Abstract
Heparin-binding EGF-like growth factor (HB-EGF) is an EGF family member that interacts with epidermal growth factor receptor (EGFR) and ERBB4. Since HB-EGF was first identified as a novel growth factor secreted from a human macrophage cell line, numerous pathological and physiological functions related to cell proliferation, migration, and inflammation have been reported. Notably, the expression of HB-EGF is sensitively upregulated by oxidative stress in the endothelial cells and functions for auto- and paracrine-EGFR signaling. Overnutrition and obesity cause elevation of HB-EGF expression and EGFR signaling in the hepatic and vascular systems. Modulations of HB-EGF signaling showed a series of protections against phenotypes related to metabolic syndrome and advanced metabolic diseases, suggesting HB-EGF as a potential target against metabolic diseases.
Keywords: HB-EGF, endothelial cells, metabolic syndrome, atherosclerosis, NAFLD
Introduction
Heparin-binding EGF-like growth factor (HB-EGF) is a primary protein in the macrophage cell supernatants that interacts with the heparin column.1 Later, it was shown to be a member of the EGF family that interacts with epidermal growth factor receptor (EGFR) and ERBB4.2–4 The HB-EGF-EGFR pathway induces multiple signaling pathways depending on cell types, including ERK1/2, PI3K-Akt, PLC-γ, and STATs.5 HB-EGF is expressed as a type I transmembrane protein on the cell surface.4,6 A list of metalloproteinases (MPs) activates the HB-EGF for auto- or paracrine-signaling.7 Mature HB-EGF still contains a heparin-binding motif; thus, it interacts with cell surface proteoglycan.1,6,8,9 Membrane-tethered HB-EGF can form a complex with neighbor cell EGFR for juxtacrine signaling.4,10 Oxidative stress inducers sensitively upregulate the HB-EGF transcription in the endothelial cells.11,12 HB-EGF expression in bone marrow stromal cells increases the proliferation of the hematopoietic stem cells and progenitor cells (HSPCs).13,14 Recent research results indicate that increased myeloid cell production in bone marrow is a significant feature in the development of metabolic diseases.15,16 The regulatory function of HB-EGF in hematopoiesis may be a connection of oxidative stress with induction of low-grade inflammation under metabolic stress environments. We also summarized recent reports on the potential of HB-EGF targeting against the advancement of metabolic diseases (Table 1). Different approaches of HB-EGF targeting induced a list of protective phenotypes in the animal and human studies, suggesting HB-EGF as a potential target for therapeutic purposes.
Table 1.
Functions of Heparin-Binding EGF-Like Growth Factor Related to Phenotypes of Metabolic Syndrome and Diseases
| Physiological and pathophysiological function | Cell type or tissues involved | Refs. |
|---|---|---|
| Interaction with EGFR and ERBB4 for cell signaling | Multiple cell types that express EGFR and ERBB4 | 1 |
| Induction of proliferation of HSPCs | Bone marrow hematopoietic and stromal cells | 13,14 |
| Development of the heart structure and function | Endothelial cells | 17 |
| Liver function | Hepatocytes | 22–24 |
| Renal function | Renal endothelial cells | 19 |
| Renal podocytes | 21 | |
| Renal tubular epithelial cells | 112,116 | |
| EGFR transactivation | Multiple cell types under stress environments | 12,29 |
| Induction of insulin resistance | Adipocytes and skeletal muscle cells | 37 |
| Interaction with adiponectin | Vascular smooth muscle cells | 82,83 |
| Induction of neointimal thickness | Vascular smooth muscle cells | 49 |
| Regulation of hepatic VLDL production | LSECs or hepatocytes | 92 |
| Regulation of inflammatory cytokine expression | Vascular endothelial cells | 34 |
| Atherosclerosis and aneurysm development | Aortic smooth muscle cells | 61,98,99,102,103 |
| Interaction with diphtheria toxin | Multiple cell types | 134 |
EGFR, epidermal growth factor receptor; HSPCs, hematopoietic stem and progenitor cells; LSECs, liver sinusoidal endothelial cells; VLDL, very-low-density lipoprotein.
Effects of the HB-EGF gene deletion and overexpression in metabolic disease phenotypes
Germline deletion of HB-EGF in systemic or vascular endothelium caused cardiac hypertrophy with gross enlargement of ventricular chambers of the heart17,18; however, the postnatal induction of HB-EGF gene deletion did not induce the deleterious effects.19–21 Although the basal level of HB-EGF expression in the hepatocytes is low, hepatocyte-specific HB-EGF gene deletion induced an increase of inflammation and fibrosis in the liver.22–24 Interestingly, HB-EGF overexpression also enhanced the induction of liver damage,25 suggesting that a physiological range of HB-EGF expression is essential for a healthy liver. The endothelial cell-specific HB-EGF gene deletion protected against both diabetic- and angiotensin-II (AngII)-induced renal disease phenotypes in animal models.19,26 The podocyte-specific HB-EGF gene deletion also induced protection against acute renal disease in a mouse model.21
HB-EGF mediates EGFR transactivation under oxidative stress
Reactive oxygen species (ROS) formations and activation of MPs are involved in EGFR transactivation in the cells and tissues under oxidative stress environments.3,12,27,28 Numerous reports indicated a crucial role of HB-EGF in the EGFR transactivation by oxidative stress inducers,3,29,30 including AngII,31 catecholamines,32 and lipid oxidation products.33,34 Differently to the canonical ligand-activated signaling, the stress-induced EGFR transactivation showed a low-level but prolonged signaling with minimal internalization or desensitization of EGFR.30 Although there are >10 ligands for EGFR, the HB-EGF almost exclusively mediates EGFR transactivation under various stress environments,4,35 suggesting that the HB-EGF is a specialized and conserved mediator for the connection of oxidative stress with cell signaling.3,36 A report indicated that the EGFR was activated in <1 min by endothelin-1, suggesting a mechanism of transcription-independent fashion for the transactivation process.37 In addition, the upregulation of HB-EGF expression would contribute to the sustained EGFR signaling.12 The transcription of HB-EGF is mainly controlled by a stress signal associated activator protein-1 (AP-1) transcriptional factor in the cells.11,38 Oxidative stress inducers also upregulated a list of MPs involved in the HB-EGF processing on the cell surface of endothelial cells.7,12,34
The obese individuals showed accumulations of oxidation products of phospholipid in the adipose and skeletal muscle tissues.39–41 The oxidation products activated AP-1 in the endothelial cells, as demonstrated by a recent Chip-Seq analysis.42 AngII is a well-known oxidative stress inducer via ROS production through the NADPH oxidase (NOX) system in the vascular smooth muscle cells.43,44 Correspondingly, AngII induces EGFR transactivation in the cells via HB-EGF mediation.7,45,46 The EGFR transactivation was an underlying mechanism for the proliferation and migration of vascular smooth muscle or glomerular mesangial cells under the stress conditions.35,45,47,48 Unsaturated lysophosphatidic acid also induced intimal thickness in the carotid artery via HB-EGF-mediated EGFR transactivation.29,30,49,50
Role of HB-EGF in the Development of Metabolic Syndrome and Low-Grade Inflammation
The metabolic syndrome is a cluster of metabolic dysfunctions, including central obesity, insulin resistance, dyslipidemia with a manifestation of hypertriglyceridemia, and hypertension.51–53 Downregulation of high-density lipoprotein (HDL) and production of small dense low-density lipoprotein (sdLDL) are frequently associated because of the enzyme activity of cholesteryl ester transfer protein, specifically in humans.53,54
Hyperlipidemia, particularly hypercholesterolemia, is closely associated with the proliferation of HSPCs and the production of myeloid cell progenitors in the bone marrow.15,55–57 Under obesity, there are increases in bone marrow-derived monocytes in the bloodstream (monocytosis) and accumulation of proinflammatory macrophages in the adipose tissue.58 There is also an increase in the local proliferation of macrophages in the atherosclerotic lesion.59
Role of HB-EGF in the development of low-grade inflammation
The oxidation products of phospholipids induced the upregulation of HB-EGF in the vascular wall, causing inflammatory responses.60,61 Also, the products of phospholipid peroxidation upregulated the expression of inflammatory cytokines (IL-8 and MCP1/CCL2) and cell adhesion molecules (e.g., ICAM1) in the endothelial cells, leading to the recruitment of bone marrow-derived monocytes into the subendothelial space.62,63
Under homeostatic conditions, the liver sinusoidal endothelial cells (LSECs) effectively clear any harmful oxidants in circulation, such as oxidized LDL particles (Ox-LDLs) and advanced glycation end products (AGEs).64–67 However, a sustained influx of oxidants overproduced in the systemic or splanchnic circulation may induce saturation and activation of the endothelial cells.68 The saturation of LSECs leads to the activation of extrahepatic endothelial cells and chronic inflammation by prolonged exposure to proinflammatory oxidants.
Krampera et al. demonstrated that HB-EGF is a crucial regulator for the self-renewal of hematopoietic stem cells (HSCs) in the bone marrow.13,14 The coordination of HB-EGF and CXCL12/SDF-1 in the hematopoietic niches is a determinant for stem cell proliferation and blood cell production in the bone marrow.14 Various exogenous and endogenous stress inducers, including phorbol myristate acetate and tumor necrosis factor-alpha, upregulated HB-EGF gene expression in the bone marrow. Bone marrow sinusoidal endothelial cells also effectively endocytose oxidants, including minimally oxidized LDLs and AGEs.64,67 The upregulation of HB-EGF expression in the sinusoidal endothelial cells may lead to the proliferation of HSPCs and the production of myeloid progenitor cells.69 The endothelial permeability also increased by oxidants,70 which would lead to the increased mobilization of the HSPCs and immune cells from the bone marrow tissues into circulation.71
Role of HB-EGF in the development of insulin resistance
Insulin resistance is a central phenotype of metabolic syndrome and is frequently associated with systemic oxidative stress and low-grade inflammation.53,72 HB-EGF was shown to be involved in the development of insulin resistance by oxidative stress inducers, including endothelin-1, thrombin, and 5-hydroxytryptamine (serotonin) in the adipocytes and skeletal muscle cells.73 Obesity is a risk factor for the accumulation of ROS in adipose, skeletal muscle tissues, and circulation.39,40,51 A list of phospholipid peroxidation products also induced insulin resistance in the primary culture of adipocytes and skeletal muscle cells.41,74–76 The obese adipose tissue is enriched with proinflammatory M1 macrophages.39 Concordantly, the HB-EGF gene expression is upregulated in adipose tissue in obese persons.77 Adiponectin is an established adipokine that is a potent insulin sensitizer.78,79 The adiponectin level inversely correlated with systemic oxidative stress and visceral obesity.51,80 Intriguingly, adiponectin directly interacts with HB-EGF for sequestering,81–83 which may partly explain the anti-atherogenic and anti-inflammatory functions of adiponectin.84
The role of HB-EGF in the induction of dyslipidemia
Dyslipidemia, as manifested by hypertriglyceridemia and the reduction of HDL, is the earliest event of metabolic syndrome in obese people.85 Hepatic very-low-density lipoprotein (VLDL) overproduction is a common feature of hypertriglyceridemia in obese individuals.53 HB-EGF is mainly expressed in the LSECs in the liver tissue.86,87 The antisense oligonucleotide (ASO) with a phosphorothioate modification is effectively uptaken by LSECs with effective induction of target gene silencing in the cells.88–91 A recent report showed that the HB-EGF ASO administration induced a competent downregulation of circulatory triglyceride (TG) levels by suppressing hepatic VLDL production in a mouse model.92 Under the obesity condition, the elevated HB-EGF expression in the LSECs may enhance VLDL production in the hepatocytes via a paracrine mechanism. As shown in several cancer cell types, the EGFR pathway may activate the sterol regulatory element-binding protein 1c (SREBP-1c) pathway for the increase of lipogenesis in the hepatocytes.93,94
The Role of HB-EGF in the Progress of Metabolic Disease Phenotypes
Role of HB-EGF in the development of atherosclerosis
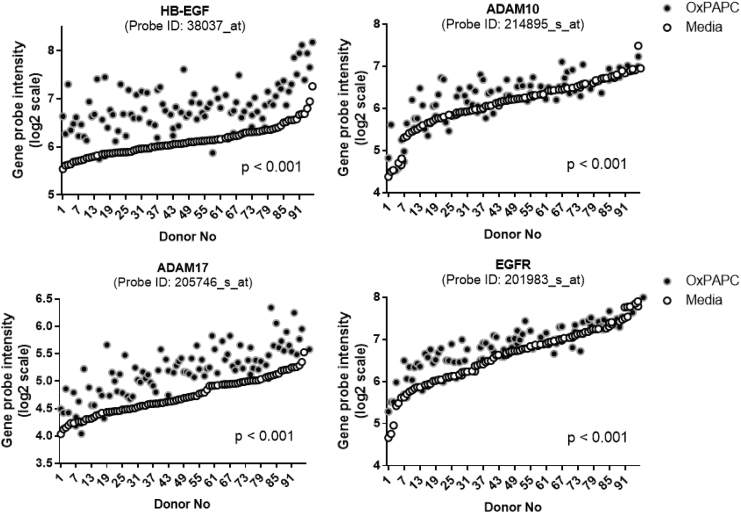
The elevation of TG-rich VLDL particles in circulation is an independent risk factor for the development of atherosclerosis and coronary artery disease.95,96 Particularly, VLDL remnant and LDL particles derived from VLDLs have optimal sizes for the infiltration into the vascular wall.62 In addition, the modification of the lipoprotein particles induced self-aggregation in the subendothelial space,97 which are associated with enhanced scavenging by the macrophages in the subendothelial space.62 The bioactive components of minimally modified LDL particles, oxidized phospholipids (Ox-PLs), increased gene transcription of HB-EGF and MPs like a disintegrin and metalloproteinase 17 (ADAM17) in the vascular endothelial cells34,63 (Fig. 1). There were positive associations of HB-EGF content in the vessel wall with the intensity of atherosclerosis in hyperlipidemic mouse and human vessels.61,98,99 AngII infusion, which elevates HB-EGF-EFR signaling, significantly increased atherosclerosis and aneurysm in hyperlipidemic animal models via HB-EGF upregulation in the vessel wall.7,100,101 Small-molecule inhibitors of EGFR also showed protection against atherosclerosis and aneurysm in hyperlipidemic animal models.102,103
FIG. 1.
Lipid peroxidation products increased the expression of genes involved in the HB-EGF-activated EGFR pathway in the vascular endothelial cells. The human aortic endothelial cells isolated from 96 different human donors were treated with vehicle and oxidized phospholipid (Ox-PAPC, 50 μg/mL) for 4 hr. The HB-EGF, ADAM10, -17, and EGFR transcript values from the microarray dataset of the donor cells were plotted by order of basal transcript levels. The openly available dataset was reanalyzed (NCBI GEO: www.ncbi.nlm.nih.gov/geo/; reference number GSE20060).63 The unique probe ID was inserted for each gene. P values mean the differential P-value from the Student's t-test for paired values of the vehicle and Ox-PAPC treatment groups after adjustment for multiple comparisons. ADAM, a disintegrin and metalloproteinase; EGFR, epidermal growth factor receptor; HB-EGF, heparin-binding EGF-like growth factor; Ox-PAPC, oxidized 1-palmitoyl-2-arachidonyl-sn-glycero phosphorylcholine.
Role of HB-EGF in the development of hepatic inflammation
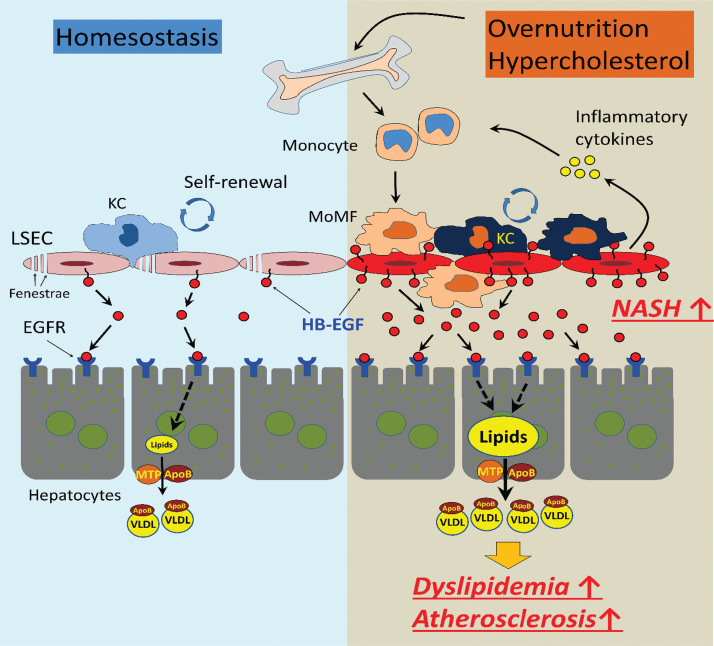
HB-EGF is mainly expressed in the sinusoidal endothelial cells in the liver.86,87 Different from the endothelial cells in the other tissues of the body, the LSECs are the scavenger endothelial cells as gatekeepers of the body.68,104 LSECs effectively scavenge oxidant wastes, including modified (heavily- or minimally oxidized) LDL particles and AGEs, which are significantly enriched in the splanchnic circulation of centrally obese individuals.51,66,105,106 The sustained influx of oxidants into the hepatic sinusoidal lumen causes the saturation and activation of LSECs for the induction of inflammatory cytokine expression.68,107,108 The upregulation of HB-EGF in the activated LSECs would induce paracrine EGFR signaling in the hepatocytes for the stimulation of VLDL production. The HB-EGF expressed on and released from the LSECs may cause dyslipidemia and low-grade inflammation in the liver (Fig. 2 for the schematic diagram for events related to chronic inflammation and dyslipidemia induced by the overnutrition). The saturation of the LSECs would also induce extrahepatic endothelial cell activation, causing low-grade systemic inflammation.
FIG. 2.
The role of HB-EGF in the regulation of the hepatic inflammation under overnutrition and obesity. In homeostatic condition, HB-EGF is constitutively expressed in and released from the LSECs for the paracrine EGFR signaling in the hepatocytes for basal VLDL production. The oxidative stress associated with nutrition excess and visceral obesity increased the production of harmful oxidants, including oxidized LDL particles in circulation. The oxidants induce saturation and activation of LSECs and upregulation of HB-EGF expression, which may cause extrahepatic endothelial cell activation and recruitment of bone marrow-derived monocyte recruitment and hepatic VLDL overproduction. The elevation of the circulatory cholesterol induces increased proliferation of the myeloid progenitor in the bone marrow, leading to monocytosis and low-grade inflammation. KC, Kupffer cell; LDL, low-density lipoprotein; LSECs, liver sinusoidal endothelial cells; MoMF, monocyte-derived macrophage; MTP, microsomal triglyceride transfer protein; VLDL, very-low-density lipoprotein. Color images are available online.
Pathological role of HB-EGF in renal diseases
Previous reports indicated that HB-EGF-mediated EGFR signaling is involved in the pathology of renal diseases.21,43,48,109–112 HB-EGF mediates the disease phenotypes associated with AngII signaling in the kidney.43 The HB-EGF-mediated EGFR transactivation signaling mediates the fibronectin and transforming growth factor-beta upregulation in renal mesangial cells induced by AngII or high glucose.47,48 Bollee et al. demonstrated that the podocyte-specific HB-EGF gene deletion or the administration of EGFR blocker induced protections against rapidly progressive glomerulonephritis in a mouse model.21 The endothelial-specific HB-EGF deletion also induced a series of protection against renal disease induced by AngII, unilateral nephrectomy,19,113–115 or ischemic reperfusion.116 In contrast, the administration of recombinant HB-EGF downregulated glomerulus filtration in the animal model of glomerulonephritis.20 Because HB-EGF is abundantly expressed in the epithelial cells in distal tubules, there were significant increases in soluble HB-EGF content in the urines under renal disease conditions.116 Overstreet et al. showed that renal tubular HB-EGF overexpression caused the development of renal fibrosis.112
The HB-EGF as a Potential Target Against Metabolic Diseases
The accumulation of lipid peroxidation products (e.g., Ox-PLs) in circulation appears to be a risk factor for the development of metabolic syndrome associated with central obesity.51,52,117,118 The inhibition of ROS production or the neutralization of the oxidants is a possible approach for the protection against metabolic syndrome and disease phenotypes.119 Considering the upregulation of the HB-EGF in the endothelium by oxidative stress, the HB-EGF targeting or modulation of the HB-EGF signaling could be another option for treatment. The clinical application of HB-EGF modulators on the development of metabolic dysfunction has not been reported yet (as far as we know). Most of the previous interventional clinical studies using HB-EGF modulators have focused on the treatment of cancer patients.3,34,120,121
Antioxidant approaches against metabolic dysfunction
Obesity is closely associated with the induction of oxidative stress in adipose tissue and circulation.51 There was a close association of oxidative stress with the development of metabolic syndrome.52,84,118 As an example of the useful therapeutic application of antioxidants, the administration of a free thiol N-acetyl-L-cysteine ameliorated the liver endothelial cell damage caused by the paracetamol overdose.122–124 However, the beneficial effects of neutralization of the oxidants using antioxidants against metabolic disease phenotypes are still under controversy.119,125 Because local transient ROS production is essential for physiological and cellular functions,125,126 nonspecific targeting of superoxide (O2−) and hydrogen peroxide (H2O2) may cause deleterious effects. Another pitfall of antioxidant therapy could be the differential reactivities of an antioxidant with oxidants due to different chemical structures and properties.119 The time point for the administration might be another determining factor for therapeutic effects.119,127 Administration of antioxidants at the end stage of metabolic diseases did not induce significant beneficial effects.125,126
HB-EGF targeting against metabolic diseases
HB-EGF blocking antibodies were evaluated for therapeutic applications for antitumor purposes.128–132 In human clinical trials, the administration of HB-EGF neutralizing antibody showed protection against cancer progression. However, the administration induced unexpected psychiatric side effects.130,133 A recent trial using another HB-EGF blocking antibody did not show the deleterious effects.132 The membrane-tethered HB-EGF is the bona fide receptor for diphtheria toxin (DTX) in primates.134 Inert DTX analog has been evaluated for the suppression of HB-EGF signaling in varying pathological conditions.121,135–137 A representative DTX analog, CRM-197, showed protection against the proliferation and metastasis of cancer cells135–139; however, the effects of the analog on the metabolic disease phenotypes have not yet been reported.
As mentioned, the administration HB-EGF ASO significantly downregulated the rate of hepatic VLDL production and effectively suppressed circulatory VLDL- and LDL-associated TG and cholesterol levels in hyperlipidemic mouse models (LDLR- or apoE-deficient mice under the Western diet).92 Concordantly, the ASO administration induced an effective suppression of atherosclerosis and aneurysm developments in the models.92,140 The inhibition of hepatic VLDL production and downregulation of innate immune cell production in the bone marrow appears to be involved in the protection.14
Recently, a list of small-molecule EGFR blockers (e.g., gefitinib and AG1478) showed protection against atherosclerosis and hyperlipidemia in animal models.102,141 The inhibitors also downregulated circulatory lipid levels in hyperlipidemic mouse models. The EGFR blocker erlotinib showed protection against diabetes in humans by suppressing inflammatory cytokine production and inflammatory cell infiltration into the pancreas.142 Gefitinib administration protected against renal, vascular, and glomerular fibrosis.109 Potentially, the re-evaluation of the clinically available EGFR blockers could be an approach for the development of therapeutic tools against metabolic diseases.12,43
Conclusion and Perspectives
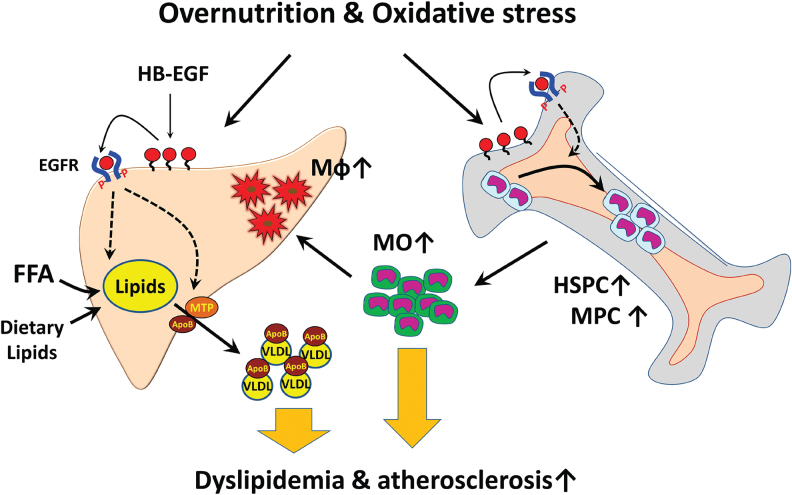
Since the first identification of the HB-EGF as a ligand of EGFR and ERBB4,1 numerous features of HB-EGF associated with cell proliferation and metabolic disease phenotypes were reported. Different from the other EGFR ligands, the HB-EGF transcription is sensitively upregulated by oxidative stresses in the endothelial cells. The role of the HB-EGF in regulating the proliferation and differentiation of HSCs could be a compelling connection of oxidative stress with the induction of low-grade inflammation in obese people. The increase of the hepatic VLDL production by the HB-EGF signaling in the hepatocytes could be the linkage of oxidative stress with dyslipidemia in obese people. As illustrated in Fig. 3, the HB-EGF upregulated in the hepatic or bone marrow sinusoidal endothelial cells by overproduction of harmful oxidants in circulation appears to be a proximal event for the induction of dyslipidemia and low-grade inflammatory responses under oxidative stress environments. The vicious cycle of accumulation of oxidants in circulation and increased myeloid cell production in the bone marrow would lead to the progress of metabolic diseases to atherosclerosis and non-alcoholic steatohepatitis. HB-EGF is also involved in the induction of suppression of insulin signaling in the adipocytes and skeletal muscle cells.
FIG. 3.
The working hypothesis of HB-EGF in metabolic disease development. Oxidants produced by the oxidative stress increased HB-EGF expression in the sinusoidal endothelial cells in the liver and bone marrow, causing increases in hepatic VLDL secretion and myeloid cell production. The bone marrow-derived monocytes can be recruited into the subendothelial space of the liver sinusoids and vessel endothelium for the development of inflammation in the liver and vessels. HB-EGF targeting would downregulate the hepatic VLDL production and reduction of the myeloid cell production. FFA, free fatty acid; HSPC, hematopoietic stem and progenitor cell; Mφ, macrophage; MO, monocyte; MPC, myeloid progenitor cell. Color images are available online.
Although the therapeutic potential of the HB-EGF modulation is a promising approach from the experimental results using animal models, still many barriers are to be overcome for the clinical applications. Previous reports indicated barriers in the translational application of the preclinical results to humans on metabolic and vascular disease phenotypes,143,144 as exemplified by the increased susceptibility of the patients with anti-inflammatory reagent canakinumab (IL-1beta blocking antibody) administration to the sepsis and infection.145 For each approach of HB-EGF targeting, a thorough evaluation of the potential side effects should be addressed. The administration of an HB-EGF blocking antibody caused psychiatric disturbances in human studies, partly because of the interruption of the cerebral function of HB-EGF.130,133 The ASO would provide a benefit of minimal side effects in the brain via the impermeability of the ASO through the blood–brain barrier146; however, the HB-EGF ASO induced buildup of the neutral lipids (TG and cholesterol ester) in the liver and potential suppression of blood cell formation in the bone marrow.92 Further evaluation of the side effects associated with liver and bone marrow functions is required. The DTX analog showed significant beneficial effects in suppressing cancer cell proliferation and metastasis. The cost of production of DTX analog and residual toxic effects of the analog could be barriers for long-term clinical usages. Collectively, considerable results indicate that the HB-EGF pathway is a target candidate for the prevention and reverse of metabolic diseases in humans, although further evaluation on potential side effects is needed.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the views of fund supporters.
Authors' Contributions
S.K. made contributions to the acquisition and interpretation of experimental data associated with the article. V.S. and A.A.-L. provided insights into the experiments and direction of the projects related to the article. S.L. originally drafted the article with an agreement to be accountable for all aspects of the work.
Author Disclosure Statement
No conflicting financial interests exist.
Funding Information
This study was supported by the National Institutes of Health K99/R00 HL105577 (S.L.) and the American Heart Association 17GRNT33700302 (S.L.).
References
- 1. Higashiyama S, Abraham JA, Miller J, et al. . A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science 1991;251:936–939 [DOI] [PubMed] [Google Scholar]
- 2. Schneider MR, Wolf E. The epidermal growth factor receptor ligands at a glance. J Cell Physiol 2009;218:460–466 [DOI] [PubMed] [Google Scholar]
- 3. Shepard HM, Brdlik CM, Schreiber H. Signal integration: A framework for understanding the efficacy of therapeutics targeting the human EGFR family. J Clin Invest 2008;118:3574–3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harris RC, Chung E, Coffey RJ. EGF receptor ligands. Exp Cell Res 2003;284:2–13 [DOI] [PubMed] [Google Scholar]
- 5. Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2001;2:127–137 [DOI] [PubMed] [Google Scholar]
- 6. Raab G, Higashiyama S, Hetelekidis S, et al. . Biosynthesis and processing by phorbol ester of the cells surface-associated precursor form of heparin-binding EGF-like growth factor. Biochem Biophys Res Commun 1994;204:592–597 [DOI] [PubMed] [Google Scholar]
- 7. Ohtsu H, Dempsey PJ, Eguchi S. ADAMs as mediators of EGF receptor transactivation by G protein-coupled receptors. Am J Physiol Cell Physiol 2006;291:C1–C10 [DOI] [PubMed] [Google Scholar]
- 8. Thompson SA, Higashiyama S, Wood K, et al. . Characterization of sequences within heparin-binding EGF-like growth factor that mediate interaction with heparin. J Biol Chem 1994;269:2541–2549 [PubMed] [Google Scholar]
- 9. Kalmes A, Vesti BR, Daum G, et al. . Heparin blockade of thrombin-induced smooth muscle cell migration involves inhibition of epidermal growth factor (EGF) receptor transactivation by heparin-binding EGF-like growth factor. Circ Res 2000;87:92–98 [DOI] [PubMed] [Google Scholar]
- 10. Iwamoto R, Mekada E. Heparin-binding EGF-like growth factor: A juxtacrine growth factor. Cytokine Growth Factor Rev 2000;11:335–344 [DOI] [PubMed] [Google Scholar]
- 11. Kayanoki Y, Higashiyama S, Suzuki K, et al. . The requirement of both intracellular reactive oxygen species and intracellular calcium elevation for the induction of heparin-binding EGF-like growth factor in vascular endothelial cells and smooth muscle cells. Biochem Biophys Res Commun 1999;259:50–55 [DOI] [PubMed] [Google Scholar]
- 12. Dreux AC, Lamb DJ, Modjtahedi H, et al. . The epidermal growth factor receptors and their family of ligands: Their putative role in atherogenesis. Atherosclerosis 2006;186:38–53 [DOI] [PubMed] [Google Scholar]
- 13. Krampera M, Pasini A, Rigo A, et al. . HB-EGF/HER-1 signaling in bone marrow mesenchymal stem cells: Inducing cell expansion and reversibly preventing multilineage differentiation. Blood 2005;106:59–66 [DOI] [PubMed] [Google Scholar]
- 14. Vinante F, Rigo A. Heparin-binding epidermal growth factor-like growth factor/diphtheria toxin receptor in normal and neoplastic hematopoiesis. Toxins 2013;5:1180–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Murphy AJ, Tall AR. Disordered haematopoiesis and athero-thrombosis. Eur Heart J 2016;37:1113–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fayad ZA, Swirski FK, Calcagno C, et al. . Monocyte and macrophage dynamics in the cardiovascular system: JACC macrophage in CVD series (Part 3). J Am Coll Cardiol 2018;72:2198–2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iwamoto R, Yamazaki S, Asakura M, et al. . Heparin-binding EGF-like growth factor and ErbB signaling is essential for heart function. Proc Natl Acad Sci U S A 2003;100:3221–3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jackson LF, Qiu TH, Sunnarborg SW, et al. . Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. EMBO J 2003;22:2704–2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zeng F, Kloepfer LA, Finney C, et al. . Specific endothelial heparin-binding EGF-like growth factor deletion ameliorates renal injury induced by chronic angiotensin II infusion. Am J Physiol Renal Physiol 2016;311:F695–F707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Feng L, Garcia GE, Yang Y, et al. . Heparin-binding EGF-like growth factor contributes to reduced glomerular filtration rate during glomerulonephritis in rats. J Clin Invest 2000;105:341–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bollee G, Flamant M, Schordan S, et al. . Epidermal growth factor receptor promotes glomerular injury and renal failure in rapidly progressive crescentic glomerulonephritis. Nat Med 2011;17:1242–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takemura T, Yoshida Y, Kiso S, et al. . Conditional loss of heparin-binding EGF-like growth factor results in enhanced liver fibrosis after bile duct ligation in mice. Biochem Biophys Res Commun 2013;437:185–191 [DOI] [PubMed] [Google Scholar]
- 23. Takemura T, Yoshida Y, Kiso S, et al. . Conditional knockout of heparin-binding epidermal growth factor-like growth factor in the liver accelerates carbon tetrachloride-induced liver injury in mice. Hepatol Res 2013;43:384–393 [DOI] [PubMed] [Google Scholar]
- 24. Huang G, Besner GE, Brigstock DR. Heparin-binding epidermal growth factor-like growth factor suppresses experimental liver fibrosis in mice. Lab Invest 2012;92:703–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guo Y, Ding Q, Chen L, et al. . Overexpression of heparin-binding epidermal growth factor-like growth factor mediates liver fibrosis in transgenic mice. Am J Med Sci 2017;354:199–210 [DOI] [PubMed] [Google Scholar]
- 26. Miyazawa T, Zeng F, Wang S, et al. . Low nitric oxide bioavailability upregulates renal heparin binding EGF-like growth factor expression. Kidney Int 2013;84:1176–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Raab G, Klagsbrun M. Heparin-binding EGF-like growth factor. Biochim Biophys Acta 1997;1333:F179–F199 [DOI] [PubMed] [Google Scholar]
- 28. Umata T. Involvement of reactive oxygen species in stimuli-induced shedding of heparin-binding epidermal growth factor-like growth factor. J UOEH 2014;36:105–114 [DOI] [PubMed] [Google Scholar]
- 29. Prenzel N, Zwick E, Daub H, et al. . EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature 1999;402:884–888 [DOI] [PubMed] [Google Scholar]
- 30. Daub H, Weiss FU, Wallasch C, et al. . Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature 1996;379:557–560 [DOI] [PubMed] [Google Scholar]
- 31. Mifune M, Ohtsu H, Suzuki H, et al. . G protein coupling and second messenger generation are indispensable for metalloprotease-dependent, heparin-binding epidermal growth factor shedding through angiotensin II type-1 receptor. J Biol Chem 2005;280:26592–26599 [DOI] [PubMed] [Google Scholar]
- 32. Maudsley S, Pierce KL, Zamah AM, et al. . The beta(2)-adrenergic receptor mediates extracellular signal-regulated kinase activation via assembly of a multi-receptor complex with the epidermal growth factor receptor. J Biol Chem 2000;275:9572–9580 [DOI] [PubMed] [Google Scholar]
- 33. Liu W, Akhand AA, Kato M, et al. . 4-hydroxynonenal triggers an epidermal growth factor receptor-linked signal pathway for growth inhibition. J Cell Sci 1999;112 (Pt 14):2409–2417 [DOI] [PubMed] [Google Scholar]
- 34. Lee S, Springstead JR, Parks BW, et al. . Metalloproteinase processing of HBEGF is a proximal event in the response of human aortic endothelial cells to oxidized phospholipids. Arterioscler Thromb Vasc Biol 2012;32:1246–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kalmes A, Daum G, Clowes AW. EGFR transactivation in the regulation of SMC function. Ann N Y Acad Sci 2001;947:42–54; discussion 54–45. [DOI] [PubMed] [Google Scholar]
- 36. Gschwind A, Zwick E, Prenzel N, et al. . Cell communication networks: Epidermal growth factor receptor transactivation as the paradigm for interreceptor signal transmission. Oncogene 2001;20:1594–1600 [DOI] [PubMed] [Google Scholar]
- 37. Chen CH, Cheng TH, Lin H, et al. . Reactive oxygen species generation is involved in epidermal growth factor receptor transactivation through the transient oxidization of Src homology 2-containing tyrosine phosphatase in endothelin-1 signaling pathway in rat cardiac fibroblasts. Mol Pharmacol 2006;69:1347–1355 [DOI] [PubMed] [Google Scholar]
- 38. Miyata K, Yotsumoto F, Nam SO, et al. . Regulatory mechanisms of the HB-EGF autocrine loop in inflammation, homeostasis, development and cancer. Anticancer Res 2012;32:2347–2352 [PubMed] [Google Scholar]
- 39. Serbulea V, Upchurch CM, Schappe MS, et al. . Macrophage phenotype and bioenergetics are controlled by oxidized phospholipids identified in lean and obese adipose tissue. Proc Natl Acad Sci U S A 2018;115:E6254–E6263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Russell AP, Gastaldi G, Bobbioni-Harsch E, et al. . Lipid peroxidation in skeletal muscle of obese as compared to endurance-trained humans: A case of good vs. bad lipids? FEBS Lett 2003;551:104–106 [DOI] [PubMed] [Google Scholar]
- 41. Prasannarong M, Santos FR, Hooshmand P, et al. . The lipid peroxidation end-product and oxidant 4-hydroxynonenal induces insulin resistance in rat slow-twitch skeletal muscle. Arch Physiol Biochem 2014;120:22–28 [DOI] [PubMed] [Google Scholar]
- 42. Hogan NT, Whalen MB, Stolze LK, et al. . Transcriptional networks specifying homeostatic and inflammatory programs of gene expression in human aortic endothelial cells. Elife 2017;6:e22536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Forrester SJ, Kawai T, O'Brien S, et al. . Epidermal growth factor receptor transactivation: Mechanisms, pathophysiology, and potential therapies in the cardiovascular system. Annu Rev Pharmacol Toxicol 2016;56:627–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Forrester SJ, Booz GW, Sigmund CD, et al. . Angiotensin II signal transduction: An update on mechanisms of physiology and pathophysiology. Physiol Rev 2018;98:1627–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yang X, Zhu MJ, Sreejayan N, et al. . Angiotensin II promotes smooth muscle cell proliferation and migration through release of heparin-binding epidermal growth factor and activation of EGF-receptor pathway. Mol Cells 2005;20:263–270 [PubMed] [Google Scholar]
- 46. Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: Linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res 2008;102:488–496 [DOI] [PubMed] [Google Scholar]
- 47. Uchiyama-Tanaka Y, Matsubara H, Nozawa Y, et al. . Angiotensin II signaling and HB-EGF shedding via metalloproteinase in glomerular mesangial cells. Kidney Int 2001;60:2153–2163 [DOI] [PubMed] [Google Scholar]
- 48. Uttarwar L, Peng F, Wu D, et al. . HB-EGF release mediates glucose-induced activation of the epidermal growth factor receptor in mesangial cells. Am J Physiol Renal Physiol 2011;300:F921–F931 [DOI] [PubMed] [Google Scholar]
- 49. Igura T, Kawata S, Miyagawa J, et al. . Expression of heparin-binding epidermal growth factor-like growth factor in neointimal cells induced by balloon injury in rat carotid arteries. Arterioscler Thromb Vasc Biol 1996;16:1524–1531 [DOI] [PubMed] [Google Scholar]
- 50. Gschwind A, Hart S, Fischer OM, et al. . TACE cleavage of proamphiregulin regulates GPCR-induced proliferation and motility of cancer cells. EMBO J 2003;22:2411–2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Furukawa S, Fujita T, Shimabukuro M, et al. . Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 2004;114:1752–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Holvoet P, De Keyzer D, Jacobs DR Jr. Oxidized LDL and the metabolic syndrome. Future Lipidol 2008;3:637–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Choi SH, Ginsberg HN. Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends Endocrinol Metab 2011;22:353–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Barter PJ, Brewer HB Jr., Chapman MJ, et al. . Cholesteryl ester transfer protein: A novel target for raising HDL and inhibiting atherosclerosis. Arterioscler Thromb Vasc Biol 2003;23:160–167 [DOI] [PubMed] [Google Scholar]
- 55. Swirski FK, Libby P, Aikawa E, et al. . Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest 2007;117:195–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gomes AL, Carvalho T, Serpa J, et al. . Hypercholesterolemia promotes bone marrow cell mobilization by perturbing the SDF-1:CXCR4 axis. Blood 2010;115:3886–3894 [DOI] [PubMed] [Google Scholar]
- 57. Tolani S, Pagler TA, Murphy AJ, et al. . Hypercholesterolemia and reduced HDL-C promote hematopoietic stem cell proliferation and monocytosis: Studies in mice and FH children. Atherosclerosis 2013;229:79–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nagareddy PR, Kraakman M, Masters SL, et al. . Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab 2014;19:821–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Robbins CS, Hilgendorf I, Weber GF, et al. . Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med 2013;19:1166–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nakano T, Raines EW, Abraham JA, et al. . Lysophosphatidylcholine upregulates the level of heparin-binding epidermal growth factor-like growth factor mRNA in human monocytes. Proc Natl Acad Sci U S A 1994;91:1069–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Reape TJ, Wilson VJ, Kanczler JM, et al. . Detection and cellular localization of heparin-binding epidermal growth factor-like growth factor mRNA and protein in human atherosclerotic tissue. J Mol Cell Cardiol 1997;29:1639–1648 [DOI] [PubMed] [Google Scholar]
- 62. Lee S, Birukov KG, Romanoski CE, et al. . Role of phospholipid oxidation products in atherosclerosis. Circ Res 2012;111:778–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Romanoski CE, Lee S, Kim MJ, et al. . Systems genetics analysis of gene-by-environment interactions in human cells. Am J Hum Genet 2010;86:399–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pitas RE, Boyles J, Mahley RW, et al. . Uptake of chemically modified low density lipoproteins in vivo is mediated by specific endothelial cells. J Cell Biol 1985;100:103–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Blomhoff R, Drevon CA, Eskild W, et al. . Clearance of acetyl low density lipoprotein by rat liver endothelial cells. Implications for hepatic cholesterol metabolism. J Biol Chem 1984;259:8898–8903 [PubMed] [Google Scholar]
- 66. Xi L, Xiao C, Bandsma RH, et al. . C-reactive protein impairs hepatic insulin sensitivity and insulin signaling in rats: Role of mitogen-activated protein kinases. Hepatology 2011;53:127–135 [DOI] [PubMed] [Google Scholar]
- 67. Qian H, Johansson S, McCourt P, et al. . Stabilins are expressed in bone marrow sinusoidal endothelial cells and mediate scavenging and cell adhesive functions. Biochem Biophys Res Commun 2009;390:883–886 [DOI] [PubMed] [Google Scholar]
- 68. Poisson J, Lemoinne S, Boulanger C, et al. . Liver sinusoidal endothelial cells: Physiology and role in liver diseases. J Hepatol 2017;66:212–227 [DOI] [PubMed] [Google Scholar]
- 69. Hassanshahi M, Hassanshahi A, Khabbazi S, et al. . Bone marrow sinusoidal endothelium as a facilitator/regulator of cell egress from the bone marrow. Crit Rev Oncol Hematol 2019;137:43–56 [DOI] [PubMed] [Google Scholar]
- 70. Birukova AA, Lee S, Starosta V, et al. . A role for VEGFR2 activation in endothelial responses caused by barrier disruptive OxPAPC concentrations. PLoS One 2012;7:e30957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Itkin T, Gur-Cohen S, Spencer JA, et al. . Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature 2016;532:323–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Samuel VT, Shulman GI. Nonalcoholic fatty liver disease as a nexus of metabolic and hepatic diseases. Cell Metab 2018;27:22–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Li Q, Hosaka T, Shikama Y, et al. . Heparin-binding EGF-like growth factor (HB-EGF) mediates 5-HT-induced insulin resistance through activation of EGF receptor-ERK1/2-mTOR pathway. Endocrinology 2012;153:56–68 [DOI] [PubMed] [Google Scholar]
- 74. Motley ED, Kabir SM, Gardner CD, et al. . Lysophosphatidylcholine inhibits insulin-induced Akt activation through protein kinase C-alpha in vascular smooth muscle cells. Hypertension 2002;39(2 Pt 2):508–512 [DOI] [PubMed] [Google Scholar]
- 75. O'Toole TE, Abplanalp W, Li X, et al. . Acrolein decreases endothelial cell migration and insulin sensitivity through induction of let-7a. Toxicol Sci 2014;140:271–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Elrayess MA, Almuraikhy S, Kafienah W, et al. . 4-hydroxynonenal causes impairment of human subcutaneous adipogenesis and induction of adipocyte insulin resistance. Free Radic Biol Med 2017;104:129–137 [DOI] [PubMed] [Google Scholar]
- 77. Matsumoto S, Kishida K, Shimomura I, et al. . Increased plasma HB-EGF associated with obesity and coronary artery disease. Biochem Biophys Res Commun 2002;292:781–786 [DOI] [PubMed] [Google Scholar]
- 78. Berg AH, Combs TP, Du X, et al. . The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 2001;7:947–953 [DOI] [PubMed] [Google Scholar]
- 79. Maeda N, Shimomura I, Kishida K, et al. . Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med 2002;8:731–737 [DOI] [PubMed] [Google Scholar]
- 80. Arita Y, Kihara S, Ouchi N, et al. . Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 1999;257:79–83 [DOI] [PubMed] [Google Scholar]
- 81. Matsuda M, Shimomura I, Sata M, et al. . Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. J Biol Chem 2002;277:37487–37491 [DOI] [PubMed] [Google Scholar]
- 82. Arita Y, Kihara S, Ouchi N, et al. . Adipocyte-derived plasma protein adiponectin acts as a platelet-derived growth factor-BB-binding protein and regulates growth factor-induced common postreceptor signal in vascular smooth muscle cell. Circulation 2002;105:2893–2898 [DOI] [PubMed] [Google Scholar]
- 83. Wang Y, Lam KS, Xu JY, et al. . Adiponectin inhibits cell proliferation by interacting with several growth factors in an oligomerization-dependent manner. J Biol Chem 2005;280:18341–18347 [DOI] [PubMed] [Google Scholar]
- 84. Matsuzawa Y. The metabolic syndrome and adipocytokines. FEBS Lett 2006;580:2917–2921 [DOI] [PubMed] [Google Scholar]
- 85. Li N, Fu J, Koonen DP, et al. . Are hypertriglyceridemia and low HDL causal factors in the development of insulin resistance? Atherosclerosis 2014;233:130–138 [DOI] [PubMed] [Google Scholar]
- 86. Hu J, Srivastava K, Wieland M, et al. . Endothelial cell-derived angiopoietin-2 controls liver regeneration as a spatiotemporal rheostat. Science 2014;343:416–419 [DOI] [PubMed] [Google Scholar]
- 87. Kiso S, Kawata S, Tamura S, et al. . Role of heparin-binding epidermal growth factor-like growth factor as a hepatotrophic factor in rat liver regeneration after partial hepatectomy. Hepatology 1995;22:1584–1590 [PubMed] [Google Scholar]
- 88. Bijsterbosch MK, Manoharan M, Rump ET, et al. . In vivo fate of phosphorothioate antisense oligodeoxynucleotides: Predominant uptake by scavenger receptors on endothelial liver cells. Nucleic Acids Res 1997;25:3290–3296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Graham MJ, Crooke ST, Lemonidis KM, et al. . Hepatic distribution of a phosphorothioate oligodeoxynucleotide within rodents following intravenous administration. Biochem Pharmacol 2001;62:297–306 [DOI] [PubMed] [Google Scholar]
- 90. Graham MJ, Crooke ST, Monteith DK, et al. . In vivo distribution and metabolism of a phosphorothioate oligonucleotide within rat liver after intravenous administration. J Pharmacol Exp Ther 1998;286:447–458 [PubMed] [Google Scholar]
- 91. Miller CM, Donner AJ, Blank EE, et al. . Stabilin-1 and Stabilin-2 are specific receptors for the cellular internalization of phosphorothioate-modified antisense oligonucleotides (ASOs) in the liver. Nucleic Acids Res 2016;44:2782–2794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kim S, Yang L, Kim S, et al. . Targeting hepatic heparin-binding EGF-like growth factor (HB-EGF) induces anti-hyperlipidemia leading to reduction of angiotensin II-induced aneurysm development. PLoS One 2017;12:e0182566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Guo D, Prins RM, Dang J, et al. . EGFR signaling through an Akt-SREBP-1-dependent, rapamycin-resistant pathway sensitizes glioblastomas to antilipogenic therapy. Sci Signal 2009;2:ra82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Cheng C, Ru P, Geng F, et al. . Glucose-mediated N-glycosylation of SCAP is essential for SREBP-1 activation and tumor growth. Cancer Cell 2015;28:569–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ginsberg HN, Maccallum PR. The obesity, metabolic syndrome, and type 2 diabetes mellitus pandemic: II. Therapeutic management of atherogenic dyslipidemia. J Clin Hypertens 2009;11:520–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chapman MJ, Ginsberg HN, Amarenco P, et al. . Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: Evidence and guidance for management. Eur Heart J 2011;32:1345–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hoff HF, O'Neil J. Lesion-derived low density lipoprotein and oxidized low density lipoprotein share a lability for aggregation, leading to enhanced macrophage degradation. Arterioscler Thromb 1991;11:1209–1222 [DOI] [PubMed] [Google Scholar]
- 98. Nakata A, Miyagawa J, Yamashita S, et al. . Localization of heparin-binding epidermal growth factor-like growth factor in human coronary arteries. Possible roles of HB-EGF in the formation of coronary atherosclerosis. Circulation 1996;94:2778–2786 [DOI] [PubMed] [Google Scholar]
- 99. Nishida M, Miyagawa J, Yamashita S, et al. . Localization of CD9, an enhancer protein for proheparin-binding epidermal growth factor-like growth factor, in human atherosclerotic plaques: Possible involvement of juxtacrine growth mechanism on smooth muscle cell proliferation. Arterioscler Thromb Vasc Biol 2000;20:1236–1243 [DOI] [PubMed] [Google Scholar]
- 100. Inagami T, Eguchi S. Angiotensin II-mediated vascular smooth muscle cell growth signaling. Braz J Med Biol Res 2000;33:619–624 [DOI] [PubMed] [Google Scholar]
- 101. Wu C, Lu H, Cassis LA, et al. . Molecular and pathophysiological features of angiotensinogen: A mini review. N Am J Med Sci 2011;4:183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Gao P, Wang XM, Qian DH, et al. . Induction of oxidative stress by oxidized LDL via meprinalpha-activated epidermal growth factor receptor in macrophages. Cardiovasc Res 2013;97:533–543 [DOI] [PubMed] [Google Scholar]
- 103. Obama T, Tsuji T, Kobayashi T, et al. . Epidermal growth factor receptor inhibitor protects against abdominal aortic aneurysm in a mouse model. Clin Sci 2015;128:559–565 [DOI] [PubMed] [Google Scholar]
- 104. Knolle PA. Role and function of liver sinusoidal endothelial cells. Liver Immunol 2007:25–39 [Google Scholar]
- 105. Van Berkel TJ, De Rijke YB, Kruijt JK. Different fate in vivo of oxidatively modified low density lipoprotein and acetylated low density lipoprotein in rats. Recognition by various scavenger receptors on Kupffer and endothelial liver cells. J Biol Chem 1991;266:2282–2289 [PubMed] [Google Scholar]
- 106. Smedsrod B, Melkko J, Araki N, et al. . Advanced glycation end products are eliminated by scavenger-receptor-mediated endocytosis in hepatic sinusoidal Kupffer and endothelial cells. Biochem J 1997;322 (Pt 2):567–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zhang Q, Liu J, Liu J, et al. . oxLDL induces injury and defenestration of human liver sinusoidal endothelial cells via LOX1. J Mol Endocrinol 2014;53:281–293 [DOI] [PubMed] [Google Scholar]
- 108. Yimin, Furumaki H, Matsuoka S, et al. . A novel murine model for non-alcoholic steatohepatitis developed by combination of a high-fat diet and oxidized low-density lipoprotein. Lab Invest 2012;92:265–281 [DOI] [PubMed] [Google Scholar]
- 109. Francois H, Placier S, Flamant M, et al. . Prevention of renal vascular and glomerular fibrosis by epidermal growth factor receptor inhibition. FASEB J 2004;18:926–928 [DOI] [PubMed] [Google Scholar]
- 110. Lautrette A, Li S, Alili R, et al. . Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: A new therapeutic approach. Nat Med 2005;11:867–874 [DOI] [PubMed] [Google Scholar]
- 111. Harris R. EGFR signaling in podocytes at the root of glomerular disease. Nat Med 2011;17:1188–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Overstreet JM, Wang Y, Wang X, et al. . Selective activation of epidermal growth factor receptor in renal proximal tubule induces tubulointerstitial fibrosis. FASEB J 2017;31:4407–4421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Homma T, Sakai M, Cheng HF, et al. . Induction of heparin-binding epidermal growth factor-like growth factor mRNA in rat kidney after acute injury. J Clin Invest 1995;96:1018–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Olivares-Reyes JA, Shah BH, Hernandez-Aranda J, et al. . Agonist-induced interactions between angiotensin AT1 and epidermal growth factor receptors. Mol Pharmacol 2005;68:356–364 [DOI] [PubMed] [Google Scholar]
- 115. Olson ER, Shamhart PE, Naugle JE, et al. . Angiotensin II-induced extracellular signal-regulated kinase 1/2 activation is mediated by protein kinase Cdelta and intracellular calcium in adult rat cardiac fibroblasts. Hypertension 2008;51:704–711 [DOI] [PubMed] [Google Scholar]
- 116. Mulder GM, Nijboer WN, Seelen MA, et al. . Heparin binding epidermal growth factor in renal ischaemia/reperfusion injury. J Pathol 2010;221:183–192 [DOI] [PubMed] [Google Scholar]
- 117. Henriksen EJ, Diamond-Stanic MK, Marchionne EM. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic Biol Med 2011;51:993–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Vaidya D, Szklo M, Cushman M, et al. . Association of endothelial and oxidative stress with metabolic syndrome and subclinical atherosclerosis: Multi-ethnic study of atherosclerosis. Eur J Clin Nutr 2011;65:818–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Hutcheson R, Rocic P. The metabolic syndrome, oxidative stress, environment, and cardiovascular disease: The great exploration. Exp Diabetes Res 2012;2012:271028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Berasain C, Perugorria MJ, Latasa MU, et al. . The epidermal growth factor receptor: A link between inflammation and liver cancer. Exp Biol Med 2009;234:713–725 [DOI] [PubMed] [Google Scholar]
- 121. Tsujioka H, Yotsumoto F, Hikita S, et al. . Targeting the heparin-binding epidermal growth factor-like growth factor in ovarian cancer therapy. Curr Opin Obstet Gynecol 2011;23:24–30 [DOI] [PubMed] [Google Scholar]
- 122. DeLeve LD, Wang X, Kaplowitz N, et al. . Sinusoidal endothelial cells as a target for acetaminophen toxicity. Direct action versus requirement for hepatocyte activation in different mouse strains. Biochem Pharmacol 1997;53:1339–1345 [DOI] [PubMed] [Google Scholar]
- 123. Mitchell JR, Jollow DJ, Potter WZ, et al. . Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther 1973;187:211–217 [PubMed] [Google Scholar]
- 124. Springstead JR, Gugiu BG, Lee S, et al. . Evidence for the importance of OxPAPC interaction with cysteines in regulating endothelial cell function. J Lipid Res 2012;53:1304–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Tiganis T. Reactive oxygen species and insulin resistance: The good, the bad and the ugly. Trends Pharmacol Sci 2011;32:82–89 [DOI] [PubMed] [Google Scholar]
- 126. Tonks NK. Protein tyrosine phosphatases: From genes, to function, to disease. Nat Rev Mol Cell Biol 2006;7:833–846 [DOI] [PubMed] [Google Scholar]
- 127. Navab M, Ananthramaiah GM, Reddy ST, et al. . The oxidation hypothesis of atherogenesis: The role of oxidized phospholipids and HDL. J Lipid Res 2004;45:993–1007 [DOI] [PubMed] [Google Scholar]
- 128. Miyamoto S, Iwamoto R, Furuya A, et al. . A novel anti-human HB-EGF monoclonal antibody with multiple antitumor mechanisms against ovarian cancer cells. Clin Cancer Res 2011;17:6733–6741 [DOI] [PubMed] [Google Scholar]
- 129. Sato S, Drake AW, Tsuji I, et al. . A potent anti-HB-EGF monoclonal antibody inhibits cancer cell proliferation and multiple angiogenic activities of HB-EGF. PLoS One 2012;7:e51964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Sarantopoulos J, Mita MM, Birrer MJ, et al. . Phase 1 study of monotherapy with KHK2866, an anti-heparin-binding epidermal growth factor-like growth factor monoclonal antibody, in patients with advanced cancer. Target Oncol 2016;11:317–327 [DOI] [PubMed] [Google Scholar]
- 131. Kasai N, Adachi M, Yamano K. Preclinical pharmacokinetics evaluation of anti-heparin-binding EGF-like growth factor (HB-EGF) monoclonal antibody using cynomolgus monkeys via (89)Zr-immuno-PET study and the determination of drug concentrations in serum and cerebrospinal fluid. Pharm Res 2016;33:476–486 [DOI] [PubMed] [Google Scholar]
- 132. Moore KN, Bendell JC, LoRusso PM, et al. . First-in-human study of the anti-HB-EGF antibody U3-1565 in subjects with advanced solid tumors. Invest New Drugs 2019;37:147–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Oyagi A, Hara H. Essential roles of heparin-binding epidermal growth factor-like growth factor in the brain. CNS Neurosci Ther 2012;18:803–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Mitamura T, Higashiyama S, Taniguchi N, et al. . Diphtheria toxin binds to the epidermal growth factor (EGF)-like domain of human heparin-binding EGF-like growth factor/diphtheria toxin receptor and inhibits specifically its mitogenic activity. J Biol Chem 1995;270:1015–1019 [DOI] [PubMed] [Google Scholar]
- 135. Yagi H, Yotsumoto F, Sonoda K, et al. . Synergistic anti-tumor effect of paclitaxel with CRM197, an inhibitor of HB-EGF, in ovarian cancer. Int J Cancer 2009;124:1429–1439 [DOI] [PubMed] [Google Scholar]
- 136. Tsujioka H, Fukami T, Yotsumoto F, et al. . A possible clinical adaptation of CRM197 in combination with conventional chemotherapeutic agents for ovarian cancer. Anticancer Res 2011;31:2461–2465 [PubMed] [Google Scholar]
- 137. Nam SO, Yotsumoto F, Miyata K, et al. . Anti-tumor effect of intravenous administration of CRM197 for triple-negative breast cancer therapy. Anticancer Res 2016;36:3651–3657 [PubMed] [Google Scholar]
- 138. Ichise T, Adachi S, Ohishi M, et al. . Humanized gene replacement in mice reveals the contribution of cancer stroma-derived HB-EGF to tumor growth. Cell Struct Funct 2010;35:3–13 [DOI] [PubMed] [Google Scholar]
- 139. Miyamoto S, Yotsumoto F, Ueda T, et al. . BK-UM in patients with recurrent ovarian cancer or peritoneal cancer: A first-in-human phase-I study. BMC Cancer 2017;17:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Kim S, Graham MJ, Lee RG, et al. . Heparin-binding EGF-like growth factor (HB-EGF) antisense oligonucleotide protected against hyperlipidemia-associated atherosclerosis. Nutr Metab Cardiovasc Dis 2019;29:306–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Lee JC, Park BK, Choung S, et al. . Amelioration of hypercholesterolemia by an EGFR tyrosine kinase inhibitor in mice with liver-specific knockout of Mig-6. PLoS One 2014;9:e114782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Fountas A, Diamantopoulos LN, Tsatsoulis A. Tyrosine kinase inhibitors and diabetes: A novel treatment paradigm? Trends Endocrinol Metab 2015;26:643–656 [DOI] [PubMed] [Google Scholar]
- 143. Maloberti A, Farina F, Carbonaro M, et al. . In healthy normotensive subjects age and blood pressure better predict subclinical vascular and cardiac organ damage than atherosclerosis biomarkers. Blood Press 2018;27:262–270 [DOI] [PubMed] [Google Scholar]
- 144. Maloberti A, Vallerio P, Triglione N, et al. . Vascular aging and disease of the large vessels: Role of inflammation. High Blood Press Cardiovasc Prev 2019;26:175–182 [DOI] [PubMed] [Google Scholar]
- 145. Ridker PM, Everett BM, Thuren T, et al. . Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131 [DOI] [PubMed] [Google Scholar]
- 146. Geary RS, Norris D, Yu R, et al. . Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv Drug Deliv Rev 2015;87:46–51 [DOI] [PubMed] [Google Scholar]