Abstract
The cranial nerves are the pathways through which environmental information (sensation) is directly communicated to the brain, leading to perception, and giving rise to higher cognition. Because cranial nerves determine and modulate brain function, invasive and non-invasive cranial nerve electrical stimulation methods have applications in the clinical, behavioral, and cognitive domains. Among other neuromodulation approaches such as peripheral, transcranial and deep brain stimulation, cranial nerve stimulation is unique in allowing axon pathway-specific engagement of brain circuits, including thalamo-cortical networks. In this review we amalgamate relevant knowledge of 1) cranial nerve anatomy and biophysics; 2) evidence of the modulatory effects of cranial nerves on cognition; 3) clinical and behavioral outcomes of cranial nerve stimulation; and 4) biomarkers of nerve target engagement including physiology, electroencephalography, neuroimaging, and behavioral metrics. Existing non-invasive stimulation methods cannot feasibly activate the axons of only individual cranial nerves. Even with invasive stimulation methods, selective targeting of one nerve fiber type requires nuance since each nerve is composed of functionally distinct axon-types that differentially branch and can anastomose onto other nerves. None-the-less, precisely controlling stimulation parameters can aid in affecting distinct sets of axons, thus supporting specific actions on cognition and behavior. To this end, a rubric for reproducible dose-response stimulation parameters is defined here. Given that afferent cranial nerve axons project directly to the brain, targeting structures (e.g. thalamus, cortex) that are critical nodes in higher order brain networks, potent effects on cognition are plausible. We propose an intervention design framework based on driving cranial nerve pathways in targeted brain circuits, which are in turn linked to specific higher cognitive processes. State-of-the-art current flow models that are used to explain and design cranial-nerve-activating stimulation technology require multi-scale detail that includes: gross anatomy; skull foramina and superficial tissue layers; and precise nerve morphology. Detailed simulations also predict that some non-invasive electrical or magnetic stimulation approaches that do not intend to modulate cranial nerves per se, such as transcranial direct current stimulation (tDCS) and transcranial magnetic stimulation (TMS), may also modulate activity of specific cranial nerves. Much prior cranial nerve stimulation work was conceptually limited to the production of sensory perception, with individual titration of intensity based on the level of perception and tolerability. However, disregarding sensory emulation allows consideration of temporal stimulation patterns (axon recruitment) that modulate the tone of cortical networks independent of sensory cortices, without necessarily titrating perception. For example, leveraging the role of the thalamus as a gatekeeper for information to the cerebral cortex, preventing or enhancing the passage of specific information depending on the behavioral state. We show that properly parameterized computational models at multiple scales are needed to rationally optimize neuromodulation that target sets of cranial nerves, determining which and how specific brain circuitries are modulated, which can in turn influence cognition in a designed manner.
Keywords: Cranial nerve, Olfactory, Optic, Vagus, Trigeminal, vestibulocochlear, Stimulation
Introduction
Cognition is conceptualized as the processing of information acquired through the senses. The central nervous system (CNS; nerves within the brain and spine) integrates and responds to signals transmitted by the peripheral nervous system (PNS; nerves outside the brain and spine), whose primary function is to connect the CNS with the rest of the body and the environment [1]. The cranial nerves are a specialized part of the PNS that emerge directly from the brain rather than through the spine and include both afferents and efferents. Afferent cranial nerve axons convey sensory information —sight, hearing, taste (gustation), touch (heat, pressure, pain, proprioception), smell (olfaction), interoception (input from the gut and internal organs), and equilibrium— to the brain. Efferent cranial nerves’ axons regulate muscles (smooth, skeletal, and cardiac) and glands (either directly or through a postganglionic axon; Table 1). In contrast to other peripheral nerves that first route through the spinal cord, cranial nerves project directly through the skull into the brain, which makes them a special target for neuromodulation. For each cranial nerve, there is a portion that is relatively accessible (extracranial), and each nerve is intimately linked to perception and regulation of CNS function, including established “bottom-up” functions in cognition and clinical disorders [2–4].
Table 1.
Summary of cranial nerve modality, conduction direction and function. The modality describes the type of information each nerve conducts. Classic modality are the designations given by anatomists; SVA – special visceral afferent; SSA – special sensory afferent; GSA – general sensory afferent; SVE – special visceral efferent; GVE –general visceral efferent; GSE – general sensory efferent, A– Afferent, E – Efferent. Cranial nerves that contain at least one major afferent branch — characterized fully in this review — are bolded.
| Cranial Nerves | Modality | Classic Modality | ↔ | Function |
|---|---|---|---|---|
| I (Olfaction) | Special sensory | SVA | A | Smell |
| II (Optic) | Special sensory | SSA | A | Vision |
| III (Oculomotor) | Parasympathetic motor Somatic motor |
GVE GSE |
E E |
Parasympathetic control of eye muscles |
| IV (Trochlear) | Somatic motor | GSE | E | Motor control of eye muscles |
| V (Trigeminal) | Somatic sensory Branchial motor |
GSA SVE |
A E |
Touch from face Motor control of mastication |
| VI (Abducens) | Somatic motor | GSE | E | Control of muscles of the eyes |
| VII (Facial) | Somatic sensory Visceral sensory Parasympathetic motor Branchial motor |
GSA SVA GVE SVE |
A A E E |
Touch from ear Taste Parasympathetic control of oral/nasal/tongue glands Muscles of the face |
| VIII (Vestibulocochlear) | Somatic sensory | SSA | A | Balance/hearing |
| IX (Glossopharyngeal) | Somatic sensory Visceral sensory Parasympathetic motor Branchial motor |
GSA SVA/GVA GVE SVE |
A A E E |
Sensation from the tongue Sensation from the carotid body and sinus; taste Parasympathetic control of glands and mucosa Control of facial muscles |
| X (Vagus) | Somatic Sensory Visceral sensory Parasympathetic motor Branchial motor |
GSA SVA/GVA GVE SVE |
A A E E |
Touch from the ear Taste; sensory info from the pharynx, larynx, abdomen, heart Parasympathetic control of smooth muscle and glands in the body and throat Motor control of the pharynx and larynx |
| XI (Accessory) | Branchial/Somatic motor | SVE | E | Control of sternocleidomastoid and trapezius muscles |
| XII (Hypoglossal) | Somatic motor | GSE | E | Muscles of the tongue |
Classic modality are the designations given by anatomists; SVA – special visceral afferent; SSA – special sensory afferent; GSA – general sensory afferent; SVE – special visceral efferent; GVE –general visceral efferent; GSE – general sensory efferent, A– Afferent, E – Efferent. Cranial nerves that contain at least a major afferent branch — characterized fully in this review — are bolded.
Here we develop a formalism to design cranial nerve stimulation by leveraging insight from modern biomedical engineering and neuroscience (i.e. biomarkers) - in order to target specific cognitive constructs and behaviors that may be linked to neuropsychiatric disorders. We focus mainly on nerves that contain a major sensory (or afferent) component, however in some cases it can be challenging to disambiguate the cognitive effects of cranial nerves stimulation on afferents vs. efferents (see sec.5). Our overall approach is to focus on each afferent cranial nerve that modulates a specific brain circuit -- including those circuits involved in lower and higher-level processes - providing a rational basis to target specific cognitive functions by optimized cranial nerve stimulation. Indeed, while transcranial approaches (e.g. TMS and tDCS) or some invasive approaches (e.g. certain forms of DBS) inevitably stimulate a complex constellation of neurons, cranial nerve stimulation allows (with limitations discussed) activation of targeted pathways into the CNS using minimally or non-invasive technology.
There is a large body of literature on the modulation of cranial nerves by electrical stimulation for both therapeutic and experimental applications; however, these studies are variable in methodology and conclusions. The clinical neuroanatomy of each cranial nerve have been explored [5], but nuance continues to emerge in anatomy and function [6, 7]. Some of the earliest applications of electrical stimulation to cranial nerves were to treat neurological disorders such as seizures [8, 9] and sensory dysfunctions [e.g., vision loss, equilibrium damage; 10, 11]. Subsequent inclusion of broader clinical indications — including neuropsychiatric disorders -- have furthered knowledge of how activity of early sensory systems through cranial nerves can influence higher cognitive processes [12–14]. This improved understanding has driven the expansion of devices geared towards a variety of applications including treatment of specific disorders as well as enhancement of cognitive and other functions [15–17].
Drawing from clinical experience, researchers have used electrical stimulation to replace a natural stimulus to measure the response of specific sensory systems. For example, using electrical stimulation to elicit flashes of light rather than using a light source [18]. While delivering light and sound stimuli to their respective sensory systems is a well characterized and more quantifiable process (allowing for a more precise psychophysical measurements), other sensory systems pose more of a challenge. Therefore, the study of touch, olfaction (smell), gustation (taste), and balance have all applied electrical stimulation, in lieu of natural stimuli, to sensory organs or directly to the nerves as a way to investigate the processing pathway of their respective sensory modalities [19–21]. Such trials are reviewed here and provide guidance on target engagement (evidence a given pathway is activated), while we also propose a framework where brain circuits can be modulated by patterns of cranial nerve stimulation independent of sensory emulation.
Neuroimaging and neurophysiological techniques can further characterize the response of cranial nerve activation, and potentially act as biomarkers of target engagement. For example, electrically induced evoked potentials (EPs) measured using electroencephalography (EEG) can be used to monitor a variety of nerve functions [22]. Auditory and visual EPs measured with electroencephalography (EEG) are used for a variety of diagnostic purposes in neurology to validate electrical stimulation of cranial nerves and as an adjunctive tool in neurosurgery [23, 24] The use of EEG measurement of EPs has been used to validate non-invasive electrical stimulation of cranial nerves as well [25–27].
Functional magnetic resonance imaging (fMRI), magnetoencephalography (MEG) and positron emission tomography (PET) can be used to examine both subcortical and cortical activation induced by targeted cranial nerve electrical stimulation [28–32]. Potential biomarkers can be developed through neural signatures evoked by electrically stimulating cranial nerve(s), in both healthy and dysfunctional subjects [2, 33], but only if they are distinct and reliable. Examples of the electrically evoked potentials and induced network effects explored as measures of cranial nerve function are summarized in Table 2 and expanded on for each nerve in the text.
Table 2.
Selected electrophysiological markers of electrical stimulation of cranial nerves. Evoked potentials are locked to the presentation of an electrical stimulus. Induced activity is not locked to a stimulus.
| CN | Reference | Marker | Population | Dose |
|---|---|---|---|---|
| Evoked Effects | ||||
| I | Ishmaru et al. (1997) | Olfactory evoked potentials | (5) healthy subjects | 2 Hz biphasic pulsed, 500 μs pulse width, 2 mA; Bipolar, silver spherical tips inserted into olfactory cleft onto olfactory mucosa |
| II | Brelen et al (2010) | Visual evoked potentials | (2) patients with retinitis pigementosa patients (1) healthy control | 0.3 Hz pulsed, 213-306 μs pulse width, 92-1040 μA; Cuff electrode around optic nerve, four platinum contacts [0°,90°,280°,270°], 0.2 mm2 contact area |
| II | Gall (2010) | Visual evoked potentials | (1) patient with optic nerve lesion | 10–30 Hz varied burst pattern, < 600 μA; Four Ag/AgCl ring electrodes around or on the eyelid, return: forearm 30–40 min daily for 10 days |
| V | Leandri (1996) | Trigeminal evoked potential | (30) patients with trigeminal neuralgia | 5 Hz pulsed, 50 μs pulse width, μ = 4 mA; Bipolar Ag ball electrodes, 2 mm, inserted into supraorbital, infraorbital, and mental foramina canals |
| V/VI I/IX |
Ohla et al. (2010) | Event related potentials | 2(4) healthy subjects | 1000 ms pulse width, 4–400 μA; Anode placed on right or left lateral edge of tongue, stainless steel 5 mm diameter |
| VIII | Wilkinson et al. (2012) | Event related potentials and EEG spectral responses | DC, 0.4-1.2 mA; Bipolar bilateral over mastoids, electrode 3 cm2 carbon-rubber | |
| VIII | Berryhill et al. (2001) | Far field potentials | (16) healthy subjects; one session | 23 Hz biphasic pulsed, 500 μs pulse width, <1 mA; Bipolar platinum-iridium |
| X | Fallgatter et al. (2003) | Far field potentials | (1) healthy subject in (5) sessions & (5) subjects in (1) session | pulsed, 100 μs pulse width, 2 s isi, 8 mA; Bipolar copper electrode, attached to bilaterally to ear at various positions, 1 cm2 |
| X | Usami et al. (2013) | Vagus nerve evoked potential | (25) patients / epilepsy implanted / VNS | 30 Hz biphasic pulsed, 130-750 μs pulse width, 670s on, 0.25-2.0 mA; Around cervical branch of vagus nerve, bipolar platinum helical coil, 2-3 mm diameter. |
| Induced Effects | ||||
| II | Fedorov et al. (2011) | Changes in cortical power spectra associated with improvements in visual function | 68 Optic Lesion Patients; 10 Sessions 40 min/day | 5-20 Hz varied burst pattern, 115-756 μA; Active: four electrodes, two electrodes placed at upper eyelid bilaterally Return: wrist of right hand |
| V | Fanselow et al. (2000) | Desynchronization of cortical and thalamic activity | 8 Pentylenetetrazole-Induced Seizure rats | 1-333 Hz pulsed, 500 μs pulse width, 3-11 mA; Bilaterally implanted on the infraorbital nerve, platinum cuff electrode, 0.5 mm wide 0.025 mm thick |
| X | Fraschini et al. (2013) | Desynchronization in gamma bands correlated with positive clinical outcomes | 10 patients with epilepsy | 30 Hz biphasic pulsed, 30s on 5 min off; Around cervical branch of vagus nerve, bipolar platinum helical coil, 2-3 mm diameter. |
CN: cranial nerve.
Sensory dysfunction has been implicated in range of neurological and psychiatric disorders. Under a hypothesis that primary sensory dysfunction has a causal role in brain disorders, then cranial nerve stimulation that acts as substitute for sensory stimuli may be therapeutic (e.g. directly treat neuro-psychiatric disorders associated with sensory/bottom-up dysregulation). Alternatively, change in sensory function (mediated by cranial nerves) functions are epiphenomena to the neuropsychiatric symptoms (e.g. non-specific neurodegeneration). In any case, the brain circuits involved in processing sensory input from a cranial nerve could overlap with those circuits underlying disease symptoms – in this sense, cranial nerve electrical stimulation provides a direct pathway to those circuits, but not necessarily dependent on sensory stimulus substitution.
We develop here a framework where cranial nerves are viewed as unique brain stimulation targets amenable to targeted therapeutic intervention. Minimally- or non-invasive cranial nerve stimulation can be used to modify higher level cortical function in a more specific fashion than is possible with other brain stimulation techniques, provide a nuanced understanding of the brain circuits specific cranial nerve activity is able to modulate. This review integrates knowledge relevant to electrical neuromodulation of cranial nerves that spans the fields of anatomy, electrophysiology, bioengineering, cognitive and clinical neuroscience, psychiatry and neurology. Advancing the science of cranial nerve stimulation as a path to modulate cognition first requires a detailed review of existing knowledge from these fields. This includes a contextual review by cranial nerve involving stimulation trials where changes in cognition were accessed (Table 3). There is an extensive medical literature on the gross anatomy and function of cranial nerves in health and disease [6, 34]. Electrophysiologists and bioengineers have characterized the biophysics and morphology of axons within these nerves and their cellular response to electrical stimulation [35–37]. Cognitive neuroscientists are ultimately interested in how signals are integrated along the sensory pathway from the periphery to the brain, and how cranial nerves effect cognition. For the most part, the cranial nerve afferents synapse in the brainstem and are then relayed to the thalamus before branching to other cortical areas [38]. The thalamus is an integral node for many cognitive networks, and drives cortical function and inherent rhythm [39].
Table 3.
Selected studies that electrically stimulate cranial nerves to modulate cognition and behavior. Outcome column lists if results are positive (+), negative, (−), or inconclusive (+/−) relative to intended rationale. Target nerve and rationale as stated in the original publication.
| CN | Citation | Device/Electrode model | Method | Duration | Dose | Rationale | Outcome |
|---|---|---|---|---|---|---|---|
| II | Sabel et al. (2011) | Noninvasive stimulation device (EBS Technologies, Kleinmachnow, Germany | rTACS (non-invasive) |
(10) sessions 40 min/session |
Varied burst pattern, <1000 μA; 4 sintered Ag/AgCl ring electrodes placed “near the eyeball” return: right wrist | Determine transorbital stimulation effect on perception and cortical function | (+) |
| II | Gall et al. (2011) | Noninvasive stimulation device (EBS Technologies, Kleinmachnow, Germany Electrode: Not Specified |
rTACS (non-invasive) |
(10) sessions 20-40 min/session | 5 - 30 Hz varied burst pattern., <500 μA; 4 periorbital gold electrodes, bilaterally superior and inferior to the eye, return: occipital pole | Improvement of visual functioning and mood in visual impaired | (+) |
| V | Basso et al. (2016) | Device/Electrode: TEN device (Thync, Inc., Los Gatos, CA) | TNS (non-invasive) | 1 week 20 min/day |
biphasic pulse-modulated High: 3-11 kHz, 5-7 mA Low: 0.5-0.75 kHz, <5 mA; Active: right temple (10/10 site F8) Return: base of the neck |
Suppressing psychophysiological and biochemical stress responses in humans | (+) |
| V | Piquet et al. (2011) | Device/Electrode: Cefaly (STX-Med., Herstal, Belgium) | TNS (non-invasive) |
(1) session 20 min |
120 Hz pulsed, 250 μs pulse width, < 14 mA; 30 mm x 94 mm bipolar electrode, placed bilaterally over the ophthalmic branch | Modulate vigilance1 | (+/−) |
| V/VII/IX | Wildenberg et al. (2011) | Device/Electrode: Tongue Display Unit Kaczmarek (2011) |
CN-NINM (non-invasive) |
(9) sessions 20 min/session |
200 Hz pulsed, 50 μs pulse width, 50 Hz burst, 3 pulses per burst, < 17 V; 12 × 12 electrode array placed on the super anterior tongue, Gold plated, 1.55 mm diameter. | Map cortical networks engaged during CN-NINM modulation of optic flow balance disorder patients | (+) |
| VIII | Wilkinson et al. (2008) | National Instruments LabVIEW 6.0 and a dual output Microstar D/A board. Electrode: ComfortEase, Empi Inc. |
GVS (non-invasive) | (1) session | 1000 Hz Gaussian noise, σ=0.25, μ = 0.8mA; Bipolar electrode placed bilaterally over mastoids, electrode 3 cm2 carbon-rubber | Improve visual memory with low levels of GVS | (+) |
| VIII | Dilda et al. (2012) | Self-designed optically isolated constant current generator Electrode: 7180, 3 M Health Care, St. Paul, MN |
GVS (non-invasive) | (1) session 641 s [CI 9.3] |
0.16-0.61 Hz noisy, ≤5mA; bilaterally over mastoids, cut electrosurgical split grounding plate electrodes coated in EMG electrode gel | Modulate cognitive function2 | (+/−) |
| X | Jacobs et al. (2015) | TENSTem dental (Schwa-medico BV, Woudenberg, The Netherlands) Electrode: Not Specified |
taVNS (non-invasive) | (1) session 17 min |
8 Hz pulsed, 200 μs pulse width, 5.0 mA; Active: left external acoustic meatus inner side of the tragus, circular clip, 10 mm diameter. Return: right arm, rectangular solid gel, 35 x 22 mm | Enhance associative memory in older individuals | (+) |
| X | Clark et al. (1999) | Device/Electrode: Implantable neurocybernetic prosthesis (Cyberonics, Inc.) | VNS (invasive) | (4) sessions 1.5 min/session |
30 Hz biphasic pulsed, 500 μs pulse width, 30s on, 0.50-1.50 mA; Wrapped around cervical branch of vagus nerve, bipolar platinum helical coil, 2-3 mm diameter. | Enhance verbal word recognition | (+) |
rTACS: repetitive transorbital alternating current stimulation; TNS: trigeminal nerve stimulation; CN-NINM: cranial nerve noninvasive neuromodulation; GVS: galvanic vestibular stimulation; tVNS: rTACS: repetitive transorbital alternating current stimulation; TNS: trigeminal nerve stimulation; CN-NINM: cranial nerve noninvasive neuromodulation; GVS: galvanic vestibular stimulation; taVNS: transcutaneous auricular vagus nerve stimulation; VNS: vagus nerve stimulation.
Psychomotor vigilance task. Critical flicker fusion frequency, D2 test
Perspective taking tasks were impaired (perspective taking, match to sample), object-based transformations were not impaired (Stroop, mental rotation, reaction time, dual tasking)
This review is focused on development of a holistic scientific understanding of cranial nerve stimulation in order to support a rational approach to neuromodulation. First, we review different approaches to the targeting of cranial nerves with transcranial electrical stimulation including both methodology (Sec. 2) and waveform parameters used in various studies (Sec. 3). We then review cranial nerves based on their composition of afferent axons and connected circuits in the brain (Sec. 4). The role of cranial nerve efferent stimulation is briefly considered (Sec. 5) followed by a detailed review of stimulation of olfactory (Sec. 6.1), optic (Sec. 6.2), trigeminal (Sec. 6.3), facial/glossopharyngeal (Sec 6.4), vestibulocochlear (Sec. 6.5) and vagus nerves (Sec. 6.6). The review concludes with a discussion of future approaches for optimization and specific targeting of cranial nerve for neuromodulation.
1. Transcranial electrical stimulation and cranial nerves
Transcranial electrical stimulation (tES) techniques include transcranial direct current stimulation (tDCS), transcranial alternating current stimulation (tACS), transcranial random noise stimulation (tRNS) and transcranial pulsed current stimulation (tPCS) [40]. TES techniques all apply current through electrodes on the scalp for the purpose of direct stimulation of the brain [40–44] in order to modulate cortical function with resultant changes in behavior and cognition [45–48]. However, the physics of tES dictates a much lower current density in the brain than in the skin [17] and skull foramina (because of skull resistance to current flow), which may result in the stimulation of cranial nerves that transverse the skin (e.g. forehead, neck) and foramina near or between the electrodes (Figure 1 & Figure 2) [49, 50].
Figure 1.
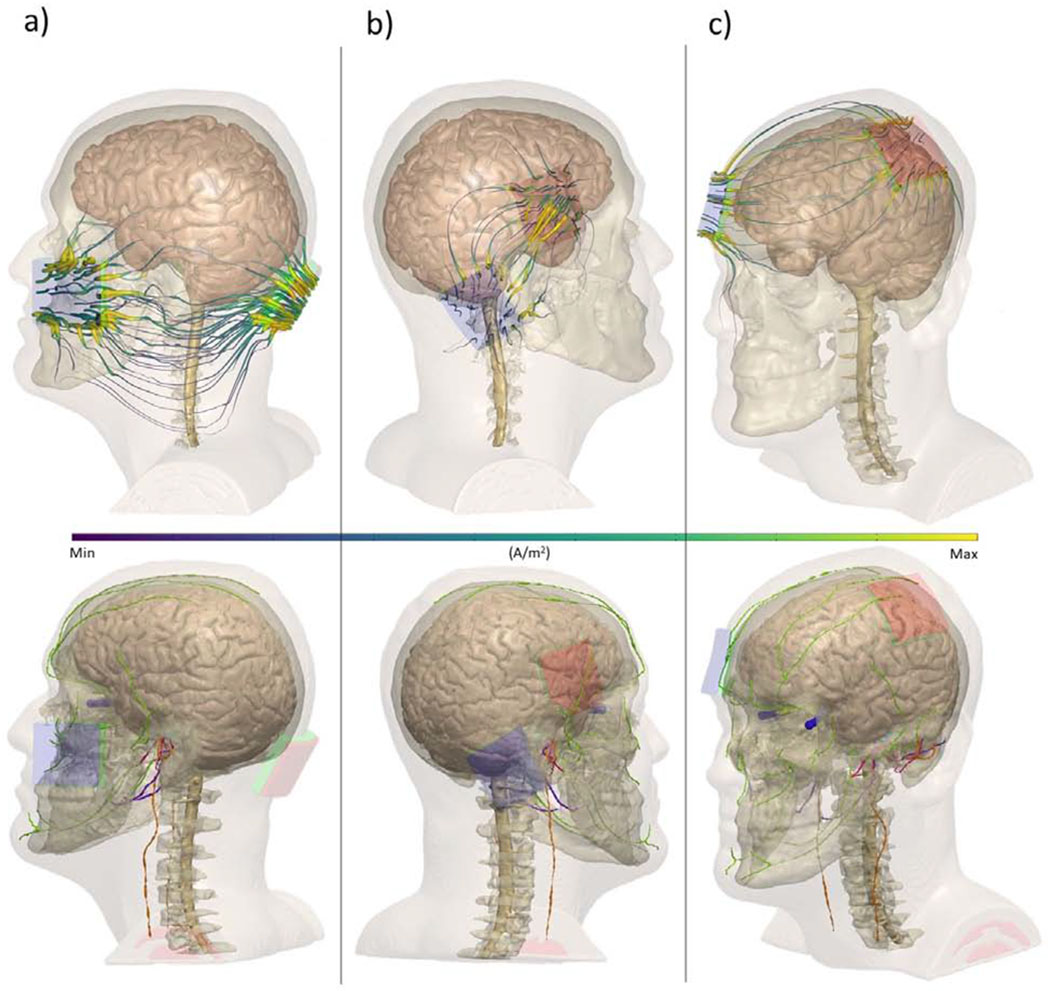
MRI-derived finite element models of electrode montages typically used in transcranial electrical stimulation (tES) studies. Surrounding tissue were segmented using automatic and manual techniques, referencing prior atlases. Each electrode montage is referenced in accordance with the International 10/20 System of Electrode Placement or other superficial anatomical marker Top row: current streamlines (purple/yellow) during electrical stimulation montages using exemplary electrode montages. Bottom row: cranial nerves overlaid with tDCS montages (trigeminal: orange, vagus: green, vestibular: green, optic: blue, glossopharyngeal: purple, intermediate branch of the facial nerve: pink). a) cerebellar stimulation with a cheek “reference” b) “right DLPFC” stimulation anode over F4 and cathode over the right mastoid (P10) c) M1-SO montage with anode over C3 and cathode over the right supra-orbital area (Fp2). These simulations show the diffuse current pattern produced by tES / tDCS electrode montages will overlap with the anatomical distribution of specific cranial nerves.
Figure 2.
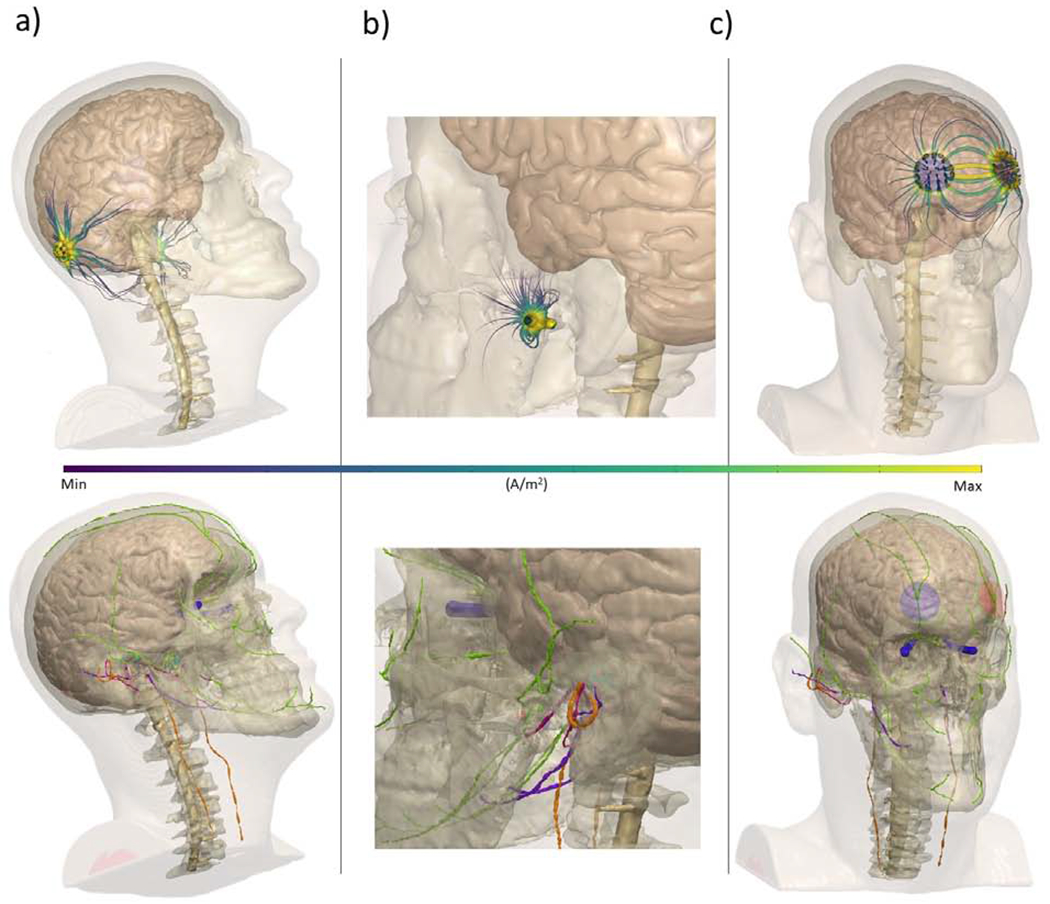
MRI-derived finite element models of non-invasive electrical stimulation. Surrounding tissue were segmented using automatic and manual techniques, referencing prior atlases. Each montage is referenced in accordance with the International 10/20 System of Electrode Placement or other superficial landmarks. Top row: current streamlines (purple/yellow) during electrical stimulation using exemplary electrode montages. Bottom row: Cranial nerves overlaid with cranial nerve stimulation montages (trigeminal: orange, vagus: green, vestibular: green, optic: blue, glossopharyngeal: purple, intermediate branch of the facial nerve: pink) a) galvanic vestibular stimulation, electrodes placed over the mastoids (P10/P9) b) transcutaneous auricular vagus nerve stimulation, electrode place on the tragus c) trigeminal nerve stimulation, electrodes roughly over Fp1 and Fp2
For example, tDCS for the treatment of major depressive disorder (MDD) is applied bilaterally to the forehead in order to target a brain region of interest, the dorsolateral prefrontal cortex (DLPFC) [51–55]. The forehead, however, is richly innervated with cranial nerves which may be unintentionally stimulated in frontal tDCS interventions (Figure 1.c & 2.c). tDCS when used for the purpose of enhancement of cognition in healthy individuals similarly employs electrodes on the forehead with the intention of targeting the frontal cortex; this may also be complicated by stimulation of cranial nerves innervating the skin of the forehead [17, 56, 57].
TES clinical trials for pain disorders — e.g., migraine [58–61], fibromyalgia [62–64], craniofacial pain [65, 66]— often target the motor cortex (M1) with an “active” electrode, while the “return” electrode is placed on the contralateral forehead (called the “supra-orbital” or SO position) (Figure 1.c.) [67], resulting in maximal current density to these skin areas with the attendant risk of stimulation of collateral cranial nerves. Some tES approaches use an extracephalic (placing the “return” electrode on the neck, face or arm ) rather than a cephalic (on the head) electrode (Figure 1.a.) [68, 69]. This method results in diffuse current through the scalp and neck [70]. Other tES current approaches place the “return” electrode on the ipsilateral mastoid (Figure 1.b, [71–73]). Direct current applied over one or both mastoids has been used to target the vestibular system(Figure 2.a, [74, 75]).
TES methods run the risk of stimulation of cranial nerves innervating areas under and between electrodes. Previous papers have reviewed the use of computational models [49, 76] and neurophysiological techniques [67, 77] to determine optimal tES electrode placements for stimulation of the cortex. Our review of the collateral effects of stimulation of cranial nerves with tES does not obviate study results consistent with direct cortical stimulation. Disambiguating the varying effects of different types of tES (i.e., tDCS, tACS, tRNS, tPCS) on cranial nerves is not within the scope of this review. Rather, we focus on a detailed consideration of the nerve anatomy, as it relates to electrode placement and function, in order to attain a more fundamental assessment of electrical stimulation treatments.
Many techniques that target the cranial nerves use dosages that overlap with “transcranial” methods. For instance, tACS, tRNS and tPCS can elicit the perception of flashes of light known as “phosphenes” [45, 78]. These phosphenes are a known effect of stimulation of the optic nerve, which often employs pulsed current and electrode placements similar to transcranial methods. It is debated where along the visual pathway these electrically induced phosphenes originate [79, 80]; several reports suggest that phosphenes originate primarily at the retina and optic nerve, regardless of the placement of the stimulation electrodes [81–83].
2. Electrical Stimulation Dosing Parameters
A complete understanding of the effects of different electrical stimulation devices and techniques on neurophysiology requires a cataloging of dosing parameters. These include electrical waveform (e.g. intensity, shape, frequency) and electrode type (e.g. material, size, location) which together make up the “dose.” Electrical waveforms are generated and then applied across electrodes. The dose determines how the pattern electrical current flow though the body, so the intensity at any given region [84]. We review below different dosing parameters used for different devices targeted for different cranial nerves to the extent that they are documented in the literature.
This section outlines the terms and reporting style used in this review when describing the dose of electrical stimulation to cranial nerves. These terms are applicable to the targeting of cranial nerves and generally electrical stimulation. While attempting to provide a uniform dose report scheme, some specialized terms may be qualified for a peculiar stimulation technique or cranial nerve target. At the beginning of each section – for each cranial nerve—the most common dose reported in the literature may be noted.
Pulses are a typical waveform used in cranial nerve stimulation. Pulses are applied repetitively in a train, where the inverse time between pulses equals the stimulation frequency. Unless otherwise specified, individual pulses are assumed to be rectangular. Individual pulses have a pulse duration (width) and amplitude. A waveform of pulses can be monophasic or biphasic. A monophasic waveform has pulses of a single polarity (Figure 3.a; z2=0), while a biphasic waveform has pulses that invert polarity, typically in paired opposite-polarity pulses [85]. Waveform types besides pulsed typically take the form of a simple periodic waveform, such as sinusoid (Figure 3.d). In the case that pulses are not evenly spaced in time, any burst patterns (Figure 3.b) or on/off times (Figure 3.c) are reported. Unless otherwise stated, the waveform for pulsed stimulation is reported as the following: pulse frequency and feature, pulse width, burst frequency, pulses per burst, time on/off, and peak amplitude (e.g., Figure 3.e–f). In this review, we systematically characterize dose to allow reproduction and reasonable comparison across studies. However, we respect dose as reported in the original papers, and do not attempt to verify the accuracy of reporting. In many cases, not all details about dose (e.g., whether the reported biphasic waveform is symmetric or asymmetric) are reported in the original paper, and so are omitted in our reference. In some cases, where the waveform description was indefinite, the phrasing used in the original report is reproduced in quotes.
Figure 3.
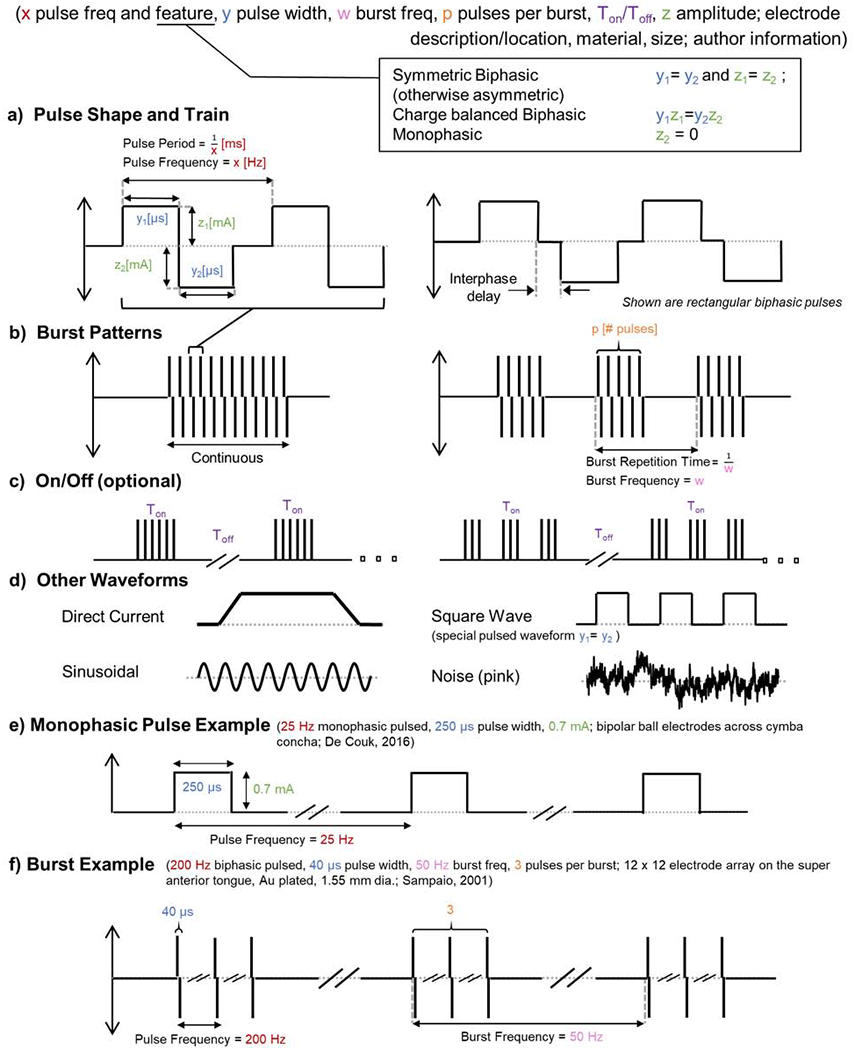
Uniform formula of stimulation waveform including as used in cranial electrical nerve stimulation. a-c address waveforms composed of rectangular pulses with expanding temporal scale, while d shows additional waveform types. a) The pulse shape includes the frequency, pulse width, amplitude, and interphase delay where applicable b) The burst stimulation pattern includes the repetition time and number of pulses or cycles per burst – if no burst pattern is reported than the stimulation pattern is continuous. c) The on/off period describes the time the stimulation pattern — continuous or burst—is active/inactive and is typically in the scale of minutes d) Direct current has a fixed amplitude but may include an on/off ramp and is, by definition, monophasic. Unless otherwise indicated, sinusoidal stimulation has a single frequency and symmetric biphasic (no DC offset). There are various types of noise-based stimulation, conventionally with no DC offset. Unless otherwise indicated, a square wave is monophasic. e) Monophasic pulse example. f) Burst example.
When waveforms are either monophasic, asymmetric biphasic, or symmetric biphasic but with importance given to the order of pulses phases, then the polarity of the waveform needs to be defined with respect to the electrodes (e.g. monophasic square wave with 5 V peak from electrode A to electrode B). In electrical stimulation, an anode electrode always indicates an electrode where, at that instant, current (defined as a positive quantity due to historical reasons, and therefore directly opposite to the physical direction taken by electrons in a circuit) enters the body and cathode electrode indicates an electrode when, at that instant, current exits the body [85]. In the context of electrical stimulation, anode / cathode always indicates an electrode location where current is entering / exiting the body – this definition which emphasizes the body-centric perspective should not be confused with how anode / cathode may be used in other specialties (e.g. batteries). However, these terms can be used in varied nuance and context.
When the waveform is monophasic an anode electrode may be defined as the electrode where current always enters the body, and a separate electrode may be defined as the “cathode electrode,” where current always exits the body. Since in all electrical stimulation there is always an anode and a cathode, the terms “anodal” stimulation can simply indicate a statement of hypothesis, that the nominal target is near the anode electrode [41]. “Cathodal” stimulation, indicating that the nominal target is near the cathode electrode. Alternatively, in some cases, like bilateral monophasic stimulation, “anodal”/ “cathodal” indicates the polarity of the waveform relative to the head (e.g. an electrode was placed on each mastoid with anodal stimulation on the right). Finally, in some applications where biphasic stimulation is used, and each electrode can alternate between anode and cathode the terms “anodic phase” and “cathodic phase” will be used (e.g. a cathodic phase pulse is followed by an anodic phase pulse). In this sense, when brain stimulation [85] is assumed to be driven by one phase, the terms “anodic stimulation” and “cathodic stimulation” are used (e.g. monopolar cathodic stimulation, where a cathode activating pulse is followed an anodic phase used for charge recovery). In this review, the limited use of anode/cathode related terms is typically qualified and influenced by how they are used in a given field of application.
Electrode shapes, sizes, positions, and materials are noted with the level of detail (and limits) as specified in the original reports. For invasive stimulation (and in electrochemistry), the electrode refers to the metal in contact with the body (tissue), while for non-invasive electrical stimulation, electrode can refer to the entire electrode assembly including electrolyte (e.g. saline, gel) which separates the metal from the skin. Here, unless otherwise indicated, the electrode (assembly) position and area indicates the surface area between the electrode (assembly) and the issue/skin – essentially the area over which current can enter/exit the body. The term “bipolar” and “unipolar” denotes electrode geometry. Unipolar electrode geometry refers to the use of a relatively small electrode placed near the target with another (larger) “return” electrode placed at a distance. However, the “return” is not inert. A bipolar electrode geometry indicates two electrodes of comparable size and proximity to the target(s). When electrodes are placed symmetrically on the head, especially to target structures in both hemispheres, the montage may also be referred to as bilateral. When two electrodes are used for a bilateral montage, it is also termed “bipolar.” To clarify, biphasic/monophasic refer to waveform and are independent of bipolar/unipolar/bilateral electrode geometry.
Beyond reporting complete dose details, in some stimulation applications specific metrics of dose are used to gauge efficacy or safety. These include current density (current / electrode area), charge (current x time per pulse), or charge density (charge / electrode area). However, reliance on such metrics does not negate the need to fully document dose.
3. Selection and Identification of Cranial Nerves as Targets for Electrical Stimulation
There are twelve cranial nerves, each emerge bilaterally from the brain. They are numbered per their anatomical organization, rostral to caudal. Each cranial nerve synapses (afferent) or originates (efferent) on a set of nuclei in the brainstem (with the exception of the olfactory and optic nerves) and exit through skull foramina, branching out onto the surface of the skull and into the neck, thorax, and abdomen (Figure 4). The cranial nerves are a part of a neuronal pathway that extends from cortical regions to/from the body (Figure 5). These pathways are made up of several connecting neurons. Sensory pathways usually begin with primary or “first order” neurons whose cell bodies are located outside the CNS and synapse in the brainstem onto second order neurons. The afferent axons of the first order neurons are axons of cranial nerves, that pass through the cranium and extend superficially to where their specific sensory information is transduced. Second order neurons then synapse in the thalamus onto third order neurons which project to the cortex. In comparison, efferent first order neurons typically originate in the cortex and synapse on secondary neurons in the brainstem, bypassing the thalamus. The efferent axons of the secondary brainstem neurons are axons of cranial nerves which exit the cranium to innervate organs and muscle. The cranial nerves are a part of the PNS (with the exception of the optic and in some cases the olfactory nerve) like the nerves that emerge from the spinal column, but unlike the spinal nerves, they relay information directly (or within one synapse) to and from the brain to the body. Therefore, cranial nerves are special targets for neuromodulation, accessible near the surface of the body and direct afferents to the cerebrum; conceptually “axons of the brain extending to the skin” (Figure 5).
Figure 4.
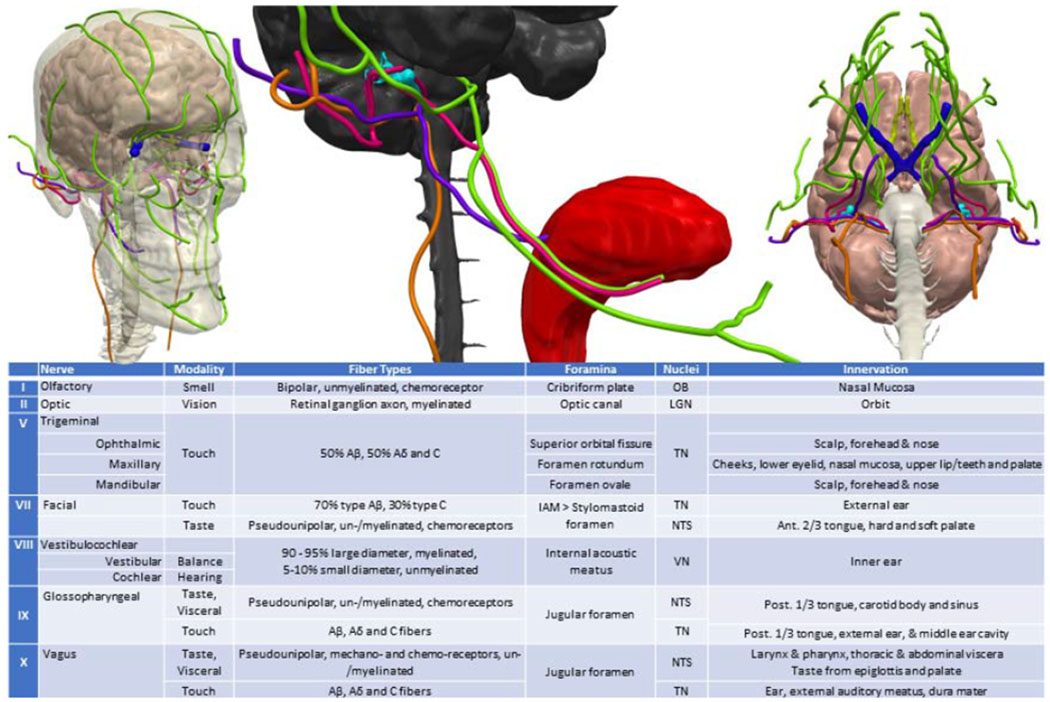
Anatomy and major axon sub-type of cranial nerves containing a major afferent component. Cranial nerve color key: olfactory tract: yellow, optic: dark blue, trigeminal: green, intermediate branch of the facial nerve: pink, vestibular: teal, glossopharyngeal: purple, vagus: orange. OB: Olfactory Bulb; LGN: Lateral Geniculate Nucleus; TN: Trigeminal Nuclei; NTS: Nucleus Tractus Solitarus; VN – Vestibular Nuclei. IAM: Internal acoustic meatus. Touch fibers (mechano-, chemo-, thermo- receptor and nociceptor) - Aβ: myelinated discriminatory touch fibers; Aβ: myelinated nociceptive thermal and mechanical fiber; C: unmyelinated mechanical, thermal, metabolic fiber.
Figure 5.
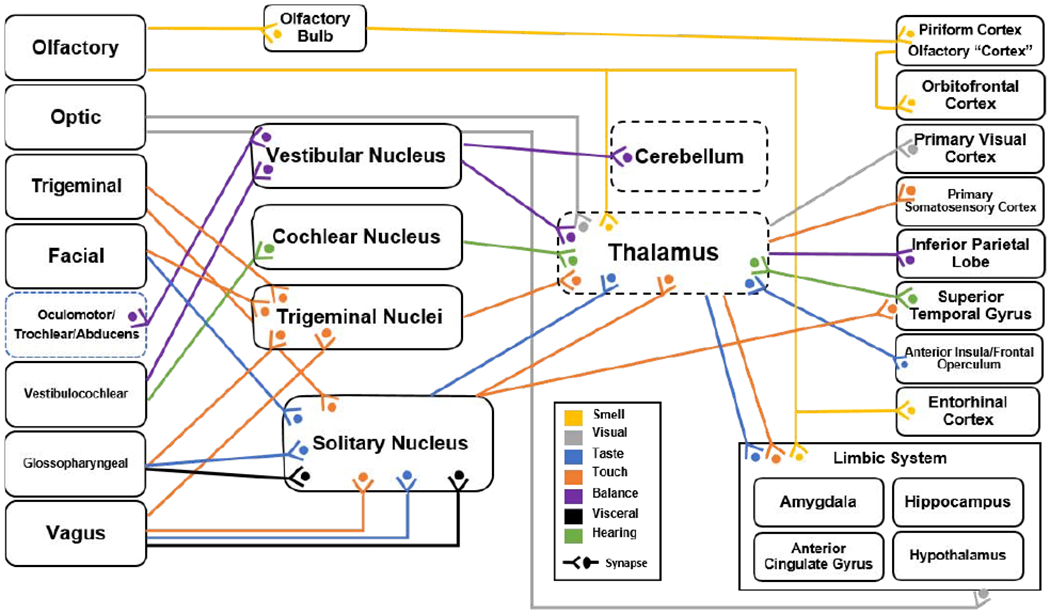
Anatomical map of connections from the cranial nerve (far left), to the brain stem nuclei (middle), to the cerebral cortex (far right). Specifically, the anatomical connections indicated provide pathways from specific cranial nerves to cortical region involved in higher order cognition as well as deep node structures involved in gating of information processing. Colors represent the sensory modality conveyed by the connection, however we propose that approaches to alter cognition can engage these pathways without necessarily inducing percepts. In this sense, sensory modalities are indicated here to illustrate functional circuit connections for neuromodulation. As explained in this review, cranial nerves offer a unique target for electrical stimulation of the brain circuits of cognition.
When considering the function of cranial nerves, it is important to characterize the types of information that the fibers of the nerve transmit. Classically, anatomists have categorized cranial nerves according to the following: afferents or efferents; special or general; and somatic or visceral. “Afferents” are fibers that send information to the CNS and “efferents” are fibers that carry information from the CNS to the rest of body. “Sensory” and “motor” can be used interchangeably with the terms afferent and efferent, respectively. “Special” nerves are functionally specific to cranial nerves (e.g. sense of smell), while “general” nerves are not functionally specific to cranial nerves (e.g. sense of touch) as this sensation also is carried by spinal nerves. Somatic refers to nerves that receive and transmit information from the skin, skeletal muscles, joints and tendons. Visceral are those nerves that innervate smooth muscles, cardiac muscle and glands [86, 87]. These classifications are in accordance with established anatomical terminology [34, 88] as summarized in Table 1. For this review, we consider in detail only the afferent branches of cranial nerves, as the flow of information is toward the brain, usually with a direct connection from the receptor to the brain, and therefore have a more direct effect on cognition, mood and behavior (Cranial nerves I, II, V, VII, VIII, IX, X). This does not exclude any possible contribution from efferent branches in altering cognition through physiological changes (e.g. heart rate) that may then secondarily affect the brain. The Cranial Nerve Efferents section (Sec 5) addresses these connections.
Each main section of this review addresses the afferents of a specified cranial nerves including: the basic anatomy (from sensory transduction to its connection with the brain); the types of electrical stimulation traditionally used to target the nerve (e.g., waveform, dose, montage, invasive/noninvasive, etc.); clinical disorders targeted for treatment by electrical stimulation; imaging/neurophysiological outcomes; and any cognitive, mood, or behavioral outcomes modulated by targeting cranial nerves by electrical stimulation.
Clarification of terminology is needed when discussing cranial nerve stimulation research, including when cranial nerves are not intentionally targeted (e.g., some tES). Afferent cranial nerves do not technically include sensory receptors, rather they are the fibers (axons) that convey signals between the receptors and the brain. However, certain cranial nerves have integrated receptors (e.g. olfactory and certain branches of somatosensory nerves). Due to the nature of the overlapping anatomy of most cranial nerves, between and within, and the heightened sensitivity of the axon terminal to electricity [89], it is unlikely that noninvasive electrical stimulation would only polarize the nerve and not the receptor system attached to it, or vice versa. If an action potential is initiated in any compartment of a cranial nerve or its sensory terminus, an action potential will be conducted along its length. In the following sections, we examine some applications that intend to target receptors (e.g., photoreceptors in the retina), yet remain cranial nerve electrical stimulation (e.g., optic nerve) for our purposes.
4. Cranial Nerve Efferents
This review is concerned primarily with the afferent pathways of cranial nerves as they offer a direct (or within one synapse) and targeted pathway to the brain. However, the efferent pathways are an important target for varied cranial nerve stimulation applications, and indeed – due to anatomical considerations -- are co-activated by approaches that target afferent pathways. As noted above, efferent nerves can be somatic (muscle) or visceral (smooth muscle, glands, cardiac muscle), some of which are part of the parasympathetic branch of the autonomic nervous system (ANS). Efferent nerves can be targeted either directly by stimulating axonal pathways from the brain to the body, or indirectly through stimulating afferents that then activate efferents through reflex pathways in the brain stem or cerebellum.
Examples of cranial nerve efferent activation include, electrical stimulation of motor cranial nerves to elicit or suppress muscle movement through efferent fibers of the hypoglossal nerve that innervate muscle, with application in the treatment for sleep apnea (30 Hz pulsed, 100 μs, 1-1.5 sec burst duration; half-cuff tripolar electrode around main trunk of hypoglossal nerve [90]). Other examples of studies that aim to stimulate efferents through direct pathways include increasing vascular blood flow (3-60 Hz monophasic pulsed, 500 μs pulse width, 5V; [91]) and treating ischemic stroke [92]. In the vestibular system, direct current stimulation applied to the mastoids can induce eye movement ([Direct current, 5mA; bilateral 1,000 mm^2 electrodes placed over mastoids; [93]) which in disordered vestibular systems may serve as a measure of remaining function (Direct current 3 sec ramp, 4 mA, 35 sec; Bilateral and unilateral (return over C7); [94]). The vestibular-ocular reflexive arc acts by modulating the vestibular nerve and/or receptors that send information to the brain stem (nuclei) and then back out via efferents that control muscles in the eye in order to stabilize visual information in the case of head or body movements [95]. The pathway from vestibular nerve to ocular nerve is only three neurons long, so while the original effector may be the afferent, the outcome may be mediated by efferents. Other signaling pathways include vagal efferents in the baroreceptor reflex arc (20 Hz retangular pulse, 200 μs pulse width, <8 V; stainless steel wire electrodes attached to right vagus nerve; [96]). Both types of stimulation, direct and reflexive are considered efferent stimulation.
There are three cranial nerves that are mixed, containing both afferents and efferents, increasing the likelihood that both pathways might be modulated by electrical stimulation. For example, the vagus nerve contains both sensory and motor nerves, some of which are anatomically adjacent. The afferent and efferent nerves travel alongside each other in the cervical branch in the carotid sheath (section 6). Typically, the afferent fibers in the cervical branch are targeted as a treatment of epilepsy, depression and other neurological and psychiatric disorders [8, 97–100]. However, it is almost certain that some vagal electrical stimulation (invasive or non-invasive) will also activate efferents. Besides inadvertent involvement, efferent axons in mixed cranial nerves can be explicitly targeted for electrical stimulation. For example, targeted electrical stimulation of efferent vagus nerve fibers invasively has been proposed as a treatment for disorders of the immune system [101–103]. Consistent with this, cervical vagus nerve stimulation increased survival in sepsis infected mice and attenuated the production of proinflammatory cytokines (1 Hz, 2 ms pulse width, 5 V, 12 min; bipolar platinum electrodes; [104]). In summary, the efferent axons of cranial nerves must be taken into consideration when designing strategies for electrical stimulation of cranial nerves for the enhancement of normal functions including mood and cognition and/or interventions for neurological and psychiatric conditions, even when afferents are the primary target of the intervention [105–110].
5. Afferent Cranial Nerves
5.1. Olfactory Nerve Stimulation
The olfactory nerve (CN I) conveys information about smell to the brain. Humans have over 350 types of odorant receptors, each of which can recognize multiple odorants [111]. Each odorant can also bind to several different receptors. The unmyelinated olfactory sensory axons project through the cribriform plate (foramina) and synapse ipsilaterally onto the olfactory bulb, a direct outcropping of the neocortex (Fig 4; yellow). Neurons that have the same receptor type synapse onto a single pair of glomeruli in the olfactory bulb. This convergence enhances the ability to detect small quantities of odorants. In the glomerulus, each olfactory sensory neuron synapses onto a tufted or mitral relay cell. From there, information projects through the olfactory tract to the piriform cortex, amygdala, anterior olfactory nucleus, olfactory tubercle, and entorhinal cortex. The areas that receive direct projections from the olfactory bulb are known collectively as the olfactory cortex [112]. The olfactory cortex has projections to the orbitofrontal cortex (OFC) and prefrontal cortical (PFC) areas [113, 114]. It also makes reciprocal connections with the brain stem nuclei — the locus coeruleus (the olfactory system’s main source of norepinephrine) and raphe nucleus, a major site of serotonin cell bodies in the brain [115, 116]. Indeed, the neurochemistry and neuroanatomy of the olfactory system highlight the important role of smell in the modulation of mood and emotion [117, 118].
The olfactory sensory nerve’s only extracranial projections are the widely dispersed cilia in the nasal mucosa These cilia synapse on the olfactory bulb, an outcropping of the cortex. This means that the olfactory nerve is one synapse away from the cortex. While there is research in animals and humans that target the olfactory nerve proper, the olfactory bulb and tract are commonly targeted for electrical stimulation when investigating the olfactory system. For the purposes of this review, the primary sensory neurons, bulb, and tract make up the olfactory system and are considered olfactory “nerve” stimulation. Noninvasive electrical stimulation targeting the olfactory system in both animals and humans generally use bipolar electrodes placed, usually unilaterally, inside the nostril to touch the nasal epithelium [19, 119, 120]. Invasive research in animals place bipolar electrodes on the exposed olfactory bulb and tract [121, 122]. This section on the olfactory nerve electrical stimulation addresses methodological and conceptual issues.
Electrical stimulation applied to rodents and non-human primates has been used to probe the structural and functional synaptic organization of the olfactory system. By studying the projections from the rat olfactory nerve using electrical stimulation, the organization of the olfactory bulb has been elucidated (0.25 Hz pulsed, 100 μs pulse width; bipolar stainless steel electrodes, placed rostral to cribriform plate; [123]). The topmost layer of the olfactory bulb, the granule cell layer, contains inhibitory interneurons and cortical structures form inhibitory connections within the olfactory bulb. Electrical stimulation of the olfactory mucosa (and thus the olfactory neurons) evokes a weak excitatory response from intracranial recordings in the olfactory bulb of rabbits cells, perhaps indicating that electrical stimulation does not activate the olfactory system in the same way as chemical olfactants (pulsed, 700 μs pulsed width, 5-20 V, 5-20 V; bipolar silver, < 0.5 μ diameter; [124]).
Olfactory receptors are somewhat indiscriminate in which odorants they bind, making the mechanism by which the olfactory system decodes odors complex to deduce. The answer lies partially in the spatiotemporal encoding of signals in the olfactory bulb, which electrical stimulation has helped to explain [125]. For example, rats taught to discriminate between points of electrical stimulation on their olfactory bulb could do so up to a relatively small area of point separation which indicates a topographically precise organization of the bulb (50 Hz sine wave, 4-20 μA; stainless steel wire in olfactory bulb; [126]). However, comparing electrically induced activation to chemically induced activation requires caution, as electrical stimuli do not always yield the same results as direct application of chemical odorants [121]. For example, the pattern of activation in rats olfactory bulb observed during electrical stimulation of the olfactory bulb is more diffuse than the discrete areas of activation induced by odors [127]. Nevertheless, electrical stimulation at the nerve and bulbar level have provided valuable information about the olfactory system’s anatomy, cell morphology, and physiology.
The olfactory nerve is the only cranial nerves to bypass the thalamus, instead olfactory information is projected to the neocortex, and therefore there are less “stops” between the sensory neurons and higher order processes. As mentioned in the introduction to this section, the olfactory system has connections with the OFC, a region imaging studies have implicated in decision making and emotion processing (for review see [113, 128]). The structures in the olfactory cortex make both direct and indirect projections to the OFC. An indirect route to the OFC through the hypothalamus in nonhuman primates was identified with recordings of extracellular potentials in the OFC and intermediate relay structures, in response to electrical stimulation of the olfactory bulb (square pulse, 10-500 μs pulse width, 2-8 V; bipolar electrodes, stainless steel (0.4-0.5 mm dia.) or tungsten (300 μm dia.); [129]). Consistent with the observation that the OFC is integral to processing olfactory information, patients with OFC lesions are unable to discriminate odors [130]. Neuroimaging has been used to functionally identify the OFC in humans during olfactory processing of chemical stimuli (for a review see [113]). More direct connections between the OFC and olfactory cortex have been identified in monkeys [131–133]. The OFC is the junction not only for olfactory information, but other sensory systems such as gustatory and visual information, implicating a multimodal role for olfaction [134].
Relating a neural signal to the onset of a stimuli underlies much of cognitive research; when EEG is used to record neural signals, these timed signals are known as event related potentials (ERPs) [33]. For instance, clinical disorders with deficits in smell can be diagnosed by signal conductance speeds tests. Typically, air puffs are applied inside the nose while measuring EEG from the scalp [135]. The neural signal evoked by the olfactory signal are disturbed in certain psychiatric disorders, such as schizophrenia and Alzheimer’s, and may aid in diagnosing a disorder [136, 137]. Olfactory dysfunction is the earliest clinical symptom of Alzheimer’s disease and quantitative assessment of olfactory system performance is suggested to serve as a potential biomarker for Alzheimer’s disease progression [138–140]. Given the critical role that smell plays in disgust, it is not surprising that smells are a potent modifier of emotion, and have been used as a probe of symptoms of posttraumatic stress disorder [117, 118, 141]. The olfactory system is the only sensory system with direct connections to the amygdala which as noted above bypass the thalamus and higher cortical areas, highlighting their role in the production of unfiltered emotion [142]. Typically, olfactometers are used to deliver chemical stimuli to a human or animal to modulated neural activity that can be measured by behavior or EEG and neuroimaging methods [143]. Electrical stimuli can alternatively be used to elicit measurable neural changes. Compared with chemical stimuli, this provides a controlled stimulus onset and discrete stimuli. This is especially important when carrying out tests where stimulus timing is important (e.g. electrical conductance tests or ERPs). In humans, electrical evoked ERPs (independent of the dynamics of transducing external chemical olfactants) were characterized following stimulation of the olfactory mucosa (2 Hz monophasic pulsed, 500 μs pulse width, 0.5-4 mA; bipolar stainless steel electrode, nasal mucosa; [19]). The characterization of the olfactory evoked potential in humans were comparable to subcutaneous EEG recording from rabbits and amphibians [119, 120, 144].
Early clinical research used electrical stimulation applied to the olfactory nerve as a tool to treat temporal lobe epilepsy (TLE) in man. Olfactory hallucinations (phantosmia) are a symptom of TLE, often occurring before the onset of an episode. In both animal models and in humans, chemical olfactants have been used as a way to attempt to control seizures with varying degrees of success [145–147]. The limbic system generates and regulates rhythmic activity in the brain, which is pathological in TLE. Application of electrical stimulation to the human mucosa was hypothesized to attenuate seizure frequency through the activation of the pyriform cortex’s connection with the limbic system and has shown promise as a possible therapeutic intervention for seizures [148]. However, we are aware of no trials in humans to treat epilepsy using electrical stimulation to the olfactory system.
In an attempt to better understand the link between seizures and olfactory hallucinations, clinicians successfully elicited phantosmia by applying intraoperative electrical stimulation to the olfactory bulb in awake patients with epilepsy [149]. The origin of this perceived olfactory phenomena is debated as either occurring in the olfactory bulb [150] or from cortical regions with projections from the olfactory bulb (e.g. entorhinal cortex, amygdala, OFC) [151, 152]. In children with epilepsy, stimulation on the orbital frontal cortex produced distinct olfactory perceptions (50 Hz biphasic pulsed, 300 μs pulse width, ≤ 5 s pulse train, 3-9 mA; subdural electrodes on the ventral surface of the frontal lobe; [153]). While transient odors can be experienced using direct olfactory bulb stimulation, subjective experience of a smell has yet to be produced using noninvasive methods of electrical stimulation, e.g. stimulation on the mucosa [154]. Noninvasive electrical stimulation in human subjects has produced changes in EEG recordings without behavioral percepts [19]. Despite no subjective experience of smell, resting state fMRI obtained following electrical stimulation of the olfactory mucosa resulted in activation in cortical olfactory structures (2-180 Hz sine wave, 90/180 Hz burst, 100 μs burst duration, 5 cycles/burst, 0.5-3s/30-50 s on/off, 50-800 μA; silver electrode, 0.7 μm dia., stimulating elec.: olfactory mucosa, reference: forehead; [31]). This may indicate that cortical neuromodulation without the concurrent subjective sensory experience is plausible during olfactory stimulation.
In summary, the olfactory nerve does not pass through the thalamus, instead it passes directly to the olfactory cortex and neocortex, including the OFC cortex [155]. Approaches to stimulate the olfactory nerve include invasive electrodes placed on the olfactory bulb or non-invasive methods that place electrode in the nostril to touch the nasal mucosa. To date, there have been few studies in humans that have applied electrical stimulation to the olfactory system outside of the olfactory mucosa. Deficits in olfaction are correlated with many psychiatric and neurological conditions (e.g. epilepsy and schizophrenia) and a selective electrical stimulation tool could be used as a way to study disordered neuropsychiatric responses to induced phantosmia [136]. However, electrical stimulation of the olfactory system is rarely used clinically. The lack of subjective smell experience following non-invasive stimulation is argued to be an indication that there is a lack of meaningful olfactory system activation, and therefore cortical activation. However, there is evidence from both EEG and fMRI studies that electrical stimulation, even to the mucosal receptors, modulates cortical activity in olfactory systems [19, 31]. This may indicate that any changes in cognition or behavior due to olfactory stimulation are not just be due to olfactory percepts. While untested, there may be potential for non-invasive electrical stimulation to modulate cortical regions that are difficult to reach using conventional electrical stimulation approaches (i.e. the limbic system), without necessarily producing percepts (a need for sensory substitution).
5.2. Optic Nerve Stimulation
The optic nerve, the second cranial nerve, conveys visual information. Light is transduced into electrical signals by photoreceptors (rods and cones) in the retina. The signal is then propagated through the second layer bipolar cells and then to retinal ganglion cells, whose axons form the optic nerve (Fig 4; blue). The axons of the ganglion cells become myelinated after exiting the back of the eyeball and leaves the orbit via the optic canal. The optic nerve carries information from the left and right retina and meets in the optic chiasm as the optic nerve enters the middle cranial fossa. There, information from the nasal and temporal half of the retina cross to the opposite optic tract before traveling around the cerebral peduncle to terminate directly onto the brain. The optic nerve does not synapse onto nuclei in the brain stem, but instead synapse directly onto the lateral geniculate nucleus (part of the thalamus), superior colliculus, the pretectal area, and the hypothalamus. Most of the information from the optic tract is conveyed to the visual cortex via the geniculate nucleus. However, some neurons from the optic nerve synapse in areas that are involved in reflexive eye movement and circadian rhythm. Both noninvasive and invasive electrical stimulations have used a range of doses to modulate optic afferents. Applications of pulsed and sinusoidal waves typically use frequencies under 40 Hz when applied non-invasively, while invasive methods of optic nerve electrical stimulation use a range of frequencies [156]. For example, invasive prostheses that directly stimulate the optic nerve use pulsed waveforms at high frequencies (up to 320 Hz) in order to obtain discrete light points [10]. Electrode placement, when applied non-invasively, is either monopolar (with “return” electrode/s placed either cephalically or extracephalically) or bipolar (with both electrodes around the orbit). Visual implants concentrate electrical stimulation epiretinally (on the retina; [157]), subretinally (beneath the retina; [158]), suprachoroidal (between the choroid and the sclera; [159]), cortically [160], and on the optic nerve [161]. Our focus here is on optic nerve stimulation, however, retinal stimulation is also discussed, as the retina contains the receptors of the optic nerve.
Use of invasive optic nerve stimulation in humans has focused on visual prosthetics that target the optic nerve with electrical current in order to rehabilitate the optic nerve [162] or to activate the remaining undamaged nerves in a pattern replicating visual patterns. Visual prosthetics that invasively target the optic nerve to create visually meaningful signals require that the visual cortex and downstream visual processing centers have retained their function, while bypassing damaged structures in the retina, such as in retinitis pigmentosa [163]. Retinitis pigmentosa causes photoreceptor cells to disappear over time, while ganglion cells and axonal processes remain intact, causing visual loss [164]. In some of these cases, a self-sizing spiral cuff is surgically attached around the optic nerve with the ability to apply either bipolar (stimulation is between two contacts on the cuff) or monopolar (the return is not on the cuff) stimulation. Unless otherwise noted, this type of cuff electrode is used when describing direct optic nerve stimulation (cuff electrode around optic nerve, four platinum contacts placed around the optic nerve at 0.2 mm2 contact area). Another form of direct optic nerve prosthesis device uses small stimulating wires placed at the location where the optic nerve leaves the eyeball to produce a more focal distribution of phosphenes (40-320 Hz biphasic pulsed, 250 μs pulse width, 5-300 μA; 3.5 mm from the limbus, three platinum wires, 0.5 mm dia.; [165]). The first steps in validating an optic nerve stimulating visual prosthesis has been to relate electrical stimulation dose and location to phosphene characteristics (location, intensity, size, etc.). The relationship between electrical stimulation site to the subjective visual perception can be approximated with volume condition models, and validated experimentally in the optic nerve (10-320 Hz biphasic pulsed, 21-400 μs pulse width, <17 pulses per burst, < 3.8 mA; [166]).
The ultimate goal of visual prostheses is not simply to elicit visual percepts, but to evoke recognizable patterns and regain some semblance of vision. By collecting data on individual’s subjective activation maps, visual stimuli can theoretically be encoded (captured by head-mounted cameras) into electrical stimuli that appear as patterns to the individual subject. In this manner, a subject with an implanted optic nerve cuff is able to decode simple shapes (1-320 Hz biphasic pulsed, 21-400 μs pulse width, 10 μA-3.8 mA; [161]). However, decoding these subjective flashes of lights into a meaningful visual field is still a daunting tasking. One way to objectively assess the function of optic nerve prostheses following implantation is by measuring visual evoked potentials over the visual cortex to determine if electrical stimulation of the optic nerve is conducting to upstream visual systems (0.3 Hz “single-charge recuperated pulses with a ratio of 1:9”, 213-426 μs pulse width, 92-1,040 μA; [167]). Even with subjective and objective measures of visual activity produced by optic nerve electrical stimulation, progress toward a clinically meaningful prosthesis has been slow. Prostheses targeting other sensory systems have had clinical success, such as with cochlear implants [168], but visual prostheses have not made the same breakthroughs.
While visual prostheses are used as a relay between damaged and healthy parts of the visual system, other forms of optic nerve electrical stimulation are used for neurorehabilitation and recovery of unhealthy sections of the optic system [169]. Following damage by either trauma or surgery, visual degeneration, as evaluated by subjective visual field (VF) loss, can be permanent [170]. In rat models, electrical stimulation to the optic nerve improves the chance of retinal ganglion cells recovery following damage (20 Hz monophasic pulsed, 50 μs pulse width, 10-70 μA, 2 hour duration; two silver ball electrodes placed on transected end of optic nerve, 1 mm dia.; [171]). In humans, application of electrical stimulation to the optic nerve following surgery to remove tumors that compressed and damaged the optic nerve increased the VF recovery post-surgery, though the recovery was not always predicted by nerve conductance tests during the surgery (25-100 Hz biphasic varied burst pattern, 250-1000 μs pulse width, 1800 μA; 3-2 gold wire implanted optic nerve bipolar electrodes, 0.1 mm^2 ellipsoid; [172, 173]). Stimulation with electrodes placed on the cornea, rather than the optic nerve itself was also explored for long term axonal survival and signal transduction to the visual cortex (20 Hz biphasic pulsed, 50 μs pulse width, 50 μA; bipolar electrodes placed on a contact lens; [174]). This technique was reported to improve measures of visual acuity in controlled human trials (20 Hz biphasic pulsed, 10 ms pulse width, current titrated to phosphene thresh.; bipolar electrodes placed on a contact lens; [175]). Research into creating an effective visual prosthesis or rehabilitation device using invasive optic nerve stimulation is long-standing and ongoing; the invasive nature of the approach evidently limits large sample-size research especially for less severely damaged visual systems, such as partial blindness due to stroke [176].
Invasive methods of optic nerve stimulation may provide a more “direct” route to modulating a nerve but involved surgery with associated complications [162]. Comparatively, non-invasive methods do not require surgery and can therefore be applied to a larger and more varied population. Because they are low resolution and applied for limited time period, non-invasive approached are directed toward neurorehabilitation applications. “Transorbital” alternative current stimulation is a noninvasive technique that targets the retina and optic nerve using electrodes that are applied to the eyelid or around the eye. Conventionally, the transorbital stimulation electrodes are either gold or sintered Ag/AgCl ring electrodes, similar to those used for EEG recordings, or 3×3 cm water-soaked pads with rubber electrode inserts. Electrodes are typically positioned around the eye (2-4 per orbit) [177–181]. In some cases of monopolar stimulation, the “return” electrode is positioned on the right upper arm, right shoulder, near the eye, or at EEG location Oz [82, 182, 183]. A few studies have investigated transcorneal electrodes that are shaped like contact lenses [175, 184–186] or transcorneal hair-like DTL electrodes [187] that directly contact the cornea [188–192] or the cornea and sclera [185].
Patients with vision loss due to optic nerve damage who received transorbital electrical stimulation were reported to display an increase in detection accuracy in their field of defective vision (AC, 2-9 pulses per burst, <1000 μA, 10 days; four sintered Ag/AgCl ring electrodes placed at or near the eye, one return electrodes on the right wrist; [193]). Additionally, these changes in detection were accompanied by changes in EEG alpha spectrum power (here defined as 7.5-12.5 Hz), indicating engagement of cortical effects. A larger (n = 446) open-label observational study of patients with optic nerve lesions similarly reported increases in VF area and visual acuity (VA) associated with changes in EEG alpha power spectra (9-37 Hz varied burst pattern, <500 μA, 25-40 min/day, 10 days; active: 4 periorbital gold electrodes, bilaterally superior and inferior to the eye, return: midline relative to occipital pole, 30 × 30 mm stainless steel; [156]). These alpha frequency changes may be mediated by thalamo-cortico-thalamic relay circuits, a pathway proposed to “sharpen” or tune receptive fields in the visual system [194–197]. Bola et al. reported that blind patients who received transorbital electrical stimulation displayed increased coherence in the high alpha/low beta frequencies (11-13 Hz) within the visual cortex and between the visual and frontal cortices [198]. These changes were associated with improvements in visual abilities. These findings point to the ability of this technique to increase inter-area coherence within a power band that may be required for effective perceptual function [199]. It is therefore possible that the entrainment phenomenon seen within certain frequency bands would be valuable for plasticity changes, particularly for remodeling required post injury. A case study of a patient of an 11 year-old optic nerve lesion reported VF and EEG changes associated with transorbital electrical stimulation, even at 1.5 years follow up, demonstrating long term cortical plasticity and rehabilitation (10–30 Hz varied burst pattern, <600 μA, 30–40 min/day for 10 days; active: four Ag/AgCl ring electrodes around or on the eyelid, return: forearm; [177]). Transorbital electrical stimulation has also reportedly improved mood rating in patients, possibly modulating non-vision related cortico-thalamic pathways (5-30 Hz varied burst pattern, < 500 μA; active: 4 periorbital gold electrodes, bilaterally superior and inferior to the eye, return: occipital pole; [12]).
Transorbital electrical stimulation and other optic nerve electrical stimulation methods, differ from “physical” optical stimulation techniques that use light. The application of light to the eye to modulate cognition and in the treatment of psychiatric and neurological disorders has a long history [200, 201]. This includes recent work in mice on showing reduction in peptide amyloid-β plaques -a hallmark of Alzheimer’s disease – following 40 Hz flickering light [202], with ongoing work addressing if this technique can be effective clinically [203]. These physical (non-electrical) studies offer a comparison for electrical approaches; in many cases the target of activation (retina/optic nerve) may be the same, especially when outcomes are considered secondary to sensory percepts. When physical and electrical approaches engage overlapping brain processes, which technique to use may be based on convenience.
As noted, the optic nerve can be targeted either invasively for prostheses development and rehabilitation, or non-invasively for rehabilitation and potentially other neuropsychiatric indications. Invasive optic nerve stimulation in humans has attempted to bypass damaged retinal cells by electrically stimulating the optic nerve directly using sensor arrays that transduce light into meaningful stimulation of the nerve, using cuff or pin electrodes. In parallel to research on optical nerves stimulation, work has been conducted on cortical visual prostheses, where visual percepts are produced by electrically stimulating the visual cortex [204–206]. Cortical prostheses offer some notional advantages to optic or retinal prostheses. The cortex has a larger surface which is considered theoretically advantageous for visual resolution by allowing for arrays of cortical stimulation electrode. Cortical implants are also hypothetical option to a wider range of visually impaired patients, those who have damage to both the retina and optic nerve. However, the technological advances necessary to decode the many synaptic connections and visual field areas is a barrier to cortical prostheses success in general. Rather, in considering more diverse cortical targets and indications for use, optic nerve modulation offers the possibility of a more nuanced behavioral effects. The optic nerve is upstream of the visual cortex and of the lateral geniculate nucleus of the thalamus, which may mean that modulation of the optic nerve could result not only in changes to visual sensory information but also to other cortical regions via the thalamo-cortical network [205, 207–209]. As such, optic nerves stimulation may produce changes in cognition independent of the visual cortex, so independent of percepts (e.g. phosphenes).
In summary, electrical stimulation of the optic nerve and retina is typically tested to treat visual system disorders [165, 210]. Therefore, studies on behavioral and cognitive effects of transorbital and optic nerve stimulation have centered on vison loss and rehabilitation. However, transorbital electrical stimulation has been linked to changes in EEG and behavior which may suggest cortical changes outside of visual functions, which in turn supports exploration of cognitive modification and clinical indications not specific to vison. Compared to invasive (surgical) approaches, noninvasive optic nerve stimulation (e.g. transorbital electrical stimulation) can more readily be applied to diverse clinical population, as well as to healthy individuals. Future applications of non-invasive optic nerve stimulation may also investigate changes to behavior and cognition independent of sensory percepts (not simply linked to phosphenes).
5.3. Trigeminal Nerve Stimulation
The trigeminal nerve (CN V) is one of the largest cranial nerves. Its afferents transmit general sensory information from the orofacial structures to the brain, while the efferents send motor control information to the muscles of mastication from the brain. The afferent branches are discussed here. There are three main branches (divisions) of the trigeminal nerve that project to three sections of the face (Fig 4; orange). The ophthalmic (CN V1) nerve exits through the superior orbital fissure and innervates the upper part of the face. The maxillary (CN V2) nerve exits through the foramen rotundum innervates the middle part of the face. Finally, the mandibular nerve (CN V3) exits through the foramen ovale and innervates the lower face. The three branches meet on the floor of the middle cranial fossa where the cell bodies of the sensory neurons are housed in the trigeminal ganglion. The central processes of the neurons synapse onto the second order neurons in the pons on the ventrolateral aspect in one of three trigeminal nuclei. Most of these fibers synapse onto third-order neurons in the ventral posteromedial nucleus (VPM) of the thalamus that relay information to the primary somatosensory cortex, but a small portion of the secondary neurons relay information back to the nucleus tractus solitarius (NTS) in the brain stem and the spinal cord.
The pseudounipolar neurons of somatic afferent nerves encode tactile information, including proprioception, temperature, touch, and pain. Generally, these are A-nerve fibers (myelinated, fast, large) and C fibers (unmyelinated, slow, small) [211–213]. These A-subtypes are subdivided by the type of information they convey: pain (nociceptive) or touch (haptic). In order of descending conduction speed, the classifications are: C (nociceptive and temperature), Aα (proprioception), Aβ (haptic), and Aδ (nociceptive and temperature) (Figure 4). Some somatic neurons contain specialized receptors, such as for pressure, but most nociceptive fibers are “free” nerve endings in the sense they are considered to have no specialized receptor type [214]. All fibers of cranial nerves or peripheral nerves that transduce haptic or pain information follow this classification (cranial nerves V, VI, IX, & X).
The trigeminal nerve has been targeted for electrical stimulation using subcutaneous (under the skin), percutaneous (through the skin) or cutaneous (on the skin) electrodes. A pulsed waveform is typically applied with a range of frequencies and intensities depending on the application. Transcutaneous electrical nerve stimulation (TENS) devices are typically adopted as pulse generators in cutaneous trigeminal nerve stimulation (TNS) [215, 216]. TENS encompasses a broad range of techniques and devices derived from pioneered work by Wall and Sweet [217] using pulsed stimulation to excite peripheral nerves (originally: 100 Hz pulsed, 100 μs pulse width, intensity at sensory threshold). Research using TENS further refined doses based on electrophysiological and behavioral studies in humans and animals [218, 219]. These TENS dose parameters were adopted by TNS researchers. Typical doses for TNS are 1-200 Hz biphasic pulsed, 50-250 μS pulse width, with a range of intensities. Surgically implanted TNS devices typically use cylindrical electrodes (as used for spinal cord stimulation) placed adjacent to the trigeminal nerve [220, 221]. Imaging, such as x-ray, can be used confirm proper placements. Externally placed TNS typically uses bipolar gel or sponge electrodes placed bilaterally over the ophthalmic branches (V1) (Figure 2c), though other electrode locations are used.
Electrical stimulation of the trigeminal nerve has been applied as a therapeutic intervention in craniofacial pain disorders, such as trigeminal neuralgia [220–223]. Surgically implanted TNS trials have been nonrandomized and small scale thus far, but report encouraging results for the treatment of refractory trigeminal neuralgia (; [221, 224, 225]). In addition to interventional use for neurological disorders, TNS (subcutaneous, percutaneous, and cutaneous) have been applied as a diagnostic tool for disorders such as neuropathic pain [225]. For example, scalp potentials over the somatosensory cortex evoked by trigeminal nerve stimulation in patients with neuralgia can be used to identify possible lesion sites along the trigeminal nerve as well as pathological related to disease progression (2 Hz monophasic pulsed, 100 μs pulse width, 8-10 mA; bipolar electrode placed on both lips; [226]). The potentials recorded from the scalp may reflect not only cortical responses from the somatosensory region, but may also represent temporally early evoked responses from the brainstem and thalamus [227–231]. Isolating these early components of the evoked potential associated with deep brainstem nuclei activation is difficult to do without confounding artifacts from muscle reflexes caused by stimulating facial muscles [232]. Myogenic artifacts can be reduced by placing stimulating needle electrodes percutaneous rather than cutaneous. If properly recorded, early deep brain signals can theoretically serve as a neural correlate (biomarker) of trigeminal nerve stimulation.
Among people with epilepsy, 30-40% of patients are unresponsive to antiepileptic medication, known as refractory epilepsy [233]. Invasive electrical stimulation of the vagus nerve (section 6) has emerged as a possible treatment for refractory epilepsy, but has device and procedural costs, and side-effects. TNS was hypothesized to have a similar effect on seizures with the benefit of reduced side-effects. In rat models of epilepsy, TNS successfully halted cortical seizure activity (1-333 Hz pulsed, 500 μs pulse width, 3-11 mA; bilaterally implanted on the infraorbital nerve, platinum cuff electrode, 0.5 mm wide 0.025 mm thick; [234]) and displayed some protective effects on cognitive function such as learning and memory (140 Hz, 500 μs pulse width, 1 min/4 min on/off, 10 m; bilateral gel-based electrodes over left and right opthalmic branches; [235]). Cutaneous TNS has also been explored as a therapeutic technique in human subjects with epilepsy [9, 236, 237]. A double-blind randomized controlled trial indicated reductions in seizure activity following cutaneous TNS, but did not show a significant decline when compared with controls (control: 2 Hz active: 120 Hz pulsed, < 250 μs pulse width, 2 s/90 s on/off, 12 h/day, 18 week duration; bipolar gel electrode over the left and right opthlamic branch; [238]), with benefit persisting in a follow up treatment phase that was comparable with control subjects (control: 2 Hz active: 120 Hz pulsed, 250 μs pulse width, 30 s/30 s on/off, 12 h/day, 12 month duration; bipolar gel electrode over the left and right opthlamic branch; [239]). Questions remain about the mechanism of action of TNS on epilepsy, but is hypothesized to act through the trigeminal nerve projections to the NTS which has limbic and cortical projections [240–242].
Following the results from TNS treated epilepsy, other neuropsychiatric disorders, including major depressive disorder (MDD), were targeted for therapeutic trigeminal nerve stimulation. Theoretical models of MDD have proposed dysfunctional network connectivity between brainstem, thalamus, and higher cortical regions [243]. Unless otherwise noted, the following clinical trials used TENS units to stimulate bilaterally over the ophthalmic branch (V1) (120 Hz pulsed, 250 μs pulse width). A randomized sham-controlled trial treating MDD patients reported decreased depressive symptom ratings following noninvasive TNS (10 days, 30 minutes/day; electrodes: 25 cm2 saline soaked sponges; [244]). These effects were maintained for at least 30-days post-stimulation, suggesting plasticity. There have been few randomized controlled studies in neuropsychiatric populations of trigeminal nerve stimulation. Uncontrolled 8-week pilot studies have tested trigeminal stimulation in MDD [245, 246], MDD-PTSD [247], migraine [248], tinnitus [249] and ADHD [250]. There have also been case studies in patients with anxiety disorders [251], posttraumatic stress disorder [252], hallucinations in schizophrenia [253], and fibromyalgia [254].
In most cases, symptom questionnaires were the only measures of improvement in trials of trigeminal nerve stimulation in psychiatric populations. However, objective cognitive measures are reported in some cases. For example, subjects with ADHD can display impulsivity that can be measured by an inability to inhibit their response on an attentional task [255]. Trigeminal nerve stimulation was reported to improve response inhibition in subjects with ADHD [250]. In healthy individuals, a double blind sham controlled study reported frequency dependent effects of trigeminal nerve stimulation on attention network modulation (High Frequency: 120 Hz Low Frequency: 2.5 Hz biphasic pulsed, 250 μS pulse width, 14 mA; electrodes 30 mm x 94 mm bilaterally cover the opthalamic branch; [256]). Subjects receiving acute high frequency stimulation had decreased reaction time on a psychomotor vigilance task (pressing a button when a dot appeared), increased subjective fatigue ratings, and decreased flicker fusion frequency (the frequency at which flickers appear as a steady light). Long term treatment with low frequency stimulation may also have a calming effect (High frequency: 3-11 kHz biphasic pulse-modulated, 5-7 mA; Low frequency: 0.5-0.75 kHz, < 5 mA; 1 week, 20 min/day; Ag/AgCl [F8, back of neck]; [257]). A potential explanation for these findings is trigeminal nerve stimulation modulation of the ascending reticular activating system (RAS), a network of nuclei that control attention and the sleep/wake cycle.
In summary, the branches of the trigeminal nerve provide cutaneous sensation for much of the face and scalp. Clinical trials of trigeminal nerve stimulation typically addressed neurological disorders such as seizure disorders, neuralgia, and migraines. However, improvements in mood ratings have led to the treatment of neuropsychiatric disorders such as MDD, anxiety disorder, post-traumatic stress disorder, and other neuropsychiatric populations. The therapeutic potential of trigeminal nerve stimulation is often compared with the outcomes of vagus nerve stimulation (see section 6), as both nerves include the NTS among their targets, with the NTS in turn projecting to the amygdala, hypothalamus, and the limbic forebrain (Figure 5). However, the nature of NTS modulation by these distinct cranial nerves and impact on specific behavioral and cognitive outcomes is not well characterized [258, 259]. Regardless of functional overlap with the vagus nerve, cortical regions that are part of the trigeminal nerve circuit are implicated in a range of complex cognitive processes. Some of these cognitive processes such as attention and vigilance have been explored within neurological and neuropsychiatric populations [255, 256], but have not have not specifically studies TNS effects on cognition as a stand-alone construct. There are thus open and compelling questions about whether trigeminal nerve stimulation has an effect on cognition at all, and if this effect acts through the NTS-cortical network.
5.4. Facial Nerve Stimulation / Glossopharyngeal Nerve Stimulation
Taste sensation from the tongue is mediated by the sensory branch of the facial nerve (CN VII) and the glossopharyngeal nerve (IX). The chorda tympani branch of the facial nerve transmits taste signals from the anterior two-thirds of the tongue, while the glossopharyngeal transmits taste from the posterior one-third (Fig 4; pink & purple, respectively). Groups of non-neural taste cells form taste buds on the tongue, soft palate, pharynx, upper esophagus, and epiglottis and detect gustatory sensation. There are five main taste qualities transduced by different mechanism in the taste cells: salty, sour, sweet, bitter, and umami. Salty and sour stimuli (tastants) activate voltage-gated ion channels; and bitter, sweet and umami activate G-protein-coupled receptors. The apical end of the taste cells contains microvilli that detect the chemicals that have been dissolved in saliva, while the basal end contacts the afferent nerves of the corresponding cranial nerve. Dysfunction of taste sensation has been linked to neurological and physiological diseases, obesity, and other disorders [260, 261].
The facial nerve (CN VII) primarily contains motor nerve efferents that innervate and control the facial muscles, but also contains sensory and parasympathetic fibers (see Figure 3 for details). These include somatic sensory fibers in the nervus intermedius that innervate the concha of the auricle, a part of the skin of the ear that are made up of 70% Aβ myelinated discriminatory touch fibers and 30% type C-nociceptive fibers [262]. Part of the sensory root of the facial nerve also forms the chorda tympani, which joins the lingual nerve —a branch of the trigeminal nerve— and conveys taste information from the anterior two-thirds of the tongue as well as general visceral sensation from the nasal cavity and soft palate. The nervus intermedius enters the skull inferiorly through the stylomastoid foramen, sandwiched between the vestibulocochlear nerve and the facial motor root, on the posterior wall of the middle ear. It then meets the geniculate ganglion in the cavity of the inner ear where the cell bodies of these first order sensory pseudounipolar neurons are located. The nervus intermedius then reenters the skull at the internal auditory meatus and synapses onto second order neurons at the junction between the pons and the medulla in the solitary nucleus (for taste) and the spinal trigeminal nucleus of the medulla (for touch sensation).
The glossopharyngeal nerve (CN IX) is a complex structure. It has four nuclei and contains general visceral afferents, general somatic afferents, special visceral afferents, special visceral efferents, and general visceral efferents (see Figure 3). The sensory fibers of the glossopharyngeal nerve are responsible for conveying taste, general touch, and visceral sensation from the posterior one-third of the tongue and the pharynx, as well as general touch sensation from the external ear. The myelinated taste fibers extend from the taste buds in the tongue to the pharyngeal branch of the glossopharyngeal nerve, entering the skull at the jugular foramen. The cell bodies of the general visceral afferent fibers and special taste fibers are located just inside the skull in the inferior glossopharyngeal ganglion. The gustatory and visceral sensory fibers synapse in the medulla on the solitary nucleus. The cell bodies of the general touch afferents are located in the superior glossopharyngeal ganglion, just above the inferior glossopharyngeal ganglion and terminate in the spinal trigeminal nucleus in the medulla.
Glossopharyngeal and facial afferents can be electrically targeted through the major sense organ for taste, the tongue. Pulsed stimulation is the most common waveform using bipolar or electrode arrays placed on the tongue. Invasive stimulation is not common in these two nerves, apart from electrophysiology studies in animals. Some studies used a device called a tongue display unit (TDU). It is made up of a flat square array of gold-plated electrodes that are placed on the superior anterior portion of the tongue. In our description of dose, the over-all electrode array dimensions are reported and the size of the individual electrodes within the array. The waveform reported may include individual pulse frequency, burst frequency, and outer burst frequency (Figure 3). For a more complete explanation of TDU dosage, refer to [263].
Gustometry is the quantification of taste perception. In humans, this is achieved by applying a stimulus to the tongue or mouth and recording the elicited subjective sensation [264]. Chemogustometry uses various concentrations of the four “primary” tastants in order to elicit a gustatory sensation [265]. Comparatively, electrogustometry is the application of electrical current to the tongue, in place of chemical stimuli, in order to induce a gustatory sensation. One prototypical device (Taste+) applies low levels of electrical current to the tongue via conductive utensils that contain electrodes in order to induce or augment taste while eating or drinking (200-600 Hz pulsed, 20-120 μA; [266]). Electrical current can be applied in discrete quanta and is believed to be more spatially specific than chemical stimuli, which in a clinical setting is desirable when diagnosing deficits in gustation [267]. Indeed, the first electrogustometry device was constructed in the 1950’s as a way to precisely assess taste functionality in a clinical population (5.75-300 μA DC; cathode: stainless steel 5 mm dia. placed lateral anterior & posterior, anode: 4x5 cm wrist; [268]). Electrogustometry is used to investigate gustatory threshold and papillary density [20], age related changes to taste [269], and other clinical disorders [270, 271]. Efforts have been made to characterize the effects of electrogustometry and its difference – if any to chemical stimulation. Anodal stimulation (with the anode inside the mouth, and cathode outside the mouth) has been reported as metallic tasting [268, 272, 273], while cathodal stimulation is subjectively described as sweet or bitter [274]. However, the specific relationship between dose and perception remains to be investigated. Recordings obtained from the rats’ chorda tympani nerves evoked by chemo- and by electro-stimuli (chemo: concentration; electro: intensity) have similar dose-responses and resultant ERP time course [275]. However, higher intensities of electrical stimulation to the tongue likely activates somatosensory afferents as well as gustatory afferents; recruiting more somatic fibers at higher intensities (anodal pulsed, 1000 ms pulse width, 4-400 μA, anode placed on right or left lateral edge of tongue, stainless steel 5 mm dia., cathode placed on the contralateral upper arm) [276]. Potentially conflated gustatory / somatosensory responses must be taken into consideration when interpreting results from electrogustometry.
Electrical stimulation of the tongue can also be used in sensory substitution. Generally, sensory substitution is a form of human-machine-interface (HMI) - transducing information from one sensory modality (using sensors) into another (for review see [277]). During tactile-visual substitution a video capture device transduces a 2D image into the corresponding tactile representation (mechanical or electrical). The tongue is a theoretically compelling target to elicit tactile stimulation as it has a high receptor density and nerves that project directly to the brain. Both subjects with vision loss and those with healthy visual systems are able to be trained to “see” a 2D image (i.e. an “E”) projected onto the surface of the tongue via an electrode matrix (200 Hz pulse, 40 μs pulse width, 50 Hz burst, 3 pulses per burst; 12 x 12 electrode array placed on the super anterior tongue, Au plated, 1.55 mm dia.; [278]). Blind individuals may be more efficient at converting this type of information than sighted individuals [279], potentially due to cortical remolding (cross modal plasticity) in the visio-tactile network that takes place in visually impaired individuals. [280]. Neuroimaging studies have reported increased activation in visual regions following sensory substitution training using electro tactile stimulation on the tongue in blind — but not sighted – individuals (Varied burst pattern, 40 μs pulse width; 3 x 3 cm array placed on superior anterior surface of tongue, 144 Au plated electrodes, 1.55 mm dia.; [281]).
Tactile-vestibular sensory substitution has also been used as an input to drive electrical stimulation of the tongue [282–285]. In this case an accelerometer is used to give real time feedback — in the form of electrical tongue stimulation — about the subject’s body sway position – allowing the body compensate, creating a closed loop system (200 Hz, 25 μs pulse width, 50 Hz burst,3 pulses per burst; 10 x 10 electrode array placed on the super anterior tongue, Au plated, 1.5 mm dia.;[286]). Several papers have reported improvements in gait that outlasted the stimulation sessions, sometimes by months [283, 286]. Based on the reported sustained improvements in gait produced by the tactile-vestibular sensory substitution, an alternate mechanism was proposed based on the cranial nerve innervation of the tongue [287–289]. Wildenberg’s group conducted experiments (using the term cranial nerve non-invasive neuromodulation; CN-NINM), under the hypothesis that electro-tactile stimulation was modulating vestibular function through afferents (Trigeminal and Facial) that project from the tongue to brainstem nuclei adjacent to the vestibular nuclei–specifically the solitary nucleus – leading to neuroplasticity and therefore may not require sensory substitution (feedback) to improve vestibular function. Both subjects with vestibular dysfunction and healthy controls demonstrated improved postural control when exposed to a sway inducing visual stimuli [290, 291] following electrical stimulation on the tongue alone (without sensory substitution) (200 Hz pulsed, 50 μs pulse width, 50 Hz burst, 3 pulses per burst, ≤ 17 V; 12 x 12 electrode array placed on the super anterior tongue, Au plated, 1.5 mm dia.; [287–289]). In addition to displaying improvements on measurements of physical movement in the previous study, patients showed normalization of the cortical network connections involved in the processing of balance by visual motion. Electrical tongue stimulation without artificial feedback mechanisms has been applied in a randomized double blind four-week trial to treat gait dysfunction in multiple sclerosis and improved patients’ gait (200 Hz pulsed, 50 μs pulse width, 50 Hz burst, 3 pulses per burst, ≤ 17 V; 12 x 12 electrode array placed on the super anterior tongue, Au plated, 1.5 mm dia.; [292]). Stimulation of the tongue may act as a tool for sensory substation (closed-loop system) or as a pathway to promote cortical remodeling that may not require feedback (open-loop system).
Direct stimulation of a branch of the glossopharyngeal nerve has been proposed as a possible target for epilepsy treatment. The glossopharyngeal nerve has an afferent branch called the nerve of Hering (HN), which shares a brainstem nucleus with the vagus nerve. The vagus nerve is a known target for therapeutic treatment of seizures using both invasive and noninvasive methods (see section 6: Vagus Nerve), inspiring exploration of a possible therapeutic role for the HN. An in vivo canine study reported seizure cessation in 75% of trials (versus 0% spontaneous cessation) following HN electrical stimulation (40 Hz pulsed, 1000 μS pulse width, 10 mA; copper wire placed dorectly on the HN; [293]). However, despite efficacy trials in human cadavers, there have been no in vivo human trials to date [293].
In summary, the tongue is a receptor dense organ that is enervated by cranial nerves VII and IX, as well as V and X. Electrical stimulation of the tongue nominally targets nerves VII and IX, reflecting applications or mechanisms linked to stimuli (taste) substitution, or nerves V and IX for sensory substitution, reflecting applications or mechanisms linked to sensory (touch) substitution. But (as emphasized throughout this review) perception is not necessarily a prerequisite for neuromodulation. The special convergence of nerves around the tongue modulate a diverse set of brain stem nuclei and connected cortices (Figure 5), providing an anatomical substrate for changing cognitive processes by electrical stimulation. Most clinical applications have addressed gait improvement and the vestibular-visual pathway. In addition to the direct innervation of the tongue by cranial nerves V, VII, IX, and X, there are other possible cranial nerve (and so brain networks) that electrical tongue stimulation may access through axon anastomoses (connection between normally discrete nerves) of anatomically adjacent nerves activated by current spread (activation of cranial nerves that do not innervate the tongue) [6, 7]. Indeed, reports of taste sensation when electrically stimulating structures within the ear [294], or other locations on the head [295] may reflect anastomoses to taste-related cranial nerves. Taken together, this suggest a framework for modulation of behavior or cognition with lingual electrical stimulation through brain circuit inclusive of and broader than the conventional gustatory system (nerves, brainstem nuclei).
5.5. Vestibulocochlear Nerve Stimulation
The vestibulocochlear nerve (CN VIII) has two distinct components: the vestibular nerve (dealing with balance) and the cochlear nerve (dealing with hearing). Cochlear stimulation, for the purpose of sensory emulation, has been reviewed extensively elsewhere [296–300]. Both nerves transduce sensory information from the inner ear to the brain via mechanoreceptors called “hair cells.” Though the vestibulocochlear nerve is conventionally classified as a purely afferent nerve, there is a small percent of efferents that project to the hair cells in both the vestibular and cochlear track [301]. The vestibular nerve has some 300-400 efferent fibers that project bilaterally from the brainstem. Experiments in mammals indicate that these have excitatory effects on vestibular afferents [302, 303], though a more heterogeneous response has been seen in other species [304, 305].
The vestibular nerve receives signals about the body’s motion from hair cells in the vestibular apparatus of the inner ear. The apparatus is comprised of a membranous sack within a bony labyrinth located within the petrous temporal bone. The vestibular sensory organs (semicircular canals and otolith organs) are filled with endolymph (low-Na+, high-K+) and are surrounded by perilymph (high-Na+, low-K+). When the head undergoes angular rotation or linear acceleration, bundles of hair cells are mechanically displaced via the flow of endolymph, effectively changing membrane potential on the attached sensory fibers. The cell bodies of the first order bipolar sensory neurons reside in the vestibular nerve ganglion (known as Scarpa’s ganglion) located in the internal auditory meatus — which also contains cell bodies of the facial nerve and the cochlear nerve. These vestibular sensory afferents are categorized into two groups with different functional and morphological characteristics: irregular firing afferents and regular firing afferents (for review see [306]). The axons of these first order neurons synapse onto the medulla and pons into four vestibular nuclei (inferior, medial, lateral, and superior). The dendrites of second order neurons synapse onto the superior, medial and inferior vestibular nuclei and form efferent and afferent feedback loops to the cerebellum. Fibers from the lateral vestibular nucleus form the vestibulospinal tract to maintain balance and posture by way of regulating muscle tone. Finally, all four of the vestibular nuclei are connected to the oculomotor, trochlear, and abducens nerves to maintain gaze and eye movement while the head is moving. There is no primary vestibular cortex like there is for audition and vision. However, the vestibular information reaches a large number of cortical regions via the thalamus (Fig 5) [29]. The Parieto-Insulo-Vestibular-Cortex (PIVC) has been identified as a possible network for vestibular processing based on single cell recordings in primates [307]. Functional imaging studies in humans have identified a similar region which includes the inferior parietal lobe, the superior temporal gyrus, the hippocampus, insula, and the anterior cingulate gyrus [29, 308, 309].
Experiments that target the vestibular system with electrical current are typically known as galvanic vestibular stimulation (GVS). The acute and robust repose to GVS has a long history. Alessandro Volta, the creator of the electrical battery, is credited as conducting one of the earliest experiments involving electrical stimulation of the vestibular nerve. In 1790, Volta placed metal rods connected to a voltage cell directly into each of his ears. He then applied 30 V and reported a spinning sensation and heard a loud noise before passing out. Purkinje — of the eponymous cerebellar cells —discussed the effects of an applied electrical current across the head inducing vertigo in his 1820 dissertation [310]. Breuer and Hitzig discovered the relationship between the ocular and vestibular system when they induced nystagmus (involuntary eye movement) by applying current across both mastoids [311, 312].
GVS differs from other types of vestibular stimulation that use non-electrical manipulation techniques [for review, see; 313]. One such method is caloric vestibular stimulation (CVS), a method that involves placing hot or cold water in the ear to induce nystagmus and perceptual changes. CVS is still used by clinicians as a tool to test the visual-ocular reflex [314–316]. Other methods used to activate vestibular afferents include the use of physical rotational devices, such as chairs or swings [313]. These non-electrical studies offer a comparison for GVS studies [317].
As with other cranial nerve techniques, there are several typical permutations of dose. Early application of electrical vestibular stimulation may have involved placing electrodes inside ears (as in Volta’s case), but many contemporary electrode placements are on the skin over the mastoids (Figure 2a) [318]. Noninvasive electrode placements can either be on both sides of the head (bilateral) or one side (unilateral). In the case of a unilateral electrode montage, a return electrode is placed either extracephalically or cephalically. Bilateral stimulation can be either bipolar, where the sides have opposite polarity waveform, or unipolar, where both sides receive the same polarity, in which case there must be a third electrode serving as the cephalic/extracephalic return. Unilateral, bilateral bipolar, and unipolar bilateral electrode montage can each use either monophasic or biphasic waveforms (asymmetric or zero mean). GVS has been applied using waveforms other than just direct current [319]. These include pulsed stimulation [320], noisy [321], and alternating current [318]. Noninvasive GVS electrodes are typically sponge pads or plastic holders filled with a conductive gel (for review see [21]). There are few examples of studies in humans that invasively stimulate the vestibular nerve directly [322].
Electrical stimulation in non-human primates has elucidated the cellular targets of vestibular stimulation. In squirrel monkeys, Goldberg et al proposed that anodal currents applied cutaneously over vestibular afferents were inhibitory while cathodal current was excitatory (10 Hz biphasic pulsed, 50 pS pulse width, ±70; Ag 0.25 diameter wire) [323]. Data suggests that there is a strong relationship between discharge regularity in vestibular nerve cells and the applied electrical current, preferentially stimulating irregularly firing cells [323, 324]. Based on the projections of irregularly firing afferents, this may indicate that GVS activates vestibulospinal, vestibulo-cerebellar and vestibulo-ocular pathways [306]. In addition to stimulating only a fraction of fiber types, only a portion of the vestibular apparatus may be activated by galvanic stimulation. Summarizing the literature from animal and human trials, Cohen and colleagues have proposed that only the otolith system contribute to the behavioral and neural responses elicited by GVS, while the semicircular canal habituates to electrical stimulation [325].
Observable disturbances in equilibrium and eye movement induced by GVS have been studied in both healthy and clinical populations. Changes in nystagmus induced by galvanic stimulation is a useful tool in diagnostic medicine, as it can indicate a disorder in the vestibular end organ [DC, 1-2 mA; bipolar electrode placed bilaterally; 326]. Clinicians using GVS noted observable body sway as a result of electrical application. Since lower currents were able to induce this response, whereas higher and more uncomfortable currents were necessary to induce nystagmus, body sway became a more comfortable indicator of vestibular activation [327, 328]. Varying dose (current amplitude, pulse width, head position, and electrode polarity) affect the changes in postural responses caused by GVS (bipolar bilateral pulsed, 20-150 ms pulse width, 75-250 μA; electrodes Ag-AgCl; [329]). The vestibular system acts as an error detector, sensing environmental or internal movements that might offset balance. In response to a direct current electrical stimulus, the vestibular system can be made to overcorrect in a predictable way (DC, 0.8 mA; bipolar aluminum electrodes applied unilaterally, return on ipsilateral arm; [11]). Typically, a subject will move toward anodal stimulation (the side of the body with the anode electrode) and move away from cathodal stimulation, though the reason for this is not completely understood.
Perturbing a system with moderate electrical stimulation to increase the propensity of neurons to fire is known as stochastic resonance [330]. This is a proposed theoretical principle behind applying “noisy” electrical signal to the vestibular system [331–333]. The vestibular system’s compensatory response to a perturbation is disordered in Parkinson’s Disease (PD) [334]. Noisy GVS is reported to improve movement dysregulation in PD [335–337]. Yamamoto et al. conducted a double-blind sham-controlled study in patients with PD or PD-like disorder in which patients received noisy GVS over 24 hours [338]. They reported increased motor performance in cognitive tasks and measurements of movement (0.01-2.0 Hz zero-mean noisy, 300 s on; unipolar bilateral, electrodes placed over mastoids, return not specified). Other studies have reported that noisy vestibular stimulation reduced movement in PD, though the effects have been comparatively small [339, 340]. It is hypothesized that dopamine independent release of GABA in the substantia nigra is a possible mechanism of action for improving movement disorders specifically using noisy vestibular stimulation [341].
In severe disorders of balance and equilibrium, long term implantable vestibular prosthetics are sometimes used (for review see [342]). Vestibular prostheses are small (~150 μm diameter) surgically implanted wires that can be placed inside sensory organs (e.g., the semicircular canals) or directly implanted on the sensory afferent. Prototypical prostheses tested in rodents and non-human primates provided hypothesized optimal dose-response parameters [320, 343–347]. Golub et al. placed the first successful vestibular implant in man in the semicircular canals in the right ear of a patient with Meniere’s disease, a disorder marked by episodes of severe vertigo followed by tinnitus [322]. During a Meniere attack, the participant was told to cycle currents of increasing intensity until the attack subsided (300-600 Hz monopolar biphasic pulsed, 100 μs pulse width, 8 μs pulse gap, 25-350 μA; trifurcating array/3 electrodes per array in semicircular canal, Pt-Ir, each electrode has 150 μm diameter and 0.25 mm length). The subject was able to successfully suppress an attack at a threshold 150 μA post-surgery. However, only one attack occurred post-operatively during the entire 63-week following. Golub et al. suggest that the damage caused to the vestibular system during the surgery that did not recover to baseline [322]. The authors postulated that the surgical implantation of the prostheses might not have worked as it did in the animal models because it was done in a diseased vestibular system instead of a healthy one.
The application of GVS is not limited to investigations of posture and balance, but extends to higher levels of cognition. Patients with vestibular dysfunction show deficits in many cognitive domains including memory, perception, and learning [4, 348]. The vestibular system plays a role in modulating spatial memory [349]. Sensory information from the vestibular system projects to the hippocampus, a brain region involved in memory (Figure 5; [350, 351]). Special spatial coordinate coincidence detectors located in the hippocampus, known as place cells, help to navigate the environment [352]. Damage to the vestibular system has reportedly led to atrophy of the hippocampus and to impaired spatial memory [353]. A randomized controlled study (n=120) reported that noisy GVS selectively impaired performance on tasks that required keeping a spatial representation in short-term memory (0.16-0.61 Hz bipolar bilateral noisy, ≤5mA; [321]). Specifically, supra-threshold (3.5 or 5 mA) versus sub-threshold (< 1 mA) stimulation decreased performance on a task where subjects had to match a sample grid to one seen later and, in a task, where subjects had to imagine themselves rotating in an environment. The stimulation did not reportedly effect performance on an object based rotation task or a task of executive control- both tasks that primarily take place in the frontoparietal lobe [354]. A functional imaging study comparing noisy GVS to rest reported deactivation in the hippocampal and parahippocampal regions, consistent with a possible inhibitory effect of GVS on spatial memory (0.1-5.0 Hz noisy, ±2.5 mA; bilateral; [355]). Mental rotation task can activate egocentric perspective taking pathways including the posterior parietal cortex, a region differentially activated by left versus right GVS [354, 356]. GVS applied during a mental rotation task reportedly improved speed on the task, but only during right anodal GVS (bipolar binaural DC, 1.0 ± mA; electrode 10 mm diameter [357]). While prior studies of GVS focused on accessing changes in spatial and bodily cognition, the differential projection of the vestibular nerve to diverse cortical regions (including those involved in learning and memory) suggest the possibility to modulate a wider range of cognitive functions.
In both human and animals studies, vestibular function may affect spatial memory [75]. The vestibular system is also intrinsically linked to the visual system which is implicated in many spatial cognition tasks. Brandit et al. proposed a reciprocal activation/deactivation mechanism between visual and vestibular stimuli for self-motion perception [358]. The theory was supported by findings from balance-impaired subjects receiving tongue stimulation that found a negative correlation between visual motion areas and areas that integrate vestibular stimuli [See Facial/Glossopharyngeal section; 289]. Noisy GVS enhanced recall in healthy subjects during a visual memory task in a polarity dependent manner (“1000 Hz noise enhanced” DC, group mean = 0.8 mA; bipolar electrode 3 cm2 carbon-rubber, bilateral; [359]). Subjects who received noisy left anodal stimulation had increased reaction time. Given the evidence of non-spatial related memory deficits in subjects with vestibular disorders, further experimentation that explicitly explore the relationship between non-spatial memory and the vestibular system stimulation are warranted.
In summary, vestibular afferents project to a distributed network in the cortex, with no single anatomical area defined as the “vestibular cortex”. Fibers synapse onto the cerebellum, spinal tract, and a range of brainstem nuclei. Some of these nuclei project to a diffusely connected cortical network involved in a range of cognitive functions [360] and others that control the body and eye reflexes [361]. It is rational that modulating the vestibular system would not only affect equilibrium, but can also affect varied cognitive domains, as seen in patients with vestibular damage. While the majority of the literature on GVS has focused on body mechanics (e.g. posture, eye movement), there is a rationale for expanded research on the cognitive effects of electrical stimulation on the vestibular system. While the immediate change in body posture or the induction of the visual-ocular reflex provides evidence of target engagement (e.g. direct current waveform), GVS approaches that do not rely on sensation (e.g. noisy waveform) may suggest sub-perceptual approaches (dose) to neuromodulation.
5.6. Vagus Nerve Stimulation
The vagus nerve (CN X) is the longest cranial nerve. It contains both sensory and motor fibers, which deliver information from the auditory canal all the way to the abdomen. The vagus nerve, like the facial and glossopharyngeal nerve, is bi-directional and carries five functional types of information; GSA, GVA, SVA, GVE, and SVE (Fig 4; orange). Though known for its role in regulating the viscera in the parasympathetic tract of the autonomic nervous system (ANS) via efferents, the vagus nerve is made up ~80% sensory afferents [362]. Efferents of the vagus also play a major role in modulation of peripheral autonomic function, including regulation of weight, heart rate, cardiovascular function and respiration [100, 363–366] as well as inflammatory responses [367–369]. The GVA pseudounipolar neurons conduct information from the viscera in the abdomen, as well as the pharynx, larynx, esophagus, and trachea. These receptors do not conduct visceral pain, but instead conduct information about stomach fullness, bladder pressure, bowel pressure, etc. (for review see [370]). GVA afferents from the thorax and abdomen ascend via sheaths within the carotid arteries and internal jugular veins in the neck, joining with afferents from the esophagus, larynx, and pharynx before all branches join at the angle of the mandible. The cell bodies of these fibers are located in the inferior ganglion, and the central processes enter the skull at the jugular foramen and synapse onto the nucleus tractus solitarius (NTS). The auricular branch of the vagus nerve (ABVN) mediates touch sensation (as well as temperature, pain) of the skin of portions of the ear including the tragus and auricle, the external auditory meatus, and the tympanic membrane. The ABVN carries sensory information through the tympanomastoid suture of the temporal bone and then traverses its surface, crossing the facial canal and then over the stylomastoid foramen, where it gives off an ascending branch that joins with the facial nerve (VII). The AVBN then enters the mastoid canaliculus in the lateral part of the jugular foramen and synapses onto the superior ganglion, where the fibers from the glossopharyngeal nerve join it before reaching the brainstem. There it joins the spinal tract of the trigeminal nerve and synapses onto the spinal nucleus of the trigeminal nerve and the NTS [371]. Finally, the SVA pseudounipolar neurons in the superior laryngeal branch (SLB) of the vagus nerve provide taste sensation to the few taste buds in the epiglottis (for a review of taste receptors see section 4). The inferior ganglion houses the cell bodies of this taste nerves. Fibers carrying taste information enters the skull at the jugular foramen and synapses onto the NTS. Studies that stimulated the vagus nerve of cats and anesthetized monkeys have also found evoked potentials in the OFC, suggesting direct connectivity [372, 373].
Invasive vagus nerve stimulation is referred to here as VNS. In animal and human VNS studies, surgically implanted stainless-steel wire cuff or hook electrodes are directly applied to the cervical branch of the vagus nerve. Animal VNS studies reported modulation in cortical activity [373] and induced cortical desynchronization (2-50 Hz pulsed, 500 μs pulse width, 1-2 V; [374] ). Chase and colleges demonstrated in cats, that different stimulation parameters correlated with changes in cortical activity linked to activation of specific vagal fiber groups [375]. Specifically, cervical VNS induced cortical synchronization (> 70 Hz, < 3 V), possibly by activating fast conducting myelinated fibers, while desynchronization is induced by a wider range of parameters (>70 Hz, > 3 V; 20 Hz, 10 V) and fiber conduction speeds (1-15 m/s) (750 μs pulse width; bipolar stainless-steel tube electrode 1/16” diameter placed on cut central end;[375, 376]). While most VNS studies apply pulsed stimulation, at least one study applied direct current (DC) stimulation in cats, where DC stimulation of the vagus nerve was reported to produce global synchronous activity followed by “resting sleep attitude” if maintained for longer than 15 seconds (0.6-1.5 mA, 15-40 sec; electrode stainless steel 0.2 mm dia.; [377]). This synchronous activity may be mediated by the NTS projection to the reticular activating system, a network of nuclei that control wakefulness [378]. Stimulating vagal nerve fibers in animals can also halt or partially halt epileptiform spiking activity induced chemically or electrically [379–381]. Still other studies have examined the dose-state response and attempted to link EEG response to fiber activation [376, 380, 382].
VNS in humans with epilepsy is typically delivered via a neurocybernetic prosthesis (Cyberonic, Inc., Webster, TX). The device is surgically implanted and the stimulating electrode is connected to the (usually left) cervical branch of the of the vagus nerve by a spiral cuff while the pulse generator (battery) is implanted in the chest via a method described by Reid [383]. The typical waveform for clinical use is as follows, though varies per patients’ tolerance: 30 Hz biphasic pulsed, 500 μs pulse width, 30s/5min on/off, 0.2-3.0 mA. In the following section, any time the “typical” waveform is referred to; it is referring to this waveform. In addition, all studies that deliver VNS, unless otherwise noted, use the neurocybernetic prosthesis system.
Dysregulation of cortical synchronization supports VNS as a therapeutic candidate for epilepsy [149]. The first therapeutic application of electrical VNS in man was used to treat patients with refractory [384] seizures (for review see [385]). Early VNS trials reported a mean reduction in seizure activity of 47% from baseline (20-50 Hz pulsed biphasic, 250-500 μs pulse width, 60-120s/60-5min on/off, 1-5 mA; [386, 387]). Two large multisite double-blind control studies reported a 28-31% reduction in seizure activity from baseline when patients received high levels of stimulation as compared with low levels of stimulation (Typical High: 30 Hz biphasic pulsed, 500 μs pulse width, 30 s/5 min on/off, 1.5 mA; Typical low: 1 Hz biphasic pulsed, 130 μs pulse width, 30 s/ 90 min on/off, 1.25 mA; [388, 389]). The FD approved VNS as a treatment for epilepsy in 1997 [390]. Partial seizures have a source of epileptic activity, often in the temporal or frontal lobe [391]. VNS may preferentially modulates synchronous activity in the prefrontal cortex [373]. An analysis of seizure loci alongside vagus nerve connectivity (Figure 5) may explain response variations in individuals receiving VNS.
Varied mechanisms have been proposed to explain the antiepileptic effects of VNS. The desynchronous EEG effects reported by Chase et al. [375] in dogs were not replicated in six awake partial seizure patients with VNS devices following 6 months of open-label treatment [392]. Stimulation of the reticular activating system and noradrenergic projections [393] has been proposed a mechanism. Specifically, a the structures within the reticular activating system, the locus coeruleus (LC), modulates thalamo-cortical oscillations [394] which may regulate seizures [395–402]. Responses from the LC in rats have been found to correlate with changes in dose parameters of VNS (0-120 Hz biphasic pulsed, 0-500 μs pulse width, 0-64 pulses per train, 0-2.5 mA; platinum-iridium bipolar electrodes; [403]).
In the course of the studies conducted to assess the efficacy of invasive VNS on epilepsy, subjects anecdotally reported improvements in mood [404]. To specifically study the effects of invasive VNS on mood, a randomly selected subpopulation was chosen from the original cohort with implanted VNS devices [405]. Over an additional 6 months of monitored VNS use, eleven subjects in this subpopulation showed improvements on self-report mood questionnaires that were independent of the treatment’s effect on seizure activity (30 Hz, 500 pS pulse width, 60/600 sec on/off, < 1.75 mA). These results were reproduced in a trial with expanded patient cohort (30 Hz biphasic pulsed, 500 μs pulse width, 30s/5 min on/off; [406]). The mechanism through which VNS improves moods may be therefore distinct from its improvements in epilepsy activity. However, a large scale (n=160) double-blind study reported only a slight improvement in quality of life (QoL) ratings following VNS and found no relationship between performance on cognitive tasks of executive function and cognitive flexibility and VNS intensity [407]. The results taken from these studies offers an incomplete picture of the effectiveness of VNS on mood and cognition in a neurologically atypical patient.
A further set of studies investigated the effects of VNS on mood in patients with treatment-resistant depression [408–413]. In randomized controlled trials patients receiving invasive VNS in addition to their usual treatment (electroconvulsive therapy and medication) reported improvements on depression ratings following 12 months of use (20 Hz pulsed biphasic, 500 μs pulse width, 30s/5min on/off, 1-3.5 mA; [408, 411]). The FDA approved invasive VNS for treatment-resistant depression in 2005 for patients who have failed at least four antidepressant trials. One proposed mechanism of action for these improvements in treatment resistant depression and mood derives from the vagus nerve projections to the prefrontal cortex and limbic system [414]. Imaging studies in patients with treatment resistant depression following treatment with invasive VNS have reported increased regional cerebral blood flow (rCBF) to the left dorsolateral prefrontal cortex (DLPFC; >20 Hz pulsed biphasic, 130-500 μs pulse width, 30s/5min on/off, 0.5-1.75 mA; [415–417]) and decreased activation in limbic structures [418]. This is in accordance with general findings in the literature indicating hypoactivity in the left DLPFC in patients with major depressive disorder (MDD) and the current therapeutic approaches to modulate activity in this region using transcranial magnetic stimulation (TMS; [419, 420]). Ongoing clinical trials are evaluating the efficacy of invasive VNS application for treatment of a number of clinical populations including anxiety, obesity, and traumatic brain injury (for review see [421]).
Early results that related VNS to changes in cognition were in memory. Researchers reported that rats receiving VNS immediately after training showed greatest memory enhancements for medium levels of stimulation, displaying an inverted U-shaped dose-response curve (20 Hz biphasic pulsed, 500 μs pulse width, 0.2/0.4./0.8 mA; 20-cm-long, electrodes: 30-gauge, kynar-insulated, silver wires; [422]). Similar dose-response relationships were found in humans. A randomized double-blind study investigating verbal memory in epilepsy patients applied VNS at various intensities during consolidation. When intensities were applied at 0.5 mA, memory was enhanced, while during sham stimulation or at intensities higher than 0.75 mA, no memory enhancement was observed (30 Hz biphasic pulsed, 500 μs pulse width, 30s on, 0.5-1.5 mA; [14]). In support of Clark et al.[14] finding that stimulation intensities higher than 0.5 mA have a null or negative effect on memory, a study using stimulation intensities greater than 1 mA found no effect of VNS on verbal memory and a negative effect on figure recognition (30 Hz biphasic pulsed, 500 μs pulse width, 4.5 min on, 1-2.5 mA; [423]). In addition to dose, time of VNS administration in relation to the tasks seems to effect whether cognition is successfully modulated. Verbal word recognition was enhanced when VNS was delivered after a word set was learned, but not before words were recalled (30 s on, 0.5 mA; [424]). The vagus nerve may also modulate decision making (30 Hz pulsed biphasic, 500 μs pulse width, 60s on, .5 mA; [425]). Cognitive tasks that have tested with VNS include executive function, cognitive flexibility, and creativity (verbal and visual) and have reported no significant relationship [407, 426].
The previous studies were carried over the course of acute application of VNS in patients. Several studies have examined the long-term effects of VNS on memory and mood. During a 1-year period, patients with Alzheimer’s disease treated with VNS had no reported changes (increases or decreases) in QoL or cognitive function as measured by clinical assessment scales (20 Hz, μs pulse width, 30s/5min on/off; [427, 428]). In one of the few studies to address the long term effects of VNS on mood and cognition using cognitive tasks rather than questionnaires, 27 treatment-resistant -depression patients received a cognitive battery of tests before, during and after 10 weeks following implantation with VNS (20-50 Hz pulsed biphasic, 250-500 μs pulse width, 30s/3-5min on/off, 0.5-1.5 mA; [429]) and reported improved performance on tasks in motor speed, psychomotor function, language, and executive function (including working memory) and no effect on measures of memory and attention. Further, these improvements did not correlate with changes in depression scores over time except on one measure of executive function, indicating that the pathways through which VNS modulates cognition may be separate from mood. A prospective one year study found no overall improvement or declines in cognitive function in subjects who received VNS when compared with other types of seizure treatments (antiepileptic drugs or cerebral resective surgery) [430]. Consistent with previous research, researchers did not find a correlation between QoL and seizure reduction.
VNS also has effects on neuroplasticity in addition to effects on learning and memory [431–440]. The vagus conveys signals to the brain from hormones and neurotransmitters in the periphery, including cortisol and epinephrine, that are known to effect cognitive functions such as learning and memory but that don’t cross the blood-brain barrier [441–448]. Adrenergic receptors are located on the vagus that can transmit information via the NTS to brain areas involved in learning and memory including the hippocampus and amygdala [449, 450]. VNS enhances memory retention with a U-shaped curve [451] acting through afferent fibers of the vagus [452] and increases norepinephrine (NE) in the basolateral nucleus of the amygdala (BLA) to concentrations capable of modulating emotional memory [450]. VNS acts through the LC and beta adrenergic receptors on the perforant pathway-CA3 region of the hippocampus to enhance synaptic transmission [453, 454] and long-term potentiation (LTP), which represents the molecular mechanism of new learning [455]. VNS also enhances hippocampal neurogenesis [456] impairment of which is an animal model for depression [457]. VNS when paired with conditioned cues facilitates extinction in animal models of classic fear conditioning [458–461] and reduces fear-like behaviors [460, 461] via modulation of connections between the medial prefrontal cortex and amygdala, suggesting a role in the treatment of anxiety [26, 432, 458–460, 462, 463]. Animal studies show that VNS enhances neural plasticity, leading to beneficial effects with pairing of VNS with an auditory tone in animal models of tinnitus [434–437] and stroke for recovery of cognitive function [438] and motor movement when paired with training [464–467]. Other studies in animals show that VNS promotes recovery from cerebral hemorrhage [468] congestive heart failure [469] and other cardiovascular events [470, 471] and reduces ventricular arrhythmia in the setting of myocardial ischemia [472] VNS promotes neuroplasticity in the frontal cortex in animals [473] and in patients with ischemic stroke, VNS sped motor recovery when paired with rehabilitation [474] and reduced tinnitus when paired with musical tones in patients with tinnitus [475]. Animal models of traumatic brain injury (TBI) show VNS acts through the LC to enhance both new learning and memory as well as synaptic plasticity and motor recovery [476, 477] and shows utility for learning and memory function in patients with Alzheimer’s Disease.[478, 479] VNS has other effects on neuroplasticity [480–484] and is beneficial in the treatment of pain [485, 486], headaches [487–490] as well as epilepsy [491–496], major depression [497–510], schizophrenia [511, 512] and obsessive-compulsive disorder [513].
5.7. Noninvasive Vagus Nerve Stimulation (nVNS)
Non-invasive applications of VNS target either the auricular branch of the vagus nerve [514–516], or the cervical branch [16, 517]. Transcutaneous auricular vagus nerve stimulation (taVNS) uses electrodes that are typically placed on the ear (Figure 2.b; [518]). Non-invasive vagus nerve stimulation (nVNS) is a broad term typically used to describe technology that places bipolar electrodes over the neck [517]. Unless otherwise noted, the devices used to apply to electrical pulses is a transcutaneous electrical stimulation (TENS) device. Auricular vagus nerve stimulation can be bilateral (applied to both ears) or unilateral (applied to just one ear); monophasic or biphasic (typically, 5-25 Hz pulsed, ≤ 500 μS pulse width, ≤ 10 mA). Non-invasive vagus nerve stimulation placed on the neck typically use approximately a 5,000 Hz pulsed bursts at 25 Hz, pulses are “approximate” sine waves [519, 520].
There are evident advantages to developing a non-surgical approach to vagus nerve stimulation. These range from reducing the risk associated with surgical implant procedures, reducing the cost of maintenance of the device and post-operative care of the patient, and supporting the adoption of the procedure across a wider range of disorders and populations. Because the auricular afferents project through a different pathway than the cervical fibers of the vagus nerve, it is possible that taVNS may act through a different mechanism than cervical VNS. Neuroimaging, behavioral, and neurophysiological studies have assessed the functionality of taVNS in comparison to cervical VNS. Results from neuroimaging studies have indicated that brain regions activated by taVNS greatly overlap with invasive cervical VNS (8 Hz pulsed, 20 μs pulse width, 5.0 ± 1.0 mA; electrode: Ag 5mm dia., anode placed inside left tragus, cathode on right arm; [28]). Namely, decreased activation in the limbic system — including the amygdala, hippocampus, and parahippocampal gyrus— and increased activity in the thalamus and insula. These effects were noted with stimulation to the anterior wall of the auditory canal, where the skin is innervated by vagus afferents (same parameters as Kraus et al. [521]) and replicated by Badran et al. using 500 μs pulse width pulsed at 25 Hz in a 30-second on/off block design [522]. Auricular projections have been traced to the NTS and spinal trigeminal nuclei in animal models [371] as well as confirmed in human neural imaging (fMRI) studies during stimulation (25 Hz monophasic pulsed, 250 μs pulsed width, 0.3-0.9 mA; two hemispheric titanium electrodes stimulating the cymba conchae; [523]). Post-mortem staining of Alzheimer’s disease patients’ brainstem identified that the only cranial nerve nuclei significantly affected by neurofibrillary tangles (NFTs) and senile plaques (SPs) were a part of the parasympathetic system, including the NTS which was particularly affected [2] and Parkinson’s disease [524]. Such specific neuropathology suggests, in addition to VNS as a therapeutic intervention, that stimulation along this nerve provide a measure of vagus nuclei function as a neuro-diagnostic tool.
There have been several studies evaluating evoked “far field EEG” potentials presumably from the NTS using taVNS (0.5 Hz bipolar unilateral pulsed, 100 μs pulse width, 8 mA; bipolar electrode wire, copper.05 mm dia., inner tragus; [514, 515]). These “far field” dose response parameters have been optimized in a healthy population and are being investigated as a possible biomarker of disease progression in Alzheimer’s disease [525–527]. However, some researchers have suggested that the source of the vagus sensory evoked potential (VSEP) recorded from the scalp is in fact myogenic in origin. In research using stimulation at the tragus with the same dose as Fallgatter et al. [528] reported that the VSEP is abolished when subjects are given muscle relaxing agents (0.5 Hz, 100 μs pulse width, 8 mA; bipolar wool wrapped steel wires stapled to a piece of silicon rubber; [529]). This is in contrast with results from subjects with VNS (which is cervical and invasive) where scalp evoked potentials do not disappear under muscle blocking agents (30 Hz biphasic pulsed, 130-750 μs pulse width, 670 s on, 0.25-2.0 mA; implanted cervical VNS device described above; [530]).
Similar to the effects seen in VNS, there have been reported effects on mood modulated by taVNS. Kraus et al. reported significant elevations in mood as measured by an adjective mood scale, a self-report instrument that assigns valences to adjectives used to describe affect [28]. In contrast to VNS, which is limited to treatment resistant depression, taVNS can be applied in less severe cases of MDD. A randomized controlled pilot study (37 patients) reported that patients with MDD improved on a self-report depression inventory questionnaire over a two-week period in both sham groups and active groups, but improved more (47%) in the group receiving taVNS (1.5 Hz pulsed, <600 μA; auri stim in ear electrode; [531]). The effects of taVNS on MDD may work through modulating the default mode network, a functionally connected network of brain areas that are engaged when the mind is reflected inward and not focused on a particular task [532]. This network has gained attention for the role it plays in several disorders include depression [533, 534]. A single-blind study reported taVNS modulated the default mode network in MDD patients following 4 weeks of electrical stimulation when compared with a sham stimulation (20 Hz, <1 ms pulse width, 4-6 mA; [535]). Further, patients reported improvements on a depression rating scale that was correlated with the changes in the degree of connectivity in the default network. There have also been reports that taVNS may modulate cognition. In an older population, associative memory was reportedly improved following a single session of taVNS (8 Hz pulsed, 200 μS pulse width, 5 mA; 10 mm dia. clip applied to the tragus; [536]). Subjects were stimulated with taVNS while being shown face-name pairs that they would have to retrieve after a short break — during which they were also stimulated. In this manner, stimulation was applied during the encoding and consolidation phases of a face recognition task. The results of this study are the first to report improvements in memory function following a single session of taVNS. Motor learning has reportedly been modulated by taVNS during suckling in neonates born preterm or with brain injury (25 Hz pulsed, 500 μs pulse width, 0.84 ± 0.17mA; clip applied to the left tragus; [537]). Other studies that have examined the cognitive effects of taVNS have reported taVNS enhances learning (25 Hz, 0.5 mA; titanium electrodes; [538]), executive function (25 Hz, 200-300 μS pulse width, 30s on/off, 0.5 mA; [539]) and cognitive control (25 Hz, 200-300 μS pulse width, 30 s on/off, 0.5 mA; [540]). These effects are a promising indication that taVNS modulates cognition. Non-invasive VNS showed promising results in pilot studies for depression [541], PTSD [542–544], and mild Traumatic Brain Injury (mTBI) [542].
The vagus nerve contains parasympathetic fibers which modulate the “rest and relax” part of the autonomic system [545]. While the primary aim of this review is to focus on afferent nerves (to the brain), the relationship between the parasympathetic response and the cognitive response during vagus nerve stimulation are intrinsically linked and may even be leveraged for more effectively vagus nerve stimulation usage. Ongoing studies suggest that modulation of the auricular branch of the vagus nerve activates the parasympathetic nervous system and induces reductions in heart rate [546]. As noted before, the vagus nerve synapses on the NTS in the brainstem, which also receives respiratory-associated signals from lung stretch receptors and central respiratory rhythm generator nuclei. In fact, inhalation and exhalation have different effects on vagal inputs to the NTS, which may gate the effects of vagus nerve stimulation [547–549]. This type of “respiratory-gated” vagal nerve stimulation has been applied in migraine patients, resulting in a respiration specific effect of taVNS (30 Hz pulsed, 450 μs pulse width, 500 ms on/off, 0.5-3.8 mA; 8 mm bipolar electrode, placed on left ear; [547]). Synching the taVNS to respiration may most strongly increase the effect of taVNS on pain processing in chronic pain patients when coupled to exhalation, the point in respiration when the NTS is receiving stronger facilitation [549]. Uhman fMRI at ultrahigh field (7T to allow improved spatial resolution for brainstem neuroimaging) has demonstrated enhanced NTS activation when taVNS is delivered during exhalation, compared to inhalation. These findings offer a new perspective on dose optimization. Most commonly researchers vary electrode placement and waveform in order to determine an optimal dose. However, the findings from respiratory-gated taVNS also suggest a crucial role of physiological-dependent effect forf vagus nerve stimulation.
Noninvasive vagus nerve stimulation (nVNS) techniques target the cervical branch of the vagus nerve rather than the auricular branch. The theory being that by targeting the cervical branch of the vagus nerve using electrodes over the neck, the pathways activated by invasive VNS can be replicated (in part) without surgical intervention [517]. Brain imaging during nVNS over the neck revealed activation of regions implicated in invasive VNS, including the NTS and cortical regions in the forebrain [550]. The overlapping activation between nVNS and VNS may imply cervical vagus nerve engagement. Therapeutically, cervical nVNS has been investigated for neurological and chronic pain disorders, indications that have been targeted by VNS but in limited populations due to the invasiveness of VNS [551, 552]. Several randomized controlled studies, including some multicenter studies have reported that nVNS had a prophylactic effect on migraines and cluster headaches [16, 553–557]. In addition to neurological application, cervical nVNS may reduce the damage caused by induced ischemic stroke (5 kHz sine wave, 1 ms duration, 25 Hz repetition, ~20 mA; Disc electrodes over the right cervical vagus nerve, 6 mm dia.; [558]). VNS was reported to decrease damage to the brain caused by ischemia, possibly through regulation of the anti-inflammatory pathway (20 Hz pulsed stimulation, 500 us pulse width, 0.5 mA; implanted cervical VNS as described above; [559]). Cervical nVNS has some overlap with invasive VNS in neurological treatment indications and in brain network activation, but without as many side effects of VNS. Noninvasive techniques that target the vagus nerve can be done by targeting the cervical branch or the auricular branch of the vagus nerve. Both of these techniques have clinical implications that may or may not differ.
In summary, there is an extensive literature in humans and animal models exploring the effects of invasive stimulation of the vagus cervical branch (VNS) on seizures, along with evidence for changes in cortical neurophysiology (e.g. desynchronization). In patients with VNS for epilepsy, clinical reports of improvements in mood, that were not necessarily correlated with changes in seizure symptoms, encouraged further clinical trials in other neuropsychiatric illnesses. This, in turn, has encouraged advancements in noninvasive vagus nerve stimulation technology, targeting either the cervical (electrodes on neck) or the auricular vagus nerve branch (electrodes on ear), that can be more readily tested across neuropsychiatric and neurological disorders as well as in healthy subjects. Electrical stimulation of the vagus nerve may modulate higher cognitive processes, such as memory and mood, as supported by studies in animals and humans [14, 422]. Given the vagus nerve’s reciprocal connections with both cortical and physiological structures involved in the parasympathetic response, a number of possible cognitive processes could be modulated by invasive or non-invasive vagus nerve stimulation in both a top-down and bottom-up manner. Yet, because of the extensive and diverse targets of the vagus nerve, disambiguating the mechanisms of action for a given constellation of cognitive and behavior changes can be complex. Similarly, optimizing intervention to modulate a specific brain circuit for targeted outcomes remains in early stages, largely driven by technology platform (VNS vs tVNS vs taVNS) rather than principled design approached, such as physiological-dependent taVNS [547, 549]. Attempts to identify dose-specific EEG biomarkers of vagus nerve stimulation -- either synchrony across the cortex or ERP associated with deep brain structures represent one principled approach, but specific results are debated. Computational models, such as for VNS [560–562] and tVNS [517] may identify which axons types can be preferentially stimulated and fMRI approaches can localize brainstem response in specific target nuclei for dose and parameter optimization. Because the vagus nerve is implicated in a wide array of functions, vagus nerve stimulation is being explored for an increasingly diverse range of applications; however, this reinforces the need to develop targeted interventions rather than expanding the same dose to produce a range of distinct outcomes. This can include electrode placement (e.g. cervical vs auricular branch) and waveform activated specific axons that influence downstream brain circuits (Figure 5) to modulate specific cognitive domains.
6. Discussion
There are multiple functional pathways to modulate higher cognitive processes, and so treat neuropsychiatric disorders involving cognitive dysregulation [244, 411] by electrically stimulating cranial nerves. Invasive cranial nerve stimulation techniques that target single cranial nerves (though nerve pathways can converge trough anastomosis and multi-modal processing; Figure 5), are explored in clinical populations, and validated through translational animal models. Noninvasive technologies make cranial nerve stimulation feasible for a broader range of patients and even for non-disordered populations. Indeed, studies in healthy subjects support a mechanistic characterizations of cranial nerve stimulation without confounding dysfunction effects [28]. With non-invasive stimulation, selective stimulation of just one cranial nerve is unlikely, and models are required to inform target engagement (Figure 4). The motivation for this review is that increased sophistication in cranial nerve stimulation techniques, that engage distributed brain-circuits through potent bottom-up mechanisms, while leveraging emerging neuroscience of cognition, can produce nuanced and enhanced tools for neuromodulation of specific cognitive domains and disorders.
We consider cranial nerve stimulation in the context of circuit-based neuromodulation, as is already informing other forms of brain stimulation such as tDCS [563–565], TMS [566–569] and DBS [570–573]. In this sense it is compelling to consider how all these techniques engage overlapping circuits but from different nodes (locations) and with different modes of influence. Transcranial stimulation approaches target cortical regions (e.g. nodes in right row Figure 5) with either a supra-threshold (e.g. TMS) or sub-threshold (e.g. tDCS) mode of influence [574–577]. Some of the earliest hypotheses followed observations in epileptic patients treated with cranial nerve stimulation (e.g. olfactory, vagus) where desynchrony could be observed across cortical activity. DBS targets deep brain regions (e.g. nodes in middle of Figure 5) with supra-threshold activation. Often, as is the case of vagus, trigeminal, or branches of the glossopharyngeal nerve, the rationale behind stimulation of sensory cranial nerves were to target their projections to deep brain structures that could modulate higher level networks in the brain via the thalamus. A detailed consideration of brain circuity (Figure 5) shows overlapping modulated circuit across brain stimulation techniques. But cranial nerves stimulation is unique in several aspects. Cranial nerve stimulation technologies can be non- or minimally invasive yet target nodes deep in the brain. This is a consequence of cranial nerves being axons of deep brain nodes that are accessible at the skin. The mode of influence by cranial nerve stimulation may also be different than techniques such as tDCS, TMS, and DBS as cranial nerve stimulation activates axon pathways that are intended to transmit information into the brain, so the brain is formed to process and adapt to information from these “natural” inputs. In this sense, cranial nerves stimulation functions like peripheral stimulation (e.g. TENS) but cranial nerves directly and specifically innervate the brain.
Here we considered how cranial nerve stimulation targeting both functionally lower-level brain regions (sensory cortices) as well as cortical regions involved in higher-level or executive functions can be rationally leveraging for stimulation strategies that are more effective and specific. Cranial nerve stimulation of lower-level regions can: (i) modulate cognition at early stages of processing; and (ii) directly treat neuro-psychiatric disorders associated with bottom-up (sensory) dysregulation. As the same time, as the direct cortical targets of cranial nerves in turn regulate distributed “higher” cortical networks, cranial nerve stimulation can: (iii) influence functional modules (circuits) of brain regions dedicated to a given modality of cognition; and (iv) treat neuro-psychiatric disorders associated with network level dysfunction. For example, disorders such as schizophrenia have been proposed to be a largely bottom-up dysregulation that has both downstream and upstream effects, causing disruption in higher cognitive function, motor function, sensory processing, and perception [578]. In such cases, cranial nerves provide a unique opportunity to access these processing streams at the early stages of information processing in a targeted manner. Conversely, modulation of upper-level connectivity may explain why seizure disorders (where the target may be unknown or diffuse) can be treated by bottom-up cranial nerve neuromodulation [8, 9, 293].
This approach to cranial nerves stimulation aligns with emerging models of brain (dys)function that stress the centrality of interaction of multiple brain regions to perform cognitive processing - in contrast to basic models that segregate the brain into discrete functional modules [579]. These brain networks include regions that are traditionally considered lower-level or higher-level regions. Cranial nerves neuromodulation of cognition and in neuropsychiatric treatment may thus consider early stage versus late stage processing, rather than low-level vs. high-level since specific brain regions.
Targeting a sensory system at an early stage of cortical processing, may seem to imply that there is a loss in the specificity of the outcome, but this is not necessarily the case. For example, cognitive tasks that require egocentric-perspective-taking were found to be selectively disrupted by targeted electrical vestibular stimulation, while allocentric-perspective-taking and general conflict resolution tasks were unaffected [321]. Indeed, compared to other neuromodulation technique (e.g. tDCS, TMS, DBS), cranial nerve stimulation activates a limited (highly targeted) set of axons. And across cranial nerves, evidence for neuromodulation independent from sensory substitution suggest nuanced circuit pathways.
While studies are often interpreted to reflect stimulation of one targeted cranial nerve, the physical overlap between cranial nerves (Figure 5) suggests outcomes related to multinerve stimulation (cross-modal), especially using noninvasive techniques. For both non-invasive and invasive techniques, mixed-nerve activation can also result from anatomical networks between cranial nerves formed during normal development (or as a result of an injury) called anastomoses [580]. Anastomoses span motor-to-sensory, motor-to-motor, and sensory-to-sensory fiber types [7]. These connections form at different points along the fiber pathways. For instance, the vestibular end-organ has been shown to respond to acoustic stimulation in guinea pigs [581] while the nervus intermedus of the facial nerve shows connections to the vestibular nerve at the Scarpa’s ganglion [582]. Depending on where along cranial nerves these connections are formed, the effect that anastomoses will have on the outcome of electrical stimulation will differ. The ability to stimulate a constellation of cranial nerves is no more a “deficit” of cranial nerve stimulation as stimulating a constellation of axonal pathways is a deficit of DBS [583–585]; rather is supports a circuit therapeutic framework for rational interventional design (Figure 5).
The leveraging of unique features of cranial nerve stimulation to target a constellation of brain regions and functions/symptoms is in line with the modern neuroscience [243, 586–588] as well as medical research directives to no longer simply treat individual symptoms in a disease like MDD or schizophrenia [589–591]. Rather than simplistically linking each brain (dys)function with one brain region and targeting that region with electrical stimulation (like a game of whack-a-mole) — the fields of neuroscience and brain stimulation are adopting concepts of network engagement and system/functional domains. In such a conceptualization, bottom-up cranial nerves stimulation offers specialized features distinct from top-down neuromodulation, such as tDCS or TMS, that target cortical regions [592–594] or invasive neuromodulation targeting deep structures [595–597]. Though modulation of networks is recognized as a key across brain stimulation techniques [598–602], only cranial nerve stimulation allow minimal- or non-invasive activation of specific brain pathways (rather than a mix of neurons and axons in a brain regions). We have emphasized evoked sensations (percepts) as demonstrating target engagement during cranial nerves stimulation, but modulation of cognition may not necessarily require sensory perception (i.e. activation of circuits independent of sensory cortex). How cranial nerve stimulation, especially over repeated or long-intervals, could reverse mal-adaptive plasticity (e.g. neuropathic pain) or promote neuro-plastic changes which may treat damaged network (e.g. stroke) should be the subject of additional future investigations [603].
Highlights:
Cranial nerves are unique neuromodulation targets in providing high specificity and non-minimally-invasive access to deep brain regions.
Stimulation of afferent cranial nerve axons activates specific brain circuits linked to specific clinical, behavioral, and cognitive domains.
Cranial nerve neuromodulation may leverage or be independent of perception, including modulation of processing through the thalamus
Acknowledgement
The contributions of M. Bikson were supported by the NIH under grants MH111896, NS101362, U54CA137788/ U54CA132378, and NS054783. Contributions of VN supported by NIH, Office of the Director (OT2-OD023867); NCCIH (P01-AT009965), and NIMH (R21-MH103468). Contributions of BWB supported by NIH/NICHD (P2CHD086844). Contributions of VPC supported by NIH/NIGMS (P30GM122734).
Disclosures
The City University of New York has inventions on tES with MB as inventor. MB has equity in Soterix Medical and serves on the scientific advisory boards or has grants from Mecta, Halo Neuroscience, Boston Scientific and GlaxoSmithKline. BWB is an inventor on tES patents and patent applications, has equity in Bodhi NeuroTech and serves as a consultant to eQuility. Vitaly Napadow has a financial interest in Cala Health which is licensing taVNS technology from Massachusetts General Hospital, and his interest was reviewed and managed by the Massachusetts General Hospital and Partners HealthCare in accordance with their institutional policies. The rest of co-authors have nothing to disclose. JDB has research grant support from ElectroCore, Inc. The rest of co-authors have nothing to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. References:
- 1.Kandel ER, Schwartz JH, Jessell TM, Siegelbaum SA, Hudspeth AJ. Principles of neural science: McGraw-hill; New York; 2000. [Google Scholar]
- 2.Parvizi J, Van Hoesen GW, Damasio A. The selective vulnerability of brainstem nuclei to Alzheimer’s disease. Annals of neurology 2001;49(1):53–66. [DOI] [PubMed] [Google Scholar]
- 3.DeGiorgio CM, Fanselow EE, Schrader LM, Cook IA. Trigeminal nerve stimulation: seminal animal and human studies for epilepsy and depression. Neurosurgery clinics of North America 2011;22(4):449–56. [DOI] [PubMed] [Google Scholar]
- 4.Hanes DA, McCollum G. Cognitive-vestibular interactions: a review of patient difficulties and possible mechanisms. Journal of Vestibular Research 2006;16(3):75–91. [PubMed] [Google Scholar]
- 5.Rea P Clinical anatomy of the cranial nerves: Academic Press; 2014. [Google Scholar]
- 6.Shoja MM, Oyesiku NM. Clinical anatomy of the cranial nerves. Clinical Anatomy 2014;27(1):2–3. [DOI] [PubMed] [Google Scholar]
- 7.Shoja MM, Oyesiku NM, Griessenauer CJ, Radcliff V, Loukas M, Chern JJ, et al. Anastomoses between lower cranial and upper cervical nerves. Clinical Anatomy 2014;27(1):118–30. [DOI] [PubMed] [Google Scholar]
- 8.George R, Salinsky M, Kuzniecky R, Rosenfeld W, Bergen D, Tarver W, et al. Vagus Nerve Stimulation for Treatment of Partial Seizures: 3. Long-Term Follow-Up on First 67 Patients Exiting a Controlled Study. Epilepsia 1994;35(3):637–43. [DOI] [PubMed] [Google Scholar]
- 9.DeGiorgio CM, Shewmon DA, Whitehurst T. Trigeminal nerve stimulation for epilepsy. Neurology 2003;61(3):421–2. [DOI] [PubMed] [Google Scholar]
- 10.Veraart C, Raftopoulos C, Mortimer JT, Delbeke J, Pins D, Michaux G, et al. Visual sensations produced by optic nerve stimulation using an implanted self-sizing spiral cuff electrode. Brain Research 1998;813(1):181–6. [DOI] [PubMed] [Google Scholar]
- 11.Coats AC. Effect of varying stimulus parameters on the galvanic body-sway response. Annals of Otology, Rhinology & Laryngology 1973;82(1):96–102. [DOI] [PubMed] [Google Scholar]
- 12.Gall C, Sgorzaly S, Schmidt S, Brandt S, Fedorov A, Sabel BA. Noninvasive transorbital alternating current stimulation improves subjective visual functioning and vision-related quality of life in optic neuropathy. Brain stimulation 2011;4(4):175–88. [DOI] [PubMed] [Google Scholar]
- 13.Wilkinson D, Ferguson HJ, Worley A. Galvanic vestibular stimulation modulates the electrophysiological response during face processing. Visual Neuroscience 2012;29(4-5):255. [DOI] [PubMed] [Google Scholar]
- 14.Clark KB, Naritoku DK, Smith DC, Browning RA, Jensen RA. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nature neuroscience 1999;2(1):94–8. [DOI] [PubMed] [Google Scholar]
- 15.Tyler WJ, Boasso AM, Charlesworth JD, Marlin MA, Aebersold K, Aven L, et al. Suppression of human psychophysiological and biochemical stress responses using high-frequency pulse-modulated transdermal electrical neurosignaling. bioRxiv 2015:015032. [Google Scholar]
- 16.Goadsby P, Grosberg B, Mauskop A, Cady R, Simmons K. Effect of noninvasive vagus nerve stimulation on acute migraine: an open-label pilot study. Cephalalgia 2014;34(12):986–93. [DOI] [PubMed] [Google Scholar]
- 17.Bikson M, Paneri B, Mourdoukoutas A, Esmaeilpour Z, Badran BW, Azzam R, et al. Limited output transcranial electrical stimulation (LOTES-2017): Engineering principles, regulatory statutes, and industry standards for wellness, over-the-counter, or prescription devices with low risk. Brain Stimulation 2017. [DOI] [PubMed] [Google Scholar]
- 18.Lazarev V, Gebodh N, Tamborino T, Bikson M, Caparelli-Daquer E. Experimental-design Specific Changes in Spontaneous EEG and During Intermittent Photic Stimulation by High Definition Transcranial Direct Current Stimulation. Neuroscience 2020;426:50–8. [DOI] [PubMed] [Google Scholar]
- 19.Ishimaru T, Shimada T, Sakumoto M, Miwa T, Kimura Y, Furukawa M. Olfactory evoked potential produced by electrical stimulation of the human olfactory mucosa. Chemical senses 1997;22(1):77–81. [DOI] [PubMed] [Google Scholar]
- 20.Miller SL, Mirza N, Doty RL. Electrogustometric thresholds: relationship to anterior tongue locus, area of stimulation, and number of fungiform papillae. Physiology & behavior 2002;75(5):753–7. [DOI] [PubMed] [Google Scholar]
- 21.Fitzpatrick RC, Day BL. Probing the human vestibular system with galvanic stimulation. Journal of applied physiology 2004;96(6):2301–16. [DOI] [PubMed] [Google Scholar]
- 22.Bosnjak R, Benedieie M. Direct epidural electrical stimulation of the optic nerve: a new method for intraoperative assessment of function. 2008. [DOI] [PubMed] [Google Scholar]
- 23.Usami K, Kawai K, Sonoo M, Saito N. Scalp-recorded evoked potentials as a marker for afferent nerve impulse in clinical vagus nerve stimulation. Brain Stimul 2012;6(4):615–23. [DOI] [PubMed] [Google Scholar]
- 24.Picton TW, Hillyard SA, Krausz HI, Galambos R. Human auditory evoked potentials. I. Evaluation of components. Electroencephalogr Clin Neurophysiol 1974;36:179–90. [DOI] [PubMed] [Google Scholar]
- 25.Fallgatter AJ, Neuhauser B, Herrmann MJ, Ehlis A-C, Wagener A, Scheuerpflug P, et al. Far field potentials from the brain stem after transcutaneous vagus nerve stimulation. J Neural Transm 2003;110:1437–43. [DOI] [PubMed] [Google Scholar]
- 26.Polak T, Markulin F, Ehlis A-C, Langer JBM, Ringel TM, Fallgatter AJ. Far field potentials from brain stem after transcutaneous vagus nerve stimulation: optimization of stimulation and recording parameters. J Neural Transm 2009;116:1237–42. [DOI] [PubMed] [Google Scholar]
- 27.Nonis R, D’Ostilio K, Schoenen J, Magis D. Evidence of activation of vagal afferents by non-invasive vagus nerve stimulation: An electrophysiological study in healthy volunteers. Cephalagia 2017:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kraus T, Hosl K, Kiess O, Schanze A, Kornhuber J, Forster C. BOLD fMRI deactivation of limbic and temporal brain structures and mood enhancing effect by transcutaneous vagus nerve stimulation. Journal of neural transmission 2007;114(11):1485–93. [DOI] [PubMed] [Google Scholar]
- 29.Bense S, Stephan T, Yousry TA, Brandt T, Dieterich M. Multisensory cortical signal increases and decreases during vestibular galvanic stimulation (fMRI). Journal of neurophysiology 2001;85(2):886–99. [DOI] [PubMed] [Google Scholar]
- 30.Karhu J, Hari R, Lu ST, Paetau R, Rif J. Cerebral magnetic fields to lingual stimulation. Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section 1991;80(6):459–68. [DOI] [PubMed] [Google Scholar]
- 31.Weiss T, Shushan S, Ravia A, Hahamy A, Secundo L, Weissbrod A, et al. From Nose to Brain: UnSensed Electrical Currents Applied in the Nose Alter Activity in Deep Brain Structures. Cerebral Cortex (New York, NY) 2016;26(11):4180–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sclocco R, Garcia RG, Kettner NW, Isenburg K, Fisher HP, Hubbard CS, et al. The influence of respiration on brainstem and cardiovagal response to auricular vagus nerve stimulation: A multimodal ultrahigh-field (7T) fMRI study. Brain Stimul 2019;12(4):911–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walsh P, Kane N, Butler S. The clinical role of evoked potentials. J Neurol Neurosurg Psychiatry 2005;76 Suppl 2:ii16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gray H, Carter H. Gray’s Anatomy: The Classic 1860 Edition: JSTOR; 2008. [Google Scholar]
- 35.Radman T, Ramos RL, Brumberg JC, Bikson M. Role of cortical cell type and morphology in subthreshold and suprathreshold uniform electric field stimulation in vitro. Brain Stimulation 2009;2(4):215–28.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nowak L, Bullier J. Axons, but not cell bodies, are activated by electrical stimulation in cortical gray matter I. Evidence from chronaxie measurements. Experimental brain research 1998;118(4):477–88. [DOI] [PubMed] [Google Scholar]
- 37.Matsushima J-I, Shepherd RK, Seldon HL, Xu S-A, Clark GM. Electrical stimulation of the auditory nerve in deaf kittens: effects on cochlear nucleus morphology. Hearing research 1991;56(1-2):133–42. [DOI] [PubMed] [Google Scholar]
- 38.Scheib CM. Brainstem Influence on Thalamocortical Oscillations during Anesthesia Emergence. Frontiers in Systems Neuroscience 2017;11:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hwang K, Bertolero MA, Liu WB, D’Esposito M. The Human Thalamus Is an Integrative Hub for Functional Brain Networks. The Journal of Neuroscience 2017;37(23):5594–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Datta A, Dmochowski JP, Guleyupoglu B, Bikson M, Fregni F. Cranial electrotherapy stimulation and transcranial pulsed current stimulation: a computer based high-resolution modeling study. Neuroimage 2013;65:280–7. [DOI] [PubMed] [Google Scholar]
- 41.Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol 2016;127(2):1031–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kenney-Jung DL, Blacker CJ, Camsari DD, Lee JC, Lewis CP. Transcranial Direct Current Stimulation: Mechanisms and Psychiatric Applications. Child Adolesc Psychiatr Clin N Am 2019;28(1):53–60. [DOI] [PubMed] [Google Scholar]
- 43.Brunoni AR, Sampaio-Junior B, Moffa AH, Aparicio LV, Gordon P, Klein I, et al. Noninvasive brain stimulation in psychiatric disorders: a primer. Braz J Psychiatry 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jog MV, Smith RX, Jann K, Dunn W, Lafon B, Truong D, et al. In-vivo Imaging of Magnetic Fields Induced by Transcranial Direct Current Stimulation (tDCS) in Human Brain using MRI. Sci Rep 2016;6:34385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kanai R, Chaieb L, Antal A, Walsh V, Paulus W. Frequency-dependent electrical stimulation of the visual cortex. Current Biology 2008;18(23):1839–43. [DOI] [PubMed] [Google Scholar]
- 46.Boggio PS, Ferrucci R, Rigonatti SP, Covre P, Nitsche M, Pascual-Leone A, et al. Effects of transcranial direct current stimulation on working memory in patients with Parkinson’s disease. Journal of the Neurological Sciences 2006;249(1):31–8. [DOI] [PubMed] [Google Scholar]
- 47.Weber MJ, Messing SB, Rao H, Detre JA, Thompson-Schill SL. Prefrontal transcranial direct current stimulation alters activation and connectivity in cortical and subcortical reward systems: A tDCS-fMRI study. Human brain mapping 2014;35(8):3673–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alam M, Truong DQ, Khadka N, Bikson M. Spatial and polarity precision of concentric high-definition transcranial direct current stimulation (HD-tDCS). Phys Med Biol 2016;61(12):4506–21. [DOI] [PubMed] [Google Scholar]
- 49.Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M. Gyri-precise head model of transcranial direct current stimulation: Improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimulation 2009;2(4):201–7.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rush S, Driscoll DA. Current distribution in the brain from surface electrodes. Anesthesia & Analgesia 1968;47(6):717–23. [PubMed] [Google Scholar]
- 51.Bikson M, Bulow P, Stiller JW, Datta A, Battaglia F, Karnup SV, et al. Transcranial direct current stimulation for major depression: a general system for quantifying transcranial electrotherapy dosage. Curr Treat Options Neurol 2008;10(5):377–85. [DOI] [PubMed] [Google Scholar]
- 52.McGirr A, Berlim MT. Clinical Usefulness of Therapeutic Neuromodulation for Major Depression: A Systematic Meta-Review of Recent Meta-Analyses. Psychiatr Clin North Am 2018;41(3):485–503. [DOI] [PubMed] [Google Scholar]
- 53.Borrione L, Moffa AH, Martin D, Loo CK, Brunoni AR. Transcranial Direct Current Stimulation in the Acute Depressive Episode: A Systematic Review of Current Knowledge. J ect 2018;34(3):153–63. [DOI] [PubMed] [Google Scholar]
- 54.Brunoni AR, Ferrucci R, Fregni F, Boggio PS, Priori A. Transcranial direct current stimulation for the treatment of major depressive disorder: a summary of preclinical, clinical and translational findings. Progress in Neuro-Psychopharmacology and Biological Psychiatry 2012;39(1):9–16. [DOI] [PubMed] [Google Scholar]
- 55.Seibt O, Brunoni AR, Huang Y, Bikson M. The pursuit of DLPFC: non-neuronavigated methods to target the left dorsolateral pre-frontal cortex with symmetric bicephalic transcranial direct current stimulation (tDCS). Brain stimulation 2015;8(3):590–602. [DOI] [PubMed] [Google Scholar]
- 56.Iyer M, Mattu U, Grafman J, Lomarev M, Sato S, Wassermann E. Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology 2005;64(5):872–5. [DOI] [PubMed] [Google Scholar]
- 57.Kincses TZ, Antal A, Nitsche MA, Bartfai O, Paulus W. Facilitation of probabilistic classification learning by transcranial direct current stimulation of the prefrontal cortex in the human. Neuropsychologia 2004;42(1):113–7. [DOI] [PubMed] [Google Scholar]
- 58.DaSilva AF, Mendonca ME, Zaghi S, Lopes M, DosSantos MF, Spierings EL, et al. tDCS-Induced Analgesia and Electrical Fields in Pain-Related Neural Networks in Chronic Migraine. Headache: The Journal of Head and Face Pain 2012;52(8):1283–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stilling JM, Monchi O, Amoozegar F, Debert CT. Transcranial Magnetic and Direct Current Stimulation (TMS/tDCS) for the Treatment of Headache: A Systematic Review. Headache 2019. [DOI] [PubMed] [Google Scholar]
- 60.Przeklasa-Muszynska A, Kocot-Kepska M, Dobrogowski J, Wiatr M, Mika J. Transcranial direct current stimulation (tDCS) and its influence on analgesics effectiveness in patients suffering from migraine headache. Pharmacol Rep 2017;69(4):714–21. [DOI] [PubMed] [Google Scholar]
- 61.Shirahige L, Melo L, Nogueira F, Rocha S, Monte-Silva K. Efficacy of Noninvasive Brain Stimulation on Pain Control in Migraine Patients: A Systematic Review and Meta-Analysis. Headache 2016;56(10):1565–96. [DOI] [PubMed] [Google Scholar]
- 62.Fregni F, Gimenes R, Valle AC, Ferreira MJL, Rocha RR, Natalle L, et al. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis & Rheumatism 2006;54(12):3988–98. [DOI] [PubMed] [Google Scholar]
- 63.Zhu CE, Yu B, Zhang W, Chen WH, Qi Q, Miao Y. Effiectiveness and safety of transcranial direct current stimulation in fibromyalgia: A systematic review and meta-analysis. J Rehabil Med 2017;49(1):2–9. [DOI] [PubMed] [Google Scholar]
- 64.Hou WH, Wang TY, Kang JH. The effects of add-on non-invasive brain stimulation in fibromyalgia: a meta-analysis and meta-regression of randomized controlled trials. Rheumatology (Oxford) 2016;55(8): 1507–17. [DOI] [PubMed] [Google Scholar]
- 65.Hagenacker T, Bude V, Naegel S, Holle D, Katsarava Z, Diener H-C, et al. Patient-conducted anodal transcranial direct current stimulation of the motor cortex alleviates pain in trigeminal neuralgia. The Journal of Headache and Pain 2014;15(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hansen N, Obermann M, Poitz F, Holle D, Diener H-C, Antal A, et al. Modulation of human trigeminal and extracranial nociceptive processing by transcranial direct current stimulation of the motor cortex. Cephalalgia 2011;31(6):661–70. [DOI] [PubMed] [Google Scholar]
- 67.Nitsche M, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. The Journal of physiology 2000;527(3):633–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Im C-H, Park J-H, Shim M, Chang WH, Kim Y-H. Evaluation of local electric fields generated by transcranial direct current stimulation with an extracephalic reference electrode based on realistic 3D body modeling. Physics in medicine and biology 2012;57(8):2137. [DOI] [PubMed] [Google Scholar]
- 69.Moliadze V, Antal A, Paulus W. Electrode-distance dependent after-effects of transcranial direct and random noise stimulation with extracephalic reference electrodes. Clinical Neurophysiology 2010;121(12):2165–71. [DOI] [PubMed] [Google Scholar]
- 70.Bikson M, Datta A, Rahman A, Scaturro J. Electrode montages for tDCS and weak transcranial electrical stimulation: role of “return” electrode’s position and size. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology 2010;121(12):1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pinchuk D, Pinchuk O, Sirbiladze K, Shuhgar O. Clinical effectiveness of primary and secondary headache treatment by transcranial direct current stimulation. Frontiers in neurology 2013;4:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beeli G, Casutt G, Baumgartner T, Jancke L. Modulating presence and impulsiveness by external stimulation of the brain. Behavioral and Brain Functions 2008;4(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zaehle T, Sandmann P, Thorne JD, Jancke L, Herrmann CS. Transcranial direct current stimulation of the prefrontal cortex modulates working memory performance: combined behavioural and electrophysiological evidence. BMC Neuroscience 2011;12(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marshall L, Molle M, Hallschmid M, Born J. Transcranial Direct Current Stimulation during Sleep Improves Declarative Memory. J Neurosci 2004;24(44):9985–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith P, Geddes L, Baek J-H, Darlington C, Zheng Y. Modulation of memory by vestibular lesions and galvanic vestibular stimulation. Frontiers in neurology 2010;1:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Truong DQ, Huber M, Xie X, Datta A, Rahman A, Parra LC, et al. Clinician accessible tools for GUI computational models of transcranial electrical stimulation: BONSAI and SPHERES. Brain Stimul 2014;7(4):521–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nitsche MA, Doemkes S, Karakose T, Antal A, Liebetanz D, Lang N, et al. Shaping the effects of transcranial direct current stimulation of the human motor cortex. J Neurophysiol 2007;97(4):3109–17. [DOI] [PubMed] [Google Scholar]
- 78.Antal A, Kincses T, Nitsche M, Paulus W. Manipulation of phosphene thresholds by transcranial direct current stimulation in man. Experimental Brain Research 2003;150(3):375–8. [DOI] [PubMed] [Google Scholar]
- 79.Paulus W On the difficulties of separating retinal from cortical origins of phosphenes when using transcranial alternating current stimulation (tACS). Clinical Neurophysiology 2010;121(7):987–91. [DOI] [PubMed] [Google Scholar]
- 80.Schwiedrzik CM. Retina or visual cortex? The site of phosphene induction by transcranial alternating current stimulation. Frontiers in integrative neuroscience 2009;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kar K, Krekelberg B. Transcranial electrical stimulation over visual cortex evokes phosphenes with a retinal origin. Journal of Neurophysiology 2012;108(8):2173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schutter DJLG, Hortensius R. Retinal origin of phosphenes to transcranial alternating current stimulation. Clinical Neurophysiology 2010;121(7):1080–4. [DOI] [PubMed] [Google Scholar]
- 83.Kanai R, Paulus W, Walsh V. Transcranial alternating current stimulation (tACS) modulates cortical excitability as assessed by TMS-induced phosphene thresholds. Clinical Neurophysiology 2010;121(9):1551–4. [DOI] [PubMed] [Google Scholar]
- 84.Peterchev AV, Wagner TA, Miranda PC, Nitsche MA, Paulus W, Lisanby SH, et al. Fundamentals of transcranial electric and magnetic stimulation dose: definition, selection, and reporting practices. Brain Stimulation 2012;5(4):435–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Merrill DR, Bikson M, Jefferys JG. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. J Neurosci Methods 2005;141(2):171–98. [DOI] [PubMed] [Google Scholar]
- 86.McCue MP, Guinan JJ Jr. Influence of efferent stimulation on acoustically responsive vestibular afferents in the cat. J Neurosci 1994;14(10):6071–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barlow RB Jr., Bolanowski SJ Jr., Brachman ML. Efferent optic nerve fibers mediate circadian rhythms in the Limulus eye. Science 1977;197(4298):86–9. [DOI] [PubMed] [Google Scholar]
- 88.Herrick CJ. An introduction to neurology: Saunders; 1918. [Google Scholar]
- 89.Chakraborty D, Truong DQ, Bikson M, Kaphzan H. Neuromodulation of Axon Terminals. Cereb Cortex 2017:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Eisele DW, Smith PL, Alam DS, Schwartz AR. Direct hypoglossal nerve stimulation in obstructive sleep apnea. Archives of Otolaryngology-Head & Neck Surgery 1997;123(1):57–61. [DOI] [PubMed] [Google Scholar]
- 91.Suzuki N, Hardebo JE, Kahrstrom J, Owman C. Selective electrical stimulation of postganglionic cerebrovascular parasympathetic nerve fibers originating from the sphenopalatine ganglion enhances cortical blood flow in the rat. Journal of Cerebral Blood Flow & Metabolism 1990;10(3):383–91. [DOI] [PubMed] [Google Scholar]
- 92.Borsody M, Sacristan E. Facial nerve stimulation as a future treatment for ischemic stroke. Brain Circulation 2016;2(4):164–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.MacDougall HG, Brizuela AE, Burgess AM, Curthoys IS. Between-subject variability and within-subject reliability of the human eye-movement response to bilateral galvanic (DC) vestibular stimulation. Experimental brain research 2002;144(1):69–78. [DOI] [PubMed] [Google Scholar]
- 94.Vailleau B, Qu’hen C, Vidal P-P, de Waele C. Probing residual vestibular function with galvanic stimulation in vestibular loss patients. Otology & Neurotology 2011;32(5):863–71. [DOI] [PubMed] [Google Scholar]
- 95.Weber KP, Rosengren SM, Michels R, Sturm V, Straumann D, Landau K. Single motor unit activity in human extraocular muscles during the vestibulo-ocular reflex. The Journal of Physiology 2012;590(13):3091–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Saku K, Kishi T, Sakamoto K, Hosokawa K, Sakamoto T, Murayama Y, et al. Afferent vagal nerve stimulation resets baroreflex neural arc and inhibits sympathetic nerve activity. Physiological Reports 2014;2(9):e12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nemeroff CB. Prevalence and management of treatment-resistant depression. J Clin Psychiatry 2007;68. [PubMed] [Google Scholar]
- 98.Sadler R, Purdy R, Rahey S. Vagal nerve stimulation aborts migraine in patient with intractable epilepsy. Cephalalgia 2002;22(6):482–4. [DOI] [PubMed] [Google Scholar]
- 99.George MS, Ward HE Jr., Ninan PT, Pollack M, Nahas Z, Anderson B, et al. A pilot study of vagus nerve stimulation (VNS) for treatment-resistant anxiety disorders. Brain Stimul 2008;1(2):112–21. [DOI] [PubMed] [Google Scholar]
- 100.Gurel NZ, Huang M, Wittbrodt MT, Jung H, Ladd SL, Shandhi MH, et al. Quantifying acute physiological biomarkers of transcutaneous cervical vagal nerve stimulation in the context of psychological stress. Brain Stimul 2019;(in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huston JM, Gallowitsch-Puerta M, Ochani M, Ochani K, Yuan R, Rosas-Ballina M, et al. Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis*. Crit Care Med 2007;35(12):2762–8. [DOI] [PubMed] [Google Scholar]
- 102.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. The Journal of clinical investigation 2007;117(2):289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000;405(6785):458–62. [DOI] [PubMed] [Google Scholar]
- 104.Guarini S, Altavilla D, Cainazzo M-M, Giuliani D, Bigiani A, Marini H, et al. Efferent vagal fibre stimulation blunts nuclear factor-KB activation and protects against hypovolemic hemorrhagic shock. Circulation 2003;107(8):1189–94. [DOI] [PubMed] [Google Scholar]
- 105.Smith LB. Cognition as a dynamic system: Principles from embodiment. Developmental Review 2005;25(3):278–98. [Google Scholar]
- 106.Jerath R, Crawford MW. How Does the Body Affect the Mind? Role of Cardiorespiratory Coherence in the Spectrum of Emotions. Adv Mind Body Med 2015;29(4):4–16. [PubMed] [Google Scholar]
- 107.Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nature reviews Neuroscience 2011;12(8): 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Targan RS, Alon G, Kay SL. Effect of long-term electrical stimulation on motor recovery and improvement of clinical residuals in patients with unresolved facial nerve palsy. Otolaryngology--Head and Neck Surgery 2000;122(2):246–52. [DOI] [PubMed] [Google Scholar]
- 109.Ando M, Katare RG, Kakinuma Y, Zhang D, Yamasaki F, Muramoto K, et al. Efferent vagal nerve stimulation protects heart against ischemia-induced arrhythmias by preserving connexin43 protein. Circulation 2005;112(2):164–70. [DOI] [PubMed] [Google Scholar]
- 110.Schwartz AR, Bennett ML, Smith PL, et al. Therapeutic electrical stimulation of the hypoglossal nerve in obstructive sleep apnea. Archives of Otolaryngology-Head & Neck Surgery 2001;127(10):1216–23. [DOI] [PubMed] [Google Scholar]
- 111.Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell 1991;65(1):175–87. [DOI] [PubMed] [Google Scholar]
- 112.Zald DH, Pardo JV. Functional neuroimaging of the olfactory system in humans. Int J Psychophysiol 2000;36(2):165–81. [DOI] [PubMed] [Google Scholar]
- 113.Gottfried JA, Zald DH. On the scent of human olfactory orbitofrontal cortex: meta-analysis and comparison to non-human primates. Brain Research Reviews 2005;50(2):287–304. [DOI] [PubMed] [Google Scholar]
- 114.Ray JP, Price JL. The organization of the thalamocortical connections of the mediodorsal thalamic nucleus in the rat, related to the ventral forebrain-prefrontal cortex topography. J Comp Neurol 1992;323(2):167–97. [DOI] [PubMed] [Google Scholar]
- 115.Shipley MT, Halloran FJ, de la Torre J. Surprisingly rich projection from locus coeruleus to the olfactory bulb in the rat. Brain research 1985;329(1):294–9. [DOI] [PubMed] [Google Scholar]
- 116.Sullivan RM, Wilson DA. The locus coeruleus, norepinephrine, and memory in newborns. Brain research bulletin 1994;35(5):467–72. [DOI] [PubMed] [Google Scholar]
- 117.Vermetten E, Schmahl C, Southwick SM, Bremner JD. Positron tomographic emission study of olfactory induced emotional recall in veterans with and without combat-related posttraumatic stress disorder. Psychopharmacol Bull 2007;40(1):8–30. [PMC free article] [PubMed] [Google Scholar]
- 118.Vermetten E, Bremner JD. Olfaction as a traumatic reminder in posttraumatic stress disorder: Case reports and review. J Clin Psychiatry 2003;64:202–7. [DOI] [PubMed] [Google Scholar]
- 119.Ishimaru T, Sakumoto M, Kimura Y, Furukawa M. Olfactory evoked potentials produced by electrical stimulation of the olfactory mucosa. Auris Nasus Larynx 1996;23(1):98–104. [DOI] [PubMed] [Google Scholar]
- 120.Yamamoto C Olfactory bulb potentials to electrical stimulation of the olfactory mucosa. Jpn J Physiol 1961;11:545–54. [DOI] [PubMed] [Google Scholar]
- 121.Coelho DH, Costanzo RM. Spatial Mapping in the Rat Olfactory Bulb by Odor and Direct Electrical Stimulation. Otolaryngol Head Neck Surg 2016;155(3):526–32. [DOI] [PubMed] [Google Scholar]
- 122.Dong Q, Du L, Zhuang L, Li R, Liu Q, Wang P. A novel bioelectronic nose based on brain-machine interface using implanted electrode recording in vivo in olfactory bulb. Biosens Bioelectron 2013;49:263–9. [DOI] [PubMed] [Google Scholar]
- 123.Greer CA, Stewart WB, Kauer JS, Shepherd GM. Topographical and laminar localization of 2-deoxyglucose uptake in rat olfactory bulb induced by electrical stimulation of olfactory nerves. Brain Research 1981;217(2):279–93. [DOI] [PubMed] [Google Scholar]
- 124.Yamamoto C, Yamamoto T, Iwama K. The inhibitory systems in the olfactory bulb studied by intracellular recording. J Neurophysiol 1963;26:403–15. [DOI] [PubMed] [Google Scholar]
- 125.Spors H, Albeanu DF, Murthy VN, Rinberg D, Uchida N, Wachowiak M, et al. Illuminating Vertebrate Olfactory Processing. The Journal of Neuroscience 2012;32(41):14102-8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mouly A-M, Vigouroux M, Holley A. On the ability of rats to discriminate between microstimulations of the olfactory bulb in different locations. Behavioural brain research 1985;17(1):45–58. [DOI] [PubMed] [Google Scholar]
- 127.Jourdan F, Duveau A, Astic L, Holley A. Spatial distribution of [14C]2-deoxyglucose uptake in the olfactory bulbs of rats stimulated with two different odours. Brain Res 1980;188(1):139–54. [DOI] [PubMed] [Google Scholar]
- 128.Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cerebral cortex 2000;10(3):295–307. [DOI] [PubMed] [Google Scholar]
- 129.Tanabe T, Yarita H, Iino M, Ooshima Y, Takagi S. An olfactory projection area in orbitofrontal cortex of the monkey. Journal of Neurophysiology 1975;38(5):1269–83. [DOI] [PubMed] [Google Scholar]
- 130.Zatorre RJ, Jones G, Marilyn. Human olfactory discrimination after unilateral frontal or temporal lobectomy. Brain 1991;114(1):71–84. [PubMed] [Google Scholar]
- 131.Mesulam MM, Mufson EJ. Insula of the old world monkey. I. Architectonics in the insulo-orbito-temporal component of the paralimbic brain. J Comp Neurol 1982;212(1):1–22. [DOI] [PubMed] [Google Scholar]
- 132.Mufson EJ, Mesulam MM. Insula of the old world monkey. II: Afferent cortical input and comments on the claustrum. J Comp Neurol 1982;212(1):23–37. [DOI] [PubMed] [Google Scholar]
- 133.Morecraft RJ, Geula C, Mesulam MM. Cytoarchitecture and neural afferents of orbitofrontal cortex in the brain of the monkey. J Comp Neurol 1992;323(3):341–58. [DOI] [PubMed] [Google Scholar]
- 134.Rolls ET, Baylis LL. Gustatory, olfactory, and visual convergence within the primate orbitofrontal cortex. Journal of Neuroscience 1994;14(9):5437–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kobal G, Hummel T. Human Electro-Olfactograms and Brain Responses to Olfactory Stimulation In: Laing DG, Doty RL, Breipohl W, editors. The Human Sense of Smell. Berlin, Heidelberg: Springer Berlin Heidelberg; 1991. p. 135–51. [Google Scholar]
- 136.Atanasova B, Graux J, El Hage W, Hommet C, Camus V, Belzung C. Olfaction: A potential cognitive marker of psychiatric disorders. Neuroscience & Biobehavioral Reviews 2008;32(7):1315–25. [DOI] [PubMed] [Google Scholar]
- 137.Mercer PBS, Witt MCZ, Pessoa RR. Olfactory dysfunction in Alzheimer’s disease Systematic review and meta-analysis. Dementia & neuropsychologia 2018;12(2):123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zou Y-m, Da Lu L-pL, Zhang H-h, Zhou Y-y. Olfactory dysfunction in Alzheimer’s disease. Neuropsychiatric disease and treatment 2016;12:869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sedghizadeh MJ, Hojjati H, Ezzatdoost K, Aghajan H, Vahabi Z, Tarighatnia H. Olfactory Response as a Marker for Alzheimer’s Disease: Evidence from Perception and Frontal Oscillation Coherence Deficit. BioRxiv 2019:840934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang J, Eslinger PJ, Doty RL, Zimmerman EK, Grunfeld R, Sun X, et al. Olfactory deficit detected by fMRI in early Alzheimer’s disease. Brain research 2010;1357:184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bremner JD, editor. Posttraumatic Stress Disorder: From Neurobiology to Treatment. 1 ed. Hoboken, New Jersey: Wiley; 2016. [Google Scholar]
- 142.LeDoux JE. The Emotional Brain: The Mysterious Underpinnings of Emotional Life. New York, N.Y.: Simon & Schuster; 1996. [Google Scholar]
- 143.Auffermann H, Mathe F, Gerull G, Mrowinski D. Olfactory Evoked Potentials and Contingent Negative Variation Simultaneously Recorded for Diagnosis of Smell Disorders. Annals of Otology, Rhinology & Laryngology 1993;102(1):6–10. [DOI] [PubMed] [Google Scholar]
- 144.Ottoson D Olfactory bulb potentials induced by electrical stimulation of the nasal mucosa in the frog. Acta Physiologica Scandinavica 1960;47(2-3):160–72. [DOI] [PubMed] [Google Scholar]
- 145.Efron R The effect of olfactory stimuli in arresting uncinate fits. Brain 1956;79(2):267–81. [DOI] [PubMed] [Google Scholar]
- 146.Ebert U, Löscher W. Strong olfactory stimulation reduces seizure susceptibility in amygdala-kindled rats. Neuroscience Letters 2000;287(3):199–202. [DOI] [PubMed] [Google Scholar]
- 147.Betts T, Fox C, MacCallum R, editors. USING OLFACTORY STIMULI TO ABORT OR PREVENT SEIZURES-COUNTERMEASURE OR CUE-CONTROLLED AROUSAL MANIPULATION-IS THERE SOMETHING SPECIAL ABOUT SMELL Epilepsia; 1995: LIPPINCOTT-RAVEN PUBL 227 EAST WASHINGTON SQ, PHILADELPHIA, PA 19106. [Google Scholar]
- 148.Li X, Fan M, Cao Y, Hong Z. Electrical stimulation of the olfactory mucosa: An alternative treatment for the temporal lobe epilepsy? Medical hypotheses 2010;74(1):24–6. [DOI] [PubMed] [Google Scholar]
- 149.Penfield W, Jasper H. Epilepsy and the functional anatomy of the human brain. 1954. [Google Scholar]
- 150.Sarnat HB, Flores-Sarnat L. Might the olfactory bulb be an origin of olfactory auras in focal epilepsy? Epileptic Disord 2016;18(4):344–55. [DOI] [PubMed] [Google Scholar]
- 151.Chen C, Shih YH, Yen DJ, Lirng JF, Guo YC, Yu HY, et al. Olfactory auras in patients with temporal lobe epilepsy. Epilepsia 2003;44(2):257–60. [DOI] [PubMed] [Google Scholar]
- 152.Ostrowsky K, Isnard J, Ryvlin P, Guenot M, Fischer C, Mauguiere F. Functional mapping of the insular cortex: clinical implication in temporal lobe epilepsy. Epilepsia 2000;41(6):681–6. [DOI] [PubMed] [Google Scholar]
- 153.Kumar G, Juhász C, Sood S, Asano E. Olfactory hallucinations elicited by electrical stimulation via subdural electrodes: effects of direct stimulation of olfactory bulb and tract. Epilepsy & Behavior 2012;24(2):264–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Straschill M, Stahl H, Gorkisch K. Effects of electrical stimulation of the human olfactory mucosa. Stereotactic and Functional Neurosurgery 1983;46(5-6):286–9. [DOI] [PubMed] [Google Scholar]
- 155.Zatorre RJ. Functional localization and lateralization of human olfactory cortex. Nature (London) 1992;360(6402):339–40. [DOI] [PubMed] [Google Scholar]
- 156.Schmidt S, Mante A, Rönnefarth M, Fleischmann R, Gall C, Brandt SA. Progressive enhancement of alpha activity and visual function in patients with optic neuropathy: A two-week repeated session alternating current stimulation study. Brain Stimulation 2013;6(1):87–93. [DOI] [PubMed] [Google Scholar]
- 157.Klauke S, Goertz M, Rein S, Hoehl D, Thomas U, Eckhorn R, et al. Stimulation with a wireless intraocular epiretinal implant elicits visual percepts in blind humans. Investigative ophthalmology & visual science 2011;52(1):449–55. [DOI] [PubMed] [Google Scholar]
- 158.Stingl K, Bartz-Schmidt KU, Besch D, Chee CK, Cottriall CL, Gekeler F, et al. Subretinal visual implant alpha IMS–clinical trial interim report. Vision research 2015;111:149–60. [DOI] [PubMed] [Google Scholar]
- 159.Fujikado T, Kamei M, Sakaguchi H, Kanda H, Morimoto T, Ikuno Y, et al. Testing of semichronically implanted retinal prosthesis by suprachoroidal-transretinal stimulation in patients with retinitis pigmentosa. Investigative ophthalmology & visual science 2011;52(7):4726–33. [DOI] [PubMed] [Google Scholar]
- 160.Schmidt E, Bak M, Hambrecht F, Kufta C, O’rourke D, Vallabhanath P. Feasibility of a visual prosthesis for the blind based on intracortical micro stimulation of the visual cortex. Brain 1996;119(2):507–22. [DOI] [PubMed] [Google Scholar]
- 161.Veraart C, Wanet-Defalque MC, Gerard B, Vanlierde A, Delbeke J. Pattern recognition with the optic nerve visual prosthesis. Artificial organs 2003;27(11):996–1004. [DOI] [PubMed] [Google Scholar]
- 162.Henrich-Noack P Electrical brain stimulation induces dendritic stripping but improves survival of silent neurons after optic nerve damage. Scientific reports 2017;7(1):627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zrenner E, Birch D. Restoring vision to the blind: the new age of implanted visual prostheses. Translational Vision Science and Technology 2015;3(7):3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. The Lancet 2006;368(9549):1795–809. [DOI] [PubMed] [Google Scholar]
- 165.Sakaguchi H, Kamei M, Fujikado T, Yonezawa E, Ozawa M, Cecilia-Gonzalez C, et al. Artificial vision by direct optic nerve electrode (AV-DONE) implantation in a blind patient with retinitis pigmentosa. Journal of Artificial Organs 2009;12(3):206–9. [DOI] [PubMed] [Google Scholar]
- 166.Delbeke J, Oozeer M, Veraart C. Position, size and luminosity of phosphenes generated by direct optic nerve stimulation. Vision research 2003;43(9):1091–102. [DOI] [PubMed] [Google Scholar]
- 167.Brelen ME, Vince V, Gerard B, Veraart C, Delbeke J. Measurement of evoked potentials after electrical stimulation of the human optic nerve. Invest Ophthalmol Vis Sci 2010;51(10):5351–5. [DOI] [PubMed] [Google Scholar]
- 168.Zeng F-G, Fay RR. Cochlear implants: Auditory prostheses and electric hearing: Springer Science & Business Media; 2013. [Google Scholar]
- 169.Okazaki Y, Morimoto T, Sawai H. Parameters of optic nerve electrical stimulation affecting neuroprotection of axotomized retinal ganglion cells in adult rats. Neuroscience Research 2008;61(2):129–35. [DOI] [PubMed] [Google Scholar]
- 170.Kerrison JB, Lynn MJ, Baer CA, Newman SA, Biousse V, Newman NJ. Stages of improvement in visual fields after pituitary tumor resection. American journal of ophthalmology 2000;130(6):813–20. [DOI] [PubMed] [Google Scholar]
- 171.Morimoto T, Miyoshi T, Fujikado T, Tano Y, Fukuda Y. Electrical stimulation enhances the survival of axotomized retinal ganglion cells in vivo. Neuroreport 2002;13(2):227–30. [DOI] [PubMed] [Google Scholar]
- 172.Bechtereva NP, Shandurina AN, Khilko VA, Lyskov EB, Matveev YK, Panin AV, et al. Clinical and physiological basis for a new method underlying rehabilitation of the damaged visual nerve function by direct electric stimulation. International Journal of Psychophysiology 1985;2(4):257–72. [DOI] [PubMed] [Google Scholar]
- 173.Bechtereva N, Bondartchuk A, Gretchin V, Iliukhina V, Kambarova D, Matveev YK, et al. Structural-functional organization of the human brain and the pathophysiology of the Parkinsonian type hyperkineses. Stereotactic and Functional Neurosurgery 1972;34(1-4):14–7. [PubMed] [Google Scholar]
- 174.Miyake K-i, Yoshida M, Inoue Y, Hata Y. Neuroprotective effect of transcorneal electrical stimulation on the acute phase of optic nerve injury. Investigative ophthalmology & visual science 2007;48(5):2356–61. [DOI] [PubMed] [Google Scholar]
- 175.Fujikado T, Morimoto T, Matsushita K, Shimojo H, Okawa Y, Tano Y. Effect of transcorneal electrical stimulation in patients with nonarteritic ischemic optic neuropathy or traumatic optic neuropathy. Japanese journal of ophthalmology 2006;50(3):266–73. [DOI] [PubMed] [Google Scholar]
- 176.Nishida K Visual Sensation by Electrical Stimulation Using a New Direct Optic Nerve Electrode Device. Brain stimulation 2015;8(3):678–81. [DOI] [PubMed] [Google Scholar]
- 177.Gall C, Fedorov AB, Ernst L, Borrmann A, Sabel BA. Repetitive transorbital alternating current stimulation in optic neuropathy. NeuroRehabilitation 2010;27(4):335–41. [DOI] [PubMed] [Google Scholar]
- 178.Schuierer G, Laub G, Huk WJ. MR angiography of the primitive trigeminal artery: report on two cases. AJNR Am J Neuroradiol 1990;11(6):1131–2. [PMC free article] [PubMed] [Google Scholar]
- 179.Sirinathsinghji DJ, Nikolarakis KE, Reimer S, Herz A. Nigrostriatal dopamine mediates the stimulatory effects of corticotropin releasing factor on methionine-enkephalin and dynorphin release from the rat neostriatum. Brain Res 1990;526(1):173–6. [DOI] [PubMed] [Google Scholar]
- 180.Kuroshima S, Hayashida N, Anju T, Matsuyama M, O S. [Proceedings: Congenital absence of the pericardium--some considerations on its diagnosis and management]. Jpn Circ J 1975;39(7):890. [PubMed] [Google Scholar]
- 181.Fedorov A, Jobke S, Bersnev V, Chibisova A, Chibisova Y, Gall C, et al. Restoration of vision after optic nerve lesions with noninvasive transorbital alternating current stimulation: a clinical observational study. Brain stimulation 2011;4(4):189–201. [DOI] [PubMed] [Google Scholar]
- 182.Kar K, Krekelberg B. Transcranial electrical stimulation over visual cortex evokes phosphenes with a retinal origin. J Neurophysiol 2012;108(8):2173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Laczo B, Antal A, Niebergall R, Treue S, Paulus W. Transcranial alternating stimulation in a high gamma frequency range applied over V1 improves contrast perception but does not modulate spatial attention. Brain Stimul 2012;5(4):484–91. [DOI] [PubMed] [Google Scholar]
- 184.Morimoto T, Fukui T, Matsushita K, Okawa Y, Shimojyo H, Kusaka S, et al. Evaluation of residual retinal function by pupillary constrictions and phosphenes using transcorneal electrical stimulation in patients with retinal degeneration. Graefes Arch Clin Exp Ophthalmol 2006;244(10):1283–92. [DOI] [PubMed] [Google Scholar]
- 185.Fujikado T, Morimoto T, Kanda H, Kusaka S, Nakauchi K, Ozawa M, et al. Evaluation of phosphenes elicited by extraocular stimulation in normals and by suprachoroidal-transretinal stimulation in patients with retinitis pigmentosa. Graefes Arch Clin Exp Ophthalmol 2007;245(10):1411–9. [DOI] [PubMed] [Google Scholar]
- 186.Oono S, Kurimoto T, Kashimoto R, Tagami Y, Okamoto N, Mimura O. Transcorneal electrical stimulation improves visual function in eyes with branch retinal artery occlusion. Clin Ophthalmol 2011;5:397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dawson WW, Trick GL, Litzkow CA. Improved electrode for electroretinography. Invest Ophthalmol Vis Sci 1979;18(9):988–91. [PubMed] [Google Scholar]
- 188.Schatz A, Pach J, Gosheva M, Naycheva L, Willmann G, Wilhelm B, et al. Transcorneal Electrical Stimulation for Patients With Retinitis Pigmentosa: A Prospective, Randomized, Sham-Controlled Followup Study Over 1 Year. Invest Ophthalmol Vis Sci 2017;58(1):257–69. [DOI] [PubMed] [Google Scholar]
- 189.Ozeki N, Shinoda K, Ohde H, Ishida S, Tsubota K. Improvement of visual acuity after transcorneal electrical stimulation in case of Best vitelliform macular dystrophy. Graefes Arch Clin Exp Ophthalmol 2013;251(7):1867–70. [DOI] [PubMed] [Google Scholar]
- 190.Naycheva L, Schatz A, Rock T, Willmann G, Messias A, Bartz-Schmidt KU, et al. Phosphene thresholds elicited by transcorneal electrical stimulation in healthy subjects and patients with retinal diseases. Invest Ophthalmol Vis Sci 2012;53(12):7440–8. [DOI] [PubMed] [Google Scholar]
- 191.Xie J, Wang GJ, Yow L, C JC, Humayun MS, Weiland JD, et al. Modeling and percept of transcorneal electrical stimulation in humans. IEEE Trans Biomed Eng 2011;58(7):1932–9. [DOI] [PubMed] [Google Scholar]
- 192.Naycheva L, Schatz A, Willmann G, Bartz-Schmidt KU, Zrenner E, Rock T, et al. Transcorneal electrical stimulation in patients with retinal artery occlusion: a prospective, randomized, sham-controlled pilot study. Ophthalmol Ther 2013;2(1):25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sabel BA, Fedorov AB, Naue N, Borrmann A, Herrmann C, Gall C. Non-invasive alternating current stimulation improves vision in optic neuropathy. Restorative neurology and neuroscience 2011;29(6):493–505. [DOI] [PubMed] [Google Scholar]
- 194.Webb BS, Tinsley CJ, Barraclough NE, Easton A, Parker A, Derrington AM. Feedback from V1 and inhibition from beyond the classical receptive field modulates the responses of neurons in the primate lateral geniculate nucleus. Vis Neurosci 2002;19(5):583–92. [DOI] [PubMed] [Google Scholar]
- 195.Murphy PC, Sillito AM. Corticofugal feedback influences the generation of length tuning in the visual pathway. Nature 1987;329(6141):727–9. [DOI] [PubMed] [Google Scholar]
- 196.Bazanova OM, Vernon D. Interpreting EEG alpha activity. Neuroscience & Biobehavioral Reviews 2014;44:94–110. [DOI] [PubMed] [Google Scholar]
- 197.Briggs F, Usrey WM. Emerging views of corticothalamic function. Current opinion in neurobiology 2008;18(4):403–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bola M, Gall C, Moewes C, Fedorov A, Hinrichs H, Sabel BA. Brain functional connectivity network breakdown and restoration in blindness. Neurology 2014;83(6):542–51. [DOI] [PubMed] [Google Scholar]
- 199.Sehatpour P, Molholm S, Schwartz TH, Mahoney JR, Mehta AD, Javitt DC, et al. A human intracranial study of long-range oscillatory coherence across a frontal–occipital–hippocampal brain network during visual object processing. Proceedings of the National Academy of Sciences 2008;105(11):4399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chellappa SL, Gordijn MC, Cajochen C. Can light make us bright? Effects of light on cognition and sleep Progress in brain research. 190: Elsevier; 2011. p. 119–33. [DOI] [PubMed] [Google Scholar]
- 201.Terman M Evolving applications of light therapy. Sleep medicine reviews 2007;11(6):497–507. [DOI] [PubMed] [Google Scholar]
- 202.laccarino HF, Singer AC, Martorell AJ, Rudenko A, Gao F, Gillingham TZ, et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 2016;540(7632):230–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ismail R, Hansen AK, Parbo P, Braendgaard H, Gottrup H, Brooks DJ, et al. The Effect of 40-Hz Light Therapy on Amyloid Load in Patients with Prodromal and Clinical Alzheimer’s Disease. Int J Alzheimers Dis 2018;2018:6852303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Dobelle W, Mladejovsky M. Phosphenes produced by electrical stimulation of human occipital cortex, and their application to the development of a prosthesis for the blind. The Journal of physiology 1974;243(2):553–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lewis PM, Rosenfeld JV. Electrical stimulation of the brain and the development of cortical visual prostheses: an historical perspective. Brain research 2016;1630:208–24. [DOI] [PubMed] [Google Scholar]
- 206.Caspi A, Dorn J, Helder JB, Katyal KD, Roy A, editors. Eye movements as a marker for visual prosthesis spatial mapping—A feasibility study using a blind patient implanted with the Argus II retinal prosthesis. Engineering in Medicine and Biology Society (EMBC), 2016 IEEE 38th Annual International Conference of the; 2016: IEEE. [DOI] [PubMed] [Google Scholar]
- 207.Muller L, Destexhe A. Propagating waves in thalamus, cortex and the thalamocortical system: Experiments and models. Journal of Physiology-Paris 2012;106(5):222–38. [DOI] [PubMed] [Google Scholar]
- 208.Sato Tatsuo K, Nauhaus I, Carandini M. Traveling Waves in Visual Cortex. Neuron 2012;75(2):218–29. [DOI] [PubMed] [Google Scholar]
- 209.Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological psychiatry 2007;62(5):429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Gall C, Schmidt S, Schittkowski MP, Antal A, Ambrus GG, Paulus W, et al. Alternating Current Stimulation for Vision Restoration after Optic Nerve Damage: A Randomized Clinical Trial. PLOS ONE 2016;11(6):e0156134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Gasser HS, Erlanger J. The role played by the sizes of the constituent fibers of a nerve trunk in determining the form of its action potential wave. American Journal of Physiology--Legacy Content 1927;80(3):522–47. [Google Scholar]
- 212.Perl ER. Ideas about pain, a historical view. Nat Rev Neurosci 2007;8(1):71–80. [DOI] [PubMed] [Google Scholar]
- 213.Vallbo AB, Hagbarth K, Torebjork H, Wallin B. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiological reviews 1979;59(4):919–57. [DOI] [PubMed] [Google Scholar]
- 214.Dubin AE, Patapoutian A. Nociceptors: the sensors of the pain pathway. The Journal of Clinical Investigation 2010;120(11):3760–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Singla S, Prabhakar V, Singla RK. Role of transcutaneous electric nerve stimulation in the management of trigeminal neuralgia. Journal of Neurosciences in Rural Practice 2011;2(2):150–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Reuter E, Krekeler G, Krainick J, Thoden U, Doerr M. Pain suppression in the trigeminal region by means of transcutaneous nerve stimulation. Deutsche zahnarztliche Zeitschrift 1976;31(3):274–6. [PubMed] [Google Scholar]
- 217.Wall PD, Sweet WH. Temporary abolition of pain in man. Science 1967;155(3758):108–9. [DOI] [PubMed] [Google Scholar]
- 218.Sluka KA, Walsh D. Transcutaneous electrical nerve stimulation: basic science mechanisms and clinical effectiveness. The Journal of Pain 2003;4(3):109–21. [DOI] [PubMed] [Google Scholar]
- 219.Jones I, Johnson MI. Transcutaneous electrical nerve stimulation. Continuing Education in Anaesthesia, Critical Care & Pain 2009;9(4):130–5. [Google Scholar]
- 220.Slavin KV, Colpan ME, Munawar N, Wess C, Nersesyan H. Trigeminal and occipital peripheral nerve stimulation for craniofacial pain: a single-institution experience and review of the literature. Neurosurgical focus 2006;21(6):1–5. [DOI] [PubMed] [Google Scholar]
- 221.Dunteman E Peripheral nerve stimulation for unremitting ophthalmic postherpetic neuralgia. Neuromodulation: Technology at the Neural Interface 2002;5(1):32–7. [DOI] [PubMed] [Google Scholar]
- 222.Katusic S, Beard CM, Bergstralth E, Kurland LT. Incidence and clinical features of trigeminal neuralgia, Rochester, Minnesota, 1945–1984. Annals of neurology 1990;27(1):89–95. [DOI] [PubMed] [Google Scholar]
- 223.Jakobs M, Unterberg A, Treede RD, Schuh-Hofer S, Ahmadi R. Subcutaneous trigeminal nerve field stimulation for refractory trigeminal pain: a cohort analysis. Acta Neurochir (Wien) 2016;158(9):1767–74. [DOI] [PubMed] [Google Scholar]
- 224.Johnson MD, Burchiel KJ. Peripheral stimulation for treatment of trigeminal postherpetic neuralgia and trigeminal posttraumatic neuropathic pain: a pilot study. Neurosurgery 2004;55(1):135–42. [PubMed] [Google Scholar]
- 225.Stidd DA, Wuollet A, Kirk Bowden D, Price T, Patwardhan A, Barker S, et al. Peripheral nerve stimulation for trigeminal neuropathic pain. Pain physician 2012;15(1):27. [PMC free article] [PubMed] [Google Scholar]
- 226.Stöhr M, Petruch F, Scheglmann K. Somatosensory evoked potentials following trigeminal nerve stimulation in trigeminal neuralgia. Annals of Neurology 1981;9(1):63–6. [DOI] [PubMed] [Google Scholar]
- 227.Soustiel JF, Feinsod M, Hafner H. Short latency trigeminal evoked potentials: normative data and clinical correlations. Electroencephalogr Clin Neurophysiol 1991;80(2):119–25. [DOI] [PubMed] [Google Scholar]
- 228.Bennett MH, Jannetta PJ. Trigeminal evoked potentials in humans. Electroencephalography and Clinical Neurophysiology 1980;48(5):517–26. [DOI] [PubMed] [Google Scholar]
- 229.Hashimoto I Trigeminal evoked potentials following brief air puff: Enhanced signal-to-noise ratio. Annals of neurology 1988;23(4):332–8. [DOI] [PubMed] [Google Scholar]
- 230.Findler G, Feinsod M. Sensory evoked response to electrical stimulation of the trigeminal nerve in humans. J Neurosurg 1982;56(4):545–9. [DOI] [PubMed] [Google Scholar]
- 231.Leandri M, Parodi CI, Favale E. Early trigeminal evoked potentials in tumours of the base of the skull and trigeminal neuralgia. Electroencephalogr Clin Neurophysiol 1988;71(2):114–24. [DOI] [PubMed] [Google Scholar]
- 232.Cruccu G, Aminoff MJ, Curio G, Guerit JM, Kakigi R, Mauguiere F, et al. Recommendations for the clinical use of somatosensory-evoked potentials. Clinical Neurophysiology 2008;119(8):1705–19. [DOI] [PubMed] [Google Scholar]
- 233.Laxer KD, Trinka E, Hirsch LJ, Cendes F, Langfitt J, Delanty N, et al. The consequences of refractory epilepsy and its treatment. Epilepsy & Behavior 2014;37:59–70. [DOI] [PubMed] [Google Scholar]
- 234.Fanselow EE, Reid AP, Nicolelis MA. Reduction of pentylenetetrazole-induced seizure activity in awake rats by seizure-triggered trigeminal nerve stimulation. The Journal of Neuroscience 2000;20(21):8160–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wang Q-Q, Zhu L-J, Wang X-H, Zuo J, He H-Y, Tian M-M, et al. Chronic trigeminal nerve stimulation protects against seizures, cognitive impairments, hippocampal apoptosis, and inflammatory responses in epileptic rats. Journal of Molecular Neuroscience 2016;59(1):78–89. [DOI] [PubMed] [Google Scholar]
- 236.DeGiorgio CM, Shewmon A, Murray D, Whitehurst T. Pilot Study of Trigeminal Nerve Stimulation (TNS) for Epilepsy: A Proof-of-Concept Trial. Epilepsia 2006;47(7):1213–5. [DOI] [PubMed] [Google Scholar]
- 237.DeGiorgio CM, Murray D, Markovic D, Whitehurst T. Trigeminal nerve stimulation for epilepsy: long-term feasibility and efficacy. Neurology 2009;72(10):936–8. [DOI] [PubMed] [Google Scholar]
- 238.DeGiorgio CM, Soss J, Cook IA, Markovic D, Gornbein J, Murray D, et al. Randomized controlled trial of trigeminal nerve stimulation for drug-resistant epilepsy. Neurology 2013;80(9):786–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Soss J, Heck C, Murray D, Markovic D, Oviedo S, Corrale-Leyva G, et al. A prospective long-term study of external trigeminal nerve stimulation for drug-resistant epilepsy. Epilepsy & Behavior 2015;42:44–7. [DOI] [PubMed] [Google Scholar]
- 240.Caous CA, Buck HdS, Lindsey CJ. Neuronal connections of the paratrigeminal nucleus: a topographic analysis of neurons projecting to bulbar, pontine and thalamic nuclei related to cardiovascular, respiratory and sensory functions. Autonomic Neuroscience 2001;94(1):14–24. [DOI] [PubMed] [Google Scholar]
- 241.de Sousa Buck H, Caous CA, Lindsey CJ. Projections of the paratrigeminal nucleus to the ambiguus, rostroventrolateral and lateral reticular nuclei, and the solitary tract. Autonomic Neuroscience 2001;87(2-3):187–200. [DOI] [PubMed] [Google Scholar]
- 242.Willoch F, Gamringer U, Medele R, Steude U, Tölle TR. Analgesia by electrostimulation of the trigeminal ganglion in patients with trigeminopathic pain: a PET activation study. PAIN® 2003;103(1):119–30. [DOI] [PubMed] [Google Scholar]
- 243.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA psychiatry 2015;72(6):603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Shiozawa P, da Silva ME, Netto GTM, Taiar I, Cordeiro Q. Effect of a 10-day trigeminal nerve stimulation (TNS) protocol for treating major depressive disorder: a phase II, sham-controlled, randomized clinical trial. Epilepsy & Behavior 2015;44:23–6. [DOI] [PubMed] [Google Scholar]
- 245.Cook IA, Schrader LM, DeGiorgio CM, Miller PR, Maremont ER, Leuchter AF. Trigeminal nerve stimulation in major depressive disorder: acute outcomes in an open pilot study. Epilepsy & Behavior 2013;28(2):221–6. [DOI] [PubMed] [Google Scholar]
- 246.Schrader LM, Cook IA, Miller PR, Maremont ER, DeGiorgio CM. Trigeminal nerve stimulation in major depressive disorder: first proof of concept in an open pilot trial. Epilepsy & Behavior 2011;22(3):475–8. [DOI] [PubMed] [Google Scholar]
- 247.Cook IA, Abrams M, Leuchter AF. Trigeminal nerve stimulation for comorbid posttraumatic stress disorder and major depressive disorder. Neuromodulation: Technology at the Neural Interface 2016. [DOI] [PubMed] [Google Scholar]
- 248.Schoenen J, Vandersmissen B, Jeangette S, Herroelen L, Vandenheede M, Gérard P, et al. Migraine prevention with a supraorbital transcutaneous stimulator A randomized controlled trial. Neurology 2013;80(8):697–704. [DOI] [PubMed] [Google Scholar]
- 249.Soleymani T, Pieton D, Pezeshkian P, Miller P, Gorgulho AA, Pouratian N, et al. Surgical approaches to tinnitus treatment: A review and novel approaches. Surg Neurol Int 2011;2:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.McGough JJ, Loo SK, Sturm A, Cowen J, Leuchter AF, Cook IA. An eight-week, open-trial, pilot feasibility study of trigeminal nerve stimulation in youth with attention-deficit/hyperactivity disorder. Brain stimulation 2015;8(2):299–304. [DOI] [PubMed] [Google Scholar]
- 251.Trevizol AP, Shiozawa P, Sato IA, de Barros Calfat EL, Alberto RL, Cook IA, et al. Trigeminal nerve stimulation (TNS) for generalized anxiety disorder: a case study. Brain stimulation 2015;8(3):659–60. [DOI] [PubMed] [Google Scholar]
- 252.Trevizol AP, Shiozawa P, Albuquerque Sato I, Da Silva ME, de Barros Calfat EL, Alberto RL, et al. Trigeminal Nerve Stimulation (TNS) for post-traumatic stress disorder: a case study. Brain stimulation 2015. [DOI] [PubMed] [Google Scholar]
- 253.Gomes JS, Akiba H, Dias Ám, Soares A, Taiar I, Gadelha AA, et al. Trigeminal Nerve Stimulation for olfactory hallucinations in schizophrenia: case study. Schizophrenia Research 2016;176(2):203–5. [DOI] [PubMed] [Google Scholar]
- 254.Shiozawa P, da Silva ME, Cordeiro Q. Trigeminal nerve stimulation (TNS) for fibromyalgia: a case study. Epilepsy & Behavior 2014;32:100–1. [DOI] [PubMed] [Google Scholar]
- 255.Fan J, Wu Y, Fossella JA, Posner MI. Assessing the heritability of attentional networks. BMC Neuroscience 2001;2(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Piquet M, Balestra C, Sava SL, Schoenen JE. Supraorbital transcutaneous neurostimulation has sedative effects in healthy subjects. BMC neurology 2011;11(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Boasso AM, Mortimore H, Silva R, Aven L, Tyler WJ. Transdermal electrical neuromodulation of the trigeminal sensory nuclear complex improves sleep quality and mood. bioRxiv 2016:043901. [Google Scholar]
- 258.Norgren R Projections from the nucleus of the solitary tract in the rat. Neuroscience 1978;3(2):207–18. [DOI] [PubMed] [Google Scholar]
- 259.Ricardo JA, Koh ET. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain research 1978;153(1):1–26. [DOI] [PubMed] [Google Scholar]
- 260.Stolbova K, Hahn A, Benes B, Andel M, Treslova L. Gustometry of diabetes mellitus patients and obese patients. The international tinnitus journal 1998;5(2):135–40. [PubMed] [Google Scholar]
- 261.Lelievre G, Le Floch J, Perlemuter L, Peynegre R, editors. [Taste in healthy subjects. Influence of alcohol and tobacco consumption]. Annales d’oto-laryngologie et de chirurgie cervico faciale: bulletin de la Societe d’oto-laryngologie des hopitaux de Paris; 1988. [PubMed] [Google Scholar]
- 262.Bruesch S The distribution of myelinated afferent fibers in the branches of the cat’s facial nerve. Journal of Comparative Neurology 1944;81(2):169–91. [Google Scholar]
- 263.Kaczmarek KA. The tongue display unit (TDU) for electrotactile spatiotemporal pattern presentation. Scientia Iranica 2011;18(6):1476–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Hinchcliffe R Clinical quantitative gustometry. Acta oto-laryngologica 1958;49(1):453–66. [DOI] [PubMed] [Google Scholar]
- 265.Kiesow F Beiträge zur physiologischen Psychologie des Geschmackssinnes: Inaugural-Dissertation: Wilhelm Engelmann; 1894. [Google Scholar]
- 266.Ranasinghe N, Lee K-Y, Suthokumar G, Do EY-L, editors. Taste+: digitally enhancing taste sensations of food and beverages. Proceedings of the 22nd ACM international conference on Multimedia; 2014: ACM. [Google Scholar]
- 267.Krarup B On the technique of gustatory examinations. Acta Oto-Laryngologica 1958;49(sup140):195–200. [DOI] [PubMed] [Google Scholar]
- 268.Krarup B Electro-gustometry: a method for clinical taste examinations. Acta oto-laryngologica 1958;49(1):294–305. [DOI] [PubMed] [Google Scholar]
- 269.Pavlidis P, Gouveris H, Anogeianaki A, Koutsonikolas D, Anogianakis G, Kekes G. Age-related changes in electrogustometry thresholds, tongue tip vascularization, density, and form of the fungiform papillae in humans. Chemical senses 2013;38(1):35–43. [DOI] [PubMed] [Google Scholar]
- 270.Tomita H, Ikeda M. Clinical use of electrogustometry: strengths and limitations. Acta Oto-Laryngologica 2002;122(4):27–38. [DOI] [PubMed] [Google Scholar]
- 271.Ovesen L, Sorensen M, Hannibal J, Allingstrup L. Electrical taste detection thresholds and chemical smell detection thresholds in patients with cancer. Cancer 1991;68(10):2260–5. [DOI] [PubMed] [Google Scholar]
- 272.Fons M, Osterhammel PA. Electrogustometry. Archives of Otolaryngology 1966;83(6):538–42. [DOI] [PubMed] [Google Scholar]
- 273.Lawless HT, Stevens DA, Chapman KW, Kurtz A. Metallic Taste from Electrical and Chemical Stimulation. Chemical Senses 2005;30(3):185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Von Békésy G Sweetness produced electrically on the tongue and its relation to taste theories. Journal of applied physiology 1964;19(6):1105–13. [DOI] [PubMed] [Google Scholar]
- 275.Herness MS. Neurophysiological and biophysical evidence on the mechanism of electric taste. The Journal of general physiology 1985;86(1):59–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Ohla K, Toepel U, le Coutre J, Hudry J. Electrical neuroimaging reveals intensity-dependent activation of human cortical gustatory and somatosensory areas by electric taste. Biological Psychology 2010;85(3):446–55. [DOI] [PubMed] [Google Scholar]
- 277.Bach-y-Rita P, Kercel SW. Sensory substitution and the human–machine interface. Trends in cognitive sciences 2003;7(12):541–6. [DOI] [PubMed] [Google Scholar]
- 278.Sampaio E, Maris S, Bach-y-Rita P. Brain plasticity:’visual’acuity of blind persons via the tongue. Brain research 2001;908(2):204–7. [DOI] [PubMed] [Google Scholar]
- 279.Chebat D-R, Rainville C, Kupers R, Ptito M. Tactile–’visual’acuity of the tongue in early blind individuals. Neuroreport 2007;18(18):1901–4. [DOI] [PubMed] [Google Scholar]
- 280.Sathian K Visual cortical activity during tactile perception in the sighted and the visually deprived. Developmental psychobiology 2005;46(3):279–86. [DOI] [PubMed] [Google Scholar]
- 281.Ptito M, Moesgaard SM, Gjedde A, Kupers R. Cross-modal plasticity revealed by electrotactile stimulation of the tongue in the congenitally blind. Brain 2005;128(3):606–14. [DOI] [PubMed] [Google Scholar]
- 282.Tyler M, Danilov Y, Bach-y-Rita P. Closing an open-loop control system: vestibular substitution through the tongue. Journal of integrative neuroscience 2003;2(02):159–64. [DOI] [PubMed] [Google Scholar]
- 283.Robinson BS, Cook JL, Richburg CM, Price SE. Use of an electrotactile vestibular substitution system to facilitate balance and gait of an individual with gentamicin-induced bilateral vestibular hypofunction and bilateral transtibial amputation. Journal of Neurologic Physical Therapy 2009;33(3):150–9. [DOI] [PubMed] [Google Scholar]
- 284.Vuillerme N, Cuisinier R. Sensory supplementation through tongue electrotactile stimulation to preserve head stabilization in space in the absence of vision. Investigative ophthalmology & visual science 2009;50(1):476–81. [DOI] [PubMed] [Google Scholar]
- 285.Vuillerme N, Pinsault N, Fleury A, Chenu O, Demongeot J, Payan Y, et al. Effectiveness of an electro-tactile vestibular substitution system in improving upright postural control in unilateral vestibular-defective patients. Gait & posture 2008;28(4):711–5. [DOI] [PubMed] [Google Scholar]
- 286.Danilov Y, Tyler M, Skinner K, Hogle R, Bach-y-Rita P. Efficacy of electrotactile vestibular substitution in patients with peripheral and central vestibular loss. Journal of Vestibular Research 2007;17(2, 3):119–30. [PMC free article] [PubMed] [Google Scholar]
- 287.Wildenberg JC, Tyler ME, Danilov YP, Kaczmarek KA, Meyerand ME. Sustained cortical and subcortical neuromodulation induced by electrical tongue stimulation. Brain imaging and behavior 2010;4(3-4):199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Wildenberg JC, Tyler ME, Danilov YP, Kaczmarek KA, Meyerand ME. High-resolution fMRI detects neuromodulation of individual brainstem nuclei by electrical tongue stimulation in balance-impaired individuals. NeuroImage 2011;56(4):2129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Wildenberg JC, Tyler ME, Danilov YP, Kaczmarek KA, Meyerand ME. Electrical Tongue Stimulation Normalizes Activity Within the Motion-Sensitive Brain Network in Balance-Impaired Subjects as Revealed by Group Independent Component Analysis. Brain Connectivity 2011;1(3):255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Lee DN, Lishman J. Visual proprioceptive control of stance. Journal of human movement studies 1975. [Google Scholar]
- 291.Peterka RJ, Benolken MS. Role of somatosensory and vestibular cues in attenuating visually induced human postural sway. Exp Brain Res 1995;105(1):101–10. [DOI] [PubMed] [Google Scholar]
- 292.Tyler ME, Kaczmarek KA, Rust KL, Subbotin AM, Skinner KL, Danilov YP. Non-invasive neuromodulation to improve gait in chronic multiple sclerosis: a randomized double blind controlled pilot trial. Journal of NeuroEngineering and Rehabilitation 2014;11:79-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Tubbs RS, Patwardhan RV, Oakes WJ. Ninth cranial nerve stimulation for epilepsy control. Pediatric neurosurgery 2002;36(5):244–7. [DOI] [PubMed] [Google Scholar]
- 294.Lobel E, Kleine JF, Le Bihan D, Leroy-Willig A, Berthoz A. Functional MRI of galvanic vestibular stimulation. Journal of Neurophysiology 1998;80(5):2699–709. [DOI] [PubMed] [Google Scholar]
- 295.Fertonani A, Ferrari C, Miniussi C. What do you feel if I apply transcranial electric stimulation? Safety, sensations and secondary induced effects. Clinical Neurophysiology 2015;126(11):2181–8. [DOI] [PubMed] [Google Scholar]
- 296.Clark G Cochlear implants. Speech processing in the auditory system: Springer; 2004. p. 422–62. [Google Scholar]
- 297.Izzo AD, Walsh JT, Jansen ED, Bendett M, Webb J, Ralph H, et al. Optical parameter variability in laser nerve stimulation: a study of pulse duration, repetition rate, and wavelength. IEEE Transactions on Biomedical Engineering 2007;54(6):1108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Gantz BJ, Dunn C, Oleson J, Hansen M, Parkinson A, Turner C. Multicenter clinical trial of the Nucleus Hybrid S8 cochlear implant: final outcomes. The Laryngoscope 2016;126(4):962–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Dettman SJ, Dowell RC, Choo D, Arnott W, Abrahams Y, Davis A, et al. Long-term communication outcomes for children receiving cochlear implants younger than 12 months: A multicenter study. Otology & Neurotology 2016;37(2):e82–e95. [DOI] [PubMed] [Google Scholar]
- 300.Stickney GS, Zeng F-G, Litovsky R, Assmann P. Cochlear implant speech recognition with speech maskers. The Journal of the Acoustical Society of America 2004;116(2):1081–91. [DOI] [PubMed] [Google Scholar]
- 301.Rasmussen GL. The olivary peduncle and other fiber projections of the superior olivary complex. Journal of Comparative Neurology 1946;84(2):141–219. [DOI] [PubMed] [Google Scholar]
- 302.Goldberg JM, Fernandez C. Efferent vestibular system in the squirrel monkey: anatomical location and influence on afferent activity. Journal of neurophysiology 1980;43(4):986–1025. [DOI] [PubMed] [Google Scholar]
- 303.Mccue MP, Guinan J. Influence of efferent stimulation on acoustically responsive vestibular afferents in the cat. The Journal of neuroscience 1994;14(10):6071–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Sugai T, Sugitani M, Ooyama H. Effects of activation of the divergent efferent fibers on the spontaneous activity of vestibular afferent fibers in the toad. The Japanese journal of physiology 1991;41(2):217–32. [DOI] [PubMed] [Google Scholar]
- 305.Rossi ML, Prigioni I, Valli P, Casella C. Activation of the efferent system in the isolated frog labyrinth: effects on the afferent EPSPs and spike discharge recorded from single fibers of the posterior nerve. Brain research 1980;185(1):125–37. [DOI] [PubMed] [Google Scholar]
- 306.Goldberg JM. Afferent diversity and the organization of central vestibular pathways. Experimental Brain Research 2000;130(3):277–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Guldin W, Grüsser O. Is there a vestibular cortex? Trends in neurosciences 1998;21(6):254–9. [DOI] [PubMed] [Google Scholar]
- 308.Best C, Stefan H, Hopfengaertner R, Dieterich M. Effects of electrical stimulation in vestibular cortex areas in humans. Journal of the Neurological Sciences 2010;290(1–2):157–62. [DOI] [PubMed] [Google Scholar]
- 309.Fasold O, von Brevern M, Kuhberg M, Ploner CJ, Villringer A, Lempert T, et al. Human Vestibular Cortex as Identified with Caloric Stimulation in Functional Magnetic Resonance Imaging. NeuroImage 2002;17(3):1384–93. [DOI] [PubMed] [Google Scholar]
- 310.Purkyně JE. Beyträge zur näheren Kenntniss des Schwindels aus heautognostischen Daten1820. [Google Scholar]
- 311.Hitzig E Über die beim Galvanisiren des Kopfes entstehenden Störungen der Muskelinnervation und der Vorstellungen vom Verhalten im Raume1871.
- 312.Breuer J Neue Versuche an den Ohrbogengangen. Arch Gesammte Physiol 1889;44:135–52. [Google Scholar]
- 313.Grabherr L, Macauda G, Lenggenhager B. The Moving History of Vestibular Stimulation as a Therapeutic Intervention. Multisensory research 2015;28(5-6):653–87. [DOI] [PubMed] [Google Scholar]
- 314.Ferrè ER, Haggard P, Bottini G, Iannetti GD. Caloric vestibular stimulation modulates nociceptive evoked potentials. Experimental brain research 2015;233(12):3393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Donevska G, Brunsdon VE, Surtees A, Ferguson HJ. Caloric vestibular stimulation facilitates spatial, but not visual, perspective-taking. 2016. [Google Scholar]
- 316.Wilkinson D, Ade KK, Rogers LL, Attix DK, Kuchibhatla M, Slade MD, et al. Preventing episodic migraine with caloric vestibular stimulation: a randomized controlled trial. Headache: The Journal of Head and Face Pain 2017;57(7):1065–87. [DOI] [PubMed] [Google Scholar]
- 317.Barany R Physiologie und Pathologie Funktions-Pmfung des Bogengang-Apparates beim Menschen: F. Deuticke; 1907. [Google Scholar]
- 318.James C, Macefield VG. Competitive interactions between vestibular and cardiac rhythms in the modulation of muscle sympathetic nerve activity. Autonomic Neuroscience 2010;158(1):127–31. [DOI] [PubMed] [Google Scholar]
- 319.Saj A, Honore J, Rousseaux M. Perception of the vertical in patients with right hemispheric lesion: Effect of galvanic vestibular stimulation. Neuropsychologia 2006;44(8):1509–12. [DOI] [PubMed] [Google Scholar]
- 320.Davidovics NS, Fridman GY, Chiang B, Della Santina CC. Effects of Biphasic Current Pulse Frequency, Amplitude, Duration and Interphase Gap on Eye Movement Responses to Prosthetic Electrical Stimulation of the Vestibular Nerve. IEEE transactions on neural systems and rehabilitation engineering : a publication of the IEEE Engineering in Medicine and Biology Society 2011;19(1):84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Dilda V, MacDougall HG, Curthoys IS, Moore ST. Effects of Galvanic vestibular stimulation on cognitive function. Experimental brain research 2012;216(2):275–85. [DOI] [PubMed] [Google Scholar]
- 322.Golub JS, Ling L, Nie K, Nowack A, Shepherd SJ, Bierer SM, et al. Prosthetic implantation of the human vestibular system. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology 2014;35(1):136–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Goldberg J, Smith CE, Fernandez C. Relation between discharge regularity and responses to externally applied galvanic currents in vestibular nerve afferents of the squirrel monkey. Journal of neurophysiology 1984;51(6):1236–56. [DOI] [PubMed] [Google Scholar]
- 324.Baird R, Desmadryl G, Fernandez C, Goldberg J. The vestibular nerve of the chinchilla. II. Relation between afferent response properties and peripheral innervation patterns in the semicircular canals. Journal of neurophysiology 1988;60(1):182–203. [DOI] [PubMed] [Google Scholar]
- 325.Cohen B, Yakushin SB, Holstein GR. What does galvanic vestibular stimulation actually activate? Frontiers in neurology 2012;2:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Pfaltz C The diagnostic importance of the galvanic test in otoneurology. ORL 1969;31(4):193–203. [DOI] [PubMed] [Google Scholar]
- 327.Coats AC, Stoltz M. The recorded body-sway response to galvanic stimulation of the labyrinth: A preliminary study. The Laryngoscope 1969;79(1):85–103. [DOI] [PubMed] [Google Scholar]
- 328.Coats A The sinusoidal galvanic body-sway response. Acta oto-laryngologica 1972;74(1-6):155–62. [DOI] [PubMed] [Google Scholar]
- 329.Nashner LM, Wolfson P. Influence of head position and proprioceptive cues on short latency postural reflexes evoked by galvanic stimulation of the human labyrinth. Brain research 1974;67(2):255–68. [DOI] [PubMed] [Google Scholar]
- 330.Collins JJ, Priplata AA, Gravelle DC, Niemi J, Harry J, Lipsitz LA. Noise-enhanced human sensorimotor function. IEEE Engineering in Medicine and Biology Magazine 2003;22(2):76–83. [DOI] [PubMed] [Google Scholar]
- 331.Fitzpatrick R, Burke D, Gandevia SC. Loop gain of reflexes controlling human standing measured with the use of postural and vestibular disturbances. Journal of neurophysiology 1996;76(6):3994–4008. [DOI] [PubMed] [Google Scholar]
- 332.Pavlik AE, Inglis JT, Lauk M, Oddsson L, Collins JJ. The effects of stochastic galvanic vestibular stimulation on human postural sway. Experimental Brain Research 1999;124(3):273–80. [DOI] [PubMed] [Google Scholar]
- 333.Scinicariello AP, Eaton K, Inglis JT, Collins J. Enhancing human balance control with galvanic vestibular stimulation. Biological cybernetics 2001;84(6):475–80. [DOI] [PubMed] [Google Scholar]
- 334.Agid Y Parkinson’s disease: pathophysiology. The Lancet 1991;337(8753):1321–4. [DOI] [PubMed] [Google Scholar]
- 335.Tran S, Shafiee M, Jones CB, Garg S, Lee S, Pasman EP, et al. Subthreshold stochastic vestibular stimulation induces complex multi-planar effects during standing in Parkinson’s disease. Brain Stimulation: Basic, Translational, and Clinical Research in Neuromodulation. [DOI] [PubMed] [Google Scholar]
- 336.Samoudi G, Jivegård M, Mulavara AP, Bergquist F. Effects of stochastic vestibular galvanic stimulation and LDOPA on balance and motor symptoms in patients with Parkinson’s disease. Brain stimulation 2015;8(3):474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Wiesenfeld K, Moss F. Stochastic resonance and the benefits of noise: from ice ages to crayfish and SQUIDs. Nature 1995;373(6509):33–6. [DOI] [PubMed] [Google Scholar]
- 338.Yamamoto Y, Struzik ZR, Soma R, Ohashi K, Kwak S. Noisy vestibular stimulation improves autonomic and motor responsiveness in central neurodegenerative disorders. Annals of neurology 2005;58(2):175–81. [DOI] [PubMed] [Google Scholar]
- 339.Pan W, Soma R, Kwak S, Yamamoto Y. Improvement of motor functions by noisy vestibular stimulation in central neurodegenerative disorders. Journal of Neurology 2008;255(11):1657–61. [DOI] [PubMed] [Google Scholar]
- 340.Pal S, Rosengren SM, Colebatch JG. Stochastic galvanic vestibular stimulation produces a small reduction in sway in Parkinson’s disease. Journal of Vestibular Research 2009;19(3, 4):137–42. [DOI] [PubMed] [Google Scholar]
- 341.Samoudi G, Nissbrandt H, Dutia MB, Bergquist F. Noisy Galvanic Vestibular Stimulation Promotes GABA Release in the Substantia Nigra and Improves Locomotion in Hemiparkinsonian Rats. PLoS ONE 2012;7(1):e29308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Wall III C, Merfeld D, Rauch S, Black F. Vestibular prostheses: the engineering and biomedical issues. Journal of Vestibular Research 2002;12(2, 3):95–113. [PubMed] [Google Scholar]
- 343.Rubinstein JTaT RS Electrical suppression of tinnitus In: Snow JB, editor. Tinnitus: Theory and Management. Hamilton, ON, Canada & Lewiston, New York, NY, USA: B. C. Decker Inc.; 2004. p. 326–35. [Google Scholar]
- 344.Merfeld DM, Haburcakova C, Gong W, Lewis RF. Chronic Vestibulo-Ocular Reflexes Evoked by a Vestibular Prosthesis. IEEE Transactions on Biomedical Engineering 2007;54(6):1005–15. [DOI] [PubMed] [Google Scholar]
- 345.Phillips JO, Bierer SM, Ling L, Nie K, Rubinstein JT, editors. Real-time communication of head velocity and acceleration for an externally mounted vestibular prosthesis. 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society; 2011 Aug. 30 2011-Sept. 3 2011. [DOI] [PubMed] [Google Scholar]
- 346.Della Santina CC, Migliaccio AA, Patel AH, editors. Electrical stimulation to restore vestibular function development of a 3-d vestibular prosthesis. 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference; 2006: IEEE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Gong W, Merfeld DM. Prototype neural semicircular canal prosthesis using patterned electrical stimulation. Annals of biomedical engineering 2000;28(5):572–81. [DOI] [PubMed] [Google Scholar]
- 348.Smith PF, Zheng Y, Horii A, Darlington CL. Does vestibular damage cause cognitive dysfunction in humans? Journal of Vestibular Research 2005;15(1):1–9. [PubMed] [Google Scholar]
- 349.Mast FW, Preuss N, Hartmann M, Grabherr L. Spatial cognition, body representation and affective processes: the role of vestibular information beyond ocular reflexes and control of posture. Frontiers in integrative neuroscience 2014;8:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Horii A, Russell NA, Smith PF, Darlington CL, Bilkey DK. Vestibular influences on CA1 neurons in the rat hippocampus: an electrophysiological study in vivo. Experimental brain research 2004;155(2):245–50. [DOI] [PubMed] [Google Scholar]
- 351.Cuthbert PC, Gilchrist DP, Hicks SL, MacDougall HG, Curthoys IS. Electrophysiological evidence for vestibular activation of the guinea pig hippocampus. Neuroreport 2000;11(7):1443–7. [DOI] [PubMed] [Google Scholar]
- 352.Moser EI, Kropff E, Moser M-B. Place cells, grid cells, and the brain’s spatial representation system. Annu Rev Neurosci 2008;31:69–89. [DOI] [PubMed] [Google Scholar]
- 353.Brandt T, Schautzer F, Hamilton DA, Brüning R, Markowitsch HJ, Kalla R, et al. Vestibular loss causes hippocampal atrophy and impaired spatial memory in humans. Brain 2005;128(11):2732–41. [DOI] [PubMed] [Google Scholar]
- 354.Zacks JM, Michelon P. Transformations of visuospatial images. Behavioral and Cognitive Neuroscience Reviews 2005;4(2):96–118. [DOI] [PubMed] [Google Scholar]
- 355.Stephan T, Deutschländer A, Nolte A, Schneider E, Wiesmann M, Brandt T, et al. Functional MRI of galvanic vestibular stimulation with alternating currents at different frequencies. Neuroimage 2005;26(3):721–32. [DOI] [PubMed] [Google Scholar]
- 356.Fink GR, Marshall JC, Weiss PH, Stephan T, Grefkes C, Shah NJ, et al. Performing allocentric visuospatial judgments with induced distortion of the egocentric reference frame: an fMRI study with clinical implications. Neuroimage 2003;20(3):1505–17. [DOI] [PubMed] [Google Scholar]
- 357.Lenggenhager B, Lopez C, Blanke O. Influence of galvanic vestibular stimulation on egocentric and object-based mental transformations. Experimental Brain Research 2008;184(2):211–21. [DOI] [PubMed] [Google Scholar]
- 358.Brandt T, Bartenstein P, Janek A, Dieterich M. Reciprocal inhibitory visual-vestibular interaction. Visual motion stimulation deactivates the parieto-insular vestibular cortex. Brain 1998;121(9):1749–58. [DOI] [PubMed] [Google Scholar]
- 359.Wilkinson D, Nicholls S, Pattenden C, Kilduff P, Milberg W. Galvanic vestibular stimulation speeds visual memory recall. Experimental brain research 2008;189(2):243–8. [DOI] [PubMed] [Google Scholar]
- 360.de Waele C, Baudonniere PM, Lepecq JC, Tran Ba Huy P, Vidal PP. Vestibular projections in the human cortex. Exp Brain Res 2001;141(4):541–51. [DOI] [PubMed] [Google Scholar]
- 361.Purves D, Augustine G, Fitzpatrick D, Katz L, LaMantia A, McNamara J, et al. Central vestibular pathways: eye, head, and body reflexes. Neuroscience 2001. [Google Scholar]
- 362.Agostoni E, Chinnock J, Daly MDB, Murray J. Functional and histological studies of the vagus nerve and its branches to the heart, lungs and abdominal viscera in the cat. The Journal of physiology 1957;135(1):182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Kukreja S, Knudson M, Tweden K, Aspinwall K, Shikora SA. Vagal Nerve Control of Appetite, Energy, Regulation, and Body Weight Global Bariatric Surgery: Springer; 2018. p. 145–58. [Google Scholar]
- 364.Katona P, Poitras JW, Barnett GO, Terry BS. Cardiac vagal efferent activity and heart period in the carotid sinus reflex. American Journal of Physiology-Legacy Content 1970;218(4):1030–7. [DOI] [PubMed] [Google Scholar]
- 365.Brock C, Brock B, Aziz Q, Møller HJ, Pfeiffer Jensen M, Drewes AM, et al. Transcutaneous cervical vagal nerve stimulation modulates cardiac vagal tone and tumor necrosis factor-alpha. Neurogastroenterol Motil 2017;29(5):1–4. [DOI] [PubMed] [Google Scholar]
- 366.Clancy JA, Mary DA, Witte KK, Greenwood JP, Deuchars SA, Deuchars J. Non-invasive vagus nerve stimulation in healthy humans reduces sympathetic nerve activity. Brain Stimul 2014;7:871–7. [DOI] [PubMed] [Google Scholar]
- 367.Vida G, Pena G, Kanashiro A, Thompson-Bonilla MdR, Palange D, Deitch EA, et al. B2-Adrenoreceptors of regulatory lymphocytes are essential for vagal neuromodulation of the innate immune system. FASEB J 2011;25:4476–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Huston JM, Gallowitsch-Puerta M, Ochani M, Ochani K, Yuan R, Rosas-Ballina M, et al. Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis. Crit Care Med 2007;35(12):2762–8. [DOI] [PubMed] [Google Scholar]
- 369.Lerman I, Hauger R, Sorkin L, Proudfoot J, Davis B, Huang A, et al. Noninvasive transcutaneous vagus nerve stimulation decreases whole blood culture-derived cytokines and chemokines: A randomized, blinded, healthy control pilot trial. Neuromodulation 2016;19(3):283–90. [DOI] [PubMed] [Google Scholar]
- 370.Leek BF. Abdominal and pelvic visceral receptors. British medical bulletin 1977;33(2):163. [DOI] [PubMed] [Google Scholar]
- 371.Nomura S, Mizuno N. Central distribution of primary afferent fibers in the Arnold’s nerve (the auricular branch of the vagus nerve): a transganglionic HRP study in the cat. Brain research 1984;292(2):199–205. [DOI] [PubMed] [Google Scholar]
- 372.MacLean DB, Wheeler F, Hayes L. Basal and stimulated release of substance P from dissociated cultures of vagal sensory neurons. Brain research 1990;519(1):308–14. [DOI] [PubMed] [Google Scholar]
- 373.Bailey P, Bremer F. A sensory cortical representation of the vagus nerve: with a note on the effects of low blood pressure on the cortical electrogram. Journal of Neurophysiology 1938;1(5):405–12. [Google Scholar]
- 374.Zanchetti A, Wang SC, Moruzzi G. The effect of vagal afferent stimulation on the EEG pattern of the cat. Electroencephalography and Clinical Neurophysiology 1952;4(3):357–61. [DOI] [PubMed] [Google Scholar]
- 375.Chase MH, Nakamura Y, Clemente CD, Sterman MB. Afferent vagal stimulation: Neurographic correlates of induced eeg synchronization and desynchronization. Brain Research 1967;5(2):236–49. [DOI] [PubMed] [Google Scholar]
- 376.Chase MH, Nakamura Y. Cortical and subcortical EEG patterns of response to afferent abdominal vagal stimulation: neurographic correlates. Physiology & Behavior 1968;3(5):605–10. [Google Scholar]
- 377.Rojas JP. Electroencephalographic synchronization resulting from direct current application to the vagus nerves. Experimental Neurology 1964;9(5):367–71. [DOI] [PubMed] [Google Scholar]
- 378.McLachlan RS. Suppression of interictal spikes and seizures by stimulation of the vagus nerve. Epilepsia 1993;34(5):918–23. [DOI] [PubMed] [Google Scholar]
- 379.Stoica I, Tudor I. Effects of vagus afferents on strychninic focus of coronal gyrus. Rev Roum Neurol 1967;4:287–95. [Google Scholar]
- 380.Woodbury DM, Woodbury JW. Effects of vagal stimulation on experimentally induced seizures in rats. Epilepsia 1990;31(s2):S7–S19. [DOI] [PubMed] [Google Scholar]
- 381.Zabara J Inhibition of experimental seizures in canines by repetitive vagal stimulation. Epilepsia 1992;33(6):1005–12. [DOI] [PubMed] [Google Scholar]
- 382.Zagon A, Kemeny AA. Slow hyperpolarization in cortical neurons: a possible mechanism behind vagus nerve simulation therapy for refractory epilepsy? Epilepsia 2000;41(11):1382–9. [DOI] [PubMed] [Google Scholar]
- 383.Reid SA. Surgical technique for implantation of the neurocybernetic prosthesis. Epilepsia 1990;31(s2). [DOI] [PubMed] [Google Scholar]
- 384.French JA. Refractory epilepsy: clinical overview. Epilepsia 2007;48(s1):3–7. [DOI] [PubMed] [Google Scholar]
- 385.Schachter SC, Saper CB. Vagus nerve stimulation. Epilepsia 1998;39(7):677–86. [DOI] [PubMed] [Google Scholar]
- 386.Uthman BM, Wilder B, Hammond EJ, Reid SA. Efficacy and safety of vagus nerve stimulation in patients with complex partial seizures. Epilepsia 1990;31(s2):S44–S50. [DOI] [PubMed] [Google Scholar]
- 387.Uthman B, Wilder B, Penry J, Dean C, Ramsay R, Reid S, et al. Treatment of epilepsy by stimulation of the vagus nerve. Neurology 1993;43(7):1338-. [DOI] [PubMed] [Google Scholar]
- 388.Ben-Menachem E, Mañon-Espaillat R, Ristanovic R, Wilder B, Stefan H, Mirza W, et al. Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. Epilepsia 1994;35(3):616–26. [DOI] [PubMed] [Google Scholar]
- 389.Handforth A, DeGiorgio C, Schachter S, Uthman B, Naritoku D, Tecoma E, et al. Vagus nerve stimulation therapy for partial-onset seizures A randomized active-control trial. Neurology 1998;51(1):48–55. [DOI] [PubMed] [Google Scholar]
- 390.Schachter SC. Vagus nerve stimulation therapy summary five years after FDA approval. Neurology 2002;59(6 suppl 4):S15–S29. [DOI] [PubMed] [Google Scholar]
- 391.Devinsky O, Kelley K, Porter RJ, Theodore WH. Clinical and electroencephalographic features of simple partial seizures. Neurology 1988;38(9):1347-. [DOI] [PubMed] [Google Scholar]
- 392.Salinsky MC. Vagus Nerve Stimulation Has No Effect on Awake EEG Rhythms in Humans. Epilepsia (Copenhagen) 1993;34(2):299–304. [DOI] [PubMed] [Google Scholar]
- 393.Henry TR. Therapeutic mechanisms of vagus nerve stimulation. Neurology 2002;59(6 suppl 4):S3. [DOI] [PubMed] [Google Scholar]
- 394.Buzsaki G Noradrenergic Control of Thalamic Oscillation: the Role of alpha-2 Receptors. The European journal of neuroscience 1991;3(3):222–9. [DOI] [PubMed] [Google Scholar]
- 395.Grimonprez A, Raedt R, Portelli J, Dauwe I, Larsen LE, Bouckaert C, et al. The antidepressant-like effect of vagus nerve stimulation is mediated through the locus coeruleus. J Psychiatr Res 2015;68:1–7. [DOI] [PubMed] [Google Scholar]
- 396.Dorr AE, Debonnel G. Effect of vagus nerve stimulation on serotonergic and noradrenergic transmission. J Pharmacol Exp Ther 2006;318(2):89–898. [DOI] [PubMed] [Google Scholar]
- 397.Follesa P, Biggio F, Gorini G, Caria S, Talani G, Dazzi L, et al. Vagus nerve stimulation increases norepinephrine concentration and the gene expression of BDNF and bFGF in the rat brain. Brain Res. 2007;1179:28–34. Brain Res 2007;1179:28-34. [DOI] [PubMed] [Google Scholar]
- 398.Hulsey DR, Riley JR, Loerwald KW, Rennaker RL, Kilgard MP, Hays SA. Parametric characterization of neural activity in the locus coeruleus in response to vagus nerve stimulation. Neurology 2017;289:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Manta S, Dong J, Debonnel G, Blier P. Enhancement of the function of rat serotonin and norepinephrine neurons by sustained vagus nerve stimulation. J Psychiatr Neurosci 2009;34:272–80. [PMC free article] [PubMed] [Google Scholar]
- 400.Manta S, Dong J, Debonnel G, Blier P. Optimization of vagus nerve stimulation parameters using the firing activity of serotonin neurons in the rat dorsal raphe. Eur Neuropsychopharmacol 2009;19:250–5. [DOI] [PubMed] [Google Scholar]
- 401.Krahl SE, Clark KB, Smith DC, Browning RA. Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. . Epilepsia 1998;39:709–14. [DOI] [PubMed] [Google Scholar]
- 402.Roosevelt RW, Smith DC, Clough RW, Jensen RA, Browning RA. Increased extracellular concentrations of norepinephrine in cortex and hippocampus following vagus nerve stimulation in the rat. Brain 2006;1119(1):124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Hulsey DR, Riley JR, Loerwald KW, Rennaker RL 2nd, Kilgard MP, Hays SA. Parametric characterization of neural activity in the locus coeruleus in response to vagus nerve stimulation. Exp Neurol 2017;289:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Harden CL, Pulver MC, Ravdin LD, Nikolov B, Halper JP, Labar DR. A Pilot Study of Mood in Epilepsy Patients Treated with Vagus Nerve Stimulation. Epilepsy & Behavior 2000;1(2):93–9. [DOI] [PubMed] [Google Scholar]
- 405.Elger G, Hoppe C, Falkai P, Rush AJ, Elger CE. Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy research 2000;42(2):203–10. [DOI] [PubMed] [Google Scholar]
- 406.Klinkenberg S, Majoie H, Van der Heijden M, Rijkers K, Leenen L, Aldenkamp A. Vagus nerve stimulation has a positive effect on mood in patients with refractory epilepsy. Clinical neurology and neurosurgery 2012;114(4):336–40. [DOI] [PubMed] [Google Scholar]
- 407.Dodrill CB, Morris GL. Effects of vagal nerve stimulation on cognition and quality of life in epilepsy. Epilepsy & Behavior 2001;2(1):46–53. [DOI] [PubMed] [Google Scholar]
- 408.George MS, Rush AJ, Marangell LB, Sackeim HA, Brannan SK, Davis SM, et al. A one-year comparison of vagus nerve stimulation with treatment as usual for treatment-resistant depression. Biological psychiatry 2005;58(5):364–73. [DOI] [PubMed] [Google Scholar]
- 409.Marangell LB, Rush AJ, George MS, Sackeim HA, Johnson CR, Husain MM, et al. Vagus nerve stimulation (VNS) for major depressive episodes: one year outcomes. Biol Psychiatry 2002;51(4):280–7. [DOI] [PubMed] [Google Scholar]
- 410.Rush AJ, George MS, Sackeim HA, Marangell LB, Husain MM, Giller C, et al. Vagus nerve stimulation (VNS) for treatment-resistant depressions: a multicenter study. Biological psychiatry 2000;47(4):276–86. [DOI] [PubMed] [Google Scholar]
- 411.Rush AJ, Marangell LB, Sackeim HA, George MS, Brannan SK, Davis SM, et al. Vagus nerve stimulation for treatment-resistant depression: a randomized, controlled acute phase trial. Biological psychiatry 2005;58(5):347–54. [DOI] [PubMed] [Google Scholar]
- 412.Sackeim HA, Rush AJ, George MS, Marangell LB, Husain MM, Nahas Z, et al. Vagus nerve stimulation (VNS™) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology 2001;25(5):713–28. [DOI] [PubMed] [Google Scholar]
- 413.Little A Treatment-resistant depression. Am Fam Physician 2009;80(2):167–72. [PubMed] [Google Scholar]
- 414.Chae J-H, Nahas Z, Lomarev M, Denslow S, Lorberbaum JP, Bohning DE, et al. A review of functional neuroimaging studies of vagus nerve stimulation (VNS). Journal of psychiatric research 2003;37(6):443–55. [DOI] [PubMed] [Google Scholar]
- 415.Conway CR, Sheline YI, Chibnall JT, George MS, Fletcher JW, Mintun MA. Cerebral blood flow changes during vagus nerve stimulation for depression. Psychiatry Research: Neuroimaging 2006;146(2):179–84. [DOI] [PubMed] [Google Scholar]
- 416.Kosel M, Brockmann H, Frick C, Zobel A, Schlaepfer TE. Chronic vagus nerve stimulation for treatment-resistant depression increases regional cerebral blood flow in the dorsolateral prefrontal cortex. Psychiatry Research: Neuroimaging 2011;191(3):153–9. [DOI] [PubMed] [Google Scholar]
- 417.Zobel A, Joe A, Freymann N, Clusmann H, Schramm J, Reinhardt M, et al. Changes in regional cerebral blood flow by therapeutic vagus nerve stimulation in depression: An exploratory approach. Psychiatry Research: Neuroimaging 2005;139(3):165–79. [DOI] [PubMed] [Google Scholar]
- 418.Henry TR, Bakay RA, Votaw JR, Pennell PB, Epstein CM, Faber TL, et al. Brain blood flow alterations induced by therapeutic vagus nerve stimulation in partial epilepsy: I. Acute effects at high and low levels of stimulation. Epilepsia 1998;39(9):983–90. [DOI] [PubMed] [Google Scholar]
- 419.Grimm S, Beck J, Schuepbach D, Hell D, Boesiger P, Bermpohl F, et al. Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: an fMRI study in severe major depressive disorder. Biological psychiatry 2008;63(4):369–76. [DOI] [PubMed] [Google Scholar]
- 420.Pascual-Leone A, Rubio B, Pallardó F, Catalá MD. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. The Lancet 1996;348(9022):233–7. [DOI] [PubMed] [Google Scholar]
- 421.Beekwilder J, Beems T. Overview of the clinical applications of vagus nerve stimulation. Journal of Clinical Neurophysiology 2010;27(2):130–8. [DOI] [PubMed] [Google Scholar]
- 422.Clark K, Smith D, Hassert D, Browning R, Naritoku D, Jensen R. Posttraining electrical stimulation of vagal afferents with concomitant vagal efferent inactivation enhances memory storage processes in the rat. Neurobiology of learning and memory 1998;70(3):364–73. [DOI] [PubMed] [Google Scholar]
- 423.Helmstaedter C, Hoppe C, Elger CE. Memory alterations during acute high-intensity vagus nerve stimulation. Epilepsy research 2001;47(1):37–42. [DOI] [PubMed] [Google Scholar]
- 424.Ghacibeh GA, Shenker JI, Shenal B, Uthman BM, Heilman KM. The influence of vagus nerve stimulation on memory. Cognitive and behavioral neurology 2006;19(3):119–22. [DOI] [PubMed] [Google Scholar]
- 425.Martin CO, Denburg NL, Tranel D, Granner MA, Bechara A. The effects of vagus nerve stimulation on decision-making. Cortex 2004;40(4):605–12. [DOI] [PubMed] [Google Scholar]
- 426.Ghacibeh GA, Shenker JI, Shenal B, Uthman BM, Heilman KM. Effect of vagus nerve stimulation on creativity and cognitive flexibility. Epilepsy & Behavior 2006;8(4):720–5. [DOI] [PubMed] [Google Scholar]
- 427.Sjögren M, Hellström P, Jonsson M, Runnerstam M, Silander HC-s, Ben-Menachem E. Cognition-enhancing effect of vagus nerve stimulation in patients with Alzheimer’s disease: a pilot study. The Journal of clinical psychiatry 2002;63(11):972–80. [DOI] [PubMed] [Google Scholar]
- 428.Merrill CA, Jonsson MA, Minthon L, Ejnell H, Hans C, Blennow K, et al. Vagus nerve stimulation in patients with Alzheimer’s disease: additional follow-up results of a pilot study through 1 year. The Journal of clinical psychiatry 2006;67(8):1171–8. [DOI] [PubMed] [Google Scholar]
- 429.Sackeim H, Keilp J, Rush A, George M, Marangell L, Dormer J, et al. The effects of vagus nerve stimulation on cognitive performance in patients with treatment-resistant depression. Neuropsychiatry, neuropsychology, and behavioral neurology 2001;14(1):53. [PubMed] [Google Scholar]
- 430.McGlone J, Valdivia I, Penner M, Williams J, Sadler RM, Clarke DB. Quality of life and memory after vagus nerve stimulator implantation for epilepsy. The Canadian Journal of Neurological Sciences 2008;35(03):287–96. [DOI] [PubMed] [Google Scholar]
- 431.Noble LJ, Meruva VB, Hays SA, Rennaker RL, Kilgard MP, McIntyre CK. Vagus nerve stimulation promotes generalization of conditioned fear extinction and reduces anxiety in rats. Brain Stimul 2018;12(1):9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Marin MF, Camprodon JA, Dougherty DD, Milad MR. Device-based brain stimulation to augment fear extinction: Implications for PTSD treatment and beyond. Depress Anxiety 2014;31(4):269–78. [DOI] [PubMed] [Google Scholar]
- 433.Nestler EJ, Lüscher C. The molecular basis of drug addiction: Linking epigenetic to synaptic and circuit mechanisms. Neuron 2019;102(1):48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Engineer CT, Engineer ND, Riley JR, Seale JD, Kilgard MP. Pairing speech sounds with vagus nerve stimulation drives stimulus-specific cortical plasticity. Brain Stimul 2015;8(3):637–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, et al. Reversing pathological neural activity using targeted plasticity. Nature 2011;470(7332):101–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Kim HJ, Shim H-J, Kwak MY, An Y-H, Kim DH, Kim YJ, et al. Feasibility and safety of transcutaneous vagus nerve stimulation paired with notched music therapy for the treatment of chronic tinnitus. J Audiol Otol 2015;18(3):159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Li T-T, Wang Z-J, Yang S-B, Zhu J-H, Zhang S-Z, Cai S-J, et al. Transcutaneous electrical stimulation at auricular acupoints innervated by auricular branch of vagus nerve pairing tone for tinnitus: Study protocol for a randomized controlled clinical trial. Trials 2015;16(101):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Liu A, Zhao F-B, Wang J, Lu YF, Tian J, Zhao Y, et al. Effects of vagus nerve stimulation on cognitive functioning in rats with cerebral ischemia reperfusion. J Transl Med 2016;14(101):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Clark KB, Naritoku DK, Smith DC, Browning RA, Jensen RA. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat Neurosci 1999;2(1):94–8. [DOI] [PubMed] [Google Scholar]
- 440.Suthana N, Fried I. Deep brain stimulation for enhancement of learning and memory. Neuroimage 2014;85:996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Cahill L, McGaugh JL. Mechanisms of emotional arousal and lasting declarative memory. Trends Neurosci 1998;21:294–9. [DOI] [PubMed] [Google Scholar]
- 442.McGaugh JL. Memory - A century of consolidation. Science 2000;287:248–51. [DOI] [PubMed] [Google Scholar]
- 443.McGaugh JL. Peripheral and central adrenergic influences on brain systems involved in the modulation of memory storage. Ann N Y Acad Sci 1985;444:150–61. [DOI] [PubMed] [Google Scholar]
- 444.Bremner JD, Vythilingam M, Vermetten E, Newcomer JW, Charney DS. Effects of dexamethasone on declarative memory function in posttraumatic stress disorder (PTSD). Psychiatry Res 2004;129(1):1–10. [DOI] [PubMed] [Google Scholar]
- 445.Bremner JD, Vythilingam M, Vermetten E, Newcomer JW, Charney DS. Effects of glucocorticoids on declarative memory function in major depression. Biol Psychiatry 2004;55(8):811–5. [DOI] [PubMed] [Google Scholar]
- 446.Cahill L, Prins B, Weber M, McGaugh JL. Beta-adrenergic activation and memory for emotional events. Nature 1994;371:702–4. [DOI] [PubMed] [Google Scholar]
- 447.Southwick SM, Horner B, Morgan CA, Bremner JD, Davis M, Cahill L, et al. Relationship of enhanced norepinephrine activity during memory consolidation to enhanced long-term memory in humans. Am J Psychiatry 2002;159:1420–2. [DOI] [PubMed] [Google Scholar]
- 448.Flood JF, Smith GE, Morley JE. Modulation of memory processing by cholecystokinin: Dependence on the vagus nerve. Science 1987;236(4803):832–4. [DOI] [PubMed] [Google Scholar]
- 449.Williams CL, Jensen RA. Effects of vagotomy on leuenkephalin-induced impairments in memory storage. Physiol Behav 1993;54(4):659–63. [DOI] [PubMed] [Google Scholar]
- 450.Hassert DL, Miyashita T, Williams CL. The effects of peripheral vagal nerve stimulation at a memory-modulating intensity on norepinephrine output in the basolateral amygdala. Behav Neurosci 2004;118(1):79–88. [DOI] [PubMed] [Google Scholar]
- 451.Clark KB, Krahl SE, Smith DC, Jensen RA. Post-training unilateral vagal stimulation enhances retention performance in the rat. Neurobiol Learn Mem 1995;63(3):213–6. [DOI] [PubMed] [Google Scholar]
- 452.Clark KB, Smith DC, Hassert DL, Browning RA, Naritoku DK, Jensen RA. Posttraining electrical stimulation of vagal afferents with concomitant vagal efferent inactivation enhances memory storage processes in the rat. Neurobiol Learn Mem 1998;70(3):364–73. [DOI] [PubMed] [Google Scholar]
- 453.Shen H, Fuchino Y, Miyamoto D, Nomura H, Matsuki N. Vagus nerve stimulation enhances perforant path-CA3 synaptic transmission via the activation of beta-adrenergic receptors and the locus coeruleus. Int J Neuropsychopharmacol 2012;4:523–30. [DOI] [PubMed] [Google Scholar]
- 454.Ura H, Sugaya Y, Ohata H, Takumi I, Sadamoto K, Shibasaki T, et al. Vagus nerve stimulation induced long-lasting enhancement of synaptic transmission and decreased granule cell discharge in the hippocampal dentate gyrus of urethane-anesthetized rats. Brain Res 2013;1492:63–71. [DOI] [PubMed] [Google Scholar]
- 455.Zuo Y, Smith DC, Jensen RA. Vagus nerve stimulation potentiates hippocampal LTP in freely-moving rats. Physiol Behav 2007;90(4):583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Revesz D, Tjernstrom M, Ben-Menachem E, Thorlin T. Effects of vagus nerve stimulation on rat hippocampal progenitor proliferation. Exp Neurol 2008;214:259–65. [DOI] [PubMed] [Google Scholar]
- 457.Duman RS. Depression: a case of neuronal life and death? Biol Psychiatry 2004;56:140–5. [DOI] [PubMed] [Google Scholar]
- 458.Pena DF, Childs JE, Willett S, Vital A, McIntyre CK, Kroener S. Vagus nerve stimulation enhances extinction of conditioned fear and modulates plasticity in the pathway from the ventromedial prefrontal cortex to the amygdala. Front Behav Neurosci 2014;8(327):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Peña DF, Engineer ND, McIntyre CK. Rapid remission of conditioned fear expression with extinction training paired with vagus nerve stimulation. Biol Psychiatry 2013;73(11):1071–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Noble IJ, Gonzalez IJ, Meruva VB, Callahan KA, Belfort BD, Ramanathan KR, et al. Effects of vagus nerve stimulation on extinction of conditioned fear and post-traumatic stress disorder symptoms in rats. Transl Psychiatry 2017;7(e1217):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Souza RR, Robertson NM, Pruitt DT, Gonzales PA, Hays SA, Rennaker RL, et al. Vagus nerve stimulation reverses the extinction impairments in a model of PTSD with prolonged and repeated trauma. Stress 2019. [DOI] [PubMed] [Google Scholar]
- 462.Agorastos A, Boel JA, Heppner PS, Hager T, Moeller-Bertram T, Haji U, et al. Diminished vagal activity and blunted diurnal variation of heart rate dynamics in posttraumatic stress disorder. Stress 2013;16(3):300–10. [DOI] [PubMed] [Google Scholar]
- 463.McLaughlin KA, Alves S, Sheridan MA. Vagal regulation and internalizing psychopathology among adolescents exposed to childhood adversity. Dev Psychobiol 2014;56(5):1036–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Hays SA. Enhancing rehabilitative therapies with vagus nerve stimulation. Neurotherapeutics 2016;13(2):382–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Hays SA, Ruiz A, Bethea T, Khodaparast N, Carmel JB, Rennaker RL, et al. Vagus nerve stimulation during rehabilitative training enhances recovery of forelimb function after ischemic stroke in aged rats. Neurobiol Aging 2016;43:111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Khodaparast N, Kilgard MP, Casavant R, Ruiz A, Qureshi I, Ganzer PD, et al. Vagus nerve stimulation during rehabilitative training improves forelimb recovery after chronic ischemic stroke in rats. Neurorehabil Neural Repair 2015;30(7):676–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Pruitt DT, Schmid A,N., Kim LL, Abe CM, Trieu JL, Choua C. Vagus nerve stimulation delivered with motor training enhances recovery of function after traumatic brain injury. J Neurotrauma 2016;33:871–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Hays SA, Khodaparast N, Hulsey DR, Ruiz A, Sloan AM, Rennaker RL, et al. Vagus nerve stimulation during rehabilitative training improves functional recovery after intracerebral hemorrhage. Stroke 2014;45(10):3097–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Li M, Zheng C, Sato T, Kawada T, Sugimachi M, Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation 2004;109(1):120–4. [DOI] [PubMed] [Google Scholar]
- 470.Groves DA, Brown VJ. Vagal nerve stimulation: a review of its applications and potential mechanisms that mediate its clinical effects. Neurosci Biobehav Rev 2005;29:493–500. [DOI] [PubMed] [Google Scholar]
- 471.Meyers R, Pearlman A, Hyman R. Beneficial effects of vagal stimulation and bradycardia during experimental acute myocardial ischemia. Circulation 1974;49:943–7. [DOI] [PubMed] [Google Scholar]
- 472.Kent KM, Smith ER, Redwood DR, Epstein SE. Electrical stability of acutely ischemic myocardium: Influences to heart rate and vagal stimulation. Circulation 1973;47:291–8. [DOI] [PubMed] [Google Scholar]
- 473.Childs JE, DeLeon J, Nickel E, Kroener S. Vagus nerve stimulation reduces cocaine seeking and alters plasticity in the extinction network. Learn Memory 2016;24(1):35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Dawson J, Pierce D, Dixit A, Kimberley TJ, Robertson M, Tarver B, et al. Safety, feasibility, and efficacy of Vagus Nerve Stimulation paired with upper-limb rehabilitation after ischemic stroke. Stroke 2016;47:143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Shim HJ, Kwak MY, An Y-H, Kim DH, Kim YJ, Kim HJ. Feasibility and safety of transcutaneous Vagus Nerve Stimulation paired with notched music therapy for the treatment of chronic tinnitus. J Audiol Otol 2015;19(3):159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Smith DC, Modglin AA, Roosevelt RW, Neese SL, Jensen RA, Browning RA, et al. Electrical stimulation of the vagus nerve enhances cognitive and motor recovery following moderate fluid percussion injury in the rat. J Neurotrauma 2005;22(12):1485–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Smith DC, Tan AA, Duke A, Neese SL, Clough RW, Browning RA, et al. Recovery of function after vagus nerve stimulation initiated 24h after fluid percussion brain injury. J Neurotrauma 2006;23(10):1549–60. [DOI] [PubMed] [Google Scholar]
- 478.Sjögren MJ, Hellstrom PT, Jonsson MA, Runnerstam M, Silander HC, Ben-Menachem E. Cognition-enhancing effect of vagus nerve stimulation in patients with Alzheimer’s disease: A pilot study. J Clin Psychiatry 2002;63(11):972–80. [DOI] [PubMed] [Google Scholar]
- 479.Merrill CA, Jonsson MA, Minthon L, Ejnell H, C-son Silander H, Blennow K, et al. Vagus nerve stimulation in patients with Alzheimer’s disease: Additional follow-up results of a pilot study through 1 year. J Clin Psychiatry 2006;67(8):1171–8. [DOI] [PubMed] [Google Scholar]
- 480.Bikson M, Unal G, Brunoni A, Loo C. What psychiatrists need to know about transcranial direct current stimulation. Psychiatric Times 2017:1–3. [Google Scholar]
- 481.Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, et al. Safety of transcranial Direct Current Stimulation: Evidence based update 2016. Brain Stimul 2016;9(5):641–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Tortella G, Casati R, Aparicio LVM, Mantovani A, Senço N, D’Urso G, et al. Transcranial direct current stimulation in psychiatric disorders. World J Psychiatr 2015;5(1):88–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol 2016;127:1031–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Schachter SC, Saper CB. Vagus nerve stimulation. Epilepsia 1998;39:677–86. [DOI] [PubMed] [Google Scholar]
- 485.Busch V, Zeman F, Heckel A, Menne F, Ellrich J, Eichhammer P. The effect of transcutaneous vagus nerve stimulation on pain perception – An experimental study. Brain Stimul 2013;6(2):202–9. [DOI] [PubMed] [Google Scholar]
- 486.Babygirija R, Sood M, Kannampalli P, Sengupta JN, Miranda A. Percutaneous electrical nerve field stimulation modulates central pain pathways and attenuates post-inflammatory visceral and somatic hyperalgesia in rats. Neuroscience 2017;356:11–21. [DOI] [PubMed] [Google Scholar]
- 487.Barbanti P, Grazzi L, Egeo G, Padovan A, Liebler E, Bussone G. Non-invasive vagus nerve stimulation for acute treatment of high-frequency and chronic migraine: an open-label study. J Headache Pain 2015;16(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Nesbitt AD, Marin JCA, Tomkins E, Ruttledge MH, Goadsby PJ. Non-invasive vagus nerve stimulation for the treatment of cluster headache: a case series. J Headache Pain 2013;14(1):1-.23566305 [Google Scholar]
- 489.Ben-Menachem E, Revesz D, Simon BJ, Silberstein S. Surgically implanted and non-invasive vagus nerve stimulation: a review of efficacy, safety and tolerability. Eur J Neurol 2015;22(9):1260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Gaul C, Magis D, Liebler EJ, Straube A. Effects of non-invasive vagus nerve stimulation on attack frequency over time and expanded response rates in patients with chronic cluster headache: A post hoc analysis of the randomized, controlled PREVA Study. J Headache Pain 2017;18(22):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Ben-Menachem E, Hellström K, Waldton C, Augustinsson LE. Evaluation of refractory epilepsy treated with vagus nerve stimulation for up to 5 years. Neurology 1999;52:1265–7. [DOI] [PubMed] [Google Scholar]
- 492.Ben-Menachem E, Mañon-Espaillat RRR, Wilder BJ, Stefan H, Mirza W, et al. Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. Epilepsia 1994;35(3):616–26. [DOI] [PubMed] [Google Scholar]
- 493.George R, Salinsky M, Kuzniecky R, Rosenfeld W, Bergen D, Tarver WB, et al. Vagus nerve stimulation for treatment of partial seizures: 3. Long-term follow-up on the first 67 patients exiting a controlled study. Epilepsia 1994;35(3):637–43. [DOI] [PubMed] [Google Scholar]
- 494.Handforth A, DeGiorgio CM, Schachter SC, Uthman BM, Naritoku DK, Tecoma ES, et al. Vagus nerve stimulation therapy for partial-onset seizures: A randomized active-control trial. Neurology 1998;51(1):48–55. [DOI] [PubMed] [Google Scholar]
- 495.Salinsky MC, Uthman BM, Ristanovic RK, Wernicke JF, Tarver WB. Vagus nerve stimulation for the treatment of medically intractable seizures. Results of a 1-year open-extension trial. The Vagus Nerve Stimulation Study Group. Arch Neurol 1999;53(11):1176–80. [DOI] [PubMed] [Google Scholar]
- 496.The Vagus Nerve Stimulation Study Group. A randomized controlled trial of chronic vagus nerve stimulation for treatment of medically intractable seizures. Neurology 1995;45(2):224–30. [DOI] [PubMed] [Google Scholar]
- 497.Berry SM, Broglio K, Bunker M, Jayewardene A, Olin B, Rush AJ. A patient-level meta-analysis of studies evaluating vagus nerve stimulation therapy for treatment-resistant depression. Med Devices (Auckl) 2013;6:17–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Brunoni AR, Moffa AH, Fregni F, Palm U, Padberg F, Blumberger DM, et al. Transcranial direct current stimulation for acute major depressive episodes: meta-analysis of individual patient data. Br J Psychiatry 2016;208(6):522–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Brunoni AR, Moffa AH, Sampaio-Junior B, Borrione L, Moreno ML, Fernandes RA, et al. Trial of electrical Direct-Current Therapy versus escitalopram for depression. N Engl J Med 2017;376(26):2523–33. [DOI] [PubMed] [Google Scholar]
- 500.Brunoni AR, Valiengo L, Baccaro A, Zanão TA, de Oliveira JF, Goulart A, et al. The sertraline versus electrical current therapy for treating depression clinical study: Results from a factorial, randomized, controlled trial. JAMA Psychiatry 2013;70(4):383–90. [DOI] [PubMed] [Google Scholar]
- 501.Dell-Osso B, Oldani L, Palazzo MC, Balossi I, Ciabatti M, Altamura AC. Vagus nerve stimulation in treatment-resistant depression: Acute and follow-up results of an Italian case series. J ECT 2013;29(1):41–4. [DOI] [PubMed] [Google Scholar]
- 502.George MS, Rush AJ, Marangell LB, Sackeim HA, Brannan SK, Davis SM, et al. A one-year comparison of Vagus Nerve Stimulation with treatment as usual for treatment-resistant depression. Biol Psychiatry 2005;58:364–73. [DOI] [PubMed] [Google Scholar]
- 503.George MS, Rush AJ, Sackeim HA, Marangell L. Vagus Nerve Stimulation (VNS): Utility in neuropsychiatric disorders. Int J Neuropsychopharmacol 2003;6:73–83. [DOI] [PubMed] [Google Scholar]
- 504.Marangell LB, Rush AJ, George MS, Sackeim HA, Johnson CR, Husain MM, et al. Vagus Nerve Stimulation (VNS) for major depressive episodes: Longer-term outcome. Biol Psychiatry 2002;51(4):280–7. [DOI] [PubMed] [Google Scholar]
- 505.Rush AJ, George MS, Sackeim HA, Marangell LB, Husain M, Giller C, et al. Vagus Nerve Stimulation (VNS) for treatment-resistant depression: A multicenter study. Biol Psychiatry 2000;47(4):276–86. [DOI] [PubMed] [Google Scholar]
- 506.Rush AJ, Marangell LB, Sackeim HA, George MS, Brannan SK, Davis SM, et al. Vagus Nerve Stimulation for treatment-resistant depression: A randomized, controlled acute phase trial. Biol Psychiatry 2005;58:347–54. [DOI] [PubMed] [Google Scholar]
- 507.Rush AJ, Sackheim HA, Marangell LB, George MS, Brannan SK, Davis SM, et al. Effects of 12 Months of Vagus Nerve Stimulation in treatment-resistant depression: A naturalistic study. Biol Psychiatry 2005;58(5):355–63. [DOI] [PubMed] [Google Scholar]
- 508.Sackeim HA, Brannan SK, Rush AJ, George MS, Marangell LB, Allen J. Durability of antidepressant response to vagus nerve stimulation (VNS). Int J Neuropsychopharmacol 2007;10:817–26. [DOI] [PubMed] [Google Scholar]
- 509.Sackeim HA, Keilp JG, Rush AJ, George MS, Marangell LB, Dormer JS, et al. The effects of vagus nerve stimulation on cognitive performance in patients with treatment-resistant depression. Neuropsychiatry Neuropsychol Behav Neurol 2001;14(1):53–62. [PubMed] [Google Scholar]
- 510.Sackeim HA, Rush AJ, George MS, Marangell LB, Husain MM, Nahas Z, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: Efficacy, side effects, and predictors of outcome. Neuropsychopharmacology 2001;25(5):713–28. [DOI] [PubMed] [Google Scholar]
- 511.Brunelin J, Mondino M, Gassab L, Haesebaert F, Lofti Gaha L, Suaud-Chagny M-F, et al. Examining transcranial direct-current stimulation (tDCS) as a treatment for hallucinations in schizophrenia. Am J Psychiatry 2012;169(7):719–24. [DOI] [PubMed] [Google Scholar]
- 512.Hasan A, Wolff-Menzler C, Pfeiffer S, Falkai P, Weidinger E, Jobst A, et al. Transcutaneous noninvasive vagus nerve stimulation (tVNS) in the treatment of schizophrenia: a bicentric randomized controlled pilot study. Eur Arch Psychiatry Clin Neurosci 2015;256(7):589–600. [DOI] [PubMed] [Google Scholar]
- 513.D’Urso G, Brunoni AR, Mazzaferro MP, Anastasia A, de Bartolomeis A, Mantovani A. Transcranial direct current stimulation for obsessive-compulsive disorder: A randomized, controlled, partial crossover trial. Depress Anxiety 2016;33(12):1132–40. [DOI] [PubMed] [Google Scholar]
- 514.Fallgatter AJ, Ehlis A-C, Ringel TM, Herrmann MJ. Age effect on far field potentials from the brain stem after transcutaneous vagus nerve stimulation. International journal of psychophysiology 2005;56(1):37–43. [DOI] [PubMed] [Google Scholar]
- 515.Fallgatter AJ, Neuhauser B, Herrmann MJ, Ehlis A-C, Wagener A, Scheuerpflug P, et al. Far field potentials from the brain stem after transcutaneous vagus nerve stimulation. Journal of Neural Transmission 2003;110(12):1437–43. [DOI] [PubMed] [Google Scholar]
- 516.Ventureyra EC. Transcutaneous vagus nerve stimulation for partial onset seizure therapy. Child’s Nervous System 2000;16(2):101–2. [DOI] [PubMed] [Google Scholar]
- 517.Mourdoukoutas AP, Truong DQ, Adair DK, Simon BJ, Bikson M. High-Resolution Multi-Scale Computational Model for Non-Invasive Cervical Vagus Nerve Stimulation. Neuromodulation 2018;21(3):261–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Badran BW, Alfred BY, Adair D, Mappin G, DeVries WH, Jenkins DD, et al. Laboratory Administration of Transcutaneous Auricular Vagus Nerve Stimulation (taVNS): Technique, Targeting, and Considerations. JoVE (Journal of Visualized Experiments) 2019(143):e58984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.FDA. K173442.pdf. 2017.
- 520.gammaCore. Instructions for Use for gammaCore®-S. 2019. [Google Scholar]
- 521.Kraus T, Kiess O, Hösl K, Terekhin P, Kornhuber J, Forster C. CNS BOLD fMRI Effects of Sham-Controlled Transcutaneous Electrical Nerve Stimulation in the Left Outer Auditory Canal – A Pilot Study. Brain Stimulation 2013;6(5):798–804. [DOI] [PubMed] [Google Scholar]
- 522.Badran BW, Dowdle LT, Mithoefer OJ, LaBate NT, Coatsworth J, Brown JC, et al. Neurophysiologic effects of transcutaneous auricular vagus nerve stimulation (taVNS) via electrical stimulation of the tragus: A concurrent taVNS/fMRI study and review. Brain Stimul 2018;11(3):492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Frangos E, Ellrich J, Komisaruk BR. Non-invasive Access to the Vagus Nerve Central Projections via Electrical Stimulation of the External Ear: fMRI Evidence in Humans. Brain Stimulation 2015;8(3):624–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Del Tredici K, Rϋb U, De Vos RA, Bohl JR, Braak H. Where does Parkinson disease pathology begin in the brain? Journal of Neuropathology & Experimental Neurology 2002;61(5):413–26. [DOI] [PubMed] [Google Scholar]
- 525.Polak T, Ehlis A-C, Langer J, Plichta M, Metzger F, Ringel T, et al. Non-invasive measurement of vagus activity in the brainstem–a methodological progress towards earlier diagnosis of dementias? Journal of neural transmission 2007;114(5):613–9. [DOI] [PubMed] [Google Scholar]
- 526.Polak T, Markulin F, Ehlis A-C, Langer JB, Ringel TM, Fallgatter AJ. Far field potentials from brain stem after transcutaneous vagus nerve stimulation: optimization of stimulation and recording parameters. Journal of neural transmission 2009;116(10):1237–42. [DOI] [PubMed] [Google Scholar]
- 527.Polak T, Markulin F, Ehlis A-C, Metzger F, Langer JB, Ringel TM, et al. Auricular vagus somatosensory evoked potentials in vascular dementia. Journal of neural transmission 2009;116(4):473–7. [DOI] [PubMed] [Google Scholar]
- 528.Fallgatter A, Neuhauser B, Herrmann M, Ehlis A-C, Wagener A, Scheuerpflug P, et al. Far field potentials from the brain stem after transcutaneous vagus nerve stimulation. Journal of neural transmission 2003;110(12):1437–43. [DOI] [PubMed] [Google Scholar]
- 529.Leutzow B, Lange J, Gibb A, Schroeder H, Nowak A, Wendt M, et al. Vagal sensory evoked potentials disappear under the neuromuscular block - an experimental study. Brain Stimul 2013;6(5):812–6. [DOI] [PubMed] [Google Scholar]
- 530.Usami K, Kawai K, Sonoo M, Saito N. Scalp-recorded evoked potentials as a marker for afferent nerve impulse in clinical vagus nerve stimulation. Brain Stimul 2013;6(4):615–23. [DOI] [PubMed] [Google Scholar]
- 531.Hein E, Nowak M, Kiess O, Biermann T, Bayerlein K, Kornhuber J, et al. Auricular transcutaneous electrical nerve stimulation in depressed patients: a randomized controlled pilot study. Journal of Neural Transmission 2013;120(5):821–7. [DOI] [PubMed] [Google Scholar]
- 532.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network. Annals of the New York Academy of Sciences 2008;1124(1):1–38. [DOI] [PubMed] [Google Scholar]
- 533.Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, Jonides J. Depression, rumination and the default network. Social cognitive and affective neuroscience 2011;6(5):548–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biological psychiatry 2011;70(4):327–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Fang J, Rong P, Hong Y, Fan Y, Liu J, Wang H, et al. Transcutaneous Vagus Nerve Stimulation Modulates Default Mode Network in Major Depressive Disorder. Biological Psychiatry 2016;79(4):266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Jacobs HI, Riphagen JM, Razat CM, Wiese S, Sack AT. Transcutaneous vagus nerve stimulation boosts associative memory in older individuals. Neurobiology of aging 2015;36(5):1860–7. [DOI] [PubMed] [Google Scholar]
- 537.Badran BW, Jenkins DD, DeVries WH, Dancy M, Summers PM, Mappin GM, et al. Transcutaneous auricular vagus nerve stimulation (taVNS) for improving oromotor function in newborns. Brain Stimulation: Basic, Translational, and Clinical Research in Neuromodulation 2018;11(5):1198–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Burger AM, Verkuil B, Van Diest I, Van der Does W, Thayer JF, Brosschot JF. The effects of transcutaneous vagus nerve stimulation on conditioned fear extinction in humans. Neurobiology of learning and memory 2016;132:49–56. [DOI] [PubMed] [Google Scholar]
- 539.Steenbergen L, Sellaro R, Stock A-K, Verkuil B, Beste C, Colzato LS. Transcutaneous vagus nerve stimulation (tVNS) enhances response selection during action cascading processes. European Neuropsychopharmacology 2015;25(6):773–8. [DOI] [PubMed] [Google Scholar]
- 540.Sellaro R, van Leusden JW, Tona K-D, Verkuil B, Nieuwenhuis S, Colzato LS. Transcutaneous vagus nerve stimulation enhances post-error slowing. Journal of cognitive neuroscience 2015. [DOI] [PubMed] [Google Scholar]
- 541.Rong P, Liu J, Wang L, Liu R, Fang J, Zhao J, et al. Effect of transcutaneous auricular vagus nerve stimulation on major depressive disorder: A nonrandomized controlled pilot study. J Affect Disord 2016;195:172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Lamb DG, Porges EC, Lewis GF, Williamson JB. Non-invasive Vagal Nerve Stimulation effects on hyperarousal and autonomic state in patients with posttraumatic stress disorder and history of mild traumatic brain injury: Preliminary evidence. Front Med 2017;4:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Gurel NZ, Mobashir HS, Bremner JD, Vaccarino V, Ladd SL, Shah A, et al. Toward closed-loop transcutaneous vagus nerve stimulation using peripheral cardiovascular physiological biomarkers: A proof-of-concept study. IEEE: Body Sensor Networks (BSN) 2018:78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Bremner JD, Gurel N, Wittbrodt M, Nye J, Alam Z, Herring I, et al. Non-invasive vagal nerve stimulation paired with stress exposure in posttraumatic stress disorder (PTSD). Brain Stimul 2019;12(2):438. [Google Scholar]
- 545.Gabella G Autonomic nervous system. e LS 2001. [Google Scholar]
- 546.Badran BW, Mithoefer OJ, Summer CE, LaBate NT, Glusman CE, Badran AW, et al. Short trains of transcutaneous auricular vagus nerve stimulation (taVNS) have parameter-specific effects on heart rate. Brain Stimulation 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Garcia RG, Lin RL, Lee J, Kim J, Barbieri R, Sclocco R, et al. Modulation of brainstem activity and connectivity by respiratory-gated auricular vagal afferent nerve stimulation in migraine patients. Pain 2017;158(8):1461–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Sclocco R, Garcia RG, Gabriel A, Kettner NW, Napadow V, Barbieri R, editors. Respiratory-gated Auricular Vagal Afferent Nerve Stimulation (RAVANS) effects on autonomic outflow in hypertension. Engineering in Medicine and Biology Society (EMBC), 2017 39th Annual International Conference of the IEEE; 2017: IEEE. [DOI] [PubMed] [Google Scholar]
- 549.Napadow V, Edwards RR, Cahalan CM, Mensing G, Greenbaum S, Valovska A, et al. Evoked pain analgesia in chronic pelvic pain patients using respiratory-gated auricular vagal afferent nerve stimulation. Pain medicine 2012;13(6):777–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Frangos E, Komisaruk BR. Access to Vagal Projections via Cutaneous Electrical Stimulation of the Neck: fMRI Evidence in Healthy Humans. Brain Stimul 2017;10(1):19–27. [DOI] [PubMed] [Google Scholar]
- 551.Mauskop A Vagus nerve stimulation relieves chronic refractory migraine and cluster headaches. Cephalalgia 2005;25(2):82–6. [DOI] [PubMed] [Google Scholar]
- 552.Kirchner A, Birklein F, Stefan H, Handwerker H. Left vagus nerve stimulation suppresses experimentally induced pain. Neurology 2000;55(8):1167–71. [DOI] [PubMed] [Google Scholar]
- 553.Gaul C, Diener HC, Silver N, Magis D, Reuter U, Andersson A, et al. Non-invasive vagus nerve stimulation for PREVention and Acute treatment of chronic cluster headache (PREVA): A randomised controlled study. Cephalalgia 2016;36(6):534–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Barbanti P, Grazzi L, Egeo G, Padovan AM, Liebler E, Bussone G. Non-invasive vagus nerve stimulation for acute treatment of high-frequency and chronic migraine: an open-label study. The Journal of Headache and Pain 2015;16(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Silberstein SD, Mechtler LL, Kudrow DB, Calhoun AH, McClure C, Saper JR, et al. Non-Invasive Vagus Nerve Stimulation for the ACute Treatment of Cluster Headache: Findings From the Randomized, Double-Blind, Sham-Controlled ACT1 Study. Headache 2016;56(8):1317–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Goadsby PJ, de Coo IF, Silver N, Tyagi A, Ahmed F, Gaul C, et al. Non-invasive vagus nerve stimulation for the acute treatment of episodic and chronic cluster headache: A randomized, double-blind, sham-controlled ACT2 study. Cephalalgia 2018;38(5):959–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Kinfe TM, Pintea B, Muhammad S, Zaremba S, Roeske S, Simon BJ, et al. Cervical non-invasive vagus nerve stimulation (nVNS) for preventive and acute treatment of episodic and chronic migraine and migraine-associated sleep disturbance: preliminary findings from a prospective observational cohort study. The journal of headache and pain 2015;16(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Yang Y, Yang LY, Orban L, Cuylear D, Thompson J, Simon B, et al. Non-invasive vagus nerve stimulation reduces blood-brain barrier disruption in a rat model of ischemic stroke. Brain stimulation 2018;11(4):689–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Ay I, Sorensen AG, Ay H. Vagus nerve stimulation reduces infarct size in rat focal cerebral ischemia: an unlikely role for cerebral blood flow. Brain research 2011;1392:110–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Helmers SL, Begnaud J, Cowley A, Corwin HM, Edwards JC, Holder DL, et al. Application of a computational model of vagus nerve stimulation. Acta Neurol Scand 2012;126(5):336–43. [DOI] [PubMed] [Google Scholar]
- 561.Goodall EV, Kosterman L, Holsheimer J, Struijk JJ. Modeling study of activation and propagation delays during stimulation of peripheral nerve fibers with a tripolar cuff electrode. IEEE transactions on rehabilitation engineering 1995;3(3):272–82. [Google Scholar]
- 562.Arle JE, Carlson KW, Mei L. Investigation of mechanisms of vagus nerve stimulation for seizure using finite element modeling. Epilepsy research 2016;126:109–18. [DOI] [PubMed] [Google Scholar]
- 563.Bikson M, Brunoni AR, Charvet LE, Clark VP, Cohen LG, Deng ZD, et al. Rigor and reproducibility in research with transcranial electrical stimulation: An NIMH-sponsored workshop. Brain Stimul 2018;11(3):465–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Truong DQ, Bikson M. Physics of Transcranial Direct Current Stimulation Devices and Their History. J ect 2018;34(3):137–43. [DOI] [PubMed] [Google Scholar]
- 565.Machado D, Unal G, Andrade SM, Moreira A, Altimari LR, Brunoni AR, et al. Effect of transcranial direct current stimulation on exercise performance: A systematic review and meta-analysis. Brain Stimul 2018. [DOI] [PubMed] [Google Scholar]
- 566.Medaglia JD, Erickson B, Zimmerman J, Kelkar A. Personalizing Neuromodulation. Int J Psychophysiol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Becker JE, Shultz EKB, Maley CT. Transcranial Magnetic Stimulation in Conditions Other than Major Depressive Disorder. Child Adolesc Psychiatr Clin N Am 2019;28(1):45–52. [DOI] [PubMed] [Google Scholar]
- 568.Barahona-Correa JB, Velosa A, Chainho A, Lopes R, Oliveira-Maia AJ. Repetitive Transcranial Magnetic Stimulation for Treatment of Autism Spectrum Disorder: A Systematic Review and Meta Analysis. Front Integr Neurosci 2018;12:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Lusicic A, Schruers KR, Pallanti S, Castle DJ. Transcranial magnetic stimulation in the treatment of obsessive-compulsive disorder: current perspectives. Neuropsychiatr Dis Treat 2018;14:1721–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Kundu B, Brock AA, Englot DJ, Butson CR, Rolston JD. Deep brain stimulation for the treatment of disorders of consciousness and cognition in traumatic brain injury patients: a review. Neurosurg Focus 2018;45(2):E14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Lozano AM, Lipsman N, Bergman H, Brown P, Chabardes S, Chang JW, et al. Deep brain stimulation: current challenges and future directions. Nat Rev Neurol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.McKinnon C, Gros P, Lee DJ, Hamani C, Lozano AM, Kalia LV, et al. Deep brain stimulation: potential for neuroprotection. Ann Clin Transl Neurol 2019;6(1):174–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Rodrigues FB, Duarte GS, Prescott D, Ferreira J, Costa J. Deep brain stimulation for dystonia. Cochrane Database Syst Rev 2019;1:Cd012405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Rahman A, Lafon B, Parra LC, Bikson M. Direct current stimulation boosts synaptic gain and cooperativity in vitro. J Physiol 2017;595(11):3535–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Reato D, Rahman A, Bikson M, Parra LC. Effects of weak transcranial alternating current stimulation on brain activity-a review of known mechanisms from animal studies. Front Hum Neurosci 2013;7:687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Cocchi L, Zalesky A, Nott Z, Whybird G, Fitzgerald PB, Breakspear M. Transcranial magnetic stimulation in obsessive-compulsive disorder: A focus on network mechanisms and state dependence. Neuroimage Clin 2018;19:661–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Wilson MT, Fulcher BD, Fung PK, Robinson PA, Fornito A, Rogasch NC. Biophysical modeling of neural plasticity induced by transcranial magnetic stimulation. Clin Neurophysiol 2018;129(6):1230–41. [DOI] [PubMed] [Google Scholar]
- 578.Javitt DC. When Doors of Perception Close: Bottom-up Models of Disrupted Cognition in Schizophrenia. Annual review of clinical psychology 2009;5:249–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron 2006;52(1):155–68. [DOI] [PubMed] [Google Scholar]
- 580.Streeter GL. The development of the cranial and spinal nerves in the occipital region of the human embryo. American Journal of Anatomy 1905;4(1):83–116. [Google Scholar]
- 581.Burian M, Gstoettner W, Zundritsch R. Saccular afferent fibers to the cochlear nucleus in the guinea pig. Archives of oto-rhino-laryngology 1989;246(5):238–41. [DOI] [PubMed] [Google Scholar]
- 582.Fisch UP. Excision of Scarpa’s ganglion. Archives of Otolaryngology 1973;97(2):147–9. [DOI] [PubMed] [Google Scholar]
- 583.Howell B, Choi KS, Gunalan K, Rajendra J, Mayberg HS, McIntyre CC. Quantifying the axonal pathways directly stimulated in therapeutic subcallosal cingulate deep brain stimulation. Hum Brain Mapp 2019;40(3):889–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Anderson DN, Duffley G, Vorwerk J, Dorval AD, Butson CR. Anodic stimulation misunderstood: preferential activation of fiber orientations with anodic waveforms in deep brain stimulation. J Neural Eng 2019;16(1):016026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Gunalan K, Howell B, McIntyre CC. Quantifying axonal responses in patient-specific models of subthalamic deep brain stimulation. Neuroimage 2018;172:263–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Olson B Treatment of refractory epilepsy. Adv stu med 2005;5:470–3. [Google Scholar]
- 587.Grace AA. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nature Reviews Neuroscience 2016;17(8):524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Ragland JD, Ranganath C, Harms MP, Barch DM, Gold JM, Layher E, et al. Functional and neuroanatomic specificity of episodic memory dysfunction in schizophrenia: a functional magnetic resonance imaging study of the relational and item-specific encoding task. JAMA psychiatry 2015;72(9):909–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Insel TR. The NIMH research domain criteria (RDoC) project: precision medicine for psychiatry. American Journal of Psychiatry 2014;171(4):395–7. [DOI] [PubMed] [Google Scholar]
- 590.Ivleva EI, Bidesi AS, Keshavan MS, Pearlson GD, Meda SA, Dodig D, et al. Gray matter volume as an intermediate phenotype for psychosis: Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). American Journal of Psychiatry 2013;170(11):1285–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591.Eack SM, Newhill CE, Anderson CM, Rotondi AJ. Quality of life for persons living with schizophrenia: more than just symptoms. Psychiatric rehabilitation journal 2007;30(3):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Chen R, Classen J, Gerloff C, Celnik P, Wassermann E, Hallett M, et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology 1997;48(5):1398–403. [DOI] [PubMed] [Google Scholar]
- 593.Geller V, Grisaru N, Abarbanel JM, Lemberg T, Belmaker RH. Slow magnetic stimulation of prefrontal cortex in depression and schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry 1997;21(1):105–10. [DOI] [PubMed] [Google Scholar]
- 594.Luo J, Ye H, Zheng H, Chen S, Huang D. Modulating the activity of the dorsolateral prefrontal cortex by tDCS alters distributive decisions behind the veil of ignorance via risk preference. Behavioural Brain Research 2017;328:70–80. [DOI] [PubMed] [Google Scholar]
- 595.Kringelbach ML, Jenkinson N, Owen SLF, Aziz TZ. Translational principles of deep brain stimulation. Nature Reviews Neuroscience 2007;8:623. [DOI] [PubMed] [Google Scholar]
- 596.Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biological psychiatry 2008;64(6):461–7. [DOI] [PubMed] [Google Scholar]
- 597.Sturm V, Lenartz D, Koulousakis A, Treuer H, Herholz K, Klein JC, et al. , editors. The nucleus accumbens: a target for Deep-brain stimulation in obsessive-compulsive and anxiety disorders Proceedings of the Medtronic Forum for Neuroscience and Neuro-Technology 2005; 2007: Springer. [DOI] [PubMed] [Google Scholar]
- 598.Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology 2008;33(2):368. [DOI] [PubMed] [Google Scholar]
- 599.Laxton AW, Tang-Wai DF, McAndrews MP, Zumsteg D, Wennberg R, Keren R, et al. A phase I trial of deep brain stimulation of memory circuits in Alzheimer’s disease. Annals of neurology 2010;68(4):521–34. [DOI] [PubMed] [Google Scholar]
- 600.Chouinard PA, Van Der Werf YD, Leonard G, Paus T. Modulating neural networks with transcranial magnetic stimulation applied over the dorsal premotor and primary motor cortices. Journal of Neurophysiology 2003;90(2):1071–83. [DOI] [PubMed] [Google Scholar]
- 601.Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biological psychiatry 2012;72(7):595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Keeser D, Meindl T, Bor J, Palm U, Pogarell O, Mulert C, et al. Prefrontal transcranial direct current stimulation changes connectivity of resting-state networks during fMRI. J Neurosci 2011;31(43):15284–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603.Hays SA, Rennaker RL, Kilgard MP. Targeting plasticity with vagus nerve stimulation to treat neurological disease. Progress in brain research 2013;207:275. [DOI] [PMC free article] [PubMed] [Google Scholar]


