Abstract
Nerve growth factor (NGF) gene therapy rescues and stimulates cholinergic neurons, which degenerate in Alzheimer's disease (AD). In a recent clinical trial for AD, intraparenchymal adeno-associated virus serotype 2 (AAV2)-NGF delivery was safe but did not improve cognition. Before concluding that NGF gene therapy is ineffective, it must be shown that AAV2-NGF successfully engaged the target cholinergic neurons of the basal forebrain. In this study, patients with clinically diagnosed early- to middle-stage AD received a total dose of 2 × 1011 vector genomes of AAV2-NGF by stereotactic injection of the nucleus basalis of Meynert. After a mean survival of 4.0 years, AAV2-NGF targeting, spread, and expression were assessed by immunolabeling of NGF and the low-affinity NGF receptor p75 at 15 delivery sites in 3 autopsied patients. NGF gene expression persisted for at least 7 years at sites of AAV2-NGF injection. However, the mean distance of AAV2-NGF spread was only 0.96 ± 0.34 mm. NGF did not directly reach cholinergic neurons at any of the 15 injection sites due to limited spread and inaccurate stereotactic targeting. Because AAV2-NGF did not directly engage the target cholinergic neurons, we cannot conclude that growth factor gene therapy is ineffective for AD. Upcoming clinical trials for AD will utilize real-time magnetic resonance imaging guidance and convection-enhanced delivery to improve the targeting and spread of growth factor gene delivery.
Keywords: AAV, AAV2-NGF, Alzheimer's disease, clinical trial, gene therapy, NGF
Introduction
Cognitive decline in Alzheimer's disease (AD) is accompanied by loss of cholinergic neurons in the basal forebrain.1,2 Nerve growth factor (NGF) is an endogenous neurotrophic factor that regulates the growth and survival of cholinergic neurons by functional activation of the TrkA receptor.3–5 Exogenous NGF prevents the death of axotomized basal forebrain cholinergic neurons in rats and monkeys,6–12 is neuroprotective and improves spatial memory in aged rats,13,14 and reverses cholinergic neuron loss in aged monkeys.15 These preclinical studies advanced NGF as a candidate neuroprotective therapy for AD.
NGF does not cross the blood–brain barrier, but gene therapy approaches can deliver NGF to the basal forebrain. In the first clinical trial of NGF gene therapy for AD, autologous NGF-expressing fibroblasts were implanted in the nucleus basalis of Meynert (nbM) of eight patients.16 Long-term NGF expression was well tolerated and significantly increased brain metabolism.16 While the trial was small and unblinded, results suggested a potential slowing of cognitive decline.16 Following the emergence of adeno-associated virus serotype 2 (AAV2) vectors for safe and long-lasting gene transfer to monkey or human brain,17–21 a dose-escalating Phase 1 clinical trial of AAV2-NGF delivery to the nbM was conducted in 10 patients with mild-to-moderate AD.22 AAV2-NGF treatment was safe, and long-term expression of biologically active NGF was observed.23
A multicenter, sham placebo-controlled Phase 2 clinical trial subsequently evaluated the safety, feasibility, and efficacy of AAV2-NGF gene therapy.24 AAV2-NGF was delivered bilaterally to the nbM in 49 patients with mild-to-moderate AD, and change from baseline on the Alzheimer's Disease Assessment Scale–cognitive subscale was examined 2 years after treatment. Although AAV2-NGF gene therapy was again safe and well tolerated, there was no significant difference in cognitive decline between the placebo and treatment groups.24
Extensive evidence supports the biological activity of NGF on cholinergic neurons and the efficacy of AAV2 for long-term neuronal gene expression. It is possible that AAV2-NGF gene therapy did not significantly improve cognition because AAV2-NGF was not successfully delivered to the nbM. To evaluate this possibility, we quantified target engagement in the brains of 3 deceased patients from the Phase 1 dose-escalation trial who collectively received 15 AAV2-NGF injections. The surgical procedure and maximum dose were identical in the Phase 1 and Phase 2 trials: each side of the brain received three stereotactic injections of AAV2-NGF targeting the nbM. In these patients, the maximum dose of 2 × 1010 vector genomes (vg) in 20 μL was delivered to each site for a total dose of 1.2 × 1011 vg per brain. We performed histological analysis to examine the spread of NGF protein around the injection site and the extent to which each AAV2-NGF injection successfully reached the nbM. Detailed analysis of target engagement is essential for interpreting clinical trial results and assessing the therapeutic potential of NGF gene therapy for AD.
Materials and Methods
AAV2-NGF Production
The AAV-NGF genome contains: two AAV2 inverted terminal repeats; the synthetic CAG promoter, which comprises the cytomegalovirus immediate-early enhancer, the chicken β-actin promoter, and a chimeric intron combining the first exon and intron of the chicken β-actin gene with the rabbit β-globin splice acceptor; the human NGF complementary DNA (cDNA) sequence; and the human growth hormone (hGH) polyadenylation signal sequence. AAV2 vectors carrying this genome were produced by triple transfection of HEK-293 cells and purified by heparin affinity chromatography, as described previously.14 The AAV2-NGF clinical product was made at Ceregene, Inc., using good manufacturing practices.
Participant Enrollment and Surgical Procedure
Patients in this study were enrolled in a dose-escalating Phase 1 clinical trial of AAV2-NGF gene therapy (ClinicalTrials.gov NCT00087789) in 2008 at the University of California, San Diego Medical Center (La Jolla, CA). The protocol was approved by the institutional review board, and written informed consent was obtained from all trial participants or their legal representatives. Consent for autopsy was obtained from the patients examined in this study. Detailed enrollment criteria were reported previously.22 Criteria included: clinical diagnosis of probable AD by the National Institute of Neurological Disorders and Stroke guidelines; age of 50–80 years; and baseline Mini-Mental State Examination score between 16 and 26. Patient characteristics are summarized in Table 1. The three patients examined in this study received the maximum dose of 1.2 × 1011 vg of AAV2-NGF, delivered in six injections of 2 × 1010 vg targeting caudal nbM (three injections per side of the brain). On each side of the brain, one injection targeted the anterolateral division of the nbM, one targeted the intermediate division, and one targeted the posterior division.
Table 1.
Patient characteristics
| Patient | Age at Diagnosis (Years) | Age at Treatment (Years) | Survival (Years) | Gender | MMSE at Entry | Braak Stage |
|---|---|---|---|---|---|---|
| 1 | 53 | 56 | 3.33 | F | 17 | V/VI |
| 2 | 61 | 62 | 7.83 | F | 26 | V/VI |
| 3 | 75 | 78 | 0.83 | M | 21 | V/VI |
MMSE, Mini-Mental State Examination.
The surgical procedure for AAV2-NGF gene delivery was described previously.22 Before surgery, injection needles were primed using high concentrations of AAV2-NGF to saturate AAV binding sites and prevent loss of vector during injection. This method was validated in preclinical studies by Ceregene, Inc. The patient was placed in a magnetic resonance imaging (MRI)-compatible head frame, MRI was performed, and MRI-based trajectories were identified. Three trajectories per side of the brain were planned to target the caudal two-thirds of the nbM at ∼2.5 mm intervals, and to avoid sulci, cortical veins, and ventricles. The needle tip was targeted ∼5 mm from the pial edge to avoid penetration of the pia and injection into the subarachnoid space.
Standard burr holes were prepared, the dura was opened, the pia was perforated, and a blunt injection needle with stylet to prevent coring was lowered along the planned stereotactic trajectory. The stylet was then removed, and 20 μL of AAV2-NGF was injected manually at 2 μL/min. The needle remained in place for 2 min after injection, was slowly withdrawn 1–2 mm, remained in place for several additional minutes, and was then withdrawn completely. The remaining five injections were performed sequentially using identical methods. Postoperative MRI was performed within 24 h to assess safety; injection sites were not visible on these scans.
Histology
Brains were fixed by immersion in 4% or 10% paraformaldehyde for 72 h and then post-fixed in a series of 10% and 20% glycerol solutions. Hemispheres were split and blocked into 2–3 cm slabs. Blocks containing the nbM were sectioned coronally at 40 μm thickness using a freezing microtome. NGF and p75 immunohistochemistry were performed on adjacent tissue sections. At minimum, every 12th tissue section was stained with each label, corresponding to a maximum interval of 480 μm between immunolabeled sections. Before NGF immunolabeling, antigen retrieval was performed by incubating sections in 0.01 M Tris-HCl (pH 9.0) at 80°C for 20 min in a hot water bath, cooling at room temperature for 30 min, and post-fixing in a freshly prepared solution of 2% paraformaldehyde and 0.2% parabenzoquinone in phosphate buffer for 5 min. Antigen retrieval was not performed before p75 immunolabeling.
Sections were washed in 0.1 M Tris-buffered saline (TBS), incubated in 0.1 M sodium metaperiodate in TBS for 20 min, and blocked in 5% heat-inactivated normal horse serum and 0.2% (NGF) or 0.25% (p75) Triton X-100 in TBS for 1 h. For NGF immunolabeling, sections were then incubated at 4°C in a 1:3,000 dilution of rabbit anti-NGF polyclonal antibody, made as previously described,25 and 5% horse serum in TBS for 72 h, washed in TBS, and incubated in a 1:300 dilution of biotin-conjugated donkey anti-rabbit IgG (No. 711-065-152; Jackson ImmunoResearch) and 5% horse serum in TBS for 2 h. For p75 immunolabeling, sections were incubated at 4°C in a 1:5,000 dilution of mouse anti-p75 monoclonal antibody clone NGFR5 (No. ab3125; Abcam), 5% horse serum, and 0.25% Triton X-100 in TBS for 96 h, washed in TBS, and incubated in a 1:300 dilution of biotin-conjugated donkey anti-mouse IgG (No. 715-065-150; Jackson ImmunoResearch), 5% horse serum, and 0.25% Triton X-100 in TBS for 2 h.
Sections were then washed in TBS, incubated in a 1:111 (NGF) or 1:250 (p75) dilution of avidin/biotin peroxidase in TBS for 90 min (No. PK6100; Vector Laboratories), washed in TBS, washed in imidazole acetate buffer (pH 7.4), and developed for 4 min in 0.05% 3,3-diamino-benzidine-HCl, 2.5% nickel ammonium sulfate, and 0.0015% H2O2 in imidazole acetate buffer. After immunolabeling, sections were washed in TBS, dehydrated, and coverslipped in DPX mounting medium (No. 06522; MilliporeSigma).
Microscopy and Analysis
Low-magnification images of whole brain sections were acquired using a Keyence BZ-X710 microscope with 10 × Plan-Apo objective and stitched using Keyence BZ-X Analyzer software. High-magnification images of injection sites are full-focus composites of z-stacks acquired using a Keyence BZ-X710 microscope with 20 × Plan-Apo objective and stitched using Keyence BZ-X Analyzer software. All quantification was performed using the “Measure” function in FIJI software (v. 2.0.0).26,27
For all sections with a detectable injection site by NGF immunolabeling, the following distances were measured: (1) the dorsoventral spread of immunolabeling; (2) the mediolateral spread of immunolabeling; (3) the shortest distance between NGF immunolabeling at the injection site and NGF immunolabeling at the nbM; and (4) the shortest distance between NGF immunolabeling at the injection site and the pial edge. For all sections with a detectable injection site by p75 immunolabeling, the shortest distance between p75 immunolabeling at the injection site and p75 immunolabeling at the nbM was measured. For each measurement, all distances at each injection site were averaged; “±” denotes 1 standard deviation from the mean. Distances are shown without standard deviation when the injection site was only detected in a single tissue section.
Results
We analyzed the brains of three deceased patients from the Phase 1 clinical trial of AAV2-NGF gene therapy for AD (Table 1). No adverse events related to gene delivery occurred in these three patients other than mild postoperative headaches that rapidly resolved.22 Cognitive decline was consistent with prior studies of mild-to-moderate AD22; in the Phase 2 trial, which used identical methods and included sham surgery controls, there was no significant difference in the rate of cognitive decline between patients that received AAV2-NGF and controls.24 Subjects 1 and 3 died 3.3 and 7.8 years after treatment, respectively, of end-stage AD. Subject 3 died 10 months after treatment from failure to thrive. No deaths were related to treatment.
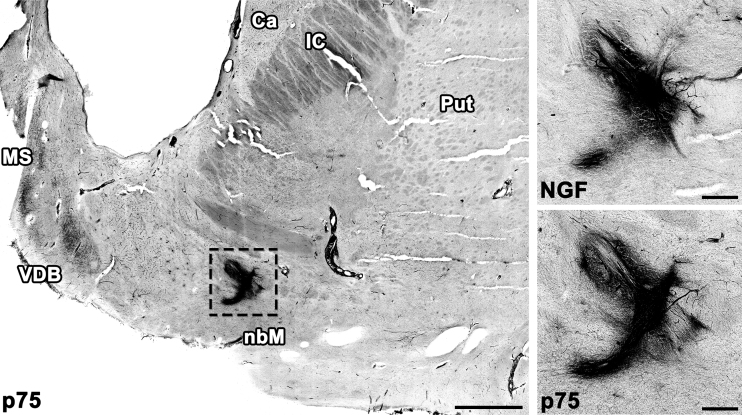
Brains underwent (1) histopathological assessment of amyloid β deposits, (2) staging of neurofibrillary tangles, and (3) scoring of neuritic plaques, together with assessments of Lewy bodies, vascular brain injury, and hippocampal sclerosis as recommended by Montine et al.28 All brains met criteria for a neuropathological diagnosis of AD, and no other pathology was identified. One half of the brain from patient 3 was reserved for biochemical analyses; a total of 15 injection sites over 5 brain halves were examined by immunolabeling of NGF and of the low-affinity NGF receptor p75. All 15 injection sites were detectable by NGF immunolabeling. NGF expression persisted more than 7 years after treatment (Fig. 1). Injection sites were innervated by axons expressing the p75 receptor, suggesting that NGF was secreted and was biologically active (Fig. 1).
Figure 1.
AAV2-NGF expression persists up to 7 years in the human brain. NGF expression and innervation by axons expressing the NGF receptor p75 were detectable at all injection sites. The left anterior injection site is shown 7 years after treatment. Scale bars: 3 mm (low magnification), 0.5 mm (high magnification). AAV2, adeno-associated virus serotype 2; Ca, caudate; IC, internal capsule; MS, medial septum; NGF, nerve growth factor; Put, putamen; VDB, vertical limb of the diagonal band of Broca.
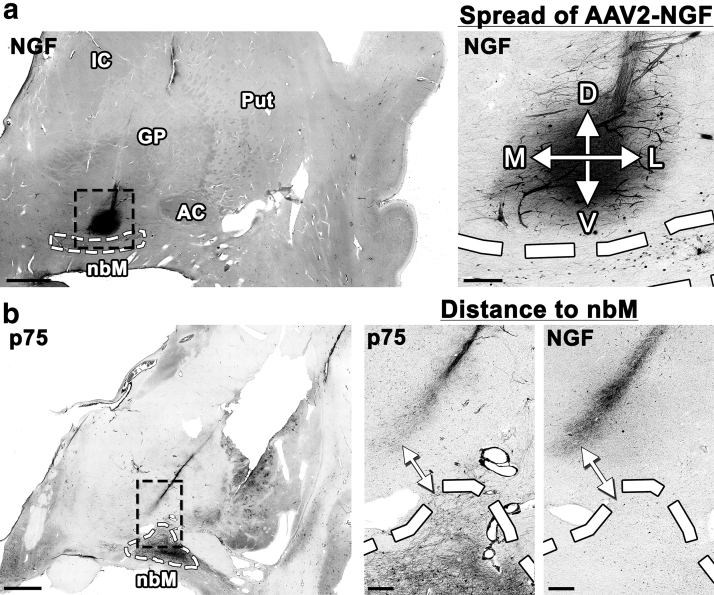
We quantified the diffusion of AAV2-NGF by measuring the dorsoventral and the mediolateral distance of NGF spread around each injection site by NGF immunolabeling of coronal brain sections (Fig. 2a). Values from all sections with a detectable injection site were averaged to determine the mean spread of AAV2-NGF around each site (Table 2). The mean spread around all 15 injection sites was 0.957 ± 0.34 mm (“±” denotes one standard deviation from the mean). NGF-expressing cells were found along the needle tract near some injection sites but were not found distant from injection sites or within cortex. No changes in cortical cholinergic innervation were detected by p75 immunolabeling.
Figure 2.
Quantification of AAV2-NGF spread and target engagement. (a) The D/V and M/L spread of NGF expression were measured by NGF immunolabeling (Table 2). The right anterior injection site is shown. (b) The shortest distance between the injection site and the nbM was measured both by NGF immunolabeling and by p75 immunolabeling (Table 3). Sites of NGF expression were innervated by axons expressing the NGF receptor p75. The left intermediate injection site is shown. Scale bars: 3 mm (low magnification), 0.5 mm (high magnification). AC, anterior commissure; D/V, dorso/ventral; GP, globus pallidus; M/L, medio/lateral; nbM, nucleus basalis of Meynert.
Table 2.
Spread of nerve growth factor expression at the injection site
| Patient | Anterior Site 1: Left | Anterior Site 1: Right | Intermediate Site 2: Left | Intermediate Site 2: Right | Posterior Site 3: Left | Posterior Site 3: Right | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | D/V | 1.48 ± 0.37 | D/V | 1.04 ± 0.21 | D/V | 1.30 ± 0.18 | D/V | 0.746 | D/V | 1.22 ± 0.14 | D/V | 0.813 ± 0.13 |
| M/L | 1.59 ± 0.38 | M/L | 1.31 ± 0.15 | M/L | 1.27 ± 0.19 | M/L | 1.09 | M/L | 1.14 ± 0.19 | M/L | 0.467 ± 0.12 | |
| 2 | D/V | 0.576 ± 0.26 | D/V | 0.878 ± 0.021 | D/V | 0.432 ± 0.069 | D/V | 0.579 | D/V | 0.520 ± 0.024 | D/V | 0.696 |
| M/L | 1.42 ± 0.054 | M/L | 0.460 ± 0.066 | M/L | 1.34 ± 0.30 | M/L | 0.372 | M/L | 0.520 ± 0.067 | M/L | 1.07 | |
| 3 | D/V | 0.527 ± 0.23 | D/V | 1.18 ± 0.31 | D/V | 1.73 ± 0.47 | ||||||
| M/L | 0.794 ± 0.22 | M/L | 0.712 ± 0.12 | M/L | 1.44 ± 0.037 | |||||||
To quantify the diffusion of AAV2-NGF, the D/V and M/L spread of NGF expression (mm ± standard deviation) were measured and averaged among all sections with a detectable injection site. The mean spread of NGF expression around the injection site was 0.957 ± 0.34 mm, less than predicted by preclinical studies.
±, 1 standard deviation; AAV2, adeno-associated virus serotype 2; D/V, dorso/ventral; M/L, medio/lateral; NGF, nerve growth factor.
We quantified target engagement by measuring the shortest distance between each injection site and the nbM using either NGF or p75 immunolabeling (Fig. 2b). No injections spread directly into the nbM by either immunolabel. Values from all sections with a detectable injection site were averaged to determine the mean distance between each injection site and the nbM (Table 3). The mean distance between NGF expression at the injection site and NGF immunolabeling of the nbM was 2.17 ± 1.3 mm. The mean distance between p75-labeled axons at the injection site and p75 immunolabeling of the nbM was 1.25 ± 1.2 mm.
Table 3.
Distance from the injection site to the nucleus basalis of Meynert
| Patient | Anterior Site 1: Left | Anterior Site 1: Right | Intermediate Site 2: Left | Intermediate Site 2: Right | Posterior Site 3: Left | Posterior Site 3: Right | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | NGF | 0.774 ± 0.21 | NGF | 1.33 ± 0.24 | NGF | 0.654 ± 0.093 | NGF | 0.640 | NGF | 0.814 ± 0.15 | NGF | 3.97 ± 0.26 |
| p75 | 0.458 ± 0.093 | p75 | 0.836 ± 0.064 | p75 | 0.557 | p75 | 0.746 | p75 | 0.594 ± 0.23 | p75 | ND | |
| 2 | NGF | 5.10 | NGF | 2.62 ± 0.61 | NGF | 3.53 ± 0.88 | NGF | 2.21 | NGF | 2.18 ± 1.0 | NGF | 2.52 |
| p75 | 4.96 ± 0.23 | p75 | 1.15 ± 0.19 | p75 | 1.82 ± 0.40 | p75 | 1.74 ± 0.054 | p75 | 0.328 ± 0.17 | p75 | 1.72 ± 0.18 | |
| 3 | NGF | 2.29 ± 0.58 | NGF | 2.44 ± 0.39 | NGF | 1.55 ± 0.21 | ||||||
| p75 | 0.806 ± 0.23 | p75 | 0.993 ± 0.057 | p75 | 0.791 ± 0.16 | |||||||
To quantify target engagement, the shortest distance (mm ± standard deviation) from the injection site to the nucleus basalis of Meynert was measured and averaged among all sections with a detectable injection site. The mean distance from NGF expression at the injection site to NGF immunolabeling of the nucleus basalis of Meynert was 2.17 ± 1.3 mm. The mean distance from p75-labeled axons at the injection site to p75 immunolabeling of the nucleus basalis of Meynert was 1.25 ± 1.2 mm. No injections spread directly to the nucleus basalis of Meynert.
ND, injection site not detected by immunolabeling; p75, low-affinity NGF receptor p75.
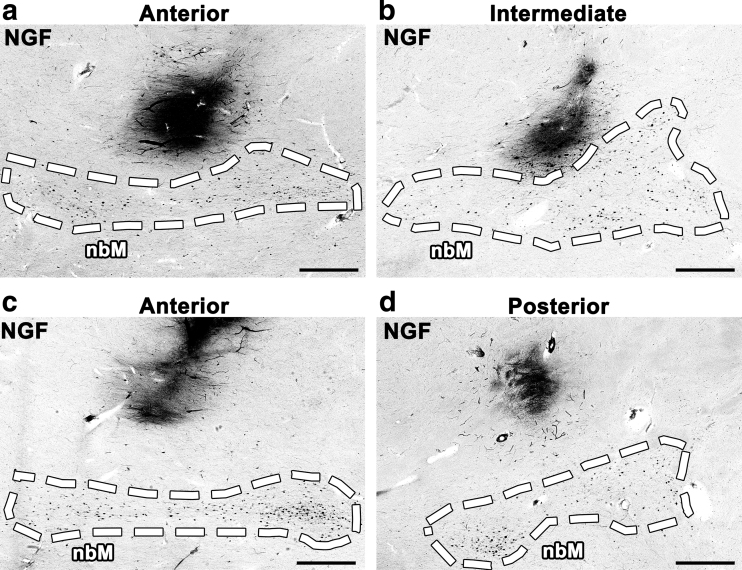
The accuracy of stereotactic injection was variable by NGF immunolabeling. Some injection sites were adjacent to the nbM; greater spread of AAV2-NGF from these injections would likely have engaged the target (Fig. 3a, b). Other injection sites were inaccurately targeted and distant from the nbM (Fig. 3c, d). During surgery, each injection was targeted using MRI-based stereotactic coordinates ∼5 mm from the pial edge to avoid penetration of the pia and injection into the subarachnoid space. We quantified injection accuracy by measuring the shortest distance from each injection site to the pial edge by NGF immunolabeling. Values from all sections with detectable NGF expression were averaged for each site. The mean distance between the infusion site and the pial edge was 4.67 ± 1.3 mm. Although injections were accurately targeted on average, 6 of the 15 injections were not targeted within 5 mm of the pial edge (Supplementary Table S1).
Figure 3.
Stereotactic injection was inconsistent. No injections spread directly into the nbM by NGF immunolabeling. Some injections were adjacent to nbM but failed to engage the target due to limited spread of AAV2-NGF (a, b), whereas other injections were more distant from nbM (c, d). Scale bars: 1 mm.
Discussion
All 15 AAV2-NGF injections examined in this study failed to reach the nbM. Some injections were mistargeted, but most were accurately targeted within 5 mm of the pial edge and 3 mm of the nbM. Failure to engage the target was primarily due to limited spread of AAV2-NGF: only two injection sites exhibited a mean spread >1.5 mm and none spread more than 2 mm. Cholinergic innervation of injection sites was detectable by p75 immunolabeling, suggesting partial access of nbM neurons to NGF, but the study protocol was intended to directly deliver AAV2-NGF to the nbM. Because AAV2-NGF did not directly engage the target, we cannot conclude that NGF is ineffective as a neuroprotective therapy in AD. Further studies that use improved gene delivery methods may be warranted.
The limited spread of AAV2-NGF was not predicted by preclinical studies. Approximately 1 mm of spread was observed surrounding injections of rat nbM with 5.3 × 108 vg of AAV2-NGF in 1 μL14; injections of AAV2-NGF in this clinical trial spread a similar distance despite containing 40 times more AAV2-NGF particles in 20 times greater volume. Injection of monkey putamen with 5.2 × 109 vg of AAV2 in 34.5 μL was reported to spread more than 11 mm29; injections of AAV2-NGF in this clinical trial contained 4 times more AAV2 particles but spread 1/10th of the distance.
This disparity may be related to the injection technique: the above rat and monkey studies used a pump to continuously infuse AAV2 at a rate of 0.5 μL/min or an average ramped rate of 0.38 μL/min, respectively.14,29 Use of a syringe pump to apply continuous positive pressure for uninterrupted infusion, termed convection-enhanced delivery, is believed to increase the spread of infusate.17,29–32 In contrast, AAV2-NGF was administered to patients by noncontinuous manual injection at an average rate of 2 μL/min22; surgeons in the trial intermittently depressed the Hamilton syringe plunger at ∼15-s intervals, but conditions of vector delivery were otherwise not precisely defined.
A Phase 2 clinical trial of AAV2-Neurturin delivery into the putamen for Parkinson's disease reported similar results: surgeons following the same instructions as the AAV2-NGF trial infused 3.4 × 1010 vg of AAV2 in 5 μL at each site by noncontinuous manual injection at an average rate of 2 μL/min.33 AAV2-Neurturin spread ∼2.5 mm in the human putamen (by measurement of a representative image of neurturin immunolabeling), again less than predicted by preclinical studies of continuous AAV2 infusion into the monkey putamen at rates enabling convection enhanced spread.29,33 Greater spread of AAV2-neurturin than AAV2-NGF may be due to the larger amount of intercalating white matter in the putamen: infused substances spread more extensively through white matter than gray matter.34
Unlike most AAV serotypes, AAV2 strongly binds heparan sulfate as a cell surface attachment receptor.35 Heparan sulfates are reported to be elevated in the AD brain, potentially reducing the spread of AAV2.36 Extracellular amyloid or tau aggregates could also affect the diffusion of AAV2 in the AD brain, although AAV2 spread does not appear to be reduced in mouse models that overexpress amyloid.37 Further study of these topics is warranted. We propose that future clinical trials that administer AAV2 by intraparenchymal brain injection should utilize continuous vector infusion and convection-enhanced delivery and should examine whenever possible the spread of gene transfer postmortem. Indeed, three other clinical trials of AAV2 gene therapy in the brain are currently using these techniques: AAV2-GDNF for Parkinson's disease, AAV2-AADC for Parkinson's disease, and AAV2-AADC for congenital dopamine deficiency.38–42
Postoperative MRIs were performed on each subject who received AAV2-NGF, but because the infusate did not contain an MRI contrast agent and was not visible by MRI, distribution of the vector could not be assessed at the time of treatment. Our upcoming clinical trial of brain-derived neurotrophic factor (BDNF) gene therapy for AD will use real-time MR guidance and convection-enhanced delivery to administer AAV2-BDNF to the entorhinal cortex. AAV2-BDNF and the MRI contrast agent gadoteridol will be co-infused while the patient is continuously scanned by MRI, enabling real-time monitoring of infusion accuracy.43,44 The spread of gadoteridol closely matches the spread of AAV2-BDNF in preclinical monkey studies44; this permits continuous monitoring of vector spread and adjustment of needle placement or infusion volume as needed for complete target engagement. MR-guided delivery addresses both the primary reason for failed target engagement in the AAV2-NGF trial, insufficient vector spread, as well as the secondary cause, variable injection accuracy.
Alternatives to intraparenchymal brain injection may further improve AAV delivery for AD. Intrathecal infusion of AAV9 drives widespread gene expression throughout cerebral cortex and basal forebrain,45,46 and the strength and subject-to-subject consistency of cortical gene transfer can be enhanced by 2 h of Trendelenburg positioning after infusion.47 This technique has strong potential for clinical translation because it is simple, minimally invasive, and eliminates the need for accurate injection targeting; a Phase 1 clinical trial of intrathecal AAV-ApoE2 gene therapy for AD in APOE ɛ4 homozygotes is expected to begin soon.48,49 Another approach combines intravenous AAV infusion with focused ultrasound to transiently open the blood–brain barrier and noninvasively deliver AAV to targeted brain regions.50,51 A third alternative is the use of novel AAVs made by directed evolution: AAVPhP.B and PhP.eB exhibit dramatically enhanced gene transfer to the mouse brain after intravenous delivery.52,53 Development of a similar AAV capsid for use in humans would permit widespread gene delivery to cerebral cortex and basal forebrain from a single noninvasive intravenous infusion. These novel approaches could allow neurotrophic factor gene therapy for AD to reach its full potential.
In conclusion, a recent sham placebo-controlled Phase 2 clinical trial of AAV2-NGF gene therapy for AD was safe and well tolerated but did not detect a difference in cognitive decline between placebo and treatment groups.24 In five brain halves from three deceased Phase 1 patients who received identical stereotactic injections of AAV2-NGF, all injections failed to spread directly to the nbM as intended; we thus cannot reliably conclude that AAV2-NGF lacked efficacy in the Phase 2 trial. Effective target engagement is critical for human gene therapy, and new methods such as MR-guided AAV infusion, intrathecal AAV infusion with Trendelenburg positioning, and directed evolution of AAV capsids may greatly improve targeting and delivery. These novel technologies will be used in upcoming trials of BDNF gene therapy for AD, which is supported by extensive preclinical evidence and remains a strong candidate for clinical translation.
Supplementary Material
Acknowledgments
We thank the participants and their families for making this study possible, and we thank Jennifer H. Yang for performing histology.
Author Disclosure
M.H.T. was the founder of Ceregene, Inc. His financial interest in the company ended in 2013. All other authors have no competing financial interests.
Funding Information
The histological analysis performed in this project was supported by the Alzheimer's Association Zenith Award (to M.H.T.), the Alzheimer's Association Research Fellowship 16-443284 (to M.J.C.), the National Institutes of Health award AG043416 (to M.H.T.), and the Veteran's Administration.
Supplementary Material
References
- 1. Whitehouse PJ, Price DL, Struble RG, et al. Alzheimer's disease and senile dementia: loss of neurons in the basal forebrain. Science 1982;215:1237–1239 [DOI] [PubMed] [Google Scholar]
- 2. Schmitz TW, Nathan Spreng R; Alzheimer's Disease Neuroimaging Initiative. Basal forebrain degeneration precedes and predicts the cortical spread of Alzheimer's pathology. Nat Commun 2016;7:13249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mobley WC, Rutkowski JL, Tennekoon GI, et al. Nerve growth factor increases choline acetyltransferase activity in developing basal forebrain neurons. Brain Res 1986;387:53–62 [DOI] [PubMed] [Google Scholar]
- 4. Holtzman DM, Li Y, Parada LF, et al. p140trk mRNA marks NGF-responsive forebrain neurons: evidence that trk gene expression is induced by NGF. Neuron 1992;9:465–478 [DOI] [PubMed] [Google Scholar]
- 5. Li Y, Holtzman DM, Kromer LF, et al. Regulation of TrkA and ChAT expression in developing rat basal forebrain: evidence that both exogenous and endogenous NGF regulate differentiation of cholinergic neurons. J Neurosci 1995;15:2888–2905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hefti F. Nerve growth factor promotes survival of septal cholinergic neurons after fimbrial transections. J Neurosci 1986;6:2155–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kromer LF. Nerve growth factor treatment after brain injury prevents neuronal death. Science 1987;235:214–216 [DOI] [PubMed] [Google Scholar]
- 8. Rosenberg MB, Friedmann T, Robertson RC, et al. Grafting genetically modified cells to the damaged brain: restorative effects of NGF expression. Science 1988;242:1575–1578 [DOI] [PubMed] [Google Scholar]
- 9. Tuszynski MH, U HS, Amaral DG, et al. Nerve growth factor infusion in the primate brain reduces lesion-induced cholinergic neuronal degeneration. J Neurosci 1990;10:3604–3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koliatsos VE, Nauta HJ, Clatterbuck RE, et al. Mouse nerve growth factor prevents degeneration of axotomized basal forebrain cholinergic neurons in the monkey. J Neurosci 1990;10:3801–3813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kordower JH, Winn SR, Liu YT, et al. The aged monkey basal forebrain: rescue and sprouting of axotomized basal forebrain neurons after grafts of encapsulated cells secreting human nerve growth factor. Proc Natl Acad Sci U S A 1994;91:10898–10902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tuszynski MH, Roberts J, Senut MC, et al. Gene therapy in the adult primate brain: intraparenchymal grafts of cells genetically modified to produce nerve growth factor prevent cholinergic neuronal degeneration. Gene Ther 1996;3:305–314 [PubMed] [Google Scholar]
- 13. Fischer W, Wictorin K, Björklund A, et al. Amelioration of cholinergic neuron atrophy and spatial memory impairment in aged rats by nerve growth factor. Nature 1987;329:65–68 [DOI] [PubMed] [Google Scholar]
- 14. Bishop KM, Hofer EK, Mehta A, et al. Therapeutic potential of CERE-110 (AAV2-NGF): targeted, stable, and sustained NGF delivery and trophic activity on rodent basal forebrain cholinergic neurons. Exp Neurol 2008;211:574–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nagahara AH, Bernot T, Moseanko R, et al. Long-term reversal of cholinergic neuronal decline in aged non-human primates by lentiviral NGF gene delivery. Exp Neurol 2009;215:153–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tuszynski MH, Thal L, Pay M, et al. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med 2005;11:551–555 [DOI] [PubMed] [Google Scholar]
- 17. Bankiewicz KS, Eberling JL, Kohutnicka M, et al. Convection-enhanced delivery of AAV vector in parkinsonian monkeys; in vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach. Exp Neurol 2000;164:2–14 [DOI] [PubMed] [Google Scholar]
- 18. During MJ, Kaplitt MG, Stern MB, et al. Subthalamic GAD gene transfer in Parkinson disease patients who are candidates for deep brain stimulation. Hum Gene Ther 2001;12:1589–1591 [PubMed] [Google Scholar]
- 19. Janson C, McPhee S, Bilaniuk L, et al. Clinical protocol. Gene therapy of Canavan disease: AAV-2 vector for neurosurgical delivery of aspartoacylase gene (ASPA) to the human brain. Hum Gene Ther 2002;13:1391–1412 [DOI] [PubMed] [Google Scholar]
- 20. Crystal RG, Sondhi D, Hackett NR, et al. Clinical protocol. Administration of a replication-deficient adeno-associated virus gene transfer vector expressing the human CLN2 cDNA to the brain of children with late infantile neuronal ceroid lipofuscinosis. Hum Gene Ther 2004;15:1131–1154 [DOI] [PubMed] [Google Scholar]
- 21. Eberling JL, Jagust WJ, Christine CW, et al. Results from a phase I safety trial of hAADC gene therapy for Parkinson disease. Neurology 2008;70:1980–1983 [DOI] [PubMed] [Google Scholar]
- 22. Rafii MS, Baumann TL, Bakay RAE, et al. A phase1 study of stereotactic gene delivery of AAV2-NGF for Alzheimer's disease. Alzheimers Dement 2014;10:571–581 [DOI] [PubMed] [Google Scholar]
- 23. Tuszynski MH, Yang JH, Barba D, et al. Nerve growth factor gene therapy: activation of Neuronal Responses in Alzheimer Disease. JAMA Neurol 2015;72:1139–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rafii MS, Tuszynski MH, Thomas RG, et al. Adeno-associated viral vector (serotype 2)—nerve growth factor for patients with Alzheimer disease. JAMA Neurol 2018;75:834–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Conner JM, Muir D, Varon S, et al. The localization of nerve growth factor-like immunoreactivity in the adult rat basal forebrain and hippocampal formation. J Comp Neurol 1992;319:454–462 [DOI] [PubMed] [Google Scholar]
- 26. Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods 2012;9:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rueden CT, Schindelin J, Hiner MC, et al. ImageJ2: imageJ for the next generation of scientific image data. BMC Bioinformatics 2017;18:529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol 2012;123:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hadaczek P, Kohutnicka M, Krauze MT, et al. Convection-enhanced delivery of adeno-associated virus type 2 (AAV2) into the striatum and transport of AAV2 within monkey brain. Hum Gene Ther 2006;17:291–302 [DOI] [PubMed] [Google Scholar]
- 30. Bobo RH, Laske DW, Akbasak A, et al. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A 1994;91:2076–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cunningham J, Oiwa Y, Nagy D, et al. Distribution of AAV-TK following intracranial convection-enhanced delivery into rats. Cell Transplant 2000;9:585–594 [DOI] [PubMed] [Google Scholar]
- 32. Sanftner LM, Sommer JM, Suzuki BM, et al. AAV2-mediated gene delivery to monkey putamen: evaluation of an infusion device and delivery parameters. Exp Neurol 2005;194:476–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marks WJ, Bartus RT, Siffert J, et al. Gene delivery of AAV2-neurturin for Parkinson's disease: a double-blind, randomised, controlled trial. Lancet Neurol 2010;9:1164–1172 [DOI] [PubMed] [Google Scholar]
- 34. Mehta AM, Sonabend AM, Bruce JN. Convection-enhanced delivery. Neurotherapeutics 2017;14:358–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huynh MB, Ouidja MO, Chantepie S, et al. Glycosaminoglycans from Alzheimer's disease hippocampus have altered capacities to bind and regulate growth factors activities and to bind tau. PLoS One 2019;14:e0209573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Summerford C, Samulski RJ. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol 1998;72:1438–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu H-Y, Hudry E, Hashimoto T, et al. Amyloid beta (Aβ) induces the morphological neurodegenerative triad of spine loss, dendritic simplification, and neuritic dystrophies through calcineurin (CaN) activation. J Neurosci 2010;30:2636–2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. ClinicalTrials.gov AAV2-GDNF for advanced Parkinson's disease. https://clinicaltrials.gov/ct2/show/NCT01621581 (last accessed February26, 2020)
- 39. Richardson RM, Kells AP, Rosenbluth KH, et al. Interventional MRI-guided putaminal delivery of AAV2-GDNF for a planned clinical trial in Parkinson's disease. Mol Ther 2011;19:1048–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. ClinicalTrials.gov Safety study of AADC gene therapy (VY-AADC01) for Parkinson's disease. https://clinicaltrials.gov/ct2/show/NCT01973543 (last accessed February26, 2020)
- 41. Christine CW, Bankiewicz KS, Van Laar AD, et al. Magnetic resonance imaging-guided phase 1 trial of putaminal AADC gene therapy for Parkinson's disease. Ann Neurol 2019;85:704–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. ClinicalTrials.gov A single-stage, adaptive, open-label, dose escalation safety and efficacy study of AADC deficiency in pediatric patients. https://clinicaltrials.gov/ct2/show/NCT02852213 (last accessed February26, 2020)
- 43. Su X, Kells AP, Aguilar Salegio EA, et al. Real-time MR imaging with gadoteridol predicts distribution of transgenes after convection-enhanced delivery of AAV2 vectors. Mol Ther 2010;18:1490–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nagahara AH, Wilson BR, Ivasyk I, et al. MR-guided delivery of AAV2-BDNF into the entorhinal cortex of non-human primates. Gene Ther 2018;25:104–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Samaranch L, Salegio EA, San Sebastian W, et al. Adeno-associated virus serotype 9 transduction in the central nervous system of nonhuman primates. Hum Gene Ther 2012;23:382–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gray SJ, Nagabhushan Kalburgi S, McCown TJ, et al. Global CNS gene delivery and evasion of anti-AAV-neutralizing antibodies by intrathecal AAV administration in non-human primates. Gene Ther 2013;20:450–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Castle MJ, Cheng Y, Asokan A, et al. Physical positioning markedly enhances brain transduction after intrathecal AAV9 infusion. Sci Adv 2018;4:eaau9859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rosenberg JB, Kaplitt MG, De BP, et al. AAVrh.10-mediated APOE2 central nervous system gene therapy for APOE4-associated Alzheimer's disease. Hum Gene Ther Clin Dev 2018;29:24–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. ClinicalTrials.gov Gene therapy for APOE4 homozygote of Alzheimer's disease. https://clinicaltrials.gov/ct2/show/NCT03634007 (last accessed February26, 2020)
- 50. Noroozian Z, Xhima K, Huang Y, et al. MRI-guided focused ultrasound for targeted delivery of rAAV to the brain. In: Castle MJ, ed. Adeno-Associated Virus Vectors. Clifton, NJ: Humana Press, 2019:177–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Weber-Adrian D, Kofoed RH, Chan JWY, et al. Strategy to enhance transgene expression in proximity of amyloid plaques in a mouse model of Alzheimer's disease. Theranostics 2019;9:8127–8137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Deverman BE, Pravdo PL, Simpson BP, et al. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat Biotechnol 2016;34:204–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chan KY, Jang MJ, Yoo BB, et al. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat Neurosci 2017;20:1172–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.