Abstract
Coenzyme Q (Qn) is a vital lipid component of the electron transport chain that functions in cellular energy metabolism and as a membrane antioxidant. In the yeast Saccharomyces cerevisiae, coq1–coq9 deletion mutants are respiratory-incompetent, sensitive to lipid peroxidation stress, and unable to synthesize Q6. The yeast coq10 deletion mutant is also respiratory-deficient and sensitive to lipid peroxidation, yet it continues to produce Q6 at an impaired rate. Thus, Coq10 is required for the function of Q6 in respiration and as an antioxidant and is believed to chaperone Q6 from its site of synthesis to the respiratory complexes. In several fungi, Coq10 is encoded as a fusion polypeptide with Coq11, a recently identified protein of unknown function required for efficient Q6 biosynthesis. Because “fused” proteins are often involved in similar biochemical pathways, here we examined the putative functional relationship between Coq10 and Coq11 in yeast. We used plate growth and Seahorse assays and LC-MS/MS analysis to show that COQ11 deletion rescues respiratory deficiency, sensitivity to lipid peroxidation, and decreased Q6 biosynthesis of the coq10Δ mutant. Additionally, immunoblotting indicated that yeast coq11Δ mutants accumulate increased amounts of certain Coq polypeptides and display a stabilized CoQ synthome. These effects suggest that Coq11 modulates Q6 biosynthesis and that its absence increases mitochondrial Q6 content in the coq10Δcoq11Δ double mutant. This augmented mitochondrial Q6 content counteracts the respiratory deficiency and lipid peroxidation sensitivity phenotypes of the coq10Δ mutant. This study further clarifies the intricate connection between Q6 biosynthesis, trafficking, and function in mitochondrial metabolism.
Keywords: ubiquinone, mitochondrial metabolism, Saccharomyces cerevisiae, lipid, yeast, coenzyme Q, CoQ synthome, Coq10, Coq11
Introduction
Coenzyme Q (ubiquinone or Q)2 is a benzoquinone lipid that functions as an essential electron carrier within the electron transport chain (1). Because of its redox activities, Q is a versatile electron acceptor in biological pathways such as cellular respiration, oxidation of proline and sulfide, fatty acid β-oxidation, and pyrimidine biosynthesis (1–3). The reduced hydroquinone form of Q (ubiquinol or QH2) also serves as an important chain-breaking antioxidant shown to alleviate lipid peroxidative damage in cellular membranes (4).
For proper functional localization, Q relies on its polyisoprenoid tail to remain anchored at the mid-plane of phospholipid bilayers. The number of isoprene units (n) that comprise the polyisoprenoid tail of Qn depends on a species-specific polyprenyl diphosphate synthase (5), with Q10 representing the major isoform in humans (6). Patients unable to produce adequate levels of Q10 display a wide variety of health issues that stem from mitochondrial dysfunction across tissues (7). Attempts to ameliorate the consequences of primary Q10 deficiency by early Q10 supplementation have been partially successful in some cases (8); however, many patients fail to demonstrate full recovery, which is related to inefficient uptake of orally-supplied Q10. Because of the striking homology between human COQ genes and those of Saccharomyces cerevisiae (7, 9), studies of Q6 biosynthesis in S. cerevisiae may provide insight into human Q10 biosynthesis, leading to the discovery of potential therapeutic targets.
In S. cerevisiae, at least 14 nuclear-encoded mitochondrial proteins (Coq1–Coq11, Yah1, Arh1, and Hfd1) drive Q6 biosynthesis (7, 9). Many Coq polypeptides (Coq3–Coq9, and Coq11) are localized to the matrix side of the mitochondrial inner membrane, where they organize into a high-molecular-weight multisubunit complex known as the “CoQ synthome” (7, 9). Several lines of evidence suggest that correct assembly of the CoQ synthome is necessary for efficient Q6 biosynthesis (9–12). In fact, deletion of certain COQ genes results in decreased levels of other Coq polypeptides and contributes to a destabilized CoQ synthome in these mutants (12, 13). Recently, a protein of unknown function encoded by the ORF YLR290C was identified to associate with the CoQ synthome, via proteomic analysis of tandem affinity-purified tagged Coq proteins (14). YLR290C copurified with Coq5, Coq7, and Coq9, in addition to Q6 and late-stage Q6-intermediates (14). Furthermore, the ylr290cΔ mutant exhibited impaired de novo Q6 biosynthesis, despite preserving growth on a nonfermentable carbon source (14). Given its effects on Q6 biosynthesis and involvement with the CoQ synthome, YLR290C was renamed Coq11 (14).
In several fungi, Coq11 and Coq10 have evolved as fusion proteins (14), suggesting that Coq11 may have a functional relationship with Coq10 (15). High-throughput genetic analyses found COQ11 to correlate with both COQ2 and COQ10 (16). Whereas the coq mutants generally lack Q6, the coq10Δ mutant is different because it produces near WT amounts of Q6 in stationary phase and only has decreased de novo Q6 biosynthesis in log phase (17, 18). Although Q6 biosynthesis is only minimally decreased in the absence of COQ10, the coq10Δ mutant has decreased NADH and succinate oxidase activity and displayed sickly growth on respiratory medium (18). The coq10Δ mutant is sensitive to lipid peroxidation initiated by exogenously supplemented polyunsaturated fatty acids (PUFAs), indicating that the Coq10 polypeptide is also required for antioxidant protection by Q6 (17, 19).
The NMR structure of a Coq10 ortholog in Caulobacter crescentus was shown to possess a steroidogenic acute regulatory protein–related lipid transfer (START) domain (20) that can directly bind Q and late-stage Q-intermediates (17). Purified Coq10 from either S. cerevisiae or Schizosaccharomyces pombe eluted with the respective species' Q isoform (18, 21). This observation has prompted speculation that Coq10 acts as a Q6 chaperone protein required for delivery of Q6 from its synthesis site to sites where Q6 functions as an antioxidant and to the respiratory complexes, thereby bridging efficient de novo Q6 biosynthesis with respiration (17). Recent studies have shown a spatial compartmentalization of the mitochondrial inner membrane with the identification of different sites, such as the inner boundary membrane, the cristae membrane, and the ER-mitochondrial contact sites (22–25). Thus, for optimal respiratory competence, newly-synthesized Q6 must move from its site of synthesis (i.e. the ER-mitochondrial contact sites (23, 24)) to the cristae membrane where the respiratory complexes are concentrated (22). The presence of Coq10–Coq11 fusions in fungal species indicates that Coq11 may have a functional association with the Coq10 chaperone to facilitate or regulate Q6 transport for respiration in yeast.
In this work, the functional relationship between Coq10 and Coq11 was investigated using a series of single- and double-knockout mutants. Deletion of COQ11 alleviated the coq10Δ respiratory defect, increased Coq polypeptides and CoQ synthome stability, and partially rescued Q6 production. Based on this evidence, we propose a novel function for Coq11 as a negative modulator of Q6 biosynthesis in the mitochondria.
Results
Coq10 and Coq11 reside in similar compartments within the mitochondria
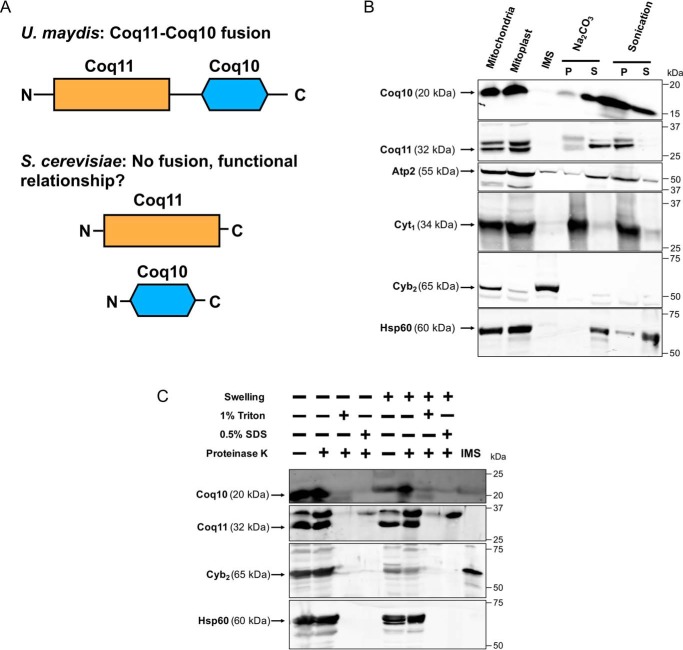
Previous phylogenetic analyses of numerous fungi revealed that Coq11-like proteins are fused to Coq10 (14). Protein fusions often indicate a functional relationship between corresponding homologs in other organisms, such as direct protein–protein interaction or operation within the same biological pathway (15). Although Coq10 and Coq11 are not physically fused in yeast (Fig. 1A), we sought to investigate whether there is a functional link between the two proteins. Because protein localization is often associated with function, we first performed mitochondrial fractionation to localize both Coq10 and Coq11. Coq10 has been localized previously (18), but fractionation was re-performed here in the context of Coq11.
Figure 1.
Coq11 and Coq10 are peripherally associated with the mitochondrial inner membrane facing the matrix, and Coq10 is additionally found in the mitochondrial matrix. A, Coq10 and Coq11 are fused in multiple fungi, suggesting an evolutionarily functional relationship between these proteins, although they are not found fused in S. cerevisiae. B, S. cerevisiae mitochondria purified from yeast strains cultured on YPGal medium were subjected to hypotonic swelling and centrifugation to separate the IMS proteins from mitoplasts. The mitoplasts were alkaline-treated (Na2CO3; pH 11.5) or sonicated and then separated by centrifugation (100,000 × g for 1 h) into supernatant (S) or pellet (P) fractions. C, intact mitochondria or mitoplasts were treated with 100 μg/ml proteinase K for 30 min on ice, with or without detergent. Mitochondrial polypeptide markers are as follows: Atp2, peripheral inner membrane protein; Cyb2, intermembrane space protein; Cyt1, integral inner membrane protein; and Hsp60, soluble matrix protein. Results are representative of two experiments.
S. cerevisiae mitochondria were fractionated as described under “Experimental procedures.” Purified mitochondria were incubated in hypotonic buffer to disrupt the outer membrane and release soluble components of the intermembrane space (IMS). The inner membrane was kept intact following hypotonic buffer treatment, protecting inner membrane and matrix proteins. Analysis of the fractions via immunoblot suggested that both Coq10 and Coq11 remained associated with the mitoplast fraction as opposed to colocalizing with the IMS marker cytochrome b2. Mitoplasts were further fractionated after sonication to separate soluble matrix components (supernatant, S) from membrane components (pellet, P). The soluble matrix marker Hsp60 was partially released into the supernatant by sonication as demonstrated in earlier work (26). Although Coq11 remained associated with the membrane fraction, Coq10 was partially dissociated in a similar manner to Hsp60 (Fig. 1B). Previous Coq10 colocalization following sonication demonstrated that Coq10 was solely associated with the membrane fraction (18). The detection of Coq10 in the supernatant shown in Fig. 1B may be due to increased sensitivity of the polyclonal antisera used in this study.
Alternatively, mitoplasts were subjected to alkaline carbonate extraction to separate peripheral membrane components (supernatant, S) from integral membrane and matrix components (pellet, P) (27). Coq10 and Coq11 were released into the supernatant following alkaline treatment (Fig. 1B), matching the peripheral inner membrane marker Atp2 (28). There was no colocalization with the pellet fraction, marked by the integral membrane protein Cyt1 (29). These results indicate that Coq10 and Coq11 are both peripheral inner membrane proteins, and Coq10 has additional localization to the matrix. The localization of Coq10 to the inner membrane is consistent with its putative role as a START domain protein possessing a hydrophobic cavity to bind and chaperone Q6 from its site of synthesis to complex III for respiration (17, 18). We hypothesize that dual mitochondrial matrix localization occurs when Coq10 is tightly bound to protein partners to decrease its hydrophobicity.
For better insight into the membrane association of Coq10 and Coq11, intact mitochondria or mitoplasts were treated with proteinase K in the absence or presence of two individual detergents (1% Triton X-100 or 0.5% SDS). Coq10 and Coq11 were both protected from protease treatment in purified mitochondria and mitoplasts, as was the matrix marker Hsp60 (Fig. 1C). When protease was used in the presence of either detergent in mitochondria or mitoplasts, all proteins became sensitive to the protease and were degraded. Expanding on the subfractionation results, these data indicate that Coq10 and Coq11 polypeptides are peripherally associated with the inner membrane facing the matrix side in yeast mitochondria, and Coq10 is also found in the mitochondrial matrix itself. The mitochondrial peripheral membrane association of these two proteins is also in agreement with their submitochondrial localization previously identified in a study of the yeast mitochondrial proteome (30).
coq10Δ respiratory defect is alleviated by deletion of COQ11
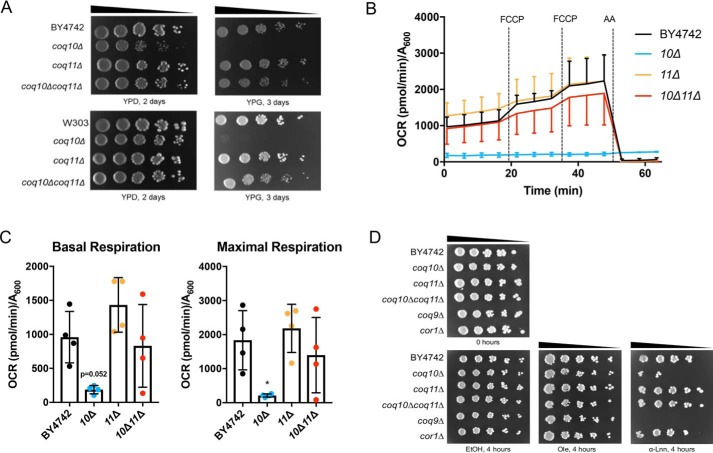
Based on similar mitochondria localization and genetic evolutionary evidence, a putative functional relationship between Coq10 and Coq11 was further probed using a series of coq10 and coq11 single- and double-knockout mutants. Strain descriptions are listed in Table 1. The Coq10 polypeptide is required for respiration in yeast, and mutants lacking coq10 have poor growth on nonfermentable carbon sources, including YPGlycerol, hereafter referred to as “YPG” (18). Unlike deletion of COQ10, coq11Δ mutants are respiratory-capable and have comparable growth to WT on nonfermentable carbon sources (14). When COQ11 was deleted in a coq10Δ mutant in two different yeast genetic backgrounds, the sickly growth of coq10Δ on nonfermentable YPG was rescued (Fig. 2A).
Table 1.
Genotype and source of yeast strains
| Strain | Genotype | Source |
|---|---|---|
| W303-1B | MAT α ade2-1 his3-1,15 leu2-,112trp1-1 ura3-1 | R. Rothsteina |
| BY4742 | MAT α his3Δ0 leu2Δ0 met15Δ0 ura3Δ0 | 53 |
| JM6 | MAT a his-4 ρ0 | 68 |
| JM8 | MAT α ade-1 ρ0 | 68 |
| BY4742 coq1Δ | MAT α his3Δ0 leu2Δ0 met15Δ0 ura3Δ0 coq1::KanMX4 | 69 |
| BY4741 coq2Δ | MAT a his3Δ0 leu2Δ0 met15Δ0 ura3Δ0 coq2::KanMX4 | 69 |
| BY4742 coq3Δ | MAT α his3Δ0 leu2Δ0 met15Δ0 ura3Δ0 coq3::KanMX4 | 69 |
| BY4742 coq4Δ | MAT α his3Δ0 leu2Δ0 met15Δ0 ura3Δ0 coq4::KanMX4 | 69 |
| BY4742 coq5Δ | MAT α his3Δ0 leu2Δ0 met15Δ0 ura3Δ0 coq5:: KanMX4 | 69 |
| BY4741 coq6Δ | MAT a his3Δ0 leu2Δ0 met15Δ0 ura3Δ0 coq6::KanMX4 | Dharmacon, Inc. |
| BY4742 coq7Δ | MAT α his3Δ0 leu2Δ0 met15Δ0 ura3Δ0 coq7::KanMX4 | 69 |
| BY4742 coq8Δ | MAT α his3Δ0 leu2Δ0 met15Δ0 ura3Δ0 coq8::KanMX4 | 69 |
| BY4742 coq9Δ | MAT αhis3Δ0 leu2Δ0 met15Δ0 ura3Δ0 coq9::KanMX4 | 69 |
| BY4742 coq10Δ | MAT α his3Δ0 leu2Δ0 met15Δ0 ura3Δ0 coq10::KanMX4 | 69 |
| BY4742 coq11Δ | MAT α his3Δ0 leu2Δ0 met15Δ0 ura3Δ0 coq11::LEU2 | This work |
| BY4742 coq10Δcoq11Δ | MAT α his3Δ0 leu2Δ0 met15Δ0 ura3Δ0 coq10::HIS3 coq11::LEU2 | This work |
| W303 coq10Δ | MAT α ade2-1 his3-1,15 leu2-3,112trp1-1 ura3-1 coq10::HIS3 | 18 |
| W303 coq10Δ | MAT a ade2-1 his3-1,15 leu2-3,112trp1-1 ura3-1 coq10::HIS3 | 18 |
| W303 coq10rev | MAT α ade2-1 his3-1,15 leu2-3,112trp1-1 ura3-1 coq10::HIS3 sup | This work |
| MB-10 | Diploid produced from W303 a coq10Δ x W303 α coq10Δrev | This work |
| W303 coq11Δ | MAT α ade2-1 his3-1,15 leu2-3,112trp1-1 ura3-1 coq11::LEU2 | This work |
| W303 coq10Δcoq11Δ | MAT α ade2-1 his3-1,15 leu2-3,112trp1-1 ura3-1 coq10::HIS3 coq11::LEU2 | This work |
| BY4741 cor1Δ | MAT a his3Δ0 leu2Δ0 met15Δ0 ura3Δ0 cor1::KanMX4 | 69 |
a Gift from Dr. Rodney Rothstein Department of Human Genetics, Columbia University.
Figure 2.
COQ11 deletion rescues the lack of growth on YPG, low-oxygen consumption rates, and lost Q6 antioxidant protection in the coq10Δ mutant. A, strains were grown overnight in 5 ml of YPD, diluted to an A600 = 0.2 with sterile PBS, and 2 μl of 5-fold serial dilutions were spotted onto fermentable (YPDextrose, YPD) or respiratory (YPGlycerol, YPG) medium, corresponding to a final A600 = 0.2, 0.04, 0.008, 0.0016, and 0.00032. Plates were incubated at 30 °C, and growth was captured after 2 or 3 days. B and C, quadruplicates of 25-ml cultures of WT, coq10Δ, coq11Δ, and coq10Δcoq11Δ yeast were grown in YPGal until they reached A600 ∼4. Yeast were diluted to an A600 = 0.1 in fresh YPGal and collected by centrifugation on poly-D-lysine-coated Seahorse XF96 microplates to assess oxygen consumption. B, representative traces of OCR of yeast strains with the XF96 extracellular flux analyzer. FCCP and antimycin A (AA) were sequentially added to evaluate mitochondrial respiratory states. Measurements were taken approximately every 4 min, as represented by points and their respective error bars. Four independent experiments were performed (Fig. S1), and each group of average traces represents 8–10 technical replicates. C, quantification of basal and maximal (maximal electron transport activity induced by the uncoupler FCCP) OCR as obtained from four independent experiments (Fig. S1). The data show the mean ± S.D., and the statistical significance as compared with WT is represented by *, p < 0.05. D, deletion of COQ11 in the coq10Δ rescues PUFA sensitivity. Results are representative of three experiments.
Quantitative respiratory capacity of each mutant was evaluated with an XF96 Extracellular Flux Analyzer (Fig. 2, B and C). Representative and normalized traces of oxygen consumption rates (OCR) of four independent experiments performed in nonrepressive medium (YPGal) are shown in Fig. 2B. Basal rates of OCR were measured prior to the addition of any small molecule inhibitors. Consistent with its slow growth on nonfermentable medium, the coq10Δ mutant had a low rate of basal oxygen consumption compared with WT (p = 0.052) (Fig. 2C). Basal OCR was rescued in the coq10Δcoq11Δ double mutant (Fig. 2C). Following the addition of two sequential injections of FCCP, a mitochondrial oxidative phosphorylation uncoupler, maximal respiration was also quantified. The maximal respiration of coq10Δcoq11Δ was rescued to that of WT (Fig. 2C). These results show that the deletion of COQ11 in a coq10Δ mutant confers a beneficial effect, such that both growth on respiratory medium and OCR are rescued to WT.
Deletion of COQ11 rescues PUFA sensitivity of the coq10Δ mutant
PUFA autoxidation is initiated by the radical-mediated abstraction of vulnerable hydrogen atoms at bis-allylic positions (31). The ensuing carbon-centered radical adds to molecular oxygen to form a lipid peroxyl radical that propagates lipid peroxidation, with the resulting lipid hydroperoxides ultimately driving cellular toxicity (32). The coq10Δ mutant is sensitive to treatment with exogenous PUFAs (Fig. 2D) (17, 19), likely because the Q6 chaperone function of Coq10 is required for the antioxidant function of Q6. Attenuated respiration in coq10Δ is rescued in the coq10Δcoq11Δ double knockout (Fig. 2, A–C), presumably through regained function of Q6 in the electron transport chain. To test whether the antioxidant capability of Q6 is also restored in the coq10Δcoq11Δ mutant, yeast strains were evaluated for sensitivity to added PUFAs (Fig. 2D). As anticipated, all strains were resistant to treatment with the monounsaturated oleic acid (Fig. 2D). Q6-less coq9Δ was sensitive to α-linolenic acid due to the lack of Q6 antioxidant protection (Fig. 2D). Conversely, the Q6-replete yet respiratory-deficient cor1Δ remained resistant to α-linolenic acid (Fig. 2D). Deletion of COQ11 rescued the α-linolenic acid sensitivity of the coq10Δ mutant, suggesting that the double knockout has restored Q6 antioxidant protection (Fig. 2D) despite the absence of Coq10 as a Q6 chaperone.
Independent coq10 revertant with rescued growth on respiratory medium harbors a mutation within COQ11
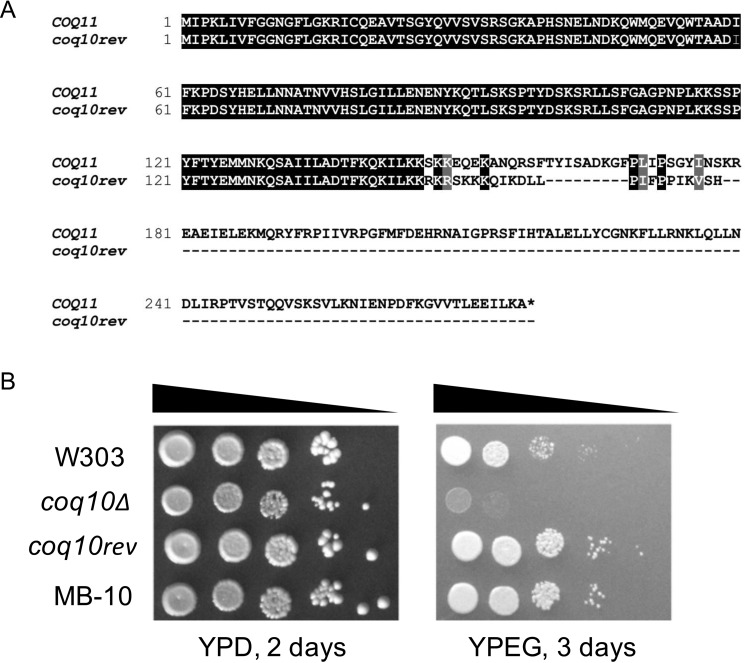
Although the coq10Δ mutant is unable to grow robustly on nonfermentable medium, an earlier study identified a spontaneous coq10 revertant (coq10rev) that arose when coq10Δ yeast was cultured for several weeks on nonfermentable medium containing ethanol and glycerol as carbon sources (18). Characterization of this revertant revealed a suppressor mutation within the COQ11 ORF, resulting in a truncated Coq11 protein that is predicted to be nonfunctional (Fig. 3A). This mutation was further assessed for dominance to determine whether it was sufficient to explain the respiratory competence of coq10rev. A haploid coq10Δ mutant crossed with haploid coq10rev produced diploid MB-10 (Table 1), which was capable of growth on respiratory medium (Fig. 3B). Illustrated growth patterns suggest that the coq11 truncated allele present in coq10rev is a dominant-negative mutation. Because the dominant mutation in coq10rev restores growth on respiratory medium via a functionally suppressive Coq11 truncation mutation, this mutant effectively validates the coq10Δcoq11Δ phenotype in an independent system.
Figure 3.
Spontaneous coq10 revertant with rescued respiratory capacity was identified to possess a base-pair deletion in COQ11, encoding a truncated Coq11 protein. A, alignment of the amino acid sequence of WT COQ11 ORF with the coq11 allele (coq10rev) present in the coq10 revertant. B, growth properties of WT were compared with coq10 mutants and diploid MB-10 (defined in Table 1). Strains were grown overnight in 8 ml of YPDextrose (YPD), diluted to an A600 = 0.2 with sterile PBS, and 2 μl of 5-fold serial dilutions were spotted onto fermentable YPD or respiratory (YPEGlycerol (YPEG)) medium, corresponding to a final A600 = 0.2, 0.04, 0.008, 0.0016, and 0.00032. Plates were incubated at 30 °C, and growth was captured after 2 or 3 days. Results are representative of three experiments.
Deletion of COQ11 fails to fully restore coq10Δ Q6 biosynthesis in whole cells
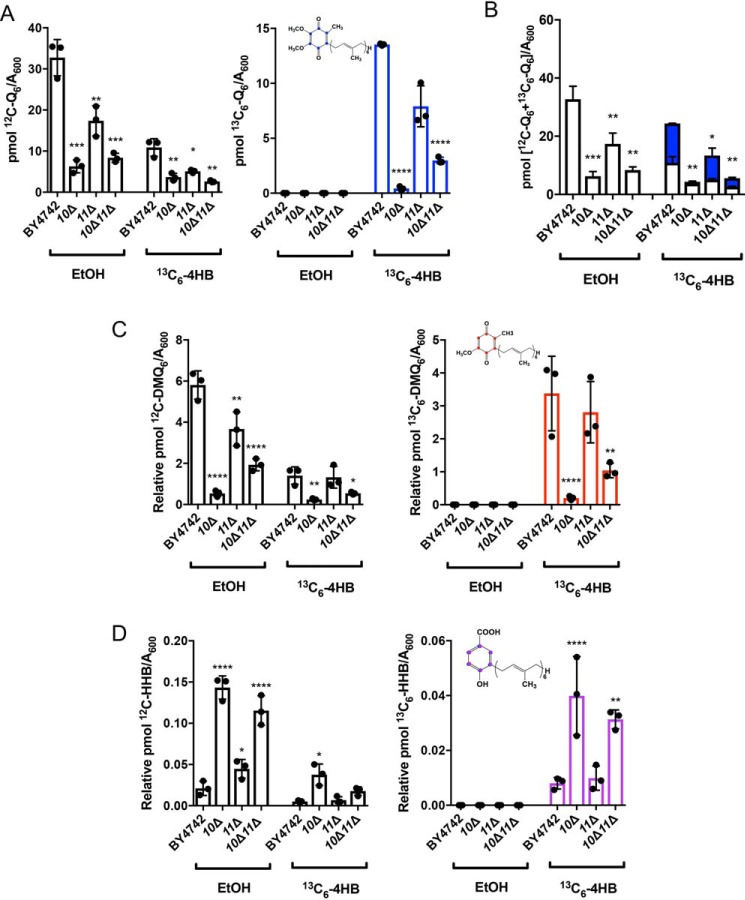
When a coq mutant displays anemic growth on respiratory medium, it is often indicative of inefficient Q6 biosynthesis (7, 9); yeast lacking COQ10 exhibit both poor growth on respiratory medium and decreased Q6 biosynthesis in log phase whole cells (17, 19). Although the coq11Δ mutant retains the ability to grow on nonfermentable medium, it is also characterized by impaired Q6 biosynthesis (14). Only a small amount of Q6 is required for growth on respiratory medium, ∼0.2–3% of the total Q6 found in WT (9, 33, 34). Because the coq10Δcoq11Δ double mutant has rescued respiration, we wanted to assess whether recovered growth was accompanied by increased Q6 biosynthesis. Whole-cell de novo–synthesized [13C6]Q6 and [12C]Q6 were measured in yeast by feeding the quinone ring-labeled precursor, [13C6]4HB, or EtOH vehicle control (Fig. 4). These analyses were performed in the fermentable, nonrepressive YPGal medium (35) to match the conditions of experiments involving purified mitochondria.
Figure 4.
Low amounts of de novo [13C6]Q6 in whole-cell lipid extracts of the coq10Δ mutant are only partially restored by deletion of COQ11. Triplicates of 6-ml cultures in YPGal were labeled at A600 ∼1 with 5 μg/ml [13C6]4HB or EtOH vehicle control, and 5 ml of each culture were collected after 4 h, lipid-extracted, and analyzed by LC-MS/MS. A, unlabeled [12C]Q6 and de novo–synthesized [13C6]Q6 (blue); B, total amount of Q6 determined from the sum of [13C6]Q6 and [12C]Q6; C, [12C]DMQ6 and [13C6]DMQ6 (red); and D, [12C]HHB and [13C6]HHB (purple) were measured from the whole-cell lipid extracts of WT and the coq10Δ, coq11Δ, and coq10Δcoq11Δ mutants. Values are the mean of three replicates. The data show mean ± S.D., and the statistical significance as compared with WT is represented by *, p < 0.05; ***, p < 0.001; and ****, p < 0.0001.
Consistent with previous results (14, 17), coq10Δ and coq11Δ had significantly decreased de novo-synthesized [13C6]Q6 and [12C]Q6 compared with WT (Fig. 4A). The coq10Δ mutant had a lower total Q6 content ([13C6]Q6 + [12C]Q6) than WT and also a lower total Q6 than coq11Δ (Fig. 4B). Deletion of COQ11 in coq10Δ yeast led to a slight increase in de novo-synthesized [13C6]Q6 and unchanged [12C]Q6 compared with coq10Δ (Fig. 4A). Therefore, the coq10Δcoq11Δ double mutant presented total Q6 contents that were significantly lower than either WT or coq11Δ (Fig. 4B). Given the robust growth of the coq10Δcoq11Δ double mutant on YPG, restored respiration, and resistance to PUFA treatment, the low Q6 concentrations observed are surprising.
Next, we quantified the concentrations of key Q6-intermediates in the same whole-cell yeast pellets. As shown previously (17, 19), the coq10Δ mutant contained lower amounts of the late-stage intermediate [13C6]DMQ6 and [12C]DMQ6 (Fig. 4C) than WT, and it accumulated the early-stage intermediate [13C6]HHB and [12C]HHB (Fig. 4D). In contrast to coq10Δ, the coq11Δ mutant mirrored WT production of both de novo-synthesized and unlabeled early- and late-stage intermediates (Fig. 4, C and D), as shown previously (14). Q6-intermediate trends in coq10Δcoq11Δ matched those of the coq10Δ mutant rather than coq11Δ (Fig. 4, C and D). The low Q6 content and accumulation of early-stage Q6-intermediates in the coq10Δcoq11Δ double knockout suggest the absence of COQ10 still produces a notable effect on Q6 biosynthesis, although respiratory capacity is rescued.
coq10Δcoq11Δ double mutant has increased mitochondrial Q6 compared with the coq10Δ single mutant
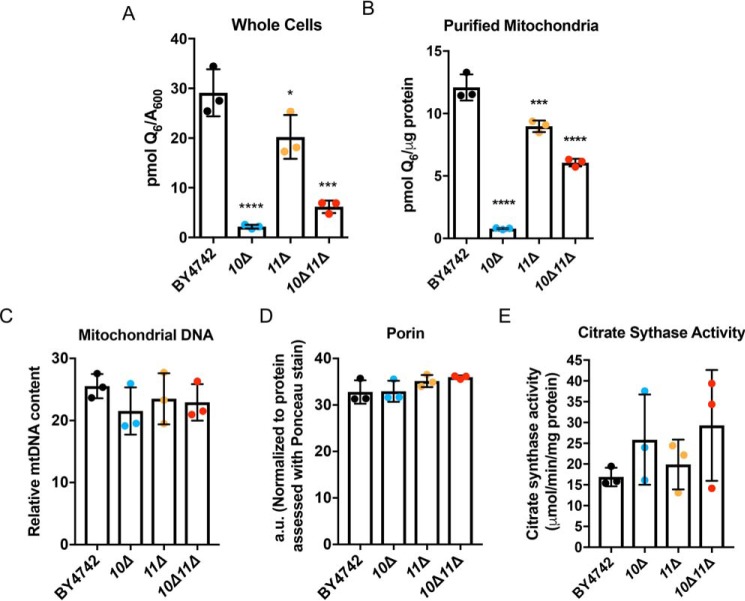
Although Q6 biosynthesis solely occurs within mitochondria, it is found in all cellular membranes (9). Therefore, Q6 was quantified in both whole cells and purified mitochondria from mutant and WT cells cultured under the same conditions (36). Whole-cell Q6 determined under mitochondrial purification conditions matched those determined in Figs. 4 and 5A. The coq10Δcoq11Δ double mutant made slightly more Q6 than the coq10Δ single mutant, but overall less Q6 compared with the coq11Δ single mutant. All mutants had lower whole-cell Q6 amounts than WT (Fig. 5A).
Figure 5.
Deletion of COQ11 in the coq10Δ mutant enhances mitochondrial Q6 content. Triplicates of 30-ml cultures of WT, coq10Δ, coq11Δ, and coq10Δcoq11Δ yeast were grown in YPGal until they reached A600 ∼4. A, 5 ml of whole cells from each culture were harvested, lipid-extracted, and analyzed by LC-MS/MS for Q6 content. Alternatively, WT, coq10Δ, coq11Δ, and coq10Δcoq11Δ yeasts were grown in YPGal until they reached A600 ∼4 and were subjected to mitochondrial preparation. B, lipids from triplicates of purified mitochondria (100 μg) were analyzed by LC-MS/MS for Q6 content. C–E, mitochondrial mass was estimated using three distinct methods. C, relative mitochondrial DNA to actin was quantified by qPCR. D, porin protein amounts were quantified by hand using ImageStudioLite following immunoblot and normalized to total protein levels evaluated by Ponceau stain. E, citrate synthase activity was determined using a colorimetric assay as outlined under “Experimental procedures.” Values are the mean of three replicates. The data show the mean ± S.D., and the statistical significance as compared with WT is represented by *, p < 0.05; ***, p < 0.001; and ****, p < 0.0001.
Similarly, mitochondrial Q6 content per microgram of mitochondrial protein was lower in coq11Δ than WT (Fig. 5B). However, deletion of COQ11 in the coq10Δ mutant increased the mitochondrial Q6 5-fold (Fig. 5B). Despite these profound differences in mitochondrial Q6 content, mitochondrial mass was consistent between strains as determined by three distinct assays (Fig. 5, C–E). Increased mitochondrial Q6 in the coq10Δcoq11Δ double mutant compared with coq10Δ indicates that the absence of COQ11 in part rescues defective Q6 synthesis in the coq10Δ mutant.
Low Coq protein content and destabilized CoQ synthome of the coq10Δ mutant are restored in the coq10Δcoq11Δ double mutant
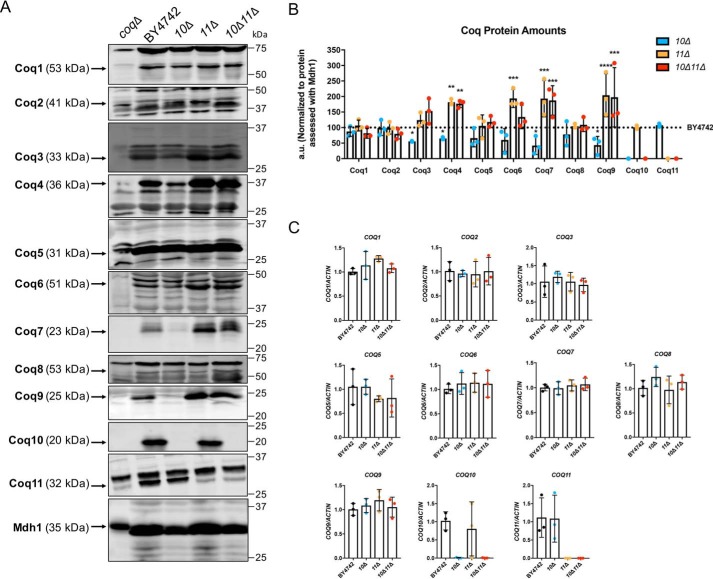
Proper formation of the CoQ synthome from component Coq polypeptides is required for efficient Q6 biosynthesis in yeast (9, 12, 13). Deletion of COQ10 causes a decrease in several other Coq polypeptides, including Coq3–Coq7, Coq9, as well as overall CoQ synthome destabilization (12, 13, 19). These results were confirmed when purified mitochondria from coq10Δ yeast were analyzed for each Coq polypeptide (Fig. 6A). The coq10Δ mutant had significantly decreased amounts of Coq3, Coq4, Coq7, and Coq9 compared to and plotted as a percentage of WT (Fig. 6B).
Figure 6.
Several Coq polypeptides have increased abundance in coq11Δ and coq10Δcoq11Δ mutants compared with WT. A, aliquots of purified mitochondria (25 μg) from WT, coq10Δ, coq11Δ, and coq10Δcoq11Δ yeasts were subjected to 10 or 12% Tris-glycine SDS-PAGE. Mitochondrial malate dehydrogenase (Mdh1) was included as a loading control, with a representative blot shown. The Coq5 protein also serves as a qualitative loading control, because the Coq5 polypeptide amounts remain unchanged across the panel of coq1–coq4 and coq6–coq10 deletion mutants (12). Aliquots of purified coqΔ mitochondria (coq1Δ–coq11Δ) were included as negative controls for immunoblotting with antisera to each of the Coq polypeptides. Black arrows highlight the location of each protein on the membrane. B, ImageStudioLite was used to quantify triplicates of each Coq protein band's intensity by hand, which were normalized to Mdh1 and plotted as a percentage of WT. The data show mean ± S.D., and the statistical significance is as compared with WT is represented by *, p < 0.05; **, p < 0.01; ***, p < 0.001; and ****, p < 0.0001. C, qPCR was used to determine COQ gene expression from whole-cell cultures of WT, coq10Δ, coq11Δ, or coq10Δcoq11Δ, and data were normalized to actin. COQ RNA levels remain unchanged in the coq10Δ, coq11Δ, or coq10Δcoq11Δ mutants as compared with WT.
In contrast to coq10Δ, the coq11Δ single mutant had elevated Coq4, Coq6, Coq7, and Coq9 (Fig. 6A), with protein quantification shown in Fig. 6B. Furthermore, the coq10Δcoq11Δ double mutant also had raised amounts of Coq4, Coq7, and Coq9 polypeptides compared with WT (Fig. 6, A and B). This increase in Coq proteins could not be explained by enhanced COQ transcription as there was no corresponding change in the concentration of the respective mRNAs, (Fig. 6C), although COQ4 mRNA was not detected.
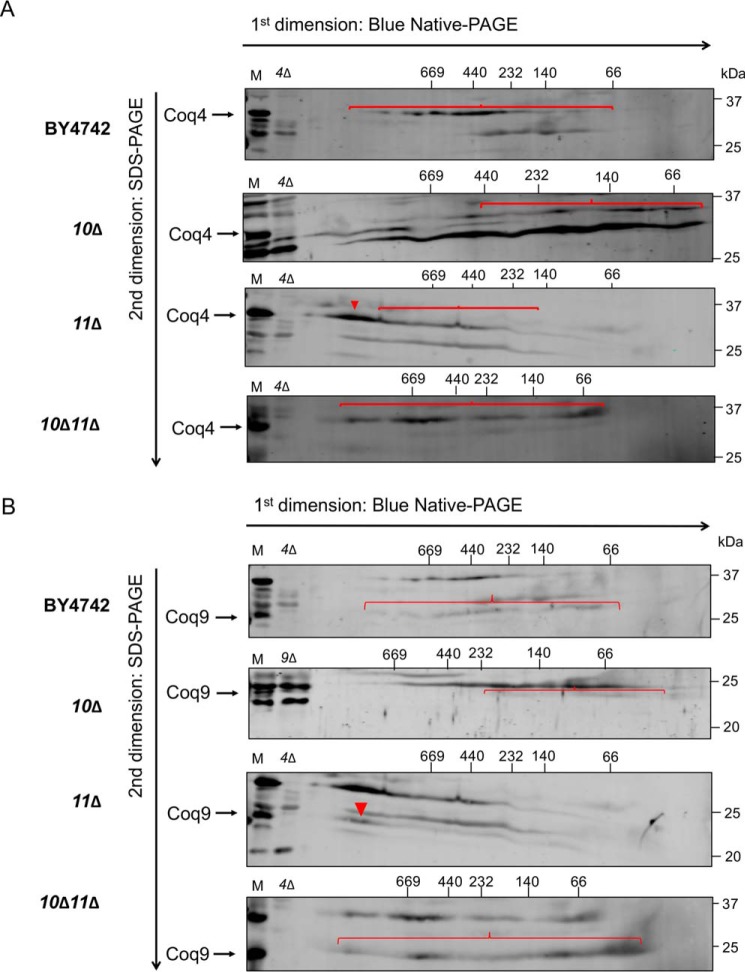
CoQ synthome formation was probed using two-dimensional blue native/SDS-PAGE (2D-BN/SDS-PAGE) with Coq4 and Coq9 serving as sensitive indicators of a high-molecular-weight complex (13). As expected, the CoQ synthome in WT yeast presented as a heterogeneous high-molecular-weight complex, spanning a range of ∼140 kDa to >1 MDa for Coq4 (Fig. 7A) and from ∼100 kDa to >1 MDa for Coq9 (Fig. 7B). Consistent with prior results (13, 19), the coq10Δ mutant displayed a highly-destabilized CoQ synthome, with a disappearance of large complexes that were replaced by lower-molecular-weight subcomplexes less than ∼440 kDa for Coq4 (Fig. 7A) and less than ∼232 kDa for Coq9 (Fig. 7B). The coq11Δ mutant had a stabilized CoQ synthome compared with WT, with high-molecular-weight complexes shifting to the left and collapsing into a more homogeneous complex spanning ∼900 kDa to >1 MDa for both Coq4 (Fig. 7A) and Coq9 (Fig. 7B). When COQ11 was deleted in combination with COQ10, there was a substantial rescue of high-molecular-weight complex formation compared with the coq10Δ single mutant (Fig. 7, A and B). The CoQ synthome of the coq10Δcoq11Δ double mutant appeared similar to that of coq11Δ complexes spanning ∼66 kDa to > 1 MDa for Coq4 (Fig. 7A) and from ∼66 kDa to > 1 MDa for Coq9 (Fig. 7B). However, the deletion of COQ11 in coq10Δ does not negate the effect from the COQ10 deletion, as it does not restore small subcomplexes <140 kDa to higher molecular weights (Fig. 7, A and B). These CoQ synthome signals for coq11 mutants were complementary to the observed increased Coq polypeptides, indicating that the absence of COQ11 enhanced the Q6 biosynthetic machinery.
Figure 7.
Deletion of COQ11 in the coq10Δ mutant restores the CoQ synthome. Aliquots (100 μg) of purified mitochondria from WT, coq10Δ, coq11Δ, and coq10Δcoq11Δ yeasts cultured in YPGal were solubilized with digitonin and separated with two-dimensional BN/SDS-PAGE. Following transfer of proteins to membranes, the CoQ synthome was visualized as a heterogeneous signal from ∼66 to ∼669 kDa in WT control with antibodies to A, Coq4, or B, Coq9. Intact mitochondria (25 μg) from each designated strain was included as a loading control (M). Aliquots of coq4Δ- or coq9Δ-purified mitochondria (25 μg) were included as a negative control for the antisera to Coq4 and Coq9, as the Coq9 polypeptide is absent from the coq4Δ mutant. Red arrowheads and brackets indicate distinct complexes.
High-copy COQ8 does not restore Q6 content in the coq10Δcoq11Δ single mutant
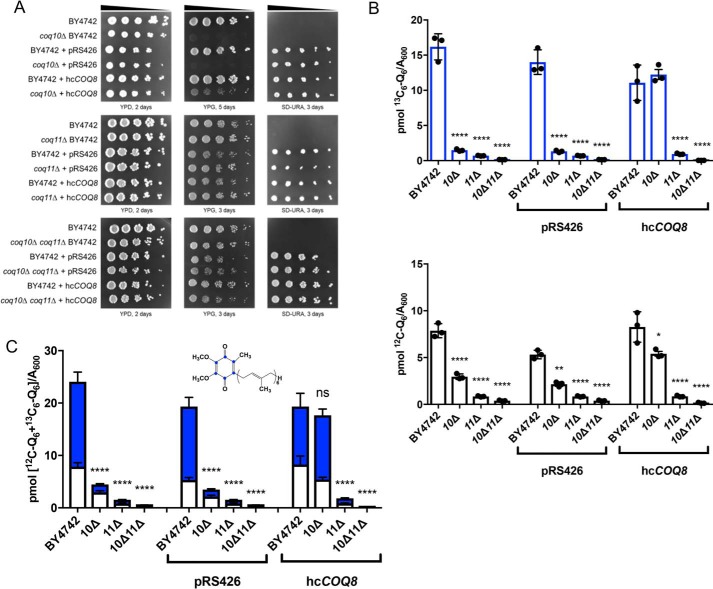
The Coq8 polypeptide is a member of an ancient atypical kinase family (37), with several conserved kinase motifs that are essential for Q biosynthesis (13, 38). Prior studies have demonstrated that overexpression of Coq8 in a coq10Δ mutant increased the otherwise low amounts of several key Coq polypeptides and stabilized CoQ synthome formation (13). This is similar to the phenotype observed when COQ11 was deleted in the coq10Δ mutant. Furthermore, Coq8 overexpression has also been shown to influence Q6 biosynthesis, including the restoration of late-stage Q6-intermediates in coq5–coq9 null mutants (39). Although the CoQ synthome of the coq10Δ mutant was stabilized by deletion of COQ11, Q6 and late-stage Q6-intermediates remained lower compared with WT and the coq11Δ single mutant (Fig. 4C). We hypothesized that the overexpression of Coq8 in the coq10Δ and coq10Δcoq11Δ mutant may restore Q6 content in both mutants.
WT, coq10Δ, coq11Δ, and coq10Δcoq11Δ were analyzed for growth on nonfermentable medium and Q6 biosynthesis upon transformation with high-copy COQ8 (hcCOQ8, Table 2) or empty vector control (Fig. 8). Similar to previous observations in a different yeast genetic background (18), coq10Δ-expressing hcCOQ8 regained the ability to grow on respiratory medium (Fig. 8A). This growth phenotype may be explained by a stabilized CoQ synthome in the coq10Δ mutant harboring hcCOQ8 (13). However, hcCOQ8 had no material effect on the growth properties of WT, coq11Δ, or coq10Δcoq11Δ strains on YPG (Fig. 8A).
Table 2.
Yeast expression vectors
Figure 8.
Overexpression of the CoQ synthome stabilizer, COQ8, has no effect on Q6 synthesis in the coq10Δcoq11Δ mutant. WT, coq10Δ, coq11Δ, and coq10Δcoq11Δ mutants were transformed with high-copy COQ8 (hcCOQ8) or empty vector (pRS426) plasmids. A, strains were grown overnight in 5 ml of selection medium, diluted to an A600 = 0.2 with sterile PBS, and 2 μl of 5-fold serial dilutions were spotted onto YPD, YPG, or selection medium (SD−Ura), corresponding to a final A600 = 0.2, 0.04, 0.008, 0.0016, and 0.00032. Plates were incubated at 30 °C, and growth was captured after 2 or 3 days. Triplicates of 5 ml of culture in selection medium were labeled with 5 μg/ml [13C6]4HB, collected after 4 h, lipid-extracted, and analyzed by LC-MS/MS. B, [12C]Q6 (white) and de novo [13C6]Q6 (blue). C, total amount of Q6 was also plotted from the sum of [13C6]Q6 and [12C]Q6. The values are the means of three replicates. The data show mean ± S.D., and the statistical significance as compared with WT is represented by *, p < 0.05; **, p < 0.01; ***, p < 0.001; and ****, p < 0.0001. The ns signifies that values are not significantly different from WT.
Each strain was grown in minimal selection medium to maintain plasmid expression and was analyzed for Q6 biosynthesis following metabolic labeling with the ring-labeled Q6 precursor, [13C6]HB (Fig. 8, B and C). Changing the growth medium from rich (i.e. YPGal) to minimal synthetic (i.e. SD and drop-out dextrose media (DOD)) changed the relative amounts of Q6 content among the mutants (Figs. 4B versus 8C). Although WT [13C6]Q6 and total Q6 content is similar in Figs. 4B and 8C, the values for coq11Δ and the double mutant are quite different. When grown in YPGal, coq10Δ had the lowest Q6 content, followed by the double mutant coq10Δcoq11Δ, with coq11Δ having the closest Q6 content to WT (Figs. 4, A and B, and 5A and Fig. S2, A and B). In contrast, when these strains are cultured in SD−Ura (Fig. 8, B and C), the double mutant coq10Δcoq11Δ had the lowest Q6 content, as compared with either the coq10Δ or coq11Δ single mutant strains. Growth on minimal dextrose medium in the absence of plasmid selection produced similar trends (Fig. S2, C and D, and Fig. S3, A and B).
Upon Coq8 overexpression, coq10Δ had increased de novo-synthesized [13C6]Q6 and [12C]Q6 (Fig. 8B), and total Q6 concentrations ([13C6]Q6 + [12C]Q6) were restored to those of WT (Fig. 8C). This finding is consistent with previous results, which also indicated that hcCOQ8 restored Q biosynthesis and amounts of Coq polypeptides and the CoQ synthome in the coq10Δ mutant (13, 17, 18). Intriguingly, expression of hcCOQ8 had no effect on de novo or unlabeled Q6 content in either the coq11Δ single mutant or the coq10Δcoq11Δ double mutant (Fig. 8B). Total Q6 contents of both coq11Δ and coq10Δcoq11Δ remained significantly decreased compared with WT (Fig. 8C). Together, these results show that the rescue of the coq10Δ mutant mediated by Coq8 overexpression requires Coq11.
Expression of low-copy COQ11 rescues only some of the phenotypes of the coq10Δcoq11Δ mutant
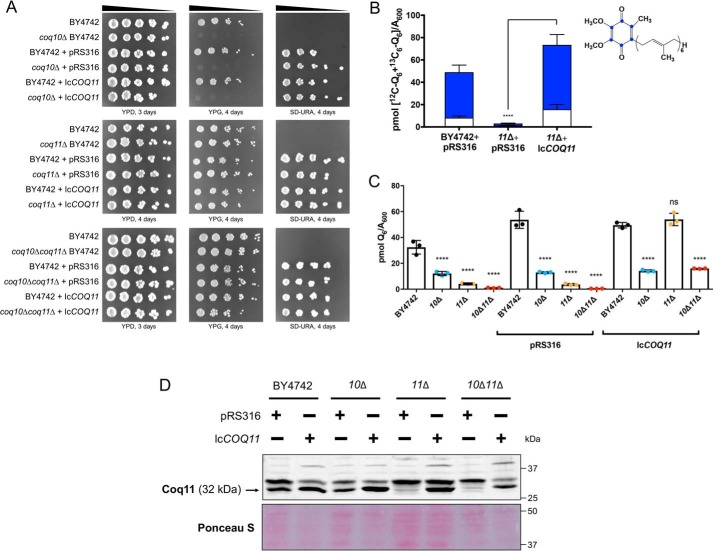
The functional complementation of coq11Δ single and coq10Δcoq11Δ double mutants with low-copy COQ11 was assessed (lcCOQ11, Table 2). As expected, the coq10Δ mutant, coq10Δ with empty vector, and coq10Δ complemented with lcCOQ11 showed slow growth on the nonfermentable carbon source YPGlycerol (Fig. 9A). Because yeast lacking COQ11 retain respiratory capacity (Fig. 2) (14), coq11Δ complemented with lcCOQ11 had no detectable change in growth phenotype compared with either the coq11Δ mutant or coq11Δ with empty vector (Fig. 9A). Intriguingly, when lcCOQ11 was expressed in the coq10Δcoq11Δ double mutant, there was no repression of growth on YPG compared with that of the coq10Δ mutant (Fig. 9A).
Figure 9.
Low-copy COQ11 rescues only some of the phenotypes of the coq10Δcoq11Δ double mutant. A low-copy plasmid expressing COQ11 (lcCOQ11) and an empty vector control (pRS316) were transformed into WT, coq10Δ, coq11Δ, and coq10Δcoq11Δ. A, strains were grown overnight in 5 ml of YPDextrose (YPD) and diluted to an A600 = 0.2 with sterile PBS, and 2 μl of 5-fold serial dilutions were spotted onto YPDextrose, YPGlycerol (YPG), or selection medium (SD−Ura), corresponding to a final A600 = 0.2, 0.04, 0.008, 0.0016, and 0.00032. Plates were incubated at 30 °C, and growth was captured after 3 or 4 days. B, rescue of mutant de novo and unlabeled Q6 production was initially demonstrated in coq11Δ. Triplicates of 6-ml cultures in selection medium were labeled with 5 μg/ml [13C6]4HB, and 5 ml of each culture was collected after 4 h, lipid-extracted, and analyzed by LC-MS/MS. Total amount of Q6 was plotted from the sum of de novo [13C6]Q6 (blue) and unlabeled [12C]Q6. Values are the mean of three replicates. C, rescue of mutant Q6 content was evaluated in each mutant strain. Triplicates of 6-ml cultures in selection medium were grown until A600 ∼4. Lipid extracts from 5 ml of each culture were analyzed by LC-MS/MS. The data show the means ± S.D., and the statistical significance as compared with WT is represented by *, p < 0.05; **, p < 0.01; ***, p < 0.001; and ****, p < 0.0001. ns signifies that values are not significantly different from WT. D, aliquots of purified mitochondria (25 μg) from WT and mutant yeast containing empty vector or lcCOQ11 were isolated in YPGal medium and were separated on 10% Tris-glycine SDS-polyacrylamide gels to determine Coq11 protein expression. Proteins stained with Ponceau stain were used as loading control.
This observation suggests that Q6 biosynthesis in coq10Δcoq11Δ may not be affected by lcCOQ11. To determine the effect of lcCOQ11 expression on mutant Q6 biosynthesis, yeast was grown in selection medium to maintain plasmid expression. We tested whether lcCOQ11 expression rescued Q6 content in the coq11Δ mutant. Expression of lcCOQ11 in coq11Δ efficiently rescued total Q6 ([13C6]Q6 + [12C]Q6) to WT amounts (Fig. 9B).
Finally, whole-cell steady-state Q6 concentrations were evaluated in all mutants. Even though lcCOQ11 complementation did not suppress coq10Δcoq11Δ growth on YPGlycerol (Fig. 9A), Q6 concentrations were increased in coq10Δcoq11Δ to a level comparable with that of coq10Δ (Fig. 9C). This implies that Coq11's role in Q6 biosynthesis is not effective when the COQ11 ORF is expressed on a single-copy plasmid in the absence of COQ10. Perhaps Coq11 expression from a plasmid does not account for multiple levels of regulation that occur through endogenous expression. Alternatively, the coq10Δcoq11Δ double mutant may have slightly lower amounts of the Coq11 polypeptide compared with coq11Δ when both are complemented by lcCOQ11 (Fig. 9D), and these lower levels may not be sufficient to suppress respiration (Fig. 9A).
Discussion
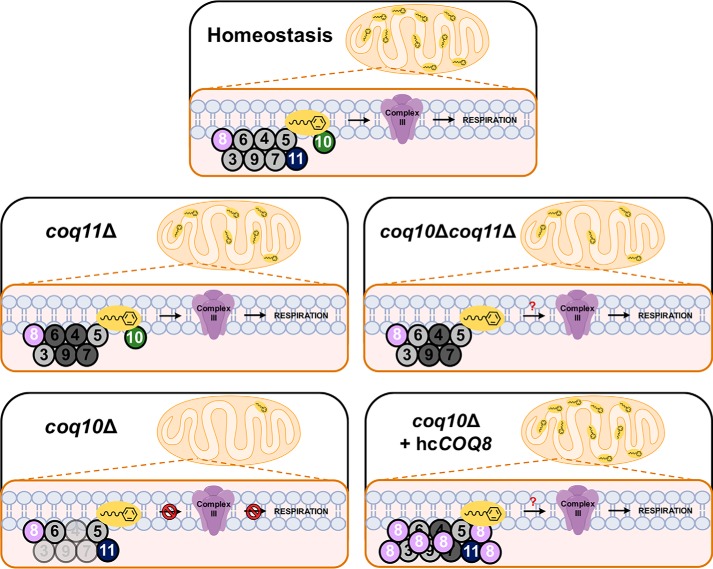
This work investigated a putative functional relationship between Coq10 and Coq11 within the S. cerevisiae Q6 biosynthetic pathway. The presence of Coq10–Coq11 fusions in several Ustilaginaceae species suggests that these proteins may directly interact or participate in the same biological pathway in yeast (Fig. 1A) (14). Yeast Coq10 and its orthologs were previously shown to be required for efficient de novo Q biosynthesis and respiration (17, 18). We were surprised to discover that the yeast coq10Δ growth defect on nonfermentable medium and oxygen consumption rates were rescued upon deletion of COQ11 (Fig. 2). Moreover, spontaneous revertants isolated from coq10Δ yeast were previously found to exhibit growth on nonfermentable medium (18). We have shown that this reversion is due to a dominant base pair deletion within the COQ11 gene, likely resulting in a nonfunctional, truncated Coq11 protein (Fig. 3). Mutants lacking both COQ10 and COQ11 when cultured on YPGal have increased de novo Q6 production (Fig. 4A) in addition to a 5-fold increase in mitochondrial Q6 content compared with the coq10Δ single knockout (Fig. 5B). Therefore, we have demonstrated that deletion of the Coq11 polypeptide in a coq10Δ mutant confers a beneficial effect on both respiration and Q6 biosynthesis (Fig. 10).
Figure 10.
Scheme postulating Coq11 as a modulator of Q6 synthesis in mitochondria. Under homeostasis, Coq11 associates with the CoQ synthome and acts as a modest negative regulator of Q6 synthesis via Coq10. In the absence of COQ11, several Coq polypeptides are increased (dark shading), and the CoQ synthome is stabilized compared with WT cells, despite a slight decrease in Q6 content. In contrast, the coq10Δ mutant is missing the Q6 chaperone protein, resulting in a decreased amount (light shading) of Coq3, Coq4, Coq7, and Coq9, a destabilized CoQ synthome, substantially decreased Q6 concentrations, and a lack of respiration. The deletion of COQ11 in the coq10Δ mutant counterbalances the destabilized CoQ synthome and decreased Q6 content phenotype of the coq10Δ mutant, allowing the coq10Δcoq11Δ double mutant to grow on YPG and respire. Expression of hcCOQ8 in the coq10Δ mutant produces many similar phenotypes to COQ11 deletion in coq10Δ cells, including increased Coq polypeptides and a stabilized CoQ synthome (13), resulting in restored growth on YPG. Unlike the coq10Δcoq11Δ double mutant, coq10Δ + hcCOQ8 has rescued Q6 content pointing to an additional role of Coq11 in Q6 biosynthesis, redox regulation, or transportation.
Enhanced Q6 content in the coq10Δcoq11Δ double mutant compared with the coq10Δ single mutant may be partially due to increased amounts of several key Coq polypeptides (Fig. 6) and CoQ synthome stabilization (Fig. 7). The ring-modifying enzymes within the Q6 biosynthetic pathway colocalize to numerous distinct “CoQ domains” in vivo, and proper assembly of the CoQ synthome components is required for the presence of these CoQ domains (23). Two recent studies demonstrated that mitochondria isolated from yeast lacking COQ10 have a reduced number of CoQ domain puncta (23, 24). This is likely due to lower levels of certain Coq polypeptides and partial CoQ synthome destabilization in the coq10Δ mutant (17, 19), which was confirmed in this work (Figs. 6 and 7). In contrast, coq11Δ yeast displayed significantly higher amounts of Coq4, Coq6, Coq7, and Coq9 polypeptides (Fig. 6). The CoQ synthome was likewise shifted to a higher molecular weight in coq11Δ mitochondria compared with WT (Fig. 7). When Coq9–yEGFP was used as a marker for CoQ domains, coq11Δ had increased CoQ domain intensity that stemmed from amplified expression of Coq9–yEGFP, although the number of domains was similar to WT. Mutants lacking essential Coq polypeptides coq1–coq9, or coq10, displayed significantly less Coq9-labeled domains (24). The CoQ synthome stabilization seen in coq11Δ via 2D-BN/SDS-PAGE analyses performed in this work (Fig. 7) agrees with the observation of increased CoQ domains and argues that the CoQ synthome is truly stabilized upon deletion of COQ11, as opposed to inducing a greater number of domains.
These observations are consistent with the biochemical data that led to the notion of a CoQ synthome whose formation relies on the presence of prenylated Q-intermediates (13, 40, 41). The coq10Δ mutant produced more early-stage intermediates (HHB) and had less late-stage intermediates (DMQ6) compared with WT (Fig. 4, C and D), resulting in less CoQ synthome formation (Fig. 7). Because coq11Δ yeast displayed comparable amounts of early- and late-stage Q6-intermediates to WT (Fig. 4, C and D), this mutant retained the ability to fully form the CoQ synthome (Fig. 7). The double knockout synthesized varying amounts of early- and late-stage Q6-intermediates that were largely in-between those of the single knockouts (Fig. 4, C and D). The CoQ synthome is thus able to form in coq10Δcoq11Δ yeast, albeit not to the efficiency of the coq11Δ single mutant (Fig. 7). We suspect that the accumulation of Coq polypeptides and restoration of the CoQ synthome in the coq10Δcoq11Δ double mutant are sufficient to allow for Q6 to escape its site of synthesis and reach the respiratory complexes, despite an absence of the Coq10 Q6 chaperone protein and lower Q6 in this strain (Fig. 10). How this occurs is presently not known. One possible explanation could be that Coq11 inhibits a currently unidentified Q6 chaperone with lower efficiency than Coq10 that is able to rescue respiration only in the absence of both Coq10 and Coq11.
Overexpression of the Q6-biosynthetic protein Coq8 also rescued coq10Δ mutant growth on nonfermentable medium (Fig. 8A) and de novo Q6 biosynthesis (Fig. 8, B and C) (17). Coq8 has been implicated in the partial extraction of Q6-intermediates out of the mitochondrial inner membrane for enzymatic modification by other Coq proteins, allowing for appropriate Q6 biosynthesis (25). Prior investigations also revealed that Coq8 overexpression in coq10Δ yeast increased Coq4 and Coq9 polypeptides and stabilized the CoQ synthome (13, 24). Despite the substantial benefit of Coq8 overexpression in a coq10Δ single mutant, Coq8 overexpression failed to enhance Q6 biosynthesis in the coq10Δcoq11Δ double knockout (Figs. 8, B and C, and 10). The absence of COQ11 in the double mutant is sufficient to restore Coq polypeptides and CoQ synthome formation (Figs. 6, A and B, 7, and 10). Coq11 and Coq8 may therefore work by different mechanisms to serve opposing functions for CoQ synthome and Coq polypeptide stabilization. Furthermore, it is clear that Coq11 is required to perform an additional function to Coq8, as hcCOQ8 requires the presence of Coq11 to restore Q6 biosynthesis (Figs. 8, B and C, and 10).
Proper CoQ synthome formation is not only required for efficient Q6 biosynthesis, but it is also vital for the establishment of ER-mitochondrial contact sites mediated by the ER-mitochondrial encounter structure (ERMES) complex (23, 24). The ERMES complex is essential for lipid exchange between the ER and mitochondria (42). Specifically, ERMES null mutants have irregular Q6 cellular distribution and a destabilized CoQ synthome (23). When COQ10 was deleted in yeast expressing Coq6–GFP, there was a significant decrease in Coq6–GFP puncta colocalization with Mdm34–mCherry, a component of the ERMES complex (23). These results indicate that CoQ synthome positioning next to the ERMES complex, and subsequent Q6 distribution from the mitochondria, depends on Coq10. Because COQ11 deletion stabilizes the CoQ synthome (Figs. 6, A and B, and 7), it is possible that coq11Δ mutants have more ER-mitochondrial contacts through the ERMES complex and improved transfer of lipids between these organelles. Therefore, one possible role of Coq11 may be an auxiliary protein mediating lipid transport between the ER and mitochondria.
In a recent study, Coq11 was named Mrx2, as part of the mitochondrial organization of gene expression (MIOREX) complex involved in the mitochondrial genetic expression system (43). Considering the proposed regulatory function of Coq11 in CoQ synthome assembly, it is tempting to speculate that Coq11 offers a mechanism to couple Q6 synthesis with the assembly of the respiratory complexes. When the synthesis of the respiratory complexes is more active, Coq11 is associated to the MIOREX complex, and Q6 synthesis at ER junctions is stimulated. Coq11 dual localization in the mitochondrial inner membrane, to the MIOREX complex and the CoQ synthome, would also explain the sensitivity of yeast cells to the number of COQ11 copies, as well as present another example of a loop system control for balanced expression of mitochondrial products (44).
Another potential explanation for the phenotypes induced by the knockout of Coq11 relates to its structural connection with the short-chain dehydrogenase/reductase (SDR) superfamily of NAD(P)(H)-dependent oxidoreductases (14, 45). These enzymes catalyze an assortment of reactions, including isomerization, decarboxylation, epimerization, imine reduction, and carbonyl–alcohol oxidoreduction (45). SDR superfamily proteins contain a conserved protein structural motif known as a Rossmann fold, a feature used in the binding of nucleotide cofactors such as FAD, FMN, and NAD(P) (46). The crystal structure of the Pseudomonas aeruginosa gene UbiX, which catalyzes the decarboxylation step in Q9 biosynthesis, revealed a Rossmann fold with a bound FMN (47). Thus, Coq11 may use its Rossmann fold in conjunction with a nucleotide cofactor to perform similar redox chemistry in S. cerevisiae Q6 biosynthesis. The ratio of QH2/Q serves as a metabolic sensor for electron transport chain efficiency (48). High QH2/Q ratios induce respiratory complex I-mediated reverse electron transport (RET) under physiological conditions in both Drosophila and mammalian cell lines (48, 49). Superoxide and secondary reactive species produced specifically through complex I RET extended Drosophila lifespan and improved mitochondrial function in a model of Parkinson's disease (49). RET induced by over-reduction of the Q pool presumably generates a superoxide-dependent signal essential for homeostasis, such that manipulation of the Q redox state is beneficial for mitochondrial function (48, 49). Mitochondrial phenotypes in the absence of COQ11, including restored respiration in coq10Δcoq11Δ and up-regulated Q6 machinery (Figs. 2, A–C, 6, and 7), seem to correlate well with the aforementioned effects of Q6H2 accumulation. Yeast coq11Δ and coq10Δcoq11Δ mutants retain antioxidant protection by Q6H2, demonstrated by their resistance to treatment with exogenously-added PUFAs (Fig. 2D). Because cells lacking the Coq11 polypeptide maintain Q6H2 as an antioxidant, it follows that Coq11 could be involved in the oxidation of Q6H2 to Q6.
The phenotypes of Coq10 and Coq11 seen in this work are similar to those in both fungi and mammalian hosts. Several fungi use Coq11 or Coq11-like proteins as NAD-dependent epimerases/dehydratases, NADH-ubiquinone oxidoreductases, and NADH dehydrogenase subunits (14). Coq11 orthologs are commonly found in plant and algae genomes, including the chloroplast-localized flavin reductase protein At1g32220 from the land plant Arabidopsis thaliana, which is thought to be involved in plastoquinone biosynthesis and storage (14, 50). The closest but distinct higher eukaryotic Coq11-like protein is the SDR subfamily protein NDUFA9 (14), an auxiliary subunit of complex I in humans (51). Patients with decreased NDUFA9 expression are unable to properly assemble complex I and may develop a degenerative infancy respiratory disorder known as Leigh syndrome (52). Although yeast cells do not possess complex I, this evidence indicates that Coq11 may play a crucial role in respiratory regulation or function, supporting the observations of this study.
The function of Coq10 is widely conserved across different organisms. Expression of the Coq10 homolog from C. crescentus (CC1736) rescues the impaired respiration and antioxidant function of Q6 in coq10 yeast mutants (17). The NMR structure of CC1736 reveals a START domain, which is known to bind lipids via a hydrophobic tunnel (20). Studies of S. pombe Coq10 demonstrate that it is able to bind Q10 (21). One proposed function of Q binding by CC1736 and Coq10 from S. pombe may be to regulate Q delivery to its proper sites in the respiratory complexes. Humans have two distinct homologs of yeast Coq10: COQ10A and COQ10B. Expression of either human protein rescues the coq10Δ respiratory deficiency and sensitivity to oxidative stress, and it restores the amounts of Coq polypeptides to WT (19). The conserved function of yeast Coq10 with human COQ10A and COQ10B suggests that the findings of this work will shed light on the role of Coq10 as a chaperone in humans, leading to a better understanding of the pathobiology of Q10 diseases.
In summary, this work reveals that Coq11 plays a regulatory role to maintain Q6 homeostasis in concert with Coq10 in S. cerevisiae (Fig. 10). The absence of COQ11 caused an augmentation of Q6 production and respiration in the coq10Δ mutant, indicating that Coq11 confers a negative effect on the CoQ synthome. Coq11 may be crucial for Q6 function in addition to Q6 biosynthesis, as total whole-cell and mitochondrial Q6 content remained lower than WT in coq11Δ and coq10Δcoq11Δ mutants.
Experimental procedures
All reagents were obtained commercially from Thermo Fisher Scientific unless otherwise specified
Yeast strains and growth medium
S. cerevisiae strains used in this study are described in Table 1. Yeast strains were derived from S288C (BY4742 (53)) or W303 (54). Growth media were prepared as described previously (55), and plate medium contained 2% bacto-agar. Growth media included the following: YPD (2% glucose, 1% yeast extract, and 2% peptone), YPGal (2% galactose, 1% yeast extract, 2% peptone, and 0.1% dextrose), YPG (3% glycerol, 1% yeast extract, and 2% peptone), and YPEG (3% glycerol, 2% ethanol, 1% yeast extract, and 2% peptone). Synthetic dextrose/selection media (SD–Complete, SD−Ura, SD-Leu, SD-His, SD-His–Leu, SD−Ura–Leu) were prepared as described previously (55) and consisted of all components minus uracil, leucine, histidine, or both uracil and leucine. Drop-out dextrose (DOD) medium was prepared as described previously (14).
COQ11 was disrupted by the one-step gene replacement method (56). The LEU2 gene from pRS305 was amplified by polymerase chain reaction (PCR), with COQ11 upstream and downstream flanking sequences 5′-GGGAAATATGTATCGTATACAAAAATACAGCTAAAGCTTGAACTG and 3′-GTACTTAACTATATACAGCTTGGTATAATTTTAAAATGGTAATAAC. Transformations of PCR products into yeast cells were performed using the Li-acetate method (57). The double coq10Δcoq11Δ mutant was constructed via disruption of COQ10 within the coq11Δ strain. The HIS3 gene from pRS313 was amplified by PCR with COQ10 upstream and downstream flanking sequences 5′-GGATAAGGAGCCAAACAATAAACGGCTAAAGATACCGTGG and 3′-CAGATAACAAAGATCATGCCATCCAGGATAAGCGTATGCA, and transformation was performed as for the COQ11 disruption. Primers were designed using SnapGene (GSL Biotech, LLC, Chicago, IL).
Mitochondria isolation from BY4742 WT and mutant yeast
Yeast cultures of BY4742, coq10Δ, coq11Δ, and coq10Δcoq11Δ were grown overnight in 5 ml of YPD. Yeast-containing plasmids were grown overnight in 5 ml of selection medium (SD−Ura). All pre-cultures were back-diluted with YPGal and grown overnight with shaking (30 °C, 250 rpm) until cell density reached an A600 ∼4. Spheroplasts were prepared with Zymolyase-20T (MP Biomedicals) and fractionated as described previously (36), in the presence of cOmpleteTM EDTA-free protease inhibitor mixture tablets (Roche Applied Science), phosphatase inhibitor mixture set I (Sigma-Aldrich), phosphatase inhibitor mixture set II (Sigma-Aldrich), and phenylmethylsulfonyl fluoride (Thermo Fisher Scientific). Nycodenz (Sigma-Aldrich) density gradient purified mitochondria were frozen in liquid nitrogen, aliquoted, and stored at −80 °C until further use. Protein concentration of mitochondria was measured by the bicinchoninic acid (BCA) assay (Thermo Fisher Scientific).
Submitochondrial localization of Coq10 and Coq11 polypeptides
Purified mitochondria from BY4742 yeast (3 mg of protein, 150 μl) were subfractionated, as described previously (13). Proteinase K treatment of purified BY4742 mitochondria was also performed as described previously (13). Proteinase K-treated mitoplasts and control samples were resuspended in SDS sample buffer (50 mm Tris, pH 6.8, 10% glycerol, 2% SDS, 0.1% bromphenol blue, and 1.33% β-mercaptoethanol); equal aliquots were separated by SDS-gel electrophoresis on 10 or 12% Tris-glycine polyacrylamide gels as detailed below. Several mitochondrial compartment markers and proteins of interest, Coq10 and Coq11, were detected with rabbit polyclonal antibodies prepared in blocking buffer at dilutions listed in Table S1.
Oxygen consumption evaluation by Seahorse
Mitochondrial function was assessed using the XF96 extracellular flux analyzer (Seahorse Bioscience, Agilent Technologies). Seahorse plates were coated with 50 μg/ml poly-d-lysine (Sigma-Aldrich), diluted 1:1 in UltraPure distilled water (Thermo Fisher Scientific). Volumes of 25 μl were added to each well for 30 min at room temperature and then aspirated before plates were dried overnight at room temperature. The Seahorse XF96 sensor cartridge was hydrated with Seahorse XF calibrant solution (Agilent) and was incubated overnight at room temperature.
Yeast cultures of BY4742, coq10Δ, coq11Δ, and coq10Δcoq11Δwere grown overnight in 25 ml of YPGal medium. On the day of measurement, all cultures were diluted to seed an A600 = 0.1 cells/well of BY4742, coq10Δ, coq11Δ, and coq10Δcoq11Δ into a Seahorse XF96 microplate in a total volume of 175 μl YPGal. Four wells containing only medium were included for background measurement. The loaded plate was centrifuged at 500 × g for 3 min at room temperature (with no brakes). Following centrifugation, the loaded plate was incubated for 30 min at 37 °C with no CO2 to aid in the transitioning of the plate into the Seahorse machine's temperature. Cells were stimulated sequentially with two injections of 4 μm FCCP in ports A and B (optimized for maximum oxygen consumption rate) (Enzo Life Sciences) and 2.5 μm antimycin A in port C (Enzo Life Sciences), delivered in YPGal. Mix, wait, and measure times were 2 min, 30 s, and 2 min, respectively. Basal respiration included four measurements, and then following each injection three measurements were made. All OCR were subtracted for nonmitochondrial respiration and normalized to A600 = 0.1. Basal respiration was calculated as an average of OCR prior to the first FCCP addition. Maximal respiration was calculated as an average of OCR following the second FCCP addition. Nonmitochondrial respiration was measured as average OCR following antimycin A addition.
Fatty acid sensitivity assay
Sensitivity of yeast cells to PUFA-induced oxidative stress was performed as described previously (19, 58, 59), with some modifications. Briefly, BY4742 WT, cor1Δ, coq9Δ, coq10Δ, coq11Δ, and coq10Δcoq11Δ were inoculated in 5 ml of YPD medium and incubated overnight at 30 °C, 250 rpm. Cultures were subinoculated to an A600 = 0.25 in 15 ml of fresh YPD medium and incubated at 30 °C, 250 rpm until they reached an A600 ∼1. Cells were harvested, washed twice with 10 ml of sterile H2O, and diluted in 0.1 m phosphate buffer with 0.2% dextrose, pH 6.2, to an A600 = 0.2. This cell suspension was divided into 5-ml aliquots and treated with an ethanol vehicle control (final concentration 0.1% v/v), ethanol-diluted oleic acid (Nu-Check Prep), or α-linolenic acid (Nu-Check Prep) to a final concentration of 200 μm. Fatty acid-treated cultures were incubated for 4 h at 30 °C, 250 rpm, after which cell viability was assessed via plate dilutions. Cell viability prior to the addition of fatty acids was determined via plate dilutions, represented in the 0-h plate.
Analysis of Q6 and Q6-intermediates
Standards of Q6 were obtained from Avanti Polar Lipids, and Q4 was from Sigma-Aldrich. Yeast cultures were grown overnight in 30 ml of YPGal, or selection medium (SD-complete or SD−Ura) for strains harboring plasmids. Cultures were diluted into triplicates of 6 ml of fresh medium to A600 = 0.5, and 5 ml of medium was harvested by centrifugation once they reached A600 ∼4. Cell pellets were stored at −20 °C. Following collection, frozen cell pellets were lipid-extracted in the presence of internal standard Q4 and analyzed for Q6 and Q6-intermediates by LC-MS/MS as described previously (19).
Stable isotope labeling for determination of de novo Q6 and Q6-intermediates
Yeast cultures were grown overnight in 30 ml of YPGal and diluted in triplicates of 6 ml of fresh medium to an A600 = 0.1. Cultures were incubated until they reached an A600 ∼1, at which point ethanol vehicle control (0.1% v/v) or 5 μg/ml of the stable isotope [13C6]4HB (Cambridge Isotope Laboratories, Inc.) was added. Cultures were allowed to grow for an additional 4 h when 5 ml of each culture was harvested by centrifugation and stored at −20 °C. Cell pellets were lipid extracted and analyzed by LC-MS/MS as described previously (19).
Mitochondrial DNA determination by qPCR analysis
DNA was extracted from yeast cells as follows. Yeast pellets (10 ml) grown in YPGal were collected at an A600 ∼4 by centrifugation at 3000 × g for 5 min, washed with 5 ml of H2O, and transferred to 2-ml screw-cap tubes. Pellets were frozen at −80 °C until DNA extraction was carried out. Cell pellets were resuspended in 200 μl of lysis buffer (10 mm Tris-Cl, pH 8.0, 2% (v/v) Triton X-100, 1 mm EDTA, 100 mm NaCl, 1% SDS), and 200 μl of acid-washed glass beads with 200 μl of phenol/chloroform/isoamyl alcohol (25:24:1) were added to the cell suspension. Cells were lysed using a bead-beater (Precellys 24; Bertin Technologies) three times for 10 s at 6500 rpm with a 45-s break between rounds at 4 °C. Tris-EDTA (TE, 200 μl) was added, and the cell suspension was centrifuged at 13,000 × g for 5 min at room temperature. The aqueous layer was removed to a new tube containing 200 μl of chloroform, mixed by inversion, and centrifuged at 13,000 × g for 5 min at room temperature. This was repeated once more. The aqueous layer was then transferred to a 2-ml screw-cap tube containing 1 ml of 95% EtOH, mixed by inversion, and centrifuged at 13,000 × g for 2 min at room temperature. The resulting pellet was resuspended in 400 μl of TE containing 30 μg of RNase A and incubated at 37 °C for 30 min. Then, 10 μl of 3 m sodium acetate and 1 ml of 95% EtOH was added, mixed by inversion, and incubated at −20 °C for 1 h. After 1 h at −20 °C, the suspension was centrifuged at 13,000 × g for 5 min, and the pellet was washed twice with 70% EtOH and air-dried. The dried pellet was resuspended in 25 μl of TE; concentration was measured by Nanodrop (Thermo Fisher Scientific), and the pellet was stored at −20 °C until use.
qPCR was performed on a CFX384 instrument (Bio-Rad) using the SensiFASTTMSYBR® NO ROX kit (Bioline) as per the manufacturer's instructions. Each sample was run in duplicate with 150 ng of total DNA used per reaction using the following thermocycling protocol (95 °C for 2 min, 95 °C for 5 s, 60 °C for 10 s, and 72 °C for 20 s, plate read and cycle repeated ×40, melt curve 40–92 °C with plate read and 40 °C for 10 s). Melting-curve analysis confirmed that all PCRs produced a single product. mtDNA-specific primers (forward (13,999), 5′-GTG CGT ATA TTT CGT TGA TGC GT-3′; reverse (14,297), 5′-TTC ACA CTG CCT GTG CTA TCT AA-3′ (60) and actin-specific primers (forward, 5′-GAA TTG AGA GTT GCC CCA GA-3′; reverse, 5′-ATC ACC GGA ATC CAA AAC AA-3) were used. The relative level of gene expression of mitochondrial DNA was normalized to the expression level of actin as described previously (61).
Citrate synthase activity
The measurement of citrate synthase activity in cells was carried out as described previously (62). Briefly, yeast pellets (10 ml) grown in YPGal were collected at an A600 ∼4 by centrifugation at 3000 × g for 5 min, washed with 5 ml of H2O, and transferred to 2-ml screw-cap tubes. Cell pellets were resuspended in 200 μl of lysis buffer (100 mm Tris-Cl, pH 7.4, 1% (v/v) Triton X-100, 1 mm EDTA, 1 mm phenylmethylsulfonyl fluoride, 1× cOmpleteTM Protease Inhibitor Mixture (Roche Applied Science)), and then 200 μl of acid-washed glass beads were added. Cells were lysed using a bead-beater (Precellys 24; Bertin Technologies) three times for 10 s at 6500 rpm with a 45-s break between rounds at 4 °C. The clarified cell lysate was collected after centrifugation at 16,000 × g for 10 min at 4 °C. The concentration of protein was determined with the BCA assay (Thermo Fisher Scientific). Cell lysates were normalized to 0.05 μg/μl protein. The colorimetric citrate synthase assay was carried out using a VersaMax plate reader (Molecular Devices) and a flat-bottom 96-well plate. First, 40 μl of 500 mm Tris-Cl, pH 7.4, 2 μl of 30 mm acetyl-CoA, 8 μl of 2.5 mm 5,5′-dithiobis(2-nitrobenzoic acid), 90 μl of H2O, and 50 μl cell lysate (2.5 μg total protein) were added into each well. Then, 10 μl of 10 mm oxaloacetic acid were added per well and mixed by pipetting up and down. A412 was measured every 30 s at 25 °C. The initial slope was calculated by using data from the first 10 min and used to determine the enzyme reaction rate using the extinction coefficient for 2-nitro-5-thiobenzoate of 14.15 mm−1 cm−1 (63).
Porin quantification
Porin content was quantified via immunoblot of yeast WT and mutant whole cells. Protein extraction from whole cells was performed (64), and 25 μg of each sample was separated by SDS-gel electrophoresis as described below. Three replicates of the immunoblots were performed and quantified by hand using ImageStudioLite software normalized to Ponceau total protein staining.
Quantitative real-time PCR (qRT-PCR)
Total RNA was isolated from yeast cells using TRIzol reagent (Invitrogen). DNA contamination from the resulting RNA was removed using the DNase TURBO kit as per the manufacturer's instructions (Invitrogen). RNA concentration was measured by Nanodrop (Thermo Fisher Scientific), and RNA was stored at −20 °C. Reverse transcription was carried out using the Superscript III first strand synthesis system using random hexamer primers (Invitrogen). cDNA was stored at −20 °C until qPCR analyses were carried out. Quantitative real-time PCR was performed on a CFX384 instrument (Bio-Rad) using the SensiFASTTMSYBR® NO-ROX kit (Bioline) in duplicate. The relative levels of gene expression were normalized to the expression level of actin. Melting curve analysis confirmed that all PCRs produced a single product. The primers (forward/reverse) used in real-time PCR were designed using Primer3 on line (RRID:SCR_003139). Primers used are given in Table S2.
SDS-PAGE and immunoblot analysis
Purified mitochondria (25 μg) were resuspended in SDS sample buffer and separated by SDS-gel electrophoresis on 10 or 12% Tris-glycine polyacrylamide gels. Proteins were transferred to a 0.45-μm polyvinylidene difluoride membrane (Millipore) and blocked with blocking buffer (0.5% BSA, 0.1% Tween 20, 0.02% SDS in PBS). Representative Coq polypeptides and loading control mitochondrial malate dehydrogenase (Mdh1) were probed with rabbit polyclonal antibodies prepared in blocking buffer at dilutions listed in Table S1. IRDye 680LT goat anti-rabbit IgG secondary antibody (LiCOR) was used at a dilution of 1:10,000. Proteins were visualized using a LiCOR Odyssey IR Scanner (LiCOR). Immunoblots are representative of three replicates and were quantified by hand using ImageStudioLite software normalized to Mdh1.
Two-dimensional Blue Native/SDS-PAGE immunoblot analysis of high-molecular-weight complexes
2D-BN/SDS-PAGE was performed as described previously (13, 65, 66). Briefly, 200 μg of purified mitochondria were solubilized at 4 mg/ml for 1 h on ice with 16 mg/ml digitonin (Biosynth) in the presence of the protease and phosphatase inhibitors used during mitochondrial isolation. Protein concentration of solubilized mitochondria was determined by BCA assay. NativePAGE 5% G-250 sample additive (Thermo Fisher Scientific) was added to a final concentration of 0.25%. Solubilized mitochondria (100 μg) were separated on NativePAGE 4–16% BisTris gels (Thermo Fisher Scientific) in the first dimension, and native gel slices were further separated on 12% Tris-glycine polyacrylamide gel in the second dimension. Following the second-dimension separation, immunoblot analyses were performed as described above, using antibodies against Coq4 and Coq9 at the dilutions indicated in Table S1. Molecular weight standards for BN gel electrophoresis and SDS gel electrophoresis were obtained from GE Healthcare (Sigma-Aldrich) and Bio-Rad, respectively.
Construction of low-copy COQ11 yeast expression vectors
Plasmids used in this study are described in Table 2. A low-copy COQ11-containing plasmid was constructed using the pRS316 low-copy empty vector. The COQ11 ORF and regions corresponding to 842 bp upstream and 256 bp downstream were cloned into pRS316 using Gibson Assembly (New England Biolabs). Clones were sequenced by Laragen, and successful clones were transformed into WT and mutant yeast, along with the corresponding empty vector (pRS316) control as described above.
Revertant isolation
As reported previously, coq10 mutant growth deficiency on nonfermentable carbon sources (YPEG) spontaneously revert due to nuclear suppression mutations (18). Here, W303 coq10Δ yeast was grown on glucose to stationary phase, and ∼10 million cells were plated on YPEG. After several weeks, a colony began to appear on this medium. The colony was purified, and its genome was sequenced.
Genome sequencing
The Wizard® genomic purification kit (Promega) was used to extract total DNA from the parental respiratory-deficient mutant W303 coq10Δ and from the spontaneous revertant W303 coq10rev. The DNA was quantified using the QUBIT DNATM high-sensitivity assay, and 1 ng of the normalized DNA was tagged by the Nextera XTTM (Illumina) protocol. The libraries were amplified and pooled as described (67). The pooled libraries were subjected to sequencing with the NextSeqTM (Illumina) equipment in the Genome Investigation and Analysis Laboratory of the Institute of Biomedical Sciences at the University of Sao Paulo. The BWA Aligner tool, version 1.1.4 (Base Space Labs-Illumina), was used to align ∼23,000,000 reads obtained from each strain with the reference genomes of S. cerevisiae. The alignments were compared using the Integrative Genomics Viewer (Base Space Labs Illumina).
Statistical analyses
All data sets were tested for normality using GraphPad Prism 7 with the Shapiro-Wilk normality test. Because a majority of sets passed the normality test (α = 0.5), statistical analyses were performed using GraphPad Prism 7 with parametric one-way analysis of variance correcting for multiple comparisons using Tukey's test, comparing the mean of each sample to the mean of its corresponding WT or empty vector control. The data show the means ± S.D., and the statistical significance as compared with WT or empty vector control is represented by the following: *, p < 0.05; **, p < 0.01; ***, p < 0.001; and ****, p < 0.0001. The denotation ns indicates values with “not significant” differences from the corresponding control.
Data availability
The MS source data for determination of Q6 and Q6 intermediates will be shared upon request. Please contact Catherine Clarke at cathy@chem.ucla.edu. All remaining data are contained within the article.
Author contributions
M. C. B., M. H. B., and C. F. C. conceptualization; M. C. B., J. N., A. A., H. S. T., O. S. S., M. H. B., and C. F. C. resources; M. C. B., K. Y., L. F.-dR., J. N., A. A., H. S. T., N. A. N., M. H. B., and C. F. C. data curation; M. C. B., J. N., A. A., and M. H. B. software; M. C. B., K. Y., L. F.-dR., J. N., A. A., H. S. T., N. A. N., R. S., M. H. B., and C. F. C. formal analysis; M. C. B., R. S., O. S. S., M. H. B., and C. F. C. supervision; M. C. B., J. N., H. S. T., M. H. B., and C. F. C. funding acquisition; M. C. B., K. Y., L. F.-dR., J. N., A. A., H. S. T., R. S., and M. H. B. validation; M. C. B., K. Y., L. F.-dR., J. N., A. A., H. S. T., N. A. N., R. S., M. H. B., and C. F. C. investigation; M. C. B., K. Y., L. F.-dR., J. N., A. A., H. S. T., N. A. N., M. H. B., and C. F. C. visualization; M. C. B., K. Y., L. F.-dR., J. N., A. A., H. S. T., N. A. N., R. S., M. H. B., and C. F. C. methodology; M. C. B. and C. F. C. writing-original draft; M. C. B., R. S., M. H. B., and C. F. C. project administration; M. C. B., K. Y., L. F.-dR., J. N., A. A., H. S. T., N. A. N., R. S., O. S. S., M. H. B., and C. F. C. writing-review and editing.
Supplementary Material
Acknowledgments
We thank the UCLA Molecular Instrumentation Core proteomics facility and Dr. Yu Chen for the use of the QTRAP4000 for lipid analysis. We thank undergraduate UCLA researcher Hope Ibarra for her contributions in assisting with experiments.
This work was supported by National Science Foundation Grant MCB-1330803 (to C. F. C.), National Institutes of Health Grant T32 GM 007185 (to H. S. T. and M. C. B.) Ruth L. Kirschstein National Service (to M. C. B. and H. S. T.), the Whitcome Individual Predoctoral Fellowship (to M. C. B.), UCLA Summer Undergraduate Research Fellowship, Department of Chemistry and Biochemistry (to K. Y.), Fundação de Amparo a Pesquisa de São Paulo–FAPESP 2013/09482-8 and 2013/07937-8 (to M. H. B.), Gates Millennium Scholars Fellowship (to J. N.), and the Eugene V. Cota Robles Fellowship (to J. N.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Figs. S1–S3, Tables S1 and S2, and supporting Refs. 1–8.
- Q
- ubiquinone
- DMQ
- demethoxy-Q
- HHB
- 3-hexaprenyl-4-hydroxybenzoic acid
- Qn
- coenzyme Qn (where n designates the number of isoprene units in the polyisoprenyl tail)
- QH2
- reduced coenzyme Q or ubiquinol
- ORF
- open reading frame
- ER
- endoplasmic reticulum
- MIOREX complex
- mitochondrial organization of gene expression complex
- IMS
- intermembrane space
- OCR
- oxygen consumption rate
- FCCP
- carbonyl cyanide p-trifluoromethoxyphenylhydrazone
- PUFA
- polyunsaturated fatty acid
- qPCR
- quantitative real-time PCR
- ERMES
- ER-mitochondrial encounter structure
- START
- steroidogenic acute regulatory protein–related lipid transfer
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- SDR
- short-chain dehydrogenase/reductase
- 2D-BN/SDS-PAGE
- two-dimensional blue native/SDS-PAGE
- DOD
- drop-out dextrose
- SD
- synthetic dextrose
- lcCOQ11
- low-copy COQ11
- RET
- reverse electron transport
- P
- pellet
- S
- supernatant.
References
- 1. Turunen M., Olsson J., and Dallner G. (2004) Metabolism and function of coenzyme Q. Biochim. Biophys. Acta 1660, 171–199 10.1016/j.bbamem.2003.11.012 [DOI] [PubMed] [Google Scholar]
- 2. Alcázar-Fabra M., Trevisson E., and Brea-Calvo G. (2018) Clinical syndromes associated with coenzyme Q10 deficiency. Essays Biochem. 62, 377–398 10.1042/EBC20170107 [DOI] [PubMed] [Google Scholar]
- 3. Desbats M. A., Lunardi G., Doimo M., Trevisson E., and Salviati L. (2015) Genetic bases and clinical manifestations of coenzyme Q10 (CoQ 10) deficiency. J. Inherit. Metab. Dis. 38, 145–156 10.1007/s10545-014-9749-9 [DOI] [PubMed] [Google Scholar]
- 4. Frei B., Kim M. C., and Ames B. N. (1990) Ubiquinol-10 is an effective lipid-soluble antioxidant at physiological concentrations. Proc. Natl. Acad. Sci. U.S.A. 87, 4879–4883 10.1073/pnas.87.12.4879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Okada K., Suzuki K., Kamiya Y., Zhu X., Fujisaki S., Nishimura Y., Nishino T., Nakagawa T., Kawamukai M., and Matsuda H. (1996) Polyprenyl diphosphate synthase essentially defines the length of the side chain of ubiquinone. Biochim. Biophys. Acta 1302, 217–223 10.1016/0005-2760(96)00064-1 [DOI] [PubMed] [Google Scholar]
- 6. Kawamukai M. (2016) Biosynthesis of coenzyme Q in eukaryotes. Biosci. Biotechnol. Biochem. 80, 23–33 10.1080/09168451.2015.1065172 [DOI] [PubMed] [Google Scholar]
- 7. Stefely J. A., and Pagliarini D. J. (2017) Biochemistry of mitochondrial coenzyme Q biosynthesis. Trends Biochem. Sci. 42, 824–843 10.1016/j.tibs.2017.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Montini G., Malaventura C., and Salviati L. (2008) Early coenzyme Q10 supplementation in primary coenzyme Q10 deficiency. N. Engl. J. Med. 358, 2849–2850 10.1056/NEJMc0800582 [DOI] [PubMed] [Google Scholar]
- 9. Awad A. M., Bradley M. C., Fernández-Del-Río L., Nag A., Tsui H. S., and Clarke C. F. (2018) Coenzyme Q10 deficiencies: pathways in yeast and humans. Essays Biochem 62, 361–376 10.1042/EBC20170106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tran U. C., Marbois B., Gin P., Gulmezian M., Jonassen T., and Clarke C. F. (2006) Complementation of Saccharomyces cerevisiae coq7 mutants by mitochondrial targeting of the Escherichia coli UbiF polypeptide: two functions of yeast Coq7 polypeptide in coenzyme Q biosynthesis. J. Biol. Chem. 281, 16401–16409 10.1074/jbc.M513267200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marbois B., Gin P., Faull K. F., Poon W. W., Lee P. T., Strahan J., Shepherd J. N., and Clarke C. F. (2005) Coq3 and Coq4 define a polypeptide complex in yeast mitochondria for the biosynthesis of coenzyme Q. J. Biol. Chem. 280, 20231–20238 10.1074/jbc.M501315200 [DOI] [PubMed] [Google Scholar]
- 12. Hsieh E. J., Gin P., Gulmezian M., Tran U. C., Saiki R., Marbois B. N., and Clarke C. F. (2007) Saccharomyces cerevisiae Coq9 polypeptide is a subunit of the mitochondrial coenzyme Q biosynthetic complex. Arch. Biochem. Biophys. 463, 19–26 10.1016/j.abb.2007.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. He C. H., Xie L. X., Allan C. M., Tran U. C., and Clarke C. F. (2014) Coenzyme Q supplementation or overexpression of the yeast Coq8 putative kinase stabilizes multi-subunit Coq polypeptide complexes in yeast coq null mutants. Biochim. Biophys. Acta 1841, 630–644 10.1016/j.bbalip.2013.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Allan C. M., Awad A. M., Johnson J. S., Shirasaki D. I., Wang C., Blaby-Haas C. E., Merchant S. S., Loo J. A., and Clarke C. F. (2015) Identification of Coq11, a new coenzyme Q biosynthetic protein in the CoQ-synthome in Saccharomyces cerevisiae. J. Biol. Chem. 290, 7517–7534 10.1074/jbc.M114.633131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marcotte E. M., Pellegrini M., Ng H. L., Rice D. W., Yeates T. O., and Eisenberg D. (1999) Detecting protein function and protein–protein interactions from genome sequences. Science 285, 751–753 10.1126/science.285.5428.751 [DOI] [PubMed] [Google Scholar]
- 16. Perocchi F., Jensen L. J., Gagneur J., Ahting U., von Mering C., Bork P., Prokisch H., and Steinmetz L. M. (2006) Assessing systems properties of yeast mitochondria through an interaction map of the organelle. PLoS Genet. 2, e170 10.1371/journal.pgen.0020170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Allan C. M., Hill S., Morvaridi S., Saiki R., Johnson J. S., Liau W. S., Hirano K., Kawashima T., Ji Z., Loo J. A., Shepherd J. N., and Clarke C. F. (2013) A conserved START domain coenzyme Q-binding polypeptide is required for efficient Q biosynthesis, respiratory electron transport, and antioxidant function in Saccharomyces cerevisiae. Biochim. Biophys. Acta 1831, 776–791 10.1016/j.bbalip.2012.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barros M. H., Johnson A., Gin P., Marbois B. N., Clarke C. F., and Tzagoloff A. (2005) The Saccharomyces cerevisiae COQ10 gene encodes a START domain protein required for function of coenzyme Q in respiration. J. Biol. Chem. 280, 42627–42635 10.1074/jbc.M510768200 [DOI] [PubMed] [Google Scholar]
- 19. Tsui H. S., Pham N. V. B., Amer B. R., Bradley M. C., Gosschalk J. E., Gallagher-Jones M., Ibarra H., Clubb R. T., Blaby-Haas C. E., and Clarke C. F. (2019) Human COQ10A and COQ10B are distinct lipid-binding START domain proteins required for coenzyme Q function. J. Lipid Res. 60, 1293–1310 10.1194/jlr.M093534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shen Y., Goldsmith-Fischman S., Atreya H. S., Acton T., Ma L., Xiao R., Honig B., Montelione G. T., and Szyperski T. (2005) NMR structure of the 18 kDa protein CC1736 from Caulobacter crescentus identifies a member of the START domain superfamily and suggests residues mediating substrate specificity. Proteins 58, 747–750 10.1002/prot.20365 [DOI] [PubMed] [Google Scholar]
- 21. Cui T. Z., and Kawamukai M. (2009) Coq10, a mitochondrial coenzyme Q binding protein, is required for proper respiration in Schizosaccharomyces pombe. FEBS J. 276, 748–759 10.1111/j.1742-4658.2008.06821.x [DOI] [PubMed] [Google Scholar]
- 22. Stoldt S., Wenzel D., Kehrein K., Riedel D., Ott M., and Jakobs S. (2018) Spatial orchestration of mitochondrial translation and OXPHOS complex assembly. Nat. Cell Biol. 20, 528–534 10.1038/s41556-018-0090-7 [DOI] [PubMed] [Google Scholar]
- 23. Eisenberg-Bord M., Tsui H. S., Antunes D., Fernández-Del-Río L., Bradley M. C., Dunn C. D., Nguyen T. P. T., Rapaport D., Clarke C. F., and Schuldiner M. (2019) The endoplasmic reticulum–mitochondria encounter structure complex coordinates coenzyme Q biosynthesis. Contact 2, 2515256418825409 10.1177/2515256418825409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Subramanian K., Jochem A., Le Vasseur M., Lewis S., Paulson B. R., Reddy T. R., Russell J. D., Coon J. J., Pagliarini D. J., and Nunnari J. (2019) Coenzyme Q biosynthetic proteins assemble in a substrate-dependent manner into domains at ER-mitochondria contacts. J. Cell Biol. 218, 1353–1369 10.1083/jcb.201808044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reidenbach A. G., Kemmerer Z. A., Aydin D., Jochem A., McDevitt M. T., Hutchins P. D., Stark J. L., Stefely J. A., Reddy T., Hebert A. S., Wilkerson E. M., Johnson I. E., Bingman C. A., Markley J. L., Coon J. J., et al. (2018) Conserved lipid and small-molecule modulation of COQ8 reveals regulation of the ancient kinase-like UbiB family. Cell Chem. Biol. 25, 154–165.e11 10.1016/j.chembiol.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reading D. S., Hallberg R. L., and Myers A. M. (1989) Characterization of the yeast HSP60 gene coding for a mitochondrial assembly factor. Nature 337, 655–659 10.1038/337655a0 [DOI] [PubMed] [Google Scholar]
- 27. Fujiki Y., Hubbard A. L., Fowler S., and Lazarow P. B. (1982) Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J. Cell Biol. 93, 97–102 10.1083/jcb.93.1.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen W. J., and Douglas M. G. (1987) Phosphodiester bond cleavage outside mitochondria is required for the completion of protein import into the mitochondrial matrix. Cell 49, 651–658 10.1016/0092-8674(87)90541-1 [DOI] [PubMed] [Google Scholar]
- 29. Ohashi A., Gibson J., Gregor I., and Schatz G. (1982) Import of proteins into mitochondria. The precursor of cytochrome c1 is processed in two steps, one of them heme-dependent. J. Biol. Chem. 257, 13042–13047 [PubMed] [Google Scholar]
- 30. Vögtle F. N., Burkhart J. M., Gonczarowska-Jorge H., Kücükköse C., Taskin A. A., Kopczynski D., Ahrends R., Mossmann D., Sickmann A., Zahedi R. P., and Meisinger C. (2017) Landscape of submitochondrial protein distribution. Nat. Commun. 8, 290 10.1038/s41467-017-00359-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yin H., Xu L., and Porter N. A. (2011) Free radical lipid peroxidation: mechanisms and analysis. Chem. Rev. 111, 5944–5972 10.1021/cr200084z [DOI] [PubMed] [Google Scholar]
- 32. Pryor W. A., and Porter N. A. (1990) Suggested mechanisms for the production of 4-hydroxy-2-nonenal from the autoxidation of polyunsaturated fatty acids. Free Radic. Biol. Med. 8, 541–543 10.1016/0891-5849(90)90153-A [DOI] [PubMed] [Google Scholar]
- 33. Heeringa S. F., Chernin G., Chaki M., Zhou W., Sloan A. J., Ji Z., Xie L. X., Salviati L., Hurd T. W., Vega-Warner V., Killen P. D., Raphael Y., Ashraf S., Ovunc B., Schoeb D. S., et al. (2011) COQ6 mutations in human patients produce nephrotic syndrome with sensorineural deafness. J. Clin. Invest. 121, 2013–2024 10.1172/JCI45693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nguyen T. P., Casarin A., Desbats M. A., Doimo M., Trevisson E., Santos-Ocaña C., Navas P., Clarke C. F., and Salviati L. (2014) Molecular characterization of the human COQ5 C-methyltransferase in coenzyme Q10 biosynthesis. Biochim. Biophys. Acta 1841, 1628–1638 10.1016/j.bbalip.2014.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Conrad M., Schothorst J., Kankipati H. N., Van Zeebroeck G., Rubio-Texeira M., and Thevelein J. M. (2014) Nutrient sensing and signaling in the yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 38, 254–299 10.1111/1574-6976.12065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Glick B. S., and Pon L. A. (1995) Isolation of highly purified mitochondria from Saccharomyces cerevisiae. Methods Enzymol. 260, 213–223 10.1016/0076-6879(95)60139-2 [DOI] [PubMed] [Google Scholar]
- 37. Stefely J. A., Reidenbach A. G., Ulbrich A., Oruganty K., Floyd B. J., Jochem A., Saunders J. M., Johnson I. E., Minogue C. E., Wrobel R. L., Barber G. E., Lee D., Li S., Kannan N., Coon J. J., et al. (2015) Mitochondrial ADCK3 employs an atypical protein kinase-like fold to enable coenzyme Q biosynthesis. Mol. Cell 57, 83–94 10.1016/j.molcel.2014.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xie L. X., Hsieh E. J., Watanabe S., Allan C. M., Chen J. Y., Tran U. C., and Clarke C. F. (2011) Expression of the human atypical kinase ADCK3 rescues coenzyme Q biosynthesis and phosphorylation of Coq polypeptides in yeast coq8 mutants. Biochim. Biophys. Acta 1811, 348–360 10.1016/j.bbalip.2011.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xie L. X., Ozeir M., Tang J. Y., Chen J. Y., Jaquinod S. K., Fontecave M., Clarke C. F., and Pierrel F. (2012) Overexpression of the Coq8 kinase in Saccharomyces cerevisiae coq null mutants allows for accumulation of diagnostic intermediates of the coenzyme Q6 biosynthetic pathway. J. Biol. Chem. 287, 23571–23581 10.1074/jbc.M112.360354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tran U. C., and Clarke C. F. (2007) Endogenous synthesis of coenzyme Q in eukaryotes. Mitochondrion 7, Suppl., S62–S71 10.1016/j.mito.2007.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang Y., and Hekimi S. (2019) The complexity of making ubiquinone. Trends Endocrinol. Metab. 30, 929–943 10.1016/j.tem.2019.08.009 [DOI] [PubMed] [Google Scholar]
- 42. Murley A., and Nunnari J. (2016) The emerging network of mitochondria-organelle contacts. Mol. Cell 61, 648–653 10.1016/j.molcel.2016.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kehrein K., Möller-Hergt B. V., and Ott M. (2015) The MIOREX complex–lean management of mitochondrial gene expression. Oncotarget 6, 16806–16807 10.18632/oncotarget.4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fontanesi F. (2013) Mechanisms of mitochondrial translational regulation. IUBMB Life 65, 397–408 10.1002/iub.1156 [DOI] [PubMed] [Google Scholar]
- 45. Marchler-Bauer A., Zheng C., Chitsaz F., Derbyshire M. K., Geer L. Y., Geer R. C., Gonzales N. R., Gwadz M., Hurwitz D. I., Lanczycki C. J., Lu F., Lu S., Marchler G. H., Song J. S., Thanki N., et al. (2013) CDD: conserved domains and protein three-dimensional structure. Nucleic Acids Res. 41, D348–D352 10.1093/nar/gks1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rossmann M. G., Moras D., and Olsen K. W. (1974) Chemical and biological evolution of nucleotide-binding protein. Nature 250, 194–199 10.1038/250194a0 [DOI] [PubMed] [Google Scholar]
- 47. Kopec J., Schnell R., and Schneider G. (2011) Structure of PA4019, a putative aromatic acid decarboxylase from Pseudomonas aeruginosa. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 67, 1184–1188 10.1107/S174430911102923X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Guarás A., Perales-Clemente E., Calvo E., Acín-Pérez R., Loureiro-Lopez M., Pujol C., Martínez-Carrascoso I., Nuñez E., García-Marqués F., Rodríguez-Hernández M. A., Cortés A., Diaz F., Pérez-Martos A., Moraes C. T., Fernández-Silva P., et al. (2016) The CoQH2/CoQ ratio serves as a sensor of respiratory chain efficiency. Cell Rep. 15, 197–209 10.1016/j.celrep.2016.03.009 [DOI] [PubMed] [Google Scholar]
- 49. Scialò F., Sriram A., Fernández-Ayala D., Gubina N., Lõhmus M., Nelson G., Logan A., Cooper H. M., Navas P., Enríquez J. A., Murphy M. P., and Sanz A. (2016) Mitochondrial ROS produced via reverse electron transport extend animal lifespan. Cell Metab. 23, 725–734 10.1016/j.cmet.2016.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. UniProt Consortium (2018) UniProt: the universal protein knowledgebase. Nucleic Acids Res. 46, 2699 10.1093/nar/gky092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pagliarini D. J., Calvo S. E., Chang B., Sheth S. A., Vafai S. B., Ong S. E., Walford G. A., Sugiana C., Boneh A., Chen W. K., Hill D. E., Vidal M., Evans J. G., Thorburn D. R., Carr S. A., and Mootha V. K. (2008) A mitochondrial protein compendium elucidates complex I disease biology. Cell 134, 112–123 10.1016/j.cell.2008.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. van den Bosch B. J., Gerards M., Sluiter W., Stegmann A. P., Jongen E. L., Hellebrekers D. M., Oegema R., Lambrichs E. H., Prokisch H., Danhauser K., Schoonderwoerd K., de Coo I. F., and Smeets H. J. (2012) Defective NDUFA9 as a novel cause of neonatally fatal complex I disease. J. Med. Genet. 49, 10–15 10.1136/jmedgenet-2011-100466 [DOI] [PubMed] [Google Scholar]
- 53. Brachmann C. B., Davies A., Cost G. J., Caputo E., Li J., Hieter P., and Boeke J. D. (1998) Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14, 115–132 [DOI] [PubMed] [Google Scholar]
- 54. Thomas B. J., and Rothstein R. (1989) Elevated recombination rates in transcriptionally active DNA. Cell 56, 619–630 10.1016/0092-8674(89)90584-9 [DOI] [PubMed] [Google Scholar]
- 55. Barkovich R. J., Shtanko A., Shepherd J. A., Lee P. T., Myles D. C., Tzagoloff A., and Clarke C. F. (1997) Characterization of the COQ5 gene from Saccharomyces cerevisiae. Evidence for a C-methyltransferase in ubiquinone biosynthesis. J. Biol. Chem. 272, 9182–9188 10.1074/jbc.272.14.9182 [DOI] [PubMed] [Google Scholar]
- 56. Rothstein R. J. (1983) One-step gene disruption in yeast. Methods Enzymol. 101, 202–211 10.1016/0076-6879(83)01015-0 [DOI] [PubMed] [Google Scholar]
- 57. Gietz R. D., and Woods R. A. (2002) Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol. 350, 87–96 10.1016/S0076-6879(02)50957-5 [DOI] [PubMed] [Google Scholar]
- 58. Hill S., Hirano K., Shmanai V. V., Marbois B. N., Vidovic D., Bekish A. V., Kay B., Tse V., Fine J., Clarke C. F., and Shchepinov M. S. (2011) Isotope-reinforced polyunsaturated fatty acids protect yeast cells from oxidative stress. Free Radic. Biol. Med. 50, 130–138 10.1016/j.freeradbiomed.2010.10.690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hill S., Lamberson C. R., Xu L., To R., Tsui H. S., Shmanai V. V., Bekish A. V., Awad A. M., Marbois B. N., Cantor C. R., Porter N. A., Clarke C. F., and Shchepinov M. S. (2012) Small amounts of isotope-reinforced polyunsaturated fatty acids suppress lipid autoxidation. Free Radic. Biol. Med. 53, 893–906 10.1016/j.freeradbiomed.2012.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Santos J. H., Mandavilli B. S., and Van Houten B. (2002) Measuring oxidative mtDNA damage and repair using quantitative PCR. Methods Mol. Biol. 197, 159–176 10.1385/1-59259-284-8:159 [DOI] [PubMed] [Google Scholar]
- 61. Gonzalez-Hunt C. P., Rooney J. P., Ryde I. T., Anbalagan C., Joglekar R., and Meyer J. N. (2016) PCR-based analysis of mitochondrial DNA copy number, mitochondrial DNA damage, and nuclear DNA damage. Curr. Protoc. Toxicol. 67, 20.11.1–20.11.25 10.1002/0471140856.tx2011s67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Guo X., Niemi N. M., Hutchins P. D., Condon S. G. F., Jochem A., Ulbrich A., Higbee A. J., Russell J. D., Senes A., Coon J. J., and Pagliarini D. J. (2017) Ptc7p dephosphorylates select mitochondrial proteins to enhance metabolic function. Cell Rep. 18, 307–313 10.1016/j.celrep.2016.12.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Eyer P., Worek F., Kiderlen D., Sinko G., Stuglin A., Simeon-Rudolf V., and Reiner E. (2003) Molar absorption coefficients for the reduced Ellman reagent: reassessment. Anal. Biochem. 312, 224–227 10.1016/S0003-2697(02)00506-7 [DOI] [PubMed] [Google Scholar]
- 64. Zhang T., Lei J., Yang H., Xu K., Wang R., and Zhang Z. (2011) An improved method for whole protein extraction from yeast Saccharomyces cerevisiae. Yeast 28, 795–798 10.1002/yea.1905 [DOI] [PubMed] [Google Scholar]
- 65. Schägger H., Cramer W. A., and von Jagow G. (1994) Analysis of molecular masses and oligomeric states of protein complexes by blue native electrophoresis and isolation of membrane protein complexes by two-dimensional native electrophoresis. Anal. Biochem. 217, 220–230 10.1006/abio.1994.1112 [DOI] [PubMed] [Google Scholar]
- 66. Wittig I., Braun H. P., and Schägger H. (2006) Blue NativePAGE. Nat. Protoc. 1, 418–428 10.1038/nprot.2006.62 [DOI] [PubMed] [Google Scholar]
- 67. Barros M. H., and Tzagoloff A. (2017) Aep3p-dependent translation of yeast mitochondrial ATP8. Mol. Biol. Cell 28, 1426–1434 10.1091/mbc.e16-11-0775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Santos-Ocaña C., Do T. Q., Padilla S., Navas P., and Clarke C. F. (2002) Uptake of exogenous coenzyme Q and transport to mitochondria is required for bc1 complex stability in yeast coq mutants. J. Biol. Chem. 277, 10973–10981 10.1074/jbc.M112222200 [DOI] [PubMed] [Google Scholar]
- 69. Winzeler E. A., Shoemaker D. D., Astromoff A., Liang H., Anderson K., Andre B., Bangham R., Benito R., Boeke J. D., Bussey H., Chu A. M., Connelly C., Davis K., Dietrich F., Dow S. W., et al. (1999) Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285, 901–906 10.1126/science.285.5429.901 [DOI] [PubMed] [Google Scholar]
- 70. Sikorski R. S., and Hieter P. (1989) A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122, 19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Christianson T. W., Sikorski R. S., Dante M., Shero J. H., and Hieter P. (1992) Multifunctional yeast high-copy-number shuttle vectors. Gene 110, 119–122 10.1016/0378-1119(92)90454-W [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The MS source data for determination of Q6 and Q6 intermediates will be shared upon request. Please contact Catherine Clarke at cathy@chem.ucla.edu. All remaining data are contained within the article.