Abstract
Current algorithms for assessing risk of atherosclerotic cardiovascular disease (ASCVD) and, in particular, the reliance on low-density lipoprotein (LDL) cholesterol in conditions where this measurement is discordant with apoB and LDL-particle concentrations fail to identify a sizeable part of the population at high risk for adverse cardiovascular events. This results in missed opportunities for ASCVD prevention, most notably in those with metabolic syndrome, prediabetes, and diabetes. There is substantial evidence that accumulation of ectopic fat and associated metabolic traits are markers for and pathogenic components of high-risk atherosclerosis. Conceptually, the subset of advanced lesions in high-risk atherosclerosis that triggers vascular complications is closely related to a set of coordinated high-risk traits clustering around a distinct metabolic phenotype. A key feature of this phenotype is accumulation of ectopic fat, which, coupled with age-related muscle loss, creates a milieu conducive for the development of ASCVD: atherogenic dyslipidemia, nonresolving inflammation, endothelial dysfunction, hyperinsulinemia, and impaired fibrinolysis. Sustained vascular inflammation, a hallmark of high-risk atherosclerosis, impairs plaque stabilization in this phenotype. This review describes how metabolic and inflammatory processes that are promoted in large measure by ectopic adiposity, as opposed to subcutaneous adipose tissue, relate to the pathogenesis of high-risk atherosclerosis. Clinical biomarkers indicative of these processes provide incremental information to standard risk factor algorithms and advanced lipid testing identifies atherogenic lipoprotein patterns that are below the discrimination level of standard lipid testing. This has the potential to enable improved identification of high-risk patients who are candidates for therapeutic interventions aimed at prevention of ASCVD.
Keywords: atherosclerosis, metabolic syndrome, ectopic adipose tissue, dyslipidemia, inflammation, lifestyle
Introduction
Despite ongoing advances in cardiovascular medicine, acute complications of atherosclerosis remain the leading causes of death worldwide.1 Of concern, common approaches relying on clustered risk factors and surrogate biomarkers fail to identify a sizeable proportion of individuals at high risk for cardiovascular events.2 Furthermore, despite management of low-density lipoprotein cholesterol (LDL-C) and other conventional risk factors, significant residual risk remains.
One explanation for this observation is the high prevalence of individuals with metabolic disorders where plasma LDL-C and atherogenic lipoprotein particle concentration, as assessed by apoB or direct particle measurement, become discordant, rendering LDL-C less predictive of atherosclerotic cardiovascular disease (ASCVD) events.3 This results in missed opportunities for prevention and intensification of lifestyle intervention and/or pharmacotherapy in the subgroup of the population with metabolic syndrome (MetS), prediabetes, and diabetes, which is at highest risk for vascular complications. Thus, assessment of adjunctive biomarkers for the presence and severity of subclinical ASCVD is of utmost importance for taking appropriate measures to prevent adverse clinical outcomes and reduce the associated economic burden of this disease.
This review discusses the biochemical principles underlying high-risk atherosclerosis within the conceptual framework of the phenotype characterized by excess ectopic fat. It furthermore provides perspective on clinical biomarkers indicative of this high-risk metabolic condition, which may add incremental information to standard risk factor algorithms for detection of high-risk patients who are candidates for interventions aimed at preventing major adverse cardiovascular events.
Pathophysiological Mechanisms Underlying High-Risk Atherosclerosis
An integrative view on atherosclerosis
Atherosclerosis is a lifelong process, and it progresses at various rates depending on genetic and nongenetic factors.4 It is initiated by the penetration and retention of apoB-containing lipoproteins within the subendothelial intima of arteries. This process is gradient driven, providing biological plausibility for the concept that the number of apoB-containing lipoprotein particles, not their aggregate cholesterol content (LDL-C), more closely tracks with risk.5–7
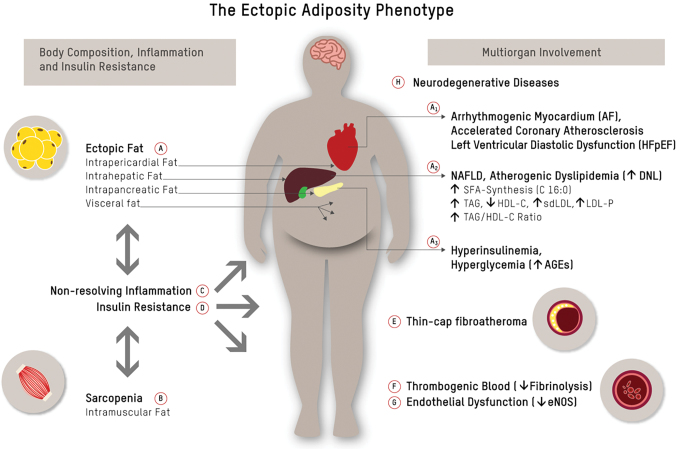
It is, however, worth noting that numerous factors modify the causality of lipoprotein-driven disease risk in ASCVD.8 High-risk atherosclerosis is closely associated with a cluster of metabolic and inflammatory features of a phenotype characterized by an increase of ectopic body fat.9,10 Figure 1 depicts a conceptual framework of how the accumulation of dysfunctional, ectopic fat in the abdominal cavity (visceral fat) and in organs (pericardium, liver, pancreas, and skeletal muscle)—in particular if accompanied by sarcopenia11,12—contributes to a proatherogenic and procoagulatory state9,10,13 and drives high-risk atherosclerosis through multiorgan/tissue involvement. Figure 1 furthermore maps the links between each of the elements within this framework.
FIG. 1.
The ectopic adiposity phenotype. Ectopic fat accumulation in the abdominal cavity (visceral fat) and in organs like pericardium, liver, and pancreas (A), and muscle wasting/intramuscular fat accumulation (B) are bidirectionally linked to chronic inflammation (C) and insulin resistance (D), both of which have been linked to conventional and novel pathways of cardiometabolic risk.9 Ectopic fat is a major driver of atherosclerosis and its acute complications: epicardial fat (A1) has been linked to AF, accelerated coronary atherosclerosis, and left ventricular diastolic dysfunction20; hepatic fat (NAFLD) (A2) causally contributes to atherogenic dyslipidemia and is closely linked to subclinical atherosclerosis22; and pancreatic fat (A3) has been linked to beta-cell dysfunction23 and concomitant postprandial and fasting hyperglycemia. Chronically elevated serum glucose levels, and postprandial glucose spikes in particular, promote oxidative stress/chronic inflammation, endothelial dysfunction, and sympathetic hyperactivity, and result in the formation of AGEs. Glycation damage has been pathophysiologically linked to numerous chronic disease states such as cardiovascular aging.24 Down arrows indicate decreased levels, and up arrows indicate increased levels. AF, arrhythmia/atrial fibrillation; AGEs, advanced glycation end products; NAFLD, non-alcoholic fatty liver disease. Color images are available online.
The role of body composition in ASCVD
Body composition, in particular accumulation of dysfunctional adipose tissue (AT)9,10 and loss of skeletal muscle,11,12 is at the core of a cluster of local and systemic pathophysiological changes that have been linked to high-risk atherosclerosis (Fig. 1A, B).
As depicted in Figure 1, excess hepatic fat production (i.e., de novo lipogenesis) may be an early common pathway of non-alcoholic fatty liver disease (NAFLD), atherogenic dyslipidemia, pancreatic β cell dysfunction, insulin resistance, and associated ASCVD risk in the high-risk phenotype.10,14
AT secretome
While subcutaneous AT is largely neutral, or in the case of lower body AT even protective with respect to cardiovascular risk,15 expansion of visceral and/or ectopic dysfunctional AT is closely linked to poor cardiometabolic health and MetS9,16 (Fig. 1A). Factors that promote AT dysfunction are chronic positive energy balance in conjunction with biochemical stressors, including physical inactivity,9 poor diet quality,9 active/passive exposure to cigarette smoke,17 and sleep deprivation.18 Free fatty acid-induced cellular stress causes remodeling of AT and encompasses a set of changes, including AT inflammation and altered secretome and modulation of the browning phenotype. Dysfunctional AT is characterized by an infiltration of macrophages and lymphocytes, and an increased abundance of senescent cells. These cells release fatty acids and proinflammatory and chemotactic compounds, which is referred to as a senescence-associated secretory phenotype. In a vicious cycle, this promotes ectopic fat accumulation and contributes to chronic inflammation, metabolic disturbances, sarcopenia, and accelerated cardiovascular aging.19 Epicardial AT (Fig. 1A1) is regarded as a paracrine transducer of the adverse effects of systemic inflammation and metabolic dysregulation on adjacent tissues, such as the underlying coronary arteries, and has accordingly been linked to arrhythmia/atrial fibrillation, accelerated coronary atherosclerosis, and left ventricular diastolic dysfunction.20 Hepatic fat accumulation/NAFLD (Fig. 1A2) causally contributes to atherogenic dyslipidemia [high plasma triglyceride and reduced high-density lipoprotein cholesterol (HDL-C)]. Increased hepatic de novo lipogenesis is furthermore associated with higher hepatic palmitic acid (C16:0) flux and enrichment of palmitic acid in very low-density lipoprotein particles (VLDL-P).14 Palmitic acid contributes to vascular inflammation through dimerization and activation of toll-like receptor (TLR) 2/4 as explained further below.21 These mechanisms provide some plausibility for the observation that NAFLD is closely linked to subclinical atherosclerosis.22 Pancreatic fat (Fig. 1A3) has been linked to β cell dysfunction23 and concomitant postprandial and fasting hyperglycemia. Chronically elevated serum glucose levels, and postprandial glucose spikes in particular, result in sympathetic hyperactivity and the formation of advanced glycation end products (AGEs). AGE, through interaction with receptor for AGEs, activate proinflammatory signaling pathways, which promote oxidative stress, chronic vascular inflammation, endothelial dysfunction, and accelerated cardiovascular aging in this phenotype.24
Lifestyle link
AT phenotype can be modified upon lifestyle interventions. Physical exercise,9 intermittent fasting,25,26 diet quality,9 and regular circadian rhythms/restorative sleep18 promote the preservation of a healthy AT phenotype and have the potential to reverse AT dysfunction and related cardiometabolic risk. These effects occur largely independent of body mass index (BMI).9,16 The CENTRAL-MRI trial demonstrated that in a group of 278 sedentary adults (age = 48 years, 89% men, BMI 30.8 kg/m2) with abdominal obesity (75%) or dyslipidemia, a Mediterranean low-carbohydrate dietary pattern was superior to a low-fat diet in decreasing intrahepatic, intrapericardial, and pancreatic fat (P < 0.05 for all), and that exercise had an independent contribution to visceral AT loss.27 Further to that, mobilization of ectopic fat depots was associated with improved cardiometabolic surrogate markers such as decreased expression of atherogenic dyslipidemia.27,28
Skeletal muscle secretome
Upon contraction, skeletal muscle fibers express and release cytokines and other peptides, which encompass a group of hormone-like substances referred to as myokines.12 Myokines exert their effects within the muscle itself (autocrine and paracrine function) and interact with remote tissues and organs (endocrine function). The muscle secretome consists of several hundred myokines that communicate with other organs, such as AT, liver, pancreas, vasculature, immune system, bones, and brain12 (Fig. 1B). Myokines such as irisin, fibroblast growth factor 21, interleukin (IL)-6, and IL-15 can improve cardiometabolic health through several mechanisms: (1) AT browning and maintenance of a functional white AT phenotype, (2) preservation of muscle mass, (3) improved endothelial function and myocardial contractility, (4) decreased inflammation, and (5) improved metabolic status, including increased insulin sensitivity and glucose homeostasis.11,12,16 This underpins the importance of maintaining or increasing muscle mass to attenuate cardiovascular aging.
Nonresolving inflammation and plaque phenotype
Inflammatory processes at the endothelial layer of the arterial wall play a fundamental role in the initiation, progression, and in particular, the clinical complications of high-risk atherosclerosis. The acute inflammatory response is divided into the two phases of initiation and resolution.29 Mounting evidence points to defects in inflammation resolution as a key causal factor in atherosclerosis (Fig. 1C).
During the initiation phase, apolipoprotein B-containing lipoproteins, when retained and modified (oxidized) in the subendothelial vascular wall, serve as damage-associated molecular patterns (DAMPs). DAMPs activate cytokine synthesis through pattern recognition receptors, for example, TLRs.30 Activation of TLR2 and the NLRP3-inflammasome by apolipoprotein C3 (ApoC3) provides one link between atherogenic lipoprotein patterns as seen in MetS and immune activation/inflammation. It is furthermore worth noting that certain saturated fatty acids (SFA) such as palmitic acid (C16:0) promote TLR activation.21,31 In the ectopic adiposity phenotype, excess dietary starch, sugar, and protein are converted into fatty acids (FA)—in particular palmitic acid (C16:0)—during a metabolic process referred to as hepatic de novo lipogenesis.14,31 This is another mechanism by which proinflammatory signaling pathways are activated in the phenotype with ectopic adiposity and NAFLD in particular. Inflammasome activation within macrophages leads to the release of proinflammatory cytokines, such as IL-1β, which are chemotactic for other inflammatory cells; this includes T cells and B cells, which further sustain the chronic inflammatory response by expression of proinflammatory cytokines and eicosanoids.29,30
During the resolution phase, in a process referred to as efferocytosis, biosynthesis of proinflammatory mediators (e.g., leukotrienes) transitions to synthesis of specialized proresolving mediators (SPMs).29 Efferocytosis—the phagocytic removal of apoptotic cells—is a crucial step in the resolution of lesional inflammation in chronic nonresolving inflammatory diseases.29 A mismatch between SPMs (low) and proinflammatory lipids such as leukotrienes (high) results in efferocytosis failure, which is, in large part, mediated by a shift in macrophage phenotype from the anti-inflammatory M2-like to proinflammatory M1-like macrophages.32 SPMs, which are predominantly produced by M2 macrophages during efferocytosis, include lipoxins biosynthesized from arachidonic acid and E-series resolvins from the eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)-derived D-series resolvins, protectins, and maresins.33 These anti-inflammatory mediators support efferocytosis by (1) limiting further neutrophil granulocyte recruitment to the site of injury and (2) enhancing macrophage uptake of cellular debris and apoptotic neutrophil granulocytes.33 One subgroup of maresins is involved in switching macrophage phenotype from the proinflammatory/proatherogenic M1 to the anti-inflammatory/antiatherogenic M2 phenotype.33 Failure to clear apoptotic cells from atherosclerotic plaques results in secondary necrosis, which generates inflammation owing to the release of DAMPs from necrotic cells.29
Lifestyle link
One group of SPMs that supports resolution of inflammation are derivatives of the long-chain marine n-3 fatty acids EPA and DHA.32 It is furthermore worth noting that DHA inhibits TLR2/4 dimerization and activation. TLRs—as discussed above—are pattern recognition receptors that can be activated by both pathogen-associated molecular patterns and nonmicrobial endogenous molecules. The inhibition of TLR2/4 dimerization and activation by DHA suggest a role for dietary components to modulate TLR-mediated immune responses.21 EPA and DHA can be supplemented or obtained by the consumption of oily fish.
In an inflammatory resolving environment, vascular smooth muscle cells that migrate from the medial layer undergo a phenotypic shift, forming a collagenous fibrous cap that overlies the lipid-rich plaque core. Plaque with net resolution is smaller, has a thicker fibrous cap (with predominantly anti-inflammatory M2-like macrophages, smooth muscle cells, and intact collagen), and has a smaller necrotic lipid core.32
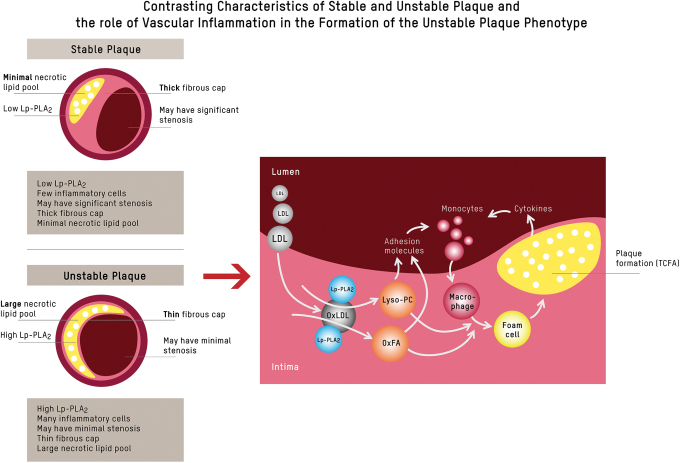
In the setting of continued inflammation, advanced lesions develop. They are larger, have a thinner fibrous cap (with predominantly proinflammatory M1-like macrophages, few smooth muscle cells, and cleaved collagen), and have a larger necrotic lipid core.32 Cells in advanced atherosclerotic plaques express a senescence-associated secretory phenotype, producing metalloproteinases that degrade the extracellular matrix, which promotes inflammation, while further weakening the fibrous cap, thus fostering a milieu conducive for a vulnerable plaque phenotype.19,29 This is commonly referred to as thin-cap fibroatheroma (TCFA) (reviewed in Kasikara et al.29, and Bäck et al.32) (Fig. 1E). One indicator for the presence of TCFA, and concomitant vascular inflammation, is lipoprotein-associated phospholipase A2 (Lp-PLA2), which plays a key role in the degradation of proinflammatory oxidized phospholipids to generate lyso-phosphatidylcholine (Lyso-PC) and oxidized fatty acids,34 as depicted in Figure 2. It is, however, important to note that, while Lp-PLA2 activity is a marker of risk, pharmacological lowering of Lp-PLA2 in patients with stable coronary heart disease (CHD) has not been shown to reduce clinical cardiovascular endpoints.35
FIG. 2.
Inflammation and plaque phenotype. When LDLs penetrate the intima of the vessel wall from the lumen, the Lp-PLA2 residing there uses oxLDL as a substrate, hydrolyzing it to Lyso-PC and OxFA.34 Lyso-PC and OxFA act as secondary messengers that stimulate the upregulation of adhesion molecules on the lumen surface, act as chemoattractants for circulating inflammatory cells, and play a role in the activation and transformation of local macrophages within the plaque lesion. As activated local macrophages take up oxidized (phospho)lipids, they transform to foam cells and subsequently express more Lp-PLA2, creating a vicious proinflammatory cycle. The expression of other cytokines like MCP-1 and adhesion molecules creates a feedback loop by attracting more monocytes to the plaque. This feedback loop generates a vicious cycle of attracting more inflammatory cells to the plaque lesion, resulting in an infiltration with an abundance of inflammatory cells, a thinning of the fibrous cap, and a growing necrotic lipid core. Although this process often results in limited luminal narrowing, it leads to the main clinical complications of ASCVD.29 ASCVD, atherosclerotic cardiovascular disease; LDL, low-density lipoprotein; Lp-PLA2, lipoprotein-associated phospholipase A2; Lyso-PC, lyso-phosphatidylcholine; MCP-1, monocyte chemoattractant protein-1; OxFA, oxidized fatty acids; oxLDL, oxidized LDL. Color images are available online.
Longitudinal observational studies have demonstrated that elevated blood levels of proinflammatory biomarkers, including high-sensitivity C-reactive protein (hsCRP) and IL-6, predict the risk of ASCVD.36 Proof of principle that lowering inflammation with pharmacotherapy reduces cardiovascular events in the absence of lipid lowering came from the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). In CANTOS, treatment with canakinumab, a human monoclonal antibody directed against the proinflammatory cytokine IL-1β, significantly reduced IL-1β, IL-6, and hsCRP and demonstrated a cardiovascular (CV) benefit.37,38 Unlike CANTOS, the Cardiovascular Inflammation Reduction Trial (CIRT), which tested low-dose methotrexate among patients with established CAD and diabetes and/or MetS, did not reduce IL-1β, IL-6, or C-reactive protein (CRP), and did not result in fewer cardiovascular events compared with placebo.39
Collectively, the evidence from CANTOS and CIRT suggests that the prognostic value of anti-inflammatory agents might strongly depend on the inflammatory pathway targeted. While inhibition of IL-1β and IL-6-signaling, which are initiated at the level of the NLRP3 inflammasome,40 effectively reduced cardiovascular events in CANTOS—with human genetic data implicating these pathways as causal in atherothrombosis40—a treatment not targeting the IL-1β and IL-6-signaling pathway (i.e., CIRT) failed to show prognostic benefit.
Lifestyle link
As further development and evaluation of pharmacological agents targeting IL-6, IL-1β, and the NLRP3 inflammasome are under way, possible alternative strategies to support resolution of inflammation to lower risk of cardiovascular disease (CVD) are (1) to increase the precursors of SPMs through dietary supplementation of the marine n-3 fatty acids EPA and/or DHA19,29 and/or (2) to address chronic inflammation by lifestyle intervention.41 Vigorous physical activity, intermittent caloric restriction, and carbohydrate restriction can be anti-inflammatory due to elevations of the ketone β-hydroxybutyrate, an endogenous inhibitor of the NLRP3 inflammasome.42 Furthermore, contracting muscle secretes hormone-like substances termed myokines, which have the potential to attenuate immunosenescence through altered tissue crosstalk.43 These effects occur largely independent of body weight.
Insulin resistance/compensatory hyperinsulinemia/prediabetes and diabetes
Insulin resistance is the inability of target tissues to coordinate a normal glucose-lowering response at a normal plasma insulin level, an effect involving suppression of endogenous glucose production, suppression of lipolysis, cellular uptake of available plasma glucose, and net glycogen synthesis.44 Compensatory hyperinsulinemia is the consequence of insulin resistance. It means that when insulin resistance is present, fasting and postprandial plasma insulin levels stay chronically elevated and increase further following a glycemic load.44 This chronic state of exaggerated postprandial dysmetabolism, including elevated plasma insulin ± glucose and free fatty acids, creates a milieu conducive to the development of high-risk atherosclerosis and type 2 diabetes mellitus (T2DM), one of the major risk factors for ASCVD.45
As depicted in Figure 1, impaired insulin signaling affects processes in various tissues and organs relevant to ASCVD, including dysregulation of glucose and lipoprotein metabolism and ectopic fat accumulation44 (Fig. 1A1–A3). Additional features that have been associated with insulin resistance include inflammation/oxidative stress and inflammasome activation as discussed above46 (Fig. 1C), procoagulation/impaired fibrinolysis (Fig. 1F), and endothelial dysfunction (Fig. 1G).47 The procoagulatory state associated with diabetes has been linked to higher concentrations of plasminogen activator inhibitor-1, which reflects a state of fibrinolytic dysfunction in this phenotype. This provides biological plausibility for the increased predisposition of individuals with MetS to develop atherothrombosis.48,49 Endothelial dysfunction, a key antecedent of age-related CVD risk, is attributed to selective vascular insulin resistance, increased superoxide-related oxidative stress, and inflammation mediated by, for example, ApoC3.30 Endothelial dysfunction results in decreased bioavailability of the vascular protective vasodilatory molecule nitric oxide.47 Furthermore, hyperinsulinemia affects kidney function in a way that promotes uric acid and sodium retention and hypertension.50
T2DM constitutes the tip of the iceberg of this vicious cycle of insulin resistance and compensatory hyperinsulinemia. It represents a model for accelerated cardiovascular aging and leads to a substantial increase in risk for ASCVD, the leading cause of death in people with T2DM.45 In this regard, it should, however, be noted that insulin resistance and compensatory hyperinsulinemia have been linked to the pathogenesis of CHD and cardiometabolic endpoints even in the absence of diabetes mellitus.51,52
Lifestyle link
Numerous lifestyle factors, dietary and nondietary, negatively affect insulin sensitivity.41 Encouragingly, insulin resistance and compensatory hyperinsulinemia, even in overt T2DM, are reversible upon reduction of intrahepatic, intrapancreatic, and intramuscular fat storage pools as a result of dietary modification and increased physical activity.23,27,53,54
Lipoprotein metabolism
LDL-C, the concentration in plasma of cholesterol in LDL particles (LDL-P), is related exponentially to risk of ASCVD, and lowering levels pharmacologically has been shown to reduce the risk of cardiovascular events proportional to the magnitude of LDL-C reduction in numerous clinical trials. As a result of these relationships, LDL-C reduction has been the cornerstone of ASCVD prevention for decades.8 However, as described below, the LDL-C-centric concept does not adequately represent the spectrum of risk attributable to atherogenic lipoproteins.8,55
Atherogenic dyslipidemia/atherogenic lipoprotein phenotype in MetS
The lipoprotein pattern referred to as atherogenic dyslipidemia is the central lipoprotein phenotype associated with MetS, insulin resistance, T2DM, and visceral adiposity56,57 (Fig. 1A2). Both clinically and pathologically, it closely tracks with high-risk atherosclerosis and ASCVD.57,58
Atherogenic dyslipidemia encompasses a constellation of lipoprotein abnormalities, including high serum triglycerides and low HDL-C [mainly due to reduced large HDL particles (HDL-P)], as well as an atherogenic lipoprotein phenotype, including a predominance of small, cholesterol-depleted LDL-P, and an accumulation of triglyceride-rich remnant lipoproteins.59,60 As opposed to elevated apoB (the structural protein of all potentially atherogenic particles, including VLDL, intermediate-density lipoproteins [IDL], and LDL), levels of LDL-C are often not increased in this syndrome. This discordance can result in significant underestimation of ASCVD risk by reliance on LDL-C, and failure to adequately manage this risk in individuals with atherogenic dyslipidemia, and, more broadly, those with visceral adiposity and other features of MetS [5–7].
Pathophysiologically, the sequence of lipoprotein changes in atherogenic dyslipidemia is induced primarily by abnormalities of hepatic fat metabolism. Increased rates of de novo lipogenesis lead to an increased hepatic triglyceride pool and overproduction of large, triglyceride-enriched VLDL-P. The accumulation of these particles in plasma results in the formation of increased levels of lipolytic remnants and ultimately smaller LDL-P, as well as decreased HDL-C due to increased HDL-P catabolism.59 The consequences with respect to the initiation and progression of high-risk atherosclerosis are twofold. First, the presence of small LDL-P in atherogenic dyslipidemia contributes to a greater total number of circulating LDL-P, even when LDL-C is normal or low. Second, there is clinically relevant evidence that small LDL-P have properties that may render them more pathologic per se.61–63 Mechanistically, this has been linked to a longer residence time of small LDL-P in the circulation due to reduced receptor-mediated clearance, which exposes the endothelial lining to proinflammatory and proatherogenic particle components such as ApoC3.30,55 This concept is supported by the notion that a common underlying trait connecting lipoprotein metabolism to heart disease risk is the extent to which the condition influences the duration of exposure to arterial tissue, due to either the properties of the LDL-P (e.g., small LDL) or to reduced hepatic LDL receptor expression (e.g., in familial hypercholesterolemia). Further traits that have been associated with potentially higher atherogenicity of small LDL-P include compositional and conformational changes that render them more prone to retention,64 aggregation,65,66 and oxidation64 in the arterial wall. Overall, there is not incontrovertible evidence supporting the concept that particle for particle, a small LDL-P (<25 nm) is more atherogenic than a large LDL-P. However, biological plausibility as well as clinical evidence, as described below, justifies consideration of small LDL-P as an informative marker of CVD risk in MetS.67
A number of studies have addressed the question as to whether the measurement of larger versus smaller LDL-P adds incremental information to standard lipid measurements with respect to cardiovascular outcomes. In the Quebec Cardiovascular Study, levels of small LDL-P were independently associated with CHD risk in 2072 men over a 13-year follow-up; in contrast, large LDL-P had no predictive value.62 In line with these data, two prospective analyses from the Atherosclerosis Risk in Communities (ARIC) and the Multi-ethnic Study of Atherosclerosis (MESA) cohorts showed a progressive increase in CHD risk over quartiles of small dense LDL cholesterol (sdLDL-C) levels. In line with the results from the Quebec Cardiovascular Study, there was no relationship with large LDL-P.61,68 In the ARIC study, among 11,419 men and women during a mean follow-up of about 11 years, sdLDL-C was significantly associated with incident CHD after adjusting for standard nonlipid CHD risk factors even in individuals with LDL-C levels <100 mg/dL.61 Similarly, in 4387 normoglycemic individuals in the MESA cohort followed for a mean of 8.5 years, elevated sdLDL-C was a risk factor for developing CHD after adjusting for standard CHD risk factors, triglycerides, and HDL-C.68 Collectively, these data support the concept of greater atherogenic risk associated with smaller versus larger LDL-P.
In patients living with MetS, sdLDL particles are strong predictors of cardiovascular and cerebrovascular events beyond traditional cardiovascular risk factors.69 In this subgroup, improving the quality of lipoproteins may represent an independent target to reduce cardiovascular risk beyond lowering the quantity of lipoproteins.70
Lifestyle link
Notably, levels of small LDL-P are primarily responsive to dietary carbohydrate intake (increase with higher carbohydrate consumption), while large LDL-P are more responsive to dietary saturated fat (increase with higher saturated fat consumption). Both weight loss and carbohydrate restriction decrease the expression of the small LDL-P pathway.71 These considerations provide some biological plausibility for the observation that in large populations, higher dietary saturated fat consumption is associated with higher LDL-C, but not with higher all-cause or CVD mortality.72 LDL-C might thus provide misleading information as to the effect of diet on ASCVD risk and may therefore be an inappropriate marker for informing dietary advice.73,74 A further level of complexity regarding recommendations for dietary fat intake is the fact that dietary fats comprise heterogeneous molecules with diverse structures even within conventional fat classes (i.e., saturated, monounsaturated, and polyunsaturated fatty acids).74 For example, odd-chain SFAs (C15:0 and C17:0) are relatively unique to dairy fat, are not synthesized by humans, and are therefore regarded as reasonable biomarkers of dairy fat consumption. Cohort studies measuring these biomarkers of dairy fat intake show associations with protection against diabetes, and meta-analytic evidence from prospective studies suggest that C17:0, but not C15:0, intake is inversely associated with the risk of CVD.74 Collectively, this does not speak to restricting all food sources of dietary saturated fat for cardiometabolic health and supports food-based, instead of nutrient-based dietary recommendations.73
HDL-C and HDL subclasses
While clinical and epidemiological evidence have consistently shown an inverse association between HDL-C levels and ASCVD,75,76 the failure of clinical trials targeting HDL-C to show prognostic benefit,77 in conjunction with genetic evidence,78 supports the notion that HDL-C is not causal in ASCVD. It is, however, worth mentioning that features of HDL other than its cholesterol content are of pathophysiological importance, most notably, the capacity of its major apoprotein component, apoAI, to promote cellular cholesterol efflux.79 While there is limited evidence that clinical measurement of HDL size subclasses provides information bearing on specific functional properties of HDL, it is of interest that small HDL-P have recently been reported to be associated with increased coronary plaque stability.80
Clinical assessment of ASCVD risk—beyond LDL-C
Lipid and lipoprotein measurements
As noted above, a measurement derived from a standard lipid panel that is more strongly related to ASCVD risk than LDL-C, particularly in patients with features of the ectopic fat phenotype, is non-HDL-C (total cholesterol minus HDL-C), which comprises the cholesterol content of VLDL and atherogenic remnant lipoproteins, in addition to LDL.72 However, measurements of plasma apoB5–7 and/or LDL-P subclasses55 provide more specific measures of atherogenic lipoprotein burden that can also serve as guides for therapeutic management of ASCVD risk. In this regard, LDL-P subclass analysis has the potential to detect atherogenic lipoprotein patterns, which are below the discrimination level of standard lipid testing in MetS.60
Another metabolic index that can be derived from standard laboratory assays is triglyceride to HDL-C (TG/HDL-C) ratio, which is associated with both insulin resistance and measures of atherogenic dyslipidemia, including smaller LDL-P diameter (also designated LDL subclass phenotype B)81 and higher remnant lipoprotein particle cholesterol.61 Furthermore, the TG/HDL-C ratio has been linked to plaque phenotype and clinical stability in coronary artery disease: the ratio was significantly higher in patients with TCFAs than in those without, and it was nonsignificantly higher in patients with multiple recurrent acute coronary syndromes than in those with long-standing stable angina.82–84 Of note, triglycerides and the TG/HDL-C ratio do not reliably predict insulin resistance in African Americans. This has been linked to the observation that insulin resistance does not impair lipoprotein lipase in this subgroup and thus does not induce hypertriglyceridemia.85
Hypertriglyceridemic waist
At any given BMI, an elevated waist circumference is predictive of visceral adiposity.13 Using waist circumference as a proxy, increased ectopic and hepatic fat22 in particular, is an independent risk factor for high-risk atherosclerosis9,10 and coronary artery disease and death in prospective studies.45 Mendelian randomization analyses indicate that triglyceride-related risk is causal in ASCVD and considering triglyceride levels along with waist circumference improves risk prediction.8,78 Hypertriglyceridemic waist, a visceral adiposity marker combining elevated waist circumference (≥90 cm) and elevated fasting plasma triglycerides (≥2 mmol/L), thus has good diagnostic accuracy for the identification of individuals at high risk of ASCVD.86
Lifestyle link
Adequate intake of EPA+DHA and/or EPA only at doses of >3 and 4 g/day, respectively, is an effective and safe option for lowering triglycerides87 and hepatic fat content in NAFLD.41,88
Inflammatory markers
While CRP is not thought to have a causal role in ASCVD, its measurement may augment overall ASCVD risk assessment, as it is a downstream marker of the IL-1β–IL-6 pathway, for which there is evidence of causality.40 Thus, measurement of high-sensitivity (hs) CRP may have a role in assessing ASCVD risk in patients with features of MetS, in whom other measurements are inconclusive.
Conclusions
While atherosclerosis is in large measure a lipoprotein-driven disease, there are numerous factors that modify that causality. In particular, in the setting of excess ectopic fat accumulation, metabolic stress fosters a milieu conducive for high-risk atherosclerosis and its clinical complications: dysfunctional AT phenotype and secretome, chronic low-grade inflammation, atherogenic dyslipidemia, impaired fibrinolysis, and endothelial dysfunction.89 This combination of high-risk metabolic and inflammatory traits can be inexpensively detected by combining anthropometric measures and clinically available biomarker panels.
Authors' Contributions
All authors listed have contributed sufficiently to the article to be included as authors, and all those who are qualified to be authors are listed in the author byline. K.L. did the literature search, and drafted the article. N.K., C.v.S., N.W., U.N., B.L., J.S., and O.W. reviewed and edited the article. A.L.M. and R.M.K contributed significantly to the writing process. R.M.K. supervised the writing process and did major revisions. All authors approved the final version of the article.
Acknowledgment
The authors are indebted to Nicola Bernhart for graphical design.
Author Disclosure Statement
K.L., N.K., N.W., U.N., B.L., J.S., and O.W. declare that no competing financial interests exist with respect to this article. A.L.M. is employed by Virta Health and has been offered stock options. C.v.S. operates Omegametrix, a laboratory for fatty acid analyses. He consults for BASF/Pronova, and Huntsworth Medical, and received speaker's honoraria from Abbott, DSM, and Norsan. R.M.K. is on the Scientific Advisory Board of Virta Health and Day Two, has grant support from Quest Diagnostics and Dairy Management, Inc., and has a licensed patent for lipoprotein particle analysis by ion mobility.
Funding Information
No funding was received for this article.
References
- 1. GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1459–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Davidson MH, Ballantyne CM, Jacobson TA, et al. Clinical utility of inflammatory markers and advanced lipoprotein testing: Advice from an expert panel of lipid specialists. J Clin Lipidol 2011;5:338–367 [DOI] [PubMed] [Google Scholar]
- 3. Mora S, Martin Seth S, Virani Salim S. Cholesterol insights and controversies from the UK Biobank Study. Circulation 2019;140:553–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: Update and therapeutic implications. Circulation 2007;116:1832–1844 [DOI] [PubMed] [Google Scholar]
- 5. Sniderman AD, Pencina M, Thanassoulis G. ApoB. Circ Res 2019;124:1425–1427 [DOI] [PubMed] [Google Scholar]
- 6. Sniderman AD, Toth PP, Thanassoulis G, et al. An evidence-based analysis of the National Lipid Association recommendations concerning non-HDL-C and apoB. J Clin Lipidol 2016;10:1248–1258 [DOI] [PubMed] [Google Scholar]
- 7. Sniderman AD, Thanassoulis G, Glavinovic T, et al. Apolipoprotein B particles and cardiovascular disease: A narrative review. JAMA Cardiol 2019. [Epub ahead of print]; DOI: 10.1001/jamacardio.2019.3780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ganda OP, Bhatt DL, Mason RP, et al. Unmet need for adjunctive dyslipidemia therapy in hypertriglyceridemia management. J Am Coll Cardiol 2018;72:330–343 [DOI] [PubMed] [Google Scholar]
- 9. Oikonomou EK, Antoniades C. The role of adipose tissue in cardiovascular health and disease. Nat Rev Cardiol 2019;16:83–99 [DOI] [PubMed] [Google Scholar]
- 10. Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med 2014;371:2237–2238 [DOI] [PubMed] [Google Scholar]
- 11. Fiuza-Luces C, Santos-Lozano A, Joyner M, et al. Exercise benefits in cardiovascular disease: Beyond attenuation of traditional risk factors. Nat Rev Cardiol 2018;15:731–743 [DOI] [PubMed] [Google Scholar]
- 12. Pedersen BK, Febbraio MA. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat Rev Endocrinol 2012;8:457–465 [DOI] [PubMed] [Google Scholar]
- 13. Despres JP. Body fat distribution and risk of cardiovascular disease: An update. Circulation 2012;126:1301–1313 [DOI] [PubMed] [Google Scholar]
- 14. Al-Mrabeh A, Zhyzhneuskaya SV, Peters C, et al. Hepatic lipoprotein export and remission of human type 2 diabetes after weight loss. Cell Metab 2019. [Epub ahead of print]; DOI: 10.1016/j.cmet.2019.11.018 [DOI] [PubMed] [Google Scholar]
- 15. Chen GC, Arthur R, Iyengar NM, et al. Association between regional body fat and cardiovascular disease risk among postmenopausal women with normal body mass index. Eur Heart J 2019;40:2849–2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Priest C, Tontonoz P. Inter-organ cross-talk in metabolic syndrome. Nat Metab 2019;1:1177–1188 [DOI] [PubMed] [Google Scholar]
- 17. Wang Z, Wang D, Wang Y. Cigarette smoking and adipose tissue: The emerging role in progression of atherosclerosis. Mediators Inflamm 2017;2017:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pagano ES, Spinedi E, Gagliardino JJ. White adipose tissue and circadian rhythm dysfunctions in obesity: Pathogenesis and available therapies. Neuroendocrinology 2017;104:347–363 [DOI] [PubMed] [Google Scholar]
- 19. Ferrucci L, Fabbri E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol 2018;15:505–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Packer M. Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol 2018;71:2360–2372 [DOI] [PubMed] [Google Scholar]
- 21. Hwang DH, Kim JA, Lee JY. Mechanisms for the activation of Toll-like receptor 2/4 by saturated fatty acids and inhibition by docosahexaenoic acid. Eur J Pharmacol 2016;785:24–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stahl EP, Dhindsa DS, Lee SK, et al. Nonalcoholic fatty liver disease and the heart. J Am Coll Cardiol 2019;73:948. [DOI] [PubMed] [Google Scholar]
- 23. White MG, Shaw JA, Taylor R. Type 2 diabetes: The pathologic basis of reversible beta-cell dysfunction. Diabetes Care 2016;39:2080–2088 [DOI] [PubMed] [Google Scholar]
- 24. Fournet M, Bonté F, Desmoulière A. Glycation damage: A possible hub for major pathophysiological disorders and aging. Aging Dis 2018;9:880–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging, and disease. N Engl J Med 2019;381:2541–2551 [DOI] [PubMed] [Google Scholar]
- 26. Di Francesco A, Di Germanio C, Bernier M, et al. A time to fast. Science 2018;362:770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gepner Y, Shelef I, Schwarzfuchs D, et al. Effect of distinct lifestyle interventions on mobilization of fat storage pools: The CENTRAL MRI randomized controlled trial. Circulation 2018;137:1143–1157 [DOI] [PubMed] [Google Scholar]
- 28. Tsaban G, Wolak A, Avni-Hassid H, et al. Dynamics of intrapericardial and extrapericardial fat tissues during long-term, dietary-induced, moderate weight loss. Am J Clin Nutr 2017;106:984–995 [DOI] [PubMed] [Google Scholar]
- 29. Kasikara C, Doran AC, Cai B, et al. The role of non-resolving inflammation in atherosclerosis. J Clin Invest 2018;128:2713–2723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zewinger S, Reiser J, Jankowski V, et al. Apolipoprotein C3 induces inflammation and organ damage by alternative inflammasome activation. Nat Immunol 2020;21:30–41 [DOI] [PubMed] [Google Scholar]
- 31. Lai HTM, de Oliveira Otto MC, Lee Y, et al. Serial plasma phospholipid fatty acids in the de novo lipogenesis pathway and total mortality, cause-specific mortality, and cardiovascular diseases in the cardiovascular health study. J Am Heart Assoc 2019;8:e012881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bäck M, Yurdagul A, Tabas I, et al. Inflammation and its resolution in atherosclerosis: Mediators and therapeutic opportunities. Nat Rev Cardiol 2019;16:389–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dalli J, Serhan C. Macrophage proresolving mediators-the when and where. Microbiol Spectr 2016;4:10..1128/microbiolspec.MCHD-0001-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tselepis AD, John Chapman M. Inflammation, bioactive lipids and atherosclerosis: Potential roles of a lipoprotein-associated phospholipase A2, platelet activating factor-acetylhydrolase. Atheroscler Suppl 2002;3:57–68 [DOI] [PubMed] [Google Scholar]
- 35. Wallentin L, Held C, Armstrong PW, et al. Lipoprotein-associated phospholipase A2 activity is a marker of risk but not a useful target for treatment in patients with stable coronary heart disease. J Am Heart Assoc 2016;5:e003407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ridker PM, Luscher TF. Anti-inflammatory therapies for cardiovascular disease. Eur Heart J 2014;35:1782–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131 [DOI] [PubMed] [Google Scholar]
- 38. Ridker PM, Libby P, MacFadyen JG, et al. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: Analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). Eur Heart J 2018;39:3499–3507 [DOI] [PubMed] [Google Scholar]
- 39. Ridker PM, Everett BM, Pradhan A, et al. Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med 2019;380:752–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Libby P, Everett B. Novel antiatherosclerotic therapies. Arterioscler Thromb Vasc Biol 2019;39:538–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lechner K, von Schacky C, McKenzie AL, et al. Lifestyle factors and high-risk atherosclerosis: Pathways and mechanisms beyond traditional risk factors. Eur J Prev Cardiol 2019. [Epub ahead of print]; DOI: 10.1177/2047487319869400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med 2015;21:263–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Duggal NA, Niemiro G, Harridge SDR, et al. Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity? Nat Rev Immunol 2019;19:563–572 [DOI] [PubMed] [Google Scholar]
- 44. Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev 2018;98:2133–2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chia CW, Egan JM, Ferrucci L. Age-related changes in glucose metabolism, hyperglycemia, and cardiovascular risk. Circ Res 2018;123:886–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shah MS, Brownlee M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ Res 2016;118:1808–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Scholz GH, Hanefeld M. Metabolic vascular syndrome: New insights into a multidimensional network of risk factors and diseases. Visc Med 2016;32:319–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Anand SS, Yi Q, Gerstein H, et al. Relationship of metabolic syndrome and fibrinolytic dysfunction to cardiovascular disease. Circulation 2003;108:420–425 [DOI] [PubMed] [Google Scholar]
- 49. Meigs JB, Mittleman MA, Nathan DM, et al. Hyperinsulinemia, hyperglycemia, and impaired hemostasis The Framingham Offspring Study. JAMA 2000;283:221–228 [DOI] [PubMed] [Google Scholar]
- 50. Quinones-Galvan A, Ferrannini E. Renal effects of insulin in man. J Nephrol 1997;10:88–191 [PubMed] [Google Scholar]
- 51. Reaven G. Insulin resistance and coronary heart disease in nondiabetic individuals. Arterioscler Thromb Vasc Biol 2012;32:1754–1759 [DOI] [PubMed] [Google Scholar]
- 52. Caporaso NE, Jones RR, Stolzenberg-Solomon RZ, et al. Insulin resistance in healthy U.S. adults: Findings from the National Health and Nutrition Examination Survey (NHANES). Cancer Epidemiol Biomarkers Prev 2020;29:157–168 [DOI] [PubMed] [Google Scholar]
- 53. Taylor R, Al-Mrabeh A, Zhyzhneuskaya S, et al. Remission of human type 2 diabetes requires decrease in liver and pancreas fat content but is dependent upon capacity for β cell recovery. Cell Metab 2018;28:547–556.e3. [DOI] [PubMed] [Google Scholar]
- 54. Taylor R, Barnes AC. Translating aetiological insight into sustainable management of type 2 diabetes. Diabetologia 2018;61:273–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Krauss RM. All low-density lipoprotein particles are not created equal. Arterioscler Thromb Vasc Biol 2014;34:959–961 [DOI] [PubMed] [Google Scholar]
- 56. Krauss RM. Insulin resistance syndrome and dyslipidemia. Endocrine Pract 2003;9 Suppl 2:67–72 [DOI] [PubMed] [Google Scholar]
- 57. Lemieux I, Pascot A, Couillard C, et al. Hypertriglyceridemic waist: A marker of the atherogenic metabolic triad (hyperinsulinemia; hyperapolipoprotein B; small, dense LDL) in men? Circulation 2000;102:179–184 [DOI] [PubMed] [Google Scholar]
- 58. Ronald M. Krauss M. Insulin resistance syndrome and dyslipidemia. Endocrine Pract 2003;9(Supplement 2):67–72 [DOI] [PubMed] [Google Scholar]
- 59. Adiels M, Olofsson SO, Taskinen MR, et al. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol 2008;28:1225–1236 [DOI] [PubMed] [Google Scholar]
- 60. Rizzo M, Berneis K. Small, dense low-density-lipoproteins and the metabolic syndrome. Diabetes Metab Res Rev 2007;23:14–20 [DOI] [PubMed] [Google Scholar]
- 61. Hoogeveen RC, Gaubatz JW, Sun W, et al. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: The Atherosclerosis Risk In Communities (ARIC) study. Arterioscler Thromb Vasc Biol 2014;34:1069–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. St-Pierre AC, Cantin B, Dagenais GR, et al. Low-density lipoprotein subfractions and the long-term risk of ischemic heart disease in men: 13-year follow-up data from the Quebec Cardiovascular Study. Arterioscler Thromb Vasc Biol 2005;25:553–559 [DOI] [PubMed] [Google Scholar]
- 63. Mora S, Caulfield MP, Wohlgemuth J, et al. Atherogenic lipoprotein subfractions determined by ion mobility and first cardiovascular events after random allocation to high-intensity statin or placebo: The justification for the use of statins in prevention: An intervention trial evaluating rosuvastatin (JUPITER) trial. Circulation 2015;132:2220–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Berneis KK, Krauss RM. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res 2002;43:1363–1379 [DOI] [PubMed] [Google Scholar]
- 65. Laufs U, Weingartner O. Pathological phenotypes of LDL particles. Eur Heart J 2018;39:2574–2576 [DOI] [PubMed] [Google Scholar]
- 66. Ruuth M, Nguyen SD, Vihervaara T, et al. Susceptibility of low-density lipoprotein particles to aggregate depends on particle lipidome, is modifiable, and associates with future cardiovascular deaths. Eur Heart J 2018;39:2562–2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tehrani DM, Zhao Y, Blaha MJ, et al. Discordance of low-density lipoprotein and high-density lipoprotein cholesterol particle versus cholesterol concentration for the prediction of cardiovascular disease in patients with metabolic syndrome and diabetes mellitus (from the Multi-Ethnic Study of Atherosclerosis [MESA]). Am J Cardiol 2016;117:1921–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tsai MY, Steffen BT, Guan W, et al. New automated assay of small dense low-density lipoprotein cholesterol identifies risk of coronary heart disease: The Multi-ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol 2014;34:196–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rizzo M, Pernice V, Frasheri A, et al. Small, dense low-density lipoproteins (LDL) are predictors of cardio- and cerebro-vascular events in subjects with the metabolic syndrome. Clin Endocrinol 2009;70:870–875 [DOI] [PubMed] [Google Scholar]
- 70. Nikolic D, Katsiki N, Montalto G, et al. Lipoprotein subfractions in metabolic syndrome and obesity: Clinical significance and therapeutic approaches. Nutrients 2013;5:928–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Krauss RM, Blanche PJ, Rawlings RS, et al. Separate effects of reduced carbohydrate intake and weight loss on atherogenic dyslipidemia. Am J Clin Nutr 2006;83:1025–1031; quiz 205. [DOI] [PubMed] [Google Scholar]
- 72. Mente A, Dehghan M, Rangarajan S, et al. Association of dietary nutrients with blood lipids and blood pressure in 18 countries: A cross-sectional analysis from the PURE study. Lancet Diabetes Endocrinol 2017;5:774–787 [DOI] [PubMed] [Google Scholar]
- 73. Astrup A, Bertram HC, Bonjour JP, et al. WHO draft guidelines on dietary saturated and trans fatty acids: Time for a new approach? BMJ 2019;366:l4137. [DOI] [PubMed] [Google Scholar]
- 74. Wu JHY, Micha R, Mozaffarian D. Dietary fats and cardiometabolic disease: Mechanisms and effects on risk factors and outcomes. Nat Rev Cardiol 2019;16:581–601 [DOI] [PubMed] [Google Scholar]
- 75. Di Angelantonio E, Sarwar N, Perry P, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA 2009;302:1993–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Acharjee S, Boden WE, Hartigan PM, et al. Low levels of high-density lipoprotein cholesterol and increased risk of cardiovascular events in stable ischemic heart disease patients: A post-hoc analysis from the COURAGE Trial (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation). J Am Coll Cardiol 2013;62:1826–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Keene D, Price C, Shun-Shin MJ, et al. Effect on cardiovascular risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: Meta-analysis of randomised controlled trials including 117,411 patients. BMJ 2014;349:g4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Holmes MV, Asselbergs FW, Palmer TM, et al. Mendelian randomization of blood lipids for coronary heart disease. Eur Heart J 2015;36:539–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Silbernagel G, Pagel P, Pfahlert V, et al. High-density lipoprotein subclasses, coronary artery disease, and cardiovascular mortality. Clin Chem 2017;63:1886–1896 [DOI] [PubMed] [Google Scholar]
- 80. Wang X, Liu X, Xie Z, et al. Small HDL subclass is associated with coronary plaque stability: An optical coherence tomography study in patients with coronary artery disease. J Clin Lipidol 2019;13:326–334.e2. [DOI] [PubMed] [Google Scholar]
- 81. McLaughlin T, Reaven G, Abbasi F, et al. Is there a simple way to identify insulin-resistant individuals at increased risk of cardiovascular disease? Am J Cardiol 2005;96:399–404 [DOI] [PubMed] [Google Scholar]
- 82. Lechner K, Halle M.. Are atherogenic lipoprotein phenotype and inflammation indicative of plaque phenotype and clinical stability in coronary artery disease? JAMA Cardiology 2019. [Epub ahead of print]; DOI: 10.1001/jamacardio.2019.2261 [DOI] [PubMed] [Google Scholar]
- 83. Vergallo R, Porto I, Crea F. Are atherogenic lipoprotein phenotype and inflammation indicative of plaque phenotype and clinical stability in coronary artery disease?-reply. JAMA Cardiol 2019;4(9):951-952 [DOI] [PubMed] [Google Scholar]
- 84. Vergallo R, Porto I, D'Amario D, et al. Coronary atherosclerotic phenotype and plaque healing in patients with recurrent acute coronary syndromes compared with patients with long-term clinical stability: An in vivo optical coherence tomography study. JAMA Cardiol 2019;4:321–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sumner AE, Finley KB, Genovese DJ, et al. Fasting triglyceride and the triglyceride-HDL cholesterol ratio are not markers of insulin resistance in African Americans. Arch Int Med 2005;165:1395–1400 [DOI] [PubMed] [Google Scholar]
- 86. LeBlanc S, Coulombe F, Bertrand OF, et al. Hypertriglyceridemic waist: A simple marker of high-risk atherosclerosis features associated with excess visceral adiposity/ectopic fat. J Am Heart Assoc 2018;7:e008139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Skulas-Ray AC, Wilson PWF, Harris WS, et al. Omega-3 fatty acids for the management of hypertriglyceridemia: A science advisory from the American Heart Association. Circulation 2019;140:e673–e691 [DOI] [PubMed] [Google Scholar]
- 88. Yan JH, Guan BJ, Gao HY, et al. Omega-3 polyunsaturated fatty acid supplementation and non-alcoholic fatty liver disease: A meta-analysis of randomized controlled trials. Medicine 2018;97:e12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ren J, Sowers JR, Zhang Y. Metabolic stress, autophagy, and cardiovascular aging: From pathophysiology to therapeutics. Trends Endocrinol Metab 2018;29:699–711 [DOI] [PMC free article] [PubMed] [Google Scholar]