Abstract
Objectives
Parkinson’s disease (PD) is known to be related to various factors, including neuroinflammation, increased oxidative stress, and brain temperature alteration. We aimed to evaluate the correlation between these factors using diffusion-weighted imaging (DWI) thermometry and blood tests of systemic inflammation.
Methods
From July 2012 to Jun 2017, 103 patients with PD (44 men and 59 women; mean age, 60.43 ± 9.12 years) and 106 sex- and age-matched healthy volunteers (48 men and 58 women; mean age, 58.16 ± 8.45 years) retrospectively underwent magnetic resonance DWI thermometry to estimate brain intraventricular temperature (Tv). Subjects were divided into three subgroups in light of their ages. The tested inflammatory markers included plasma nuclear DNA, mitochondrial DNA, apoptotic leukocytes, and serum adhesion molecules. The correlations among the Tv values, clinical severity, and systemic inflammatory markers were then calculated.
Results
The PD patients did not show a natural trend of decline in Tv with age. Comparisons among the different age groups revealed that the younger PD subjects had significantly lower Tv values than the younger controls, but the older subjects had no significant group differences. Overall, the PD patients exhibited lower Tv values than the controls, as well as increased oxidative stress. The brain temperature showed positive correlations with inflammatory markers, including plasma nuclear DNA and L-selectin levels, in all the subjects.
Conclusions
Possible pathophysiological correlations between systemic inflammation and brain temperature were indicated by the results of this study, a finding which may aid us in investigating the underlying pathogenesis of PD.
Electronic supplementary material
The online version of this article (10.1007/s10072-019-04217-3) contains supplementary material, which is available to authorized users.
Keywords: Oxidative stress, Diffusion-weighted MRI, Autonomic diseases, Mitochondrial disorders
Introduction
The pathophysiological basis of the Parkinson’s disease (PD) is the degeneration of dopaminergic neurons in the substantia nigra and the presence of Lewy bodies, which are made of α-synuclein, in those neurons [1]. In PD patients, the activity of mitochondrial complex I, which is one of the components of the electron transport chain, is impaired in various regions, including the substantia nigra, skeletal muscles, and platelets [2]. The structural changes in complex I also make the dopaminergic neurons more susceptible to neurotoxins [3] and results in the chronic production of reactive oxygen species (ROS), increased oxidative stress [4], and subsequent neuroinflammation.
In addition to the motor symptoms in PD, α-synuclein aggregates and Lewy bodies can be seen in autonomic regulatory regions, including the hypothalamus, sympathetic system, and parasympathetic system, and the damage to this circuit is believed to play a central role in the autonomic dysfunctions of these patients [5]. In addition, the brains of patients with mitochondrial diseases have previously been reported to display a protected hypothermic conditions [6] aimed at reducing the oxidative stress and promoting cellular survival [7]. Disturbed mitochondrial function and a disturbed autonomic system [8] will alter the thermal regulation of PD patients [8], especially as the patients grow older or when the disease status progresses.
Recently, neuroinflammation is considered to be one of the major factors in the progression of PD [9], but the role of inflammatory markers, such as apoptotic leukocytes, plasma DNA, and adhesion molecules in peripheral blood, in correlation with the brain temperature has not been well investigated. We hypothesized that the increased circulation of inflammatory markers in patients with PD is correlated with the alteration of brain temperature. Thus, this study aimed to evaluate the brain temperature using diffusion-weighted imaging (DWI) thermometry in order to further explore the pathophysiology of PD. To accomplish this task, the levels of systemic inflammatory markers and brain temperature were investigated in different age groups of patients with PD as well as healthy controls. Furthermore, the relationships between brain temperature and systemic inflammation were investigated.
Materials and methods
Participants
This cross-sectional retrospective study targeted patients with idiopathic PD. One hundred and three patients with idiopathic PD (44 men and 59 women; mean age, 60.43 ± 9.12 years), and one hundred and six sex- and age-matched healthy volunteers (48 men and 58 women; mean age, 58.16 ± 8.45 years) were recruited. These participants were enrolled in our previous studies [9–11], and the data were collected from past records. The exclusion criteria for this study included age < 16 or > 80 years, evidence of alcoholism, a history of other neurologic or psychiatric illness potentially affecting the central nervous system, and severe recent life events which could affect the results of the neuropsychiatric or neuroimaging surveys. The protocols of this study were approved by the relevant local ethics committee. Written informed consent was collected from each participant prior to his or her participation in this study.
Idiopathic PD was diagnosed according to the Parkinson’s Disease Society’s criteria by an experienced neurology specialist and only in the event that the given patient had no prior history of other neurologic or psychiatric illnesses and psychotropic medications. Patients with idiopathic PD were treated at the neurology department of a hospital. In each case, the duration of the disease and treatment, and the current daily dosage of levodopa were recorded. The clinical evaluations for disease severity were performed in the “OFF” status. The Unified Parkinson’s Disease Rating Scale (UPDRS) was applied via clinical observation and interview to evaluate multiple aspects of PD. The modified Hoehn and Yahr (H&Y) staging scale was also used to provide an evaluation of functional disability and track the progression of PD. Furthermore, the Schwab and England (S&E) scale was used to record the subjects’ abilities.
All of the subjects were also divided into three subgroups according to their age: subgroup 1, including participants 30–54 years old; subgroup 2, including participants 55–64 years old; and subgroup 3, including participants 65–79 years old.
Blood sampling and laboratory investigations
Systemic inflammation and oxidative stress were evaluated in some of the subjects in terms of the percentage of apoptotic peripheral leukocytes, the plasma levels of nuclear DNA, mitochondrial DNA, and serum adhesion molecules. Blood samples were collected from some of the patients with PD and some of the controls by venipuncture of forearm veins on the same day as the MRI study and neuropsychological testing. Specifically, 99 patients with PD and 59 controls underwent blood sampling. Thus, the further statistical analyses and graph plots related to inflammatory markers were done with this limited number of PD patients and controls. The procedural details were as described previously [9, 10, 12], and are summarized in the supplementary material.
Leukocytes and their subtypes were identified based on the intensity of CD45 expression. Apoptotic cells were defined as those that were positive for APO 2.7.
The plasma nuclear DNA was measured by a real-time quantitative polymerase chain reaction (RT-PCR) assay (Roche LightCycler, Roche, Grenzach-Wyhlen, Germany) for the β-globin and ND2 genes as plasma nuclear and mitochondrial DNA.
Serum adhesion molecules, including serum ICAM-1, VCAM-1, E-selectin, L-selectin, and P-selectin levels were assessed using commercially available enzyme-linked immunosorbent assays (R&D Systems, Minneapolis, MN, USA).
MR imaging
Data acquisition
The MRI scan of each participant was performed using a 3.0-Tesla whole-body GE Signa MRI system (General Electric Healthcare, Milwaukee, WI, USA) equipped with an eight-channel head coil. DTI was conducted along the anterior-posterior commissure line (AC-PC line) in the axial plane using a single-shot spin-echo echoplanar imaging (EPI) sequence. The DTI gradient encoding schemes included 13 noncollinear directions with a b value of 1000 s/mm2 and a nondiffusion-weighted image volume (b value 0 s/mm2).
Temperature estimation
The kinetic theory states that a direct relationship exists between the absolute temperature and the diffusion coefficient. The diffusion coefficient of non-restricted water molecules can be reliably measured using MRI. Studies by Mills and Kozak et al. have shown that the cerebrospinal fluid temperature can be estimated using this relationship [13, 14]. Based on previous studies, we calculated the diffusion constant using the following equation:
where D is the diffusion constant (mm2/s), b is the applied diffusion weighting (s/mm2), and S0 and S are the voxel signal intensities of the reference and DWIs, respectively. The D value was converted to the corresponding temperature by the following equation:
where T is the temperature (°C). The temperature estimation was only considered within the lateral ventricles, since this method is only applicable to nonrestricted water. This DWI-based MR thermometry of the ventricles was calculated using an automated method developed by Sakai et al. [15].
Statistical analyses
Analysis of demographic data between groups
The statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) software package (version 17, SPSS Inc. Chicago, IL, USA). Age and sex were compared between the study groups by the independent t test and Pearson chi-square test. An analysis of covariance (ANCOVA) model with age and gender as covariates was used to determine group differences in the oxidative stress parameters in terms of the percentage of apoptotic peripheral leukocytes and the plasma levels of nuclear DNA, mitochondrial DNA, and serum adhesion molecules. Statistical significance was set at p < 0.05.
Analysis of brain temperature between groups
ANCOVA with age and gender as potential confounding variables was used to compare the PD and control groups in terms of intraventricular brain temperature (Tv). ANCOVA with age as the covariant was used to compare the group differences of Tv between the PD and control groups in the male and female populations, respectively. ANCOVA with gender as the covariant was used to compare the group differences of Tv among the three age subgroups. Statistical significance was set at p < 0.05.
Correlations among brain temperature, clinical severity, and inflammatory parameters
Partial correlation analysis adjusted for age and gender was performed with respect to the clinical severity, inflammatory parameters, and Tv. The threshold for statistical significance was set at p < 0.05 with a Bonferroni correction accounting for multiple regions of interest comparisons.
Results
Demographic characteristics of the participants and the disease severity of the PD patients
The demographic characteristics of the 103 PD cases and 106 controls are listed in Table 1. There were no significant differences in age and sex between the two groups (age: p = 0.064; gender: p = 0.781).
Table 1.
Demographic characteristics of patients with PD and control subjects
| Variable | PD | Normal | p value |
|---|---|---|---|
| Number | 103 | 106 | |
| Gender (M/F) | 44/59 | 48/58 | 0.781 |
| Age (year) | 60.43 ± 9.12 | 58.16 ± 8.45 | 0.064 |
| Male | 60.16 ± 11.01 | 57.81 ± 9.88 | 0.284 |
| Female | 60.63 ± 7.51 | 58.45 ± 7.12 | 0.110 |
| Disease duration (year) | 3.10 ± 3.31 | ||
| Treatment duration (month) | 16.12 ± 20.10 | ||
| Current daily dose (mg) | 424.04 ± 402.90 | ||
| UPDRS I | 3.17 ± 2.55 | ||
| UPDRS II | 10.24 ± 7.64 | ||
| UPDRS III | 24.70 ± 16.19 | ||
| UPDRS 176 | 37.23 ± 24.28 | ||
| Modified H&Y stage | 2.10 ± 1.11 | ||
| S&E scale | 82.60 ± 18.24 | ||
| Inflammatory parameters | n = 99 | n = 59 | |
| Apoptotic neutrophils (%) | 1.11 ± 0.89 | 0.74 ± 0.56 | 0.005* |
| Apoptotic monocytes (%) | 5.30 ± 6.30 | 2.97 ± 4.05 | 0.012* |
| Apoptotic lymphocytes (%) | 0.73 ± 0.52 | 0.49 ± 0.40 | 0.002* |
| Total apoptotic leukocytes (%) | 1.73 ± 1.20 | 1.13 ± 0.74 | 0.001* |
| Nuclear DNA (ng/mL) | 32.69 ± 26.36 | 19.99 ± 14.41 | 0.002* |
| Mitochondrial DNA (ng/mL) | 42.51 ± 36.34 | 51.41 ± 120.47 | 0.393 |
| ICAM-1 | 207.77 ± 77.39 | 213.09 ± 59.46 | 0.734 |
| VCAM-1 | 738.10 ± 286.68 | 640.92 ± 143.86 | 0.025* |
| P-selectin | 96.30 ± 21.17 | 84.47 ± 20.58 | 0.001* |
| L-selectin | 860.98 ± 175.11 | 955.84 ± 218.81 | 0.008* |
| E-selectin | 36.53 ± 18.53 | 34.54 ± 14.81 | 0.526 |
Data are presented as mean ± standard deviation. Age data were compared by independent t test. Gender data were compared by Pearson chi-square test. The inflammatory parameters data were compared by analysis of covariance (ANCOVA) after controlling for age and gender
UPDRS, Unified Parkinson Disease Rating Scale; H&Y stage, Hoehn and Yahr stage; S&E scale, Schwab and England scale
*p < 0.05
Comparison of inflammatory parameters between groups
The percentages of total apoptotic leukocytes and their subsets, including neutrophils, monocytes, and lymphocytes, were significantly higher in the PD group than in the controls. The PD patients exhibited higher nuclear DNA levels (p = 0.002), VCAM-1 levels (p = 0.002), and P-selectin levels (p = 0.001), as well as lower L-selectin levels (p = 0.008), compared with the controls.
Comparison of brain temperature in groups and subgroups
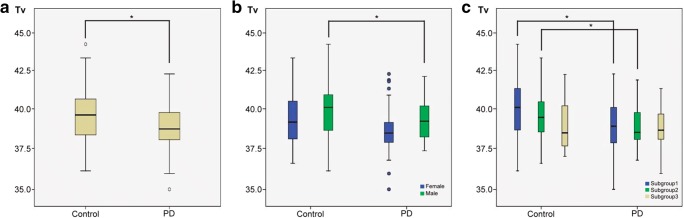
The box and whisker plot of measured Tv in the two groups is shown in Fig. 1. The Tv of the patients with PD was lower than that of the controls (p = 0.006). In the male population, the Tv in the patient group was slightly lower than that in the control group (p = 0.039). In the female population, the difference in Tv between the patient and control groups did not reach a statistically significant level (p = 0.052).
Fig. 1.
a Measured brain intraventricular temperatures (Tv) in patients with PD (n = 103) compared with healthy controls (n = 106). The Tv values of the patients with PD were significantly lower than those of the controls (p = 0.006) after controlling for age and gender. b Measured Tv in male and female patients with PD (male, n = 44; female, n = 59) compared with male and female healthy controls (male, n = 48; female, n = 58), respectively. The Tv values of the male patients with PD were significantly lower than those of the male controls (p = 0.039) after controlling for age. c Measured Tv in different age subgroups of patients with PD (subgroup 1, 30–54 years old, n = 24, Tv = 39.01 ± 1.72 °C; subgroup 2, 55–64 years old, n = 37, Tv = 38.83 ± 1.20 °C; subgroup 3, 65–79 years old, n = 41, Tv = 38.88 ± 1.19 °C; p = 0.273) compared with different age subgroups of healthy controls (subgroup 1, n = 30, Tv = 40.12 ± 1.90 °C; subgroup 2, n = 54, Tv = 39.52 ± 1.52 °C; subgroup 3, n = 22, Tv = 39.03 ± 1.52 °C; p = 0.072), respectively. In the age subgroups of the controls, the Tv values of subgroup 1 were significantly higher than those of subgroup 2 (p = 0.031) and subgroup 3 (p = 0.027). The Tv values of subgroups 1 and 2 patients with PD were significantly lower than those of the controls (p = 0.042 and 0.024) after controlling for gender
Among the different age subgroups of the PD patients, there was no significant difference in Tv (p = 0.273). Among the different age subgroups of the controls, the Tv of subgroup 1 was significantly higher than those of subgroup 2 (p = 0.031) and subgroup 3 (p = 0.027). In the comparison between the PD and controls, the Tv values of the subgroup 1 and 2 PD patients were significantly lower than those of the controls (p = 0.042 and p = 0.024).
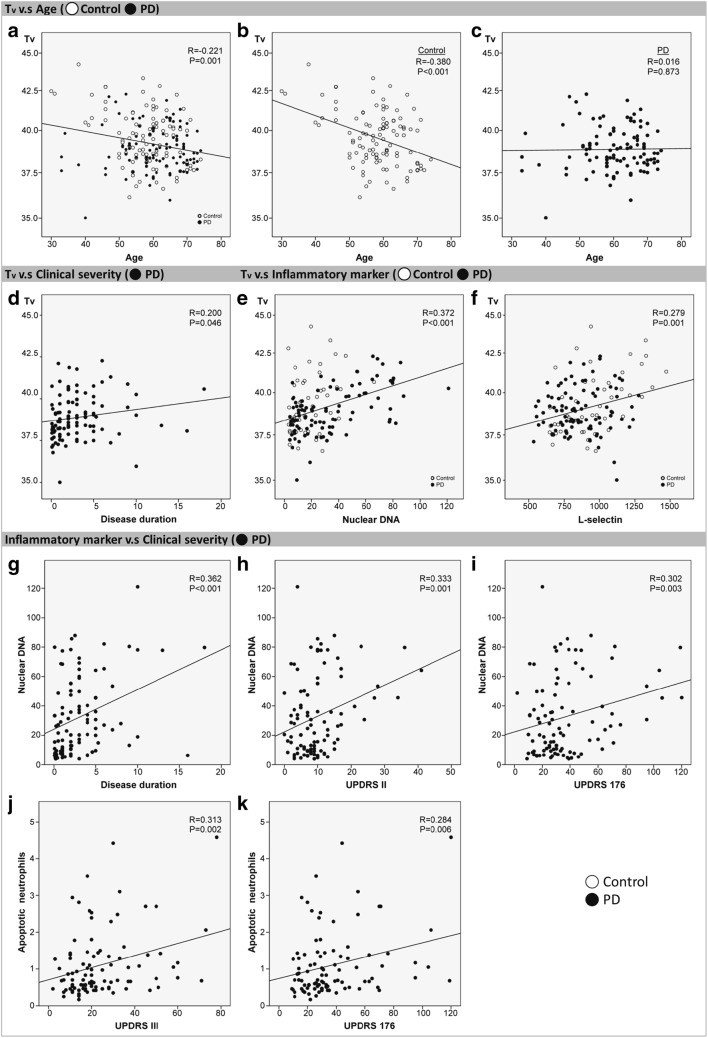
Relationships between brain temperature and inflammatory parameters
The results of the correlation analysis between brain temperature and inflammatory parameters are also presented in Table 2 and Fig. 2e, f. Both the plasma nuclear DNA level and L-selectin level revealed statistically positive correlations with Tv (p < 0.001 and p = 0.001) in all the subjects. Meanwhile, the plasma nuclear DNA level revealed a significant positive correlation with Tv (p < 0.001) in the PD group, but no statistical correlation (p = 0.071) in the control group. In contrast, the L-selectin level revealed a significant positive correlation with Tv (p = 0.005) in the control group, but no statistical correlation (p = 0.275) in the PD group.
Table 2.
Correlations among brain intraventricular temperature, clinical severity, and systemic inflammatory parameters
| Variables | Tv | |
|---|---|---|
| R | p | |
| Clinical data | ||
| Disease duration | 0.200 | 0.046* |
| Treatment duration | 0.095 | 0.345 |
| Current daily dose | 0.031 | 0.761 |
| UPDRS I | 0.157 | 0.120 |
| UPDRS II | 0.136 | 0.181 |
| UPDRS III | 0.074 | 0.468 |
| UPDRS 176 | 0.109 | 0.284 |
| Modified H&Y stage | 0.141 | 0.165 |
| S&E scale | − 0.197 | 0.052 |
| Inflammatory parameters | ||
| Apoptotic neutrophils | − 0.138 | 0.090 |
| Apoptotic monocytes | − 0.062 | 0.444 |
| Apoptotic lymphocytes | − 0.018 | 0.826 |
| Total apoptotic leukocytes | − 0.131 | 0.108 |
| Nuclear DNA | 0.372 | < 0.001* |
| VCAM-1 | − 0.054 | 0.520 |
| P-selectin | − 0.021 | 0.299 |
| L-selectin | 0.279 | 0.001* |
Partial correlation analysis adjusted for age and gender was performed to correlate the Tv with the clinical severity and inflammatory parameters
*p < 0.0, threshold for statistical significance
Fig. 2.
Correlations among age, clinical severity, inflammatory markers, and intraventricular temperature. The line indicates the result of linear fitting. Intraventricular temperature in all subjects (a) and controls (b) tended to decrease with age, with a significant correlation seen after controlling for gender. There was no correlation between age and Tv in the PD patients (c) after controlling for gender. The Tv was positively correlated with disease duration (d), nuclear DNA (e), and L-selectin (f) after controlling for age and gender. The level of plasma nuclear DNA revealed statistically positive correlations with disease duration (g), UPDRS II (h), and UPDRS 176 (i). The percentage of apoptotic neutrophils revealed statistically positive correlations with UPDRS III (j) and UPDRS 176 (k)
Relationship between brain temperature and age
Correlation analysis was used to evaluate the relationship between age and brain temperature in the different groups (Fig. 2a–c. A significant negative correlation was evident between the Tv and age in all the subjects (R = − 0.221; p = 0.001) and in the control group (R = − 0.380, p < 0.001). However, the Tv revealed no statistically significant correlation with age in the PD group (R = 0.016, p = 0.873).
Relationship between brain temperature and clinical severity
Correlation analysis was used to evaluate the relationship between clinical severity and brain temperature in the PD group (Table 2; Fig. 2d). The Tv revealed a significant positive correlation with disease duration (p = 0.046).
Relationships between clinical severity and inflammatory parameters
Correlation analysis was used to evaluate the relationship between clinical severity and inflammatory markers in the PD group (Table 3; Fig. 2g–k). The percentage of apoptotic neutrophils revealed statistically positive correlations with UPDRS III (p = 0.002) and UPDRS 176 (p = 0.006). The level of plasma nuclear DNA revealed statistically positive correlations with disease duration (p < 0.001), UPDRS II (p = 0.001), and UPDRS 176 (p = 0.003).
Table 3.
Correlations between clinical severity and systemic inflammatory parameters
| Variables | Apoptotic neutrophils | Apoptotic monocytes | Apoptotic lymphocytes | Total apoptotic leukocytes | Nuclear DNA | VCAN-1 | P-selectin | L-selectin | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | p | R | p | R | p | R | p | R | p | R | p | R | p | R | p | |
| Disease duration | 0.056 | 0.594 | 0.017 | 0.870 | − 0.059 | 0.572 | 0.066 | 0.533 | 0.362 | < 0.001* | − 0.014 | 0.895 | 0.119 | 0.248 | 0.078 | 0.448 |
| Treatment duration | 0.057 | 0.587 | 0.091 | 0.387 | 0.262 | 0.011 | 0.206 | 0.048 | − 0.068 | 0.513 | 0.245 | 0.016 | − 0.257 | 0.011 | − 0.167 | 0.103 |
| Current daily dosage | 0.097 | 0.353 | − 0.005 | 0.965 | − 0.062 | 0.557 | 0.124 | 0.238 | 0.154 | 0.133 | 0.205 | 0.046 | 0.004 | 0.967 | 0.042 | 0.685 |
| UPDRS I | 0.157 | 0.138 | − 0.111 | 0.297 | − 0.132 | 0.214 | 0.089 | 0.404 | 0.220 | 0.033 | 0.009 | 0.930 | 0.061 | 0.562 | − 0.086 | 0.411 |
| UPDRS II | 0.203 | 0.053 | 0.022 | 0.837 | − 0.041 | 0.699 | 0.201 | 0.056 | 0.333 | 0.001* | 0.115 | 0.269 | 0.198 | 0.056 | 0.060 | 0.568 |
| UPDRS III | 0.313 | 0.002* | − 0.027 | 0.799 | − 0.084 | 0.431 | 0.227 | 0.031 | 0.270 | 0.009 | 0.171 | 0.098 | 0.083 | 0.428 | 0.065 | 0.532 |
| UPDRS 176 | 0.284 | 0.006* | − 0.022 | 0.832 | − 0.081 | 0.445 | 0.219 | 0.037 | 0.302 | 0.003* | 0.148 | 0.153 | 0.121 | 0.244 | 0.052 | 0.619 |
| Modified H&Y stage | 0.229 | 0.029 | − 0.067 | 0.530 | − 0.138 | 0.194 | 0.156 | 0.140 | 0.257 | 0.012 | 0.192 | 0.064 | − 0.052 | 0.617 | 0.029 | 0.779 |
| S&E scale | − 0.248 | 0.018 | 0.054 | 0.611 | 0.090 | 0.394 | − 0.156 | 0.140 | − 0.255 | 0.013 | − 0.105 | 0.312 | 0.008 | 0.943 | 0.040 | 0.700 |
Partial correlation analysis adjusted for age and gender was performed to correlate the inflammatory parameters with the clinical severity
*p < 0.05, threshold for statistical significance with a Bonferroni correction accounting for multiple region of interest comparisons
Discussion
The regulation of brain temperature is dependent on various factors, including cerebral metabolism, cerebral blood flow, and body core temperature. Heat production in the brain is closely related to the cerebral metabolic rates of glucose and oxygen, and the age-dependent decrease in metabolism can lead to temperature decline (Figs. 1c and 2b) [16]. A natural decline in Tv among normal subjects have been repeatedly shown, but the PD subjects failed to reveal this descent in Tv. Another characteristic finding was that PD patients tended to have lower temperature compared with controls, which was especially evident in the younger age subgroups (Figs. 1c and 2c). For the older subjects (subgroup 3, 65–79 years old), the temperature difference was not significant (Fig. 1c).
Significantly higher levels of circulating apoptotic leukocytes, nuclear DNA, VCAM-1, and P-selectin in PD patients were shown in the present study. These markers are important indicators supporting the presence of underlying systemic inflammation (Table 1). Those markers were also associated with increased disease severity, suggesting that they may play critical roles in the pathophysiology of PD (Table 3; Fig. 2g–k). It is well known that in brain tissue, dysfunction of the blood-brain barrier accompanied by peripheral immune cell infiltration/invasion leads to the loss of dopaminergic neurons caused by programmed cell death [17].
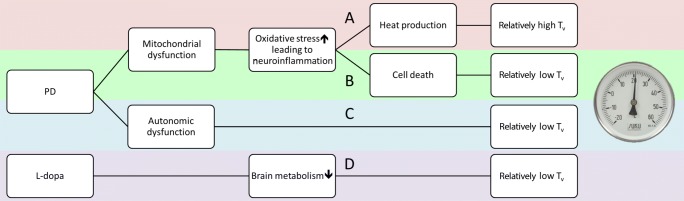
This study is the first to report an association between brain temperature and circulating inflammatory markers in PD patients. As the temperature of the brain should be determined by multiple different factors, a simplified summary of those factors is provided in Fig. 3. Heat in the brain is produced mostly by mitochondrial oxidative chemical reactions [6]. As PD patients have impaired mitochondrial biogenesis (mitobiogenesis), this would directly affect the brain temperature of PD patients, and it is thought to be one of the major causes for temperature decline.
Fig. 3.
The pathophysiologic model involving oxidative stress and thermal regulation in PD in this study. Mitochondria are responsible for maintaining the cellular energy reserves with heat production. Mitochondrial dysfunction is involved in activated neuroinflammation and increased oxidative stress. The injury from oxidative stress might induce cell apoptosis or death, which would in turn result in the loss of heat production and the associated relatively low Tv (pathway B). However, in the condition of cell damage but survival, the resulting hyper-metabolism under oxidative stress would promote heat generation and the associated relatively high Tv (pathway A). The dynamic regulation of pro- and anti-inflammatory cytokines could potentially promote neuroprotection and lead to the initiation of thermal modulation. Other etiologies, such as autonomic dysfunction and dopamine agonists, might also be related to thermal dysregulation in PD (pathways C and D)
Oxidative stress and neuroinflammation also play an important role in determining brain temperature, as they can either elevate or lower it (Fig. 3a, b). Mitochondrial ROS is known to activate signaling pathways that control the initiation of cell death [18]. Increased plasma nuclear DNA is one of the inflammatory markers, and in this study, it was found to have positive correlations with higher brain temperature, disease duration (Fig. 2g), and UPDRS scores (Fig. 2i–k).
Mitochondrial and plasma membranes are the most temperature-sensitive cellular elements. Hyperthermia can further potentiate the cytotoxic effects of ROS into a vicious cycle [19]. In contrast, hypothermia is known to reduce superoxide anion production [20], in addition to preventing hydroxyl radical formation. The injury from oxidative stress might induce cell apoptosis or death, which would result in the loss of heat production and associated hypothermia (Fig. 3, pathway B). However, in the condition of cell damage but survival, the hyper-metabolism under oxidative stress would promote heat generation and associated hyperthermia (Fig. 3, pathway A).
The dynamic regulation of pro- and anti-inflammatory cytokines is suggested to be potentially useful in promoting neuroprotection [21], and it could lead to the initiation of thermal modulation. Since the alteration of temperature and the mechanisms underlying the relationship between oxidative stress and thermoregulation are complex, further studies of animal models might be required to fully clarify the causal relationships among those factors. Other etiologies, such as autonomic dysfunction and dopamine agonists [8], might also play some role in thermal dysregulation (Fig. 3, pathways C and D). Both of them were known to topographically involve similar temperature regulation area and should be further evaluated in the future.
Even though there are many mechanisms to regulate the human core temperature (Fig. 3), PD is ultimately an irreversible neurodegenerative disease. Therefore, the overall Tv in the PD patients presented in our study was lower than that of the normal controls. However, overlaps in Tv between the PD patients and the controls were observed. This might have been due to the combined results of cell inflammation with different degrees of the disease in the PD patients, as well as other variations among the physiological effects on the individual subjects, such as variations in the effects on their individual metabolic rates. The higher oxidative stress in the PD patients seen in our results might reflect both anti- and pro-inflammatory processes in their early disease [22] that lead to the overlap with the normal controls in Tv presentation. As the disease progresses, more oxidative stress induced in neurons undergoing pro-inflammatory processes leads to cell apoptosis, which in turn eventually causes a drop in the overall central temperature. The trajectory of oxidative stress seen in the present study was similar to the trajectory presented in a previous report [23] about ramified microglia transforming into anti-inflammatory and pro-inflammatory phenotypes. However, we could not verify the phenotypes of oxidative stress in our results, so a further validation study should therefore be conducted.
Although this study has yielded useful findings, the interpretation of its results must be done carefully. First, it should be noted that the brain temperature is dependent on the body core temperature, but this latter temperature was not recorded as this was a retrospective study. Second, the ventricular temperatures of all the participants were slightly higher than expected, possibly because the pulsatile movement of the cerebrospinal fluid led to the overestimation of these temperature levels. In any case, the method used was thus only applicable for comparison between the groups. Whether this technique can also be applied to monitor a patient for longitudinal follow-up remains to be tested in future studies. Third, the effects of medication on brain temperature remain unknown. Quite naturally, almost all of the PD patients were undergoing treatment with various medications depending on their clinical symptoms. Last but not the least, the individuals in this group of PD patients were at multiple different stages of disease progression, and therefore, the data used in this study do not constitute an ideal set of data to elucidate the true nature of disease progression. This study was a cross-sectional one, which limits the degree to which its results can be interpreted as representing the full picture of PD. Thus, the causal relationships between inflammation and the alteration of brain temperature in PD still remain to be elucidated. It is hoped, nonetheless, that this study can serve as a basis for further research.
The results of this study support our hypothesis that increased inflammation in patients with PD correlates with the alteration of brain temperature and provide a possible pathophysiological explanation for the associated systemic inflammation and thermoregulation. This model could assist us in investigating the underlying pathogenesis of PD in future research.
Electronic supplementary material
(DOCX 49 kb)
Abbreviations
- ANCOVA
Analysis of covariance
- Tv
Brain intraventricular temperature
- DWI
Diffusion-weighted imaging
- H&Y
Modified Hoehn and Yahr scale
- PD
Parkinson’s disease
- ROS
Reactive oxygen species
- S&E
Schwab and England scale
- UPDRS
Unified Parkinson’s Disease Rating Scale
Authors’ contributions
HLC, KY, and CHL were the main contributors in the writing of the literature review. KS, CHL, and WCL were the main contributors in the conducting of the clinical and experimental aspects of the study. HLC, KY, and MHC were the main contributors in the conducting of the statistical analysis. HLC, KY, and WCL were the main contributors in the editing of the manuscript. All authors read and approved the final manuscript.
Funding information
This work was supported by funds from the National Science Council (MOST 106-2314-B-182A-031-MY2 to W-C Lin and MOST 104-2314-B-182A-053 to H-L Chen) and Chang Gung Memorial Hospital (CMRPG891511 and CMRPG8B0831 to W-C Lin and CMRPG8E0621 to M-H Chen).
Compliance with ethical standards
Ethics approval
The protocols of this study were approved by the Local Ethics Committee on Human Research of Kaohsiung Chang Gung Memorial Hospital in Taiwan.
Informed consent
Written informed consent was collected from each participant prior to his or her participation in this study.
Consent for publication
Not applicable.
Availability of data and material
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Competing interests
The authors declare that they have no competing interests
Footnotes
Key point
Possible links between systemic inflammation and brain temperature were revealed, providing further insights into the underlying pathogenesis of Parkinson’s disease
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hsiu-Ling Chen, Email: suring.tw@gmail.com.
Kei Yamada, Email: kyamada@koto.kpu-m.ac.jp.
Koji Sakai, Email: sakai3@koto.kpu-m.ac.jp.
Cheng-Hsien Lu, Email: chlu99@adm.cgmh.org.tw.
Meng-Hsiang Chen, Email: sperfect@msn.com.
Wei-Che Lin, Email: linwc137@gmail.com.
References
- 1.Bose A, Beal MF. Mitochondrial dysfunction in Parkinson’s disease. J Neurochem. 2016;139(Suppl 1):216–231. doi: 10.1111/jnc.13731. [DOI] [PubMed] [Google Scholar]
- 2.Chaturvedi RK, Beal MF. Mitochondrial approaches for neuroprotection. Ann N Y Acad Sci. 2008;1147:395–412. doi: 10.1196/annals.1427.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perier C, Bove J, Dehay B, Jackson-Lewis V, Rabinovitch PS, Przedborski S, Vila M. Apoptosis-inducing factor deficiency sensitizes dopaminergic neurons to parkinsonian neurotoxins. Ann Neurol. 2010;68(2):184–192. doi: 10.1002/ana.22034. [DOI] [PubMed] [Google Scholar]
- 4.Hunot S, Hirsch EC. Neuroinflammatory processes in Parkinson’s disease. Ann Neurol. 2003;53(Suppl 3):S49–S58. doi: 10.1002/ana.10481. [DOI] [PubMed] [Google Scholar]
- 5.Micieli G, Tosi P, Marcheselli S, Cavallini A. Autonomic dysfunction in Parkinson’s disease. Neurol Sci. 2003;24(Suppl 1):S32–S34. doi: 10.1007/s100720300035. [DOI] [PubMed] [Google Scholar]
- 6.Rango M, Arighi A, Bonifati C, Del Bo R, Comi G, Bresolin N. The brain is hypothermic in patients with mitochondrial diseases. J Cereb Blood Flow Metab. 2014;34(5):915–920. doi: 10.1038/jcbfm.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwon JY, Bacher A, Deyo DJ, Grafe MR, Disterhoft JF, Uchida T, Zornow MH. Effects of hypothermia and lamotrigine on trace-conditioned learning after global cerebral ischemia in rabbits. Exp Neurol. 1999;159(1):105–113. doi: 10.1006/exnr.1999.7130. [DOI] [PubMed] [Google Scholar]
- 8.Iodice V, Low DA, Vichayanrat E, Mathias CJ. Cardiovascular autonomic dysfunction in MSA and Parkinson’s disease: similarities and differences. J Neurol Sci. 2011;310(1–2):133–138. doi: 10.1016/j.jns.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Yu CC, Chen MH, Lu CH, Huang YC, Chen HL, Tsai NW, Wang HC, Yang IH, Li SH, Lin WC. Altered striatocerebellar metabolism and systemic inflammation in Parkinson’s disease. Oxidative Med Cell Longev. 2016;2016:1810289. doi: 10.1155/2016/1810289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin WC, Tsai NW, Huang YC, Cheng KY, Chen HL, Li SH, Kung CT, Su YJ, Lin WM, Chen MH, Chiu TM, Yang IH, Lu CH. Peripheral leukocyte apoptosis in patients with parkinsonism: correlation with clinical characteristics and neuroimaging findings. Biomed Res Int. 2014;2014:635923. doi: 10.1155/2014/635923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen MH, Chen PC, Lu CH, Chen HL, Chao YP, Li SH, Chen YW, Lin WC. Plasma DNA mediate autonomic dysfunctions and white matter injuries in patients with Parkinson’s disease. Oxidative Med Cell Longev. 2017;2017:7371403. doi: 10.1155/2017/7371403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen HL, Lu CH, Lin HC, Chen PC, Chou KH, Lin WM, Tsai NW, Su YJ, Friedman M, Lin CP, Lin WC. White matter damage and systemic inflammation in obstructive sleep apnea. Sleep. 2015;38(3):361–370. doi: 10.5665/sleep.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mills R. Self-diffusion in normal and heavy water in the range 1–45. deg. J Phys Chem. 1973;77(5):685–688. [Google Scholar]
- 14.Kozak LR, Bango M, Szabo M, Rudas G, Vidnyanszky Z, Nagy Z. Using diffusion MRI for measuring the temperature of cerebrospinal fluid within the lateral ventricles. Acta Paediatr. 2010;99(2):237–243. doi: 10.1111/j.1651-2227.2009.01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakai K, Yamada K, Sugimoto N. Calculation methods for ventricular diffusion-weighted imaging thermometry: phantom and volunteer studies. NMR Biomed. 2012;25(2):340–346. doi: 10.1002/nbm.1755. [DOI] [PubMed] [Google Scholar]
- 16.Sakai K, Yamada K, Mori S, Sugimoto N, Nishimura T. Age-dependent brain temperature decline assessed by diffusion-weighted imaging thermometry. NMR Biomed. 2011;24(9):1063–1067. doi: 10.1002/nbm.1656. [DOI] [PubMed] [Google Scholar]
- 17.Stolp HB, Dziegielewska KM. Review: role of developmental inflammation and blood-brain barrier dysfunction in neurodevelopmental and neurodegenerative diseases. Neuropathol Appl Neurobiol. 2009;35(2):132–146. doi: 10.1111/j.1365-2990.2008.01005.x. [DOI] [PubMed] [Google Scholar]
- 18.Martin LJ. Mitochondrial and cell death mechanisms in neurodegenerative diseases. Pharmaceuticals (Basel) 2010;3(4):839–915. doi: 10.3390/ph3040839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiyatkin EA. Brain temperature homeostasis: physiological fluctuations and pathological shifts. Front Biosci (Landmark Ed) 2010;15:73–92. doi: 10.2741/3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maier CM, Sun GH, Cheng D, Yenari MA, Chan PH, Steinberg GK. Effects of mild hypothermia on superoxide anion production, superoxide dismutase expression, and activity following transient focal cerebral ischemia. Neurobiol Dis. 2002;11(1):28–42. doi: 10.1006/nbdi.2002.0513. [DOI] [PubMed] [Google Scholar]
- 21.Bhalala US, Koehler RC, Kannan S. Neuroinflammation and neuroimmune dysregulation after acute hypoxic-ischemic injury of developing brain. Front Pediatr. 2014;2:144. doi: 10.3389/fped.2014.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiang PL, Chen HL, Lu CH, Chen YS, Chou KH, Hsu TW, Chen MH, Tsai NW, Li SH, Lin WC. Interaction of systemic oxidative stress and mesial temporal network degeneration in Parkinson’s disease with and without cognitive impairment. J Neuroinflammation. 2018;15(1):281. doi: 10.1186/s12974-018-1317-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan Z, Brooks DJ, Okello A, Edison P. An early and late peak in microglial activation in Alzheimer’s disease trajectory. Brain. 2017;140(3):792–803. doi: 10.1093/brain/aww349. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 49 kb)