Abstract
The goal of this study was to develop a quantitative detection system for severe acute respiratory syndrome-associated coronavirus (SARS-CoV), targeting the nucleocapsid protein (NP), to determine the presence and degree of infection in suspected individuals. Because the NP is the viral protein shed during infection and its template mRNA is the most abundant subgenomic RNA, it is a suitable candidate for developing antibodies for diagnostic applications. In this study, we have prepared full-length SARS-CoV NP expressed in Escherichia coli and purified. Full-length NP was used for the preparation of mouse monoclonal antibody and chicken polyclonal IgY antibodies for the development of heterosandwich ELISA for early diagnostics of SARS-suspected individuals. The sensitivity of the developed heterosandwich ELISA can detect the viral antigen at 18.5 pg/mL of recombinant NP. This study describes ultrasensitive ELISA using 19B6 monoclonal antibody as the capture antibody and IgY as the detecting antibody against the most abundant SARS-CoV NP antigens. One of the most important findings was the use of inexpensive polyclonal IgY antibody to increase the sensitivity of the detection system for SARS-CoV at the picogram level. Furthermore, the immunoassay of SARS-CoV NP antigen developed could be an effective and sensitive method of diagnosing SARS-suspected individuals during a future SARS-CoV outbreak.
Key words: SARS-CoV, nucleocapsid protein, 19B6 monoclonal antibody, immunoglobulin Y, heterosandwich ELISA
INTRODUCTION
Severe acute respiratory syndrome (SARS), a viral respiratory illness caused by a coronavirus, called SARS-associated coronavirus (SARS-CoV), has caused serious worldwide epidemics. Because it is highly contagious, potentially life-threatening, and spreads rapidly, the US Center for Disease Control and Prevention and the World Health Organization continue to closely monitor the SARS situation to strictly control its spread. Because it is an airborne disease, and is easily transmitted from person to person, several measures have been taken to prevent the recurrence of another SARS outbreak. Since the outbreak of SARS-CoV in 2002 and 2003, there is growing demand for diagnostic tools for early, accurate, and safe laboratory diagnosis of SARS-CoV. Thus, the development and evaluation of specific and early detection of SARS-CoV may contribute to its risk management in the future. The 3 major diagnostic methods currently available are (i) viral RNA detection using real-time reverse-transcription PCR (Yam et al., 2003; Jiang et al., 2004; Poon et al., 2004), (ii) antibody detection (Chan et al., 2004; Li et al., 2005), and (iii) antigen detection (Che et al., 2004; Lau et al., 2004; Li et al., 2005). The most predominant SARS-CoV-derived protein throughout the infection is the nucleocapsid protein (NP) due to the comparatively high levels of mRNA expressed upon infection (Hiscox et al., 1995). The NP is also reported to be high in humans infected with SARS-CoV. The NP of SARS-CoV exhibits low homology with other members of the coronavirus family (Marra et al., 2003). This unique feature makes it a suitable candidate for developing a monoclonal antibody (mAb) for ultrasensitive SARS diagnostics. It has also been demonstrated that there is a significant amount of circulating shed NP antigen in serum samples from infected patients ranging from 100 pg/mL to 3.2 ng/mL. Detection of the NP antigen by ELISA was peaked at d 6 to 10 with 71% of patients being positive for the antigen (Che et al., 2004). It has also been reported that the NP was detectable from SARS-CoV-infected patients in other body fluids, such as nasopharyngeal aspirate, urine, and fecal samples, collected between d 2 to 33 after the onset of symptoms (Peiris et al., 2003; Lau et al., 2004).
The detection of NP by mAb will be more useful than the detection of other viral markers for rapid and early diagnosis of SARS in patients. During the first 10 d after disease onset, the NP was detected in 90% of the patients compared with the detection of IgG and RNA (Li et al., 2005). The detection rate of the NP between d 11 to 15 of the disease was significantly higher than those of anti-SARS-CoV IgG and RNA. Hence, there is a need for the development of sensitive and highly specific diagnostic kits that can be used in the field. Traditionally, polyclonal antibodies (pAb) and mAb are used for various clinical diagnostic applications. Highly pathogenic strains of bacteria and viruses can be detected by exploiting the pAb technique for its higher sensitivity due to its ability to bind to multiple epitopes.
Though development of pAb has been carried out in rodent and other mammalian species, pAb raised in the chicken have some unique advantages. Avian pAb raised against different targets produces chicken IgY in abundant amounts compared with the amount in mammalian and rodent species. Chicken IgY can also be obtained by noninvasive methods by the collection of antibodies in the egg yolks of hens hyperimmunized with the specific antigen. Chicken IgY is structurally similar to mammalian IgG in its affinity, but its molecular weight (˜180 kDa) is comparatively higher than IgG (˜150 kDa). This is due to its additional heavy-chain constant domain and carbohydrate chains. It has been demonstrated in the literature that IgY has been used in several applications, which includes quantitation of tumor biomarkers (Xiao et al., 2008) as immunotherapeutic agents against Candida albicans (Ibrahim et al., 2008), and detection of E. coli O157 (Sunwoo et al., 2006) and Salmonella (Terzolo et al., 1998; Lee et al., 2002).
Our current objective was to develop an ultrasensitive heterosandwich ELISA diagnostic method using mouse mAb and chicken egg yolk IgY directed against SARS-CoV NP antigen.
MATERIALS AND METHODS
Materials
Acrylamide:bisacrylamide (40%), prestained low-range protein molecular weight markers and protein assay reagent were purchased from Bio-Rad Laboratories Ltd. (Ontario, Canada). Western-blotting reagents were purchased from Amersham Pharmacia Biotech (Baie d'Urfé, Quebec, Canada). Bovine serum albumin, rabbit anti-chicken IgY-horseradish peroxidase (HRP), 2-2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid), and other general reagents were purchased from Sigma (St. Louis, MO). Microtiter 96-well plates were purchased from Costar Inc. (Cambridge, MA) and 3,3′,5,5′-tetramethylbenzidine (TMB) substrate from KPL Inc. (Frederick, MD). The ELISA Vmax kinetic microplate reader was obtained from Molecular Devices Corp. (Sunnyvale, CA).
Protein Preparation
Full-length SARS-CoV NP was expressed in Escherichia coli and purified as described previously (Das and Suresh, 2006). Full-length NP was used for chicken immunization as well as an antigen for the development of the heterosandwich ELISA.
Generation of mAb
Five 4- to 6-wk-old Balb/C mice were injected intraperitoneally with 25 μg of NP antigen emulsified with an equal volume of Freund's complete adjuvant and subsequently boostered with Freund's incomplete adjuvant 2 wk apart. A final booster injection with 10 μg of NP in PBS was given 2 d before hybridoma fusion. Mice were killed and spleens were harvested for hybridoma fusion, according to our published methods (Shahhosseini et al., 2007). We have generated and characterized 5 mAb directed against SARS-CoV NP (unpublished data).
Chicken Immunization
Immunization of hens was carried out as described previously (Sunwoo et al., 1996). The SARS-CoV NP (100 μg of protein/mL) was suspended in PBS (pH 7.3) and emulsified with an equal volume of Freund's incomplete adjuvant. Two 23-wk-old Single-Comb White Leghorn chickens were intramuscularly injected with the emulsified antigen (0.25 mL) at 4 different sites in the breast muscles. A booster immunization was given after 2 wk of the initial immunization. Hen eggs were collected daily and stored at 4°C until further purification and characterization of the antibodies.
Purification of Anti-SARS-CoV NP IgY Polyclonal Antibody
The water-soluble fraction (WSF) containing specific IgY against SARS-CoV NP was prepared from an egg yolk using the modified water dilution method (Sunwoo et al., 2002). The egg yolk was separated from the egg white and mixed with 8 volumes of cold distilled water (acidified with 0.1 M HCl). Cold acidified distilled water (pH 2.0) was then added slowly to make the final dilution of 1:10, and the mixture was adjusted to a pH of 5.0 to 5.2 and incubated at 4°C for 12 h. The WSF was separated by centrifugation (3,125 × g at 4°C) for 20 min. The IgY-rich WSF was mixed with 60% saturation of ammonium sulfate to precipitate the IgY and was purified by Sephacryl S-300 gel chromatography. The purified IgY was titrated by indirect ELISA. Nonspecific IgY obtained from nonimmunized eggs was also prepared to serve as a control.
Titer of Specific IgY by ELISA
The specific IgY was assayed by an ELISA procedure as described previously (Sunwoo et al., 2002). Microtiter 96-well plates were coated with 150 μL (10 μg/mL of NP) of SARS-CoV NP in carbonate-bicarbonate buffer (0.05 M, pH 9.6) and incubated at 37°C for 90 min. The plates were then washed 4 times with PBS containing 0.05% Tween 20 (PBS-T). After washing, 200 μL of 1% BSA solution (wt/vol) in PBS-T was added to each well and the plates were again incubated at 37°C for 45 min. After washing, specific IgY or nonspecific IgY as a control at a dilution of 1:5,000 in PBS-T was added to each well (150 µL/well), and the plates were further incubated at 37°C for 1 h. After washing, 100 μL of rabbit anti-chicken IgY-HRP (diluted 1:5,000 in PBS-T) was added to each well and incubated at 37°C for 90 min. The plates were washed 4 times with PBS-T, followed by the addition of 100 μL of freshly prepared 2-2′-azino-bis substrate solution in 0.05 M phosphate citrate buffer (pH 5.0) containing 30% hydrogen peroxide. An optical density reading at 405 nm was taken after 30 min using an ELISA Vmax kinetic microplate reader. The ELISA value of specific IgY activity was determined by subtracting the value of the control IgY from that of specific IgY.
SDS-PAGE and Western Blot Analysis
Purified IgY was electrophoresed on SDS-PAGE using 10% polyacrylamide gel to check the purity according to a published method (Laemmli, 1970). Recombinant NP was electrophoresed and then electroblotted onto Hybond ECL nitrocellulose membranes (Towbin et al., 1979). The membrane was blocked with 5% skim milk in PBS-T for 1 h. The membrane was washed 4 times with PBS-T and incubated for 1 h with anti-SARS-CoV NP IgY (1 μg/mL). After washing 4 times with PBS-T, the nitrocellulose membrane was incubated with rabbit anti-chicken IgY-HRP for 1 h. All incubations were carried out at room temperature. Finally, the membrane was washed with PBS 4 times and electrochemiluminescence detection was performed to visualize the specific binding.
Heterosandwich ELISA
The 5 anti-NP mAb (P140.20B7, P140.19B6, P140.1C7, P140.19D3, and P140.14D6) were coated with 100 µL (10 µg/mL) on microtiter 96-well plates overnight at 4°C. After washing 5 times with PBS-T, the plate was incubated with 2% BSA for 2 h at room temperature to block nonspecific binding. After washing, 100 μL of (10 μg/mL of NP in 1% BSA) antigens was added and incubated for 1 h at room temperature. The plate was washed 4 times with PBS-T and incubated with 100 μL of IgY (5 μg/mL diluted in 1% BSA) for 30 min at 37°C. The wells were washed with PBS-T and incubated with 100 μL of rabbit anti-chicken IgY-HRP (1:5,000 diluted in 1% BSA) for 45 min at 37°C. After washing, TMB substrate and hydrogen peroxide (1:1) were added and optical density was measured at 650 nm after 5 min using an ELISA Vmax kinetic microplate reader. The wells containing capture mAb without NP antigen were used to determine a nonspecific adsorption.
Ultrasensitive ELISA
The best anti-NP mAb that were developed by hybridoma technology as capture antibody and the chicken IgY as the detecting antibody were used in the development of an ultrasensitive ELISA for SARS-CoV NP detection. To accomplish this, first we have optimized the NP antigen and detecting IgY antibody concentrations as follows. To optimize the NP antigen concentrations, ELISA plates were coated with 100 μL of 10 μg/mL of mAb (19B6 or 19C7) in PBS pH 7.3 overnight at 4°C. After washing 5 times with PBS-T, the plate was blocked with 2% BSA for 2 h at room temperature. After washing 4 times with PBS-T, 100 μL of a serially diluted concentrations of NP antigens diluted in 1% BSA was added and incubated for 1 h at room temperature. The plate was washed 4 times with PBS-T and incubated with 100 μL of IgY (5 μg/mL diluted in 1% BSA) for 1 h at 37°C. To optimize the detecting antibody concentrations, ELISA plates were first coated with the mAb as capture antibody and blocked as described above. After washing, 100 μL of 1 μg/mL concentrations of NP antigens diluted in 1% BSA was added and incubated for 1 h at room temperature. After washing, the plate was incubated with 100 μL of different concentrations of IgY diluted in 1% BSA for 1 h at 37°C. After incubation, the wells were washed and incubated with rabbit anti-chicken IgY-HRP (100 μL of 1:5,000 diluted in 1% BSA) for 45 min at 37°C. The color was developed with TMB substrate and hydrogen peroxide (1:1) for 5 min at room temperature. The well containing capture mAb without antigen was used as a negative control. The optical density was measured at 650 nm using an ELISA Vmax kinetic microplate reader. A Student t-test was used to analyze the significance differences (P < 0.05) between the sample and control.
RESULTS
Immunization of hens was carried out with SARS-CoV NP antigen, and eggs were collected daily. Specific activities of IgY from chickens immunized with SARS-CoV NP antigen were monitored by indirect ELISA during the period of immunization (Figure 1 ). The IgY titer estimated by indirect ELISA indicated a primary response characterized by slight increase in its activity. Following a booster injection, a rapid secondary response was shown with high SARS-CoV NP specific IgY activity at a peak of 8 wk. As a result, a high antigen-binding specificity of anti-SARS-CoV NP IgY could be obtained from the egg yolks collected from immunized eggs during the immunization period of 6 to 10 wk. The WSF of the egg yolk was purified and analyzed for purity and specificity to the NP antigen. The crude egg yolk IgY WSF showed only 38% purity (data not shown), indicating that the rest of the fraction was contaminated with alpha and gamma livetins, lipoproteins, and fatty acid molecules. The crude IgY WSF from the immunized hens were further purified by ammonium sulfate precipitation and subsequently by Sephacryl S-300 gel chromatography. The purified IgY was then analyzed by SDS-PAGE under reducing conditions, which revealed that the purity of IgY was greater than 95% (Figure 2A ). The specificity of the immune IgY was determined by Western blotting, which demonstrated that IgY strongly binds to the NP in the Western blot (Figure 2C). There was no cross reaction with other viral proteins, Dengue virus, or Ebola virus (data not shown).
Figure 1.
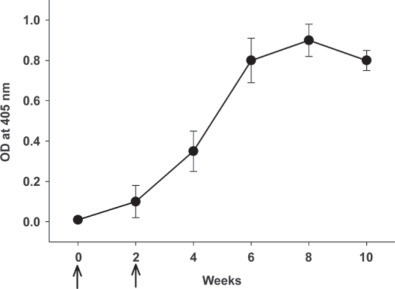
The change of specific activity of IgY in the egg yolk of chickens immunized with severe acute respiratory syndrome-associated coronavirus nucleocapsid antigen. The level of IgY activity in a 3,000-fold dilution of IgY was measured by ELISA using severe acute respiratory syndrome-associated coronavirus nucleocapsid as an antigen and expressed as the optical density (OD) value at 405 nm. Values are the means of quadruple samples. Vertical bars indicate the SD. Arrows indicate the week of immunization.
Figure 2.
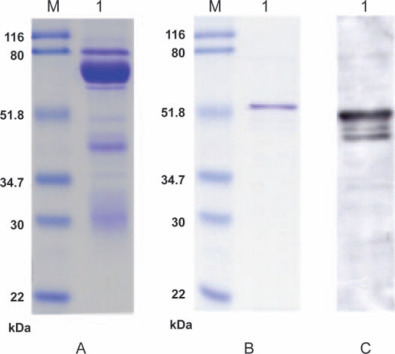
SDS-PAGE and Western blot assay. A) SDS-PAGE of purified IgY (lane M, standard protein molecular weight markers; lane 1, IgY antibody); B) SDS-PAGE of purified nucleocapsid antigen (lane M, standard protein molecular weight markers; lane 1, nucleocapsid antigen); C) Western blot assay of nucleocapsid antigen probed with anti-severe acute respiratory syndrome-associated coronavirus nucleocapsid IgY (lane 1, nucleocapsid antigen). Color version available in the online PDF.
The heterosandwich assay was developed using different mAb as the capture antibody and IgY as the detecting antibody. It was clearly demonstrated that all 5 mAb worked well for heterosandwich when used as capture antibodies with NP as the antigen and IgY as the detecting antibody with different relative affinity (Figure 3 ). The best heterosandwich pair was when 19B6 and 19C7 mAb were used as capture antibodies, respectively, with IgY as the detecting antibody. There were no significantly different affinities between the pairs. Hence, we have selected the best pair (19B6 as the capture antibody and IgY as the detecting antibody) for the development of ultrasensitive ELISA.
Figure 3.
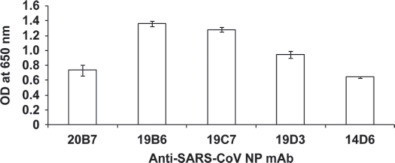
Heterosandwich ELISA with 5 anti-severe acute respiratory syndrome-associated coronavirus monoclonal antibodies (SARS-CoV mAB): anti-severe acute respiratory syndrome-associated coronavirus nucleocapsid antibodies were coated in the ELISA plate. Ten micrograms per milliliter of nucleocapsid antigen was added and detected by chicken IgY. Values are the means of quadruple samples. Vertical bars indicate the SD. OD = optical density.
A series of different anti-SARS-CoV NP IgY concentrations (n = 4) were used to select the optimum concentration of anti-SARS-CoV NP IgY, which could be used in the heterosandwich ELISA and also increase the sensitivity of the assay (limit of detection). The level of SARS-CoV NP IgY chosen for further studies was 2.5 µg/mL (Figure 4 ).
Figure 4.
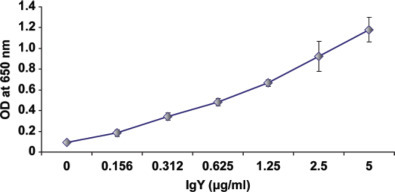
IgY optimization assay: P140.19B6 monoclonal antibody was coated in the ELISA plate. One microgram per milliliter of nucleocapsid antigen was added and detected by different concentrations of chicken IgY. Values are the means of quadruple samples. Vertical bars indicate the SD. Color version available in the online PDF. OD = optical density.
The optimized anti-SARS-CoV NP IgY concentrations (2.5 µg/mL) were used to develop heterosandwich ELISA with NP antigen (in serial dilutions from 2.5 µg/mL to 9.2 pg/mL; n = 3) with 19B6 as the capture antibody (10 µg/mL). When 2.5 µg/mL of anti-SARS-CoV NP IgY (as the detecting antibody) was used in the assay with 19B6 mAb as the capture antibody, 18.5 pg/mL of NP antigen could be detected, which was found to be statistically significant (P < 0.05) as compared with the other 4 mAb studied (Figure 5 ).
Figure 5.
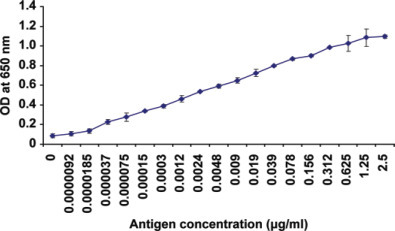
Ultrasensitive ELISA: P140.19B6 monoclonal antibody was coated in the ELISA plate. Each serially diluted nucleocapsid antigen (from 2.5 µg/mL to 9.2 pg/mL) was added and detected by chicken IgY. Values are the means of quadruple samples. Vertical bars indicate the SD. Color version available in the online PDF. OD = optical density.
DISCUSSION
Severe acute respiratory syndrome-CoV causes a severe lower respiratory tract infection, resulting in acute respiratory distress syndrome (Lo et al., 2006) with a fatality rate of 10 to 15% with > 60% being in elderly patients (Drosten et al., 2003; Holmes, 2003; Ksiazek et al., 2003). The NP is the most predominant virus derived protein throughout the infection, because the NP mRNA levels are amplified 3 to 10 times higher at 12 h postinfection (Hiscox et al., 1995) compared with other structural genes. This feature makes it a suitable candidate for developing a diagnostic kit in suspected individuals. In many viral diseases, virus shedding is greatest during the early symptomatic phase. The detection of viral RNA could be done by real-time, reverse-transcription PCR because it is very sensitive but is expensive and can cause false positives from cross-contamination. Whereas the whole process of extraction of mRNA from different specimens can be labor intensive and relies on special technical expertise (Tan et al., 2004), an ELISA assay is an accurate and sensitive detection system, leading to early therapeutic diagnosis.
In this study, we developed heterosanwich ELISA with the use of both mouse mAb and chicken IgY for the achievement of a highly sensitive detection system for SARS-CoV infection, targeting the NP antigen. The chicken was used to generate the antibody employed in this study, because it has been reported that the avian maternal antibodies are transferred from serum to egg yolk to confer passive immunity to their offspring in the form of an IgY antibody. This process of accumulating antibodies in the egg yolk by transfer of IgG from serum to egg yolk has been exploited, which resulted in increased use of chicken egg yolk for production of antibodies to various antigens, such as viral antigens (Piela et al., 1984) and bacteria (Sunwoo et al., 2002). In addition, the high amount of chicken IgY generated within a short period of time is an advantage to develop significant antigen-specific IgY compared with mammalian serum antibodies (Schade et al., 1994). It was also shown that IgY was relatively stable under different conditions, including heat, pressure, alkalinity, acidity (> pH 3.5), and in the presence of proteolytic enzymes, such as trypsin and chymotrypsin (Shimizu et al., 1992). Therefore, the ability to produce high amounts of antigen-specific IgY with reasonably high stability makes production of IgY against various viral antigens a viable option to be used in diagnostics (Erhard et al., 2000), antibiotic-alternative therapy (Carlander et al., 2000), and therapeutics (Schade et al., 2007).
In our previous study, the mAb and IgY base qualitative immunoswab assay developed was as sensitive as 10 pg/mL, which was 3 times more sensitive than the rapid immunochromatographic test developed by Kogaki et al. that had the detection for NP at 31 pg/mL in nasopharyngeal aspirate samples of SARS patients (Kogaki et al., 2005). In this study, we targeted the development of quantitative detection for NP antigen by heterosandwich ELISA for clinical laboratory purposes. Recent ELISA studies using pAb have reported the sensitivity of recombinant NP spiked in human sera to be 50 pg/mL (Che et al., 2004). The use of IgY in this study together with mAb against NP antigen, gave a sensitivity of 18 pg/mL, which was 3 times more sensitive than the literature value mentioned earlier. Another ELISA system developed from pAb in rabbit and guinea pig sera could detect nanogram levels of NP antigen in nasopharyngeal aspirate specimens as early as d 6 from symptom onset (Lau et al., 2004). In this study, we have significantly lowered the detection limit to the picogram level, which should allow detection of the NP antigen before the onset of symptoms by using a combination of IgY and mAb. The match of both of the antibodies was appropriate for the development of an effective immunological diagnostic tool, because pAb are capable of binding to many epitopes on the antigen that is beneficial for high sensitivity but has inherent specificity limitations. Hence, the availability of suitable highly specific mAb is essential.
For the extension of this study, biological fluids such as human mucus from nasopharyngeal aspirates of SARS-infected patients would need to be optimized for the extraction of the NP antigen methodology to give minimal antigen loss and false positives. The relative ease of noninvasively accessing nasopharyngeal aspirate from patients gives it an added advantage for quantitatively testing the suspected individuals on the presence as well as severity of infection within a short period of time during a future SARS outbreak in a clinical healthcare setting.
Conclusion
In this study we have developed a SARS-CoV diagnostic tool by heterosandwich ELISA for the detection of the NP antigen using mAb with high affinity and specificity for NP coupled with IgY (chicken egg yolk polyclonal antibody) for antigen detection with a sensitivity of 18.5 pg/mL of recombinant NP antigen. The effective and sensitive assay could be translated to other infectious diseases and essentially replace other detection system sources of the expensive polyclonal or monoclonal antibodies. The immunoassay method of detecting SARS-CoV NP antigen developed in the laboratory could be an effective and easy way of quantitatively detecting the degree of SARS infection in suspected individuals during a future SARS-CoV outbreak.
Acknowledgments
This work was supported by a research grant (5U01AI061233-02) from the National Institute of Allergy and Infectious Diseases, part of the National Institutes of Health (Bethesda, MD).
REFERENCES
- Carlander D., Kollberg H., Wejaker P.E., Larsson A. Peroral immunotherapy with yolk antibodies for the prevention and treatment of enteric infections. Immunol. Res. 2000;21:1–6. doi: 10.1385/IR:21:1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P.K., Ng K.C., Chan R.C., Lam R.K., Chow V.C., Hui M., Wu A., Lee N., Yap F.H., Cheng F.W., Sung J.J., Tam J.S. Immunofluorescence assay for serologic diagnosis of SARS. Emerg. Infect. Dis. 2004;10:530–532. doi: 10.3201/eid1003.030493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che X.Y., Qiu L.W., Pan Y.X., Wen K., Hao W., Zhang L.Y., Wang Y.D., Liao Z.Y., Hua X., Cheng V.C.C., Yuen K.Y. Sensitive and specific monoclonal antibody-based capture enzyme immunoassay for detection of nucleocapsid antigen in sera from patients with severe acute respiratory syndrome. J. Clin. Microbiol. 2004;42:2629–2635. doi: 10.1128/JCM.42.6.2629-2635.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das D., Suresh M.R. Copious production of SARS-CoV nucleocapsid protein employing codon optimized synthetic gene. J. Virol. Methods. 2006;137:343–346. doi: 10.1016/j.jviromet.2006.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A., Berger A., Burguiere A.M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.C., Muller S., Rickerts V., Sturmer M., Vieth S., Klenk H.D., Osterhaus A.D., Schmitz H., Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Erhard M.H., Schmidt P., Zinsmeister P., Hofmann A., Munster U., Kaspers B., Wiesmuller K.H.B., Bessler W.G., Stangassinger M. Adjuvant effects of various lipopeptides and interferon-γ on the humoral immune response of chickens. Poult. Sci. 2000;79:1264–1270. doi: 10.1093/ps/79.9.1264. [DOI] [PubMed] [Google Scholar]
- Hiscox J.A., Cavanagh D., Britton P. Quantification of individual subgenomic mRNA species during replication of the coronavirus transmissible gastroenteritis virus. Virus Res. 1995;36:119–130. doi: 10.1016/0168-1702(94)00108-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K.V. SARS-associated coronavirus. N. Engl. J. Med. 2003;348:1948–1951. doi: 10.1056/NEJMp030078. [DOI] [PubMed] [Google Scholar]
- el-Ibrahim S.M., Rahman A.K., Isoda R., Umeda K., Van Sa N., Kodama Y. In vitro and in vivo effectiveness of egg yolk antibody against Candida albicans (anti-CA IgY) Vaccine. 2008;26:2073–2080. doi: 10.1016/j.vaccine.2008.02.046. [DOI] [PubMed] [Google Scholar]
- Jiang S.S., Chen T.C., Yang J.Y., Hsiung C.A., Su I.J., Liu Y.L., Chen P.C., Juang J.L. Sensitive and quantitative detection of severe acute respiratory syndrome coronavirus infection by real-time nested polymerase chain reaction. Clin. Infect. Dis. 2004;38:293–296. doi: 10.1086/380841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogaki H., Uchida Y., Fujii N., Kurano Y., Miyake K., Kido Y., Kariwa H., Takashima I., Tamashiro H., Ling A.E., Okada M. Novel rapid immunochromatographic test based on an enzyme immunoassay for detecting nucleocapsid antigen in SARS-associated coronavirus. J. Clin. Lab. Anal. 2005;19:150–159. doi: 10.1002/jcla.20070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.E., Humphrey C.D., Shieh W.J., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J.Y., Cox N., Hughes J.M., LeDuc J.W., Bellini W.J., Anderson L.J. A novel coronavirus 383 associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lau S.K.P., Woo P.C.Y., Wong B.H.L., Tsoi H.W., Woo G.K.S., Poon R.W.S., Chan K.H., Wei W.I., Peiris J.S.M., Yuen K.Y. Detection of severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein in SARS patients by enzyme-linked immunosorbent assay. J. Clin. Microbiol. 2004;42:2884–2889. doi: 10.1128/JCM.42.7.2884-2889.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E.N., Sunwoo H.H., Menninen K., Sim J.S. In vitro studies of chicken egg yolk antibody (IgY) against Salmonella enteritidis and Salmonella typhimurium. Poult. Sci. 2002;81:632–641. doi: 10.1093/ps/81.5.632. [DOI] [PubMed] [Google Scholar]
- Li Y.H., Li J., Liu X.E., Wang L., Li T., Zhou Y.H., Zhuang H. Detection of the nucleocapsid protein of severe acute respiratory syndrome coronavirus in serum: Comparison with results of other viral markers. J. Virol. Methods. 2005;130:45–50. doi: 10.1016/j.jviromet.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo A.W., Tang N.L., To K.F. How the SARS coronavirus causes disease: Host or organism? J. Pathol. 2006;208:142–151. doi: 10.1002/path.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra M.A., Jones S.J.M., Astell C.R., Holt R.A., Brooks-Wilson A., Butterfield Y.S.N., Khattra J., Asano J.K., Barber S.A., Chan S.Y., Cloutier A., Coughlin S.M., Freeman D., Girn N., Griffith O.L., Leach S.R., Mayo M., McDonald H., Montgomery S.B., Pandoh P.K., Petrescu A.S., Robertson A.G., Schein J.E., Siddiqui A., Smailus D.E., Stott J.M., Yang G.S., Plummer F., Andonov A., Artsob H., Bastien N., Bernard K., Booth T.F., Bowness D., Czub M., Drebot M., Fernando L., Flick R., Garbutt M., Gray M., Grolla A., Jones S., Feldmann H., Meyers A., Kabani A., Li Y., Normand S., Stroher U., Tipples G.A., Tyler S., Vogrig R., Ward D., Watson B., Brunham R.C., Krajden M., Petric M., Skowronski D.M., Upton C., Roper R.L. The genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–1404. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- Peiris J.S., Chu C.M., Cheng V.C., Chan K.S., Hung I.F., Poon L.L., Law K.I., Tang B.S., Hon T.Y., Chan C.S., Chan K.H., Ng J.S., Zheng B.J., Ng W.L., Lai R.W., Guan Y., Yuen K.Y. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: A prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piela T.H., Gulka C.M., Yates V.J., Chang P.W. Use of egg yolk in serological test (ELISA and HI) to detect antibody to Newcastle disease, infectious bronchitis, and Mycoplasma gallisepticum. Avian Dis. 1984;28:877–883. [PubMed] [Google Scholar]
- Poon L.L., Chan K.H., Wong O.K., Cheung T.K., Ng I., Zheng B., Seto W.H., Yuen K.Y., Guan Y., Peiris J.S. Detection of SARS coronavirus in patients with severe acute respiratory syndrome by conventional and real-time quantitative reverse-transcription PCR assays. Clin. Chem. 2004;50:67–72. doi: 10.1373/clinchem.2003.023663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schade R., Burger W., Schoneberg T., Schniering A., Schwarzkopf C., Hlinak A., Kobilke H. Avian egg yolk antibodies. The egg laying capacity of hens following immunization with antigens of different kind and origin and the efficiency of egg yolk antibodies in comparison to mammalian antibodies. ALTEX. 1994;11:75–84. [PubMed] [Google Scholar]
- Schade R., Zhang X., Terzolo H. Use of IgY antibodies in human and veterinary medicine. In: Houopalahti R., Lopez-Fandino R., Anton M., Schade R., editors. Bioactive Egg Compounds. Springer-Verlag; Berlin Heidelberg, Germany.: 2007. pp. 213–222. [Google Scholar]
- Shahhosseini S., Das D., Qiu X., Feldmann H., Jones S.M., Suresh M.R. Production and characterization of monoclonal antibodies against different epitopes of Ebola virus antigens. J. Virol. Methods. 2007;143:29–37. doi: 10.1016/j.jviromet.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Shimizu M., Nagashima H., Sano K., Hashimoto K., Ozeki M., Tsuda K., Hatta H. Molecular stability of chicken and rabbit immunoglobulin G. Biosci. Biotechnol. Biochem. 1992;56:270–274. doi: 10.1271/bbb.56.270. [DOI] [PubMed] [Google Scholar]
- Sunwoo H.H., Lee E.N., Menninen K., Suresh M.R., Sim J.S. Growth inhibitory effect of chicken egg yolk antibody (IgY) on Escherichia coli O157:H7. J. Food Sci. 2002;67:1486–1494. [Google Scholar]
- Sunwoo H.H., Nakano T., Dixon W.T., Sim J.S. Immune responses in 453 chickens against lipopolysaccharide of Escherichia coli and Salmonella typhimurium. Poult. Sci. 1996;75:342–345. doi: 10.3382/ps.0750342. [DOI] [PubMed] [Google Scholar]
- Sunwoo H.H., Wang W.W., Sim J.S. Detection of Escherichia coli O157:H7 using chicken immunoglobulin Y. Immunol. Lett. 2006;106:191–193. doi: 10.1016/j.imlet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Tan Y.J., Goh P.Y., Fielding B.C., Shen S., Chou C.F., Fu J.L., Leong H.N., Leo Y.S., Ooi E.E., Ling A.E., Lim S.G., Hong W. Profiles of antibody responses against severe acute respiratory syndrome coronavirus recombinant proteins and their potential use as diagnostic markers. Clin. Diagn. Lab. Immunol. 2004;11:362–371. doi: 10.1128/CDLI.11.2.362-371.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzolo H.R., Sandoval V.E., Caffer M.I., Terragno R., Alcain A. Agglutination of hen egg-yolk immunoglobulins (IgY) against Salmonella enterica serovar Enteritidis. Rev. Argent. Microbiol. 1998;30:84–92. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., Gao X., Gannot G., Emmert-Buck M.R., Srivastava S., Wagner P.D., Amos M.D., Barker P.E. Quantitation of HER2 and telomerase biomarkers in solid tumors with IgY antibodies and nanocrystal detection. Int. J. Cancer. 2008;122:2178–2186. doi: 10.1002/ijc.23320. [DOI] [PubMed] [Google Scholar]
- Yam W.C., Chan K.H., Poon L.L., Guan Y., Yuen K.Y., Seto W.H., Peiris J.S. Evaluation of reverse-transcription PCR assays for rapid diagnosis of severe acute respiratory syndrome associated with a novel coronavirus. J. Clin. Microbiol. 2003;41:4521–4524. doi: 10.1128/JCM.41.10.4521-4524.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


