Abstract
Uric acid (UA) is the end product of the metabolic breakdown of purine nucleotides. Recent studies have measured UA in saliva in relation to obesity and chronic disease risk. Given the increasing prevalence of metabolic syndrome among Latino youth, we examined gender and age differences in salivary uric acid (sUA) and weight in a sample of Mexican-origin children (n = 65, 2 months to 18 years, 49% female) and adults (n = 46, 19–58 years, 72% female). We measured weight, height, waist, and hip circumference and collected saliva samples (later assayed for sUA). Structural equation models estimated the relationship between age, developmental stage, and weight outcomes in relation to sUA levels between genders, while controlling for race. Results demonstrate that increased sUA levels were related to higher BMI percentiles in females of all ages (β = 0.43, p < .001). There were significant differences in sUA levels between developmental stages for girls, with female toddlers having the highest sUA levels (β = .28, p = .02). In an interaction between BMI z-score and gender between youth and adults, BMI has a larger effect on increasing sUA levels among younger girls (β = 0.27, p < .03) and adult women (β = 0.33, p = .02). Levels of sUA may be gender-specific in relation to BMI and developmental stage.
Keywords: Hispanics/Latinos, infants and children, body mass index, gender, salivary uric acid
1 |. INTRODUCTION
Uric acid (UA) is proving to be a valuable biomarker for hypertension and metabolic syndrome (De Oliveira & Burini, 2012). UA is an end product of dietary and endogenous metabolic breakdown of purine nucleotides (Voruganti et al., 2009). At higher levels (e.g., hyperuricemia) the probability increases that uric acid crystals may form in the joints, leading to acute gout, or in the urinary tract, leading to kidney stones. High levels of UA may be caused by high dietary intake of high fructose corn syrup and table sugar, fasting, or rapid weight loss, and/or reduced excretion from the kidneys (see Mueller, Kasl, Brooks, & Cobb, 1970 for review). Also, higher levels of UA can be indicative of oxidative stress (Baldree & Stapleton, 1997; De Oliveira & Burini, 2012; De Oliveira, Moreto, Silveira, & Burini, 2013; Ishizaka, Yamakado, Toda, Tani, & Ishizaka, 2014), which among other effects potentially reduces telomere length, accelerating cellular aging (Epel, 2009). There is also evidence to suggest that acute and chronic psychosocial stressors are related to increased UA levels (Acheson, 1969; Aschbacher et al., 2013; Guney et al., 2014; Kasl, Gore, & Cobb, 1975; Mueller et al., 1970; Thomas, Goodwin, & Goodwin, 1985). Researchers and clinicians should take interest monitoring UA levels in youth because evidence suggests that it is a suitable predictor of individual differences in cardiovascular disease risks in adolescence and adulthood (Jones, Richey, Alpert, & Li, 2008; Mellen et al., 2006; Wang, Chen, Hsu, Tang, Wu, & Pei, 2012).
Numerous studies link individual differences in circulating UA to higher blood pressure, triglyceride levels, high-density lipoprotein, fasting blood glucose levels, and body mass index (BMI) (Ford, Li, Cook, & Choi, 2007; Hayden & Tyagi, 2004). Other research suggests that UA secretion has a developmental pattern, with levels increasing from adolescence to adulthood (Gillum, 1987; Harlan, Cornoni-Hartley, Leaverton, 1979). Studies suggest the increase in UA during the transition to adulthood may be explained by the presence of pubertal hormones, vary by gender, and is associated with body weight (Feig, Kang, & Johnson, 2008; Garbagnati, 1996; Garbagnati & Boschetti, 1994). There are very few studies examining UA among children younger than 4 years old (with the exception of Grivna, Pruša, & Janda, 1997) and much less with racial/ethnic minorities in the United States (US).
There are few studies reporting UA levels in Latino adults and children (with the exception of Voruganti et al., 2009 and Modino et al., 2012). Yet, according to the NHANES 2011–12 data, the prevalence of obesity among Mexican children ages 2–11 was the highest at 20.9%, in comparison to Whites (13.3%) and Blacks (18.8%) (Kaur, Lamb, & Ogden, 2015). Similarly, Latino adults have a higher prevalence of obesity than non-Hispanic Whites (Flegal, Carroll, Ogden, & Curtin, 2010). Latino adolescents have a higher prevalence of metabolic syndrome in comparison to other racial and ethnic groups in the US population (Cook et al., 2003; Sun, Pei, Lue, & Chen, 2015). Having more adiposity and higher levels of uric acid in youth is associated with metabolic syndrome in children and adolescents (Ford et al., 2007; Tailor et al., 2010).
The use of salivary biomarkers in biobehavioral health research, and among such studies that include predominantly minority participants in particular, is attracting increasing attention because the collection of saliva is perceived as minimally invasive, and may be more culturally acceptable for some, than traditional biospecimens (i.e., blood, urine, or tissue samples; Gorodischer, Burtin, Hwang, Levine, & Koren, 1994). The measurement of UA in saliva is a relatively new development in salivary bioscience. Studies report a strong positive association between UA measured in serum and in saliva (Riis et al., under review; Zhao & Huang, 2015), raising the possibility that sUA has the potential to be a promising biomarker to examine the relationship between psychosocial and oxidative stress, and to identify chronic disease risk in children. To the best of our knowledge, however, we have yet to learn whether patterns of uric acid are the same in different age groups and in a racially diverse group, such as Mexican infants, children, and their parents. Studies investigating the relationship between gender, BMI, and uric acid have not been conducted on Latino children younger than 6 years.
1.1 |. Present study
This is the first study (to the best of our knowledge) to examine sUA in Mexican-origin youth. Aims were to: 1) explore how sUA levels differ among Mexican-origin children, at different developmental stages between the ages of 2 months to 18 years, and their adult parents; and 2) examine relationships between sUA and BMI percentile between females and males. By developmental stage we are referring to different periods of infancy, childhood, and adolescence where children will have varying levels of dietary and behavioral independence.
2 |. METHODS
2.1 |. Study design and recruitment
This was a cross-sectional, exploratory study. Participants were Latino families with at least one immigrant parent. The research team collected anthropometric, demographic, psychosocial, and salivary analyte data. Data collection took place in Phoenix, AZ, and all of the families that agreed to participate were Mexican-origin. Cluster probability sampling was employed, in which we conducted a simple random selection of 30 census tracts with a high location quotient of foreign-born Hispanics/Latinos between 1.8 and 3.6 (range 0.4–3.6). Although we did not use a formal registry of Hispanic/Latino families in the city of Phoenix, there is an estimated 6,775 Hispanic/Latino households with at least one Hispanic or Latino householder in the 10 census tracts from which we recruited our families (U.S. Census Bureau, 2014). The research team identified potential families by going door-to-door in these randomly selected census tracts. We used a Spanish recruitment script that briefly described the purpose and procedures of the study.
Families were able to participate in the study if they self-identified as Hispanic/Latino and had at least one parent who was an immigrant from a Spanish-speaking, Latin American country. We excluded families from participating if the head of household was under the age of 18 years, or if the head of household was incapable of providing consent for themselves or their children. Following recommendations by Granger et al. (2012), we excluded families who had one family member that: just visited the dentist in the last 24 hr; smoked or chewed tobacco; had open mouth sores or abrasions; was ill with an acute condition or chronic disease; had a fever; or was taking any medication, including NSAIDs, hormonal contraceptives, or beta-blockers (Granger, Hibel, Fortunato, & Kapelewski, 2009).
Upon the head of household and individual family members consenting to participate, we gave each family a $50 gift card. Children under the age of six provided an oral assent and children above the age of six provided a written assent form. The families also chose a pseudonym for which we used to communicate with them via phone. The university institutional review board approved this study.
2.2 |. Saliva collection and assay
We collected 1–1.8 ml of whole unstimulated saliva from each family member (Granger et al., 2007). Participants could not have eaten food, brushed their teeth, or drank liquids, other than water, within an hour of providing sample (Granger et al., 2012). Children over the age of five provided whole saliva using the passive drool technique, while children under the age of six sat on their parent’s lap while the research team held a saliva child swab in their mouth for 3 min (Granger et al., 2007). The saliva was expressed from the swab with a needleless 5 cc syringe into a 2.0 ml cryovial on site in an effort to ensure sufficient sample volumes were donated.
After providing saliva, samples were immediately stored and transported on dry ice. At the end of each day, participants’ de-identified saliva samples were transported to the Institute for Interdisciplinary Salivary Bioscience Research (IISBR) where they were frozen at −80 °C until the day of assay.
Saliva samples were assayed in duplicate for sUA using a commercially available enzymatic reaction kit specifically designed for use with saliva (Salimetrics, Carlsbad, CA). The assay used 10 μl test volume, had a range of sensitivity from 0.78 to 25 mg/dl, average inter- and intra-assay coefficients of variation were less than 10% and 5%. The average of the duplicate tests were used in the descriptive analyses and for the structural equation models.
2.3 |. Anthropometric and survey measures
After collecting the saliva samples, each family member’s weight (SECA 876 Portable Scale) and height (SECA Portable Stadiometer) were determined. We also measured the smallest part of their waist and the widest part of their hips with a Gulick II measuring tape. Adults and children age 2 or older were measured for height standing, while children under the age of two were measured recumbent for length. The weight of children under the age of 2 was taken by having one of the parents be weighed first, in order to tare the scale, and then the parent was weighed again with the child in their arms. The difference between the parent’s weight from the child’s was shown on the display of the SECA scale. All measures were recorded in metric units. The participants took off their shoes and heavier clothing and only had form-fitting clothing when we weighed and measured them. Anthropometric data were entered on site into a secure online database.
We collected weight, height, or length, in the case of infants, in order to determine each participant’s body mass index (BMI) percentile. BMI percentiles were used in place of BMI scores because children have fluctuating body weights as a result of their growth (Lindkvist, Ivarsson, Silfverdal, & Eurenius, 2015). BMI percentiles were determined for infants by entering their weight and length into an online calculator (Medscape, 2011) based on age-sex-specific Centers for Disease Control and Prevention (CDC) Growth Charts (Kuczmarski et al., 2002). We determined the BMI percentile of youth between the ages of 2 and 20 by using a specific Stata program calculating age-sex-specific BMI percentiles based on the 2000 CDC Growth Charts (Vidmar, Carlin, Hesketh, & Cole, 2004). We determined sex-age-specific BMI percentiles for each adult by entering his/her age, gender, weight and height into an online calculator that also used the 2000 CDC/NCHS Data as the reference population.
After collecting the saliva samples and anthropometric measures of each family member, we administered a survey to the head of household in the language of their choice (English/Spanish). We used a tablet-based, interviewer-administered survey that included validated psychosocial scales (evaluating the head of household’s psychosocial health) and demographic items on family composition, housing status, language use in various settings, the self-rated health of the head of household and their family’s health, and the health insurance status of all family members. We also queried the head of household’s current employment status, education, and literacy. The home visit took approximately 2 hr.
In order to assess the family’s ancestry we first asked the head of household their country of origin. If the head of household was US-born then we also asked for the immigrant family member’s country of origin. We augmented the race item on the survey to include the four US Office of Management and Budget categories (i.e., White, non-Hispanic, Black non-Hispanic, etc.) and the Latin American caste system categories, which takes into consideration racial miscegenation. White and Black racial mixture persons self-identify as mulatto, those mixed with White and Indian identify as mestizo, those that were mixed with Indian and African descent identified as zambo, and those that identified as African descent were moreno.
2.4 |. Statistical methods
Since this was the first study (to our knowledge) examining the relationship between anthropometric measures and sUA in Mexican-origin children, we lacked pre-existing data on standard deviations, means and standard errors in youth’s sUA levels to calculate power and estimate a required sample size. Nonetheless, methodologists (Anderson & Gerbing, 1984; Iacobucci, 2010) suggest a sample size of at least 100 for convergence in a structural equation model, where we had a total of 111 participants. Kreft and De Leeuw (1998) suggest that the second-level group sizes of multilevel models (in this case, family) should be no lower than 25—we had 30 families.
Our preliminary analyses included bivariate analysis of sUA with the demographic and anthropometric variables (See Table 3). We noticed that sUA differed by developmental stage: 0–2 (infancy), 3–5 (early childhood), 6–12 (middle childhood), 13–18 (adolescence), and 19+ years (adulthood) (see Figure 1). Our subsequent sUA models analyzed developmental stage and age separately. For development stages, we created a dummy variable to examine its relationship to sUA, while controlling for BMI percentile, gender, and race.
TABLE 3.
BMI percentile categories for Mexican-origin children and adults, N = 102 This table reports BMI percentile categories for Mexican-origin children and adults in this study.
| All youth (2–20) | Boys | Girls | Adults (21+) | Men | Women | Total | |
|---|---|---|---|---|---|---|---|
| BMI percentile categories (N[%]) | |||||||
| Normal weight (<85th) | 38 (63.3%) | 20 (68.9%) | 18 (58.1%) | 30 (68.1%) | 11 (84.6%) | 19 (63.6%) | 68 (65.4%) |
| Overweight (85th–94th) | 10 (16.7%) | 3 (10.3%) | 7 (22.6%) | 5 (11.4%) | 1 (7.7%) | 4 (12.1%) | 15 (14.4%) |
| Obese (≥95th) | 12 (20.0%) | 6 (20.7%) | 6 (19.4%) | 9 (20.5%) | 1 (7.7%) | 8 (24.2%) | 21 (20.2%) |
| Total | N = 60 | N = 29 | N = 31 | N = 44 | N = 13 | N = 31 | |
| 57.7% | 27.9% | 29.8% | 42.3% | 12.5% | 29.8% |
N = 102, no infants included one girl and one adult male missing.
FIGURE 1.
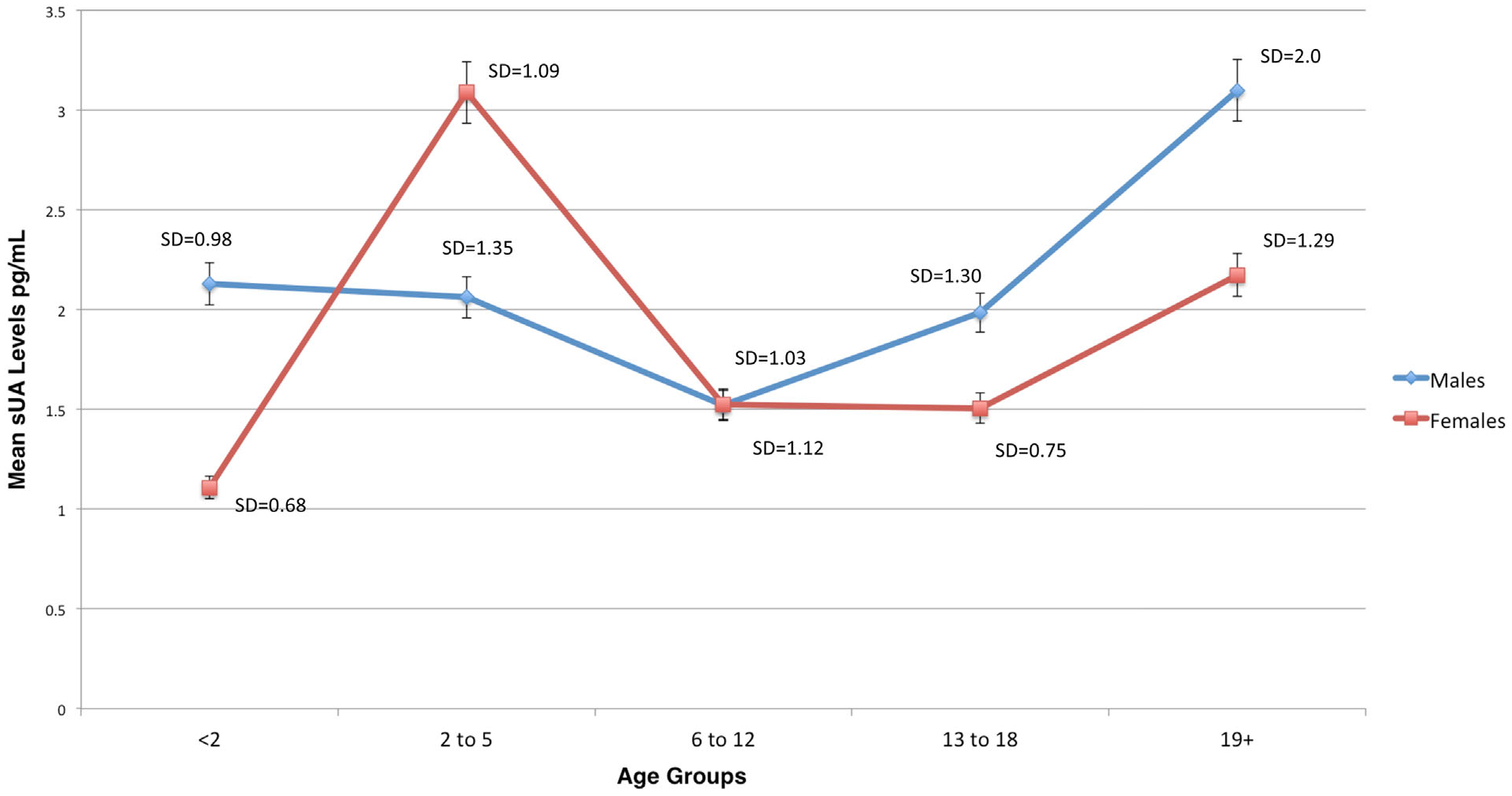
Mean salivary uric acid (sUA) levels (mg/ml) by age group among female and male Mexican-origin youth and adults in Phoenix, AZ
To explore patterns of sUA among Mexican-origin children and their parents, we estimated a comprehensive structural equation model (SEM) with multiple factors (e.g., age, BMI percentile, gender, race, waist-to-hip circumference) that are associated with levels of sUA for all individuals and in a two-group model to examine gender differences. We excluded children younger than 2 years from the SEM models because 1) there were few in the sample (n = 7), 2) they were measured recumbent, unlike the other participants who were standing, and 3) their BMI percentiles are based on a different growth grid that is not used in the Stata function that transforms youth’s anthropometric data to BMI categories, percentiles and z-scores.
The models were estimated using full information maximum likelihood (Enders, 2010) and checking the fit between the model and observed data was acceptable (i.e., CFI/TLI was greater than0.95 [0.90], RMSEA was less than .05 [.08], and SRMR was less than [.05]).
3 |. RESULTS
3.1 |. Sample characteristics
The sample (N = 111) consisted of 30 Mexican-origin, low-income families where 53.5% reported an annual family income of <$20,000/year. The typical family size was three to four persons, with the largest family having eight persons. The average number of children in each home was two to three, with a maximum of six. Although these families were typically headed by a single-mother, half lived with a male partner, who was often not the biological father of the child(ren). The majority (36.7%) of families identified as mestizo, zambo (23.3%), and moreno (16.7%). We excluded the Black racial category from the models because only one person self-identified as Black (Table 1).
TABLE 1.
Socio-demographic family characteristics
| Families, N = 30 | |
|---|---|
| Family size (R, [M]) | 2–8 persons (4.2) |
| Number of children | 1–6 children (2.3) |
| Years in USA (M ± SD) | 10.69 ± 7.49 |
| Years in phoenix | 9.91 ± 6.75 |
| Race | |
| White | 7 (23.3%) |
| Moreno (black, hispanic-origin) | 5 (16.7%) |
| Mestizo (indian and white) | 11 (36.7%) |
| Zambo (black and indian) | 7 (23.3%) |
| Marital statusa | |
| Married | 16 |
| Living w/ partner | 10 |
| Divorced/separated | 4 |
| Annual family income | |
| <$20,000 | 16 (53.5%) |
| $20,000–$34,999 | 11 (36.5%) |
| $35,000–$49,999 | 3 (10.0%) |
| Home ownership | |
| Rent | 28 (93.3%) |
| Own | 2 (6.7%) |
Head of household.
Our sample included 65 females and 46 males. There were 65 youth (ages 2 months to 17 years) in this sample. Most children were in the middle childhood developmental stage (N = 24 children, 12 females). Female adults had the highest BMIs (32.51 kg/cm2). There was very little variance in waist to hip circumference (0.97 ± .04 cm2) so, this variable was not used in estimating the structural equation models. Since mean sUA levels were positively skewed, we used the natural log transformation for statistics where a normal distribution was needed (See Table 2). A little over a third of the sample (34.6%) was overweight/obese with BMIs at or above the 85th percentile, with 24% of those in the overweight/obese categories being female (see Table 3).
TABLE 2.
Anthropometric measures and salivary uric acid (sUA) levels for Mexican-origin children and adults by developmental stage
| Infants | Early childhood | Middle childhood | Adolescence | Adult | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <2 years | 2–5 years | 6–12 years | 13–18 years | 19+ years | ||||||
| Gender | M | F | M | F | M | F | M | F | M | F |
| N = 111 | 4 | 3 | 9 | 12 | 12 | 12 | 8 | 5 | 12 | 33 |
| BMI Percentileab (M ± SD) (Range) | 0.43 ± 0.38 (0.13–0.99) | 0.82 ± 0.23 (0.55–0.97) | 0.55 ± 0.36 (0.02–0.95) | 0.73 ± 0.33 (0.03–0.99) | 0.71 ± 0.32 (0.04–0.99) | 0.71 ± 0.21 (0.39–0.99) | 0.66 ± 0.17 (0.45–0.99) | 0.59 ± 0.41 (0.05–0.94) | 0.70 ± 0.20 (0.23–0.93) | 0.71 ± 0.23 (0.19–0.99) |
| Waist:Hip (cm) (M ± SD) (Range) | 0.99 ± 0.04 (.932–1.02) | 0.96 ± 0.03 (.927–0.98) | 0.95 ± 0.05 (0.89–1.03) | 0.95 ± 0.06 (0.86–1.06) | 0.92 ± 0.07 (0.79–1.02) | 0.87 ± 0.05 (0.79–0.99) | 0.91 ± 0.07 (0.79–0.98) | 0.86 ± 0.04 (0.81–0.90) | 0.97 ± 0.04 (0.92–1.06) | 0.87 ± 0.06 (0.69–1.02) |
| sUA (mg/ml) (M ± SD) (Range) | 2.12 ± 0.98 (1.31–3.51) | 1.11 ± 0.68 (0.46–1.82) | 2.06 ± 1.35 (0.70–5.09) | 3.09 ± 1.09 (1.18–5.34) | 1.52 ± 1.03 (0.37–4.04) | 1.53 ± 1.12 (0.35–4.38) | 1.98 ± 1.30 (0.096–3.81) | 1.51 ± 0.75 (0.80–2.72) | 3.10 ± 2.00 (0.81–7.86) | 2.17 ± 1.29 (0.41–5.45) |
BMI, body mass index; M, male; F, female. Descriptive statistics calculated in Stata 12.1.
CDC/NCHS age-sex-specific growth charts youth 2–20 years of age, Available online at: http://www.cdc.gov/nccdphp/dnpa/growthcharts/training/modules/module2/text/module2print.pdf
Adult BMI percentiles based on NHANES 2007–2008 data, Available online at: http://198.246.102.49/nchs/data/hestat/obesity_adult_07_08/obesity_adult_07_08.pdf
Examining Figure 1, the highest mean sUA levels were detected in female toddlers (3.09 mg/dl) and adult males (3.10 mg/dl). In this sample, males in infant and early childhood stage have similar mean sUA levels (2.12 and 2.06 mg/dl, respectively), but males in middle childhood have the lowest levels (1.52 mg/dl). In contrast, females in middle childhood and adolescence have similar mean sUA levels. There were only significant gender differences in mean sUA levels among males and females in early childhood, ages 2–5 years (t = −2.19, p < .05, assuming unequal variance).
Log mean sUA levels were positively correlated to BMI percentiles (ρ = 0.22, p < .05) and those identifying as zambo (ρ = 0.18, p < .0.5). There was an inverse relationship between log mean sUA levels and identifying as mestizo (ρ = −.17, p < .05). Mestizos have lower log mean sUA levels than those who identified as zambo. BMI percentiles were positively correlated with age (ρ = 0.75, p < .05) and developmental stage (ρ = 0.78, p < .05) (See Table 4).
TABLE 4.
Correlations between natural log of mean sUA levels with study covariates, n = 110
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| 1. LsUA | — | ||||
| 2. BMI percentile | 0.22* | — | |||
| 3. Age | 0.15 | 0.75** | — | ||
| 4. Developmental stagea | 0.07 | 0.78** | 0.95** | — | |
| 5. Gendera | 0.02 | 0.12 | 0.15 | 0.20** | — |
| 6. Blacka | −0.14 | −0.04 | 0.03 | 0.10 | 0.08 |
| 7. Mestizoa | −0.17* | 0.01 | −0.11 | −0.12 | −0.05 |
| 8. Morenoa | 0.05 | 0.01 | 0.05 | 0.03 | −0.15 |
| 9. Zamboa | 0.18* | −0.03 | −0.09 | −0.10 | 0.03 |
L, natural log transformed variable.
Spearman rank correlation.
p < 0.10,
p ≤ 0.05.
In the comprehensive structural equation model, with all participants, we find that log mean sUA levels are higher among those with weights at higher BMI percentiles (β = 0.28, p < .01) (See Table 5, Model 1), while controlling for age, gender, and racial caste. Noting the gender differences in mean sUA levels, we estimated whether the relationship between BMI percentile and log mean sUA levels remained significant between females and males. While examining gender separately, females with weights at higher BMI percentiles had higher mean log sUA levels (β = 0.43, p < .01). For females, for each increase in BMI percentile there is an increase of 1.54 mg/ml in mean sUA levels. Older males had significantly higher mean log sUA levels (β = 0.57, p < .04). For males, with each increase in age there is a 1.76 mg/dl increase in mean sUA levels (See Table 5, Model 2).
TABLE 5.
Structural equation models examining the relationship between BMI, age, developmental stage, and race in relation to log mean salivary uric acid (sUA)a
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 104 | Males, N = 42 | Females, N = 61 | N = 104 | Males, N = 42 | Females, N = 61 | Youth (<21), N = 60 | Adults (21+), N = 44 | |||||||||||||||||
| β | SE | p | β | SE | p | β | SE | p | β | SE | p | β | SE | p | β | SE | p | β | SE | p | β | SE | p | |
| BMI percentile | 0.28 | 0.09 | .002 | 0.03 | 0.15 | 0.82 | 0.43 | 0.08 | 0.01 | 0.30 | 0.09 | .001 | 0.13 | 0.15 | 0.40 | 0.43 | 0.09 | .001 | — | — | — | — | — | — |
| BMI z-score | — | — | — | — | — | — | — | — | — | — | — | — | — | 0.23 | 0.12 | 0.01 | 0.36 | 0.10 | 0.01 | |||||
| Genderb | 0.03 | 0.09 | 0.80 | −.02 | 0.09 | 0.82 | — | — | — | — | — | — | 0.11 | 0.12 | 0.39 | −.29 | 0.13 | 0.03 | ||||||
| Age | 0.13 | 0.09 | 0.17 | 0.57 | 0.11 | 0.04 | 0.29 | 0.12 | 0.19 | — | — | — | — | — | — | — | — | — | −.27 | 0.12 | 0.03 | 0.09 | 0.14 | 0.53 |
| Developmental stage | ||||||||||||||||||||||||
| Early (2–5) childhood | — | — | — | 0.37 | 0.32 | .001 | −.17 | 0.13 | 0.20 | 0.28 | 0.10 | 0.02 | ||||||||||||
| Middle (6–12) childhood | — | — | — | −.38 | 0.11 | .001 | −.24 | 0.19 | 0.21 | −.46 | 0.12 | 0.01 | ||||||||||||
| Adolescence (13–18) | — | — | — | −.29 | 0.10 | .005 | −.26 | 0.18 | 0.15 | −.19 | 0.12 | 0.10 | ||||||||||||
| Adult (19+) | — | — | — | −.14 | −.12 | 0.23 | 0.05 | 0.20 | 0.79 | −.32 | 0.13 | 0.02 | ||||||||||||
| Mestizo | −.16 | 0.10 | 0.15 | −.21 | 0.10 | 0.04 | −.28 | 0.19 | 0.13 | −.11 | 0.11 | 0.33 | ||||||||||||
| Moreno | 0.05 | 0.11 | 0.69 | −.01 | 0.11 | 0.94 | −.16 | 0.20 | 0.45 | 0.20 | 0.12 | 0.09 | ||||||||||||
| Zambo | 0.18 | 0.10 | 0.11 | 0.12 | 0.11 | 0.26 | −.02 | 0.19 | 0.90 | 0.25 | 0.12 | 0.03 | ||||||||||||
| R2 = 0.15 | R2 = 0.09 | R2 = 0.21 | R2 = 0.25 | R2 = 0.175 | R2 = 0.45 | R2 = 0.14 | R2 = 0.19 | |||||||||||||||||
| RV = 0.85** | RV = 0.91** | RV = 0.80** | RV = 0.75** | RV = 0.83** | RV = 0.55** | RV = 0.86** | RV = 0.81** | |||||||||||||||||
RV, residual variance
Consecutive dashes (—) indicate the exclusion of a variable in a model. Infants (<2 years old) not included in these analyses.
Model 1: Comprehensive model examining age-gender-specific BMI percentile, developmental stage, and race in relation to log mean sUA levels.
Model 2: Parsimonious model examining age-gender-specific BMI percentile and age in relation to sUA log mean sUA levels between males and females.
Model 3: Model examining age-gender-specific BMI percentile, developmental stage, and race in relation to log mean sUA levels.
Model 4: Model examining age-gender-specific BMI percentile, developmental stage, and race in relation to sUA between males and females
Model 5: Parsimonious model examining age-gender-specific BMI z-score, Gender, and Age in relation to log mean sUA levels between youth (<21 years) and adults (21+).
Standardized results reported from MPlus 7.2.
0, male; 1, female.
p ≤ 0.01.
Acknowledging differences between developmental stages in the bivariate analysis, we examined differences in log mean sUA levels in relationship to developmental stages and BMI percentiles. We found that the relationship between BMI percentile on log mean sUA remained strong (β = 0.30, p < .001) in the presence of developmental stages and racial caste categories. Also, belonging to the early childhood stage was related to higher mean log sUA levels (β = 0.37, p < .01), in relationship to the other developmental stages, in which lower log mean sUA levels was related to belonging to the middle childhood (β = −.38, p < .001) or the adolescent (β = −.29, p < .001) stage. (Table 5, Model 3). However, in the bivariate analysis, the highest levels of mean sUA levels were not only seen in females in the early childhood developmental stage, but also adult males. So, in the proceeding model we examined differences in log mean sUA levels in relationship to developmental stages and BMI percentile between males and females.
Examining the relationship between BMI percentile, developmental stage, and race on log mean sUA levels by gender (Table 5, Model 4), BMI percentile remained significantly related to increased log mean sUA levels in females (β = 0.43, p < .01), but lost its effect on males’ log mean sUA levels (β = 0.13, p = 0.40). By splitting the sample by gender we also found that females who identified as zambo had significantly higher log mean sUA levels (β = 0.25, p = .03), in comparison to other racial castes. By splitting the sample by gender, we found developmental differences in females only. There were significant differences in mean log sUA levels between females in each development stage, where sUA levels appear to be significantly lower for youth in middle childhood (β = −.46, p = .01), in comparison to girls in early childhood (95% CI: −1.14 to −.34). Among females, BMI percentile, developmental stage and race accounted for 45% of the variance in log mean sUA levels (RV = 0.55) (Table 5, Model 4).
In the end, we wanted to discern which variable, weight, age, or gender, is most related to levels of sUA in Mexican-origin youth and adults. The final model (Table 5, Model 5) examines the relationship between age, BMI z-scores, gender and log mean sUA levels between youth and adults. Results demonstrate that in both youth and adults, BMI is significantly related to increases in log mean sUA levels. In youth, for every increase in BMI z-score, there is an expected increase of 1.26 mg/ml of sUA (95% CI: −.497 to −.030). In adults, for every increase in BMI z-score, there is an expected increase of 1.43 mg/dl of sUA (95% CI: 0.103–0.62). Among youth, we found younger age (β = −.27, p = .03) was significantly related to log mean sUA levels. The older youth in this sample were, the lower their log mean sUA levels, where a decrease of 0.96 mg/ml in mean sUA levels (95% CI: .002–0.46) was estimated for each year of age. Lastly, among adults being male was strongly related to higher log mean sUA levels (β = −.29, p = .03, 95% CI: −.79 to −.27), which is consistent with our bivariate analysis (See Figure 1).
Given these results, an interaction effect was created to examine if gender modified the effect of BMI on sUA in youth and adults, while controlling for age. An interaction of BMI z-scores with gender, between youth and adults, is consistent with our observations in the previous models that the effect of higher BMI on increasing sUA levels differs between genders and among youth in this sample. BMI matters more in increasing sUA levels among younger girls (βintx = 0.27, βage = −.26 p < .03) (95% CI: .04–0.49) and adult women (βintx = 0.33, p = .02) (CI: 95% CI: .07–0.60) (results omitted from Table 5.).
4 |. DISCUSSION
In this study, we found that there were significant differences in sUA levels in relation to developmental stages among female youth, with sUA levels being lower for girls in middle childhood and adolescence, even after controlling for BMI percentile and race. We also found that larger body mass is positively related to sUA levels in youth and adults, particularly among females. Similar to serum uric acid, sUA levels appear to increase between adolescence and adulthood among both females and males, but with males having higher increases with age. In previous studies with adolescent and adult males, increased serum uric acid has been attributed to androgens and higher BMI (Feig et al., 2008; Garbagnati, 1996; Garbagnati & Boschetti, 1994).
Another distinct finding in our study was discovering that the highest sUA levels in this sample were detected among females in early childhood (ages 2–5). Other studies (Suzuki et al., 2012; Tanaka et al., 2015) have identified the gender-specificity of serum uric acid in adult populations, but few among youth. One possible explanation for the high sUA levels detected in girls in early childhood (ages 2–5) is that they had significantly higher BMI percentiles than their male counterparts. Yet, both males and females in early childhood had higher sUA levels than those in middle childhood and adolescence. Specifically, sUA levels were significantly lower for girls in older childhood developmental stages. Since uric acid is also a protective antioxidant, children in the early childhood developmental stage may have more UA to protect against infections (Grivna et al., 1997).
We did find a significant relationship between sUA levels and identifying with one of the Latin American racial caste categories, particularly zambo females. Some research reports that there are higher levels of serum uric acid in older racial and ethnic minorities (age 45–80) in the US, than among Whites (Alderman, Cohen, Madhavan, & Kivlighn, 1999). However, we would need a larger, more diverse sample to detect differences in individual sUA levels between Mexicans and Latinos that self-identify with other US or Latin American racial categories. Further studies will be needed to determine whether higher sUA levels are also detected in very young children belonging to other racial/ethnic groups and in other countries.
4.1 |. Limitations and future directions
Future studies of sUA would benefit if they had comparable numbers of females and males in the sample, a larger sample size for each developmental stage, and multiple time points of saliva collection in order to conduct a moderated mediation analysis of developmental stage predicting body mass, and in turn, sUA levels by gender to identify patterns of sUA. Having a small sample size limits the generalizability of these results and increases the likelihood of committing a Type II error.
It is well known that absorbent materials used to collect saliva have the potential to influence the concentration of some salivary analytes (see Granger et al., 2012 for review). This fact raises the possibility that the developmental difference observed here in sUA levels is an artifact of the different sample collection methods used with children (absorbent swab) and adults (passive drool). Fortunately, we have confirmed that the two methods used to collect saliva in the present study do not differentially affect sUA levels.
Flow rates were not collected to establish sUA concentration, but in a study just submitted for publication elsewhere, Riis et al. (unpublished) measured total protein in saliva (an indirect measure of flow rate). The serum-saliva association for UA was unchanged after covariation of total protein. Salivary flow rate does not appear to explain a significant portion of the variance in sUA levels. Therefore, we have confidence that the interpretation of the age-related differences here are not likely to be due to differential collection methods. Individual serum UA levels increase after the consumption of purine-rich proteins and fructose (Choi et al., 2005; Choi, Ford, Gao, & Choi, 2008; Viazzi, Genovesi, Ambruzzi, & Giussani, 2015), and decreases with the consumption of dairy in adults (Choi et al., 2005) and breast milk in infants (Kuchan, Ostrom, Smith, & Hu, 2000). Given the strong association between serum and salivary UA levels, it is possible that sUA levels are also influenced by diet. Logistical and practical constraints coordinating home visits with this group of participants made it impossible to expect that families in the study could fast before saliva collection. Thus, one alternative explanation for the differences observed here in sUA levels might be related to differential diet. Incorporating diet quality data, or requiring participants to fast, or both in future studies of sUA would seem well worthwhile.
4.2 |. Concluding remarks
This study makes three important contributions. To date, there are few studies examining uric acid among very young, US Mexican children. Latinos are the fastest growing ethnic group in the US population, yet most US epidemiological studies continue to compare uric acid differences between African Americans and Whites (Alper et al., 2005; Gillum, 1987). We find developmental differences in sUA levels in females in comparison to males. Unlike previous studies (Baldree & Stapleton, 1997; Epel, 2009; Lu et al., 2012) we found that BMI percentile or BMI is related to sUA levels, but more so in females. Taken together, these preliminary findings underscore the potential utility of sUA as an index of chronic disease risk in studies of obesity, metabolic syndrome, and cardiovascular disease among Mexican-origin youth and adults.
ACKNOWLEDGMENTS
We would like to the manuscript reviewers for their valuable feedback. We would like to thank the laboratory staff at the Institute for Interdisciplinary Salivary Bioscience Research for their assistance on this project. We would like to thank Elizabeth Reifsnider for her advice on BMI percentiles in infants, children and adults. Lastly, we would also like to thank Supriya Gaitonde at Salimetrics for her assistance with our questions regarding sUA assays and saliva collection. This project received funding from the ASU College of Liberal Arts and Sciences Seed Funding Mechanism and a Program for Transborder Communities seed grant. We would like to thank the laboratory staff at the Institute for Interdisciplinary Salivary Bioscience Research. We also would like to thank the families who participated in this study.
Funding information
ASU College of Liberal Arts and Sciences Seed Funding Mechanism; Program for Transborder Communities Seed Grant
Footnotes
DISCLOSURES
In the interest of full disclosure, DAG is founder and chief scientific and strategy advisor at Salimetrics LLC and SalivaBio LLC and these relationships are managed by the policies of the committees on conflict of interest at the Johns Hopkins University School of Medicine and the University of California at Irvine.
REFERENCES
- Acheson RM (1969). Social class gradients and serum uric acid in males and females. British Medical Journal, 4, 65–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JC, & Gerbing DW (1984). The effect of sampling error on convergence, improper solutions, and goodness-of-fit indices for maximum likelihood confirmatory factor analysis. Psychometrika, 49, 155–173. [Google Scholar]
- Aschbacher K, O’Donovan A, Wolkowitz OM, Dhabhar FS, Su Y, & Epel ES (2013). Good stress, bad stress and oxidative stress: Insights from anticipatory cortisol reactivity. Psychoneuroendocrinology, 38, 1698–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderman MH, Cohen H, Madhavan S, & Kivlighn S (1999). Serum uric acid and cardiovascular events in successfully treated hypertensive patients. Hypertension, 34, 144–150. [DOI] [PubMed] [Google Scholar]
- Alper AB, Chen W, Yau L, Srinivasan SR, Berenson GS, & Hamm LL (2005). Childhood uric acid predicts adult blood pressure: The Bogalusa Heart Study. Hypertension, 45, 34–48. [DOI] [PubMed] [Google Scholar]
- Baldree LA, & Stapleton FB (1997). Uric acid metabolism in children. Pediatric Clinics of North America, 37, 391–418. [DOI] [PubMed] [Google Scholar]
- Choi JWJ, Ford ES, Gao X, & Choi HK (2008). Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: The third national health and nutrition examination survey. Arthritis Care & Research, 59, 109–116. [DOI] [PubMed] [Google Scholar]
- Choi HK, Liu S, & Curhan G (2005). Intake of purine-rich foods, protein, and dairy products and relationship to serum levels of uric acid: The Third National Health and Nutrition Examination Survey. Arthritis & Rheumatism, 52, 283–289. [DOI] [PubMed] [Google Scholar]
- Cook S, Weitzman M, Auinger P, Nguyen M, & Dietz WH (2003). Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988–1994. Archives of Pediatrics & Adolescent Medicine, 157, 821–827. [DOI] [PubMed] [Google Scholar]
- De Oliveira EP, & Burini RC (2012). High plasma uric acid concentration: Causes and consequences. Diabetology and Metabolic Syndrome, 4, 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Oliveira EP, Moreto F, Silveira LV, & Burini RC (2013). Dietary, anthropometric, and biochemical determinants of uric acid in free-living adults. Nutrition Journal, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders CK (2010). Applied missing data analysis. New York: Guilford Press. [Google Scholar]
- Epel ES (2009). Psychological and metabolic stress: A recipe for accelerated cellular aging? Hormones, 8, 7–22. [DOI] [PubMed] [Google Scholar]
- Feig DI, Kang D, & Johnson RJ (2008). Uric acid and cardiovascular risk. The New England Journal of Medicine, 359, 1811–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, & Curtin LR (2010). Prevalence and trends in obesity among US adults, 1999–2008. Journal of the American Medical Association, 303, 235–241. [DOI] [PubMed] [Google Scholar]
- Ford ES, Li C, Cook S, & Choi HK (2007). Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation, 115, 2526–2532. [DOI] [PubMed] [Google Scholar]
- Garbagnati E, & Boschetti M (1994). Uric acid homeostasis in lean and obese girls during pubertal development. Metabolism, 43, 819–821. [DOI] [PubMed] [Google Scholar]
- Garbagnati E (1996). Urate changes in lean and obese boys during pubertal development. Metabolism, 45, 203–205. DOI: 10.1016/S0026-0495(96)90054-2 [DOI] [PubMed] [Google Scholar]
- Gillum RF (1987). The association of the ratio of waist to hip girth with blood pressure, serum cholesterol and serum uric acid in children and youths aged 6–17 years. Journal of Chronic Diseases, 40, 413–420. [DOI] [PubMed] [Google Scholar]
- Gorodischer R, Burtin P, Hwang P, Levine M, & Koren G (1994). Saliva versus blood sampling for therapeutic drug monitoring in children: Patient and parental preferences and an economic analysis. Therapeutic Drug Monitoring, 16, 437–443. [DOI] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT, Fortunato C, Harmon AG, Hibel LC, Schwartz EB, & Whembolua GL (2007). Integration of salivary biomarkers into developmental and behaviorally-oriented research: Problems and solutions for collecting specimens. Physiological Behavior, 92, 583–590. [DOI] [PubMed] [Google Scholar]
- Granger DA, Hibel LC, Fortunato CK, & Kapelewski CH (2009). Medication effects on salivary cortisol: Tactics and strategy to minimize impact in behavioral and developmental science. Psychoneuroendocrinology, 34, 1437–1448. [DOI] [PubMed] [Google Scholar]
- Granger DA, Fortunato CK, Beltzer EK, Virag M, Bright MA, & Out D (2012). Focus on methodology: Salivary bioscience and research on adolescence: An integrated perspective. Journal of Adolescence, 35, 1081–1095. [DOI] [PubMed] [Google Scholar]
- Grivna M, Pruša R, & Janda J (1997). Urinary uric acid excretion in healthy male infants. Pediatric Nephrology, 11, 623–624. DOI: 10.1007/s004670050350. [DOI] [PubMed] [Google Scholar]
- Guney E, Ceylan MF, Tektas A, Alisik M, Ergin M, Goker Z, & Erel O (2014). Oxidative stress in children and adolescents with anxiety disorders. Journal of Affective Disorders, 156, 62–66. [DOI] [PubMed] [Google Scholar]
- Harlan WR, Cornoni-Huntley J, & Leaverton PE (1979). Physiologic determinants of serum urate levels in adolescence. Pediatrics, 63, 569–575. [PubMed] [Google Scholar]
- Hayden MR, & Tyagi SC (2004). Uric acid: A new look at an old risk marker for cardiovascular disease, metabolic syndrome, and type 2 diabetes mellitus: The urate redox shuttle. Nutritional Metabolism, 1, 10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacobucci D (2010). Structural equations modeling: Fit indices, sample size, and advanced topics. Journal of Consumer Psychology, 20, 90–98. [Google Scholar]
- Ishizaka Y, Yamakado M, Toda A, Tani M, & Ishizaka N (2014). Relationship between serum uric acid and serum oxidative stress markers in the japanese general population. Nephronological Clinical Practice, 128, 49–56. [DOI] [PubMed] [Google Scholar]
- Jones DP, Richey PA, Alpert BS, & Li R (2008). Serum uric acid and ambulatory blood pressure in children with primary hypertension. Pediatric Research, 64, 556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasl SV, Gore S, & Cobb S (1975). The experience of losing a job: Reported changes in health, symptoms and illness behavior. Psychosomatic Medicine, 37, 106–122. [DOI] [PubMed] [Google Scholar]
- Kaur J, Lamb MM, & Ogden CL (2015). The association between food insecurity and obesity in children—The National Health and Nutrition Examination Survey. Journal of the Academy of Nutrition and Dietetics, 115, 751–758. [DOI] [PubMed] [Google Scholar]
- Kreft IGG, & De Leeuw J (1998). Introducing multilevel modeling. Newbury Park, CA: Sage Publications. [Google Scholar]
- Kuchan MJ, Ostrom KM, Smith C, & Hu PE (2000). Influence of purine intake on uric acid excretion in infants fed soy infant formulas. Journal of the American College of Nutrition, 19, 16. [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, & Johnson CL (2002). 2000 CDC Growth Charts for the United States: Methods and development. Vital and health statistics. Series 11, Data from the national health survey, 246, 1–190. [PubMed] [Google Scholar]
- Lindkvist M, Ivarsson A, Silfverdal SA, & Eurenius E (2015). Associations between toddlers’ and parents’ BMI, in relation to family socio-demography: A cross-sectional study. BMC Public Health, 15, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Song K, Wang Y, Zhang Q, Li W, Jiao H, … Huang G (2012). Relationship between serum uric acid and metabolic syndrome: An analysis by structural equation modeling. Journal of Clinical Lipidology, 6, 159–167. [DOI] [PubMed] [Google Scholar]
- Medscape Calculator. CDC/NCHS Infant Weight for Length Percentiles (<36 months). Retrieved at: http://reference.medscape.com/calculator/infant-weight-length-percentile
- Mellen PB, Bleyer AJ, Erlinger TP, Evans GW, Nieto FJ, Wagenknecht LE, … Herrington DM (2006). Serum uric acid predicts incident hypertension in a bi-ethnic cohort: The atherosclerosis risk in communities in communities study. Hypertension, 49, 1037–1042. [DOI] [PubMed] [Google Scholar]
- Modino SC, de Armas MGG, Mejías SM, Martínez JMM, Bolaños PI, Viveros MM, & Quesada JML (2012). Hyperuricemia and metabolic syndrome in children with overweight and obesity. Endocrinología y Nutrición (English Edition), 59, 533–538. [DOI] [PubMed] [Google Scholar]
- Mueller EF, Kasl SV, Brooks G, & Cobb S (1970). Psychosocial correlates of serum urate levels. Psychological Bulletin, 73, 238. [DOI] [PubMed] [Google Scholar]
- Riis JL, Bryce CI, Hand T, Bayer J, Matin MJ Stebbins JL, … Granger DA (n.d.) Validity, stability, and utility of measuring uric acid in saliva (sUA): A salivary bioscience-biobehavioral research interface. Manuscript submitted for publication.
- Sun HL, Pei D, Lue KH, & Chen YL (2015). Uric acid levels can predict metabolic syndrome and hypertension in adolescents: A 10-year longitudinal study. PLoS ONE, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Sagara K, Otsuka T, Matsuno S, Funada R, Uejima T, … Yamashita T (2012). Gender-specific relationship between serum uric acid level and atrial fibrillation prevalence. Circulation Journal, 76, 607–611. [DOI] [PubMed] [Google Scholar]
- Tailor AM, Peeters PH, Norat T, Vineis P, & Romaguera D (2010). An update on the prevalence of the metabolic syndrome in children and adolescents. International Journal of Pediatric Obesity, 5, 202–213. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Ogata S, Tanaka H, Omura K, Honda C, Hayakawa K, & Osaka Twin Research Group. (2015). The relationship between body mass index and uric acid: A study on Japanese adult twins. Environmental Health and Preventive Medicine, 20, 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas PD, Goodwin JM, & Goodwin JS (1985). Effect of social support on stress-related changes in cholesterol level, uric acid level, and immune function in an elderly sample. American Journal of Psychiatry, 142, 735–737. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau. 2010–2014. American Community Survey 5-year Estimates. B1101I-Household Type (Including Living Alone) (Hispanic or Latino). Retrieved 20 July 2016 at: http://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=ACS_14_5YR_B11001I&prodType=table
- Viazzi F, Genovesi S, Ambruzzi M, & Giussani M (2015). Sugar, fructose, uric acid and hypertension in children and adolescents. Italian Journal of Pediatrics, 41, 72–76.26444666 [Google Scholar]
- Vidmar S, Carlin J, Hesketh K, & Cole T (2004). Standardizing anthropometric measures in children and adolescents with new functions for egen. Stata Journal, 4, 50–55. [Google Scholar]
- Voruganti VS, Nath SD, Cole SA, Thameem F, Jowett JB, Bauer R, … Arar NH (2009). Genetics of variation in serum uric acid and cardiovascular risk factors in Mexican-Americans. Journal of Clinical Endocrinological Metabolism, 94, 632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JY, Chen YL, Hsu CH, Tang SH, Wu CZ, & Pei D (2012). Predictive value of serum uric acid levels for the diagnosis of metabolic syndrome in adolescents. The Journal of Pediatrics, 161, 753–756. [DOI] [PubMed] [Google Scholar]
- Zhao J, & Huang Y (2015). Salivary uric acid as a noninvasive biomarker for monitoring the efficacy of urate-lowering therapy in a patient with chronic gouty arthropathy. Clinica Chimica Acta, 450, 115–120. [DOI] [PubMed] [Google Scholar]


