Abstract
The aim of present study was to assess the effects and safety of a dry Phaseoli vulgari pericarpi-um (PVP) extract on postprandial glycemia in healthy participants. A randomized crossover experiment where participants received either PVP extract or placebo. Chemical compounds in dry extract were assessed by established methods. Eighteen healthy participants (9 male and 9 female) aged 29 ±4,8 years, body mass index (BMI) 23 ±3,7 kg/m2 were recruited among students and staff at the Faculty of Pharmacy, University of Belgrade. All participants were able to follow the study protocol without difficulty. The participants received either PVP extract or placebo 30 minutes before a 50g oral glucose tolerance test (OGTT). The protocol followed the guidelines for the OGTT with blood samples drawn at 0,15, 30, 60, 90 and 120 min. This study demonstrated that there was no significantly effect of the PVP extract on incremental blood glucose (IBG) and their areas under the curve (AUC) neither male nor female participants. However, IBG together with AUC changes were significantly lower in male compared with female participants in treated and untreated groups. The presence of chrome, soluble fiber, vitamin C, protein, glucose and lectins were also quantified. The applied amount of PVP extract was unable to produce the postprandial hypoglycemia. We assumed that amounts of chrome, soluble fiber, vitamin C which have beneficial effects on diabetes treatment were sufficient to produce hypoglycemia.
Keywords: alternative medicine, Phaseoli vulgari pericarpium, postprandial, glucose
INTRODUCTION
Diabetes is a major health problem in the world. According to report of WHO there were about 157 millions diabetic patients in 2000, and by the year 2025 about 268 millions people are expected to be diabetic in the world (1). Diabetes mellitus is a chronic metabolic disease that in the majority of patients arises from defects in both peripheral insulin action (insulin resistance) and insulin secretion (beta-cell dysfunction) (2). The disease often co-exists with hypertension and dyslipidaemia with dangerous consequences to the cardiovascular system (3). Although there are several medicaments that regulate blood glucose, level of lipids and blood pressure, and many patients are more inclined to use alternative therapies, which include diet, food supplements and herbal medicine. That is why it becomes necessary to investigate active components and clinical safety of herbal medicine. Animal studies showed that variety plants which compound peptide (S-alilcistein), alkaloid (leurozin, vindolin), cumarin, steroid (fenu-grekin), vitamin E, lectins, polysaccharide (acarbose) as well as inorganic ions (Cr, Mn, Mg) might have hypoglycemic effect (4). Phaseoli vulgaris pericarpium (PVP) also contains components which might have beneficial effects on diabetes treatment such as trigonelline, ar-ginin, titosin, levicin, lysine, tryptophane, asparagine, chrome, vitamin C, hemicelluloses, soluble fibers (5, 6). Commission E categorised this herbal as drug used in supportive treatment for inability to urinate, but not as a drug used in diabetes treatment (7). Hypoglycemic effect on humans is not clarified, whereas results on animals demonstrated that administration of PVP rapidly decreases blood glucose (8, 9, 10). Due to fact that dry plant PVP is available in pharmacy stores across Serbia as antidiabetic herbal preparation, the aim of the this study was to assess the effects and safety of a dry PVP extract on postprandial glycemia in healthy humans.
MATERIAL AND METHODS
PARTICIPANTS
Healthy participants were recruited among students and staff at the Faculty of Pharmacy, University of Belgrade. Exclusion criteria included previously diagnosed dysglycemia, liver or kidney disease, major surgery in the last 6 months, < 18 or >60 years of age. Eighteen participants (9 male and 9 female) aged 29 ±4,8 years, body mass index (BMI) 23 ±3,7 kg/m2 were chosen who met these criteria. All gave informed written consent and the study was approved by the medical ethic committee.
TREATMENT
Participants received either capsule with dry extract or a placebo capsule in randomized cross-over design. Each capsule contains 126,2 ± 0,3 mg of dry extract. The protocol followed the WHO guidelines for the administration of the oral glucose tolerance test (11). The participants attended the Department of Broma-tology at Faculty of Pharmacy on two separate mornings after a 12 hour overnight fast. A minimum of 3 days, washout period, separated each visit to minimize carry-over effects. The PVP capsules together with the placebo capsules were administered with exactly 100 ml tap water. After 30 minutes, the participants provided a blood sample (0 minute) and then consumed a 50g oral glucose load (dissolved in 200 ml tap water) over exactly 3 minutes. A finger-prick capillary blood sample was collected by using Roche’s Lancet. Additional finger-prick blood samples were obtained 15, 30, 60, 90 and 120 minutes after the start of the challenge.
BLOOD GLUCOSE ANALYSIS PROCEDURE
The glucose concentration from capillary blood samples were determined by Accuchek advantage system, Roche.
DRY PLANT EXTRACTION PROCEDURE
Dry plant material of the PVP was extracted with water in ratio 1 g dry plant / 10 ml water solution. After 30 minutes at 15°C in ultrasonic mixer, the extract was filtered by filter paper and water was evaporated on a vacuum rotatory evaporator at a temperature of 60°C. An anti-diabetic herbal preparation with PVP, which is available in pharmacy stores, recommended a 2,5g dry plant dissolved in cup of boiled water. Because of this recommendation, PVP was extracted 2,5g per capsule. The yield of extraction was 5,05%. Each capsule contained 126,2 ± 0,3 mg of dry extract.
DRY PLANT EXTRACT ANALYSIS
Chrome analysis. The chrome concentration in dry plant extract of PVP was measured using atomic absorption spectrophotometry (12). Solublefiber analysis. The determination of soluble fiber in dry plant extract was based on enzymatic digestion (α-amylase, protease and amilo-glycosidase) of starch and protein, which doesn’t effect on fiber and determined by gravimetric methode (13). Vitamin C analysis. The determination of vitamin C was based on extraction by three chloracetic acid and their reduction with Thillmans reagent (2,6 dichlorphenol indophenol pH 8) (14). Protein analysis. The total protein content analysis of the PVP was conducted with the Improved Kjeldahl Method for Nitrate-Free Samples (15). Glucose analysis. The glucose amount was determined by a hexokinase test (16). Lectin analysis. Lectin is a trace component in dry plant material. Due to this fact, quality determination by agglutination test was conducted (17).
STATISTICAL ANALYSIS
Blood glucose curves were represented as the incremental change in blood glucose from time 0 min, and positive incremental area under the curve (AUC) was calculated geometrically for each participants, ignoring areas below the fasting blood glucose value (18). Incremental blood glucose (IBG) concentrations were used to control for baseline (fasting) differences between the treatments. Statistical analyses were then performed. The significance of the effect was assessed by the use of two-way ANOVA test.
RESULTS
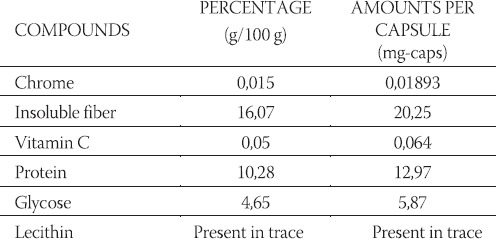
All participants were able to follow the study protocol without difficulty. No adverse effects from the PVP extract or placebo administration were reported by the participants during or after testing sessions. There were also no differences in reported symptoms of bloating, nausea, dizziness, headache, diarrhea, insomnia, anxiety or thirst between placebo and PVP treatment during testing and washout period. The effect of dry plant extract of PVP on the glycemic response to 50g oral glucose load at 0,15, 30, 60, 90 and 120 min is compared in Figure 1. Repeated-measures two-way ANOVA showed that there are not significantly differences in IBG changes, between control and test group which were treated with dry extract of PVP in female as well as male healthy individuals. However, incremental changes in glucose are significantly different between gender (p < 0,001) in treated and untreated groups. Tukey test showed that, glycemic response to the oral glucose load was significantly lower after administration of dry plant extract of PVP at 60 and 90 min compared with glycemic response at 30 min (p < 0,05) in male and female individuals. The effect of dry plant extract of PVP on the AUC after glucose load at 0, 15, 30, 60, 90 and 120 min is compared in Figure 2. Repeated-measures two-way ANOVA showed that there are not significantly differences on AUC, between control and test group which were treated with dry plant extract of PVP in female as well as male healthy individuals. Results also showed significantly differences on AUC between gender (p < 0,001). Pair wise comparisons using Tukey test showed that AUC was significantly lower in male group (p < 0,05). Expressed as a percentage of female group, AUC of male group were 53,2% and 61,8% in control and test group, respectively. Table 1. showed the some herbal compounds in dry plant extract of PVP which might have beneficial effect in diabetes treatment. Although data showed chemical composition of PVP (5, 6, 7) but not composition of its extract, the main criteria in selection of chemical compounds in present study was their hypoglycemic effect and solubility in water. Contents of compounds were expressed as percentages in dry plant extract (g/100g) and amounts per capsule (mg/caps).
FIGURE 1.
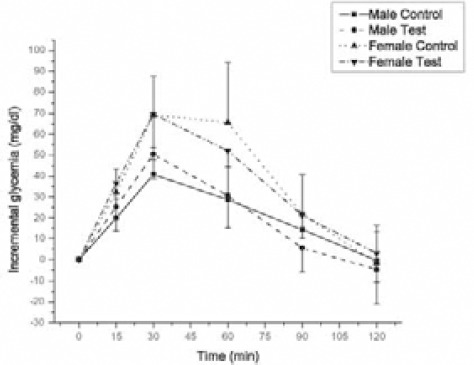
Effect of dry extract of PVP on incremental changes in glycemia at selected time intervals (0,15,30,60,90 and 120 min) after oral glucose load in 9 male and 9 females subjects without diabetes. All values are expressed as mean ± SD.
FIGURE 2.
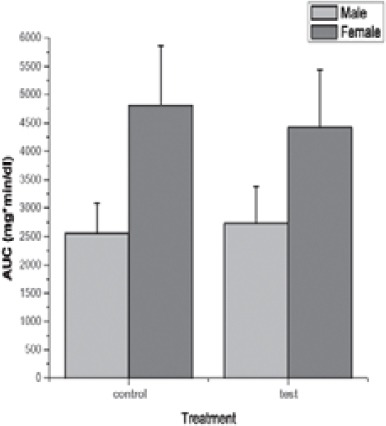
Effect of dry extract of PVP on glycemic area under the curve after oral glucose load in male and female subjects without diabetes. All values are expressed as mean ± SD.
TABLE 1.
Composition of dry extract of PVP. Contents of compounds were expressed as percentages in dry plant extract (g / 100g) and amounts per capsule (mg / caps).
DISCUSSION
The use of ethno-medicinal plants in managing of diabetes mellitus is in progress all over the world. In this manner extract of PVP has been used for a long time in the Balkan (19). Animal studies demonstrated that administration of PVP rapidly decreases blood glucose (8, 9, 10), in spite of the fact that there is no scientific affirmation of the hypoglycemic effect in the human population. Thus, it becomes necessary to investigate effect and safety of administration of PVP which is used in alternative diabetes treatment and these were the aims of present study. Our results showed that there are not significantly differences in IBG changes in blood glucose, as well as AUC in control and test group of both gender. Although the test group was treated with herbal preparation, glucose response to oral glucose load was similar when compared to control group. Glucose level reached peak at 30 minutes after which it was decreasing and reaching basal level. This shape of glucose curves fitted with insulin concentration after postprandial glycemia (20). In order to find reasons why dry extract of PVP didn’t demonstrate hypoglycemic effect, we determined its chemical compounds which showed that effect in other studies, as well as beneficial effects on diabetes treatment (21, 22). On other hand, in order to estimate its safety administration we also determined toxic chemical compound. Following chemical compounds were found soluble fibers, chrome, vitamin C, glucose, protein, lectins (Table 1). As it could be seen in Table 1 chrome is present in dry extract capsules. After administration of dietary inorganic chrome, it must be converted into a biologically active form in order to function physiologically, called the Glucose Tolerance Factor (GTF) by Evans (23). The purpose of biologically active chrome is to act as a co-factor of insulin. It stimulates the oxidation of glucose in-vitro in the presence of insulin, but is otherwise ineffective in its absence (24). Data also showed that GTF decreases plasma glucose levels in diabetic mice (25), as well as absence of chromium in diabetic patients on total parenteral nutrition (TPN) developed weight loss and hyperglycemia (26). Our results showed lower glycemic response to the oral glucose load after administration of dry extract of PVP at 60 and 90 min compared with glycemic response at 30 min (p < 0,05) in male and female individuals. It is possible that decrease in blood glucose upon the dry extract capsules intake is due to the effect of chrome, which produces maximum decrease of glucose levels 1 or 2 hours after an oral intake (27). If it is known that Recommended Daily Allowances for chrome as 50-200 μg/day for adult men and women (28), and it could be concluded that capsules of the dry extract which contained 18,93 μg of chrome (Table 1), are not enough to bring the blood glucose levels down in all patients due to their individual differences in metabolic functions. Viscous soluble fibers are compounds of dry plant extract (Table 1). They tend to flatten blood glucose and insulin levels postprandially (21). The effects are related to viscosity, general delay in gastric emptying and slow small intestinal uptake of sugars, amino acids. But lower incremental changes in glucose in test compared to control group did not appear, because the amount of soluble fibers in dry plant extract (20,25 μg) was sufficient to produce significant decrease in postprandial glycemia. Study performed on human showed that most diabetics suffer from deficient intracellular vitamin C, because insulin facilitates the transport of vitamin C into cells (22). Results showed that intracellular vitamin C deficiency leads to increasing capillary permeability, poor wound healing, elevations in cholesterol levels (29, 30). Data also showed that vitamin C is principal modulator of free radical activity in diabetes, reduces the accumulation of sorbitol within cells (31) and inhibits the glycosylation of proteins which are linked to complications of diabetes (32). Available data haven’t showed that vitamin C produce hypoglycemic effect. On the basis of these effects of vitamin C one may suggest that long-term oral administration of extract PVP, which contain it, might have beneficial effect in diabetes treatment. In order to estimate safety administration of dry plant extract, we determined presence of lectin, which are contained in seeds of common bean, PVP. Data also showed that it contains a family of plant defense proteins arcelin (ARL) and α-amylase inhibitor (α-AI), which were not analysed in present study but have similar structure with lectin (33). Although comparison of the amino acid sequences of lectins and lectin-like proteins (ARL, α-AI) shows that all four sequences have a high degree of both identity and homology, loops involved in the carbohydrate-binding sites of lectins are absent in the ARL and α-AI (34). Lectin is not degraded in digestive tract due to its structure, and it is able to bind epithelial cells and can cause dramatic changes in the cellular morphology and metabolism of stomach (35). Therefore, its ability to inhibit various intestinal and brush borders hydrolases such as sucrase, isomaltase, maltase glucoamylase, lactase, aminopeptidase, lectin is classified in antinutritional factor (35). According to these findings administration of extract of PVP must be controlled. We also assumed that lectin-like proteins arcelin and α-amylase inhibitor, might be found in dry extract of PVP together with lectin. Data showed that α-AI is a glycoprotein and inhibits pancreatic α-amy-lase in mammals; maximum inhibition is reached after 30 min in contact (33, 36). Although starch was not administered in present study so α-AI could not influence postprandial glycaemia, but its inhibition activity should be take into consideration during use of PVP extract used in alternative diabetes treatment. By looking into the influence of gender on AUC our data showed that male have lower glycemic response to 50g oral glucose load compared to female individuals. There are evidences to support a role of gender in postprandial glycemia. Studies demonstrated that female had the higher postprandial glucose appearance in plasma, as well as higher insulin dependent glucose disposal compared the male individuals. Therefore, it might be concluded that female exhibit greater whole body insulin sensitivity than male individuals. Study performed on animals also showed that postprandial insulin concentrations were higher in male compared to female individuals. The observations that dry extract of PVP tend to produce hypoglycaemic effect in female but not in male individuals can be explained by the presence of chrome which might have greater effect on hypoglycemia in females with lower postprandial insulin concentration.
CONCLUSION
Taking into account all our observation, we could conclude that administration of PVP in dose commonly used in Serbia has no effect on postprandial glycemia either in male or in female healthy individuals. It tends to produce hypoglycemic effect more in female than male individuals due to sufficient amount of chrome in recommended dose which have greater effect on hypoglycemia in females with lower postprandial insulin concentration compared to males. The presence of vitamin C as well as soluble fibers which have beneficial effects on treatment of diabetes, opens the door to further investigations in which the amount of dry plant extract needs to be increased but, consequently, the toxicity of lecithin must be taken into account.
REFERENCES
- 1.Meteljko Z. Organization of diabetes health care in Croatia-“Cro-atian model”. Medicus. 1997;6:243–253. [Google Scholar]
- 2.Ferrannini E. Insulin resistance versus insulin deficiency in non-insulin-dependent diabetes mellitus: problems and perspectives. Endocrin. Rev. 1998;19:491–503. doi: 10.1210/edrv.19.4.0336. [DOI] [PubMed] [Google Scholar]
- 3.Fagan TC, Sowers J. Type 2 diabetes mellitus: greater cardiovascular risk and greater benefits of therapy. Arch. Inter. Med. 1999;159:1033–1034. doi: 10.1001/archinte.159.10.1033. [DOI] [PubMed] [Google Scholar]
- 4.Petlovski R, Hadžić M, Slijepčević M, Juretić D. Biljke u lečenju šećerne bolesti. Biochemica medica. 2001;3:63–70. [Google Scholar]
- 5.Manolova L. Natural Pharmacy, Health & Fitness. 2004 [Google Scholar]
- 6.Casanas F, Pujola M, Bosch L, Sanchez E, Nuez F. Chemical basis for the low sensory perception of the Ganxet bean (Phaseo-lus vulgaris) seed coat. J. Sci. Food and Agricul. 2002;82:1282–1286. [Google Scholar]
- 7.Blumenthal M, Busse WR, Goldberg A, Gruenwald J, Hall T, Riggins CW. The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines. The American Botanical Council, Austin, TX. 1998 [Google Scholar]
- 8.Roman-Ramos R, Flores-Saenz JL, Partida-Hernandez G. Experimental study of the hypoglycemic effect of some antidiabetic plants. Arch. Invest. Med. (Mex) 1991;22:87–93. [PubMed] [Google Scholar]
- 9.Khaleeva LD, Maloshtan LN, Sytnik AG. Comparative evaluation of the hypoglycemic activity of the vegetal complex of Phaseolus vulgaris and chlorpropamide in experimental diabetes. Probl. Endokrinol. (Mosk) 1987;33:69–71. [PubMed] [Google Scholar]
- 10.Petlevski L, Hadzija M, Slijepcevic M, Juretic D. Effect of ‘anti-diabetis’ herbal preparation on serum glucose and fructosamine in NOD mice. J. Ethnopharmacology. 2001;75:181–184. doi: 10.1016/s0378-8741(01)00177-5. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization Study Group. Prevention of diabetes mellitus. WHO Technical Report seriers no 844. Geneva: WHO; 1994. [PubMed] [Google Scholar]
- 12.Horwitz W. AOAC official methods of analysis. 13th Edition. Washington DC: Association of official analytical chemists; 1980. Plants: Metals, atomic absorption method-official first action; p. 31. [Google Scholar]
- 13.Prosky L, Asp N-G, Schweizer TF, DeVries JW, Furda I. Determination of Insoluble, Soluble and Total Dietary Fibre in Foods and Food Products: Interlaboratory Study. J. Assoc. Off. Anal. Chem. 1988;71:1017–1023. [PubMed] [Google Scholar]
- 14.Trajković J, Mirić M, Baras J, Siler S. Analiza životnih namirnica, Tehnološko-metalurškifakultet. Univerzitet u Beogradu. 1983 [Google Scholar]
- 15.Horwitz W. AOAC official methods of analysis. 13th Edition. Washington DC: Association of official analytical chemists; 1980. Fertilizers: Improved Kjeldahl method for nitratefree samples-official; p. 15. [Google Scholar]
- 16.Tietz NW. Textbook of Clinical Chemistry. Philadelphia: W.B. Saunders Company; 1986. [Google Scholar]
- 17.Sudakevitz D, Imberty A, Gilboa-Garber N. Production, properties and specificity of a new bacterial L-fucose- and D-arabi-nose binding lectin of the plant aggressive pathogen ralstonia solanacearum, and its comparison to related plant and microbial lectinc. J. Biochem. 2002;132:353–358. doi: 10.1093/oxfordjournals.jbchem.a003230. [DOI] [PubMed] [Google Scholar]
- 18.Vuksan V, Sievenpiper J, Wong J, Xu Z, Beljen-Zdravković U, Arnason J, et al. American ginseng (panax quinquefolius L.) attenuates postprandial glycemia in time-dependent but not dose-dependent manner in healthy individuals. Am. J. Clin. Nutr. 2001;73:753–758. doi: 10.1093/ajcn/73.4.753. [DOI] [PubMed] [Google Scholar]
- 19.Tucakov J. Lečenje biljem, Rad, Beograd. 1990 [Google Scholar]
- 20.Collings P, Williams C, MacDonald I. Effect of cooking on serum glucose and insulin responses to starch. Br. Med. J. 1981;28:1032. doi: 10.1136/bmj.282.6269.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallaher DD, Scneeman BO. Dietary fiber. In: Ziegler EE, Filer LJ, editors. Present knowledge in nutrition. Washington, DC: ILSI Press; 1996. pp. 87–97. [Google Scholar]
- 22.Sinclair AJ, Taylor PB, Lunec J, Girling AJ, Barnett AH. Low plasma ascorbate in patients with type 2 diabetes mellitus consuming adequate dietary vitamin C. Diabet. Med. 1994;11:893–898. doi: 10.1111/j.1464-5491.1994.tb00375.x. [DOI] [PubMed] [Google Scholar]
- 23.Evans GW, Mertz W, Roginski EE. Interaction of the glucose tolerance factor (GTF) with insulin. Biochem. Biophys. Res. Commun. 1973;5:718–722. doi: 10.1016/0006-291x(73)91303-x. [DOI] [PubMed] [Google Scholar]
- 24.Mertz W, Roginski EE, Schwarz K. Effect of trivalent chromium complexes on glucose uptake by epidymal fat tissue of rats. J. Biol. Chem. 1961;236:318–322. [PubMed] [Google Scholar]
- 25.Tuman RW, Doisy RJ. Metabolic effects of the glucose tolerance factor (GTF) in normal and genetically diabetic mice. Diabetes. 1977;26:820–826. doi: 10.2337/diab.26.9.820. [DOI] [PubMed] [Google Scholar]
- 26.Jeejeebhoy KN, Chu RC, Marliss EB, Greenberg GR, BruceRobertson A. Chromium deficiency, glucose intolerance, and neuropathy reversed by chromium supplementation, in a patient receiving long-term total parental nutrition. Am. J. Clin. Nutr. 1977;30:531–538. doi: 10.1093/ajcn/30.4.531. [DOI] [PubMed] [Google Scholar]
- 27.Vinson JA, Hsiao K-H. Comparative effect of various forms of chrome on serum glucose: an assay for biologically active chromium. Nutritional Reports International. 32:1985. [Google Scholar]
- 28.Committee on dietary allowances, food and nutrition board national research council. Recommended Dietary Allowance (9th rewEdition) Washington DC: National Academy Press; 1980. [Google Scholar]
- 29.Eriksson J, Kohvakka A. Magnesium and ascorbic acid supplementation in diabetes mellitus. Ann. Nutr. Metab. 1995;39:217–223. doi: 10.1159/000177865. [DOI] [PubMed] [Google Scholar]
- 30.Seghieri G, Martinoli L, Miceli M, Ciuti M, D’Alessandri G, Gironi A, et al. Renal excretion of ascorbic acid in insulin dependent diabetes mellitus. Int. J. Vitam. Nutr. Res. 1994;64:119–124. [PubMed] [Google Scholar]
- 31.Cunningham JJ, Mearkle PL, Brown RG. Vitamin C: An aldose reductase inhibitor the normalizes erythrocyte sorbitol in insulin-dependent diabetes mellitus. J. Am. Coll. Nutr. 1994;13:344–350. doi: 10.1080/07315724.1994.10718420. [DOI] [PubMed] [Google Scholar]
- 32.Paolisso G, Balbi V, Volpe C, Varricchio G, Gambardella A, Saccomanno F, et al. Metabolic benefits deriving from chronic vitamin C supplementation in aged non-insulin dependent diabetics. J. Am. Coll. Nutr. 1995;14:387–392. doi: 10.1080/07315724.1995.10718526. [DOI] [PubMed] [Google Scholar]
- 33.Bompard-Gilles C, Rousseau P, Rouge P, Payan F. Substrate mimicry in the active center of a mammalian alpha-amylase: structural analysis of an enzyme-inhibitor complex. Structure. 1996;4:1441–1452. doi: 10.1016/s0969-2126(96)00151-7. [DOI] [PubMed] [Google Scholar]
- 34.Rouge P, Barre A, Causse H, Chatalain C, Porthe G. Arcelin and alpha-amylase inhibitor from the seeds of common bean Phaseo-lus vulgaris are truncated lectins. Biochem. Syst. Ecol. 1993;21:695–703. [Google Scholar]
- 35.Vasconcelos IM, Tadeu J, Oliveira A. Antinutritional properties of plant lectins. Toxicon. 2004;44:385–403. doi: 10.1016/j.toxicon.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Koukiekolo R, Berre-Anton VL, Desseaux V, Moreau Y, Rougé P, Marchis-Mouren G, et al. Mechanism of porcine pancreatic alfa-amylase. Eur. J. Biochem. 1999;265:20–26. doi: 10.1046/j.1432-1327.1999.00611.x. [DOI] [PubMed] [Google Scholar]
- 37.Robertson MD, Livesey G, Mathers JC. Quantitative kinetics of glucose appearance and disposal following a 13C-labelled starchrich meal: comparison of male and female subjects. Br. J. Nutr. 2002;87:569–577. doi: 10.1079/BJNBJN2002586. [DOI] [PubMed] [Google Scholar]
- 38.Sugden MC, Holness MJ. Gender-specific programming of insulin secretion and action. J. Endocinology. 2002;175:757–767. doi: 10.1677/joe.0.1750757. [DOI] [PubMed] [Google Scholar]


