Abstract
A checklist of world species of Microgastrinae parasitoid wasps (Hymenoptera: Braconidae) is provided. A total of 81 genera and 2,999 extant species are recognized as valid, including 36 nominal species that are currently considered as species inquirendae. Two genera are synonymized under Apanteles. Nine lectotypes are designated. A total of 318 new combinations, three new replacement names, three species name amendments, and seven species status revised are proposed. Additionally, three species names are treated as nomina dubia, and 52 species names are considered as unavailable names (including 14 as nomina nuda). A total of three extinct genera and 12 extinct species are also listed. Unlike in many previous treatments of the subfamily, tribal concepts are judged to be inadequate, so genera are listed alphabetically. Brief diagnoses of all Microgastrinae genera, as understood in this paper, are presented. Illustrations of all extant genera (at least one species per genus, usually more) are included to showcase morphological diversity. Primary types of Microgastrinae are deposited in 108 institutions worldwide, although 76% are concentrated in 17 collections. Localities of primary types, in 138 countries, are reported. Recorded species distributions are listed by biogeographical region and by country. Microgastrine wasps are recorded from all continents except Antarctica; specimens can be found in all major terrestrial ecosystems, from 82°N to 55°S, and from sea level up to at least 4,500 m a.s.l. The Oriental (46) and Neotropical (43) regions have the largest number of genera recorded, whereas the Palaearctic region (28) is the least diverse. Currently, the highest species richness is in the Palearctic region (827), due to more historical study there, followed by the Neotropical (768) and Oriental (752) regions, which are expected to be the most species rich. Based on ratios of Lepidoptera and Microgastrinae species from several areas, the actual world diversity of Microgastrinae is expected to be between 30,000–50,000 species; although these ratios were mostly based on data from temperate areas and thus must be treated with caution, the single tropical area included had a similar ratio to the temperate ones. Almost 45,000 specimens of Microgastrinae from 67 different genera (83% of microgastrine genera) have complete or partial DNA barcode sequences deposited in the Barcode of Life Data System; the DNA barcodes represent 3,545 putative species or Barcode Index Numbers (BINs), as estimated from the molecular data. Information on the number of sequences and BINs per genus are detailed in the checklist. Microgastrinae hosts are here considered to be restricted to Eulepidoptera, i.e., most of the Lepidoptera except for the four most basal superfamilies (Micropterigoidea, Eriocranioidea, Hepialoidea and Nepticuloidea), with all previous literature records of other insect orders and those primitive Lepidoptera lineages being considered incorrect. The following nomenclatural acts are proposed: 1) Two genera are synonymyzed under Apanteles: Cecidobracon Kieffer & Jörgensen, 1910, new synonym and Holcapanteles Cameron, 1905, new synonym; 2) Nine lectotype designations are made for Alphomelondisputabile (Ashmead, 1900), Alphomelonnigriceps (Ashmead, 1900), Cotesiasalebrosa (Marshall, 1885), Diolcogasterxanthaspis (Ashmead, 1900), Dolichogenideaononidis (Marshall, 1889), Glyptapantelesacraeae (Wilkinson, 1932), Glyptapantelesguyanensis (Cameron, 1911), Glyptapantelesmilitaris (Walsh, 1861), and Pseudapantelesannulicornis Ashmead, 1900; 3) Three new replacement names are a) Diolcogasteraurangabadensis Fernandez-Triana, replacing Diolcogasterindicus (Rao & Chalikwar, 1970) [nec Diolcogasterindicus (Wilkinson, 1927)], b) Dolichogenideaincystatae Fernandez-Triana, replacing Dolichogenidealobesia Liu & Chen, 2019 [nec Dolichogenidealobesia Fagan-Jeffries & Austin, 2019], and c) Microplitisvitobiasi Fernandez-Triana, replacing Microplitisvariicolor Tobias, 1964 [nec Microplitisvaricolor Viereck, 1917]; 4) Three names amended are Apantelesirenecarrilloae Fernandez-Triana, 2014, Cotesiaayerzai (Brèthes, 1920), and Cotesiariverai (Porter, 1916); 5) Seven species have their status revised: Cotesiaarctica (Thomson, 1895), Cotesiaokamotoi (Watanabe, 1921), Cotesiaukrainica (Tobias, 1986), Dolichogenideaappellator (Telenga, 1949), Dolichogenideamurinanae (Capek & Zwölfer, 1957), Hypomicrogasteracarnas Nixon, 1965, and Nyererianigricoxis (Wilkinson, 1932); 6) New combinations are given for 318 species: Alloplitiscongensis, Alloplitisdetractus, Apantelesasphondyliae, Apantelesbraziliensis, Apantelessulciscutis, Choerasaper, Choerasapollion, Choerasdaphne, Choerasfomes, Choerasgerontius, Choerashelle, Choerasirates, Choeraslibanius, Choeraslongiterebrus, Choerasloretta, Choerasrecusans, Choerassordidus, Choerasstenoterga, Choerassuperbus, Choerassylleptae, Choerasvacillatrix, Choerasvacillatropsis, Choerasvenilia, Cotesiaasavari, Cotesiabactriana, Cotesiabambeytripla, Cotesiaberberidis, Cotesiabhairavi, Cotesiabiezankoi, Cotesiabifida, Cotesiacaligophagus, Cotesiacheesmanae, Cotesiacompressithorax, Cotesiadelphinensis, Cotesiaeffrena, Cotesiaeuphobetri, Cotesiaelaeodes, Cotesiaendii, Cotesiaeuthaliae, Cotesiaexelastisae, Cotesiahiberniae, Cotesiahyperion, Cotesiahypopygialis, Cotesiahypsipylae, Cotesiajujubae, Cotesialesbiae, Cotesialevigaster, Cotesializeri, Cotesiamalevola, Cotesiamalshri, Cotesiamenezesi, Cotesiamuzaffarensis, Cotesianeptisis, Cotesianycteus, Cotesiaoeceticola, Cotesiaoppidicola, Cotesiaopsiphanis, Cotesiapachkuriae, Cotesiapaludicolae, Cotesiaparbhanii, Cotesiaparvicornis, Cotesiapratapae, Cotesiaprozorovi, Cotesiapterophoriphagus, Cotesiaradiarytensis, Cotesiarangii, Cotesiariverai, Cotesiaruficoxis, Cotesiasenegalensis, Cotesiaseyali, Cotesiasphenarchi, Cotesiasphingivora, Cotesiatransuta, Cotesiaturkestanica, Diolcogasterabengouroui, Diolcogasteragama, Diolcogasterambositrensis, Diolcogasteranandra, Diolcogasterannulata, Diolcogasterbambeyi, Diolcogasterbicolorina, Diolcogastercariniger, Diolcogastercincticornis, Diolcogastercingulata, Diolcogastercoronata, Diolcogastercoxalis, Diolcogasterdipika, Diolcogasterearina, Diolcogasterepectina, Diolcogasterepectinopsis, Diolcogastergrangeri, Diolcogasterheterocera, Diolcogasterhomocera, Diolcogasterindica, Diolcogasterinsularis, Diolcogasterkivuana, Diolcogastermediosulcata, Diolcogastermegaulax, Diolcogasterneglecta, Diolcogasternigromacula, Diolcogasterpalpicolor, Diolcogasterpersimilis, Diolcogasterplecopterae, Diolcogasterplutocongoensis, Diolcogasterpsilocnema, Diolcogasterrufithorax, Diolcogastersemirufa, Diolcogasterseyrigi, Diolcogastersubtorquata, Diolcogastersulcata, Diolcogastertorquatiger, Diolcogastertristiculus, Diolcogasterturneri, Diolcogastervulcana, Diolcogasterwittei, Distatrixanthedon, Distatrixcerales, Distatrixcuspidalis, Distatrixeuproctidis, Distatrixflava, Distatrixgeometrivora, Distatrixmaia, Distatrixtookei, Distatrixtermina, Distatrixsimulissima, Dolichogenideaagamedes, Dolichogenideaaluella, Dolichogenideaargiope, Dolichogenideaatreus, Dolichogenideabakeri, Dolichogenideabasiflava, Dolichogenideabersa, Dolichogenideabiplagae, Dolichogenideabisulcata, Dolichogenideacatonix, Dolichogenideachrysis, Dolichogenideacoffea, Dolichogenideacoretas, Dolichogenideacyane, Dolichogenideadiaphantus, Dolichogenideadiparopsidis, Dolichogenideadryas, Dolichogenideaearterus, Dolichogenideaensiger, Dolichogenideaeros, Dolichogenideaevadne, Dolichogenideafalcator, Dolichogenideagelechiidivoris, Dolichogenideagobica, Dolichogenideahyalinis, Dolichogenideairiarte, Dolichogenidealakhaensis, Dolichogenidealampe, Dolichogenidealaspeyresiella, Dolichogenidealatistigma, Dolichogenidealebene, Dolichogenidealucidinervis, Dolichogenideamalacosomae, Dolichogenideamaro, Dolichogenideamendosae, Dolichogenideamonticola, Dolichogenideanigra, Dolichogenideaolivierellae, Dolichogenideaparallelis, Dolichogenideapelopea, Dolichogenideapelops, Dolichogenideaphaenna, Dolichogenideapisenor, Dolichogenidearoepkei, Dolichogenideascabra, Dolichogenideastatius, Dolichogenideastenotelas, Dolichogenideastriata, Dolichogenideawittei, Exoryzaasotae, Exoryzabelippicola, Exoryzahylas, Exoryzamegagaster, Exoryzaoryzae, Glyptapantelesaggestus, Glyptapantelesagynus, Glyptapantelesaithos, Glyptapantelesamenophis, Glyptapantelesantarctiae, Glyptapantelesanubis, Glyptapantelesarginae, Glyptapantelesargus, Glyptapantelesatylana, Glyptapantelesbadgleyi, Glyptapantelesbataviensis, Glyptapantelesbistonis, Glyptapantelesborocerae, Glyptapantelescacao, Glyptapantelescadei, Glyptapantelescinyras, Glyptapanteleseryphanidis, Glyptapanteleseuproctisiphagus, Glyptapanteleseutelus, Glyptapantelesfabiae, Glyptapantelesfulvigaster, Glyptapantelesfuscinervis, Glyptapantelesgahinga, Glyptapantelesglobatus, Glyptapantelesglyphodes, Glyptapantelesguierae, Glyptapanteleshorus, Glyptapantelesintricatus, Glyptapanteleslamprosemae, Glyptapanteleslefevrei, Glyptapantelesleucotretae, Glyptapanteleslissopleurus, Glyptapantelesmadecassus, Glyptapantelesmarquesi, Glyptapantelesmelanotus, Glyptapantelesmelissus, Glyptapantelesmerope, Glyptapantelesnaromae, Glyptapantelesnepitae, Glyptapantelesnigrescens, Glyptapantelesninus, Glyptapantelesnkuli, Glyptapantelesparasundanus, Glyptapantelespenelope, Glyptapantelespenthocratus, Glyptapantelesphilippinensis, Glyptapantelesphilocampus, Glyptapantelesphoebe, Glyptapantelesphytometraduplus, Glyptapantelespropylae, Glyptapantelespuera, Glyptapantelesseydeli, Glyptapantelessiderion, Glyptapantelessimus, Glyptapantelesspeciosissimus, Glyptapantelesspilosomae, Glyptapantelessubpunctatus, Glyptapantelesthespis, Glyptapantelesthoseae, Glyptapantelesvenustus, Glyptapanteleswilkinsoni, Hypomicrogastersamarshalli, Iconellacajani, Iconelladetrectans, Iconellajason, Iconellalynceus, Iconellapyrene, Iconellatedanius, Illidopsazamgarhensis, Illidopslamprosemae, Illidopstrabea, Keylimepiestriatus, Microplitisadisurae, Microplitismexicanus, Neoclarkinellaariadne, Neoclarkinellacurvinervus, Neoclarkinellasundana, Nyereriaituriensis, Nyererianioro, Nyereriaproagynus, Nyereriataoi, Nyereriavallatae, Parapantelesaethiopicus, Parapantelesalternatus, Parapantelesaso, Parapantelesatellae, Parapantelesbagicha, Parapantelescleo, Parapantelescyclorhaphus, Parapantelesdemades, Parapantelesendymion, Parapantelesepiplemicidus, Parapantelesexpulsus, Parapantelesfallax, Parapantelesfolia, Parapantelesfurax, Parapanteleshemitheae, Parapanteleshyposidrae, Parapantelesindicus, Parapantelesjavensis, Parapantelesjhaverii, Parapantelesmaculipalpis, Parapantelesmaynei, Parapantelesneocajani, Parapantelesneohyblaeae, Parapantelesnydia, Parapantelesprosper, Parapantelesprosymna, Parapantelespunctatissimus, Parapantelesregalis, Parapantelessarpedon, Parapantelessartamus, Parapantelesscultena, Parapantelestransvaalensis, Parapantelesturri, Parapantelesxanthopholis, Pholetesoracutus, Pholetesorbrevivalvatus, Pholetesorextentus, Pholetesoringenuoides, Pholetesorkuwayamai, Promicrogasterapidanus, Promicrogasterbriareus, Promicrogasterconopiae, Promicrogasteremesa, Promicrogastergrandicula, Promicrogasterorsedice, Promicrogasterrepleta, Promicrogastertyphon, Sathonbekilyensis, Sathonflavofacialis, Sathonlaurae, Sathonmikeno, Sathonruandanus, Sathonrufotestaceus, Venanidesastydamia, Venanidesdemeter, Venanidesparmula, and Venanidessymmysta.
Keywords: Microgastrinae, world fauna, checklist, nomenclature changes, genus diagnosis, genus illustration, distribution, Lepidoptera
Introduction
With almost 3,000 described species and estimates of up to 46,000+ worldwide (Rodriguez et al. 2013), the parasitoid wasp subfamily Microgastrinae (Hymenoptera: Ichneumonoidea, Braconidae) is an important and hyperdiverse group, which has long played a central role in our understanding of insect parasitism in the context of many areas of ecological, agricultural, and basic science (Whitfield et al. 2018). Because of their diversity, prevalence in most terrestrial habitats, and the fact that species are exclusively parasitoids of larval Lepidoptera across nearly the full range of families within the taxon (Eulepidoptera, sensuAarvik et al. 2017), microgastrine wasps are one of the most important groups in the biological control of agricultural and forestry lepidopterous pests worldwide (Whitfield 1997).
A world checklist of Microgastrinae has never been published, although Shenefelt (1972, 1973) listed the species as part of his monumental work cataloguing the world species of Braconidae. Unfortunately, those papers are outdated, especially since Mason (1981) published a seminal study that changed the generic and tribal classifications. In addition to taxonomic changes (many nominal species had been placed in synonymy), the number of newly described species has increased dramatically since Shenefelt’s catalogue: 1,446 new species of Microgastrinae (48.2%) were described between 1974 and 2019. In the past six years alone (2014–2019), 720 new species have been described (an average of 120 new species/year), which represents, by far, the largest increase in species for any subfamily of Braconidae in that time span (data extracted from this paper and Yu et al. 2016).
The database Taxapad, originally produced as a CD (Yu et al. 2005), and later available as a USB drive (Yu et al. 2012, 2016) or, partially, as a web product (now offline), has been used as the de facto catalogue of Ichneumonoidea (and associated data comprising some 350,000 names) for almost fifteen years. It is important to understand that it is essentially a compilation of all published information, whether correct or not. Nevertheless, Taxapad is an extraordinary product that contains copious information about the taxonomy, distribution, hosts and associated host plants, morphology, etc., of Ichneumonoidea that is easy to collate and analyze. As a result, it is widely consulted by researchers worldwide, and it has been adopted and (unfortunately uncritically) used in many other databases, websites, and publications pertinent to Ichneumonoidea.
However, for Microgastrinae, Taxapad follows a classification based on van Achterberg (2003), which is far from being universally accepted. A different classification, based on an older, more comprehensive paper (Mason 1981), is the one preferred and used by most researchers worldwide (e.g., Papp 1988, Kotenko 2007a, Shaw 2012, Broad et al. 2016 in the Palearctic; Whitfield 1995a, Fernandez-Triana 2010 in the Nearctic; Whitfield 1997, Fernandez-Triana et al. 2014e in the Neotropical region; Rousse and Gupta 2013 in the Afrotropical region; Chen and Song 2004, Liu et al. 2017, 2018 in the Oriental region; Austin and Dangerfield 1992 in Australasia). Thus, the Microgastrinae arrangement in Taxapad conflicts with that used by most taxonomists working on the subfamily, a situation that becomes even more confusing for ecologists, biocontrol researchers and other non-taxonomist users of Taxapad.
To complicate matters further, neither Mason (1981) nor van Achterberg (2003) treated all world species, having left many nominal species without checking their generic placement, especially those described in older literature. As a result, many of those species have remained where they were originally described or as Nixon (1965) interpreted them, usually in one of the three traditional genera historically considered to constitute practically all Microgastrinae: Apanteles Foerster, Microgaster Latreille, and Microplitis Foerster; or they were placed as part of an expanded Apanteles and Protapanteles Ashmead (sensuvan Achterberg 2003). Some exceptions fared slightly better, e.g., Papp (1988) assigned many European species to Mason’s (1981) genera, Whitfield (1995a) did the same for North America, and Austin and Dangerfield (1992) for Australasia.
In this paper we a) summarize general information about Microgastrinae, including a historical outline of the internal classification, estimates of specific and generic diversity, distribution at local and world levels, advances in regional taxonomic studies, and general trends in host use; b) characterize all 81 currently accepted genera of extant Microgastrinae, including brief morphological diagnostic features, colour illustrations, available DNA barcodes and general comments on known host families; c) revise, to the best of our knowledge, the generic placement of all described species of Microgastrinae; d) compile an updated checklist of the extant and fossil world species of Microgastrinae, including recorded geographical distribution and taxonomic notes; and e) provide all information as a supplementary Excel file, to facilitate future use of the data. As work on Microgastrinae advances, we hope to provide updates in future versions of this checklist.
Materials and methods
We used the last two versions of Taxapad (Yu et al. 2012, 2016) as the starting point to compile a list of world genera and species of Microgastrinae and their recorded geographical distribution. Because the last version of Taxapad includes only information published up to the end of 2015, with some data from early 2016 (Yu, pers. comm.), we checked Zoological Record and Google Scholar for all papers published after 2015. The information presented in this paper has the cut off date of 31 December 2019.
We also compiled information from some of the world’s largest collections of Microgastrinae. All primary types (representing almost 500 species) of the Canadian National Collection of Insects (Ottawa, Canada) were studied, and unpublished information on the distribution of many species and genera was extracted from that collection, probably the largest depository of world Microgastrinae, with 120,000+ pinned specimens. We examined all primary types (representing almost 500 species of Microgastrinae) in The Natural History Museum (London, United Kingdom). Most of the primary types (representing almost 400 species of Microgastrinae) in the National Museum of Natural History (Washington, United States) were either examined or studied from images (available at http://www.usnmhymtypes.com/). Types and non-type material were extensively studied in the Finnish Museum of Natural History (Helsinki, Finland), the National Museums of Scotland (Edinburgh, United Kingdom), four major Japanese collections (Hokkaido University, Sappporo; Kobe University, Kobe; Meijo University, Nagoya; and the Osaka Museum of Natural History, Osaka), the New Zealand Arthropod Collection (Auckland, New Zealand), Naturalis (Leiden, the Netherlands), the Hungarian Natural History Museum (Budapest, Hungary), and the Austrian Natural History Museum (Vienna, Austria). Extensive non-type material, representing thousands of specimens worldwide, were borrowed for study from several institutions in Canada, Costa Rica, France, Sweden, Thailand, and the United States. Several online databases such as the Barcoding of Life Data Systems (http://v4.boldsystems.org/) and Area de Conservación Guanacaste (ACG), Costa Rica (http://janzen.sas.upenn.edu/caterpillars/database.lasso) were searched as well. The final data were input into an Excel file, which is provided here as a supplementary file to facilitate access to all information for personal use and editing (Suppl. material 1). We also provide an index of all available species names of Microgastrinae in strict alphabetical order; with the valid names in bold and italics, and the synonyms, homonyms, and nomina dubia just in italics (Suppl. material 2).
After the initial list was compiled, all species were assessed as comprehensively as possible, including: a) examination of primary types whenever possible (in a few cases we examined high quality illustrations of the primary types, which were sufficient to establish their generic placement unambiguously; in those cases we clearly indicate the source of the illustrations); b) study of secondary types and/or authenticated specimens (= specimens in collections identified by experts on the group; in those cases we mention the name of the expert identifying the species); and c) checking relevant literature, either the original description (including illustrations whenever available) or subsequent references where the species was treated (e.g., taxonomic revision, regional checklist, etc.). Throughout the checklist, “not examined but original description checked” or “not examined but subsequent treatment of the species checked” means that one of us checked those references. For every species, we detail how we assessed its status, as it is evident that the conclusion will be more reliable if the primary type was examined as opposed to secondary types, authenticated specimens, or the reading of a description. For species where we could neither examine specimens nor check for relevant literature we (explicitly) maintain the original generic combination.
For a few species, mostly in Apanteles and Microgaster, the available information (usually only the original description) was enough to suggest that they belonged to a different genus, but not enough to confidently place them in another genus (usually because several alternatives were possible, or none was clear). In those cases we considered the species as species inquirendae and add a question mark before the genus name it was originally described in (e.g., ? Apanteles) to indicate the questionable generic placement.
In the checklist, at the beginning of each genus we detail its author, year of publication and page (of the original description of the genus), gender of the genus name, type species, genus synonyms, and comments (if needed). As far as we know, the gender of every Microgastrinae genus has not been stated in a single publication before (e.g., Shenefelt (1972, 1973) did not address that; Mason (1981) only discussed the gender of some of the new genera described there; Yu et al. (2016) did not present that information either). For our checklist we follow the original publication (if the gender was stated there), or expert advice from an ICZN commissioner (Doug Yanega, pers. comm.).
For each species in the checklist we provide current name, original combination, synonyms, homonyms, and details of the primary type (including sex, holding institution, and country of the type locality), as well as details of the recorded geographical distribution of the species. Where necessary, additional comments are added at the end of the species’ treatment under “Notes”. We do not include full details on the combination history of the species name or further taxonomic details (other than the ones detailed above). For such details, Taxapad (Yu et al. 2012, 2016) and Shenefelt (1972, 1972) must be consulted.
The spelling of some author’s last names was found to vary in the literature: de Saeger/De Saeger, de Santis/De Santis, Fernandez-Triana/Fernandez-Triana, Foerster/Förster, van Achterberg/Van Achterberg. For the sake of consistency, in this paper we are using the first alternative in each of the above cases. The only exception is María Teresa Oltra Moscardó (Spain), as she has recorded her last name in several publications as either Oltra (referring to species authorship and also as paper authorship for most of her papers) or Oltra-Moscardó (only applying to one paper cited in our checklist: Oltra-Moscardó and Jiménez-Peydró 2005). In this case we use the appropriate alternative according to the corresponding reference cited, but for all eight species that she has described we refer to her as Oltra.
The availability of species names was assessed following the latest version of the International Commission on Zoological Nomenclature (ICZN); throughout the text any reference to ICZN articles follows the online version (https://www.iczn.org/the-code/the-international-code-of-zoological-nomenclature/the-code-online/).
Details on species distribution are first presented by biogeographical regions, and then by countries within biogeographical regions, in both cases arranged in alphabetical order. For biogeographical boundaries we follow the O’Hara et al. (2009) approach of combining the Australasian and Oceanian regions into one, with the name of the former. Throughout the text we use six regions (there are no Microgastrinae recorded from their Antarctic region), abbreviated as follows: NEO Neotropical (sometimes referred to as Neotropics), NEA Nearctic, PAL Palaearctic, OTL Oriental, AFR Afrotropical (sometimes referred to as Afrotropics), and AUS Australasian.
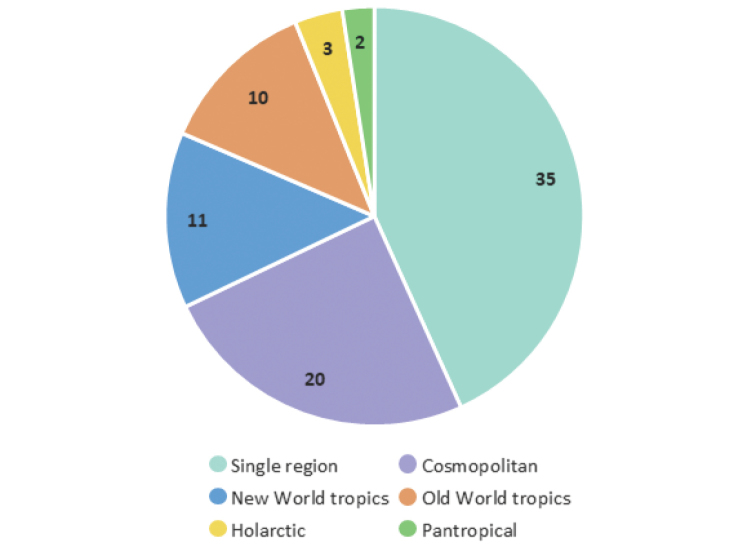
Occasionally, we use wider terms such as Holarctic (NEA and PAL), New World (NEA and NEO), Old World tropics (AFR, OTL and AUS), and pantropical (NEO, AFR, OTL, AUS). Some of these terms can be vague or hard to define precisely (e.g., some of the Australasian or southern Neotropical taxa are not really “tropical”, and the southern limits of the Holarctic region have a mix of temperate and subtropical taxa). However, they are used throughout the paper as a way to discuss trends in generic distribution and are not meant to be taken as strictly defined boundaries.
The list of countries follows the Standard ISO 3166 (codes for names of countries and their subdivisions: https://www.iso.org/obp/ui/#search). Throughout the text, we abbreviate United States of America as USA. For the six largest countries by area (Russia, Canada, China, USA, Brazil and Australia) we also present finer species distributions by country subdivisions (provinces, republics, states, territories, etc.). For Australian states and territories, we follow http://www.bda-online.org.au/help/bda-conventions/abbreviations-states/. For states of the USA and for Canadian provinces and territories, acronyms consisting of two capital letters are used, following Canada Post (http://www.canadapost.ca/tools/pg/manual/PGaddress-e.asp). We follow Standard ISO 3166 for China provinces (https://www.iso.org/obp/ui/#iso:code:3166:CN) and Brazil states (https://www.iso.org/obp/ui/#iso:code:3166:BR). For Russia subdivisions we mostly follow Standard ISO 3166 (https://www.iso.org/obp/ui/#iso:code:3166:RU), but see next paragraph for explanation on exceptions.
In most cases the information on species distribution per subdivisions was summarized from Yu et al. (2016), with updates from publications after that date. For Brazil we followed Shimbori et al. (2019). For Russia we mostly followed Yu et al. (2016), but we also added information from a recent update from Belokobylskij et al. (2019). However, Belokobylskij et al. (2019) combined several of the Russian subdivisions (according to the Standard ISO 3166, followed by Yu et al. 2016 and also by us in this paper) into broader categories, its “geoscheme for Russia” being different. As a result, some species recorded from Russia have its distribution detailed only to the level of those broader categories, as dealt with by Belokobylskij et al. (2019). The acronyms for those categories are as follow: C Centre, E East, N North, NC North Caucasus, NW North-West and S South, in the “European Part of Russia”; IR Irkutsk Province, in “Eastern Siberia”; UR Ural in the “Ural” (no province or territory detailed); KA Kamchatka Territory and PR Primorskii Territory, in the “Far East” (for more details see Belokobylskij et al. 2019: 9, fig. 1 on page 10).
Some countries have political units located in different biogeographical regions (or, in some cases, islands which are separate from the continent where the country is located), we considered those units as separate entities in our checklist (and the “country” in those cases is recorded as the separate entity and not the actual country it politically belongs to). Those cases are: Chile (Juan Fernández Islands), France (French Guiana, Guadeloupe, Marquesas Islands, Réunion, Society Islands), Japan (Ryukyu Islands), the Netherlands (Netherlands Antilles), Portugal (Azores, Madeira Islands, Selvagens Islands), Spain (Canary Islands), United Kingdom (British Virgin Islands, Saint Helena), and USA (American Samoa, Hawaiian Islands, and the USA Virgin Islands).
For all species historically recorded from the former Czechoslovakia we were able to separate the records that belong to either Czech Republic or Slovakia, based on Capek and Lukas (1989). However, for some species historically recorded from the former Yugoslavia (currently six or seven different countries, depending on the source) and also from the former Sudan (currently two countries: Sudan and South Sudan), the sources of the species records did not contain enough information to determine to which country they currently belong; therefore we annotate those records just as Yugoslavia and Sudan respectively.
Apart from some general comments on Microgastrinae hosts, we have not attempted to add host information for particular species; we intend to publish a critical assessment of Microgastrinae host records at a later date. We do, however, state general trends in host parasitization on a generic level. We follow the arrangement in Aarvik et al. (2017) when referring to families and superfamilies of Lepidoptera. Taxapad (Yu et al. 2016) gives almost complete information on published host records up to the end of 2015, but that source is inevitably very far from a reliable indication of true host associations. A complete and critical analysis of those records would require a huge effort, and in many cases it might be very difficult to determine unambiguously which ones are correct. In this respect, the amount of misinformation in the general literature is far larger than generally realised and can completely mask any real understanding of a parasitoid’s host range; Noyes (1994), Shaw (1994) and Shaw and Aeschlimann (1994) discuss this with examples.
For collection acronyms we mostly follow the website “Insect and Spider Collections of the World” (http://hbs.bishopmuseum.org/codens/codens-r-us.html). In cases where institutions were not listed there, we propose codens based on some abbreviation of the institution name. The complete list of institutions mentioned in this paper is:
AEIC American Entomological Institute, Utah State University, Logan, USA
AMNH American Museum of Natural History, New York, New York, USA
AMUZ Aligarh Muslim University, Zoological Museum, Aligarh, Uttar Pradesh, India
ANIC Australian National Insect Collection, CSIRO, Canberra City, Australia
ANSP Academy of Natural Sciences, Philadelphia, Pennsylvania, USA
BAMU Dr. Babasaheb Ambedkar Marathwada University, Aurangabad, India
BGM Beth Gordon Agriculture and Nature Study Institute, Deganya, Israel
BPBM Bernice P. Bishop Museum, Honolulu, Hawaii, USA
CAS California Academy of Sciences, San Francisco, California, USA
CBGP Centre de Biologie pour la Gestion des Populations, Montpellier, France
CFRB Chinese Academy of Forestry, Forest Research Institute, Beijing, China
CNC Canadian National Collection of Insects, Ottawa, Canada
CUIC Cornell University, Ithaca, New York, USA
DCBU Departamento de Ecologia e Biologia Evolutiva, Universidad Federal de São Carlos, São Carlos, Brazil
DCMP Universidade Federal do Paraná, Curitiba, Paraná, Brazil
DPBA Departamento de Patologia Vegetal, Buenos Aires, Argentina
DPPZ Department of Plant Protection, University of Zabol, Zabol, Iran
DZCU Department of Zoology, University of Calicut, Kerala, India
DZUC University of Ceylon, Department of Zoology, Colombo, Sri Lanka
EBW Deutsches Entomologisches Institut, Eberswalde, Germany
EIHU Hokkaido University, Sapporo, Hokkaido, Japan
ESUW University of Wyoming, Laramie, USA
FAFU Fujian Agriculture and Forestry University, Fuzhou, China
FNIC Fiji National Insect Collection, Suva, Fiji
FSCA Florida State Collection of Arthropods, Division of Plant Industry, Gainesville, USA
GUGC Guizhou University, Guiyang, China
HNHM Hungarian Natural History Museum, Budapest, Hungary
HUNAU Hunan Agricultural University, Changsha, China
IAVH Instituto Alexander von Humboldt, Bogotá, Colombia
IEAS Academia Sinica, Institute of Entomology, Shanghai, Shanghai, China
IEBR Institute of Ecology and Biological Resources, Hanoi, Vietnam
IECA Institute of Entomology, Ceské Budejovice, Czech Republic
IFRI Indian Forest Research Institute, Dehradun, Uttarakhand, India
IIAF Instituto de Investigaciones Agropecuarias y Forestales, Universidad Michoacana San Nicolás de Hidalgo, México
INBio Instituto Nacional de Biodiversidad, Santo Domingo de Heredia, Costa Rica
INHS Illinois Natural History Survey, Champaign, Illinois, USA
INPC National Pusa Collections, Indian Agricultural Research Institute, New Delhi, India
KUEC Kyushu University, Fukuoka, Japan
LNKD Landessammlung für Naturkunde, Karlsruhe, Germany
LSUK The Linnean Society of London, London, United Kingdom
LUNZ Lincoln University, Lincoln, New Zealand
MACN Museo Argentino de Ciencias Naturales, Buenos Aires, Argentina
MCZ Museum of Comparative Zoology, Harvard University, Cambridge, USA
MHNG Muséum d'Histoire Naturelle, Geneva, Switzerland
MIUP Museo de Invertebrados Graham Bell Fairchild, Universidad de Panamá, Panama
MLP Museo de La Plata, La Plata, Argentina
MMBC Moravske Muzeum [Moravian Museum], Brno, Czech Republic
MNCN Museo Nacional de Ciencias Naturales, Madrid, Spain
MNHN Muséum National d'Histoire Naturelle, Paris, France
MNNC Museo Nacional de Historia Natural, Santiago, Chile
MUSM Museo de Historia Natural, Universidad Nacional Mayor de San Marcos, Lima, Peru
MVMMA Museums Victoria, Melbourne Museum, Melbourne, Australia
MZH Finnish Museum of Natural History, Helsinki, Finland
MZLU Lund University, Lund, Sweden
MZUSP Museum of Zoology, University of São Paulo, Brazil
NBAIR National Bureau of Agricultural Insect Resources, Bangalore, India
NHMO Zoological Museum, University of Oslo, Oslo, Norway
NHMUK Natural History Museum, London, United Kingdom
NHMW Naturhistorisches Museum Wien, Vienna, Austria
NHRS Naturhistoriska Riksmuseet, Stockholm, Sweden
NIAES National Institute for Agro-Environmental Sciences, Tsukuba, Japan
NMID National Museum of Ireland, Dublin, Ireland
NMKE National Museum of Kenya, Nairobi, Kenya
NZAC New Zealand Arthropod Collection, Landcare Research, Auckland, New Zealand
NZSI National Zoological Collection, Zoological Survey of India, Kolkata, West Bengal, India
OUMNH Museum of Natural History, Oxford University, United Kingdom
PCMAG Plymouth City Museum and Art Gallery, Plymouth, United Kingdom
PPRI Plant Protection Research Institute, Pretoria, Gauteng, South Africa
QM Queensland Museum, South Brisbane, Queensland, Australia
QSBG Queen Sirikit Botanic Garden, Chaing Mai, Thailand
QCAZ Pontificia Universidad Católica del Ecuador, Quito, Ecuador
RBINS Royal Belgian Institute of Natural Sciences, Brussels, Belgium
RMCA Musée Royal de l'Afrique Centrale, Tervuren, Belgium
RMNH Naturalis Biodiversity Centre, Leiden, Netherlands
RSME National Museums of Scotland, Edinburgh, United Kingdom
SAMA South Australian Museum, Adelaide, South Australia, Australia
SAMC Iziko Museum of Capetown, Cape Town, South Africa
SAUC Shandong Agricultural University, Tai'an, China
SCAC South China Agricultural College, Guangzhou, Guangdong, China
SEMC Snow Entomological Museum, University of Kansas, Lawrence, Kansas, USA
SIZK Schmalhausen Institute of Zoology, Kiev, Ukraine
SJCA St. John's College, Agra, Uttar Pradesh, India
SMF Forschungsinstitut und Naturmuseum Senckenberg, Frankfurt-am-Main, Germany
SUKI Shivaji University, Kolhapur, India
TARI Taiwan Agricultural Research Institute, Taichung, Taiwan, China
TFRI Insect Museum, Tropical Forest Research Institute, Jabalpur, Madhya Pradesh, India
TMAG Tasmanian Museum and Art Gallery, Hobart, Tasmania, Australia
TMSA Ditsong National Museum of Natural History, Pretoria, Gauteng, South Africa
TMUC Department of Entomology, Tarbiat Modares University, Tehran, Iran
TUDTG Technische Universität Dresden, Department of Forest Science, Tharandt, Germany
UCDC R.M. Bohart Museum of Entomology, University of California, Davis, California, USA
UFSM Universidade Federal de Santa Maria, Rio Grande do Sul, Brazil
UFVB Universidade Federal de Viçosa, Museum of Entomology, Viçosa, Minas Gerais, Brazil
UKM Universiti Kebangsaan, Bangi, Selangor, Malaysia
UKZMP Universiti Kebangsaan, Bangi, Selangor, Malaysia
ULQC University of Laval, Quebec City, Canada
USNM National Museum of Natural History, Washington, USA
UUZM Uppsala University, Uppsala, Sweden
UVS University of Valencia, Valencia, Spain
VNMN Vietnam National Museum of Nature, Vietnam Academy of Science and Technology, Hanoi, Vietnam
WAM Western Australian Museum, Perth, Western Australia, Australia
ZIN Zoological Institute, Russian Academy of Sciences, St. Petersburg, Russia
ZJUH Parasitic Hymenoptera Collection, Zhejiang University, Hangzhou, China
ZMHB Museum für Naturkunde der Humboldt-Universität, Berlin, Germany
ZMTU Zoological Museum, Trakya University, Turkey
ZMUC Zoological Museum, University of Copenhagen, Copenhagen, Danmark
ZMUK Zoologisches Museum, Universität Kiel, Kiel, Germany
ZSM Zoologische Staatssammlung, Munich, Germany
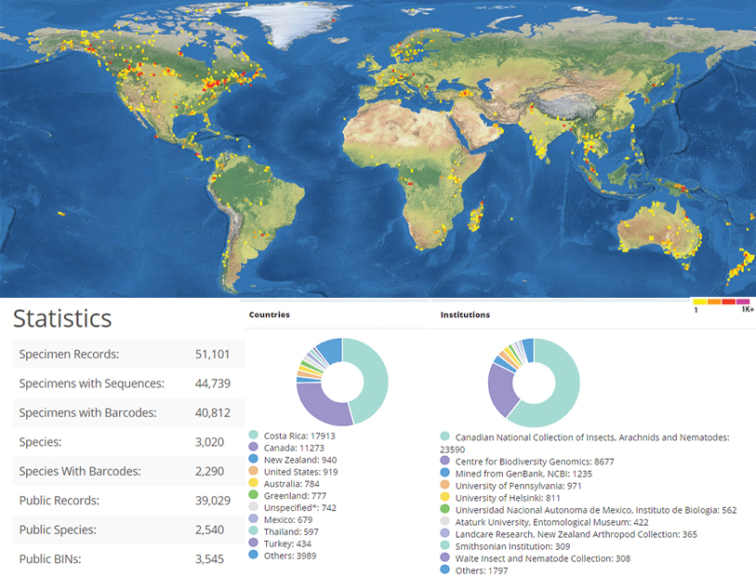
The concept of DNA barcoding as a tool for species discovery and identification was proposed approximately 15 years ago (Hebert et al. 2003a, 2003b). A short DNA sequence, approximately 650 base pairs (bp) in the mitochondrial gene encoding cytochrome c oxidase subunit 1 (CO1), has been accepted as a practical and standardized DNA barcode for many groups of animals (e.g., Kress et al. 2015). The Barcode Index Number (BIN) System uses DNA barcodes to indicate possible species limits (see more details on the BIN concept in Ratnasingham and Hebert 2013), and it has been used in taxonomic studies of Microgastrinae (e.g., Fernandez-Triana and Boudreault 2018, Fagan-Jeffries et al. 2018b). In the checklist below we provide details of the number of DNA barcode sequences and BINs for every genus of Microgastrinae currently available in the Barcoding of Life Data Systems (BOLD, see also http://v4.boldsystems.org/index.php) as of 31 December 2019. Sequences were considered as “barcode compliant” if they fulfilled the requirements set in Ratnasingham and Hebert (2007), namely: the sequence has at least 500 nucleotides with fewer than 1% ambiguous base calls (Ns); it has a species name (assigned by an expert taxonomist) or a provisional name; it has a unique specimen identifier, information related to the voucher specimen (including the name of the institution storing the voucher), and a collection record (e.g., collector, collection date, collection location, geospatial coordinates); and it has the sequence of PCR primers used to generate the CO1 amplicon and the trace files (Santschi et al. 2013).
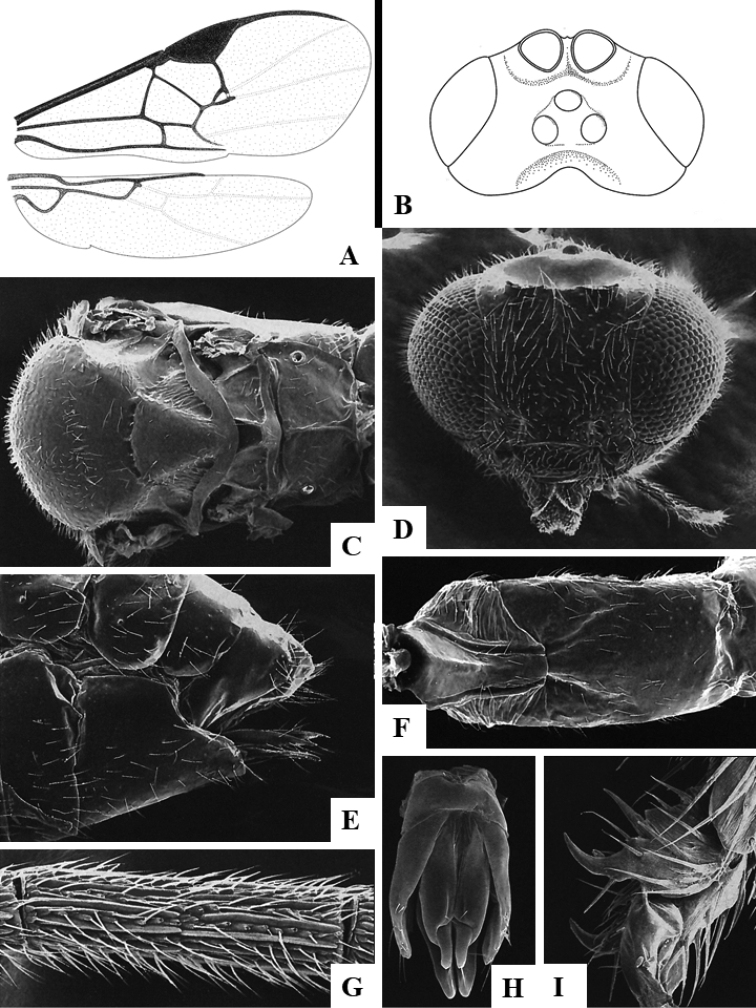
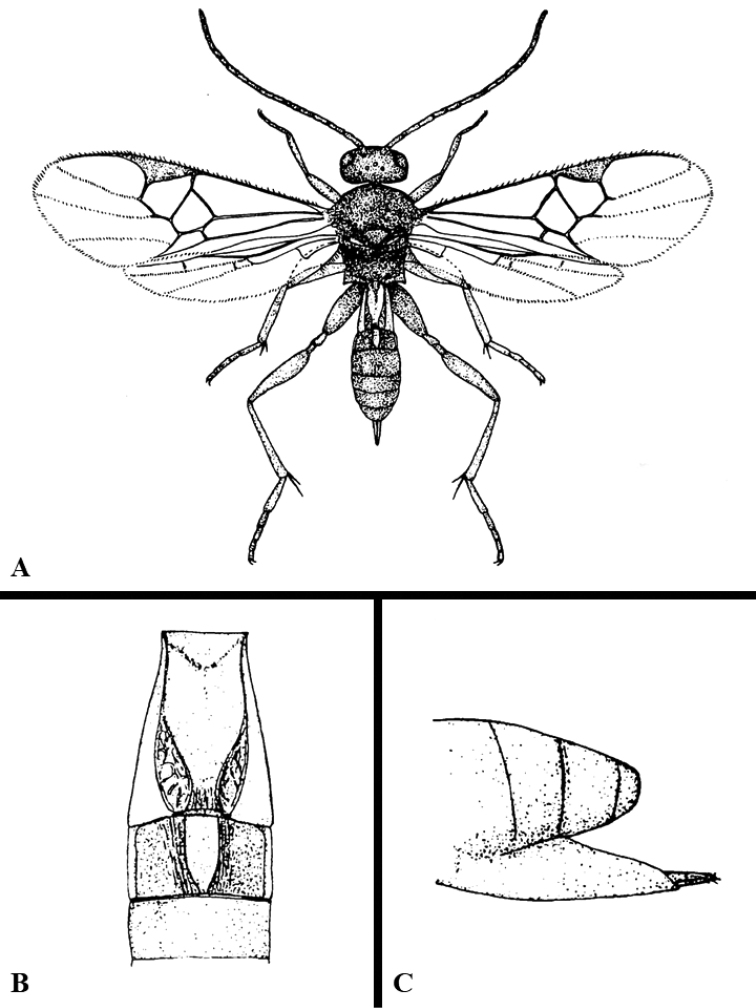
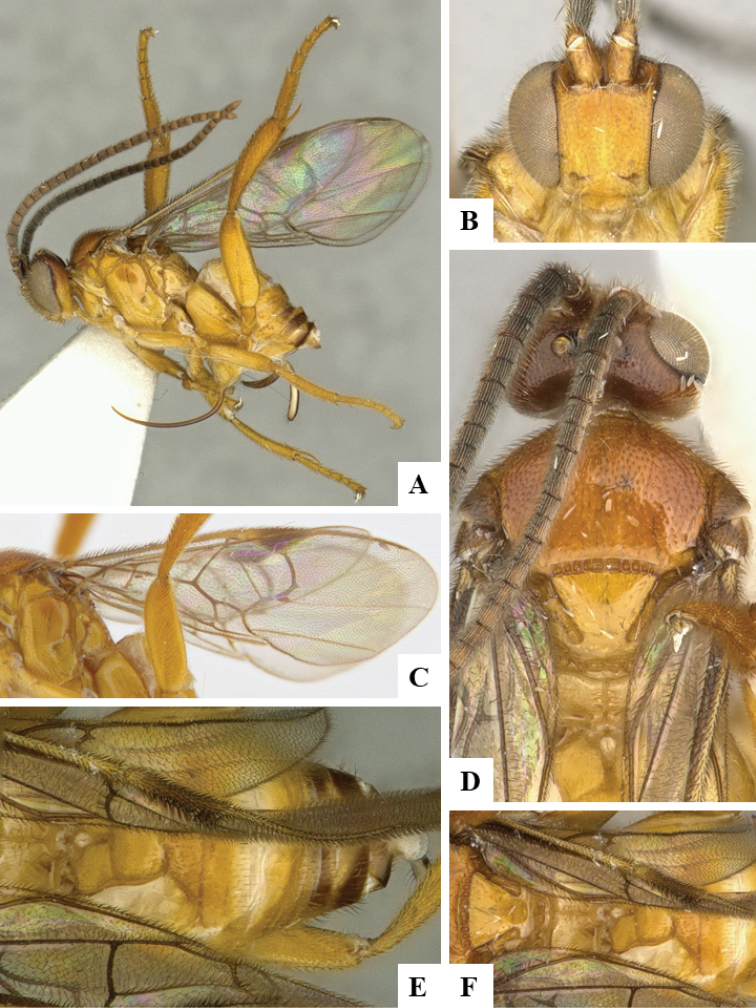
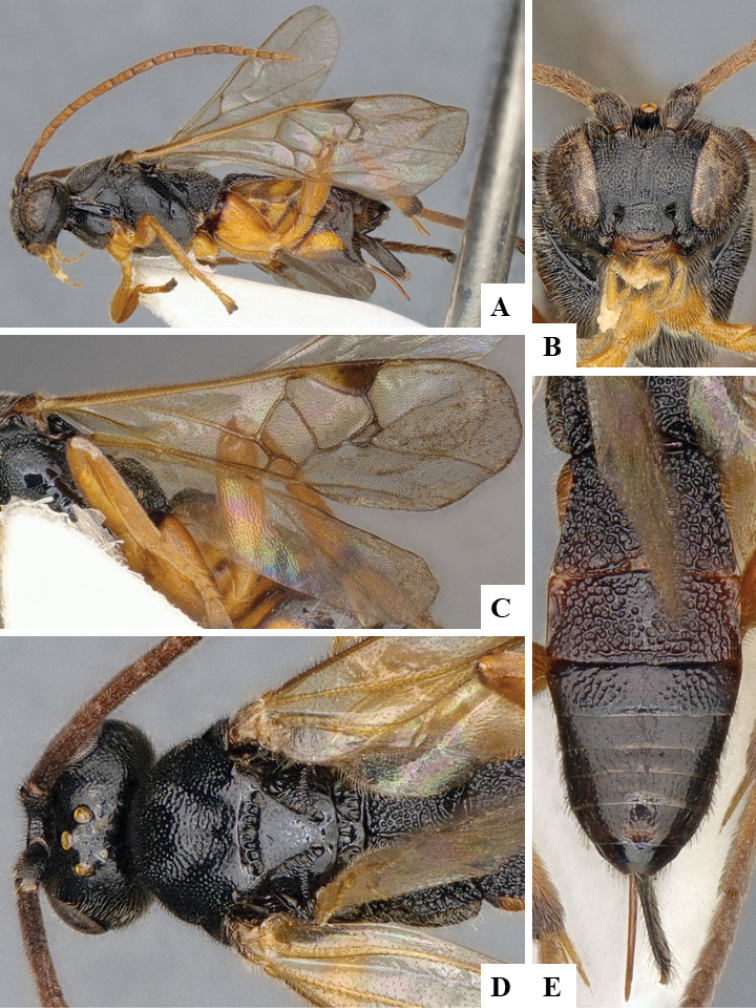
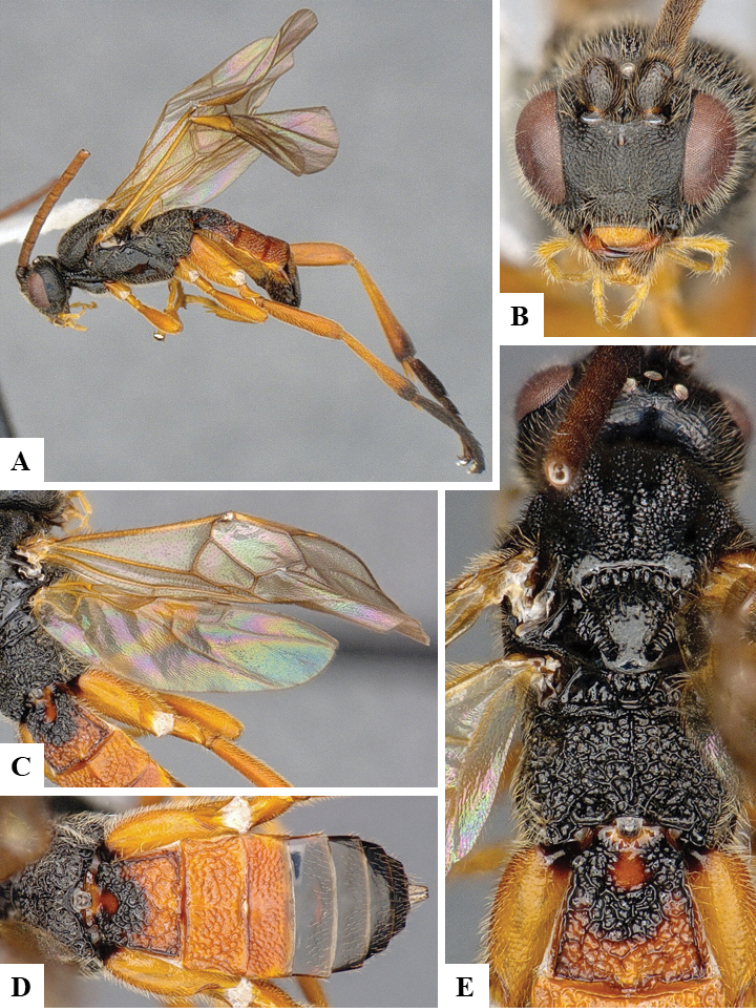
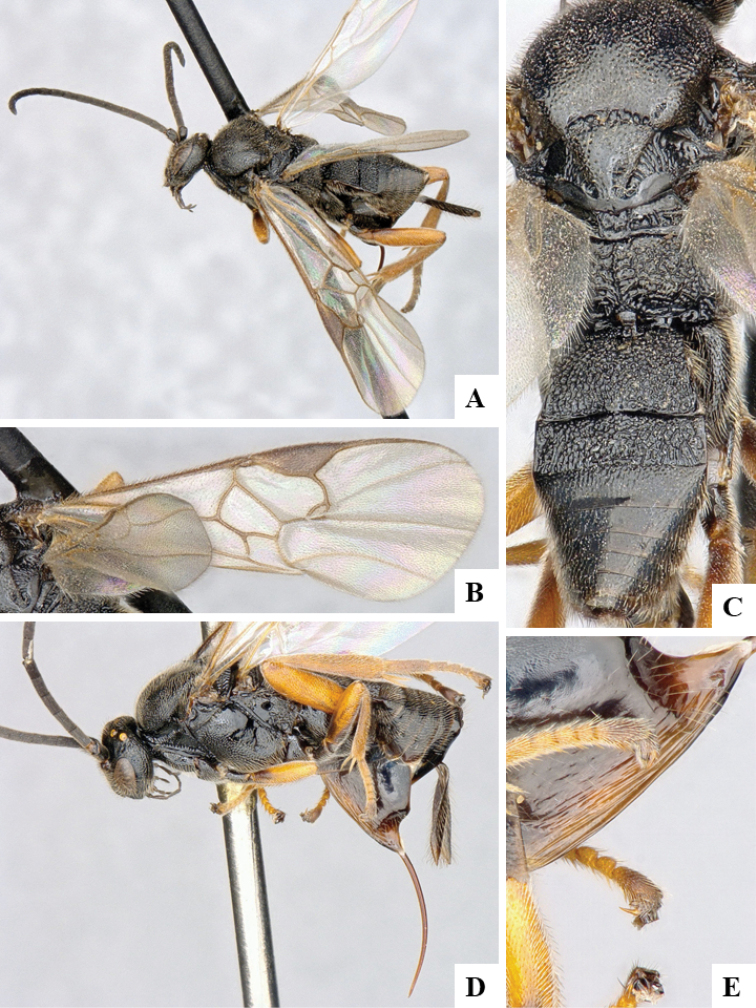
We provide brief morphological diagnostic features and colour illustrations for all 81 valid genera of Microgastrinae (at least one species per genus is illustrated, usually more). For morphological terms we follow several published references (Huber and Sharkey 1993, Sharkey and Wharton 1997, Karlsson and Ronquist 2012, Fernandez-Triana et al. 2014e) as well as the Hymenoptera Anatomy Ontology (HAO) website (http://portal.hymao.org/projects/32/public/ontology/). We use the abbreviations T1, T2, and T3 for metasomal mediotergites 1, 2, and 3; and the fore wing second submarginal cell is mentioned throughout the text as areolet for the sake of brevity.
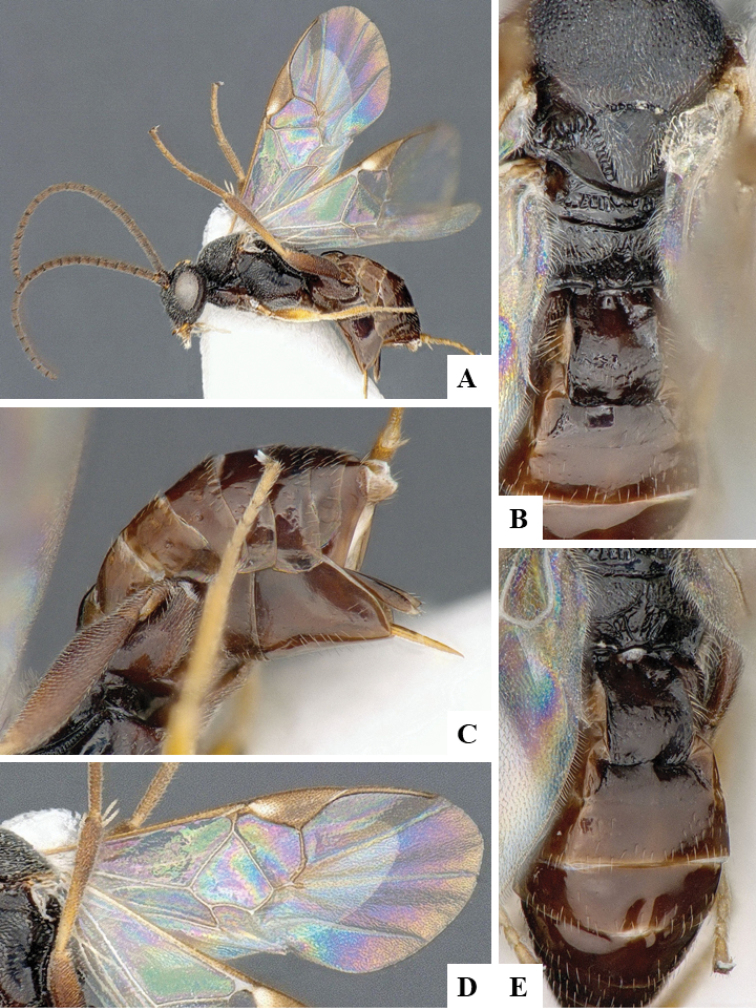
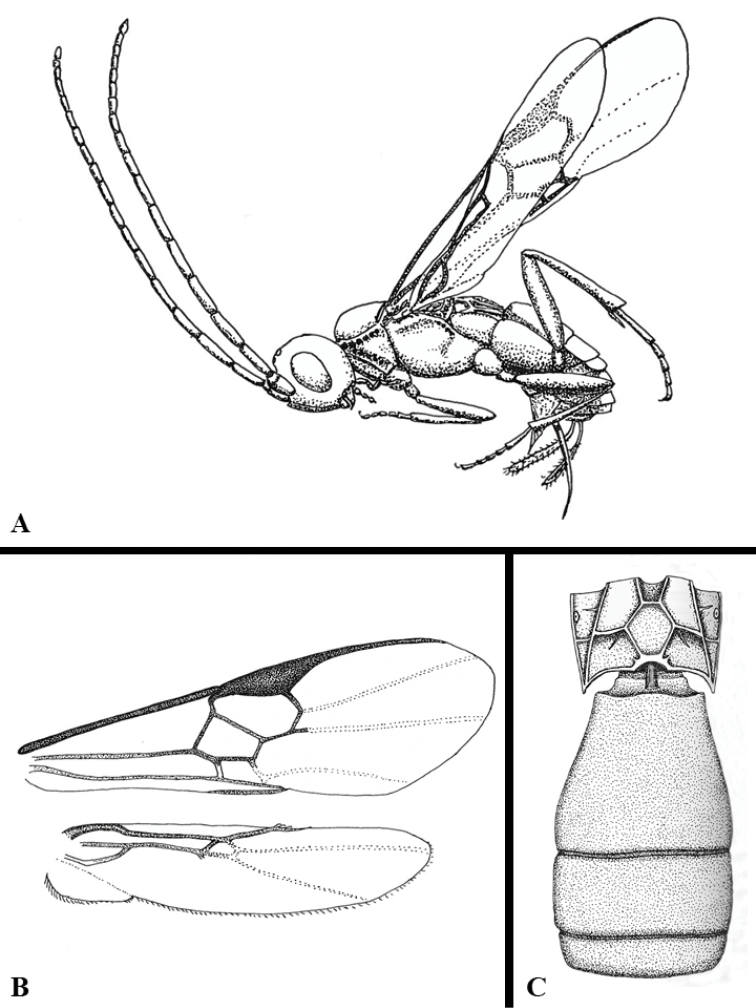
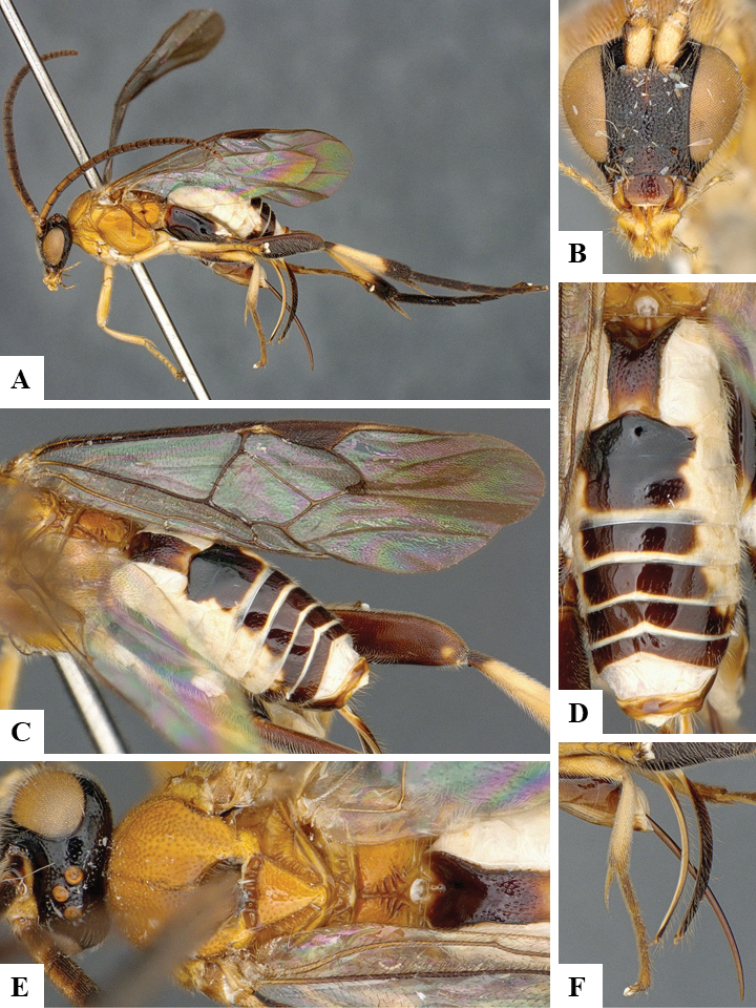
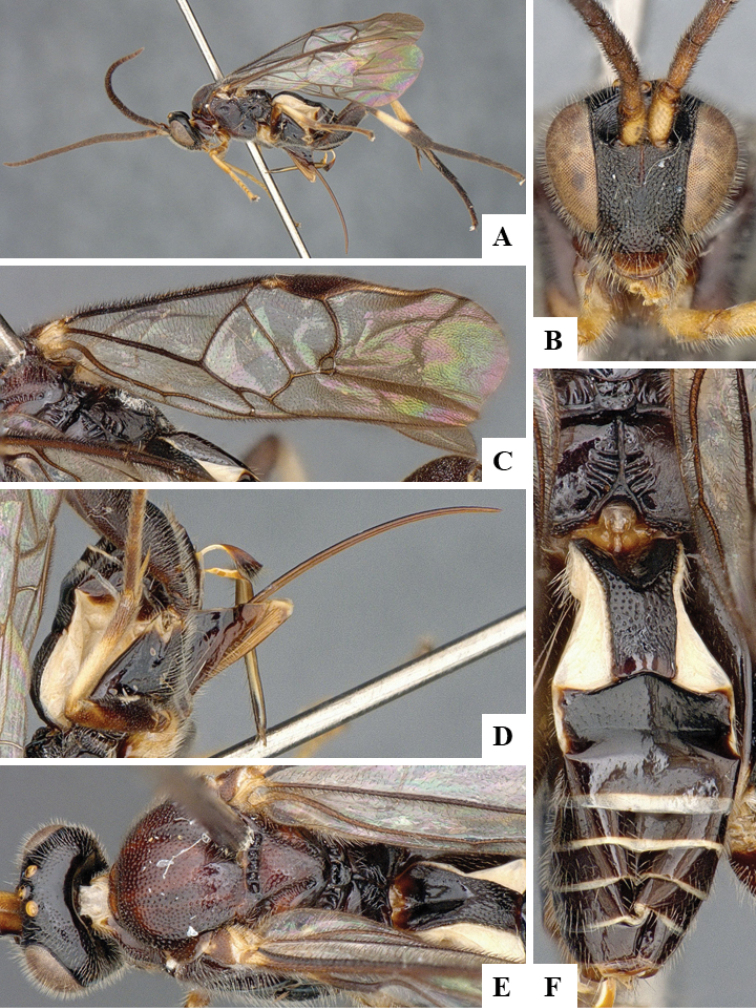
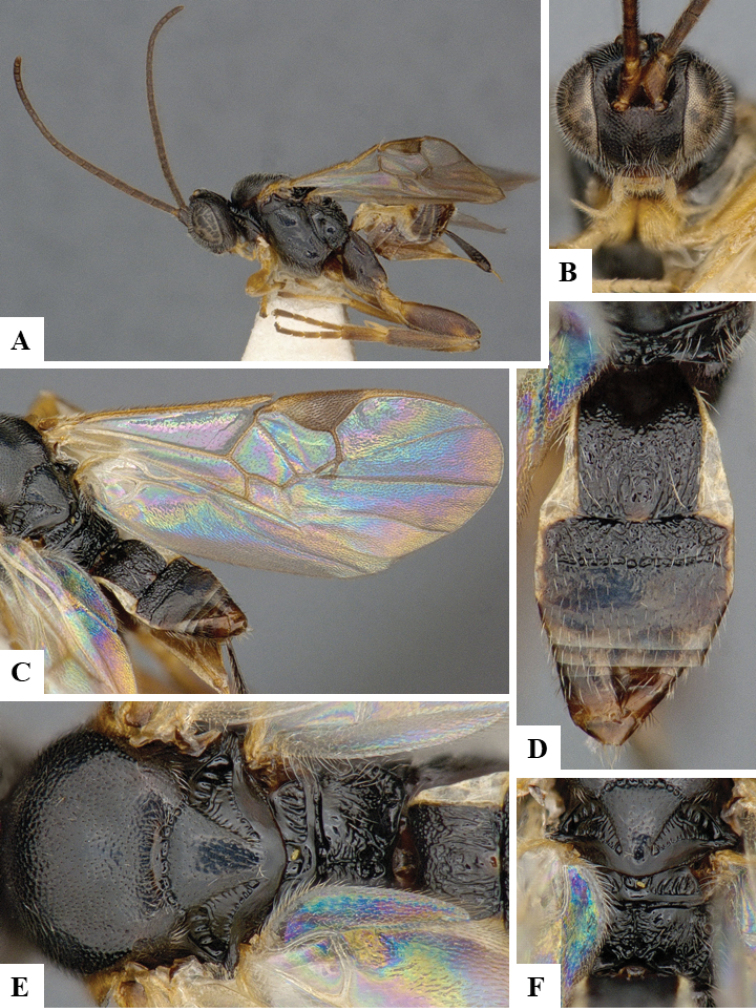
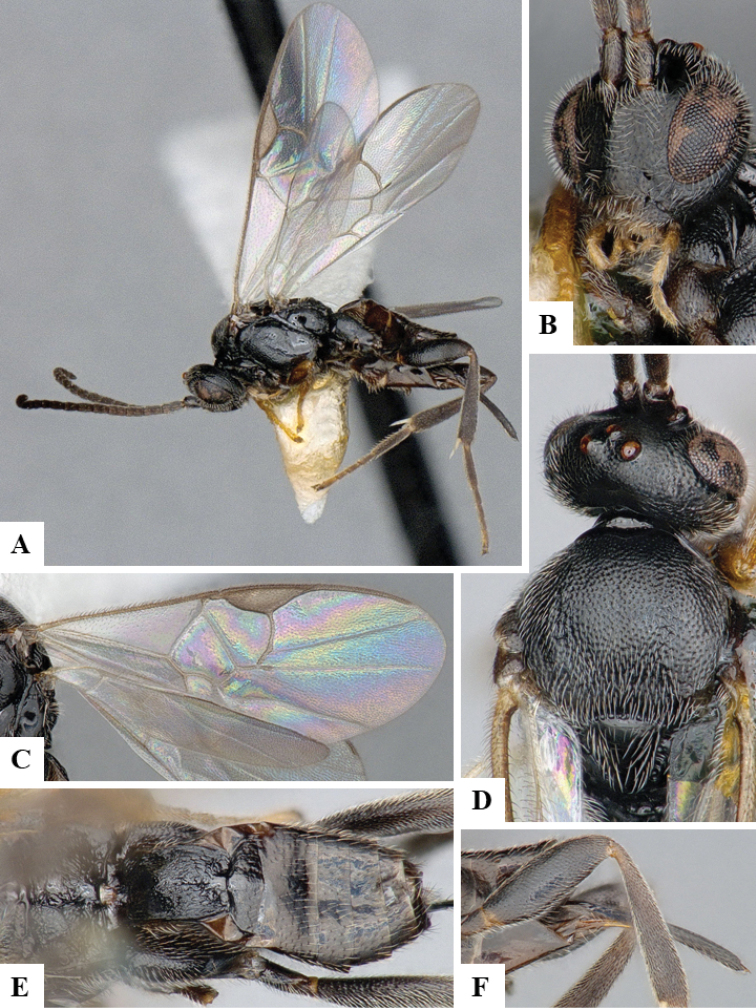
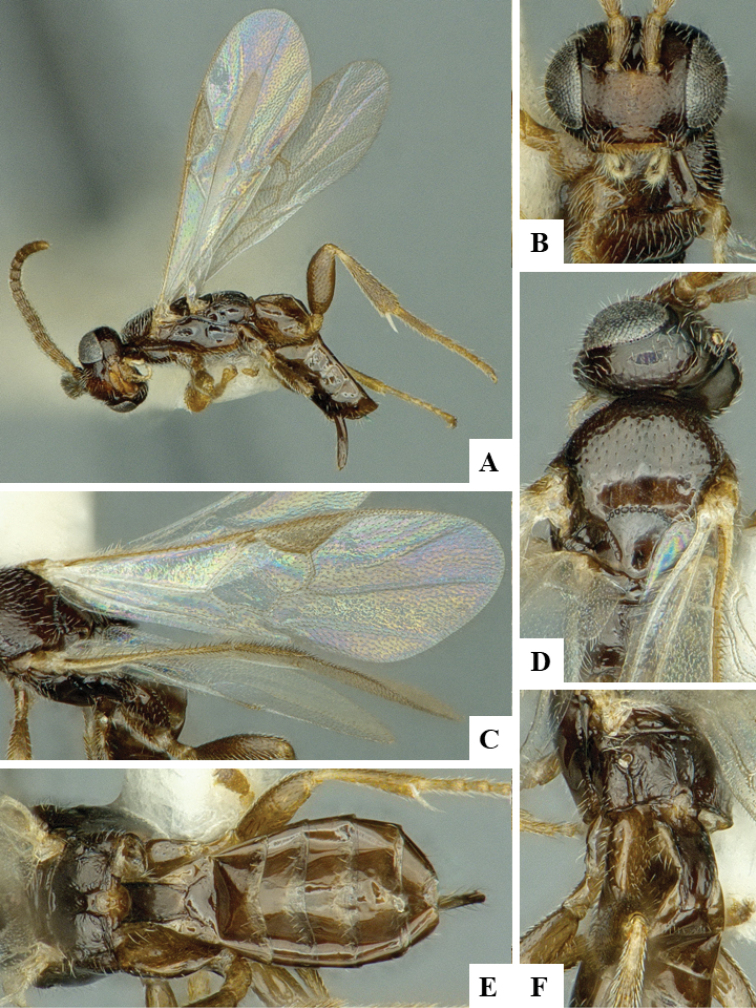
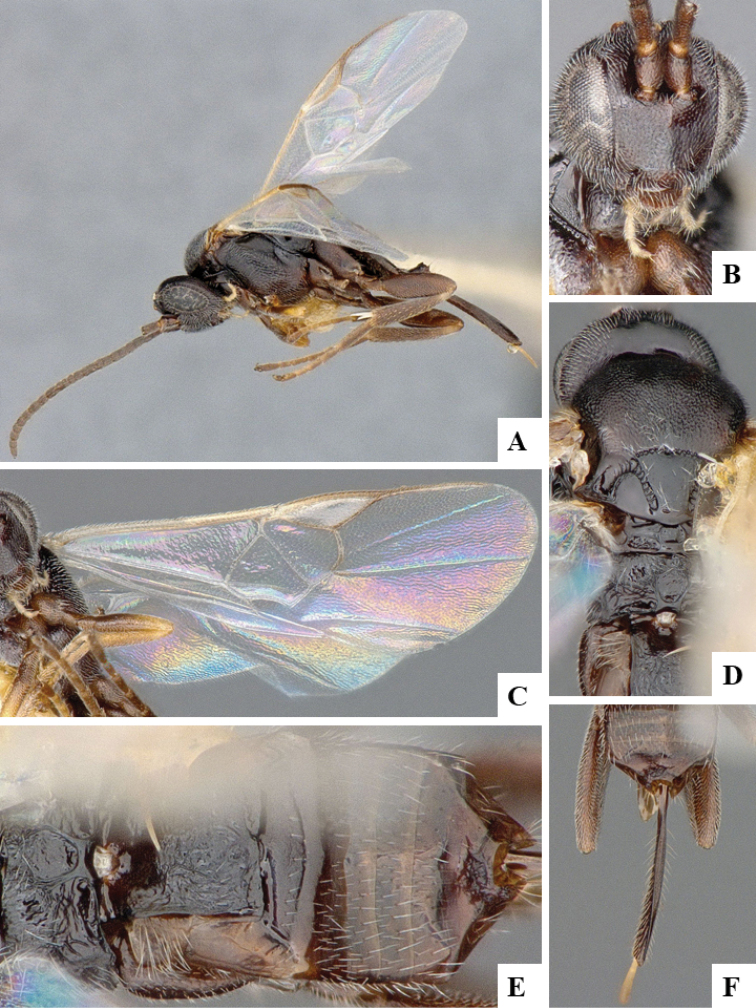
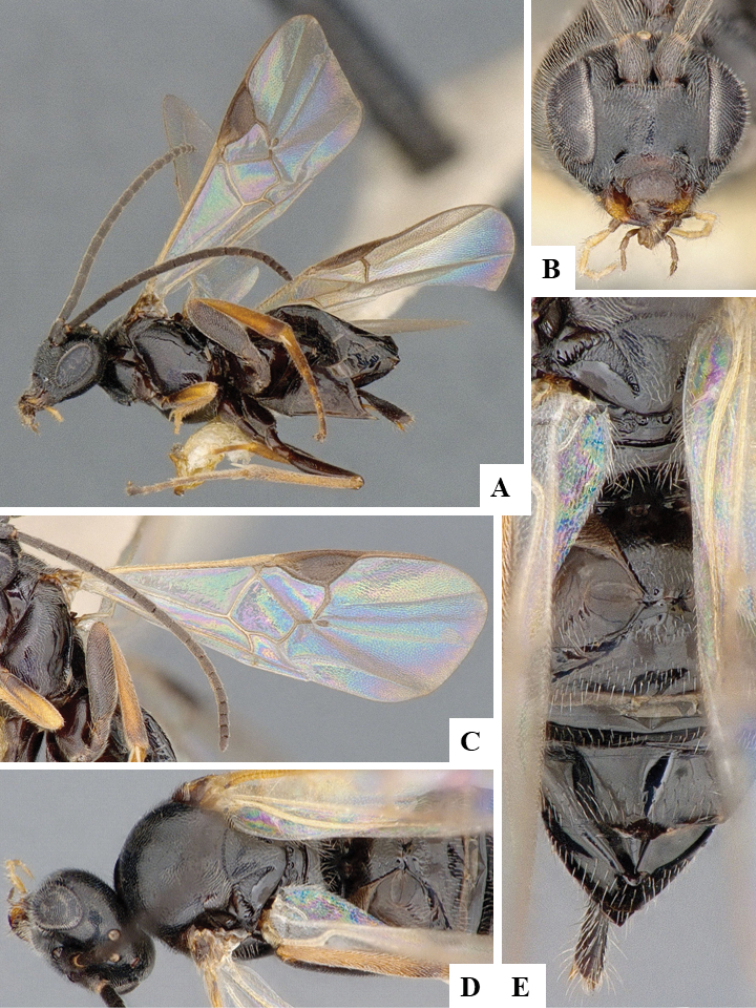
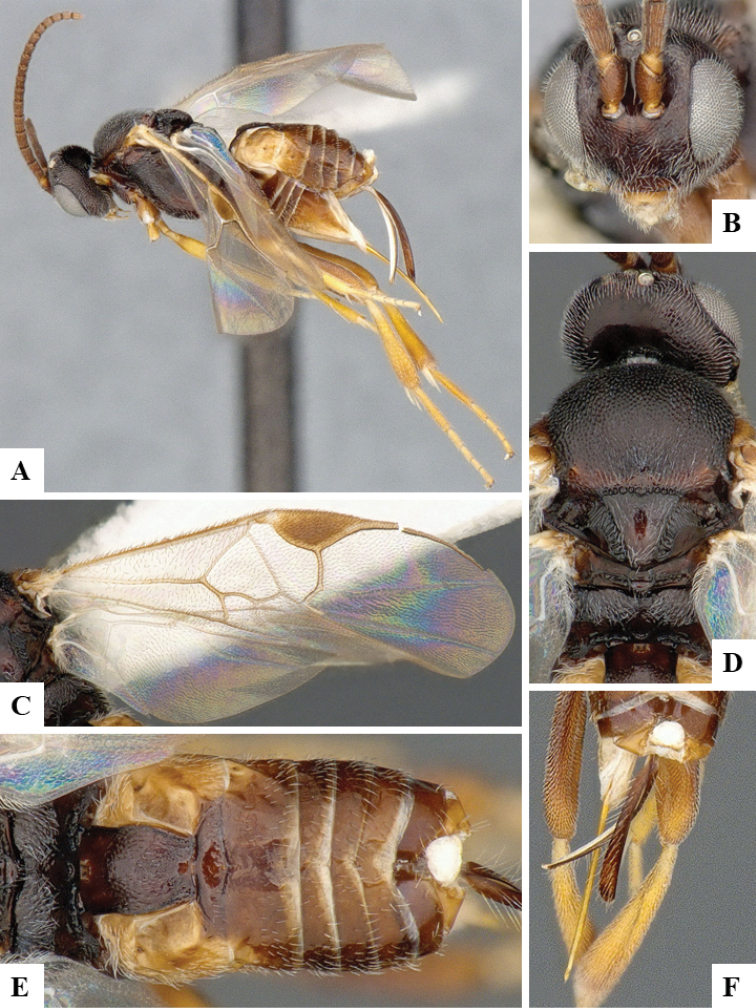
Photographs were taken with either a Keyence VHX-1000 Digital Microscope or with a Leica camera on a Leica M165 C Microscope, using lenses with a range of 10–130 ×. Multiple images were taken of a structure through the focal plane and then combined to produce a single in-focus image using the software associated with the Keyence System or, for the images taken with the Leica camera, the Zerene Stacker program (http://zerenesystems.com/cms/stacker). Images were corrected using Adobe Photoshop CS4 and Gimp 2.10.12; the plates were prepared using Microsoft PowerPoint 2010 and later saved as .tiff files. For seven figures in our paper we used other sources, all of which are acknowledged in the corresponding figure caption and in the Acknowledgements section below.
In the Results section, we discuss several topics concerning Microgastrinae before providing the checklist of world species. These include a detailed explanation of the generic concepts used here, geographical patterns, general overview of host data in the subfamily, extinct taxa, and limitations of both Taxapad and our checklist. It is very important to understand the limitations, as the user must be aware of the areas where Taxapad and/or our list lack strong support, e.g., critical review of host data, and/or missing information, such as examination of primary types. Further, there will undoubtedly be some yet to be recognised synonymy. We hope future versions of our world checklist will address some of the shortcomings of the present one. We also hope to prepare an online version that is continuously updated, probably in the style of a similar effort currently outdated (http://microgastrinae.myspecies.info/).
Results
Overview of the present paper and its limitations
In the checklist below, a total of 81 genera and 2,999 extant species are recognized as valid, including 36 nominal species that are currently considered to be species inquirendae.
Two genera are synonymized under Apanteles: Cecidobracon Kieffer & Jörgensen, 1910 syn. nov., and Holcapanteles Cameron, 1905 syn. nov. Nine lectotypes are designated. A total of 318 new combinations, three new replacement names, three species name amendments, and seven species status revised are proposed. Additionally, three species names are treated as nomina dubia, and 52 species names are considered to be unavailable (including 14 as nomina nuda), listed at the end of the checklist.
Extinct taxa, only known as fossils (three genera and 12 species) are listed in a separate section below (Table 3).
Table 3.
Extinct genera and species of Microgastrinae, compiled from Yu et al. (2012, 2016) and Belokobylskij (2014).
| Genera only known from fossils | Species only known from fossils |
| Dacnusites Cockerell, 1921 | Apantelesconcinnus Statz, 1938 |
| Eocardiochiles Brues, 1933 | Apantelesmacrophthalmus Statz, 1938 |
| Palaeomicrogaster Belokobylskij, 2014 | Dacnusitesreductus Cockerell, 1921 |
| Dacnusitessepultus Cockerell, 1921 | |
| Eocardiochilesfritschii Brues, 1933 | |
| Microplitiselegans Timon-David, 1944 | |
| Microplitisprimordialis (Brues, 1906) | |
| Microplitisvesperus Brues, 1910 | |
| Semionisnixoni Tobias, 1987 | |
| Semioniswightensis Belokobylskij, 2014 | |
| Snelleniussuccinalis Brues, 1933 | |
| Palaeomicrogasteroculatus Belokobylskij, 2014 |
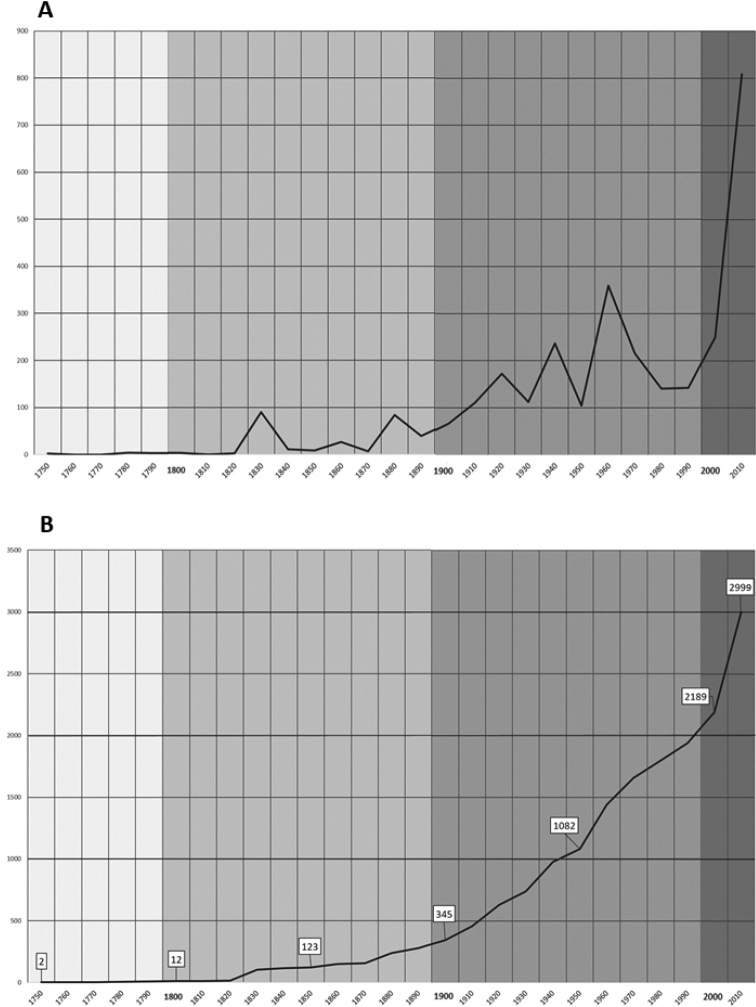
The pace of species description in Microgastrinae has been steadily increasing since the first species was described in 1758 and has shown no signs of slowing down (Fig. 1). The total number of genera has also increased substantially, especially since 1965; the information is summarized in Whitfield et al. (2018), Fernandez-Triana and Boudreault (2018), and below.
Figure 1.
Microgastrinae species described since 1758 based on data in present paper A Total numbers per decade B Cumulative number (1758–2019).
Primary types of Microgastrinae are deposited in 108 institutions worldwide, although 76% of those types are concentrated in seventeen collections (Table 1), seven of which have more than 100 primary types each. Localities of primary types are reported from 138 different countries.
Table 1.
World collections with the largest numbers of primary types of Microgastrinae (data from valid species as recognized in the present paper).
| Collection code | Country | Number of primary types |
| NHMUK | UK | 491 |
| CNC | Canada | 476 |
| USNM | USA | 380 |
| ZJUH | China | 160 |
| RMCA | Belgium | 122 |
| ZIN | Russia | 113 |
| HNHM | Hungary | 108 |
| MNHN | France | 84 |
| FAFU | China | 63 |
| ANIC | Australia | 52 |
| SIZK | Ukraine | 44 |
| ZMHB | Germany | 40 |
| MACN | Argentina | 36 |
| RMNH | The Netherlands | 35 |
| AEIC | USA | 32 |
| EIHU | Japan | 29 |
| HUNAU | China | 29 |
Microgastrine wasps have been recorded in most countries and all continents except Antarctica. Only 16 countries do not yet have any recorded species of Microgastrinae: Bahrain, Botswana, Bhutan, Cambodia, Djibouti, Equatorial Guinea, Gabon, Gambia, Guinea, Guinea-Bissau, Kuwait, Laos, Liberia, Mauritania, Qatar, and Swaziland. This is of course just an artifact of insufficient collecting and/or lack of studies in those countries; each is expected to harbour many species.
The current data (Table 2) show two countries with 400+ Microgastrinae species each (China with 448 and Costa Rica with 427), another two with 300+ species each (Russia with 388 and Hungary with 327) and five with 200+ species each (USA, Germany, India, United Kingdom, and Canada). Overall, 34 countries have more than 100 described species recorded, although those numbers can be misleading. For example, the reason Hungary ranks so high is because of extensive studies in that country, done over many years by Jenö Papp while working in the Hungarian Natural History Museum. A similar situation applies to both the United Kingdom and Germany, where a long European tradition of experts on the group coupled with extensive collecting have provided figures that are close to the actual diversity in those countries. While the microgastrine fauna of those three countries is relatively well known, the opposite occurs in large and/or mostly tropical countries, where more species are still undescribed. For example, in Costa Rica, DNA barcoding has already identified more than 1,200 species just in ACG (Janzen and Hallwachs 2016). And the figures for China and India (which are considered to be “megadiverse countries”, sensuMyers et al. 2000), are still very far from being complete, as both countries should easily reach more than 1,000 species each. Other megadiverse countries such as Australia, Brazil, Colombia, Democratic Republic of Congo, Indonesia, Madagascar, Mexico, Peru, Malaysia, Papua New Guinea and USA are all likely to have similar (in some cases higher) totals, but studies thus far have been insufficient, leading to most of those countries having “only” a hundred species or fewer recorded at present.
Table 2.
Alphabetic list of countries with described species of Microgastrinae (data based on this paper). Countries with political units located in different biogeographical regions (mostly islands) have species recorded from those entities listed separately below; those species are not added to the total for the country to which the entities belong politically.
| Countries | No. of Species | Countries | No. of Species |
|---|---|---|---|
| Afghanistan | 20 | Lithuania | 70 |
| Albania | 7 | Luxembourg | 1 |
| Algeria | 7 | Macedonia | 37 |
| Andorra | 2 | Madagascar | 67 |
| Angola | 1 | Malawi | 11 |
| Argentina | 68 | Malaysia | 70 |
| Armenia | 105 | Mali | 1 |
| Australia | 129 | Malta | 18 |
| Austria | 97 | Mauritius | 12 |
| Azerbaijan | 126 | Mexico | 54 |
| Bahamas | 1 | Moldova | 113 |
| Bangladesh | 11 | Mongolia | 161 |
| Barbados | 2 | Montenegro | 23 |
| Belarus | 23 | Morocco | 14 |
| Belgium | 61 | Mozambique | 7 |
| Belize | 7 | Myanmar | 9 |
| Benin | 3 | Namibia | 1 |
| Bolivia | 10 | Nepal | 6 |
| Bosnia and Herzegovina | 6 | Netherlands | 105 |
| Brazil | 120 | Netherlands (Netherlands Antilles) | 1 |
| Brunei | 1 | New Zealand | 27 |
| Bulgaria | 128 | Nicaragua | 5 |
| Burkina Faso | 1 | Niger | 1 |
| Burundi | 1 | Nigeria | 16 |
| Cape Verde | 32 | Norway | 15 |
| Cameroon | 13 | Oman | 1 |
| Canada | 213 | Pakistan | 20 |
| Central African Republic | 2 | Panama | 22 |
| Chad | 1 | Papua New Guinea | 47 |
| Chile | 21 | Paraguay | 10 |
| Chile (Juan Fernández Islands) | 2 | Peru | 39 |
| China | 448 | Philippines | 90 |
| Colombia | 31 | Poland | 170 |
| Comoros | 1 | Portugal | 7 |
| Democratic Republic of Congo | 135 | Portugal (Azores) | 3 |
| Costa Rica | 427 | Portugal (Madeira Islands) | 14 |
| Croatia | 70 | Portugal (Selvagens Islands) | 2 |
| Cuba | 20 | Romania | 174 |
| Cyprus | 11 | Russia | 388 |
| Czech Republic | 90 | Rwanda | 59 |
| Denmark | 20 | Saint Kitts & Nevis | 2 |
| Dominica | 3 | Saint Lucia | 2 |
| Dominican Republic | 5 | Saint Vincent | 18 |
| Ecuador | 101 | Saudi Arabia | 2 |
| Egypt | 12 | Senegal | 51 |
| El Salvador | 1 | Serbia | 95 |
| Eritrea | 3 | Sierra Leone | 3 |
| Estonia | 12 | Singapore | 11 |
| Ethiopia | 11 | Slovakia | 161 |
| Fiji | 29 | Slovenia | 18 |
| Findland | 162 | Solomon Islands | 5 |
| France | 122 | Somalia | 2 |
| France (French Guiana) | 6 | South Africa | 98 |
| France (Guadeloupe) | 2 | Spain | 103 |
| France (Marquesas Islands) | 1 | Spain (Canary Islands) | 18 |
| France (Réunion) | 34 | Sri Lanka | 37 |
| France (Society Islands) | 2 | Sudan | 8 |
| Gambia | 1 | Suriname | 5 |
| Georgia | 73 | Sweden | 121 |
| Germany | 248 | Switzerland | 166 |
| Ghana | 6 | Syria | 2 |
| Greece | 92 | Tajikistan | 42 |
| Grenada | 15 | Tanzania | 23 |
| Guatemala | 6 | Thailand | 30 |
| Guyana | 12 | Togo | 3 |
| Haiti | 2 | Tonga | 2 |
| Honduras | 8 | Trinidad & Tobago | 19 |
| Hungary | 327 | Tunisia | 40 |
| Iceland | 5 | Turkey | 173 |
| India | 245 | Turkmenistan | 63 |
| Indonesia | 63 | Uganda | 35 |
| Iran | 109 | Ukraine | 154 |
| Iraq | 2 | United Arab Emirates | 3 |
| Ireland | 81 | United Kingdom | 242 |
| Israel | 72 | United Kingdom (British Virgin Islands) | 1 |
| Italy | 149 | United Kingdom (Saint Helena) | 1 |
| Ivory Coast | 16 | United States | 299 |
| Jamaica | 6 | United States (American Samoa) | 3 |
| Japan | 96 | United States (Hawaiian Islands) | 14 |
| Japan (Ryukyu Islands) | 7 | United States (USA Virgin Islands) | 1 |
| Jordan | 10 | Uruguay | 11 |
| Kazakhstan | 121 | Uzbekistan | 72 |
| Kenya | 30 | Vanuatu | 8 |
| Korea | 130 | Venezuela | 21 |
| Kyrgyzstan | 18 | Vietnam | 137 |
| Latvia | 37 | Western Samoa | 10 |
| Lebanon | 2 | Yemen | 17 |
| Lesotho | 1 | Zambia | 3 |
| Libya | 2 | Zimbabwe | 7 |
There are three main limitations in our paper that we want to point out. The first relates to the coverage of primary types in our study. We were able to examine primary types for 1,394 species (46.5%), and for another 1,568 species (52.3%) we studied authenticated specimens, checked original descriptions, or read subsequent revisions. However, for 37 species (1.2%) we could not check any source of information, or it was considered inadequate, and they are left in the genus in which they were originally described (or as species inquirendae), with explanatory annotations. In future versions, we aim to increase the number of species for which we have examined primary types, but for the present paper the reader must consider the relatively large number of species still needing to be thoroughly studied. It is especially important to keep in mind that for some of those species for which we could only study descriptions (which may not be detailed or clear enough), the generic placement made in this paper might be incorrect.
A second limitation is the coverage of references concerning Microgastrinae. In the References section we tried to list all papers where original descriptions of Microgastrinae were published (those references in turn are cited under the corresponding treatment of every species in the checklist below). However, our list is not complete and we are aware of some omissions; in that sense, the latest versions of Taxapad (Yu et al. 2012, 2016) have more comprehensive lists of references than our paper. Especially important and comprehensive is Yu et al. (2016), which lists 6,200+ references related to Microgastrinae.
A third limitation of our paper is that we do not treat host records in detail. We expect to present host data for microgastrine species with verified information in a subsequent version of the world checklist, although it is improbable that we will be able to comment with reliability on all published records. The latest versions of Taxapad (Yu et al. 2012, 2016) provide the best coverage of references on hosts of Microgastrinae; however, that is only an uncritical compilation of literature, and many of those references report incorrect data. The reader is strongly advised to double check host references and be very cautious in interpreting information from secondary sources.
Fossil Microgastrinae taxa
Extinct genera and species of Microgastrinae have been found in Eocene and Oligocene deposits, from 37–44 million years ago (MYA). Many specimens from the Miocene (20–30 MYA) are known from Dominican and Chiapas ambers, but most appear to be undescribed representatives of extant genera (Murphy et al. 2008). Belokobylskij (2014) revised the taxonomic status of all previously known taxa of fossil Microgastrinae and described one new genus as well as two new species. The origin of Microgastrinae has been estimated at ~ 54 MYA (Murphy et al. 2008).
Unlike previous work (Mason 1981, Yu et al. 2005, 2012, 2016), we exclude fossil genera or species from our world checklist. Instead, we tabulate in this section the three genera and 12 species of fossil Microgastrinae currently described (Table 3).
Generic limits and taxonomic arrangement of the subfamily Microgastrinae
Microgastrinae was originally described at family rank, as ‘Microgasteroidae’, by Foerster (1863). At that time, it comprised only three genera: Microgaster Latreille, 1804 (the genus that provides the root for the subfamily name, meaning “small abdomen”, in reference to the relatively short length of the metasoma compared to other Braconidae), as well as two genera described by Foerster (1863): Microplitis (which means “small sword” or “small weapon”, referring to the generally short ovipositor in that genus) and Apanteles (meaning “incomplete”, in reference to the fore wing lacking the second intercubitus, leaving the second submarginal cell open or incomplete). Fornicia, although described by Brullé (1846) before Foerster’s work, was at the time considered to belong to other subfamilies in Braconidae (e.g., Dalla Torre (1898) placed the genus in Cheloninae; Ashmead (1900a) placed it in Sigalphinae; Granger (1949) placed it in Triaspidinae), and it was not recognized to be part of Microgastrinae until a century later (Baltazar 1962, Nixon 1965).
The high diversity of Microgastrinae quickly became evident, and so attempts to split the group into further genera started shortly after Foerster’s (1863) paper, e.g., by Reinhard (1880). Many additional genera (15 recognized in this paper) were described between 1882 and 1958, although some were not associated with Microgastrinae at the time, and others were not accepted as valid genera by some authors of the period, e.g., Muesebeck (1921) and Telenga (1955).
This view changed with two seminal works in 1965 and 1981. Nixon (1965) reclassified the subfamily limits and provided some structure to what was being recognized as a huge assemblage of parasitoids of Lepidoptera. He recognized 20 genera, eight of which were new, and reclassified the species within Apantelessensu lato into a more practical and useful array of 44 species groups to facilitate identification. Mason (1981) fundamentally changed the taxonomy of Microgastrinae by recognizing 50 genera (23 of which he described as new), including numerous taxa that mostly corresponded to particular species groups of Nixon (1965, 1973), and additionally proposing new combinations for some 350 species.
Since Mason (1981) 32 genera have been described. Whitfield et al. (2018: fig. 2) graphically showed the increase in description of new genera during the past 150 years. Nevertheless, there are still many more genera of Microgastrinae that remain to be described, e.g., Fernandez-Triana and Boudreault (2018). Additionally, several genera, as currently understood, are probably polyphyletic and need to be split, e.g., Diolcogaster and Glyptapanteles. A comprehensive phylogenetic analysis of the subfamily is needed before we can achieve a clearer picture. However, just based on the material we have seen in collections, we estimate that the Microgastrinae is likely to comprise close to one hundred genera.
For the past few years the main problem with the generic concepts is that two different classifications of Microgastrinae have been proposed and are widely used: those based on Mason (1981) and on van Achterberg (2003). For a visual depiction of how the two classifications differ (based on the number of species assigned to each of the most speciose genera), see Figure 2.
Figure 2.
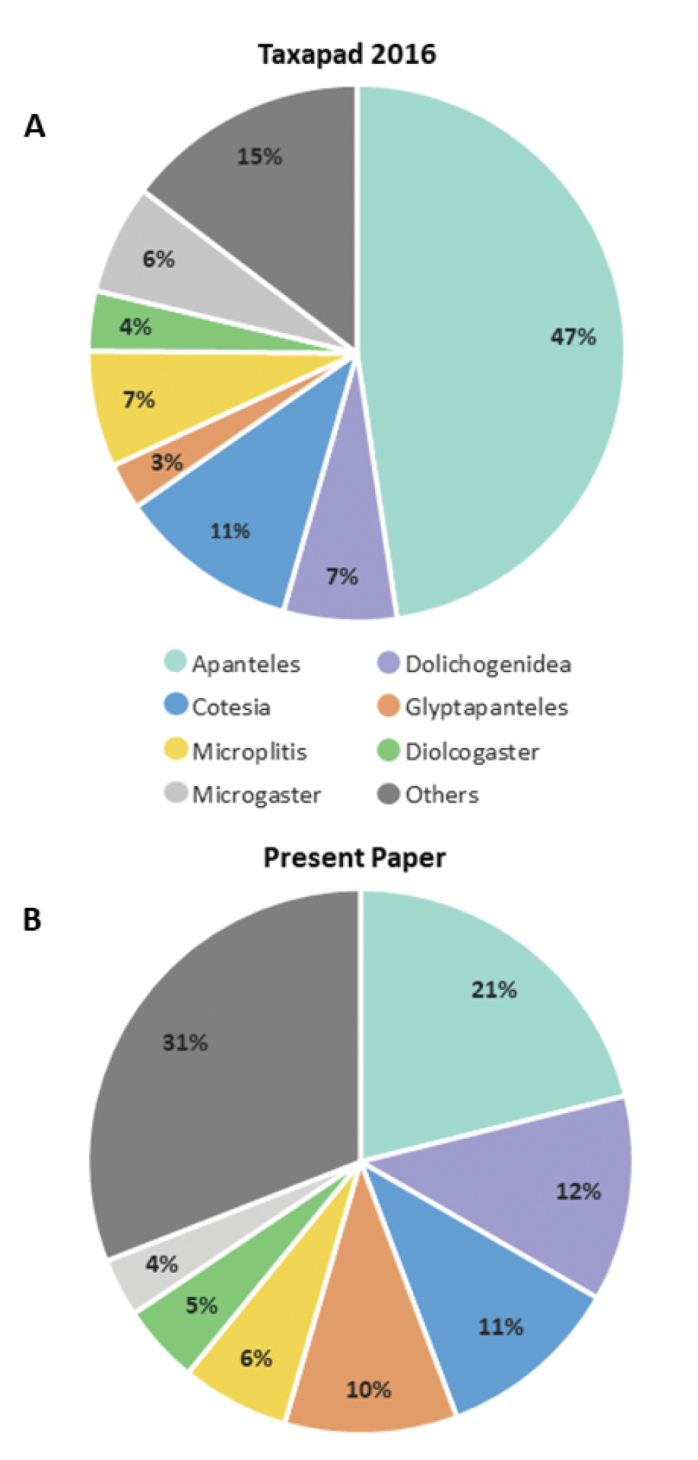
Number of extant species per larger genera of MicrogastrinaeA Data from Taxapad 2016, which is mostly an update, with slight modifications, of van Achterberg (2003), total number of species: 2,710 B Data from present paper, which is mostly based on Mason (1981) but extensively updated, total number of species: 2,999.
The classification proposed by Mason (1981) had a narrower concept of Apanteles and Protapanteles, which resulted in a larger number of Microgastrinae genera treated as valid. Many of the new combinations resulting from that classification are in Mason (1981), although not all species have been properly transferred to the corresponding genus. Mason’s system has been followed by most researchers (see examples cited in the Introduction) and has remained largely stable for the past 38+ years, with a few exceptions: his genus Teremys was synonymized under Pholetesor (Whitfield 2006); and his arrangement of genera within tribes, largely based on phylogenetic grounds, has not been universally accepted (Austin 1990, Austin and Dangerfield 1992, Whitfield 1995a, Fernandez-Triana 2010; see also Walker et al. 1990 for further criticism of tribes within Microgastrinae). Mason (1981) based his paper on studies of the world fauna; however, a careful examination of the CNC collection (Mason’s base) and other material available to him at the time shows that specimens from the Afrotropical, Oriental, and Australasian regions were much more poorly represented than the remaining regions. Thus, most of the new genera from those regions described by Mason (1981) have later been found to have a wider distribution and greater morphological variation than originally thought, and some of those genera will need redefinition. Another consequence of the limited geographical coverage of the studied specimens is that the keys to tribes and genera in Mason (1981) work reasonably well for the temperate areas, but not as well for the tropical areas, especially the Old World tropics.
The classification proposed by van Achterberg (2003) reduced the number of genera by treating eleven genera recognised by Mason as synonyms or subgenera of Apanteles and Protapanteles. That system was later implemented in Taxapad (Yu et al. 2005, 2012, 2016) and other, mostly European, databases, e.g., Fauna Europaea (https://fauna-eu.org/) and Dyntaxa (https://www.dyntaxa.se/). Shortcomings of this approach have been pointed out by other authors, e.g., Broad et al. (2016) and Whitfield et al. (2018). The main issue with van Achterberg’s approach is that his classification was based mainly on the European species, a region with relatively little diversity in genera and species (see sections below), and is thus clearly insufficient to capture the rich fauna of Microgastrinae worldwide. Second, and more worrisome, van Achterberg’s generic concepts were applied in Taxapad to the entire world fauna, effectively producing numerous (perhaps hundreds) of new name combinations which have never been formally published, let alone critically assessed. The validity of those names may be questionable, but van Achterberg’s classification has been embraced uncritically by some users of Taxapad.
To complicate things further, generic concepts changed slightly in Taxapad from the 2012 to the 2016 version (Table 4). For example, Taxapad 2016 considers some taxa as subgenera that the 2012 version had listed as synonyms of Apanteles (Dolichogenidea, Exoryza, Iconella, Illidops, and Pholetesor) or as synonyms of Protapanteles (Nyereria, Rasivalva, Sathon, and Venanides). Other genera were treated differently, e.g., Distatrix is treated as a valid genus in the 2012 version but as a subgenus of Protapanteles in 2016, and Glyptapanteles is a synonym of Protapanteles in 2012 but a valid genus in 2016. Some of those decisions may have merit, but three are highly questionable:
Table 4.
Microgastrinae arrangement (genera, subgenera, subtribes, and tribes) used in the 2012 and 2016 versions of Taxapad (Yu et al. 2012, 2016) and the present paper. Each column is independent of the others, so the lists must be read vertically only, as they are not comparable horizontally.
| Taxapad 2012 | Taxapad 2016 | Present paper |
|---|---|---|
| MICROGASTRINAE Foerster, 1862 | MICROGASTRINAE Foerster, 1863 | MICROGASTRINAE Foerster, 1863 |
| MICROGASTRINI Foerster, 1863 | (No tribes) | |
| APANTELINI Viereck, 1918 | APANTELINA Viereck, 1918 | (No subtribes) |
| Alphomelon Mason, 1981 | Alphomelon Mason, 1981 | Agupta Fernandez-Triana, 2018 |
| Apanteles (Apanteles) Foerster, 1862 | Apanteles (Apanteles) Foerster, 1863 | Alloplitis Nixon, 1965 |
| Dolichogenidea Viereck, 1911 | Napamus Papp, 1993 | Alphomelon Mason, 1981 |
| Iconella Mason, 1981 | Apanteles (Choeras) Mason, 1981 | Apanteles Foerster, 1863 |
| Illidops Mason, 1981 | Apanteles (Dolichogenidea) Viereck, 1911 | Austinicotesia Fernandez-Triana, 2018 |
| Napamus Papp, 1993 | Apanteles (Exoryza) Mason, 1981 | Austrocotesia Austin & Dangerfield, 1992 |
| Apanteles (Choeras) Mason, 1981 | Apanteles (Iconella) Mason, 1981 | Beyarslania Koçak & Kemal, 2009 |
| Apanteles (Exoryza) Mason, 1981 | Apanteles (Illidops) Mason, 1981 | Billmasonius Fernandez-Triana, 2018 |
| Austrocotesia Austin & Dangerfield, 1992 | Apanteles (Pholetesor) Mason, 1981 | Buluka de Saeger, 1948 |
| Exulonyx Mason, 1981 | Austrocotesia Austin & Dangerfield, 1992 | Carlmuesebeckius Fernandez-Triana, 2018 |
| Miropotes Nixon, 1965 | Dasylagon Muesebeck, 1958 | Chaoa Luo & You, 2004 |
| Papanteles Mason, 1981 | Exulonyx Mason, 1981 | Choeras Mason, 1981 |
| Parapanteles Ashmead, 1900 | Miropotes Nixon, 1965 | Clarkinella Mason, 1981 |
| Pelicope Mason, 1981 | Papanteles Mason, 1981 | Cotesia Cameron, 1891 |
| Pholetesor Mason, 1981 | Parapanteles Ashmead, 1900 | Cuneogaster Choi & Whitfield, 2006 |
| Promicrogaster Brues & Richardson, 1913 | Promicrogaster Brues & Richardson, 1913 | Dasylagon Muesebeck, 1958 |
| Sendaphne Nixon, 1965 | Sendaphne Nixon, 1965 | Deuterixys Mason, 1981 |
| Xanthapanteles Whitfield, 1995 | Xanthapanteles Whitfield, 1995 | Diolcogaster Ashmead, 1900 |
| COTESIINI Mason, 1981 | COTESIINA Mason, 1981 | Distatrix Mason, 1981 |
| Buluka de Saeger, 1948 | Buluka de Saeger, 1948 | Dodogaster Rousse, 2013 |
| Chaoa Luo & You, 2004 | Chaoa Luo & You, 2004 | Dolichogenidea Viereck, 1911 |
| Cotesia Cameron, 1891 | Cotesia Cameron, 1891 | Eripnopelta Xiong, van Achterberg & Chen, 2017 |
| Cuneogaster Choi & Whitfield, 2006 | Cuneogaster Choi & Whitfield, 2006 | Exix Mason, 1981 |
| Deuterixys Mason, 1981 | Deuterixys Mason, 1981 | Exoryza Mason, 1981 |
| Diolcogaster Ashmead, 1900 | Diolcogaster Ashmead, 1900 | Exulonyx Mason, 1981 |
| Distatrix Mason, 1981 | Exix Mason, 1981 | Fornicia Brullé, 1846 |
| Exix Mason, 1981 | Glyptapanteles Ashmead, 1904 | Gilbertnixonius Fernandez-Triana, 2018 |
| Larissimus Nixon, 1965 | Larissimus Nixon, 1965 | Glyptapanteles Ashmead, 1904 |
| Lathrapanteles Williams, 1985 | Lathrapanteles Williams, 1985 | Hygroplitis Thomson, 1895 |
| Parenion Nixon, 1965 | Nyereria Mason, 1981 | Hypomicrogaster Ashmead, 1898 |
| Protapanteles (Protapanteles) Ashmead, 1898 | Parenion Nixon, 1965 | Iconella Mason, 1981 |
| Glyptapanteles Ashmead, 1904 | Protapanteles (Protapanteles) Ashmead, 1898 | Illidops Mason, 1981 |
| Protapanteles (Nyereria) Mason, 1981 | Protapanteles (Distatrix) Mason, 1981 | Janhalacaste Fernandez-Triana, 2018 |
| Protapanteles (Rasivalva) Mason, 1981 | Protapanteles (Rasivalva) Mason, 1981 | Jenopappius Fernandez-Triana, 2018 |
| Protapanteles (Sathon) Mason, 1981 | Protapanteles (Sathon) Mason, 1981 | Jimwhitfieldius Fernandez-Triana, 2018 |
| Protapanteles (Venanides) Mason, 1981 | Protapanteles (Venanides) Mason, 1981 | Keylimepie Fernandez-Triana, 2016 |
| Protomicroplitis Ashmead, 1898 | Protomicroplitis Ashmead, 1898 | Kiwigaster Fernandez-Triana, Ward & Whitfield, 2011 |
| Pseudovenanides Xiao & You, 2002 | Pseudovenanides Xiao & You, 2002 | Kotenkosius Fernandez-Triana, 2018 |
| Venanus Mason, 1981 | Venanus Mason, 1981 | Larissimus Nixon, 1965 |
| Wilkinsonellus Mason, 1981 | Wilkinsonellus Mason, 1981 | Lathrapanteles Williams, 1985 |
| MICROGASTRINI Foerster, 1862 | MICROGASTRINA Foerster, 1863 | Mariapanteles Whitfield & Fernandez-Triana, 2012 |
| Beyarslania Koçak & Kemal, 2009 | Beyarslania Koçak & Kemal, 2009 | Markshawius Fernandez-Triana, 2018 |
| Cecidobracon Kieffer & Jörgensen, 1910 | Cecidobracon Kieffer & Jörgensen, 1910 | Microgaster Latreille, 1804 |
| Clarkinella Mason, 1981 | Clarkinella Mason, 1981 | Microplitis Foerster, 1863 |
| Dasylagon Muesebeck, 1958 | Ectadiophatnus Cameron, 1913 | Miropotes Nixon, 1965 |
| Ectadiophatnus Cameron, 1913 | Holcapanteles Cameron, 1905 | Napamus Papp, 1993 |
| Holcapanteles Cameron, 1905 | Hygroplitis Thomson, 1895 | Neoclarkinella Rema & Narendran, 1996 |
| Hygroplitis Thomson, 1895 | Hypomicrogaster Ashmead, 1898 | Nyereria Mason, 1981 |
| Hypomicrogaster Ashmead, 1898 | Lissogaster Bengtsson, 1926 | Ohenri Fernandez-Triana, 2018 |
| Lissogaster Bengtsson, 1926 | Mariapanteles Whitfield & Fernandez-Triana, 2012 | Papanteles Mason, 1981 |
| Microgaster Latreille, 1804 | Microgaster Latreille, 1804 | Parapanteles Ashmead, 1900 |
| Neoclarkinella Rema & Narendran, 1996 | Neoclarkinella Rema & Narendran, 1996 | Parenion Nixon, 1965 |
| Paroplitis Mason, 1981 | Paroplitis Mason, 1981 | Paroplitis Mason, 1981 |
| Prasmodon Nixon, 1965 | Prasmodon Nixon, 1965 | Pelicope Mason, 1981 |
| Pseudapanteles Ashmead, 1898 | Pseudapanteles Ashmead, 1898 | Philoplitis Nixon, 1965 |
| Rhygoplitis Mason, 1981 | Rhygoplitis Mason, 1981 | Pholetesor Mason, 1981 |
| Xanthomicrogaster Cameron, 1911 | Shireplitis Fernandez-Triana & Ward, 2013 | Prasmodon Nixon, 1965 |
| MICROPLITINI Mason, 1981 | Xanthomicrogaster Cameron, 1911 | Promicrogaster Brues & Richardson, 1913 |
| Alloplitis Nixon, 1965 | MICROPLITINI Mason, 1981 | Protapanteles Ashmead, 1898 |
| Microplitis Foerster, 1862 | Alloplitis Nixon, 1965 | Protomicroplitis Ashmead, 1898 |
| Philoplitis Nixon, 1965 | Microplitis Foerster, 1863 | Pseudapanteles Ashmead, 1898 |
| Snellenius Westwood, 1882 | Philoplitis Nixon, 1965 | Pseudofornicia van Achterberg, 2015 |
| FORNICIINI Mason, 1981 | Snellenius Westwood, 1882 | Pseudovenanides Xiao & You, 2002 |
| Fornicia Brullé, 1846 | FORNICIINI Mason, 1981 | Qrocodiledundee Fernandez-Triana, 2018 |
| SEMIONINI Tobias, 1987 | Fornicia Brullé, 1846 | Rasivalva Mason, 1981 |
| Semionis Nixon, 1965 | Pseudofornicia van Achterberg, 2015 | Rhygoplitis Mason, 1981 |
| Kiwigaster Fernandez-Triana, Whitfield & Ward, 2011 | SEMIONINI Tobias, 1987 | Sathon Mason, 1981 |
| Pelicope Mason, 1981 | Semionis Nixon, 1965 | |
| Semionis Nixon, 1965 | Sendaphne Nixon, 1965 | |
| Dodogaster Rousse, 2013 | Shireplitis Fernandez-Triana & Ward, 2013 | |
| Keylimepie Fernandez-Triana, 2016 | Snellenius Westwood, 1882 | |
| Kiwigaster Fernandez-Triana, Whitfield & Ward, 2011 | Silvaspinosus Fernandez-Triana, 2018 | |
| Tobleronius Fernandez-Triana, 2018 | ||
| Ungunicus Fernandez-Triana, 2018 | ||
| Venanides Mason, 1981 | ||
| Venanus Mason, 1981 | ||
| Wilkinsonellus Mason, 1981 | ||
| Xanthapanteles Whitfield, 1995 | ||
| Xanthomicrogaster Cameron, 1911 | ||
| Ypsilonigaster Fernandez-Triana, 2018 | ||
| Zachterbergius Fernandez-Triana, 2018 |
a) Rasivalva should never have been considered to be part of Protapanteles as it has a complete areolet in the fore wing (a character not present in any Protapanteles or related genera);
b) Ectadiophatnus is listed as a genus of Microgastrinae in both the 2012 and 2016 versions, following Shenefelt (1973), despite having been published as belonging to the subfamily Blacinae since at least 1935 (Ferrière 1935, Mani 1938, Varshney 1976, Mason 1981) [van Achterberg (pers. comm.) has examined the type species and found that it is a new synonym of Eubazus Nees, in Brachistinae-Brachistini];
c) the species listed under Lissogaster have since 1988 been transferred back to Microgaster (see more details about that in Mason (1986) and in the checklist below, in the introductory comments to the genus Microgaster).
The rationale for the changes between versions of Taxapad is not always evident and, as far as we are aware, has never been explained in a published paper. As a result, it is difficult to follow the different arrangements of genera and subgenera, a problem which is further compounded by the use of tribes in the 2012 version, while the 2016 version added sub-tribes (Table 4).
We believe that the classification proposed by Mason (1981), although not entirely free from problems and shortcomings, provides the best framework currently available to deal with the world diversity of Microgastrinae and provides a solid and clear foundation from which to work towards future improvements. In this paper we largely follow that system, except for dividing the subfamily into tribes, as we do not think the tribes proposed by Mason properly reflect the phylogenetic relationships within the subfamily. We here classify the world species in 81 genera of Microgastrinae (Table 4 and checklist below).
Brief diagnosis of all Microgastrinae genera as they are understood in this paper
The last two published keys to world genera of Microgastrinae were in Nixon (1965) and Mason (1981). Nixon (1965) recognized 19 genera in his key, whereas Mason (1981) included 50 genera (although Mason’s paper started with a key to tribes, and then genera within each tribe are keyed out and treated separately). Some regional generic keys have been published since, e.g., Tobias (1986) for the former Soviet Union, Austin and Dangerfield (1992) for the Australasian region, Whitfield (1997) for the New World, Chen and Song (2004) for China, and Kotenko (2007a) for the Russian Far East. However, with 81 genera considered in this paper, the information to recognize them in the aforementioned references is clearly outdated, and an updated key to world genera is badly needed.
Unfortunately, we still lack a robust phylogeny for the subfamily, which would be needed to provide a useful and comprehensive key. The limits of some genera at present are not well defined, and at times are contradictory; moreover, it is likely that future work will change many groups as currently understood. We anticipate that a few genera will end up as synonyms while several others, which are paraphyletic or polyphyletic as currently defined, will be split. This should likely result in an overall increase in the total number of genera as compared to present (e.g., see Fernandez-Triana and Boudreault 2018).
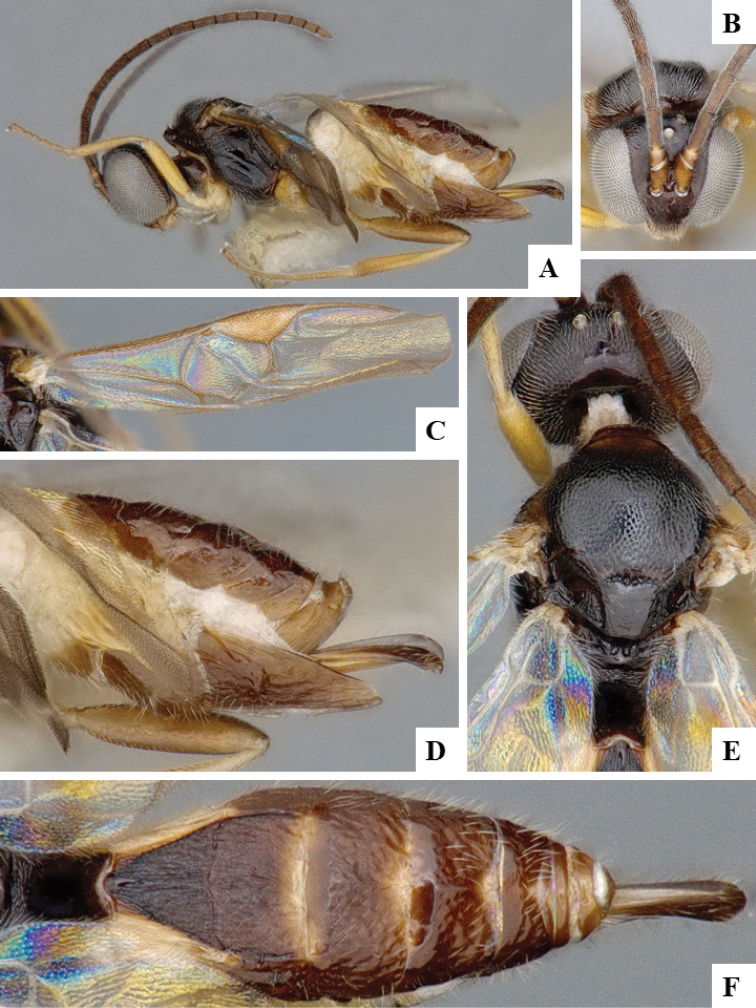
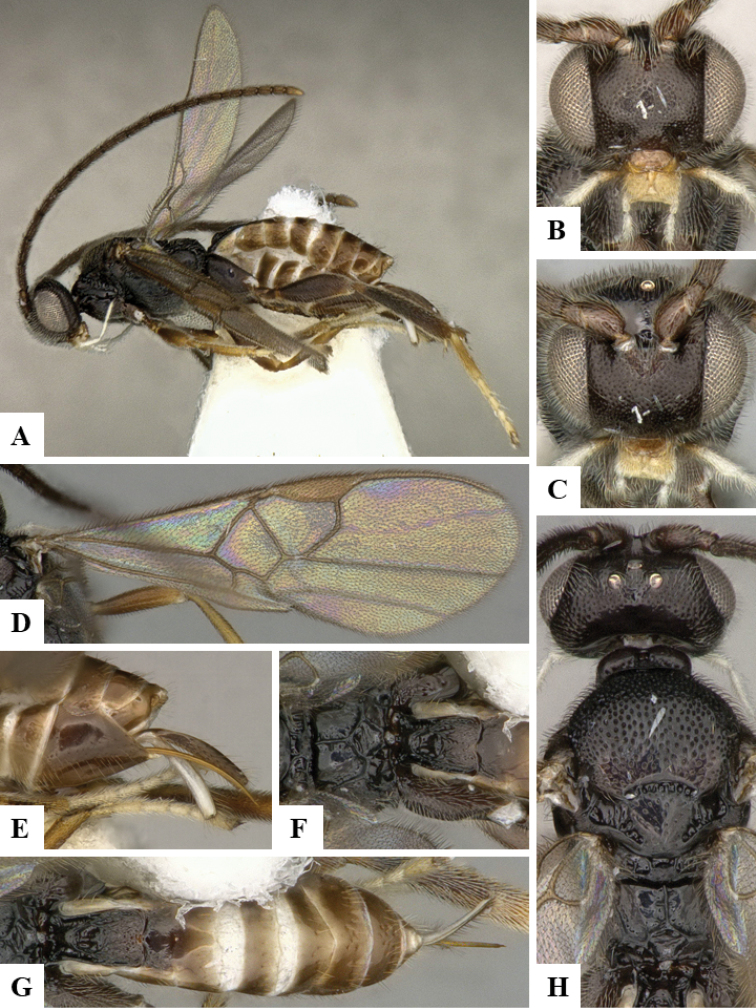
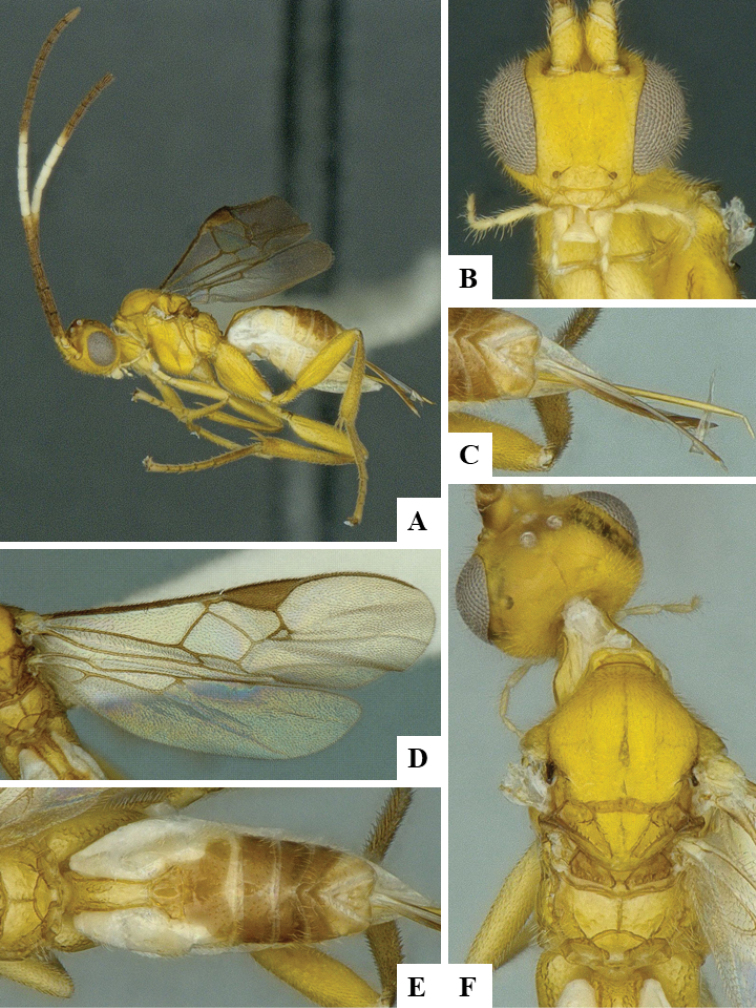
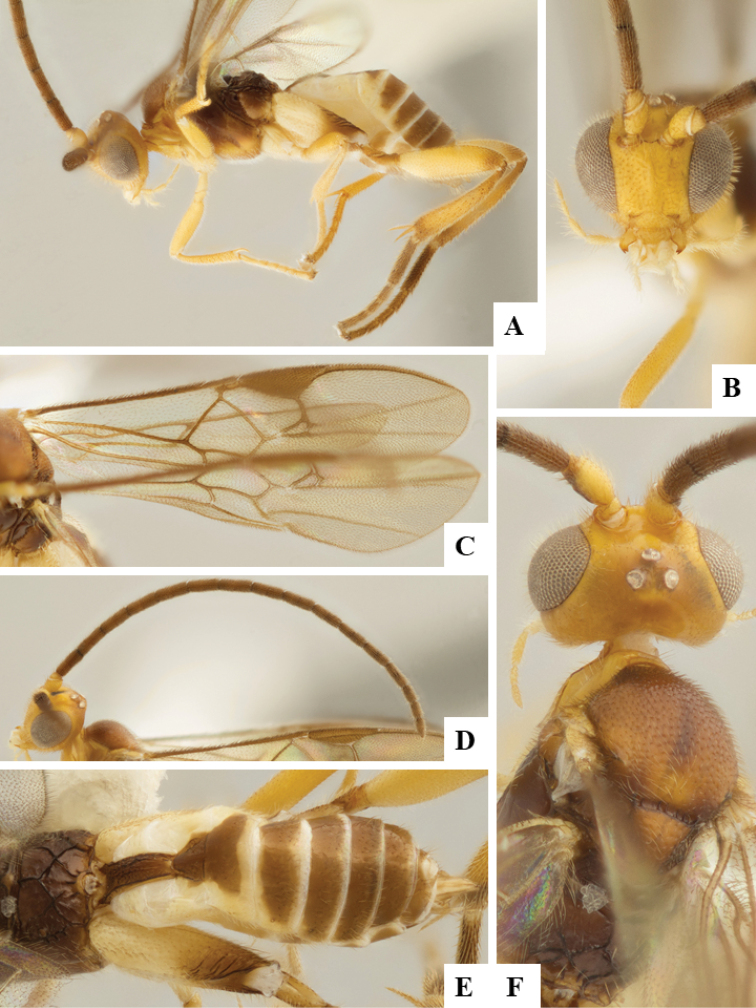
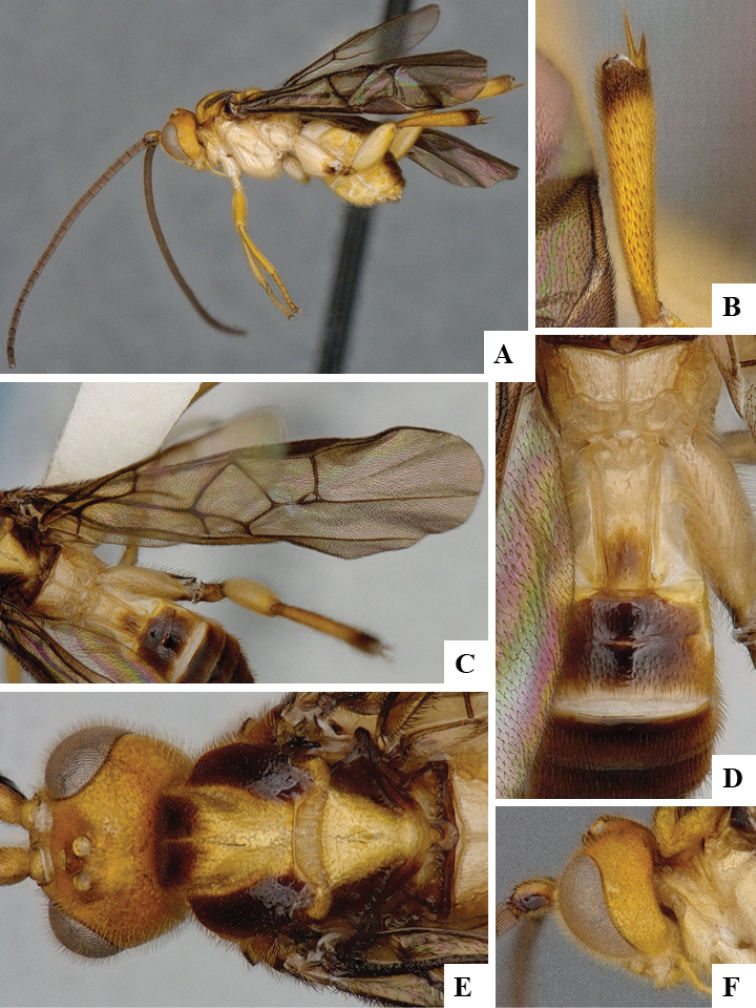
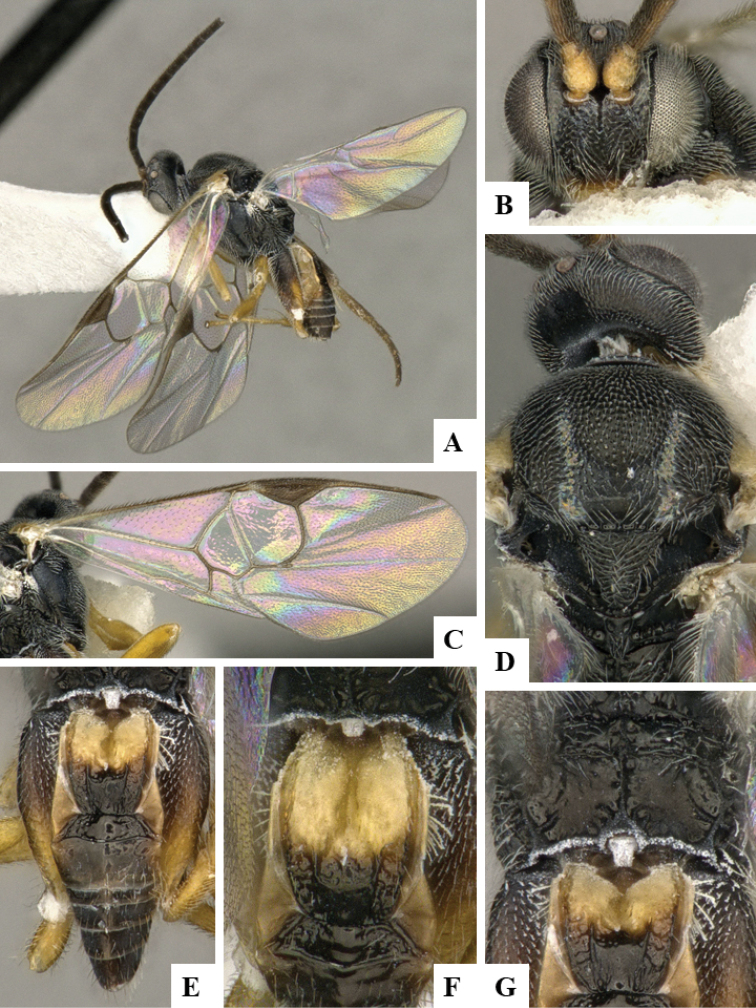
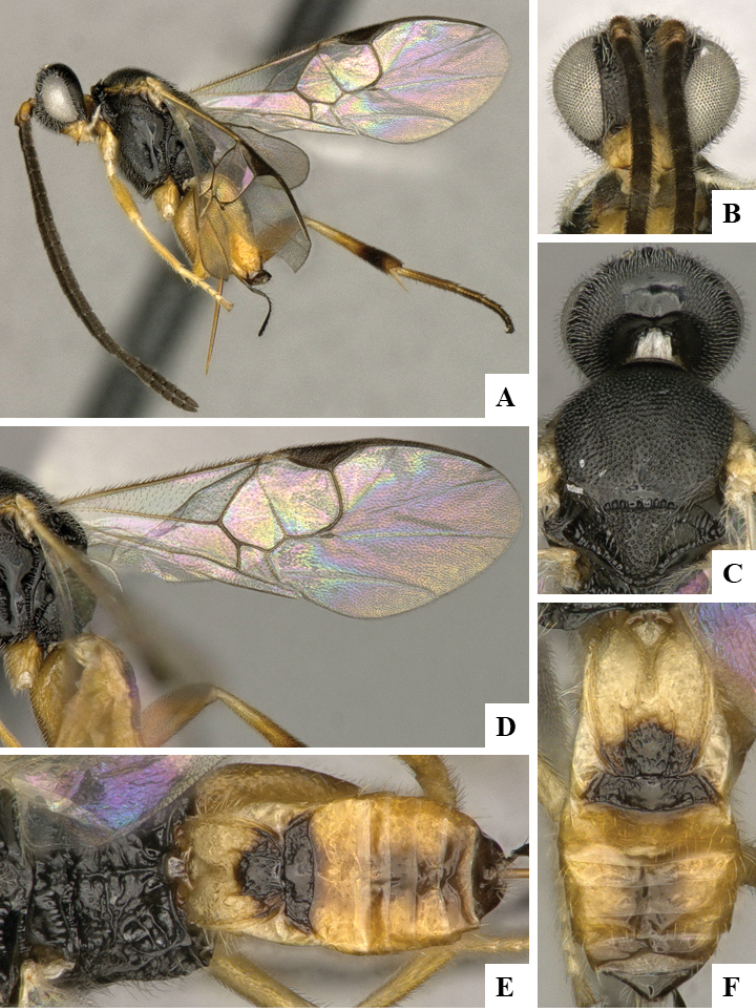
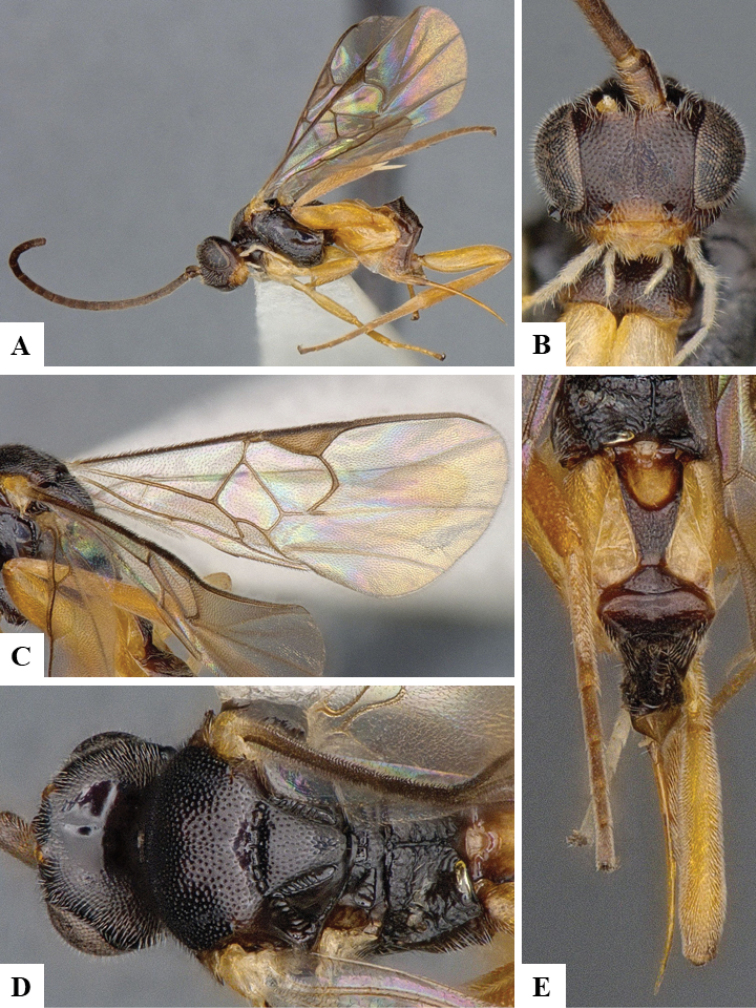
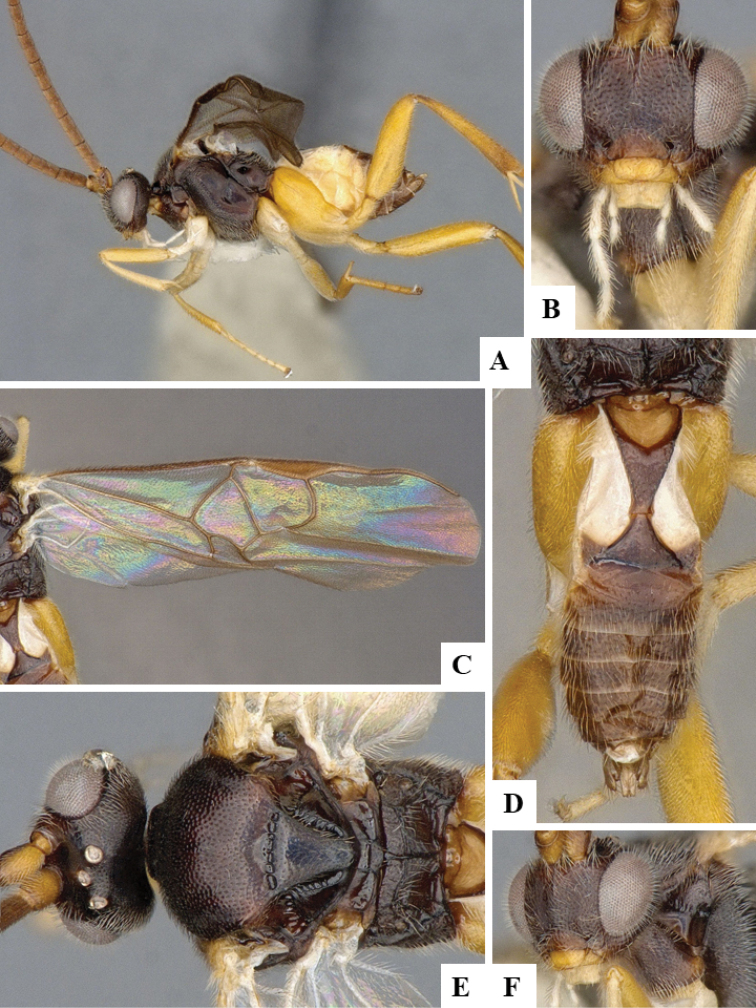
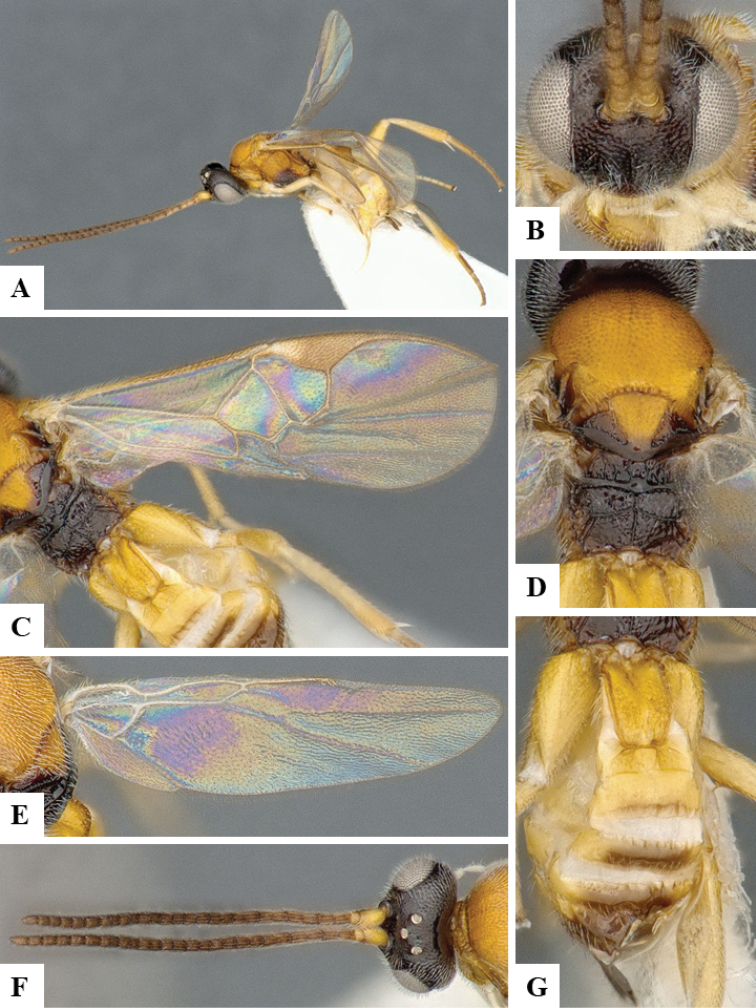
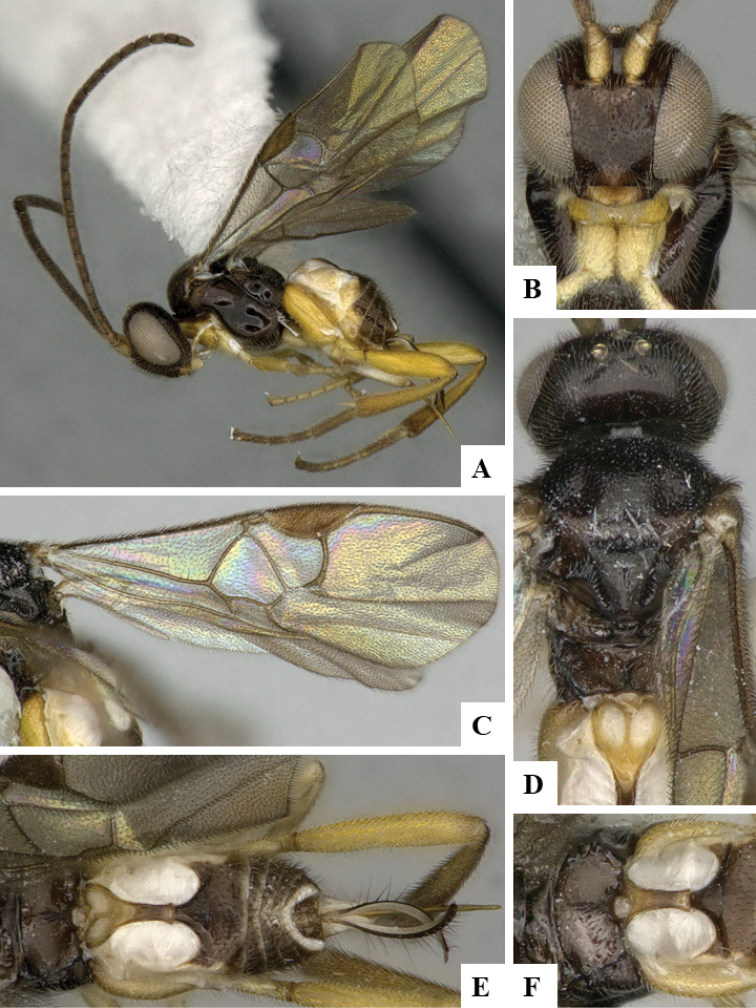
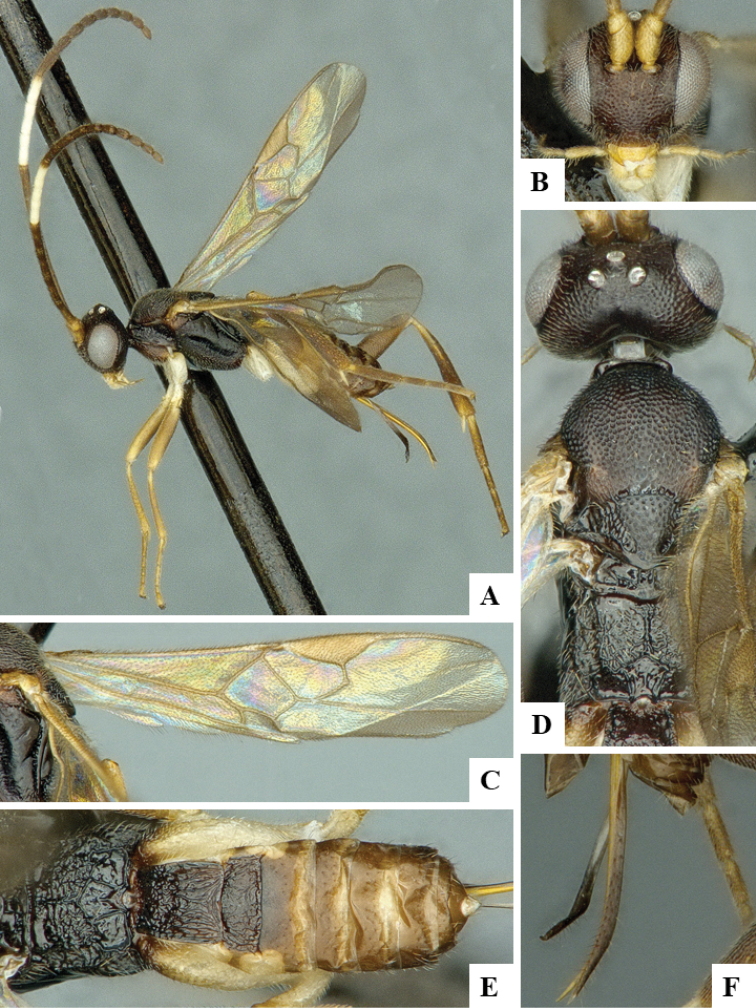
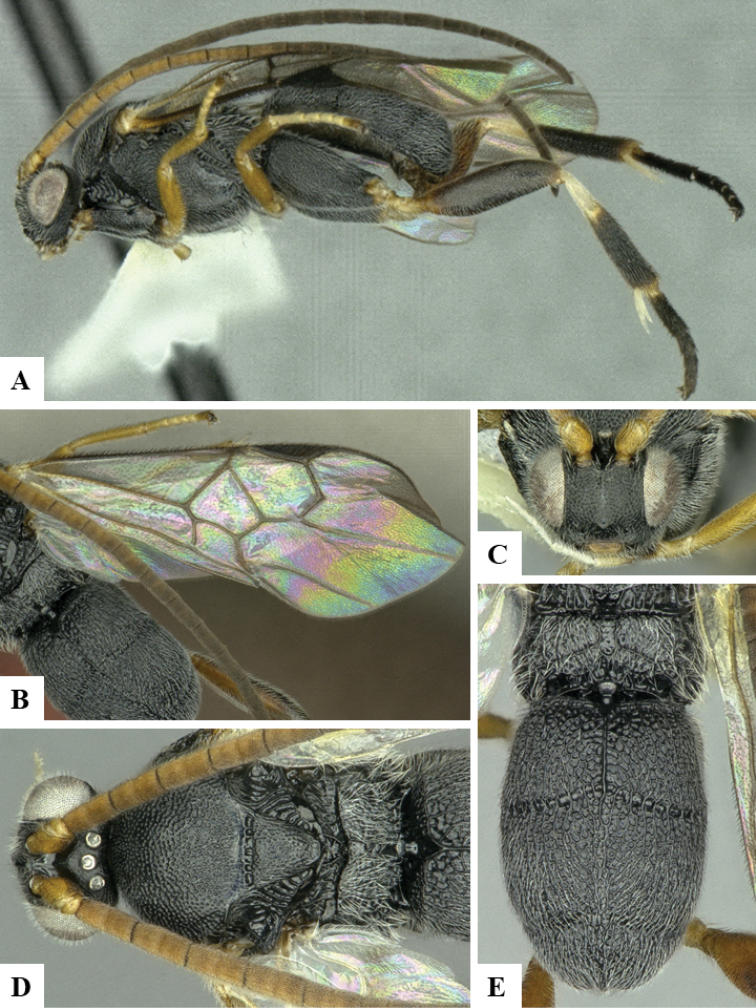
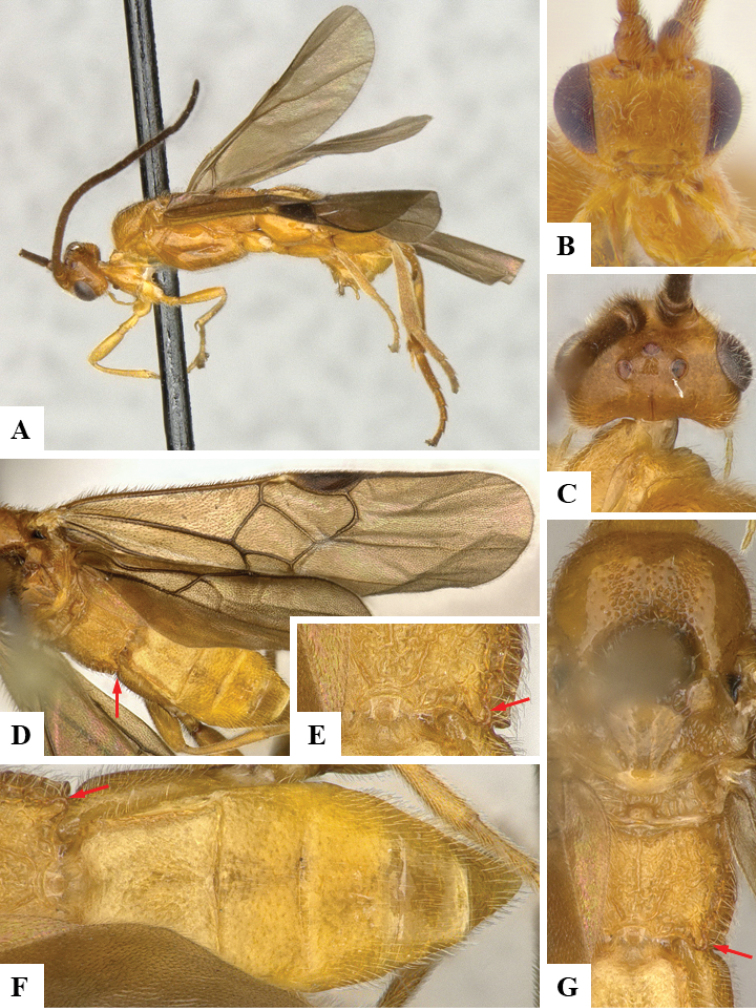
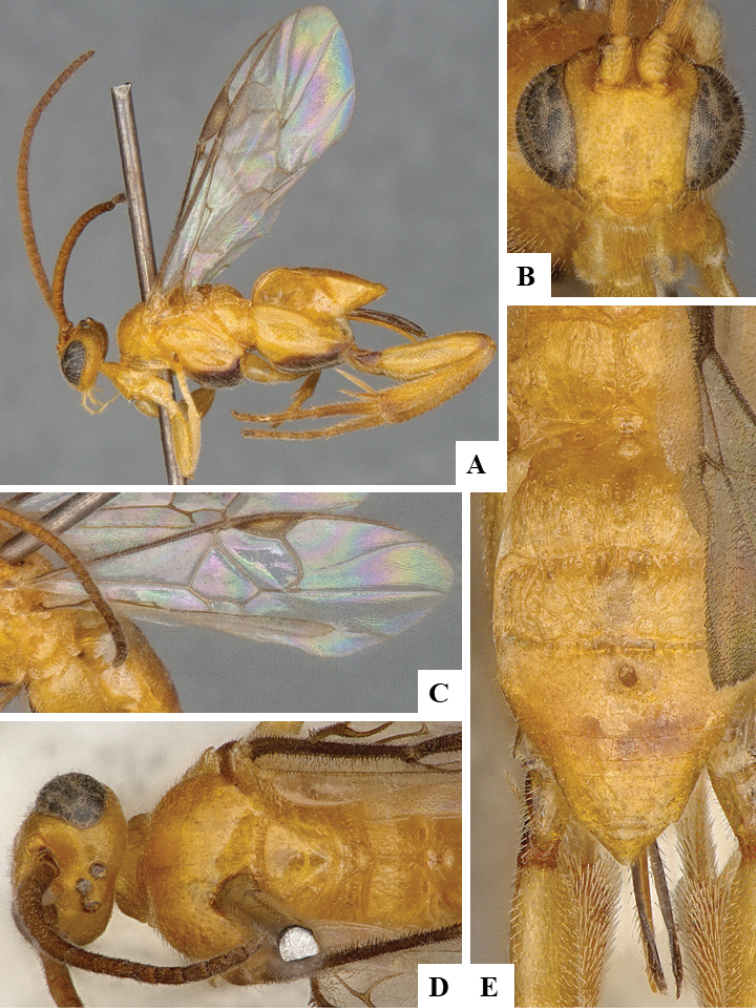
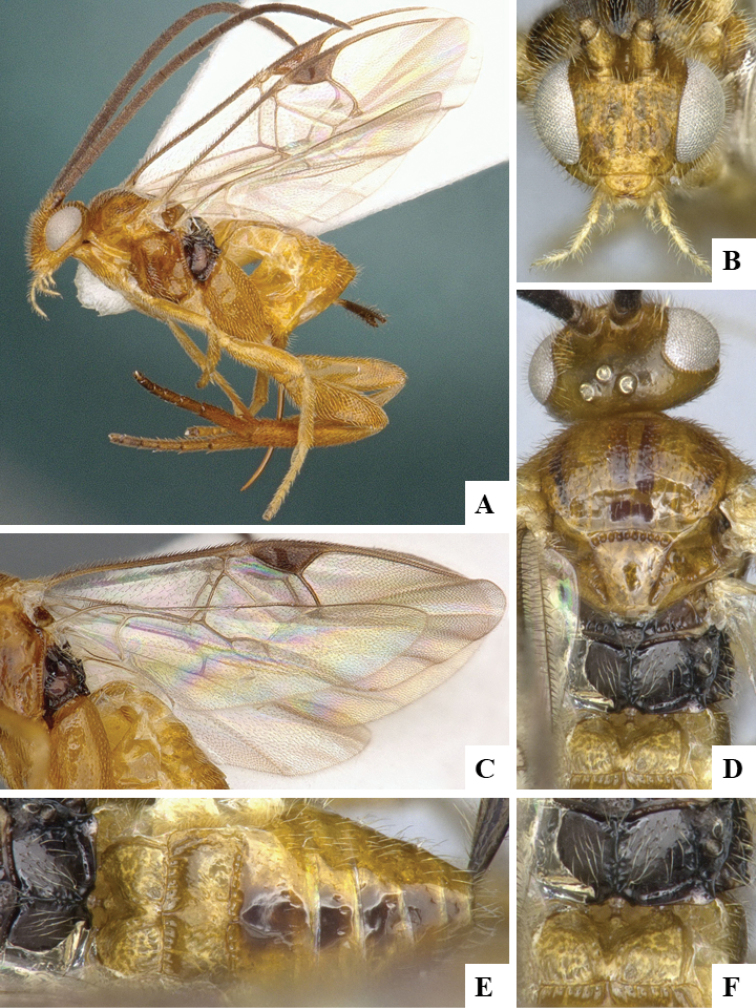
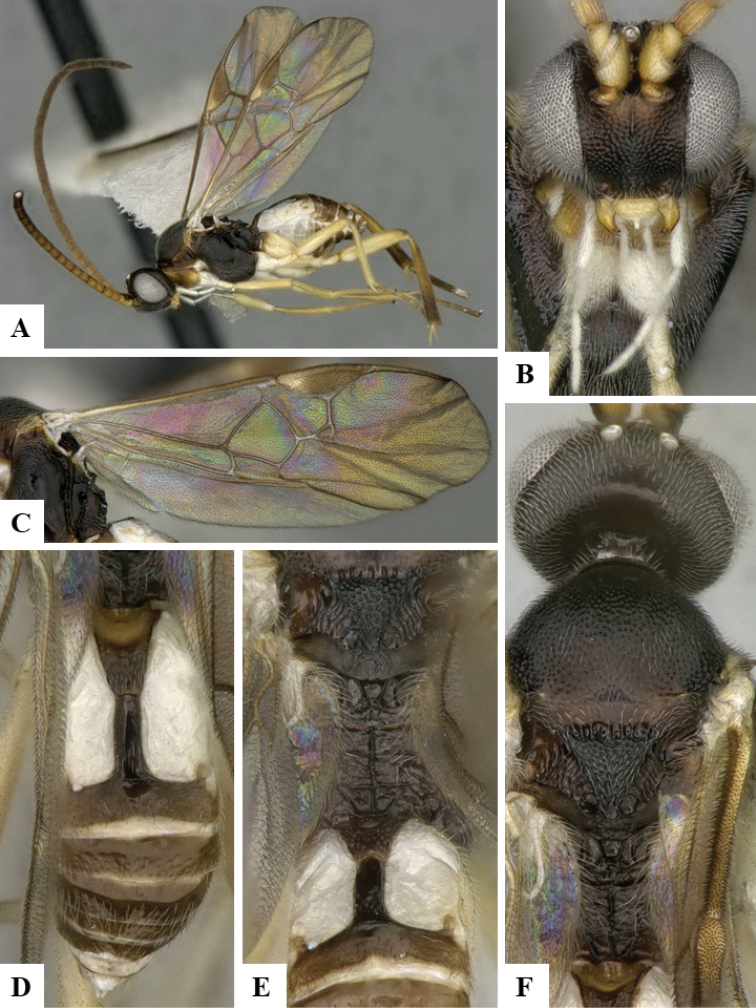
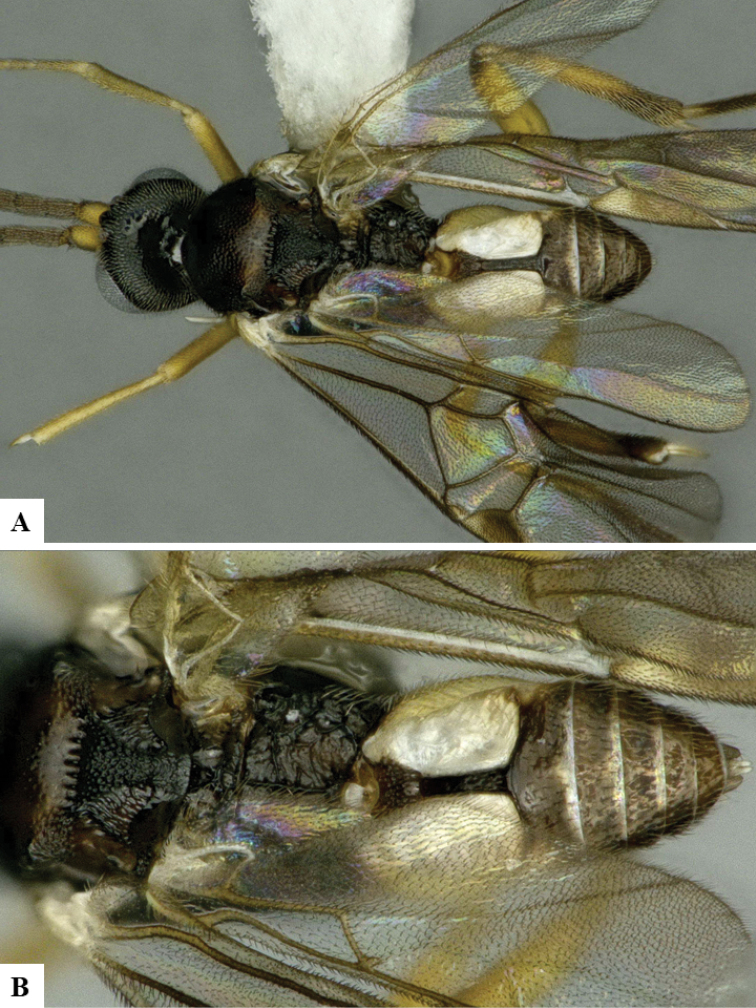
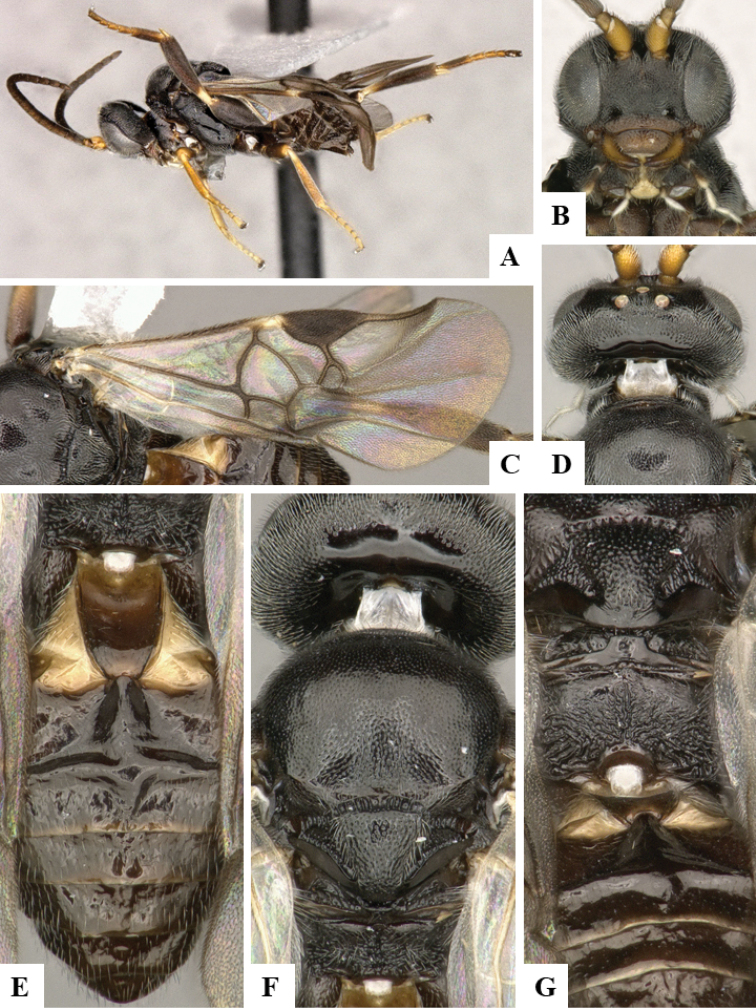
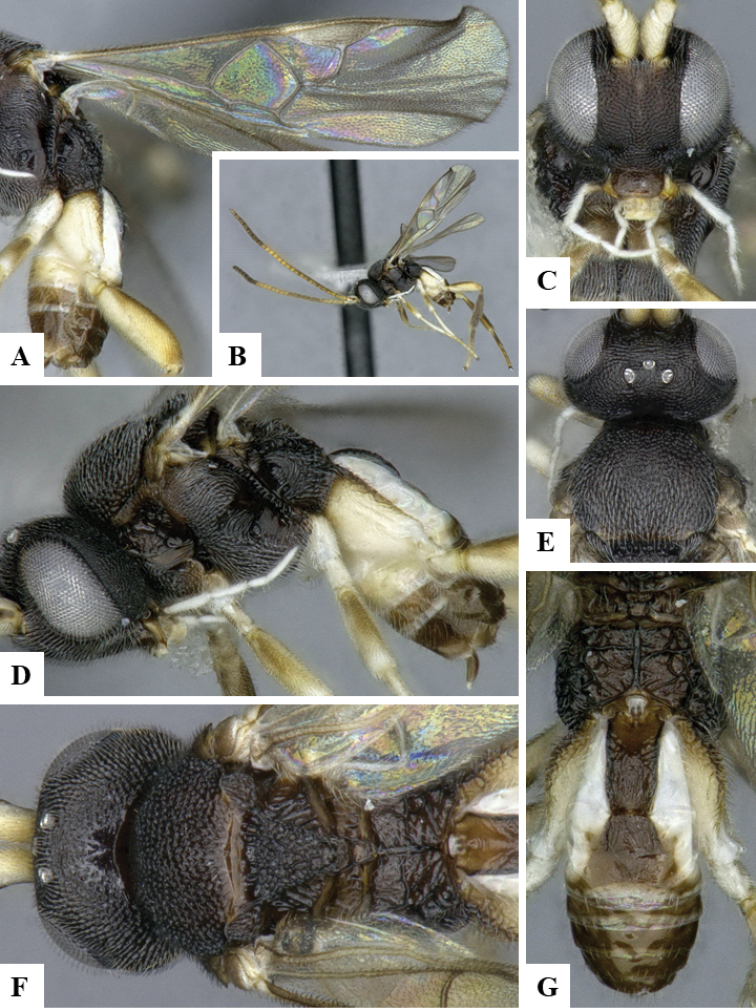
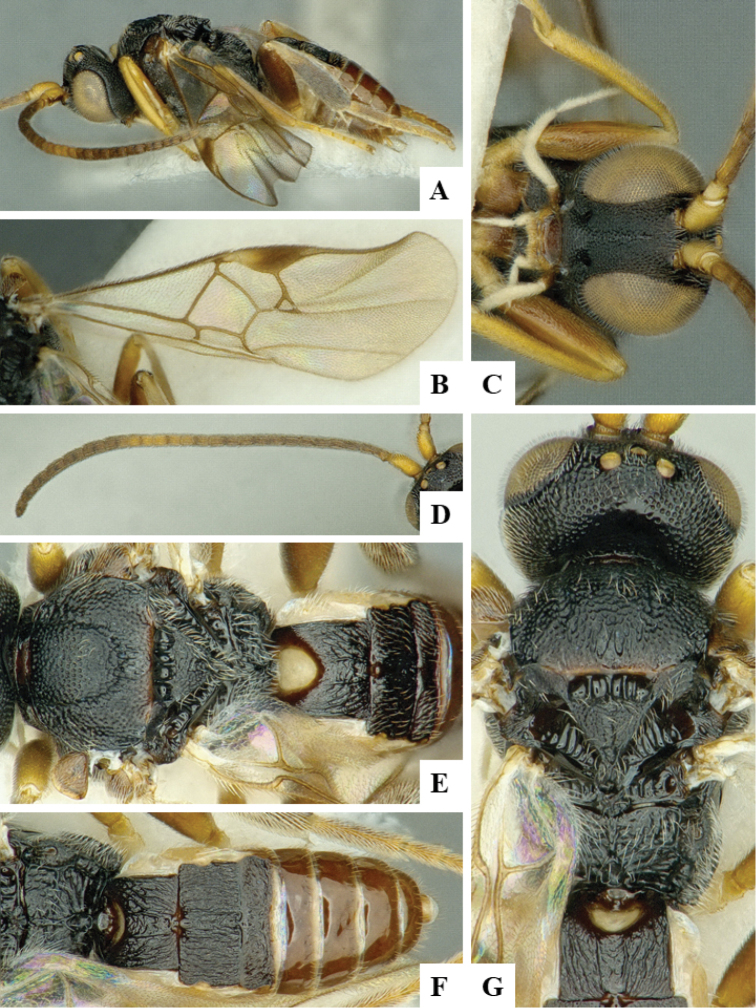
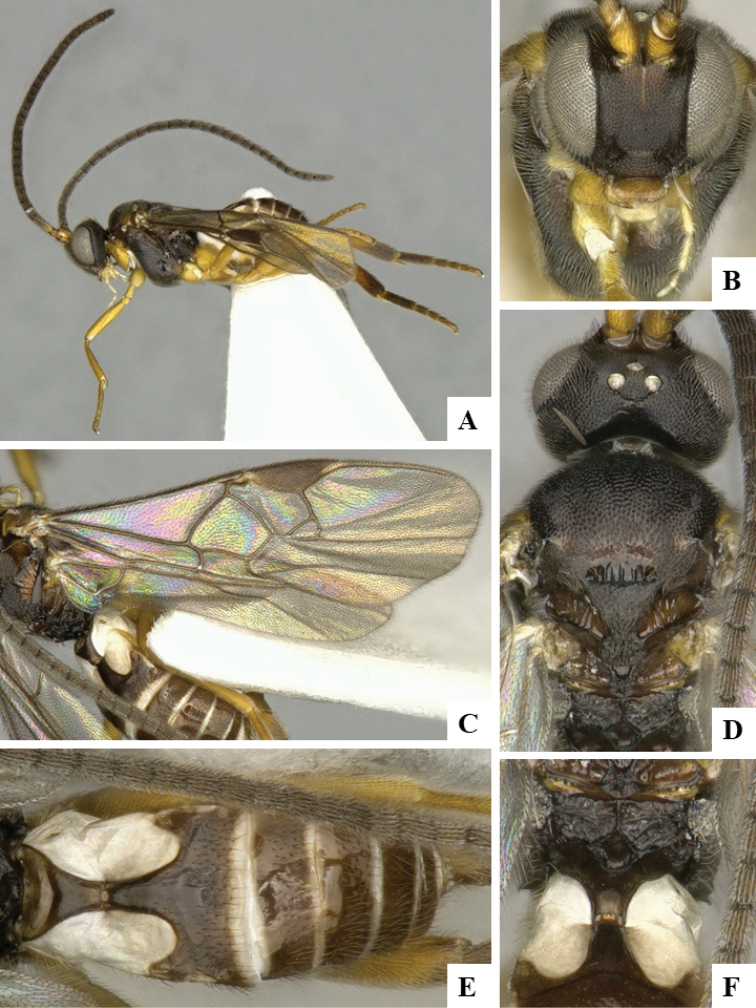
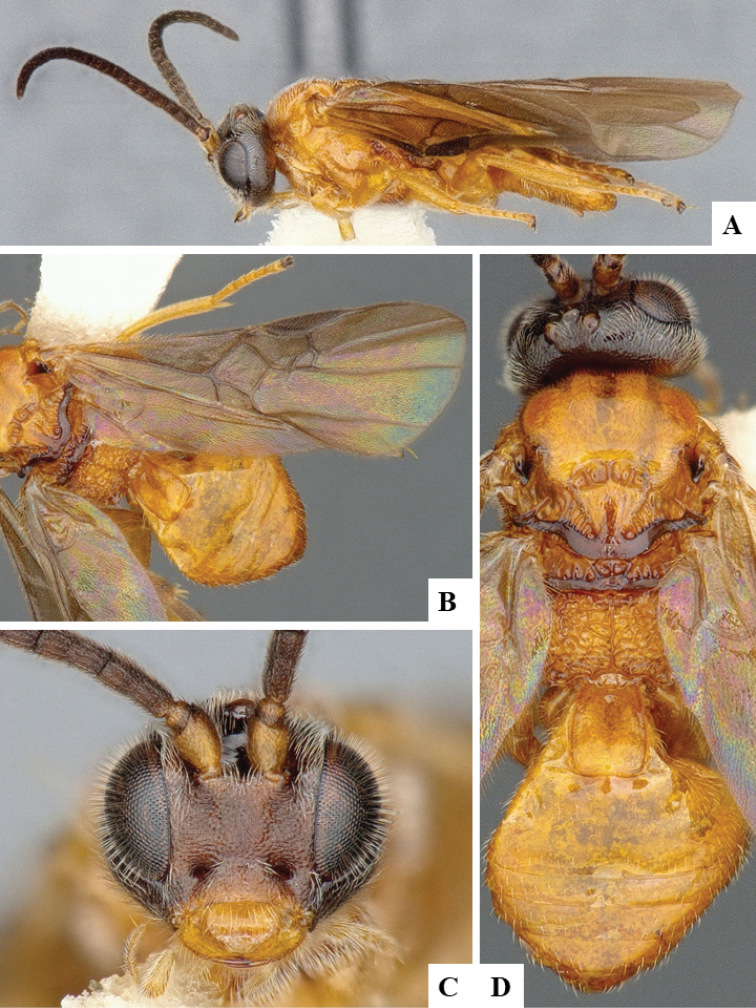
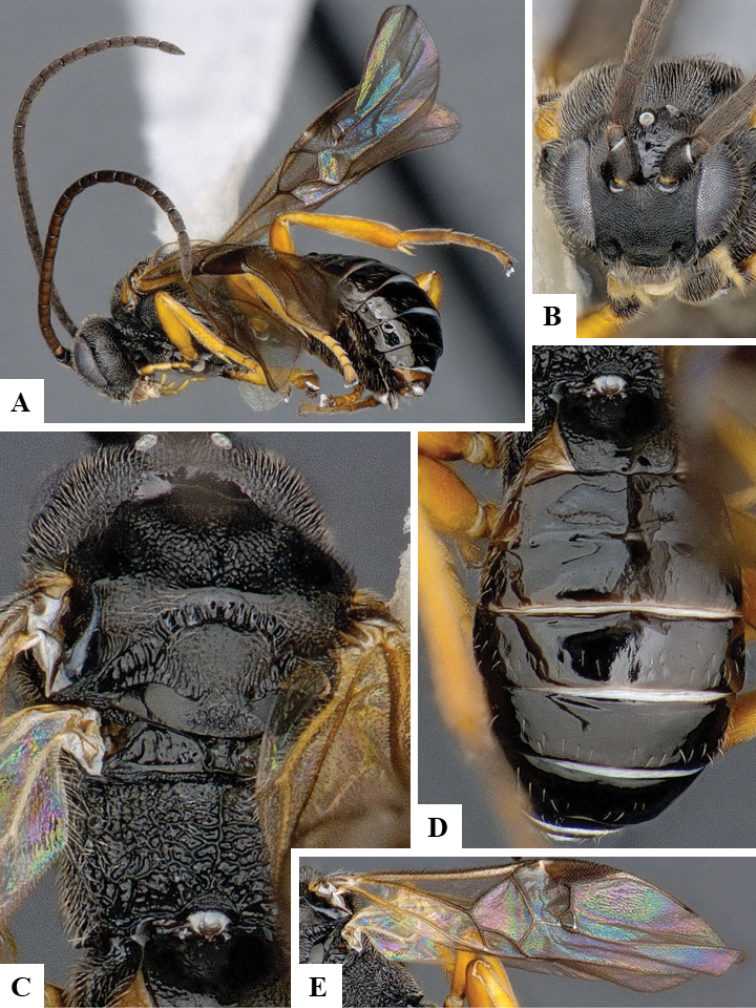
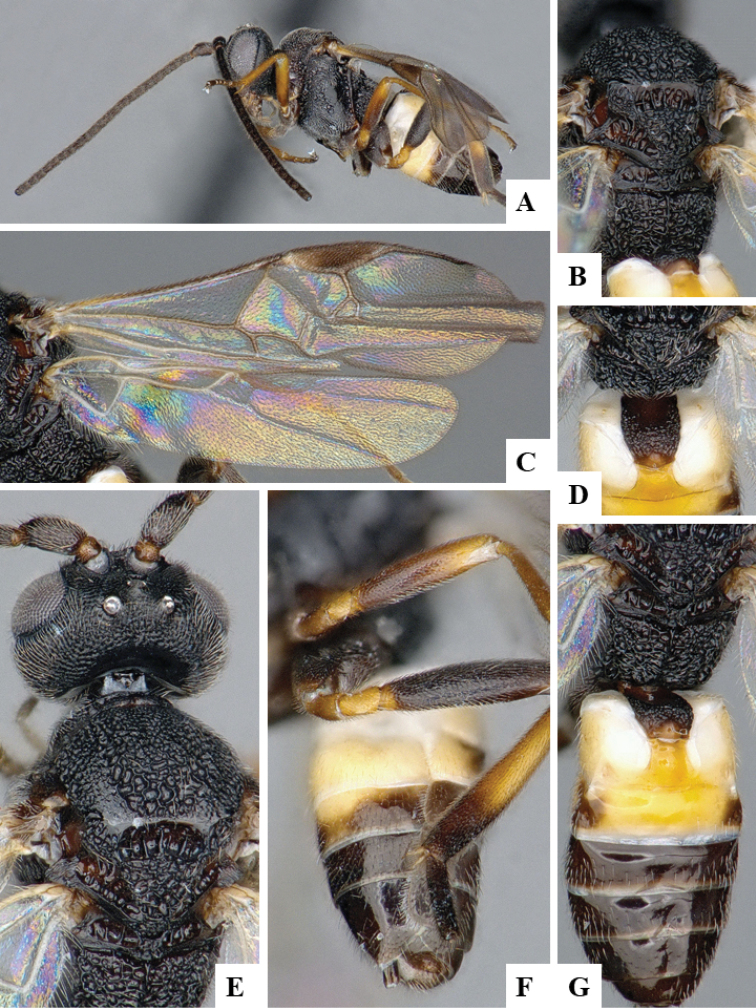
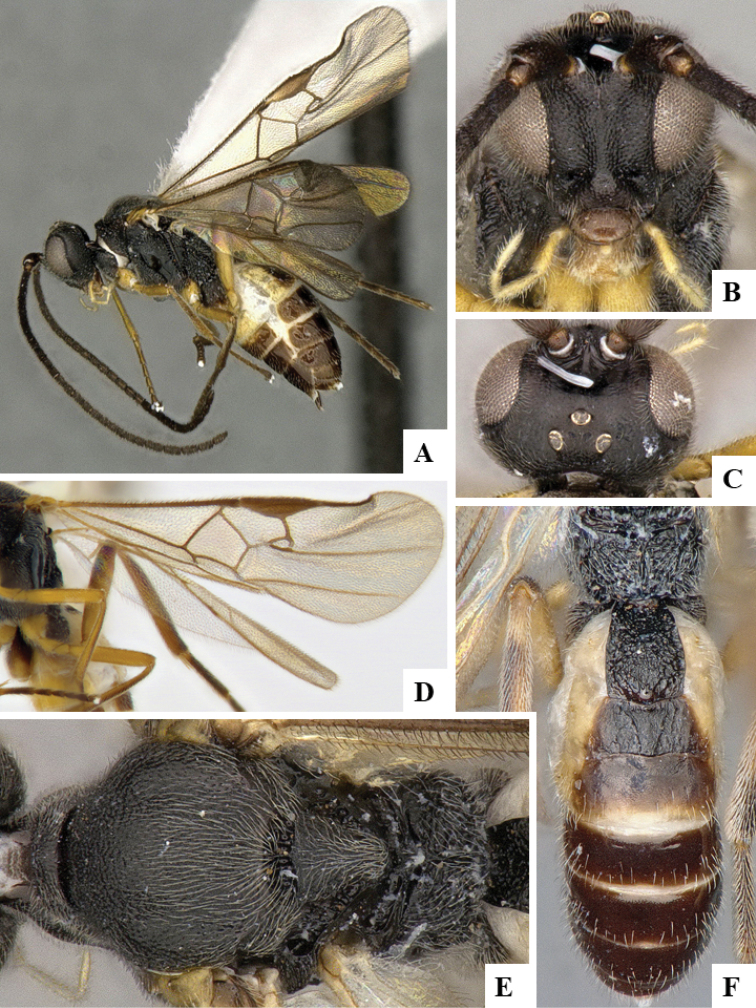
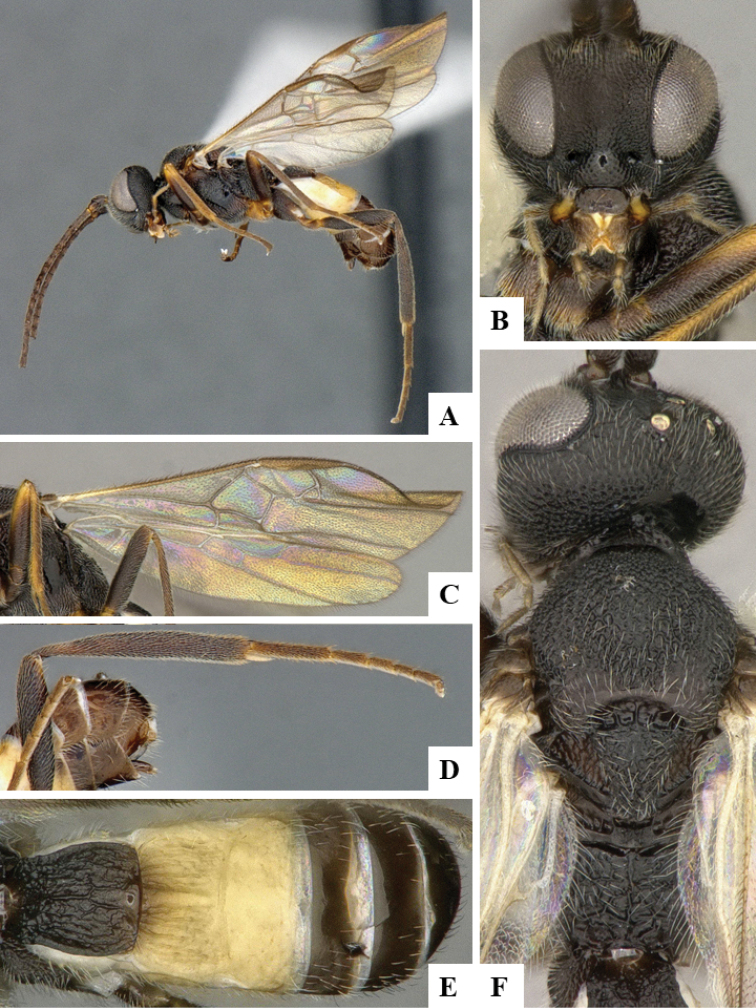
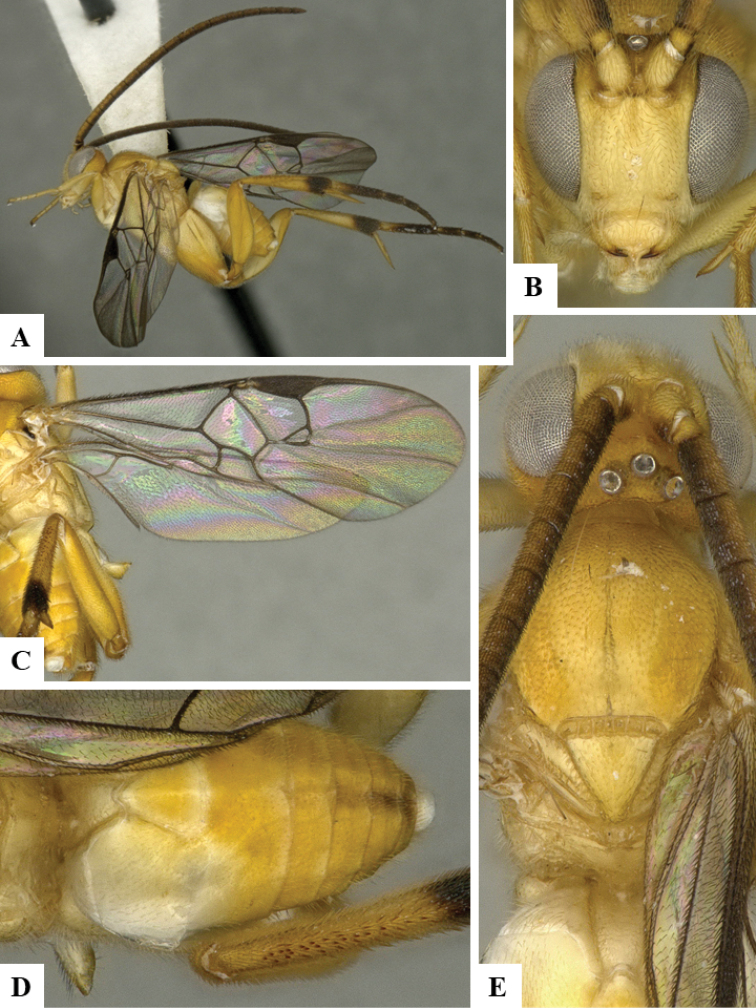
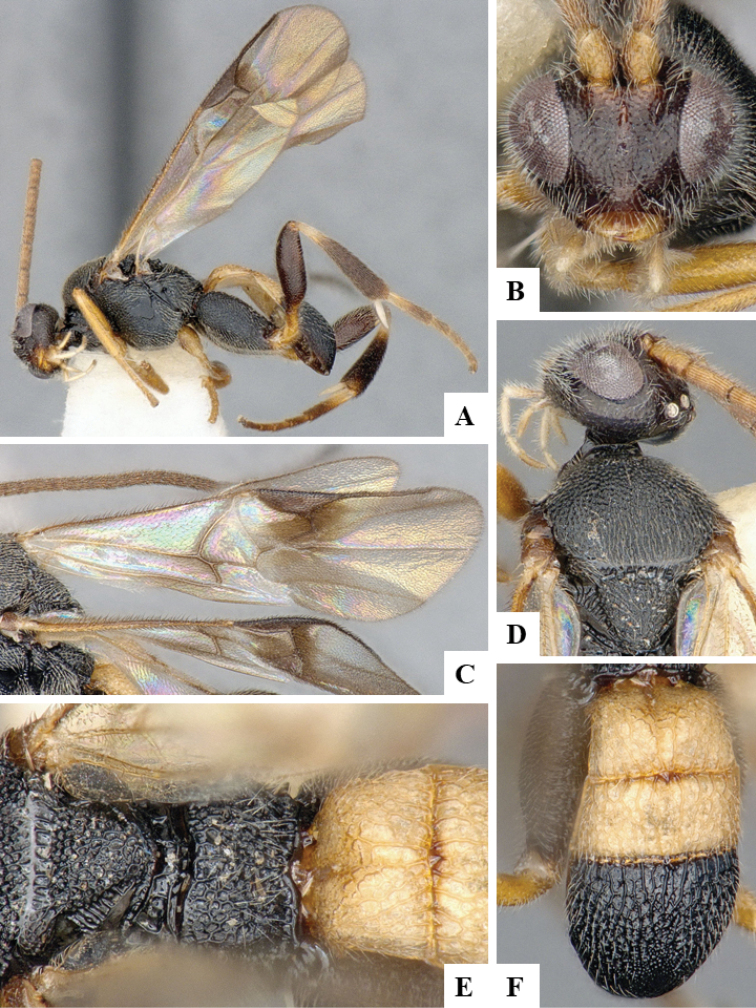
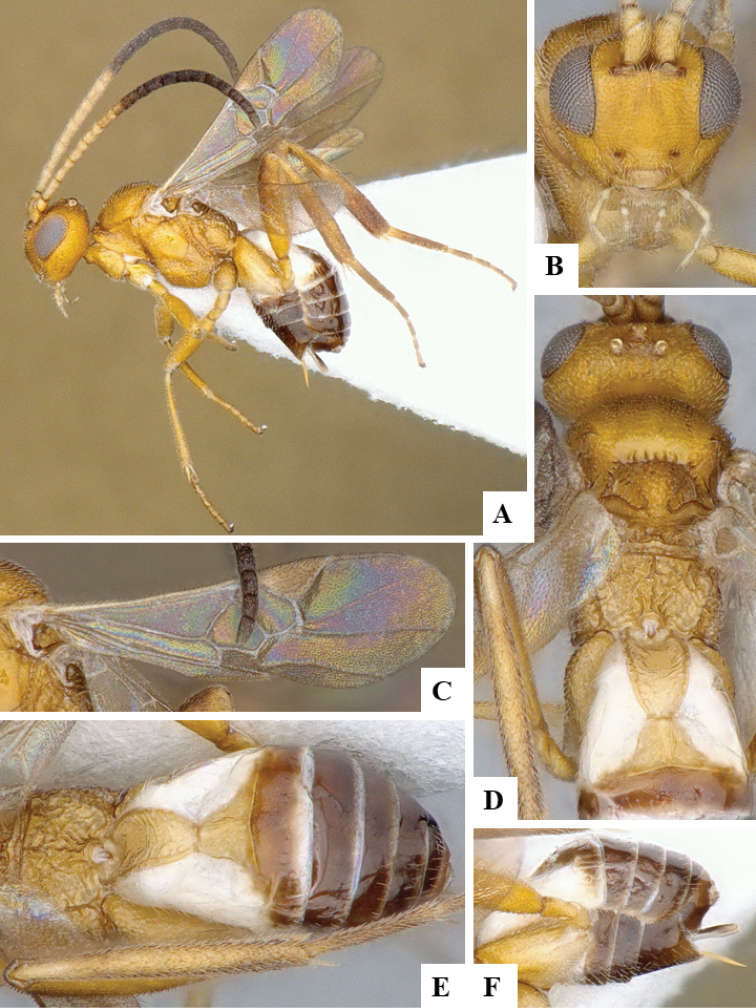
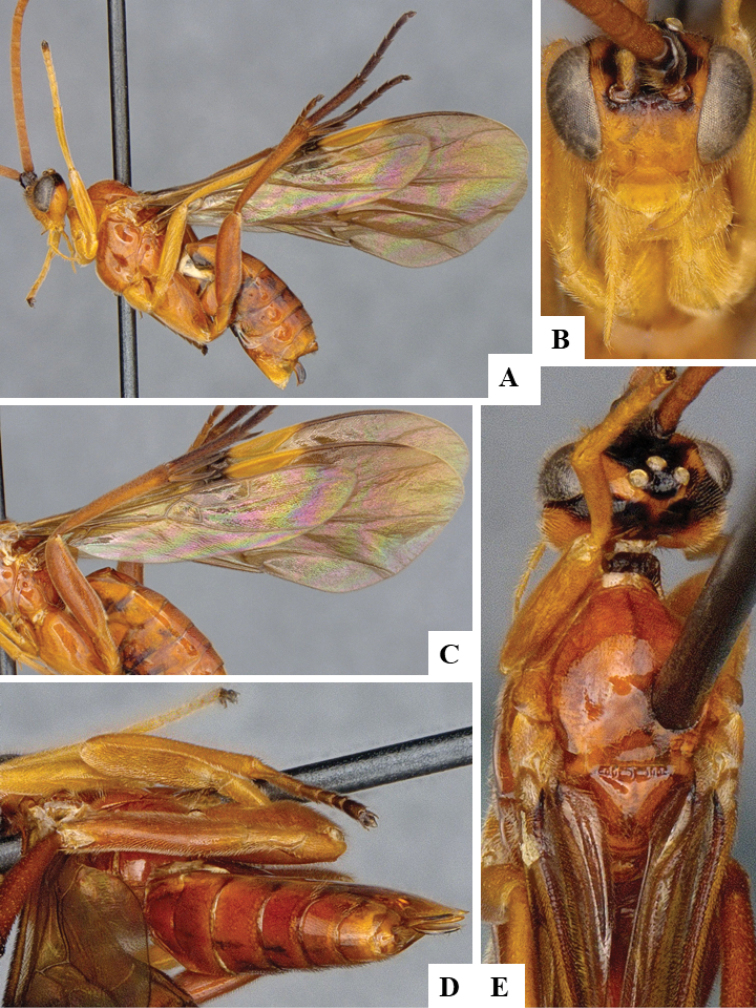
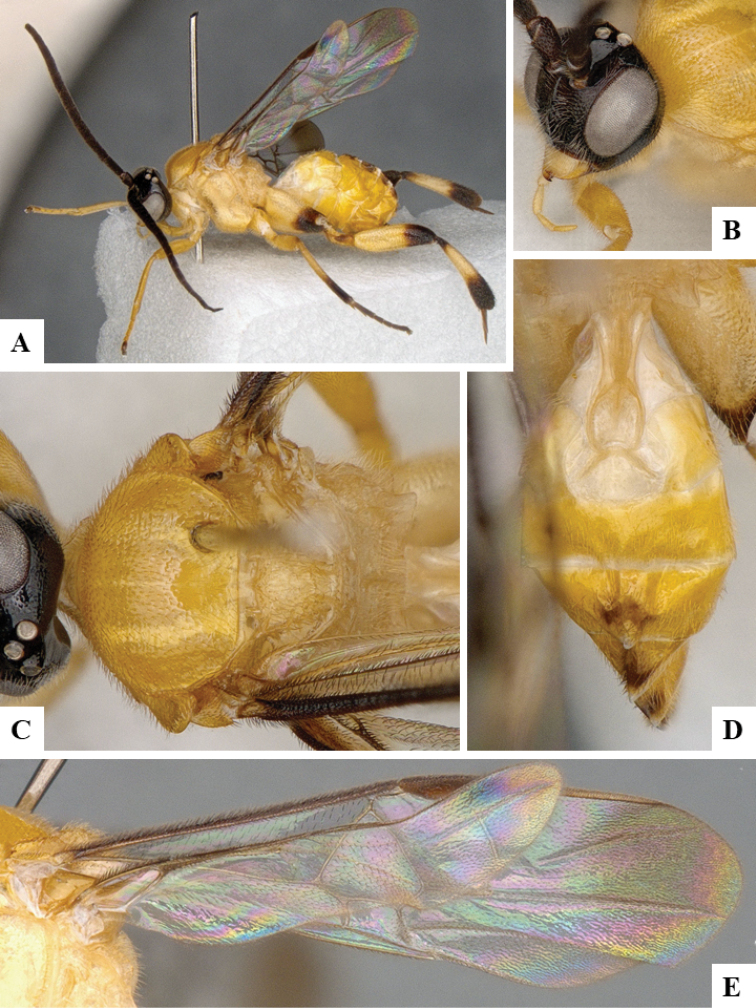
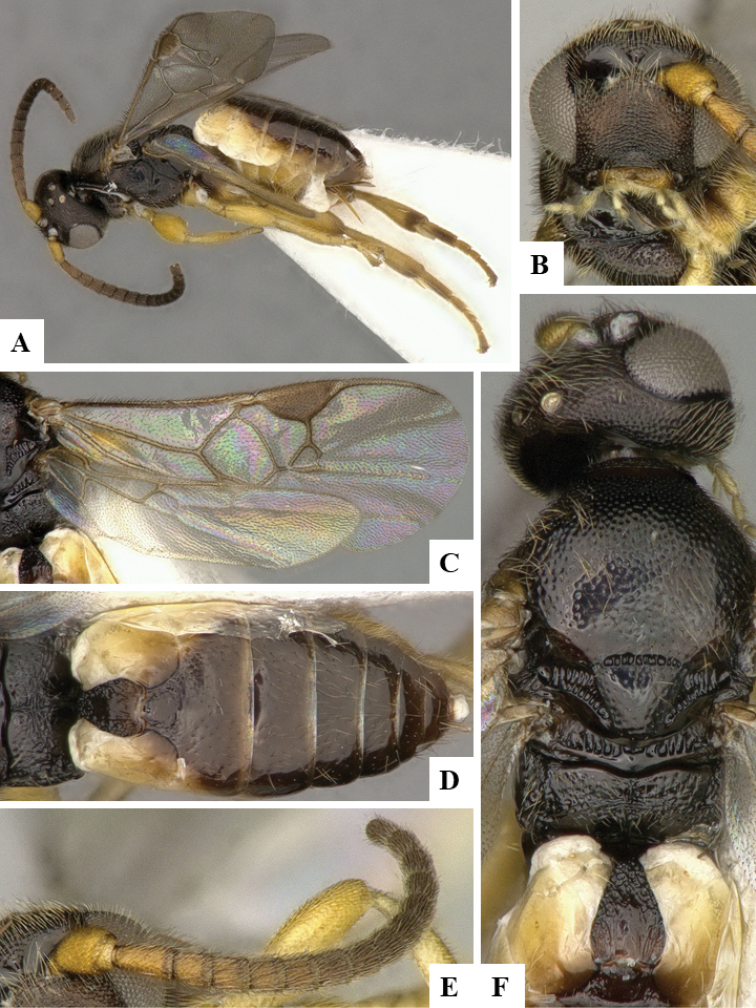
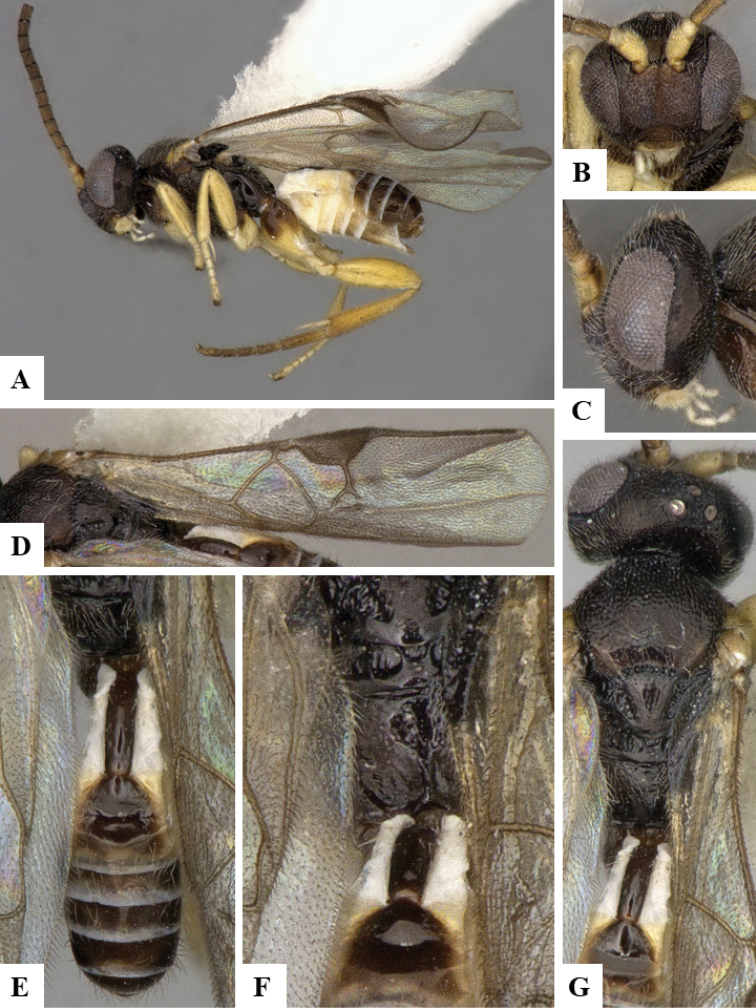
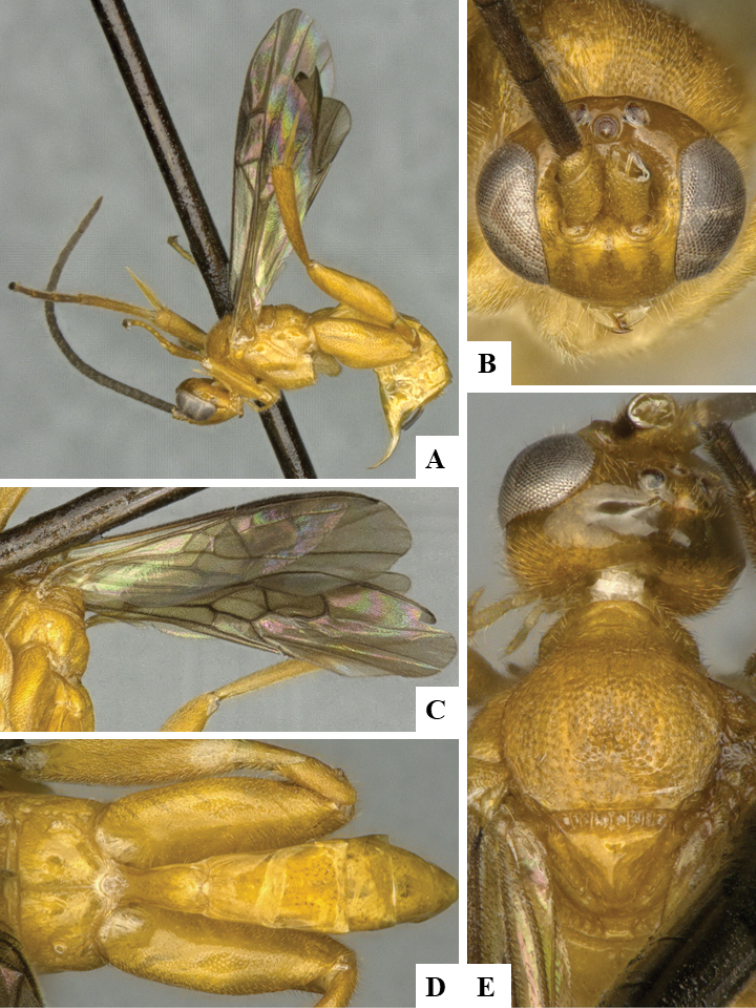
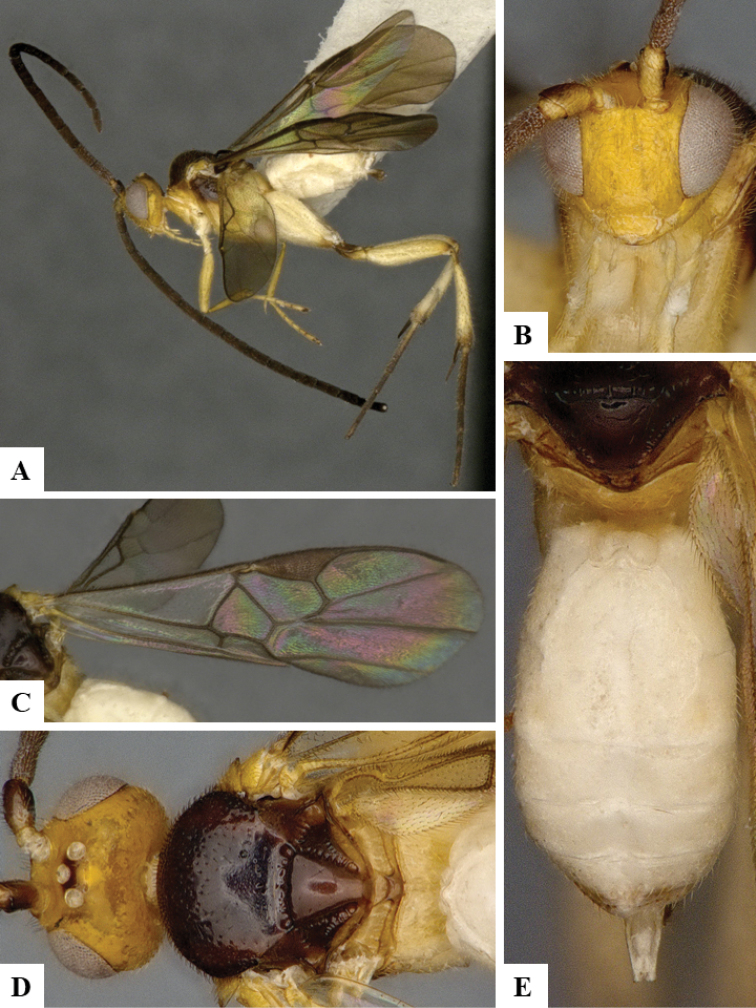
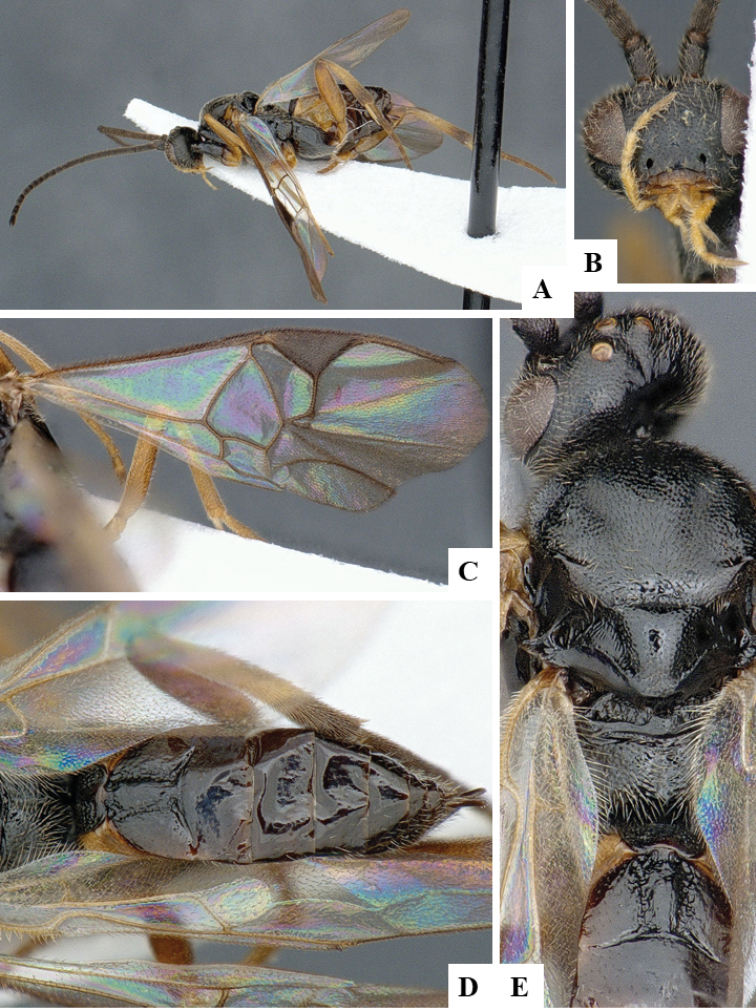
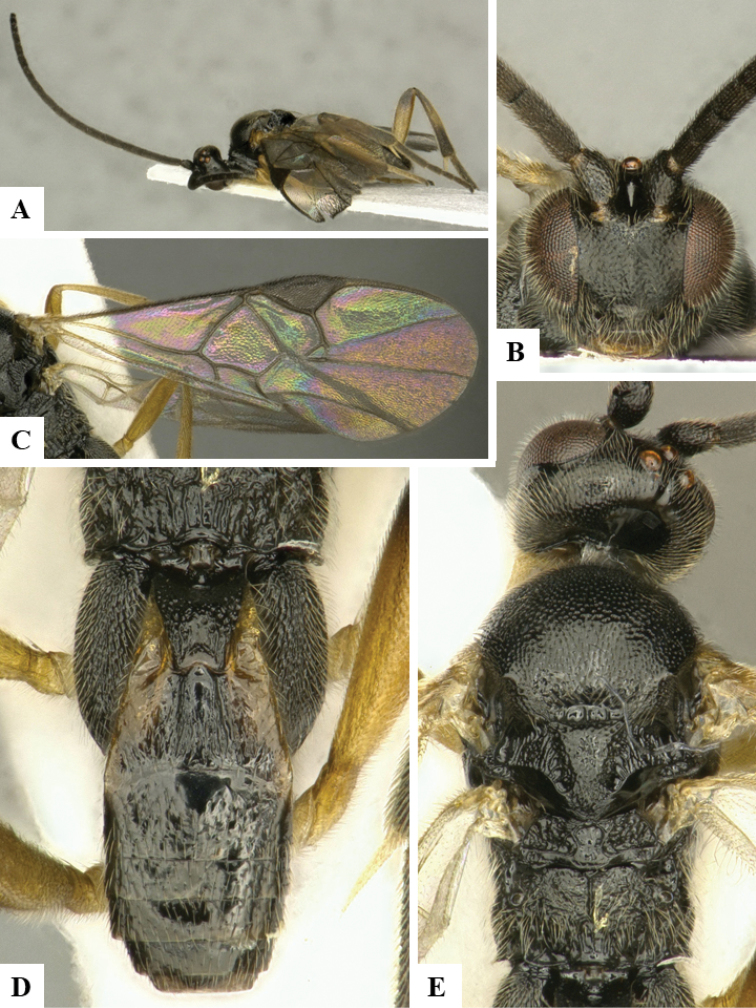
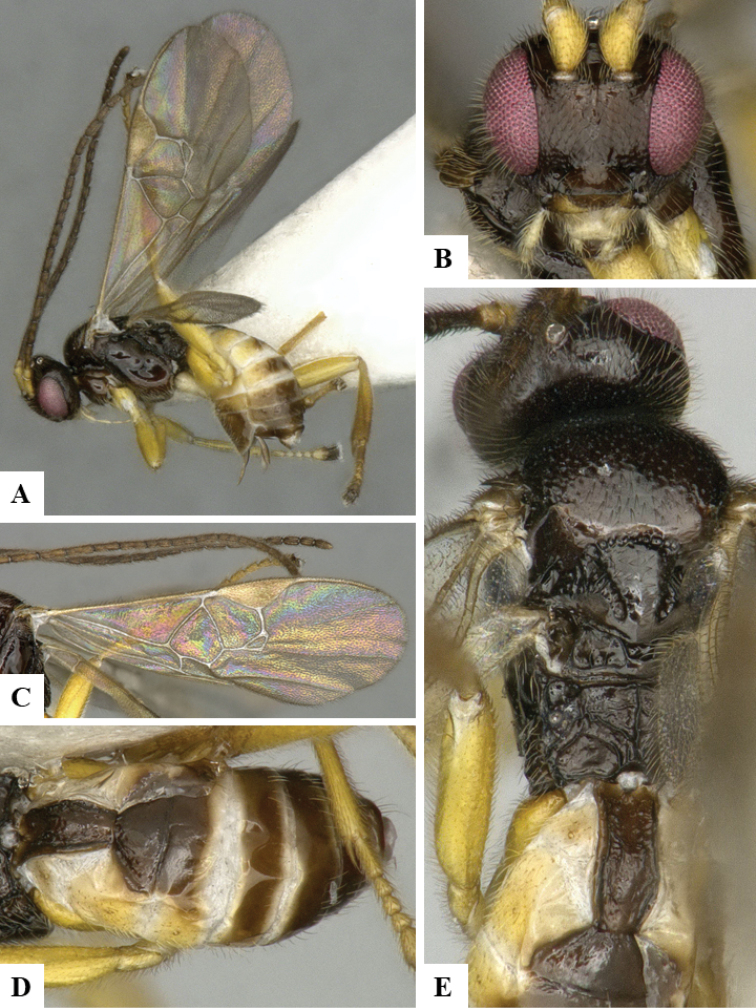
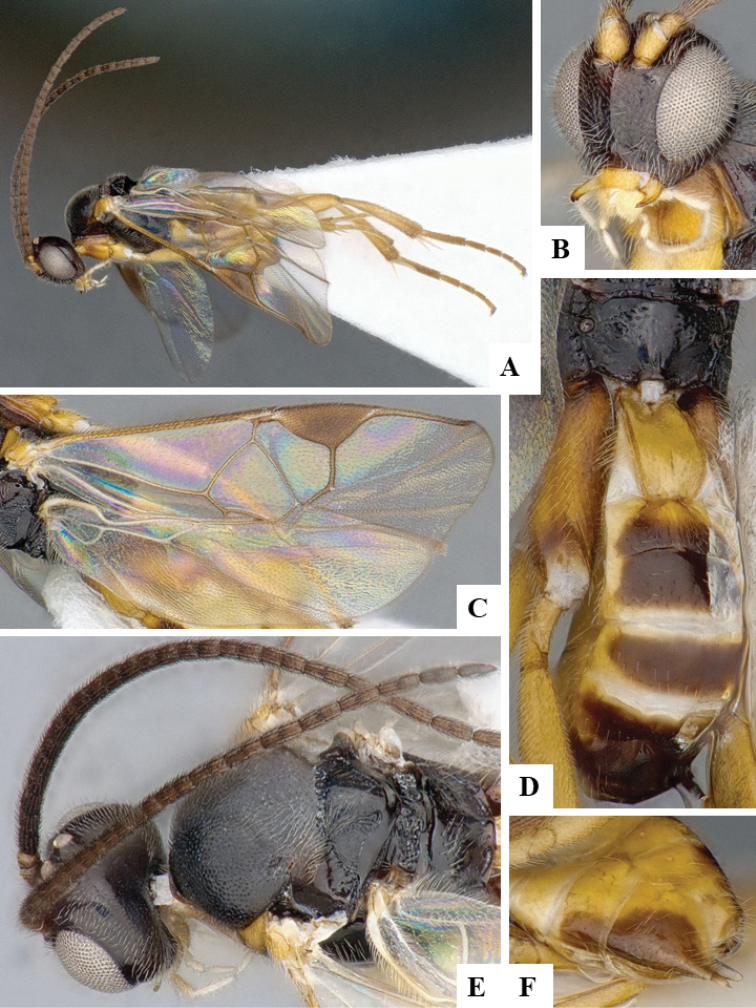
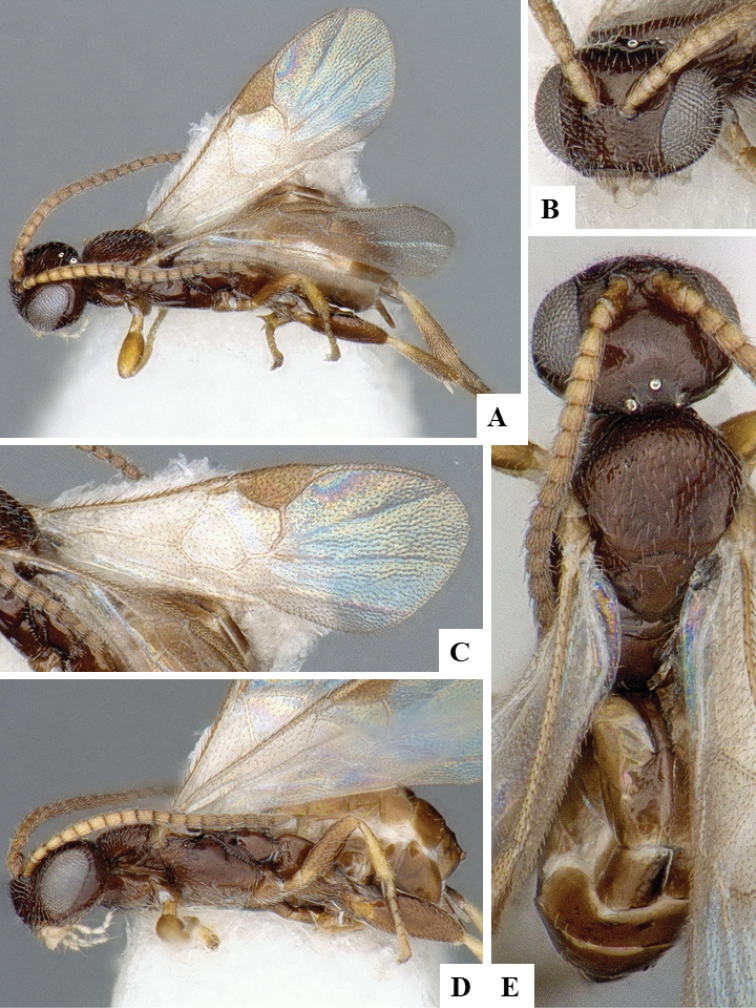
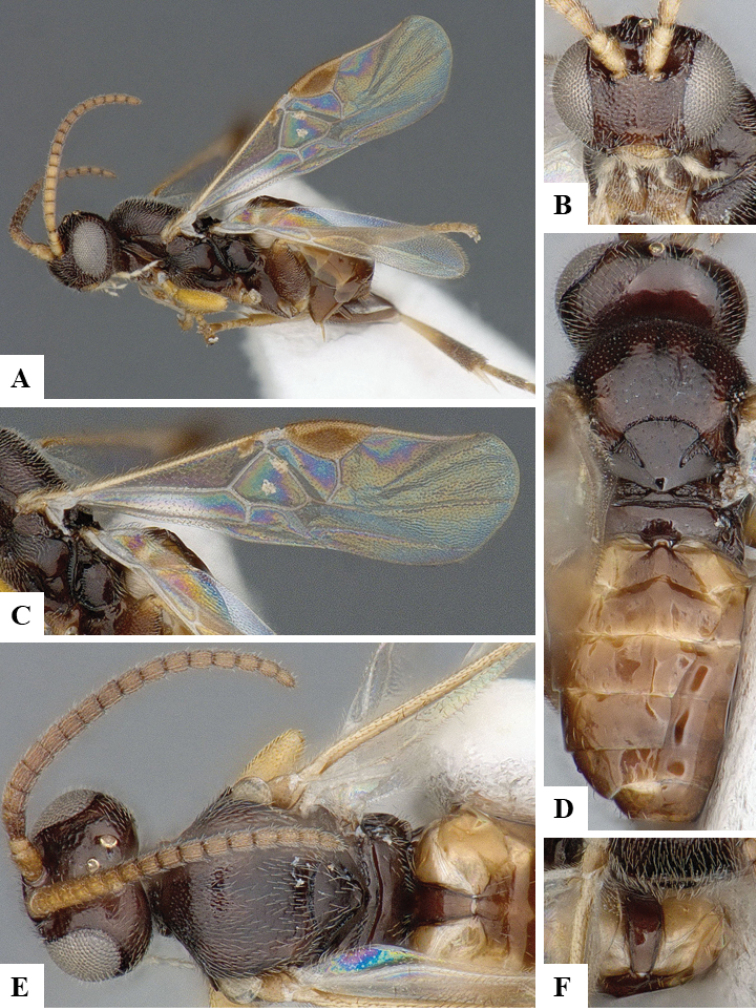
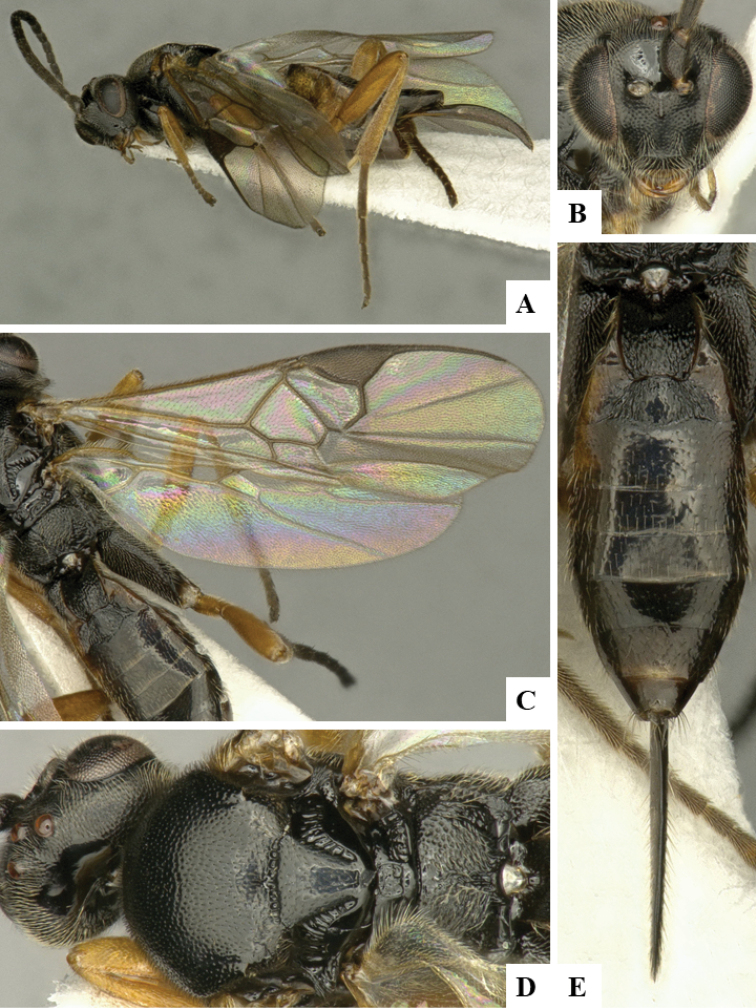
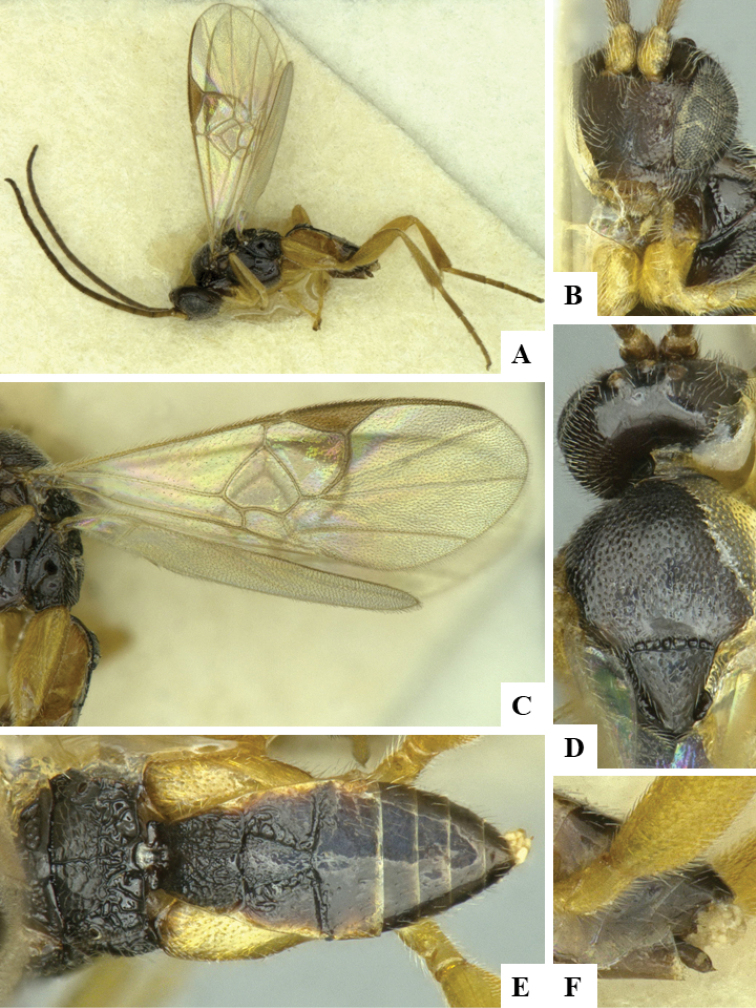
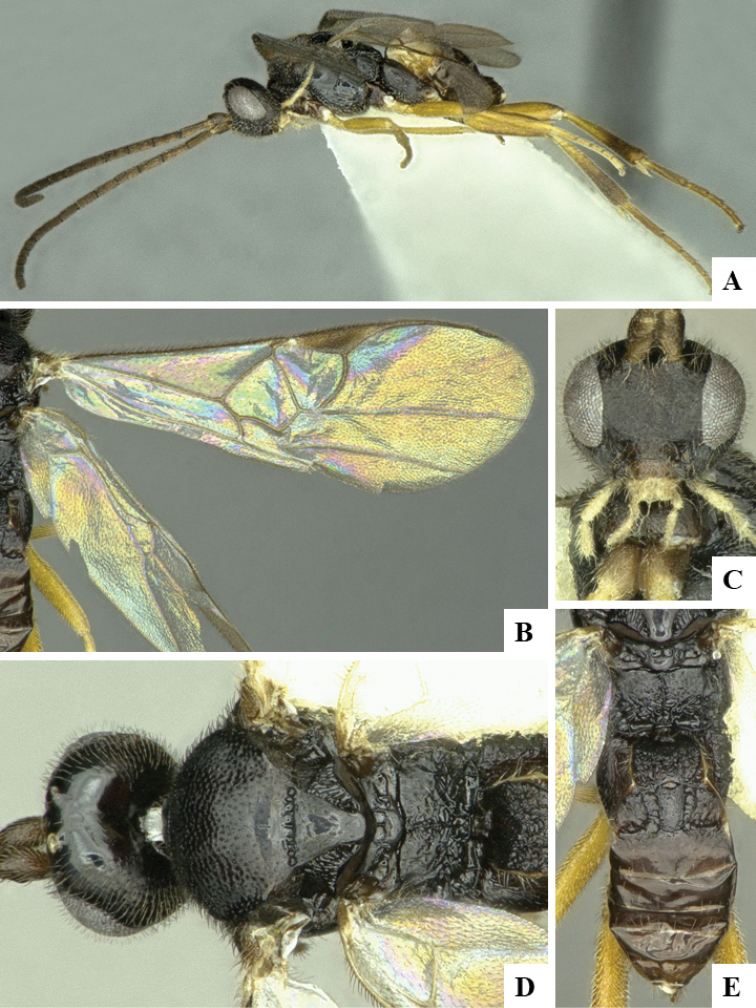
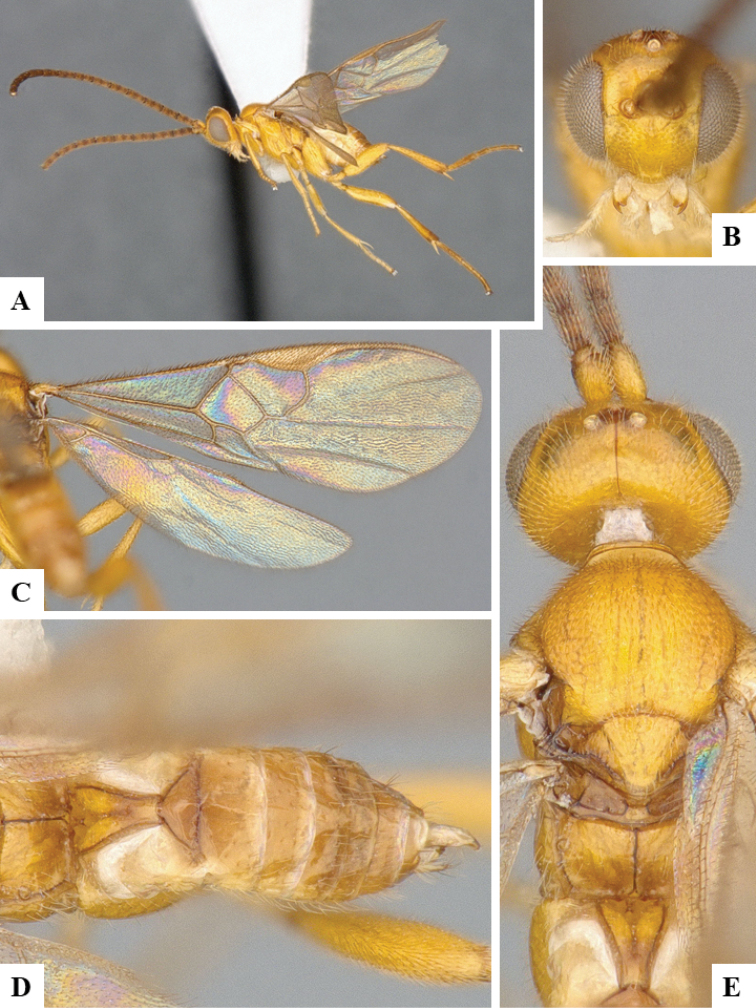
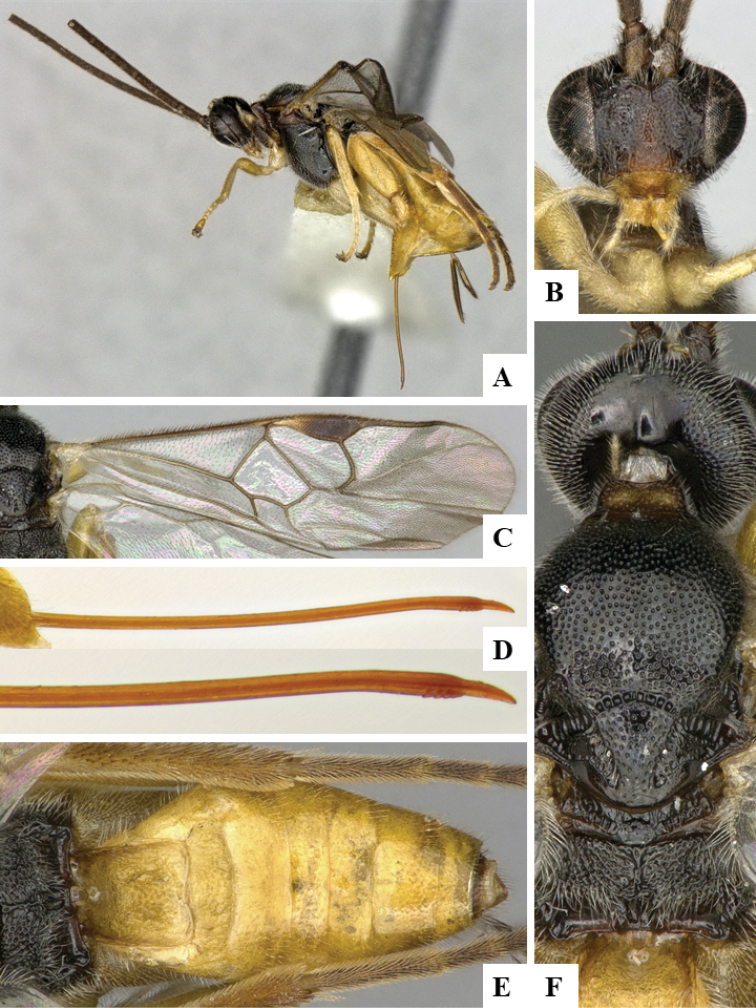
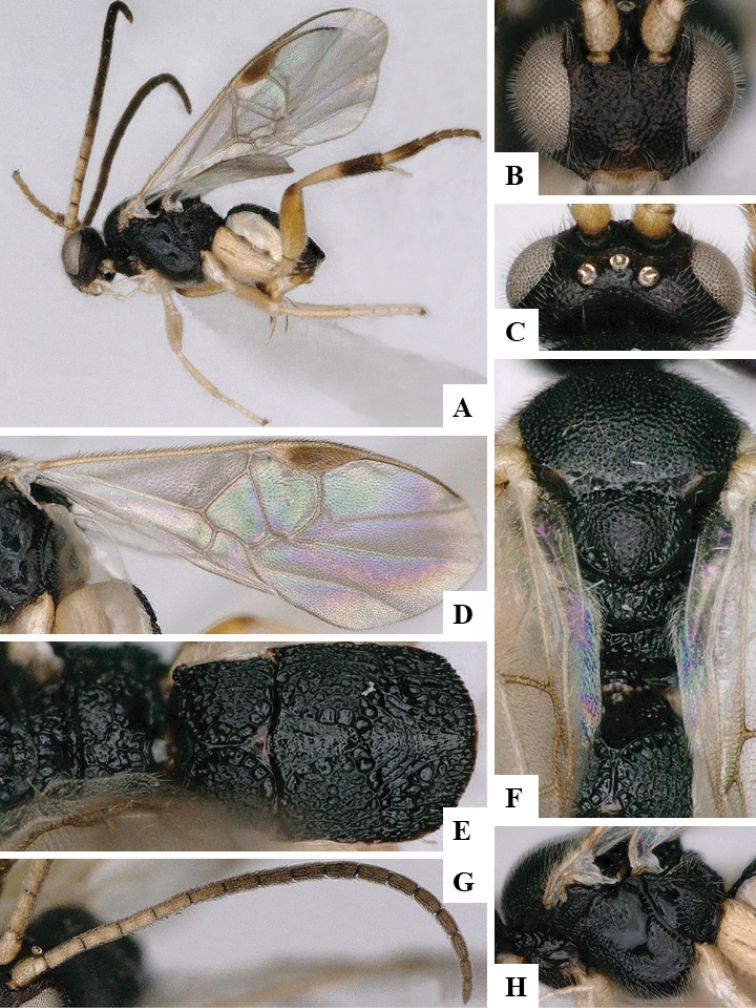
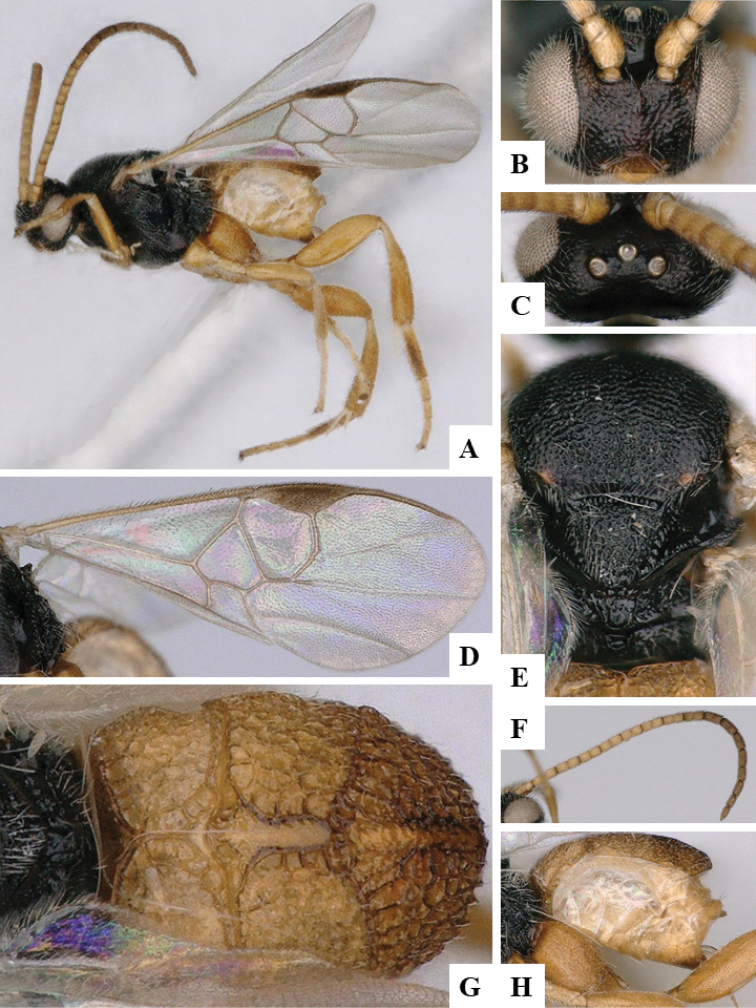
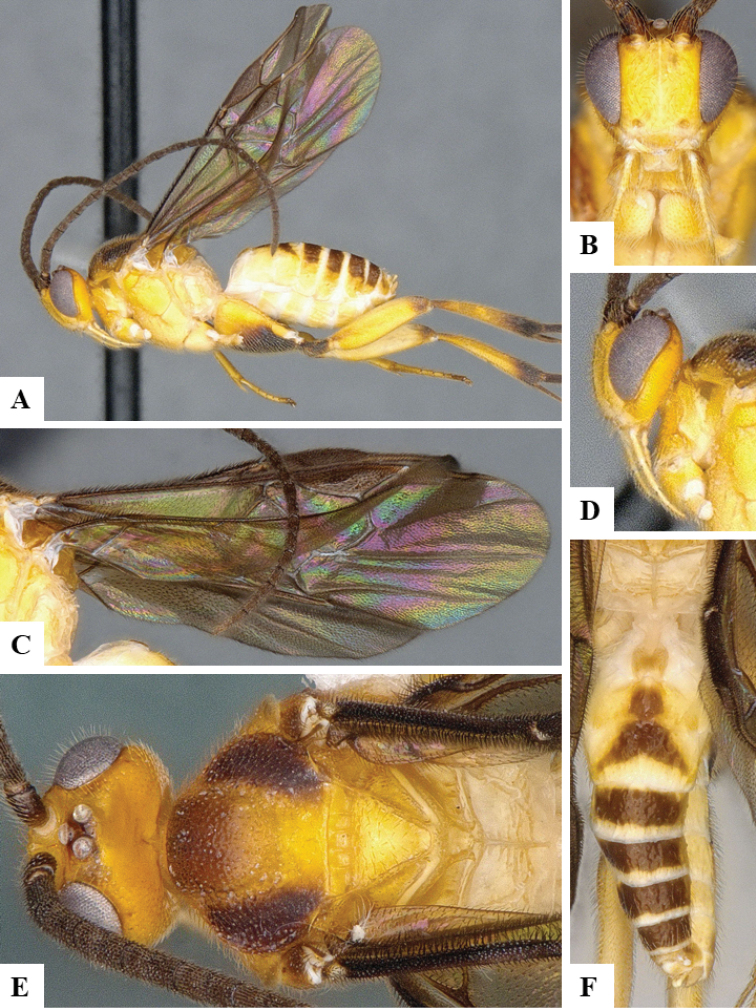
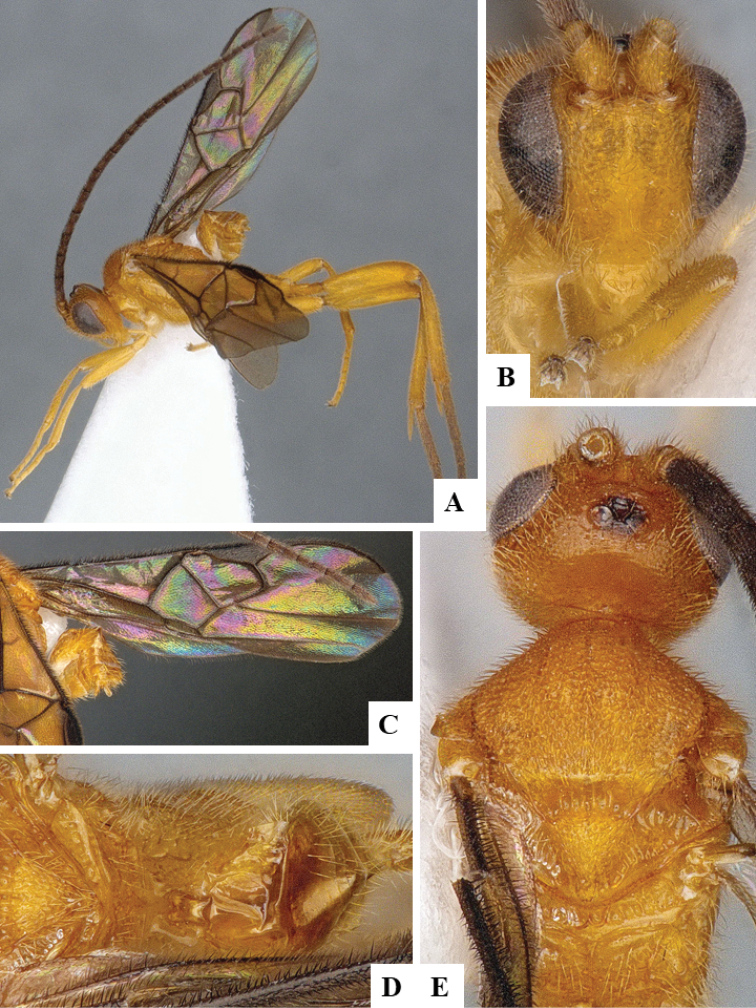
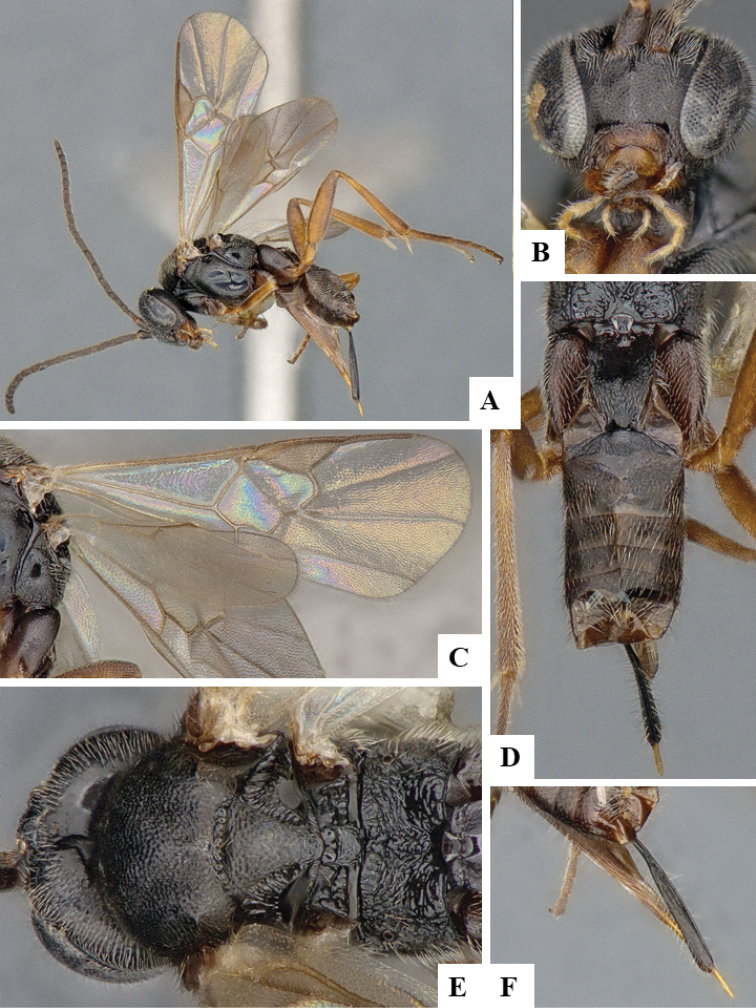
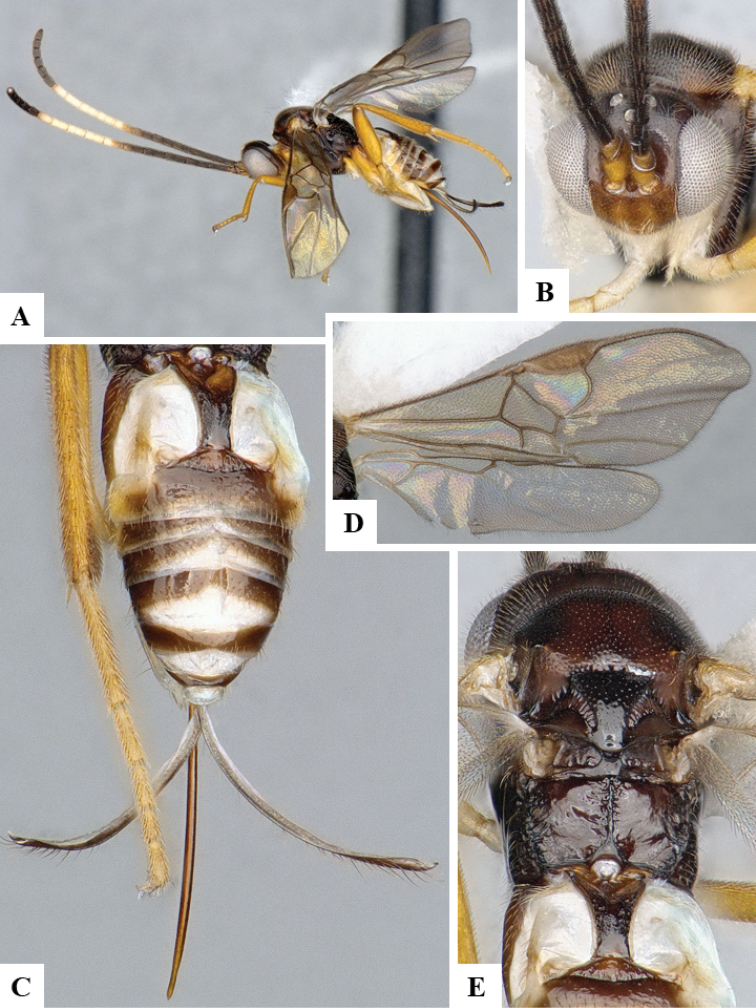
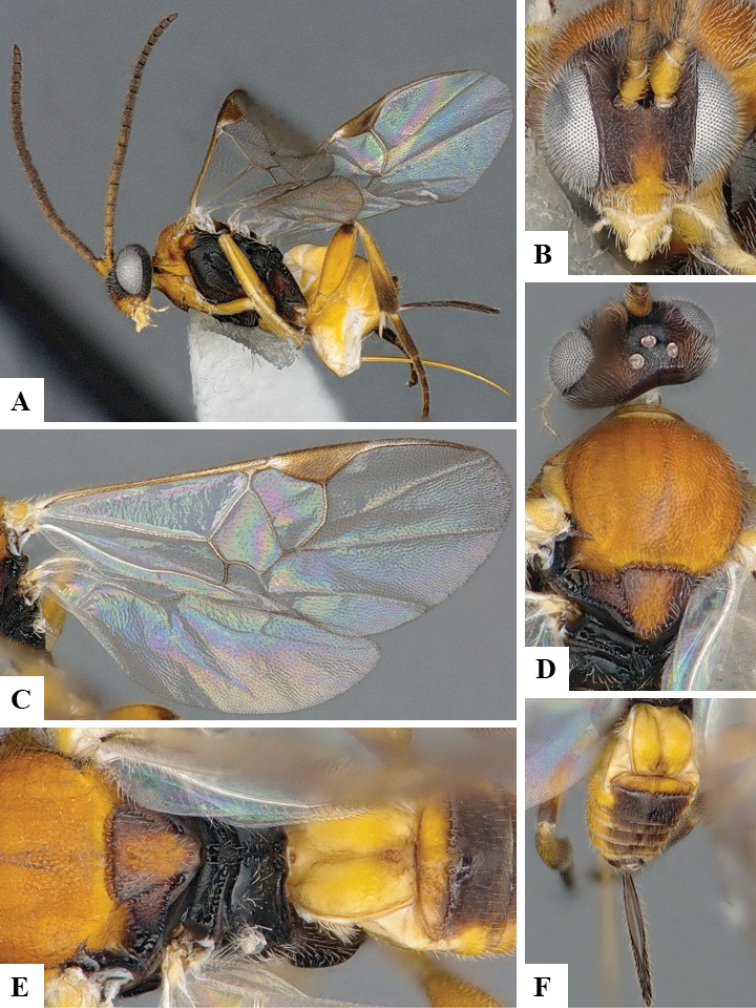
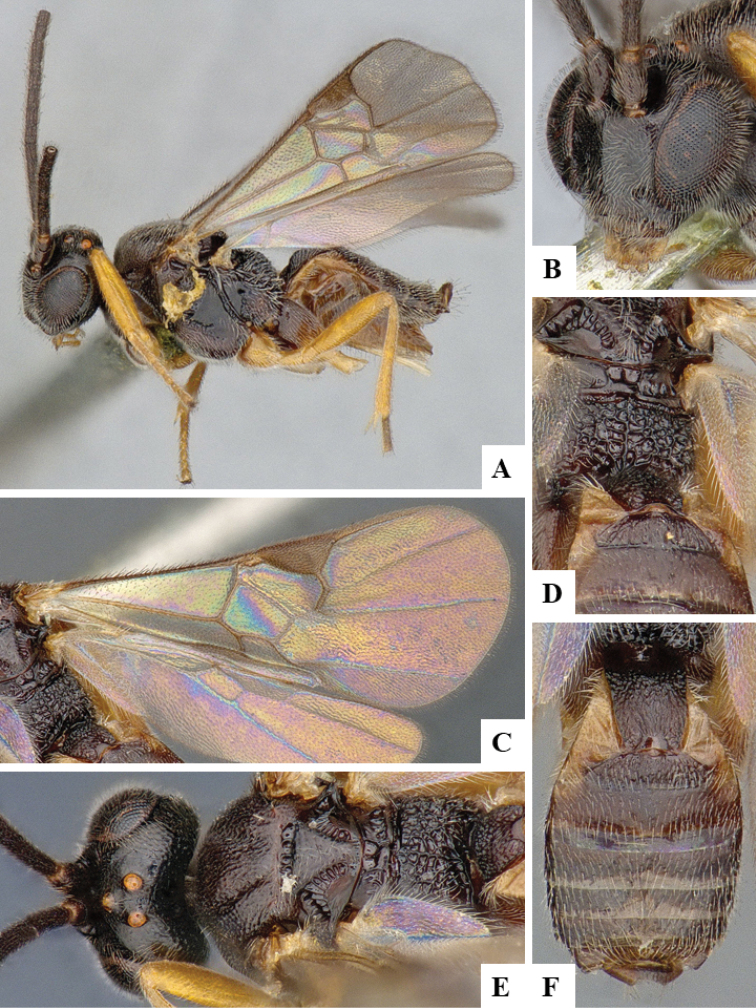
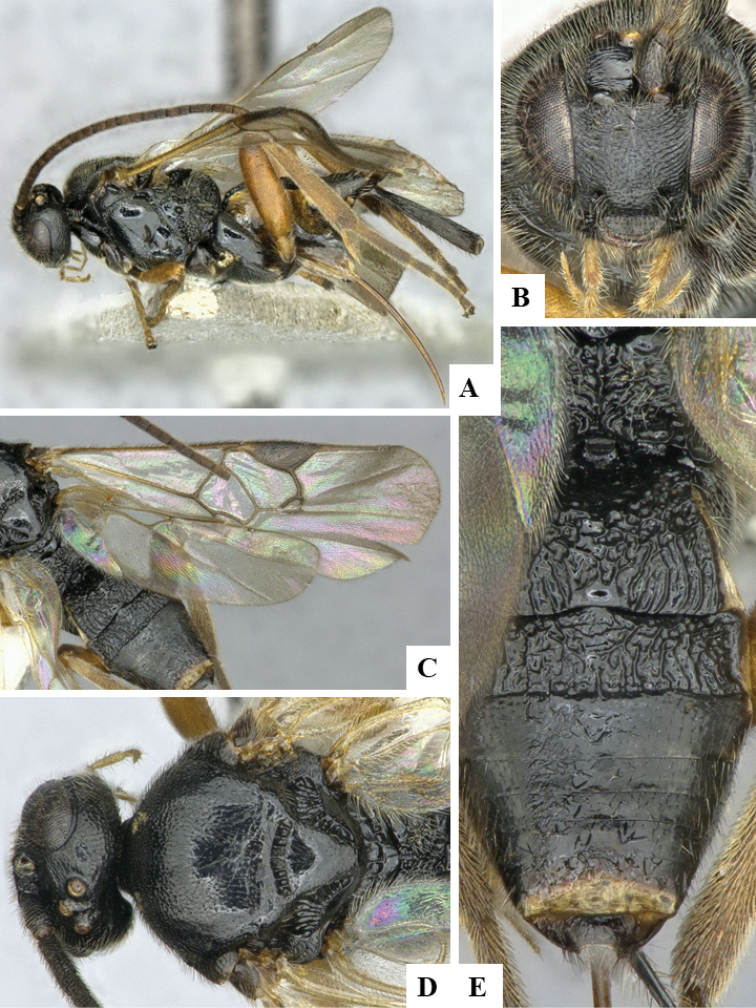
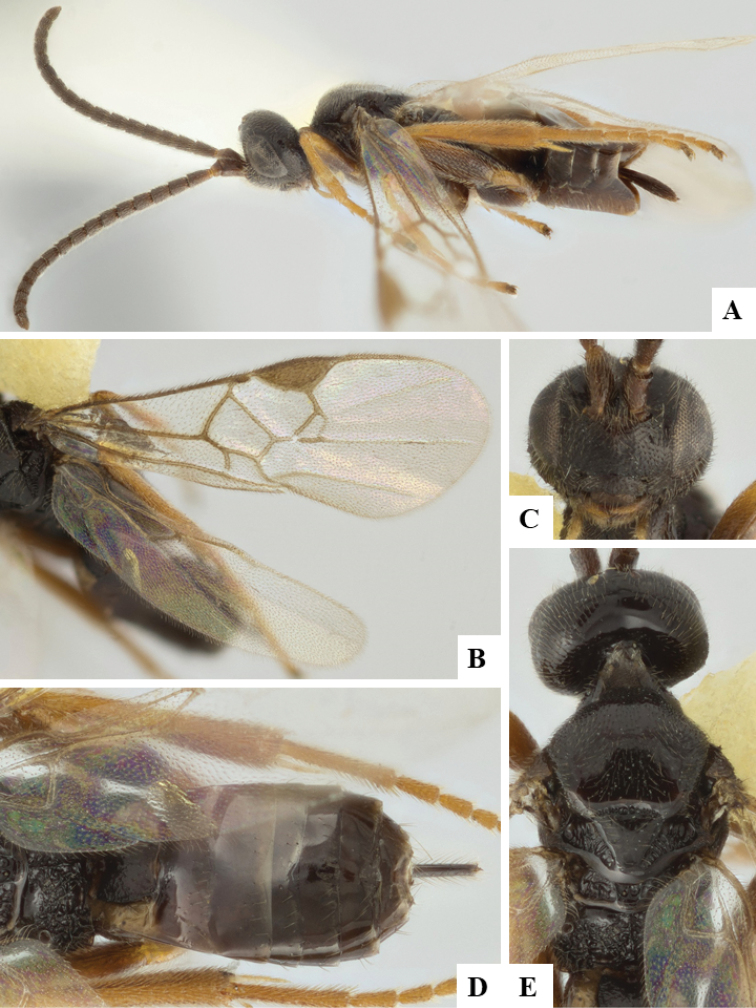
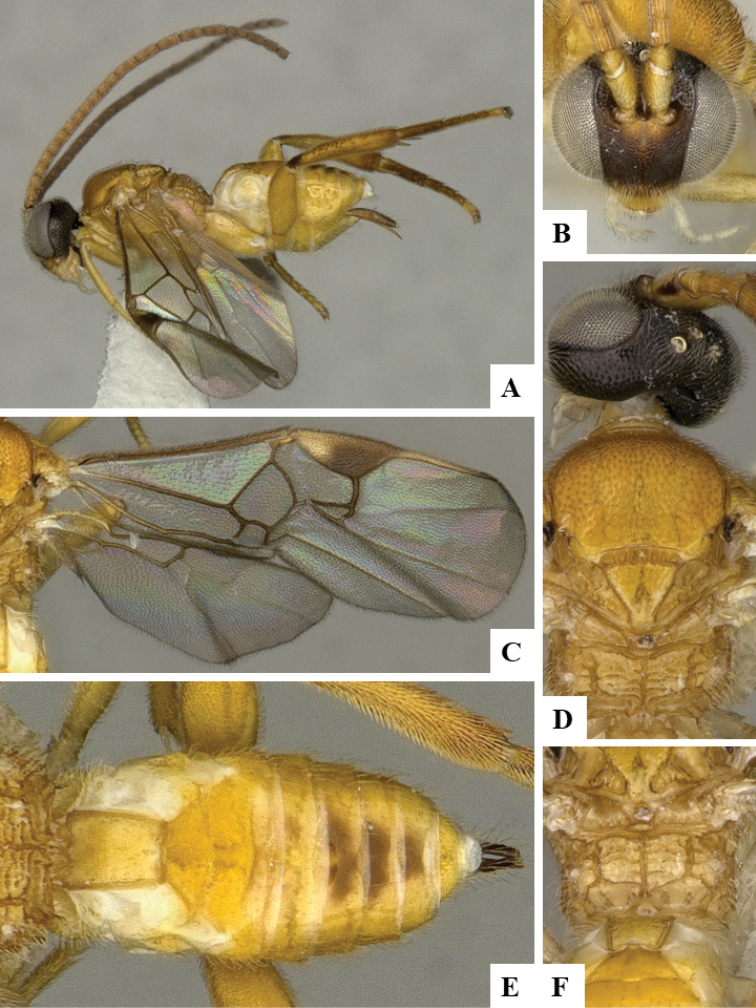
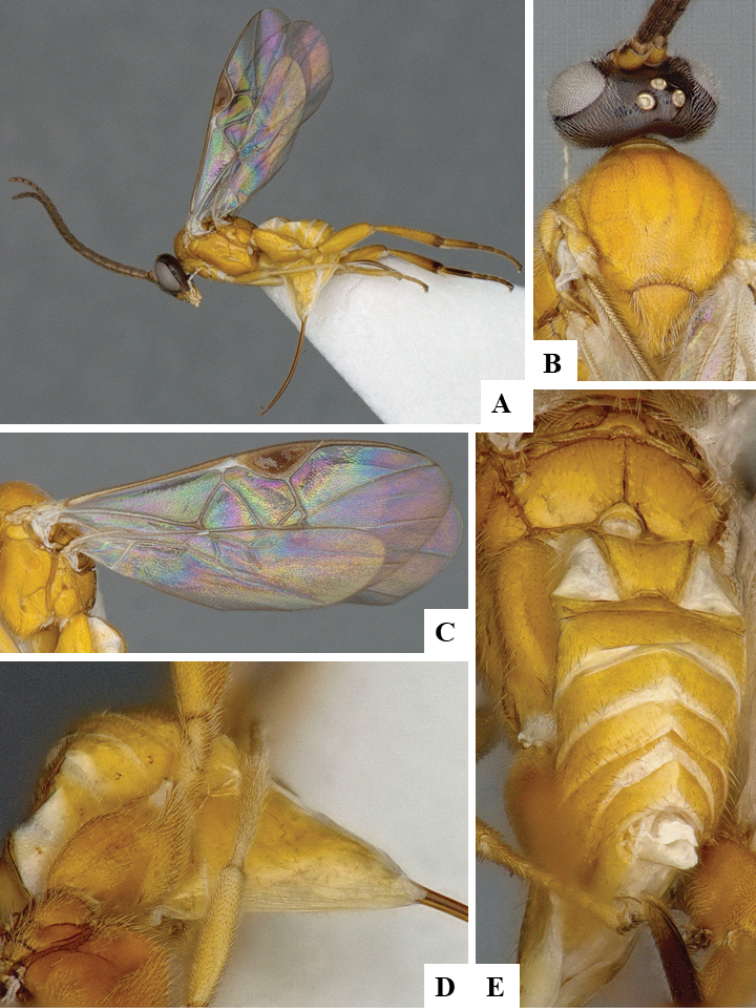
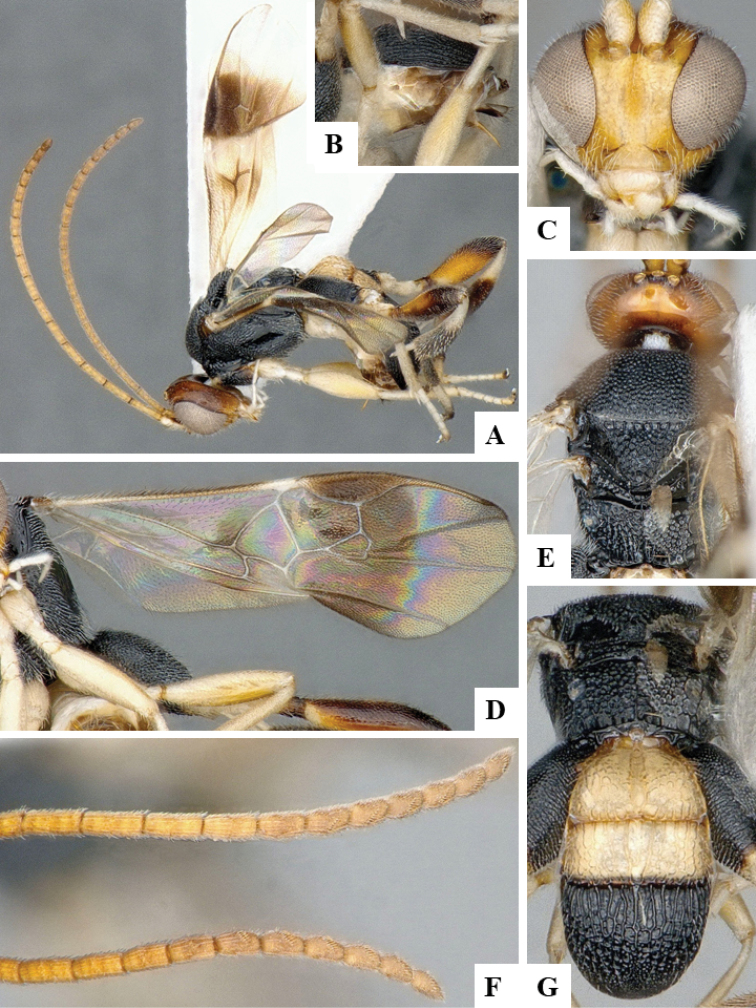
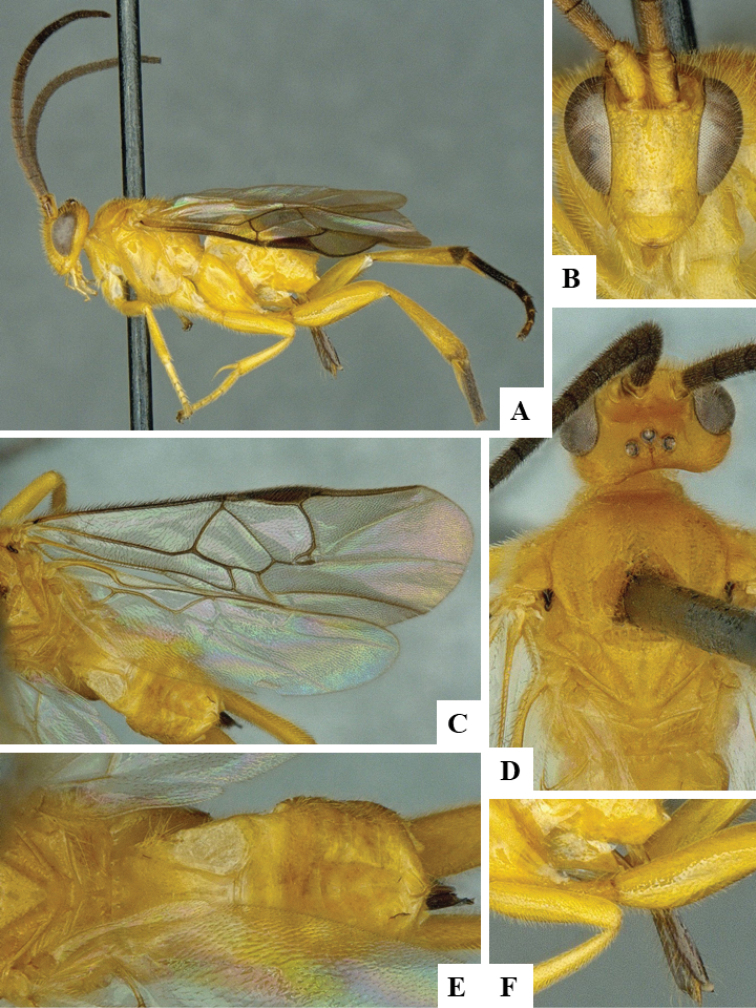
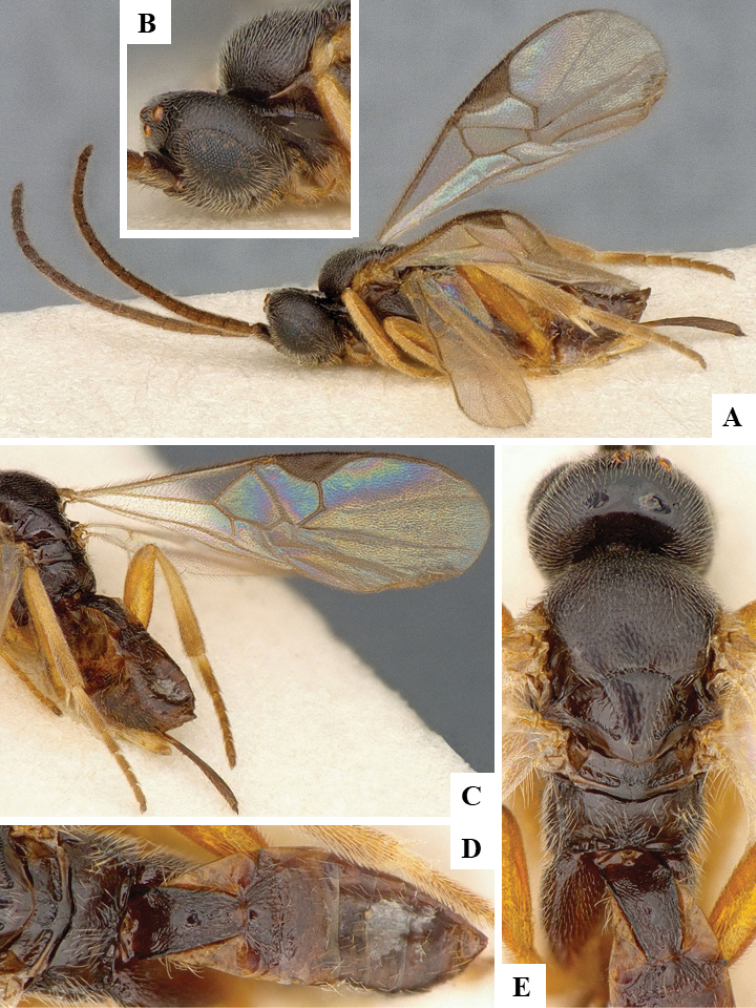
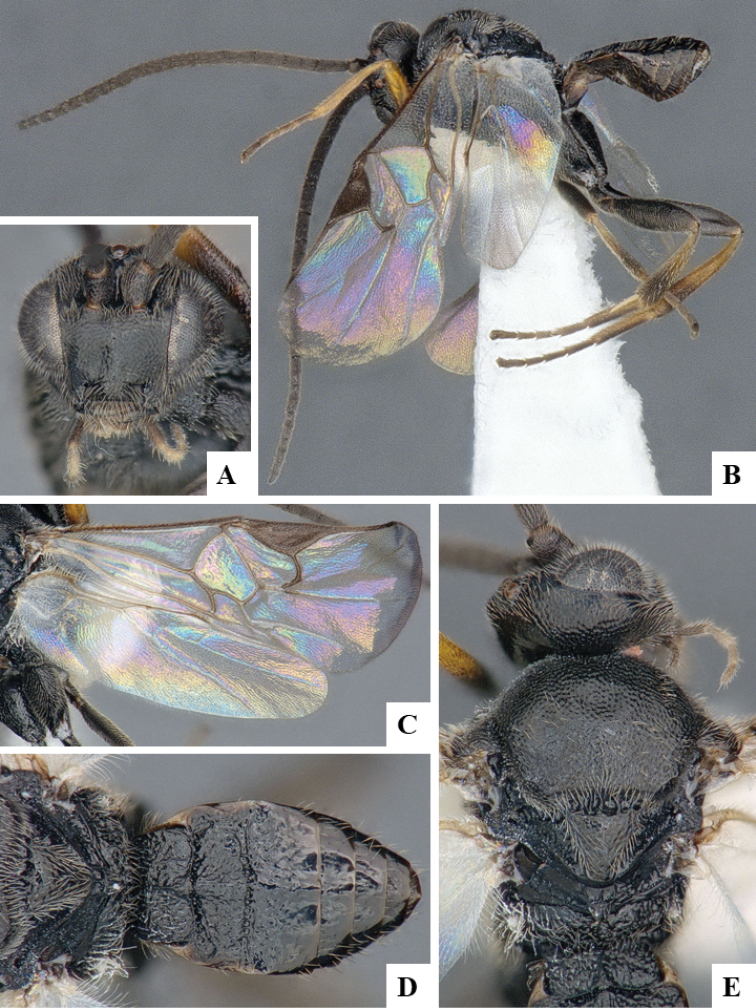
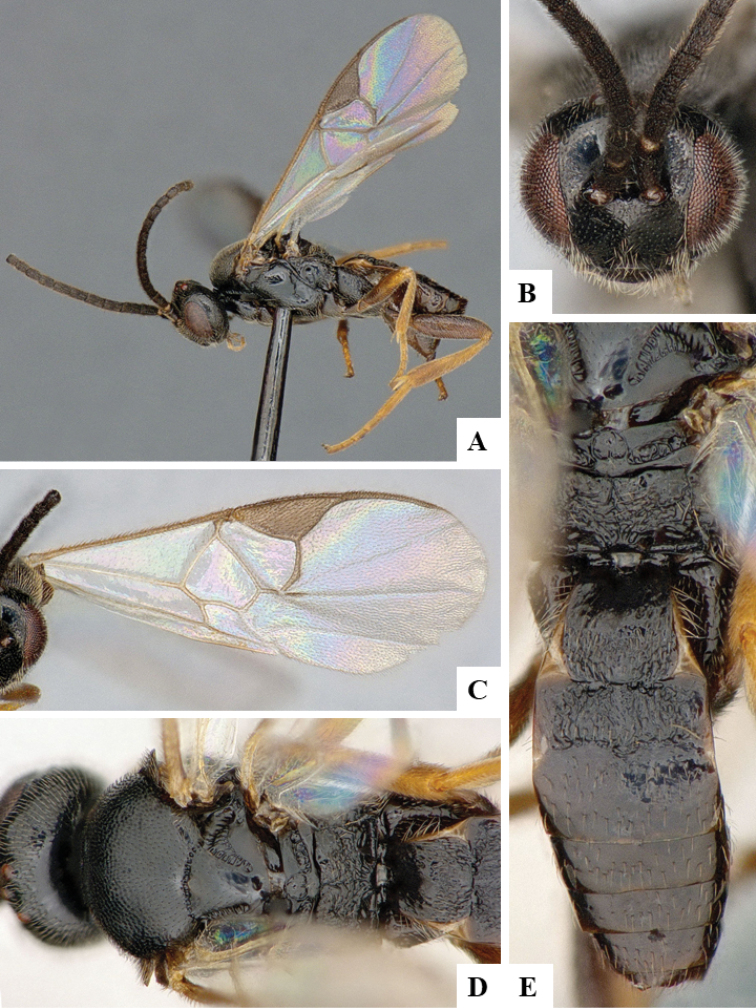
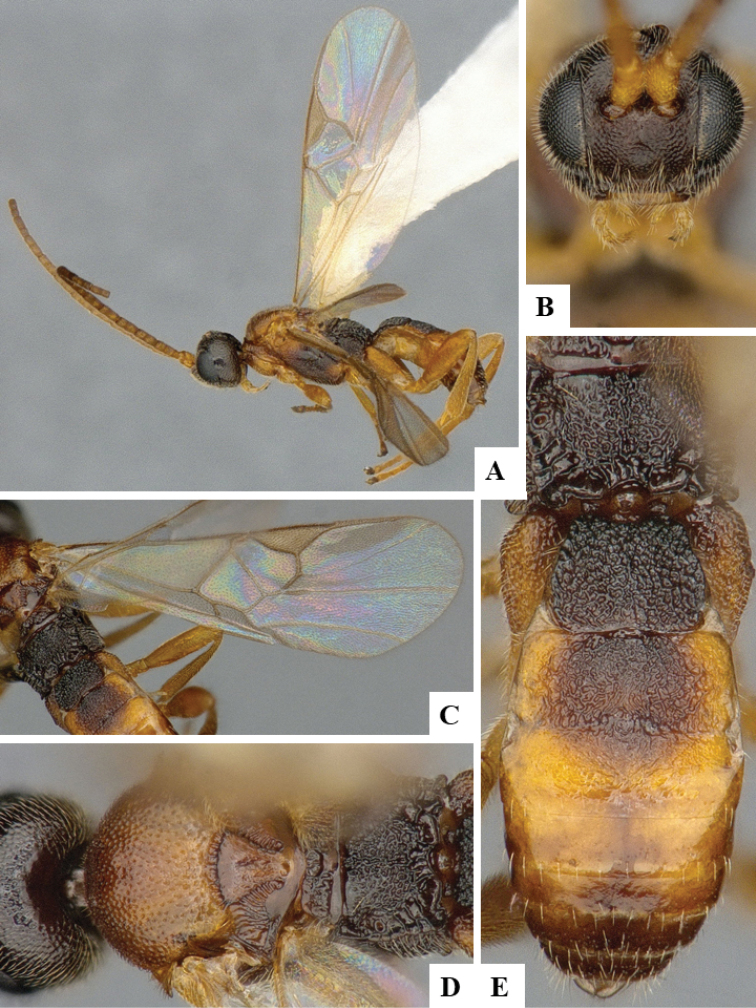
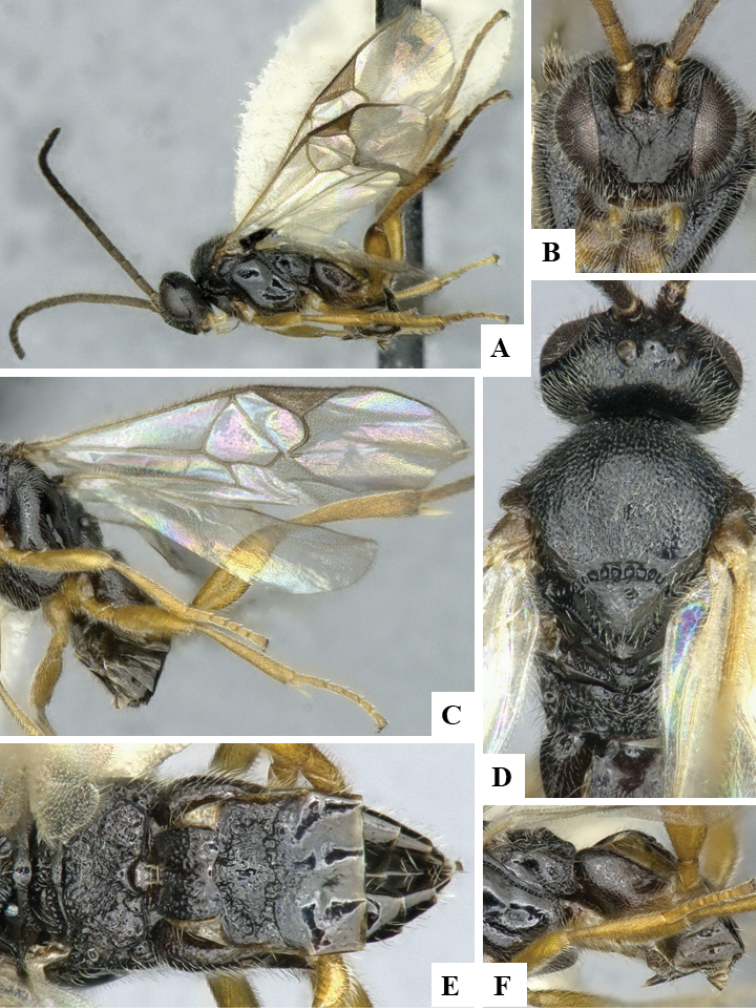
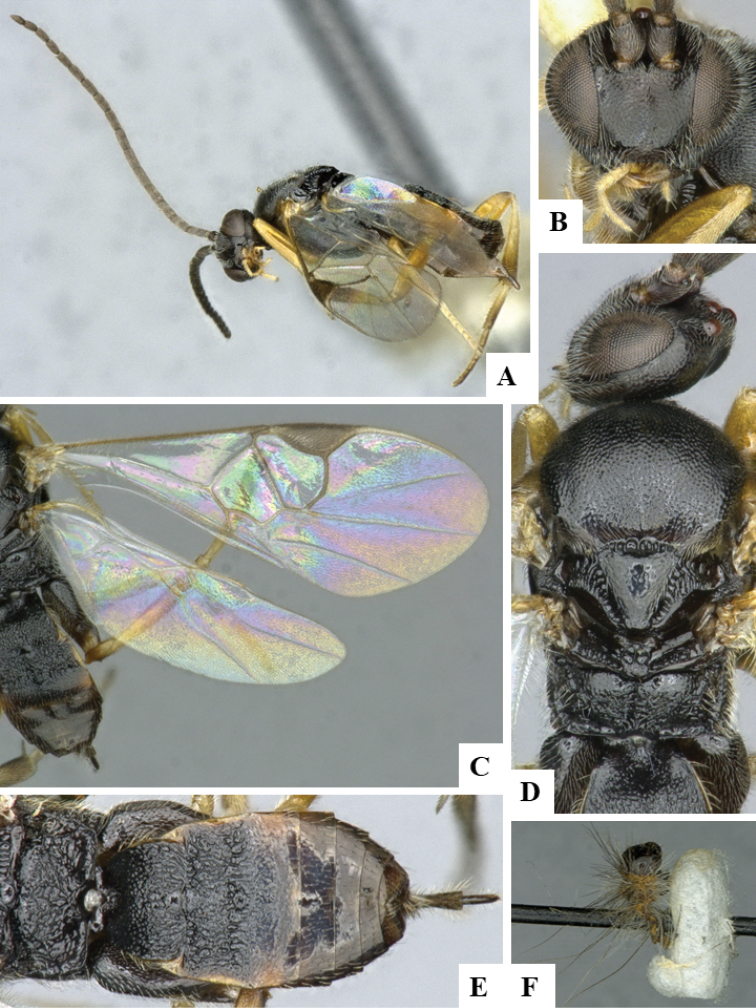
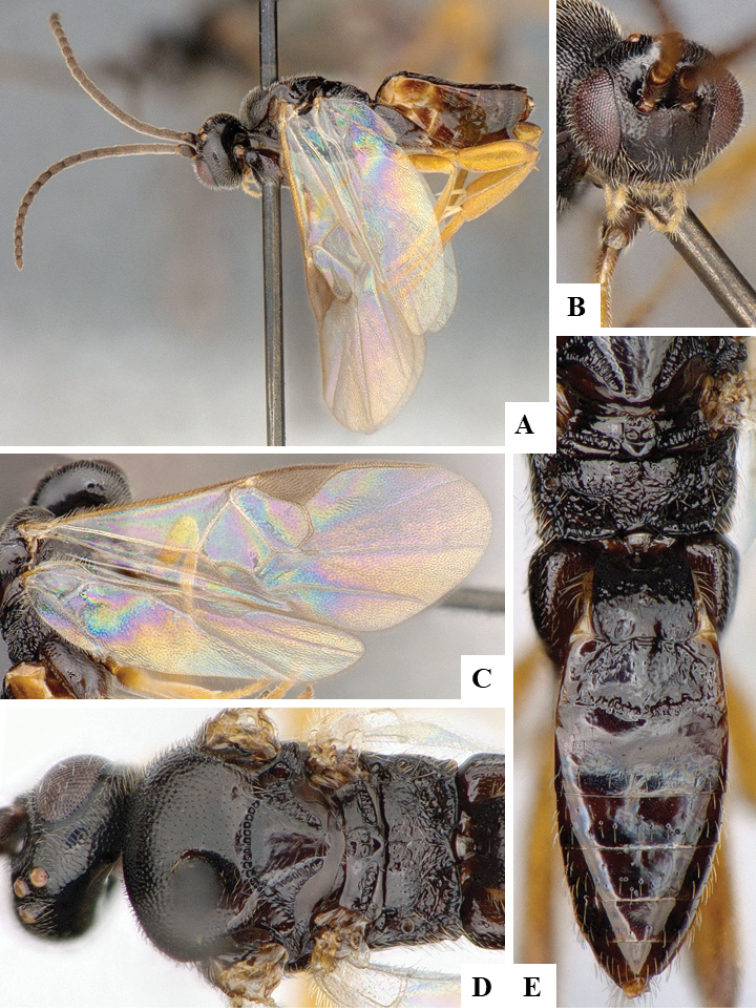
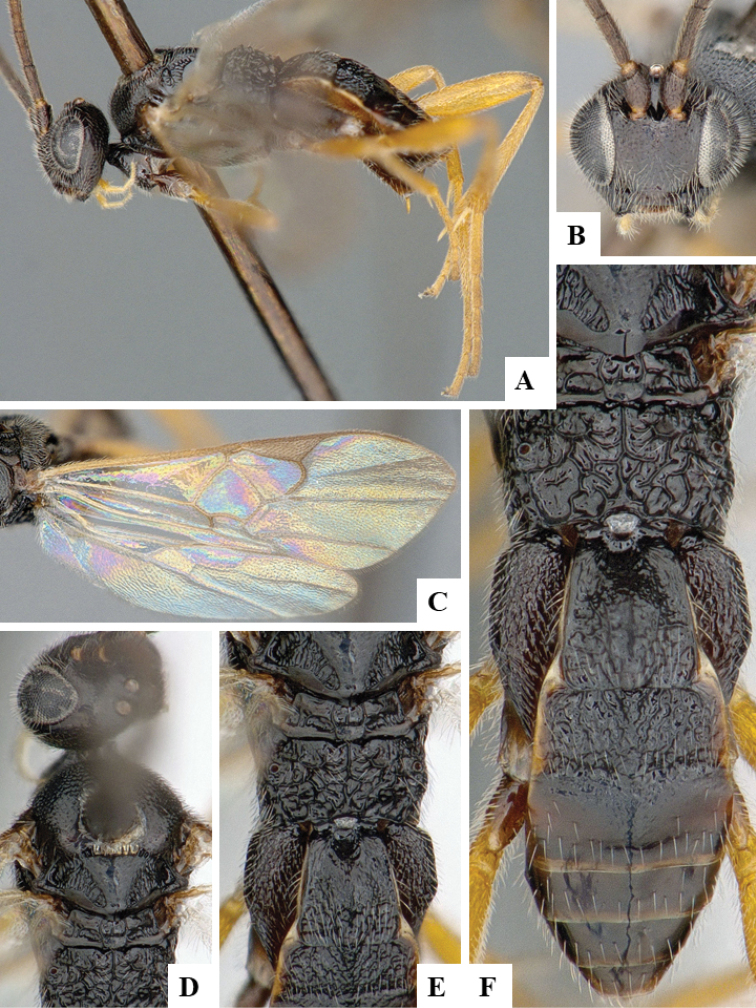
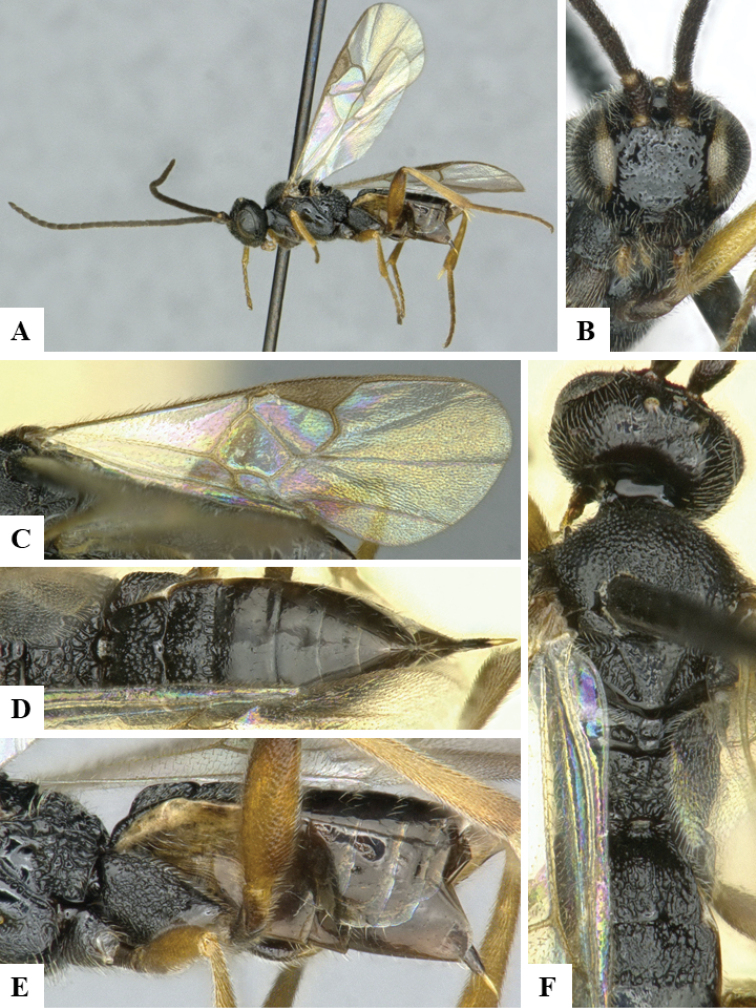
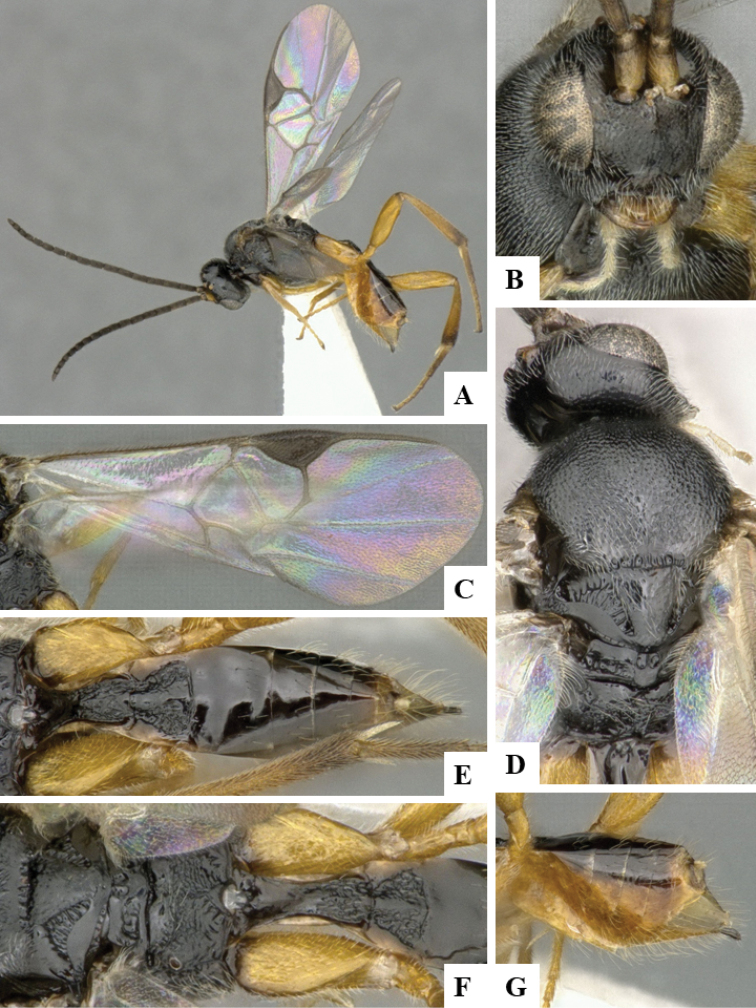
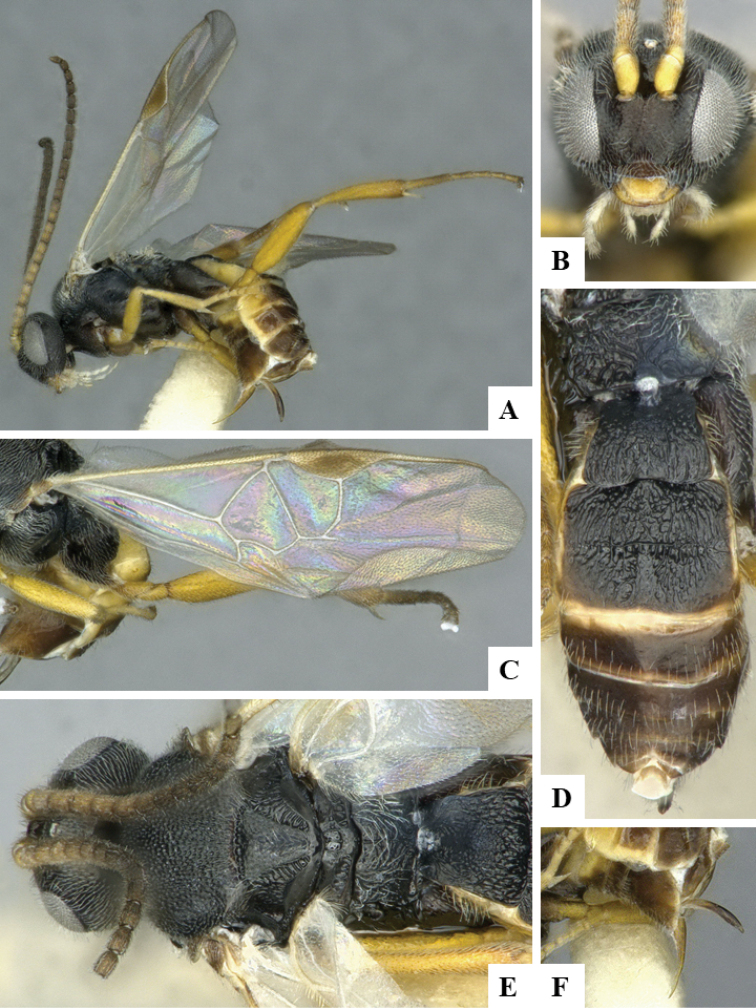
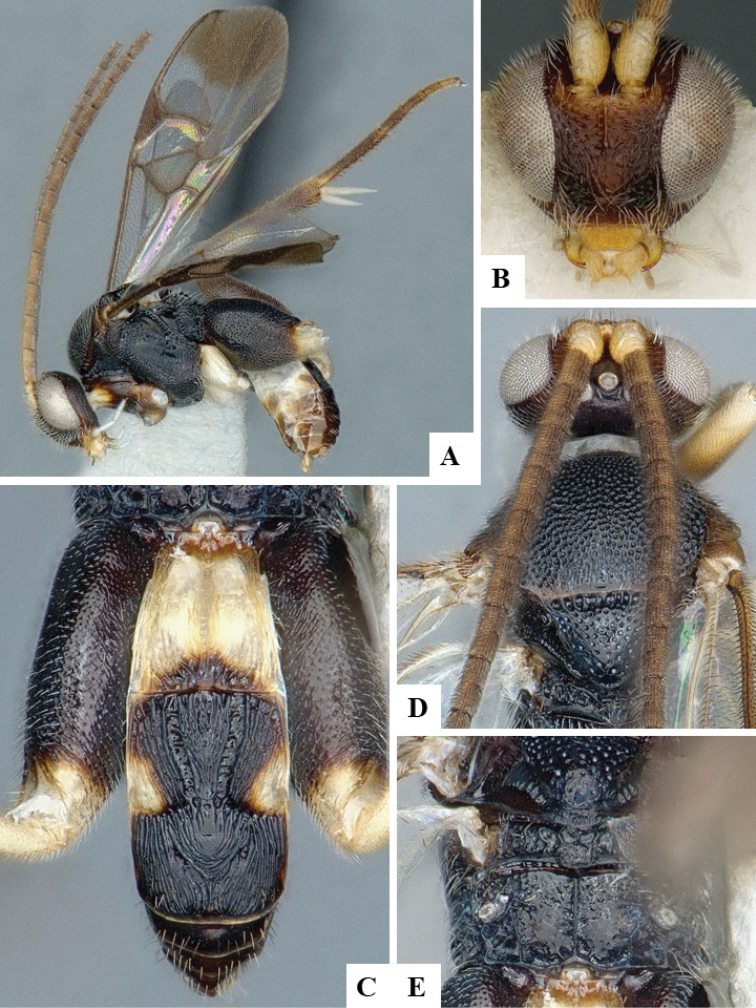
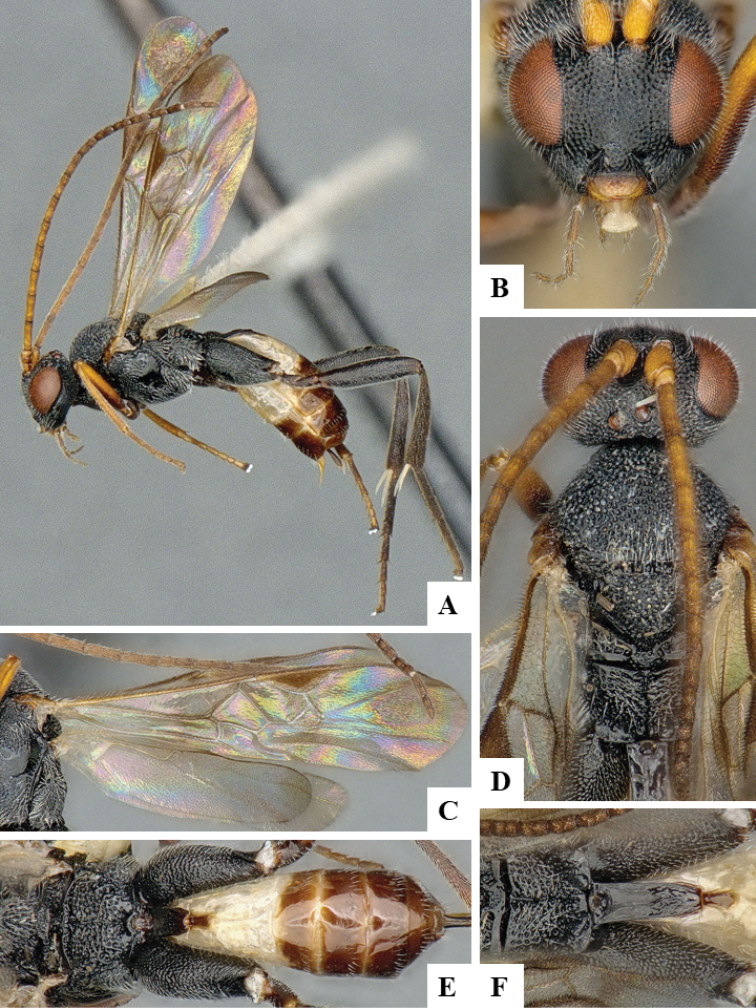
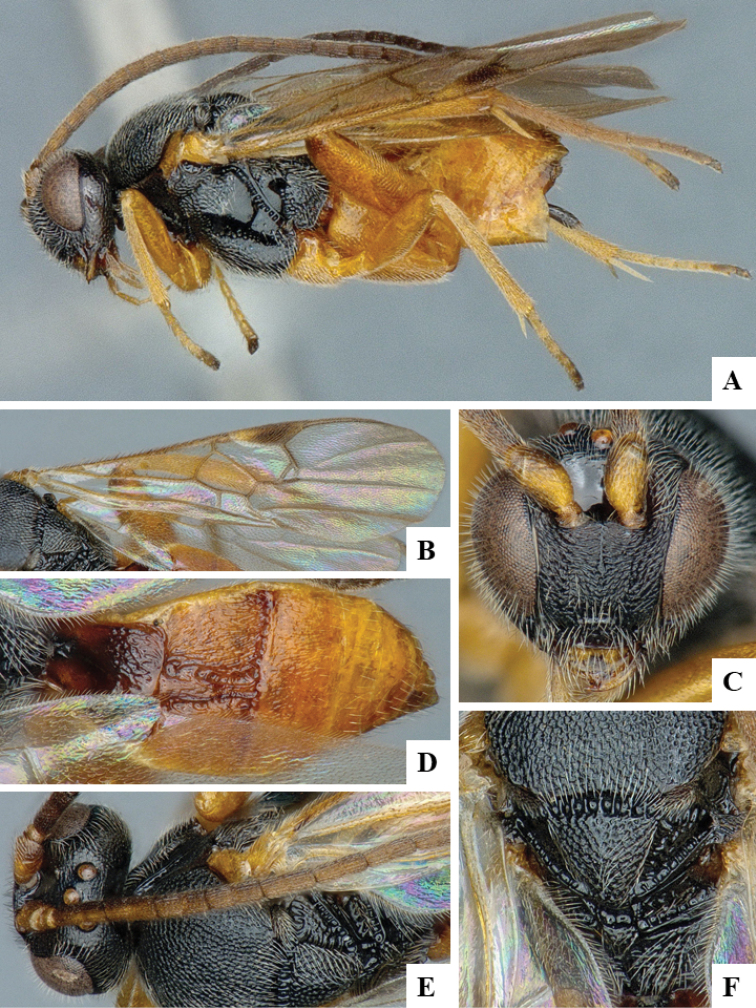
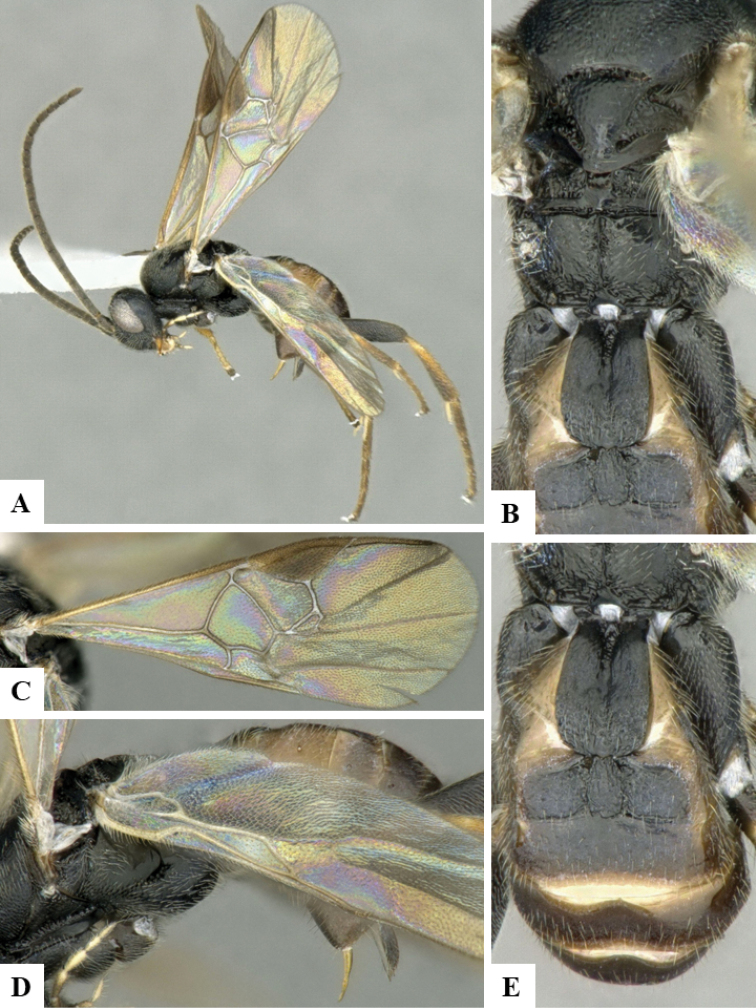
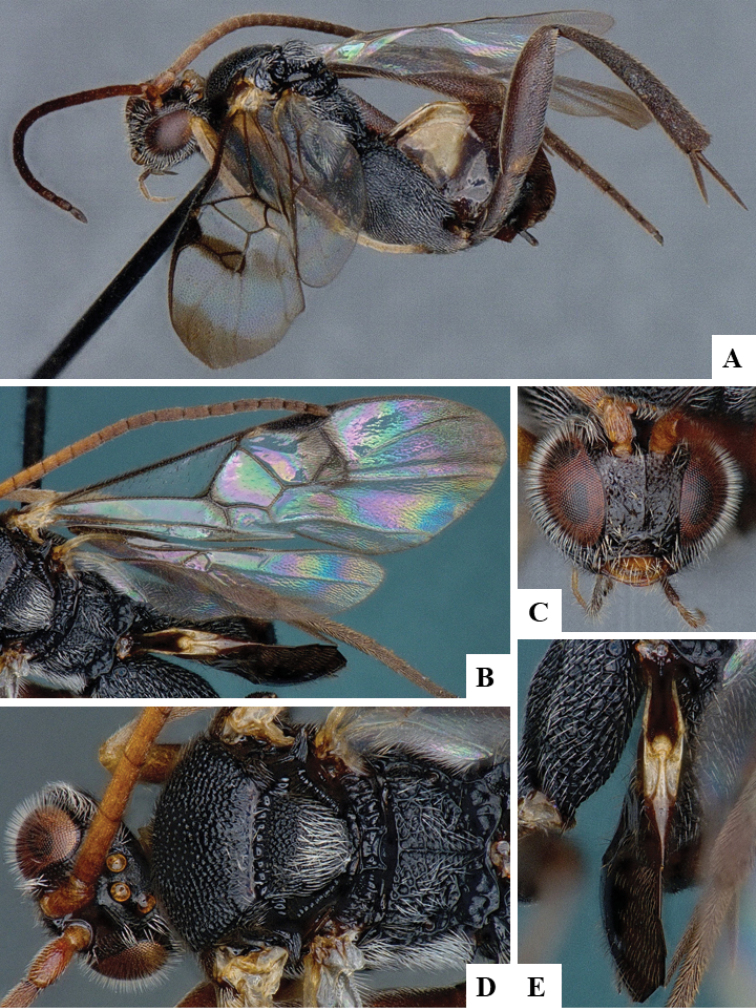
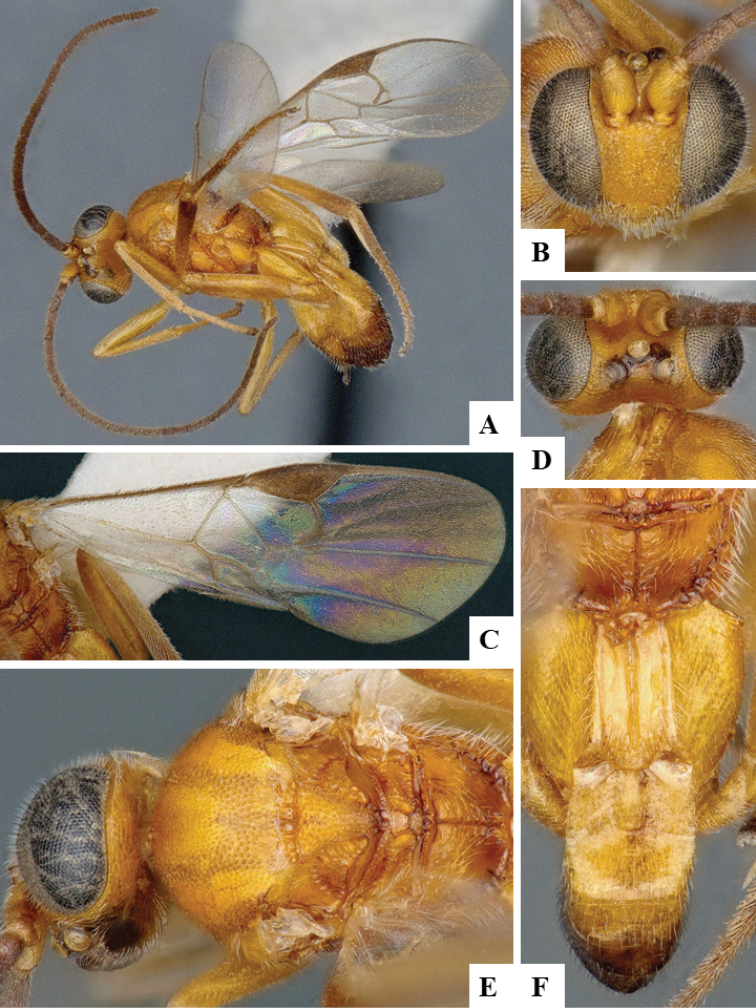
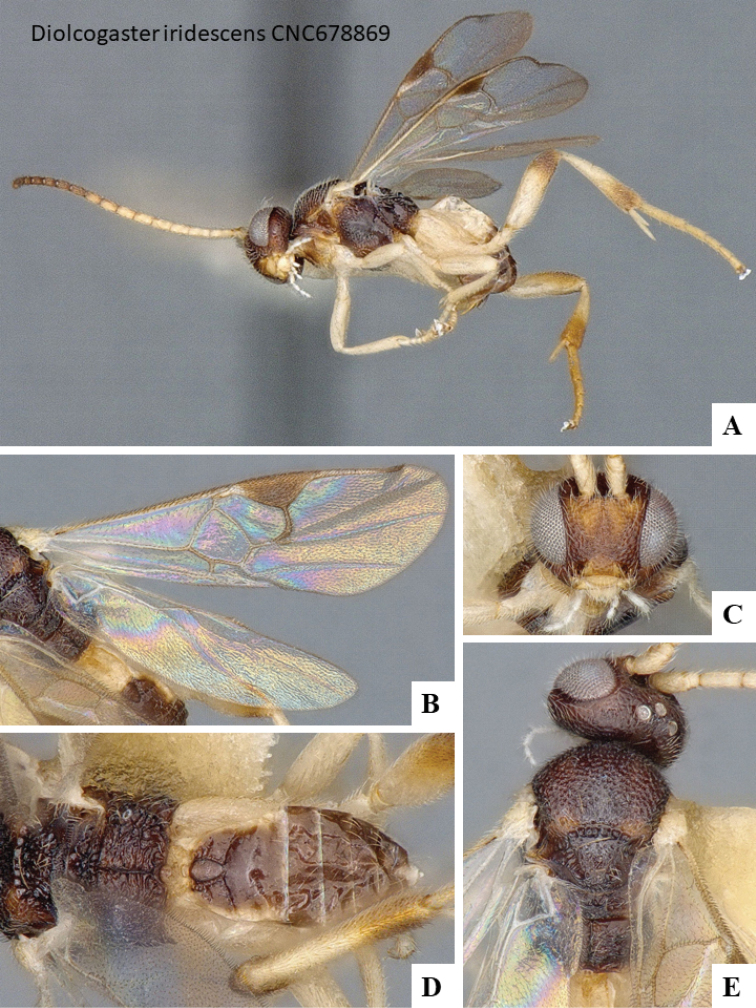
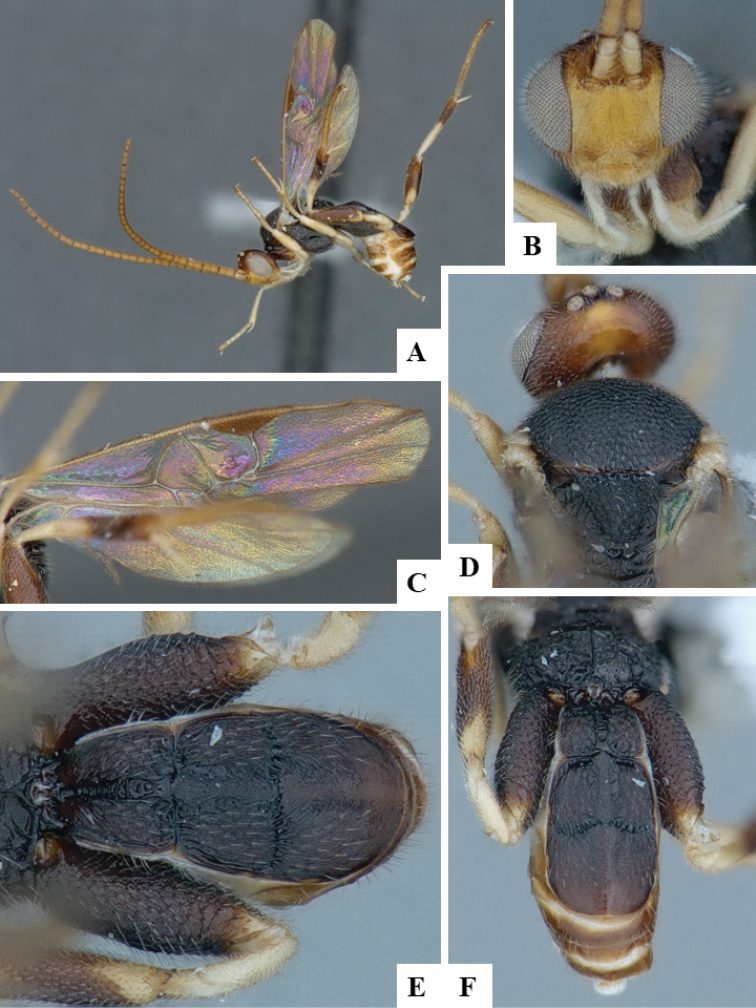
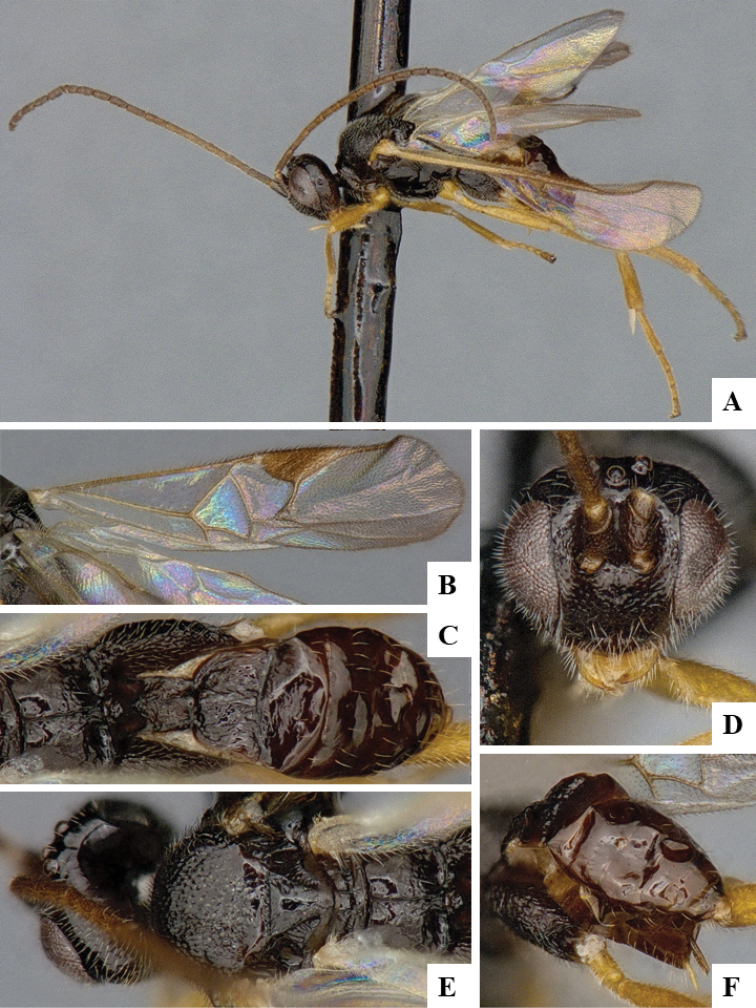
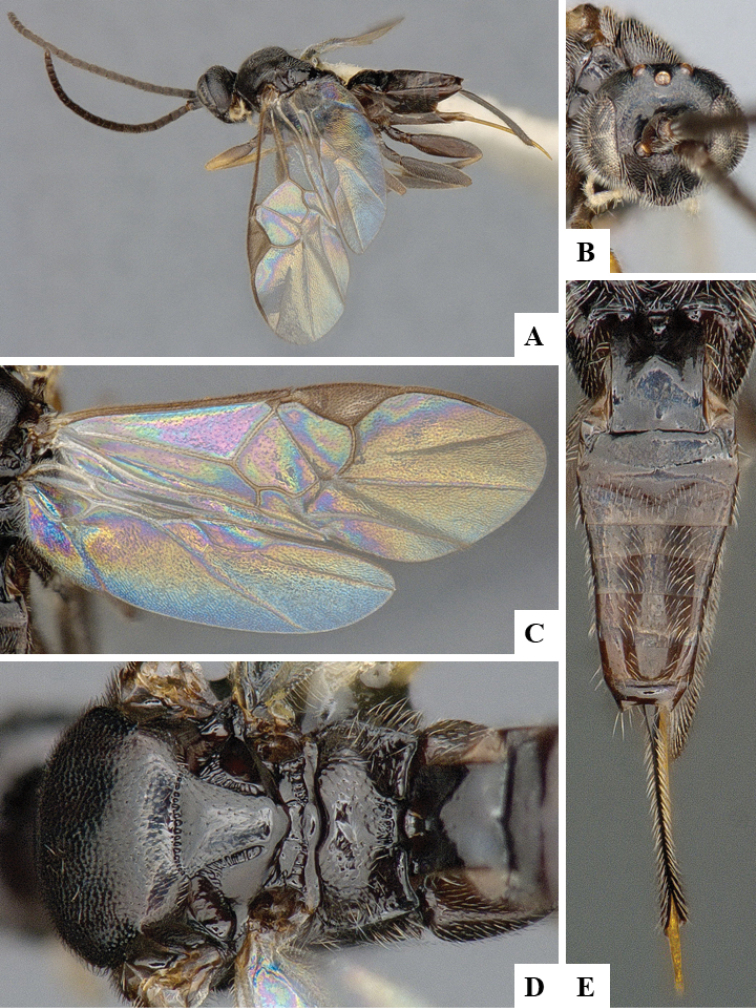
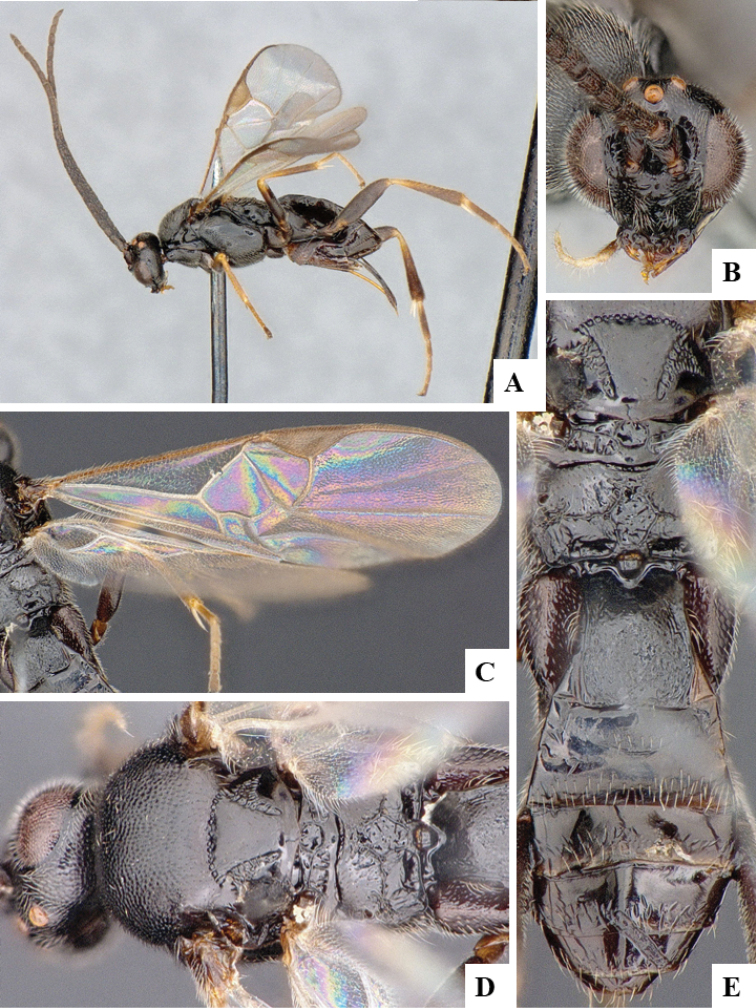
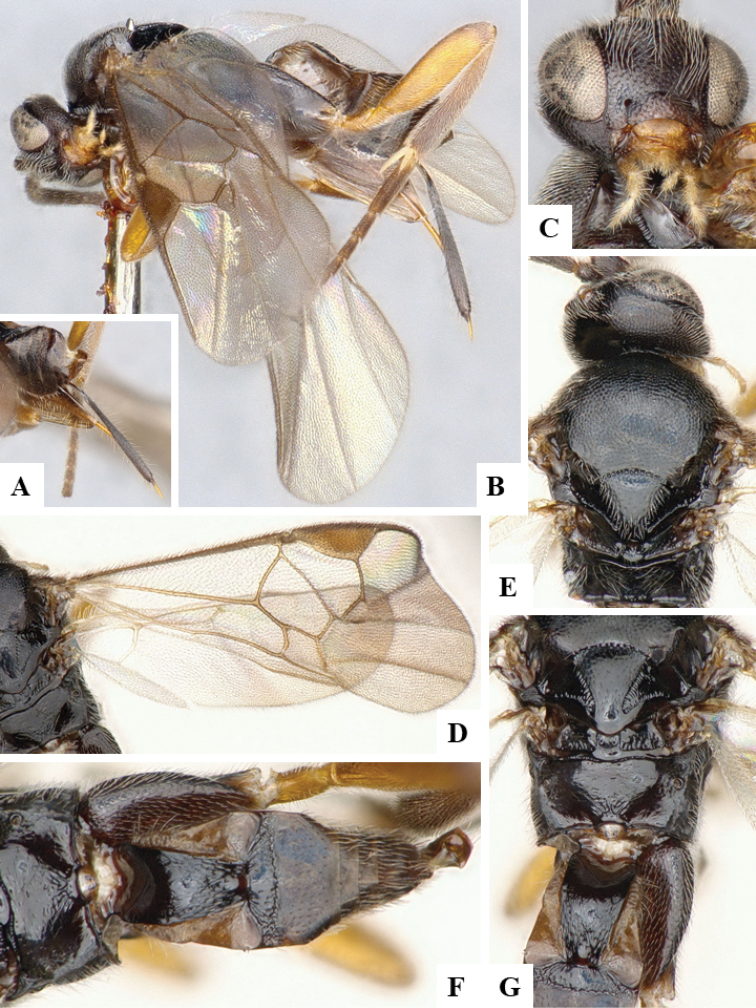
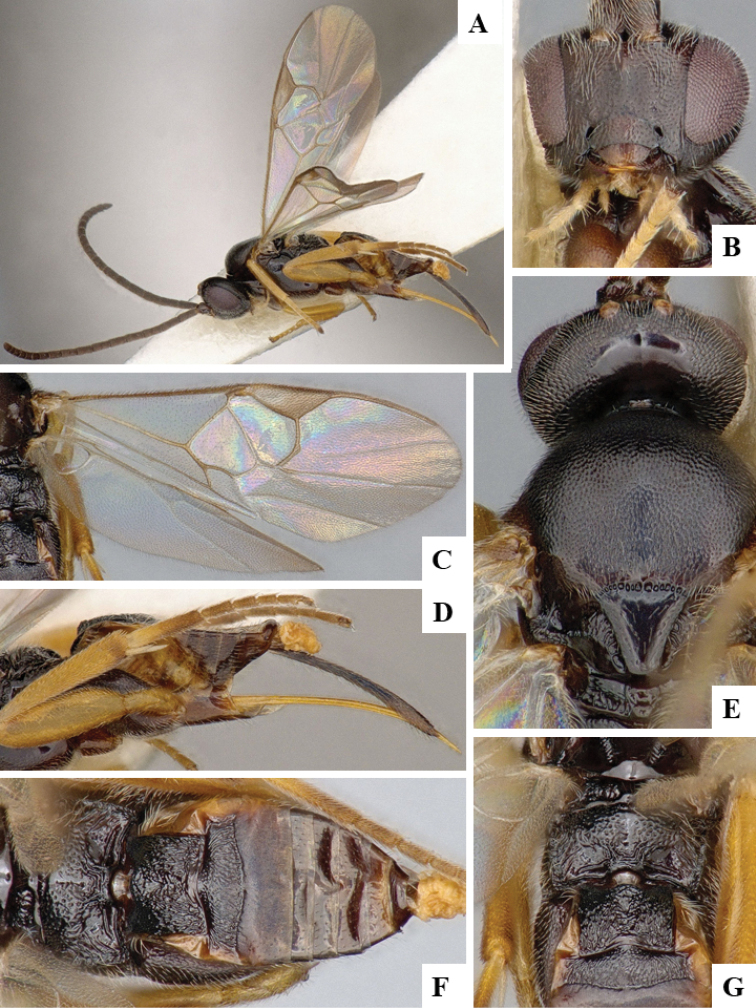
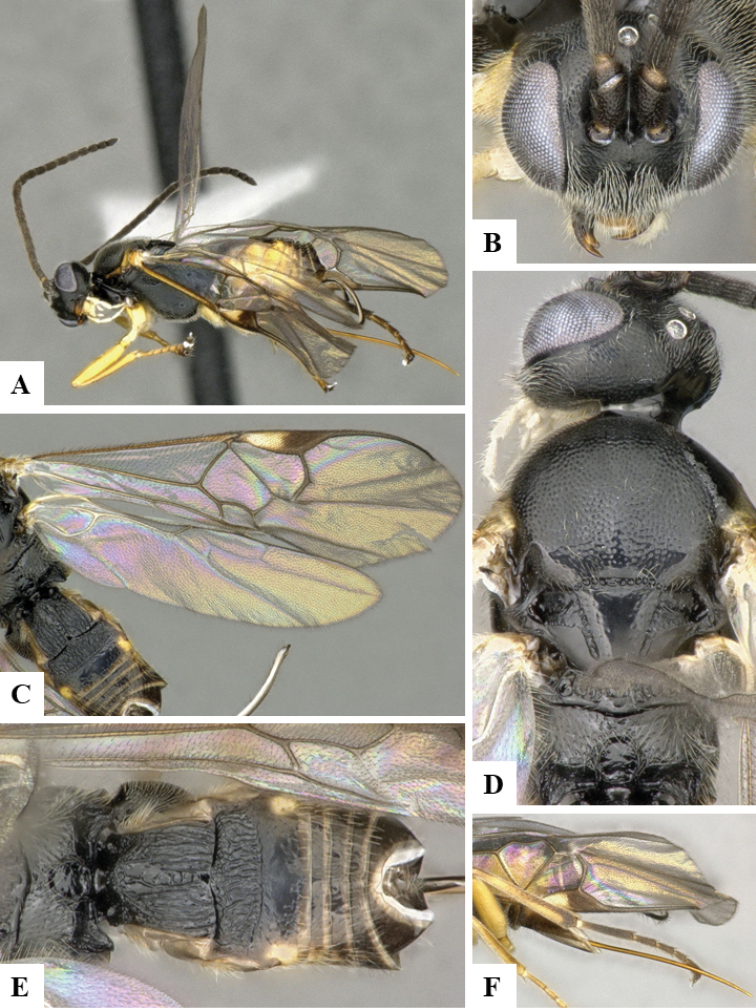
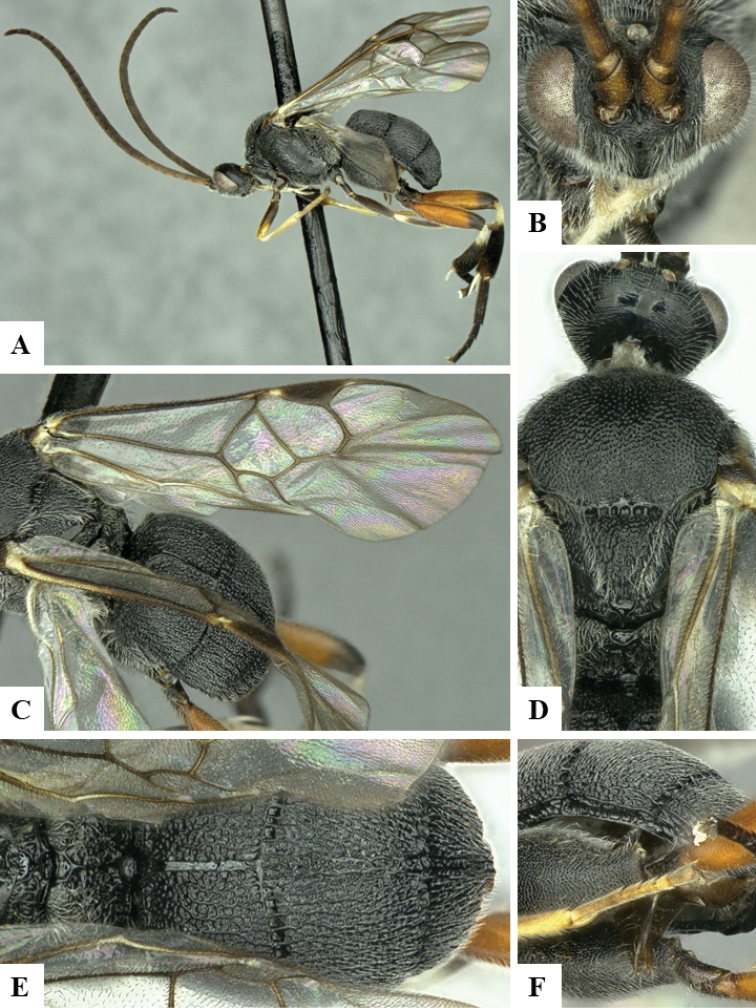
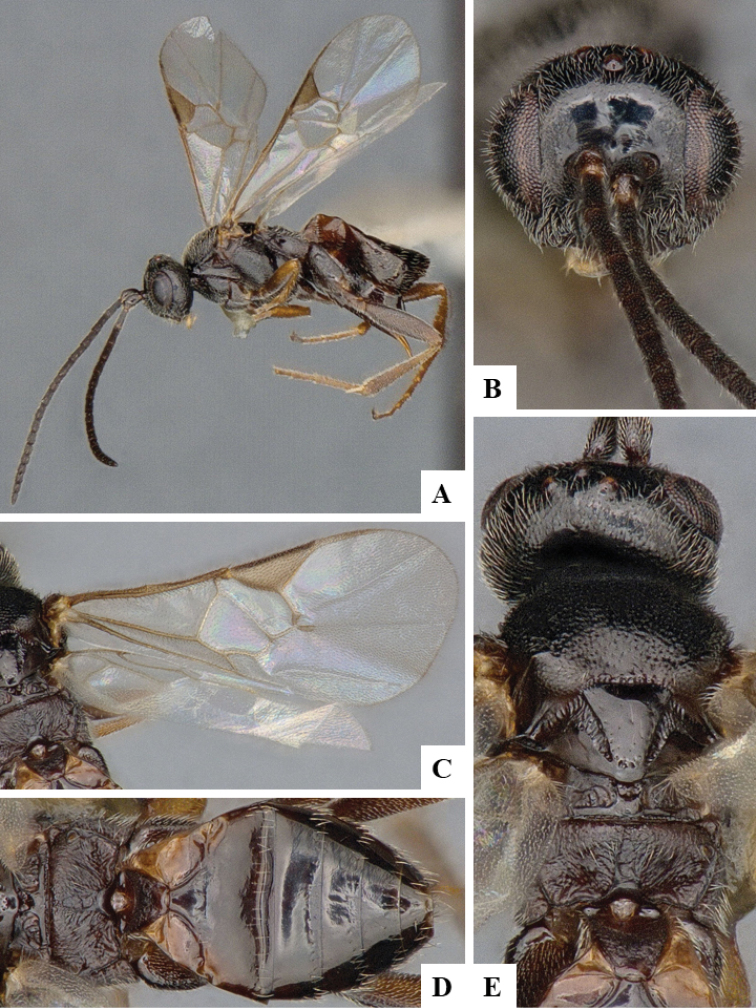
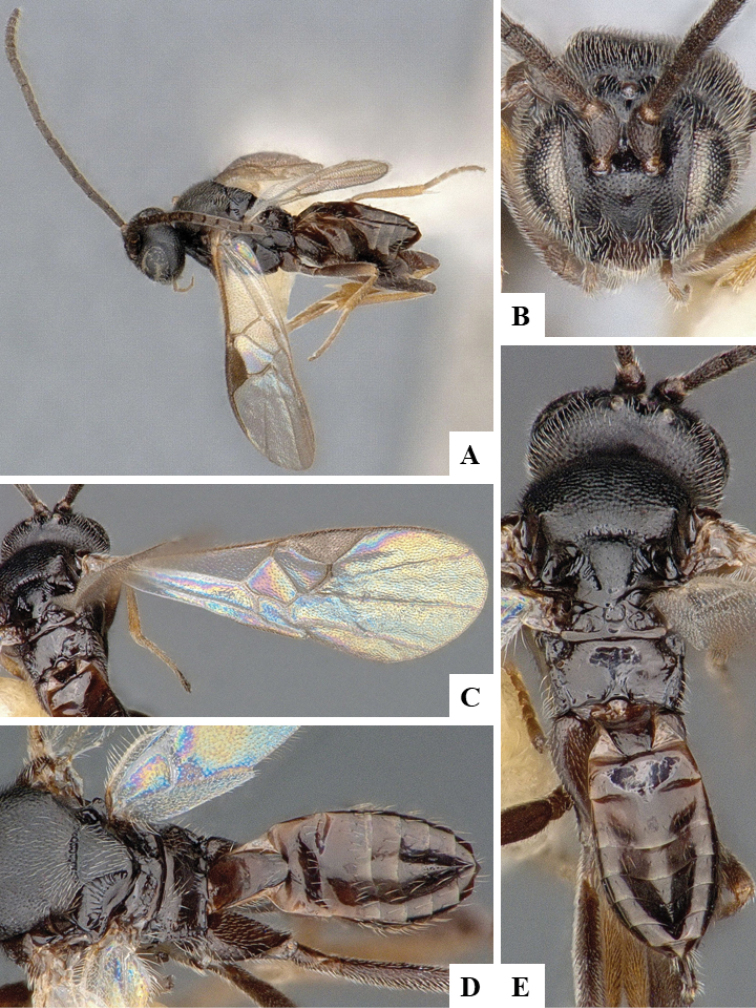
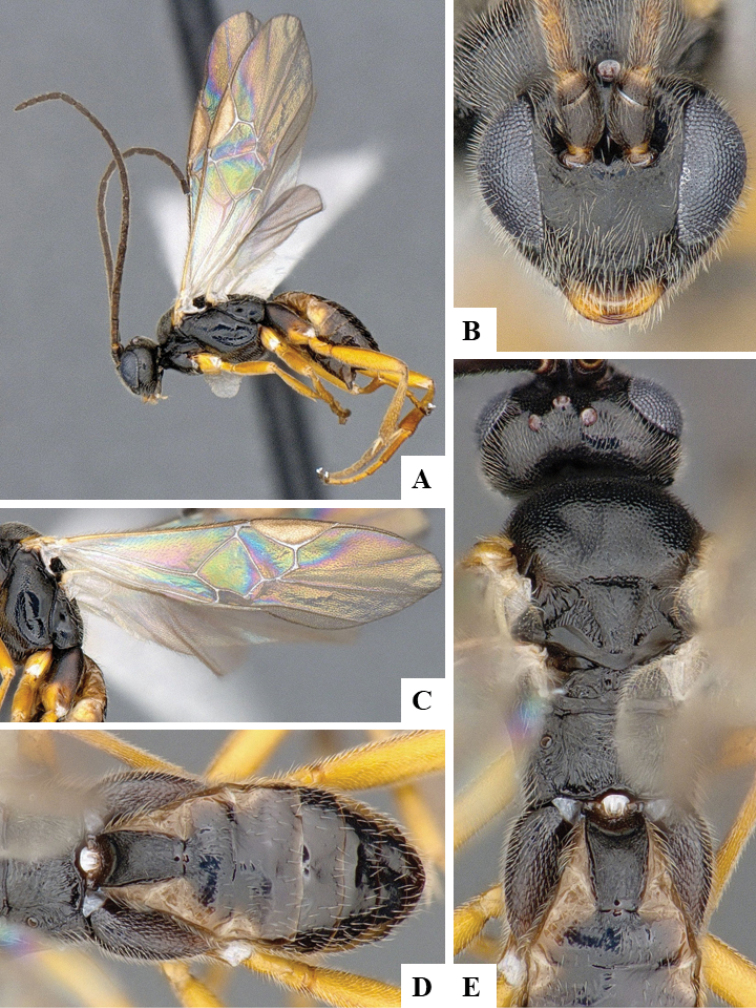
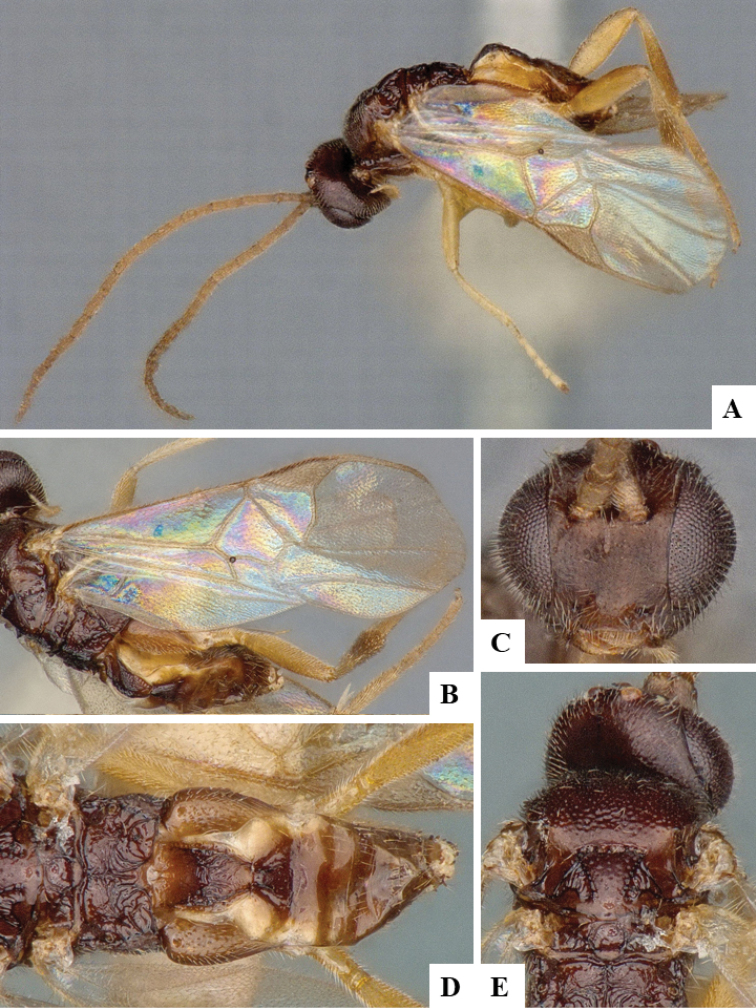
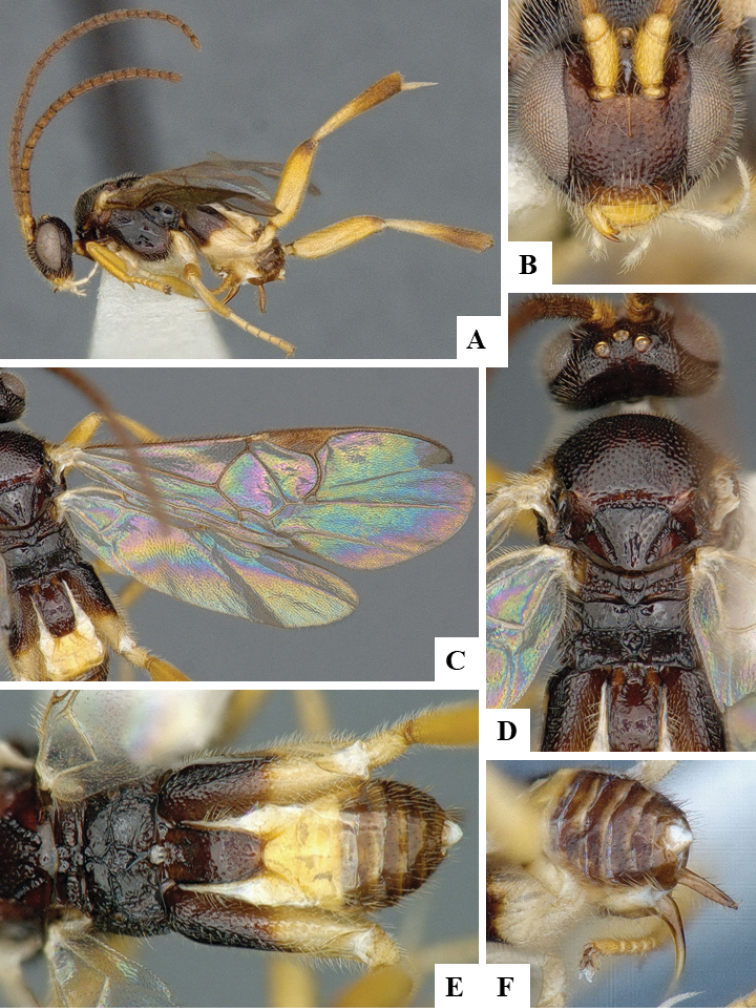
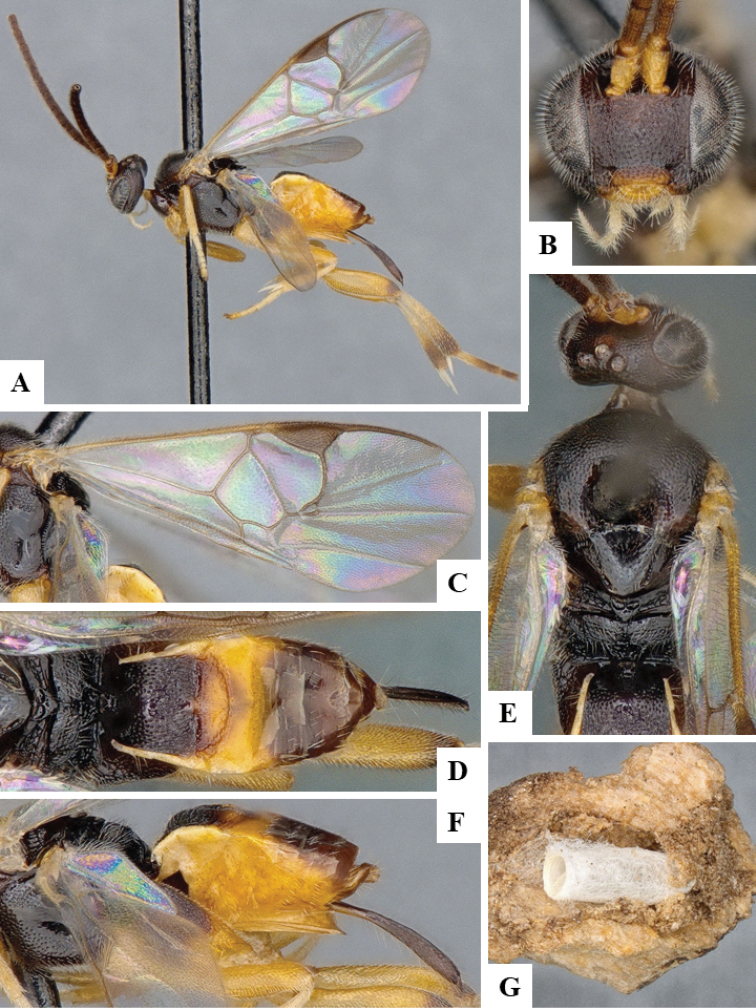
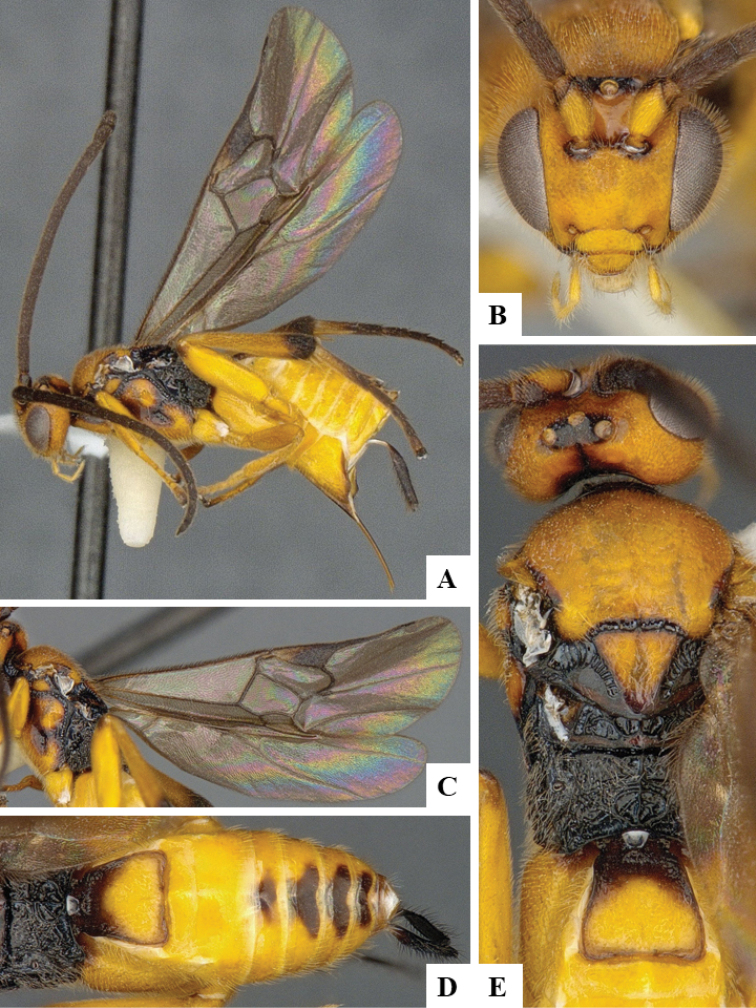
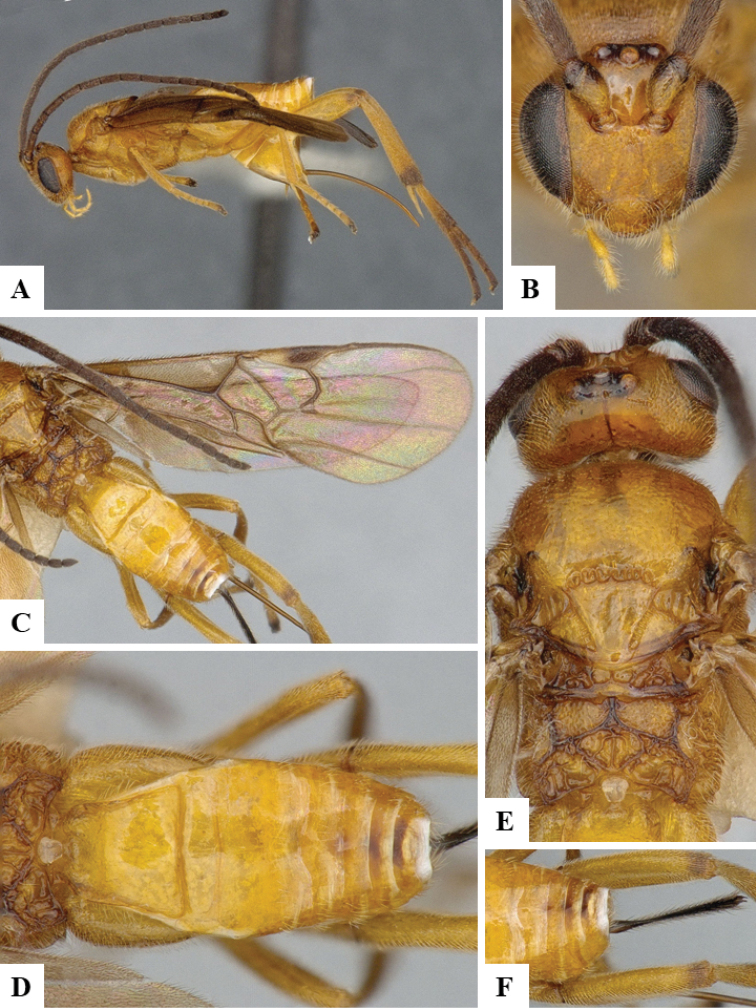
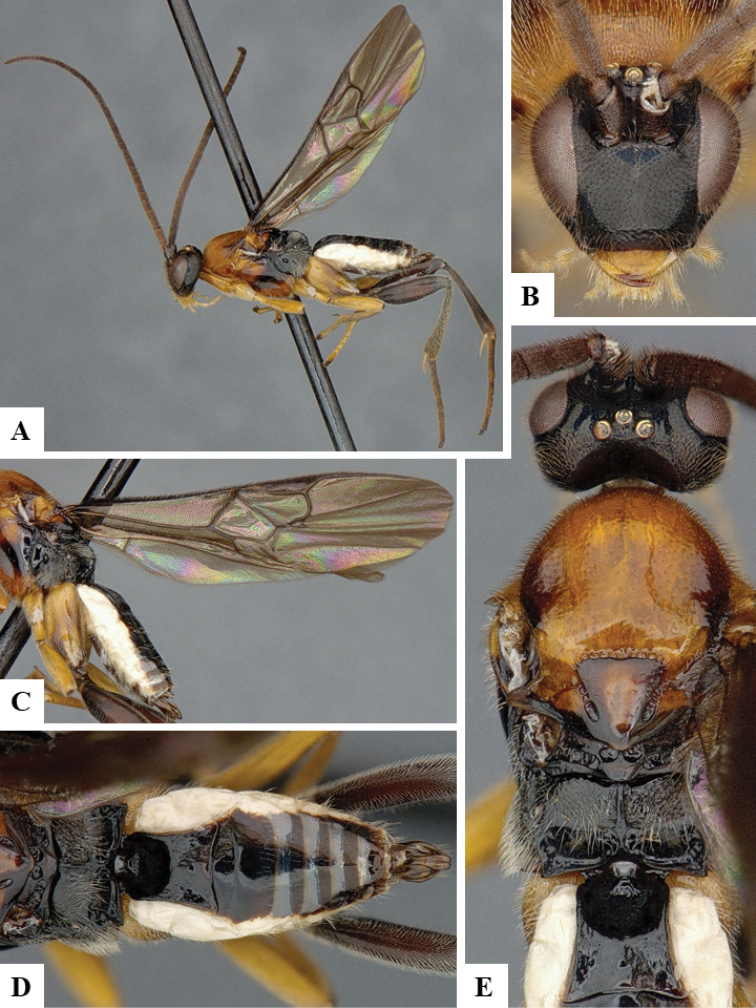
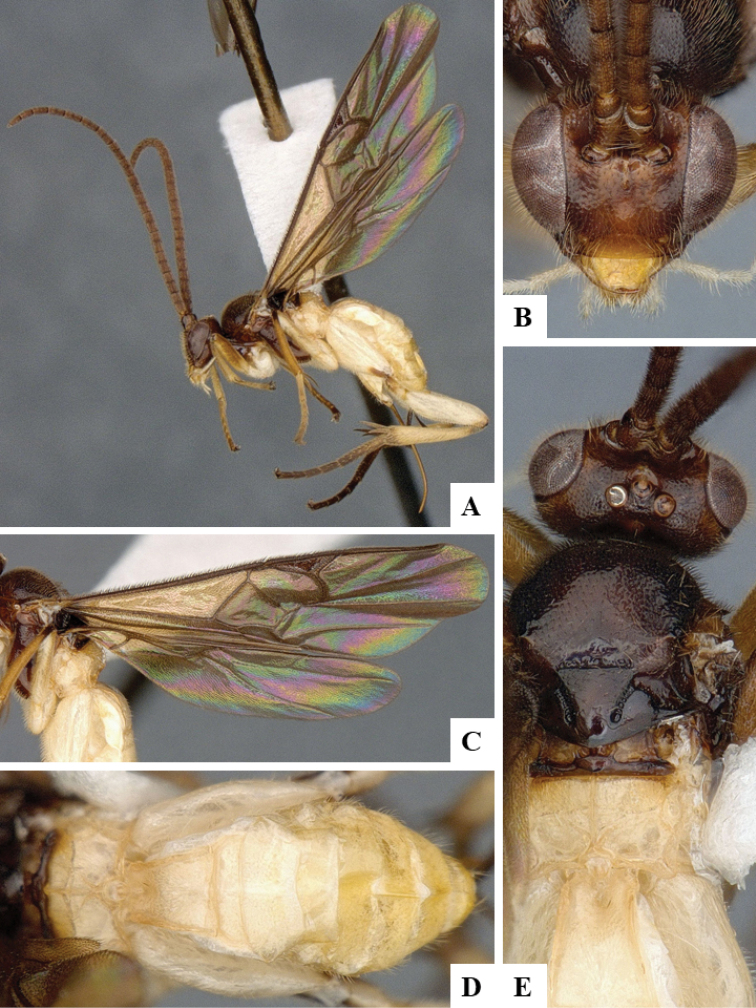
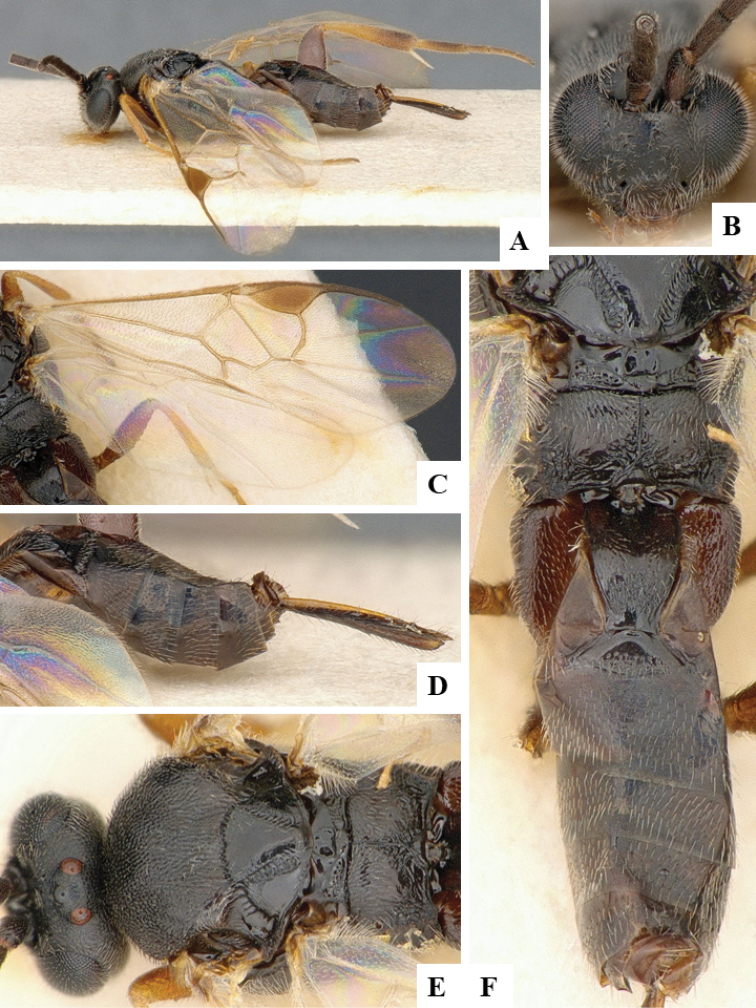
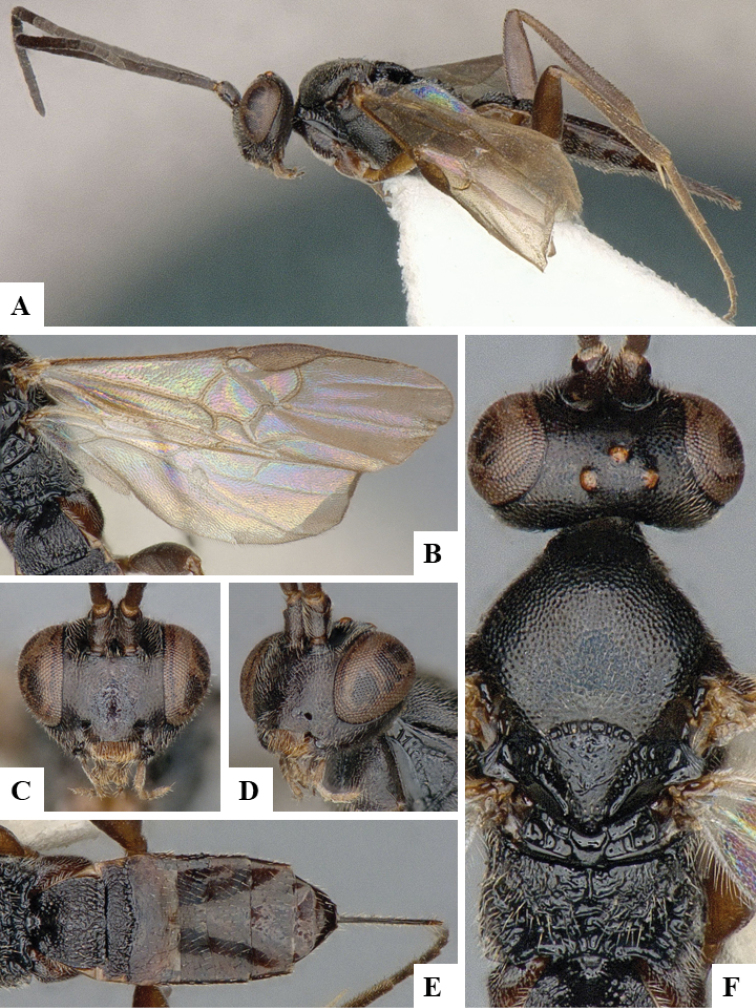
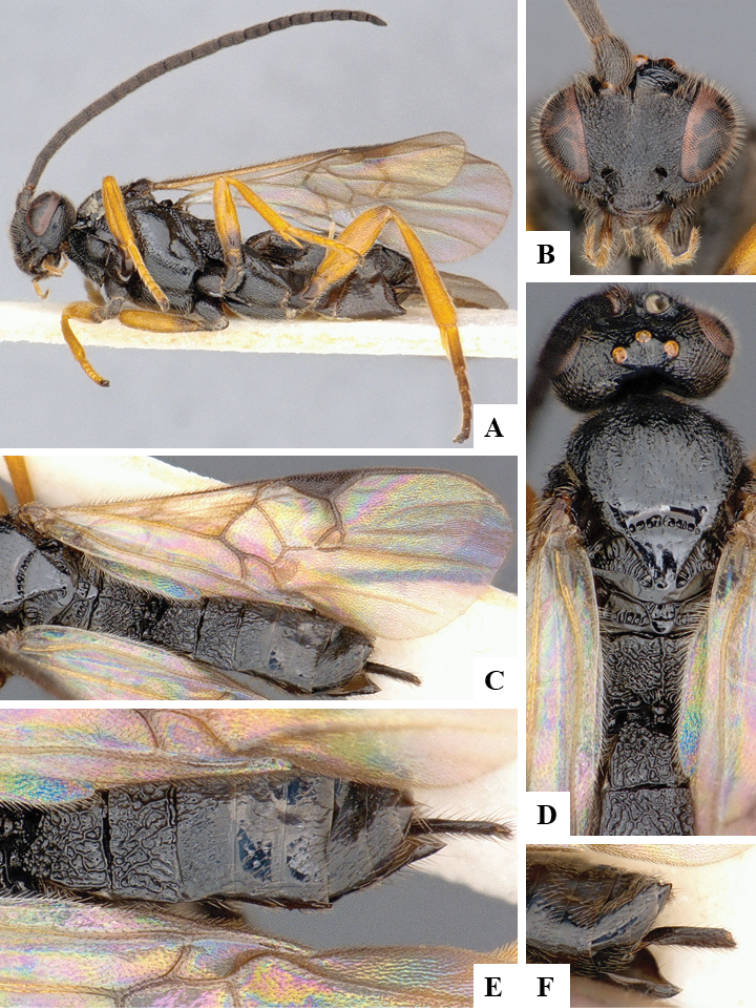
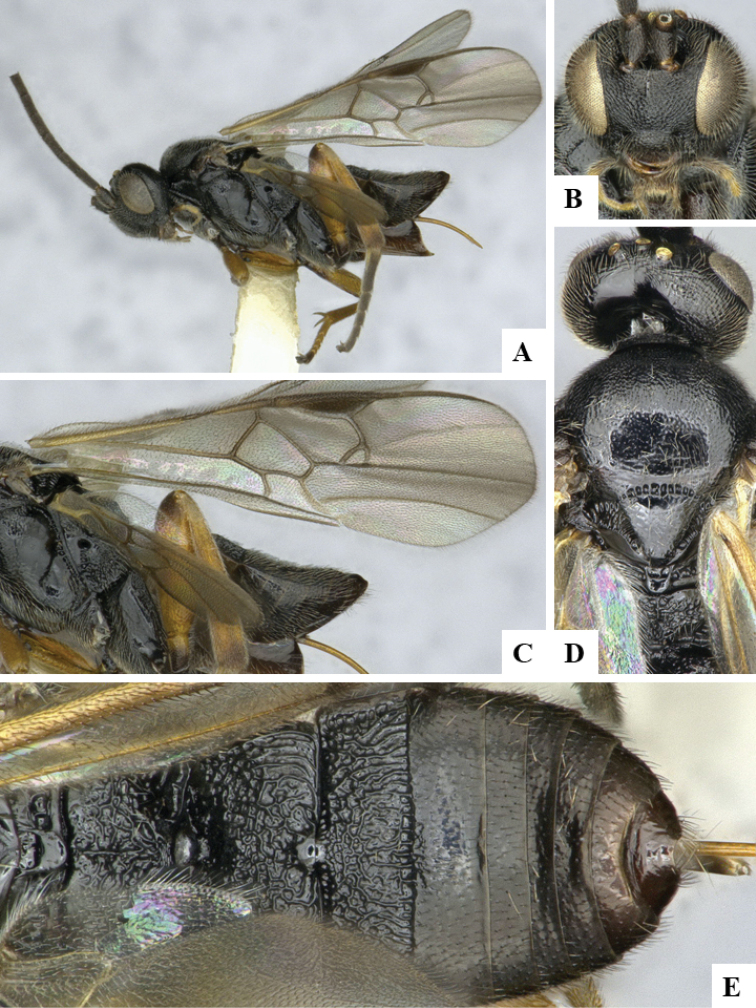
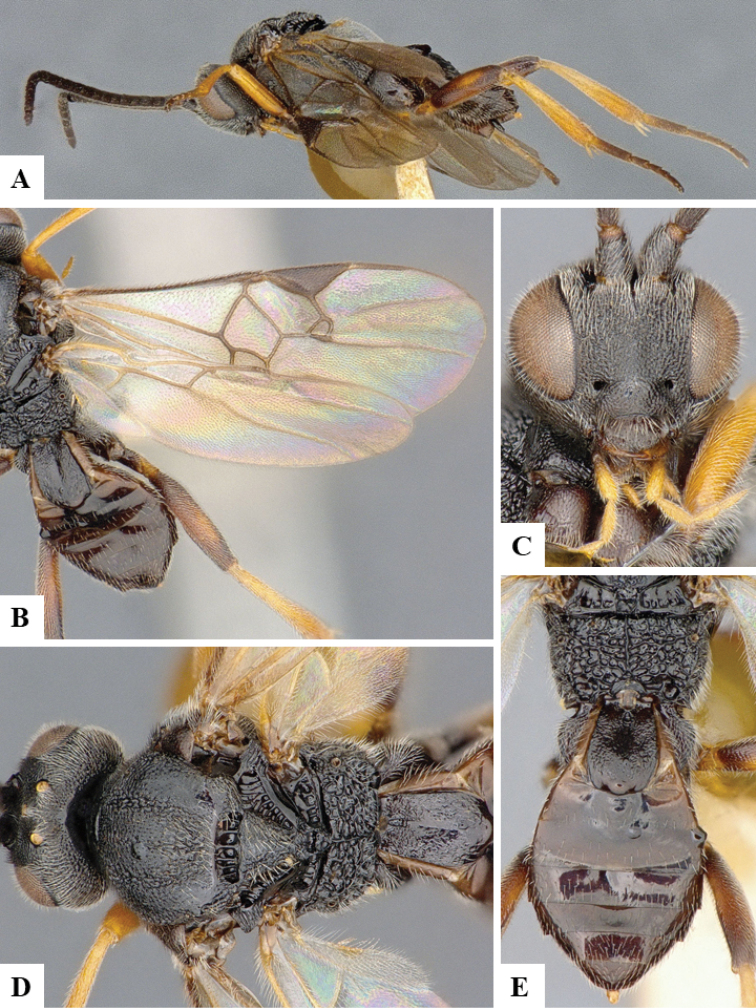
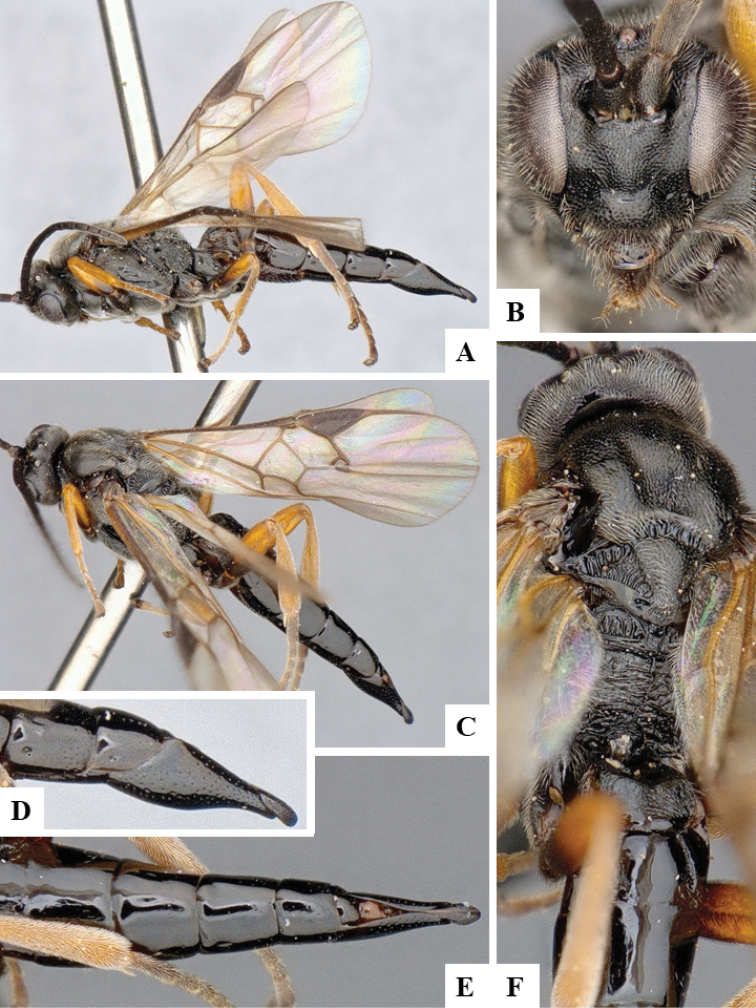
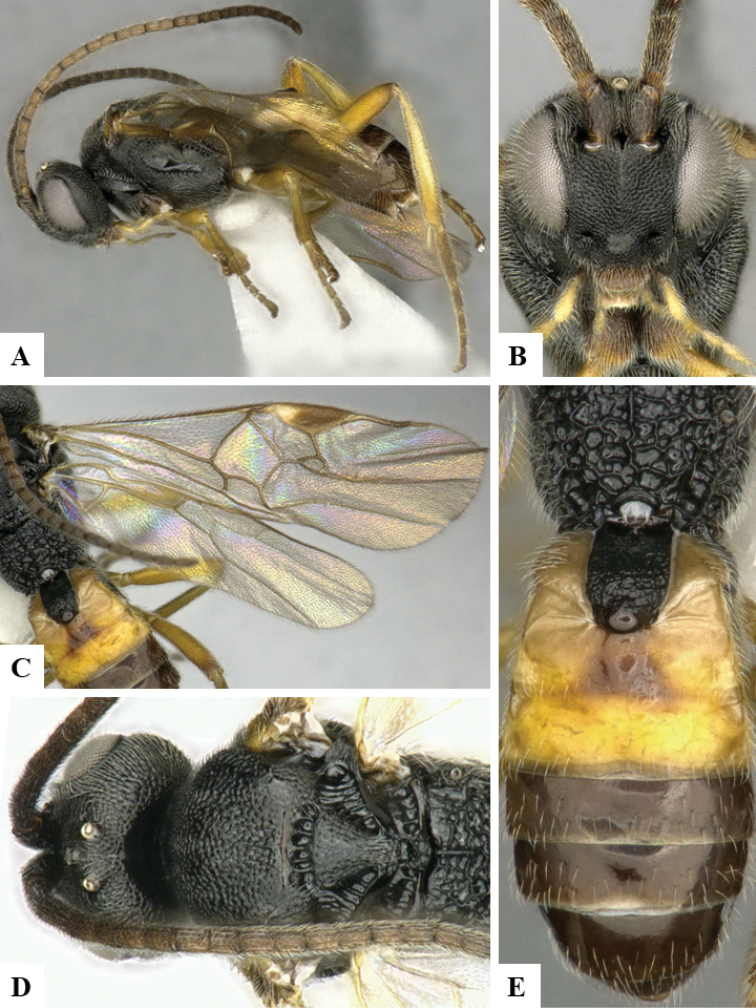
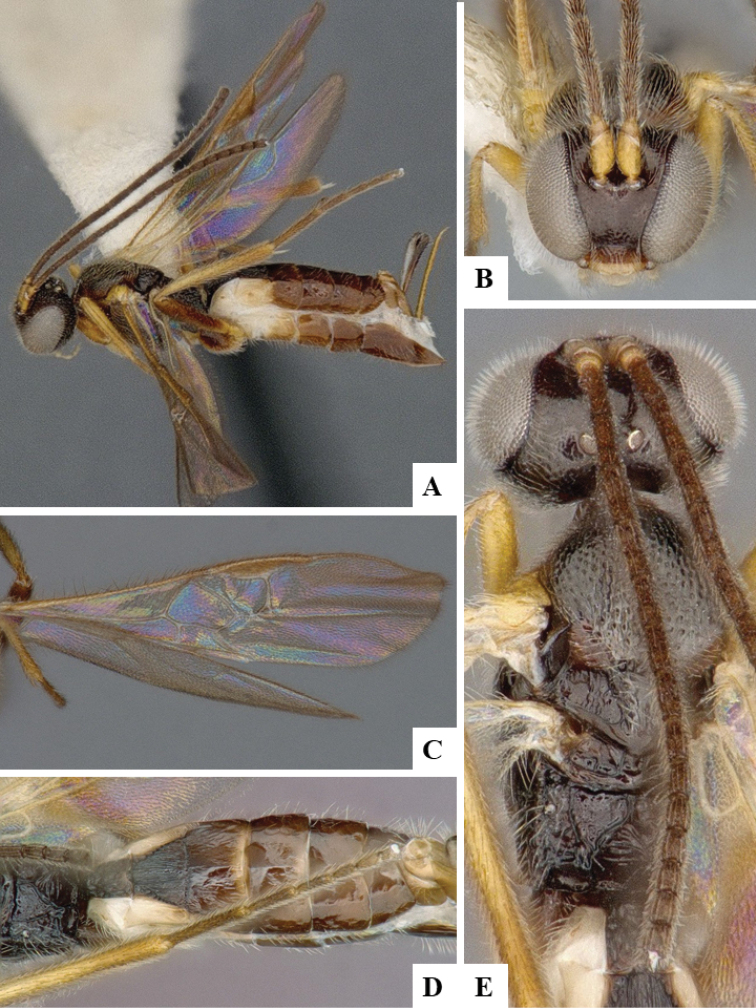
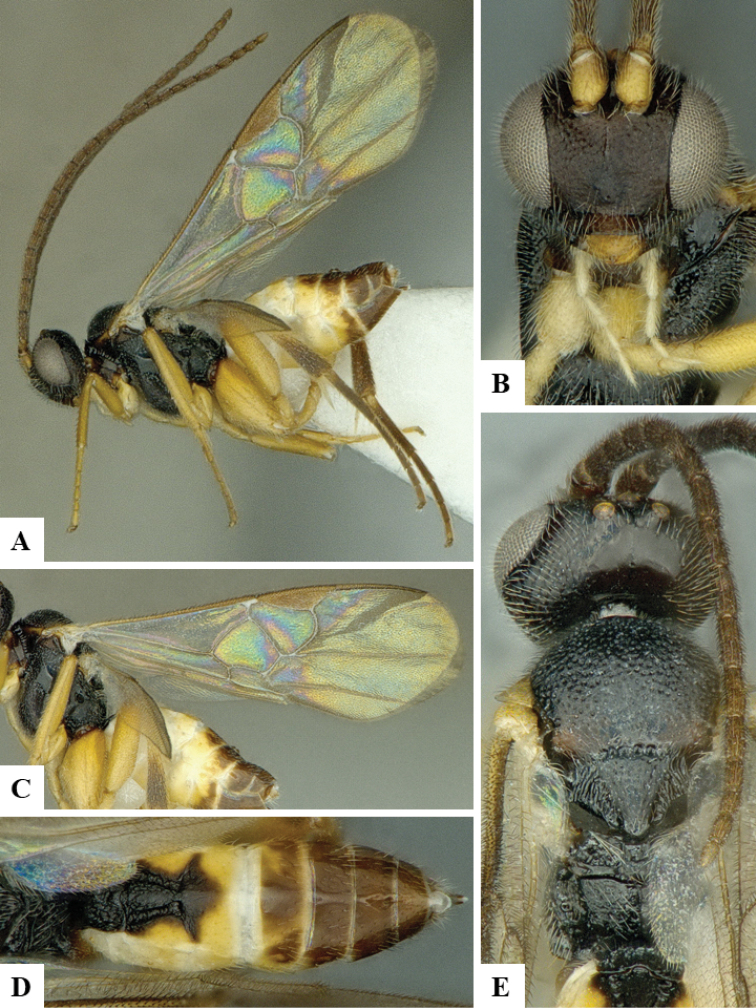
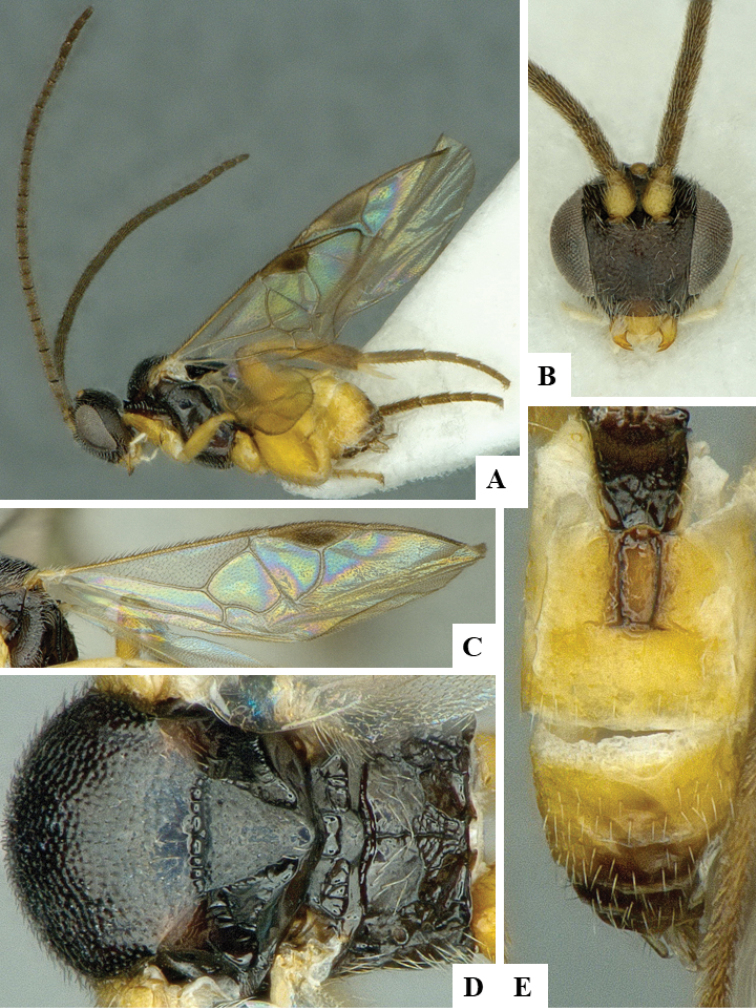
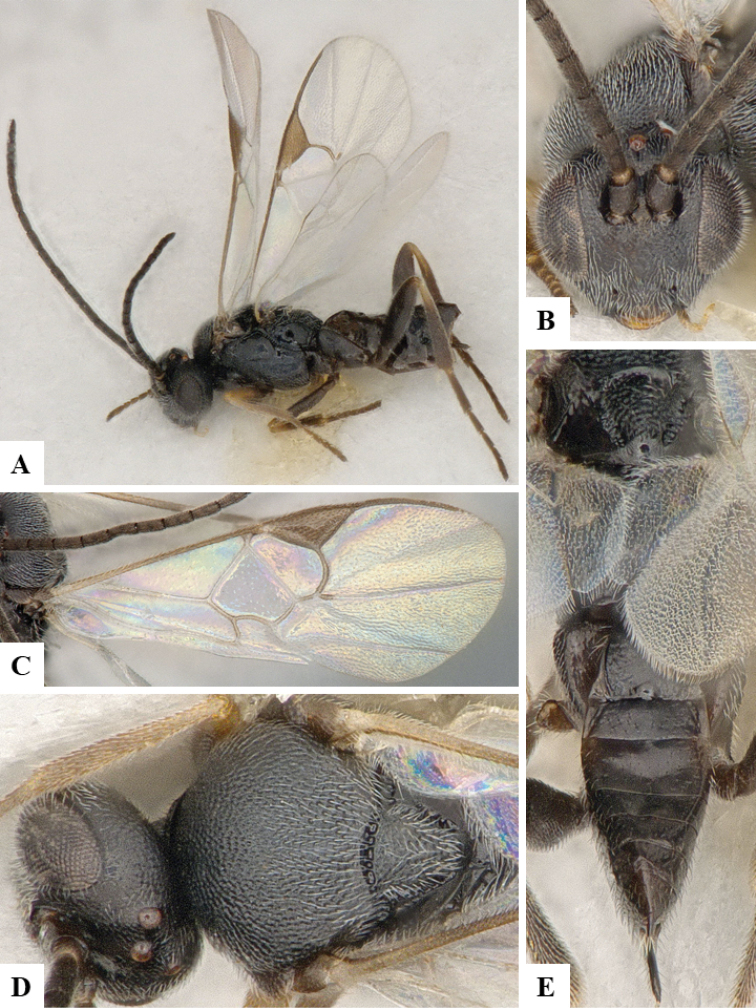
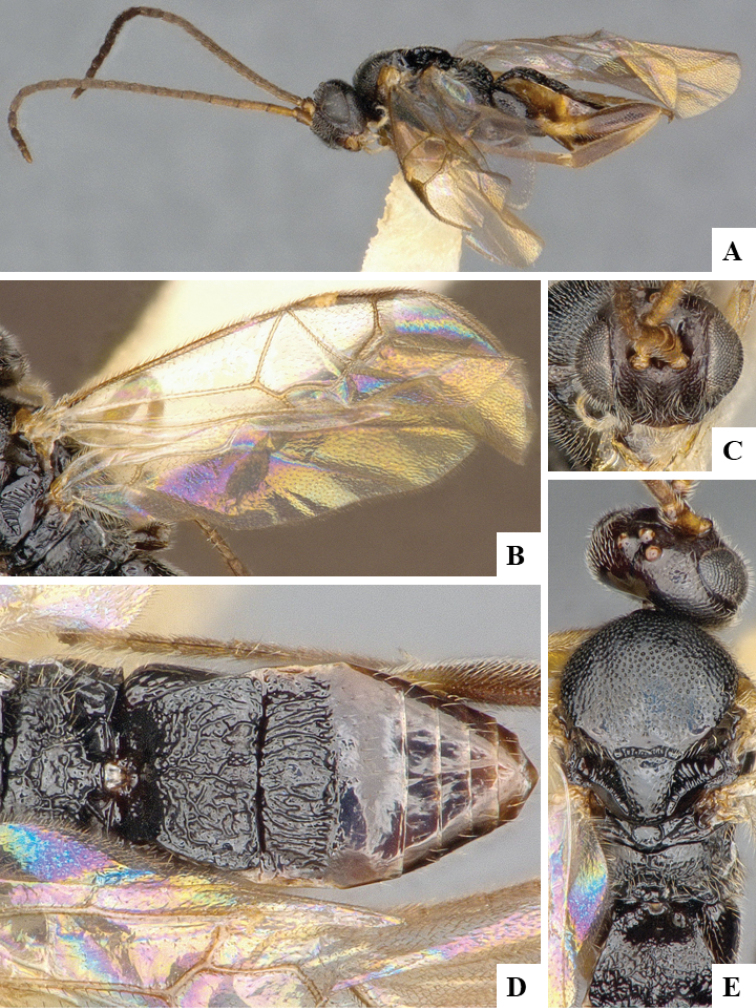
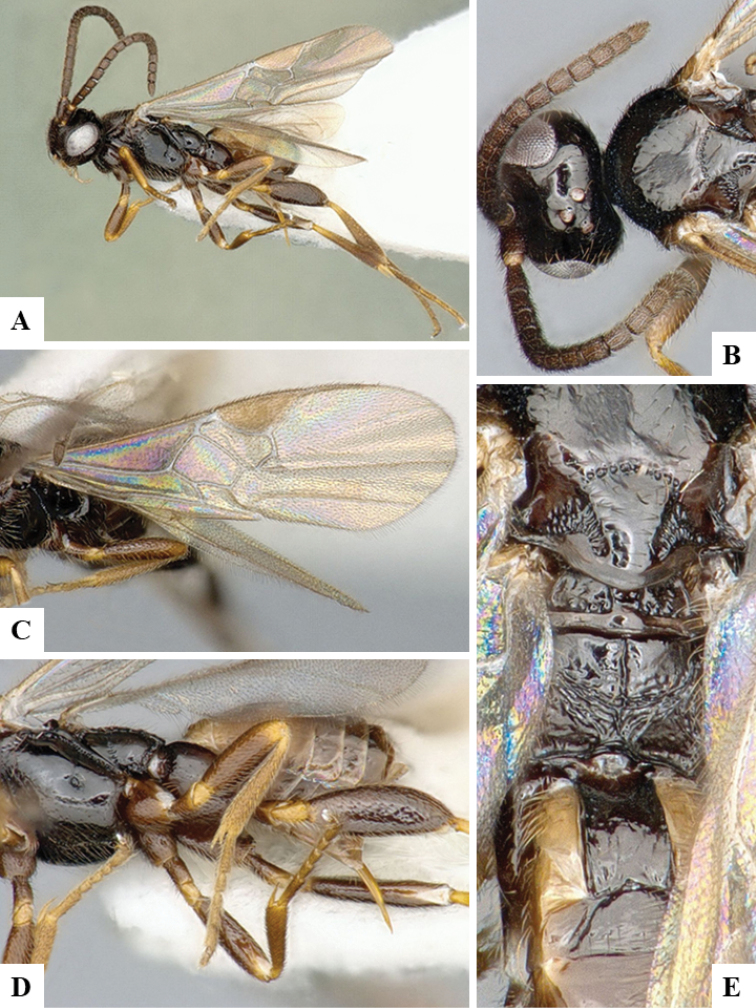
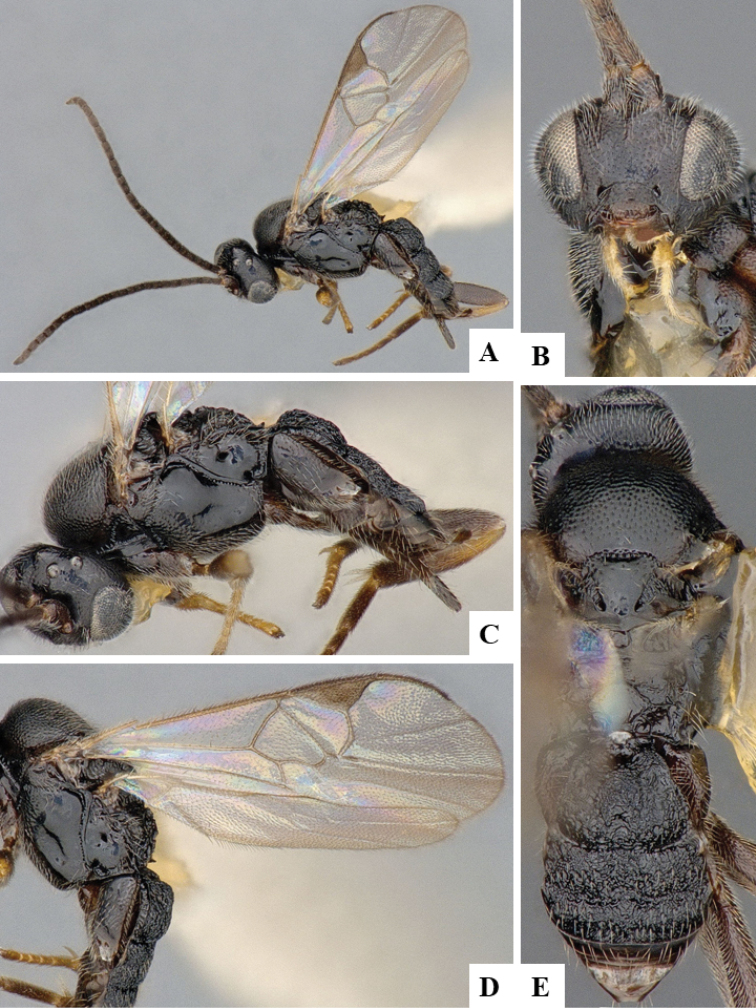
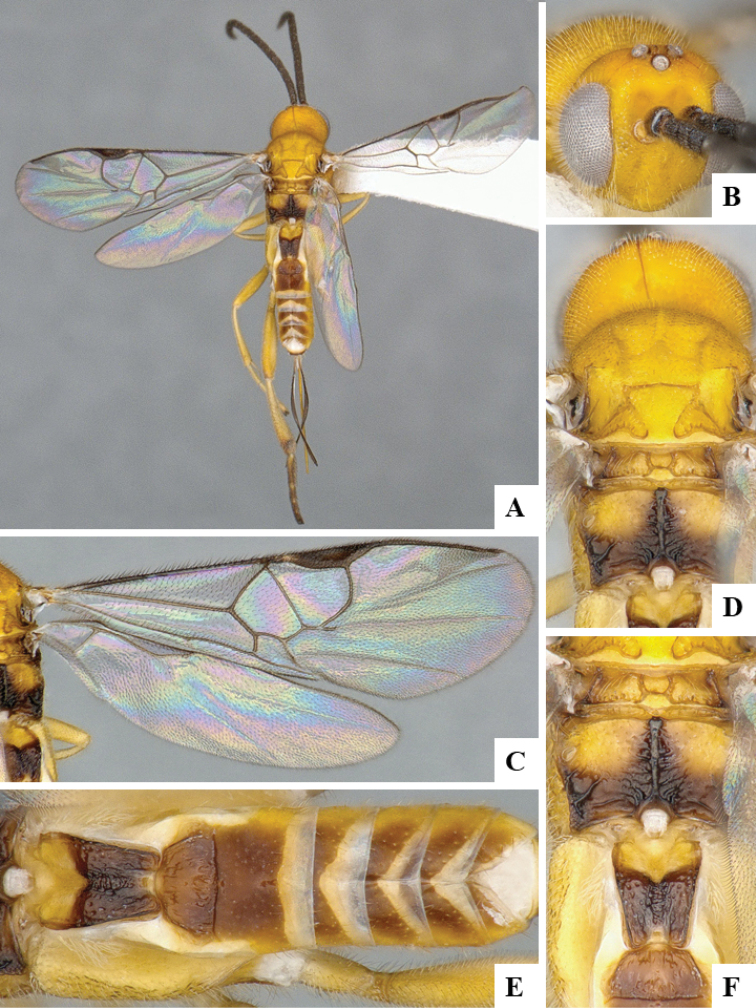
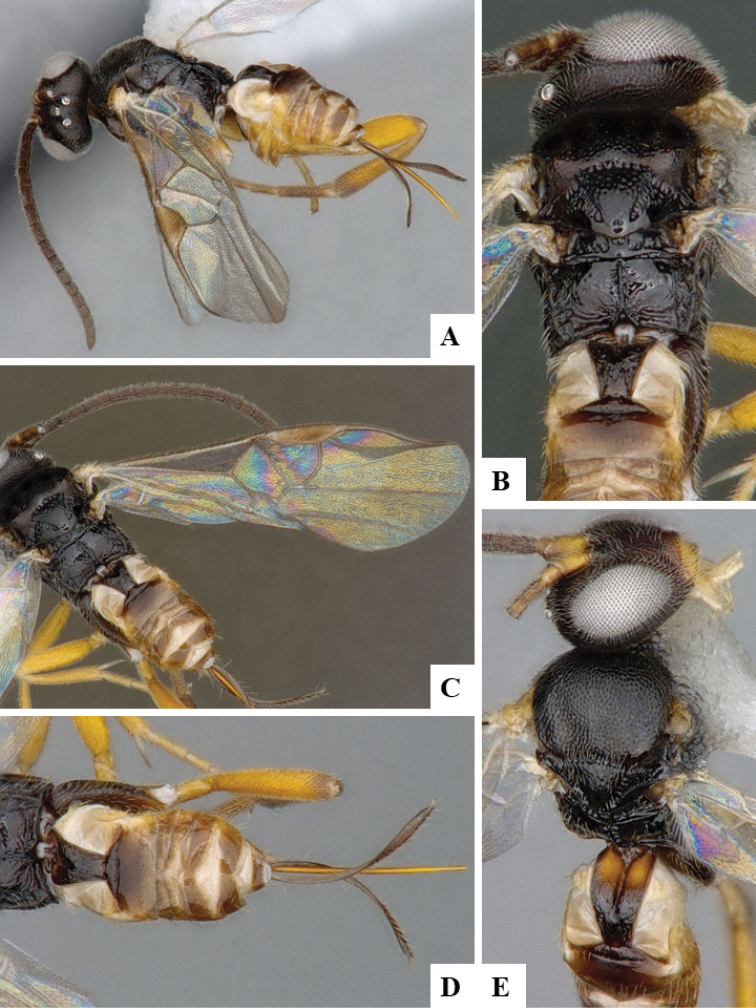
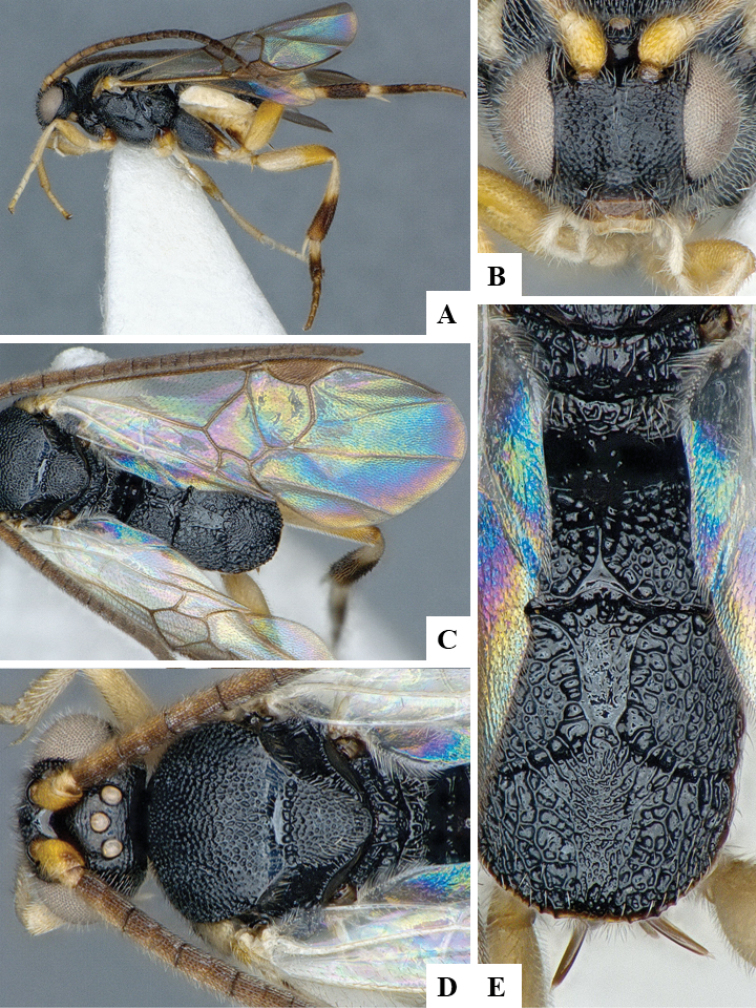
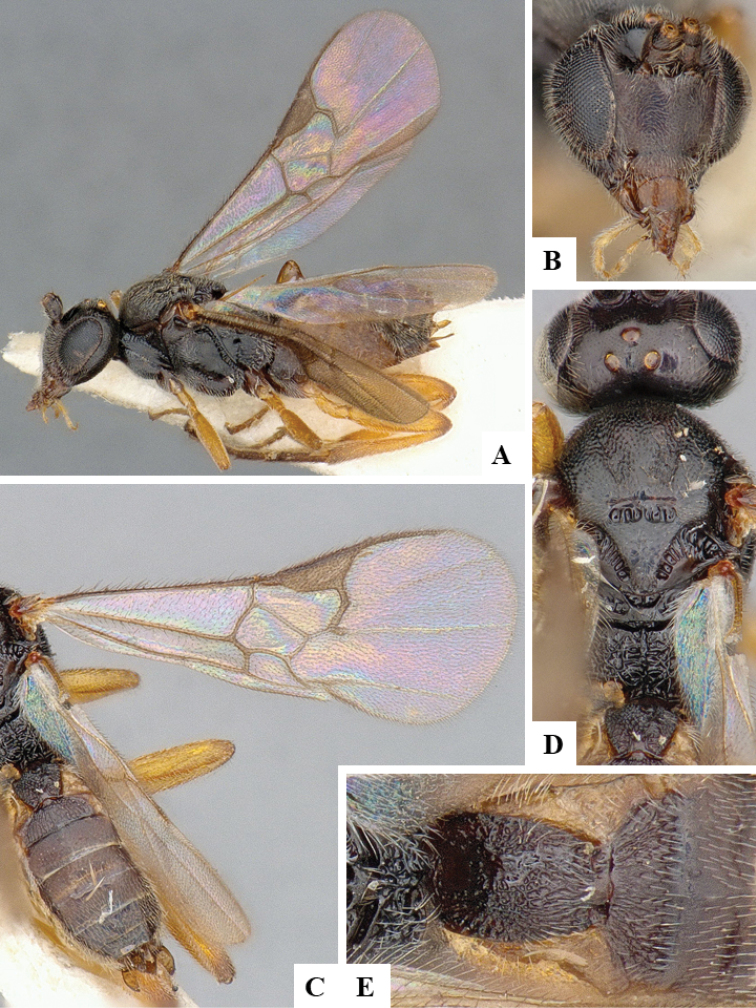
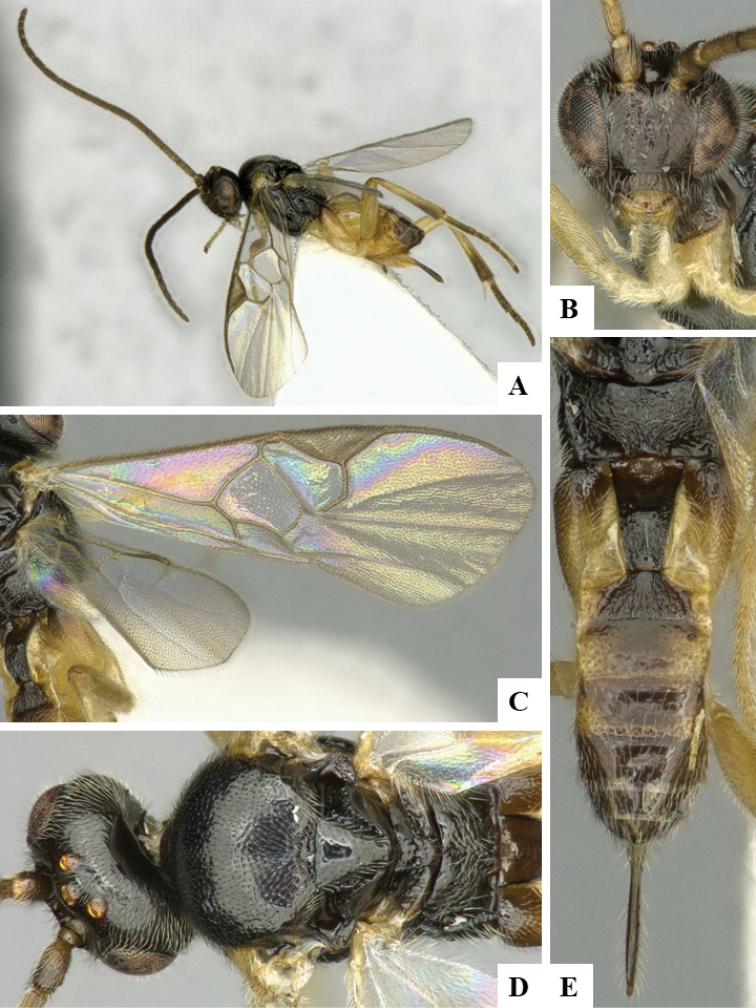
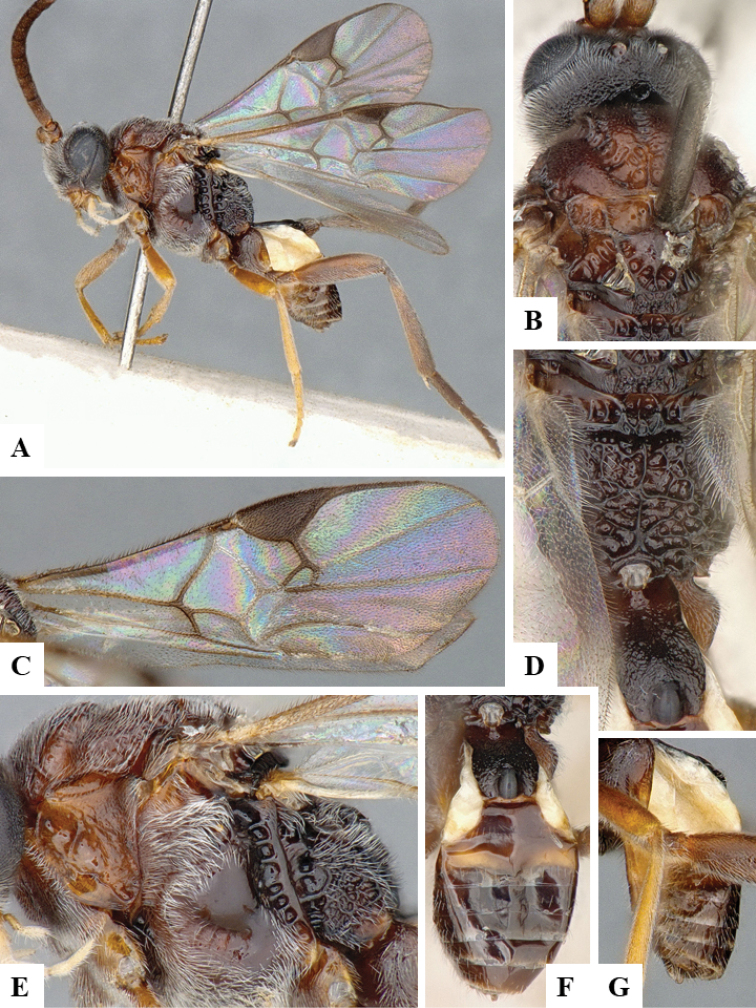
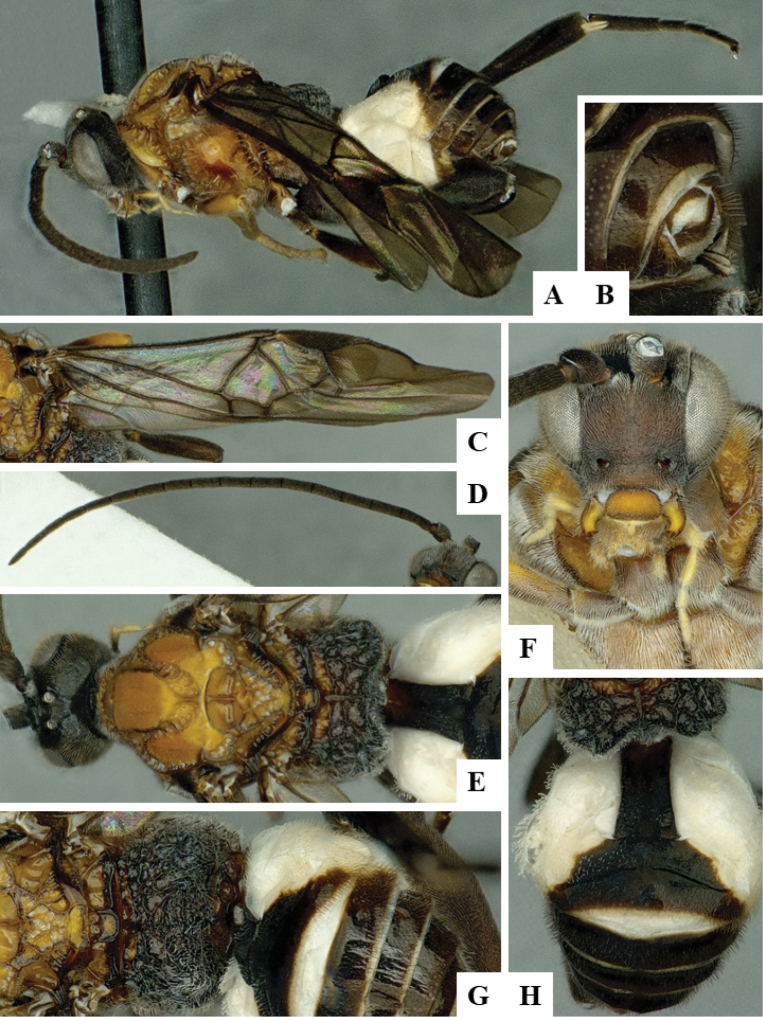
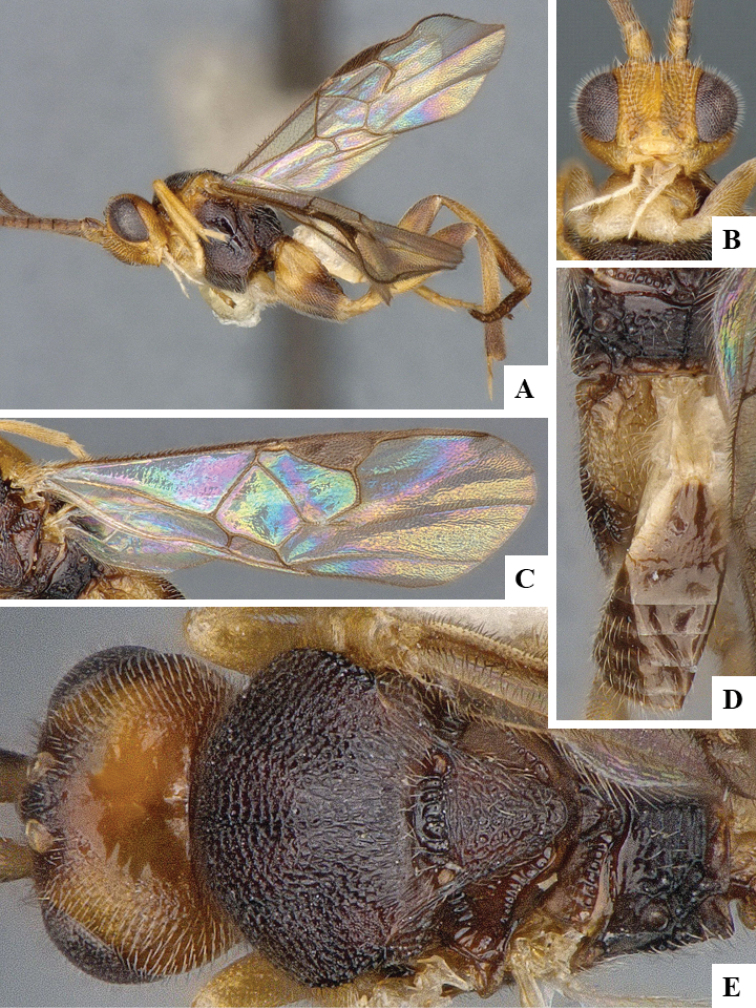
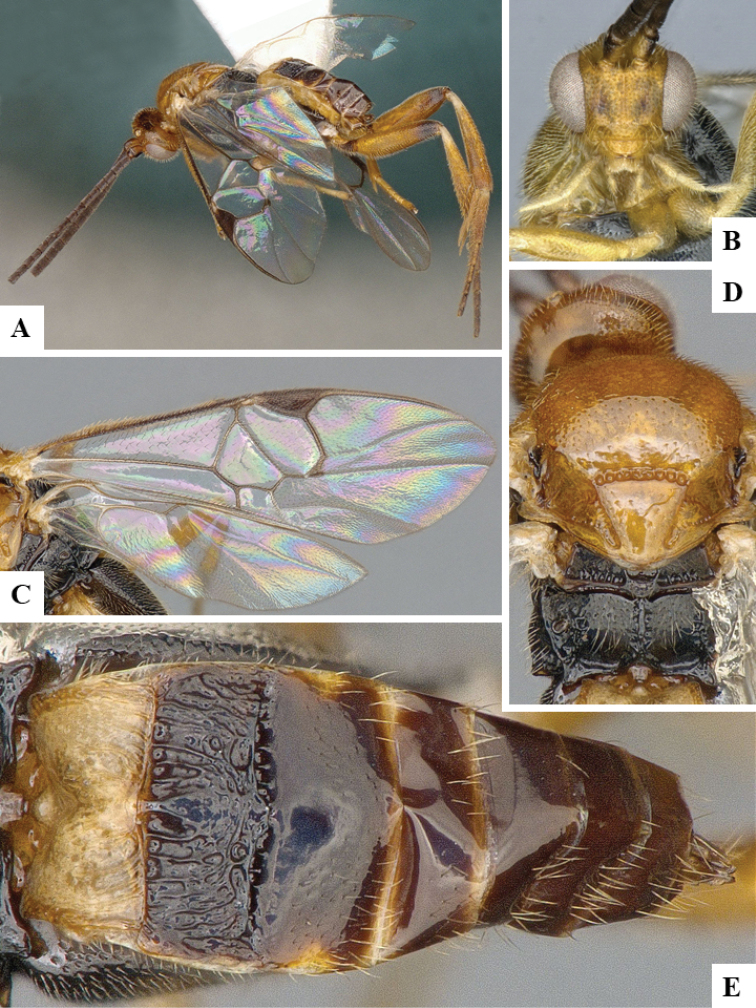
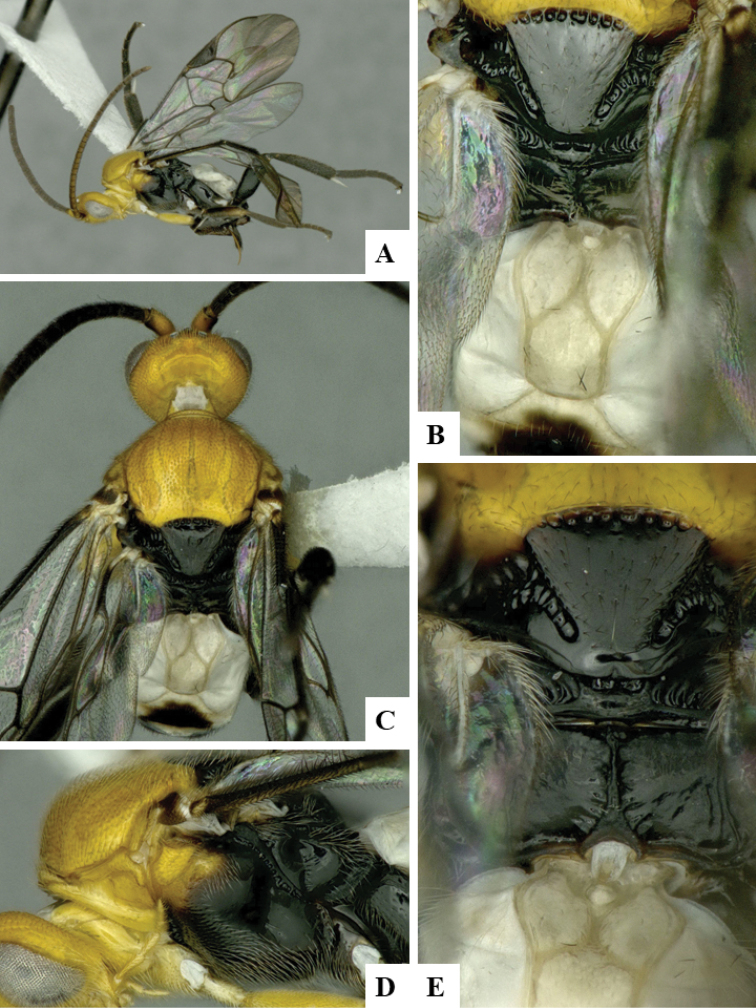
We divide the 81 genera recognized in this paper into four groups and characterize each group and singular genus with brief morphological diagnoses. We emphasize that these groups are not to be considered as monophyletic, and we caution that the discussion below is not to be taken as a new phylogeny for the subfamily, which is beyond the scope of the present paper. We do not present the information below as a surrogate key either; to key out Microgastrinae genera the reader is advised to initially consider the works mentioned at the beginning of this section. Our only intention here is to provide the reader with some basic information on the concepts we have followed when making decisions about generic placement of species, especially in the new combinations we propose in the checklist below. Besides comments on morphological diagnoses, we also provide illustrations for every Microgastrinae genus (at least one species per genus, usually more), the first time that has been done for the entire subfamily.
We separate Microgastrinae into four broadly defined groups:
a) unplaced genera, all of which have unique morphological characters that make them very distinctive, although they do not share any character in common per se, comprising 18 genera: Austinicotesia, Austrocotesia, Beyarslania, Billmasonius, Clarkinella, Exulonyx, Fornicia, Janhalacaste, Kiwigaster, Mariapanteles, Miropotes, Neoclarkinella, Pelicope, Prasmodon, Qrocodiledundee, Semionis, Xanthomicrogaster, and Zachterbergius;
b) Microplitis group, which includes the Microplitini (sensuMason 1981) and four additional genera described by Fernandez-Triana and Boudreault (2018), for a total of eight genera: Alloplitis, Gilbertnixonius, Jenopappius, Microplitis, Philoplitis, Silvaspinosus, Snellenius, and Tobleronius;
c) Cotesia group, which includes most but not all of the Cotesiini (sensuMason 1981), with 29 genera: Buluka, Carlmuesebeckius, Chaoa, Cotesia, Cuneogaster, Deuterixys, Diolcogaster, Distatrix, Eripnopelta, Exix, Glyptapanteles, Jimwhitfieldius, Keylimepie, Larissimus, Lathrapanteles, Markshawius, Nyereria, Ohenri, Parenion, Protapanteles, Protomicroplitis, Pseudofornicia, Pseudovenanides, Rasivalva, Sathon, Ungunicus, Venanides, Venanus, and Wilkinsonellus;
d) Apanteles group, which includes most but not all of the Apantelini + Microgastrini (sensuMason 1981) with 26 genera: Agupta, Alphomelon, Apanteles, Choeras, Dasylagon, Dodogaster, Dolichogenidea, Exoryza, Hygroplitis, Hypomicrogaster, Iconella, Illidops, Kotenkosius, Microgaster, Napamus, Papanteles, Parapanteles, Paroplitis, Pholetesor, Promicrogaster, Pseudapanteles, Rhygoplitis, Sendaphne, Shireplitis, Xanthapanteles, and Ypsilonigaster.
a) Unplaced genera
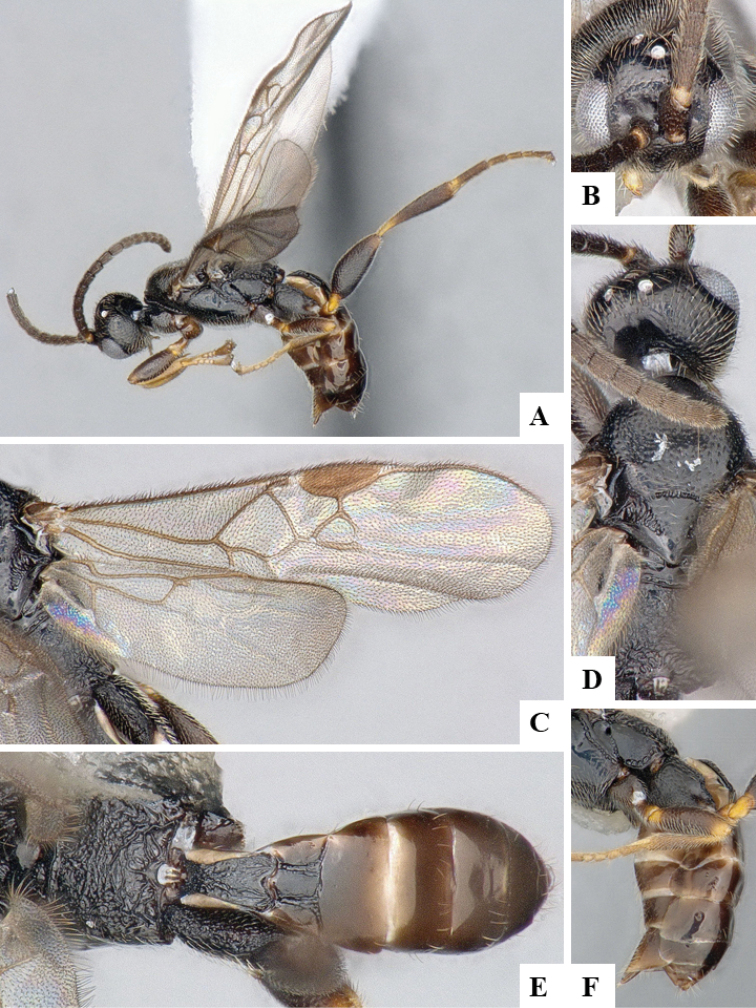
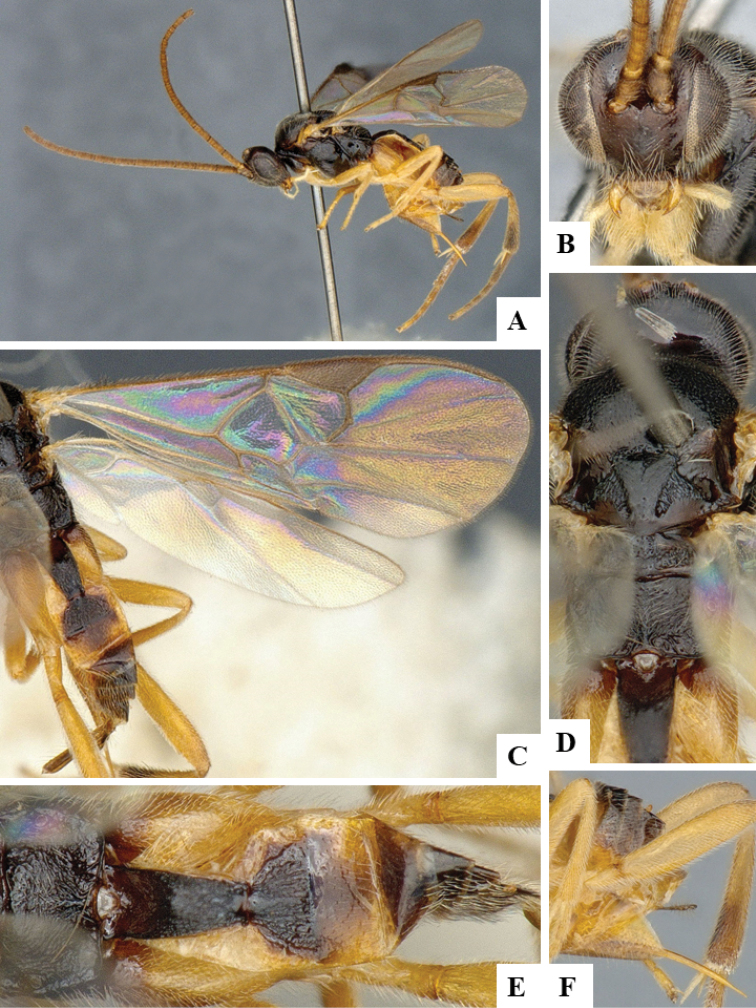
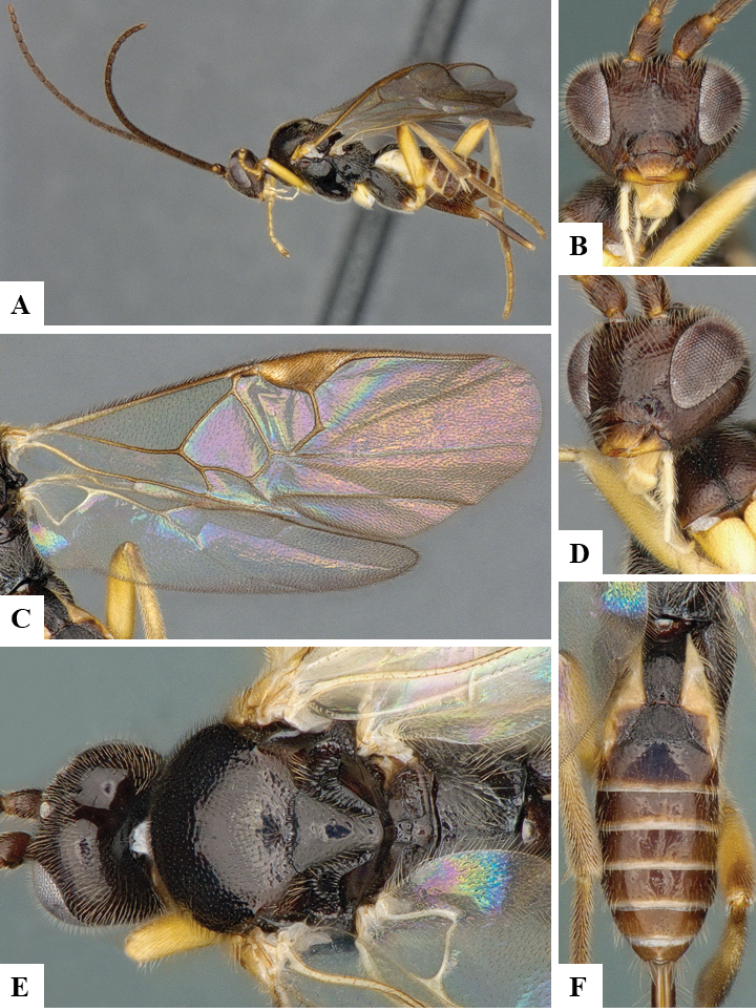
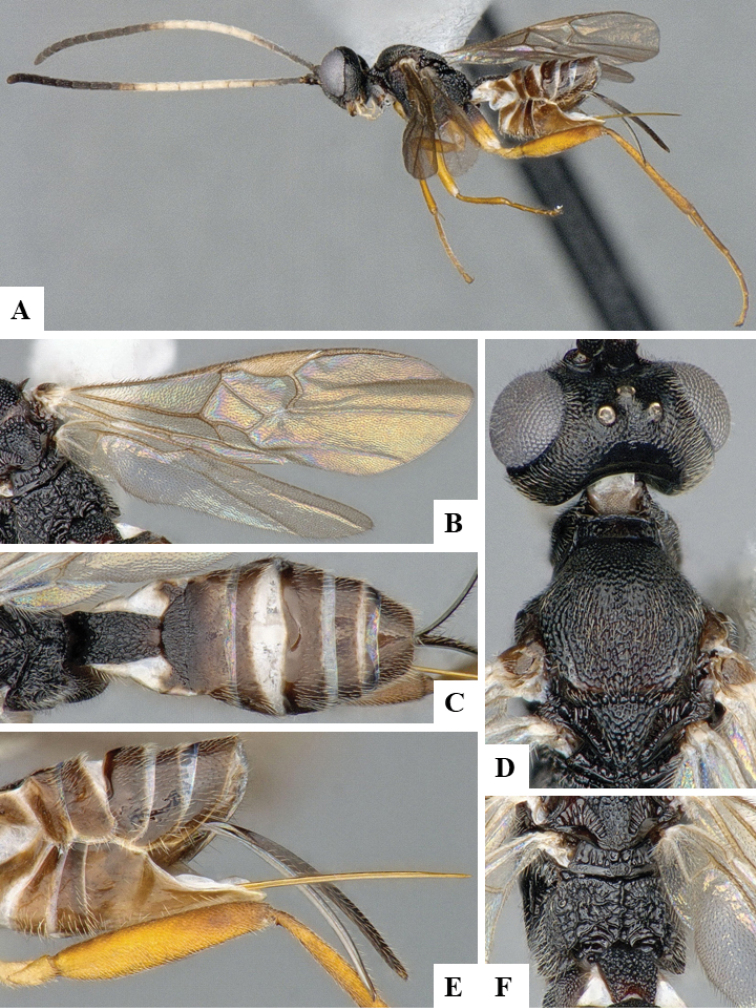
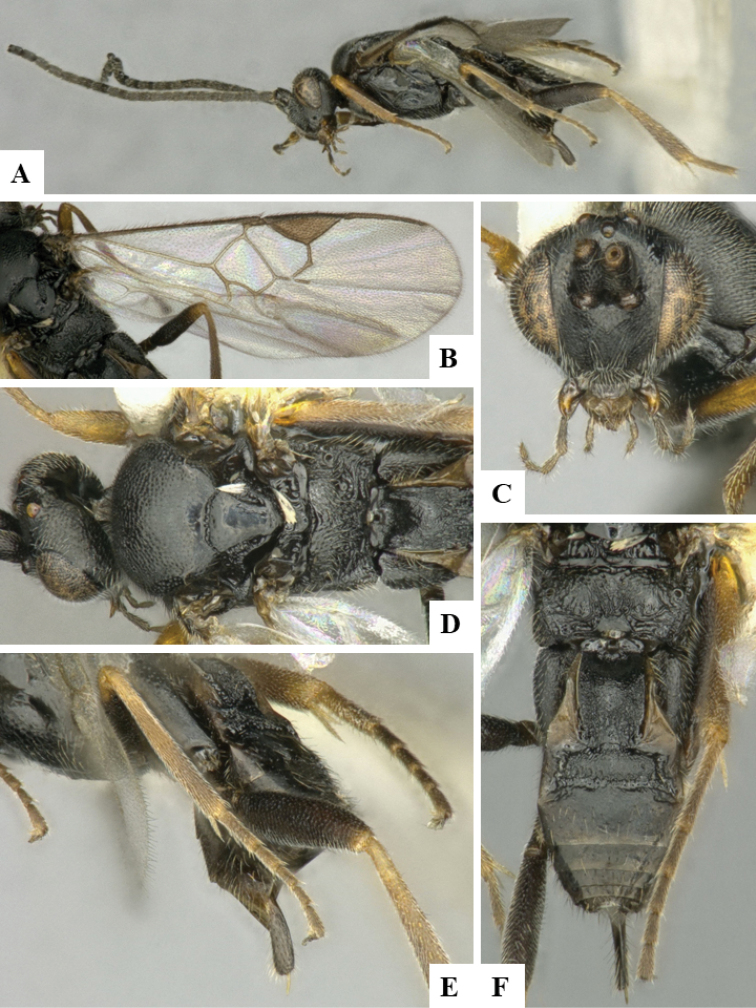
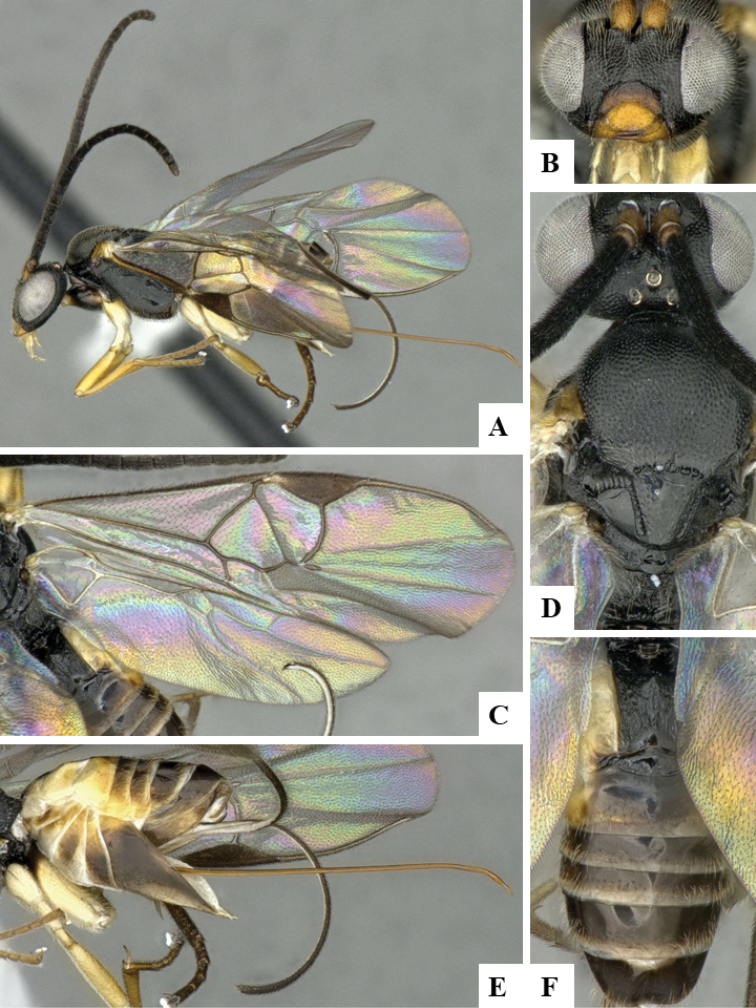
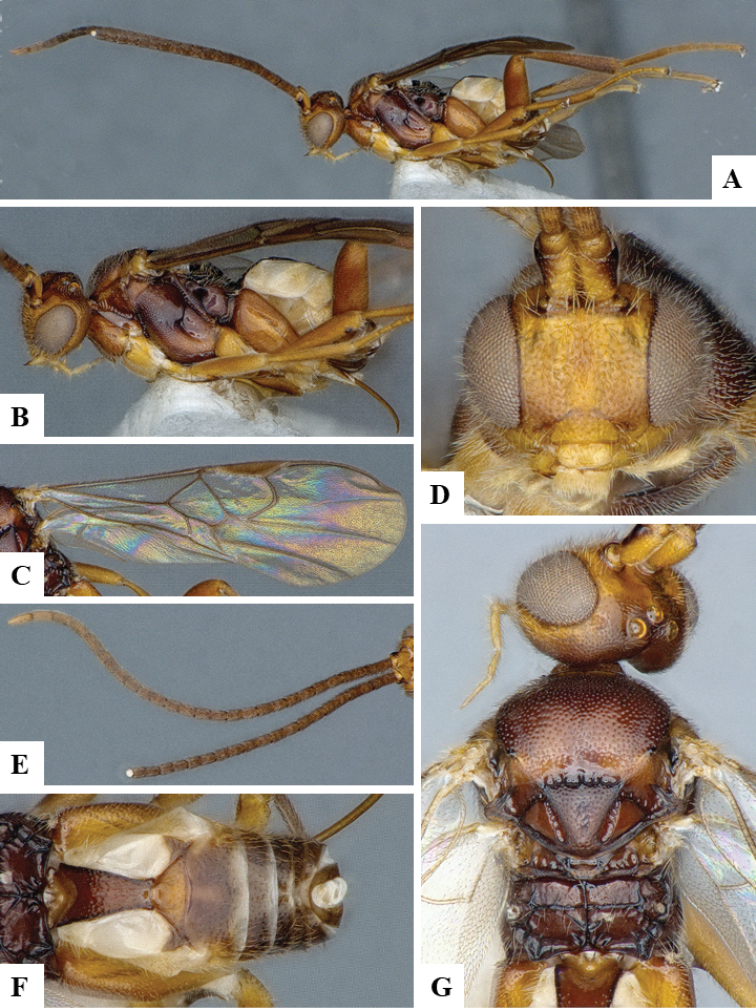
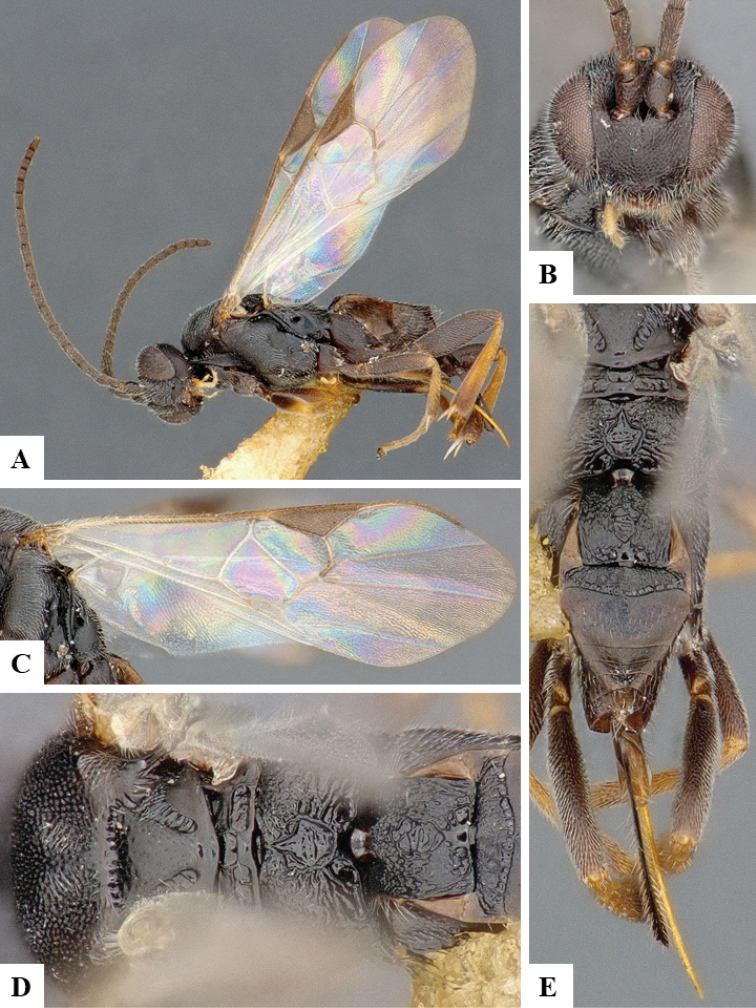
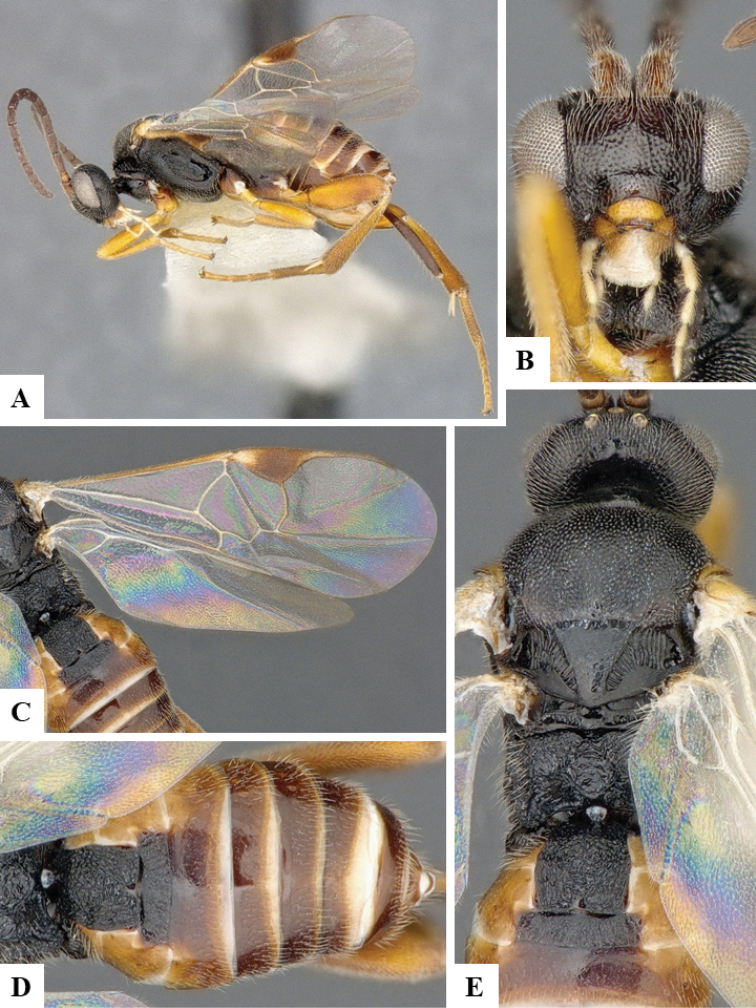
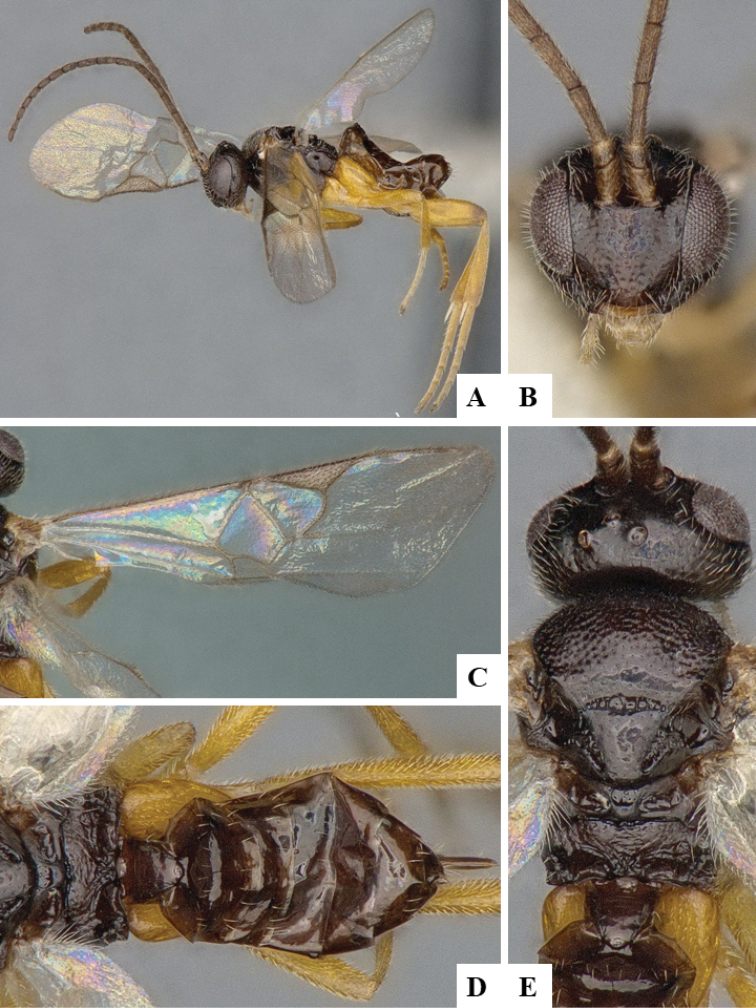
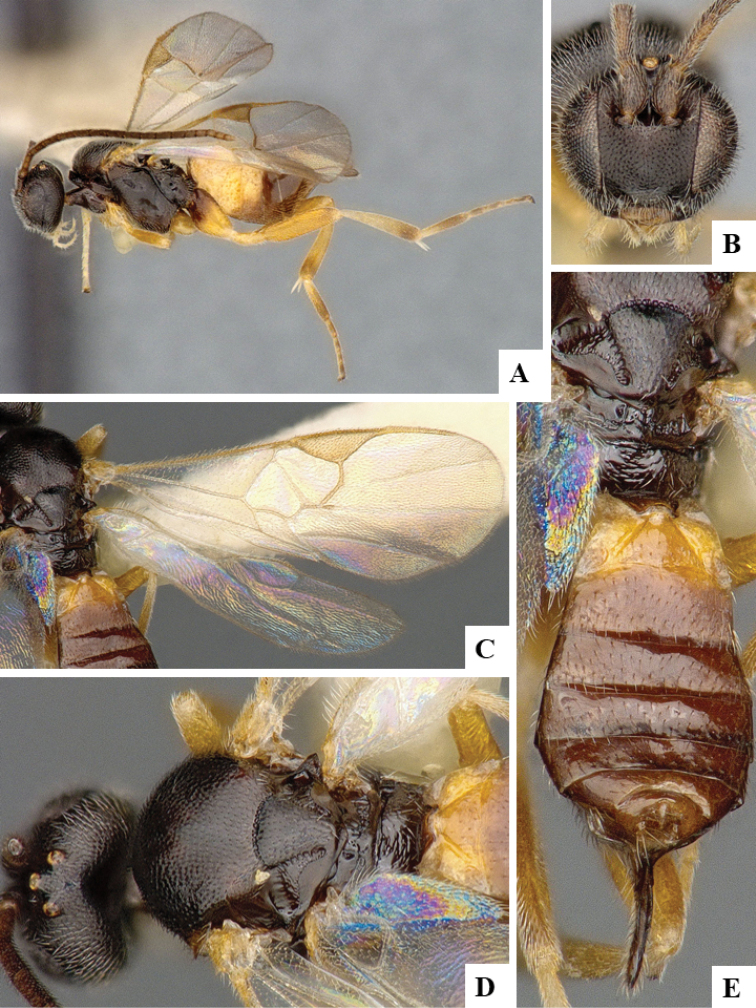
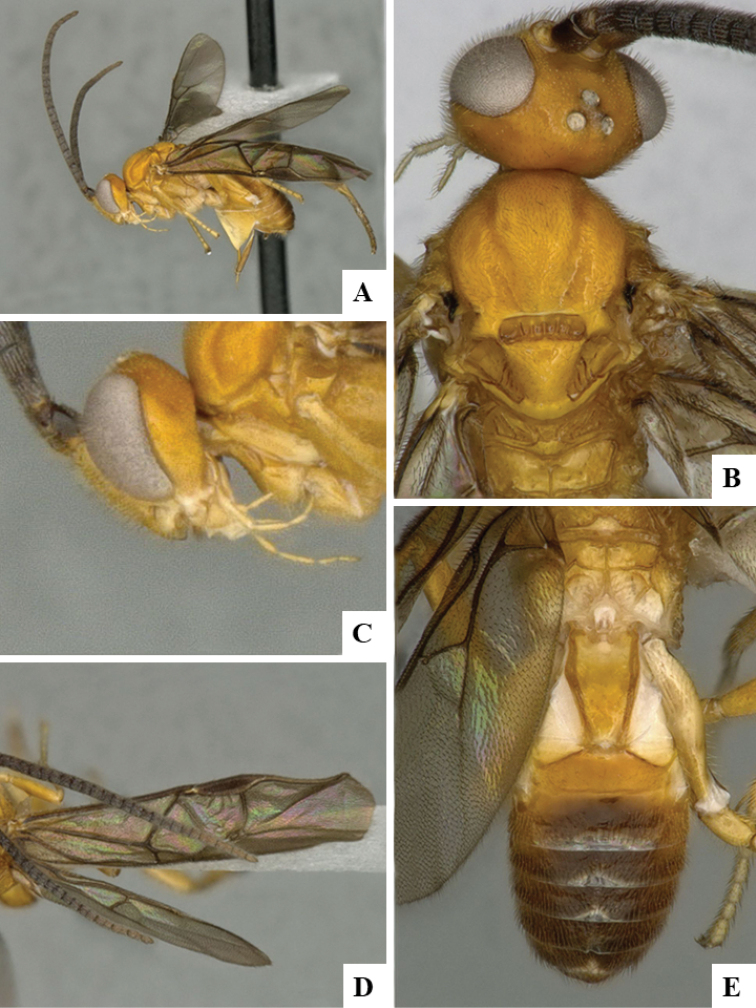
Kiwigaster (Figs 136–137) is the only genus of Microgastrinae with sexual dimorphism in the number of antennal segments; females have 17 flagellomeres and males have 18 (Fernandez-Triana et al. 2011). All other known microgastrines have 16 flagellomeres in both sexes.
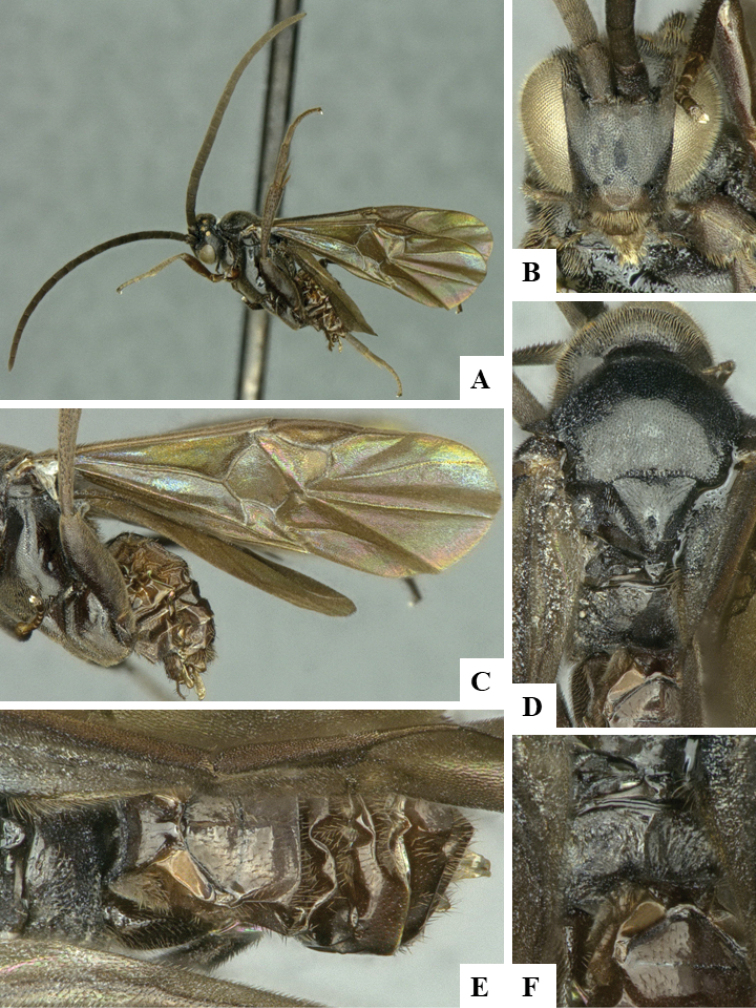
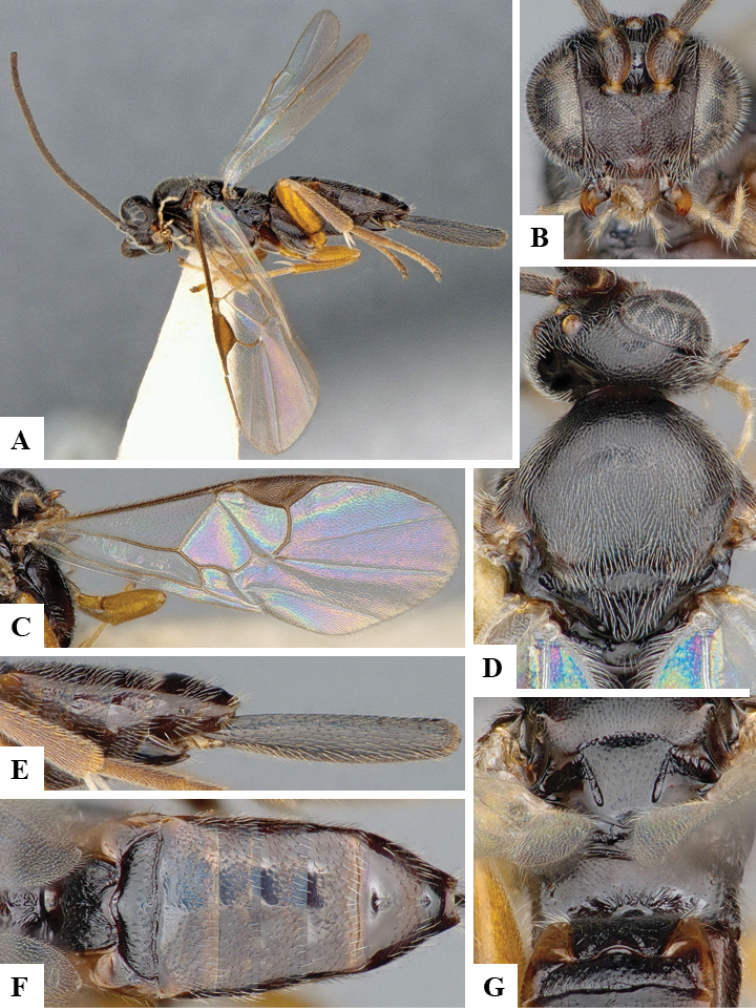
Figure 136.
Kiwigastervariabilis female AMNZ71859 A Habitus, lateral B Head, frontal C Fore wing D Propodeum, dorsal E Metasoma, dorsal F Head and mesosoma, dorsal.
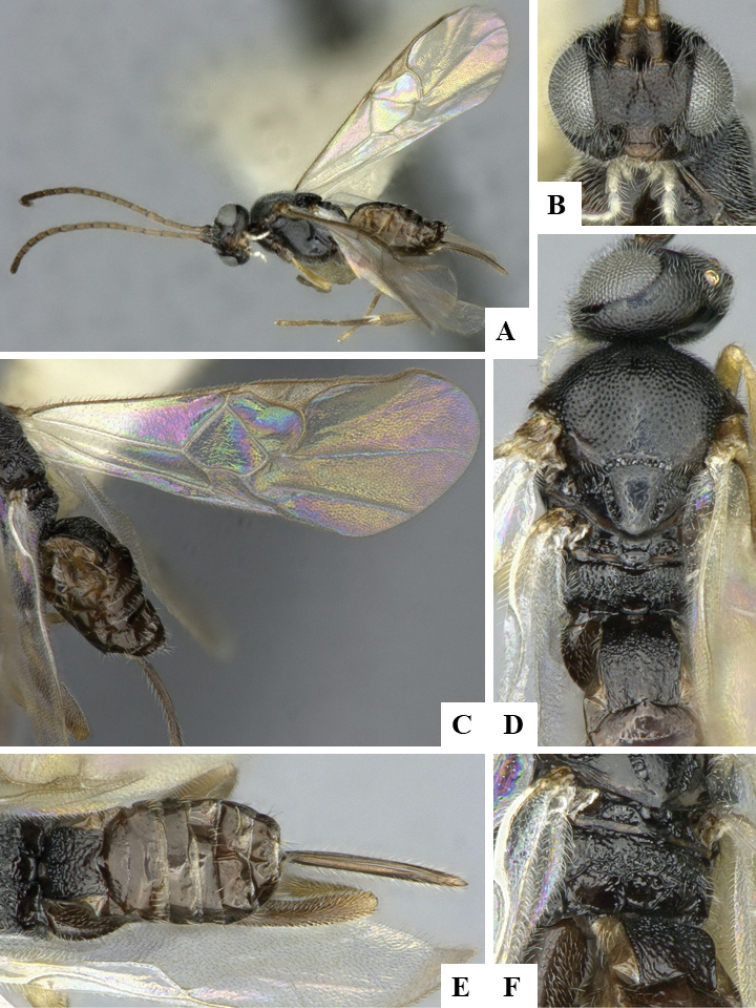
Figure 137.
Kiwigastervariabilis female AMNZ71861 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Head and mesosoma, dorsal E Propodeum and metasoma, dorsal F Ovipositor and ovipositor sheaths.
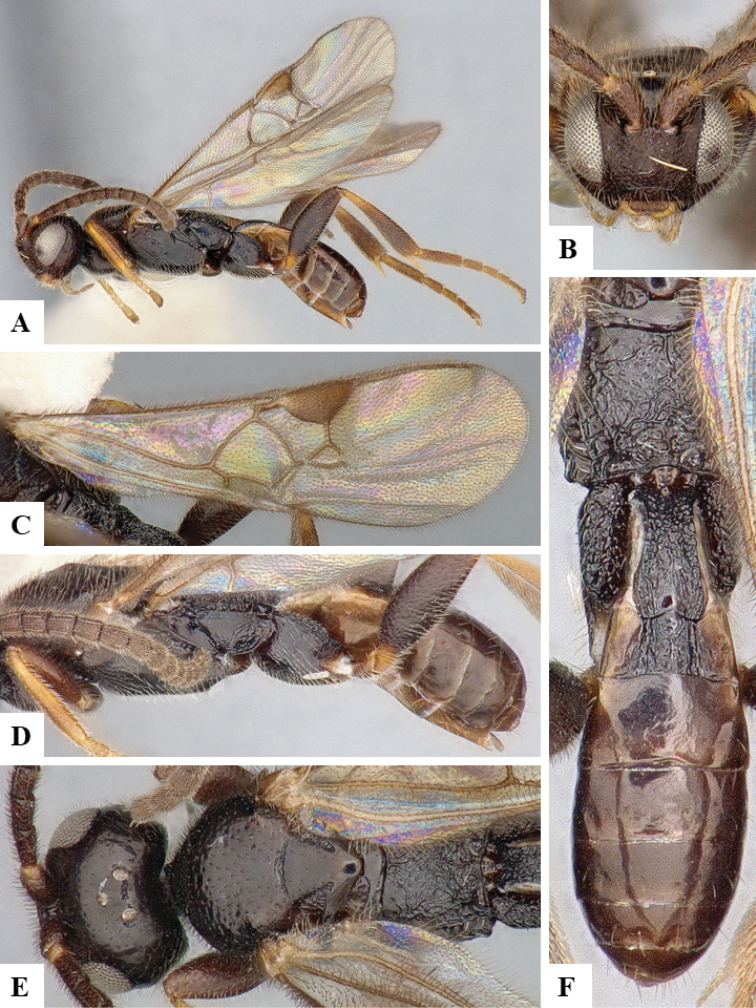
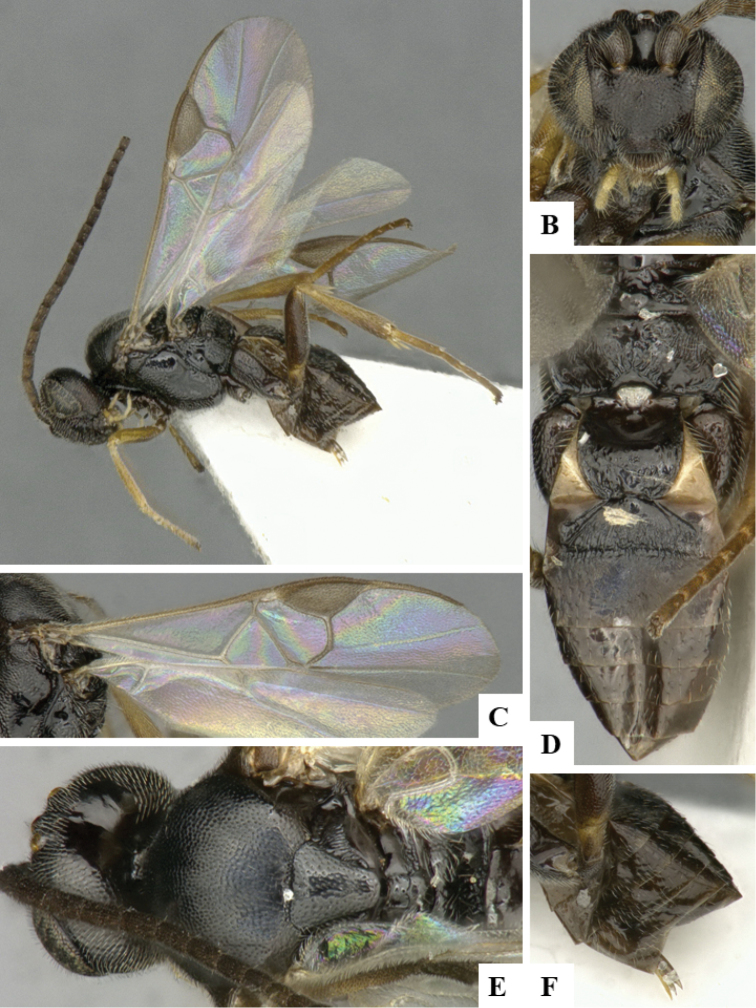
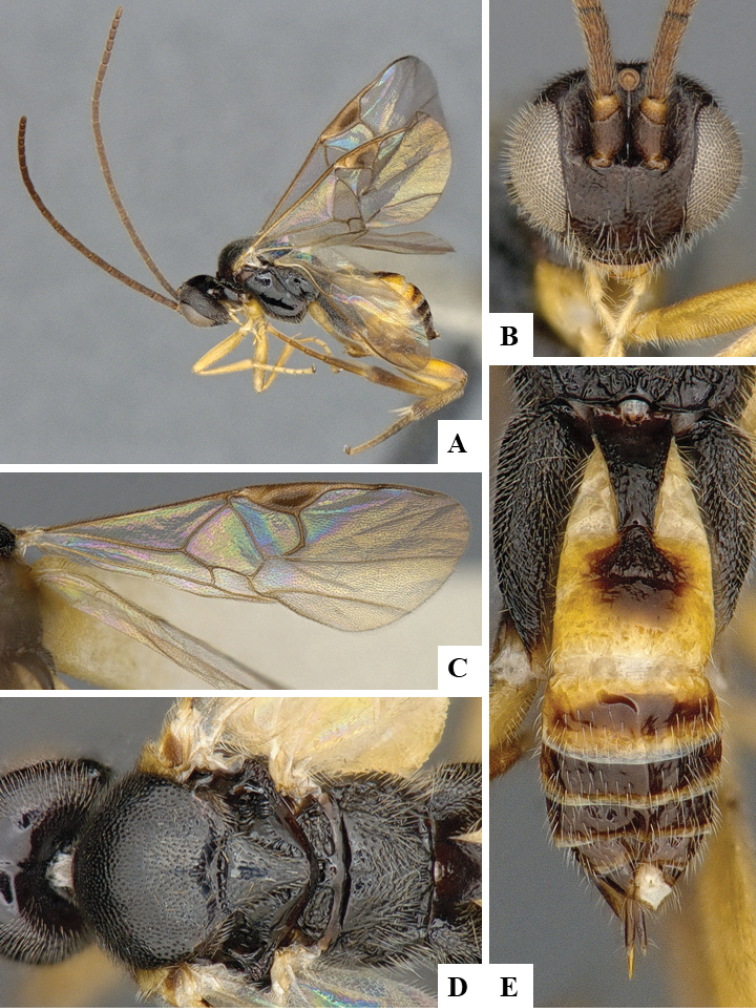
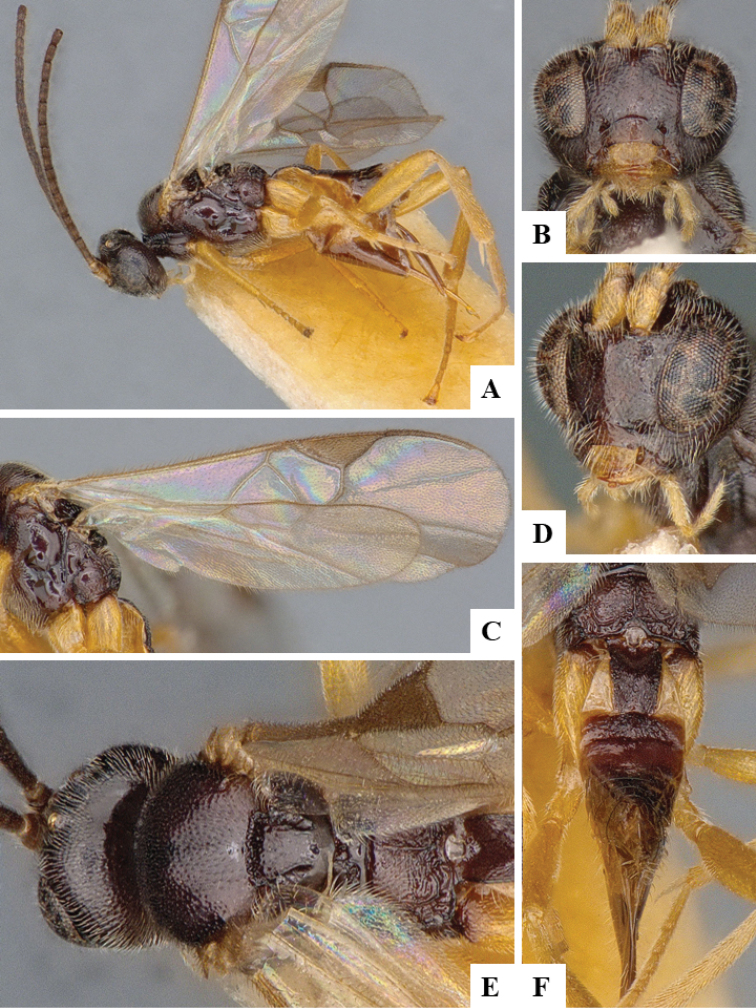
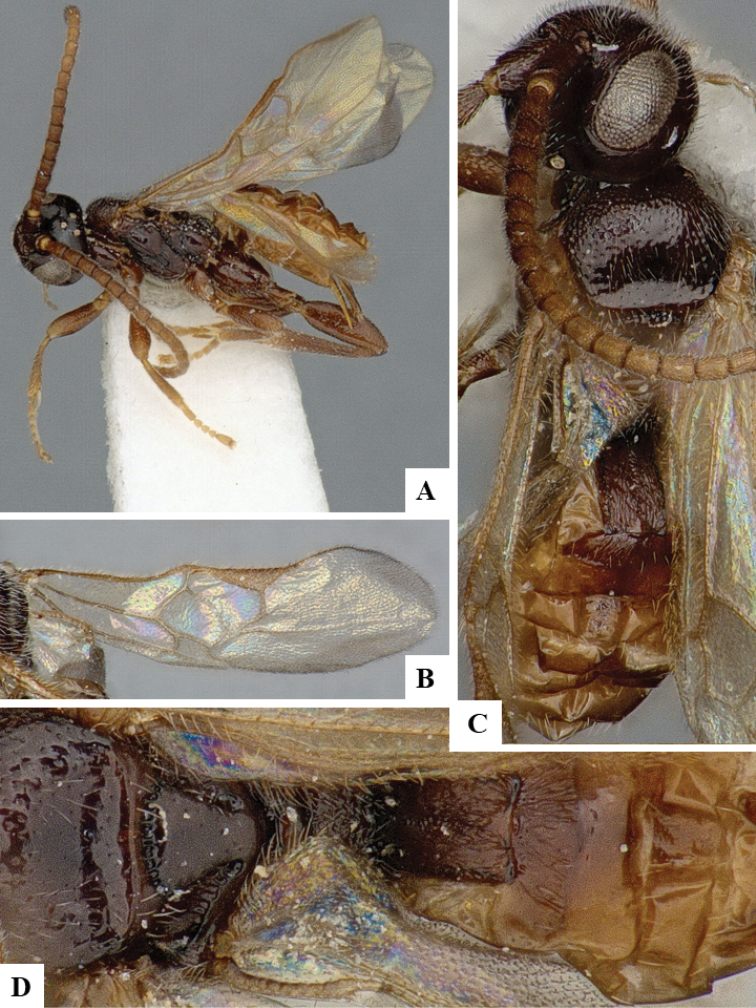
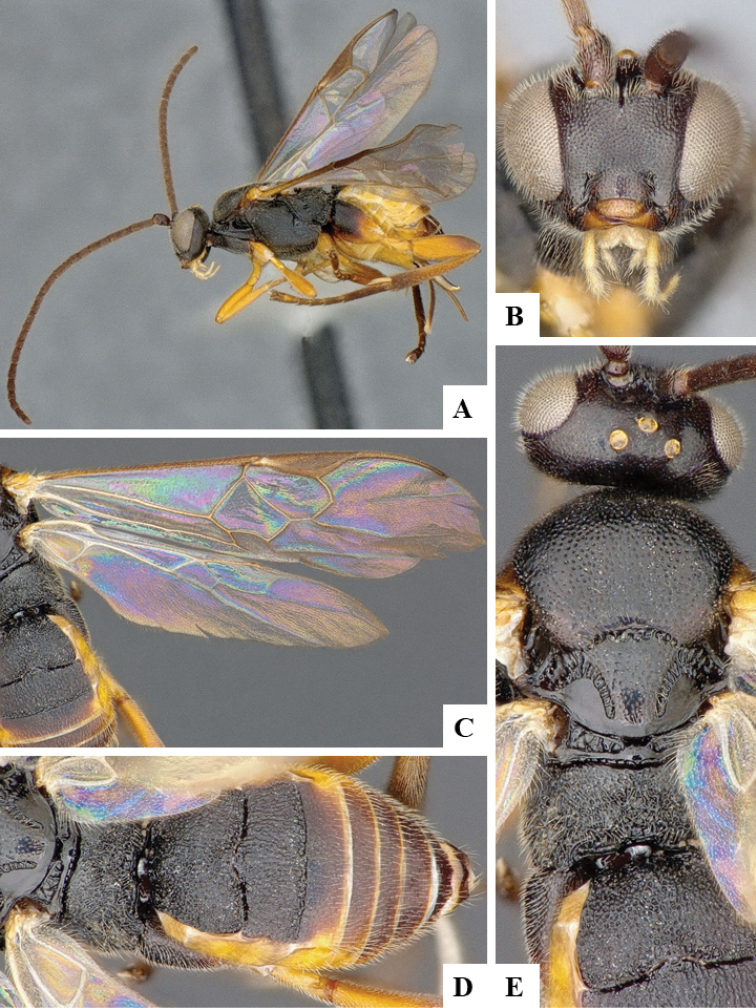
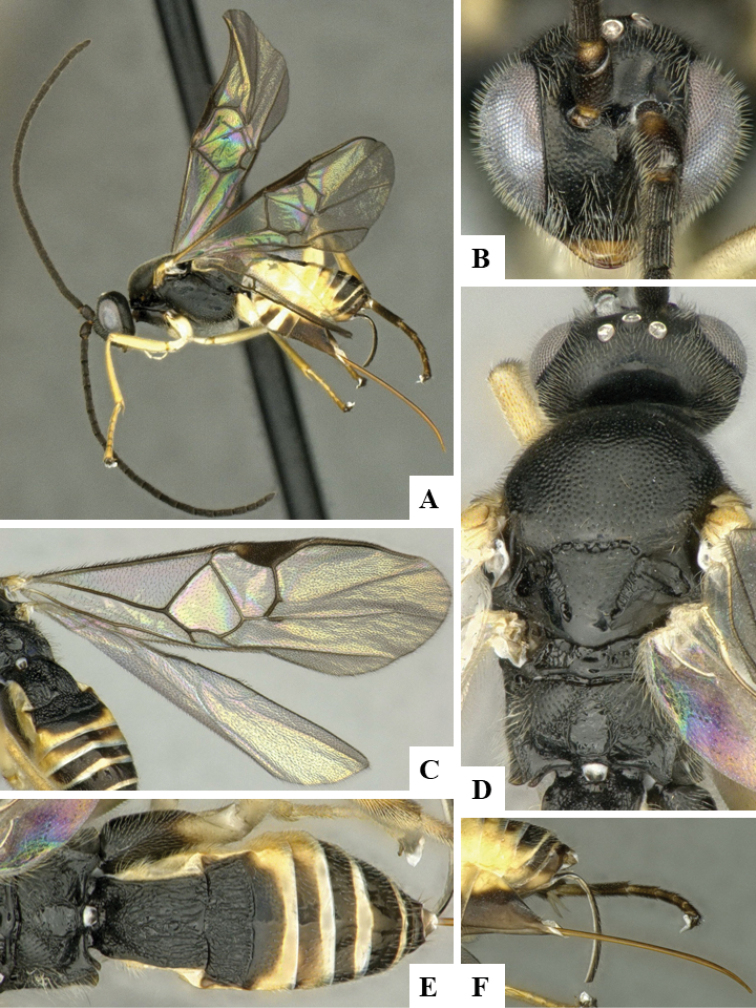
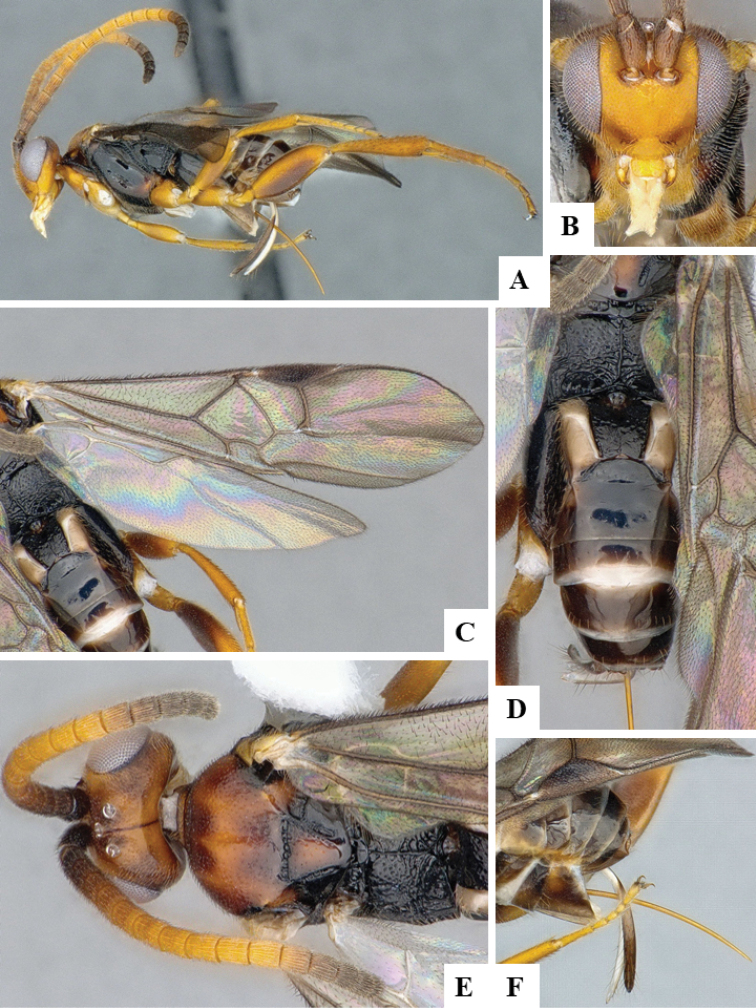
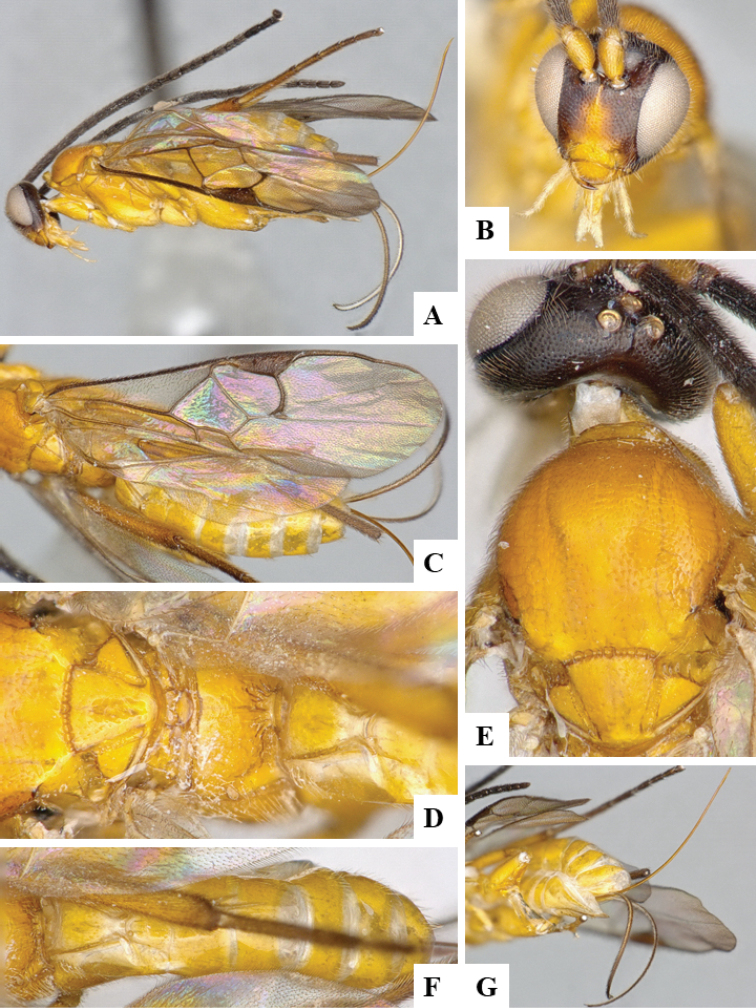
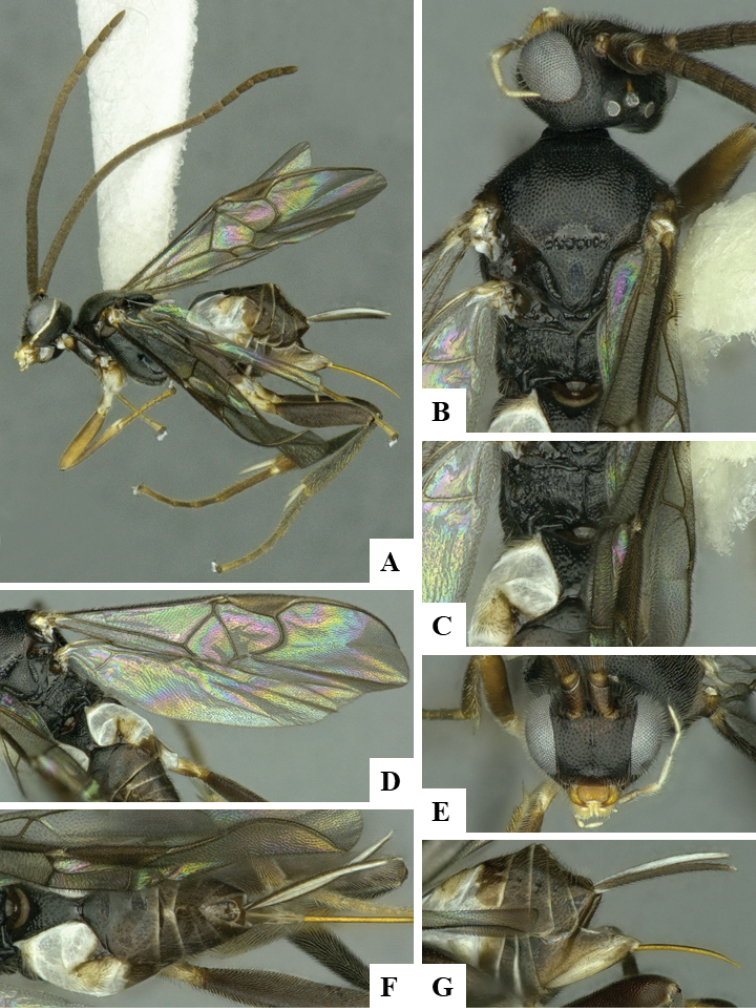
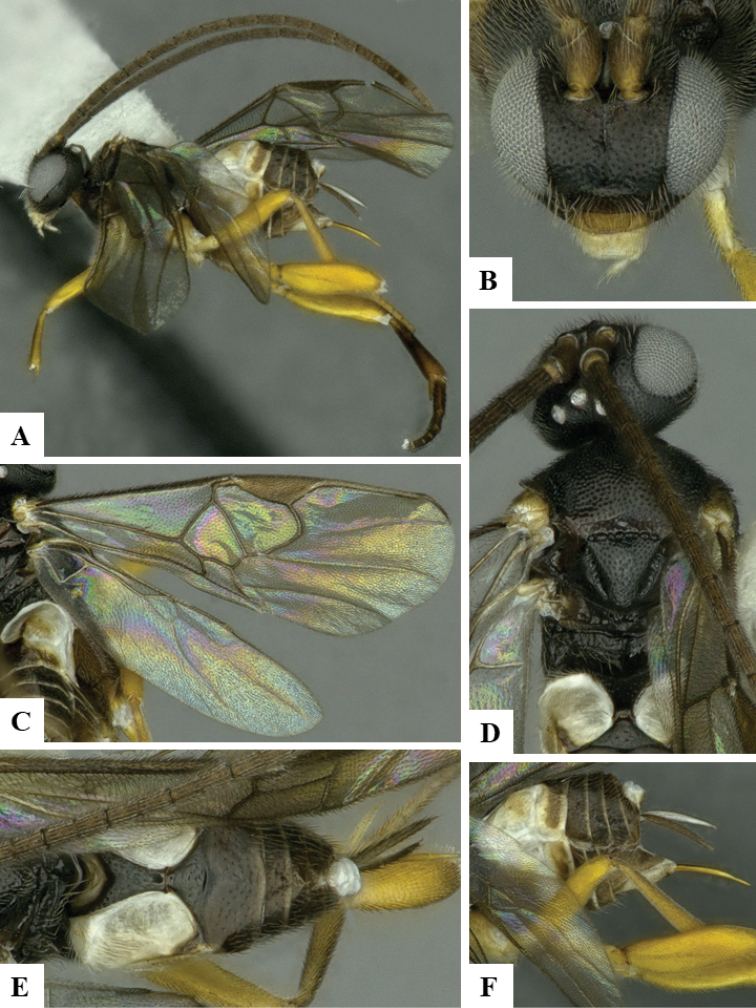
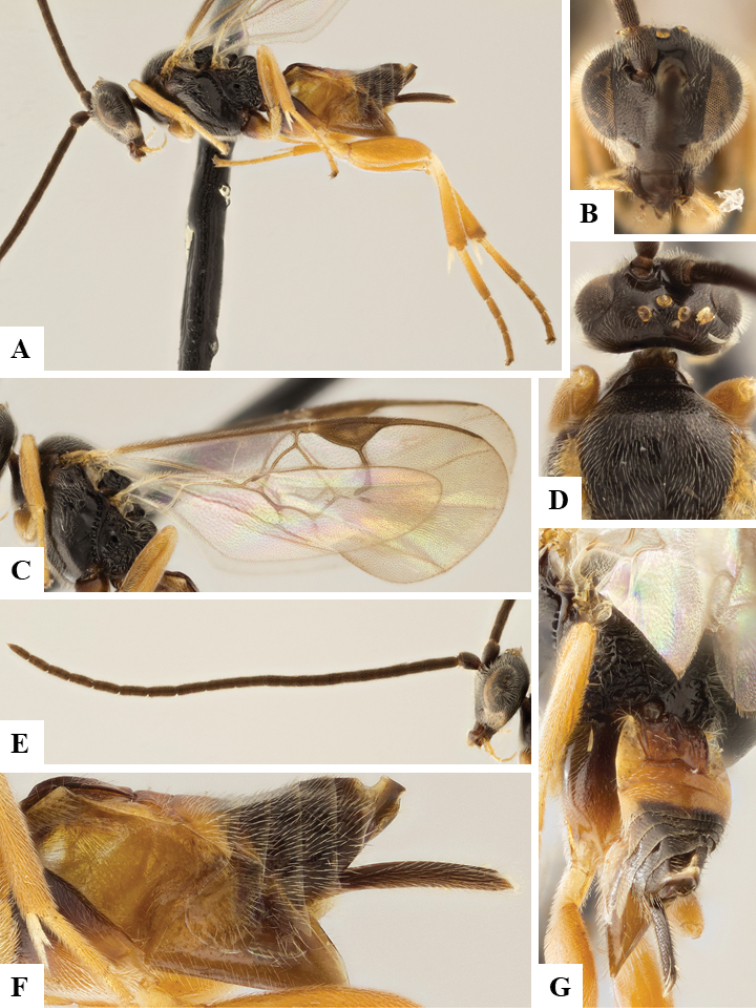
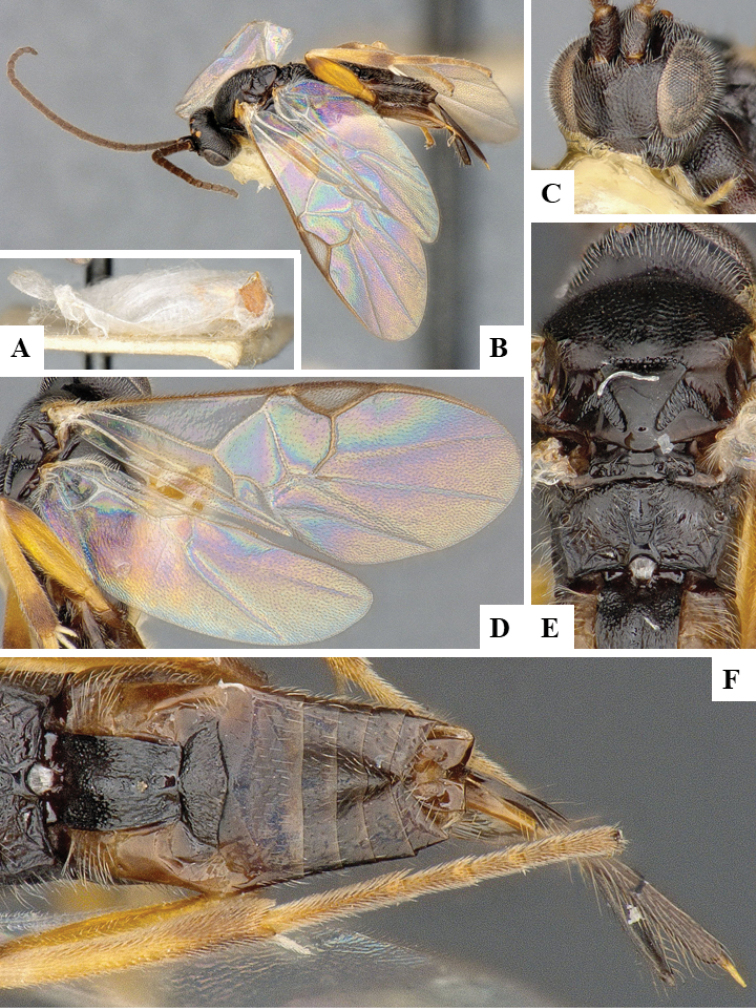
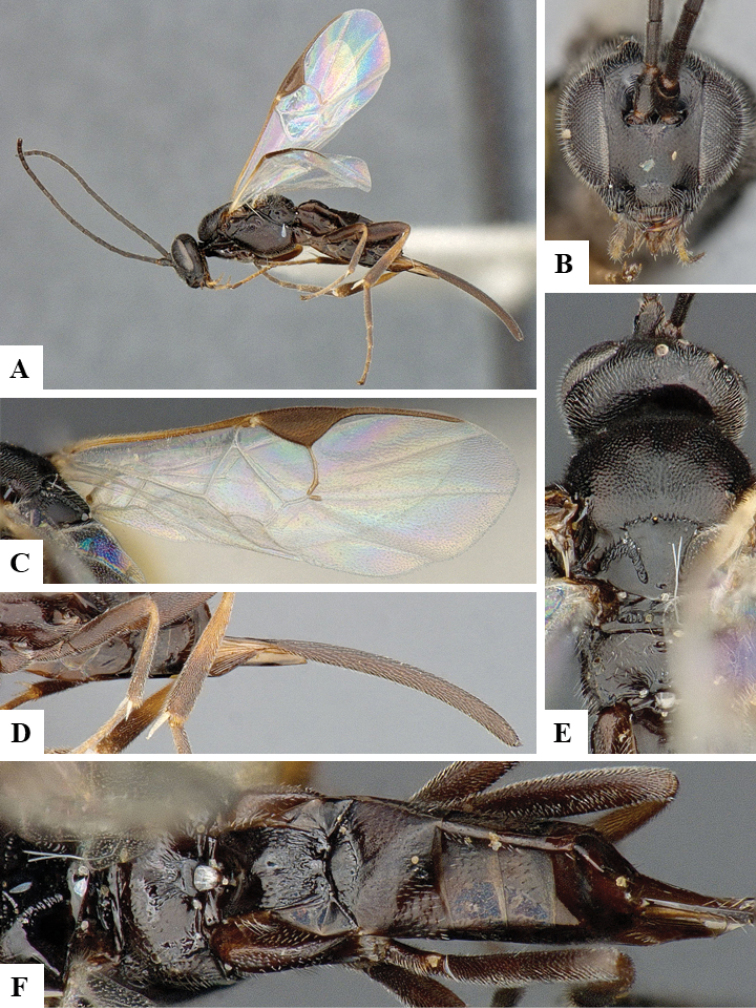
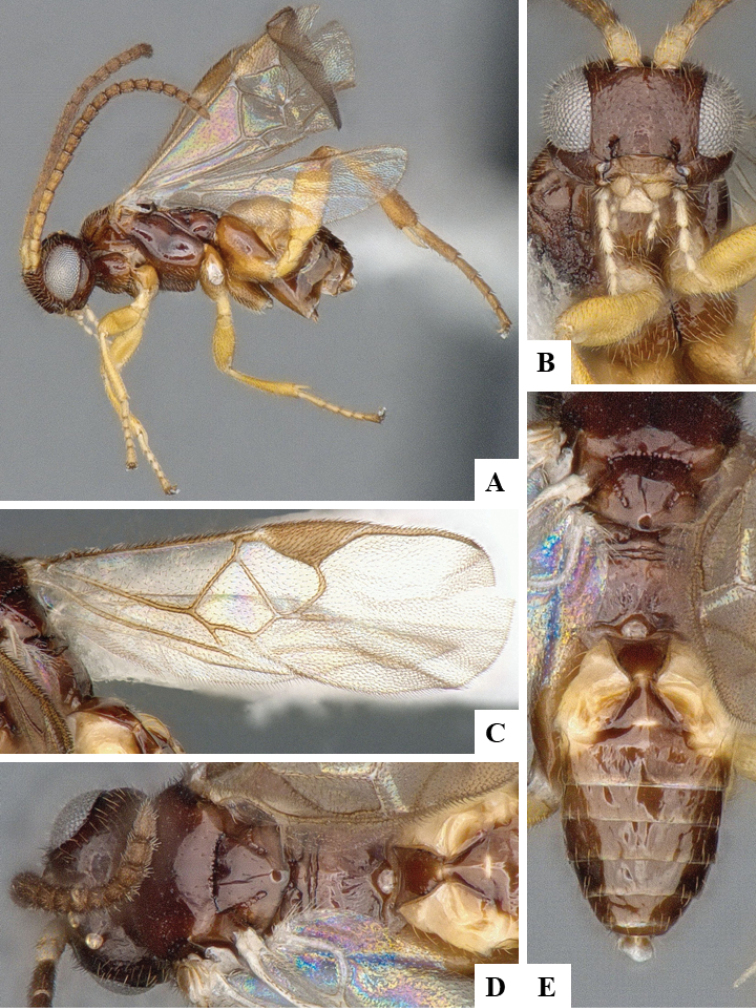
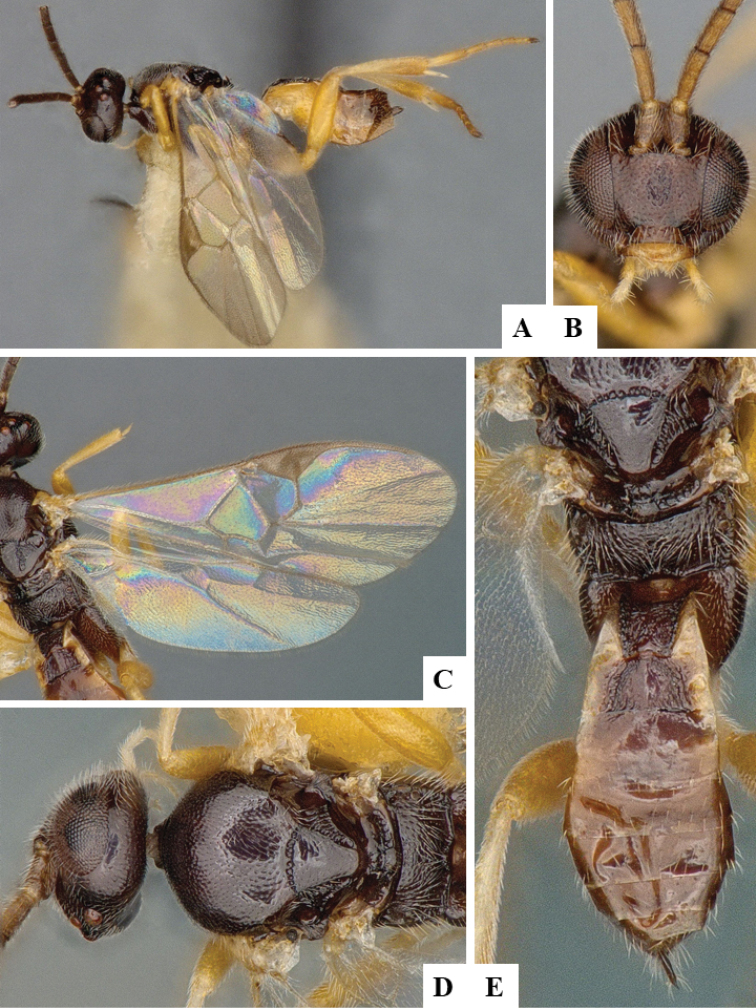
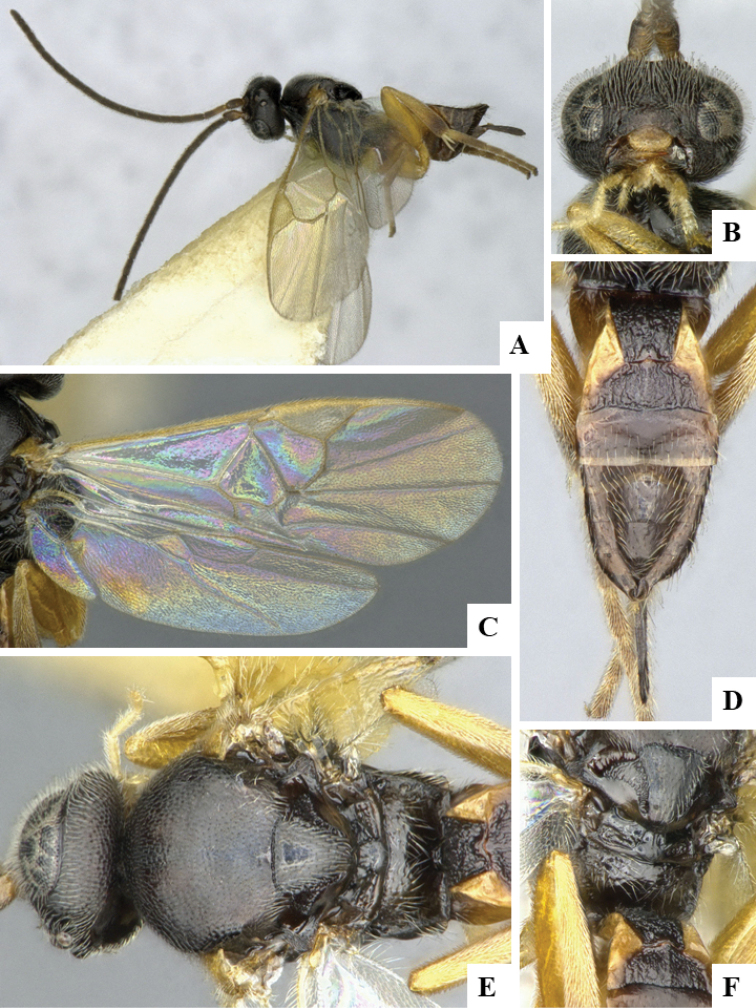
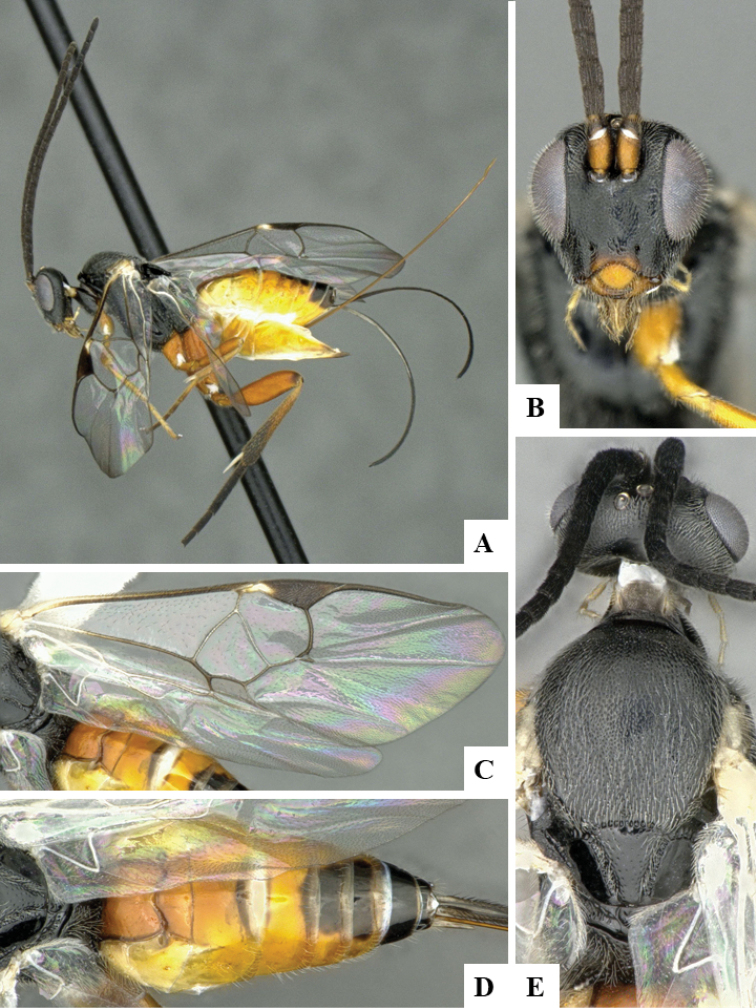
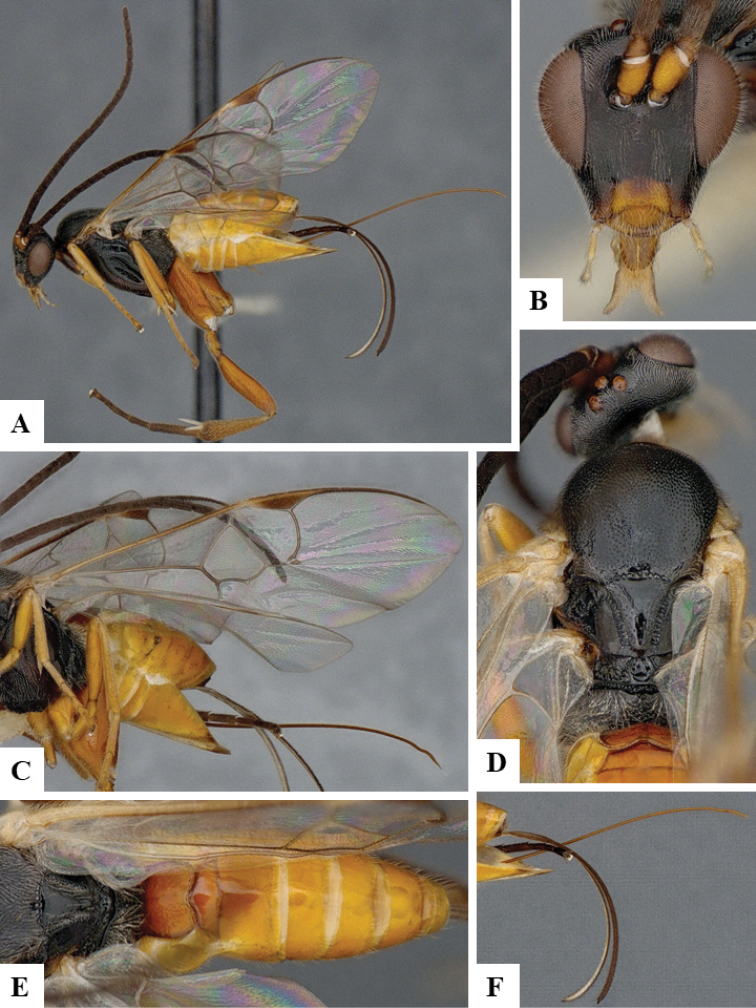
Only five genera of Microgastrinae, Austinicotesia, Austrocotesia, Miropotes, Pelicope, and Semionis, have hind wings without vein 2r-m (all other known Microgastrinae have that vein present, although often weakly pigmented).
Pelicope and Semionis can be recognized within this group because both have the fore wing areolet very large (while the other three genera are without an areolet or have a very small areolet). Pelicope (Fig. 181) has the propodeum unsculptured, notauli at least partially marked, and eyes in frontal view slightly divergent ventrally. Semionis (Figs 221, 222) has the propodeum with a partial transverse carina and many fine striations radiating from the nucha, the notauli not marked, and the eyes in frontal view are not divergent ventrally (Nixon 1965, Mason 1981).
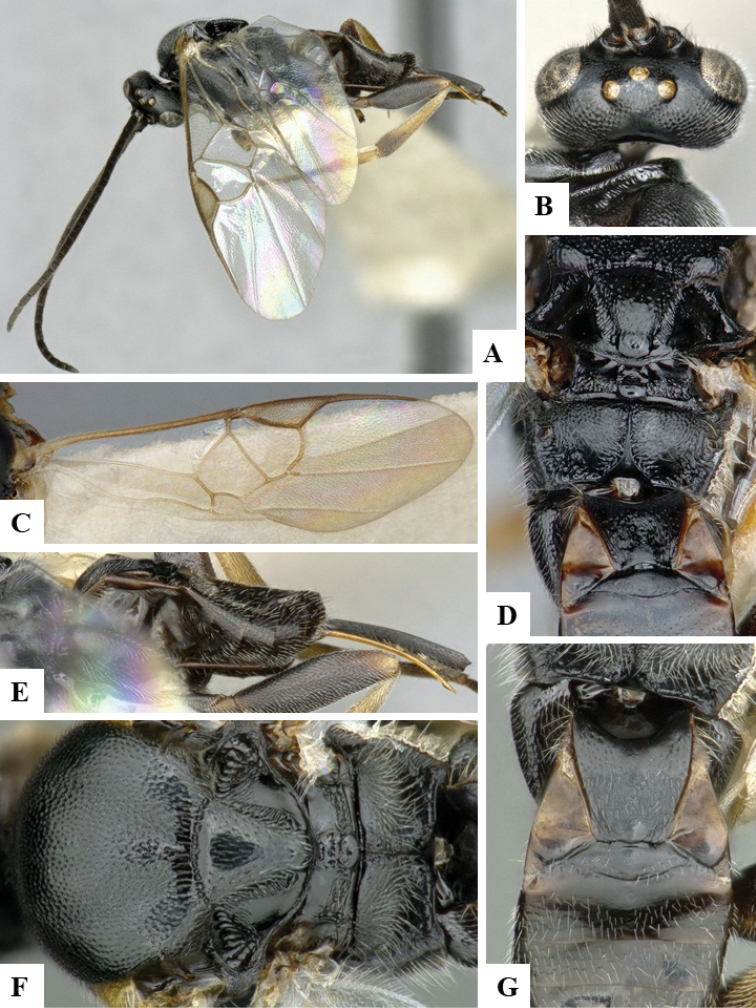
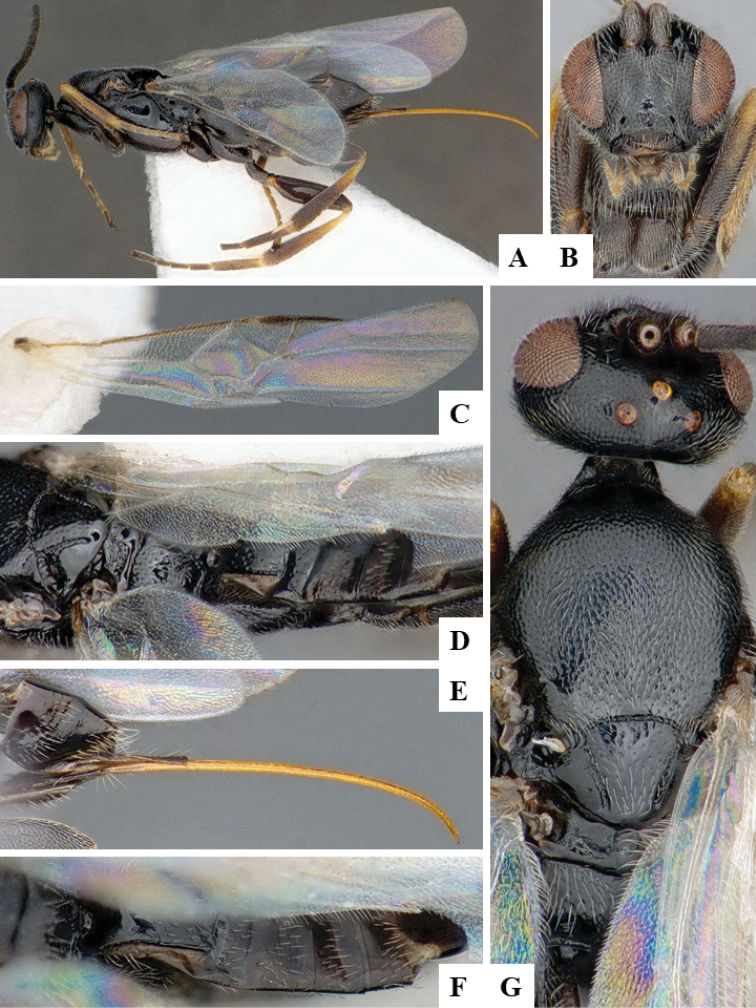
Figure 181.
Pelicopeyuccamica male CNC309859 A Habitus, lateral B Head, dorsal C Fore wing and hind wing D Mesosoma, dorsal E Metasoma, dorsal F Propodeum and tergites 1–2, dorsal.
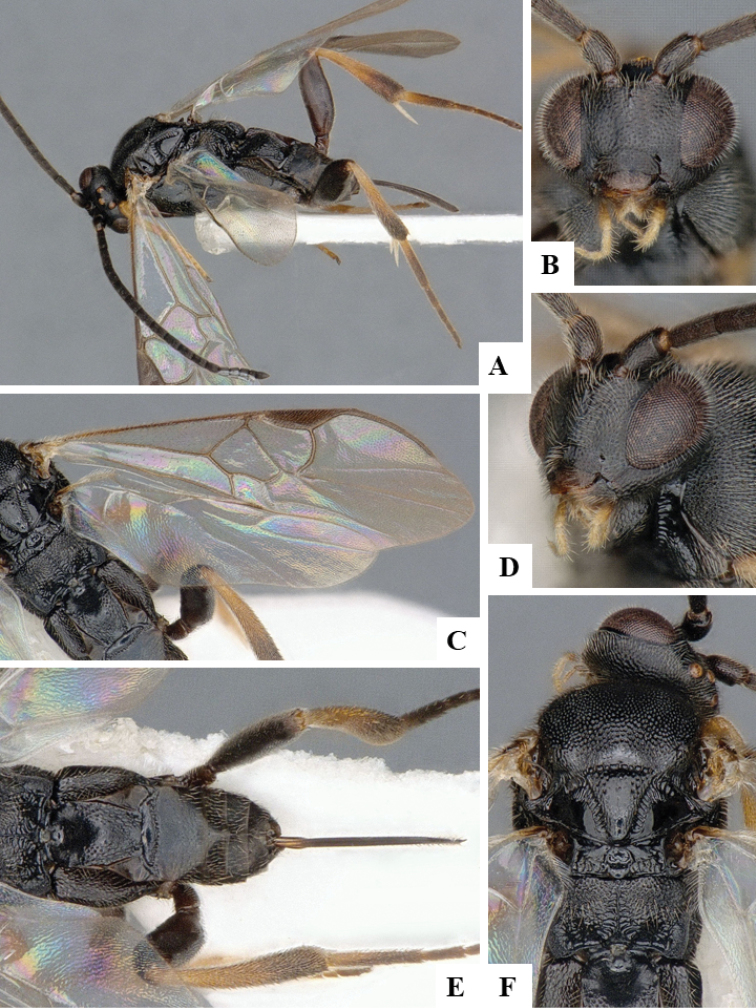
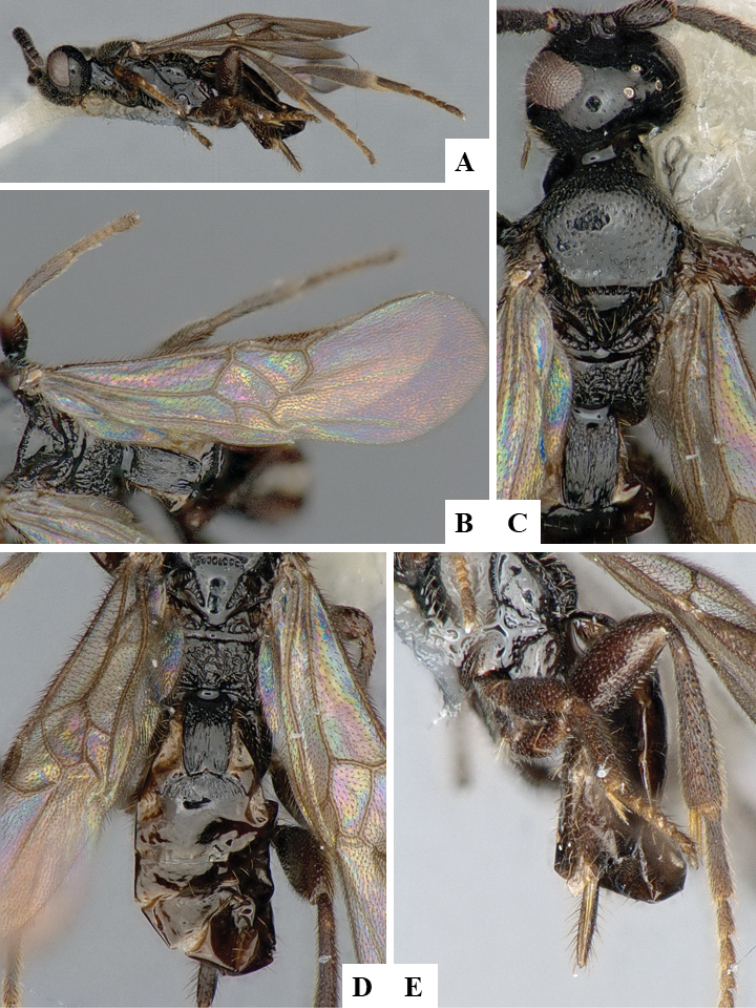
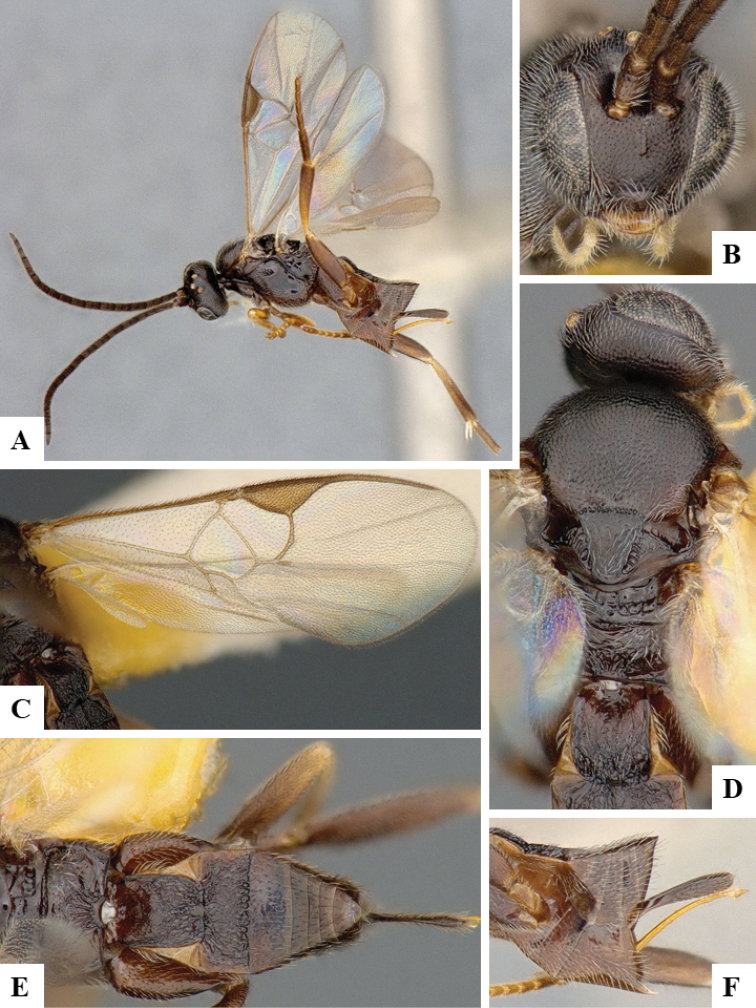
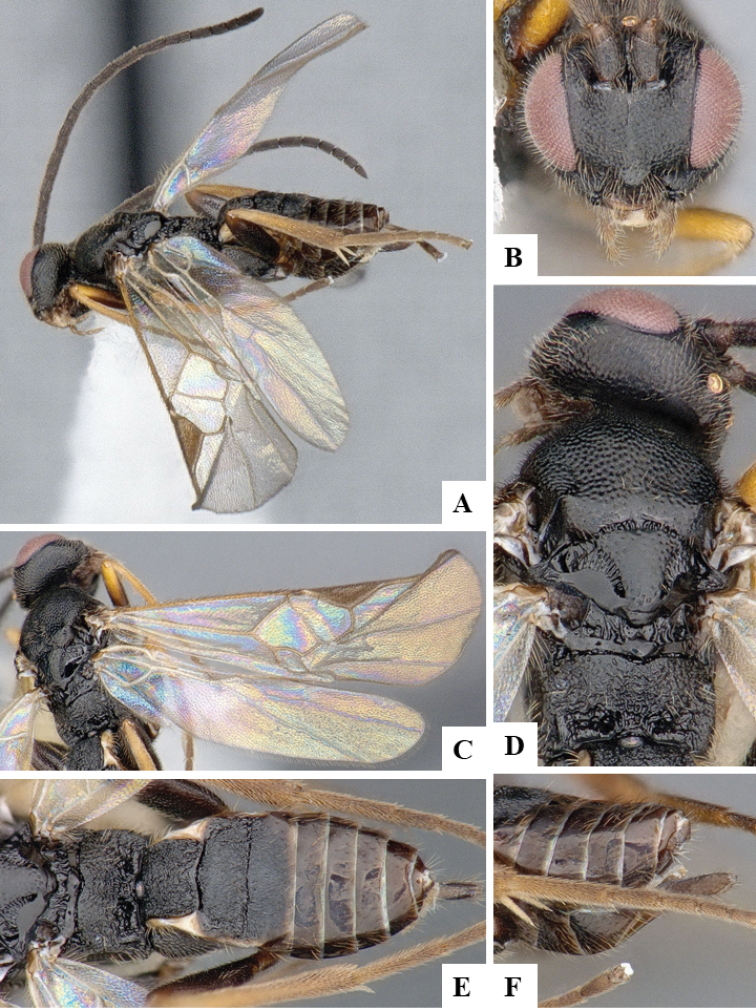
Figure 221.
Semionisrarus male holotype A Habitus, lateral B Head, frontal C Fore wing D Antennae.
Figure 222.
Semionisrarus male holotype A Habitus, lateral B Head and mesosoma, dorsal C Propodeum and metasoma, dorsal.
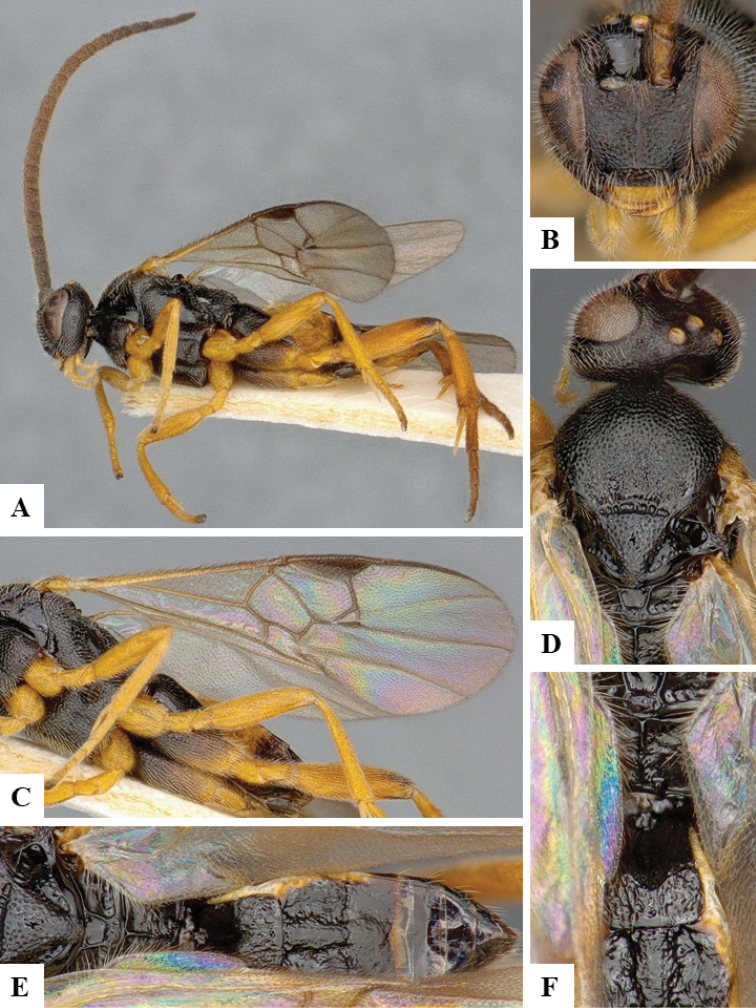
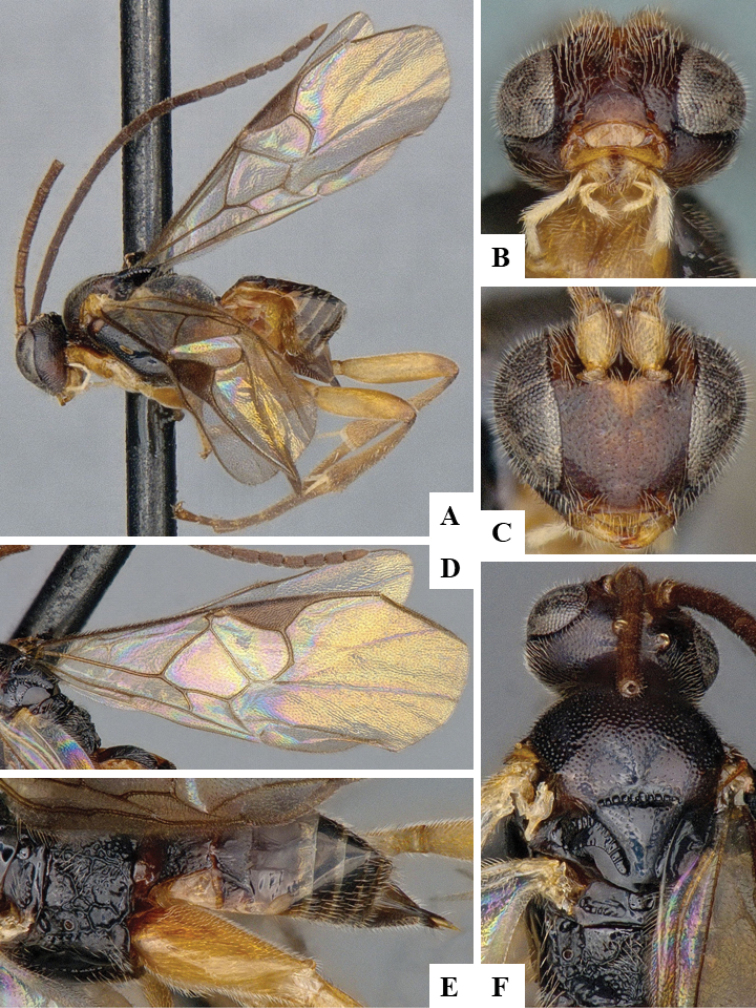
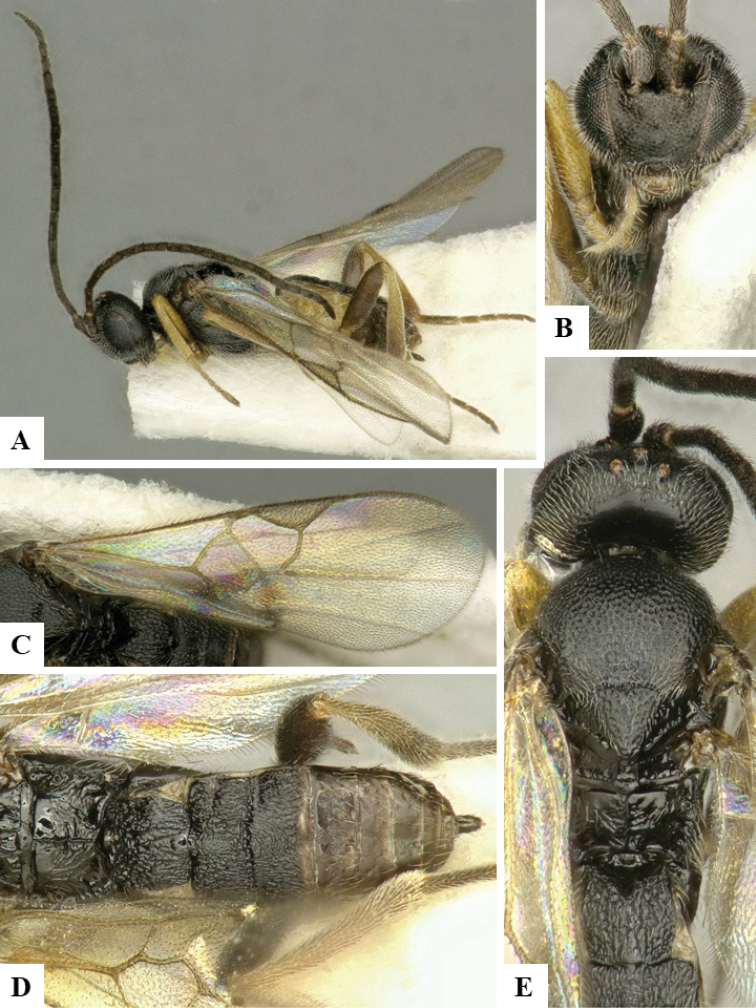
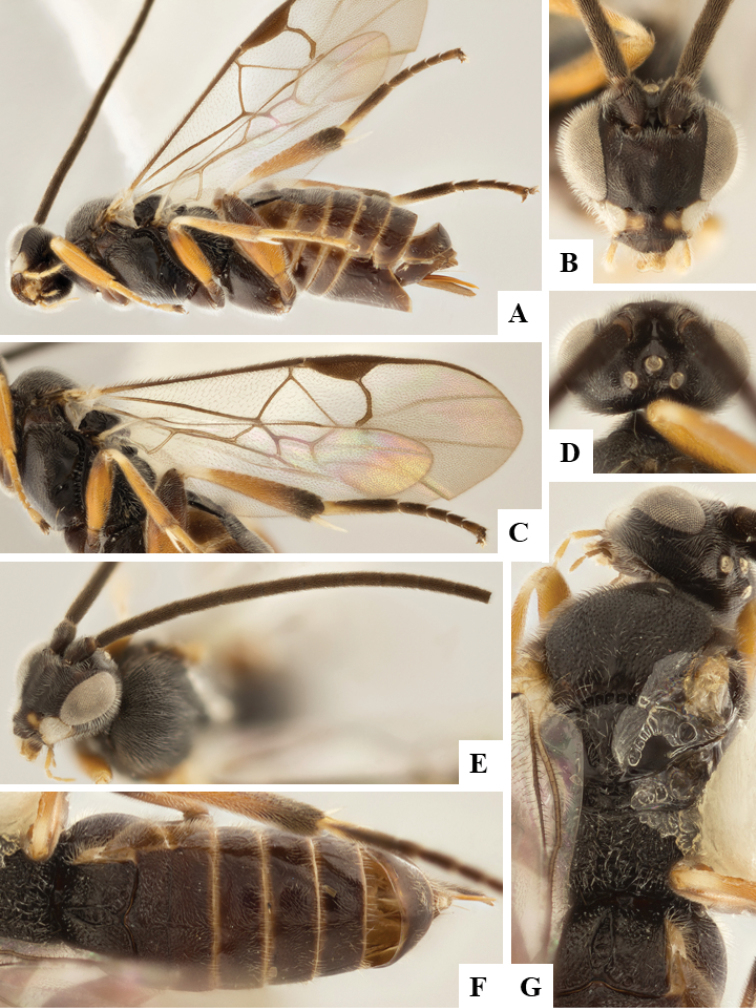
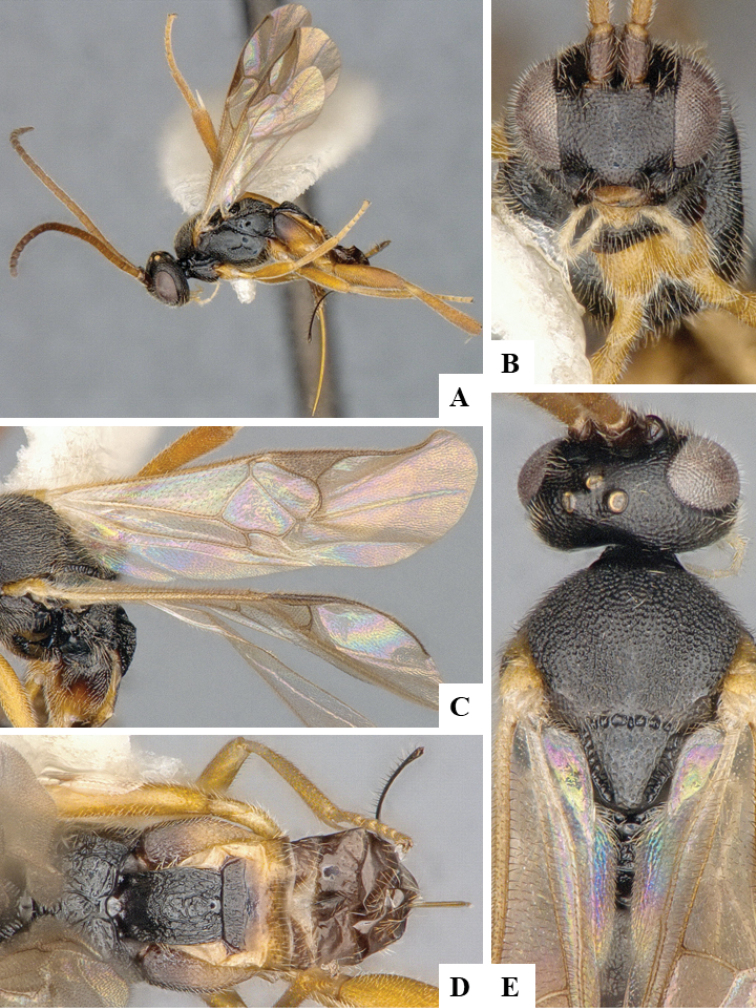
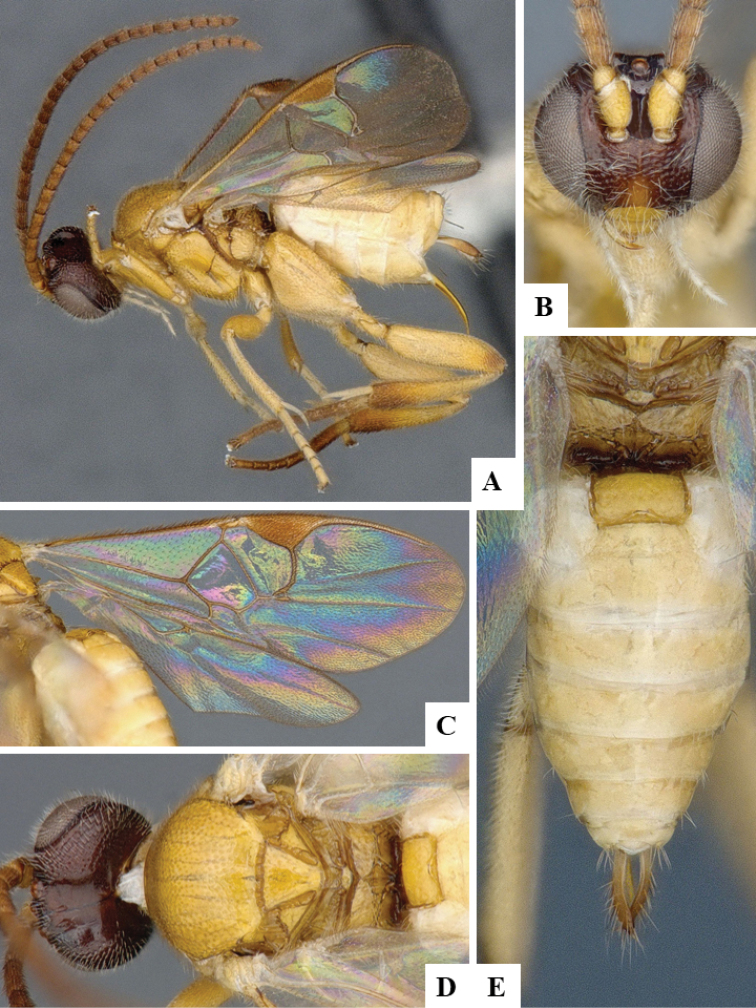
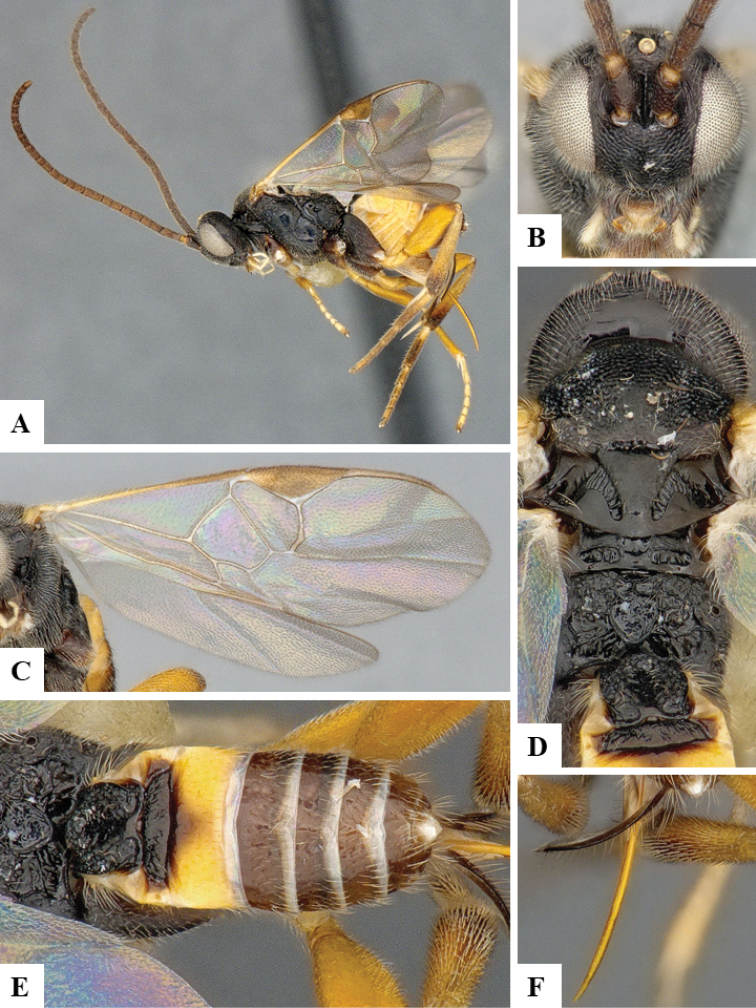
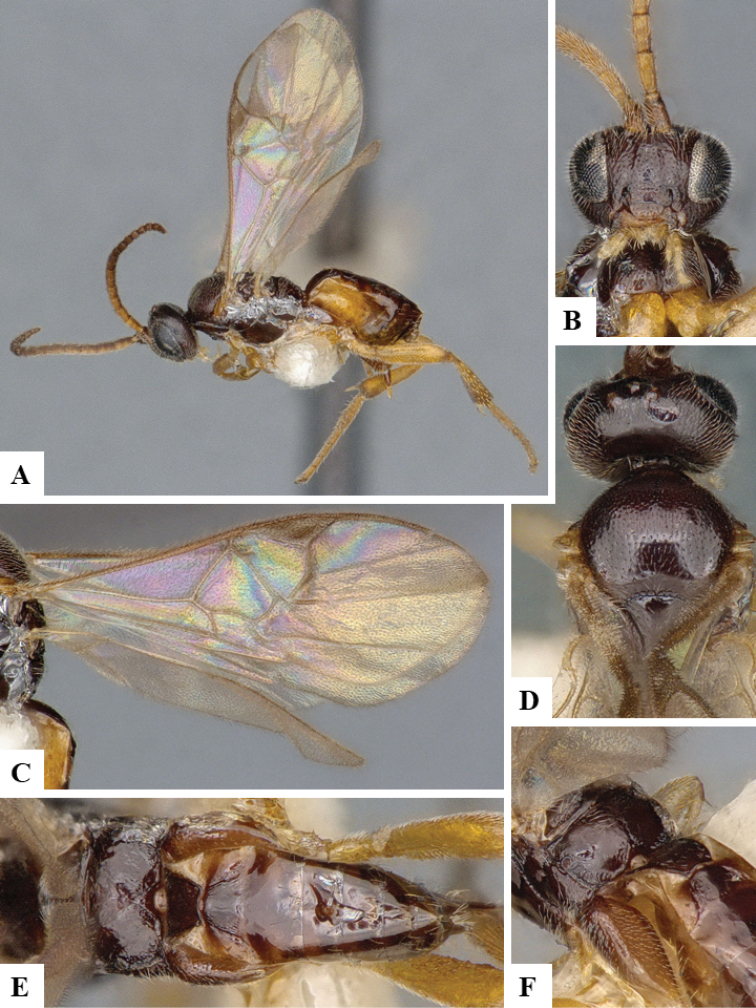
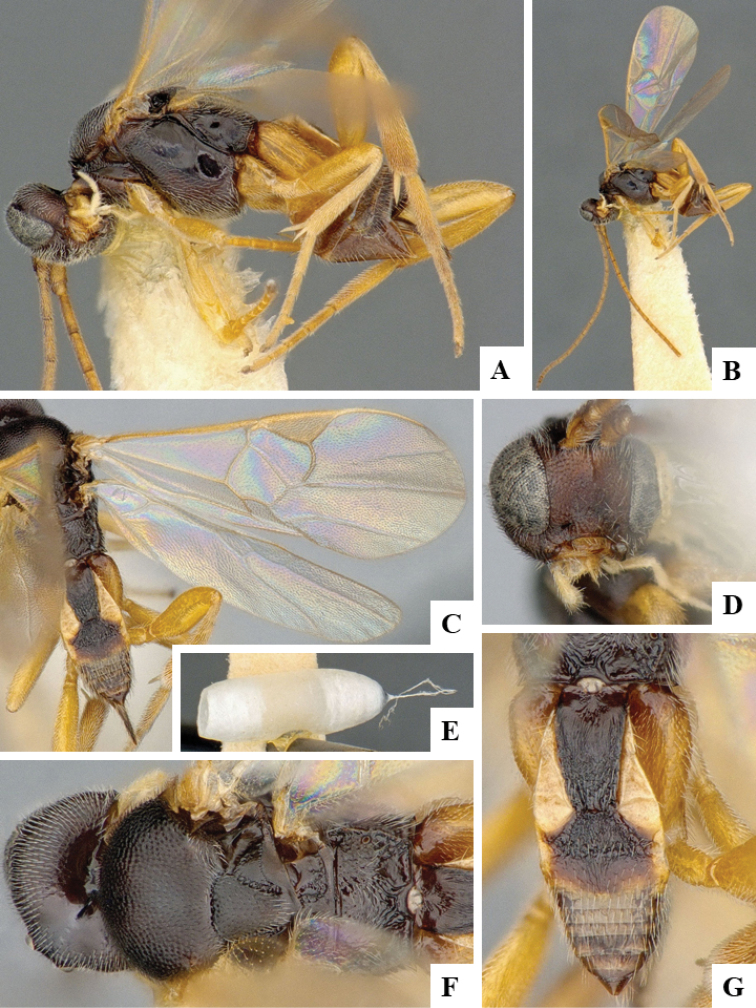
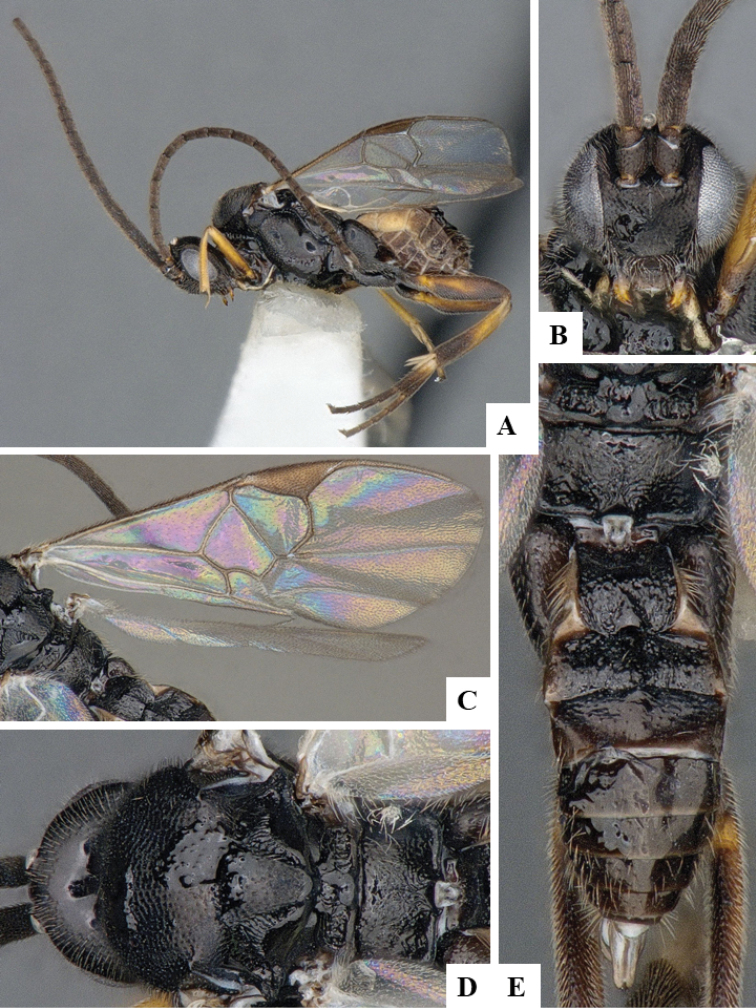
Miropotes (Figs 157–159) differs from the other genera by the ovipositor sheaths and ovipositor with a unique shape, in most species strongly bent; eyes enlarged and strongly convergent with malar space totally or almost totally obliterated; metacoxa small and metatibial spurs very short (Fernandez-Triana et al. 2014d).
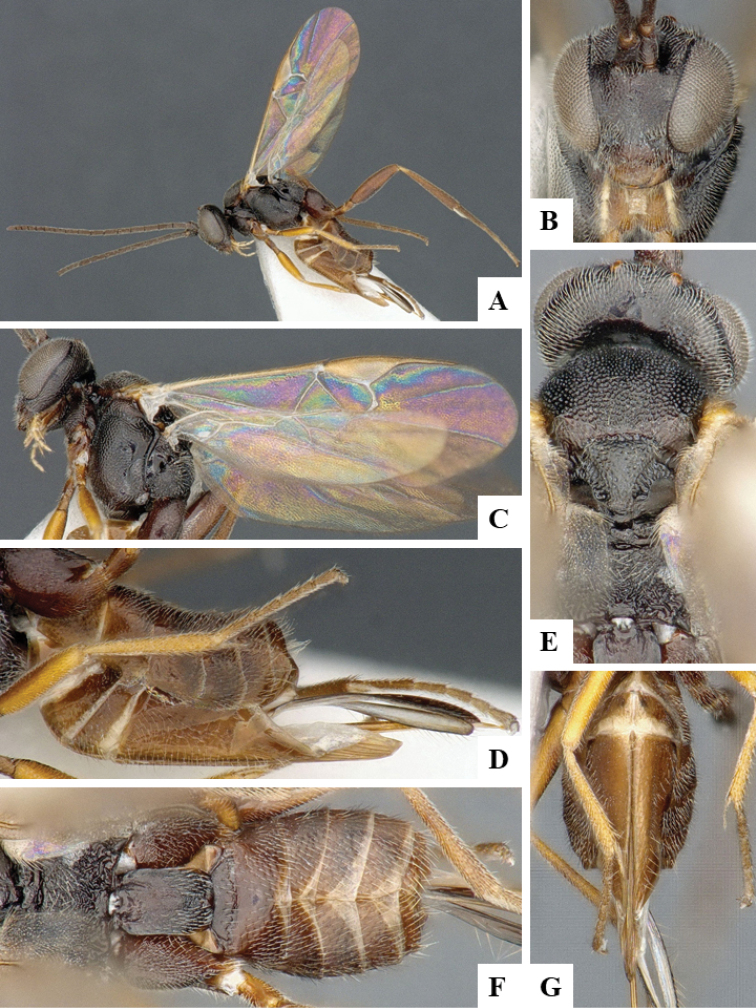
Figure 157.
Miropotesaustini female holotype A Habitus, lateral B Head, frontal C Fore wing D Metasoma, lateral E Head and mesosoma, dorsal F Metasoma, dorsal.
Figure 159.
Miropotesorientalis female paratype CNCH2114 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Metasoma, dorsal E Mesosoma, dorsal F Apex of metasoma, lateral.
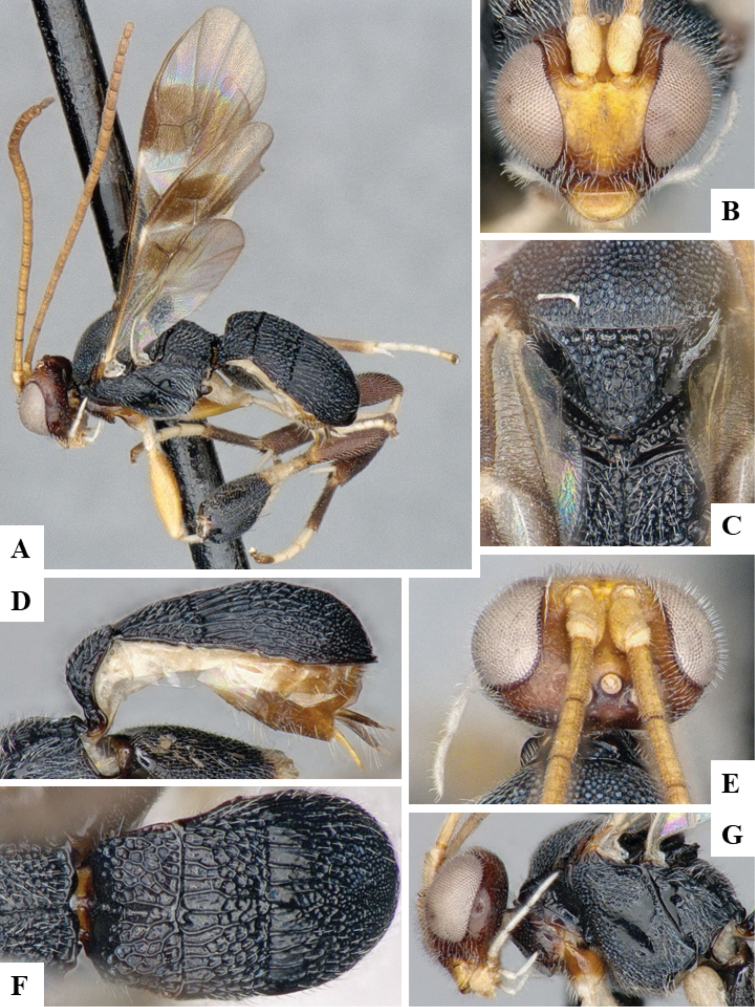
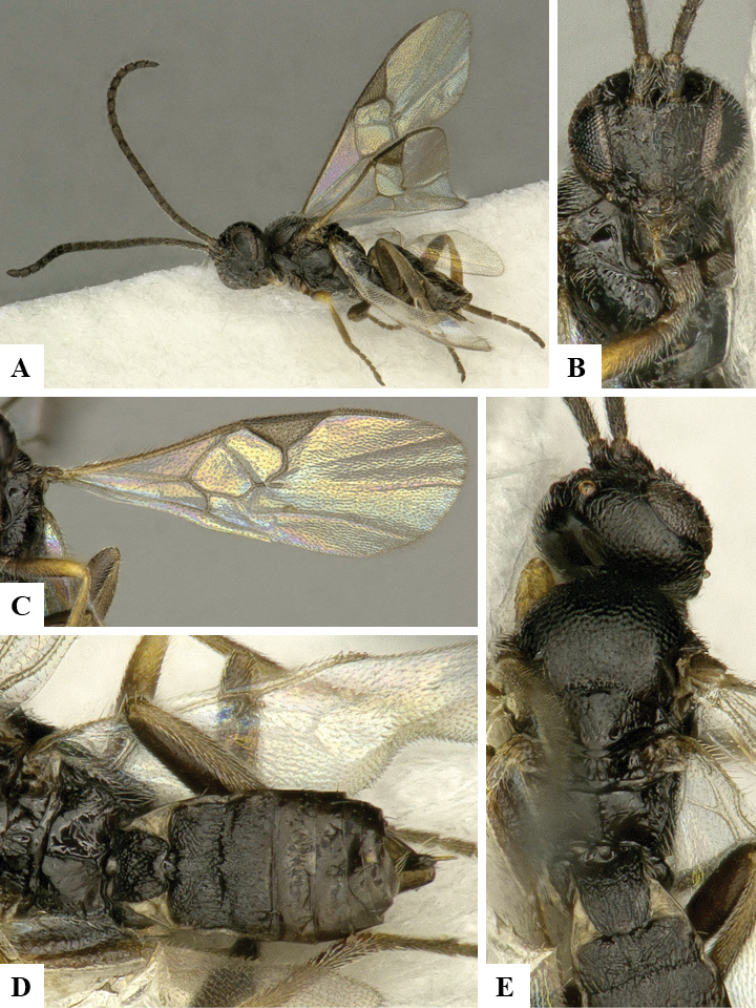
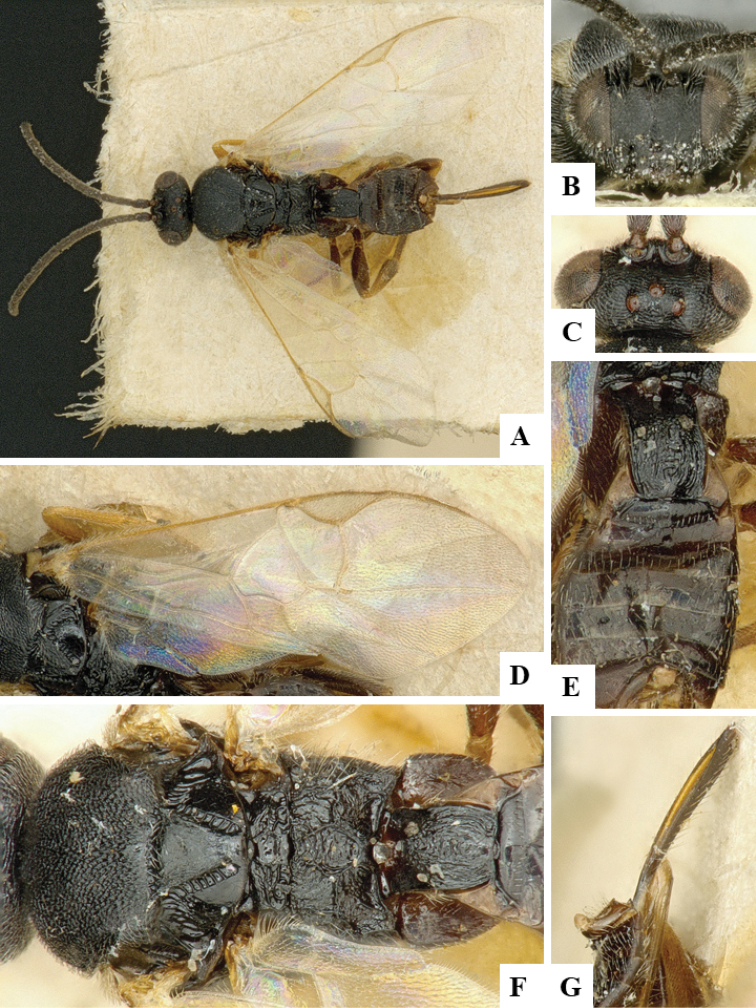
Austinicotesia (Figs 27, 28) and Austrocotesia (Figs 29–32) are similar to each other in several features (Austin and Dangerfield 1992, Fernandez-Triana and Boudreault 2018) but differ as follows: Austinicotesia has the fore wing without areolet (with areolet in Austrocotesia); fore wing with pterostigma relatively thin and long, 3.5 × as long as wide (pterostigma much less than 3.0 × as long as wide in Austrocotesia); fore wing vein 2RS much longer, ca. 1.5 ×, than vein r (fore wing vein 2RS much shorter, ca. 0.5 ×, than vein r in Austrocotesia); metafemur relatively thick and stout (of more normal proportions in Austrocotesia); T1 widening towards posterior margin and with strong hump followed by deeply excavated area and strong carinae (T1 more or less parallel-sided or narrowing towards posterior margin and without hump or excavate area in Austrocotesia); and T2 mostly smooth (usually mostly sculptured in Austrocotesia).
Figure 27.
Austinicotesiaindonesiensis female holotype A Habitus, lateral B Head, frontoventral C Head, frontal D Fore wing E Ovipositor and ovipositor sheaths F Propodeum and tergite 1, dorsal G Metasoma, dorsal; Head and mesosoma, dorsal.
Figure 28.
Austinicotesiapapuanus female holotype A Habitus, lateral B Head, frontal C Fore wing and hind wing D Propodeum and metasoma, dorsal E Mesosoma, dorsal.
Figure 29.
Austrocotesiacroizati female CNC280750 A Habitus, lateral B Head, frontal C Ovipositor and ovipositor sheaths D Fore wing and hind wing E Metasoma, dorsal F Head and mesosoma, dorsal.
Figure 32.
Austrocotesiarenei female holotype A Habitus, lateral B Head, frontal C Fore wing D Antenna E Propodeum and metasoma, dorsal F Mesosoma, dorsolateral.
Only six genera of Microgastrinae have the propodeum mostly smooth except for complete longitudinal and transverse carinae: Beyarslania, Clarkinella, Janhalacaste, Neoclarkinella, Mariapanteles, and Prasmodon. We place them together because of the diagnostic value of that unique carination pattern, but it is clear that these genera do not constitute a monophyletic group.
Prasmodon (Figs 191–193) is the only genus in this subgroup with notauli strongly marked and fore wing areolet relatively large (Fernandez-Triana et al. 2014f).
Figure 191.
Prasmodonalmasolisae female holotype A Habitus, lateral B Head and mesosoma, dorsal C Fore wing and hind wing D Apex of antennae E Hind tibia, lateral F Metasoma, dorsal.
Figure 193.
Prasmodonsubfuscus male holotype A Habitus, lateral B Hind tibia, lateral C Fore wing D Propodeum and metasoma, dorsal E Head and mesosoma, dorsal F Head, lateral.
Clarkinella and Janhalacaste also have a fore wing areolet (although very small, almost obliterated) and can be distinguished from each other as follows. Clarkinella (Figs 46, 47) has the scutellar disc with a smooth posteromedian band, T1 without a median longitudinal carina, and hypopygium mostly inflexible with only a sharp fold posteriorly (Mason 1981), whereas Janhalacaste (Figs 128, 129) has the scutellar disc with a coarse posteromedian band, T1 with a longitudinal sulcus on the anterior 0.6–0.7 of its length and posterior 0.3 with two short carinae centrally delimiting a slightly raised area, and hypopygium folded medially and with several pleats (Fernandez-Triana and Boudreault 2018).
Figure 46.
Clarkinellacanadensis female holotype A Habitus, lateral B Head, frontal C Fore wing D Head, dorsal E Mesosoma, dorsal F Propodeum and metasoma, dorsal.
Figure 47.
Clarkinellaedithae female holotype A Habitus, lateral B Head, frontal C Fore wing D Mesosoma, dorsal E Head and antenna, lateral F Metasoma, dorsal.
Figure 128.
Janhalacastedanieli male holotype A Habitus, lateral B Head, frontal C Fore wing D Mesosoma, dorsal E Metasoma, dorsal F Tergites 1–2, dorsal G Propodeum and tergite 1, dorsal.
Figure 129.
Janhalacastewinnieae female holotype A Habitus, lateral B Head, frontal C Mesosoma, dorsal D Fore wing E Propodeum and metasoma, dorsal F Metasoma, dorsal.
Neoclarkinella (Figs 161–165), Mariapanteles, and Beyarslania all lack a fore wing areolet. Neoclarkinella can be recognized because it has a very distinctive T1 which sharply narrows towards posterior margin and has a wide depression on the anterior half, and a hypopygium with multiple pleats (Chen and Song 2004, Veena et al. 2014).
Figure 161.
Neoclarkinellaariadne female CNCHYM01447 A Habitus, lateral B Head, frontal C Fore wing D Mesosoma, dorsal E Metasoma, dorsal.
Figure 165.
Neoclarkinellasundanus male CNCHYM01464 A Habitus, lateral B Head, frontal C Fore wing D Metasoma, dorsal E Head and mesosoma, dorsal F Head, frontolateral.
Mariapanteles and Beyarslania have the hypopygium mostly inflexible, with a posteromedian translucent fold where only a few or no pleats are visible; and T1 has a sharply defined median, longitudinal sulcus, at least on the anterior half. Mariapanteles (Figs 143, 144) has the ovipositor sheaths much longer (0.7 × as long as the metatibia length), and ovipositor mostly straight to slightly curved (Whitfield et al. 2012), whereas Beyarslania (Fig. 33) has the ovipositor sheaths relatively very short (less than 0.3 × metatibial length), and the ovipositor strongly downcurved (Mason 1981, at the time referring to the genus as Xenogaster). Mariapanteles is also the only genus in this group with the propodeum having some additional, small and short transverse carinae that radiate from the median carina (but, nevertheless, the propodeum still appears as if it is crossed by the median and transverse carinae, the defining trait of this group).
Figure 143.
Mariapantelesdapkeyae female holotype A Habitus, lateral B Fore wing C Ovipositor and ovipositor sheaths D Head and mesosoma, dorsal E Propodeum and metasoma, dorsal F Propodeum and tergites 1–3, dorsal.
Figure 144.
Mariapantelesfelipei female holotype A Habitus, dorsal B Head, frontal C Fore wing D Head and mesosoma, dorsal E Ovipositor and ovipositor sheaths F Metasoma, dorsal G Propodeum and tergite 1, dorsal.
Figure 33.
Beyarslaniainsolens female WAM 0141 A Habitus, lateral B Head, frontal C Fore wing D Mesosoma, dorsal E Hind wing F Antenna and head, dorsal G Mesosoma, dorsal.
The remaining six genera in this group cannot easily be associated with any other genus and are discussed below in alphabetical order.
Billmasonius (Fig. 34) is recognized by T1 with a unique shape and desclerotization, with a relatively wide anterior 0.6 and very narrow posterior 0.4, so that widest part of tergite, near anterior margin, is around 4.0 × the narrowest width, along posterior 0.4, and with anterior 0.6 mostly desclerotized, only with lateral margins and narrow central strip sclerotized; T2 is also diagnostic, with a partially sclerotized area surrounding each spiracle on laterotergite 2 the same colour as T2, giving the impression of T2 having three peaks, the largest and central one being the actual T2, the two smaller lateral ones being the area surrounding the spiracles on each laterotergite (Fernandez-Triana and Boudreault 2018).
Figure 34.
Billmasoniuscienci female holotype A Habitus, lateral B Head, frontal C Fore wing D Mesosoma, dorsal E Metasoma, dorsal F Propodeum and tergite 1, dorsal.
Exulonyx (Fig. 95) has a unique combination of features within Microgastrinae: propodeum with a partial median, longitudinal carina on anterior 0.6 and complete areola on posterior 0.4, hypopygium inflexible, ovipositor curving downwards on posterior 0.3, and T1 and T2 coarsely sculptured (Mason 1981).
Figure 95.
Exulonyxcamma female CNCHYM01205 A Habitus, lateral B Head, frontal C Fore wing D Head and mesosoma, dorsal E Propodeum and metasoma, dorsal F Ovipositor and ovipositor sheaths.
Fornicia (Figs 96–98) is the only Microgastrinae genus with the epicnemial carina complete and the fore wing areolet absent; also, the head in lateral view is relatively small (compared to the mesosoma) (Austin and Dangerfield 1992), and T1–T3 form a carapace covering the entire dorsal surface of the metasoma. Only a few species in the Microplitis group (see below) have a partial to complete epicnemial carina, but all those genera have the fore wing with an areolet (usually relatively large), and the head of normal proportions.
Figure 96.
Forniciajarmilae female holotype A Habitus, lateral B Head, frontal C Fore wing D Metasoma, dorsal E Mesosoma, dorsal.
Figure 98.
Fornicia sp. male JMIC 0049 A Habitus, lateral B Fore wing C Head, frontal D Head and mesosoma, dorsal E Propodeum and metasoma, dorsal.
Qrocodiledundee (Fig. 212) can easily be recognized by its propodeal apophysis, unique among Microgastrinae, as well as the flattened mesosoma, metafemur short and stout, pronotum dorsally enlarged, and the propodeum with a median carina and a partially defined areola (Fernandez-Triana and Boudreault 2018).
Figure 212.
Qrocodiledundeeoutbackense male holotype A Habitus, lateral B Head, frontal C Head, dorsal D Fore wing E Propodeum, dorsal F Metasoma, dorsal G Mesosoma, dorsal. Red arrow shows the propodeal aphophysis.
Xanthomicrogaster (Figs 246–249) is unique because of the following combination of features: hind wing with vein 1cu-a strongly sinuous and first submarginal cell tall (height at least 2 × its width), fore wing with a very small areolet, metacoxa very large (almost as large as the metasoma length), propodeum mostly smooth but with a strong and sharp median longitudinal carina, T1 very wide and with a strong median longitudinal sulcus, T2 rectangular and usually sculptured, hypopygium inflexible, and ovipositor sheaths relatively long (more than 0.5 × metatibia length) and with numerous setae. Some of these morphological features would suggest this genus could be placed within the Cotesia group, contrary to Mason’s (1981) opinion when he grouped it within his Microgastrini. However, Xanthomicrogaster has many other features that are so different to both our Cotesia group and Mason’s Microgastrini that we prefer to maintain it as an unplaced genus.
Figure 246.
Xanthomicrogasterfortipes female CNCHYM07148 A Habitus, lateral B Head, frontal C Fore wing D Head and mesosoma, dorsal E Propodeum and metasoma, dorsal.
Figure 249.
Xanthomicrogaster sp. female CNC492880 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Head and mesosoma, dorsal E Propodeum and metasoma, dorsal F Propodeum and tergite 1, dorsal.
Zachterbergius (Figs 253, 254) has the longest and thinnest T2 among all known Microgastrinae, with T2 length 4.0 × its width at base and apex, 0.7–0.8 × as long as T1 length, and around 1.5× as long as T3 length. Also, the propodeum has a clearly defined median carina, partially defined transverse carina, and the posterior part of an areola; the antennal scape is very transverse, and the labial palpi are very long, extending to the mesopleuron (Fernandez-Triana and Boudreault 2018).
Figure 253.
Zachterbergiustenuitergum male holotype A Habitus, lateral B Head, frontal C Fore wing D Metasoma, dorsal E Propodeum F Head and mesosoma, dorsal.
Figure 254.
Zachterbergiustenuitergum male paratype JMIC 0538 A Habitus, dorsal B Mesosoma and metasoma, dorsal.
b) Microplitis group
This is one of the best-defined groups of genera within Microgastrinae (see Mason 1918), and most likely to be monophyletic. It is characterized by: tentorial pits relatively large, head mostly coarsely sculptured, stemmaticum usually very well defined and slightly to strongly raised from the surrounding areas, anteromesoscutum and scutellar disc usually coarsely sculptured, notauli almost always defined (often very clearly), propodeum always sculptured and with several strongly defined carinae, fore wing with areolet usually large, metacoxa relatively small, metatibial spurs short, T1 with median longitudinal sulcus, hypopygium inflexible and almost always relatively short, ovipositor sheaths with few setae that are mostly limited to the apex, and ovipositor almost always very short (much shorter than 0.5 × metatibia length).
Philoplitis (Figs 182, 183) has a unique combination of features including an enormous scutellum conically prolonged posteriorly over the propodeum (Mason 1981, Fernandez-Triana and Goulet 2009, Ranjith et al. 2019). It also has an occipital carina, and ocelli forming a very low triangle, to the point that the anterior ocellus seems almost on the same line as the posterior ones.
Figure 182.
Philoplitispunctatus female CNC309861 A Habitus, lateral B Head, frontal C Fore wing D Head and mesosoma, dorsal E Propodeum and metasoma, dorsal F Propodeum and tergites 1–2, dorsal.
Figure 183.
Philoplitisstriatus male holotype A Habitus, lateral B Head, frontal C Fore wing D Head and mesosoma, dorsal E Antenna F Metasoma, dorsal G Mesosoma, dorsal.
Silvaspinosus (Fig. 227) has the clypeus extremely long and thin, the malar line extremely short (almost absent), the mandible base separated from the rest of the head by a desclerotized area that looks almost like an opening, and mandibles relatively stout and large. The shape of the clypeus, and the separation of the mandible from the rest of the head by a desclerotized area are unique among Microgastrinae (Fernandez-Triana and Boudreault 2018). It also has the fore tarsus with a spine-like seta, and the scutellar disc with the posteromedian band smooth; both of which are unique and distinctive among the Microplitis group.
Figure 227.
Silvaspinosusvespa female holotype A Habitus, lateral B Head, frontal C Fore wing D Head, dorsal E Metasoma, dorsal F Mesosoma, dorsal G Propodeum and tergites 1–4, dorsal.
Gilbertnixonius (Fig. 99), is the only genus in this group that has the propodeum with both longitudinal and transverse carinae but without an areola (Alloplitis and Tobleronius have those carinae, although sometimes incomplete, but they also have a complete areola on the propodeum). Gilbertnixonius also has an epicnemial carina (otherwise only present in some species of Snellenius and in all species of the unrelated genus Fornicia) and an incomplete occipital carina (otherwise only present in Alloplitis, Philoplitis, and Tobleronius) (Fernandez-Triana and Boudreault 2018).
Figure 99.
Gilbertnixoniusbiem female holotype A Fore wing B Habitus, lateral C Head, frontal D Head, mesosoma and metasoma, lateral E Head and mesosoma, dorsal F Mesosoma, dorsal G Propodeum and metasoma, dorsal.
Alloplitis and Tobleronius are somewhat similar morphologically and distinguished from the other six genera in this group by the propodeum with a complete areola (in addition to partial longitudinal and transverse carinae). Alloplitis (Figs 7, 8) has T1 more or less parallel-sided or slightly widening towards the posterior margin, and T2 more or less rectangular; whereas Tobleronius (Fig. 233) has T1 strongly narrowing towards the posterior margin (width at posterior margin 0.3 × or less of width at anterior margin) and T2 very long and thin (although slightly widening towards the posterior margin) and with the area surrounding the spiracles on laterotergite 2 partially sclerotized and the same colour as T2 giving the impression of T2 having three peaks, the largest and central one being the actual T2, the two smaller lateral ones being the area surrounding the spiracle on each laterotergite (Fernandez-Triana and Boudreault 2018).
Figure 7.
Alloplitiscompletus female holotype A Habitus, lateral B Head, frontal C Fore wing D Antenna and head, lateral E Metasoma, dorsal F Head, mesosoma and propodeum, dorsal.
Figure 8.
Alloplitis sp. female CNC1065631 A Habitus, lateral B Fore wing C Head, frontal D Antenna E Mesosoma and tergite 1, dorsal F Metasoma, dorsal G Head and mesosoma, dorsal.
Figure 233.
Tobleroniusorientalis male holotype A Habitus, lateral B Head, frontal C Fore wing and hind wing D Head and mesosoma, dorsal E Metasoma, dorsal F Propodeum and tergites 1–2, dorsal.
Microplitis (Figs 151–156) and Snellenius (Figs 228–232) are very similar and form one of the most morphologically distinct groups of Microgastrinae (Nixon 1965, Mason 1981, Walker et al. 1990, Shaw and Huddleston 1991, Austin and Dangerfield 1993, Fernandez-Triana et al. 2015b) with the following shared diagnostic features: propodeum with coarse sculpturing and a strong median carina and T2 and T3 with a poorly defined separation between them. Most species of Snellenius are easily distinguished by having the notauli and the scutellar disc strongly excavated and sculptured, and by having the scutoscutellar sulcus very wide and deep; both cases represent the most extreme examples within Microgastrinae. Additionally, the propodeum is divided into two distinct areas (faces) clearly marked by a strong angulation (observed in lateral view) and a transverse carina (observed in dorsal view). The main difficulty when trying to distinguish both genera is that those features appear to grade, from strongly excavated and sculptured notauli and scutellar disc (most Snellenius) to less excavated and less sculptured (a few Snellenius, most Microplitis), to basically smooth and unexcavated (some Microplitis). The only reliable feature to separate the two genera is the presence of an epicnemial carina in Snellenius, which is absent in Microplitis (Mason 1981, Austin and Dangerfield 1992, 1993, Fernandez-Triana et al. 2015b), although in practice it may be difficult to distinguish the epicnemial carina due to setae and/or sculpture on the epicnemium and mesopleuron.
Figure 151.
Microplitischacoensis female CNCHYM01728 A Habitus, lateral B Fore wing C Head, frontal D Habitus, dorsal.
Figure 156.
Microplitisocellatae female MRSJFT0076 A Habitus, lateral B Head, frontal C Mesosoma, dorsal D Metasoma, dorsal E Fore wing.
Figure 228.
Snelleniusisidrochaconi male holotype A Habitus, lateral B Metasoma, dorsal C Fore wing D Mesosoma, dorsal E Head and mesosoma, dorsal.
Figure 232.
Snelleniusvickifunkae female holotype A Habitus, lateral B Mesosoma, dorsolateral C Fore wing and hind wing D Propodeum and tergites 1–2, dorsal E Head and mesosoma, dorsal F Metasoma, lateral G Propodeum and metasoma, dorsal.
Jenopappius (Figs 130–131) resembles Microplitis but with T2 strongly sculptured and rectangular, and T1 mostly sculptured and with a median depression anteriorly. Some Alloplitis may also have a somewhat similar sculpture on either T1 or T2 but the shape of those tergites is very different, and Alloplitis always has the propodeum with a complete areola, defined by strongly raised carinae. The combination of the sculptured propodeum without an areola, T1 with an anteromedian depression, and T1 and T2 with strong sculpture are very unusual and will separate Jenopappius from any other genus of Microgastrinae (Fernandez-Triana and Boudreault 2018).
Figure 130.
Jenopappiusaethiopica female CNC878534 A Habitus, lateral B Head, frontal C Head, dorsal D Fore wing E Mesosoma, dorsal F Metasoma, dorsal.
Figure 131.
Jenopappiusmagyarmuzeum female holotype A Habitus, lateral B Head, frontal C Fore wing and hind wing D Hind leg and apex of metasoma, lateral E Metasoma, dorsal F Mesosoma, dorsal.
c) Cotesia group
We place here genera with a completely inflexible hypopygium, ovipositor sheaths relatively short (less than 0.5 × metatibial length, usually much less) and mostly without setae (except apically in some cases). Most of the 29 genera considered here also have the propodeum without a complete areola (although some have it, and others have a complex arrangement of carinae and sculpture where a partial to complete areola can sometimes be defined). Although these features work well to recognize most members of the group, a few species of Sathon, Lathrapanteles, Glyptapanteles, and Ohenri have relatively long ovipositor sheaths, but in these cases the hypopygium is still always inflexible. Most or perhaps all the species within the Cotesia group posses a suite of characters indicative of parasitism of “macrolepidoptera” (sensuMason 1981: 25), but the group is probably not monophyletic. From the Cotesiini (sensuMason 1981) we exclude here Parapanteles and instead transfer it to the Apanteles group (see details under that group); the main reason being that this genus, as it had been understood, apparently includes two different sets of taxa: one that seems to be Cotesia species misidentified as Parapanteles (Valerio et al. 2009, Parks 2018, Freitas et al. 2019), and another (representing the majority of the genus, as currently understood, including the type species) that are more related to Dolichogenidea and Apanteles than to any genus in the Cotesia group. We also add here Sathon, which we consider to be closer to Glyptapanteles and related genera, unlike Mason (1981), who considered it to be part of his Microgastrini group.
The Cotesia group can be broadly split into two subgroups, based on whether the fore wing has an areolet (Buluka, Cuneogaster, Diolcogaster, Eripnopelta, Exix, Jimwhitfieldius, Keylimepie, Larissimus, Markshawius, Parenion, Protomicroplitis, Rasivalva, Ungunicus, Venanus) or does not have an areolet (Carlmuesebeckius, Chaoa, Cotesia, Deuterixys, Distatrix, Glyptapanteles, Lathrapanteles, Nyereria, Ohenri, Protapanteles, Pseudofornicia, Pseudovenanides, Sathon, Venanides, Wilkinsonellus).
Among the genera with a fore wing areolet, Jimwhitfieldius (Figs 132, 133) has the metatrochantellus with a unique shape (Fig. 133), the head with a strong depression behind the occiput, the metatibia with a very long and thick inner spur, and the ovipositor and ovipositor sheaths extremely short, probably the shortest in the entire subfamily (Fernandez-Triana and Boudreault 2018).
Figure 132.
Jimwhitfieldiusjamesi female holotype A Habitus, lateral B Head, frontal C Fore wing and hind wing D Metasoma, dorsal E Head and mesosoma, dorsal.
Figure 133.
Jimwhitfieldiusjamesi female holotype A Antennal flagellomeres 1–4 B Hind leg C Hypopygium and ovipositor, lateral D Hypopygium and ovipositor, ventral E Inner and outer spines of metatibia.
Venanus (Figs 237–240) is quite distinctive, and comprises small species, often with the body slightly depressed, face with a triangular flange between the antennal sockets, fore wing with a relatively large areolet, T2 with strongly defined lateral sulci, and ovipositor sheaths with very few and minute setae (Mason 1981).
Figure 237.
Venanusjohnnyrosalesi female holotype A Habitus, lateral B Head, frontal C Fore wing and hind wing D Mesosoma, dorsal E Propodeum and metasoma, dorsal F Metasoma, lateral.
Figure 240.
Venanusrandallgarciai female holotype A Habitus, lateral B Head, frontal C Fore wing D Mesosoma and metasoma, lateral E Head and mesosoma, dorsal F Propodeum and metasoma, dorsal.
The remaining genera in the subgroup seem to share one or several morphological features with Diolcogaster (whether those features are homoplastic or not). Diolcogaster (Figs 66–77), as currently understood, is most likely a polyphyletic genus that will need to be split into several genera. Until then, it is difficult to define unequivocally. Instead, we discuss the remaining genera in this subgroup in alphabetical order, with the features that distinguish them from Diolcogaster.
Figure 66.
Diolcogasterabdominalis male CNCHYM00768 A Habitus, lateral B Fore wing C Head, frontal D Propodeum and metasoma, dorsal E Head and mesosoma, dorsal.
Figure 77.
Diolcogasterscotica male CNC474705 A Habitus, lateral B Head, frontal C Fore wing D Head and mesosoma, dorsal E Propodeum and metasoma, dorsal F Propodeum and tergites 1–2, dorsal.
Buluka (Figs 35–37) has T1–T3 forming a carapace and occupying the entire dorsal surface of the metasoma, the fore wing has a complete areolet, and females have part of the ventral surface of the distal six or seven flagellomeres without longitudinal placodes, instead having an oblique groove bounded on one side by a row of bent-tipped sensilla (Austin 1989). The carapace is shared with Fornicia and very few species of other genera, e.g., Deuterixys, Pholetesor, none of which have a fore wing areolet. The basimacula species group of Diolcogaster (sensuSaeed et al. 1999) have both the carapace and areolet, but the antenna does not have the special groove and sensilla.
Figure 35.
Bulukaachterbergi female paratype CNCHYM00244 A Habitus, lateral B Head, frontal C Mesosoma, dorsal D Metasoma, lateral E Head, dorsal F Metasoma, dorsal G Mesosoma, lateral.
Figure 37.
Bulukastraeleni male CNCHYM00245 A Habitus, lateral B Head, frontal C Fore wing D Mesosoma, dorsal E Propodeum and tergite 1, dorsal F Metasoma, dorsal.
Cuneogaster (Figs 61, 62) resembles Diolcogaster but it has the glossa long and apically bilobed, T1 wedge-shaped, and the scutellar disc with the medioposterior band smooth (Choi and Whitfield 2006) whereas in Diolcogaster the glossa is not elongated, T1 is usually not wedge-shaped, and the scutellar disc has a medioposterior band of rugosity in most species.
Figure 61.
Cuneogasterinae female holotype (except H that is a paratype image) based on modified drawings and SEM images from the original descriptions of the species (Choi and Whitfield 2006) A Fore wing and hind wing B Head, dorsal C Mesosoma, dorsal D Head, frontal E Hypopygium and ovipositor sheaths F Tergites 1–3 G Fifth antennal segment, dorsal H Male genitalia, dorsal I Hind tarsal claw, lateral.
Figure 62.
Cuneogaster sp. male CNC1065632 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Propodeum and metasoma, dorsal E Mesosoma, dorsal.
Eripnopelta (Figs 87, 88) could be considered an atypical Diolcogaster, but the pronotal lateral surface does not have distinct furrows, the scutellar disc has a smooth and protruding medioposterior band, T1 does not have a distinct median groove on the basal half, and the fore wing areolet is very small, almost obliterated (Xiong et al. 2017).
Figure 87.
Eripnopeltaithyvena female holotype based on modified images from the original descriptions of the species (Xiong et al. 2017) A Habitus, dorsal B Fore wing and hind wing.
Figure 88.
Eripnopeltaithyvena female holotype (except F and H that are male paratype images) based on modified images from the original descriptions of the species (Xiong et al. 2017) A Head and mesosoma, lateral B Head, frontal C Mesosoma, dorsal D Head, dorsal E Propodeum and tergites 1–2, dorsal F Metasoma of paratype, lateral G Hind leg H Propodeum and basal part of metasoma of paratype, dorsal I Antenna, basal segments and apical segments.
Exix (Figs 89, 90) also seems morphologically related to Diolcogaster, but it is defined by T2 large and smooth, without submedian grooves, the hind wing has the vannal lobe concave and lacking setae, and the hind wing nervellus is externally concave (Mason 1981).
Figure 89.
Exixbahia female holotype A Habitus, lateral B Head, frontal C Fore wing and hind wing D Mesosoma, laterodorsal E Metasoma, laterodorsal.
Figure 90.
Exixcolumbica female holotype A Habitus, lateral B Head, frontal C Fore wing and hind wing D Propodeum and metasoma, dorsal E Head and mesosoma, dorsal.
Keylimepie (Figs 134, 135) can be recognized by the reduced wings in females, relatively small eyes and long malar space. The shape and sculpture of the head, mesosoma sculpture, shape and sculpture of T2, and ovipositor are all similar to some Diolcogaster, but Keylimepie has a T1 without a median sulcus and instead it has the anterior 0.5 rather depressed and concave, and the posterior 0.5 with strong transversal striations (Fernandez-Triana and Boudreault 2016).
Figure 134.
Keylimepiehadhramautensis female holotype A Habitus, lateral B Head, frontal C Fore wing D Head, mesosoma and tergites 1–3, dorsal E Propodeum and metasoma, dorsal F Metasoma, lateral.
Figure 135.
Keylimepiepeckorum female CNC483615 A Habitus, lateral B Head, frontal C Antenna D Head and mesosoma, dorsal E Fore wing F Mesosoma and metasoma, lateral G Metasoma, dorsal.
Larissimus (Figs 139, 140) is another genus related to Diolcogaster but it can be recognized by the greatly reduced vannal lobe in the hind wing with, almost entirely smooth body, and the only described species is the largest known species of Microgastrinae, with a body and fore wing length of 7–8 mm (Nixon 1965, Mason 1981).
Figure 139.
Larissimuscassander female CNC281020 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Metasoma, laterodorsal E Head and mesosoma, dorsal.
Figure 140.
Larissimus sp. female CNC666286 A Habitus, lateral B Head, frontolateral C Mesosoma, dorsal D Metasoma, dorsal E Fore wing and hind wing.
Markshawius (Figs 145, 146) has a unique set of features (Fernandez-Triana and Boudreault 2018) which together are very distinctive (although some, but not all, are shared with other genera). The female head is elongated and strongly concave posteriorly, modified to be tightly appressed to the anterior margin of the pronotum (following its contour); the face has its upper margin produced dorsally between the antennal insertions into a triangular flange; the frons is very elongated, with ocelli clearly much higher than normal; the antenna is very short (much shorter than body length, usually shorter than the combined length of the head and mesosoma), with all flagellomeres except the first having a single row of placodes; the propodeum has a median carina (defined posteriorly) and transverse rugosity which includes a poorly and partially defined transverse carina; and T1 is either extremely long and thin, with length at least 6.0× its width centrally, or very thin on the anterior 0.3–0.4, then strongly widening posteriorly, its width at the posterior margin around 3.0 × its width centrally.
Figure 145.
Markshawiuserucidoctus female holotype A Habitus, lateral B Head, frontal C Fore wing and hind wing D Metasoma, dorsal E Antenna F Mesosoma and tergite 1, dorsal.
Figure 146.
Markshawiusfrancescae female holotype A Habitus, lateral B Head, frontal C Head, lateral D Fore wing E Metasoma, dorsal F Propodeum and tergites 1–3, dorsal G Mesosoma and tergites 1–2, dorsal.
Parenion (Figs 176, 177) can only be confused with some Diolcogaster, but is distinguished by having T2 and T3 smooth and barely or not separated, scutellar disc with the medioposterior band smooth and very small lunules on its lateral surface (Mason 1981).
Figure 176.
Parenionkokodana female CNCHYM01939 A Habitus, lateral B Head, frontal C Fore wing D Propodeum and metasoma, dorsal E Head and mesosoma, dorsal.
Figure 177.
Parenion sp. male CNCHYM01945 A Habitus, lateral B Head, frontal C Fore wing D Head and mesosoma, dorsal E Metasoma, dorsal.
Protomicroplitis (Figs 201, 202) is closely related to Diolcogaster, both morphologically and molecularly, and some of the criteria used to define it may need revision. The genus is defined by some flagellomeres having three rows of placodes, relatively large fore wing areolet, and T1 very long and narrow (Mason 1981, Fernandez-Triana 2015), although the last two features are also present in a few Diolcogaster species.
Figure 201.
Protomicroplitiscalliptera female CNCH1333 A Habitus, lateral B Head, frontal C Head and mesosoma, dorsal D Head, dorsal E Fore wing F Propodeum, dorsal G Metasoma, dorsal H Metasoma, lateral.
Figure 202.
Protomicroplitiscentroamericanus female holotype A Habitus, lateral B Head, frontal C Head and mesosoma, dorsal D Fore wing E Propodeum, dorsal F Apex of metasoma, lateral G Metasoma, dorsal.
Rasivalva (Figs 213, 214) is characterized by the ovipositor sheaths lacking setae, or with very few and minute setae (Mason 1981, Chen and Song 2004, Kotenko 2007b). This separates it from Diolcogaster, which has relatively long setae on the ovipositor sheaths, including a few strong and thickened setae in many species. Other distinguishing features that appear in some species are the scutellar disc with the medioposterior band smooth, body sculpture smoother overall than in Diolcogaster, and propodeum with a median, longitudinal carina that is sometimes reduced or absent.
Figure 213.
Rasivalvacalceata female CNC474694 A Habitus, lateral B Head, frontal C Fore wing D Metasoma, dorsal E Mesosoma and tergites 1–2, dorsal.
Figure 214.
Rasivalvamarginata male CNC638380 A Habitus, lateral B Head, frontal C Fore wing D Metasoma, dorsal E Mesosoma, dorsal.
Ungunicus (Fig. 234) has remarkable and very distinctive tarsal claws, with a very large basal tooth longer than the apex of the tarsal claw, and a median lobe with setae arising from its margin, which seems slightly bilobate. These claws are unique within Microgastrinae (Fernandez-Triana and Boudreault 2018).
Figure 234.
Ungunicusvietnamensis female holotype A Habitus, lateral B Head, frontal C Fore wing D Metasoma, dorsal E Head and mesosoma, dorsal.
Among the genera without the fore wing areolet, Chaoa (Fig. 39) was described from a single specimen (Luo et al. 2004), with little information provided. Based on the original description and illustrations of the holotype, this genus might just represent a species of Glyptapanteles, or perhaps Nyereria but without examining the type we cannot conclude and therefore retain it as a valid genus for the time being.
Figure 39.
Chaoaflavipes female holotype based on modified drawings from the original descriptions of the species (Luo, You & Xiao, 2004) A Habitus, dorsal B Tergites 1–3, dorsal C Apex of metasoma, lateral.
Carlmuesebeckius (Fig. 38) has the ovipositor and ovipositor sheaths relatively long, and the propodeum with a complete areola, unlike most other genera in this subgroup. Other unique features are T1 with a strong and raised median carina for most of its length, and the ovipositor bulging near apex and with two subapical serrate teeth on the lower (first) valvulae (Fernandez-Triana and Boudreault 2018).
Figure 38.
Carlmuesebeckiussmithsonian female holotype A Habitus, lateral B Head, frontal C Fore wing D Head and mesosoma, dorsal E Metasoma, dorsal.
Cotesia (Figs 48–60) is a relatively uniform genus morphologically, long considered the easiest group to recognize among all segregates from Apantelessensu lato (Mason 1981: 113). Defining characters are: fore wing without areolet; T1 and T2 usually mostly to entirely sculptured, T3 also often at least partially sculptured or, more rarely, completely sculptured; T1 either widening towards its posterior margin (very often), more or less parallel-sided or barrel-shaped (often), slightly widening towards the posterior 0.7–0.8 of the tergite length and from that point slightly narrowing towards the posterior margin which is more or less rounded (rarely), or medially constricted (extremely rare), but never completely narrowing towards the posterior margin; ovipositor and ovipositor sheaths are very short to short, very rarely moderately long. The propodeum varies considerably but has a well defined median longitudinal carina (very often), although the median carina may be difficult to distinguish on its own in species with the propodeum strongly sculptured with an irregular pattern of carinae (often), or the median carina may be partially absent (rarely), or the median carina may be combined with a partial to complete areola partially defined by a transverse carina (rarely), or the median carina is absent and/or the propodeal surface is shiny overall and almost without any sculpture (rarely). The only other genus that could be confused here would be Protapanteles, which may eventually be considered as just a species group within Cotesia, with smoother propodeum and T1–T3.
Figure 48.
Cotesiaaffinis male CNCHYM00340 A Head frontal B Habitus, lateral C Fore wing D Metasoma, dorsal E Mesosoma and tergite 1, dorsal.
Figure 60.
Cotesiatyphae female CNC634434 A Habitus, lateral B Head, frontal C Fore wing D Propodeum and metasoma, dorsal E Mesosoma, tergites 1–2, dorsal.
Protapanteles (Figs 198–200) usually has T1 either slightly widening towards the posterior 0.7–0.8 of the tergite length and then slightly narrowing towards the posterior margin which is more or less rounded (often), more or less parallel-sided or barrel-shaped (rarely), or slightly widening towards the posterior margin (rarely). The propodeum is variously sculptured, usually having a median longitudinal carina that may be partially or completely defined, and rarely lacking the median carina. A character commonly used to define this genus, a modified spine on the fore tarsus (Nixon 1965, 1972, 1973, 1976, Mason 1981), is present in some species of many related genera, e.g., Cotesia, Glyptapanteles, Distatrix, Nyereria, and even in some non-related genera such as Silvaspinosus, and thus does not have the same diagnostic value as expressed by Mason (1981). Some species may be considered as borderline between Cotesia and Protapanteles, and others may be considered as borderline between Glyptapanteles and Protapanteles; thus, it is difficult to clearly define these three genera. Differences between Protapanteles and Cotesia were given in the previous paragraph. Differences with Glyptapanteles are mostly related to the shape of T1. In Glyptapanteles, T1 is either parallel-sided anteriorly and then strongly narrowing posteriorly, or its sides are gradually to strongly converging posteriorly when compared to Protapanteles which has T1 parallel-sided throughout, except for a strongly rounded apex, and propodeum sculpture that is usually, but not always, more rugose and carinated than in Glyptapanteles. Additionally, Protapanteles larvae have mandibles with a row of 12 or fewer large teeth concentrated distally on the blade, and its species distribution is almost completely confined to the Holarctic region (Mason 1981). However, the morphological features mentioned above vary considerably among different species (Arias-Penna et al. 2019).
Figure 198.
Sathonfausta female CNC474693 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Mesosoma, dorsal E Propodeum and metasoma, dorsal F Ovipositor and ovipositor sheaths.
Figure 200.
Protapantelespopularis female CNC309903 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Propodeum and metasoma, dorsal E Mesosoma, dorsal F Metasoma, lateral.
Glyptapanteles (Figs 100–110) is most likely a polyphyletic assemblage, and may eventually be split into several genera. As a result, it is difficult to define (Arias-Penna et al. 2019). Some of its species may be confused with Protapanteles, Sathon, Lathrapanteles and, to a lesser extent, also Distatrix, Venanides, and Nyereria. The main features defining Glyptapanteles are: fore wing without an areolet; propodeum that is either completely smooth (often) to more or less rugose (more rarely), with a median longitudinal carina that is entirely absent (often), partially defined posteriorly (often) to complete and strong (rarely), or no median carina but instead a series of very short carinae radiating from the nucha (rarely); T1 narrows towards the posterior margin, usually strongly (almost always), or more parallel-sided, or rounded at apex, as in some species of Protapanteles (rarely); T2 is almost always subtriangular or trapezoidal (rarely shaped differently); ovipositor and ovipositor sheaths are relatively short (usually) to moderately long (rarely); setae at apex of ovipositor sheaths relatively long (as long or longer than setae on hypopygium). The differences from Protapanteles were given in the previous paragraph. Sathon has the ovipositor sheaths longer and male specimens have enlarged external genitalia; however, a few Glyptapanteles species have females with longer ovipositor sheaths, and a very few other species have males with external genitalia similarly enlarged; whether those species should be transferred to Sathon requires further study. Lathrapanteles has similar characters to Sathon (see more about those two genera below) and can be separated in the same manner from Glyptapanteles. Distatrix has the pronotum with only one furrow laterally, eyes enlarged and ovipositor sheaths without setae or with very few minute setae, whereas Glyptapanteles has the pronotum with two furrows, eyes that are almost never enlarged (but see Fernandez-Triana 2018, for one exception) and the ovipositor sheaths have much longer setae. Venanides can in turn be separated from Glyptapanteles based on having similar ovipositor sheaths to Distatrix (Mason 1981).
Figure 100.
Glyptapantelesaliphera female CNCHYM01229 A Habitus, lateral B Head, frontal C Fore wing D Mesosoma, dorsal E Metasoma, dorsal.
Figure 110.
Glyptapantelesvafer female CNCHYM03251 A Habitus, lateral B Head, frontoventral C Head, frontal D Fore wing E Propodeum and metasoma, laterodorsal F Head and mesosoma, dorsal.
Distatrix (Figs 78, 79) is similar to Venanides, but it has two rows of placodes in the flagellomeres in females, and T2 has a characteristic shape, with the lateral margins widely diverging (Mason 1981, Grinter et al. 2009).
Figure 78.
Distatrixcarolinae female holotype A Habitus, lateral B Head, frontal C Fore wing D Metasoma, dorsal E Head and mesosoma, dorsal.
Figure 79.
Distatrixyemeniticus female paratype WAM 0138 A Habitus, lateral B Head, frontolateral C Fore wing and hind wing D Propodeum and metasoma, dorsal E Mesosoma, dorsal F Ovipositor and ovipositor sheaths.
Venanides (Figs 235, 236) can be differentiated from Distatrix because it has only a single row of placodes in the flagellomeres in females, and T2 has less diverging lateral margins (Mason 1981). Additionally, Venanides specimens tend to be smaller and have a dorsoventrally compressed body that is also generally mostly smooth and shiny.
Figure 235.
Venanidessupracompressus female holotype A Habitus, lateral B Head, frontal C Fore wing D Head, mesosoma and metasoma, lateral E Head, mesosoma and metasoma, dorsal.
Figure 236.
Venanidestenuitergus female paratype WAM 0128 A Habitus, lateral B Head, frontal C Fore wing D Head, mesosoma and metasoma, dorsal E Head and mesosoma, dorsal F Tergite 1, dorsal.
Sathon (Figs 218–220) is distinguished mainly by the enlarged external genitalia in males and relatively long ovipositor sheaths in females; some species probably have the longest sheaths among the entire Cotesiini (sensuMason 1981). However, these features are not unique: a few Glyptapanteles species have similarly enlarged male genitalia, and all described Lathrapanteles species (Figs 141, 142) are also very similar to Sathon (e.g., Williams 1985, 1988). The limits of Lathrapanteles and Sathon need revision and it is possible that one will eventually be placed in synonymy with the other.
Figure 218.
Sathoncircumflexus female holotype A Habitus, lateral B Head, frontal C Fore wing D Antennae E Propodeum and metasoma, dorsal.
Figure 220.
Sathonfalcatus female CNC638313 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Mesosoma, dorsal E Metasoma, dorsal.
Figure 141.
Lathrapantelesampyx female paratype CNCHYM01560 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Head, laterofrontal E Mesosoma, dorsal F Metasoma, dorsal.
Figure 142.
Lathrapantelespapaipemae female CNC807785 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Head, frontolateral E Mesosoma, dorsal F Metasoma, dorsal.
Deuterixys (Figs 64, 65) is a very distinctive genus on account of its T1–T3 sculpture and shape (there appears to be a second constriction between T2 and T3), the propodeum being smooth and shiny and with a complete and strong median, longitudinal carina, and the relatively small body length (Mason 1981, Whitfield 1985, Zeng et al. 2011).
Figure 64.
Deuterixyscarbonaria female CNC878801 A Habitus, lateral B Head, frontal C Fore wing D Propodeum and metasoma, dorsal E Head and mesosoma, dorsal.
Figure 65.
Deuterixysrimulosa female CNC638336 A Habitus, lateral B Head, frontal C Fore wing D Propodeum and metasoma, dorsal E Mesosoma, dorsal.
Nyereria (Figs 166–169) has T2 divided into three sections by two deep, usually crenulated, longitudinal grooves delimiting a raised, median area that is not wider than long (Mason 1981). This genus can only be confused with a few species of Cotesia and Glyptapanteles that have their T2 with a similar raised, median area, although in those cases T2 is never as strongly defined by grooves.
Figure 166.
Nyereriamlanje female CNCHYM01901 A Habitus, lateral B Head, frontolateral C Fore wing and hind wing D Mesosoma, dorsal E Propodeum and metasoma, dorsal F Ovipositor sheaths.
Figure 169.
Nyereria sp. female CNCHYM01906 A Habitus, lateral B Fore wing C Head, frontal D Head and mesosoma, dorsal E Metasoma, dorsal.
Pseudovenanides (Fig. 211) has very scarce information available, but from the original description (Xiao and You 2002) it is clear that it is related to Glyptapanteles and, to a lesser extent, to Venanides. Apparently, T1 with a strongly marked longitudinal sulcus on most of the tergite is the defining feature of this genus.
Figure 211.
Pseudovenanides sp. male CNC661265 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Metasoma, dorsal E Head and mesosoma, dorsal.
Ohenri (Fig. 170) has many unique features and is only tentatively considered to be part of this subgroup lacking the fore wing areolet. The pronotum is considerably enlarged dorsally, the ovipositor has its lower valvulae with four subapical teeth, the tarsal claws have large teeth, and the propodeum has a median carina with a partially defined areola (Fernandez-Triana and Boudreault 2018).
Figure 170.
Ohenrigouletarum female holotype A Habitus, lateral B Head, frontal C Fore wing D Ovipositor and apex of ovipositor E Propodeum and metasoma, dorsal F Head and mesosoma, dorsal.
Pseudofornicia (Figs 208–210) superficially resembles the (probably) unrelated Fornicia because its metasoma mostly forms a dorsal carapace, but it differs in lacking the epicnemial carina, the fore wing does not have an areolet, and T1 is movably joined to T2, whereas Fornicia has an epicnemial carina, fore wing with an areolet, and T1 and T2 are immovably joined (van Achterberg et al. 2015).
Figure 208.
Pseudofornicianigrisoma female holotype based on modified images from the original descriptions of the species (van Achterberg et al. 2015) A Habitus, lateral B Head, frontal C Head, dorsal D Fore wing E Propodeum and metasoma, dorsal F Mesosoma, dorsal G Antenna H Mesosoma, lateral.
Figure 210.
Pseudoforniciavanachterbergi female holotype based on modified images from van Achterberg et al. (2015)A Habitus, lateral B Head, frontal C Head, dorsal D Fore wing E Mesosoma, dorsal F Antenna G Metasoma, dorsal H Metasoma, lateral.
Wilkinsonellus (Figs 241–244) is a very recognizable genus, with T1 very long and thin, propodeum with distinctive sculpture and carination pattern, and fore wing with veins r and 2RS strongly angled (Mason 1981, Long & van Achterberg 2011, Arias-Penna et al. 2013, 2014).
Figure 241.
Wilkinsonellusalexsmithi male DHJPAR0047147 A Habitus, lateral B Head, frontal C Fore wing D Head, lateral E Head and mesosoma, dorsal F Propodeum and metasoma, dorsal.
Figure 244.
Wilkinsonellustomi male CNC309943 A Habitus, lateral B Head, frontal C Fore wing D Metasoma, dorsal E Head and mesosoma, dorsal.
d) Apanteles group
Mason (1981) proposed the tribes Apantelini and Microgastrini to accommodate species with ovipositor sheaths mostly setose and relatively long (at least 0.5 × metatibial length), hypopygium with ventral margin usually flexible and either with one (rarely) or several (commonly) pleats. The latter is the most diagnostic feature for this group; however, there are exceptions (all Alphomelon, most Hygroplitis, and a few species of Apanteles and Microgaster) where the hypopygium is mostly to entirely inflexible. In this paper we combine most of the genera included in the two tribes into a single Apanteles group composed of 26 genera. The group is clearly not monophyletic. Most, if not all, of the species included here have the “microlepidoptera suite of characters” sensu Mason (see further discussion in Mason 1981, Walker et al. 1990). Here we separate the group into several subgroups that can be recognized on simple morphological features, although the genera included in each subgroup are not necessarily related.
The largest subgroup includes 13 genera that lack a fore wing areolet: Alphomelon, Apanteles, Dolichogenidea, Exoryza, Iconella, Illidops, Napamus, Parapanteles, Pholetesor, Pseudapanteles, Rhygoplitis, Shireplitis, and Xanthapanteles. Another two genera could be placed here, at least partially: some species of Choeras lack a fore wing areolet; however, most of the species have a complete or partial areolet so we consider Choeras to be better placed with the subgroup of genera with a complete or partial fore wing areolet; and a similar situation occurs with Promicrogaster, where smaller species tend to lack the areolet whereas the larger species have a complete areolet, and we similarly place that genus in the subgroup with an areolet. These two genera exemplify the challenges of delimiting precise groups in Microgastrinae (a frustration also shared by Mason 1981: 77).
Among the genera without a fore wing areolet, four have the propodeum either with a median longitudinal carina (Iconella, Pseudapanteles, Rhygoplitis) or with a complex pattern that includes full sculpturing and a series of short carinae radiating medially on the posterior 0.2–0.3 near the nucha (Illidops). A fifth genus, Napamus, could also be included in this subgroup, as one of its two described species has the propodeum with a median, longitudinal carina; however, the other species does not (Papp 1993: 170). Nevertheless, Napamus (Fig. 160) can be characterized by its mouth parts elongate, fore wing vein R1 very short (shorter than pterostigma length), inner metatibial spur much longer (1.3 ×) than the outer spur, body and legs black, and wings strongly infumate.
Figure 160.
Napamus sp. male CNCHYM01899 A Habitus, lateral B Head, frontal C Fore wing D Mesosoma, dorsal E Metasoma, dorsal F Propodeum, dorsal.
Iconella (Figs 122–124) was described by Mason (1981) as a new genus based on the hind wing with a sinuous vein cu-a as a plesiomorphic character that suggests its unique status among similar genera. Fernandez-Triana et al. (2013a, 2014e) also considered the presence of a median longitudinal carina on the propodeum as strong support for its generic status. However, some Oriental species (with large body size and large and bilobate glossae) currently assigned to Iconella may eventually be placed in a different genus.
Figure 122.
Iconellacanadensis female holotype A Habitus, lateral B Head, dorsal C Fore wing D Propodeum and tergites 1 to 2, dorsal E Metasoma, lateral F Mesosoma, dorsal G Tergites 1–4, dorsal.
Figure 124.
Iconellavindicia female CNCHYM01472 A Habitus, dorsolateral B Head, frontal C Fore wing and hind wing D Head, frontolateral E Metasoma, dorsal F Mesosoma, dorsal.
Illidops (Figs 125–127) includes species that have the scutellar disc with a medioposterior band of rugosity, fore wing vein R1 shortened, and the propodeum with a series of short carinae medially on its posterior 0.2–0.3, near the nucha (Fernandez-Triana et al. 2014e). In some, but not all species the lower margins of the eyes converge, and T3–T7 are weakly sclerotized (Mason 1981).
Figure 125.
Illidopsalbostigmalis female holotype A Habitus, lateral B Head, frontal C Fore wing and hind wing D Metasoma, lateral E Mesosoma, dorsal F Propodeum and metasoma, dorsal G Hypopygium, ventral.
Figure 127.
Illidopsterrestris female paratype CNCHYM01522 A Habitus, lateral B Head, frontal C Fore wing D Metasoma, dorsal E Mesosoma, dorsal F Ovipositor and ovipositor sheaths.
Pseudapanteles (Figs 203–207) is characterized by the glossa elongate and strongly bilobed apically, propodeum with a strongly defined median longitudinal carina but no transverse carina (traces of a transverse carina are very rarely present in a few Neotropical species), and T1 with a sharp median sulcus (Mason 1981, Whitfield 1997, Fernandez-Triana et al. 2014a).
Figure 203.
Pseudapanteleschristinafigueresae female holotype A Habitus, lateral B Head, frontal C Metasoma, dorsal D Fore wing and hind wing E Mesosoma and tergites 1–2, dorsal.
Figure 207.
Pseudapantelesteofilodelatorrei female holotype A Habitus, lateral B Head, frontal C Fore wing and hind wing D Head and mesosoma, dorsal E Tergites 1–2, dorsal F Metasoma, dorsolateral.
Rhygoplitis (Figs 215–217) is the only genus in this subgroup with notauli relatively well defined. It also has the propodeum coarsely sculptured (in addition to a median, longitudinal carina), and fore wing with very short vein R1 (Mason 1981, Whitfield 1997).
Figure 215.
Rhygoplitisaciculatus female. The specimen photographed is the type of Pseudapantelessancti-vincenti (Ashmead, 1900), which is currently a synonym of Rhygoplitisaciculatus (see comments under that species in this paper, as well as details in Fernandez-Triana et al. 2014e) A Habitus, lateral B Head, frontolateral C Fore wing and hind wing D Propodeum and tergites 1–3, dorsal E Head and mesosoma, dorsal F Metasoma, dorsal.
Figure 217.
Rhygoplitis sp. female DHJPAR0012570 A Habitus, lateral B Fore wing and hind wing C Metasoma, dorsal D Head and mesosoma, dorsal E Ovipositor and ovipositor sheaths F Propodeum, dorsal.
The other eight genera without a fore wing areolet have the propodeum with a complete to partial areola, although in large genera such as Apanteles, Dolichogenidea, and Pholetesor, some species have lost all carinae and the propodeum is mostly smooth.
Shireplitis (Figs 225, 226) has the propodeum entirely sculptured, without median or transverse carina, but with the areola defined on the posterior 0.5 by two lateral carinae, ovipositor sheaths relatively short (0.4–0.5 × metatibia length), and legs short and robust – with the metafemur usually less than 3.0 × as long as wide (Fernandez-Triana et al. 2013b).
Figure 225.
Shireplitismeriadoci female holotype A Habitus, lateral B Fore wing C Mesosoma and metasoma, dorsal D Scutellar disc, propodeum (partially), and mediotergites 1–5, dorsal.
Figure 226.
Shireplitistolkieni female holotype A Habitus, lateral B Fore wing C Head and mesosoma, dorsal D Propodeum and metasoma, dorsal E Metasoma, lateral.
Alphomelon (Figs 9–11) has the gena with a white/pale spot that is relatively large and very distinctive (Mason 1981, Deans et al. 2003). A few other Microgastrinae genera have some species with a similar pale spot, but it is usually much smaller. Alphomelon is distinguished from the other Microgastrinae with white/pale spot on gena by its ovipositor sheaths being relatively long (much shorter in Cotesia, Glyptapanteles, Protapanteles), mesoscutum anteriorly without strong notauli (strong notauli in Prasmodon), propodeum without a median, longitudinal carina (strong median, longitudinal carina in Pseudapanteles), and the hypopygium inflexible and unpleated (almost always flexible and with several pleats in Apanteles).
Figure 9.
Alphomelonrhyssocerus female holotype A Habitus, lateral B Head, frontal C Fore wing D Head, dorsal E Antenna and head, frontolateral F Metasoma, dorsal G Mesosoma, dorsal.
Figure 11.
Alphomelonwinniewertzae female CNCHYM00025 A Habitus, lateral B Head and mesosoma, frontolateral C Head and mesosoma, dorsal D Propodeum and metasoma, dorsal.
Apanteles (Figs 12–26) is currently the most speciose genus in Microgastrinae and has some morphological variability. It usually has the propodeum fully to partially areolated, rarely smooth and never with a median longitudinal carina; fore wing without an areolet; hind wing with the vannal lobe usually strongly concave or straight (see next paragraph for more details on that); ovipositor sheaths relatively long; and the hypopygium almost always flexible and pleated. This genus could only be confused with Pholetesor or Dolichogenidea (which seem to be related to one another, see below) and Parapanteles. Most Apanteles species can be distinguished from both Pholetesor and Parapanteles by the flexible, pleated hypopygium and relatively long ovipositor sheaths (usually at least 0.5 × length of metatibia). In contrast, Parapanteles and Pholetesor have the hypopygium either entirely inflexible or at most with a small, translucent area near the posterior margin (which may look like a pleat in a few species); and the ovipositor sheaths are relatively short (less than half the metatibia length, usually much less). However, a few species of Apanteles have relatively short ovipositor sheaths, and very few species may even have an inflexible hypopygium (e.g., Fernandez-Triana et al. 2014e); the generic placement of those species may be revisited, but at present those exceptions make for a more difficult separation of these three genera.
Figure 12.
Apantelesalejandromasisi female holotype A Habitus, lateral B Mesosoma and metasoma, dorsal C Fore wing and hind wing D Head, frontal.
Figure 26.
Apantelesstagmatophorae female CNCHYM00217 A Habitus, lateral B Head, frontal C Fore wing D Mesosoma, dorsal E Ovipositor sheaths F Metasoma, dorsal G Propodeum, dorsal.
Dolichogenidea (Figs 81–86) is even more difficult to distinguish from Apanteles, as there is some overlap in some species groups of both genera (e.g., Mason 1981: 53, 54). The differences are frequently subtle and, at times it is very difficult to assign a species to one or other genus depending on the interpretation of morphological features alone. Apanteles has the hind wing with the vannal lobe usually strongly concave or, more rarely, straight to very slightly convex; the central part of the vannal lobe lacks any setae or has few, sparse setae that are often minute and not continuous. In contrast, Dolichogenidea has the vannal lobe convex to slightly straight; the central part of the vannal lobe is more or less entirely setose so that a continuous fringe of setae is almost always visible (although setae may be small in a few species). The fringe of setae (or lack of them) is the only morphological character that almost always seems to work in distinguishing these genera from each other; we are aware of very few species currently assigned to Dolichogenidea where the fringe is not complete and could lead to the species being placed within Apanteles, despite molecular data strongly suggesting the best generic placement is Dolichogenidea. Other features function only partially and seem to represent trends that are far from being universally present in one genus or the other. For example, the anteromesoscutum punctures (when present) tend to be partially or completely fused near the scutoscutellar sulcus in Apanteles, whereas in Dolichogenidea, which usually does not have punctures on the anteromesoscutum anteriorly and very rarely has them near scutoscutellar sulcus, the punctures never fuse. The scutoscutellar sulcus in many Dolichogenidea species tends to be very narrow and sometimes looks almost obliterated, whereas the sulcus in Apanteles is usually wider. Despite the rather subtle morphological differences, DNA barcodes tend to cluster both genera clearly apart (Smith et al. 2013, Fernandez-Triana et al. 2014e).
Figure 81.
Dolichogenideabaoris female CNCHYM00980 A Habitus, lateral B Head, frontal C Fore wing D Mesosoma, dorsal E Metasoma, dorsal F Propodeum, dorsolateral.
Figure 86.
Dolichogenideavictoriata female CNCHYM01172 A Habitus, lateral B Head, frontal C Fore wing D Propodeum, dorsal E Ovipositor F Metasoma, dorsal G Head and mesosoma, dorsal.
Dolichogenidea tends to cluster near Pholetesor (Figs 184–190) and these genera seem to be closer to each other than either is to Apanteles. Dolichogenidea has a flexible, pleated hypopygium and relatively long ovipositor sheaths (usually at least 0.5 × metatibia length) whereas Pholetesor has the hypopygium entirely inflexible or with a small, translucent area near the posterior margin that could look like a pleat in a few species, and the ovipositor sheaths are relatively short, less than half the metatibia length (Mason 1981, Whitfield 2006).
Figure 184.
Pholetesorbedelliae female CNCHYM03137 A Habitus, lateral B Head, frontal C Fore wing D Mesosoma, dorsal E Propodeum and metasoma, dorsal F Ovipositor and ovipositor sheaths.
Figure 190.
Pholetesorviminetorum female CNC678004 A Habitus, lateral B Fore wing C Head, frontal D Mesosoma, dorsal E Ovipositor sheaths F Propodeum and metasoma, dorsal.
The status of Exoryza (Figs 92–94) as a valid genus has been questioned by many authors (Valerio et al. 2004, Rousse and Gupta 2013, Fernandez-Triana et al. 2014e, 2016c). Mason (1981) characterized it as having T1 and T2 heavily sculptured, and the propodeum coarsely rugose, with an areola present but obscured by heavy sculpture. However, the distinction between Exoryza and Dolichogenidea may be particularly difficult because many species of the latter genus have the propodeum sculptured, with or without an areola, and T1 is occasionally sculptured, although not as strongly as in Exoryza (Fernandez-Triana et al. 2014e, 2016c).
Figure 92.
Exoryzaoryzae female CNCHYM01202 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Propodeum and metasoma, dorsal E Head and mesosoma, dorsal.
Figure 94.
Exoryzaritaashleyae female DHJPAR0031500 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Head and mesosoma, dorsal E Propodeum and metasoma, dorsal F Ovipositor and ovipositor sheaths.
Parapanteles (Figs 172–175) is a very difficult genus to understand at present. Parks (2018) found it to be paraphyletic. Some species of “Parapanteles” with available DNA barcodes cluster within Dolichogenidea and could just be considered as species within that genus, with short ovipositor sheaths and an inflexible hypopygium (similar to Pholetesor and the few borderline species of Apanteles mentioned above). Another group of Parapanteles seems to represent misidentifications of Cotesia (e.g., Valerio et al. 2009, Freitas et al. 2019). Whether a group of species that could be considered true Parapanteles actually exists remains to be seen. For the present, the genus can be defined as having the propodeum completely to mostly areolated (usually with well defined carinae), ovipositor sheaths short, and an inflexible hypopygium.
Figure 172.
Parapantelesaletiae female CNCHYM01930 A Habitus, lateral B Head, frontal C Fore wing D Propodeum and metasoma, dorsal E Mesosoma, dorsal.
Figure 175.
Parapanteles sp. female WAM 0186 A Habitus, lateral B Mesosoma and tergites 1–3, dorsal C Metasoma, lateral D Fore wing E Propodeum and metasoma, laterodorsal.
Xanthapanteles (Fig. 245) is a very distinctive genus, on the basis of the propodeum fully areolated with strongly defined and raised carinae, T1 very large and wide, T1–T3 sculpture like a finely pebble-grained surface (unlike any other Microgastrinae), flagellomere placodes arranged irregularly and fore wing relatively slender and much longer than body length (Whitfield 1995b).
Figure 245.
Xanthapantelescameronae female holotype based on modified drawings from the original descriptions of the species (Whitfield 1995) A Habitus, lateral B Fore wing and hind wing C Propodeum and metasoma, dorsal.
Another subgroup within the Apanteles group includes six genera, Agupta, Dasylagon, Hypomicrogaster, Papanteles, Promicrogaster, and Sendaphne, that can be recognized by the fore wing with a very small areolet, sometimes almost obliterated. They also share (except for Agupta, see below) having the scutellum with lunules relatively high, more than 0.5 × the height of its lateral face. These genera are separated from each other based on different propodeal carination patterns, and T1 and T2 shapes and sculptures. Some described species of Choeras, almost exclusively from the Oriental region, have a very small areolet and thus could be included in this group. However, these are exceptions and are very likely to be transferred elsewhere or classified separately. For now, we place Choeras (see below) within the subgroup with a large fore wing areolet.
Agupta (Figs 5, 6) does not have enlarged lunules; however, it can be recognized by several unusual features: in males (and sometimes in females) the antenna has the first few flagellomeres with placodes irregularly distributed in three rows or no row can be clearly defined; the propodeum has a strongly raised median carina with small radiating carinae across its length; T1 shape (narrowing for first half, then parallel-sided) and T1 sculpture (anterior half mostly smooth, strongly concave and with central sulcus, posterior half punctured and with a polished area on posterior margin) are distinctive; and the body length is among the largest in Microgastrinae (second only to the unrelated genus Larissimus) (Fernandez-Triana and Boudreault 2018). Some large specimens of Choeras in the Oriental region (see previous paragraph) might end being placed within Agupta when more studies are done in the future.
Figure 5.
Aguptadanyi female holotype A Habitus, lateral B Head, frontal C Fore wing D Metasoma, dorsal E Head and mesosoma, dorsal F Ovipositor and ovipositor sheaths.
Figure 6.
Aguptajeanphilippei female holotype A Habitus, lateral B Head, frontal C Fore wing D vipositor and ovipositor sheaths E Head and mesosoma, dorsal F Propodeum and metasoma, dorsal.
Promicrogaster and Sendaphne can be recognized by the following combination of features: glossa elongate and bilobate, metacoxa very long (0.8–1.0 × metafemur length and 0.6–0.8 × metatibia length), and ovipositor and ovipositor sheaths very long – among the longest in Microgastrinae usually 2.0 × as long as the metatibia or even longer. Most species have the body length longer than the fore wing length, usually by 0.2–0.4 mm (the majority of Microgastrinae species have the fore wing slightly longer than the body length). These two genera are very closely related and may eventually be treated as a single genus. Promicrogaster (Figs 194–197) has the ovipositor apically sinuate; propodeum sculptured and usually with some carination (which may include a complete or partial median longitudinal carina, or an indication of a partial areola posteriorly); T1 parallel-sided to slightly narrowing towards the posterior margin; and T2 transverse, its width at the posterior margin 3.0–4.5 × (rarely 2.0 ×) its length medially (Fernandez-Triana et al. 2016b, Fernandez-Triana 2019). Sendaphne (Figs 223, 224) has the ovipositor straight apically, propodeum mostly smooth and without carina (with the rare exception of having sparse punctures and a few rugae near the nucha), T1 strongly narrowing towards the posterior margin, and T2 subtriangular (Fernandez-Triana et al. 2014h).
Figure 194.
Promicrogasterbrandondinartei female DHJPAR0031326 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Head and mesosoma, dorsal E Metasoma, lateral F Metasoma, dorsal.
Figure 197.
Promicrogasterpablouzagai female DHJPAR0025926 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Propodeum and metasoma, dorsal E Head and mesosoma, dorsal F Ovipositor and ovipositor sheaths.
Figure 223.
Sendaphneanitae female holotype A Habitus, lateral B Head, frontolateral C Fore wing D Propodeum and tergites 1–2, dorsal E Head and mesosoma, dorsal F Metasoma, dorsal G Ovipositor and ovipositor sheaths.
Figure 224.
Sendaphnerogerblancoi female holotype A Habitus, lateral B Fore wing and hind wing C Head, frontal D Head and mesosoma, dorsal E Ovipositor and ovipositor sheaths F Metasoma, dorsal G Propodeum and tergite 1, dorsal.
Dasylagon (Fig. 63) has the propodeum fully areolated (defined by strong carinae), T1 comparatively very wide and large (in dorsal view more than 0.3 of entire metasoma), T2 very transverse, metasomal terga entirely smooth, hind wing with a sinuous vein cu-a, and ovipositor and ovipositor sheaths relatively very long (more than 1.5 × metatibia length) (Mason 1981).
Figure 63.
Dasylagonaegeriae female holotype A Habitus, lateral B Habitus, magnified C Mesosoma, dorsal D Metasoma, dorsal.
Hypomicrogaster (Figs 113–121) has the propodeum with a complex carination pattern, which includes a median carina and a more or less complete areola, although some species have all carinae reduced, but still the propodeum would be mostly sculptured. The head is relatively transverse, i.e., wider than in most other genera of Microgastrinae, and T1 and T2 are mostly to entirely smooth (Mason 1981, Valerio and Whitfield 2015).
Figure 113.
Hypomicrogastercrocinus female holotype A Habitus, lateral B Head, frontal C Fore wing and hind wing D Head and mesosoma, dorsal E Metasoma, dorsal.
Figure 121.
Hypomicrogasterzonaria female CNCHYM01436 A Habitus, lateral B Fore wing and hind wing C Head, frontal D Head and mesosoma, dorsal E Metasoma, dorsal.
Papanteles (Fig. 171) has the propodeum fully areolated, T1 relatively long (ca. 2.0 × its width at posterior margin), T1 and T2 strongly sculptured, T2 and T3 comparatively narrow and not occupying the entire dorsal surface of the segment (dorsal width of T2 and T3 half the width of T5 and following terga), and the ovipositor sheaths are approximately the same length as the metatibia length (Mason 1981).
Figure 171.
Papantelespeckorum female holotype A Habitus, lateral B Head, frontal C Fore wing D Head and mesosoma, dorsal E Metasoma, dorsal F Antennae G Propodeum, dorsal.
The remaining eight genera in the Apanteles group all have the fore wing areolet relatively large; even when some species may have a relatively smaller areolet, it never appears almost obliterated.
Ypsilonigaster (Figs 250–252) has a very characteristic T1, with a median sulcus shaped like an inverted Y, a unique feature to recognize the genus (Fernandez-Triana and Boudreault 2018).
Figure 250.
Ypsilonigasternaturalis female holotype A Habitus, lateral B Head, frontal C Fore wing D Head and mesosoma, dorsal E Metasoma, dorsal F Propodeum, dorsal.
Figure 252.
Ypsilonigasterzuparkoi male holotype A Habitus, lateral B Head, frontal C Fore wing and hind wing D Metasoma, dorsal E Genitalia F Metatibia, lateral G Head and mesosoma, dorsal H Tergites 1–2, dorsal.
Hygroplitis and Microgaster have the propodeum with a median carina, fore wing areolet relatively large, anteromesoscutum anteriorly mostly smooth, T1 and T2 heavily sculptured (also T3, partially or entirely), T1 relatively large and wide (width at posterior margin greater than width at anterior margin), and T2 mostly rectangular. The two genera are very closely related and DNA barcodes suggest Hygroplitis may eventually be synonymized under Microgaster. Hygroplitis (Figs 111, 112) has the body somewhat depressed dorsoventrally, notauli more strongly impressed, flagellomeres with three rows of placodes, and the hypopygium usually inflexible although in some cases it is weakly but distinctly pleated (Mason 1981); whereas Microgaster (Figs 147–150) does not have the body dorsoventrally depressed, the notauli are barely visible, flagellomeres are usually (but not always) with two rows of placodes, and the hypopygium is usually (but not always) flexible and pleated (Mason 1981).
Figure 111.
Hygroplitisbasarukini female paratype CNCHYM01362 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Head and mesosoma, dorsal E Metasoma, dorsal.
Figure 112.
Hygroplitispseudorussata male MRSJFT0057 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Metasoma, dorsal E Mesosoma and tergite 1, dorsal.
Figure 147.
Microgasterdeductor female CNC752604 (for images A, B and C) and female CNC752606 (for images D and E) A Habitus, lateral B Fore wing C Mesosoma and metasoma, dorsal D Habitus, lateral E Hypopygium, ventrolateral.
Figure 150.
Microgastersubcompleta female CNCHYM01657 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Head and mesosoma, dorsal E Propodeum and metasoma, dorsal.
Paroplitis (Figs 178–180) species are relatively small, with a body length of 2.5 mm or less; legs, especially the metafemur, short and robust; antenna short, with flagellomeres in females having only a single row of placodes; hypopygium almost entirely sclerotized but with a sharp fold medially; propodeum rarely entirely sculptured but almost always with a median longitudinal carina, at least on the anterior 0.5, and sometimes also with a complete or partial transverse carina; and T2 usually smooth, rarely sculptured (Mason 1981, Fernandez-Triana et al. 2013b).
Figure 178.
Paroplitisberingianus female holotype A Habitus, lateral B Fore wing C Head, frontal D Metasoma, dorsal E Head and mesosoma, dorsal.
Figure 180.
Paroplitiswesmaeli female CNCHYM01946 A Habitus, lateral B Fore wing C Mesosoma and metasoma, lateral D propodeum and metasoma, dorsal.
Kotenkosius (Fig. 138) has a unique propodeal carination pattern that includes three complete longitudinal carinae, one medially, the other two sublaterally, and a complete transverse carina near posterior 0.6, with additional small striae radiating from the median and sublateral longitudinal carinae, and most carinae strongly defined and raised (Fernandez-Triana and Boudreault 2018).
Figure 138.
Kotenkosiustricarinatus female holotype A Habitus, lateral B Head, frontal C Fore wing and hind wing D Mesosoma, dorsal E Metasoma, dorsal F Propodeum, dorsal.
Choeras (Figs 40–45), as presently understood, is clearly a polyphyletic assemblage of species, some of which may eventually be placed in different genera. It is one of the few Microgastrinae genera that has some species without a fore wing areolet (although the shape of the remaining veins r, 2RS, and 2M usually indicate a partially defined areolet), and other species with a complete areolet that can vary from very small in some species to large in others (van Achterberg 2002, Fagan-Jeffries and Austin 2018b). The propodeum also varies, from having a complete longitudinal median carina to having a partial one, to not having any visible median carina, or having just minute carinae radiating from the nucha. T1 is mostly rectangular (slightly narrowing towards the posterior margin in some species), but never much wider on the posterior margin than on the anterior margin, and T2 is mostly transverse. Many Oriental species of “Choeras” most likely represent different lineages from the temperate species and may warrant placement in different genera, e.g., some of the species may be better placed in Agupta.
Figure 40.
Choerasafrotropicalis female holotype A Habitus, lateral B Head and mesosoma, dorsal C Fore wing and hind wing D Metasoma, lateral E Propodeum and metasoma, dorsolateral.
Figure 45.
Choerastiro female CNC474677 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Metasoma, dorsal E Mesosoma, dorsal F Propodeum, dorsal.
Dodogaster (Fig. 80) has a unique set of features in the Apanteles group. The propodeum has a more or less complete areola and a partial median carina, the fore wing has a relatively large areolet, and T1–T3 are heavily sculptured and almost form a carapace (Rousse and Gupta 2013).
Figure 80.
Dodogastergrangeri female paratype (CNC841340) A Habitus, lateral B Mesosoma, dorsal C Mesosoma, lateral D Habitus, dorsal.
Diversity and distribution of Microgastrinae genera at world and regional scales
Microgastrinae are present in all continents except Antarctica. Specimens can be found in all major terrestrial ecosystems, from 82°30'N (Canada, Nunavut, Ellesmere Island, Alert) to 55°S (Argentina and Chile, Tierra del Fuego) in the New World and 50°S (New Zealand, Auckland Islands) in the Old World, and from sea level up to at least 4,500 m (Fernandez-Triana 2018). The information currently available allows us to make preliminary comments on species diversity and distribution at the generic level (Table 5 and Fig. 2).
Table 5.
World genera of Microgastrinae, based on the present paper. The column Species richness details the current number of described species and estimated total, for each genus, the two figures separate by a slash. The estimated total is very conservative and is based on specimens we have seen in collections. For many genera, more species are to be expected. World region keys: NEO Neotropical, NEA Nearctic, PAL Palaearctic, OTL Oriental, AFR Afrotropical, AUS Australasian (including Oceanian). X Genus present in specific region. X* New record for that region (based on undescribed species seen in collections). X- Introduced into that region, not native. X? Questionable record for a region. The column Host data tallies the genera that have at least one lepidopteran host recorded (although no critical assessment of how accurate those host records was made). The column DNA barcodes records all genera for which there is at least one DNA barcode available; Yes- denotes a genus with only partial sequence(s) available, without fulfilling the criteria for DNA-barcode compliant sequences (see Materials and methods for definition of a barcode-compliant sequence).
| Genera | Species richness | NEO | NEA | PAL | OTL | AFR | AUS | Host data | DNA bar-codes |
|---|---|---|---|---|---|---|---|---|---|
| Agupta | 4/30+ | X | X | No | Yes | ||||
| Alloplitis | 8/30+ | X | X* | No | Yes | ||||
| Alphomelon | 19/50+ | X | X | Yes | Yes | ||||
| Apanteles | 633/3,000+ | X | X | X | X | X | X | Yes | Yes |
| Austinicotesia | 2/5 | X | No | Yes | |||||
| Austrocotesia | 5/10 | X? | X | No | Yes- | ||||
| Beyarslania | 1/2 | X | No | Yes | |||||
| Billmasonius | 1/1 | X | No | Yes | |||||
| Buluka | 11/20 | X | X | X | Yes | Yes | |||
| Carlmuesebeckius | 1/1 | X | No | No | |||||
| Chaoa | 1/1 | X | No | No | |||||
| Choeras | 80/100+ | X* | X | X | X | X | X | Yes | Yes |
| Clarkinella | 2/5+ | X | X | No | Yes | ||||
| Cotesia | 328/1500+ | X | X | X | X | X | X | Yes | Yes |
| Cuneogaster | 1/5 | X | No | No | |||||
| Dasylagon | 2/5 | X | Yes | No | |||||
| Deuterixys | 18/20+ | X | X | X | X | X | Yes | Yes | |
| Diolcogaster | 141/1,000+ | X | X | X | X | X | X | Yes | Yes |
| Distatrix | 32/40+ | X | X | X | X | X | Yes | Yes | |
| Dodogaster | 1/1 | X | No | No | |||||
| Dolichogenidea | 366/700+ | X | X | X | X | X | X | Yes | Yes |
| Eripnopelta | 1/1 | X | No | No | |||||
| Exix | 7/10 | X | X | No | Yes- | ||||
| Exoryza | 15/20+ | X | X | X | X | X | Yes | Yes | |
| Exulonyx | 1/1 | X | No | No | |||||
| Fornicia | 32/50+ | X | X | X | X | Yes | Yes | ||
| Gilbertnixonius | 1/1 | X | No | Yes | |||||
| Glyptapanteles | 307/3,000+ | X | X | X | X | X | X | Yes | Yes |
| Hygroplitis | 9/10+ | X | X | X | Yes | Yes | |||
| Hypomicrogaster | 48/200+ | X | X | Yes | Yes | ||||
| Iconella | 38/50+ | X | X | X | X | X | Yes | Yes | |
| Illidops | 37/50+ | X | X | X | X | X | X- | Yes | Yes |
| Janhalacaste | 3/5 | X | Yes | Yes | |||||
| Jenopappius | 3/5+ | X | No | Yes | |||||
| Jimwhitfieldius | 2/5+ | X | No | Yes | |||||
| Keylimepie | 4/10 | X* | X | X | No | Yes- | |||
| Kiwigaster | 1/1 | X | No | Yes | |||||
| Kotenkosius | 1/2+ | X | No | Yes | |||||
| Larissimus | 1/5+ | X | Yes | Yes | |||||
| Lathrapanteles | 4/10+ | X | X | Yes | Yes | ||||
| Mariapanteles | 2/10+ | X | No | Yes | |||||
| Markshawius | 3/5 | X | No | Yes | |||||
| Microgaster | 104/200+ | X | X | X | X | X | X | Yes | Yes |
| Microplitis | 192/500+ | X | X | X | X | X | X | Yes | Yes |
| Miropotes | 15/20 | X | X* | X | Yes | Yes | |||
| Napamus | 2/2 | X | Yes | No | |||||
| Neoclarkinella | 7/50+ | X* | X | X* | No | Yes | |||
| Nyereria | 29/50+ | X | X | X | Yes | Yes | |||
| Ohenri | 1/1 | X | No | No | |||||
| Papanteles | 2/5 | X | Yes | Yes | |||||
| Parapanteles | 62/100+? | X | X | X* | X | X | X | Yes | Yes |
| Parenion | 3/5+ | X | X | No | Yes | ||||
| Paroplitis | 5/10 | X | X | X | Yes | Yes | |||
| Pelicope | 1/1 | X | Yes | Yes | |||||
| Philoplitis | 9/10+ | X* | X | X | No | Yes | |||
| Pholetesor | 57/100+ | X | X | X | X | X* | X | Yes | Yes |
| Prasmodon | 18/30+ | X | Yes | Yes | |||||
| Promicrogaster | 46/100+ | X | X* | X* | X* | X* | X* | Yes | Yes |
| Protapanteles | 25/30+ | X | X | X | Yes | Yes | |||
| Protomicroplitis | 3/5 | X | X | Yes | Yes | ||||
| Pseudapanteles | 36/100+ | X | X | Yes | Yes | ||||
| Pseudofornicia | 4/5+ | X | X | No | No | ||||
| Pseudovenanides | 1/5+ | X* | X | Yes | No | ||||
| Qrocodiledundee | 1/1 | X | No | No | |||||
| Rasivalva | 12/20+ | X* | X | X | X | X | X* | Yes | Yes |
| Rhygoplitis | 4/10+ | X | X | Yes | Yes | ||||
| Sathon | 23/30+ | X | X | X | X | X* | X | Yes | Yes |
| Semionis | 1/1 | X | No | No | |||||
| Sendaphne | 11/20 | X | No | Yes | |||||
| Shireplitis | 6/6 | X | No | Yes | |||||
| Silvaspinosus | 1/2+ | X | No | Yes | |||||
| Snellenius | 41/50+ | X | X | X | X* | X | Yes | Yes | |
| Tobleronius | 1/2+ | X | No | Yes | |||||
| Ungunicus | 1/1 | X | No | Yes | |||||
| Venanides | 14/20+ | X | X | X* | X | X | X | Yes | Yes |
| Venanus | 11/15+ | X | X | Yes | Yes | ||||
| Wilkinsonellus | 23/50+ | X | X | X | X | Yes | Yes | ||
| Xanthapanteles | 1/1 | X | No | No | |||||
| Xanthomicrogaster | 6/30+ | X | Yes | Yes | |||||
| Ypsilonigaster | 6/10+ | X | No | Yes | |||||
| Zachterbergius | 1/1 | X | No | Yes |
The most species-rich genera are Apanteles (in its restricted sense) and Glyptapanteles. The latter is probably the largest, but it may eventually be split into several genera. In contrast, Apanteles, although also likely to have some species reclassified into other genera, is a much more cohesive group and might end up being the larger group if many species are removed from the current Glyptapanteles. Regardless, the diversity of both genera will likely comprise a few thousand species each.
Apanteles already contains more than 630 described species (see checklist below); just in ACG, Costa Rica, 186 new species were recently described (Fernandez-Triana et al. 2014e). The world fauna of Apanteles could number many more than 3,000 species. The genus is notably absent from New Zealand (although a few species have been introduced there), where it is replaced by Dolichogenidea and an undescribed genus. It also has not been found in the high Arctic (Fernandez-Triana et al. 2017b).
Glyptapantes contains more than 300 species, with hundreds of undescribed species from all biogeographical regions seen in collections; we estimate that the world total could be more than 3,000 species. However, the generic limits are controversial (see previous section) and it may eventually be restricted to a slightly smaller, although still substantial, number of species. Regardless, its status as one of the two largest genera of Microgastrinae is certain.
The following genera are also very speciose: Cotesia, Diolcogaster, Dolichogenidea, Hypomicrogaster, and Microplitis. Among these, Diolcogaster is clearly the largest, and it could attain more than 1,000 species. But it will almost certainly be split into several genera and thus it could potentially end up having just a few hundred species. Cotesia, already with more than 320 described species, will also attain more than 1,000 species (Mason (1981) estimated between 1,500–2,000 species), and is a more cohesive group, unlikely to be severely split. The other three genera will certainly surpass 500 species each, probably substantially (e.g., Dolichogenidea already has more than 360 described species). Diolcogaster and Hypomicrogaster are more speciose in tropical areas, whereas Cotesia, Dolichogenidea and Microplitis tend to be richer in temperate areas.
Other relatively large genera are Microgaster, Choeras, and Pholetesor in temperate areas, and Parapanteles and Pseudapanteles in tropical areas. All of them are likely to have more than one hundred (in most cases several hundred) species. A few other genera might be equally large, but the material in collections is not comprehensive enough to provide estimates.
In regional composition, the tropical areas have a larger representation than temperate areas (as expected) with the Oriental (46 genera) and Neotropical (43 genera) regions being of comparable diversity, and the Afrotropical (36 genera) and Australasian (28 genera) regions following. Furthermore, we have seen in collections several putative additional (undescribed) genera from all tropical regions. In temperate areas, the Nearctic region (33 genera, including several Neotropical genera having a few species entering North America) has the highest generic diversity and the Palaearctic region (28 genera, including some Oriental genera that have a few species entering the southernmost areas of the Palearctic) has the lowest diversity. Considered as a whole, the entire Holarctic region would have a relatively high diversity of 39 genera.
The distribution of individual genera worldwide (Fig. 3) shows that 20 genera (24.7%) are cosmopolitan or almost so: 15 are present in all biogeographical regions (Apanteles, Choeras, Cotesia, Diolcogaster, Dolichogenidea, Glyptapanteles, Illidops, Microgaster, Microplitis, Parapanteles, Pholetesor, Promicrogaster, Rasivalva, Sathon, and Venanides) while another five are present in five out of the six biogeographical regions (Deuterixys, Distatrix, Exoryza, Iconella, and Snellenius). A few additional genera may eventually be found to be cosmopolitan.
Figure 3.
Biogeographical distribution of the 81 Microgastrinae genera currently known worldwide. Data from the present paper.
Eleven genera (13.6%) are restricted to the New World tropics (Neotropical region): Cuneogaster, Dasylagon, Janhalacaste, Larissimus, Mariapanteles, Papanteles, Prasmodon, Sendaphne, Venanus, Xanthapanteles, and Xanthomicrogaster. Another nine genera (Alphomelon, Clarkinella, Exix, Hypomicrogaster, Lathrapanteles, Protomicroplitis, Pseudapanteles, Rhygoplitis, and Venanus) are almost exclusively found in the Neotropics, with few species reaching the Nearctic. The only genus that can be considered as a Nearctic endemic is Pelicope.
Ten genera (12.3%) are relatively widespread in, but restricted to, the Old World tropics: Agupta, Alloplitis, Buluka, Miropotes, Parenion, and Pseudofornicia. We also consider here Neoclarkinella, Nyereria, Philoplitis, and Pseudovenanides as almost exclusively present in the Old World tropics, as only a few species reach the southernmost areas of the Palaearctic.
Only two genera (2.5%) (Fornicia and Wilkinsonellus) seem to be pantropical, and completely absent in the Holarctic. Because almost all undescribed genera of Microgastrinae in collections are from tropical areas, this proportion could increase. No genus has a strictly Holarctic distribution, but three genera almost fulfill that criterion, as just a few species of each reach the northern limits of either the Oriental region (Hygroplitis and Paroplitis) or the Neotropical region (Rhygoplitis).
A total of 35 genera (43.2%) are presently known only from a single biogeographical region, with the Neotropical and Oriental regions each having ten endemic genera, respectively, and the Afrotropical having eight (Table 5). However, some of those genera will almost certainly be found to have a wider distribution.
DNA barcoding and Microgastrinae
During the past 12+ years, an extensive library of DNA barcodes for Microgastrinae has been assembled (Smith et al. 2013), resulting in the subfamily comprising 37% of all DNA sequences of Braconidae and almost 5% of all Hymenoptera sequences currently available in BOLD. At present, 44,739 specimens of Microgastrinae have sequences deposited in BOLD; 40,812 of those specimens have DNA barcodes representing 3,545 public BINs (http://v4.boldsystems.org/index.php/Taxbrowser_Taxonpage?taxid=2099). The number of BINs will certainly increase, as most of the Microgastrinae specimens currently in BOLD come from just two countries: Canada and Costa Rica (Fig. 4).
Figure 4.
Overview of Microgastrinae data in the Barcoding of Life Data System (BOLD) as of 31 December 2019.
BINs usually match well with putative species (as identified by an expert taxonomist), and thus could be used as a surrogate for analyses of species diversity, like other Operational Taxonomic Units (e.g., Ratnasingham and Hebert 2013, Fagan-Jeffries et al. 2018b). Based on our unpublished data, the correspondence between BINs and putative species in Microgastrinae may exceed 90%. For example, the number of Microgastrinae public BINs from Canada and Alaska (combined) currently found in BOLD is 551, very similar to the 550 species estimated for that area by Fernandez-Triana (2010; see also Fagan-Jeffries et al. 2018b). Even with the limited geographical coverage presently available, the total number of worldwide MicrogastrinaeBINs already surpasses the total of described species in our checklist by almost 200.
At the genus level, a significant proportion (67 genera or 83%) have some DNA data (Table 5). In most cases (64 genera or 79%) that includes at least one barcode compliant sequence, usually many more. Many of the 14 genera without molecular data in BOLD include taxa that are very rare in collections, i.e., only known from one or very few specimens, and/or the available specimens are very old (collected many decades ago) and did not yield any DNA. However, for at least a few of those genera it is expected that it will soon be possible to have DNA data.
Estimating species richness in Microgastrinae
With 2,999 valid species of Microgastrinae recognized here, an interesting question is how many species remain undescribed, whether or not known from collections. The actual species richness of Microgastrinae worldwide has been variously estimated during the past 35 years. At the lower end, Dolphin and Quicke (2001) extrapolated species richness of Braconidae based on data from butterflies and (primarily) mammals, arriving at an estimated 3,617–4,178 species of Microgastrinae. Jones et al. (2009) obtained similar results by comparing taxonomic revision data, with their estimates ranging from 3,900–5,500 species. Mason (1981) thought that 5,000–10,000 species would be a reasonable estimate, based on museum specimens he had seen. At the higher end of the spectrum, Rodriguez et al. (2013) compared the number of Lepidoptera and Microgastrinae species in several areas to arrive at estimates ranging from 17,000 to 46,000+ species.
Obviously, these estimates vary considerably: if the lowest one (3,617) were accurate, then we would already know 82% of the Microgastrinae species; if the highest one (46,620) were accurate, then the described species would represent only 6% of the actual diversity worldwide. Which estimate is more likely to be correct?
While a definite answer cannot be provided, some refinement of the current estimates is possible. The lowest range (3,000–5,000 species) is clearly too low based on what is currently known (2,999 described, valid species are recognized in this paper). As mentioned in the previous section, and despite its limited geographical coverage, Microgastrinae public BINs already represent 3,545 putative species. But, even if DNA data is disregarded, we have certainly seen in collections a few thousand undescribed species, which are clearly distinct based on morphological features alone. In that sense, Mason’s estimate of 10,000 species seems very reasonable.
But could the figures from Rodriguez et al. (2013) also be considered reasonable, or are they way off the mark? Although this might be seen just as a numbers game, the implications are significant. If indeed there were 30-, 40- or even 50,000 species of Microgastrinae worldwide, that could extrapolate to the entire family Braconidae having at least 150–200,000 species, and the entire Hymenoptera having much more than one million species. Those values are an order of magnitude higher than the values presently known for subfamily, family, and order, although they agree with estimates of the entire Hymenoptera suggested by other authors (e.g., LaSalle and Gauld 1991, Hanson and Gauld 1995, Foottit and Adler 2017).
Rodriguez et al. (2013) based their estimates on what Fernandez-Triana (2010) had referred to as the Lepidoptera/Microgastrinae ratio (L/M). Briefly explained, the ratio between lepidopteran and microgastrine species (where sufficient data are available) seems to be surprisingly similar in different regions, regardless of the area and diversity of such regions. The initial calculations were limited and only included three separate areas in Canada (Table 2 in Fernandez-Triana 2010). Based on the average ratio calculated from those three areas (L/M = 12/1) it was concluded that the richness of Microgastrinae in Canada and Alaska would be approximately 550 species. Rodriguez et al. (2013: Table 1) expanded the dataset to eleven different regions, mostly from North America and Europe, but also including New Zealand and ACG in Costa Rica; the resulting L/M ratios were still remarkably close, mostly ranging from 10/1 to 20/1, with an average of 16.4/1.
But just a few years later, some of the numbers used by Fernandez-Triana (2010) and Rodriguez et al. (2013) are already outdated. For Microgastrinae, the species richness in Ottawa, based on Fernandez-Triana et al. (2016a) and subsequent unpublished data, is now approaching 180 species, which represents a 20% increase compared to the total published in 2010; ACG in Costa Rica has surpassed 1,200 species, a 50% increase (based on Janzen and Hallwachs 2016); the Canadian High Arctic now has 26 recorded species or 30% more than initially reported (based on Fernandez-Triana et al. 2017b); the New Zealand fauna will increase by more than 25% compared to previous estimates (Fernandez-Triana & Ward, unpublished); even for the UK, arguably the most thoroughly studied region, the microgastrine count increased by at least 15% (based on Broad et al. 2016). Those revised figures all share one element in common: the species richness of Microgastrinae in those areas was underestimated by both Fernandez-Triana (2010) and Rodriguez et al. (2013).
Thus, the updated L/M ratios calculated for the above regions decreased, from an average of 16/1 in Rodriguez et al. (2013) to around 10/1 at present (also including Finland, where comprehensive data have become available since the Rodriguez et al. paper was published). But the lower the L/M ratio the higher the actual species richness of Microgastrinae. For example, assuming an estimated world number of Lepidoptera between 300,000 (Kristensen et al. 2007) and 500,000 species (Foottit and Adler 2017), and a world average L/M ratio of 10/1, the estimated number of Microgastrinae would then range from 30,000–50,000 species. If anything, the current data still seem to support higher, rather than lower, estimates for the subfamily.
As far as we know, there is only one major caveat in using L/M ratios to extrapolate and calculate the world fauna of Microgastrinae: at present all known figures come from temperate areas, with the sole exception of ACG. There is no other tropical area in the world with sufficient data to allow for meaningful L/M ratios to be calculated. Thus, it may be argued that if different ratios were prevalent in temperate areas compared to the tropics, which harbour, by far, the highest richness of Microgastrinae, then the overall world estimates could not be as high as Rodriguez et al. (2013) suggested. Only more data will allow this to be answered in a definite way; however, for the present it is worth noting that the L/M ratio in ACG (10/1) is actually very similar to those of temperate areas.
Overview of regional taxonomic studies on Microgastrinae
As with many insect groups, knowledge of Microgastrinae has been historically concentrated on the Northern Hemisphere temperate fauna. However, numerous recent studies are starting to shift focus to the tropics, with most new species in the past few years being described from the hitherto poorly worked Neotropical and Oriental regions, chiefly Costa Rica, China, and India.
In the Western Palearctic subregion, papers from the 1960s–1990s from Nixon and Papp treated most of the Microgastrinae species known up to that time, following careful work by Wilkinson from the 1920s–1940s aimed largely at interpreting poorly understood names (see papers of these three authors cited in the References section). Recent works have described a relatively small number of new species, although their papers sometimes included detailed accounts of species biology, and there is an ongoing concomitant deposition of DNA barcodes, etc. (Oltra and Michelena 1989, Oltra et al. 1995, 1996, Oltra-Moscardó & Jiménez-Peydró 2005, Shaw 1992, 2004, 2007, 2009, 2012b, van Achterberg 2002, Fernandez-Triana et al. 2014c). The Eastern Palearctic subregion is less well known, although progress has also been made (Tobias 1986, Kotenko 1981, 1986, 1992, 1993, 2004, 2007a, 2007b), and most of the new Palaearctic species to be discovered will probably come from the Eastern Palearctic. Some southern areas of the Palearctic, e.g., Iran, Turkey, and the Palearctic area of the Arabian Peninsula have also seen an increase in the number of publications in the last few years (Inanç 1992, 2002a, 2002b, Inanç and Çetin Erdogan 2004, Gadallah et al. 2015, Farahani et al. 2016, Ghahari and van Achterberg 2016, Fernandez-Triana and van Achterberg 2017, Ghafouri Moghaddam et al. 2018, 2019, Samin et al. 2018, Abdoli et al. 2019a, 2019b, Zargar et al. 2019); however, there have been few taxonomic revisions, with most of the work being biodiversity estimates, local checklists, or isolated species descriptions. With 827 described species of Microgastrinae, the Palearctic is currently the most speciose region, although it will almost certainly become the least when more studies in the other regions are undertaken.
In the Nearctic region progress has been slower than in the Palearctic. After two seminal papers from Muesebeck (1921, 1922), most of the new taxa have been described in isolated papers, mostly treating species of biocontrol relevance (Marsh 1975, 1978, 1979b, 1979c, Wharton 1983, Whitfield et al. 1999, Fernandez-Triana 2010, 2018; cf. other papers from Muesebeck cited in the References section), with some taxonomic revisions also produced (Whitfield 1985, 2006, Whitfield et al. 2011, Grinter et al. 2009, Valerio et al. 2009, Valerio and Whitfield 2015, Fernandez-Triana 2015, 2019,Fernandez-Triana and Boudreault 2016, Fernandez-Triana et al. 2013a). Hundreds of additional species from this region have been revealed by DNA barcoding, but the southernmost areas and west coast, which also happen to be the most species rich, have barely been studied (Fernandez-Triana 2018). It is expected that the actual numbers in the Nearctic will be several times higher than the current 350 described species.
The Neotropical region has been the focus of recent efforts, including the description of more than 400 new species and revision of many genera. However, most of those papers deal almost exclusively with the fauna of ACG, Costa Rica (Janzen et al. 2003, Valerio and Whitfield 2003, Valerio et al. 2005a, Fernandez-Triana 2015, Fernandez-Triana et al. 2013a, 2014a, 2014e, 2014f, 2014g, 2014h, 2015b, 2016b, 2016c), with only some marginal coverage of other countries (Austin and Dangerfield 1989, Penteado-Dias 1995, Penteado-Dias et al. 2000, 2002, 2011, Valerio and Whitfield 2005, 2015, Valerio et al. 2004, 2009, Choi and Whitfield 2006, Grinter et al. 2009, Arias-Penna et al. 2014, 2019, Salgado-Neto et al. 2018, 2019). Large collections have been amassed in South America, e.g., French Guiana, Colombia, Brazil, Ecuador, and Peru, but an impediment to assessing that material is the difficulty in exchanging specimens with colleagues from other countries. In general, most of the Neotropics are extremely understudied, with several thousand species awaiting description but only 768 species described so far. For Microgastrinae, this is likely to be the most speciose region of the world.
The Oriental region, with 752 described species, currently ranks third after the Palearctic and Neotropical regions. It also contains thousands of undescribed species and may rival the Neotropical region as the most speciose. Recent advances have mostly been made in China and India, but we are also aware of large collections of specimens from other countries such as Indonesia, Malaysia, Thailand and Vietnam, which have already resulted in several publications (Austin 1987, 1989, Chen et al. 1994, Long and van Achterberg 2003, 2008, 2011, 2013, 2014, 2015, Chen and Song 2004, Long 2007, 2010, 2015, Fernandez-Triana and Goulet 2009, Fernandez-Triana et al. 2014d, Zeng et al. 2011a, 2011b, Gupta 2013a, 2013b, Gupta and Kalesh 2012, Gupta and Fernandez-Triana 2014, 2015, Gupta et al. 2011, 2013a, 2013b, 2014a, 2014b, 2016a, 2016b, Liu et al. 2014, 205, 2016, 2018, Veena et al. 2014, van Achterberg et al. 2015, Xiong et al. 2017, Zhang et al. 2017, Ranjith et al. 2015a, 2015b, 2019; cf. papers from authors Chen, Sathe, Song, Xu, and You cited in the References section). The main problem (other than difficulties in exchanging material) is the lack of revisions covering the entire region; the available taxonomic keys and papers tend to cover single countries, with few efforts to coordinate work at a larger (regional) scale. There is also a number of species described from India in publications that do not comply with ICZN Article 16, and thus those names are unavailable (see section Unavailable names below).
No significant progress has been made in the Afrotropical region for the past half a century. The very few exceptions include recent papers on the fauna of Réunion (Rousse and Gupta 2013), the Afrotropical area of the Arabian Peninsula (Fernandez-Triana and van Achterberg 2017), and some new species of importance in biocontrol (Kaiser et al. 2017, Fiaboe et al. 2017), or more general papers not specifically devoted to the Afrotropics (Walker 1994, Valerio et al. 2009, Fernandez-Triana and Goulet 2009, Fernandez-Triana and Boudreault 2018). However, relatively large collections from Kenya, Madagascar, Republic of Congo, and South Africa have been amassed during the past few years (Fernandez-Triana and Boudreault 2018), and there is potential to add hundreds, if not thousands of new species. Although the current total of described species is just 429 it is estimated that this will be the third most species-rich region of the planet for Microgastrinae.
Since the 1990s, several papers have treated the Australasian species (Austin 1990, Austin and Dangerfield 1992, 1993, Walker 1996, Saeed et al. 1999, Fernandez-Triana et al. 2011, 2013b, Fagan-Jeffries and Austin 2018, Fagan-Jeffries et al. 2018a, 2018b, 2019), but progress has been comparatively slow. At present 222 species are described from this region. Work on Pacific islands is basically non-existent but, when done, may reveal many more new and interesting taxa.
Hosts of Microgastrinae
The host range of a parasitoid is one of its most important features, linking its evolutionary past with its present autecology (Shaw 1994, Shaw and Aeschlimann 1994). Through knowledge of the host range it is possible to understand and to predict a parasitoid’s behaviour within current ecosystems (Shaw 2017b), and also gain some understanding of the speciation processes that brought them into existence (Shaw 2003).
Microgastrinae are the single most important group of parasitoids of Lepidoptera in the world, both in economic terms and in species richness (Whitfield 1995a, 1997). They are all koinobiont endoparasitoids and parasitize almost the entire taxonomic and biological spectrum of Lepidoptera (Shaw and Huddleston 1991, Whitfield 1997, Whitfield et al. 2018), with the probable exception of the four most basal superfamilies.
Adult female wasps typically oviposit into early instar larvae (with a few species known to oviposit into host eggs), within which the wasp eggs hatch and larval development takes place with the aid of venom and polydnavirus (PDV) effects on the host’s immune and endocrine system (summarized in Whitfield et al. 2018). All microgastrines fully depend on mutualistic PDVs to successfully parasitize hosts, the relationship between wasps and PDVs being the most remarkable known example of the evolution of a mutualistic endosymbiotic association between eukaryotes and viruses (Strand and Burke 2012, 2014).
Numerous literature records of non-Lepidoptera as hosts of Microgastrinae exist (Table 6), comprising at least 29 families within five orders of Insecta (data compiled from Yu et al. 2012). However, these records are wrong or at the very least highly questionable.
Table 6.
Historical account of Microgastrinae hosts that are not Lepidoptera, based on the compilation of Yu et al. (2012).
| Order | Families |
| Coleoptera | Anobiidae, Anthomyiidae, Attelabidae, Bostrichidae, Buprestidae, Cerambycidae, Chrysomelidae, Coccinellidae, Curculionidae, Melandryidae, Phalacridae, Scirtidae |
| Diptera | Agromyzidae, Cecidomyiidae, Chloropidae, Muscidae, Syrphidae, Tephritidae |
| Hymenoptera | Apidae, Argidae, Cimbicidae, Cynipidae, Diprionidae, Eurytomidae, Pteromalidae, Tenthredinidae, Vespidae |
| Mantodea | Mantidae |
| Trichoptera | Limnephilidae |
For example, the record of Apidae (Bombus sp.) as “host” of Microgastrinae can be easily rejected. Bombus nests have associated case-bearing moth caterpillars (Tineidae) feeding within the nest and the three known species of Microgastrinae that emerge from those nests actually parasitize the caterpillars, not the bees (Whitfield and Cameron 1993, Whitfield et al. 2001).
Two other recent examples are equally illustrative. The record of Enoicylapusilla (Burmeister) (Trichoptera: Limnophilidae) as a host of the microgastrine Choerasgielisi (van Achterberg 2002) was at times considered to be a reliable example of a non-Lepidoptera host record; however, subsequent examination of the situation has called that record into doubt as it was reared from a substrate from which the host remains were not recovered (Shaw 2017a). Similarly, Kopelke (2011) reported two different species of Dolichogenidea (each from a single specimen), as part of his extensive rearing of inhabitants of 34,210 galls of nematine sawflies (Hymenoptera: Tenthredinidae) in Europe; he asserted those two cases to be accidental, but genuine (Kopelke 2011: 9). Unfortunately, it is not clear from the publication if host remains were available (in those two specific cases) to confirm host identity, and under such circumstances we consider it appropriate to regard those records as highly dubious. Sawfly galls are nutritious and frequently fed on by caterpillars. It is relatively easy for a small parasitized lepidopteran larva to enter such a structure to die and become practically entirely consumed by the parasitoid, leaving almost only the head capsule. This happens with most Dolichogenidea species, which have a final external feeding period that leaves the host remains easily overlooked or misinterpreted. Many similar deductions concerning other recorded supposed non-lepidopteran hosts are easily made.
Even if examples of parasitization of other insect orders by Microgastrinae are well founded, we consider such cases would be highly abnormal. Shaw (1994) provided a conceptual definition for the host range of a particular parasitoid species, which should include only those species of potential hosts that the parasitoid is usually able to attack successfully, following a pattern of searching behaviour enabling it to encounter them regularly. That rather loose definition implies that some perfectly correct rearing records should be excluded from the host range if they represent only freak events of no importance to the autecology of the parasitoid or the host, and lack phylogenetic significance. It also implies that some hosts within the host range may be intrinsically more important than others that are encountered less frequently, or attacked less enthusiastically, or with a less successful outcome.
We also consider that there is no convincing evidence that the four most basal superfamilies of Lepidoptera (sensuAarvik et al. 2017) are parasitized by Microgastrinae. There is no published record of Microgastrinae parasitizing Micropterigoidea and Eriocranioidea, and the few literature records of hosts in Hepialoidea and Nepticuloidea are highly questionable; we discuss and reject them below.
Sathonfalcatus (Nees, 1834) was recorded in two broods (of 45 and 37 individuals) parasitizing Hepialushumulis (Linnaeus, 1758) (Hepialidae) in the United Kingdom (Hammond and Smith 1957). We have located those specimens in the NHMUK but, although the relevant cocoon masses are present, there are no host remains. Sathonfalcatus is a known parasitoid of the noctuid moth Apameamonoglypha (Hufnagel, 1766), whose larvae are superficially very similar to those of Hepialushumuli. Thus, we distrust the record strongly enough to refute it. It should also be noted that the rearings were not done by Hammond, who was the expert on Lepidoptera larvae.
The other known record is for Cotesiaspuria (Wesmael, 1837) parasitizing Triodiasylvina (Linnaeus, 1761) (Hepialidae), published by Telenga (1955) with no details whatsoever, i.e., no information was provided on who identified the host or the parasitoid, or where and when the sample was collected, nor the depository of specimens. Cotesiaspuria does have a wide host range, but confirmed hosts are all folivorous macrolepidoptera. Under these circumstances it is best to simply refute the record; although of course, if a rearing is repeated with appropriate credentials the refuted record could be recalled to stand as a possible previous instance.
The two published records of Nepticuloidea as hosts are also highly suspicious. Nixon’s (1976) record of Fomoriaweaveri (Stainton, 1855) (Nepticulidae) as a host of Apantelescontaminatus (Haliday, 1834) has recently been refuted by Shaw (2012b), who commented on the rearing. The inflated mines of F.weaveri are superficially similar to those Epinotianemorivaga (Tengström, 1848) (Tortricidae) from which A.contaminatus has been reliably reared; thus, in this case an error in host identification was almost certainly involved. Unfortunately, the specimen could not be found in the cited depository.
Gates et al. (2002: 221) recorded Stigmella?variella (Nepticulidae) being parasitized by DolichogenideatischeriaeViereck (1912b) from a leaf mine on oak (Quercusagrifolia Née). However, that record is quoted as “parasitoids lot-reared from more than one leafmines from a single plant” (see caption of Table 2 on page 230 of Gates et al. 2002), and in that same Table other Lepidoptera families were recorded from that host plant, including several species of Gracillariidae and Tischeriidae, both of which had been reported as hosts of D.tischeriae in other papers and most likely represent the actual host(s). In that case, it is clear that the sample (leaves with mines) contained several lepidopteran species, and that Stigmella was wrongly assigned as a host of D.tischeriae.
Adeloidea and Tischerioidea are the most basal superfamilies of Lepidoptera (and the only non-Ditrysia groups) for which there is reasonably solid evidence supporting them as being hosts of Microgastrinae. There is reliable data showing that a few Microgastrinae indeed parasitize species of Adelidae (Shaw 2012b), Incurvariidae (Fernandez-Triana 2010), Prodoxidae (Nixon 1972, Shaw 2012b, Whitfield et al. 2005), Tischeriidae (Shaw 2012b) and even Heliozelidae (Fernandez-Triana et al., unpublished data).
Ditrysia (sensuKristensen and Skalski 1999, Roe et al. 2009) constitutes the most derived clade of Lepidoptera, comprising more than 98% of all lepidopteran species, and representing by far the group most commonly parasitized by Microgastrinae. Eulepidoptera (sensuAarvik et al. 2017) consists of Adeloidea + Tischerioidea + Ditrysia, which are the three groups for which we have solid evidence of parasitism by Microgastrinae. Thus, in this paper we propose that Microgastrinae hosts are restricted to Eulepidoptera, i.e., most of the Lepidoptera except for the four most basal superfamilies: Micropterigoidea, Eriocranioidea, Hepialoidea and Nepticuloidea. We consider all previous literature records of other insect orders and of the four early branching lineages of Lepidoptera as incorrect. Claims for hosts other than Eulepidoptera, which are made with conviction from time to time, are in our experience never supported by the recovery and preservation of associated host remains for careful assessment.
The published sources we compiled so far include Lepidoptera host data for 44 genera (54%) and around 1,250 species (42%) of Microgastrinae. Although the coverage is insufficient, those records include 3,200+ species of Lepidoptera and represent 5,500+ supposed host/parasitoid associations. In addition, there is a large amount of unpublished but databased host information (e.g., http://janzen.sas.upenn.edu/caterpillars/database.lasso; http://www.caterpillars.org/), with hundreds of additional host/parasitoid records from currently undescribed microgastrine species (e.g., Whitfield et al. 2009, 2018, Hrcek et al. 2013). Still, more than half of the described species of microgastrines lack any information about their hosts. Even worse, an unknown but probably very large proportion of the published associations are also almost certainly wrong. Clearly, there is much to be learned, and for the existing information to be a good basis for understanding host records there needs to be a critically examination of the data to (try to) prune out wrong host/parasitoid associations, an effort that would require years of work, and even then would leave much uncertainty. A better approach to secure real knowledge may be to ensure that higher standards of data collection and specimen deposition take place for the future: in fact, without that we cannot expect much improvement in our understanding.
From the data presently available, the top ten families of Lepidoptera (as per number of species recorded as host) which are parasitized by Microgastrinae are Noctuidae, Tortricidae, Pyralidae, Crambidae, Geometridae, Gracillariidae, Depressariidae, Hesperiidae, Gelechiidae, and Nymphalidae. Altogether those families account for two-thirds of all known host/microgastrine parasitoid associations, which is not surprising given that they are also among the most species- rich Lepidoptera families. That probably also reflects a bias in collecting effort: these families provide most of the economically important crop and forestry pests, which are accordingly the most intensely sampled taxa for their parasitoids. Further, in some of these families there are large and/or spectacular caterpillars that are the most often seen and reared by hobbyists. Other groups such as stem borers, leaf litter, and canopy caterpillars tend to be less commonly reared.
Earlier compilations for species within particular microgastrine genera are dominated by records from the northern temperate region which are unlikely to reflect the complete spectrum of host associations when the ongoing (but currently mostly unpublished) massive number of rearings from tropical surveys are taken into account, e.g., Whitfield et al. (2018). In addition, there is a need to recognise phenological aspects of host range, especially in temperate climates: many parasitoid species are plurivoltine yet use univoltine hosts, each available to only one generation of the parasitoid; sometimes it happens that the parasitoid is, at least locally, entirely dependent on a single host species at one time of year but able to use another host or a wider range of hosts at another (see Shaw and Aeshlimann 1994). Last but not least, a parasitoid’s realized host range may not be constant in either space or time unless, of course, the parasitoid is strictly monophagous, and thus the relative abundance of co-occurring hosts will also vary (Shaw 2006). Recognition of the realized host range at a point in space and time is often of more practical significance for population dynamics, conservation biology, or biological control (Shaw 1994, 2003, 2017).
Despite the constraints mentioned above and the relatively poor state of knowledge, some general comments can be made for some of the most speciose Microgastrinae genera. For example, most Microgaster, Choeras, Apanteles, and Dolichogenidea species parasitize more or less concealed host larvae, allowing the final instar larvae of these parasitoids to carry out their external feeding phase in a sheltered environment, and host Lepidoptera with this amenable larval biology overwhelmingly belong to the families of the so-called microlepidoptera. Other genera such as Pholetesor and Deuterixys specialize on leaf-miners and parasitize hosts that feed in at least moderate concealment, as is required by the final external tissue-feeding phase of their parasitoid larvae. This is correlated with their use of hosts primarily from microlepidopteran families, which tend to be small, resulting in most of the parasitoids of microlepidoptera being solitary. In contrast, genera such as Microplitis, Cotesia, Distatrix, Diolcogaster, Protapanteles, and Glyptapanteles are fully endophagous and well-suited to parasitize exposed Lepidoptera larvae, such as those of many macrolepidoptera, which tend to be large and are thus more suited to support gregariousness, which is much more expressed in these microgastrine genera. There are exceptions, but they can often be understood in autecological terms, e.g., the few Microgaster that parasitize macrolepidopterans have hosts that feed or rest in concealed sites (Shaw 2004); the few Cotesia that parasitize microlepidopterans are usually associated with semi-exposed hosts in webs which feed partly exposed (see Nixon 1974); the flavipes group of Cotesia parasitizes stem borers in the families Pyralidae and Crambidae (e.g., Fujie et al. 2018).
Whenever comprehensive data are available, be it in temperate (e.g., in Europe and especially the United Kingdom), or tropical areas (e.g., ACG), patterns emerge. Often, they show that many species within most genera of Microgastrinae appear to have a high host specificity, often having been recorded from only a single or very few taxonomically closely related species. An alternative is having ecologically similar hosts (Shaw 2003, Fernandez-Triana 2018). Earlier studies often did not differentiate these levels of host specificity clearly, partly due to the presence of many morphologically cryptic species in large genera of Microgastrinae but also because it is very much harder to discover all or most of the hosts of a particular parasitoid species than it is to discover all or most of the parasitoid species using a given host. Only recently have they been detected through integrative taxonomy that incorporates DNA barcoding and other molecular methods, as well as much greater levels of field ecological data (Shaw 2017b, Whitfield et al. 2018).
However, some species of Microgastrinae seem to be much less restricted. Examples include Glyptapantelesvitripennis (Curtis, 1830), an incredibly polyphagous species with an immense host range of mainly (but not entirely) exposed macrolepidoptera found on trees and bushes in Europe (Nixon 1973, Shaw unpublished data), or Glyptapantelespseudotsugae Fernandez-Triana, 2018, which parasitizes several lepidopteran species (Geometridae and Erebidae) feeding on Douglas fir across a range of 2,500 km in western North America (Fernandez-Triana 2018).
A few Microgastrinae genera seem to be restricted to only one host Lepidoptera family, e.g., Alphomelon (only reared from Hesperiidae), Fornicia (Limacodidae), Janhalacaste (Depressariidae), Papanteles and Xanthomicrogaster (Crambidae), and Pelicope (Prodoxidae). However, these microgastrine genera are not very species rich and it is difficult to know whether more data would extend their apparent associations.
For more speciose genera, the patterns are less clear or consistent, as the number of host families increases, in some cases dramatically. This may in part be a consequence of some Microgastrinae genera not being well defined, comprising at present an arrangement of different lineages that may be separated into different genera in future, e.g., Choeras, Diolcogaster, Glyptapanteles, and Hypomicrogaster. But some large and relatively well-defined genera, e.g., Apanteles, Cotesia, Dolichogenidea, Microplitis, and Microgaster, have large host ranges, including both early and more recently branching lepidopteran families, and ecological factors in their radiations have clearly been of importance.
There is no comprehensive account of the impact of Microgastrinae in biological control. Whitfield (1997) estimated that more than one hundred species had been studied and used in biocontrol programs against caterpillar pests worldwide, but he did not provide details or references to support that number. We have compiled the available information and have found that 800+ species of Lepidoptera considered as pests of some sort in agriculture and forestry are parasitized by Microgastrinae (Fernandez-Triana et al. unpublished data; host data for individual species of Microgastrinae is not presented in this paper, see next paragraph). That includes 110+ major pests, highlighting the importance of this group of parasitoid wasps in biological control programs anywhere.
In summary, Microgastrinae are the most abundant and diverse taxon of hymenopteran parasitoids reared from lepidopteran caterpillars worldwide. However, our current level of knowledge is still poor, as more than half of the wasp species have no host association records, and of the records that do exist, many of them are doubtful or plainly wrong. Considerable effort will be needed before we have a better and more accurate picture of the host/parasitoid associations of most species of Microgastrinae. Thus, in this paper we only provide general comments; details on individual host/parasitoid associations are intentionally omitted to avoid repeating and perpetuating inaccurate information.
Checklist of world genera and species of Microgastrinae
[Genera, and species within each genus, are arranged in alphabetical order. At the end of the list we place the species we consider as species inquirendae, nomina dubia, and nomina nuda, also in alphabetical order. For a complete list of all Microgastrinae available names in strict alphabetical order see also Suppl. material 1, 2]
Genus Agupta Fernandez-Triana, 2018
Agupta Fernandez-Triana, 2018: 28. Gender: neuter. Type species: Aguptajeanphilippei Fernandez-Triana & Boudreault, 2018, by original designation.
Four species are described from the Oriental region (Fernandez-Triana and Boudreault 2018); those authors stated that there are dozens of undescribed species, based on collection holdings and specimens with available DNA barcodes, from the Australasian and Oriental regions. No revision of the genus has yet been produced. No host data are currently available for this genus. There are dozens of DNA-barcode compliant sequences of Agupta in BOLD, representing more than 25 different BINs (but none of those sequences have been identified in BOLD as belonging to Agupta, see Fernandez-Triana and Boudreault (2018) for more details on that).
Aguptadanyi Fernandez-Triana & Boudreault, 2018
Aguptadanyi Fernandez-Triana & Boudreault, 2018.
Type information. Holotype female, RMNH (examined). Country of type locality: Malaysia.
Geographical distribution.OTL.
OTL: Malaysia.
Aguptajeanphilippei Fernandez-Triana & Boudreault, 2018
Aguptajeanphilippei Fernandez-Triana & Boudreault, 2018.
Type information. Holotype female, RMNH (examined). Country of type locality: Malaysia.
Geographical distribution.OTL.
OTL: Malaysia.
Aguptaraymondi Fernandez-Triana & Boudreault, 2018
Aguptaraymondi Fernandez-Triana & Boudreault, 2018.
Type information. Holotype female, RMNH (examined). Country of type locality: Malaysia.
Geographical distribution.OTL.
OTL: Malaysia.
Aguptasolangeae Fernandez-Triana & Boudreault, 2018
Aguptasolangeae Fernandez-Triana & Boudreault, 2018.
Type information. Holotype female, RMNH (examined). Country of type locality: Malaysia.
Geographical distribution.OTL.
OTL: Malaysia.
Genus Alloplitis Nixon, 1965
Alloplitis Nixon, 1965: 268. Gender: masculine. Type species: Alloplitisguapo Nixon, 1965, by original designation.
Eight species are currently described from the Oriental and Afrotropical regions, but we have seen in collections (CNC, RMNH) numerous additional species from those regions. No revision of the genus has been produced, although a key to all four species known from Vietnam (Long & van Achterberg 2008) covers half of the described species. No host data are currently available for the genus. There are 20 DNA-barcode compliant sequences of Alloplitis in BOLD representing eight different BINs, most of them undescribed species from Thailand.
Alloplitisalbiventris Long & van Achterberg, 2008
Alloplitisalbiventris Long & van Achterberg, 2008.
Type information. Holotype female, IEBR (not examined but original description checked). Country of type locality: Vietnam.
Geographical distribution.OTL.
OTL: Vietnam.
Alloplitiscompletus Mason, 1981
Alloplitiscompletus Mason, 1981.
Type information. Holotype female, CNC (examined). Country of type locality: Malaysia.
Geographical distribution.OTL.
OTL: Malaysia.
Alloplitiscongensis (de Saeger, 1944), new combination
Microplitiscongensis de Saeger, 1944.
Type information. Holotype male, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. Even in the original description (de Saeger 1944), this species was considered not likely to belong to Microplitis. Without examining the holotype (and only known specimen), the best generic placement at present would be Alloplitis based on the propodeal areola, T1 with an impression on the basal third and striae on lateral margins, T2 rectangular in shape, and T3 shorter than T2.
Alloplitisdetractus (Walker, 1860), new combination
Microgasterdetractus Walker, 1860.
Type information. Holotype male, NHMUK (examined). Country of type locality: Sri Lanka.
Geographical distribution.OTL.
OTL: Sri Lanka.
Notes. From the original description and subsequent treatment of the species (Wilkinson 1927, 1929), it is clear that this species does not belong to Microgaster. After examining the holotype, we here transfer detractus to Alloplitis based on its short metatibial spurs, propodeum with a complete areola defined by strong carinae, T1 with a broad impression on anterior half, T2 broadly rectangular, and anteromesoscutum, scutellar disc, T1 and T2 heavily sculptured.
Alloplitisguapo Nixon, 1965
Alloplitisguapo Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines, Vietnam.
Alloplitislaevigaster Long & van Achterberg, 2008
Alloplitislaevigaster Long & van Achterberg, 2008.
Type information. Holotype male, IEBR (not examined but original description checked). Country of type locality: Vietnam.
Geographical distribution.OTL.
OTL: Vietnam.
Alloplitistyphon Nixon, 1965
Alloplitistyphon Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Alloplitisvietnamicus Long & van Achterberg, 2008
Alloplitisvietnamicus Long & van Achterberg, 2008.
Type information. Holotype female, IEBR (not examined but original description checked). Country of type locality: Vietnam.
Geographical distribution.OTL.
OTL: Vietnam.
Genus Alphomelon Mason, 1981
Alphomelon Mason, 1981: 54. Gender: neuter. Type species: Urogasternigriceps Ashmead, 1900, by original designation.
Known from 19 described species from the New World (mostly Neotropical, with a few extending north into the Nearctic). The revision by Deans et al. (2003) is outdated; we have seen in collections (CNC) dozens of additional species, and the genus will easily surpass 50 species with additional study of the Neotropical fauna. All data currently available suggest that Alphomelon species may exclusively be parasitoids of Hesperiidae. There are 1,200+ DNA-barcode compliant sequences of this genus in BOLD, representing 32 BINs, most of them undescribed species from Costa Rica.
Alphomelonarecaphile Deans, 2003
Alphomelonarecaphile Deans, 2003.
Type information. Holotype female, USNM (not examined but paratype examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Brazil (PA), Costa Rica.
Alphomelonbrachymacher Deans, 2003
Alphomelonbrachymacher Deans, 2003.
Type information. Holotype female, USNM (not examined but authoritatively identified specimens examined). Country of type locality: Colombia.
Geographical distribution.NEO.
NEO: Brazil (ES, MT, PA, SC), Colombia, Costa Rica, Ecuador, Peru.
Notes. The specimens we studied were identified by the author of the species.
Alphomelonbrasiliensis Shimabukuro & Penteado-Dias, 2003
Alphomelonbrasiliensis Shimabukuro & Penteado-Dias, 2003.
Type information. Holotype female, DCBU (not examined but original description checked). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (MG, SP, RS).
Alphomelonbromeliphile Deans, 2003
Alphomelonbromeliphile Deans, 2003.
Type information. Holotype female, USNM (not examined but paratype examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica, Mexico.
Alphomeloncitroloma Deans, 2003
Alphomeloncitroloma Deans, 2003.
Type information. Holotype female, USNM (not examined but paratype examined). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina, Bolivia, Brazil (PE, RJ, RO), Costa Rica, Ecuador, Panama, Paraguay, Trinidad & Tobago, Venezuela.
Alphomelonconforme (Muesebeck, 1958)
Apantelesconformis Muesebeck, 1958.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: Venezuela.
Geographical distribution.NEO.
NEO: Brazil (RJ), Costa Rica, Venezuela.
Notes. This species was transferred from Apanteles to Alphomelon by Deans et al. (2003), although the new combination was not clearly formalized (but is implicit, see pages 1 and 18 of that paper). Deans et al. (2003) did not change the ending of the species name to agree in gender with the generic name (Article 34.2 of the ICZN). The genus Alphomelon was described by Mason (1981) as neuter, but conformis is a masculine adjective, and thus it must be changed to the neuter form conforme. Until now, no published paper had ever referred to this species as Alphomelonconforme although Taxapad (Yu et al. 2012, 2016) correctly did so.
Alphomeloncrocostethus Deans, 2003
Alphomeloncrocostethus Deans, 2003.
Type information. Holotype female, USNM (not examined but paratype examined). Country of type locality: Jamaica.
Geographical distribution.NEO.
NEO: Bolivia, Brazil (ES, MG, RJ), Colombia, Jamaica, Puerto Rico.
Alphomelondisputabile (Ashmead, 1900), lectotype designation
Urogasterdisputabilis Ashmead, 1900.
Type information. Lectotype male, NHMUK (examined). Country of type locality: Grenada.
Geographical distribution.NEA, NEO.
NEA: USA (KS, TX); NEO: Argentina, Belize, Bolivia, Brazil (ES, MT, PA, RJ, SC), Costa Rica, Cuba, Dominica, Ecuador, Grenada, Guatemala, Mexico, Panama, Paraguay, Puerto Rico, Saint Vincent, Trinidad & Tobago, Venezuela.
Notes.Ashmead (1900c: 286) did not designate a type in the original description of the species, which was based on 'several specimens'. Subsequent references to the species (e.g., Muesebeck 1921, Shenefelt 1972, Marsh et al. 1979) did not address that either. In the most complete nomenclatural account of the species (Shenefelt 1972: 494), it is implied that the type series, including both male and female specimens, was deposited in London (NHMUK), and could be from either Grenada or Saint Vincent. Much later Deans et al. (2003) mentioned that they had examined the holotype of the species, which they wrote was a male and was deposited in the USNM (with USNM type #6446). However, there cannot be a 'holotype' when Ashmead’s paper makes it clear that the species description was based on a series of specimens. From the Introduction section of the original paper (Ashmead 1900c: 207) it is also clear that the specimens studied were loaned to him from London (NHMUK). Thus, what likely happened was that, after studying the loaned material, Ashmead retained one specimen in Washington from the original type series and returned the rest to London. That means that the male specimen examined by Deans et al. (2003) in Washington is a syntype. The Washington specimen cannot be considered as the lectotype either, following ICZN Article 74.7 “Lectotype designation after 1999”, which clearly states that “To be valid, a lectotype designation made after 1999 must, 74.7.1. employ the term “lectotype” or an exact translation (e.g., “lectotypus” but not “the type”), 74.7.2. contain information sufficient to ensure recognition of the specimen designated, and 74.7.3. contain an express statement of deliberate designation (merely citing a specimen as “lectotype” is insufficient)”. For the sake of clarity, here we designate a male specimen as the lectotype [NHMUK, type number 3c.2395, specimen number 010636228, ‘St’ Vincent, | W.I. | H.H. Smith’, ‘W. Indies | 99-331.’]. There are an additional four paralectotype males in NHMUK, three from Grenada and one from St. Vincent, that from St. Vincent labelled by Ashmead as ‘Type male’ and with a yellow ‘co-type’ label. The specimen designated lectotype here is in better condition, albeit lacking its antennae.
Alphomelonmelanoscelis Deans, 2003
Alphomelonmelanoscelis Deans, 2003.
Type information. Holotype female, ESUW (not examined but paratype examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Belize, Brazil (AL, MT), Costa Rica, Mexico, Venezuela.
Alphomelonnanosoma Deans, 2003
Alphomelonnanosoma Deans, 2003.
Type information. Holotype female, USNM (not examined but authoritatively identified specimens examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Brazil (MT), Costa Rica, Ecuador, Mexico, Panama, Trinidad & Tobago.
Notes. The specimens we studied were identified by the author of the species.
Alphomelonnigriceps (Ashmead, 1900), lectotype designation
Urogasternigriceps Ashmead, 1900.
Type information. Lectotype female, NHMUK (examined). Country of type locality: Saint Vincent.
Geographical distribution.NEA, NEO.
NEA: USA (FL, NC, TX); NEO: Argentina, Belize, Brazil (RO), Colombia, Cuba, Dominica, Grenada, Netherlands Antilles, Peru, Saint Lucia, Saint Vincent, Trinidad & Tobago, Venezuela.
Notes.Ashmead (1900c: 284) did not designate a type in the original description of the species, which was based on eight female specimens. Subsequent references to the species (e.g., Szépligeti 1904, Muesebeck 1921, Shenefelt 1972, Marsh et al. 1979) did not address that either. The most complete nomenclatural account of the species (Shenefelt 1972: 580) mentioned that the type series was in London (NHMUK), and a female specimen, with code 3c.1125 is referred to as the type. Much later, Deans et al. (2003) mentioned that they had examined the holotype of the species, which they wrote was a female and was deposited in the USNM (with USNM type #6443). Deans et al. (2003) probably overlooked Shenefelt’s account, but in any case, there cannot be a holotype when the original paper makes clear that it was a series of specimens. From the Introduction section of the original paper (Ashmead 1900c: 207) it is clear that the specimens studied were loaned to him from London (NHMUK). Thus, what likely happened was that, after studying the loaned material, Ashmead retained one specimen in Washington from the original type series and returned the rest to London. That means that the female specimen that Deans et al. (2003) saw in Washington is a syntype. We have seen in London the specimen referred to by Shenefelt (1972) with code 3c.1125. It is a female in good condition and, in addition to the standard type label from the NHMUK, it also has an additional, handwritten label that reads “Urogasternigriceps, ? type, Ash.” For the sake of clarity, here we designate that female specimen as the lectotype; the female specimen examined by Deans et al. (2003) and deposited in the USNM, as well as the rest of the female specimens deposited in NHMUK are thus to be considered as paralectotypes.
Alphomelonpaurogenum Deans, 2003
Alphomelonpaurogenum Deans, 2003.
Type information. Holotype female, MCZ (not examined but original description checked). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina, Chile.
Alphomelonpyrrhogluteum Deans, 2003
Alphomelonpyrrhogluteum Deans, 2003.
Type information. Holotype female, MCZ (not examined but original description checked). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina.
Alphomelonrhyssocercus Deans, 2003
Alphomelonrhyssocercus Deans, 2003.
Type information. Holotype female, CNC (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Argentina, Costa Rica, Ecuador, Panama, Peru, Trinidad & Tobago, Venezuela.
Alphomelonrugosum Shimabukuro & Penteado-Dias, 2003
Alphomelonrugosum Shimabukuro & Penteado-Dias, 2003.
Type information. Holotype female, DCBU (not examined but original description checked). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (DF, SP).
Alphomelonsimpsonorum Deans, 2003
Alphomelonsimpsonorum Deans, 2003.
Type information. Holotype female, CNC (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (PR, SC), Costa Rica, Paraguay.
Alphomelontalidicida (Wilkinson, 1931)
Apantelestalidicida Wilkinson, 1931.
Type information. Holotype female, NHMUK (examined). Country of type locality: Guyana.
Geographical distribution.NEO.
NEO: Belize, Brazil, Colombia, Costa Rica, Ecuador, Guyana, Mexico, Panama, Peru, Trinidad & Tobago, Venezuela.
Alphomelonwinniewertzae Deans, 2003
Alphomelonwinniewertzae Deans, 2003.
Type information. Holotype female, USNM (not examined but authoritatively identified specimens examined). Country of type locality: USA.
Geographical distribution.NEA, NEO.
NEA: Canada (ON, QC), USA (AR, DC, FL, KS, MA, MI, NC, OH, TN, TX, VA); NEO: Costa Rica, Mexico.
Notes. The specimens we studied were identified by the author of the species.
Alphomelonxestopyga Deans, 2003
Alphomelonxestopyga Deans, 2003.
Type information. Holotype female, USNM (not examined but paratype examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Genus Apanteles Foerster, 1863
Apanteles Foerster, 1863: 245. Gender: masculine. Type species: Microgasterobscurus Nees, 1834, by original designation and monotypy.
Urogaster Ashmead, 1898: 166. Type species: Urogastervulgaris Ashmead, 1898, by subsequent designation (Viereck 1914).
Holcapanteles Cameron, 1905: 44. Type species: Holcapantelessulciscutis Cameron, 1905, by monotypy. New synonymy.
Xestapanteles Cameron, 1910: 447. Type species: Xestapanteleslatiannulatus Cameron, 1910, by monotypy.
Cecidobracon Kieffer & Jörgensen, 1910: 436. Type species: Cecidobraconasphondyliae Kieffer & Jörgensen, 1910, by monotypy. New synonymy.
Allapanteles Brèthes, 1915: 404. Type species: Allapantelescecidiptae Brèthes, 1915, by monotypy.
The year of publication of Foerster’s paper, with the original description of Apanteles, was until recently almost universally cited as 1862 (e.g., Dalla Torre 1898, Szépligeti 1904, Shenefelt 1972, Marsh 1979a, Yu et al. 2012); however, it has been shown that the actual year of publication was 1863 (Foley et al. 2003), which has been followed by Yu et al. (2016) and it is also accepted here.
The type species of Holcapanteles is H.sulciscutis Cameron, 1905, from Indonesia. The holotype is apparently lost (Shenefelt 1973, van Achterberg 1980, Mason 1981). The type species of Cecidobracon is C.asphondyliae Kieffer & Jörgensen, 1910, from Argentina. Unfortunately, the type depository was never stated in the original description, and the specimen has not been located subsequently (Shenefelt 1973, Mason 1981). A second species, Cecidobraconbraziliensis Kieffer & Tavares, 1925, was described from Brazil a few years later, but the type depository is also unknown. Without seeing the type specimens it may never be possible to establish with certainty the validity of Holcapanteles and Cecidobracon as Microgastrinae genera; however, based on the original descriptions, Mason (1981: 26, 27) considered that both genera were likely to be synonyms of Apanteles, although he did not formally synonymize the names. After reading the three original descriptions (Cameron 1905a: 44, Kieffer and Jörgensen 1910: 436–437, Kieffer and Tavares 1925: 48), including the associated illustrations of the wings of the two Cecidobracon species, we concur with Mason’s opinion and thus formally synonymize both genera under Apanteles for the sake of clarity and stability. The three species are also formally transferred below.
Currently Apanteles is the largest genus of Microgastrinae with 633 described species from all biogeographical regions (although, interestingly, there are no native species in New Zealand and the genus has not been recorded from the high Arctic). Several regional revisions are available, but some are very outdated and the taxonomic coverage of world species is far from complete. We have seen a large number of undescribed species in collections, mostly from tropical areas, and the actual species richness may well attain several thousand species. The name Apanteles was traditionally applied to all species with the fore wing areolet open: subsequently Apanteles auctt. has been split into numerous genera starting as early as 1880 and resulting in more than two dozen new genera being proposed since (see Mason 1981, Whitfield et al. 2002b, and Fernandez-Triana et al. 2014e for summaries of the history of Apanteles and its different concepts). van Achterberg (2003) synonymised several of these genera under Apanteles, thus potentially increasing the number of described species to 1,290 (Fig. 2A; see also Yu et al. 2016); however, we do not follow that arrangement here (Fig. 2B; also, see above, under the section Brief diagnosis of all Microgastrinae genera as they are understood in this paper, a more detailed discussion on the generic limits of the subfamily). Even with the restricted generic concept that we use in this paper, Apanteles is still a huge and varied assemblage of species. Nixon (1965) proposed 44 species groups for the world fauna (although that was before Mason (1981) split the genus, meaning some of those groups are not currently in Apanteles); and Fernandez-Triana et al. (2014e) proposed 30 new species groups just for Mesoamerica. Many of the Apanteles species groups represent monophyletic or at least morphologically cohesive groups, but others are poorly defined, and some are just containers for species that do not fit into any other group. Many families of Lepidoptera have been recorded as hosts for Apanteles, but many records are likely to be incorrect and/or need further verification. In Costa Rica most of the known hosts belong to three families: Crambidae, Depresariidae, and Hesperiidae (Fernandez-Triana et al. 2014e; in that paper depressarids were treated as elachistids). There are 7,800+ DNA-barcode compliant sequences of Apanteles in BOLD representing almost 600 different BINs, mostly from Costa Rica and North America.
Apantelesabdera Nixon, 1965
Apantelesabdera Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: Cape Verde, South Africa.
Apantelesabditus Muesebeck, 1957
Apantelesabditus Muesebeck, 1957.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (SP), Uruguay, Venezuela.
Apantelesacoris Nixon, 1965
Apantelesacoris Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Apantelesacutissimus Granger, 1949
Apantelesacutissimus Granger, 1949.
Type information. Syntypes female and male, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Notes. The original description mentions 15 female and 16 male specimens but does not explicitly designate a holotype, thus all are here considered to be syntypes.
Apantelesadelinamoralesae Fernandez-Triana, 2014
Apantelesadelinamoralesae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesadoxophyesi Minamikawa, 1954
Apantelesadoxophyesi Minamikawa, 1954.
Type information. Holotype female, depository unknown (not examined but authoritatively identified specimens examined). Country of type locality: Japan.
Geographical distribution.OTL, PAL.
OTL: China (ZJ); PAL: China (AH, SD), Japan.
Notes. Our concept of Apantelesadoxophyesi is based on two female specimens we examined (EIHU), presumably identified by Watanabe. The digital collection of TARI also contains images of this species, although we could not confirm the accuracy of that identification (https://digiins.tari.gov.tw/tarie/treelist003E.php?id=Brac11122001&lev1=3&lev2=0/1/7/&lev3=01&page=5).
Apantelesadreus Nixon, 1965
Apantelesadreus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Apantelesadrianachavarriae Fernandez-Triana, 2014
Apantelesadrianachavarriae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesadrianaguilarae Fernandez-Triana, 2014
Apantelesadrianaguilarae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesadrianguadamuzi Fernandez-Triana, 2014
Apantelesadrianguadamuzi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesafer Wilkinson, 1932
Apantelesafer Wilkinson, 1932.
Type information. Holotype female, NHMUK (examined). Country of type locality: Uganda.
Geographical distribution.AFR.
AFR: Uganda.
Apantelesagatillus Nixon, 1965
Apantelesagatillus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Apantelesaglaope Nixon, 1965
Apantelesaglaope Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Indonesia.
Geographical distribution.OTL.
OTL: Indonesia.
Apantelesaglaus Nixon, 1965
Apantelesaglaus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Fiji.
Geographical distribution.AUS.
AUS: Fiji.
Apantelesagrus Nixon, 1965
Apantelesagrus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Apantelesaichagirardae Fernandez-Triana, 2014
Apantelesaichagirardae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesaidalopezae Fernandez-Triana, 2014
Apantelesaidalopezae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesalaspharus Nixon, 1965
Apantelesalaspharus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Apantelesalastor de Saeger, 1944
Apantelesalastor de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Apantelesalazoni Lozan, 2008
Apantelesalazoni Lozan, 2008.
Type information. Holotype female, IECA (not examined but original description checked). Country of type locality: Canary Islands.
Geographical distribution.PAL.
PAL: Canary Islands.
Apantelesalbanjimenezi Fernandez-Triana, 2014
Apantelesalbanjimenezi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesalbinervis (Cameron, 1904)
Urogasteralbinervis Cameron, 1904.
Apantelesalbinervicam Shenefelt, 1972.
Type information. Holotype male, NHMUK (examined). Country of type locality: Mexico.
Geographical distribution.NEO.
NEO: Mexico.
Apantelesalejandromasisi Fernandez-Triana, 2014
Apantelesalejandromasisi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesalejandromorai Fernandez-Triana, 2014
Apantelesalejandromorai Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesalexanderi Brèthes, 1922
Apantelesalexanderi Brèthes, 1922.
Type information. Lectotype female, MACN (not examined but subsequent treatment of the species checked). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina, Uruguay.
Notes. Our concept of Apantelesalexanderi is based on Martinez et al. (2012), who examined and designated the lectotype, and provided images and DNA barcodes of the species.
Apantelesallofulvigaster Long, 2007
Apantelesallofulvigaster Long, 2007.
Type information. Holotype female, VNMN (not examined but original description checked). Country of type locality: Vietnam.
Geographical distribution.OTL.
OTL: Vietnam.
Notes. The holotype depository was not stated in the English version of the original description (Long 2007). That paper was written in two languages, the first part in Vietnamese, followed by a second part in English; based on the extent of both versions, we suspect that the English part is just a translation from the Vietnamese. However, we do not know if it is a literal translation or just a summarized (= shorter) version; thus, we do not know if the holotype depository is mentioned in the Vietnamese part of the paper. If the holotype was not stated in the Vietnamese version, then this species name would be unavailable (a subsequent paper (Long and Achterberg 2014) records the holotype depository; however, that alone does not comply with the ICZN requirements and would not make the name available). Although we have not been able to establish with certainty what is stated in the Vietnamese part of Long (2007), we provisionally consider here the species name as available.
Apantelesalvarougaldei Fernandez-Triana, 2014
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesanabellecordobae Fernandez-Triana, 2014
Apantelesanabellecordobae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesanamarencoae Fernandez-Triana, 2014
Apantelesanamarencoae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesanamartinezae Fernandez-Triana, 2014
Apantelesanamartinezae Fernandez-Triana, 2014.
Apantelesanamartinesae Fernandez-Triana, 2014 [incorrect original spelling].
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Notes. In the paper where this species was originally described, the name was spelled in two different ways: as anamartinezae (in the species list of Table 3, species description, references to ZooBank and caption of Figure 227) or as anamartinesae (in the Abstract, key to species, and caption to Figure 25). The correct spelling is obviously anamartinezae, as the species was named after Ana Martínez, and it is the one to be preserved, following Article 32 of the ICZN.
Figure 25.
Apantelessodalis female MIC000013 A Habitus, lateral B Head, frontolateral C Fore wing and hind wing D Mesosoma, dorsal E Metasoma, dorsal F Ovipositor and ovipositor sheaths.
Apantelesanariasae Fernandez-Triana, 2014
Apantelesanariasae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesanatole Nixon, 1965
Apantelesanatole Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Notes. The holotype specimen has the vannal lobe with very few, very sparse setae across lobe length.
Apantelesandreacalvoae Fernandez-Triana, 2014
Apantelesandreacalvoae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesangaleti Muesebeck, 1956
Apantelesangaleti Muesebeck, 1956.
Type information. Holotype female, USNM (examined). Country of type locality: India.
Geographical distribution.AFR, OTL, PAL.
AFR: Kenya; OTL: China (SN, ZJ), India, Indonesia, Pakistan, Vietnam; PAL: Iraq.
Notes. Introduced into Mexico and the USA (e.g., Mcgough and Noble 1957, Bartlett et al. 1978). In total more than 150,000 specimens were released but the species was never recovered in any of the USA states where it was released (Mcgough and Noble 1957), and a subsequent citation of the species from Mexico (Coronado-Blanco et al. 2004) is merely a repetition of the information cited in older references, not a confirmation of the species’ presence in the country. Thus, in this paper we do not consider A.angaleti as an established species in the Nearctic or Neotropical regions.
Apantelesangelsolisi Fernandez-Triana, 2014
Apantelesangelsolisi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesangulatus Granger, 1949
Apantelesangulatus Granger, 1949.
Type information. Syntypes female and male, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Apantelesangustibasis Gahan, 1925
Apantelesangustibasis Gahan, 1925.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: China (HN), India, Malaysia, Pakistan, Philippines, Vietnam.
Notes. This species was transferred to Cotesia by Gupta and Pawar (1992), a non-taxonomic paper, in which it could be argued that those authors did not study the holotype. We have studied the holotype as well as illustrations of specimens from Malaysia identified by C. Watanabe that are deposited in EIHU. Both the holotype and the Malaysian specimens are clearly not Cotesia but Apanteles, and thus we restore the combination of this species here.
Apantelesanodaphus Nixon, 1965
Apantelesanodaphus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Papua New Guinea.
Geographical distribution.AUS.
AUS: Papua New Guinea.
Apantelesansata Song & Chen, 2004
Apantelesansata Song & Chen, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Apantelesanthozelae de Saeger, 1941
Apantelesanthozelae de Saeger, 1941.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Apantelesanticlea Nixon, 1965
Apantelesanticlea Nixon, 1965
Type information. Holotype female, USNM (examined). Country of type locality: Malaysia.
Geographical distribution.OTL.
OTL: Malaysia.
Apantelesantilla Nixon, 1965
Apantelesantilla Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Apantelesarachidis Risbec, 1951
Apantelesarachidis Risbec, 1951.
Type information. Holotype male, MNHN (not examined but original description checked). Country of type locality: Senegal.
Geographical distribution.AFR.
AFR: Senegal.
Notes. The original description is not clear enough to determine the correct generic placement of the species, thus is best kept in the genus it was originally described. Future study of the type specimen may change its current generic status.
Apantelesaraeceri Wilkinson, 1928
Apantelesaraeceri Wilkinson, 1928.
Type information. Holotype female, NHMUK (examined). Country of type locality: Indonesia.
Geographical distribution.OTL.
OTL: India, Indonesia, Malaysia.
Apantelesaragatzi Tobias, 1976
Apantelesaragatzi Tobias, 1976.
Type information. Holotype female, depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Armenia.
Geographical distribution.PAL.
PAL: Armenia, Russia (KDA), Sweden, Turkey.
Notes. Our concept of the species is based on the descriptions provided by Papp (1984a) and Tobias (1986).
Apantelesarielopezi Fernandez-Triana, 2014
Apantelesarielopezi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesarion Nixon, 1965
Apantelesarion Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Apantelesariovistus Nixon, 1965
Apantelesariovistus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Indonesia.
Geographical distribution.OTL.
OTL: Indonesia.
Apantelesaristaeus Nixon, 1965
Apantelesaristaeus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: China (TW), India, Indonesia.
Apantelesaristoteliae Viereck, 1912
Apantelesaristoteliae Viereck, 1912.
Apantelesgelechiae Viereck, 1912.
Type information. Holotype male, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (NB, ON, QC), USA (AZ, CA, CO, CT, KS, LA, MI, NJ, NY, NC, OH, OR, PA, TX, UT, VT, WA).
Apantelesarsanes Nixon, 1965
Apantelesarsanes Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Kenya.
Geographical distribution.AFR.
AFR: Kenya.
Notes. Despite its relatively short ovipositor sheaths, we are retaining this species in Apanteles because of its pleated hypopygium, strongly concave vannal lobe lacking setae, and anteromesoscutum punctures which are fusing near scutoscutellar disc.
Apantelesarticas Nixon, 1965
Apantelesarticas Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Senegal.
Geographical distribution.AFR, PAL.
AFR: Senegal; PAL: Israel, Tunisia, Turkey.
Apantelesartustigma Liu & Chen, 2015
Apantelesartustigma Liu & Chen, 2015.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (GD, ZJ).
Apantelesarundinariae de Saeger, 1944
Apantelesarundinariae de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Rwanda.
Apantelesasphondyliae (Kieffer & Jörgensen, 1910), new combination
Cecidobraconasphondyliae Kieffer & Jörgensen, 1910.
Type information. Holotype male, lost (not examined but original description checked). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina.
Notes. The type depository was not stated in the original description, and the specimen has never been located (Shenefelt 1973, Mason 1981). See comments at the beginning of Apanteles for details on the decision to transfer this species to Apanteles.
Apantelesassis Nixon, 1965
Apantelesassis Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines, Vietnam.
Apantelesatrocephalus Granger, 1949
Apantelesatrocephalus Granger, 1949.
Type information. Holotype female, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Notes. Based on some morphological features described by Granger (1949), e.g., the areolated propodeum, shape and sculpture of T1–T3, acute hypopygium, ovipositor sheaths half the metatibia length, we think that this species could potentially be placed in one of the following genera: Apanteles, Parapanteles, or Cotesia. Because the original description (the only source available, apart from the single known specimen, which we could not examine), is not sufficient to determine the correct generic placement, we maintain atrocephalus within the genus in which it was originally described.
Apantelesattevae Yousuf, Hassan & Singh, 2008
Apantelesattevae Yousuf, Hassan & Singh, 2008.
Type information. Holotype female, TFRI (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Apantelesaudens Kotenko, 1986
Apantelesaudens Kotenko, 1986.
Type information. Holotype female?, ZIN (not examined but original description checked). Country of type locality: Georgia.
Geographical distribution.PAL.
PAL: Georgia, Russia (NC).
Notes. The paper in which the original description is included does not clarify the sex of the type material, nor is it specified if there is a holotype (or syntypes) on which the species description was based (Tobias 1986: 805). Without examining the actual specimen(s) is impossible to determine its sex or type status; however, in the Foreword section of the paper (Tobias 1986: page numbered as ix) it is stated that, to comply with nomenclature rules, the type material is specified for all species. The author then explicitly says that the paper includes lectotype and paralectotype designations for species described from the USSR in the past. Such a statement allows the assumption that all new species descriptions must have been based on holotypes – and not a type series (syntypes) as was presumably done in the past. Thus, we are assuming that there is a holotype for Apantelesaudens Kotenko, 1986. Regarding the sex of the type, again only assumptions can be made until the specimen is examined, but the key is based on female specimens, including a brief original description that mentions the ovipositor sheaths. Thus, we consider here as very likely that the holotype is a female but add a question mark to clarify that it is only an educated guess.
Apantelesaurangabadensis Rao & Chalikwar, 1970
Apantelesaurangabadensis Rao & Chalikwar, 1970.
Type information. Holotype male, NZSI (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Apantelesazollae Sumodan & Sevichan, 1989
Apantelesazollae Sumodan & Sevichan, 1989.
Type information. Holotype female, RMNH (not examined but subsequent treatment of the species checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. See van Achterberg and Narendran (1997) for details about the type, and for the generic placement of the species. Apantelesazollae has been misspelled twice, as azolae and azolla, as previously noted by Yu et al. (2016).
Apantelesbajariae Papp, 1975
Apantelesbajariae Papp, 1975.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Hungary.
Geographical distribution.PAL.
PAL: Bulgaria, Canary Islands, Greece, Hungary, Montenegro, Turkey.
Notes. Based on the position this species occupies in the key of Papp (1984a), it is possible that bajariae would actually belong to Dolichogenidea. However, the details in both the original description and Papp (1984a) are not definite to conclude with certainty, thus it is here kept in the genus it was originally described.
Apantelesbaldufi Muesebeck, 1968
Apantelesbaldufi Muesebeck, 1968.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (ON), USA (MI, MN).
Apantelesbalteatae Lal, 1942
Apantelesbalteatae Lal, 1942.
Type information. Holotype male, INPC (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Apantelesbalthazari (Ashmead, 1900)
Urogasterbalthazari Ashmead, 1900.
Urogastermeridionalis Ashmead, 1900.
Apantelesmeridionalis Ashmead, 1900.
Type information. Holotype female, NHMUK (examined). Country of type locality: Saint Vincent.
Geographical distribution.NEO.
NEO: Brazil (CE, PA, PB, PE, RN, SP), Cuba, Grenada, Saint Vincent.
Notes. The original description (Ashmead 1900c) does not match the holotype, as his description of the T1 shape, T2 sculpture and colouration of meso- and metafemora are completely different from the actual specimen examined (see Fernandez-Triana et al. 2014e).
Apantelesbannaensis Song, Chen & Yang, 2001
Apantelesbannaensis Song, Chen & Yang, 2001.
Type information. Holotype female, FAFU (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (YN).
Notes. Our species concept is based on Chen and Song (2004).
Apantelesbaoli Risbec, 1951
Apantelesbaoli Risbec, 1951.
Type information. Holotype male, depository unknown (not examined but original description checked). Country of type locality: Senegal.
Geographical distribution.AFR.
AFR: Senegal.
Apantelesbasicavus Liu & Chen, 2015
Apantelesbasicavus Liu & Chen, 2015.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (JL, LN).
Apantelesbellatulus de Saeger, 1944
Apantelesbellatulus de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Apantelesbernardoespinozai Fernandez-Triana, 2014
Apantelesbernardoespinozai Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesbernyapui Fernandez-Triana, 2014
Apantelesbernyapui Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesbettymarchenae Fernandez-Triana, 2014
Apantelesbettymarchenae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesbienvenidachavarriae Fernandez-Triana, 2014
Apantelesbienvenidachavarriae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesbiroicus Papp, 1973
Apantelesbiroicus Papp, 1973.
Type information. Holotype female, HNHM (not examined but paratype examined). Country of type locality: Hungary.
Geographical distribution.PAL.
PAL: Hungary, Romania, Tunisia.
Notes. This species was transferred from Apanteles to Illidops by Papp (1988), but examination of two paratype specimens in the CNC revealed that those specimens do not have a median band of rugosity posteriorly on the scutellum, and the propodeum sculpture is also different from that found in Illidops (sensuFernandez-Triana et al. 2014e). Thus, here we transfer the species back to Apanteles.
Apantelesbitalensis de Saeger, 1944
Apantelesbitalensis de Saeger, 1944.
Type information. Syntypes female, RMCA (not examined but original description checked). Country of type locality: Rwanda.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Rwanda.
Apantelesbordagei Giard, 1898
Apantelesbordagei Giard, 1898.
Type information. Type lost (not examined but original description checked). Country of type locality: Réunion.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Kenya, Réunion, Tanzania.
Notes. The year of description for this species has been incorrectly cited as 1902 by most authors (e.g., Granger 1949, Shenefelt 1972, Rousse and Gupta 2013, Yu et al. 2016), in all cases based on Giard (1902: 22). Having read that paper, it is clear that it only refers to the species as being described by the author in a previous work (Giard 1898: 202, which we have also read). This was correctly mentioned by de Saeger (1944: 316) and Wilkinson (1934: 150). Wilkinson comprehensively redescribed the species, based on specimens from Kenya and Tanzania, and he considered the type(s) to be lost based on his enquiry to a curator of the MNHN at the time, who could not find the specimen(s). Subsequent authors have provided shorter redescriptions, based on specimens from Democratic Republic of Congo (de Saeger 1944), Madagascar (Granger 1949), or Réunion (Rousse and Gupta 2013). Our species concept is based on Wilkinson (1934). We accept the following comments from Madl and van Achterberg (2014): “Known from the Afrotropical region. The record from Madagascar mentioned in Risbec (1960: 629) is doubtful. Brénière (1965b: 347) mentions Apantelesbordagei from Madagascar, citing Granger (1949: 359) as reference, but Granger recorded this species only from Réunion and Africa. The record from Madagascar in Appert et al. (1969: 568) is based on Brénière (1965b)”. Consequently, here we do not consider Madagascar as a country where this species is found.
Apantelesbrachmiae Bhatnagar, 1950
Apantelesbrachmiae Bhatnagar, 1950.
Type information. Holotype female, INPC (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. The year of publication of the Bhatnagar paper was until recently commonly cited as 1948 and/or 1950 (e.g., Chen and Song 2004, Yu et al. 2016), probably following Shenefelt (1972) who referred to this paper as “Bhatnagar (1948) 1950”. While the intended year for Volume X, Parts I & II of the Indian Journal of Entomology was 1948, the actual dates of publication were June 1950 (Part I) and October 1950 (Part II), as clearly shown on the cover page of the Volume, which we have checked. Because the dates of publication are the ones to be considered, and for the sake of clarity, we hereby revise the species year of description to 1950.
Apantelesbraziliensis (Kieffer & Tavares, 1925), new combination
Cecidobraconbraziliensis Kieffer & Tavares, 1925.
Type information. Type and depository unknown (not examined but original description checked). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (BA).
Notes. The type depository was not given in the original description, and the specimen has never been located (Shenefelt 1973, Mason 1981). See comments at the beginning of Apanteles for details on the decision to transfer this species to Apanteles (p 74, 75).
Apantelesbredoi de Saeger, 1941
Apantelesbredoi de Saeger, 1941.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Senegal.
Apantelesbrethesi Porter, 1917
Apantelesbrethesi Porter, 1917.
Type information. Type and depository unknown (not examined). Country of type locality: Chile.
Geographical distribution.NEO.
NEO: Chile.
Apantelesbrevicarinis Song, 2002
Apantelesbrevicarinis Song, 2002.
Type information. Holotype female, FAFU (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (HB).
Notes. Our concept of this species is based on Chen and Song (2004).
Apantelesbrevimetacarpus Hedqvist, 1965
Apantelesbrevimetacarpus Hedqvist, 1965.
Illidopsmetacarpus Hedqvist, 1965 [subsequent misspelling (Papp 2003)].
Type information. Holotype female, MZH (examined). Country of type locality: Cape Verde.
Geographical distribution.AFR, PAL.
AFR: Cape Verde; PAL: Tunisia.
Notes.Papp (2003: 145) transferred this species to Illidops (although he misspelled the species name as metacarpus). A subsequent paper, also treating the species and reporting it for the first time from Tunisia, continued to place it within Illidops (Papp 2014). We examined the female holotype and a male paratype, and they clearly are not Illidops. The only feature that would suggest placement in that genus is the short vein R1 (metacarp), but that is known in several species of both Apanteles and Dolichogenidea. The posteromedian band of the scutellum is smooth. The propodeum, although without an areola, has a weak impression in its place, and its overall weak sculpture is not like that found in Illidops. Based on the hind wing, with a slightly concave vannal lobe lacking setae, the best generic placement for this species is Apanteles. This concurs with Forshage et al. (2016), although those authors were probably not aware of the two papers by Papp and were following the treatment of the original description. In any case, the statement by Forshage et al. (2016) that the holotype and paratype were missing is here updated, as in 2018 we found the specimens in the MZH.
Apantelesbrevivena Liu & Chen, 2015
Apantelesbrevivena Liu & Chen, 2015.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (XJ, LN, JL, NM, SD).
Apantelesbruchi Blanchard, 1941
Apantelesbruchi Blanchard, 1941.
Type information. Type lost (not examined but subsequent treatment of the species checked). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina, Peru.
Notes. Our concept of this species is based on Aquino et al. (2010), including details on the fate of the type material.
Apantelesbrunnistigma Abdinbekova, 1969
Apantelesbrunnistigma Abdinbekova, 1969.
Apantelessotades Nixon, 1976.
Type information. Holotype female, ZIN (not examined but authoritatively identified specimens examined). Country of type locality: Azerbaijan.
Geographical distribution.NEA, PAL.
NEA: Canada (MB, NL, NT, ON, YT); PAL: Azerbaijan, Canary Islands, Czech Republic, Finland, France, Germany, Hungary, Iran, Italy, Korea, Lithuania, Russia (ZAB, PRI, TOM), Sweden, Switzerland, Turkey, United Kingdom, Ukraine.
Notes. Our concept of this species is based on Fernandez-Triana et al. (2014c). We have also examined the type of Apantelessotades Nixon. New data from specimens with sequences in BOLD expand the species distribution within the Nearctic (northwestern Canada) as well as the Palearctic (Germany, Ukraine).
Apantelesbrunnus Rao & Chalikwar, 1976
Apantelesbrunnus Rao & Chalikwar, 1976.
Type information. Holotype female, BAMU (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Apantelesburunganus de Saeger, 1944
Apantelesburunganus de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. The original description does not provide enough detail to place this species in a genus unambiguously (it could be Apanteles but also Dolichogenidea). Until the type series is studied, we retain it in the genus in which it was originally described.
Apantelescaesar Wilkinson, 1938
Apantelescaesar Wilkinson, 1938.
Type information. Holotype female, NHMUK (examined). Country of type locality: Namibia.
Geographical distribution.AFR.
AFR: Namibia, South Africa.
Notes. This species bears some resemblance to the two described species currently placed within Napamus. It shares with them the dark colour, infumate wings, elongate mouth parts (especially very long glossa and galea), and relatively short fore wing vein R1 (although not as short as in the two described Napamus). However, we retain caesar within Apanteles because it has some differences in propodeum sculpture (which is mostly smooth, having only small carinae near the nucha), metatibial spines (which are not as long as in Napamus) and the disparate geographic distribution of the known species.
Apantelescalixtomoragai Fernandez-Triana, 2014
Apantelescalixtomoragai Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelescalycinae Wilkinson, 1928
Apantelescalycinae Wilkinson, 1928.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India, Vietnam.
Apantelescamilla Nixon, 1965
Apantelescamilla Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Apantelescamirus Nixon, 1965
Apantelescamirus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Apantelescanarsiae Ashmead, 1898
Apantelescanarsiae Ashmead, 1898.
Apanteleshousatannuckorum Viereck, 1917.
Apantelesmaquinnai Viereck, 1917.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (ON, QC), USA (AR, CT, DC, IL, IN, IA, KS, NY, VA).
Notes. We examined the holotype female of housatannuckorum and the holotype male of maquinnai, both currently considered as synonyms of A.canarsiae. All three holotypes are in the USNM and not in INHS as stated in Yu et al. (2016).
Apantelescarloscastilloi Fernandez-Triana, 2014
Apantelescarloscastilloi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelescarlosguadamuzi Fernandez-Triana, 2014
Apantelescarlosguadamuzi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelescarlosrodriguezi Fernandez-Triana, 2014
Apantelescarlosrodriguezi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelescarlosviquezi Fernandez-Triana, 2014
Apantelescarlosviquezi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelescarloszunigai Fernandez-Triana, 2014
Apantelescarloszunigai Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelescarolinacanoae Fernandez-Triana, 2014
Apantelescarolinacanoae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelescarpatus (Say, 1836)
Microgastercarpata Say, 1836.
Urogastersolitarius Ashmead, 1900.
Protapanteleshawaiiensis Ashmead, 1901.
Urogasterfuscicornis Cameron, 1910.
Apantelespiceoventris Muesebeck, 1921.
Apantelesigae Watanabe, 1932.
Apantelessarcitorius Telenga, 1955.
Apantelesultericus Telenga, 1955.
Type information. Holotype female, lost (not examined but subsequent treatment of the species checked). Country of type locality: USA.
Geographical distribution.AFR, AUS, NEA, NEO, OTL, PAL.
AFR: Democratic Republic of Congo, Ghana, Mozambique, South Africa, Tanzania; AUS: Australia (QLD), Fiji, Hawaiian Islands, New Zealand; NEA: Canada (AB, BC, NB, NL, ON, PE, QC, SK), USA (CO, CT, DE, IL, IN, MD, MA, MI, MO, NH, NJ, NY, SC, TX, VA); NEO: Argentina, Bermuda, Brazil (SP), Cuba, Grenada, Peru, Puerto Rico; OTL: China (SN, TW, ZJ), Malaysia, Vietnam; PAL: Armenia, Croatia, Finland, France, Germany, Greece, Hungary, Iran, Israel, Japan, Kazakhstan, Latvia, Lithuania, Malta, Moldova, Mongolia, Poland, Romania, Russia (AMU, AST, KHA, PRI, SAK), Serbia, Spain, Switzerland, Turkey, Turkmenistan, United Kingdom, Uzbekistan.
Notes. We examined the types of two of the seven currently accepted synonyms of carpatus: hawaiiensis (in USNM) and solitarius (in NHMUK). If Apantelescarpatus is ever going to be split into several species, the type of hawaiiensis would be a candidate to be considered as a different species, supported by morphological differences when compared to other Apantelescarpatus specimens and also through different host associations. We also examined one female (in EIHU, identified by Muesebeck) which also looks different to the traditional carpatus and could represent yet another species. Determining the limits of A.carpatus is beyond the scope of this paper and at present we leave all of the examined specimens as a single species.
Apantelescassiae Chalikwar & Rao, 1982
Apantelescassiae Chalikwar & Rao, 1982.
Type information. Type and depository unknown (not examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Apantelescato de Saeger, 1944
Apantelescato de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Rwanda.
Apantelescavatiptera Chen & Song, 2004
Apantelescavatiptera Chen & Song, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, YN).
Apantelescavatithoracicus Chen, 2001
Apantelescavatithoracica Chen, 2001.
Type information. Holotype female, FAFU (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, HB).
Notes. For the generic placement of this species we follow Chen and Song (2004).
Apantelescavifrons Nixon, 1965
Apantelescavifrons Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Apantelescebes Nixon, 1965
Apantelescebes Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Apantelescecidiptae (Brèthes, 1916)
Allapantelescecidiptae Brèthes, 1916.
Type information. Syntypes female and male, MACN (not examined). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina.
Apantelescerberus Nixon, 1965
Apantelescerberus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Apantelescestius Nixon, 1965
Apantelescestius Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Apanteleschalcomelas Nixon, 1965
Apanteleschalcomelas Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Apanteleschanghingensis Chu, 1937
Apanteleschanghingensis Chu, 1937.
Type information. Holotype female, depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, ZJ).
Notes. For the generic placement of this species we follow Chen and Song (2004).
Apantelescharacomae Risbec, 1951
Apantelescharacomae Risbec, 1951.
Type information. Holotype male, depository unknown (not examined but original description checked). Country of type locality: Ivory Coast.
Geographical distribution.AFR.
AFR: Ivory Coast.
Apanteleschatterjeei Sharma & Chatterjee, 1970
Apanteleschatterjeei Sharma & Chatterjee, 1970.
Type information. Holotype female, IFRI (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Apanteleschloris Nixon, 1965
Apanteleschloris Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines, Vietnam.
Apanteleschristianzunigai Fernandez-Triana, 2014
Apanteleschristianzunigai Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelescingulicornis Granger, 1949
Apantelescingulicornis Granger, 1949.
Type information. Syntypes female, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Apantelescinthiabarrantesae Fernandez-Triana, 2014
Apantelescinthiabarrantesae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesciriloumanai Fernandez-Triana, 2014
Apantelesciriloumanai Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesclita Nixon, 1965
Apantelesclita Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: China (FJ), India, Vietnam.
Apantelescockerelli Muesebeck, 1921
Apantelescockerelli Muesebeck, 1921.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CA, ID, IA, MI, MO, NE, NM, OR, SD, TX).
Apantelescocotis Wilkinson, 1934
Apantelescocotis Wilkinson, 1934.
Type information. Holotype female, NHMUK (examined). Country of type locality: Indonesia.
Geographical distribution.OTL.
OTL: Indonesia, Vietnam.
Apantelescoedicius Nixon, 1965
Apantelescoedicius Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines, Vietnam.
Apantelescoffeellae Muesebeck, 1958
Apantelescoffeellae Muesebeck, 1958.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: Guadeloupe.
Geographical distribution.NEO.
NEO: Guadeloupe, Puerto Rico.
Apantelescoilus Nixon, 1965
Apantelescoilus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Apantelesconanchetorum Viereck, 1917
Apantelesconanchetorum Viereck, 1917.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (NS, ON), USA (AR, CT, DC, IL, IA, KS, MI, MO, NY, OH, PA, SC, SD, WV, WI).
Notes. Specimens of Apantelesconanchetorum that have rendered DNA barcodes comprise two BINS: BOLD:AAC5506 (eastern North America) and BOLD:AAC5507 (principally western Canada, but some records from ON, PE). Whether they represent two different species or not has been mentioned in the past (Fernandez-Triana et al. 2014b), but for the time being all known specimens are kept as one species.
Apantelesconcordalis Cameron, 1911
Apantelesconcordalis Cameron, 1911.
Type information. Holotype female, NHMUK (examined). Country of type locality: Guyana.
Geographical distribution.NEO.
NEO: Guyana, Peru.
Notes. Based on the carination and sculpture pattern of propodeum and fore wing venation, this species belongs to the leucostigmus group (sensuFernandez-Triana et al. 2014e).
Apantelesconon Nixon, 1965
Apantelesconon Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Indonesia.
Geographical distribution.OTL, PAL.
OTL: China (HB, TW), Indonesia, Philippines; PAL: Korea.
Notes. It is possible this is actually a Dolichogenidea species. The hind wing vannal lobes are not clearly visible (they are folded in both wings in the holotype) but what can be seen suggests they may be setose. Additionally, the anteromesoscutum punctures near the scutoscutellar sulcus do not fuse. However, because we cannot see the vannal lobe clearly, we refrain from transferring the species in this paper.
Apantelesconspicabilis de Saeger, 1944
Apantelesconspicabilis de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Rwanda.
Apantelescontactus Papp, 1977
Apantelescontactus Papp, 1977.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Mongolia.
Geographical distribution.PAL.
PAL: Mongolia, Russia (ZAB).
Apantelescontaminatus (Haliday, 1834)
Microgastercontaminatus Haliday, 1834.
Type information. Neotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Ireland, Italy, Netherlands, United Kingdom.
Apantelescontemptus Nixon, 1965
Apantelescontemptus Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Singapore.
Geographical distribution.OTL.
OTL: Singapore.
Apantelescordoi de Santis, 1980
Apantelescordoi de Santis, 1980.
Type information. Holotype female, MLP (not examined but subsequent treatment of the species checked). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina.
Notes. Our concept of this species is based on Aquino et al. (2010).
Apantelescornicula Chen & Song, 2004
Apantelescornicula Chen & Song, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Apantelescosmopterygivorus Liu & Chen, 2014
Apantelescosmopterygivorus Liu & Chen, 2014.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (ZJ).
Apantelescoxalis Szépligeti, 1911
Apantelescoxalis Szépligeti, 1911.
Type information. Holotype female, ZMHB (not examined but subsequent treatment of the species checked). Country of type locality: Tanzania.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Malawi, Senegal, Tanzania.
Notes. Our species concept is based on the redescription provided by Wilkinson (1932a), who was able to study the holotype.
Apantelescrassicornis (Provancher, 1886)
Microgastercrassicornis Provancher, 1886.
Microgastercrassicornis Provancher [primary junior homonym of Microgastercrassicornis Ruthe, 1860].
Type information. Lectotype female, ULQC (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (AB, ON, QC, SK), USA (AZ, IL, IN, IA, KS, MD, MA, MI, MN, MO, NJ, NY, OH, PA).
Apantelescrates Nixon, 1965
Apantelescrates Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines, Vietnam.
Apantelescrispulae Blanchard, 1943
Apantelescrispulae Blanchard, 1943.
Type information. Holotype female, MACN (not examined but original description checked). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina.
Apantelescristianalemani Fernandez-Triana, 2014
Apantelescristianalemani Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelescrius Nixon, 1965
Apantelescrius Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Notes. The ovipositor sheaths are short, and the hypopygium has only one median fold (not pleated), similar to that of Pholetesor. However, the species otherwise resembles Apanteles and thus we have decided to maintain this species in the latter genus.
Apantelescroceicornis Muesebeck, 1958
Apantelescroceicornis Muesebeck, 1958.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: Peru.
Geographical distribution.NEO.
NEO: Peru.
Apantelescrocidolomiae Ahmad, 1945
Apantelescrocidolomiae Ahmad, 1945.
Type information. Holotype female, INPC (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Apantelescrouzeli Blanchard, 1947
Apantelescrouzeli Blanchard, 1947.
Apantelescrouzelae de Santis, 1967 [unjustified emendation].
Type information. Holotype female, MACN (not examined but original description checked). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina.
Apantelescuneiformis Song & Chen, 2004
Apantelescuneiformis Song & Chen, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, YN).
Apantelescurvicaudatus Granger, 1949
Apantelescurvicaudatus Granger, 1949.
Type information. Syntypes female and male, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Apantelescynthiacorderoae Fernandez-Triana, 2014
Apantelescynthiacorderoae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelescyprioides Nixon, 1965
Apantelescyprioides Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.AFR, OTL.
AFR: South Africa; OTL: China (FJ, HN), Philippines, Singapore.
Apantelescypris Nixon, 1965
Apantelescypris Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL, PAL.
OTL: Bangladesh, China (FJ, GD, GX, GZ, HI, HK, HB, HN, JS, JX, SH, SN, TW, YN, ZJ), India, Indonesia, Malaysia, Nepal, Pakistan, Philippines, Singapore, Sri Lanka, Vietnam; PAL: China (AH, HA, SN, SD), Japan.
Apantelesdaimenes Nixon, 1965
Apantelesdaimenes Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Fiji.
Geographical distribution.AUS.
AUS: Fiji.
Apantelesdakotae Muesebeck, 1921
Apantelesdakotae Muesebeck, 1921.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (ID, SD).
Apantelesdecoloratus Granger, 1949
Apantelesdecoloratus Granger, 1949.
Type information. Syntypes female, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Apantelesdeifiliadavilae Fernandez-Triana, 2014
Apantelesdeifiliadavilae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesdelhiensis Muesebeck & Subba Rao, 1958
Apantelesdelhiensis Muesebeck & Subba Rao, 1958.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Apantelesdentatus Muesebeck, 1958
Apantelesdentatus Muesebeck, 1958.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (SP).
Apantelesdeplanatus Muesebeck, 1957
Apantelesdeplanatus Muesebeck, 1957.
Type information. Holotype female, USNM (not examined but subsequent treatment of the species checked). Country of type locality: Mexico.
Geographical distribution.NEO.
NEO: Mexico.
Notes. Our species concept is based on Austin and Dangerfield (1989) and Fernandez-Triana et al. (2014e).
Apantelesdepressariae Muesebeck, 1931
Apantelesdepressariae Muesebeck, 1931.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (NS, ON, QC), USA (IA, ME, MA, VT).
Apantelesderivatus Long, 2010
Apantelesderivatus Long, 2010.
Type information. Holotype female, IEBR (not examined but original description checked). Country of type locality: Vietnam.
Geographical distribution.OTL.
OTL: Vietnam.
Apantelesdesantisi Blanchard, 1947
Apantelesdesantisi Blanchard, 1947.
Type information. Holotype female, MACN (not examined but original description checked). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina.
Apantelesdespectus Nixon, 1965
Apantelesdespectus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Thailand.
Geographical distribution.OTL.
OTL: Thailand.
Apantelesdiatraeae Muesebeck, 1921
Apantelesdiatraeae Muesebeck, 1921.
Type information. Holotype female, USNM (not examined but subsequent treatment of the species checked). Country of type locality: Cuba.
Geographical distribution.NEA, NEO.
NEA: USA (AZ, FL); NEO: Colombia, Cuba, Dominican Republic, Grenada, Guatemala, Haiti, Honduras, Jamaica, Mexico, Nicaragua, Panama, Puerto Rico, Trinidad & Tobago, Venezuela.
Notes. Our species concept is based on Austin and Dangerfield (1989) and Fernandez-Triana et al. (2014e). Yu et al. (2016) recorded France as a country for the species, based on one reference (Paddock 1933). However, we have read that paper, and there is no mention of A.diatraea there. Because we have not found any other source citing or supporting the presence of this species for France, we consider that record to be incorrect. Other country records (for introductions of diatraea) can be found in Bartlett et al. (1978).
Apantelesdickyui Fernandez-Triana, 2014
Apantelesdickyui Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesdictys Nixon, 1965
Apantelesdictys Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Indonesia.
Geographical distribution.OTL.
OTL: Indonesia.
Apantelesdidiguadamuzi Fernandez-Triana, 2014
Apantelesdidiguadamuzi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesdido Nixon, 1965
Apantelesdido Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Apantelesdiegoalpizari Fernandez-Triana, 2014
Apantelesdiegoalpizari Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesdiegotorresi Fernandez-Triana, 2014
Apantelesdiegotorresi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesdiniamartinezae Fernandez-Triana, 2014
Apantelesdiniamartinezae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesdiocles Nixon, 1965
Apantelesdiocles Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: China (HN), India, Indonesia, Philippines, Vietnam.
Apantelesdiourbeli Risbec, 1951
Apantelesdiourbeli Risbec, 1951.
Type information. Holotype male, depository unknown (not examined but original description checked). Country of type locality: Senegal.
Geographical distribution.AFR.
AFR: Senegal.
Apantelesdissimilis Nixon, 1965
Apantelesdissimile Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL, PAL.
OTL: China (FJ, HB), Philippines, Vietnam; PAL: China (JL).
Notes. Both versions of Taxapad (Yu et al. 2012, 2016) correctly spell the name of this species as Apantelesdissimilis. However, the original description of the species (Nixon 1965) and most of the subsequent, published references (e.g., Shenefelt 1972, Long et al. 2004, Chen and Song 2004) incorrectly refer to the species as Apantelesdissimile, which is the neuter rather than the masculine form of the adjective, and therefore violates ICZN Article 31.2. The Code-compliant spelling must be dissimilis, regardless of the original spelling (Doug Yanega, pers. comm.), and it is the one we follow here. The specimen is in poor condition, with detached metasoma and one pair of wings glued to a second card (underneath the point with the specimen). Because the wings were detached, the vannal lobe was torn and its shape and setation patterns can no longer be determined. But the punctures on the posterior margin of the anteromesoscutum are fused, thus we consider this species to belong to Apanteles.
Apantelesdores Nixon, 1965
Apantelesdores Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Malaysia.
Geographical distribution.OTL.
OTL: Malaysia, Vietnam.
Apantelesdotus Nixon, 1965
Apantelesdotus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Sri Lanka.
Geographical distribution.OTL.
OTL: Philippines, Sri Lanka, Vietnam.
Apantelesdrupes Nixon, 1965
Apantelesdrupes Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Apantelesdumosus Liu & Chen, 2014
Apantelesdumosus Liu & Chen, 2014.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (JL, LN).
Apantelesduniagarciae Fernandez-Triana, 2014
Apantelesduniagarciae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesduplicatus Brèthes, 1922
Apantelesduplicatus Brèthes, 1922.
Type information. Holotype female, MACN (not examined). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina.
Apantelesduvalierbricenoi Fernandez-Triana, 2014
Apantelesduvalierbricenoi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesedgarjimenezi Fernandez-Triana, 2014
Apantelesedgarjimenezi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesedithlopezae Fernandez-Triana, 2014
Apantelesedithlopezae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apanteleseduardoramirezi Fernandez-Triana, 2014
Apanteleseduardoramirezi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesedwardsii Riley, 1889
Apantelesedwardsii Riley, 1889.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (ON, QC), USA (CT, IL, IA, ME, MA, MI, MN, NY, OH).
Apantelesedwinapuii Fernandez-Triana, 2014
Apantelesedwinapuii Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apanteleselagabalus Nixon, 1965
Apanteleselagabalus Nixon, 1965.
Type information. Holotype female, AEIC (not examined but original description checked). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Apanteleseldarayae Fernandez-Triana, 2014
Apanteleseldarayae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apanteleseliethcantillanoae Fernandez-Triana, 2014
Apanteleseliethcantillanoae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesepiblemae Muesebeck, 1935
Apantelesepiblemae Muesebeck, 1935.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CA, DE, FL, GA, KS).
Apantelesepijarbi Rao, 1953
Apantelesepijarbi Rao, 1953.
Type information. Holotype female, SJCA (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Apantelesepinotiae Viereck, 1912
Apantelesepinotiae Viereck, 1912.
Type information. Holotype male, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (ON), USA (CT, FL, IL, KS, KY, ME, MD, MA, MO, NE, NJ, NY, OH, OK, PA, SC, TX, VA, WV).
Apanteleserickduartei Fernandez-Triana, 2014
Apanteleserickduartei Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apanteleseriphyle Nixon, 1965
Apanteleseriphyle Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Apanteleserse Nixon, 1965
Apanteleserse Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Indonesia.
Geographical distribution.OTL.
OTL: Indonesia, Malaysia.
Apantelesesthercentenoae Fernandez-Triana, 2014
Apantelesesthercentenoae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apanteleseublemmae Nixon, 1965
Apanteleseublemmae Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Tanzania.
Geographical distribution.AFR.
AFR: Kenya, South Africa, Tanzania.
Apanteleseugeniaphilipsae Fernandez-Triana, 2014
Apanteleseugeniaphilipsae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apanteleseulogiosequeirai Fernandez-Triana, 2014
Apanteleseulogiosequeirai Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apanteleseupolis Nixon, 1965
Apanteleseupolis Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Apanteleseurynome Nixon, 1965
Apanteleseurynome Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Fiji.
Geographical distribution.AUS.
AUS: Fiji.
Apanteleseurytergis de Saeger, 1941
Apanteleseurytergis de Saeger, 1941.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Cape Verde, Democratic Republic of Congo.
Apantelesevadnix Shenefelt, 1972
Apantelesevadnix Shenefelt, 1972.
Apantelesevadne Nixon, 1965 [primary junior homonym of Apantelesevadne Nixon, 1955].
Type information. Holotype female, NHMUK (examined). Country of type locality: Uganda.
Geographical distribution.AFR.
AFR: Uganda.
Apantelesevanidus Papp, 1975
Apantelesevanidus Papp, 1975.
Apantelescalpurnia Nixon, 1976.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Hungary.
Geographical distribution.PAL.
PAL: Finland, Hungary, Moldova, Russia (S), Sweden, Turkey, Ukraine.
Apantelesevansi Nixon, 1971
Apantelesevansi Nixon, 1971.
Type information. Holotype female, NHMUK (examined). Country of type locality: Kenya.
Geographical distribution.AFR.
AFR: Cape Verde, Kenya.
Apantelesfaustina Nixon, 1965
Apantelesfaustina Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Mauritius.
Geographical distribution.AFR.
AFR: Mauritius.
Apantelesfedericomatarritai Fernandez-Triana, 2014
Apantelesfedericomatarritai Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesfelipechavarriai Fernandez-Triana, 2014
Apantelesfelipechavarriai Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesfelixcarmonai Fernandez-Triana, 2014
Apantelesfelixcarmonai Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesfeltiae Viereck, 1912
Apantelesfeltiae Viereck, 1912.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (SK), USA (AZ, CA, ID, IN, IA, MI, OH, SD, UT).
Apantelesfernandochavarriai Fernandez-Triana, 2014
Apantelesfernandochavarriai Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesfirmus Telenga, 1949
Apantelesfirmus Telenga, 1949.
Apantelesfirmusrufipes Telenga, 1955.
Type information. Holotype and depository unknown not examined but authoritatively identified specimens examined). Country of type locality: Tajikistan.
Geographical distribution.PAL.
PAL: Armenia, Azerbaijan, France, Hungary, Kazakhstan, Korea, Mongolia, Romania, Russia (YAR), Tajikistan, Ukraine, Yugoslavia.
Notes. We have examined a female paratype, donated to the CNC. That specimen looks like Dolichogenidea (the vannal lobes in both hind wings are broken but they appear to be setose, although it is not entirely clear). The descriptions and comments by Nixon (1973) and Papp (1984a) also suggest this species could be placed in Dolichogenidea. However, without examining more specimens we refrain to transfer the species in this paper and prefer to maintain it in Apanteles for the time being.
Apantelesflavicapus Liu & Chen, 2014
Apantelesflavicapus Liu & Chen, 2014.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (GD).
Apantelesflavicentrus Long, 2010
Apantelesflavicentrus Long, 2010.
Type information. Holotype female, IEBR (not examined but original description checked). Country of type locality: Vietnam.
Geographical distribution.OTL.
OTL: Vietnam.
Notes. This species might not be Apanteles, but the original description does not provide enough details to determine its placement, so we retain it within Apanteles.
Apantelesflavigaster Long, 2010
Apantelesflavigaster Long, 2010.
Type information. Holotype female, IEBR (not examined but original description checked). Country of type locality: Vietnam.
Geographical distribution.OTL.
OTL: Vietnam.
Notes. This species might not be Apanteles, but the original description does not provide enough details to determine its placement, so we retain it within Apanteles.
Apantelesfloralis Tobias, 1966
Apantelesfloralis Tobias, 1966.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Turkmenistan.
Geographical distribution.PAL.
PAL: Kazakhstan, Mongolia, Turkmenistan.
Notes. Our species concept is based on Papp (1984a) and Tobias (1986).
Apantelesflormoralesae Fernandez-Triana, 2014
Apantelesflormoralesae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesflorus Nixon, 1965
Apantelesflorus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (GD, HN).
Apantelesfluitantis de Santis, 1980
Apantelesfluitantis de Santis, 1980.
Type information. Holotype female, MLP (not examined but subsequent treatment of the species checked). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina.
Notes. Our concept of this species is based on Aquino et al. (2010).
Apantelesfontinalis de Saeger, 1944
Apantelesfontinalis de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Rwanda.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Réunion, Rwanda.
Apantelesforbesi Viereck, 1910
Apantelesforbesi Viereck, 1910.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (MB, NS, ON), USA (AZ, CT, FL, IL, IN, IA, KS, KY, MD, MA, MO, NY, OR, SD).
Apantelesfranciscopizarroi Fernandez-Triana, 2014
Apantelesfranciscopizarroi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesfranciscoramirezi Fernandez-Triana, 2014
Apantelesfranciscoramirezi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesfreddyquesadai Fernandez-Triana, 2014
Apantelesfreddyquesadai Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesfreddysalazari Fernandez-Triana, 2014
Apantelesfreddysalazari Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesfredi Austin & Dangerfield, 1989
Apantelesfredi Austin & Dangerfield, 1989.
Type information. Holotype female, NHMUK (not examined but original description checked). Country of type locality: Guatemala.
Geographical distribution.NEO.
NEO: Guatemala.
Apantelesfrersi (Brèthes, 1917)
Coelothoraxfrersi Brèthes, 1917.
Type information. Holotype female, MACN (not examined). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina.
Apantelesfumiferanae Viereck, 1912
Apantelesfumiferanae Viereck, 1912.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA, PAL.
NEA: Canada (BC, MB, NB, NL, NT, ON, QC), USA (AK, CO, ID, ME, MA, MI, MN, MT, NM, NY, OR, SC, SD, WA, WI); PAL: Poland.
Notes. We consider A.fumiferanae as a Nearctic species (Fernandez-Triana 2010, Fernandez-Triana and Huber 2010). The single record from the Palearctic is based on one publication compiling the Hymenoptera from Poland (Huflejt 1997), and it is very likely to be incorrect; however, we refrain to remove that record until more studies are done. The generic placement of this species is also somewhat controversial as the female holotype has the hind wings with a straight vannal lobe with small setae which are just slightly sparser than proximal and distal margins of lobe, and the (shallow and sparse) punctures on the anteromesoscutum are not fused near posterior margin. These two features are borderline with Dolichogenidea and more studies, combining morphology, biology, and molecular data, will be needed.
Apantelesfundulus Nixon, 1965
Apantelesfundulus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Australia.
Geographical distribution.AUS, OTL.
AUS: Australia (QLD); OTL: Vietnam.
Apantelesgabrielagutierrezae Fernandez-Triana, 2014
Apantelesgabrielagutierrezae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesgalatea Nixon, 1965
Apantelesgalatea Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Apantelesgalleriae Wilkinson, 1932
Apantelesgalleriae Wilkinson, 1932.
Type information. Holotype female, NHMUK (examined). Country of type locality: France.
Geographical distribution.AFR, NEA, NEO, OTL, PAL.
AUS: Hawaiian Islands, New Zealand; AFR: Mauritius, Réunion; NEA: Canada (BC), USA (GA, NC, OH, SC); NEO: Argentina, Brazil (SP); OTL: China (GZ, HN, TW, ZJ), India, Pakistan; PAL: Armenia, Bulgaria, France, Greece, Hungary, Iran, Italy, Japan, Malta, Romania, Russia (PRI), Spain, Turkey, United Kingdom.
Notes. Distribution in Brazil based on de Santis (1964) and Shimbori (pers. comm.).
Apantelesgandoensis de Saeger, 1944
Apantelesgandoensis de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Rwanda.
Geographical distribution.AFR.
AFR: Rwanda.
Apantelesgarygibsoni Fernandez-Triana, 2014
Apantelesgarygibsoni Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesgaytotini Blanchard, 1959
Apantelesgaytotini Blanchard, 1959.
Type information. Holotype female, MACN (not examined). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina.
Apantelesgerardobandoi Fernandez-Triana, 2014
Apantelesgerardobandoi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesgerardosandovali Fernandez-Triana, 2014
Apantelesgerardosandovali Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesghesquierei de Saeger, 1941
Apantelesghesquierei de Saeger, 1941.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Senegal.
Apantelesgialamensis Long, 2007
Apantelesgialamensis Long, 2007.
Type information. Holotype female, IEBR (not examined but original description checked). Country of type locality: Vietnam.
Geographical distribution.OTL.
OTL: Vietnam.
Apantelesgitebe de Saeger, 1944
Apantelesgitebe de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Apantelesgladysrojasae Fernandez-Triana, 2014
Apantelesgladysrojasae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesglenriverai Fernandez-Triana, 2014
Apantelesglenriverai Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesgloriasihezarae Fernandez-Triana, 2014
Apantelesgloriasihezarae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesgoron Nixon, 1965
Apantelesgoron Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Malaysia.
Geographical distribution.OTL.
OTL: Malaysia.
Apantelesgracilicorne Song & Chen, 2004
Apantelesgracilicorne Song & Chen, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Apantelesgracilipes Song & Chen, 2004
Apantelesgracilipes Song & Chen, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, GD, HI, HB, YN).
Apantelesguadaluperodriguezae Fernandez-Triana, 2014
Apantelesguadaluperodriguezae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesguamensis (Holmgren, 1868)
Microgasterguamensis Holmgren, 1868.
Type information. Type and depository unknown (not examined but authoritatively identified specimens examined). Country of type locality: Guam.
Geographical distribution.AUS.
AUS: Guam.
Notes. The last two versions of Taxapad (Yu et al. 2012, 2016) have this species listed as Microgaster. However, we examined a female homotype in the CNC, previously studied by William Mason, and the species clearly belongs in Apanteles, which agrees with Shenefelt (1972) who had also transferred the species to that genus. For clarity we revise the combination of this species here. The type(s) details and depository are presently unknown but Shenefelt (1972: 527) recorded the female sex as part of the original description, although without elaborating.
Apantelesguillermopereirai Fernandez-Triana, 2014
Apantelesguillermopereirai Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apanteleshainanensis Liu & Chen, 2015
Apanteleshainanensis Liu & Chen, 2015.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (HI).
Apanteleshalfordi Ullyett, 1946
Apanteleshalfordi Ullyett, 1946.
Apanteleseriophyes Nixon, 1965.
Type information. Holotype female, TMSA (not examined but original description checked). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Notes. We examined the type of Apanteleseriophyes Nixon, 1965.
Apanteleshapaliae de Saeger, 1941
Apanteleshapaliae de Saeger, 1941.
Type information. Holotype male, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Apantelesharryramirezi Fernandez-Triana, 2014
Apantelesharryramirezi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesharti Viereck, 1910
Apantelesharti Viereck, 1910.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (ON), USA (CT, DC, IL, IA, KS, MD, MI, MO, NJ, OH, TN).
Apanteleshatinhensis Long, 2010
Apanteleshatinhensis Long, 2010.
Type information. Holotype female, IEBR (not examined but original description checked). Country of type locality: Vietnam.
Geographical distribution.OTL.
OTL: Vietnam.
Apanteleshaywardi Blanchard, 1947
Apanteleshaywardi Blanchard, 1947.
Type information. Holotype female, MACN (not examined but original description checked). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina, Brazil (SP).
Apanteleshazelcambroneroae Fernandez-Triana, 2014
Apanteleshazelcambroneroae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apanteleshebrus Nixon, 1965
Apanteleshebrus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Apanteleshectorsolisi Fernandez-Triana, 2014
Apanteleshectorsolisi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apanteleshedwigi Shenefelt, 1972
Apanteleshedwigi Shenefelt, 1972.
Apantelesareolaris Hedwig, 1961 [primary junior homonym of Apantelesareolaris Blanchard, 1947].
Type information. Holotype female, LNKD (not examined but original description checked). Country of type locality: Afghanistan.
Geographical distribution.PAL.
PAL: Afghanistan.
Notes. The original description alone is not sufficient to unambiguously establish the generic placement for this species (it could be Apanteles, Dolichogenidea, Pholetesor, or perhaps even another genus). Until study of the only known specimen is done, we retain the species under Apanteles.
Apantelesheichinensis Sonan, 1942
Apantelesheichinensis Sonan, 1942.
Type information. Holotype male, TARI (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (HN, TW, ZJ); PAL: China (AH).
Notes. For the generic placement of this species we follow Chen and Song (2004).
Apanteleshellulae Risbec, 1951
Apanteleshellulae Risbec, 1951.
Apanteleshellulaecrocidolomiae Risbec, 1951 [primary junior homonym of Apantelescrocidolomiae Ahmad, 1945].
Type information. Syntypes female and male, depository unknown (not examined but original description checked). Country of type locality: Senegal.
Geographical distribution.AFR.
AFR: Senegal.
Apanteleshemara Nixon, 1965
Apanteleshemara Nixon, 1965.
Apantelescaboverdensis Hedqvist, 1965.
Apantelesproalastor Hedqvist, 1965.
Apantelesbulgaricus Balevski & Tobias, 1980.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.AUS, AFR, OTL, PAL.
AUS: Australia (ACT); AFR: Cape Verde, Egypt, Kenya, Madagascar, Mauritius, Republic of the Congo, Senegal, South Africa, Yemen; OTL: China (HN), India, Pakistan, Vietnam; PAL: Bulgaria, Canary Islands, Cyprus, France, Greece, Iran, Israel, Italy, Madeira Islands, Oman, Russia (PRI), Spain, Saudi Arabia, Turkey, United Arab Emirates, Yugoslavia.
Notes. In Fernandez-Triana et al. (2017a: 3) this species is incorrectly listed as occurring in the Democratic Republic of Congo, when that record was actually from the Republic of Congo. Additional country distributions are also reported here, based on collections and DNA barcoding.
Apanteleshemiaurantius van Achterberg & Ng, 2009
Apanteleshemiaurantius van Achterberg & Ng, 2009.
Type information. Holotype female, UKM (not examined but original description checked). Country of type locality: Malaysia.
Geographical distribution.OTL.
OTL: Malaysia.
Apanteleshersilia Nixon, 1965
Apanteleshersilia Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Apantelesholmgreni Shenefelt, 1972
Apantelesholmgreni Shenefelt, 1972.
Microgastercarbonarius Holmgren, 1868 [primary junior homonym of Microgastercarbonarius Wesmael, 1837].
Type information. Holotype female, NHRS (not examined but subsequent treatment of the species checked). Country of type locality: Mauritius.
Geographical distribution.AFR.
AFR: Mauritius.
Notes. Our species concept is based on the comments that Wilkinson (1932a: 323) made on this species. However, examination of the type will be needed in the future to corroborate its generic placement.
Apanteleshoraeus Kotenko, 1986
Apanteleshoraeus Kotenko, 1986.
Type information. Holotype female, SIZK (not examined but original description checked). Country of type locality: Ukraine.
Geographical distribution.PAL.
PAL: Russia (S), Ukraine.
Apanteleshuberi Fernandez-Triana, 2010
Apanteleshuberi Fernandez-Triana, 2010.
Type information. Holotype female, CNC (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (BC).
Apanteleshumbertolopezi Fernandez-Triana, 2014
Apanteleshumbertolopezi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apanteleshyalinatus Granger, 1949
Apanteleshyalinatus Granger, 1949.
Type information. Syntypes female and male, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Apanteleshymeniae Wilkinson, 1935
Apanteleshymeniae Wilkinson, 1935.
Type information. Holotype female, NHMUK (examined). Country of type locality: Fiji.
Geographical distribution.AUS, OTL.
AUS: Fiji, Vietnam; OTL: Vietnam.
Apantelesicarti Blanchard, 1960
Apantelesicarti Blanchard, 1960.
Apantelesicartae de Santis, 1967 [unjustified emendation].
Type information. Holotype female, DPBA (not examined). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina.
Apantelesimitandus Muesebeck, 1954
Apantelesimitandus Muesebeck, 1954.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (SP).
Apantelesimpiger Muesebeck, 1958
Apantelesimpiger Muesebeck, 1958.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: Puerto Rico.
Geographical distribution.NEO.
NEO: Cuba, Puerto Rico.
Apantelesimportunus Wilkinson, 1928
Apantelesimportunus Wilkinson, 1928.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: China (GX), India.
Apantelesimpunctatus Muesebeck, 1933
Apantelesimpunctatus Muesebeck, 1933.
Type information. Holotype female, USNM (not examined but subsequent treatment of the species checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (LA).
Notes. Our species concept is based on Austin and Dangerfield (1989).
Apantelesinaron Nixon, 1965
Apantelesinaron Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Apantelesincurvus Liu & Chen, 2014
Apantelesincurvus Liu & Chen, 2014.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (NX).
Apantelesinesolisae Fernandez-Triana, 2014
Apantelesinesolisae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesinops Nixon, 1965
Apantelesinops Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Apantelesinsignicaudatus Granger, 1949
Apantelesinsignicaudatus Granger, 1949.
Type information. Syntypes female and male, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Apantelesinsularis Muesebeck, 1921
Apantelesinsularis Muesebeck, 1921.
Urogastergrenadensis Ashmead, 1900 [secondary homonym of Cotesiagrenadensis Ashmead, 1900].
Type information. Holotype female, NHMUK (examined). Country of type locality: Grenada.
Geographical distribution.NEO.
NEO: Grenada, Saint Vincent.
Notes. We also examined the type of Urogastergrenadensis in the NHMUK.
Apantelesinunctus Nixon, 1965
Apantelesinunctus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Malaysia.
Geographical distribution.OTL.
OTL: Malaysia.
Apantelesione Nixon, 1965
Apantelesione Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Apantelesippeus Nixon, 1965
Apantelesippeus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Australia.
Geographical distribution.AUS, OTL.
AUS: Australia (ACT, NSW, QLD); OTL: Vietnam.
Apantelesirenecarrilloae Fernandez-Triana, 2014, name amended
Apantelesirenecarrilloi Fernandez-Triana, 2014 [incorrect original spelling].
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Notes. The original spelling of the species Apantelesirenecarrilloi is incorrect, as the species was named after Irene Carrillo, a woman, and thus its ending should be -ae instead of -i. The correct spelling is here amended to Apantelesirenecarrilloae.
Apantelesisaacbermudezi Fernandez-Triana, 2014
Apantelesisaacbermudezi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesisander Nixon, 1965
Apantelesisander Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR, OTL.
AFR: South Africa; OTL: Vietnam.
Apantelesisidrochaconi Fernandez-Triana, 2014
Apantelesisidrochaconi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesisidrovillegasi Fernandez-Triana, 2014
Apantelesisidrovillegasi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesivondroensis Granger, 1949
Apantelesivondroensis Granger, 1949.
Type information. Holotype female, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Apantelesivonnetranae Fernandez-Triana, 2014
Apantelesivonnetranae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesjairomoyai Fernandez-Triana, 2014
Apantelesjairomoyai Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesjaviercontrerasi Fernandez-Triana, 2014
Apantelesjaviercontrerasi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesjavierobandoi Fernandez-Triana, 2014
Apantelesjavierobandoi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesjaviersihezari Fernandez-Triana, 2014
Apantelesjaviersihezari Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesjenniferae Fernandez-Triana, 2010
Apantelesjenniferae Fernandez-Triana, 2010.
Type information. Holotype female, CNC (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (NB, ON, QC).
Apantelesjesusbrenesi Fernandez-Triana, 2014
Apantelesjesusbrenesi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesjesusugaldei Fernandez-Triana, 2014
Apantelesjesusugaldei Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesjimmychevezi Fernandez-Triana, 2014
Apantelesjimmychevezi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesjohanvargasi Fernandez-Triana, 2014
Apantelesjohanvargasi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesjorgecortesi Fernandez-Triana, 2014
Apantelesjorgecortesi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesjorgehernandezi Fernandez-Triana, 2014
Apantelesjorgehernandezi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesjosecalvoi Fernandez-Triana, 2014
Apantelesjosecalvoi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesjosecortezi Fernandez-Triana, 2014
Apantelesjosecortezi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesjosediazi Fernandez-Triana, 2014
Apantelesjosediazi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesjosejaramilloi Fernandez-Triana, 2014
Apantelesjosejaramilloi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesjosemonteroi Fernandez-Triana, 2014
Apantelesjosemonteroi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesjoseperezi Fernandez-Triana, 2014
Apantelesjoseperezi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesjoserasi Fernandez-Triana, 2014
Apantelesjoserasi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesjuanapuii Fernandez-Triana, 2014
Apantelesjuanapuii Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesjuancarrilloi Fernandez-Triana, 2014
Apantelesjuancarrilloi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesjuangazoi Fernandez-Triana, 2014
Apantelesjuangazoi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesjuanhernandezi Fernandez-Triana, 2014
Apantelesjuanhernandezi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesjuanlopezi Fernandez-Triana, 2014
Apantelesjuanlopezi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesjuanmatai Fernandez-Triana, 2014
Apantelesjuanmatai Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesjuanvictori Fernandez-Triana, 2014
Apantelesjuanvictori Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesjubmeli Hedqvist, 1972
Apantelesjubmeli Hedqvist, 1972.
Type information. Holotype female, NHRS (examined). Country of type locality: Sweden.
Geographical distribution.PAL.
PAL: Sweden.
Apantelesjuliodiazi Fernandez-Triana, 2014
Apantelesjuliodiazi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesjuniorlopezi Fernandez-Triana, 2014
Apantelesjuniorlopezi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apanteleskeineraragoni Fernandez-Triana, 2014
Apanteleskeineraragoni Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apanteleskivuensis de Saeger, 1941
Apanteleskivuensis de Saeger, 1941.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Apanteleskubensis Abdinbekova, 1969
Apanteleskubensis Abdinbekova, 1969.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Azerbaijan.
Geographical distribution.PAL.
PAL: Azerbaijan, Hungary, Korea, Moldova, Mongolia, Russia (NC, S), Turkey.
Notes. Our species concept is based on Papp (1980a) and Tobias (1986).
Apanteleslacteus (Nees, 1834)
Microgasterlacteus Nees, 1834.
Type information. Holotype female, lost (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Armenia, Azerbaijan, Finland, Germany, Greece, Iran, Israel, Italy, Kazakhstan, Moldova, Poland, Romania, Russia (ORE, ROS, RYA, TAM), Slovakia, Sweden, Tajikistan, Tunisia, Turkey, Ukraine, United Kingdom, Uzbekistan.
Notes. This species was first transferred to Dolichogenidea (as D.lacteus) by Halperin (1986), following Papp’s identification of those specimens. Since then it has been variously treated as Apanteles or Dolichogenidea (e.g., Papp 1988, Shaw 2012b, Belokobylskij et al. 2003, Yu et al. 2012, 2016, Liu et al. 2015, Broad et al. 2016). For the sake of clarity, we revise the combination of this species here. The specimens we have examined all have a strongly concave hind wing vannal lobe, being clearly Apanteles.
Apanteleslaevicoxis Muesebeck, 1921
Apanteleslaevicoxis Muesebeck, 1921.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (MS).
Apanteleslanassa Nixon, 1965
Apanteleslanassa Nixon, 1965.
Type information. Holotype female, NHMUK (examined). County of type locality: Malaysia.
Geographical distribution.OTL.
OTL: Malaysia.
Apanteleslangenburgensis Szépligeti, 1911
Apanteleslangenburgensis Szépligeti, 1911.
Type information. Syntypes female and male, ZMHB (not examined but subsequent treatment of the species checked). Country of type locality: Tanzania.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Ivory Coast, Malawi, Rwanda, Senegal, Tanzania.
Notes. Information about type specimens taken from Shenefelt (1972). Our concept of this species is based on Wilkinson (1932a), de Saeger (1944) and Risbec (1951).
Apanteleslaricellae Mason, 1959
Apanteleslaricellae Mason, 1959.
Type information. Holotype female, CNC (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (NB, ON, QC), USA (WI).
Apanteleslatericarinatus Song & Chen, 2001
Apanteleslatericarinatus Song & Chen, 2001.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, YN).
Apanteleslatisulca Chen & Song, 2004
Apanteleslatisulca Chen & Song, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Apanteleslaurahuberae Fernandez-Triana, 2014
Apanteleslaurahuberae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apanteleslaurenmoralesae Fernandez-Triana, 2014
Apanteleslaurenmoralesae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apanteleslavignei Blanchard, 1959
Apanteleslavignei Blanchard, 1959.
Type information. Holotype female, MACN (not examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (BA).
Notes. The holotype was part of the Blanchard collection, which we assume is now deposited in the MACN.
Apanteleslaxus de Saeger, 1944
Apanteleslaxus de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Apanteleslectus Tobias, 1964
Apanteleslectus Tobias, 1964.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Kazakhstan.
Geographical distribution.PAL.
PAL: Kazakhstan, Lithuania, Macedonia, Mongolia, Russia (C, S), Yugoslavia.
Notes. Our species concept is based on Nixon (1976).
Apanteleslenea Nixon, 1976
Apanteleslenea Nixon, 1976.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Austria, Bulgaria, Czech Republic, France, Germany, Hungary, Ireland, Italy, Korea, Romania, Russia (ZAB, PRI, SAK), Serbia, Slovakia, Spain, Sweden, Switzerland, Turkey, United Kingdom.
Apantelesleninguadamuzi Fernandez-Triana, 2014
Apantelesleninguadamuzi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesleonelgarayi Fernandez-Triana, 2014
Apantelesleonelgarayi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesleptothecus (Cameron, 1907)
Pseudapantelesleptothecus Cameron, 1907.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. The holotype is in very poor condition, missing the entire metasoma, the head badly smashed, and with the micropin (which was pinned through the mesosoma) corroding. However, the propodeum is clearly visible, as well as the vannal lobe of one hind wing. Based on that, it is still possible to corroborate the placement of this species within Apanteles, although probably the type would be mostly useless for a better characterization of the species.
Apantelesleptoura Cameron, 1909
Apantelesleptoura Cameron, 1909.
Type information. Holotype female, NHMUK (examined). Country of type locality: Sri Lanka.
Geographical distribution.OTL.
OTL: China (HB, HN), Malaysia, Sri Lanka.
Apantelesleucochiloneae Cameron, 1911
Apantelesleucochiloneae Cameron, 1911.
Type information. Syntypes female and male, NHMUK (examined). Country of type locality: Guyana.
Geographical distribution.NEO.
NEO: Guyana.
Notes.Yu et al. (2016) recorded the type as being female; however, we examined one female and one male specimens, both glued on the same card that has a type label, and thus are to be considered as syntypes, as correctly implied by Shenefelt (1972: 553).
Apantelesleucopus (Ashmead, 1900)
Urogasterleucopus Ashmead, 1900.
Type information. Holotype female, NHMUK (examined). Country of type locality: Grenada.
Geographical distribution.NEO.
NEO: Grenada, Saint Vincent.
Apantelesleucostigmus (Ashmead, 1900)
Urogasterleucostigmus Ashmead, 1900.
Type information. Holotype female, NHMUK (examined). Country of type locality: Saint Vincent.
Geographical distribution.NEO, NEA.
NEA: USA (FL); NEO: Cuba, Grenada, Puerto Rico, Saint Vincent.
Apanteleslilliammenae Fernandez-Triana, 2014
Apanteleslilliammenae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apanteleslineodos Cameron, 1911
Apanteleslineodos Cameron, 1911.
Type information. Holotype male, NHMUK (examined). Country of type locality: Guyana.
Geographical distribution.NEO.
NEO: Guyana.
Notes. Based on the carination and sculpture pattern of the propodeum and fore wing venation, this species belongs to the leucostigmus group (sensuFernandez-Triana et al. 2014e).
Apanteleslinus Nixon, 1965
Apanteleslinus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Apantelesliopleuris Szépligeti, 1914
Apantelesliopleuris Szépligeti, 1914.
Type information. Holotype male, MNHN (not examined but original description checked). Country of type locality: Tanzania.
Geographical distribution.AFR.
AFR: Tanzania.
Notes. Only known from the male holotype (Wilkinson 1932a: 324). Without examining the type, it is not possible to conclude on the generic placement of this species.
Apanteleslisabearssae Fernandez-Triana, 2014
Apanteleslisabearssae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apanteleslongiantenna Chen & Song, 2004
Apanteleslongiantenna Chen & Song, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Apanteleslongicaudatus You & Zhou, 1991
Apanteleslongicaudatus You & Zhou, 1991.
Type information. Holotype female, HUNAU (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, JX, ZJ).
Notes. The depository acronym was chosen from the English website of the Hunan Agricultural College (now Hunan Agricultural University). Our species concept is based on Liu et al. (2014).
Apanteleslongirostris Chen & Song, 2004
Apanteleslongirostris Chen & Song, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, YN).
Apanteleslongistylus de Saeger, 1944
Apanteleslongistylus de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Rwanda.
Notes. Based on the illustration of the ovipositor and ovipositor sheaths (de Saeger 1944), this could be either an Apanteles or Dolichogenidea. Until the vannal lobe of specimens are examined, it is not possible to conclude, thus we retain the species in the genus in which it was originally described.
Apanteleslongitergiae Rao & Kurian, 1950
Apanteleslongitergiae Rao & Kurian, 1950.
Type information. Holotype female, NZSI (not examined but subsequent treatment of the species checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. Our species concept is based on Rao and Kurian (1950), Rao (1961), and Rao and Chalikwar (1971). The original description mentions “ovipositor sheaths short, exserted” which may suggest this species belongs to Parapanteles; however, because no other details are clear to conclude, we prefer to retain the species in Apanteles until specimens can be examined.
Apantelesluciariosae Fernandez-Triana, 2014
Apantelesluciariosae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesluisbrizuelai Fernandez-Triana, 2014
Apantelesluisbrizuelai Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesluiscanalesi Fernandez-Triana, 2014
Apantelesluiscanalesi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesluiscantillanoi Fernandez-Triana, 2014
Apantelesluiscantillanoi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesluisgarciai Fernandez-Triana, 2014
Apantelesluisgarciai Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesluisgaritai Fernandez-Triana, 2014
Apantelesluisgaritai Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesluishernandezi Fernandez-Triana, 2014
Apantelesluishernandezi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesluislopezi Fernandez-Triana, 2014
Apantelesluislopezi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesluisvargasi Fernandez-Triana, 2014
Apantelesluisvargasi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apanteleslunata Song & Chen, 2004
Apanteleslunata Song & Chen, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (FJ, HB); PAL: China (JL).
Apantelesluteocinctus de Saeger, 1941
Apantelesluteocinctus de Saeger, 1941.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Rwanda.
Apantelesluzmariaromeroae Fernandez-Triana, 2014
Apantelesluzmariaromeroae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apanteleslycidas Nixon, 1965
Apanteleslycidas Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Apanteleslyridice Nixon, 1965
Apanteleslyridice Nixon, 1965.
Type information. Holotype female, AEIC (not examined but original description checked). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines, Vietnam.
Apantelesmachaeralis Wilkinson, 1928
Apantelesmachaeralis Wilkinson, 1928.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: China (GD), India, Myanmar, Vietnam.
Apantelesmacromphaliae Silva Figueroa, 1917
Apantelesmacromphaliae Silva Figueroa, 1917.
Type information. Syntypes female, MNNC (not examined but subsequent treatment of the species checked). Country of type locality: Chile.
Geographical distribution.NEO.
NEO: Argentina, Chile.
Notes.Shenefelt (1972: 563) stated that the original description of the species mentioned female and male specimens, which would mean that they were syntypes; however, Yu et al. (2016) recorded the type of this species as a female; neither publications specified the depository of the specimens. We have found a local reference in Spanish that clearly states that the species was described based on 13 female syntypes, deposited in the MNNC (Camousseight 1975: 5), and we are following this source here. The generic placement of this species is impossible to define until the original material is examined.
Apantelesmagnioculus Liu & Chen, 2015
Apantelesmagnioculus Liu & Chen, 2015.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (GZ).
Apantelesmalleus Liu & Chen, 2014
Apantelesmalleus Liu & Chen, 2014.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (HE).
Apantelesmamitus Nixon, 1965
Apantelesmamitus Nixon, 1965.
Type information. Holotype female, AEIC (not examined but original description checked). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: China (FJ, JX, TW), India, Philippines, Vietnam.
Apantelesmanuelarayai Fernandez-Triana, 2014
Apantelesmanuelarayai Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesmanuelpereirai Fernandez-Triana, 2014
Apantelesmanuelpereirai Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesmanuelriosi Fernandez-Triana, 2014
Apantelesmanuelriosi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesmanuelzumbadoi Fernandez-Triana, 2014
Apantelesmanuelzumbadoi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesmarcobustosi Fernandez-Triana, 2014
Apantelesmarcobustosi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesmarcogonzalezi Fernandez-Triana, 2014
Apantelesmarcogonzalezi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesmarcovenicioi Fernandez-Triana, 2014
Apantelesmarcovenicioi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesmariachavarriae Fernandez-Triana, 2014
Apantelesmariachavarriae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesmariaguevarae Fernandez-Triana, 2014
Apantelesmariaguevarae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesmarialuisariasae Fernandez-Triana, 2014
Apantelesmarialuisariasae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesmariamendezae Fernandez-Triana, 2014
Apantelesmariamendezae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesmarianopereirai Fernandez-Triana, 2014
Apantelesmarianopereirai Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesmariatorrentesae Fernandez-Triana, 2014
Apantelesmariatorrentesae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesmarisolarroyoae Fernandez-Triana, 2014
Apantelesmarisolarroyoae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesmarisolnavarroae Fernandez-Triana, 2014
Apantelesmarisolnavarroae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesmarvinmendozai Fernandez-Triana, 2014
Apantelesmarvinmendozai Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesmasoni Chen & Song, 2004
Apantelesmasoni Chen & Song, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, HI, YN).
Apantelesmauriciogurdiani Fernandez-Triana, 2014
Apantelesmauriciogurdiani Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesmedioexcavatus Granger, 1949
Apantelesmedioexcavatus Granger, 1949.
Type information. Syntypes female and male, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Apantelesmedioimpressus Granger, 1949
Apantelesmedioimpressus Granger, 1949.
Type information. Syntypes female and male, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Apantelesmedon Nixon, 1965
Apantelesmedon Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Malaysia.
Geographical distribution.OTL.
OTL: Malaysia, Vietnam.
Apantelesmegastidis Muesebeck, 1958
Apantelesmegastidis Muesebeck, 1958.
Type information. Holotype female, USNM (examined). Country of type locality: Trinidad & Tobago.
Geographical distribution.NEO.
NEO: Trinidad & Tobago.
Apantelesmegathymi Riley, 1881
Apantelesmegathymi Riley, 1881.
Type information. Syntypes male, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA, NEO.
NEA: USA (AZ, CA, NC, SC); NEO: Mexico.
Apantelesmehdialii Rao & Chalikwar, 1970
Apantelesmehdialii Rao & Chalikwar, 1970.
Type information. Holotype female, depository unknown (not examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Apantelesmelpomene Nixon, 1965
Apantelesmelpomene Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Malaysia.
Geographical distribution.OTL.
OTL: Malaysia.
Notes. The propodeum sculpture is somewhat atypical for Apanteles, as noted by Nixon (1965); nevertheless, we think at present this is still the best generic placement for the species.
Apantelesmenes Nixon, 1965
Apantelesmenes Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Apantelesmeriones Nixon, 1965
Apantelesmeriones Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Apantelesmetacarpalis (Thomson, 1895)
Microgastermetacarpalis Thomson, 1895.
Type information. Lectotype female, MZLU (not examined but subsequent treatment of the species checked). Country of type locality: Sweden.
Geographical distribution.PAL.
PAL: Azerbaijan, China (SN), Czech Republic, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Korea, Malta, Moldova, Mongolia, Romania, Russia (PRI), Serbia, Spain, Sweden, Tajikistan, Tunisia, United Kingdom, Ukraine, Uzbekistan.
Notes. Our species concept is based on Nixon (1965, 1973) and Liu et al. (2014).
Apantelesmetacarpellatus Blanchard, 1963
Apantelesmetacarpellatus Blanchard, 1963.
Type information. Holotype female, MACN (not examined but original description checked). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina.
Apantelesmetagenes Nixon, 1965
Apantelesmetagenes Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Apantelesmetellus Nixon, 1965
Apantelesmetellus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Apantelesmilenagutierrezae Fernandez-Triana, 2014
Apantelesmilenagutierrezae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesmilleri Mason, 1974
Apantelesmilleri Mason, 1974.
Type information. Holotype female, CNC (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (BC, NB, NT, ON, QC), USA (MT).
Apantelesmimoristae Muesebeck, 1922
Apantelesmimoristae Muesebeck, 1922.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (FL, TX).
Apantelesminatchy Rousse & Gupta, 2013
Apantelesminatchy Rousse & Gupta, 2013.
Type information. Holotype female, MNHN (not examined but original description checked). Country of type locality: Réunion.
Geographical distribution.AFR.
AFR: Réunion.
Apantelesminator Muesebeck, 1957
Apantelesminator Muesebeck, 1957.
Type information. Holotype female, USNM (examined). Country of type locality: Argentina.
Geographical distribution.NEA, NEO.
NEA: USA (TX); NEO: Argentina, Bolivia, Peru.
Apantelesminor Fahringer, 1938
Apantelesminor Fahringer, 1938.
Type information. Holotype male, depository unknown (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (JS).
Notes.Williams (1988: 562) considered Apantelesfalcatusminor Fahringer, 1938, originally described as a subspecies of Sathonfalcatus (Nees, 1834), to be a different species. Williams elevated it to species rank and placed it in Apanteles; however, he also wrote that the Apantelesminor type had “a sparsely setose vannal lobe of the hind wing”. While this might indicate that the species is better placed in Dolichogenidea instead of Apanteles, for the time being we prefer to follow Williams (1988), as we have not been able to study the type of minor.
Apantelesminorcarmonai Fernandez-Triana, 2014
Apantelesminorcarmonai Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesminornavarroi Fernandez-Triana, 2014
Apantelesminornavarroi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesmiramis Nixon, 1976
Apantelesmiramis Nixon, 1976.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Finland, United Kingdom.
Apantelesmohandasi Sumodan & Narendran, 1990
Apantelesmohandasi Sumodan & Narendran, 1990.
Type information. Holotype female, RMNH (not examined but subsequent treatment of the species checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes.van Achterberg and Narendran (1997) transferred the species to Dolichogenidea. Later, Gupta et al. (2011) transferred it back to Apanteles based on the hind wing vannal lobe being concave and without setae.
Apantelesmonicachavarriae Fernandez-Triana, 2014
Apantelesmonicachavarriae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesmontezumae Sánchez, Figueroa & Whitfield, 2015
Apantelesmontezumae Sánchez, Figueroa & Whitfield, 2015.
Type information. Holotype female, IIAF (not examined but original description checked). Country of type locality: Mexico.
Geographical distribution.NEO.
NEO: Mexico.
Notes. The last name of the first author of the paper is Sánchez-García, as spelled on the title page (Sánchez-García et al. 2015: 10). However, for the species description in the Systematics section, only Sánchez was used (Sánchez-García et al. 2015: 11); thus, the authors of the species must be considered to be Sánchez, Figueroa and Whitfield.
Apantelesmorrisi Mason, 1974
Apantelesmorrisi Mason, 1974.
Type information. Holotype female, CNC (examined). Country of type locality: Canada.
Geographical distribution.NEA, PAL.
NEA: Canada (BC, MB, NB, ON, QC), USA (MI, WI) PAL: Germany.
Apantelesmorroensis Nixon, 1955
Apantelesmorroensis Nixon, 1955.
Type information. Holotype female, NHMUK (examined). Country of type locality: Juan Fernández Islands.
Geographical distribution.NEO.
NEO: Juan Fernández Islands.
Notes. Both the original description and references afterwards (e.g., Shenefelt 1972, Yu et al. 2016) refer to the type to be deposited in “the University of Santiago, Chile”. However, we have examined the type which is in NHMUK.
Apantelesmujtabai Bhatnagar, 1950
Apantelesmujtabai Bhatnagar, 1950.
Type information. Holotype male, INPC (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. The original description suggests that this species may not belong to Apanteles, based on the relatively short ovipositor sheaths; however, there are not enough details in the rest of the description to come to a conclusion, so in this paper we retain mujtabai in the genus it was originally described until specimens can be studied. The year of publication of the Bhatnagar paper was until recently commonly cited as 1948 and/or 1950 (e.g., Chen and Song 2004, Yu et al. 2016), probably following Shenefelt (1972) who referred to this paper as “Bhatnagar (1948) 1950”. While the intended year for Volume X, Parts I & II of the Indian Journal of Entomology was 1948, the actual dates of publication were June 1950 (Part I) and October 1950 (Part II), as clearly shown on the cover page of the Volume, which we have checked. Because the dates of publication are the ones to be considered, and for the sake of clarity, we hereby revise the year of description for this species to 1950.
Apantelesmunnarensis Sumodan & Narendran, 1990
Apantelesmunnarensis Sumodan & Narendran, 1990.
Type information. Holotype female, RMNH (not examined but subsequent treatment of the species checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. Our species concept is based on van Achterberg and Narendran (1997).
Apantelesmurcia Nixon, 1965
Apantelesmurcia Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Singapore.
Geographical distribution.OTL.
OTL: Singapore.
Apantelesmuticiculus Liu & Chen, 2014
Apantelesmuticiculus Liu & Chen, 2014.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Apantelesmutilia Nixon, 1965
Apantelesmutilia Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Sudan.
Geographical distribution.AFR.
AFR: Sudan.
Apantelesmycerinus Nixon, 1965
Apantelesmycerinus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR, OTL.
AFR: South Africa; OTL: Vietnam.
Notes. Before 2014, this species was only known from three specimens from South Africa (Nixon 1965: 57). A recent record of this species from Vietnam (Long and van Achterberg 2014) should be considered suspicious because that paper does not claim to be the first record of the species for Vietnam (but no other published reference can be found), the authors did not see the type material of the species, and the geographical distribution of the specimens is disparate.
Apantelesmycetophilus Wilkinson, 1931
Apantelesmycetophilus Wilkinson, 1931.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Apantelesmyrsus Nixon, 1965
Apantelesmyrsus Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Apantelesnamkumensis Gupta, 1957
Apantelesnamkumensis Gupta, 1957.
Type information. Holotype female, FSCA? (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. The original description refers to the Gupta collection, which we assume to be currently deposited in FSCA.
Apantelesnatras Nixon, 1965
Apantelesnatras Nixon, 1965.
Type information. Holotype female, AEIC (not examined but original description checked). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Apantelesnavius Nixon, 1965
Apantelesnavius Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Apantelesnemesis Nixon, 1965
Apantelesnemesis Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Apantelesneotaeniaticornis Yousuf & Ray, 2010
Apantelesneotaeniaticornis Yousuf & Ray, 2010.
Type information. Holotype female, IFRI (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Apantelesnepe Nixon, 1965
Apantelesnepe Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Notes. The holotype (only known specimen) has all the wings glued together, so it is not possible to see the vannal lobe in the hind wings with clarity.
Apantelesnephereus Nixon, 1965
Apantelesnephereus Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Apantelesnephoptericis (Packard, 1864)
Microgasternephoptericis Packard, 1864.
Apantelesephestiae Baker, 1895.
Type information. Holotype sex unknown, MCZ (not examined but subsequent treatment of the species checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (ON), USA (AR, CA, CO, FL, IL, IN, IA, KS, NV, NJ, NY, OH, OR).
Notes. Our species concept is based on Whitfield et al. (2001). Shenefelt (1972: 578) stated that the type consisted of only one fore wing. Thus, it is not possible to determine the sex of the type.
Apantelesnephus Papp, 1974
Apantelesnephus Papp, 1974.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Hungary.
Geographical distribution.PAL.
PAL: Hungary, Russia (PRI), Ukraine.
Apantelesniceppe Nixon, 1965
Apantelesniceppe Nixon, 1965.
Type information. Holotype female, AEIC (not examined but original description checked). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines, Vietnam.
Apantelesnidophilus Whitfield & Cameron, 2001
Apantelesnidophilus Whitfield & Cameron, 2001.
Type information. Holotype female, USNM (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Brazil (AM, SP), Colombia, Ecuador, Peru.
Notes. The holotype is missing the head and part of one of the hind legs.
Apantelesnigrofemoratus Granger, 1949
Apantelesnigrofemoratus Granger, 1949.
Type information. Syntypes female and male, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar, Réunion.
Apantelesninigretorum Viereck, 1917
Apantelesninigretorum Viereck, 1917.
Type information. Type lost (not examined but subsequent treatment of the species checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CT).
Notes. Our species concept is based on Muesebeck (1921).
Apantelesnitidus de Saeger, 1944
Apantelesnitidus de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Rwanda.
Geographical distribution.AFR.
AFR: Rwanda.
Notes.Dolichogenidea cannot be discarded as a potential generic placement for this species; however, until the hind wing vannal lobe of the holotype can be examined, we prefer to retain it in Apanteles.
Apantelesnivellus Nixon, 1965
Apantelesnivellus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Ghana.
Geographical distribution.AFR.
AFR: Ghana.
Apantelesnixoni Song, 2002
Apantelesnixoni Song, 2002.
Apantelesnixoni Song, 2002 [primary junior homonym of Apantelesnixoni Papp, 1971].
Type information. Holotype female, FAFU (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (FJ, HB); PAL: China (JL).
Notes. Our species concept is based on Chen and Song (2004).
Apantelesnoronhai de Santis, 1975
Apantelesnoronhai de Santis, 1975.
Type information. Holotype female, MLP (not examined but subsequent treatment of the species checked). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (Fernando de Noronha Is, PE).
Notes. Our concept of this species is based on Aquino et al. (2010).
Apantelesnovatus Nixon, 1965
Apantelesnovatus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Apantelesnycon Nixon, 1965
Apantelesnycon Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Apantelesnymphis Nixon, 1965
Apantelesnymphis Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Indonesia.
Geographical distribution.OTL.
OTL: Indonesia.
Apantelesoatmani Marsh, 1979
Apantelesoatmani Marsh, 1979.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: Colombia.
Geographical distribution.NEO.
NEO: Colombia.
Apantelesobscurus (Nees, 1834)
Microgasterobscura Nees, 1834.
Microgasterarenarius Haliday, 1834.
Type information. Lectotype female, ZMHB (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Albania, Armenia, Azerbaijan, Belgium, Croatia, Denmark, Finland, France, Georgia, Germany, Greece, Hungary, Iran, Ireland, Israel, Italy, Kazakhstan, Lithuania, Macedonia, Moldova, Mongolia, Montenegro, Netherlands, Poland, Romania, Russia (KDA, KYA, PRI, SAK, SPE, YAR), Serbia, Slovakia, Slovenia, Spain, Sweden, Switzerland, Tunisia, Turkey, United Kingdom.
Notes. Our species concept is based on Wilkinson (1945), Nixon (1976), Papp (1980a), and Kotenko (2007a).
Apantelesoculatus Tobias, 1967
Apantelesoculatus Tobias, 1967.
Type information. Holotype male, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Turkmenistan.
Geographical distribution.PAL.
PAL: Turkmenistan, Uzbekistan.
Notes. Our species concept is based on Papp (1984a).
Apantelesodites Nixon, 1965
Apantelesodites Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: China (ZJ), Philippines.
Apantelesoenone Nixon, 1965
Apantelesoenone Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Australia.
Geographical distribution.AUS, OTL.
AUS: Australia (NT, QLD, WA); OTL: Vietnam.
Apantelesolorus Nixon, 1965
Apantelesolorus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Apantelesopacus (Ashmead, 1905)
Urogasteropacus Ashmead, 1905.
Apantelesderogatae Watanabe, 1935.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.AUS, OTL, PAL.
AUS: Hawaiian Islands; OTL: China (FJ, GX, HN, JS, SH, SN, ZJ), India, Indonesia, Malaysia, Philippines, Vietnam; PAL: China (SD), Japan.
Notes. The species was recorded from the Hawaiian Islands as an adventive species (Nishida 2002), but has been later found to be common (Howarth et al. 2012).
Apantelesopuntiarum Martínez & Berta, 2012
Apantelesopuntiarum Martínez & Berta, 2012.
Type information. Holotype female, MACN (not examined but original description checked). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina.
Apantelesorientalis Szépligeti, 1913
Apantelesorientalis Szépligeti, 1913.
Type information. Holotype male, HNHM (not examined but subsequent treatment of the species checked). Country of type locality: Tanzania.
Geographical distribution.AFR, OTL.
AFR: Tanzania; OTL: India.
Notes. Our species concept is based on Papp (2004).
Apantelesoritias Nixon, 1965
Apantelesoritias Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: China (ZJ), India.
Apantelesoroetes Nixon, 1965
Apantelesoroetes Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Apantelesorphne Nixon, 1965
Apantelesorphne Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Fiji.
Geographical distribution.AUS.
AUS: Fiji.
Apantelesortia Nixon, 1965
Apantelesortia Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Solomon Islands.
Geographical distribution.AUS.
AUS: Solomon Islands.
Apantelesorus Nixon, 1965
Apantelesorus Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Apantelesoryzicola Watanabe, 1967
Apantelesoryzicola Watanabe, 1967.
Type information. Holotype female, KUEC (not examined but paratype examined). Country of type locality: Japan.
Geographical distribution.PAL.
PAL: Japan.
Apantelesoscarchavezi Fernandez-Triana, 2014
Apantelesoscarchavezi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesoscus Nixon, 1965
Apantelesoscus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Apantelesosvaldoespinozai Fernandez-Triana, 2014
Apantelesosvaldoespinozai Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelespablotranai Fernandez-Triana, 2014
Apantelespablotranai Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelespabloumanai Fernandez-Triana, 2014
Apantelespabloumanai Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelespablovasquezi Fernandez-Triana, 2014
Apantelespablovasquezi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelespachycarinatus Song & Chen, 2002
Apantelespachycarinatus Song & Chen, 2002.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Apantelespainei Nixon, 1965
Apantelespainei Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Papua New Guinea.
Geographical distribution.AUS.
AUS: Papua New Guinea.
Apantelesparaglaope Long, 2010
Apantelesparaglaope Long, 2010.
Type information. Holotype female, IEBR (not examined but original description checked). Country of type locality: Vietnam.
Geographical distribution.OTL.
OTL: Vietnam.
Apantelesparaguayensis Brèthes, 1924
Apantelesparaguayensis Brèthes, 1924.
Type information. Type unknown, MACN (not examined but original description checked). Country of type locality: Paraguay.
Geographical distribution.NEO.
NEO: Paraguay.
Notes. The original description is insufficient to conclude on the generic placement of this species.
Apantelesparalus Nixon, 1965
Apantelesparalus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Apantelesparanthrenidis Muesebeck, 1921
Apantelesparanthrenidis Muesebeck, 1921.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA, NEO.
NEA: USA (CA, DC, FL, MS, NY, OK, PA); NEO: Mexico.
Apantelesparapholetesor Liu & Chen, 2015
Apantelesparapholetesor Liu & Chen, 2015.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (LN).
Apantelesparkeri Muesebeck, 1954
Apantelesparkeri Muesebeck, 1954.
Type information. Holotype female, USNM (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (MG).
Apantelesparsodes Nixon, 1965
Apantelesparsodes Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Apantelesparvus Liu & Chen, 2014
Apantelesparvus Liu & Chen, 2014.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (FJ, GD, ZJ); PAL: China (HA, SN).
Apantelespashmina Rousse, 2013
Apantelespashmina Rousse, 2013.
Type information. Holotype female, MNHN (not examined but original description checked). Country of type locality: Réunion.
Geographical distribution.AFR.
AFR: Réunion.
Apantelespastranai Blanchard, 1960
Apantelespastranai Blanchard, 1960.
Type information. Holotype female, MACN (not examined). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina.
Apantelespatens Nixon, 1965
Apantelespatens Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Apantelespaulaixcamparijae Fernandez-Triana, 2014
Apantelespaulaixcamparijae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelespeisonis Fischer, 1965
Apantelespeisonis Fischer, 1965.
Apantelessubfirmus Abdinbekova, 1969.
Type information. Holotype female, NHMW (not examined but subsequent treatment of the species checked). Country of type locality: Austria.
Geographical distribution.PAL.
PAL: Austria, Azerbaijan, Romania, Russia (NC).
Notes. Our species concept is based on Papp (1984a).
Apantelespellucipterus Song & Chen, 2001
Apantelespellucipterus Song & Chen, 2001.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Notes. Our species concept is based on Song et al. (2001) and Chen and Song (2004).
Apantelespentagonalis Blanchard, 1963
Apantelespentagonalis Blanchard, 1963.
Type information. Holotype male, MACN (not examined but original description checked). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina.
Apantelespentagonius de Saeger, 1944
Apantelespentagonius de Saeger, 1944.
Apanteleswilkinsoni de Saeger, 1941 [homonym of Apanteleswilkinsoni Fahringer, 1936].
Type information. Syntypes female and male, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Apantelesperidoneus Papp, 1974
Apantelesperidoneus Papp, 1974.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Hungary.
Geographical distribution.PAL.
PAL: Hungary.
Apantelespersephone Nixon, 1965
Apantelespersephone Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (WA).
Apantelespertiades Nixon, 1965
Apantelespertiades Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Solomon Islands.
Geographical distribution.AUS.
AUS: Papua New Guinea, Solomon Islands.
Apantelespetilicaudium Chen, Song & Yang, 2002
Apantelespetilicaudium Chen, Song & Yang, 2002.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (YN).
Apantelespetronariosae Fernandez-Triana, 2014
Apantelespetronariosae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesphalis Nixon, 1965
Apantelesphalis Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Apantelesphtorimoeae Risbec, 1951
Apantelesphtorimoeae Risbec, 1951.
Apantelesheliopae Risbec, 1951.
Type information. Holotype female, depository unknown (not examined but original description checked). Country of type locality: Senegal.
Geographical distribution.AFR.
AFR: Senegal.
Apantelesphycodis Viereck, 1913
Apantelesphycodis Viereck, 1913.
Type information. Holotype female, USNM (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India, Vietnam.
Apantelespiceotrichosus Blanchard, 1947
Apantelespiceotrichosus Blanchard, 1947.
Type information. Holotype female, MACN (not examined but original description checked). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina, Brazil (RS), Chile.
Apantelespilosus Telenga, 1955
Apantelespilosus Telenga, 1955.
Type information. Lectotype female, ZIN (not examined but original description checked). Country of type locality: Turkmenistan.
Geographical distribution.PAL.
PAL: Kazakhstan, Turkmenistan, Uzbekistan.
Notes. Our species concept is based on the original description and Papp (1984a). We suspect that this species might belong to Dolichogenidea (or perhaps even Pholetesor), based on the description of the hypopygium, ovipositor sheaths, shapes of T1 and T2, and sculpture of anteromesoscutum; however, the hind wing vannal lobe is not described or illustrated in the sources we studied. Thus, we follow here Papp (1988) who kept the species in Apanteles.
Apantelesplatyptiliophagus Shenefelt, 1972
Apantelesplatyptiliophagus Shenefelt, 1972.
Apantelesplatyptiliae Rao & Kurian, 1950 [homonym of Apantelesplatyptiliae Cameron, 1909].
Type information. Holotype male, NZSI (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Apantelesplatyptiliovorus Blanchard, 1965
Apantelesplatyptiliovorus Blanchard, 1965.
Type information. Holotype female, MACN (not examined but original description checked). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina.
Notes. The original description suggests this species may belong to Choeras (based on the host species as well as the comparison the author made with Choerasadjuntcus). However, the details of the propodeum, T1–T3, and ovipositor cannot be interpreted unambiguously as being similar to Choeras (other genera could also be considered, including Apanteles). Thus, until specimens can be studied, we think is better to retain the species in the genus in which it was originally described.
Apantelesplesius Viereck, 1912
Apantelesplesius Viereck, 1912.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (ON), USA (IL, MO, NJ, WI).
Notes. The holotype is currently missing the metasoma, three legs, and tips of antenna. We are following previous references to quote the holotype sex.
Apantelespolychrosidis Viereck, 1912
Apantelespolychrosidis Viereck, 1912.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (BC, MB, NB, ON, QC), USA (AK, DC, FL, IL, KS, MI, MN, MO, NY, NC, OH, OR, PA, SD, WA, WI).
Apantelespongamiae Sumodan & Narendran, 1990
Apantelespongamiae Sumodan & Narendran, 1990.
Type information. Holotype female, RMNH (not examined but subsequent treatment of the species checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. Our species concept is based on van Achterberg and Narendran (1997).
Apantelesprinoptus Papp, 1984
Apantelesprinoptus Papp, 1984.
Apantelesmetaclypealis Tobias & Kotenko, 1986.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Hungary.
Geographical distribution.PAL.
PAL: Germany, Hungary, Russia (S), Ukraine.
Notes. Our species concept is based on the original description and also comments from Kotenko (2006).
Apantelesprocoxalis Hedqvist, 1965
Apantelesprocoxalis Hedqvist, 1965.
Type information. Holotype female, MZH (examined). Country of type locality: Cape Verde.
Geographical distribution.AFR.
AFR: Cape Verde.
Notes.Forshage et al. (2016) considered the type material to be lost; however, in 2017 it was found by the senior author of this paper in another section of the MZH collection.
Apantelesprosopis Risbec, 1951
Apantelesprosopis Risbec, 1951.
Type information. Holotype female, depository unknown (not examined but original description checked). Country of type locality: Senegal.
Geographical distribution.AFR.
AFR: Senegal.
Apantelesprusias Nixon, 1965
Apantelesprusias Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Sri Lanka.
Geographical distribution.OTL.
OTL: Sri Lanka.
Notes. The propodeum sculpture is somewhat atypical for Apanteles, as noted by Nixon (1965); nevertheless, we think at present this is still the best generic placement for the species.
Apantelespsenes Nixon, 1965
Apantelespsenes Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Malaysia.
Geographical distribution.OTL.
OTL: Malaysia, Vietnam.
Apantelespseudoglossae Muesebeck, 1921
Apantelespseudoglossae Muesebeck, 1921.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (QC), USA (IL, MD, MI, MN).
Apantelespseudomacromphaliae Havrylenko & Winterhalter, 1949
Apantelespseudomacromphaliae Havrylenko & Winterhalter, 1949.
Apantelesmacromphaliae Blanchard, 1942 [nomen nudum].
Type information. Type and depository unknown (not examined). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina.
Apantelespusaensis Lal, 1942
Apantelespusaensis Lal, 1942.
Type information. Holotype female, INPC (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Apantelespycnos Nixon, 1965
Apantelespycnos Nixon, 1965.
Type information. Holotype female, AEIC (not examined but original description checked). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Apantelespyrodercetus de Saeger, 1941
Apantelespyrodercetus de Saeger, 1941.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Apantelesquadratus Anjum & Malik, 1978
Apantelesquadratus Anjum & Malik, 1978.
Type information. Holotype female, UKZMP (not examined). Country of type locality: Pakistan.
Geographical distribution.PAL.
PAL: Pakistan.
Notes. The depository acronym (UKZMP) was selected based on the institution name: University of Karachi, Zoological Museum, Pakistan.
Apantelesquadrifacies Papp, 1984
Apantelesquadrifacies Papp, 1984.
Type information. Holotype female, HNHM (not examined but subsequent treatment of the species checked). Country of type locality: Hungary.
Geographical distribution.PAL.
PAL: Hungary.
Notes. Our species concept is based on Papp (1984a).
Apantelesquinquecarinis Song & Chen, 2003
Apantelesquinquecarinis Song & Chen, 2003.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (JX).
Apantelesracilla Nixon, 1965
Apantelesracilla Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Apantelesraesus Nixon, 1965
Apantelesraesus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Apantelesrandallgarciai Fernandez-Triana, 2014
Apantelesrandallgarciai Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesrandallmartinezi Fernandez-Triana, 2014
Apantelesrandallmartinezi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesraulacevedoi Fernandez-Triana, 2014
Apantelesraulacevedoi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesraulsolorsanoi Fernandez-Triana, 2014
Apantelesraulsolorsanoi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesraviantenna Chen & Song, 2004
Apantelesraviantenna Chen & Song, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (FJ, HB); PAL: China (JL).
Apantelesrhipheus Nixon, 1965
Apantelesrhipheus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Apantelesrhomboidalis (Ashmead, 1900)
Urogasterrhomboidalis Ashmead, 1900.
Type information. Holotype female, NHMUK (examined). Country of type locality: Saint Vincent.
Geographical distribution.NEO.
NEO: Saint Vincent.
Apantelesricardocaleroi Fernandez-Triana, 2014
Apantelesricardocaleroi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesricini Bhatnagar, 1950
Apantelesricini Bhatnagar, 1950.
Type information. Holotype male, INPC (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. The year of publication of the Bhatnagar paper was until recently commonly cited as 1948 and/or 1950 (e.g., Chen and Song 2004, Yu et al. 2016), probably following Shenefelt (1972) who referred to this paper as “Bhatnagar (1948) 1950”. While the intended year for Volume X, Parts I & II of the Indian Journal of Entomology was 1948, the actual dates of publication were June 1950 (Part I) and October 1950 (Part II), as clearly shown on the cover page of the Volume, which we have checked. Because the dates of publication are the ones to be considered, and for the sake of clarity, we hereby revise the species year of description to 1950.
Apantelesriograndensis Brèthes, 1920
Apantelesriograndensis Brèthes, 1920.
Type information. Holotype female, MACN (not examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (RS).
Notes. The following information is based on Eduardo Shimbori (pers. comm.), and we use it as the most reliable source to conclude on the species distribution and type locality: a) there is no clear indication that Apantelesriograndensis Brèthes, 1920 is from Argentina, as far as we know, Taxapad (Yu et al. 2016) is the only source that states that, and it may be just an error based on the museum where the holotype is deposited; b) the title of the paper containing the original description is: “Insectos utiles y dañinos de Rio Grande do Sul y de la Plata” (Brèthes 1920), Rio Grande do Sul is certainly a Brazilian state (although there is a Rio Grande in Argentina, it is located in the Isla Grande de Tierra del Fuego, Patagonia, a place very far removed from La Plata); c) another paper (Ronna 1924) about insects of that same Brazilian state also mentions A.riograndensis; d) the catalogue on Hymenoptera Brasilenos (de Santis 1980) also cite A.riograndensis as from Rio Grande do Sul. Based on the information above we here consider Apantelesriograndensis Brèthes, 1920 as a Brazilian species, and not present in Argentina.
Apantelesrisbeci de Saeger, 1942
Apantelesrisbeci de Saeger, 1942.
Type information. Holotype female?, RMCA (not examined but subsequent treatment of the species checked). Country of type locality: Senegal.
Geographical distribution.AFR.
AFR: Senegal.
Notes. We could not read the original description, but subsequent treatments of the species (de Saeger 1944, Risbec 1951) stated that the species was described based on the female sex (although it is not clear if one or more female specimens were studied). Thus, we have added a question mark after the holotype to denote the uncertainty.
Apantelesrobertmontanoi Fernandez-Triana, 2014
Apantelesrobertmontanoi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesrobertoespinozai Fernandez-Triana, 2014
Apantelesrobertoespinozai Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesrobertovargasi Fernandez-Triana, 2014
Apantelesrobertovargasi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesrobustus Hedqvist, 1965
Apantelesrobustus Hedqvist, 1965.
Type information. Holotype female, MZH (examined). Country of type locality: Cape Verde.
Geographical distribution.AFR.
AFR: Cape Verde.
Notes.Forshage et al. (2016) considered the type material to be lost; however, in 2017 it was found by the senior author of this paper in another section of the MZH collection.
Apantelesrodrigogamezi Fernandez-Triana, 2014
Apantelesrodrigogamezi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesrogerblancoi Fernandez-Triana, 2014
Apantelesrogerblancoi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesrolandoramosi Fernandez-Triana, 2014
Apantelesrolandoramosi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesrolandovegai Fernandez-Triana, 2014
Apantelesrolandovegai Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesromei Rousse, 2013
Apantelesromei Rousse, 2013.
Type information. Holotype female, MNHN (not examined but original description checked). Country of type locality: Réunion.
Geographical distribution.AFR.
AFR: Réunion.
Apantelesronaldcastroi Fernandez-Triana, 2014
Apantelesronaldcastroi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesronaldgutierrezi Fernandez-Triana, 2014
Apantelesronaldgutierrezi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesronaldmurilloi Fernandez-Triana, 2014
Apantelesronaldmurilloi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesronaldnavarroi Fernandez-Triana, 2014
Apantelesronaldnavarroi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesronaldquirosi Fernandez-Triana, 2014
Apantelesronaldquirosi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesronaldzunigai Fernandez-Triana, 2014
Apantelesronaldzunigai Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesrosaces Nixon, 1965
Apantelesrosaces Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Apantelesrosibelelizondoae Fernandez-Triana, 2014
Apantelesrosibelelizondoae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesrostermoragai Fernandez-Triana, 2014
Apantelesrostermoragai Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesroughleyi Fernandez-Triana, 2010
Apantelesroughleyi Fernandez-Triana, 2010.
Type information. Holotype female, CNC (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (BC).
Apantelesrufithorax Hedqvist, 1965
Apantelesrufithorax Hedqvist, 1965.
Type information. Holotype female, MZH (not examined but original description checked). Country of type locality: Cape Verde.
Geographical distribution.AFR.
AFR: Cape Verde.
Notes.Forshage et al. (2016) considered the type material to be lost; however, in 2017 it was found by the senior author in the MZH.
Apantelesrugiceps Wilkinson, 1934
Apantelesrugiceps Wilkinson, 1934.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Apantelesruthfrancoae Fernandez-Triana, 2014
Apantelesruthfrancoae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesrutilans Nixon, 1965
Apantelesrutilans Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Kenya.
Geographical distribution.AFR, OTL.
AFR: Kenya; OTL: Vietnam.
Apantelessaegeri Risbec, 1951
Apantelessaegeri Risbec, 1951.
Apantelessaegeribambeyi Risbec, 1951.
Apantelessaegeriduplosenegalensis Shenefelt, 1972 [new name for Apantelessaegerisenegalensis Risbec, 1951, a homonym of Apantelessenegalensis Risbec, 1951].
Type information. Syntypes female, depository unknown (not examined but original description checked). Country of type locality: Senegal.
Geographical distribution.AFR.
AFR: Senegal.
Apantelessagax Wilkinson, 1929
Apantelessagax Wilkinson, 1929.
Type information. Holotype female, NHMUK (examined). Country of type locality: Tanzania.
Geographical distribution.AFR.
AFR: Cameroon, Democratic Republic of Congo, Ivory Coast, Nigeria, Senegal, Tanzania, Togo, Uganda.
Apantelessalutifer Wilkinson, 1931
Apantelessalutifer Wilkinson, 1931.
Type information. Holotype female, NHMUK (examined). Country of type locality: Thailand.
Geographical distribution.OTL, PAL.
OTL: China (FJ, HB, YN), India, Myanmar, Thailand, Vietnam; PAL: Japan, Korea.
Apantelessamedovi Abdinbekova, 1969
Apantelessamedovi Abdinbekova, 1969.
Apanteleslencoranicus Abdinbekova, 1969.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Azerbaijan.
Geographical distribution.PAL.
PAL: Azerbaijan.
Notes. Our species concept is based on Papp (1980a) and Tobias (1986).
Apantelessamoanus Fullaway, 1940
Apantelessamoanus Fullaway, 1940.
Type information. Holotype female, BPBM (not examined but subsequent treatment of the species checked). Country of type locality: American Samoa.
Geographical distribution.AUS.
AUS: American Samoa, Fiji.
Notes. Our species concept is based on Austin and Dangerfield (1992).
Apantelessaravus Nixon, 1965
Apantelessaravus Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Apantelessauros Nixon, 1965
Apantelessauros Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Apantelesschneideri Nixon, 1965
Apantelesschneideri Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Indonesia.
Geographical distribution.OTL.
OTL: Indonesia.
Apantelesschoutedeni de Saeger, 1941
Apantelesschoutedeni de Saeger, 1941.
Type information. Syntypes female and male, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Senegal.
Apantelessergiocascantei Fernandez-Triana, 2014
Apantelessergiocascantei Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelessergioriosi Fernandez-Triana, 2014
Apantelessergioriosi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesseyrigi Wilkinson, 1936
Apantelesseyrigi Wilkinson, 1936.
Type information. Holotype female, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Apantelessigifredomarini Fernandez-Triana, 2014
Apantelessigifredomarini Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelessignificans (Walker, 1860)
Microgastersignificans Walker, 1860.
Type information. Holotype male, NHMUK (examined). Country of type locality: Sri Lanka.
Geographical distribution.OTL.
OTL: China (FJ), India, Pakistan, Philippines, Singapore, Sri Lanka, Vietnam.
Apantelessingaporensis Szépligeti, 1905
Apantelessingaporensis Szépligeti, 1905.
Type information. Lectotype female, HNHM (not examined but subsequent treatment of the species checked). Country of type locality: Singapore.
Geographical distribution.OTL.
OTL: India, Singapore.
Notes. Our species concept is based on Wilkinson (1928b).
Apantelessmerdis Nixon, 1965
Apantelessmerdis Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Apantelessodalis (Haliday, 1834)
Microgastersodalis Haliday, 1834.
Microgastercarbonarius Ratzeburg, 1848 [homonym of Microgastercarbonarius Wesmael, 1837].
Microgasterater Ratzeburg, 1852.
Microgasterlugens Ratzeburg, 1852.
Apanteleslindbergi Hedqvist, 1965.
Type information. Type lost (not examined but subsequent treatment of the species checked). Country of type locality: United Kingdom.
Geographical distribution.AFR, NEA, PAL, OTL.
AFR: Cape Verde; NEA: Canada (BC, NB, NL); PAL: Armenia, Azerbaijan, Bulgaria, China (SN), Czech Republic, France, Germany, Greece, Hungary, Ireland, Italy, Japan, Kazakhstan, Korea, Latvia, Lithuania, Moldova, Netherlands, Poland, Romania, Russia (KHA, KDA, MOS, PRI, SAK, SAM, SAR), Serbia, Slovakia, Sweden, Switzerland, Turkey, Ukraine, United Kingdom; OTL: China (GD, ZJ).
Notes. Our species concept is based on Fernandez-Triana and Huber (2010). The specimens of Apantelessodalis that have yielded DNA barcodes comprise two BINs, BOLD:AAM7223 (from Canada: BC, NL) and BOLD:AAN1859 (Canada: BC). Whether they represent two different species or not was mentioned by Fernandez-Triana et al. (2014b) but no further study has been conducted so in this paper all known specimens are kept as one species.
Apantelessolox Nixon, 1965
Apantelessolox Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Singapore.
Geographical distribution.OTL.
OTL: Singapore.
Apantelessosis Nixon, 1965
Apantelessosis Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR, OTL.
AFR: South Africa; OTL: Vietnam.
Apantelessparsus Liu & Chen, 2015
Apantelessparsus Liu & Chen, 2015.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (GD).
Apantelesspicicula Chen & Song, 2004
Apantelesspicicula Chen & Song, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Apantelesstagmatophorae Gahan, 1919
Apantelesstagmatophorae Gahan, 1919.
Type information. Holotype female, USNM (not examined but subsequent treatment of the species checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (MD).
Notes. Our species concept is based on Muesebeck (1921) and Nixon (1972).
Apantelesstarki Mason, 1960
Apantelesstarki Mason, 1960.
Type information. Holotype female, CNC (examined). Country of type locality: Canada.
Geographical distribution.NEA, OTL, PAL.
NEA: Canada (AB, BC), USA (ID, UT); OTL: China (HB); PAL: China (NX).
Apantelesstegenodactylae Cameron, 1909
Apantelesstegenodactylae Cameron, 1909.
Type information. Holotype male, NHMUK (examined). Country of type locality: Sri Lanka.
Geographical distribution.OTL.
OTL: Sri Lanka.
Notes.Wilkinson (1928b: 137) considered that this species should probably be synonymized under Apantelessubductus Walker, 1860. The two species were described from a single male each, which were collected in the same island (Sri Lanka), and both are in relatively poor condition. However, after examining both specimens we do not think that is advisable to do what Wilkinson suggested. Although both specimens indeed share some resemblance morphologically, subductus lacks any biological information, the type locality is only stated as Ceylon (currently Sri Lanka), and the wings are partially shredded and glued together on the card, making it impossible to see any details on the hind wing. In contrast, stegenodactylae is slightly better preserved, has information about the type locality, and it also has preserved the wasp cocoon (and associated host information). Until more material from Sri Lanka is more comprehensively studied, we prefer to maintain both species as separate.
Apantelesstennos Nixon, 1965
Apantelesstennos Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Apantelesstenomae Muesebeck, 1958
Apantelesstenomae Muesebeck, 1958.
Type information. Holotype female, USNM (examined). Country of type locality: Venezuela.
Geographical distribution.NEO.
NEO: Brazil (SP), Venezuela.
Notes. Distribution in Brazil from de Santis (1967b).
Apantelesstictipes Chen & Song, 2004
Apantelesstictipes Chen & Song, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Apantelesstriatopleurus Hedqvist, 1965
Apantelesstriatopleurus Hedqvist, 1965.
Type information. Holotype female, MZH (examined). Country of type locality: Cape Verde.
Geographical distribution.AFR.
AFR: Cape Verde.
Apantelessubaltus de Saeger, 1944
Apantelessubaltus de Saeger, 1944.
Type information. Syntypes female and male, RMCA (not examined but original description checked). Country of type locality: Rwanda.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Rwanda.
Apantelessubandinus Blanchard, 1947, restored combination
Apantelessubandinus Blanchard, 1947.
Type information. Holotype female, MACN (not examined but original description checked). Country of type locality: Argentina.
Geographical distribution.AFR, AUS, NEO.
AFR: Réunion, South Africa; AUS: Australia (ACT, NSW, QLD, SA, TAS, VIC, WA), New Zealand; NEO: Argentina, Brazil (PR), Peru, Uruguay.
Notes. Under this species name there is likely a complex of species, some of them not even related. We have seen in the CNC two different species, one of them (from the USA, CA, and reared from the Gelechiidae moth Phthorimaeaoperculella) clearly belongs to Apanteles, and perhaps represents the true Apantelessubandinus. Another species (from Venezuela, reared from the same host) clearly belongs to Dolichogenidea, as it differs in the shape and fully setose vannal lobe of the hind wing, as well as the shape and sculpture of T1. Additionally, in BOLD, there are two BINs with the same name Apantelessubandinus but they are far apart from each other. BINBOLD:AAM4042 contains the Venezuelan specimens (as well as other specimens from Chile, also deposited in the CNC but with no associated host records); that BIN is close to species of Dolichogenidea and not Apanteles. The second BINBOLD:AAV2170 contains specimens from Colombia and New Zealand (with no host record available); that BIN is close to species of Apanteles and not Dolichogenidea. To complicate things further, Rousse and Gupta (2013: 534) considered the species as “unambiguously belonging to the genus Glyptapanteles” and thus transferred it to that genus; however, from their own figures in that paper (Rousse and Gupta 2013: fig. 15g-i) it is evident that the single female specimen they saw is not Glyptapanteles (e.g., see length and shape of the ovipositor sheaths and the hypopygium shown there in their fig. 15g). Solving the complexities of this species is beyond the scope of the present paper, but for now we transfer the species back to Apanteles, the best placement that it can be currently assigned to.
Apantelessubcamilla Long, 2007
Apantelessubcamilla Long, 2007.
Type information. Holotype female, IEBR (not examined but original description checked). Country of type locality: Vietnam.
Geographical distribution.OTL.
OTL: Vietnam.
Apantelessubcristatus Blanchard, 1936
Apantelessubcristatus Blanchard, 1936.
Type information. Holotype female, MACN (not examined but original description checked). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina, Chile, Uruguay.
Apantelessubductus (Walker, 1860)
Microgastersubductus Walker, 1860.
Type information. Holotype male, NHMUK (examined). Country of type locality: Sri Lanka.
Geographical distribution.OTL.
OTL: Sri Lanka.
Notes. See comments above (under the species Apantelesstegenodactylae Cameron, 1909) for more comments on both species.
Apantelessubrugosus Granger, 1949
Apantelessubrugosus Granger, 1949.
Type information. Syntypes female, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Apantelessulciscutis (Cameron, 1905), new combination
Holcapantelessulciscutis Cameron, 1905.
Type information. Type lost (not examined but original description checked). Country of type locality: Indonesia.
Geographical distribution.OTL.
OTL: Indonesia.
Notes. See comments at the beginning of Apanteles for more details on the decision to transfer this species to Apanteles.
Apantelessyleptae Ferrière, 1925
Apantelessyleptae Ferrière, 1925.
Type information. Holotype female, MHNG (not examined but subsequent treatment of the species checked). Country of type locality: Sudan.
Geographical distribution.AFR.
AFR: Chad, Democratic Republic of Congo, Egypt, Kenya, Nigeria, Senegal, Sudan, Tanzania, Togo.
Notes. Our species concept is based on Nixon (1965). The species was recorded from India by Abraham et al. (1973); however, we consider that source as questionable (based on the previously known distribution of the species and different host species), pending further corroboration we prefer to exclude that record for the time being.
Apantelessylvaticus de Saeger, 1944
Apantelessylvaticus de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Rwanda.
Geographical distribution.AFR.
AFR: Rwanda.
Apantelessymithae Bhatnagar, 1950
Apantelessymithae Bhatnagar, 1950.
Type information. Holotype female, INPC (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. The year of publication of the Bhatnagar paper was until recently commonly cited as 1948 and/or 1950 (e.g., Chen and Song 2004, Yu et al. 2016), probably following Shenefelt (1972) who referred to this paper as “Bhatnagar (1948) 1950”. While the intended year for Volume X, Parts I & II of the Indian Journal of Entomology was 1948, the actual dates of publication were June 1950 (Part I) and October 1950 (Part II), as clearly shown on the cover page of the Volume, which we have checked. Because the dates of publication are the ones to be considered, and for the sake of clarity, we hereby revise the species year of description to 1950.
Apantelestachardiae Cameron, 1913
Apantelestachardiae Cameron, 1913.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: China (HN), India.
Apantelestaeniaticornis Wilkinson, 1928
Apantelestaeniaticornis Wilkinson, 1928.
Type information. Holotype female, NHMUK (examined). Country of type locality: Indonesia.
Geographical distribution.OTL.
OTL: Indonesia.
Apantelestaiticus (Holmgren, 1868)
Microgastertaiticus Holmgren, 1868.
Type information. Holotype female, NHRS (not examined). Country of type locality: Society Islands.
Geographical distribution.AUS.
AUS: Society Islands.
Apantelestalinum Risbec, 1951
Apantelestalinum Risbec, 1951.
Type information. Syntypes female and male, depository unknown (not examined but original description checked). Country of type locality: Senegal.
Geographical distribution.AFR.
AFR: Senegal.
Apantelestapatapaoanus Fullaway, 1946
Apantelestapatapaoanus Fullaway, 1946.
Apantelesbedelliae Fullaway, 1941 [homonym of Apantelesbedelliae Viereck, 1911].
Type information. Holotype female, BPBM (not examined but subsequent treatment of the species checked). Country of type locality: Western Samoa.
Geographical distribution.AUS.
AUS: American Samoa, Western Samoa.
Notes. Our species concept is based on Austin and Dangerfield (1992).
Apantelestaragamae Viereck, 1912
Apantelestaragamae Viereck, 1912.
Apantelesplusiae Viereck, 1913.
Apanteleshomonae Rohwer, 1922.
Type information. Holotype female, USNM (examined). Country of type locality: India.
Geographical distribution.AUS, OTL, PAL.
AUS: Papua New Guinea; OTL: China (FJ, GX, GZ, HB, HN, TW, ZJ), India, Indonesia, Sri Lanka, Thailand, Vietnam; PAL: Japan, Korea.
Notes. We examined the types of Apantelesplusiaeplusiae Viereck, 1913, and Apanteleshomonae Rohwer, 1922, both synonyms of Apantelestaragamae.
Apantelestelon Nixon, 1965
Apantelestelon Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Pakistan.
Geographical distribution.PAL.
PAL: Pakistan.
Apantelesthoracartus Liu & Chen, 2015
Apantelesthoracartus Liu & Chen, 2015.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (GD).
Apantelesthurberiae Muesebeck, 1921
Apantelesthurberiae Muesebeck, 1921.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA, NEO.
NEA: USA (AZ, TX); NEO: Colombia, Nicaragua, Trinidad & Tobago, Venezuela.
Apantelestiapi Risbec, 1952
Apantelestiapi Risbec, 1952.
Apanteleslongicornis Risbec, 1951 [homonym of Apanteleslongicornis Provancher, 1886].
Type information. Holotype male, depository unknown (not examined but original description checked). Country of type locality: Senegal.
Geographical distribution.AFR.
AFR: Senegal.
Notes.Yu et al. (2016) listed the date of Risbec publication as 1951, but in fact that was for Apanteleslongicornis; the replacement name, tiapi, was proposed a year later (Risbec 1952: 701).
Apantelestiboshartae Fernandez-Triana, 2014
Apantelestiboshartae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelestigasis Nixon, 1965
Apantelestigasis Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Indonesia.
Geographical distribution.OTL.
OTL: Indonesia.
Apantelestirathabae Wilkinson, 1928
Apantelestirathabae Wilkinson, 1928.
Type information. Holotype female, NHMUK (examined). Country of type locality: Malaysia.
Geographical distribution.AUS, OTL.
AUS: Fiji, Solomon Islands; OTL: Indonesia, Malaysia, Philippines, Vietnam.
Apantelestownesi Nixon, 1965
Apantelestownesi Nixon, 1965.
Type information. Holotype female, AEIC (not examined but original description checked). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Apantelestranstergum Liu & Chen, 2014
Apantelestranstergum Liu & Chen, 2014.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (HE).
Apantelestriareus Nixon, 1965
Apantelestriareus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Apantelestricoloripes Granger, 1949
Apantelestricoloripes Granger, 1949.
Type information. Syntypes female, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Apantelestrifasciatus Muesebeck, 1946
Apantelestrifasciatus Muesebeck, 1946.
Type information. Holotype female, USNM (examined). Country of type locality: Hawaiian Islands.
Geographical distribution.AUS.
AUS: Fiji, Hawaiian Islands.
Apantelestrochanteratus Szépligeti, 1911
Apantelestrochanteratus Szépligeti, 1911.
Type information. Holotype female, ZMHB (not examined but subsequent treatment of the species checked). Country of type locality: Tanzania.
Geographical distribution.AFR.
AFR: Senegal, Tanzania.
Notes. Our species concept is based on Wilkinson (1932a).
Apantelestulis Nixon, 1965
Apantelestulis Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Notes. The holotype (only known specimen) is missing the metasoma, hind legs, and wings (one fore wing is glued to the card, and a pair of wings is loose in the unit tray). The species was described as “not very distinctive” (Nixon 1965: 78), and no illustration is available. The only remnants of this species are thus the holotype head (antenna missing apical flagellomeres) and the mesosoma.
Apantelesuchidai Watanabe, 1934
Apantelesuchidai Watanabe, 1934.
Type information. Holotype female, EIHU (not examined but authoritatively identified specimens examined). Country of type locality: Japan.
Geographical distribution.PAL.
PAL: Japan.
Notes. In EIHU there is a specimen with a label that reads “?Type Apantelesuchida Watanabe”. However, that label is white, unlike all other labels of primary types in EIHU which are red, and the writing on it is not from Watanabe; thus, it was presumably added later by someone else, and perhaps indicates that the actual type is lost (or at least not clearly marked). In any case, the specimen is in very poor condition, as the only remains are the mesosoma (with a micropin through it, so some characters are not visible), two metacoxae, one metafemur, one fore wing, and the two hind wings.
Apantelesunguifortis Song & Chen, 2004
Apantelesunguifortis Song & Chen, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (HB).
Apantelesupis Nixon, 1965
Apantelesupis Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Apantelesuroxys de Saeger, 1941
Apantelesuroxys de Saeger, 1941.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Rwanda, Senegal.
Apantelesusipetes Nixon, 1965
Apantelesusipetes Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Malaysia.
Geographical distribution.OTL.
OTL: Malaysia.
Notes. The holotype is missing the metasoma, the fore wings and the hind legs. The hind wing vannal lobe is more or less straight with some setae visible, especially on the left hind wing where they seem to occupy most of the central part of the lobe, although the poor condition of the specimen makes it difficult to conclude. The presence of setae in the vannal lobe (also mentioned in the key to the species group in the original description (Nixon 1965: 39), although that paper only referred to few, sparse setae), would suggest that the species is better placed in Dolichogenidea. However, due to the poor condition of the type (only known specimen), we prefer to retain the species in the genus it was originally described.
Apantelesussuriensis Telenga, 1955
Apantelesussuriensis Telenga, 1955.
Type information. Type and depository unknown (not examined but original description checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (PRI).
Apantelesvacillans Nixon, 1965
Apantelesvacillans Nixon, 1965.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Apantelesvala Nixon, 1965
Apantelesvala Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (QLD).
Apantelesvalvatus de Saeger, 1944
Apantelesvalvatus de Saeger, 1944.
Apantelesvalvatusrwindicus de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Rwanda.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Rwanda.
Notes. This species could also be considered to belong to Dolichogenidea, but the original description does not detail the vannal lobe of the hind wing. Thus, until the specimens are examined, it is not possible to conclude, and we prefer to retain the species in Apanteles for the time being.
Apantelesvalvulae Rao & Kurian, 1951
Apantelesvalvulae Rao & Kurian, 1951.
Type information. Holotype female, NZSI (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Apantelesvannesabrenesae Fernandez-Triana, 2014
Apantelesvannesabrenesae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesverticalis Song & Chen, 2004
Apantelesverticalis Song & Chen, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, HB).
Apantelesvictorbarrantesi Fernandez-Triana, 2014
Apantelesvictorbarrantesi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesvivax de Saeger, 1944
Apantelesvivax de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Apantelesvulgaris (Ashmead, 1900)
Urogastervulgaris Ashmead, 1900.
Urogasterxanthopus Ashmead, 1900.
Type information. Holotype male, NHMUK (examined). Country of type locality: Saint Vincent.
Geographical distribution.NEO.
NEO: Argentina, Brazil (SP), Grenada, Puerto Rico, Saint Vincent, Uruguay.
Apanteleswadyobandoi Fernandez-Triana, 2014
Apanteleswadyobandoi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apanteleswaldymedinai Fernandez-Triana, 2014
Apanteleswaldymedinai Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apanteleswanei Risbec, 1951
Apanteleswanei Risbec, 1951.
Type information. Syntypes female and male, depository unknown (not examined but original description checked). Country of type locality: Senegal.
Geographical distribution.AFR.
AFR: Senegal.
Apantelesweitenweberi (Amerling, 1862)
Microgasterweitenweberi Amerling, 1862.
Type information. Type and depository unknown (not examined but original description checked). Country of type locality: Czech Republic.
Geographical distribution.PAL.
PAL: Czech Republic, Italy.
Notes. The original description, which is very brief and does not detail much, states that the species is close to Sathonfalcatus (Nees, 1834). Thus, it is likely that weitenweberi does not belong to Apanteles; however, without examining specimens we cannot conclude and prefer to retain it in the genus it was described.
Apanteleswilbertharayai Fernandez-Triana, 2014
Apanteleswilbertharayai Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apanteleswilliamcamposi Fernandez-Triana, 2014
Apanteleswilliamcamposi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apanteleswuyiensis Song & Chen, 2002
Apanteleswuyiensis Song & Chen, 2002.
Type information. Holotype female, FAFU (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Notes. Our species concept is based on Chen and Song (2004).
Apantelesxanthostigma (Haliday, 1834)
Microgasterxanthostigma Haliday, 1834.
Microgasterochrostigma Wesmael, 1837.
Apantelesxanthocarpus Szépligeti, 1901.
Type information. Neotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.AFR, NEA, PAL.
AFR: Uganda; NEA: Canada (BC, MB, NL, SK); PAL: Armenia, Austria, Azerbaijan, Belarus, Belgium, Bulgaria, Czech Republic, Faroe Islands, Finland, France, Germany, Hungary, Ireland, Italy, Japan, Kazakhstan, Latvia, Lithuania, Madeira Islands, Moldova, Mongolia, Netherlands, Poland, Portugal, Romania, Russia (ALT, ZAB, IRK, KAM, KHA, KIR, KDA, MOS, NGR, OMS, PRI, ROS, SAK, SPE, STA, YAR), Slovakia, Spain, Sweden, Switzerland, Tajikistan, Tunisia, Turkey, Ukraine, United Kingdom.
Notes. See Fernandez-Triana et al. (2014c) for a recent discussion of this species and its rather broad range of hosts.
Apantelesxerophila Risbec, 1951
Apantelesxerophila Risbec, 1951.
Type information. Holotype female, depository unknown (not examined but original description checked). Country of type locality: Senegal.
Geographical distribution.AFR.
AFR: Senegal.
Apantelesyeissonchavesi Fernandez-Triana, 2014
Apantelesyeissonchavesi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesyilbertalvaradoi Fernandez-Triana, 2014
Apantelesyilbertalvaradoi Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apantelesyolandarojasae Fernandez-Triana, 2014
Apantelesyolandarojasae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apanteleszeneidabolanosae Fernandez-Triana, 2014
Apanteleszeneidabolanosae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Apanteleszhangi Song & Chen, 2003
Apanteleszhangi Song & Chen, 2003.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Apanteleszizaniae Muesebeck, 1957
Apanteleszizaniae Muesebeck, 1957.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (DE, DC).
Apantelesznoikoi Tobias, 1976
Apantelesznoikoi Tobias, 1976.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Azerbaijan.
Geographical distribution.PAL.
PAL: Armenia, Azerbaijan.
Notes. Our species concept is based on Papp (1984a).
Genus Austinicotesia Fernandez-Triana, 2018
Austinicotesia Fernandez-Triana, 2018: 43. Gender: neuter. Type species: Austinicotesiaindonesiensis Fernandez-Triana & Boudreault, 2018, by original designation.
Two species were recently described from the Australasian region (Fernandez-Triana and Boudreault 2018), and in that same paper it was mentioned that one or two additional species had been seen in collections (but not described because the material was not sufficient). No host data are currently available for this genus. There are seven DNA-barcode compliant sequences of Austinicotesia in BOLD, representing one BIN.
Austinicotesiaindonesiensis Fernandez-Triana & Boudreault, 2018
Austinicotesiaindonesiensis Fernandez-Triana & Boudreault, 2018.
Type information. Holotype female, RMNH (examined). Country of type locality: Indonesia.
Geographical distribution.OTL.
OTL: Indonesia.
Austinicotesiapapuanus Fernandez-Triana & Boudreault, 2018
Austinicotesiapapuanus Fernandez-Triana & Boudreault, 2018.
Type information. Holotype female, MNHN (examined). Country of type locality: Papua New Guinea.
Geographical distribution.AUS.
AUS: Papua New Guinea.
Genus Austrocotesia Austin & Dangerfield, 1992
Austrocotesia Austin & Dangerfield, 1992: 11. Gender: feminine. Type species: Austrocotesiaexigua Austin & Dangerfield, 1992, by original designation.
Five species are currently described from the Australasian and Neotropical regions, which can be separated using the key by Valerio and Whitfield (2005). However, it is possible that the Australasian and South American specimens actually represent different genera. In the original description of the genus, Austin and Dangerfield (1992: 11–12 and their figures 19 & 20) considered the lack of vein 2r-m in the hind wing as one of the main characters defining Austrocotesia (and indeed that is an important feature, as it is present in very few genera of Microgastrinae). However, the two Neotropical species defined by Valerio and Whitfield (2005, see their figures 1F & 2F) actually have such a vein. Further study of specimens from both regions will be needed to draw firm conclusions. In any case, it does not seem that Austrocotesia is very species rich, although a few additional species remain in collections. No host data are currently available. There are no DNA-barcode compliant sequences of the genus in BOLD, but there are two shorter sequences (minibarcodes) from paratypes of A.croizati.
Austrocotesiacroizati Valerio & Whitfield, 2005
Austrocotesiacroizati Valerio & Whitfield, 2005.
Type information. Holotype female, IAVH (not examined but original description checked). Country of type locality: Colombia.
Geographical distribution.NEO.
NEO: Colombia, Ecuador.
Notes. This species is likely to represent a different genus (see a detailed explanation above, on comments about Austrocotesia). However, pending further study of the Neotropical fauna, we prefer to maintain it here for the time being.
Austrocotesiadelicata Austin & Dangerfield, 1992
Austrocotesiadelicata Austin & Dangerfield, 1992.
Type information. Holotype female, CNC (examined). Country of type locality: Papua New Guinea.
Geographical distribution.AUS.
AUS: Australia (QLD), Papua New Guinea.
Austrocotesiaexigua Austin & Dangerfield, 1992
Austrocotesiaexigua Austin & Dangerfield, 1992.
Type information. Holotype female, CNC (examined). Country of type locality: Papua New Guinea.
Geographical distribution.AUS.
AUS: Papua New Guinea.
Austrocotesiaparadoxa Austin & Dangerfield, 1992
Austrocotesiaparadoxa Austin & Dangerfield, 1992.
Type information. Holotype female, AEIC (not examined but original description checked). Country of type locality: Papua New Guinea.
Geographical distribution.AUS.
AUS: Papua New Guinea.
Austrocotesiarenei Valerio & Whitfield, 2005
Austrocotesiarenei Valerio & Whitfield, 2005.
Type information. Holotype female, CNC (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Notes. This species is likely to represent a different genus (see a detailed explanation above, on comments about Austrocotesia). However, pending further study of the Neotropical fauna, we prefer to maintain it here for the time being.
Genus Beyarslania Koçak & Kemal, 2009
Beyarslania Koçak & Kemal, 2009: 14. Gender: feminine. Type species: Apantelesinsolens Wilkinson, 1930 by original designation (Mason 1981: 71).
Xenogaster Mason, 1981, preoccupied by Xenogaster Wasmann, 1891.
Only known from one species in the Afrotropical region (but see comments under that species about the possibility of a second, potentially new species). The genus was originally described by Mason (1981) as Xenogaster, but the name was later found to be a junior homonym of Xenogaster Wasmann, 1891 (Coleoptera) and thus subsequently changed to its current name (Koçak and Kemal 2009). No host data are currently available for this genus. There is one DNA-barcode compliant sequence in BOLD, its corresponding BIN characterizing the genus and species.
Beyarslaniainsolens (Wilkinson, 1930)
Apantelesinsolens Wilkinson, 1930.
Type information. Neotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: Rwanda, South Africa, Yemen.
Notes.Fernandez-Triana and van Achterberg (2017) recorded B.insolens from Yemen, based on a single female specimen. In spite of the relatively large geographical separation (the record from Fernandez-Triana and van Achterberg (2017) expanded 2,000 km northwards the distribution of Beyarslania in Africa), those authors still considered it to belong to the same species previously recorded from South Africa (Wilkinson 1930b) and Rwanda (de Saeger 1944). After that 2017 paper was published, we have been able to study the holotype of insolens in the NHMUK, and two female and two male specimens deposited in the USNM (three from the type locality in South Africa, Cape Province, Mossel Bay; and one specimen from another, close locality, George, also in Cape Province). The five South African specimens seem different (although mostly in colouration) to the female from Yemen (deposited in the RMNH), which in turn is similar to another female specimen from Rwanda (deposited in the CNC). After comparing those seven specimens, we now think that the most northernly records in Africa (Rwanda and Yemen) may represent a different species, distinct from the South African one. However, until that is studied further (and the potential new species is properly described) we consider here only one species for Africa.
Genus Billmasonius Fernandez-Triana, 2018
Billmasonius Fernandez-Triana, 2018: 28. Gender: neuter. Type species: Billmasoniuscienci Fernandez-Triana & Boudreault, 2018, by original designation.
The only known species was recently described from the Oriental region (Fernandez-Triana and Boudreault 2018). No host data are currently available for this genus. There is one DNA-barcode compliant sequences of Billmasonius in BOLD, representing one BIN (although that sequence has not been identified in BOLD as belonging to Billmasonius, see Fernandez-Triana and Boudreault 2018 for that).
Billmasoniuscienci Fernandez-Triana & Boudreault, 2018
Billmasoniuscienci Fernandez-Triana & Boudreault, 2018.
Type information. Holotype female, QSBG (examined). Country of type locality: Thailand.
Geographical distribution.OTL.
OTL: Thailand.
Genus Buluka de Saeger, 1948
Buluka de Saeger, 1948: 64. Gender: neuter (see below). Type species: Bulukastraeleni de Saeger, 1948, by original designation.
Known from eleven described species, mostly from the Oriental region, with a couple of taxa reaching the Afrotropical and Australasian regions. The revision by Austin (1989) is outdated. We have seen a few undescribed species in collections (CNC, RMNH) but the genus does not seem to be very diverse. Two Lepidoptera have been recorded as hosts, Immathyriditis Meyrick, 1906 (Immidae) and Psimadaquadripennis Walker, 1858 (Noctuidae) (Austin 1989, Gupta & Fernandez-Triana 2014). There is one DNA-barcode compliant sequence of Buluka in BOLD, from one undescribed species from Thailand.
Neither the etymology nor the gender of this genus was stated in the original description (de Saeger 1948) and, as far as we know, has never been discussed. Buluka was described based on a single species from the Belgian Congo, currently the Democratic Republic of the Congo, in central Africa. There is a word “Buluka” in the Xitsonga or Tsonga (a Bantu language spoken by the Tsonga people in central Africa), meaning “Explode. Burst. Blast” in Xitsonga (https://www.xitsonga.org/grammar/past?_=buluka); although it is not clear to us if that was the source de Saeger used for the genus name. Because of that, we here propose to consider the gender of this Microgastrinae genus to be neuter.
Bulukaachterbergi Austin, 1989
Bulukaachterbergi Austin, 1989.
Type information. Holotype female, AEIC (not examined but original description checked). Country of type locality: Malaysia.
Geographical distribution.OTL.
OTL: Malaysia.
Bulukacollessi Austin & Dangerfield, 1992
Bulukacollessi Austin & Dangerfield, 1992.
Type information. Holotype male, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (QLD).
Bulukahorni Gupta, 2013
Bulukahorni Gupta, 2013.
Type information. Holotype female, NBAIR (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Bulukahuddlestoni Austin, 1989
Bulukahuddlestoni Austin, 1989.
Type information. Holotype female, NHMUK (examined). Country of type locality: Solomon Islands.
Geographical distribution.AUS.
AUS: Solomon Islands.
Bulukanoyesi Austin, 1989
Bulukanoyesi Austin, 1989.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Bulukaorientalis Chou, 1985
Bulukaorientalis Chou, 1985.
Type information. Holotype female, TARI (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (TW).
Notes. Our species concept is based on Austin (1989).
Bulukaquickei Ranjith, 2015
Bulukaquickei Ranjith, 2015.
Type information. Holotype female, DZCU (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Bulukastraeleni de Saeger, 1948
Bulukastraeleni de Saeger, 1948.
Type information. Holotype female, RMCA (not examined but subsequent treatment of the species checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Cameroon, Democratic Republic of Congo, South Africa.
Notes. Our species concept is based on Austin (1989).
Bulukataiwanensis Austin, 1989
Bulukataiwanensis Austin, 1989.
Type information. Holotype male, TARI (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (TW).
Bulukatownesi Austin, 1989
Bulukatownesi Austin, 1989.
Type information. Holotype female, AEIC (not examined but original description checked). Country of type locality: Malaysia.
Geographical distribution.OTL.
OTL: India, Malaysia.
Bulukavuquangensis Long, 2015
Bulukavuquangensis Long, 2015.
Type information. Holotype female, VNMN (not examined but original description checked). Country of type locality: Vietnam.
Geographical distribution.OTL.
OTL: Vietnam.
Genus Carlmuesebeckius Fernandez-Triana, 2018
Carlmuesebeckius Fernandez-Triana, 2018: 28. Gender: neuter. Type species: Carlmuesebeckiussmithsonian Fernandez-Triana & Boudreault, 2018, by original designation.
The only known species was recently described from the Afrotropical region (Fernandez-Triana and Boudreault 2018). No host data are currently available for this genus. There are no DNA barcodes of Carlmuesebeckius in BOLD.
Carlmuesebeckiussmithsonian Fernandez-Triana & Boudreault, 2018
Carlmuesebeckiussmithsonian Fernandez-Triana & Boudreault, 2018.
Type information. Holotype female, CNC (examined). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Notes. In the original description the holotype depository was stated to be the CAS (Fernandez-Triana and Boudreault 2018: 54); however, that is a mistake as the specimen belongs to and is deposited in the CNC.
Genus Chaoa Luo & You, 2004
Chaoa Luo & You, 2004: 339. Gender: neuter. Type species: Chaoaflavipes Luo, You & Xiao, 2004, by original designation.
One known species from the Oriental region, described from a single female from China. No host data are currently available for this genus. There are no DNA barcodes of Chaoa in BOLD. The only reference available is the original description, which included three line drawings showing the species habitus dorsally, and some details of the metasoma. We suspect the validity of this genus, as it seems to us to be just a species of Glyptapanteles. The appearance of mediotergite II (divided into three sections by a pair of longitudinal grooves delimiting a smooth, medial are) was considered by Luo et al. (2004) to be unique to Chaoa but in fact it is quite similar to that found in all or some species of several Microgastrinae genera (e.g., Cotesia, Diolcogaster, Distatrix, Glyptapanteles, Nyereria, Rasivalva). However, pending a reassessment of Glyptapanteles (which, as currently understood, seems to be polyphyletic), and without having examined the single known specimen of Chaoa, we refrain from changing its generic status for the time being.
Chaoaflavipes Luo, You & Xiao, 2004
Chaoaflavipes Luo, You & Xiao, 2004.
Type information. Holotype female, HUNAU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Notes. The information on the type depository was confirmed to us by Kees van Achterberg (pers. comm.), who examined the specimen.
Genus Choeras Mason, 1981
Choeras Mason, 1981. Gender: masculine. Type species: Apantelesconsimilis Viereck, 1911, by original designation (Mason 1981: 76).
Currently with 80 described species, the genus is known from all biogeographical regions. No revision of Choeras has ever been produced, but most of the European/Palearctic species can be separated using the keys from van Achterberg (2002), Kotenko (2007a), Song et al. (2014), and Abdoli et al. (2019b). This is one of the most variable genera of Microgastrinae and, as currently understood, is probably polyphyletic. Even in the original description of the genus it was acknowledged that its limits might be changed in the future (Mason 1981: 77). The Holarctic species are relatively distinctive and uniform, but even in that region the species richness is much larger than documented at present. In tropical areas Choeras includes a mix of several unrelated groups, some of which might better be placed within separate genera. Depending on the generic concept that is adopted following future phylogenetic studies of Microgastrinae, Choeras may end up having several hundred species or just a few dozen. The host data are also very variable, with approximately 15 different families of Lepidoptera recorded so far, but records must be suspected in many cases. There are 820+ DNA-barcode compliant sequences of this genus in BOLD, representing 113 BINs, most of them from Canada and Thailand.
Choerasachterbergi Narendran, 1998
Choerasachterbergi Narendran, 1998.
Type information. Holotype female, RMNH (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Choerasadjunctus (Nees, 1834)
Microgasteradjunctus Nees, 1834.
Type information. Neotype female ZMHB (examined). Country of type locality: Germany.
Geographical distribution.OTL, PAL.
OTL: China (SN); PAL: Denmark, Germany, Netherlands, Sweden, United Kingdom.
Notes. Transferred from Microgaster to Apanteles by Reinhard (1881), then from Apanteles to Dolichogenidea by Papp (1988), and then from Dolichogenidea to Choeras by Shaw (2012b). The type from the original description was from Germany, we have not been able to determine the country of the neotype locality.
Choerasafrotropicalis Fernandez-Triana & van Achterberg, 2017
Choerasafrotropicalis Fernandez-Triana & van Achterberg, 2017.
Type information. Holotype female, RMNH (examined). Country of type locality: Yemen.
Geographical distribution.AFR.
AFR: Yemen.
Choerasalmus (Tobias & Kotenko, 1984)
Apantelesalmus Tobias & Kotenko, 1984.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (PRI).
Notes. Our species concept is based on Kotenko (2007a).
Choerasangustus Song & Chen, 2014
Choerasangustus Song & Chen, 2014.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, HI, HN, ZJ).
Choerasaper (Nixon, 1965), new combination
Apantelesaper Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (QLD).
Notes. Even in the original description it was recognized that this species was not at all typical of Apanteles (Nixon 1965: 62). The holotype (only known specimen of the species) has a propodeum with a coarse and irregular pattern of carinae and sculpture. A median, longitudinal carina is visible for most of the propodeum length; two shorter carinae near the nucha are also distinguished (suggesting the posterior half of an areola, although there are more carinae across the propodeum). The metanotum is strongly retracted from the scutellum, exposing the phragma. The ovipositor and sheaths are withdrawn into the hypopygium, but it is evident that the hypopygium is flexible (and supposedly pleated). The above characters strongly suggest the species does not belong in Apanteles. The best generic placement we can propose at present would be in Choeras (another candidate genus, Sathon, has an inflexible hypopygium), but study of additional specimens (if more are ever found) may change that in the future. The specimen was collected in an important area of wet subtropical rainforest habitats, with many endemic and/or significant species found there.
Choerasapo (Wilkinson, 1929)
Microgasterapo Wilkinson, 1929.
Type information. Syntypes female and male, ZMHB (not examined but original description checked). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Choerasapollion (Nixon, 1965), new combination
Hypomicrogasterapollion Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Notes. This species is clearly not an Hypomicrogaster, the most obvious characters to exclude it from that genus would be the large areolet in the fore wing, and the shapes of T1 and T2. The best generic placement at present is in Choeras.
Choerasarene (Nixon, 1973)
Apantelesarene Nixon, 1973.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Germany, Hungary, Ireland, Russia (ZAB, SAK), Spain, United Kingdom.
Choerasavus (Tobias & Kotenko, 1984)
Apantelesavus Tobias & Kotenko, 1984.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (ZAB, IRK, MAG, PRI).
Notes. Our species concept is based on Kotenko (2007a).
Choerasbatrachedrae (Kotenko, 1992)
Apantelesbatrachedrae Kotenko, 1992.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (ZAB, PRI).
Notes. Our species concept is based on Kotenko (2007a).
Choerasbotydis (Wilkinson, 1930)
Microgsterbotydis Wilkinson, 1930.
Type information. Holotype female, NHMUK (examined). Country of type locality: Indonesia.
Geographical distribution.OTL, PAL.
OTL: Indonesia; PAL: Japan, Russia (SAK).
Choerasbrevinervus Song & Chen, 2014
Choerasbrevinervus Song & Chen, 2014.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (ZJ); PAL: China (GS, XJ).
Choerasbushblitz Fagan-Jeffries & Austin, 2019
Choerasbushblitz Fagan-Jeffries & Austin, 2019.
Type information. Holotype female, TMAG (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (TAS).
Choerascalacte (Nixon, 1965)
Promicrogastercalacte Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (ACT, VIC).
Notes. This species is morphologically different from the typical Choeras that are usually found in temperate areas, specially the shape of the fore wing areolet. However, pending further study on “Choerassensu lato”, it is best kept as Choeras for the time being.
Choerasceto (Nixon, 1965)
Hypomicrogasterceto Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (ACT).
Notes. The ending of the species name has been variously treated as cetus (e.g., Austin and Dangerfield 1992, Fagan-Jeffries and Austin 2018), or ceto (e.g., Yu et al. 2016). Because the name is to be considered as a noun under ICZN Article 31.2.1, it must retain its original spelling and remain as ceto.
Choerasciscaucasicus (Tobias, 1971)
Apantelesciscaucasicus Tobias, 1971.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Lithuania, Russia (AD, PRI).
Notes. Our species concept is based on Kotenko (2007a).
Choerascompressifemur Chen & Song, 2004
Choerascompressifemur Chen & Song, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, HB).
Choerasconsimilis (Viereck, 1911)
Apantelesconsimilis Viereck, 1911.
Microgasterlateralis Provancher, 1886 [homonym of Microgasterlateralis Haliday, 1834].
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA, OTL.
NEA: Canada (MB, NB, ON, QC), USA (AR, MI, NY, OH, PA, VA); OTL: China (HB).
Notes. This species was treated as Dolichogenideaconsimilis by Yu et al. (2012, 2016), following the decision by Chen and Song (2004) of transferring the species to that genus. However, after examining the holotype, numerous specimens deposited in the CNC, DNA barcodes, and other references (e.g., Fernandez-Triana 2010), all available evidence clearly indicates that this species belongs to Choeras.
Choerasdaphne (Nixon, 1965), new combination
Apantelesdaphne Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Notes. The holotype is missing the head but is otherwise in good condition. We place this species within Choeras based on the complete median carina on the propodeum and the shapes and sculpture of T1 and T2, although the fore wing venation is not that of a typical Choeras. It might be that this species represents a different genus, but with only one specimen available we prefer to maintain it in Choeras, the best placement at present.
Choerasdissors (Nixon, 1965)
Promicrogasterdissors Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (ACT).
Choerasdorsalis (Spinola, 1808)
Microgasterdorsalis Spinola, 1808.
Microgastercruciatus Ratzeburg, 1844.
Microgastersuffolciensis Morley, 1902.
Type information. Type and depository unknown (not examined but authoritatively identified specimens examined). Country of type locality: Italy.
Geographical distribution.PAL.
PAL: Armenia, Austria, Azerbaijan, Belgium, Bulgaria, Canary Islands, Cyprus, Egypt, France, Georgia, Germany, Greece, Hungary, Iran, Israel, Italy, Jordan, Lithuania, Madeira Islands, Malta, Moldova, Poland, Romania, Russia (DA), Slovakia, Spain, Switzerland, Tunisia, Turkey, Ukraine, United Kingdom, Uzbekistan.
Notes.Shenefelt (1973: 703) summarized very well the status of the species when he wrote: “Much confusion exists regarding the species to which literature refers. Conflicting statements occur and some authors have simply chosen to ignore certain of the older names. It appears best for the present merely to list the references as found and hope that intensive study and corrections may be made later to rectify erroneous citations”. Unfortunately, not much progress has been achieved since, and here we restrict ourselves to citing the information as recorded in Yu et al. (2016). We examined the type of Microgastersuffolciensis Morley (which is missing the metasoma and the hind legs except for the metacoxae); we found that the pterostigma is notably wide (i.e., pterostigma height ca. two thirds its length), a character that may be unique among other Holarctic described species of Choeras.
Choerasepaphus (Nixon, 1965)
Hypomicrogasterepaphus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (ACT, QLD).
Choerasflavicorpus Song & Chen, 2014
Choerasflavicorpus Song & Chen, 2014.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (HI, YN).
Choerasfomes (Nixon, 1965), new combination
Hypomicrogasterfomes Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Notes. This species is clearly not an Hypomicrogaster, the most obvious characters to exclude it from that genus would be the large areolet in the fore wing, and the shapes of T1 and T2. The best generic placement at present is in Choeras.
Choerasformosus Abdoli & Fernandez-Triana, 2019
Choerasformosus Abdoli & Fernandez-Triana, 2019.
Type information. Holotype female, TMUC (examined). Country of type locality: Iran.
Geographical distribution.PAL.
PAL: Iran.
Choerasfujianensis Song & Chen, 2014
Choerasfujianensis Song & Chen, 2014.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Choerasfulviventris Fernandez-Triana & Abdoli, 2019
Choerasfulviventris Fernandez-Triana & Abdoli, 2019.
Type information. Holotype female, TMUC (examined). Country of type locality: Iran.
Geographical distribution.PAL.
PAL: Iran.
Choerasgerontius (Nixon, 1965), new combination
Hypomicrogastergerontius Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Notes. This species is clearly not an Hypomicrogaster, the most obvious characters to exclude it from that genus would be the large areolet in the fore wing, and the shapes of T1 and T2. The best generic placement at present is in Choeras.
Choerasgielisi van Achterberg, 2002
Choerasgielisi van Achterberg, 2002.
Type information. Holotype female, RMNH (not examined but original description checked). Country of type locality: Netherlands.
Geographical distribution.PAL.
PAL: France, Netherlands.
Choerasgnarus (Tobias & Kotenko, 1984)
Apantelesgnarus Tobias & Kotenko, 1984.
Type information. Holotype female, SIZK (not examined but subsequent treatment of the species checked). Country of type locality: Ukraine.
Geographical distribution.PAL.
PAL: Belarus, Russia (C, NC), Ukraine.
Notes. Our species concept is based on van Achterberg (2002).
Choerasgrammatitergitus Song & Chen, 2014
Choerasgrammatitergitus Song & Chen, 2014.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (SN); PAL: China (NX).
Choerashelespas Walker, 1996
Choerashelespas Walker, 1996.
Type information. Holotype female, LUNZ (not examined but original description checked). Country of type locality: New Zealand.
Geographical distribution.AUS.
AUS: New Zealand.
Choerashelle (Nixon, 1965), new combination
Hypomicrogasterhelle Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Sierra Leone.
Geographical distribution.AFR.
AFR: Nigeria, Sierra Leone.
Notes. This species is clearly not an Hypomicrogaster, the most obvious characters to exclude it from that genus would be the large areolet in the fore wing, and the shapes of T1 and T2. The best generic placement at present is in Choeras.
Choerasinfirmicarinatus Song & Chen, 2014
Choerasinfirmicarinatus Song & Chen, 2014.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, GD, ZJ).
Choerasinsignis (Muesebeck, 1938)
Apantelesinsignis Muesebeck, 1938.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (BC), USA (CA).
Choerasirates (Nixon, 1965), new combination
Hypomicrogasterirates Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. This species is clearly not an Hypomicrogaster, the most obvious characters to exclude it from that genus would be the large areolet in the fore wing, and the shapes of T1 and T2. The best generic placement at the time is in Choeras but it must be noted that this is one of the Oriental species of Choeras that probably will need a different, perhaps new, genus. Because that is beyond the scope of this paper, for the time being the species is transferred to Choeras.
Choeraskoalascatocola Fagan-Jeffries & Austin, 2017
Choeraskoalascatocola Fagan-Jeffries & Austin, 2017.
Type information. Holotype female, QM (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (QLD).
Choeraslibanius (Nixon, 1965), new combination
Hypomicrogasterlibanius Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Notes. This species was synonymized under Microgasterpsarae Wilkinson, 1927 (now in Choeras) by Valerio and Whitfield (2015). Here we raise libanius from synonymy and consider it to be a valid species, different from psarae (see below under psarae for more details on morphological differences between them). Also, we transfer libanius to Choeras, based on its pleated hypopygium, relatively long ovipositor sheaths, and T1 without a longitudinal sulcus. This species clearly belongs to a group of Oriental Choeras such as apo, nephta, psarae and many undescribed species we have seen in collections, with very large body size; they may be transferred to a different genus in the future (see discussion below, under C.nephta).
Choeraslongiterebrus (Rao & Chalikwar, 1976), new combination
Protomicroplitislongiterebrus Rao & Chalikwar, 1976.
Type information. Holotype female, BAMU (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. This species was transferred to Diolcogaster by Zeng et al. (2009). Based on the original description and extensive illustrations there, this species is clearly not Diolcogaster, based on the following: T1 excavate anteriorly but without median, longitudinal sulcus; ovipositor longer than metatibia; ovipositor sheaths long and moderately setose, but without thick setae apically; scutellar disc lacking a posteromedian band of rugosity; other characters (size of fore wing areolet, mostly smooth propodeum, T1 and T2) are not commonly present in Diolcogaster. The best generic placement at present would be in Choeras, although future study of the Oriental species currently placed in that genus may change its status. Following Article 31.2.1 of the ICZN the name is to be considered as a noun phrase in apposition, and the original spelling longiterebrus must be retained.
Choeraslongitergitus Song & Chen, 2014
Choeraslongitergitus Song & Chen, 2014.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, GZ, HI, HN, GD, ZJ).
Choeraslongus Song & Chen, 2014
Choeraslongus Song & Chen, 2014.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, ZJ).
Choerasloretta (Nixon, 1965), new combination
Hypomicrogasterloretta Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Notes. This species is clearly not an Hypomicrogaster, the most obvious characters to exclude it from that genus would be the large areolet in the fore wing, and the shapes of T1 and T2. The best generic placement at present is in Choeras.
Choerasmorialta Fagan-Jeffries & Austin, 2017
Choerasmorialta Fagan-Jeffries & Austin, 2017.
Type information. Holotype female, SAMA (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia.
Choerasnephta (Nixon, 1965)
Hypomicrogasternephta Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Notes. A very large species (body length 5.6 mm, fore wing length 6.2 mm). It belongs to a group of large Choeras from the Oriental region (e.g., apo, libanius, psarae, and many undescribed species we have seen in collection) which in the future may be better placed in a different genus. For the time being they are all kept in Choeras, until a better phylogenetic understanding of this group is gained.
Choeraspapua (Wilkinson, 1936)
Microgasterpapua Wilkinson, 1936.
Type information. Holotype female, NHMUK (examined). Country of type locality: Papua New Guinea.
Geographical distribution.AUS.
AUS: Indonesia, Papua New Guinea.
Choerasparabolus Kotenko, 2007
Choerasparabolus Kotenko, 2007.
Type information. Holotype female, SIZK (not examined but original description checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (PRI).
Choerasparasitellae (Bouché, 1834)
Microgasterparasitellae Bouché, 1834.
Microgasterflavilabris Ratzeburg, 1844.
Microgasterrufilabris Ratzeburg, 1844.
Apanteleslictorius Reinhard, 1880.
Apantelespolypori Gautier & Bonnamour, 1930.
Type information. Holotype female, ZMHB (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.NEA, PAL.
NEA: Canada (Ontario); PAL: Austria, Belgium, Czech Republic, Finland, France, Georgia, Germany, Hungary, Iran, Israel, Italy, Korea, Latvia, Moldova, Netherlands, Poland, Romania, Russia (DA, PRI, SAK, SPE, TOM, YAR), Serbia, Slovakia, Spain, Sweden, Switzerland, Turkey, Ukraine, United Kingdom, Uzbekistan.
Notes. Our species concept is based on van Achterberg (2002) and Fernandez-Triana et al. (2016a).
Choerasparasonium Kotenko, 2007
Choerasparasonium Kotenko, 2007.
Type information. Holotype female, ZIN (not examined but original description checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (KAM).
Choerasparviocellus Song & Chen, 2014
Choerasparviocellus Song & Chen, 2014.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (GZ, HI, SN, TW, YN); PAL: China (NM, NX).
Choerasparvoculus Fagan-Jeffries & Austin, 2019
Choerasparvoculus Fagan-Jeffries & Austin, 2019.
Type information. Holotype female, TMAG (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (TAS).
Choeraspsarae (Wilkinson, 1927)
Microgasterpsarae Wilkinson, 1927.
Type information. Holotype female, NHMUK (examined). Country of type locality: Malaysia.
Geographical distribution.OTL, PAL.
OTL: China (TW), India, Malaysia, Sri Lanka, Thailand; PAL: Korea.
Notes. We consider the record of psarae from Korea (Papp 1987: 439), which is also reported in Yu et al. (2012, 2016), as very suspicious, although we do not remove it from the present checklist. The short description of the single Korean specimen (as provided by Papp 1987) is different from the psarae type we have examined, and the known distribution in Korea (near Pyongyang) is fully Palearctic, whereas all other known records of this species are in the Oriental region. Also, a literature record from Taiwan (Papp 1987: 439) had been overlooked by recent authors but it is accepted here. Mason (1981) transferred Microgasterpsarae to Choeras, a decision followed by several other authors (see Yu et al. 2016 for list of references). Then Valerio and Whitfield (2015) transferred the species to Diolcogaster and synonymized Hypomicrogasterlibanius Nixon, 1965 under psarae. After examining the types and original description, we consider both decisions from Valerio and Whitfield (2015) incorrect. Here we transfer psarae back to Choeras based on its pleated hypopygium, relatively long ovipositor sheaths (around two thirds metatibia length), T1 smooth and without longitudinal sulcus, T2 smooth, transversely subtriangular and without median field, and propodeum mostly smooth, with a strong, median longitudinal carina with several small carinae radiating from it. This species clearly belongs to a group of Oriental Choeras such as apo, libanius, nephta, and many undescribed species we have seen in collections, with very large body size; they may be transferred to a different genus in the future (see discussion above, under C.nephta). We also remove H.libanius from synonym with psarae and consider it as a valid species, as the differences between them are significant: psarae has a yellow metasoma (except for T2 brown on posterior half and T3 with small brown), all coxae yellow (except for brown spot on posterior third ventrally), T1 comparatively narrower at posterior margin (as compared to anterior margin), and smaller body size (ca. 4.5 mm); whereas libanius has the metasoma mostly dark brown, all coxae dark brown to black, T1 comparatively wider at the posterior margin, and much larger body size (ca. 6.2 mm) (see Nixon 1965 for more differences).
Choerasqazviniensis Fernandez-Triana & Talebi, 2019
Choerasqazviniensis Fernandez-Triana & Talebi, 2019.
Type information. Holotype female, TMUC (examined). Country of type locality: Iran.
Geographical distribution.PAL.
PAL: Iran.
Choerasrecusans (Walker, 1860), new combination
Microgasterrecusans Walker, 1860.
Type information. Holotype female, NHMUK (examined). Country of type locality: Sri Lanka.
Geographical distribution.OTL.
OTL: India, Sri Lanka.
Notes. This species was transferred to Apanteles by Wilkinson (1927: 178) and treated under that genus by most authors (but see Yu et al. 2016 for a different treatment). We have examined the holotype and it has a strong median, longitudinal carina, which precludes the species from belonging in Apanteles or related genera. Based on the mesosoma sculpture, T1 wide and with a shallow excavation anteriorly and relatively long ovipositor sheaths (as long as the metatibia), this species is better placed in Choeras.
Choerasruficornis (Nees, 1834)
Microgasterruficornis Nees, 1834.
Apanteleshedymeles Nixon, 1973.
Type information. Neotype female, RBINS (not examined but authoritatively identified specimens examined). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Belgium, Finland, France, Georgia, Germany, Hungary, Italy, Latvia, Netherlands, Norway, Poland, Romania, Russia (AMU, PRI, SAK), Slovakia, Sweden, Switzerland, United Kingdom.
Notes. We examined the type of Apanteleshedymeles Nixon.
Choerasrugulosus Song & Chen, 2014
Choerasrugulosus Song & Chen, 2014.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (GZ, YN, ZJ); PAL: China (HA).
Choerassemele (Nixon, 1965)
Hypomicrogastersemele Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Morocco.
Geographical distribution.PAL.
PAL: Canary Islands, Greece, Israel, Italy, Malta, Morocco, Spain.
Choerassemilunatus Song & Chen, 2014
Choerassemilunatus Song & Chen, 2014.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (GD).
Choerassemirugosus Song & Chen, 2014
Choerassemirugosus Song & Chen, 2014.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (HI, HN, YN, ZJ); PAL: China (HE).
Choerassordidus (Ashmead, 1900), new combination
Apantelessordidus Ashmead, 1900.
Microplitiscarinata Ashmead, 1900.
Type information. Holotype male, NHMUK (examined). Country of type locality: Saint Vincent.
Geographical distribution.NEO.
NEO: Saint Vincent.
Notes.Muesebeck (1958b: 427) transferred this species from Apanteles to Microplitis, a decision accepted by other authors (Shenefelt 1972, Fernandez-Triana et al. 2015b). However, after we examined the male holotype it became clear that is not Microplitis, as it has an enlarged metacoxa (at least two thirds as long as entire metasoma), T1 does not have a median sulcus (the anterior half is broadly hollowed whereas the posterior half is rugose), the scutellar disc does not have a posteromedian band of rugosity, and the head and mesosoma are almost totally unsculptured (including completely smooth propodeum which has only a median longitudinal carina). We examined one female and two males of the type series of Microplitiscarinata Ashmead, 1900 which are also in the NHMUK; those specimens are very similar and clearly conspecific with the sordidus holotype. The female has a relatively long ovipositor and a pleated hypopygium. Based on the morphological characters discussed above, we consider this species to belong to the genus Choeras.
Choerasstenoterga (de Saeger, 1944), new combination
Microgasterstenoterga de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. Based on the original description (de Saeger 1944), the best generic placement at present would be in Choeras. However, study of the type specimen will be needed in the future.
Choerassuperbus (de Saeger, 1944), new combination
Microgastersuperba de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. Based on the original description, the best generic placement at present would be in Choeras, based on the propodeum with median carina, pleated hypopygium, length of ovipositor sheaths, and shapes of T1 and T2 (as described and illustrated in de Saeger 1944: 103–105).
Choerassylleptae (de Saeger, 1942), new combination
Microgastersylleptae de Saeger, 1942.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Ivory Coast, Rwanda.
Notes. Based on the original description and illustrations provided there (de Saeger 1942), the best generic placement at present would be in Choeras, based on the propodeum with median carina, pleated hypopygium, and length of the ovipositor sheaths.
Choerastaftanensis Ghafouri Moghaddam & van Achterberg, 2018
Choerastaftanensis Ghafouri Moghaddam & van Achterberg, 2018.
Type information. Holotype female, DPPZ (not examined but original description checked). Country of type locality: Iran.
Geographical distribution.PAL.
PAL: Iran.
Choerastakeuchii (Watanabe, 1937)
Microgastertakeuchii Watanabe, 1937.
Type information. Holotype female, EIHU (examined). Country of type locality: Japan.
Geographical distribution.PAL.
PAL: Japan, Russia (PRI).
Choerastarasi Kotenko, 2007
Choerastarasi Kotenko, 2007.
Type information. Holotype female, SIZK (not examined but original description checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (SAK).
Choerastedellae (Nixon, 1961)
Apantelestedellae Nixon, 1961.
Apantelesepinotiae Fischer, 1962 [homonym of Apantelesepinotiae Viereck, 1912].
Apantelesepinoticida Fischer, 1966.
Type information. Holotype female, MMBC (not examined but subsequent treatment of the species checked). Country of type locality: Czech Republic.
Geographical distribution.PAL.
PAL: Austria, Bulgaria, Croatia, Czech Republic, Denmark, Finland, Germany, Greece, Hungary, Iran, Israel, Korea, Madeira Islands, Moldova, Netherlands, Poland, Romania, Russia (ZAB, PRI, YAR), Slovakia, Sweden, Switzerland, United Kingdom.
Notes. Our species concept is based on van Achterberg (2002).
Choerastegularis (Szépligeti, 1905)
Microgastertegularis Szépligeti, 1905.
Type information. Type lost (not examined but subsequent treatment of the species checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (NSW, WA).
Notes. Our species concept is based on Austin and Dangerfield (1992), Papp (2004) and Fernandez-Triana (2015). The later author commented on what we know about the status of this species: “Because the male holotype is lost (Austin and Dangerfield 1993, Papp 2004) the correct identity of this species may never be established unambiguously. However, it is clear that this species is not Protomicroplitis, based on the illustrations of the fore wing in Nixon (1965: fig. 304), and propodeum and mediotergites 1–3 in Austin and Dangerfield (1992: fig. 27). We agree with Austin and Dangerfield (1992) that it is most likely to be Choeras”.
Choerastenuialatus Song & Chen, 2014
Choerastenuialatus Song & Chen, 2014.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (ZJ).
Choerastiro (Reinhard, 1880)
Microgastertiro Reinhard, 1880.
Type information. Type and depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.NEA, PAL.
NEA: Canada (NL, NS, PE); PAL: Austria, Bulgaria, France, Germany, Greece, Hungary, Iran, Israel, Poland, Romania, Russia (SAK, SAR), Slovakia, Spain, Switzerland, United Kingdom.
Notes. Location of type doubtful (see Nixon 1965). Our species concept is based on van Achterberg (2002) and Abdoli et al. (2019b).
Choerastumidus Song & Chen, 2014
Choerastumidus Song & Chen, 2014.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (GZ, HB, SN); PAL: China (NX).
Choerasvacillatrix (Wilkinson, 1930), new combination
Microgastervacillatrix Wilkinson, 1930.
Type information. Holotype female, NHMUK (examined). Country of type locality: Uganda.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Uganda.
Notes. Transferred to Choeras based on the fore wing having an areolet (vein r-m transparent but clearly visible), propodeum with median, longitudinal carina, hypopygium flexible and with several pleats, ovipositor sheaths relatively long and entirely setose, and the shapes of T1 and T2 in agreement with many other species in this genus.
Choerasvacillatropsis (de Saeger, 1944), new combination
Microgastervacillatropsis de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. Based on the original description (de Saeger 1944), the best generic placement at present would be Choeras. This species was considered by de Saeger (1944: 97) as morphologically similar to Microgastervacillatrix Wilkinson, 1930, which is also being transferred to Choeras in the present paper (see notes under that species above).
Choerasvalidicarinatus Song & Chen, 2014
Choerasvalidicarinatus Song & Chen, 2014.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (NX).
Choerasvalidus (Thomson, 1895)
Microgastervalidus Thomson, 1895.
Type information. Neotype female, MZLU (not examined but subsequent treatment of the species checked). Country of type locality: Sweden.
Geographical distribution.PAL.
PAL: France, Hungary, Italy, Netherlands, Russia (SAK), Slovakia, Sweden, Switzerland, United Kingdom.
Notes. Our species concept is based on van Achterberg (2002).
Choerasvaricolor Song & Chen, 2014
Choerasvaricolor Song & Chen, 2014.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, GD, GX, GZ, HI, SN, YN, ZJ).
Choerasvenilia (Nixon, 1965), new combination
Apantelesvenilia Nixon, 1965.
Type information. Holotype female, AEIC (not examined but original description checked). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Notes. We place this species within Choeras based on the complete median carina on the propodeum, and the fact it was keyed out by Nixon (1965) in the same couplet as Apantelesdaphne Nixon (a species for which we were able to examine the holotype and are transferring in this paper to Choeras, see above under that species for more details). Both venilia and daphne may be part of a different genus related to Choeras but, pending a comprehensive study of this genus, we consider the best placement at present is the one we propose here.
Choerasyunnanensis Song & Chen, 2014
Choerasyunnanensis Song & Chen, 2014.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (YN).
Choeraszerovae Kotenko, 2007
Choeraszerovae Kotenko, 2007.
Type information. Holotype female, SIZK (not examined but original description checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (PRI).
Choeraszygon Fagan-Jeffries & Austin, 2019
Choeraszygon Fagan-Jeffries & Austin, 2019.
Type information. Holotype female, QM (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (QLD).
Genus Clarkinella Mason, 1981
Clarkinella Mason, 1981: 66. Gender: feminine. Type species: Clarkinellacanadensis Mason, 1981, by original designation.
This is a New World genus, with two species currently described from the Nearctic and Neotropical regions. We have seen a few additional species in collections (CNC) but Clarkinella does not seem to be very species rich. No host data are currently available. There are eight DNA-barcode compliant sequences of this genus in BOLD, representing five BINs.
Clarkinellacanadensis Mason, 1981
Clarkinellacanadensis Mason, 1981.
Type information. Holotype female, CNC (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (ON).
Clarkinellaedithae Mason, 1981
Clarkinellaedithae Mason, 1981.
Type information. Holotype female, CNC (examined). Country of type locality: Trinidad & Tobago.
Geographical distribution.NEO.
NEO: Brazil (MG. RJ), Trinidad & Tobago.
Genus Cotesia Cameron, 1891
Cotesia Cameron, 1891: 185. Gender: feminine. Type species: Cotesiaflavipes Cameron, 1891, by monotypy.
Cryptapanteles Viereck, 1909: 209. Type species: Cryptapantelesrileyanus Viereck, 1909 (= Apantelesemarginatus Riley, not Nees), by original designation and monotypy.
A cosmopolitan genus, with 328 described species known from all biogeographical regions of the planet, and perhaps 1,500–2,000 species (Mason 1981). Many European species were revised by Nixon and Papp in several papers from the 1970s and 1980s, as well as more recently by Shaw (2007, 2009, 2012a, 2012b, 2017b, Shaw et al. 2009, 2015). The Chinese species are keyed out in Chen and Song (2004). Other revisions include the species from Greenland (van Achterberg 2006), Réunion (Rousse and Gupta 2013), stem-borer parasitoids in Africa (van Achterberg and Polaszek 1996, van Achterberg and Walker 1998), the flavipes species group worldwide (Fujie et al. 2018) but overall the taxonomic coverage of the world species is far from complete. We have seen hundreds of undescribed species in collections, from both temperate and tropical areas. This is also one of the most morphologically distinctive genera of Microgastrinae, with perhaps only a few species of Protapanteles that might be confused with part of the genus. More than 30 families of Lepidoptera have been recorded as hosts for Cotesia, but many records are likely to be incorrect and/or need further verification. From a biological control perspective this is probably the most significant and well-studied genus of Microgastrinae in the world. In Costa Rica (ACG) most of the known hosts belong to three families: Nymphalidae, Saturniidae, and Sphingidae (unpublished information extracted from BOLD and ACG databases). There are almost 5,000 DNA-barcode compliant sequences of this genus in BOLD, representing 320 BINs, mostly from Canada and Costa Rica.
Cotesiaabdinbekovae Papp, 2009
Cotesiaabdinbekovae Papp, 2009.
Apantelesrufiventris Abdinbekova, 1969 [secondary homonym of Apantelesrufiventris Bingham, 1906].
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Azerbaijan.
Geographical distribution.PAL.
PAL: Azerbaijan, Croatia, Russia (S), Turkmenistan.
Notes. Our species concept is based on Papp (1987a, 2009).
Cotesiaabjecta (Marshall, 1885)
Apantelesabjectus Marshall, 1885.
Apantelescomplanatus Lyle, 1916.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Croatia, Finland, France, Germany, Hungary, Iran, Ireland, Israel, Italy, Mongolia, Poland, Romania, Russia (N), Slovakia, Switzerland, United Kingdom, Yugoslavia.
Notes.Shenefelt (1972: 431) did not provide details for the type location and was even doubtful of the type being in London (NHMUK). However, Nixon (1974: 484 and especially 485) referred to the type specimen both in the species description and in additional comments added at the end of the species treatment. We have examined the type of abjectus (a female with number 3c.29, which is exactly the same code mentioned by Shenefelt) and also the type series of Apantelescomplanatus (Lyle, 1916), which was synonymized by Nixon (1974: 484), a decision we agree with. The species distribution in Iran and Russia is based on Belokobylskij et al. (2019).
Cotesiaacaudus (Provancher, 1886)
Microgasteracaudus Provancher, 1886.
Apanteleshydriae Muesebeck, 1921.
Type information. Lectotype female, ULQC (not examined but subsequent treatment of the species checked). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (NS, ON, QC), USA (CT, MA, NJ, NY, PA, RI, VA, WV, WI).
Notes. Our species concept is based on Muesebeck (1921), Mason (1981), Papp (1987a) and Fernandez-Triana (2010). The ending of the species name has been variously treated; following Article 31.2.1 of the ICZN the name is a noun in apposition and the original spelling acaudus must be retained.
Cotesiaacerbiae Shaw & Vikberg, 2015
Cotesiaacerbiae Shaw & Vikberg, 2015.
Type information. Holotype female, RSME (examined). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (YAN).
Cotesiaacronyctae (Riley, 1870)
Microgasteracronyctae Riley, 1870.
Apantelesorgyiae Ashmead, 1893.
Type information. Holotype male, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (AB, ON, SK), USA (CA, CO, CT, IL, IN, IA, MD, MA, MO, NH, NJ, OH).
Notes. The male specimen (USNM type number 2770) has one fore wing and the head detached (but glued to a piece of wood on the pin). The sex of the holotype had not been detailed before (e.g., Shenefelt 1972: 433 listed it as “?”) so it is here recorded for the first time. This species was first mentioned in Riley (1870: 120) as Microgasteracronyctae. From a footnote in that same page it appears that Riley intended to describe the species in a different manuscript; however, the 1870 publication provides details of the wasp larvae and cocoons, as well as comments on its Lepidoptera host, thus making that paper the de facto original description of acronyctae. This has been accepted by subsequent authors when recording the author and year of the species (e.g., Shenefelt 1972, Marsh 1979, Whitfield 1995, Fernandez-Triana 2010; also by Yu et al. 2016, although those authors considered 1871 (not 1870) as the year of publication). The wasp species was described in a more comprehensive way, including details of the adult wasp, differences with other Microgastrinae species, and a repetition of the biological information presented in 1870, in Riley (1881: 312–313), this time with the name Apantelesacronyctae.
Cotesiaacuminata (Reinhard, 1880)
Apantelesacuminatus Reinhard, 1880.
Apantelescultrator Marshall, 1885.
Type information. Syntypes female and male, ZMHB (not examined but authoritatively identified specimens examined). Country of type locality: Germany.
Geographical distribution.OTL, PAL.
OTL: China (FJ); PAL: Armenia, Austria, China (BJ), Czech Republic, Finland, France, Georgia, Germany, Hungary, Israel, Romania, Russia (BU, PRI), Slovakia, Spain, Sweden, Tajikistan, Ukraine, Uzbekistan.
Notes. We examined the type of Apantelescultrator (Marshall, 1885). The species distribution in Uzbekistan is based on Belokobylskij et al. (2019).
Cotesiaacutula (Tobias, 1973)
Apantelesacutulus Tobias, 1973.
Type information. Holotype female, ZIN (examined). Country of type locality: Lithuania.
Geographical distribution.PAL.
PAL: Hungary, Lithuania, Russia (NW).
Cotesiaadippevora Shaw, 2009
Cotesiaadippevora Shaw, 2009.
Type information. Holotype female, RSME (examined). Country of type locality: Italy.
Geographical distribution.PAL.
PAL: Finland, Italy.
Cotesiaaffinis (Nees, 1834)
Microgasteraffinis Nees, 1834.
Microgastereuphorbiae Bouché, 1834.
Microgastervinulae Bouché, 1834.
Apantelesharpyiae Niezabitowski, 1910.
Apantelesplanus Watanabe, 1932.
Type information. Neotype female, ZMHB (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.AFR, OTL, PAL.
AFR: Cape Verde; OTL: China (GZ, HN, ZJ); PAL: Armenia, Austria, China (HL, LN, NM, NX, SN), France, Germany, Hungary, Italy, Japan, Kazakhstan, Korea, Latvia, Poland, Romania, Russia (PRI, ROS, YAR), Serbia, Slovakia, Spain, Sweden, Switzerland, Ukraine, United Kingdom.
Notes. Our species concept is based on Nixon (1974), Papp (1987a), Chen and Song (2004), and Kotenko (2007a).
Cotesiaagricola (Viereck, 1917)
Apantelesagricola Viereck, 1917.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CT).
Cotesiaalgonquinorum (Viereck, 1917)
Apantelesalgonquinorum Viereck, 1917.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CT).
Cotesiaalia (Muesebeck, 1958)
Apantelesalius Muesebeck, 1958.
Type information. Holotype female, USNM (examined). Country of type locality: Venezuela.
Geographical distribution.NEO.
NEO: Brazil (SP), Peru, Venezuela.
Notes. The species name must be treated as an adjective and not as a noun (Doug Yanega, pers. comm.) and thus it must match the gender of the genus name.
Cotesiaalternicolor (You & Zhou, 1988)
Apantelesalternicolor You & Zhou, 1988.
Type information. Holotype female, HUNAU (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (SD).
Notes. Our species concept is based on Chen and Song (2004).
Cotesiaalypiae (Muesebeck, 1922)
Apantelesalypiae Muesebeck, 1922.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CT).
Cotesiaamericana (Lepeletier, 1825)
Microgasteramericanus Lepeletier, 1825.
Microgasterflaviventris Cresson, 1865.
Apantelesmexicanus Ashmead, 1895.
Type information. Syntypes female and male, depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Martinique.
Geographical distribution.NEA, NEO.
NEA: USA (AZ, FL, OK, TX); NEO: Cuba, Dominican Republic, Guyana, Haiti, Jamaica, Martinique, Mexico, Puerto Rico.
Notes. Our species concept is based on Muesebeck (1921) and Wilkinson (1930c).
Cotesiaamesis (Nixon, 1974)
Apantelesamesis Nixon, 1974.
Type information. Holotype female, NHMUK (examined). Country of type locality: Switzerland.
Geographical distribution.PAL.
PAL: Poland, Slovakia, Switzerland.
Cotesiaammalonis (Muesebeck, 1926)
Apantelesammalonis Muesebeck, 1926.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (NJ, NY).
Cotesiaamphipyrae (Watanabe, 1934)
Apantelesamphipyrae Watanabe, 1934.
Type information. Holotype female, EIHU (examined). Country of type locality: Japan.
Geographical distribution.PAL.
PAL: Japan.
Cotesiaanalis (Nees, 1834)
Microgasteranalis Nees, 1834.
Microgasterpraetextata Haliday, 1834.
Microgastermediana Ratzeburg, 1852.
Apantelesleucaniae Wilkinson, 1937.
Type information. Neotype female, ZMHB (not examined but authoritatively identified specimens examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Armenia, Belgium, Czech Republic, France, Georgia, Germany, Hungary, Ireland, Italy, Netherlands, Russia (IRK), Sweden, Switzerland, United Kingdom.
Notes. We examined the type of A.leucaniae (Wilkinson).
Cotesiaancilla (Nixon, 1974)
Apantelesancilla Nixon, 1974.
Type information. Holotype female, NHMUK (examined). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Armenia, Austria, Bulgaria, Croatia, Germany, Greece, Hungary, Iran, Israel, Italy, Japan, Macedonia, Mongolia, Netherlands, Russia (PRI), Slovakia, Spain, Switzerland, Turkey, Yugoslavia.
Notes. The holotype (with code 3c.1790) is deposited in the NHMUK and not in Berlin (ZHMB), as stated in Yu et al. (2016). The species distribution in Armenia is based on Belokobylskij et al. (2019).
Cotesiaanisotae (Muesebeck, 1921)
Apantelesanisotae Muesebeck, 1921.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (NB, ON), USA (AR, CT, FL, MD, MA, NJ, NY, RI, TX, VA).
Cotesiaanomidis (Watanabe, 1942)
Apantelesanomidis Watanabe, 1942.
Type information. Holotype female, EIHU (examined). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (HN, JS, ZJ), Vietnam; PAL: China (LN, SN).
Cotesiaanthelae (Wilkinson, 1928)
Apantelesanthelae Wilkinson, 1928.
Type information. Holotype female, NHMUK (not examined but subsequent treatment of the species checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (NSW, VIC).
Notes. Our concept of this species is based on Austin and Dangerfield (1992).
Cotesiaaphae (Watanabe, 1934)
Apantelesaphae Watanabe, 1934.
Type information. Holotype female, EIHU (examined). Country of type locality: Japan.
Geographical distribution.PAL.
PAL: Japan.
Cotesiaarctica (Thomson, 1895), status revised
Microgasterarcticus Thomson, 1895.
Type information. Holotype female, MZLU (not examined but subsequent treatment of the species checked). Country of type locality: Norway.
Geographical distribution.PAL.
PAL: Norway.
Notes. Because of the confusion surrounding the application of this name and despite its possible synonymy (summarised by Shaw 2007, see also Broad et al. 2016) it seems best to regard arcticus as a valid species for the time being, especially because Marshall (1899) redescribed it without reference to any similarity with his own species astrarches.
Cotesiaargynnidis (Riley, 1889)
Apantelesargynnidis Riley, 1889.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CA, CT, DC, IL, KY, MA, NJ, NY, TX, WV).
Cotesiaasavari (Sathe, 1989), new combination
Apantelesasavari Sathe, 1989.
Type information. Holotype female, SUKI (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. The original description is the only reference available for this species. Even though is not clear or consistent (e.g., compare the drawing of the propodeum with its description) it is evident that the species is not an Apanteles. Based on the short ovipositor, the drawing of the propodeum, and the recorded host, the best placement at present will be in Cotesia.
Cotesiaastrarches (Marshall, 1889)
Apantelesastrarches Marshall, 1889.
Apantelesgenalis Tobias, 1964.
Type information. Lectotype female, PCMAG (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Afghanistan, Azerbaijan, Croatia, Cyprus, Finland, France, Georgia, Germany, Greece, Hungary, Kazakhstan, Macedonia, Moldova, Norway, Poland, Russia (C, NC, S), Slovakia, Slovenia, Spain, United Kingdom, Yugoslavia.
Note. In this paper we are removing Microgasterarcticus Thomson, 1895 (currently in Cotesia) from synonymy with astrarches and considering it as a distinct, valid species (see more details under Cotesiaarctica above).
Cotesiaatalantae (Packard, 1881)
Microgasteratalantae Packard, 1881.
Type information. Syntypes female and male, depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (AB, MB, ON, QC, SK), USA (CO, CT, MA, MI, NH, NJ, NY, PA, RI, VT, WV, WY).
Notes.Shenefelt (1972: 448) mentioned the need to designate a lectotype but, as far as we know, none has been designated yet. Our species concept is based on Muesebeck (1921) and Papp (1987a).
Cotesiaaururus (Telenga, 1955)
Apantelesaururus Telenga, 1955.
Type information. Lectotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Georgia, Russia (ORE).
Notes. Our species concept is based on Tobias (1986) and Papp (1987a). The ending of the species name has been variously treated; following Article 31.2.1 of the ICZN the name is a noun in apposition and the original spelling aururus must be retained.
Cotesiaaustraliensis (Ashmead, 1900)
Apantelesaustraliensis Ashmead, 1900.
Type information. Holotype female, USNM (examined). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (VIC).
Notes. The holotype has a rather smooth propodeum, with only a trace of a median carina on the posterior 0.3, T1 is parallel-sided and T2 looks more like Protapanteles. Overall, the specimen looks more like a Protapanteles species than Cotesia, but we refrain from transferring it here until future studies better resolve the relationships between the two genera (see above under the section Brief diagnosis of all Microgastrinae genera as they are understood in this paper, for a discussion of Protapanteles as just a potential species group of Cotesia).
Cotesiaautographae (Muesebeck, 1921)
Apantelesautographae Muesebeck, 1921.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (MB, NL, QC), USA (FL, GA, LA, MD, MI, OR, SC, SD, TN, TX, VA).
Cotesiaautumnatae Shaw, 2013
Cotesiaautumnatae Shaw, 2013.
Type information. Holotype female, RSME (examined). Country of type locality: Finland.
Geographical distribution.PAL.
PAL: Finland.
Cotesiaayerzai (Brèthes, 1920), name amended
Apantelesayerzai Brèthes, 1920.
Apanteleswilliamsoni Blanchard, 1935.
Apantelesayerza Blanchard, 1920 [incorrect subsequent spelling].
Type information. Lectotype female, MACN (not examined but subsequent treatment of the species checked). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina.
Notes. The original name of the species Apantelesayerzai was meant to honor Dr. Abel Ayerza, a man, as clearly mentioned in the original description, and also mentioned in a subsequent treatment of the species (Blanchard 1947: 8). Thus, the correct specific epithet must end with an i. That spelling of the species name was followed by most of the Spanish-speaking authors and is the one used in the catalogue of parasitic Hymenoptera from Argentina (de Santis 1967a: 135). However, the name was incorrectly spelled subsequently as ayerza by English-speaking authors, e.g., the catalogue of world Braconidae (Shenefelt 1972: 450) and the lectotype designation (Sharkey et al. 2000). Because the scientific literature about the species contains examples of both uses of the name within the past 50 years, it cannot be considered that the incorrect subsequent spelling is in prevailing use (cf. Article 33.3.1 of the ICZN) and thus there is no need to preserve that subsequent spelling. For that reason, we amend here the species name to its original spelling ayerzai.
Cotesiabactriana (Telenga, 1955), new combination
Apantelesbactrianus Telenga, 1955.
Type information. Lectotype female, ZIN (not examined but original description checked). Country of type locality: Uzbekistan.
Geographical distribution.PAL.
PAL: Uzbekistan.
Notes. The original description as well as subsequent papers (Tobias 1986, Papp 1987a) clearly indicate this species belongs to Cotesia.
Cotesiaballi Oltra & Michelena, 1989
Cotesiaballi Oltra & Michelena, 1989.
Type information. Holotype female, UVS (not examined but original description checked). Country of type locality: Spain.
Geographical distribution.PAL.
PAL: Spain.
Notes. The type material is probably in the Instituto Cavanilles de Biodiversitat y Biología Evolutiva, University of Valencia, Spain. But we have not been able to verify that information yet.
Cotesiabambeytripla (Shenefelt, 1972), new combination
Apantelesbambeytriplus Shenefelt, 1972.
Apantelesdiacrisiae Risbec, 1951 [primary junior homonym of Apantelesdiacrisiae Gahan, 1917].
Apantelesbambeyi Risbec, 1952 [primary junior homonym of Apantelesbambeyi Risbec, 1951].
Type information. Syntypes female and male, depository unknown (not examined but original description checked). Country of type locality: Senegal.
Geographical distribution.AFR.
AFR: Senegal.
Notes. From the original description, as well as a drawing of propodeum and T1–T2 included there, it is clear that this species belongs in Cotesia. From a nomenclatural point of view, this species has had a rather complicated story, including having different names throughout the years. For the sake of clarity we briefly outline it here. It was originally described as Apantelesdiacrisiae by Risbec (1951). One year later, to avoid homonymy with Apantelesdiacrisiae (Gahan, 1917), it was changed to Apantelesbambeyi by Risbec (1952). Surprisingly, Risbec overlooked his own Apantelesbambeyi Risbec, 1951 (described in the same paper in which he had originally described Apantelesdiacrisiae!). Thus his 1952 paper created another junior homonym. To correct that, Shenefelt (1972) proposed a replacement name, Apantelesbambeytriplus.
Cotesiaberberidis (Rudow, 1910), new combination
Microgasterberberidis Rudow, 1910.
Type information. Type and depository unknown (not examined but original description checked). Country of type locality: Austria.
Geographical distribution.PAL.
PAL: Austria.
Notes. The original description provides only a brief description of the cocoon mass shape and colour, and the number of wasps emerged (Rudow 1910: 230). We here transfer berberidis to Cotesia based on the statement that the species is similar to glomeratus, which has long been placed in Cotesia. In the original description it is stated that the host is a sawfly, Argeberberidis Schrank, 1802 (Argidae), but we deem that record likely to be incorrect, as the author reared many lepidopteran larvae alongside (as stated in that paper and others authored by him). In fact, the cocoons of berberidis are described as a sulfur yellow mass, which matches the shape and colour of cocoons of Cotesia species on Aporiacrataegi (Linnaeus, 1758) (Pieridae) (e.g., see http://www.lepiforum.de/lepiwiki.pl?Aporia_Crataegi), a lepidopteran treated by Rudow in the previous paragraph of his paper (we suspect that is the actual host of berberidis). Examination of the specimens will be needed in the future.
Cotesiaberberis (Nixon, 1974)
Apantelesberberis Nixon, 1974.
Type information. Holotype female, NHMUK (examined). Country of type locality: Switzerland.
Geographical distribution.PAL.
PAL: Finland, Netherlands, Switzerland.
Cotesiabhairavi (Sathe & Inamdar, 1991), new combination
Parenionbhairavi Sathe & Inamdar, 1991.
Type information. Holotype female, SUKI (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. Based on the original description and drawings included there, it is very clear that this species does not belong to Parenion. Without examining the type material is difficult to conclude, but the best generic placement at present would be Cotesia, based on the sculpture of propodeum, T1-T3 shape, and hypopygium and ovipositor (as described and illustrated by the authors).
Cotesiabiezankoi (Blanchard, 1960), new combination
Apantelesbiezankoi Blanchard, 1960.
Type information. Type and depository unknown (not examined but original description checked). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (RS).
Notes. The original reference to this species appears to be in Biezanko (1960), where the wasp is mentioned as described by Everard E. Blanchard (in a letter he sent to Biezanko after examining the specimens he had sent to Blanchard). The paper from Biezanko (1960: 9) transcribed part of the information received, where the species name and species author (Blanchard) are clearly stated, the specimens collecting place and date, and Lepidoptera host are provided, and a brief comparison of metasoma color to differentiate adults of the new species from three other previously described Apanteles (all of those species currently placed in Cotesia) is also presented. Although the details in Biezanko (1960) are relatively scarce, they nevertheless satisfy the provisions of Articles 11 and 13 of the ICZN for a species name (published after 1930 but before 1961) to be available, and thus we include this species in our checklist. The new combination here proposed is based on the host information (the only known Microgastrinae wasps parasitizing Opsiphanes are two other Cotesia species), as well as the comparisons made with three other species that have long been placed in Cotesia (see previous sentences).
Cotesiabifida (Sharma, 1973), new combination
Apantelesbifida Sharma, 1973.
Type information. Holotype female, IFRI (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. Based on the drawings that are part of the original description, this species does not belong to Deuterixys (as had been proposed by Zeng et al. 2009). At present we consider the best generic placement to be Cotesia, based on the drawing of the propodeum as well as associated host record. Future study of the type material will be needed to conclude (as there is also a possibility that it could be Parapanteles).
Cotesiabignellii (Marshall, 1885)
Apantelesbignellii Marshall, 1885.
Type information. Lectotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Finland, France, Germany, Greece, Hungary, Ireland, Italy, Romania, Russia (ROS), Spain, Sweden, United Arab Emirates, United Kingdom, Yugoslavia.
Notes.Wilkinson (1945: 91) designated a lectotype (at the time referred to only as "the type") among four specimens and a cocoon mass, all glued to the same card, which is numbered as 1603. He marked the lectotype with a cross, which corresponds to the specimens to the top right on that card.
Cotesiabonariensis (Brèthes, 1916)
Protapantelesbonariensis Brèthes, 1916.
Type information. Syntypes female and male, MACN (not examined). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina.
Cotesiabosei (Bhatnagar, 1950)
Apantelesbosei Bhatnagar, 1950.
Type information. Holotype female, INPC (not examined but subsequent treatment of the species checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: China (FJ, HN, SN), India.
Notes. Our species concept is based on Chen and Song (2004). The year of publication of the Bhatnagar paper was until recently commonly cited as 1948 and/or 1950 (e.g., Chen and Song 2004, Yu et al. 2016), probably following Shenefelt (1972) who referred to this paper as “Bhatnagar (1948) 1950”. While the intended year for Volume X, Parts I & II of the Indian Journal of Entomology was 1948, the actual dates of publication were June 1950 (Part I) and October 1950 (Part II), as clearly shown on the cover page of the Volume, which we have checked. Because the dates of publication are the ones to be considered, and for the sake of clarity, we hereby revise the species year of description to 1950.
Cotesiabrachycera (Thomson, 1895)
Microgaster (Microplitis) brachycera Thomson, 1895.
Microgaster (Apanteles) brachycerus Thomson, 1895 [primary junior homonym of Microgaster (Microplitis) brachycera Thomson, 1895].
Type information. Lectotype female, MZLU (not examined but subsequent treatment of the species checked). Country of type locality: Sweden.
Geographical distribution.PAL.
PAL: Sweden.
Notes.Thomson (1895: 2237–2238) considered a single genus Microgaster with four subgenera (Microgaster, HygroplitisMicroplitis, and Apanteles), and described six new species within that framework. He described Microgaster (Microplitis) brachycera (page 2252 of his paper) and and a different species Microgaster (Apanteles) brachycerus (page 2259); as first revisers we designate the latter as a primary junior homonym as it appears later in the publication. Our species concept is based on van Achterberg (1997: 68), who stated that the species Apanteles/Cotesiapraepotens (sensuWilkinson 1940, Nixon 1974, Papp 1988) was a misidentification and actually corresponded to Cotesiabrachycera (Thomson, 1895), a decision we agree with and follow here. The names of several species of Cotesia (brachycera, juniperatae, praepotens, sericea, sessilis, tetrica) have a complicated and somewhat interrelated history, which we attempt to detail below. While van Achterberg (1997: 68) deemed C.brachycera (Thomson, 1895) to be a valid species, some authors (e.g., Papp 1988: 153–156, Kotenko 2007: 186) considered brachycera to be a synonym of C.praepotens (Haliday, 1834), whereas others (e.g., Belokobylskij et al. 2003: 387, Broad et al. 2016: 243) considered brachycera to be a synonym of C.sericea (Nees, 1834). The name praepotens itself has been interpreted in two different ways: a) as the Haliday species (e.g., Papp 1988, Belokobylskij et al. 2003, Kotenko 2007, Broad et al. 2016); b) as a misidentification of brachycera (Thomson, 1895) (e.g., Wilkinson 1940, Nixon 1974, Papp 1988). The name sericea also has been interpreted in different ways: Lyle (1916: 186, 206–208) stated that Nees, subsequent to describing sericea, said later that it was the same species as C.juniperatae (Bouché, 1834), but then Reinhard (1881: 34) and Marshall (1885: 184) subsequently misinterpreted that. Thus, there has also been confusion regarding the status of juniperatae:
a) Yu et al. (2016) listed juniperatae as a synonym of Cotesiasessilis (Geoffroy, 1785) and cited Papp (1988) as the author of that synonymy, when in fact the opposite occurred, as Papp (1988: 154, also see 155) actually placed sessilis as a synonym of juniperatae, albeit with a question mark;
b) Belokobylskij et al. (2003: 387) considered juniperatae as a valid species and sessilis as its synonym;
c) Broad et al. (2016) also regarded juniperatae as valid but did not refer to sessilis as its synonym. Similarly, the name sessilis has been interpreted in different ways: Yu et al. (2016) considered it a valid species, with both juniperatae and C.tetrica (Reinhard, 1880) as synonyms; Papp (1988) and Kotenko (2007) considered tetrica a valid species with sessilis as a synonym (with a question mark); and Belokobylskij et al. (2003) and Broad et al (2016) deemed tetrica to be a valid species, but not with sessilis as a synonym of tetrica. Lastly, ongoing studies involving DNA barcoding, biology and morphology (Shaw, Quicke and Fernandez-Triana, unpublished data) indicate that there is the potential for other species/names to be involved, e.g., some of the current synonyms of praepotens (as accepted in this paper, see below under that species) may represent additional species, related to the ones we have mentioned in this paragraph and/or even other Palearctic Cotesia species. Because that is beyond the scope of the present paper, we do not expand on that here, but the reader must be aware that the situation with all these species is far from being resolved. For the sake of clarity, we detail here the arrangement that we are following in this paper, where we consider valid species brachycera, juniperatae, praepotens, sericea, and tetrica, whereas sessilis is listed as a nomen dubium.
Cotesiabrevicornis (Wesmael, 1837)
Microgasterbrevicornis Wesmael, 1837.
Apantelescleoceridis Marshall, 1889.
Type information. Holotype female, RBINS (not examined but subsequent treatment of the species checked). Country of type locality: Belgium.
Geographical distribution.NEA, PAL.
NEA: Canada (AB); PAL: Azerbaijan, Belgium, Croatia, Finland, Germany, Hungary, Iceland, Ireland, Korea, Lithuania, Poland, Romania, Russia (YAR), Slovakia, Sweden, Switzerland, Turkey, Ukraine, United Kingdom, Yugoslavia.
Notes. Our species concept is based on Nixon (1974) and Papp (1986).
Cotesiacajae (Bouché, 1834)
Microgastercajae Bouché, 1834.
Microgasterdifficilis Nees, 1834.
? Microgasterperspicuus Nees, 1834.
Type information. Lectotype female, ZMHB (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Azerbaijan, Belarus, Belgium, China (XJ), Croatia, Czech Republic, Finland, France, Georgia, Germany, Hungary, Italy, Japan, Kazakhstan, Latvia, Moldova, Netherlands, Poland, Romania, Russia (AD, AST, IRK, KGD, KDA, ORL, PRI, ROS, SAK, SAM, SPE, TA, VOR, YAR), Slovakia, Spain, Sweden, Switzerland, Tajikistan, Ukraine, United Kingdom, Uzbekistan, Yugoslavia.
Notes. The name perspicuus (Nees, 1834) has sometimes been regarded as a senior synonym of both cajae (Bouché, 1834) and ofella (Nixon, 1974) (e.g., Yu et al. 2012, 2016). However, Marshall (1885: 183) had listed cajae as the senior name, and Papp (1988: 153) also considered cajae as a valid species. There has been little consensus on the correct application of the Nees name, the type of which is lost (see also comments under Cotesiaofella below). The arrangement proposed by Papp (1988), i.e., maintaining both cajae and ofella as valid species (and not as synonyms of perspicuus) has been subsequently followed by Papp (2005) and Broad et al. (2016), and it is also followed here. The species distribution in Japan is based on Belokobylskij et al. (2019).
Cotesiacaligophagus (Blanchard, 1964), new combination
Apantelescaligophagus Blanchard, 1964.
Type information. Holotype female, MACN (not examined but original description checked). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina.
Notes. The generic placement of the species within Cotesia is clear from the original description and the accompanying drawings. Following Article 31.2.1 of the ICZN the name is a noun in apposition and the original spelling caligophagus must be retained.
Cotesiacallimone (Nixon, 1974)
Apantelescallimone Nixon, 1974.
Apantelesscelerata Tobias, 1986.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Bulgaria, Finland, Hungary, Iran, Ireland, Mongolia, Russia (KIR), Serbia, Slovakia, Switzerland, Turkey, Ukraine, United Kingdom.
Notes. The species’ distribution in Iran is based on Belokobylskij et al. (2019).
Cotesiacalodetta (Nixon, 1974)
Apantelescalodetta Nixon, 1974.
Type information. Holotype female, NHMUK (examined). Country of type locality: Sweden.
Geographical distribution.PAL.
PAL: Russia (ALT), Sweden, Turkey.
Cotesiacapucinae (Fischer, 1961)
Apantelescapucinae Fischer, 1961.
Type information. Holotype female, NHMW (not examined but subsequent treatment of the species checked). Country of type locality: Macedonia.
Geographical distribution.PAL.
PAL: Macedonia, Netherlands, Serbia.
Notes. Our concept of this species is based on Papp (1988) and Aquino et al. (2010).
Cotesiacarduicola (Packard, 1881)
Microgastercarduicola Packard, 1881.
Type information. Syntypes female and male, depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (ON), USA (CT, IL, MA, NJ, TX).
Notes.Shenefelt (1972: 464) mentioned the need to designate a lectotype but, as far as we know, none has been designated yet. Our species concept is based on Muesebeck (1921), Mason (1981) and Fernandez-Triana (2010).
Cotesiacerurae (Muesebeck, 1926)
Apantelescerurae Muesebeck, 1926.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (ON, QC), USA (CT, MD, NJ).
Cotesiacharadrae (Muesebeck, 1921)
Apantelescharadrae Muesebeck, 1921.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CT, DC, MA).
Cotesiachares (Nixon, 1965)
Apanteleschares Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Hungary, Mongolia, Slovakia, United Kingdom.
Cotesiacheesmanae (Wilkinson, 1928), new combination
Apantelescheesmanae Wilkinson, 1928.
Type information. Holotype female, NHMUK (examined). Country of type locality: Society Islands.
Geographical distribution.AUS.
AUS: Society Islands.
Notes. This species belongs to Cotesia, based on the strong, median longitudinal carinae of propodeum, shape and sculpture of T1 and T2, inflexible hypopygium, and short length of the ovipositor sheaths.
Cotesiachiloluteelli (You, Xiong & Wang, 1985)
Apanteleschiloluteelli You, Xiong & Wang, 1985.
Apanteleschiloluteelli You, Xiong & Wang, 1985 [incorrect original spelling].
Type information. Holotype female, HUNAU (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (HN).
Notes. Our species concept is based on Zeng (2012). The emendation of the incorrect original spelling was done by You (1986).
Cotesiachiloniponellae (You & Wang, 1990)
Apanteleschiloniponellae You & Wang, 1990.
Type information. Holotype female, HUNAU (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (HB, HN).
Notes. Our species concept is based on Chen and Song (2004).
Cotesiachilonis (Munakata, 1912)
Apanteleschilonis Munakata, 1912.
Apanteleschilocida Viereck, 1912.
Type information. Neotype female, EIHU (not examined but subsequent treatment of the species checked). Country of type locality: Japan.
Geographical distribution.OTL, PAL.
OLT: China (FJ, GZ, HB, HN, JS, JX, SN, ZJ), India, Indonesia, Myanmar; PAL: China (AH), Iran, Japan, Korea.
Notes. An account of the rather complicated history of the species name and its year of publication was provided by Fernandez-Triana et al. (2015a). We also examined the type specimen, a female, of Apanteleschilocida (Viereck, 1912).
Cotesiachinensis (Wilkinson, 1930)
Apanteleschinensis Wilkinson, 1930.
Type information. Holotype female, NHMUK (examined). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, HB, HN, ZJ).
Cotesiachrysippi (Viereck, 1911)
Apanteleschrysippi Viereck, 1911.
Type information. Holotype female, USNM (examined). Country of type locality: Mozambique.
Geographical distribution.AFR.
AFR: Madagascar, Mozambique, Nigeria, South Africa.
Cotesiacingiliae (Muesebeck, 1931)
Apantelescingiliae Muesebeck, 1931.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (AB, BC, NB, NS, ON, QC), USA (MA).
Cotesiacirphicola (Bhatnagar, 1950)
Apantelescirphicola Bhatnagar, 1950.
Type information. Holotype female, INPC (not examined but subsequent treatment of the species checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: China (HN), India, Vietnam.
Notes. Our concept of this species is based on Chen and Song (2004). The year of publication of the Bhatnagar paper was until recently commonly cited as 1948 and/or 1950 (e.g., Chen and Song 2004, Yu et al. 2016), probably following Shenefelt (1972) who referred to this paper as “Bhatnagar (1948) 1950”. While the intended year for Volume X, Parts I & II of the Indian Journal of Entomology was 1948, the actual dates of publication were June 1950 (Part I) and October 1950 (Part II), as clearly shown on the cover page of the Volume, which we have checked. Because the dates of publication are the ones to be considered, and for the sake of clarity, we hereby revise the species year of description to 1950.
Cotesiacleora (Nixon, 1974)
Apantelescleora Nixon, 1974.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Czech Republic, Hungary, United Kingdom.
Cotesiaclepta (Tobias, 1986)
Apantelesclepta Tobias, 1986.
Type information. Holotype female, ZIN (not examined but original description checked). Country of type locality: Moldova.
Geographical distribution.PAL.
PAL: Hungary, Moldova, Serbia, Sweden.
Cotesiaclethrogynae Long, 2014
Cotesiaclethrogynae Long, 2014.
Type information. Holotype female, IEBR (not examined but original description checked). Country of type locality: Vietnam.
Geographical distribution.OTL.
OTL: Vietnam.
Cotesiaclisiocampae (Ashmead, 1903)
Apantelesclisiocampae Ashmead, 1903.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (ON), USA (CT, NH, NJ, NY).
Cotesiacompressithorax (Hedqvist, 1965), new combination
Apantelescompressithorax Hedqvist, 1965.
Type information. Holotype female, MZH (examined). Country of type locality: Cape Verde.
Geographical distribution.AFR.
AFR: Cape Verde.
Notes. This species was considered a junior synonym of Cotesiapistrinariae (Wilkinson, 1929) by Koponen (1989) and Forshage et al. (2016). However, we have examined the types (and other specimens) from both species and they are different species. Cotesiacompresithorax has a shorter malar space (as long as pedicel in the wording of the original description); thorax compressed and flattened; propodeum nearly smooth all over (only posteriorly with punctures); T1 not narrowing medially; T2 much more transverse (its width at posterior margin more than twice its median length); and darker colouration (most of legs and sternites brown). Cotesiapistrinariae has a longer malar space (at least twice the length of pedicel and slightly longer than mandible base width); thorax of normal appearance; propodeum mostly sculptured, with transverse striation centrally and a partial median carina (defined on posterior half of propodeum); T1 strongly narrowing medially; T2 much less transverse (its width at posterior margin around 1.5 × its median length); and lighter coloration (most of legs and sternites are yellow to light yellow-brown). Thus, here we remove compressithorax from synonymy with pistrinariae and transfer it from Apanteles to Cotesia.
Cotesiacongestiformis (Viereck, 1923)
Apantelescongestiformis Viereck, 1923.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (AK).
Cotesiacongregata (Say, 1836)
Microgastercongregata Say, 1836.
Microgasterutilis French, 1880.
Apantelesaugustus Viereck, 1917.
Type information. Neotype female, USNM (not examined but authoritatively identified specimens examined). Country of type locality: USA.
Geographical distribution.NEA, NEO.
NEA: Canada (MB, NB, ON, PE), USA (AL, CO, CT, DC, FL, GA, IL, IA, KS, KY, MD, MA, MI, MS, MO, NH, NJ, NY, NC, PA, RI, SC, TN, VT, VA, WV); NEO: Brazil (SP), Honduras, Jamaica, Nicaragua, Peru, Puerto Rico.
Notes. We examined the female type and a paratype male of Apantelesaugustus (Viereck, 1917), currently a synonym of C.congregata.
Cotesiacorylicolus (Tobias, 1986)
Apantelescorylicolus Tobias, 1986.
Type information. Holotype female, ZIN (not examined but original description checked). Country of type locality: Azerbaijan.
Geographical distribution.PAL.
PAL: Azerbaijan, Hungary, Netherlands, Russia (NC), Serbia.
Notes. Following Article 31.2.2 of the ICZN, in the absence of an original statement that the epithet is adjectival, the name is to be treated as a noun in apposition and the original spelling corylicolus must be retained.
Cotesiacoryphe (Nixon, 1974)
Apantelescoryphe Nixon, 1974.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: United Kingdom.
Notes.Papp (1987a) synonymized C.coryphe under C.rubripes (Haliday, 1834), which was refuted by Broad et al. (2016), in part based on the host data given by Nixon (1974), a decision we accept and follow here.
Cotesiacrambi (Weed, 1887)
Apantelescrambi Weed, 1887.
Type information. Lectotype female, INHS (not examined but subsequent treatment of the species checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (ON, QC), USA (CT, IL, KS, KY, MD, MO, NJ, OH, SD, TN).
Notes. Our species concept is based on Muesebeck (1921), Papp (1986) and Fernandez-Triana et al. (2014c).
Cotesiacrassifemorata van Achterberg, 2006
Cotesiacrassifemorata van Achterberg, 2006.
Type information. Holotype female, ZMUC (not examined but original description checked). Country of type locality: Greenland.
Geographical distribution.NEA.
NEA: Greenland.
Cotesiacultellata (Tobias, 1966)
Apantelescultellatus Tobias, 1966.
Type information. Holotype female, ZIN (not examined but original description checked). Country of type locality: Uzbekistan.
Geographical distribution.PAL.
PAL: Uzbekistan.
Cotesiacuprea (Lyle, 1925)
Apantelescupreus Lyle, 1925.
Type information. Syntypes female and male, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Azerbaijan, Bulgaria, Canary Islands, Finland, France, Germany, Greece, Hungary, Iran, Lithuania, Mongolia, Netherlands, Poland, Romania, Russia (NW), Slovakia, Spain, Switzerland, Turkey, United Kingdom.
Notes. The species distribution in Iran is based on Belokobylskij et al. (2019).
Cotesiacyaniridis (Riley, 1889)
Apantelescyaniridis Riley, 1889.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (ON, QC), USA (AZ, CO, CT, IL, IA, NJ, NY, WV).
Cotesiacynthiae (Nixon, 1974)
Apantelescynthiae Nixon, 1974.
Type information. Holotype female, NHMUK (examined). Country of type locality: Austria.
Geographical distribution.PAL.
PAL: Austria, Bulgaria, France, Hungary, Iran, Switzerland, Turkey.
Cotesiadanaisae (Hedqvist, 1965)
Apantelesdanaisae Hedqvist, 1965.
Type information. Holotype female, MZH (not examined but subsequent treatment of the species checked). Country of type locality: Cape Verde.
Geographical distribution.AFR.
AFR: Cape Verde.
Notes.Forshage et al. (2016) considered the type material to be lost; however, in 2017 it was found by the senior author of this paper in another section of the MZH collection.
Cotesiadecaryi (Granger, 1949)
Apantelesdecaryi Granger, 1949.
Type information. Syntypes female and male, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Notes. According to a recent catalogue on Braconidae from Madagascar (Madl and van Achterberg 2014), this species was transferred to Cotesia back in 2002 (Kuklinski and Borgemeister 2002). While Kuklinski and Borgemeister mentioned the species as Cotesia in their paper, there is no formal transfer there, nor an explanation as to why. After reading the original description, we concur that the species indeed belongs in Cotesia, and for the sake of clarity we revise its combination here.
Cotesiadeliadis (Bingham, 1906)
Apantelesdeliadis Bingham, 1906.
Type information. Syntypes female and male, OUMNH (not examined but subsequent treatment of the species checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (QLD).
Notes. Our species concept is based on Wilkinson (1928a).
Cotesiadelicata (Howard, 1897)
Apantelesdelicatus Howard, 1897.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CT, DC, MD, NJ, NY).
Notes. Apart from the original description, our species concept is based on Muesebeck (1921).
Cotesiadelphinensis (Granger, 1949), new combination
Apantelesdelphinensis Granger, 1949.
Type information. Syntypes female and male, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Notes. This species is clearly not an Apanteles; although the original description is not clear or detailed enough to conclude, we consider that the best generic placement at present would be in Cotesia. Further examination of the type series will be needed in the future.
Cotesiadepressa (Viereck, 1912)
Apantelesdepressus Viereck, 1912.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (QC), USA (IN).
Notes. Most of the holotype specimen is missing (the point has remnants of one leg glued, and the pin has all associated labels, but the rest of the specimen is missing from the unit tray).
Cotesiadepressithorax (Tobias, 1964)
Apantelesdepressithorax Tobias, 1964.
Type information. Holotype female, ZIN (not examined but original description checked). Country of type locality: Kazakhstan.
Geographical distribution.PAL.
PAL: Kazakhstan.
Notes. Apart from the original description, our species concept is also based on Tobias (1986) and Papp (1987a).
Cotesiadiacrisiae (Gahan, 1917)
Apantelesdiacrisiae Gahan, 1917.
Type information. Holotype female, USNM (not examined but subsequent treatment of the species checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (ON, QC), USA (CO, DE, DC, IL, KS, LA, MD, MS, MO, NJ, OK, SC, VA, WV).
Notes. Our species concept is based on Muesebeck (1921), Papp (1987a), Whitfield (1995a), Fernandez-Triana (2010), and images of the holotype available at http://www.usnmhymtypes.com/.
Cotesiadictyoplocae (Watanabe, 1940)
Apantelesdictyoplocae Watanabe, 1940.
Type information. Holotype female, EIHU (examined). Country of type locality: Japan.
Geographical distribution.OTL, PAL.
OTL: China (FJ, HN, YN, ZJ), India; PAL: China (LN), Japan, Korea.
Cotesiadisparis (Tobias, 1986)
Apantelesdisparis Tobias, 1986.
Type information. Holotype female, ZIN (not examined but paratype examined). Country of type locality: Azerbaijan.
Geographical distribution.PAL.
PAL: Azerbaijan, Hungary.
Notes. We examined one female paratype from the same cocoon mass than the (not examined by us) holotype.
Cotesiadiurnii Rao & Nikam, 1984
Cotesiadiurnii Rao & Nikam, 1984.
Type information. Holotype female, NZSI (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Cotesiadiversa (Muesebeck & Walkely, 1951)
Apantelesdiversa Muesebeck & Walkely, 1951.
Apantelescoxalis Muesebeck, 1926 [homonym of Apantelescoxalis Szépligeti, 1911].
Type information. Holotype female, USNM (not examined but subsequent treatment of the species checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (MB), USA (CT).
Notes. Our species concept is based on Muesebeck and Walkley (1951), Mason (1981), Whitfield (1995a) and Fernandez-Triana (2010).
Cotesiaeffrena (Wilkinson, 1928), new combination
Apanteleseffrenus Wilkinson, 1928.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. Examination of the holotype reveals that this species belongs in Cotesia, based on the propodeum with complete median, longitudinal carina, partial transverse carina, shape and sculpture of T1 and T2, inflexible hypopygium and short ovipositor sheaths.
Cotesiaelaeodes (de Saeger, 1944), new combination
Apanteleselaeodes de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. Based on the original description and drawings (de Saeger 1944), the best generic placement is in Cotesia.
Cotesiaelectrae (Viereck, 1912)
Apanteleselectrae Viereck, 1912.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA, NEO.
NEA: Canada (BC), USA (AZ, CA, CO, NM, TX, UT); NEO: Mexico.
Notes. According to Papp (1987a) this species looks similar to C.euchaetis. But, after examining the holotypes of both species we found that there are many differences to clearly separate them.
Cotesiaeliniae Papp, 1989
Cotesiaeliniae Papp, 1989.
Type information. Holotype female, HNHM (not examined but subsequent treatment of the species checked). Country of type locality: Greenland.
Geographical distribution.NEA.
NEA: Canada (NT, NU), Greenland.
Notes. Our species concept is based on van Achterberg (2006) and Fernandez-Triana et al. (2017b).
Cotesiaelongata Zargar & Gupta, 2019
Cotesiaelongata Zargar & Gupta, 2019.
Type information. Holotype female, NBAIR (not examined but original description checked). Country of type locality: Iran.
Geographical distribution.PAL.
PAL: Iran.
Cotesiaempretiae (Viereck, 1913)
Apantelesempretiae Viereck, 1913.
Apantelessibinidis Rohwer, 1915.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA, NEO.
NEA: USA (AL, DE, DC, FL, IL, LA, MD, MA, MO, NJ, VA); NEO: Ecuador.
Notes. We also examined the type of Apantelessibinidis (Rohwer, 1915), a female specimen.
Cotesiaendii (Sathe & Ingawale, 1995), new combination
Apantelesendii Sathe & Ingawale, 1995.
Type information. Holotype female, NZSI (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. The original description of the species is problematic, and drawings are clearly wrong (e.g., the depiction of the fore wing) or are in contradiction with the written description (e.g., is not clear in fig. 1h of Sathe and Ingawale (1995) what are the anterior and posterior margins of T1, as the sculpture described in the text does not match the drawing, and neither does the drawn shape match the text description of T1 as barrel-shaped). What is very clear is that the species is not an Apanteles (based on the propodeum sculpture, unpleated hypopygium, and setose vannal lobe in hind wings). Based on the overall description and recorded host, the best generic placement that can be proposed for this species at present is within Cotesia (coincidentally, the authors stated that the species is similar to Apantelescirphicola, which has long been considered as belonging in Cotesia). Further study of the specimens will be needed to unambiguously confirm the generic identity of the species.
Cotesiaenypiae (Mason, 1959)
Apantelesenypiae Mason, 1959.
Type information. Holotype female, CNC (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (BC).
Cotesiaerionotae (Wilkinson, 1928)
Apanteleserionotae Wilkinson, 1928.
Type information. Holotype female, NHMUK (not examined but subsequent treatment of the species checked). Country of type locality: Malaysia.
Geographical distribution.AFR, AUS, OTL.
AFR: Mauritius; AUS: Guam, Hawaiian Islands, Papua New Guinea; OTL: China (TW), India, Indonesia, Malaysia, Thailand.
Notes. Our concept of this species is based on Austin and Dangerfield (1992). The only Afrotropical record so far is from an introduction for biological control purposes (from Malaysia (Sabah) to Mauritius, see Madl and van Achterberg 2014); as far as we know there is no published information confirming if the species was established or not. Additional comments on distribution and biology of the species can be found in Cock (2015).
Cotesiaerrator (Nixon, 1974)
Apanteleserrator Nixon, 1974.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Austria, Switzerland, Russia (NW), United Kingdom.
Cotesiaeuchaetis (Ashmead, 1898)
Apanteleseuchaetis Ashmead, 1898.
Type information. Syntypes female and male, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CT, IL, MA, NH, NJ, NY, PA, RI, TX, VA, WV).
Notes. By all accounts this species seems to better be placed in Protapanteles than Cotesia, but we refrain from transferring it here until future studies resolve better the relationships between those two genera (see above under the section Brief diagnosis of all Microgastrinae genera as they are understood in this paper, for a discussion of Protapanteles as just a potential species group of Cotesia; p 35, 36. The whole body of euchaetis is unusually smooth (although the female syntype has many body parts covered in glue, artificially increasing the shiny and smooth appearance); T1 is parallel-sided, and especially T2 is subtriangular (trapezoidal) and rather small and narrow (very much unlike Cotesia and more like Protapanteles); the propodeum has only a short, apically defined, median carina (discernible in the female only) without any other carinae visible on the propodeum (although perhaps the glue obscures the sculpture, if there is any carination, it would still be very faint). Interestingly, Michel-Salzat and Whitfield (2004) in a phylogenetic analysis of 25 species of Cotesia clearly recovered euchaetis as part of Cotesia, with strong support (those results might indirectly support the opinion that Protapanteles species represent just a species group within Cotesia). It must also be noted that Papp (1987a) provided a rather poor diagnosis of the species in his key, not likely to work properly as a diagnostic tool (and best avoided when studying that group of species).
Cotesiaeulipis (Nixon, 1974)
Apanteleseulipis Nixon, 1974.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Bulgaria, Finland, Germany, Greece, Hungary, Sweden, United Kingdom.
Cotesiaeunomiae Shaw, 2009
Cotesiaeunomiae Shaw, 2009.
Type information. Holotype female, RSME (examined). Country of type locality: Belgium.
Geographical distribution.PAL.
PAL: Belgium.
Cotesiaeuphobetri (Blanchard, 1935), new combination
Apanteleseuphobetri Blanchard, 1935.
Type information. Syntypes female and male, MACN (not examined but original description checked). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina.
Notes. This species was described based on specimens from the Blanchard collection, which we believe is currently deposited in the MACN. The original description and illustrations there (scutellar disc, propodeum, T1–T2, part of fore wing, tip of antenna), strongly suggest that this species belongs in Cotesia. The species has the propodeum with a median, longitudinal carina, in addition to a more or less complete transverse carina forking around the spiracles, T1 slightly widening posteriorly, T2 is sub-rectangular, and the ovipositor sheaths barely protrude.
Cotesiaeuphydryidis (Muesebeck, 1921)
Apanteleseuphydryidis Muesebeck, 1921.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (MD, NJ, NY, VA).
Cotesiaeuprocti Sathe, 2005
Cotesiaeuprocti Sathe, 2005.
Type information. Holotype female, SUKI (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Cotesiaeuryale (Nixon, 1974)
Apanteleseuryale Nixon, 1974.
Type information. Holotype female, NHMUK (examined). Country of type locality: France.
Geographical distribution.PAL.
PAL: Bulgaria, Czech Republic, France, Greece, Hungary, Iran, Macedonia, Mongolia, Netherlands, Russia (C, IR, NW, S), Switzerland, Yugoslavia.
Notes. The presence of this species in the United Kingdom was questioned by Broad et al. (2016), a decision we accept and follow here. The presence of the species in Iran is based in Belokobylskij et al. (2019).
Cotesiaeuthaliae (Bhatnagar, 1950), new combination
Apanteleseuthaliae Bhatnagar, 1950.
Type information. Holotype female, INPC (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. Transferred to Cotesia based on the propodeum having a strong, median longitudinal carina, T1 parallel-sided, T2 more or less rectangular and as long as T3, and ovipositor sheaths very short. The year of publication of the Bhatnagar paper was until recently commonly cited as 1948 and/or 1950 (e.g., Chen and Song 2004, Yu et al. 2016), probably following Shenefelt (1972) who referred to this paper as “Bhatnagar (1948) 1950”. While the intended year for Volume X, Parts I & II of the Indian Journal of Entomology was 1948, the actual dates of publication were June 1950 (Part I) and October 1950 (Part II), as clearly shown on the cover page of the Volume, which we have checked. Because the dates of publication are the ones to be considered, and for the sake of clarity, we hereby revise the species year of description to 1950.
Cotesiaevagata (Papp, 1973)
Apantelesevagatus Papp, 1973.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Turkmenistan.
Geographical distribution.PAL.
PAL: Jordan, Turkmenistan.
Cotesiaexelastisae (Bhatnagar, 1950), new combination
Apantelesexelastisae Bhatnagar, 1950.
Type information. Holotype male, INPC (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. Because the only known specimen is male, it is difficult to conclude with certainty, but the original description makes clear this species is not Apanteles. Here we transfer it to Cotesia based on the propodeum having a strong, median longitudinal carina, metatibial spurs of equal size and relatively short, and T1 mostly parallel-sided (although posterior 0.2 narrows towards posterior margin). Additional support to consider exelastisae in Cotesiacomes from the original description, which considered it morphologically close to Apanteleserionotae Wilkinson (currently placed in Cotesia) and also the host species being Pterophoridae (Bhatnagar 1950). The year of publication of the Bhatnagar paper was until recently commonly cited as 1948 and/or 1950 (e.g., Chen and Song 2004, Yu et al. 2016), probably following Shenefelt (1972) who referred to this paper as “Bhatnagar (1948) 1950”. While the intended year for Volume X, Parts I & II of the Indian Journal of Entomology was 1948, the actual dates of publication were June 1950 (Part I) and October 1950 (Part II), as clearly shown on the cover page of the Volume, which we have checked. Because the dates of publication are the ones to be considered, and for the sake of clarity, we hereby revise the species year of description to 1950.
Cotesiafascifemorata van Achterberg, 2006
Cotesiafascifemorata van Achterberg, 2006.
Type information. Holotype female, ZMUC (not examined but original description checked). Country of type locality: Greenland.
Geographical distribution.NEA.
NEA: Greenland.
Cotesiaferruginea (Marshall, 1885)
Apantelesferrugineus Marshall, 1885.
Type information. Lectotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Belgium, Germany, Hungary, Italy, Korea, Lithuania, Netherlands, Romania, Russia (PRI), Slovakia, Switzerland, Turkey, Ukraine, United Kingdom.
Notes.Wilkinson (1945) stated that the specimen was in the Essex Museum of Natural History, but it is currently in the NHMUK.
Cotesiafiskei (Viereck, 1910)
Apantelesfiskei Viereck, 1910.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (AB, BC, MB, NB, NL, NS, ON, SK), USA (CT, KS, MA, MT, OR, WI).
Cotesiaflagellator (Wilkinson, 1930)
Apantelesflagellator Wilkinson, 1930.
Type information. Holotype female, NHMUK (examined). Country of type locality: Uganda.
Geographical distribution.AFR.
AFR: Uganda.
Cotesiaflagitata (Papp, 1971)
Apantelesflagitatus Papp, 1971.
Apantelesjaicus Tobias, 1986.
Type information. Holotype female, HNHM (not examined but subsequent treatment of the species checked). Country of type locality: Mongolia.
Geographical distribution.PAL.
PAL: Kazakhstan, Mongolia.
Notes. Our species concept is based on Papp (1987a).
Cotesiaflaviconchae (Riley, 1881)
Apantelesflaviconchae Riley, 1881.
Type information. Lectotype female, USNM (not examined but subsequent treatment of the species checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (ON), USA (AR, CA, CT, DC, IL, IA, LA, MD, MA, MN, MO, OK, TX, UT, VA, WA, WV).
Notes. Our species concept is based on Muesebeck (1921), Mason (1981), Whitfield (1995a) and Fernandez-Triana (2010).
Cotesiaflavicornis (Riley, 1889)
Apantelesflavicornis Riley, 1889.
Type information. Syntypes female and male, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (MB, ON), USA (CT, MO, NJ, TX).
Cotesiaflavipes Cameron, 1891
Cotesiaflavipes Cameron, 1891.
Apanteles (Stenopleura) nonagriae Viereck, 1913.
Apantelessimplicis Viereck, 1913.
Apantelesflavatus Ishida, 1915.
Type information. Holotype male, NHMUK (examined). Country of type locality: India.
Geographical distribution.AFR, AUS, NEA, NEO, OTL, PAL.
AFR: Ethiopia, Kenya, Madagascar, Mauritius, Mozambique, Réunion, Tanzania, Uganda; AUS: Australia (NSW, QLD), Papua New Guinea; NEA: USA (FL, TX); NEO: Barbados, Brazil, Costa Rica, Guadeloupe, Jamaica, Mexico, Peru, Saint Kitts & Nevis, Trinidad & Tobago, Venezuela; OTL: Bangladesh, China (FJ, GD, GX, GZ, HI, HK, HB, HN, JS, JX, SN, TW, YN, ZJ), India, Indonesia, Malaysia, Myanmar, Nepal, Pakistan, Philippines, Ryukyu Islands, Sri Lanka, Thailand, Vietnam); PAL: China (AH), Japan.
Notes. We examined the type of A.simplicis (Viereck, 1913), a female specimen from Taiwan considered to be a synonym of C.flavipes since at least Wilkinson (1928a). The female type of A.simplicis looks slightly different to other specimens of C.flavipes we have examined, and we caution that it might represent another species within the flavipes species-complex (see Fujie et al. 2018). We also examined the type of Apanteles (Stenopleura) nonagriae Viereck, 1913, another female specimen from Taiwan, which looks similar to A.simplicis (but is not related to Apantelesnongriae Olliff, 1893).
Cotesiafluvialis (Balevski, 1980)
Apantelesfluvialis Balevski, 1980.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Bulgaria.
Geographical distribution.PAL.
PAL: Bulgaria.
Notes. We follow Papp (1986: 232), who based his species concept on the original description only, as he could not see any specimen.
Cotesiagabera Papp, 1990
Cotesiagabera Papp, 1990.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Korea.
Geographical distribution.PAL.
PAL: Korea.
Cotesiagades (Nixon, 1974)
Apantelesgades Nixon, 1974.
Type information. Holotype female, NHMUK (examined). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Germany, Hungary, Macedonia, United Kingdom, Yugoslavia.
Cotesiagastropachae (Bouché, 1834)
Microgastergastropachae Bouché, 1834.
Type information. Holotype male, ZMHB (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.OTL, PAL.
OTL: China (ZJ); PAL: Azerbaijan, Bulgaria, China (SD, SN), Czech Republic, Finland, France, Germany, Hungary, Israel, Japan, Kazakhstan, Lithuania, Moldova, Mongolia, Montenegro, Poland, Romania, Russia (ZAB, KHA, KIR, KYA, ORE, PRI, SAK, SPE, VOR), Slovakia, Turkey, United Kingdom, Uzbekistan.
Notes. Our species concept is based on Nixon (1974) and Papp (1990a).
Cotesiageometricae Austin, 2000
Cotesiageometricae Austin, 2000.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (ACT, VIC).
Cotesiageryonis (Marshall, 1885)
Apantelesgeryonis Marshall, 1885.
Type information. Lectotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Bulgaria, Germany, Hungary, Iran, Italy, Korea, Mongolia, Poland, Romania, Russia (PRI), Slovakia, Spain, Switzerland, Turkey, Ukraine, United Kingdom.
Notes. The presence of the species in Iran is based in Belokobylskij et al. (2019).
Cotesiagillettei (Baker, 1895)
Apantelesgillettei Baker, 1895.
Type information. Holotype female, USNM (not examined but subsequent treatment of the species checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (AZ, CO, NJ).
Notes. Our species concept is based on Muesebeck (1921), Mason (1981) and Whitfield (1995a).
Cotesiaglabrata (Telenga, 1955)
Apantelesglabratus Telenga, 1955.
Type information. Lectotype female, ZIN (examined). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Bulgaria, Georgia, Germany, Hungary, Iran, Israel, Kazakhstan, Russia (DA, KDA, RYA, SPE, YAR), Turkey, Turkmenistan, Ukraine.
Notes. Our species concept is based on Shaw et al. (2009). The species distribution in Turkmenistan is based on Belokobylskij et al. (2019).
Cotesiaglomerata (Linnaeus, 1758)
Ichneumonglomeratus Linnaeus, 1758.
Ichneumonglomerator Thunberg, 1822.
Microgasternigriventris Nees, 1834.
Microgasterrecondita Nees, 1834.
Microgasterstellatarum Bouché, 1834.
Microgastercrataegi Ratzeburg, 1844.
Microgasteroleracea Taylor, 1860.
Microgasterpieridis Packard, 1881 [homonym of Microgasterpieridis Bouché, 1834].
Microgasterpieridivora Riley, 1882.
Apantelesaporiae Ivanov, 1899.
Glyptapantelesnawaii Ashmead, 1906.
Apantelesaporiae Matsumura, 1908).
Apantelesheterotergis Fahringer, 1936.
Type information. Type unknown, NHRS (not examined but subsequent treatment of the species checked). Country of type locality: unclear.
Geographical distribution.AUS, NEA, NEO, OTL, PAL.
AUS: Australia (ACT, NSW, QLD), Fiji, Hawaiian Islands, New Zealand; NEA: Canada (BC, NB, ON, QC), USA (CA, CO, CT, DC, FL, IL, IA, LA, MD, MA, MI, MN, NH, NJ, NY, OR, PA, SC, VT, VA, WA, WI); NEO: Barbados, Brazil (SP), Chile, Uruguay; OTL: China (GZ, HN, JS, SH, SN, TW, ZJ), India, Pakistan, Vietnam; PAL: Armenia, Azerbaijan, Azores, Belarus, Belgium, Bulgaria, Canary Islands, China (BJ, HE, HA, JL, LN, NM, NX, SN, XJ), Croatia, Cyprus, Czech Republic, Denmark, Egypt, Estonia, Finland, France, Georgia, Germany, Hungary, Iran, Ireland, Israel, Italy, Japan, Jordan, Kazakhstan, Korea, Latvia, Lithuania, Macedonia, Malta, Moldova, Mongolia, Morocco, Netherlands, Poland, Portugal, Romania, Russia (AD, AST, BU, KGD, KAM, KHA, KIR, KDA, KRS, MOS, PRI, ROS, SAK, SPE, SAR, TAM, VGG, VLG, YAR), Serbia, Slovakia, Spain, Sweden, Switzerland, Syria, Turkey, Ukraine, United Kingdom, Uzbekistan.
Notes. Our species concept is based on Shaw et al. (2009). Information about type(s) status for this species was discussed by Shenefelt (1972) and Fitton (1978). The species distribution in Armenia, Georgia, Jordan, Kazakhstan and Mongolia is based on Belokobylskij et al. (2019).
Cotesiagonopterygis (Marshall, 1898)
Apantelesgonopterygis Marshall, 1898.
Type information. Holotype female, PCMAG (not examined but subsequent treatment of the species checked). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Germany, Hungary, Japan, Romania, Russia (C, S), Slovakia, Switzerland, Turkey, United Kingdom.
Notes. Our species concept is based on Shaw et al. (2009).
Cotesiagordii (Muesebeck, 1926)
Apantelesgordii Muesebeck, 1926.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CT).
Cotesiagramini Sathe & Rokade, 2005
Cotesiagramini Sathe & Rokade, 2005.
Type information. Holotype female, depository unknown (not examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. This species may not be valid as we suspect that no type depository was specified. However, because we could not read the original description to confirm that, we retain it as valid species for the time being.
Cotesiagregalis Yang & Wei, 2002
Cotesiagregalis Yang & Wei, 2002.
Type information. Holotype female, CFRB (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (HE, LN, SD, TJ).
Notes. Our species concept is based on Zeng (2012).
Cotesiagriffini (Viereck, 1911)
Apantelesgriffini Viereck, 1911.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (AB, NB, QC), USA (AR, KS, MA, NY, OK, SC, SD, TX, WA).
Cotesiahadenae (Muesebeck, 1926)
Apanteleshadenae Muesebeck, 1926.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (MI, NJ).
Cotesiahalisidotae (Muesebeck, 1931)
Apanteleshalisidotae Muesebeck, 1931.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (BC, MB, ON, QC), USA (MA, NH, NY, VT).
Cotesiahallii (Packard, 1877)
Microgasterhallii Packard, 1877.
Type information. Holotype male, ANSP (not examined but subsequent treatment of the species checked). Country of type locality: Greenland.
Geographical distribution.NEA.
NEA: Canada (NT, NU), Greenland.
Notes. Our species concept is based on van Achterberg (2006) and Fernandez-Triana et al. (2017b). Shenefelt (1972: 528) recorded the type as male but with a question mark.
Cotesiahanshouensis (You & Xiong, 1983)
Apanteleshanshouensis You & Xiong, 1983.
Type information. Holotype female, HUNAU (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (HB, HN, JS, JX).
Notes. Our species concept is based on Chen and Song (2004).
Cotesiaharteni Papp, 2003
Cotesiaharteni Papp, 2003.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Cape Verde.
Geographical distribution.AFR.
AFR: Cape Verde.
Cotesiahemileucae (Riley, 1881)
Apanteleshemileucae Riley, 1881.
Type information. Syntypes female and male, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (NB, ON), USA (CT, FL, KS, MA, MN, MO, NY, OR).
Cotesiahesperidivora (Viereck, 1912)
Apanteleshesperidivorus Viereck, 1912.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (AZ, CA, CT, FL, MA).
Cotesiahiberniae (Kurdjumov, 1912), new combination
Apanteleshiberniae Kurdjumov, 1912.
Type information. Syntypes female and male, depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Ukraine.
Geographical distribution.PAL.
PAL: Ukraine.
Notes. Our concept of this species is based on Fahringer (1936) and Telenga (1955). We are placing this species in Cotesia based on the shape of T1-T3, short ovipositor, and the placement those authors gave to this species in their respective papers (Fahringer placed it in the Wilkinson group F, which comprises mostly species of what is currently Cotesia; Telenga’s key had hiberniae within a large group of Cotesia as well).
Cotesiahispanica (Oltra & Falco, 1996)
Protapanteleshispanica Oltra & Falco, 1996.
Type information. Holotype female, UVS (not examined but original description checked). Country of type locality: Spain.
Geographical distribution.PAL.
PAL: Spain.
Notes. The type material is probably in the Instituto Cavanilles de Biodiversitat y Biología Evolutiva, University of Valencia, Spain, but we have not been able to verify that information yet. Although the species was originally described in Protapanteles, Yu et al. (2016) treated it as Cotesia, a generic placement with agree with.
Cotesiahonora Papp, 1990
Cotesiahonora Papp, 1990.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Korea.
Geographical distribution.PAL.
PAL: Korea.
Cotesiahyperion (de Saeger, 1944), new combination
Apanteleshyperion de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Rwanda.
Notes. Based on the details from the original description (de Saeger 1944), the best generic placement would be in Cotesia.
Cotesiahyphantriae (Riley, 1887)
Apanteleshyphantriae Riley, 1887.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA, NEO, PAL.
NEA: Canada (BC, MB, NB, NS, ON, QC), USA (AR, CO, CT, DE, DC, LA, MD, MA, MI, MO, NJ, NM, NY, SC, TZ, VA, WV); NEO: Mexico; PAL: Bulgaria, Czech Republic, Germany, Greece, Hungary, Iran, Japan, Korea, Moldova, Netherlands, Poland, Romania, Russia (KDA), Serbia, Slovakia, Switzerland, Turkey, Ukraine, United Kingdom.
Cotesiahypopygialis (Granger, 1949), new combination
Apanteleshypopygialis Granger, 1949.
Type information. Syntypes female and male, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Notes. The details provided in the original description clearly show this species belongs in Cotesia.
Cotesiahypsipylae (Wilkinson, 1928), new combination
Apanteleshypsipylae Wilkinson, 1928.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. Examination of the holotype reveals the species belongs in Cotesia, based on the propodeum with a complete median, longitudinal carina, partial transverse carina, shapes and sculptures of T1 and T2, inflexible hypopygium, and short ovipositor sheaths.
Cotesiaicipe Fernandez-Triana & Fiaboe, 2017
Cotesiaicipe Fernandez-Triana & Fiaboe, 2017.
Type information. Holotype female, NMK (examined). Country of type locality: Kenya.
Geographical distribution.AFR, PAL.
AFR: Kenya, Madagascar, South Africa, Yemen. PAL: Saudi Arabia.
Notes. In the original description of the species, its distribution was recorded as only present in the Afrotropical region. However, in this paper we follow O’Hara et al. (2009) for boundaries of regions, and Saudi Arabia is considered to belong to the Palearctic, so the distribution above reflects that.
Cotesiaindica Sathe & Rokade, 2005
Cotesiaindica Sathe & Rokade, 2005.
Type information. Holotype female, depository unknown (not examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. This species may not be valid as we suspect that no type depository was specified. However, because we could not read original description to confirm that, we consider it as valid species for the time being.
Cotesiainducta (Papp, 1973)
Apantelesinductus Papp, 1973.
Apantelestenuivalvis Tobias, 1986.
Type information. Holotype female, HNHM (not examined but subsequent treatment of the species checked). Country of type locality: Hungary.
Geographical distribution.PAL.
PAL: Bulgaria, Hungary, Ireland, Israel, Korea, Moldova, Russia (KDA, PRI), Slovakia, Spain, Turkey, Ukraine, United Kingdom, Uzbekistan.
Notes. Our species concept is based on Shaw (2007).
Cotesiaintermixta (Balevski, 1980)
Apantelesintermixtus Balevski, 1980.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Bulgaria.
Geographical distribution.PAL.
PAL: Bulgaria.
Notes. We follow Papp (1986: 227) who based his species concept on the original description only, as he could not examine any specimens.
Cotesiainvirae Salgado-Neto & Whitfield, 2019
Cotesiainvirae Salgado-Neto & Whitfield, 2019.
Type information. Holotype female, UFSM (not examined but original description checked). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (RS).
Cotesiaishizawai (Watanabe, 1939)
Apantelesishizawai Watanabe, 1939.
Type information. Syntypes female and male?, EIHU (examined). Country of type locality: Japan.
Geographical distribution.PAL.
PAL: Japan.
Notes. In the EIHU collection there are two specimens, one female and one male, both with red labels marked as Type. That suggests they are actually syntypes, but we could not read the original description from Watanabe to corroborate if indeed several specimens were part of the original description with none being designated as a holotype (i.e., they are indeed syntypes) or if a holotype was designated. For the time being, and based on the specimens and labels we examined, we consider the specimens to be syntypes.
Cotesiaisolde (Nixon, 1974)
Apantelesisolde Nixon, 1974.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Finland, Germany, Hungary, Poland, Slovakia, Switzerland, United Kingdom.
Cotesiaitororensis Sousa-Lopes & Whitfield, 2019
Cotesiaitororensis Sousa-Lopes & Whitfield, 2019.
Type information. Holotype female, MZUSP (not examined but original description checked). Country of type locality: Brazil.
Geographical distribution. NEO.
NEO: Brazil (MG).
Cotesiajayanagarensis (Bhatnagar, 1950)
Apantelesjayanagarensis Bhatnagar, 1950.
Type information. Holotype female, INPC (not examined but subsequent treatment of the species checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: China (HN, SN, ZJ), India.
Notes. Our species concept is based on Chen and Song (2004). The year of publication of the Bhatnagar paper was until recently commonly cited as 1948 and/or 1950 (e.g., Chen and Song 2004, Yu et al. 2016), probably following Shenefelt (1972) who referred to this paper as “Bhatnagar (1948) 1950”. While the intended year for Volume X, Parts I & II of the Indian Journal of Entomology was 1948, the actual dates of publication were June 1950 (Part I) and October 1950 (Part II), as clearly shown on the cover page of the Volume, which we have checked. Because the dates of publication are the ones to be considered, and for the sake of clarity, we hereby revise the species year of description to 1950.
Cotesiajucunda (Marshall, 1885)
Apantelesjucundus Marshall, 1885.
Microgasternigrinervis Thomson, 1895.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Armenia, Austria, Azerbaijan, Bulgaria, Croatia, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iran, Ireland, Moldova, Mongolia, Poland, Romania, Russia (PRI), Serbia, Slovakia, Spain, Sweden, Switzerland, Turkey, United Kingdom.
Notes. The species distribution in Azerbaijan is based on Belokobylskij et al. (2019).
Cotesiajudaica (Papp, 1970)
Apantelesjudaica Papp, 1970.
Apantelesdzhanybeki Tobias, 1986.
Type information. Holotype female, BGM (not examined but original description checked). Country of type locality: Israel.
Geographical distribution.PAL.
PAL: Hungary, Israel, Italy, Kazakhstan, Russia (S), Tunisia, Ukraine.
Notes. The depository of the holotype was stated to be the “Beth Gordon Agriculture and Nature Study Institute, Deganya, Israel”. The depository acronym we provide here (BGM) is based on the way the museum is referred to in its Hebrew website as Beit Gordon Museum.
Cotesiajujubae (Wilkinson, 1929), new combination
Apantelesjujubae Wilkinson, 1929.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. The holotype has very short ovipositor sheaths, inflexible hypopygium, T1 coarsely sculptured and widening posteriorly, and T2 more or less rectangular in shape and with similar sculpture to T1. All those features clearly indicate that this species belongs in Cotesia. The carination pattern on the propodeum is rather unusual for the genus, with a median longitudinal carina that is weakly defined anteriorly, transverse carina present and an areola that is partially defined by carinae. However, that pattern is present in other Cotesia species (see Gupta et al. 2016b for a discussion of examples and illustrations of other species with similar carination patterns).
Cotesiajuniperatae (Bouché, 1834)
Microgasterjuniperatae Bouché, 1834.
Type information. Lectotype male, ZSM (not examined but original description checked). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: United Kingdom.
Notes. In this paper we follow Broad et al. (2016) decision to consider juniperatae as a valid species, but see notes under Cotesiabrachycera for more details on the history and use of related names and species.
Cotesiajunoniae (Riley, 1889)
Apantelesjunoniae Riley, 1889.
Type information. Holotype male, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CT, KS, MO, NJ).
Cotesiakamiyai (Watanabe, 1934)
Apanteleskamiyai Watanabe, 1934.
Type information. Syntypes female and male, EIHU (examined). Country of type locality: Japan.
Geographical distribution.OTL, PAL.
OTL: China (GZ, ZJ); PAL: Japan.
Notes. Despite previous references (e.g., Shenefelt 1972, Yu et al. 2016), the original description did not designate a holotype, and instead is based on a series of six female and two male specimens (Watanabe 1934: 134–135). We examined a male from the syntype series, in the Hokkaido collection.
Cotesiakariyai (Watanabe, 1937)
Apanteleskariyai Watanabe, 1937.
Apantelespurgata (Telenga, 1955).
Type information. Syntypes female and male, EIHU (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.PAL, OTL.
OTL: China (FJ, GX, GZ, HB, HN, JS, JX, SN, TW, YN, ZJ), Vietnam; PAL: China (AH, BJ, HL, HA, JL, LN, NX, SD, SN), Japan, Korea, Russia (PRI).
Notes. Our species concept is based on Papp (1986) and Chen and Song (2004). We also examined specimens from the EIHU collection.
Cotesiakarviri Sathe & Rokade, 2005
Cotesiakarviri Sathe & Rokade, 2005.
Type information. Holotype female, depository unknown (not examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. This species may not be valid as we suspect that no type depository was specified. However, because we could not read the original description to confirm that, we retain it as valid species for the time being.
Cotesiakasparyani (Tobias, 1976)
Apanteleskasparyani Tobias, 1976.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (KHA, PRI).
Notes. Our species concept is based on Papp (1987a) and Kotenko (2007a).
Cotesiakazak (Telenga, 1949)
Apanteleskazak Telenga, 1949.
Type information. Lectotype female, ZIN (examined). Country of type locality: Tajikistan.
Geographical distribution.AUS, OTL, PAL.
AUS: Australia (VIC, WA), New Zealand; OTL: India; PAL: Armenia, Azerbaijan, Bulgaria, Croatia, Greece, Iran, Israel, Kazakhstan, Mongolia, Morocco, Portugal, Russia (ROS), Spain, Tajikistan, Tunisia, Turkey, Turkmenistan, Uzbekistan.
Notes. Our species concept is based on Telenga (1955) and Papp (1986, 1987).
Cotesiakhuzestanensis Zargar & Gupta, 2019
Cotesiakhuzestanensis Zargar & Gupta, 2019.
Type information. Holotype female, NBAIR (not examined but original description checked). Country of type locality: Iran.
Geographical distribution.PAL.
PAL: Iran.
Cotesiakoebelei (Riley, 1889)
Apanteleskoebelei Riley, 1889.
Type information. Syntypes female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (BC), USA (CA, NE).
Notes. One of the syntypes is missing the head and metasoma, thus is impossible to know its sex with certainty. All other syntypes are female specimens.
Cotesiakraussi (Muesebeck, 1958)
Apanteleskraussi Muesebeck, 1958.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: Mexico.
Geographical distribution.NEO.
NEO: Mexico.
Cotesiakurdjumovi (Telenga, 1955)
Apanteleskurdjumovi Telenga, 1955.
Apanteleslaverna Nixon, 1974.
Type information. Lectotype female, ZIN (not examined but authoritatively identified specimens examined). Country of type locality: Ukraine.
Geographical distribution.PAL.
PAL: Bulgaria, Germany, Hungary, Israel, Lithuania, Moldova, Mongolia, Montenegro, Russia (C, S), Spain, Turkey, Turkmenistan, Ukraine, United Kingdom.
Notes. We examined the type of Apanteleslaverna Nixon.
Cotesialaeviceps (Ashmead, 1890)
Apanteleslaeviceps Ashmead, 1890.
Apantelesleviceps Dalla Torre, 1898 [unjustified emendation].
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (AB, BC, MB, NB, ON, QC, SK), USA (CA, CO, CT, GA, IL, IA, MO, NM, NY, UT).
Notes. The holotype is in poor condition, the only wing remaining is the right hind wing, antennae are missing, and the hind legs are embedded in glue.
Cotesialangei (Muesebeck, 1938)
Apanteleslangei Muesebeck, 1938.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CA).
Cotesialepidopteri Sathe & Rokade, 2005
Cotesialepidopteri Sathe & Rokade, 2005.
Type information. Holotype female, depository unknown (not examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. This species may not be valid as we suspect that no type depository was specified. However, because we could not read the original description to confirm that, we retain it as valid species for the time being.
Cotesialesbiae (Blanchard, 1947), new combination
Apanteleslesbiae Blanchard, 1947.
Apantelesgrioti Blanchard, 1943 [nomen nudum].
Type information. Holotype female, MACN (not examined but original description checked). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina.
Notes. The generic placement of the species within Cotesia is clear from the original description and the accompanying drawings.
Cotesialevigaster (Granger, 1949), new combination
Apanteleslevigaster Granger, 1949.
Type information. Syntypes female and male, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Notes. The original description (which includes a drawing of T1 and T2) strongly suggests that this species does not belong to Apanteles. Based on the described shape of T1-T2, hypopygium, length of ovipositor sheaths, recorded hosts, and (to a lesser extent) propodeum sculpture, the best generic placement at present would be Cotesia.
Cotesialimbata (Marshall, 1885)
Apanteleslimbatus Marshall, 1885.
Apanteleskawadai Watanabe, 1934.
Type information. Syntypes females, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Bulgaria, Croatia, France, Germany, Hungary, Japan, Moldova, Mongolia, Poland, Romania, Russia (AD, MOS), Serbia, Slovakia, Switzerland, United Kingdom.
Notes.Shenefelt (1972: 554) recorded the syntype specimens as female only.
Cotesialimenitidis (Riley, 1871)
Microgasterlimenitidis Riley, 1871.
Type information. Syntypes female and male, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (NS, ON), USA (CT, IL, IA, MD, MA, MO, NJ, NY, OH, PA, UT).
Cotesialineola (Curtis, 1830)
Microgasterlineola Curtis, 1830.
Microgasterlineola Curtis, 1829. [nomen nudum].
Apantelesgabrielis Gautier & Riel, 1919.
Type information. Syntypes female and male, MVMMA (not examined but subsequent treatment of the species checked). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Armenia, Bulgaria, Czech Republic, Finland, France, Germany, Hungary, Latvia, Moldova, Romania, Russia (SPE), Spain, Turkey, United Kingdom.
Notes. Our species concept is based on Wilkinson (1945), Nixon (1974), and Papp (1987a).
Cotesializeri (Blanchard, 1947), new combination
Apanteleslizeri Blanchard, 1947.
Type information. Holotype female, MACN (not examined but original description checked). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina.
Notes. From the original description and accompanying drawings, it is clear that this species is not an Apanteles. There is a small chance it could be placed within Parapanteles (examination of the specimens will be needed in the future to conclude); however, we consider that it is much more likely the species belongs in Cotesia, based on the short ovipositor sheaths, the propodeum carination and the available host data. An indirect support (but far from conclusive) for this species being placed in Cotesia is that all of its reported hosts are moths of the family Lasiocampidae (see Yu et al. 2016 for references). There is only one Parapanteles species recorded as a parasitoid of Lasiocampidae (Valerio et al. 2009) whereas there are numerous records of Cotesia parasitizing that family (Yu et al. 2016).
Cotesialuminata Chen & Song, 2004
Cotesialuminata Chen & Song, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (XZ).
Cotesialunata (Packard, 1881)
Microgasterlunatus Packard, 1881.
Type information. Neotype male, EIHU (not examined but subsequent treatment of the species checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (QC), USA (CA, CT, FL, GA, IL, IA, KS, MA, MO, NJ, NY, NC, TX, WA).
Notes. Our species concept is based on Muesebeck (1921), Mason (1981), Whitfield (1995a) and Fernandez-Triana (2010).
Cotesialyciae (Muesebeck, 1926)
Apanteleslyciae Muesebeck, 1926.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (QC), USA.
Cotesialycophron (Nixon, 1974)
Apanteleslycophron Nixon, 1974.
Type information. Holotype female, NHMUK (examined). Country of type locality: France.
Geographical distribution.PAL.
PAL: France, Hungary, Israel, Netherlands.
Cotesiamahoniae (Mason, 1975)
Apantelesmahoniae Mason, 1975.
Type information. Holotype female, CNC (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (BC), USA (ID).
Cotesiamalevola (Wilkinson, 1929), new combination
Apantelesmalevolus Wilkinson, 1929.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India, Myanmar.
Notes. This species belongs in Cotesia. It has very short ovipositor sheaths, inflexible hypopygium, strongly sculptured T1 and T2, and propodeum with both longitudinal and transverse carinae.
Cotesiamalshri (Sathe & Inamdar, 1991), new combination
Glyptapantelesmalshri Sathe & Inamdar, 1991.
Type information. Holotype female, SUKI (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. Based on the original description and drawings included there, it is very clear that this species does not belong to Glyptapanteles. Without examining the type material it is difficult to conclude, but the best generic placement at present would be Cotesia, based on the sculpture of the propodeum, T1–T3 shape and hypopygium and ovipositor.
Cotesiamarginiventris (Cresson, 1865)
Microgastermarginiventris Cresson, 1865.
Apantelesgrenadensis Ashmead, 1900.
Apanteleslaphygmae Ashmead, 1901 [nomen nudum].
Apantelesharnedi Viereck, 1912.
Type information. Holotype male, ANSP (not examined but authoritatively identified specimens examined). Country of type locality: Cuba.
Geographical distribution.AFR, AUS, NEA, NEO, OTL, PAL.
AFR: Cape Verde; AUS: Fiji, Hawaiian Islands; NEA: USA (AL, AZ, AR, CA, DE, FL, GA, IL, IN, IA, KS, KY, LA, MD, MS, MO, NC, OH, OK, SC, TN, TX, VA, WI); NEO: Argentina, Bermuda, Brazil (PR, RS, SC), Chile, Cuba, Grenada, Honduras, Mexico, Nicaragua, Peru, Puerto Rico, Suriname, Uruguay, Venezuela; OTL: India; PAL: Spain.
Notes. We have examined the types of Apantelesgrenadensis (Ashmead, 1900) (in the NHMUK), and Apantelesharnedi (Viereck, 1912) (in the USNM), as well as numerous specimens from several collections. There is considerable morphological variation, as well as wide host data; we suspect that Cotesiamarginiventris is likely to be a complex of cryptic species.
Cotesiamayaguezensis (Viereck, 1913)
Apantelesmayaguezensis Viereck, 1913.
Type information. Holotype female, USNM (examined). Country of type locality: Puerto Rico.
Geographical distribution.NEO.
NEO: Puerto Rico.
Cotesiamedicaginis (Muesebeck, 1947)
Apantelesmedicaginis Muesebeck, 1947.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (AZ, CA, ID, KS, MO, NV, NM, OK).
Cotesiameghrangini Dawale, Bhosale & Sathe, 1993
Cotesiameghrangini Dawale, Bhosale & Sathe, 1993.
Type information. Holotype female, NZSI (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Cotesiamelanoscelus (Ratzeburg, 1844)
Microgastermelanoscelus Ratzeburg, 1844.
Microgastersolitarius Ratzeburg, 1844.
Apantelescreata Balevski, 1980.
Type information. Type and depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.NEA, OTL, PAL.
NEA: Canada (BC, NB, NL, NS, ON, PE, QC), USA (CT, MD, MA, NH, NJ, NY, OR, PA, RI, VT, VA, WA, WV); OTL: China (FJ, HB, HN), India; PAL: Armenia, Azerbaijan, Austria, Belarus, Belgium, Bulgaria, China (BJ, HL, JL, LN), Czech Republic, Denmark, Finland, France, Germany, Hungary, Iran, Italy, Japan, Kazakhstan, Korea, Latvia, Lithuania, Moldova, Mongolia, Netherlands, Poland, Portugal, Romania, Russia (ALT, AMU, ZAB, KGD, KHA, KIR, KDA, PRI, YAR), Serbia, Slovakia, Spain, Sweden, Switzerland, Tajikistan, Turkey, Ukraine, United Kingdom.
Notes. Our species concept is based on Muesebeck (1921), Nixon (1974), Papp (1987a), and Chen and Song (2004). Following Article 31.2.1 of the ICZN the name is a noun in apposition and the original spelling melanoscelus must be retained. The species distribution in Armenia and Azerbaijan is based on Belokobylskij et al. (2019).
Cotesiamelitaearum (Wilkinson, 1937)
Apantelesmelitaearum Wilkinson, 1937.
Apantelesmelittaearum Wilkinson, 1937 [subsequent misspelling (Nixon 1974)].
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Armenia, Azerbaijan, China (BJ), Estonia, Finland, France, Germany, Hungary, Ireland, Italy, Kazakhstan, Korea, Moldova, Poland, Romania, Russia (BU, ZAB, PRI), Slovakia, Spain, Sweden, Turkey, United Kingdom, Uzbekistan.
Notes. The species distribution in Armenia is based on Belokobylskij et al. (2019).
Cotesiamendicae (Tobias, 1986)
Apantelesmendicae Tobias, 1986.
Type information. Holotype female, ZIN (not examined but paratype examined). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Kazakhstan, Russia (VOR).
Notes. We examined one female paratype from the same cocoon mass than the (unexamined by us) holotype.
Cotesiamenezesi (de Santis & Redolfi, 1976), new combination
Apantelesmenezesi de Santis & Redolfi, 1976.
Type information. Holotype female, MLP (not examined but subsequent treatment of the species checked). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO:Brazil (SP).
Notes. Transferred to Cotesia based on the following details, all mentioned in the original description: propodeum with median longitudinal carina; T1 barrel-shaped (described as wide but slightly narrowing at anterior and posterior margins); T2 twice as wide as long; short ovipositor sheaths; and species being considered as close to Apantelesschini Muesebeck, 1958 (currently placed in Cotesia). Yu et al. (2016) referred to the second author of the species as del Carmen Redolfi but the last name should be just Redolfi, as correctly stated by Aquino et al. (2010).
Cotesiamicrosomus (Tobias, 1986)
Apantelesmicrosomus Tobias, 1986.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Belarus.
Geographical distribution.PAL.
PAL: Belarus.
Notes. Our species concept is based on Tobias (1986) and Papp (1988, 1990). Following Article 31.2.1 of the ICZN the name is a noun phrase in apposition and the original spelling microsomus is retained.
Cotesiamiyoshii (Watanabe, 1932)
Apantelesmiyoshii Watanabe, 1932.
Apantelessmerinthii Matsumura, 1925 [primary homonym of Apantelessmerinthii Riley, 1881].
Type information. Syntypes female and male, EIHU (examined). Country of type locality: Japan.
Geographical distribution.OTL, PAL.
OTL: China (HB, JS, SN, ZJ); PAL: China (BJ, LN, SD), Japan, Korea.
Cotesiamurtfeldtae (Ashmead, 1898)
Apantelesmurtfeldtae Ashmead, 1898.
Type information. Holotype female, USNM (not examined but subsequent treatment of the species checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (MB, ON, QC), USA (CT, MD, MA, MO, NH, NY, NC, VT, VA, WV).
Notes. Our species concept is based on Muesebeck (1921) and Papp (1987a).
Cotesiamuzaffarensis (Lal, 1939), new combination
Apantelesmuzaffarensis Lal, 1939.
Type information. Holotype male, INPC (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. The original description, although very brief, provides enough information to unequivocally place this species in Cotesia. The propodeum has a complete median longitudinal carina, the ovipositor sheaths are short, and the shape and sculpture of T1–T2 (as illustrated in a dorsal habitus provided in the original description) are all typical of this genus. Additionally, Lal (1939: 53) mentioned that muzaffarensis is morphologically close to Cotesiasalebrosa.
Cotesianemoriae (Ashmead, 1898)
Apantelesnemoriae Ashmead, 1898.
Apanteleswinkleyi Viereck, 1917.
Type information. Holotype female, USNM (not examined but subsequent treatment of the species checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (MB, NL, NS, ON, QC, SK), USA (CA, CT, DC, KS, MA, MO, NH, NY, TN).
Notes. Our species concept is based on Muesebeck (1921) and Fernandez-Triana (2010). We also examined the female type of Apanteleswinkleyi (Viereck, 1917).
Cotesianeptisis (Watanabe, 1934), new combination
Apantelesneptisis Watanabe, 1934.
Type information. Lectotype female, EIHU (examined). Country of type locality: Japan.
Geographical distribution.PAL.
PAL: Japan.
Notes. From Shenefelt (1972: 579), it could be implied that a lectotype for this species was designated by him; however, no details were provided as to which specimen should be considered as the lectotype. After we examined the available specimens, we believe that the lectotype specimen is a female missing its head and two legs (one fore and one mid leg). We also examined one female and one male specimen from the same locality (Sapporo), as well as a cocoon mass with the same label as those specimens (which presumably belongs to this species). Kotenko (2007a) had transferred this species from Apanteles to Protapanteles, but we found that the propodeum sculpture rather clearly indicates that this species is better placed in Cotesia than Protapanteles (although T1 and the central part of T2 are rather smooth, they are in line with other Cotesia with relatively smooth sculpture). Also, Kotenko (2007a) mentioned the species from Russia but that seems to be a mistake; thus, here we consider the species to be restricted to Japan only.
Cotesianeustriae (Tobias, 1986)
Apantelesneustriae Tobias, 1986.
Type information. Holotype female, ZIN (not examined but paratype examined). Country of type locality: Moldova.
Geographical distribution.PAL.
PAL: Kazakhstan, Moldova, Russia (KDA, RYA, SAR, VGG, VOR), Turkey, Ukraine.
Notes. Our species concept is based on examined female paratypes, with the same host and locality data than the holotype – except for the collecting date being the following year compared to the holotype.
Cotesianigritibialis (Tobias, 1986)
Apantelesnigritibialis Tobias, 1986.
Type information. Holotype female, ZIN (not examined but original description checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Hungary, Korea, Russia (KDA).
Notes. Our species concept is based on Tobias (1986) and Papp (1988, 1990).
Cotesianikami Kurhade & Nikam, 1998
Cotesianikami Kurhade & Nikam, 1998.
Type information. Holotype female, BAMU (not examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Cotesianitens (Muesebeck, 1921)
Apantelesnitens Muesebeck, 1921.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CA, CO, OR, UT, WY).
Cotesianoctuidiphagus (Muesebeck, 1926)
Apantelesnoctuidiphagus Muesebeck, 1926.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CT).
Notes. Following Article 31.2.1 of the ICZN the name is a noun in apposition and the original spelling noctuidiphagus must be retained.
Cotesianonagriae (Olliff, 1893)
Apantelesnonagriae Olliff, 1893.
Apanteles (Stenopleura) nonagriae Viereck, 1913 [primary homonym of Apantelesnonagriae Olliff, 1893].
Cotesiaparthenayae Kittel, 2016 [unnecessary replacement name for Cotesianonagriae (Viereck, 1913)].
Type information. Syntypes female and male, depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (NSW, QLD).
Notes.Wilkinson (1928b: 136) mentioned that this species was described based on female and male specimens, which was also corroborated by Muirhead et al. (2008: 38–43). However, recent efforts by Muirhead et al. (2008) could not locate those specimens in Australian collections and it is likely that the syntypes are lost (or the depository remains unknown at present). In the literature there are two species named as Apantelesnonagriae, the oldest one (Olliff 1893) applies to this species, whereas the youngest one (Viereck 1913) has long being considered as a synonym of CotesiaflavipesCameron 1891. Because Viereck’s name is not related to Olliff’s the replacement name proposed by Kittel (2016) is unnecessary.
Cotesianothus (Marshall, 1885)
Apantelesnothus Marshall, 1885.
Type information. Lectotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Germany, Greece, Hungary, Iran, Italy, Korea, Mongolia, Romania, Russia (SPE), Slovakia, Turkey, United Kingdom.
Notes. Following Article 31.2.2 of the ICZN, in the absence of an original statement that the epithet is adjectival, the name is to be treated as a noun in apposition and the original spelling nothus must be retained (Doug Yanega, pers. comm.). The species distribution in Iran is based on Belokobylskij et al. (2019).
Cotesianuellorum Whitfield, 2018
Cotesianuellorum Whitfield, 2018.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: TX.
Cotesianumen (Nixon, 1974)
Apantelesnumen Nixon, 1974.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Czech Republic, Denmark, France, Germany, Hungary, Mongolia, Slovakia, Turkey, United Kingdom.
Cotesianycteus (de Saeger, 1944), new combination
Apantelesnycteus de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Rwanda.
Notes. Based on the details provided in the original description (de Saeger 1944), the best generic placement would be in Cotesia. Following Article 31.2.1 of the ICZN, the name is a noun in apposition and the original spelling nycteus must be retained.
Cotesiaobscuricornis (Viereck, 1917)
Apantelesobscuricornis Viereck, 1917.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CT, MD).
Notes. This species is rather similar to C.marginiventris (and other partially yellowish Cotesia) and may be part of a species complex.
Cotesiaocneriae (Ivanov, 1899)
Apantelesocneriae Ivanov, 1899.
Type information. Type and depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Ukraine.
Geographical distribution.PAL.
PAL: Austria, Hungary, Moldova, Poland, Romania, Russia (S), Serbia, Ukraine.
Notes. Our species concept is based on Telenga (1955), Tobias (1986) and Papp (1987a, 1990). The information about the type is taken from Shenefelt (1972: 585).
Cotesiaoeceticola (Blanchard, 1935), new combination
Apantelesoeceticola Blanchard, 1935.
Type information. Holotype female, MACN (not examined but original description checked). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina.
Notes. This species was described based on specimens from the Blanchard collection, which we believe is currently deposited in the MACN. The original description and illustrations (scutellar disc, propodeum, T1–T2, part of fore wing, tip of antenna), strongly suggest that this species belongs in Cotesia. The species has the propodeum with a median, longitudinal carina in addition to a more or less complete transverse carina, T1 and T2 are sculptured, T1 slightly widens towards the posterior margin, T2 is sub-rectangular, and the ovipositor sheaths barely protrude.
Cotesiaofella (Nixon, 1974)
Apantelesofella Nixon, 1974.
? Microgasterperspicuus Nees, 1834.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Belgium, Finland, Germany, Hungary, Iran, Israel, Italy, Netherlands, Poland, Serbia, Slovakia, Spain, Switzerland, Turkey, Ukraine, United Kingdom.
Notes. The name perspicuus (Nees, 1834) has sometimes been regarded as a senior synonym of both cajae (Bouché, 1834) and ofella (Nixon, 1974) (e.g., Yu et al. 2012, 2016). However, Marshall (1885: 183) had listed cajae as the senior name; and Papp (1988: 155) had tentatively suggested that perspicuus might be a synonym of ofella (Nixon, 1974), though without elaborating further. There has been little consensus on the correct application of the Nees name, the type of which is lost (see also comments under Cotesiacajae above). The arrangement proposed by Papp (1988), i.e., maintaining both cajae and ofella as valid species (and not as synonyms of perspicuus) has been subsequently followed by Papp (2005) and Broad et al. (2016), and it is also followed here.
Cotesiaogara Papp, 1990
Cotesiaogara Papp, 1990.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Korea.
Geographical distribution.PAL.
PAL: Korea.
Cotesiaokamotoi (Watanabe, 1921), status revised
Apantelesokamotoi Watanabe, 1921.
Type information. Holotype male, EIHU (examined). Country of type locality: Japan.
Geographical distribution.PAL.
PAL: Japan.
Notes. This species was synonymized under Cotesiaaffinis (Nees, 1834) by Papp (1987a), who wrote that synonymy was based on examination and comparison of authenticated specimens as well as original descriptions. However, one female of okamotoi (from the EIHU collection) that we have examined (which is placed together with the male holotype and another male, the three specimens collected in the same locality and with same label data) is clearly not affinis, and differs in practically all characters mentioned in Papp’s work, e.g., the length/width of T1 and T2 is different (T1 width at posterior margin is actually larger than T1 length, and T2 is 3.0 x as wide at posterior margin as long), T3 is not longer than T2 but both are of the same length (although in males T3 is longer), and vein r arises from the pterostigma closer to the end. The Japanese specimens have yellow T2–T6. Based on all of the above, we resurrect this species from synonymy with C.affinis and consider okamotoi to be a valid species. Cotesiaokamotoi looks similar to C.miyoshii, as stated in the comments for both species by Watanabe (1932); however, the differences in T1–T3 shape and sculpture are diagnostic, as well as different tegula colour, and in addition they parasitize different hosts and are found in different habitats.
Cotesiaolenidis (Muesebeck, 1922)
Apantelesolenidis Muesebeck, 1922.
Type information. Holotype female, USNM (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (BC).
Cotesiaonaspis (Nixon, 1974)
Apantelesonaspis Nixon, 1974.
Apantelesavetyanae Tobias, 1976.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Armenia, Finland, Hungary, Slovakia, United Kingdom.
Cotesiaoppidicola (Granger, 1949), new combination
Apantelesoppidicola Granger, 1949.
Type information. Syntypes female and male, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Notes. Based on the details provided in the original description, we consider that the best generic placement at present would be in Cotesia, but further examination of the type series will be needed in the future.
Cotesiaopsiphanis (Schrottky, 1909), new combination
Apantelesopsiphanis Schrottky, 1909.
Type information. Type and depository unknown (not examined but original description checked). Country of type locality: Paraguay.
Geographical distribution.NEO.
NEO: Ecuador, Paraguay.
Notes. Here we transfer the species to Cotesia based on the original description, which mentions a coarsely rugose propodeum, T1 and T2, as well as short ovipositor sheaths (Schrottky 1909: 211). Those morphological details, as well as images and details of the wasp cocoon mass (Waterston 1923) and the record of two Nymphalidae as hosts (Schrottky 1909, Waterston 1923, Malo and Willis 1961), strongly indicate that the best generic placement of the species at present is in Cotesia. However, examination of specimens will be needed in the future.
Cotesiaordinaria (Ratzeburg, 1844)
Microgasterordinarius Ratzeburg, 1844.
Apantelesdendrolimi Matsumura, 1926.
Apantelesdendrolimusi Matsumura, 1926.
Type information. Syntypes female and male, depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.OTL, PAL.
OTL: China (GX, HN, JS, ZJ); PAL: China (HL, JL, LN, SN), Czech Republic, Germany, Hungary, Iran, Israel, Italy, Japan, Korea, Mongolia, Poland, Romania, Russia (AMU, IRK, KYA, PRI, SAK, TOM, TY, YAR), Turkey, Ukraine.
Notes. Our species concept is based on Wilkinson (1945), Nixon (1974) and Papp (1987a). Wilkinson (1945: 71-72) wrote about the type series, which he examined, at the time deposited in the Forestry College of Eberswalde (Forstlichen Hochschule Eberswalde). Unfortunately, that collection was mostly destroyed during the Second World War; however, five drawers with Hymenoptera specimens, among them type species of Ratzeburg were spared and are now safe at the Senckenberg Deutsches Entomologisches Institut (SDEI) in Müncheberg, Germany (Schulz et al. 2018: 285-286). We do not know if the syntype specimens of Microgasterordinarius Ratzeburg are at present in Müncheberg. The species distribution in Iran is based on Belokobylskij et al. (2019).
Cotesiaorestes (Nixon, 1974)
Apantelesorestes Nixon, 1974.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Finland, Germany, Hungary, Korea, Netherlands, Russia (TVE), Turkey, United Kingdom.
Cotesiaorientalis Chalikwar & Nikam, 1984
Cotesiaorientalis Chalikwar & Nikam, 1984.
Type information. Holotype female, NZSI (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Cotesiaornatricis (Muesebeck, 1958)
Apantelesornatricis Muesebeck, 1958.
Type information. Holotype female, USNM (examined). Country of type locality: Colombia.
Geographical distribution.NEO.
NEO: Brazil (SP), Colombia.
Cotesiaorobenae (Forbes, 1883)
Apantelesorobenae Forbes, 1883.
Type information. Lectotype female, INHS (not examined but subsequent treatment of the species checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (AZ, CA, DC, FL, IL, LA, SC, VA).
Notes. Our species concept is based on Muesebeck (1921) and Papp (1987a).
Cotesiapachkuriae (Bhatnagar, 1950), new combination
Apantelespachkuriae Bhatnagar, 1950.
Type information. Holotype female, INPC (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. Transferred to Cotesia based on the propodeum with strong, median longitudinal carina and a transverse carina (near anterior), T1 sculptured and widening towards posterior margin, T2 slightly sculptured and around same length as T3 (Bhatnagar 1950). The year of publication of the Bhatnagar paper was until recently commonly cited as 1948 and/or 1950 (e.g., Chen and Song 2004, Yu et al. 2016), probably following Shenefelt (1972) who referred to this paper as “Bhatnagar (1948) 1950”. While the intended year for Volume X, Parts I & II of the Indian Journal of Entomology was 1948, the actual dates of publication were June 1950 (Part I) and October 1950 (Part II), as clearly shown on the cover page of the Volume, which we have checked. Because the dates of publication are the ones to be considered, and for the sake of clarity, we hereby revise the species year of description to 1950.
Cotesiapaludicolae (Cameron, 1909), new combination
Apantelespaludicolae Cameron, 1909.
Apantelesplatyptiliae Cameron, 1909.
Type information. Syntypes female, NHMUK (examined). Country of type locality: Sri Lanka.
Geographical distribution.AFR, OTL.
AFR: Sudan, Uganda; OTL: India, Sri Lanka.
Notes. This species belongs in Cotesia based on sculpture and carination of the propodeum, shapes of T1 and T2, inflexible hypopygium and relatively short ovipositor sheaths. Wilkinson (1928a, 1932a) correctly associated this species with the glomeratus group.
Cotesiapaphi (Schrottky, 1902)
Apantelespaphi Schrottky, 1902.
Type information. Syntypes female and male, depository unknown (not examined). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina, Brazil (SP), Peru, Uruguay.
Notes. The depository is presumed to be the MACN, but we have not been able to corroborate that.
Cotesiapappi Inanç, 2002
Cotesiapappi Inanç, 2002.
Type information. Holotype female, ZMTU (not examined but original description checked). Country of type locality: Turkey.
Geographical distribution.PAL.
PAL: Turkey.
Cotesiaparastichtidis (Muesebeck, 1921)
Apantelesparastichtidis Muesebeck, 1921.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (AB, BC, MB, NB, NS, ON, YT), USA (AK, MI, NY, TN).
Cotesiaparbhanii (Rao, 1969), new combination
Apantelesparbhanii Rao, 1969.
Type information. Holotype female, NZSI (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. From the original description it is clear that this species belongs to Cotesia, based on propodeum with median longitudinal carina, shapes of T1 and T2, and length of ovipositor sheaths. Rao (1969: 222) mentioned that although the specimens were in his personal collection, they would be deposited in the NZSI, information that we tentatively follow here when recording the type depository.
Cotesiaparijati Sathe, 2003
Cotesiaparijati Sathe, 2003.
Type information. Holotype female, SUKI (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Cotesiaparvicornis (de Saeger, 1944), new combination
Apantelesparvicornis de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Rwanda.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Rwanda, Senegal.
Notes. Based on the details of the original description (de Saeger 1944), as well as the known host record, the best generic placement at present would be in Cotesia; however, future examination of the holotype will be needed (as there is a small chance that the species might belong to Glyptapanteles).
Cotesiapeltoneni (Papp, 1987)
Apantelespeltoneni Papp, 1987.
Type information. Holotype female, MZH (not examined but original description checked). Country of type locality: Finland.
Geographical distribution.PAL.
PAL: Finland.
Cotesiaphiloeampus (Cameron, 1911)
Apantelesphiloeampus Cameron, 1911.
Type information. Lectotype female, NHMUK (not examined but subsequent treatment of the species checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (NSW).
Notes. Our species concept is based on Austin and Dangerfield (1992). There is some confusion with this species name and for the sake of clarity we provide more details here. Cameron (1911a: 342) described an Australian species as Apantelesphiloeampus. In another paper published that same year, Cameron (1911b: 327) also described a different species from Guyana as Apantelesphilocampus. Wilkinson (1928a: 96) correctly pointed that out; however, the two names being so similar (and being published by the same author in the same year, albeit in two different publications) have brought unintentional confusion over the years when dealing with them. For example, Austin and Dangerfield (1992: 22), when transferring the Australian species from Apanteles to Cotesia mentioned that Apantelesphilocampus was being transferred when they really meant Apantelesphiloeampus. The species from Guyana is also transferred from Apanteles in the present paper (see below under Glyptapantelesphilocampus).
Cotesiaphobetri (Rohwer, 1915)
Apantelesphobetri Rohwer, 1915.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (AB, NL, ON), USA (IL, IN, KS, KY, MD, MA, MO, NH, NJ, NY, NC, RI, VT, VA, WV).
Cotesiapholisorae (Riley, 1889)
Apantelespholisorae Riley, 1889.
Type information. Syntypes female and male, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CT, DC, IL, KS, MD, MO, SC, WV).
Cotesiapieridis (Bouché, 1834)
Microgasterpieridis Bouché, 1834.
Type information. Type lost (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Armenia, Azerbaijan, China (SD), Georgia, Germany, Hungary, Israel, Kazakhstan, Lithuania, Moldova, Mongolia, Romania, Russia (PRI, SAK, VLA, VOR), Slovakia, Tajikistan, Turkey, Uzbekistan.
Notes. Our species concept is based on Nixon (1974), Papp (1987a), and Chen and Song (2004). Nixon (1974: 493) considered the type to be lost, based on unpublished notes from Wilkinson, who had examined the Bouché collection. The species distribution in Israel is based on Belokobylskij et al. (2019).
Cotesiapilicornis (Thomson, 1895)
Microgasterpilicornis Thomson, 1895.
Apantelespiliflagellaris Tobias, 1986.
Type information. Holotype female, MZLU (not examined but subsequent treatment of the species checked). Country of type locality: Sweden.
Geographical distribution.PAL.
PAL: Bulgaria, Croatia, Finland, Germany, Hungary, Italy, Moldova, Romania, Russia (C, E, NC, S), Slovakia, Sweden, Switzerland, Turkey, United Kingdom.
Notes. Our species concept is based on Nixon (1974) and Papp (1987a). Broad et al. (2016) commented on the morphological variation of specimens they had examined, which they considered as conspecific anyway.
Cotesiapistrinariae (Wilkinson, 1929)
Apantelespistrinariae Wilkinson, 1929.
Apantelespistrinariaenyasaensis Wilkinson, 1929.
Type information. Holotype female, NHMUK (examined). Country of type locality: Nigeria.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Eritrea, Ethiopia, Malawi, Nigeria, Rwanda, South Africa.
Notes. The record of this species from Cape Verde (e.g., Koponen 1989, Forshage et al. 2016, Gupta et al. 2016b) is based on specimens of Cotesiacompressithorax (which was considered a synonym of pistrinariae until this paper, where we consider both as valid species, see more comments about differences between these two species under the Notes for compressithorax; p 292, 293). Thus, here we remove that country from the geographical distribution of pistrinariae.
Cotesiaplanula Song & Chen, 2004
Cotesiaplanula Song & Chen, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (NX).
Cotesiaplathypenae (Muesebeck, 1921)
Apantelesplathypenae Muesebeck, 1921.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (BC, MB), USA (ID, IL, IN, IA, KS, MO, NY, OH, SD, WA).
Cotesiapodunkorum (Viereck, 1912)
Apantelespodunkorum Viereck, 1912.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CT, OH, VA, WV).
Cotesiapraepotens (Haliday, 1834)
Microgasterpraepotens Haliday, 1834.
Microgasterplacidus Haliday, 1834.
Apantelesmemnon Nixon, 1974.
Apantelesacutivalvis Balevski, 1980.
Apantelesbeshtaui Tobias, 1986.
Type information. Lectotype female, NMID (not examined but authoritatively identified specimens examined). Country of type locality: Ireland.
Geographical distribution.PAL.
PAL: Afghanistan, Armenia, Azerbaijan, Bulgaria, Croatia, Czech Republic, Finland, Germany, Greece, Hungary, Iran, Ireland, Italy, Kazakhstan, Lithuania, Macedonia, Moldova, Mongolia, Poland, Romania, Russia (ZAB, KDA, PRI, SAR, STA, VOR), Slovakia, Spain, Sweden, Switzerland, Tajikistan, Turkey, Turkmenistan, United Kingdom, Uzbekistan, Yugoslavia.
Notes. We examined the type of Apantelesmemnon Nixon. Our species concept is based on van Achterberg (1997), but see notes under Cotesiabrachycera for more details on the history and use of related names and species.
Cotesiapratapae (Ashmead, 1896), new combination
Apantelespratapae Ashmead, 1896.
Type information. Holotype female, USNM (examined). Country of type locality: Sri Lanka.
Geographical distribution.OTL.
OTL: Sri Lanka.
Notes. Transferred to Cotesia based on the following: inflexible hypopygium, ovipositor sheaths short, propodeum reticulated (although without clear median carina), T1 mostly parallel-sided, but slightly narrowing medially, and T2 rectangular and rugulose. The shape of T1 in this species is similar to that of C.trabalae Gupta, 2016, a very rare feature in Cotesia (see Gupta et al. 2016b for more details).
Cotesiaprenidis (Muesebeck, 1921)
Apantelesprenidis Muesebeck, 1921.
Type information. Holotype female, USNM (examined). Country of type locality: Puerto Rico.
Geographical distribution.NEO.
NEO: Puerto Rico.
Cotesiaprogahinga (Hedqvist, 1965)
Apantelesprogahinga Hedqvist, 1965.
Type information. Holotype female, MZH (examined). Country of type locality: Cape Verde.
Geographical distribution.AFR.
AFR: Cape Verde.
Notes.Forshage et al. (2016) considered the type material to be lost; however, in 2017 the holotype was found by the senior author in another section of the MZH collection.
Cotesiaprozorovi (Telenga, 1955), new combination
Apantelesprozorovi Telenga, 1955.
Type information. Syntypes female and male, ZIN? (not examined but original description checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (IRK, PRI).
Notes. Our species concept is based on the original description and Papp (1987a). It is not clear that the type specimens are deposited in the ZIN, but it is an educated guess based on Tobias (1986).
Cotesiapterophoriphagus (Shenefelt, 1972), new combination
Apantelespterophoriphagus Shenefelt, 1972.
Apantelespterophori Risbec, 1951 [homonym of Apantelespterophori Muesebeck, 1926].
Type information. Holotype female, depository unknown (not examined but original description checked). Country of type locality: Senegal.
Geographical distribution.AFR.
AFR: Senegal.
Notes. Transferred to Cotesia based on the propodeum with a strong median carina (in addition to lateral and transverse carinae), shape and sculpture of T1–T2, acute hypopygium and short ovipositor sheaths (Risbec 1951: 435–437, see also figure 13 in that paper). The original description also compares this species with sphenarchi (Risbec, 1951), also currently placed in Cotesia. Because the name is to be considered as a noun under ICZN Article 31.2.1, it must retain its original spelling and remain as pterophoriphagus.
Cotesiapyralidis (Muesebeck, 1921)
Apantelespyralidis Muesebeck, 1921.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (AR, DC, IL, MD, MO, NC, OH, WI).
Cotesiapyraustae (Viereck, 1912)
Apantelespyraustae Viereck, 1912.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (ON), USA (CT, MO, NJ).
Notes. The holotype is missing the metasoma, two legs, one pair of wings and the apical half of the antennae.
Cotesiapyrophilae (Muesebeck, 1926)
Apantelespyrophilae Muesebeck, 1926.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (ON), USA (CT, MA, RI).
Cotesiaradiantis (Wilkinson, 1929)
Apantelesradiantis Wilkinson, 1929.
Type information. Holotype female, NHMUK (not examined but subsequent treatment of the species checked). Country of type locality: Australia.
Geographical distribution.AUS, OTL.
AUS: Australia (QLD); OTL: China (HN).
Notes. Our concept of this species is based on Austin and Dangerfield (1992).
Cotesiaradiarytensis (Shenefelt, 1972), new combination
Apantelesradiarytensis Shenefelt, 1972.
Apantelesradiatus Niezabitowski, 1910 [homonym of Apantelesradiatus Ashmead, 1898].
Type information. Holotype female, depository unknown (not examined but original description checked). Country of type locality: Poland.
Geographical distribution.PAL.
PAL: Poland.
Notes. Our species concept is based on the original description, as well as the key and comments provided by Telenga (1955). From those two sources, it is clear that this species is not Apanteles, based on the very short ovipositor, and the position in Telenga’s key (near many species of Cotesia and far from all true Apanteles keyed out in that paper). Without examining an actual specimen (and we note that there is no information about the whereabouts of the holotype), this species cannot be unambiguously assigned to genus. However, Cotesia seems the most reasonable choice, and is the one we propose here (other alternatives based on elements from the original description, such as Protapanteles, Glyptapanteles and even Nyereria, are much less plausible).
Cotesiarangii (Bhatnagar, 1950), new combination
Apantelesrangii Bhatnagar, 1950.
Type information. Holotype male, INPC (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. Transferred to Cotesia based on the followings details provided in the original description (Bhatnagar 1950: 165–166, also figs 40, 84 in that paper): propodeum rugulose and with transverse and median longitudinal carinae (both strongly marked), as well as other carinae (around the median one) running upwards; T1 relatively broad (length less than 2.0 x its width) but mostly parallel-sided; T2 as long as T3 and with curved margins laterally. The median, longitudinal carina in the propodeum excludes this species from Apanteles, whereas the strong transverse carina and the shapes of T1 and T2 exclude it from Glyptapanteles. Bhatnagar (1950) considered rangii to come close to Apantelessundanus (Wilkinson, 1930) [which in this paper is placed within the genus Neoclarkinella, see notes under that species in its treatment below], probably due to the presence of both longitudinal and transverse carinae in both taxa. However, rangii cannot be placed in Neoclarkinella as that genus has T1 strongly narrowing towards the posterior margin (T1 width at anterior margin being several times that of width at posterior margin), and T2 is much smaller than T3; also, the veins r and 2RS in Neoclarkinella are curved in a very characteristic way (e.g., see Figs 161–165 in present paper) whereas the shape of those veins in rangii are very different (see fig. 40 in Bhatnagar 1950). Thus, we consider that the available evidence strongly indicates Cotesia as the best generic placement at present. The year of publication of the Bhatnagar paper was until recently commonly cited as 1948 and/or 1950 (e.g., Chen and Song 2004, Yu et al. 2016), probably following Shenefelt (1972) who referred to this paper as “Bhatnagar (1948) 1950”. While the intended year for Volume X, Parts I & II of the Indian Journal of Entomology was 1948, the actual dates of publication were June 1950 (Part I) and October 1950 (Part II), as clearly shown on the cover page of the Volume, which we have checked. Because the dates of publication are the ones to be considered, and for the sake of clarity, we hereby revise the species year of description to 1950.
Cotesiarisilis (Nixon, 1974)
Apantelesrisilis Nixon, 1974.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Greece, Hungary, Iran, Italy, Mongolia, Montenegro, Netherlands, Romania, Slovakia, Turkey, United Kingdom.
Cotesiariverai (Porter, 1916), name amended and new combination
Apantelesriverae Porter, 1916 [incorrect original spelling].
Type information. Syntypes female and male, depository unknown (not examined but original description checked). Country of type locality: Chile.
Geographical distribution.NEO.
NEO: Chile.
Notes. The original spelling of the species Apantelesriverae is incorrect, as the species was named after M.J. Rivera, a man (Porter 1916: 96), and thus its ending should be i instead of ae. The correct spelling is here amended to riverai. After reading the original description, we consider that the evidence there strongly supports this species as belonging to Cotesia. Porter (1916: 96–98) mentioned a median longitudinal carina on the propodeum, a quadrate T1, a T2 with a median field (smoother than the rest of the tergite), and very short ovipositor sheaths that barely project beyond the metasoma. He also provided illustrations of the fore wing and hind leg. Additionally, the host of the type series (Erebidae) and the gregarious wasp cocoons are common, although not exclusive, features of Cotesia.
Cotesiarubecula (Marshall, 1885)
Apantelesrubecula Marshall, 1885.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.AUS, NEA, OTL, PAL.
AUS: New Zealand; NEA: Canada (BC, ON, QC), USA (MA, MI, MN, OR, VT, VA, WA); OTL: China (FJ, HB, ZJ); PAL: Austria, Bulgaria, China (BJ, HE, JL, LN, SN), France, Germany, Hungary, Iran, Macedonia, Moldova, Netherlands, Poland, Romania, Russia (IRK, KHA, KDA, MOS, PRI, ROS, RYA, SAK), Slovakia, Spain, Switzerland, Ukraine, United Kingdom, Yugoslavia.
Cotesiarubripes (Haliday, 1834)
Microgasterrubripes Haliday, 1834.
Type information. Lectotype female, MVMMA (not examined but subsequent treatment of the species checked). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Belarus, Bulgaria, Croatia, Czech Republic, France, Germany, Hungary, Israel, Italy, Japan, Kazakhstan, Korea, Lithuania, Mongolia, Morocco, Poland, Romania, Russia (KDA, KYA, MOS, PRI, TOM, VOR, YAR), Serbia, Switzerland, Turkey, Ukraine, United Kingdom, Uzbekistan, Yugoslavia.
Notes. Our species concept is based on Wilkinson (1945), Nixon (1974), Papp (1987a), Kotenko (2007a), and Broad et al. (2016). The species distribution in Japan and Turkey is based on Belokobylskij et al. (2019).
Cotesiaruficoxis (Hedwig, 1962), new combination
Apantelesruficoxis Hedwig, 1962.
Type information. Holotype female, depository unknown (not examined but original description checked). Country of type locality: Greece.
Geographical distribution.PAL.
PAL: Greece.
Notes. Based on the original description, this species is clearly not Apanteles, due to a median longitudinal carina on the propodeum and the short ovipositor. Those characters, coupled with the shape and sculpture of T1 and T2 (also taken from the original description), strongly suggest the best generic placement at present to be in Cotesia. However, until the holotype (only known specimen) is found and studied, this decision must be considered as provisional.
Cotesiaruficrus (Haliday, 1834)
Microgasterruficrus Haliday, 1834.
Apantelesantipoda Ashmead, 1900.
Apantelesmanilae Ashmead, 1904.
Apantelessydneyensis Cameron, 1911.
Apantelesnarangae Viereck, 1913.
Microgastercontextus Imhof & Labram, 1836.
Apantelessesamiae Risbec, 1956 [nomen nudum].
Type information. Lectotype female, MVMMA (not examined but subsequent treatment of the species checked). Country of type locality: unknown.
Geographical distribution.AFR, AUS, NEO, OTL, PAL.
AFR: Cameroon, Cape Verde, Ethiopia, Kenya, Ivory Coast, Madagascar, Mauritius, Nigeria, Réunion, Senegal, Somalia, South Africa, Sudan, Tanzania, Uganda, Yemen, Zimbabwe; AUS: Australia (NSW, QLD), Fiji, Hawaiian Islands, New Zealand; NEO: Trinidad & Tobago; OTL: Bangladesh, China (FJ, GD, GX, GZ, HI, HK, HB, HN, JS, JX, SH, SN, TW, YN, ZJ), India, Indonesia, Malaysia, Pakistan, Philippines, Ryukyu Islands, Sri Lanka, Thailand, Vietnam; PAL: Afghanistan, Algeria, Armenia, Azerbaijan, Belarus, Belgium, Bulgaria, China (AH, BJ, HE, HL, HA, JL, LN, SN, SD), Cyprus, Egypt, Finland, France, Georgia, Germany, Hungary, Iceland, Iran, Iraq, Ireland, Israel, Italy, Japan, Kazakhstan, Korea, Libya, Lithuania, Malta, Moldova, Mongolia, Nepal, Netherlands, Poland, Romania, Russia (AD, AST, ZAB, KAM, KDA, NIZ, PNZ, PRI, ROS, SAK, YAR), Slovakia, Slovenia, Spain, Sweden, Switzerland, Turkey, Turkmenistan, Ukraine, United Kingdom, Yugoslavia.
Notes. The country of the lectotype was not specified by van Achterberg (1997: 74), it could be either Ireland or the United Kingdom. We examined the female holotype of Apantelesantipoda (Ashmead, 1900), and indeed it looks like C.ruficrus. We also examined the type, a female specimen, of Apantelesnarangae (Viereck, 1913), another synonym of ruficrus. van Achterberg (2014) synonymized Microgastercontextus (Imhof & Labram, 1836) under C.rufricus based on the figure and biology detailed in the original description of contextus. The species distribution in Afghanistan is based on Belokobylskij et al. (2019).
Cotesiarufiventris (Bingham, 1906)
Apantelesrufiventris Bingham, 1906.
Type information. Lectotype female, OUMNH (not examined but subsequent treatment of the species checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (QLD).
Notes. Our species concept is based on Wilkinson (1928a, 1930a), Austin and Dangerfield (1992) and van Achterberg & O’Toole (1993).
Cotesiarufocoxalis (Riley, 1881)
Apantelesrufocoxalis Riley, 1881.
Type information. Syntypes female and male, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (NS), USA (AL, CT, DC, FL, LA, MO, NJ, NY, TN, TX, VA).
Notes. The type series is on a single pin, which has a piece of card containing 32+ specimens and the cocoon mass.
Cotesiarugosa (Szépligeti, 1914)
Apantelesrugosus Szépligeti, 1914.
Type information. Holotype male, MNHN (not examined but subsequent treatment of the species checked). Country of type locality: Kenya.
Geographical distribution.AFR.
AFR: Kenya.
Notes. Our species concept is based on Wilkinson (1932a) and Papp (2008).
Cotesiaruidus (Wilkinson, 1928)
Apantelesruidus Wilkinson, 1928.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Cotesiasalebrosa (Marshall, 1885), lectotype designation
Apantelessalebrosus Marshall, 1885.
Apantelescallunae Nixon, 1974.
Type information. Lectoype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Bulgaria, Finland, France, Germany, Hungary, Iran, Italy, Korea, Lithuania, Mongolia, Norway, Poland, Russia (YAR), Sweden, Switzerland, Turkey, Ukraine, United Kingdom.
Notes. We have examined what by all indications seem to be the type series from Marshall, which is in the NHMUK with number 3c.25. It contains two female and two male specimens and the code agrees with that reported for this species by Shenefelt (1972: 621), although Shenefelt reported the specimens to be all female. However, there is another label attached to those specimens from Nixon (with date of 1952) and that label states that those specimens are not the type of salebrosus Marshall but rather A.solitarus Ratz (a reference to Microgastersolitarius Ratzeburg, 1844, which is currently considered to be a synonym of Cotesiamelanoscela Ratzeburg, 1844). However, having examined other specimens of both Cotesiamelanoscela and C.salebrosa (e.g., see Ruohomäki et al. 2013), we think that the specimens from Marshall that are deposited in the NHMUK belong to the latter species, in that sense disagreeing with Nixon’s label from 1952. As far as we know no specimen from that card has ever been designated as the lectotype, thus we designate one here. The card has the two male specimens towards the left side, close to a single cocoon which is glued near them. The right side of the card contains the female specimens, with the bottom one having the metasoma and one hind leg detached (but glued nearby). The female at the top right of the card is the only specimen in the series that is in very good condition, and thus is the one we select as the lectotype. We also examined the type of Apantelescallunae Nixon. The species distribution in Iran is based on Belokobylskij et al. (2019).
Cotesiasaltator (Thunberg, 1822)
Ichneumonsaltator Thunberg, 1822.
Ichneumonsalsator Thunberg, 1822 [incorrect original spelling].
Ichneumonsaltator Thunberg, 1824 [justified emendation and homonym of Ichneumonsaltator Müller, 1776].
Type information. Syntypes male, UUZM (not examined but subsequent treatment of the species checked). Country of type locality: Sweden.
Geographical distribution.PAL.
PAL: Armenia, Bulgaria, France, Germany, Hungary, Iran, Israel, Lebanon, Mongolia, Poland, Russia (SPE), Slovakia, Sweden, Turkey, Ukraine, Yugoslavia.
Notes. Our species concept is based on Shaw et al. (2009).
Cotesiasaltatoria (Balevski, 1980)
Apantelessaltatorius Balevski, 1980.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Bulgaria.
Geographical distribution.PAL.
PAL: Bulgaria, Croatia, France, Germany, Hungary, Macedonia, Mongolia, Serbia, Slovakia, Spain, Turkey, United Kingdom.
Notes. Our species concept is based on Shaw (2007).
Cotesiasasakii (Watanabe, 1932)
Apantelessasakii Watanabe, 1932.
Type information. Holotype female, EIHU (examined). Country of type locality: Japan.
Geographical distribution.PAL.
PAL: Japan, Korea.
Notes. The female holotype is missing the metasoma, but we have examined six other female specimens from the same date and locality which are in better condition. The species is rather characteristic in having ovipositor sheaths with long setae, much longer than the sheath width.
Cotesiasatunini (Tobias, 1986)
Apantelessatunini Tobias, 1986.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Azerbaijan.
Geographical distribution.PAL.
PAL: Azerbaijan.
Notes. Our species concept is based on Tobias (1986) and Papp (1988, 1990).
Cotesiascabricula (Reinhard, 1880)
Apantelesscabriculus Reinhard, 1880.
Apanteleseguchii Watanabe, 1935.
Type information. Holotype female, depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Austria.
Geographical distribution.OTL, PAL.
OTL: China (HB, HN, SN, ZJ); PAL: Armenia, Austria, China (HE, SD, SN), Germany, Hungary, Iran, Italy, Korea, Macedonia, Moldova, Mongolia, Romania, Russia (KDA), Serbia, Slovakia, Switzerland.
Notes. Our species concept is based on Nixon (1974) and Papp (1987a). The species distribution in Iran is based on Belokobylskij et al. (2019).
Cotesiaschaeferi (Marsh, 1979)
Apantelesschaeferi Marsh, 1979.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: Japan.
Geographical distribution.PAL.
PAL: China (BJ), Japan, Korea.
Cotesiaschaffneri (Muesebeck, 1931)
Apantelesschaffneri Muesebeck, 1931.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (DE, NJ, PA, TX, VA).
Cotesiaschini (Muesebeck, 1958)
Apantelesschini Muesebeck, 1958.
Type information. Holotype female, USNM (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (SP).
Cotesiaschizurae (Ashmead, 1898)
Apantelesschizurae Ashmead, 1898.
Type information. Holotype female, USNM (not examined but subsequent treatment of the species checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (ON), USA (AZ, AR, CA, CT, FL, IL, MD, MA, MS, MO, NH, NY, VA, WI).
Notes. Our species concept is based on Muesebeck (1921), Papp (1987a), Mason (1981), and Fernandez-Triana (2010).
Cotesiascitula (Riley, 1881)
Apantelesscitulus Riley, 1881.
Apantelesemarginata Riley, 1889.
Apantelesparorgyiae Ashmead, 1898.
Cryptapantelesrileyana Viereck, 1910.
Type information. Syntypes female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (NS, ON), USA (CT, DC, FL, IL, KS, KY, LA, MD, MO, NE, NH, NJ, TN, TX, WI).
Notes. We have also examined the type of A.rileyanus (Viereck, 1910).
Cotesiascotti (Valerio & Whitfield, 2009)
Parapantelesscotti Valerio & Whitfield, 2009.
Type information. Holotype female, INBio (not examined but original description checked). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Notes. Our species concept is based on Freitas et al. (2019).
Cotesiaselenevora Shaw, 2009
Cotesiaselenevora Shaw, 2009.
Type information. Holotype female, RSME (examined). Country of type locality: Belgium.
Geographical distribution.PAL.
PAL: Belgium, Finland, Sweden.
Cotesiasenegalensis (Risbec, 1951), new combination
Apantelessenegalensis Risbec, 1951.
Type information. Holotype female, depository unknown (not examined but original description checked). Country of type locality: Senegal.
Geographical distribution.AFR.
AFR: Senegal.
Notes. From the details provided in the original description it is clear that this species belongs to Cotesia.
Cotesiasericea (Nees, 1834)
Microgastersericeus Nees, 1834.
Type information. Type and depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Azerbaijan, Belgium, France, Georgia, Germany, Italy, Mongolia, Russia (KDA, NGR, SPE, SAR, YAR), Tajikistan, Turkmenistan, Ukraine, United Kingdom, Uzbekistan.
Notes. Our species concept is based on Belokobylskij et al. (2003), which was followed by Broad et al. (2016), but see Notes under Cotesiabrachycera for more details on the history and use of related names and species.
Cotesiasesamiae (Cameron, 1906)
Apantelessesamiae Cameron, 1906.
Type information. Type and depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: South Africa.
Geographical distribution.AFR, OTL.
AFR: Benin, Burkina Faso, Cameroon, Central African Republic, Comoros, Democratic Republic of Congo, Eritrea, Ethiopia, Ghana, Ivory Coast, Kenya, Lesotho, Madagascar, Malawi, Mauritius, Mozambique, Nigeria, Réunion, Senegal, South Africa, Sudan, Tanzania, Uganda, Zambia, Zimbabwe; OTL: India.
Notes. Our species concept is based on Kaiser et al. (2017). According to Madl & van Achterberg (2014), this species was successfully introduced to Comoros as a biological control agent.
Cotesiasetebis (Nixon, 1974)
Apantelessetebis Nixon, 1974.
Apanteleskhibinica Tobias, 1986.
Type information. Holotype female, NHMUK (examined). Country of type locality: Sweden.
Geographical distribution.PAL.
PAL: Bulgaria, Czech Republic, Greece, Hungary, Iran, Mongolia, Russia (MUR, SVE), Slovakia, Sweden, Switzerland, Turkey.
Cotesiaseyali (Risbec, 1951), new combination
Apantelesseyali Risbec, 1951.
Type information. Type and depository unknown (not examined but original description checked). Country of type locality: Senegal.
Geographical distribution.AFR.
AFR: Senegal.
Notes. Transferred to Cotesia based on the propodeum with a median carina (in addition to lateral and transverse carinae), shape and sculpture of T1–T2. In the original description seyali is presented as very similar (morphologically) with sphenarchi (Risbec, 1951), also described in that same paper and currently placed in Cotesia.
Cotesiashemachaensis (Tobias, 1976)
Apantelesshemachaensis Tobias, 1976.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Azerbaijan.
Geographical distribution.PAL.
PAL: Azerbaijan, Hungary, Kazakhstan.
Notes. Our species concept is based on Tobias (1986) and Papp (1987a).
Cotesiashrii Sathe, Ingawale & Bhosale, 1994
Cotesiashrii Sathe, Ingawale & Bhosale, 1994.
Type information. Type and depository unknown (not examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Cotesiasibyllarum (Wilkinson, 1936)
Apantelessibyllarum Wilkinson, 1936.
Apantelessibyllarumnipponensis Watanabe, 1942.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.NEA, PAL.
NEA: USA (MA); PAL: Czech Republic, Germany, Hungary, Japan, Slovakia, United Kingdom.
Cotesiasimurae (You & Zhou, 1989)
Apantelessimurae You & Zhou, 1989.
Type information. Holotype female, HUNAU (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (YN).
Notes. Our species concept is based on Chen and Song (2004).
Cotesiasmerinthi (Riley, 1881)
Apantelessmerinthi Riley, 1881.
Type information. Syntypes female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (BC, ON, QC), USA (CA, CO, DC, IN, MD, MA, MO, NH, NJ, TX).
Cotesiasorghiellae (Muesebeck, 1933)
Apantelessorghiellae Muesebeck, 1933.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (AR, MO, TX).
Notes. The female holotype has the head detached.
Cotesiaspecularis (Szépligeti, 1896)
Apantelesspecularis Szépligeti, 1896.
Apantelesbalcanica Balevski, 1980.
Type information. Lectotype female, HNHM (not examined but subsequent treatment of the species checked). Country of type locality: Hungary.
Geographical distribution.PAL.
PAL: Bulgaria, Germany, Greece, Hungary, Iran, Israel, Jordan, Kyrgyzstan, Moldova, Romania, Russia (PRI), Spain, Tajikistan, Turkey, Uzbekistan.
Notes. Our species concept is based on Shaw et al. (2009). The species distribution in Israel and Tajikistan is based on Belokobylskij et al. (2019).
Cotesiasphenarchi (Risbec, 1951), new combination
Apantelessphenarchi Risbec, 1951.
Type information. Syntypes female and male, depository unknown (not examined but original description checked). Country of type locality: Senegal.
Geographical distribution.AFR.
AFR: Senegal.
Notes. Transferred to Cotesia based on the propodeum with a median carina (in addition to lateral and transverse carinae), shape and sculpture of T1–T2 and short ovipositor sheaths (Risbec 1951: 433–435, fig. 11). The original description also compares this species with ruficrus (Haliday, 1834) and nycteus (de Saeger, 1944), both currently placed in Cotesia.
Cotesiasphingivora (Granger, 1949), new combination
Apantelessphingivorus Granger, 1949.
Type information. Syntypes female and male, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Notes. Transferred to Cotesia based on the original description mentioning the propodeum rugose with more or less defined areola and costulae, ovipositor sheaths short (0.7 × metatarsus length), and T1–T3 shape and sculpture, as illustrated and described in Granger (1949: 270–271, fig. 281). The host is reported to be Sphingidae, and the wasp cocoons form a dense, white mass, both features also common in (although not exclusive from) Cotesia. A record of this species from Réunion was later considered as doubtful (Madl and van Achterberg 2014), a decision we also follow here and thus we consider the species to be present only in Madagascar.
Cotesiaspuria (Wesmael, 1837)
Microgasterspurius Wesmael, 1837.
Microgasterinsidens Ratzeburg, 1844.
Type information. Lectotype female, RBINS (examined). Country of type locality: Belgium.
Geographical distribution.PAL.
PAL: Afghanistan, Armenia, Austria, Azerbaijan, Belgium, Bulgaria, China (JL), Croatia, Finland, France, Germany, Greece, Hungary, Iran, Ireland, Israel, Italy, Japan, Kazakhstan, Korea, Latvia, Lithuania, Moldova, Poland, Romania, Russia (AD, KDA, NVS, PRI, ROS, RYA, SAM, VOR), Serbia, Slovakia, Slovenia, Sweden, Switzerland, Tajikistan, Turkey, Ukraine, United Kingdom, Uzbekistan.
Notes. The species distribution in Israel is based on Belokobylskij et al. (2019).
Cotesiasubancilla (Balevski, 1980)
Apantelessubancilla Balevski, 1980.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Bulgaria.
Geographical distribution.PAL.
PAL: Bulgaria, Greece, Hungary, Slovakia.
Notes. Our species concept is based on Tobias (1986) and Papp (1987a).
Cotesiasubordinaria (Tobias, 1976)
Apantelessubordinarius Tobias, 1976.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Georgia.
Geographical distribution.PAL.
PAL: Azerbaijan, Georgia, Netherlands, Russia (NC), United Kingdom.
Notes. Our species concept is based on Shaw (2012a, 2012b).
Cotesiasuvernii Sathe, Ingawale & Bhosale, 1994
Cotesiasuvernii Sathe, Ingawale & Bhosale, 1994.
Type information. Type and depository unknown (not examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Cotesiasuzumei (Watanabe, 1932)
Apantelessuzumei Watanabe, 1932.
Type information. Holotype male, EIHU (examined). Country of type locality: Japan.
Geographical distribution.PAL.
PAL: Japan.
Notes. We examined the holotype and it is a male specimen, not a female as it had been considered until now (e.g., Shenefelt, 1972). We also examined another six specimens and the remnants of a parasitized lepidopteran larva with rather loose wasp cocoons.
Cotesiataprobanae (Cameron, 1897)
Apantelestaprobanae Cameron, 1897.
Apantelesstauropi Viereck, 1912.
Apantelesformosae Viereck, 1913.
Type information. Lectotype female, OUMNH (not examined but authoritatively identified specimens examined). Country of type locality: Sri Lanka.
Geographical distribution.OTL.
OTL: China (FJ, GD, HI, TW, ZJ), India, Indonesia, Sri Lanka, Vietnam.
Notes. We examined the types of Apantelesstauropi, A.formosae, and the two female paralectotypes of taprobanae deposited in the NHMUK.
Cotesiatatehae (Watanabe, 1932)
Apantelestatehae Watanabe, 1932.
Type information. Holotype female, EIHU (examined). Country of type locality: Japan.
Geographical distribution.PAL.
PAL: Japan.
Cotesiategera (Papp, 1977)
Apantelestegerus Papp, 1977.
Type information. Holotype female, HNHM (not examined but subsequent treatment of the species checked). Country of type locality: Mongolia.
Geographical distribution.PAL.
PAL: Mongolia.
Notes. Our species concept is based on Papp (1986, 1990, 2009).
Cotesiateleae (Muesebeck, 1926)
Apantelesteleae Muesebeck, 1926.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (AB, BC), USA (CT, MD, PA).
Notes. The holotype has a mostly smooth T1 (only the apical 0.3 with shallow punctures) and an almost entirely smooth T2. The propodeum is also mostly smooth, with a few short carinae near the nucha, but without a median longitudinal carina, although there are traces (laterally) of the transverse carina, which forks around the spiracles. Overall this is a relatively very smooth species of Cotesia which could be considered to be a Protapanteles. However, because Protapanteles may represent just a species group of Cotesia (see above under the section Brief diagnosis of all Microgastrinae genera as they are understood in this paper for a discussion) we retain teleae within Cotesia for the time being.
Cotesiatelengai (Tobias, 1972)
Apantelestelengai Tobias, 1972.
Apantelesamabilis Nixon, 1974.
Type information. Holotype female, ZIN (not examined but authoritatively identified specimens examined). Country of type locality: Armenia.
Geographical distribution.PAL.
PAL: Afghanistan, Albania, Algeria, Armenia, Azerbaijan, Bosnia and Herzegovina, Bulgaria, Croatia, Georgia, Germany, Greece, Hungary, Iran, Israel, Italy, Kazakhstan, Moldova, Morocco, Netherlands, Poland, Russia (VLA), Slovakia, Spain, Switzerland, Tajikistan, Tunisia, Turkey, Turkmenistan, United Kingdom, Uzbekistan.
Notes. We examined the type of Apantelesamabilis Nixon. The species distribution in Israel is based on Belokobylskij et al. (2019), that paper also recorded India and New Zealand as country records for the wasp species; however, we have not been able to find any published source supporting that and thus those records are excluded from our checklist until further evidence is available.
Cotesiatenebrosa (Wesmael, 1837)
Microgastertenebrosus Wesmael, 1837.
Type information. Type lost (not examined but subsequent treatment of the species checked). Country of type locality: Belgium.
Geographical distribution.PAL.
PAL: Andorra, Azerbaijan, Belgium, Croatia, Finland, France, Georgia, Germany, Greece, Hungary, Iran, Israel, Kazakhstan, Korea, Macedonia, Moldova, Mongolia, Poland, Russia (PRI), Serbia, Spain, Sweden, Switzerland, Tajikistan, Turkey, Ukraine, United Kingdom, Uzbekistan.
Notes. Our species concept is based on Shaw (2007). Additional comments on this species are in Broad et al. (2016). The species distribution in Azerbaijan, Georgia, Iran, Kazakhstan, Tajikistan and Uzbekistan is based on Belokobylskij et al. (2019).
Cotesiatestacea Fujie, Shimizu & Fernandez-Triana, 2018
Cotesiatestacea Fujie, Shimizu & Fernandez-Triana, 2018.
Type information. Holotype female, NIAES (examined). Country of type locality: Japan.
Geographical distribution.PAL.
PAL: Japan, Korea.
Notes. The original description discussed the possibility that a previous record of Cotesiaferruginea from Russia Far East in Primorsky Krai (Kotenko 2007a) might actually represent a specimen of C.testacea, as C.ferruginea is restricted to the Western Palaearctic.
Cotesiatetrica (Reinhard, 1880)
Apantelestetricus Reinhard, 1880.
Microgasteropaculus Thomson, 1895.
Type information. Holotype female, ZMHB (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Montenegro, United Kingdom.
Notes. Our species concept is based on Nixon (1974), Belokobylskij et al. (2003), and Broad et al. (2016), but see notes under Cotesiabrachycera for more details on the history and use of related names and species.
Cotesiathapinthotha Papp, 1990
Cotesiathapinthotha Papp, 1990.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Korea.
Geographical distribution.PAL.
PAL: Korea.
Cotesiatheae (Sonan, 1942)
Apantelestheae Sonan, 1942.
Type information. Syntypes female and male, TARI (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (TW).
Cotesiatheclae (Riley, 1881)
Apantelestheclae Riley, 1881.
Type information. Syntypes female and male, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA, NEO.
NEA: USA (AL, CA, CO, CT, DC, GA, ID, KS, MO, NJ, OK, TX); NEO: Mexico.
Cotesiatibialis (Curtis, 1830)
Microgastertibialis Curtis, 1830.
Microgasteratrator Curtis, 1829 [nomen nudum].
Microgastergracilis Curtis, 1829 [nomen nudum].
Microgastertibialis Curtis, 1829 [nomen nudum].
Microgastercongesta Nees, 1834.
Microgasterintricata Haliday, 1834.
Microgastergracilipes Thomson, 1895.
Apantelessimilis Szépligeti, 1901.
Microgasteratratrix Schulz, 1906.
Apantelesaranearum Goureau, 1908 [nomen nudum].
Apantelesmamestrae Matsumura, 1908.
Apantelesimulans (Lyle, 1917).
Apantelesclaustrata (Gautier & Bonnamour, 1923).
Type information. Holotype male, MVMMA (not examined but subsequent treatment of the species checked). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Afghanistan, Armenia, Austria, Azerbaijan, Belgium, Bulgaria, Canary Islands, China (SN, XJ), Croatia, Czech Republic, Estonia, Finland, France, Georgia, Germany, Greece, Hungary, Iran, Ireland, Israel, Italy, Japan, Kazakhstan, Kyrgyzstan, Latvia, Lithuania, Macedonia, Moldova, Mongolia, Montenegro, Netherlands, Poland, Romania, Russia (AD, ZAB, IRK, KGD, KDA, KYA, MOS, PRI, SPE, TA, VLA, VGG, YAR), Serbia, Slovakia, Spain, Sweden, Switzerland, Tajikistan, Turkey, Turkmenistan, Ukraine, United Kingdom, Uzbekistan.
Notes. Our species concept is based on Nixon (1974), Papp (1986) and Tobias (1986). The information about the type is taken from Nixon (1974: 496). The species distribution in Tajikistan is based on Belokobylskij et al. (2019).
Cotesiatiracolae (Ashmead, 1896)
Apantelestivacolae Ashmead, 1896.
Apantelestivacholae Ashmead, 1896 [original misspelling].
Apantelestiracholae Wilkinson, 1928 [unjustified emendation].
Apantelestiracolae Thompson, 1953 [justified emendation].
Type information. Holotype female, USNM (not examined but subsequent treatment of the species checked). Country of type locality: Sri Lanka.
Geographical distribution.OTL.
OTL: Sri Lanka.
Notes. Our species concept is based on Gupta & Fernandez-Triana (2014).
Cotesiatmetocerae (Muesebeck, 1921)
Apantelestmetocerae Muesebeck, 1921.
Type information. Holotype female, USNM (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (NS).
Cotesiatrabalae Gupta, 2016
Cotesiatrabalae Gupta, 2016.
Type information. Holotype female, NBAIR (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Cotesiatransuta (de Saeger, 1944), new combination
Apantelestransutus de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Rwanda.
Notes. Based on the details provided in the original description, the best generic placement would be in Cotesia.
Cotesiatuita Papp, 2009
Cotesiatuita Papp, 2009.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Mongolia.
Geographical distribution.PAL.
PAL: Mongolia.
Cotesiaturkestanica (Telenga, 1955), new combination
Apantelesturkestanicus Telenga, 1955.
Type information. Lectotype female, ZIN (not examined but original description checked). Country of type locality: Uzbekistan.
Geographical distribution.PAL.
PAL: Uzbekistan.
Notes. The original description and the key to species there, as well as the works of Tobias (1986) and Papp (1987a), make clear that this species belongs in Cotesia. We transfer it here based on the propodeum with a distinct, median longitudinal carina, shape and sculpture of T1 and T2, small hypopygium and very short ovipositor sheaths.
Cotesiatyphae Fernandez-Triana, 2017
Cotesiatyphae Fernandez-Triana, 2017.
Type information. Holotype female, CBGP (examined). Country of type locality: Kenya.
Geographical distribution.AFR.
AFR: Ethiopia, Kenya, Tanzania.
Cotesiaukrainica (Tobias, 1986), status revised
Apantelesukrainicus Tobias, 1986.
Type information. Holotype female, ZIN (examined). Country of type locality: Ukraine.
Geographical distribution.PAL.
PAL: Ukraine.
Notes.Papp (1988, his footnote 24 on page 154) synonymized ukrainicus under Cotesiamelitaearum (Wilkinson, 1937). However, after examining the holotype we consider it not conspecific with melitaearum but actually more related to the Cotesiavestalis and C.ruficrus species complex. Thus, here we resurrect ukrainicus from synonymy with melitaearum and consider it a valid species.
Cotesiaunicolor (Curtis, 1835)
Microgasterunicolor Curtis, 1835.
Type information. Holotype male, NHMUK? (not examined but original description checked). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (NU).
Notes. The information about this species is sparse and probably in need of further revision. The holotype (and only known specimen) was considered by Muesebeck (1922: 43) to be probably deposited in the NHMUK. Muesebeck transferred that species from Microgaster to Apanteles and he even commented that the species could be related to Cotesiayakutatensis (at that time the generic concept of Apanteles included Cotesia). Later, Mason (1981) transferred the species to Cotesia. Over the years several authors have cited the species (e.g., Shenefelt 1972, Marsh 1979, Whitfield 1995a) for North America, but without providing details on where it was found, the only information available so far is Arctic North America. The species was described by Curtis (1835: 62, with Roman numeral lxii) in an Appendix on Natural History that was part of the book about J. C. Ross’s second voyage in search of a North-west Arctic passage. We have carefully read the original description and agree that the species very likely belongs to Cotesia. Not considered before now, the original description actually mentions some information about the actual host: “A male was bred from a cluster of cocoons, enveloped in a silky ball, resembling those containing the eggs of some spiders”. That very likely refers to the hibernacula built by the larvae of the arctic woolly bear moth (Gynaephoragroenlandica, Lepidoptera: Erebidae), which looks like a silky ball from a spider egg sac. Two Cotesia species have already been recorded (Fernandez-Triana et al. 2017b) parasitizing larvae of Gynaephora spp. in the High Arctic: Cotesiahalli and an undetermined species with provisional name of Cotesia sp. 1; it may well be that Cotesiaunicolor is actually one of those species, but study of the holotype will be needed to form a firm conclusion. As for the actual collecting locality of the type, no details are provided in the original description. However, the second voyage of Ross was spent in areas of what is today considered to be Canadian territory of Nunavut, thus we provide all that information for the sake of the checklist completion.
Cotesiaurabae Austin & Allen, 1989
Cotesiaurabae Austin & Allen, 1989.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (SA, TAS), New Zealand.
Cotesiavanessae (Reinhard, 1880)
Apantelesvanessae Reinhard, 1880.
Type information. Type and depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.AFR, NEA, PAL.
AFR: Ethiopia; NEA: Canada (AB, ON); PAL: Afghanistan, Armenia, Austria, Azerbaijan, Bulgaria, Canary Islands, China (XJ), Czech Republic, Finland, France, Georgia, Germany, Greece, Hungary, Iran, Ireland, Israel, Italy, Japan, Kazakhstan, Korea, Latvia, Moldova, Montenegro, Morocco, Netherlands, Poland, Romania, Russia (BEL, BU, IRK, OMS, PRI, SAK, TOM), Serbia, Spain, Tunisia, Turkey, Ukraine, United Kingdom, Uzbekistan.
Notes. Our species concept is based on Shaw et al. (2009). The species distribution in Azerbaijan, Georgia, Israel, Japan, and Korea is based on Belokobylskij et al. (2019).
Cotesiavestalis (Haliday, 1834)
Microgastervestalis Haliday, 1834.
Apantelesplutellae Kurdjumov, 1912.
Type information. Lectotype female, NMID (examined). Country of type locality: Ireland.
Geographical distribution.AFR, AUS, NEA, NEO, OTL, PAL.
AFR: Benin, Cape Verde, Kenya, Mauritius, Réunion, Saint Helena, Senegal, South Africa, Tanzania, Zimbabwe; AUS: Hawaiian Islands, New Zealand, Papua New Guinea, Western Samoa; NEA: USA (TX); NEO: Argentina, Brazil (PE); OTL: Bangladesh, China (GD, HN, SN, TW, ZJ), India, Malaysia, Pakistan, Philippines, Ryukyu Islands, Singapore, Sri Lanka, Thailand, Vietnam; PAL: Afghanistan, Armenia, Azerbaijan, Azores, Belgium, Bulgaria, China (BJ), Czech Republic, Finland, France, Germany, Greece, Hungary, Iran, Ireland, Israel, Italy, Japan, Kazakhstan, Korea, Kyrgyzstan, Latvia, Libya, Macedonia, Malta, Moldova, Mongolia, Morocco, Netherlands, Poland, Romania, Russia (AMU, ZAB, KDA, MOS, PRI, ROS, SAK, SPE, STA, VGG, YAR), Serbia, Spain, Sweden, Switzerland, Tajikistan, Tunisia, Turkey, Turkmenistan, Ukraine, United Kingdom, Uzbekistan.
Notes. Besides of examining the lectotype, our species concept is based on Shaw et al. (2009). New country data from Kant (2019), Ngowi et al. (2019), and Sithole et al. (2019).
Cotesiavillana (Reinhard, 1880)
Apantelesvillanus Reinhard, 1880.
Apantelesfasciatae Gautier & du Dresnay, 1926.
Apantelesrubroides Papp, 1971.
Type information. Holotype female, ZMHB (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Croatia, Finland, France, Germany, Greece, Hungary, Iran, Mongolia, Poland, Romania, Russia (ZAB, PRI), Slovakia, Switzerland, Turkey, United Kingdom.
Notes. Our species concept is based on Wilkinson (1945), Nixon (1974), and Papp (1986).
Cotesiaviridanae (Tobias, 1986)
Apantelesviridanae Tobias, 1986.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (VOR).
Notes. Our species concept is based on Tobias (1986) and Papp (1990a).
Cotesiaxavieri Rousse, 2013
Cotesiaxavieri Rousse, 2013.
Type information. Holotype female, MNHN (not examined but original description checked). Country of type locality: Réunion.
Geographical distribution.AFR.
AFR: Réunion.
Cotesiaxylina (Say, 1836)
Microgasterxylina Say, 1836.
Apantelescushmani Viereck, 1912.
Apantelesoxyacanthoidis Viereck, 1912.
Apanteleslanifica Viereck, 1917.
Type information. Type lost (not examined but authoritatively identified specimens examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (AB, MB, NS, ON, QC), USA (CO, CT, DC, FL, IL, IN, KS, MA, NH, NJ, UT, VA, WV).
Notes. We have also examined the types of Apantelescushmani (Viereck, 1912), a male specimen, Apanteleslanifica (Viereck, 1917), a male specimen, and Apantelesoxyacanthoidis (Viereck, 1912), a female specimen, all currently synonyms of C.xylina.
Cotesiayakutatensis (Ashmead, 1902)
Apantelesyakutatensis Ashmead, 1902.
Apanteleshyslopi Viereck, 1910.
Type information. Holotype female, USNM (not examined but authoritatively identified specimens examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (BC, MB, QC, NU), Greenland, USA (AK, CA, ID, OR, UT, WA).
Notes. We have examined the type of Apanteleshyslopi (Viereck, 1910), a female specimen.
Cotesiazagrosensis Zargar & Gupta, 2019
Cotesiazagrosensis Zargar & Gupta, 2019.
Type information. Holotype female, NBAIR (not examined but original description checked). Country of type locality: Iran.
Geographical distribution.PAL.
PAL: Iran.
Cotesiazygaenarum (Marshall, 1885)
Apanteleszygaenarum Marshall, 1885.
Type information. Lectotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.OTL, PAL.
OTL: China (HB); PAL: Albania, Armenia, Austria, Azerbaijan, Czech Republic, Finland, France, Germany, Greece, Hungary, Iran, Ireland, Israel, Italy, Japan, Kazakhstan, Korea, Macedonia, Moldova, Mongolia, Poland, Romania, Russia (DA, KDA, OMS, PRI, RYA, VOR), Serbia, Slovakia, Switzerland, Turkey, Tunisia, United Kingdom.
Notes. The species distribution in Israel and Kazakhstan is based on Belokobylskij et al. (2019).
Genus Cuneogaster Choi & Whitfield, 2006
Cuneogaster Choi & Whitfield, 2006: 120. Gender: feminine. Type species: Cuneogasterinae Choi & Whitfield, 2006, by original designation.
Only known from a single species from the Neotropical region (Choi and Whitfield 2006). We have seen in collections (CNC) a few additional species from South America, but the genus does not seem to be species rich. Cuneogaster is part of a group of genera (some described and some as yet undescribed) related to Diolcogaster; future phylogenetic studies of Microgastrinae may change the status of, and relationships between, some of those taxa. No host data are currently available for Cuneogaster. There are no DNA barcodes of this genus in BOLD.
Cuneogasterinae Choi & Whitfield, 2006
Cuneogasterinae Choi & Whitfield, 2006.
Type information. Holotype female, IAVH (not examined but original description checked). Country of type locality: Colombia.
Geographical distribution.NEO.
NEO: Colombia, Panama, Venezuela.
Genus Dasylagon Muesebeck, 1958
Dasylagon Muesebeck, 1958: 424. Gender: feminine. Type species: Dasylagonaegeriae Muesebeck, 1958, by original designation.
Only known from two described species from the Neotropical region. We have seen in collections (CNC) a few specimens that might represent additional species from South America, but the genus does not seem to be very speciose. Two families of Lepidoptera, Sesiidae, and Thyrididae, have been recorded as hosts of Dasylagon. There are no DNA barcodes of this genus in BOLD.
Dasylagonaegeriae Muesebeck, 1958
Dasylagonaegeriae Muesebeck, 1958.
Type information. Holotype female, USNM (examined). Country of type locality: Colombia.
Geographical distribution.NEO.
NEO: Colombia.
Dasylagonsimulans Muesebeck, 1958
Dasylagonsimulans Muesebeck, 1958.
Type information. Holotype male, USNM (examined). Country of type locality: Honduras.
Geographical distribution.NEO.
NEO: Brazil (BA), Honduras.
Genus Deuterixys Mason, 1981
Deuterixys Mason, 1981: 123. Gender: feminine. Type species: Microgastercarbonarius Wesmael, 1837, by original designation (Mason 1981: 123).
Known from 18 described species from all biogeographical regions except for Africa (the lack of species recorded from the Afrotropical region is probably due to insufficient collecting and study there). Several revisions of the genus are available for the Nearctic (Whitfield 1985), Neotropics (Whitfield et al. 2004), Russia (Kotenko 2007a), and China (Zeng et al. 2011a). We have seen a few additional species in collections but the genus does not seem to be very large. The vast majority of the known host records belong to the family Bucculatricidae, the few other families cited in older literature must be considered as likely to be wrong. There are 30 DNA-barcode compliant sequences of this genus in BOLD, representing seven BINs.
Deuterixysanica Austin & Dangerfield, 1992
Deuterixysanica Austin & Dangerfield, 1992.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (NSW, QLD, VIC).
Deuterixysbennetti Whitfield, 1985
Deuterixysbennetti Whitfield, 1985.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA, NEO.
NEA: USA (FL); NEO: Cuba, Dominican Republic, Jamaica.
Deuterixysbifossalis Zeng & Chen, 2011
Deuterixysbifossalis Zeng & Chen, 2011.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (HI, ZJ).
Deuterixyscarbonaria (Wesmael, 1837)
Microgastercarbonarius Wesmael, 1837.
Apantelesanomalus Lyle, 1925.
Type information. Lectotype female, RBINS (not examined but subsequent treatment of the species checked). Country of type locality: Belgium.
Geographical distribution.PAL.
PAL: Austria, Belgium, Czech Republic, Finland, France, Germany, Hungary, Italy, Japan, Korea, Lithuania, Mongolia, Netherlands, Poland, Romania, Russia (MOS, PRI, SAK, YAR), Slovenia, United Kingdom, Yugoslavia.
Notes. Our species concept is based on Nixon (1965), Papp (1983a), and Zeng et al. (2011a). The species distribution in Japan is based on Belokobylskij et al. (2019).
Deuterixyscolombiana Whitfield & Oltra, 2005
Deuterixyscolombiana Whitfield & Oltra, 2005.
Type information. Holotype female, CNC (examined). Country of type locality: Colombia.
Geographical distribution.NEO.
NEO: Colombia, Ecuador, Peru.
Deuterixyscondarensis (Tobias, 1960)
Apantelescondarensis Tobias, 1960.
Apantelesnixoni Papp, 1971.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Tajikistan.
Geographical distribution.PAL.
PAL: Japan, Korea, Mongolia, Russia (PRI), Tajikistan.
Notes. Our species concept is based on Papp (1983a), Kotenko (2007a), and Zeng et al. (2011a). The species distribution in Japan is based on Belokobylskij et al. (2019).
Deuterixyscurticalcar Zeng & Chen, 2011
Deuterixyscurticalcar Zeng & Chen, 2011.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (GD, GZ, HN, JX, YN); PAL: China (HA, NX).
Deuterixyserythrocephala Whitfield & Oltra, 2005
Deuterixyserythrocephala Whitfield & Oltra, 2005.
Type information. Holotype female, CNC (examined). Country of type locality: Trinidad & Tobago.
Geographical distribution.NEO.
NEO: Argentina, Dominican Republic, Trinidad & Tobago.
Deuterixyshansoni Whitfield & Oltra, 2005
Deuterixyshansoni Whitfield & Oltra, 2005.
Type information. Holotype female, ESUW (not examined but original description checked). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Bolivia, Costa Rica.
Deuterixyspacifica Whitfield, 1985
Deuterixyspacifica Whitfield, 1985.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA, NEO.
NEA: Canada (BC), USA (CA, NM, WY); NEO: Mexico.
Deuterixyspatro (Nixon, 1965)
Apantelespatro Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Notes. This species represents one of the smallest Microgastrinae so far described (body and fore wing lengths 1.6–1.7 mm).
Deuterixysplugarui (Tobias, 1975)
Apantelesplugarui Tobias, 1975.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Moldova.
Geographical distribution.PAL.
PAL: Hungary, Moldova, Russia (S), Ukraine, United Kingdom.
Notes. Our species concept is based on Papp (1983a), Kotenko (2007a), Zeng et al. (2011a), and Shaw (2012b).
Deuterixysquercicola Whitfield, 1985
Deuterixysquercicola Whitfield, 1985.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA, NEO.
NEA: USA (CA); NEO: Mexico.
Deuterixysrimulosa (Niezabitowski, 1910)
Apantelesrimulosus Niezabitowski, 1910.
Apantelescomes Wilkinson, 1940.
Type information. Syntypes female and male, depository unknown (not examined but authoritatively identified specimens examined). Country of type locality: Poland.
Geographical distribution.PAL.
PAL: Azerbaijan, Croatia, Germany, Greece, Hungary, Iran, Kazakhstan, Mongolia, Poland, Russia (VOR), Slovakia, Spain, Turkmenistan, United Kingdom, Uzbekistan.
Notes. We examined the type of Apantelescomes Wilkinson, 1940. The species distribution in Turkmenistan is based on Belokobylskij et al. (2019).
Deuterixyssvetlanae Kotenko, 2007
Deuterixyssvetlanae Kotenko, 2007.
Type information. Holotype female, SIZK (not examined but original description checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (PRI).
Deuterixystehuantepeca Whitfield & Oltra, 2005
Deuterixystehuantepeca Whitfield & Oltra, 2005.
Type information. Holotype female, CNC (examined). Country of type locality: Guatemala.
Geographical distribution.NEO.
NEO: Guatemala, Mexico.
Deuterixystenuiconvergens Zargar & Gupta, 2019
Deuterixystenuiconvergens Zargar & Gupta, 2019.
Type information. Holotype female, NBAIR (not examined but original description checked). Country of type locality: Iran.
Geographical distribution.PAL.
PAL: Iran.
Deuterixysx-formis Papp, 2012
Deuterixysx-formis Papp, 2012.
Type information. Holotype female, RMNH (not examined but original description checked). Country of type locality: Cape Verde.
Geographical distribution.AFR.
AFR: Cape Verde.
Genus Diolcogaster Ashmead, 1900
Diolcogaster Ashmead, 1900: 132. Gender: feminine. Type species: Microgasterbrevicaudus Provancher, 1886, by subsequent designation and monotypy (Viereck 1914: 46).
Zadiolcogaster Viereck, 1913: 366. Type species: Zadiolcogasteranomus Viereck, 1913, by original designation.
A cosmopolitan genus, with 141 described species known from all biogeographical regions of the planet. Relatively recent revisions of the genus are available for the Australasian region (Saeed et al. 1999), Russia (Kotenko 2007a), China (Zeng et al. 2011b), and India (Gupta & Fernandez-Triana 2015), but overall the taxonomic coverage of the world species is far from complete. We have seen hundreds of undescribed species in collections, mostly from tropical areas. This is one of the most variable genera of Microgastrinae and, as currently defined, it is certainly polyphyletic. Depending on the generic concept adopted following future phylogenetic studies of Microgastrinae, Diolcogaster may end up having several hundred species or even more than one thousand. Around 15 families of Lepidoptera have been recorded as hosts, but many records are likely to be incorrect and/or need further verification. There are almost 4,000 DNA-barcode compliant sequences of this genus in BOLD, representing 270 BINs, most of them from Costa Rica and Canada.
Diolcogasterabdominalis (Nees, 1834)
Microgasterabdominalis Nees, 1834.
Type information. Holotype male, depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Azerbaijan, Belgium, France, Georgia, Germany, Hungary, Ireland, Israel, Italy, Kazakhstan, Korea, Macedonia, Moldova, Mongolia, Montenegro, Poland, Romania, Russia (BU, ZAB, PRI), Serbia, Slovakia, Spain, Switzerland, United Kingdom.
Notes. Our species concept is based on Nixon (1965), Kotenko (2007), Shaw et al. (2009), and Shaw (2012b). The species distribution in Israel is based on Belokobylskij et al. (2019).
Diolcogasterabengouroui (Risbec, 1951), new combination
Microgasterabengouroui Risbec, 1951.
Type information. Syntypes male, depository unknown (not examined but original description checked). Country of type locality: Ivory Coast.
Geographical distribution.AFR.
AFR: Ivory Coast.
Notes. The original description includes a drawing of propodeum, T1, and T2, which clearly shows that this species belongs to Diolcogaster.
Diolcogasteradiastola Saeed, Austin & Dangerfield, 1999
Diolcogasteradiastola Saeed, Austin & Dangerfield, 1999.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (ACT, NSW, QLD, TAS).
Diolcogasteragama (de Saeger, 1944), new combination
Microgasteragama de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. The original description and key place this species in the basimacula group, defined by T1–T3 forming a carapace (e.g., Mason 1981, Saeed et al. 1999, Fernandez-Triana 2015).
Diolcogasteralce (Nixon, 1965)
Protomicroplitisalce Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (SC).
Diolcogasteralkingara Saeed, Austin & Dangerfield, 1999
Diolcogasteralkingara Saeed, Austin & Dangerfield, 1999.
Type information. Holotype female, CNC (examined). Country of type locality: Papua New Guinea.
Geographical distribution.AUS.
AUS: Australia (QLD), Papua New Guinea.
Diolcogasteralvearia (Fabricius, 1798)
Ichneumonalvearius Fabricius, 1798.
Ichneumonaleuarius Fabricius, 1798 [incorrect original spelling].
Anomalonaphidum Panzer, 1804.
Ichneumonalveator Thunberg, 1822.
Microgasterbicolor Curtis, 1830.
Apantelesareolata Szépligeti, 1896.
Type information. Syntypes sex undetermined, ZMUK (not examined but original description checked). Country of type locality: France.
Geographical distribution.PAL.
PAL: Austria, Bulgaria, China (GS), Croatia, France, Germany, Greece, Hungary, Iran, Israel, Italy, Moldova, Netherlands, Romania, Russia (KDA, MOS), Slovakia, Slovenia, Spain, Switzerland, Turkey, United Kingdom, Yugoslavia.
Notes. The original description mentions two specimens, but no details of their sex is provided. The original species name (alvearius, currently alvearia), was misspelled in the original description as aleuarius, and it was also subsequently misspelled in a variety of ways, e.g., aluearius, alevarius, and even alveolaris (see Yu et al. 2016 for a compilation of references on those misspellings). The species distribution in Israel is based on Belokobylskij et al. (2019).
Diolcogasterambositrensis (Granger, 1949), new combination
Microgasterambositrensis Granger, 1949.
Type information. Holotype female, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Notes. Transferred to Diolcogaster based on the illustrations of the fore wing and T1–T3 provided in the original description.
Diolcogasteranandra (de Saeger, 1944), new combination
Microgasteranandra de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. Based on the details provided in the original description, the best generic placement would be in Diolcogaster.
Diolcogasterandamanensis Gupta & Fernandez-Triana, 2015
Diolcogasterandamanensis Gupta & Fernandez-Triana, 2015.
Type information. Holotype female, NBAIR (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Diolcogasterannulata (Granger, 1949), new combination
Microgasterannulata Granger, 1949.
Type information. Holotype female, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Notes. Transferred to Diolcogaster based on the illustrations of the fore wing and T1–T3 provided in the original description.
Diolcogasteranoma (Viereck, 1913)
Zadiolcogasteranomus Viereck, 1913.
Type information. Holotype female, ZMHB (not examined but subsequent treatment of the species checked). Country of type locality: Paraguay.
Geographical distribution.NEO.
NEO: Paraguay.
Notes. Our species concept is based on Mason (1981). The species name must be treated as an adjective and not as a noun (Doug Yanega, pers. comm.) and thus it must match the gender of the genus name.
Diolcogasterashmeadi Saeed, Austin & Dangerfield, 1999
Diolcogasterashmeadi Saeed, Austin & Dangerfield, 1999.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (NSW, QLD, TAS, VIC).
Diolcogasteraurangabadensis Fernandez-Triana, 2019, new replacement name
Protomicroplitisindicus Rao & Chalikwar, 1970.
Diolcogasterindica (Rao & Chalikwar, 1970) [secondary homonym of Diolcogasterindica (Wilkinson, 1927)].
Type information. Holotype female, NZSI (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes.Diolcogasterindica (Rao & Chalikwar, 1970) is a secondary homonym of Diolcogasterindica (Wilkinson, 1927). The replacement name refers to the city where the holotype was collected.
Diolcogasterauripes (Provancher, 1886)
Microgasterauripes Provancher, 1886.
Type information. Lectotype female, ULQC (not examined but subsequent treatment of the species checked). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (NB, ON, QC), USA (IL, IA, KS, KY, MD, MI, MO, NE, NJ, NY, OH, VA).
Notes. Our species concept is based on Muesebeck (1922), Nixon (1965), and Fernandez-Triana (2010).
Diolcogasteraustrina (Wilkinson, 1929)
Microgasteraustrina Wilkinson, 1929.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: Cameroon, Cape Verde, Democratic Republic of Congo, Ivory Coast, Réunion, Rwanda, South Africa, Uganda.
Notes. Madl & van Achterberg (2014) noted of this species: “Known from the Afrotropical Region. As the record from Réunion is probably a misidentification (Rousse and Gupta 2013: 519), the material should be checked”. However, the species has been found in other countries from the region (e.g., Wilkinson 1927, 1929, de Saeger 1944), information we accept and follow here.
Diolcogasterbakeri (Muesebeck, 1922)
Microgasterbakeri Muesebeck, 1922.
Type information. Holotype female, USNM (not examined but subsequent treatment of the species checked). Country of type locality: USA.
Geographical distribution.NEA, NEO.
NEA: Canada (ON, QC, SK), USA (AR, FL, GA, IL, IA, KS, LA, TX); NEO: Peru.
Notes. Our species concept is based on Muesebeck (1922), Mason (1981), Whitfield (1995a), and Fernandez-Triana (2010).
Diolcogasterbambeyi (Risbec, 1951), new combination
Microgasterbambeyi Risbec, 1951.
Type information. Syntypes female and male, depository unknown (not examined but original description checked). Country of type locality: Senegal.
Geographical distribution.AFR.
AFR: Senegal.
Notes. From the original description (and drawings there of propodeum, T1 and part of the fore wing) it is clear this species belongs to Diolocogaster.
Diolcogasterbasimacula (Cameron, 1905)
Apantelesbasimacula Cameron, 1905.
Type information. Syntypes male, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Madagascar, South Africa.
Notes. We have examined the two male specimens (mentioned by Wilkinson 1929a: 103) that were both labelled as Type in Cameron's writing. Those specimens are mounted on separate cards but share the same code in the NHMUK: 3c.988, and are from the type locality (Grahamstown) which was also mentioned by Wilkinson. We consider those two specimens to be syntypes. Additionally, we examined a third male specimen, also in the type collection of the NHMUK, and with code 3c.986; that specimen is mentioned by Wilkinson to have an additional label (Stellenbosch) not written by Cameron, and most likely was not part of the type series in the original description.
Diolcogasterbelokobylskiji Kotenko, 2007
Diolcogasterbelokobylskiji Kotenko, 2007.
Type information. Holotype female, ZIN (not examined but original description checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (PRI).
Diolcogasterbicolorina (Shenefelt, 1973), new combination
Microgasterbicolorinus Shenefelt, 1973.
Microgasterbicolor Szépligeti, 1911 [primary homonym of Microgasterbicolor Nees, 1834].
Type information. Holotype female, ZMHB (not examined but original description checked). Country of type locality: Kenya.
Geographical distribution.AFR.
AFR: Kenya, Tanzania.
Notes. Based on the sculpture pattern of T1 and T2, as well as the setae on the ovipositor sheaths, which are clearly depicted in the redescription of the species (Wilkinson 1929a), the species belongs in Diolcogaster.
Diolcogasterbifurcifossa Zeng & Chen, 2011
Diolcogasterbifurcifossa Zeng & Chen, 2011.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, GD, GX, HI, ZJ), Japan, Vietnam.
Diolcogasterbrevicaudus (Provancher, 1886)
Microgasterbrevicaudus Provancher, 1886.
Type information. Lectotype female, ULQC (not examined but subsequent treatment of the species checked). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (QC), USA (IL, NJ, NY, PA).
Notes. Our species concept is based on Muesebeck (1922), Mason (1981), Whitfield (1995a), and Fernandez-Triana (2010). Because the name is to be considered as a noun under ICZN Article 31.2.1, it must retain its original spelling and remain as brevicaudus.
Diolcogasterbreviterebrus (Rao & Chalikwar, 1970)
Protomicroplitisbreviterebrus Rao & Chalikwar, 1970.
Type information. Holotype female, NZSI (not examined but subsequent treatment of the species checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. Our species concept is based on Fernandez-Triana (2015).
Diolcogasterbrevivena Zeng & Chen, 2011
Diolcogasterbrevivena Zeng & Chen, 2011.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (YN).
Diolcogastercariniger (Granger, 1949), new combination
Microgastercariniger Granger, 1949.
Type information. Syntypes female and male, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Notes. Transferred to Diolcogaster based on the illustrations of the fore wing and T1–T3 provided in the original description.
Diolcogasterchaoi (Luo & You, 2003)
Caracallatuschaoi Luo & You, 2003.
Type information. Holotype female, HUNAU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, GZ, HI YN), Vietnam.
Diolcogastercincticornis (de Saeger, 1944), new combination
Microgastercincticornis de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. Based on the details provided in the original description, the best generic placement would be in Diolcogaster.
Diolcogastercingulata (Granger, 1949), new combination
Microgastercingulata Granger, 1949.
Type information. Holotype female, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Notes. Transferred to Diolcogaster based on the illustrations of the fore wing and T1–T3 provided in the original description.
Diolcogasterclaritibia (Papp, 1959)
Microgasterclaritibia Papp, 1959.
Protomicroplitisorontes Nixon, 1965.
Type information. Holotype female, HNHM (examined). Country of type locality: Hungary.
Geographical distribution.NEA, PAL.
NEA: Canada (AB, MB, ON); PAL: Afghanistan, Armenia, Austria, Azerbaijan, Belarus, Finland, Georgia, Greece, Hungary, Iran, Kazakhstan, Lithuania, Macedonia, Moldova, Russia (ZAB, KDA), Spain, Tunisia, Turkey, Turkmenistan, Ukraine, Yugoslavia.
Notes. We have also examined the type of Protomicroplitisorontes Nixon, 1965. The species distribution in Iran and Turkmenistan is based on Belokobylskij et al. (2019).
Diolcogastercoenonymphae (Watanabe, 1937)
Microgastercoenonymphae Watanabe, 1937.
Type information. Holotype female, EIHU (examined). Country of type locality: Japan.
Geographical distribution.PAL.
PAL: Japan.
Notes.Nixon (1965) transferred Microgastercoenonymphae to Protomicroplitis, then Fernandez-Triana (2015) transferred it to Diolcogaster. Although Watanabe (1937a: 102) mentioned in the original description that there was a holotype and seven paratypes (all females, reared from the same caterpillar), we have examined the material and found four females from the type series, all with a small, thin label that reads Co-type; thus is not clear which specimens is the actual holotype.
Diolcogasterconnexa (Nees, 1834)
Microgasterconnexus Nees, 1834.
Microgasterconsularis Haliday, 1834.
Microgasterdiluta Ratzeburg, 1852.
Type information. Type lost (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Austria, Finland, France, Germany, Hungary, Italy, Korea, Netherlands, Poland, Romania, Russia (PRI, TY), Ukraine, United Kingdom.
Notes. Our species concept is based on Nixon (1965) and Kotenko (2007a). The type is presumed lost (Nixon 1965: 248). The country of the type locality is presumed by us to be Germany.
Diolcogastercoronata (de Saeger, 1944), new combination
Microgastercoronata de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Rwanda.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. Based on the details provided in the original description (de Saeger 1944), this species belongs to Diolcogaster and the basimacula species group within that genus. In the original description no details were provided on the etymology of the species name; as first revisers we consider it as a noun in apposition and thus its gender to be neuter, following Article 31.2.2 of the ICZN.
Diolcogastercoxalis (de Saeger, 1944), new combination
Microgastercoxalis de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. We transfer here coxalis to Diolcogaster based on the details from original description and an illustration of the fore wing (partially) also provided there. This species was regarded by de Saeger (1944) as morphologically similar to palpicolor, another species described in the same paper and also transferred here to Diolcogaster, see more details under Notes for that species below.
Diolcogastercurticornis (Granger, 1949)
Microgastercurticornis Granger, 1949.
Type information. Syntypes female and male, MNHN (not examined but subsequent treatment of the species checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar, Mauritius, Réunion.
Notes. Our species concept is based on Rousse and Gupta (2013).
Diolcogasterdichromus Saeed, Austin & Dangerfield, 1999
Diolcogasterdichromus Saeed, Austin & Dangerfield, 1999.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (QLD).
Diolcogasterdipika (Bhatnagar, 1950) , new combination
Apantelesdipika Bhatnagar, 1950.
Type information. Holotype male, lost (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. This species was transferred to Microplitis (Rao 1953, Shenefelt 1973) but a recent paper (Gupta 2013a: 451) considered it as incertae sedis. However, the original description is detailed enough to prove that this species actually belongs to Diolcogaster. The illustrations of T1–T3 and the fore wing in Bhatnagar (1950: 136, 156) clearly belong to Diolcogaster, also the metatibia spurs are described as relatively long (inner spur three quarters and outer spur half the length of the first metatarsus segment), which would exclude the species from Microplitis. The year of publication of the Bhatnagar paper was until recently commonly cited as 1948 and/or 1950 (e.g., Chen and Song 2004, Yu et al. 2016), probably following Shenefelt (1972) who referred to this paper as “Bhatnagar (1948) 1950”. While the intended year for Volume X, Parts I & II of the Indian Journal of Entomology was 1948, the actual dates of publication were June 1950 (Part I) and October 1950 (Part II), as clearly shown on the cover page of the Volume, which we have checked. Because the dates of publication are the ones to be considered, and for the sake of clarity, we hereby revise the species year of description to 1950.
Diolcogasterduocolor Gupta & Fernandez-Triana, 2015
Diolcogasterduocolor Gupta & Fernandez-Triana, 2015.
Type information. Holotype female, NBAIR (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Diolcogasterduris (Nixon, 1965)
Protomicroplitisduris Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Mexico.
Geographical distribution.NEO.
NEO: Mexico.
Diolcogasterearina (Wilkinson, 1929), new combination
Microgasterearina Wilkinson, 1929.
Type information. Holotype female, NHMUK (examined). Country of type locality: Nigeria.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Nigeria.
Notes. We transfer this species to Diolcogaster based on the inflexible hypopygium, short ovipositor sheaths, T1 with a strong longitudinal sulcus, large metacoxae and metatibial spurs.
Diolcogastereclectes (Nixon, 1965)
Protomicroplitiseclectes Nixon, 1965.
Protomicroplitiseclectesextentus Papp, 1974.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.AUS, OTL, PAL.
AUS: Australia (QLD), Papua New Guinea; OTL: Malaysia, Philippines, Singapore; PAL: Korea.
Notes. Belongs to the basimacula species group.
Diolcogasterepectina (de Saeger, 1944), new combination
Microgasterepectina de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. Based on the details provided in the original description, the best generic placement would be in Diolcogaster and, within that genus, in the basimacula species group.
Diolcogasterepectinopsis (de Saeger, 1944), new combination
Microgasterepectinopsis de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. Based on the details provided in the original description, the best generic placement would be in Diolcogaster and, within that genus, in the basimacula species group.
Diolcogastererro (Nixon, 1965)
Protomicroplitiserro Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (SC).
Notes. This species belongs to the Diolcogasterbasimacula species group (Fernandez-Triana 2015).
Diolcogastereuterpe (Nixon, 1965)
Protomicroplitiseuterpe Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Indonesia.
Geographical distribution.AUS.
AUS: Indonesia, Papua New Guinea.
Notes.Saeed et al. (1999) pointed out some of the unique features of this species, which may be transferred to a different genus following future studies of Diolcogastersensu lato.
Diolcogasterfacetosa (Weed, 1888)
Microgasterfacetosus Weed, 1888.
Microgastersolidaginis Viereck, 1917.
Type information. Syntypes female and male, ANSP (not examined but subsequent treatment of the species checked). Country of type locality: USA.
Geographical distribution.NEA, OTL.
NEA: Canada (AB, BC, ON, QC), USA (AR, CO, CT, DE, GA, IL, IA, KS, KY, MD, MA, MI, MO, NH, NJ, NY, OH, OK, PA, SC, TN, VT, VA, WA, WV); OTL: China (FJ).
Notes. Our species concept is based on Nixon (1965), Chen and Song (2004) and Fernandez-Triana (2010). The latest version of Taxapad (Yu et al. 2016) lists the type material as deposited in the USNM. However, those specimens should be in the ANSP (e.g., Shenefelt 1972: 777). The online database on Hymenoptera Holotypes of the Smithsonian Institution (http://www.usnmhymtypes.com/default.asp) also confirms that the type material for this species is not in the USNM. We have also examined the type of Microgastersolidaginis (Viereck, 1917), a synonym of D.facetosa.
Diolcogasterfasciipennis (Gahan, 1918)
Microgasterfasciipennis Gahan, 1918.
Type information. Holotype female, USNM (not examined but subsequent treatment of the species checked). Country of type locality: Uganda.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Nigeria, Uganda.
Notes. Our species concept is based on Wilkinson (1929a), de Saeger (1944), and Nixon (1965).
Diolcogasterflammea Salgado-Neto & Fernandez-Triana, 2018
Diolcogasterflammeus Salgado-Neto & Fernandez-Triana, 2018.
Type information. Holotype female, UFVB (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (MG).
Notes. The species name must be treated as an adjective and not as a noun (Doug Yanega, pers. comm.) and thus it must match the gender of the genus name.
Diolcogasterflavipes (Haliday, 1834)
Microgasterflavipes Haliday, 1834.
Type information. Lectotype female, NMID (not examined but subsequent treatment of the species checked). Country of type locality: Ireland.
Geographical distribution.PAL.
PAL: Armenia, Austria, Finland, Germany, Hungary, Ireland, Italy, Poland, Russia (AMU, BU, ZAB, PRI, SAK, TY), United Kingdom.
Notes. Our species concept is based on van Achterberg (1997) and Kotenko (2007a). The species distribution in Armenia is based on Belokobylskij et al. (2019).
Diolcogastergalazia Kotenko, 2007
Diolcogastergalazia Kotenko, 2007.
Type information. Holotype male, SIZK (not examined but original description checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (ZAB).
Diolcogastergarmani (Ashmead, 1900)
Protomicroplitisgarmani Ashmead, 1900.
Protomicroplitisgermani Ashmead, 1900 [incorrect original spelling].
Type information. Holotype female, USNM (not examined but subsequent treatment of the species checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (ON), USA (DC, IL, KS, KY, LA, MD, NY, TX, VA).
Notes. Our species concept is based on Muesebeck (1922), Mason (1981), Whitfield (1995a), and Fernandez-Triana (2010, 2015).
Diolcogastergefidra Kotenko, 2007
Diolcogastergefidra Kotenko, 2007.
Type information. Holotype male, SIZK (not examined but original description checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (PRI).
Diolcogasterglaphyra (de Saeger, 1944)
Microgasterglaphyra de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but subsequent treatment of the species checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Ethiopia.
Notes.Nixon (1965) transferred Microgasterglaphyra to Protomicroplitis, then Fernandez-Triana (2015) transferred it to Diolcogaster. It belongs to the Diolcogasterbasimacula species group (Fernandez-Triana 2015).
Diolcogastergrammata Zeng & Chen, 2011
Diolcogastergrammata Zeng & Chen, 2011.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (GD, HI, HN), Vietnam.
Diolcogastergrangeri (Shenefelt, 1973), new combination
Microgastergrangeri Shenefelt, 1973.
Microgastercrenulatus Granger, 1949 [primary homonym of Microgastercrenulatus Provancher, 1888].
Type information. Syntypes female and male, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Notes. The best generic placement of this species at this time is within Diolcogaster, based on the original description of the scutoscutellar sulcus, propodeum sculpture, fore wing venation, shape and sculpture of T1, and length of the ovipositor. However, the description does not closely match that of a typical Diolcogaster, which is a very variable genus; thus, further study of the type series will be required.
Diolcogasterhadrommata Saeed, Austin & Dangerfield, 1999
Diolcogasterhadrommatus Saeed, Austin & Dangerfield, 1999.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (NSW, NT, QLD, SA, WA).
Notes. The species name must be treated as an adjective and not as a noun (Doug Yanega, pers. comm.) and thus it must match the gender of the genus name.
Diolcogasterharrisi Saeed, Austin & Dangerfield, 1999
Diolcogasterharrisi Saeed, Austin & Dangerfield, 1999.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (NSW, TAS, VIC).
Diolcogasterheterocera (de Saeger, 1944), new combination
Microgasterheterocera de Saeger, 1944.
Microgasterheterocera de Saeger, 1944 [primary homonym of Microgasterheterocerus Ruthe, 1860].
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Rwanda.
Notes. Based on the original description (de Saeger 1944), the best generic placement would be in Diolcogaster.
Diolcogasterhinzi (Nixon, 1965)
Protomicroplitishinzi Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Finland, Germany, Hungary, Kazakhstan, Russia (KAM, SAK), United Kingdom.
Notes.Yu et al. (2016) recorded the holotype to be in Munich (ZSM); however, we have examined a specimen in the NHMUK that clearly is the type, including a handwritten label by Nixon stating so (type number: 3c.2114).
Diolcogasterhomocera (de Saeger, 1944), new combination
Microgasterhomocera de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. Based on the original description (de Saeger 1944), the best generic placement would be in Diolcogaster.
Diolcogasterichiroi Fernandez-Triana, 2018
Diolcogasterichiroi Fernandez-Triana, 2018.
Type information. Holotype female, CNC (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (FL).
Diolcogasterindica (Wilkinson, 1927), new combination
Microgasterindica Wilkinson, 1927.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India, Indonesia, Myanmar.
Notes. We transfer this species to Diolcogaster based on the inflexible hypopygium, very short ovipositor sheaths with a few thickened setae at the apex, T1 with a strong longitudinal sulcus, and T2 with the median field clearly defined.
Diolcogasterineminens Zeng & Chen, 2011
Diolcogasterineminens Zeng & Chen, 2011.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, GD, ZJ), Vietnam.
Diolcogasterinsularis (Hedqvist, 1965), new combination
Microgasterinsularis Hedqvist, 1965.
Type information. Holotype female, MZH (examined). Country of type locality: Cape Verde.
Geographical distribution.AFR.
AFR: Cape Verde.
Notes. A recent treatment of the species (Forshage et al. 2016) reported that the female holotype and male paratype were apparently deposited in the Lindberg collection (not MZH) and were apparently missing. However, we found those two specimens in the MZH collection and were able to study them. The species belongs to the genus Diolcogaster.
Diolcogasterintegra (Wilkinson, 1929)
Microgasterintegra Wilkinson, 1929.
Type information. Holotype male, NHMUK (examined). Country of type locality: Uganda.
Geographical distribution.AFR.
AFR: Uganda.
Notes.Nixon (1965) transferred Microgasterintegra to Protomicroplitis, then Fernandez-Triana (2015) transferred it to the basimacula species group in the genus Diolcogaster.
Diolcogasterippis (Nixon, 1965)
Protomicroplitisippis Nixon, 1965.
Type information. Holotype male, NHMUK (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (SC).
Diolcogasteriqbali Saeed, Austin & Dangerfield, 1999
Diolcogasteriqbali Saeed, Austin & Dangerfield, 1999.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (NSW, NT, QLD, SA, WA).
Diolcogasteriridescens (Cresson, 1865)
Microgasteriridescens Cresson, 1865.
Type information. Holotype female, ANSP (not examined but subsequent treatment of the species checked). Country of type locality: Cuba.
Geographical distribution.NEA, NEO.
NEA: USA (FL); NEO: Cuba.
Notes. Our species concept is based on Muesebeck (1922) and Mason (1981). Shenefelt (1973: 715) considered the holotype to be female; however, the last version of Taxapad (Yu et al. 2016) records the type as a male specimen.
Diolcogasterkasachstanica (Tobias, 1964)
Hygroplitiskasachstanica Tobias, 1964.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Kazakhstan.
Geographical distribution.PAL.
PAL: Kazakhstan, Russia (ZAB).
Notes. Our species concept is based on Tobias (1986) and Kotenko (2007a).
Diolcogasterkasparyani Kotenko, 2007
Diolcogasterkasparyani Kotenko, 2007.
Type information. Holotype female, ZIN (not examined but original description checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (YEV).
Diolcogasterkivuana (de Saeger, 1944), new combination
Microgasterkivuana de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. Based on the original description (de Saeger 1944), the best generic placement would be in Diolcogaster.
Diolcogasterlaetimedia Zeng & Chen, 2011
Diolcogasterlaetimedia Zeng & Chen, 2011.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, GD, HI, ZJ), Vietnam.
Diolcogasterlelaps (Nixon, 1965)
Protomicroplitislelaps Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Mexico.
Geographical distribution.NEO.
NEO: Mexico.
Diolcogasterlongistria Gupta & Fernandez-Triana, 2015
Diolcogasterlongistria Gupta & Fernandez-Triana, 2015.
Type information. Holotype female, NBAIR (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Diolcogasterlucindae Saeed, Austin & Dangerfield, 1999
Diolcogasterlucindae Saeed, Austin & Dangerfield, 1999.
Type information. Holotype female, AEIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (QLD, TAS).
Diolcogastermalabarensis Narendran & Sheeba, 2003
Diolcogastermalabarensis Narendran & Sheeba, 2003.
Type information. Holotype female, NZSI (not examined but subsequent treatment of the species checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. Gupta & Fernandez-Triana (2015) mentioned the publication date of the original description as 2005, and other references online also consider 2005 as the publication year. However, the original paper, which we have examined, is dated 2003; and the online contents of the Journal of Bio-Sciences (https://www.banglajol.info/index.php/JBS) also indicate 2003 as the date for Volume 11, where the species was originally described.
Diolcogastermasoni Saeed, Austin & Dangerfield, 1999
Diolcogastermasoni Saeed, Austin & Dangerfield, 1999.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (NSW, QLD).
Diolcogastermayae (Shestakov, 1932)
Microgastermayae Shestakov, 1932.
Microgasteriranensis Hedwig, 1957.
Type information. Holotype female, depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: unknown.
Geographical distribution.AFR, PAL.
AFR: Yemen; PAL: Afghanistan, Algeria, Armenia, Azerbaijan, Iran, Israel, Kazakhstan, Mongolia, Romania, Russia (NC, S), Tajikistan, Turkey, Turkmenistan, Uzbekistan.
Notes. Our species concept is based on Tobias (1986) and Kotenko (2007a). Shenefelt (1973: 716) considered the type to be a female specimen; he also recorded the type locality as Chiva: Ravatt. We believe that probably refers to the Ravat village, near the city of Khiva, in the Xorazm region of Uzbekistan. However, at present there is no certainty about the type locality. The published record of this species from Russia (Ghafouri Moghaddam et al. 2019) did not specify the subdivisions from that country where the specimens were collected.
Diolcogastermediosulcata (Granger, 1949), new combination
Microgastermediosulcatus Granger, 1949.
Type information. Syntype female and male, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Notes. Transferred to Diolcogaster based on the illustrations of the fore wing and T1–T3 provided in the original description.
Diolcogastermedon (Nixon, 1965)
Protomicroplitismedon Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Notes. This species belongs to the Diolcogasterxanthaspis species group (Fernandez-Triana 2015).
Diolcogastermegaulax (de Saeger, 1944), new combination
Microgastermegaulax de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. Based on the original description, the best generic placement would be in Diolcogaster.
Diolcogastermellea (Nixon, 1965)
Protomicroplitismelleus Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Notes. This species belongs to the Diolcogasterxanthaspis species group (Fernandez-Triana 2015). The fore wing areolet is not visible, as veins are thickened in the area that the areolet should be (see original description for more details and drawing of fore wing). A mostly honey-yellow coloured species. The species name must be treated as an adjective and not as a noun (Doug Yanega, pers. comm.) and thus it must match the gender of the genus name.
Diolcogastermerata Saeed, Austin & Dangerfield, 1999
Diolcogastermerata Saeed, Austin & Dangerfield, 1999.
Type information. Holotype female, AEIC (not examined but original description checked). Country of type locality: Papua New Guinea.
Geographical distribution.AUS.
AUS: Papua New Guinea.
Diolcogastermiamensis Fernandez-Triana, 2018
Diolcogastermiamensis Fernandez-Triana, 2018.
Type information. Holotype female, CNC (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (FL).
Diolcogasterminuta (Reinhard, 1880)
Microgasterminutus Reinhard, 1880.
Type information. Syntypes female and male, depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: unknown.
Geographical distribution.PAL.
PAL: Armenia, Czech Republic, Finland, Germany, Lithuania, Poland, Romania, Russia (ZAB, IRK, TY, YAR), Switzerland, Turkey, Turkmenistan, Ukraine, United Kingdom.
Notes. Our species concept is based on Nixon (1968) and Tobias (1986). The species distribution in Turkey is based on Belokobylskij et al. (2019).
Diolcogastermuzaffari Saeed, Austin & Dangerfield, 1999
Diolcogastermuzaffari Saeed, Austin & Dangerfield, 1999.
Type information. Holotype female, AEIC (not examined but original description checked). Country of type locality: Papua New Guinea.
Geographical distribution.AUS.
AUS: Papua New Guinea.
Diolcogasternarendrani Rema & Sheeba, 2004
Diolcogasternarendrani Rema & Sheeba, 2004.
Type information. Holotype female, depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. Our species concept is based on Gupta & Fernandez-Triana (2015).
Diolcogasternaumanni Saeed, Austin & Dangerfield, 1999
Diolcogasternaumanni Saeed, Austin & Dangerfield, 1999.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (WA).
Diolcogasterneglecta (de Saeger, 1944), new combination
Microgasterneglecta de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Rwanda.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Rwanda.
Notes. Based on the details provided in the original description, the best generic placement would be in Diolcogaster and, within that genus, in the basimacula species group. In the original description, no details were provided on the etymology of the species name; as first revisers we consider it as a noun in apposition and thus its gender to be neuter, following Article 31.2.2 of the ICZN.
Diolcogasternephele (Nixon, 1965)
Protomicroplitisnephele Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (SC).
Notes. It belongs to the Diolcogasterbasimacula species group (Fernandez-Triana 2015).
Diolcogasternewguineaensis Saeed, Austin & Dangerfield, 1999
Diolcogasternewguineaensis Saeed, Austin & Dangerfield, 1999.
Type information. Holotype female, AEIC (not examined but original description checked). Country of type locality: Papua New Guinea.
Geographical distribution.AUS.
AUS: Papua New Guinea.
Diolcogasternigromacula (de Saeger, 1944), new combination
Microgasternigromacula de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. Based on the original description, this species belongs to the Diolcogasterbasimacula species group. In the original description, no details were provided on the etymology of the species name; as first revisers we consider it as a noun in apposition and thus its gender to be neuter, following Article 31.2.2 of the ICZN.
Diolcogasternixoni Saeed, Austin & Dangerfield, 1999
Diolcogasternixoni Saeed, Austin & Dangerfield, 1999.
Type information. Holotype female, AEIC (not examined but original description checked). Country of type locality: Papua New Guinea.
Geographical distribution.AUS.
AUS: Australia (QLD), Papua New Guinea.
Diolcogasternotopecktos Saeed, Austin & Dangerfield, 1999
Diolcogasternotopecktos Saeed, Austin & Dangerfield, 1999.
Type information. Holotype female, AEIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (QLD), Papua New Guinea.
Diolcogasterorientalis (Rao & Chalikwar, 1970)
Protomicroplitisorientalis Rao & Chalikwar, 1970.
Type information. Holotype female, NZSI (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Diolcogasterpalpicolor (de Saeger, 1944), new combination
Microgasterpalpicolor de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. Based on the original description and illustrations provided there of T1–T3, fore wing (partially), apical half of metasoma and details of the ovipositor sheaths, this species clearly belongs in Diolcogaster. The hypopygium is inflexible, the ovipositor is short and strongly curved downwards, the ovipositor sheaths have strong setae apically, the T1 has a strong median sulcus, and the shape of the areolet in the fore wing is typical of the genus.
Diolcogasterperiander (Nixon, 1965)
Protomicroplitisperiander Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Diolcogasterperniciosa (Wilkinson, 1929)
Microgasterperniciosa Wilkinson, 1929.
Type information. Holotype female, NHMUK (examined). Country of type locality: Australia.
Geographical distribution.AUS, OTL.
AUS: Australia (ACT, NSW, QLD, SA, TAS, VIC, WA), New Zealand; OTL: China (FJ, GZ, YN, ZJ).
Diolcogasterpersimilis (Wilkinson, 1929), new combination
Microgasterpersimilis Wilkinson, 1929.
Type information. Holotype female, NHMUK (examined). Country of type locality: Uganda.
Geographical distribution.AFR.
AFR: Uganda.
Notes. We transfer this species to Diolcogaster based on the inflexible hypopygium, short ovipositor sheaths, T1 with a strong longitudinal sulcus, and large metacoxae and metatibial spurs. The metasoma and one hind leg of the holotype are in a gelatin capsule adjacent to the pinned specimen.
Diolcogasterplecopterae (Wilkinson, 1929), new combination
Microgasterplecopterae Wilkinson, 1929.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. Transferred to Diolcogaster here based on its inflexible hypopygium, short ovipositor sheaths with some thickened setae on apex, T1 with a strong longitudinal sulcus, T2 with median field (although weakly defined), very large metacoxae, and large metatibial spurs.
Diolcogasterpluriminitida Zeng & Chen, 2011
Diolcogasterpluriminitida Zeng & Chen, 2011.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (GD, GZ, HN, ZJ), Vietnam.
Diolcogasterplutocongoensis (Shenefelt, 1973), new combination
Microgasterplutocongoensis Shenefelt, 1973.
Microgasterpluto de Saeger, 1944 [primary homonym of Microgasterpluto Morley, 1936].
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Rwanda.
Notes. Transferred to Diolcogaster based on the original description and illustrations provided there. T1 has a strong median sulcus, T2 has a median field defined by sulci that narrow towards posterior margin of tergite, the hypopygium is inflexible, the ovipositor sheaths are short and at least with one thick seta apically, and the ovipositor is strongly curved downwards.
Diolcogasterpraritas Zeng & Chen, 2011
Diolcogasterpraritas Zeng & Chen, 2011.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (YN, ZJ).
Diolcogasterprocris (Fischer, 1964)
Microgasterprocris Fischer, 1964.
Type information. Holotype female, MHNG (not examined but subsequent treatment of the species checked). Country of type locality: Austria.
Geographical distribution.PAL.
PAL: Austria.
Notes. Our species concept is based on Papp (1991b) and Shaw (2012b).
Diolcogasterpsilocnema (de Saeger, 1944), new combination
Microgasterpsilocnema de Saeger, 1944.
Type information. Holotype male, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Rwanda.
Notes. Based on the original description, the best generic placement would be in Diolcogaster, based on the shapes of T1 and T2. However, the description is based on a male specimen only and it is not clear enough to conclude with certainty.
Diolcogasterpunctata (Rao & Chalikwar, 1976)
Protomicroplitispunctata Rao & Chalikwar, 1976.
Type information. Holotype male, BAMU (not examined but subsequent treatment of the species checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. Our species concept is based on Gupta & Fernandez-Triana (2015).
Diolcogasterpunctatiscutum Zeng & Chen, 2011
Diolcogasterpunctatiscutum Zeng & Chen, 2011.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (GD).
Diolcogasterpyrene (Nixon, 1965)
Protomicroplitispyrene Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Notes. This species belongs to the Diolcogasterxanthaspis species group (Fernandez-Triana 2015).
Diolcogasterreales (Nixon, 1965)
Protomicroplitisreales Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Diolcogasterrixosa (Wilkinson, 1929)
Microgasterrixosa Wilkinson, 1929.
Type information. Holotype female, NHMUK (examined). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (ACT, NSW, QLD, SA, VIC, WA).
Diolcogasterrobertsi Saeed, Austin & Dangerfield, 1999
Diolcogasterrobertsi Saeed, Austin & Dangerfield, 1999.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (QLD).
Diolcogasterrufithorax (Granger, 1949), new combination
Microgasterrufithorax Granger, 1949.
Type information. Syntypes female, MNHN (not examined but illustrations of the holotype examined). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Notes. The generic placement of this species has been determined based on information from the original description and low-resolution images of the holotype (taken with a cell phone) which we have examined. Transferred to Diolcogaster based on fore wing areolet, propodeum with median carina, large metacoxa and metatibial spurs, T1 with median sulcus, ovipositor sheaths short, and hypopygium inflexible.
Diolcogasterrufula Papp, 1991
Diolcogasterrufula Papp, 1991.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Hungary.
Geographical distribution.PAL.
PAL: Hungary.
Diolcogasterrugosicoxa (Papp, 1959)
Microgasterrugosicoxa Papp, 1959.
Protomicroplitismeges Nixon, 1965.
Type information. Holotype female, HNHM (examined). Country of type locality: Hungary.
Geographical distribution.PAL.
PAL: Austria, Hungary, Italy, Romania, Switzerland.
Notes. We also examined the type of Protomicroplitismeges Nixon.
Diolcogasterrugulosa (Rao & Chalikwar, 1970)
Protomicroplitisrugulosus Rao & Chalikwar, 1970.
Type information. Holotype male, NZSI (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. The species name must be treated as an adjective and not as a noun (Doug Yanega, pers. comm.) and thus it must match the gender of the genus name.
Diolcogasterschizurae (Muesebeck, 1922)
Microgasterschizurae Muesebeck, 1922.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (BC, ON), USA (AR, DC, KS, MD, MA, MI, NJ, OH, VA, WV).
Diolcogasterscotica (Marshall, 1885)
Microgasterscoticus Marshall, 1885.
Type information. Holotype male, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.NEA, PAL.
NEA: Canada (BC, QC), USA (MI); PAL: Finland, Germany, Hungary, Mongolia, Poland, Romania, Russia (ZAB, IRK), Slovakia, Switzerland, United Kingdom.
Diolcogastersemirufa (de Saeger, 1944), new combination
Microgastersemirufa de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. Based on the original description and illustrations provided there of the fore wing (partially) and posterior half of metasoma (de Saeger 1944: 79–80), the species is transferred to Diolcogaster, due to its propodeum with strong, median longitudinal carina, inflexible hypopygium and ovipositor sheaths with at least one thick seta on apex.
Diolcogasterseriphus (Nixon, 1965)
Protomicroplitisseriphus Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Notes.Kotenko (2007a: 163) transferred Protomicroplitisseriphus to Diolcogaster, although it was done within a key to species, without explicitly stating the new combination, and without placing the author’s name between parentheses. Yu et al. (2012: Taxapad) revised that combination, transferring the species back to Protomicroplitis. Fernandez-Triana (2015) then transferred seriphus back to Diolcogaster, as a member of the xanthaspis species group.
Diolcogasterseyrigi (Granger, 1949), new combination
Microgasterseyrigi Granger, 1949.
Type information. Syntypes female, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Notes. Transferred to Diolcogaster based on T1 with median longitudinal carina, T2 with delimited median field, fore wing with areolet, and short ovipositor sheaths (as detailed in the original description and illustrations there of T1–T3 and part of the fore wing).
Diolcogastersolitaria Gupta & Fernandez-Triana, 2015
Diolcogastersolitarium Gupta & Fernandez-Triana, 2015.
Type information. Holotype female, NBAIR (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Diolcogastersons (Wilkinson, 1932)
Microgastersons Wilkinson, 1932.
Type information. Holotype male, NHMUK (examined). Country of type locality: Australia.
Geographical distribution.AUS, OTL.
AUS: Australia (ACT, QLD, TAS, WA), New Caledonia; OTL: Indonesia.
Notes. This species belongs to the Diolcogasterbasimacula species group.
Diolcogasterspreta (Marshall, 1885)
Microgasterspreta Marshall, 1885.
Type information. Type lost (not examined but subsequent treatment of the species checked). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Bulgaria, China (SN), Denmark, Germany, Greece, Hungary, Iran, Moldova, Romania, Russia (NC, S), Slovakia, Spain, Turkey, United Kingdom.
Notes. Our species concept is based on Nixon (1965), Zeng et al. (2011b), and Ghafouri Moghaddam et al. (2019).
Diolcogasterstepposa (Tobias, 1964)
Hygroplitisstepposa Tobias, 1964.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Kazakhstan.
Geographical distribution.PAL.
PAL: Kazakhstan.
Notes. Our species concept is based on Nixon (1968) and Tobias (1986).
Diolcogastersubtorquata (Granger, 1949), new combination
Microgastersubtorquata Granger, 1949.
Type information. Syntype female and male, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Notes. Transferred to Diolcogaster based on T2 with delimited median field, fore wing with areolet, and short ovipositor sheaths (as detailed in the original description and illustrations there of T1–T3 and part of the fore wing).
Diolcogastersulcata (de Saeger, 1944), new combination
Microgastersulcata de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. Based on the original description, this species belongs to the Diolcogasterbasimacula species group. In the original description no details were provided on the etymology of the species name; as first revisers we consider it as a noun in apposition and thus its gender to be neuter, following Article 31.2.2 of the ICZN.
Diolcogastertearae (Wilkinson, 1929)
Microgastertearae Wilkinson, 1929.
Type information. Lectotype female, NHMUK (examined). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (NSW, SA, VIC, WA).
Notes.Shenefelt (1973: 732) designated a female specimen as lectotype, from a type series that included three female specimens (Wilkinson 1929a: 107-108). However, Saeed et al. (1999: 167 and especially 169) wrongly mentioned a holotype, which they even considered to be the only known specimen of the species, until their revision added more specimens and localities. The host is also recorded as two potentially different species by Saeed et al. (1999); it is not clear if there is a reason for that, as their paper does not mention any extra host record. Thus, the host species associated with D.tearae need further verification.
Diolcogastertegularia (Papp, 1959)
Microgastertegularius Papp, 1959.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Hungary.
Geographical distribution.PAL.
PAL: Hungary.
Diolcogastertomentosae (Wilkinson, 1930)
Microgastertomentosae Wilkinson, 1930.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes.Watanabe (1937a: 101) recorded Diolcogastertomentosae from Taiwan, although he also noticed that the specimens from that area were slightly different from the original description of the species (which was based on Indian specimens). We have examined the material from Watanabe (deposited in Hokkaido) and it is clear that they represent a different species (to be eventually described). Therefore, we consider D.tomentosae to be present only in India.
Diolcogastertorquatiger (Granger, 1949), new combination
Microgastertorquatiger Granger, 1949.
Type information. Holotype female, MNHN (not examined but illustrations of the holotype examined). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Notes. The generic placement of this species has been determined based on information from the original description and low-resolution images of the holotype (taken with a cell phone) which we have examined. Transferred to Diolcogaster here based on fore wing areolet, propodeum with median longitudinal carina, metacoxa large, metatibial spurs relatively long, T1 with median sulcus, T2 with partially defined median field, hypopygium inflexible and ovipositor sheaths short.
Diolcogastertranslucida Zeng & Chen, 2011
Diolcogastertranslucida Zeng & Chen, 2011.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (FJ, GD, HN, ZJ), Vietnam; PAL: China (HA).
Diolcogastertristiculus (Granger, 1949), new combination
Microgastertristiculus Granger, 1949.
Type information. Holotype female, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Notes. Based on the original description and illustrations provided there of the fore wing (partially) and T1–T3 (Granger 1949: 222, 225–227), the species is transferred to Diolcogaster, due to its fore wing with areolet, inflexible hypopygium and ovipositor sheaths with at least one thick seta on apex.
Diolcogastertropicalus Saeed, Austin & Dangerfield, 1999
Diolcogastertropicalus Saeed, Austin & Dangerfield, 1999.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (QLD), Papua New Guinea.
Diolcogasterturneri (Wilkinson, 1929), new combination
Microgasterturneri Wilkinson, 1929.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Notes. Transferred to Diolcogaster here based on its inflexible hypopygium, short ovipositor sheaths with some thickened setae at the apex, T1 with a strong longitudinal sulcus, large metacoxae, and large metatibial spurs.
Diolcogasterurios (Nixon, 1965)
Protomicroplitisurios Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Malaysia.
Geographical distribution.OTL.
OTL: Malaysia.
Notes. This species belongs to the Diolcogasterxanthaspis species group (Fernandez-Triana 2015).
Diolcogastervulcana (de Saeger, 1944), new combination
Microgastervulcana de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Rwanda.
Notes. Based on the original description, the best generic placement would be in Diolcogaster.
Diolcogastervulpina (Wilkinson, 1929)
Microgastervulpina Wilkinson, 1929.
Type information. Holotype female, NHMUK (examined). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (NSW, NT, QLD, SA, VIC, WA).
Diolcogasterwalkerae Saeed, Austin & Dangerfield, 1999
Diolcogasterwalkerae Saeed, Austin & Dangerfield, 1999.
Type information. Holotype female, MCZC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (NSW, WA).
Diolcogasterwittei (de Saeger, 1944), new combination
Microgasterwittei de Saeger, 1944.
Type information. Holotype male, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Rwanda.
Notes. Based on the original description, the best generic placement would be in Diolcogaster. However, the description mentions that the ovipositor sheaths lack “appendix” (from the original description: “dépourvues d'appendice”) which could be interpreted as lacking setae, at least on the apex of the sheaths. The genus Rasivalva looks like Diolcogaster except for the ovipositor sheaths lacking visible setae, thus there is a small chance that this species could actually belong to Rasivalva. Without examining specimens, it is impossible to conclude, but because at present there is only one Rasivalva species recorded from the Afrotropical region (versus dozens of Diolcogaster), the probability of the species belonging to Rasivalva is much smaller.
Diolcogasterxanthaspis (Ashmead, 1900), lectotype designation
Apantelesxanthaspis Ashmead, 1900.
Type information. Lectotype female, NHMUK (examined). Country of type locality: Saint Vincent.
Geographical distribution.NEO.
NEO: Grenada, Saint Vincent.
Notes. The original description (Ashmead 1900c: 280) was based on a series of two female and four male specimens. Later, Shenefelt (1973: 782) referred to the type as a female, with BMNH code 3c.992; however, he did not designate it as the lectotype (although Shenefelt did that for many other species dealt with in his catalogue of world Braconidae). For the sake of completion, it is here formally designated. We have examined that female specimen, which is currently missing the head but otherwise is in reasonably good condition.
Diolcogasteryousufi Saeed, Austin & Dangerfield, 1999
Diolcogasteryousufi Saeed, Austin & Dangerfield, 1999.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (ACT, NSW, QLD, SA, TAS, WA).
Genus Distatrix Mason, 1981
Distatrix Mason, 1981: 93. Gender: feminine. Type species: Apantelespapilionis Viereck, 1912, by original designation.
A total of 32 described species from all biogeographical regions except for Australasia. The New World species were revised recently (Grinter et al. 2009), but the taxonomic coverage of the world species is far from complete. Based on additional specimens we have seen in collections, the genus may well exceed 50 species. Nine families of Lepidoptera, mainly Geometridae and Papilionidae, have been recorded as hosts of Distatrix; some of those families may prove to be wrong. There are 50 DNA-barcode compliant sequences of this genus in BOLD, representing 22 BINs.
Distatrixanthedon (Nixon, 1965), new combination
Apantelesanthedon Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Notes. Transferred to Distatrix based on the pronotum laterally with a single furrow, T2 lateral sulcus only marked on the anterior half of the tergite, fore wing venation, and ovipositor sheaths with only very minute setae near the very apex. In the original description, Nixon (1965) did not provide any details on the etymology of the species name; as first revisers we consider it as a noun in apposition and thus its gender to be neuter, following Article 31.2.2 of the ICZN.
Distatrixantirrheae Whitfield & Grinter, 2009
Distatrixantirrheae Whitfield & Grinter, 2009.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Distatrixbelliger (Wilkinson, 1929)
Apantelesbelliger Wilkinson, 1929.
Type information. Holotype female, NHMUK (examined). Country of type locality: Mauritius.
Geographical distribution.AFR.
AFR: Madagascar, Mauritius, Réunion.
Distatrixcarolinae Fernandez-Triana, 2010
Distatrixcarolinae Fernandez-Triana, 2010.
Type information. Holotype female, CNC (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (QC).
Distatrixcerales (Nixon, 1965), new combination
Apantelescerales Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Uganda.
Geographical distribution.AFR.
AFR: Uganda.
Notes. Transferred to Distatrix based on the pronotum laterally with a single furrow, T2 lateral sulcus only marked on the anterior half of the tergite, fore wing venation, and ovipositor sheaths with only very minute setae near the very apex.
Distatrixcuspidalis (de Saeger, 1944), new combination
Apantelescuspidalis de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. Based on the original description and drawings, the best generic placement is Distatrix. However, it could also be Glyptapanteles and examination of the specimens would be needed to conclude.
Distatrixeuproctidis (Ullyett, 1946), new combination
Apanteleseuproctidis Ullyett, 1946.
Type information. Holotype female, SAMC (not examined but original description checked). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Notes. The original description strongly suggest that this species belongs to Distatrix, based on the entirely smooth propodeum, shape and sculpture of T1 and T2, and shape of the hypopygium. Ullyett (1946) also placed euproctidis close to another Distatrix species (in a modified couplet of a key from Wilkinson (1932a) that Ullyett discussed in his paper). Another plausible alternative could be Glyptapanteles; however, the distinguishing characters between these two genera (number of sulci on the pronotum laterally and setation pattern on ovipositor sheaths) were not detailed by Ullyett (1946). Although only a study of the type series can establish this unambiguously, we still consider Distatrix to be the best generic placement, based on T2 having lateral margins (diverging sulci) not extending to the apex of the tergite, a strong character of Distatrix.
Distatrixflava (Fernandez-Triana & van Achterberg, 2017), new combination
Venanidesflavus Fernandez-Triana & van Achterberg, 2017.
Type information. Holotype female, RMNH (examined). Country of type locality: Yemen.
Geographical distribution.AFR.
AFR: Yemen.
Notes. This species clearly belongs to Distatrix, but it was wrongly placed within Venanides in the original description. The species name must be treated as an adjective and not as a noun (Doug Yanega, pers. comm.) and thus it must match the gender of the genus name.
Distatrixformosa (Wesmael, 1837)
Microgasterformosus Wesmael, 1837.
Apantelesmarshallii Bignell, 1901.
Type information. Holotype female, RBINS (not examined but subsequent treatment of the species checked). Country of type locality: Belgium.
Geographical distribution.PAL.
PAL: Armenia, Belgium, France, Germany, Hungary, Italy, Japan, Korea, Netherlands, Poland, Romania, Russia (KEM, PRI), Switzerland, Ukraine, United Kingdom.
Notes. Our species concept is based on Nixon (1965, 1974), Papp (1983a), and Kotenko (2007a). Recent references treat this species as Distatrix (e.g., Mason 1981, Kotenko 2007a, Broad et al. 2016). But Taxapad (Yu et al. 2012, 2016) and references following Taxapad treat it as Protapanteles. For the sake of clarity we revise its combination here. The species distribution in is based on Belokobylskij et al. (2019).
Distatrixgeometrivora (de Saeger, 1944), new combination
Apantelesgeometrivora de Saeger, 1941.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Uganda.
Notes. Based on the original description (de Saeger 1941a) and that of Nixon (1965), the best generic placement would be in Distatrix. The original name of the species was spelled as geometrivora (de Saeger 1941a) although in a following paper, the same author spelled it as geometrivorus (de Saeger 1944).
Distatrixgratiosa (Wilkinson, 1930)
Apantelesgratiosus Wilkinson, 1930.
Type information. Holotype female, NHMUK (examined). Country of type locality: Uganda.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Uganda.
Distatrixiglesiasi (Viereck, 1913)
Apantelesiglesiasi Viereck, 1913.
Type information. Holotype female, USNM (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (SP).
Distatrixiraklii (Kotenko, 1986)
Apantelesiraklii Kotenko, 1986.
Type information. Holotype female, SIZK (not examined but original description checked). Country of type locality: Georgia.
Geographical distribution.PAL.
PAL: Georgia.
Notes.Papp (1988) had placed this species in the genus Distatrix, a decision we agree with after reading the original description and key provided in Kotenko (1986: 658) where several characters that define this genus (especially the pronotum laterally with a single, ventral furrow) are clearly stated.
Distatrixloretta Grinter, 2009
Distatrixloretta Grinter, 2009.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Distatrixmaia (Nixon, 1965), new combination
Apantelesmaia Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Notes. Transferred to Distatrix based on the pronotum laterally with a single furrow, T2 lateral sulcus marked only on the anterior half of the tergite, fore wing venation, and ovipositor sheaths with only very minute setae near the very apex.
Distatrixmalloi (Blanchard, 1942)
Apantelesmalloi Blanchard, 1942.
Type information. Syntypes female and male, MACN (not examined but subsequent treatment of the species checked). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina.
Notes. Our species concept is based on Mason (1981).
Distatrixpallidocinctus (Gahan, 1918)
Apantelespallidocinctus Gahan, 1918.
Type information. Holotype female, USNM (not examined but subsequent treatment of the species checked). Country of type locality: Uganda.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Kenya, Uganda, Zimbabwe.
Notes. Our species concept is based on Nixon (1965) and Mason (1981). Under ICZN Article 31.2.2, this species name must be treated as a noun and thus retain its original spelling (Doug Yanega, pers. comm.).
Distatrixpandora Grinter, 2019
Distatrixpandora Grinter, 2019.
Distatrixpandora Grinter, 2009 [unavailable name].
Type information. Holotype female, MIUP (not examined but original description checked). Country of type locality: Panama.
Geographical distribution.NEO.
NEO: Costa Rica, Ecuador, Panama.
Notes. The original description (Grinter et al. 2009: 14) did not detail where the holotype was deposited. As such, under ICZN Article 16.4.2, the name Distratrixpandora Grinter, 2009 must be considered unavailable. However, the species name was validated in a subsequent paper (Grinter and Whitfield 2019).
Distatrixpapilionis (Viereck, 1912)
Apantelespapilionis Viereck, 1912.
Apantelesagamemnonis Wilkinson, 1928.
Type information. Holotype female, USNM (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: China (YN), India, Indonesia, Malaysia, Myanmar.
Distatrixpitillaensis Grinter, 2009
Distatrixpitillaensis Grinter, 2009.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Distatrixpompelon (Nixon, 1965)
Apantelespompelon Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Japan.
Geographical distribution.PAL.
PAL: Japan, Korea, Slovakia, Switzerland.
Notes. This species is considered by most authors to belong to Distatrix (e.g., Papp 1988, 1990, Capek and Lukas 1989, van Achterberg and Rezbanyai-Reser 2001, Kotenko 2007a), a decision we agree with, based on our study of the holotype. However, Yu et al. (2016) list it in Protapanteles, following van Achterberg (2003). For the sake of clarity, we revise the species combination here.
Distatrixsancus (Nixon, 1965)
Apantelessancus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: France.
Geographical distribution.PAL.
PAL: Azerbaijan, Bulgaria, France, Germany, Hungary, Russia (NC, S), Spain, Ukraine.
Notes. This species is considered by most authors to belong to Distatrix (e.g., Papp 1988, 2005, Schurian et al. 1993, Jiménez et al. 1996), a decision we agree with, based on our study of the holotype. However, Yu et al. (2016) list it in Protapanteles, following van Achterberg (2003). For the sake of clarity, we revise the species combination here.
Distatrixsimulissima (de Saeger, 1944), new combination
Apantelessimulissimus de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Ivory Coast.
Notes. Based on the original description and that of Nixon (1965), the best generic placement would be in Distatrix. However, it could also be Glyptapanteles but examination of specimens would be needed to conclude. The species name must be treated as an adjective and not as a noun (Doug Yanega, pers. comm.) and thus it must match the gender of the genus name.
Distatrixsolanae Whitfield, 1996
Distatrixsolanae Whitfield, 1996.
Type information. Holotype female, UCDC (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CA, OR).
Distatrixteapae (Nixon, 1965)
Apantelesteapae Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Mexico.
Geographical distribution.NEO.
NEO: Mexico.
Distatrixtookei (Shenefelt, 1972), new combination
Apantelestookei Shenefelt, 1972.
Apantelesflaviventris Ullyett, 1946 [secondary homonym of Apantelesflaviventris Cresson, 1865].
Type information. Holotype female, SAMC (not examined but original description checked). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Notes. The original description strongly suggest that this species belongs to Distatrix, based on the entirely smooth propodeum, shape and sculpture of T1–T2, and shape of the hypopygium. Ullyett (1946) also mentioned two previously described species of Distatrix as the closest to the new taxon, and placed the new species in a key by Wilkinson (1932a) with other species of the genus. Another plausible alternative could be Glyptapanteles; however, the distinguishing characters between these two genera (number of sulci on the pronotum laterally and setation pattern on the ovipositor sheaths) were not detailed by Ullyett (1946). Although only study of the type series can establish this unambiguously, we still consider Distatrix to be the best generic placement, based on T2 having lateral margins (diverging sulci) not extending to the apex of the tergite, a strong character of Distatrix.
Distatrixtormina (Nixon, 1965), new combination
Apantelestormina Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. Transferred to Distatrix based on the pronotum laterally with a single furrow, T2 lateral sulcus only marked on the anterior half of the tergite, fore wing venation, and ovipositor sheaths with only very minute setae near the very apex.
Distatrixugandaensis (Gahan, 1918)
Apantelesugandaensis Gahan, 1918.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: Uganda.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Ivory Coast, Kenya, Rwanda, Uganda.
Notes. The type series, from Uganda, was reared from a pyralid on Hibiscus (Wilkinson 1934a: 146); however, that same paper cites a few dozen specimens from three collecting events, reared from the tortricid host Choristoneuraoccidentalis. That is the only reference to a tortricid as host for the entire genus Distatrix and should be considered as a record needing further verification.
Distatrixvigilis Grinter, 2009
Distatrixvigilis Grinter, 2009.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Distatrixxanadon Grinter, 2009
Distatrixxanadon Grinter, 2009.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Distatrixyemenitica van Achterberg & Fernandez-Triana, 2017
Distatrixyemeniticus van Achterberg & Fernandez-Triana, 2017.
Type information. Holotype female, RMNH (examined). Country of type locality: Yemen.
Geographical distribution.AFR.
AFR: Yemen.
Notes. The species name must be treated as an adjective and not as a noun (Doug Yanega, pers. comm.) and thus it must match the gender of the genus name.
Distatrixyunae Rousse & Gupta, 2013
Distatrixyunae Rousse & Gupta, 2013.
Type information. Holotype female, MNHN (not examined but original description checked). Country of type locality: Réunion.
Geographical distribution.AFR.
AFR: Réunion.
Genus Dodogaster Rousse, 2013
Dodogaster Rousse, 2013: 522. Gender: feminine. Type species: Dodogastergrangeri Rousse, 2013, by original designation.
Only known from a single species from the Afrotropical region, described from two females from Réunion (Rousse and Gupta 2013). Its relationship within Microgastrinae is unclear. No host data are currently available for this genus. There are no DNA barcodes of Dodogaster in BOLD.
Dodogastergrangeri Rousse, 2013
Dodogastergrangeri Rousse, 2013.
Type information. Holotype female, MNHN (not examined but original description checked). Country of type locality: Réunion.
Geographical distribution.AFR.
AFR: Réunion.
Notes. In addition to the original description, we also examined the female paratype (in the CNC).
Genus Dolichogenidea Viereck, 1911
Dolichogenidea Viereck, 1911: 173. Gender: feminine. Type species: Apanteles (Dolichogenidea) banksi Viereck, 1911, by monotypy.
Originally described as a subgenus of Apanteles, but elevated to generic rank by Mason (1981). This is a cosmopolitan genus, with 366 described species known from all biogeographical regions. Many European species were revised by Wilkinson, Nixon and Papp in several papers from the middle part of the 20th century, and a few more recent papers treat the fauna of China (Chen and Song 2004), the Russian Far East (Kotenko 2007a), and a species group in Costa Rica (Fernandez-Triana et al. 2019). Overall, the taxonomic coverage of the world species is far from complete; we have seen at least the same number of undescribed species in collections, from both tropical and temperate areas, and it is likely that the genus will approach one thousand species. The concept of Dolichogenidea and its separation from Apanteles has been controversial (e.g., Mason 1981, van Achterberg 2003, Fernandez-Triana et al. 2014e), but we consider it as a valid genus. More than 30 families of Lepidoptera have been recorded as hosts for Dolichogenidea, but many records are likely to be incorrect and/or need further verification. In Costa Rica (ACG) most of the known hosts belong to four families: Depressariidae, Thyrididae, Tortricidae, and Mimallonidae (unpublished information extracted from BOLD and ACG databases). There are almost 3.500 DNA-barcode compliant sequences of Dolichogenidea in BOLD representing 372 different BINs, mostly from Costa Rica and North America.
Dolichogenideaaberrantenna Liu & Chen, 2018
Dolichogenideaaberrantenna Liu & Chen, 2018.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (SC).
Dolichogenideaabsona (Muesebeck, 1965)
Apantelesabsonus Muesebeck, 1965.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (AB, BC, MB, NB, NL, NS, ON, PE, QC), USA (CO, MT, NM, OR, WA, WI).
Notes. Our species concept is based on Muesebeck (1965), Nixon (1972), Mason (1974) and Fernandez-Triana and Huber (2010).
Dolichogenideaacratos (Nixon, 1967)
Apantelesacratos Nixon, 1967.
Type information. Holotype female, NHMUK (examined). Country of type locality: Papua New Guinea.
Geographical distribution.AUS, OTL.
AUS: Papua New Guinea; OTL: Vietnam.
Dolichogenideaacrobasidis (Muesebeck, 1921)
Apantelesacrobasidis Muesebeck, 1921.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (FL, MD, MS).
Dolichogenideaacron (Nixon, 1967)
Apantelesacron Nixon, 1967.
Type information. Holotype female, NHMUK (examined). Country of type locality: Thailand.
Geographical distribution.OTL.
OTL: China (ZJ), Thailand.
Notes. After examining the holotype we had decided to transfer this species to Dolichogenidea based on the entirely setose hind wing vannal lobe, and the anteromesoscutum with punctures that do not fuse near the posterior margin. However, the species was transferred right before our paper by Liu et al. (2019), who also recorded the species from China based on one female specimen, an information we incorporate here.
Dolichogenideaaegeriphagous Liu & Chen, 2018
Dolichogenideaaegeriphagous Liu & Chen, 2018.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (HL, HA, JL, LN, SD, XJ).
Dolichogenideaagamedes (Nixon, 1965), new combination
Apantelesagamedes Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Mauritius.
Geographical distribution.AFR, OTL.
AFR: Mauritius; OTL: Vietnam.
Notes. After examining the holotype, we believe the best generic placement for this species is in Dolichogenidea, based on the relatively coarse punctures on the anteromesoscutum (which do not fuse near the scutoscutellar sulcus), and the entirely setose vannal lobe in the hind wing. Yu et al. (2016) listed this species’ distribution as Oceanic and Oriental. However, Mauritius is better considered as part of the Afrotropical region, and thus here we record the species distribution as only AFR and OTL.
Dolichogenideaagilis (Ashmead, 1905)
Pseudapantelesagilis Ashmead, 1905.
Apanteleshidaridis Rohwer, 1922.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: China (GD), India, Indonesia, Philippines, Vietnam.
Notes. We also examined the type of Apanteleshidaridis Rohwer, 1922, a female specimen. The species distribution in China is based in Liu et al. (2019).
Dolichogenideaagilla (Nixon, 1972)
Apantelesagilla Nixon, 1972.
Apantelespiraticus Papp, 1977.
Type information. Holotype female, MZH (not examined but paratype examined). Country of type locality: Finland.
Geographical distribution.PAL.
PAL: Finland, Greece, Hungary, Iran, Mongolia, Russia (ZAB, PRI), United Kingdom.
Notes. Both the holotype and paratype are from Finland. Nixon (1972) points out that the difference between the two specimens may be significant. The English specimen recorded by Shaw (2012b) agrees closely with the paratype.
Dolichogenideaagonoxenae (Fullaway, 1941)
Apantelesagonoxenae Fullaway, 1941.
Apantelesorelia Nixon,1967.
Type information. Holotype female, BPBM (not examined but authoritatively identified specimens examined). Country of type locality: Western Samoa.
Geographical distribution.AUS, OTL.
AUS: Fiji, Tonga, Western Samoa; OTL: Vietnam.
Notes. We studied the female type of Apantelesorelia Nixon, which was synonymized under agonoxenae by Walker (1989), a reference we also read.
Dolichogenideaalaria (Kotenko, 1986)
Apantelesalarius Kotenko, 1986.
Type information. Holotype female, SIZK (not examined but original description checked). Country of type locality: Azerbaijan.
Geographical distribution.PAL.
PAL: Azerbaijan.
Dolichogenideaalbipennis (Nees, 1834)
Microgasteralbipennis Nees, 1834.
Type information. Type lost (not examined but subsequent treatment of the species checked). Country of type locality: unknown.
Geographical distribution.PAL.
PAL: Afghanistan, Albania, Azerbaijan, Belarus, Denmark, France, Georgia, Germany, Hungary, Italy, Kazakhstan, Kyrgyzstan, Lithuania, Moldova, Mongolia, Netherlands, Poland, Romania, Russia (DA, KDA, MOS, PRI, ROS, SPE, TA, VOR, YAR), Sweden, Turkey, Turkmenistan, Ukraine, United Kingdom.
Notes. Our species concept is based on Papp (1978a, 1988), Tobias (1986) and Kotenko (2007a).
Dolichogenideaalejandromasisi Fernandez-Triana & Boudreault, 2019
Dolichogenideaalejandromasisi Fernandez-Triana & Boudreault, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Dolichogenideaalophogaster Liu & Chen, 2019
Dolichogenideaalophogaster Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Dolichogenideaaltithoracica Liu & Chen, 2019
Dolichogenideaaltithoracica Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Dolichogenideaaluella (Nixon, 1967), new combination
Apantelesaluella Nixon, 1967.
Type information. Holotype female, NHMUK (examined). Country of type locality: Indonesia.
Geographical distribution.OTL.
OTL: Indonesia, Malaysia, Philippines.
Notes. It is clear, from the original description (also, from Austin 1987) that this species belongs to Dolichogenidea based on its uniformly setose hind wing vannal lobe and the anteromesoscutum punctures that do not fuse near the scutoscutellar sulcus. However, it was never transferred to that genus – Austin (1987) mentioned that it belonged to DolichogenideasensuMason (1981) but he preferred to retain it under an Apantelessensu lato at the time. Thus, here we formally transfer the species.
Dolichogenideaalutacea (Balevski, 1980)
Apantelesalutaceus Balevski, 1980.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Bulgaria.
Geographical distribution.PAL.
PAL: Bulgaria.
Dolichogenideaamaris (Nixon, 1967)
Apantelesamaris Nixon, 1967.
Type information. Holotype female, NHMUK (examined). Country of type locality: Thailand.
Geographical distribution.OTL.
OTL: China (YN), Thailand.
Dolichogenideaanarsiae (Faure & Alabouvette, 1924)
Apantelesanarsiae Faure & Alabouvette, 1924.
Type information. Syntypes female and male, depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: France.
Geographical distribution.PAL.
PAL: Azerbaijan, Bulgaria, France, Georgia, Hungary, Italy, Moldova, Romania, Russia (KDA, PRI), Switzerland, Turkey.
Notes. Our species concept is based on Wilkinson (1945), Nixon (1976), Papp (1981) and Tobias (1986).
Dolichogenideaancylotergita Liu & Chen, 2018
Dolichogenideaancylotergita Liu & Chen, 2018.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (SC); PAL: China (NX).
Dolichogenideaangelagonzalezae Fernandez-Triana & Boudreault, 2019
Dolichogenideaangelagonzalezae Fernandez-Triana & Boudreault, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Dolichogenideaangularis Song, Chen & Yang, 2006
Dolichogenideaangularis Song, Chen & Yang, 2006.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Dolichogenideaannularis (Haliday, 1834)
Microgasterannularis Haliday, 1834.
Type information. Lectotype female, NMID (not examined but subsequent treatment of the species checked). Country of type locality: Ireland.
Geographical distribution.PAL.
PAL: Germany, Hungary, Ireland, Italy, Poland, Russia (AMU, BU, SPE), Sweden, Switzerland, United Kingdom.
Notes. Our species concept is based on Nixon (1972), Papp (1980a) and Tobias (1986).
Dolichogenideaanterocava Liu & Chen, 2019
Dolichogenideaanterocava Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, GD, HI).
Dolichogenideaanteruga Liu & Chen, 2018
Dolichogenideaanteruga Liu & Chen, 2018.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (SC, ZJ); PAL: China (HA).
Dolichogenideaapicurvus Liu & Chen, 2019
Dolichogenideaapicurvus Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Dolichogenideaappellator (Telenga, 1949), status revised
Apantelesappellator Telenga, 1949.
Apantelessalverdensis Hedqvist, 1965.
Apanteleslitae Nixon, 1972.
Apanteleslitaevar.operculellae Nixon, 1972.
Type information. Holotype female, depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Tajikistan.
Geographical distribution.AFR, PAL.
AFR: Cape Verde, Ghana, Senegal; PAL: Afghanistan, Armenia, Azerbaijan, Belarus, Bulgaria, China (NM, XJ), Croatia, Cyprus, Egypt, Germany, Hungary, Iran, Israel, Italy, Jordan, Kazakhstan, Malta, Moldova, Mongolia, Romania, Russia (KDA, MOS, PRI, ROS, VGG, YAR), Selvagens Islands, Spain, Switzerland, Tajikistan, Tunisia, Turkey, Turkmenistan, Ukraine, United Kingdom, Uzbekistan, Yugoslavia.
Notes.Shaw (2012b) synonymized Dolichogenidealitae within D.appellator, and provided comments on the rationale for that. Additional comments were also provided in Broad et al. (2016). Papp (2015) brought Dolichogenidealitae back from synonymy and considered it to be a valid species, based on subtle morphological differences as well as ecological traits, including the distribution (of litae) mainly in marine environments. However, we have obtained DNA barcodes of specimens attributed to both species and they do not differ. We have also examined the holotype and many paratypes of D.litae, and a rather long series of specimens in the NHMUK identified as D.appellator and we cannot find enough differences to justify separation as two species. Thus, here we revise the species status and sink D.litae back into synonymy with D.appellator. An additional problem that remains to be solved is the status of what Nixon (1972) referred to as Apanteleslitaevar.operculellae; we have examined those specimens and they might represent a different species. However, resolving that is beyond the scope of the present paper.
Dolichogenideaargiope (Nixon, 1965), new combination
Apantelesargiope Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR, AUS, OTL, PAL.
AFR: South Africa; AUS: Australia (QLD) Fiji, Vanuatu; OTL: India, Indonesia, Malaysia, Philippines, Singapore; PAL: Korea.
Notes. The holotype has a uniformly setose vannal lobe (clearly visible on the right hind wing, less clear on the left hind wing) and the anteromesoscutum has coarse punctures that do not fuse to each other near the scutoscutellar sulcus. Both characters indicate this species is better placed in Dolichogenidea.
Dolichogenideaartissima (Papp, 1971)
Apantelesartissimus Papp, 1971.
Apantelesabila Nixon, 1972.
Type information. Holotype female, HNHM (examined). Country of type locality: Hungary.
Geographical distribution.PAL.
PAL: Finland, Germany, Hungary, Mongolia, Russia (PRI), Sweden, United Kingdom.
Notes. Our species concept is based on Shaw (2012b). In addition to the type of artissima, we have examined the female type of its synonym, A.abila (Nixon 1972), deposited in the NHMUK, which resembles Pholetesor, based on its inflexible hypopygium, relatively short ovipositor sheaths, and the shape and coarse sculpture of T2. Other species currently placed in Dolichogenidea (e.g., bres, coniferoides, and mycale, see notes under those species as well) also resemble Pholetesor because of their relatively short ovipositor and inflexible hypopygium. The available host records for those microgastrines often include species whose early instars are leaf miners; thus, there might be some convergence in species that oviposit into leaf miners on important functional things, e.g., short length of ovipositor and the associated relative robustness (lack of multiple creases) in the hypopygium. Until more evidence is available, especially based on molecular studies, we refrain to transfer those species in this paper and retain them in Dolichogenidea.
Dolichogenideaartusicarina Song & Chen, 2004
Dolichogenideaartusicarina Song & Chen, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Dolichogenideaashoka Rousse, 2013
Dolichogenideaashoka Rousse, 2013.
Type information. Holotype female, MNHN (not examined but original description checked). Country of type locality: Réunion.
Geographical distribution.AFR.
AFR: Réunion.
Dolichogenideaatarsi Liu & Chen, 2019
Dolichogenideaatarsi Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (GZ).
Dolichogenideaate (Nixon, 1973)
Apantelesate Nixon, 1973.
Type information. Holotype female, NHMUK (examined). Country of type locality: Sweden.
Geographical distribution.PAL.
PAL: Hungary, Poland, Sweden, United Kingdom.
Dolichogenideaatreus (Nixon, 1973), new combination
Apantelesatreus Nixon, 1973.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Bulgaria, Czech Republic, Denmark, Germany, Greece, Hungary, Italy, Russia (NC), Turkey, United Kingdom.
Notes. Transferred to Dolichogenidea based on the entirely setose hind wing vannal lobe, and the anteromesoscutum with punctures not fusing near the scutoscutellar sulcus. The holotype has a mostly inflexible hypopygium and relatively short ovipositor sheaths (ca. half the length of the metatibia), which might indicate that Pholetesor would be a better generic placement. However, we have decided to transfer this species to Dolichogenidea as it belongs to a group of species which are all currently placed in that genus. Furthermore, all known hosts of this wasp belong to Momphidae, a Lepidoptera family which has only been recorded as a host for a few other Dolichogenidea species (e.g., see Yu et al. 2016), and one species in the unrelated genus Microgaster (Shaw 2012b).
Dolichogenideaazovica (Kotenko, 1986)
Apantelesazovicus Kotenko, 1986.
Type information. Holotype female, SIZK (not examined but original description checked). Country of type locality: Ukraine.
Geographical distribution.PAL.
PAL: Poland, Russia (PRI), Ukraine.
Dolichogenideabakeri (Wilkinson, 1932), new combination
Apantelesbakeri Wilkinson, 1932.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: China (TW), Philippines.
Notes. After examining the holotype, it is clear this species belongs to Dolichogenidea based on its slighly convex and uniformly setose vannal lobe.
Dolichogenideabambusae (Wilkinson, 1928)
Apantelesbambusae Wilkinson, 1928.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: China (HI), India, Vietnam.
Notes. The holotype has one hind wing left, but its vannal lobe is entirely setose, which indicates this species belongs in Dolichogenidea. The species was transferred to this genus right before our paper by Liu et al. (2019), who also recorded the species from China based several female specimens, an information we incorporate here.
Dolichogenideabanksi (Viereck, 1911)
Apantelesbanksi Viereck, 1911.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (DC, MD, MI, NY, PA, VA).
Dolichogenideabaoris (Wilkinson, 1930)
Apantelesbaoris Wilkinson, 1930.
Apantelesparnarae Watanabe, 1935.
Type information. Holotype female, NHMUK (examined). Country of type locality: Malaysia.
Geographical distribution.OTL, PAL.
OTL: Bangladesh, China (FJ, GD, GX, GZ, HI, HK, HN, JX, SC, SH, SN, TW, YN, ZJ), India, Malaysia, Pakistan, Philippines, Ryukyu Islands, Sri Lanka, Thailand, Vietnam; PAL: China (AH, HA, HB, JL, JS, LN, SN, SD), Japan, Nepal.
Notes. The species distribution in China is based in Liu et al. (2019).
Dolichogenideabasiflava (Papp, 1974), new combination
Apantelesbasiflavus Papp, 1974.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Korea.
Geographical distribution.PAL.
PAL: Korea.
Notes. Both the original description and a subsequence treatment of the species (Papp 1978a) considered Apantelesbasiflavus within a group of species now placed in Dolichogenidea; the original description also provides enough detail to transfer this species to that genus.
Dolichogenideabenevolens (Papp, 1973)
Apantelesbenevolens Papp, 1973.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Italy.
Geographical distribution.PAL.
PAL: Italy.
Dolichogenideabenkevitshi (Kotenko, 1986)
Apantelesbenkevitshi Kotenko, 1986.
Type information. Holotype female, SIZK (not examined but original description checked). Country of type locality: Ukraine.
Geographical distribution.PAL.
PAL: Russia (NC), Ukraine.
Notes. The original description (Kotenko 1986: 785) referred to the hind wing vannal lobe as straight and without a clear fringe of setae, which suggests this species might not belong in Dolichogenidea. However, all other characters mentioned in the original description agree with that genus, as does the position of the species in the key provided by Kotenko (where D.benkevitshi is keyed out in a section with several couplets that include many other Dolichogenidea species). It could well be that the setae are present but are small and difficult to see, as it is the case with other borderline species of Dolichogenidea such as D.murinanae and D.petrovae (see below for more comments on those species). Without seeing the type it is impossible to conclude, thus we prefer to maintain the species in Dolichogenidea for the time being, which is also where Papp (1988) placed it when he re-assigned all European Apantelessensu lato to the generic framework proposed by Mason (1981).
Dolichogenideabersa (Papp, 1976), new combination
Apantelesbersus Papp, 1976.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Mongolia.
Geographical distribution.PAL.
PAL: Mongolia, Russia (ZAB).
Notes. From the original description and Papp (1978a) it is clear this species belongs to Dolichogenidea. The spelling of the species name follows Belokobylskij et al. (2019).
Dolichogenideabetheli (Viereck, 1911)
Apantelesbetheli Viereck, 1911.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CO).
Notes.Muesebeck (1921) mentioned that the type series had three specimens, all deposited in the USNM; however, we could only find the female holotype, and no other specimen was found in the regular collection. Also, in Muesebeck's key (1921) this species is described as having a strongly compressed metasoma, but the holotype does not have such a strongly compressed metasoma. The holotype has the ovipositor sinuous, not at the tip as in Promicrogaster species, but sinuous throughout its entire length.
Dolichogenideabicolor Song & Chen, 2004
Dolichogenideabicolor Song & Chen, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (JL).
Dolichogenideabiconcava Liu & Chen, 2018
Dolichogenideabiconcava Liu & Chen, 2018.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (GD).
Dolichogenideabilecikensis Inanç & Cetin Erdogan, 2004
Dolichogenideabilecikensis Inanç & Cetin Erdogan, 2004.
Type information. Holotype female, ZMTU (not examined but original description checked). Country of type locality: Turkey.
Geographical distribution.PAL.
PAL: Turkey.
Dolichogenideabimacula Song & Chen, 2004
Dolichogenideabimacula Song & Chen, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Dolichogenideabiplagae (Fischer, 1968), new combination
Apantelesbiplagae Fischer, 1968.
Type information. Holotype female, MHNG (not examined but original description checked). Country of type locality: Ivory Coast.
Geographical distribution.AFR.
AFR: Ivory Coast.
Notes. Transferred to Dolichogenidea based on the original description mentioning the hind wing with vannal lobe convex and setose.
Dolichogenideabiroi (Szépligeti, 1905)
Apantelesbiroi Szépligeti, 1905.
Type information. Lectotype female, HNHM (not examined but authoritatively identified specimens examined). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (NSW).
Notes. We studied a female and a male paralectotype deposited in the NHMUK, incorrectly labelled with the primary type code 3c.1055.
Dolichogenideabisulcata (Cameron, 1909), new combination
Apantelesbisulcatus Cameron, 1909.
Type information. Holotype female, NHMUK (examined). Country of type locality: Sri Lanka.
Geographical distribution.OTL.
OTL: China (TW), India, Sri Lanka.
Notes. The hind wing vannal lobe of this species is fully setose. Although the lobe in the right wing is partially and slightly folded behind in the holotype, giving the impression of not having setae, careful examination at higher magnification and study of the left hind wing corroborates the setosity of the vannal lobe. Also, the anteromesoscutum punctures do not fuse.
Dolichogenideabonbonensis Fagan-Jeffries & Austin, 2019
Dolichogenideabonbonensis Fagan-Jeffries & Austin, 2019.
Type information. Holotype female, SAMA (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (SA).
Dolichogenideaborysthenica (Kotenko, 1986)
Apantelesborysthenicus Kotenko, 1986.
Type information. Holotype female, SIZK (not examined but original description checked). Country of type locality: Ukraine.
Geographical distribution.PAL.
PAL: Russia (S), Ukraine.
Dolichogenideabrabyi Fagan-Jeffries & Austin, 2019
Dolichogenideabrabyi Fagan-Jeffries & Austin, 2019.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (ACT).
Dolichogenideabres (Nixon, 1973)
Apantelesbres Nixon, 1973.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: United Kingdom.
Notes. The setae on the hind wing vannal lobe are very small and not easily visible, but otherwise this species mostly agrees with the Dolichogenidea concept we follow in this paper. Although the hypopygium is evenly sclerotized, which would suggest Pholetesor as a better generic placement than Dolichogenidea, we refrain to transfer those species in this paper until more evidence is available, especially based on molecular studies (see also additional comments under D.artisima above).
Dolichogenideabreviattenuata Liu & Chen, 2019
Dolichogenideabreviattenuata Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (GD, YN).
Dolichogenideabrevicarinata Chen & Song, 2004
Dolichogenideabrevicarinata Chen & Song, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (FJ, GZ, ZJ); PAL: China (HE, JL, LN, SD).
Notes. The species distribution in China is based in Liu et al. (2019).
Dolichogenideabrevifacialis Liu & Chen, 2018
Dolichogenideabrevifacialis Liu & Chen, 2018.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (GD, ZJ).
Dolichogenideabreviventris (Ratzeburg, 1848)
Microgasterbreviventris Ratzeburg, 1848.
Apantelesmesoxanthus Ruschka, 1917.
Apantelesnilae Telenga, 1961.
Type information. Neotype female, EBW (not examined but original description checked). Country of type locality: Germany.
Geographical distribution.NEA, OTL, PAL.
NEA: Canada (NL); OTL: China (ZJ); PAL: Czech Republic, Egypt, Finland, Germany, Hungary, Ireland, Italy, Korea, Moldova, Netherlands, Poland, Romania, Russia (VOR), Serbia, Slovakia, Sweden, Switzerland, Turkey, United Kingdom.
Dolichogenideabritannica (Wilkinson, 1941)
Apantelesbritannicus Wilkinson, 1941.
Apantelesmasmithi Fernandez-Triana, 2010.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.NEA, PAL.
NEA: Canada (MB, NS, ON); PAL: Armenia, Greece, Hungary, Iran, Israel, Malta, Russia (PRI), Slovakia, Tajikistan, Ukraine, United Kingdom.
Notes. In the holotype the hind wing vannal lobe is more or less straight (at most very slightly convex) and the fringe of setae is not very visible (as setae are minute). The species distribution in Israel is based on Belokobylskij et al. (2019).
Dolichogenideabroadi Rousse, 2013
Dolichogenideabroadi Rousse, 2013.
Type information. Holotype female, MNHN (not examined but original description checked). Country of type locality: Réunion.
Geographical distribution.AFR.
AFR: Réunion.
Dolichogenideabushnelli (Muesebeck, 1933)
Apantelesbushnelli Muesebeck, 1933.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CA, FL, IA, NE).
Dolichogenideacacoeciae (Riley, 1881)
Apantelescacoeciae Riley, 1881.
Pseudapantelesgallaediploppi Ashmead, 1899 [Nomen nudum].
Type information. Syntypes female and male, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (ON, QC), USA (CO, IL, KY, MD, MI, MO, NJ, NY, PA, SC, SD).
Notes. The type series is on a single pin, which has a piece of card cut into eight points. Three of the eight syntypes are missing, and another syntype is missing the head and metasoma.
Dolichogenideacalifornica (Muesebeck, 1921)
Apantelescalifornicus Muesebeck, 1921.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (AB, BC, ON, QC), USA (CA, ID, OR).
Dolichogenideacameroonensis Walker, 1994
Dolichogenideacameroonensis Walker, 1994.
Type information. Holotype female, MNHN (not examined but original description checked). Country of type locality: Cameroon.
Geographical distribution.AFR.
AFR: Cameroon.
Dolichogenideacandidata (Haliday, 1834)
Microgastercandidatus Haliday, 1834.
Microgasterterebrator Ratzeburg, 1852.
Type information. Lectotype female, NMID (not examined but subsequent treatment of the species checked). Country of type locality: Ireland.
Geographical distribution.AFR, PAL.
AFR: Cape Verde; PAL: Azerbaijan, Bulgaria, Germany, Greece, Hungary, Ireland, Macedonia, Mongolia, Romania, Russia (KHA, KDA, PRI, SAK), Serbia, Sweden, Tajikistan, Turkmenistan, United Kingdom, Uzbekistan.
Notes. Our species concept is based on Papp (2005, 2009a, 2009b), Kotenko (2007) and Shaw (2012b). Here we consider Dolichogenidealongicauda (Wesmael, 1837) as a separate, different species, following Fernandez-Triana and Huber (2010) and Fernandez-Triana et al. (2014b), although that decision has not been followed by most authors (see further notes under our treatment of longicauda below).
Dolichogenideacaniae (Wilkinson, 1928)
Apantelescaniae Wilkinson, 1928.
Type information. Holotype female, NHMUK (examined). Country of type locality: Indonesia.
Geographical distribution.OTL.
OTL: China (FJ, GD, GX, GZ, YN, ZJ), India, Indonesia, Sri Lanka, Thailand.
Notes. The species distribution in China is based in Liu et al. (2019).
Dolichogenideacarborugosa Liu & Chen, 2019
Dolichogenideacarborugosa Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (ZJ); PAL: China (HB).
Dolichogenideacarlosmanuelrodriguezi Fernandez-Triana & Boudreault, 2019
Dolichogenideacarlosmanuelrodriguezi Fernandez-Triana & Boudreault, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Dolichogenideacarposinae (Wilkinson, 1938)
Apantelescarposinae Wilkinson, 1938.
Type information. Holotype female, NHMUK (examined). Country of type locality: New Zealand.
Geographical distribution.AUS.
AUS: New Zealand.
Dolichogenideacatonix (Shenefelt, 1972), new combination
Apantelescatonix Shenefelt, 1972.
Apantelescato Nixon, 1967 [primary junior homonym of Apantelescato de Saeger, 1944].
Type information. Holotype female, NHMUK (examined). Country of type locality: Malaysia.
Geographical distribution.OTL.
OTL: Malaysia.
Notes. We transfer the species to Dolichogenidea based on the anteromesoscutum having punctures that do not fuse near the scutoscutellar sulcus, and the entirely setose hind wing vannal lobe.
Dolichogenideacauda Song & Chen, 2004
Dolichogenideacauda Song & Chen, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (JL).
Dolichogenideacelsa (Papp, 1975)
Apantelescelsus Papp, 1975.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Hungary.
Geographical distribution.PAL.
PAL: Bosnia and Herzegovina, Hungary, Montenegro, Tunisia.
Dolichogenideacerialis (Nixon, 1976)
Apantelescerialis Nixon, 1976.
Apantelesareolaris Balevski & Tobias, 1980 [primary junior homonym of Apantelesareolaris Blanchard, 1947].
Type information. Holotype female, ZSM (not examined but original description checked). Country of type locality: Italy.
Geographical distribution.PAL.
PAL: Bulgaria, Hungary, Israel, Italy, Kazakhstan, Russia (S), Spain.
Notes. The species distribution in Kazakhstan and Russia is based on Belokobylskij et al. (2019).
Dolichogenideachangbaiensis Liu & Chen, 2018
Dolichogenideachangbaiensis Liu & Chen, 2018.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (JL).
Dolichogenideacheles (Nixon, 1972)
Apantelescheles Nixon, 1972.
Type information. Holotype female, NHMUK (not examined but original description checked). Country of type locality: Sweden.
Geographical distribution.PAL.
PAL: Finland, Hungary, Poland, Russia (NW), Sweden, Turkey.
Notes. We could not find the holotype in the NHMUK collection (its assigned number is 3c.1760). In the place where it is supposed to be, there is a note from Annette K. Walker, dated August 1998, stating that the type was not present in the collection at that time either.
Dolichogenideachrysis (Nixon, 1973), new combination
Apanteleschrysis Nixon, 1973.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: United Kingdom.
Notes. Transferred to Dolichogenidea based on the vannal lobe entirely setose, and the anteromesoscutum with punctures not fusing near the scutoscutellar sulcus.
Dolichogenideacinerosa (Papp, 1971)
Apantelescinerosus Papp, 1971.
Type information. Holotype female, HNHM (not examined but subsequent treatment of the species checked). Country of type locality: Mongolia.
Geographical distribution.PAL.
PAL: Belgium, Hungary, Mongolia, Russia (PRI), Serbia.
Notes. Our species concept is based on Papp (1978a) and Tobias (1986).
Dolichogenideacinnarae Gupta, Lokhande & Soman, 2013
Dolichogenideacinnarae Gupta, Lokhande & Soman, 2013.
Type information. Holotype female, NBAIR (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Dolichogenideaclaniae (You & Zhou, 1990)
Apantelesclaniae You & Zhou, 1990.
Type information. Holotype female, HUNAU (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (FJ, JX); PAL: China (JL).
Notes. Our species concept is based on Chen and Song (2004).
Dolichogenideaclausa Liu & Chen, 2019
Dolichogenideaclausa Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (HI).
Dolichogenideaclavata (Provancher, 1881)
Microgasterclavatus Provancher, 1881.
Type information. Lectotype female, ULQC (not examined but subsequent treatment of the species checked). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (ON, QC), USA (AR, CA, CT, GA, KY, MO, NJ, OH, OR, SC, TN, WI).
Notes. Our species concept is based on Nixon (1972) and Papp (1978a).
Dolichogenideacoequata (Nixon, 1967)
Apantelescoequatus Nixon, 1967.
Type information. Holotype female, NHMUK (examined). Country of type locality: Tonga.
Geographical distribution.AUS, OTL.
AUS: Tonga; OTL: Vietnam.
Dolichogenideacoffea (Wilkinson, 1930), new combination
Apantelescoffea Wilkinson, 1930.
Type information. Holotype female, NHMUK (examined). Country of type locality: Uganda.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Kenya, Uganda.
Note. This species has the hind wing with an entirely setose vannal lobe, indicating it belongs to Dolichogenidea and not Apanteles. The ovipositor is apically sinuate, something very rarely present in Microgastrinae (very few species outside of the unrelated genus Promicrogaster). Other notable features of this species are T1 and T2 almost entirely smooth and shiny, and yellow metasoma and legs (except for metacoxa).
Dolichogenideacolchica (Tobias, 1976)
Apantelescolchicus Tobias, 1976.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Georgia.
Geographical distribution.PAL.
PAL: Georgia.
Notes. Our species concept is based on Papp (1978a) and Tobias (1986).
Dolichogenideacoleophorae (Wilkinson, 1938)
Apantelescoleophorae Wilkinson, 1938.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.NEA, PAL.
NEA: Canada (NL); PAL: Azerbaijan, Finland, Hungary, Poland, Romania, Russia (KHA, VOR, YAR), Slovakia, Switzerland, Tajikistan, Tunisia, Turkey, United Kingdom, Uzbekistan.
Dolichogenideaconcentrica Liu & Chen, 2018
Dolichogenideaconcentricus Liu & Chen, 2018.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (ZJ).
Notes. The species name must be treated as an adjective and not as a noun (Doug Yanega, pers. comm.) and thus it must match the gender of the genus name.
Dolichogenideaconiferae (Haliday, 1834)
Microgasterconiferae Haliday, 1834.
Type information. Lectotype female, NMID (not examined but subsequent treatment of the species checked). Country of type locality: Ireland.
Geographical distribution.AFR, PAL.
AFR: Cape Verde; PAL: Azerbaijan, Germany, Hungary, Mongolia, Romania, Russia (KDA), Sweden, Tajikistan, Turkmenistan, United Kingdom, Uzbekistan.
Notes. Our species concept is based on Shaw (2012b) and Broad et al. (2016).
Dolichogenideaconiferoides (Papp, 1972)
Apantelesconiferoides Papp, 1972.
Apantelestrogos Nixon, 1973.
Type information. Holotype female, HNHM (not examined but subsequent treatment of the species checked). Country of type locality: Hungary.
Geographical distribution. PAL
PAL: Hungary, Sweden, Turkey.
Notes. We examined the type of Apantelestrogos Nixon. Our species concept is based on Shaw (2012b), but see also additional comments under D.artisima above.
Dolichogenideaconpuncta Liu & Chen, 2019
Dolichogenideaconpuncta Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (GD, HI).
Dolichogenideacontergita Song & Chen, 2004
Dolichogenideacontergita Song & Chen, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (JL).
Dolichogenideacordiae Ahmad, 2019
Dolichogenideacontergita Ahmad & Pandey, 2019.
Type information. Holotype female, AMUZ (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. The authorship of the species names was not made clear in the original description paper, thus here we follow Ahmad (pers. comm.) for that information.
Dolichogenideacoretas (Nixon, 1965), new combination
Apantelescoretas Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Vanuatu.
Geographical distribution.AUS.
AUS: Vanuatu.
Notes. We transfer the species to Dolichogenidea based on the anteromesoscutum having punctures that do not fuse near the scutoscutellar sulcus, and the hind wing vannal lobe entirely setose.
Dolichogenideacorvina (Reinhard, 1880)
Apantelescorvinus Reinhard, 1880.
Apanteleslucidus Szépligeti, 1896.
Apantelesrasteratus Fahringer, 1936.
Apantelesaptus Papp, 1977.
Type information. Lectotype female, ZMHB (not examined but original description checked). Country of type locality: Germany.
Geographical distribution.NEA, PAL.
NEA: Canada (NL); PAL: Azerbaijan, Bulgaria, China (NX), Czech Republic, Finland, Georgia, Germany, Greece, Hungary, Iran, Ireland, Japan, Kazakhstan, Lithuania, Moldova, Mongolia, Netherlands, Poland, Romania, Russia (KDA, ROS), Sweden, Tajikistan, Turkmenistan, Ukraine, United Kingdom, Uzbekistan.
Notes. This species has been almost always been treated as Apantelescorvinus in the literature, even by authors recognizing Dolichogenidea as a valid genus (e.g., Broad et al. 2016). The only reference we have found that consider this species to belong to Dolichogenidea is Evenhuis and Vlug (1983). We have examined numerous specimens of this species and found that the vannal lobe indeed corresponds to Dolichogenidea.
Dolichogenideacrassa Liu & Chen, 2019
Dolichogenideacrassa Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (SD).
Dolichogenideacredne (Nixon, 1973)
Apantelescredne Nixon, 1973.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Germany, Poland, Russia (NW), Slovakia, United Kingdom.
Dolichogenideacucurbita Liu & Chen, 2019
Dolichogenideacucurbita Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Dolichogenideacultriformis Song & Chen, 2004
Dolichogenideacultriformis Song & Chen, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (FJ); PAL: China (JL).
Dolichogenideacyamon (Nixon, 1967)
Apantelescyamon Nixon, 1967.
Type information. Holotype female, NHMUK (examined). Country of type locality: Vanuatu.
Geographical distribution.AUS.
AUS: Vanuatu.
Dolichogenideacyane (Nixon, 1965), new combination
Apantelescyane Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Fiji.
Geographical distribution.AUS.
AUS: Fiji.
Notes. We transfer this species to Dolichogenidea based on the hind wing with the vannal lobe being convex and entirely setose, and the anteromesoscutum mostly smooth, with punctures that do not fuse near scutoscutellar sulcus.
Dolichogenideacytherea (Nixon, 1972)
Apantelescytherea Nixon, 1972.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Greece, Hungary, Mongolia, Poland, Russia (S), Serbia, Slovakia, Switzerland, Ukraine, United Kingdom.
Dolichogenideadecora (Haliday, 1834)
Microgasterdecorus Haliday, 1834.
Apanteleslineatus Reinhard, 1880.
Apantelessibiricus Fahringer, 1938.
Type information. Lectotype female, NMID (not examined but subsequent treatment of the species checked). Country of type locality: Ireland.
Geographical distribution.OTL, PAL.
OTL: China (JS); PAL: Bulgaria, Czech Republic, Estonia, Finland, Georgia, Germany, Greece, Hungary, Iran, Ireland, Kazakhstan, Lithuania, Poland, Romania, Russia (DA, IRK, KHA, KDA, NGR), Slovakia, Spain, Sweden, Turkmenistan.
Notes. Our species concept is based on Papp (1979a) and Tobias (1986).
Dolichogenideadiaphantus (Nixon, 1965), new combination
Apantelesdiaphantus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. We transfer the species to Dolichogenidea based on the anteromesoscutum having punctures that do not fuse near the scutoscutellar sulcus, and the hind wing vannal lobe setose (although the setae are relatively smaller and sparser than in typical members of the genus, the vannal lobe is still uniformly setose).
Dolichogenideadilecta (Haliday, 1834)
Microgasterdilectus Haliday, 1834.
Microgasterfemoralis Bouché, 1834.
Type information. Lectotype female, NMID (not examined but subsequent treatment of the species checked). Country of type locality: Ireland.
Geographical distribution.OTL, PAL.
OTL: China (FJ); PAL: Armenia, Austria, Bulgaria, Czech Republic, Finland, France, Germany, Hungary, Ireland, Italy, Japan, Korea, Moldova, Netherlands, Poland, Romania, Russia (PRI, SAK), Slovakia, Sweden, Switzerland, Turkey, Ukraine, United Kingdom.
Notes. Our species concept is based on Wilkinson (1945), Nixon (1972), Papp (1978a), Tobias (1986), and Chen and Song (2004).
Dolichogenideadioryctriphagous Liu & Chen, 2018
Dolichogenideadioryctriphagous Liu & Chen, 2018.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (HN, ZJ); PAL: China (AH, HA, JL).
Dolichogenideadiparopsidis (Lyle, 1927), new combination
Apantelesdiparopsidis Lyle, 1927.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Ivory Coast, Malawi, Nigeria, South Africa, Tanzania, Uganda, Zimbabwe.
Notes. Transferred to Dolichogenidea based on the hind wing with the vannal lobe entirely setose, and the anteromesoscutum with punctures not fusing near the scutoscutellar sulcus.
Dolichogenideadiscreta (Szépligeti, 1914)
Apantelesdiscretus Szépligeti, 1914.
Type information. Holotype female, MNHN (not examined but subsequent treatment of the species checked). Country of type locality: Kenya.
Geographical distribution.AFR.
AFR: Kenya, Madagascar.
Notes. Our species concept is based on Wilkinson (1932a), Granger (1949), and Madl & van Achterberg (2014).
Dolichogenideadolichocephalus (Muesebeck, 1921)
Apantelesdolichocephalus Muesebeck, 1921.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (IL, MI, VA).
Notes. Following Article 31.2.1 of the ICZN the name is a noun phrase in apposition and the original spelling dolichocephalus must be retained.
Dolichogenideadrusilla (Nixon, 1972)
Apantelesdrusilla Nixon, 1972.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Bulgaria, Germany, Hungary, Italy, Mongolia, Russia (PRI), Slovakia, Sweden, Turkey, Ukraine, United Kingdom.
Dolichogenideadryas (Nixon, 1965), new combination
Apantelesdryas Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (BJ).
Notes. Transferred to Dolichogenidea based on the hind wing with the vannal lobe being more or less straight but setose, and the anteromesoscutum with coarse punctures that do not fuse.
Dolichogenideaearterus (Wilkinson, 1930), new combination
Apantelesearterus Wilkinson, 1930.
Type information. Holotype female, NHMUK (examined). Country of type locality: Sudan.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Senegal, Sudan.
Notes. The holotype has the hind wing vannal lobe entirely setose, indicating the species belongs to Dolichogenidea.
Dolichogenideaeleagnellae (Tobias, 1976)
Apanteleseleagnellae Tobias, 1976.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Armenia.
Geographical distribution.PAL.
PAL: Armenia, Russia (NC).
Notes. Our species concept is based on Papp (1979a) and Tobias (1986). The holotype is presumed to be in the ZIN based on Tobias (1986).
Dolichogenideaemarginata (Nees, 1834)
Microgasteremarginatus Nees, 1834.
Microgasterscapularis Bouché, 1834.
Type information. Type lost (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Armenia, Austria, Azerbaijan, Belgium, Bulgaria, France, Germany, Hungary, Iran, Ireland, Israel, Italy, Kazakhstan, Lithuania, Moldova, Poland, Romania, Russia (ALT, KHA, PRI, ROS, SPE, YAR), Slovakia, Sweden, Switzerland, Turkey, United Kingdom.
Notes. Our species concept is based on Nixon (1972), Papp (1978a), and Tobias (1986).
Dolichogenideaensiformis (Ratzeburg, 1844)
Microgasterensiformis Ratzeburg, 1844.
Type information. Neotype female, SMF (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Germany, Hungary, Italy, Latvia, Mongolia, Poland, Romania, Russia (NW), Slovakia, Spain, Tunisia.
Notes. Our species concept is based on Papp (1979a) and Tobias (1986).
Dolichogenideaensiger (Say, 1836), new combination
Microgasterensiger Say, 1836.
Microgasterfemurnigrum Provancher, 1886.
Apantelestrachynotus Viereck, 1912.
Apantelesnipmuckorum Viereck, 1917.
Type information. Neotype female, USNM (not examined but authoritatively identified specimens examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (AB, MB, NB, NL, NS, NT, ON, PE, QC, SK), USA (AK, AL, CO, CT, DE, DC, FL, GA, IL, IN, IA, KS, LA, MA, MD, MO, MT, NH, NJ, NY, NC, TN).
Notes. We could not find the neotype of ensiger in the USNM collection; however, we have studied many authoritatively named specimens of this species available in the CNC and USNM collections: a) One male paratype of ensiger (with a red USNM Paratype label no. 14707), which has a hind wing vannal lobe typical of Dolichogenidea; b) The types of two other species currently considered as synonyms of ensiger, both in the USNM: A.trachynotus Viereck, 1912, a male specimen; and A.nipmuckorum Viereck, 1917, a female specimen; c) Non-type material from another synonym, Microgasterfemurnigrum Provancher, 1886 (in the USNM); d) Many specimens of ensiger (in the CNC and USNM), determined by Viereck, Muesebeck or Mason. All of those specimens clearly belong to Dolichogenidea. Apart from the morphological evidence (hind wing vannal lobe setose) of the specimens we examined, DNA barcoding also supports the same generic placement. More than 500 sequences of Apantelesensiger are currently available in BOLD, they comprise two BINS: BOLD:ACE6783 (Canada: ON, MB) and BOLD:AAA3764 (Canada: AB, ON, SK and some localities of southern USA), and both BINs cluster near many other species of Dolichogenidea and far apart from Apanteles sequences. Whether those BINs represent two different species or not has been mentioned in the past (Fernandez-Triana et al. 2014b) but no further study has been conducted so far, thus all known specimens are kept as one species for the time being.
Dolichogenideaerasmi (Nixon, 1972)
Apanteleserasmi Nixon, 1972.
Type information. Holotype female, ZSM (not examined but original description checked). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Germany, Italy, Slovakia.
Dolichogenideaerdoesi (Papp, 1973)
Apanteleserdoesi Papp, 1973.
Apantelesnegativus Tobias, 1976.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Hungary.
Geographical distribution.PAL.
PAL: Azerbaijan, Hungary, Russia (NC), Ukraine.
Notes.Dolichogenideaerdoesi is related to several species of Dolichogenidea, and it was transferred to that genus by Papp (1988). However, Kotenko (2007a) placed it within Iconella. After carefully reading the original description and subsequent treatment of the species, it is clear to us that it does not belong to Iconella, as it does not have a median, longitudinal carina on the propodeum. Thus, here we are revising the species combination back to Dolichogenidea.
Dolichogenideaerevanica (Tobias, 1976)
Apanteleserevanicus Tobias, 1976.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Armenia.
Geographical distribution.PAL.
PAL: Armenia, Bulgaria, Serbia.
Notes. Our species concept is based on Papp (1981) and Tobias (1986). The holotype is presumed to be in the ZIN based on Tobias (1986).
Dolichogenideaeros (Wilkinson, 1932), new combination
Apanteleseros Wilkinson, 1932.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Notes. The holotype has the hind wing vannal lobe entirely setose, indicating the species belongs to Dolichogenidea.
Dolichogenideaeucalypti Austin & Allen, 1989
Dolichogenideaeucalypti Austin & Allen, 1989.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (SA).
Dolichogenideaevadne (Nixon, 1955), new combination
Apantelesevadne Nixon, 1955.
Type information. Holotype female, NHMUK (examined). Country of type locality: Juan Fernández Islands.
Geographical distribution.NEO.
NEO: Juan Fernández Islands.
Notes. The holotype has the hind wing vannal lobe entirely setose, indicating the species belongs to Dolichogenidea. Yu et al. (2016) stated that the holotype is deposited in Chile, but we examined a female specimen with code 3c.1465 in the NHMUK, which has a label with Nixon’s handwriting stating that is the female holotype. We have based our assessment of this species based on that specimen.
Dolichogenideaevonymellae (Bouché, 1834)
Microgasterevonymellae Bouché, 1834.
Apantelesiarbas Nixon, 1972.
Type information. Holotype female, ZMHB (not examined but authoritatively identified specimens examined). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Armenia, Azerbaijan, Belarus, Bulgaria, Czech Republic, Germany, Hungary, Italy, Lebanon, Netherlands, Portugal, Romania, Russia (C, NC, NW, S), Serbia, United Kingdom.
Notes. We studied the type of Apantelesiarbas Nixon (in the NHMUK). The species’ presence in UK and Russia is based on Žiki et al. (2013) and Belokobylskij et al. (2019) respectively.
Dolichogenideaexcellentis Liu & Chen, 2019
Dolichogenideaexcellentis Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (GD, GZ, HI, SC, YN); PAL: China (HA, HE).
Dolichogenideaexilis (Haliday, 1834)
Microgasterexilis Haliday, 1834.
Type information. Lectotype female, NMID (examined). Country of type locality: Ireland.
Geographical distribution.PAL.
PAL: Bulgaria, Germany, Hungary, Ireland, Sweden, United Kingdom.
Notes. This species was transferred from Apanteles to Dolichogenidea by Shaw (2012b).
Dolichogenideafakhrulhajiae (Mahdihassan, 1925)
Apantelesfakhrulhajiae Mahdihassan, 1925.
Apantelesrufulus Wilkinson, 1930.
Apantelesfakhrulhajiae nagoliensis Mahdihassan, 1955.
Type information. Type and depository unknown (not examined but authoritatively identified specimens examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India, Vietnam.
Note. We have examined the type of A.rufulus Wilkinson, deposited in the NHMUK.
Dolichogenideafalcator (Ratzeburg, 1852), new combination
Microgasterfalcator Ratzeburg, 1852.
Type information. Type lost (not examined but subsequent treatment of the species checked). Country of type locality: unknown.
Geographical distribution.PAL.
PAL: Germany, Italy, Netherlands, Poland.
Notes. Our species concept is based on Telenga (1955) and van Achterberg (1976). The species is transferred to Dolichogenidea based on the entirely setose hind wing vannal lobe (van Achterberg 1976: fig. 54).
Dolichogenideafaucula (Nixon, 1972)
Apantelesfaucula Nixon, 1972.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Hungary, Finland, Poland, Russia (C, PR), United Kingdom.
Dolichogenideafernandeztrianai Abdoli & Talebi, 2019
Dolichogenideafernandeztrianai Abdoli & Talebi, 2019.
Type information. Holotype female, TMUC (not examined but original description checked). Country of type locality: Iran.
Geographical distribution.PAL.
PAL: Iran.
Dolichogenideaficicola Donaldson, 1991
Dolichogenideaficicola Donaldson, 1991.
Type information. Holotype female, TMSA (not examined but original description checked). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Dolichogenideafinchi Fagan-Jeffries & Austin, 2018
Dolichogenideafinchi Fagan-Jeffries & Austin, 2018.
Type information. Holotype female, WAM (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (NSW, QLD, VIC, WA).
Dolichogenideaflavigastrula Chen & Song, 2004
Dolichogenideaflavigastrula Chen & Song, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Dolichogenideaflavostriata (Papp, 1977)
Apantelesflavostriatus Papp, 1977.
Type information. Holotype female, HNHM (not examined but subsequent treatment of the species checked). Country of type locality: Hungary.
Geographical distribution.PAL.
PAL: Greece, Hungary.
Notes. Our species concept is based on Papp (1977b, 1980), and Tobias (1986).
Dolichogenideaflexisulcus Liu & Chen, 2019
Dolichogenideaflexisulcus Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (GD, ZJ); PAL: China (JS).
Dolichogenideaflexitergita Liu & Chen, 2019
Dolichogenideaflexitergita Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (YN).
Dolichogenideafluctisulcus Liu & Chen, 2019
Dolichogenideafluctisulcus Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (YN).
Dolichogenideaforrestae Fagan-Jeffries & Austin, 2019
Dolichogenideaforrestae Fagan-Jeffries & Austin, 2019.
Type information. Holotype female, SAMA (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (SA).
Dolichogenideafrustrata (Papp, 1975)
Apantelesfrustratus Papp, 1975.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Mongolia.
Geographical distribution.PAL.
PAL: Mongolia.
Dolichogenideafumea Liu & Chen, 2018
Dolichogenideafumeus Liu & Chen, 2018.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (FJ, GZ, HB, SC, ZJ); PAL: China (AH, HE, HA, SD).
Notes. The species name must be treated as an adjective and not as a noun (Doug Yanega, pers. comm.) and thus it must match the gender of the genus name.
Dolichogenideafunalicauda Liu & Chen, 2018
Dolichogenideafunalicauda Liu & Chen, 2018.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, GD, GZ, YN).
Dolichogenideafurtim (Papp, 1977)
Apantelesfurtim Papp, 1977.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Hungary.
Geographical distribution.PAL.
PAL: Azerbaijan, Greece, Hungary, Russia (S).
Dolichogenideafuscivora Walker, 1994
Dolichogenideafuscivora Walker, 1994.
Type information. Holotype female, NHMUK (examined). Country of type locality: Ethiopia.
Geographical distribution.AFR.
AFR: Cameroon, Ethiopia, Kenya.
Dolichogenideagagates (Nees, 1834)
Microgastergagates Nees, 1834.
Type information. Neotype female, RBINS (not examined but subsequent treatment of the species checked). Country of type locality: Belgium.
Geographical distribution.PAL.
PAL: Austria, Belgium, Bulgaria, Estonia, Finland, France, Georgia, Germany, Hungary, Italy, Latvia, Lithuania, Poland, Romania, Russia (MOS, YAR), Spain, Sweden, Switzerland, United Kingdom.
Notes. Our species concept is based on Nixon (1972), Papp (1979a), and Shaw (2012b).
Dolichogenideagallicola (Giraud, 1869)
Microgastergallicolus Giraud, 1869.
Type information. Holotype female, MNHN (not examined but subsequent treatment of the species checked). Country of type locality: France.
Geographical distribution.PAL.
PAL: Algeria, Tunisia.
Notes. Our species concept is based on Papp (1978a).
Dolichogenideagansuensis Liu & Chen, 2018
Dolichogenideagansuensis Liu & Chen, 2018.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (GS).
Dolichogenideagarytaylori Fagan-Jeffries & Austin, 2019
Dolichogenideagarytaylori Fagan-Jeffries & Austin, 2019.
Type information. Holotype female, SAMA (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (SA).
Dolichogenideagelechiidivoris (Marsh, 1975), new combination
Apantelesgelechiidivoris Marsh, 1975.
Type information. Holotype female, USNM (examined). Country of type locality: Colombia.
Geographical distribution.NEO.
OTL: Chile, Colombia, Peru.
Notes. Examination of the holotype and paratypes show that they have the hind wing vannal lobe entirely setose, clearly indicating they are best placed within Dolichogenidea.
Dolichogenideagentilis (Nixon, 1967)
Apantelesgentilis Nixon, 1967.
Type information. Holotype female, NHMUK (examined). Country of type locality: Papua New Guinea.
Geographical distribution.AUS, OTL.
AUS: Papua New Guinea, Solomon Islands; OTL: Vietnam.
Dolichogenideagenuarnunezi Fernandez-Triana & Boudreault, 2019
Dolichogenideagenuarnunezi Fernandez-Triana & Boudreault, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Dolichogenideaglabra (Papp, 1978)
Apantelesglaber Papp, 1978.
Type information. Holotype female, HNHM (not examined but subsequent treatment of the species checked). Country of type locality: Finland.
Geographical distribution.PAL.
PAL: Finland, United Kingdom.
Notes. Our species concept is based on Papp (1981), Tobias (1986) and Shaw (2012b).
Dolichogenideagleditsia Liu & Chen, 2019
Dolichogenideagleditsia Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (HN, ZJ); PAL: China (LN).
Dolichogenideagobica (Papp, 1976), new combination
Apantelesgobicus Papp, 1976.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Mongolia.
Geographical distribution.PAL.
PAL: Mongolia.
Notes. Although neither the original description nor a subsequent treatment of the species (Papp 1976b, 1984a) described the vannal lobe in the hind wing, both papers placed gobicus as very similar morphologically and very close to Apantelespelopea Nixon (which has been transferred in this paper to Dolichogenidea after we examined its holotype, see below under that species). Furthermore, other species considered by Papp to be relatively close morphologically to gobicus (based on how they were keyed out in both papers from Papp) have all been transferred to Dolichogenidea as well. The rest of the gobicus description fits well with it being Dolichogenidea (although no character is as conclusive as describing the setation pattern on the hind wing vannal lobe). Based on all available information and secondary evidence, we here formally transfer the species to Dolichogenidea.
Dolichogenideagobustanica (Kotenko, 1986)
Apantelesgobustanicus Kotenko, 1986.
Type information. Holotype female, SIZK (not examined but original description checked). Country of type locality: Azerbaijan.
Geographical distribution.PAL.
PAL: Azerbaijan.
Dolichogenideagolovushkini (Kotenko, 1992)
Apantelesgolovushkini Kotenko, 1992.
Type information. Holotype female, SIZK (not examined but subsequent treatment of the species checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (ZAB).
Notes. Our species concept is based on Kotenko (2006).
Dolichogenideagracilariae (Wilkinson, 1940)
Apantelesgracilariae Wilkinson, 1940.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Armenia, Austria, Azerbaijan, Bulgaria, Czech Republic, Germany, Hungary, Iran, Kazakhstan, Moldova, Poland, Romania, Russia (KDA), Serbia, Slovakia, Spain, Sweden, Switzerland, Turkey, United Kingdom, Uzbekistan.
Notes. The species distribution in Iran and Uzbekistan is based on Belokobylskij et al. (2019).
Dolichogenideagracilituba Song & Chen, 2004
Dolichogenideagracilituba Song & Chen, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Dolichogenideagrata (Kotenko, 1986)
Apantelesgratus Kotenko, 1986.
Type information. Holotype female, SIZK (not examined but original description checked). Country of type locality: Ukraine.
Geographical distribution.PAL.
PAL: Russia (NC), Ukraine.
Notes. The species name must be treated as an adjective and not as a noun (Doug Yanega, pers. comm.) and thus it must match the gender of the genus name.
Dolichogenideahalidayi (Marshall, 1872)
Apanteleshalidayi Marshall, 1872.
Apanteleshalidaii Marshall, 1872 [incorrect original spelling].
Microgasteralbipennis Haliday, 1834 [primary junior homonym of Microgasteralbipennis Nees, 1834].
Type information. Neotype female, MZLU (not examined but subsequent treatment of the species checked). Country of type locality: Sweden.
Geographical distribution.PAL.
PAL: Armenia, Croatia, Finland, Germany, Greece, Hungary, Iran, Ireland, Macedonia, Madeira Islands, Romania, Russia (MOS, NGR), Sweden, Ukraine, United Kingdom, Yugoslavia.
Notes.Wilkinson (1941b: 73) considered the original type series to be lost, and thus designated a neotype from Ringsjön, Sweden, which is deposited in the MZLU, and he provided ample explanation on the rationale to do so. However, van Achterberg (1997: 13) designated a lectotype from the Haliday material, presumably from Ireland, which is deposited in the NMID.
Dolichogenideahamakii (Watanabe, 1932)
Apanteleshamakii Watanabe, 1932.
Type information. Holotype female, EIHU (examined). Country of type locality: Japan.
Geographical distribution.PAL.
PAL: Japan.
Notes. A transfer of this species to the genus Dolichogenidea was proposed by Liu et al. (2018), but it was only stated in the abstract of that paper. We have examined the female holotype and concur with them as the hind wing vannal lobe is fully setose.
Dolichogenideahanoii (Tobias & Long, 1990)
Apanteleshanoii Tobias & Long, 1990.
Type information. Holotype female, ZIN (not examined but original description checked). Country of type locality: Vietnam.
Geographical distribution.OTL.
OTL: Vietnam.
Notes. Our species concept is based on Tobias and Long (1990) and Long and Belokobylskij (2004).
Dolichogenideahasorae Wilkinson, 1928
Apanteleshasorae Wilkinson, 1928.
Type information. Holotype female, NHMUK (examined). Country of type locality: Indonesia.
Geographical distribution.OTL.
OTL: India, Indonesia.
Dolichogenideahedyleptae (Muesebeck, 1958)
Apanteleshedyleptae Muesebeck, 1958.
Type information. Holotype female, USNM (examined). Country of type locality: Puerto Rico.
Geographical distribution.NEO.
NEO: Puerto Rico, Trinidad & Tobago.
Dolichogenideahelleni (Nixon, 1972)
Apanteleshelleni Nixon, 1972.
Type information. Holotype female, MZH (not examined but original description checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Bulgaria, Finland, Germany, Hungary, Russia (KR), Ukraine.
Dolichogenideahemerobiellicida (Fischer, 1966)
Apanteleshemerobiellicida Fischer, 1966.
Type information. Holotype female, NHMW (not examined but subsequent treatment of the species checked). Country of type locality: Austria.
Geographical distribution.PAL.
PAL: Austria.
Notes. Our species concept is based on Papp (1980a) and Tobias (1986).
Dolichogenideahemituba Liu & Chen, 2019
Dolichogenideahemituba Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, GD, ZJ).
Dolichogenideaheterusiae (Wilkinson, 1928)
Apantelesheterusiae Wilkinson, 1928.
Type information. Holotype female, NHMUK (examined). Country of type locality: Sri Lanka.
Geographical distribution.AUS, OTL, PAL.
AUS: Fiji; OTL: China (FJ, GX, HN, TW, ZJ), India, Sri Lanka, Vietnam; PAL: China (AH, BJ, CQ, GS, HB, JL, JS, SD, SN).
Dolichogenideahexagona Liu & Chen, 2019
Dolichogenideahexagona Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (YN, ZJ); PAL: China (SD).
Dolichogenideahilaris (Haliday, 1834)
Microgasterhilaris Haliday, 1834.
Type information. Lectotype female, NMID (not examined but subsequent treatment of the species checked). Country of type locality: Ireland.
Geographical distribution.PAL.
PAL: Ireland.
Notes. Transferred to Dolichogenidea by Broad et al. (2016), although the revised combination was not clearly stated.
Dolichogenideahomoeosomae (Muesebeck, 1933)
Apanteleshomoeosomae Muesebeck, 1933.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: Cuba.
Geographical distribution.NEA, NEO.
NEA: Canada (SK), USA (CA, MS, MO, SD, TX, WA); NEO: Cuba.
Dolichogenideahyalinis (Hedqvist, 1965), new combination
Apanteleshyalinis Hedqvist, 1965.
Type information. Holotype male, MZH (examined). Country of type locality: Cape Verde.
Geographical distribution.AFR.
AFR: Cape Verde.
Notes.Forshage et al. (2016) considered that the type was missing and not found in Helsinki (MZH), as they wrote for a number of other Hedqvist types of Microgastrinae. Although Forshage et al. (2016) were unable to find those types, they are all present in the MZH collection, but were placed in a separate section of the collection and only recently found by the senior author of the present paper in 2017. The male holotype of hyalinis is missing one fore and one hind wing, one antenna and flagellomeres 15–16 of the other, but it is otherwise in reasonably good condition. The hind wing vannal lobe is clearly that of Dolichogenidea, slightly concave and entirely setose and thus the species is transferred to this genus here.
Dolichogenideahyblaeae (Wilkinson, 1928)
Apanteleshyblaeae Wilkinson, 1928.
Type information. Holotype female, NHMUK (examined). Country of type locality: Western Samoa.
Geographical distribution.AUS, OTL.
AUS: Fiji, Western Samoa; OTL: China (GD), India, Indonesia, Vietnam.
Notes. The holotype is missing the metasoma.
Dolichogenideailione (Nixon, 1967)
Apantelesilione Nixon, 1967.
Type information. Holotype female, NHMUK (examined). Country of type locality: Fiji.
Geographical distribution.AUS.
AUS: Fiji.
Dolichogenideaimmissa (Papp, 1977)
Apantelesimmissus Papp, 1977.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Hungary.
Geographical distribution.PAL.
PAL: Germany, Hungary, Slovakia, Turkey.
Dolichogenideaimperator (Wilkinson, 1939)
Apantelesimperator Wilkinson, 1939.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Armenia, Austria, Azerbaijan, Czech Republic, Germany, Hungary, Italy, Kazakhstan, Lithuania, Moldova, Netherlands, Romania, Russia (C, E, NC, NW, S), Switzerland, Turkmenistan, Tajikistan, United Kingdom, Uzbekistan.
Notes. The species distribution in Turkmenistan, Tajikistan and Uzbekistan is based on Belokobylskij et al. (2019).
Dolichogenideaimpura (Nees, 1834)
Microgasterimpurus Nees, 1834.
Type information. Type lost (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Austria, Azerbaijan, Belgium, Bulgaria, Czech Republic, France, Germany, Greece, Hungary, Italy, Latvia, Lithuania, Mongolia, Poland, Russia (KGD, YAR), Sweden, Switzerland.
Notes. Our species concept is based on Papp (1978a) and Tobias (1986). Broad et al. (2016) stated that this species is not present in the United Kingdom or Ireland. Additionally, they considered the name as uncertainly interpreted, although they did not elaborate more on that, and thus the species is here retained as valid for the time being. The species distribution in Azerbaijan is based on Belokobylskij et al. (2019).
Dolichogenideaincompleta (Szépligeti, 1914)
Apantelesincompletus Szépligeti, 1914.
Type information. Holotype female, MNHN (not examined but subsequent treatment of the species checked). Country of type locality: Kenya.
Geographical distribution.AFR.
AFR: Kenya, Tanzania.
Notes. Our species concept is based on Papp (2008).
Dolichogenideaincystatae Fernandez-Triana, 2019, new replacement name
Dolichogenidealobesia Liu & Chen, 2019 [junior primary homonym of Dolichogenidealobesia Fagan-Jeffries & Austin, 2019].
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (YN).
Notes.Dolichogenidealobesia Liu & Chen, 2019 is a junior primary homonym of Dolichogenidealobesia Fagan-Jeffries & Austin, 2019. Both names represent different wasp species, named after two different host caterpillars in the genus Lobesia (Tortricidae). The replacement name was selected after the specific epithet of the host, Lobesiaincystata Liu & Yang, 1987, as mentioned in the original description of the Chinese wasp (Liu et al. 2019).
Dolichogenideaindicaphagous Liu & Chen, 2018
Dolichogenideaindicaphagous Liu & Chen, 2018.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, GD, GX, HI, JX, ZJ).
Dolichogenideainfima (Haliday, 1834)
Microgasterinfimus Haliday, 1834.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Azerbaijan, Czech Republic, Finland, France, Georgia, Germany, Hungary, Ireland, Italy, Kazakhstan, Lithuania, Macedonia, Mongolia, Netherlands, Poland, Romania, Russia (ZAB, KDA, PRI, YAR), Sweden, Switzerland, Turkey, United Kingdom, Uzbekistan, Yugoslavia.
Dolichogenideainfirmus Liu & Chen, 2019
Dolichogenideainfirmus Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, GD, GZ, HN, ZJ).
Dolichogenideainquisitor (Wilkinson, 1928)
Apantelesinquisitor Wilkinson, 1928.
Type information. Holotype female, NHMUK (examined). Country of type locality: Malaysia.
Geographical distribution.AUS, OTL.
AUS: Fiji; OTL: China (TW), Malaysia, Vietnam.
Dolichogenideainterpolata (Papp, 1975)
Apantelesinterpolatus Papp, 1975.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Hungary.
Geographical distribution.PAL.
PAL: Hungary.
Dolichogenideairanica (Telenga, 1955)
Apantelesiranicus Telenga, 1955.
Type information. Type and depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Iran.
Geographical distribution.PAL.
PAL: Iran, Kazakhstan, Mongolia.
Notes. Our species concept is based on Papp (1978a) and Tobias (1986).
Dolichogenideairiarte (Nixon, 1965), new combination
Apantelesiriarte Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Notes. Transferred to Dolichogenidea based on the hind wing vannal lobe being more or less straight and setose, and the posterior margin of anteromesoscutum with punctures that do not fuse with each other. The label of the type spells the name as iriate; however, the proper name, as spelled in the original description and accounts of this species after that, is iriarte.
Dolichogenideaiulis (Nixon, 1967)
Apantelesiulis Nixon, 1967.
Type information. Holotype female, NHMUK (examined). Country of type locality: Papua New Guinea.
Geographical distribution.AUS, OTL.
AUS: Papua New Guinea; OTL: Vietnam.
Dolichogenideajaroshevskyi (Tobias, 1976)
Apantelesjaroshevskyi Tobias, 1976.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Ukraine.
Geographical distribution.PAL.
PAL: Armenia, Russia (S), Ukraine.
Notes. Our species concept is based on Papp (1981) and Tobias (1986). The holotype is presumed to be in the ZIN based on Tobias (1986).
Dolichogenideajilinensis Chen & Song, 2004
Dolichogenideajilinensis Chen & Song, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (JL).
Dolichogenideajosealfredohernandezi Fernandez-Triana & Boudreault, 2019
Dolichogenideajosealfredohernandezi Fernandez-Triana & Boudreault, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Dolichogenideakelleri Fagan-Jeffries & Austin, 2019
Dolichogenideakelleri Fagan-Jeffries & Austin, 2019.
Type information. Holotype female, SAMA (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (SA).
Dolichogenideakunhi Gupta & Kalesh, 2012
Dolichogenideakunhi Gupta & Kalesh, 2012.
Type information. Holotype female, NBAIR (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Dolichogenideakurosawai (Watanabe, 1940)
Apanteleskurosawai Watanabe, 1940.
Type information. Holotype female, EIHU (examined). Country of type locality: Japan.
Geographical distribution.OTL, PAL.
OTL: China (FJ, GD, ZJ); PAL: China (NM), Japan.
Notes. After examining the holotype and other specimens (in the Hokkaido collection) we had decided to transfer this species to Dolichogenidea based on the hind wing vannal lobe being entirely setose. However, the species was transferred right before our paper by Liu et al. (2019), who also provided additional distribution records from China, an information we incorporate here.
Dolichogenidealabaris (Nixon, 1967)
Apanteleslabaris Nixon, 1967.
Type information. Holotype female, NHMUK (examined). Country of type locality: Fiji.
Geographical distribution.AUS.
AUS: Fiji.
Dolichogenidealacteicolor (Viereck, 1911)
Apanteleslacteicolor Viereck, 1911.
Apantelesconspersae Fiske, 1911.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA, OTL, PAL.
NEA: Canada (NB, NS, QC), USA (CO, CT, MA, NH); OTL: China (FJ, GX, GZ, HN, TW, YN, ZJ); PAL: Afghanistan, Armenia, Austria, Azerbaijan, Bulgaria, China (HA, SX), Finland, France, Germany, Hungary, Iran, Israel, Italy, Japan, Kazakhstan, Korea, Lithuania, Moldova, Mongolia, Poland, Portugal, Romania, Russia (KIR, KDA, PRI, SAK, VOR, YAR), Slovakia, Spain, Switzerland, Tajikistan, Turkey, Ukraine, United Kingdom, Uzbekistan, Yugoslavia.
Dolichogenidealacteipennis (Curtis, 1830)
Microgasterlacteipennis Curtis, 1830.
Apanteleslissonotus Tobias, 1964.
Microgasterlacteipennis Curtis, 1829 [nomen nudum].
Type information. Holotype male, MVMMA (not examined but subsequent treatment of the species checked). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Czech Republic, Germany, Italy, Kazakhstan, Latvia, Mongolia, Poland, Russia (C, NW), Slovakia, United Kingdom.
Notes. Our species concept is based on Papp (1978a, 1988).
Dolichogenidealaevigata (Ratzeburg, 1848)
Microgasterlaevigatus Ratzeburg, 1848.
Microgasterhoplites Ratzeburg, 1848.
Apantelescalcaratus Ivanov, 1899.
Type information. Holotype male, ZMHB (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.OTL, PAL.
OTL: China (FJ); PAL: Armenia, Azerbaijan, Bulgaria, China (SN), Finland, France, Georgia, Germany, Hungary, Israel, Italy, Kazakhstan, Korea, Latvia, Lithuania, Moldova, Netherlands, Poland, Romania, Russia (ALT, MOS, PRI, ROS, SPE, VOR), Serbia, Slovakia, Spain, Sweden, Switzerland, Turkey, Ukraine, United Kingdom, Uzbekistan.
Notes. Our species concept is based on Wilkinson (1945), Nixon (1972), Papp (1978a) and Chen and Song (2004).
Dolichogenidealaevigatoides (Nixon, 1972)
Apanteleslaevigatoides Nixon, 1972.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Czech Republic, Germany, Hungary, Poland, Russia (C, S), Switzerland, United Kingdom.
Dolichogenidealaevissima (Ratzeburg, 1848)
Microgasterlaevissimus Ratzeburg, 1848.
Apanteleslevissimus Dalla Torre, 1898 [unjustified emendation].
Apantelestersus Papp, 1973.
Type information. Holotype male, depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: France.
Geographical distribution.PAL.
PAL: Czech Republic, France, Germany, Hungary, Romania, Ukraine, United Kingdom.
Notes. Our species concept is based on Wilkinson (1945), Nixon (1973), Papp (1984a) and Tobias (1986). The holotype specimen was deposited in the Forestry College of Eberswalde (Forstlichen Hochschule Eberswalde). Unfortunately, that collection was mostly destroyed during the Second World War; however, five drawers with Hymenoptera specimens, among them type species of Ratzeburg were spared and are now safe at the Senckenberg Deutsches Entomologisches Institut (SDEI) in Müncheberg, Germany [see a detailed story in Schulz et al. (2018: 285-286)]. We do not know if the holotype of this species is at present in Müncheberg.
Dolichogenidealakhaensis (Ray & Yousuf, 2009), new combination
Apanteleslakhaensis Ray & Yousuf, 2009.
Type information. Holotype female, IFRI (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. Based on the original description and drawings (which clearly show a setose vannal lobe in the hind wing), this species is better placed within Dolichogenidea. Additional characters and details provided in the original description, e.g., anteromesoscutum and scutellar disc sculpture, and its similarity with another species of Dolichogenidea (D.hyblaea), also support the generic placement that we propose here.
Dolichogenidealampe (Nixon, 1965), new combination
Apanteleslampe Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Notes. Transferred to Dolichogenidea based on the hind wing vannal lobe being more or less straight but uniformly setose, and the posterior margin of the anteromesoscutum with few punctures that do not fuse with each other.
Dolichogenidealaspeyresiae (Viereck, 1913)
Apanteleslaspeyresiae Viereck, 1913.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (BC), USA (CA, ID, OR).
Dolichogenidealaspeyresiella (Papp, 1972), new combination
Apanteleslaspeyresiella Papp, 1972.
Type information. Holotype female, HNHM (not examined but paratype examined). Country of type locality: Hungary.
Geographical distribution.PAL.
PAL: Austria, Azerbaijan, Belarus, Bulgaria, China (NX), Hungary, Iran, Romania, Russia (PRI), Turkey.
Notes. We have examined one female and one male paratypes deposited in the CNC, and we think that the best generic placement is in Dolichogenidea. The propodeum lacks the strongly defined median carina that characterizes Iconella (instead it has several rugae near the nucha, defining a partial areola posteriorly); and the hind wing vein cu-a is not sinuous but straight. The original description and subsequent treatment of the species (Papp 1972, Nixon 1976) also suggest this species belongs to Dolichogenidea. The species distribution in Azerbaijan is based on Belokobylskij et al. (2019).
Dolichogenidealaticauda Chen & Song, 2004
Dolichogenidealaticauda Chen & Song, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Dolichogenidealatistigma (Papp, 1977), new combination
Apanteleslatistigma Papp, 1977.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Mongolia.
Geographical distribution.PAL.
PAL: Mongolia.
Notes. The original description and illustrations, as well as comparison with similar European species in the key by Papp (1978a), strongly support latistigma to be a species of Dolichogenidea.
Dolichogenidealatitergita Liu & Chen, 2019
Dolichogenidealatitergita Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (ZJ); PAL: China (HL, JL, LN, SC, SD).
Dolichogenidealebene (Nixon, 1967), new combination
Apanteleslebene Nixon, 1967.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. Transferred to Dolichogenidea based on the hind wing vannal lobe being entirely setose.
Dolichogenidealemariei (Nixon, 1961)
Apanteleslemariei Nixon, 1961.
Type information. Holotype female, MMBC (not examined but original description checked). Country of type locality: Czech Republic.
Geographical distribution.PAL.
PAL: Czech Republic, Hungary, Poland, Russia (SPE), United Kingdom.
Dolichogenidealevifida (Kotenko, 1992)
Apanteleslevifidus Kotenko, 1992.
Type information. Holotype female, SIZK (not examined but subsequent treatment of the species checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (ZAB).
Notes. Our species concept is based on Kotenko (2006).
Dolichogenidealincostulata Liu & Chen, 2019
Dolichogenidealincostulata Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Notes. Based on the short ovipositor sheaths and hypopygium, as well as the similarities that Liu et al. (2019) stated lincostulata had with Apanteleshyposidrae Wilkinson, 1928 (transferred to Parapanteles by us, see below under that species), it is likely that lincostulata ends placed in a different genus in the future. However, until more study is done, we prefer here to retain it in Dolichogenidea.
Dolichogenidealineipes (Wesmael, 1837)
Microgasterlineipes Wesmael, 1837.
Type information. Holotype female, RBINS (not examined but subsequent treatment of the species checked). Country of type locality: Belgium.
Geographical distribution.PAL.
PAL: Austria, Belgium, Bulgaria, Croatia, Czech Republic, Finland, France, Germany, Hungary, Israel, Italy, Latvia, Mongolia, Poland, Romania, Russia (SPE), Slovakia, Switzerland, United Kingdom.
Notes. Our species concept is based on Nixon (1972), Papp (1980a) and Tobias (1986).
Dolichogenidealipsis (Nixon, 1967)
Apanteleslipsis Nixon, 1967.
Type information. Holotype female, NHMUK (examined). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (WA).
Dolichogenidealissos (Nixon, 1967)
Apanteleslissos Nixon, 1967.
Type information. Holotype female, NHMUK (examined). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (GD, HI, SC).
Notes. After examining the holotype we had decided to transfer this species to Dolichogenidea based on the hind wing vannal lobe being entirely setose and the punctures on the anteromesoscutum not fusing near scutoscutellar sulcus. However, the species was transferred right before our paper by Liu et al. (2019), who also provided additional distribution records from China, an information we incorporate here. Because the name is to be considered as a noun under ICZN Article 31.2.1, it must retain its original spelling and remain as lissos.
Dolichogenidealobesiae Fagan-Jeffries & Austin, 2019
Dolichogenidealobesiae Fagan-Jeffries & Austin, 2019.
Type information. Holotype female, QM (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (QLD).
Dolichogenidealocastrae (You & Tong, 1987)
Apanteleslocastrae You & Tong, 1987.
Type information. Holotype female, HUNAU (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (HN, ZJ).
Notes. Our species concept is based on Chen and Song (2004).
Dolichogenidealongialba Liu & Chen, 2019
Dolichogenidealongialba Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (XJ).
Dolichogenidealongicalcar (Thomson, 1895)
Microgasterlongicalcar Thomson, 1895.
Type information. Lectotype female, MZLU (not examined but subsequent treatment of the species checked). Country of type locality: Sweden.
Geographical distribution.PAL.
PAL: Czech Republic, Finland, Hungary, Korea, Russia (KR, PRI), Sweden, Switzerland, United Kingdom.
Notes. Our species concept is based on Nixon (1973), Papp (1980a) and Tobias (1986).
Dolichogenidealongicauda (Wesmael, 1837)
Microgasterlongicauda Wesmael, 1837.
Apanteleslongicaudis Marshall, 1885 [unjustified emendation].
Apanteleslongicauderra Shenefelt, 1972.
Type information. Holotype female, RBINS (not examined but subsequent treatment of the species checked). Country of type locality: Belgium.
Geographical distribution.NEA, PAL.
NEA: Canada (BC), USA (WA); PAL: Afghanistan, Armenia, Austria, Azerbaijan, Belarus, Belgium, Bulgaria, Estonia, Finland, France, Georgia, Germany, Hungary, Italy, Korea, Latvia, Lithuania, Moldova, Mongolia, Netherlands, Poland, Romania, Russia (BU, KDA, SPE), Serbia, Slovakia, Slovenia, Spain, Switzerland, Turkey, Turkmenistan, Ukraine, United Kingdom.
Notes. This species was synonymized under D.candidata by van Achterberg (1997), a decision that has been subsequently followed by most authors (e.g., Belokobylskij et al. 2003, Kotenko 2007, Papp 2009a, Broad et al. (2016). However, Fernandez-Triana and Huber (2010) and Fernandez-Triana et al. (2014b) considered it a valid species, based on Mason (1981) and Whitfield (1995a). Here we are considering them as separate species until further study allow us to conclude on this.
Dolichogenidealongimagna Liu & Chen, 2019
Dolichogenidealongimagna Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (SC).
Dolichogenidealongipalpis (Reinhard, 1880)
Apanteleslongipalpis Reinhard, 1880.
Apantelestadzhicus Telenga, 1949.
Type information. Type and depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.OTL, PAL.
OTL: China (JS); PAL: Armenia, Finland, Germany, Greece, Hungary, Iran, Poland, Romania, Russia (S, NC), Slovakia, Tajikistan, Turkey, Ukraine, United Kingdom.
Notes. Our species concept is based on Nixon (1965), Papp (1981) and Shaw (2012b). Papp (1981) reported that the type series of Apantelestadzhicus includes two species (lacteus and longipalpis) but he did not select a lectotype; Belokobylskij et al. (2003) treated the name as a synonym of longipalpis. The species distribution in Greece and Iran are based on Belokobylskij et al. (2019) and Kavallieratos et al. (2019).
Dolichogenidealongituba Song & Chen, 2004
Dolichogenidealongituba Song & Chen, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Dolichogenidealongivena Liu & Chen, 2018
Dolichogenidealongivena Liu & Chen, 2018.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, GD, GX, HI, SC, ZJ).
Dolichogenidealucidinervis (Wilkinson, 1928), new combination
Apanteleslucidinervis Wilkinson, 1928.
Urogasteralbinervis Ashmead, 1905 [primary junior homonym of Urogasteralbinervis Cameron, 1904].
Type information. Holotype male, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Notes. We examined the holotype and it clearly belongs to Dolichogenidea, based on its convex, uniformly setose vannal lobe in the hind wing, and the anteromesoscutum with punctures that do not fuse near the scutoscutellar sulcus.
Dolichogenidealuctifica (Papp, 1971)
Apantelesluctificus Papp, 1971.
Apantelesanfitrion Nixon, 1972.
Type information. Holotype female, HNHM (not examined but subsequent treatment of the species checked). Country of type locality: Mongolia.
Geographical distribution.PAL.
PAL: Finland, Hungary, Mongolia, Russia (PRI, SPE), Yugoslavia.
Notes. This species was described from Mongolia by Papp (1971) as Apantelesluctificus. A year later, Nixon (1972) described the species Apantelesanfitrion from Finland and Yugoslavia, the Finnish specimen being the holotype. Papp (1978a: 278) was able to examine the type material of those two species and synonymized anfitrion under luctificus. However, the specimen from Finland was collected on the island of Tytärsaari (currently Bolshoi Tyuters), which became part of Russia after 1940. Thus, the record of this species as part of the Finnish fauna is questionable at present.
Dolichogenidealumba Rousse & Gupta, 2013
Dolichogenidealumba Rousse & Gupta, 2013.
Type information. Holotype female, MNHN (not examined but original description checked). Country of type locality: Réunion.
Geographical distribution.AFR.
AFR: Réunion.
Dolichogenidealunata Liu & Chen, 2019
Dolichogenidealunatus Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (YN).
Dolichogenideamaetoi Fernandez-Triana & Shimizu, 2018
Dolichogenideamaetoi Fernandez-Triana & Shimizu, 2018.
Type information. Holotype female, NIAES (examined). Country of type locality: Japan.
Geographical distribution.PAL.
PAL: Japan.
Notes. The paper describing this species incorrectly stated the holotype as deposited in the CNC (Fernandez-Triana et al. 2018), when it is in fact deposited in the NIAES. This is corrected here.
Dolichogenideamalacosomae (Pandey, Ahmad, Haider & Shujauddin, 2004), new combination
Apantelesmalacosomae Pandey, Ahmad, Haider & Shujauddin, 2004.
Type information. Holotype female, AMUZ (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. Transferred to Dolichogenidea based on the original description mentioning and illustrating an entirely setose hind wing vannal lobe (fig. 2 in Pandey et al. 2004).
Dolichogenideamarica (Nixon, 1972)
Apantelesmarica Nixon, 1972.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Hungary, Switzerland, United Kingdom.
Dolichogenideamaro (Nixon, 1967), new combination
Apantelesmaro Nixon, 1967.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. Transferred to Dolichogenidea based on the hind wing with the vannal lobe entirely setose, and the anteromesoscutum with punctures that do not fuse near the posterior margin. In the original description, no details on the etymology of the species name were provided; as first revisers we consider it as a noun in apposition and thus its gender to be neuter, following Article 31.2.2 of the ICZN.
Dolichogenideamarokkana (Fahringer, 1936)
Apantelesmarokkanus Fahringer, 1936.
Type information. Type and depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Morocco.
Geographical distribution.PAL.
PAL: Morocco.
Notes. Our species concept is based on Papp (1981) and Liu et al. (2015).
Dolichogenideamasoni Pandey, Ahmad, Haider & Shujauddin, 2005
Dolichogenideamasoni Pandey, Ahmad, Haider & Shujauddin, 2005.
Type information. Holotype female, AMUZ (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Dolichogenideamedicava Liu & Chen, 2019
Dolichogenideamedicava Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Dolichogenideamediocaudata Fagan-Jeffries & Austin, 2018
Dolichogenideamediocaudata Fagan-Jeffries & Austin, 2018.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (NSW).
Dolichogenideamelaniamunozae Fernandez-Triana & Boudreault, 2019
Dolichogenideamelaniamunozae Fernandez-Triana & Boudreault, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Dolichogenideamelanopus (Viereck, 1917)
Apantelesmelanopus Viereck, 1917.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (AB, BC, MB, NL, PE, QC, SK, YT), USA (AK, CT).
Notes. The holotype is in relatively poor condition, missing the left pair of wings and most of the antennae. Following Article 31.2.1 of the ICZN the name is a noun phrase in apposition and the original spelling melanopus must be retained.
Dolichogenideamendosae (Wilkinson, 1929), new combination
Apantelesmendosae Wilkinson, 1929.
Type information. Holotype female, NHMUK (examined). Country of type locality: Malaysia.
Geographical distribution.OTL.
OTL: Malaysia.
Notes. The holotype has the hind wing vannal lobe entirely setose, indicating the species belongs in Dolichogenidea.
Dolichogenideamesocanalis Liu & Chen, 2018
Dolichogenideamesocanalis Liu & Chen, 2018.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (YN).
Dolichogenideametesae (Nixon, 1967)
Apantelesmetesae Nixon, 1967.
Type information. Holotype female, NHMUK (examined). Country of type locality: Malaysia.
Geographical distribution.OTL, PAL.
OTL: China (GX, HI, ZJ), Malaysia, Vietnam; PAL: China (SH).
Dolichogenideamiantonomoi (Viereck, 1917)
Apantelesmiantonomoi Viereck, 1917.
Apantelespequodorum Viereck, 1917.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CT, MI).
Notes. We also examined the type of A.pequodorum (synonym of A.miantonomoi).
Dolichogenideamidas (Nixon, 1972)
Apantelesmidas Nixon, 1972.
Type information. Holotype female, MZH (not examined but original description checked). Country of type locality: Finland.
Geographical distribution.PAL.
PAL: Finland, Hungary, Mongolia, Russia (BA, PRI).
Dolichogenideamimi (Papp, 1974)
Apantelesmimi Papp, 1974.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Hungary.
Geographical distribution.PAL.
PAL: Hungary, Moldova, Ukraine.
Dolichogenideaminuscula Liu & Chen, 2019
Dolichogenideaminuscula Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, ZJ).
Dolichogenideamira (Papp, 1977)
Apantelesmirus Papp, 1977.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Hungary.
Geographical distribution.PAL.
PAL: Hungary.
Dolichogenideamiris (Nixon, 1967)
Apantelesmiris Nixon, 1967.
Type information. Holotype female, NHMUK (examined). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (ACT).
Dolichogenideamolestae (Muesebeck, 1933)
Apantelesmolestae Muesebeck, 1933.
Type information. Holotype female, USNM (examined). Country of type locality: Japan.
Geographical distribution.OTL, PAL.
OTL: China (TW), Ryukyu Islands; PAL: Japan, Korea.
Notes. After examining the holotype we had decided to transfer this species to Dolichogenidea based on the entirely setose hind wing vannal lobe (the specimen also has a very distinctive T1 with strong longitudinal striation). However, the species was transferred just before our paper by Liu et al. (2019).
Dolichogenideamonticola (Ashmead, 1890), new combination
Apantelesmonticola Ashmead, 1890.
Type information. Holotype male, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CO, CT, ID, NM).
Notes. Transferred to Dolichogenidea because of the convex and fully setose hind wing vannal lobe. The specimen is missing both antenna, one pair of wings and some legs.
Dolichogenideamulticolor Liu & Chen, 2019
Dolichogenideamulticolor Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (GD).
Dolichogenideamurinanae (Capek & Zwölfer, 1957), status revised
Apantelesmurinanae Capek & Zwölfer, 1957.
? Apantelesdioryctriae Wilkinson, 1938.
? Apantelesmagnus Telenga, 1955.
Type information. Holotype female, MMBC (not examined but subsequent treatment of the species checked). Country of type locality: Slovakia.
Geographical distribution.PAL.
PAL: Austria, Czech Republic, Finland, France, Germany, Italy, Lithuania, Mongolia, Morocco, Poland, Romania, Russia (SA), Slovakia, Switzerland, Turkey, United Kingdom.
Notes.Apantelesmurinanae Capek & Zwölfer, altogether with A.dioryctriae Wilkinson and A.magnus Telenga, were all synonymized under Apantelespetrovae Walley, 1937 by Papp (1980a: 253), who based his decision on the study of one female and one male specimens of petrovae sent to him by Mason (CNC). However, Capek (1989) reexamined the situation in more detail, by studying host relations, geographical distribution, and larval taxonomy of the species involved, and he concluded that the synonyms were not warranted. After that, some authors (e.g., Shaw 2012b, Broad et al. 2016) have considered all involved species as junior synonyms of petrovae, while others have considered murinanae as a valid species (e.g., Mills and Kenis 1991, van Achterberg 2014, Yu et al. 2016). However, those references did not assess the species involved, but just followed either Papp (1980a) or Capek (1989). We have examined the holotype, paratypes and additional specimens of Apantelespetrovae (all deposited in the CNC), and have compared them versus Apantelesmurinanae specimens (also deposited in the CNC, some of that material collected by Zwölfer and apparently identified by Capek; the rest of the material coming from France and apparently part of the specimens studied by Mills and Kenis 1991). There are slight morphological differences among those two groups of specimens, but most importantly, there are also substantial differences in DNA barcodes. There are more than 15 petrovae specimens with sequences available, representing BINBOLD:AAA6374; whereas the only barcode compliant sequence of murinanae (there are two other specimens with sequences available for this species, they are just minibarcodes, with only 144 bp) represents BINBOLD:AAZ7315. Both BINs are the closest between each other in BOLD, but still have 6% bp difference, which suggest they represent two different species. It may even be possible that they are part of a complex of morphologically cryptic species, but study of more specimens from the range of petrovaesensu lato would be needed, including obtaining more DNA barcodes. In this paper we restrict the name petrovae to American specimens, while considering the Palearctic specimens to represent a different species, murinanae. If all European specimens would actually end up belonging to just one species, then the proper name should actually be Apantelesdioryctriae Wilkinson, 1938 (the oldest, senior synonym). However, because we have not been able to study more specimens, we prefer to use Apantelesmurinanae Capek & Zwölfer, 1957 for the time being, as it has been more widely used than dioryctriae or magnus (these last two names have been considered as junior synonyms since 1980). Because it is impossible to conclude on the status of dioryctriae and magnus with the evidence available at present (they could be synonyms of murinanae or petrovae, or even valid species on their own); here we provisionally include them as synonyms of both murinanae and petrovae, with question marks to indicate this matter will require further investigation. In addition to the nomenclatural changes discussed above, we have also assessed the best generic placement for both murinanae and petrovae, and have decided to maintain them within Dolichogenidea. In the specimens we have studied (which include the type of dioryctriae in the NHMUK, non-type material from murinanae in the CNC, type and non-type material of petrovae in the CNC) the vannal lobe is more or less straight and with very minute setae that are sparse but still look like a fringe. Both species could be considered borderline between Apanteles and Dolichogenidea, but we have based our decision not only on morphology but also on DNA barcodes, which cluster with dozens of other Dolichogenidea species in BOLD, far apart from Apanteles.
Dolichogenideamycale (Nixon, 1972)
Apantelesmycale Nixon, 1972.
Type information. Holotype female, NHMUK (examined). Country of type locality: Sweden.
Geographical distribution.OTL, PAL.
OTL: China (GZ, SC); PAL: Bulgaria, China (JL, LN), Czech Republic, Finland, Hungary, Poland, Slovakia, Sweden, Tunisia, Turkey.
Notes. Our species concept is based on Shaw (2012b).
Dolichogenideamyron (Nixon, 1973)
Apantelesmyron Nixon, 1973.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Austria, Finland, Germany, Greece, Switzerland, Turkey, United Kingdom.
Notes.Broad et al. (2016: 227-228) wrote that this species was being “Transferred from Apanteles in anticipation of publication by Jose Fernandez-Triana”, although the new combination was not made explicit in that paper. The holotype has the hind wing vannal lobe setose.
Dolichogenideanigra (Muesebeck, 1921), new combination
Apantelesniger Muesebeck, 1921.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (DC, ID, KS, MI, MN, NY, SD, VA).
Notes. After examining the type and several other specimens (in the USNM and CNC) determined by Muesebeck, we consider this species to belong to Dolichogenidea. The vannal lobe on the hind wing is fully setose, and the anteromesoscutum has very few and shallow puncture which do not fuse near the scutoscutellar sulcus.
Dolichogenideanixosiris (Papp, 1976)
Apantelesnixosiris Papp, 1976.
ApantelesosirisNixon 1972 [primary junior homonym of Apantelesosiris de Saeger, 1944].
Type information. Holotype female, MZH (not examined but original description checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: China (HE, HL, NM, XJ), Finland, Hungary, Mongolia, Russia (ZAB, KR, NVS, PRI), Turkmenistan.
Dolichogenideanovoguineensis (Szépligeti, 1905)
Apantelesnovo-guineensis Szépligeti, 1905.
Type information. Lectotype female, HNHM (not examined but subsequent treatment of the species checked). Country of type locality: Papua New Guinea.
Geographical distribution.AUS.
AUS: Papua New Guinea.
Notes. Our species concept is based on Austin and Dangerfield (1992) and Papp (2004).
Dolichogenideanumenes (Nixon, 1967)
Apantelesnumenes Nixon, 1967.
Type information. Holotype female, NHMUK (examined). Country of type locality: Indonesia.
Geographical distribution.OTL.
OTL: China (HI), Indonesia, Vietnam.
Notes. The species distribution in China is based on Liu et al. (2019).
Dolichogenideaoblicarina Chen & Song, 2004
Dolichogenideaoblicarina Chen & Song, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (JL).
Dolichogenideaobscurugosa Liu & Chen, 2018
Dolichogenideaobscurugosus Liu & Chen, 2018.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (ZJ); PAL: China (NM).
Notes. The species name must be treated as an adjective and not as a noun (Doug Yanega, pers. comm.) and thus it must match the gender of the genus name.
Dolichogenideaobsoleta Liu & Chen, 2019
Dolichogenideaobsoleta Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (ZJ).
Dolichogenideaobstans (Papp, 1971)
Apantelesobstans Papp, 1971.
Type information. Holotype female, HNHM (not examined but subsequent treatment of the species checked). Country of type locality: Mongolia.
Geographical distribution.PAL.
PAL: Kazakhstan, Mongolia, Slovakia.
Notes. Our species concept is based on Papp (1978a) and Tobias (1986).
Dolichogenideaoehlkei (Papp, 1982)
Apantelesoehlkei Papp, 1982.
Type information. Holotype female, EBW (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Germany.
Notes. Our species concept is based on Papp (1982, 1988) and Tobias (1986).
Dolichogenideaoidaematophori (Muesebeck, 1929)
Apantelesoidaematophori Muesebeck, 1929.
Apantelesoidematophori Muesebeck, 1929 [incorrect original spelling].
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (ID, WI).
Dolichogenideaolivierellae (Wilkinson, 1936), new combination
Apantelesolivierellae Wilkinson, 1936.
Type information. Holotype female, NHMUK (examined). Country of type locality: Algeria.
Geographical distribution.PAL.
PAL: Algeria, Morocco, Israel.
Notes. Since its original description, this species has been recognized to be a “remarkable species of Apanteles, since it possesses more than one character not previously described in the genus” (Wilkinson 1936b: 85). After examining the holotype we agree that there are some unique features, some not or very rarely present in Apanteles: strongly emarginate clypeus; mandible base separated from the head by a desclerotized area that looks like an opening; anteromesoscutum mostly smooth and shiny, the few punctures that are discernible (mostly on the anterior half of the anteromesoscutum) are shallow and sparse, never fusing with each other; scutoscutellar sulcus very narrow and shallow, almost imperceptible; propodeum almost entirely smooth and shiny, only with very short carinae near the nucha; ovipositor sheaths relatively short (ca. half the metatibia length), and fully setose; hind wing vannal lobe slightly convex to straight, with small setae that do not form a full fringe, but nevertheless cover more or less the entire area of the vannal lobe; hypopygium with narrow translucent area (more evident on the posterior third of the hypopygium, but very narrowly present on the anterior two thirds as well), the translucent area with one or two pleats barely visible. Wilkinson (1936b) mentioned other important features such as spines at the base of the ovipositor sheaths (which we could not see in the holotype), as well as the short antennae, overall smooth and shiny body, head shape, and uniqueness of the host caterpillar. It is clear that this species does not belong to Apanteles and, after studying the holotype, we think the best generic placement for the species is in Dolichogenidea.
Dolichogenideaononidis (Marshall, 1889), lectotype designation
Apantelesononidis Marshall, 1889.
Type information. Lectotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Germany, United Kingdom.
Notes. The original description was based on female and male specimens. We have examined a female in the NHMUK with a type label and a code 3c.45, which we here designate as the lectotype. Marshall (1889) described the species from Gracillariaononidis (now Parechtopaononidis (Zeller, 1839) (Gracillariidae)), and Coleophorasalinella Stainton, 1859 (Coleophoridae). The lectotype lacks host data but agrees with specimens we examined in the RSME which were reared in UK from P.ononidis.
Dolichogenideaopacifinis Liu & Chen, 2019
Dolichogenideaopacifinis Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (HB, ZJ).
Dolichogenideaovata Liu & Chen, 2019
Dolichogenideaovata Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Dolichogenideapallidalata (Tobias, 1964)
Apantelespallidalatus Tobias, 1964.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Kazakhstan.
Geographical distribution.PAL.
PAL: Kazakhstan, Russia (S), Ukraine.
Notes. Our species concept is based on Papp (1981) and Tobias (1986).
Dolichogenideapalpator (Tobias, 1960)
Apantelespalpator Tobias, 1960.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Tajikistan.
Geographical distribution.PAL.
PAL: Tajikistan.
Notes. Our species concept is based on Papp (1978a) and Tobias (1986).
Dolichogenideaparacostulae Liu & Chen, 2018
Dolichogenideaparacostulae Liu & Chen, 2018.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (ZJ).
Dolichogenideaparalechiae (Muesebeck, 1932)
Apantelesparalechiae Muesebeck, 1932.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (NB, ON, QC), USA (CA, MA, MI, NH, NY, OH, PA, TN, WI).
Dolichogenideaparallelis (Ashmead, 1900), new combination
Protapantelesparallelis Ashmead, 1900.
Type information. Holotype female, NHMUK (examined). Country of type locality: Saint Vincent.
Geographical distribution.NEO.
NEO: Saint Vincent.
Notes. The information about this species has been very limited. For his revision of Nearctic Apanteles, Muesebeck could not examine the only known specimen (the holotype being in London) and instead had to key it out based just on the original description (Muesebeck 1921: 491 and especially 523, see also Ashmead 1900c: 281). The only other source of information for the species is Shenefelt (1972: 595), who just lists the previous two references. Taxapad (Yu et al. 2012, 2016) refers to this species as Cotesiaparallelis, probably because most of the Apanteles in the part of the Muesebeck key where parallelis is placed are currently considered to belong to Cotesia. We have examined the female holotype (NHMUK) and conclude that it belongs to Dolichogenidea, as it has a fully setose hind wing vannal lobe. The species has a propodeum that is relatively smooth, but with a short carina near the nucha which partially defines an areola posteriorly. T1 and T2 are relatively smooth but T1 has some shallow longitudinal striations near the lateral margins. The anteromesoscutum and scutellar disc are mostly shiny and smooth (sparse, very shallow punctures on the anteromesoscutum). The ovipositor sheaths are slightly longer than the metatibia length. The antennae (except for scapes) are missing in the holotype. The overall colouration is about as described by Ahsmead (1900).
Dolichogenideaparallodorsum Liu & Chen, 2019
Dolichogenideaparallodorsum Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, GD, ZJ).
Dolichogenideaparametacarp Liu & Chen, 2018
Dolichogenideaparametacarp Liu & Chen, 2018.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (FJ, GD, HI, HN, YN, ZJ); PAL: China (HL, JL, LN).
Dolichogenideaparanthrenea (You & Dang, 1987)
Apantelesparanthreneus You & Dang, 1987.
Type information. Holotype female, HUNAU (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (FJ), PAL: China (SN).
Notes. Our species concept is based on Chen and Song (2004).
Dolichogenideaparasae (Rohwer, 1922)
Apantelesparasae Rohwer, 1922.
Urogasterphilippinensis Ashmead, 1904 [primary junior homonym of Apantelesphilippinensis Ashmead, 1904].
Type information. Holotype female, USNM (examined). Country of type locality: Indonesia.
Geographical distribution.OTL.
OTL: China (GD, HI, HN, JX, TW, ZJ), India, Indonesia, Malaysia, Philippines, Sri Lanka, Thailand.
Dolichogenideapartergita Liu & Chen, 2018
Dolichogenideapartergita Liu & Chen, 2018.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (GD, GZ, HI, TW, YN, ZJ); PAL: China (JL, LN, SD).
Dolichogenideapelopea (Nixon, 1973), new combination
Apantelespelopea Nixon, 1973.
Type information. Holotype female, NHMUK (examined). Country of type locality: Italy.
Geographical distribution.PAL.
PAL: China (NM), Italy, Mongolia.
Notes. Transferred to Dolichogenidea based on the hind wing vannal lobe being entirely setose, and the anteromesoscutum with punctures not fusing near the scutoscutellar sulcus. Liu et al. (2014) had already recognized the entirely setose vannal lobe, but the species had never been transferred to Dolichogenidea until now.
Dolichogenideapelops (de Saeger, 1944), new combination
Apantelespelops de Saeger, 1944.
Apantelespelopsbambeyduplus Shenefelt, 1972 [new name for Apantelespelopsbambeyi Risbec, 1951, a homonym of Apantelesbambeyi Risbec, 1951].
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Rwanda, Senegal.
Notes. Here transferred to Dolichogenidea based on the anteromesoscutum punctures not fusing near the scutoscutellar sulcus, as well as the shape of the hypopygium and the length and shape of the ovipositor sheaths. Also, the original description compares this species as close to Apantelescaniae Wilkinson (placed in Dolichogenidea by Chen and Song 2004), and Apanteleswittei de Saeger (similarly placed in Dolichogenidea by us, see below under that species).
Dolichogenideapentgona Liu & Chen, 2019
Dolichogenideapentgona Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (HI).
Dolichogenideapetrovae (Walley, 1937)
Apantelespetrovae Walley, 1937.
? Apantelesdioryctriae Wilkinson, 1938.
? Apantelesmagnus Telenga, 1955.
Type information. Holotype female, CNC (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (AB, BC, MB, NB, NL, ON, QC, SK), USA (CA, CO, MI, MN, SC, WI)
Notes. This species has been variously treated as Apanteles or Dolichogenidea (Mason 1974 & 1981, Papp 1988, Whitfield 1995a, van Achterberg 2003, Fernandez-Triana 2010, Shaw 2012b). Fernandez-Triana (2010) mentioned that the morphological and molecular evidence was controversial (mostly pointing towards the species belonging to Dolichogenidea); however, he decided to maintain the species within Apanteles based on the examination of the holotype vannal lobe. After re-examination of the available evidence, including holotype, paratypes and other specimens, as well as DNA barcodes, we now consider that the best generic placement would be in Dolichogenidea (see more details and comments under D.murinanae above; both species could be considered borderline between Apanteles and Dolichogenidea).
Dolichogenideaphaenna (Nixon, 1965), new combination
Apantelesphaenna Nixon, 1965.
Type information. Holotype female, AEIC (not examined but paratype checked). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Notes. In the CNC there is a female paratype and two male specimens identified as this species. They are all from the same locality as the holotype and were all collected on the same date (May 11, 1954). All three CNC specimens have the vannal lobe slightly convex and uniformly setose, which indicates the species belongs to Dolichogenidea. The paratype has a blue label indicating its status and another label with the species identification (made by Nixon himself). The two male specimens were identified by Mason and indeed seem to be the same species as the paratype female (although there are slight differences in colour of legs and apical sculpture of T1, but those differences are rather normal between male and female specimens in Microgastrinae). The three specimens differ from the original description of A.phaenna as the wings are not that dark; rather, they look very slightly infumate.
Dolichogenideaphaloniae (Wilkinson, 1940)
Apantelesphaloniae Wilkinson, 1940.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Azerbaijan, Finland, Georgia, Germany, Hungary, Ireland, Israel, Italy, Lithuania, Madeira Islands, Moldova, Poland, Romania, Russia (KDA, MOS), Slovakia, United Kingdom.
Notes. The species distribution in Azerbaijan is based on Belokobylskij et al. (2019).
Dolichogenideaphaola (Nixon, 1972)
Apantelesphaola Nixon, 1972.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.OTL, PAL.
OTL: China (FJ, HB); PAL: Hungary, Russia (PR), Sweden, United Kingdom.
Notes. The holotype is missing the metasoma, one antenna and one hind leg.
Dolichogenideaphthorimaeae (Muesebeck, 1921)
Apantelesphthorimaeae Muesebeck, 1921.
Type information. Holotype female, USNM (not examined but subsequent treatment of the species checked). Country of type locality: USA.
Geographical distribution.NEA, NEO.
NEA: Canada (ON), USA (FL, LA); NEO: Honduras.
Notes. This species was transferred from Apanteles to Dolichogenidea by Mason (1981), also followed by other authors (e.g., Whitfield 1995a). However, You et al. (2002b) referred to it as Alphomelon in a phylogenetic analysis of the subfamily. Fernandez-Triana (2010) transferred the species back to Dolichogenidea, but he did not state that would represent a revised combination. For the sake of clarity, here we revise the combination of phthorimaeae and retain it within Dolichogenidea.
Dolichogenideapiliventris (Tobias, 1966)
Apantelespiliventris Tobias, 1966.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Turkmenistan.
Geographical distribution.PAL.
PAL: Turkmenistan.
Notes. Our species concept is based on Tobias (1986) and Papp (1988).
Dolichogenideapisenor (Nixon, 1965), new combination
Apantelespisenor Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Vanuatu.
Geographical distribution.AUS, OTL.
AUS: Vanuatu; OTL: Vietnam.
Notes. Based on the hind wing vannal lobe entirely setose, and anteromesoscutum with relatively coarse and deep punctures that do not fuse near the scutoscutellar disc, this species clearly belongs to Dolichogenidea.
Dolichogenideaplatyedrae (Wilkinson, 1928)
Apantelesplatyedrae Wilkinson, 1928.
Type information. Holotype female, NHMUK (examined). Country of type locality: Fiji.
Geographical distribution.AUS, OTL.
AUS: Fiji; OTL: Vietnam.
Notes. The holotype is missing its antennae, but otherwise is in good condition.
Dolichogenideapolaszeki Walker, 1994
Dolichogenideapolaszeki Walker, 1994.
Type information. Holotype female, NHMUK (examined). Country of type locality: Nigeria.
Geographical distribution.AFR.
AFR: Benin, Cameroon, Ethiopia, Ghana, Kenya, Malawi, Mozambique, Nigeria, Tanzania, Uganda, Zambia.
Dolichogenideapoliobrevis Liu & Chen, 2018
Dolichogenideapoliobrevis Liu & Chen, 2018.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (FJ, ZJ); PAL: China (HL, LN, XJ, XZ).
Dolichogenideapolitiventris (Muesebeck, 1958)
Apantelespolitiventris Muesebeck, 1958.
Type information. Holotype female, USNM (examined). Country of type locality: Puerto Rico.
Geographical distribution.NEO.
NEO: Puerto Rico.
Dolichogenideapolystinelliphagous Liu & Chen, 2018
Dolichogenideapolystinelliphagous Liu & Chen, 2018.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (JL, LN, SN, SX).
Dolichogenideapraetor (Marshall, 1885)
Apantelespraetor Marshall, 1885.
Type information. Lectotype male, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Armenia, Finland, France, Hungary, Mongolia, Romania, Russia (YAR), Slovakia, Sweden, Switzerland, United Kingdom.
Dolichogenideapraetoria (Tobias, 1976)
Apantelespraetorius Tobias, 1976.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (KDA).
Notes.Papp (1978a, 1988; also followed by Yu et al. 2016) considered Apantelespraetorius Tobias, 1976 to be a junior synonym of Apantelespropinquus Papp, 1975. However, Tobias (1986: 769) stated that both species were clearly different, based on examination of the praetorius holotype – whereas Papp (1978: 293) had only examined a paratype of that species. Tobias species concepts were followed by Belokobylskij et al. (2019) and are also accepted by us here, thus we treat both species as distinct in our checklist.
Dolichogenideaprinceps (Wilkinson, 1941)
Apantelesprinceps Wilkinson, 1941.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Azerbaijan, Hungary, Italy, Korea, Malta, Mongolia, Romania, Russia (PRI), Serbia, Slovakia, Spain, Tunisia, Turkey, Ukraine, United Kingdom.
Notes. The species distribution in Azerbaijan and Mongolia are based on Belokobylskij et al. (2019).
Dolichogenideaprisca (Nixon, 1967)
Apantelespriscus Nixon, 1967.
Dolichogenideaacutituba Song, Chen & Yang, 2006.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL, PAL.
OTL: China (FJ, GD, GX, GZ, HN, SC, YN, ZJ), India, Malaysia, Sri Lanka, Vietnam; PAL: China (HA, SH).
Notes. We follow Liu et al. (2019) for the synonymy of acutituba under prisca, and also for additional distribution of the species in China.
Dolichogenideaprobata (Papp, 1973)
Apantelesprobatus Papp, 1973.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Hungary.
Geographical distribution.PAL.
PAL: Hungary.
Notes. The species name must be treated as an adjective and not as a noun (Doug Yanega, pers. comm.) and thus it must match the gender of the genus name.
Dolichogenideaprodeniae (Viereck, 1912)
Apantelesprodeniae Viereck, 1912.
Type information. Holotype male, USNM (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: China (GX), India, Thailand, Vietnam.
Notes. The USNM collection contains nine female and five male specimens. One of the male specimens is labeled as the type, whereas the other 13 specimens each have labels stating that they all are paratypes. All of those 14 specimens have the same USNM code (14310), and they seem to come from the same collecting event (as all have the same labels, reared from Spodopteralittoralis). Shenefelt’s (1972) catalogue also confirms that there is a male holotype. It is unfortunate that a male was chosen as the species name bearer. In the future it would be advisable to photograph and provide details of the female specimens, which should better characterize the species as compared to the chosen male.
Dolichogenideapropinqua (Papp, 1975)
Apantelespropinquus Papp, 1975.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Hungary.
Geographical distribution.PAL.
PAL: Greece, Hungary, Madeira Islands, Netherlands, Poland, Switzerland.
Notes. See notes under Dolichogenideapraetoria (Tobias, 1976) for an explanation on why we consider these two species to be different.
Dolichogenideapterophori (Muesebeck, 1926)
Apantelespterophori Muesebeck, 1926.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (MA).
Dolichogenideapulchra (Telenga, 1955)
Apantelespulcher Telenga, 1955.
Type information. Type unknown, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Kazakhstan.
Geographical distribution.PAL.
PAL: Kazakhstan.
Notes. Details on a type specimen or specimens are not provided in the original description (Tobias 1986). However female and male characters are used in two couplets of the key (where the species is described), and thus it is reasonable to assume that the author studied both sexes for the species description. Additionally, in the introductory sections of that book (Tobias 1986: foreword) the intention to detail holotypes for all new species (and to designate lectotypes/paralectotypes from species previously described from the former USSR) is clearly stated, so we can also assume that a holotype for D.pulchra was designated, even if not clearly stated in the actual description. It is likely that the holotype is a female specimen, but until the specimens are examined is not possible to confirm.
Dolichogenideapunctiger (Wesmael, 1837)
Microgasterpunctiger Wesmael, 1837.
Apantelesitea Nixon, 1972.
Type information. Holotype female, RBINS (not examined but authoritatively identified specimens examined). Country of type locality: Belgium.
Geographical distribution.PAL.
PAL: Belgium, Croatia, Czech Republic, Denmark, France, Germany, Hungary, Ireland, Italy, Netherlands, Poland, Russia (BEL, RYA), Slovakia, Sweden, Switzerland, Ukraine, United Kingdom.
Notes. We examined the type of Apantelesitea Nixon.
Dolichogenideapunctipila Liu & Chen, 2019
Dolichogenideapunctipila Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, GD, GZ, ZJ).
Dolichogenideapurdus (Papp, 1977)
Apantelespurdus Papp, 1977.
Type information. Holotype female, HNHM (not examined but subsequent treatment of the species checked). Country of type locality: Hungary.
Geographical distribution.OTL, PAL.
OTL: China (HB); PAL: China (JL, SN), Hungary, Turkey.
Notes. Our species concept is based on Papp (1979a, 1988). Yu et al. (2016) treated this species as Dolichogenideapurda. However, the original description did not give an etymology and it is neither Latin nor Greek, so following ICZN Article 31.2.3, it must be treated as a noun in apposition and the original spelling purdus is retained.
Dolichogenidearectivena Liu & Chen, 2019
Dolichogenidearectivena Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (ZJ).
Dolichogenideareicharti (Papp, 1974)
Apantelesreicharti Papp, 1974.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Hungary.
Geographical distribution.PAL.
PAL: Hungary.
Dolichogenidearenata (Kotenko, 1986)
Apantelesrenatus Kotenko, 1986.
Type information. Holotype female, SIZK (not examined but original description checked). Country of type locality: Tajikistan.
Geographical distribution.PAL.
PAL: Mongolia, Tajikistan.
Dolichogenidearenaulti (Mason, 1974)
Apantelesrenaulti Mason, 1974.
Type information. Holotype female, CNC (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (NB, NS, ON, QC).
Dolichogenidearoepkei (Shenefelt, 1972), new combination
Apantelesroepkei Shenefelt, 1972.
Apantelesthoseae Roepke, 1935 [homonym of Apantelesthoseae Wilkinson, 1934].
Type information. Syntypes female and male, depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Indonesia.
Geographical distribution.OTL.
OTL: Indonesia.
Notes. Our species concept is based on Austin (1987), who recognized the species belonged to Dolichogenidea (sensuMason 1981) but stopped short of transferring the species. For the sake of clarity, we do that here.
Dolichogenidearogerblancoi Fernandez-Triana & Boudreault, 2019
Dolichogenidearogerblancoi Fernandez-Triana & Boudreault, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Dolichogenidearufescentis Chen & Song, 2004
Dolichogenidearufescentis Chen & Song, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (JL).
Dolichogenideasagus (Kotenko, 1986)
Apantelessagus Kotenko, 1986.
Type information. Holotype female, ZIN (not examined but original description checked). Country of type locality: Turkmenistan.
Geographical distribution.PAL.
PAL: Turkmenistan.
Dolichogenideasandwico Liu & Chen, 2018
Dolichogenideasandwico Liu & Chen, 2018.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (FJ, GD, HN, SC, ZJ); PAL: China (NX).
Dolichogenideascabra (Tobias, 1977), new combination
Apantelesscaber Tobias, 1977.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (PRI).
Notes. Our species concept is based on Papp (1978a). Based on his description of the sculpture and shape of T2–T3, this species is unique among Holarctic species of Dolichogenidea. The species name must be treated as an adjective and not as a noun (Doug Yanega, pers. comm.) and thus it must match the gender of the genus name.
Dolichogenideascabipuncta Chen & Song, 2004
Dolichogenideascabipuncta Chen & Song, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (FJ, JX, TW); PAL: China (JL).
Dolichogenideaseriphia (Nixon, 1972)
Apantelesseriphia Nixon, 1972.
Type information. Holotype female, ZSM (not examined but original description checked). Country of type locality: Italy.
Geographical distribution.PAL.
PAL: Greece, Hungary, Iran, Italy, Montenegro, Poland, Russia (S), Slovakia, Spain, Tunisia, Turkey.
Notes. The species distribution in Iran and Russia are based on Belokobylskij et al. (2019).
Dolichogenideasicaria (Marshall, 1885)
Apantelessicarius Marshall, 1885.
Apanteleschrysostictus Marshall, 1889.
Apantelescrudelis Papp, 1971.
Type information. Lectotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.AUS, NEA, OTL, PAL.
AUS: New Zealand; NEA: Canada (NU), Greenland; OTL: China (SC, ZJ); PAL: Azerbaijan, Belarus, China (HE, LN, NM, SN, XJ, XZ, ZJ), Czech Republic, Estonia, Finland, France, Georgia, Germany, Greece, Hungary, Iran, Kazakhstan, Kyrgyzstan, Macedonia, Moldova, Mongolia, Montenegro, Morocco, Netherlands, Poland, Romania, Russia (AMU, ZAB, KAM, KHA, MOS, OMS, PRI, YAR), Serbia, Slovakia, Spain, Switzerland, Tunisia, Turkey, Ukraine, United Kingdom.
Notes. The species distribution in Iran and Kyrgyzstan are based on Belokobylskij et al. (2019).
Dolichogenideasimulata (Papp, 1974)
Apantelessimulatus Papp, 1974.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Korea.
Geographical distribution.PAL.
PAL: Korea, Russia (PRI).
Dolichogenideasingularis Yang & You, 2002
Dolichogenideasingularis Yang & You, 2002.
Type information. Holotype female, CFRB (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (ZJ); PAL: China (HE, SD, SN, TJ).
Dolichogenideasisenna (Nixon, 1972)
Apantelessisenna Nixon, 1972.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: United Kingdom.
Dolichogenideasoikai (Nixon, 1972)
Apantelessoikai Nixon, 1972.
Type information. Holotype female, NHMUK (examined). Country of type locality: Italy.
Geographical distribution.PAL.
PAL: Bulgaria, Greece, Hungary, Italy, Russia (S), Switzerland, Tunisia, Turkey, United Kingdom.
Dolichogenideasolenobiae (Walley, 1935)
Apantelessolenobiae Walley, 1935.
Type information. Holotype female, CNC (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (ON, QC), USA (PA).
Dolichogenideasonani (Watanabe, 1932)
Apantelessonani Watanabe, 1932.
Type information. Holotype female, EIHU (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (GD, GX, GZ, SC, TW, ZJ).
Notes. Our species concept is based on Watanabe (1937a), Song and Chen (2004) and Liu et al. (2019).
Dolichogenideasophiae (Papp, 1972)
Apantelessophiae Papp, 1972.
Type information. Holotype female, HNHM (not examined but subsequent treatment of the species checked). Country of type locality: Hungary.
Geographical distribution.OTL, PAL.
OTL: China (ZJ); PAL: Armenia, Georgia, Hungary, Moldova, Russia (ZAB), Slovakia, Turkey, Ukraine.
Notes. Our species concept is based on Papp (1972, 1978).
Dolichogenideaspanis Chen & Song, 2004
Dolichogenideaspanis Chen & Song, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, HB).
Dolichogenideaspinulicula Liu & Chen, 2018
Dolichogenideaspinulicula Liu & Chen, 2018.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (GZ, ZJ); PAL: China (LN, NX).
Dolichogenideastantoni (Ashmead, 1904)
Urogasterstantoni Ashmead, 1904.
Apantelesfistulae Wilkinson, 1928.
Type information. Holotype female, USNM (not examined but authoritatively identified specimens examined). Country of type locality: Philippines.
Geographical distribution.AUS, OTL.
AUS: Fiji, Papua New Guinea; OTL: China (FJ, GD, GX, GZ, TW, ZJ), India, Malaysia, Philippines, Vietnam.
Notes. We examined the type of A.fistulae Wilkinson, which is missing the head and metasoma.
Dolichogenideastatius (Nixon, 1965), new combination
Apantelesstatius Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Notes. We transfer this species to Dolichogenidea based on its uniformly setose vannal lobe and anteromesoscutum with punctures that do not fuse near the scutoscutellar sulcus.
Dolichogenideastenosis Song & Chen, 2004
Dolichogenideastenosis Song & Chen, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Dolichogenideastenotelas (Nixon, 1965), new combination
Apantelesstenotelas Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Vanuatu.
Geographical distribution.AUS, OTL.
AUS: Vanuatu, OTL: Vietnam.
Notes. After examining the holotype, we believe the best generic placement for this species is in Dolichogenidea, based on the relatively coarse punctures on the anteromesoscutum (which do not fuse near the scutoscutellar sulcus), and the entirely setose vannal lobe in the hind wing (although setae are rather small and a magnification more than 100 × is recommended to see the setation pattern).
Dolichogenideastictoscutella Liu & Chen, 2018
Dolichogenideastictoscutella Liu & Chen, 2018.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (GD, ZJ).
Dolichogenideastriata (van Achterberg & Ng, 2009), new combination
Apantelesstriatus van Achterberg & Ng, 2009.
Type information. Holotype female, UKM (not examined but original description checked). Country of type locality: Malaysia.
Geographical distribution.OTL.
OTL: Malaysia.
Notes. A drawing from the original description clearly shows a setose vannal lobe in the hind wing, and thus the species is here transferred to Dolichogenidea. The species name must be treated as an adjective and not as a noun (Doug Yanega, pers. comm.) and thus it must match the gender of the genus name.
Dolichogenideasubemarginata (Abdinbekova, 1969)
Apantelessubemarginatus Abdinbekova, 1969.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Azerbaijan.
Geographical distribution.PAL.
PAL: Armenia, Azerbaijan, Hungary, Turkey.
Notes. Our species concept is based on Papp (1980a) and Tobias (1986).
Dolichogenideasubgentilis (Tobias & Long, 1990)
Apantelessubgentilis Tobias & Long, 1990.
Type information. Holotype female, ZIN (not examined but original description checked). Country of type locality: Vietnam.
Geographical distribution.OTL.
OTL: Vietnam.
Dolichogenideasublabene (Tobias & Long, 1990)
Apantelessublabene Tobias & Long, 1990.
Type information. Holotype female, ZIN (not examined but original description checked). Country of type locality: Vietnam.
Geographical distribution.OTL.
OTL: Vietnam.
Dolichogenideasugae (Watanabe, 1932)
Apantelessugae Watanabe, 1932.
Type information. Holotype female, EIHU (examined). Country of type locality: Japan.
Geographical distribution.PAL.
PAL: Japan, Korea.
Notes. The sex of the holotype had never been detailed before (e.g., Shenefelt 1972), and thus it is clarified here. The female holotype is missing the metasoma, head and three legs. But other female and one male specimens (plus a pin with a lepidopteran larva and wasp cocoon mass) are associated with the holotype and help to recognize the species. The entirely setose and slightly convex vannal lobe indicates this species belongs to Dolichogenidea. The transfer of the species to Dolichogenidea was proposed by Liu et al. (2018) in the abstract but those authors did not provide further details nor explanation in their paper. For the sake of clarity, the species combination is revised here.
Dolichogenideasyngramma Ahmad, 2019
Dolichogenideasyngramma Ahmad & Pandey, 2019.
Type information. Holotype female, AMUZ (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. The authorship of the species names was not made clear in the original description paper, thus here we follow Ahmad (pers. comm.) for that information.
Dolichogenideaszalayi (Papp, 1977)
Apantelesszalayi Papp, 1977.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Hungary.
Geographical distribution.PAL.
PAL: Hungary.
Dolichogenideaszelenyii (Papp, 1972)
Apantelesszelenyii Papp, 1972.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Hungary.
Geographical distribution.PAL.
PAL: Hungary.
Dolichogenideataiwanensis (Sonan, 1942)
Apantelestaiwanensis Sonan, 1942.
Type information. Syntypes female and male, TARI (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (HI, TW).
Notes. Our species concept is based on Chen and Song (2004). Type information from Shenefelt (1972), depository information from Yu et al. (2016).
Dolichogenideatasmanica (Cameron, 1912)
Apantelestasmanica Cameron, 1912.
Type information. Syntypes female and male, NHMUK (examined). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (ACT, QLD, SA, TAS, VIC), New Zealand.
Notes.Yu et al. (2016) recorded the type as being female; however, we have examined one female and one male specimens, which both have a type label, and are thus to be considered as syntypes, as correctly stated by Shenefelt (1972: 648).
Dolichogenideatestacea Liu & Chen, 2018
Dolichogenideatestacea Liu & Chen, 2018.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (ZJ).
Dolichogenideathujae (Muesebeck, 1935)
Apantelesthujae Muesebeck, 1935.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (ON, QC).
Notes. The holotype is missing the head but it is otherwise in good condition.
Dolichogenideatischeriae Viereck, 1912
Dolichogenideatischeriae Viereck, 1912.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (QC), USA (CA, CT, DE, DC, KS, MO, NY, OH, WI).
Dolichogenideatobiasi (Balevski, 1980)
Apantelestobiasi Balevski, 1980.
Type information. Holotype female, ZIN (examined). Country of type locality: Bulgaria.
Geographical distribution.PAL.
PAL: Bulgaria, Russia (S), Ukraine.
Notes. Our species concept is based on Tobias (1986) and Papp (1988).
Dolichogenideatrachalus (Nixon, 1965)
Apantelestrachalus Nixon, 1965.
Apantelessevocatus Papp, 1975.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Hungary, Ireland, Syria, United Kingdom.
Dolichogenideatranscarinata Liu & Chen, 2019
Dolichogenideatranscarinata Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (ZJ).
Dolichogenideatuliemensis (Tobias & Long, 1990)
Apantelestuliemensis Tobias & Long, 1990.
Type information. Holotype female, ZIN (not examined but original description checked). Country of type locality: Vietnam.
Geographical distribution.OTL.
OTL: Vietnam.
Dolichogenideaturcmenica (Tobias, 1967)
Apantelesturcmenicus Tobias, 1967.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Turkmenistan.
Geographical distribution.PAL.
PAL: Mongolia, Turkmenistan, Uzbekistan.
Notes. Our species concept is based on Tobias (1986) and Papp (1978a, 1988).
Dolichogenideaturionellae (Nixon, 1971)
Apantelesturionellae Nixon, 1971.
Type information. Holotype female, NHMUK (examined). Country of type locality: Austria.
Geographical distribution.PAL.
PAL: Austria, Ukraine.
Dolichogenideaturkmenus (Telenga, 1955)
Apantelesturkmenus Telenga, 1955.
Type information. Syntypes female and male, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Turkmenistan.
Geographical distribution.PAL.
PAL: Armenia, China (XJ), Jordan, Kazakhstan, Turkey, Turkmenistan, Uzbekistan.
Notes. Our species concept is based on Telenga (1955), Tobias (1986) and Papp (1978a, 1988). Type information from Shenefelt (1972), depository information from Tobias (1986). Following Article 31.2.3 of the ICZN the name is neither Latin nor Greek and must be treated as a noun in apposition, so the original spelling turkmenus is retained (the suffix -us is not definitively adjectival, unlike -icus).
Dolichogenideaultima (Kotenko, 1986)
Apantelesultimus Kotenko, 1986.
Type information. Holotype female, SIZK (not examined but original description checked). Country of type locality: Ukraine.
Geographical distribution.PAL.
PAL: China (JL), Russia (S), Ukraine.
Dolichogenideaultor (Reinhard, 1880)
Apantelesultor Reinhard, 1880.
Microgasterlactipennis Ratzeburg, 1852 [primary homonym of Microgasterlacteipennis Curtis, 1830].
Type information. Lectotype female, depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Azerbaijan, Czech Republic, Georgia, Germany, Hungary, Italy, Poland, Romania, Russia (IN, DA, KDA, STA), Serbia, Slovakia, Slovenia, Switzerland, Ukraine, United Kingdom.
Notes. Our species concept is based on Wilkinson (1945), Nixon (1976), and Papp (1981). The type series was deposited in the Forestry College of Eberswalde (Forstlichen Hochschule Eberswalde). Unfortunately, that collection was mostly destroyed during the Second World War; however, five drawers with Hymenoptera specimens, among them type species of Ratzeburg were spared and are now safe at the Senckenberg Deutsches Entomologisches Institut (SDEI) in Müncheberg, Germany [See a detailed story of that in Schulz et al. (2018: 285-286)]. We do not know whether the lectotype of this species is at present in Müncheberg. The species distribution in Azerbaijan is based on Belokobylskij et al. (2019).
Dolichogenideaunicarina Liu & Chen, 2018
Dolichogenideaunicarina Liu & Chen, 2018.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (GZ, ZJ); PAL: China (NX, SN, SD).
Dolichogenideaupoluensis (Fullaway, 1941)
Apantelesupoluensis Fullaway, 1941.
Type information. Holotype male, BPBM (not examined but subsequent treatment of the species checked). Country of type locality: Western Samoa.
Geographical distribution.AUS.
AUS: Western Samoa.
Notes. Our species concept is based on Austin and Dangerfield (1992).
Dolichogenideauru Rousse & Gupta, 2013
Dolichogenideauru Rousse & Gupta, 2013.
Type information. Holotype female, MNHN (not examined but original description checked). Country of type locality: Réunion.
Geographical distribution.AFR.
AFR: Réunion.
Dolichogenideavadosulcus Liu & Chen, 2019
Dolichogenideavadosulcus Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, GD, GX, HI, ZJ).
Dolichogenideavarifemur (Abdinbekova, 1969)
Apantelesvarifemur Abdinbekova, 1969.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Azerbaijan.
Geographical distribution.PAL.
PAL: Azerbaijan, Lithuania, Russia (NC).
Notes. Our species concept is based on Papp (1978a) and Tobias (1986).
Dolichogenideavernaliter (Wilkinson, 1932)
Apantelesvernaliter Wilkinson, 1932.
Type information. Holotype female, NHMUK (examined). Country of type locality: Indonesia.
Geographical distribution.OTL.
AUS: Vanuatu; OTL: Indonesia, Vietnam.
Dolichogenideavictor (Wilkinson, 1941)
Apantelesvictor Wilkinson, 1941.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: United Kingdom.
Dolichogenideavictoria Liu & Chen, 2019
Dolichogenideavictoria Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, GD, GX, ZJ).
Dolichogenideavictoriae (Muesebeck, 1921)
Apantelesvictoriae Muesebeck, 1921.
Type information. Holotype female, USNM (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (BC).
Notes. The only know specimen is the holotype, which is in good condition, except for one fore wing being detached from the body but glued to the same point.
Dolichogenideavictoriata (Kotenko, 1986)
Apantelesvictoriatus Kotenko, 1986.
Type information. Holotype female, SIZK (not examined but original description checked). Country of type locality: Ukraine.
Geographical distribution.PAL.
PAL: Mongolia, Russia (S), Ukraine.
Dolichogenideavillemantae Rousse, 2013
Dolichogenideavillemantae Rousse, 2013.
Type information. Holotype female, MNHN (not examined but original description checked). Country of type locality: Réunion.
Geographical distribution.AFR.
AFR: Réunion.
Dolichogenideawangi Liu & Chen, 2019
Dolichogenideawangi Liu & Chen, 2019.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (YN).
Dolichogenideawittei (de Saeger, 1944), new combination
Apanteleswittei de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Rwanda.
Notes. Here transferred to Dolichogenidea based on the anteromesoscutum punctures not fusing near scutoscutellar sulcus, as well as the shape of the hypopygium and the length and shape of the ovipositor sheaths. Additionally, the original description compares this species as close to Apantelesbaoris Wilkinson (placed in Dolichogenidea by Chen and Song 2004, among other authors), as well as Apantelesearterus Wilkinson and Apantelespelops de Saeger (both species similarly placed in Dolichogenidea by us, see more details under those two species above).
Dolichogenideaxenomorph Fagan-Jeffries & Austin, 2018
Dolichogenideaxenomorph Fagan-Jeffries & Austin, 2018.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (NSW, WA).
Dolichogenideayamini Sathe & Rokade, 2005
Dolichogenideayamini Sathe & Rokade, 2005.
Type information. Holotype female, depository unknown (not examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. This species name may not be valid as we suspect that no type depository was specified. However, because we could not check original description to confirm that, we retain it as valid species for the time being.
Dolichogenideayeimycedenoae Fernandez-Triana & Boudreault, 2019
Dolichogenideayeimycedenoae Fernandez-Triana & Boudreault, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Dolichogenideazerafai Papp, 2015
Dolichogenideazerafai Papp, 2015.
Type information. Holotype female, RSME (examined). Country of type locality: Malta.
Geographical distribution.PAL.
PAL: Malta.
Dolichogenideazeris Papp, 2012
Dolichogenideazeris Papp, 2012.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Cape Verde.
Geographical distribution.AFR.
AFR: Cape Verde.
Genus Eripnopelta Xiong, van Achterberg & Chen, 2017
Eripnopelta Xiong, van Achterberg & Chen, 2017: 392. Gender: feminine. Type species: Eripnopeltaithyvena Xiong, van Achterberg and Chen 2017, by original designation.
The only known species was recently described from the Oriental region (Xiong et al. 2017). No host data are currently available for this genus. There are no DNA barcodes of Eripnopelta in BOLD.
Eripnopeltaithyvena Xiong, van Achterberg & Chen, 2017
Eripnopeltaithyvena Xiong, van Achterberg & Chen, 2017.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (ZJ, NX, GZ).
Genus Exix Mason, 1981
Exix Mason, 1981: 116. Gender: feminine. Type species: Exixmexicana Mason, 1981, by original designation.
This is a New World genus, with seven species currently described from the Nearctic and Neotropical regions and revised by Mason (1981). A few species may remain undescribed, but the genus does not seem very speciose. No host data are currently available. There are no DNA-barcode compliant sequences of this genus in BOLD, but four specimens have mini-barcodes of 110–120 bp.
Exixbahia Mason, 1981
Exixbahia Mason, 1981.
Type information. Holotype female, CNC (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (BA).
Exixcolorados Mason, 1981
Exixcolorados Mason, 1981.
Type information. Holotype female, CNC (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Exixcolumbica Mason, 1981
Exixcolumbica Mason, 1981.
Type information. Holotype female, CNC (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (BC).
Exixitatiaia Souza-Gessner, Bortoni & Penteado-Dias, 2016
Exixitatiaia Souza-Gessner, Bortoni & Penteado-Dias, 2016.
Type information. Holotype female, DCBU (not examined but original description checked). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (MG, RJ).
Exixmexicana Mason, 1981
Exixmexicana Mason, 1981.
Type information. Holotype female, CNC (examined). Country of type locality: Mexico.
Geographical distribution.NEO.
NEO: Mexico.
Exixschunkei (Nixon, 1965)
Protomicroplitisschunkei Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Peru.
Geographical distribution.NEO.
NEO: Peru.
Exixtinalandica Mason, 1981
Exixtinalandica Mason, 1981.
Type information. Holotype female, CNC (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Genus Exoryza Mason, 1981
Exoryza Mason, 1981: 40. Gender: feminine. Type species: Apantelesschoenobii Wilkinson, 1932, by original designation.
Known from 15 described species from all biogeographical regions except for Australasian (the lack of species recorded from Australasian is likely an artefact due to insufficient collecting there). All known species were dealt with in a recent revision (Fernandez-Triana et al. 2016c). The status of Exoryza as a valid genus separate from Dolichogenidea has been questioned by many authors (e.g., Valerio et al. 2004, Rousse and Gupta 2013, Fernandez-Triana et al. 2014e, 2016c), but until a comprehensive phylogenetic study of Microgastrinae is available we have decided to maintain its present status. Host data include four families of Lepidoptera: Choreutidae, Crambidae, Depressariidae and Gelechiidae; and at least one species is an important biocontrol agent of stem-boring Lepidoptera in rice fields in Asia (Fernandez-Triana et al. 2016c). There are 46 DNA-barcode compliant sequences of Exoryza in BOLD representing three different BINs, although one of those BINs actually contains three nominal species (see Fernandez-Triana et al. 2016c for more details).
Exoryzaasotae (Watanabe, 1932), new combination
Apantelesasotae Watanabe, 1932.
Type information. Holotype female, EIHU (examined). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (FJ, GD, TW, ZJ); PAL: China (HL, SC), Japan.
Notes. After examining the holotype and two paratypes (female and male) we transfer asotae to Exoryza based on the entirely setose vannal lobe and T2 with strong longitudinal striae. The species distribution in China is based in Liu et al. (2019).
Exoryzabelippicola (Liu & You, 1988), new combination
Apantelesbelippicola Liu & You, 1988.
Type information. Holotype female, HUNAU (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (SN, ZJ).
Notes. Our species concept is based on Chen and Song (2004) and Liu et al. (2019). The species is transferred to Exoryza based on T1–T2 strongly rugose (cf. figure 11e in Liu et al. 2019).
Exoryzahylas (Wilkinson, 1932), new combination
Apanteleshylas Wilkinson, 1932.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Notes. Transferred to Exoryza based on the entirely setose vannal lobe and T2 with strong longitudinal striae.
Exoryzamariabustosae Fernandez-Triana, 2016
Exoryzamariabustosae Fernandez-Triana, 2016.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Exoryzamegagaster (de Saeger, 1944), new combination
Apantelesmegagaster de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Rwanda.
Notes. Based on the original description, the best generic placement would be in Exoryza, based on the shape and sculpture of T2.
Exoryzaminnesota Mason, 1981
Exoryzaminnesota Mason, 1981.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (ON), USA (MN).
Exoryzamonocavus Valerio & Whitfield, 2004
Exoryzamonocavus Valerio & Whitfield, 2004.
Type information. Holotype female, INBio (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Exoryzaoryzae (Walker, 1994), new combination
Dolichogenideaoryzae Walker, 1994.
Type information. Holotype female, NHMUK (examined). Country of type locality: Senegal.
Geographical distribution.AFR.
AFR: Gambia, Ivory Coast, Niger, Senegal.
Notes.Fernandez-Triana et al. (2016c) considered this species to belong to Exoryza, based on the available evidence (shape and sculpture of T2, as well as host data). However, they stopped short of transferring the species to that genus due to the possibility that future phylogenetic studies would find that Exoryza is just a synonym of Dolichogenidea. While that possibility still exists, in this paper we are considering Exoryza as a valid genus, and for the sake of consistency we are placing here all species which currently fit that genus concept.
Exoryzareticarina Song & Chen, 2003
Exoryzareticarina Song & Chen, 2003.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (YN).
Exoryzarichardashleyi Fernandez-Triana, 2016
Exoryzarichardashleyi Fernandez-Triana, 2016.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Exoryzaritaashleyae Fernandez-Triana, 2016
Exoryzaritaashleyae Fernandez-Triana, 2016.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Exoryzarosamatarritae Fernandez-Triana, 2016
Exoryzarosamatarritae Fernandez-Triana, 2016.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Exoryzasafranum Rousse & Gupta, 2013
Exoryzasafranum Rousse & Gupta, 2013.
Type information. Holotype female, MNHN (not examined but original description checked). Country of type locality: Réunion.
Geographical distribution.AFR.
AFR: Réunion.
Exoryzaschoenobii (Wilkinson, 1932)
Apantelesschoenobii Wilkinson, 1932.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: Bangladesh, China (FJ, GD, GX, GZ, HI, HB, HN, JS, JX, SN, TW, YN, ZJ), India, Malaysia, Philippines, Sri Lanka, Vietnam.
Exoryzayeimycedenoae Fernandez-Triana, 2016
Exoryzayeimycedenoae Fernandez-Triana, 2016.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Genus Exulonyx Mason, 1981
Exulonyx Mason, 1981: 33. Gender: masculine. Type species: Apantelescamma Nixon, 1965, by original designation.
Only known from a single, very divergent species from the Afrotropical region (Nixon 1965, Mason 1981). No host data are currently available for this genus. There are no DNA barcodes of Exulonyx in BOLD.
Exulonyxcamma (Nixon, 1965)
Apantelescamma Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Genus Fornicia Brullé, 1846
Fornicia Brullé, 1846: 511. Gender: feminine. Type species: Forniciaclathrata Brullé, 1846, by monotypy.
Odontofornicia Enderlein, 1912: 260. Type species: Odontoforniciaarata Enderlein, 1912, by monotypy and original designation.
This is a pantropical genus with 32 species recorded from all regions except for the Holarctic. It` is one of the most distinctive genera of Microgastrinae from a morphological perspective. We have seen in collections many more undescribed species. All known host records are from Limacodidae. There are 67 DNA-barcode compliant sequences of Fornicia in BOLD representing 19 different BINs.
Forniciaachterbergi Yang & Chen, 2006
Forniciaachterbergi Yang & Chen, 2006.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Forniciaafricana Wilkinson, 1930
Forniciaafricana Wilkinson, 1930.
Type information. Holotype female, NHMUK (examined). Country of type locality: Zimbabwe.
Geographical distribution.AFR.
AFR: Nigeria, Zimbabwe.
Forniciaafrorum de Saeger, 1942
Forniciaafrorum de Saeger, 1942.
Type information. Holotype female, RMCA (not examined but subsequent treatment of the species checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. Our species concept is based on de Saeger (1948).
Forniciaalbalata Ma & Chen, 1994
Forniciaalbalata Ma & Chen, 1994.
Type information. Holotype male, ZJUH (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (SN).
Notes. Our species concept is based on Chen and Song (2004).
Forniciaandamanensis Sharma, 1984
Forniciaandamanensis Sharma, 1984.
Type information. Holotype female, depository unknown (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Forniciaannulipes Ashmead, 1905
Forniciaannulipes Ashmead, 1905.
Type information. Holotype male, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Notes. Specimen with legs and one antenna broken but within the tray that contains the holotype.
Forniciaarata (Enderlein, 1912)
Odontoforniciaarata Enderlein, 1912.
Type information. Holotype female, depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (GZ, SN, TW, ZJ); PAL: China (AH).
Notes. Our species concept is based on Watanabe (1937a) and Mason (1981).
Forniciaballoui Muesebeck, 1958
Forniciaballoui Muesebeck, 1958.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: Venezuela.
Geographical distribution.NEO.
NEO: French Guiana, Suriname, Venezuela.
Forniciaborneana (Cushman, 1929)
Odontoforniciaborneanus Cushman, 1929.
Type information. Holotype female, USNM (not examined but illustrations of the holotype examined). Country of type locality: Malaysia.
Geographical distribution.OTL.
OTL: Malaysia.
Forniciabrachymetacarpa Luo & You, 2006
Forniciabrachymetacarpa Luo & You, 2006.
Type information. Holotype female, HUNAU (not examined). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (HN).
Forniciaceylonica Wilkinson, 1928
Forniciaceylonica Wilkinson, 1928.
Type information. Holotype female, NHMUK (examined). Country of type locality: Sri Lanka.
Geographical distribution.OTL.
OTL: China (TW), India, Indonesia, Philippines, Sri Lanka, Thailand.
Forniciachalcoscelidis Wilkinson, 1936
Forniciachalcoscelidis Wilkinson, 1936.
Type information. Holotype female, NHMUK (examined). Country of type locality: Malaysia.
Geographical distribution.OTL.
OTL: Indonesia, Malaysia.
Forniciaclathrata Brullé, 1846
Forniciaclathrata Brullé, 1846.
Type information. Type and depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Brazil.
Geographical distribution.NEO, OTL.
NEO: Brazil (BA, MG), Guyana, Peru, Venezuela; OTL: Indonesia.
Notes. Our species concept is based on Muesebeck (1958b) and Mason (1981).
Forniciaghesquierei de Saeger, 1942
Forniciaghesquierei de Saeger, 1942.
Type information. Holotype male, RMCA (not examined but subsequent treatment of the species checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. Our species concept is based on de Saeger (1948).
Forniciaimbecilla Chen & He, 1994
Forniciaimbecilla Chen & He, 1994.
Type information. Holotype female, ZJUH (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (ZJ).
Notes. Our species concept is based on Chen and Song (2004).
Forniciajarmilae Mason, 1981
Forniciajarmilae Mason, 1981.
Type information. Holotype female, CNC (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Fornicialongiantenna Luo & You, 2008
Fornicialongiantenna Luo & You, 2008.
Type information. Holotype female, GUGC (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (GZ).
Forniciamacistigma Luo & You, 2006
Forniciamacistigma Luo & You, 2006.
Type information. Holotype female, HUNAU (not examined). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (HN).
Forniciamicrocephala Granger, 1949
Forniciamicrocephala Granger, 1949.
Type information. Holotype female, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Forniciaminis He & Chen, 1994
Forniciaminis He & Chen, 1994.
Type information. Holotype male, ZJUH (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (ZJ).
Notes. Our species concept is based on Chen and Song (2004).
Forniciamoronis (Cushman, 1929)
Odontoforniciamoronis Cushman, 1929.
Type information. Holotype female, USNM (not examined but subsequent treatment of the species checked). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Notes. Our species concept is based on Papp (1980c).
Forniciamuluensis Austin, 1987
Forniciamuluensis Austin, 1987.
Type information. Holotype female, NHMUK (examined). Country of type locality: Malaysia.
Geographical distribution.OTL.
OTL: Brunei, Malaysia.
Forniciaobscuripennis Fahringer, 1934
Forniciaobscuripennis Fahringer, 1934.
Type information. Holotype male, NHRS (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, GX, GZ, HN, JS, SN, TW, ZJ).
Notes. Our species concept is based on Papp (1980c).
Forniciapenang (Cushman, 1929)
Odontoforniciapenang Cushman, 1929.
Type information. Holotype female, USNM (not examined but subsequent treatment of the species checked). Country of type locality: Malaysia.
Geographical distribution.OTL.
OTL: China (TW), Indonesia, Malaysia.
Notes. Our species concept is based on Papp (1980c). Type information from Shenefelt (1973).
Forniciapilosa Cushman, 1931
Forniciapilosa Cushman, 1931.
Type information. Holotype female, USNM (not examined but subsequent treatment of the species checked). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Brazil (PA), Costa Rica.
Notes. Our species concept is based on Papp (1980c).
Forniciaprominentis Chen & He, 1994
Forniciaprominentis Chen & He, 1994.
Type information. Holotype female, ZJUH (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (GX).
Notes. Our species concept is based on Chen and Song (2004).
Forniciarixata Papp, 1980
Forniciarixata Papp, 1980.
Type information. Holotype male, HNHM (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (TW).
Forniciaseyrigi Granger, 1949
Forniciaseyrigi Granger, 1949.
Type information. Holotype female, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Forniciasurinamensis Muesebeck, 1958
Forniciasurinamensis Muesebeck, 1958.
Type information. Holotype female, USNM (examined). Country of type locality: Suriname.
Geographical distribution.NEO.
NEO: Suriname.
Forniciatagalog (Cushman, 1929)
Odontoforniciatagalog Cushman, 1929.
Type information. Holotype female, USNM (not examined but subsequent treatment of the species checked). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Notes. Our species concept is based on Papp (1980c).
Forniciatergiversata Papp, 1980
Forniciatergiversata Papp, 1980.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (TW).
Forniciathoseae Wilkinson, 1930
Forniciathoseae Wilkinson, 1930.
Type information. Holotype female, NHMUK (examined). Country of type locality: Indonesia.
Geographical distribution.OTL.
OTL: Indonesia.
Genus Gilbertnixonius Fernandez-Triana, 2018
Gilbertnixonius Fernandez-Triana, 2018: 56. Gender: neuter. Type species: GilbertnixoniusbiemFernandez-Triana and Boudreault 2018, by original designation.
The only known species was recently described from the Oriental region (Fernandez-Triana and Boudreault 2018). No host data are currently available for this genus. There is one DNA-barcode compliant sequence of Gilbertnixonius in BOLD.
Gilbertnixoniusbiem Fernandez-Triana & Boudreault, 2018
Gilbertnixoniusbiem Fernandez-Triana & Boudreault, 2018.
Type information. Holotype female, QSBG (examined). Country of type locality: Thailand.
Geographical distribution.OTL.
OTL: Thailand.
Genus Glyptapanteles Ashmead, 1904
Glyptapanteles Ashmead, 1904: 147. Gender: masculine. Type species: (Glyptapantelesmanilae Ashmead, 1904) = Apantelesashmeadi Wilkinson, 1928, by monotypy.
Viereck (1914: 62) correctly noted that Ashmead (1900a: 131) was intending to refer to this genus in his 1900 key in the second half of couplet 10, where Protapanteles is separated from another genus, the name of which is (accidentally ?) omitted, but it is clear that it would have been Glyptapanteles. Thus, technically Ashmead’s (1900a) would be the first intention to mention the name Glyptapanteles in a published paper, but because the actual name never appeared there due to an omission, the first official reference to the genus must be considered Ashmead (1904b). In any case the 1900 paper did not designate any type species, so the 1904 paper is the one that matters for that purpose (as Viereck also correctly noted). Glyptapanteles is a cosmopolitan genus, with 307 described species known from all biogeographical regions. Many European species were revised by Nixon and Papp in several papers from the 1970s and 1980s, following earlier work by Wilkinson (1945); and a recent paper dealt with 136 Neotropical species (Arias-Penna et al. 2019), which represents almost half of all described species in the genus. Overall, the taxonomic coverage of the world species is far from complete; we have seen hundreds of undescribed species in collections, mostly from tropical areas, and it is likely that the actual richness will reach several thousand species. The concept of Glyptapanteles and its separation from Protapanteles has been controversial (e.g., Mason 1981, van Achterberg 2003, Broad et al. 2016), but we consider it as a valid genus, although future studies on Microgastrinae phylogeny may split the genus into several. More than 25 families of Lepidoptera have been recorded as hosts for Glyptapanteles, but many records are likely to be incorrect and/or need further verification. There are almost 5,000 DNA-barcode compliant sequences of this genus in BOLD, representing 504 BINs.
Glyptapantelesacasta (Nixon, 1973)
Apantelesacasta Nixon, 1973.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Bulgaria, Finland, Germany, Greece, Hungary, Poland, Russia (ALT, ZAB), Slovakia, Switzerland, Turkey, United Kingdom.
Glyptapantelesacherontiae (Cameron, 1907)
Apantelesacherontiae Cameron, 1907.
Apantelesacherontiae Muesebeck, 1927 [homonym of Apantelesacherontiae Cameron, 1907].
Type information. Syntypes female, NHMUK (examined). Country of type locality: Sri Lanka.
Geographical distribution.OTL.
OTL: China (FJ, HN), India, Sri Lanka.
Glyptapantelesacraeae (Wilkinson, 1932), lectotype designation
Apantelesacraeae Wilkinson, 1932.
Type information. Lectotype female, NHMUK (examined). Country of type locality: Uganda.
Geographical distribution.AFR.
AFR: South Africa, Uganda.
Notes. The species was described from female and male specimens. We have examined a female specimen with a type label and code 3c.1027 in the NHMUK and are designating it here as the lectotype.
Glyptapantelesafiamaluanus (Fullaway, 1941)
Apantelesafiamaluana Fullaway, 1941.
Type information. Holotype female, BPBM (not examined but subsequent treatment of the species checked). Country of type locality: Western Samoa.
Geographical distribution.AUS.
AUS: Western Samoa.
Notes. Our species concept is based on Austin and Dangerfield (1992).
Glyptapantelesafricanus (Cameron, 1911)
Apantelesafricanus Cameron, 1911.
Apantelesbeneficus Viereck, 1911.
Apantelescameroni Brues, 1924.
Type information. Holotype female, TMSA (not examined but subsequent treatment of the species checked). Country of type locality: South Africa.
Geographical distribution.AFR, OTL.
AFR: Ghana, Kenya, Malawi, Mali, Mozambique, Nigeria, South Africa, Uganda, Zimbabwe; OTL: India, Pakistan.
Notes. Our species concept is based on Wilkinson (1932a), van Achterberg and Polaszek (1996), and van Achterberg and Walker (1998). We examined the type, a female specimen, of Apantelesbeneficus (Viereck, 1911), currently a synonym of G.africanus.
Glyptapantelesaggestus (Granger, 1949), new combination
Glytapantelesaggestus Granger, 1949.
Type information. Syntypes female, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Notes. This species clearly is not an Apanteles. Based on the original description it is provisionally transferred to Glyptapanteles until examination of the syntype series allows a more definitive identification.
Glyptapantelesagrotivorus Whitfield, 2002
Glyptapantelesagrotivorus Whitfield, 2002.
Type information. Holotype female, USNM (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Notes. The holotype is dirty and not in good condition.
Glyptapantelesagynus (de Saeger, 1944), new combination
Apantelesagynus de Saeger, 1944.
Type information. Holotype male, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. Based on the original description, which is the only reference available for this species, the best generic placement at present would be in Glyptapanteles. However, the only known specimen is a male and the description is not clear enough to rule out the genus Distatrix. Examination of the specimen will be needed to conclude.
Glyptapantelesaithos (Sharma, 1973), new combination
Apantelesaithos Sharma, 1973.
Type information. Holotype female, IFRI (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. This species is clearly not an Apanteles. The original description does not provide enough information to determine the generic identity in a conclusive way but Glyptapanteles seems to be the best match (although Distatrix might be another possibility). Examination of the type series will be needed to conclude on its generic status.
Glyptapantelesalejandrovalerioi Arias-Penna, 2019
Glyptapantelesalejandrovalerioi Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesaletta (Nixon, 1973)
Apantelesaletta Nixon, 1973.
Type information. Holotype female, MZH (not examined but original description checked). Country of type locality: Finland.
Geographical distribution.PAL.
PAL: Belarus, Finland, Hungary, Korea, Slovakia, Switzerland.
Glyptapantelesalexborisenkoi Arias-Penna, 2019
Glyptapantelesalexborisenkoi Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesalexwildi Arias-Penna, 2019
Glyptapantelesalexwildi Arias-Penna, 2019.
Type information. Holotype male, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesaliphera (Nixon, 1973)
Apantelesaliphera Nixon, 1973.
Apantelessublateralis Tobias, 1976.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Armenia, Azerbaijan, Finland, France, Georgia, Germany, Greece, Hungary, Iran, Israel, Netherlands, Poland, Romania, Russia (KDA), Slovakia, Sweden, Switzerland, United Kingdom.
Glyptapantelesalticola (Ashmead, 1902)
Protapantelesalticola Ashmead, 1902.
Type information. Holotype male, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (BC, NB), USA (AK, CA, CO, ID, NH, OR, UT).
Notes. The holotype is a male, not a female as stated by Shenefelt (1972) and Yu et al. (2012). The metasoma is separate from the head and mesosoma, but it is glued to the same point; only the right wings are present. Muesebeck (1921) mentioned a type series, which we have not seen, and also provided a brief description of the species as part of his key to ‘Apantelessensu lato’. According to that key, Muesebeck states that the species has metafemur ‘dark reddish testaceous, usually edged with blackish’ and also ‘stigma and veins of forewing dark brown’ (Muesebeck 1921: 493). However, the holotype has yellow metafemur and the pterostigma is very pale brown. Other than that, the holotype resembles many ‘Glyptapanteles’ from the northern Nearctic in colour, propodeum sculpture (which has a faint median carina on posterior 0.5), and shape and sculpture of T1–T3.
Glyptapantelesalvarowillei Arias-Penna, 2019
Glyptapantelesalvarowillei Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesamenophis (de Saeger, 1944), new combination
Apantelesamenophis de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. From the original description it is clear that this species does not belong in Apanteles (due to the short ovipositor and shape of the hypopygium). When describing it, de Saeger (1944) stated that it would come close to Apantelesparallelus (Lyle, 1971), which is currently placed within Protapanteles. We consider that amenophis is better placed within Glyptapanteles for the time being, but future examination of the specimens may change that.
Glyptapantelesandrewdebeveci Arias-Penna, 2019
Glyptapantelesandrewdebeveci Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesandybennetti Arias-Penna, 2019
Glyptapantelesandybennetti Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesandydeansi Arias-Penna, 2019
Glyptapantelesandydeansi Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesandysuarezi Arias-Penna, 2019
Glyptapantelesandysuarezi Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesandywarreni Arias-Penna, 2019
Glyptapantelesandywarreni Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesankitaguptae Arias-Penna, 2019
Glyptapantelesankitaguptae Arias-Penna, 2019.
Type information. Holotype male, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesannettewalkerae Arias-Penna, 2019
Glyptapantelesannettewalkerae Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesantarctiae (Blanchard, 1935), new combination
Apantelesantarctiae Blanchard, 1935.
Apantelesantarctiaevar.fusca Blanchard, 1935.
Type information. Syntypes male, MACN (not examined but original description checked). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina.
Notes. The type material belongs to the Blanchard collection, deposited in MACN. The descriptions and illustrations (scutellar disc, propodeum, T1–T2, part of fore wing, tip of antenna) provided in the original description and a following paper (Blanchard 1935, 1936), strongly suggest that this species belongs to Glyptapanteles. The species has the propodeum mostly smooth and without carinae (although the illustration for a male specimen shows a weakly defined median, longitudinal carina), T1 anterior half is parallel-sided while posterior half slightly narrows towards posterior margin, T2 is trapezoidal, and ovipositor sheaths are barely protruding.
Glyptapantelesantinoe (Nixon, 1973)
Apantelesantinoe Nixon, 1973.
Type information. Holotype female, NHMW (not examined but original description checked). Country of type locality: Austria.
Geographical distribution.PAL.
PAL: Austria, Germany, Hungary, Turkey.
Glyptapantelesantsirabensis (Granger, 1949)
Apantelesantsirabensis Granger, 1949.
Type information. Holotype female, MNHN (not examined but subsequent treatment of the species checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar, Réunion.
Notes. Our species concept is based on Rousse and Gupta (2013).
Glyptapantelesanubis (de Saeger, 1944), new combination
Apantelesanubis de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. From the original description it is clear that this species does not belong to Apanteles (due to the short ovipositor and propodeum with median carina). When describing it, de Saeger (1944) stated that, in the Wilkinson key to African species, anubis would come close to Apantelespallidocinctus (Gahan, 1918), which is currently placed within the genus Distarix. However, anubis should not be placed in that genus, due to having a rugose and carinated propodeum. Examination of the holotype will eventually be needed to conclude but, based on all information available in the original description (the only published source of information for this species), the best generic placement at present would be within Glyptapanteles.
Glyptapantelesarcuatus (Telenga, 1955)
Apantelesarcuatus Telenga, 1955.
Type information. Syntypes female and male, depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Germany, Russia (PRI).
Notes. Our species concept is based on Telenga (1955), Papp (1983a) and Kotenko (2007a).
Glyptapantelesarginae (Bhatnagar, 1950), new combination
Apantelesarginae Bhatnagar, 1950.
Type information. Holotype female, INPC (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. Based on the original description this species is clearly not an Apanteles. The description of the propodeum with a faint median longitudinal carina, short ovipositor sheaths, T1 smooth and narrowing towards apex, and T2 smooth and subtriangular shaped, all suggest that the best generic placement for this species at present would be within Glyptapanteles. However, examination of the holotype and paratypes may change that in the future. The year of publication of the Bhatnagar paper was until recently commonly cited as 1948 and/or 1950 (e.g., Chen and Song 2004, Yu et al. 2016), probably following Shenefelt (1972) who referred to this paper as “Bhatnagar (1948) 1950”. While the intended year for Volume X, Parts I & II of the Indian Journal of Entomology was 1948, the actual dates of publication were June 1950 (Part I) and October 1950 (Part II), as clearly shown on the cover page of the Volume, which we have checked. Because the dates of publication are the ones to be considered, and for the sake of clarity, we hereby revise the species year of description to 1950.
Glyptapantelesargus (de Saeger, 1944), new combination
Apantelesargus de Saeger, 1944.
Type information. Holotype male, RMCA (not examined but original description checked). Country of type locality: Rwanda.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Rwanda.
Notes. The original description states this species is very close to A.intricatus (de Saeger, 1944), which in turn was described as very close to several species currently placed within Glyptapanteles. The drawings of the original description of A.intricatus indeed confirm it belongs to Glyptapanteles, and thus argus is also placed in that genus.
Glyptapantelesaristolochiae (Wilkinson, 1928)
Apantelesaristolochiae Wilkinson, 1928.
Type information. Holotype female, NHMUK (examined). Country of type locality: Sri Lanka.
Geographical distribution.OTL.
OTL: India, Sri Lanka.
Glyptapantelesartonae (Rohwer, 1926)
Apantelesartonae Rohwer, 1926.
Type information. Holotype female, USNM (examined). Country of type locality: Malaysia.
Geographical distribution.AUS, OTL.
AUS: Fiji; OTL: Indonesia, Malaysia.
Notes. See Austin and Dangerfield (1992) for details questioning the distibution of this species in Fiji. Yu et al. (2016) have the NHMUK as the type depository, but we have found and examined the holotype in Washington (USNM).
Glyptapantelesashmeadi (Wilkinson, 1928)
Apantelesashmeadi Wilkinson, 1928.
Glyptapantelesmanilae Ashmead, 1904 [secondary homonym of Apantelesmanilae Ashmead, 1904].
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Glyptapantelesatylana (Nixon, 1965), new combination
Apantelesatylana Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Indonesia.
Geographical distribution.OTL.
OTL: Indonesia.
Notes. See comments on G.siderion (Nixon, 1965) below for more details on the rationale to provisionally place these two species in Glyptapanteles.
Glyptapantelesaucklandensis (Cameron, 1909)
Apantelesaucklandensis Cameron, 1909.
Type information. Holotype male, NHMUK (examined). Country of type locality: New Zealand.
Geographical distribution.AUS.
AUS: New Zealand.
Glyptapantelesbadgleyi (Wilkinson, 1928), new combination
Apantelesbadgleyi Wilkinson, 1928.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. This species is placed in Glyptapanteles based on very short ovipositor sheaths, inflexible hypopygium, T1 narrowing towards posterior margin, and T2 subtriangular (trapezoidal).
Glyptapantelesbarneyburksi Arias-Penna, 2019
Glyptapantelesbarneyburksi Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesbataviensis (Rohwer, 1919), new combination
Apantelesbataviensis Rohwer, 1919.
Type information. Holotype female, USNM (examined). Country of type locality: Indonesia.
Geographical distribution.OTL.
OTL: India, Indonesia, Vietnam.
Notes. The species was transferred from Apanteles to Cotesia by Long et al. (2004). However, after examining the holotype, we find it clearly belongs to Glyptapanteles as it has an entirely smooth propodeum and T1 is strongly narrowing towards posterior margin.
Glyptapantelesbetogarciai Arias-Penna, 2019
Glyptapantelesbetogarciai Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesbidentatus (Sharma, 1972)
Apantelesbidentatus Sharma, 1972.
Type information. Holotype male, IFRI (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Glyptapantelesbillbrowni Arias-Penna, 2019
Glyptapantelesbillbrowni Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesbimus Papp, 1990
Glyptapantelesbimus Papp, 1990.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Korea.
Geographical distribution.PAL.
PAL: Korea.
Notes. Our species concept is based on Papp (1990b) and Kotenko (2007a).
Glyptapantelesbistonis (Watanabe, 1934), new combination
Apantelesbistonis Watanabe, 1934.
Type information. Holotype male, EIHU (examined). Country of type locality: Japan.
Geographical distribution.PAL.
PAL: Japan.
Notes. We examined the male holotype and another pin which carries a cocoon mass. This species is clearly Glyptapanteles (as Papp had recognized in a label he wrote in 1992, although he never published that new combination).
Glyptapantelesbobhanneri Arias-Penna, 2019
Glyptapantelesbobhanneri Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesbobkulai Arias-Penna, 2019
Glyptapantelesbobkulai Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesbobwhartoni Arias-Penna, 2019
Glyptapantelesbobwhartoni Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesboharti Arias-Penna, 2019
Glyptapantelesboharti Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesborocerae (Granger, 1949), new combination
Apantelesborocerae Granger, 1949.
Type information. Syntypes female and male, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Notes. This species is not Apanteles. Based on the original description as well as host information, the species is provisionally transferred to Glyptapanteles until examination of the syntype series allows a more definitive identification.
Glyptapantelesbourquini (Blanchard, 1936)
Apantelesbourquini Blanchard, 1936.
Apanteleselegans Blanchard, 1936.
Type information. Holotype female, MACN (not examined but subsequent treatment of the species checked). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina, Brazil (RS) Chile, Ecuador, Peru, Uruguay.
Notes. The type belongs to the Blanchard collection, which we assume is deposited in the MACN. Our species concept is based on Whitfield et al. (2002a). The record from Brazil is based on Shimbori et al. (2019).
Glyptapantelesbreviscuta Song & Chen, 2004
Glyptapantelesbreviscuta Song & Chen, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Glyptapantelesbrianestjaquesae Arias-Penna, 2019
Glyptapantelesbrianestjaquesae Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelescaberatae (Muesebeck, 1956)
Apantelescaberatae Muesebeck, 1956.
Type information. Holotype female, USNM (not examined but subsequent treatment of the species checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CA).
Notes. Our species concept is based on Whitfield (1995a).
Glyptapantelescacao (Wilkinson, 1934), new combination
Apantelescacao Wilkinson, 1934.
Type information. Holotype female, NHMUK (examined). Country of type locality: Sri Lanka.
Geographical distribution.OTL.
OTL: Sri Lanka.
Notes. This species is placed in Glyptapanteles based on the short ovipositor sheaths, inflexible hypopygium, T1 narrowing towards posterior margin, and T2 subtriangular (trapezoidal).
Glyptapantelescadei (Risbec, 1951), new combination
Apantelescadei Risbec, 1951.
Type information. Holotype male, depository unknown (not examined but original description checked). Country of type locality: Senegal.
Geographical distribution.AFR.
AFR: Senegal.
Notes. The original description (and the included drawings of the propodeum, T1 and T2) suggests this species does not belong to Apanteles, and it is better placed in Glyptapanteles for the time being. However, the information available is not enough to conclude with absolute certainty on the generic status of the species, and study of the single male specimen will be required to clarify its status in the future.
Glyptapantelescaffreyi (Muesebeck, 1921)
Apantelescaffreyi Muesebeck, 1921.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA, NEO.
NEA: USA (AZ); NEO: Mexico, Peru.
Glyptapantelescallidus (Haliday, 1834)
Microgastercallidus Haliday, 1834.
Apantelesurolus Papp, 1983.
Type information. Lectotype female, NMID (not examined but subsequent treatment of the species checked). Country of type locality: unknown.
Geographical distribution.PAL.
PAL: Armenia, Austria, Belgium, Bulgaria, Czech Republic, Finland, France, Georgia, Germany, Hungary, Ireland, Israel, Lithuania, Netherlands, Poland, Romania, Russia (AMU, SAK), Slovakia, Sweden, Switzerland, Turkey, Ukraine, United Kingdom.
Notes.van Achterberg (1997) reinterpreted this name and treated it only sensuHaliday (1834). The species called callidus by Nixon (1973) and Papp (1983a) are now considered to be Glyptapantelesmajalis (Wesmael, 1837) (e.g., van Achterberg 1997, Broad et al. 2016).
Glyptapantelescapeki (Györfi, 1955)
Apantelescapeki Györfi, 1955.
Type information. Holotype female, depository unknown (not examined). Country of type locality: Slovakia.
Geographical distribution.PAL.
PAL: Slovakia.
Glyptapantelescarinachicaizae Arias-Penna, 2019
Glyptapantelescarinachicaizae Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelescarinatus (Szépligeti, 1913)
Apantelescarinatus Szépligeti, 1913.
Type information. Holotype male, HNHM (not examined but subsequent treatment of the species checked). Country of type locality: Tanzania.
Geographical distribution.AFR.
AFR: Tanzania.
Notes. Our species concept is based on Papp (2004).
Glyptapantelescarlhuffakeri Arias-Penna, 2019
Glyptapantelescarlhuffakeri Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelescarlossarmientoi Arias-Penna, 2019
Glyptapantelescarlossarmientoi Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelescarlrettenmeyeri Arias-Penna, 2019
Glyptapantelescarlrettenmeyeri Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelescassianus (Riley, 1881)
Apantelescassianus Riley, 1881.
Type information. Syntypes female and male, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CO, CT, IL, IA, MO, NJ, TX).
Glyptapantelescelsoazevedoi Arias-Penna, 2019
Glyptapantelescelsoazevedoi Arias-Penna, 2019.
Type information. Holotype male, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelescharlesmicheneri Arias-Penna, 2019
Glyptapantelescharlesmicheneri Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelescharlesporteri Arias-Penna, 2019
Glyptapantelescharlesporteri Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapanteleschidra Rousse & Gupta, 2013
Glyptapanteleschidra Rousse & Gupta, 2013.
Type information. Holotype female, MNHN (not examined but original description checked). Country of type locality: Réunion.
Geographical distribution.AFR.
AFR: Réunion.
Glyptapanteleschrisdarlingi Arias-Penna, 2019
Glyptapanteleschrisdarlingi Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapanteleschrisgrinteri Arias-Penna, 2019
Glyptapanteleschrisgrinteri Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapanteleschristerhanssoni Arias-Penna, 2019
Glyptapanteleschristerhanssoni Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelescinyras (de Saeger, 1944), new combination
Apantelescinyras de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Rwanda.
Geographical distribution.AFR.
AFR: Rwanda.
Notes. Based on the original description, the best generic placement would be in Glyptapanteles.
Glyptapantelesclanisae Gupta, 2013
Glyptapantelesclanisae Gupta, 2013.
Type information. Holotype female, NBAIR (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Glyptapantelesclaudiamartinezae Arias-Penna, 2019
Glyptapantelesclaudiamartinezae Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelescolemani (Viereck, 1912)
Apantelescolemani Viereck, 1912.
Type information. Holotype female, USNM (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: China (GX), India, Vietnam.
Glyptapantelescompressiventris (Muesebeck, 1921)
Apantelescompressiventris Muesebeck, 1921.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA, PAL.
NEA: Canada (MB, NT, NU, QC), USA (NH); PAL: Armenia, Azerbaijan, Croatia, Czech Republic, Finland, Germany, Hungary, Italy, Kazakhstan, Lithuania, Macedonia, Moldova, Netherlands, Romania, Russia (KAM, PRI, SAK, SPE, VOR), Serbia, Slovakia, Spain, Switzerland, Turkey, United Kingdom.
Glyptapantelescompressus (Muesebeck, 1919)
Apantelescompressus Muesebeck, 1919.
Type information. Holotype female, USNM (not examined but subsequent treatment of the species checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (MA, NH, RI, VA, WV).
Notes. Our species concept is based on Muesebeck (1921), Mason (1981) and Whitfield (1995a).
Glyptapantelesconcinnus (Muesebeck, 1958)
Apantelesconcinnus Muesebeck, 1958.
Apantelesconcinnus Muesebeck, 1958 [primary homonym of Apantelesconcinnus Statz, 1938].
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (SP).
Glyptapantelescorbetti (Wilkinson, 1928)
Apantelescorbetti Wilkinson, 1928.
Type information. Holotype female, NHMUK (examined). Country of type locality: Malaysia.
Geographical distribution.OTL.
OTL: China (GX), Malaysia.
Glyptapantelescorriemoreauae Arias-Penna, 2019
Glyptapantelescorriemoreauae Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelescreatonoti (Viereck, 1912)
Apantelescreatonoti Viereck, 1912.
Type information. Holotype female, USNM (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: Bangladesh, India, Malaysia.
Glyptapantelesdalosoma de Santis, 1987
Glyptapantelesdalosoma de Santis, 1987.
Type information. Holotype female, MLP (not examined but subsequent treatment of the species checked). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (SP).
Notes. Our species concept is based on Aquino et al. (2010).
Glyptapantelesdarjeelingensis (Sharma & Chatterjee, 1970)
Apantelesdarjeelingensis Sharma & Chatterjee, 1970.
Type information. Holotype male, FSCA? (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. The original description refers to the Gupta collection, which we assume to be currently deposited in the FSCA (at least the Braconidae part); however, there is also the possibility that the type of this species is deposited elsewhere.
Glyptapantelesdaveroubiki Arias-Penna, 2019
Glyptapantelesdaveroubiki Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesdaveschindeli Arias-Penna, 2019
Glyptapantelesdaveschindeli Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesdavesmithi Arias-Penna, 2019
Glyptapantelesdavesmithi Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesdavidwahli Arias-Penna, 2019
Glyptapantelesdavidwahli Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesdeliasa Austin & Dangerfield, 1992
Glyptapantelesdeliasa Austin & Dangerfield, 1992.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (SA).
Glyptapantelesdiegocamposi Arias-Penna, 2019
Glyptapantelesdiegocamposi Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesdistatus Papp, 1990
Glyptapantelesdistatus Papp, 1990.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Korea.
Geographical distribution.PAL.
PAL: Korea.
Glyptapantelesdonquickei Arias-Penna, 2019
Glyptapantelesdonquickei Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesdorislagosae Arias-Penna, 2019
Glyptapantelesdorislagosae Arias-Penna, 2019.
Type information. Holotype male, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesecuadorius Whitfield, 2002
Glyptapantelesecuadorius Whitfield, 2002.
Type information. Holotype female, USNM (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Notes. Holotype specimen is relatively dirty and not in good condition.
Glyptapantelesedgardpalacioi Arias-Penna, 2019
Glyptapantelesedgardpalacioi Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesedwinnarvaezi Arias-Penna, 2019
Glyptapantelesedwinnarvaezi Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapanteleseowilsoni Arias-Penna, 2019
Glyptapanteleseowilsoni Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapanteleserictepei Arias-Penna, 2019
Glyptapanteleserictepei Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapanteleseryphanidis (Whitfield, 2011), new combination
Protapanteleseryphanidis Whitfield, 2011.
Type information. Holotype male, USNM (not examined but original description checked). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Notes. The original description (Greeney et al. 2011) was based on a single male, usually the more difficult sex to work with in Microgastrinae (Whitfield 1997). However, the illustrations in the original description clearly show T1 and T2 smooth, with T1 narrowing towards the posterior margin and T2 subtriangular; and the propodeum lacks a median carina and only has a few short carinulae radiating from the nucha. All of those characters suggest that the species is better placed within Glyptapanteles instead of Protapanteles, a decision we make here. More evidence, if only weak, comes from biology, something that even the authors recognized and mentioned in the paper (Greeney et al. 2011: 1087) when they acknowledged that the host family (Nymphalidae) had never been recorded for Protapanteles (e.g., Mason 1981, Whitfield 1997, Whitfield et al. 1999).
Glyptapanteleseucosmae (Wilkinson, 1929)
Apanteleseucosmae Wilkinson, 1929.
Apantelessalensis Hedqvist, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Nigeria.
Geographical distribution.AFR, PAL.
AFR: Cape Verde, Democratic Republic of Congo, Nigeria, Senegal, Uganda, Zambia; PAL: China (LN), Mongolia.
Glyptapanteleseuproctisiphagus (Ahmad, 1945), new combination
Apanteleseuproctisiphagus Ahmad, 1945.
Type information. Holotype female, INPC (not examined but subsequent treatment of the species checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. Our species concept is based on Papp (1983a), who placed euproctisiphagus within a key comprising other Glyptapanteles, and also provided illustrations of the species.
Glyptapanteleseutelus (de Saeger, 1941), new combination
Apanteleseutelus de Saeger, 1941.
Type information. Holotype male, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Ivory Coast, Rwanda, Senegal.
Notes. Based on the original description (de Saeger 1941b), the best generic placement would be in Glyptapanteles.
Glyptapantelesfabiae (Wilkinson, 1928), new combination
Apantelesfabiae Wilkinson, 1928.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. This species is placed in Glyptapanteles based on the very short ovipositor sheaths, inflexible hypopygium, T1 narrowing towards posterior margin, and T2 subtriangular (trapezoidal).
Glyptapantelesfelipesotoi Arias-Penna, 2019
Glyptapantelesfelipesotoi Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesfemoratus Ashmead, 1906
Glyptapantelesfemoratus Ashmead, 1906.
Type information. Lectotype male, USNM (not examined but subsequent treatment of the species checked). Country of type locality: Japan.
Geographical distribution.OTL, PAL.
OTL: China (HB, ZJ); PAL: Japan, Korea.
Notes. Our species concept is based on Watanabe (1932, 1937) and Chen and Song (2004).
Glyptapantelesferfernandezi Arias-Penna, 2019
Glyptapantelesferfernandezi Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesficus (Granger, 1949)
Apantelesficus Granger, 1949.
Type information. Holotype female, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar, Réunion.
Notes. Our species concept is based on Granger (1949) and Rousse and Gupta (2013).
Glyptapantelesflavicoxis (Marsh, 1979)
Apantelesflavicoxis Marsh, 1979.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Glyptapantelesflavovariatus (Muesebeck, 1921)
Apantelesflavovariatus Muesebeck, 1921.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (BC, ON), USA (MI, OR, SD).
Glyptapantelesfloridanus (Muesebeck, 1921)
Apantelesfloridanus Muesebeck, 1921.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (FL).
Glyptapantelesfraternus (Reinhard, 1880)
Apantelesfraternus Reinhard, 1880.
Type information. Holotype female, ZMHB (not examined but subsequent treatment of the species checked). Country of type locality: Austria.
Geographical distribution.PAL.
PAL: Austria, Bosnia and Herzegovina, Croatia, Czech Republic, France, Germany, Hungary, Kazakhstan, Moldova, Mongolia, Poland, Romania, Russia (ZAB, TY), Slovakia, Switzerland, Turkmenistan, Ukraine, United Kingdom, Uzbekistan, Yugoslavia.
Notes. Our species concept is based on Wilkinson (1945), Nixon (1973), Papp (1983a), Tobias (1986) and Kotenko (2007a). The species distribution in Turkmenistan is based on Belokobylskij et al. (2019).
Glyptapantelesfullawayi Austin & Dangerfield, 1992
Glyptapantelesfullawayi Austin & Dangerfield, 1992.
Apantelespolitus Fullaway, 1941 [primary homonym of Apantelespolitus Riley, 1881].
Type information. Holotype male, BPBM (not examined but original description checked). Country of type locality: Western Samoa.
Geographical distribution.AUS.
AUS: Western Samoa.
Glyptapantelesfulvigaster (Granger, 1949), new combination
Apantelesfulvigaster Granger, 1949.
Type information. Syntypes female and male, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Notes. This species is clearly not an Apanteles, based on the short ovipositor sheaths. In the original description it is considered to be related to Apantelesbelliger Wilkinson, which is now placed within Distatrix. However, Granger (1949) did not mention in his description that fulvigaster has ovipositor sheaths lacking setae (which could be argued to be a noticeable feature and would have indeed shown the species to belong to Distatrix). The original description does not mention any details on the lateral sulci on pronotum either, which would have helped to clarify the generic position of the species. Due to all of the above, we take the conservative approach of transferring fulvigaster to Glyptapanteles, which fits better with the available description. However, we caution that the species could be within Distatrix once the specimens from the type series can be examined further.
Glyptapantelesfulvipes (Haliday, 1834)
Microgasterfulvipes Haliday, 1834.
Type information. Lectotype female, NMID (not examined but subsequent treatment of the species checked). Country of type locality: Ireland.
Geographical distribution.NEA, PAL.
NEA: Canada (AB, NT, NU, QC), Greenland; PAL: Armenia, Austria, Azerbaijan, Belarus, Belgium, Bulgaria, Canary Islands, Croatia, Czech Republic, Faroe Islands, Finland, France, Georgia, Germany, Hungary, Iceland, Ireland, Italy, Japan, Kazakhstan, Korea, Lithuania, Macedonia, Moldova, Mongolia, Netherlands, Poland, Romania, Russia (AMU, ZAB, DA, AL, KDA, MOS, PRI, SAK, SPE, YAR), Serbia, Slovakia, Slovenia, Spain, Sweden, Switzerland, Turkey, Ukraine, United Kingdom.
Notes. Our species concept is based on Nixon (1973), Papp (1983a), Tobias (1986), Kotenko (2007a, van Achterberg (2006) and Fernandez-Triana et al. (2017b).
Glyptapantelesfuscinervis (Cameron, 1911), new combination
Apantelesfuscinervis Cameron, 1911.
Type information. Holotype male, TMSA (not examined but original description checked). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: Rwanda, South Africa.
Notes. Based on the description of Wilkinson (1932a) the best generic placement at present would be in Glyptapanteles.
Glyptapantelesgahinga (de Saeger, 1944), new combination
Apantelesgahinga de Saeger, 1944.
Type information. Syntypes female and male, RMCA (not examined but original description checked). Country of type locality: Rwanda.
Geographical distribution.AFR.
AFR: Rwanda.
Notes. Based on the original description, the best generic placement would be in Glyptapanteles.
Glyptapantelesgarygibsoni Arias-Penna, 2019
Glyptapantelesgarygibsoni Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesgavinbroadi Arias-Penna, 2019
Glyptapantelesgavinbroadi Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesgenorodriguezae Arias-Penna, 2019
Glyptapantelesgenorodriguezae Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesgerarddelvarei Arias-Penna, 2019
Glyptapantelesgerarddelvarei Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesglobatus (Linnaeus, 1758), new combination
Ichneumonglobatus Linnaeus, 1758.
Type information. Syntypes female and male, LSUK (not examined but illustrations of the type series examined). Country of type locality: Sweden.
Geographical distribution.PAL.
PAL: Sweden.
Notes. The use of the name Ichneumonglobatus Linnaeus, 1758 has been problematic for a long time, as it was mostly associated with the genus Microgaster in Europe (e.g., Yu et al. 2012, 2016, Broad et al. 2016; see also van Achterberg 2014 and Scaramozzino et al. 2017, for more details on the topic). Because the type series of globatus clearly does not belong to Microgaster, van Achterberg (2014) proposed to use the name Microgasterrufipes Nees, 1834 (the oldest available name) for the historical references to that Microgaster species in Europe, a decision we accept and follow here (see our rationale to do that in the Notes we provide in this paper under the species Microgasterrufipes). As for the type series of globatus, those specimens are deposited in The Linnean Society, and two photos of those syntypes are shown in their website (http://linnean-on1ine.org/16250/). After examining those images (at least four specimens are distinguishable in the two photos, one clearly being a female), we think that the best generic placement at present would be in Glyptapanteles, and propose this new combination here, based on the T1 narrowing towards posterior margin and T2 subtriangular (as evident from one the specimens photographed that are on the cocoon mass) and the short ovipositor sheaths (as evident on the female specimen also photographed on the cocoon mass, the specimen being the closest to the pin holding the mass). The name Glyptapantelesglobatus (Linnaeus, 1758), as we propose here, would be limited for the time being to the specimens from the Linnaeus series, which are supposedly from Sweden (e.g., see Linnaeus 1761: 411, specimen 1645). Future studies of those specimens will be needed to place this species within the larger context of European and Palearctic Glyptapanteles.
Glyptapantelesglyphodes (Wilkinson, 1932), new combination
Apantelesglyphodes Wilkinson, 1932.
Type information. Holotype female, NHMUK (examined). Country of type locality: Uganda.
Geographical distribution.AFR.
AFR: Uganda.
Notes. This species is placed in Glyptapanteles based on the very short ovipositor sheaths, inflexible hypopygium, T1 narrowing towards posterior margin, and T2 subtriangular (trapezoidal).
Glyptapantelesgowdeyi (Gahan, 1918)
Apantelesgowdeyi Gahan, 1918.
Type information. Holotype female, USNM (not examined but subsequent treatment of the species checked). Country of type locality: Uganda.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Uganda.
Notes. Our species concept is based on Wilkinson (1932a), de Saeger (1944) and Mason (1981).
Glyptapantelesgrantgentryi Arias-Penna, 2019
Glyptapantelesgrantgentryi Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesguierae (Risbec, 1951), new combination
Apantelesguierae Risbec, 1951.
Type information. Syntypes female and male, depository unknown (not examined but original description checked). Country of type locality: Senegal.
Geographical distribution.AFR.
AFR: Senegal.
Notes. From the original description is evident that this species is not Apanteles, based on the sculpture of propodeum and shapes of T1 and T2, the best generic placement at present would be in Glyptapanteles. That is also supported by the original description, where Risbec (1951: 423) considered the species to be related to Apanteleseucosmae (Wilkinson, 1929) which has long been placed within Glyptapanteles (e.g., Mason 1981).
Glyptapantelesgunnarbrehmi Arias-Penna, 2019
Glyptapantelesgunnarbrehmi Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesguyanensis (Cameron, 1911), lectotype designation
Apantelesguyanensis Cameron, 1911.
Type information. Lectotype female, NHMUK (examined). Country of type locality: Guyana.
Geographical distribution.NEO.
NEO: Guyana.
Notes. The type series has four female specimens, all glued on the same card. Shenefelt (1972: 527) mentioned the need to designate a lectotype but did not formally propose it (as he did for many other species in that paper). For the sake of completion, here we designate the lectotype. It is the female placed at the extreme left of the card, which is not only the best-preserved specimen but also has an X below it, which works to clearly mark the lectotype specimen among the series. Taxapad (Yu et al. 2012, 2016) reported the species as occurring in Guyana and Australia, with the latter country being based on Wilkinson (1930c). However, Austin and Dangerfield (1992) considered the Australian specimens to be different from the type series (Guyana). Here we agree with Austin and Dangerfield (1992) and consider Glyptapantelesguyanensis as strictly Neotropical (Guyana). A recent paper (Gallardo-Covas 2005) mentioned the possibility of this species also being in Puerto Rico (the species being reported as “probably guayanensis”, the species name being misspelled throughout the manuscript), and even mentions Pseudoplusiaincludens (Noctuidae) as its host in the island. However, Gallardo-Covas (2005) did not mention how the specimens were identified and thus we consider here that the Puerto Rico record must be confirmed before being formally listed as part of the species distribution.
Glyptapantelesharoldgreeneyi Arias-Penna, 2019
Glyptapantelesharoldgreeneyi Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesharrisinae (Muesebeck, 1953)
Apantelesharrisinae Muesebeck, 1953.
Type information. Holotype female, USNM (not examined but subsequent treatment of the species checked). Country of type locality: USA.
Geographical distribution.NEA, NEO.
NEA: USA (AZ, CA, CT, FL); NEO: Mexico.
Notes. Our species concept is based on Papp (1984a) and Whitfield (1995a).
Glyptapanteleshelmuthaguirrei Arias-Penna, 2019
Glyptapanteleshelmuthaguirrei Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapanteleshenryhespenheidei Arias-Penna, 2019
Glyptapanteleshenryhespenheidei Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapanteleshenrytownesi Arias-Penna, 2019
Glyptapanteleshenrytownesi Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesherbertii (Ashmead, 1900)
Apantelesherbertii Ashmead, 1900.
Type information. Holotype female, NHMUK (examined). Country of type locality: Saint Vincent.
Geographical distribution.NEA, NEO.
NEA: USA (FL); NEO: Argentina, Belize, Colombia, Cuba, Ecuador, Grenada, Mexico, Nicaragua, Peru, Saint Vincent, Venezuela.
Glyptapanteleshorus (de Saeger, 1944), new combination
Apanteleshorus de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Rwanda.
Notes. Based on the original description, the best generic placement would be in Glyptapanteles.
Glyptapanteleshowelldalyi Arias-Penna, 2019
Glyptapanteleshowelldalyi Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapanteleshugokonsi Arias-Penna, 2019
Glyptapanteleshugokonsi Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapanteleshydroeciae (You & Xiong, 1983)
Apanteleshydroeciae You & Xiong, 1983.
Type information. Holotype female, HUNAU (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (SN).
Notes. Our species concept is based on Chen and Song (2004).
Glyptapanteleshypermnestrae Gupta & Pereira, 2012
Glyptapanteleshypermnestrae Gupta & Pereira, 2012.
Type information. Holotype female, NBAIR (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Glyptapantelesiangauldi Arias-Penna, 2019
Glyptapantelesiangauldi Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesianyarrowi Arias-Penna, 2019
Glyptapantelesianyarrowi Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesilarisaaksjarvi Arias-Penna, 2019
Glyptapantelesilarisaaksjarvi Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesinclusus (Ratzeburg, 1844)
Microgasterinclusus Ratzeburg, 1844.
Microgastercurvulus Thomson, 1895.
Apantelesrectinervis Telenga, 1955.
Type information. Lectotype female, ZMHB (not examined but subsequent treatment of the species checked). Country of type locality: unknown.
Geographical distribution.AFR, PAL.
AFR: Cape Verde; PAL: Austria, Azerbaijan, Bulgaria, China (SD, SN), Denmark, France, Germany, Ireland, Italy, Japan, Kazakhstan, Korea, Mongolia, Poland, Romania, Russia (ZAB, IRK, PRI, TY), Slovakia, Switzerland, Ukraine, United Kingdom.
Notes. Our species concept is based on Wilkinson (1945), Nixon (1973), Papp (1983a), Tobias (1986), and Chen and Song (2004). The species distribution in Japan and Mongolia is based on Belokobylskij et al. (2019).
Glyptapantelesindiensis (Marsh, 1979)
Apantelesindiensis Marsh, 1979.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: India.
Geographical distribution.NEA, OTL.
NEA: USA (PA), OTL: India.
Glyptapantelesintermedius (Balevski, 1980)
Apantelesintermedius Balevski, 1980.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Bulgaria.
Geographical distribution.PAL.
PAL: Bulgaria, Ukraine.
Notes. Our species concept is based on Tobias (1986) and Kotenko (2006).
Glyptapantelesintricatus (de Saeger, 1944), new combination
Apantelesintricatus de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Rwanda.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Rwanda.
Notes. The original description of A.intricatus contains drawings that show this species is better placed within Glyptapanteles. See also comments above under Glyptapantelesargus (de Saeger, 1944).
Glyptapantelesjacklonginoi Arias-Penna, 2019
Glyptapantelesjacklonginoi Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesjamesrobertsoni Arias-Penna, 2019
Glyptapantelesjamesrobertsoni Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesjaquioconnorae Arias-Penna, 2019
Glyptapantelesjaquioconnorae Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesjeremydewaardi Arias-Penna, 2019
Glyptapantelesjeremydewaardi Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesjerrypowelli Arias-Penna, 2019
Glyptapantelesjerrypowelli Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesjesusugaldei Arias-Penna, 2019
Glyptapantelesjesusugaldei Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesjimmilleri Arias-Penna, 2019
Glyptapantelesjimmilleri Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesjjrodriguezae Arias-Penna, 2019
Glyptapantelesjjrodriguezae Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesjohnburnsi Arias-Penna, 2019
Glyptapantelesjohnburnsi Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesjohnheratyi Arias-Penna, 2019
Glyptapantelesjohnheratyi Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesjohnlasallei Arias-Penna, 2019
Glyptapantelesjohnlasallei Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesjohnnoyesi Arias-Penna, 2019
Glyptapantelesjohnnoyesi Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesjohnstiremani Arias-Penna, 2019
Glyptapantelesjohnstiremani Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesjosesimbanai Arias-Penna, 2019
Glyptapantelesjosesimbanai Arias-Penna, 2019.
Type information. Holotype male, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesjuanvargasi Arias-Penna, 2019
Glyptapantelesjuanvargasi Arias-Penna, 2019.
Type information. Holotype male, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesjumamuturii Arias-Penna, 2019
Glyptapantelesjumamuturii Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapanteleskeithwillmotti Arias-Penna, 2019
Glyptapanteleskeithwillmotti Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapanteleskevinjohnsoni Arias-Penna, 2019
Glyptapanteleskevinjohnsoni Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapanteleskyleparksi Arias-Penna, 2019
Glyptapanteleskyleparksi Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapanteleslamborni (Wilkinson, 1928)
Apanteleslamborni Wilkinson, 1928.
Type information. Holotype female, NHMUK (examined). Country of type locality: Malaysia.
Geographical distribution.OTL.
OTL: China (GZ, HN, TW, YN), Malaysia.
Glyptapanteleslamprosemae (Wilkinson, 1928), new combination
Apanteleslamprosemae Wilkinson, 1928.
Type information. Holotype female, NHMUK (examined). Country of type locality: Malaysia.
Geographical distribution.OTL.
OTL: Malaysia.
Notes. This species is placed in Glyptapanteles based on the very short ovipositor sheaths, inflexible hypopygium, T1 narrowing towards posterior margin, and T2 subtriangular (trapezoidal).
Glyptapanteleslaxatus (Wilkinson, 1930)
Apanteleslaxatus Wilkinson, 1930.
Type information. Holotype female, NHMUK (examined). Country of type locality: Uganda.
Geographical distribution.AFR.
AFR: Uganda.
Glyptapanteleslefevrei (de Saeger, 1941), new combination
Apanteleslefevrei de Saeger, 1941.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Rwanda.
Geographical distribution.AFR.
AFR: Burundi, Rwanda.
Notes. Here transferred to Glyptapanteles based on propodeum with median, longitudinal carina (defined on posterior half of propodeum), short ovipositor sheaths, and shape and sculpture of T1 and T2 (de Saeger 1941a: 333–335).
Glyptapantelesleucotretae (Ullyett, 1946), new combination
Apantelesleucotretae Ullyett, 1946.
Type information. Holotype female, TMSA (not examined but original description checked). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Notes. Based on the original description, the best generic placement is in Glyptapanteles, due to the propodeum having a partial median carina, the shapes of T1 and T2, acute hypopygium and length of ovipositor sheaths. Ullyett (1946) also mentions the species as being close to Glyptapantelesfuscinervis Cameron.
Glyptapanteleslinghsiuae Arias-Penna, 2019
Glyptapanteleslinghsiuae Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesliparidis (Bouché, 1834)
Microgasterliparidis Bouché, 1834.
Microgasternemorum Hartig, 1838.
Microgasterliparidis Ratzeburg, 1844 [primary homonym of Microgasterliparidis Bouché, 1834].
Glyptapantelesjaponicus Ashmead, 1906.
Glyptapantelespolitus Ashmead, 1906.
Apantelesposticae Sonan, 1927.
Apantelesawanomeigae Watanabe, 1942.
Type information. Holotype female, ZMHB (not examined but authoritatively identified specimens examined). Country of type locality: Germany.
Geographical distribution.OTL, PAL.
OTL: China (HN, TW, ZJ), India; PAL: Austria, Belarus, Bulgaria, China (BJ, HL, JL, LN, NM, SN), Czech Republic, Finland, France, Germany, Hungary, Iran, Italy, Japan, Kazakhstan, Korea, Lithuania, Moldova, Mongolia, Poland, Romania, Russia (ZAB, IRK, KGD, KHA, KDA, NVS, PRI, SAK, SPE, SAR, TOM, VOR, YAR), Serbia, Slovakia, Spain, Sweden, Switzerland, Ukraine.
Notes. We examined the female type of A.japonicusAshmead (1906) in the USNM and most of the specimens of Apantelesawanomeigae (Watanabe, 1942) which were seen and determined by Watanabe.
Glyptapanteleslissopleurus (de Saeger, 1944), new combination
Apanteleslissopleurus de Saeger, 1944.
Type information. Holotype male, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. Based on the original description, which is the only reference available for this species, the best generic placement at present would be in Glyptapanteles. However, the only known specimen is a male and the description is not clear enough to rule out the genus Distatrix. Examination of the specimen will be needed to conclude.
Glyptapanteleslongiantennatus (You & Xiong, 1987)
Apanteleslongiantennatus You & Xiong, 1987.
Type information. Holotype female, HUNAU (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (HN).
Notes. Our species concept is based on Kotenko (2007a).
Glyptapanteleslongistigma Chen & Song, 2004
Glyptapanteleslongistigma Chen & Song, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
OTL: China (HB).
Glyptapanteleslongivena Chen & Song, 2004
Glyptapanteleslongivena Chen & Song, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Glyptapanteleslubomasneri Arias-Penna, 2019
Glyptapanteleslubomasneri Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesluchosalagajei Arias-Penna, 2019
Glyptapantelesluchosalagajei Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesluciana (Nixon, 1973)
Apantelesluciana Nixon, 1973.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Armenia, Bulgaria, Finland, Germany, Greece, Hungary, Korea, Madeira Islands, Netherlands, Romania, Slovakia, Switzerland, United Kingdom.
Glyptapanteleslucidus (Sharma, 1972)
Apanteleslucidus Sharma, 1972.
Apanteleslucidus Sharma, 1972 [primary junior homonym of Apanteleslucidus Szépligeti].
Type information. Holotype female, IFRI (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Glyptapantelesluteipennis (Muesebeck, 1921)
Apantelesluteipennis Muesebeck, 1921.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (VA).
Notes. After examining the holotype, we believe the specimen may be better placed in Protapanteles, because of the sculpture and carination of propodeum. However, the fore tarsus does not have a thick seta (usual for Protapanteles) and the ovipositor sheaths are hidden inside the hypopygium so it is not clear if they have setae or not. Because only the holotype is known, we refrain from transferring the species here and prefer to retain it in Glyptapanteles, as Mason (1981) suggested, although future studies may change that.
Glyptapantelesmaculitarsis (Cameron, 1905)
Apantelesmaculitarsis Cameron, 1905.
Apantelescapensis Cameron, 1907.
Apantelesafricanus Viereck, 1911 [primary homonym of Apantelesafricanus Cameron, 1911].
Apantelestestaceioventris Cameron, 1911.
Apantelestestaceolineatus Cameron, 1911.
Apantelestestaceiventris Brues, 1926 [emendation].
Type information. Holotype female, depository unknown (not examined but authoritatively identified specimens examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: Ethiopia, Kenya, Malawi, Nigeria, Senegal, Sierra Leone, South Africa, Tanzania, Uganda.
Notes. We examined the type, a female specimen, of Apantelesafricanus (Viereck, 1911), currently a synonym of G.maculitarsis.
Glyptapantelesmadecassus (Granger, 1949), new combination
Glyptapantelesmadecassus Granger, 1949.
Type information. Syntypes female and male, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Notes. This species is not an Apanteles. Based on the original description (including an illustration of T1-T3), as well as host information, the species is provisionally transferred to Glyptapanteles until examination of the syntype series allows a more definitive identification.
Glyptapantelesmajalis (Wesmael, 1837)
Microgastermajalis Wesmael, 1837.
Microgastercallidus Haliday, 1834 [misidentification].
Type information. Syntypes female and male, RBINS (not examined but subsequent treatment of the species checked). Country of type locality: Belgium.
Geographical distribution.PAL.
PAL: Belgium, Germany, United Kingdom.
Notes.Van Achterberg (1997) treated majalis as the valid name for the species called callidus by Nixon (1973) and Papp (1983a). We follow Broad et al. (2016) for the generic placement of this species.
Glyptapantelesmalleyneae Arias-Penna, 2019
Glyptapantelesmalleyneae Arias-Penna, 2019.
Type information. Holotype male, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesmalloryvanwyngaardenae Arias-Penna, 2019
Glyptapantelesmalloryvanwyngaardenae Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesmalthacae (Muesebeck, 1958)
Apantelesmalthacae Muesebeck, 1958.
Type information. Holotype female, USNM (examined). Country of type locality: Mexico.
Geographical distribution.NEO.
NEO: Mexico.
Glyptapantelesmamiae Arias-Penna, 2019
Glyptapantelesmamiae Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesmarcelotavaresi Arias-Penna, 2019
Glyptapantelesmarcelotavaresi Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesmarcepsteini Arias-Penna, 2019
Glyptapantelesmarcepsteini Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesmarcpolleti Arias-Penna, 2019
Glyptapantelesmarcpolleti Arias-Penna, 2019.
Type information. Holotype male, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesmarjorietownesae Arias-Penna, 2019
Glyptapantelesmarjorietownesae Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesmarkshawi Arias-Penna, 2019
Glyptapantelesmarkshawi Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesmarquesi (Brèthes, 1924), new combination
Protapantelesmarquesi Brèthes, 1924.
Type information. Holotype female, MACN (not examined but authoritatively identified specimens examined). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina, Brazil (SC).
Notes. Since its description within Protapanteles, this species has been variously treated as Apanteles (Shenefelt 1972) or as Cotesia (Yu et al. 2016). We have examined a relatively large series of 23 specimens from Brazil, which are deposited in the CNC and were identified to species by William Mason in 1978, after he compared them versus the type. Those specimens clearly belong to Glyptapanteles, based on the metasoma dorsally smooth, T1 narrowing towards posterior margin, T2 subtriangular, and propodeum mostly smooth and without carinae. Two of those specimens (with voucher codes CNCHYM 01307 and CNCHYM 01308 in BOLD) rendered partial DNA barcodes, which cluster near other species of Neotropical Glyptapanteles, corroborating the generic placement we propose here.
Glyptapantelesmarshawheelerae Arias-Penna, 2019
Glyptapantelesmarshawheelerae Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesmayberenbaumae Arias-Penna, 2019
Glyptapantelesmayberenbaumae Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesmeganmiltonae Arias-Penna, 2019
Glyptapantelesmeganmiltonae Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesmegistusocellus Song & Chen, 2004
Glyptapantelesmegistusocellus Song & Chen, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (JL).
Glyptapantelesmehrdadhajibabaei Arias-Penna, 2019
Glyptapantelesmehrdadhajibabaei Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesmelanotus (de Saeger, 1944), new combination
Apantelesmelanotus de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. Based on the original description, the best generic placement would be in Glyptapanteles.
Glyptapantelesmelissus (de Saeger, 1944), new combination
Apantelesmelissus de Saeger, 1944.
Type information. Holotype male, RMCA (not examined but original description checked). Country of type locality: Rwanda.
Geographical distribution.AFR.
AFR: Rwanda.
Notes. Based on the original description, the best generic placement would be in Glyptapanteles.
Glyptapantelesmenander (Nixon, 1973)
Apantelesmenander Nixon, 1973.
Type information. Holotype female, MZH (not examined but original description checked). Country of type locality: Finland.
Geographical distribution.PAL.
PAL: Finland, United Kingdom.
Glyptapantelesmerope (Nixon, 1965), new combination
Apantelesmerope Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Malaysia.
Geographical distribution.OTL.
OTL: Malaysia.
Notes. This species is placed in Glyptapanteles based on the propodeum with strong and complete median carina, T1 narrowing towards posterior margin, T2 subtriangular, inflexible hypopygium and short ovipositor sheaths.
Glyptapantelesmichelleduennesae Arias-Penna, 2019
Glyptapantelesmichelleduennesae Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesmikegatesi Arias-Penna, 2019
Glyptapantelesmikegatesi Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesmikepoguei Arias-Penna, 2019
Glyptapantelesmikepoguei Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesmikeschauffi Arias-Penna, 2019
Glyptapantelesmikeschauffi Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesmikesharkeyi Arias-Penna, 2019
Glyptapantelesmikesharkeyi Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesmilitaris (Walsh, 1861), lectotype designation
Microgastermilitaris Walsh, 1861.
Type information. Lectotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.AUS, NEA, NEO, PAL.
AUS: Hawaiian Islands; NEA: Canada (MB, NB, ON, QC), USA (AZ, AR, CA, CT, DC, FL, IL, IN, IA, KS, LA, MD, MA, MI, MN, MO, NJ, NM, NY, OK, TN, TX, VA); NEO: Argentina, Honduras, Mexico, Puerto Rico; PAL: Azores, Madeira Islands.
Notes. There is a single card piece on the pin, with seven cuts where each syntype is glued. Four syntypes are in relatively poor condition: one has only three legs glued to the card, another has only some legs and metasoma left, a third is missing the head (there is one head loose in the unit tray where the specimens are placed), and a fourth is missing the metasoma. The remaining three syntypes are mostly in good condition (although only two specimens each have one complete antenna remaining). The fourth specimen, from left to right, is a female in relatively fair condition (with one antenna complete and another antenna broken before the middle) and here we designate it as the lectotype; it is placed between a complete specimen to its left and a specimen missing the metasoma to its right.
Glyptapantelesminor Ashmead, 1906
Glyptapantelesminor Ashmead, 1906.
Type information. Lectotype female, USNM (examined). Country of type locality: Japan.
Geographical distribution.OTL, PAL.
OTL: China (GZ, TW, ZJ); PAL: Japan, Korea.
Notes.Yu et al. (2016) transferred the species to Protapanteles based on an unpublished PhD thesis on Chinese Cotesiini (Zeng 2012). However, after examining the lectotype in the USNM as well as six female and two male specimens in the EIHU collection, we found that they clearly belong to Glyptapanteles (based on smooth propodeum, T1 and T2, as well as shapes of T1 and T2), which is in agreement with other authors (e.g., Papp 1990b, Chen and Song 2004, Kotenko 2007a). Thus, for the sake of clarity the species combination is revised here.
Glyptapantelesmnesampela Austin, 2000
Glyptapantelesmnesampela Austin, 2000.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (ACT).
Glyptapantelesmontywoodi Arias-Penna, 2019
Glyptapantelesmontywoodi Arias-Penna, 2019.
Type information. Holotype male, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesmuesebecki (Blanchard, 1947)
Apantelesmuesebecki Blanchard, 1947.
Type information. Holotype female, MACN (not examined but subsequent treatment of the species checked). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina, Brazil (PR), Paraguay, Peru.
Notes. Our species concept is based on Blanchard (1947) and Whitfield et al. (2002a).
Glyptapantelesmygdonia (Nixon, 1973)
Apantelesmygdonia Nixon, 1973.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Bulgaria, Finland, France, Germany, Hungary, Iran, Ireland, Italy, Korea, Madeira Islands, Russia (KDA, PRI), Slovakia, Spain, Switzerland, Turkey, United Kingdom.
Glyptapantelesnaromae (Risbec, 1951), new combination
Apantelesnaromae Risbec, 1951.
Type information. Syntypes female and male, depository unknown (not examined but original description checked). Country of type locality: Senegal.
Geographical distribution.AFR.
AFR: Senegal.
Notes. Based on the original description (including a drawing of propodeum and T1-T2), the best generic placement of this species is in Glyptapanteles.
Glyptapantelesnataliaivanovae Arias-Penna, 2019
Glyptapantelesnataliaivanovae Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesnealweberi Arias-Penna, 2019
Glyptapantelesnealweberi Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesneoliparidis Chen & Song, 2004
Glyptapantelesneoliparidis Chen & Song, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Glyptapantelesnepitae (Wilkinson, 1934), new combination
Apantelesnepitae Wilkinson, 1934.
Type information. Holotype female, NHMUK (examined). Country of type locality: Sri Lanka.
Geographical distribution.OTL.
OTL: Sri Lanka.
Notes. After examining the holotype, we place this species in Glyptapanteles based on the inflexible hypopygium, short ovipositor sheaths with a few setae, T1 mostly parallel-sided but narrowing towards posterior margin on apical third, and T2 subtriangular (trapezoidal) in shape. However, this species is not typical within the genus, as the propodeum has two short carinae near the nucha, which appear to represent a partial areola (but just very short). Most Glyptapanteles species, when they have some carination it is mostly a complete (or partial) median, longitudinal carina, or a few, very short carinae near nucha that do not appear to represent a partial areola. But, other than those carinae, the specimen fits well within Glyptapanteles and thus we transfer it to that genus here.
Glyptapantelesnigerrimus (Roman, 1924)
Apantelesnigerrimus Roman, 1924.
Type information. Lectotype female, NHMO (not examined but subsequent treatment of the species checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Poland, Romania, Russia (ARK), Yugoslavia.
Notes.Shenefelt (1972: 579) recorded the type material for this species (female and male specimens) as being deposited in the NHRS in Stockholm, Sweden. On the other hand, Nixon (1973: 185) referred to the type of the species as being found in the NHMUK London among material previously borrowed by Wilkinson; and Nixon stated that the type was being returned to the NHMO in Oslo, Norway, where it had been originally borrowed from. We follow Nixon for the depository of this species type. However, the type cannot be a holotype, as it was part of a series in the original description (Roman 1924: 19), thus the specimen that Nixon is referring to as type would actually be the lectotype.
Glyptapantelesnigrescens (Cameron, 1906), new combination
Protapantelesnigrescens Cameron, 1906.
Type information. Holotype male, NHMUK (examined). Country of type locality: Pakistan.
Geographical distribution.OTL.
OTL: Pakistan.
Notes. The holotype, with code 3c.1032, is a male specimen and not a female as previously stated. The confusion is likely due to the relatively small size of the specimen (2.1 mm body length) and the fact that one of the gonoforceps is slightly pulled outwards, more than the rest of the external genitalia, giving the impression of being a very short ovipositor sheath. That must have been very difficult to appreciate with older microscopes and also explains why Wilkinson (1928a: 92) considered the ovipositor sheaths to be shorter than even the metatibial spurs. We have re-examined the specimen (which is in relatively poor condition, covered by metallic rust from the micropin through the mesosoma), and it is evident that is not Apanteles but Glyptapanteles (which agrees with Wilkinson’s (1928a) assessment of nigriscens being related to creatonoti, another Glyptapanteles species). Also, the type locality (only known locality for the species) is currently in Pakistan, not India (as older references mentioned, and still reflected in Yu et al. 2016).
Glyptapantelesnigricornis (Muesebeck, 1921)
Apantelesnigricornis Muesebeck, 1921.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CA, VT).
Glyptapantelesninazitaniae Arias-Penna, 2019
Glyptapantelesninazitaniae Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesninus (de Saeger, 1944), new combination
Apantelesninus de Saeger, 1944.
Type information. Syntypes female and male, RMCA (not examined but original description checked). Country of type locality: Rwanda.
Geographical distribution.AFR.
AFR: Rwanda.
Notes. Based on the original description, the best generic placement would be in Glyptapanteles.
Glyptapantelesnivalis (Papp, 1983)
Apantelesnivalis Papp, 1983.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Switzerland.
Geographical distribution.PAL.
PAL: Italy, Switzerland.
Glyptapantelesnkuli (de Saeger, 1941), new combination
Apantelesnkuli de Saeger, 1941.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Rwanda.
Geographical distribution.AFR.
AFR: Rwanda.
Notes. Based on the original description (de Saeger 1941a), the best generic placement would be in Glyptapanteles.
Glyptapantelesobliquae (Wilkinson, 1928)
Apantelesobliquae Wilkinson, 1928.
Apantelesobliquaeniger Wilkinson, 1928 [homonym of Apantelesniger Muesebeck, 1921)].
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: Bangladesh, China (GX), India, Nepal.
Glyptapantelesoctonarius (Ratzeburg, 1852)
Microgasteroctonarius Ratzeburg, 1852.
Apantelesstauropodis Marshall, 1889 [nomen nudum].
Apanteleslucifugus Lyle, 1917.
Type information. Type lost (not examined but subsequent treatment of the species checked). Country of type locality: unknown.
Geographical distribution.PAL.
PAL: Azerbaijan, Croatia, Georgia, Germany, Hungary, Ireland, Italy, Lithuania, Netherlands, Poland, Romania, Russia (PRI, TAM), Slovakia, Ukraine, United Kingdom, Yugoslavia.
Notes. Our species concept is based on Wilkinson (1945), Nixon (1973), Papp (1983a) and Tobias (1986). We examined the type series of Apanteleslucifugus (Lyle, 1917). The species distribution in Azerbaijan is based on Belokobylskij et al. (2019).
Glyptapantelesoperculinae (Fullaway, 1941)
Apantelesoperculinae Fullaway, 1941.
Type information. Holotype female, BPBM (not examined but subsequent treatment of the species checked). Country of type locality: Western Samoa.
Geographical distribution.AUS.
AUS: American Samoa, Western Samoa.
Notes. Our species concept is based on Austin and Dangerfield (1992).
Glyptapantelespachopinasi Arias-Penna, 2019
Glyptapantelespachopinasi Arias-Penna, 2019.
Type information. Holotype male, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelespalabundus (Tobias, 1986)
Apantelespalabundus Tobias, 1986.
Type information. Holotype female, ZIN (not examined but original description checked). Country of type locality: Ukraine.
Geographical distribution.PAL.
PAL: Ukraine.
Glyptapantelespallipes (Reinhard, 1880)
Apantelespallipes Reinhard, 1880.
Apantelespallidipes Marshall, 1885.
Microgasterlongicornis Provancher, 1886.
Apantelesradiatus Ashmead, 1898.
Apantelesreinhardi Wilkinson, 1936.
Type information. Lectotype female, ZMHB (not examined but subsequent treatment of the species checked). Country of type locality: Austria.
Geographical distribution.NEA, PAL.
NEA: Canada (BC, NB, ON, QC), Greenland, USA (AK, CT, IL, MA, NH, NY, OH, VA); OTL: China (FJ, HN, SH), India; PAL: Armenia, Austria, Azerbaijan, Belgium, Bulgaria, China (GS, LN), Czech Republic, Denmark, Finland, France, Germany, Hungary, Ireland, Italy, Japan, Korea, Latvia, Lithuania, Macedonia, Mongolia, Poland, Romania, Russia (KGD, MOS, PRI, SAK, VLG, VOR), Spain, Switzerland, Ukraine, United Kingdom, Yugoslavia.
Notes. Our species concept is based on Nixon (1965, 1973), Papp (1983a), Tobias (1986), Chen and Song (2004), van Achterberg (2006) and Fernandez-Triana et al. (2017b).
Glyptapantelespamitchellae Arias-Penna, 2019
Glyptapantelespamitchellae Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesparasundanus (Bhatnagar, 1950), new combination
Apantelesparasundanus Bhatnagar, 1950.
Type information. Holotype female, INPC (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. The best generic placement for this species is Glyptapanteles, based on propodeum having weak, median longitudinal carina but lacking a tranverse carina; T1 parallel-sided on anterior 0.7 but then strongly narrowing towards posterior margin; T2 smooth, trapezoidal in shape and shorter than T3 length; and ovipositor sheaths short. The year of publication of the Bhatnagar paper was until recently commonly cited as 1948 and/or 1950 (e.g., Chen and Song 2004, Yu et al. 2016), probably following Shenefelt (1972) who referred to this paper as “Bhatnagar (1948) 1950”. While the intended year for Volume X, Parts I & II of the Indian Journal of Entomology was 1948, the actual dates of publication were June 1950 (Part I) and October 1950 (Part II), as clearly shown on the cover page of the Volume, which we have checked. Because the dates of publication are the ones to be considered, and for the sake of clarity, we hereby revise the species year of description to 1950.
Glyptapantelespaulhansoni Arias-Penna, 2019
Glyptapantelespaulhansoni Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelespaulheberti Arias-Penna, 2019
Glyptapantelespaulheberti Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelespaulhurdi Arias-Penna, 2019
Glyptapantelespaulhurdi Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelespenelope (Nixon, 1965), new combination
Apantelespenelope Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Malaysia.
Geographical distribution.OTL.
OTL: Malaysia.
Notes. At present, the best generic placement for this species is Glyptapanteles, based on its inflexible hypopygium and short ovipositor sheaths. In the holotype a median sulcus on T1 is partially visible, as well as traces of transverse carinae laterally on propodeum (near spiracles).
Glyptapantelespenelopeus (Tobias, 1986)
Apantelespenelopeus Tobias, 1986.
Type information. Holotype female, ZIN (not examined but original description checked). Country of type locality: Moldova.
Geographical distribution.PAL.
PAL: Moldova.
Glyptapantelespenthocratus (Austin, 1987), new combination
Apantelespenthocratus Austin, 1987.
Type information. Holotype female, NHMUK (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Notes. The original description makes clear that this species belongs to Glyptapanteles, and even a comment from the author explicitly says so (Austin 1987: 149). After examining the holotype we here formally transfer it to Glyptapanteles, based on inflexible hypopygium, shapes of T1 and T2, and very short ovipositor sheaths with only setae near apex.
Glyptapantelespetermarzi Arias-Penna, 2019
Glyptapantelespetermarzi Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesphildevriesi Arias-Penna, 2019
Glyptapantelesphildevriesi Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesphilippinensis (Ashmead, 1904), new combination
Apantelesphilippinensis Ashmead, 1904.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Notes. This species is clearly not Apanteles. The holotype has a mostly smooth propodeum, although a median carina is visible on posterior 0.4, as well as two lateral carinae (at both sides of the median carina) which seem to define a partial areola on posterior 0.3 of propodeum; T1 is smooth and mostly parallel-sided but narrowing on posterior 0.3; T2 is smooth and trapezoidal in shape; the ovipositor and ovipositor sheaths are very short (less than 0.2 metatibia length) and the sheaths are mostly without setae (but with a few setae near apex, those setae being as long as the setae on the hypopygium). Most of those features could be associated with Glyptapanteles (shapes of T1 and T2; mostly smooth propodeum, T1, and T2, ovipositor and sheaths), but what appears to be a partially defined areola on posterior 0.3 of the propodeum would be closer to Cotesia (and in fact, there are Cotesia species with similar shape and sculpture of T1 and T2 and mostly smooth propodeum, e.g., see Figure 53 in this paper, showing Cotesiahispanica). We prefer to transfer the species to Glyptapanteles because Wilkinson (1928a: 91), who was able to examine a female paratype of the species, considered it as very close to Apantelesphytometrae Wilkinson, which is now placed in Glyptapanteles.
Figure 53.
Cotesiahispanica female, specimen without voucher code A Habitus, lateral B Habitus, magnified C Propodeum and tergite 1–2, dorsal D Mesosoma and metasoma, dorsal.
Glyptapantelesphilocampus Cameron, 1911, new combination
Apantelesphilocampus Cameron, 1911.
Type information. Syntypes female, NHMUK (examined). Country of type locality: Guyana.
Geographical distribution.NEO.
NEO: Guyana.
Notes. After examining the type series, it is evident that this species belongs to the genus Glyptapanteles (based on the sort ovipositor sheaths, inflexible hypopygium, subtriangular (trapezoidal) shape of T2 and propodeum mostly shiny and with only small carinae near nucha). Both the original description (Cameron 1911b: 327) and Shenefelt (1972: 599) mention that the type series was composed of female and male; however, after carefully examining it, we found that the five syntypes are female (the ovipositor and sheaths on the extreme left specimen are barely visible because of being covered by glue, which might have been overlooked by earlier authors).
Glyptapantelesphilwardi Arias-Penna, 2019
Glyptapantelesphilwardi Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesphoebe (Nixon, 1965), new combination
Apantelesphoebe Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Malaysia.
Geographical distribution.OTL.
OTL: Malaysia, Philippines.
Notes. Transferred to Glyptapanteles based on subtriangular T2, inflexible hypopygium and short ovipositor sheaths.
Glyptapantelesphragmataeciae (You & Zhou, 1990)
Apantelesphragmataeciae You & Zhou, 1990.
Type information. Holotype female, HUNAU (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (HN).
Notes. Our species concept is based on Chen and Song (2004).
Glyptapantelesphytometraduplus (Shenefelt, 1972), new combination
Apantelesphytometraduplus Shenefelt, 1972.
Apantelesphytometrae Risbec, 1951 [homonym of Apantelesphytometrae Wilkinson, 1928].
Type information. Holotype female, depository unknown (not examined but original description checked). Country of type locality: Senegal.
Geographical distribution.AFR.
AFR: Senegal.
Notes. Based on the original description (and associated drawing of propodeum, T1, and T2) the species is best placed in Glyptapanteles. The original description (Risbec 1951) is based on the female, but it does not detail the number of specimens actually examined by the author. However, we make the assumption that only one specimen was seen, as other descriptions in that paper mention the total number of specimens when it is more than one.
Glyptapantelesphytometrae (Wilkinson, 1928)
Apantelesphytometrae Wilkinson, 1928.
Type information. Holotype female, NHMUK (examined). Country of type locality: Western Samoa.
Geographical distribution.AUS, OTL.
AUS: Fiji, Western Samoa; OTL: Bangladesh, Indonesia.
Glyptapantelespinicola (Lyle, 1917)
Apantelespinicola Lyle, 1917.
Type information. Lectotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Hungary, Italy, Madeira Islands, Romania, Russia (KIR, KRS), Slovakia, Switzerland, United Kingdom.
Glyptapantelespolitus (Riley, 1881)
Apantelespolitus Riley, 1881.
Type information. Syntypes female and male, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (FL, IL, MO, NJ).
Glyptapantelespopovi (Telenga, 1955)
Apantelespopovi Telenga, 1955.
Type information. Lectotype female, depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Turkmenistan.
Geographical distribution.PAL.
PAL: Turkey, Turkmenistan.
Notes. Our species concept is based on Papp (1983a), Tobias (1986).
Glyptapantelesporthetriae (Muesebeck, 1928)
Apantelesporthetriae Muesebeck, 1928.
Type information. Holotype female, USNM (examined). Country of type locality: Hungary.
Geographical distribution.OTL, PAL.
OTL: India; PAL: Armenia, Austria, Azerbaijan, Bulgaria, China (JL), Croatia, Finland, France, Georgia, Germany, Greece, Hungary, Iran, Israel, Italy, Korea, Moldova, Morocco, Poland, Portugal, Romania, Russia (ZAB, DA, MOS, PRI, VOR, YAR), Serbia, Slovakia, Spain, Switzerland, Turkey, Ukraine, United Kingdom.
Notes. The species distribution in Israel is based on Belokobylskij et al. (2019).
Glyptapantelespraesens (Muesebeck, 1947)
Apantelespraesens Muesebeck, 1947.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.AUS, NEA.
AUS: Hawaiian Islands; NEA: USA (CA).
Glyptapantelespropylae (de Saeger, 1941), new combination
Apantelespropylae de Saeger, 1941.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. Based on the original description (de Saeger 1941a), the best generic placement would be in Glyptapanteles.
Glyptapantelespseudacraeae Donaldson, 1991
Glyptapantelespseudacraeae Donaldson, 1991.
Type information. Holotype female, TMSA (not examined but original description checked). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Glyptapantelespseudotsugae Fernandez-Triana, 2018
Glyptapantelespseudotsugae Fernandez-Triana, 2018.
Type information. Holotype female, CNC (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (AB, BC), USA (AZ, CA, OR).
Glyptapantelespuera (Wilkinson, 1928), new combination
Apantelespuera Wilkinson, 1928.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India, Myanmar.
Notes. This species is placed in Glyptapanteles based on the very short ovipositor sheaths, inflexible hypopygium, T1 narrowing towards posterior margin, and T2 subtriangular (trapezoidal).
Glyptapantelesrafamanitioi Arias-Penna, 2019
Glyptapantelesrafamanitioi Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesripus (Papp, 1983)
Apantelesripus Papp, 1983.
Type information. Holotype female, ZMHB (not examined but original description checked). Country of type locality: Slovakia.
Geographical distribution.PAL.
PAL: Germany, Hungary, Korea, Macedonia, Poland, Russia (TVE), Slovakia, Spain, Yugoslavia.
Glyptapantelesrobbinthorpi Arias-Penna, 2019
Glyptapantelesrobbinthorpi Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesronaldzunigai Arias-Penna, 2019
Glyptapantelesronaldzunigai Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesroysnellingi Arias-Penna, 2019
Glyptapantelesroysnellingi Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesrubens (Reinhard, 1880)
Apantelesrubens Reinhard, 1880.
Type information. Holotype male, ZMHB (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Germany, Israel, Russia (MOS), Ukraine.
Notes. Our species concept is based on Papp (1983a), Tobias (1986). The species distribution in Israel is based on Belokobylskij et al. (2019).
Glyptapantelessagmaria (Nixon, 1965)
Apantelessagmaria Nixon, 1965.
Type information. Holotype female, AEIC (not examined but original description checked). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Glyptapantelessalepus (Papp, 1983)
Apantelessalepus Papp, 1983.
Type information. Holotype female, RMNH (not examined but original description checked). Country of type locality: Netherlands.
Geographical distribution.PAL.
PAL: Greece, Netherlands, United Kingdom.
Glyptapantelessarrothripae (Weed, 1887)
Apantelessarrothripae Weed, 1887.
Type information. Lectotype female, INHS (not examined but subsequent treatment of the species checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (BC, NS, ON), USA (CT, DC, IL, MD, MA, MI, MO, NJ, NY, OH, RI, VA).
Notes. Our species concept is based on Muesebeck (1921), Mason (1981), Papp (1983a), Whitfield (1995a) and Fernandez-Triana (2010).
Glyptapantelesscottmilleri Arias-Penna, 2019
Glyptapantelesscottmilleri Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesscottshawi Arias-Penna, 2019
Glyptapantelesscottshawi Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesseydeli (de Saeger, 1941), new combination
Apantelesseydeli de Saeger, 1941.
Type information. Syntypes female and male, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. Based on the original description, the best generic placement at present is in Glyptapanteles, due to the sculpture and carination pattern of propodeum, shape and sculpture of T1–T2, and the short ovipositor sheaths.
Glyptapantelesshelbystedenfeldae Arias-Penna, 2019
Glyptapantelesshelbystedenfeldae Arias-Penna, 2019.
Type information. Holotype male, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelessibiricus (Papp, 1983)
Apantelessibiricus Papp, 1983.
Apantelessibiricus Papp, 1983 [homonym of Apantelessibiricus Fahringer, 1938].
Type information. Holotype female, ZMHB (not examined but original description checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Germany, Russia, Serbia.
Notes. The species distribution in Russia is only quoted as Siberia (Papp 1983a, Belokobylskij et al. 2019).
Glyptapantelessiderion (Nixon, 1965), new combination
Apantelessiderion Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Indonesia.
Geographical distribution.OTL.
OTL: Indonesia.
Notes. This species is clearly not an Apanteles, based on the inflexible hypopygium and very short, mostly glabrous, ovipositor sheaths. The best generic placement at present would be in Glyptapanteles; however, the propodeum has a complete transverse carina (in addition to the median one), and T1 has a weakly defined longitudinal sulcus on the anterior 0.3 of tergite. It is likely that this species, together with Apantelesatylana Nixon (which is similar to siderion) and several undescribed species we have seen in collections from the Oriental region, will be placed in a different, new genus (related to the Cotesiini group of genera; see section above Brief diagnosis of all Microgastrinae genera as they are understood in this paper, for details of our current concepts on Microgastrinae groups) in the future. Pending the resolution of these species in a future paper, here we transfer siderion and atylana to Glyptapanteles.
Glyptapantelessimus (de Saeger, 1944), new combination
Apantelessimus de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Rwanda.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Rwanda.
Notes. Based on the original description, the best generic placement at present would be in Glyptapanteles. However, the ovipositor sheaths shown in the drawing and in part of the original description also look similar to those found in Pholetesor. Further study of the specimens will be needed to conclude.
Glyptapantelessondrawardae Arias-Penna, 2019
Glyptapantelessondrawardae Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesspeciosissimus (Granger, 1949), new combination
Apantelesspeciosissimus Granger, 1949.
Type information. Syntypes female and male, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Notes. Based on the propodeum sculpture, shapes of T1 and T2, and the short length of the ovipositor sheaths (all detailed in the original description), this species is better placed in Glyptapanteles.
Glyptapantelesspilosomae (de Saeger, 1941), new combination
Apantelesspilosomae de Saeger, 1941.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Angola.
Geographical distribution.AFR.
AFR: Angola, Democratic Republic of Congo.
Notes. Based on the original description (de Saeger 1941a), the best generic placement would be in Glyptapanteles.
Glyptapantelesspodopterae Ahmad, 2009
Glyptapantelesspodopterae Ahmad, 2009.
Type information. Holotype female, AMUZ (not examined but subsequent treatment of the species checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. Our species concept is based on Gupta & Fernandez-Triana (2014).
Glyptapantelesstackelbergi (Telenga, 1955)
Apantelesstackelbergi Telenga, 1955.
Type information. Lectotype female, depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Uzbekistan.
Geographical distribution.PAL.
PAL: Uzbekistan.
Notes. Our species concept is based on Telenga (1955), Papp (1983a) and Tobias (1986).
Glyptapantelesstephaniecluttsae Arias-Penna, 2019
Glyptapantelesstephaniecluttsae Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesstephaniekirkae Arias-Penna, 2019
Glyptapantelesstephaniekirkae Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelessubpunctatus (Granger, 1949), new combination
Apantelessubpunctatus Granger, 1949.
Type information. Syntypes female and male, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Notes. Based on the propodeum sculpture, shapes of T1 and T2, and the short length of the ovipositor sheaths (all from the original description), the best generic placement for this species is in Glyptapanteles.
Glyptapantelessujeevanratnasinghami Arias-Penna, 2019
Glyptapantelessujeevanratnasinghami Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelessuniae Arias-Penna, 2019
Glyptapantelessuniae Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelessureshnaiki Arias-Penna, 2019
Glyptapantelessureshnaiki Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelessuzannegreenae Arias-Penna, 2019
Glyptapantelessuzannegreenae Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelessydneycameronae Arias-Penna, 2019
Glyptapantelessydneycameronae Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelestaniaariasae Arias-Penna, 2019
Glyptapantelestaniaariasae Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelestanyadapkeyae Arias-Penna, 2019
Glyptapantelestanyadapkeyae Arias-Penna, 2019.
Type information. Holotype male, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelestaylori (Wilkinson, 1928)
Apantelestaylori Wilkinson, 1928.
Type information. Holotype female, NHMUK (examined). Country of type locality: Indonesia.
Geographical distribution.OTL.
OTL: Indonesia.
Glyptapantelestheivorae (Shenefelt, 1972)
Apantelestheivorae Shenefelt, 1972.
Apantelesgracilariae Sonan, 1942 [primary homonym of Apantelesgracilariae Wilkinson, 1940].
Type information. Type and depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (GZ, HN, TW, YN, ZJ).
Notes. Our species concept is based on Sonan (1942) and Chen and Song (2004).
Glyptapantelesthespis (de Saeger, 1944), new combination
Apantelesthespis de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Rwanda.
Notes. Even in the original description, de Saeger (1944) suspected that this species did not belong to Apanteles, based on the ovipositor sheaths. The median longitudinal carina on the propodeum, also clearly excludes the species from Apanteles. Without examining the specimens, it is impossible to conclude but we consider the best generic placement at present to be in Glyptapanteles.
Glyptapantelesthibautdelsinnei Arias-Penna, 2019
Glyptapantelesthibautdelsinnei Arias-Penna, 2019.
Type information. Holotype male, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesthomaspapei Arias-Penna, 2019
Glyptapantelesthomaspapei Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesthompsoni (Lyle, 1927)
Apantelesthompsoni Lyle, 1927.
Type information. Holotype female, NHMUK (examined). Country of type locality: France.
Geographical distribution.AFR, OTL, PAL.
AFR: Cameroon; OTL: China (TW, ZJ); PAL: Belgium, France, Hungary, Iran, Japan, Korea, Moldova, Romania, Russia (NGR, PRI, SAK).
Glyptapantelesthoseae (Wilkinson, 1934), new combination
Apantelesthoseae Wilkinson, 1934.
Type information. Syntypes female and male, NHMUK (examined). Country of type locality: Indonesia.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Rwanda.
Notes. This species is placed in Glyptapanteles based on the short ovipositor sheaths, inflexible hypopygium, T1 narrowing towards posterior margin, and T2 subtriangular (= trapezoidal).
Glyptapantelestoluagunbiadeae Arias-Penna, 2019
Glyptapantelestoluagunbiadeae Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelestomwallai Arias-Penna, 2019
Glyptapantelestomwallai Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelestrilochae Gupta, 2013
Glyptapantelestrilochae Gupta, 2013.
Type information. Holotype female, NBAIR (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Glyptapantelesvafer (Nixon, 1965)
Apantelesvafer Nixon, 1965.
Type information. Holotype female, AEIC (not examined but original description checked). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Glyptapantelesvenustus (de Saeger, 1944), new combination
Apantelesvenustus de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Rwanda.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Rwanda, Senegal.
Notes. Based on the original description (de Saeger 1944), the best generic placement at present would be in Glyptapanteles.
Glyptapantelesvictoriapookae Arias-Penna, 2019
Glyptapantelesvictoriapookae Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesvitripennis (Curtis, 1830)
Microgastervitripennis Curtis, 1830.
Microgastervitripennis Curtis, 1829 [nomen nudum].
Microgasterfulcriger Wesmael, 1837.
Apantelesimpavidus Gautier & du Dresnay, 1926.
Type information. Lectotype female, MVMMA (not examined but subsequent treatment of the species checked). Country of type locality: United Kingdom.
Geographical distribution.OTL, PAL.
OTL: India, Pakistan; PAL: Azerbaijan, Belgium, Bulgaria, Czech Republic, Finland, France, Georgia, Germany, Greece, Hungary, Ireland, Italy, Kazakhstan, Kyrgyzstan, Latvia, Poland, Romania, Russia (IRK, MOS, PRI, SPE), Serbia, Slovakia, Spain, Sweden, Switzerland, Tajikistan, Turkey, Turkmenistan, Ukraine, United Kingdom, Uzbekistan.
Notes. Our species concept is based on Nixon (1973) and Tobias (1986). The species distribution in Turkmenistan is based on Belokobylskij et al. (2019).
Glyptapanteleswebsteri (Muesebeck, 1921)
Apanteleswebsteri Muesebeck, 1921.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (AB, NB, QC), USA (AR, NC, OH).
Glyptapanteleswilkinsoni (Fahringer, 1936), new combination
Apanteleswilkinsoni Fahringer, 1936.
Apantelesplutellae Wilkinson, 1931 [primary homonym of Apantelesplutellae Kurdjumov, 1912].
Type information. Holotype female, NHMUK (examined). Country of type locality: Indonesia.
Geographical distribution.OTL.
OTL: Indonesia.
Notes. This species is placed in Glyptapanteles based on the short ovipositor sheaths, inflexible hypopygium, T1 slightly narrowing towards posterior margin, and T2 subtriangular (trapezoidal).
Glyptapanteleswilmersimbanai Arias-Penna, 2019
Glyptapanteleswilmersimbanai Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapanteleswonyoungchoi Arias-Penna, 2019
Glyptapanteleswonyoungchoi Arias-Penna, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Glyptapantelesyalizhangae Arias-Penna, 2019
Glyptapantelesyalizhangae Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Glyptapantelesyanayacuensis Arias-Penna, 2019
Glyptapantelesyanayacuensis Arias-Penna, 2019.
Type information. Holotype female, QCAZ (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Genus Hygroplitis Thomson, 1895
Hygroplitis Thomson, 1895: 2244. Gender: masculine. Type species: Microgasterrussatus Haliday, 1834, by subsequent designation (Viereck 1914: 73).
Originally described as a subgenus of Microgaster but elevated to the generic rank by Viereck (1914). Known from nine described species, mostly from the Palaearctic region, with a few taxa reaching the Oriental and Nearctic regions. We have seen a few additional species in collections. Revisions are available for species of China (Xu and Han 2007) and Russia (Kotenko 2007a). The known host records are mostly from three families of Lepidoptera (Crambidae, Noctuidae and Tortricidae). There are 18 DNA-barcode compliant sequences of Hygroplitis in BOLD, representing two BINs; molecular data suggest that this genus might be just a group of Microgaster, but the evidence is not conclusive at present. The gender of Hygroplitis has been treated historically as feminine, but that is incorrect (Doug Yanega, pers. comm.), as the name is based on the Greek noun ?p??t?? (oplitis), which is masculine; accordingly, species names are changed below to match the gender of the genus.
Hygroplitisbasarukini Kotenko, 1993
Hygroplitisbasarukini Kotenko, 1993.
Type information. Holotype female, SIZK (not examined but subsequent treatment of the species checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (SAK).
Notes. Our species concept is based on Kotenko (2006, 2007).
Hygroplitismelligaster (Provancher, 1886)
Microgastermelligaster Provancher, 1886.
Microgasterrubricoxa Provancher, 1888.
Type information. Lectotype female, ULQC (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (MB, NB, NS, ON, PE, QC), USA (IA, MA, MI, NJ, NY, VA).
Hygroplitisnigritus Luo & You, 2005
Hygroplitisnigrita Luo & You, 2005.
Type information. Holotype female, GUGC (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (GZ).
Notes. Our species concept is based on Xu and Han (2007) and Kotenko (2007a).
Hygroplitispseudorussatus Shaw, 1992
Hygroplitispseudorussata Shaw, 1992.
Type information. Holotype female, RSME (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Netherlands, United Kingdom.
Hygroplitisrugulosus (Nees, 1834)
Microgasterrugulosus Nees, 1834.
Microgasterinfumata Haliday, 1834.
Microgasteropaca Ruthe, 1858.
Type information. Type and depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Czech Republic, Finland, Germany, Hungary, Ireland, Italy, Netherlands, Poland, Russia (C, NW), Sweden, Switzerland, Turkey, Ukraine, United Kingdom.
Notes. Our species concept is based on Shaw (2012b).
Hygroplitisruinosus Kotenko, 2007
Hygroplitisruinosa Kotenko, 2007.
Type information. Holotype female, ZIN (not examined but original description checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (PRI).
Hygroplitisrussatus (Haliday, 1834)
Microgasterrussatus Haliday, 1834.
Microgasterdimidiata Wesmael, 1837.
Microgasterbasalis Stephens, 1846.
Microgasteraomoriensis Matsumura, 1910.
Type information. Lectotype male, NHMUK (examined). Country of type locality: unknown.
Geographical distribution.OTL, PAL.
OTL: China (FJ, GX, GZ, HB, HN, JS, JX, SN, TW, YN, ZJ), Vietnam; PAL: Belgium, China (AH, BJ, HA, LN, SN, SD), Finland, France, Germany, Hungary, Ireland, Japan, Korea, Moldova, Netherlands, Poland, Russia (ALT, SA), Sweden, Turkey, Ukraine, United Kingdom.
Notes. The lectotype specimen is missing its head (except for the antennae, which are glued to the card) and the anterior part of mesosoma.
Hygroplitissinicus (Xu & He, 2000)
Microgastersinicus Xu & He, 2000.
Type information. Holotype female, ZJUH (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL. PAL.
OTL: China (FJ); PAL: China (JL).
Notes. Our species concept is based on Xu and Han (2007).
Hygroplitistoritarsis Song & Chen, 2004
Hygroplitistoritarsis Song & Chen, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Genus Hypomicrogaster Ashmead, 1898
Hypomicrogaster Ashmead, 1898: 166. Gender: feminine. Type species: Microgasterzonaria Say, 1836, by subsequent designation and monotypy (Ashmead 1900a: 132).
Known from 48 species, Hypomicrogaster may end up as just a New World genus, with the majority of species found in the Neotropical region. Species from the Old World tropics previously assigned to this genus seem to represent different lineages, and they are all assigned to different genera in this paper. A recent revision of the world species (Valerio and Whitfield 2015) has a number of inaccuracies and does not work well for all species. In addition to that, we have seen more than 100 undescribed species in collections. More than 15 families of Lepidoptera have been recorded as hosts for Hypomicrogaster, but many records are likely to be incorrect and/or need further verification. There are 2,100+ DNA-barcode compliant sequences of this genus in BOLD, representing 148 BINs. The gender of Hypomicrogaster has at times being treated as masculine; however, all genera ending in gaster are feminine, without exception (Doug Yanega, pers. comm., see also Article 30.1.2 of the ICZN). Accordingly, a large number of adjectival epithets in Hypomicrogaster are incorrect and are changed below.
Hypomicrogasteracarnas Nixon, 1965, status revised
Hypomicrogasteracarnas Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (SC).
Notes.Valerio and Whitfield (2015) synonymized H.acarnas under H.tydeus, at the same time misspelling the name acarnas as arcanas. After examining the types of both tydeus and acarnas (both in the NHMUK) we consider that there are sufficient morphological features to support both as different species, and thus here we remove acarnas from synonym with tydeus and treat both as separate species. Additionally, we provide some morphological details to separate them.
1) H.acarnas: T1 length 1.8 x its width at posterior margin; T1 almost entirely smooth (only very few, shallow, and scattered punctures near posterior margin); T2 width at posterior margin 2.1 × its length; propleuron, pronotum laterally and metacoxa entirely yellow; ovipositor sheaths 0.36 × metatibia length; body length 2.4 mm and fore wing length 2.5 mm.
2) H.tydeus: T1 length 1.3 × its width at posterior margin; posterior 0.3 of T1 with punctures; T2 width at posterior margin 3.1 × its length; propleuron, pronotum laterally and anterior half of metacoxa brown; ovipositor sheaths 0.62 × metatibia length; body length and fore wing length 2.8 mm.
Hypomicrogasteraodoa Valerio, 2015
Hypomicrogasteraodus Valerio, 2015.
Type information. Holotype female, IAVH (not examined but original description checked). Country of type locality: Colombia.
Geographical distribution.NEO.
NEO: Colombia.
Notes.Valerio and Whitfield (2015) stated that the name was adjectival; it therefore must change spelling in compliance with ICZN Article 31.2.
Hypomicrogasteraplebis Valerio, 2015
Hypomicrogasteraplebis Valerio, 2015.
Type information. Holotype female, MCZC (not examined but original description checked). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (MT).
Hypomicrogasterareolaris (Blanchard, 1947)
Apantelesareolaris Blanchard, 1947.
Microgasterblanchardi Muesebeck, 1958 [replacement name].
Hypomicrogasterdiaphaniae Muesebeck, 1958.
Hypomicrogasteracontes Nixon, 1965.
Hypomicrogastermetris Nixon, 1965.
Hypomicrogastermoscus Nixon, 1965.
Hypomicrogastersolox Nixon, 1965.
Type information. Holotype female, MACN (not examined but authoritatively identified specimens examined). Country of type locality: Argentina.
Geographical distribution.NEA, NEO.
NEA: USA (FL); NEO: Argentina, Brazil (DF, SC), Costa Rica, El Salvador, Mexico.
Notes. The original name, Apantelesareolaris Blanchard, 1947, was transferred to Microgaster and then became a secondary junior homonym of Microgasterareolaris Thomson, 1895; so Muesebeck (1958b) changed the name to Microgasterblanchardi Muesebeck, 1958. Then Valerio and Whitfield (2015) transferred the species to Hypomicrogaster as H.areolaris (Blanchard). Valerio and Whitfield (2015) also synonymized under 'areolaris' four other species of Hypomicrogaster that had been considered as valid species until that moment (see synonyms above). The type belongs to the Blanchard collection, which we assume is deposited in the MACN. We have examined the types of H.acontes Nixon, H.metris Nixon (which is broken in pieces, glued to two points on the same pin), H.moscus Nixon and H.solox Nixon (all in the NHMUK), and we consider that at least some of the synonyms proposed by Valerio and Whitfield (2015) are not justified, i.e., we think some of those species should be considered as valid. However, pending a reassessment of Hypomicrogaster in the New World, we refrain from changing the status of those species names in this paper.
Hypomicrogastercernus Valerio, 2015
Hypomicrogastercernus Valerio, 2015.
Type information. Holotype female, IAVH (not examined but original description checked). Country of type locality: Colombia.
Geographical distribution.NEO.
NEO: Colombia.
Notes.Valerio and Whitfield (2015) stated that the name was adjectival, but this is not an actual Latin adjective; it therefore must be treated as indeclinable under ICZN Article 31.2.3.
Hypomicrogastercrocina Valerio, 2015
Hypomicrogastercrocinus Valerio, 2015.
Type information. Holotype female, CNC (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (PE).
Notes.Valerio and Whitfield (2015) stated that the name was adjectival; it therefore must change spelling in compliance with ICZN Article 31.2.
Hypomicrogasterdaktulios Valerio, 2015
Hypomicrogasterdaktulios Valerio, 2015.
Type information. Holotype female, ESUW (not examined but original description checked). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Hypomicrogasterdeltis Valerio, 2015
Hypomicrogasterdeltis Valerio, 2015.
Type information. Holotype female, CNC (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (MT, RJ, RO).
Hypomicrogasterduo Valerio, 2015
Hypomicrogasterduo Valerio, 2015.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: Honduras.
Geographical distribution.NEO.
NEO: Honduras.
Hypomicrogasterecus Nixon, 1965
Hypomicrogasterecus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (SC).
Hypomicrogasterepipagis Valerio, 2015
Hypomicrogasterepipagis Valerio, 2015.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: Uruguay.
Geographical distribution.NEO.
NEO: Bolivia, Uruguay.
Hypomicrogasterespera Valerio, 2015
Hypomicrogasterespera Valerio, 2015.
Type information. Holotype female, ESUW (not examined but original description checked). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Hypomicrogasterevrys Valerio, 2015
Hypomicrogasterevrys Valerio, 2015.
Type information. Holotype female, INBio (not examined but original description checked). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Hypomicrogasterguille Valerio, 2015
Hypomicrogasterguille Valerio, 2015.
Type information. Holotype female, CNC (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Hypomicrogasterhektos Valerio, 2015
Hypomicrogasterhektos Valerio, 2015.
Type information. Holotype female, CNC (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (RJ).
Hypomicrogasterhupsos Valerio, 2015
Hypomicrogasterhupsos Valerio, 2015.
Type information. Holotype female, CNC (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Hypomicrogasterimitator (Ashmead, 1900)
Urogasterimitator Ashmead, 1900.
Type information. Holotype female, NHMUK (examined). Country of type locality: Saint Vincent.
Geographical distribution.NEO.
NEO: Grenada, Saint Vincent.
Hypomicrogasteringensis Valerio, 2015
Hypomicrogasteringensis Valerio, 2015.
Type information. Holotype female, CNC (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (RJ).
Hypomicrogasterinsolita Valerio, 2015
Hypomicrogasterinsolitus Valerio, 2015.
Type information. Holotype female, CNC (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (MT).
Notes.Valerio and Whitfield (2015) stated that the name was adjectival; it therefore must change spelling in compliance with ICZN Article 31.2.
Hypomicrogasterinversalis Valerio, 2015
Hypomicrogasterinversalis Valerio, 2015.
Type information. Holotype male, CNC (examined). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina.
Hypomicrogasterkoinos Valerio, 2015
Hypomicrogasterkoinos Valerio, 2015.
Type information. Holotype female, AEIC (not examined but original description checked). Country of type locality: Costa Rica.
Geographical distribution.NEA, NEO.
NEA: USA (MI); NEO: Brazil (PA, RJ), Costa Rica, Colombia, Ecuador, Mexico, Trinidad & Tobago, Venezuela.
Hypomicrogasterlarga Valerio, 2015
Hypomicrogasterlargus Valerio, 2015.
Type information. Holotype female, CNC (examined). Country of type locality: Ecuador.
Geographical distribution.NEA, NEO.
NEA: Canada (ON), USA (OH); NEO: Argentina, Belize, Brazil (MT, PR, SP), Cayman Islands, Colombia, Costa Rica, Dominican Republic, Ecuador, Guatemala, Mexico, Panama.
Notes.Valerio and Whitfield (2015) stated that the name was adjectival; it therefore must change spelling in compliance with ICZN Article 31.2.
Hypomicrogasterlaxa Valerio & Mason, 2015
Hypomicrogasterlaxus Valerio & Mason, 2015.
Type information. Holotype female, AEIC (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (ON), USA (KS, TX).
Notes.Valerio and Whitfield (2015) stated that the name was adjectival; it therefore must change spelling in compliance with ICZN Article 31.2.
Hypomicrogasterlinearis Valerio, 2015
Hypomicrogasterlinearis Valerio, 2015.
Type information. Holotype female, INBio (not examined but original description checked). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Hypomicrogasterlineata Valerio, 2015
Hypomicrogasterlineatus Valerio, 2015.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (NY, VA).
Notes.Valerio and Whitfield (2015) stated that the name was adjectival; it therefore must change spelling in compliance with ICZN Article 31.2.
Hypomicrogasterluisi Valerio, 2015
Hypomicrogasterluisi Valerio, 2015.
Type information. Holotype female, MCZC (not examined but original description checked). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina, Brazil (MT), Colombia, Costa Rica, Ecuador, Mexico, Peru.
Hypomicrogastermasoni Valerio, 2015
Hypomicrogastermasoni Valerio, 2015.
Type information. Holotype female, CNC (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (RJ, SP).
Hypomicrogastermesos Valerio, 2015
Hypomicrogastermesos Valerio, 2015.
Type information. Holotype female, CNC (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (SC).
Hypomicrogastermikrosus Valerio, 2015
Hypomicrogastermikrosus Valerio, 2015.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina.
Notes.Valerio and Whitfield (2015) stated that the name was adjectival, but this is not an actual Greek adjective; it therefore must be treated as indeclinable under ICZN Article 31.2.3.
Hypomicrogastermulta Valerio, 2015
Hypomicrogastermultus Valerio, 2015.
Type information. Holotype female, CNC (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Argentina, Brazil (RJ), Venezuela.
Notes.Valerio and Whitfield (2015) stated that the name was adjectival; it therefore must change spelling in compliance with ICZN Article 31.2.
Hypomicrogasterpablouzagai (Fernandez-Triana & Boudreault, 2016)
Promicrogasterpablouzagai Fernandez-Triana & Boudreault, 2016.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Hypomicrogasterpectinata Valerio, 2015
Hypomicrogasterpectinatus Valerio, 2015.
Type information. Holotype male, CNC (examined). Country of type locality: Bolivia.
Geographical distribution.NEO.
NEO: Bolivia.
Notes.Valerio and Whitfield (2015) stated that the name was adjectival; it therefore must change spelling in compliance with ICZN Article 31.2.
Hypomicrogasterplagios Valerio, 2015
Hypomicrogasterplagios Valerio, 2015.
Type information. Holotype female, INBio (not examined but original description checked). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Hypomicrogasterpollex Valerio, 2015
Hypomicrogasterpollex Valerio, 2015.
Type information. Holotype female, IAVH (not examined but original description checked). Country of type locality: Colombia.
Geographical distribution.NEO.
NEO: Colombia, Ecuador.
Hypomicrogasterrugosa Valerio, 2015
Hypomicrogasterrugosus Valerio, 2015.
Type information. Holotype female, INBio (not examined but original description checked). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Bolivia, Brazil (RO), Colombia, Costa Rica, Ecuador, Mexico, Panama, Peru.
Notes.Valerio and Whitfield (2015) stated that the name was adjectival; it therefore must change spelling in compliance with ICZN Article 31.2.
Hypomicrogastersamarshalli (Fernandez-Triana, 2010), new combination
Apantelessamarshalli Fernandez-Triana, 2010.
Type information. Holotype female, CNC (examined). Country of type locality: USA.
Geographical distribution.NEA, NEO.
NEA: Canada (ON), USA (FL); NEO: Costa Rica, Mexico.
Notes. A critical re-examination of the many available specimens (including the holotype), as well as the numerous DNA barcodes available, clearly indicates that this species is better placed within Hypomicrogaster. Among the main morphological characters that suggest so, the propodeum has an irregular pattern of carinae radiating from the nucha, as well as coarse sculpture (over most of propodeum), which have been observed in other species of Hypomicrogaster; also, the fore wing venation suggests a very small (basically obliterated) areolet, which would clearly exclude the species from Apanteles. The DNA barcodes cluster with many species of Hypomicrogaster and relatively far from other species of Apanteles, further supporting the decision to transfer the species.
Hypomicrogasterscindus Valerio, 2015
Hypomicrogasterscindus Valerio, 2015.
Type information. Holotype female, CNC (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (RJ).
Notes.Valerio and Whitfield (2015) stated that the name was adjectival, but this is not an actual Latin adjective; it therefore must be treated as indeclinable under ICZN Article 31.2.3.
Hypomicrogastersicingens Valerio, 2015
Hypomicrogastersicingens Valerio, 2015.
Type information. Holotype female, CNC (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (RJ).
Hypomicrogastersicpollex Valerio, 2015
Hypomicrogastersicpollex Valerio, 2015.
Type information. Holotype female, CNC (examined). Country of type locality: Mexico.
Geographical distribution.NEO.
NEO: Mexico.
Hypomicrogastersicscindus Valerio, 2015
Hypomicrogastersicscindus Valerio, 2015.
Type information. Holotype female, AEIC (not examined but original description checked). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (RJ).
Notes.Valerio and Whitfield (2015) stated that the name was adjectival, but this is not an actual Latin adjective; it therefore must be treated as indeclinable under ICZN Article 31.2.3.
Hypomicrogastersiderion Valerio, 2015
Hypomicrogastersiderion Valerio, 2015.
Type information. Holotype female, CNC (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Hypomicrogasterspatulae Valerio, 2015
Hypomicrogasterspatulae Valerio, 2015.
Type information. Holotype female, CNC (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (AM, PE, RO), Ecuador.
Hypomicrogasterspecialis Valerio, 2015
Hypomicrogasterspecialis Valerio, 2015.
Type information. Holotype female, MCZC (not examined but original description checked). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Bolivia, Brazil (AM, DF), Colombia, Costa Rica, Ecuador, Panama, Paraguay.
Hypomicrogastertantilla Valerio, 2015
Hypomicrogastertantillus Valerio, 2015.
Type information. Holotype female, CNC (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Argentina, Brazil (BA, RJ).
Notes.Valerio and Whitfield (2015) stated that the name was adjectival; it therefore must change spelling in compliance with ICZN Article 31.2.
Hypomicrogastertetra Valerio, 2015
Hypomicrogastertetra Valerio, 2015.
Type information. Holotype female, IAVH (not examined but original description checked). Country of type locality: Colombia.
Geographical distribution.NEO.
NEO: Colombia.
Hypomicrogastertydeus Nixon, 1965
Hypomicrogastertydeus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (SC).
Notes. See comments under H.acarnas for a justification to consider both species as separate, including morphological details.
Hypomicrogasterzan Valerio, 2015
Hypomicrogasterzan Valerio, 2015.
Type information. Holotype female, INBio (not examined but original description checked). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Brazil (SC), Costa Rica.
Hypomicrogasterzonaria (Say, 1836)
Microgasterzonaria Say, 1836.
Microgastercincta Provancher, 1881.
Protapantelesrecurvariae Ashmead, 1903.
Hypomicrogasterecdytolophae Muesebeck, 1922.
Hypomicrogasterjocarae Muesebeck, 1958.
Hypomicrogasterhypsipylae de Santis, 1972.
Type information. Type lost (not examined but subsequent treatment of the species checked). Country of type locality: USA.
Geographical distribution.NEA, NEO.
NEA: Canada (NB, NS, ON, QC), USA (AR, CO, CT, DE, DC, FL, IL, IN, IA, KS, KY, LA, MD, MA, MO, NE, NH, NJ, NY, OH, OK, PA, TX, VA, WV, WI); NEO: Costa Rica, Cuba, Guatemala, Puerto Rico.
Notes.Valerio and Whitfield (2015: 31) mentioned the species names Protapantelesrecurviriae Ashmead, 1903 and Microgasterrecurvitae (Ashmead) Muesebeck, 1920 as associated names to H.zonaria but both are typographical errors of recurvariae (and the correct year for the Muesebeck citation is 1921). In any case, H.zonaria (sensuValerio and Whitfield 2015) seems to comprise a large assemblage of species dumped altogether, but DNA and host records strongly suggest they may represent several distinct species. However, resolution of this is beyond the scope of this paper.
Genus Iconella Mason, 1981
Iconella Mason, 1981: 74. Gender: feminine. Type species: Apantelesetiellae Viereck, 1911, by original designation.
A cosmopolitan genus, with 38 described species known from all biogeographical regions except Australasian. There are revisions available for China (Chen and Song 2004), the Palaearctic (Kotenko 2007b), and the New World (Fernandez-Triana et al. 2013a), but we have seen in collections additional species, mostly from tropical areas. The genus may be split into several following more studies on the phylogeny of Microgastrinae (especially the species from the Old World tropics). The concept of Iconella and its separation from Apanteles has been controversial (e.g., Mason 1981, van Achterberg 2003, Fernandez-Triana et al. 2014e), but we consider it as a valid genus. Host data include mostly Crambidae and Pyralidae, with a couple of records from Tortricidae. There are 49 DNA-barcode compliant sequences of Iconella in BOLD, representing 12 BINs.
Iconellaaeolus (Nixon, 1965)
Apantelesaeolus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Armenia, Germany, Russia (MOS), Turkey, Ukraine, United Kingdom.
Notes. Because the name is to be considered as a noun under ICZN Article 31.2.1, it must retain its original spelling and remain as aeolus.
Iconellaalbinervis (Tobias, 1964)
Apantelesalbinervis Tobias, 1964.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Kazakhstan.
Geographical distribution.PAL.
PAL: Azerbaijan, Hungary, Kazakhstan, Moldova, Russia (S), Turkey, Ukraine.
Notes. Our species concept is based on Papp (1982), Tobias (1986), Kotenko (2007b).
Iconellaalfalfae (Nixon, 1960)
Apantelesalfalfae Nixon, 1960.
Type information. Holotype female, NHMUK (examined). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (SA).
Notes. This species was transferred to Iconella by Mason (1981), and it was also considered to belong to that genus by Austin and Dangerfield (1992). However, Yu et al. (2016) treated it as an Apanteles. After examining the female holotype, we agree it belongs to Iconella, and for the sake of clarity we revise its combination here.
Iconellaandydeansi Fernandez-Triana, 2013
Iconellaandydeansi Fernandez-Triana, 2013.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Iconellaargante (Nixon, 1976)
Apantelesargante Nixon, 1976.
Type information. Holotype female, MZH (not examined but original description checked). Country of type locality: Finland.
Geographical distribution.PAL.
PAL: Finland, Kazakhstan, Russia (PRI), Ukraine.
Iconellaassabensis (Shenefelt, 1972)
Apantelesassabensis Shenefelt, 1972.
Apanteleslacteipennis Szépligeti, 1913 [secondary homonym of Apanteleslacteipennis Curtis, 1830].
Type information. Lectotype female, HNHM (not examined but subsequent treatment of the species checked). Country of type locality: Eritrea.
Geographical distribution.AFR.
AFR: Eritrea, Tanzania.
Notes. Our species concept is based on Papp (2004).
Iconellacajani (Wilkinson, 1928), new combination
Apantelescajani Wilkinson, 1928.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. This species is placed in Iconella based on the propodeum having a complete median, longitudinal carina; the scutellar lunules maximum height being more than 0.7 x the maximum height of the lateral face of the scutellum, and the hind wing having a sinuous vein cu-a.
Iconellacanadensis Fernandez-Triana, 2013
Iconellacanadensis Fernandez-Triana, 2013.
Type information. Holotype female, CNC (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (NB, ON, QC).
Notes.Fernandez-Triana et al. (2013a) considered that a record of Iconella from Virginia, USA (reported in Yu et al. 2012) probably belongs to I.canadensis, but specimen examination is needed to conclude.
Iconellacompressiabdominis (You & Tong, 1991)
Apantelescompressiabdominis You & Tong, 1991.
Type information. Holotype female, HUNAU (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (HN).
Notes. Our species concept is based on Chen and Song (2004) and Kotenko (2007b).
Iconelladetrectans (Wilkinson, 1928), new combination
Apantelesdetrectans Wilkinson, 1928.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.AFR, OTL.
AFR: Sudan; OTL: India.
Notes. This species is placed in Iconella based on the propodeum having a complete median, longitudinal carina; the scutellar lunules maximum height being more than 0.7 x the maximum height of the lateral face of the scutellum, and the hind wing having a sinuous vein cu-a.
Iconellaetiellae (Viereck, 1911)
Apantelesetiellae Viereck, 1911.
Apantelesiselyi Cushman, 1919.
Type information. Holotype male, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA, NEO.
NEA: USA (AZ, AR, CA, CO, IA, KS, NM, OK, TX, UT, VA, WA); NEO: Mexico.
Notes. The record of this species from Mexico (Muesebeck 1958, Coronado-Blanco et al. 2004) probably refers to a different species, but specimen examination is needed to conclude.
Iconellafedtschenkoi (Kotenko, 1986)
Apantelesfedtschenkoi Kotenko, 1986.
Type information. Type and depository unknown (not examined but original description checked). Country of type locality: Uzbekistan.
Geographical distribution.PAL.
PAL: Uzbekistan.
Iconellainula Papp, 2012
Iconellainula Papp, 2012.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Cape Verde.
Geographical distribution.AFR.
AFR: Cape Verde.
Iconellaisolata (Muesebeck, 1955)
Apantelesetiellae Muesebeck, 1955.
Apantelesetiellaeisolatus Muesebeck, 1955.
Type information. Holotype female, USNM (not examined but subsequent treatment of the species checked). Country of type locality: Trinidad & Tobago.
Geographical distribution.NEO.
NEO: British Virgin Islands, Cayman Islands, Dominica, Grenada, Guyana, Montserrat, Puerto Rico, Saint Kitts & Nevis, Trinidad & Tobago.
Notes. Our species concept and geographical distribution is based on Fernandez-Triana et al. (2013a).
Iconellaisus (Nixon, 1965)
Apantelesisus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Hungary.
Geographical distribution.PAL.
PAL: Armenia, Hungary, Iran, Israel, Kazakhstan, Russia (C, S), Serbia, Spain, Uzbekistan.
Notes. The species distribution in Iran, Israel, Kazakhstan and Russia is based on Belokobylskij et al. (2019).
Iconellajason (Nixon, 1965), new combination
Apantelesjason Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Malaysia.
Geographical distribution.OTL.
OTL: Indonesia, Malaysia.
Notes. Transferred to Iconella based on the well defined, strong median carina.
Iconellajayjayrodriguezae Fernandez-Triana, 2013
Iconellajayjayrodriguezae Fernandez-Triana, 2013.
Type information. Holotype female, USNM (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica, Mexico.
Iconellalacteoides (Nixon, 1965)
Apanteleslacteoides Nixon, 1965.
Apantelesmemorabilis Alexeev, 1971.
Type information. Holotype female, NHRS (not examined but original description checked). Country of type locality: Sweden.
Geographical distribution.PAL.
PAL: Armenia, Azerbaijan, Germany, Greece, Hungary, Italy, Kazakhstan, Mongolia, Poland, Russia (PRI, ROS), Slovakia, Sweden, Turkey, Turkmenistan, Ukraine, Uzbekistan.
Iconellalynceus (Nixon, 1965), new combination
Apanteleslynceus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Notes. Transferred to Iconella based on the well defined, strong median carina, with some smaller striae radiating from it.
Iconellamasallensis (Abdinbekova, 1969)
Apantelesmasallensis Abdinbekova, 1969.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Azerbaijan.
Geographical distribution.PAL.
PAL: Azerbaijan, Tajikistan.
Notes. Our species concept is based on Kotenko (1981), Papp (1982) and Tobias (1986).
Iconellamemorata Kotenko, 2007
Iconellamemorata Kotenko, 2007.
Type information. Holotype female, SIZK (not examined but original description checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (PRI).
Iconellamera (Kotenko, 1992)
Apantelesmerus Kotenko, 1992.
Type information. Holotype female, SIZK (not examined but original description checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (ZAB).
Notes. Our species concept is based on Kotenko (1992, 2007). The species name must be treated as an adjective and not as a noun (Doug Yanega, pers. comm.) and thus it must match the gender of the genus name.
Iconellamerata (Kotenko, 1981)
Apantelesmeratus Kotenko, 1981.
Type information. Holotype female, SIZK (not examined but original description checked). Country of type locality: Ukraine.
Geographical distribution.PAL.
PAL: Russia (S), Ukraine.
Iconellamerula (Reinhard, 1880)
Apantelesmerula Reinhard, 1880.
Type information. Holotype female, ZMHB (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Austria, Belgium, Bulgaria, Finland, Germany, Hungary, Israel, Poland, Romania, Russia (S), Slovakia, Turkey, Ukraine.
Notes. Our species concept is based on Nixon (1968, 1976), Kotenko (1981), Papp (1982), and Tobias (1986). The species distribution in Israel is based on Belokobylskij et al. (2019).
Iconellameruloides (Nixon, 1965)
Apantelesmeruloides Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Turkey.
Geographical distribution.PAL.
PAL: Hungary, Iran, Israel, Jordan, Malta, Romania, Turkey.
Iconellamongashtensis Zargar & Gupta, 2019
Iconellamongashtensis Zargar & Gupta, 2019.
Type information. Holotype female, TMUC (not examined but original description checked). Country of type locality: Iran.
Geographical distribution.PAL.
PAL: Iran.
Iconellamyeloenta (Wilkinson, 1937)
Apantelesmyeloenta Wilkinson, 1937.
Type information. Holotype female, NHMUK (examined). Country of type locality: Cyprus.
Geographical distribution.PAL.
PAL: Cyprus, Greece, Iran, Israel, Moldova, Russia (NC, S), Spain, Tunisia, Turkey, Turkmenistan.
Notes. The holotype is missing its head, but otherwise is in good condition.
Iconellanagyi (Papp, 1975)
Apantelesnagyi Papp, 1975.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Romania.
Geographical distribution.PAL.
PAL: Romania.
Notes. We suspect this species does not belong to Iconella, as it does not have a median longitudinal carinae on the propodeum, one of the main defining characters of the genus. Examination of specimens will be needed to conclude on that.
Iconellaoppugnator (Papp, 1974)
Apantelesoppugnator Papp, 1974.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Korea.
Geographical distribution.PAL.
PAL: Korea.
Iconellapyrene (Nixon, 1965), new combination
Apantelespyrene Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Notes. Transferred to Iconella based on the well-defined, strong, median carina on the propodeum.
Iconellarudolphae (Kotenko, 1986)
Apantelesrudolphae Kotenko, 1986.
Type information. Type and depository unknown (not examined but original description checked). Country of type locality: Kazakhstan.
Geographical distribution.PAL.
PAL: Kazakhstan, Russia (S).
Iconellasimilus Zargar & Gupta, 2019
Iconellasimilus Zargar & Gupta, 2019.
Type information. Holotype female, TMUC (not examined but original description checked). Country of type locality: Iran.
Geographical distribution.PAL.
PAL: Iran.
Iconellasubcamilla (Tobias, 1976)
Apantelessubcamilla Tobias, 1976.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Azerbaijan.
Geographical distribution.PAL.
PAL: Azerbaijan, Cape Verde, Iran, Israel.
Notes. Our species concept is based on Tobias (1986) and Kotenko (1981, 2007).
Iconellatedanius (Nixon, 1965), new combination
Apantelestedanius Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Notes. Transferred to Iconella based on the propodeum with a complete median carina. Also, Nixon (1965) placed the species within the merula species group, which comprises other Iconella species.
Iconellaturanica (Telenga, 1955)
Apantelesturanicus Telenga, 1955.
Apantelessubtilis Alexeev, 1971.
Type information. Holotype female, depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: unknown.
Geographical distribution.PAL.
PAL: Tajikistan, Turkmenistan.
Notes. Our species concept is based on Telenga (1955), Papp (1982) and Tobias (1986).
Iconellavaliko Kotenko, 2007
Iconellavaliko Kotenko, 2007.
Type information. Holotype female, SIZK (not examined but original description checked). Country of type locality: Kyrgyzstan.
Geographical distribution.PAL.
PAL: Kyrgyzstan.
Iconellaverae (Tobias, 1976)
Apantelesverae Tobias, 1976.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Armenia.
Geographical distribution.PAL.
PAL: Armenia.
Notes. Our species concept is based on Papp (1984a), Tobias (1986) and Kotenko (2007b).
Iconellavindicius (Nixon, 1965)
Apantelesvindicius Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Italy.
Geographical distribution.PAL.
PAL: Bulgaria, Georgia, Hungary, Italy, Korea, Russia (ZAB, DA, PRI), Turkey, Ukraine.
Notes. Because the name is to be considered as a noun under ICZN Article 31.2.1, it must retain its original spelling and remain as vindicius.
Genus Illidops Mason, 1981
Illidops Mason, 1981: 56. Gender: masculine. Type species: Apantelesbutalidis Marshall, 1889, by original designation.
A cosmopolitan genus, with 37 described species known from all biogeographical regions except Australasian (one species has been introduced to Hawaii). A few species from the Neotropical region, India, and Russia Far East have been keyed out (Penteado-Dias et al. 2000, Ahmad et al. 2005a, Kotenko 2007a), but we have seen in collections many additional species, from both temperate and tropical areas. The concept of Illidops and its separation from Apanteles has been controversial (e.g., Mason 1981, van Achterberg 2003, Fernandez-Triana et al. 2014e), but we consider it a valid genus. Host data include the families Gelechiidae and Scythrididae, but they may need verification. There are 112 DNA-barcode compliant sequences of this genus in BOLD, representing 12 BINs.
Illidopsalbostigmalis van Achterberg & Fernandez-Triana, 2017
Illidopsalbostigmalis van Achterberg & Fernandez-Triana, 2017.
Type information. Holotype female, RMNH (examined). Country of type locality: Yemen.
Geographical distribution.AFR.
AFR: United Arab Emirates, Yemen.
Illidopsaridus Penteado-Dias & Scatolini, 2000
Illidopsaridus Penteado-Dias & Scatolini, 2000.
Type information. Holotype female, DCBU (not examined but original description checked). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (SP).
Illidopsassimilis (Papp, 1976)
Apantelesassimilis Papp, 1976.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Mongolia.
Geographical distribution.PAL.
PAL: Mongolia.
Illidopsazamgarhensis (Ahmad, 2005), new combination
Apantelesazamgarhensis Ahmad, 2005.
Type information. Holotype female, AMUZ (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. This species was described as Apanteles (Illidops) azamgarhensis, as the authors of the paper considered Illidops to be a subgenus within Apanteles (Ahmad et al. 2005: 229). As far as we know, no other publication has dealt with this species, except for Taxapad, which last two versions treated Illidops as a synonym (Yu et al. 2012) or as a subgenus of Apanteles (Yu et al. 2016). Thus, until now all available references had placed this species within Apanteles. In the original description, the presence or absence of a postero-median band of rugosity on the scutellar disc is not discussed, but the details of the propodeum sculpture, metasoma and fore wing venation seem to suggest that this species belongs to Illidops, thus the new combination is here proposed.
Illidopsbarcinonensis (Marshall, 1898)
Apantelesbarcinonensis Marshall, 1898.
Apantelesrhamphus Marshall, 1898.
Type information. Lectotype female, MNCN (not examined but subsequent treatment of the species checked). Country of type locality: Spain.
Geographical distribution.PAL.
PAL: Spain.
Notes. Our species concept is based on Papp (1986, 1988).
Illidopsbellicosus (Papp, 1977)
Apantelesbellicosus Papp, 1977.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Mongolia.
Geographical distribution.PAL.
PAL: Mongolia.
Illidopsblandus (Tobias & Kotenko, 1986)
Apantelesblandus Tobias & Kotenko, 1986.
Type information. Holotype female, SIZK (not examined but original description checked). Country of type locality: Tajikistan.
Geographical distribution.PAL.
PAL: Tajikistan.
Illidopsbutalidis (Marshall, 1889)
Apantelesbutalidis Marshall, 1889.
Type information. Holotype female, PCMAG (not examined but subsequent treatment of the species checked). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Bulgaria, Croatia, Germany, Hungary, Mongolia, Romania, Russia (ZAB, PRI), Serbia, Slovakia, Spain, Sweden, Tunisia, Turkey, Ukraine, United Kingdom.
Notes. Our concept of this species is based on Wilkinson (1945), Nixon (1965, 1976), Papp (1981) and Kotenko (2007a).
Illidopsbuteonis (Kotenko, 1986)
Apantelesbuteonis Kotenko, 1986.
Type information. Holotype female, SIZK (not examined but original description checked). Country of type locality: Ukraine.
Geographical distribution.PAL.
PAL: Russia (S), Ukraine.
Illidopscloelia (Nixon, 1965)
Apantelescloelia Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Switzerland.
Geographical distribution.PAL.
PAL: Austria, Hungary, Korea, Russia (E, NC), Slovakia, Switzerland, Tajikistan, Yugoslavia.
Notes. The distribution in Tajikistan is based in Belokobylskij et al. (2019).
Illidopsdauricus Kotenko, 2007
Illidopsdauricus Kotenko, 2007.
Type information. Holotype female, SIZK (not examined but original description checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (ZAB).
Illidopselectilis (Tobias, 1964)
Apanteleselectilis Tobias, 1964.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Kazakhstan.
Geographical distribution.PAL.
PAL: Croatia, Hungary, Kazakhstan, Russia (S), Serbia, Tunisia.
Notes. Our species concept is based on Nixon (1976), Papp (1981) and Tobias (1986).
Illidopskeralensis (Narendran & Sumodan, 1992)
Chelonuskeralensis Narendran & Sumodan, 1992.
Type information. Holotype female, HNHM (not examined but subsequent treatment of the species checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. Our species concept is based on van Achterberg and Narendran (1997).
Illidopskostjuki (Kotenko, 1986)
Apanteleskostjuki Kotenko, 1986.
Type information. Holotype female, SIZK (not examined but original description checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (ALT).
Illidopskostylevi (Kotenko, 1986)
Apanteleskostylevi Kotenko, 1986.
Type information. Holotype female, SIZK (not examined but original description checked). Country of type locality: Ukraine.
Geographical distribution.PAL.
PAL: Russia (ROS), Ukraine.
Illidopslamprosemae (Ahmad, 2005), new combination
Apanteleslamprosemae Ahmad, 2005.
Apanteleslamprosemae Ahmad, 2005 [primary junior homonym of Apanteleslamprosemae Wilkinson, 1928].
Type information. Holotype female, AMUZ (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. This species was described as Apanteles (Illidops) lamprosemae Ahmad, 2005, as the authors of the paper considered Illidops to be a subgenus within Apanteles (Ahmad et al. 2005: 229). As far as we know, no other publication has dealt with this species, except for Taxapad, which last two versions treated Illidops as a synonym (Yu et al. 2012) or as a subgenus of Apanteles (Yu et al. 2016). Thus, until now all available references had placed this species within Apanteles. In the original description, the presence or absence of a postero-median band of rugosity on the scutellar disc is not discussed, but the details of the propodeum sculpture, metasoma and fore wing venation seem to suggest that this species belongs to Illidops, thus the new combination is here proposed.
Illidopsmutabilis (Telenga, 1955)
Apantelesmutabilis Telenga, 1955.
Apantelesszaboi Papp, 1972.
Type information. Syntypes female and male, depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Ukraine.
Geographical distribution.PAL.
PAL: Austria, Bulgaria, Georgia, Hungary, Kazakhstan, Mongolia, Romania, Russia (KDA), Serbia, Slovakia, Spain, Tunisia, Turkey, Ukraine.
Notes. Our species concept is based on Papp (1981), Tobias (1986) and Kotenko (2007a).
Illidopsnaso (Marshall, 1885)
Apantelesnaso Marshall, 1885.
Apantelescontortus Tobias, 1964.
Apantelescrantor Nixon, 1965.
Apantelesevander Nixon, 1965.
Apantelescoresia Nixon, 1973.
Type information. Holotype male, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Afghanistan, Armenia, Azerbaijan, Bulgaria, Croatia, Finland, Georgia, Greece, Hungary, Iran, Kazakhstan, Korea, Kyrgyzstan, Macedonia, Moldova, Mongolia, Romania, Russia (KC, VOR), Serbia, Slovakia, Switzerland, Turkey, Turkmenistan, United Kingdom, Uzbekistan.
Notes. The distribution in Turkmenistan is based in Belokobylskij et al. (2019).
Illidopsnigritegula (Tobias & Kotenko, 1986)
Apantelesnigritegula Tobias & Kotenko, 1986.
Type information. Type and depository unknown (not examined but original description checked). Country of type locality: Kazakhstan.
Geographical distribution.PAL.
PAL: Kazakhstan, Russia (S).
Illidopsparanaensis Penteado-Dias & Scatolini, 2000
Illidopsparanaensis Penteado-Dias & Scatolini, 2000.
Type information. Holotype female, DCMP (not examined but original description checked). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (PR).
Illidopsperseveratus (Papp, 1977)
Apantelesperseveratus Papp, 1977.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Mongolia.
Geographical distribution.PAL.
PAL: Mongolia.
Illidopsplaniscapus (Tobias, 1976)
Apantelesplaniscapus Tobias, 1976.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (DA).
Notes. Our species concept is based on Papp (1988) and Tobias (1988). Type depository inferred from Tobias (1986).
Illidopsrostratus (Tobias, 1976)
Apantelesrostratus Tobias, 1976.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Armenia, Russia (KDA), Uzbekistan.
Notes. Our species concept is based on Papp (1988) and Tobias (1988). Type depository inferred from Tobias (1986).
Illidopsscutellaris (Muesebeck, 1921)
Apantelesscutellaris Muesebeck, 1921.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.AUS, NEA, NEO, PAL.
AUS: Hawaiian Islands; NEA: USA (AZ, CA, FL, TX); NEO: Mexico; PAL: Bulgaria, Cyprus, Greece, Hungary, Iran.
Illidopssophrosine (Nixon, 1976)
Apantelessophrosine Nixon, 1976.
Type information. Holotype female, NHMUK (examined). Country of type locality: Italy.
Geographical distribution.PAL.
PAL: Bulgaria, Hungary, Italy, Russia (ZAB, PRI).
Illidopssplendidus (Papp, 1974)
Apantelessplendidus Papp, 1974.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Hungary.
Geographical distribution.PAL.
PAL: Hungary, Russia (C).
Illidopssubversor (Tobias & Kotenko, 1986)
Apantelessubversor Tobias & Kotenko, 1986.
Type information. Holotype female, SIZK (not examined but original description checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (NVS).
Illidopssuevus (Reinhard, 1880)
Apantelessuevus Reinhard, 1880.
Apantelesminutus Szépligeti, 1896.
Apantelespolonicus Fahringer, 1936.
Apantelesbrevisternis Tobias, 1964.
Apantelessuspicax Tobias, 1964.
Apantelesdion Nixon, 1965.
Apantelessesostris Nixon, 1976.
Type information. Holotype female, ZMHB (not examined but authoritatively identified specimens examined). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Armenia, Austria, Bulgaria, Croatia, Czech Republic, France, Germany, Greece, Hungary, Iran, Kazakhstan, Korea, Macedonia, Malta, Moldova, Mongolia, Montenegro, Poland, Romania, Russia (IRK), Serbia, Slovakia, Switzerland, United Kingdom.
Notes. We examined the type of Apantelessesostris Nixon. The species distribution in Iran is based in Belokobylskij et al. (2019).
Illidopssuffectus (Tobias & Kotenko, 1986)
Apantelessuffectus Tobias & Kotenko, 1986.
Type information. Holotype female, ZIN (not examined but original description checked). Country of type locality: Kazakhstan.
Geographical distribution.PAL.
PAL: Kazakhstan.
Illidopsterrestris Wharton, 1983
Illidopsterrestris Wharton, 1983.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CA, FL, GA, TX).
Illidopstigris (Kotenko, 1986)
Apantelestigris Kotenko, 1986.
Type information. Holotype female, SIZK (not examined but original description checked). Country of type locality: Tajikistan.
Geographical distribution.PAL.
PAL: Tajikistan, Turkmenistan.
Illidopstoreicus Kotenko, 2007
Illidopstoreicus Kotenko, 2007.
Type information. Holotype female, SIZK (not examined but original description checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (ZAB).
Illidopstrabea (Nixon, 1965), new combination
Apantelestrabea Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Notes. The holotype has its eyes converging ventrally, scutellar disc with posteromedian band of rugosity, propodeum entirely strongly rugulose, and short vein R1 in the fore wing.
Illidopsurgens Kotenko, 2004
Illidopsurgens Kotenko, 2004.
Type information. Holotype female, SIZK (not examined but subsequent treatment of the species checked). Country of type locality: Kazakhstan.
Geographical distribution.PAL.
PAL: Kazakhstan, Russia (SAR).
Notes. Our species concept is based on Kotenko (2006).
Illidopsurgo (Nixon, 1965)
Apantelesurgo Nixon, 1965.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Greece.
Geographical distribution.PAL.
PAL: Armenia, Azerbaijan, Croatia, Greece, Hungary, Iran, Mongolia, Russia (S), Slovakia, Turkey.
Notes. The species distribution in Armenia and Russia are based in Belokobylskij et al. (2019).
Illidopsuvidus Penteado-Dias & Scatolini, 2000
Illidopsuvidus Penteado-Dias & Scatolini, 2000.
Type information. Holotype female, DCBU (not examined but original description checked). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (PR, SP).
Illidopsvitobiasi Kotenko, 2004
Illidopsvitobiasi Kotenko, 2004.
Type information. Holotype female, SIZK (not examined but subsequent treatment of the species checked). Country of type locality: Turkmenistan.
Geographical distribution.PAL.
PAL: Turkmenistan.
Notes. Our species concept is based on Kotenko (2006).
Genus Janhalacaste Fernandez-Triana, 2018
Janhalacaste Fernandez-Triana, 2018: 59. Gender: neuter. Type species: Janhalacastewinnieae Fernandez-Triana & Boudreault, 2018, by original designation.
Known from three species recently described from the Neotropical region (Fernandez-Triana and Boudreault 2018). We are aware of at least one additional species in collections. All known host records are from Depressariidae. There are 12 DNA-barcode compliant sequences of Janhalacaste in BOLD, representing three BINs.
Janhalacastedanieli Fernandez-Triana & Boudreault, 2018
Janhalacastedanieli Fernandez-Triana & Boudreault, 2018.
Type information. Holotype male, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Janhalacasteguanacastensis Fernandez-Triana & Boudreault, 2018
Janhalacasteguanacastensis Fernandez-Triana & Boudreault, 2018.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Janhalacastewinnieae Fernandez-Triana & Boudreault, 2018
Janhalacastewinnieae Fernandez-Triana & Boudreault, 2018.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Genus Jenopappius Fernandez-Triana, 2018
Jenopappius Fernandez-Triana, 2018: 67. Gender: neuter. Type species: JenopappiusmagyarmuzeumFernandez-Triana and Boudreault 2018, by original designation.
Known from three species from the Afrotropical region, which were recently revised (Fernandez-Triana and Boudreault 2018). We are aware of additional species in collections. No host data are currently available for this genus. There are eleven DNA-barcode compliant sequences of Jenopappius in BOLD, representing one BIN (although those sequences have not been identified in BOLD as belonging to Jenopappius, see Fernandez-Triana and Boudreault 2018 for that).
Jenopappiusaethiopicus (de Saeger, 1944)
Microplitisaethiopicus de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Kenya, Rwanda.
Jenopappiusmagyarmuzeum Fernandez-Triana & Boudreault, 2018
Jenopappiusmagyarmuzeum Fernandez-Triana & Boudreault, 2018.
Type information. Holotype female, CNC (examined). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of the Congo.
Jenopappiusniger (de Saeger, 1944)
Microplitisniger de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Genus Jimwhitfieldius Fernandez-Triana, 2018
Jimwhitfieldius Fernandez-Triana, 2018: 75. Gender: neuter. Type species: JimwhitfieldiusjamesiFernandez-Triana and Boudreault 2018, by original designation.
Known from two species from the Oriental region, which were recently revised (Fernandez-Triana and Boudreault 2018). We are aware of additional species in collections. No host data are currently available for this genus. There are 19 DNA-barcode compliant sequences of Jimwhitfieldius in BOLD, representing five BINs (although those sequences have not been identified in BOLD as belonging to Jimwhitfieldius, see Fernandez-Triana and Boudreault 2018 for that).
Jimwhitfieldiusjamesi Fernandez-Triana & Boudreault, 2018
Jimwhitfieldiusjamesi Fernandez-Triana & Boudreault, 2018.
Type information. Holotype female, QSBG (examined). Country of type locality: Thailand.
Geographical distribution.OTL.
OTL: Thailand, Vietnam.
Jimwhitfieldiussydneyae Fernandez-Triana & Boudreault, 2018
Jimwhitfieldiussydneyae Fernandez-Triana & Boudreault, 2018.
Type information. Holotype female, QSBG (examined). Country of type locality: Thailand.
Geographical distribution.OTL.
OTL: Thailand.
Genus Keylimepie Fernandez-Triana, 2016
Keylimepie Fernandez-Triana, 2016: 96. Gender: neuter. Type species: Keylimepiepeckorum Fernandez-Triana, 2016, by original designation.
Four species from the Nearctic and Afrotropical regions (Fernandez-Triana 2016, Fernandez-Triana & van Achterberg 2017). We have seen a few additional species in collections, including from the Neotropics, but the genus does not seem to be very speciose. The known species were collected in relatively hot and dry environments. No host data are currently available for this genus. There are no DNA-barcode compliant sequences of Keylimepie in BOLD, but the two African species have mini-barcodes of 276–278 bp.
Keylimepiehadhramautensis van Achterberg & Fernandez-Triana, 2017
Keylimepiehadhramautensis van Achterberg & Fernandez-Triana, 2017.
Type information. Holotype female, RMNH (examined). Country of type locality: Yemen.
Geographical distribution.AFR.
AFR: Yemen.
Keylimepiepeckorum Fernandez-Triana, 2016
Keylimepiepeckorum Fernandez-Triana, 2016.
Type information. Holotype female, CNC (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (FL).
Keylimepiesanaaensis van Achterberg & Fernandez-Triana, 2017
Keylimepiesanaaensis van Achterberg & Fernandez-Triana, 2017.
Type information. Holotype female, RMNH (examined). Country of type locality: Yemen.
Geographical distribution.AFR.
AFR: Yemen.
Keylimepiestriatus (Muesebeck, 1922), new combination
Microplitisstriatus Muesebeck, 1922.
Type information. Holotype male, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (IL, MI, TX).
Notes. Here we place this species in Keylimepie. The male holotype is not in great condition but fits well within the current genus concept (including shape and sculpture of the head, large tentorial pits, fore wing areolet, and T1 and T2 shapes and sculptures). In the USNM collection there are two other males of the genus, both identified as M.striatus by Muesebeck, but clearly representing different, undescribed species (with different venation patterns and body colouration from striatus).
Genus Kiwigaster Fernandez-Triana, Ward & Whitfield, 2011
Kiwigaster Fernandez-Triana, Ward & Whitfield, 2011: 25. Gender: feminine. Type species: Kiwigastervariabilis Fernandez-Triana & Ward, 2011, by original designation.
Only known from a single, very unique species from the Australasian region (Fernandez-Triana et al. 2011). No host data are currently available for this genus. There is one DNA-barcode compliant sequence in BOLD, that BIN characterizing the genus and species. In the original description of Kiwigaster, its gender was incorrectly stated to be masculine (Fernandez-Triana et al. 2011: 25); however all genera ending in gaster are feminine, without exception (Doug Yanega, pers. comm., see also Article 30.1.2 of the ICZN); thus here we correct that previous mistake.
Kiwigastervariabilis Fernandez-Triana & Ward, 2011
Kiwigastervariabilis Fernandez-Triana & Ward, 2011.
Type information. Holotype female, NZAC (examined). Country of type locality: New Zealand.
Geographical distribution.AUS.
AUS: New Zealand.
Genus Kotenkosius Fernandez-Triana, 2018
Kotenkosius Fernandez-Triana, 2018: 84. Gender: neuter. Type species: KotenkosiustricarinatusFernandez-Triana and Boudreault 2018, by original designation.
Known from one recently described species from the Oriental region (Fernandez-Triana and Boudreault 2018). We are aware of at least one additional species in collections. No host data are currently available for this genus. There are at least three DNA-barcode compliant sequences of Kotenkosius in BOLD, representing one BIN, with another potential, undescribed species, having a BIN (see Fernandez-Triana and Boudreault 2018 for more details).
Kotenkosiustricarinatus Fernandez-Triana & Boudreault, 2018
Kotenkosiustricarinatus Fernandez-Triana & Boudreault, 2018.
Type information. Holotype female, RMNH (examined). Country of type locality: Vietnam.
Geographical distribution.OTL.
OTL: Bangladesh, Malaysia, Taiwan, Thailand, Vietnam.
Genus Larissimus Nixon, 1965
Larissimus Nixon, 1965: 204. Gender: masculine. Type species: Larissimuscassander Nixon, 1965, by original designation.
One described species from the Neotropical region (Nixon 1965, Mason 1981). We have seen in collections (CNC) a few additional species from South America, but the genus does not seem to be very speciose. The described species has been reared from Erebidae (Arctiinae). There is one DNA-barcode compliant sequence of this genus in BOLD, representing one BIN, which corresponds to the described species; additionally, there are seven shorter sequences from specimens which represent at least one other species.
Larissimuscassander Nixon, 1965
Larissimuscassander Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (SC, SP).
Genus Lathrapanteles Williams, 1985
Lathrapanteles Williams, 1985: 1963. Gender: masculine. Type species: Apantelespapaipemae Muesebeck, 1921, by original designation.
This is a New World genus, with four species currently described from the Nearctic and Neotropical regions, which were revised by Williams (1985). We have seen a few additional species in collections (CNC), mostly from tropical areas, but Lathrapanteles does not seem to be very speciose. Host data include the family Noctuidae, with one record from Pyralidae. There are 41 DNA-barcode compliant sequences of this genus in BOLD, representing six BINs.
Lathrapantelesampyx Williams, 1985
Lathrapantelesampyx Williams, 1985.
Type information. Holotype female, USNM (examined). Country of type locality: Colombia.
Geographical distribution.NEO.
NEO: Colombia, Peru.
Lathrapantelesfuscus Williams, 1985
Lathrapantelesfuscus Williams, 1985.
Type information. Holotype female, CNC (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (BC, MB, NT, NS, QC), USA (CO, MN).
Lathrapantelesheleios Williams, 1985
Lathrapantelesheleios Williams, 1985.
Type information. Holotype female, CNC (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (ON, QC).
Notes. The Canadian record from Quebec (Aylmer) is from Fernandez-Triana et al. (2016a), a paper where that specimen was wrongly reported to be from Ontario.
Lathrapantelespapaipemae (Muesebeck, 1921)
Apantelespapaipemae Muesebeck, 1921.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (NL, ON, QC), USA (IL, IN, IA, KS, MA, MI, MO, NY, OH, OR).
Genus Mariapanteles Whitfield & Fernandez-Triana, 2012
Mariapanteles Whitfield & Fernandez-Triana, 2012: 66. Gender: masculine. Type species: Mariapantelesfelipei Whitfield, 2012, by original designation.
This is a Neotropical genus with two species currently described (Whitfield et al. 2012). We have seen a few additional species in collections (CNC), mostly from tropical areas, but Mariapanteles does not seem to be very speciose. No host data are currently available for this genus. There are four DNA-barcode compliant sequences of this genus in BOLD, representing two BINs.
Mariapantelesdapkeyae Fernandez-Triana, 2012
Mariapantelesdapkeyae Fernandez-Triana, 2012.
Type information. Holotype female, CNC (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (GO, MT).
Mariapantelesfelipei Whitfield, 2012
Mariapantelesfelipei Whitfield, 2012.
Type information. Holotype female, USNM (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Genus Markshawius Fernandez-Triana, 2018
Markshawius Fernandez-Triana, 2018: 88. Gender: neuter. Type species: MarkshawiuserucidoctusFernandez-Triana and Boudreault 2018, by original designation.
Known from three recently described species from the Oriental region (Fernandez-Triana and Boudreault 2018). We are aware of at least one additional species in collections. No host data are currently available for this genus. There is one DNA-barcode compliant sequence of Markshawius in BOLD, representing one BIN (although that sequence has not been identified in BOLD as belonging to Markshawius, see Fernandez-Triana and Boudreault 2018 for that).
Markshawiuserucidoctus Fernandez-Triana & Boudreault, 2018
Markshawiuserucidoctus Fernandez-Triana & Boudreault, 2018.
Type information. Holotype female, RMNH (examined). Country of type locality: Vietnam.
Geographical distribution.OTL.
OTL: Vietnam.
Markshawiusfrancescae Fernandez-Triana & Boudreault, 2018
Markshawiusfrancescae Fernandez-Triana & Boudreault, 2018.
Type information. Holotype female, QSBG (examined). Country of type locality: Thailand.
Geographical distribution.OTL.
OTL: Nepal, Thailand, Vietnam.
Markshawiusthailandensis Fernandez-Triana & Boudreault, 2018
Markshawiusthailandensis Fernandez-Triana & Boudreault, 2018.
Type information. Holotype female, QSBG (examined). Country of type locality: Thailand.
Geographical distribution.OTL.
OTL: Thailand.
Genus Microgaster Latreille, 1804
Microgaster Latreille, 1804: 175. Gender: feminine. Type species: Microgasteraustralis Thomson, 1895, by subsequent designation (ICZN 1988).
Liganira Walker, 1860: 308 [Name mentioned as previous error and suppressed as Microgaster (Shenefelt 1973: 694), see also Mason (1981: 80)]. Type species: Microgaster detractus Walker, 1860.
Lissogaster Bengtsson, 1926: 64. Type species: Microgasterpolita, Marshall, 1885, by subsequente designation (Muesebeck and Walkley 1951).
This was the first genus of Microgastrinae to be described and is the basis for the subfamily name. Until relatively recently, there was some confusion with the application of the name Microgaster and its type species (e.g., see van Achterberg 1982, Papp 1984c, Mason 1986, Tobias 1986, Whitfield 1987, Yu et al. 2012, 2016), which had the potential to complicate and confuse the treatment of many species used in biological control. Following van Achterberg’s (1982) examination of the lectotype of Ichneumondeprimator Fabricius, designated as the type species of Microgaster, which turned out to be a species of Microplitis, the generic name Microgaster was applied to what had been called Microplitis, and the junior synonym Lissogaster was brought into play for Microgaster auctt. Mason (1986) applied to ICZN and it was reversed by a 1988 ICZN Opinion (1510) by setting aside previous designations (i.e., deprimator) and making Microgasteraustralis Thomson the type species of Microgaster (which returned Lissogaster to synonymy under Microgaster and restored the traditional use of Microplitis). But, for a short period of time (1982–1988), the name Lissogaster was in legitimate use for Microgaster, and Microgaster for Microplitis (e.g., Papp 1984c). As currently understood, Microgaster is a cosmopolitan genus, with 104 described species. We have seen many additional species in collections, mostly from temperate areas. There are some revisions available for certain regions and/or countries, but most are outdated and even the most recent revisions do not take into account the hidden diversity that is revealed by DNA barcoding and biological data. Approximately 25 families of Lepidoptera have been recorded as hosts for Microgaster, but many records are likely to be incorrect and/or need further verification. There are 1,000+ DNA-barcode compliant sequences of this genus in BOLD, representing 67 BINs.
Microgasteracilius Nixon, 1968
Microgasteracilius Nixon, 1968.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: United Kingdom.
Notes. Reinstated as a valid species by Shaw (2012b), a decision we agree with and follow here.
Microgasteralbomarginata Fahringer, 1935
Microgasteralbomarginata Fahringer, 1935.
Type information. Holotype female, depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (GZ, SN).
Notes. Our species concept is based on Xu and Han (2007).
Microgasteralebion Nixon, 1968
Microgasteralebion Nixon, 1968.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Czech Republic, Finland, Germany, Hungary, Italy, Poland, Romania, Russia (KR), Serbia, Switzerland, Turkey, United Kingdom.
Microgasterarchboldensis Fernandez-Triana, 2018
Microgasterarchboldensis Fernandez-Triana, 2018.
Type information. Holotype female, CNC (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (FL).
Microgasterarctostaphylica Shaw, 2012
Microgasterarctostaphylica Shaw, 2012.
Type information. Holotype female, RSME (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: United Kingdom.
Microgasterareolaris Thomson, 1895
Microgasterareolaris Thomson, 1895.
Type information. Type unknowm, MZLU (not examined but subsequent treatment of the species checked). Country of type locality: Sweden.
Geographical distribution.PAL.
PAL: Armenia, Bosnia and Herzegovina, Finland, Germany, Hungary, Ireland, Mongolia, Montenegro, Norway, Poland, Romania, Russia (STA), Sweden, Switzerland, Ukraine, United Kingdom.
Notes. Our species concept is based on Nixon (1968) and Papp (1976c).
Microgasterasramenes Nixon, 1968
Microgasterasramenes Nixon, 1968.
Type information. Holotype female, NHMUK (examined). Country of type locality: Turkey.
Geographical distribution.OTL, PAL.
OTL: China (ZJ); PAL: Georgia, Hungary, Italy, Korea, Poland, Romania, Russia (PRI), Turkey.
Microgasteratropa de Saeger, 1944
Microgasteratropa de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Microgasterauriculata (Fabricius, 1804)
Ichneumonauriculatus Fabricius, 1804.
Microgasterauriculatrix Schulz, 1906 [unjustified emendation].
Type information. Type and depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Austria.
Geographical distribution.PAL.
PAL: Austria, Germany, Italy, Russia (NC, S).
Notes. Our species concept and geographical distribution is based on Nixon (1968) and Papp (1976c), but we exclude it from the UK based on Broad et al. (2016). The species is treated as a member of Ichneumonidae, as Scolobatesauriculatus (Fabricius, 1804) in Yu et al. (2016), but the status of this species (and the history of the name use) will require further clarification. The issue is currently under investigation for publication (Ghafouri Moghaddam, pers. comm.), and thus for the time being we present the basic information for this species as it concerns Microgastrinae.
Microgasteraustralis Thomson, 1895
Microgasteraustralis Thomson, 1895.
Type information. Type and depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Italy.
Geographical distribution.PAL.
PAL: Georgia, Germany, Greece, Hungary, Iran, Italy, Kazakhstan, Latvia, Moldova, Mongolia, Montenegro, Poland, Russia (PRI), Slovenia, Spain, Turkey, Turkmenistan.
Notes. Our species concept is based on Shaw et al. (2009). The species distribution in Georgia, Turkey and Turkmenistan is based on Belokobylskij et al. (2019).
Microgasterbalearica Marshall, 1898
Microgasterbalearica Marshall, 1898.
Type information. Syntypes female and male, depository unknown (not examined but original description checked). Country of type locality: Spain.
Geographical distribution.PAL.
PAL: Spain.
Notes. Our species concept is based on Marshall (1898) and Telenga (1955).
Microgasterbiaca Xu & He, 1998
Microgasterbiaca Xu & He, 1998.
Type information. Holotype female, ZJUH (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (ZJ).
Notes. Our species concept is based on Xu and Han (2007).
Microgasterbreviterebrae Xu & He, 2003
Microgasterbreviterebrae Xu & He, 2003.
Type information. Holotype female, ZJUH (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (HL, JL, LN).
Notes. Our species concept is based on Xu and Han (2007).
Microgasterbrittoni Viereck, 1917
Microgasterbrittoni Viereck, 1917.
Type information. Holotype male, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (ON), USA (CT, GA, IA, MA, MI, MN, NY, WI).
Microgastercampestris Tobias, 1964
Microgastercampestris Tobias, 1964.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Kazakhstan.
Geographical distribution.PAL.
PAL: Azerbaijan, China (HA, LN), Kazakhstan, Russia (S), Serbia, Uzbekistan.
Notes. Our species concept is based on Papp (1976c), Tobias (1986) and Xu and Han (2007).
Microgastercanadensis Muesebeck, 1922
Microgastercanadensis Muesebeck, 1922.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (AB, BC, MB, NB, NS, ON, PE, QC, SK), USA (AR, CO, MA, MI, NY, OR).
Microgastercaris Nixon, 1968
Microgastercaris Nixon, 1968.
Type information. Holotype female, NHMW (not examined but original description checked). Country of type locality: Austria.
Geographical distribution.PAL.
PAL: Austria, China (JL), Czech Republic, Hungary, Russia (C, PR), Slovakia, Switzerland.
Microgasterchrysosternis (Tobias, 1986)
Lissogasterchrysosternis Tobias, 1986.
Type information. Holotype female, ZIN (not examined but original description checked). Country of type locality: Moldova.
Geographical distribution.PAL.
PAL: Moldova.
Microgastercongregatiformis Viereck, 1917
Microgastercongregatiformis Viereck, 1917.
Type information. Holotype male, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (AB, MB, ON), USA (CA, CT, MA, MI, NJ, NY).
Microgasterconsors Nixon, 1968
Microgasterconsors Nixon, 1968.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Hungary, Slovakia, United Kingdom.
Microgastercrassicornis Ruthe, 1860
Microgastercrassicornis Ruthe, 1860.
Type information. Holotype female, depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Finland, Germany, Hungary, Ireland, Poland, Romania, Russia (BEL, YAR), Serbia, Sweden, Switzerland, United Kingdom.
Notes. Our species concept is based on Nixon (1968) and Papp (1976c).
Microgasterdebilitata Papp, 1976
Microgasterdebilitata Papp, 1976.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Mongolia.
Geographical distribution.PAL.
PAL: Mongolia.
Microgasterdeceptor Nixon, 1968
Microgasterdeceptor Nixon, 1968.
Type information. Holotype female, MZH (examined). Country of type locality: Finland.
Geographical distribution.PAL.
PAL: Finland, Slovenia.
Microgasterdeductor Nixon, 1968
Microgasterdeductor Nixon, 1968.
Type information. Holotype female, MZH (not examined but original description checked). Country of type locality: Finland.
Geographical distribution.NEA, PAL.
NEA: Canada (MB, NT, YT), USA (AK); PAL: Finland, Poland, Sweden.
Notes. The record from Poland was questioned by Fernandez-Triana (2014) as a possible misidentification, but is still kept here until more evidence is found.
Microgasterdiscoidus Xu & He, 2000
Microgasterdiscoidus Xu & He, 2000.
Type information. Holotype female, ZJUH (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (SN).
Notes. Our species concept is based on Chen and Song (2004) and Xu and Han (2007).
Microgasterductilis Nixon, 1968
Microgasterductilis Nixon, 1968.
Type information. Holotype female, MZH (examined). Country of type locality: Finland.
Geographical distribution.PAL.
PAL: Finland, Georgia, Hungary, Korea, Mongolia, Russia (PRI), United Kingdom.
Microgasterdudichi Papp, 1961
Microgasterdudichi Papp, 1961.
Type information. Holotype female, ZMHB (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Germany.
Notes. Our species concept is based on Nixon (1968), Papp (1976c) and Tobias (1986).
Microgasterelegans Herrich-Schäffer, 1838
Microgasterelegans Herrich-Schäffer, 1838.
Type information. Type and depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Germany, Netherlands.
Notes. Our species concept is based on Herrich-Schäffer (1838), Shenefelt (1973) and Belokobylskij et al. (2003). We have examined the colour plates from the original source (Herrich-Schäffer 1838) and there are three plates (numbered as 153.13, 153.14 and 153.15) which all correspond to Microgastrinae genera. Those plates are detailed enough to allow us to assign each to a genus with a high degree of certainty: plate 13 corresponds to Microgaster, 14 to Glyptapanteles, and 15 to either Dolichogenidea (most likely) or Apanteles. However, both catalogues of Szépligeti (1904: 150) and Shenefelt (1973: 705) record M.elegans as being described in plate 14. That is likely to be a mistake, as that plate is clearly not Microgaster (but the previous one definitely is).
Microgasterepagoges Gahan, 1917
Microgasterepagoges Gahan, 1917.
Type information. Holotype female, USNM (not examined but subsequent treatment of the species checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (BC, ON, QC), USA (CO, IL, IN, IA, MA, MO, NY, OH, PA, SC, TN, VA).
Notes. Our species concept is based on Muesebeck (1922), Nixon (1968) and Fernandez-Triana and Huber (2010).
Microgastererro Nixon, 1968
Microgastererro Nixon, 1968.
Type information. Holotype female, MZH (examined). Country of type locality: Finland.
Geographical distribution.PAL.
PAL: Finland, Germany, Hungary, Kazakhstan, Mongolia, Russia (KR, PRI), Serbia, Slovakia, Sweden, Switzerland.
Notes. The species distribution in Kazakhstan is based on Belokobylskij et al. (2019).
Microgastereupolis Nixon, 1968
Microgastereupolis Nixon, 1968.
Type information. Holotype female, NHMW (not examined but original description checked). Country of type locality: Austria.
Geographical distribution.PAL.
PAL: Austria, Germany, Italy, Serbia, Switzerland.
Microgasterfamula Nixon, 1968
Microgasterfamula Nixon, 1968.
Type information. Holotype female, NHMW (not examined but original description checked). Country of type locality: Austria.
Geographical distribution.PAL.
PAL: Austria, Croatia, Hungary, Moldova, Romania, Russia (C), Serbia, Slovakia, Switzerland, Turkey.
Microgasterfemoralamericana Shenefelt, 1973
Microgasterfemoralamericana Shenefelt, 1973.
Microgasterfemoralis Muesebeck, 1922 [primary homonym of Microgasterfemoralis Bouché, 1834].
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CA, ID, OR, WA).
Notes. Our species concept is based on Muesebeck (1922).
Microgasterferruginea Xu & He, 2000
Microgasterferruginea Xu & He, 2000.
Type information. Holotype male, ZJUH (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (ZJ); PAL: China (SD).
Notes. Our species concept is based on Chen and Song (2004) and Xu and Han (2007).
Microgasterfilizinancae Koçak & Kemal, 2013
Microgasterfilizinancae Koçak & Kemal, 2013.
Microgastergracilis Inanç, 1992 [primary homonym of Microgastergracilis Curtis, 1830].
Type information. Holotype female, ZMTU (not examined but subsequent treatment of the species checked). Country of type locality: Turkey.
Geographical distribution.PAL.
PAL: Turkey.
Notes.Koçak and Kemal (2013) proposed the name Microgasterfilizinancae as a replacement for M.gracilis Inanç, 1992, junior primary homonym of Microgastergracilis Curtis, 1830.
Microgasterfischeri Papp, 1960
Microgasterfischeri Papp, 1960.
Type information. Holotype male, NHMW (not examined but subsequent treatment of the species checked). Country of type locality: Austria.
Geographical distribution.PAL.
PAL: Austria, Hungary, Moldova, Mongolia, Russia (KDA, PRI), Turkey.
Notes. Our species concept is based on Shaw (2012b). We also examined two male paratypes.
Microgasterflaviventris Xu & He, 2002
Microgasterflaviventris Xu & He, 2002.
Microgasterflaviventris Xu & He, 2002 [primary homonym of Microgasterflaviventris Cresson, 1865].
Type information. Holotype female, ZJUH (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (HL).
Notes. Our species concept is based on Xu and Han (2007).
Microgasterfulvicrus Thomson, 1895
Microgasterfulvicrus Thomson, 1895.
Microgasterstriatoscutellaris Kiss, 1927.
Type information. Syntypes female and male, MZLU (not examined but subsequent treatment of the species checked). Country of type locality: Sweden.
Geographical distribution.PAL.
PAL: Finland, Germany, Hungary, Ireland, Japan, Korea, Moldova, Montenegro, Romania, Russia (DA, PRI), Serbia, Slovakia, Sweden, Turkey, United Kingdom, Uzbekistan.
Notes. Our species concept is based on Nixon (1968), Papp (1976c), and Tobias (1986). The species distribution in Japan and Uzbekistan is based on Belokobylskij et al. (2019).
Microgasterfusca Papp, 1959
Microgasterfusca Papp, 1959.
Microgasterphryne Nixon, 1968.
Type information. Holotype female, HNHM (not examined but paratype examined). Country of type locality: Hungary.
Geographical distribution.PAL.
PAL: Hungary, Macedonia, Moldova, Romania, Russia (C), Yugoslavia.
Notes. We also examined the type of Microgasterphryne Nixon.
Microgastergelechiae Riley, 1869
Microgastergelechiae Riley, 1869.
Microgastergelechia Riley, 1869 [incorrect original spelling].
Microgastergelechiaetrichotaphae Walley, 1932.
Type information. Holotype male, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (ON, QC), USA (CO, CT, DC, IL, LA, MD, MA, MO, NJ, NY, NC, ND, VA, WI).
Microgasterglabritergites Xu & He, 2000
Microgasterglabritergites Xu & He, 2000.
Type information. Holotype female, ZJUH (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (HL).
Notes. Our species concept is based on Xu and Han (2007).
Microgastergregaria (Schrank, 1781)
Ichneumongregarius Schrank, 1781.
Type information. Type and depository unknown (not examined but original description checked). Country of type locality: unknown.
Geographical distribution.PAL.
PAL: Austria.
Microgasterharnedi Muesebeck, 1922
Microgasterharnedi Muesebeck, 1922.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (IN, MA, MI, MS, SC, VA, WA).
Microgasterhimalayensis Cameron, 1910
Microgasterhimalayensis Cameron, 1910.
Type information. Holotype female, depository unknown (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. From the original description, as well as subsequent treatment of the species (Wilkinson 1927), it is not clear if this species actually belongs to Microgaster. We suspect it does not, but until further study of the type is done, it is not possible to establish with certainty the generic placement of the species, so we leave it in the genus in which it was originally described.
Microgasterhospes Marshall, 1885
Microgasterhospes Marshall, 1885.
Microgastercomptanae Viereck, 1911.
Type information. Syntypes female and male, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.NEA, PAL.
NEA: Canada (ON, QC), USA (CO, IA, KS, MD, NJ, NY, OH, UT, VA); PAL: Bulgaria, Czech Republic, Finland, Georgia, Germany, Hungary, Ireland, Italy, Lithuania, Moldova, Mongolia, Netherlands, Poland, Romania, Russia (BU, KR, PRI), Slovakia, Switzerland, United Kingdom, Uzbekistan.
Notes. We also examined the type of Microgastercomptanae Viereck, 1911, a female specimen.
Microgasterhungarica Szépligeti, 1896
Microgasterhungarica Szépligeti, 1896.
Type information. Lectotype male, HNHM (not examined but subsequent treatment of the species checked). Country of type locality: Hungary.
Geographical distribution.PAL.
PAL: Austria, Azerbaijan, Hungary, Kyrgyzstan, Moldova, Mongolia, Romania, Russia (KDA, KYA), Ukraine.
Notes. Our species concept is based on Papp (1976c, 2004). The species distribution in Azerbaijan and Kyrgyzstan is based on Belokobylskij et al. (2019).
Microgasterhyalina Cresson, 1865
Microgasterhyalina Cresson, 1865.
Type information. Holotype female, ANSP (not examined but subsequent treatment of the species checked). Country of type locality: Cuba.
Geographical distribution.NEO.
NEO: Cuba.
Notes. Our species concept is based on Muesebeck (1921).
Microgasterintercus (Schrank, 1781)
Ichneumonintercus Schrank, 1781.
Type information. Holotype female, depository unknown (not examined but original description checked). Country of type locality: Austria.
Geographical distribution.PAL.
PAL: Austria.
Microgasterkuchingensis Wilkinson, 1927
Microgasterkuchingensis Wilkinson, 1927.
Type information. Holotype female, NHMUK (examined). Country of type locality: Malaysia.
Geographical distribution.AUS, OTL, PAL.
AUS: Papua New Guinea; OTL: China (FJ, TW, ZJ), India, Malaysia, Philippines; PAL: China (JL), Japan.
Notes. The holotype is missing the head, the metasoma is detached (but glued to a card) and the micropin is full of rust.
Microgasterlatitergum Song & Chen, 2004
Microgasterlatitergum Song & Chen, 2004.
Type information. Holotype female, FAFU (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (FJ, HB); PAL: China (JL).
Notes. Our species concept is based on Xu and Han (2007).
Microgasterleechi Walley, 1935
Microgasterleechi Walley, 1935.
Type information. Holotype female, CNC (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (BC, MB, ON, QC), USA (FL, MD, MA, MI, OH, OR, PA).
Microgasterlongicalcar Xu & He, 2003
Microgasterlongicalcar Xu & He, 2003.
Microgasterlongicalcar Xu & He, 2003 [homonym of Microgasterlongicalcar Thomson, 1895].
Type information. Holotype female, ZJUH (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (HB).
Notes. Our species concept is based on Xu and Han (2007).
Microgasterlongicaudata Xu & He, 2000
Microgasterlongicaudata Xu & He, 2000.
Type information. Holotype female, ZJUH (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (ZJ).
Notes. Our species concept is based on Xu and Han (2007).
Microgasterlongiterebra Xu & He, 2000
Microgasterlongiterebra Xu & He, 2000.
Type information. Holotype female, ZJUH (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (YN).
Notes. Our species concept is based on Xu and Han (2007).
Microgasterluctuosa Haliday, 1834
Microgasterluctuosus Haliday, 1834.
Microgastercurvicrus Thomson, 1895.
Type information. Holotype male, NMID (not examined but original description checked). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Austria, Azerbaijan, Bulgaria, Croatia, Finland, Germany, Greece, Hungary, Ireland, Israel, Moldova, Mongolia, Poland, Romania, Russia (KR, KIR, PRI, SPE, YAR), Serbia, Sweden, Switzerland, Tunisia, Turkey, Turkmenistan, United Kingdom, Uzbekistan.
Notes. The original description (Haliday 1834: 239) is based on a single male specimen. Not only is that clearly stated, but the actual description, which we thoroughly checked, undoubtedly refers to a male specimen as there is no mention of an ovipositor (all previous and subsequent descriptions in that paper, when based on female specimens, mention the ovipositor as aculeus and provide details on its length, but that is missing in the description of luctuosus). Shenefelt (1973: 734) also refers to the type as male. However, van Achterberg (1997: 54–55) in his revision of Haliday collection of Braconidae mentions the type as female, which is also referred to by Taxapad (Yu et al. 2016). We follow here the original description in considering the holotype to be a male. The species distribution in Israel and Turkey is based on Belokobylskij et al. (2019).
Microgastermagnifica Wilkinson, 1929
Microgastermagnifica Wilkinson, 1929.
Type information. Holotype female, NHMUK (examined). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (QLD).
Microgastermemorata Papp, 1971
Microgastermemorata Papp, 1971.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Mongolia.
Geographical distribution.PAL.
PAL: Mongolia.
Microgastermeridiana Haliday, 1834
Microgastermeridiana Haliday, 1834.
Microgasterspinolae Haliday, 1834 [primary homonym of Microgasterspinolae Nees, 1834 (?)].
Microgasteralexis Curtis, 1837.
Microgastergrandis Thomson, 1895.
Microgastercontubernalis Marshall, 1898.
Type information. Lectotype female, NMID (examined). Country of type locality: unknown.
Geographical distribution.PAL.
PAL: Bulgaria, Canary Islands, Czech Republic, Finland, Germany, Hungary, Ireland, Italy, Kazakhstan, Latvia, Lithuania, Moldova, Poland, Romania, Russia (IRK, KR, PRI, RYA, SPE, YAR), Slovakia, Spain, Sweden, Switzerland, Turkey, Ukraine, United Kingdom.
Notes. Our species concept is based on Shaw (2012b), who also removed Microgasteracilia Nixon, 1968 from synonym and reinstated it as a valid species.
Microgastermessoria Haliday, 1834
Microgastermessoria Haliday, 1834.
Microgastertibialis Nees, 1834 [primary homonym of Microgastertibialis Curtis, 1830].
Microgasterambigua Ruthe, 1860.
Microgastermaculata Ruthe, 1860.
Microgastervulgaris Ruthe, 1860.
Microgasterpluto Morley, 1936.
Type information. Lectotype female, NMID (examined). Country of type locality: unknown.
Geographical distribution.NEA, PAL.
NEA: Canada (ON, QC); PAL: Armenia, Austria, Azerbaijan, Bulgaria, Canary Islands, China (JL, SN, XJ), Croatia, Czech Republic, Finland, France, Georgia, Germany, Hungary, Ireland, Italy, Japan, Kazakhstan, Latvia, Macedonia, Malta, Moldova, Montenegro, Netherlands, Poland, Romania, Russia (DA, KAM, KIR, KDA, MOS, ORE, PRI, SAK, SPE, VGG, YAR), Serbia, Spain, Sweden, Switzerland, Turkey, Turkmenistan, Ukraine, United Kingdom, Uzbekistan.
Notes. We also examined the type of Microgasterpluto Morley.
Microgasternerione Nixon, 1968
Microgasternerione Nixon, 1968.
Type information. Holotype female, NHMUK (examined). Country of type locality: Mexico.
Geographical distribution.NEO.
NEO: Mexico.
Microgasternigricans Nees, 1834
Microgasternigricans, Nees, 1834.
Type information. Holotype male, ZMHB (not examined but subsequent treatment of the species checked). Country of type locality: Sweden.
Geographical distribution.PAL.
PAL: Germany, Hungary, Mongolia, Russia (PRI), Sweden, United Kingdom.
Notes. Our species concept is based on Papp (2016). Broad et al. (2016) considered the species as of doubtful status in the United Kingdom, but nevertheless listed in their account, a decision we accept and follow here.
Microgasternitidula Wesmael, 1837
Microgasternitidula Wesmael, 1837.
Type information. Lectotype female, RBINS (not examined but subsequent treatment of the species checked). Country of type locality: Belgium.
Geographical distribution.PAL.
PAL: Belgium, France, Germany, Poland, Romania, Russia (DA, SPE, YAR), Sweden.
Notes. Our species concept is based on Papp (1976c) and Tobias (1986).
Microgasternixalebion Shaw, 2004
Microgasternixalebion Shaw, 2004.
Type information. Holotype female, RSME (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Belgium, France, Greece, United Kingdom.
Microgasternixoni Austin & Dangerfield, 1992
Microgasternixoni Austin & Dangerfield, 1992.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (NSW, TAS).
Microgasternobilis Reinhard, 1880
Microgasternobilis Reinhard, 1880.
Microgasternobiliscompressifemur Fahringer, 1937.
Type information. Holotype male, depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Armenia, Canary Islands, France, Germany, Hungary, Moldova, Romania, Russia (RYA), Switzerland, Ukraine.
Notes. Our species concept is based on Shaw et al. (2009). The species distribution in Armenia is based on Belokobylskij et al. (2019).
Microgasternovicia Marshall, 1885
Microgasternovicia Marshall, 1885.
Microgasterswammerdamiae Muesebeck, 1922.
Type information. Syntypes female and male, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.NEA, OTL, PAL.
NEA: USA (CT); OTL: China (GD, ZJ); PAL: Finland, Germany, Hungary, Mongolia, Russia (NW), Serbia, Switzerland, United Kingdom.
Notes. We also examined the type of Microgasterswammerdamiae Muesebeck, 1922, a female specimen: it has a relatively short ovipositor and the hypopygium is not pleated but inflexible.
Microgasternoxia Papp, 1976
Microgasternoxia Papp, 1976.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Mongolia.
Geographical distribution.PAL.
PAL: Mongolia.
Microgasterobscuripennata You & Xia, 1992
Microgasterobscuripennata You & Xia, 1992.
Type information. Holotype female, HUNAU (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (HN, ZJ).
Notes. Our species concept is based on Xu and Han (2007).
Microgasteropheltes Nixon, 1968
Microgasteropheltes Nixon, 1968.
Type information. Holotype female, NHMUK (examined). Country of type locality: Macedonia.
Geographical distribution.PAL.
PAL: Ireland, Italy, Macedonia, Romania, Turkey, Yugoslavia.
Microgasterostriniae Xu & He, 2000
Microgasterostriniae Xu & He, 2000.
Type information. Holotype female, ZJUH (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (HN, ZJ); PAL: China (LN, SD).
Notes. Our species concept is based on Xu and Han (2007).
Microgasterpantographae Muesebeck, 1922
Microgasterpantographae Muesebeck, 1922.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA, PAL.
NEA: Canada (ON), USA (IL, IA, MA, MI, MO, NY, OH, PA, VA); PAL: United Kingdom.
Microgasterparvistriga Thomson, 1895
Microgasterparvistriga Thomson, 1895.
Microgasterparvistrigis Marshall, 1897 [unjustified emendation].
Type information. Type unknown, MZLU (not examined but subsequent treatment of the species checked). Country of type locality: Sweden.
Geographical distribution.PAL.
PAL: Armenia, Bulgaria, Finland, Germany, Greece, Hungary, Iran, Korea, Mongolia, Poland, Romania, Russia (PRI), Slovakia, Sweden, Switzerland, United Kingdom.
Notes. Our species concept is based on Papp (1976c) and Shaw (2012b). The species distribution in Iran is based on Belokobylskij et al. (2019).
Microgasterperoneae Walley, 1935
Microgasterperoneae Walley, 1935.
Type information. Holotype female, CNC (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (BC, NB, NL, NS, ON, QC), USA (AK, DC, MI, OH, WA).
Microgasterphthorimaeae Muesebeck, 1922
Microgasterphthorimaeae Muesebeck, 1922.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CA, OR, WA).
Microgasterplaniabdominalis You, 2002
Microgasterplaniabdominalis You, 2002.
Type information. Holotype female, IEAS (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (QH).
Microgasterpolita Marshall, 1885
Microgasterpolita Marshall, 1885.
Microgastercarinata Bengtsson, 1926 [primary homonym of Microgastercarinata Packard, 1881].
Microgasterbengtssoni Fahringer, 1937.
Type information. Holotype male, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Armenia, Finland, Germany, Hungary, Ireland, Kazakhstan, Korea, Lithuania, Norway, Poland, Romania, Russia (KAM, PRI, SAK, SPE), Sweden, Switzerland, United Kingdom.
Notes. The species distribution in Kazakhstan is based on Belokobylskij et al. (2019).
Microgasterpostica Nees, 1834
Microgasterpostica Nees, 1834.
Microgastermarginella Wesmael, 1837.
Type information. Holotype female, ZMHB (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Belgium, Czech Republic, France, Germany, Hungary, Netherlands, Poland, Romania, Russia (PRI).
Notes. Our species concept is based on Papp (1976c) and Tobias (1986).
Microgasterprocera Ruthe, 1860
Microgasterprocerus Ruthe, 1860.
Microgasterintermedia Ivanov, 1899.
Type information. Type and depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Austria, Finland, Germany, Hungary, Ireland, Mongolia, Netherlands, Poland, Romania, Russia (SPE), Spain, Ukraine.
Notes. Our species concept is based on Nixon (1968), Papp (1976c) and Shaw (2012b).
Microgasterpseudotibialis Fahringer, 1937
Microgasterpseudotibialis Fahringer, 1937.
Microgastertibialis Brullé, 1832 [primary homonym of Microgastertibialis Curtis, 1830].
Type information. Type and depository unknown (not examined but original description checked). Country of type locality: unknown.
Geographical distribution.PAL.
PAL: Algeria, Greece.
Microgasterpunctithorax Xu & He, 2000
Microgasterpunctithorax Xu & He, 2000.
Type information. Holotype male, ZJUH (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (LN).
Notes. Our species concept is based on Xu and Han (2007).
Microgasterraschkiellae Shaw, 2012
Microgasterraschkiellae Shaw, 2012.
Type information. Holotype female, RSME (examined). Country of type locality: United Kingdom.
Geographical distribution.NEA, PAL.
NEA: Canada (MB); PAL: United Kingdom.
Microgasterrava You & Zhou, 1996
Microgasterravus You & Zhou, 1996.
Type information. Holotype female, HUNAU (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (GX).
Notes. Our species concept is based on Chen and Song (2004), Xu and Han (2007), and Kotenko (2007a). The species name must be treated as an adjective and not as a noun (Doug Yanega, pers. comm.) and thus it must match the gender of the genus name.
Microgasterreticulata Shestakov, 1940
Microgasterreticulata Shestakov, 1940.
Type information. Holotype female, NHRS (not examined but subsequent treatment of the species checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (PRI).
Notes. Our species concept is based on Nixon (1968) and Kotenko (2007a).
Microgasterrubricollis Spinola, 1851
Microgasterrubricollis Spinola, 1851.
Type information. Type and depository unknown (not examined but original description checked). Country of type locality: Chile.
Geographical distribution.NEO.
NEO: Chile.
Microgasterrufipes Nees, 1834
Microgasterrufipes Nees, 1834. [= Microgasterglobata auctt., not Linnaeus, 1758].
Ichneumongossypinus Retzius, 1783.
Ichneumonglobator Thunberg, 1822.
Microgasteranthomyiarum Bouché, 1834.
Microgasteramentorum Ratzeburg, 1844.
Microgastersubincompleta Ratzeburg, 1852.
Microgasterlaeviscuta Thomson, 1895.
Microgasterincurvata Papp, 1976.
Type information. Type and depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: unknown.
Geographical distribution.PAL.
PAL: Albania, Armenia, Austria, Azerbaijan, Belgium, Bulgaria, Canary Islands, China (JL), Croatia, Czech Republic, Finland, France, Georgia, Germany, Greece, Hungary, Iran, Ireland, Italy, Japan, Kazakhstan, Korea, Latvia, Lithuania, Moldova, Mongolia, Montenegro, Netherlands, Norway, Poland, Romania, Russia (IRK, KAM, KC, MOS, PRI, ROS, SAK, SPE, SAR, VGG, VOR, YAR), Serbia, Slovakia, Slovenia, Spain, Sweden, Switzerland, Turkey, Turkmenistan, Ukraine, United Kingdom.
Notes. We accept and follow here the decision made by van Achterberg (2014) to apply the name Microgasterrufipes Nees, 1834 to what historically has been considered Microgasterglobata Linnaeus, 1758. Because of its importance and future implications, we reproduce verbatim what van Achterberg (2014: 207) wrote:
“Ichneumonglobatus Linnaeus, 1758 has been for long a problematic species and was mostly associated with the genus Microgaster Latreille, 1804. This is contradicted by the biological data supplied in the original description and the specimens in the Linnean Society (http://linnean-on1ine.org/16250/). They may be from the original Linnean collection and are labelled (by Linnaeus?) as «globatus ?». It concerns specimens of a gregarious species with yellow hind coxae on a white cocoon-mass, probably belonging to the genus Protapanteles Ashmead, 1900. This disagrees with Linnaeus' (1761) statement that the host is Papiliobrassicae and therewith implying that this species is Cotesiaglomerata (Linnaeus, 1758), the common gregarious parasitoid of the cabbage white (Pierisbrassicae (Linnaeus, 1758)). The oldest available name for Ichneumonglobatus auctt. is Microgasterrufipes Nees, 1834”.
After we examined the two photos of the purportedly syntypes of Ichneumonglobatus Linnaeus, 1758, as depicted in the web link mentioned by van Achterberg (2014), we agree that the specimens shown there do not belong to Microgaster; we think that the best generic placement at present would be in Glyptapanteles. That implies that all historical references to Microgasterglobata Linnaeus, which were commonly associated to any Microgaster with reddish femora/legs (e.g., see comments on that by Scaramozzino et al. 2017) should now be referred to Microgasterrufipes Nees, 1834, which is the oldest available name, as van Achterberg (2014) correctly proposed [For more details on this, see the treatment of globata by older sources; e.g., rufipes was considered as one of three varieties of globata in the Hymenoptera Catalogue of Dalla Torre (1898: 153), with the two other names listed as varieties of globata being junior to rufipes)]. However, there remains a tangle of species that have been, in some cases almost certainly incorrectly, synonymized under globata (e.g., as discussed by Shaw 2012b and Broad et al. 2016), so it is too simple a solution to suggest that by accepting and following van Achterberg’s (2014) decision, all records from Europe that were previously cited as globata (i.e., all countries listed for globata in Yu et al. 2012, Yu et al. 2016, Broad et al. 2016) should just be transferred to rufipes. [One example of the problems that remain is the name Microgasterlaeviscuta Thomson, 1895. Papp (1976c) synonymized it under Ichneumonglobatus Linnaeus, 1758; however, Shaw (2012b) questioned this, based on material from the NMS he had seen, and instead suggested that laeviscuta was probably a different species but more study was required before both species were recognized as separate; subsequently, Yu et al. (2016) considered both as different species. Until more evidence is available, here we are following Shaw (2012b) and thus list laeviscuta as a questionable synonym of rufipes (= globata auctt.)]. For the morphological concept of rufipes we follow Papp (1976c), Tobias (1986), Kotenko (2007a), and Xu and Han (2007); we also read the original descriptions of the names involved (Linnaeus 1758, Nees 1834). In addition to this, we here propose that the specimens from Linnaeus be considered as a different species, for now restricted to Sweden (e.g., see Linnaeus 1761: 411, specimen 1645), and to be placed in the genus Glyptapanteles (see further comments and rationale for our decision under the Notes for the species Glyptapantelesglobatus (Linnaeus, 1758)).
Microgasterruralis Xu & He, 1998
Microgasterruralis Xu & He, 1998.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, HB, JL, LN).
Microgasterscopelosomae Muesebeck, 1926
Microgasterscopelosomae Muesebeck, 1926.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (MA).
Microgastershennongjiaensis Xu & He, 2001
Microgastershennongjiaensis Xu & He, 2001.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (HB).
Microgasterstictica Ruthe, 1858
Microgasterstictica Ruthe, 1858.
Microgasterconfusa Papp, 1971.
Type information. Type and depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: unknown.
Geographical distribution.PAL.
PAL: Bulgaria, Croatia, Czech Republic, Finland, Germany, Hungary, Ireland, Italy, Korea, Mongolia, Netherlands, Poland, Romania, Russia (PRI), Slovakia, Spain, Sweden, Switzerland, Turkey, United Kingdom, Yugoslavia.
Notes. Our species concept is based on Nixon (1968), Papp (1986) and Kotenko (2007a).
Microgastersubcompleta Nees, 1834
Microgastersubcompleta Nees, 1834.
Microgasterannulipes Curtis, 1830 [nomen nudum].
Microgastercarinata Packard, 1881.
Type information. Type and depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.NEA, PAL.
NEA: USA (IL, MA, NH, NJ, NY); OTL: China (GX); PAL: Armenia, Austria, Azerbaijan, Belarus, Belgium, Bulgaria, China (HL, JL), Croatia, Czech Republic, France, Georgia, Germany, Hungary, Ireland, Italy, Japan, Korea, Lithuania, Macedonia, Moldova, Netherlands, Poland, Romania, Russia (BEL, KHA, KDA, MOS, NGR, PRI, RYA, SAK, SPE, VOR, YAR), Slovakia, Spain, Switzerland, Turkey, Ukraine, United Kingdom, Yugoslavia.
Notes. Our species concept is based on Nixon (1968), Papp (1976c), Kotenko (2007a), Xu and Han (2007) and Shaw et al. (2009).
Microgastersubtilipunctata Papp, 1959
Microgastersubtilipunctata Papp, 1959.
Microgasterobsepiens Nixon, 1968.
Type information. Holotype female, HNHM (not examined but subsequent treatment of the species checked). Country of type locality: Hungary.
Geographical distribution.PAL.
PAL: Austria, Germany, Hungary, Moldova, Romania, Russia (NC, S), Switzerland, Turkey.
Notes. Our species concept is based on Nixon (1968) and Papp (1976c).
Microgastersyntopic Fernandez-Triana, 2018
Microgastersyntopic Fernandez-Triana, 2018.
Type information. Holotype female, CNC (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (FL, GA).
Microgasterszelenyii Papp, 1974
Microgasterszelenyii Papp, 1974.
Type information. Holotype female, HNHM (not examined but paratype examined). Country of type locality: Korea.
Geographical distribution.OTL, PAL.
OTL: China (GZ, ZJ); PAL: China (HA, JL, LN), Korea, Russia (PRI).
Microgastertaishana Xu, He & Chen, 1998
Microgastertaishana Xu, He & Chen, 1998.
Type information. Holotype female, SAUC (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (SD).
Notes. Our species concept is based on Chen and Song (2004), Xu and Han (2007) and Kotenko (2007a). The depository acronym is based on the institution name, Shandong Agricultural University, China.
Microgastertianmushana Xu & He, 2001
Microgastertianmushana Xu & He, 2001.
Type information. Holotype female, ZJUH (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (ZJ).
Notes. Our species concept is based on Xu and Han (2007).
Microgastertjibodas Wilkinson, 1927
Microgastertjibodas Wilkinson, 1927.
Type information. Holotype female, NHMUK (examined). Country of type locality: Indonesia.
Geographical distribution.OTL.
OTL: Indonesia.
Microgastertortricis (Schrank, 1781)
Ichneumontortricis Schrank, 1781.
Type information. Type and depository unknown (not examined but original description checked). Country of type locality: Austria.
Geographical distribution.PAL.
PAL: Austria.
Microgastertremenda Papp, 1971
Microgastertremenda Papp, 1971.
Type information. Holotype female, HNHM (not examined but subsequent treatment of the species checked). Country of type locality: Mongolia.
Geographical distribution.PAL.
PAL: Mongolia.
Notes. Our species concept is based on Kotenko (2007a).
Microgasteruliginosa Thomson, 1895
Microgasteruliginosus Thomson, 1895.
Type information. Holotype female, MZLU (not examined but subsequent treatment of the species checked). Country of type locality: Sweden.
Geographical distribution.PAL.
PAL: Finland, Netherlands, Poland, Romania, Russia (NW), Sweden.
Notes. Our species concept is based on Papp (1976c).
Microgasterutibilis Papp, 1976
Microgasterutibilis Papp, 1976.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Mongolia.
Geographical distribution.PAL.
PAL: Mongolia.
Notes. Our species concept is based on Papp (1976c) and Kotenko (2007a).
Microgastervaricornis Rondani, 1872
Microgastervaricornis Rondani, 1872.
Type information. Type and depository unknown (not examined). Country of type locality: Italy.
Geographical distribution.PAL.
PAL: Italy.
Microgasteryichunensis Xu & Chen, 2002
Microgasteryichunensis Xu & Chen, 2002.
Type information. Holotype female, ZJUH (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (HL).
Notes. Our species concept is based on Xu and Han (2007).
Microgasteryunnanensis Xu & He, 1999
Microgasteryunnanensis Xu & He, 1999.
Type information. Holotype female, ZJUH (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (YN).
Notes. Our species concept is based on Xu and Han (2007).
Microgasterzhaoi Xu & He, 1997
Microgasterzhaoi Xu & He, 1997.
Type information. Holotype female, ZJUH (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Notes. Our species concept is based on Xu and Han (2007).
Genus Microplitis Foerster, 1863
Microplitis Foerster, 1863: 245. Gender: masculine. Type species: Microgastersordipes Nees, 1834, by original designation.
This is a cosmopolitan genus, with 192 described species, but we have seen many additional species in collections, mostly from temperate areas, and the actual number of species could be at least twice that currently known. There are some revisions available for certain regions and/or countries, but most are outdated and even the most recent ones do not take into account the hidden diversity that is revealed by DNA barcoding and biological data. Approximately 12 families of Lepidoptera have been recorded as hosts for Microplitis, but many records are likely to be incorrect and/or need further verification. There are almost 4,000 DNA-barcode compliant sequences of this genus in BOLD, representing 212 BINs.
Microplitisabrs Austin & Dangerfield, 1993
Microplitisabrs Austin & Dangerfield, 1993.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS, OTL.
AUS: Australia (QLD); OTL: Vietnam.
Microplitisadelaidensis Austin & Dangerfield, 1993
Microplitisadelaidensis Austin & Dangerfield, 1993.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (SA).
Microplitisadisurae (Subba Rao & Sharma, 1960), new combination
Microgasteradisurae Subba Rao & Sharma, 1960.
Type information. Holotype female, INPC (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. Transferred to Microplitis based on the original descriptions and illustrations provided, which show short ovipositor and ovipositor sheaths (the sheaths with only few setae concentrated at apex), T1 narrowing towards posterior margin and more than 2.5 × as long medially as its width at posterior margin, T2 subtriangular, metatibial spurs less than half length of first segment of metatarsus. All these characters exclude the species from being Microgaster and strongly indicate the best generic placement at present to be in Microplitis.
Microplitisadrianguadamuzi Fernandez-Triana & Whitfield, 2015
Microplitisadrianguadamuzi Fernandez-Triana & Whitfield, 2015.
Type information. Holotype female, USNM (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Microplitisaduncus (Ruthe, 1860)
Microgasteraduncus Ruthe, 1860.
Microgasterbrachycerus Thomson, 1895.
Type information. Holotype female, NHMUK (examined). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Bulgaria, Finland, Georgia, Germany, Hungary, Iran, Korea, Mongolia, Netherlands, Poland, Russia (KAM), Selvagens Islands, Serbia, Sweden, Switzerland, Tunisia, Turkmenistan, United Kingdom.
Notes. The holotype is in relatively poor condition, missing the antennae and with the hind wings glued over the body, obscuring or impeding the observation of features of part of the mesosoma and most of the metasoma. The species distribution in Georgia, Korea, and Turkmenistan is based on Belokobylskij et al. (2019).
Microplitisajmerensis Rao & Kurian, 1950
Microplitisajmerensis Rao & Kurian, 1950.
Type information. Holotype female, NZSI (not examined but subsequent treatment of the species checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. Our species concept is based on Gupta (2013a) and Ranjith et al. (2015a).
Microplitisalajensis Telenga, 1955, restored combination
Microplitisalajensis Telenga, 1955.
Type information. Lectotype female, depository unknown (not examined but original description checked). Country of type locality: Kyrgyzstan.
Geographical distribution.PAL.
PAL: Kyrgyzstan, Mongolia.
Notes. Our species concept is based on Telenga (1955), Papp (1984c) and Tobias (1986). This species was at times considered to belong to Microgaster, e.g., Papp (1984c) and Tobias (1986), as part of the confusion with the application and use of the Microplitis and Microgaster names, which was only solved after 1988 (see more details and comments under our introduction to the genus Microgaster above, p 717). The correct generic placement at present would be in Microplitis, which is corroborated by the original description and images, as well as the key and images provided by Papp (1984c). Because some of the more recent references (e.g., Yu et al. 2016) still refer to the species within Microgaster, for the sake of clarity we restore its status here.
Microplitisalaskensis Ashmead, 1902
Microplitisalaskensis Ashmead, 1902.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (AB, BC, MB, NS, ON, QC), USA (AK, AZ, CA, CO, IL, KS, MA, MT, NY, OR, WA).
Microplitisalbipennis Abdinbekova, 1969
Microplitisalbipennis Abdinbekova, 1969.
Type information. Holotype male, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Azerbaijan.
Geographical distribution.PAL.
PAL: Azerbaijan, Hungary, Mongolia, Poland, Russia (NC), Turkey.
Notes. Our species concept is based on Papp (1984c) and Tobias (1986).
Microplitisalbotibialis Telenga, 1955
Microplitisalbotibialis Telenga, 1955.
Type information. Type and depository unknown (not examined but original description checked). Country of type locality: Russia.
Geographical distribution.OTL, PAL.
OTL: China (HN); PAL: China (HA, JL, LN), Hungary, Korea, Mongolia, Russia (ZAB, PRI).
Microplitisalexanderrojasi Fernandez-Triana & Whitfield, 2015
Microplitisalexanderrojasi Fernandez-Triana & Whitfield, 2015.
Type information. Holotype female, USNM (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Microplitisaltissimus Fernandez-Triana, 2018
Microplitisaltissimus Fernandez-Triana, 2018.
Type information. Holotype female, CNC (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CO).
Microplitisamplitergius Xu & He, 2002
Microplitisamplitergius Xu & He, 2002.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (GZ, ZJ); PAL: China (LN, NX).
Microplitisaprilae Austin & Dangerfield, 1993
Microplitisaprilae Austin & Dangerfield, 1993.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS, OTL.
AUS: Australia (NSW, NT, QLD); OTL: Vietnam.
Microplitisareyongensis Austin & Dangerfield, 1993
Microplitisareyongensis Austin & Dangerfield, 1993.
Type information. Holotype female, AEIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS, OTL.
AUS: Australia (NT); OTL: India, Vietnam.
Microplitisariatus Papp, 1979
Microplitisariatus Papp, 1979.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Tunisia.
Geographical distribution.PAL.
PAL: Canary Islands, Tunisia.
Microplitisatamiensis Ashmead, 1906
Microplitisatamiensis Ashmead, 1906.
Type information. Holotype male, USNM (examined). Country of type locality: Japan.
Geographical distribution.PAL.
PAL: Japan, Korea.
Microplitisautographae Muesebeck, 1922
Microplitisautographae Muesebeck, 1922.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (AB, ON, QC), USA (AZ, ID, KS, NM).
Microplitisbamagensis Austin & Dangerfield, 1993
Microplitisbamagensis Austin & Dangerfield, 1993.
Type information. Holotype female, AEIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (QLD).
Microplitisbasalis (Bingham, 1906)
Microgasterbasalis Bingham, 1906.
Microgasterbasalis Bingham, 1906 [primary homonym of Microgasterbasalis Stephens, 1846].
Type information. Holotype female, OUMNH (not examined but subsequent treatment of the species checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (QLD).
Notes. Our species concept is based on Austin and Dangerfield (1993).
Microplitisbasipallescentis Song & Chen, 2008
Microplitisbasipallescentis Song & Chen, 2008.
Type information. Holotype female, HUNAU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (HB).
Microplitisbeyarslani Inanç, 2002
Microplitisbeyarslani Inanç, 2002.
Type information. Holotype female, ZMTU (not examined). Country of type locality: Turkey.
Geographical distribution.PAL.
PAL: Turkey.
Notes. The depository acronym was selected based on the institution name (Zoological Museum, Trakya University, Turkey).
Microplitisbicoloratus Xu & He, 2003
Microplitisbicoloratus Xu & He, 2003.
Microplitisbicoloratus Chen, 2004 [primary homonym of Microplitisbicoloratus Xu & He, 2003].
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (FJ, HB, ZJ), India; PAL: China (SD).
Microplitisblascoi Papp & Shaw, 2001
Microplitisblascoi Papp & Shaw, 2001.
Type information. Holotype female, RSME (examined). Country of type locality: Spain.
Geographical distribution.PAL.
PAL: Spain.
Microplitisbomiensis Zhang, 2019
Microplitisbomiensis Zhang, 2019.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (XZ).
Microplitisborealis Xu & He, 2000
Microplitisborealis Xu & He, 2000.
Microplitisborealis Xu & He, 2000 [primary homonym of Microplitisborealis Marshall, 1885].
Type information. Holotype male, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (JL, LN, XJ).
Microplitisbradleyi Muesebeck, 1922
Microplitisbradleyi Muesebeck, 1922.
Type information. Holotype female, CUIC (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (AB, BC), USA (CA, OR, UT).
Microplitisbrassicae Muesebeck, 1922
Microplitisbrassicae Muesebeck, 1922.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (AZ, CA, CO, NE, NM, TX).
Microplitisbrevispina Song & Chen, 2008
Microplitisbrevispina Song & Chen, 2008.
Type information. Holotype female, HUNAU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Microplitiscapeki Nixon, 1970
Microplitiscapeki Nixon, 1970.
Type information. Holotype female, MMBC (not examined but original description checked). Country of type locality: Czech Republic.
Geographical distribution.PAL.
PAL: Czech Republic, Germany, Hungary.
Microplitiscarinatus Song & Chen, 2008
Microplitiscarinata Song & Chen, 2008.
Microplitiscarinata Song & Chen, 2008 [primary homonym of Microplitiscarinata Ahsmead, 1900].
Type information. Holotype female, HUNAU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (HB).
Microplitiscarinicollis (Cameron, 1905)
Microgaster (?) carinicollis Cameron, 1905.
Type information. Type and depository unknown (not examined but original description checked). Country of type locality: Sri Lanka.
Geographical distribution.OTL.
OTL: India, Sri Lanka.
Microplitiscarteri Walley, 1932
Microplitiscarteri Walley, 1932.
Type information. Holotype male, CNC (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (AB).
Microplitiscebes Nixon, 1970
Microplitiscebes Nixon, 1970.
Type information. Holotype female, NHMUK (examined). Country of type locality: Switzerland.
Geographical distribution.PAL.
PAL: Austria, Croatia, Germany, Greece, Hungary, Mongolia, Poland, Serbia, Slovakia, Spain, Switzerland, Turkey.
Microplitisceratomiae Riley, 1881
Microplitisceratomiae Riley, 1881.
Microplitiswaldeni Viereck, 1917.
Microplitisceratomiaeactuosus Riley, 1881.
Type information. Syntypes female and male, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (NB, NS, ON, QC, SK), USA (AR, CA, CO, CT, DC, IL, KS, MA, MI, MO, NJ, NM, NY, OR, RI, TX).
Notes.Janzen et al. (2003) stated that ceratomiae could actually represent a complex of morphologically similar but biologically distinct species. We have examined the type of Microplitiswaldeni Viereck, 1917, currently a synonym of M.ceratomiae, and found it to be different based on a) larger body size, b) darker colour, c) coarser sculpture of frons, clypeus, anteromesoscutum, and mesopleuron, and d) slight differences in the shape of T1 and the setae pattern on metasomal terga. However, we refrain here to reinstate waldeni as a valid species until more study of specimens allows for a better sorting of their distribution (as well as clarifying if other potential cryptic species exist under the ceratomiae name).
Microplitischacoensis (Cameron, 1908)
Microgasterchacoensis Cameron, 1908.
Microplitisayerzai Brèthes, 1910.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.NEO, PAL.
NEO: Argentina, Brazil, Paraguay, Trinidad & Tobago, Uruguay, Venezuela; PAL: United Kingdom.
Microplitischangbaishanus Song & Chen, 2008
Microplitischangbaishanus Song & Chen, 2008.
Type information. Holotype female, HUNAU (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (JL).
Microplitischivensis Telenga, 1955, restored combination
Microplitischivensis Telenga, 1955.
Type information. Lectotype male, ZIN (not examined but original description checked). Country of type locality: Uzbekistan.
Geographical distribution.PAL.
PAL: Uzbekistan.
Notes. Our species concept is based on Telenga (1955), Papp (1984c) and Tobias (1986). Transferred back to Microplitis because of the short ovipositor and metatibial spurs shorter than half the length of first segment of metatarsus. The reference to this species as Microgaster in papers after the original description (e.g., Papp 1984c, Tobias 1986, Yu et al. 2012, 2016) is only due to the confusion with the application of the Microgaster name and its type species (see details on that above, under the introduction to the genus Microgaster).
Microplitischoui Xu & He, 2000
Microplitischoui Xu & He, 2000.
Type information. Holotype female, ZJUH (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (GS, SN).
Notes. Our species concept is based on Xu and He (2003b).
Microplitischrysostigma Tobias, 1964, restored combination
Microplitischrysostigma Tobias, 1964.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Kazakhstan.
Geographical distribution.PAL.
PAL: Kazakhstan.
Notes. Our species concept is based on Papp (1984c) and Tobias (1986); both authors dealt with this taxon in their keys to Microplitis, and the closest species (morphologically) in these papers are all Microplitis. The reference to this species as Microgaster in papers after the original description (e.g., Papp 1984c, Tobias 1986, Yu et al. 2012, 2016) is only due to the confusion with the application of the name Microgaster and its type species (see details above, under the introduction to the genus Microgaster; p 717).
Microplitischui Xu & He, 2002
Microplitischui Xu & He, 2002.
Type information. Holotype female, ZJUH (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (ZJ).
Notes. Our species concept is based on Chen and Song (2004), Kotenko (2007a) and Ranjith et al. (2015a).
Microplitiscoactus (Lundbeck, 1896)
Microgastercoactus Lundbeck, 1896.
Type information. Lectotype female, ZMUC (not examined but subsequent treatment of the species checked). Country of type locality: Greenland.
Geographical distribution.NEA, PAL.
NEA: Canada (NU), Greenland; PAL: Iceland.
Notes. Our species concept is based on Muesebeck (1922), Papp (1984c), van Achterberg (2006), and Fernandez-Triana et al. (2017b).
Microplitiscombinatus (Papp, 1984)
Microgastercombinata Papp, 1984.
Type information. Holotype female, ZSM (not examined but original description checked). Country of type locality: Austria.
Geographical distribution.PAL.
PAL: Austria, Germany.
Microplitisconfusus Muesebeck, 1922
Microplitisconfusus Muesebeck, 1922.
Type information. Holotype male, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (NB, ON), USA (IN, MD, MI, NY, TX).
Microplitiscrassiantenna Song & Chen, 2008
Microplitiscrassiantenna Song & Chen, 2008.
Type information. Holotype female, HUNAU (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (JL).
Microplitiscrassifemoralis Alexeev, 1971
Microplitiscrassifemoralis Alexeev, 1971.
Type information. Holotype female, ZIN (not examined but original description checked). Country of type locality: Turkmenistan.
Geographical distribution.PAL.
PAL: Turkmenistan.
Microplitiscrenulatus (Provancher, 1888)
Microgastercrenulatus Provancher, 1888.
Type information. Lectotype female, ULQC (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (QC), USA (MA, MI).
Microplitiscroceipes (Cresson, 1872)
Microgastercroceipes Cresson, 1872.
Microplitisnigripennis Ashmead, 1905.
Type information. Holotype female, ANSP (not examined but subsequent treatment of the species checked). Country of type locality: USA.
Geographical distribution.AUS, NEA.
AUS: New Zealand; NEA: USA (AL, AZ, AR, CO, GA, IL, KS, MD, MS, MO, NJ, NM, NC, OK, OR, SC, TN, UT, VA).
Notes. Our species concept is based on Muesebeck (1922), Papp (1986) and Papp and Shaw (2001).
Microplitiscubitellanus Xu & He, 2000
Microplitiscubitellanus Xu & He, 2000.
Type information. Holotype male, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (JL, XJ).
Microplitisdaitojimensis Sonan, 1940
Microplitisdaitojimensis Sonan, 1940.
Type information. Type and depository unknown (not examined). Country of type locality: Ryukyu Islands.
Geographical distribution.OTL.
OTL: Ryukyu Islands.
Microplitisdecens Tobias, 1964
Microplitisdecens Tobias, 1964.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Kazakhstan.
Geographical distribution.PAL.
PAL: Finland, Germany, Hungary, Italy, Kazakhstan, Korea, Mongolia, Montenegro, Netherlands, Russia (S), Serbia, Spain, Switzerland, Turkey, United Kingdom.
Notes. The presence of this species in the United Kingdom has been questioned by Shaw (2012b).
Microplitisdecipiens Prell, 1925
Microplitisdecipiens Prell, 1925.
Type information. Lectotype female, TUDTG (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Azerbaijan, Germany, Hungary, Iran, Kazakhstan, Lithuania, Moldova, Poland, Russia (C, NW), Turkey.
Notes. Our species concept is based on Papp (1984c). The depository acronym (TUDTG) is based on the institution name: Technische Universität Dresden, Department of Forest Science in Tharandt, Germany.
Microplitisdemolitor Wilkinson, 1934
Microplitisdemolitor Wilkinson, 1934.
Type information. Holotype female, NHMUK (examined). Country of type locality: Australia.
Geographical distribution.AUS, OTL.
AUS: Australia (NSW, NT, QLD, SA, WA); OTL: India, Pakistan, Vietnam.
Microplitisdeprimator (Fabricius, 1798)
Ichneumondeprimator Fabricius, 1798.
Microgasteringratus Haliday, 1834.
Microgasterdeprimatrix Schulz, 1906.
Type information. Lectotype male, ZMUC (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Armenia, Austria, Azerbaijan, Belgium, Bulgaria, Cyprus, Czech Republic, Finland, France, Georgia, Germany, Hungary, Iran, Ireland, Italy, Kazakhstan, Korea, Latvia, Moldova, Mongolia, Netherlands, Norway, Poland, Romania, Russia (DA, KYA, RYA, SPE, SAR), Serbia, Spain, Switzerland, Turkey, Ukraine, United Kingdom.
Notes. Our species concept is based on Papp (1984c) and Kotenko (2007a). The species distribution in Kazakhstan is based on Belokobylskij et al. (2019).
Microplitisdesertorum Telenga, 1955, restored combination
Microplitisdesertorum Telenga, 1955.
Type information. Lectotype female, ZIN (not examined but original description checked). Country of type locality: Kazakhstan.
Geographical distribution.PAL.
PAL: Kazakhstan.
Notes. Our species concept is based on Telenga (1955), Papp (1984c) and Tobias (1986). Transferred back to Microplitis because of the short ovipositor and metatibial spurs shorter than half the length of first segment of metatarsus. The reference to this species as Microgaster in papers after the original description (e.g., Papp 1984c, Tobias 1986, Yu et al. 2012, 2016) is only due to the confusion with the application of the Microgaster name and its type species (see details above, under the introduction to the genus Microgaster; p 717).
Microplitisdesertus Alexeev, 1977
Microplitisdesertus Alexeev, 1977.
Type information. Holotype female, ZIN (not examined but original description checked). Country of type locality: Turkmenistan.
Geographical distribution.PAL.
PAL: Turkmenistan.
Microplitisdocilis Nixon, 1970
Microplitisdocilis Nixon, 1970.
Type information. Holotype female, MZH (not examined but original description checked). Country of type locality: Finland.
Geographical distribution.PAL.
PAL: Bulgaria, Croatia, Finland, Germany, Hungary, Russia (BU, PRI), Serbia, Sweden, Turkey.
Microplitisdornator (Papp, 1987)
Microgasterdornator Papp, 1987.
Type information. Holotype female, HNHM (not examined but subsequent treatment of the species checked). Country of type locality: Korea.
Geographical distribution.PAL.
PAL: Korea, Russia (PRI).
Notes. Our species concept is based on Kotenko (2007a).
Microplitiseminius (Papp, 1987)
Microgastereminius Papp, 1987.
Type information. Holotype female, HNHM (not examined but subsequent treatment of the species checked). Country of type locality: Korea.
Geographical distribution.PAL.
PAL: Korea.
Notes. Our species concept is based on Kotenko (2007a).
Microplitiseremitus Reinhard, 1880
Microplitiseremitus Reinhard, 1880.
Type information. Lectotype male, ZMHB (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Armenia, Austria, Azerbaijan, Croatia, Finland, France, Germany, Hungary, Kazakhstan, Korea, Lithuania, Mongolia, Netherlands, Poland, Russia (ZAB, IRK, PRI, SPE, VOR, YAR), Serbia, Spain, Sweden, Switzerland, Turkey, Ukraine, Uzbekistan.
Notes. Our species concept is based on Nixon (1970), Papp (1984c) and Kotenko (2007a).
Microplitiserythrogaster Abdinbekova, 1969
Microplitiserythrogaster Abdinbekova, 1969.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Azerbaijan.
Geographical distribution.PAL.
PAL: Azerbaijan, Denmark, Germany, Hungary, Russia (NC, S), Tajikistan, Turkmenistan.
Notes. Our species concept is based on Papp (1984c) and Tobias (1986).
Microplitisespinachi Walker, 2003
Microplitisespinachi Walker, 2003.
Type information. Holotype female, NHMUK (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Notes.Janzen et al. (2003) indicated that the holotype and an unspecified number of paratypes for M.espinachi were deposited in the USNM. However, Fernandez-Triana et al. (2015b) could not locate any of the specimens in that collection, unit trays did not exist for any specimen of M.espinachi in the USNM, and there was no record of their existence in any USNM database. Because of that, Fernandez-Triana et al. (2015b) considered it unlikely that specimens of this species were ever deposited in the USNM and they speculated that the type might be misplaced or lost. The finding of the holotype for this species in London (NHMUK) is thus very important as it clarifies its situation. Also, it will allow for future studies about the limits between M.espinachi and M.adrianguadamuzi Fernandez-Triana & Whitfield, and the validity of the latter species, something that was not possible until the M.figueresi Walker type series was also found (see Fernandez-Triana et al. 2015b for more details about these three species).
Microplitisexcisus Telenga, 1955
Microplitisexcisus Telenga, 1955.
Type information. Lectotype female, ZIN (not examined but original description checked). Country of type locality: Ukraine.
Geographical distribution.PAL.
PAL: Azerbaijan, Russia (NC, S), Ukraine.
Microplitisfeltiae Muesebeck, 1922
Microplitisfeltiae Muesebeck, 1922.
Type information. Holotype male, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (AL, AZ, CA, CO, ID, IL, IN, KS, LA, MO, OK, TN, TX, WA).
Microplitisfigueresi Walker, 2003
Microplitisfigueresi Walker, 2003.
Type information. Holotype female, NHMUK (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Notes. Until this paper, the type of M.figueresi was considered lost or misplaced (Fernandez-Triana et al. 2015b). The finding of the holotype for this species in London (NHMUK) is thus very important as it clarifies its situation, and will allow for future studies about the limits between M.espinachi Walker and M.adrianguadamuzi Fernandez-Triana & Whitfield, and the validity of the latter species, something that was not possible until the M.figueresi Walker type series was found. See Fernandez-Triana et al. (2015b) for more details about these three species, and also comments above, under Microplitisespinachi.
Microplitisflavipalpis (Brullé, 1832)
Microgasterflavipalpis Brullé, 1832.
Microplitisruricola Lyle, 1918.
Type information. Lectotype male, MNHN (not examined but authoritatively identified specimens examined). Country of type locality: Greece.
Geographical distribution.PAL.
PAL: Algeria, Armenia, Bulgaria, Finland, France, Germany, Greece, Hungary, Israel, Kazakhstan, Korea, Lithuania, Moldova, Mongolia, Poland, Russia (ZAB, PRI), Serbia, Slovakia, Spain, Switzerland, Tunisia, Turkey, United Kingdom.
Notes. We examined the type of Microplitisruricola Lyle, 1918. The species distribution in Israel is based on Belokobylskij et al. (2019).
Microplitisfordi Nixon, 1970
Microplitisfordi Nixon, 1970.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Austria, Bulgaria, Croatia, Czech Republic, Germany, Greece, Hungary, Israel, Italy, Jordan, Macedonia, Mongolia, Russia (C), Switzerland, Tunisia, Turkey, United Kingdom, Yugoslavia.
Notes.Papp (1984c) suggested that this species might be a junior synonym of Microplitissemicircularis (Ratzeburg, 1834), but nevertheless retained the species as valid (also Broad et al. 2016), a decision we follow here.
Microplitisfrancopupulini Fernandez-Triana & Whitfield, 2015
Microplitisfrancopupulini Fernandez-Triana & Whitfield, 2015.
Type information. Holotype female, USNM (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Microplitisfraudulentus (Papp, 1984)
Microgasterfraudulenta Papp, 1984.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Mongolia.
Geographical distribution.PAL.
PAL: Mongolia, Russia (ZAB).
Microplitisfujianicus Song & Zhang, 2017
Microplitisfujianica Song & Zhang, 2017.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Notes. The species name must be treated as an adjective and not as a noun (Doug Yanega, pers. comm.) and thus it must match the gender of the genus name.
Microplitisfulvicornis (Wesmael, 1837)
Microgasterfulvicornis Wesmael, 1837.
Microplitispallidicornis Marshall, 1898.
Type information. Lectotype male, RBINS (not examined but subsequent treatment of the species checked). Country of type locality: Belgium.
Geographical distribution.PAL.
PAL: Belgium, Croatia, Czech Republic, Finland, Germany, Hungary, Iran, Ireland, Netherlands, Poland, Romania, Russia (RYA, SAR), Serbia, Slovakia, Switzerland, Turkey, United Kingdom.
Notes. Our species concept is based on Papp (1984c) and Tobias (1986).
Microplitisgalinarius Kotenko, 2007
Microplitisgalinarius Kotenko, 2007.
Type information. Holotype female, SIZK (not examined but original description checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (ZAB).
Microplitisgerulus Papp, 1980, restored combination
Microplitisgerulus Papp, 1980.
Type information. Holotype female, HNHM (not examined but subsequent treatment of the species checked). Country of type locality: Mongolia.
Geographical distribution.PAL.
PAL: Mongolia.
Notes. Our species concept is based on Papp (1984c) and Tobias (1986). This species was at times considered to belong to Microgaster, e.g., Papp (1984c) and Tobias (1986), as part of the confusion with the application and use of the Microplitis and Microgaster names (which was only solved after 1988, see more details and comments under our introduction to the genus Microgaster above). The correct generic placement at present would be in Microplitis, which is also corroborated by the description and images in Papp (1984c). Because some of the more recent references (e.g., Kotenko 2007a, Yu et al. 2016) still refer to the species within Microgaster, for the sake of clarity we restore its status here.
Microplitisgidjus Austin & Dangerfield, 1993
Microplitisgidjus Austin & Dangerfield, 1993.
Type information. Holotype female, AEIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS, OTL.
AUS: Australia (NT); OTL: Vietnam.
Microplitisglabrior Alexeev, 1971, restored combination
Microplitisglabrior Alexeev, 1971.
Type information. Holotype female, ZIN (not examined but original description checked). Country of type locality: Turkmenistan.
Geographical distribution.PAL.
PAL: Turkmenistan.
Notes. Our species concept is based on Alexeev (1971), Papp (1984c), and Tobias (1986). This species was at times considered to belong to Microgaster, e.g., Papp (1984c) and Tobias (1986), as part of the confusion with the application and use of the Microplitis and Microgaster names (which was only solved after 1988, see more details and comments under our introduction to the genus Microgaster above). The correct generic placement at present would be in Microplitis, which is also corroborated by the description and images in Alexeev (1971) and Papp (1984c). Because some of the more recent references (e.g., Yu et al. 2016) still refer to the species within Microgaster, for the sake of clarity we restore its status here.
Microplitisgortynae Riley, 1881
Microplitisgortynae Riley, 1881.
Type information. Syntypes female and male, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (ON), USA (CO, IL, IA, KS, MS, MO, NH, NJ, NY, OH, OR, PA, VA, WI).
Microplitisgoughi Austin & Dangerfield, 1993
Microplitisgoughi Austin & Dangerfield, 1993.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (ACT, NSW, SA, TAS, VIC, WA).
Microplitishebertbakeri Fernandez-Triana & Whitfield, 2015
Microplitishebertbakeri Fernandez-Triana & Whitfield, 2015.
Type information. Holotype female, USNM (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Microplitishelicoverpae Xu & He, 2000
Microplitishelicoverpae Xu & He, 2000.
Type information. Holotype male, ZJUH (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (HB); PAL: China (HE).
Notes. Our species concept is based on Chen and Song (2004).
Microplitisheterocerus (Ruthe, 1860)
Microgasterheterocerus Ruthe, 1860.
Type information. Holotype female, NHMUK (examined). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Croatia, Germany, Hungary, Israel, Italy, Korea, Poland, Romania, Russia (IN, ROS, VGG), Slovakia, Spain, Turkey, Ukraine, Yugoslavia.
Notes. The holotype is missing the metasoma.
Microplitishirtifacialis Song & You, 2008
Microplitishirtifacialis Song & You, 2008.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (HB); PAL: China (JL).
Notes. The shape and sculpture of T2 is uncommon for Microplitis, but until specimens can be examined, we prefer to retain the species in this genus.
Microplitishispalensis Marshall, 1898
Microplitishispalensis Marshall, 1898.
Microgasterserotinus Papp, 1984.
Type information. Lectotype female, MNCN (not examined but subsequent treatment of the species checked). Country of type locality: Spain.
Geographical distribution.PAL.
PAL: France, Spain.
Notes. Our species concept is based on Papp (1984c) and Shaw (2012b).
Microplitishova Granger, 1949
Microplitishova Granger, 1949.
Type information. Syntypes female and male, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Microplitishyalinipennis Alexeev, 1971
Microplitishyalinipennis Alexeev, 1971.
Type information. Holotype male, ZIN (not examined but original description checked). Country of type locality: Turkmenistan.
Geographical distribution.PAL.
PAL: Turkmenistan.
Microplitishyphantriae Ashmead, 1898
Microplitishyphantiae Ashmead, 1898.
Microplitishyphantiae Ashmead, 1898 [incorrect original spelling].
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (AB, ON, QC), USA (AR, IL, IN, MD, MA, MI, MO, NH, NJ, NY, OH, TX, WV).
Notes.Yu et al. (2012, 2016) listed the holotype of this species as being in the INHS collection. However, we have examined it in the USNM, which should be recorded as the correct depository for the holotype.
Microplitisidia Nixon, 1970
Microplitisidia Nixon, 1970.
Type information. Holotype female, NHMUK (examined). Country of type locality: Sweden.
Geographical distribution.PAL.
PAL: Germany, Hungary, Israel, Russia (NW), Sweden, Turkey.
Microplitisimpressus (Wesmael, 1837)
Microgasterimpressus Wesmael, 1837.
Microgastersispes Nixon, 1970.
Type information. Lectotype male, RBINS (not examined but authoritatively identified specimens examined). Country of type locality: Belgium.
Geographical distribution.NEA, PAL.
NEA: Canada (MB, ON, QC); PAL: Belgium, France, Germany, Hungary, Poland, Slovakia, United Kingdom.
Notes. We have examined the type of Microgastersispes Nixon.
Microplitisimprovisus (Papp, 1984)
Microgasterimprovisa Papp, 1984.
Type information. Holotype female, RMNH (not examined but paratype examined). Country of type locality: Netherlands.
Geographical distribution.PAL.
PAL: Netherlands.
Notes. We examined female and male paratypes.
Microplitisincurvatus Xu & He, 2002
Microplitisincurvata Xu & He, 2002.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (XJ).
Microplitisindicus Marsh, 1978
Microplitisindicus Marsh, 1978.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Microplitisinfula (Kotenko, 1994)
Microgasterinfula Kotenko, 1994.
Type information. Holotype female, SIZK (not examined but subsequent treatment of the species checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (ZAB, PRI).
Notes. Our species concept is based on Kotenko (2006, 2007).
Microplitisjamesi Austin & Dangerfield, 1993
Microplitisjamesi Austin & Dangerfield, 1993.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (NSW).
Microplitisjiangsuensis Xu & He, 2000
Microplitisjiangsuensis Xu & He, 2000.
Type information. Holotype male, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (JS).
Microplitisjorgehernandezi Fernandez-Triana & Whitfield, 2015
Microplitisjorgehernandezi Fernandez-Triana & Whitfield, 2015.
Type information. Holotype female, USNM (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Microplitisjorgeluisi Fernandez-Triana, 2018
Microplitisjorgeluisi Fernandez-Triana, 2018.
Type information. Holotype female, CNC (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (TX).
Microplitisjuanmanueli Fernandez-Triana, 2018
Microplitisjuanmanueli Fernandez-Triana, 2018.
Type information. Holotype female, CNC (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (BC), USA (CO).
Microplitisjulioalbertoi Fernandez-Triana, 2018
Microplitisjulioalbertoi Fernandez-Triana, 2018.
Type information. Holotype female, CNC (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CT, GA).
Microplitiskarakurti Rossikov, 1904
Microplitiskara-kurti Rossikov, 1904.
Type information. Type and depository unknown (not examined). Country of type locality: unknown.
Geographical distribution.PAL.
PAL: Kazakhstan, Turkmenistan.
Microplitiskaszabi Papp, 1980
Microplitiskaszabi Papp, 1980.
Type information. Holotype female, HNHM (not examined but subsequent treatment of the species checked). Country of type locality: Mongolia.
Geographical distribution.PAL.
PAL: Korea, Mongolia, Russia (PRI).
Notes. Our species concept is based on Papp (1984c) and Kotenko (2007a).
Microplitiskewleyi Muesebeck, 1922
Microplitiskewleyi Muesebeck, 1922.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (AB, MB, NB, NL, NS, ON, PE, QC), USA (CA, DC, IA, MD, MI, NJ, NY, WI).
Microplitiskurandensis Austin & Dangerfield, 1993
Microplitiskurandensis Austin & Dangerfield, 1993.
Type information. Holotype male, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (QLD).
Microplitislacteus Austin & Dangerfield, 1993
Microplitislacteus Austin & Dangerfield, 1993.
Type information. Holotype female, CNC (examined). Country of type locality: Papua New Guinea.
Geographical distribution.AUS.
AUS: Papua New Guinea.
Microplitislaticinctus Muesebeck, 1922
Microplitislaticinctus Muesebeck, 1922.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (QC), USA (AL, DC, IL, IA, MA, NY, OH, VA).
Microplitislatistigmus Muesebeck, 1922
Microplitislatistigmus Muesebeck, 1922.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (MD).
Microplitisleoniae Niezabitowski, 1910
Microplitisleoniae Niezabitowski, 1910.
Type information. Syntypes female and male, depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Poland.
Geographical distribution.PAL.
PAL: Georgia, Hungary, Korea, Poland, Russia (PRI).
Notes. Our species concept is based on Papp (1984c) and Kotenko (2007a). The species distribution in Korea is based on Belokobylskij et al. (2019).
Microplitisleucaniae Xu & He, 2002
Microplitisleucaniae Xu & He, 2002.
Type information. Holotype female, ZJUH (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (FJ, GX, JS, ZJ); PAL: China (XJ).
Notes. Our species concept is based on Chen and Song (2004), Kotenko (2007a) and Ranjith et al. (2015a).
Microplitislineatus Austin & Dangerfield, 1993
Microplitislineatus Austin & Dangerfield, 1993.
Type information. Holotype female, AEIC (not examined but original description checked). Country of type locality: Papua New Guinea.
Geographical distribution.AUS.
AUS: Papua New Guinea.
Microplitislongicaudus Muesebeck, 1922
Microplitislongicaudus Muesebeck, 1922.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CA, CO, ID, NV, OR).
Microplitislongiradiusis Xu & He, 2003
Microplitislongiradiusis Xu & He, 2003.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (HL).
Microplitislongwangshanus Xu & He, 2000
Microplitislongwangshana Xu & He, 2000.
Type information. Holotype female, ZJUH (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, HB, ZJ).
Notes. Our species concept is based on Chen and Song (2004) and Ranjith et al. (2015a).
Microplitislugubris (Ruthe, 1860)
Microgasterlugubris Ruthe, 1860.
Microplitisborealis Marshall, 1885.
Microgastercoracinus Thomson, 1895.
Microplitisrutheana Fahringer, 1937.
Type information. Holotype female, NHMUK (examined). Country of type locality: Poland.
Geographical distribution.NEA, PAL.
NEA: Canada (MB, NU), Greenland; PAL: Armenia, Finland, Germany, Hungary, Ireland, Lithuania, Mongolia, Poland, Russia (TA, YAR), Serbia, Sweden, Switzerland, Turkey, United Kingdom.
Notes. We also examined the type of M.borealis Marshall. A new distribution record (from Canada, MB, Churchill, at ca. 59° N), which had been named Microplitis jft01 in previous papers (Fernandez-Triana 2010, Fernandez-Triana et al. 2011) expands considerably the southernmost distribution of the species within the Nearctic region.
Microplitislugubroides van Achterberg, 2006
Microplitislugubroides van Achterberg, 2006.
Type information. Holotype female, ZMUC (not examined but original description checked). Country of type locality: Greenland.
Geographical distribution.NEA.
NEA: Greenland.
Microplitismahunkai (Papp, 1979)
Glabromicroplitismahunkai Papp, 1979.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Tunisia.
Geographical distribution.PAL.
PAL: Tunisia.
Microplitismalimba (Papp, 1984)
Microgastermalimba Papp, 1984.
Type information. Holotype female, RMNH (not examined but paratype examined). Country of type locality: Netherlands.
Geographical distribution.PAL.
PAL: Netherlands, Russia (PRI), Ukraine, United Kingdom.
Notes. According to Shaw (2012b) and Broad et al. (2016), the interpretation by (Nixon 1970) of Microplitistrochanterata (not tuberculifer, of which trochanterata is a junior synonym, see below under that species in our checklist) is actually referable to malimba. Because the name is to be considered as a noun under ICZN Article 31.2.1, it must retain its original spelling and remain as malimba. We examined one female paratype.
Microplitismamestrae Weed, 1887
Microplitismamestrae Weed, 1887.
Type information. Lectotype female, INHS (not examined but subsequent treatment of the species checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (BC), USA (CT, DC, IL, KS, MA, MI, NH, NJ, NM, NY, OH, UT, WI).
Notes. Our species concept is based on Muesebeck (1922) and Whitfield (1995a).
Microplitismandibularis (Thomson, 1895)
Microgastermandibularis Thomson, 1895.
Type information. Lectotype female, MZLU (not examined but subsequent treatment of the species checked). Country of type locality: Sweden.
Geographical distribution.NEA, PAL.
NEA: Greenland; PAL: Armenia, Azerbaijan, Croatia, Finland, Georgia, Germany, Hungary, Macedonia, Mongolia, Netherlands, Russia (PRI, SAK), Serbia, Slovakia, Spain, Sweden, Switzerland, Tunisia, Turkey, United Kingdom.
Notes. Our species concept is based on van Achterberg (2006) and Kotenko (2007a). The species distribution in Armenia, Azerbaijan and Georgia is based on Belokobylskij et al. (2019).
Microplitismanilae Ashmead, 1904
Microplitismanilae Ashmead, 1904.
Type information. Holotype female, USNM (not examined but subsequent treatment of the species checked). Country of type locality: Philippines.
Geographical distribution.AUS, OTL, PAL.
AUS: Australia (QLD), Papua New Guinea; OTL: China (GD, TW, ZJ), India, Malaysia, Philippines, Ryukyu Islands, Thailand, Vietnam; PAL: Korea.
Notes. Our species concept is based on Austin and Dangerfield (1993), Gupta (2013a) and Ranjith et al. (2015a).
Microplitismariamargaritae Fernandez-Triana, 2018
Microplitismariamargaritae Fernandez-Triana, 2018.
Type information. Holotype female, CNC (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CO).
Microplitismarini Whitfield, 2003
Microplitismarini Whitfield, 2003.
Type information. Holotype female, USNM (examined). Country of type locality: Costa Rica.
Geographical distribution.NEA, NEO.
NEA: USA (AZ); NEO: Costa Rica.
Notes. The record of this species from Arizona (US) was questioned by Fernandez-Triana et al. (2015b) as the available information suggests it may represent a different (most likely undescribed) species. However, to conclude, examination of the US specimen would be needed; thus, for the time being they are listed under marini.
Microplitismarshallii Kokujev, 1898
Microplitismarshallii Kokujev, 1898.
Type information. Lectotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Georgia.
Geographical distribution.OTL, PAL.
OTL: China (FJ, HB, YN); PAL: Armenia, Azerbaijan, China (JL), Finland, Georgia, Hungary, Iran, Moldova, Romania, Russia (KEM, ROS, STA), Turkey.
Notes. Our species concept is based on Papp (1984c), Chen and Song (2004), Kotenko (2007a) and Ranjith et al. (2015a).
Microplitismasneri Austin & Dangerfield, 1993
Microplitismasneri Austin & Dangerfield, 1993.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (QLD).
Microplitismaturus Weed, 1888
Microplitismaturus Weed, 1888.
Microplitiscincta Ashmead, 1891.
Microgastertuckeri Viereck, 1905.
Type information. Holotype male, ANSP (not examined but subsequent treatment of the species checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (BC, ON, QC), USA (AR, CT, FL, GA, IL, IA, KS, LA, MD, MI, MO, NJ, NY, SD, VT).
Notes. The information about the holotype is taken from Shenefelt (1973: 750). We have also examined the holotype of M.cincta in the USNM, a male specimen.
Microplitismediator (Haliday, 1834)
Microgastermediator Haliday, 1834.
Microgastermedianus Ruthe, 1860 [primary homonym of Microgastermedianus Ratzeburg, 1852].
Microplitishalidayi Fahringer, 1937.
Microplitispseudomedianus Fahringer, 1937.
Type information. Lectotype male, NMID (examined). Country of type locality: unknown.
Geographical distribution.NEA, NEO, OTL, PAL.
NEA: Greenland, NEO: Brazil (PR); OTL: China (HN, JS, ZJ), Pakistan; PAL: Albania, Armenia, Austria, Azerbaijan, Belgium, Bulgaria, China (HE, HL, HA, LN, NM, SD, SN, XJ), Croatia, Czech Republic, Finland, France, Georgia, Germany, Hungary, Iran, Ireland, Italy, Japan, Kazakhstan, Korea, Latvia, Lithuania, Moldova, Mongolia, Netherlands, Norway, Poland, Romania, Russia (AST, ZAB, KIR, KDA, MOS, ORE, PRI, RYA, SAK, SAR, STA, YAR), Serbia, Slovakia, Slovenia, Spain, Sweden, Switzerland, Turkey, Ukraine, United Kingdom, Uzbekistan.
Notes.van Achterberg (1997) revised the Haliday collection of Braconidae and designated a lectotype for Microplitismediator. Unfortunately, the type locality or country for the lectotype specimen are not clearly specified (van Achterberg 1997: 57). Based on the first few sections of van Achterberg’s paper (where he detailed the process he used to recognize Haliday’s type specimens, publication dates, and list of taxa described), Ireland seems to be the most likely country of the lectotype. We also examined the type of Microgastermedianus Ruthe. The species distribution in Armenia, Georgia, and Iran is based on Belokobylskij et al. (2019).
Microplitismelianae Viereck, 1911
Microplitismelianae Viereck, 1911.
Type information. Holotype male, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (AB, ON), USA (IL, IA, KS, NY, OH, OK, TN, TX).
Microplitismencianus Xu & He, 1999
Microplitismenciana Xu & He, 1999.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (HL).
Notes. The species name must be treated as an adjective and not as a noun (Doug Yanega, pers. comm.) and thus it must match the gender of the genus name.
Microplitismexicanus (Cameron, 1887), new combination
Microgastermexicana Cameron, 1887.
Type information. Holotype female, NHMUK (examined). Country of type locality: Mexico.
Geographical distribution.NEO.
NEO: Mexico.
Notes. After the original description, the only taxonomist that has commented on this species was Muesebeck (1922: 42). He stated that he did not know that species but guessed that it did not belong to Microgaster, and then correctly guessed that it could be Microplitis, based on the description from Cameron. After examining the female holotype, we formally transfer the species here to Microplitis, based on its inflexible hypopygium, very short ovipositor sheaths, T1 very narrow and with polished knob at apex, T2 subtriangular, and metatibial spurs shorter than half the length of the first metatarsus segment.
Microplitisminutus Alexeev, 1977
Microplitisminutus Alexeev, 1977.
Type information. Holotype female, ZIN (not examined). Country of type locality: Turkmenistan.
Geographical distribution.PAL.
PAL: Turkmenistan.
Microplitismoestus (Ratzeburg, 1852)
Microgaster möstus Ratzeburg, 1852.
Microplitismaestus Dalla Torre, 1898 [unjustified emendation].
Type information. Type and depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Austria, Germany, Hungary, Netherlands, United Kingdom.
Notes. Our species concept is based on Papp (1984c).
Microplitismongolicus Papp, 1967
Microplitismongolicus Papp, 1967.
Type information. Holotype male, HNHM (not examined but subsequent treatment of the species checked). Country of type locality: Mongolia.
Geographical distribution.PAL.
PAL: Hungary, Israel, Jordan, Mongolia, Russia (ZAB).
Notes. Our species concept is based on Papp (1984c).
Microplitismontanus Muesebeck, 1922
Microplitismontanus Muesebeck, 1922.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CA, MO, NV).
Microplitismurkyi Gupta, 2013
Microplitismurkyi Gupta, 2013.
Type information. Holotype female, NBAIR (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Microplitismurrayi Austin & Dangerfield, 1993
Microplitismurrayi Austin & Dangerfield, 1993.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (ACT, NSW, QLD, TAS, VIC, WA).
Microplitisnaenia Nixon, 1970
Microplitisnaenia Nixon, 1970.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Hungary, Russia (C, NW), Slovakia, Turkey, United Kingdom.
Microplitisnarendrani Ranjith & Nasser, 2015
Microplitisnarendrani Ranjith & Nasser.
Type information. Holotype female, DZUC (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. The depository acronym (DZUC) was selected based on Ranjith et al. (2015a) and not the Insect and Spider Collections of the World website, which lists a different institution under that same acronym.
Microplitisnecopinatus (Papp, 1984)
Microgasternecopinata Papp, 1984.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Finland.
Geographical distribution.PAL.
PAL: Finland.
Microplitisnewguineaensis Austin & Dangerfield, 1993
Microplitisnewguineaensis Austin & Dangerfield, 1993.
Type information. Holotype female, CNC (examined). Country of type locality: Papua New Guinea.
Geographical distribution.AUS.
AUS: Papua New Guinea.
Microplitisnielseni Austin & Dangerfield, 1993
Microplitisnielseni Austin & Dangerfield, 1993.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (WA).
Microplitisnigrifemur Xu & He, 2006
Microplitisnigrifemur Xu & He, 2006.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (HE).
Microplitisnigritus Muesebeck, 1922
Microplitisnigritus Muesebeck, 1922.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CO).
Microplitisobscuripennatus Xu & He, 1999
Microplitisobscuripennatus Xu & He, 1999.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Notes. The illustration of the mesosoma and metasoma in the original description suggest that this species might belong to Snellenius, based on the deep notauli, the deeply impressed scutellar disc, and the shape of T1. However, the English translation (Xu and He 1999: 67, 68) that follows the Chinese description makes no mention of an epicnemial carina, the most distinguishing character of Snellenius; thus, we retain the species under the genus in which it was originally described.
Microplitisocellatae (Bouché, 1834)
Microgasterocellatae Bouché, 1834.
Microgastercanaliculatus Wesmael, 1837.
Type information. Type and depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.OTL, PAL.
OTL: China (JS); PAL: Belgium, China (LN), Croatia, Czech Republic, Finland, France, Germany, Hungary, Italy, Japan, Moldova, Netherlands, Poland, Romania, Russia (ZAB, SAK), Serbia, Ukraine, United Kingdom.
Notes. Our species concept is based on Nixon (1970), Papp (1984c) and Kotenko (2007a).
Microplitisochraceus Szépligeti, 1896
Microplitisochraceus Szépligeti, 1896.
Microplitisflaviventris Ivanov, 1898.
Type information. Holotype female, HNHM (not examined but subsequent treatment of the species checked). Country of type locality: Hungary.
Geographical distribution.PAL.
PAL: Azerbaijan, Greece, Hungary, Iran, Kazakhstan, Moldova, Mongolia, Romania, Russia (KDA, ROS), Ukraine, Uzbekistan.
Notes. Our species concept is based on Papp (1984c) and Kotenko (2007a).
Microplitispaizhensis Zhang, 2019
Microplitispaizhensis Zhang, 2019.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (XZ).
Microplitispallidipennis Tobias, 1964
Microplitispallidipennis Tobias, 1964.
Type information. Holotype male, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Kazakhstan.
Geographical distribution.PAL.
PAL: Kazakhstan, Mongolia, Russia (S).
Notes. Our species concept is based on Papp (1984c), Tobias (1986), and Kotenko (2007a).
Microplitispallidipes Szépligeti, 1902
Microplitispallidipes Szépligeti, 1902.
Type information. Holotype male, HNHM (not examined but subsequent treatment of the species checked). Country of type locality: Singapore.
Geographical distribution.OTL, PAL.
OTL: China (FJ, HN, TW, ZJ), Singapore, Vietnam; PAL: China (SD), Korea, Russia (PRI, SAK).
Notes. Our species concept is based on Wilkinson (1930a), Long and Belokobylski (2004), and Kotenko (2007a).
Microplitispellucidus Telenga, 1955
Microplitispellucidus Telenga, 1955.
Type information. Lectotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Denmark, Germany, Hungary, Korea, Netherlands, Russia (ALT, PRI), Serbia.
Notes. Our species concept is based on Telenga (1955), Papp (1984c), Tobias (1986) and Kotenko (2007a).
Microplitispennatulae Ranjith & Nasser, 2015
Microplitispennatulae Ranjith & Nasser.
Type information. Holotype female, DZUC (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Microplitisperelegans (Bingham, 1906)
Microgasterperelegans Bingham, 1906.
Type information. Holotype female, OUMNH (not examined but subsequent treatment of the species checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (NT, QLD, WA).
Notes. Our species concept is based on Austin and Dangerfield (1992, 1993).
Microplitispipus Austin & Dangerfield, 1993
Microplitispipus Austin & Dangerfield, 1993.
Type information. Holotype male, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (QLD).
Microplitisplutellae Muesebeck, 1922
Microplitisplutellae Muesebeck, 1922.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA, OTL, PAL.
NEA: Canada (AB, ON, PE, QC, SK), USA (CA, CO, ID, IA, MI, MN, NY, ND, OH, SC, TX, UT); OTL: China (TW); PAL: Egypt, Russia (MUR).
Microplitisprodeniae Rao & Kurian, 1950
Microplitisprodeniae Rao & Kurian, 1950.
Microplitisbicoloratus Chen, 2004 [M.bicoloratus Chen, 2004 is also a primary homonym of Microplitisbicoloratus Xu & He, 2003].
Microplitiskovalevskayae Kittel, 2016 [unnecessary replacement name for M.bicoloratus Chen, 2004].
Type information. Holotype female, NZSI (not examined but subsequent treatment of the species checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: China (GD, GX), India, Vietnam.
Notes. Our species concept is based on Gupta (2013a), Gupta & Fernandez-Triana (2014), and Ranjith et al. (2015a). The status of Microplitisbicoloratus (Chen, 2004) as a synonym of M.prodenia Rao & Kurian, 1950, and as a junior synonym of M.bicoloratus Xu & He, 2003 was established by Zhang et al. (2017). Thus, there is no need to replace the name Microplitisbicoloratus (Chen, 2004) with Microplitiskovalevskayae Kittel, 2016 as proposed by Kittel (2016).
Microplitispseudomurinus Abdinbekova, 1969
Microplitispseudomurina Abdinbekova, 1969.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Azerbaijan.
Geographical distribution.PAL.
PAL: Azerbaijan, Bulgaria, Georgia, Greece, Hungary, Kazakhstan, Moldova, Russia (ZAB, PRI), Turkey.
Notes. Our species concept is based on Papp (1984c), Tobias (1986), and Kotenko (2007a). The species name must be treated as an adjective and not as a noun (Doug Yanega, pers. comm.) and must match the gender of the genus name.
Microplitispseudoochraceus Alexeev, 1977
Microplitispseudoochraceus Alexeev, 1977.
Type information. Holotype female, ZIN (not examined but original description checked). Country of type locality: Turkmenistan.
Geographical distribution.PAL.
PAL: Turkmenistan.
Microplitisquadridentatus (Provancher, 1886)
Microgaster 4-dentatus Provancher, 1886.
Microplitisterminatus Weed, 1888.
Type information. Lectotype male, ULQC (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (ON), USA (IL, IN, MA, NH, NY, SD).
Microplitisquintilis Viereck, 1917
Microplitisquintilis Viereck, 1917.
Type information. Holotype male, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CT, MO).
Microplitisratzeburgii (Ruthe, 1858)
Microgasterratzeburgii Ruthe, 1858.
Microgasterspinolae Ratzeburg, 1852 [homonym of Microgasterspinolae Nees, 1834].
Microplitiscerurae Matsumura, 1921.
Type information. Holotype female, NHMUK (examined). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Armenia, Bulgaria, Denmark, Finland, France, Germany, Israel, Italy, Japan, Poland, Russia (ZAB, PRI, SAK), Serbia, Ukraine.
Notes. Our species concept is based on Shenefelt (1973: 756-757). Nixon (1970) referred to this species as ratzeburgi but the original and correct spelling is ratzeburgii (Ruthe 1858: 6).
Microplitisretentus (Papp, 1984)
Microgasterretenta Papp, 1984.
Type information. Holotype female, ZSM (not examined but subsequent treatment of the species checked). Country of type locality: France.
Geographical distribution.PAL.
PAL: France.
Notes. Our species concept is based on Papp (1984c) and Shaw et al. (2009).
Microplitisrufipes Dutu-Lacatusu, 1961
Microplitisrufipes Dutu-Lacatusu, 1961.
Type information. Holotype female, depository unknown (not examined). Country of type locality: Romania.
Geographical distribution.PAL.
PAL: Romania.
Notes. The information about the holotype is taken from Shenefelt (1973: 757).
Microplitisrufiventris Kokujev, 1914
Microplitisrufiventris Kokujev, 1914
Type information. Type and depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Uzbekistan.
Geographical distribution.OTL, PAL.
OTL: China (HN); PAL: Afghanistan, Cyprus, Egypt, Iran, Israel, Jordan, Romania, Russia (NC, S), Turkey, Turkmenistan, Uzbekistan.
Notes. Our species concept is based on Papp (1984c) and Tobias (1986).
Microplitisschmidti Austin & Dangerfield, 1993
Microplitisschmidti Austin & Dangerfield, 1993.
Type information. Holotype female, AEIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (NT, South WA).
Microplitisscrophulariae Szépligeti, 1898
Microplitisscrophulariae Szépligeti, 1898.
Type information. Lectotype female, HNHM (not examined but subsequent treatment of the species checked). Country of type locality: Hungary.
Geographical distribution.PAL.
PAL: Armenia, Azerbaijan, Bulgaria, Croatia, Czech Republic, France, Georgia, Greece, Hungary, Iran, Kazakhstan, Korea, Mongolia, Romania, Russia (ZAB, IRK, KEM, SPE, YAR), Serbia, Slovakia, Sweden, Turkey, United Kingdom.
Notes. Our species concept is based on Papp (2004).
Microplitisscutellatus Muesebeck, 1922
Microplitisscutellatus Muesebeck, 1922.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (AB, ON), USA (ID, IA, KS, MI, MT, NY, OR, SD, WA).
Notes. The female holotype has the scutellar disc with deep impressions close to and around the margins (like Snellenius species that have that feature not so pronounced); however, there is no epicnemial carina nor deeply marked notauli, so we retain this species in Microplitis.
Microplitissemicircularis (Ratzeburg, 1844)
Microgastersemicircularis Ratzeburg, 1844.
Type information. Type and depository unknown (not examined but original description checked). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Germany, Hungary.
Notes. Our species concept is based on the descriptions and comments from Ratzeburg (1844), Fahringer (1937) and Papp (1984c). See comments under the species Microplitisfordi Nixon above, and also Broad et al. (2016) for more details on these two species.
Microplitissimilis Lyle, 1921
Microplitissimilis Lyle, 1921.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: Bangladesh, India, Indonesia, Vietnam.
Microplitissofron Nixon, 1970
Microplitissofron Nixon, 1970.
Type information. Holotype female, NHMUK (examined). Country of type locality: Sweden.
Geographical distribution.NEA, PAL.
NEA: Greenland; PAL: Armernia, Azerbaijan, Bulgaria, Denmark, Finland, France, Germany, Greece, Hungary, Iran, Ireland, Italy, Kazakhstan, Netherlands, Norway, Russia (ZAB, SPE), Serbia, Spain, Sweden, Switzerland, Turkey, Turkmenistan, United Kingdom.
Notes.Papp (1984c) suggested that this species might be a junior synonym of Microplitisstigmaticus (Ratzeburg, 1844), but nevertheless maintained the species as valid (also Broad et al. 2016), a decision we follow here. The species distribution in Armenia and Turkmenistan is based in Belokobylskij et al. (2019).
Microplitissordipes (Ziegler, 1834)
Microgastersordipes Ziegler, 1834.
Microgastertau Ratzeburg, 1852.
Type information. Holotype male, lost (not examined but subsequent treatment of the species checked). Country of type locality: Austria.
Geographical distribution.PAL.
PAL: Albania, Armenia, Austria, Azerbaijan, Belgium, Czech Republic, Finland, France, Georgia, Germany, Hungary, Italy, Kazakhstan, Lithuania, Moldova, Mongolia, Poland, Romania, Russia (IRK, KL, KIR, KDA, RYA, SPE, VOR, YAR), Slovakia, Sweden, Switzerland, Turkey, Turkmenistan, Ukraine, United Kingdom, Uzbekistan, Yugoslavia.
Notes. Our species concept is based on Papp (2016), who also provided details and emendation of the species author name (the species author name was previously considered to be Nees, 1834 by most references, e.g., Yu et al. 2016).
Microplitisspectabilis (Haliday, 1834)
Microgasterspectabilis Haliday, 1834.
Microgasterfossulatus Bouché, 1834.
Microgasterparvulus Ruthe, 1860.
Microgasterseuratii Marshall, 1898.
Dapsilotomatestaceipes Cameron, 1906.
Type information. Lectotype female, NMID (not examined but authoritatively identified specimens examined). Country of type locality: Ireland.
Geographical distribution.OTL, PAL.
OTL: Pakistan; PAL: Armenia, Austria, Azerbaijan, Belgium, Bulgaria, Croatia, Finland, France, Germany, Greece, Hungary, Iran, Ireland, Israel, Italy, Kazakhstan, Latvia, Lithuania, Madeira Islands, Malta, Moldova, Mongolia, Morocco, Poland, Romania, Russia (AD, ZAB, DA, IRK, KIR, KDA, PRI, RYA, SAK, VOR, YAR), Slovakia, Sweden, Switzerland, Tajikistan, Tunisia, Turkey, Turkmenistan, Ukraine, United Kingdom, Uzbekistan.
Notes. We examined the type of Microgasterparvulus Ruthe. The species distribution in Israel is based in Belokobylskij et al. (2019).
Microplitisspinolae (Nees, 1834)
Microgasterspinolae Nees, 1834.
Microplitissapporoensis Ashmead, 1906.
Microplitisradiorimatus Telenga, 1955.
Type information. Neotype female, ZMHB (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Armenia, Austria, Azerbaijan, Belgium, Bosnia and Herzegovina, Bulgaria, Croatia, Czech Republic, Finland, France, Georgia, Germany, Greece, Hungary, Iran, Ireland, Italy, Japan, Kazakhstan, Korea, Kyrgyzstan, Lithuania, Macedonia, Moldova, Netherlands, Poland, Romania, Russia (KAM, KDA, NGR, PRI, SAK, SPE, VOR, YAR, ZAB), Serbia, Slovakia, Sweden, Switzerland, Tajikistan, Turkey, Ukraine, United Kingdom, Uzbekistan.
Notes. Our species concept is based on Nixon (1970), Tobias (1986), Kotenko (2007a) and Shaw (2012b).
Microplitisspodopterae Rao & Kurian, 1950
Microplitisspodopterae Rao & Kurian, 1950.
Type information. Holotype female, NZSI (not examined but subsequent treatment of the species checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. Our species concept is based on Gupta (2013a), Gupta & Fernandez-Triana (2014), and Ranjith et al. (2015a).
Microplitissteinbergi Tobias, 1964, restored combination
Microplitissteinbergi Tobias, 1964.
Type information. Holotype female, ZIN (not examined but original description checked). Country of type locality: Kazakhstan.
Geographical distribution.PAL.
PAL: Kazakhstan, Russia (S).
Notes. Our species concept is based on Tobias (1964, 1986) and Papp (1984c). This species was at times considered to belong to Microgaster, e.g., Papp (1984c) and Tobias (1986), as part of the confusion with the application and use of the Microplitis and Microgaster names, which was only solved after 1988 (see more details and comments under our introduction to the genus Microgaster above, p 717). The correct generic placement at present would be in Microplitis, which is also corroborated by the description and images in Tobias (1964, 1986). Because some of the more recent references (e.g., Yu et al. 2016) still refer to the species within Microgaster, for the sake of clarity we restore its status here.
Microplitisstigmaticus (Ratzeburg, 1844)
Microgasterstigmaticus Ratzeburg, 1844.
Microplitisstigmaticus Ratzeburg, 1844 [secondary homonym of Microplitisstigmaticus Muesebeck, 1922].
Microplitisstigmativetus Shenefelt, 1973.
Type information. Type and depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: unknown.
Geographical distribution.PAL.
PAL: Armenia, Azerbaijan, Finland, Germany, Italy, Kazakhstan, Latvia, Poland, Romania, Russia (ALT, KDA, SPE, SAR), Serbia, Turkmenistan, Ukraine, Uzbekistan.
Notes. Our species concept is based on Telenga (1955) and Tobias (1986).
Microplitisstoreyi Austin & Dangerfield, 1993
Microplitisstoreyi Austin & Dangerfield, 1993.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (QLD).
Microplitisstrenuus Reinhard, 1880
Microplitisstrenuus Reinhard, 1880.
Microgastergracilis Ruthe, 1860 [primary homonym of Microplitisgracilis Curtis, 1830].
Type information. Holotype female, NHMUK (examined). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Afghanistan, Armenia, Azerbaijan, China (GS, SN), Croatia, Czech Republic, Germany, Hungary, Kazakhstan, Moldova, Mongolia, Netherlands, Poland, Russia (ZAB, KDA, PRI, YAR), Serbia, Sweden, Switzerland, Turkey, Ukraine, United Kingdom, Uzbekistan.
Microplitissuavis Alexeev, 1971
Microplitissuavis Alexeev, 1971.
Type information. Holotype female, ZIN (not examined but original description checked). Country of type locality: Turkmenistan.
Geographical distribution.PAL.
PAL: Turkmenistan.
Microplitissubsulcatus Granger, 1949
Microplitissubsulcatus Granger, 1949.
Type information. Holotype female, MNHN (not examined but subsequent treatment of the species checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar, Réunion.
Notes. Our species concept is based on Granger (1949) and Rousse and Gupta (2013).
Microplitistadzhicus Telenga, 1949
Microplitistadzhicus Telenga, 1949.
Microplitismurina Telenga, 1955.
Microplitisintermedius Hedwig, 1961.
Type information. Type and depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Tajikistan.
Geographical distribution.OTL, PAL.
OTL: China (JS); PAL: Afghanistan, Azerbaijan, China (SD), France, Hungary, Kazakhstan, Korea, Russia (UR), Tajikistan, Turkmenistan, Uzbekistan.
Notes. Our species concept is based on Telenga (1955), Papp (1984c), Tobias (1986) and Chen and Song (2004).
Microplitistaptor (Papp, 1987)
Microgastertaptor Papp, 1987.
Type information. Holotype female, HNHM (not examined but subsequent treatment of the species checked). Country of type locality: Korea.
Geographical distribution.PAL.
PAL: Korea, Russia (PRI).
Notes. Our species concept is based on Kotenko (2007a).
Microplitistasmaniensis Austin & Dangerfield, 1993
Microplitistasmaniensis Austin & Dangerfield, 1993.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (TAS).
Microplitistaylori Austin & Dangerfield, 1993
Microplitistaylori Austin & Dangerfield, 1993.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (ACT, NSW, QLD, VIC).
Microplitisteba (Kotenko, 1994)
Microgasterteba Kotenko, 1994.
Type information. Holotype female, SIZK (not examined but subsequent treatment of the species checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (ZAB).
Notes. Our species concept is based on Kotenko (2006, 2007).
Microplitistestaceicornis Niezabitowski, 1910
Microplitistestaceicornis Niezabitowski, 1910.
Type information. Type and depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Poland.
Geographical distribution.PAL.
PAL: Poland.
Notes. Our species concept is based on Telenga (1955) and Papp (1984c).
Microplitistobiasi Kotenko, 2007
Microplitistobiasi Kotenko, 2007.
Type information. Holotype female, SIZK (not examined but original description checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (PRI).
Microplitistristis (Nees, 1834)
Microgastertristis Nees, 1834.
Microplitisdolens Marshall, 1885
Type information. Type lost (not examined but authoritatively identified specimens examined). Country of type locality: unknown.
Geographical distribution.PAL.
PAL: Azerbaijan, Belgium, Croatia, France, Germany, Hungary, Kazakhstan, Kyrgyzstan, Lithuania, Moldova, Mongolia, Netherlands, Poland, Romania, Russia (PRI), Slovakia, Switzerland, Turkey, Ukraine, United Kingdom.
Notes. We have examined the type of Microplitisdolens Marshall, 1885, which is deposited in the NHMUK with code 3c.18. The species distribution in Azerbaijan and Kyrgyzstan is based in Belokobylskij et al. (2019).
Microplitistuberculatus (Bouché, 1834)
Microgastertuberculatus Bouché, 1834.
Microgasterfumipennis Ratzeburg, 1852.
Type information. Holotype female, ZMHB (not examined but subsequent treatment of the species checked). Country of type locality: unknown.
Geographical distribution.PAL.
PAL: Armenia, Azerbaijan, Finland, Georgia, Germany, Hungary, Ireland, Israel, Italy, Moldova, Mongolia, Poland, Romania, Russia (IN, ZAB, KYA, ROS, RYA, VOR), Slovakia, Sweden, Switzerland, Ukraine, United Kingdom.
Notes. Our species concept is based on Papp (1984c), Tobias (1986) and Kotenko (2007a). The species distribution in Israel is based in Belokobylskij et al. (2019).
Microplitistuberculifer (Wesmael, 1837)
Microgastertuberculifer Wesmael, 1837.
Microgastercalcaratus Thomson, 1895.
Microgastertrochanteratus Thomson, 1895.
Microplitismanevali Gautier & Bonnamour, 1939.
Type information. Lectotype female, RBINS (examined). Country of type locality: Belgium.
Geographical distribution.OTL, PAL.
OTL: China (FJ, GZ, HB, SN, TW, ZJ), India; PAL: Armenia, Austria, Azerbaijan, Belarus, Belgium, Bulgaria, China (BJ, HE, HL, HA, JL, LN, SD, XJ), Croatia, Czech Republic, Estonia, Finland, France, Georgia, Germany, Greece, Hungary, Iran, Ireland, Israel, Italy, Japan, Kazakhstan, Korea, Kyrgyzstan, Latvia, Lithuania, Moldova, Mongolia, Morocco, Netherlands, Poland, Romania, Russia (ARK, ZAB, KAM, KEM, KDA, MOS, NGR, PRI, RYA, SAK, SPE, STA, SA, YAR), Serbia, Slovakia, Spain, Sweden, Switzerland, Turkey, Ukraine, United Kingdom, Uzbekistan, Vietnam.
Notes. The species distribution in Iran and Israel is based in Belokobylskij et al. (2019).
Microplitistunetensis Marshall, 1901
Microplitistunetensis Marshall, 1901.
Type information. Lectotype female, MNHN (not examined but subsequent treatment of the species checked). Country of type locality: Tunisia.
Geographical distribution.PAL.
PAL: Hungary, Tunisia.
Notes. Our species concept is based on Papp (1984c).
Microplitisvaricolor Viereck, 1917
Microplitisvaricolor Viereck, 1917.
Type information. Holotype male, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (MB, NB, ON, QC), USA (AL, CO, CT, DC, FL, IL, LA, MI, MO, NY, OK, PA, SC, TN, TX).
Notes.Choi and Kim (2018) reported this species from Korea, and also considered that the species was previously distributed in other countries of the Palearctic region (Bulgaria, China, Finland, Germany, Japan, Norway, and Russia), but without citing any references to support those claims. Because the illustrations of the paper clearly show a male specimen (and not a female, as referred to by the authors), there are no details on the expert identifying the specimens, and the previous distribution of the species in other Palearctic countries has no supporting evidence, we strongly suspect that Choi and Kim (2018) misidentified the species they collected and refute their claims that varicolor is a Palearctic species. Those specimens likely belong to Microplitismediator, a widespread Palearctic species which seems morphologically and molecularly (DNA barcodes) similar to M.varicolor (Fernandez-Triana, unpublished data).
Microplitisvaripes (Ruthe, 1860)
Microgastervaripes Ruthe, 1860.
Microplitisvariipes Dalla Torre, 1898 [unjustified emendation].
Type information. Holotype female, NHMUK (examined). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Austria, Azerbaijan, China (QH, XJ), Finland, Georgia, Germany, Hungary, Italy, Kazakhstan, Malta, Moldova, Mongolia, Montenegro, Netherlands, Poland, Russia (ZAB, KDA, RYA, SPE, YAR), Serbia, Slovakia, Switzerland, Turkey, Ukraine.
Notes. The species distribution in Georgia is based in Belokobylskij et al. (2019).
Microplitisviduus (Ruthe, 1860)
Microgasterviduus Ruthe, 1860.
Type information. Holotype female, NHMUK (examined). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Armenia, Azerbaijan, Croatia, Cyprus, Czech Republic, Finland, Georgia, Germany, Greece, Hungary, Iran, Israel, Italy, Kazakhstan, Macedonia, Moldova, Mongolia, Netherlands, Poland, Romania, Russia (ZAB, DA, PRI, SAR, YAR), Serbia, Switzerland, Turkey, Ukraine, United Kingdom, Uzbekistan.
Notes. The species distribution in Armenia, Georgia, Kyrgyzstan and Turkmenistan is based in Belokobylskij et al. (2019).
Microplitisvitobiasi Fernandez-Triana, 2019, new replacement name
Microplitisvariicolor Tobias, 1964 [junior primary homonym of Microplitisvaricolor Viereck, 1917].
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Kazakhstan.
Geographical distribution.PAL.
PAL: Azerbaijan, Kazakhstan, Mongolia, Russia (S), Ukraine.
Notes. Our species concept is based on Papp (1984c) and Kotenko (2006). Microplitisvariicolor Tobias, 1964 is a junior primary homonym of Microplitisvaricolor Viereck, 1917 under ICZN Article 58.15 (they differ only in the presence or absence of a connecting -i before a suffix). The replacement name is a combination of the initials and last name of V.I. Tobias, the author originally describing the species.
Microplitisxanthopus (Ruthe, 1860)
Microgasterxanthopus Ruthe, 1860.
Microgastertenuipes Thomson, 1895.
Type information. Holotype female, NHMUK (examined). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Belarus, Bulgaria, Croatia, Czech Republic, Finland, Georgia, Germany, Hungary, Iran, Ireland, Italy, Kazakhstan, Moldova, Poland, Romania, Russia (IRK, KDA, SAK, SPE, VGG, YAR), Serbia, Sweden, Switzerland, Ukraine, United Kingdom.
Notes. The species distribution in Iran is based in Belokobylskij et al. (2019).
Microplitiszhaoi Xu & He, 2000
Microplitiszhaoi Xu & He, 2000.
Type information. Holotype female, ZJUH (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, ZJ), India.
Notes. Our species concept is based on Ranjith et al. (2015a).
Genus Miropotes Nixon, 1965
Miropotes Nixon, 1965: 200. Gender: feminine. Type species: Miropotescreon Nixon, 1965, by original designation.
Known from 15 described species from the Oriental, Australasian and Afrotropical regions. We have seen additional species in collections but Miropotes does not seem to be very speciose. There are 34 DNA-barcode compliant sequences of this genus in BOLD, representing 12 BINs.
Miropotesaustini Fernandez-Triana & Whitfield, 2014
Miropotesaustini Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (NSW).
Miropotesboothis Austin, 1990
Miropotesboothis Austin, 1990.
Type information. Holotype female, ANIC (not examined but subsequent treatment of the species checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (QLD).
Notes. Our species concept is based on Fernandez-Triana et al. (2014d).
Miropotesburringbaris Austin, 1990
Miropotesburringbaris Austin, 1990.
Type information. Holotype female, ANIC (not examined but subsequent treatment of the species checked). Country of type locality: Australia.
Geographical distribution.AUS, OTL.
AUS: Australia (ACT, NSW, QLD, TAS, VIC), Papua New Guinea; OTL: Indonesia.
Notes. Our species concept is based on Fernandez-Triana et al. (2014d).
Miropotescadgeis Austin, 1990
Miropotescadgeis Austin, 1990.
Type information. Holotype female, ANIC (not examined but subsequent treatment of the species checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (NSW, QLD).
Notes. Our species concept is based on Fernandez-Triana et al. (2014d).
Miropoteschookolis Austin, 1990
Miropoteschookolis Austin, 1990.
Type information. Holotype female, ANIC (not examined but subsequent treatment of the species checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (QLD).
Notes. Our species concept is based on Fernandez-Triana et al. (2014d).
Miropotescreon Nixon, 1965
Miropotescreon Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (TAS).
Miropotesgoobitis Austin, 1990
Miropotesgoobitis Austin, 1990.
Type information. Holotype female, ANIC (not examined but subsequent treatment of the species checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (NT, QLD, WA).
Notes. Our species concept is based on Fernandez-Triana et al. (2014d).
Miropotesinexpectatus van Achterberg & Fernandez-Triana, 2017
Miropotesinexpectatus van Achterberg & Fernandez-Triana, 2017.
Type information. Holotype female, RMNH (examined). Country of type locality: Yemen.
Geographical distribution.AFR.
AFR: Yemen.
Miropoteskatois Austin, 1990
Miropoteskatois Austin, 1990.
Type information. Holotype female, ANIC (not examined but subsequent treatment of the species checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (SA).
Notes. Our species concept is based on Fernandez-Triana et al. (2014d).
Miropoteskilkulunis Austin, 1990
Miropoteskilkulunis Austin, 1990.
Type information. Holotype female, ANIC (not examined but subsequent treatment of the species checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (NT).
Notes. Our species concept is based on Fernandez-Triana et al. (2014d).
Miropoteslordhowensis Fernandez-Triana & Whitfield, 2014
Miropoteslordhowensis Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (NSW, TAS).
Miropotesneglectus Fernandez-Triana & Whitfield, 2014
Miropotesneglectus Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Papua New Guinea.
Geographical distribution.AUS.
AUS: Papua New Guinea.
Miropotesorientalis Fernandez-Triana & van Achterberg, 2014
Miropotesorientalis Fernandez-Triana & van Achterberg, 2014.
Type information. Holotype female, RMNH (examined). Country of type locality: Vietnam.
Geographical distribution.OTL.
OTL: Thailand, Vietnam.
Miropotespetiolaris (Szépligeti, 1905)
Microgasterpetiolaris Szépligeti, 1905.
Type information. Lectoype lost (not examined but subsequent treatment of the species checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (ACT, NSW, NT, QLD, SA, WA).
Notes. Our species concept is based on Fernandez-Triana et al. (2014d). The female lectotype is considered to be lost (Austin and Dangerfield 1993: 1156).
Miropotesthuraris Austin, 1990
Miropotesthuraris Austin, 1990.
Type information. Holotype female, ANIC (not examined but subsequent treatment of the species checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (NSW, NT, QLD, SA, TAS, VIC, WA), New Caledonia, Papua New Guinea, Vanuatu.
Notes. Our species concept is based on Fernandez-Triana et al. (2014d).
Genus Napamus Papp, 1993
Napamus Papp, 1993: 168. Gender: masculine. Type species: Apantelesvipio Reinhard, 1880, by original designation.
Known from two described species (Papp 1993), but the limits of this genus are not clear at present. One of the species has been reared from Scythrididae and Tineidae. There are no DNA barcode sequences of Napamus in BOLD.
Napamusvipio (Reinhard, 1880)
Apantelesvipio Reinhard, 1880.
Type information. Syntypes female and male, depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: unknown.
Geographical distribution.PAL.
PAL: Austria, Croatia, France, Germany, Hungary, Israel, Italy, Romania, Russia (C), Spain, Turkey.
Notes. Our species concept is based on Papp (1993). The species distribution in Israel is based in Belokobylskij et al. (2019).
Napamuszomborii Papp, 1993
Napamuszomborii Papp, 1993.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Armenia.
Geographical distribution.PAL.
PAL: Armenia.
Genus Neoclarkinella Rema & Narendran, 1996
Neoclarkinella Rema & Narendran, 1996: 264. Gender: feminine. Type species: Apantelesnilamburensis Sumodan & Narendran, 1990, by original designation.
There are seven described species of Neoclarkinella, all from the Oriental region, but the genus has never been revised and we have seen many undescribed species in collections, including species from the Afrotropical, Oriental, and Palearctic regions. No host data are currently available for this genus. There are 130 DNA-barcode compliant sequences of this genus in BOLD, representing 32 BINs.
Neoclarkinellaariadne (Nixon, 1965), new combination
Apantelesariadne Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Sri Lanka.
Geographical distribution.OTL.
OTL: China (GX), India, Sri Lanka.
Notes. This species was transferred to Iconella by Mason (1981) based on its strong median longitudinal carina on the propodeum. However, the propodeum also has a transverse carina (near the anterior margin), T1 has a wide depression in the anterior half (in addition to a median, longitudinal sulcus throughout the entire tergite), and the veins r and 2RS have the characteristic shape found in Neoclarkinella (e.g., Figs 161C, 162C, 163D, 164C, 165C). Based on these characters, we here transfer the species to that genus.
Figure 162.
Neoclarkinella sp. female CNC924600 A Habitus, lateral B Habitus magnified, lateral C Fore wing D Head, frontal E Antennae F Metasoma, dorsal G Mesosoma, dorsal.
Figure 163.
Neoclarkinella sp. female CNCH1454 A Habitus, lateral B Mesosoma, dorsal C Propodeum, dorsal D Fore wing and hind wing E Head, frontal F Metasoma, dorsal G Ovipositor and ovipositor sheaths.
Figure 164.
Neoclarkinella sp. female CNCH2005 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Mesosoma, dorsal E Metasoma, dorsal F Ovipositor and ovipositor sheaths.
Neoclarkinellacurvinervus (Song & Chen, 2014), new combination
Choerascurvinervus Song & Chen, 2014.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, GD, GZ, HI, HN, SN, YN, ZJ).
Notes. Transferred to Neoclarkinella based on the curved veins r and 2RS in the fore wing, shape of T1, and propodeum carination.
Neoclarkinellajanakikkadensis Veena, 2014
Neoclarkinellajanakikkadensis Veena, 2014.
Type information. Holotype female, DZUC (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Neoclarkinellanarendrani Veena, 2014
Neoclarkinellanarendrani Veena, 2014.
Type information. Holotype female, DZUC (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Neoclarkinellapunctata Ahmad, Pandey, Haider & Shujauddin, 2005
Neoclarkinellapunctata Ahmad, Pandey, Haider & Shujauddin, 2005.
Type information. Holotype female, AMUZ (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Neoclarkinellasundana (Wilkinson, 1930), new combination
Apantelessundanus Wilkinson, 1930.
Type information. Holotype female, NHMUK (examined). Country of type locality: Indonesia.
Geographical distribution.OTL.
OTL: Indonesia.
Notes. This species was transferred to Iconella by Mason (1981), based on the median longitudinal carina on the propodeum. However, we have examined the holotype and there is also an almost complete transverse carina (only interrupted centrally), and the fore wing venation and shape of T1 clearly show this species is better placed in Neoclarkinella.
Neoclarkinellavitellinipes (You & Zhou, 1990)
Apantelesvitellinipes You & Zhou, 1990.
Apantelesnilamburensis Sumodan & Narendran, 1990.
Type information. Holotype female, HUNAU (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ, GX, HB, YN), India.
Notes. Our species concept is based on van Achterberg and Narendran (1997), Chen and Song (2004), Veena et al. (2014) and Gupta & Fernandez-Triana (2014).
Genus Nyereria Mason, 1981
Nyereria Mason, 1981: 108. Gender: feminine. Type species: Apantelesmlanje Wilkinson, 1929, by original designation.
There are 29 described species of Nyereria, but the genus has never been revised and we have seen many undescribed species in collections, including species from the Afrotropical, Oriental and Palearctic regions. Five families of Lepidoptera have been recorded as hosts of Nyereria, but they require further verification. There are 26 DNA-barcode compliant sequences of this genus in BOLD, representing five BINs.
Nyereriaachaeus (de Saeger, 1944)
Apantelesachaeus de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Rwanda.
Geographical distribution.AFR.
AFR: Rwanda.
Notes. Because the name is to be considered as a noun under ICZN Article 31.2.1, it must retain its original spelling and remain as achaeus.
Nyereriaalbicentrus (Long & van Achterberg, 2008)
Protapantelesalbicentrus Long & van Achterberg, 2008.
Type information. Holotype female, IEBR (not examined but original description checked). Country of type locality: Vietnam.
Geographical distribution.OTL.
OTL: Vietnam.
Nyereriaankaratrensis (Granger, 1949)
Apantelesankaratrensis Granger, 1949.
Type information. Holotype female, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Nyereriaareatus (Granger, 1949)
Apantelesareatus Granger, 1949.
Type information. Syntypes female and male, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Notes. Because the name is to be considered as a noun under ICZN Article 31.2.1, it must retain its original spelling and remain as areatus.
Nyereriabicolorata Long & van Achterberg, 2015
Nyereriabicolorata Long & van Achterberg, 2015.
Type information. Holotype female, VNMN (not examined but original description checked). Country of type locality: Vietnam.
Geographical distribution.OTL.
OTL: Vietnam.
Nyereriabifissa (de Saeger, 1944)
Apantelesbifissus de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Nyereriacircinus (de Saeger, 1944)
Apantelescircinus de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. Because the name is to be considered as a noun under ICZN Article 31.2.1, it must retain its original spelling and remain as circinus.
Nyereriaepaphus (de Saeger, 1944)
Apantelesepaphus de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Rwanda.
Notes. Because the name is to be considered as a noun under ICZN Article 31.2.1, it must retain its original spelling and remain as epaphus.
Nyereriaflavotorquata (Granger, 1949)
Apantelesflavotorquatus Granger, 1949.
Type information. Syntypes female, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Nyereriaforensis (Tobias, 1977)
Apantelesforensis Tobias, 1977.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Korea, Russia (KHA).
Notes. Our species concept is based on Kotenko (2007a). The species name was misspelled as forensic by Belokobylskij et al. (2019).
Nyereriaganges Rousse & Gupta, 2013
Nyereriaganges Rousse & Gupta, 2013.
Type information. Holotype female, MNHN (not examined but original description checked). Country of type locality: Réunion.
Geographical distribution.AFR.
AFR: Réunion.
Nyereriageometrae (Granger, 1949)
Apantelesgeometrae Granger, 1949.
Type information. Syntypes female and male, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Nyereriahiero (de Saeger, 1944)
Apanteleshiero de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Nyereriaituriensis (de Saeger, 1941), new combination
Apantelesituriensis de Saeger, 1941.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. The original description and drawing of T1 and T2 are clear enough to allow us to place the species within the genus Nyereria.
Nyereriamayurus Rousse & Gupta, 2013
Nyereriamayurus Rousse & Gupta, 2013.
Type information. Holotype female, MNHN (not examined but original description checked). Country of type locality: Réunion.
Geographical distribution.AFR.
AFR: Réunion.
Nyereriamenuthias (Wilkinson, 1935)
Apantelesmenuthias Wilkinson, 1935.
Type information. Holotype female, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Nyereriamlanje (Wilkinson, 1929)
Apantelesmlanje Wilkinson, 1929.
Apantelesmlanjeflaviventris Risbec, 1951.
Apantelesmlanjepallidus Risbec, 1951.
Type information. Holotype female, NHMUK (examined). Country of type locality: Malawi.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Malawi, Senegal.
Notes. This species has long been considered to be very variable. In the original description of Apantelesmlanjenigricoxis, Wilkinson (1932a) provided details on some of the differences, mostly in colour, between the new taxon (from Uganda) and the type series of A.mlanje (which was also described by Wilkinson in 1929, from Malawi), but for some reason he decided to retain nigricoxis as a subspecies of mlanje. Other authors working on the African fauna of Microgastrinae also found specimens related to (but morphologically different from) mlanje. De Saeger (1944) described three taxa from the Democratic Republic of Congo, which he considered the same as Wilkinson species (mlanje) but awarded them infraspecific status as “aberrations”; those three names were mentioned by Shenefelt (1972: 573-574) but treated as excluded names in his Braconidae catalogue. Similarly, Risbec (1951) mentioned at least two “groups” or “forms” from Senegal, which he called Apantelesmlanjeflaviventris and Apantelesmlanjepallidus; those two names were not referred to by Shenefelt (1972). Both de Saeger and Risbec found considerable variation within mlanjesensu lato, and they detailed differences beyond colouration, e.g., sculpture, fore wing venation, shape of T2; Risbec (1951: 431) even acknowledged that the range of variation in the species seemed to be considerably more than in other species of Microgastrinae. Regardless of that, until now these specimens have all been kept as one species. After examining the holotypes of Apantelesmlanje Wilkinson, 1929 (from Malawi) and A.mlanjenigricoxis Wilkinson, 1932 (from Uganda), both deposited in the NHMUK, we consider them to represent distinct species. The differences in colour are substantial, and the variation in shapes of T1 and T2 (especially the shape of the raised, central area of T2) are also significant. Thus, we elevate nigricoxis to species status (see below, under that species, for more details; p 822, 823). As for the other forms or subspecies proposed by de Saeger and Risbec, we suspect some may represent additional species (especially the specimens from Senegal, in Western Africa, which are far from all other specimens in Central Africa and seem to have lighter colouration). However, we cannot make any decisions based only on the original descriptions alone; until we have studied these specimens we prefer to leave them as Nyereriamlanje.
Nyererianeavei (Wilkinson, 1929)
Apantelesneavei Wilkinson, 1929.
Type information. Holotype female, NHMUK (examined). Country of type locality: Malawi.
Geographical distribution.AFR, OTL.
AFR: Democratic Republic of Congo, Malawi; OTL: China (FJ, YN).
Nyererianeleus (de Saeger, 1944)
Apantelesneleus de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. Because the name is to be considered as a noun under ICZN Article 31.2.1, it must retain its original spelling and remain as neleus.
Nyererianigricoxis (Wilkinson, 1932), status revised
Apantelesmlanjenigricoxis Wilkinson, 1932.
Type information. Holotype female, NHMUK (examined). Country of type locality: Uganda.
Geographical distribution.AFR.
AFR: Uganda.
Notes. Until now this was considered a subspecies of Nyereriamlanje (Wilkinson, 1929). After comparing the holotypes of both taxa, we consider them to be distinct species (see more comments above under mlanje). Nyererianigricoxis has darker legs (especially metacoxa and metatibia), T1 narrower at the posterior margin, and T2 with a median raised area much thinner than in mlanje. The fore wing venation also differs, specially the proportional lengths of veins r and 2RS.
Nyererianioro (Risbec, 1951), new combination
Apantelesnioro Risbec, 1951.
Type information. Syntypes female and male, depository unknown (not examined but original description checked). Country of type locality: Senegal.
Geographical distribution.AFR.
AFR: Senegal.
Notes. From the original description and drawings there, it is clear that this species is not an Apanteles. The best generic placement at present is in Nyereria, based on the shape and sculpture of T2, and also on comments made by Risbec (1951) on its closest relatives (which are also Nyereria species).
Nyereriaosiris (de Saeger, 1944)
Apantelesosiris de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Cameroon, Democratic Republic of Congo, Rwanda.
Nyereriaproagynus (Hedqvist, 1965), new combination
Apantelesproagynus Hedqvist, 1965.
Type information. Holotype male, MZH (examined). Country of type locality: Cape Verde.
Geographical distribution.AFR.
AFR: Cape Verde.
Notes.Forshage et al. (2016) considered the type material to be lost; however, it was found by the senior author of this paper in another section of the MZH collection. We examined the holotype and paratype, both male specimens in relatively good condition. They clearly belong to the genus Nyereria based on the carination pattern of propodeum and the median field in T2. Because the name is considered as a noun under ICZN Article 31.2.1, it must retain its original spelling and remain as proagynus.
Nyereriarageshri Sathe, 1988
Nyereriarageshri Sathe, 1988.
Type information. Holotype female, NZSI (not examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Nyereriataoi (Watanabe, 1935), new combination
Apantelestaoi Watanabe, 1935.
Type information. Holotype female, EIHU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (JX, ZJ); PAL: China (SD).
Notes. Here transferred to Nyereria based in the original description mentioning T2 having sulci enclosing a smooth median area, short ovipositor sheaths, acute hypopygium, and the author’s statement that taoi closely resembles Apantelesmlanje Wilkinson, a species long placed in Nyereria.
Nyereriatereus (de Saeger, 1944)
Apantelestereus de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Rwanda.
Geographical distribution.AFR.
AFR: Rwanda.
Notes. Because the name is to be considered as a noun under ICZN Article 31.2.1, it must retain its original spelling and remain as tereus.
Nyereriatriptolemus (de Saeger, 1944)
Apantelestriptolemus de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Ivory Coast, Rwanda.
Notes. Because the name is to be considered as a noun under ICZN Article 31.2.1, it must retain its original spelling and remain as triptolemus.
Nyereriavallatae (Watanabe, 1934), new combination
Apantelesvallatae Watanabe, 1934.
Type information. Syntypes female and male, EIHU (examined). Country of type locality: Japan.
Geographical distribution.PAL.
PAL: Japan.
Notes. The original description (Watanabe 1934: 132–133) was based on five female specimens and did not designate a holotype. We have examined four of those specimens, in the EIHU collection, all with red labels that have the word Type written, and also a second, smaller, white label that reads Cotype. Thus, we consider that they are all syntypes (and that there is no holotype, as stated by other sources, e.g., Shenefelt 1972: 658; Yu et al. 2016). Furthermore, one of the specimens is a male, its relatively small genitalia might have been difficult to see clearly in 1934. There is also a fifth pin with the cocoon mass on a plant twig. One of the syntypes had lost its metasoma, but the other three have their metasomae intact; in two of those cases T2 is relatively narrow and delimited by strong, parallel sulci, clearly similar to other Nyereria species. That agrees with Watanabe’s statement, in his original description, that the species belongs to Wilkinson’s mlanje subgroup (which is currently considered to belong to the genus Nyereria). The third syntype has an intact metasoma has T2 with a slightly different shape (slightly widening towards posterior margin), but overall is very similar to the other two specimens.
Nyereriayenthuyensis (Long & van Achterberg, 2008)
Protapantelesyenthuyensis Long & van Achterberg, 2008.
Type information. Holotype female, IEBR (not examined but original description checked). Country of type locality: Vietnam.
Geographical distribution.OTL.
OTL: Vietnam.
Genus Ohenri Fernandez-Triana, 2018
Ohenri Fernandez-Triana, 2018: 98. Gender: neuter. Type species: Ohenrigouletorum Fernandez-Triana & Boudreault, 2018, by original designation.
Known from a single species from the Afrotropical region, which was recently described (Fernandez-Triana and Boudreault 2018). No host data are currently available for this genus. There are no DNA barcode sequences of Ohenri in BOLD.
Ohenrigouletorum Fernandez-Triana & Boudreault, 2018
Ohenrigouletorum Fernandez-Triana & Boudreault, 2018.
Type information. Holotype female, CNC (examined). Country of type locality: Nigeria.
Geographical distribution.AFR.
AFR: Nigeria.
Genus Papanteles Mason, 1981
Papanteles Mason, 1981: 47. Gender: masculine. Type species: Papantelespeckorum Mason, 1981, by original designation.
Known from two described species from the Neotropics; we have seen a few more in collections but the genus does not seem to be species rich. Although no host information has ever been published for Papanteles, the ACG caterpillar database records a few species of Crambidae as hosts. There are 56 DNA-barcode compliant sequences of this genus in BOLD, representing three BINs.
Papantelespeckorum Mason, 1981
Papantelespeckorum Mason, 1981.
Type information. Holotype female, CNC (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Belize, Brazil (RJ), Ecuador, Mexico, Panama, Trinidad & Tobago.
Papantelesvirbius (Nixon, 1965)
Hypomicrogastervirbius Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (SC).
Genus Parapanteles Ashmead, 1900
Parapanteles Ashmead, 1900: 131. Gender: masculine. Type species: Apantelesaletiae Riley, 1881, by original designation and monotypy.
A recent revision of the genus (Valerio et al. 2009) is now considered to be outdated, as we recognize 62 described species of Parapanteles (including a relatively large number transferred in the present paper). However, the limits of this genus are highly controversial (see discussion above on section Brief diagnosis of all Microgastrinae genera as they are understood in this paper, for more details on p 41), and it is difficult to estimate the potential species richness. Regardless of that, we have seen many undescribed species in collections, from all regions. Approximately a dozen Lepidoptera host families have been recorded in the literature, but many of those records may be wrong. There are almost 1,000 DNA-barcode compliant sequences of this genus in BOLD, representing 97 BINs, but many of those sequences are likely to represent other genera.
Parapantelesaethiopicus (Wilkinson, 1931), new combination
Dolichogenideaaethiopicus Wilkinson, 1931.
Apantelesprocerae Risbec, 1951.
Type information. Holotype female, NHMUK (examined). Country of type locality: Uganda.
Geographical distribution.AFR.
AFR: Cameroon, Democratic Republic of Congo, Egypt, Ethiopia, Ivory Coast, Kenya, Rwanda, Senegal, Sierra Leone, Somalia, South Africa, Sudan, Tanzania, Uganda.
Notes. Based on the relatively short ovipositor sheaths (approximately one third as long as metatibia length), inflexible hypopygium, and fully areolated propodeum, this species is placed in Parapanteles.
Parapantelesaletiae (Riley, 1881)
Apantelesaletiae Riley, 1881.
Type information. Syntypes female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA, NEO.
NEA: USA (AL, FL); NEO: Cuba, Puerto Rico.
Notes.Valerio et al. (2009: 12) mentioned a female holotype and two paratypes of this species in the USNM. We have examined the same material and found that the three specimens (mounted on individual points) are all on the same pin, which also contains a fourth point with the three cocoons. The red label attached to that pin shows that it is USNM Type number 2771, which agrees with both Valerio et al. (2009) and Shenefelt's catalogue (1972). None of the available labels associated with those specimens (nor any other data or published papers that we are aware of) suggest that a lectotype was designated from among the three syntypes, so we consider them all to be syntypes; in any case, it is obvious that there cannot be a holotype for this species. At the time one of us (JFT) examined the syntypes, in October 2017, the first specimen (the top point) was almost entirely missing, with only parts of two legs glued to that point. The other two specimens were both missing the entire metasoma (and one of them was also missing one antenna). That leaves the entire type series as currently having only two syntypes with missing metasomae. Additionally, the drawing of the propodeum from Mason (1981), reproduced by Valerio et al. (2009), does not entirely reflect the two syntypes we examined, which have the areola wider at the posterior end, i.e., the carinae meet the nucha more separated from each other than is depicted by Mason or Valerio.
Parapantelesalternatus (Papp, 1973), new combination
Apantelesalternatus Papp, 1973.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. The original description and drawings provided there clearly show that this species belongs to Parapanteles.
Parapantelesarka Gupta, 2014
Parapantelesarka Gupta, 2014.
Type information. Holotype female, NBAIR (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Parapantelesaso (Nixon, 1967), new combination
Apantelesaso Nixon, 1967.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: China (YN), India.
Notes. This species was transferred from Apanteles to Dolichogenidea by Chen and Song (2004). However, we have examined the holotype, which has an inflexible hypopygium and very short ovipositor sheaths (less than 0.3 × metatibia length). Those characters suggest this species is better placed in Parapanteles, as is the case with two related taxa (Apanteleshyposidrae Wilkinson, 1928 and Apantelescleo Nixon, 1967). These three species were keyed out together in the same section of the key to Indo-Australian species of the ultor group by Nixon (1967) and are all transferred to Parapanteles in the present paper.
Parapantelesatellae (Wilkinson, 1932), new combination
Apantelesatellae Wilkinson, 1932.
Type information. Holotype female, NHMUK (examined). Country of type locality: Uganda.
Geographical distribution.AFR.
AFR: Uganda.
Notes. Based on the relatively short ovipositor sheaths (approximately one third as long as metatibia length), inflexible hypopygium and fully areolated propodeum, this species is placed in Parapanteles.
Parapantelesathamasae Gupta, Khot & Chorge, 2014
Parapantelesathamasae Gupta, Khot & Chorge, 2014.
Type information. Holotype female, NBAIR (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Parapantelesbagicha (Narayanan & Subba Rao, 1961), new combination
Apantelesbagicha Narayanan & Subba Rao, 1961.
Type information. Holotype female, INPC (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. Based on the original description and drawings included there, this species is better placed within Parapanteles, based on the areolated propodeum but very short ovipositor sheaths.
Parapantelescleo (Nixon, 1967), new combination
Apantelescleo Nixon, 1967.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India, Vietnam.
Notes. This species was transferred from Apanteles to Dolichogenidea by Long and Belokobylskij (1990). However, we have examined the holotype, which has an inflexible hypopygium and very short ovipositor sheaths (less than 0.3 × metatibia length). Those characters suggest this species is better placed in Parapanteles, as is the case with two related taxa (Apanteleshyposidrae Wilkinson, 1928 and Apantelesaso Nixon, 1967). These three species were keyed out together in the same section of the key to Indo-Australian species of the ultor group by Nixon (1967) and are all transferred to Parapanteles in the present paper.
Parapantelescomplexus Valerio & Janzen, 2009
Parapantelescomplexus Valerio & Janzen, 2009.
Type information. Holotype male, INBio (not examined but original description checked). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Parapantelescontinuus Valerio & Whitfield, 2009
Parapantelescontinua Valerio & Whitfield, 2009.
Type information. Holotype female, INBio (not examined but original description checked). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Notes. The species name must be treated as an adjective and not as a noun (Doug Yanega, pers. comm.) and thus it must match the gender of the genus name.
Parapantelescovino Rousse, 2013
Parapantelescovino Rousse, 2013.
Type information. Holotype female, MNHN (not examined but original description checked). Country of type locality: Réunion.
Geographical distribution.AFR.
AFR: Réunion.
Parapantelescyclorhaphus (de Saeger, 1944), new combination
Apantelescyclorhaphus de Saeger, 1944.
Type information. Syntypes female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. Based on the original description, the best generic placement is in Parapanteles.
Parapantelesdarignac Rousse, 2013
Parapantelesdarignac Rousse, 2013.
Type information. Holotype female, MNHN (not examined but original description checked). Country of type locality: Réunion.
Geographical distribution.AFR.
AFR: Réunion.
Parapantelesdemades (Nixon, 1965), new combination
Apantelesdemades Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Malaysia.
Geographical distribution.OTL.
OTL: Malaysia, Vietnam.
Notes. Based on the propodeal areola, hypopygium mostly inflexible and unpleated (but with small area postero-ventrally slightly translucent) and short ovipositor sheaths, this species is better placed in the genus Parapanteles.
Parapantelesecheriae Gupta, Pereira & Churi, 2013
Parapantelesecheriae Gupta, Pereira & Churi, 2013.
Type information. Holotype female, NBAIR (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Parapantelesem Valerio & Whitfield, 2009
Parapantelesem Valerio & Whitfield, 2009.
Type information. Holotype female, INBio (not examined but original description checked). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Parapantelesendymion (Wilkinson, 1932), new combination
Apantelesendymion Wilkinson, 1932.
Type information. Holotype female, NHMUK (examined). Country of type locality: Uganda.
Geographical distribution.AFR.
AFR: Uganda.
Notes. Based on the relatively short ovipositor sheaths (approximately one third as long as metatibial lengths), inflexible hypopygium and fully areolated propodeum, this species is placed in Parapanteles.
Parapantelesepiplemicidus (de Saeger, 1941), new combination
Apantelesepiplemicidus de Saeger, 1941.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. Transferred to Parapanteles based on the propodeum with pentagonal areolet and very short ovipositor sheaths.
Parapanteleseros Gupta, 2014
Parapanteleseros Gupta, 2014.
Type information. Holotype female, NBAIR (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Parapantelesesha Gupta, 2014
Parapantelesesha Gupta, 2014.
Type information. Holotype female, NBAIR (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Parapantelesexpulsus (Turner, 1919), new combination
Apantelesexpulsus Turner, 1919.
Apantelesmendanae Wilkinson, 1928.
Type information. Holotype female, NHMUK (examined). Country of type locality: Fiji.
Geographical distribution.AUS, OTL.
AUS: Fiji, Marquesas Islands, Western Samoa; OTL: China (FJ, GD, GX, HI, ZJ), Sri Lanka, Vietnam.
Notes. The holotype has an inflexible ovipositor, very short ovipositor sheaths (less than 0.3 x metatibial lengths), and the propodeum has a complete areola defined by strong carinae. All of this suggests this species is better placed in Parapanteles. We have also examined the type of A.mendanae Wilkinson, in the NHMUK. The species distribution in China is based in Liu et al. (2019).
Parapantelesfallax (de Saeger, 1944), new combination
Apantelesfallax de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. Based on the original description, the best generic placement is in Parapanteles.
Parapantelesfolia (Nixon, 1965), new combination
Apantelesfolia Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Malaysia.
Geographical distribution.AUS, OTL.
AUS: Australia (QLD), Papua New Guinea; OTL: China (GD, TW), India, Malaysia, Philippines.
Notes. The holotype is missing the antennae and the micropin is full of rust, but nevertheless most of the morphological features are visible. Based on the propodeal areola, hypopygium mostly inflexible and unpleated (but with small area postero-ventrally slightly translucent), and short ovipositor sheaths, this species is better placed in the genus Parapanteles. This species most likely contains a complex of species, also suggested by Nixon (1965).
Parapantelesfurax (de Saeger, 1944), new combination
Apantelesfurax de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Rwanda.
Notes. Based on the original description, the best generic placement would be in Parapanteles.
Parapantelesgerontogeae Donaldson, 1991
Parapantelesgerontogeae Donaldson, 1991.
Type information. Holotype female, TMSA (not examined but original description checked). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Parapanteleshemitheae (Wilkinson, 1928), new combination
Apanteleshemitheae Wilkinson, 1928.
Type information. Holotype female, NHMUK (examined). Country of type locality: Malaysia.
Geographical distribution.OTL, PAL.
OTL: China (FJ, GD, GX, TW, ZJ), Malaysia, Vietnam; PAL: China (JS).
Notes. This species was transferred to Dolichogenidea by Long and Belokobylskij (2004), as part of their listing of Braconidae from Vietnam. We have examined the holotype and consider it would be better placed in a different genus. The ovipositor sheaths are very short (shorter than 0.3 x metatibia length), the hypopygium is mostly inflexible (with only a small translucent area near the apex, where no pleat is discernible), and T1, T2 and the anterior half of T3 are strongly sculptured. Those characters are very unusual (if at all present) in Dolichogenidea. Although some features would suggest Pholetesor, the host caterpillar recorded by Wilkinson (1928b) for the type series is Geometridae, a Lepidoptera family that has never been reported as host for Pholetesor. Thus, we believe that the best generic placement at present would be in Parapanteles, based on the complete areola on the propodeum, inflexible hypopygium, short ovipositor sheaths and known host. More studies of this and other Oriental species of Parapanteles may change that in the future (a similar situation might also apply to the species Parapantelesexclusus and P. hyposidrae). The species distribution in China is based in Liu et al. (2019).
Parapanteleshyposidrae (Wilkinson, 1928), new combination
Apanteleshyposidrae Wilkinson, 1928.
Type information. Holotype female, NHMUK (examined). Country of type locality: Indonesia.
Geographical distribution.AUS, OTL.
AUS: Australia (QLD), Papua New Guinea; OTL: China (FJ, GD, GX, HB, HN, TW, YN, ZJ), India, Indonesia, Malaysia, Myanmar, Vietnam.
Notes. This species was considered to belong to Dolichogenidea by Yu et al. (2016) and Liu et al. (2019). However, we have examined the holotype and it has an inflexible hypopygium, very short ovipositor sheaths (less than 0.2 x metatibia length), and the propodeum has a complete areola defined by strong carinae; these features suggest this species is better placed in Parapanteles.
Parapantelesindicus (Bhatnagar, 1950), new combination
Apantelesindica Bhatnagar, 1950.
Type information. Holotype female, INPC (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. Transferred to Parapanteles based on the propodeum with a quadrate areola and ovipositor sheaths very short (Bhatnagar, 1950: 178–179). The year of publication of the Bhatnagar paper was until recently commonly cited as 1948 and/or 1950 (e.g., Chen and Song 2004, Yu et al. 2016), probably following Shenefelt (1972) who referred to this paper as “Bhatnagar (1948) 1950”. While the intended year for Volume X, Parts I & II of the Indian Journal of Entomology was 1948, the actual dates of publication were June 1950 (Part I) and October 1950 (Part II), as clearly shown on the cover page of the Volume, which we have checked. Because the dates of publication are the ones to be considered, and for the sake of clarity, we hereby revise the species year of description to 1950.
Parapantelesjavensis (Rohwer, 1919), new combination
Apantelesjavensis Rohwer, 1919.
Type information. Holotype female, USNM (examined). Country of type locality: Indonesia.
Geographical distribution.OTL, PAL.
OTL: China (FJ, GX, HB, SN), India, Indonesia, Sri Lanka, Thailand, Vietnam; PAL: Japan.
Notes. The holotype is more reddish, when compared to the paratype illustrated in Gupta & Fernandez-Triana (2014), which looks more black. The holotype also has transverse striation on the middle of the hypopygium (very unusual and nothing to do with the hypopygium pleats, as it is actually oriented perpendicular to the hypopygium margin). Based on the inflexible hypopygium lacking pleats, we transfer this species to Parapanteles.
Parapantelesjhaverii (Bhatnagar, 1950), new combination
Apantelesjhaverii Bhatnagar, 1950.
Type information. Holotype female, INPC (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. Transferred to Parapanteles based on the propodeum with an areola, T1 with longitudinal carina, and very short ovipositor sheaths (Bhatnagar, 1950: 172–174). The year of publication of the Bhatnagar paper was until recently commonly cited as 1948 and/or 1950 (e.g., Chen and Song 2004, Yu et al. 2016), probably following Shenefelt (1972) who referred to this paper as “Bhatnagar (1948) 1950”. While the intended year for Volume X, Parts I & II of the Indian Journal of Entomology was 1948, the actual dates of publication were June 1950 (Part I) and October 1950 (Part II), as clearly shown on the cover page of the Volume, which we have checked. Because the dates of publication are the ones to be considered, and for the sake of clarity, we hereby revise the species year of description to 1950.
Parapanteleslincolnii Valerio & Whitfield, 2009
Parapanteleslincolnii Valerio & Whitfield, 2009.
Type information. Holotype male, INHS (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (MO).
Parapantelesmaculipalpis (de Saeger, 1941), new combination
Apantelesmaculipalpis de Saeger, 1941.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. Transferred to Parapanteles based on the relatively very short ovipositor sheaths, areolated propodeum, and also the comments by de Saeger (1941b: 261) about maculipalpis being very close to Apantelesatellae Wilkinson, a species that we have also transferred to Parapanteles in this paper, after examining its holotype.
Parapantelesmariae Valerio & Whitfield, 2009
Parapantelesmariae Valerio & Whitfield, 2009.
Type information. Holotype female, INBio (not examined but original description checked). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Parapantelesmasoni Austin & Dangerfield, 1992
Parapantelesmasoni Austin & Dangerfield, 1992.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (NT).
Parapantelesmaynei (de Saeger, 1941), new combination
Apantelesmaynei de Saeger, 1941.
Type information. Holotype male, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Senegal.
Notes. Transferred to Parapanteles based on the relatively short ovipositor sheaths, areolated propodeum, and also the comments by de Saeger (1941b: 256) about maynei being close to Apantelesaethipicus Wilkinson and Apantelesprosper Wilkinson, two species that we have also transferred to Parapanteles in this paper, after examining their holotypes.
Parapantelesneocajani (Yousuf & Ray, 2010), new combination
Apantelesneocajani Yousuf & Ray, 2010.
Type information. Holotype female, IFRI (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. The original description and drawings included there show a hind wing with vannal lobe fully setose, an inflexible hypopygium, and very short ovipositor sheaths (its length equal to the first segment of the metatarsus). Based on those characters, this species is clearly not an Apanteles but is better placed in Parapanteles.
Parapantelesneohyblaeae (Ray & Yousuf, 2009), new combination
Apantelesneohyblaeae Ray & Yousuf, 2009.
Type information. Holotype female, IFRI (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. The species was described as Apanteles, but the very small ovipositor and ovipositor sheaths indicate it does not belong to that genus. The original description does not provide any details about the propodeum, which would have helped considerably to assess the genus to which this species belongs. Without examining the specimens, the best generic placement at present is in Parapanteles.
Parapantelesnephos Valerio & Whitfield, 2009
Parapantelesnephos Valerio & Whitfield, 2009.
Type information. Holotype female, USNM (examined). Country of type locality: Peru.
Geographical distribution.NEO.
NEO: Peru.
Parapantelesnoae Valerio & Whitfield, 2009
Parapantelesnoae Valerio & Whitfield, 2009.
Type information. Holotype female, INBio (not examined but original description checked). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Parapantelesnydia (Nixon, 1967), new combination
Apantelesnydia Nixon, 1967.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. This species exemplifies the sometimes-blurred lines separating Dolichogenidea from Parapanteles. The holotype has the hind wing vannal lobe entirely setose, the anteromesoscutum punctures do not fuse near the scutoscutellar sulcus, the ovipositor sheaths are approximately half the length of the metatibia, and the hypopygium is mostly inflexible (although with a minor fold, seen as a translucent area ventro-posteriorly, but with no pleats marked). With the current understanding of both genera we think at present there is more support for the species to be transferred to Parapanteles, a decision we adopt here, but we note that future research on Microgastrinae may change that.
Parapantelesparadoxus (Muesebeck, 1958)
Apantelesparadoxus Muesebeck, 1958.
Type information. Holotype female, USNM (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Parapantelespolus Valerio & Whitfield, 2009
Parapantelespolus Valerio & Whitfield, 2009.
Type information. Holotype female, INBio (not examined but original description checked). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Parapantelesprosper (Wilkinson, 1932), new combination
Apantelesprosper Wilkinson, 1932.
Type information. Holotype female, NHMUK (examined). Country of type locality: Uganda.
Geographical distribution.AFR.
AFR: Uganda.
Notes. Based on the relatively very short ovipositor sheaths (less than 0.3 × metatibia length), inflexible hypopygium, and areolated propodeum (although the areola is poorly defined anteriorly), this species is placed in Parapanteles.
Parapantelesprosymna (Nixon, 1965), new combination
Apantelesprosymna Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Malaysia.
Geographical distribution.OTL.
OTL: Malaysia.
Notes. Based on the propodeal areola, hypopygium mostly inflexible and short ovipositor sheaths, this species is better placed in the genus Parapanteles.
Parapantelespunctatissimus (Granger, 1949), new combination
Apantelespunctatissimus Granger, 1949.
Type information. Syntypes female and male, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Notes. Transferred to Parapanteles based on the original description mentioning the propodeum with a complete areola, ovipositor sheaths very short, and T1–T3 shape and sculpture, as illustrated and described in Granger (1949: 269–270, fig. 280).
Parapantelesrarus Valerio & Whitfield, 2009
Parapantelesrarus Valerio & Whitfield, 2009.
Type information. Holotype female, INHS (not examined but original description checked). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Parapantelesregale Gupta, 2014
Parapantelesregale Gupta, 2014.
Type information. Holotype female, NBAIR (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Parapantelesregalis (de Saeger, 1941), new combination
Apantelesregalis de Saeger, 1941.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. Transferred to Parapanteles based on the original description mentioning the propodeum with a complete areola (in addition to a partially defined median carina), ovipositor sheaths very short, and T1–T3 shapes and sculptures as illustrated and described in de Saeger (1941: 218–220, fig. 7). The presence of a partial median carina would suggest Cotesia as another possible genus; however, the shapes of T1 (anterior 0.4 more or less parallel-sided, posterior 0.6 strongly narrowing towards posterior margin of tergite) and T2 (subtriangular) precludes the species to be considered in that genus, and Parapanteles is a much better generic placement. Future study of this species may be needed.
Parapantelesrooibos Valerio, Whitfield & Kole, 2005
Parapantelesrooibos Valerio, Whitfield & Kole, 2005.
Type information. Holotype female, PPRI (not examined but original description checked). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Parapantelessarpedon (de Saeger, 1944), new combination
Apantelessarpedon de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Rwanda.
Notes. Based on the original description, the best generic placement would be in Parapanteles because of the inflexible hypopygium, relatively short ovipositor sheaths, and propodeum with areola.
Parapantelessartamus (Nixon, 1965), new combination
Apantelessartamus Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Notes. Here transferred to Parapanteles, based on the propodeal areola complete and the short ovipositor sheaths (Nixon 1965).
Parapantelesscultena (Nixon, 1965), new combination
Apantelesscultena Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Malaysia.
Geographical distribution.OTL.
OTL: Malaysia.
Notes. We place this species in Parapanteles, based on the propodeal areola, short ovipositor sheaths and hypopygium inflexible and unfolded.
Parapantelesshivranginii Sathe & Ingawale, 1989
Parapantelesshivranginii Sathe & Ingawale, 1989.
Type information. Holotype female, NZSI (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Parapantelessicpolus Valerio & Whitfield, 2009
Parapantelessicpolus Valerio & Whitfield, 2009.
Type information. Holotype female, INBio (not examined but original description checked). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Parapantelessireeshaae Ahmad & Akhtar, 2010
Parapantelessireeshaae Ahmad & Akhtar, 2010.
Type information. Holotype female, INPC (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Parapantelestessares Valerio & Whitfield, 2009
Parapantelestessares Valerio & Whitfield, 2009.
Type information. Holotype female, INBio (not examined but original description checked). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Parapantelesthrix Valerio & Whitfield, 2009
Parapantelesthrix Valerio & Whitfield, 2009.
Type information. Holotype female, INHS (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (MO).
Parapantelestlinea Valerio & Whitfield, 2009
Parapantelestlinea Valerio & Whitfield, 2009.
Type information. Holotype female, INHS (not examined but original description checked). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Parapantelestransvaalensis (Cameron, 1911), new combination
Apantelestransvaalensis Cameron, 1911.
Type information. Holotype female, TMSA (not examined but subsequent treatment of the species checked). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: Malawi, South Africa.
Notes. Our species concept is based on Wilkinson (1932a: 320–321), who redescribed the species after examining the female holotype (the only specimen known). Transferred to Parapanteles based on the relatively very short ovipositor (shorter than the first segment of the metatarsus), truncate hypopygium, and fully areolated propodeum.
Parapantelesturri (Rao & Chalikwar, 1976), new combination
Apantelesturri Rao & Chalikwar, 1976.
Type information. Holotype female, BAMU (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. The drawings in the original description suggest that this species is better placed in Parapanteles, based on its short ovipositor sheaths and unpleated hypopygium. The authors even considered the species to have a “superficial resemblance with Apantelesfolia (Nixon, 1965)” (Rao and Chalikwar 1976a: 185), which is an indirect confirmation of the generic placement, since Apantelesfolia is also transferred to Parapanteles in the present paper.
Parapantelesxanthopholis (de Saeger, 1944), new combination
Apantelesxanthopholis de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Rwanda.
Notes. Based on the original description (de Saeger 1944), the best generic placement would be in Parapanteles.
Genus Parenion Nixon, 1965
Parenion Nixon, 1965: 208. Gender: feminine. Type species: Microgasterkokodana Wilkinson, 1936, by original designation.
Three described species are known from Australasia, but we have seen a few more in collections. No host data are currently available for this genus. There are four DNA-barcode compliant sequences of this genus in BOLD, representing one BIN. The gender of Parenion is not stated in the original description, but it is here assumed to be feminine based on the way Nixon (1965) treated the name of the only species known (at the time the genus was described).
Parenionbeelaronga Austin & Dangerfield, 1992
Parenionbeelaronga Austin & Dangerfield, 1992.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (QLD).
Parenionbootha Austin & Dangerfield, 1992
Parenionbootha Austin & Dangerfield, 1992.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (QLD).
Parenionkokodana (Wilkinson, 1936)
Microgasterkokodana Wilkinson, 1936.
Type information. Holotype female, NHMUK (examined). Country of type locality: Papua New Guinea.
Geographical distribution.AUS.
AUS: Australia (QLD), Papua New Guinea.
Genus Paroplitis Mason, 1981
Paroplitis Mason, 1981: 68. Gender: masculine. Type species: Paroplitisberingianus Mason, 1981, by original designation.
Five described species were recently revised (Fernandez-Triana et al. 2013b) but we have seen more species in collections. The genus is essentially Holarctic, but occasionally reaching the northern limits of the Oriental region. Host records representing four Lepidoptera families have been reported for one species of Paroplitis, but only Crambidae (Scopariinae) has been confirmed (Shaw 2012b). There are 32 DNA-barcode compliant sequences of this genus in BOLD, representing one BIN.
Paroplitisberingianus Mason, 1981
Paroplitisberingianus Mason, 1981.
Type information. Holotype female, CNC (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (BC), USA (AK).
Paroplitisluzonicus Mason, 1981
Paroplitisluzonicus Mason, 1981.
Type information. Holotype female, AEIC (not examined but subsequent treatment of the species checked). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Notes. Our species concept is based on Fernandez-Triana et al. (2013b).
Paroplitisrugosus Papp, 1991
Paroplitisrugosus Papp, 1991.
Type information. Holotype female, HNHM (not examined but subsequent treatment of the species checked). Country of type locality: Austria.
Geographical distribution.PAL.
PAL: Austria.
Notes. Our species concept is based on Fernandez-Triana et al. (2013b).
Paroplitisvietnamensis van Achterberg & Fernandez-Triana, 2013
Paroplitisvietnamensis van Achterberg & Fernandez-Triana, 2013.
Type information. Holotype female, RMNH (examined). Country of type locality: Vietnam.
Geographical distribution.OTL.
OTL: Vietnam.
Notes. A recent PhD thesis (Ahmed 2017) recorded this species from India (from two different districts in the state of Jammu and Kashmir). The accompanying images in that paper strongly indicate that this is a different, undescribed species, based on different sculpture of T1 and T2, and also the fact that the Indian localities are more than 3,500 km from the type locality in Vietnam. Thus, we here consider P. vietnamensis not to be present in India.
Paroplitiswesmaeli (Ruthe, 1860)
Microgasterwesmaeli Ruthe, 1860.
Microgasterpicipes Wesmael, 1837 [primary homonym of Microgasterpicipes Bouché, 1834].
Type information. Holotype female, RBINS (not examined but subsequent treatment of the species checked). Country of type locality: Belgium.
Geographical distribution.PAL.
PAL: Azerbaijan, Belgium, Finland, France, Germany, Hungary, Poland, Romania, Russia (KDA), Sweden, Switzerland, Ukraine, United Kingdom.
Notes. Our species concept is based on Shaw (2012b) and Fernandez-Triana et al. (2013b).
Genus Pelicope Mason, 1981
Pelicope Mason, 1981: 57. Gender: feminine. Type species: Pelicopeyuccamica Mason, 1981, by original designation.
Only known from one species in the Nearctic region. The parasitoid has been reared from Prodoxidae. There is one DNA-barcode compliant sequence of Pelicope in BOLD, representing one BIN.
Pelicopeyuccamica Mason, 1981
Pelicopeyuccamica Mason, 1981.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CA).
Genus Philoplitis Nixon, 1965
Philoplitis Nixon, 1965: 267. Gender: masculine. Type species: Philoplitisconiferens Nixon, 1965 by original designation and monotypy.
Philoplitis has been revised twice in the past ten years (Fernandez-Triana and Goulet 2009, Ranjith et al. 2019), with the latest paper recording nine species. We suspect a few more will be found when more collections are studied, but the genus does not seem to be species rich. Philoplitis species are mainly found in the Oriental region, but it also reaches the Afrotropics and one species marginally reaches the southernmost limits of the Palearctic region (Ranjith et al. 2019). No host data are currently available for this genus. There are seven DNA-barcode compliant sequences of this genus in BOLD, representing four BINs.
Philoplitisadustipalpus Ahmad, 2005
Philoplitisadustipalpus Ahmad, 2005.
Type information. Holotype female, AMUZ (not examined but subsequent treatment of the species checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. Our species concept is based on Fernandez-Triana and Goulet (2009) and Ranjith et al. (2019).
Philoplitisconiferens Nixon, 1965
Philoplitisconiferens Nixon, 1965.
Type information. Holotype female, USNM (not examined but subsequent treatment of the species checked). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: China (GD, GX), Philippines.
Notes. Our species concept is based on Fernandez-Triana and Goulet (2009) and Ranjith et al. (2019).
Philoplitisdzangasangha Fernandez-Triana & Ranjith, 2019
Philoplitisdzangasangha Fernandez-Triana & Ranjith, 2019.
Type information. Holotype male, CNC (examined). Country of type locality: Central African Republic.
Geographical distribution.AFR.
AFR: Central African Republic.
Philoplitiskeralensis Ranjith & Fernandez-Triana, 2019
Philoplitiskeralensis Ranjith & Fernandez-Triana, 2019.
Type information. Holotype female, DZUC (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Philoplitismargalla Fernandez-Triana & Ranjith, 2019
Philoplitismargalla Fernandez-Triana & Ranjith, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Pakistan.
Geographical distribution.PAL.
PAL: Pakistan.
Philoplitismasneri Fernandez-Triana & Goulet, 2009
Philoplitismasneri Fernandez-Triana & Goulet, 2009.
Type information. Holotype male, CNC (examined). Country of type locality: Kenya.
Geographical distribution.AFR.
AFR: Kenya.
Philoplitispunctatus Fernandez-Triana & Goulet, 2009
Philoplitispunctatus Fernandez-Triana & Goulet, 2009.
Type information. Holotype male, CNC (examined). Country of type locality: Thailand.
Geographical distribution.OTL.
OTL: Thailand.
Philoplitisstriatus Fernandez-Triana & Goulet, 2009
Philoplitisstriatus Fernandez-Triana & Goulet, 2009.
Type information. Holotype male, CNC (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India, Sri Lanka.
Philoplitistrifoveatus Ranjith & Fernandez-Triana, 2019
Philoplitistrifoveatus Ranjith & Fernandez-Triana, 2019.
Type information. Holotype female, DZUC (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Genus Pholetesor Mason, 1981
Pholetesor Mason, 1981: 37. Gender: masculine. Type species: Apantelesornigis Weed, 1887, by original designation.
This is a cosmopolitan genus, with 57 described species, but we have seen many additional species in collections, mostly from temperate areas. There are some revisions available for the Nearctic (Whitfield 2006) and Palearctic regions (see works of Nixon and Papp in the References section below), but most can be considered as outdated because none of them take into account the hidden diversity that is revealed by DNA barcoding and biological data. Around two dozen families of Lepidoptera have been recorded as hosts for Pholetesor, but many records are likely to be incorrect and/or need further verification. There are 1,000+ DNA-barcode compliant sequences of this genus in BOLD, representing 50 BINs.
Pholetesoracricauda Liu & Chen, 2016
Pholetesoracricauda Liu & Chen, 2016.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (SN); PAL: China (HA).
Pholetesoracutus (Papp, 1971), new combination
Apantelesacutus Papp, 1971.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Mongolia.
Geographical distribution.PAL.
PAL: Mongolia.
Notes. From the original description it is evident that this species is not Apanteles. Here we transfer acutus to Pholetesor based on the short length of the ovipositor sheaths, the inflexible and unpleated hypopygium, the shapes of T1 and T2, the propodeum sculpture, and the fact that Papp (1971: 311) considered the species to be closely related to Apantelesingenuus (which is currently placed within Pholetesor). In a subsequent paper illustrating Apantelesacutus (Papp 1984a: 290, figure 21), the drawing seems to show much longer ovipositor sheaths, although that may be a mistake, as the key to species in that same paper places acutus with other species which have “Ovipositor sheath short, in lateral view at most as long as first joint of hind tarsus” (quoted from couplet 17 in Papp 1984a: 267). We suspect that many of the species placed within the metacarpalis group by Papp (1984a), which includes species mostly described by Papp and Tobias, belong to Pholetesor.
Pholetesorambiguus (Papp, 1977)
Apantelesambiguus Papp, 1977.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Mongolia.
Geographical distribution.PAL.
PAL: Mongolia.
Pholetesorargyresthiae Liu & Chen, 2016
Pholetesorargyresthiae Liu & Chen, 2016.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (GS).
Pholetesorarisba (Nixon, 1973)
Apantelesarisba Nixon, 1973.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.AUS, OTL, PAL.
AUS: New Zealand; OTL: China (FJ, GZ, ZJ); PAL: Austria, Bulgaria, China (NX), Czech Republic, Denmark, Egypt, Germany, Greece, Hungary, Israel, Italy, Netherlands, Norway, Russia (NC), Serbia, Spain, Ukraine, United Kingdom.
Notes. The species distribution in Israel is based in Belokobylskij et al. (2019).
Pholetesorartusisulcus Liu & Chen, 2016
Pholetesorartusisulcus Liu & Chen, 2016.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (ZJ); PAL: China (NX).
Pholetesorbedelliae (Viereck, 1911)
Apantelesbedelliae Viereck, 1911.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.AUS, NEA, NEO, PAL.
AUS: Hawaiian Islands; NEA: Canada (AB, BC, MB, NS, ON, QC, SK), USA (AK, AZ, AR, CA, CT, DC, FL, IL, IA, KA, LA, MO, NJ, NY, OR, VA); NEO: Bermuda, Peru; PAL: Finland.
Notes. This species was introduced to the Hawaiian Islands (Fullaway 1950). There is also a record from Peru (de Huiza 1995) which should be considered as suspicious, but we retain it here as we could not examine it in more detail. The species is probably Holarctic in distribution.
Pholetesorbicolor (Nees, 1834)
Microgasterbicolor Nees, 1834.
Microgasterardeaepenellae Bouché, 1834.
Apantelesschillei Niezabitowski, 1910.
Apanteleslongicauda Fahringer, 1938.
Apantelespedias Nixon, 1973.
Type information. Neotype female, ZMHB (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.AUS, NEA, OTL, PAL.
AUS: New Zealand; NEA: Canada (ON); OTL: China (JS); PAL: Belgium, Bulgaria, Canary Islands, China (NX), Croatia, Finland, France, Georgia, Germany, Greece, Hungary, Iran, Ireland, Israel, Italy, Japan, Kyrgyzstan, Lithuania, Moldova, Mongolia, Poland, Romania, Russia (KAM, MOS, PRI, SPE, YAR), Serbia, Slovakia, Spain, Switzerland, Tunisia, Turkmenistan, Ukraine, United Kingdom.
Notes. According to Papp (1983a: remark on pages 253–254, not page 267 as referenced in footnote on page 251), Microgasterbicolor Curtis, 1830 is a nomen nudum, so Microgasterbicolor Nees, 1834 is valid. That statement was accepted by Shaw (2012b) and Broad et al. (2016) and it is also followed here, where we consider Pholetesorbicolor (Nees, 1834) as a valid name and a valid species. However, Wilkinson (1938d) listed bicolor (= pediassensu Nixon) as a synonym of circumscriptus, a species he interpreted widely and considered very variable in some characters. Shaw (2012b) has added strong evidence (biological and morphological data) that support bicolor and circumscriptus being considered as different species. Thus, all the synonyms listed by Wilkinson (1938d) need to be re-apportioned between bicolor and circumscriptus. Here we consider as synonyms of bicolor: a) Microgasterardeaepenellae (Bouché, 1834), following Papp (1983a, 1988), who saw the type; b) Apantelesschillei (Niezabitowski, 1910) is tentatively placed (with a question mark) under bicolor, following Papp (1988: 148), who did not see the type of that species; c) Apanteleslongicauda (Fahringer, 1938), following the original description (Fahringer 1938: 10); and d) Apantelespedias Nixon, 1973, based on our study of the type. We consider synonyms of circumscriptus: e) Microgasterexiguus (Haliday, 1834), based on our study of the lectotype and also van Achterberg (1997); f) Microgasterumbellatarum (Haliday, 1834), following Papp (1988), who did not see the type of that species [but also note that van Achterberg (1997), who did not see the type either, placed umbellatarum as a synonym of bicolor]; g) Microgasterblancardellae (Bouché, 1834), following Papp (1983a, 1988) who saw the type; h) Microgasterlividipes (Wesmael, 1837), following Papp (1988), although it is not clear to us if he saw that type; i) Microgasterflavolimbatus (Ratzeburg, 1848), following Papp (1983a, 1988), it is not clear to us if he saw that type; j) Apanteleslautellus (Marshall, 1885), based on our study of the type. In addition to the above, material determined by Nixon as exiguus Haliday (see Nixon 1973) is a different species, the status of which is still unresolved; Shaw (2012b) thought that species (exiguussensu Nixon nec Haliday) was probably a northern form of laetus Marshall, partly on the basis of rearing experiments, but ongoing research involving DNA barcoding will be needed before a conclusion can be reached. Additionally, Shaw (2012b) rejected the statement by van Achterberg (1997) that exiguussensu Nixon is the same as salalicus Mason, a position we follow here. Because of the convoluted story of the use and application of the names bicolor and circumscriptus (and corresponding synonyms), it is very difficult to determine with certainty the actual distribution of the two species which, based on current data, seem to overlap for the most part (e.g., see Yu et al. 2016). Both species seem to be rather broadly distributed in the Palearctic region, also reaching into the northern part of the Oriental region (China); however, until comprehensive studies of the specimens mentioned in the historical literature are done it will not be possible to untangle the distributional information. Similarly, there are a few references to these two species in New Zealand and North America (e.g., Bartlett et al. 1978, Valentine and Walker 1991, Fernandez-Triana 2010), mostly as introductions for biological control. DNA barcodes are equally confusing at present, as among dozens of specimens in BOLD which are labelled as either Pholetesorcircumscriptus, Pholetesorexiguuus, or Pholetesor (with some interim names), there seems to be a complex of molecularly (DNA barcodes) related species. Solving these problems is beyond the scope of the present paper, and thus we are limited here to pointing out the difficulties and unknowns related to these species. The species distribution in Israel and Kyrgyzstan are based in Belokobylskij et al. (2019).
Pholetesorbrevivalvatus (Balevski & Tobias, 1980), new combination
Apantelesbrevivalvatus Balevski & Tobias, 1980.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Bulgaria.
Geographical distribution.PAL.
PAL: Bulgaria.
Notes. This species is clearly not an Apanteles, based on the short ovipositor sheaths (and, to a lesser extent, also based on the shapes of T1 and T2, which is not commonly found in Apanteles). Papp (1984a) considered this species to be related to Pholetesoringenuus (Tobias, 1964), based on a number of features; the available drawings for both species indeed look similar. Examination of the type specimen will be needed to conclude, but for the time being we follow Papp's suggestion and transfer the species from Apanteles to Pholetesor.
Pholetesorbucculatricis (Muesebeck, 1921)
Apantelesbucculatricis Muesebeck, 1921.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CA).
Pholetesorcaloptiliae Whitfield, 2006
Pholetesorcaloptiliae Whitfield, 2006.
Type information. Holotype female, CNC (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (ON), USA (CT, IN, NY, OH).
Pholetesorchiricahuensis Whitfield, 2006
Pholetesorchiricahuensis Whitfield, 2006.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (AZ, CA, CO, FL, NM).
Pholetesorcircumlatus Kotenko, 2007
Pholetesorcircumlatus Kotenko, 2007.
Type information. Holotype female, SIZK (not examined but original description checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (SAK).
Pholetesorcircumscriptus (Nees, 1834)
Microgastercircumscriptus Nees, 1834.
Microgasterexiguus Haliday, 1834.
Microgasterumbellatarum Haliday, 1834.
Microgasterblancardellae Bouché, 1834.
Microgasterlividipes Wesmael, 1837.
Microgasterflavolimbatus Ratzeburg, 1848.
Apanteleslautellus Marshall, 1885.
Type information. Neotype female, ZMHB (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.AUS, NEA, OTL, PAL.
AUS: New Zealand; NEA: USA (AK); OTL: China (SN, YN, ZJ); PAL: Armenia, Austria, Azerbaijan, Belgium, Bulgaria, China (SD), Croatia, Czech Republic, Finland, Georgia, Germany, Greece, Hungary, Iran, Ireland, Israel, Italy, Japan, Kazakhstan, Korea, Latvia, Lithuania, Madeira Islands, Malta, Moldova, Netherlands, Poland, Romania, Russia (IRK, KEM, KHA, KDA, MOS, PRI, ROS, SAK, SPE, VLA, VOR), Slovakia, Spain, Switzerland, Ukraine, United Kingdom, Yugoslavia.
Notes. See notes under Pholetesorbicolor above for detailed explanations on the history of names used for these two species, their synonyms, distribution and molecular data. The species distribution in Japan and Kazakhstan are based in Belokobylskij et al. (2019).
Pholetesorconfusus Liu & Chen, 2016
Pholetesorconfusus Liu & Chen, 2016.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (LN).
Pholetesordixianus Whitfield, 2006
Pholetesordixianus Whitfield, 2006.
Type information. Holotype female, SEMC (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (NC, TX).
Pholetesordmitriyi Kotenko, 2007
Pholetesordmitriyi Kotenko, 2007.
Type information. Holotype female, SIZK (not examined but original description checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (PRI).
Pholetesorelpis (Nixon, 1973)
Apanteleselpis Nixon, 1973.
Apantelesgirkanus Tobias, 1976.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Austria, Azerbaijan, Bulgaria, Croatia, Finland, Germany, Greece, Hungary, Iran, Korea, Mongolia, Netherlands, Poland, Russia (MAG, PRI, SAK), Serbia, Slovakia, Ukraine, United Kingdom.
Pholetesorerrans (Nixon, 1973)
Pholetesorerrans Nixon, 1973.
Apantelesarenicola Papp, 1973.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Hungary, United Kingdom.
Pholetesorextentus (Papp, 1977), new combination
Apantelesextentus Papp, 1977.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Mongolia.
Geographical distribution.PAL.
PAL: Mongolia.
Notes. Based on the original description and illustrations provided there, this species is clearly not an Apanteles. The relatively short ovipositor sheaths and shapes of T1 and T2 strongly suggest the best generic placement would be Pholetesor (although future examination of the specimens in the HNHM may show Dolichogenidea as a possible alternative).
Pholetesorflavigleba Liu & Chen, 2016
Pholetesorflavigleba Liu & Chen, 2016.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (ZJ); PAL: China (HE, LN, SN).
Pholetesorflaviparvus Liu & Chen, 2016
Pholetesorflaviparvus Liu & Chen, 2016.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (JS).
Pholetesorglacialis (Ashmead, 1902)
Protapantelesglacialis Ashmead, 1902.
Type information. Holotype male, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (BC), USA (AK).
Pholetesorhanniae (Valerio & Whitfield, 2003)
Teremyshanniae Valerio & Whitfield, 2003.
Type information. Holotype female, INBio (not examined but original description checked). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Pholetesorhayati Akhtar, 2010
Pholetesorhayati Akhtar, 2010.
Type information. Holotype female, INPC (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Pholetesoringenuoides (Papp, 1971), new combination
Apantelesingenuoides Papp, 1971.
Apantelesfrater Tobias, 1976.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Mongolia.
Geographical distribution.PAL.
PAL: Armenia, Bulgaria, Croatia, France, Germany, Greece, Hungary, Iran, Korea, Mongolia, Montenegro, Turkey.
Notes. Our concept for this species is based on Papp (1971, 1984). The descriptions and illustrations in those two papers strongly suggest this species is not an Apanteles. Until the type material can be examined, we consider that the best generic placement at present would be in Pholetesor, based on the shapes of T1 and T2, smooth propodeum, short hypopygium, and relatively short ovipositor sheaths. Another line of supporting evidence is that Papp (1971: 318) considered the species to be closely related to ingenuus (Tobias, 1964), which is currently placed within Pholetesor. However, ingenuoides could also be placed in Dolichogenidea; the two genera are closely related and unfortunately the papers we have consulted do not provide enough details to corroborate or refute that possibility.
Pholetesoringenuus (Tobias, 1964)
Apantelesingenuus Tobias, 1964.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Kazakhstan.
Geographical distribution.PAL.
PAL: Hungary, Kazakhstan, Mongolia.
Notes. Our species concept is based on Papp (1984a), Tobias (1986) and Kotenko (2007a).
Pholetesorintercedens (Tobias, 1977)
Apantelesintercedens Tobias, 1977.
Type information. Holotype female, ZIN (not examined). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (PRI).
Pholetesorkuwayamai (Watanabe, 1932), new combination
Apanteleskuwayamai Watanabe, 1932.
Type information. Holotype female, EIHU (examined). Country of type locality: Japan.
Geographical distribution.PAL.
PAL: Japan, Korea.
Notes. This species is clearly not Apanteles but Pholetesor. Papp had recognized that (based on a label he wrote in 1992 and attached to the specimen, although that combination was never published). The female holotype is in poor condition, missing the metasoma and some legs, but two other females (supposedly paratypes, because they have the same labels) are in relatively good condition. In the same collection there is also a gelatin capsule with some other specimens and cocoons.
Pholetesorlaetus (Marshall, 1885)
Apanteleslaetus Marshall, 1885.
Apantelesmetallicus Jakimavicius, 1972.
? Microgasterexiguus Haliday, 1834 [misidentification by Nixon (1973)].
? Apantelessalalicus Mason, 1959 [misidentification by van Achterberg (1997)].
Type information. Lectotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.OTL, PAL.
OTL: China (FJ, GD, HI, HN, SN, YN, ZJ); PAL: Austria, Bulgaria, Germany, Hungary, Japan, Lithuania, Netherlands, Poland, Romania, Russia (ZAB, IRK, PRI, SAK), Slovenia, Switzerland, United Kingdom, Yugoslavia.
Notes.Wilkinson (1945: 155–156) designated a type for this species, which should be considered as the lectotype, but until now no reference to that specimen as the lectotype had been made. Originally, the specimen was stated to be in the Essex Museum of Natural History, but currently is in the NHMUK. For more details on laetus and the probable misidentifications of exiguus and salalicus see Shaw (2012b) and Broad et al. (2016).
Pholetesorlithocolletis Liu & Chen, 2016
Pholetesorlithocolletis Liu & Chen, 2016.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (SD).
Pholetesorlongicoxis Whitfield, 2006
Pholetesorlongicoxis Whitfield, 2006.
Type information. Holotype female, CNC (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (QC), USA (MI).
Pholetesorlyonetiae Liu & Chen, 2016
Pholetesorlyonetiae Liu & Chen, 2016.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (SN).
Pholetesormaritimus (Wilkinson, 1941)
Apantelesmaritimus Wilkinson, 1941.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.OTL, PAL.
OTL: China (JS); PAL: China (AH, XJ), Denmark, France, Germany, Hungary, Kyrgyzstan, Poland, Russia (C, NW), Slovakia, United Kingdom.
Notes. The species distribution in Kyrgyzstan and Russia are based in Belokobylskij et al. (2019).
Pholetesormasneri (Mason, 1981)
Teremysmasneri Mason, 1981.
Type information. Holotype female, CNC (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (ON), USA (CT).
Pholetesormasoni Whitfield, 2006
Pholetesormasoni Whitfield, 2006.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA, NEO.
NEA: Canada (AB, BC, MB, NS, ON, QC, YT), USA (AZ, CA, IN, MD, MA, MI, MN, NY, NC, OH, WY); NEO: Mexico.
Notes. Because of its fully areolated propodeum, if this species indeed is to be placed in Pholetesor, then many Parapanteles that have similar propodeum areola could be considered to have the same generic placement. An alternative would be that Pholetesormasoni should be transferred to Parapanteles. More study on those species (including DNA and biological data) will be needed before a conclusion on the topic can be reached; for the time being we retain this species where it was originally described.
Pholetesormoczari Papp, 2014
Pholetesormoczari Papp, 2014.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Tunisia.
Geographical distribution.PAL.
PAL: Tunisia.
Notes. In the original description the generic position of this species and also that of Pholetesorrufulus (Tobias, 1964) are discussed; it is implied that both species could equally be placed in a different genus (Glyptapanteles) and that the “generic assignment depends mainly on the deliberation that which generic feature composition is considered more decisive to Pholetesor or to Glyptapanteles” (Papp 2014: 164). After reading the original description and studying the drawings that are provided there, we agree that the status of those two species is ambiguous at present. However, we refrain to transfer them to Glyptapanteles until specimens can be examined.
Pholetesornanus (Reinhard, 1880)
Apantelesnanus Reinhard, 1880.
Apantelesszoecsi Papp, 1973.
Type information. Syntypes female and male, depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: unknown.
Geographical distribution.NEA, PAL.
NEA: Canada (ON); PAL: Austria, Czech Republic, Finland, Germany, Hungary, Italy, Lithuania, Netherlands, Poland, Romania, Russia (C, IR, KA, NW), Serbia, Sweden, Switzerland, Ukraine, United Kingdom.
Notes. Our species concept is based on Nixon (1973), Papp (1983a), Kotenko (2007a), Shaw (2012b) and Fernandez-Triana et al (2016a).
Pholetesorornigis (Weed, 1887)
Apantelesornigis Weed, 1887.
Microgasterrobiniae Fitch, 1859.
Protapantelestortricis Ashmead, 1898.
Apantelesbraunae Viereck, 1912.
Apanteleslithocolletidis Viereck, 1912.
Type information. Lectotype female, INHS (not examined but authoritatively identified specimens examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (MB, NB, NS, ON, QC), USA (AR, CA, CT, DC, IL, IN, KS, KY, MA, MI, MN, MO, NH, NY, NC, OR, PA, TX, UT, VT, VA, WV, WI).
Notes. We examined the types of Apantelesbraunae (Viereck, 1912), a male specimen, and Apanteleslithocolletidis (Viereck, 1912), a male specimen, currently synonyms of P. ornigis and both deposited in the USNM.
Pholetesorphaetusa (Nixon, 1973)
Apantelesphaetusa Nixon, 1973.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Bulgaria, Germany, Hungary, Korea, Mongolia, Netherlands, Romania, Russia (SAK), Ukraine, United Kingdom.
Pholetesorpinifoliellae Whitfield, 2006
Pholetesorpinifoliellae Whitfield, 2006.
Type information. Holotype female, CNC (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (ON, QC), USA (CA, MD).
Pholetesorpowelli Whitfield, 2006
Pholetesorpowelli Whitfield, 2006.
Type information. Holotype female, CAS (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (CA, OR).
Notes. The female holotype has the ovipositor sheaths ca. half as long as metatibia; the hypopygium has a translucent median fold, where one or two pleats are visible (second pleat not clearly defined); the hind wing vannal lobe is convex and entirely setose; the propodeum is rugose, without areola or median carina; and T1 and T2 are rugose. Most of those features could also be interpreted as being Dolichogenidea, especially the hypopygium pleats, but more study will be required, so the for the time being we prefer to retain this species in Pholetesor.
Pholetesorpseudocircumscriptus Abdoli, 2019
Pholetesorpseudocircumscriptus Abdoli, 2019.
Type information. Holotype female, TMUC (not examined but original description checked). Country of type locality: Iran.
Geographical distribution.PAL.
PAL: Iran.
Pholetesorrhygoplitoides Whitfield, 2006
Pholetesorrhygoplitoides Whitfield, 2006.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (AB, NL, ON, QC), USA (AZ, ID, MN).
Pholetesorrohweri (Muesebeck, 1921)
Apantelesrohweri Muesebeck, 1921.
Apantelesnigripes Rohwer, 1913 [homonym of Apantelesnigripes Ratzeburg, 1844].
Type information. Holotype female, USNM (not examined but authoritatively identified specimens examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (NB, ON), USA (NY, PA, VA).
Notes. We examined the type of Apantelesnigripes (Rohwer, 1913), a female specimen deposited in the USNM.
Pholetesorrufulus (Tobias, 1964)
Apantelesrufulus Tobias, 1964.
Apantelesrufulus Tobias, 1964 [homonym of Apantelesrufulus Wilkinson, 1930].
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Kazakhstan.
Geographical distribution.PAL.
PAL: Azerbaijan, Hungary, Kazakhstan, Turkey, Uzbekistan.
Notes. Our species concept is based on Papp (1983a) and Tobias (1986).
Pholetesorsalalicus (Mason, 1959)
Apantelessalalicus Mason, 1959.
Type information. Holotype female, USNM (not examined but subsequent treatment of the species checked). Country of type locality: USA.
Geographical distribution.NEA, OTL, PAL.
NEA: Canada (BC, QC), USA (CA, OR); OTL: China (GZ, JS, ZJ); PAL: Finland, Netherlands, Norway, United Kingdom.
Notes. Our species concept is based on Whitfield (2006).
Pholetesorsalicifoliellae (Mason, 1959)
Apantelessalicifoliellae Mason, 1959.
Type information. Holotype female, CNC (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (BC, MB, NB, NS, NT, ON, QC, YT), USA (AK, CA, NY, OR, UT).
Pholetesorspinadensus Liu & Chen, 2016
Pholetesorspinadensus Liu & Chen, 2016.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (GZ, SN).
Pholetesortaiwanensis Liu & Chen, 2016
Pholetesortaiwanensis Liu & Chen, 2016.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (TW).
Pholetesorteresitergum Liu & Chen, 2016
Pholetesorteresitergum Liu & Chen, 2016.
Type information. Holotype female, ZJUH (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (ZJ); PAL: China (XJ).
Pholetesorterneicus Kotenko, 2007
Pholetesorterneicus Kotenko, 2007.
Type information. Holotype female, ZIN (not examined but original description checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (PRI).
Pholetesorthuiellae Whitfield, 2006
Pholetesorthuiellae Whitfield, 2006.
Type information. Holotype female, CNC (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (NB, ON, QC), USA (CT, NY).
Pholetesorvariabilis Whitfield, 2006
Pholetesorvariabilis Whitfield, 2006.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (AB, BC, ON, SK), USA (CA, CO, ID, MI, NV, OR, UT).
Pholetesorviminetorum (Wesmael, 1837)
Microgasterviminetorum Wesmael, 1837.
Microgasterfuliginosus Wesmael, 1837.
Type information. Neotype female, ZMHB (not examined but subsequent treatment of the species checked). Country of type locality: Belgium.
Geographical distribution.NEA, OTL, PAL.
NEA: Canada (AB, BC, MB, NB, NL, NS, NT, ON, SK, YT), USA (AK, CO, ID, MI, MN, NH, SD, UT, WA, WY); OTL: China (SN); PAL: Azerbaijan, Belarus, Belgium, Croatia, Czech Republic, Estonia, Finland, France, Georgia, Germany, Hungary, Iran, Ireland, Italy, Japan, Kazakhstan, Kyrgyzstan, Moldova, Netherlands, Poland, Romania, Russia (AD, IRK, KEM, KDA, MOS, ORE, ROS, SPE, SMO, STA, VOR, YAR), Slovenia, Spain, Sweden, Switzerland, Ukraine, United Kingdom, Uzbekistan, Yugoslavia.
Notes. Our species concept is based on Whitfield (2006). The species distribution in Japan and Kyrgyzstan is based in Belokobylskij et al. (2019).
Pholetesorzelleriae Whitfield, 2006
Pholetesorzelleriae Whitfield, 2006.
Type information. Holotype female, CNC (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (MB, ON, QC), USA (MI).
Pholetesorzherikhini Kotenko, 2007
Pholetesorzherikhini Kotenko, 2007.
Type information. Holotype female, ZIN (not examined but original description checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (PRI).
Genus Prasmodon Nixon, 1965
Prasmodon Nixon, 1965: 205. Gender: masculine. Type species: Prasmodoneminens Nixon, 1965, by original designation.
This Neotropical genus was recently revised (Fernandez-Triana et al. 2014f) and at present comprises 18 described species, but we have seen a few more in collections. Most of the host records include the family Crambidae, with other two families recorded (Fernandez-Triana et al. 2014f, and ACG data available online) which require further verification. There are 204 DNA-barcode compliant sequences of this genus in BOLD, representing 15 BINs.
Prasmodonalmasolisae Fernandez-Triana & Whitfield, 2014
Prasmodonalmasolisae Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Prasmodonaureus Fernandez-Triana & Whitfield, 2014
Prasmodonaureus Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Prasmodonbobpoolei Fernandez-Triana & Whitfield, 2014
Prasmodonbobpoolei Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Prasmodonbobrobbinsi Fernandez-Triana & Whitfield, 2014
Prasmodonbobrobbinsi Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Prasmodondondavisi Fernandez-Triana & Whitfield, 2014
Prasmodondondavisi Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Prasmodoneminens Nixon, 1965
Prasmodoneminens Nixon, 1965.
Type information. Holotype male, NHMUK (examined). Country of type locality: Peru.
Geographical distribution.NEO.
NEO: Brazil (AM), Costa Rica, Ecuador, Peru.
Prasmodonerenadupontae Braet & Fernandez-Triana, 2014
Prasmodonerenadupontae Braet & Fernandez-Triana, 2014.
Type information. Holotype female, MNHN (examined). Country of type locality: French Guiana.
Geographical distribution.NEO.
NEO: Brazil (MT), French Guiana.
Prasmodonjohnbrowni Fernandez-Triana & Whitfield, 2014
Prasmodonjohnbrowni Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Prasmodonmasoni Fernandez-Triana & Whitfield, 2014
Prasmodonmasoni Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (AM, MT).
Prasmodonmikepoguei Fernandez-Triana & Whitfield, 2014
Prasmodonmikepoguei Fernandez-Triana & Whitfield, 2014.
Type information. Holotype male, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Prasmodonnixoni Fernandez-Triana & Whitfield, 2014
Prasmodonnixoni Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Peru.
Geographical distribution.NEO.
NEO: French Guiana, Peru.
Prasmodonpaulgoldsteini Fernandez-Triana & Whitfield, 2014
Prasmodonpaulgoldsteini Fernandez-Triana & Whitfield, 2014.
Type information. Holotype male, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Prasmodonscottmilleri Fernandez-Triana & Whitfield, 2014
Prasmodonscottmilleri Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Prasmodonsilvatlanticus Fernandez-Triana & Whitfield, 2014
Prasmodonsilvatlanticus Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (RJ).
Prasmodonsubfuscus Fernandez-Triana & Whitfield, 2014
Prasmodonsubfuscus Fernandez-Triana & Whitfield, 2014.
Type information. Holotype male, CNC (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (RJ).
Prasmodontijucaensis Fernandez-Triana & Whitfield, 2014
Prasmodontijucaensis Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (RJ).
Prasmodonverhoogdenokus Braet & Fernandez-Triana, 2014
Prasmodonverhoogdenokus Braet & Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (MT), Colombia, Ecuador, French Guiana, Peru, Suriname.
Prasmodonzlotnicki Valerio & Rodriguez, 2005
Prasmodonzlotnicki Valerio & Rodriguez, 2005.
Type information. Holotype female, INBio (not examined but subsequent treatment of the species checked). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Notes. Our species concept is based on Fernandez-Triana et al. (2014f).
Genus Promicrogaster Brues & Richardson, 1913
Promicrogaster Brues & Richardson, 1913: 499. Gender: feminine. Type species: Promicrogasterterebrator Brues & Richardson, 1913, by original designation.
Until very recently (e.g., Fernandez-Triana et al. 2016b), this genus was considered restricted to the New World. However, during the preparation of this paper we found evidence than this taxon is cosmopolitan, reported below. Currently, there are 46 described species of Promicrogaster, with recent reviews of the Mesoamerican (Fernandez-Triana et al. 2016b) and North American species (Fernandez-Triana 2019). We have seen many more species in collections, mostly from the Neotropical region. Known hosts are from the families Sessidae and Tineidae. There are 134 DNA-barcode compliant sequences of this genus in BOLD, representing 37 BINs.
Promicrogasteralexmartinezi Fernandez-Triana & Boudreault, 2016
Promicrogasteralexmartinezi Fernandez-Triana & Boudreault, 2016.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Promicrogasterandreyvallejosi Fernandez-Triana & Boudreault, 2016
Promicrogasterandreyvallejosi Fernandez-Triana & Boudreault, 2016.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Promicrogasterapharea Nixon, 1965
Promicrogasterapharea Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Mexico.
Geographical distribution.NEO.
NEO: Brazil (SC), Mexico.
Promicrogasterapidanus (Nixon, 1965), new combination
Apantelesapidanus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Notes. Although the well defined, strong median carina on the propodeum, and the species group to which Nixon (1965) assigned this species might suggest it belongs to Iconella, other characters indicate a different genus. The ovipositor tip is sinuate (versus straight in Iconella); the ovipositor and sheaths are relatively very long (versus ca. twice metatibia length, much longer than in described species of Iconella); and the hind wing vein cu-a is straight (versus sinuate in Iconella). We consider that the available evidence provides more support for this species to be placed in Promicrogaster.
Promicrogasterbrandondinartei Fernandez-Triana & Boudreault, 2016
Promicrogasterbrandondinartei Fernandez-Triana & Boudreault, 2016.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica, Panama.
Promicrogasterbriareus (Nixon, 1965), new combination
Apantelesbriareus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (QLD), Vanuatu.
Notes.Austin and Dangerfield (1992) transferred this species from Apanteles to Iconella. However, briareus lacks the two main characters defining Iconella: the hind wing does not have the vein cu-a sinuate; and its propodeum does not have a median, longitudinal carina but instead has a few, shorter carinae radiating from the nucha which seem to partially define an areola (Austin and Dangerfield (1992: 36) referred to that as “more diffuse posterior striae”). After examining the holotype, we found that the ovipositor tip is sinuate, the ovipositor length is almost twice that of the metatibia, and the polished area of the lateral face of the scutellar disc occupies most of the face. All those characters suggest this species is better placed in Promicrogaster, a genus that was recently considered to be found only in the New World (e.g., Fernandez-Triana et al. 2016b). The report in this paper of species from the Afrotropical and Australasian regions indicate a much wider distribution of Promicrogaster worldwide.
Promicrogastercara Nixon, 1965
Promicrogastercarus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (BA).
Notes. The species name must be treated as an adjective and not as a noun (Doug Yanega, pers. comm.) and thus it must match the gender of the genus name.
Promicrogasterconopiae (Watanabe, 1934), new combination
Apantelesconopiae Watanabe, 1934.
Type information. Holotype female, EIHU (examined). Country of type locality: Japan.
Geographical distribution.OTL, PAL.
OTL: Malaysia; PAL: China (QH), Japan, Korea.
Notes. We have examined the holotype and several more specimens in the EIHU collection. They look similar to the described species from the New World, based on the sinuate ovipositor tip, shapes of T1 andT2, large metacoxa and relatively high polished area of the lateral face of the scutellum. The only differences we observed were that the Japanese specimens (which are relatively large, at least by Promicrogaster standards) do not have a bilobate glossa and the fore wing lacks an areolet; in the New World, the currently described species all have an elongate and bilobate glossa, and all large species have a small areolet in the fore wing (with only a few small species lacking the areolet in the fore wing). These differences are minor and thus we consider that the best generic placement for this species is in Promicrogaster. The known host data for this species (Sesiidae) also agree with the very few host records known from the New World (Fernandez-Triana et al. 2016b).
Promicrogasterdaniellopezi Fernandez-Triana & Boudreault, 2016
Promicrogasterdaniellopezi Fernandez-Triana & Boudreault, 2016.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Promicrogasterdaretrizoi Fernandez-Triana & Boudreault, 2016
Promicrogasterdaretrizoi Fernandez-Triana & Boudreault, 2016.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Promicrogastereddycastroi Fernandez-Triana & Boudreault, 2016
Promicrogastereddycastroi Fernandez-Triana & Boudreault, 2016.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Promicrogastereimyobandoae Fernandez-Triana & Boudreault, 2016
Promicrogastereimyobandoae Fernandez-Triana & Boudreault, 2016.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Promicrogasteremesa (Nixon, 1965), new combination
Apantelesemesa Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Notes. We transfer this species to Promicrogaster based on the ovipositor length being more than twice the metatibia length, sinuate ovipositor tip, propodeum with irregular carinae radiating from the nucha, a large polished area of the lateral face of scutellum, and relatively large metacoxae.
Promicrogastererigone Nixon, 1965
Promicrogastererigone Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (SC).
Promicrogasterfabiancastroi Fernandez-Triana & Boudreault, 2016
Promicrogasterfabiancastroi Fernandez-Triana & Boudreault, 2016.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Promicrogasterfabriciocambroneroi Fernandez-Triana & Boudreault, 2016
Promicrogasterfabriciocambroneroi Fernandez-Triana & Boudreault, 2016.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Promicrogasterfloridakeys Fernandez-Triana, 2019
Promicrogasterfloridakeys Fernandez-Triana, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (FL).
Promicrogastergainesvillensis Fernandez-Triana, 2019
Promicrogastergainesvillensis Fernandez-Triana, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (FL).
Promicrogastergrandicula (Wilkinson, 1929), new combination
Apantelesgrandiculus Wilkinson, 1929.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: China (FJ), India, Vietnam.
Notes. We transfer this species to Promicrogaster based on the ovipositor length ca. twice the metatibia length, sinuate ovipositor tip, propodeum with irregular carinae radiating from the nucha, a large polished area of the lateral face of the scutellum, and relatively large metacoxae. The species name must be treated as an adjective and not as a noun (Doug Yanega, pers. comm.) and thus it must match the gender of the genus name.
Promicrogasterhillaryvillafuerteae Fernandez-Triana & Boudreault, 2016
Promicrogasterhillaryvillafuerteae Fernandez-Triana & Boudreault, 2016.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Promicrogasterhuachuca Fernandez-Triana, 2019
Promicrogasterhuachuca Fernandez-Triana, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (AZ).
Promicrogasterjaymeae Fernandez-Triana, 2019
Promicrogasterjaymeae Fernandez-Triana, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (ON), USA (MA).
Promicrogasterkevinmartinezi Fernandez-Triana & Boudreault, 2016
Promicrogasterkevinmartinezi Fernandez-Triana & Boudreault, 2016.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Promicrogasterkiralycastilloae Fernandez-Triana & Boudreault, 2016
Promicrogasterkiralycastilloae Fernandez-Triana & Boudreault, 2016.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Promicrogasterleilycastilloae Fernandez-Triana & Boudreault, 2016
Promicrogasterleilycastilloae Fernandez-Triana & Boudreault, 2016.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Promicrogasterliagrantae Fernandez-Triana & Boudreault, 2016
Promicrogasterliagrantae Fernandez-Triana & Boudreault, 2016.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Promicrogasterluismendezi Fernandez-Triana & Boudreault, 2016
Promicrogasterluismendezi Fernandez-Triana & Boudreault, 2016.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Promicrogastermadreanensis Fernandez-Triana, 2019
Promicrogastermadreanensis Fernandez-Triana, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (AZ).
Promicrogastermerella Nixon, 1965
Promicrogastermerella Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (SC).
Promicrogastermiranda Muesebeck, 1958
Promicrogastermiranda Muesebeck, 1958.
Type information. Holotype female, USNM (not examined but subsequent treatment of the species checked). Country of type locality: Panama.
Geographical distribution.NEO.
NEO: Panama, Trinidad & Tobago.
Notes. Our species concept is based on Fernandez-Triana et al. (2016b).
Promicrogastermonteverdensis Fernandez-Triana & Boudreault, 2016
Promicrogastermonteverdensis Fernandez-Triana & Boudreault, 2016.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Promicrogastermunda Muesebeck, 1958
Promicrogastermunda Muesebeck, 1958.
Type information. Holotype female, USNM (not examined but subsequent treatment of the species checked). Country of type locality: Honduras.
Geographical distribution.NEO.
NEO: Costa Rica, Honduras, Mexico, Panama.
Notes. Our species concept is based on Fernandez-Triana et al. (2016b). According to those authors, P. munda may actually represent a species complex.
Promicrogasternaomiduarteae Fernandez-Triana & Boudreault, 2016
Promicrogasternaomiduarteae Fernandez-Triana & Boudreault, 2016.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Promicrogasterorsedice (Nixon, 1965), new combination
Apantelesorsedice Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Papua New Guinea.
Geographical distribution.AUS, OTL.
AUS: Papua New Guinea; OTL: Vietnam.
Notes. We transfer this species to Promicrogaster based on the ovipositor length more than twice the metatibia length, sinuate ovipositor tip, propodeum with irregular carinae radiating from the nucha, a large polished area of the lateral face of scutellum, and relatively large metacoxae.
Promicrogasterpolyporicola Muesebeck, 1958
Promicrogasterpolyporicola Muesebeck, 1958.
Type information. Holotype female, USNM (examined). Country of type locality: Panama.
Geographical distribution.NEO.
NEO: Panama.
Promicrogasterprater Nixon, 1965
Promicrogasterprater Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (SC).
Promicrogasterrepleta (Papp, 1990), new combination
Iconellarepleta Papp, 1990.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Korea.
Geographical distribution.PAL.
PAL: Korea.
Notes. Transferred to Promicrogaster based on the the sinuate ovipositor tip, shape of T1–T2, large metacoxa and relatively high polished area on the lateral face of scutellum (Papp 1990b).
Promicrogasterrondeau Fernandez-Triana, 2019
Promicrogasterrondeau Fernandez-Triana, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (ON).
Promicrogasterronycastilloi Fernandez-Triana & Boudreault, 2016
Promicrogasterronycastilloi Fernandez-Triana & Boudreault, 2016.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Promicrogastersebastiancambroneroi Fernandez-Triana & Boudreault, 2016
Promicrogastersebastiancambroneroi Fernandez-Triana & Boudreault, 2016.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Promicrogasterspilopterus Nixon, 1965
Promicrogasterspilopterus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (SC).
Promicrogastersterope Nixon, 1965
Promicrogastersterope Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (SC).
Promicrogasterterebrator Brues & Richardson, 1913
Promicrogasterterebrator Brues & Richardson, 1913.
Type information. Holotype female, AMNH (examined). Country of type locality: Guyana.
Geographical distribution.NEO.
NEO: Guyana.
Notes. Our species concept is based on Brues and Richardson (1913), Muesebeck (1958b) and Mason (1981).
Promicrogastertracyvindasae Fernandez-Triana & Boudreault, 2016
Promicrogastertracyvindasae Fernandez-Triana & Boudreault, 2016.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Promicrogastertyphon (Nixon, 1965), new combination
Apantelestyphon Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Togo.
Geographical distribution.AFR.
AFR: South Africa, Togo.
Notes. The ovipositor tip is sinuate, the ovipositor length is almost twice that of metatibia, the propodeum has a series of short carinae radiating from the nucha, and the polished area of the lateral face of the scutellar disc occupies most of the face. These characters indicate that this species is better placed in Promicrogaster.
Promicrogastervirginiana Fernandez-Triana, 2019
Promicrogastervirginianus Fernandez-Triana, 2019.
Type information. Holotype female, CNC (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (VA).
Notes. The species name must be treated as an adjective and not as a noun (Doug Yanega, pers. comm.) and thus it must match the gender of the genus name.
Genus Protapanteles Ashmead, 1898
Protapanteles Ashmead, 1898: 166. Gender: masculine. Type species: (Protapantelesephyrae Ashmead, 1898) = Apantelespaleacritae Riley, 1881, by subsequent designation (Viereck 1914: 123).
We record 25 described species, although the limits of this genus are controversial (see discussion above in section Brief diagnosis of all Microgastrinae genera as they are understood in this paper for more details, p 35, 36), and it is difficult to even estimate the potential species richness. As considered in this paper, Protapanteles is essentially Holarctic, occasionally reaching the Oriental region. Many Lepidoptera families have been recorded as hosts but, as the limits of this genus have varied considerably (e.g., Mason, 1981, Yu et al. 2016, present paper), all records need to be verified. There are 481 DNA-barcode compliant sequences of this genus in BOLD, representing 26 BINs, but some of those sequences are likely to represent other genera.
Protapantelesalaskensis Ashmead, 1902, restored combination
Protapantelesalaskensis Ashmead, 1902.
Type information. Holotype male, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (BC, MB, ON, QC, NL), USA (AK, CA, MI).
Notes. Both Shenefelt (1972: 437) and Yu et al. (2016) referred to the holotype of this species as a female specimen, and Shenefelt even recorded the type number (5704). However, examination of the holotype (which indeed has the same type number that Shenefelt mentioned) clearly shows that it is a male specimen, and thus we are correcting that information here. Yu et al. (2016) listed this species as belonging to the genus Cotesia, without any valid (published) paper to support that change. After studying the holotype and other specimens, we think this species is better placed in Protapanteles, based on the propodeum mostly smooth, with a few short striae near the nucha, T1 smooth and mostly parallel-sided (but slightly narrowing on posterior 0.2), and T2 subtriangular to trapezoidal in shape and with a smooth, poorly defined median area. For the sake of clarity, we restore the species combination here.
Protapantelesanchisiades (Nixon, 1973)
Apantelesanchisiades Nixon, 1973.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Bulgaria, Czech Republic, Finland, Germany, Hungary, Italy, Korea, Mongolia, Netherlands, Norway, Poland, Russia (KAM, PRI, SAK), Slovakia, Sweden, Switzerland, Turkey, Ukraine, United Kingdom.
Protapantelesandromica (Nixon, 1976)
Apantelesandromica Nixon, 1976.
Type information. Holotype female, ZSM (not examined but original description checked). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Germany, Hungary, Poland, Russia (C, S), Slovakia.
Protapantelesarmeniacus (Tobias, 1976)
Apantelesarmeniacus Tobias, 1976.
Type information. Holotype female, depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Armenia.
Geographical distribution.PAL.
PAL: Armenia.
Notes. Our species concept is based on Papp (1984a) and Tobias (1986).
Protapantelesbuzurae (You, Xiong & Zhou, 1987)
Apantelesbuzurae You, Xiong & Zhou, 1987.
Type information. Holotype female, HUNAU (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (YN).
Notes. Our species concept is based on Chen and Song (2004) and Kotenko (2007a).
Protapantelesdelitutus (Papp, 1984)
Apantelesdelitutus Papp, 1984.
Type information. Holotype female, RBINS (not examined but original description checked). Country of type locality: Belgium.
Geographical distribution.PAL.
PAL: Belgium, Germany, Hungary, Netherlands, Slovakia.
Protapantelesendemus (Nixon, 1965)
Apantelesendemus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: France, Hungary, Kazakhstan, Russia (ZAB, SPE), Switzerland, Ukraine, United Kingdom.
Notes. Until the limits of Protapanteles are clearly established (the validity of this genus is questionable), we prefer not to transfer species to other genera. But it is likely that endemus will be placed in Cotesia because it has a propodeum with a transverse carina (in addition to other sculpture), and the shapes of T1 and T2 are closer to typical Cotesia than to Protapanteles. The holotype does not have a specialized seta on the protarsus.
Protapantelesenephes (Nixon, 1965)
Apantelesenephes Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Germany, Hungary, Korea, Russia (AMU, PRI, SAK), Slovakia, Sweden, Switzerland, Turkmenistan, Ukraine, United Kingdom.
Notes. Until the limits of Protapanteles are clearly established (the validity of this genus is questionable), we prefer not to transfer species to other genera. But it is likely that enephes will be placed in Cotesia. This species was recently recorded from Brazil (Penteado-Dias et al. 2011), which would represent a significant range expansion, as Protapantelesenephes was only known from the Palearctic region (e.g., Yu et al. 2016). We have examined the holotype as well as many European specimens (deposited in the CNC) versus the illustrations and description in Penteado-Dias et al. (2011), and it is clear that the Brazilian specimens, although sharing with enephes the relatively unusual pale spot on the gena, actually represent a completely different species. The Brazilian species remains undescribed at present, and we are not even sure of its generic status (as the images of propodeum, T1, and T2 suggest it could be a species of Cotesia or perhaps even Nyereria).
Protapanteleshirtariae (Kotenko & Tobias, 1986)
Apanteleshirtariae Kotenko & Tobias, 1986.
Type information. Holotype female, SIZK (not examined but subsequent treatment of the species checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (VGG), United Kingdom.
Notes. Our species concept is based on Tobias (1986), Papp (1988), Kotenko (2006) and Shaw (2012b).
Protapantelesiapetus (Nixon, 1976)
Apantelesiapetus Nixon, 1976.
Type information. Holotype female, ZSM (not examined but original description checked). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Germany.
Protapantelesimmunis (Haliday, 1834)
Microgasterimmunis Haliday, 1834.
Type information. Lectotype female, NMID (not examined but subsequent treatment of the species checked). Country of type locality: Ireland.
Geographical distribution.NEA, PAL.
NEA: Greenland; PAL: Armenia, Austria, Bulgaria, Croatia, Estonia, Finland, Germany, Hungary, Ireland, Italy, Kazakhstan, Korea, Lithuania, Moldova, Netherlands, Norway, Poland, Romania, Russia (ZAB, NVS, PRI, SAK, TOM, VOR), Serbia, Slovakia, Sweden, Switzerland, Tunisia, Ukraine, United Kingdom.
Notes. Our species concept is based on Nixon (1976), Papp (1984a), Tobias (1986), van Achterberg (2006) and Kotenko (2007a).
Protapantelesincertus (Ruthe, 1859)
Microgasterincertus Ruthe, 1859.
Apantelescaberae Marshall, 1885.
Apantelesjugosus Lyle, 1916.
Apantelesmihalyii Papp, 1973.
Type information. Holotype male, NHMW (not examined but authoritatively identified specimens examined). Country of type locality: Iceland.
Geographical distribution.PAL.
PAL: Austria, Azerbaijan, Georgia, Germany, Hungary, Iceland, Italy, Mongolia, Poland, Romania, Russia (VOR), Slovakia, Sweden, Switzerland, Ukraine, United Kingdom, Yugoslavia.
Notes. We examined the female type of Apantelescaberae (Marshall, 1885) and the type series (syntypes) of Apantelesjugosus (Lyle, 1916), which are deposited in the NHMUK. The species distribution in Azerbaijan and Georgia is based on Belokobylskij et al. (2019).
Protapanteleslymantriae (Marsh, 1979)
Apanteleslymantriae Marsh, 1979.
Type information. Holotype female, USNM (examined). Country of type locality: Japan.
Geographical distribution.PAL.
PAL: Japan.
Notes. This species was described as Apanteles, as it predated the paper by Mason (1981) where Apanteles was split into many genera. After the original description, Maeto (1996) referred to the species as Protapanteles, although he did not specify that as a new combination. Yu et al. (2016) considered the species to belong to Cotesia, although there is no published reference that we are aware of for the treatment of lymantriae in that genus. We have examined the holotype and the best generic placement at present is in Protapanteles, based on the sculpture of propodeum, T1, and T2, the shapes of T1 and T2, and presence of a spine on the fore tarsus. For the sake of clarity, we revise the species combination here.
Protapantelesmandanis (Nixon, 1965)
Apantelesmandanis Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Finland, Germany, Hungary, Switzerland.
Notes. Until the limits of Protapanteles are clearly established (the validity of this genus is questionable), we prefer not to transfer species to other genera. But it is very likely that mandanis will be placed in Glyptapanteles because it has a propodeum without any strong carinae or sculpture (at most a few short carinae radiating from the nucha), and the shapes of T1 and T2 are closer to typical Glyptapanteles than to Protapanteles. Additionally, the holotype does not have a specialized seta on the protarsus.
Protapantelesneparallelus Kotenko, 2007
Protapantelesneparallelus Kotenko, 2007.
Type information. Holotype female, SIZK (not examined but original description checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (ZAB).
Protapantelespaleacritae (Riley, 1881)
Apantelespaleacritae Riley, 1881.
Protapantelesephyrae Ashmead, 1898.
Type information. Lectotype male, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (BC, MB, NL, NS, ON), USA (AR, DE, GA, IL, KS, MD, MA, MO, NH, NJ, NY, VT, VA, WV).
Notes.Shenefelt (1972: 591) designated a lectotype from the four available syntypes and stated that it was “The specimen on point no. 2 directly ahead of the cocoon on the card”. We have examined the series, and the lectotype is a male specimen, a correction reflected in the type details we present here. Of the three remaining specimens (paralectotypes) one is entirely missing (except for two legs glued to the card) and the other two are missing the metasoma. Apart from the type material of P. paleacritae, we also examined the male holotype of P. ephyrae Ashmead, 1898 (currently a synonym of P. paleacritae), which has the metasoma detached but glued to another card.
Protapantelesparallelus (Lyle, 1917)
Apantelesparallelus Lyle, 1917.
Apantelesparallelus Lyle, 1917 [secondary homonym of Cotesiaparallelis (Ashmead, 1900)].
Apanteleslylei Shenefelt, 1972 [new name for secondary homonym].
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Germany, Hungary, Russia (ZAB), United Kingdom.
Protapantelesphigaliae (Muesebeck, 1919)
Apantelesphigaliae Muesebeck, 1919.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (NB, ON), USA (MA, NJ).
Notes. This species was transferred to Protapanteles by Mason (1981), also followed by Whitfield (1995a) and Fernandez-Triana (2010). However, Yu et al. (2016) considered the species to belong to Cotesia, although there is no published reference that we are aware of for this treatment of phigaliae. We have examined the holotype and the best generic placement at present is in Protapanteles, based on the mostly smooth propodeum, T1 and T2, as well as shape of T1 (mostly parallel-sided, but narrowing towards apex on posterior 0.3), and shape of T2 (subtriangular). For the sake of clarity, we revise the species combination here.
Protapantelesphlyctaeniae (Muesebeck, 1929)
Apantelesphlyctaeniae Muesebeck, 1929.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (ON), USA (IL, KS, OH).
Notes. This species was transferred to Protapanteles by Mason (1981), also followed by Whitfield (1995a) and Fernandez-Triana (2010). However, Yu et al. (2016) considered the species to belong to Cotesia, although there is no published reference that we are aware of for the treatment of phlyctaeniae in that genus. We have examined the holotype and the best generic placement at present is in Protapanteles, based on the shape of T1 (mostly parallel-sided, but narrowing towards apex on posterior 0.3) and the fore tarsus with a spine. Available DNA barcodes (with sequence lengths 164–422 bp, from three authenticated specimens) also place phlyctaeniae close to other Protapanteles species and not Cotesia. For the sake of clarity, we revise the species combination here.
Protapantelespopularis (Haliday, 1834)
Microgasterpopularis Haliday, 1834.
Type information. Neotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.OTL, PAL.
OTL: China (JS); PAL: Finland, France, Germany, Hungary, Ireland, Mongolia, Netherlands, Romania, Russia (YAR), Slovakia, Turkmenistan, United Kingdom.
Protapantelespraecipuus Papp, 1993
Protapantelespraecipuus Papp, 1993.
Type information. Holotype female, HNHM (not examined but original description checked). Country of type locality: Italy.
Geographical distribution.PAL.
PAL: Italy.
Protapantelesquerceus (Tobias, 1986)
Apantelesquerceus Tobias, 1986.
Type information. Holotype female, ZIN (not examined but original description checked). Country of type locality: Ukraine.
Geographical distribution.PAL.
PAL: Russia (S), Ukraine.
Protapantelessantolinae Oltra, 1995
Protapantelessantolinae Oltra, 1995.
Type information. Holotype female, UVS (not examined but original description checked). Country of type locality: Spain.
Geographical distribution.PAL.
PAL: Spain.
Protapantelestriangulator (Wesmael, 1837)
Microgastertriangulator Wesmael, 1837.
Type information. Syntypes female and male, RBINS (not examined but subsequent treatment of the species checked). Country of type locality: Belgium.
Geographical distribution.PAL.
PAL: Belgium, Czech Republic, France, Germany, Hungary, Ireland, Italy, Poland, Romania, Russia (YAR), Serbia, Slovakia, Sweden, Ukraine, United Kingdom.
Notes. We follow Broad et al. (2016) for the generic placement of this species.
Protapantelesyunnanensis (You & Xiong, 1987)
Apantelesyunnanensis You & Xiong, 1987.
Type information. Holotype female, HUNAU (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL, PAL.
OTL: China (YN); PAL: Korea, Russia (PRI).
Notes. Our species concept is based on Chen and Song (2004) and Kotenko (2007a). The species distribution in Korea is based on Belokobylskij et al. (2019).
Genus Protomicroplitis Ashmead, 1898
Protomicroplitis Ashmead, 1898: 167. Gender: masculine. Type species: Microgastermediatus Cresson, 1865, by subsequent designation and monotypy (Viereck 1914: 124).
A recent review (Fernandez-Triana 2015) restricted the genus to three species in the New World. We have seen at least one other undescribed species in collections. The only known host records are from the family Noctuidae. There are six DNA-barcode compliant sequences of this genus in BOLD, representing three BINs.
Protomicroplitiscalliptera (Say, 1836)
Microgastercalliptera Say, 1836.
Microgastermaculipennis Cresson, 1872.
Type information. Type lost (not examined but subsequent treatment of the species checked). Country of type locality: USA.
Geographical distribution.NEA, NEO.
NEA: Canada (ON), USA (AL, AR, CO, FL, GA, IN, IA, KS, LA, MD, MS, NE, NJ, NY, NC, SC, SD, TN, TX); NEO: Mexico.
Notes. Our species concept is based on Fernandez-Triana (2015).
Protomicroplitiscentroamericanus Fernandez-Triana 2015
ProtomicroplitiscentroamericanusFernandez-Triana 2015.
Type information. Holotype female, CNC (examined). Country of type locality: Mexico.
Geographical distribution.NEO.
NEO: Costa Rica, Mexico.
Protomicroplitismediatus (Cresson, 1865)
Microgastermediatus Cresson, 1865.
Type information. Holotype male, ANSP (not examined but subsequent treatment of the species checked). Country of type locality: Cuba.
Geographical distribution.NEA, NEO.
NEA: USA (FL); NEO: Bahamas, Cuba.
Notes. Our species concept is based on Fernandez-Triana (2015). The specimens recorded from Mexico in the older literature (Muesebeck 1958a, Nixon 1965) actually belong to P. calliptera (Fernandez-Triana 2015). We found two males from the Bahamas (San Salvador island, 12-14.vi.1978, coll. N. Elliot) in the USNM collection, which we record in this paper because they represent the first Microgastrinae ever recorded from that country. [In the USNM there are also specimens from the Florida Keys and Miami (USA, FL) and several localities in Cuba, all of them representing new records of the species, but those details will be published elsewhere.]
Genus Pseudapanteles Ashmead, 1898
Pseudapanteles Ashmead, 1898: 166. Gender: masculine. Type species: Pseudapantelesannulicornis Ashmead, 1900, by subsequent designation (Viereck 1911b: 177).
This genus is widely distributed in the New World, with most of the species found in the Neotropics and just a few extending north into the Nearctic Region. A recent paper provided a key to all 36 known species (Fernandez-Triana et al. 2014a), but we have seen dozens of undescribed species in collections, and the genus is likely to surpass one hundred species. Six Lepidoptera families have been recorded as hosts. There are 676 DNA-barcode compliant sequences of this genus in BOLD, representing 55 BINs.
Pseudapantelesabantidas (Nixon, 1965)
Apantelesabantidas Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (SC).
Pseudapantelesalfiopivai Fernandez-Triana & Whitfield, 2014
Pseudapantelesalfiopivai Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Pseudapantelesalvaroumanai Fernandez-Triana & Whitfield, 2014
Pseudapantelesalvaroumanai Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Pseudapantelesanalorenaguevarae Fernandez-Triana & Whitfield, 2014
Pseudapantelesanalorenaguevarae Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Pseudapantelesannulicornis Ashmead, 1900, lectotype designation
Pseudapantelesannulicornis Ashmead, 1900.
Type information. Lectotype female, NHMUK (examined). Country of type locality: Saint Vincent.
Geographical distribution.NEO.
NEO: Panama, Saint Vincent.
Notes.Fernandez-Triana et al. (2014a: 19) mentioned that they had examined the female holotype. That is incorrect, as the original description was based on four specimens, female and male (Ashmead 1900c: 292). Thus, Fernandez-Triana et al. (2014a) only examined a female syntype from the original type series, but because that specimen was fully illustrated (Fernandez-Triana et al. 2014: 48, figs 24–31), is in good condition, and perfectly matches the original description we are here designating it as the lectotype. It has the code 3c.1077.
Pseudapantelesbrunneus Ashmead, 1900
Pseudapantelesbrunneus Ashmead, 1900.
Type information. Holotype male, NHMUK (examined). Country of type locality: Saint Vincent.
Geographical distribution.NEO.
NEO: Saint Vincent.
Pseudapantelescarlosespinachi Fernandez-Triana & Whitfield, 2014
Pseudapantelescarlosespinachi Fernandez-Triana & Whitfield, 2014.
Type information. Holotype male, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Pseudapantelescarlosrodriguezi Fernandez-Triana & Whitfield, 2014
Pseudapantelescarlosrodriguezi Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Pseudapanteleschristianafigueresae Fernandez-Triana & Whitfield, 2014
Pseudapanteleschristianafigueresae Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Pseudapantelesdignus (Muesebeck, 1938)
Apantelesdignus Muesebeck, 1938.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.AUS, NEA, NEO.
AUS: Hawaiian Islands; NEA: USA (CA, FL); NEO: Argentina, Bermuda, Cuba, Mexico, Puerto Rico, US Virgin Islands.
Pseudapantelesgouleti Fernandez-Triana, 2010
Pseudapantelesgouleti Fernandez-Triana, 2010.
Type information. Holotype female, CNC (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (ON, QC).
Pseudapanteleshernanbravoi Fernandez-Triana & Whitfield, 2014
Pseudapanteleshernanbravoi Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Pseudapantelesjorgerodriguezi Fernandez-Triana & Whitfield, 2014
Pseudapantelesjorgerodriguezi Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Pseudapantelesjosefigueresi Fernandez-Triana & Whitfield, 2014
Pseudapantelesjosefigueresi Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Pseudapanteleslaurachinchillae Fernandez-Triana & Whitfield, 2014
Pseudapanteleslaurachinchillae Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Pseudapanteleslipomeringis (Muesebeck, 1958)
Apanteleslipomeringis Muesebeck, 1958.
Type information. Holotype female, USNM (examined). Country of type locality: Panama.
Geographical distribution.NEO.
NEO: Panama.
Pseudapantelesluisguillermosolisi Fernandez-Triana & Whitfield, 2014
Pseudapantelesluisguillermosolisi Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Pseudapantelesmargaritapenonae Fernandez-Triana & Whitfield, 2014
Pseudapantelesmargaritapenonae Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Pseudapantelesmariobozai Fernandez-Triana & Whitfield, 2014
Pseudapantelesmariobozai Fernandez-Triana & Whitfield, 2014.
Type information. Holotype male, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Pseudapantelesmariocarvajali Fernandez-Triana & Whitfield, 2014
Pseudapantelesmariocarvajali Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Pseudapantelesmaureenballesteroae Fernandez-Triana & Whitfield, 2014
Pseudapantelesmaureenballesteroae Fernandez-Triana & Whitfield, 2014.
Type information. Holotype male, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Pseudapantelesmoerens (Nixon, 1965)
Apantelesmoerens Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (SC).
Pseudapantelesmunifigueresae Fernandez-Triana & Whitfield, 2014
Pseudapantelesmunifigueresae Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Pseudapantelesnerion (Nixon, 1965)
Apantelesnerion Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (SC).
Pseudapantelesnigrovariatus (Muesebeck, 1921)
Apantelesnigrovariatus Muesebeck, 1921.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (GA, PA).
Notes. The specimen photographed by Fernandez-Triana et al. (2014a) was not the holotype. We examined the actual holotype in 2016, and it has dark orange metanotum and propodeum, unlike the specimen studied for the 2014 paper, which had those areas black. This slightly modifies the species concept and key presented by Fernandez-Triana et al. (2014a) but, other than that, the holotype looks mostly similar.
Pseudapantelesoscarariasi Fernandez-Triana & Whitfield, 2014
Pseudapantelesoscarariasi Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Pseudapantelesottonsolisi Fernandez-Triana & Whitfield, 2014
Pseudapantelesottonsolisi Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Pseudapantelespedroleoni Fernandez-Triana & Whitfield, 2014
Pseudapantelespedroleoni Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Pseudapantelesraulsolorzanoi Fernandez-Triana & Whitfield, 2014
Pseudapantelesraulsolorzanoi Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Pseudapantelesrenecastroi Fernandez-Triana & Whitfield, 2014
Pseudapantelesrenecastroi Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Pseudapantelesrodrigogamezi Fernandez-Triana & Whitfield, 2014
Pseudapantelesrodrigogamezi Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Pseudapantelesrosemarykarpinskiae Fernandez-Triana & Whitfield, 2014
Pseudapantelesrosemarykarpinskiae Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Pseudapantelesruficollis (Cameron, 1911)
Xanthomicrogasterruficollis Cameron, 1911.
Type information. Lectotype female, NHMUK (examined). Country of type locality: Guyana.
Geographical distribution.NEO.
NEO: Costa Rica, Cuba, Guyana.
Pseudapantelessesiae (Viereck, 1912)
Apantelessesiae Viereck, 1912.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (ON), USA (DC, FL, IN, NJ, TX, VA).
Pseudapantelessoniapicadoae Fernandez-Triana & Whitfield, 2014
Pseudapantelessoniapicadoae Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Pseudapantelesteofilodelatorrei Fernandez-Triana & Whitfield, 2014
Pseudapantelesteofilodelatorrei Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Genus Pseudofornicia van Achterberg, 2015
Pseudofornicia van Achterberg, 2015: 91. Gender: feminine. Type species: Pseudofornicianigrisoma van Achterberg & Long, 2015, by original designation.
The four described species, from the Oriental and Australasian regions, were recently revised (van Achterberg et al. 2015), but we have seen at least one additional, undescribed, species in collections. No host data are currently available for this genus. There are no DNA barcodes of Pseudofornicia in BOLD.
Pseudoforniciacommoni (Austin & Dangerfield, 1992)
Forniciacommoni Austin & Dangerfield, 1992.
Type information. Holotype female, ANIC (not examined but subsequent treatment of the species checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (QLD).
Notes. Our species concept is based on van Achterberg et al. (2015).
Pseudoforniciaflavoabdominis (He & Chen, 1994)
Forniciaflavoabdominis He & Chen, 1994.
Type information. Holotype female, ZJUH (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (ZJ).
Notes. Our species concept is based on van Achterberg et al. (2015).
Pseudofornicianigrisoma van Achterberg & Long, 2015
Pseudofornicianigrisoma van Achterberg & Long, 2015.
Type information. Holotype female, IEBR (not examined but original description checked). Country of type locality: Vietnam.
Geographical distribution.OTL.
OTL: Vietnam.
Pseudoforniciavanachterbergi Long, 2015
Pseudoforniciavanachterbergi Long, 2015.
Forniciaachterbergi Long, 2007 [primary homonym of Forniciaachterbergi Yang & Chen, 2006].
Type information. Holotype female, IEBR (not examined but subsequent treatment of the species checked). Country of type locality: Vietnam.
Geographical distribution.OTL.
OTL: Vietnam.
Notes. Our species concept is based on van Achterberg et al. (2015).
Genus Pseudovenanides Xiao & You, 2002
Pseudovenanides Xiao & You, 2002: 616. Gender: masculine. Type species: Pseudovenanideshunanus Xiao & You, 2002, by original designation and monotypy.
Apart from the single known species, we have seen a few more in collections, from the Oriental and Palearctic regions. The described species was reared from Gelechiidae. There are no DNA barcodes of Pseudovenanides in BOLD.
Pseudovenanideshunanus Xiao & You, 2002
Pseudovenanideshunanus Xiao & You, 2002.
Type information. Holotype female, HUNAU (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (GX, HN).
Genus Qrocodiledundee Fernandez-Triana, 2018
Qrocodiledundee Fernandez-Triana, 2018: 101. Gender: neuter. Type species: Qrocodiledundeeoutbackense Fernandez-Triana & Boudreault, 2018, by original designation.
Only known from a single described species in the Australasian region. No host data are currently available for this genus. There are no DNA barcodes of Qrocodiledundee in BOLD.
Qrocodiledundeeoutbackense Fernandez-Triana & Boudreault, 2018
Qrocodiledundeeoutbackense Fernandez-Triana & Boudreault, 2018.
Type information. Holotype male, CNC (examined). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (QLD).
Genus Rasivalva Mason, 1981
Rasivalva Mason, 1981: 91. Gender: feminine. Type species: Microplitisstigmaticus Muesebeck, 1922, by original designation.
Twelve species are recognized here, but many undescribed ones remain in collections. The genus is essentially Holarctic, occasionally reaching the Afrotropical and Oriental regions. The only known host records are all from Geometridae, but future studies may change that. There are 68 DNA-barcode compliant sequences of this genus in BOLD, representing 12 BINs, although several species and BINs are currently misidentified in BOLD as Diolcogaster and may actually represent Rasivalva.
Rasivalvacalceata (Haliday, 1834)
Microgastercalceatus Haliday, 1834.
Microgasterpubescens Ratzeburg, 1844.
Type information. Type lost (not examined but subsequent treatment of the species checked). Country of type locality: Ireland.
Geographical distribution.PAL.
PAL: Germany, Hungary, Ireland, Italy, Netherlands, Poland, Romania, Russia (C), Slovakia, Sweden, Switzerland, United Kingdom.
Notes. Our species concept is based on Nixon (1965), Tobias (1986), Oltra-Moscardó & Jiménez-Peydró (2005), and Kotenko (2007a).
Rasivalvacircumvecta (Lyle, 1918)
Diolcogastercircumvectus Lyle, 1918.
Type information. Lectotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Finland, Russia (C), United Kingdom.
Notes. The species was originally described based on four specimens (mounted on individual cards), which are all deposited in the NHMUK, and share the same type number 3c.31. There is a fifth pin with a label that has written: “Type to be selected from the above 4 specimens”. That fifth label was presumably added by Nixon, because when he dealt with that species he mentioned “Type in British Museum” (Nixon 1965: 256). In fact, one of the female specimens (the specimen occupying the top left corner in the unit tray at the NHMUK) has a Holotype round label added. Article 74.5 of the ICZN “Lectotype designations before 2000” stipulates that: “the term lectotype, or an exact translation or equivalent expression (e.g., the type), must have been used”, and also states that “a subsequent use of the term holotype does not constitute a valid lectotype designation unless the author, when wrongly using that term, explicitly indicated that he or she was selecting from the type series that particular specimen to serve as the name-bearing type.” Both situations clearly apply to Nixon (1965), as he referred to a type which he also unambiguously selected among the available specimens (even adding to that specimen an extra label marked as holotype). Thus, Nixon’s designation is to be considered valid, although that specimen should be considered as the lectotype and not the holotype, and the remaining three specimens are paralectotypes.
Rasivalvadesueta Papp, 1989
Rasivalvadesueta Papp, 1989.
Type information. Holotype female, HNHM (examined). Country of type locality: Hungary.
Geographical distribution.PAL.
PAL: Switzerland.
Rasivalvakaradagi Tobias, 1986
Rasivalvakaradagi Tobias, 1986.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Russia.
Geographical distribution.PAL.
PAL: Russia (NC), Ukraine.
Notes. Our species concept is based on Oltra-Moscardó & Jiménez-Peydró (2005).
Rasivalvaleleji Kotenko, 2007
Rasivalvaleleji Kotenko, 2007.
Type information. Holotype female, SIZK (not examined but original description checked). Country of type locality: Ukraine.
Geographical distribution.PAL.
PAL: Russia (SAK).
Rasivalvalepelleyi (Wilkinson, 1934)
Microgasterlepelleyi Wilkinson, 1934.
Type information. Holotype female, NHMUK (examined). Country of type locality: Kenya.
Geographical distribution.AFR.
AFR: Kenya.
Rasivalvalongivena Song & Chen, 2004
Rasivalvalongivena Song & Chen, 2004.
Type information. Holotype female, FAFU (not examined but original description checked). Country of type locality: China.
Geographical distribution.PAL.
PAL: China (HB).
Rasivalvamarginata (Nees, 1834)
Microgastermarginatus Nees, 1834.
Type information. Holotype female, depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.OTL, PAL.
OTL: Philippines; PAL: Austria, Finland, Germany, Hungary, Poland, Russia (KR, PRI, RYA, SPE, YAR), Slovenia, Sweden, Switzerland, United Kingdom, Yugoslavia.
Notes. Our species concept is based on Oltra-Moscardó & Jiménez-Peydró (2005).
Rasivalvaperplexa (Muesebeck, 1922)
Microplitisperplexus Muesebeck, 1922.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (BC, NB, ON), USA (IN, MI).
Rasivalvapyrenaica Oltra & Jiménez, 2005
Rasivalvapyrenaica Oltra & Jiménez, 2005.
Type information. Holotype female, UVS (not examined but original description checked). Country of type locality: Andorra.
Geographical distribution.PAL.
PAL: Andorra.
Rasivalvarugosa (Muesebeck, 1922)
Microplitisrugosus Muesebeck, 1922.
Microplitiscoloradensis Muesebeck, 1922.
Type information. Holotype male, MCZC (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (ON, QC), USA (CO, MI, MN, NJ).
Rasivalvastigmatica (Muesebeck, 1922)
Microplitisstigmaticus Muesebeck, 1922.
Microplitismuesebecki Marsh, 1974 [replacement name for Microplitisstigmaticus Muesebeck, 1922].
Type information. Holotype female, MCZC (not examined but original description checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (AB, BC, QC), USA (CA, CO, ID, NH, WA).
Genus Rhygoplitis Mason, 1981
Rhygoplitis Mason, 1981: 81. Gender: masculine. Type species: Apanteles (Pseudapanteles) terminalis Gahan, 1912, by original designation.
This genus is distributed in the New World, with four described species but several more remain in collections undescribed, mostly from the Neotropical region. Known hosts are mostly Crambidae, but more studies are needed. There are 294 DNA-barcode compliant sequences of this genus in BOLD, representing 13 BINs.
Rhygoplitisaciculatus (Ashmead, 1900)
Urogasteraciculatus Ashmead, 1900.
Pseudapantelessancti-vincenti Ashmead, 1900.
Apantelesthoracicus Muesebeck, 1921.
Type information. Holotype male, NHMUK (examined). Country of type locality: Grenada.
Geographical distribution.NEA, NEO.
NEA: USA (KS, TX); NEO: Costa Rica, Grenada, Panama, Saint Vincent.
Notes. We have examined the female type of P. sancti-vincenti and indeed it is the same species as the male type of U.aciculatus. Thus, although the name bearer for this species is the male specimen (following the rules of the ICZN), the female specimen of P. sancti-vincenti should be considered as useful, if not more useful, in any further study of the genus, as most of Microgastrinae taxonomy is based on female specimens.
Rhygoplitischoreuti (Viereck, 1912)
Apanteleschoreuti Viereck, 1912.
Type information. Holotype female, USNM (not examined but subsequent treatment of the species checked). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (FL, IA, NJ, SC, TX, VA).
Notes. Our species concept is based on Mason (1981) and Whitfield (1995a).
Rhygoplitissanctivincenti (Ashmead, 1900)
Apantelessanctivincenti Ashmead, 1900.
Type information. Type lost (not examined but subsequent treatment of the species checked). Country of type locality: Saint Vincent.
Geographical distribution.NEO.
NEO: Saint Vincent.
Notes. Our species concept is based on Fernandez-Triana et al. (2014e).
Rhygoplitisterminalis (Gahan, 1912)
Apantelesterminalis Gahan, 1912.
Type information. Holotype male, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (AR, CO, FL, GA, IL, IA, KY, MD, NY, TX).
Genus Sathon Mason, 1981
Sathon Mason, 1981: 78. Gender: masculine. Type species: Apantelesneomexicanus Muesebeck, 1921, by original designation.
This genus is distributed in all biogeographical regions, with 23 described species, although is not highly species rich anywhere. We have seen additional species in collections, but it is difficult to estimate the actual diversity due to some species being similar to other genera (e.g., Glyptapanteles and Lathrapanteles). Williams’ (1988) revision is the most up to date and comprehensive work for this genus but is now outdated. Five families of Lepidoptera have been reported as hosts, but in most cases those records need further verification. There are 266 DNA-barcode compliant sequences of Sathon in BOLD, representing 27 BINs.
Sathonaggeris Williams, 1988
Sathonaggeris Williams, 1988.
Type information. Holotype female, CNC (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Sathonalbicoxus Austin & Dangerfield, 1992
Sathonalbicoxus Austin & Dangerfield, 1992.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (NSW, TAS, VIC).
Sathonbekilyensis (Granger, 1949), new combination
Microgasterbekilyensis Granger, 1949.
Type information. Holotype female, MNHN (not examined but illustrations of the holotype examined). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Notes. The generic placement of this species has been determined based on information from the original description and low-resolution images of the holotype (taken with a cell phone) which we have examined. The species is clearly not Microgaster, and we are transferring it to Sathon here based on propodeum with a median longitudinal carina, T2 subtriangular or trapezoidal and with lateral margins well defined by sulcus, ovipositor sheaths moderately long (almost half metatibia length), and hypopygium inflexible. The type also has antenna with some central flagellomeres white-yellow, and the body is mostly yellow. This species seems to be related to Microgasterrufotestacea Granger, also transferred in this paper to Sathon. We have seen in collections several undescribed species from Africa which share similar morphological features to these two species, and the best generic placement at present would be in Sathon. However, future study of this group of species may change that, and we suspect that they may represent an undescribed genus.
Sathonbelippae (Rohwer, 1919)
Apantelesbelippae Rohwer, 1919.
Type information. Holotype female, USNM (examined). Country of type locality: Indonesia.
Geographical distribution.AUS, OTL.
AUS: Fiji; OTL: India, Indonesia.
Sathoncinctiformis (Viereck, 1911)
Apantelescinctiformis Viereck, 1911.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (ON, QC), USA (IN, IA, MD, MI, NJ, NY, NC, OH, PA, RI, VT, VA, WI).
Sathoncircumflexus Williams, 1988
Sathoncircumflexus Williams, 1988.
Type information. Holotype female, CNC (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: USA (AZ, CO, NM).
Sathoneugeni (Papp, 1972)
Apanteleseugeni Papp, 1972.
Apantelesmagnicoxis Jakimavicius, 1972.
Type information. Holotype female, HNHM (examined). Country of type locality: Hungary.
Geographical distribution.PAL.
PAL: Austria, Bulgaria, Finland, Germany, Hungary, Italy, Latvia, Lithuania, Netherlands, Russia (C, NW), Slovakia, Sweden, Switzerland, Turkey, United Kingdom.
Notes. Our species concept is based on Williams (1988), which considered it part of the lateralis group (see more comments about this species group in the Notes provided under S.lateralis). The species distribution in Russia is based on Belokobylskij et al. (2019).
Sathonfalcatus (Nees, 1834)
Microgasterfalcatus Nees, 1834.
Microgasterequestris Haliday, 1834.
Apantelesgladiator Szépligeti, 1901.
Apantelespriapus Gautier & Cleu, 1927.
Type information. Neotype female, ZMHB (not examined but authoritatively identified specimens examined). Country of type locality: unknown.
Geographical distribution.OTL, PAL.
OTL: Indonesia; PAL: Afghanistan, Austria, Armenia, Azerbaijan, Belarus, Bosnia and Herzegovina, China, Croatia, Czech Republic, Denmark, Egypt, Estonia, Finland, France, Georgia, Germany, Hungary, Ireland, Italy, Japan, Kazakhstan, Kyrgyzstan, Korea, Latvia, Lithuania, Luxembourg, Macedonia, Mongolia, Montenegro, Netherlands, Poland, Romania, Russia (ZAB, DA, IRK, KAM, KR, KIR, KYA, SAK, SPE, VLG, YAR), Serbia, Slovenia, Spain, Sweden, Switzerland, Tajikistan, Turkey, United Kingdom, Uzbekistan.
Notes. We examined the type of M.equestris (Haliday) which is in the NHMUK, as well as numerous authenticated specimens in the CNC, MZH and RSME. Apantelespriapus Gautier & Cleu was considered by Telenga (1955: 54) to be a valid species, not a synonym of falcatus, based on differences in body size and sculpture, as well as ovipositor size; however, Telenga did not examine specimens of the priapus type series (from France), his species concept was only based on the original description. Yu et al. (2016), following Telenga, also considered priapus to be a valid species. However, Wilkinson (1945: 133–137) actually examined two cotypes of the priapus series and considered them to be the same species than falcatus; Wilkinson also designated a neotype for falcatus and was able to study a large number of specimens from different localities and collections. Thus, we consider Wilkinson (1945) a more accurate account and here we accept his decision to synonymize priapus under falcatus, which has also been accepted and followed by most authors (e.g., Shenefelt 1972, Papp 1982, 1988, Williams 1998, Kotenko 2007). The species distribution in Afghanistan, Armenia, Azerbaijan, China, Georgia, Kyrgyzstan, and Tajikistan is based on Belokobylskij et al. (2019).
Sathonfausta (Nixon, 1973)
Apantelesfausta Nixon, 1973.
Type information. Holotype female, NHMUK (examined). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: Austria, Finland, Germany, Slovakia, Sweden, Switzerland, United Kingdom.
Notes. This species was placed in Sathon by Mason (1981) when he described the genus. The only comprehensive revision ever done of Sathon (Williams 1988) also treated fausta within the genus (although as a synonym of S.eugeni Papp, 1972). Since then, fausta has been variously treated as Protapanteles (van Achterberg 2003), Glyptapanteles (Papp 1988, Belokobylskij et al. 2003, Shaw 2012, Broad et al. 2016), or Sathon (Capek and Hofmann 1997). Here we follow Williams (1988) study and consider at present the best generic placement for fausta to be in Sathon (as part of lateralis group of species, see more comments about this species group in the Notes provided under S.lateralis). The status of fausta as a valid species has varied during the years (e.g., Papp 1983a, 1998), and we suspect it is only a synonym of eugeni (e.g., Shaw 2012, Broad et al. 2016), but because that will require further investigation, for the time being we retain it as a valid species in this paper.
Sathonflavofacialis (Granger, 1949), new combination
Microgasterflavofacialis Granger, 1949.
Type information. Syntypes female, MNHN (not examined but illustrations of the holotype examined). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Notes. The generic placement of this species has been determined based on information from the original description and low-resolution images of the holotype (taken with a cell phone) which we have examined. The species is clearly not Microgaster, and we are transferring it to Sathon here based on propodeum with median longitudinal carina, T2 subtriangular or trapezoidal and with lateral margins well defined by sulcus, ovipositor sheaths moderately long (almost half metatibia length), and hypopygium inflexible. The type also has antenna with some central flagellomeres white-yellow, and the head, propleuron, most of legs, and sternites are orange-yellow. We have seen in collections several undescribed species from Africa which share similar morphological features to this species, and the best generic placement at present would be in Sathon. However, future study of this group of species may change that and we suspect that they may represent an undescribed genus.
Sathonlaevidorsum Williams, 1988
Sathonlaevidorsum Williams, 1988.
Type information. Holotype female, CNC (examined). Country of type locality: Mexico.
Geographical distribution.NEO.
NEO: Mexico.
Sathonlateralis (Haliday, 1834)
Microgasterlateralis Haliday, 1834.
Type information. Lectotype female, NHMUK (examined). Country of type locality: unknown.
Geographical distribution.PAL.
PAL: Armenia, Azerbaijan, Belgium, Finland, France, Georgia, Germany, Hungary, Ireland, Italy, Kazakhstan, Lithuania, Madeira Islands, Moldova, Netherlands, Romania, Russia (C, NC, S), Serbia, Slovakia, Spain, Sweden, Switzerland, Turkey, Ukraine, United Kingdom.
Notes. This species was placed in Sathon by Mason (1981) when he described the genus. The only comprehensive revision ever done of the genus (Williams 1988) also considered lateralis to be in Sathon, as part of a newly created lateralis group of species, which also comprises three other species: eugeni Papp, 1972, fausta (Nixon, 1973), and papilionae Williams, 1988. These four species were differentiated from the more derived falcatus group, which comprises the rest of Sathon (sensuWilliams 1988), based on the propodeum sculpture, shape and sculpture of T2, hypopygium shape, straight ovipositor sheaths, males without enlarged genitalia and host data. The lateralis group resembles Glyptapanteles with relatively long ovipositor sheaths (longer than the majority of the described species in that genus), which has likely influenced the decision of many subsequent authors to treat some of the species in the lateralis group as Glyptapanteles (e.g., Papp 1988, Belokobylskij et al. 2003, Shaw 2012, Broad et al. 2016) or Protapantelessensu lato (van Achterberg 2003); although other authors continued to treat those species as Sathon (Oltra and Michelena 1988, Maetô 1996, Capek and Hofmann 1997). Until a more robust phylogenetic framework for Microgastrinae is available, we prefer to maintain the lateralis group in Sathon, as we consider Williams (1988) the most detailed study currently available. It should also be noted that in large neighbour-joining trees with thousands of Microgastrinae DNA barcodes (e.g., Smith et al. 2013), the described species of Sathon, from both lateralis and falcatus groups, cluster together.
Sathonlaurae (de Saeger, 1944), new combination
Microgasterlaurae de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, South Africa.
Notes. Based on the original description (de Saeger 1944), the best generic placement at present is in Sathon, based on the propodeum with a strong median carina, ovipositor and ovipositor sheaths relatively long, and hypopygium supposedly unpleated. We consider the hypopygium of this species as lacking pleats because it is depicted as such in figure 22 of the paper (de Saeger 1944: 62), although that detail was not mentioned in the written part of the description. However, we deem this a fair assumption because in the same paper, illustrations of other species with pleated hypopygium were clearly drawn as such, and often also explicitly mentioned in the written part of the descriptions.
Sathonmasoni Williams, 1988
Sathonmasoni Williams, 1988.
Type information. Holotype female, CNC (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (NT, NU), USA (AK, ID, MN).
Sathonmikeno (de Saeger, 1944), new combination
Microgastermikeno de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. Based on the original description, this species is clearly not Microgaster. The best generic placement at present would be in Sathon, as many of the characters described would correspond to that genus, e.g., ovipositor and ovipositor sheaths relatively long (as long as metatibia), inflexible hypopygium, and propodeum with strong median carina. A few characters differ from previously described species of Sathon: a) the male specimens from the type series studied by de Saeger (1944) were not described as having large external genitalia, one of the most distinctive traits of Sathon, although that trait is not always present (see Williams 1988 for a discussion of that character); b) the head (described by de Saeger as more transverse and globose than normal, and with face rugose and prominent) is not like in typical species of Sathon, but is similar to some species of several genera (e.g., Cotesia, Diolcogaster, Keylimepie, Venanides), where it seems to be related to some specialized activity; c) T2, as illustrated in de Saeger (1944: fig. 78) is not as in typical Sathon, in the sense of being more transverse. However, we do not consider these differences as sufficient to invalidate our decision to transfer mikeno to Sathon.
Sathonmorata (Wilkinson, 1929)
Microgastermorata Wilkinson, 1929.
Type information. Holotype female, NHMUK (examined). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (ACT, SA, VIC, WA).
Sathonnaryciae Austin & Dangerfield, 1992
Sathonnaryciae Austin & Dangerfield, 1992.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (VIC).
Sathonneomexicanus (Muesebeck, 1921)
Apantelesneomexicanus Muesebeck, 1921.
Apantelescaudatus Muesebeck, 1922.
Type information. Holotype female, USNM (examined). Country of type locality: USA.
Geographical distribution.NEA.
NEA: Canada (AB, BC, MB, NL, NT, ON, PE), USA (AK, AZ, CA, CO, ID, MI, MN, MT, NV, NM, OR, SD, UT, WA, WI, WY).
Sathonoreo Fagan-Jeffries & Austin, 2019
Sathonoreo Fagan-Jeffries & Austin, 2019.
Type information. Holotype female, SAMA (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (SA).
Sathonpapilionae Williams, 1988
Sathonpapilionae Williams, 1988.
Type information. Holotype female, CNC (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (BC), USA (AK).
Notes. Our species concept is based on Williams (1988), which considered it part of the lateralis group (see more comments about this species group in the Notes provided under S.lateralis).
Sathonresplendens (Wilkinson, 1929)
Microgasterresplendens Wilkinson, 1929.
Type information. Holotype female, NHMUK (examined). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (TAS).
Notes. The holotype is in relatively poor condition, broken into several pieces all glued together, with the consequence that some morphological details are lost.
Sathonruandanus (de Saeger, 1944), new combination
Microgasterruandana de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Rwanda.
Notes. Based on the original description, the best generic placement at present would be in Sathon, as many of the characters described would correspond to that genus, e.g., ovipositor and ovipositor sheaths relatively long (as long as metatibia), inflexible hypopygium, and propodeum with median carina.
Sathonrufotestaceus (Granger, 1949), new combination
Microgasterrufotestacea Granger, 1949.
Type information. Holotype female, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Notes. The generic placement of this species has been determined based on information from the original description. The species is clearly not Microgaster, and we are transferring it to Sathon based on the propodeum being mostly smooth but with a strong median longitudinal carina, T1 and T2 shapes and sculptures (as detailed in Granger 1949: 225, fig. 218), and ovipositor sheaths moderately long (as long as metafemur). This species seems to be related to Microgasterbekilyensis Granger, also transferred to Sathon in this paper. We have seen in collections several undescribed species from Africa which share similar morphological features to these two species, and the best generic placement at present would be in Sathon. However, future study of this group of species may change that; we suspect that they may represent an undescribed genus.
Genus Semionis Nixon, 1965
Semionis Nixon, 1965: 206. Gender: masculine. Type species: Semionisrarus Nixon, 1965, by original designation and monotypy.
There is only one extant species (Nixon 1965, Mason 1981), which is very distinctive, with a fossil species also recently described (Belokobylskij 2014). No host data are currently available for this genus. There are no DNA barcodes of Semionis in BOLD.
Semionisrarus Nixon, 1965
Semionisrarus Nixon, 1965.
Type information. Holotype male, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Genus Sendaphne Nixon, 1965
Sendaphne Nixon, 1965: 203. Gender: feminine. Type species: Sendaphneolearus Nixon, 1965, by original designation.
This is a strictly Neotropical genus, recently revised (Fernandez-Triana et al. 2014h) with eleven species recorded, but also several additional, undescribed species in collections. No host data are currently available for Sendaphne. There are seven DNA-barcode compliant sequences of this genus in BOLD (with 24 additional, shorter sequences ranging from 102 to 420 bp.), representing two BINs.
Sendaphneanitae Fernandez-Triana & Whitfield, 2014
Sendaphneanitae Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Sendaphnebennetti Fernandez-Triana & Whitfield, 2014
Sendaphnebennetti Fernandez-Triana & Whitfield, 2014.
Type information. Holotype male, CNC (examined). Country of type locality: Mexico.
Geographical distribution.NEO.
NEO: Mexico.
Sendaphnebrasilianus Penteado-Dias, 1995
Sendaphnebrasilianus Penteado-Dias, 1995.
Type information. Holotype female, DCBU (not examined but subsequent treatment of the species checked). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (DF).
Notes. Our species concept is based on Fernandez-Triana et al. (2014h).
Sendaphnebroadi Fernandez-Triana & Whitfield, 2014
Sendaphnebroadi Fernandez-Triana & Whitfield, 2014.
Type information. Holotype male, CNC (examined). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Sendaphnedianariaspennae Fernandez-Triana & Whitfield, 2014
Sendaphnedianariaspennae Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (PE, RJ, SC), Colombia.
Sendaphnejatai Penteado-Dias, 1995
Sendaphnejatai Penteado-Dias, 1995.
Type information. Holotype female, DCBU (not examined but subsequent treatment of the species checked). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (MT, SP), Ecuador, French Guiana.
Notes. Our species concept is based on Fernandez-Triana et al. (2014h).
Sendaphneolearus Nixon, 1965
Sendaphneolearus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (SC), French Guiana, Peru.
Sendaphneparanaensis Scatolini & Penteado-Dias, 1999
Sendaphneparanaensis Scatolini & Penteado-Dias, 1999.
Type information. Holotype female, DCMP (not examined but subsequent treatment of the species checked). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (ES, PR, RJ), Paraguay.
Notes. Our species concept is based on Fernandez-Triana et al. (2014h).
Sendaphnepenteadodiasae Fernandez-Triana & Whitfield, 2014
Sendaphnepenteadodiasae Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (PR).
Sendaphnerogerblancoi Fernandez-Triana & Whitfield, 2014
Sendaphnerogerblancoi Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Sendaphnesulmo Nixon, 1965
Sendaphnesulmo Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Mexico.
Geographical distribution.NEO.
NEO: Mexico.
Genus Shireplitis Fernandez-Triana & Ward, 2013
Shireplitis Fernandez-Triana & Ward, 2013: 556. Gender: masculine. Type species: Shireplitisbilboi Fernandez-Triana & Ward, 2013, by original designation.
The genus was recently described, to include six species limited to New Zealand (Fernandez-Triana et al. 2013b). We are not aware of any undescribed species in collections. No host data are currently available for Shireplitis. There are three DNA-barcode compliant sequences of this genus in BOLD, representing one BIN.
Shireplitisbilboi Fernandez-Triana & Ward, 2013
Shireplitisbilboi Fernandez-Triana & Ward, 2013.
Type information. Holotype female, NZAC (examined). Country of type locality: New Zealand.
Geographical distribution.AUS.
AUS: New Zealand.
Shireplitisfrodoi Fernandez-Triana & Ward, 2013
Shireplitisfrodoi Fernandez-Triana & Ward, 2013.
Type information. Holotype female, NZAC (examined). Country of type locality: New Zealand.
Geographical distribution.AUS.
AUS: New Zealand.
Shireplitismeriadoci Fernandez-Triana & Ward, 2013
Shireplitismeriadoci Fernandez-Triana & Ward, 2013.
Type information. Holotype female, NZAC (examined). Country of type locality: New Zealand.
Geographical distribution.AUS.
AUS: New Zealand.
Shireplitisperegrini Fernandez-Triana & Ward, 2013
Shireplitisperegrini Fernandez-Triana & Ward, 2013.
Type information. Holotype female, NZAC (examined). Country of type locality: New Zealand.
Geographical distribution.AUS.
AUS: New Zealand.
Shireplitissamwisei Fernandez-Triana & Ward, 2013
Shireplitissamwisei Fernandez-Triana & Ward, 2013.
Type information. Holotype female, NZAC (examined). Country of type locality: New Zealand.
Geographical distribution.AUS.
AUS: New Zealand.
Shireplitistolkieni Fernandez-Triana & Ward, 2013
Shireplitistolkieni Fernandez-Triana & Ward, 2013.
Type information. Holotype female, NZAC (examined). Country of type locality: New Zealand.
Geographical distribution.AUS.
AUS: New Zealand.
Genus Silvaspinosus Fernandez-Triana, 2018
Silvaspinosus Fernandez-Triana, 2018: 102. Gender: neuter. Type species: SilvaspinosusvespaFernandez-Triana and Boudreault 2018, by original designation.
A single species from the Afrotropical region was recently described, but in collections there is at least one additional species (Fernandez-Triana & Boudreault, 2018). No host data are currently available for this genus. There are no full DNA barcodes of Silvaspinosus in BOLD, but two short sequences.
Silvaspinosusvespa Fernandez-Triana & Boudreault, 2018
Silvaspinosusvespa Fernandez-Triana & Boudreault, 2018.
Type information. Holotype female, CAS (examined). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Genus Snellenius Westwood, 1882
Snellenius Westwood, 1882: 19. Gender: masculine. Type species: Snelleniusvollenhovii Westwood, 1882, by original designation and monotypy.
The 41 described species of this genus are distributed in all regions except the Nearctic (although that might be due to relatively little collecting effort and fewer studies of Microgastrinae in southwestern North America, where a few species may be found). Although some revisions are available (e.g., Austin and Dangerfield 1993, Long & van Achterberg 2013, Fernandez-Triana et al. 2015b, Perez and Berta 2017) there are many undescribed species in collections and the genus is far from being completely understood from a taxonomic perspective. All known host records are from three Lepidoptera families (Erebidae, Noctuidae, Sphingidae). There are 185 DNA-barcode compliant sequences of this genus in BOLD, representing 25 BINs.
Snelleniusatratus Shenefelt, 1968
Snelleniusatratus Shenefelt, 1968.
Type information. Holotype female, depository unknown (not examined but original description checked). Country of type locality: Peru.
Geographical distribution.NEO.
NEO: Peru.
Notes. The original description (Shenefelt 1968) mentions the holotype as deposited in the collection of the author. We do not know where that collection is stored at present but suspect it might be in the USNM.
Snelleniusbasalis (Walker, 1874)
Proteropsbasalis Walker, 1874.
Type information. Holotype female, NHMUK (examined). Country of type locality: Japan.
Geographical distribution.PAL.
PAL: Japan.
Notes. Van Achterbeg & de Chenon (2009) transferred the species to Snellenius, after the authors were able to examine the holotype; at that time the authors included a synonym within that species, Snelleniustheretrae (Watanabe, 1937). Subsequently, Long & van Achterberg (2013) revised this and removed S.theretrae from synonymy, to be considered as a valid species, a decision we follow here.
Snelleniusbicolor Shenefelt, 1968
Snelleniusbicolor Shenefelt, 1968.
Type information. Holotype female, depository unknown (not examined but original description checked). Country of type locality: Peru.
Geographical distribution.NEO.
NEO: Argentina, Bolivia, Peru.
Notes. The original description (Shenefelt 1968) mentions the holotype as deposited in the collection of the author. We do not know where that collection is stored at present but suspect it might be in the USNM.
Snelleniusbillburgeri Fernandez-Triana & Whitfield, 2015
Snelleniusbillburgeri Fernandez-Triana & Whitfield, 2015.
Type information. Holotype male, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Snelleniusbobdressleri Fernandez-Triana & Whitfield, 2015
Snelleniusbobdressleri Fernandez-Triana & Whitfield, 2015.
Type information. Holotype female, USNM (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Snelleniusclavitergum Austin & Dangerfield, 1993
Snelleniusclavitergum Austin & Dangerfield, 1993.
Type information. Holotype female, AEIC (not examined but original description checked). Country of type locality: Papua New Guinea.
Geographical distribution.AUS.
AUS: Papua New Guinea.
Snelleniusdonstonei Fernandez-Triana & Whitfield, 2015
Snelleniusdonstonei Fernandez-Triana & Whitfield, 2015.
Type information. Holotype female, USNM (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Snelleniusfelipechavarriai Fernandez-Triana & Whitfield, 2015
Snelleniusfelipechavarriai Fernandez-Triana & Whitfield, 2015.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Snelleniusgelleus Nixon, 1965
Snelleniusgelleus Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (FJ).
Snelleniusgerardoherrerai Fernandez-Triana & Whitfield, 2015
Snelleniusgerardoherrerai Fernandez-Triana & Whitfield, 2015.
Type information. Holotype female, USNM (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Snelleniusguizhouensis Luo & You, 2005
Snelleniusguizhouensis Luo & You, 2005.
Type information. Holotype female, GUGC (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (GZ).
Notes. Our species concept is based on Long and van Achterberg (2013).
Snelleniushippotionus Austin & Dangerfield, 1993
Snelleniushippotionus Austin & Dangerfield, 1993.
Type information. Holotype male, ANIC (not examined but original description checked). Country of type locality: Papua New Guinea.
Geographical distribution.AUS.
AUS: Papua New Guinea.
Snelleniusirenebakerae Fernandez-Triana & Whitfield, 2015
Snelleniusirenebakerae Fernandez-Triana & Whitfield, 2015.
Type information. Holotype female, USNM (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Snelleniusisidrochaconi Fernandez-Triana & Whitfield, 2015
Snelleniusisidrochaconi Fernandez-Triana & Whitfield, 2015.
Type information. Holotype male, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica, Panama.
Snelleniusjohnkressi Fernandez-Triana & Whitfield, 2015
Snelleniusjohnkressi Fernandez-Triana & Whitfield, 2015.
Type information. Holotype female, USNM (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Snelleniusjorgecampabadali Fernandez-Triana & Whitfield, 2015
Snelleniusjorgecampabadali Fernandez-Triana & Whitfield, 2015.
Type information. Holotype female, INBio (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Snelleniusjorgegomezlauritoi Fernandez-Triana & Whitfield, 2015
Snelleniusjorgegomezlauritoi Fernandez-Triana & Whitfield, 2015.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Snelleniusjosesarukhani Fernandez-Triana & Whitfield, 2015
Snelleniusjosesarukhani Fernandez-Triana & Whitfield, 2015.
Type information. Holotype male, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Snelleniuskerrydresslerae Fernandez-Triana & Whitfield, 2015
Snelleniuskerrydresslerae Fernandez-Triana & Whitfield, 2015.
Type information. Holotype female, USNM (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Snelleniuslatigenus Luo & You, 2005
Snelleniuslatigenus Luo & You, 2005.
Type information. Holotype female, GUGC (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (GZ).
Notes. Our species concept is based on Long and van Achterberg (2013).
Snelleniuslucindamcdadeae Fernandez-Triana & Whitfield, 2015
Snelleniuslucindamcdadeae Fernandez-Triana & Whitfield, 2015.
Type information. Holotype female, USNM (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Snelleniusluisdiegogomezi Fernandez-Triana & Whitfield, 2015
Snelleniusluisdiegogomezi Fernandez-Triana & Whitfield, 2015.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica, Panama.
Snelleniusmaculipennis (Szépligeti, 1900)
Microplitismaculipennis Szépligeti, 1900.
Microplitiseusirus Lyle, 1921.
Microplitisophiusae Ramakrishna Ayyar, 1921.
Type information. Type lost (not examined but authoritatively identified specimens examined). Country of type locality: Papua New Guinea.
Geographical distribution.AUS, OTL.
AUS: Australia (QLD), Papua New Guinea; OTL: India, Thailand, Vietnam.
Notes. The female holotype is considered to be lost (see details in Austin and Dangerfield 1993: 1156). Although several authors have placed this species in Snellenius, Gupta (2013a) transferred the species to Microplitis, despite the illustrations of her paper clearly showing the presence of an epicnemial carina in that species, which would place it within Snellenius. Ranjith et al. (2015a) also followed Gupta (2013a). We have examined the type of Microplitiseusirus Lyle, and it also has an epicnemial carina, in addition to having the scutellar disc strongly impressed, as is typical of species of Snellenius. Thus, we revise the species combination here back to Snellenius.
Snelleniusmariakuzminae Fernandez-Triana & Whitfield, 2015
Snelleniusmariakuzminae Fernandez-Triana & Whitfield, 2015.
Type information. Holotype male, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Snelleniusmariamartachavarriae Fernandez-Triana & Whitfield, 2015
Snelleniusmariamartachavarriae Fernandez-Triana & Whitfield, 2015.
Type information. Holotype male, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Snelleniusnigellus Long & van Achterberg, 2013
Snelleniusnigellus Long & van Achterberg, 2013.
Type information. Holotype male, VNMN (not examined but original description checked). Country of type locality: Vietnam.
Geographical distribution.OTL.
OTL: Vietnam.
Snelleniusperuensis Shenefelt, 1968
Snelleniusperuensis Shenefelt, 1968.
Type information. Holotype female, depository unknown (not examined but original description checked). Country of type locality: Peru.
Geographical distribution.NEO.
NEO: Peru.
Notes. The original description (Shenefelt 1968) mentions the holotype as deposited in the collection of the author. We do not know where that collection is stored at present, but suspect it might be in the USNM.
Snelleniusphildevriesi Fernandez-Triana & Whitfield, 2015
Snelleniusphildevriesi Fernandez-Triana & Whitfield, 2015.
Type information. Holotype female, USNM (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Snelleniusphilippinensis (Ashmead, 1904)
Microplitisphilippinensis Ashmead, 1904.
Microplitisbimaculatus Cameron, 1909.
Type information. Holotype male, USNM (not examined but subsequent treatment of the species checked). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Indonesia, Malaysia, Philippines, Vietnam.
Notes. Our species concept is based on Long and van Achterberg (2013).
Snelleniusquiricojimenezi Fernandez-Triana & Whitfield, 2015
Snelleniusquiricojimenezi Fernandez-Triana & Whitfield, 2015.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Snelleniusradicalis (Wilkinson, 1929)
Microplitisradicalis Wilkinson, 1929.
Type information. Syntypes female and male, ZMHB (not examined but subsequent treatment of the species checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (TW).
Notes. The information about type specimens and depository we follow here is from the original description (Wilkinson 1945: 206-207); however, Nixon (1965: 270) mentioned that the type was deposited in the NHMUK.
Snelleniusrobertoespinozai Fernandez-Triana & Whitfield, 2015
Snelleniusrobertoespinozai Fernandez-Triana & Whitfield, 2015.
Type information. Holotype male, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Snelleniussandyknappae Fernandez-Triana & Whitfield, 2015
Snelleniussandyknappae Fernandez-Triana & Whitfield, 2015.
Type information. Holotype female, USNM (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Snelleniussedlaceki Austin & Dangerfield, 1993
Snelleniussedlaceki Austin & Dangerfield, 1993.
Type information. Holotype female, AEIC (not examined but original description checked). Country of type locality: Papua New Guinea.
Geographical distribution.AUS.
AUS: Papua New Guinea.
Snelleniussimilis Long & van Achterberg, 2013
Snelleniussimilis Long & van Achterberg, 2013.
Type information. Holotype female, VNMN (not examined but original description checked). Country of type locality: Vietnam.
Geographical distribution.OTL.
OTL: Vietnam.
Snelleniustheretrae (Watanabe, 1937)
Microplitistheretrae Watanabe, 1937.
Type information. Holotype female, EIHU (not examined but subsequent treatment of the species checked). Country of type locality: Japan.
Geographical distribution.PAL.
PAL: Japan, Korea.
Notes. The status of this species was revised by Long & van Achterberg (2013), who removed the species from synonymy with Snelleniusbasalis (Walker, 1874), a decision we accept and follow.
Snelleniustricolor Shenefelt, 1968
Snelleniustricolor Shenefelt, 1968.
Type information. Holotype female, USNM (examined). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina.
Snelleniusvelvaruddae Fernandez-Triana & Whitfield, 2015
Snelleniusvelvaruddae Fernandez-Triana & Whitfield, 2015.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Snelleniusvickifunkae Fernandez-Triana & Whitfield, 2015
Snelleniusvickifunkae Fernandez-Triana & Whitfield, 2015.
Type information. Holotype female, USNM (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Snelleniusvollenhovii Westwood, 1882
Snelleniusvollenhovii Westwood, 1882.
Type information. Holotype male, OUMNH (not examined but subsequent treatment of the species checked). Country of type locality: Papua New Guinea.
Geographical distribution.AUS.
AUS: Papua New Guinea.
Notes. Our species concept is based on Nixon (1965), Austin and Dangerfield (1993), and Long and van Achterberg (2013).
Snelleniuswarrenwagneri Fernandez-Triana & Whitfield, 2015
Snelleniuswarrenwagneri Fernandez-Triana & Whitfield, 2015.
Type information. Holotype female, USNM (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Genus Tobleronius Fernandez-Triana, 2018
Tobleronius Fernandez-Triana, 2018: 108. Gender: neuter. Type species: TobleroniusorientalisFernandez-Triana and Boudreault 2018, by original designation.
One species was recently described from the Oriental region (Fernandez-Triana & Boudreault, 2018), but at least another one may exist in collections. No host data are currently available for this genus. There are two DNA-barcode compliant sequences of Tobleronius in BOLD, representing two BINs (although those sequences have not been identified in BOLD as belonging to Tobleronius; see Fernandez-Triana and Boudreault 2018).
Tobleroniusorientalis Fernandez-Triana & Boudreault, 2018
Tobleroniusorientalis Fernandez-Triana & Boudreault, 2018.
Type information. Holotype male, RMNH (examined). Country of type locality: Vietnam.
Geographical distribution.OTL.
OTL: Thailand, Vietnam.
Genus Ungunicus Fernandez-Triana, 2018
Ungunicus Fernandez-Triana, 2018: 113. Gender: neuter. Type species: Ungunicusvietnamensis Fernandez-Triana & Boudreault, 2018, by original designation.
One species was recently described from the Oriental region (Fernandez-Triana and Boudreault 2018); we are not aware of additional species in collections. No host data are currently available for this genus. There is one DNA-barcode compliant sequence of Ungunicus in BOLD, representing one BIN (although that sequence has not been identified in BOLD as belonging to Ungunicus, see Fernandez-Triana and Boudreault 2018 for that).
Ungunicusvietnamensis Fernandez-Triana & Boudreault, 2018
Ungunicusvietnamensis Fernandez-Triana & Boudreault, 2018.
Type information. Holotype female, RMNH (examined). Country of type locality: Vietnam.
Geographical distribution.OTL.
OTL: Vietnam.
Genus Venanides Mason, 1981
Venanides Mason, 1981: 99. Gender: masculine. Type species: Venanidesxeste Mason, 1981, by original designation.
A cosmopolitan genus, with 14 described species and several more undescribed and found in collections. No revision of this genus is currently available. Host records are from six families of Lepidoptera, but some are questionable. There are 89 DNA-barcode compliant sequences of this genus in BOLD, representing five BINs.
Venanidesastydamia (Nixon, 1965), new combination
Apantelesastydamia Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Notes. Transferred to Venanides based on pronotum laterally with a single furrow, venation of fore wing, smooth propodeum, T1 and T2 shapes, and ovipositor sheaths without setae. In the original description, Nixon (1965) did not provide any detail on the etymology of the species name. As first revisers, we thus consider its gender to be neuter.
Venanidescaspius Abdoli, Fernandez-Triana & Talebi, 2019
Venanidescaspius Abdoli, Fernandez-Triana & Talebi, 2019.
Type information. Holotype female, TMUC (examined). Country of type locality: Iran.
Geographical distribution.PAL.
PAL: Iran.
Venanidescongoensis (de Saeger, 1941)
Apantelescongoensis de Saeger, 1941.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Cameroon, Democratic Republic of Congo, Uganda.
Venanidescurticornis (Granger, 1949)
Apantelescurticornis Granger, 1949.
Type information. Holotype female, MNHN (not examined but original description checked). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar, Réunion.
Venanidesdemeter (Wilkinson, 1934), new combination
Apantelesdemeter Wilkinson, 1934.
Type information. Holotype female, NHMUK (examined). Country of type locality: New Zealand.
Geographical distribution.AUS.
AUS: New Zealand.
Notes. This species had been transferred to Glyptapanteles by Mason (1981), but it is placed in Venanides in this work because the pronotum laterally has only one ventral sulcus (two sulci in Glyptapanteles) and the propodeum is mostly sculptured, including numerous, relatively long carinae radiating from nucha (propodeum not like that in Glyptapanteles). DNA barcodes obtained from this species suggest that it might even belong to a different genus on its own but solving that will require further study beyond the scope of this paper; for the time being the best generic placement is Venanides.
Venanideslongifrons Fernandez-Triana & van Achterberg, 2017
Venanideslongifrons Fernandez-Triana & van Achterberg, 2017.
Type information. Holotype female, RMNH (examined). Country of type locality: Yemen.
Geographical distribution.AFR.
AFR: Yemen.
Venanidesparmula (Nixon, 1965), new combination
Apantelesparmula Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Notes. Transferred to Venanides based on pronotum laterally with a single furrow, venation of fore wing, smooth propodeum, T1 and T2 shapes, and ovipositor sheaths without setae.
Venanidesplancina (Nixon, 1965)
Apantelesplancina Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: India.
Geographical distribution.OTL.
OTL: China (HN), India.
Venanidespyrogrammae (Nixon, 1965)
Apantelespyrogrammae Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Papua New Guinea.
Geographical distribution.AUS.
AUS: Australia (QLD), Papua New Guinea.
Venanidessupracompressus Fernandez-Triana & van Achterberg, 2017
Venanidessupracompressus Fernandez-Triana & van Achterberg, 2017.
Type information. Holotype female, RMNH (examined). Country of type locality: Yemen.
Geographical distribution.AFR.
AFR: Yemen.
Venanidessymmysta (Nixon, 1965), new combination
Apantelessymmysta Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Notes. Transferred to Venanides based on pronotum laterally with a single furrow, venation of fore wing, smooth propodeum, T1 and T2 shapes, and ovipositor sheaths without setae.
Venanidestenuitergitus Fernandez-Triana & van Achterberg, 2017
Venanidestenuitergitus Fernandez-Triana & van Achterberg, 2017.
Type information. Holotype female, RMNH (examined). Country of type locality: Yemen.
Geographical distribution.AFR.
AFR: Yemen.
Venanidesvanharteni Fernandez-Triana & van Achterberg, 2017
Venanidesvanharteni Fernandez-Triana & van Achterberg, 2017.
Type information. Holotype female, RMNH (examined). Country of type locality: Yemen.
Geographical distribution.AFR.
AFR: Yemen.
Venanidesxeste Mason, 1981
Venanidesxeste Mason, 1981.
Type information. Holotype female, CNC (examined). Country of type locality: Canada.
Geographical distribution.NEA, NEO.
NEA: Canada (MB, ON), USA (AZ, AR, CT, IA, MI, MN, MO, NY, NC, TX); NEO: Brazil (SC), Saint Lucia.
Genus Venanus Mason, 1981
Venanus Mason, 1981: 94. Gender: masculine. Type species: Venanuspinicola Mason, 1981, by original designation.
This genus seems to be restricted to the New World, with most of the eleven described species being found in the Neotropical region. A recent revision of Venanus (Whitfield et al. 2011) covered most of the known species, but we have seen in collections a few additional ones. Known host records include the families Gelechiidae and Gracillariidae. There are 71 DNA-barcode compliant sequences of this genus in BOLD, representing five BINs.
Venanuschilensis Mason, 1981
Venanuschilensis Mason, 1981.
Type information. Holotype male, CNC (examined). Country of type locality: Chile.
Geographical distribution.NEO.
NEO: Chile.
Venanusgreeneyi Whitfield & Arias-Penna, 2011
Venanusgreeneyi Whitfield & Arias-Penna, 2011.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Venanusheberti Fernandez-Triana, 2010
Venanusheberti Fernandez-Triana, 2010.
Type information. Holotype male, CNC (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (NS, PE, QC).
Notes.Mason (1981) described the new genus Venanus, with the type species being Venanuspinicola, a species widespread in Canada, and also Idaho, USA. Mason (1981: 97) reported V.pinicola as parasitizing “Gracillariaasplenifoliella” (Gracillaridae). Fernandez-Triana (2010) considered V.pinicola specimens (sensu Mason) to actually comprise two different species, pinicola (restricted to western Canada and Idaho) and a new species he described as Venanusheberti (from eastern Canada). Some of the pinicola specimens (sensu Mason) were transferred to heberti, and when doing so, Fernandez-Triana (2010) spelled the host name as “Caloptiliaasplenifoliella”; and Fernandez-Triana (2014) repeated that same information. The actual name of the host is Caloptiliaasplenifoliatella (Darlington, 1949), thus, both Mason (1981) and Fernandez-Triana (2010, 2014) spelled the specific name incorrectly. Summarizing, the correct identity of the known hosts for both species of Venanus are: Coleotechnitesmilleri (Busck, 1914) and Coleotechnitesstarki (Freeman, 1957) (both Gelechiidae) for Venanuspinicola; and Caloptiliaasplenifoliatella (Darlington, 1949) for Venanusheberti.
Venanushelavai Mason, 1981
Venanushelavai Mason, 1981.
Type information. Holotype male, CNC (examined). Country of type locality: Colombia.
Geographical distribution.NEO.
NEO: Colombia, Ecuador.
Venanusjohnnyrosalesi Fernandez-Triana & Whitfield, 2014
Venanusjohnnyrosalesi Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Venanuskusikuyllurae Rasmussen & Whitfield, 2011
Venanuskusikuyllurae Rasmussen & Whitfield, 2011.
Type information. Holotype female, MUSM (not examined but original description checked). Country of type locality: Peru.
Geographical distribution.NEO.
NEO: Peru.
Venanusminutalis (Muesebeck, 1958)
Microplitisminutalis Muesebeck, 1958.
Type information. Holotype female, USNM (examined). Country of type locality: Chile.
Geographical distribution.NEO.
NEO: Chile.
Venanusperuensis Mason, 1981
Venanusperuensis Mason, 1981.
Type information. Holotype female, CNC (examined). Country of type locality: Peru.
Geographical distribution.NEO.
NEO: Peru.
Venanuspinicola Mason, 1981
Venanuspinicola Mason, 1981.
Type information. Holotype female, CNC (examined). Country of type locality: Canada.
Geographical distribution.NEA.
NEA: Canada (AB, BC, NS, QC, YT), USA (ID).
Notes. See notes on Venanusheberti above for details on the correct identity of the hosts for these two species of Venanus.
Venanusrandallgarciai Fernandez-Triana & Whitfield, 2014
Venanusrandallgarciai Fernandez-Triana & Whitfield, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Venanusyanayacuensis Arias-Penna & Whitfield, 2011
Venanusyanayacuensis Arias-Penna & Whitfield, 2011.
Type information. Holotype female, USNM (not examined but original description checked). Country of type locality: Ecuador.
Geographical distribution.NEO.
NEO: Ecuador.
Genus Wilkinsonellus Mason, 1981
Wilkinsonellus Mason, 1981: 122. Gender: masculine. Type species: Apantelesiphitus Nixon, 1965, by original designation.
Several recent papers on this genus have increased the total of described species to 23, but there are still many undescribed species in collections. The genus seems to be pantropical. A single host record is known, from Crambidae. There are 55 DNA-barcode compliant sequences of this genus in BOLD, representing 14 BINs.
Wilkinsonellusalexsmithi Arias-Penna & Whitfield, 2013
Wilkinsonellusalexsmithi Arias-Penna & Whitfield, 2013.
Type information. Holotype female, CNC (not examined but original description checked). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Wilkinsonellusamplus Austin & Dangerfield, 1992
Wilkinsonellusamplus Austin & Dangerfield, 1992.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (NT, QLD).
Wilkinsonellusarabicus van Achterberg & Fernandez-Triana, 2017
Wilkinsonellusarabicus van Achterberg & Fernandez-Triana, 2017.
Type information. Holotype female, RMNH (examined). Country of type locality: Yemen.
Geographical distribution.AFR.
AFR: Yemen.
Wilkinsonelluscorpustriacolor Arias-Penna, Zhang & Whitfield, 2014
Wilkinsonelluscorpustriacolor Arias-Penna, Zhang & Whitfield, 2014.
Type information. Holotype female, FNIC (not examined but original description checked). Country of type locality: Fiji.
Geographical distribution.AUS.
AUS: Fiji.
Wilkinsonellusdaira (Nixon, 1965)
Apantelesdaira Nixon, 1965.
Type information. Holotype male, NHMUK (examined). Country of type locality: Papua New Guinea.
Geographical distribution.AUS.
AUS: Papua New Guinea.
Wilkinsonellusfijiensis Arias-Penna, Zhang & Whitfield, 2014
Wilkinsonellusfijiensis Arias-Penna, Zhang & Whitfield, 2014.
Wilkinsonellusfijienis Arias-Penna, Zhang & Whitfield, 2014 [original misspelling].
Type information. Holotype female, FNIC (not examined but original description checked). Country of type locality: Fiji.
Geographical distribution.AUS.
AUS: Fiji.
Wilkinsonellusflavicrus Long & van Achterberg, 2011
Wilkinsonellusflavicrus Long & van Achterberg, 2011.
Type information. Holotype female, IEBR (not examined but original description checked). Country of type locality: Vietnam.
Geographical distribution.OTL.
OTL: Vietnam.
Wilkinsonellusgranulatus Ahmad, Pandey, Haider & Shujauddin, 2005
Wilkinsonellusgranulatus Ahmad, Pandey, Haider & Shujauddin, 2005.
Type information. Holotype female, AMUZ (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Wilkinsonellushenicopus (de Saeger, 1944)
Apanteleshenicopus de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Kenya.
Wilkinsonellusiphitus (Nixon, 1965)
Apantelesiphitus Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: China (HI, TW), Philippines.
Wilkinsonelluskogui Arias-Penna & Whitfield, 2013
Wilkinsonelluskogui Arias-Penna & Whitfield, 2013.
Type information. Holotype male, IAVH (not examined but original description checked). Country of type locality: Colombia.
Geographical distribution.NEO.
NEO: Colombia.
Wilkinsonelluslongicentrus Long & van Achterberg, 2003
Wilkinsonelluslongicentrus Long & van Achterberg, 2003.
Type information. Holotype female, IEBR (not examined but original description checked). Country of type locality: Vietnam.
Geographical distribution.OTL.
OTL: Vietnam.
Wilkinsonellusmasoni Long & van Achterberg, 2011
Wilkinsonellusmasoni Long & van Achterberg, 2011.
Type information. Holotype female, IEBR (not examined but original description checked). Country of type locality: Vietnam.
Geographical distribution.OTL.
OTL: Vietnam.
Wilkinsonellusnarangahus Rousse & Gupta, 2013
Wilkinsonellusnarangahus Rousse & Gupta, 2013.
Type information. Holotype female, MNHN (not examined but original description checked). Country of type locality: Réunion.
Geographical distribution.AFR.
AFR: Réunion.
Wilkinsonellusnescalptura Arias-Penna, Zhang & Whitfield, 2014
Wilkinsonellusnescalptura Arias-Penna, Zhang & Whitfield, 2014.
Type information. Holotype female, FNIC (not examined but original description checked). Country of type locality: Fiji.
Geographical distribution.AUS.
AUS: Fiji.
Wilkinsonellusnigratus Long & van Achterberg, 2011
Wilkinsonellusnigratus Long & van Achterberg, 2011.
Type information. Holotype male, IEBR (not examined but original description checked). Country of type locality: Vietnam.
Geographical distribution.OTL.
OTL: Vietnam.
Wilkinsonellusnigrocentrus Long & van Achterberg, 2011
Wilkinsonellusnigrocentrus Long & van Achterberg, 2011.
Type information. Holotype female, IEBR (not examined but original description checked). Country of type locality: Vietnam.
Geographical distribution.OTL.
OTL: Vietnam.
Wilkinsonelluspanamaensis Arias-Penna & Whitfield, 2013
Wilkinsonelluspanamaensis Arias-Penna & Whitfield, 2013.
Type information. Holotype female, CNC (not examined but original description checked). Country of type locality: Panama.
Geographical distribution.NEO.
NEO: Panama.
Wilkinsonellusparamplus Long & van Achterberg, 2003
Wilkinsonellusparamplus Long & van Achterberg, 2003.
Type information. Holotype female, IEBR (not examined but original description checked). Country of type locality: Vietnam.
Geographical distribution.OTL.
OTL: China (GD, GX), Vietnam.
Wilkinsonellusstriatus Austin & Dangerfield, 1992
Wilkinsonellusstriatus Austin & Dangerfield, 1992.
Type information. Holotype female, ANIC (not examined but original description checked). Country of type locality: Australia.
Geographical distribution.AUS.
AUS: Australia (QLD), Papua New Guinea.
Wilkinsonellusthyone (Nixon, 1965)
Apantelesthyone Nixon, 1965.
Type information. Holotype female, USNM (examined). Country of type locality: Philippines.
Geographical distribution.OTL.
OTL: Philippines.
Wilkinsonellustobiasi Long, 2007
Wilkinsonellustobiasi Long, 2007.
Type information. Holotype female, IEBR (not examined but original description checked). Country of type locality: Vietnam.
Geographical distribution.OTL.
OTL: Vietnam.
Wilkinsonellustomi Austin & Dangerfield, 1992
Wilkinsonellustomi Austin & Dangerfield, 1992.
Type information. Holotype female, AEIC (not examined but original description checked). Country of type locality: Papua New Guinea.
Geographical distribution.AUS.
AUS: Australia (QLD), Papua New Guinea.
Genus Xanthapanteles Whitfield, 1995
Xanthapanteles Whitfield, 1995: 879. Gender: masculine. Type species: Xanthapantelescameronae Whitfield, 1995, by original designation and monotypy.
Only one species is known, from the Neotropics (Whitfield 1995b). No host data are currently available for this genus. There are no DNA barcodes of Xanthapanteles in BOLD.
Xanthapantelescameronae Whitfield, 1995
Xanthapantelescameronae Whitfield, 1995.
Type information. Holotype female, MCZC (not examined but original description checked). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina.
Genus Xanthomicrogaster Cameron, 1911
Xanthomicrogaster Cameron, 1911: 324. Gender: feminine. Type species: Xanthomicrogasterfortipes Cameron, 1911, by subsequent designation (Viereck 1914).
This genus seems to be restricted to the Neotropical region. Apart from the six described species, there are many more undescribed in collections. No host data are currently available for this genus. There are 112 DNA-barcode compliant sequences of this genus in BOLD, representing 23 BINs.
Xanthomicrogasterfortipes Cameron, 1911
Xanthomicrogasterfortipes Cameron, 1911.
Type information. Holotype female, NHMUK (examined). Country of type locality: Guyana.
Geographical distribution.NEO.
NEO: Brazil (MG, PA, SP), Guyana, Suriname.
Xanthomicrogastermaculata Penteado-Dias, Shimabukuro & van Achterberg, 2002
Xanthomicrogastermaculatus Penteado-Dias, Shimabukuro & van Achterberg, 2002.
Type information. Holotype female, DCBU (not examined but original description checked). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (MG, SP).
Notes. The species name must be treated as an adjective and not as a noun (Doug Yanega, pers. comm.) and thus it must match the gender of the genus name.
Xanthomicrogasterotamendi Martínez, 2018
Xanthomicrogasterotamendi Martínez, 2018.
Type information. Holotype female, MACN (not examined but original description checked). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina.
Xanthomicrogasterpelides Nixon, 1965
Xanthomicrogasterpelides Nixon, 1965.
Type information. Holotype female, NHMUK (examined). Country of type locality: Brazil.
Geographical distribution.NEO.
NEO: Brazil (SC).
Xanthomicrogastersayjuhu Martínez, 2018
Xanthomicrogastersayjuhu Martínez, 2018.
Type information. Holotype female, MACN (not examined but original description checked). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina.
Xanthomicrogasterseres Nixon, 1965
Xanthomicrogasterseres Nixon, 1965.
Type information. Holotype male, NHMUK (examined). Country of type locality: Mexico.
Geographical distribution.NEO.
NEO: Mexico.
Genus Ypsilonigaster Fernandez-Triana, 2018
Ypsilonigaster Fernandez-Triana, 2018: 116. Gender: feminine. Type species: Ypsilonigastertiger Fernandez-Triana & Boudreault, 2018, by original designation.
Six species were recently described (Fernandez-Triana and Boudreault 2018), but we have seen a few undescribed ones in collections. No host data are currently available for this genus. There are two DNA-barcode compliant sequences of this genus in BOLD, representing two BINs (although those sequences have not been identified in BOLD as belonging to Ypsilonigaster, see Fernandez-Triana and Boudreault 2018 for that). In the original description of Ypsilonigaster, its gender was incorrectly stated to be neuter (Fernandez-Triana & Boudreault, 2018: 117); however, all genera ending in gaster are feminine, without exception (Doug Yanega, pers. comm., see also Article 30.1.2 of the ICZN), so here we correct that previous mistake.
Ypsilonigasterbumbana (de Saeger, 1942)
Microgasterbumbana de Saeger, 1942.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Ypsilonigasternaturalis Fernandez-Triana & Boudreault, 2018
Ypsilonigasternaturalis Fernandez-Triana & Boudreault, 2018.
Type information. Holotype female, RMNH (examined). Country of type locality: Malaysia.
Geographical distribution.OTL.
OTL: Malaysia.
Ypsilonigasterpteroloba (de Saeger, 1944)
Microgasterpteroloba de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Ypsilonigastersharkeyi Fernandez-Triana & Boudreault, 2018
Ypsilonigastersharkeyi Fernandez-Triana & Boudreault, 2018.
Type information. Holotype male, CNC (examined). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of the Congo.
Ypsilonigastertiger Fernandez-Triana & Boudreault, 2018
Ypsilonigastertiger Fernandez-Triana & Boudreault, 2018.
Type information. Holotype female, QSBG (examined). Country of type locality: Thailand.
Geographical distribution.OTL.
OTL: Thailand.
Ypsilonigasterzuparkoi Fernandez-Triana & Boudreault, 2018
Ypsilonigasterzuparkoi Fernandez-Triana & Boudreault, 2018.
Type information. Holotype male, CAS (examined). Country of type locality: Madagascar.
Geographical distribution.AFR.
AFR: Madagascar.
Genus Zachterbergius Fernandez-Triana, 2018
Zachterbergius Fernandez-Triana, 2018: 129. Gender: neuter. Type species: Zachterbergiustenuitergum Fernandez-Triana & Boudreault, 2018, by original designation.
Only one species is known, from the Oriental region. No host data are currently available for this genus. There is a single DNA-barcode compliant sequence of this genus in BOLD, representing one BIN (although that sequence has not been identified in BOLD as belonging to Zachterbergius, see Fernandez-Triana and Boudreault 2018 for that).
Zachterbergiustenuitergum Fernandez-Triana & Boudreault, 2018
Zachterbergiustenuitergum Fernandez-Triana & Boudreault, 2018.
Type information. Holotype male, QSBG (examined). Country of type locality: Thailand.
Geographical distribution.OTL.
OTL: Thailand.
Species inquirendae
Below we treat 36 species for most of which we could not examine the types or any other specimens; the original descriptions, if available to us, were insufficient to determine a correct generic placement (in the case of Apantelessanctivicenti Ashmead, 1900, the type was in poor condition; and in the case of Apantelesanapiedrae Fernandez-Triana, 2014 more studies on the holotype and paratypes are required). Until types of those species can be examined and/or more studies are done, we consider them here as species inquirendae – they can almost certainly be placed with further research. Additionally, Gupta (2013a: 451) had proposed two Indian species of Microplitis to be incertae sedis; however, in this paper we consider that one of those species actually belongs to Diolcogaster (see D.dipika above, p 398, 399), whereas the other should be listed as an unavailable name (see Microplitisbageshri in the section Other unavailable names below, p 1033).
? Apantelesacaciae Risbec, 1951
Apantelesacaciae Risbec, 1951.
Type information. Holotype male, depository unknown (not examined but original description checked). Country of type locality: Senegal.
Geographical distribution.AFR.
AFR: Senegal.
Notes. This species is likely not an Apanteles, but the original description, based on a single male specimen, is not clear enough to determine the correct generic placement.
? Apantelesahmednagarensis Kurhade & Nikam, 1997
Apantelesahmednagarensis Kurhade & Nikam, 1997.
Type information. Holotype female, BAMU (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. The original description does not provide enough details to confirm the species placement. The low-quality figures provided seem to indicate that the propodeum and T1 are not as in Apanteles.
? Apantelesanapiedrae Fernandez-Triana, 2014
Apantelesanapiedrae Fernandez-Triana, 2014.
Type information. Holotype female, CNC (examined). Country of type locality: Costa Rica.
Geographical distribution.NEO.
NEO: Costa Rica.
Notes. This species is likely not an Apanteles, as stated even in the original description (Fernandez-Triana et al. 2014e). We have re-examined the holotype and many paratypes in the CNC; in addition to the inflexible hypopygium and relatively short ovipositor and sheaths (noted in the original description), we have also found that the vannal lobe is mostly setose, which would exclude this species from Apanteles. However, we cannot conclude on which genus would be the best placement at the moment, as other morphological traits are variable between Dolichogenidea, Pholetesor or even Parapanteles; and molecular data (DNA barcodes) is not conclusive either.
? Apantelesautomeridis Brèthes, 1926
Apantelesautomeridis Brèthes, 1926.
Type information. Type and depository unknown (not examined). Country of type locality: Colombia.
Geographical distribution.NEO.
NEO: Colombia.
Notes.Shenefelt (1972: 450) could not find the original description or the type material and neither could we, so we cannot confirm the generic placement of this species. Here we consider the type and depository of this species as unknown.
? Apantelesbarrosi Porter, 1926
Apantelesbarrosi Porter, 1926.
Type information. Holotype female, depository unknown (not examined but original description checked). Country of type locality: Chile.
Geographical distribution.NEO.
NEO: Chile.
Notes. The short original description is insufficient to determine the correct generic placement, but it is clear that this species does not belong to Apanteles. Porter (1926: 143) stated that the species has a very short ovipositor and it is morphologically related to Apantelesriverae Porter (a species transferred to Cotesia in our paper, see under that species, p 351, 352).
? Apantelesbauhiniae Risbec, 1951
Apantelesbauhiniae Risbec, 1951.
Type information. Holotype male, depository unknown (not examined but original description checked). Country of type locality: Senegal.
Geographical distribution.AFR.
AFR: Senegal.
Notes. The original description is not detailed enough to determine the correct generic placement. The species does not seem to belong to Apanteles, based on the illustration of the propodeum with a complete median carina bisecting a complete areola (Risbec 1951: 460).
? Apantelescamachoi Silva Figueroa, 1917
Apantelescamachoi Silva Figueroa, 1917.
Type information. Holotype female, MNNC (not examined but original description checked). Country of type locality: Chile.
Geographical distribution.NEO.
NEO: Chile.
Notes. The original description is not detailed enough to determine the correct generic placement of this species.
? Apantelesdeepica Rao & Chalikwar, 1971
Apantelesdeepica Rao & Chalikwar, 1971.
Type information. Holotype male, BAMU (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. The original description (based on three male specimens) is not detailed enough to determine the correct generic placement, but this species does not belong to Apanteles; it could belong either to Glyptapanteles or Sathon.
? Apantelesdirphiae Silva Figueroa, 1917
Apantelesdirphiae Silva Figueroa, 1917.
Type information. Syntypes female and male, MNNC (not examined but original description checked). Country of type locality: Chile.
Geographical distribution.NEO.
NEO: Chile.
Notes. The original description is not detailed enough to determine the correct generic placement of this species.
? Apantelesespinosai Porter, 1920
Apantelesespinosai Porter, 1920.
Type information. Type and depository unknown (not examined but original description checked). Country of type locality: Chile.
Geographical distribution.NEO.
NEO: Chile.
Notes. The original description is not detailed enough to determine the correct generic placement of this species, but it is evident that is not Apanteles, because it mentions a median longitudinal carina on the propodeum. The only known specimen, not even clear if it is a female or male, was deposited in Porter’s personal collection, but we are not aware of the current depository of that collection or if the specimen still exists.
? Apanteleshoffmanni Silva Figueroa, 1917
Apanteleshoffmanni Silva Figueroa, 1917.
Type information. Holotype female, MNNC (not examined but original description checked). Country of type locality: Chile.
Geographical distribution.NEO.
NEO: Chile.
Notes. The original description is not detailed enough to determine the correct generic placement of this species.
? Apanteleslaorae Porter, 1921
Apanteleslaorae Porter, 1921.
Type information. Syntypes female and male, depository unknown (not examined but original description checked). Country of type locality: Chile.
Geographical distribution.NEO.
NEO: Chile.
Notes. The original description is not detailed enough to determine the correct generic placement of this species. There are no details of the number of specimens studied or the depository, but Porter mentions many specimens from the host larva, so we infer that the type series must have included both females and males.
? Apanteleslatiannulatus (Cameron, 1910)
Xestapanteleslatiannulatus Cameron, 1910.
Type information. Holotype female, ZMHB (not examined but subsequent treatment of the species checked). Country of type locality: Mozambique.
Geographical distribution.AFR.
AFR: Mozambique.
Notes.Wilkinson (1932a: 324) explained why it may never be possible to establish the status of this species, due to the very poor condition of the two known specimens.
? Apantelesmontanus de Saeger, 1944
Apantelesmontanus de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. The original description is not detailed enough (or characters are uninformative) to determine the correct generic placement of this species. It does not belong to Apanteles because a median longitudinal carina on the propodeum is mentioned. Other features such as a pleated hypopygium and relatively long ovipositor sheaths, would suggest Choeras as the likely genus, but the shape of T2 would be very unusual (as compared to other known species in that genus).
? Apantelesnecator (Bechstein & Scharfenberg, 1805)
Ichneumonnecator Bechstein & Scharfenberg, 1805.
Microgasternecatrix Schulz, 1906.
Ichneumonnecator Bechstein & Scharfenberg, 1805 [junior primary homonym of Ichneumonnecator Fabricius, 1777].
Type information. Holotype male, ZMUC (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Germany.
Notes. Our species concept is based on descriptions provided in the historical literature (Haliday 1834, Fahringer 1837). Both authors mentioned the ovipositor as not visible (or hidden), an indication that the ovipositor and sheaths are very short, which excludes necator from Apanteles. The rest of the information provided in those papers is too general, e.g., the description of antenna length, colour of body, wings and veins, to help determine the correct generic placement. Based on the general colour of the body, host data (Pterophoridae) and the number of wasp cocoons (forming a cocoon mass), this species could be placed either in Cotesia (most likely) or Glyptapanteles.
? Apantelesnigripes (Ratzeburg, 1844)
Microgasternigripes Ratzeburg, 1844.
Type information. Type and depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Bulgaria, Germany, Latvia.
Notes. Like many of Ratzeburg’s types, the nigripes type is lost or presumed destroyed. Shenefelt (1972) treated this species as Apanteles (sensu lato), but the actual generic placement of this species is unknown. Besides Germany, supposedly the country of the type locality, the other countries cited for this species, Bulgaria and Latvia (see Yu et al. 2016 for details) should be considered as suspicious. Broad et al. (2016) excluded the species from UK, based on Papp (1988) and van Achterberg (2003), a decision we accept and follow here.
? Apantelesreedi Porter, 1920
Apantelesreedi Porter, 1920.
Type information. Type and depository unknown (not examined but original description checked). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina, Chile.
Notes. This species is not an Apanteles, based on the very short ovipositor. The original description is not detailed enough to allow us to determine the correct generic placement.
? Apantelessanctivicenti Ashmead, 1900
Apantelessanctivicenti Ashmead, 1900.
Rhygoplitissanctivincenti Ashmead, 1900. See Fernandez-Triana et al. (2014e).
Type information. Holotype male, NHMUK (examined). Country of type locality: Saint Vincent.
Geographical distribution.NEO.
NEO: Saint Vincent.
Notes.Fernandez-Triana et al. (2014e) discussed in detail problems with this species name and tentatively classified it in Rhygoplitis. At the time, the type was thought to be lost and all of the assumptions were based on the original description. However, we recently found the type specimen in NHMUK and have been able to examine it in detail. Unfortunately a large drop of glue covers most of the propodeum and T1 and thus it is not possible to determine with certainty its generic status. But it is now evident that the species does not belong to Rhygoplitis as it does not have visible notauli, T2 is smooth, and vein R1 in the fore wing is relatively very large (longer than pterostigma length and several times longer than the distance between its end and the end of vein RS). Those features are unlike any known species of Rhygoplitis (a genus characterized, among other things, by strong notauli, T2 strongly sculptured, and relatively short vein R1 in fore wing). Based on the mostly smooth anteromesoscutum, thin scutoscutellar sulcus, T1 shape and hind wing with vannal lobe apparently fully setose (but vannal lobe not totally clear because of glue obscuring its view) this species could be placed within Dolichogenidea (but it could also be Apanteles if the hind wing vannal lobe is interpreted differently). The main problem in placing this species in Dolichogenidea (or Apanteles for that matter) is that the original description mentions a median longitudinal carina in the propodeum, which would exclude it from either genus. But it is possible that Ashmead (1900) misinterpreted the presence of a median longitudinal carina (indeed, if he examined the specimen after it was glued, it would have not been possible to see it, especially with the microscope available at that time). Another possibility would be Pseudapanteles (a genus with a median longitudinal carina), but what can be seen from propodeum and T1 (both relatively sculptured) does not match well with our current concept of Pseudapanteles. Because of that, it is not possible to establish with certainty the generic identity of sanctivincenti until the type is unglued from the pin for re-examination and/or DNA is extracted.
? Apantelesshrii Sathe & Ingawale, 1995
Apantelesshrii Sathe & Ingawale, 1995.
Type information. Holotype female, NZSI (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. The original description and illustrations are very deficient and the elements provided therein do not allow to establish with any accuracy to which genus the species belongs, but it is very clear that this species does not belong to Apanteles. The described sculpture of propodeum, unpleated hypopygium, and length of ovipositor sheaths all suggest it could be Parapanteles; but the authors also stated that the antennal flagellomeres are three segmented and that the ovipositor sheaths have no setae, both features not known in any described species of Parapanteles. The authors mentioned that the new taxon is similar to two previously described Microgastrinae species, one of which belongs to Cotesia and the other to Dolichogenidea (two unrelated genera with many different morphological features). The illustrations provided are somewhat inaccurate, e.g., the venation of the hind wing, and the proportions of the metacoxa and metafemur are different in the drawing as compared to what is detailed in the written description. The specimens on which the species description was based (45 female and 20 male specimens) were all reared from Eariasvittella (Fabricius, 1794) (Nolidae). That host record cannot be attributed unequivocally to any specific genus of Microgastrinae (four genera: Apanteles, Cotesia, Diolcogaster, and Dolichogenidea all have species previously recorded as parasitizing the genus Earias). All of the above evidence indicates a rather poorly characterized species with insufficient information to establish its generic placement, other than not belonging in Apanteles.
? Apantelestineaephagus Bhatnagar, 1950
Apantelestineaephagus Bhatnagar, 1950.
Type information. Holotype female, INPC (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. The original description is not detailed enough to determine the correct generic placement; we consider that it could be either Parapanteles, Apanteles or Dolichogenidea. The year of publication of the Bhatnagar paper was until recently commonly cited as 1948 and/or 1950 (e.g., Chen and Song 2004, Yu et al. 2016), probably following Shenefelt (1972) who referred to this paper as “Bhatnagar (1948) 1950”. While the intended year for Volume X, Parts I & II of the Indian Journal of Entomology was 1948, the actual dates of publication were June 1950 (Part I) and October 1950 (Part II), as clearly shown on the cover page of the Volume, which we have checked. Because the dates of publication are the ones to be considered, and for the sake of clarity, we hereby revise the species year of description to 1950.
? Choeraspappi Narendran, 1998
Choeraspappi Narendran, 1998.
Type information. Holotype female, RMNH (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes. The original description is not detailed enough to determine the correct generic placement. But it mentions a very short ovipositor (half metacoxal length), which indicates that this species does not belong in Choeras. This is also corroborated by the illustration of veins r and 2RS in the fore wing (Narendran 1998: fig. 5), which do not look like those of any other described species of Choeras.
? Cotesiapicipes (Bouché, 1834)
Microgasterpicipes Bouché, 1834.
Type information. Type and depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: unknown.
Geographical distribution.PAL.
PAL: Azerbaijan, France, Germany, Hungary, Italy, Russia (SPE, VLA), Tajikistan, Uzbekistan.
Notes. The type of picipes is presumed to be lost. Broad et al (2016), based on van Achterberg (2003) not citing picipes for the Western Palearctic, excluded the species from their United Kingdom list. Broad et al (2016) considered that picipes might be a synonym or a nomen dubium. However, at least two sources (Belokobylskij et al. 2003, Papp 2005) considered the species as valid, and actually belonging to Cotesia, in contrast with Papp (1987a), who provisionally considered picipes to be a synonym of Apantelesxanthostigmus.
? Glyptapantelesconopomorphae Tsang & You, 2007
Glyptapantelesconopomorphae Tsang & You, 2007.
Type information. Holotype female, SCAC (not examined but original description checked). Country of type locality: China.
Geographical distribution.OTL.
OTL: China (GD).
Notes. Based on the illustrations from the original description, this species is not likely to be Glyptapanteles (the ovipositor sheaths are relatively long, the propodeum shows a partial areola defined apically, and T2 is relatively transverse, unlike most described Glyptapanteles). We believe this species could be better placed in Dolichogenidea; however, the vannal lobe in the hind wing and the hypopygium are not clearly visible to help determine the correct generic placement.
? Microgasteralvearifex (Schrank, 1781)
Ichneumonalvearifex Schrank, 1781.
Ichneumonalveariformis Geoffroy, 1785.
Type information. Type and depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: unknown.
Geographical distribution.PAL.
PAL: Austria, Germany, Italy.
Notes. We read the very short comments and/or descriptions in Gmelin (1790: 2712), Haliday (1834: 257) and Fahringer (1837: 368), mostly reproducing what Schrank (1781) wrote about the species. The species was considered to be part of “Microgastersensu lato, including Apanteles and Microplitis” (see Fahringer (1937), which would include most of the Microgastrinae at the time. The available details, about the cocoons (white and forming a mass like a honeycomb) as well as general colour of the adult wasp (body black, legs reddish), are not sufficient to place this species correctly to genus but seem to indicate that is probably not Microgaster. For example, the shape of the cocoon mass is stated to be similar to those of Ichneumonalvearius Fabricius, 1798 (a species currently in Diolcogaster) and we are aware of a similar cocoon mass made by Sathonfalcatus (Nees, 1834).
? Microgasterannulipesiduo Shenefelt, 1973
Microgasterannulipesiduo Shenefelt, 1973.
Microgasterannulipes Motschoulsky, 1863 [primary homonym of Microgasterannulipes Curtis, 1830].
Type information. Holotype male, depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Sri Lanka.
Geographical distribution.OTL.
OTL: Sri Lanka.
Notes.Wilkinson (1927: 173) thought this species did not belong in Microgaster, a statement we agree with.
? Microgasterduvauae Brèthes, 1916
Microgasterduvauae Brèthes, 1916.
Type information. Holotype female, MACN (not examined but original description checked). Country of type locality: Argentina.
Geographical distribution.NEO.
NEO: Argentina.
Notes. The original description is not clear enough to conclude, but it seems that duvauae does not belong to Microgaster, based on details of T1 and T2, body size, wing length, and presumed host. Until further study of the type is done, it is not possible to establish with certainty the generic placement of the species.
? Microgastereurygaster (Cameron, 1911)
Apanteleseurygaster Cameron, 1911.
Type information. Holotype male, TMSA (not examined but subsequent treatment of the species checked). Country of type locality: South Africa.
Geographical distribution.AFR.
AFR: South Africa.
Notes. Based on the original description of the holotype male (only known specimen) and the subsequent treatment (Cameron 1911c, Wilkinson 1929a, de Saeger 1944) this species is clearly not a Microgaster. However, it is not possible to place it in any genus with any degree of certainty, as the description is not conclusive.
? Microgastermortuorum (Rossi, 1792)
Ichneumonmortuorum Rossi, 1792.
Type information. Type and depository unknown (not examined). Country of type locality: unknown.
Geographical distribution.PAL.
PAL: Italy.
Notes.Yu et al. (2012) list this species with a question mark regarding its status and generic placement. Other than the original description, which we have not been able to see, very few references, all of them catalogues, treat this species.
? Microgasternigricornis Motschoulsky, 1863
Microgasternigricornis Motschoulsky, 1863.
Type information. Type and depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Sri Lanka.
Geographical distribution.OTL.
OTL: Sri Lanka.
Notes.Wilkinson (1927: 173) thought this species did not possibly belong in Microgaster, a statement we agree with. Shenefelt (1973: 718) added a male sign when referring to the original description of the species, but it is not clear if that means a single specimen (which would then be considered as the holotype), or a series of male specimens (which would then be syntypes); thus, we consider the type status as unknown for the time being. Based on the key by Wilkinson (1927), we suspect that this species does not belong to Microgaster, but until further study(ies) of the type(s), it is not possible to establish with certainty the generic placement of the species.
? Microgasterpictipes Marshall, 1898
Microgasterpictipes Marshall, 1898.
Type information. Holotype male, depository unknown (not examined but original description checked). Country of type locality: Spain.
Geographical distribution.PAL.
PAL: France, Spain.
Notes. Based on the original description, this species is not Microgaster, as T2 is relatively very short (half the length of T3) and T2 has oblique divergent grooves delimiting a triangular area. The original description, and a similar translation by Telenga (1955) are not clear enough to determine if this species should be better placed in Microplitis or Diolcogaster (or even Rasivalva).
? Microgasterpinos Cresson, 1865
Microgasterpinos Cresson, 1865.
Type information. Holotype female, ANSP (not examined but original description checked). Country of type locality: Cuba.
Geographical distribution.NEO.
NEO: Cuba.
Notes. The original description is not clear enough to determine the correct generic placement. Cresson (1865: 67) considered the fore wing venation of pinos to be similar to that of Microgastermarginiventris Cresson (i.e., without an areolet), a species currently classified in Cotesia. This strongly suggest that pinos does not belong in Microgaster (in the modern sense), as all Microgaster have a large areolet whereas all Cotesia lack an areolet. Muesebeck (1921: 11), even though he did not see the type of pinos, placed it within Apanteles (which, at the time included Cotesia, but not Microgaster). After analyzing all available evidence, we conclude that pinos very likely belongs to one of the Apantelessensu lato genera.
? Microgasterruficoxis Ruthe, 1858
Microgasterruficoxis Ruthe, 1858.
Type information. Type and depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: Germany.
Geographical distribution.PAL.
PAL: Germany.
Notes. The original description is not sufficiently detailed to determine the correct generic placement. Our species concept is based on Telenga (1955). This species is very unlikely to belong to Microgaster, as the metacoxa is described as very long, half the metasoma length. It could likely belong to Diolcogaster.
? Microplitisbambusanus de Saeger, 1944
Microplitisbambusanus de Saeger, 1944.
Type information. Holotype female, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo, Rwanda.
Notes. This species almost certainly does not belong in Microplitis. The original description provides some features that suggest it could belong to Jenopappius, but other characters are slightly different.
? Microplitisisis de Saeger, 1944
Microplitisisis de Saeger, 1944.
Type information. Holotype male, RMCA (not examined but original description checked). Country of type locality: Democratic Republic of Congo.
Geographical distribution.AFR.
AFR: Democratic Republic of Congo.
Notes. This species almost certainly does not belong in Microplitis. The original description provides some features that suggest it could belong to Alloplitis, but other characters are slightly different.
? Promicrogastersaraswatii Sathe & Bhoje, 1998
Promicrogastersaraswatii Sathe & Bhoje, 1998.
Type information. Holotype female, depository unknown (not examined but original description checked). Country of type locality: India.
Geographical distribution.OTL.
OTL: India.
Notes.Fernandez-Triana et al. (2016b) provided several reasons to treat this species as incertae sedis; however, here we think it is more appropriate to consider it as a species inquirenda. The host record associated with this species in the original description is highly suspicious.
? Venanidesmoldavicus (Tobias, 1975)
Apantelesmoldavicus Tobias, 1975.
Type information. Holotype female, ZIN (not examined but subsequent treatment of the species checked). Country of type locality: Moldova.
Geographical distribution.PAL.
PAL: Armenia, Korea, Moldova, Russia (VOR), Slovakia, Ukraine, United Kingdom.
Notes. The drawings in Tobias (1975, 1986) suggest that this species belongs to Venanides, the same generic placement reported by Capek and Lukas (1989). However, Papp (1988, 1990b), Belokobylskij et al. (2019), Shaw (2012b), and Broad et al. (2016) placed it in Pholetesor although the latter two papers considered that as a provisional or even questionable generic placement. Specimens of moldavicus we examined seem to fit better within the Cotesini group (sensuMason 1981), which contains genera such as Venanides and Glyptapanteles (two genera that we consider are the best candidates for moldavicus). Morphological evidence is not sufficient to determine the correct generic placement but ongoing molecular studies of those specimens should help determine this.
Nomina dubia
Apantelesanomalon (Curtis, 1830)
Microgasteranomalon Curtis, 1830.
Type information. Type unknown, MVMMA (not examined but subsequent treatment of the species checked). Country of type locality: United Kingdom.
Geographical distribution.PAL.
PAL: United Kingdom.
Notes.Shenefelt (1972: 443) gave England as the country where this species is found, information accepted by most researchers afterwards (see Yu et al. 2016 for complete list of historical references). However, Broad et al (2016) did not consider this species to be present in the United Kingdom, adding the following: “This name appeared in Huddleston (1978) but is not listed by Papp (1988) or van Achterberg (2003c) and remains uninterpreted”. Because this species had been only recorded from the United Kingdom, its status will require further investigation.
Cotesiasessilis (Geoffroy, 1785)
Evaniasessilis Geoffroy, 1785.
Evaniasessilis (Fabricius, 1793).
Apantelestetrica (Reinhard, 1880).
Microgasteropacula (Thomson, 1895).
Type information. Type and depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: unknown.
Geographical distribution.PAL.
PAL: Armenia, Austria, Azerbaijan, Belarus, Belgium, Croatia, Czech Republic, Estonia, Finland, France, Germany, Greece, Hungary, Iran, Ireland, Italy, Kazakhstan, Latvia, Lithuania, Moldova, Norway, Poland, Romania, Russia (ALT, DA, KGD, KDA, MOS, PRI, RYA, SPE, SAR, VLA, VOR, YAR), Serbia, Sweden, Switzerland, Tajikistan, Turkey, Uzbekistan.
Notes. The name sessilis has been interpreted in different ways: a) Yu et al. (2016) considered it a valid species, with both C.juniperatae (Bouché, 1834) and C.tetrica (Reinhard, 1880) as its synonyms; b) Papp (1988) and Kotenko (2007) considered tetrica as a valid species, with sessilis as its synonym, with a question mark; c) Belokobylskij et al. (2003) deemed juniperatae as a valid species, with sessilis as its synonym; and d) Broad et al (2016) deemed juniperatae and tetrica to be a valid species, but did not list sessilis as a synonym of either. With the type and depository unknown, and the evidence available to us being contradictory (for more details see our Notes under Cotesiabrachycera in the checklist above, p 285–287) we consider it impossible to conclude on the status of the sessilis name for the time being and thus consider it as a nomen dubium. The distribution of sessilis detailed above is taken from Yu et al. (2016), which is a compilation of historical references; however, that is very likely to be inaccurate, due to the many potential species linked to this name over the years.
Microgastersubcutanea (Linnaeus, 1758)
Ichneumonsubcutaneus Linnaeus, 1758.
Type information. Type and depository unknown (not examined but subsequent treatment of the species checked). Country of type locality: unknown.
Geographical distribution.PAL.
PAL: Finland, Norway.
Notes. We have studied a) the original description (Linnaeus 1758: 568); b) a lateral habitus of the species illustrated in DeGeer (1752, plate 30, figure 21), a paper that predates Linnaeus work, but which is supposed to be the source used by Linnaeus to describe the species (see Fitton 1978: 379 for a discussion on that topic); and c) Zetterstedt’s (1838: 404–405) redescription of the species. The illustration from DeGeer (1752; fig. 21) indeed seems to represent a braconid wasp (as recognized by Fitton 1978), but it does not look like a Microgastrinae, as there appears to be a closed marginal cell in the fore wing (defined by a complete vein RS) and the vein M is also very long, almost reaching the apex of the wing. That same illustration also shows what appears to be an elongate glossa (not common but present in a few species of several genera in Microgastrinae), and the overall appearance of the metasoma looks somewhat different from a typical microgastrine wasp; however, we are hesitant to make a decision based just on an old drawing which may not be accurate enough to be meaningful. The other source we read, the description from Zetterstedt (1838), is actually more in line with the Microgastrinae concept of that time (where all species were considered to belong to the genus Microgaster), and it seems to support the idea of the species belonging to that subfamily. With the evidence available to us being contradictory and relatively very old (a drawing from 1752, a description from 1838) we consider it impossible to conclude on the status of this species for the time being, thus we are here following Fitton (1978) who considered it as a nomen dubium. It is also worth mentioning that, according to Fitton (1978), material from the species can still exist in the NHRS in Stockholm and its future study may clarify the status of this name.
Nomina nuda
Apantelesargentinensis Blanchard, 1937
Apantelesargentinensis Blanchard, 1937.
Notes. This species name is mentioned in Bourquin (1937) as a manuscript name and must be considered as nomen nudum (see also de Santis 1967a).
Apantelesdeltinea Blanchard, 1961
Apantelesdeltinea Blanchard, 1961.
Notes. This species name is mentioned in Bourquin (1961) as a manuscript name and must be considered as nomen nudum (see also de Santis 1967a).
Apantelesgeometraephagus Blanchard, 1939
Apantelesgeometraephagus Blanchard, 1939.
Notes. This species name is mentioned in Blanchard (1939) as a manuscript name and must be considered as nomen nudum (see also de Santis 1967a).
Apanteleskoehleri Blanchard, 1942
Apantelesgeometraephagus Blanchard, 1942.
Notes. This species name is mentioned in Blanchard (1942b) as a manuscript name and must be considered as nomen nudum (see also de Santis 1967a).
Apantelesmysticus Blanchard, 1961
Apantelesmysticus Blanchard, 1961.
Notes. This species name is mentioned in Bourquin (1961) as a manuscript name and must be considered as nomen nudum (see also de Santis 1967a).
Apantelesplatystigma Blanchard, 1938
Apantelesplatystigma Blanchard, 1938.
Notes. This species name is mentioned in Blanchard (1938) as a manuscript name and must be considered as nomen nudum (see also de Santis 1967a).
Apantelessericeoneesi Papp, 1974
Apantelessericeoneesi Papp 1974.
Notes. This name was mentioned in two papers by Papp (1974c, 1976a). In both cases the name is only cited in the caption of figure 54, a drawing of T1–T3. No other details, description, or depository are available, and thus the species name is to be treated as a nomen nudum. In the CNC there is a female specimen donated by Papp with the same species name, and the specimen metasoma agrees with the drawing in Papp (1974c, 1976a). The CNC specimen was sampled for DNA barcoding, and the resulting, partial sequence (144 base pairs) is deposited in BOLD (voucher code CNCHYM 00707, sequence code: HYCNE518-11).
Apantelesspeocropiae Blanchard, 1941
Apantelesspeocropiae Blanchard, 1941.
Notes. This species name is mentioned in de Santis (1941) as a manuscript name and must be considered as nomen nudum (see also de Santis 1967a).
Apantelesveronesi Blanchard, 1940
Apantelesveronesi Blanchard, 1940
Notes. This species name is mentioned in Blanchard (1940) as a manuscript name and must be considered as nomen nudum (see also de Santis 1967a).
Cotesiaferventis Kotenko, 2007
Cotesiaferventis Kotenko, 2007.
Notes.Kotenko (2007a: 185) mentioned this name as part of his treatment of Microgastrinae of the Russian Far East. However, the name is not accompanied by any description or any other detail.
Glyptapantelesobvius Kotenko, 2007
Glyptapantelesobvius Kotenko, 2007.
Notes.Kotenko (2007a: 185) mentioned this name as part of his treatment of Microgastrinae of the Russian Far East. However, the name is not accompanied by any description or any other detail.
Glyptapantelesurios Kotenko, 2007
Glyptapantelesurios Kotenko, 2007.
Notes.Kotenko (2007a: 185) mentioned this name as part of his treatment of Microgastrinae of the Russian Far East. However, the name is not accompanied by any description or any other detail.
Microgastereuchthoniae Blanchard, 1939
Microgastereuchthoniae Blanchard, 1939.
Notes. This species name is mentioned in Bourquin (1939) as a manuscript name and must be considered as nomen nudum (see also de Santis 1967a).
Microplitisparapsidalis Blanchard, 1950
Microplitisparapsidalis Blanchard, 1950.
Notes. This species name is mentioned in Ratkovich (1950) as a manuscript name and must be considered as nomen nudum (see also de Santis 1967a).
Other unavailable names
Below we list 38 species names that were described in post 1999 publications that did not state the type depository – thus they do not fulfill the requirements of ICZN Article 16.4.2 and must be considered as unavailable names. Additionally, in BOLD (http://v4.boldsystems.org/), there are some Microgastrinae sequences with associated names that have never been described in a publication and do not fulfill most of the requirements of ICZN Article 16 to be considered as available names (most of those cases are in the genus Glyptapanteles). However, we do not list those names here because they have never been published (BOLD, being an online database, is not considered to be a publication, sensuICZN Article 8 “What constitutes published work”), but we caution against using those names in future publications, as currently they cannot be considered as available.
Apantelesindica Chougale, 2016.
Notes. The type and depository are not specified in the original publication.
Apantelesmultani Sathe, Inamdar & Dawale, 2003.
Notes. The depository of the type is not specified in the original publication.
Cotesiaanari Sathe & Bhoje, 2000.
Notes. The depository of the type is not specified in the original publication.
Cotesiaarachi Sathe & Bhoje, 2000.
Notes. The depository of the type is not specified in the original publication.
Cotesiabazari Sathe & Bhoje, 2000.
Notes. The depository of the type is not specified in the original publication
Cotesiachiloi Sathe & Bhoje, 2000 .
Notes. The depository of the type is not specified in the original publication.
Cotesiahandhwani Sathe, Inamdar & Dawale, 2003.
Notes. The depository of the type is not specified in the original publication.
Cotesiajanati Sathe & Bhoje, 2000.
Notes. The depository of the type is not specified in the original publication.
Cotesiamangiferi Sathe & Bhoje, 2000.
Notes. The depository of the type is not specified in the original publication.
Cotesiaparnari Sathe & Bhoje, 2000.
Notes. The depository of the type is not specified in the original publication.
Cotesiasunflowari Sathe & Bhoje, 2000.
Notes. The depository of the type is not specified in the original publication.
Cotesiatuski Sathe & Bhoje, 2000.
Notes. The depository of the type is not specified in the original publication.
Dolichogenideabageshri Sathe, Inamdar & Dawale, 2003.
Notes. The depository of the type is not specified in the original publication.
Dolichogenideadarbari Sathe, Inamdar & Dawale, 2003.
Notes. The depository of the type is not specified in the original publication.
Dolichogenideaexiguvi Sathe & Bhoje, 2000.
Notes. The depository of the type is not specified in the original publication.
Dolichogenidealycopersi Sathe & Bhoje, 2000.
Notes. The depository of the type is not specified in the original publication.
Dolichogenideamythimna Sathe & Bhoje, 2000.
Notes. The depository of the type is not specified in the original publication.
Dolichogenideaoryzae Bhoje & Sathe, 2002.
Notes. The depository of the type is not specified in the original publication. Additionally, this species name is a secondary homonym of Dolichogenideaoryzae Walker, 1994.
Dolichogenideaparijatki Sathe & Rokade, 2005.
Notes. The depository of the type is not specified in the original publication.
Dolichogenidearevatl Sathe & Rokade, 2005.
Notes. The depository of the type is not specified in the original publication.
Dolichogenideasathei Sathe & Rokade, 2005.
Notes. The depository of the type is not specified in the original publication.
Dolichogenideasushili Bhoje & Sathe, 2002.
Notes. The depository of the type is not specified in the original publication.
Dolichogenideasunflowari Sathe & Bhoje, 2000.
Notes. The depository of the type is not specified in the original publication.
Dolichogenideatarvadi Sathe & Rokade, 2005.
Notes. The depository of the type is not specified in the original publication.
Dolichogenideaujlai Sathe & Rokade, 2005.
Notes. The depository of the type is not specified in the original publication.
Glyptapantelesbhupali Sathe, Inamdar & Dawale, 2003.
Notes. The depository of the type is not specified in the original publication.
Glyptapantelesmalshri Sathe, Inamdar & Dawale, 2003.
Notes. This same species was described previously by two of the authors (as Glyptapantelesmalshri Sathe & Inamdar, 1991; that species is valid and is treated in this paper – see notes under Cotesiamalshri above for more details on that species, p 328). In any case, the name Glyptapantelesmalshri Sathe, Inamdar & Dawale, 2003 must be considered as an unavailable name because the depository of the type is not specified in the original (2003) publication.
Glyptapantelesmelentis Sathe & Bhoje, 2000.
Notes. The depository of the type is not specified in the original publication.
Hypomicrogasterminari Sathe & Bhoje, 2000.
Notes. The depository of the type is not specified in the original publication.
Microplitisbageshri Sathe, Inamdar & Dawale, 2003.
Notes. The depository of the type is not specified in the original publication.
Microplitisvitellipedis Li, Tan & Song, 2009.
Notes. The depository of the type is not specified in the original description. Ranjith et al. (2015a) mentioned that the holotype of this species is deposited in the HUNAU collection, but that was only an assumption (Ranjith, pers. comm.). They also stated that “the type specimen of this species could not be examined” and that instead they based their species description and illustration on specimens from India which they actually examined. Ranjith et al. (2015a) does not fulfill ICZN Article 16.1, and thus it does not make the species name available.
Parenionbhairavi Sathe, Inamdar & Dawale, 2003.
Notes. This same species was described previously by two of the authors (as Parenionbhairavi Sathe & Inamdar, 1991; that species is valid and is treated in this paper – see notes under Cotesiabhairavi above for more details, p 283, 284). In any case, the name Parenionbhairavi Sathe, Inamdar & Dawale, 2003 must be considered as an unavailable name because the depository of the type is not specified in the original (2003) publication.
Pholetesorrangini Sathe, Inamdar & Dawale, 2003.
Notes. The depository of the type is not specified in the original publication.
Promicrogastervachaspati Sathe, Inamdar & Dawale, 2003.
Notes. The depository of the type is not specified in the original publication.
Protomicroplitisindus Ahmed & Usmani, 2016.
Notes. The depository of the type is not specified in the original publication.
Protomicroplitisshivrangini Sathe, Inamdar & Dawale, 2003.
Notes. The depository of the type is not specified in the original publication.
Rhygoplitispahadi Sathe, Inamdar & Dawale, 2003.
Notes. The depository of the type is not specified in the original publication.
Semionismadhuvanti Sathe, Inamdar & Dawale, 2003.
Notes. The depository of the type is not specified in the original publication.
Figure 10.
Alphomelonsimpsonorum female holotype A Habitus, lateral B Head, frontal C Fore wing D Head, dorsal E Antenna and head, lateral, F- Metasoma and ovipositor sheaths, lateral G Propodeum and metasoma, dorsal.
Figure 13.
Apantelesaristoteliae female CNCHYM00068 A Habitus, lateral B Head, frontal C Fore wing D Mesosoma, propodeum and tergite 1, dorsal E Metasoma, dorsal.
Figure 14.
Apantelesbaldufi female MIC000024 A Habitus, lateral B Head, frontal C Fore wing D Metasoma, ovipositor and ovipositor sheaths, dorsal E Propodeum and tergite 1, dorsal F Mesosoma, dorsolateral G Antenna and head, dorsal H Cocoon.
Figure 15.
Apantelesbiroicus female CNC280546 A Habitus, lateral B Head, lateral C Fore wing D Mesosoma and tergite 1, dorsal E Propodeum and metasoma, dorsal F Metasoma and ovipositor sheaths, lateral.
Figure 16.
Apantelescanarsiae female MIC000030 A Cocoon B Habitus, lateral C Head, frontolateral D Fore wing and hind wing E Mesosoma and propodeum, dorsal F Propodeum and metasoma, dorsal.
Figure 17.
Apantelescarpatus female MIC000036 A Habitus, lateral B Head, frontoventral C Fore wing and hind wing D Metasoma, dorsal E Mesosoma and propodeum, dorsal.
Figure 18.
Apantelescockerelli female MIC000043 A Habitus, lateral B Head, frontal C Fore wing D Hypopygium and ovipositor sheaths, lateral E Mesosoma, dorsal F Propodeum and metasoma, dorsal.
Figure 19.
Apantelesconanchetorum female MIC000060 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Mesosoma and propodeum, dorsal E Metasoma, dorsal F Ovipositor and ovipositor sheaths.
Figure 20.
Apantelesconcordalis female holotype A Habitus, dorsal B Head, frontal C Head, dorsal D Fore wing E Metasoma, dorsal F Mesosoma and propodeum, dorsal G Ovipositor and ovipositor sheaths.
Figure 21.
Apantelesdeplanatus female CNC280564 A Habitus, lateral B Head, frontal C Fore wing D Mesosoma, dorsal E Metasoma, dorsal F Propodeum, dorsolateral.
Figure 22.
Apantelesepinotiae female CNC280581 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Mesosoma, dorsal E Propodeum and metasoma, dorsal F Ovipositor and ovipositor sheaths.
Figure 23.
Apantelesfeltiae female MIC000097 A Habitus, lateral B Head, frontal C Fore wing D Mesosoma, dorsal E Metasoma, dorsal.
Figure 24.
Apantelesgalleriae female MIC000116 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Mesosoma, dorsal E Metasoma, dorsal F Ovipositor and ovipositor sheaths.
Figure 30.
Austrocotesiadelicata female holotype A Habitus, lateral B Head, frontal C Antenna D Metasoma, dorsal E Mesosoma, dorsal.
Figure 31.
Austrocotesiaexigua female holotype A Habitus, lateral B Head, frontal C Fore wing and hind wing D Mesosoma, dorsal E Metasoma, dorsal.
Figure 36.
Buluka sp. female CNC281638 A Habitus, lateral B Ovipositor and ovipositor sheaths C Head, frontal D Fore wing E Mesosoma, dorsal F Apex of antennae F Metasoma and propodeum, dorsal.
Figure 41.
Choerasapo female CNC280754 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Head and mesosoma, dorsal E Metasoma, dorsal F Ovipositor and ovipositor sheaths.
Figure 42.
Choerasparasitellae female CNC474678 A Habitus, lateral B Head, frontal C Fore wing D Mesosoma, dorsal E Metasoma, lateral F Metasoma, dorsal.
Figure 43.
Choerasruficornis female CNC280764 A Habitus, lateral B Fore wing and hind wing C Head, frontal D Ovipositor and ovipositor sheaths E Metasoma, dorsal F Mesosoma, dorsal.
Figure 44.
Choerastedellae female CNC474677 A Habitus, lateral B Head, lateral C Fore wing D Propodeum and metasoma, dorsal E Head and mesosoma, dorsal.
Figure 49.
Cotesiaancilla male CNC735671 A Head, frontal B Habitus, lateral C Fore wing and hind wing D Metasoma, dorsal E Mesosoma, dorsal.
Figure 50.
Cotesiaeulipis female CNC474686 A Habitus, lateral B Head, frontal C Fore wing D Mesosoma, dorsal E Propodeum and metasoma, dorsal.
Figure 51.
Cotesiaferruginea male CNCHYM00455 A Habitus, lateral B Head, frontal C Fore wing D Mesosoma, dorsal E Propodeum and metasoma, dorsal.
Figure 52.
Cotesiaglomerata female CNC722382 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Head and mesosoma, dorsal E Propodeum and metasoma, dorsal F Ovipositor and ovipositor sheaths.
Figure 54.
Cotesiahyphantriae female CNC721970 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Mesosoma, dorsal E Propodeum and metasoma, dorsal F Cocoon and host larvae.
Figure 55.
Cotesiaisolde female CNC475164 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Mesosoma, dorsal E Propodeum and metasoma, dorsal.
Figure 56.
Cotesiaofella female CNC474690 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Mesosoma, dorsal E Propodeum and tergites 1–2, dorsal F Propodeum and metasoma, dorsal.
Figure 57.
Cotesiaordinaria female CNC280830 A Habitus, lateral B Head, frontal C Fore wing D Propodeum and metasoma, dorsal E Metasoma, lateral F Head and mesosoma, dorsal.
Figure 58.
Cotesiapistrinariae female CNC841352 A Habitus, lateral B Head, frontal C Fore wing D Mesosoma, dorsal E Metasoma, dorsal F Propodeum and tergites 1–2, dorsal G Ovipositor and ovipositor sheaths.
Figure 59.
Cotesiaschaeferi female CNC280847 A Habitus, lateral B Head, frontal C Fore wing D Metasoma, dorsal E Head and mesosoma, dorsal F Ovipositor and ovipositor sheaths.
Figure 67.
Diolcogasteralkingara male CNCHYM00775 A Habitus, lateral B Head, frontal C Metasoma, dorsal D Head and mesosoma, dorsal E Propodeum, dorsal.
Figure 68.
Diolcogasterashmeadi female CNCHYM00785 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Head and mesosoma, dorsal E Propodeum and metasoma, dorsal F Propodeum and tergites 1–2, dorsal.
Figure 69.
Diolcogasterauripes female CNC475093 A Habitus, lateral B Fore wing C Head, frontal D Metasoma, dorsal E Head, dorsal F Mesosoma, dorsal.
Figure 70.
Diolcogasterclaritibia female HYM00000437 A Habitus, lateral B Mesosoma and tergites 1–2, dorsal C Fore wing D Mesosoma and metasoma, lateral E Metasoma, dorsal.
Figure 71.
Diolcogasterichiroi female holotype A Habitus, lateral B Head, frontal C Fore wing and hind wing D Metasoma, dorsal E Head and mesosoma, dorsal.
Figure 72.
Diolcogasterippis female CNCHYM00835 A Habitus, lateral B Fore wing and hind wing C Head, frontal D Head and mesosoma; dorsal E Metasoma, dorsal.
Figure 73.
Diolcogasteriqbali female CNCHYM00833 A Habitus, lateral B Head, frontal C Fore wing D Head, dorsal E Mesosoma, dorsal F Propodeum and metasoma, dorsal.
Figure 74.
Diolcogasteriridescens female CNC678869 A Habitus, lateral B Fore wing and hind wing C Head, frontal D Propodeum and metasoma, dorsal E Head and mesosoma, dorsal.
Figure 75.
Diolcogastermiamensis male paratype CNC489838 A Habitus, lateral B Head, frontal C Fore wing D Mesosoma, dorsal E Metasoma, dorsal F Propodeum and metasoma, dorsal.
Figure 76.
Diolcogasterminuta female CNC280884 A Habitus, lateral B Fore wing C Propodeum and metasoma, dorsal D Head, frontal E Mesosoma, dorsal F Ovipositor and ovipositor sheaths.
Figure 82.
Dolichogenideacandidata female CNC677699 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Mesosoma, dorsal E Metasoma, dorsal.
Figure 83.
Dolichogenideacoleophorae female CNC475168 A Habitus, lateral B Head, frontal C Fore wing D Mesosoma, dorsal E Propodeum and metasoma, dorsal.
Figure 84.
Dolichogenideadilecta female CNCHYM01030 A Ovipositor and ovipositor sheaths B Habitus, lateral C Head, frontal D Fore wing and hind wing E Mesosoma, dorsal F Propodeum and metasoma, dorsal G Propodeum and tergites 1–2, dorsal.
Figure 85.
Dolichogenideasolenobiae female CNCHYM01140 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Metasoma, lateral E Head and mesosoma, dorsal F Propodeum and metasoma, dorsal G Propodeum and tergites 1–2, dorsal.
Figure 91.
Exixtinalandica female holotype A Habitus, lateral B Head, frontal C Fore wing D Head, dorsal E Antenna F Propodeum and metasoma, dorsal G Mesosoma, dorsal.
Figure 93.
Exoryzarichardashleyi female DHJPAR0031507 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Mesosoma, dorsal E Propodeum and metasoma, dorsal F Ovipositor.
Figure 97.
Fornicia sp. female CNCHYM01223 A Habitus, lateral B Head, frontal C Fore wing D Mesosoma, dorsal E Propodeum and metasoma, dorsal F Ovipositor and ovipositor sheaths.
Figure 101.
Glyptapantelesbourquini female CNCHYM01239 A Habitus, lateral B Head, frontodorsal C Fore wing D Propodeum and metasoma, dorsal E Mesosoma, dorsal.
Figure 102.
Glyptapantelesfraternus female CNC497049 A Habitus, lateral B Head, frontal C Fore wing D Mesosoma and metasoma, dorsal E Mesosoma and metasoma, dorsal.
Figure 103.
Glyptapantelesfulvipes female MRSJFT0427 A Habitus, lateral B Head, frontal C Fore wing D Metasoma, dorsal E Head, mesosoma and tergites 1–2, dorsal.
Figure 104.
Glyptapantelesindiensis female paratype CNCHYM03231 A Habitus, lateral B Head, frontal C Fore wing D Mesosoma, dorsolateral E Propodeum and metasoma, dorsal.
Figure 105.
Glyptapantelesluteipennis female paratype CNC679221 A Habitus, lateral B Fore wing C Head, frontal D Propodeum and metasoma, dorsal E Mesosoma, dorsal.
Figure 106.
Glyptapantelesmilitaris female CNC679219 A Habitus, lateral B Head, frontal C Fore wing D Propodeum and metasoma, dorsal E Head and mesosoma, dorsal.
Figure 107.
Glyptapantelespolitus female CNCH1334 A Habitus, lateral B Head, frontal C Fore wing D Mesosoma and tergites 1–2, dorsal E Propodeum and metasoma, dorsal.
Figure 108.
Glyptapantelessarrothripae female CNC679326 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Mesosoma, dorsal E Propodeum and metasoma, dorsal.
Figure 109.
Glyptapantelesthompsoni female CNCHYM01350 A Habitus, lateral B Head, frontal C Fore wing D Head and mesosoma, dorsal E Propodeum and metasoma, dorsal F Propodeum, dorsolateral.
Figure 114.
Hypomicrogasterdeltis female holotype A Habitus, lateral B Head, frontal C Fore wing and hind wing D Head and mesosoma, dorsal E Propodeum and metasoma, dorsal F Ovipositor and ovipositor sheaths.
Figure 115.
Hypomicrogasterecdytolophae female CNC482258 A Habitus, lateral B Head, frontal C Fore wing D Propodeum and metasoma, dorsal E Mesosoma, dorsal F Mesosoma, metasoma and ovipositor sheaths, lateral G Cocoon.
Figure 116.
Hypomicrogasterguille female holotype A Habitus, lateral B Fore wing C Head, frontal D Head and mesosoma, dorsal E Metasoma, dorsal.
Figure 117.
Hypomicrogasterhektos female holotype A Habitus, lateral B Head, frontal C Fore wing and hind wing D Metasoma, dorsal E Head, mesosoma and tergite 1, dorsal.
Figure 118.
Hypomicrogastermultus female holotype A Habitus, lateral B Head, frontal C Fore wing D Metasoma, dorsal E Head and mesosoma, dorsal F Ovipositor sheaths.
Figure 119.
Hypomicrogasterpectinatus male holotype A Habitus, lateral B Head, frontal C Fore wing D Propodeum and metasoma, dorsal E Head and mesosoma, dorsal.
Figure 120.
Hypomicrogastersiderion female holotype A Habitus, lateral B Head, frontal C Fore wing and hind wing D Propodeum and metasoma, dorsal E Head and mesosoma, dorsal.
Figure 123.
Iconellamerula female CNC474671 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Metasoma, lateral E Head and mesosoma, dorsal F Propodeum and metasoma, dorsal.
Figure 126.
Illidopssuevus female CNCHYM01526 A Habitus, lateral B Fore wing and hind wing C Head, frontal D Head, frontolateral E Metasoma, dorsal F Head and mesosoma, dorsal.
Figure 148.
Microgastermeridiana female CNC474707 A Habitus, lateral B Head, frontal C Fore wing D Head and mesosoma, dorsal E Metasoma, dorsal F Hypopygium and ovipositor sheaths.
Figure 149.
Microgastermessoria female CNCHYM01635 A Habitus, lateral B Head, frontal C Fore wing D Mesosoma, dorsal E Propodeum and metasoma, dorsal.
Figure 152.
Microplitisflavipalpis female CNCHYM01748 A Habitus, lateral B Fore wing and hind wing C Head, frontal D Head and mesosoma, dorsal E Propodeum and metasoma, dorsal.
Figure 153.
Microplitisjuanmanueli female holotype A Habitus, lateral B Head, frontal C Fore wing D Apex of metasoma, lateral E Metasoma, dorsal F Mesosoma and tergites 1–3, laterodorsal.
Figure 154.
Microplitismanilae female CNC776776 A Habitus, lateral B Fore wing C Head, frontal D Head and mesosoma, dorsal E Metasoma, dorsal.
Figure 155.
Microplitismediator female CNC677799 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Head and mesosoma, dorsal E Propodeum and metasoma, dorsal.
Figure 158.
Miropoteslordhowensis female holotype A Habitus, lateral B Head, frontal C Fore wing and hind wing D Metasoma, dorsal E Head and mesosoma, dorsal.
Figure 167.
Nyereria sp. female CNCH0835 A Habitus, lateral B Head, frontal C Fore wing D Metasoma, dorsal E Head and mesosoma, dorsal.
Figure 168.
Nyereria sp. male CNCH0837 A Habitus, lateral B Head, frontal C Fore wing D Mesosoma, dorsal E Metasoma, dorsal.
Figure 173.
Parapantelesgerontogeae female CNC309845 A Habitus, lateral B Head, frontal C Fore wing D Mesosoma, dorsal E Metasoma, dorsal.
Figure 174.
Parapantelesparadoxus female CNCHYM01936 A Habitus, lateral B Fore wing and hind wing C Head, frontal D Propodeum and metasoma, dorsal E Mesosoma, dorsal.
Figure 179.
Paroplitisvietnamensis female holotype A Habitus, lateral B Head and mesosoma, dorsal C Fore wing D Mesosoma and metasoma, ventrolateral E Mesosoma and tergites 1–3, dorsal.
Figure 185.
Pholetesorbucculatricis female CNCHYM03147 A Habitus, lateral B Head, frontal C Mesosoma and metasoma, lateral D Fore wing and hind wing E Mesosoma and metasoma, dorsal.
Figure 186.
Pholetesorexiguous female CNCHYM03168 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Head and mesosoma, dorsal E Propodeum and metasoma, dorsal.
Figure 187.
Pholetesorlaetus female CNCHYM03170 A Habitus, lateral B Head, frontoventral C Fore wing and hind wing D Metasoma, dorsal E Mesosoma, dorsal F Propodeum and tergites 1–2, dorsolateral.
Figure 188.
Pholetesormaritimus female MRSJFT0464 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Mesosoma, dorsal E Propodeum and metasoma, dorsal F Apex of metasoma, lateral.
Figure 189.
Pholetesorsalalicus female CNC483619 A Habitus magnified, lateral B Habitus, lateral C Fore wing and hind wing D Head, frontal E Cocoon F Mesosoma, dorsal G Metasoma, dorsal.
Figure 192.
Prasmodonbobrobbinsi female holotype A Habitus, lateral B Head and mesosoma, dorsal C Head, lateral D Fore wing and hind wing E Propodeum and metasoma, dorsal.
Figure 195.
Promicrogasterfabriciocambroneroi female DHJPAR0012588 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Metasoma, dorsal E Mesosoma, dorsal.
Figure 196.
Promicrogastermiranda female CNCHYM01980 A Habitus, lateral B Head, frontal C Fore wing D Head and mesosoma, dorsal E Metasoma, dorsal F Ovipositor and ovipositor sheaths.
Figure 199.
Protapantelesimmunis male CNC841408 A Habitus, lateral B Head, frontal C Fore wing D Mesosoma, dorsal E Propodeum and metasoma, dorsal.
Figure 204.
Pseudapantelesmargaritapenonae female holotype A Habitus, lateral B Head, frontal C Fore wing D Metasoma, dorsal E Head and mesosoma, dorsal.
Figure 205.
Pseudapantelesmariocarvajali female holotype A Habitus, dorsal B Head, frontal C Fore wing and hind wing D Mesosoma, dorsal E Metasoma, dorsal F Propodeum and tergites 1–2, dorsal.
Figure 206.
Pseudapantelesrenecastroi female holotype A Habitus, lateral B Mesosoma and tergites 1–3, dorsal C Fore wing D Metasoma, dorsal E Mesosoma and tergites 1–3, laterodorsal.
Figure 209.
Pseudofornicia sp. female CNC92461 A Habitus, lateral B Head, frontal C Fore wing D Head and mesosoma, dorsal E Metasoma, dorsal.
Figure 216.
Rhygoplitisaciculatus male holotype A Habitus, lateral B Head, frontal C Fore wing D Head and mesosoma, dorsal E Tergites 1–3, dorsal.
Figure 219.
Sathoneugeni female CNCHYM01255 A Habitus, lateral B Head, frontal C Fore wing D Head and mesosoma, dorsal E Propodeum and metasoma, dorsal.
Figure 229.
Snelleniusmariakuzminae male holotype A Habitus, lateral B Propodeum, dorsal C Fore wing D Metasoma, lateral E Tergites 1–4, dorsal F Head and mesosoma, dorsal G Metasoma, dorsal.
Figure 230.
Snelleniusrobertoespinozai male holotype A Habitus, lateral B Mesosoma, dorsal C Fore wing D Propodeum and tergite 1, dorsal E Mesosoma, lateral F Metasoma, dorsal G Metasoma, lateral.
Figure 231.
Snellenius sp. female CNCH1580 A Habitus, lateral B Apex of metasoma, dorsolateral C Fore wing D Antenna E Mesosoma, dorsal F Head, frontal G Propodeum and part of metasoma, dorsal H Metasoma, dorsal.
Figure 238.
Venanusperuensis female holotype A Habitus, lateral B Head frontal C Fore wing and hind wing D Head dorsal E Antenna F Propodeum and metasoma, dorsal G Mesosoma dorsal.
Figure 239.
Venanuspinicola female holotype A Habitus, lateral B Head, frontal C Head, dorsal D Fore wing E Mesosoma, dorsal F Propodeum and metasoma, dorsal G Antenna.
Figure 242.
Wilkinsonellushenicopus male CNCHYM03452 A Habitus, lateral B Head, frontal C Fore wing D Propodeum and metasoma, dorsolateral E Mesosoma, dorsal.
Figure 243.
Wilkinsonellusstriatus male CNCH2428 A Habitus, lateral B Head, frontal C Head and mesosoma, dorsal D Fore wing and metasoma, lateral E Metasoma, dorsal.
Figure 247.
Xanthomicrogasterpelides female CNCHYM07146 A Habitus, lateral B Head frontal C Fore wing D Head and mesosoma, dorsal E Propodeum and metasoma, dorsal.
Figure 248.
Xanthomicrogaster sp. male CNC492878 A Habitus, lateral B Head, frontal C Fore wing and hind wing D Mesosoma, dorsal E Metasoma, dorsal.
Figure 251.
Ypsilonigastertiger female holotype A Habitus, lateral B Tergite 1, dorsal C Mesosoma, dorsal D Mesosoma, lateral E Propodeum, dorsal.
Acknowledgements
Many people and institutions were of capital importance in the completion of this work. First of all, we thank Dicky Yu and Kees van Achterberg for their extraordinary work preparing the different versions of Taxapad (2005, 2012, 2016), which were heavily consulted and provided an important foundation for this paper (including the many references, sometimes difficult to find, that Dicky and Kees were able to retrieve and kindly share with the wider scientific community). Even if we did not always agree with some decisions made in Taxapad about generic limits, the importance of that database cannot be overstated, as well as the kindness and collaborative spirit of its authors. The entire Hymenoptera Unit of the CNC, especially John Huber, helped and advised the senior author over the years on how to complete this work, including endless discussions about this paper and revisions of the manuscript at several stages of its completion. We benefited from the extremely useful revisions of the manuscript done by the Editor (Kees van Achterberg), the Reviewers selected by Kees to revise this manuscript when submitted to ZooKeys (Eduardo Shimbori, Avinjikkattu Parambil Ranjith, Andrew Austin, Ankita Gupta, James Whitfield, and an anonymous reviewer) and the ZooKeys Copy Editor (Nathalie Yonow). Another extra-official review by ICZN Commissioner Doug Yanega considerably improved our approach to many nomenclature issues; we are especially thankful to Doug for his kindness and time spent. Many braconid experts have supported us over the years, as colleagues, mentors, and friends; other researchers who preceded us (and thus we never met) also significantly influenced our work here with their contributions to the knowledge of Braconidae and Microgastrinae, in alphabetical order: Kees van Achterberg, Andrew Austin, William Mason, Carl Muesebeck, Gilbert Nixon, Jeno Papp, James Whitfield, and Douglas Wilkinson. Additionally, many other colleagues, curators and technicians of many institutions worldwide arranged for visits to their institutions, sent material for study or checked specimens for us, kindly shared information, publications, DNA barcodes and other resources; we recognize them in alphabetical order, and apologize if we are forgetting anyone: Parisa Abdoli, Diana Carolina Arias-Penna, Andrew Austin, Frederique Bakker, Kevin Barber, Sergey Belokobylskij, Noubar Bostanian, Yves Braett, Matthew Buffington, Xue-Xin Chen, Wouter Dekoninck, Rachel Diaz-Bastin, Charley Eiseman, Cecilia Escobar, Erinn Fagan-Jeffries, Shunpei Fujie, Mostafa Ghafouri Moghaddam, Christopher Grinter, Ankita Gupta, Winnie Hallwachs, Lars Ove Hansen, Paul Hanson, Antonius van Harten, Paul Hebert, Daniel Janzen, Martti Koponen, Anatoly Kotenko, Robert Kula, Christine Lebeau, Kaoru Maeto, Stephen Marshall, José Luis Nieves-Aldrey, James O’Connor, Juho Paukkunen, Kyle Parks, Angélica Maria Penteado-Dias, Donald Quicke, Avinjikkattu Parambil Ranjith, Josephine Rodriguez, Pascal Rousse, Michael Sharkey, Eduardo Shimbori, So Shimizu, Derek Sikes, Alex Smith, Jayme Sones, Alejandro Valerio, Kees van Achterberg, Gergely Varkonyi, Zoltan Vas, Claire Villemant, Darren Ward, James Whitfield, Alejandro Zaldívar-Riverón, Robert Zuparko, and members of the Swedish Malaise Trap Program. Also helpful were discussions with members of the Taxacom and Parahym lists. The following scientists kindly shared information or pictures about a few Microgastrinae types deposited in several institutions, which were useful to us when studying those species: Sergey Belokobylskij and Andrew Bennett (specimens in the MNHN); Mostafa Ghafouri Moghaddam (specimens deposited in HNHM); Robert Kula (specimens in the USNM); Darren Ward (specimens in the NZAC); Kees van Achterberg (types in China). Seven of the figures used in this paper were adapted from other works and authors, which are acknowledged here: 1) Figure 39 (based on modified drawings from Luo et al. 2004); 2) Figure 61 (mostly based on modified drawings and SEM images from Choi and Whitfield 2006); 3) Figures 87 and 88 (based on modified images from Xiong et al. 2017); 4) Figures 208 and 210 (based on modified images from van Achterberg et al. 2015); 5) Figure 245 (based on modified drawings from Whitfield 1995b). Lyubomir Penev, Yordanka Banalieva, and colleagues at Pensoft were very helpful and understanding with the difficulties of assembling this paper, their help is greatly appreciated. This work was supported by Projects J-001283 “Arthropod Systematics” and 3199 “Systematics of beneficial arthropods in support of resilient agroecosystems”, from Agriculture and Agri-Food Canada.
Citation
Fernandez-Triana J, Shaw MR, Boudreault C, Beaudin M, Broad GR (2020) Annotated and illustrated world checklist of Microgastrinae parasitoid wasps (Hymenoptera, Braconidae). ZooKeys 920: 1–1090. https://doi.org/10.3897/zookeys.920.39128
Supplementary materials
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Jose Fernandez-Triana, Mark R. Shaw, Caroline Boudreault, Melanie Beaudin, Gavin R. Broad
Supplementary table 1
Data type: species data
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Jose Fernandez-Triana, Mark R. Shaw, Caroline Boudreault, Melanie Beaudin, Gavin R. Broad
Supplementary table 2
Data type: species data
References
- Aarvik L, Bengtsson BÅ, Elven H, Ivinskis P, Jürivete U, Karshol O, Mutanen M, Savenkov N. (2017) Nordic-Baltic Checklist of Lepidoptera. Norwegian Journal of Entomology 3: 1–236. [Google Scholar]
- Abdinbekova AA. (1969) New braconid wasps (Hymenoptera, Braconidae) in the fauna of Azerbaidjan. [in Russian] Doklady Akademii Nauk Azerbaidzhanskoi SSR 25(6): 72–77. [Google Scholar]
- Abdoli P, Talebi AA, Farahani S. (2019a) Dolichogenideafernandeztrianai sp. nov. (Hymenoptera: Braconidae, Microgastrinae) from Iran. Journal of Agricultural Science and Technology 21(3): 647–658. 10.2478/aemnp-2019-0046 [DOI]
- Abdoli P, Talebi AA, Farahani S, Fernandez-Triana J. (2019b) Three new species of the genus Choeras Mason, 1981 (Hymenoptera: Braconidae, Microgastrinae) from Iran. Zootaxa 4545(1): 77–92. 10.11646/zootaxa.4545.1.4. [DOI] [PubMed] [Google Scholar]
- Abdoli P, Talebi AA, Farahani S, Fernandez-Triana J. (2019c) Venanidescaspius sp. nov. from Iran, the first species of Venanides (Hymenoptera: Braconidae) described from the Palaearctic Region. Acta Entomologica Musei Nationalis Pragae 59(2): 543–548. [Google Scholar]
- Abraham CC, Mathew KP, Das NM. (1973) Records of Hymenopterous parasites of the rice leaf folder Cnaphalocrocismedinalis Guen. in Kerala. Agricultural Research Journal Kerala 11(1): 81. [Google Scholar]
- Ahmad M. (1945) Some new species of parasitic Hymenoptera from India. Indian Journal of Entomology 7(1-2): 5–11. [Google Scholar]
- Ahmad Z, Haider AA. (2005a) Two new species of Apanteles (Illidops) Mason (Hymenoptera: Braconidae) from India. Oriental Insects 39: 229–232. 10.1080/00305316.2005.10417437 [DOI] [Google Scholar]
- Ahmad Z, Pandey K, Haider AA. (2005b) A new Philoplitis species (Hymenoptera: Braconidae) from India. Zoos' Print Journal 20(1): 1736. 10.11609/JoTT.ZPJ.1128.1736 [DOI] [Google Scholar]
- Ahmad Z, Pandey K, Haider AA. (2005c) Description of a new species of the genus Neoclarkinella Rema & Narendran (Hymenoptera: Braconidae) from India. Journal of the Bombay Natural History Society 102(2): 208–209. [Google Scholar]
- Ahmad Z, Pandey K, Haider AA. (2005d) Discovery of the genus Wilkinsonellus Mason (Hymenoptera: Braconidae) from India. Zoos' Print Journal 20(3): 1804. 10.11609/JoTT.ZPJ.1098.1804 [DOI] [Google Scholar]
- Ahmad Z, Hussain KZ, Shanthi R. (2009) Glyptapantelesspodopterae Ahmad sp. nov. (Hymenoptera: Braconidae: Microgastrinae) parasitic on larvae of Spodopteralitura in India. Journal of Entomological Research 33(1): 97–99. [Google Scholar]
- Ahmad Z, Al Ghramh HA, Pandey K, Khan F. (2019) Two new species of Dolichogenidea (Hymenoptera: Braconidae: Microgastrinae) parasitoids of leafminer Lepidoptera from India. Biologia: 1–5. 10.2478/s11756-019-00351-7 [DOI]
- Ahmed I. (2017) Taxonomic Studies on Braconidae (Hymenoptera: Insecta) from Jammu Division of the State Jammu and Kashmir, India. Doctoral dissertation, Aligarh Muslim University, 542 pp. [Google Scholar]
- Ahmed I, Usmani MK. (2016) A new species of the genus Protomicroplitis (Braconidae: Microgastrinae) from India. Journal of Entomology and Zoology Studies 4(1): 540–542. [Google Scholar]
- Akhtar MS, Ahmad Z, Ramamurthy VV. (2010) Description of two new species of Microgastrini (Hymenoptera: Braconidae) from India. Zootaxa 2608: 57–62. 10.11646/zootaxa.2608.1.4Alexeev JI (1971) New species of Braconids (Hymenoptera, Braconidae) from Turkmenia. Entomologicheskoye Obozreniye 50(2): 404–415. [DOI] [Google Scholar]
- Alexeev YI. (1977) New species of the genus Microplitis Foerst. (Hymenoptera, Braconidae). Zoologicheskii Zhurnal 56(19): 1417–1419. [Google Scholar]
- Amerling (1862) Eine neue Species Microgaster. Lotos 12: 107.
- Anjum Q, Malik KF. (1978) A new species of the genus Apanteles (Hymenoptera: Braconidae) parasite of the pink bollworm Pectinophoragossypiella, from Pakistan. Pakistan Journal of Zoology 10(2): 273–277. [Google Scholar]
- Aquino DA, Gaddi AL, Hernandez EP, Martinez JJ. (2010) The types of Braconidae and Ichneumonidae (Hymenoptera: Ichneumonoidea) in the Museo de La Plata, Argentina. Zootaxa 2487: 43–51. 10.11646/zootaxa.2487.1.4 [DOI] [Google Scholar]
- Arias-Penna DC, Whitfield JB, Janzen DH, Hallwachs W. (2013) Three new species in the genus Wilkinsonellus (Braconidae, Microgastrinae) from the Neotropics, and the first host record for the genus. ZooKeys 302: 79–95. 10.3897/zookeys.302.4962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias-Penna DC, Zhang Y, Whitfield JB. (2014) First record of the genus Wilkinsonellus (Hymenoptera, Braconidae, Microgastrinae) from Fiji with description of three new species. ZooKeys 397: 25–47. 10.3897/zookeys.397.7195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias-Penna DC, Whitfield JB, Janzen DH, Hallwachs W, Dyer LA, Smith MA, Hebert PDN, Fernandez-Triana JL. (2019) A species-level taxonomic review and host associations of Glyptapanteles (Hymenoptera, Braconidae, Microgastrinae) with an emphasis on 136 new reared species from Costa Rica and Ecuador. ZooKeys 890: 1–685. 10.3897/zookeys.890.35786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmead WH. (1890) On the Hymenoptera of Colorado; descriptions of new species, notes and a list of the species found in the State. Bulletin of the Colorado Biological Association 1: 1–47. [Google Scholar]
- Ashmead WH. (1898) Part 2. Descriptions of new parasitic Hymenoptera. Proceedings of the Entomological Society of Washington 4: 155–171. [Google Scholar]
- Ashmead WH. (1900a) Classification of the Ichneumon flies, or the superfamily Ichneumonoidea. Proceedings of the United States National Museum 23(1206): 1–220. 10.5479/si.00963801.23-1206.1 [DOI] [Google Scholar]
- Ashmead WH. (1900b) Notes on some New Zealand and Australian parasitic Hymenoptera with description of new genera and new species. Proceedings of the Linnean Society of New South Wales 25: 327–360. 10.5962/bhl.part.12157 [DOI] [Google Scholar]
- Ashmead WH. (1900c) Report upon the Aculeate Hymenoptera of the islands of St. Vincent and Grenada, with additions to the parasitic Hymenoptera and a list of the described Hymenoptera of the West Indies. Transactions of the Entomological Society of London 1900: 207–367. 10.1111/j.1365-2311.1900.tb02379.x [DOI] [Google Scholar]
- Ashmead WH. (1902) Papers from the Harriman Alaska Expedition XXVIII. Hymenoptera. Proceedings of the Washington Academy of Science 4: 117–268. 10.5962/bhl.part.18572 [DOI] [Google Scholar]
- Ashmead WH. (1904a) A list of Hymenoptera of the Philippine Islands with descriptions of new species. Journal of the New York Entomological Society 12: 1–22. [Google Scholar]
- Ashmead WH. (1904b) Descriptions of new genera and species of Hymenoptera from the Philippine Islands. Proceedings of the United States National Museum 28(1387): 127–158. 10.5479/si.00963801.28-1387.127 [DOI] [Google Scholar]
- Ashmead WH. (1905a) New Hymenoptera from the Philippines. Proceedings of the United States National Museum 29(1416): 107–119. 10.5479/si.00963801.29-1416.107 [DOI] [Google Scholar]
- Ashmead WH. (1905b) New Hymenoptera from the Philippine Islands. The Canadian Entomologist 37(1): 3–8. 10.4039/Ent373-1 [DOI] [Google Scholar]
- Ashmead WH. (1906) Descriptions of new Hymenoptera from Japan. Proceedings of the United States National Museum 30: 169–201. 10.5479/si.00963801.30-1448.169 [DOI] [Google Scholar]
- Austin AD. (1987) Braconidae. A review of the Braconidae (Hymenoptera) that parasitize Limacodidae in south-east Asia, particularly those associated with coconut and oil palm. In: Cook MJW, Godfray HCJ, Holloway JD (Eds) Slug and Nettle Caterpillars: the Biology, Taxonomy and Control of the Limacodidae of Economic Importance on Palms in Southeast Asia, 139–164.
- Austin AD. (1989) Revision of the genus Buluka de Saeger (Hymenoptera: Braconidae: Microgastrinae). Systematic Entomology 14(2): 149–163. 10.1111/j.1365-3113.1989.tb00273.x [DOI] [Google Scholar]
- Austin AD. (1990) Revision of the enigmatic Australasian genus Miropotes Nixon (Hymenoptera: Braconidae: Microgastrinae), with comments on the phylogenetic importance of the female ovipositor system. Systematic Entomology 15: 43–68. 10.1111/j.1365-3113.1990.tb00302.x [DOI] [Google Scholar]
- Austin AD, Allen GR. (1989) Parasitoids of Urabalugens Walker (Lepidoptera: Noctuidae) in South Australia, with description of Braconidae. Transactions of the Royal Society of South Australia 113(4): 169–184. 10.1017/S0007485300018642 [DOI] [Google Scholar]
- Austin AD, Dangerfield PC. (1989) The taxonomy of New World microgastrine braconids (Hymenoptera) parasitic on Diatraea spp. (Lepidoptera: Pyralidae). Bulletin of Entomological Research 79(1): 131–144. [Google Scholar]
- Austin AD, Dangerfield PC. (1992) Synopsis of Australasian Microgastrinae (Hymenoptera: Braconidae), with a key to genera and description of new taxa. Invertebrate Taxonomy, 6(1): 1–76. 10.1071/IT9920001 [DOI] [Google Scholar]
- Austin AD, Dangerfield PC. (1993) Systematics of Australian and New Guinean Microplitis Foerster and Snellenius Westwood (Hymenoptera: Braconidae: Microgastrinae), with a review of their biology and host relationships. Invertebrate Taxonomy 7(5): 1097–1166. 10.1071/IT9931097 [DOI] [Google Scholar]
- Baker CF. (1895) Two new Apanteles. Entomological News 6: 201–203. [Google Scholar]
- Balduf WV. (1968) Bionomic notes on the hexapodous parasites of Acrobasisrubrifasciella. Annals of the Entomological Society of America 61(2): 463–476. 10.1093/aesa/61.2.463 [DOI] [Google Scholar]
- Balevski NA. (1980) New species of Apanteles Foerst. (Hymenoptera, Braconidae) from Bulgaria. [In Russian with English summary] Entomologicheskoye Obozreniye 59(2): 350–362. [Google Scholar]
- Balevski NA, Tobias VI. (1980) Three new species of the genus Apanteles Foerst. (Hymenoptera, Braconidae) from southwestern Bulgaria. [In Russian with English summary]. Entomologicheskoye Obozreniye 59: 363–367. [Google Scholar]
- Baltazar CR. (1962) The genera of parasitic Hymenoptera in the Philippines, Part 1. Pacific Insects 4(4): 737–771. [Google Scholar]
- Bartlett BR, Clausen CP, DeBach P, Goeden RD, Legner EF, McMurtry JA, Oatman ER. (1978) Introduced parasites and predators of arthropod pests and weeds: A world review. Agricultural Research Service. United States Department of Agriculture, Agriculture Handbook No. 480, 545 pp. [Google Scholar]
- Bechstein JM, Scharfenberg GL. (1805) Naturgeschichte der Schädlichen Forstinsekten. III. Leipzig, 605–1046.
- Belokobylskij SA. (2014) Family Braconidae Nees, 1812. In: Antropov AV, Belokobylskij SA, Compton SG, Dlussky GM, Khalaim AI, Kolyada VA, Kozlov MA, Perfilieva KS, Rasnitsyn AP (2014). The wasps, bees and ants (Insecta: Vespida = Hymenoptera) from the Insect Limestone (Late Eocene) of the Isle of Wight, UK. Earth and Environmental Science Transactions of the Royal Society of Edinburgh 104(3–4): 335–446. 10.1017/S1755691014000103 [DOI] [Google Scholar]
- Belokobylskij SA, Samartsev KG, Il'inskaya AS. (2019) Annotated catalogue of the Hymenoptera of Russia. Volume II. Apocrita: Parasitica. Proceedings of the Zoological Institute, Russian Academy of Sciences, St Petersburg, Supplement 8, 594 pp. [Google Scholar]
- Belokobylskij SA, Taeger A, van Achterberg C, Haeselbarth E, Riedel M. (2003) Checklist of the Braconidae (Hymenoptera) of Germany. Beiträge zur Entomologie 53(2): 341–435. [Google Scholar]
- Bhatnagar SP. (1950) Studies on Apanteles Förster (Vipionidae: parasitic Hymenoptera) from India. Indian Journal of Entomology 10(2): 133–203. [Google Scholar]
- Bhoje PM, Sathe TV. (2002a) A new species of Dolichogenidea Viereck (Hymenoptera: Braconidae) from India. Journal of Nature Conservation 14(1): 63–66. [Google Scholar]
- Bhoje PM, Sathe TV. (2002b) On a new species of Dolichogenidea Viereck (Hymenoptera: Braconidae) from India. Uttar Pradesh Journal of Zoology 22(1): 81–83. [Google Scholar]
- Biezanko CM. (1960) Satyridae, Morphidae et Brassolidae da Zona Sueste do Rio Grande do Sul. Arquivos de Entomologia da Escola de Agronomia ‘Eliseu Maciel” (Inst. Agron. Sul, Pelotas, RS) (A) 4: 1–12. [Google Scholar]
- Bingham CT. (1906) New species of Braconidae and Chalcididae from N. Queensland, bred by F.P. Dodd. Transactions of the Entomological Society of London 124–130.
- Blanchard EE. (1935) Microgastrinos argentinos nuevos y poco conocidos. Physis Buenos Aires 11(40): 459–471. [Google Scholar]
- Blanchard EE. (1936) Microgastrinos argentinos nuevos y poco conocidos. Segunda parte. Physis Buenos Aires 12: 137–152. [Google Scholar]
- Blanchard EE. (1938) Insectos útiles. Insectos nocivos. Hiperparásitos. Boletín Informativo Dirección Sanidad Vegetal 1(4): 43–48. [Google Scholar]
- Blanchard EE. (1939) Insectos útiles. Insectos nocivos. Boletín Informativo Dirección Sanidad Vegetal 2(7): 36–38. [Google Scholar]
- Blanchard EE. (1940) Insectos útiles. Boletín Informativo Dirección Sanidad Vegetal 3(12): 26–28. [Google Scholar]
- Blanchard EE. (1941) Una especie nueva de Apanteles (Hymenopt.) parásito de Melittiabergi, Edwards (Lepid. Aegeriidae). Notas del Museo de La Plata 6: 153–155. [Google Scholar]
- Blanchard EE. (1942a) Parásitos de Alabamaargillacea Hbn. en la República Argentina. Estudio preliminar. Anales de la Sociedad Cientifica Argentina 134: 54–63, 94–128.
- Blanchard EE. (1942b) Division de zoología agrícola. Boletin Informativo Dirección Sanidad Vegetal 5(19): 14–15. [Google Scholar]
- Blanchard EE. (1943) Un díptero y seis himenópteros argentinos, nuevos para la ciencia. Revista de la Sociedad Entomologica Argentina 12: 92–104. [Google Scholar]
- Blanchard EE. (1947) Descripciones y anotaciones de Microgastrinos argentinos (Hymenoptera). Arthropoda 1(1): 6–22. [Google Scholar]
- Blanchard EE. (1959) Dos nuevos microgastrinos útiles para la agricultura (Hym. Braconidae). Revista de Investigaciones Agrícolas 13: 151–155. [Google Scholar]
- Blanchard EE. (1960) Himenópteros parásitos de arctiidos argentinos. Revista de Investigaciones Agrícolas 14: 19–23. [Google Scholar]
- Blanchard EE. (1964) Un nuevo Microgastrino Argentino (Hym. Braconidae). Revista de la Sociedad Entomológica Argentina 26(1963): 41–42. [Google Scholar]
- Blanchard EE. (1965) Un nuevo parasito de Pterophoridae Argentino (Hym. Braconidae). Revista de la Sociedad Entomológica Argentina 27(1964): 117–118. [Google Scholar]
- Blanchard EE, de Santis L. (1963) Himenópteros argentinos parásitos de Coleophorahaywardi. Revista de Investigaciones Agrícolas 17: 114–124. [Google Scholar]
- Bourquin F. (1937) Metamorfosis de Speocropiasmilacis Hayw. (Lep. Aerynyctinae). Revista de la Sociedad Entomológica Argentina 9: 67–71. [Google Scholar]
- Bourquin F. (1939) Metamorfosis de Phthorimaeaeuchthonia Meyrick, 1939 (Microlep. Gelechiidae). Revista de Entomologia, Rio de Janeiro 10: 637–640. [Google Scholar]
- Bouché PF. (1834) Naturgeschichte der Insekten, besonders in Hinsicht ihrer ersten Zustande als Larven und Puppen. Berlin, p. 216.
- Bourquin F. (1939) Metamorfosis de Phthorimaeaeuchthonia Meyrick, 1939 (Microlep. Gelechiidae). Revista de Entomologia, Rio de Janeiro 10: 637–640. [Google Scholar]
- Bourquin F. (1961) Notas sobre la metamorfosis de Deltinaacostalimai Pastrana, Tortricidae (Lep.). Revista de la Sociedad Entomológica Argentina 23: 51–53. [Google Scholar]
- Brèthes J. (1916) Hyménoptères parasites de l'Amérique méridionale. Anales del Museo Nacional de Historia Natural de Buenos Aires 27: 401–430. [Google Scholar]
- Brèthes J. (1917) Descripción de dos nuevos Himenópteros de Buenos Aires. Physis Buenos Aires 3: 90–91. [Google Scholar]
- Brèthes J. (1920) Insectos útiles y dañinos de Rio Grande do Sul y de la Plata. Anales de la Sociedad Rural Argentina 54: 281–290, 307–308.
- Brèthes J. (1922) Himenópteros y Dípteros de varias procedencias. Anales de la Sociedad Científica Argentina 93: 119–146. [Google Scholar]
- Brèthes J. (1924a) Quelques insectes du Paraguay. Revista Chilena de Historia Natural 28: 67–72. [Google Scholar]
- Brèthes J. (1924b) Varios Himenopteros de la America de Sud. Nunquam Otiosus. 1924: 6–16, 145–175.
- Broad G, Shaw M, Godfray H. (2016) Checklist of British and Irish Hymenoptera – Braconidae. Biodiversity Data Journal 4: e8151. 10.3897/BDJ.4.e8151 [DOI] [PMC free article] [PubMed]
- Brullé MA. (1832) Expédition scientifique de Morée. Section des sciences physiques. Tome III. 1re partie. Zoologie. F.G. Levrault, Paris, 400 pp. [Google Scholar]
- Brullé MA. (1846) Tome Quatrième. Des Hyménoptères. Les Ichneumonides. In: Lepeletier de Saint-Fargeau A. Histoire Naturelles des Insectes. Paris, 680 pp.
- Brues CT, Richardson CH. (1913) Descriptions of new parasitic Hymenoptera from British Guiana. Bulletin of the American Museum of Natural History 32: 485–503. [Google Scholar]
- Brues CT. (1926) Studies on Ethiopian Braconidae with a catalogue of the African species. Proceedings of the American Academy of Arts and Sciences 61: 206–436. 10.2307/20026158 [DOI] [Google Scholar]
- Cameron GX. (2018) Two new species of Xanthomicrogaster (Hymenoptera: Braconidae) from Argentina, with a key to species of the genus. Zootaxa 4370(3): 295–300. 10.11646/zootaxa.4370.3.10 [DOI] [PubMed] [Google Scholar]
- Cameron P. (1887) Hymenoptera. In: Godman FD, Salvin O. (Eds) Biologia Centrali-Americana; or, Contributions to the knowledge of the fauna and flora of Mexico and Central America.Zoology 1: 329–422, 471–472.
- Cameron P. (1891) Hymenopterological notices. I. On some Hymenoptera parasitic in Indian injurious insects. Memoirs and Proceedings of the Manchester Literary and Philosophical Society (4)4: 182–194.
- Cameron P. (1897) HymenopteraOrientalia, or contribution to a knowledge of the Hymenoptera of the Oriental Zoological Region. Part V. Memoirs and Proceedings of the Manchester Literary and Philosophical Society 41(4): 1–144. [Google Scholar]
- Cameron P. (1904) Description of a new genus and species of Hymenoptera from Mexico. Transactions of the American Entomological Society 30: 251–267. [Google Scholar]
- Cameron P. (1905a) On some Australian and Malay Parasitic Hymenoptera in the Museum of the R. Zool. Soc. "Natura artis magistra" at Amsterdam. Tijdschrift voor Entomologie 48: 33–47. [Google Scholar]
- Cameron P. (1905b) On the Hymenoptera of the Albany Museum, Grahamstown, South Africa. Record of the Albany Museum 1(1904): 161–176. [Google Scholar]
- Cameron P. (1905c) On the phytophagous and parasitic Hymenoptera collected by Mr. E.Green in Ceylon. Spolia Zeylanica 3: 67–143. [Google Scholar]
- Cameron P. (1906a) On the Hymenopterous parasites of the mealie stalk borer (Sesamiafusca, Hampson). Transactions of the South African Philosophical Society 16(4): 334–336. 10.1080/21560382.1905.9526068 [DOI] [Google Scholar]
- Cameron P. (1906b) On the Tenthredinidae and parasitic Hymenoptera collected in Baluchistan by Major C.G. Nurse. Part I. Journal of the Bombay Natural History Society 17: 89–107. [Google Scholar]
- Cameron P. (1907) On the parasitic Hymenoptera collected by Major C.G. Nurse in the Bombay presidency. Journal of the Bombay Natural History Society 17: 584–595, 1011–1012.
- Cameron P. (1908b) Description of a new species of Apanteles from Ceylon. Spolia Zeylanica 5: 17–18. 10.5962/bhl.part.26462 [DOI] [Google Scholar]
- Cameron P. (1908a) Description of a new species of Microgaster (Braconidae) from the Paraguayan Chaco, South America (Hym.). Deutsche Entomologische Zeitschrift 1908: 686. 10.1002/mmnd.48019080603 [DOI]
- Cameron P. (1909a) Article V. – Descriptions of four species of Hymenoptera from Auckland Island. In: Chilton C. (Ed.) The Subantarctic Islands of New Zealand, Vol.1. Philosophical Institute of Canterbury, Christchurch, 75–77.
- Cameron P. (1909b) On some undescribed Ichneumonidae and Braconidae reared by Mr. T. Bainbrigge Fletcher from Ceylonese Lepidoptera (Pterophoridae). Spolia Zeylanica 6(21): 40–43. [Google Scholar]
- Cameron P. (1910a) On some African species of the subfamilies Exothecinae, Aphrastobraconinae, Cheloninae, Doryctinae, Cardiochilinae and Microgasterinae in the Royal Berlin Zoological Museum. Zeitschrift für Naturwissenschaft 81: 433–450. [Google Scholar]
- Cameron P. (1910b) On some Asiatic species of the Braconid subfamilies Rhogadinae, Agathinae, and Macrocentrinae and of the Alysiidae. Wiener Entomologische Zeitschrift 29: 1–10. 10.5962/bhl.part.23337 [DOI] [Google Scholar]
- Cameron P. (1911a) On a collection of parasitic Hymenoptera (chiefly bred) made by Mr. W.W. Froggatt, F.L.S., in New South Wales, with descriptions of new genera and species. Part i. Proceedings of the Linnean Society of New South Wales 36: 333–346. 10.5962/bhl.part.21902 [DOI] [Google Scholar]
- Cameron P. (1911b) On the Hymenoptera of the Georgetown Museum, British Guiana. Part II. Timehri 1: 306–330. [Google Scholar]
- Cameron P. (1911c) On the parasitic Hymenoptera collected by Mr. A.J.T. Janse, Transvaal. Annals of the Transvaal Museum 2: 173–217. [Google Scholar]
- Cameron P. (1912) On a collection of parasitic Hymenoptera (chiefly bred) made by Mr. W.W. Froggatt, F.L.S., in New South Wales, with descriptions of new genera and species. Part iii. Proceedings of the Linnean Society of New South Wales 37: 172–219. 10.5962/bhl.part.22341 [DOI] [Google Scholar]
- Cameron P. (1913) On the parasitic Hymenoptera reared at Dehra Dun, Northern India from Lac (Tachardia) and Sal insects. Indian Forest Records 4: 1–20. [Google Scholar]
- Camousseight A. (1975) Las especies descritas por el Prof. Carlos Silva Figueroa. Noticiario Mensual del Museo Nacional de Historia Natural, Santiago de Chile 19(222-223): 3–5. [Google Scholar]
- Capek M. (1989) The identity of Apantelesdioryctriae Wilk. and A.murinanae Cap. & Zwölf. (Hym.: Braconidae). Lesnictvi (Prague), 35(LXII): 995–1001.
- Capek M, Zwölfer H. (1957) Apantelesmurianae nov. spec. (Braconidae, Hym.), ein neuer Parasit des Tannentriebwicklers. Mitteilungen der Schweizerischen Entomologischen Gesellschaft 30(2): 119–126.
- Capek M, Lukas J. (1989) ApocritaParasitica, Ichneumoidea, Braconidae. Acta Faunistica Entomologica Musei Nationalis Pragae 19: 27–44. [Google Scholar]
- Capek M, Hofmann C. (1997) The Braconidae (Hymenoptera) in the collections of the Musée cantonal de Zoologie, Lausanne. Litterae Zoologicae (Lausanne) 2: 25–162. [Google Scholar]
- Chalikwar MR, Rao S. (1982) A new Indian species of the genus Apanteles Forster (Hymenoptera, Braconidae). Proceedings of the Indian Science Congress 69(3): 126. [Google Scholar]
- Chalikwar MR, Rao SN, Nikam PK. (1985) Two new species of Cotesia from India (Hymenoptera: Braconidae). Oriental Insects 18(1984): 17–23 10.1080/00305316.1984.10432190 [DOI] [Google Scholar]
- Chen J, Song D. (2004) Systematic studies on Microgastrinae of China (Hymenoptera: Braconidae). Fujian Scientific Publisher, Fuchow, China, 354 pp. [Google Scholar]
- Chen J, Yang J, Huang J, Song D. (2001) Description of a new Apanteles from China. [In Chinese with English summary] Journal of Fujian Agricultural University 30(3): 290–292. [Google Scholar]
- Chen J, Song D, Yang J. (2002) A new species of Apanteles Forster (Hymenoptera: Braconidae) from China. [In Chinese with English summary] Entomotaxonomia 24(2): 206–208. [Google Scholar]
- Chen XX, He JH, Ma Y. (1994) Five new species of the genus Fornicia Brulle (Hymenoptera: Braconidae: Microgastrinae) from China. [In Chinese with English summary] Entomotaxonomia 16(2): 127–134. [Google Scholar]
- Choi WY, Whitfield JB. (2006) Cuneogaster, a new genus of the subfamily Microgastrinae (Hymenoptera: Braconidae) from the Neotropical region. Proceedings of the Entomological Society of Washington 108(1): 119–124. [Google Scholar]
- Choi S, Kim H. (2018) A New Record of Species of the Microplitis (Hymenoptera: Braconidae: Microgastrinae) in Korea. Animal Systematics, Evolution and Diversity 34(3): 159. [Google Scholar]
- Chou LY. (1985) A new species of Buluka (Hymenoptera: Braconidae) from Taiwan. Chinese Journal of Entomology 5: 85–88. [Google Scholar]
- Chougale TM. (2016) A new species of the genus Apanteles Foerster (Hymenoptera: Braconidae) from India. Thorax 2(14): 1–25. [Google Scholar]
- Chu JT. (1937) Notes on the Hymenopterous parasites of the pine caterpillar Dendrolimuspunctatus Walker in China. [In Chinese with English summary] Entomology and Phytopathology, 5: 56103.
- Cock MJ. (2015) A critical review of the literature on the pest Erionota spp. (Lepidoptera, Hesperiidae): taxonomy, distribution, food plants, early stages, natural enemies and biological control. CAB Reviews, 10(007): 1–30. 10.1079/PAVSNNR201510007 [DOI] [Google Scholar]
- Coronado-Blanco JM, Ruiz-Cancino E, Varela-Fuentes SE. (2004) Adenda a Braconidae (Hymenoptera). In: Llorente J, Morrone JJ, Yanez O, Vargas I. (Eds) , Biodiversidad, taxonomía y biogeografía de artrópodos de Mexico: hacia una síntesis de su conocimiento, Vol.IV. Universidad Autónoma de México, México DF, 713–720.
- Cresson ET. (1865) On the Hymenoptera of Cuba. Proceedings of the Entomological Society of Philadelphia 4: 1–200. [Google Scholar]
- Cresson ET. (1872) Hymenoptera Texana. Transactions of the American Entomological Society 4: 153–292. 10.2307/25076272 [DOI] [Google Scholar]
- Curtis J. (1829) Guide to an arrangement of British insects. London, 256 pp.
- Curtis J. (1830) British Entomology; being illustrations and descriptions of the genera of insects found in Great Britain and Ireland, 7: 321.
- Curtis J. (1835) Descriptions, & c. of the insects brought home by Commander James Clark Ross, R.N., F.R.S., &c. In: Ross J. Appendix to the narrative of a second voyage in search of a North-West Passage and of a residence in the Arctic Regions during the years 1829, 1830, 1831, 1832, 1833. Webster, London, 61-64.
- Cushman RA. (1929) Baker's Entomologica Malayana. The braconid genera Fornicia Brullé and Odontofornica Enderlein. Philippine Journal of Science 40: 233–237. [Google Scholar]
- Cushman RA. (1931) Description of thirteen new American and Asiatic Ichneumon-flies with taxonomic Notes. Proceedings of the United States National Museum 79(2880): 1–16. 10.5479/si.00963801.79-2880.1 [DOI] [Google Scholar]
- Dalla Torre CG. (1898) Catalogus Hymenopterorum. Volumen IV. Braconidae. Guilelmi Engelmann, Lipsiae, 323 pp. [Google Scholar]
- Dawale RK, Bhosale YA, Sathe TV. (1993) A new species of the genus Cotesia Cameron (Hymenoptera: Braconidae) from India. Bioved 4(2): 263–266. [Google Scholar]
- de Saeger H. (1941a) Le genre Apanteles au Congo Belge (Hym. Braconidae). Revue de Zoologie et de Botanique Africaines 34: 322–347. [Google Scholar]
- de Saeger H. (1941b) Le genre Apanteles au Congo Belge (Hym. Braconidae). Revue de Zoologie et de Botanique Africaines 35: 218–268. [Google Scholar]
- de Saeger H. (1942a) Description d'une nouvelle espèce du genre Fornicia (Hym. Bracon.). Revue de Zoologie et de Botanique Africaines 35(1941): 328–331 [Google Scholar]
- de Saeger H. (1942b) Le genre Microgaster au Congo belge (Hym. Braconidae). Revue de Zoologie et de Botanique Africaines 35(1941): 332–341. [Google Scholar]
- de Saeger H. (1942c) Un nouvel Apanteles du Sénégal (Hym.: Braconidae). Revue de Zoologie et de Botanique Africaines 36: 61–63. [Google Scholar]
- de Saeger H. (1944) Microgasterinae (Hymenoptera: Apocrita). Exploration du Parc National Albert. Mission G.F. de Witte, 47: 1–342. [Google Scholar]
- de Saeger H. (1948) Cardiochilinae et Sigalphinae (Hymenoptera: Apocrita) Fam. Braconidae. Exploration du Parc National Albert. Mission G.F. de Witte, 53: 1–272. [Google Scholar]
- de Santis L. (1941) Lista de Himenopteros parásitos y predatores de los insectos de la República Argentina. Boletim da Sociedade Brasileira de Agronomia 4: 1–66. [Google Scholar]
- de Santis L. (1964) Sobre dos especies brasileñas del genero Elasmus (Insecta: Hymenoptera: Elasmidae). Revista Agrícola, Buenos Aires 39(2): 89–92. [Google Scholar]
- de Santis L. (1967a) Catálogo de los Himenópteros Argentinos de la Serie Parasitica, incluyendo Bethyloidea. Comision de Investigaciones Cientificas, Provincia de Buenos Aires Gobernacion, La Plata, 337 pp. [Google Scholar]
- de Santis L. (1967b) Himenópteros parasitos de Lepidopteros estenomidos en Brasil. Revista Brasileira de Entomologia 12: 155.
- de Santis L. (1975) Himenópteros Icneumonoideos de la Isla Fernando de Noronha (Hym.: Braconidae, Ichneumonidae). Studia Entomologica 18: 251–258. [Google Scholar]
- de Santis L. (1980) Catálogo de los himenopteros brasileños de la serie Parasitica, incluyendo Bethyloidea. Editora da Universidade Federal do Paraná, Curitiba, 395 pp. [Google Scholar]
- de Santis L. (1987) Himenopteros parasitoides e hiperparasitoides de Anacraga sp. (Lepidoptera, Dalceridae) en Brasil. Revista Brasileira de Entomologia 31(1): 97–99. [Google Scholar]
- de Santis L, del Carmen Redolfi I. (1976) Un nuevo Apanteles (Hym., Braconidae) brasileño parasitoide de Hylesia (Lep.). Revista Brasileira de Biologia 36(1): 185–186. [Google Scholar]
- de Sousa-Lopes B, Whitfield JB, Salgado-Neto G, Del-Claro K. (2019) Cotesiaitororensis sp. nov. from Brazilian savanna: a new reared microgastrine wasp (Hymenoptera: Braconidae) described using an integrative taxonomic approach. Zootaxa 4544(3): 437–445. 10.11646/zootaxa.4544.3.9. [DOI] [PubMed] [Google Scholar]
- Deans AR, Whitfield JB, Janzen DH. (2003) Taxonomy and natural history of the Microgastrinae genus Alphomelon Mason (Hymenoptera: Braconidae). Journal of Hymenoptera Research 12(1): 1–41. [Google Scholar]
- DeGeer C. (1752) Mémoires pour servir a l’histoire des insectes, 1. L’imprimerie de LL Grefing, Stockholm, 707 pp. 10.5962/bhl.title.14802 [DOI] [Google Scholar]
- Deloach CJ, Cordo HA, Ferrer R, Runnacles J. (1980) Acigonainfusella, a potential biological control agent for water hyacinth observations in Argentina (with descriptions of two new species of Apanteles by L. de Santis). Annals of the Entomological Society of America 73(2): 138–146. 10.1093/aesa/73.2.138 [DOI] [Google Scholar]
- Dolphin K, Quicke DLJ. (2001) Estimating the global species richness of an incompletely described taxon: an example using parasitoid wasps (Hymenoptera: Braconidae). Biological Journal of the Linnean Society 73: 279–286. 10.1111/j.1095-8312.2001.tb01363.x [DOI] [Google Scholar]
- Donaldson JS. (1991) Three new species of Microgastrinae (Hymenoptera: Braconidae) from South Africa with notes on Glyptapantelesacraeae (Wilkinson). Journal of the Entomological Society of Southern Africa 54(1): 29–37. [Google Scholar]
- Enderlein G. (1912) H. Sauter's Formosa-Ausbeute. Braconidae, Proctotrupidae und Evaniidae (Hym.). Entomologische Mitteilungen 1: 257–267. 10.5962/bhl.part.25896 [DOI] [Google Scholar]
- Evenhuis HH, Vlug HJ. (1983) The Hymenopterous parasites of leaf-feeding apple Tortricids (Lepidoptera, Tortricidae) in the Netherlands. Tijdschrift voor Entomologie 126(6): 109–135. [Google Scholar]
- Fabricius JC. (1798) Supplementum entomologiae systematicae. Hafniae, 572 pp. 10.5962/bhl.title.123559 [DOI]
- Fagan-Jeffries EP, Austin AD. (2018) Synopsis of the parasitoid wasp genus Choeras Mason (Hymenoptera: Braconidae: Microgastrinae) from Australasia, with the description of two new species. Austral Entomology 57: 349–358. 10.1111/aen.12283. [DOI] [Google Scholar]
- Fagan-Jeffries EP, Cooper SJ, Austin AD. (2018a) Three new species of Dolichogenidea Viereck (Hymenoptera, Braconidae, Microgastrinae) from Australia with exceptionally long ovipositors. Journal of Hymenoptera Research 64: 177–190. 10.3897/jhr.64.25219 [DOI] [Google Scholar]
- Fagan-Jeffries EP, Cooper SJ, Bertozzi T, Bradford TM, Austin AD. (2018b) DNA barcoding of microgastrine parasitoid wasps (Hymenoptera: Braconidae) using high-throughput methods more than doubles the number of species known for Australia. Molecular Ecology Resources 18(5): 1132–1143. 10.1111/1755-0998.12904 [DOI] [PubMed] [Google Scholar]
- Fagan-Jeffries EP, Cooper SJ, Austin AD. (2019) New species of Australian microgastrine parasitoid wasps (Hymenoptera: Braconidae: Microgastrinae) documented through the ‘Bush Blitz’ surveys of national reserves. Zootaxa 4560(3): 401–440. 10.11646/zootaxa.4560.3.1. [DOI] [PubMed] [Google Scholar]
- Fahringer J. (1934) Opuscula braconologica. Band 3. Palaearktischen Region. Lieferung 5-8. Opuscula braconologica, Fritz Wagner, Wien, 321–594. 10.11646/zootaxa.4560.3.1 [DOI]
- Fahringer J. (1935) Schwedisch-chinesische wissenschaftliche Expedition nach den nordwestlichen Provinzen Chinas, 26. Hymenoptera. 4. Braconidae Kirby. Arkiv foer Zoologi 27A(12) (1934): 1–15.
- Fahringer J. (1936) Opuscula braconologica. Band 4. Palaearktischen Region. Lieferung 1-3. Opuscula braconologica. (1935) Fritz Wagner, Wien, 1–256.
- Fahringer J. (1937) Opuscula braconologica. Band 4. Palaearktische region. Lieferung 4-6. Opuscula braconologica. Fritz Wagner, Wien, 257–520.
- Fahringer J. (1938) Beiträge zur Kenntnis der Braconidenfauna Chinas. Arkiv foer Zoologi 30A(12): 1–16.
- Farahani S, Talebi AA, Rakhshani E. (2016) Iranian Braconidae (Insecta: Hymenoptera: Ichneumonoidea): diversity, distribution and host association. Journal of Insect Biodiversity and Systematics 2(1): 1–92. [Google Scholar]
- Faure JC, Alabouvette L. (1924) Note sur l'Anarsialineatella Zell. et ses parasites dans la région de Lyon en 1924. Revue de Zoologie Agricole et Appliquee 23: 279–288. [Google Scholar]
- Ferrière C. (1935) The chalcidoid parasites of lac-insects. Bulletin of Entomological Research 26(3): 391–406. [PubMed] [Google Scholar]
- Fernandez-Triana J. (2010) Eight new species and an annotated checklist of Microgastrinae (Hymenoptera: Braconidae) from Canada and Alaska. ZooKeys 63: 1–53. 10.3897/zookeys.63.565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Triana J. (2015) A revision of the genus Protomicroplitis Ashmead (Hymenoptera, Braconidae, Microgastrinae), with the description of a new species. Zootaxa 4039(4): 529–542. 10.11646/zootaxa.4039.4.3 [DOI] [PubMed] [Google Scholar]
- Fernandez-Triana J. (2018) Ten unique and charismatic new species of Microgastrinae wasps (Hymenoptera, Braconidae) from North America. ZooKeys 730: 123–150. 10.3897/zookeys.730.22869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Triana J. (2019) Revision of the North American species of Promicrogaster (Hymenoptera, Braconidae, Microgastrinae), with an updated key to all described species in North and Meso America. Journal of Hymenoptera Research 70: 89–112. 10.3897/jhr.70.35555 [DOI] [Google Scholar]
- Fernandez-Triana J, Goulet H. (2009) A review of the genus Philoplitis Nixon (Hymenoptera: Braconidae: Microgastrinae). ZooKeys 20: 285–298. 10.3897/zookeys.20.84 [DOI] [Google Scholar]
- Fernandez-Triana JL, Huber JT. (2010) Braconid parasitoids (Hymenoptera: Braconidae) of Nearctic Choristoneura species (Lepidoptera: Tortricidae), with a summary of other parasitoid families attacking Choristoneura. The Canadian Entomologist 142(4): 295–343. 10.4039/n10.025 [DOI] [Google Scholar]
- Fernandez-Triana J, Boudreault C. (2016) Keylimepiepeckorum gen. n. and sp. n., (Hymenoptera, Braconidae) from southern Florida, U.S., the first known brachypterous member of the subfamily Microgastrinae. ZooKeys 584: 95–107. 10.3897/zookeys.584.8319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Triana J, van Achterberg C. (2017) Microgastrinae (Hymenoptera: Braconidae) from the Arabian Peninsula. Arthropod fauna of the UAE, 6: 275–321. [Google Scholar]
- Fernandez-Triana J, Boudreault C. (2018) Seventeen new genera of microgastrine parasitoid wasps (Hymenoptera: Braconidae) from tropical areas of the world. Journal of Hymenoptera Research 64: 25–140. 10.3897/jhr.64.25453 [DOI] [Google Scholar]
- Fernandez-Triana J, Ward DF, Whitfield JB. (2011) Kiwigaster gen. nov. (Hymenoptera: Braconidae) from New Zealand: the first Microgastrinae with sexual dimorphism in number of antennal segments. Zootaxa 2932: 24–32. 10.11646/zootaxa.2932.1.2 [DOI] [Google Scholar]
- Fernandez-Triana J., Cardinal S, Whitfield JB, Hallwachs W, Smith MA, Janzen DH. (2013a) A review of the New World species of the parasitoid wasp Iconella (Hymenoptera: Braconidae, Microgastrinae). ZooKeys 321: 65–87, 10.3897/zookeys.321.5160 [DOI] [PMC free article] [PubMed]
- Fernandez-Triana J, Ward DF, Cardinal S, van Achterberg C. (2013b) A review of Paroplitis (Braconidae, Microgastrinae), and description of a new genus from New Zealand, Shireplitis, with convergent morphological traits. Zootaxa 3722(4): 549–568. 10.11646/zootaxa.3722.4.6 [DOI] [PubMed] [Google Scholar]
- Fernandez-Triana J, Janzen DH, Hallwachs W, Whitfield JB, Smith MA, Kula R. (2014a) Revision of the genus Pseudapanteles (Hymenoptera: Braconidae, Microgastrinae), with emphasis on the species in Area de Conservación Guanacaste, northwestern Costa Rica. ZooKeys 446: 1–82. 10.3897/zookeys.446.8195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Triana J, Penev L, Ratnasingham S, Smith M, Sones J, Telfer A, deWaard J, Hebert P. (2014b) Streamlining the use of BOLD specimen data to record species distributions: a case study with ten Nearctic species of Microgastrinae (Hymenoptera: Braconidae). Biodiversity Data Journal 2: e4153. 10.3897/BDJ.2.e4153 [DOI] [PMC free article] [PubMed]
- Fernandez-Triana J, Shaw M, Cardinal S, Mason P. (2014c) Contributions to the study of the Holarctic fauna of Microgastrinae (Hymenoptera, Braconidae). I. Introduction and first results of transatlantic comparisons. Journal of Hymenoptera Research 37: 61–76. 10.3897/jhr.37.7186 [DOI] [Google Scholar]
- Fernandez-Triana J, van Achterberg K, Whitfield JB. (2014d) Australasian endemic no more: four new species of Miropotes Nixon (Hymenoptera, Braconidae, Microgastrinae), with the first record from the Oriental region. Tijdschrift voor Entomologie (Netherlands Journal of Entomology) 157(1): 59–77. 10.1163/22119434-00002034 [DOI] [Google Scholar]
- Fernandez-Triana J, Whitfield JB, Rodriguez JJ, Smith MA, Janzen DH, Hallwachs W, Hajibabaei M, Burns JM, Solis MA, Brown J, Cardinal S, Goulet H, Hebert PDN. (2014e) Review of Apantelessensu stricto (Hymenoptera, Braconidae, Microgastrinae) from Area de Conservación Guanacaste, northwestern Costa Rica, with keys to all described species from Mesoamerica. ZooKeys 383: 1–565. 10.3897/zookeys.383.6418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Triana J, Whitfield JB, Smith MA, Braet Y, Hallwachs W, Janzen DH. (2014f) Review of the Neotropical genus Prasmodon (Hymenoptera, Braconidae, Microgastrinae), with emphasis on species from Area de Conservación Guanacaste, northwestern Costa Rica. Journal of Hymenoptera Research 37: 1–52. 10.3897/JHR.37.6748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Triana J, Whitfield JB, Smith MA, Hallwachs W, Janzen DH. (2014g) First record of the genus Venanus (Hymenoptera: Braconidae: Microgastrinae) in Mesoamerica, with the description of two new species from Costa Rica. Biodiversity Data Journal, 2: e4167. 10.3897/BDJ.2.e4167 [DOI] [PMC free article] [PubMed]
- Fernandez-Triana J, Whitfield JB, Smith MA, Hallwachs W, Janzen DH. (2014h) Revision of the neotropical genus Sendaphne Nixon (Hymenoptera: Braconidae: Microgastrinae). Journal of Hymenoptera Research 41: 1–29. 10.3897/JHR.41.8586 [DOI] [Google Scholar]
- Fernandez-Triana J, Noyes JS, Polaszek A, Yu DSK. (2015a) Clarification of the author and year of publication of Cotesiachilonis, a species used widely for biological control of Chilo stem borers. Journal of Hymenoptera Research 45: 113–123. 10.3897/JHR.45.5274 [DOI] [Google Scholar]
- Fernandez-Triana JL, Whitfield JB, Smith MA, Kula RR, Hallwachs W, Janzen DH. (2015b) Revision of the genera Microplitis and Snellenius (Hymenoptera, Braconidae, Microgastrinae) from Area de Conservacion Guanacaste, Costa Rica, with a key to all species previously described from Mesoamerica. Deutsche Entomologische Zeitschrift 62(2): 137–201. 10.3897/dez.62.5276 [DOI] [Google Scholar]
- Fernandez-Triana J, Boudreault C, Buffam J, Mclean R. (2016a) A biodiversity hotspot for Microgastrinae (Hymenoptera: Braconidae) in North America: annotated species checklist for Ottawa, Canada. ZooKeys 633: 1–93. 10.3897/zookeys.633.10480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Triana J, Boudreault C, Dapkey T, Smith MA, Rodriguez JJ, Hallwachs W, Janzen DH. (2016b) Revision of the genus Promicrogaster (Hymenoptera, Braconidae, Microgastrinae) from Area de Conservación Guanacaste, Costa Rica, with a key to all species previously described from Mesoamerica. Journal of Hymenoptera Research 50: 25–79. 10.3897/JHR.50.8220 [DOI] [Google Scholar]
- Fernandez-Triana JL, Whitfield JB, Smith MA, Dapkey T, Hallwachs W, Janzen DH. (2016c) Review of the world species of Exoryza (Hymenoptera, Braconidae, Microgastrinae), with description of five new species. Deutsche Entomologische Zeitschrift 63(2): 195–210. 10.3897/dez.63.8977 [DOI] [Google Scholar]
- Fernandez-Triana J, Beaudin M, van Achterberg K, Agbodzavu MK, Othim STO, Nyamu FW, Fiaboe KKM. (2017a) DNA barcodes, expanded distribution, and redescription of Apanteleshemara Nixon, 1965 (Hymenoptera, Braconidae, Microgastrinae), a potential biocontrol species against amaranth leaf-webbers in Africa. Journal of Hymenoptera Research 58: 1–15. 10.3897/jhr.58.13361 [DOI] [Google Scholar]
- Fernandez-Triana J, Buffam J, Beaudin M, Davis H, Fernandez-Galliano A, Griffin E, Lin SY, McAulay MK, Richter R, Rodriguez F, Várkonyi G. (2017b) An annotated and illustrated checklist of Microgastrinae wasps (Hymenoptera: Braconidae) from the Canadian Arctic Archipelago and Greenland. ZooKeys 691: 49–101. 10.3897/zookeys.691.14491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Triana J, Sakagami K, Shimizu S. (2018) Dolichogenideamaetoi sp. nov. (Hymenoptera: Braconidae) from Japan, the first parasitoid wasp recorded from Hyblaeafortissima (Lepidoptera). Acta Entomologica Musei Nationalis Pragae 58(1): 167–175. 10.2478/aemnp-2018-0014 [DOI] [Google Scholar]
- Ferrière C. (1935) The chalcidoid parasites of lac-insects. Bulletin of Entomological Research 26(3): 391–406. 10.1017/S0007485300036725 [DOI] [PubMed] [Google Scholar]
- Fiaboe KKM, Fernandez-Triana J, Nyamu FW, Agbodzavu MK. (2017) Cotesiaicipe sp. n., a new Microgastrinae wasp (Hymenoptera, Braconidae) of importance in the biological control of Lepidopteran pests in Africa. Journal of Hymenoptera Research 61: 49–64. 10.3897/jhr.61.21015 [DOI] [Google Scholar]
- Fischer M. (1961) Eine neue Apanteles-Art aus Jugoslawien (Hymenoptera, Braconidae). Zeitschrift der Arbeitsgemeinschaft Österreichischer Entomologen 13: 4–5. 10.1007/BF02371915 [DOI] [Google Scholar]
- Fischer M. (1964) Zwei neue gezüchtete Braconiden (Hymenoptera). Entomophaga 9(1): 39–44. 10.1007/BF02375737 [DOI] [Google Scholar]
- Fischer M. (1965) Eine neue Apanteles-Art aus dem Burgenland. Nachrichtenblatt der Bayerischen Entomologen 14: 121–123. [Google Scholar]
- Fischer M. (1966) Gezüchtete Braconiden aus Niederösterreich und aus dem Burgenland (Hymenoptera). Zeitschrift für Angewandte Zoologie 53: 385–402. [Google Scholar]
- Fischer M. (1968) Über gezüchtete Raupenwespen (Hymenoptera, Braconidae). Pflanzenschutz Berichte 37(7/8/9): 97–140.
- Fiske WF. (1903) A study of the parasites of the American tent caterpillar. New Hampshire Agricultural Experiment Station. Technical Bulletin 6: 184–230. [Google Scholar]
- Fitch EA. (1859) Flattened locust leaf-miner, Anacampsisrobiniella, new species (Lepidoptera. Yponomeutidae. ) Transactions of the New York State Agricultural Society 18(1858): 834–836. [Google Scholar]
- Fitton MG. (1978) The species of "Ichneumon" (Hymenoptera) described by Linnaeus. Biological Journal of the Linnean Society 10: 361–383. 10.1111/j.1095-8312.1978.tb00022.x [DOI] [Google Scholar]
- Foerster A. (1863) Synopsis der Familien und Gattungen der Braconiden. Verhandlungen des Naturhistorischen Vereins der Preussischen Rheinlande und Westfalens, 19(1862): 225–288. [Google Scholar]
- Foley IA, Ivie MA, Spies M. (2003) Pachystigmus Hellén, 1927, a substitute name for Noserus Foerster, 1863 (Hymenoptera: Braconidae), not Noserus LeConte, 1862 (Coleoptera: Zopheridae). Insecta Mundi 17(1–2): 103–106. [Google Scholar]
- Foottit RG, Adler PH. (2017) Insect biodiversity: science and society, volume I. Second Edition. Wiley-Blackwell, 904 pp. 10.1002/9781118945568 [DOI]
- Forbes (1883) Report of the noxious insects of Illinois. 12(1882): 104. [Google Scholar]
- Forshage M, Broad GR, Papilloud ND-S, Vårdal H. (2016) Insect species described by Karl-Johan Hedqvist. Journal of Hymenoptera Research 51: 101–158. 10.3897/jhr.51.9296 [DOI] [Google Scholar]
- Fourcroy AF. (1785) Entomologia Parisiensis, sive catalogus insectorum quae in agro Parisiensi reperiuntur. Paris, 544 pp. 10.5962/bhl.title.36528 [DOI]
- Freitas JG, Takahashi TA, Figueiredo LL, Fernandes PM, Camargo LF, Watanabe IM, Foerster LA, Fernandez-Triana J, Shimbori EM. (2019) First record of Cotesiascotti (Valerio and Whitfield, 2009) (Hymenoptera: Braconidae: Microgastrinae) comb. nov. parasitising Spodopteracosmioides (Walk, 1858) and Spodopteraeridania (Stoll, 1782) (Lepidoptera: Noctuidae) in Brazil. Revista Brasileira de Entomologia 63(3): 238–244. 10.1016/j.rbe.2019.05.001 [DOI]
- Fujie S, Shimizu S, Fernandez-Triana J. (2018) A new species and a key to world species of the flavipes species-group of the genus Cotesia Cameron, 1891 (Hymenoptera: Braconidae: Microgastrinae) from Japan. Zootaxa 4527(3), 372–380. 10.11646/zootaxa.4527.3.6 [DOI] [PubMed]
- Fullaway DT. (1940) New species from the Bishop Museum collection of Samoan parasitic Hymenoptera. Proceedings of the Hawaiian Entomological Society 10: 399–410. [Google Scholar]
- Fullaway DT. (1941) A checklist of the parasitic Hymenoptera of the Samoan Islands with descriptions of new species appended. Proceedings of the Hawaiian Entomological Society 11: 41–49. [Google Scholar]
- Fullaway DT. (1946) Apantelestapatapaoanus Fullaway. Proceedings of the Hawaiian Entomological Society 12: 486.
- Fullaway DT. (1950) Apanteles in Hawaii. Proceedings of the Hawaiian Academy of Sciences 25: 7.
- Gadallah NS, Ghahari H, Peris-Felipo FJ. (2015) Catalogue of the Iranian Microgastrinae (Hymenoptera: Braconidae). Zootaxa 4043(1): 1–69. 10.11646/zootaxa.4043.1.1 [DOI] [PubMed] [Google Scholar]
- Gahan AB. (1912) Descriptions of two new genera and six new species of parasitic Hymenoptera. Proceedings of the Entomological Society of Washington 14: 2–8. [Google Scholar]
- Gahan AB. (1917) Descriptions of some new parasitic Hymenoptera. Proceedings of the United States National Museum 53(2197): 195–217. 10.5479/si.00963801.53-2197.195 [DOI] [Google Scholar]
- Gahan AB. (1918) Four new African parasitic Hymenoptera belonging to the sub-family Microgasterinae. Proceedings of the United States National Museum 54: 587–590. 10.5479/si.00963801.54-2252.587 [DOI] [Google Scholar]
- Gahan AB. (1919) New reared parasitic Hymenoptera, with some notes on synonymy. Proceedings of the United States National Museum 55(2261): 113–128. 10.5479/si.00963801.55-2261.113 [DOI] [Google Scholar]
- Gahan AB. (1925) A second lot of parasitic Hymenoptera from the Philippines. Philippine Journal of Science 27: 83–109. [Google Scholar]
- Gallardo-Covas F. (2005) Parasitoids of Pseudoplusiaincludens Walker (Lepidoptera: Noctuidae) larvae on the south coast of Puerto Rico. The Journal of Agriculture of the University of Puerto Rico 89(1-2): 119–22. [Google Scholar]
- Gates MW, Heraty JM, Schauff ME, Wagner DL, Whitfield JB, Wahl DB. (2002) Survey of the parasitic Hymenoptera on leafminers in California. Journal of Hymenoptera Research 11(2): 213–270. [Google Scholar]
- Gautier C, Cleu H. (1927) Hyménoptères Braconides: description d'une nouvelle espèce d'Apanteles parasite de Hadenalateritia Hfn. (Lep. Noctuidae). Annales de la Société Entomologique de France 96: 85–91. [Google Scholar]
- Ghafouri Moghaddam M, Rakhshani E, van Achterberg C, Mokhtari A. (2018) A study of the Iranian species of Choeras Mason (Hymenoptera: Braconidae: Microgastrinae), with the description of a new species. Zootaxa 4446(4): 455–476. 10.11646/zootaxa.4446.4.3 [DOI] [PubMed] [Google Scholar]
- Ghafouri Moghaddam M, Rakhshani E, van Achterberg C, Mokhtari A. (2019) A taxonomic review of the genus Diolcogaster Ashmead (Hymenoptera, Braconidae, Microgastrinae) in Iran, distribution and morphological variability. Zootaxa 4590(1): 95–124. 10.11646/zootaxa.4590.1.4 [DOI] [PubMed] [Google Scholar]
- Ghahari H, van Achterberg C. (2016) A contribution to the study of subfamilies Microgastrinae and Opiinae (Hymenoptera: Braconidae) from the Arasbaran Biosphere Reserve and vicinity, Northwestern Iran. Natura Somogyiensis 28: 23–32. [Google Scholar]
- Giard A. (1898) Sur l’existence de Cemiostomacoffeella (Guérin-Mén.) [LÉP.] à l’île de la Réunion. Bulletin de la Société entomologique de France, Paris: 201–203.
- Giard A. (1902) Sur un moyen de lutte contre les insectes nuisibles à habitat très étendu. Bulletin d'Agriculture Colonial 1: 21–23. [Google Scholar]
- Giraud J. (1869) Observations hyménoptèrologiques. Annales de la Société Entomologique de France 4(9): 469–488. [Google Scholar]
- Gmelin JF. (1790) Caroli a Linne Systema Naturae (Ed. XIII). Tom I. G.E. Beer, Lipsiae, 2225–3020.
- Granger C. (1949) Braconides de Madagascar. Mémoires de l'Institut Scientifique de Madagascar (A) 2: 1–428. [Google Scholar]
- Greeney HF, Whitfield JB, Stireman JO III, Penz CM, Dyer LA. (2011) Natural History of Eryphanisgreeneyi (Lepidoptera: Nymphalidae) and its enemies, with a description of a new species of braconid parasitoid and notes on its tachinid parasitoid. Annals of the Entomological Society of America 104(6): 1078–1090. 10.1603/AN10064 [DOI] [Google Scholar]
- Grinter CC, Whitfield JB. (2019) Validation of Distatrixpandora Grinter, 2009 (Hymenoptera: Braconidae, Microgastrinae). Journal of Hymenoptera Research (68): 17–9. 10.3897/jhr.68.33598 [DOI]
- Grinter CC, Whitfield JB, Connahs H, Dyer LA, Hallwachs W, Janzen DH. (2009) A key to New World Distatrix Mason (Hymenoptera: Braconidae), with descriptions of six new reared Neotropical species. Journal of Insect Science 9(29): 1–17. 10.1673/031.009.2901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A. (2013a) Revision of the Indian Microplitis Foerster (Hymenoptera: Braconidae: Microgastrinae), with description of one new species. Zootaxa 3620(3): 429–452. 10.11646/zootaxa.3620.3.5 [DOI] [PubMed] [Google Scholar]
- Gupta A. (2013b) Three new species of reared parasitic wasps (Hymenoptera: Braconidae: Microgastrinae) from India. Zootaxa 3701(3): 365–380. 10.11646/zootaxa.3701.3.6 [DOI] [PubMed] [Google Scholar]
- Gupta A, Kalesh S. (2012) Reared parasitic wasps attacking hesperiids from Western Ghats (Kerala, India) with description of a new species of Dolichogenidea (Hymenoptera: Braconidae) as a larval parasitoid of Thoressaevershedi (Evans) (Lepidoptera: Hesperiidae). Zootaxa 3413: 29–43. 10.11646/zootaxa.3413.1.3 [DOI] [Google Scholar]
- Gupta A, Fernandez-Triana J. (2014) Diversity, host association and cocoon variability of reared Indian Microgastrinae (Hymenoptera: Braconidae). Zootaxa 3800(1): 1–101. 10.11646/zootaxa.3800.1.1 [DOI] [PubMed] [Google Scholar]
- Gupta A, Fernandez-Triana J. (2015) Four new species of the genus Diolcogaster Ashmead (Hymenoptera: Braconidae: Microgastrinae) from South East Asia with a key to the Indian species. Systematic Parasitology 90(3): 285–300. 10.1007/s11230-014-9546-8 [DOI] [PubMed] [Google Scholar]
- Gupta A, Ghosh A, Baby NL, Jalali SK. (2011) Morphological and molecular characterization of Apantelesmohandasi Sumodan & Narendran (Hymenoptera: Braconidae), a solitary endoparasitoid of Pammenecritica Meyrick (Lepidoptera: Tortricidae), with notes on biology from India. Entomological News 122: 354–365. 10.3157/021.122.0409 [DOI] [Google Scholar]
- Gupta A, Lokhande SA, Soman A. (2013a) Parasitoids of Hesperiidae from peninsular India with description of a new species of Dolichogenidea (Hymenoptera: Braconidae) parasitic on catarpillar of Borbocinnara (Wallace) (Lepidoptera: Hesperiidae). Zootaxa 3701(2): 277–290. 10.11646/zootaxa.3701.2.8 [DOI] [PubMed] [Google Scholar]
- Gupta A, Pereira B, Churi PV. (2013b) A new species of Parapanteles Ashmead (Hymenoptera: Braconidae) from India reared from Abisaraecheria Stoll (Lepidoptera: Riodinidae) with key to the Indian Parapanteles species. Zootaxa 3709(4): 363–370. 10.11646/zootaxa.3709.4.4 [DOI] [PubMed] [Google Scholar]
- Gupta A, Churi PV, Sengupta A, Mhartre S. (2014a) Lycaenidae parasitoids from peninsular India with description of four new species of microgastrine wasps (Hymenoptera: Braconidae) along with new insights on host relationships. Zootaxa 3827(4): 439–470. 10.11646/zootaxa.3827.4.2 [DOI] [PubMed] [Google Scholar]
- Gupta A, Khot R, Chorge S. (2014b) A new species of Parapanteles Ashmead, 1900 (Hymenoptera: Braconidae: Microgastrinae) parasitic on Charaxesathamas (Drury) (Lepidoptera: Nymphalidae) in India. Systematic Parasitology 88(3): 273–279. 10.1007/s11230-014-9492-5 [DOI] [PubMed] [Google Scholar]
- Gupta A, Saji K, Manoj P. (2016a) Parasitoids of butterflies: reassignment of Dolichogenideahasorae (Wilkinson, 1928) as a new combination along with new host-parasitoid linkages and notes on host specificity from Kerala, India. Journal of Biological Control 30(2): 61–67. 10.18641/jbc/30/2/79953 [DOI] [Google Scholar]
- Gupta A, Shaw M, Cardinal S, Fernandez-Triana J. (2016b) A review of unusual species of Cotesia (Hymenoptera, Braconidae, Microgastrinae) with the first tergite narrowing at midlength. ZooKeys 580: 29–44. 10.3897/zookeys.580.8090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta M, Pawar AD. (1992) Biological wealth in rice ecosystem. Plant Protection Bulletin (Faridabad) 44(3): 6–15. [Google Scholar]
- Gupta VK. (1957) Some species of Apanteles Förster and their hyperparasites from India with descriptions of new species (parasitic Hymenoptera). Indian Journal of Entomology 19: 101–106. [Google Scholar]
- Györfi J. (1955) Eine neue Brackwespe – Apantelescapeki aus der Slowakei. Zoologické a Entomologické Listy (Folia Zoologica and Entomologica) 4: 33–34. [Google Scholar]
- Hanson PE, Gauld ID. (1995) The Hymenoptera of Costa Rica, Oxford University Press, 893 pp.
- Haliday AH. (1834) Essay on parasitic Hymenoptera. Entomological Magazine 2(iii): 225–259.
- Halperin J. (1986) Braconidae (Hymenoptera) associated with forest and ornamental trees and shrubs in Israel. Phytoparasitica 14(2): 119–135. 10.1007/BF02980898 [DOI] [Google Scholar]
- Havrylenko D, Winterhalter JJ. (1949) Insectos del Parque Nacional Nahuel Huapí. Buenos Aires, 53.
- He JH, Chen XX, Lou XM, You LS. (1992) Braconidae. In: Peng JW, Liu YQ. (Eds) Iconography of Forest Insects in Hunan, China.Hunan Science and Technology Press, Changsha, 1250–1267.
- Hebert PD, Cywinska A, Ball SL, deWaard JR. (2003a) Biological identifications through DNA barcodes. Proceedings of the Royal Society B 270: 313–321. 10.1098/rspb.2002.2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert PD, Ratnasingham S, de Waard JR. (2003b) Barcoding animal life: cytochrome c oxidase subunit 1 divergences among closely related species. Proceedings of the Royal Society of London. Series B: Biological Sciences 270: S96–S99. 10.1098/rsbl.2003.0025 [DOI] [PMC free article] [PubMed]
- Hedqvist KJ. (1965) Braconidae from the Cape Verde Islands. Commentationes Biologicae (Helsinki) 28: 1–28. [Google Scholar]
- Hedqvist KJ. (1972) Notes on the parasites of green leaf miner (Epinotiananana Treitschke) (Hym., Ichneumonidae and Braconidae). Entomologisk Tidskrift 93(1-3): 60–64. [Google Scholar]
- Hedwig K. (1962) Mitteleuropäische Schlupfwespen und ihre Wirte. Nachrichten des Naturwissenschaftlichen Museums der Stadt Aschaffenburg 68: 87–97. [Google Scholar]
- Herrich-Schäffer GAW. (1838) Faunae Insectorum Germaniae initiae oder Deutschlands Insecten. Hymenoptera. Uebersicht der Gattung Coelinius Nees. Uebersicht der Gattung Eubadizon Nees. Auseinandersetzung der Gattung Sigalphus Latr. Regensburg. Heft 153.
- Holmgren AE. (1868) Hymenoptera. Species novas descripsit. Kongliga Svenska Fregatten Eugenies Resa omkring jorden. Zoologi 6: 391–442. [Google Scholar]
- Howard LO. (1897) A study in insect parasitism: a consideration of the parasites of the white-marked tussock moth, with an account of their habits and interrelations, and with descriptions of new species. United States Department of Agriculture. Bulletin of Entomology 5: 57. 10.1126/science.5.126.848 [DOI]
- Howard LO, Ashmead WH. (1896) On some reared parasitic Hymenopterous insects from Ceylon. Proceedings of the United States National Museum 18: 633–648. 10.5479/si.00963801.18-1092.633 [DOI] [Google Scholar]
- Howarth FG, Preston DJ, Pyle R. (2012) Surveying for terrestrial arthropods (insects and relatives) occurring within the Kahului Airport environs, Maui, Hawaii: Synthesis Report. Bishop Museum Technical Report 58, 215 pp.
- Hrcek J, Miller SE, Whitfield JB, Shima H, Novotny V. (2013) Parasitism rate, parasitoid community composition and host specificity on exposed and semi-concealed caterpillars from a tropical rainforest. Oecologia 173(2): 521–532. 10.1007/s00442-013-2619-6 [DOI] [PubMed] [Google Scholar]
- Huber JT, Sharkey MJ. (1993) Structure. In: Goulet H, Huber JT. (Eds) Hymenoptera of the world: an identification guide to families.Agriculture Canada Research Branch, Monograph No. 1894E, Ottawa, 13–59.
- Huflejt T. (1997) Ichneumonoidea. In: Razowski J [Ed.] Checklist of animals of Poland. Vol. 5: part 32/24 Hymenoptera – postscript. Wydawnictwa Instytutu Systematyki i Ewolucji Zwierzat PAN, Krakow, 75–114.
- Inanç F. (1992) Microgastergracilis sp. nov. aus der Turkei (Hymenoptera, Braconidae, Microgasterinae). Entomofauna 13(32): 537–541.
- Inanç F. (2002a) A new species of Microplitis (Hymenoptera: Braconidae, Microgastrinae) from Turkey. Biologia (Bratislava) 57(5): 563–566. [Google Scholar]
- Inanç F. (2002b) Cotesiapappi sp. n. (Hymenoptera, Braconidae: Microgastrinae) from Turkey. Acta Zoologica Academiae Scientiarum Hungaricae 48(2): 157–160. [Google Scholar]
- Inanç F, Çetin Erdogan O. (2004) Contribution to the Microgastrinae (Hymenoptera, Braconidae) fauna of Turkey, with description of a new species of Dolichogenidea. Biologia (Bratislava) 59(5): 547–551. [Google Scholar]
- Ivanov P. (1899) Braconids cryptogastres et aréolaires des environs de Koupiansk avec tableaux synoptiques des genres et des espèces de ces insectes. [In Ukrainian] Trudy Obshchestva Ispytatelei Prirody pri Imperatorskom Khar'kovskom Universitete, 33: 273–382. [Google Scholar]
- Janzen DH, Hallwachs W. (2016) DNA barcoding the Lepidoptera inventory of a large complex tropical conserved wildland, Area de Conservacion Guanacaste, northwestern Costa Rica. Genome 59(9): 641–660. [DOI] [PubMed] [Google Scholar]
- Janzen DH, Walker AK, Whitfield JB, Delvare G, Gauld ID. (2003) Host-specific and hyperparasitoids of three new Costa Rican species of Microplitis Foerster (Hymenoptera: Braconidae: Microgastrinae), parasitoids of sphingid caterpillars. Journal of Hymenoptera Research 12(1): 42–76. 10.1139/gen-2016-0005 [DOI] [Google Scholar]
- Jiménez R, Falcó JV, Moreno J, Oltra MT. (1996) Approximation of the braconids study (Hymenoptera, Ichneumonoidea) from the "Parque Natural de las Sierras de Cazorla, Segura y Las Villas". Aproximacion al estudio de los braconidos (Hymenoptera, Ichneumonoidea) del Parque Natural de las Sierras de Cazorla, Segura y Las Villas. Boletin de la Asociacion Española de Entomologia 20(3–4): 9–17. [Google Scholar]
- Jones OR, Purvis A, Baumgart E, Quicke DLJ. (2009) Using taxonomic revision data to estimate the geographic and taxonomic distribution of undescribed species richness in the Braconidae (Hymenoptera: Ichneumonoidea). Insect Conservation and Diversity 2: 204–212. 10.1111/j.1752-4598.2009.00057.x [DOI] [Google Scholar]
- Kaiser L, Fernandez-Triana J, Capdevielle-Dulac C, Chantre C, Bodet M, Kaoula F, Benoist R, Calatayud P, Dupas S, Herniou EA, Jeannette R, Obonyo J, Silvain JF, Le Ru B. (2017) Systematics and biology of Cotesiatyphae sp. n. (Hymenoptera, Braconidae, Microgastrinae), a potential biological control agent against the noctuid Mediterranean corn borer, Sesamianonagrioides. ZooKeys 682: 105–136. 10.3897/zookeys.682.13016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant R. (2019) Parasitism of diamondback moth Plutellaxylostella by the solitary parasitoid wasp Cotesiavestalis in Samoa. New Zealand Plant Protection 72: 283. 10.30843/nzpp.2019.72.324 [DOI]
- Karlsson D, Ronquist F. (2012) Skeletal morphology of Opiusdissitus and Biosterescarbonarius (Hymenoptera: Braconidae), with a discussion of terminology. PLoS ONE 7(4). 10.1371/journal.pone.0032573 [DOI] [PMC free article] [PubMed]
- Kavallieratos NG, Stankovic SS, Schwarz M, Alissandrakis E, Athanassiou CG, Floros GD, Žikic V. (2019) A survey of parasitoids from Greece with new associations. ZooKeys 817: 25–40. 10.3897/zookeys.817.3011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer JJ, Jörgensen P. (1910) Gallen und Gallentiere aus Argentinien. Zentralblatt für Bakteriologie und Parasitenkunde (2)27: 362–442.
- Kieffer JJ, Tavares JS. (1925) Nova contribuição para o conhecimento da cecidologia brasileira. Brotéria, 22: 5–55. [Google Scholar]
- Kittel R. (2016) Eighty-nine Replacement Names for Braconidae and Ichneumonidae (Insecta: Hymenoptera: Ichneumonoidea). Japanese Journal of Systematic Entomology 22(2): 161–174. [Google Scholar]
- Koçak AO, Kemal M. (2009) A replacement name in the family Braconidae (Hymenoptera). Centre for Entomological Studies Miscellaneous Papers 14: 147–148. [Google Scholar]
- Koçak AO, Kemal M. (2013) Nomenclatural notes on the Asiatic Ichneumonoidea (Hymenoptera), Miscellaneous Papers, Centre for Entomological Studies Ankara 160: 7.
- Kokujev NR. (1914) Hymenoptera parasitic nove fauna turanica V.I. Platnikov collecta. Revue Russe d'Entomologie 13: 513–514. [Google Scholar]
- Kopelke JP. (2011) . Community structure and mortality in European populations of Phyllocolpa leaf-gallers (Hymenoptera: Tenthredinidae: Nematinae). Entomologia Generalis 33(1/2): 1–34. 10.1127/entom.gen/33/2011/1 [DOI]
- Kotenko AG. (1981) A new species of the Apanteles Foerster genus of the merula group (Hymenoptera, Braconidae) from the Black Sea Reservation. [In Russian with English summary]. Vestnik Zoologii 2: 26–30. [Google Scholar]
- Kotenko AG. (1986) New and little-known species of the genus Apanteles (Hymenoptera, Braconidae) from the USSR, Vestnik Zoologii 3: 19–25.
- Kotenko AG. (1992) A contribution to the fauna of Braconidae (Hymenoptera) of Dauria. In: Amirkhanov AM. (Ed.) Insects of Dauria and adjacent territories.Izdatelstvo Tsentralnoi Nauchno issledovatelskoi laboratorii okhotnichego khozyaistva i zapovednikov. Moskva, 94–107.
- Kotenko AG. (1993) A new species of the genus Hygroplitis (Hymenoptera, Braconidae) from Sakhalin. Vestnik Zoologii 3: 31–34. [Google Scholar]
- Kotenko AG. (1994) On the braconid-fauna (Hymenoptera, Braconidae) of the Dahuria. Report 2. In: Kotenko AG. (Ed.) Hymenopteran insects of Siberia and Far East: memoirs of the Daursky Nature Reserve, no.3. Institut zoologii NAN Ukrainy. Kiev, 79–89.
- Kotenko AG. (2004) Two new species of Illidops Mason (Hymenoptera: Braconidae, Microgastrinae) from Turkmenia, Kazakhstan and Russia. [In Russian with English summary] Trudy Russkogo Entomologicheskogo Obshchestva 75(1): 118–121. [Google Scholar]
- Kotenko AG. (2006) The types of Ichneumonoidea, Cynipoidea, and Chalcidoidea (Hymenoptera, Apocrita) deposited in the collection of the Schmalhausen Institute of Zoology of National Academy of Sciences of Ukraine. Family Braconidae. Vestnik Zoologii, Supplement 20: 29–42. [Google Scholar]
- Kotenko AG. (2007a) Microgastrinae. In: Lelej AS. (Ed.) Key to the insects of Russia Far East.Vol. IV. Neuropteroidea, Mecoptera, Hymenoptera. Pt 5. Vladivostok: Dalnauka, 134–192.
- Kotenko AG. (2007b) Review Palaearctic species of the genus Iconella (Hymenoptera, Braconidae, Microgastrinae): Species with propodeum lacking the median longitudinal carina [In Russian with English summary] Vestnik Zoologii 41(4): 315–325.
- Kress WJ, García-Robledo C, Uriarte M, Erickson DL. (2015) DNA barcodes for ecology, evolution, and conservation. Trends in Ecology & Evolution 30(1): 25–35. 10.1016/j.tree.2014.10.008 [DOI] [PubMed] [Google Scholar]
- Kristensen NP, Skalski AW. (1999) Phylogeny and palaeontology. In: Kristensen NP (Ed.), Handbook of Zoology, Volume 1: Evolution, Systematics, and Biogeography, Walter de Gruyter, Berlin, New York: 7–25.
- Kristensen NP, Scoble MP, Karsholt O. (2007) Lepidoptera phylogeny and systematics: the state of inventorying moth and butterfly diversity. Zootaxa 1668: 699–747. 10.11646/zootaxa.1668.1.30 [DOI] [Google Scholar]
- Kuklinski F, Borgemeister C. (2002) Cotton pests and their natural enemies in Madagascar. Journal of Applied Entomology 126: 55–65. 10.1046/j.1439-0418.2002.00622.x [DOI] [Google Scholar]
- Kurdjumov NV. (1912) Hyménoptères-parasites nouveaux ou peu connus. Revue Russe d'Entom 12: 223–240. [Google Scholar]
- Kurhade SM, Nikam PK. (1997) A new species of the genus Apanteles Foerster (Hymenoptera: Braconidae) from India. Journal of the Bombay Natural History Society 94(1): 124–126. [Google Scholar]
- Kurhade SM, Nikam PK. (1998) Report on a new species of genus Cotesia (Cameron) from India (Hymenoptera: Braconidae). Journal of Animal Morphology and Physiology 45(1-2): 145–146. [Google Scholar]
- Lacatusu M. (1961) Braconide (Hymenoptera-Braconidae) din fauna Republicii Populare Romine. Studii si Cercetari de Biologie. Seria Biologie Animala 13: 173–188. [Google Scholar]
- Lal KB. (1939) Some new species of Hymenoptera from India. Indian Journal of Entomology 1: 49–58. [Google Scholar]
- Lal KB. (1942) Description of two new and redescription of a third species of Apanteles from India. Indian Journal of Entomology 4: 163–166. [Google Scholar]
- LaSalle J, Gauld ID. (1991) Parasitic Hymenoptera and the biodiversity crisis. Redia 74(3): 315–334. [Google Scholar]
- Latreille PA. (1804) Histoire naturelle, générale et particulière, des Crustacés et des Insectes. Tome treizième. Paris (1802–1804), 432 pp.
- Lepeletier M, Serville JG. (1825) (Paxylomma on p. 23. Peltaste on pp. 36-44.) In: Latreille M. Encyclopédie méthodique. Histoire naturelle. Entomologie, ou histoire naturelle des Crustacés, des Arachnides et des Insectes. Tome dixième. Paris, 833 pp. [Google Scholar]
- Li XY, Tan JC, Song DB. (2009) A new species of Microplitis Foerster (Hymenoptera: Braconidae: Microgastrinae) of China. Entomotaxonomia 31(3): 225–229. [Google Scholar]
- Linnaeus C. (1758) Systema naturae per regna tria naturae, secundum classes, ordines, genera, species cum characteribus, differentiis, synonymis locis. Tomus I. Editio decima, reformata. Laurnetii Salvii, Holmiae, 824 pp. 10.5962/bhl.title.542 [DOI] [Google Scholar]
- Liu LR, You LS. (1988) A new species of the genus Apanteles Foerster from Hengduan Mountains, China (Hymenoptera: Braconidae). [In Chinese with English summary] Acta Entomologica Sinica 31(3): 318–320. [Google Scholar]
- Liu Z, He JH, Chen XX. (2014) The grandiculus- and metacarpalis-group of the genus Apanteles Foerster, 1862 (Hymenoptera, Braconidae, Microgastrinae) from China, with descriptions of eight new species. Zootaxa 3765(5): 435–457. 10.11646/zootaxa.3765.5.3 [DOI] [PubMed] [Google Scholar]
- Liu Z, He JH, Chen XX. (2015) The lacteus-, laspeyresiella- and mycetophilus-groups of Apanteles Foerster, 1862 (Hymenoptera, Braconidae, Microgastrinae) in China, with descriptions of eight new species. Zootaxa 3949(3): 370–392. 10.11646/zootaxa.3949.3.4 [DOI] [PubMed] [Google Scholar]
- Liu Z, He JH, Chen XX. (2016) The genus Pholetesor Mason, 1981 (Hymenoptera, Braconidae, Microgastrinae) from China, with descriptions of eleven new species. Zootaxa 4150(4): 351–87. 10.11646/zootaxa.4150.4.1 [DOI] [PubMed] [Google Scholar]
- Liu Z, He JH, Chen XX. (2018) The laevigata-group of the genus Dolichogenidea Mason, 1981 from China, with descriptions of 26 new species. Zootaxa 4436(1): 1–74. 10.11646/zootaxa.4436.1.1 [DOI] [PubMed] [Google Scholar]
- Liu Z, He JH, Chen XX, Gupta A, Ghafouri Moghaddam M. (2019) The ultor-group of the genus Dolichogenidea Viereck (Hymenoptera, Braconidae, Microgastrinae) from China with the descriptions of thirty-nine new species. Zootaxa 4710(1), 1–134. 10.11646/zootaxa.4710.1.1 [DOI] [PubMed]
- Long KD. (2007) Three new species of the subfamily Microgastrinae (Hymenoptera: Braconidae) from Viet Nam. Tap Chi Sinh Hoc (Journal of Biology) 29(2): 35–43. 10.15625/0866-7160/v29n2.5371 [DOI] [Google Scholar]
- Long KD. (2010) Five new species of the genus Apanteles Foerster (Hymenoptera: Braconidae: Microgastrinae) from Vietnam. Tap Chi Sinh Hoc (Journal of Biology) 32(4): 69–79. 10.15625/0866-7160/v32n4.723 [DOI] [Google Scholar]
- Long KD. (2015) New record of the genus Buluka de Saeger (Hymenoptera: Braconidae: Microgastrinae) with description of a new species from Vietnam. Academia Journal of Biology 37(3): 282–287. 10.15625/0866-7160/v37n3.6761 [DOI] [Google Scholar]
- Long KD, van Achterberg C. (2003) Two new species of the genus Wilkinsonellus Mason (Hymenoptera: Braconidae: Microgastrinae) from northern Vietnam. Zoologische Mededelingen Leiden 77(10): 221–227. [Google Scholar]
- Long KD, Belokobylskij SA. (2004) A preliminary list of the Braconidae (Hymenoptera) of Vietnam. Russian Entomological Journal 12(4): 385–398. [Google Scholar]
- Long KD, van Achterberg C. (2008) Two genera and one species newly recorded with description of five species of the subfamily Microgastrinae (Hymenoptera: Braconidae) from Vietnam. Tap Chi Sinh Hoc (Journal of Biology) 30(3): 78–87. 10.15625/0866-7160/v30n3.5438 [DOI] [Google Scholar]
- Long KD, van Achterberg C. (2011) Review of the genus Wilkinsonellus Mason, 1981 (Hymenoptera, Braconidae, Microgastrinae) from Vietnam, with a key to species and four new species. Deutsche Entomologische Zeitschrift 58(1): 123–133. 10.1002/mmnd.201100009 [DOI] [Google Scholar]
- Long KD, van Achterberg C. (2013) New records of the genus Snellenius Westwood, 1882 (Hymenoptera: Braconidae: Microgastrinae) from Vietnam, with description of two new species. Tap Chi Sinh Hoc (Journal of Biology) 35(3): 272–280. 10.15625/0866-7160/v35n3.3371 [DOI] [Google Scholar]
- Long KD, Dzung DT. (2014) Synopsis of Cotesia species, biological agents for pest control on vegetables in Vietnam, with description of one new species. Tijdschrift voor Entomologie 157(2–3): 83–93. 10.1163/22119434-00002042 [DOI] [Google Scholar]
- Long KD, van Achterberg C. (2014) An additional list with new records of Braconid wasps of the family Braconidae (Hymenoptera) from Vietnam. Academia Journal of Biology 36(4): 397–415. 10.15625/0866-7160/v36n4.5979 [DOI] [Google Scholar]
- Long KD, van Achterberg C. (2015) A new species of the genus Nyereria Mason (Hymenoptera: Braconidae: Microgastrinae) from Vietnam. Tap Chi Sinh Hoc 37(3): 288–295. 10.15625/0866-7160/v37n3.6945 [DOI] [Google Scholar]
- Lozan A. (2008) A new braconid parasitoid of the tortricid moth Cydiaalazon (Diakonoff, 1976) from the Canary Islands, Spain (Hymenoptera, Braconidae: Microgastrinae). Entomologist's Monthly Magazine 144(1727–1729): 103–107. [Google Scholar]
- Lundbeck W. (1897) Hymenoptera groenlandica. Videnskabelige Meddelelser fra den Naturhistoriske Forening i Kjøbenhaven 1896: 220–251. [Google Scholar]
- Luo QH, You LS. (2005a) Description of a new species and a new combination of Microgastrinae (Hymenoptera: Braconidae) from China. Entomotaxonomia 27(1): 50–56. [Google Scholar]
- Luo QH, You LS. (2005b) Descriptions of two new species of the genus Snellenius Westwood (Hymenoptera, Braconidae, Microgastrinae) from China. [In Chinese with English summary] Acta Zootaxonomica Sinica 30(1): 170–174. [Google Scholar]
- Luo QH, You LS. (2008) A new species of the genus Fornicia Brulle (Hymenoptera, Braconidae, Microgastrinae) from China. [In Chinese with English summary] Acta Zootaxonomica Sinica 33(1): 184–186. [Google Scholar]
- Luo QH, You LS, Xiao ZS. (2004) A new genus of Microgastrinae from China (Hymenoptera, Braconidae). Acta Zootaxonomica Sinica 29(2): 339–341. [Google Scholar]
- Lyle GT. (1917) Contributions to our knowledge of the British Braconidae. No. 3. Microgasteridae. Entomologist 50: 193–201. 10.5962/bhl.part.3487 [DOI] [Google Scholar]
- Lyle GT. (1918) Contributions to our knowledge of the British Braconidae. Entomologist 51: 104–111. [Google Scholar]
- Lyle GT. (1921) On three new species of Indian Braconidae. Bulletin of Entomological Research 12: 129–132. 10.1017/S0007485300044965 [DOI] [Google Scholar]
- Lyle GT. (1925) Some Braconidae new to Britain. Entomologist's Monthly Magazine 61: 119–123. [Google Scholar]
- Lyle GT. (1927) Two new species of Apanteles (Hym., Braconidae). Bulletin of Entomological Research 17: 415–416. 10.1017/S0007485300019520 [DOI] [Google Scholar]
- Madl M, van Achterberg C. (2014) A catalogue of the Braconidae (Hymenoptera: Ichneumonoidea) of the Malagasy subregion. Linzer Biologische Beitraege 46(1): 5–220. [Google Scholar]
- Maeto K. (1996) Inter-generic variation in the external male genitalia of the subfamily Microgastrinae (Hymenoptera, Braconidae), with a reassessment of Mason's tribal system. Journal of Hymenoptera Research 5: 38–52. [Google Scholar]
- Mahdihassan S. (1925) Some insects associated with lac and a symbolic representation of their inter-relationship. Journal of the Vizianagram Science Association 2: 64–88. [Google Scholar]
- Malo F, Willis ER. (1961) Life history and biological control of Caligoeurilochus, a pest of banana. Journal of Economic Entomology 54: 530–536. 10.1093/jee/54.3.530 [DOI] [Google Scholar]
- Mani MS. (1938) Catalogue of Indian Insects, Part 23: Chalcidoidea. ICAR, New Delhi, 1–174.
- Marsh PM. (1975) A new species of Apanteles from South America being introduced into California. Pan-Pacific Entomologist 51(2): 143–146. [Google Scholar]
- Marsh PM. (1978) The braconid parasites (Hymenoptera) of Heliothis species (Lepidoptera: Noctuidae). Proceedings of the Entomological Society of Washington 80(1): 15–36. [Google Scholar]
- Marsh PM. (1979a) Braconidae. Aphidiidae. Hybrizontidae. In: Krombein KV, Hurd Jr. PD, Smith DR, Burks BD (Eds) Catalog of Hymenoptera in America north of Mexico. Smithsonian Institution Press, Washington, 144–313.
- Marsh PM. (1979b) Description of new Braconidae (Hymenoptera) parasitic on the potato tuberworm and on related Lepidoptera from Central and South America. Journal of the Washington Academy of Sciences 69(1): 12–17. [Google Scholar]
- Marsh PM. (1979c) The braconid (Hymenoptera) parasites of the gypsy moth, Lymantriadispar (Lepidoptera, Lymantriidae). Annals of the Entomological Society of America 72(6): 794–810. 10.1093/aesa/72.6.794 [DOI] [Google Scholar]
- Marshall TA. (1872) A catalogue of British Hymenoptera; Chrysididae, Ichneumonidae, Braconidae, and Evanidae. A. Napier, London, The Entomological Society of London, 136 pp. [Google Scholar]
- Marshall TA. (1885) Monograph of British Braconidae. Part I. Transactions of the Entomological Society of London 1885: 1–280. 10.1111/j.1365-2311.1885.tb00886.x [DOI] [Google Scholar]
- Marshall TA. (1890) Les Braconides. In: André E (Ed.) Espèces des Hyménoptères d'Europe et d'Algérie. Beaune, Tome 4, 609 pp. [Google Scholar]
- Marshall TA. (1899) Descriptions de Braconides. Bulletin du Muséum national d'Histoire naturelle, Paris 5: 372–373. [Google Scholar]
- Marshall TA. (1900) Les Braconides (Supplément). In: André E (Ed.) Espèces des Hyménoptères d'Europe et d'Algérie. Paris, Tome 5, 369 pp. [Google Scholar]
- Marshall TA. (1901) Description de deux espèces nouvelles de Braconides. Bulletin du Muséum national d'Histoire naturelle, Paris 6(1900): 363–364. [Google Scholar]
- Martinez JJ, Berta C, Varone L, Logarzo G, Zamudio P, Zaldivar-Riveron A, Aguilar-Velasco RG. (2012) DNA barcodes and morphological identification of the parasitoids of cactus feeding moths (Lepidoptera: Pyralidae: Phycitinae). Invertebrate Systematics 26: 435–444. 10.1071/IS12060 [DOI] [Google Scholar]
- Mason WRM. (1959) Some new Braconidae (Hymenoptera). The Canadian Entomologist 91: 42–50. 10.4039/Ent9142-1 [DOI] [Google Scholar]
- Mason WRM. (1960) New Hymenopterous parasites of lodgepole pine needle miners. The Canadian Entomologist 92: 140–147. 10.4039/Ent92140-2 [DOI] [Google Scholar]
- Mason WRM. (1974) The Apanteles species (Hymenoptera: Braconidae attacking Lepidoptera in the micro-habitat of the spruce budworm (Lepidoptera: Tortricidae). The Canadian Entomologist 106: 1087–1102. 10.4039/Ent1061087-10 [DOI] [Google Scholar]
- Mason WRM. (1975) A new Nearctic Apanteles (Hymenoptera: Braconidae) from Oregon grape (Berberidaceae). The Canadian Entomologist 107: 1133–1135. 10.4039/Ent1071133-10 [DOI] [Google Scholar]
- Mason WRM. (1981) The polyphyletic nature of Apanteles Foerster (Hymenoptera: Braconidae): A phylogeny and reclassification of Microgastrinae. Memoirs of the Entomological Society of Canada 113(S115): 1–147. 10.4039/entm113115fv [DOI]
- Mason WRM. (1986) Microgaster Latreille, 1804. (Insecta, Hymenoptera): proposed designation of Microgasteraustralis Thomson, 1895 as type species. Z.N.(S.) 2397. Bulletin of Zoological Nomenclature 43(2): 173–174. 10.5962/bhl.part.413 [DOI]
- McGough JM, Noble LW. (1957) Summary of work at Brownsville, Texas with imported pink bollworm parasites and an aphid predator. Journal of Economic Entomology 50(4): 514. 10.1093/jee/50.4.514 [DOI] [Google Scholar]
- Michel-Salzat A, Whitfield JB. (2004) Preliminary evolutionary relationships within the parasitoid wasp genus Cotesia (Hymenoptera: Braconidae: Microgastrinae): combined analysis of four genes. Systematic Entomology 29(3): 371–382. 10.1111/j.0307-6970.2004.00246.x [DOI] [Google Scholar]
- Mills NJ, Kenis M. (1991) A study of the parasitoid complex of the European fir budworm, Choristoneuramurinana (Lepidoptera: Tortricidea), and its relevance for biological control of related hosts. Bulletin of Entomological Research 81: 429–436. 10.1017/S0007485300031990 [DOI] [Google Scholar]
- Minamikawa J. (1954) On the Hymenopterous parasites of the tea-leafrollers found in Japan and Formosa. Mushi 26: 35–46. [Google Scholar]
- Motschoulsky V. (1863) Essai d'un catalogue des insectes de l'ile Ceylan. Bulletin de la Société Impériale des Naturalistes, Moscou 36: 1–153. [Google Scholar]
- Muesebeck CFW. (1919) Three new species of Braconidae. The Canadian Entomologist 51: 113–116. 10.4039/Ent51113-5 [DOI] [Google Scholar]
- Muesebeck CFW. (1921) A revision of the North American species of ichneumon-flies belonging to the genus Apanteles. Proceedings of the United States National Museum 58: 483–576. 10.5479/si.00963801.2349.483 [DOI] [Google Scholar]
- Muesebeck CFW. (1922) A revision of the North American ichneumon-flies, belonging to the subfamilies Neoneurinae and Microgasterinae. Proceedings of the United States National Museum 61: 1–76. ttps:// 10.5479/si.00963801.61-2436.1 [DOI]
- Muesebeck CFW. (1926) Descriptions of new reared parasitic Hymenoptera and some notes on synonymy. Proceedings of the United States National Museum 69(7): 1–18. 10.5479/si.00963801.2633 [DOI] [Google Scholar]
- Muesebeck CFW. (1928) A new European species of Apanteles parasitic on the gipsy moth. Proceedings of the Entomological Society of Washington 30(1): 8–9. [Google Scholar]
- Muesebeck CFW. (1929) Two new species of Apanteles (Hymenoptera: Braconidae). Proceedings of the Entomological Society of Washington 31(6): 118–120. [Google Scholar]
- Muesebeck CFW. (1931) Descriptions of a new genus and eight new species of ichneumon-flies, with taxonomic Notes. Proceedings of the United States National Museum 79(2882): 1–16. 10.5479/si.00963801.79-2882.1 [DOI] [Google Scholar]
- Muesebeck CFW. (1933a) Five new Hymenopterous parasites of the Oriental fruit moth. Proceedings of the Entomological Society of Washington 35(4): 48–54. [Google Scholar]
- Muesebeck CFW. (1933b) Seven new species of reared Braconidae (Hymenoptera). Proceedings of the Entomological Society of Washington 33(9): 193–200. [Google Scholar]
- Muesebeck CFW. (1935) Three new reared parasitic Hymenoptera, with some notes on synonymy. Journal of the Washington Academy of Sciences 25: 279–283. [Google Scholar]
- Muesebeck CFW. (1938) Three new reared species of Apanteles from California (Hymenoptera: Braconidae). Proceedings of the Entomological Society of Washington 40(7): 201–204. [Google Scholar]
- Muesebeck CFW. (1946) A new Apanteles from Hawaii (Hymenoptera: Braconidae). Proceedings of the Hawaiian Entomological Society 12: 615–616. [Google Scholar]
- Muesebeck CFW. (1947) Two new species of Apanteles from California (Hymenoptera: Braconidae). Pan-Pacific Entomologist 23(1): 21–24. [Google Scholar]
- Muesebeck CFW. (1953) Three new reared Braconidae (Hymenoptera). Proceedings of the Entomological Society of Washington 55(3): 149–151. [Google Scholar]
- Muesebeck CFW. (1955) New reared Braconidae from Trinidad (Hymenoptera). Proceedings of the Entomological Society of Washington 57(4): 161–164. [Google Scholar]
- Muesebeck CFW. (1956a) Some braconid parasites of the pink bollworm Pectinophoragossypiella (Saunders). Bollettino del Laboratorio di Zoologia Generale e Agraria, Portici 33: 57–68. [Google Scholar]
- Muesebeck CFW. (1956b) Two new braconid parasites of the avocado looper (Hymenoptera: Braconidae). Pan-Pacific Entomologist 32(1): 25–28. [Google Scholar]
- Muesebeck CFW. (1957) New World Apanteles parasitic on Diatraea (Hymenoptera: Braconidae). Entomological News 68: 19–25. [Google Scholar]
- Muesebeck CFW. (1958a) Family Braconidae. In: Krombein KV. (Ed.) Hymenoptera of America North of Mexico synoptic catalog (Agriculture Monograph No.2), first supplement. United States Government Printing Office, Washington DC, USA, 18–36.
- Muesebeck CFW. (1958b) New Neotropical wasps of the family Braconidae (Hymenoptera) in the U.S. National Museum. Proceedings of the United States National Museum 107: 405–461. 10.5479/si.00963801.108-3389.405 [DOI] [Google Scholar]
- Muesebeck CFW. (1965) Two new Braconid parasites of the spruce budworm (Hymenoptera). Entomological News 76: 71–74. [Google Scholar]
- Muesebeck CFW, Walkley LM. (1951) Family Braconidae. In: Muesebeck C. F.W., Krombein K.V. & Townes H.K. (Eds.) " Hymenoptera of America North of Mexico – Synoptic catalog." U.S. Dept. Agriculture Monograph No.2: 90–184.
- Muesebeck CFW, Subba Rao BR. (1958) A new braconid parasite of Hymeniarecurvalis (Fabricius). Indian Journal of Entomology 20: 27–28. [Google Scholar]
- Muirhead K, Austin A, Sallam M. (2008) The systematics and biology of Cotesianonagriae (Olliff) stat. rev. (Hymenoptera: Braconidae: Microgastrinae), a newly recognized member of the Cotesiaflavipes species complex. Zootaxa 1846: 35–46. 10.11646/zootaxa.1846.1.3 [DOI] [Google Scholar]
- Munakata T. (1912) Investigation into the Chilosuppressalis (Walker) of Aomori Prefecture. Extra Report Agricultural Experimental Station Aomori 2: 67–76. [Google Scholar]
- Murphy N, Banks JC, Whitfield JB, Austin AD. (2008) Phylogeny of the parasitic microgastroid subfamilies (Hymenoptera: Braconidae) based on sequence data from seven genes, with an improved time estimate of the origin of the lineage. Molecular Phylogenetics and Evolution 47(1): 378–395. 10.1016/j.ympev.2008.01.022 [DOI] [PubMed] [Google Scholar]
- Myers N, Mittermeier RA, Mittermeier CG, Da Fonseca GA, Kent J. (2000) Biodiversity hotspots for conservation priorities. Nature 403(6772), 853. 10.1038/35002501 [DOI] [PubMed]
- Narayanan ES, Subba Rao BR. (1961) New species of encyrtid and braconid parasites. Indian Journal of Entomology 22(1960): 75–79. [Google Scholar]
- Narendran TC. (1998) On Indian species of Choeras Mason (Hymenoptera: Braconidae). Journal of Entomological Research 22(1): 89–96. [Google Scholar]
- Narendran TC, Sheeba M. (2005) A new Diolcogaster Ashmead (Hymenoptera: Braconidae: Microgastrinae) from Kerala, India. Journal of Bio-Sciences 11: 1–3. [Google Scholar]
- Narendran TC, Sumodan PK, Rema CG. (1992) A study of Indian species of Chelonus Panzer (Hymenoptera: Braconidae). Journal of the Zoological Society of Kerala 2(2): 1–9. [Google Scholar]
- Nees von Esenbeck CG. (1834) Hymenopterorum Ichneumonibus affinium monographiae, genera Europaea et species illustrantes. 1. Stuttgartiae et Tubingae, 320. 10.5962/bhl.title.26555 [DOI]
- Ngowi BV, Tonnang HEZ, Khamis F, Mwangi EM, Nyambo B, Ndegwa PN, Subramanian S. (2019) Seasonal abundance of Plutellaxylostella (Lepidoptera: Plutellidae) and diversity of its parasitoids along altitudinal gradients of the eastern Afromontane. Phytoparasitica 47: 375–391. 10.1007/s12600-019-00732-3 [DOI] [Google Scholar]
- Nishida GM. (2002) Hawaiian Terrestrial Arthropod Checklist: Fourth Edition. Hawaii Biological Survey, Bishop Museum Technical Report No. 22, 313 pp. [Google Scholar]
- Niezabitowski EL. (1910) Materyaly do fauny Brakonidow Polski. Braconidae, zebrane w Galicyi. Sprawozdania Akademii Umiejetnosci w Krakowie 44: 47–106. [Google Scholar]
- Nixon GEJ. (1955) Los insectos de las Islas Juan Fernández. 26. Braconidae (Hymenoptera). Revista Chilena de Entomología 4: 159–165. [Google Scholar]
- Nixon GEJ. (1960) A new species of Apanteles associated with lucerne in Australia (Hym.: Braconidae). Annals and Magazine of Natural History (13)2: 303–304. 10.1080/00222935908650868 [DOI]
- Nixon GEJ. (1961) Two new European species of Apanteles (Hymenoptera, Braconidae). Proceedings of the Royal Entomological Society of London (B) 30(3–4): 50–52. 10.1111/j.1365-3113.1961.tb00161.x [DOI] [Google Scholar]
- Nixon GEJ. (1965) A reclassification of the tribe Microgasterini (Hymenoptera: Braconidae). Bulletin of the British Museum (Natural History), Entomology Series, Supplement 2: 1–284. [Google Scholar]
- Nixon GEJ. (1967) The Indo-Australian species of the ultor-group of Apanteles Förster (Hymenoptera: Braconidae). Bulletin of the British Museum (Natural History), Entomology Series 21(1): 1–34. [Google Scholar]
- Nixon GEJ. (1968) A revision of the genus Microgaster Latreille (Hymenoptera: Braconidae). Bulletin of the British Museum (Natural History), Entomology Series 22: 33–72. 10.5962/bhl.part.9950 [DOI] [Google Scholar]
- Nixon GEJ. (1970) A revision of the n.w. European species of Microplitis Förster (Hymenoptera: Braconidae). Bulletin of the British Museum (Natural History), Entomology Series 25(1): 1–30. [Google Scholar]
- Nixon GEJ. (1971) Two new species of Apanteles from Europe and Africa with a redescription of the African Apantelesrutilans Nixon (Hymenoptera: Braconidae). Journal of Natural History 5: 361–365. 10.1080/00222937100770271 [DOI] [Google Scholar]
- Nixon GEJ. (1972) A revision of the north-western European species of the laevigatus-group of Apanteles Förster (Hymenoptera, Braconidae). Bulletin of Entomological Research 61: 701–743. 10.1017/S0007485300047544 [DOI] [Google Scholar]
- Nixon GEJ. (1973) A revision of the north-western European species of the vitripennis, pallipes, octonarius, triangulator, fraternus, formosus, parasitellae, metacarpalis and circumscriptus- groups of Apanteles Förster (Hymenoptera: Braconidae). Bulletin of Entomological Research 63: 169–228. 10.1017/S0007485300039006 [DOI] [Google Scholar]
- Nixon GEJ. (1974) A revision of the north-western European species of the glomeratus-group of Apanteles Foerster (Hymenoptera, Braconidae). Bulletin of Entomological Research 64: 453–524. 10.1017/S0007485300031333 [DOI] [Google Scholar]
- Nixon GEJ. (1976) A revision of the north-western European species of the merula, lacteus, vipio, ultor, ater, butalidis, popularis, carbonarius and validus-groups of Apanteles Förster (Hym.: Braconidae). Bulletin of Entomological Research 65: 687–732. 10.1017/S0007485300006386 [DOI] [Google Scholar]
- Noyes JS. (1994) The reliability of published host-parasitoid records: a taxonomist’s view. Norwegian Journal of Agricultural Sciences 16: 59–69. [Google Scholar]
- O’Hara JE, Shima H, Zhang C. (2009) Annotated catalogue of the Tachinidae (Insecta: Diptera) of China. Zootaxa 2190(1): 1–236. 10.11646/zootaxa.2190.1.1 [DOI] [Google Scholar]
- Olliff AS. (1893) Report on a visit to the Clarence River District for the purpose of ascertaining the nature and extent of insect ravages in the sugar-cane crop. Agricultural Gazette of New South Wales 4: 373–386. [Google Scholar]
- Oltra MT, Michelena JM. (1988) Contribution to the knowledge of the Microgastrinae in the Iberian Peninsula (Hymenoptera, Braconidae): IV. Lissogastrini. Boletin de la Asociacion Española de Entomologia 12: 353–358. [Google Scholar]
- Oltra MT, Michelena JM. (1989) Cotesiaballi, new species from Spain (Hymenoptera, Braconidae). Nouvelle Revue d'Entomologie 6(2): 131–134. [Google Scholar]
- Oltra-Moscardó MT, Jiménez-Peydró R. (2005) The taxon Rasivalva (Hymenoptera: Braconidae) in the palaearctic region and description of Rasivalvapyrenaica new species from Andorra. Journal of Entomological Science 40(4): 438–445. 10.18474/0749-8004-40.4.438 [DOI] [Google Scholar]
- Oltra MT, Dominquez M, Baixeras J. (1995) A new Iberian species of Protapanteles (Hymenoptera: Braconidae) associated with the endemic moth Heliotheadiscoidaria (Lepidoptera: Geometridae). Entomological News 106(2): 87–96. [Google Scholar]
- Oltra MT, Dominguez M, Falco JV. (1996) Considerations on Protapanteles Ashmead, 1898 and a description of P. hispanica a new species from the Iberian Peninsula (Hymenoptera, Braconidae). Bulletin de la Société Entomologique de France 101(2): 145–150. [Google Scholar]
- Packard AS. (1864) Microgasternephoptericis n. sp. Proceedings of the Essex Institute 4: 122.
- Packard AS. (1877) Explorations of the Polaris Expedition to the North Pole. American Naturalist 11: 51–53. [Google Scholar]
- Packard AS. (1881) Descriptions of some new Ichneumon parasites of North American butterflies. Proceedings of the Boston Society of Natural History 21: 18–38. [Google Scholar]
- Paddock FB. (1933) Further notes on the bee moth Galleriamellonella L. Journal of Economic Entomology 26: 177–181. 10.1093/jee/26.1.177 [DOI] [Google Scholar]
- Pandey K, Ahmad Z, Haider AA. (2004) Apantelesmalacosomae sp. nov., (Hymenoptera: Braconidae) parasitic on the tent caterpillar, Malacosomaindica Wlk. (Lepidoptera: Lasiocampidae) in India. Journal of Entomological Research 28(1): 51–54. [Google Scholar]
- Pandey K, Ahmad Z, Haider AA. (2005) Description of a new species of the genus Dolichogenidea Viereck (Hymenoptera: Braconidae) from India. Journal of the Bombay Natural History Society 102(3): 324–325. 10.11609/JoTT.ZPJ.1098.1804 [DOI] [Google Scholar]
- Papp J. (1959) The Microgaster Latr., Microplitis Foerst., and Hygroplitis Thoms. species of the Carpathian Basin (Hymenoptera, Braconidae). Annales Historico-Naturales Musei Nationalis Hungarici 51: 397–413. [Google Scholar]
- Papp J. (1960) Zur Kenntnis der Microgaster Latr.- und Microplitis Först.-Arten Österreichs (Hym., Braconidae). Zeitschrift der Arbeitsgemeinschaft Österreichischer Entomologen 12: 117–128. [Google Scholar]
- Papp J. (1961) Untersuchungen über drei Microgaster-Arten (Hymenoptera: Braconidae). Beiträge zur Entomologie 11(1/2): 154–159.
- Papp J. (1967) Ergebnisse der zoologischen Forschungen von Dr. Z. Kaszab in der Mongolei Braconidae (Hymenoptera). Acta Zoologica Academiae Scientiarum Hungaricae 13: 191–226. [Google Scholar]
- Papp J. (1970) A contribution of the Braconid fauna of Israel (Hymenoptera). Israel Journal of Entomology 5: 63–76. [Google Scholar]
- Papp J. (1971) Ergebnisse der zoologischen Forschungen von Dr. Z. Kaszab in der Mongolei. 215. Braconidae (Hym.) 3. Annales Historico-Naturales Musei Nationalis Hungarici 63: 307–363. [Google Scholar]
- Papp J. (1972) New Apanteles Först. species from Hungary (Hymenoptera, Braconidae: Microgasterinae), I. Annales Historico-Naturales Musei Nationalis Hungarici 64: 335–345. [Google Scholar]
- Papp J. (1973a) Three new Apanteles Först. species of the Old World (Hymenoptera: Braconidae, Microgasterinae). Acta Zoologica Hungarica 19: 361–367. [Google Scholar]
- Papp J. (1973b) New Apanteles Först. species from Hungary (Hymenoptera, Braconidae: Microgasterinae), II. Annales Historico-Naturales Musei Nationalis Hungarici 65: 287–304. [Google Scholar]
- Papp J. (1974a) Braconidae (Hymenoptera) from Korea, I. Acta Zoologica Hungarica 20: 165–175. [Google Scholar]
- Papp J. (1974b) New Apanteles Först. species from Hungary (Hymenoptera, Braconidae: Microgasterinae), III. Annales Historico-Naturales Musei Nationalis Hungarici 66: 325–337. [Google Scholar]
- Papp J. (1974c) On the classification of the species Apanteles Först. with special respect to the species living in Hungary (Hym. Braconidae: Microgasterinae) [In Hungarian with English summary]. Animal Communications, Akadémiai Kiadó, Budapest 41(1–4): 86–100. [Google Scholar]
- Papp J. (1975a) Braconidae (Hymenoptera) from Mongolia, IV. Acta Zoologica Hungarica 21: 115–118. [Google Scholar]
- Papp J. (1975b) New Apanteles Först. species from Hungary (Hymenoptera, Braconidae: Microgasterinae), IV. Annales Historico-Naturales Musei Nationalis Hungarici 67: 237–255. [Google Scholar]
- Papp J. (1976a) A survey of the European species of Apanteles Först. (Hymenoptera, Braconidae: Microgasterinae), I. The species groups. Annales Historico-Naturales Musei Nationalis Hungarici 68: 251–274. [Google Scholar]
- Papp J. (1976b) Braconidae (Hymenoptera) from Mongolia, V. Annales Historico-Naturales Musei Nationalis Hungarici 68: 227–249. [Google Scholar]
- Papp J. (1976c) Key to the European Microgaster Latr. species, with a new species and taxonomical remarks (Hymenoptera: Braconidae; Microgasterinae). Acta Zoologica Academiae Scientiarum Hungaricae 22: 97–117. [Google Scholar]
- Papp J. (1977a) Braconidae (Hymenoptera) from Mongolia VII. Annales Historico-Naturales Musei Nationalis Hungarici 69: 219–240. [Google Scholar]
- Papp J. (1977b) New Apanteles Först. species from Hungary (Hymenoptera, Braconidae: Microgasterinae), V. Annales Historico-Naturales Musei Nationalis Hungarici 69: 201–217. [Google Scholar]
- Papp J. (1978a) A survey of the European species of Apanteles Först. (Hymenoptera, Braconidae: Microgasterinae), II. The laevigatus-group, 1. Annales Historico-Naturales Musei Nationalis Hungarici 70: 265–301. [Google Scholar]
- Papp J. (1978b) Apantelesglaber sp.n. from Finland (Hymenoptera, Braconidae, Microgasterinae). Annales Entomologici Fennici 44: 113–114.
- Papp J. (1979a) A survey of the European species of Apanteles Först. (Hymenoptera, Braconidae: Microgasterinae), III. The laevigatus-group, 2. Annales Historico-Naturales Musei Nationalis Hungarici 71: 235–250. [Google Scholar]
- Papp J. (1979b) Braconidae (Hymenoptera) from Tunisia, 1. Folia Entomologica Hungarica 32(2): 175–187. [Google Scholar]
- Papp J. (1980a) A survey of the European species of Apanteles Först. (Hymenoptera, Braconidae: Microgasterinae), IV. The lineipes-, obscurus- and ater-group. Annales Historico-Naturales Musei Nationalis Hungarici 72: 241–272. [Google Scholar]
- Papp J. (1980b) Braconidae (Hymenoptera) from Mongolia VIII. Acta Zoologica Hungarica 26: 401–413. [Google Scholar]
- Papp J. (1980c) On the genus Fornicia Brullé with two new Oriental species (Hymenoptera, Braconidae: Microgasterinae). Folia Entomologica Hungarica 41(33)(2): 305–311.
- Papp J. (1981) A survey of the European species of Apanteles Först. (Hymenoptera, Braconidae: Microgastrinae), V. The lacteus-, longipalpis-, ultor-, butalidis- and vipio-group. Annales Historico-Naturales Musei Nationalis Hungarici 73: 263–291. [Google Scholar]
- Papp J. (1982) A survey of the European species of Apanteles Först. (Hymenoptera, Braconidae: Microgastrinae), VI. The laspeyresiella-, merula-, falcatus- and validus-group. Annales Historico-Naturales Musei Nationalis Hungarici 74: 255–267. [Google Scholar]
- Papp J. (1983a) A survey of the European species of Apanteles Först. (Hymenoptera, Braconidae: Microgastrinae), VII. The carbonarius-, circumscriptus-, fraternus-, pallipes-, parasitellae-, vitripennis-, liparidis-, octonarius- and thompsoni- group. Annales Historico-Naturales Musei Nationalis Hungarici 75: 247–283. [Google Scholar]
- Papp J. (1983b) Contributions to the braconid fauna of Hungary, IV. Microgastrinae. (Hymenoptera: Braconidae). Folia Entomologica Hungarica 44: 125–138. [Google Scholar]
- Papp J. (1984a) A survey of the European species of Apanteles Först. (Hymenoptera, Braconidae: Microgastrinae) VIII. The metacarpalis, formosus, popularis and suevus-groups. Annales Historico-Naturales Musei Nationalis Hungarici 76: 265–295. [Google Scholar]
- Papp J. (1984b) Contributions to the braconid fauna of Hungary, VI. Microgasterinae. (Hymenoptera: Braconidae). Folia Entomologica Hungarica 45(2): 157–168. [Google Scholar]
- Papp J. (1984c) Palaearctic species of Microgaster Latreille (= Microplitis Förster) with description of seven new species (Hymenoptera, Braconidae, Microgastrinae). Entomologische Abhandlungen 47: 95–140. [Google Scholar]
- Papp J. (1986) A survey of the European species of Apanteles Först. (Hymenoptera, Braconidae: Microgastrinae). IX. The glomeratus-group, 1. Annales Historico-Naturales Musei Nationalis Hungarici 78: 225–247. [Google Scholar]
- Papp J. (1987a) A survey of the European species of Apanteles Förster (Hymenoptera, Braconidae: Microgastrinae), X. The glomeratus-group 2 and the cultellatus-group. Annales Historico-Naturales Musei Nationalis Hungarici 79: 207–258. [Google Scholar]
- Papp J. (1987b) Braconidae (Hymenoptera) from Korea. IX. Acta Zoologica Hungarica 33: 435–456. [Google Scholar]
- Papp J. (1988) A survey of the European species of Apanteles Först. (Hymenoptera, Braconidae: Microgastrinae). 11. "Homologization" of the species-groups of Apanteles s.l. with Mason's generic taxa. Checklist of genera. Parasitoid/host list 1. Annales Historico-Naturales Musei Nationalis Hungarici 80: 145–175. [Google Scholar]
- Papp J. (1989a) Contribution to the Braconid wasp of Greenland, Denmark (Hymenoptera: Braconidae). Folia Entomologica Hungarica 100: 95–104. [Google Scholar]
- Papp J. (1989b) Three new braconid species from central Switzerland (Hymenoptera, Braconidae). Mitteilungen der Schweizerischen Entomologischen Gesellschaft 62: 269–278. [Google Scholar]
- Papp J. (1990a) A survey of the European species of Apanteles Först. (Hymenoptera, Braconidae: Microgastrinae) XII. Supplement to the key of the glomeratus-group. Parasitoid/host list 2. Annales Historico-Naturales Musei Nationalis Hungarici 81: 159–203. [Google Scholar]
- Papp J. (1990b) Braconidae (Hymenoptera) from Korea. XII. Acta Zoologica Hungarica 36(1–2): 87–119. [Google Scholar]
- Papp J. (1991a) New Braconid wasps (Hymenoptera, Braconidae) in the Hungarian Natural History Museum, 2. Annales Historico-Naturales Musei Nationalis Hungarici 83: 145–167. [Google Scholar]
- Papp J. (1991b) Second survey of the braconid wasps in the Bátorliget Nature Conservation Areas, Hungary (Hymenoptera: Braconidae). Studia Naturalia 1(2): 639–674. [Google Scholar]
- Papp J. (1993) New Braconid wasps (Hymenoptera, Braconidae) in the Hungarian Natural History Museum, 4. Annales Historico-Naturales Musei Nationalis Hungarici 84: 155–180. [Google Scholar]
- Papp J. (2003) Braconid wasps from the Cape Verde Islands (Insecta: Hymenoptera: Braconidae) 2. Doryctinae, Braconinae, Hormiinae, Rogadinae, Gnamptodontinae, Homolobinae, Opiinae, Alysiinae, Cheloninae, Adeliinae and Microgastrinae. Faunistische Abhandlungen. (Dresden) 24: 137–167. [Google Scholar]
- Papp J. (2004) Type specimens of the braconid species by Gy. Szépligeti deposited in the Hungarian Natural History Museum (Hymenoptera: Braconidae). Annales Historico-Naturales Musei Nationalis Hungarici 96: 153–223. [Google Scholar]
- Papp J. (2005) A checklist of the Braconidae of Hungary (Hymenoptera). Folia Entomologica Hungarica 66: 137–194. [Google Scholar]
- Papp J. (2008) Szepligeti's braconid type specimens deposited in the Museum National d'Histoire Naturelle, Paris (Hymenoptera, Braconidae). Revue Francaise d'Entomologie (Nouvelle Serie) 30(1): 1–12. [Google Scholar]
- Papp J. (2009a) Braconidae (Hymenoptera) from Mongolia, XVII. Eleven subfamilies. Acta Zoologica Academiae Scientiarum Hungaricae 55(2): 139–173. [Google Scholar]
- Papp J. (2009b) Contribution to the braconid fauna of the former Yugoslavia, V. Ten subfamilies (Hymenoptera, Braconidae). Entomofauna 30(1): 1–35. [Google Scholar]
- Papp J. (2012) Braconid wasps from the Cape Verde Islands 3. Braconinae, Cheloninae, Hormiinae, Microgastrinae and Opiinae (Hymenoptera: Braconidae). Mitteilungen des Internationalen Entomologischen Vereins E.V. Frankfurt A.M. 37(3): 117–138. [Google Scholar]
- Papp J. (2014) Braconidae (Hymenoptera) from Tunisia, 4. Fourteen subfamilies. Folia Entomologica Hungarica 75: 143–66. 10.17112/FoliaEntHung.2014.75.143 [DOI] [Google Scholar]
- Papp J. (2015) First contribution to the knowledge of the braconid wasps (Hymenoptera, Braconidae) of Malta. Bulletin of the Entomological Society of Malta 7: 93–108. [Google Scholar]
- Papp J. (2016) Species of braconid wasps described by Christian Godfried Nees ab Esenbeck in 1811, 1812, 1816, 1818, 1834. A revisionary checklist (Hymenoptera, Braconidae). Zootaxa 4169(3): 401–34. 10.11646/zootaxa.4169.3.1 [DOI] [PubMed] [Google Scholar]
- Papp J, Shaw MR. (2001) A new species of Microplitis Foerster (Hym., Braconidae, Microgastrinae) from Spain. Entomologist's Monthly Magazine 137: 53–58. [Google Scholar]
- Parks KS. (2018) Phylogenetics of Parapanteles (Braconidae: Microgastrinae) wasps, an underused tool for their identification, and an exploration of the evolution of their symbiotic viruses. Doctoral dissertation, University of Illinois at Urbana-Champaign, USA.
- Penteado-Dias AM. (1995) Duas espécies novas de Sendaphne Nixon (Hymenoptera, Braconidae, Microgastrinae) do Brasil. Revista Brasileira de Zoologia 12(2): 251–254. 10.1590/S0101-81751995000200004 [DOI] [Google Scholar]
- Penteado-Dias AM, Scatolini D, Braga SM. (2000) First report of Illidops Mason (Hymenoptera: Braconidae: Microgastrinae) from the Neotropical region. Zoologische Mededelingen Leiden 74(1-17): 219–224. [Google Scholar]
- Penteado-Dias AM, Shimabukuro PHF, van Achterberg C. (2002) A new species of the genus Xanthomicrogaster Cameron (Hymenoptera: Braconidae: Microgastrinae) from Brazil. Zoologische Mededelingen Leiden 76(3): 41–44. [Google Scholar]
- Penteado-Dias AM, Fernandes LBR, Iemma LGR, Dias MM. (2011) First occurrence of Protapanteles (Protapanteles) enephes (Nixon, 1965) (Hymenoptera, Braconidae, Microgastrinae) in Brazil and new biological data. Brazilian Journal of Biology 71(3): 735–738. 10.1590/S1519-69842011000400019 [DOI] [PubMed] [Google Scholar]
- Perez EC, Berta DC. (2017) Redescription of Snelleniusbicolor and S.tricolor (Hymenoptera: Braconidae) and new distribution in South America. Revista de la Sociedad Entomológica Argentin 71(3–4): 293–299. [Google Scholar]
- Porter CE. (1916) Descripción de un nuevo Himenóptero parásito de Notophusantiqua. Revista Chilena de Historia Natural 20: 96–98. [Google Scholar]
- Porter CE. (1918) Notas brevos de entomologica agricola. 9. Descripción de un nuevo Bracónido. Anales Zoología Aplicada (Chile) 4(1917): 44–46. [Google Scholar]
- Porter CE. (1920a) Entomologia Chilena. Un nuevo Bracónido del sur de Chile. Boletín Museo Nacional, Chile 11: 215–216. [Google Scholar]
- Porter CE. (1920b) Sobre dos Bracónidos argentinos. Revista Chilena de Historia Natural 24: 33–34. [Google Scholar]
- Porter CE. (1923) Un nuevo Bracónido chileno. Revista Chilena de Historia Natural 25(1921): 26–27. [Google Scholar]
- Porter CE. (1926) Otro Apanteles nuevo de Chile. Revista Chilena de Historia Natural 30: 143.
- Prell H. (1925a) Grüne Schlupfwespenkokons in Kieferneulenrevieren. Anzeiger für Schädlingskunde 1: 54–55. 10.1007/BF02628588 [DOI] [Google Scholar]
- Prell H. (1925b) Zur Biologie eines bisher verkannten Kieferneulenschmarotzers (Microplitisdecipiens sp.n.). Zeitschrift für Wissenschaftliche Insektenbiologie 20: 137–147. [Google Scholar]
- Provancher L. (1881) Faune canadienne. Les insectes – Hyménoptères. Naturaliste Canadien 12(139): 193–207. [Google Scholar]
- Provancher L. (1886) Additions et corrections au Volume II de la Faune Entomologique du Canada. Traitant des Hyménoptères. Québec, 29–157.
- Provancher L. (1889) Additions à la faune hyménoptérologique. Naturaliste Canadien 17: 273–440. [Google Scholar]
- Ranjith AP, Rajesh KM, Nasser M. (2015a) Taxonomic studies on Oriental Microplitis Foerster (Hymenoptera: Braconidae, Microgastrinae) with description of two new species from South India. Zootaxa 3963(3): 369–415. 10.11646/zootaxa.3963.3.4 [DOI] [PubMed] [Google Scholar]
- Ranjith AP, Veena T, Dharma Rajan P, Nasser M. (2015b) Revision of Buluka de Saeger (Hymenoptera: Microgastrinae) with the description of one new species from South India. Biosystematica 9: 29–38. [Google Scholar]
- Ranjith AP, Fernandez-Triana J, Veena T, Priyadarsanan DR, Nasser M. (2019) Four new species of Philoplitis Nixon (Braconidae, Microgastrinae) with an updated key and illustrations of all described species. ZooKeys 841: 125–150. 10.3897/zookeys.841.33549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SN. (1953) Notes on some parasitic Hymenoptera from India with the description of a new species, Apantelesepijarbi. Indian Journal of Entomology 15(1): 23–28. [Google Scholar]
- Rao SN. (1961) Key to the Oriental species of Apanteles Foerster (Hymenoptera). Proceedings of the National Academy of Sciences India 31B: 32–46.
- Rao SN. (1969) Studies on Indian Ichneumonidae (HymenopteraParasitica). Journal of the Bombay Natural History Society 66(1): 222–226. [Google Scholar]
- Rao SN, Kurian C. (1950) Descriptions of eleven new and records of fifteen known species of Ichneumonoidea (HymenopteraParasitica) from India. Indian Journal of Entomology 12: 167–190. [Google Scholar]
- Rao SN, Chalikwar MR. (1970a) Four new species of the Braconid genus Protomicroplitis Ashmead from India and a key to the Oriental species. Bulletin of Entomology, India 11: 102–115. [Google Scholar]
- Rao SN, Chalikwar MR. (1970b) Studies on Indian parasitic Hymenoptera (Braconidae) from Marathwada – I. Marathwada University Journal of Sciences 9: 107–112. [Google Scholar]
- Rao SN, Chalikwar MR. (1971) Studies on parasitic Hymenoptera (Braconidae) from Marathwada – V. Marathwada University Journal of Sciences 10(3): 181–186. [Google Scholar]
- Rao SN, Chalikwar MR. (1976a) Studies on Indian parasitic HymenopteraBraconidae with special reference to Apanteles Forster from Marathwada. Marathwada University Journal of Sciences 14(7): 183–188. [Google Scholar]
- Rao SN, Chalikwar MR. (1976b) Two new species of Protomicroplitis Ashmead (Hymenoptera, Braconidae: Microgasterinae). Oriental Insects 9(4): (1975): 439–445. 10.1080/00305316.1975.10434512 [DOI]
- Ratkovich M. (1950) Primera lista de insectos tucumanos útiles. Publicaciones Misceláneas de la Estación Experimental Agrícola, Tucuman 5: 1–33. [Google Scholar]
- Ratnasingham S, Hebert PD. (2007) Bold: The Barcode of Life Data System (http://www.barcodinglife.org). Molecular Ecology Notes 7(3): 355–364. 10.1111/j.1471-8286.2007.01678.x [DOI] [PMC free article] [PubMed]
- Ratnasingham S, Hebert PD. (2013) A DNA-based registry for all animal species: the Barcode Index Number (BIN) system. PloS ONE 8(7): e66213. 10.1371/journal.pone.0066213 [DOI] [PMC free article] [PubMed]
- Ratzeburg JTC. (1844) Die Ichneumonen der Forstinsecten in forstlicher und entomologischer Beziehung. Berlin, 224 pp. 10.5962/bhl.title.11094 [DOI]
- Ratzeburg JTC. (1848) Die Ichneumonen der Forstinsecten in forstlicher und entomologischer Beziehung. Zweiter Band. Berlin, 238 pp.
- Ratzeburg JTC. (1852) Die Ichneumonen der Forstinsecten in forstlicher und entomologischer Beziehung. Dritter Band. Berlin, 272 pp.
- Ray P, Yousuf M. (2009) New Descriptions – Record of two new species of Apanteles Foerster (Braconidae: Microgastrinae) from Central India. Journal of the Bombay Natural History Society 106(3): 335–338. [Google Scholar]
- Redolfi de Huiza I. (1995) Diversidad de Braconidae (Hymenoptera) en el Peru. [Diversity of Braconidae (Hymenoptera) in Peru. ] Revista Peruana de Entomología 37: 11–22. [Google Scholar]
- Reinhard H. (1880) Beiträge zur Kenntniss einiger Braconiden-Gattungen. Fünftes Stück. XVI. Zur Gattung Microgaster, Latr. (Microgaster, Microplitis, Apanteles). Deutsche Entomologische Zeitschrift 24: 353–370. 10.1002/mmnd.4800240215 [DOI] [Google Scholar]
- Reinhard H. (1881) Beiträge zur Kenntniss einiger Braconiden-Gattungen. (Schluss) Deutsche Entomologische Zeitschrift 25(1): 33–52. 10.1002/mmnd.48018810102 [DOI] [Google Scholar]
- Rema CG, Narendran TC. (1996) A remarkable new genus of Braconidae (Hymenoptera) from India. Journal of the Bombay Natural History Society 93: 264–267. [Google Scholar]
- Rema CG, Sheeba M. (2004) A new species of Diolcogaster Ashmead (Hymenoptera: Braconidae: Microgastrinae) from Kerala, India with a key to Indian species. In: Rajmohan K, Sudheer K, Girish Kumar P, Santhosh S. (Eds) Perspectives on Biosystematics and Biodiversity.Harvest Media Services. Calicut, India, 509–513.
- Riley CV. (1869) First annual report on the noxious, beneficial and other insects, of the state of Missouri. Ellwood Kirby, Jefferson City, 182 pp. [Google Scholar]
- Riley CV. (1871) Third annual report on the noxious, beneficial and other insects, of the state of Missouri. Horace Wilcox, Jefferson City, 176 pp. [Google Scholar]
- Riley CV. (1881) Notes on North American Microgasters, with descriptions of new species. Transactions of the Academy of Science of St. Louis 4: 296–315. [Google Scholar]
- Riley CV. (1887) True parasites of the web-worm. Report of the Entomologist, USDA, 1886: 530–536. [Google Scholar]
- Riley CV. (1889) Family Braconidae. Subfamily Microgasterinae. In: Scudder SH. (Ed.) Butterflies of Eastern U.S. and Canada. Vol. III, 1897–1911.
- Risbec J. (1951) Les Microgasterinae de l'Afrique occidentale française. Mémoires de l'Institut Francais d'Afrique Noire 13: 411–473. [Google Scholar]
- Risbec J. (1952) Les Microgasteridae d'A.O.F. (rectifications). Bulletin de l'Institut Français d'Afrique Noire 14: 701.
- Rodriguez JJ, Fernandez-Triana J, Smith MA, Janzen DH, Hallwachs W, Erwin T, Whitfield JB. (2013) Extrapolations from field studies and known faunas converge on dramatically increased estimates of global microgastrine parasitoid wasp species richness (Hymenoptera: Braconidae). Insect Conservation and Diversity 6(4): 530–536. 10.1111/icad.12003 [DOI] [Google Scholar]
- Roe AD, Weller SJ, Baixeras J, Brown J, Cummings MP, Davis D, Kawahara AY, Parr C, Regier JC, Rubinoff D, Simonsen TJ, Wahlberg N, Zwick A. (2009) Evolutionary Framework for Lepidoptera Model Systems. In: Goldsmith M, Marec F. (Eds) Molecular Biology and Genetics of Lepidoptera.CRC Press, Boca Raton, 1–24. 10.1201/9781420060201-c1 [DOI]
- Roepke WKJ. (1935) De slakrupsenplaag op het Molukken-elland Batjan. Meded. LandbHoogesch 39: 1–38. [Google Scholar]
- Rohwer SA. (1915) Descriptions of new species of Hymenoptera. Proceedings of the United States National Museum 49: 205–249. 10.5479/si.00963801.2105.205 [DOI] [Google Scholar]
- Rohwer SA. (1919) Descriptions and notes on some Ichneumon-flies from Java. Proceedings of the United States National Museum 54(2249): 563–570. 10.5479/si.00963801.2249.563 [DOI] [Google Scholar]
- Rohwer SA. (1922) Descriptions of Javanese Braconidae (Hym.) received from Mr. S. Leefmans. Treubia 3: 53–55. [Google Scholar]
- Rohwer SA. (1926) Description of a new braconid parasite of Artonacatoxantha (Hymenoptera). Proceedings of the Entomological Society of Washington 28: 188–189. [Google Scholar]
- Roman A. (1924) Ichneumoniodea. Report of the Scientific Results of the Norwegian Expedition to Novaja Zemlya 1921. Kristiania 14: 11–25. [Google Scholar]
- Ronna E. (1924) Apontamentos da microfauna Rio-Grandense. Egatea 9(3): 267–272. [Google Scholar]
- Rondani C. (1872) Degli Insetti parassiti e delle loro vittime. Bollettino della Societa Entomologica Italiana 4: 41–78, 229–258.
- Rossi P. (1792) Mantissa insectorum exhibens species nuper in Etruria collectas. Adiectis faunae Etruscae illustrationinus, ac emendationibus. Tom. I. Pisis, 111–126. 10.5962/bhl.title.49449 [DOI]
- Rossikoff KN. (1904) Lathrodectustredecimguttatus Rossi s. Kara-kurt. Trudy Byuro po Entomologii 5(2), 232 pp.
- Rousse P, Gupta A. (2013) Microgastrinae (Hymenoptera: Braconidae) of Reunion Island: a catalogue of the local species, including 18 new taxa and a key to species. Zootaxa 3616(6): 501–547. 10.11646/zootaxa.3616.6.1 [DOI] [PubMed] [Google Scholar]
- Rudow F. (1910) Einige Zucht- und Sammelergebnisse des letzten Sommers. Internationale Entomologische Zeitschrift 3: 229–231. [Google Scholar]
- Ruohomäki K, Klemola T, Shaw MR, Snäll N, Sääksjärvi IE, Veijalainen A, Wahlberg N. (2013) Microgastrinae (Hymenoptera: Braconidae) parasitizing Epirritaautumnata (Lepidoptera: Geometridae) larvae in Fennoscandia with description of Cotesiaautumnatae Shaw sp. n. Entomologica Fennica 24: 65–80. 10.33338/ef.8340 [DOI] [Google Scholar]
- Ruthe JF. (1858) Beiträge zur Kenntnis der Braconiden. Berliner Entomologische Zeitschrift 2: 1–9. 10.1002/mmnd.18580020103 [DOI] [Google Scholar]
- Ruthe JF. (1859) Verzeichniss der von Dr. Staudinger im Jahre 1856 auf Island gesammelten Hymenopteren. Stettiner Entomologische Zeitung 20: 305–322. [Google Scholar]
- Ruthe JF. (1860) Deutsche Braconiden. Erstes Stück. Berliner Entomologische Zeitschrift 4: 105–160. [Google Scholar]
- Saeed A, Austin AD, Dangerfield PC. (1999) Systematics and host relationships of Australasian Diolcogaster (Hymenoptera: Braconidae: Microgastrinae). Invertebrate Taxonomy 13: 117–178. 10.1071/IT97033 [DOI] [Google Scholar]
- Salgado-Neto G, Fernandez-Triana JL, de Souza Tavares W, Zanuncio JC. (2018) Diolcogasterflammeus sp. nov. from Brazil, a new Microgastrinae wasp (Hymenoptera: Braconidae) of importance in biological control. Revista Brasileira de Entomologia 62(3): 232–236. 10.1016/j.rbe.2018.06.001 [DOI] [Google Scholar]
- Salgado-Neto G, Whitfield JB, Garcia FR. (2019) Cotesiainvirae, sp. nov., from South Brazil: a new gregarious microgastrine wasp (Hymenoptera: Braconidae) reared from Opsiphanesinvirae (Nymphalidae) feeding on palms. Revista Brasileira de Entomologia 63(2): 136–140. 10.1016/j.rbe.2019.02.003 [DOI] [Google Scholar]
- Samin N, Coronado-Blanco JM, Fischer M, van Achterberg K, Sakenin H, Davidian E. (2018) Updated checklist of Iranian Braconidae (Hymenoptera: Ichneumonoidea) with twenty-three new records. Natura Somogyiensis (32), 21–36.
- Sánchez-García JA, Figueroa JI, Whitfield JB, Pineda S, Martínez AM. (2015) A new species of Apanteles Foerster (Hymenoptera: Braconidae) parasitic of two blackberry leafrollers (Lepidoptera: Tortricidae) in Mexico. Journal of the Kansas Entomological Society 88(1): 10–15. 10.2317/JKES1407.02.1 [DOI] [Google Scholar]
- Santschi L, Hanner R, Ratnasingham S, Riconscente M, Imondi R. (2013) . Barcoding Life's Matrix: Translating Biodiversity Genomics into High School Settings to Enhance Life Science Education. PLoS Biology. 11. e1001471. 10.1371/journal.pbio.1001471 [DOI] [PMC free article] [PubMed]
- Sathe TV. (2003) On a new species of Cotesia Cameron (Hymenoptera: Braconidae) from India. Rivista di Parassitologia 22(64)(2): 129–133.
- Sathe TV. (2005) On a new species of Cotesia Cameron (Hymenoptera: Braconidae) from India. Journal of Advanced Zoology 26(2): 73–75. [Google Scholar]
- Sathe TV, Inamdar SA. (1988) A new species of the genus Nyereria Wilkinson (Hymenoptera: Braconidae) from India. Journal of Advanced Zoology 9(2): 128–131. [Google Scholar]
- Sathe TV, Inamdar SA. (1989) A new species of the genus Apanteles Forster (Hymenoptera: Braconidae), from western Maharashtra (India). Oikoassay 6(1): 5–8. [Google Scholar]
- Sathe TV, Inamdar SA. (1991) Two new species of the genus Glytapanteles Ashmead and Parenion Nixon (Hymenoptera: Braconidae) from India. Hexapoda (Insecta Indica) 3(1–2): 89–93. [Google Scholar]
- Sathe TV, Ingawale DM. (1995) Two new species of the genus Apanteles Foerster (Hymenoptera: Braconidae) from India. Journal of the Bombay Natural History Society 92(1): 81–84. [Google Scholar]
- Sathe TV, Bhoje PM. (1998) On a new species of the genus Promicrogaster Brues & Richardson (Hymenoptera: Braconidae). Journal of Advanced Zoology 19(2): 105–106. [Google Scholar]
- Sathe TV, Bhoje PM. (2000) Biological Pest Control. Daya Publishing House, India, 122 pp. [Google Scholar]
- Sathe TV, Rokade AG. (2005) Biodiversity of braconids, the biocontrol agents of insect pests in Maharashtra. In: Kumar A. (Ed.) Biodiversity and conservation.APH Publishing Corporation, New Delhi, 209–259.
- Sathe TV, Dawale RK, Ingawale DM. (1989) A new species of the genus Parapanteles Ashmead (Hymenoptera: Braconidae) from India. Indian Journal of Parasitology 13(2): 211–213. [Google Scholar]
- Sathe TV, Ingawale DM, Bhosale YA. (1994) Two species of the genus Cotesia Cameron (Hymenoptera: Braconidae) from India. Hexapoda (Insecta Indica) 6(2): 65–71. [Google Scholar]
- Sathe TV, Inamdar SA, Dawale Daya RK. (2003) Indian pest parasitoids. Daya Publishing House, India, 145 pp. [Google Scholar]
- Say T. (1835) Descriptions of new North American Hymenoptera, and observations on some already described. Boston Journal of Natural History 1(3): 210–305. [Google Scholar]
- Scaramozzino PL, Loni A, Lucchi A. (2017) A review of insect parasitoids associated with Lobesiabotrana (Denis & Schiffermüller, 1775) in Italy. 1. DipteraTachinidae and HymenopteraBraconidae (Lepidoptera, Tortricidae). ZooKeys 647: 67–100. 10.3897/zookeys.647.11098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrank F. (1781) Enumeratio insectorum austriae indigenorum. Augustae Vindelicorum, 548 pp.
- Schrottky C. (1902) Neue argentinische Hymenoptera. Anales del Museo Nacional de Buenos Aires 8: 91–117. [Google Scholar]
- Schrottky C. (1909) Hymenoptera nova. Anales de la Sociedad Científica Argentina 67: 209–228. [Google Scholar]
- Schulz U, Linde A, Möller J. (2018) EBERSWALDE: Zoological Collections of Eberswalde: Like Phoenix from the Ashes? Zoological Collections of Germany. Springer, 281–294. 10.1007/978-3-319-44321-8_25 [DOI]
- Schumacher RK, Austin AD, Floyd RB. (2000) Parasitoids of the autumn gum moth, Mnesampelaprivata (Guenee) (Lepidoptera: Geometridae) in south-eastern Australia, with description of two new larval parasitoids. Transactions of the Royal Society of South Australia 124(1): 1–15. [Google Scholar]
- Schurian KG, Fiedler K, Maschwitz U. (1993) Parasitoids exploit secretions of myrmecophilous Lycaenid butterfly caterpillars (Lycaenidae). Journal of the Lepidopterists' Society 47(2): 150–154. [Google Scholar]
- Sharkey MJ, Wharton RA. (1997) Morphology and Terminology. In: Wharton RA, Marsh PM, Sharkey MJ. (Eds) Manual of the New World genera of the family Braconidae (Hymenoptera).Special Publication of the International Society of Hymenopterists 1: 19–37.
- Sharkey MJ, Finnell KA, Leathers JA, Frana JO. (2000) Microgastrine (Hymenoptera: Braconidae) parasitoids of Coliaslesbia (Fabricius)(Lepidoptera: Pieridae). Journal of Hymenopteran Research 91: 108–10. [Google Scholar]
- Sharma V. (1972) Taxonomic studies on Apanteles Foerster (Hymenoptera: Braconidae: Microgasterinae) from India III. The vitripennis group. Oriental Insects 6(4): 553–560. 10.1080/00305316.1972.10434195 [DOI] [Google Scholar]
- Sharma V. (1973) Taxonomic studies on Apanteles Foerster (Hymenoptera: Braconidae: Microgasterinae) from India IV. The carbonarius group. Oriental Insects 7(1): 119–126. 10.1080/00305316.1973.10434208 [DOI] [Google Scholar]
- Sharma V. (1984) A new species of genus Fornicia (Hymenoptera, Braconidae, Microgasterinae). Reichenbachia 22(29): 209–211. [Google Scholar]
- Sharma V, Chatterjee PN. (1970a) A new species of Apanteles (Hymenoptera: Braconidae) from India. Oriental Insects 4(2): 165–168. 10.1080/00305316.1970.10433951 [DOI] [Google Scholar]
- Sharma V, Chatterjee PN. (1970b) Description of Apanteleschatterjeei sp. nov. (Hymenoptera; Braconidae) from Nilambur, Madras, India. Indian Forester 96: 322–325. [Google Scholar]
- Shaw MR. (1992) A new species of Hygroplitis Thomson in England (Hymenoptera: Braconidae, Microgastrinae). Entomologist's Gazette 43: 283–288. [Google Scholar]
- Shaw MR. (1994) Parasitoid host ranges. In: Hawkins BA, Sheehan W. (Eds) Parasitoid community ecology.Oxford University Press, 111–144.
- Shaw MR. (2003) Host ranges of Aleiodes species (Hymenoptera: Braconidae), and an evolutionary hypothesis. In: Melika G & Thuroczy C (Eds) Parasitic wasps: evolution, systematics, biodiversity and biological control: 321–327.
- Shaw MR. (2004) Microgasteralebion Nixon and its 'var A': description of a new species and biological notes (Hymenoptera: Braconidae, Microgastrinae). Entomologist's Gazette 55: 217–224. [Google Scholar]
- Shaw MR. (2006) Habitat considerations for parasitic wasps (Hymenoptera). Journal of Insect Conservation 10: 117–127. 10.1007/s10841-006-6288-1 [DOI] [Google Scholar]
- Shaw MR. (2007) The species of Cotesia Cameron (Hymenoptera: Braconidae: Microgastrinae) parasitising Lycaenidae (Lepidoptera) in Britain. British Journal of Entomology and Natural History 20(4): 255–269. [Google Scholar]
- Shaw MR. (2009) Cotesia Cameron (Hymenoptera: Braconidae: Microgastrinae) parasitoids of Heliconiinae (Lepidoptera: Nymphalidae) in Europe, with description of three new species. British Journal of Entomology and Natural History 22: 133–146. [Google Scholar]
- Shaw MR. (2012a) Larval parasitoids of Rivulasericealis (Scopoli) (Lepidoptera: Noctuidae) in Britain, including notes on the biology of Cotesiasubordinaria (Tobias) (Hymenoptera: Braconidae, Microgastrinae), a solitary-cum-gregarious parasitoid. Entomologist's Gazette 63: 251–257. [Google Scholar]
- Shaw MR. (2012b) Notes on some European Microgastrinae (Hymenoptera: Braconidae) in the National Museums of Scotland, with twenty species new to Britain, new host data, taxonomic changes and remarks, and descriptions of two new species of Microgaster Latreille. Entomologist’s Gazette 63: 173–201. [Google Scholar]
- Shaw MR. (2017a) A few recommendations on recording host information for reared parasitoids. Hamuli 8(1): 7–9. [Google Scholar]
- Shaw MR. (2017b) Anatomy, reach and classification of the parasitoid complex of a common British moth, Anthophilafabriciana (L.) (Choreutidae). Journal of Natural History 51(19–20): 1119–1149. 10.1080/00222933.2017.1315837 [DOI] [Google Scholar]
- Shaw MR, Huddleston T. (1991) Classification and biology of Braconid wasps (Hymenoptera: Braconidae). Handbooks for the Identification of British Insects 7(11): 1–126. [Google Scholar]
- Shaw MR, Aeschlimann JP. (1994) Host ranges of parasitoids (Hymenoptera: Braconidae and Ichneumonidae) reared from Epermeniachaerophyllella (Goeze) (Lepidoptera: Epermeniidae) in Britain, with description of a new species of Triclistus (Ichneumonidae). Journal of Natural History 28(4): 619–629. 10.1080/00222939400770281 [DOI] [Google Scholar]
- Shaw MR, Stefanescu C, van Nouhuys S. (2009) Parasitoids of European butterflies. In: Settele J, Shreeve T, Konvicka M, van Dyck H. (Eds) Ecology of Butterflies in Europe.Cambridge University Press, 130–156.
- Shaw MR, Vikberg V, Malinen P. (2015) Cotesiaacerbia sp.nov. (Hymenoptera: Braconidae, Microgastrinae), a gregarious parasitoid of Acerbiaalpina (Quensel, 1802) (Lepidoptera: Erebidae, Arctiinae) in Polar Ural, Russia. Entomologist's Gazette 66: 131–137. [Google Scholar]
- Shenefelt RD. (1968) Snellenius in the Neotropical Region. Proceedings of the Entomological Society of Washington 70(4): 339–345. [Google Scholar]
- Shenefelt RD. (1972) Braconidae 4. Microgasterinae: Apanteles. Hymenopterorum Catalogus (nova editio) 7: 429–668. [Google Scholar]
- Shenefelt RD. (1973) Braconidae 5. Microgasterinae & Ichneutinae. Hymenopterorum Catalogus (nova editio) 9: 669–812. [Google Scholar]
- Shestakov A. (1932) Zur Kenntnis der asiatischen Braconiden. Zoologische Annalen, Würzburg 99: 255–263. [Google Scholar]
- Shestakov A. (1940) Zur Kenntnis der Braconiden Ostsibiriens. Arkiv foer Zoologi 32A(19): 1–21.
- Shimabukuro PHF, Penteado-Dias AM. (2003) Two new species of Alphomelon Mason, 1981 (Hymenoptera, Braconidae, Microgastrinae) from Brazil. Revista Brasileira de Entomologia 47(2): 197–199. 10.1590/S0085-56262003000200007 [DOI] [Google Scholar]
- Shimbori EM, Gadelha SS, Tavares MT, Fernandes DRR. (2019) Braconidae in Catálogo Taxonômico da Fauna do Brasil. PNUD. 10.1044/leader.PPL.24082019.26 [Accessed on 26 August 2019] [DOI]
- Silva Figueroa C. (1917) La Dirphiaamphimone (F.) Berg y sus parásitos. Boletín Museo Nacional, Chile 10: 105–128. [Google Scholar]
- Silva Figueroa C. (1918) La Macromphaladedecora y sus parásitos. Anales Zoología Aplicada (Chile) 4(1917): 55–71. [Google Scholar]
- Sithole R, Nyamukondiwa C, Chinwada P, Lohr B. (2019) Population dynamics of the diamondback moth and its parasitoids in Zimbabwe. Biological control 133: 66–74. 10.1016/j.biocontrol.2019.03.008 [DOI] [Google Scholar]
- Smith MA, Fernandez-Triana J, Eveleigh E, Gómez J, Guclu C, Hallwachs W, Hebert PDN, Hrcek J, Huber JT, Janzen DH, Mason PG, Miller SE, Quicke D, Rodriguez JJ, Rougerie R, Shaw MR, Varkonyi G, Ward D, Whitfield JB, Zaldívar-Riverón A. (2013) DNA barcoding and the taxonomy of Microgastrinae wasps (Hymenoptera, Braconidae): impacts after eight years and nearly 20,000 sequences. Molecular Ecology Resources 13: 168–176. 10.1111/1755-0998.12038 [DOI] [PubMed] [Google Scholar]
- Sonan J. (1940) M. Yanagihara's collection from Daito-Islands, Okinawa: Hymenoptera. Transactions of the Natural History Society of Formosa. Taihoku 30: 369–375. [Google Scholar]
- Sonan J. (1942a) Three new species of parasitic Hymenoptera from Formosa. Transactions of the Natural History Society of Formosa. Taihoku 32: 217–220. [Google Scholar]
- Sonan J. (1942b) Two new species of Apanteles from Formosa (Hymenoptera: Braconidae). Transactions of the Natural History Society of Formosa. Taihoku 32: 245–246. [Google Scholar]
- Song D. (2002) Two new species of Apanteles Foerster from China (Hymenoptera: Braconidae). Mitteilungen des Internationalen Entomologischen Vereins E.V. Frankfurt A.M. 27(1-2): 1–8. [Google Scholar]
- Song D, Chen J. (2002) One new species of genus Apanteles Foerster. [In Chinese with English summary] Journal of Jilin Agricultural University 24(1): 40–46. [Google Scholar]
- Song D, Chen J. (2003a) A study on the genus Exoryza Mason from China with the description of one new species (Hymenoptera: Braconidae: Microgastrinae). [In Chinese with English summary] Shanghai Jiaotong Daxue Xuebao Nongye Kexue Ban. 21(4) General series 77: 286–288.
- Song D, Chen J. (2003b) Two new species of the genus Apanteles Foerster s. str. from China (Hymenoptera: Braconidae: Microgastrinae). Serangga 8(1–2): 1–12. [Google Scholar]
- Song D, Chen J. (2004) A study on Microgaster Latreille from China with description of a new species (Hymenoptera: Braconidae: Microgastrinae). In: Rajmohan K, Sudheer K, Girish Kumar P, Santhosh S. (Eds.) Perspectives on Biosystematics and Biodiversity.Harvest Media Services, Calicut, India, 315–325.
- Song D, Chen J. (2008) Five new species of the genus Microplitis (Hymenoptera: Braconidae: Microgastrinae) from China. Florida Entomologist 91(2): 283–293. 10.1653/0015-4040(2008)91[283:FNSOTG]2.0.CO;2 [DOI]
- Song D, Chen J, Yang J. (2001) A new species of Apanteles Foerster in China (Hymenoptera: Braconidae). [In Chinese with English summary]. Journal of Huazhong Agricultural University 20(6): 535–538. [Google Scholar]
- Song D, Chen J, Yang J. (2002) One new species of Apanteles Foerster from Mt. Wuyi (Hymenoptera: Braconidae). [In Chinese with English summary] Journal of Fujian Agriculture and Forestry University 22(2): 117–119. [Google Scholar]
- Song D, Chen J, Yang J. (2006) Two new species of the genus Dolichogenidea Viereck (Hymenoptera, Braconidae, Microgastrinae) from China. Acta Zootaxonomica Sinica 31(1): 200–205. [Google Scholar]
- Song SN, He JH, Chen XX. (2014) The subgenusChoeras Mason, 1981 of genus Apanteles Foerster, 1862 (Hymenoptera, Braconidae, Microgastrinae) from China, with descriptions of eighteen new species. Zootaxa 3754(5): 501–554. 10.11646/zootaxa.3754.5.1 [DOI] [PubMed] [Google Scholar]
- Souza-Gessner CS, Bortoni MA, Penteado-Dias AM. (2016) New Species of Exix Mason, 1981 (Hymenoptera: Braconidae, Microgastrinae) from Brazil. Entomological News 125(5): 351–357. 10.3157/021.125.0506 [DOI] [Google Scholar]
- Spinola M. (1808) Insectorum Liguriae species novae aut rariores, quas in agro Ligustico nuper detexit, descripsit, et iconibus illustravit (Hymenoptera). 2. Genua, 262 pp.
- Spinola M. (1851) Icneumonitos. Zoologia. 6: 471–550. In: Gay C. Historia física y politica de Chile. Paris, 572 pp.
- Strand MR, Burke GR. (2012) Polydnaviruses as symbionts and gene delivery systems. PLoS Pathogens 8(7), e1002757. 10.1371/journal.ppat.1002757 [DOI] [PMC free article] [PubMed]
- Strand MR, Burke GR. (2014) Polydnaviruses: nature's genetic engineers. Annual Review of Virology 1: 333–354. 10.1146/annurev-virology-031413-085451 [DOI] [PubMed] [Google Scholar]
- Subba Rao BR, Sharma AK. (1960) Three new species of Braconidae from India. Proceedings of the Indian Academy of Sciences 51(B): 82–88.
- Sumodan PK, Sevichan PJ. (1989) A new species of Apanteles Foerster (Hymenoptera: Braconidae) reared from a pyralid pest of Azolla. Journal of Ecobiology 1(4): 319–322. [Google Scholar]
- Sumodan PK, Narendran TC. (1990) Five new species of Apanteles Foerster (Hymenoptera: Braconidae) from Kerala, India. Journal of Ecobiology 2(3): 239–248. [Google Scholar]
- Szépligeti G. (1896) Beiträge zur Kenntnis der ungarischen Braconiden. (Zweiter Theil). Természetrajzi Füzetek 19: 285–321 [Hungarian], 359–386 [German].
- Szépligeti G. (1898) Beiträge zur Kenntnis der ungarischen Braconiden, 3. Theil. Természetrajzi Füzetek 21: 381–396 [Hungarian], 396–408 [German].
- Szépligeti G. (1900) Braconiden aus New-Guinea in der Sammlung des Ungarischen National Museums. Természetrajzi Füzetek 23: 49–65. [Google Scholar]
- Szépligeti G. (1902) Tropischen Cenocoeliden und Braconiden aus der Sammlung des Ungarischen National-Museums. Természetrajzi Füzetek 25: 39–84. [Google Scholar]
- Szépligeti G. (1904) Hymenoptera. Fam. Braconidae. Genera Insectorum 22: 1–253. [Google Scholar]
- Szépligeti G. (1905) Exotische Braconiden aus den aethiopischen, orientalischen und australischen Regionen. Annales Musei Nationalis Hungarici 3: 25–55. [Google Scholar]
- Szépligeti G. (1906) Neue exotische Ichneumoniden aus der Sammlung des Ungarischen National Museums. Annales Musei Nationalis Hungarici 4: 119–156. [Google Scholar]
- Szépligeti G. (1911) Braconidae der I. Zentral-Afrika-Expedition. Wissenschaftliche Ergebnisse Deutschen Zentral-Afrika Expedition 3: 393–418. [Google Scholar]
- Szépligeti G. (1913) Neue afrikanische Braconiden aus der Sammlung der Ungarischen National-Museum. Annales Historico-Naturales Musei Nationalis Hungarici 11: 592–608. [Google Scholar]
- Szépligeti G. (1914) Braconidae. In: Voyage de Ch. Alluaud et R. Jeannel en Afrique orientale 1911–1912 – Résultats scientifiques, Hymenoptera 4: 168–198.
- Telenga NA. (1949) Faunal list of parasites of the family Braconidae (Hymenoptera). Tadzhikistan. Entomologicheskoye Obozreniye 30(3-4): 381–388. [Google Scholar]
- Telenga NA. (1955) Braconidae, subfamily Microgasterinae, subfamily Agathinae. Fauna USSR, Hymenoptera 5(4): 1–311. [Google Scholar]
- Thomson CG. (1895) LII. Bidrag till Braconidernas Kännedom. Opuscula Entomologica 20: 2141–2339. [Google Scholar]
- Thunberg CP. (1822) Ichneumonidea, InsectaHymenoptera illustrata. Mémoires de l'Académie Imperiale des Sciences de Saint Petersbourg 8: 249–281. [Google Scholar]
- Tobias VI. (1960) Novye vidy naczdnikov-brakonid (Hymenoptera, Braconidae) iz srendnei Azii. Izvestiya Akademii Nauk Tadzhik SSR. Otd. Sel'skokhoz i Biol. Nauk 2: 85–90. [Google Scholar]
- Tobias VI. (1964) New species and genus of Braconids (Hymenoptera, Braconidae) from Kazakhstan. Trudy Zoologicheskogo Instituta, Leningrad 34: 177–234. [Google Scholar]
- Tobias VI. (1966) New species and genus of Braconids (Hymenoptera, Braconidae) from Turkmenia and adjacent territories. Trudy Zoologicheskogo Instituta, Leningrad 37: 111–131. [Google Scholar]
- Tobias VI. (1967) Middle Asian species of braconids (Hymenoptera, Braconidae) taken with light quartz lamp. [In Russian] Trudy Zoologicheskogo Instituta. Proceedings of the Zoological Institute, Leningrad 38: 382–396. [Google Scholar]
- Tobias VI. (1971) Review of the Braconidae (Hymenoptera) of the U.S.S.R. [In Russian] Trudy Vsesoyuznogo Entomologicheskogo Obshchestva 54: 156–268. [Google Scholar]
- Tobias VI. (1975) Two new species of braconids from the genus Apanteles Forst. (Hymenoptera, Braconidae) parasites of the moth Bucculatrixulmella Z. [In Russian] Izvestiya Akademii Nauk Moldav. SSR (Biol. Khim). 1975(3): 60–63. [Google Scholar]
- Tobias VI. (1976) Braconids of the Caucasus (Hymenoptera, Braconidae) [In Russian] Opred. Faune SSSR. Nauka Press. Leningrad, 110: 1–286. [Google Scholar]
- Tobias VI. (1977) New species of braconids of the genus Apanteles Forst. (Hymenoptera, Braconidae) from the Far East. Trudy Zoologicheskogo Instituta, Leningrad 67(1976): 90–96. [Google Scholar]
- Tobias VI. (1986) Acaeliinae, Cardiochilinae, Microgastrinae, Miracinae. In: Medvedev GS. (Ed.) Opredelitel Nasekomych Evrospeiskoi Tsasti SSSR 3, Peredpontdatokrylye 4. Opr.Faune SSSR, 145: 1–501.
- Tobias VI, Alexeev YI. (1972) Braconidae (Hymenoptera)- parasites of lepidopterous pests in the Middle Asia (Key for identification). [In Russian] Trudy Vsesoyuznogo Entomologicheskogo Obshchestva 55: 267–283. [Google Scholar]
- Tobias VI, Jakimavicius AB. (1973) Supplementary data about the braconid (Hymenoptera, Braconidae) fauna of Lithuania [In Russian with English summary] Acta Entomologica Lituanica 2: 23–38.
- Tobias VI, Kotenko AG. (1984) Three new species of genus Apanteles Foerster of the parasitellae-group (Hymenoptera, Braconidae). Taxonomy and Zoogeography of Insects. Kiev, 61–66.
- Tobias VI, Long KD. (1990) New species of Apanteles Foerster of the A.ultor group (Hymenoptera, Braconidae) from Vietnam. Trudy Zoologicheskogo Instituta 209: 107–114. [Google Scholar]
- Tsang W, You LS, Liang GW. (2007) A new species of Glyptapanteles Ashmead Foerster attacking litch fruit borer Conopomorphasinensis Bradley (Hymenoptea: Braconidae). [In Chinese with English summary] Journal of Hunan Agricultural University 33(1): 65–67. [Google Scholar]
- Turner RE. (1919) The Hymenoptera of Fiji. Transactions of the Entomological Society of London 1918: 334–346. 10.1111/j.1365-2311.1919.tb02599.x [DOI] [Google Scholar]
- Ullyett GC. (1946) New species of Apanteles (Hym. Bracon.) and new host records from South Africa. Journal of the Entomological Society of South Africa 9: 28–35. [Google Scholar]
- Valentine EW, Walker AK. (1991) Annotated catalogue of New Zealand Hymenoptera. D.S.I.R. Plant Protection Report No. 4.
- Valerio AA, Whitfield JB. (2003) A new species of the enigmatic genus Teremys Mason, T.hanniae, from Costa Rica (Hymenoptera: Braconidae). Zootaxa 364: 1–9. 10.11646/zootaxa.364.1.1 [DOI] [Google Scholar]
- Valerio AA, Whitfield JB. (2005) Two new species of the genus Austrocotesia Austin & Dangerfield (Hymenoptera: Braconidae) from the Andean region of South America. Zootaxa 888: 1–11. 10.11646/zootaxa.888.1.1 [DOI] [Google Scholar]
- Valerio AA, Whitfield JB. (2015) Taxonomic review of the genus Hypomicrogaster Ashmead (Hymenoptera: Braconidae: Microgastrinae), with descriptions of 40 new species. Zootaxa 3979(1): 1–98. 10.11646/zootaxa.3979.1.1 [DOI] [PubMed] [Google Scholar]
- Valerio AA, Deans AR, Whitfield JB. (2004) Review of the microgastrine braconid wasp genus Exoryza, with description of a new species, E.monocavus, from Central America. Zootaxa 526: 1–11. 10.11646/zootaxa.526.1.1 [DOI] [Google Scholar]
- Valerio AA, Rodriguez JJ, Whitfield JB, Janzen DH. (2005a) Prasmodonzlotnicki, a new Neotropical species of the genus Prasmodon Nixon (Braconidae: Microgastrinae) from Costa Rica, with the first host records for the genus. Zootaxa 1016: 29–38. 10.11646/zootaxa.1016.1.4 [DOI]
- Valerio AA, Whitfield JB, Kole M. (2005b) Parapantelesrooibos, n. sp. (Hymenoptera: Braconidae: Microgastrinae): the first record of the genus from the African continent. Zootaxa 855: 1–8. 10.11646/zootaxa.855.1.1 [DOI] [Google Scholar]
- Valerio AA, Whitfield JB, Janzen DH. (2009) Review of world Parapanteles Ashmead (Hymenoptera: Braconidae: Microgastrinae), with description of fourteen new Neotropical species and the first description of the final instar larvae. Zootaxa 2084: 1–49. 10.11646/zootaxa.2084.1.1 [DOI] [Google Scholar]
- van Achterberg C. (1976) A preliminary key to the subfamilies of the Braconidae (Hymenoptera). Tijdschrift voor Entomologie 119(3): 33–78. [Google Scholar]
- van Achterberg C. (1980) The Cameron types of Braconidae in the Netherlands (Hymenoptera, Ichneumonoidea). Bulletin Zoologisch Museum Universiteit van Amsterdam 7(21): 209–214. [Google Scholar]
- van Achterberg C. (1982) Notes on some type-species described by Fabricius of the subfamilies Braconinae, Rogadinae, Microgastrinae and Agathidinae (Hymenoptera: Braconidae). Entomologische Berichten, Amsterdam 42: 133–139. [Google Scholar]
- van Achterberg C. (1997) Revision of the Haliday collection of Braconidae (Hymenoptera). Zoologische Verhandelingen 314: 115.
- van Achterberg C. (2002) Apanteles (Choeras) gielisi spec. nov. (Hymenoptera: Braconidae: Microgastrinae) from the Netherlands and the first report of Trichoptera as host of Braconidae. Zoologische Mededelingen Leiden 76(5): 53–60. [Google Scholar]
- van Achterberg C. (2003) Western Palaearctic genera of the subfamily Microgastrinae: a reappraisal of the generic and tribal division (Hymenoptera: Braconidae). In: Melika G, Thuróczy G. (Eds) Parasitic Wasps: Evolution, Systematics, Biodiversity and Biological Control.Agroinform, Budapest, 19–35.
- van Achterberg C. (2006) The Braconidae (Hymenoptera) of Greenland. Zoologische Mededelingen Leiden 80–1(2): 13–62.
- van Achterberg C. (2014) Notes on the checklist of Braconidae (Hymenoptera) from Switzerland. Mitteilungen der Schweizerischen Entomologischen Gesellschaft 87: 191–213. [Google Scholar]
- van Achterberg C, O'Toole C. (1993) Annotated catalogue of the types of Braconidae (Hymenoptera) in the Oxford University Museum. Zoologische Verhandelingen 287: 48.
- van Achterberg C, Polaszek A. (1996) The parasites of cereal stem borers (Lepidoptera: Cossidae, Crambidae, Noctuidae, Pyralidae) in Africa, belonging to the family Braconidae (Hymenoptera: Ichneumonoidea). Zoologische Verhandelingen 304, 123 pp.
- van Achterberg C, Narendran TC. (1997) Notes on the types and type depositories of Braconidae (Insecta: Hymenoptera) described by T.C. Narendran and students. Zoologische Mededelingen 71(16): 177–179. [Google Scholar]
- van Achterberg C, Walker AK. (1998) 17 Braconidae. In: Polaszek A. (Ed.) African Cereal Stem Borers.Economic importance, taxonomy, natural enemies and control. CAB International. Wallingford, UK, 137–185, 448–483.
- van Achterberg C, Rezbanyai-Reser L. (2001) Zur Insektenfauna der Umgebung von Lauerz, Kanton Schwyz. 1. Sägel (455 m) und Schuttwald (480 m). IV Hymenopter 1: Braconidae (Brackwespen). Entomologische Berichte Luzern 45: 109–122. [Google Scholar]
- van Achterberg C, Hosaka T, Ng YF, Ghani Idris BA. (2009) The braconid parasitoids (Hymenoptera: Braconidae) associated with seeds of Dipterocaroaceae in Malaysia. Journal of Natural History 43(11-12): 635–686. 10.1080/00222930802610501 [DOI] [Google Scholar]
- van Achterberg C, Long KD, Chen XX, You LS. (2015) Pseudofornicia gen. n. (Hymenoptera, Braconidae, Microgastrinae), a new Indo-Australian genus and one new species from Vietnam. ZooKeys 524: 89–102. 10.3897/zookeys.524.6158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney RK. (1976) A check-list of insect parasites associated with lac. Oriental Insects 10(1): 55–78. 10.1080/00305316.1976.10432322 [DOI] [Google Scholar]
- Vayssière P, Mimeur J. (1925) Les Pyrales du cotonnier (Syleptaderogata F. & Glyphodesindica Saund.) en Afrique occidentale française. Agronomie Coloniale 12(90): 255–268. [Google Scholar]
- Veena T, Ranjith AP, Santosh S, Kishore L. (2014) Review of the Oriental genus Neoclarkinella Rema and Narendran, 1996 (Hymenoptera: Braconidae, Microgastrinae) with the description of two new species from India. Zootaxa 3857(3): 423–432. 10.11646/zootaxa.3857.3.5 [DOI] [PubMed] [Google Scholar]
- Viereck HL. (1910a) Descriptions of new species of Ichneumon flies. Proceedings of the United States National Museum 38(1754): 379–384. 10.5479/si.00963801.38-1754.379 [DOI] [Google Scholar]
- Viereck HL. (1910b) Hymenoptera for the New Jersey list of insects, and other Hymenoptera. Proceedings of the Entomological Society of Washington 11: 208–211. [Google Scholar]
- Viereck HL. (1911a) Descriptions of one new genus and eight new species of Ichneumon flies. Proceedings of the United States National Museum 40(1832): 475–480. 10.5479/si.00963801.1832.475 [DOI] [Google Scholar]
- Viereck HL. (1911b) Descriptions of six new genera and thirty-one new species of Ichneumon flies. Proceedings of the United States National Museum 40(1812): 173–196. 10.5479/si.00963801.1812.173 [DOI] [Google Scholar]
- Viereck HL. (1912a) Contributions to our knowledge of bees and Ichneumon-flies, including descriptions of twenty-one new genera and fifty-seven new species of Ichneumon-flies. Proceedings of the United States National Museum 42(1920): 613–648. 10.5479/si.00963801.42-1920.613 [DOI] [Google Scholar]
- Viereck HL. (1912b) Descriptions of five new genera and twenty-six new species of Ichneumon-flies. Proceedings of the United States National Museum 42: 139–153. 10.5479/si.00963801.1888.139 [DOI] [Google Scholar]
- Viereck HL. (1912c) Descriptions of one new family, eight new genera, and thirty-three new species of Ichneumonidae. Proceedings of the United States National Museum 43: 575–593. 10.5479/si.00963801.1942.575 [DOI] [Google Scholar]
- Viereck HL. (1913) Descriptions of ten new genera and twenty-three new species of Ichneumon-flies. Proceedings of the United States National Museum 44: 555–568. 10.5479/si.00963801.1968.555 [DOI] [Google Scholar]
- Viereck HL. (1914) Type species of the genera of Ichneumon flies. United States National Museum Bulletin No.83, 186 pp. 10.5479/si.03629236.83.1 [DOI]
- Viereck HL. (1917) Guide to the insects of Connecticut. Part III. The Hymenoptera, or wasp-like insects of Connecticut. Ichneumonoidea. State of Connecticut. State Geological and Natural History Survey. Bulletin No. 22(1916) 1–824.
- Viereck HL. (1923) Insects, Arachnids and Chilopods of the Pribilof Islands, Alaska; Hymenoptera. North American Fauna 46: 229–236. 10.3996/nafa.46.0002 [DOI] [Google Scholar]
- Walker AK. (1994) Species of Microgastrinae (Hymenoptera: Braconidae) parasitizing lepidopterous cereal stem borers in Africa. Bulletin of Entomological Research 84: 421–434. 10.1017/S0007485300032557 [DOI] [Google Scholar]
- Walker AK. (1996) A new species of Choeras (Braconidae: Microgastrinae) widespread in New Zealand. New Zealand Entomologist 19: 43–48. 10.1080/00779962.1996.9722020 [DOI] [Google Scholar]
- Walker AK, Kitching IJ, Austin AD. (1990) A reassessment of the phylogenetic relationships within the Microgastrinae (Hymenoptera: Braconidae). Cladistics 6: 291–306. 10.1111/j.1096-0031.1990.tb00546.x [DOI] [Google Scholar]
- Walker F. (1860) Characters of some apparently undescribed Ceylon insects. Annals and Magazine of Natural History (3)5: 304–311. 10.1080/00222936008697221 [DOI]
- Walker F. (1874) Descriptions of some Japanese Hymenoptera. Cistula Entomologica 1: 301–310. [Google Scholar]
- Walley GS. (1932) Host records and new species of Canadian Hymenoptera. The Canadian Entomologist 64: 181–189. 10.4039/Ent64181-8 [DOI] [Google Scholar]
- Walley GS. (1935) Five new species of Braconidae with host records of additional species. The Canadian Entomologist 67: 55–61. 10.4039/Ent6755-3 [DOI] [Google Scholar]
- Walley GS. (1937) New Canadian Ichneumon flies with some notes on synonymy (Hymen.: Ichneumonoidea). The Canadian Entomologist 69: 189–193. 10.4039/Ent69189-9 [DOI] [Google Scholar]
- Walsh BD. (1861) Primary and secondary parasites of the army-worm. Transactions of the Illinois State Agricultural Society 4: 368–369. [Google Scholar]
- Watanabe C. (1932) Notes on the Braconidae. III. Apanteles. Insecta Matsumurana 7: 74–102. [Google Scholar]
- Watanabe C. (1934) Notes on Braconidae of Japan. IV. Apanteles (First Supplement). Insecta Matsumurana 8(3): 132–143. [Google Scholar]
- Watanabe C. (1935) On some species of Braconidae from North China and Korea. Insecta Matsumurana 10: 43–51. [Google Scholar]
- Watanabe C. (1937a) A contribution to the knowledge of the Braconid fauna of the Empire of Japan. Journal of the Faculty of Agriculture, Hokkaido (Imp. ) University 42: 1–188. [Google Scholar]
- Watanabe C. (1937b) On some species of Braconidae from Manchoukuo (Contributions to the knowledge of the Braconid fauna of Manchoukuo, I). Insecta Matsumurana 12(1): 39–44. [Google Scholar]
- Watanabe C. (1939) A new species of Apanteles bred from Daimio tethys Ménétriès, with notes on other species. Insecta Matsumurana 13: 129–131. [Google Scholar]
- Watanabe C. (1940a) Description of a new Apanteles-species bred from Dictyoplocajaponica (Moore) Butler (Hymenoptera: Braconidae). Insecta Matsumurana 15: 51–52. [Google Scholar]
- Watanabe C. (1940b) Hymenopterous parasites of the mulberry pyralid moth, Margaroniapyloalis Walker, in Japan (I). Insecta Matsumurana 14(2-3): 85–94. [Google Scholar]
- Watanabe C. (1942) Descriptions of a new Apanteles-species bred from Anomisfimbriago Stephen in Manchoukuo. Insecta Matsumurana 16: 169–170. [Google Scholar]
- Watanabe C. (1967) Notes on Braconidae caught in a sweep-net at paddy fields, Part I. Mushi 40: 189–198. [Google Scholar]
- Waterston J. (1923) Notes on parasitic Hymenoptera. Bulletin of Entomological Research 14: 103–118. 10.1017/S0007485300028248 [DOI] [Google Scholar]
- Weed CM. (1887) Notes on some Illinois Microgasters, with descriptions of new species. Bulletin of the Illinois State Laboratory of Natural History 3(1896): 1–8. [Google Scholar]
- Weed CW. (1888) Descriptions of some new or little known Microgasterinae. Transactions of the American Entomological Society 15: 294–297. 10.2307/25076507 [DOI] [Google Scholar]
- Wesmael C. (1837) Monographie des Braconides de Belgique. (Suite. ) Nouveaux Mémoires de l'Academie Royale des Sciences et Belles-Lettres de Bruxelles 10: 1–68. [Google Scholar]
- Westwood JO. (1882) Descriptions of new or imperfectly known species of Ichneumones Adsciti. Tijdschrift voor Entomologie 25: 17–48. [Google Scholar]
- Wharton RA. (1983) New species of Illidops and Bracon (Hymenoptera: Braconidae) of potential use in biological control. The Canadian Entomologist 115: 667–672. 10.4039/Ent115667-6 [DOI] [Google Scholar]
- Whitfield JB. (1985) The Nearctic species of Deuterixys Mason (Hymenoptera: Braconidae). Pan-Pacific Entomologist 61(1): 60–67. [Google Scholar]
- Whitfield JB. (1987) Comment on the proposed designation of Microgasteraustralis Thomson, 1895 as type of Microgaster Latreille, 1804 (Insecta, Hymenoptera) (Case 2397). Bulletin of Zoological Nomenclature 44(1): 47. [Google Scholar]
- Whitfield JB. (1995a) Annotated checklist of the Microgastrinae of North America north of Mexico (Hymenoptera: Braconidae). Journal of the Kansas Entomological Society 68(3): 245–262. [Google Scholar]
- Whitfield JB. (1995b) Xanthapanteles, a new genus of Microgastrinae (Hymenoptera: Braconidae) from South America. Proceedings of the Entomological Society of Washington 97(4): 879–883. [Google Scholar]
- Whitfield JB. (1997) Subfamily Microgastrinae. Manual of the New World genera of the family Braconidae (Hymenoptera). International Society of Hymenopterists. Special Publication (1): 333–364.
- Whitfield JB. (2006) Revision of the Nearctic species of the genus Pholetesor Mason (Hymenoptera: Braconidae). Zootaxa 1144: 3–94. 10.11646/zootaxa.1144.1.1 [DOI] [Google Scholar]
- Whitfield JB, Cameron SA. (1993) Comparative notes on hymenopteran parasitoids in bumble bee and honey bee colonies (Hymenoptera: Apidae) reared adjacently. Entomological News 104(5): 240–48. [Google Scholar]
- Whitfield JB, Scaccia B. (1996) A new species of Distatrix (Hymenoptera: Braconidae) from California, with biological Notes. Proceedings of the Entomological Society of Washington 98(2): 308–313. [Google Scholar]
- Whitfield JB, Oltra MT. (2004) The Neotropical species of Deuterixys Mason (Hymenoptera: Braconidae). Journal of Hymenoptera Research 13(1): 134–148. [Google Scholar]
- Whitfield JB, Marquis RJ, Le Corff J. (1999) Braconid parasitoids associated with caterpillars feeding on oaks in the Missouri Ozarks. Entomological News 110(4): 225–230. [Google Scholar]
- Whitfield JB, Cameron SA, Ramírez SR, Roesch K, Messinger S, Taylor OM, Cole D. (2001) Review of the Apanteles species (Hymenoptera: Braconidae) attacking Lepidoptera in Bombus (Fervidobombus) (Hymenoptera: Apidae) colonies in the New World, with description of a new species from South America. Annals of the Entomological Society of America 94(6): 851–857. 10.1603/0013-8746(2001)094[0851:ROTASH]2.0.CO;2 [DOI]
- Whitfield JB, Benzing A, Ponce F. (2002a) Review of the Glyptapanteles species (Hymenoptera: Braconidae, Microgastrinae) attacking noctuids in field crops in the Neotropical region, with descriptions of two new species from the Ecuadorian Andes. Journal of Hymenoptera Research 11(1): 152–165. [Google Scholar]
- Whitfield JB, Mardulyn P, Austin AD, Dowton M. (2002b) Phylogenetic relationships among microgastrine braconid wasp genera based on data from the 16S, COI and 28S genes and morphology. Systematic Entomology 27(3): 337–359. 10.1046/j.1365-3113.2002.00183.x [DOI] [Google Scholar]
- Whitfield JB, Valerio AA, Choi WY. (2005) Redescription of Pelicopeyuccamica Mason (Hymenoptera: Braconidae: Microgastrinae), with notes on its unusual biology and relationships. Journal of Hymenoptera Research 14(2): 200–205. [Google Scholar]
- Whitfield JB, Rodriguez JJ, Masonick PK. (2009) Reared microgastrine wasps (Hymenoptera: Braconidae) from Yanayacu Biological Station and environs (Napo Province, Ecuador): diversity and host specialization. Journal of Insect Science 9(1): 31. 10.1673/031.009.3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield JB, Rasmussen C, Arias-Penna DC. (2011) Review of the New World genus Venanus (Hymenoptera: Braconidae: Microgastrinae), with a new key and descriptions of three new reared Neotropical species. Annals of the Entomological Society of America 104(6): 1119–1127. 10.1603/AN10048 [DOI] [Google Scholar]
- Whitfield JB, Fernandez-Triana J, Janzen DH, Hallwachs W, Smith MA, Cardinal S. (2012) Mariapanteles (Hymenoptera, Braconidae), a new genus of Neotropical microgastrine parasitoid wasp discovered through biodiversity inventory. ZooKeys 208: 61–80. 10.3897/zookeys.208.3326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield JB, Austin A, Fernandez-Triana JL. (2018) Systematics, Biology, and Evolution of Microgastrine Parasitoid Wasps. Annual Review of Entomology 63: 389–406. 10.1146/annurev-ento-020117-043405 [DOI] [PubMed] [Google Scholar]
- Whitfield JB, Nuelle Jr RJ, Nuelle III RJ. (2018) A new species of Cotesia Cameron (Hymenoptera, Braconidae, Microgastrinae) reared from the hickory horned devil, Citheroniaregalis, and luna moth, Actiasluna, in east Texas. ZooKeys 740: 35–44. 10.3897/zookeys.740.242226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DS. (1927) On the Indo-Malayan species of the genus Microgaster (Hymenoptera: Braconidae). Bulletin of Entomological Research 18: 171–178. 10.1017/S0007485300019878 [DOI] [Google Scholar]
- Wilkinson DS. (1928a) A revision of the Indo-Australian species of the genus Apanteles (Hym. Bracon.). Part I. Bulletin of Entomological Research 19: 79–105. 10.1017/S0007485300028856 [DOI] [Google Scholar]
- Wilkinson DS. (1928b) A revision of the Indo-Australian species of the genus Apanteles (Hym. Bracon.). Part II. Bulletin of Entomological Research 19: 109–146. 10.1017/S0007485300020393 [DOI] [Google Scholar]
- Wilkinson DS. (1929a) A revision of the Indo-Australian and Ethiopian species of the genus Microgaster (Hym. Bracon.). Transactions of the Royal Entomological Society of London 77: 99–123. 10.1111/j.1365-2311.1929.tb00681.x [DOI] [Google Scholar]
- Wilkinson DS. (1929b) New parasitic Hymenoptera and notes on other species. Bulletin of Entomological Research 20(1): 103–114. 10.1017/S000748530002099X [DOI] [Google Scholar]
- Wilkinson DS. (1929c) New species and host records of Braconidae. Bulletin of Entomological Research 19: 205–208. 10.1017/S0007485300021131 [DOI] [Google Scholar]
- Wilkinson DS. (1929d) Seven new species of Braconidae. Bulletin of Entomological Research 20: 443–455. 10.1017/S0007485300021416 [DOI] [Google Scholar]
- Wilkinson DS. (1930a) A revision of the Indo-Australian species of the genus Microplitis (Hym. Bracon.). Bulletin of Entomological Research 21: 23–27. 10.1017/S0007485300021519 [DOI] [Google Scholar]
- Wilkinson DS. (1930b) New Braconidae and other Notes. Bulletin of Entomological Research 21(3): 275–285. 10.1017/S0007485300021799 [DOI] [Google Scholar]
- Wilkinson DS. (1930c) New species and host records of Ichneumonidae and Braconidae. Bulletin of Entomological Research 21(2): 147–158. 10.1017/S0007485300021660 [DOI] [Google Scholar]
- Wilkinson DS. (1931) Braconidae: notes and new species. Bulletin of Entomological Research 22: 75–82. 10.1017/S000748530002976X [DOI] [Google Scholar]
- Wilkinson DS. (1932a) A revision of the Ethiopian species of the genus Apanteles (Hym. Bracon.). Transactions of the Entomological Society of London 80: 301–344. 10.1111/j.1365-2311.1932.tb03312.x [DOI] [Google Scholar]
- Wilkinson DS. (1932b) Four new Apanteles (Hym. Bracon.). Stylops 1: 139–144. 10.1111/j.1365-3113.1932.tb01372.x [DOI] [Google Scholar]
- Wilkinson DS. (1932c) Three new Braconids (Hym.). Stylops 1: 85–88. 10.1111/j.1365-3113.1932.tb01358.x [DOI] [Google Scholar]
- Wilkinson DS. (1934a) On some Apanteles (Hym. Bracon.). Stylops 3: 145–156. 10.1111/j.1365-3113.1934.tb01568.x [DOI] [Google Scholar]
- Wilkinson DS. (1934b) On some Microgasterinae (Hymenoptera: Braconidae). Stylops 3(5): 118–120. 10.1111/j.1365-3113.1934.tb01562.x [DOI] [Google Scholar]
- Wilkinson DS. (1935) Two new Apanteles (Hym. Braconidae). Stylops 4: 266–269. 10.1111/j.1365-3113.1935.tb00658.x [DOI] [Google Scholar]
- Wilkinson DS. (1936a) A new Palaearctic species of Apanteles (Hym. Brac.). Proceedings of the Royal Entomological Society of London (B) 5(9): 174–176. 10.1111/j.1365-3113.1936.tb00618.x [DOI] [Google Scholar]
- Wilkinson DS. (1936b) Microgasterinae: notes and new species (Hym. Brac.). Proceedings of the Royal Entomological Society of London (B) 5(4): 81–88. 10.1111/j.1365-3113.1936.tb01320.x [DOI] [Google Scholar]
- Wilkinson DS. (1936c) On two braconids (Hym.) bred from economic hosts. Bulletin of Entomological Research 27: 385–388. 10.1017/S0007485300058247 [DOI] [Google Scholar]
- Wilkinson DS. (1937a) A new species of Apanteles (Hym. Brac.) bred from Myeloisceratoniae attacking carobs in Cyprus. Bulletin of Entomological Research 28: 463–466. 10.1017/S0007485300038918 [DOI] [Google Scholar]
- Wilkinson DS. (1937b) On two new Palaearctic species of Apanteles (Hym., Braconidae). Proceedings of the Royal Entomological Society of London 6(4): 65–72. 10.1111/j.1365-3113.1937.tb00300.x [DOI] [Google Scholar]
- Wilkinson DS. (1938a) A new species of Apanteles (Hym. Brac.) from South Africa. Proceedings of the Royal Entomological Society of London Series B 7(6): 131–133. 10.1111/j.1365-3113.1938.tb01263.x [DOI] [Google Scholar]
- Wilkinson DS. (1938b) A new species of Apanteles (Hymenoptera: Braconidae) bred from Carposinaadreptella attacking raspberry in New Zealand. Bulletin of Entomological Research 29: 247–249. 10.1017/S0007485300035586 [DOI] [Google Scholar]
- Wilkinson DS. (1938c) On a further two new palearctic species of Apanteles (Hym. Brac.). Proceedings of the Royal Entomological Society of London, Series B 7(10): 222–227. 10.1111/j.1365-3113.1938.tb01231.x [DOI] [Google Scholar]
- Wilkinson DS. (1938d) On the identity of Apantelescircumscriptus Nees (Hym. Braconidae). Proceedings of the Royal Entomological Society of London, Series B 7(2): 41–51. 10.1111/j.1365-3113.1938.tb01244.x [DOI] [Google Scholar]
- Wilkinson DS. (1939) On the identity of Apantelesinfimus Haliday and of Apantelesinfimus Haliday of Marshall (Hym. Bracon.). Proceedings of the Royal Entomological Society of London, Series B 8(4): 53–60. 10.1111/j.1365-3113.1939.tb00491.x [DOI] [Google Scholar]
- Wilkinson DS. (1940) New species of Apanteles (Hym. Brac.). 1. Proceedings of the Royal Entomological Society of London, Series B 9(2): 23–29. 10.1111/j.1365-3113.1940.tb00336.x [DOI] [Google Scholar]
- Wilkinson DS. (1941a) New species of Apanteles (Hym. Brac.). 2. Proceedings of the Royal Entomological Society of London, Series B 10(2): 28–34. 10.1111/j.1365-3113.1941.tb00687.x [DOI] [Google Scholar]
- Wilkinson DS. (1941b) On the identity of Apantelesalbipennis Haliday non Nees and of Apantelesalbipennis Haliday of Marshall (Hym. Brac.). Proceedings of the Royal Entomological Society of London, Series B 10(5): 71–81. 10.1111/j.1365-3113.1941.tb00698.x [DOI] [Google Scholar]
- Wilkinson DS. (1945) Description of Palaearctic species of Apanteles (Hymen., Braconidae). Transactions of the Entomological Society of London 95: 35–226. 10.1111/j.1365-2311.1945.tb00436.x [DOI] [Google Scholar]
- Williams DJM. (1985) The New World genus Lathrapanteles n. gen.: Phylogeny and placement in the Microgastrinae (Hymenoptera: Braconidae: Cotesini). Canadian Journal of Zoology 63: 1962–1981. 10.1139/z85-289 [DOI] [Google Scholar]
- Williams DJM. (1988) Classification, phylogeny and zoogeographic studies of species of Sathon Mason (Hymenoptera: Braconidae). Quaestiones Entomologicae 24: 529–638. [Google Scholar]
- Xiao ZS, You LS. (2002) A new genus of Microgastrinae from China (Hymenoptera: Braconidae). Acta Zootaxonomica Sinica 27(3): 616–620. [Google Scholar]
- Xiong Y, Achterberg CV, Chen X. (2017) A new genus of the tribe Cotesiini Mason (Hymenoptera: Braconidae: Microgastrinae) from China. Zootaxa 4324(2): 391–400. 10.11646/zootaxa.4324.2.12 [DOI] [Google Scholar]
- Xu WA, He JH. (1997) A new species of Microgaster Latreille from China (Hym.: Microgasterinae). [In Chinese with English summary] Wuyi Science Journal 13: 76–79. [Google Scholar]
- Xu WA, He JH. (1998a) A new species of Microgaster Latreille (Hymenoptera: Braconidae) from China. [In Chinese with English summary] Journal of Shandong Agricultural University 29(2): 161–164. [Google Scholar]
- Xu WA, He JH. (1998b) Hymenoptera: Braconidae (II): Microgastrinae. In: Wu H. (Ed.) Insects of Longwangshan Nature Reserve.China Forestry Publishing House, Beijing, 395–397.
- Xu WA, He JH. (1999a) A new species of Microgaster Latreille from Yunnan Province of China (Hymenoptera: Braconidae: Microgasterinae). [In Chinese with English summary] Entomological Journal of East China 8(2): 7–9. [Google Scholar]
- Xu WA, He JH. (1999b) A new species of Microplitis Foerster from China (Hymenoptera: Braconidae, Microgastrinae). [In Chinese with English summary] Entomological Journal of East China 8(1): 1–3. [Google Scholar]
- Xu WA, He JH. (1999c) A new species of Microplitis Foerster (Hymenoptera: Braconidae: Microgastrinae) from Fujian, China. [In Chinese with English summary] Entomotaxonomia, 21(1): 64–68. [Google Scholar]
- Xu WA, He JH. (2000a) A new species and a new record species of Microplitis Foerster (Hymenoptera: Braconidae: Microgastrinae) from China. [In Chinese with English summary] Entomological Journal of East China 9(2): 5–8. [Google Scholar]
- Xu WA, He JH. (2000b) A new species and a new record of Microplitis Foerster from China (Hymenoptera: Braconidae: Microgastrinae). [In Chinese with English summary] Acta Entomologica Sinica 43(2): 193–197. [Google Scholar]
- Xu WA, He JH. (2000c) A new species of Microplitis Foerster from China (Hymenoptera: Braconidae: Microgastrinae). [In Chinese with English summary] Acta Zootaxonomica Sinica 25(2): 195–198. [Google Scholar]
- Xu WA, He JH. (2000d) Two new species of the genus Microgaster Latreille (Hymenoptera: Braconidae: Microgastrinae) from China. Entomological Journal of East China 9(2): 1–4. [Google Scholar]
- Xu WA, He JH. (2000e) Two new species of the genus of the genus Microgaster Latreille (Hymenoptera: Braconidae: Microgastrinae) from China. [In Chinese with English summary] In: Zhang Y-L. (Ed.) Systematic and faunistic research on Chinese insects.Proceedings of the.5th National Congress of Insect Taxonomy. China Agriculture Press, 246–250.
- Xu WA, He JH. (2000f) Two new species of Microplitis Foerster (Hymenoptera: Braconidae) from China. [In Chinese with English summary] Entomotaxonomia 22(3): 204–208. [Google Scholar]
- Xu WA, He JH. (2000g) Two new species of Microplitis Foerster (Hymenoptera: Braconidae) from China. Entomologia Sinica 7(2): 107–112. 10.1111/j.1744-7917.2000.tb00346.x [DOI] [Google Scholar]
- Xu WA, He JH. (2002a) Two new species of Microplitis Foerster (Hymenoptera: Braconidae, Microgastrinae) from China. [In Chinese with English summary] Acta Entomologica Sinica 45 (Supplement): 99–102
- Xu WA, He JH. (2002b) Two new species of Microplitis Foerster from China (Hymenoptera: Braconidae: Microgastrinae). [In Chinese with English summary] Acta Zootaxonomica Sinica 27(1): 153–157. [Google Scholar]
- Xu WA, He JH. (2002c) Two new species of the genus Microgaster Latreille (Hymenoptera: Braconidae, Microgastrinae) from China. Acta Entomologica Sinica 45 (Supplement): 103–106.
- Xu WA, He JH. (2003a) Two new species of Microgaster Latreille from China (Hymenoptera, Braconidae, Microgastrinae). Acta Zootaxonomica Sinica 28(3): 525–529. [Google Scholar]
- Xu WA, He JH. (2003b) Two new species of Microplitis Foerster from China (Hymenoptera, Braconidae, Microgastrinae). Acta Zootaxonomica Sinica 28(4): 724–728. [Google Scholar]
- Xu WA, He JH. (2006) One new species of Microplitis Foerster (Hymenoptera: Braconidae: Microgastrinae) from China. Entomotaxonomia 28(3): 227–230. [In Chinese with English summary] [Google Scholar]
- Xu WA, Han HY. (2007) A survey of the genera of Microgaster Latreille and Hygroplitis Thomson (Hymenoptera: Braconidae: Microgastrinae) from China. Phytoparasitica 35(1): 86–99. 10.1007/BF02981063 [DOI] [Google Scholar]
- Xu WA, He JH, Chen XX. (1998) A new species of Microgaster Latreille from China (Hymenoptera: braconidae: Microgasterinae). Acta Zootaxonomica Sinica 23(3): 302–305. [Google Scholar]
- Xu WA, He JH, Chen XX. (2001a) Hymenoptera: Braconidae: Microgasterinae [In Chinese with English summary] In: Wu H, Pan C. (Eds) Insects of Tianmushan National Nature Reserve.Science Press, Beijing, 734–736.
- Xu WA, He JH, Li SJ. (2001b) One new species and a new record species of Microgaster Latreille (Hymenoptera) from China. [In Chinese with English summary] Journal of Shandong Agricultural University 32(2): 143–146. [Google Scholar]
- Yang JQ, Chen JH. (2006) A new species of the genus Fornicia Brulle (Hymenoptera, Braconidae) from China. Acta Zootaxonomica Sinica 31(3): 627–629. [In Chinese with English summary] [Google Scholar]
- Yang ZQ, Wei JR, You LS. (2002) Two new braconid species parasitizing larva of fall webworm from China (Hymenoptera: Braconidae). Acta Zootaxonomica Sinica 27(3): 608–615. [Google Scholar]
- You LS. (1986) Emendation of the species name Apanteleschiloluteelli You, Xiong et Wang. Acta Zootaxonomica Sinica 11: 208.
- You LS, Xiong SL. (1983) Two new species of Apanteles Foerster (Hymenoptera: Braconidae) from China. Entomotaxonomia 5: 225–229. [Google Scholar]
- You LS, Zhou ZH. (1988) A new species of Apanteles from reed field in Shandong, China (Hymenoptera: Braconidae). Acta Zootaxonomica Sinica 13(3): 305–307. [Google Scholar]
- You LS, Zhou ZH. (1989) A new species of paddy field Apanteles Foerster from Yunan, China (Hymenoptera: Braconidae). ] [In Chinese with English summary] Entomotaxonomia 11(4): 307–309. [Google Scholar]
- You LS, Zhou ZH. (1990) Two new species of Apanteles Foerster (Hymenoptera: Braconidae: Microgasterinae) from South China. [In Chinese with English summary] Entomotaxonomia 12(2): 151–155. [Google Scholar]
- You LS, Zhou ZH. (1996) A new species of Microgaster Latreille from China (Hymenoptera: Braconidae: Microgasterinae). [In Chinese with English summary] Acta Zootaxonomica Sinica 21(2): 214–217. [Google Scholar]
- You LS, Wei MC. (2006) Fauna Hunan HymenopteraBraconidae. [In Chinese with English summary] Hunan Press of Science and Technology. ChangSha, Hunan, China, 285 pp. [Google Scholar]
- You LS, Xiong SL, Wang ZD. (1985) A new species of Apanteles Foerster (Hymenoptera, Braconidae) from China. [In Chinese with English summary] Acta Zootaxonomica Sinica 10: 421–423. [Google Scholar]
- You LS, Xiong SL, Dang XD, Tong XW. (1987a) Four new species of Apanteles Forster from China. (Hymenoptera: Braconidae, Microgasterinae). [In Chinese with English summary] Entomotaxonomia 9(4): 275–281. [Google Scholar]
- You LS, Xiong SL, Zhou ZH. (1987b) On a new species of Apanteles Foerster from Yunnan Province (China) (Hymenoptera: Braconidae: Microgasterinae). [In Chinese with English summary] Acta Zootaxonomica Sinica 12(4): 424–426. [Google Scholar]
- You LS, Wang ZD, Zhou ZH. (1990) New species and new records of Apanteles Forster from China (Hymenoptera: Braconidae: Microgasterinae). [In Chinese with English summary] Acta Entomologica Sinica 33(2): 237–242. [Google Scholar]
- You LS, Zhou ZH, Tong XW. (1991) Two new species of Apanteles Foerster from China (Hymenoptera: Braconidae: Microgasterinae). [In Chinese with English summary] Entomotaxonomia 13(1): 39–42. [Google Scholar]
- You LS, Zhang WN, Luo QH. (2002a) A new species of Microgaster Latreille from China (Hymenoptera: Braconidae: Microgastrinae). [In Chinese with English summary] Journal of Hunan Agricultural University 28(6): 514–515. [Google Scholar]
- You LS, Xiao ZS, Bo LY, Zhou ZH. (2002b) On the external male genitalia and phylogenetic relationships of the tribal taxa of Microgastrinae (Hymenoptera: Braconidae). [In Chinese with English summary] Acta Entomologica Sinica 45(6): 794–804. [Google Scholar]
- Yousuf M, Hassan ME, Singh RB. (2008) Record of Apanteles sp. (Hymenoptera: Braconidae): a larval parasitoid of Attevafabriciella Swederus. Bulletin of Pure and Applied Sciences A Zoology 27A(1): 31–34.
- Yousuf M, Ray P. (2010) Description of two new species of Apanteles Foerster (Hymenoptera: Braconidae: Microgastrinae) from Chhattisgarh, India. Entomon 35(1): 23–30. [Google Scholar]
- Yu DSK, van Achterberg C, Horstmann K. (2005) World Ichneumonoidea 2004. Taxonomy, Biology, Morphology and Distribution. CD/DVD. Taxapad, Vancouver, Canada.
- Yu DSK, van Achterberg C, Horstmann K. (2012) Taxapad 2012, Ichneumonoidea 2011. Database on flash-drive. Ottawa, Ontario. http://ww.taxapad.com
- Yu DSK, van Achterberg C, Horstmann K. (2016) Taxapad 2016, Ichneumonoidea 2015. Database on flash-drive. Nepean, Ontario. http://www.taxapad.com
- Zargar M, Gupta A, Talebi AA, Farahani S. (2019) A review of the Iranian species of genus Iconella Mason (Hymenoptera: Braconidae: Microgastrinae) with description of two new species. Zootaxa 4586(3): 491–504. 10.11646/zootaxa.4586.3.6 [DOI] [PubMed] [Google Scholar]
- Zargar M, Gupta A, Talebi AA, Farahani S. (2019) Three new species and two new records of the genus Cotesia Cameron (Hymenoptera: Braconidae) from Iran. European Journal of Taxonomy 571, 10.5852/ejt.2019.571 [DOI]
- Zeng A, You L, Bai L. (2009) The classification of Braconidae and application of their male genitalia. [In Chinese with English summary]. Hunan Science & Technology Press, Changsha, Hunan, China, 317 pp. [Google Scholar]
- Zeng J. (2012) A taxonomic study on the tribe Cotesiini (Hymenoptera: Braconidae: Microgastrinae) from China. [In Chinese] Doctoral Thesis of Zhejiang University, 379 pp.
- Zeng J, He JH, Chen XX. (2011a) The genera Deuterixys Mason, 1981 and Wilkinsonellus Mason, 1981 (Hymenoptera, Braconidae, Microgastrinae) from China, with description of two new species. ZooKeys 120: 27–40. 10.3897/zookeys.120.891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J, He JH, Chen XX. (2011b) The genus Diolcogaster Ashmead, 1900 (Hymenoptera, Braconidae, Microgastrinae) from China. ZooKeys 129: 49–87. 10.3897/zookeys.129.1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterstedt JW. (1838) Insecta Lapponica. Sectio secunda. Hymenoptera. Lipsiae 358–408.
- Zhang W, Song D, Chen J. (2017) Revision of Microplitis species from China with description of a new species. Zootaxa 4231(2): 296–300. 10.11646/zootaxa.4231.2.12 [DOI] [PubMed] [Google Scholar]
- Žikic V, Stankovic SS, Ilic M, Kavallieratos NG. (2013) Braconid parasitoids (Hymenoptera: Braconidae) on poplars and aspen (Populus spp.) in Serbia and Montenegro. North-Western Journal of Zoology 9(2): 264–275. [Google Scholar]
- Žikic V, Lazarevic M, Stankovic SS, Miloševic MI. (2015) New data on Microgastrinae in Serbia and Montenegro (Hymenoptera: Braconidae) and their hosts. Biologica Nyssana 6(1): 41–48. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Jose Fernandez-Triana, Mark R. Shaw, Caroline Boudreault, Melanie Beaudin, Gavin R. Broad
Supplementary table 1
Data type: species data
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Jose Fernandez-Triana, Mark R. Shaw, Caroline Boudreault, Melanie Beaudin, Gavin R. Broad
Supplementary table 2
Data type: species data