Abstract
d-Allulose, a C-3 epimer of d-fructose, is a rare sugar and a non-caloric sweetener. d-Allulose is reported to have several health benefits, such as suppressing a rise in postprandial glucose levels and preventing fat accumulation in rodents and humans. Additionally, low HDL-cholesterol levels post-d-allulose feeding were observed in humans but it is unclear how d-allulose decreased HDL-cholesterol levels. It is necessary to research the mechanism of HDL-cholesterol reduction by d-allulose ingestion because low HDL-cholesterol levels are known to associate with increased atherosclerosis risk. We therefore investigated the mechanism by which d-allulose lowers HDL-cholesterol using rat’s primary hepatocytes. Sprague Dawley rats were fed an AIN-93G based diet containing 3% d-allulose for 2 weeks. Thereafter, primary hepatocytes were isolated by perfusion of collagenase. We measured the ability of HDL-cholesterol uptake in hepatocytes and the protein levels of scavenger receptor class B type 1 (SR-B1) as a HDL-cholesterol receptor. d-Allulose enhanced hepatocyte uptake of HDL-cholesterol, with a concurrent increase in hepatic SR-B1 protein levels. The results suggest that d-allulose enhances HDL-cholesterol uptake into the liver by increasing SR-B1 expression. It is estimated that HDL-cholesterol levels decreased accordingly. Since SR-B1 overexpression would decrease HDL-cholesterol levels, reportedly preventing atherosclerosis development, d-allulose could be a useful sweetener for atherosclerosis prevention.
Keywords: d-Allulose, HDL-cholesterol, SR-B1, Primary hepatocytes
Introduction
d-Allulose is a rare sugar, and an epimer of d-fructose. When d-allulose is ingested, 70% d-allulose is absorbed in the small intestine and is excreted in the urine within a few hours in humans (Iida et al. 2010). It has been observed that d-allulose was present in the kidney and the liver until excretion in the urine after absorption in rats (Tsukamoto et al. 2014). d-Allulose is expected to function well as a sweetener for diabetics or individuals needing low-calorie diets, as it possesses sweetness similar to that of sucrose (70%), but has almost zero-calories. Several studies indicate that d-allulose improves glucose metabolism (Matsuo and Izumori 2006, 2009; Hossain et al. 2011). Hossain et al. (2011) reported that d-allulose suppressed postprandial serum glucose levels by enhancing glucokinase translocation. Matsuo and Izumori (2009) reported that d-allulose suppressed α-glucosidase and consequently reduced postprandial glucose levels. d-Allulose has been shown to exert anti-obesity effects (Matsuo et al. 2001; Chung et al. 2012; Ochiai et al. 2013, 2014; Nagata et al. 2015; Han et al. 2016); for example, d-allulose reduced abdominal fat accumulation by decreasing enzyme activities related to fatty acid synthesis or enhancing energy expenditure in rats (Matsuo et al. 2001; Ochiai et al. 2014; Nagata et al. 2015). In human studies, d-allulose reportedly decreased postprandial glucose levels (Hayashi et al. 2010), and reduced weight gain and abdominal fat accumulation (Han et al. 2018). These reports suggest that d-allulose can be used as a sweetener with anti-diabetic and anti-obesity effects.
Recently, it is reported that d-allulose intake provided low levels of HDL-cholesterol even though total cholesterol levels were not altered in normal human subjects (Hayashi et al. 2010). Low levels of HDL-cholesterol may lead to atheroma formation, thus increasing the risk of developing coronary heart disease or cardiovascular disease (Rubins et al. 2003; Eran et al. 2012). HDL plays a role in the collection of redundant cholesterol from peripheral tissues, such as the vascular wall to the liver, generally termed reverse cholesterol transport (RCT). Since the accumulation of cholesterol in the vascular wall induces atheroma formation, the maintenance of moderate HDL-cholesterol levels has gained consequently attention in the last few decades for the prevention of atherosclerosis. Few studies have been able to elucidate the effect of d-allulose on cholesterol metabolism, and thus further research is required.
Scavenger receptor class B type 1 (SR-B1), HDL receptor, is one of key protein associated with RCT. It is reported that low levels of HDL-cholesterol resulting from SR-B1 overexpression do not cause atherosclerosis (Kozarsky et al. 2000). SR-B1 is regulated by several factors, such as insulin, leptin, vitamin A, oxysterols (Leiva et al. 2011) and nuclear receptors (Lambert et al. 2003; Zhang and Edwards 2008).
In this study, we hypothesized that d-allulose may lower HDL-cholesterol by up-regulating RCT via enhancing HDL-cholesterol uptake into the liver, and investigated the mechanism by which d-allulose lowers HDL-cholesterol level.
Materials and methods
Experiment 1
Animal study
All experimental procedures were performed in accordance with nationally prescribed ethical guidelines, and ethical approval was granted by the Animal Experiment Committee of Matsutani Chemical Industry Co., Ltd. (No. 191001). Four-week-old male Sprague Dawley (Jcl: SD) rats were purchased from CLEA Japan Inc. (Tokyo, Japan), and housed individually in cages with free access to a commercial diet (CE2: CLEA Japan Inc.) and purified water for a week to acclimatize to the environment controlled room (temperature: 22–24 °C, light/dark cycle: 7:00–19:00). Thereafter the rats were divided into 2 groups (n = 6/group) according to body weights. Rats were fed AIN-93G-based diets with/without 3% d-allulose ad-libitum for 1 or 2 weeks. As a test substance, d-allulose was obtained from Matsutani Chemical Industry Co., Ltd (Itami, Japan) with a purity greater than 99%. After the feeding periods, rats were anesthetized with isoflurane in the non-fasting status, and bloods were collected from the abdominal aorta. The serum were obtained from the collected bloods by centrifugation (1693×g, 15 min) to be measured cholesterol in each lipoproteins (chylomicron, VLDL, LDL, and HDL) by the LipoSEARCH service (Skylight Biotech Inc., Akita, Japan) using gel-filtration high performance liquid chromatography.
Experiment 2
Isolation of primary hepatocytes
All experimental procedures were performed in accordance with nationally prescribed ethical guidelines, and ethical approval was granted by the Animal Experiment Committee of Matsutani Chemical Industry Co., Ltd. (No. 181220). Six-week-old male SD rats were purchased from CLEA Japan, Inc. Rats were fed a commercial diet and purified water ad-libitum for 1 week to acclimatize rats to the controlled environment as described above. Rats were divided into 2 groups (n = 8/group) according to their body weights and were fed the AIN-93G based diets with/without 3% d-allulose ad-libitum for 2 weeks. Then, rats were anesthetized with isoflurane and were used for hepatocytes isolation using the collagenase perfusion method of Berry and Friend (1969). The perfusion tubing was inserted via the portal vein, and then the abdominal portion of vena cava was cut. Thereafter 0.1% collagenase solution (kept at 37 °C) were perfused after the pre-perfusion for blood removal. Isolated primary hepatocytes with an 80% or greater viability were used in this experiment (control group, n = 8; d-allulose group, n = 6). The hepatocytes were suspended in Williams’ E (WE) cell culture medium (Sigma-Aldrich Co. LLC., MO, USA) containing 10% fetal bovine serum (FBS) (Biological Industries Ltd., Beit Haemek, Israel) (3.0 × 104 cells/well in 96-well plate and 2.1 × 105 cells/well in 12-well plate), and then cultured in collagen-coated plates (Nippi Inc., Tokyo, Japan). Following a 3-h incubation period, the hepatocytes were washed with PBS and cultured overnight in WE medium with 5% FBS. Thereafter, the media was changed to WE media containing or not-containing 10 mM d-allulose, followed by subsequent overnight incubation.
HDL uptake assay
Uptake of HDL was measured using an HDL uptake assay kit (Bio Vision, Inc., CA, USA). When the hepatocytes reached confluency after 2 days from the seeding, the cell culture media was removed, and cells were washed 3 times using the assay buffer. Fluorescent-labeled HDL (5 µg/well) in FBS-free WE media was added to the hepatocytes, and cultivated for 6 h. Thereafter, the media was discarded and the fluorescence intensity was measured. Total protein levels were measured using a Protein Assay BCA Kit (Wako Pure Chemical Industries, Ltd., Osaka, Japan).
Measurement of SR-B1 and FXR
Once the 12-well plates reached confluency after 2 days from the seeding, the hepatocytes were washed 3 times with PBS, and total proteins were extracted using a Minute™ Total Protein Extraction Kit (Invent Biotechnologies, Inc., MN, USA). The extracted proteins were used to measure levels of SR-B1 using a Scavenger Receptor Class B Member 1 ELISA Kit (Cell Biolabs, Inc., CA, USA). FXR protein levels were measured using an enzyme-linked immunosorbent assay (ELISA) kit for farnesoid X receptor (Cloud-Clone Corp., TX, USA). Total protein levels were measured using the commercial kit described above.
MTT assay for cellular viability
MTT (3-[4,5-dimethylthiazol-2-yl]-2,5diphenyltetrazoliumbromide) assays were performed to determine the quality of isolated primary hepatocytes. After 48 h post-cultivation in WE media with/without d-allulose, cellular viabilities were measured using a commercial kit (CytoSelect™ MTT Cell Proliferation Assay; Cell Biolabs, Inc., CA, USA).
Statistical analysis
All data are presented as mean ± SE. Data were statistically analyzed using the Student’s t-test. The values were considered statistically significant when the p-value was less than 0.05.
Results and discussion
A previous study showed that successive ingestion of d-allulose significantly reduced HDL-cholesterol levels in human subjects (Hayashi et al. 2010). We therefore studied the mechanism of lowering HDL-cholesterol levels by d-allulose ingestion in this study. In the Experiment 1, d-allulose did not affect final body weights and food intakes between the 2 groups in both feeding periods. Although 1 week feeding of d-allulose did not affect the levels of cholesterol, serum total, LDL and HDL cholesterol levels were significantly reduced by 2 weeks d-allulose feeding (Table 1). The result of HDL-cholesterol levels in rats fed d-allulose for 2 weeks resembles the result of the human trial showing that HDL-cholesterol levels are reduced in normal human subjects following ingestion of d-allulose for a period of 3 months (Hayashi et al. 2010).
Table 1.
Growth parameters and serum cholesterol levels in rats (Experiment 1)
| 1 week | 2 week | |||
|---|---|---|---|---|
| Control | d-Allulose | Control | d-Allulose | |
| Final body weight (g) | 208 ± 4 | 199 ± 4 | 271 ± 7 | 257 ± 3 |
| Food intake (g/day) | 21.0 ± 0.2 | 20.0 ± 0.4 | 21.9 ± 0.7 | 21.5 ± 0.4 |
| Cholesterol (mg/dL) | ||||
| Total | 71.5 ± 5.1 | 70.0 ± 4.5 | 69.5 ± 2.5 | 57.3 ± 1.8** |
| Chylomicron | 0.59 ± 0.11 | 0.70 ± 0.08 | 0.82 ± 0.17 | 1.22 ± 0.26 |
| VLDL | 7.9 ± 1.2 | 10.1 ± 1.3 | 10.3 ± 1.2 | 14.2 ± 1.5 |
| LDL | 17.3 ± 1.4 | 20.3 ± 2.9 | 17.0 ± 1.2 | 13.2 ± 1.0* |
| HDL | 45.7 ± 3.8 | 38.8 ± 1.8 | 41.4 ± 1.9 | 28.6 ± 1.0** |
Values are expressed as mean ± SE (n = 6/group)
Asterisk(s) indicate significant differences between two groups with the Student’s t-test (*p < 0.05, **p < 0.01)
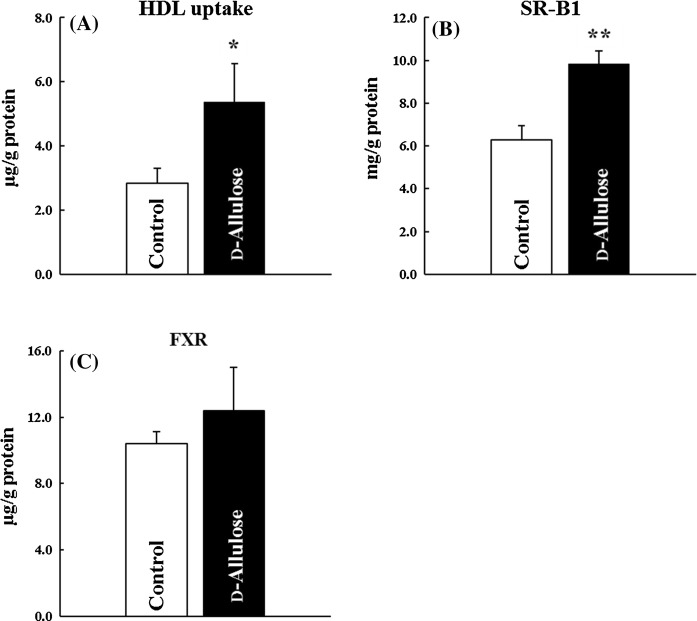
SR-B1 is generally known as a receptor of HDL-cholesterol and one of the regulators of HDL-cholesterol levels. Varban et al. (1998) reported increased HDL-cholesterol level through a decrease in hepatic HDL-cholesterol uptake in SR-B1-deficient mice. In the Experiment 2, we evaluated the effect of d-allulose on hepatocyte uptake of HDL-cholesterol using rat primary hepatocytes. Hepatocytes vitality of control group and d-allulose group were 87.9 ± 1.4% and 86.5 ± 1.2%, respectively. MTT activities in hepatocytes, growth parameters, were unaffected by d-allulose presence (data not shown). d-Allulose treatment significantly increased the transfer of fluorescent-labeled HDL from the culture media to rat primary hepatocytes (Fig. 1a). Since d-allulose enhanced the uptake of HDL-cholesterol into the liver, we measured SR-B1 protein levels. d-Allulose treatment significantly increased hepatic SR-B1 protein levels (p < 0.01) (Fig. 1b). These results suggest that d-allulose enhances RCT by increasing SR-B1 levels, and consequently, d-allulose may reduce HDL-cholesterol levels in humans.
Fig. 1.
Effects of d-allulose on HDL-cholesterol uptake into the hepatocytes. a Fluorescently-labeled HDL uptake, b SR-B1 protein levels, c FXR protein levels: white bars; control, black bars; d-allulose. Data are shown as mean ± SE (control group, n = 8; d-allulose group, n = 6). Columns marked with asterisk(s) are significantly different (*p < 0.05, **p < 0.01), analyzed using the Student’s t-test
FXR is known to regulate SR-B1 expression (Lambert et al. 2003; Zhang and Edwards 2008). Because bile acids are natural FXR agonists (Makishima et al. 1999; Parks et al. 1999), altering bile acid metabolism could contribute to low levels of HDL-cholesterol. A previous study has shown that d-allulose reduces alkaline phosphatase (ALP) and gamma-glutamyltranspeptidase (γ-GTP) levels in humans (Hayashi et al. 2010). Since these parameters are known as the biliary enzymes, d-allulose is expected to modulate bile or/and bile acid metabolism. Thus we measured FXR protein levels in rat’s primary hepatocytes. Contrary to our expectation, d-allulose did not affect hepatic FXR protein levels (Fig. 1c). It is suggested that FXR are not directly induced by d-allulose and the increase in the protein levels of SR-B1 by d-allulose ingestion were not caused by FXR. There are several possibilities that d-allulose regulates the SR-B1 protein levels by other factors. For example, polyunsaturated fatty acids and oxysterols are known as up-regulators of hepatic SR-B1 expression (Leiva et al. 2011). More detailed study of d-allulose on the mechanism of the increase in SR-B1 levels is needed in the future.
It is reported that the low levels of HDL-cholesterol, caused by an overexpression of SR-B1, did not lead to atherosclerosis onset (Kozarsky et al. 2000). Lower HDL-cholesterol levels and decreased fatty streak lesions in the aorta were observed in high SR-B1/apoB transgenic mice (Ueda et al. 2000). Overexpression of SR-B1 reportedly reduced atherosclerosis in LDL receptor-deficient mice (Arai et al. 1999; Kozarsky et al. 2000). On the other hand, a loss of SR-B1 expression leads to atherosclerotic coronary artery disease in apoE-deficient mice (Braun et al. 2002). Probucol, used for the treatment of atherosclerosis, was reported to reduce HDL-cholesterol, a suggested consequence of RCT enhancement (Sawayama et al. 2002). In addition, d-allulose is reported to inhibit the expression of monocyte chemoattractant protein-1 (MCP-1), which is found in macrophage-rich areas of atherosclerotic lesions (Murao et al. 2007). Accordingly, there is a possibility that continuous ingestion of d-allulose could prevent the development of atherosclerosis by increasing SR-B1 protein levels, though further research is required using atherosclerotic animal models.
In this study, the ability of HDL-cholesterol uptake and related protein levels were evaluated to elucidate the mechanism by which d-allulose lowers HDL-cholesterol. We revealed that d-allulose enhanced RCT, thereby decreasing HDL-cholesterol levels. This finding leads to the suggestion that d-allulose could be a sweetener which might contribute to preventing the onset of atherosclerosis. d-Allulose is expected to play a role not only in the anti-diabetes and anti-obesity field but also in the anti-atherosclerosis field. However, the reason of increasing SR-B1 and the effects of d-allulose on atherosclerosis development are still unclear. Furthermore, we need to examine whether d-allulose affects HDL-cholesterol regulators such as nascent HDL formation relating with apolipoprotein A1 and ATP-binding cassette A1 (Ji et al. 2012) in addition to HDL uptake. It is also well known that physiological sex differences affect metabolic status including cholesterol metabolism. Hepatic SR-B1 expression is reduced in male rats injected subcutaneously with estrogen (Stangl et al. 2002). Therefore, using female rats, whether the enhancement of HDL uptake by d-allulose is disturbed by estrogen would be of interest for future study. In the future, further studies will be required to investigate the relation between cholesterol metabolism and atherosclerosis by d-allulose ingestion.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Author contributions
AK wrote the manuscript. AK and TI participated in the experimental work and collected and analyzed data. KM, BS and MS supervised the study and commented on the manuscript. All authors have read and approved the final version of the manuscript.
Conflict of interest
The study was funded by Matsutani Chemical Industry Co. Ltd. (Itami, Japan). A.K. and T.I. are employees for this company.
References
- Arai T, Wang N, Bezouevski M, et al. Decreased atherosclerosis in heterozygous low density lipoprotein receptor-deficient mice expressing the scavenger receptor BI transgene. J Biol Chem. 1999;274:2366–2371. doi: 10.1074/jbc.274.4.2366. [DOI] [PubMed] [Google Scholar]
- Berry MN, Friend DS. High-yield preparation of isolated rat liver parenchymal cells. A biochemical and fine structural study. J Cell Biol. 1969;43:506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A, Trigatti BL, Post MJ, et al. Loss of SR-BI expression leads to the early onset of occlusive atherosclerotic coronary artery disease, spontaneous myocardial infarctions, severe cardiac dysfunction, and premature death in apolipoprotein E-deficient mice. Circ Res. 2002;90:270–276. doi: 10.1161/hh0302.104462. [DOI] [PubMed] [Google Scholar]
- Chung YM, Hyun Lee J, Youl Kim D, et al. Dietary D-psicose reduced visceral fat mass in high-fat diet-induced obese rats. J Food Sci. 2012;77:53–58. doi: 10.1111/j.1750-3841.2011.02571.x. [DOI] [PubMed] [Google Scholar]
- Eran E, Yilmaz N, Aydin O. High density lipoprotein and it’s dysfunction. Open Biochem J. 2012;6:78–93. doi: 10.2174/1874091x01206010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Han HJ, Kim AH, et al. D-Allulose supplementation normalized the body weight and fat-pad mass in diet-induced obese mice via the regulation of lipid metabolism under isocaloric fed condition. Mol Nutr Food Res. 2016;60:1695–1706. doi: 10.1002/mnfr.201500771. [DOI] [PubMed] [Google Scholar]
- Han Y, Kwon E, Yu MK, et al. A preliminary study for evaluating the dose-dependent effect of D-allulose for fat mass reduction in adult humans: A randomized, double-blind, placebo-controlled trial. Nutrients. 2018;10:160. doi: 10.3390/nu10020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi N, Iida T, Yamada T, et al. Study on the postprandial blood glucose suppression effect of D-psicose in borderline diabetes and the safety of long-term ingestion by normal human subjects. Biosci Biotechnol Biochem. 2010;74:510–519. doi: 10.1271/bbb.90707. [DOI] [PubMed] [Google Scholar]
- Hossain MA, Kitagaki S, Nakano D, et al. Rare sugar D-psicose improves insulin sensitivity and glucose tolerance in type 2 diabetes Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Biochem Biophys Res Commun. 2011;405:7–12. doi: 10.1016/j.bbrc.2010.12.091. [DOI] [PubMed] [Google Scholar]
- Iida T, Hayashi N, Yamada T, et al. Failure of D-psicose absorbed in the small intestine to metabolize into energy and its low large intestinal fermentability in humans. Metabolism. 2010;59:206–214. doi: 10.1016/j.metabol.2009.07.018. [DOI] [PubMed] [Google Scholar]
- Ji A, Wroblewski JM, Cai L, et al. Nascent HDL formation in hepatocytes and role of ABCA1, ABCG1, and SR-B1. J Lipid Res. 2012;53:446–455. doi: 10.1194/jlr.M017079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozarsky KF, Donahee MH, Glick JM, et al. Gene transfer and hepatic overexpression of the HDL receptor SR-BI reduces atherosclerosis in the cholesterol-fed LDL receptor-deficient mouse. Arterioscler Thromb Vasc Biol. 2000;20:721–727. doi: 10.1161/01.ATV.20.3.721. [DOI] [PubMed] [Google Scholar]
- Lambert G, Amar MJA, Guo G, et al. The farnesoid X-receptor is an essential regulator of cholesterol homeostasis. J Biol Chem. 2003;278:2563–2570. doi: 10.1074/jbc.M209525200. [DOI] [PubMed] [Google Scholar]
- Leiva A, Verdejo H, Benítez ML, et al. Mechanisms regulating hepatic SR-BI expression and their impact on HDL metabolism. Atherosclerosis. 2011;217:299–307. doi: 10.1016/j.atherosclerosis.2011.05.036. [DOI] [PubMed] [Google Scholar]
- Makishima M, Okamoto AY, Repa JJ, et al. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- Matsuo T, Izumori K. Effects of dietary D-psicose on diurnal variation in plasma glucose and insulin concentrations of rats. Biosci Biotechnol Biochem. 2006;70:2081–2085. doi: 10.1271/bbb.60036. [DOI] [PubMed] [Google Scholar]
- Matsuo T, Izumori K. D-Psicose inhibits intestinal α-glucosidase and suppresses the glycemic response after ingestion of carbohydrates in rats. J Clin Biochem Nutr. 2009;45:202–206. doi: 10.3164/jcbn.54. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Matsuo T, Baba Y, Hashiguchi M, et al. Dietary D-psicose, a C-3 epimer of D-fructose, suppresses the activity of hepatic lipogenic enzymes in rats. Asia Pac J Clin Nutr. 2001;10:233–237. doi: 10.1046/j.1440-6047.2001.00246.x. [DOI] [PubMed] [Google Scholar]
- Murao K, Yu X, Cao WM, et al. D-Psicose inhibits the expression of MCP-1 induced by high-glucose stimulation in HUVECs. Life Sci. 2007;81:592–599. doi: 10.1016/j.lfs.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Nagata Y, Kanasaki A, Tamaru S, Tanaka K. D-Psicose, an epimer of D-fructose, favorably alters lipid metabolism in sprague-dawley rats. J Agric Food Chem. 2015;63:3168–3176. doi: 10.1021/jf502535p. [DOI] [PubMed] [Google Scholar]
- Ochiai M, Nakanishi Y, Yamada T, et al. Inhibition by dietary D-psicose of body fat accumulation in adult rats fed a high-sucrose diet. Biosci Biotechnol Biochem. 2013;77:1123–1126. doi: 10.1271/bbb.130019. [DOI] [PubMed] [Google Scholar]
- Ochiai M, Onishi K, Yamada T, et al. D-Psicose increases energy expenditure and decreases body fat accumulation in rats fed a high-sucrose diet. Int J Food Sci Nutr. 2014;65:245–250. doi: 10.3109/09637486.2013.845653. [DOI] [PubMed] [Google Scholar]
- Parks DJ, Blanchard SG, Bledsoe RK, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. J Cardiopulm Rehabil. 2003;20:66. doi: 10.1097/00008483-200001000-00015. [DOI] [PubMed] [Google Scholar]
- Sawayama Y, Shimizu C, Maeda N, et al. Effects of probucol and pravastatin on common carotid atherosclerosis in patients with asymptomatic hypercholesterolemia: Fukuoka atherosclerosis trial (FAST) J Am Coll Cardiol. 2002;39:610–616. doi: 10.1016/S0735-1097(01)01783-1. [DOI] [PubMed] [Google Scholar]
- Stangl H, Graf GA, Yu L, et al. Effect of estrogen on scavenger receptor BI expression in the rat. J Endocrinol. 2002;175:663–672. doi: 10.1677/joe.0.1750663. [DOI] [PubMed] [Google Scholar]
- Tsukamoto I, Akram H, Fuminori Y, et al. Intestinal absorption, organ distribution, and urinary excretion of the rare sugar D-psicose. Drug Des Devel Ther. 2014;8:1955–1964. doi: 10.2147/DDDT.S60247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Gong E, Royer L, et al. Relationship between expression levels and atherogenesis in scavenger receptor class B, type I transgenics. J Biol Chem. 2000;275:20368–20373. doi: 10.1074/jbc.M000730200. [DOI] [PubMed] [Google Scholar]
- Varban ML, Rinninger F, Wang N, et al. Targeted mutation reveals a central role for SR-BI in hepatic selective uptake of high density lipoprotein cholesterol. Proc Natl Acad Sci USA. 1998;95:4619–4624. doi: 10.1073/pnas.95.8.4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Edwards PA. FXR signaling in metabolic disease. Fed Eur Biochem Soc. 2008;582:10–18. doi: 10.1016/j.febslet.2007.11.015. [DOI] [PubMed] [Google Scholar]