Abstract
The craving for multiphase materials with adjustable properties for mammalian cell encapsulation persists despite intensive research on 3D cell culture and tissue engineering. This interest is incited by the complex interaction between cells and different materials, various manufacturing methods, cell chip applications, and the aspiration to abolish animal experiments. This study aims to show the feasibility of preparing a stable multiphase material for prolonged mammalian cell embedment and 3D cell culture. The material comprises silica as the solid phase, cell culture medium with serum as the main liquid phase and air as the gas phase. The silica sol-cell culture medium-serum mixture was foamed, and it turned into a stable foamed hydrogel. The stability, flow properties and foaming parameters were studied by rheological and surface tension measurements. The viability of embedded cells was studied by measuring the metabolic activity at different time points. Their sensitivity to the surrounding conditions was compared to cells grown in monolayers by exposing them to a toxic compound. A stable foamed hydrogel with cell culture medium as the main liquid phase was prepared. Based on oscillatory measurements, the foamed hydrogel stays stable for at least 6–7 weeks and the embedded mammalian cells remain viable for the same time period. Appropriate surface tension and viscosity were crucial for an at least twofold volume increase by foaming, which is necessary for the mammalian cells to survive and proliferate. A test with a toxic compound reveals a difference in the sensitivity of cells in monolayer cultures versus embedded cells.
Keywords: Silica, Mammalian cell, Foamed hydrogel, 3D cell culture
Introduction
Mammalian cell embedment, i.e., integration of living cells within a (bio)material (typically porous scaffolds or hydrogels), of synthetic and/or of natural origin, is a widely studied topic within tissue engineering and cell therapy. Other application areas include testing of various biologically active agents in three-dimensional (3D, also 2.5D) cell cultures in vitro (e.g., drug testing, testing of environmental poisons, and general research of molecular and cell biology etc.) (Fernandez-Yague et al. 2015; Liu et al. 2013; Shamir and Ewald 2014; Suna et al. 2006). There is also a growing interest to use 3D matrices in high-throughput screening (HTS) as miniaturized 3D models. Challenges are then to adapt the chemical analysis methods to 3D environment or to develop automated image analysis for phenotypic screening of cells (Härmä et al. 2014).
When the embedment of cells in the biomaterial is used for testing the effect of different chemical compounds in vitro in order to simulate their effect on living organisms, the in vitro model system has to imitate the target organ/tissue to a high degree. Traditional in vitro 2D culture systems without the use of supporting (bio)materials, which are widely used for batch screening of drugs, fail to imitate the physiological and biochemical features of cells in the original tissue (Antoni et al. 2015; Langhans 2018). Differences between the 2D-cell culture models and in vivo tissues cause dissimilarities in cell response and hence 2D-cultures have a poor predictive quality for drug response. For example some tumor properties are more realistically represented in 3D-models as compared to 2D culture models (Lovitt et al. 2014; Pampaloni et al. 2007), including oxygen and nutrient gradients, cell-to-cell interactions, non-uniform exposure of cells within a 3D structure to drug/compound, ECM-to-cell signaling and different rates of cellular proliferation throughout the 3D structure. The development of 3D models, which are more biologically relevant in vitro models may ultimately result in improved translation towards the patient and a reduction in number of the animal models required in drug discovery programs (Hickman et al. 2014; Lovitt et al. 2014; Pampaloni et al. 2007; Edmondson et al. 2014).
Immobilized mammalian and microbial cells are also used as biocatalysts in bioprocesses to produce therapeutic cells, biopharmaceuticals, and vaccines (de Soure et al. 2016; Warnock and Al-Rubeai 2006; Baeshen et al. 2015). The cells are immobilized either in or on microcarriers, or embedded in hydrogels for flow reactors, which resemble scaffolds, hydrogels and reactor systems used in tissue engineering.
One of the benefits of the hydrogels is an easy integration with the cells, because the cells can be added to the system before a sol or a solution turns into a gel. It is also easy to inject cells into the hydrogel, but it is not the best possible way to ensure homogeneous distribution of the cells and substantial cell survival over time is not guaranteed. On the other hand, solid porous scaffolds offer more opportunities with respect to biomechanical properties and surface modification is easier. Nanotechnology has accelerated the development of smart biomaterials and it has offered more options to utilize biomimetic approach in the design of scaffolds for tissue engineering. Adjustable biomechanical properties (e.g., by mimicking tissue structures using composites), surface modification (e.g., to enhance tissue integration and regeneration), biochemical functionalization (e.g., localized delivery of biological agents to guide cell growth) and stimuli responsive biomaterials (e.g., responsive to changing pH in tissue) are some examples that are studied to better match the properties of the scaffolds with the properties and functions of tissue (Fernandez-Yague et al. 2015). Both solid macroporous scaffolds and injectable hydrogels are used to develop new and smart biomimetic materials. They can also be combined, e.g., a porous scaffold can be impregnated with the sol that turns into hydrogel in the pores of the scaffold, and this requires detailed knowledge about the rheology. A hydrogel may contain a biological agent that is released in controlled manner, or the use of hydrogel may also be the method of adding the cells into the porous scaffolds and to enhance the retention of the cells in the scaffold.
The fast development of additive manufacturing methods is a driving force for the new solutions in materials science and cell encapsulation. The 3D printing, and even 4D printing (3D printed or other material with variable properties, e.g., structural changes due to external stimuli) are under a fast development phase, especially from the viewpoint of techniques (Zhu et al. 2016; Gladman et al. 2016), but 3D printed material options for tissue engineering and 3D cell culture are mostly concentrated on organic polymers and their post-processing and there is also need for more detailed knowledge about rheological properties. Different multiphase materials, e.g., dispersions such as composites, suspensions (pastes), gels, and foams containing several types of materials and phases with different properties are common in general material processing, as intermediate products or end products, and they are becoming more common also in 3D printing for tissue engineering. The use of complex dispersions is also increasing due to expanding use and research of paste extruding 3D printers or direct ink writing (Amza et al. 2015; Quan et al. 2015). The setting of the dispersions typically occurs due to coagulation, a sol–gel transfer, or by a corresponding phase transfer mechanism, which emphasizes good knowledge of rheology. It also means that melting or separate methods to support setting are not necessarily needed and the whole process can be conducted at low temperatures.
Silica and silica-based materials (e.g., bioactive glasses) are widely studied biomaterials both in controlled release of drugs and therapeutic agents, and in tissue repair and regeneration. They are mostly used in an amorphous and biodegradable form and they are known to affect the biological processes and regeneration of tissue. Dissolution of bioactive glasses has been studied in detail and it is known that, e.g., Si is an essential element in the formation and calcification of bone tissue (Kortesuo et al. 2002; Kangasniemi et al. 2009; Xynos et al. 2000; Hoppe et al. 2011). Also plain, sol–gel derived silica dissolves in the body fluid conditions and the dissolution rate can be adjusted at the large scale mainly by adjusting the number of OH groups (Viitala et al. 2005a, b).
Silica and silica-based materials also are challenging materials from the viewpoint of mechanical properties, because they are inorganic, ceramic materials, or glasses. They are mostly used in solid form, as macroscopic 3D compacts, but more often as small particles in suspensions with a liquid or in composites with organic polymers as particulate fillers or as fibers with reinforcing or bioactive properties. However, certain multiphase materials, such as foamed silica hydrogels, i.e., stable dispersions of nanoscale particles, gas and liquid phase, provide new options. They are viscoelastic and at the same time it is possible to utilize the beneficial regenerative properties of dissolving silica.
Foamed scaffolds, including sol–gel based, and foam-derived silica-based scaffolds are well known and they have already been developed for tissue engineering (Sepulveda et al. 2002; Jones et al. 2007; Wua et al. 2011; Cabanas-Polo et al. 2016), but the end products are not commonly foams, or foamed (hydro)gels, but rather ceramic materials or composites forming a macroporous scaffold prepared by a process, where the hydrogel formation and/or foaming has been one of the unit processes. In other words, foams are intermediate products to create prefabricated, macroporous ceramic or polymeric or ceramic-polymer composite scaffolds.
As already noted, rheology is one of the main challenges in the development of new complex multiphase materials for 3D printing. In addition, minimally invasive administration of biomaterials, e.g., thin-needle injections in drug delivery, is becoming more common. The rheological requirements of the thin needle injections resemble those of 3D printing. In 3D systems for high-throughput screening, the rheology of the hydrogel does not only affect the cell behavior, e.g., proliferation, but it has also a technical effect, e.g., proper viscosity and predictable gelation time are needed when applying hydrogels into wells (Engel et al. 2015).
The aim of this study is to show that it is possible to prepare stable multiphase silica-based foamed hydrogels (with cell culture medium as the main liquid phase) with controlled rheological properties for prolonged 3D mammalian cell culture. The structural stability and flow properties of the foamed silica hydrogels and the optimal parameters for the sols to be foamed are studied by rheological measurements and injections tests. The feasibility to maintain mammalian cell lines for a prolonged period inside the foamed silica hydrogel is examined by measuring the metabolic activity of the cells at different time points. In addition, the sensitivity to the surrounding conditions of the embedded mammalian cells is compared to that of cells grown in monolayers, by exposing them to a toxic compound.
Materials and methods
Preparation of foamed silica hydrogels
Tetraethyl orthosilicate (TEOS, Sigma-Aldrich, 131903, 99.0% purity 86578) was used to prepare the silica sol. TEOS was added dropwise under mixing into deionized water in molar ratio R70 (R = n(H2O)/n(TEOS)) and the pH was adjusted to pH2 by 1.0 M HCl (VWR) to ensure the hydrolysis of TEOS. After the hydrolysis of TEOS, the ethanol amount was reduced to low and cell-compatible level (< 1 m- % in the silica sol, determined by headspace gas chromatography; the amount is further diluted after addition of cell culture medium and after the foaming) by continuous evaporation (at 60 °C) combined with a continuous addition of water to keep the total volume constant, or alternatively by adding in advance a known (tested in advance) amount of water at once to end up with the same total liquid volume as prior to the addition of the water. The resulting, low-alcohol silica sol was then cooled down, kept 24 h at 4 °C (to ensure the homogeneity of the several separate silica sol batches used) and used on the fifth day after production), after which it was combined with a medium made out of cell culture medium (DMEM, Gibco, 31330-038), Fetal Bovine Serum (FBS, Gibco 10500-056 or Sigma-Aldrich, F9665) and 0.1 M NaOH (Chemlab) in the volume ratios of Silica sol:DMEM (with 10% FBS):NaOH = 18:6:0.45 (medium = DMEM F12 1:1 (Gibco, 31330-038) supplemented with 10% fetal bovine serum (Gibco), 1% penicillin–streptomycin (Gibco) and 1 mL Fungizone (250 µg/mL amphotericin B and 205 µg/mL sodium deoxycholate, Gibco). The liquid in the silica sol and the cell culture medium formed together the final liquid phase in the foamed gel.
After the addition of the medium into the silica sol (which also increased the pH to the cell-compatible level, ca. to pH6.5–7), the mixture was foamed in a beaker by adding air with an Ultra-Turrax T25 (with stator S25N-18G) homogenizer inside a laminar flow hood. Homogenizer is turned on for 5 min at 8000 rpm after which the stator is removed and the foam is left to solidify (the sol turns into a gel within the foamed structure).
Rheological and surface tension measurements
The properties of the silica sol-cell culture medium (DMEM supplemented with FBS and NaOH or only with NaOH) mixture was also studied prior to the gel formation to find out the optimal process parameters for foaming. The mixture properties were monitored as a function time by measuring surface tension with a Force Tensiometer (K6, Krüss) and dynamic viscosity (Anton-Paar, MCR 102) with the rotational test method under constant shear rate (CSR at 10 1/s) at 24 °C and 28 °C near to the point where the mixture turns into a gel. Both 24 °C and 28 °C were studied, because the use Ultra-Turrax homogenizer increases the temperature 3–4 °C during the foaming. Four parallel silica sol- cell culture medium (DMEM supplemented with FBS and NaOH or only with NaOH) mixtures were used both in the surface tension and viscosity measurements.
The foamed silica gel without the cells were used to study the structural stability with a rheometer (Anton-Paar, MCR 102) by oscillatory measurements. The foamed silica gels were incubated in the same medium, which also was used in the preparation of the foamed hydrogels (DMEM supplemented with FBS and NaOH) for 42 days at 37 °C and the medium was changed weekly. The stability of the incubated foamed hydrogels was studied as a function of time, at 7 different time points during the 3 days (1 h, 2 h, 3 h, 4 h, 24 h, 48 h and 72 h), and further after 10, 16, 22, 29, 34 and 42 days of incubation by measuring the rheological properties in the oscillatory mode. The linear viscoelastic region was determined in advance for every time point. At 1–4 h the optimal strain was 0.05%, and after that 1% for all other time points. In the actual follow-up measurements the frequency sweep was conducted at the angular frequency of 0.01–100 rad/s. The damping (loss) factor (G″/G′ = loss modulus/storage modulus) and complex modulus, G* at angular frequency of 1 rad/s (the results were practically identical at 0.25, 1 and 10 rad/s) were used to express the stability of the foamed hydrogels as a function of time. The rheological measurements were conducted at room temperature.
The effect of the presence of cells on the rheological properties was studied separately in a shorter follow-up study for 30 days in the corresponding conditions to determine whether the cells have an influence on the rheological properties. The follow-up was conducted by following the loss and storage modulus at several time points up to 5 days of incubation and then by measuring the moduli after 30 days of incubation.
Injection experiments
The injectability was tested by injecting the foam (as fresh and at the steady state, i.e., when the damping factor was stabilized) from a syringe (Henke Sass Wolf) through a 25G needle (Terumo, diameter 0.5 mm).
Cell maintenance
Human breast cancer cells (MCF-7/GFP Cell Biolabs, Inc. AKR-211) and Human Embryonic Kidney cells (HEK 293S, Courtesy of UGent) were grown in T185 tissue culture flasks in DMEM F12 1:1 (Gibco, 31330-038) supplemented with 10% FBS (Gibco, 10500-056), 1% penicillin–streptomycin (Gibco, 15140-122) and 1 mL Fungizone (250 µg/mL amphotericin B and 205 µg/mL sodium deoxycholate, Gibco, 15290-026). Seeded cell density for MCF-7 was 1.6 × 104 cells/cm2 (2.96 × 106 cells/flask) and for HEK293S, 104 cells/cm2 (1.85 × 106 cells/flask). All cells were incubated for 3 days at 37 °C and in a humidified 5% CO2 atmosphere.
Cells were trypsinized with Trypsin/EDTA 0.05% (Gibco, 25300-054) solution and pooled. Cells were centrifuged (5702R, Eppendorf) for 5 min at 1000 rpm, room temperature and resuspended in 2 mL DMEM growth medium. Cells were counted (50× dilution) with a Moxi Z cell counter (Orflo, S-cassette). Cell suspension of 1.2 × 107 cells/mL was prepared.
3D cell culture and cell viability
The foamed silica hydrogels were prepared in a beaker of 50 mL with the diameter of 4.2 cm and height of 6.0 cm as described above. At least 10 min after the foam was cut into cubes with a scalpel with dimensions of approximately L5 × W5 × H3 mm and placed in a 24-well suspension plate (Greiner, 662102). For the cytotoxicity assays, a biopsy punch (Kai Medical, ref. BP-60F) was used. Silica foams were cylinder shaped, 6 mm in diameter and 6 mm in height. A total amount of 50 µL 1.2 × 107 cells/mL (6 × 105 cells) was administered in at least five consecutive injections per foam cube or cylinder using a 1 mL syringe (Terumo) and 19G needle (Terumo). The 24-well plate was incubated for 20 min at 37 °C and in a humidified 5% CO2 atmosphere. Afterwards, 1 mL cell culture medium (formulation see above) was added to each well. Medium in all wells was refreshed every 2 to 3 days.
Sliced foams without cells and 2D cell monolayers were used as negative controls. Cells were observed using an inverted fluorescence microscope (Motic AE31 with fluorescence unit, MHG-100B). Photos were taken using Moticam Pro 252A camera.
Metabolic activity of embedded cells was measured using a resazurin assay (Prestoblue, Invitrogen, A13262). 112 µL of Prestoblue (10% total volume) was added to each well. After 5 h of incubation, fluorescence was measured with a Victor X2 Multimode Plate Reader (PerkinElmer) with excitation 560 nm and emission 590 nm. The foams with embedded cells were washed (DPBS, Oxoid, BR0014G) and transferred to a new 24-well plate prior to viability measurements to ensure the measurement of cell viability of embedded cells only. Cell viability was determined after 1, 3, 7, 10, 14, 17, 21, 24, 28, 31, and 49 days of incubation. Control wells with 2D monolayer cells were measured up to 31 days of incubation.
Cytotoxicity assay and chemotherapeutic compound testing
Curcumin, obtained from Sigma–Aldrich (79%; C1386), was dissolved in DMSO at a stock concentration of 50 mM and 10 mM, shielded from direct light using aluminum foil and stored at − 20 °C.
MCF-7/GFP cells (5 × 104 cells/foam or 24-well) were grown in foamed silica hydrogels (see 3D cell culture and cell viability) or in a 24-well plate (2D) for 14 days. During this procedure DMEM F12 1:1 with Glutamax was used (Gibco, 31331028) for production of the foam and for cell maintenance. After 9 days, cell viability of the cell injected foams was measured using Prestoblue (Invitrogen, A13262). After 14 days, the foams with cells were placed in a new 24-well plate and curcumin (final concentrations of 7.5/15/30/45/60 µM) or mock solutions (DMSO, triton X-100) were added to 3D and 2D cultures and incubated at 37 °C and 5% CO2 for 72 h. Metabolic activity was measured using Prestoblue (Invitrogen, A13262).
Results
Surface tension and viscosity of silica sol–medium mixtures
Properties of the silica sol-cell culture medium (DMEM supplemented with FBS and NaOH or with NaOH only) mixture were studied in detail in order to find optimal process parameters for foaming, and to find out whether the FBS addition affects foaming. In the practical foaming experiments for the silica sol-DMEM-FBS-NaOH, it was observed that a too short or too long aging time made the foaming difficult, i.e., a homogenous multiphase foam was not formed. Too early start of the foaming (e.g., at 4–5 min after the mixing of the silica sol and the medium mixture) resulted in a short-aged foam, i.e., the gas phase was separated, and the volume increase due to gas phase was minimal. Too late start of the foaming (e.g., at 7–8 min after the mixing of the silica sol and the medium mixture) resulted in the solidification of the material during the foaming and the structure was disintegrated into arbitrary pieces of solidified foam. The foaming was also tested without FBS, in this case only short-age foams (phase separation occurs fast and the volume does not increase as such) were formed, the separation occurred in minutes and the volume increase during the foaming was minimal.
To determine the optimal properties and time slot for the foaming both surface tension and dynamic viscosity were measured as a function of time. The surface tension results for the foaming process are shown in Fig. 1. The starting point of the foaming is 6 min after the addition of DMEM-FBS-NaOH or DMEM-NaOH into the silica sol. The aging of 6 min for the silica sol-DMEM-FBS-NaOH mixture was based on several foaming tests with the same formulation, where the foaming at 6–11 min resulted in a stable foam. If FBS was not used as a supplement, a stable foam could not be formed. The resulting foam collapsed within some minutes and it contained large visible bubbles (that were not observed in formulations containing FBS) that burst very fast.
Fig. 1.
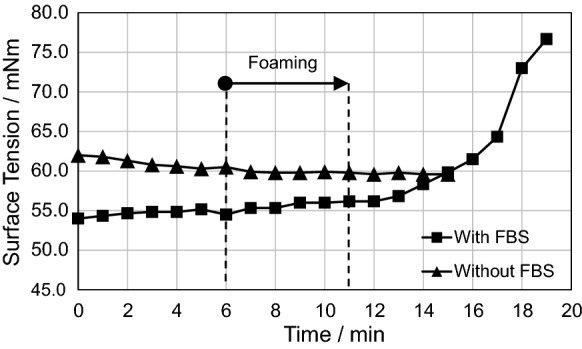
Average Surface tension of 3 parallel samples of silica sol-DMEM-NaOH mixture with and without FBS
At 0–6 min, i.e., during the aging (prior to the foaming) the surface tension of the silica sol-DMEM-FBS-NaOH mixture was quite constant, ca. 55 mN/m and about 10% lower than that of the sol-DMEM-NaOH mixture (60.5–62.0 mN/m). Hence, the presence of FBS lowered the surface tension. Surface tension was also quite constant during the foaming both with and without FBS in the mixture and a steeper increase starts only at ca. 15 min, when the foaming has already been conducted. However, the dynamic viscosity measurements for the silica sol-DMEM-FBS-NaOH mixture (Fig. 2a) show an increase during the foaming, at 6–11 min. The first actual and slow increase of viscosity starts at 2–3 min (to ca. 10 mPas), at 6–10 min the viscosity increases from 10 to 40–50 mPas and steeper increase (from 40–50 to 150–200 mPas) occurs between 10 and 11 min and after that the viscosity increases very fast. The viscosity measurements were conducted both at 24 and 28 °C, because the temperature rises during the foaming, but the difference between the results at the two temperatures was insignificant.
Fig. 2.
a Dynamic viscosity (with the constant shear rate (CSR)) for 4 parallel samples of the silica sol-DMEM-FBS-NaOH mixture after the mixing (0–6 min) and during the foaming (6–11 min at 24–28 °C); b damping factor (loss tangent) vs. follow-up time at 1 rad/s and 37 °C (without cells); c complex modulus and the linear trend line vs. follow-up time at 1 rad/s and 37 °C
Oscillatory measurements for foamed silica hydrogels and injectability
Before the longer follow-up study, a shorter rheological study (oscillation) was conducted with and without the cells and it was found that the presence of the cells does not affect the rheological properties, the difference in the rheological results was minimal. The damping (loss) factor for the foam with the cells showed steady values below 0.1 after 1 day and stayed almost constant up to 5 days and in the measurement conducted after 30 days (Fig. 2b). There was no significant difference compared with the corresponding foam without the cells, i.e., the structural stability of the foams as a function of time in a cell culture medium can also be studied without the cells. However, a small difference was observed in the beginning of the study: the foam seemed to be stabilized (indicated by the constant value for the damping factor) a bit faster with the cells (in 1 day) than without cells, but the resulting steady state damping factor after few days was the same with and without the cells.
The foamed silica hydrogels were stored in air-tight containers in an incubator at 37 °C immersed in a cell culture medium (DMEM supplemented with FBS) and the stability of the foams was monitored for 42 days by measuring the rheological properties in the oscillatory mode. The main part of liquid phase of the foamed silica hydrogels consisted also of DMEM supplemented with FBS, the rest is excess water originating from the sol–gel synthesis of silica.
The damping or loss factor (tan δ), also called loss tangent, at different time points (at the angular frequency of 0.1 rad/s) is shown in Fig. 2b. The selected angular frequency (ω) of 1 rad/s represents the average damping factors received from the frequency sweeps at 0.1 to 10 rad/s. The damping factor results for the foamed hydrogel structure are clearly below 1, which indicates a non-flowing and gel-like or solid state material. The results also indicate some development of the structure during the first days of the immersion, but the structure is quite well stabilized after few days, and more clearly after 10 days (tan δ = 0.06 ± 0.005 at 10–42 d) of immersion in the cell culture medium at 37 °C. However, another quantity summarizing the same oscillatory signal, the complex modulus (G* at ω = 1 rad/s shown in Fig. 2c) suggests that there is a slightly changing trend towards the end of the follow-up study. The increasing complex modulus (average complex modulus G* = 3.2 kPa between 2 and 29 days and the maximum G* = 7 kPa at day 42) indicates slightly increasing stiffness or rigidity of the foamed silica gel. The increase started after 29 days of immersion, and it is also seen in the increase of the storage (G′) and loss modulus (G″). The average storage modulus was ca. 3 kPa between 2 and 29 days of immersion and increased then to ca. 5.5 kPa after 34 days of immersion and to ca. 7.5 after 42 days of immersion. However, the change from the average complex modulus (at 2–29 days), G* = 3.2 kPa to the maximum G* = 7 kPa (at day 42) did not result in visible and practical differences of the foam structure and it did not affect the viability of the cells either.
The injectability of the foam was tested by injecting the fresh foam through a syringe opening and through the 25G needle. The injection test showed that the non-flowing foamed gel turned into flowing and injectable material, when shear stress was applied by injection. The foamed gel came easily through both the syringe opening and the 25G needle, and it also resumed its 3D compact form after the injection.
3D cell culture
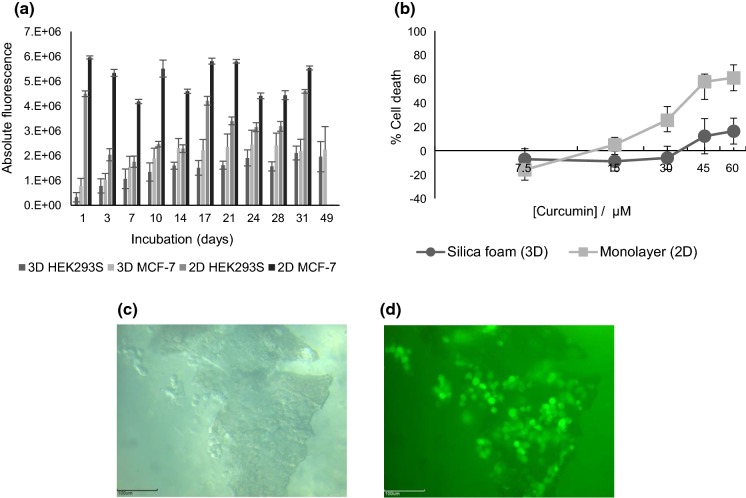
As shown in Fig. 3a, mammalian cell lines embedded in the foamed silica hydrogel survive for at least 7 weeks as shown by their metabolic activity. The metabolic activity increases until day 14, due to increase in cell number. After day 14, this metabolic activity stagnates as the foam cubes start eroding, due to frequent medium refreshment. Monolayer control wells were confluent after 1 day, due to the high plating density (6 × 105 cells/well). Variation in the control wells is explained by the limited growth surface and possibly also to insufficient nutrient availability. Variation in HEK293S monolayers is even higher because of their non-adherent nature, they partly disappear during medium refreshment.
Fig. 3.
a Viability of HEK293S and MCF-7/GFP mammalian cell lines in 3D silica hydrogel foam and 2D monolayers (control) as evaluated by resazurin assay. Data are represented as mean ± SE with n = 6 (3D test wells) or n = 3 (2D control wells) samples/group; b dose response curve of curcumin on MCF-7/GFP cells in silica hydrogel foam (3D) and in monolayer culture (2D) after 72 h of incubation. Data are represented as mean ± SE with n = 3 (3D test wells) samples/group; c fluorescence and d light microscopic image of a sliced cell encapsulated foam cube after 32 days of incubation. Scale bar, 100 µm
Overall higher metabolic activity of MCF-7 as compared to HEK293S cells, is due to the better adherence properties of MCF-7 as compared to HEK293S cells, which are normally grown in suspension, whereas MCF-7 cells are adherent cells. It was observed that MCF-7 even attached to the non-tissue culture treated suspension plates.
Macroscopic evaluation of the foam (e.g., cutting of the foam into cubes) revealed a difference in mechanical properties between the foam cubes with embedded cells as compared to the empty foam cubes suggesting connection between cells and foam. However, no difference was observed in the oscillation measurements for the general stiffness of the foam with and without the cells.
Another visual observation is that foam squares without cells float in the medium, partly underneath the liquid surface, partly above, a longer time (before sinking to the bottom of the plate) than the foam cubes with embedded cells. However, this varied also between the parallel samples of the foams without the cells. Air bubbles are present at the outside of the foam after 1 day of incubation with cells, but they disappear after few days. This might indicate the diffusion of the cell culture medium into the pores of the foam replacing the air bubbles present in the foam, and thereby increasing the density of the 3D structure.
Microscopic evaluation of a foam cube, which was cut in two halves, reveals the presence of living cells inside the foam cube at different time points, visualized by their production of GFP. In Fig. 3c, d, data of the embedded MCF-7 cells at day 32 are shown. The foam cube was cut in two parts with a scalpel and rinsed with PBS prior to visualization. Images of the cut surface with encapsulated cells inside the foam cube are represented. Cells are evenly distributed throughout the foam which is indicated by their presence over the complete surface and at different focal planes.
Response to chemotherapeutic compound
To address the difference in response of 2D- and 3D-cultures to compounds with known anti-tumor effects (Larasati et al. 2018), both culture types of MCF-7 cells were exposed to different concentrations of curcumin (7.5/15/30/45/60 µM) for 72 h after 14 days of cultivation. Mock treatment with DMSO was performed to correct for solvent effects and with Triton X-100 to determine 100% cell death signal. A clear difference can be seen between the cells cultured in monolayer versus the cells encapsulated in the foamed silica hydrogel (Fig. 3b). Up to 61% of cells grown in monolayer die under the maximum applied curcumin concentration (60 µM) versus only 16% of cells grown in the foam. Repetition of the experiment under the same conditions, with a new batch of sol/foam and a new batch of cells, gives the same pattern. Up to 82% of cells grown in monolayer die under 60 µM curcumin versus 27% of cells in the foam. Increasing the curcumin concentration to 240 µM gives 100% cell death in monolayer and 45% in the foam (data not shown). This points towards a greater resistance to toxic compounds such as curcumin of cells in the 3D structure as compared to cells grown in monolayers, i.e. the traditional way of cell culturing. This can be explained by less efficient diffusion of the compounds throughout the 3D structure, comparable to the in vivo situation of a real tumor, caused by the presence of the foamed silica hydrogel and the primary assembly of extracellular matrix (ECM) by cells growing in three dimensions. Proteins in the ECM form a physical barrier for diffusional transport into the tumor cells (McLaughlin et al. 2014; Au et al. 2016).
A dose–response effect is observed for curcumin in both 2D- and 3D-conditions, however, in neither setup the highest concentration was sufficient to reach 100% cell death. A remark should be made about the total number of cells, which is higher in the monolayer culture as judged from the absolute fluorescence value (three times higher in monolayer cultures, both for cells treated or untreated with DMSO). It was observed that cells do divide better in the monolayer cultures. On top of that, in the 3D cultures, the foamed silica hydrogel slowly dissolves causing clumps of silica foam with cells detaching from the structure. To perform metabolic activity measurements, the 3D structure is brought in a new plate, explaining the extra cell loss as compared to the 2D condition. Despite the much higher cell number, the effect of curcumin is stronger in monolayer cell cultures pointing towards a higher sensitivity to the toxic compound.
Discussion
It is generally known and showed (Brinker et al. 1990) that the increase in viscosity of the silica sols (e.g., at pH 2–7) is connected with the increase in aggregate size, i.e., accelerating aggregation of nanoscale species, nanoscale particles and/or smaller aggregates. The results show that both surface tension and dynamic viscosity increase as the aggregate size grows, but the increase in surface tension does not occur during the foaming. It occurs when the viscosity is already so high that it may disturb the actual measurements of surface tension. When these results are combined with the practical foaming experiments (too early start vs. no or short-age foam or too late start vs. pieces of solidified foam), it can be concluded that the foaming process is very sensitive to certain viscosities. Viscosity above ca. 200 mPas is already too high for the foaming, but also foaming at 4–5 to 9–10 min (η = ca. 10–40 mPas) did not result in a stable foam. The steep increase in the viscosity from 10 to 11 min with viscosity of ca. 40 to 150 mPas seems to be of importance for the foaming and for the formation of a stable foam.
It is important to remember that the viscosity measurements are for the silica sol-DMEM-FBS-NaOH mixture, while the real dispersion during foaming is changing, i.e., the amount of dispersed air in the mixture is increasing. Hence, direct interpretation of the viscosity after the very beginning of the foaming is not straightforward. On the other hand, the viscosity was approximately at the same level both at 4 min and 6 min, but the difference in the foaming and foam stability was clear. This suggests that the observed viscosity changes in the silica sol-DMEM-FBS-NaOH mixture are indicative also for the same mixture with increasing air content. In addition, the results from the oscillatory measurements show that in spite of the presence of air in the structure, there is a continuum in a structure. In other words, the aggregation continues and a foamed silica sol turns into a foamed gel (which is non-flowing at rest), which is observed in its viscoelastic properties.
Without FBS only short-aged foams were formed and the volume increase during the foaming was minimal. Thus, the surface-active molecules of FBS, most presumably proteins, have a role in the foaming. They decrease surface tension, which helps in the foam formation (when dispersing air into the silica sol-medium mixture), and this was clearly observed in the volume increase (from liquid phase silica sol mixture to the foam). Proteins may also have a positive effect on foam stability, but not without a high enough viscosity, because the same mixture did not result in a stable foam if the foaming started at 4 min, but a stable foam was formed as the foaming started at 6 min. Hence, the foaming process was very sensitive to the sol properties (viscosity and surface tension) and dependent on FBS addition in order to establish a volume increase (at least twofold) by foaming, which was observed to be necessary for the formation of porous structure in which the mammalian cells can reside, survive and proliferate.
However, while FBS seems to be crucial to the foaming process, it must be considered that FBS batches show substantial differences from lot to lot. Special attention needs to go to the use of uniform serum batches when comparing results of experiments using these foams as an in vitro model. In future work, chemically defined medium, i.e. xenofree standard media, should be considered, especially with regard to potential in vivo applications. It is also possible to use recombinant proteins for foaming instead of serum. Albumins, e.g., bovine serum albumin (BSA) is known to enable formation of stable foams (Krzan et al. 2013). There are also many other surface-active proteins, and synthetic molecules, such as in chemically defined media common polyvinyl alcohol (PVA) that act as a foaming agent. Corresponding consideration holds true also for the use of antibiotics and antimycotics during cell culture maintenance and foaming experiments. They can be omitted in future experiments if all manipulations are performed with extra care regarding sterility.
The rheological results with relatively low moduli indicate that the material also remains injectable. It was observed that non-flowing foamed gels turned into a flowing and injectable material, when shear stress was applied by the injection through the syringe opening and further through the 25G needle. The injected fresh foam also resumed its 3D compact form (without phase separation) after the injection, but the viscoelastic properties were not studied in detail. Some phase separation was observed for the foams that had reached the steady state. This is in accordance with the rheological properties that show non-flowing properties already at day 1, but the damping factors are higher (indicating more flow properties) for the fresh foams than for the foam that has reached the steady state. Hence, as the injectability with a thin needle (i.e., shear-thinning under shear) is good, and even the recovery to the non-flowing form is observed after the injection, the fresh foams also have a potential in extrusion, 3D printing and in dosing systems, where flow properties under shear and recovery to non-flowing 3D form are desired.
The increasing rigidity, as shown by the increasing complex modulus and also storage and loss modulus after immersion of the foams in cell culture medium, might have an effect on cell health over time. Since this increase only starts after 29 days after immersion, it is important to perform experiments with foams, for instance drug testing experiments, before this timepoint. The cytotoxicity experiment with curcumin was initiated after 14 days, the optimal time point for cell density and was finished at day 17.
The viability and curcumin results are promising for the use of the foamed silica hydrogel with embedded mammalian cells as an in vitro model system for testing potential therapeutic compounds in a pre-clinical phase. However, more cell studies are needed because there are no statistical results available on reproducibility of the system, nor on suitability for other cell types or other compounds. Therefore, in first instance the model should be tested with different cell types and with a limited number of compounds with known toxic effects to reveal its usability in drug testing.
The system in its current form is not optimized for the high-throughput screening, which is a common challenge for most 3D cell cultures (Rimann and Graf-Hausner 2012; Edmondson et al. 2014; Montanez-Sauri et al. 2015; Ryan et al. 2016). Producing the foam and encapsulating the cells is a labor-intensive process, which is not scaled down to a 96-well format, not to mention 384 or 1536 wells. However, as noted above, the studied rheological properties and injectability tests suggest flow properties under shear (during injection) and formation of a non-flowing 3D structure (re-gelling) after the shear. In other words, the results suggest that the foamed silica hydrogels also have a potential to be further developed for substrate deposition or 3D printing systems to create arrays used in high-throughput screening. In addition, the paste extrusion 3D printing in general is currently in the fast development phase and low-temperature processing and controlled rheology also have a potential for other applications of multiphase materials comprising sensitive agents, such as controlled delivery of viral vectors, vaccine antigens and biopharmaceuticals.
Conclusions
It was shown that it is possible to prepare a stable foamed silica hydrogel with a cell culture medium as the main liquid phase. The foamed silica hydrogel stays structurally stable and the metabolic activity of the embedded mammalian cells show that they remain viable for at least 6–7 weeks inside the foams under the normal cell culture conditions. The rheological properties of the foamed silica-based hydrogel are suitable for thin needle injection, and consequently also potentially for other corresponding types of form-giving methods with similar rheological prerequisites, such as 3D printing.
It was also shown that the foaming process is very sensitive to the sol properties (surface tension and viscosity) in order to establish a volume increase (at least twofold) by foaming, which is crucial for the formation of a porous structure in which the mammalian cells can reside, survive and proliferate. The foaming process was dependent on the addition of serum and its surface-active molecules in the cell culture medium, because no stable foam could be prepared with cell culture medium only.
A test with a toxic compound reveals a difference in the sensitivity of cells grown in traditional monolayer cultures versus cells embedded in the foamed silica hydrogel. This is promising with respect to use of the latter as an in vitro model system for therapeutic compound testing, however, further downscaling and extensive testing of more cell lines and compounds are necessary.
Acknowledgments
This research was partly funded by the PWO fund of the Artesis Plantijn University College.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Amza CG, Zapciu A, Popescu D. Paste extruder—hardware add-on for desktop 3D printers. Technologies. 2015;5:50. doi: 10.3390/technologies5030050. [DOI] [Google Scholar]
- Antoni D, Burckel H, Josset E, Noel G. Three-dimensional cell culture: a breakthrough in vivo. Int J Mol Sci. 2015;16:5517–5527. doi: 10.3390/ijms16035517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au JLS, Yeung BZ, Wientjes MG, Lu Z, Wientjes MG. Delivery of cancer therapeutics to extracellular and intracellular targets: Determinants, barriers, challenges and opportunities. Adv Drug Del Rev. 2016;97:280–301. doi: 10.1016/j.addr.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeshen MN, Al-Hejin AM, Bora RS, Ahmed MMM, Ramadan HAI, Saini KS, Baeshen NA, Redwan EM. Production of biopharmaceuticals in E. coli: current scenario and future perspectives. J Microbiol Biotechnol. 2015;25:953–962. doi: 10.4014/jmb.1412.12079. [DOI] [PubMed] [Google Scholar]
- Brinker CJ, Scherer GW. Sol-gel science: the physics and chemistry of sol-gel processing. New York: Academic Press; 1990. [Google Scholar]
- Cabanas-Polo S, Philippart A, Boccardi E, Hazur J, Boccaccini AR. Facile production of porous bioactive glass scaffolds by the foam replica technique combined with sol–gel/electrophoretic deposition. Cer Int. 2016;42:5772–5777. doi: 10.1016/j.ceramint.2015.12.115. [DOI] [Google Scholar]
- de Soure AM, Fernandes-Platzgummer A, da Silva CL, Cabral JMS. Scalable microcarrier-based manufacturing of mesenchymal stem/stromal cells. J Biotechnol. 2016;236:88–109. doi: 10.1016/j.jbiotec.2016.08.007. [DOI] [PubMed] [Google Scholar]
- Edmondson R, Broglie JJ, Adcock AF, Yang L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol. 2014;12:207–218. doi: 10.1089/adt.2014.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel BJ, Constantinou PE, Sablatura LK, Doty NJ, Carson DD, Farach-Carson MC, Harrington DA, Zarembinski TI. Multi-layered, hyaluronic acid-based hydrogel formulations suitable for automated 3D high throughput drug screening of cancer-stromal cell co-cultures. Adv Healthc Mater. 2015;4:1664–1674. doi: 10.1002/adhm.201500258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Yague MA, Abbah SA, McNamara L, Zeugolis DI, Pandit A, Biggs MJ. Biomimetic approaches in bone tissue engineering: integrating biological and physicomechanical strategies. Adv Drug Deliv Rev. 2015;84:1–29. doi: 10.1016/j.addr.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Gladman AS, Matsumoto EA, Nuzzo RG, Mahadevan L, Lewis JA. Biomimetic 4D printing. Nat Mat. 2016;15:413–418. doi: 10.1038/nmat4544. [DOI] [PubMed] [Google Scholar]
- Härmä V, Schukov H-P, Happonen A, Ahonen I, Virtanen J, Siitari H, Åkerfelt M, Lötjönen J, Nees M. Quantification of dynamic morphological drug responses in 3d organotypic cell cultures by automated image analysis. PLoS ONE. 2014;9:e96426. doi: 10.1371/journal.pone.0096426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman JA, Graeser R, de Hoogt R, Vidic S, Brito C, Gutekunst M, van der Kuip H. Three-dimensional models of cancer for pharmacology and cancer cell biology: capturing tumor complexity in vitro/ex vivo. Biotechnol J. 2014;9:1115–1128. doi: 10.1002/biot.201300492. [DOI] [PubMed] [Google Scholar]
- Hoppe A, Güldal NS, Boccaccini AR. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32:2757–2774. doi: 10.1016/j.biomaterials.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Jones JR, Poologasundarampillai G, Atwood RC, Bernard D, Lee PD. Non-destructive quantitative 3D analysis for the optimisation of tissue scaffolds. Biomaterials. 2007;28:1404–1413. doi: 10.1016/j.biomaterials.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Kangasniemi L, Koskinen M, Jokinen M, Toriseva M, Ala-Aho R, Kähäri V-M, Jalonen H, Ylä-Herttuala S, Moilanen H, Stenman U-H, Diaconu I, Kanerva A, Pesonen S, Hakkarainen T, Hemminki A. Extended release of adenovirus from silica implants in vitro and in vivo. Gene Ther. 2009;16:103–110. doi: 10.1038/gt.2008.142. [DOI] [PubMed] [Google Scholar]
- Kortesuo P, Ahola M, Kangas M, Jokinen M, Leino T, Vuorilehto L, Laakso S, Kiesvaara J, Yli-Urpo A, Marvola M. Effect of synthesis parameters of the sol-gel-processed spray-dried silica gel microparticles on the release rate of dexmedetomidine. Biomaterials. 2002;23:2795–2801. doi: 10.1016/S0142-9612(02)00016-9. [DOI] [PubMed] [Google Scholar]
- Krzan M, Caps H, Vandewalle N. High stability of the bovine serum albumine foams evidenced in Hele-Shaw cell. Coll Surf A. 2013;438:112–118. doi: 10.1016/j.colsurfa.2013.01.012. [DOI] [Google Scholar]
- Langhans SA. Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Front Pharmacol. 2018;9:6. doi: 10.3389/fphar.2018.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larasati YA, Yoneda-Kato N, Nakamae I, Yokoyama T, Meiyanto E, Kato JY. Curcumin targets multiple enzymes involved in the ROS metabolic pathway to suppress tumor cell growth. Sci Rep. 2018;8:2039–2051. doi: 10.1038/s41598-018-20179-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Lim J, Teoh S-H. Review: development of clinically relevant scaffolds for vascularised bone tissue engineering. Biotechnol Adv. 2013;3:688–705. doi: 10.1016/j.biotechadv.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Lovitt CJ, Shelper TB, Avery VM. Advanced cell culture techniques for cancer drug discovery. Biology. 2014;3:345–367. doi: 10.3390/biology3020345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin RL, Newitt DC, Wilmes LJ, Jones EF, Wisner DJ, Kornak J, Proctor E, Joe BN, Hylton NM. High resolution in vivo characterization of apparent diffusion coefficient at the tumor-stromal boundary of breast carcinomas: A pilot study to assess treatment response using proximity-dependent diffusion-weighted imaging. J Magn Reson Imaging. 2014;39:1308–1313. doi: 10.1002/jmri.24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanez-Sauri SI, Beebe DJ, Sung KE. Microscale screening systems for 3D cellular microenvironments: platforms, advances, and challenges. Cell Mol Life Sci. 2015;72:237–249. doi: 10.1007/s00018-014-1738-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampaloni F, Reynaud EG, Stelzer EH. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol. 2007;8:839–845. doi: 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- Quan Z, Wu A, Keefe M, Qin X, Yu J, Suhr J, Byun J-H, Kim B-S, Chou T-W. Additive manufacturing of multidirectional preforms for composites: opportunities and challenges. Mat Today. 2015;18:9. doi: 10.1016/j.mattod.2015.05.001. [DOI] [Google Scholar]
- Rimann M, Graf-Hausner U. Synthetic 3D multicellular systems for drug development. Curr Opin Biotechnol. 2012;23:803–809. doi: 10.1016/j.copbio.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Ryan SL, Baird AM, Vaz G, Urquhart AJ, Senge M, Richard DJ, O’Byrne KJ, Davies AM. Drug discovery approaches utilizing three-dimensional cell culture. Assay Drug Dev Technol. 2016;14:19–28. doi: 10.1089/adt.2015.670. [DOI] [PubMed] [Google Scholar]
- Sepulveda P, Jones JR, Hench LL. Bioactive sol-gel foams for tissue repair. J Biomed Mater Res. 2002;59:340–348. doi: 10.1002/jbm.1250. [DOI] [PubMed] [Google Scholar]
- Shamir ER, Ewald AJ. Three-dimensional organotypic culture: experimental models of mammalian biology and disease. Nat Rev Mol Cell Biol. 2014;15:647–664. doi: 10.1038/nrm3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suna T, Jackson S, Haycock JW, MacNeil S. Culture of skin cells in 3D rather than 2D improves their ability to survive exposure to cytotoxic agents. J Biotechnol. 2006;122:372–381. doi: 10.1016/j.jbiotec.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Viitala R, Jokinen M, Tuusa S, Rosenholm JB. Adjustably bioresorbable sol-gel derived SiO2 matrices for release of large biologically active molecules. J Sol–Gel Sci Technol. 2005;36:147–156. doi: 10.1007/s10971-005-5286-1. [DOI] [Google Scholar]
- Viitala R, Jokinen M, Maunu SL, Jalonen H, Rosenholm JB. Chemical characterization of bioresorbable sol–gel derived SiO2 matrices prepared at protein-compatible pH. J Non-Cryst Sol. 2005;351:3225–3234. doi: 10.1016/j.jnoncrysol.2005.08.023. [DOI] [Google Scholar]
- Warnock JN, Al-Rubeai M. Bioreactor systems for the production of biopharmaceuticals from animal cells. Biotechnol Appl Biochem. 2006;45:1–12. doi: 10.1042/BA20050233. [DOI] [PubMed] [Google Scholar]
- Wua ZY, Hill RG, Yue S, Nightingale D, Lee PD, Jones JR. Melt-derived bioactive glass scaffolds produced by a gel-cast foaming technique. Acta Biomater. 2011;7:1807–1816. doi: 10.1016/j.actbio.2010.11.041. [DOI] [PubMed] [Google Scholar]
- Xynos ID, Edgar AJ, Buttery LDK, Hench LL, Polak JM. Ionic products of bioactive glass dissolution increase proliferation of human osteoblasts and induce insulin-like growth factor II mRNA expression and protein synthesis. Biochem Biophys Res Comm. 2000;276:461–465. doi: 10.1006/bbrc.2000.3503. [DOI] [PubMed] [Google Scholar]
- Zhu W, Ma X, Gou M, Mei D, Zhang K, Chen S. 3D printing of functional biomaterials for tissue engineering. Curr Op Biotechnol. 2016;40:103–112. doi: 10.1016/j.copbio.2016.03.014. [DOI] [PubMed] [Google Scholar]