Abstract
Rationale: Decreasing medication overuse represents an opportunity to avoid harm and costs in the era of value-based purchasing. Studies of inhaled corticosteroids (ICS) overuse in chronic obstructive pulmonary disease (COPD) have examined prevalent use. Understanding initiation of low-value ICS among complex patients with COPD may help shape deadoption efforts.
Objectives: Examine ICS initiation among a cohort with low exacerbation risk COPD and test for associations with markers of patient and health system complexity.
Methods: Between 2012 and 2016, we identified veterans with COPD from 21 centers. Our primary outcome was first prescription of ICS. We used the care assessment needs (CAN) score to assess patient-level complexity as the primary exposure. We used a time-to-event model with time-varying exposures over 1-year follow-up. We tested for effect modification using selected measures of health system complexity.
Results: We identified 8,497 patients with COPD without an indication for ICS and did not have baseline use (inception cohort). Follow-up time was four quarters. Patient complexity by a continuous CAN score was associated with new dispensing of ICS (hazard ratio = 1.17 per 10-unit change; 95% confidence interval = 1.13–1.21). This association demonstrated a dose–response when examining quartiles of CAN score. Markers of health system complexity did not modify the association between patient complexity and first use of low-value ICS.
Conclusions: Patient complexity may represent a symptom burden that clinicians are attempting to mitigate by initiating ICS. Lack of effect modification by health system complexity may reflect the paucity of structural support and low prioritization for COPD care.
Keywords: chronic obstructive pulmonary disease, inhaled corticosteroids, care quality
Inhaled corticosteroids (ICSs) were among the first long-term therapies developed for treatment of chronic obstructive pulmonary disease (COPD), and early studies demonstrated a reduced risk of COPD exacerbations (1–4). As a result, ICSs are among the most commonly prescribed inhaled medications for the condition. In these initial and subsequent trials, investigators noted an increased risk of pneumonia that waned with discontinuation (5–7). Additional observational studies demonstrated increased risk of osteoporotic fractures, poor diabetes control, and cataracts from ICS use (8–11). Subsequent comparative efficacy studies demonstrate marginal benefit from adding ICS to other long-acting inhaled bronchodilators for treatment of stable, low–exacerbation risk COPD. Collectively, this led to the narrowing of the indications for ICS for patients with COPD in recent updates to the GOLD (Global Initiative for Chronic Obstructive Lung Disease) societal statement in 2017 (2).
Several studies have found that the guideline recommendations for prescribing ICS in COPD are not followed in clinical practice (12). Current estimates suggest that ICSs are commonly prescribed in up to 40% of patients with COPD (13), and that 47–89% of existing ICS prescriptions are potentially unnecessary (“potential overuse and low value”) (14, 15). Deadopting ICS has great potential for harm reduction to patients and cost savings to health systems. Given this interest in reducing harm and costs, a few studies of ICS overuse have been performed using cohorts of prevalent users (16, 17). Although these studies have shown important barriers to deadoption, the findings are subject to prevalent user bias (18), and do not describe why low-value medications are initiated. Identifying an inception cohort of new users mitigates this bias to provide a new understanding of the potential drivers of ICS overuse. These insights may help health systems curtail low-value prescribing practices as they navigate value-based purchasing.
Both patient- and system-level complexity have the potential to influence the prescription of low-value ICS. Patients with COPD have multiple mechanisms and risk factors for dyspnea that a clinician may attribute to COPD and respond by initiating ICS. They also have multiple comorbidities (19, 20), frailty (21, 22), mental health disorders (23, 24), lower health literacy, education (25, 26), and socioeconomic status (27, 28). The Department of Veterans Affairs (VA) health system uses a unique measure, the care assessment needs (CAN) score, that encompasses these factors and reflects complexity at the patient level (29). The concept of complexity extends beyond the patient to the level of the healthcare system. System-level complexity reflects available services and the center’s ability to deliver highly coordinated, effective care (30–32). As patients and systems become more complex, however, the number of opportunities for failures in healthcare delivery likely increases (33, 34). We evaluated the association between patient complexity and incident prescription of ICS in a national cohort of Veterans with low–exacerbation risk COPD who did not have an indication for ICS. We hypothesized that greater patient complexity would be associated with initiation of low-value ICS. Given the known differences in the complexity of health care systems, we explored whether center complexity modified the association between patient-level complexity and initiation of low-value ICS.
Methods
Cohort and Participants
We conducted a cohort study of Veterans with COPD who received a pulmonary function test (PFT) from 2012 to 2016. We included patients with a clinical diagnosis of COPD by International Classification of Diseases (ICD)-9/10 code (35, 36) and airflow obstruction on PFT (post-bronchodilator forced expiratory volume in 1 second [FEV1]/forced vital capacity < 0.7). The cohort entry date was the date of the first PFT and patients were followed for 1 year or until death.
To create the inception cohort without an indication for ICS, we excluded patients prescribed ICS in the year before entry (prevalent users) and those missing a CAN score. We excluded patients with an indication for ICS, including an asthma ICD9/10 diagnosis in the 10 prior years, and those experiencing two or more outpatient and/or one or more inpatient exacerbation in the year before entry. Inpatient exacerbations were defined by ICD 9/10 codes consistent with COPD at discharge. The outpatient exacerbations were defined by outpatient visits with an ICD code of COPD plus a prescription for a corticosteroid and/or respiratory antibiotic (37).
Data Collection
We used the CAN score as our primary exposure. The CAN score was developed to estimate the probabilities of hospitalization and death, and are subsequently ranked into scores between 0 and 100 (29). Scores between 95 and 100 represent the 5% of patients with the highest probability for hospitalization or death. The CAN uses approximately 50 characteristics, including demographics, vital signs, health factors, utilization, and medications. The CAN score is used clinically and calculated automatically each week for all patients seen in VA primary care. We calculated an average CAN score at baseline and over each quarter for 1 year of follow-up.
Our main outcome was first prescription of low-value ICS. We assessed incident prescription of ICS in each quarter after the cohort entry date for 1 year of follow-up time. The outcome was dichotomized (initiated vs. did not initiate) for each quarter.
We used two markers from Strategic Analytics for Improvement and Learning (SAIL) report data to characterize system-level complexity (38). The SAIL report includes structural data on COPD-specific hospitalization volume and care-complexity designations. Using SAIL report data, we characterized each site as high versus low site-complexity (VHA care complexity designation 1a–1c = high site complexity; 2–3 = low site complexity) and high versus low site volume (≥250 annual COPD hospitalizations = high site volume; <250 hospitalizations = low site volume). We used SAIL report data from 2016 due to its completeness in that year for COPD-related metrics.
Statistical Analysis
To assess the association between CAN scores with ICS initiation, we modeled a time-to-event analysis using Cox proportional hazards with a time-varying CAN exposure. Failure was defined as the first prescription of ICS for each quarter of follow-up time. Veterans were censored at the time of death or at the end of follow-up, whichever came first. In light of the competing risk of death, and to account for COPD exacerbations in the year after index, we also used a competing risk regression model created by Fine and Gray (39). This accounted for the subdistribution of competing risk from death and allowed censoring for patients who experienced an exacerbation, and therefore became eligible for initiation of ICS.
We tested both a continuous CAN score, and a CAN score categorized by quartiles given the high-score–skewed distribution of the scores. We included patient age in the model and did not include additional covariates due to concerns with model overfit from the comprehensive nature of the CAN score. Each model was clustered by site to account for correlated data.
To assess effect modification by health system complexity, we tested two models. The first model included the binary site complexity parameter plus an interaction term for site complexity and continuous CAN score. The second included the binary site-volume parameter plus an interaction term for site-volume and CAN score. The interaction terms were examined for statistical significance. The proportional hazards assumption was met in all models with Schoenfeld residuals.
We performed sensitivity analyses to address the changes in the 2017 GOLD statement recommendations regarding indications for ICS (2). At the time of this cohort, the 2011 GOLD statement was in use and ICSs were recommended for severe COPD by FEV1 (≤50% predicted). Starting in 2017, spirometric severity was not included in the GOLD recommendations for ICS, and therefore we excluded patients with severe COPD by FEV1.
We used STATA version 16 (StataCorp LLC) for analysis. This study was conducted as a quality improvement project under the VA Quality Enhancement Research Initiative program sponsored by the Office of Specialty Care Services.
Results
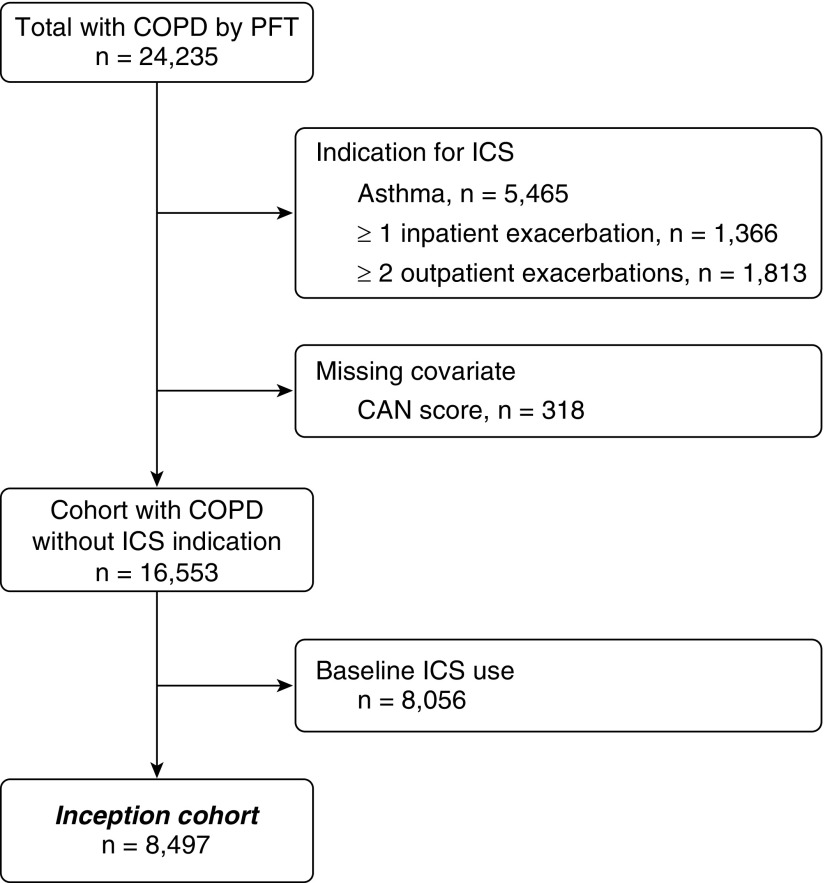
Overall, 24,235 Veterans with a COPD diagnosis and airflow obstruction were available for review. We excluded 1,366 patients with a COPD hospital admission and 1,813 patients with two or more outpatient COPD exacerbations in the year before cohort entry. We also excluded an additional 5,465 patients diagnosed with asthma, because ICS may be beneficial for this population. A total of 8,056 patients received ICS at baseline and were excluded as prevalent users; 318 patients were excluded for missing CAN scores. Exclusion criteria were not mutually exclusive. After all exclusions, 8,497 patients with COPD at a low exacerbation risk remained for analysis for whom the mean follow-up was four quarters (Figure 1).
Figure 1.
Cohort selection diagram. CAN = care assessment needs; COPD = chronic obstructive pulmonary disease; ICS = inhaled corticosteroids; PFT = pulmonary function test.
At baseline, the sample had a mean age of 68.4 years (SD = 8.8). The baseline characteristics of Veterans are presented, stratified by CAN score in quartiles (Table 1). The quartiles were unevenly distributed due to repeated values and ties within the CAN scores. Those in the highest quartile of patient complexity were more likely to be older and not married. Subjects with greater complexity also had a greater proportion of comorbidities, including severe COPD by FEV1, and greater utilization of healthcare services. The distribution of high-complexity patients varied significantly across the 21 sites (range of proportion, 9–32%). There were 654 deaths (7.7%) during follow-up.
Table 1.
Baseline characteristics of the cohort stratified by quartiles of care assessment needs score score
| Cohort (n = 8,497) | CAN0–25% (n = 2,212) | CAN25–50% (n = 2,302) | CAN50–75% (n = 1,865) | CAN75–100% (n = 2,118) | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age at PFT, mean (SD), yr | 68.4 (8.8) | 67.4 (8.2) | 68.2 (8.5) | 68.4 (8.9) | 69.7 (9.4) |
| Sex, male | 8,257 (97) | 2,165 (98) | 2,227 (98) | 1,812 (97) | 2,053 (97) |
| Race | |||||
| White | 7,055 (83) | 1,870 (85) | 1,928 (84) | 1,543 (83) | 1,714 (81) |
| Black | 827 (10) | 156 (7) | 213 (9) | 192 (11) | 260 (12) |
| Marital status | |||||
| Married | 3,970 (47) | 1,310 (59) | 1,093 (48) | 773 (42) | 794 (38) |
| Service connected | 4,539 (53) | 1,152 (52) | 1,259 (55) | 1,029 (55) | 31,098 (52) |
| Body mass Index | |||||
| Underweight | 306 (4) | 65 (3) | 67 (3) | 72 (4) | 102 (5) |
| Normal | 2,580 (30) | 660 (30) | 678 (30) | 561 (30) | 681 (32) |
| Overweight | 2,744 (32) | 763 (35) | 741 (32) | 560 (30) | 680 (32) |
| Obese | 2,840 (34) | 713 (32) | 806 (35) | 669 (36) | 652 (31) |
| Smoking status, current | 3,367 (44) | 880 (45) | 932 (45) | 721 (43) | 834 (42) |
| COPD severity | |||||
| Severe COPD by FEV1 | 2,067 (24) | 511 (23) | 564 (25) | 431 (23) | 561 (27) |
| Long-acting β-agonist | 428 (5) | 121 (5) | 121 (5) | 91 (5) | 98 (5) |
| Long-acting muscarinic antagonist | 1,705 (20) | 422 (19) | 492 (21) | 395 (21) | 396 (19) |
| Comorbidities | |||||
| Congestive heart failure | 1,222 (14) | 45 (2) | 153 (7) | 275 (15) | 749 (35) |
| Diabetes | 2,297 (27) | 371 (17) | 566 (25) | 577 (31) | 783 (37) |
| Utilization, n (%) | |||||
| Pulmonary visit | 750 (9) | 83 (4) | 159 (7) | 194 (10) | 314 (15) |
| Cardiology visit | 1,039 (12) | 81 (4) | 159 (7) | 248 (13) | 551 (26) |
| Primary care visit | 3,651 (43) | 943 (43) | 994 (43) | 780 (42) | 934 (44) |
Definition of abbreviations: CAN = care assessment needs score; COPD = chronic obstructive pulmonary disease; FEV1 = forced expiratory volume in 1 second; PFT = pulmonary function test; SD = standard deviation.
All values are presented as n (%) unless otherwise indicated.
Over the follow-up period, we identified 1,889 (22.2%) patients with a first ICS prescription. Only 48 of those patients (2.5% of 1,889) were on dual long-acting antimuscarinic and long-acting β-agonist before initiation of ICS as third-line therapy. In the model testing CAN score as a continuous measure, higher CAN score was significantly associated with first ICS prescription (hazard ratio [HR] = 1.17 per 10 CAN units; 95% confidence interval [CI], 1.13–1.21), adjusted for age. This association demonstrated a dose–response when analyzing CAN scores by quartile (Table 2). In sensitivity analyses, the association of CAN score with incident ICS did not change after excluding subjects with severe COPD by FEV1.
Table 2.
Risk of initiation of low-value inhaled corticosteroids by care assessment needs scores
| CAN | Incident ICS (n) | Person-Time at Risk, Person-Quarters | Incident ICS Rate per Person-Quarter | HR (95% CI) |
|---|---|---|---|---|
| First quartile | 416 | 7,736 | 0.054 | 1.00 (reference) |
| Second quartile | 463 | 8,433 | 0.055 | 1.39 (1.20–1.61) |
| Third quartile | 459 | 6,185 | 0.074 | 1.69 (1.44–1.97) |
| Fourth quartile | 551 | 6,936 | 0.079 | 2.02 (1.75–2.34) |
| CAN score (linear) | 1,889 | 29,290 | 0.064 | 1.017 (1.013–1.021) |
Definition of abbreviations: CAN = care assessment needs score; CI = confidence interval; HR = hazard ratio; ICS = inhaled corticosteroids.
Values are presented as hazard ratios with 95% CIs and are adjusted for age and clustered by site.
In the year after index, 212 patients (2.5% of 8,497) were hospitalized for COPD and another 344 (4.0% of 8,497) had two or more outpatient exacerbations, and therefore became eligible for ICS. When accounting for these exacerbations during follow-up and for the competing risk of death, the association between CAN score and initiation of ICS persisted (HR, 1.030; 95% CI, 1.016–1.025).
The 21 healthcare sites in this study represented diverse geographic areas across the regions of the United States with near complete representation from the 19 Veterans Integrated Service Networks. The initiation of low-value ICS varied significantly across the 21 sites (range of proportion, 0–34%). Four sites were designated as “low site complexity,” and seven sites were assigned “high site volume.” The annual COPD hospitalization volume ranged from 42 to 560. Neither site complexity nor site volume significantly modified the association between CAN score and incident low-value ICS (Table 3).
Table 3.
Tests of effect modification by health system complexity and chronic obstructive pulmonary disease–volume
| Variable | HR (95% CI) | Interaction P Value |
|---|---|---|
| Model 1 | ||
| CAN score | 1.02 (1.01–1.03) | |
| Site complexity | 1.38 (0.88–2.16) | |
| CAN × site complexity | 0.997 (0.99–1.01) | 0.45 |
| Model 2 | ||
| CAN score | 1.02 (1.01–1.02) | |
| Site COPD-volume | 1.38 (0.85–2.24) | |
| CAN × site COPD-volume | 0.997 (0.99–1.00) | 0.24 |
Definition of abbreviations: CAN = care assessment needs score; CI = confidence interval; COPD = chronic obstructive pulmonary disease; HR = hazard ratio.
Values are presented as HR with 95% CIs. Each model is adjusted for age and clustered by site.
Discussion
In a large, nationwide cohort with COPD and low exacerbation risk, higher patient complexity was associated with incident prescription of low-value ICS. Our markers of system-level complexity did not modify the association between patient level complexity and low-value ICS prescription. This is the first study to describe the factors associated with initiating low-value ICS in COPD. As health systems grapple with how to reduce overuse of ICS, our results suggest that processes to mitigate ICS overuse will need to take patient complexity into account. We hypothesize that patient complexity may reflect higher symptom burden or greater diagnostic uncertainty, and may lead to incident ICS prescription.
Comparison to Prior Literature and Novel Findings
Our work is in general agreement with previous studies and highlights the difficulty of delivering high-value care to complex patients with COPD. An analysis of the SPIROMICS cohort found that 50% of patients with COPD were prescribed ICS, and that 92% of overuse occurred among patients with a low exacerbation risk (GOLD groups A and B) (12). The authors postulated that “clinical inertia” may explain their findings, especially when patients are clinically stable; a concept documented for not only ICS, but for other inhaled therapies (16, 17). Our results add to the existing literature by examining an inception cohort.
Possible Explanations for Low-Value ICS in COPD
The reasons complex patients with COPD receive low-value ICS are likely to derive from a complex mechanism. One potential explanation for initiating low-value ICS in complex patients with COPD and low exacerbation risk is the now outdated GOLD recommendation to prescribe ICS for severe deficits in lung function by FEV1. Before 2017, GOLD recommended the use of ICS for both frequent exacerbators and patients with severe COPD by FEV1. The 2017 statement did not reaffirm that recommendation to offer ICS to patients with severe COPD (15). When we excluded patients with severe COPD by FEV1, patient complexity remained associated with low-value ICS, suggesting that severity by FEV1 could not fully explain our findings.
Another hypothesis for initiating low-value ICS in complex patients with COPD may reflect provider efforts to treat uncontrolled symptoms of dyspnea. The 2014 VA/Department of Defense clinical practice guideline carries a weak recommendation to add ICS as a third-line therapy for uncontrolled dyspnea. Our study found that ICS was added as a third-line agent in the minority of cases (2.5%) and mostly does not align with the VA/Department of Defense recommendation. Meanwhile, palliative care and pulmonary rehabilitation (PR) are recommended by GOLD for patients with COPD and a high symptom burden to improve breathlessness (40). Studies have shown that referral to PR and to palliative care for patients with COPD is underused, thought to be due to multiple barriers at the patient level and the health system (41, 42). Lack of availability to PR and palliative care may lead to new prescriptions of low-value ICS to mitigate symptoms.
Effect of Health System Complexity
We found no evidence that our markers of health system complexity modified the effect of patient complexity and low-value ICS prescription. Unlike congestive heart failure, few if any, system-level quality improvement interventions exist to improve COPD care quality, and may explain the lack of an effect of system complexity (43). Another explanation is also that we measured only two site-level characteristics that were indirect measures of system complexity. We also did not have measures of provider-level quality of care nor measures of provider knowledge and attitudes toward ICS prescribing practices that could explain our findings.
Limitations and Strengths
Our study has some potential limitations. Patients in this cohort may have become eligible for ICS treatment by experiencing an indication not captured within VA data, such as a COPD hospital admission at a non-VA facility. Patient-reported symptoms were also not available in this dataset. Eosinophils were also not available, though their use as a biomarker in low–exacerbation risk patients with COPD, such as this cohort, has not been yet shown to be beneficial. This study was conducted within a VA population, which may limit generalizability. However, studies have shown that the quality of care delivered at the VA often outperforms non-VA systems, and therefore should not bias our findings (44–47). For these potential weaknesses, we had important strengths. The availability of spirometry to confirm airflow obstruction and reduce diagnostic misclassification of COPD is often not available in observational studies using administrative data. We also had a geographically diverse cohort representing nearly all Veterans Integrated Service Networks within the continental United States. Moreover, we had complete pharmacy records to analyze prescriptions over time, limiting selection and ascertainment biases.
Conclusions
Healthcare continues to move toward value-based purchasing that will increasingly transfer the risk of healthcare services to healthcare systems. Preferred provider status for large contracts will also include demonstration of provision of high-quality, cost-effective approaches to population health management. Targeting overuse of ICS represents an opportunity to improve care value by mitigating risk of harm to patients, avoiding potential costs from treating iatrogenic harms, and increasing savings from reduced medication acquisition.
Supplementary Material
Footnotes
Supported by Department of Veterans Affairs Quality Enhancement Research Initiative grant I01 HX002113-01, National Institutes of Health/National Heart, Lung, and Blood Institute grants F32HL140685-01, K23HL111116, and 5K12HL137940.
The views expressed here are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs. None of the funding sources was involved in the design, conduct, or analysis of this project.
This manuscript served as the basis of L.J.S.’s thesis for a master’s degree in epidemiology.
Author Contributions: L.J.S. was primarily responsible for study design, analysis, interpretation, and drafting this manuscript. L.M.D., M.F.G., T.K., L.C.F., N.L.S., and D.H.A. provided substantial contributions to study design, analysis, interpretation, and critical revisions. All authors approved this final version and agree to be held accountable.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Yang IA, Clarke MS, Sim EH, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;(7):CD002991. doi: 10.1002/14651858.CD002991.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Initiative for Chronic Obstructive lung Disease (GOLD) Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease: 2019 Report. 2019 [accessed 2019 Feb 8]. Available from: www.goldcopd.org.
- 3.Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, Maslen TK. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ. 2000;320:1297–1303. doi: 10.1136/bmj.320.7245.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 5.Crim C, Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, et al. Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study results. Eur Respir J. 2009;34:641–647. doi: 10.1183/09031936.00193908. [DOI] [PubMed] [Google Scholar]
- 6.Suissa S, Patenaude V, Lapi F, Ernst P. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax. 2013;68:1029–1036. doi: 10.1136/thoraxjnl-2012-202872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suissa S, Coulombe J, Ernst P. Discontinuation of inhaled corticosteroids in COPD and the risk reduction of pneumonia. Chest. 2015;148:1177–1183. doi: 10.1378/chest.15-0627. [DOI] [PubMed] [Google Scholar]
- 8.Loke YK, Cavallazzi R, Singh S. Risk of fractures with inhaled corticosteroids in COPD: systematic review and meta-analysis of randomised controlled trials and observational studies. Thorax. 2011;66:699–708. doi: 10.1136/thx.2011.160028. [DOI] [PubMed] [Google Scholar]
- 9.Singh S, Loke YK. An overview of the benefits and drawbacks of inhaled corticosteroids in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2010;5:189–195. doi: 10.2147/copd.s6942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suissa S, Kezouh A, Ernst P. Inhaled corticosteroids and the risks of diabetes onset and progression. Am J Med. 2010;123:1001–1006. doi: 10.1016/j.amjmed.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 11.Slatore CG, Bryson CL, Au DH. The association of inhaled corticosteroid use with serum glucose concentration in a large cohort. Am J Med. 2009;122:472–478. doi: 10.1016/j.amjmed.2008.09.048. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh S, Anderson WH, Putcha N, Han MK, Curtis JL, Criner GJ, et al. Alignment of inhaled chronic obstructive pulmonary disease therapies with published strategies: analysis of the GOLD recommendations in SPIROMICS. Ann Am Thorac Soc. 2018;16:200–208. doi: 10.1513/AnnalsATS.201804-283OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suissa S, Barnes PJ. Inhaled corticosteroids in COPD: the case against. Eur Respir J. 2009;34:13–16. doi: 10.1183/09031936.00190908. [DOI] [PubMed] [Google Scholar]
- 14.Corrado A, Rossi A. How far is real life from COPD therapy guidelines? An Italian observational study. Respir Med. 2012;106:989–997. doi: 10.1016/j.rmed.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Rinne ST, Wiener RS, Chen Y, Rise P, Udris E, Feemster LC, et al. Impact of guideline changes on indications for inhaled corticosteroids among veterans with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;198:1226–1228. doi: 10.1164/rccm.201803-0554LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Contoli M, Corsico AG, Santus P, Di Marco F, Braido F, Rogliani P, et al. Use of ICS in COPD: from blockbuster medicine to precision medicine. COPD. 2017;14:641–647. doi: 10.1080/15412555.2017.1385056. [DOI] [PubMed] [Google Scholar]
- 17.Solem CT, Lee TA, Joo MJ, Lambert BL, Walton SM, Pickard AS. Complexity of medication use in newly diagnosed chronic obstructive pulmonary disease patients. Am J Geriatr Pharmacother. 2012;10:110–122.e111. doi: 10.1016/j.amjopharm.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158:915–920. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 19.Fabbri LM, Luppi F, Beghé B, Rabe KF. Complex chronic comorbidities of COPD. Eur Respir J. 2008;31:204–212. doi: 10.1183/09031936.00114307. [DOI] [PubMed] [Google Scholar]
- 20.Spece LJ, Epler EM, Donovan LM, Griffith MF, Collins MP, Feemster LC, et al. Role of comorbidities in treatment and outcomes after chronic obstructive pulmonary disease exacerbations. Ann Am Thorac Soc. 2018;15:1033–1038. doi: 10.1513/AnnalsATS.201804-255OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiss CO, Boyd CM, Yu Q, Wolff JL, Leff B. Patterns of prevalent major chronic disease among older adults in the United States. JAMA. 2007;298:1160–1162. doi: 10.1001/jama.298.10.1160-b. [DOI] [PubMed] [Google Scholar]
- 22.Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med. 2002;162:2269–2276. doi: 10.1001/archinte.162.20.2269. [DOI] [PubMed] [Google Scholar]
- 23.Dowson C, Laing R, Barraclough R, Town I, Mulder R, Norris K, et al. The use of the hospital anxiety and depression scale (HADS) in patients with chronic obstructive pulmonary disease: a pilot study. N Z Med J. 2001;114:447–449. [PubMed] [Google Scholar]
- 24.Brenes GA. Anxiety and chronic obstructive pulmonary disease: prevalence, impact, and treatment. Psychosom Med. 2003;65:963–970. doi: 10.1097/01.psy.0000097339.75789.81. [DOI] [PubMed] [Google Scholar]
- 25.Puente-Maestu L, Calle M, Rodríguez-Hermosa JL, Campuzano A, de Miguel Díez J, Álvarez-Sala JL, et al. Health literacy and health outcomes in chronic obstructive pulmonary disease. Respir Med. 2016;115:78–82. doi: 10.1016/j.rmed.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention (CDC) Chronic obstructive pulmonary disease among adults—United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:938–943. [PubMed] [Google Scholar]
- 27.Gershon AS, Dolmage TE, Stephenson A, Jackson B. Chronic obstructive pulmonary disease and socioeconomic status: a systematic review. COPD. 2012;9:216–226. doi: 10.3109/15412555.2011.648030. [DOI] [PubMed] [Google Scholar]
- 28.Gershon AS, Hwee J, Victor JC, Wilton AS, To T. Trends in socioeconomic status-related differences in mortality among people with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11:1195–1202. doi: 10.1513/AnnalsATS.201403-094OC. [DOI] [PubMed] [Google Scholar]
- 29.Wang L, Porter B, Maynard C, Evans G, Bryson C, Sun H, et al. Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Med Care. 2013;51:368–373. doi: 10.1097/MLR.0b013e31827da95a. [DOI] [PubMed] [Google Scholar]
- 30.Yen TW, Pezzin LE, Li J, Sparapani R, Laud PW, Nattinger AB. Effect of hospital volume on processes of breast cancer care: a National Cancer Data Base study. Cancer. 2017;123:957–966. doi: 10.1002/cncr.30413. [DOI] [PubMed] [Google Scholar]
- 31.Landovitz RJ, Desmond KA, Gildner JL, Leibowitz AA. Quality of care for HIV/AIDS and for primary prevention by HIV specialists and nonspecialists. AIDS Patient Care STDS. 2016;30:395–408. doi: 10.1089/apc.2016.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korom-Djakovic D, Canamucio A, Lempa M, Yano EM, Long JA. Organization complexity and primary care providers’ perceptions of quality improvement culture within the Veterans Health Administration. Am J Med Qual. 2016;31:139–146. doi: 10.1177/1062860614559743. [DOI] [PubMed] [Google Scholar]
- 33.Sjoding MW, Prescott HC, Wunsch H, Iwashyna TJ, Cooke CR. Hospitals with the highest intensive care utilization provide lower quality pneumonia care to the elderly. Crit Care Med. 2015;43:1178–1186. doi: 10.1097/CCM.0000000000000925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valley TS, Sjoding MW, Goldberger ZD, Cooke CR. ICU use and quality of care for patients with myocardial infarction and heart failure. Chest. 2016;150:524–532. doi: 10.1016/j.chest.2016.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooke CR, Joo MJ, Anderson SM, Lee TA, Udris EM, Johnson E, et al. The validity of using ICD-9 codes and pharmacy records to identify patients with chronic obstructive pulmonary disease. BMC Health Serv Res. 2011;11:37. doi: 10.1186/1472-6963-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stein BD, Bautista A, Schumock GT, Lee TA, Charbeneau JT, Lauderdale DS, et al. The validity of International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis codes for identifying patients hospitalized for COPD exacerbations. Chest. 2012;141:87–93. doi: 10.1378/chest.11-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joo MJ, Lee TA, Weiss KB. Geographic variation in chronic obstructive pulmonary disease exacerbation rates. J Gen Intern Med. 2007;22:1560–1565. doi: 10.1007/s11606-007-0354-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.National Center for Veterans Analysis and Statistics. Strategic Analytics for Improvement and Learning (SAIL) Value Model. 2019 [accessed 2019 Feb 8]
- 39.Gray Fa. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 40.McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;(2):CD003793. doi: 10.1002/14651858.CD003793.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spitzer KA, Stefan MS, Priya A, Pack QR, Pekow PS, Laqu T, et al. Participation in pulmonary rehabilitation after hospitalization for chronic obstructive pulmonary disease among Medicare beneficiaries. Ann Am Thorac Soc. 2018;16:99–106. doi: 10.1513/AnnalsATS.201805-332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown CE, Jecker NS, Curtis JR. Inadequate palliative care in chronic lung disease: an issue of health care inequality. Ann Am Thorac Soc. 2016;13:311–316. doi: 10.1513/AnnalsATS.201510-666PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rinne ST, Liu CF, Wong ES, Hebert PL, Heidenreich P, Bastian LA, et al. Organizational structure for chronic heart failure and chronic obstructive pulmonary disease. Am J Manag Care. 2016;22:e82–e87. [PubMed] [Google Scholar]
- 44.O’Hanlon C, Huang C, Sloss E, Anhang Price R, Hussey P, Farmer C, et al. Comparing VA and non-VA quality of care: a systematic review. J Gen Intern Med. 2017;32:105–121. doi: 10.1007/s11606-016-3775-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blay E, Jr, DeLancey JO, Hewitt DB, Chung JW, Bilimoria KY. Initial public reporting of quality at Veterans Affairs vs non–Veterans Affairs hospitals. JAMA Intern Med. 2017;177:882–885. doi: 10.1001/jamainternmed.2017.0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nuti SV, Qin L, Rumsfeld JS, Ross JS, Masoudi FA, Normand SL, et al. Association of admission to Veterans Affairs hospitals vs non–Veterans Affairs hospitals with mortality and readmission rates among older men hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA. 2016;315:582–592. doi: 10.1001/jama.2016.0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anhang Price R, Sloss EM, Cefalu M, Farmer CM, Hussey PS. Comparing quality of care in Veterans Affairs and non–Veterans Affairs settings. J Gen Intern Med. 2018;33:1631–1638. doi: 10.1007/s11606-018-4433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.