Abstract
Striated myocytes compose about half of the cells of the heart, while contributing the majority of the heart’s mass and volume. In response to increased demands for pumping power, including in diseases of pressure and volume overload, the contractile myocytes undergo non-mitotic growth, resulting in increased heart mass, i.e. cardiac hypertrophy. Myocyte hypertrophy is induced by a change in the gene expression program driven by the altered activity of transcription factors and co-repressor and co-activator chromatin-associated proteins. These gene regulatory proteins are subject to diverse post-translational modifications and serve as nuclear effectors for intracellular signal transduction pathways, including those controlled by cyclic nucleotides and calcium ion. Scaffold proteins contribute to the underlying architecture of intracellular signaling networks by targeting signaling enzymes to discrete intracellular compartments, providing specificity to the regulation of downstream effectors, including those regulating gene expression. Muscle A-kinase anchoring protein β (mAKAPβ) is a well-characterized scaffold protein that contributes to the regulation of pathological cardiac hypertrophy. In this review, we discuss the mechanisms how this prototypical scaffold protein organizes signalosomes responsible for the regulation of class IIa histone deacetylases and cardiac transcription factors such as NFAT, MEF2, and HIF-1α, as well as how this signalosome represents a novel therapeutic target for the prevention or treatment of heart failure.
Keywords: Kinase, AKAP, gene transcription, cardiac hypertrophy, cAMP
INTRODUCTION
An increase in the mass of the heart known as “cardiac hypertrophy” is the primary response of that organ to stress in disease and a major risk factor for the development of heart failure, a leading cause of death and a problem of increasing public health significance worldwide [1, 2]. According to the latest statistics from the American Heart Association, an estimated 6.2 million Americans have heart failure[3]. This number is estimated to increase 46% by 2030. Furthermore, the 5-year mortality rate of patients diagnosed with heart failure is ~50%, demonstrating the need for better therapeutics to treat this syndrome. The main contributor to the increased heart size of the hypertrophic heart is hypertrophy of the cardiac myocytes, an increase in the volume of the contractile cells in the absence of cellular proliferation. Fundamental to the development of hypertrophy is a dramatic altering of the myocyte gene expression program, that besides the change in morphology also results in disease in changes in cellular metabolism, contractility, and survival, and the release of paracrine factors that promote myocardial interstitial fibrosis [4]. Research over the last 20 years has revealed that select transcription factors, including nuclear factor of activated T-cells (NFAT) and myocyte enhancer factor 2 (MEF2) family members, nucleate the chromatin activating and repressive transcriptional complexes that direct pathological cardiac gene expression [4]. In addition, changes in the activity of histone deactylases (HDACs) and other chromatin modifying enzymes are integral to the induction of hypertrophy [5]. As these gene regulatory proteins are tightly regulated by intracellular signal transduction pathways, the elucidation of the upstream signals that control transcription factor and epigenetic modifier activity remains an important area of heart failure research. Extensive research has shown the relevance of mitogen-activated protein kinase (MAPK), cyclic nucleotide, calcium, hypoxia and phosphoinositide-dependent pathways to the regulation of gene regulatory protein post-translational modification in hypertrophy [6]. However, the mechanisms of how these pathways selectively control relevant gene regulatory proteins in disease remains unclear, especially given the separate role that cyclic nucleotides and calcium play in the regulation of excitation-contraction coupling.
One mechanism that has evolved to confer specificity to signaling networks is compartmentation by scaffolding proteins [7]. These typically non-enzymatic proteins function to co-localize signaling enzymes into discrete complexes with both their upstream activators and their downstream effector substrates, providing enhanced substrate specificity while increasing the kinetics of signaling events. In addition, as scaffold proteins are often multivalent and bind to enzymes from multiple signaling pathways, “signalosomes” organized by scaffold proteins serve to integrate multiple upstream signals and facilitate crosstalk between different relevant signaling pathways, thereby providing a combinatorial regulation of downstream signaling events. As discussed herein, research concerning the scaffold protein muscle A-kinase anchoring protein β (mAKAPβ) has revealed that mAKAPβ signalosomes are central to the orchestration of gene regulatory proteins controlling pathological cardiac remodeling.
A-Kinase anchoring proteins (AKAPs) are scaffold proteins that bind the cAMP-dependent Protein Kinase A (PKA) [8]. Directing PKA to discrete cellular compartments via localization domains unique to each AKAP and binding multiple other signaling enzymes such as phosphatases, phosphodiesterases, and other kinases, AKAPs serve important roles in the facilitation of cross-talk between different signaling pathways, including G-protein coupled receptor signaling in the heart [9]. The first demonstration of the importance of AKAPs for cardiac physiology utilized a peptide to globally disrupt AKAP/PKA binding [10]. Incubation of cardiac myocytes with a PKA anchoring disruptor peptide showed that AKAPs play a role in the β-adrenergic stimulation of calcium influx and contraction. Since these original findings, AKAP have been shown to regulate many cellular events such as potassium channel currents, sarcoplasmic calcium cycling, and G-protein coupled signaling [11–13]. Additionally, several AKAPs are involved in the induction of cardiac disease [9]. This review will focus on the function of mAKAPβ signalosomes in the induction of pathological cardiac hypertrophy through the orchestrated regulation of myocyte gene expression.
mAKAP – A perinuclear scaffold protein that regulates cardiac myocyte hypertrophy
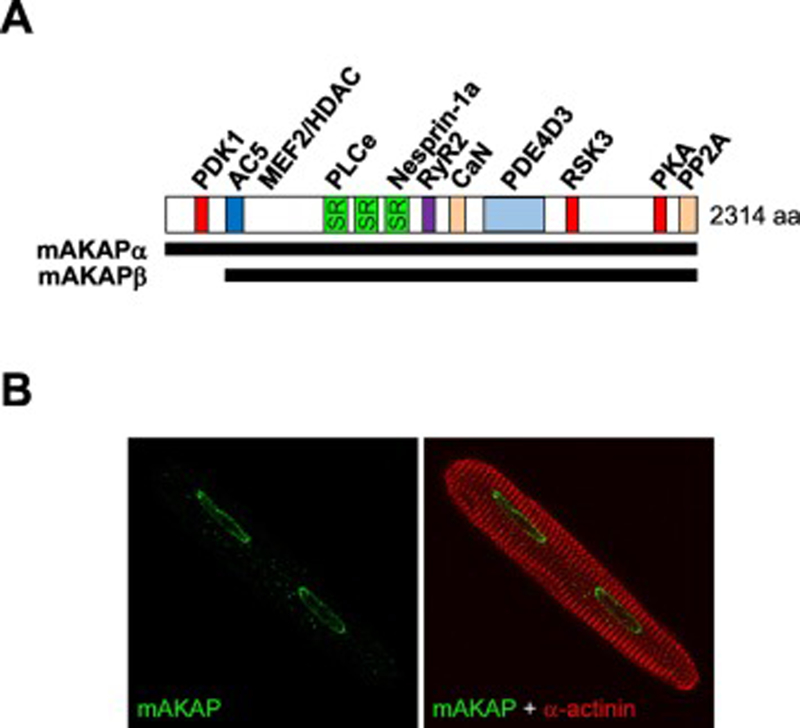
The mAKAP (AKAP6) gene is alternatively-spliced and expressed as the 260kDa α-isoform in neurons and the 230 kDa β-isoform in striated myocytes, such that the mAKAPβ protein is identical to mAKAPα aa residues 245–2314 due to initiation of translation at an internal residue (Figure 1A) [14, 15]. Although initially misconstrued to be localized to the sarcoplasmic reticulum [16, 17], in terminally differentiated striated myocytes, mAKAPβ is constitutively localized in myocytes to the nuclear envelope by a series of spectrin-like repeat domains (amino acids 772–1187, numbering per mAKAPα, Figure 1B) [14, 15, 18–20]. This discrete mAKAPβ localization has been confirmed using multiple different mAKAP antibodies and by the lack of staining in both cultured myocytes depleted of mAKAPβ and in mice with a cardiac myocyte-specific mAKAPβ gene deletion [18, 21]. Binding of the third mAKAP spectrin repeat directly to the Klarsicht, ANC-1, Syne Homology (KASH) domain protein nesprin-1α is necessary and sufficient for nuclear envelope localization [22, 23]. Consistent with most studies addressing KASH domain protein localization and their nuclear envelope-bridging interactions with inner nuclear membrane Sad1, UNC-84 (SUN) domain proteins [24], digitonin-solubilization experiments have shown that mAKAPβ and nesprin-1α are present on the outer nuclear membrane where they are exposed to the cytoplasm (M.S.K., unpublished observations and [25]). Albeit controversial, there is also evidence that through lamin-A and SUN protein nucleoplasmic domain binding, nesprins may localize to the inner nuclear membrane as well [26, 27], making it is plausible that mAKAP also extends into the nucleoplasm. Further work will be required to define the topology of mAKAP localization, especially given its relevance to the regulation of gene expression.
Figure 1. mAKAP is a perinuclear scaffold protein.
A. mAKAPβ is identical to residues 245–2314 of the mAKAPα isoform expressed in neurons, such that mAKAP residues are numbered according to mAKAPα (rat). Binding sites are shown for those mAKAP binding partners for which there is evidence of direct binding: PDK1, 3-phosphoinositide-dependent kinase-1 [15], AC5, adenylyl cyclase 5 [52], MEF2 and HDAC [31, 50], PLCε, phospholipase Cε [29], nesprin-1α [22], RyR, ryanodine receptor [84], calcineurin [64], PDE4D3, phosphodiesterase 4D3 [51], RSK3, p90 ribosomal S6 kinase 3 [85], PKA, protein kinase A [14], PP2A, protein phosphatase 2A [55]. B. mAKAPβ is localized to the myocyte nuclear envelope regardless of developmental stage or stress conditions [14, 18]. An adult rat ventricular myocyte is shown [18].
The recognition that mAKAPβ was localized to the nuclear envelope and was restricted in expression to excitable cells immediately raised the question whether the scaffold was involved in the regulation of myocyte-specific gene expression, including that controlling myocyte hypertrophy [19]. Initial evidence showing the requirement for mAKAPβ in myocyte hypertrophy was obtained by RNA interference (RNAi) using cultured primary neonatal rat ventricular myocytes stimulated with α-adrenergic, β-adrenergic, or gp130/leukemia inhibitor factor receptor agonists [21, 28]. mAKAPβ depletion inhibited morphologic hypertrophy (cell size and myofibril organization), de novo protein synthesis and fetal gene expression. Subsequent studies extended these results to myocytes treated with angiotensin II and endothelin-1 [29, 30]. The role of mAKAPβ in cardiac myocytes may be compared with that in skeletal muscle and retina. mAKAPβ in skeletal myocytes has been shown to be important for muscle regeneration in injury and for myoblast differentiation, consistent with its expression only in terminally differentiated myocytes [31, 32]. In contrast, mAKAPα in retinal ganglion cells was required for neurite extension in vitro and neuronal survival in vivo, as tested in an optic nerve crush model that induces retrograde cell death of these central nervous system neurons [33]. While the functions of mAKAPβ in cardiac and skeletal muscle may be reconciled as related to the elaboration of the terminally differentiated myocyte phenotype, the apparently divergent role of mAKAPα in the nervous system will require further explanation.
The function of mAKAPβ suggested by in vitro experimentation using primary neonatal rat ventricular myocytes is further supported by data obtained in vivo. Conditional, cardiac myocyte-specific mAKAP gene deletion in mice attenuated both the pathological myocyte hypertrophy induced by pressure overload and by chronic isoproterenol infusion, as well as the physiological myocyte hypertrophy induced by swimming training [18]. Notably, the induction of interstitial fibrosis and myocardial apoptosis by chronic transverse aortic constriction was inhibited by mAKAP conditional knock-out, correlating with the prevention of heart failure and associated mortality in these mice. This work, taken together with the earlier in vitro studies, strongly implicates mAKAPβ signalosomes in the regulation of pathological cardiac remodeling. Furthermore, as mAKAPβ localization and expression are similar in human myocytes [14, 19], these results suggest that targeting of mAKAPβ signalosomes may be therapeutically beneficial for the prevention or treatment of human heart failure. Development of any such mAKAPβ-targeted therapies will be facilitated by an in-depth understanding of the mechanisms by which mAKAPβ signalosomes control myocyte hypertrophy, as discussed below.
mAKAP Signalosomes and Regulation of Class IIa HDACs
When present in the nucleus, class IIa HDACs (HDACs 4, 5, 7, and 9), which have weak intrinsic HDAC activity, organize co-repressor complexes that suppress the activity of site-specific transcription factors [34, 35]. Nuclear export of class IIa HDACs permits the expression of genes activated by the MEF2 family of transcription factors key to the development of pathological cardiac remodeling, while in normal cardiac physiology class IIa HDAC-mediated repression maintains normal cardiac structure and function. For example, ablation of the Hdac5 and Hdac9 mouse genes resulted in spontaneous cardiac hypertrophy, as well as an exaggerated response to pathologic pressure overload stress [36, 37] Class IIa HDACs have conserved regulatory and deacetylase domains (Figure 2). Phosphorylation of serine residues (e.g. HDAC5 Ser-259/498/661, with the latter site apparently less important) by protein kinase D (PKD), salt inducible kinase 1 (SIK1), and Ca2+/calmodulin-dependent kinases creates docking sites for 14–3-3 proteins that mask the intervening nuclear localization signal (NLS) and favors Crm1-dependent nuclear export [38–44]. In contrast, PKA-dependent phosphorylation (HDAC5 Ser-279) promotes nuclear import [45–49]. Recent work has demonstrated that class IIa HDAC phosphorylation by both PKA and PKD is regulated in cardiac myocytes by mAKAPβ signalosomes in response to cAMP signaling [50].
Figure 2. Structure of a class IIa histone deacetylase.
HDAC5 is similar to other class IIa family members [86, 87]. CtBP, HP1 - binding sites for these co-repressors; MEF2 - binding site for the transcription factor; NLS and NES - nuclear localization and export signals. Phosphorylation sites and cysteine residues regulated by oxidation (Ox) are indicated.
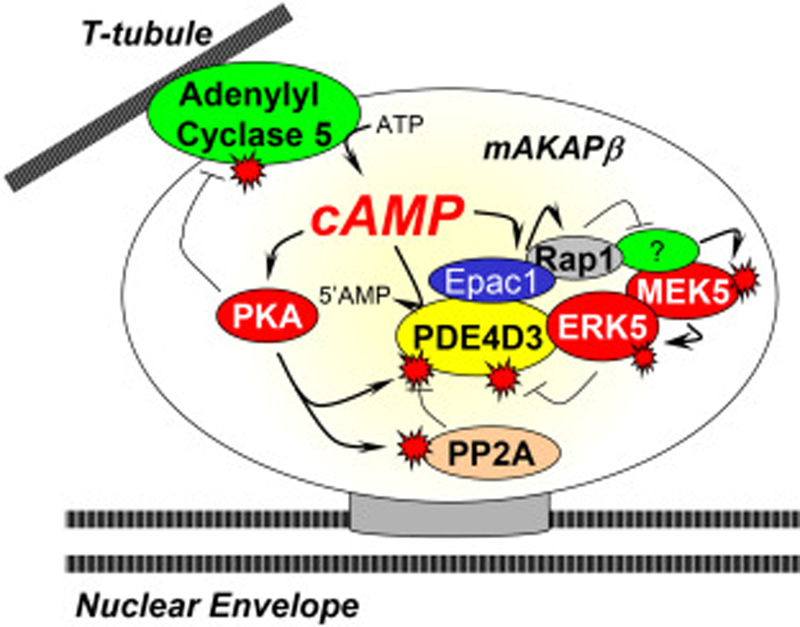
By binding an adenylyl cyclase (AC), cAMP effectors, and a phosphodiesterase (PDE), mAKAPβ signalosomes can produce, utilize and hydrolyze cAMP within an autonomous perinuclear myocyte signaling compartment (Figure 3). mAKAP was initially identified in a screen for new PKA binding proteins [16] and was the first AKAP to be shown to bind a PDE [51]. Subsequent research revealed that mAKAPβ also bound AC in myocytes, as well as a second cAMP effector, the guanine nucleotide exchange factor Epac1 [28, 52]. mAKAP binds specific PDE and AC isoforms, namely the cAMP-specific type 4D3 PDE and type 5 AC [51, 52], with functional consequence. The mAKAP PDE binding domain (within amino acids 1286–1831) binds directly the N-terminal 4D3 peptide of PDE4D3 [53], such that other PDE4D alternatively-spliced isoforms like PDE4D5 do not bind the scaffold [51]. PKA-catalyzed phosphorylation of PDE4D3 Ser-54 increases cAMP hydrolysis, while phosphorylation at Ser-13 increases the affinity of the PDE for mAKAPβ [53, 54]. Additional feedback and feed-forward regulation is conferred by the association of Epac1, ERK5, and protein phosphatase 2A (PP2A) with the complex [28, 55]. Thus, formation of PKA-mAKAP-PDE4D3 complexes creates the potential for tightly regulated local cAMP signaling that can restrict local PKA activity and uniquely define cAMP-signaling dynamics within mAKAP compartments. Interestingly, recent work has identified human mAKAP single nucleotide, non-synonymous polymorphisms (SNP) that affect the binding affinity of PDE4D3 and PKA, potentially changing the dynamics of cAMP hydrolysis and PKA signaling at the complex [56, 57]. Whether these polymorphisms affect cardiac signaling under physiologic or pathologic conditions deserves further insight. Currently, these are the only investigated human mutations of mAKAPβ, but a genome-wide association study (GWAS) identified that a mAKAP SNP (rs12885467) was associated with a higher BMI in humans [58]. Further investigation of these human mAKAP variants may contribute to our understanding of the molecular mechanism of human disease.
Figure 3. Regulation of perinuclear cAMP by mAKAPβ signalosomes.
AC5 presumably located on transverse tubules adjacent to the nuclear envelope can locally produce cAMP, followed by PKA phosphorylation that inhibits AC5 and activates PDE4D3 activity, resulting in decreased cAMP accumulation [54, 88]. Activation of ERK5 by MEK5 will lead to PDE4D3 inhibition and increased PKA activity [28]. When high cAMP levels activate the guanine nucleotide exchange factor Epac1, Rap1 will inhibit the ERK5 pathway, reversing the ERK5-mediated inhibition of PDE4D3 and limiting downstream signaling. In addition, there is an incoherent feedforward loop that will oppose PKA phosphorylation of PDE4D3 resulting from PKA phosphorylation and activation of PP2A in the complex [55].
cAMP can be locally synthesized at mAKAPβ signalosomes by bound AC5 [52]. While mAKAP can bind either AC2 and AC5 when expressed together in heterologous cells, AC5 is the primary cyclase associated with mAKAPβ in the heart [52]. Complex formation is through the binding of the N-terminal C1 and C2 catalytic AC5 domains to a discrete N-terminal mAKAPβ domain (aa 275–340). PKA phosphorylation of PDE4D3 increases its activity, but PKA phosphorylation of AC5 inhibits cyclase activity (Figure 3) [59]. The importance of PKA-AC5 negative feedback regulation was demonstrated using mAKAP-derived anchoring disruptor peptides to compete signalosome formation in neonatal rat ventricular myocytes [52]. Disruption of AC5 binding to mAKAPβ (and potentially AC5 in other compartments bound similarly to other AKAPs) increased cAMP concentration to a level detectable in whole cell lysates, inducing hypertrophy in the absence of any additional stimulation. Consistent with the role that excessive β-adrenergic signaling can play in the development of pathological cardiac hypertrophy [60], these results showed the importance of properly restricted AC activity in the cardiac myocyte.
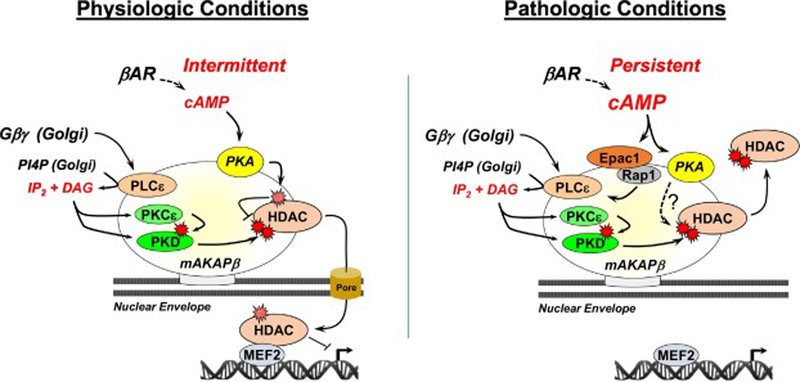
Both cAMP effectors at mAKAPα signalosomes, Epac 1 and PKA, are relevant to class IIa HDAC regulation, the former associated with HDAC nuclear export and the latter with HDAC nuclear import. In myocytes both Epac1 and Gβγ protein complexes can activate mAKAPβ-bound phospholipase Cε (PLCε, Figure 4) [29, 61]. In turn, PLCε bound to mAKAPβ hydrolyzes phosphatidylinositol 4-phosphate (PI4P) on adjacent Golgi apparatus, resulting in diacylglycerol-dependent activation of mAKAPβ-bound protein kinase Cε and PKD and phosphorylation and nuclear export of class IIa HDACs [62]. Evidence supporting the relevance of this model includes that the first Ras-association domain of PLCε binds directly the first spectrin repeat of mAKAPβ, such that disruption of that interaction will inhibit myocyte hypertrophy in rat neonatal cardiac myocytes [29]. In addition, this pathway has been confirmed in vivo by PLCε and mAKAPβ gene deletion in mice [18, 62]. Notably, myocyte-specific mAKAPβ gene deletion in mice inhibited HDAC4 and HDAC5 phosphorylation in response to pressure overload [18]. Moreover, both HDAC4 and HDAC5 bind mAKAPβ in striated myocytes [18, 50]. Disruption of HDAC5 binding to mAKAPβ blocked PKD-mediated phosphorylation in neonatal rat ventricular myocytes, similarly to decreased expression of mAKAPβ in these cells [50]. Furthermore, α-adrenergic receptor-induced myocyte hypertrophy was inhibited when HDAC binding to mAKAPβ was disrupted, confirming the importance of this local PI4P signaling module for class IIa HDAC nuclear export.
Figure 4. A model for bidirectional regulation of class IIa HDACc by mAKAPβ signalosomes.
Gβγ-activated PLCε at perinuclear mAKAPβ signalosomes will result in DAG and IP2 production from PI4P on adjacent Golgi apparatus [89]. DAG activates PKCε and PKD [62], inducing HDAC nuclear export promoting MEF2-dependent gene expression. Intermittent, acute βAR stimulation inhibits PKD phosphorylation and HDAC nuclear export by inducing PKA HDAC phosphorylation at Ser-279 [50]. Chronic, persistent βAR stimulation results in greater cAMP elevation and further activation of PLCε by Epac1 and the small g-protein Rap1 that drives with PKD-dependent HDAC nuclear export [29]. Chronic, persistent βAR stimulation promotes myocyte hypertrophy by additional PKA-dependent pathways [21], potentially involving HDACs.
Although chronic β-adrenergic stimulation of myocytes will induce HDAC5 nuclear export and myocyte hypertrophy, it has been recognized that acute β-adrenergic stimulation will oppose Gq-coupled receptor-dependent HDAC4/5 nuclear export (Figure 4, left panel) [48], presumably by separate mechanisms. PKA-dependent phosphorylation of HDAC4 Ser-266 and HDAC5 Ser-279 promotes nuclear import [45–49], such that a HDAC5 Ser-279 phosphomimetic mutation blocked nuclear export in response to α-adrenergic or endothelin-1 receptor stimulation of adult cardiac myocytes [46, 47]. Conversely, a Ser-279 phosphoablative mutation blocked HDAC5 nuclear import in response to acute β-adrenergic or forskolin stimulation [46, 49]. We have found that mAKAPβ is the primary scaffold mediating class IIa HDAC – PKA association in rat neonatal ventricular myocytes and that PKA binding to mAKAPβ was required for the inhibitory effects of acute β-adrenergic stimulation on class IIa HDAC nuclear export and PKD-dependent HDAC phosphorylation [50]. The inhibition of HDAC nuclear export by acute β-adrenergic signaling has been proposed as a mechanism to prevent the inappropriate induction of cardiac hypertrophy during a fight-or-flight sympathetic response [46]. In conjunction with the feedback regulation conferred by PKA-dependent phosphorylation of AC5 and PDE4D3, both Epac1- and Gβγ-dependent HDAC nuclear export pathway should be restricted under physiologic conditions, especially since Epac1 requires higher concentrations of cAMP for activation than PKA [63].
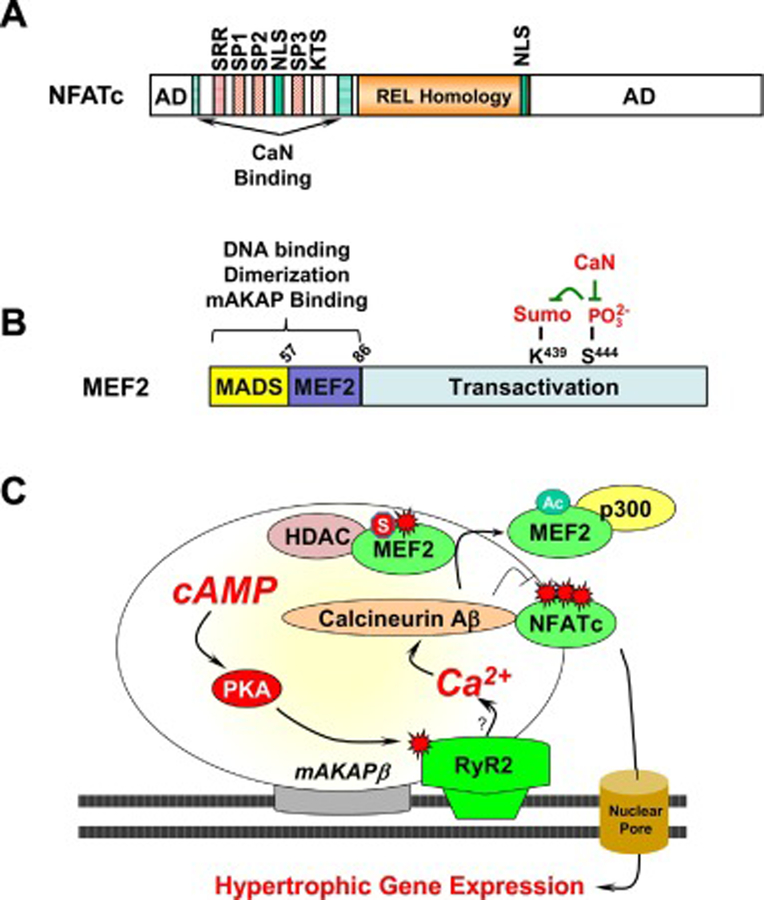
It is important to note that PKA bound to mAKAP can also be pro-hypertrophic. Loss of PKA binding to mAKAPβ as provided by RNAi-rescue in rat neonatal ventricular myocytes with a full-length mAKAPβ PKA-binding mutant showed that association of the kinase with the complex is required for the induction of myocyte hypertrophy in response to chronic α- or β- adrenergic signaling (Figure 4, right panel) [21]. Additional targets for mAKAPβ-bound PKA are likely relevant under conditions of persistent cAMP elevation. mAKAPβ-bound PKA can phosphorylate a small pool of perinuclear type II ryanodine receptors (RyR) also associated with mAKAPβ and nesprin-1α (Figure 5) [17, 19, 22]. Calcium released from perinuclear stores through this channel is likely responsible for local activation of the Ca2+/calmodulin phosphatase calcineurin (PP2B, CaN) that binds a discrete domain within mAKAPβ (aa 1286–1345 [64]) and that can in turn induce NFAT and MEF2-dependent gene expression associated with hypertrophy [64], as discussed in the following sections.
Figure 5. Regulation of calcineurin-dependent transcription factors by mAKAPβ signalosomes.
A. Conserved Domains in NFATc Transcription Factors. AD, transactivation domain; NLS, nuclear localization signal; SRR, SP1, SP2 SP3, and KTS are serine-rich domains that are de-phosphoyrlated by calcineurin. The REL Homology Domain binds DNA. B. MEF2 structure and post-translational modification. C. RyR2 at mAKAPβ signalosomes is phosphorylated by bound PKA [19], presumably enhancing perinuclear Ca2+ levels that can activate local calcineurin Aβ [64]. We propose that MEF2D dynamically associates with chromatin, such that transient association with mAKAPβ facilitates its regulation by the phosphatase. Calcineurin-catalyzed de-phosphorylation of MEF2 results in MEF2 desumoylation and acetylation and an exchange of HDAC and p300 binding [90]. mAKAPβ-bound calcineurin also dephosphorylates NFATc transcription factors promoting their nuclear localization and hypertrophic gene expression [64].
mAKAPβ and NFAT
The NFAT family of transcription factors, in particular NFATc2 and NFATc3, are key regulators of cardiac hypertrophy (Figure 5A) [65, 66]. They are present in the cytoplasm of resting cells due to multiple sites of phosphorylation, but translocate to the nucleus following dephosphorylation by calcineurin [67]. The mAKAP complex has been shown to regulate the activity of multiple members of the NFAT family. NFATc3 co-precipitates with mAKAPβ isolated from rat cardiac extracts, although it may not be a direct interaction [64]. Decreased expression of mAKAPβ in neonatal rat ventricular myocytes prevented the nuclear translocation of both NFATc1 and NFATc3 after adrenergic stimulation [21, 64]. This is suggested to be due to the associated calcineurin, as disruption of calcineurin binding to mAKAPβ prevented the dephosphorylation of NFATc3 and inhibited myocyte hypertrophy [64]. Accordingly, mAKAPβ is required for NFAT-activated gene transcription in vivo as well, as NFATc2 dephosphorylation and the expression of NFAT-dependent genes was significantly decreased in mice containing the mAKAPβ cardiac myocyte-specific knockout following pressure overload [18]. Therefore, mAKAPβ regulates multiple NFAT family members contributing to the gene transcription required for pathological remodeling. This suggests that mAKAPβ acts as a focal point to focus the upstream stimulators of pathological gene transcription and that targeting this complex will halt multiple transcriptional pathways involved in induction of disease.
mAKAPβ REGULATION OF MEF2
The myocyte enhancer factor 2 (MEF2) family of transcription factors (MEF2A, MEF2B, MEF2C and MEF2D) are important for both heart development and induction of cardiac remodeling, as well as skeletal myoblast differentiation [68–70]. For example, conditional MEF2D gene deletion attenuated pathological cardiac remodeling in response to pressure overload and chronic catecholamine infusion [71]. MEF2D is an effector for mAKAPβ signalosomes, and MEF2D-mAKAPβ complexes can be isolated from both adult heart and the skeletal myoblast cell line C2C12 [31]. Biochemical studies have revealed that the conserved MADS domain of MEF2A and MEF2D can both bind an N-terminal domain of mAKAPβ (Figure 5B) [31]. Importantly, disruption of MEF2D-mAKAPβ binding in C2C12 cells blunted the differentiation- induced increase in MEF2 transcription. This correlated with the inhibited differentiation of skeletal myoblasts into mature myocytes (myotubes) and the decreased expression of the endogenous MEF2 target genes MF20 and myogenin both in C2C12 cells and in vivo in mice lacking mAKAPβ expression [31, 32]. These findings are in parallel to results obtained for the mAKAPβ cardiac myocyte-specific conditional knockout mouse subjected to pressure overload, where MEF2-dependent gene expression was significantly down-regulated [18]. Hence, the interaction MEF2 with the mAKAPβ scaffold is critical for stimulation of MEF2 transcriptional activities.
An important inducer of MEF2-dependent gene expression during myogenic differentiation is calcineurin (Figure 5C) [72]. In skeletal muscle, inhibition of phosphatase activity during differentiation prevented MEF2-dependent transcription and myoblast differentiation, while expression of a constitutively active calcineurin mutant protein in C2C12 cells induced differentiation and MEF2 transcriptional activity in the absence of differentiation signals [73]. We propose that MEF2D moving in and out of the nucleus in striated myocytes transiently docks at mAKAPβ signalosomes where MEF2D post-translational modification is controlled by mAKAPβ-bound calcineurin. Similar to the inhibition of NFAT nuclear import, disruption of calcineurin binding to mAKAPβ via competing peptides blocked both MEF2- dependent gene transcription and differentiation of C2C12 cells [73].
An elegant model has been proposed for the activation of MEF2 in which calcineurin- catalyzed dephosphorylation of MEF2A Ser-408 and MEF2D Ser-444 promotes the desumoylation and acetylation of Lys-403 and Lys-439, respectively (Figure 5C) [74–76]. We recently demonstrated that in cardiac myocytes, the regulation of MEF2D post-translational modifications by calcineurin requires mAKAPβ signalosome formation [77]. MEF2D phosphorylation and sumoylation resulted in increased association with HDAC5, promoting transcriptional repression, while dephosphorylation of Ser-444 resulted in Lys-439 acetylation and increased binding of the co-activator p300 histone acetylase, correlating with increased MEF2D-dependent gene transcription [77]. Importantly, disruption of calcineurin binding or lack of mAKAPβ expression blocked the calcineurin-mediated MEF2D dephosphorylation, preventing the sumoylation to acetylation switch and halting the differentiation process in C2C12 cells [77]. Taken together, these results highlight the importance of mAKAPβ signalosomes for orchestrating MEF2D-dependent activating and repressive chromatin complexes in cardiac and skeletal muscle myocytes. Furthermore, these mechanistic insights suggest that modulating calcineurin or MEF2D binding to mAKAPβ may be beneficial for preventing the increase in MEF2D-dependent gene transcription required for pathological remodeling.
HIF-1-α and mAKAPβ
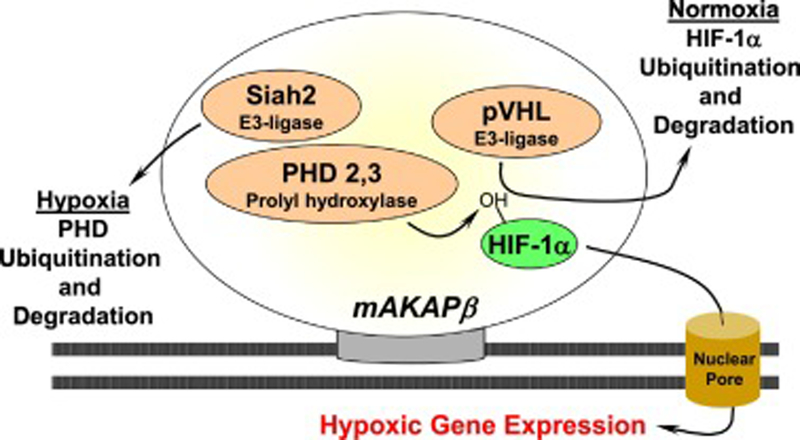
The central role for mAKAPβ in hypertrophic gene transcription has raised the question whether mAKAPβ can regulate additional transcription factors involved in other forms of heart disease. Biochemical studies have revealed that mAKAPβ binds the transcription factor hypoxia-inducible factor 1-alpha (HIF-1α) and related regulatory proteins in cardiac myocytes (Figure 6) [78]. This basic helix-loop PAS domain containing protein is a critical regulator of the cellular response to hypoxia [79]. In normoxic conditions, HIF-1-α concentration is low due to ubiquitin-mediated proteosomal degradation resulting from HIF-α hydroxylation by a family of oxygen-sensitive dioxygenases called prolyl hydroxylases (PHDs) [80]. However, under hypoxic conditions, degradation is decreased due to deactivation of PHDs and their degradation by Siah2-mediated ubiquitination and proteosomal degradation. As a result, HIF-1-α moves to the nucleus, dimerizes with HIF-1β, and stimulates gene transcription of proangiogenic, metabolic and antiapoptotic genes that promote cell survival during hypoxia. Besides HIF-1α, mAKAPβ binds von Hippel–Lindau protein (pVHL), PHD2/3 and Siah2, thereby regulating HIF-1α levels under both normoxic and hypoxic conditions in myocytes [78]. For example, cellular depletion of mAKAPβ, disrupting the nuclear location of mAKAPβ, and preventing HIF-1α binding to mAKAPβ all thwarted the stabilization of the transcription factor and associated hypoxia-stimulated gene expression in neonatal rat ventricular myocytes [78]. Whether HIF-1α regulation by the mAKAPβ signalosomes provides a protective mechanism for myocyte survival in ischemic heart disease has not yet been studied in vivo, making compelling future work investigating the role of the mAKAPβ in ischemia-reperfusion injury.
Figure 6. Regulation of HIF-1α by mAKAPβ signalosomes.
The association of E3-ubuiquitin ligase and HIF-1α with mAKAPβ confers bidirectional regulation of HIF-1α [78].
A role for mAKAPβ in intranuclear PKA regulation?
Most of the research concerning mAKAPβ has been premised upon the idea that regulation of mAKAPβ effector proteins occurs within a discrete perinuclear compartment. It is possible however, that mAKAPβ-associated PKA might have effects distal from the scaffold. Work performed in adult rat ventricular myocytes found that a nuclear pool of cAMP was activated by both β1- and β2-adrenergic receptors, while only the former receptor increased nucleoplasmic PKA activity and the expression of the gene for pro-apoptotic factor inducible cAMP early repressor (ICER)[20]. This finding correlates with the identification of β1, but not β2 receptors on the myocyte nucleus [81, 82]. Notably, depletion of mAKAPβ using shRNA induced a partial decrease in β1-stimulated nucleoplasmic (but not cytosolic) PKA activity, as well as inhibiting β2-adrenergic receptor-induced nucleoplasmic PKA activity that could be elicited by PDE4 inhibition [20]. This suggests that mAKAPβ may plays a role in the β1-dependent regulation of intranuclear PKA that regulates gene expression, although whether mAKAPβ regulates ICER expression and its function in apoptosis has not been investigated.
Perspective
It is well-established in the basic cardiovascular sciences literature that induction of pathological cardiac remodeling requires an altered myocyte gene expression program. How chromatin-associated factors such as MEF2, NFAT, HIF-1α, and class IIa HDACs are individually regulated has been well-studied, but much remains to be understood regarding their coordinated regulation by upstream second messenger systems. The discovery of mAKAPβ signalosomes has provided a unique insight into the organization of the hypertrophic signaling network that regulates pathological myocyte gene expression. Extensive biochemical and physiological experiments have characterized the interactions between mAKAPβ and gene regulatory factors and provided molecular details regarding the orchestration of their control by the scaffold protein. However, many of these pathways have been studied in isolation, and the interactions between pathways, and composition of the mAKAPβ complex under physiological and pathological conditions, has yet to be investigated. An in-depth proteomic study of mAKAPβ complexes may provide a better understanding of how to target it’s signaling pathways.
The pathophysiological relevance of mAKAPβ signalosomes has been validated by research using genetically modified mice and primary cardiac myocyte cultures. Notably, cardiac myocyte-specific mAKAPβ gene deletion inhibited pressure overload-induced cardiac hypertrophy, including an attenuation in associated MEF2 and NFAT-dependent gene expression and the development of heart failure [18]. To our knowledge, mAKAPβ is the only AKAP whose ablation confers a survival benefit in the fact of pathological stress. These findings suggest that targeting mAKAPβ signalosomes would provide a novel therapeutic approach for the prevention and/or treatment of cardiovascular diseases that exhibit pathological cardiac remodeling and progress to heart failure.
In part due to the pleiotropy of many candidate drug targets, with rare exception the pursuit of new heart failure drugs has been remarkably unfruitful. A notable example of an elusive heart failure drug target is calcineurin, whose inhibition results in immunosuppression and worse outcome (at least in mice) subjected to ischemia-reperfusion injury [83]. Targeting the scaffold protein instead of the signaling enzyme may provide an opportunity to inhibit cellular signaling with greater specificity. Strategies to target mAKAPβ signalosomes might include the use of mAKAP siRNA or shRNA to facilitate RNA interference of mAKAPβ expression and the expression of anchoring disruptor peptides to displace key regulatory molecules from mAKAPβ signalosomes, as utilized in past basic science studies [21, 50]. Although mAKAPβ may contribute to physiological hypertrophy and the prevention of inappropriate remodeling [18, 50], lack of mAKAPβ expression seems to be well tolerated, as no deleterious effect of mAKAPβ gene targeting was observed in mice [18]. The use of anchoring disruptor peptides may be more complicated, as an anchoring disruptor peptide might have effects beyond mAKAPβ signalosomes. This may be alleviated in part by the use of gene therapy vectors to express peptides specifically in the cardiac myocyte, as opposed to the systemic delivery of synthetic peptides, stapled peptides, or even small molecule inhibitors, which could incur significant side effects due to actions at other organ systems. Given that the premise for targeting mAKAPβ signalosomes is well supported by a deep understanding of mAKAPβ-related mechanisms, translational research to determine whether mAKAPβ signalosomes can be safely and effectively targeted in cardiovascular disease is now highly compelling.
HIGHLIGHTS.
Muscle A-kinase anchoring protein β (mAKAPβ) is a well-characterized scaffold protein localized to the nuclear envelope in striated muscle
Expression of mAKAPβ contributes to the induction of pathological hypertrophy
mAKAPβ orchestrates the regulation of gene transcription required for induction of cardiac hypertrophy
mAKAPβ is responsible for the regulation of class IIa HDAC enzymes as well as cardiac transcription factors such as NFAT, MEF2, and HIF-1α
Acknowledgments:
This work was funded, in whole or in part, by National Institutes of Health Grants HL126825 and HL146111 (K.D.K. and M.S.K.) and HL126950 and EY026766 (M.S.K), funds provided by The Regents of the University of California, Research Grants Program Office, Tobacco-Related Diseases Research Program, Grant Number No. 27IR-0045 (M.S.K.), and by an American Heart Association Predoctoral Grant 18PRE34030209 to MG. The opinions, findings, and conclusions herein are those of the authors and not necessarily represent those The Regents of the University of California, The National Institutes of Health, or any of their programs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: Dr. Kapiloff owns equity in Anchored RSK3 Inhibitors, LLC, and Cardiac RSK3 Inhibitors, LLC, companies interested in developing mAKAPβ-based therapies.
References
- 1.Burchfield JS, Xie M, and Hill JA, Pathological ventricular remodeling: mechanisms: part 1 of 2. Circulation, 2013. 128(4): p. 388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin EJ, et al. , Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation, 2018. 137(12): p. e67–e492. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, et al. , Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation, 2019. 139(10): p. e56–e528. [DOI] [PubMed] [Google Scholar]
- 4.van Berlo JH, Maillet M, and Molkentin JD, Signaling effectors underlying pathologic growth and remodeling of the heart. J Clin Invest, 2013. 123(1): p. 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKinsey TA, Vondriska TM, and Wang Y, Epigenomic regulation of heart failure: integrating histone marks, long noncoding RNAs, and chromatin architecture. F1000Res, 2018. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heineke J and Molkentin JD, Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol, 2006. 7(8): p. 589–600. [DOI] [PubMed] [Google Scholar]
- 7.Scott JD and Pawson T, Cell signaling in space and time: where proteins come together and when they’re apart. Science, 2009. 326(5957): p. 1220–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott JD, Dessauer CW, and Tasken K, Creating order from chaos: cellular regulation by kinase anchoring. Annu Rev Pharmacol Toxicol, 2013. 53: p. 187–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diviani D, et al. , A-kinase anchoring proteins: scaffolding proteins in the heart. Am J Physiol Heart Circ Physiol, 2011. 301(5): p. H1742–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fink MA, et al. , AKAP-mediated targeting of protein kinase a regulates contractility in cardiac myocytes. Circ Res, 2001. 88(3): p. 291–7. [DOI] [PubMed] [Google Scholar]
- 11.Marx SO, et al. , Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science, 2002. 295(5554): p. 496–9. [DOI] [PubMed] [Google Scholar]
- 12.Lygren B, et al. , AKAP complex regulates Ca2+ re-uptake into heart sarcoplasmic reticulum. EMBO Rep, 2007. 8(11): p. 1061–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardner LA, et al. , AKAP79-mediated targeting of the cyclic AMP-dependent protein kinase to the beta1-adrenergic receptor promotes recycling and functional resensitization of the receptor. J Biol Chem, 2006. 281(44): p. 33537–53. [DOI] [PubMed] [Google Scholar]
- 14.Kapiloff MS, et al. , mAKAP: an A-kinase anchoring protein targeted to the nuclear membrane of differentiated myocytes. J Cell Sci, 1999. 112 ( Pt 16): p. 2725–36. [DOI] [PubMed] [Google Scholar]
- 15.Michel JJ, et al. , Spatial restriction of PDK1 activation cascades by anchoring to mAKAPalpha. Mol Cell, 2005. 20(5): p. 661–72. [DOI] [PubMed] [Google Scholar]
- 16.Mccartney S, et al. , Cloning and Characterization of a-Kinase Anchor Protein-100 (Akap100) - a Protein That Targets a-Kinase to the Sarcoplasmic-Reticulum. Journal of Biological Chemistry, 1995. 270(16): p. 9327–9333. [DOI] [PubMed] [Google Scholar]
- 17.Marx SO, et al. , PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell, 2000. 101(4): p. 365–76. [DOI] [PubMed] [Google Scholar]
- 18.Kritzer MD, et al. , The scaffold protein muscle A-kinase anchoring protein beta orchestrates cardiac myocyte hypertrophic signaling required for the development of heart failure. Circ Heart Fail, 2014. 7(4): p. 663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kapiloff MS, Jackson N, and Airhart N, mAKAP and the ryanodine receptor are part of a multi-component signaling complex on the cardiomyocyte nuclear envelope. J Cell Sci, 2001. 114(Pt 17): p. 3167–76. [DOI] [PubMed] [Google Scholar]
- 20.Bedioune I, et al. , PDE4 and mAKAPbeta are nodal organizers of beta2-ARs nuclear PKA signalling in cardiac myocytes. Cardiovasc Res, 2018. 114(11): p. 1499–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pare GC, et al. , The mAKAP complex participates in the induction of cardiac myocyte hypertrophy by adrenergic receptor signaling. J Cell Sci, 2005. 118(Pt 23): p. 5637–46. [DOI] [PubMed] [Google Scholar]
- 22.Pare GC, et al. , Nesprin-1alpha contributes to the targeting of mAKAP to the cardiac myocyte nuclear envelope. Exp Cell Res, 2005. 303(2): p. 388–99. [DOI] [PubMed] [Google Scholar]
- 23.Zhou C, et al. , Nesprin-½: roles in nuclear envelope organisation, myogenesis and muscle disease. Biochem Soc Trans, 2018. 46(2): p. 311–320. [DOI] [PubMed] [Google Scholar]
- 24.Sosa BA, et al. , LINC complexes form by binding of three KASH peptides to domain interfaces of trimeric SUN proteins. Cell, 2012. 149(5): p. 1035–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou C, et al. , Novel nesprin-1 mutations associated with dilated cardiomyopathy cause nuclear envelope disruption and defects in myogenesis. Hum Mol Genet, 2017. 26(12): p. 2258–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haque F, et al. , Mammalian SUN protein interaction networks at the inner nuclear membrane and their role in laminopathy disease processes. J Biol Chem, 2010. 285(5): p. 3487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris GE and Randles KN, Nesprin isoforms: are they inside or outside the nucleus? Biochem Soc Trans, 2010. 38(Pt 1): p. 278–80. [DOI] [PubMed] [Google Scholar]
- 28.Dodge-Kafka KL, et al. , The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature, 2005. 437(7058): p. 574–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L, et al. , Phospholipase C epsilon scaffolds to muscle-specific A kinase anchoring protein (mAKAPbeta) and integrates multiple hypertrophic stimuli in cardiac myocytes. J Biol Chem, 2011. 286(26): p. 23012–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo H, et al. , The role of mAKAPbeta in the process of cardiomyocyte hypertrophy induced by angiotensin II. Int J Mol Med, 2015. 35(5): p. 1159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vargas MA, et al. , Myocyte enhancer factor 2 (MEF2) tethering to muscle selective A-kinase anchoring protein (mAKAP) is necessary for myogenic differentiation. Cell Signal, 2012. 24(8): p. 1496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee SW, et al. , AKAP6 inhibition impairs myoblast differentiation and muscle regeneration: Positive loop between AKAP6 and myogenin. Sci Rep, 2015. 5: p. 16523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, et al. , Muscle A-Kinase Anchoring Protein-alpha is an Injury-Specific Signaling Scaffold Required for Neurotrophic- and Cyclic Adenosine Monophosphate-Mediated Survival. EBioMedicine, 2015. 2(12): p. 1880–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie M and Hill JA, HDAC-dependent ventricular remodeling. Trends Cardiovasc Med, 2013. 23(6): p. 229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright LH and Menick DR, A class of their own: exploring the nondeacetylase roles of class IIa HDACs in cardiovascular disease. Am J Physiol Heart Circ Physiol, 2016. 311(1): p. H199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang CL, et al. , Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell, 2002. 110(4): p. 479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang S, et al. , Histone deacetylases 5 and 9 govern responsiveness of the heart to a subset of stress signals and play redundant roles in heart development. Mol Cell Biol, 2004. 24(19): p. 8467–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKinsey TA, et al. , Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature, 2000. 408(6808): p. 106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vega RB, et al. , Protein kinases C and D mediate agonist-dependent cardiac hypertrophy through nuclear export of histone deacetylase 5. Mol Cell Biol, 2004. 24(19): p. 8374–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carnegie GK, et al. , AKAP-Lbc mobilizes a cardiac hypertrophy signaling pathway. Mol Cell, 2008. 32(2): p. 169–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McKinsey TA, Zhang CL, and Olson EN, Activation of the myocyte enhancer factor-2 transcription factor by calcium/calmodulin-dependent protein kinase-stimulated binding of 14–3-3 to histone deacetylase 5. Proc Natl Acad Sci U S A, 2000. 97(26): p. 14400–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grozinger CM and Schreiber SL, Regulation of histone deacetylase 4 and 5 and transcriptional activity by 14–3-3-dependent cellular localization. Proc Natl Acad Sci U S A, 2000. 97(14): p. 7835–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang AH, et al. , Regulation of histone deacetylase 4 by binding of 14–3-3 proteins. Mol Cell Biol, 2000. 20(18): p. 6904–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berdeaux R, et al. , SIK1 is a class II HDAC kinase that promotes survival of skeletal myocytes. Nat Med, 2007. 13(5): p. 597–603. [DOI] [PubMed] [Google Scholar]
- 45.Liu Y and Schneider MF, Opposing HDAC4 nuclear fluxes due to phosphorylation by beta-adrenergic activated protein kinase A or by activity or Epac activated CaMKII in skeletal muscle fibres. J Physiol, 2013. 591(14): p. 3605–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang CW, et al. , Acute beta-adrenergic activation triggers nuclear import of histone deacetylase 5 and delays G(q)-induced transcriptional activation. J Biol Chem, 2013. 288(1): p. 192–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ha CH, et al. , PKA phosphorylates histone deacetylase 5 and prevents its nuclear export, leading to the inhibition of gene transcription and cardiomyocyte hypertrophy. Proc Natl Acad Sci U S A, 2010. 107(35): p. 15467–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sucharov CC, et al. , beta-Adrenergic receptor stimulation and activation of protein kinase A protect against alpha1-adrenergic-mediated phosphorylation of protein kinase D and histone deacetylase 5. J Card Fail, 2011. 17(7): p. 592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weeks KL, et al. , beta-Adrenergic Stimulation Induces Histone Deacetylase 5 (HDAC5) Nuclear Accumulation in Cardiomyocytes by B55alpha-PP2A-Mediated Dephosphorylation. J Am Heart Assoc, 2017. 6(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dodge-Kafka KL, et al. , Bidirectional regulation of HDAC5 by mAKAPbeta signalosomes in cardiac myocytes. J Mol Cell Cardiol, 2018. 118: p. 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dodge KL, et al. , mAKAP assembles a protein kinase A/PDE4 phosphodiesterase cAMP signaling module. EMBO J, 2001. 20(8): p. 1921–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kapiloff MS, et al. , An adenylyl cyclase-mAKAPbeta signaling complex regulates cAMP levels in cardiac myocytes. J Biol Chem, 2009. 284(35): p. 23540–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carlisle Michel JJ, et al. , PKA-phosphorylation of PDE4D3 facilitates recruitment of the mAKAP signalling complex. Biochem J, 2004. 381(Pt 3): p. 587–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sette C and Conti M, Phosphorylation and activation of a cAMP-specific phosphodiesterase by the cAMP-dependent protein kinase. Involvement of serine 54 in the enzyme activation. J Biol Chem, 1996. 271(28): p. 16526–34. [DOI] [PubMed] [Google Scholar]
- 55.Dodge-Kafka KL, et al. , cAMP-stimulated protein phosphatase 2A activity associated with muscle A kinase-anchoring protein (mAKAP) signaling complexes inhibits the phosphorylation and activity of the cAMP-specific phosphodiesterase PDE4D3. J Biol Chem, 2010. 285(15): p. 11078–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suryavanshi SV, et al. , Human muscle-specific A-kinase anchoring protein polymorphisms modulate the susceptibility to cardiovascular diseases by altering cAMP/PKA signaling. Am J Physiol Heart Circ Physiol, 2018. 315(1): p. H109–H121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rababa’h A, et al. , Protein kinase A and phosphodiesterase-4D3 binding to coding polymorphisms of cardiac muscle anchoring protein (mAKAP). J Mol Biol, 2013. 425(18): p. 3277–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Horikoshi M, et al. , Discovery and Fine-Mapping of Glycaemic and Obesity-Related Trait Loci Using High-Density Imputation. PLoS Genet, 2015. 11(7): p. e1005230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dessauer CW, Adenylyl cyclase--A-kinase anchoring protein complexes: the next dimension in cAMP signaling. Mol Pharmacol, 2009. 76(5): p. 935–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bristow MR, Treatment of chronic heart failure with beta-adrenergic receptor antagonists: a convergence of receptor pharmacology and clinical cardiology. Circ Res, 2011. 109(10): p. 1176–94. [DOI] [PubMed] [Google Scholar]
- 61.Malik S, et al. , G protein betagamma subunits regulate cardiomyocyte hypertrophy through a perinuclear Golgi phosphatidylinositol 4-phosphate hydrolysis pathway. Mol Biol Cell, 2015. 26(6): p. 1188–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang L, et al. , Phospholipase Cepsilon hydrolyzes perinuclear phosphatidylinositol 4-phosphate to regulate cardiac hypertrophy. Cell, 2013. 153(1): p. 216–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Enserink JM, et al. , A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol, 2002. 4(11): p. 901–6. [DOI] [PubMed] [Google Scholar]
- 64.Li J, et al. , The mAKAPbeta scaffold regulates cardiac myocyte hypertrophy via recruitment of activated calcineurin. J Mol Cell Cardiol, 2010. 48(2): p. 387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilkins BJ, et al. , Targeted disruption of NFATc3, but not NFATc4, reveals an intrinsic defect in calcineurin-mediated cardiac hypertrophic growth. Mol Cell Biol, 2002. 22(21): p. 7603–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bourajjaj M, et al. , NFATc2 is a necessary mediator of calcineurin-dependent cardiac hypertrophy and heart failure. J Biol Chem, 2008. 283(32): p. 22295–303. [DOI] [PubMed] [Google Scholar]
- 67.Wilkins BJ and Molkentin JD, Calcium-calcineurin signaling in the regulation of cardiac hypertrophy. Biochem Biophys Res Commun, 2004. 322(4): p. 1178–91. [DOI] [PubMed] [Google Scholar]
- 68.Black BL and Olson EN, Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu Rev Cell Dev Biol, 1998. 14: p. 167–96. [DOI] [PubMed] [Google Scholar]
- 69.Naya FJ, et al. , Transcriptional activity of MEF2 during mouse embryogenesis monitored with a MEF2-dependent transgene. Development, 1999. 126(10): p. 2045–52. [DOI] [PubMed] [Google Scholar]
- 70.Potthoff MJ and Olson EN, MEF2: a central regulator of diverse developmental programs. Development, 2007. 134(23): p. 4131–40. [DOI] [PubMed] [Google Scholar]
- 71.Kim Y, et al. , The MEF2D transcription factor mediates stress-dependent cardiac remodeling in mice. J Clin Invest, 2008. 118(1): p. 124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu H, et al. , Activation of MEF2 by muscle activity is mediated through a calcineurin-dependent pathway. EMBO J, 2001. 20(22): p. 6414–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li J, et al. , Regulation of MEF2 transcriptional activity by calcineurin/mAKAP complexes. Exp Cell Res, 2013. 319(4): p. 447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shalizi A, et al. , A calcium-regulated MEF2 sumoylation switch controls postsynaptic differentiation. Science, 2006. 311(5763): p. 1012–7. [DOI] [PubMed] [Google Scholar]
- 75.Flavell SW, et al. , Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science, 2006. 311(5763): p. 1008–12. [DOI] [PubMed] [Google Scholar]
- 76.Gregoire S, et al. , Control of MEF2 transcriptional activity by coordinated phosphorylation and sumoylation. J Biol Chem, 2006. 281(7): p. 4423–33. [DOI] [PubMed] [Google Scholar]
- 77.Li J, et al. , Muscle A-kinase-anchoring protein-beta-bound calcineurin toggles active and repressive transcriptional complexes of myocyte enhancer factor 2D. J Biol Chem, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wong W, et al. , mAKAP compartmentalizes oxygen-dependent control of HIF-1alpha. Sci Signal, 2008. 1(51): p. ra18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang GL, et al. , Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A, 1995. 92(12): p. 5510–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ohh M, et al. , Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol, 2000. 2(7): p. 423–7. [DOI] [PubMed] [Google Scholar]
- 81.Boivin B, et al. , Functional beta-adrenergic receptor signalling on nuclear membranes in adult rat and mouse ventricular cardiomyocytes. Cardiovasc Res, 2006. 71(1): p. 69–78. [DOI] [PubMed] [Google Scholar]
- 82.Buu NT, Hui R, and Falardeau P, Norepinephrine in neonatal rat ventricular myocytes: association with the cell nucleus and binding to nuclear alpha 1- and beta-adrenergic receptors. J Mol Cell Cardiol, 1993. 25(9): p. 1037–46. [DOI] [PubMed] [Google Scholar]
- 83.Bueno OF, et al. , Calcineurin Abeta gene targeting predisposes the myocardium to acute ischemia-induced apoptosis and dysfunction. Circ Res, 2004. 94(1): p. 91–9. [DOI] [PubMed] [Google Scholar]
- 84.Marx SO, et al. , Phosphorylation-dependent regulation of ryanodine receptors: a novel role for leucine/isoleucine zippers. J Cell Biol, 2001. 153(4): p. 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Passariello CL, et al. , p90 ribosomal S6 kinase 3 contributes to cardiac insufficiency in alpha-tropomyosin Glu180Gly transgenic mice. Am J Physiol Heart Circ Physiol, 2013. 305(7): p. H1010–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rusnak F and Mertz P, Calcineurin: form and function. Physiol Rev, 2000. 80(4): p. 1483–521. [DOI] [PubMed] [Google Scholar]
- 87.Martin M, Kettmann R, and Dequiedt F, Class IIa histone deacetylases: regulating the regulators. Oncogene, 2007. 26(37): p. 5450–67. [DOI] [PubMed] [Google Scholar]
- 88.Iwami G, et al. , Regulation of adenylyl cyclase by protein kinase A. J Biol Chem, 1995. 270(21): p. 12481–4. [DOI] [PubMed] [Google Scholar]
- 89.Madukwe JC, et al. , G protein betagamma subunits directly interact with and activate phospholipase C. J Biol Chem, 2018. 293(17): p. 6387–6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li J, et al. , Muscle A-kinase-anchoring protein-beta-bound calcineurin toggles active and repressive transcriptional complexes of myocyte enhancer factor 2D. J Biol Chem, 2019. 294(7): p. 2543–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]