Abstract
Background
Panax ginseng is a marvelous herbal remedy for all ailments of body. That may be why it is called Panax, which means “cure for all”. Melanin is a pigment that gives color to our skin; however, increased melanin production can lead to tumor formation. Human exposure to ultraviolet B radiation has increased extensively owing to the increased sunlight due to global warming. Consequently, a phenomenon called photoaging has been observed for all skin colors and types. As a result of this phenomenon, a set of enzymes called matrix metalloproteinases, which serve as degradation enzymes for extracellular matrix proteins, mainly collagen, is increased, causing depletion of collagen and resulting in early wrinkle formation.
Methods
Therefore, in our study, we used the murine melanoma cell line B16/F10 to study the inhibition of melanogenesis by Korean Red Ginseng (KRG) extract in vitro and HRM-2 hairless mice exposed to artificial ultraviolet B to examine the efficacy of KRG in vivo. We prepared a 3% red ginseng extract cream and evaluated its effects on human skin.
Results
Our results demonstrated that KRG induced potent suppression of tyrosinase activity and melanin production in B16/F10 cells; moreover, it reduced the transcription and translation of components involved in the melanin production pathway. In the in vivo experiments, KRG potently suppressed the expression of matrix metalloproteinases, reduced wrinkle formation, and inhibited collagen degradation. On human skin, ginseng cream increased skin resilience and skin moisture and enhanced skin tone.
Conclusion
Therefore, we conclude that KRG is an excellent skin whitening and antiaging product.
Keywords: Antiaging, Human trials, Korean Red Ginseng extract, Melanogenesis, Wrinkles
1. Introduction
Melanin is a compound responsible for skin pigmentation, and the variety of skin colors in the human race is attributable to the amount of melanin-producing cells present in the skin [1]. The process responsible for the formation of melanin is called melanogenesis. In this process, α-melanocyte–stimulating hormone (α-MSH) binds to its receptor (i.e., melanocortin 1 receptor) causing elevated levels of cAMP,which in turn activates microphthalmia-associated factor (MITF) through various pathways, such as cyclic Adenosine Monophosphate (cAMP) response element binding protein, extracellular-regulated kinase, and protein kinase B (AKT), causing its degradation. Owing to this degradation, the rate-limiting step in the process of melanogenesis (i.e., the action of the enzyme tyrosinase, TYR) is affected and becomes activated. TYR is responsible for the conversion of tyrosine to L-3,4-dihydroxyphenylalanine (L-DOPA), which forms melanin. Tyrosinase-related protein 1 (TRP-1) and tyrosinase-related protein 2 (TRP-2) are further downstream factors of TYR and MITF that are activated and are involved in the formation of melanin. Consequently, throughout the whole process, it is TYR that is the most important component of the regulation of melanin production in skin cells [2], [3], [4].
Panax ginseng is a wonder herb that has been consumed widely in eastern Asia for over 1,000 years as it has many beneficial health effects. It is available in a variety of forms, including drinks, capsules, and tablets [5], [6]. Past studies have revealed the noteworthy effects of ginseng when used to reduce the incidence of various types of tumors, many mental anomalies, diabetes, hypertension, hyperlipidemia, and inflammation [7], [8], [9], [10], [11], [12], [13]. In particular, in the Korean peninsula, ginseng supplements form part of a normal diet. Commercially, ginseng is available in the form of whole ginseng root extract, single ginsenoside extracts, or capsules. Ginsenosides are the constituent active compounds present in the whole ginseng root that are responsible for the efficacious health-enhancing properties of ginseng [14].
There have been many studies on the effects of single ginsenoside on melanin production and melasma [15]. However, at present, no study has reported the antimelanogenic effects of Korean Red Ginseng (KRG) extract on melanin production and examined its skin whitening and antiaging effects, particularly in humans. Therefore, we investigated the TYR inhibition and melanin production inhibition by KRG in vitro in the B16/F10 melanoma cell line via a mechanistic study of the pathways involved in this process. Our results indicated that KRG markedly inhibited TYR activity and decreased melanin content via the MITF degradation pathway. Moreover, our in vivo study using an ultraviolet B (UVB)–irradiated hairless mouse (HRM-2) model of photoaging and hyperpigmentation revealed that the production of melanin in HRM-2 mice was substantially and markedly reduced by the application of KRG (150 and 300mg/kg). Furthermore, the 3% red ginseng extract cream showed excellent antiwrinkle and skin-whitening qualities in humans. Thus, we concluded that KRG and KRG formulations as a cream should be useful in the cosmetic industry as a skin-whitening and antiaging agent.
2. Materials and methods
2.1. Chemicals and reagents
Dulbecco's modified Eagle's medium (WelGene Co, Korea); fetal bovine serum (WelGene Co., Korea); streptomycin and penicillin (Lonza, MD, USA); TRIzol reagent (Invitrogen, Carlsbad, CA, USA); oligodT, MITF, TYR, TRP-1, TRP-2, and β-actin primers were obtained from (Bioneer, Daejeon, Korea). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide was purchased from Sigma-Aldrich. Antibodies for MITF, TYR, TRP-1, and TRP-2 were obtained (Santa Cruz Biotechonology, Santa Cruz, Inc., TX, USA).Mushroom TYR and L-DOPA were purchased from Sigma (St. Louis, MO, USA). All other reagents were of local analytical grade.
2.2. Sample preparation
KRG was kindly provided by the Korea Ginseng Cooperation that consisted of the following 11 ginsenosides composition (mg/g): Rb1 6.67, Rb2 2.79, Rc 1.01, Rd 1.01, Rg3s 2.22, Rg3r 0.79, Re 1.97, Rf 1.40, Rg1 1.67, Rg2s 1.23, and Rh1 0.77 as analyzed by High Performance Liquid Chromatography (HPLC) analysis. While 3% red ginseng cream (the composition of ginsenosides was same as described previously) was prepared by Kyungnam University, Changwon, Republic of Korea. Briefly, a mixture of 80 mL of purified water and 30 mL of either sweet almond oil or sunflower seed oil was heated at 80°C. Thereafter, purified water was added again and emulsified by using a blender. Subsequently, tocopherol was added as an antioxidant, and 3% red ginseng extract was added as the active ingredient; the mixture was termed red ginseng cream.
2.3. Evaluation of the stability of red ginseng cream
The following tests were performed to evaluate of the stability of 3% red ginseng cream:
-
(1)
pH test
The pH of the red ginseng cream was measured on Days 1, 7, 15, and 30 of the 30-day experiment. The pH of the red ginseng cream was slightly acidic and showed almost no change over the 30 days (Table 2).
-
(2)
Discoloration test
Table 2.
Measure of oil content in the experimental group and the control group
| Groups | Oil content | Forehead | Right cheek ① |
Right cheek ② |
Left cheek ① |
Left cheek ② |
Average |
|---|---|---|---|---|---|---|---|
| 0 weeks | 36.8 ± 25.67 | 37.0 ± 33.50 | 40.4 ± 24.60 | 22.2 ± 12.95 | 26.6 ± 20.96 | 32.6 ± 7.78 | |
| Experimental group | 2 weeks | 85.0 ± 44.54 | 94.0 ± 68.19 | 123.2 ± 10.57 | 82.0 ± 69.65 | 106.2 ± 13.86 | 98.08 ± 16.91 |
| 4 weeks | 104.20 ± 44.64 | 112.20 ± 104.48 | 114.40 ± 83.73 | 94.00 ± 67.92 | 77.20 ± 55.36 | 100.4 ± 15.24 | |
| Variation | +67.4 | +75.2 | +74 | +71.8 | +50.6 | 67.8 ± 10.06 | |
| 0 weeks | 98.0 ± 44.12 | 80.8 ± 45.19 | 116.4 ± 74.93 | 91.4 ± 41.42 | 66.8 ± 24.04 | 90.68 ± 18.60 | |
| Control group | 2 weeks | 173.4 ± 112.74 | 199.8 ± 171.67 | 115.2 ± 56.96 | 124 ± 51.78 | 114.6 ± 70.72 | 145.4 ± 38.93 |
| 4 weeks | 144.6 ± 48.41* | 143.0 ± 64.25 | 177.6 ± 57.86 | 116.8 ± 77.16 | 99.4 ± 91.78 | 136.28 ± 29.84 | |
| Variation | +46.6 | +62.2 | +61.2 | +25.4 | +32.6 | 45.6 ± 16.56 |
The degree of discoloration of red ginseng cream was measured on Days 1, 7, 15, and 30. No change in color was observed over the 30-day experimental period (Supplemental Fig. 1).
-
(3)
Patch test
To check for an allergic response to ginseng cream, 1 mL of red ginseng cream was applied to the dorsal central back area (1 cm × 1 cm) of the volunteers, and the skin reaction was evaluated after 24 h. Various types of skin reactions were evaluated, as shown in Supplemental Table. 3A-B, and no erythema, edema, or untoward reaction was observed. Therefore, this cream was further tested for other parameters on skin. Furthermore, the degree of skin irritation was evaluated, as shown in Supplemental Table. 4, to yield the primary skin irritation index.
2.4. Red ginseng cream treatment regimen and evaluation of effects on oil and moisture content, elasticity, and whitening of skin
To assess the effects of red ginseng cream on the oil content, moisture content, elasticity, and whitening of the skin, 10 women (approximately 40 years of age, nonsmokers, with no past history of skin disease or facial treatment) were divided into two groups each containing five individuals: the control group, in which cream without red ginseng extract was applied (non-KRG), and the experimental group, in which cream with red ginseng extract was applied (KRG-treated group). The number of individuals were calculated by using the statistical software, G*Power 3.1 omicX; the coefficient of significance was 0.05, the power was 80, the number of groups was 2, and the number of repeated measurements was 6.
Ten individuals (five in the experimental group and five in the control group) were selected to account for a dropout rate of 20%. The human trial experiments were permitted by the Institutional Review Board of Kyungnam University (IRB # 1040460-A-2017-040).
Every day for 1 month, the cream was applied to the skin 1 h after cleansing. Thereafter, the aforementioned parameters were measured in the skin on Weeks 0, 2, and 4.
The cream was applied on the T-zone area (1) of the face, which is located 1 cm above the upper part of the middle of the T on the forehead between the eyebrows and on the right and the left sides for 3–5 seconds. The U-zone (2) was drawn horizontally from the nose and vertically down from the tail of the eye, as shown in Supplemental Fig. 2. To standardize the measurement conditions, the research was performed at 24 ° C ± 1 ° C and 55% ± 10% relative humidity.
The oil content, water content, elasticity, and melanin content were measured by using a sebumeter SM815 (CK electronics, Germany), corneometer CM825 (CK electronics, Germany), mexameter MX18 (CK electronics, Germany), and a cutometer (CK electronics, Germany), respectively, by touching these instruments to the skin for 3–5 seconds.
The moisture and oil content and skin tones of the skin were analyzed by T-test. An empirical analysis of the study results was considered to indicate statistically significant changes for p values of <0.05. After the end of the application period, the satisfaction of the product assessed through a questionnaire was converted into a score for each response.
2.5. Cell culture
The murine melanoma B16/F10 cell line was sourced from the American Type Culture Collection and cultured in Dulbecco's Modified Eagle's Medium supplemented with 8% fetal bovine serum (WelGene Co, Daejeon), 100 IU/mL of penicillin, and 100 μg/mL of streptomycin sulfate (Lonza, MD, USA). The cells were maintained in a humidified 5% CO2 incubator at 37°C.
2.6. Cell-free TYR inhibition assay
The assay was performed using a slightly modified protocol as previously described [3]. Briefly, 10 μL of KRG was placed in triplicate in a 96-well plate and mixed with 60 μL of 50mmol/L of phosphate buffer on ice (pH 6.8). Subsequently, 20 μL of 0.9mg/mL L-DOPA was added to each well. Finally, 10 μL mushroom TYR was added to each well, and the plate was incubated at 27°C for 10 min. After incubation, dopachrome production was measured spectrophotometrically at 450nm by using a microplate reader (Versamax; Molecular Devices, LLC, CA, USA); in this experiment, kojic acid was used as the positive control [16].
2.7. Melanin inhibition assay
The B16/F10 cells were seeded in 6-well culture plates at a density of 2.5 × 103 cells/well and incubated for 5 days. After the cells reached the desired confluency, KRG treatment was applied and the cells were stimulated with α-MSH. The cells were then incubated for 3 days, harvested by using 0.25% trypsin–ethylenediaminetetraacetic acid solution, and transferred to 1.5-mL microcentrifuge tubes. The tubes were then centrifuged at 10,000rpm for 10 min, and the pellet was dissolved in 2mol/L NaOH for 15min at 60°C. The mixture was then transferred to 96-well plates, and the absorbance of each well at 450nm was measured by using a microplate reader (Versamax; Molecular Devices, LLC, CA, USA). The absorbance was compared with that of standard curves produced from synthetic melanin (Sigma).
2.8. Animal experiment and grouping
Six-week-old male HRM-2 melanin-possessing hairless mice were obtained from Central Lab Animal Inc. (Seoul, South Korea) and were housed in a controlled room (23°C ± 1°C, 55% ± 5% relative humidity, 12h light/dark cycle) and given ad libitum access to water and feed. All animal experimentations were performed strictly in accordance with the Institutional Animal Care and Use Committee of Daejeon University (Daejeon, Korea) (permission number: DJUARB2017-033).After an acclimation period of 1 week, the mice were randomly divided into five groups, each containing five animals: the normal group, which received no treatment; the control UVB-irradiated group; the positive control group, which received 0.01% sunblock and UVB irradiation; the lower-dose KRG group, which received KRG 150 mg/kg p.o. with UVB irradiation; and the higher-dose KRG group, which received KRG 300 mg/kg p.o. with UVB irradiation.
2.9. UVB irradiation and induction of photoaging
The dorsal skin of HRM-2 mice was irradiated by an UVB lamp (15 W; maximum wavelength, 312 nm; UV intensity, 100 μW cm−2; IedaBoeki Co., Tokyo, Japan). To evaluate the effects of the positive control (0.01% sunblock) and KRG on wrinkle formation and pigmentation, HRM-2 mice were irradiated with 100 mJ/cm2 UVB (1 minimal erythematic dose = 100 mJ/cm2) daily for Week 1 and then 200 mJ/cm2 UVB from Weeks 2–5. The mice were monitored three times per week. The dietary intake and body weight were measured every week for 12 weeks.
2.10. Effect on melanin production in HRM-2 mice
To evaluate the melanin production that resulted from exposure of the mice to UVB, the dorsal area of mice was divided into right and left sides. In all groups except the normal group, the left side received UVB (control), sunblock, or KRG and UVB irradiation for 5 weeks. The right side was left untreated. UVB irradiation was applied three times per week for 5 weeks (as described above), and the pigmentation status of the skin was analyzed in Weeks 1, 3, and 5 by using the digital camera (D70 model; Nikon, Tokyo, Japan) after the mice were anesthetized with ether. Image analysis of the dorsal skin was performed by using software (Bio-Rad, USA) on the digital camera photographs. The degree of melanin deposition was analyzed from the difference between the pigmented, UVB-exposed, and sample-treated area on the left side compared with the untreated and unpigmented area on the right side.
2.11. Skin wrinkle measurement
The degree of skin aging induced by UVB was measured through observation of the wrinkle formation. To evaluate the formation of wrinkles, the HRM-2 mice were anesthetized by the intraperitoneal injection of chloral hydrate (0.1 mL of 7% chloral hydrate/25 g mouse) in Week 5. Exposure to UVB and sample treatment was as described in the previous section. Skin wrinkles were evaluated in Weeks 3, 4, and 5 by using DETAX System II (MIXPAC) and Double-Stick Disc (3M Healthcare, Germany) after UVB irradiation. Double-Stick Disc (sprayed with DETAX System II) was attached to the mouse skin and removed after 2–3 minutes. Disc wrinkles were evaluated in accordance with the scoring system of Bissett [17]: grade 0, absence of wrinkles; grade 1, several shallow wrinkles; grade 2, some wrinkles; and grade 3, some deep wrinkles. After the disc was removed and the skin was cleaned with 70% ethanol, the skin was photographed by using a USB Digital Microscope ( × 400; CE FOROHS, China) and a visual analysis of skin wrinkles was performed.
2.12. Enzyme-linked immunosorbent assay
To investigate the effect of UVB-induced wrinkle-related genes (i.e., matrix metalloproteinase; MMP-2), the skin from all treated groups was harvested and the proteins were analyzed by using the enzyme-linked immunosorbent assay (ELISA) kit in accordance with manufacturer's instructions (MMP-2 ELISA kit; R&D Systems, USA).
2.13. Histological observation of skin
The skin tissues extracted from each experimental group were fixed in 10% formalin solution for 48 h and then stained with hematoxylin and eosin in accordance with the method of Cardiff [18] to determine the epidermal thickness. For collagen visualization, Masson's trichome staining was performed in accordance with established protocols [19].
2.14. RNA extraction and real-time polymerase chain reaction
Total RNA was extracted from the B16/F10 cells and UVB-irradiated mouse skin after they were treated with KRG and stimulated with α-MSH by using TRIzol in accordance with the manufacturer's instructions. RNA (2 μg) was annealed with oligodT (Bioneer Co, Daejeon) for 10 min at 70°C and cooled for 5 min on ice, reverse transcribed using reverse transcriptase premix (Bioneer Co., Daejeon) in 20 μL of reaction mixture, and run for 90 min at 42.5°C in a thermal cycler. The reaction was terminated at 95°C for 5 min to inactivate the reverse transcriptase. The reverse transcription polymerase chain reaction (PCR) was performed on aliquots of cDNA obtained from Reverse Transcriptase (RT) reaction in a PCR premix (Bioneer Co, Daejeon). The PCR products were then electrophoresed on a 1% agarose gel, stained with ethidium bromide, and visualized by using ImageQuant LAS 500 (GE Healthcare Life sciences, Seoul, South Korea). The intensity of band densities was normalized to that of Glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and the primer sequences are provided in Supplemental Table. 1. The expression of MMP-2, MMP-9, and interleukin (IL)-1β was analyzed by using real-time quantitative PCR using an Applied Biosystems 7500 Real-Time PCR system (Applied Biosystems, USA).
2.15. Western blot analysis
B16/F10 cells were treated with KRG in the presence of α-MSH (10μM). Total proteins from cells and UVB-irradiated mouse skin were extracted in accordance with the instructions of the PRO-PREP lysis buffer (iNtRON Biotechnology, Korea). Protein concentration was then measured by using the PRO-MEASURE assay kit (PRO-PREP; iNtRON Biotechnology, Korea), and equal amounts of protein were separated by 10% polyacrylamide gels by using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto Polyvinylidene difluoride (PVDF ) membranes (Immobilon-P; Millipore, Billerica MA, USA). Nonspecific binding to the PVDF membranes was minimized by incubation of the membrane in blocking buffer containing 5% nonfat dry milk and 0.1% Tween-20 in Tris buffered-saline (TBS). The membranes were then incubated overnight at 4°C with specific primary antibodies, followed by incubation for 1 h with horseradish peroxidase–conjugated antirabbit antibody (1: 3000 dilution). Bound antibodies were visualized by the application of enhanced chemiluminescence solution (Supex, Daegu, Korea), and images were analyzed by using ImageJ software. β-Actin was used as the internal control.
2.16. Statistical analysis
The data were presented as the mean ± Standard Error of the Mean (SEM). One-way Analysis of variance (ANOVA), Dunnett's test, and unpaired Student's t-test were applied for the statistical evaluation of data or where specifically otherwise indicated. Statistical analysis results of***p < 0.001, **p < 0.05, and *p < 0.01 were considered significant.
3. Results
3.1. KRG inhibited TYR activity and suppressed melanin content in the cell-free system
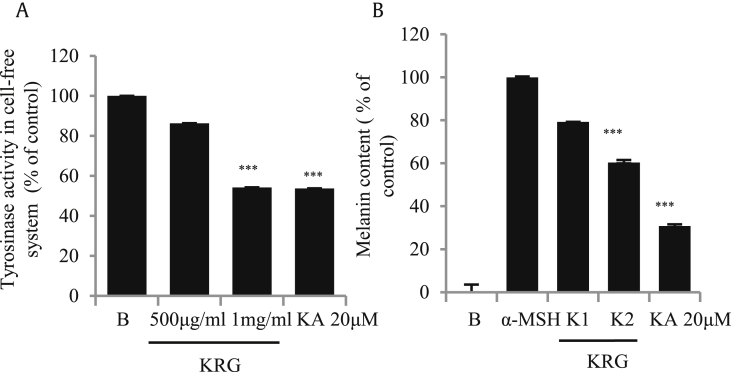
The TYR enzyme participates in the rate-limiting step of melanogenesis [20], [21]. Therefore, to check if KRG was able to suppress the production of this enzyme, we performed a cell-free mushroom TYR assay. As shown in Fig. 1A, KRG potently inhibited TYR production. B16/F10 cells were treated with KRG and stimulated with α-MSH to induce melanin secretion, and it was found that it was efficiently suppressed by KRG, as shown in Fig. 1B. Kojic acid, a known whitening agent, was used as the positive control in both experiments.
Fig. 1.
Inhibition of mushroom tyrosinase and melanin production by KRG. (A) KRG inhibited the mushroom tyrosinase activity in a cell-free system. (B) Melanin content in crude lysates was suppressed by KRG when B16/F10 cells were stimulated with α-MSH (K1= KRG 500μg/mL and K2= KRG 1mg/mL). The values in the bar graph are the mean ± SEM of at least four independent experiments. ***p < 0.001 compared with the untreated group.
KRG, Korean Red Ginseng; α-MSH, α-melanocyte–stimulating hormone.
3.2. Effects of KRG on the mRNA and protein expressions of MITF and TRPs
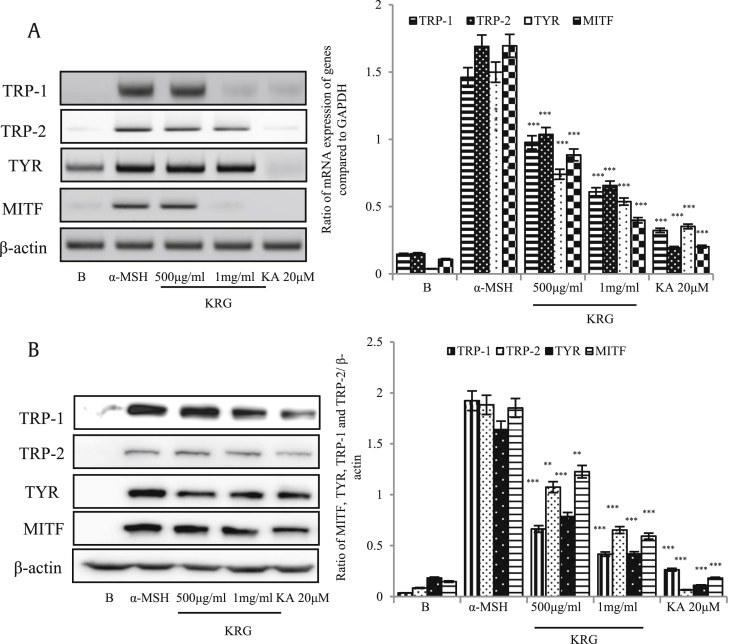
The aforementioned results indicated the effects of KRG on the TYR and melanin content. However, to elucidate the mechanism of melanin inhibition by KRG, we evaluated the transcription and translation of genes related to the melanogenesis pathway: TRP-1 and TRP-2, TYR, and microphthalmia-associated transcription factor (MITF). As shown in Fig. 2A and B, all four components of the melanogenesis pathway were strongly inhibited by KRG. Therefore, KRG was confirmed as an effect whitening agent in the in vitro study.
Fig. 2.
Decreased expression of MITF pathway genes induced by KRG. B16/F10 cells were seeded in 6-well plates, treated with the indicated concentrations of KRG, and then stimulated with α-MSH (10μM). Subsequently, RNA and proteins were extracted and MITF, TYR, TRP-1, and TRP-2 expression was measured by Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) (A) and western blotting (B). GAPDH was used as the internal control qRT-PCR and β-actin was used as the internal control for western blotting. ***p < 0.001 and **p < 0.05 were considered as statistically significant against the α-MSH-treated group only.
KRG, Korean Red Ginseng; α-MSH, α-melanocyte–stimulating hormone; MITF, microphthalmia-associated factor; TYR, tyrosinase; TRP-1, tyrosinase-related protein 1; TRP-2, tyrosinase-related protein 2.
3.3. Effect of oral supplementation of KRG on body weight and food intake
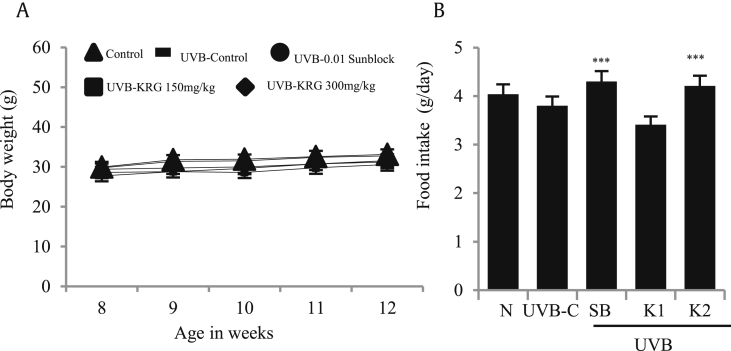
Exposure to artificial UVB induces stress in animals, which may result in a loss of appetite; therefore, we evaluated the dietary intake and dietary efficiency of HRM-2 mice exposed to UVB for 5 weeks, treated with sunblock and orally administered KRG. As shown in Fig. 3A, there was no significant change in the body weight in the normal, control UVB, or treatment groups. Food intake was also found to be significantly increased in the positive control and KRG-treated groups, as shown in Fig. 3B. These results show that although UVB induced stress in mice, KRG treatment effectively prevented the loss of appetite and weight in mice.
Fig. 3.
Effects of KRG on body weight and food intake. Food intake and body weight was measured daily for 5 weeks in HRM-2 mice. (A) No significant weight changes were observed in the control and treatment groups. (B) Daily food intake was significantly higher in the positive control and KRG-treated groups (N= HRM-2 control mice, UVB-C = control UVB-irradiated group, SB = 0.01% sunblock, K1= KRG 150mg/kg, and K2= KRG 300mg/kg). The values are expressed as the mean ± SEM from three independent experiments. ***p < 0.001 compared with UVB control. HRM-2 N = normal mice, UVB-C= HRM-2 mice control, exposed to UVB.
KRG, Korean Red Ginseng; UVB, ultraviolet B.
3.4. Analysis of expression of wrinkle-related genes in UVB-irradiated HRM-2 mice
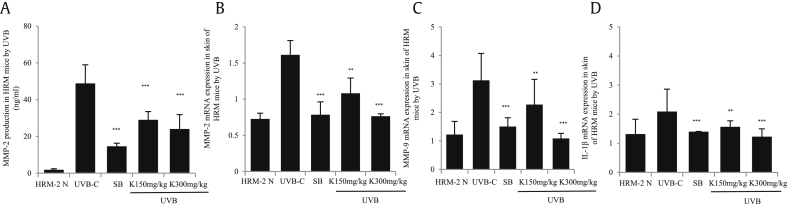
To measure the effect of KRG on the formation of skin wrinkles, HRM-2 mice were irradiated with UVB to induce wrinkles. After 5 weeks of irradiation, the skin tissues were isolated and the expression of wrinkle-related genes (i.e. IL-1β, MMP-2, and MMP-9) was analyzed. As shown in Fig. 4A and B, the protein expression of MMP-2, as determined by ELISA, and the mRNA expression, as determined by real-time PCR, were significantly lower in the positive control group and the KRG treatment groups than those in the control UVB-irradiated group. MMP-9 and IL-1β levels were also significantly decreased in the KRG-treated groups compared with the control UVB-irradiated group, as shown by real-time PCR (Fig. 4C and D).
Fig. 4.
Analysis of wrinkle-related gene expression in UVB-irradiated skin. After 5 weeks, the skin tissues were isolated from HRM-2 mice and MMP-2, MMP-9, and IL-1β gene expression was analyzed. (A) MMP-2 production was significantly reduced by KRG, as determined by ELISA. (B) MMP-2 mRNA expressions was significantly decreased in both KRG-treated groups, as determined by qRT-PCR. (C) MMP-9 mRNA expressions was significantly decreased by KRG, as determined by qRT-PCR. (D) IL-1β expression was significantly decreased by KRG, as determined by qRT-PCR (K= KRG). The values in bar graphs are the mean ± SEM from three independent experiments. ***p < 0.001 and **p < 0.05 compared with the control UVB irradiated mice.
KRG, Korean Red Ginseng; MMP, matrix metalloproteinase; IL, interleukin; ELISA, enzyme-linked immunosorbent assay.
3.5. Effects of KRG on melanogenesis in UVB-irradiated skin damage
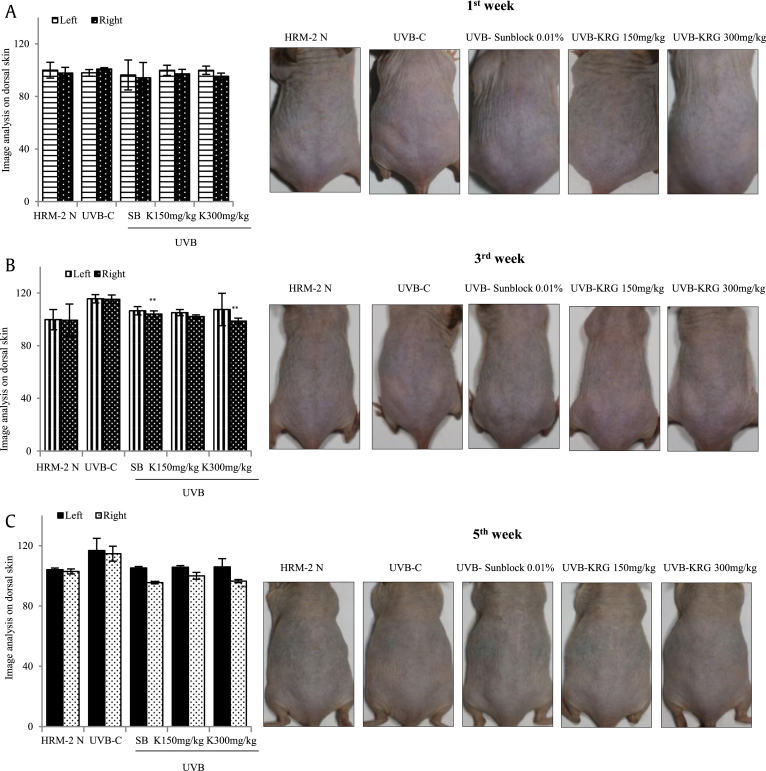
To confirm the whitening efficacy of KRG, the difference in the formation of melanin in Weeks 1, 3, and 5 of UVB irradiation was examined by dividing the dorsal skin of HRM-2 mice into left (untreated) and right (UVB and sample-treated sections). As shown in Fig. 5A, during Week 1 of exposure of the mice to UVB with the treatment of sunblock and KRG, there was no significant decrease in the production of melanin at both KRG doses. In week 3, there was a minor decrease in melanin production in the positive control sunblock-treated group and KRG groups compared with the control UVB-irradiated group, as shown in Fig. 5B. In week 5, as clearly shown in Fig. 5C, the positive control sunblock-treated group and both KRG-treated groups showed a significant decrease in melanin production. These results indicated that KRG was an effective skin-whitening agent.
Fig. 5.
Effects of KRG on melanogenesis in UVB-irradiated skin. (A) No significant reduction was found in melanin production in any groups in Week 1. (B) A minor reduction was found in the melanin production in the KRG-treated group in Week 3. (C) A significant reduction was found in the melanin production in both KRG-treated groups in Week 5. The values in the bar graphs are the mean ± SEM from three independent experiments. ***p < 0.001and **p < 0.05 when compared with the control UVB-irradiated group. [HRM-2 N = normal mice, UVB-C= HRM-2 mice control (exposed to UVB), SB = 0.01% sunblock, and K= KRG].
KRG, Korean Red Ginseng; UVB, ultraviolet B.
3.6. Analysis of effects of KRG on wrinkle formation in HRM-2 mice
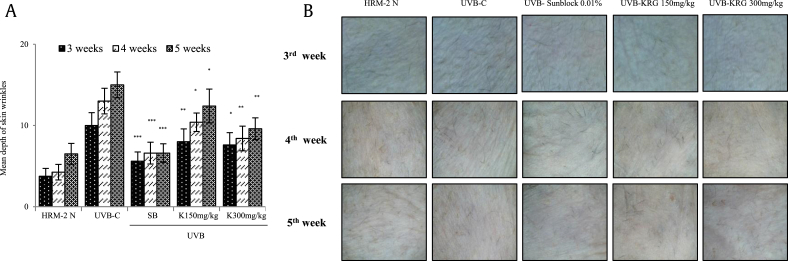
To determine the effects of KRG on the formation and depth of wrinkles, HRM-2 mice were irradiated with UVB to induce photoaging, and the skin wrinkle depth was measured using a 3D analyzer, as described in the Materials and method section (2.11), in Weeks 3, 4, and 5. As shown in Fig. 6A and B, the positive control sunblock-treated group and both KRG-treated groups showed significant decreases in wrinkle formation and depth.
Fig. 6.
Effects of KRG on wrinkle depth and formation in HRM-2 mice. (A) A significant reduction was found in the depth of wrinkles in Weeks 3, 4, and 5 of KRG treatment. The values in bar graphs are the mean ± SEM from three independent experiments. ***p < 0.001, **p < 0.05, and *p < 0.01 compared with the control UVB-irradiated groups. (B) Wrinkle formation in Week 3, 4, and 5 of KRG treatment. HRM-2 N = normal mice, UVB-C= HRM-2 mice control (exposed to UVB), SB = 0.01% sunblock, and K= KRG.
KRG, Korean Red Ginseng; UVB, ultraviolet B.
3.7. Effect of KRG on decrease in epidermal thickness and increase in collagen
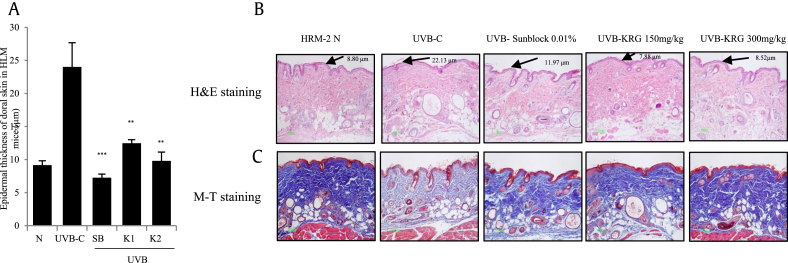
UV radiation can increase epidermal thickness, causing the skin to become thicker and rough. Moreover, continuous exposure to UVB can degrade the MMPs and cause a loss in collagen, which is essential for good skin health [22]. As shown in Fig. 7A, the epidermal thickness induced by UVB was potently reduced by the positive control sunblock-treated group and both KRG-treated groups. Moreover, in Fig. 7B, it is shown that the epidermal thickness, as visualized by hematoxylin and eosin staining, was clearly decreased in the positive control sunblock-treated group and both KRG-treated groups. Masson's trichome staining, performed to visualize the matrix components in the skin of HRM-2 mice, revealed reduced staining intensity in the control UVB-irradiated group resulting from the degradation of collagen fibers; conversely, in mice treated with sunblock and both KRG doses, the intensity of staining was dramatically increased compared with the UVB control group (Fig. 7C). These results clearly showed that KRG is a safe and efficacious treatment with skin-whitening and antiaging effects.
Fig. 7.
Effects of KRG on epithelial thickness and collagen fiber changes in UVB-irradiated HRM-2 mice. After 5 weeks, the skin tissues were stained with H&E and M-T staining and epithelial thickness was measured (A, B and C). The values in the bar graphs are the mean ± SEM from three independent experiments. ***p < 0.001 and **p < 0.05 compared with the UVB control. (N= HRM-2 control mice, UVB-C= ultraviolet-exposed control, SB = 0.01% sunblock, K1= KRG 150 mg/kg, and K2= KRG 300 mg/kg).
KRG, Korean Red Ginseng; M-T, Masson's trichome; H&E, hematoxylin and eosin; UVB, ultraviolet B.
3.8. Effects of red ginseng cream on human skin
Skin elasticity (also called as resilience) was measured as shown in Table 1. An average increase in resilience of 0.03386 was observed in the experimental group that received the application of the cream containing 3% red ginseng. The control group, which received cream without ginseng, showed an average increase of resilience of 0.0255.
Table 1.
Measure of resilience in the experimental group and the control group
| Groups | Resilience | Forehead | Right cheek ① |
Right cheek ② |
Left cheek ① |
Left cheek ② | Average |
|---|---|---|---|---|---|---|---|
| 0 weeks | 0.7378 ± 00.07 | 0.7844 ± 0.05 | 0.7324 ± 0.05 | 0.7719 ± 0.12 | 0.7673 ± 0.11 | 0.75876 ± 0.02 | |
| Experimental group | 2 weeks | 0.8193 ± 0.11 | 0.8467 ± 0.09 | 0.8242 ± 0.10 | 0.8173 ± 0.09 | 0.8255 ± 0.05 | 0.8266 ± 0.01 |
| 4 weeks | 0.8105 ± 0.07 | 0.8419 ± 0.03 | 0.7906 ± 0.05 | 0.7822 ± 0.03 | 0.7379 ± 0.08 | 0.79262 ± 0.04 | |
| Variation | +0.0727 | +0.0575 | +0.0582 | +0.0103 | −0.0294 | 0.03386 ± 0.04 | |
| Control Group | 0 weeks | 0.8503 ± 0.13 | 0.7940 ± 0.08 | 0.7619 ± 0.06 | 0.8445 ± 0.03 | 0.6774 ± 0.08 | 0.78562 ± 0.07 |
| 2 weeks | 0.8695 ± 0.08 | 0.7724 ± 0.07 | 0.7649 ± 0.03 | 0.8446 ± 0.07 | 0.7813 ± 0.11 | 0.80654 ± 0.05 | |
| 4 weeks | 0.8666 ± 0.08 | 0.8185 ± 0.08 | 0.7448 ± 0.05 | 0.8476 ± 0.05 | 0.7781 ± 0.05 | 0.81112 ± 0.05 | |
| Variation | +0.0163 | +0.0245 | −0.0171 | +0.0031 | +0.1007 | 0.0255 ± 0.04 |
The oil content in the experimental group was increased to 67.8% compared with the control group, in which the oil content was 45.6%, as shown in Table 2. In detail, the results showed that the oil content was increased by 50% in all experimental groups: 46.6% in the hemisphere, 25.4% in the left cheek, and 32.6% overall.
The moisture content was decreased in both the control and experimental groups, as shown in Table 3. This was considered to be attributable to the change in weather conditions between October and November in the Republic of Korea.
Table 3.
Measure of moisture content in the experimental group and the control group
| Groups | Moisture content | Forehead | Right cheek ① |
Right cheek ② |
Left cheek ① |
Left cheek ② |
Average |
|---|---|---|---|---|---|---|---|
| 0 weeks | 61.1 ± 11.01 | 55.2 ± 10.51 | 58.8 ± 14.59 | 74.4 ± 7.24 | 71.0 ± 7.15 | 64.1 ± 8.22 | |
| Experimental group | 2 weeks | 51.7 ± 16.00 | 38.2 ± 15.24 | 35.8 ± 17.28 | 51.5 ± 19.47 | 39.4 ± 21.89 | 43.32 ± 7.67 |
| 4 weeks | 54.34 ± 8.94 | 39.02 ± 16.32 | 35.48 ± 19.05 | 61.80 ± 11.33 | 57.18 ± 18.54 | 49.564 ± 11.62 | |
| Variation | −6.76 | −16.18 | −23.32 | −12.6 | −13.82 | −14.536 ± 6.01 | |
| 0 weeks | 63.5 ± 13.23 | 48.42 ± 12.02 | 47.02 ± 11.89 | 73.62 ± 4.37 | 75.30 ± 6.23 | 61.57 ± 13.44 | |
| Control group | 2 weeks | 55.16 ± 15.06 | 41.58 ± 12.84 | 44 ± 10.32 | 64.72 ± 4.42 | 64.14 ± 9.21 | 53.92 ± 10.88 |
| 4 weeks | 54.60 ± 10.25 | 41.42 ± 19.92 | 47.98 ± 9.96* | 54.06 ± 19.45 | 59.08 ± 16.50 | 51.428 ± 6.85 | |
| Variation | −8.9 | −7 | +0.96 | −19.56 | −16.22 | −10.14 ± 8.07 |
The change in skin tone in the experimental group was an average decrease of 8.24; conversely, the control group showed an increase of 2.12 (Table 4). In detail, the skin tone of the forehead was increased in both the control and experimental groups. In the experimental group, the skin tone decreased on both the right cheek and left cheek; conversely, in the control group, there skin tone increased on both the forehead and right cheek, which indicated that the skin had darkened. Therefore, we conclude that ginseng cream decreased the tendency of skin to lose its color; in other words, it enhanced the brightening effect.
Table 4.
Measure of skin tone in the experimental group and the control group
| Groups | Skin tone | Forehead | Right cheek ① |
Right cheek ② |
Left cheek ① |
Left cheek ② |
Average |
|---|---|---|---|---|---|---|---|
| 0 weeks | 101.0 ± 15.81 | 91.4 ± 28.16 | 97.0 ± 10.65 | 112.6 ± 25.74 | 123.0 ± 31.36 | 105 ± 12.72 | |
| Experimental group | 2 weeks | 107.0 ± 22.55 | 80.4 ± 28.80 | 77.2 ± 17.27 | 109.8 ± 24.01 | 108.8 ± 21.49 | 96.64 ± 16.36 |
| 4 weeks | 103.80 ± 7.79 | 76.40 ± 20.70 | 85.80 ± 12.07 | 105.00 ± 47.92 | 112.80 ± 15.99 | ±96.76 ± 15.08 | |
| Variation | +2.8 | −15 | −11.2 | −7.6 | −10.2 | −8.24 ± 6.72 | |
| 0 weeks | 95.2 ± 22.86 | 71.2 ± 19.64 | 65 ± 14.61 | 99.6 ± 25.72 | 85.8 ± 27.31 | 83.36 ± 14.96 | |
| Control group | 2 weeks | 118.6 ± 77.98 | 61.2 ± 10.87 | 66.4 ± 24.52 | 86 ± 7.31 | 86.2 ± 31.89 | 83.68 ± 22.56 |
| 4 weeks | 107.2 ± 32.77 | 71.8 ± 13.79 | 70.4 ± 8.71* | 93.4 ± 22.02 | 84.6 ± 20.26 | 85.48 ± 15.41 | |
| Variation | +12 | +0.6 | +5.4 | −6.2 | −1.2 | 2.12 ± 6.91 |
The average erythema of the skin was reduced by 33.8 in the experimental group and 3.6 in the control group (Table 5). These results strongly suggest the positive effects of red ginseng extract in improving the overall condition of skin without inducing any side effects.
Table 5.
Measure of erythema in the experimental group and the control group
| Groups | Erythema | Forehead | Right cheek ① |
Right cheek ② |
Left cheek ① |
Left cheek ② |
Average |
|---|---|---|---|---|---|---|---|
| 0 weeks | 286.8 ± 40.27 | 300.6 ± 68.81 | 273.4 ± 67.16 | 241.8 ± 66.65 | 235.6 ± 70.88 | 267.64 ± 28.20 | |
| Experimental group | 2 weeks | 259.2 ± 45.03 | 238.4 ± 64.35 | 221.6 ± 48.88 | 229.0 ± 47.71 | 225.6 ± 35.91 | 234.76 ± 15.01 |
| 4 weeks | 251.40 ± 31.07 | 249.00 ± 44.65 | 231.80 ± 63.24 | 225.60 ± 42.12 | 211.40 ± 47.31 | 233.84 ± 16.69 | |
| Variation | −35.4 | −51.6 | −41.6 | −16.2 | −24.2 | −33.8 ± 13.98 | |
| 0 weeks | 244.0 ± 24.77 | 260.6 ± 31.10 | 248.2 ± 34.24 | 216.4 ± 30.75 | 215.8 ± 56.81 | 237 ± 20.03 | |
| Control group | 2 weeks | 242.8 ± 34.75 | 246.6 ± 55.90 | 237.2 ± 37.27 | 197.2 ± 12.03 | 207.8 ± 22.21 | 226.32 ± 22.32 |
| 4 weeks | 236.4 ± 45.92 | 265.0 ± 51.56 | 243.4 ± 22.88 | 211.6 ± 7.37 | 210.6 ± 38.60 | 233.4 ± 22.93 | |
| Variation | −7.6 | +4.4 | −4.8 | −4.8 | −5.2 | −3.6 ± 4.62 |
4. Discussion
Melanocytes are the specialized melanin-producing cells found in the basal layer of the epidermis. These cells are involved in the production and transportation of melanin to the neighboring keratinocytes and the consequent formation of a uniform layer of skin pigmentation. Melanin is an essential pigment that serves two functions. The first, and most important, function is to pigment skin, and the second function is to protect the skin from harmful UV radiation by decreasing the amount of reactive oxygen species [23], [24]. When the skin is exposed to UV, α-MSH hormone is activated, which causes the production of melanin from melanocytes [25]. The key regulators of this whole process of melanin production are MITF, a transcriptional factor for the regulation of TYR, and tyrosine-related proteins (TRP-1 and TRP-2) [4], [26], [27], [28]. These components are involved in the production of melanin and skin pigmentation.
Skin aging can be broadly classified into two types [29]. Intrinsic (or endogenous) aging is an inevitable aging phenomenon that occurs with human aging. The clinical features of this type of aging are relatively mild and include fine lines, dry skin, and reduced elasticity [30]. Photoaging (exogenous aging) refers to the aging phenomenon observed in skin exposed to sunlight for a long time. The element responsible for damage is UVB in sunlight. This type of photoaging can be prevented by the application of commercially available sunblock before going out into sun and minimizing the exposure time to the sun. The clinical features of exogenous aging are deleterious, such as very rough, dry skin with reduced elasticity and the formation of deep wrinkles with severe skin sagging. Photoaged skin is also very prone to pigmentation diseases, such as solar lentigo [31], [32].
Moreover, when the skin is overexposed to sunlight that contains UVB, the amount of extracellular MMPs increases, causing degradation of the matrix proteins, of which collagen is a major component [33]. Collagen is globally accepted to be a required element for skin elasticity, and its degradation is known to cause early wrinkle formation and to reduce skin elasticity. After exposure to UVB, increases in MMPs degrade collagen and other substrate proteins [34]. Thus, this is a type of wound inflicted by the sun's radiation on the skin, and our body makes efforts to heal wounds through the synthesis of new collagen. However, as the process of wound healing is not always perfect, continuous exposure of the skin to UVB will result in clinical symptoms of aging, including wrinkles.
Henceforth, to avoid the damage caused by UVB as a result of exposure to sunlight, many herbal and pharmaceutical remedies are available, such as sunblock creams, lotions, and sprays. In addition to these, when going out into the sun, it is advised to cover the head (e.g., a cap) and body parts with clothes. Our research has demonstrated that the oral administration and topical application of a herbal KRG-based cream effectively reduced the formation of wrinkles and decreased the dermal thickness induced by UVB exposure. Moreover, these products also decreased the production of MMPs and the subsequent degradation of collagen.
Although melanocytes release a large number of soluble components, prostaglandin E2, prostaglandin F2α, adrenocorticotropic hormone, and NO are considered regulators of melanogenesis [35], [36], [37], [38]. However, the effects of cytokines on the process of melanogenesis are rather complicated. For example, among the IL family, IL-1α/1β and granulocyte-macrophage colony-stimulating factor are involved in the stimulation of melanogenesis; however, IL-6, Transforming growth factor beta 1 (TGF-β1), and Tissue Necrosis Factor α (TNF-α) suppress melanin production. Our results showed that IL-1β was increased in the control UVB-irradiated group but was significantly decreased by KRG treatment. In conclusion, KRG and KRG cream exhibited potent antimelanogenic and skin-whitening properties and may be developed into a commercially available cosmetic agent.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by a grant from the Korean Society of Ginseng (2017).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2019.05.003.
Contributor Information
Seong-Soo Roh, Email: ddede@dhu.ac.kr.
Man Hee Rhee, Email: rheemh@knu.ac.kr.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Brenner M., Hearing V.J. The protective role of melanin against UV damage in human skin. Photochem Photobiol. 2008;84:539–549. doi: 10.1111/j.1751-1097.2007.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim D.S., Jeong Y.M., Park I.K., Hahn H.G., Lee H.K., Kwon S.B., Jeong J.H., Yang S.J., Sohn U.D., Park K.C. A new 2-imino-1,3-thiazoline derivative, KHG22394, inhibits melanin synthesis in mouse B16 melanoma cells. Biol Pharm Bull. 2007;30:180–183. doi: 10.1248/bpb.30.180. [DOI] [PubMed] [Google Scholar]
- 3.Kim D.S., Kim S.Y., Park S.H., Choi Y.G., Kwon S.B., Kim M.K., Na J.I., Youn S.W., Park K.C. Inhibitory effects of 4-n-butylresorcinol on tyrosinase activity and melanin synthesis. Biol Pharm Bull. 2005;28:2216–2219. doi: 10.1248/bpb.28.2216. [DOI] [PubMed] [Google Scholar]
- 4.Kim H.R., Kim H., Jung B.J., You G.E., Jang S., Chung D.K. Lipoteichoic acid isolated from Lactobacillus plantarum inhibits melanogenesis in B16F10 mouse melanoma cells. Mol Cells. 2015;38:163–170. doi: 10.14348/molcells.2015.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldstein B. Ginseng: its history, dispersion, and folk tradition. Am J Chin Med (Gard City N Y) 1975;3:223–234. doi: 10.1142/s0192415x75000244. [DOI] [PubMed] [Google Scholar]
- 6.Chevallier A. Dorling kindersley pty limited; St Leonards. New South Wales: 1996. Encyclopedia of medicinal plants. [Google Scholar]
- 7.Kim B.M., Kim D.H., Park J.H., Na H.K., Surh Y.J. Ginsenoside Rg3 induces apoptosis of human breast cancer (MDA-MB-231) cells. J Cancer Prev. 2013;18:177–185. doi: 10.15430/JCP.2013.18.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim E.-K., Lee J.-H., Cho S.-H., Shen G.-N., Jin L.-G., Myung C.-S., Oh H.-J., Kim D.-H., Yun J.-D., Roh S.-S. Preparation of black panax ginseng by new methods and its antitumor activity. The Korea Journal of Herbology. 2008;23:85–92. [Google Scholar]
- 9.Saba E., Jeong D.H., Roh S.S., Kim S.H., Kim S.D., Kim H.K., Rhee M.H. Black ginseng-enriched Chong-Myung-Tang extracts improve spatial learning behavior in rats and elicit anti-inflammatory effects in vitro. J Ginseng Res. 2017;41:151–158. doi: 10.1016/j.jgr.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saba E., Kim S.-H., Kim S.-D., Park S.-J., Kwak D.-M., Oh J.-H., Park C.-K., Rhee M.H. Alleviation of diabetic complications by ginsenoside Rg3-enriched red ginseng extract in western diet-fed LDL–/–mice. J Ginseng Res. 2017;42:352–355. doi: 10.1016/j.jgr.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saba E., Son Y., Jeon B.R., Kim S.E., Lee I.K., Yun B.S., Rhee M.H. Acetyl eburicoic acid from laetiporus sulphureus var. miniatus suppresses inflammation in murine macrophage RAW 264.7 cells. Mycobiology. 2015;43:131–136. doi: 10.5941/MYCO.2015.43.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai D., Zhang C.F., Williams S., Yuan C.S., Wang C.Z. Ginseng on cancer: potential role in modulating inflammation-mediated angiogenesis. Am J Chin Med. 2017;45:13–22. doi: 10.1142/S0192415X17500021. [DOI] [PubMed] [Google Scholar]
- 13.Saba E., Jeon B.R., Jeong D.H., Lee K., Goo Y.K., Kim S.H., Sung C.K., Roh S.S., Kim S.D., Kim H.K. Black ginseng extract ameliorates hypercholesterolemia in rats. J Ginseng Res. 2016;40:160–168. doi: 10.1016/j.jgr.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christensen L.P. Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res. 2009;55:1–99. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- 15.Song M., Mun J.H., Ko H.C., Kim B.S., Kim M.B. Korean red ginseng powder in the treatment of melasma: an uncontrolled observational study. J Ginseng Res. 2011;35:170–175. doi: 10.5142/jgr.2011.35.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cabanes J., Chazarra S., Garcia-Carmona F. Kojic acid, a cosmetic skin whitening agent, is a slow-binding inhibitor of catecholase activity of tyrosinase. J Pharm Pharmacol. 1994;46:982–985. doi: 10.1111/j.2042-7158.1994.tb03253.x. [DOI] [PubMed] [Google Scholar]
- 17.Bissett D.L., Hannon D.P., Orr T.V. An animal model of solar-aged skin: histological, physical, and visible changes in UV-irradiated hairless mouse skin. Photochem Photobiol. 1987;46:367–378. doi: 10.1111/j.1751-1097.1987.tb04783.x. [DOI] [PubMed] [Google Scholar]
- 18.Cardiff R.D., Miller C.H., Munn R.J. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb Protoc. 2014;2014:655–658. doi: 10.1101/pdb.prot073411. [DOI] [PubMed] [Google Scholar]
- 19.Chang J.Y., Kessler H.P. Masson trichrome stain helps differentiate myofibroma from smooth muscle lesions in the head and neck region. J Formos Med Assoc. 2008;107:767–773. doi: 10.1016/S0929-6646(08)60189-8. [DOI] [PubMed] [Google Scholar]
- 20.Ohguchi K., Tanaka T., Iliya I., Ito T., Iinuma M., Matsumoto K., Akao Y., Nozawa Y. Gnetol as a potent tyrosinase inhibitor from genus Gnetum. Biosci Biotechnol Biochem. 2003;67:663–665. doi: 10.1271/bbb.67.663. [DOI] [PubMed] [Google Scholar]
- 21.Yokota T., Nishio H., Kubota Y., Mizoguchi M. The inhibitory effect of glabridin from licorice extracts on melanogenesis and inflammation. Pigment Cell Res. 1998;11:355–361. doi: 10.1111/j.1600-0749.1998.tb00494.x. [DOI] [PubMed] [Google Scholar]
- 22.Jablonska-Trypuc A., Matejczyk M., Rosochacki S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J Enzyme Inhib Med Chem. 2016;31:177–183. doi: 10.3109/14756366.2016.1161620. [DOI] [PubMed] [Google Scholar]
- 23.Englaro W., Bertolotto C., Brunet A., Pagès G., Ortonne J.-P., Ballotti R. Inhibition of the mitogen-activated protein kinase pathway triggers B16 melanoma cell differentiation. Journal of Biological Chemistry. 1998;273:9966–9970. doi: 10.1074/jbc.273.16.9966. [DOI] [PubMed] [Google Scholar]
- 24.Tam I., Stepien K. Melanocytes-immunocompetent pigmented cells. Postepy Dermatologii I Alergologii. 2007;24:188. [Google Scholar]
- 25.Lee H.J., Lee W.J., Chang S.E., Lee G.Y. Hesperidin, a popular antioxidant inhibits melanogenesis via erk1/2 mediated MITF degradation. Int J Mol Sci. 2015;16:18384–18395. doi: 10.3390/ijms160818384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu Y.H., Liu X., Jia Y.L., Guo Y.J., Wang Q., Chen Q.X. Inhibitory kinetics of chlorocinnamic acids on mushroom tyrosinase. J Biosci Bioeng. 2014;117:142–146. doi: 10.1016/j.jbiosc.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Masamoto Y., Ando H., Murata Y., Shimoishi Y., Tada M., Takahata K. Mushroom tyrosinase inhibitory activity of esculetin isolated from seeds of Euphorbia lathyris L. Biosci Biotechnol Biochem. 2003;67:631–634. doi: 10.1271/bbb.67.631. [DOI] [PubMed] [Google Scholar]
- 28.Wu M., Hemesath T.J., Takemoto C.M., Horstmann M.A., Wells A.G., Price E.R., Fisher D.Z., Fisher D.E. c-Kit triggers dual phosphorylations, which couple activation and degradation of the essential melanocyte factor Mi. Genes Dev. 2000;14:301–312. [PMC free article] [PubMed] [Google Scholar]
- 29.Tobin D.J. Introduction to skin aging. Journal of Tissue Viability. 2017;26:37–46. doi: 10.1016/j.jtv.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Helfrich Y.R., Sachs D.L., Voorhees J.J. Overview of skin aging and photoaging. Dermatol Nurs. 2008;20:177–183. quiz 184. [PubMed] [Google Scholar]
- 31.Fisher G.J., Kang S., Varani J., Bata-Csorgo Z., Wan Y., Datta S., Voorhees J.J. Mechanisms of photoaging and chronological skin aging. Archives of Dermatology. 2002;138:1462–1470. doi: 10.1001/archderm.138.11.1462. [DOI] [PubMed] [Google Scholar]
- 32.El-Domyati M., Attia S., Saleh F., Brown D., Birk D., Gasparro F., Ahmad H., Uitto J. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Experimental Dermatology. 2002;11:398–405. doi: 10.1034/j.1600-0625.2002.110502.x. [DOI] [PubMed] [Google Scholar]
- 33.Brennan M., Bhatti H., Nerusu K.C., Bhagavathula N., Kang S., Fisher G.J., Varani J., Voorhees J.J. Matrix metalloproteinase-1 is the major collagenolytic enzyme responsible for collagen damage in UV-irradiated human skin. Photochemistry and Photobiology. 2003;78:43–48. doi: 10.1562/0031-8655(2003)078<0043:mmitmc>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 34.Quan T., Qin Z., Xia W., Shao Y., Voorhees J.J., Fisher G.J. Matrix-degrading metalloproteinases in photoaging. J Investig Dermatol Symp Proc. 2009;14:20–24. doi: 10.1038/jidsymp.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gillbro J.M., Olsson M.J. The melanogenesis and mechanisms of skin-lightning agents--existing and new approaches. Int J Cosmet Sci. 2011;33:210–221. doi: 10.1111/j.1468-2494.2010.00616.x. [DOI] [PubMed] [Google Scholar]
- 36.Roméro-Graillet C., Aberdam E., Clément M., Ortonne J.-P., Ballotti R. Nitric oxide produced by ultraviolet-irradiated keratinocytes stimulates melanogenesis. Journal of Clinical Investigation. 1997;99:635. doi: 10.1172/JCI119206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wakabayashi Y., Nakajima H., Imokawa G. Abrogating effect of N-linked carbohydrate modifiers on the stem cell factor and endothelin-1-stimulated epidermal pigmentation in human epidermal equivalents. Journal of Dermatological Science. 2013;69:215–228. doi: 10.1016/j.jdermsci.2012.11.590. [DOI] [PubMed] [Google Scholar]
- 38.Mizoguchi M. Melanocyte development: with a message of encouragement to young women scientists. Pigment Cell Res. 2004;17:533–544. doi: 10.1111/j.1600-0749.2004.00163.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.