Abstract
Bioactive gibberellins (GAs, diterpenes) are essential hormones in land plants, controlling many aspects of plant growth and developments. In flowering plants, 13-OH (low bioactivity; such as GA1) and 13-H GAs (high bioactivity; such as GA4) frequently coexist. However, the bona fide GA 13-hydroxylase and its physiological functions in Arabidopsis remain unknown. Here, we report that novel cytochrome P450 genes (CYP72A9 and its homologs) encode active GA 13-hydroxylases in Brassicaceae plants. CYP72A9-overexpressing plants exhibited semi-dwarfism, which was caused by significant reduction in GA4 levels. Biochemical assays revealed that recombinant CYP72A9 protein catalyzed the conversion from 13-H GAs to the corresponding 13-OH GAs. CYP72A9 was expressed predominantly in developing seeds in Arabidopsis. Freshly harvested seeds of cyp72a9 mutants germinated more quickly than wild-type, while long-term storage and stratification-treated seeds did not. The evolutionary origin of GA 13-oxidases from the CYP72A subfamily also was investigated and discussed here.
INTRODUCTION
Gibberellins (GAs), biosynthesized from geranylgeranyl diphosphate (GGDP) via multiple enzymatic steps, are essential phytohormones for plant growth and development. Among more than 130 discovered GAs to date, GA4, GA1 (also known as 13-OH GA4), GA7 and GA3 (also known as 13-OH GA7) are common bioactive GAs in flowering plants. The bioactivity of GA1 is approximately 1000-fold lower than GA4 in plants1–5. The plant gibberellin biosynthetic pathway and its regulation have been well elucidated, mainly in Arabidopsis and rice6 (Fig. 1). Recent studies have shown that not only GA biosynthesis, but also GA deactivation is important for GA homeostasis in planta. Thus far, three types of GA deactivation enzymes and their encoding genes have been identified, including GA 2-oxidase (soluble 2-oxoglutarate-dependent dixoygenase; seven GA2ox genes in Arabidopsis and ten GA2ox genes in rice), GA methyltransferase (GAMT1/2 from Arabidopsis), and GA 16,17-oxidase (CYP714D1/EUI from rice)7–9. More recently, reverse genetic studies in rice have suggested a predominant role for CYP714B1 and CYP714B2 (CYP714D1 homologs) in reducing GA activity through 13-hydroxylation of GA12 to form GA533. Interestingly, both cyp714d1 mutant and cyp714b1/cyp714b2 double mutant rice display an elongated uppermost internode, in where these three P450s are highly expressed, at the heading stage.
Fig. 1. The GA biosynthesis pathway in Arabidopsis.
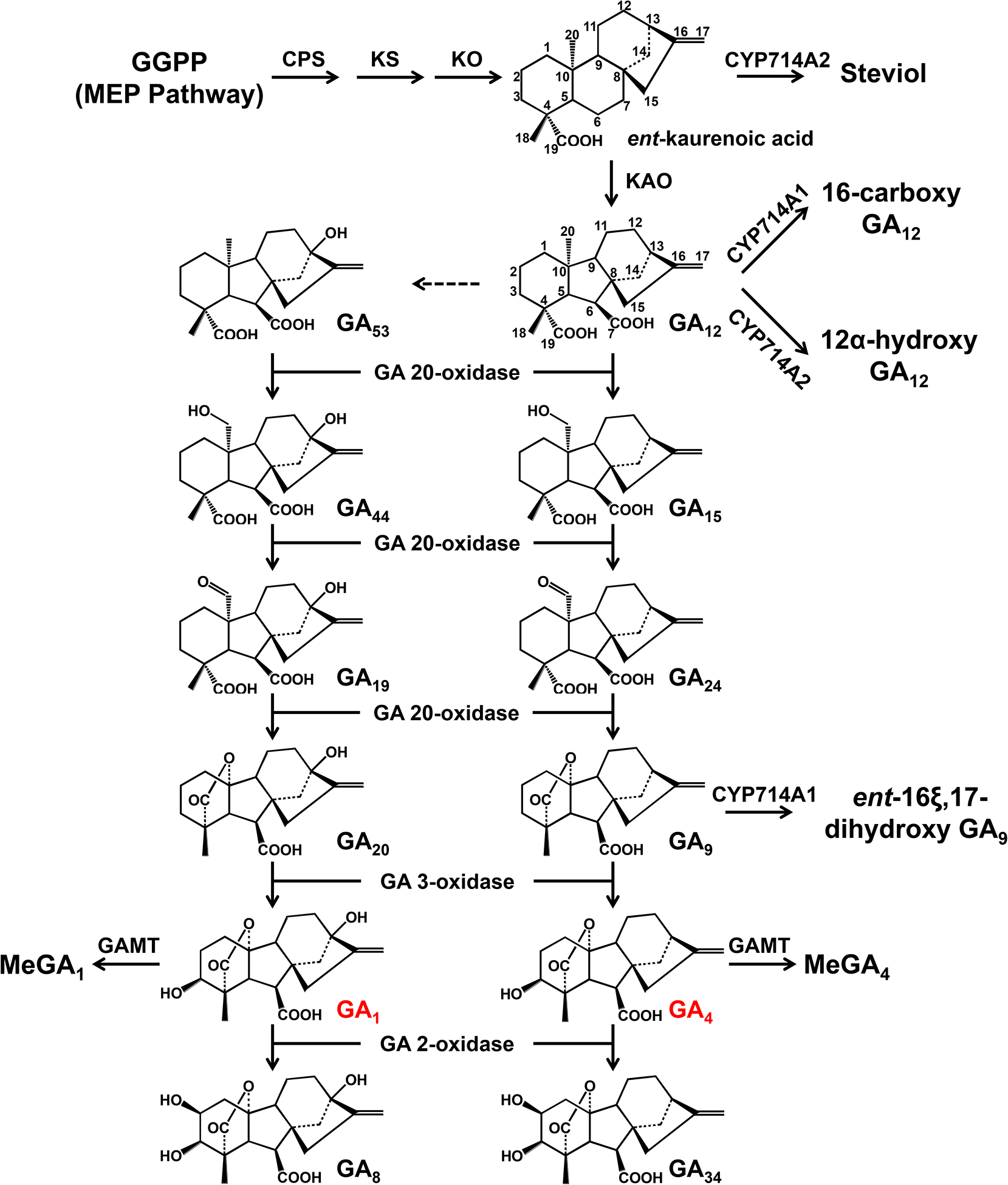
All enzymes mapped to the GA biosynthesis pathway were verified with enzymatic assays and chemical profiling of loss-of-function mutants. The dashed line indicates uncharacterized enzymatic step in Arabidopsis. The carbon backbone of GA12 is labeled with numbers, and the bioactive GAs (GA1 and GA4) are marked in red. CPS, ent-copalyl diphosphate synthase; GAMT, GA methyltransferase; GGPP, geranylgeranyl diphosphate; KAO, ent-kaurenoic acid oxidase; KO, ent-kaurene oxidase; KS, ent-kaurenoic acid synthase; MEP, 2-C-methyl-D-erythritol 4-phosphate.
In Arabidopsis, GA4 is the predominant bioactive GA in most tissues while GA1 accumulates at relatively high levels in developing seeds/siliques8,10,11. These results suggest that 13-hydroxylation of GA4 to form GA1 plays an important role in seed maturation and germination, at least in Arabidopsis. Two Arabidopsis EUI homologs (CYP714A1 and CYP714A2) had been shown to catalyze distinct oxidations events in GA12 in vitro and in planta: CYP714A1 converts GA12 to 16-carboxylated GA12, while CYP714A2 catalyzes the hydroxylation at the C12 or C13 (only one twentieth of C12 hydroxylase activity) position of GA12 and its upstream precursor, ent-kaurenoic acid12. However, the C19-GA profiles, including bioactive GA4 and GA1, of both cyp714a1 and cyp714a2 single mutants are comparable to those in wild-type13. These results suggest that other genes should encode bona fide GA 13-hydroxylase in Arabidopsis.
In this study, we report that one member of the CYP72A subfamily, CYP72A9, encodes gibberellin 13-oxidase, which catalyzes the conversion of 13-H GAs (GA12, GA9 and GA4) to the corresponding13-OH GAs (GA53, GA20 and GA1). CYP72A9 is predominantly expressed in developing seeds. cyp72a9 mutants show a deficiency in GA1 and an increase in the concentration of GA4, suggesting that CYP72A9 plays a key role in the 13-hydroxylation of bioactive GAs in Arabidopsis thaliana. We further demonstrated that the conversion of GA4 to GA1 is an indispensable factor for primary seed dormancy in Brassicaceae plants.
RESULTS
Overexpression of CYP72A9 in Arabidopsis Results in Dwarf Phenotypes Caused by GA4 Deficiency
The Arabidopsis thaliana genome contains one geranylfarnesyl pyrophosphate synthase (C25)-sesterterpene synthase-P450 (GFPPS-sesterTPS-P450) gene cluster, in which eight tandem duplicated CYP72As are functionally unknown14–16. To elucidate the biochemical and biological functions of each member of the CYP72A subfamily (CYP72A7, At3g14610; A8, At3g14620; A9, At3g14630; A10, At3g14640; A11, At3g14650; A13, At3g14660; A14, At3g14680; A15, At3g14690) in Arabidopsis, we firstly generated transgenic Arabidopsis overexpressing each CYP72A gene (Supplementary Fig. 1). It is noteworthy that we failed to generate CYP72A15-overexpressing plants, in which CYP72A15 was upregulated only by 2–3-fold (Supplementary Fig. 1). Among these CYP72A-overexpressing Arabidopsis lines, CYP72A9-overexpressing plants exhibited a semi-dwarf and late-flowering phenotype at the mature stage (Fig. 2A and Supplementary Fig. 2), which phenocopied the gibberellin deficiency mutants (like ga1-t (SALK_023192), Col-0 ecotype) or plants overexpressing GA deactivation genes3,8,12,17. To determine whether the bioactive GA deficiency caused the phenotypes of CYP72A9-overexpressing plants, we treated CYP72A9-overexpressing seedlings with 2 μM bioactive GA3, which was able to reverse this semi-dwarf phenotype (Fig. 2B), indicating a defect in GA metabolism.
Fig. 2. Overexpression of CYP72A9 results in a dwarf phenotype and decreases endogenous GA4 levels.
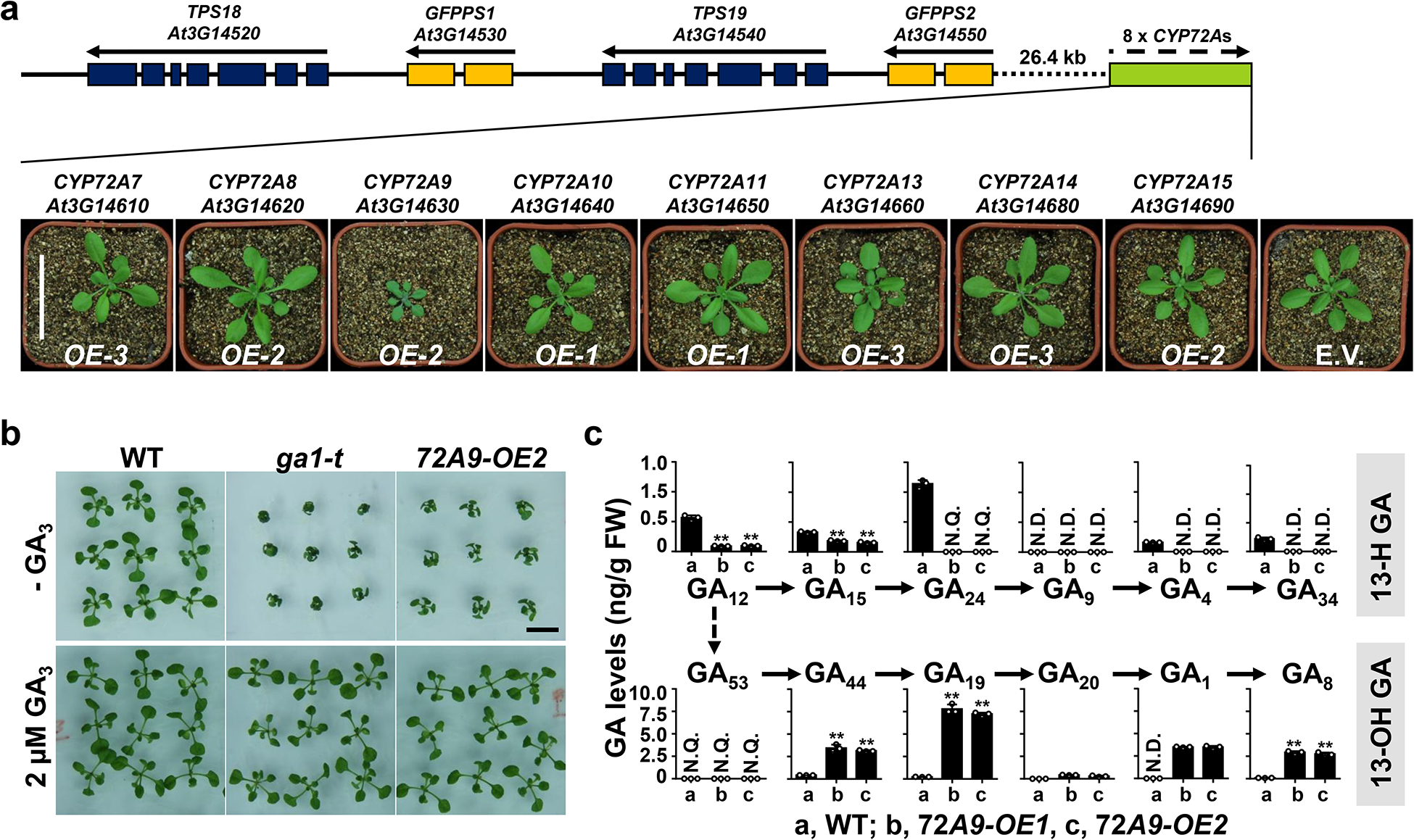
a. GFPPS-sesterTPS-P450 gene cluster and phenotypes of Arabidopsis with increased expression level of each AtCYP72 (CYP72A7, A8, A9, A10, A11, A13, A14, and A15). All plants were grown under the same growth conditions and images were taken at 24 days after germination. This experiment was repeated at least three times with similar results. E.V., empty vector; Scale bar = 5 cm.
b. Exogenous applied GA3 rescues the growth of ga1-t mutant and CYP72A9-overexpressing Arabidopsis. All one-week-old seedlings, which grown on ½ MS agar medium, were transferred onto ½ MS agar medium and grown for another week with or without 2 μM bioactive GA3. This experiment was repeated three times with similar results. WT, wild type. Scale bar = 1 cm.
c. Profile of endogenous GAs in WT and two independent CYP72A9-overexpressing lines. The GA levels in the rosette leaves of 4-week-old Arabidopsis are presented as the means ± SDs (n = 3 biologically independent samples). **, significant difference from WT (P < 0.01; two-tailed Student’s t-test). N.D., not detectable; N.Q., detected, but not quantifiable due to low abundance.
We further applied UPLC-QQQ-MS/MS (ultra-high-performance liquid chromatography coupled to a triple quadrupole mass spectrometer; MRM (multiple reaction monitoring) mode, for detailed parameters see Supplementary Table 1) to profile the endogenous GAs in wild-type (WT, Col-0 ecotype) and two independent CYP72A9-overexpressing lines (72A9 OE-1 and 72A9 OE-2; Supplementary Fig. 1). The results showed that 13-H GAs (GA12, GA15, GA24, GA9, GA4, and GA34) were greatly reduced in both CYP72A9-overexpressing lines, while 13-OH GAs (GA44, GA19, GA20, GA1, and GA8) were greatly increased compared with those in WT (Fig. 2c). GA3 and GA7 were not detected in any of the tested plants. The expression levels of many genes involved in GA metabolism and signaling also were significantly altered in the CYP72A9-overexpressing lines, with those involved in biosynthesis (GA20ox1–3 and GA3ox1) significantly upregulated, while those involved in catabolism (GA2ox1, 2, 6, CYP714A1, CYP714A2 and GAMT2) or signaling (RGA and GID1c) were significantly downregulated (Supplementary Fig. 3). Altogether, these data suggested that CYP72A9 played a role in the production of 13-OH GA in Arabidopsis.
Biochemical Characterization of CYP72A9
To clarify the biochemical functions of CYP72A9 in GA metabolism, we expressed each CYP72A9 gene (pESC-Leu vector) in the yeast strain WAT11, which has the Arabidopsis cytochrome P450 reductase 1 (AtCPR1, At4G24520) gene integrated into its chromosome. We incubated 13-H GAs (bioactive GA4, GA9, and GA12) in the heterologous culture and checked the resulting products by GC-MS (gas chromatography-mass spectrometry, and selected ion mode) after extraction and derivation. The results clearly showed that CYP72A9 was promiscuous; hydroxylating GA4, GA9, and GA12 at C13 position to produce GA1, GA20, and GA53, respectively (Fig. 3 and Supplementary Fig. 4a, c). Additionally, CYP72A9 converted ent-kaurenoic acid to produce both steviol (ent-13-hydroxy kaurenoic acid, minor product) and ent-16β,17-dihydroxy kaurenoic acid (main product; Supplementary Fig. 4d and Supplementary Fig. 5).
Fig. 3. CYP72A9 is a GA 13-hydroxylase.
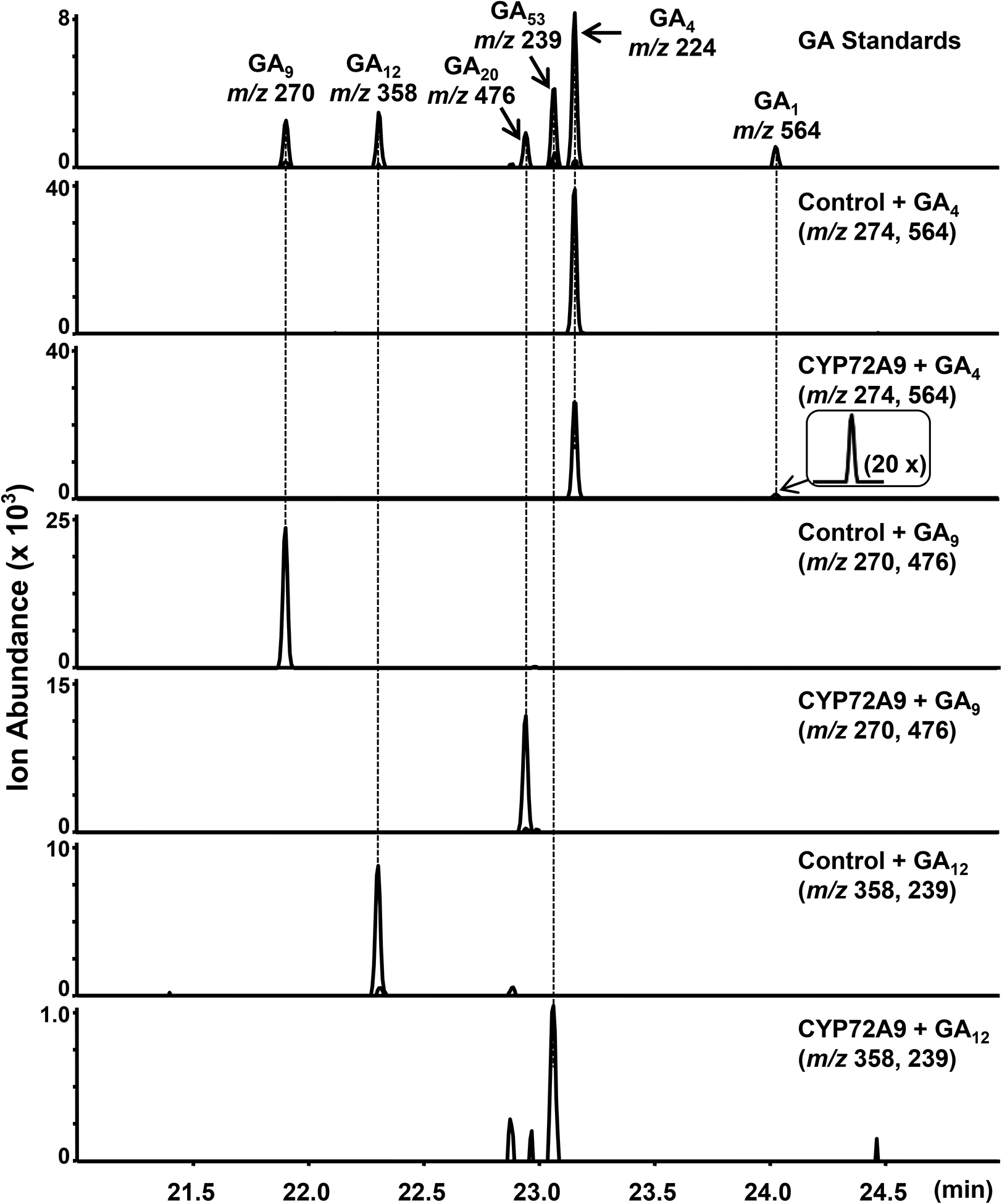
CYP72A9 converted GA4, GA9, and GA12 to GA1, GA20, and GA53, as verified by comparison to authentic standards. Chromatogram of selected ions of m/z 546 for GA1, m/z 224 for GA4, m/z 270 for GA9, m/z 358 for GA12, m/z 476 for GA20, and m/z 239 for GA53. It is noteworthy that the y axis scale for each reaction is arbitrary for clarity, and the GA1 region has been amplified 20 times to allow easier visualization (inlet window). Control, yeast strain harboring pESC-Leu empty vector. This experiment was repeated at least three times with similar results.
CYP72A9 Predominantly Expressed in Developing Seeds and Involved in Primary Seed Dormancy
To determine the more specific physiological functions of CYP72A9 in Arabidopsis, we checked the tissue-specificity of CYP72A9 using qRT-PCR. CYP72A9 was predominantly expressed in developing seeds/siliques and was barely detected in other tested tissues (Fig. 4a). Consistent with the RT-PCR results, upon transformation with a P72A9::GUS construct, strong GUS activity was mainly detected in the developing seeds (Fig. 4b). Subcellular localization of 72A9:GFP fusion protein in protoplasts indicated that CYP72A9 is a prototypical CYP, which usually is an endoplasmic reticulum (ER)-bound protein (Fig. 4c). Two mutant alleles were obtained, the first, designated cyp72a9–1, was a T-DNA insertion (SALK_130811C; Supplementary Fig. 6), while the second, designated cyp72a9–2, was generated using the CRISPR/Cas9 technique (Supplementary Fig. 7). Note that CYP72A9 transcripts were still present at a low level (approximately 5% of WT) in the cyp72a9–1 mutant, which likely caused by the T-DNA insertion at the fourth intron of CYP72A9 genomic DNA (Supplementary Fig. 6a, c). GA profiling of developing seeds/siliques of WT and the two independent cyp72a9 mutants indicated that bioactive GA4 (0.84 ± 0.016 ng/g fresh weight in WT vs 1.46 ± 0.032 ng/g fresh weight in cyp72a9–1 and 2.20 ± 0.26 ng/g fresh weight in cyp72a9–2, n = 3) was significantly increased in both cyp72a9 mutants, while 13-OH GAs were decreased compared with WT (Fig. 4d). Indeed, GA20 and GA1 were present at levels below the detection limits in the cyp72a9 mutants (Fig. 4d). These results strongly indicate that CYP72A9 plays a regulatory role in the homeostasis of bioactive GA4 in the developing seeds/siliques of Arabidopsis thaliana.
Fig. 4. Tissue-specific expression and subcellular localization of CYP72A9.
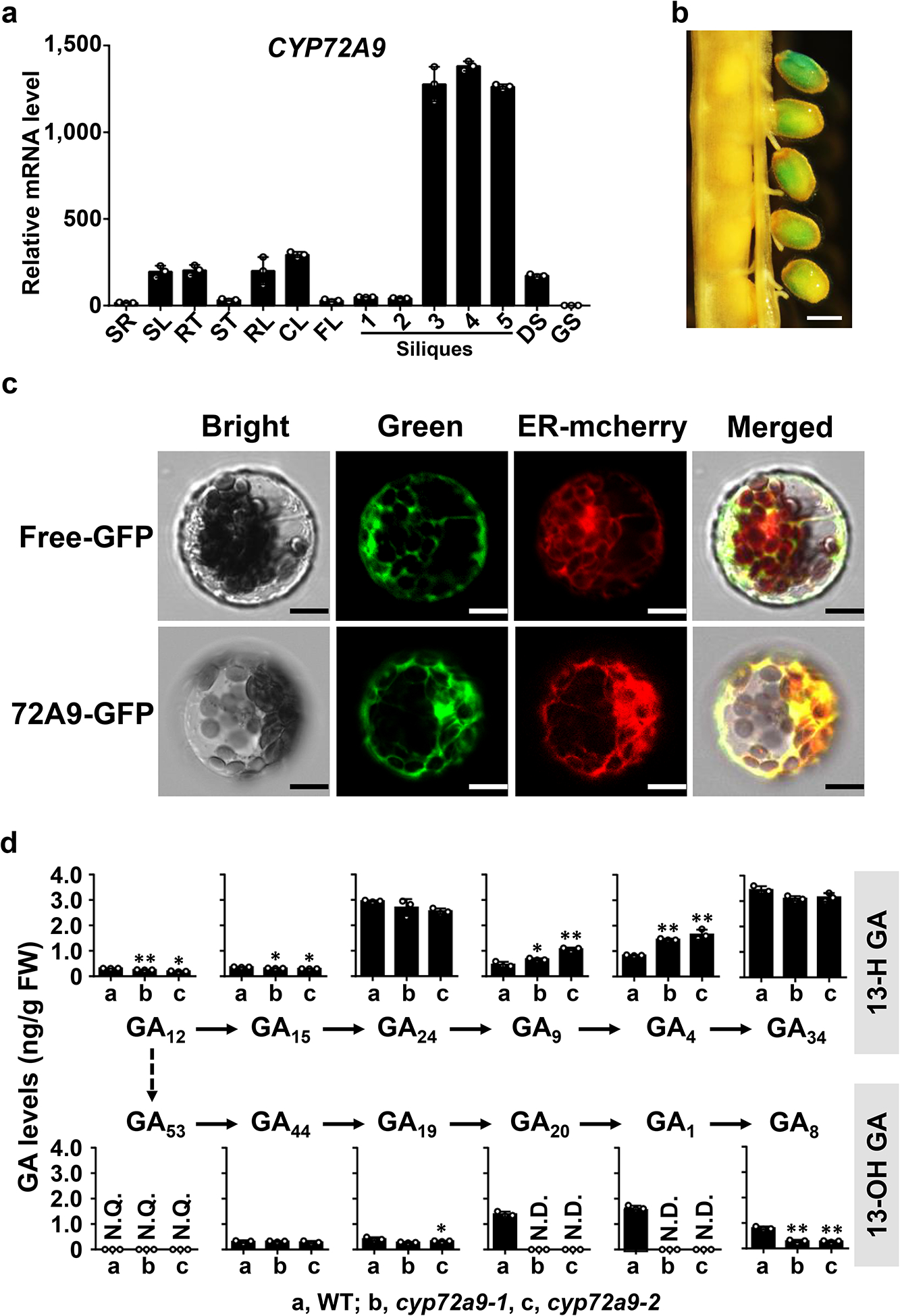
a. qRT-PCR analysis of CYP72A9 transcript levels in different tissues. Error bars represent the SD of three independent experiments. SR, root of 10-day-old seedlings; SL, leaf of 10-day-old seedlings; RO, root of mature plants; RL, rosette leaf; CL, cauline leaf; FL, flowers; GS, germinating seeds; DS, dry seeds; Silique samples were prepared followed the reference by Varbanova et al8. At1g13320, At2g28390, and At4g34270 were used as reference genes in this analysis. The lowest level of CYP72A9 transcript in germinating seeds was set as 1.0.
b. Histochemical GUS staining of siliques (stages 9 and 10) from Pro72A9:GUS transgenic plants. Scale bar = 0.2 mm. Silique samples had been stained for 24 h before imaging. This experiment was repeated two times with similar results.
c. Subcellular localization of CYP72A9 in Arabidopsis leaf-mesophyll protoplasts. The ER was revealed by mCherry marker protein (Nelson et al., 2007). Scale bar = 5 μm. This experiment was repeated two times with similar results.
d. Profiles of endogenous GAs in WT and two independent cyp72a9 mutants. The GA levels in the developing seeds/siliques of mature Arabidopsis are presented as the means ± SDs (n = 3 biologically independent samples). **, significant difference from WT (P < 0.01; two-tailed Student’s t-test); *, (P < 0.05; two-tailed Student’s t-test). N.D., not detectable; N.Q., detected, but not quantifiable due to low abundance.
The expression levels of a number of genes involved in GA biosynthesis, including GA20ox1, GA20ox2 and GA3ox1, were significantly downregulated in the developing seeds/siliques of cyp72a9–1 mutants. However, the expression levels of the seed-predominant GA deactivation genes CYP714A1/2 and GAMT1/2 are not significantly changed (Supplementary Fig. 8b). Moreover, the GA signaling genes GID1a and GID1c were significantly downregulated (Supplementary Fig. 8c). Overall, the pattern of changes in expression for genes involved in GA metabolism from the cyp72a9–1 mutant were opposite of those observed in the CYP72A9-overexpressing lines.
Previous studies have demonstrated that bioactive GA4 plays a key role in primary seed dormancy and germination18,19. Enzymatic deactivation of GA4 by CYP72A9 and predominant expression of CYP72A9 in the developing seeds inspired us to ascertain the involvement of CYP72A9 in primary seed dormancy and germination. Freshly harvested seeds from WT and the two cyp72a9 mutants were used to investigate the effect of cyp72a9 on seed dormancy. The germination ratio of cyp72a9 seeds was significantly higher than that of WT in the absence of stratification (exposure to 4 °C for three days), even at 5 days after sowing (55% for WT versus 78% for cyp72a9–1 and 80% for cyp72a9–2; Fig. 5a). Previous studies have demonstrated that primary seed dormancy could be released by a period of dry storage and stratification, which induced the de novo biosynthesis of GA19. We did not observe any difference when all seeds were subjected to stratification (4°C for 3 days) or dry storage for six months prior to germination with the germination ratio of all three tested lines reaching almost 100% at 3 days after sowing (Fig. 5b, c). Moreover, cyp72a9 seeds in developing siliques also germinated more quickly than WT (Fig. 5d). These results suggested that the disruption of CYP72A9 reduced the primary seed dormancy in Arabidopsis. Thus, the conversion of GA4 to GA1 during seed maturation appears to be necessary for primary seed dormancy.
Fig. 5. Decreased primary seed dormancy of cyp72a9 mutants.
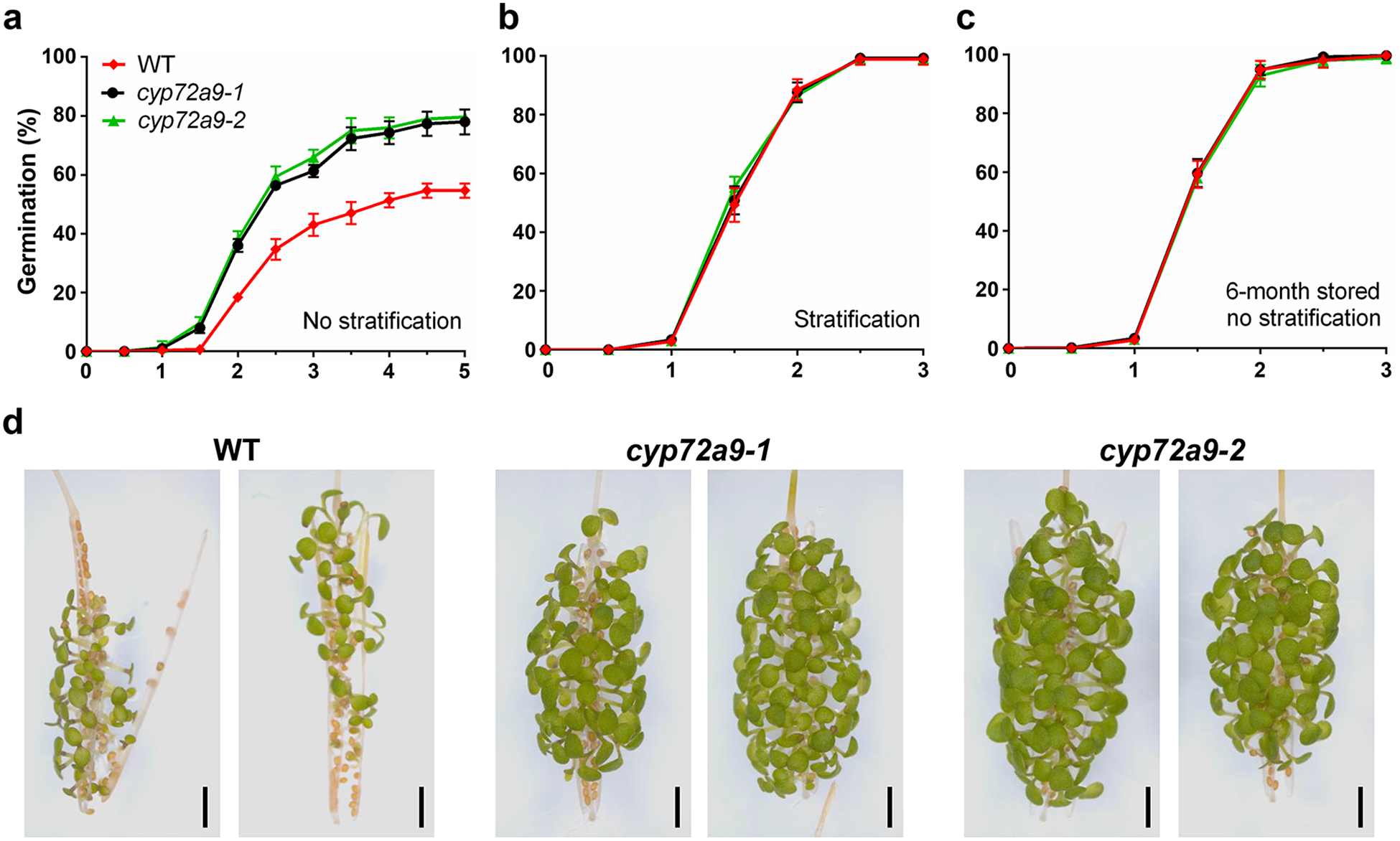
a. Germination of fresh harvested WT and cyp72a9 seeds on water-saturated filter paper without stratification treatment (4 °C for 3 days). Each data point represents the means ± SDs (n = 5 biologically independent experiments).
b. Germination of fresh harvested WT and cyp72a9 seeds on water-saturated filter paper with stratification treatment (4 °C for 3 days). Each data point represents the means ± SDs (n = 5 biologically independent experiments).
c. Germination of 6-month dry storage WT and cyp72a9 seeds on water-saturated filter paper without stratification treatment. Each data point represents the means ± SDs (n = 5 biologically independent experiments).
d. Representative images of precocious germination tests of WT and cyp72a9 siliques. Pictures were taken 14 days after plating on ½MS medium.
Biochemical Screening Other Plant CYP72As
The CYP72A subfamily was expanded with a lineage-specific pattern, mainly due to independent tandem gene-duplication events. Thus, it is very difficult to predict the biochemical activities of CYP72As based only on comparisons of primary protein sequences20. The presence of both the CYP72A subfamily and 13-OH GAs in flowering plants inspired us to test whether CYP72A from other plant species also displayed GA 13-oxidase activity. Using the abovementioned yeast system, we further tested all CYP72As from five representative plant species, including three Brassicales plants (8 CYP72As from Arabidopsis thaliana, 5 CYP72As from Capsella rubella and 3 CYP72As from Brassica rapa), one Fabales plant (12 CYP72As from Glycine max), and one Poales plant (13 CYP72As from Oryza sativa; Fig. 6). A total twelve CYP72As from these 41 candidates, including CYP72A9, were found to utilize at least one of the tested substrates (ent-kaurenoic acid, GA12, GA9, and GA4). All twelve active CYP72As had the ability to convert ent-kaurenoic acid to ent-16β,17-dihydroxy kaurenoic acid as a main product (Fig. 6 and Supplementary Fig. 9). Moreover, CYP72A262 from Brassica rapa had a similar activity profile to CYP72A9, including hydroxylating GA4 at C13 position to produce GA1 (Supplementary Fig. 10), while CYP72A15 from Arabidopsis, CYP72A272 from Brassica rapa, CYP72A484 from Capsella rubella, and CYP72A135 from Glycine max utilized ent-kaurenoic acid, GA12 and GA9 as substrates rather than GA4 (Supplementary Figs.11–14). Interestingly, both CYP72A262 and CYP72A484 showed a silique/seed-predominant expression pattern, like CYP72A9, in Brassica rapa and Capsella rubella (Supplementary Fig. 15). AtCYP72B1 (also named BAS1), which is the closest relative to CYP72As (around 40% identity at protein level), inactivates castasterone and brassinolide (both are triterpenoids) via C26 hydroxylation21. However, CYP72B1 did not show activity toward four tested diterpenoids in this study (Fig. 6 and Supplementary Fig. 16).
Fig. 6. Phylogenetic and biochemical analysis of CYP72A proteins from three Brassicaceae plants, rice, and soybean.
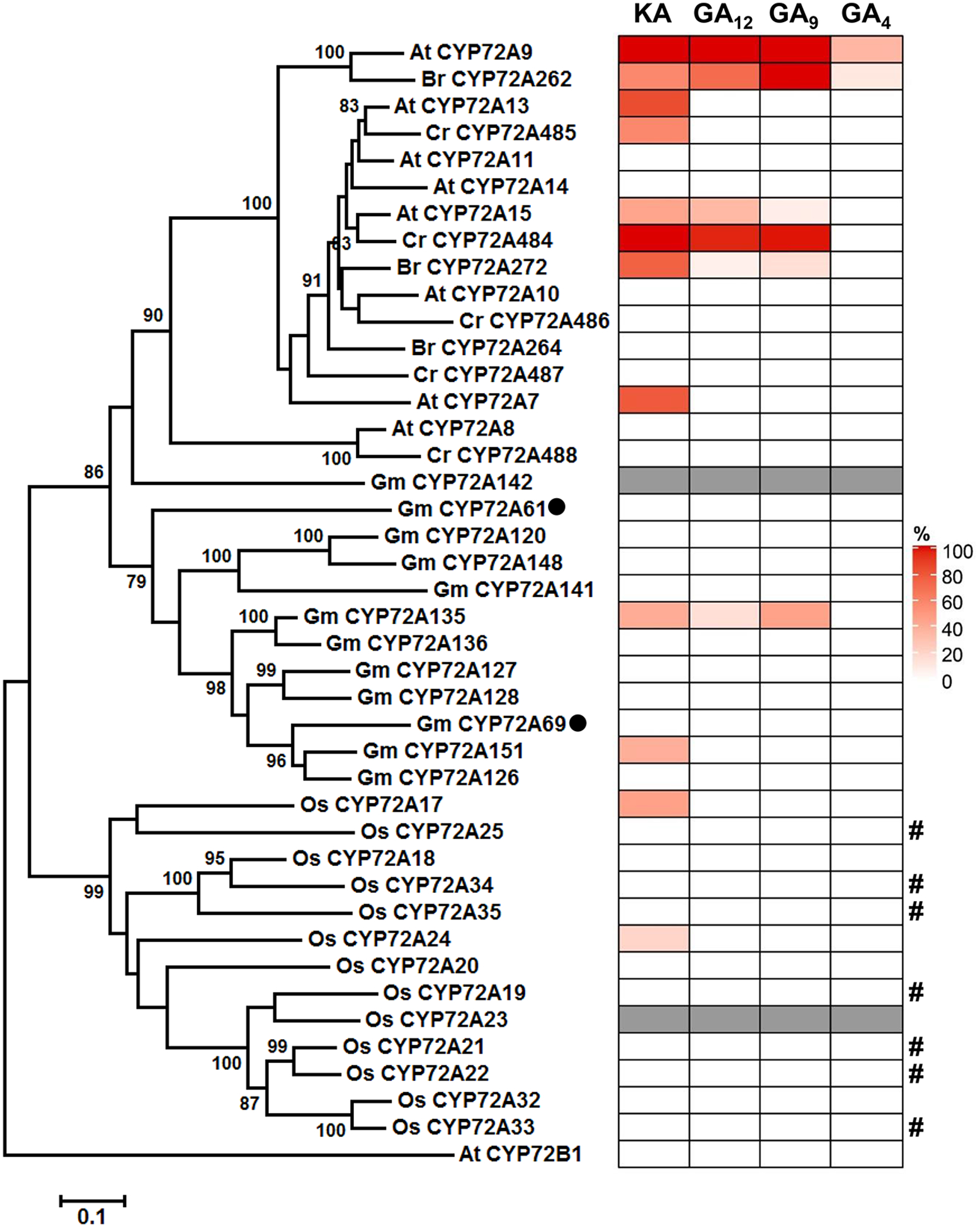
Gray color means no gene cloned in this study. GmCYP72A61 and GmCYP72A69, previously identified as triterpene oxidase, are indicated by black circles. Relative activity of various CYP72A proteins is expressed as the substrate conversion ratio (%). Values represent means from two independent experiments. It is noteworthy that the product of AtCYP72A15 and GmCYP72A135 using GA9 as the substrate is a mixture of GA20 and one unidentified hydroxylated GA9 (Supplementary Figs. 12 and 13). #, Inactivity with tested GA substrates might just reflect unsuccessful P450 protein expression in WAT11 yeast strain, which not detected by western blot (Supplementary Fig. 16).
DISCUSSION
GA1 Formation via a Distinct Biosynthetic Pathway in Arabidopsis Seeds
Both GA1 (weak bioactivity) and GA4 (strong bioactivity) coexist in flowering plants. It is well-established that GA12 and GA53 are converted to GA4 and GA1 via two parallel pathways, which are catalyzed by GA 20-oxidases (GA20ox) and 3-oxidases (GA3ox; Fig. 1). The parallel formation of GA1 and GA4 was also supported by GA profiling in the cyp714b1/cyp714b2 rice mutant (both CYP714B1 and CYP714B2 catalyze 13-hydroxylation of GA12, rather than GA9 and GA4): all examined 13-OH GAs were significantly decreased3. In this study, we discovered a GA4 13-hydroxylase, encoded by CYP72A9, in Arabidopsis thaliana. GA20 and GA1 were not detected in the developing seeds/siliques of cyp72a9 mutants, while their upstream intermediates (GA19 and GA44) were almost unchanged in cyp72a9 mutants. These results suggested that the efficiency of converting GA19 to GA20 by GA20ox was very low, at least in developing silique/seeds tissue of Arabidopsis. This proposal is consistent with the previous biochemical assays of GA20ox from pumpkin (Cucurbita maxima L.). The pumpkin GA20ox catalyzed the three-step conversions of GA53 to GA44 to GA19 to GA17 (a tricarboxylic acid GA) and GA20; however, the conversion of GA19 to GA20 was 20 times lower than that of GA19 to GA1722. Thus far, only GA20ox1 from Arabidopsis has been partially characterized using GA53 and GA19 as substrates23. The detailed catalytic efficiencies of all GA20oxs toward various GA substrates (five GA20ox genes in Arabidopsis and four GA20ox genes in rice) are needed to explain the difference in GA metabolism between Arabidopsis and rice24. Nevertheless, the increased GA4 observed in the cyp72a9 mutants indicates that CYP72A9 is mainly responsible for the formation of GA1 (from GA4) and GA20 (from GA9) in Arabidopsis thaliana. It is noteworthy that, as shown in Fig. 4d, GA8 was detected in the cyp72a9 mutants with a comparable level to wild-type although GA1 was almost undetectable. One possible explanation is that there are one or several uncharacterized oxidases (e.g. P450 or 2-oxoglutarate-dependent dioxygenaes etc), which catalyzed the 13-hydroxylation of GA34 to from GA8 in Arabidopsis. Thus, we have updated the GA metabolic network in Arabidopsis, as summarized in Fig. 7.
Fig. 7. Updated GA metabolism in developing seeds/silique of Arabidopsis.
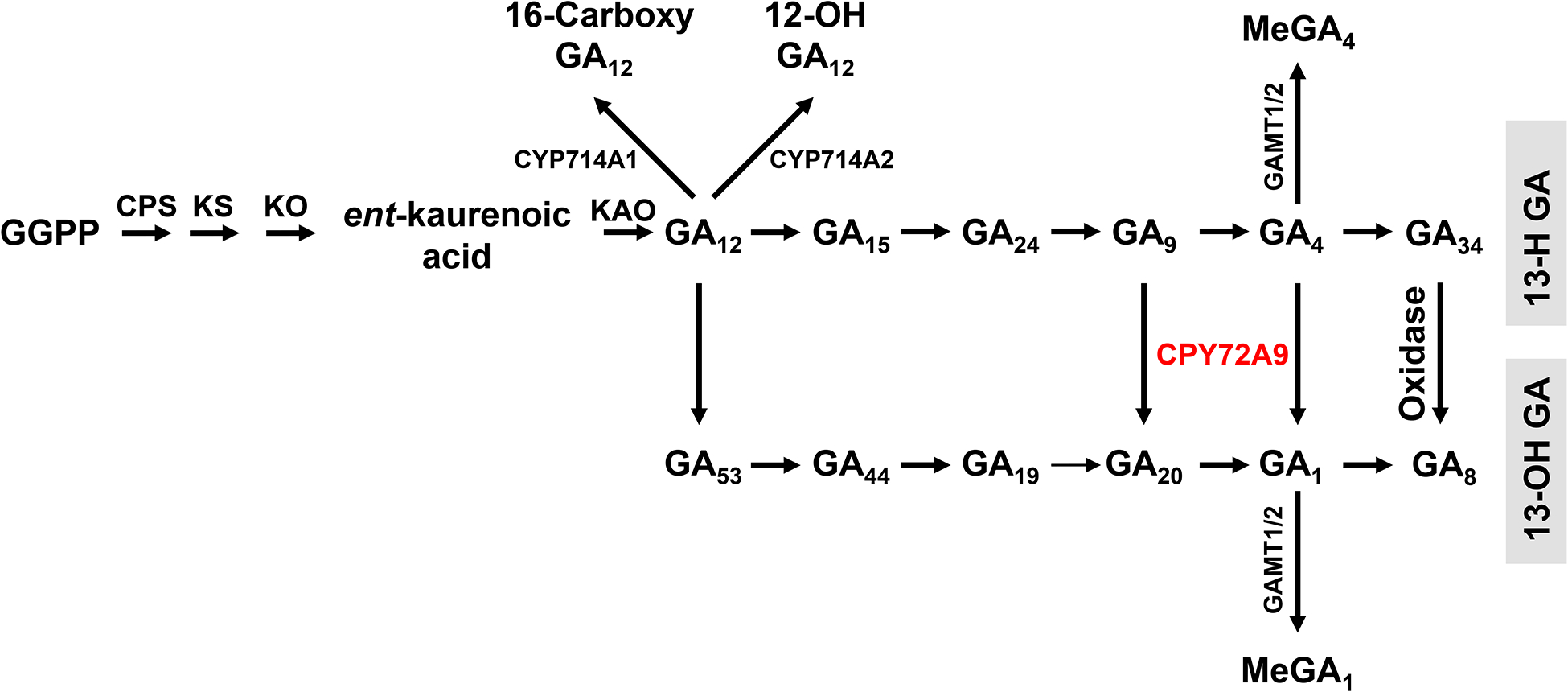
CYP72A is highlighted in red. The arrow between GA19 and GA20 (in Arabidopsis pathway) is thinner than the others to show the low catalytic efficiency of this reaction in Arabidopsis, at least in developing seeds/silique. Uncharacterized oxidase(s) catalyzed the conversion from GA34 to GA8.
GA Deactivation and Primary Seed Dormancy
It is well-known that the ABA level is increases and GA level decreases during seed maturation to maintain primary seed dormancy. Although there are many lines of evidence supporting the importance of ABA biogenesis in primary seed dormancy, the mechanism by which GA biogenesis is regulated during seed maturation has been largely unknown19,25. Recent GA profiling across seed development stages has clearly revealed that the presence of strong bioactive GA4 in the early stages of seed development, which is dramatically decreased in later stages. In contrast, 13-OH GAs, such as GA1, GA3, GA8, GA20, and GA29 (2-hydroxylation of GA20) are highly accumulated in mid-development during seed maturation10, which is tightly correlated with the CYP72A9 expression pattern (Fig. 4a, b). Disruption of CYP72A9 significantly reduced the primary seed dormancy, most likely by increasing GA4 level (cyp72a-1 had 1.74 folds and cyp72a-2 had 2.62 folds higher than WT; Fig. 4c). Interestingly, all GA deactivation genes identified in Arabidopsis thaliana to date, including CYP714A1, CYP714A2, two GAMTs, and CYP72A9 (as shown here), are highly expressed in developing seeds8,13, suggesting that GA4 deactivation plays an important role in seed development and germination. Consistent with this hypothesis, CYP714A1, GAMT1, and GAMT2 null mutants showed higher seed germination ratio than WT, although only in the presence of inhibitors of GA de novo biosynthesis (paclobutrazol or ancymidol)8,13. It is notable that dry stored seeds, rather than fresh harvested seeds, were used in these experiments. Further germination assays with fresh harvested seeds are needed to ascertain whether CYP714A1, GAMT1, and GAMT2, like CYP72A9, are also involved in primary seed dormancy in Arabidopsis thaliana. Furthermore, the conservation of GA-13 hydroxylase activity and seed-predominant expression pattern of CYP72A262 (from Brassica rapa) and CYP72A484 (from Capsella rubella) suggests to us that GA deactivation is indispensable for primary seed dormancy in Brassicaceae plants.
Divergence of Terpene Oxidase among the CYP72A Subfamily
The CYP72A subfamily has expanded dramatically in some plant species during plant evolution20,26. Previously characterized CYP72A subfamily members utilized monoterpene (CYP72A1 from Catharanthus roseus) or triterpenes as substrates27–30. Here, we found at least one diterpene oxidase (ent-kaurenoic acid or GA) from the CYP72A subfamily in the examined flowing plants, in which this subfamily was expanded mainly due to tandem gene duplication. These results suggested that the common ancestor of the CYP72A subfamily might have encoded a diterpene oxidase, as ent-kaurene and ent-kaurenoic acid are widely detected in flowering plants31. The coexistence of diterpene oxidase (CYP72A135 and CYP72A151) and triterpene oxidase (CYP72A61 and CYP72A69) from this subfamily in soybean further suggests that CYP72A subfamily underwent rapid functional divergence in specific plant lineages. Parallel gain of GA9 13-hydroxylase activity by CYP72A9/CYP72A15/CYP72A262/CYP72A272/CYP72A484 in Brassicales and CYP72A135 in Fabales also would support this conclusion. It should be noted that the tandem CYP72A members are linked to GFPPS-sesterTPS to form a gene cluster in Brassicaceae plants16 (Fig. 2). It seems plausible to suggest that one or more of these AtCYP72As are involved in the oxidation of the sesterterpene backbones14,15, and metabolomic analysis of the transgenic plants generated in this study will shed light on the further functional assignation of the CYP72A subfamily in Arabidopsis.
In summary, we conclude that GA deactivation (GA4 to GA1) by CYP72As has an important molecular function in fine-tuning GA homeostasis, which is particularly important for primary seed dormancy in Brassicaceae plants. The identification and functional characterization of the various CYP72A genes encoding diterpene (GA) oxidases in this study pave the way for further understanding of the functional diversity of the CYP72A subfamily in flowering plants.
METHODS
Plant Materials and Chemicals
The Arabidopsis thaliana lines (both WT and transgenic plants are Col-0 ecotype) and other plant species (Brassica rapa and Capsella rubella) mentioned in this study were grown on soil at 22 °C under a 16-h light/8-h dark cycle. The ga1-t mutant (SALK_023192) was a gift from Dr. Xiangdong Fu (Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing). The T-DNA insertion line cyp72a9–1(SALK_130811C) was ordered from ABRC (http://abrc.osu.edu/). To construct transgenic plants overexpressing AtCYP7A7-AtCYP7A15, coding regions of AtCYP7A7-AtCYP7A15 were amplified from cDNA clones using sets of primers (Supplementary Table 1). The PCR products were first ligated into pENTR/D-TOPO vector (Life Technologies Corporation, USA) and then ligated into the binary vector pCHF3 by the LR reaction. The cyp72a9–2 mutant was generated using the CRISPR/Cas9 technique. Cas9 editing target site primers were designed (Supplementary Table 1) and ligated into pCAMBIA 1300-pYAO-cas9.
All chemicals used in this study were purchased from Sigma-Aldrich (St. Louis, MO), except gibberellins (GA1, GA4, GA9 and GA20), which were purchased from OlChemIm company (http://www.olchemim.cz/) and ent-16β,17-dihydroxy kaurenoic acid, which was purchased from BOS Science (http://www.bocsci.com/).
Quantitative RT-PCR Analysis and Promoter: GUS Staining
RNA extraction, reverse transcription reaction and quantitative RT-PCR were performed as described previously32,33. Two sets of reference genes (three for each set) were selected for different qRT-PCR assay by following previous studies9,34. The annealing temperature, specificity and amplification efficiency of all primers used in the study were tested and are listed in Supplementary Table 1.
The promoter fragments for CYP72A9 (644 bp upstream of the ATG codon) were subcloned into the pMDC162 GATEWAY binary vector using a PCR-mediated technique (see Supplementary Table 1 for primer information). Arabidopsis transformation, screening for homozygous plants and GUS staining were performed as described previously35.
Subcellular Localization of CYP72A9
Constructs of the CYP72A9-GFP fusion protein (pJIT163-hGFP vector), Arabidopsis leaf protoplast preparations, transformation, and image collection using a laser scanning confocal microscope were all performed as described previously36. The plasmid harboring the gene encoding ER-localized mCherry marker protein (plasmid# CD3–959)37 was ordered from ABRC (http://abrc.osu.edu/). For detailed primer information, see Supplementary Table 1.
Heterologous Expression in Yeast and Identification of CYP72As Products
All CYP72A genes tested in this study were subcloned into the pESC-Leu vector for expression of Myc-tagged CYP72A in the WAT11 yeast strain38. Related primers used in the constructs are listed in Supplementary Table 1. The resulting positive yeast clones were cultured in 5 mL of SD dropout medium (-Ura, -Leu), and then harvested and induced with SG dropout medium (-Ura, -Leu) at 30°C for one day in a shaking incubator (200 rpm). The medium was diluted with fresh SG medium to OD595 = 0.4, 5 mL of the diluted medium was incubated with 1.0 μg of the different GA substrates for an additional 16 h. Ethyl acetate extracts of these recombinant cultures were evaporated to dryness by nitrogen flux. The dried chemicals were then derivatized with N-methyl-N-(trimethylsilyl) trifluoroacetamide (MSTFA) before analysis by GC-MS. One microliter of analytical sample was loaded onto the GC-MS for analysis. The initial oven temperature was held at 60°C for 1 min and then ramped at 10°C/min to 325°C and maintained at 325°C for 10 min. The inlet temperature was 270°C. Positive mode EI ionization was used. The temperatures of the ion source and quadrupole were set at 230°C and 150°C, respectively. Mass spectra were acquired within a scanning range of m/z 50–600.
Western Blot Assay
Yeast microsome was prepared as previously described39. Briefly, collected yeast cells (30 mL culture) were suspended in 4 mL of TE buffer (50 mM Tris-HCL, pH 7.4, 1 mM EDTA with 1 mM protease inhibitor Cocktail) and were disrupted with glass beads by vortexing. The broken cells were centrifuged at 10,000 g for 15 min, and the resulting supernatant was further centrifuged at 100,000 g for 2 h. The microsomal proteins were re-suspended in 200 μl TE buffer adding 1% Triton X-100 and quantified using Bradford assay. Twenty μg microsomal proteins were separated by 12% SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and blotted with anti-Myc tag primary antibody (1:5,000; ZSGB-BIO, China) and goat anti-mouse igG (H+L)-HRP as the second antibody (1:10,000).
Germination Assay and Vivipary Testing
For the Arabidopsis germination test, freshly harvested seeds were sown on water-saturated filter paper and then germinated under a 16-h light/8-h dark photoperiod at 22 °C. The number of germinated seeds was counted every 12 h until approximately 100% of the seeds had germinated. Radicle protrusion was used to indicate germination and was scored at the indicated time points. More than 3 biological replicates consisting of 50 seeds per replicate were used for each experiment. The vivipary assay was performed as previously described40. Briefly, developing siliques at the long-green stage were collected, sterilized with 70% ethanol for 1 min and 25% bleach for 10 min and then plated on ½ MS medium for germination.
Quantification of Endogenous Gibberellins in Arabidopsis
The quantification of endogenous GAs levels was performed as reported previously with modifications of the sample pretreatment41. Briefly, 200 mg of the ground plant material powder was extracted with 5 mL of 90% aqueous methanol. Simultaneously, 2 ng of each D-labeled GA compound was added to the extracting solvents as internal standards for GA content measurement. The MAX cartridge (Waters Corporation, Milford, USA) was activated and equilibrated with MeOH, water, 5% NH4OH, and 90% MeOH in turn, while MCX (Waters Corporation, Milford, USA) was equilibrated with MeOH, water and 90% MeOH. Subsequently, the crude extracts were loaded onto the tandem cartridges connected to an adapter. The MAX cartridge was then disconnected and rinsed with 5% NH4OH in 5% MeOH, MeOH in turn. Finally, GA compounds were eluted with 90% MeOH containing 2% FA. The eluent was dried under a N2 stream and redissolved in 150 μL of 40% MeOH prior to UPLC-MS/MS analysis. GA analysis was performed on a quadrupole linear ion trap hybrid mass spectrometer (QTRAP 6500, AB SCIEX, Foster City, CA) equipped with an electrospray ionization (ESI) source and coupled to a UPLC (Waters, Milford, MA, USA). The UPLC inlet method and ESI source parameters were set as previously reported41. GAs were detected in negative multiple reaction monitoring (MRM) mode. Two transitions were monitored for each GA compound, including one as a quantifier and the other as a qualifier. The MRM transitions and the corresponding collision energies were listed in Supplementary Table 2.
Molecular Phylogenetic Analysis
The protein sequences of the CYP72A subfamily from three Brassicaceae plants (Arabidopsis thaliana, Capsella rubella, and Brassica rapa), rice (Oryza sativa) and soybean (Glycine max L.) were extracted from a previous report20. A maximum likelihood tree was constructed using MEGA6.0 software with 1,000 boot-strap replicates42.
Supplementary Material
Acknowledgements
We cordially thank Dr. Xiangdong Fu (Institute of Genetics and Developmental Biology, Chinese Academy of Sciences) for providing the ga1-t mutant seeds and Dr. Qi Xie (Institute of Genetics and Developmental Biology, Chinese Academy of Sciences) for providing the vector pCAMBIA 1300-pYAO-cas9. We would also like to thank Dr. Jianqiang Wu (Kunming Institute of Botany, Chinese Academy of Sciences) for giving us the GA standard. This work was financially supported by the National Key R&D Program of China (Grant No.2018YFA0900600) to G.W., “Priority Research Program” of the Chinese Academy of Science (Grant No. ZDRW-ZS-2019-2) to G.W., and the State Key Laboratory of Plant Genomics of China (SKLPG2016A-15) to G.W., and National Natural Science Foundation of China (Grant No. 31770398) to J.C.
Footnotes
Data availability
All data and materials generated during this study are available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
References
- 1.Cowling RJ, Kamiya Y, Seto H & Harberd NP Gibberellin dose-response regulation of GA4 gene transcript levels in Arabidopsis. Plant Physiol. 117, 1195–1203 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang YY et al. Effects of gibberellins on seed germination of phytochrome deficient mutants of Arabidopsis thaliana. Plant Cell Physiol. 36, 1205–1211 (1995). [PubMed] [Google Scholar]
- 3.Magome H et al. CYP714B1 and CYP714B2 encode gibberellin 13-oxidases that reduce gibberellin activity in rice. Proc. Natl. Acad. Sci. USA 110, 1947–1952 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blazquez MA, Green R, Nilsson O, Sussman MR & Weigel D Gibberellins promote flowering of Arabidopsis by activating the LEAFY promoter. Plant Cell 10, 791–800 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eriksson S, Bohlenius H, Moritz T & Nilsson O GA4 is the active gibberellin in the regulation of LEAFY transcription and Arabidopsis floral initiation. Plant Cell 18, 2172–2181 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hedden P & Thomas SG Gibberellin biosynthesis and its regulation. Biochem. J 444, 11–25 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Zhu YY et al. ELONGATED UPPERMOST INTERNODE encodes a cytochrome P450 monooxygenase that epoxidizes gibberellins in a novel deactivation reaction in rice. Plant Cell 18, 442–456 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varbanova M et al. Methylation of gibberellins by Arabidopsis GAMT1 and GAMT2. Plant Cell 19, 32–45 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rieu I et al. Genetic analysis reveals that C19-GA 2-oxidation is a major gibberellin inactivation pathway in Arabidopsis. Plant Cell 20, 2420–2436 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu YL et al. Gibberellins play an essential role in late embryogenesis of Arabidopsis. Nature Plants 4, 289–298 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Kanno Y et al. Comprehensive hormone profiling in developing Arabidopsis seeds: examination of the site of ABA biosynthesis, ABA transport and hormone interactions. Plant Cell Physiol. 51, 1988–2001 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Zhang YY et al. Two Arabidopsis cytochrome P450 monooxygenases, CYP714A1 and CYP714A2, function redundantly in plant development through gibberellin deactivation. Plant J. 67, 342–353 (2011). [DOI] [PubMed] [Google Scholar]
- 13.Nomura T et al. Functional analysis of arabidopsis CYP714A1 and CYP714A2 reveals that they are distinct gibberellin modification enzymes. Plant Cell Physiol. 54, 1837–1851 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Wang C et al. Structural analyses of short-chain prenyltransferases identify an evolutionarily conserved GFPPS clade in Brassicaceae plants. Mol. Plant 9, 195–204 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Shao J et al. (+)-Thalianatriene and (−)-retigeranin B catalyzed by sesterterpene synthases from Arabidopsis thaliana. Org. Lett 19, 1816–1819 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Chen Q et al. Recently duplicated sesterterpene (C25) gene clusters in Arabidopsis thaliana modulate root microbiota. Sci. China Life Sci doi: 10.1007/s11427-019-9521-2 (2019, in press). [DOI] [PubMed] [Google Scholar]
- 17.Jiang CF, Gao XH, Liao L, Harberd NP & Fu XD Phosphate starvation root architecture and anthocyanin accumulation responses are modulated by the gibberellin-DELLA signaling pathway in Arabidopsis. Plant Physiol. 145, 1460–1470 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seo M et al. Regulation of hormone metabolism in Arabidopsis seeds: phytochrome regulation of abscisic acid metabolism and abscisic acid regulation of gibberellin metabolism. Plant J. 48, 354–366 (2006). [DOI] [PubMed] [Google Scholar]
- 19.Finkelstein R, Reeves W, Ariizumi T & Steber C Molecular aspects of seed dormancy. Annu. Rev. Plant Biol 59, 387–415 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Prall W, Hendy O & Thornton LE Utility of a phylogenetic perspective in structural analysis of CYP72A enzymes from flowering plants. PLoS One 11, e0163024 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turk EM et al. CYP72B1 inactivates brassinosteroid hormones: An intersection between photomorphogenesis and plant steroid signal transduction. Plant Physiol. 133, 1643–1653 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lange T, Hedden P & Graebe JE Expression cloning of a gibberellin 20-oxidase, a multifunctional enzyme involved in gibberellin biosynthesis. Proc. Natl. Acad. Sci. USA 91, 8552–8556 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu YL et al. The ga5 locus of Arabidopsis thaliana encodes a multifunctional gibberellin 20-oxidase – molecular cloning and functional expression. Proc. Natl. Acad. Sci. USA 92, 6640–6644 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamaguchi S Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol 59, 225–251 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Shu K, Liu XD, Xie Q & He ZH Two faces of one seed: hormonal regulation of dormancy and germination. Mol. Plant 9, 34–45 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Nelson D & Werck-Reichhart D A P450-centric view of plant evolution. Plant J. 66, 194–211 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Yano R et al. Metabolic switching of astringent and beneficial triterpenoid saponins in soybean is achieved by a loss-of-function mutation in cytochrome P450 72A69. Plant J. 89, 527–539 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Irmler S et al. Indole alkaloid biosynthesis in Catharanthus roseus: new enzyme activities and identification of cytochrome P450CYP72A1 as secologanin synthase. Plant J. 24, 797–804 (2000). [DOI] [PubMed] [Google Scholar]
- 29.Itkin M et al. Biosynthesis of antinutritional alkaloids in solanaceous crops is mediated by clustered genes. Science 341, 175–179 (2013). [DOI] [PubMed] [Google Scholar]
- 30.Umemoto N et al. Two cytochrome P450 monooxygenases catalyze early hydroxylation steps in the potato steroid glycoalkaloid biosynthetic pathway. Plant Physiol. 171 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zi JC, Mafu S & Peters RJ To gibberellins and beyond! Surveying the evolution of (Di)terpenoid metabolism. Annu. Rev. Plant Biol 65, 259–286 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang G et al. Terpene biosynthesis in glandular trichomes of hop. Plant Physiol. 148, 1254–1266 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li W et al. Nicotinate O-glucosylation is an evolutionarily metabolic trait important for seed germination under stress conditions in Arabidopsis thaliana. Plant Cell 27, 1907–1924, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Czechowski T, Stitt M, Altmann T, Udvardi MK & Scheible WR Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol. 139, 5–17 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang G & Pichersky E Nicotinamidase participates in the salvage pathway of NAD biosynthesis in Arabidopsis. Plant J. 49, 1020–1029 (2007). [DOI] [PubMed] [Google Scholar]
- 36.Xu H et al. Characterization of the formation of branched short-chain fatty acid:CoAs for bitter acid biosynthesis in hop glandular trichomes. Mol. Plant 6, 1301–1317 (2013). [DOI] [PubMed] [Google Scholar]
- 37.Nelson BK, Cai X & Nebenfuhr A A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J. 51, 1126–1136 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Urban P, Mignotte C, Kazmaier M, Delorme F & Pompon D Cloning, yeast expression, and characterization of the coupling of two distantly related Arabidopsis thaliana NADPH-cytochrome P450 reductases with P450 CYP73A5. J. Biol. Chem 272, 19176–19186 (1997). [DOI] [PubMed] [Google Scholar]
- 39.Pompon D, Louerat B, Bronine A & Urban P Yeast expression of animal and plant P450s in optimized redox environments. Methods Enzymol. 272, 51–64 (1996). [DOI] [PubMed] [Google Scholar]
- 40.Martinez-Andujar C et al. Induction of 9-cis-epoxycarotenoid dioxygenase in Arabidopsis thaliana seeds enhances seed dormancy. Proc. Natl. Acad. Sci. USA 108, 17225–17229 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma XD et al. CHR729 is a CHD3 protein that controls seedling development in rice. PLoS One 10, e0138934 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tamura K, Stecher G, Peterson D, Filipski A & Kumar S MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol 30, 2725–2729 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


