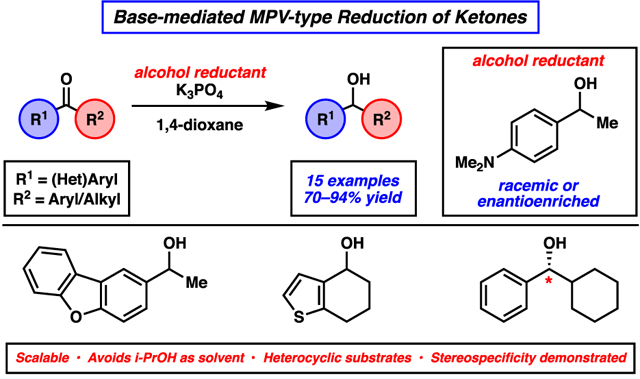
Abstract
An experimental protocol to achieve the Meerwein–Ponndorf–Verley (MPV) reduction of ketones under mildly basic conditions is reported. The transformation is tolerant of a range of ketone substrates, including O- and S-containing heterocycles, is scalable, and shows potential to be used as a platform to access enantioenriched products. These studies provide a general method for achieving the reduction of ketones under mildly basic conditions and offer an alternative protocol to more well-known Al-based MPV reduction conditions.
Graphical Abstract
Graphical abstract
The Meerwein–Ponndorf–Verley (MPV) reaction is an important and powerful tool for the reduction of ketones and aldehydes because of its chemoselectivity, mild reaction conditions, scalability, and low operational cost.1 Discovered nearly a century ago,2 the traditional MPV reduction employs an aluminum alkoxide catalyst generated from a secondary alcohol (most commonly isopropanol) to achieve the reversible transfer hydrogenation of carbonyl substrates (Figure 1).3 This venerable reaction has been featured in the syntheses of several natural products4 and spurred numerous experimental5 and computational studies.6 Despite the synthetic utility of the traditional MPV reduction, several drawbacks exist. These include long reaction times, the need for a large excess of reducing agent, competing side reactions such as aldol condensation and the Tishchenko reduction of aldehydes, and low enantioselectivities in the case of intermolecular asymmetric variants.1,3 Methodological advances to address these limitations include the use of additives,7 microwave irradiation,8 and the development of novel aluminum,9 organoboron,10 and metal alkoxide catalysts (i.e., transition11 and lanthanide12). A particularly efficient aluminum siloxide catalyst has been reported by the Krempner group.9c
Figure 1.
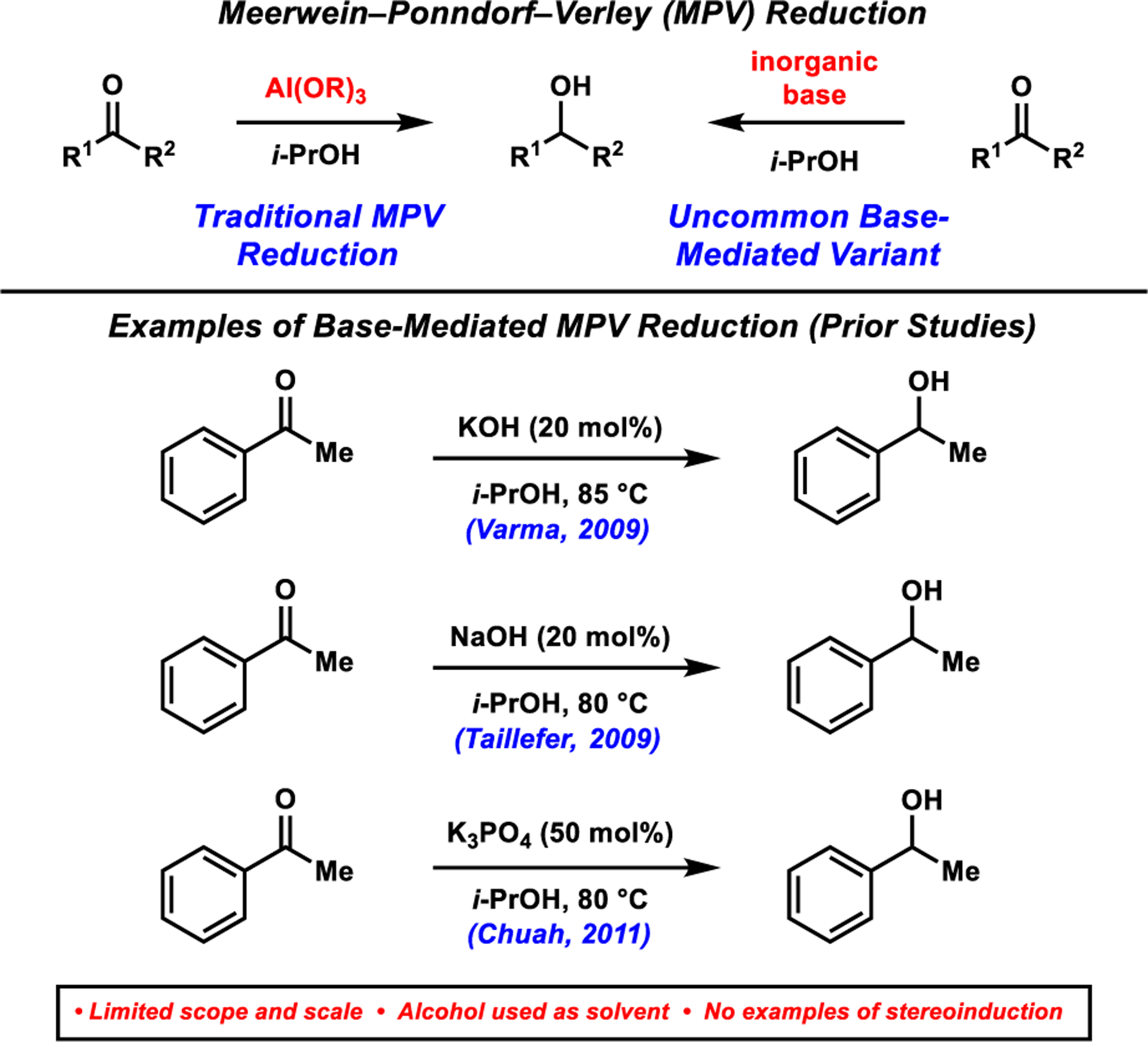
Traditional MPV reduction of ketones and base-mediated variant (prior studies).
A largely unexplored approach to the MPV-type reduction of carbonyls uses simple alkali metal alkoxides (Figure 1).13,14 This variant of the MPV reaction has several benefits including its avoidance of transition and main group metal catalysts, operational simplicity, and compatibility with heteroatoms known to inhibit metal catalysis.3,13 Specifically, isopropoxide catalysts generated from strong alkali bases, such as NaOH13a and KOH13b and milder bases such as K3PO4,13c have been employed in the reduction of aldehydes and ketones. Nevertheless, a number of limitations of the base-mediated MPV reduction remain unaddressed including a limited scope and the reliance on i-PrOH as the solvent and hydride source.15 Additionally, no examples of stereoselective base-mediated MPV reactions exist. We report the use of a simple potassium alkoxide reductant, generated in situ from the corresponding alcohol and K3PO4, for the reduction of a wide range of aromatic ketones. This methodology is tolerant of heterocycles, is scalable, and shows potential for the asymmetric reduction of alkyl–aryl ketones.
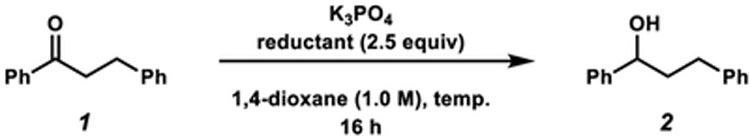
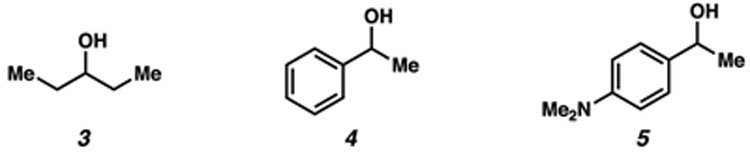
To initiate our studies, we examined the reduction of dihydrochalcone (1) using alkyl–alkyl secondary alcohols and K3PO4, a readily available and mild base (Table 1).16 Subjecting 1 to catalytic K3PO4 using isopropanol or 3-pentanol (3, 2.5 equiv) in 1,4-dioxane at 80 °C provided none of the desired alcohol product 2 (entries 1 and 2).16–18 Owing to the potential reversibility of the reaction,1a–c,16 we turned to the use of aryl–alkyl reductants to bias the reaction equilibrium. Importantly, this class of alcohols enabled greater control of the redox properties of the reductant. We evaluated alcohol 4 and the more electron-rich derivative 5 as reductants,19 anticipating that the stability of the respective aryl ketone and doubly vinylogous amide byproducts would drive the forward reaction to yield 2. Gratifyingly, the use of 2.5 equiv of 4 or 5 provided 2 in 40% and 61% yield, respectively (entries 3 and 4). Employing reductant 5 at 120 °C furnished desired product 2 in 92% yield (entry 5). Finally, alcohol 2 was obtained in near-quantitative yield by utilizing excess base (entry 6).
Table 1.
Optimization of Reaction Conditions
 | ||||
|---|---|---|---|---|
| entry | reductant | equiv K3PO4 | temp (°C) | yield |
| 1 | i-PrOH | 0.50 | 80 | 0% |
| 2 | 3 | 0.50 | 80 | 0% |
| 3 | 4 | 0.50 | 80 | 40% |
| 4 | 5 | 0.50 | 80 | 61% |
| 5 | 5 | 0.50 | 120 | 92% |
| 6 | 5 | 4.0 | 120 | 99% |
 | ||||
General conditions unless otherwise stated: substrate 1 (1.0 equiv, 0.10 mmol), K3PO4 (0.50–4.0 equiv), reductant (2.5 equiv), and 1,4-dioxane (1.0 M) heated at 80–120 °C for 16 h in a sealed vial under an atmosphere of N2. Yields determined by 1H NMR analysis using 1,3,5-trimethoxybenzene as an external standard.
With optimized conditions in hand, we examined a range of aryl ketone substrates in the reduction (Figure 2). Linear and α-branched substrates smoothly underwent reduction, giving rise to alcohols 2 and 6–8 in good yields. Of note, steric bulk on the alkyl substituent of the ketone was tolerated, as shown by the successful reduction of tert-butyl phenyl ketone to furnish alcohol 8 in 83% yield. The reduction of α-tetralone to give α-tetralol (9) in 86% yield demonstrates competence of a cyclic ketone substrate in this transformation. Notably, we found that electron-rich aromatic ketones and those highly decorated with heteroatom substituents underwent facile reduction, as demonstrated by the formation of alcohols 10 and 11 in 81% and 87% yield, respectively. Finally, both electron-rich and electron-deficient benzophenone derivatives were suitable substrates, as shown by the production of alcohol products 12 and 13 in good yields.
Figure 2.
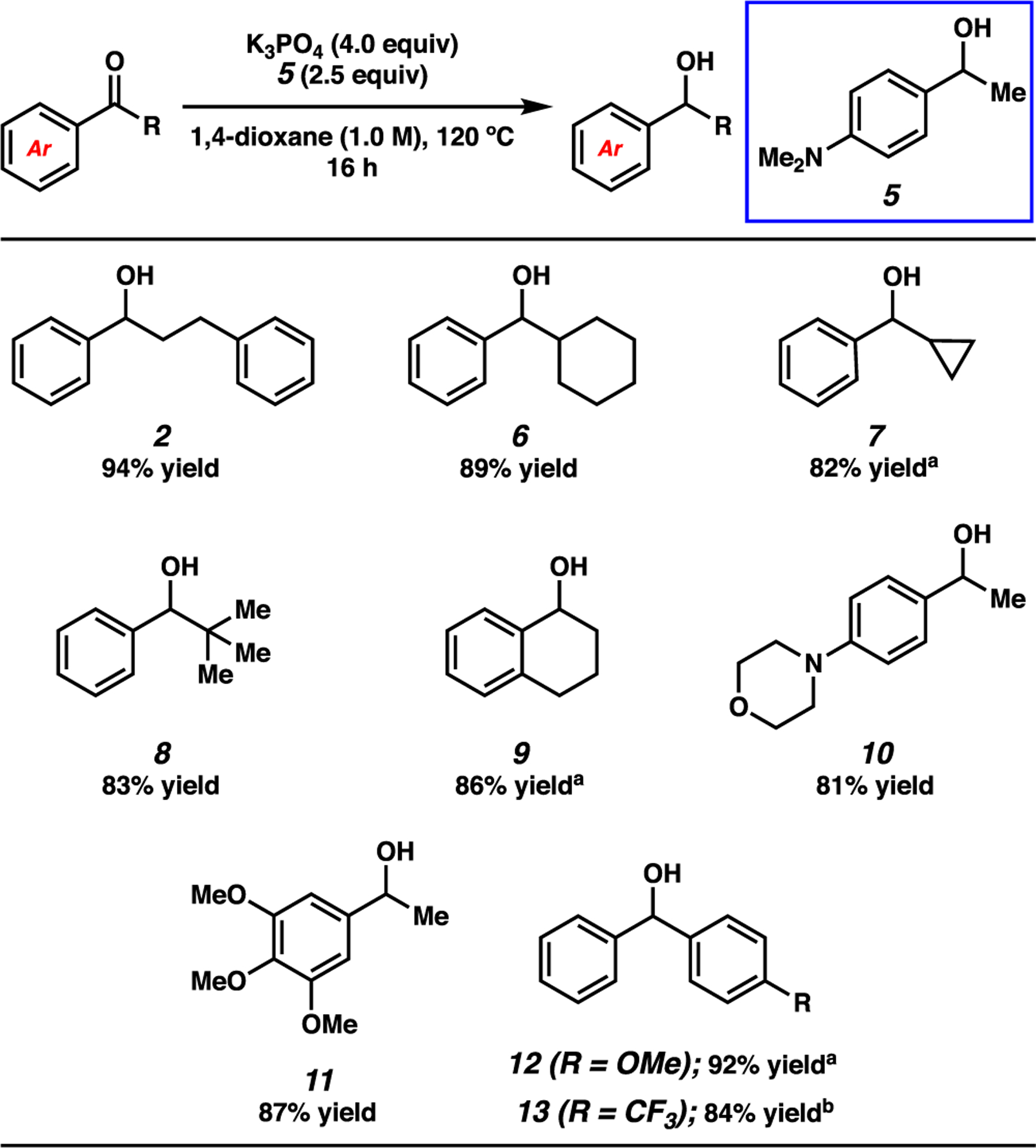
Scope of the base-mediated MPV reduction of aromatic ketones. Conditions: substrate (1.0 equiv, 0.10 mmol), K3PO4 (4.0 equiv), reductant (2.5 equiv), and 1,4-dioxane (1.0 M) heated at 120 °C for 16 h in a sealed vial under an atmosphere of N2. Unless otherwise noted, yields reflect the average of two isolation experiments. a Yield determined by 1H NMR analysis using hexamethylbenzene as an external standard. b Reaction heated at 80 °C for 16 h.
We next set out to evaluate the reactivity of a number of heterocyclic ketone substrates, as only a few examples of base-mediated MPV reductions of heterocyclic ketones have been previously reported (Figure 3).20 Benzofuran- and dibenzofuran-containing ketones underwent reduction to provide alcohols 14 and 15 in 73% and 76% yield, respectively. Benzodioxole and benzodioxane moieties were also well tolerated, as seen by the formation of alcohols 16 and 17 in good yields. Lastly, ketones bearing thiophenes were successfully employed, as judged by the formation of benzothiophene 18 and tetrahydrobenzothiophene 19 in 70% and 73% yield, respectively.21
Figure 3.
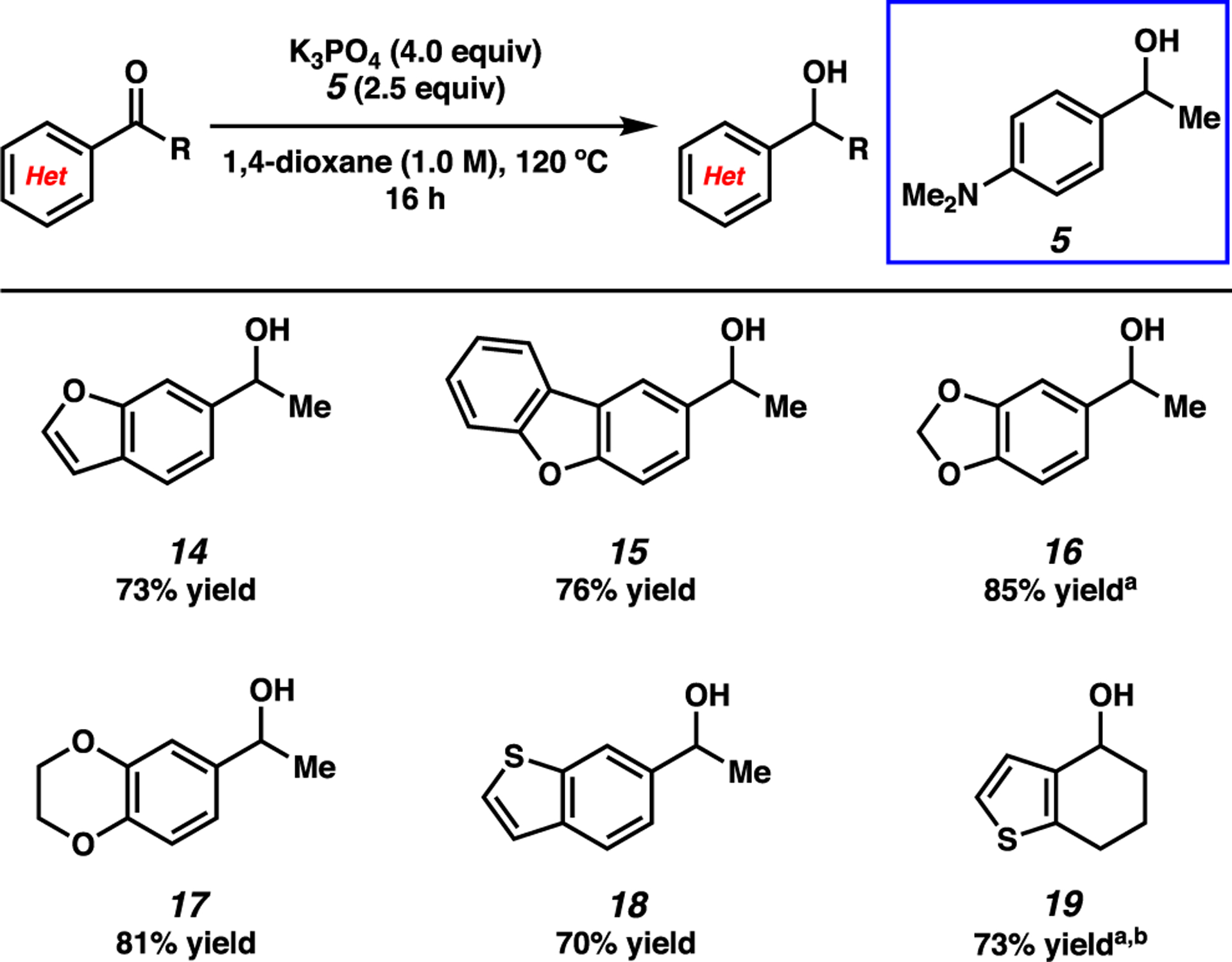
Scope of the base-mediated MPV reduction of heteroaromatic ketones. Conditions: substrate (1.0 equiv, 0.10 mmol), K3PO4 (4.0 equiv), reductant (2.5 equiv), and 1,4-dioxane (1.0 M) heated at 120 °C for 16 h in a sealed vial under an atmosphere of N2. Unless otherwise noted, yields reflect the average of two isolation experiments. a Yield determined by 1H NMR analysis using hexamethylbenzene as an external standard. b Reaction heated at 130 °C for 16 h.
As a demonstration of the utility of the base-mediated MPV reduction of ketones, we performed the additional studies shown in Figure 4. In the first, we performed a gram-scale reduction of acetyldibenzofuran 20.22 Carrying out the reaction at 130 °C for 24 h delivered alcohol 15 in 66% yield, thus demonstrating the scalability of this methodology. Next, we questioned whether this reaction could be used for the synthesis of enantioenriched alcohols. Toward this end, we performed the reduction of phenylcyclohexyl ketone 21 using enantioenriched (R)-5. This proceeded to give alcohol (S)-6 with 50% stereochemical transfer (96% ee of (R)-5 → 48% ee (S)-6). This result underscores the potential of the base-mediated MPV reduction to generate enantioenriched products.1e,12d,23
Figure 4.
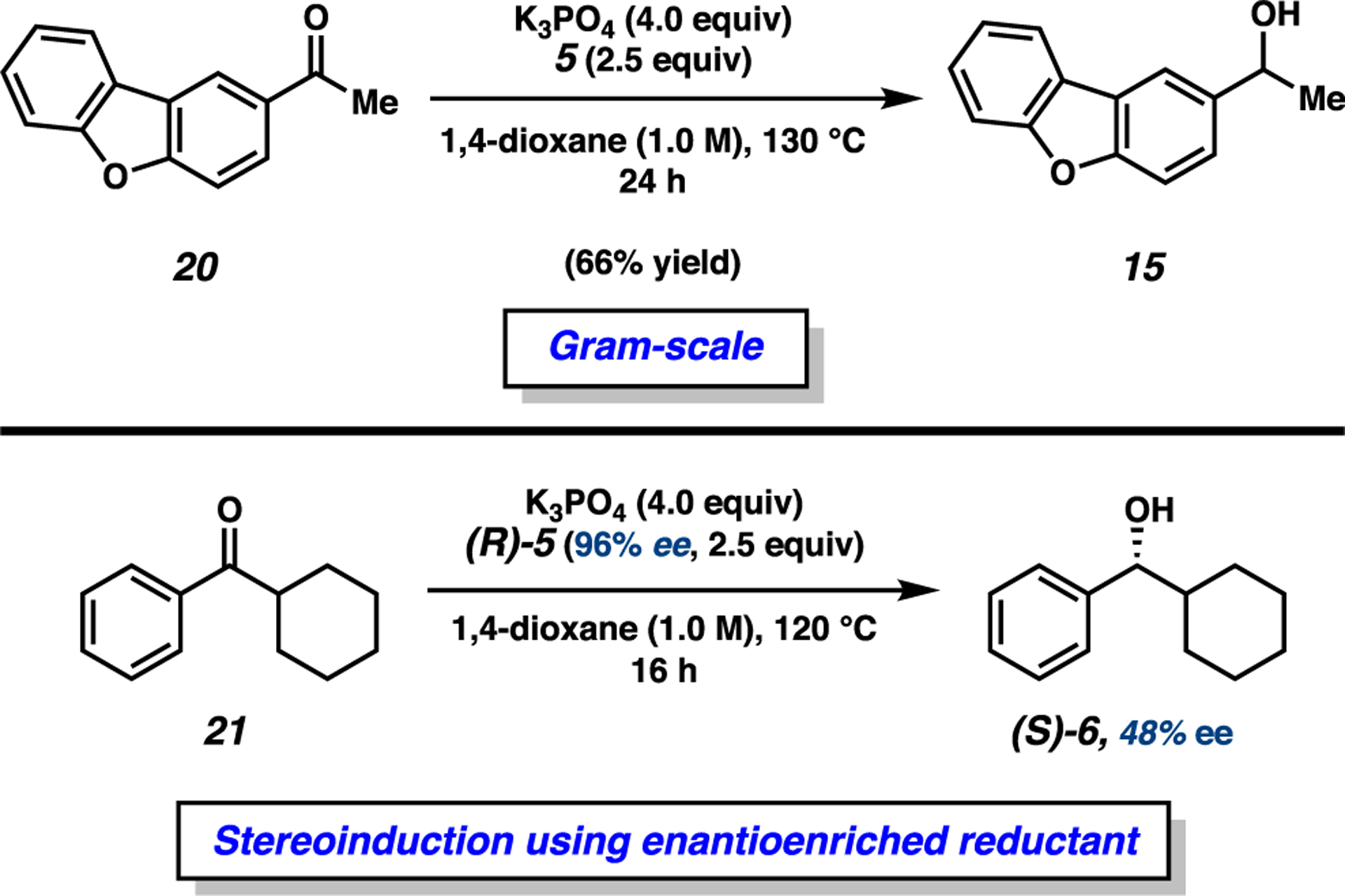
Gram-scale reduction and stereochemical transfer studies demonstrating the synthetic utility of the base-mediated MPV reduction. Conditions: substrate (1.0 equiv), K3PO4 (4.0 equiv), reductant (2.5 equiv), and 1,4-dioxane (1.0 M) heated at the indicated temperature and time in a sealed vial under an atmosphere of N2.
In summary, we have developed the base-mediated MPV reduction of aromatic and heteroaromatic ketones.24 This methodology employs the simple combination of K3PO4 as a mild base and secondary alcohol 5 as the reductant. The transformation is tolerant of a range of ketone substrates, including O- and S-containing heterocycles, and avoids the hydride source being used as the solvent. The reduction has been demonstrated on gram scale and shows potential to be used as a platform to provide enantioenriched products. These studies provide a general platform for achieving the reduction of ketones under mildly basic MPV conditions and offer an alternative protocol to the more classic Al-based MPV reduction. We hope this study will enable the greater utilization of the uncommon base-mediated variant of the MPV reduction in chemical synthesis.
Supplementary Material
■ ACKNOWLEDGMENTS
The authors thank the NIH-NIGMS (R01-GM117016 to N.K.G.), the California Tobacco-Related Disease Research Program (28DT-0006 to T.B.B.), the Trueblood Family (N.K.G.), and the University of California, Los Angeles, for financial support. Colleen Hui (UCLA) is acknowledged for assistance in performing trace metal analysis. Dr. Junyong Kim is acknowledged for experimental assistance. We thank the Nelson laboratory (UCLA) for use of instrumentation. These studies were supported by shared instrumentation grants from the NSF (CHE-1048804) and the NIH NCRR (S10RR025631).
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.orglett.9b02342.
Experimental details and compound characterization data (PDF)
The authors declare no competing financial interest.
■ REFERENCES
- (1).For reviews, see:Johnstone RAW; Wilby AH; Entwistle ID Heterogeneous catalytic transfer hydrogenation and its relation to other methods for reduction of organic compounds. Chem. Rev 1985, 85, 129–170.Cha JS Recent developments in the Meerwein–Ponndorf–Verley and related reactions for the reduction of organic functional groups using aluminum, boron, and other metal reagents: A review. Org. Process Res. Dev 2006, 10, 1032–1053.de Graauw CF; Peters JA; van Bekkum H; Huskens J Meerwein–Ponndorf–Verley reductions and Oppenauer oxidations: An integrated approach. Synthesis 1994, 1007–1017.97.2091Inch TD Asymmetric Synthesis. Synthesis 1970, 466–473.Nishide K; Node M Recent development of asymmetric syntheses based on the Meerwein–Ponndorf–Verley reduction. Chirality 2002, 14, 759–767.Ooi T; Miura T; Itagaki Y; Ichikawa H; Maruoka K Catalytic Meerwein–Ponndorf–Verley (MPV) and Oppenauer (OPP) reactions: Remarkable acceleration of hydride transfer by powerful bidentate aluminum alkoxides. Synthesis 2002, 279–291.Zassinovich G; Mestroni G Asymmetric hydrogen transfer reactions promoted by homogeneous transition metal catalysts. Chem. Rev 1992, 92, 1051–1069.Wilds AL Reduction with aluminum alkoxides. Org. React. 2011, 2, 178–223. (i) Djerassi, C. The Oppenauer oxidation. Org. React 2011, 6, 207–272.
- (2).For seminal publications, see:Meerwein H; Schmidt R Ein neues Verfahren zur Reduktion von Aldehyden und Ketonen. Liebigs Ann. 1925, 444, 221–238.Verley A The exchange of functional groups between two molecules: The passage of ketones to alcohols and the reverse. Bull. Soc. Chim. Fr 1925, 37, 871–874.Ponndorf WZ The reversible exchange of oxygen between aldehydes or ketones on the one hand and primary or secondary alcohols on the other hand. Angew. Chem 1926, 39, 138–143.
- (3).Woodward RB; Wendler NL; Brutschy FJ Quininone. J. Am. Chem. Soc 1945, 67, 1425–1429. [Google Scholar]
- (4).(a) Gammill RB The synthesis and chemistry of functionalized furochromones. 2. The synthesis, Sommelet-Hauser rearrangement, and conversion of 4,9-dimethoxy-7-[(methylthio)methyl]-5H-furo-(3,2-g)benzopyran-5-one to ammiol. J. Org. Chem 1984, 49, 5035–5041. [Google Scholar]; (b) Sano T; Toda J; Maehara N; Tsuda Y Synthesis of erythrina and related alkaloids. 17. Total synthesis of dl-coccuvinine and dl-coccolinine. Can. J. Chem 1987, 65, 94–98. [Google Scholar]; (c) Evans DA; Rieger DL; Jones TK; Kaldor SW Assignment of stereochemistry in the oligomycin/rutamycin/cytovaricin family of antibiotics. Asymmetric synthesis of the rutamycin spiroketal synthon. J. Org. Chem 1990, 55, 6260–6268. [Google Scholar]; (d) Toyota M; Odashima T; Wada T; Ihara M Application of palladium-catalyzed cycloalkenylation reaction to C20 gibberellin synthesis: Formal syntheses of GA12, GA111, and GA112. J. Am. Chem. Soc 2000, 122, 9036–9037. [Google Scholar]
- (5).(a) Doering W v. E.; Aschner, T. C. Mechanism of the alkoxide-catalyzed carbinol–carbonyl equilibrium. J. Am. Chem. Soc 1953, 75, 393–397. [Google Scholar]; (b) Moulton WN; Van Atta RE; Ruch RR Mechanism of the Meerwein–Ponndorf–Verley reduction. J. Org. Chem 1961, 26, 290–292. [Google Scholar]; (c) Yager BJ; Hancock CK. Equilibrium and kinetic studies of the Meerwein–Ponndorf–Verley–Oppenauer (MPVO) reaction. J. Org. Chem 1965, 30, 1174–1179. [Google Scholar]; (d) Screttas CG; Cazianis CT Mechanism of Meerwein–Ponndorf–Verley type reductions. Tetrahedron 1978, 34, 933–940. [Google Scholar]; (e) Ashby EC; Argyropoulos JN Single electron transfer in the Meerwein–Ponndorf–Verley reduction of benzophenone by lithium alkoxides. J. Org. Chem 1986, 51, 3593–3597. [Google Scholar]; (f) Shiner VJ Jr.; Whittaker D Kinetics of the Meerwein–Ponndorf–Verley reaction. J. Am. Chem. Soc 1969, 91, 394–398. [Google Scholar]
- (6).(a) Cohen R; Graves CR; Nguyen ST; Martin JML; Ratner MA The mechanism of aluminum-catalyzed Meerwein–Schmidt–Ponndorf–Verley reduction of carbonyls to alcohols. J. Am. Chem. Soc 2004, 126, 14796–14803. [DOI] [PubMed] [Google Scholar]; (b) Boronat M; Corma A; Renz M Mechanism of the Meerwein–Ponndorf–Verley–Oppenauer (MPVO) redox equilibrium on Sn– and Zr–beta zeolite catalysts. J. Phys. Chem. B 2006, 110, 21168–21174. [DOI] [PubMed] [Google Scholar]; (c) Sominsky L; Rozental E; Gottlieb H; Gedanken A; Hoz S Uncatalyzed Meerwein–Ponndorf–Oppenauer–Verley reduction of aldehydes and ketones under supercritical conditions. J. Org. Chem 2004, 69, 1492–1496. [DOI] [PubMed] [Google Scholar]
- (7).(a) Kow R; Nygren R; Rathke MW Rate enhancement of the Meerwein–Ponndorf–Verley–Oppenauer reaction in the presence of proton acids. J. Org. Chem 1977, 42, 826–827. [Google Scholar]; (b) Akamanchi KG; Varalakshmy NR Aluminium isopropoxide - TFA, a modified catalyst for highly accelerated Meerwein–Ponndorf–Verley (MPV) reduction. Tetrahedron Lett. 1995, 36, 3571–3572. [Google Scholar]; (c) Akamanchi KG; Varalakshmy NR Truly catalytic Meerwein–Ponndorf–Verley (MPV) reduction. Tetrahedron Lett. 1995, 36, 5085–5088. [Google Scholar]
- (8).Barbry D; Torchy S Accelerated reduction of carbonyl compounds under microwave irradiation. Tetrahedron Lett. 1997, 38, 2959–2960. [Google Scholar]
- (9).(a) Graves CR; Scheidt KA; Nguyen ST Enantioselective MSPV reduction of ketimines using 2-propanol and (BINOL)AlIII. Org. Lett 2006, 8, 1229–1232. [DOI] [PubMed] [Google Scholar]; (b) Campbell EJ; Zhou H; Nguyen ST The asymmetric Meerwein–Schmidt–Ponndorf–Verley reduction of prochiral ketones with iPrOH catalyzed by Al catalysts. Angew. Chem., Int. Ed 2002, 41, 1020–1022. [DOI] [PubMed] [Google Scholar]; (c) McNerney B; Whittlesey B; Cordes DB; Krempner C. A well-defined monomeric aluminum complex as an efficient and general catalyst in the Meerwein-Ponndorf-Verley reduction. Chem. - Eur. J 2014, 20, 14959–14964. [DOI] [PubMed] [Google Scholar]
- (10).(a) Midland MM; Tramontano A B-Alkyl-9-borabicyclo-[3.3.1]nonanes as mild, chemoselective reducing agents for aldehydes. J. Org. Chem 1978, 43, 1470–1471. [Google Scholar]; (b) Chandrasekharan J; Ramachandran PV; Brown HC Diisopinocampheylchloroborane, a remarkably efficient chiral reducing agent for aromatic prochiral ketones. J. Org. Chem 1985, 50, 5446–5448. [Google Scholar]
- (11).(a) Krohn K; Knauer B The diastereoselectivity of zirconium alkoxide catalysed Meerwein–Ponndorf–Verley reductions. Liebigs Ann. 1995, 1347–1351. [Google Scholar]; (b) Battilocchio JM; Hawkins SV; Ley A Mild and Efficient Flow Procedure for the Transfer Hydrogenation of Ketones and Aldehydes using Hydrous Zirconia C. Org. Lett 2013, 15, 2278–2281. [DOI] [PubMed] [Google Scholar]
- (12).(a) Kagan HB; Namy JL Lanthanides in organic synthesis. Tetrahedron 1986, 42, 6573–6614. [Google Scholar]; (b) Namy JL; Souppe J; Collin J; Kagan HB New preparations of lanthanide alkoxides and their catalytical activity in Meerwein–Ponndorf–Verley–Oppenauer reactions. J. Org. Chem 1984, 49, 2045–2049. [Google Scholar]; (c) Okano T; Matsuoka M; Konishi H; Kiji J Meerwein–Ponndorf–Verley reduction of ketones and aldehydes catalyzed by lanthanide tri-2-propoxides. Chem. Lett 1987, 16, 181–184. [Google Scholar]; (d) Evans DA; Nelson SG; Gagné MR; Muci AR A chiral samarium-based catalyst for the asymmetric Meerwein–Ponndorf–Verley reduction. J. Am. Chem. Soc 1993, 115, 9800–9801. [Google Scholar]; (e) Molander GA; McKie JA Samarium(II) iodide induced sequential intramolecular nucleophilic acyl substitution and stereospecific intramolecular Meerwein–Ponndorf–Verley reduction/Oppenauer oxidation. J. Am. Chem. Soc 1993, 115, 5821–5822. [Google Scholar]; (f) Xianming H; Kellogg RM Asymmetric reduction and Meerwein–Ponndorf–Verley reaction of prochiral aromatic ketones in the presence of optically pure 1-aryl-2,2-dimethylpropane-1,3-diols. Recl. Trav. Chim. Pays-Bas 1996, 115, 410–417. [Google Scholar]
- (13).(a) Ouali A; Majoral J-P; Caminade A-M; Taillefer M NaOH-promoted hydrogen transfer: Does NaOH or traces of transition metals catalyze the reaction? ChemCatChem 2009, 1, 504–509. [Google Scholar]; (b) Polshettiwar V; Varma RS Revisiting the Meerwein–Ponndorf–Verley reduction: a sustainable protocol for transfer hydrogenation of aldehydes and ketones. Green Chem. 2009, 11, 1313–1316. [Google Scholar]; (c) Radhakrishan R; Do DM; Jaenicke S; Sasson Y; Chuah G-K Potassium phosphate as a solid base catalyst for the catalytic transfer hydrogenation of aldehydes and ketones. ACS Catal. 2011, 1, 1631–1636. [Google Scholar]
- (14).Mojtahedi MM; Akbarzadeh E; Sharifi R; Abaee MS Lithium bromide as a flexible, mild, and recyclable reagent for solvent-free Cannizzaro, Tishchenko, and Meerwein–Ponndorf–Verley reactions. Org. Lett 2007, 9, 2791–2793. [DOI] [PubMed] [Google Scholar]
- (15). Under the conditions reported by Chuah and co-workers (ref 12c), only cyclohexanone, 4-tert-butyl cyclohexanone, and acetophe-none were evaluated for reactivity using K3PO4/i-PrOH affording the respective alcohol products in 55%, 30%, and 38% yield.
- (16). Subjecting dihydrochalcone (1) to previously reported conditions for the reduction of ketones using catalytic K3PO4 using i-PrOH as a solvent gave the desired product 2 in only 47% yield.
- (17). Although 1,4-dioxane was chosen for these studies, we found other solvents could be employed (see SI for details).
- (18).K3PO4 is roughly 103 less basic than NaOH and KOH: for the pKa of KH2PO4 and H2O, respectively, see:Bruice PY Organic Chemistry, 6th ed; Prentice Hall: Boston, 2011.Bordwell FG Equilibrium acidities in dimethylsulfoxide solution. Acc. Chem. Res 1988, 21, 456–463.
- (19). Using DFT calculations (M06–2X/6–31G(d)), we estimate that the conversion of 1 and 5 to 2 and reduced 5 is thermodynamically favorable by ~2 kcal/mol.
- (20). Previous reports on the base-mediated MPV reductions of ketones using NaOH and KOH have shown only a handful of heterocyclic substrates undergoing reduction (see refs 12a, b). The base-mediated MPV reduction of heterocyclic ketones using K3PO4 has not been previously reported.
- (21). Subjecting N-containing heterocyclic ketones such as N-MOM 4-acetyl indole and 4-acetyl pyridine to base-mediated MPV reduction conditions led to lower yields of the products (20% and 11% yield, respectively).
- (22).Dibenzofurans have found application in OLEDs, bioactive molecules, and chemical probes:Kim S-Y; Hwang S-H; Kim Y-K; Jung H-J; Lim J-O; Han S-H; Jeong E-J; Park J-H; Lee E-Y; Lee B-R; Lee J-H Condensed-cyclic compound and organic light-emitting device. U.S. Patent 20180248127, Jul. 25, 2012.Patpi SR; Pulipati L; Yogeeswari P; Sriram D; Jain N; Sridhar B; Murthy R; Devi TA; Kalivendi SV; Kantevari S Design, synthesis, and structure–activity correlations of novel dibenzo-[b,d]furan, dibenzo[b,d]thiophene, and N-methylcarbazole clubbed 1,2,3-triazoles as potent inhibitors of Mycobacterium tuberculosis. J. Med. Chem 2012, 55, 3911–3922.Liu L-X; Wang X-Q; Yan J-M; Li Y; Sun C-J; Chen W; Zhou H-B; Yang X-D Synthesis and antitumor activities of novel dibenzo[b,d]furan–imidazole hybrid compounds. Eur. J. Med. Chem 2013, 66, 423–437.Lusic H; Uprety R; Deiters A Improved synthesis of the two-photon caging group 3-nitro-2-ethyldibenzofuran and its application to a caged thymidine phosphoramidite. Org. Lett 2010, 12, 916–919.
- (23).For select examples of intermolecular asymmetric MPV reductions of ketones, see:Doering WE; Young RW Partially asymmetric Meerwein–Ponndorf–Verley reductions. J. Am. Chem. Soc 1950, 72, 631.Nandi P; Solovyov A; Okrut A; Katz A AlIII–calix[4]arene catalysts for asymmetric Meerwein–Ponndorf–Verley reduction. ACS Catal. 2014, 4, 2492–2495.Wu W; Zou S; Lin L; Ji J; Zhang Y; Ma B; Liu X; Feng X Catalytic asymmetric Meerwein–Ponndorf–Verley reduction of glyoxylates induced by a chiral N,N′-dioxide/Y(OTf)3 complex. Chem. Commun. 2017, 53, 3232–3235.Ooi T; Miura T; Maruoka K Highly efficient, catalytic Meerwein–Ponndorf–Verley reduction with a novel bidentate aluminum catalyst. Angew. Chem., Int. Ed 1998, 37, 2347–2349.
- (24). Based on a prior mechanistic proposal by Chuah and coworkers (ref 13c) and our observation that the reaction does not proceed in the presence of non-basic potassium salts, the term “base-mediated” was deemed an appropriate descriptor for this methodology. However, proposing a well-supported mechanism for this transformation would be premature.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


