Abstract
Many inland waters exhibit complete or partial desiccation, or have vanished due to global change, exposing sediments to the atmosphere. Yet, data on carbon dioxide (CO2) emissions from these sediments are too scarce to upscale emissions for global estimates or to understand their fundamental drivers. Here, we present the results of a global survey covering 196 dry inland waters across diverse ecosystem types and climate zones. We show that their CO2 emissions share fundamental drivers and constitute a substantial fraction of the carbon cycled by inland waters. CO2 emissions were consistent across ecosystem types and climate zones, with local characteristics explaining much of the variability. Accounting for such emissions increases global estimates of carbon emissions from inland waters by 6% (~0.12 Pg C y−1). Our results indicate that emissions from dry inland waters represent a significant and likely increasing component of the inland waters carbon cycle.
Subject terms: Carbon cycle, Hydrology
Many inland waters seasonally or permanently dry up, thus exposing sediments to the atmosphere. Here the authors show that a substantial amount of CO2 is emitted from these dry sediments, increasing current inland water carbon flux estimates by 6%.
Introduction
Both natural and human-made inland waters are frequently impacted by drying1–3. Such ecosystems may partially or fully desiccate temporarily, and in some cases inland waters have even desiccated permanently4,5. Drying can result from natural hydrological factors (e.g. snowmelt driven lake-level fluctuations6, or the seasonal desiccation of intermittent streams or rivers7) or from anthropogenic factors8 (e.g. agricultural diversions, or water level fluctuation in reservoirs9). Indeed, climate change and increased water abstraction are together expected to exacerbate the widespread prevalence of dry inland waters10. Two-thirds of the planet’s first-order mid-latitude (below 60°) streams are estimated to flow only temporarily, as are one-third of larger, fifth-order rivers11. Furthermore, seasonal desiccation affects 18% (~800,000 km2) of the global surface area covered by inland waters, exposing previously submerged sediments to the atmosphere10. Such hydrologically dynamic environments are typically excluded from inland aquatic carbon (C) budgets and not explicitly accounted for in the terrestrial budgets, representing a potential blind spot in global C cycling estimates12. In accordance with previous work12, we define dry inland waters as the areas of lotic and lentic aquatic ecosystems on the Earth’s land masses where surface water is absent, and sediments are exposed to the atmosphere.
Gaseous C emissions from inland waters to the atmosphere play an important role in the global C cycle11,13–15. However, recent studies have shown that exposed sediments following the desiccation of inland waters can contribute CO2 emissions to the atmosphere at greater rates than those measured from the water surface during inundated periods16–18. Initial estimates predicted that these emissions may be relevant at a global scale12,19. Specifically, if the fluxes from desiccated areas were added to existing global estimates of CO2 emissions from inland waters11,20,21 they would result in 0.4–10% higher estimates of inland CO2 emissions to the atmosphere. However, these emission estimates from desiccated areas were based on a small number of localised studies, and convincing evidence for the global importance of this pathway is still lacking. Many inland water ecosystems are affected by water diversion, water abstraction and climate change8,22, leading to likely future increases in exposed sediment areas. Therefore, there is an urgent need to quantify the global CO2 emission from dry inland waters and to deepen our understanding of the environmental factors regulating them.
We hypothesised that CO2 emissions from dry inland waters are above reported mean aquatic rates, thus making emissions from dry inland waters globally relevant. We further hypothesised that sediment-atmosphere emissions vary as a function of parameters controlling CO2 production rates (such as organic matter supply, temperature and moisture) and parameters controlling the transport of gas to the atmosphere (e.g. sediment texture) as well as geographical properties of the sampling locations, which influence the biogeochemical conditions. To test these hypotheses, we conducted a global survey in which we quantified CO2 fluxes from 196 dry inland waters distributed across all continents except Antarctica, representing diverse inland water ecosystem types (rivers, lakes, reservoirs and ponds) and climate zones (tropical, arid, temperate, continental and polar). We compared the magnitude of these fluxes to those measured at adjacent uphill soils as well as global estimates for inundated water bodies compiled from the literature. To investigate potential drivers, we modelled the influence of environmental variables on the magnitude of CO2 emissions from the sediments to the atmosphere. Because dry inland waters are environments in between aquatic and terrestrial ecosystems, we aimed to disentangle whether CO2 emissions from dry inland waters were closer in value to those from aquatic or terrestrial ecosystems to improve the accuracy of current upscaling models of global CO2 emissions.
Results
Magnitude of CO2 emissions from dry inland waters
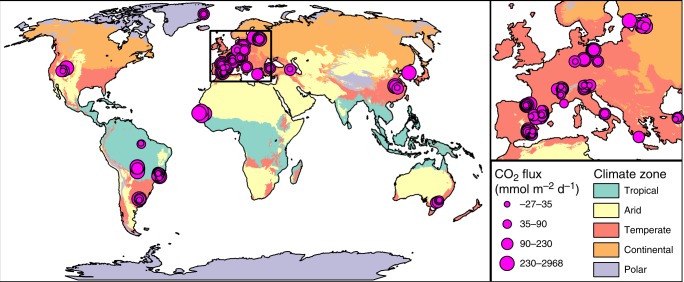
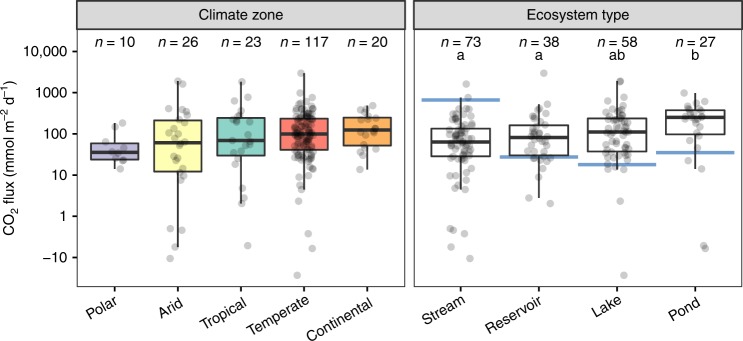
Sediment CO2 fluxes ranged from −27 to 2968 mmol m−2 d−1 (mean ± SD = 186 ± 326, median = 93, n = 196, Fig. 1; negative values indicate a net flux from the atmosphere to the sediments). This study provides the first data confirming that elevated CO2 emissions from desiccated sediments reported in prior localised studies17,19 (Supplementary Table 1) are globally prevalent and an intrinsic characteristic of dry inland waters. The sampled sites include a great diversity of environmental conditions (Fig. 1), although the collaborative nature of the study precluded an even geographical distribution of sampling efforts, and sites in the temperate zone dominate the dataset. Measured CO2 emissions from dry inland waters to the atmosphere were an order of magnitude higher than average water surface emissions (water-to-atmosphere) previously reported for lentic waters (27 mmol m−2 d−1), but lower than average emissions reported for lotic waters (663 mmol m−2 d−1) (Fig. 2; Supplementary Table 1).
Fig. 1. Global distribution of CO2 fluxes from dry inland waters.
Size of pink dots indicates magnitude of measured CO2 fluxes. Background colours indicate climate zones according to the Köppen–Geiger climate classification system52. Inset illustrates the spatial distribution within the most densely sampled area.
Fig. 2. CO2 fluxes separated by climate zones and ecosystem types.
Box = 25th and 75th percentiles, whiskers = 1.5* inter-quartile range. Black line = median. Blue lines represent average estimates of CO2 emissions for inland waters as reported in the literature11, 20, 21. Colours refer to climate zones as defined in Fig. 1. Note that the y-axis is presented on a log10 scale to show a wide range of flux values. Letters indicate significant differences between ecosystem types (Kruskal–Wallis test and Dunn’s post hoc test, P < 0.05).
Higher CO2 emissions to the atmosphere from exposed sediments relative to lentic inland water surface emissions are likely due to a closer coupling of CO2 production and gas flux in dry sediments (due to the lack of an intervening layer of water) as well as increased CO2 production rates due to increased oxygen availability, as oxygenation can stimulate enzymatic activity and overall microbial growth23. In aquatic environments, CO2 fluxes are typically controlled by diffusion and the accumulation of CO2 is buffered by the carbonate system24,25. Streams and rivers typically show higher gas fluxes than lentic ecosystems due to higher turbulence and, thus, higher gas exchange coefficients26.
CO2 emissions from dry inland waters (mean = 186 mmol m−2 d−1) were in the same range, but significantly lower, than those from adjacent uphill soils which had not been previously inundated (mean ± SD = 222 ± 277 mmol m−2 d−1, median = 144, n = 196) (Wilcoxon signed rank test, P < 0.05) (Supplementary Fig. 1). Previously inundated sediments and terrestrial (uphill) soils are distinct environments in terms of their physical structure, biogeochemical dynamics, and biological communities16,27,28. Therefore, one plausible explanation for the observed difference in CO2 emissions is the possible potential for higher root respiration in soils compared with desiccated sediments. Root respiration typically accounts for 50% of total soil respiration but may reach up to 90%29,30. Furthermore, organic matter content, which would fuel CO2 production, was greater in uphill soils (mean ± SD = 8 ± 8%) than in dry inland waters (mean ± SD = 6 ± 7%) (Kruskal–Wallis Test, P < 0.001).
We observed CO2 uptake by the exposed sediments at eight sites (4% of total) and by the uphill soils at five sites (3% of total). In soils, a net uptake of atmospheric CO2 has been related to the dissolution of CO2 in pore water and carbonate weathering31, but direct evidence from dry inland waters supporting these mechanisms is currently missing12.
Homogeneity among climate zones and ecosystem types
Our global study did not reveal significant differences in CO2 fluxes between climate zones (Fig. 2). Nonetheless, this result needs to be interpreted with caution due to the unbalanced sampling sizes and the underrepresentation of sites in the polar zone. CO2 emissions from polar (mean ± SD = 60 ± 58 mmol m−2 d−1, median = 36), continental (mean ± SD = 174 ± 140 mmol m−2 d−1, median = 125), temperate (mean ± SD = 178 ± 308 mmol m−2 d−1, median = 99), arid (mean ± SD = 233 ± 470 mmol m−2 d−1, median = 61) and tropical sites (mean ± SD = 236 ± 403 mmol m−2 d−1, median = 69) all fell within the same range (Fig. 2). CO2 emissions from temperate sites experiencing dry winters (16% of temperate sites) were significantly lower than emissions from temperate sites located in either dry-summer locations (13%) or those lacking dry seasons (71%) (Kruskal–Wallis Test, P < 0.05). This result indicates an effect of the interaction between temperature and moisture with hot and wet conditions facilitating high gas fluxes.
All studied lentic ecosystem types (i.e. reservoirs, lakes and ponds) showed higher CO2 emissions from dry sediments than globally estimated for their inundated stages (Fig. 2). CO2 emissions from dry sediments of ponds (mean ± SD = 267 ± 221 mmol m−2 d−1, median = 252) were significantly higher than those from streams (mean ± SD = 128 ± 218 mmol m−2 d−1, median = 64) and reservoirs (mean ± SD = 194 ± 478 mmol m−2 d−1, median = 82) (Kruskal–Wallis Test, P < 0.05) and marginally higher than those from lakes (mean ± SD = 215 ± 353 mmol m−2 d−1, median = 111) (Fig. 2). This result emphasises the global importance of small waterbodies17,18,21,32, which are extremely prevalent global biogeochemical hotspots21,33, and which furthermore frequently exist as only temporary ecosystems, increasing the proportional relevance of their dry fluxes17. Possible reasons for higher CO2 emissions from dry ponds compared with other ecosystem types may be high temperature and a large perimeter to area ratio which leads to organic matter accumulation in their sediments. Indeed, higher CO2 emissions from ponds match the higher content of organic material we found at desiccated pond sites (18 ± 20%) compared with streams (3 ± 4%, Kruskal–Wallis Test, P < 0.05), lakes (14 ± 17%), and reservoirs (10 ± 11%).
Variation in CO2 fluxes from dry inland waters was higher between sites than between climate zones or between the studied ecosystem types (Fig. 2). Hence, local conditions prevailed over geographical patterns, indicating that the drivers of CO2 emissions in dry inland waters might be universal, thus facilitating the evaluation of this process at the global scale.
Drivers of CO2 emissions from dry inland waters
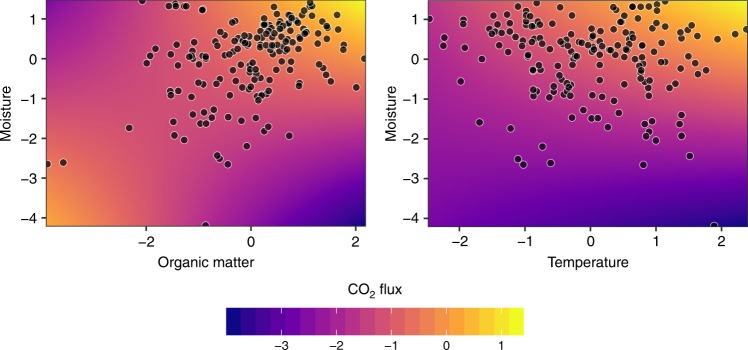
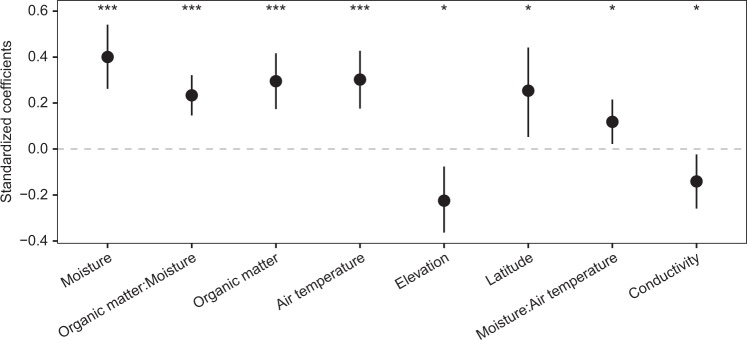
The relationships between CO2 fluxes and environmental variables were modelled using a linear mixed-effects model (LMM) (Fig. 3). LMM modelling of CO2 fluxes explained 39% of the total variance by the fixed effects and 52% by the entire model (Supplementary Table 2). Organic matter content, moisture, temperature and the interaction between organic matter content and moisture were the strongest predictors of CO2 fluxes from dry inland waters (analysis of variance, P < 0.001; Fig. 3, Supplementary Table 2), followed by the interaction of temperature with moisture and elevation, latitude and conductivity (analysis of variance, P < 0.05; Fig. 4). These results indicate that there is a universal control mechanism across ecosystems and climates. Under low-moisture conditions, neither the organic matter content of the sediments nor their temperature affected CO2 emissions, because microbial activity is inhibited by water limitation34 (Fig. 3, Supplementary Table 2). Hence, an increase in organic matter or temperature alone is not enough to produce high CO2 emissions. In contrast, high moisture facilitates the contact between microorganisms and available labile organic matter, but high moisture in combination with limited availability of organic matter to fuel CO2 production results in low CO2 emissions (Fig. 3). The same effect can be observed when low temperature limits microbial activity. Beyond the joint influence of moisture and organic matter on CO2 emissions induced by respiration, abiotic processes depending on pore water characteristics can affect the C cycle of drying sediments35. Abiotic CO2 emissions linked to carbonate precipitation and dissolution can be a potent source of total C emissions36. Sediment pore water can additionally lead to an uncoupling of CO2 production and emissions in dry sediments due to reduced physical gas transfer rates26.
Fig. 3. Response of CO2 fluxes to environmental variables.
Left, moisture against organic matter. Right, moisture against temperature. Original values of moisture (%), organic matter (%) and CO2 flux (mmol m−2 d−1) are shown in a log10-transformed and z-transformed scale. Original values of temperature (°C) are shown in a z-transformed scale. Relationships arise from the linear mixed-effects model analysis.
Fig. 4. Resulting coefficients from the linear mixed-effects model.
Error bars indicate 95% confidence interval. Variables are shown in decreasing order of significance (analysis of variance, ***P < 0.001, *P < 0.05). Moisture, elevation and conductivity have been log10-transformed and all variables have been z-transformed prior to analysis. Colons indicate interaction between the respective variables.
Elevation, latitude and conductivity likely represent local geographical conditions as well as small-scale patterns, which were not included in our sampling design. These could be, for instance, organic matter quality/lability, the presence of terrestrial vegetation (primary production), CO2 inputs via groundwater discharge, composition of the microbial community, or carbonate formation, which previous studies have identified as being potentially important16,17. Finally, antecedent conditions such as the time since desiccation or the past input of organic matter into the system may also influence CO2 emissions37,38.
Discussion
Our study encompasses 196 dry inland waters (and adjacent uphill terrestrial sites), spanning all major lotic and lentic aquatic ecosystem types and global climate zones. We show that drivers of CO2 emissions from desiccated sediments to the atmosphere are globally consistent, and are better predictors of CO2 emissions compared with regional variability associated with climate and ecosystem type. CO2 emissions from dry inland waters were generally lower than those reported for flowing streams and rivers11, but higher than from lentic waters11,20,21. This pattern is consistent for most ecosystems across all climate zones. These results strongly indicate that dry inland waters are significant and globally prevalent sources of CO2 to the atmosphere12.
Desiccated areas are usually excluded from global inventories of water bodies39 and so their contribution is missing in current global C budgets of inland waters11,14,20. A global upscaling of our measured CO2 emissions results in global C emissions from dry inland waters of 0.12 ± 0.13 Pg C y−1 (Supplementary Table 3), which is equivalent to 6 ± 6% of the currently estimated global C emissions from inland waters (2.1, range = 1.56 – 2.94 Pg C y−1)11. Because of the considerable variation of global CO2 emissions from dry inland waters, a final evaluation of their contribution to global CO2 emissions from inland waters remains difficult. However, partial exposure of sediments might become disproportionally more relevant in regions with a projected increase in water stress due to global change22,40. Hence, CO2 emissions from dry inland waters could increase significantly in more arid regions, and other climate zones subject to large seasonality such as monsoon climates, even if the increase in global emissions remains modest.
In any case, the net effect of including desiccated areas in current global inventories of C emissions from inland waters would depend on how desiccated areas have been considered in former studies, which is not always traceable. For instance, excluding CO2 emissions from dry inland waters, as done in recent studies11 would at first sight imply an underestimation of current inland waters CO2 emissions to the atmosphere. However, the mistaken assignment of an intermittent stream as a permanent flow area may instead result in an overestimation of fluxes, as flowing waters appear to generally emit more CO2 than the dry phases of intermittent rivers. On the contrary, dry areas of ponds, lakes and reservoirs, which global CO2 flux assessments assigned wrongly as wetted areas would likely result in an underestimation of net fluxes. Recent global emission inventories have either disregarded desiccated areas11,41 (i.e. likely underestimating emissions) or incorporated intermittent streams using rough approaches, probably underestimating their area19,38 (i.e. likely overestimating emissions). Certainly, no current global estimate considers desiccated areas in ponds, lakes and reservoirs, and thus these fluxes are likely to be underestimated. In sum, an assessment of the impact of desiccated areas on the global inland waters C inventory requires a much more accurate estimate of temporarily and permanently desiccated areas. Recent developments in remote sensing10 may help to incorporate desiccated areas from lakes, reservoirs and large rivers, but an accurate estimate of intermittent stream and pond area is still a challenging endeavour considering most desiccated areas in vast regions of the world are obscured by cover (e.g. dense trees, clouds). This should be a research priority if CO2 emissions from stream, rivers and ponds are to be accurately incorporated into global inland water C flux estimates.
We also note that our global estimates of dry CO2 emissions are likely to be conservative as the global surface area of desiccated inland waters is likely underestimated12. Furthermore, rewetting events are short periods of high biogeochemical activity that may contribute significantly to CO2 fluxes42 and are not purposely included in our estimates. Rapid pulses of CO2 production following rewetting have been observed in a variety of soil ecosystems42,43 as well as in dry river beds37,38.
The substantial variation between sites demands a better understanding of the underlying mechanisms driving CO2 emissions from dry inland waters to the atmosphere. Further research is necessary to determine the effect of temporal and seasonal variability on CO2 emissions from dry inland waters, to link these emissions with the consumptive loss of sediment organic matter and to assess the role of growing vegetation on net CO2 emissions. Furthermore, little is known about the emissions of other GHGs such as methane (CH4) or nitrous oxide (N2O) from dry sediments of inland waters. While desiccation and subsequent oxygenation of the sediment might minimise emissions of CH4 from dry sediments44, there are nevertheless reports of high CH4 emissions immediately after drying3,42. In addition, we expect desiccation to have a major impact on nitrogen cycling with consequences for N2O emissions; that is lower denitrification but higher nitrification, with both processes contributing to N2O production45. Further research is necessary to improve our understanding of the magnitude and drivers of the emissions of these GHGs from dry inland waters.
Upscaling CO2 emissions from dry inland waters for global estimates is particularly relevant because dry areas are predicted to increase in the future due to the observed and predicted decline in inland water levels following projected trends in global climate22,40 and human activities10,46. An improved understanding of the global patterns and drivers of desiccated sediment CO2 emissions to the atmosphere is thus crucial for an accurate understanding of contemporary landscape C cycling, as well as predictions of future atmospheric CO2 concentrations due to anthropogenic activities.
Methods
Sampling design
To obtain a global data set of CO2 fluxes and sediment and soil characteristics, measurements were performed by 24 teams in 17 countries. The methodology was defined in a standardised sampling protocol. The objective of this study was to record a dataset with the best possible geographical coverage. Therefore, and to enable all partners to conduct the sampling campaigns, we chose parameters and methods that were relatively easy to measure and to apply. All sites were chosen by the local teams, who ensured that sites were independent and not hydrologically connected in a direct upstream–downstream relationship. Sampling was performed at two locations on each site, the dry sediment of the water body and the adjacent uphill soil. The measurements of CO2 flux and additional soil and sediment parameters were performed at three plots, typically separated by a few metres, within each site. In cases where the whole ecosystem had dried up (e.g. small ponds, ephemeral streams), measurements were performed at representative parts of the bare sediment. In case of partial drying, measurements were performed at the emerged sediments at the shore. All raw data were collected and centrally analysed. The sampling sites were classified into four inland water ecosystem types, based on the information provided by the local sampling teams. We defined a stream as a natural watercourse that flows permanently or intermittently47, a lake as a naturally occurring low point in the landscape that contains standing water at least during certain periods48, a reservoir as a human-made lake48 and a pond as a standing surface water body type that is considerably smaller than a lake or reservoir49.
CO2 flux
Closed chamber measurements were performed to measure the CO2 flux directly. Opaque chambers connected to an infra-red gas analyser were inserted about 1 cm into the sediment. The CO2 concentration within the chamber was monitored for <5 min and the flux was determined by a linear regression based on the change in CO2 partial pressure (pCO2) over time. The CO2 flux (mmol m−2 d−1) was calculated according to Eq. (1), where dpCO2/dt is the slope of the change in pCO2 with time [µatm d−1], V is the volume of the chamber [m3], S is the surface area covered by the chamber [m2], T is the air temperature [K] and R is the ideal gas constant = 8.314 l atm K−1 mol−1.
| 1 |
When intrusion of the chamber to the ground was prevented (e.g. by a stony surface), the chamber was sealed to the ground using clay50. Chamber placement was restricted to plots with bare ground and sampling of vegetated surface was avoided. Positive values represent emissions from the sediment to the atmosphere while negative values indicate an inflow from the atmosphere to the ground.
Environmental variables
A set of 14 environmental variables was estimated for each site. Of these, ten variables were measured in situ or determined locally. We measured air and sediment temperature, determined sediment texture following the FAO manipulative test51 and collected sediment samples at every measurement plot. For measuring sediment temperature, the sensing head of a thermometer was inserted 2–3 cm into the sediment. In the laboratory, one part of fresh sediment sample was mixed with 2.5 parts distilled water and pH and conductivity were measured in the suspension using conventional electrodes. Furthermore, we determined water content and organic matter gravimetrically by drying 5 g of fresh sediment at 105 °C until constant weight, followed by combustion at 500 °C.
Five major climate zones were assigned to sites based on their location using the ‘World Maps Of Köppen-Geiger Climate Classification’ dataset52: tropical (Köppen–Geiger group A), arid (Köppen–Geiger group B), temperate (Köppen–Geiger group C), continental (Köppen–Geiger group D) and polar (Köppen–Geiger group E). For an in-depth analysis of temperate sites, the 2nd-order sub-groups dry-summer (Köppen–Geiger group Cs), dry-winter (Köppen–Geiger group Cw) and without-dry-seasons (Köppen–Geiger group Cf) were additionally distinguished. Annual mean temperature and annual precipitation for each site were taken from the WorldClim database53.
Data analysis
We tested the influence of environmental variables (Supplementary Table 4) on CO2 emissions from dry inland waters by fitting LMM to the response variable CO2 flux. This was done using the function lmer of the lme4 package54 of R55. We selected air temperature, organic matter content, texture, moisture, conductivity, latitude, elevation, type of ecosystem (i.e. stream, lake, reservoir, pond) pH, climate zone, annual mean temperature and annual precipitation as well as 2nd order interactions between moisture, temperature and organic matter as fixed effects. Air temperature was included instead of sediment temperature because of the high correlation between these parameters (r = 1). We included the team performing the analysis as a random effect to account for unmeasured team-level variation (random intercepts). Afterwards the model was simplified by removing non-significant predictors from the model (Supplementary Table 4).
For all steps of the analysis, one value per parameter was obtained per location and site by averaging the three measured plots. We log-transformed CO2 flux (x + 28), conductivity, organic matter content, moisture (x + 0.1) and elevation to meet the condition of normality and homogeneity of variance. All statistical analyses were conducted using R version 3.4.455. Statistical tests were considered significant at P < 0.05.
Supplementary information
Acknowledgements
This study was made possible thanks to a large collective effort of a global research network called dryflux (www.ufz.de/dryflux). We would like to thank numerous helpers for their assistance during field work. This research was inspired by GLEON (Global Lake Ecological Observatory Network). This work was supported by the German Research Foundation (DFG, KO1911/6-1 and GR1540/23-1) to P.S.K. and H.P.G., the Spanish Ministry of Science, Innovation and Universities (C-HYDROCHANGE, CGL2017-86788-C3-3-P and CGL2017-86788-C3-2-P) to B.O. and R.M., the Spanish Government (CGL2016-77487-R), the Basque Government (IT951-16), the BBVA Foundation (06417) to D.vS. and A.E., the European Research Council (FP7/2007-2013, ERC grant agreement 336642) to A.L. and R.F.M., CNPq (310033/2017-9) to A.M.A., the Carlsberg Foundation (CF16-0325) to T.R. and A.P., the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO, Veni Grant 86312012) to S.K., the Estonian Ministry of Education and Research (IUT 21-02) and the Estonian Research Council grant (PUT PSG32, PUT1598) to A.L. and E-I.R., the National Research Foundation of Korea (2017R1D1A1B06035179) to J-H.P., German Federal Ministry of Education and Research (BMBF) CLIENT programme (grant: 2WCL1337A) and German Academic Exchange Service (DAAD, grant 57218695) to M.A.F., the Seneca Foundation (20645/JLI/18) to M.M.S.M. and M.I.A. N.C. was supported by Beatriu de Pinós grant (2016-00215), A.P. by the Ramón Areces Foundation postgraduate studies programme, R.dC. by the University of Murcia (FPU R-269/2014), E.S.O.J. by the Erasmus+ Programme NON-EU 2017/2018, J.R.P. by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES Finance Code 001). C.M-L. by the French Agency for Biodiversity (ONEMA-AFB, Action 13, ‘Colmatage, échange snappe-rivière et processus biogéochimiques’), R.M. by the project C-HydroChange, funded by the Spanish Agencia Estatal de Investigación (AEI) and Fondo Europeo de Desarrollo Regional (FEDER) under the contract FEDER-MCIU-AEI/ CGL2017-86788-C3, P.S.K. by a grant for a short‐term scientific mission within the COST Action CA15113 (SMIRES, Science and Management of Intermittent Rivers and Ephemeral Streams, www.smires.eu) and GLEON (student travel grant). In memory of our esteemed colleague and friend Julia Howitt, who passed away after this paper was accepted. She was full of enthusiasm for this work and will be deeply missed by her colleagues around the world.
Source data
Author contributions
R.M., N.C., D.vS., H-P.G., B.O., and M.K. initiated the project and designed the sampling campaign. P.S.K., N.C., D.vS., H-P.G., M.K., B.O., M.A.F., N.K., N.B., J.A.H., C.M-L., A.P., G.F., R.A., T.R., M.I.A., G.O., J.R.P., A.L., R.dC., A.M.A., S.C-F., S.B., J.C., R.F.M., F.R., E-I.R., T.D., F.R., A.L., U.O., J-H.P., H.W., S.K., R.G., C.F., A.E., M.M.S-M., C.M.F., M.M., E.S.O.J., C.C.M., L.G-G., C.L., Q.Z., R.M. measured CO2 fluxes, sampled field data and processed this material. P.S.K. carried out the data compilation and database management. P.S.K., N.C and R.M. performed the data analyses. P.S.K. led the writing of the manuscript with notable contributions by N.C., D.vS., H-P.G., B.O., M.K. and R.M. All the other authors commented on and contributed to revising draft versions. Their order was computed randomly.
Data availability
The source data underlaying Figs. 1–4, Supplementary Fig. 1 and Supplementary Tables 2–4 are provided as a Source data file.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Kerri Finlay and the other, anonymous, reviewer(s) for their contributions to the peer review of this work. Peer review reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Deceased: J. A. Howitt.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-020-15929-y.
References
- 1.Bolpagni R, Folegot S, Laini A, Bartoli M. Role of ephemeral vegetation of emerging river bottoms in modulating CO2 exchanges across a temperate large lowland river stretch. Aquat. Sci. 2017;79:149–158. doi: 10.1007/s00027-016-0486-z. [DOI] [Google Scholar]
- 2.Gilbert, P. J., Cooke, D. A., Deary, M., Taylor, S. & Jeffries, M. J. Quantifying rapid spatial and temporal variations of CO2 fluxes from small, lowland freshwater ponds. Hydrobiologia793, 83–93 (2017).
- 3.Jin H, et al. Enhanced greenhouse gas emission from exposed sediments along a hydroelectric reservoir during an extreme drought event. Environ. Res. Lett. 2016;11:124003. doi: 10.1088/1748-9326/11/12/124003. [DOI] [Google Scholar]
- 4.Micklin P. The future Aral Sea: hope and despair. Environ. Earth Sci. 2016;75:844. doi: 10.1007/s12665-016-5614-5. [DOI] [Google Scholar]
- 5.Khazaei B, et al. Climatic or regionally induced by humans? Tracing hydro-climatic and land-use changes to better understand the Lake Urmia tragedy. J. Hydrol. 2019;569:203–217. doi: 10.1016/j.jhydrol.2018.12.004. [DOI] [Google Scholar]
- 6.Flaim G, Nishri A, Camin F, Corradini S, Obertegger U. Shift from nival to pluvial recharge of an aquifer-fed lake increases water temperature. Inland Waters. 2019;9:261–274. doi: 10.1080/20442041.2019.1582958. [DOI] [Google Scholar]
- 7.Larned ST, Datry T, Arscott DB, Tockner K. Emerging concepts in temporary-river ecology. Freshw. Biol. 2010;55:717–738. doi: 10.1111/j.1365-2427.2009.02322.x. [DOI] [Google Scholar]
- 8.Wurtsbaugh WA, et al. Decline of the world’s saline lakes. Nat. Geosci. 2017;10:816–821. doi: 10.1038/ngeo3052. [DOI] [Google Scholar]
- 9.Beaulieu, J. J. et al. Effects of an experimental water-level drawdown on methane emissions from a eutrophic reservoir. Ecosystems21, 657–674 (2018). [DOI] [PMC free article] [PubMed]
- 10.Pekel J-F, Cottam A, Gorelick N, Belward AS. High-resolution mapping of global surface water and its long-term changes. Nature. 2016;540:418–422. doi: 10.1038/nature20584. [DOI] [PubMed] [Google Scholar]
- 11.Raymond PA, et al. Global carbon dioxide emissions from inland waters. Nature. 2013;503:355–359. doi: 10.1038/nature12760. [DOI] [PubMed] [Google Scholar]
- 12.Marcé R, et al. Emissions from dry inland waters are a blind spot in the global carbon cycle. Earth-Sci. Rev. 2019;188:240–248. doi: 10.1016/j.earscirev.2018.11.012. [DOI] [Google Scholar]
- 13.Cole JJ, et al. Plumbing the global carbon cycle: integrating inland waters into the terrestrial carbon budget. Ecosystems. 2007;10:172–185. doi: 10.1007/s10021-006-9013-8. [DOI] [Google Scholar]
- 14.DelSontro T, Beaulieu Jake J, Downing John A. Greenhouse gas emissions from lakes and impoundments: upscaling in the face of global change. Limnol. Oceanogr. Lett. 2018;3:64–75. doi: 10.1002/lol2.10073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tranvik LJ, et al. Lakes and reservoirs as regulators of carbon cycling and climate. Limnol. Oceanogr. 2009;54:2298–2314. doi: 10.4319/lo.2009.54.6_part_2.2298. [DOI] [Google Scholar]
- 16.Gómez-Gener L, et al. When water vanishes: magnitude and regulation of carbon dioxide emissions from dry temporary streams. Ecosystems. 2016;19:710–723. doi: 10.1007/s10021-016-9963-4. [DOI] [Google Scholar]
- 17.Obrador B, et al. Dry habitats sustain high CO2 emissions from temporary ponds across seasons. Sci. Rep. 2018;8:3015. doi: 10.1038/s41598-018-20969-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Catalan N, et al. Carbon dioxide efflux during the flooding phase of temporary ponds. Limnetica. 2014;33:349–359. [Google Scholar]
- 19.von Schiller D, et al. Carbon dioxide emissions from dry watercourses. Inland Waters. 2014;4:377–382. doi: 10.5268/IW-4.4.746. [DOI] [Google Scholar]
- 20.Deemer BR, et al. Greenhouse gas emissions from reservoir water surfaces: a new global synthesis. BioScience. 2016;66:949–964. doi: 10.1093/biosci/biw117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holgerson MA, Raymond PA. Large contribution to inland water CO2 and CH4 emissions from very small ponds. Nat. Geosci. 2016;9:222–226. doi: 10.1038/ngeo2654. [DOI] [Google Scholar]
- 22.Dai A. Increasing drought under global warming in observations and models. Nat. Clim. Change. 2013;3:52–58. doi: 10.1038/nclimate1633. [DOI] [Google Scholar]
- 23.Fromin N, et al. Impact of seasonal sediment desiccation and rewetting on microbial processes involved in greenhouse gas emissions. Ecohydrology. 2010;3:339–348. doi: 10.1002/eco.115. [DOI] [Google Scholar]
- 24.Cole JJ, Caraco NF. Atmospheric exchange of carbon dioxide in a low-wind oligotrophic lake measured by the addition of SF6. Limnol. Oceanogr. 1998;43:647–656. doi: 10.4319/lo.1998.43.4.0647. [DOI] [Google Scholar]
- 25.Wanninkhof R. Relationship between wind speed and gas exchange over the ocean revisited. Limnol. Oceanogr. Methods. 2014;12:351–362. doi: 10.4319/lom.2014.12.351. [DOI] [Google Scholar]
- 26.Gómez-Gener L, et al. Hot spots for carbon emissions from Mediterranean fluvial networks during summer drought. Biogeochemistry. 2015;125:409–426. doi: 10.1007/s10533-015-0139-7. [DOI] [Google Scholar]
- 27.Steward AL, von Schiller D, Tockner K, Marshall JC, Bunn SE. When the river runs dry: human and ecological values of dry riverbeds. Front. Ecol. Environ. 2012;10:202–209. doi: 10.1890/110136. [DOI] [Google Scholar]
- 28.Arce MI, et al. A conceptual framework for understanding the biogeochemistry of dry riverbeds through the lens of soil science. Earth-Sci. Rev. 2019;188:441–453. doi: 10.1016/j.earscirev.2018.12.001. [DOI] [Google Scholar]
- 29.Cable JM, Ogle K, Williams DG, Weltzin JF, Huxman TE. Soil texture drives responses of soil respiration to precipitation pulses in the Sonoran desert: implications for climate change. Ecosystems. 2008;11:961–979. doi: 10.1007/s10021-008-9172-x. [DOI] [Google Scholar]
- 30.Larionova AA, Sapronov DV, Lopez de Gerenyu VO, Kuznetsova LG, Kudeyarov VN. Contribution of plant root respiration to the CO2 emission from soil. Eurasia. Soil Sci. 2006;39:1127–1135. doi: 10.1134/S1064229306100103. [DOI] [Google Scholar]
- 31.Ma J, Wang Z-Y, Stevenson BA, Zheng X-J, Li Y. An inorganic CO2 diffusion and dissolution process explains negative CO2 fluxes in saline/alkaline soils. Sci. Rep. 2013;3:2025. doi: 10.1038/srep02025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinsen KT, Kragh T, Sand-Jensen K. Carbon dioxide fluxes of air-exposed sediments and desiccating ponds. Biogeochemistry. 2019;144:165–180. doi: 10.1007/s10533-019-00579-0. [DOI] [Google Scholar]
- 33.Downing JA, et al. The global abundance and size distribution of lakes, ponds, and impoundments. Limnol. Oceanogr. 2006;51:2388–2397. doi: 10.4319/lo.2006.51.5.2388. [DOI] [Google Scholar]
- 34.Manzoni S, Schimel JP, Porporato A. Responses of soil microbial communities to water stress: results from a meta-analysis. Ecology. 2012;93:930–938. doi: 10.1890/11-0026.1. [DOI] [PubMed] [Google Scholar]
- 35.Marcé R, et al. Carbonate weathering as a driver of CO2 supersaturation in lakes. Nat. Geosci. 2015;8:107–111. doi: 10.1038/ngeo2341. [DOI] [Google Scholar]
- 36.Rey A. Mind the gap: non-biological processes contributing to soil CO2 efflux. Glob. Change Biol. 2015;21:1752–1761. doi: 10.1111/gcb.12821. [DOI] [PubMed] [Google Scholar]
- 37.von Schiller D, et al. Sediment respiration pulses in intermittent rivers and ephemeral streams. Glob. Biogeochem. Cycles. 2019;33:1251–1263. doi: 10.1029/2019GB006276. [DOI] [Google Scholar]
- 38.Datry T, et al. A global analysis of terrestrial plant litter dynamics in non-perennial waterways. Nat. Geosci. 2018;11:497–503. doi: 10.1038/s41561-018-0134-4. [DOI] [Google Scholar]
- 39.Verpoorter C, Kutser T, Seekell DA, Tranvik LJ. A global inventory of lakes based on high-resolution satellite imagery. Geophys. Res. Lett. 2014;41:6396–6402. doi: 10.1002/2014GL060641. [DOI] [Google Scholar]
- 40.Wang J, et al. Recent global decline in endorheic basin water storages. Nat. Geosci. 2018;11:926–932. doi: 10.1038/s41561-018-0265-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lauerwald R, Laruelle GG, Hartmann J, Ciais P, Regnier PAG. Spatial patterns in CO2 evasion from the global river network. Glob. Biogeochem. Cycles. 2015;29:534–554. doi: 10.1002/2014GB004941. [DOI] [Google Scholar]
- 42.Kosten S, et al. Extreme drought boosts CO2 and CH4 emissions from reservoir drawdown areas. Inland Waters. 2018;8:329–340. doi: 10.1080/20442041.2018.1483126. [DOI] [Google Scholar]
- 43.Austin AT, et al. Water pulses and biogeochemical cycles in arid and semiarid ecosystems. Oecologia. 2004;141:221–235. doi: 10.1007/s00442-004-1519-1. [DOI] [PubMed] [Google Scholar]
- 44.Wang, H., Lu, J., Wang, W., Yang, L. & Yin, C. Methane fluxes from the littoral zone of hypereutrophic Taihu Lake, China. J. Geophys. Res. Atmos. 111, D17109 (2006).
- 45.Arce, M. I. et al. Drying and rainfall shape the structure and functioning of nitrifying microbial communities in riverbed sediments. Front. Microbiol. 9, 2794 (2018). [DOI] [PMC free article] [PubMed]
- 46.Jaeger KL, Olden JD, Pelland NA. Climate change poised to threaten hydrologic connectivity and endemic fishes in dryland streams. Proc. Natl Acad. Sci. 2014;111:13894–13899. doi: 10.1073/pnas.1320890111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allan, J. D. & Castillo, M. M. Stream Ecology: Structure and Function of Running Waters (Springer, Netherlands, 2007).
- 48.Hayes NM, Deemer BR, Corman JR, Razavi NR, Strock KE. Key differences between lakes and reservoirs modify climate signals: a case for a new conceptual model. Limnol. Oceanogr. Lett. 2017;2:47–62. doi: 10.1002/lol2.10036. [DOI] [Google Scholar]
- 49.Downing, J. A. & Duarte, C. M. Abundance and size distribution of lakes, ponds and impoundments. in encyclopedia of inland waters 469–478 (Elsevier, 2009).
- 50.Lesmeister L, Koschorreck M. A closed-chamber method to measure greenhouse gas fluxes from dry aquatic sediments. Atmos. Meas. Tech. 2017;10:2377–2382. doi: 10.5194/amt-10-2377-2017. [DOI] [Google Scholar]
- 51.Food and Agriculture Organization (FAO). Soil texture.
- 52.Kottek M, Grieser J, Beck C, Rudolf B, Rubel F. World Map of the Köppen–Geiger climate classification updated. Meteorol. Z. 2006;15:259–263. doi: 10.1127/0941-2948/2006/0130. [DOI] [Google Scholar]
- 53.Fick SE, Hijmans RJ. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017;37:4302–4315. doi: 10.1002/joc.5086. [DOI] [Google Scholar]
- 54.Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
- 55.R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2018).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The source data underlaying Figs. 1–4, Supplementary Fig. 1 and Supplementary Tables 2–4 are provided as a Source data file.