, 2005), as well as expand on the evaluation of cognitive heterogeneity in ADHD to include heterogeneity within subprocesses of other key domains (e.g., inhibitory control). Studies of this type will further refine cognitive models of ADHD and improve our understanding of the equifinal nature of the disorder.
Supplementary Material
Acknowledgements:
This work was supported in part by National Institutes of Health grants (R34 MH102499–01, R01 MH115048; PI: Kofler). During the preparation of the manuscript, JSR was supported in part by grants from NIH (R21 MH112002), National Science Foundation (CNS1532061), and The Children’s Trust (#1914–7561). The sponsors had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest: The authors have no conflicts of interest to report.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional review board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent and assent were obtained from all individual participants included in the study.
Though the current project focuses on the central executive demands of a task, the term ‘working memory’ is maintained in task descriptions because all tasks require both short-term storage and central executive processes.
Children in the healthy control group did not exhibit significantly different performance than the clinical control group in any working memory domain (BF10= 0.32–2.54).
References
- Baddeley A. (2003). Working memory: looking back and looking forward. Nature Reviews Neuroscience, 4, 829–839. [DOI] [PubMed] [Google Scholar]
- Baddeley A. (2012). Working memory: Theories, models, and controversies. Annual Review of Psychology, 63, 1–29. [DOI] [PubMed] [Google Scholar]
- Bari A, & Robbins TW. (2013). Inhibition and impulsivity: behavioral and neural basis of response control. Progress in Neurobiology, 108, 44–79. [DOI] [PubMed] [Google Scholar]
- Coghill DR, Seth S, & Matthews K. (2014). A comprehensive assessment of memory, delay aversion, timing, inhibition, decision making and variability in attention deficit hyperactivity disorder: Advancing beyond the three-pathway models. Psychological Medicine, 44, 1989–2001. [DOI] [PubMed] [Google Scholar]
- Conway AR, Kane MJ, Bunting MF, Hambrick DZ, Wilhelm O, & Engle RW. (2005). Working memory span tasks: A methodological review and user’s guide. Psychonomic Bulletin & Review, 12, 769–786. [DOI] [PubMed] [Google Scholar]
- Denney CB, Rapport MD, & Chung K. (2005). Interactions of task and subject variables among continuous performance tests. Journal of Child Psychology and Psychiatry, 46, 420–435. [DOI] [PubMed] [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, & Fletcher JM. (2009). Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society, 15, 331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M, & Postle BR. (2015). The cognitive neuroscience of working memory. Annual Review of Psychology, 66, 115–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiStefano C, Zhu M, & Mindrila D. (2009). Understanding and using factor scores: Considerations for the applied researcher. Practical Assessment, Research & Evaluation, 14, 1–11. [Google Scholar]
- Dvorsky MR, & Langberg JM. (2016). A review of factors that promote resilience in youth with ADHD and ADHD symptoms. Clinical Child and Family Psychology Review, 19, 368–391. [DOI] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, & Reid R. (2016). ADHD rating scale- 5 for children and adolescents: checklists, norms, and clinical interpretation. NY: Guilford Press. [Google Scholar]
- Engle RW, Tuholski SW, Laughlin JE, & Conway AR. (1999). Working memory, short-term memory, and general fluid intelligence: A latent-variable approach. Journal of Experimental Psychology: General, 128, 309. [DOI] [PubMed] [Google Scholar]
- Fair DA, Bathula D, Nikolas MA, & Nigg JT. (2012). Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with ADHD. Proceedings of the National Academy of Sciences, 109, 6769–6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feczko E, Miranda-Dominguez O, Marr M, Graham AM, Nigg JT, & Fair DA. (2019). The heterogeneity problem: Approaches to identify psychiatric subtypes. Trends in Cognitive Sciences. Advance online publication. doi: 10.1016/j.tics.2019.03.009 [DOI] [PMC free article] [PubMed]
- Friedman LM, Rapport MD, Raiker JS, Orban SA, & Eckrich SJ. (2017). Reading comprehension in boys with ADHD: The mediating roles of working memory and orthographic conversion. Journal of Abnormal Child Psychology, 45, 273–287. [DOI] [PubMed] [Google Scholar]
- Halperin JM, Trampush JW, Miller CJ, Marks DJ, & Newcorn JH. (2008). Neuropsychological outcome in adolescents/young adults with childhood ADHD: profiles of persisters, remitters and controls. Journal of Child Psychology and Psychiatry, 49, 958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawk LW Jr., Fosco WD, Colder CR, Waxmonsky JG, Pelham WE Jr., & Rosch KS. (2018). How do stimulant treatments for ADHD work? Evidence for mediation by improved cognition. Journal of Child Psychology and Psychiatry, 59, 1271–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson E, Bavin E, Efron D, & Sciberras E. (2012). A comparison of working memory profiles in school-aged children with specific language impairment, attention deficit/hyperactivity disorder, comorbid SLI and ADHD and their typically developing peers. Child Neuropsychology, 18, 190–207. [DOI] [PubMed] [Google Scholar]
- Jacobson NS, & Truax P. (1991). Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. Journal of Consulting and Clinical Psychology, 59, 12–19. [DOI] [PubMed] [Google Scholar]
- JASP Team (2017). JASP. Version 0.8.5. [Computer Software].
- Karalunas SL, Gustafsson HC, Dieckmann NF, Tipsord J, Mitchell SH, & Nigg JT. (2017). Heterogeneity in development of aspects of working memory predicts longitudinal attention deficit hyperactivity disorder symptom change. Journal of Abnormal Psychology, 126, 774–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper LJ, Alderson RM, & Hudec KL. (2012). Moderators of working memory deficits in children with attention-deficit/hyperactivity disorder (ADHD): a meta-analytic review. Clinical Psychology Review, 32, 605–617. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U…Ryan N. (1997). K-SADS-PL: Initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry, 36, 980–988. [DOI] [PubMed] [Google Scholar]
- Kaufman AS & Kaufman NL. (2014). Technical and Interpretive Manual: Kaufman Test of Educational Achievement (3rd ed.). Bloomington, MN: Pearson. [Google Scholar]
- Kofler MJ, Irwin LN, Soto EF, Groves NB, Harmon SL, & Sarver DE. (2019). Executive functioning heterogeneity in pediatric ADHD. Journal of Abnormal Child Psychology, 47, 273–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler MJ, Rapport MD, Bolden J, Sarver DE, & Raiker JS. (2010). ADHD and working memory: The impact of central executive deficits and exceeding storage/rehearsal capacity on observed inattentive behavior. Journal of Abnormal Child Psychology, 38, 149–161. [DOI] [PubMed] [Google Scholar]
- Kofler MJ, Sarver DE, Spiegel JA, Day TN, Harmon SL, & Wells EL. (2017). Heterogeneity in ADHD: Neurocognitive predictors of peer, family, and academic functioning. Child Neuropsychology, 23, 733–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler MJ, Spiegel JA, Austin KE, Irwin LN, Soto EF, & Sarver DE. (2018). Are episodic buffer processes intact in ADHD? Experimental evidence and linkage with hyperactive behavior. Journal of Abnormal Child Psychology, 46, 1171–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, & Rounsaville BJ. (1992). Sensitivity of psychiatric diagnosis based on the best estimate procedure. The American Journal of Psychiatry, 149, 1225–1227. [DOI] [PubMed] [Google Scholar]
- Larson K, Russ SA, Kahn RS, & Halfon N. (2011). Patterns of comorbidity, functioning, and service use for US children with ADHD, 2007. Pediatrics, 127, 462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinussen R, Hayden J, Hogg-Johnson S, & Tannock R. (2005). A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 44, 377–384. [DOI] [PubMed] [Google Scholar]
- McQuade JD, Murray-Close D, Shoulberg EK, & Hoza B. (2013). Working memory and social functioning in children. Journal of Experimental Child Psychology, 115, 422–435. [DOI] [PubMed] [Google Scholar]
- Melby-Lervåg M, & Hulme C. (2013). Is working memory training effective? A meta-analytic review. Developmental Psychology, 49, 270. [DOI] [PubMed] [Google Scholar]
- Miller M, & Hinshaw SP. (2010). Does childhood executive function predict adolescent functional outcomes in girls with ADHD? Journal of Abnormal Child Psychology, 38, 315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, & Wager TD. (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology, 41, 43–100. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Willcutt EG, Doyle AE, & Sonuga-Barke ES. (2005). Causal heterogeneity in Attention-Deficit/ Hyperactivity Disorder: Do we need neuropsychologically impaired subtypes?. Biological Psychiatry, 57, 1224–1230. [DOI] [PubMed] [Google Scholar]
- Nyberg L, & Eriksson J. (2016). Working memory: maintenance, updating, and the realization of intentions. Cold Spring Harbor Perspectives in Biology, 8, a021816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham WE Jr., Fabiano GA, & Massetti GM. (2005). Evidence-based assessment of ADHD in children and adolescents. Journal of Clinical Child and Adolescent Psychology, 34, 449–476 [DOI] [PubMed] [Google Scholar]
- Rapport MD, Alderson RM, Kofler MJ, Sarver DE, Bolden J, & Sims V. (2008). Working memory deficits in boys with Attention-deficit/Hyperactivity Disorder: The contribution of central executive and subsystem processes. Journal of Abnormal Child Psychology, 36, 825–837. [DOI] [PubMed] [Google Scholar]
- Rapport MD, Bolden J, Kofler MJ, Sarver DE, Raiker JS, Alderson RM. (2009). Hyperactivity in boys with Attention-deficit/Hyperactivity Disorder: A ubiquitous core symptom or manifestation of working memory deficits? Journal of Abnormal Child Psychology, 37, 521–534. [DOI] [PubMed] [Google Scholar]
- Rapport MD, Orban SA, Kofler MJ, & Friedman LM. (2013). Do programs designed to train working memory, other executive functions, and attention benefit children with ADHD? A meta-analytic review of cognitive, academic, and behavioral outcomes. Clinical Psychology Review, 33, 1237–1252. [DOI] [PubMed] [Google Scholar]
- Reynolds CR, & Kamphaus RW. (2004). BASC-2 Behavior Assessment for Children Manual. Circle Pines, MN: American Guidance Service. [Google Scholar]
- Reynolds CR, & Kamphaus RW. (2015). Behavior assessment system for children–Third Edition (BASC-3). Bloomington, MN: Pearson. [Google Scholar]
- Rottschy C, Langner R, Dogan I, Reetz K, Laird AR, Schulz JB, … & Eickhoff SB. (2012). Modelling neural correlates of working memory: a coordinate-based meta-analysis. Neuroimage, 60, 830–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouder JN, & Morey RD. (2012). Default Bayes factors for model selection in regression. Multivariate Behavioral Research, 47, 877–903. [DOI] [PubMed] [Google Scholar]
- Sarver DE, Rapport MD, Kofler MJ, Raiker JS, & Friedman LM. (2015). Hyperactivity in attention-deficit/hyperactivity disorder (ADHD): Impairing deficit or compensatory behavior?. Journal of Abnormal Child Psychology, 43, 1219–1232. [DOI] [PubMed] [Google Scholar]
- Sattler JM, Dumont R, & Coalson DL. (2016). Assessment of children: WISC-V and WPPSI-IV. San Diego, CA: Jerome M. Sattler, Publisher. [Google Scholar]
- Schmiedek F, Lövdén M, & Lindenberger U. (2014). A task is a task is a task: Putting complex span, n-back, and other working memory indicators in psychometric context. Frontiers in Psychology, 5, 1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, … Wise SP. (2008). Neurodevelopmental trajectories of the human cerebral cortex. Journal of Neuroscience, 28, 3586–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone AN, Marks DJ, Bédard AC, & Halperin JM. (2018). Low working memory rather than ADHD symptoms predicts poor academic achievement in school-aged children. Journal of Abnormal Child Psychology, 46, 277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, Miyake A, & Hankin BL. (2015). Advancing understanding of executive function impairments and psychopathology: Bridging the gap between clinical and cognitive approaches. Frontiers in Psychology, 6, 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsworth N, & Engle RW. (2007). On the division of short-term and working memory: An examination of simple and complex span and their relation to higher order abilities. Psychological Bulletin, 133, 1038. [DOI] [PubMed] [Google Scholar]
- Wager TD, & Smith EE. (2003). Neuroimaging studies of working memory. Cognitive, Affective, & Behavioral Neuroscience, 3, 255–274. [DOI] [PubMed] [Google Scholar]
- Wagenmakers EJ, Marsman M, Jamil T, Ly A, Verhagen AJ, Love J, & Morey RD. (2016). Bayesian statistical inference for psychological science. Part I: Theoretical advantages and practical ramifications. Psychonomic Bulletin & Review, 25, 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wåhlstedt C, Thorell LB, & Bohlin G. (2009). Heterogeneity in ADHD: Neuropsychological pathways, comorbidity and symptom domains. Journal of Abnormal Child Psychology, 37, 551–564. [DOI] [PubMed] [Google Scholar]
- Wechsler D. (2014). WISC-V: Technical and interpretive manual. NCS Pearson, Incorporated. [Google Scholar]
- Wells EL, Kofler MJ, Soto EF, Schaefer HS, & Sarver DE. (2018). Assessing working memory in children with ADHD: Minor administration and scoring changes may improve digit span backward’s construct validity. Research in Developmental Disabilities, 72, 166–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall PH, Johnson WO, & Utts JM. (1997). A Bayesian perspective on the Bonferroni adjustment. Biometrika, 84, 419–427. [Google Scholar]
- Wilens TE, Biederman J, Brown S, Tanguay S, Monuteaux MC, Blake C, & Spencer TJ. (2002). Psychiatric comorbidity and functioning in clinically referred preschool children and school-age youths with ADHD. Journal of the American Academy of Child & Adolescent Psychiatry, 41, 262–268. [DOI] [PubMed] [Google Scholar]
- Willoughby MT, & Blair CB. (2016). Measuring executive function in early childhood: A case for formative measurement. Psychological Assessment, 28, 319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.