Treatment-free remission (TFR) has become a new frontier in the treatment of patients with chronic myeloid leukemia (CML). Prospective clinical trials have shown that around 50% of patients in sustained deep molecular response can successfully discontinue their tyrosine kinase inhibitor (TKI) therapy, without losing major molecular response (defined as BCR-ABL1 real-time quantitative PCR [(RT-qPCR) of ≤0.1% International scale (IS)] for a number of years. Interim analysis of the largest stopping study, EURO-SKI, has identified duration of MR4 (BCR-ABL1 RT-qPCR of <0.01% IS) and duration of TKI treatment as the only strong predictive factors associated with TFR.1 Current recommendations for a safe TFR attempt advise that this approach should be avoided in presence of resistance and/or BCR-ABL1 kinase domain mutation (KDm),2 however comprehensive data on the outcome following TKI discontinuation in patients with history of KDm are lacking.
We performed a retrospective analysis of 10 CML patients, followed-up at our institution, with previous KDm who stopped TKI due to intolerance having been in MR4 for at least 1 year. Molecular monitoring3 and standard response definitions were used as previously described.4 Mutational screening of KDm followed currently available guidelines.5 This study was approved by our internal review board and all patients gave informed consent.
Molecular recurrence-free survival (MRFS) was defined as the probability of remaining alive in stable MR3 (or deeper) after TKI cessation. Kaplan-Meier function was used to determine MRFS and patients were censored at last follow-up.
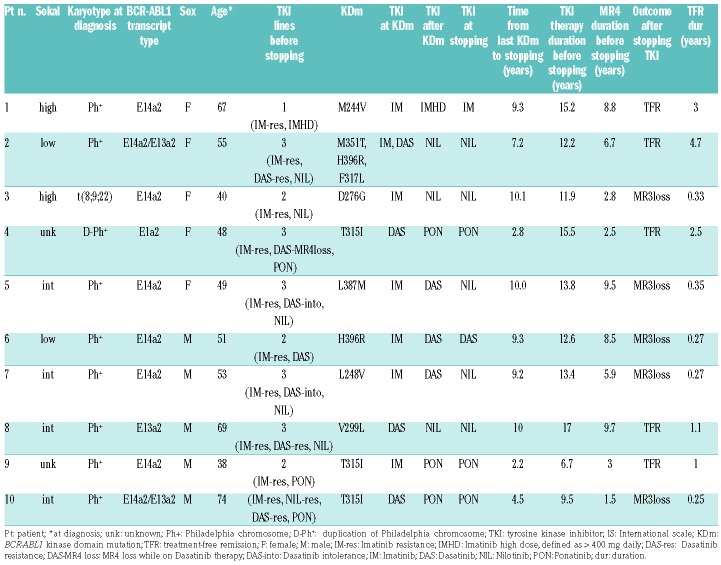
Patient characteristics are shown in Table 1. Karyotype analysis at diagnosis revealed the classical Ph translocation as the only abnormality in the majority of patients (n=8), while a variant t(8;9;22)(p22;q34;q11.2) (n=1) and duplication of the Philadelphia chromosome (n=1) occurred in others.
Table 1.
Patient characteristics.
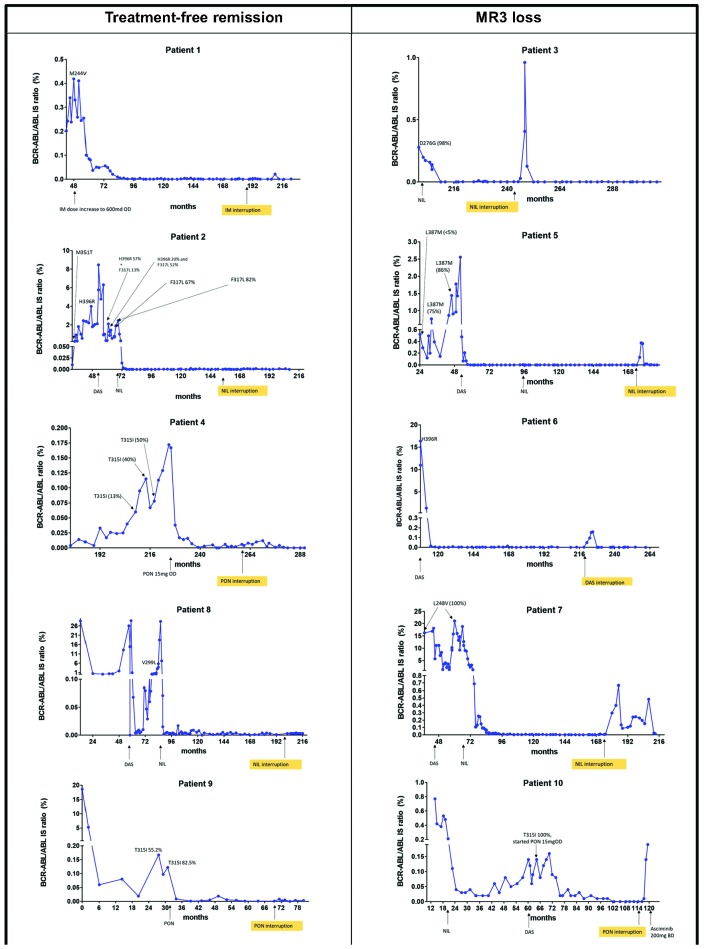
A total of nine different KD mutations were detected through Sanger sequencing and one patient had three consecutive different KDm throughout her disease course. Values of BCR-ABL1/ABL1 at the time of detection of KDm are shown in Figure 1. Change of TKI was the preferred choice after the detection of KDm in nine patients, while for one patient increasing the imatinib dose was the only available option.
Figure 1.
Evaluation of molecular response before and after tyrosine kinase inhibitor discontinuation due to intolerance in 10 chronic myeloid leukemia patients with previously detected BCR-ABL1 kinase domain mutations (KDm). See Table 1 for greater detail. In each graph, time is indicated in months from CML diagnosis. Patient 4: raw BCR-ABL1/ABL1 ratios are not reported on the International Scale (IS), given the atypical BCR-ABL1 transcript (e1a2). Variant allele frequency of the KDm is reported when Pyrosequencing or Next Generation Sequencing was also performed in addition to Sanger sequencing, in order to follow the kinetics of the mutant clone.
The median duration of TKI therapy and MR4 before stopping treatment were 13 years (range 6.7-15.5) and 6.3 years (range 1.5-9.7), respectively. The TKI at time of discontinuation due to intolerance was imatinib (n=1), dasatinib (n=1), nilotinib (n=5) or ponatinib (n=3). All patients had a history of resistance to at least one TKI as previously defined by the ELN consensus group.6
Five patients (50%) lost MR3 at a median of 3.3 months (range 3-4.2) off therapy, but stayed in complete cytogenetic response throughout. Four patients regained MR3 after a median time of 2.7 months (range 2-12) (two patients on the same TKI, after resolution of non-hematological toxicity and dose reduction, and two on an alternative TKI); none of them experienced disease progression and all were in MR4 or better response at last contact, after a median of 40.2 months (range 16.3–63.5) from TKI interruption. No molecular follow-up is yet available for one patient (patient 10) who started Asciminib 200 mg BD after having lost MR3.
MRFS at one year was 50% (95% confidence interval [CI]: 46.9-53.1). The median follow-up in TFR for patients without loss of MR3 was 2.1 years (1-4.7).
The emergence of mutations within the kinase domain of BCR-ABL1 is a frequent association with TKI resistance7 and correlates with inferior long-term outcome.8–10 The detection of KDm at any time during follow-up is a sufficient single criterion to define treatment failure according to ELN recommendations.6 The T315I in particular has a negative impact on failure free and overall survival,11 and even ponatinib, which is the single currently licensed TKI available against this mutation, is only effective in achieving deep molecular response in ~40% of cases.12
At present, there is no consensus on the clinical variables that determine patient suitability for a TFR attempt. Criteria for TKI interruption2 include chronic phase disease without history of accelerated or blast phase, TKI therapy of at least 3 years and MR4 level sustained for at least 2 years, however TKI resistance is no longer excluded in the current update of these recommendations.13 Two independent studies, DADI14 and STOP 2G-TKI,15 showed that previous resistance to TKI was associated with a higher rate of relapse after stopping. The DADI trial excluded patients with dasatinib-resistant KDm and no information is forthcoming regarding the outcome on other KDm. In the STOP 2G-TKI study, although 4 of 13 TKI resistant patients had a previous KDm, the TFR outcome for these patients is not provided in detail.
We report five patients who have successfully maintained a prolonged TFR (up to 4.7 years), despite a previous history of TKI failure and presence of KDm, including T315I. Also, the durability of TFR according to the patient mutation status and after ponatinib cessation has not been reported previously.
It is reasonable to speculate that when an effective alternative TKI is started promptly after KDm detection, the achievement of a deep molecular response overcomes the traditionally accepted adverse patient outcomes. TFR appears feasible in patients with previous KDm, however a larger number of cases are required to determine the prognostic impact of KDm on the TFR probability and on the safety of this approach. Stopping TKI outside clinical trials in patients with KDm currently needs to be reserved for those patients with significant TKI-related toxicity in the absence of alternative therapy and to be approached with caution.
These observations are of importance for the CML physician and patient community in order to provide clinical experience to optimally manage patients, some of whom may be unduly suffering from complications of their therapy.
Footnotes
Funding: JFA and DM acknowledge the support of the Imperial College NIHR Biomedical Research Centre.
The views expressed in this article are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Saussele S, Richter J, Guilhot J, et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): a prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. 2018;19(6):747–757. [DOI] [PubMed] [Google Scholar]
- 2.Hughes TP, Ross DM. Moving treatment-free remission into mainstream clinical practice in CML. Blood. 2016;128(1):17–23. [DOI] [PubMed] [Google Scholar]
- 3.Foroni L, Wilson G, Gerrard G, et al. Guidelines for the measurement of BCR-ABL1 transcripts in chronic myeloid leukaemia. Br J Haematol. 2011;153(2):179–190. [DOI] [PubMed] [Google Scholar]
- 4.Cross NC, White HE, Muller MC, Saglio G, Hochhaus A. Standardized definitions of molecular response in chronic myeloid leukemia. Leukemia. 2012;26(10):2172–2175. [DOI] [PubMed] [Google Scholar]
- 5.Soverini S, Hochhaus A, Nicolini FE, et al. BCR-ABL kinase domain mutation analysis in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors: recommendations from an expert panel on behalf of European LeukemiaNet. Blood. 2011;118(5):1208–1215. [DOI] [PubMed] [Google Scholar]
- 6.Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122(6):872–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apperley JF. Part I. mechanisms of resistance to imatinib in chronic myeloid leukaemia. Lancet Oncol. 2007;8(11):1018–1029. [DOI] [PubMed] [Google Scholar]
- 8.Khorashad JS, de Lavallade H, Apperley JF, et al. Finding of kinase domain mutations in patients with chronic phase chronic myeloid leukemia responding to imatinib may identify those at high risk of disease progression. J Clin Oncol. 2008;26(29):4806–4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jabbour E, Jones D, Kantarjian HM, et al. Long-term outcome of patients with chronic myeloid leukemia treated with second-generation tyrosine kinase inhibitors after imatinib failure is predicted by the in vitro sensitivity of BCR-ABL kinase domain mutations. Blood. 2009;114(10):2037–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muller MC, Cortes JE, Kim DW, et al. Dasatinib treatment of chronic-phase chronic myeloid leukemia: analysis of responses according to preexisting BCR-ABL mutations. Blood. 2009;114(24):4944–4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicolini FE, Ibrahim AR, Soverini S, et al. The BCR-ABLT315I mutation compromises survival in chronic phase chronic myelogenous leukemia patients resistant to tyrosine kinase inhibitors, in a matched pair analysis. Haematologica. 2013;98(10):1510–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cortes JE, Kim DW, Pinilla-Ibarz J, et al. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood. 2018;132(4):393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radich JP, Deininger M, Abboud CN, et al. Chronic Myeloid Leukemia, Version 1.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16(9):1108–1135. [DOI] [PubMed] [Google Scholar]
- 14.Imagawa J, Tanaka H, Okada M, et al. Discontinuation of dasatinib in patients with chronic myeloid leukaemia who have maintained deep molecular response for longer than 1 year (DADI trial): a multicentre phase 2 trial. Lancet Haematol. 2015;2(12):e528–535. [DOI] [PubMed] [Google Scholar]
- 15.Rea D, Nicolini FE, Tulliez M, et al. Discontinuation of dasatinib or nilotinib in chronic myeloid leukemia: interim analysis of the STOP 2G-TKI study. Blood. 2017;129(7):846–854. [DOI] [PubMed] [Google Scholar]