SUMMARY
Cancer cells rely on altered metabolism to support abnormal proliferation. We performed a CRISPR/Cas9 functional genomic screen targeting metabolic enzymes and identified PDXK– an enzyme that produces pyridoxal phosphate (PLP) from vitamin B6– as an acute myeloid leukemia (AML)-selective dependency. PDXK kinase activity is required for PLP production and AML cell proliferation, and pharmacological blockade of the vitamin B6 pathway at both PDXK and PLP levels recapitulated PDXK disruption effects. PDXK disruption reduced intracellular concentrations of key metabolites needed for cell division. Furthermore, disruption of PLP-dependent enzymes ODC1 or GOT2 selectively inhibited AML cell proliferation and their downstream products partially rescued PDXK disruption induced proliferation blockage. Our work identifies the vitamin B6 pathway as a pharmacologically actionable dependency in AML.
Graphical Abstract

In Brief/eTOC
In a CRISPR/Cas9 functional screen targeting metabolic enzymes, Lowe et al. identify PDXK, which produces pyridoxal phosphate (PLP) from vitamin B6, as an AML dependency. PLP-dependent enzymes ODC1 and GOT2 support AML proliferation. Blockade of the vitamin B6 metabolic pathway exhibits anti-leukemic activity.
INTRODUCTION
Most cancer cells display alterations in metabolism that are associated with tumorigenesis, although whether this metabolic reprograming is a cause or consequence of cancer phenotypes remains a topic of debate (Cairns et al., 2011; Cantor and Sabatini, 2012; Pavlova and Thompson, 2016; Vander Heiden, 2011). Studies over the last decade indicate that genetic drivers of tumor development (mutated oncogenes or tumor suppressors) can, among other things, hijack cellular metabolism to support cancer cell proliferation and survival. Further emphasizing the importance of metabolic reprograming during tumorigenesis, a subset of cancer-causing mutations directly affect metabolic processes. These include missense mutations in isocitrate dehydrogenase (IDH) occurring in leukemia, glioma, sarcoma, and cholangiocarcinoma, loss-of-function mutation of succinate dehydrogenase (SDH) in pheochromocytoma and paraganglioma, and hereditary mutations in fumarate hydratase (FH) occurring in renal cell cancer. While the clinical success of agents capable of inhibiting the mutant IDH proteins highlights the therapeutic utility of targeting deregulated metabolism in cancer, the vast majority of oncogenic drivers alter metabolism through indirect mechanisms and, as such, metabolic dependencies cannot be inferred from genomic analyses alone.
A number of metabolic processes relevant to cancer are controlled by vitamins, which are essential nutrients needed in limited amounts to support organismal health. For example, vitamin C and D have recently been implicated in controlling a variety of cancer associated processes, and perturbations in these processes can modulate various cancer phenotypes (Agathocleous et al., 2017; Cimmino et al., 2017; Sherman et al., 2014; Yun et al., 2015). One class of vitamins relevant to cell proliferation that has not been directly linked to cancer is vitamin B6. This family consists of 6 structurally related small metabolites: pyridoxine (PN), pyridoxamine (PM), pyridoxal (PL) and their phosphorylated forms pyridoxine 5-phosphate (PNP), pyridoxamine 5-phosphate (PMP) and pyridoxal 5-phosphate (PLP) (Eliot and Kirsch, 2004; Galluzzi et al., 2013). Of these, the active form of vitamin B6 is PLP, which is a cofactor for over 160 enzymes, including those involved in production and degradation of many amino acids and nucleic acids, as well as those controlling glucose, sphingolipid and fatty acid metabolism(Ueland et al., 2015).
In plants and microorganisms, vitamin B6 is synthesized through de novo pathways, while in humans it is obtained from the diet. The major circulating forms of vitamin B6 are PL and PLP, although PN is more stable and included in most common cell culture media formulations. PLP cannot cross the cell membrane and circulating PLP first becomes dephosphorylated to PL by tissue-resident alkaline phosphatases, then, upon entry into the cell, all B6 forms are rapidly phosphorylated by pyridoxal kinase (PDXK). Phosphorylation captures the intracellular vitamin B6 and then a second enzyme, pyridoxine 5-phosphate oxidase (PNPO), converts PNP into the bioactive form PLP. (McCormick and Chen, 1999). Despite the established role of vitamin B6 in regulating normal cellular metabolism, its role in cancer cell proliferation and maintenance is poorly understood.
The goal of this study was to identify and validate selective metabolic dependencies in acute myeloid leukemia (AML).
RESULTS
CRISPR/Cas9 functional genomics identified PDXK as a selective leukemia dependency
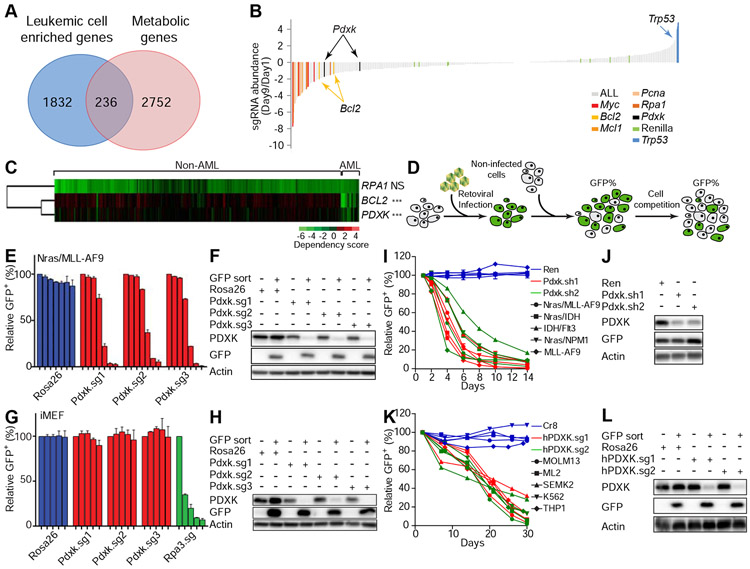
To identify metabolic dependencies in AML, we performed a focused CRISPR/Cas9 “drop out” screen using a single guide RNA (sgRNA) library targeting metabolic genes highly expressed in AML cells (Figure 1A and S1A). 2,752 genes encoding metabolic enzymes and transporters were analyzed and 236 genes were found to be abundantly expressed in leukemic cells (Figure 1A and Table S1). To functionally characterize whether these genes are essential for leukemic cell proliferation, a focused CRISPR/Cas9 sgRNA library specifically targeting these 236 genes was constructed and introduced as a pool into Nras(G12D)/MLL-AF9 leukemic cells. To monitor the robustness of our genetic screen, additional sgRNAs targeting genes that are known to be important in leukemogenesis, as well as neutral sgRNAs targeting Renilla luciferase, were also included. The abundance of each sgRNA from day 1 to 9 of continuous culture was quantified by deep sequencing (Figure S1A). As expected, control sgRNAs targeting genes known to be essential for AML cell proliferation were depleted in the screen (e.g. Myc, Bcl2, Mcl1, Pcna, and Rpa1) (Zuber et al., 2011b), whereas sgRNAs that target the tumor suppressor Trp53 were enriched and the abundance of neutral sgRNAs (e.g. targeting Renilla luciferase) remained largely unchanged (Figure 1B). These results confirm our screen was robust.
Figure 1. CRISPR/Cas9 functional genomics screen identifies pyridoxal kinase as a selective leukemia dependency.
(A) Schematic showing the numbers of metabolic genes enriched in AML leukemic cells. Among 2752 metabolic genes analyzed, 236 genes were enriched in AML leukemic cells in comparison to normal hematopoietic stem and progenitor cells. (B) Nras(G12D)/MLL–AF9 leukemic cells were infected with viral sgRNA pool. The ratios of integrated sgRNA reads on day 9 over day 1 are shown. (C) The dependency of indicated cell lines on listed genes was analyzed from previously published data (Rauscher et al., 2017; Wang et al., 2017). Each row represents a gene, while each column represents a cell line. AML or non-AML cell lines are denoted on the top panel. Gene dependency score was calculated by taking the average of Log2FC (abundance fold-change) of all corresponding sgRNAs. This average Log2FC number was color-coded. Green represents depletion, black represents no change, and red represents an enrichment of indicated cell lines. Un-supervised clustering was used to cluster genes or experiments with similar phenotypes. ’NS’ represents no statistical significance and ‘***’represents p<0.001 of Wilcox test. (D) Schematic diagram showing the in vitro competition assay. (E-H) Nras(G12D)/MLL-AF9 leukemic cells (E and F) or iMEF cells (G and H) were infected with viruses encoding indicated sgRNAs. The percentage of GFP+ infected cells were counted on day 2, 4, 6, 8, 10, 12 and 14 (E) or on day 2, 7, 13, 18 and 24 (G). The individual bar in histograms represents the GFP+ percentage on each day. Data are presented as average and standard deviation (SD) (n=2). Western blot analysis showing the expression levels of PDXK in sorted GFP+ and GFP− cells (F and H). (I-L) Indicated mouse (I and J) or human (K and L) leukemic cell lines were infected with viruses encoding indicated shRNAs/sgRNAs. The percentage of GFP+ infected cells were counted from day 1 to 14 (I) or day 2 to 30 (K). Western blot was performed to measure the expression levels of PDXK in mouse Nras(G12D)/MLL-AF9 cells (J) and human MOLM13 cells (L). The average and SD of relative GFP+ percentage are shown (n=2). See also Figure S1-S3 and Table S1-S4.
Of the 236 metabolic genes included in the screen, we focused on 23 where the representation of at least two independent sgRNAs was decreased by at least 50% (Figure 1B and Table S2). These 23 genes regulate diverse metabolic processes. For example, Rrm2 is involved in nucleotide production, Gmppb is involved in mannose metabolism, Slc25a37 and Slc2a1 encode transporters for small molecules, Idua controls glycan degradation, and Sult1c2 is involved in sulfate metabolism. To prioritize these genes for follow-up experiments, we analyzed publicly available data from genome-wide CRISPR/Cas9 screens in human cancer cells (Rauscher et al., 2017; Wang et al., 2017). Interestingly, across over 400 cell lines, sgRNAs targeting pyridoxal kinase (PDXK) were selectively depleted in leukemia relative to other normal and cancer cell types (Figure 1C and S1B). Indeed, the requirement for PDXK to support proliferation across a large number of cell lines was similar to BCL2 (Figure 1C, S1B and S1C), the target of an FDA-approved anti-leukemia drug (Yogarajah and Stone, 2018a). In contrast, sgRNAs targeting the universally essential gene Rpa1 exhibited potent but non-specific depletion in all cell lines (Figure 1C).
To validate these findings, we generated individual sgRNAs and short hairpin RNAs (shRNAs) that target PDXK and performed competition assays in multiple murine and human AML cell lines, where GFP is encoded on the same transcript as the sgRNA/shRNA and thus is used to mark cells in which PDXK is suppressed (Figure 1D). Consistent with CRISPR/Cas9 screening results, knockout of Pdxk using individual sgRNAs inhibited proliferation of Nras(G12D)/MLL-AF9 leukemic cells, but not immortalized mouse embryonic fibroblasts (iMEFs) (Figure 1E-1H). The validity of these findings was reinforced by shRNA-mediated knockdown of Pdxk as an orthogonal approach (Figure 1I and 1J). Although the rate at which PDXK sgRNAs were depleted from AML cells was slower than observed for sgRNAs targeting essential genes such as PCNA and RPA1, their effects were more specific for leukemic cells. Accordingly, in contrast to sgRNAs targeting PCNA and RPA1, PDKXsgRNAs showed modest to no anti-proliferative effects in many non-leukemia cancer cells (Figure 1C and Figure S1B).
Knockdown of Pdxk inhibited the proliferation of 4 additional mouse AML cell lines produced by different oncogenic events (e.g. Nras(G12D)/IDH, IDH/Flt3, Nras(G12D)/NPM1, and MLL-AF9) (Figure 1I and 1J), as well as a panel of human leukemic cell lines (MOLM13, ML2, SEMK2, K562, and THP1) (Figure 1K and 1L). In contrast, PDXK depletion had no influence on the proliferation of additional non-AML murine and human cell lines including 3T3 cells and sarcoma cells (Figure S1D-S1F). Through further analysis of publically available data, we noticed that PDXK is also required for the proliferation of some acute lymphoblastic leukemia (ALL; PF382 and SUPT1) and chronic myeloid leukemia (CML; K562 and KBM7) cell lines (Figure S1G), which was similar to BCL2 but not universal essential genes (RPA1 or PCNA). We experimentally validated this sensitivity in 4 additional ALL cell lines (Figure S1H-S1K).
Importantly, depletion of Pdxk had only subtle effects on bone marrow HSPCs in both in vitro (Figure S1L-S1N) and in vivo competition assays (Figure S1O and S1P). Of note, PDXK depletion did not trigger leukemic cell differentiation (Figure S2A and S2B) but instead impaired cell cycle progression (Figure S2C-S2E) and increased apoptosis (Figure S2F). Gene set enrichment analysis of RNA-seq data obtained following Pdxk knockout revealed a significant downregulation of genes associated with “cell cycle progression”, “DNA replication”, and “nucleotide metabolism” (Figure S3A-S3D and Table S3 and S4). Collectively, these results establish Pdxk as a genetic vulnerability in AML.
PDXK is required for leukemia disease maintenance in vivo
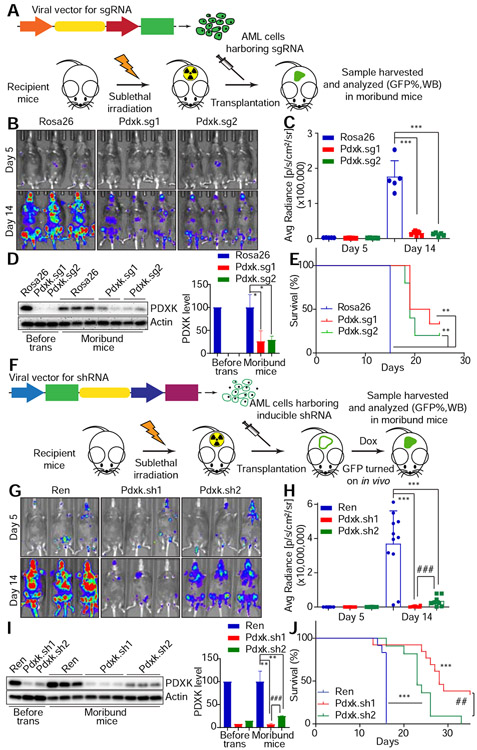
To test the in vivo significance of PDXK for leukemia disease progression, we evaluated the consequences of suppressing PDXK in established leukemia in vivo (Figure 2A). We previously generated an AML mouse model where Nras(G12D) was co-expressed with luciferase to allow for monitoring of disease progression with bioluminescence imaging (Zuber et al., 2011a). These Nras(G12D)/MLL-AF9 leukemic cells were first transduced with viruses encoding control sgRNA or sgRNAs targeting Pdxk with a co-expressed GFP reporter, and subsequently transplanted into sub-lethally irradiated recipient mice. Pdxk depletion significantly delayed disease progression and extended overall animal survival (Figure 2B-2E). Consistent with the decreased luciferase intensity (Figure 2B and 2C), the percentage of GFP+ cells with Pdxk sgRNA in bone marrow was also decreased, compared with control sgRNA harboring cells. This result suggested an in vivo AML growth disadvantage when PDXK is depleted. Of note, flow cytometry analysis of bone marrow cells from moribund mice revealed a marked reduction in GFP+ population compared to the initial transplanted AML population (Figure S3E). While the ineffective inhibition of Pdxk in lethal leukemia cells could arise from several mechanisms, including selection for cells that did not inactivate Pdxk following sgRNA expression, leukemic cells escaping Pdxk depletion were responsible for progressive disease (Figure 2D).
Figure 2. PDXK is required for leukemia disease maintenance in vivo.
(A) Schematic showing the in vivo leukemia mouse model with sgRNA infected AML cells. (B-E) Nras(G12D)/MLL-AF9 cells infected with viruses encoding sgRNAs targeting Rosa26 (n=5) or Pdxk (Pdxk. sg1, n=7 and Pdxk.sg2, n=5) were transplanted to sub-lethally irradiated mice. On day 5 and day 14, luciferase signals were detected (B), quantified and represented as average and SD (C). ‘***’ represents p<0.001 of t-test between Rosa26 and Pdxk sgRNAs. Western blot was performed to measure the PDXK level of virus infected Nras(G12D)/MLL–AF9 cells before transplantation or taken from moribund mice, and the intensity was quantified and normalized to the corresponding Rosa26 sgRNA (D). “*” represents p<0.05 of t-test between Rosa26 and Pdxk sgRNAs (Rosa26 sgRNA, n=3; Pdxk.sg1 and 2, n=2). Survival curves are shown (E). “**” represents p<0.01 of Log-rank (Mantel-Cox) test between Rosa26 and Pdxk sgRNAs. (F) The schematic diagram shows the in vivo leukemia mouse model with shRNA infected AML cells. Doxycycline (Dox) was supplemented 5 days after transplantation. (G-J) Nras(G12D)/MLL–AF9 cells infected with viruses encoding shRNAs targeting Renilla luciferase (Ren, n=12) or Pdxk (Pdxk.sh1, n=13 and Pdxk.sh2, n=10) were transplanted to sub-lethally irradiated mice. On day 5 and day 14, luciferase signals were detected (G), quantified and represented as average and SD (H). ‘***’ represents p<0.001 of t-test between Ren and Pdxk shRNAs and ‘###’ represents p<0.001 of t-test between Pdxk shRNAs. Western blot was performed to measure the PDXK level of viruses infected Nras(G12D)/MLL–AF9 cells before transplantation or taken from moribund mice, and the intensity was quantified and normalized to the corresponding Ren shRNA (I). “**” represents p<0.01 of t-test between Ren and Pdxk shRNAs and “###” represents p<0.001 of t-test between Pdxk shRNAs (n=3). Survival curves are shown (J). ‘***’ represents p<0.001 of Log-rank (Mantel-Cox) test between Ren and Pdxk shRNAs and ‘##’ represents p<0.01 of Log-rank (Mantel-Cox) test between Pdxk shRNAs.
We next validated this in vivo dependency for Pdxk using a mouse model of AML that permits inducible expression of transduced shRNAs (Fellmann et al., 2013; Zuber et al., 2011a) (Figure 2F). Similar to the sgRNA approach, Nras(G12D)/MLL-AF9 leukemic cells harboring a reverse tetracycline transactivator (rtTA) were transduced with vectors expressing control or Pdxk shRNAs placed downstream of a tetracycline-responsive element (TRE) promoter and physically linked to GFP. These cells were then transplanted into sub-lethally irradiated recipient mice (Zuber et al., 2009). Induction of Pdxk shRNAs following doxycycline treatment significantly inhibited PDXK expression, delayed disease progression and extended overall animal survival in a dose-dependent manner (Figure 2G-2J). Consistent with the decrease of luciferase intensity (Figure 2G and 2H), the percentage of GFP+ cells harboring Pdxk shRNAs in bone marrow was also decreased (Figure S3F). Together, the results further confirm that PDXK is required for leukemia maintenance in vivo.
Detection of intracellular PLP
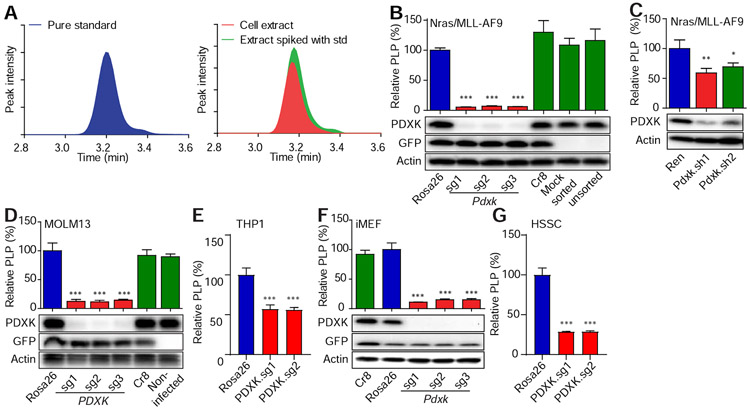
The PDXK product PLP is a cofactor for multiple enzymes involved in amino acid, nucleic acid, and lipid metabolism (Ueland et al., 2015). To determine whether genetic blockade of PDXK activity affected PLP levels in leukemic cells, we developed a high-performance liquid chromatography-mass spectrometry (LC-MS) method to measure intracellular PLP. Vitamin B6 species in cell pellets were extracted with trichloroacetic acid (TCA) and analyzed by LC-MS. Peak identities of vitamin B6 species were confirmed by comparison to pure standards (Figure 3A).
Figure 3. LC-MS method measures intracellular PLP.
(A) Chromatograms showing LC-MS detection of PLP (C8H10NO6P, extracted with a 20 ppm window). Detection of PLP in cells was confirmed by mass and retention time relative to the pure standard. (B-C) Nras(G12D)/MLL–AF9 leukemic cells were infected with viruses encoding indicated sgRNAs (Rosa26, n=3; Pdxk.sg1, n=4; Pdxk.sg2, n=3; Pdxk.sg3, n=3) (B) or shRNAs (Ren, n=4; Pdxk.sh1 and 2, n=3) (C). (D-G) MOLM13 leukemic cells (n=5) (D), THP1 leukemic cells (Rosa26, n=6; PDXK.sg1 and 2, n=4)(E), iMEF cells (Rosa26, n=5; Pdxk.sg1, n=2; Pdxk.sg2, n=5; Pdxk.sg3, n=4) (F) or human sarcoma (HSSC) (Rosa26, n=6; PDXK.sg1 and 2, n=4) cells (G) were infected with viruses encoding indicated sgRNAs. After infections (8 to 11 days for sgRNAs and 3 days for shRNAs), the PLP levels were measured by LC-MS and normalized to the level of Rosa26 sgRNA or Ren shRNA group. Western blot was performed to measure the expression levels of PDXK. ‘*’ represents p<0.05, ‘**’ represents p<0.01 and ‘***’ represents p<0.001 of t-test. Data are presented as average and SD.
Using this LC-MS assay, we first measured vitamin B6 species levels in leukemia cell lines following PDXK inhibition. As anticipated, the reduced proliferation of murine and human leukemia cells following genetic inhibition of PDXK was accompanied by a dramatic decrease in PLP levels (Figure 3B-3E). iMEFs and human sarcoma cells displayed a similar reduction in PLP levels following PDXK disruption (Figure 3F and 3G), despite retaining the ability to proliferate (Figures 1G and S1E). These results confirm that PDXK disruption reduces intracellular PLP levels and imply that different cell types can have a different biological response to this perturbation.
Vitamin B6 selectively supports leukemic cell proliferation
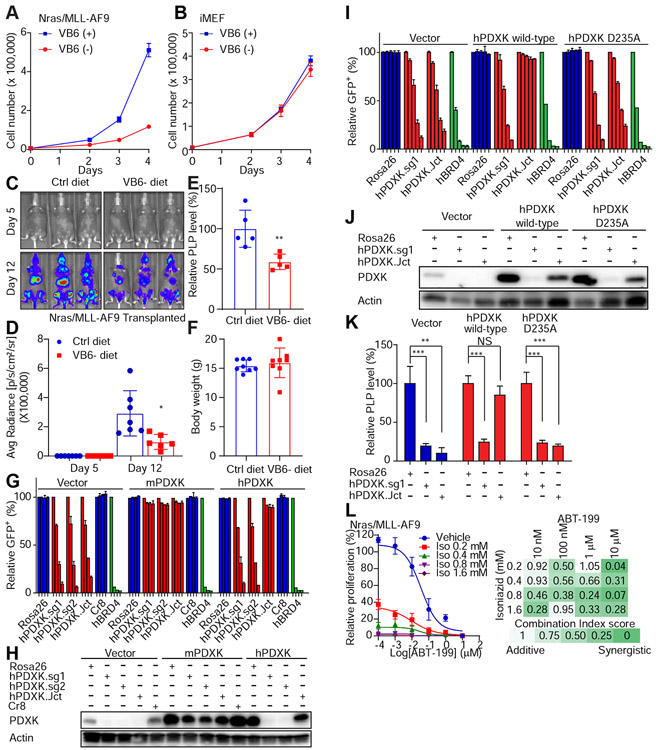
We next performed a series of experiments to examine the contribution of the vitamin B6-PDXK-PLP network to supporting leukemic cell proliferation. Consistently, depletion of pyridoxine, the absorbable form of vitamin B6 present in tissue culture media, suppressed proliferation of mouse and human AML cells (Figure 4A and S4A), but had no effect on the proliferation of immortalized fibroblasts (Figure 4B). While supplementation of vitamin B6 rescued leukemic cell growth in pyridoxine-deficient medium, it was unable to restore the proliferation of leukemia cells following PDXK depletion (Figure S4B-S4D). This vitamin B6 dependency was assessed in vivo by subjecting mice to a vitamin B6 deficient diet, injecting murine AML cells, and monitoring leukemia progression and survival. While producing only an ~50% reduction in PLP (likely owing to the short duration of dietary intervention), vitamin B6 depleted animals showed decreased disease progression without any obvious systemic toxicity as determined by body weight (Figure 4C-4F). These results support a model whereby conversion of vitamin B6 to PLP by PDXK is preferentially required for leukemic cell proliferation.
Figure 4. Vitamin B6 selectively supports leukemic cell proliferation.
(A-B) Nras(G12D)/MLL–AF9 leukemic cells (A) or iMEF cells (B) were cultured with or without 1 μg/ml of vitamin B6 (pyridoxine) using dialyzed FBS. The cell number was counted over 4 days. The average and SD are shown (n=6). (C-F) Nras(G12D)/MLL–AF9 cells were transplanted to sub-lethally irradiated mice. The mice were supplied with the control diet (n=7) or VB6 deficient diet (n=6). On day 5 and day 12, luciferase signals were detected (C), quantified and represented as average and SD (D). ‘*’ represents p<0.05 of t-test. The PLP levels in plasma (n=5) (E) and the body weight (n=8) (F) were measured on day 16. PLP levels were measured using a commercial vitamin B6 enzymatic assay. ‘**’ represents p<0.01 of t-test. Data are presented as average and SD. (G-K) Human MOLM13 leukemic cells were infected with viruses encoding vector, mouse or human WT/mutant PDXK and the hygromycin resistant gene. After hygromycin selection, leukemic cells were infected with viruses encoding indicated sgRNAs. hPDXK.sg1 and .sg2 target the exon regions of PDXK, while hPDXK.Jct targets the intron-exon junction of PDXK. GFP+ percentages were monitored on day 4, 16, 22 and 30 (G) or on day 6, 10, 16, 22 and 28 (I). The individual bar in histograms represents the GFP+ percentage on each day. The average and SD of relative GFP+ percentages are shown (n=2) (G and I). Western blot analysis was performed to show PDXK expression levels (H and J). After 11 days of infections, the PLP levels were measured by LC-MS and normalized to the level of the Rosa26 sgRNA group (K). The average and SD are shown in the histogram (n=4). ‘NS’ represents no statistical significance, ‘**’ represents p<0.01 and ‘***’ represents p<0.001 of t-test. (L) Nras(G12D)/MLL-AF9 cells were treated with the indicated combination of isoniazid (Iso) and BCL2 inhibitor venetoclax (ABT-199). The number of cells was counted on day 5 and normalized to the vehicle group. The average and SD of relative proliferation are shown (n=3). Combination Index values were calculated with CompuSyn Software. The synergistic effect is denoted with colors. See also Figure S4 and S5.
To further test this hypothesis, we assessed whether the PDXK kinase activity is needed to support leukemia cell proliferation. Wild-type PDXKs or a kinase dead human PDXK mutant were co-expressed with a series of human PDXK-targeting sgRNAs in Cas9-expressing human MOLM13 leukemia cells (Figure 4G-4K). It has been shown previously that mutation of the aspartic acid residue at position 235 in human PDXK to alanine (D235A) eliminates the kinase activity of PDXK while maintaining the overall structure and expression of the protein (Gandhi et al., 2009). As expected, enforced expression of wild-type PDXK restored PLP levels and rescued the proliferative defects associated with genetic PDXK reduction (Figure 4G, 4H and 4K). By contrast, enforced expression of the kinase dead PDXK mutant (which was expressed at comparable levels to wild-type PDXK) or PDXK with a corresponding deletion did not rescue the proliferative defects or restore PLP levels (Figure 4I-4K and Figure S4E-S4G). These results further validated PDXK kinase activity as a molecular dependency in AML.
To further confirm PLP-dependence of the leukemia cell inhibitory phenotype, we took advantage of a small molecule drug isoniazid, which was initially identified based on its ability to suppress tuberculosis (TB) growth and is an FDA-approved drug to treat this disease. Isoniazid inhibits TB by suppressing the activity of enoyl-acyl carrier protein reductase, thereby blocking mycolic acids and mycobacterial cell wall synthesis (Unissa et al., 2016). Isoniazid also reacts with PLP and sequesters it from downstream enzymes and has produced symptoms of vitamin B6 deficiency in some patients (Lainé-Cessac et al., 1997). Indeed, isoniazid treatment reduced intracellular PLP levels of AML cell lines and human iPS derived HSPCs (Figure S5A-S5K), although the effects were less robust than the genetic approaches, and the results are complicated by the potential for a post-lysis reaction between residual isoniazid and PLP, which was minimized by washing the cells with PBS prior to metabolite extraction. Similar to PDXK depletion, isoniazid blocked the proliferation of leukemic cell lines (Figure S5L) and primary patient AML cells (Figure S5M). In contrast, isoniazid had no effect on the proliferation of iMEFs or human sarcoma cells in culture (Figure S5N), and produced only a subtle decrease in proliferation of primary mouse and human HSPCs in culture (Figure S5O and S5M).
Lastly, we tested whether isoniazid would show combinatorial activity with the FDA-approved BCL2 inhibitor, venetoclax (ABT-199). Indeed, treatment of murine and human AML cell lines led to a synergistic inhibition of leukemia cell proliferation (Figure 4L and S5P). Collectively, these data define a mechanism whereby vitamin B6 conversion to PLP by PDXK is required to selectively sustain leukemia cell proliferation and hint that targeting the vitamin B6 pathway – alone or in combination with existing agents - could be a pharmacologically accessible anti-leukemia strategy.
Vitamin B6 network is conditionally required for leukemic cell proliferation
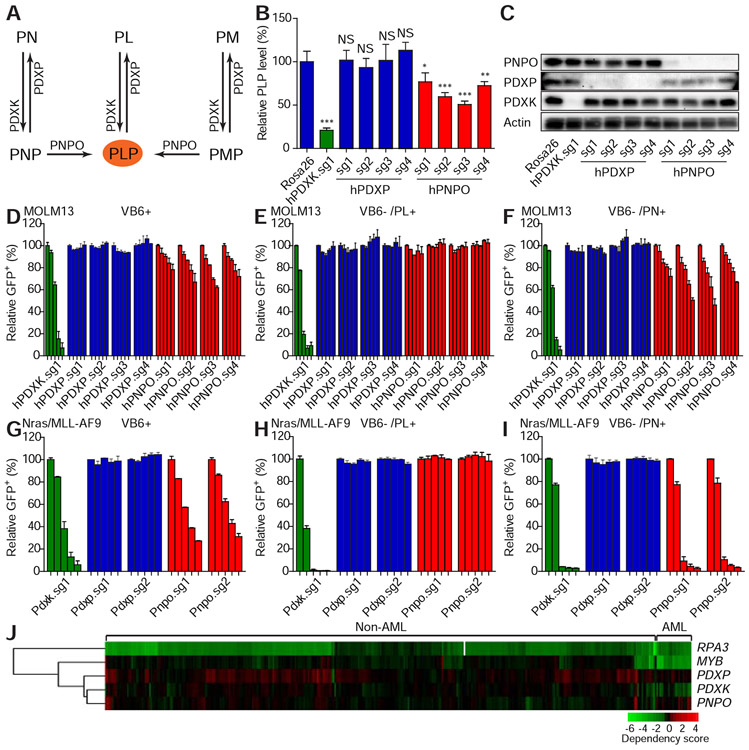
Humans rely on dietary uptake and hepatic metabolism to maintain tightly regulated levels of PL and PLP in the systemic circulation. In addition to PDXK, two enzymes, pyridoxamine phosphate oxidase (PNPO) and pyridoxal phosphatase (PDXP) are part of vitamin B6 network: PNPO catalyzes the conversion of PNP and PMP to PLP, while PDXP dephosphorylates PLP, PNP, and PMP (Figure 5A). To better understand the involvement of the vitamin B6 network enzymes in leukemic cell proliferation, we genetically depleted PNPO and PDXP with sgRNAs and assessed the impact on PLP levels and leukemia cell proliferation.
Figure 5. Vitamin B6 network is conditionally required for leukemic cell proliferation.
(A) The schematic diagram shows the vitamin B6 network. (B-C) MOLM13 leukemic cells were infected with viruses encoding indicated sgRNAs. After 11 days of infections, the intracellular PLP levels were measured by LC-MS and normalized to the level of the Rosa26 sgRNA group (B). The average and SD are shown in the histogram (n=4). ‘NS’ represents no statistical significance, ‘*’ represents p<0.05, ‘**’ represents p<0.01, and ‘***’ represents p<0.001 of t-test. Western blot was performed to measure the expression levels of PDXK, PDXP, and PNPO (C). (D-I) MOLM13 (D-F) or Nras(G12D)/MLL-AF9 (G-I) leukemic cells were cultured in complete medium (VB6 containing; D and G), VB6 deficient medium (VB6 free RPMI with dialyzed FBS) with PL (E and H) or PN (F and I). The cells were infected with viruses encoding indicated sgRNAs. The percentage of GFP+ infected cells were monitored on day 4, 9, 16, 21, and 30 (D, E and F) or on day 3, 6, 10, 12, and 14 (G, H, and I). The individual bar in histograms represents the GFP+ percentage on each day. The average and SD of relative GFP+ percentage are shown (n=2). (J) The dependency of indicated cell lines on listed genes was analyzed from the previously published data (Rauscher et al., 2017; Wang et al., 2017). Each row represents a gene. Each column represents a cell line. AML or non-AML cell lines are denoted on the top panel. Gene dependency score was calculated by taking the average of Log2FC (abundance fold-change) of all corresponding sgRNAs. This average Log2FC number was color-coded. Green represents depletion, black represents no change, and red represents an enrichment of indicated cell lines. Un-supervised clustering was used to cluster genes or experiments with similar phenotypes.
Depletion of PNPO decreased PLP, although to a lesser extent than PDXK depletion, while depletion of PDXP did not influence PLP levels (Figure 5B and 5C). Consistent with the role of PNPO in converting PNP to PLP, we predicted that PNPO would be required for leukemic cell proliferation in vitro where PN is the vitamin B6 supplied. To our surprise, PNPO depletion was rescued by PM (Figure S5Q and S5R). This may be explained by the fact that PM can react with carbonyl compounds such as keto acids, resulting in conversion to PL independent of PNPO activity (Schnellbaecher et al., 2019). However, it is noteworthy that neither PN nor PM was able to rescue the anti-proliferative effect caused by PDXK suppression (Figure 5D-5I and Figure S5Q and S5R). In agreement, analysis of data obtained from genome wide-CRISPR screens revealed that, in contrast to PDXK, PNPO and PDXP are not required for AML cell proliferation (Figure 5J). Additionally, decreases in PLP levels correlate with reduced cell proliferation (Figure 5B and 5D). These results demonstrate that PDXK is crucial for sustaining intracellular PLP levels.
PDXK inhibition reduces the levels of key metabolites in leukemia cells
We next explored the downstream mechanism by which PLP contributes to leukemia maintenance. Metabolomic profiling showed that genetic depletion of PDXK in human MOLM13 leukemia cells altered a wide range of metabolites with nucleotides, their precursors and the polyamine putrescine among the most decreased (Figure 6A and S6A). Genetic depletion of Pdxk in mouse Nras(G12D)/MLL-AF9 leukemia cells displayed qualitatively similar changes (Figure 6B). Changes in TCA cycle intermediates and glycolytic metabolites were also observed upon PDXK depletion (Figure S6B and S6C).
Figure 6. PLP-dependent enzymes are selectively required for leukemic cell proliferation.
(A-B) MOLM13 (A) or Nras(G12D)/MLL-AF9 (B) leukemic cells infected with viruses encoding control, PDXK or Pdxk targeting sgRNAs were collected for LC-MS analysis (Control n=12 and PDXK sgRNA n=9 for panel A; Control n=18 and Pdxk sgRNA n=14 for panel B). The row Z-Score is calculated by measuring the SD of corresponding sgRNA treatment from the mean of each metabolite and represented with color. The most statistically significant down-regulated metabolites are shown in rank order concerning to the level of change. ‘*’ represents p<0.05, ‘**’ represents p<0.01, and ‘***’ represents p<0.001 of t-test. (C) The expression levels of PLP-dependent genes in indicated mouse and human leukemic cells are shown. Essential AML cell proliferation genes (identified in Figure S6H) are denoted in red. (D) Leukemic cells or iMEF cells were infected with viruses encoding indicated sgRNAs. The Log2 ratios of GFP+ percentage on day 14 (Nras(G12D)/MLL-AF9) or day 24 (iMEF) over day 2 corresponding to each sgRNA are shown. (E-H) Nras(G12D)/MLL-AF9 leukemic cells were infected with viruses encoding indicated shRNA. The cells were cultured in the absence or presence of indicated concentrations of aspartate (n=3) (E), uridine (n=5) (F), putrescine (n=4) (G) or their combination (n=6) (H). The percentage of GFP+ infected cells were counted on day 7 and normalized to each vehicle respectively. The average with 95% CI of relative GFP+ fold is shown. ‘NS’ represents no statistical significance, ‘*’ represents p<0.05, ‘**’ represents p<0.01 and ‘***’ represents p<0.001 of t-test. (I) Nras(G12D)/MLL–AF9 cells were cultured with indicated concentrations of isoniazid for 5 days. The PLP levels were measured by LC-MS and normalized to the level in the vehicle group. The average and SD (n=4) are shown in the histogram. (J-M) Nras(G12D)/MLL–AF9 leukemic cells were cultured in the presence of 0.8 mM isoniazid and indicated concentrations of aspartate (n=4) (J), uridine (n=4) (K), putrescine (n=6) (L) or their combinations (n=3) (M; 1.6 mM isoniazid with Asp (mM)/uridine (μM)/putrescine (nM) C1: 2/10/10, C2: 5/30/30, C3: 10/100/100). The number of cells were counted on day 4 and normalized to the vehicle respectively. The average with 95% CI is shown. ‘*’ represents p<0.05, ‘**’ represents p<0.01 and ‘***’ represents p<0.001 of one-way ANOVA test. (N) Schematic of metabolites downstream of GOT2 and ODC1, significantly down-regulated metabolites are denoted in green. (O-P) Stable isotope labeling of UTP from [15N]-alpha-Gln (O) and N-acetylputrescine from U-[13C]-Gln (P). iMEF cells or Nras(G12D)/MLL-AF9 cells were infected with the viruses encoding indicated sgRNAs for 8 days and cultured with the respective tracer for 8 hr prior to harvesting. Data are presented as average and SD (n=3). See also Figure S6.
We performed a similar Pdxk genetic depletion experiment in immortalized MEFs and interestingly, while putrescine levels were reduced, the metabolic impact of PDXK depletion in iMEF cells was less severe (Figure S6D and S6E). Pharmacological inhibition of PLP-dependent metabolism using isoniazid or 4'-O-methylpyridoxine (OMP), an analog of PL that directly binds the PDXK kinase domain and inhibits its activity in vitro (Gandhi et al., 2012), caused a similar change in both metabolic and gene expression profiles as genetic depletion of Pdxk, suggesting that, though isoniazid undoubtedly targets other activities, its effects are at least in part mediated by changes in vitamin B6-dependent metabolism (Figure S6F and S6G). These data imply that PDXK inhibition can have distinct effects on downstream metabolism depending on the cellular context.
Select PLP-dependent enzymes are required for leukemic cell proliferation
In parallel with the metabolomic analyses, we performed a focused CRISPR/Cas9 screen in order to determine whether any of the 27 PLP-dependent enzymes expressed in both murine Nras(G12D)/MLL-AF9 and human MOLM13 AML cells were important for leukemic cell proliferation (Figure 6C). sgRNAs targeting eight of these enzymes were reproducibly depleted, including glutamic-oxaloacetic transaminase 2 (GOT2), delta-aminolevulinate synthase (ALAS1), cysteine desulfurase (NFS1), serine palmitoyltransferase (SPTLC1 and SPTLC2), antizyme inhibitor 1 (AZIN1), ornithine decarboxylase (ODC1), and O-phosphoseryl-tRNA selenium transferase (SEPSECS) (Figure 6C and Figure S6H). Among these, sgRNAs targeting Got2, Alas1, Sptlc1, Sptlc2, Azin1, and Odc1 depleted in leukemic cells but not iMEFs (Figure 6D and Figure S6H and S6I). Analysis of publicly available datasets from genome wide CRISPR/Cas9 screens (Wang et al., 2017) as well as in vitro competition assays (Figure S6J-S6L), confirmed that these same enzymes are required for the proliferation of human AML cells in culture. These results demonstrate multiple PLP-dependent enzymes are required for leukemic cell growth.
To further explore the functional relevance of these PLP-dependent enzymes in leukemic cell maintenance, we assessed whether addition of exogenous metabolites could rescue cell proliferation following PLP depletion. ODC1 is a decarboxylase that catalyzes the formation of putrescine from ornithine, while AZIN1 binds to ornithine decarboxylase antizyme and stabilizes ODC1. GOT2 is a transaminase that plays essential roles in both the malate-aspartate shuttle and generating aspartate for nucleotide biosynthesis. Each of these enzymes has been identified as a candidate cancer drug target in other contexts (Hogarty et al., 2008; Son et al., 2013). Exogenous aspartate, putrescine or uridine could partially rescue the proliferative defect created by PDXK disruption (Figure 6E-6H) or isoniazid treatment (Figure 6I-6M). However, the combinatorial treatment of cells with all three metabolites did not enhance this effect (Figure 6H and 6M). While the incomplete rescue of leukemic cell proliferation by these downstream metabolites makes it likely that additional PLP-related mechanisms not identified in our screen are important, these observations identify ODC1 and GOT2 as PLP-dependent enzymes required for leukemia maintenance (Figure 6N).
To determine how PDXK inhibition impacts the enzymatic activity of ODC1 or GOT2 we then used 15N-and 13C-stable isotopically labeled substrates to qualitatively profile flux through these PLP-dependent reactions. Consistent with decreased GOT2 activity, entry of glutamine labeled at the alpha- or amide-nitrogen (alpha- or amide-15N-glutamine) into newly synthesized nucleotides including UTP, CTP and ATP was decreased (Figure 6O and S6M-S6O). Consistent with decreased ODC1 activity, entry of uniformly labeled 13C-glutamine or glucose (U-13C-glutamine or glucose) into newly synthesized derivatives, N-acetyl-putrescine, was also decreased (Figure 6P and S6P) upon Pdxk knockout. Interestingly, neither the pool size nor the proportion of newly synthesized aspartate labeled by these tracers were altered upon PDXK depletion (Figure S6Q), suggesting the existence of a more complex regulatory network. In fact, two prior studies have shown that depletion of GOT1, also a PLP-dependent enzyme, substantially increases aspartate levels (Birsoy et al., 2015; Son et al., 2013). A reduction in GOT1 activity may therefore offset the effects of decreased GOT2 activity on total cellular aspartate synthesis. Nonetheless, attenuation of GOT2 appears sufficient to reduce nucleotide synthesis, perhaps owing to distinct metabolite pools or cellular compartmentalization for downstream utilization.
Pharmacological inhibition of the vitamin B6 pathway exerts anti-leukemic activity
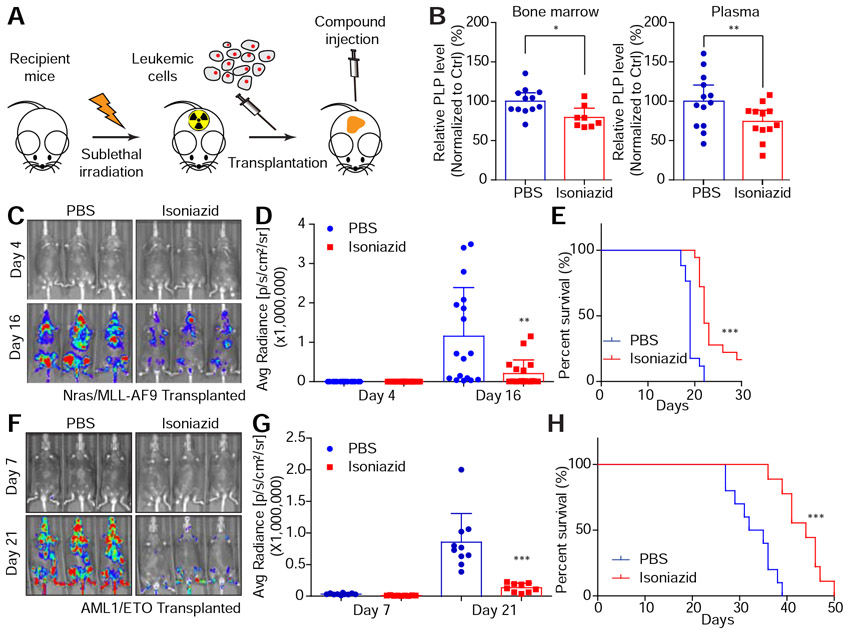
The demonstration that PDXK functions as an upstream node controlling multiple enzymes needed for leukemia maintenance supports our observation that its genetic ablation has potent anti-leukemia effects. Isoniazid is capable of mimicking the effects of genetic PDXK depletion in vitro, but the in vivo pharmacology is such that effective doses for TB patients produce only a ~40% reduction in serum PLP levels (Visser et al., 2004). Nevertheless, we decided to test whether isoniazid could inhibit leukemia cell expansion in mice. We transplanted Nras(G12D)/MLL-AF9 cells co-expressing luciferase into sublethally irradiated recipient mice to establish leukemia in vivo (Zuber et al., 2011a) (Figure 7A). Using a dose schedule of 90 mg/kg/day, we were able to achieve a ~25% reduction in systemic PLP levels and in leukemia cells isolated from bone marrow 12 days post treatment (Figure 7B). Despite this modest enzyme inhibition, isoniazid significantly enhanced survival (Figure 7C-7E). We also tested a less aggressive leukemia harboring the AML-ETO9a fusion, and observed a more substantial survival advantage (Figure 7F-7H). At these doses, isoniazid had little if any effect on normal peripheral blood cell populations (Figure S7A).
Figure 7. Pharmacological blockade of the vitamin B6 pathway exhibits anti-leukemia activity in vivo.
(A) The schematic diagram shows the in vivo leukemia mouse model. (B-E) Nras(G12D)/MLL–AF9 cells were transplanted to sub-lethally irradiated mice. PBS or 90 mg/kg isoniazid was intraperitoneally injected daily. The bone marrow (n=12 for PBS group and n=8 for isoniazid group) and plasma (n=13 for PBS group and n=12 for isoniazid group) were collected on day 12. PLP levels were measured by LC-MS and normalized to the PBS injection group (B). The average and SD are shown in the histogram. ‘*’represents p<0.05 and ‘**’represents p<0.01 of t-test. On day 4 and day 16, luciferase signals were detected (C), quantified and represented as average and SD (n=17 for PBS group and n=18 for isoniazid group) (D). ‘**’ represents differences with p<0.01 of t-test. Survival curves are shown (E). ‘***’ represents differences with p<0.001 of Log-rank (Mantel-Cox) test. (F-H) AML1/ETO cells were transplanted to sub-lethally irradiated mice. PBS (n=10) or 90 mg/kg isoniazid (n=9) was intraperitoneally injected daily. On day 7 and day 21, luciferase signals were detected (F), quantified and represented as average and SD (G). ‘***’ represents differences with p<0.001 of t-test. Survival curves are shown (H). ‘***’ represents p<0.001 of Log-rank (Mantel-Cox) test. See also Figure S7.
While our experiments with isoniazid provide proof-of-principle that targeting PLP levels in leukemia can be therapeutically beneficial, the modest decrease in intracellular PLP and the likelihood of off-target activities make it an unsuitable starting point for therapeutic drug development. An alternative strategy involves direct targeting of the enzyme with OMP. OMP potently inhibited intracellular PLP production in vitro (Figure S7B and S7C) and that this effect correlated with its ability to inhibit the proliferation of murine and human leukemic cells harboring different genetic drivers (Figure S7D), as well as of primary leukemia cells from patients (Figure S7E). As with genetic inhibition of PDXK, OMP treatment had minimal effects on the proliferation of iMEFs (Figure S7F), human sarcoma cells (Figure S7G), or primary mouse or human HSPCs (Figure S7E and S7H), despite reducing PLP levels (Figure S7C).
Owing to its poor in vivo pharmacology (t ½ < 1 hr (Kobayashi et al., 2015)), we did not expect OMP to substantially enhance the survival of leukemia bearing mice. Nonetheless, a dose schedule of 7 mg/kg twice daily, was able to inhibit leukemia expansion in both the Nras(G12D)/MLL-AF9 and AML-ETO leukemia models (Figure S7I-S7N), without disrupting normal hematopoiesis (Figure S7A). These results further indicate that the vitamin B6 pathway is pharmacologically actionable and provide a molecular starting point for further drug development.
DISCUSSION
In this study, we identified PDXK as a molecular dependency in acute myeloid leukemia. PDXK was identified in a focused CRISPR/Cas9 screen to probe metabolic enzymes in AML, and its broad relevance to leukemia maintenance – but not other cancer cell types – was confirmed through analysis of publicly available screening datasets and validated in vitro and in vivo. Mechanistic studies using a combination of genetic, pharmacologic, and biochemical approaches confirmed the underlying mechanism for this dependence involves the ability of PDXK to phosphorylate vitamin B6 to PLP which, in turn, regulates enzymes needed for the production of key amino and nucleic acids needed for AML proliferation. Although current agents to target PDXK are sub-optimal, their ability to exert anti-leukemic effects in vivo validates the vitamin B6 pathway as a pharmacologically actionable pathway in AML.
It is well-established that vitamin B6 is an essential nutrient at the organism level, and that its active product PLP is important for the regulation of enzymes that contribute key metabolites needed for cell metabolism (Eliot and Kirsch, 2004). While the consequences of vitamin B6 deficiency are well studied and produce clinical symptoms such as nausea, depression, confusion, and skin rashes (Vilter et al., 1953), the physiologic consequences of PDXK inhibition on key metabolites, and the relative contribution of PLP regulated enzymes to cell proliferation has not been well studied. The biochemical and genetic analyses we performed indicate that cells are highly dependent on PDXK to produce PLP, and that PDXK inhibition can produce dramatic decreases in cell metabolites such as nucleic acids and polyamines, among others. Nevertheless, PDXK inhibition produced different effects based on the cell type examined, having particularly profound effects in AML cells.
PDXK inhibition led to a dramatic reduction in putrescine levels in both AML cells and MEFs, whereas nucleotide levels were more dramatically reduced in AML cells than MEFs. Conversely, genetic perturbation of ODC1, which is a PLP-dependent enzyme needed for putrescine levels, had a greater effect on AML cells than MEFs. AML cells also showed a greater dependence on the PLP-dependent GOT2 enzyme, needed for the production of nucleotides. Collectively, these results indicate that cell types can differ in their dependence on PLP-dependent metabolic pathways and it may be that leukemic cells rely more on PLP-dependent pathways to produce certain metabolites. This is consistent with the observation that, compared to normal cells, cancer cells depend more on GOT2 (Son et al., 2013).
Our results indicate that leukemic cells are addicted to the vitamin B6 pathway, such that its inhibition selectively impairs leukemic cell proliferation compared to other normal and cancer cell types. Moreover, at therapeutically active doses, PLP and PDXK inhibitors have no obvious effects on normal hematopoiesis. While this differential dependency may involve the qualitatively different effects of PDXK inhibition between cell types mentioned above, PDXK is highly expressed in AML blasts, perhaps to satisfy their increased requirements for nucleotides to sustain their extraordinarily rapid proliferation. Besides AML, we found that PDXK may be important in other leukemias such as acute lymphoblastic leukemia. The dependence of leukemia cells on vitamin B6 metabolism is consistent with previous epidemiological observations that plasma vitamin B6 levels are decreased in leukemic patients, perhaps suggesting increased utilization in AML cells to support leukemia proliferation (Pais et al., 1990). Regardless of the precise mechanism, the requirement of PDXK to support cancer cell proliferation is similar to BCL2, the target of venetoclax, a recently approved drug for leukemia, where the mechanism of drug selectivity for leukemia is also not entirely clear (Yogarajah and Stone, 2018b).
Vitamins are essential nutrients that organisms require in limited amounts. While deficiencies in vitamins have long been known to produce certain pathologies, their role in modulating other pathological states is less understood. The fact that leukemia cells show a greater dependence than normal cells for vitamin B6 is consistent with recent epidemiological studies suggesting that vitamin B6 is not chemopreventive but instead may increase cancer risk (Brasky et al., 2017). Together with emerging roles of other vitamins in cancer, including vitamin D in pancreatic cancer (Sherman et al., 2014) and vitamin C in colorectal cancer (Yun et al., 2015) and leukemia (Agathocleous et al., 2017; Cimmino et al., 2017), our results emphasize that vitamins are critical regulators of cancer cell proliferation and maintenance, and that through understanding their action, new cancer targets can be identified.
In conclusion, our results identify the PDXK kinase as a new anti-leukemia target. While neither isoniazid nor 4'-O-methylpyridoxine have the pharmacological properties to be directly used as anti-leukemic agents, the crystal structure of 4'-O-methylpyridoxine bound to PDXK should provide a starting point for further drug development, and in anecdotal studies, use of a pyridoxine competitive inhibitor did induce remissions in some leukemia patients (Weir and Morningstar, 1954). In addition, drugs targeting the VB6 network can synergize with a BCL2 inhibitor to further impede leukemic cell propagation. Taken together, our study implies that the vitamin B6 pathway coordinates multiple activities that are critical for cancer cell division and maintenance and that PDXK inhibitors, by simultaneously attenuating these pathways, could become useful anti-leukemia agents.
STAR METHODS
LEAD CONTACT AND MATERIALS AVAILABILITY
Further information and requests for resources and reagents should be directed to the Lead Contact: Lingbo Zhang (lbzhang@cshl.edu). This study did not generate new unique plasmid vectors. Plasmids and recombinant DNA used in this study are detailed in the key resources table and available upon request. The sequences of all used sgRNA and shRNA are listed in Table S5. This study did not generate new experimental animal models. Cell lines used and generated in this study are listed in the key resources table and available upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit Anti-PDXK antibody | Sigma | Cat# HPA030196; RRID:AB_10599735 |
| Anti-GFP | Hypromatrix | HM2020 |
| Mouse Anti-Actin, beta Monoclonal Antibody, Horseradish Peroxidase Conjugated, Clone AC-15 | Sigma | Cat# A3854; RRID:AB_262011 |
| Anti c-Kit | BioLegend | Cat# 105811, RRID:AB_313220 |
| Anti Mac-1 | BioLegend | Cat# 101211, RRID:AB_312794 |
| Bacterial and Virus Strains | ||
| pLKO5.sgRNA.EFS.GFP vector | (Heckl et al., 2014) | Addgene plasmid # 57822 |
| RT3GEN (pQCXIX) vector | (Fellmann et al., 2013) | N/A |
| miR-E based MLS retroviral vector | (Fellmann et al., 2013) | N/A |
| LentiCas9-Blast | (Sanjana et al., 2014) | Addgene Plasmid #52962 |
| LentiCRISPR v2 vector | (Sanjana et al., 2014) | Addgene plasmid # 52961 |
| Biological Samples | ||
| PBSCs from healthy donor | Fred Hutchinson Cancer Research Center | https://www.fredhutch.org/en.html |
| Primary samples from AML patients | Memorial Sloan Kettering Cancer Center | https://www.mskcc.org |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Isoniazid | Sigma | I3377 |
| 4’-O-methoxypyridoxine | BOC Sciences | B17LM09262 |
| ABT-199 | LC Laboratory | V-3579 |
| Murine recombinant SCF | PEPROTECH | 250-03 |
| Murine recombinant IL-3 | PEPROTECH | 213-13 |
| Murine recombinant IL-6 | PEPROTECH | 216-16 |
| Doxycycline hyclate | Sigma | D9891 |
| Polybrene | Millipore | TR-1003-G |
| Putrescine dihydrochloride | Sigma | P7505 |
| Uridine | Sigma | U6381 |
| Pyridoxal-5-phosphate | Sigma | P3657-1G |
| Pyridoxine | Sigma | P2680-10G |
| Pyridoxamine | Sigma | P9380-1G |
| Pyridoxal | Sigma | 271748-5G |
| [3D]-pyridoxal phosphate | IsoSciences | 7017 |
| [3D]-pyridoxal | IsoSciences | 7098 |
| [3D]-pyridoxine | IsoSciences | P200798 |
| [3D]-pyridoxamine | IsoSciences | 7099 |
| Ammonium chloride | STEMCELL | 07850 |
| Annexin-APC | BioLegend | 640930 |
| Puromycin dihydrochloride from Streptomyces alboniger,>=98% (TLC), powder | Sigma-Aldrich | P7255-25MG |
| Blasticidin S HCl (10 mg/mL) | Life Technologies | a11139-03 |
| 2-Mercaptoethanol | Sigma-Aldrich | M3148 |
| Luciferin | GOLDBIO | LUCK-1G |
| Critical Commercial Assays | ||
| Biotin mouse lineage panel | BD Biosciences | 559971 |
| CD117 MicroBeads, mouse | Miltenyi Biotec | 130-091-224 |
| StemSpan™ SFEM | STEMCELL | 09600 |
| 4–15% Mini-PROTEAN® TGX™ Precast Protein Gels | Bio-rad | 4561084 |
| QuikChange Lightning Multi Site-Directed Mutagenesis Kit | Agilent Technologies | 210515 |
| RNeasy Mini Kit (RNA extraction) | Qiagen | 74106 |
| QIAquick Gel Extraction Kit (cloning) | Qiagen | 28704 |
| QIAquick PCR purification Kit ( cloning) | Qiagen | 28106 |
| Flow cytometry absolute count standard | Bangs Laboratories | 580 |
| EdU Alexa Fluor 647 Flow Cytometry Assay Kit | Invitrogen | C10634 |
| A/C Enzymatic Vitamin B6 Assay | A/C Diagnostics | 510k #0111260 |
| Deposited Data | ||
| All deep sequencing data | This paper | GSE139908, GSE139892, GSE139905 |
| Experimental Models: Cell Lines | ||
| Mouse: Nras(G12D)/MLL–AF9 | (Chen et al., 2013) | N/A |
| Mouse: Nras(G12D)/IDH | (Chen et al., 2013) | N/A |
| Mouse: Nras(G12D)/NPM1 | (Dovey et al., 2017) | N/A |
| Mouse: IDH/Flt3 | (Chen et al., 2013) | N/A |
| Mouse: AML1/ETO | (Zuber et al., 2009) | N/A |
| Mouse: MLL-AF9-cas9 | (Shi et al., 2015) | N/A |
| Human: MOLM13 cell line | DSMZ | Cat# ACC-554, RRID:CVCL_2119 |
| Human: MOLM13 -cas9-blast -empty-hygro | This paper | N/A |
| Human: MOLM13 -cas9-blast -mPdxk cDNA- hygro | This paper | N/A |
| Human: MOLM13 -cas9-blast -hPDXK cDNA- hygro | This paper | N/A |
| Human: MOLM13 -cas9-blast -hPDXK del 232-235 cDNA- hygro | This paper | N/A |
| Human: MOLM13 -cas9-blast -hPDXK D235A cDNA- hygro | This paper | N/A |
| Human: ML-2 cell line | DSMZ | Cat# ACC-15, RRID:CVCL_1418 |
| Human: SEMK2 cell line | (Pocock et al., 1995) | N/A |
| Human: THP-1 cell line | ATCC | Cat# TIB-202, RRID:CVCL_0006 |
| Human: K-562 cell line | ATCC | Cat# CCL-243, RRID:CVCL_0004 |
| Mouse: iMEF | (Huang et al., 2014) | N/A |
| Mouse: NIH 3T3 cell line | ATCC | Cat# CRL-1658, RRID:CVCL_0594 |
| Human: sarcoma cell (HSSC) | (Banito et al., 2018) | N/A |
| Human: HEK293T cell line | ATCC | Cat# CRL-3216, RRID:CVCL_0063 |
| Human: pluripotent stem cell | (Doulatov et al., 2013) | N/A |
| Experimental Models: Organisms/Strains | ||
| Mouse: NCI B6-Ly5.1/Cr | Charles River | B6/SJL(CD45.1) |
| Mouse: C57BL/6J | Jackson | 000664 |
| Oligonucleotides | ||
| sgRNA for CRISPR/Cas9 functional genomics screen, see Table S5 | This paper | N/A |
| shRNA or sgRNA sequence for PLP dependent enzyme screening, see Table S5 | This paper | N/A |
| Recombinant DNA | ||
| MSCV Hygro-PGK-Hygro vector | Clontech | 634401 |
| MSCV-PGK-Puro vector | (Shi et al., 2015) | N/A |
| MSCV empty-PGK-Hygro | This paper | N/A |
| MSCV mPDXK-PGK-Hygro | This paper | N/A |
| MSCV hPDXK-PGK-Hygro | This paper | N/A |
| MSCV hPDXK D235A-PGK-Hygro | This paper | N/A |
| MSCV hPDXK Del 232-235-PGK-Hygro | This paper | N/A |
| MSCV-miRE-SV40-GFP/mCherry (LMS) | (Zhao et al., 2015) | N/A |
| Human PDXK cDNA | Dharmacon | OHS6085-213574600 |
| Mouse Pdxk cDNA | Dharmacon | MMM1013-202768267 |
| Software and Algorithms | ||
| Guavasoft software 3.3 | Millipore | https://www.luminexcorp.com/guava-easycyte-software/ |
| Living Image software 4.5 | PerkinElmer | https://www.perkinelmer.com/lab-products-and-services/resources/in-vivo-imaging-software-downloads.html |
| Image J 1.49v | NIH | https://imagej.nih.gov/ij/download.html |
| MassHunter software | Agilent | https://www.agilent.com/en/products/liquid-chromatography-mass-spectrometry-lc-ms/lc-ms-instruments/triple-quadrupole-lc-ms/ultivo-triple-quadrupole-lc-ms |
| GraphPad PRISM 7 | GraphPad | https://www.graphpad.com/scientific-software/prism/ |
| DAVID | NIH | https://david.ncifcrf.gov/ |
| GSEA | Broad Institute | http://www.broadinstitute.org/gsea |
| STAR | (Dobin et al., 2013) | https://github.com/alexdobin/STAR |
| Subread | (Liao et al., 2014) | http://subread.sourceforge.net/ |
| DESeq2 | (Love et al., 2014) | https://bioconductor.org/packages/release/bioc/html/DESeq2.html |
| Trimmomatic | (Bolger et al., 2014) | http://www.usadellab.org/cms/?page=trimmomatic |
| CompuSyn Software | ComboSyn, Inc. | http://www.combosyn.com/index.html |
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Cell Lines
Mouse leukemic cell lines including Nras(G12D)/MLL–AF9, Nras(G12D)/IDH, IDH/Flt3, Nras(G12D)/NPM1, and MLL-AF9 were cultured in RPMI medium supplemented with 10 % Fetal Bovine Serum (FBS), L-glutamine, penicillin streptomycin, recombinant mouse SCF, IL-3, and IL-6. Human leukemic cell lines MOLM13, ML2, SEMK2, K562 and THP1 were cultured in RPMI medium supplemented with 10 % FBS, L-glutamine, penicillin streptomycin. Human HEK293 cells, human sarcoma cells were cultured in DMEM medium supplemented with 10 % FBS, L-glutamine, penicillin streptomycin. Mouse AML cell lines, human AML cell lines, iMEF cell line, 3T3 cell line and human sarcoma cell line were infected with virus encoding Cas9 (Addgene 52962) followed with blastcitidin selection to generate Cas9 cell lines. Human hematopoietic progenitor cells were differentiated from human pluripotent stem cell (hPSC) based system and then transduced with a combination of HSC transcription factors (5 Factors: HOXA9, ERG, RORA SOX4 and MYB) that lock progenitors in a stem cell fate (Doulatov et al., 2013).
Primary cells
The primary PBSCs from healthy donors were obtained from Fred Hutchinson Cancer Research Center. The primary cells from AML patients were obtained from Memorial Sloan Kettering Cancer Center. This study received approval from the Institutional Review Board at MSKCC (IRB Protocol 15-188). The consent and research authorization for the use of the biospecimens are waived as per 45 CFR 46.116(d) and 45 CFR 164.512(i)(2)(ii). All primary cells were cultured in StemSpan (STEMCELL) with indicated concentration of isoniazid or 4'-O-methylpyridoxine.
Animal Studies
For xenograft AML mouse models, NCI B6-Ly5.1/Cr (B6.SJL-PtprcaPepcb/BoyCr) mice were purchased from Charles River. 5~6 weeks old mice were sublethally irradiated (450 cGy) and transplanted with leukemic cells through tail vein injection. For genetic Pdxk depletion or knockdown, Nras(G12D)/MLL-AF9 leukemic cells were infected with viruses encoding sgRNAs or shRNAs either targeting Renilla luciferase or Pdxk before transplantation. For in vivo competitive proliferation assay, C57BL/6J mice were purchased from Jackson Laboratory. 7 weeks old mice were lethally irradiated (850 cGy) and transplanted with leukemic cells through tail vein injection. Before transplantation, bone marrow cells were isolated from syngeneic donors and infected with viruses encoding shRNAs targeting Renilla luciferase or Pdxk with mCherry or GFP in the same transcript. All animal studies were adherence to the NIH Guide for the Care and Use of Laboratory Animals and conducted following protocols approved by the ethics committee at Cold Spring Harbor Laboratory (566729-10).
METHOD DETAILS
CRISPR/Cas9 sgRNA library construction and functional genomics screen
The expression levels of all the genes in both AML leukemic cells and CD34+ normal hematopoietic stem and progenitor cells were downloaded from previously published report (Valk et al., 2004). The ratios of gene expression level corresponding to each gene were calculated as their expression levels in AML leukemic cells divided by their expression levels in CD34+ normal hematopoietic stem and progenitor cells. A total of 1,832 genes were found to be enriched in AML leukemic cells with a ratio of gene expression level larger than 2. Among 2,752 genes encoding metabolic enzymes and transporters analyzed, 236 genes were found to be enriched. The CRISPR/Cas9 screening was designed to target these 263 metabolic genes enriched in AML leukemic cells.
sgRNAs targeting Renilla luciferase were included as negative control. sgRNAs targeting Bcl2, Mcl1, Myc, Pcna, Rpa1, and Rpa3 were included as control for positive regulators of leukemic cell proliferation. sgRNA sequences targeting Trp53 were included as control for negative regulators of leukemic cell proliferation. sgRNA sequences targeting above control genes and 236 metabolic genes were adapted from previous work (Koike-Yusa et al., 2014). sgRNA inserts were designed as GTGGAAAGGACGAAACACCG (U6 promoter) + 20 nt sgRNA + GTTTTAGAGCTAGAAATAGC (tracrRNA) + GGCCCTGGGGGATCTTT (barcode). sgRNA inserts were synthesized by CustomArray and were first round PCR amplified with forward primer: AAAGATCCCCCAGGGCC, and reverse primer: TTATATATCTTGTGGAAAGGACGAAACACC, and were then second round PCR amplified with forward primer: TAGCCTTATTTTAACTTGCTATTTCTAGCTCTAAAAC, and reverse primer: ATTTCTTGGCTTTATATATCTTGTGGAAAGG. Second round PCR products were then treated with T4 Polynucleotide Kinase followed with a Gibson assembly reaction with LentiCRISPR v2 vector (Addgene #52961), which was digested with BsmBI and treated with alkaline phosphatase. Transformation was performed by using electrocompetent cells (Lucigen), and bacterial colonies were then collected together for plasmids extraction (QIAGEN Plasmid Maxi Kit).
For functional genomic screening, viral sgRNA pools were produced by transfecting HEK239 cells with lentiCRISPR v2 sgRNA libraries with VSVG and PAK2 packaging vectors. Two days after transfection, viral sgRNA pool supernatants were collected and filtered. Nras(G12D)/MLL-AF9 leukemic cells were infected with viral sgRNA pool supernatants at 37°C and 1,500 rpm for 60 min. Viral infection was performed at a viral titer so that the majority of leukemic cells were with only one sgRNA integration. After spin infection, viral supernatants were substituted with leukemic cell culture medium. On day 1 and day 9 of cell culture, leukemic cells were collected respectively and genomic DNAs were extracted. The integrated sgRNAs were PCR amplified and were combined for a pooled deep sequencing. Deep sequencing reads corresponding to each sgRNA at each day were identified after demultiplexing of deep sequencing result.
Gene dependency analysis
The collection of published human cancer cell line whole genome sgRNA screen results was downloaded from http://genomecrispr.dkfz.de/ (Rauscher et al., 2017). The data used for all heatmaps was from the 06-Nov-2017 collection https://www.dkfz.de/signaling/crispr-downloads/GENOMECRISPR/ with the file name GenomeCRISPR_full05112017.csv. The information for each individual sgRNA in each experiment is in the csv file. The detailed explanation of the structure of the raw data is listed at http://genomecrispr.dkfz.de/#!/download. Whole genome straight lethal experiments as specified in the condition and cas columns, but not drug treatment combination experiments, were selected in the analysis. The Log2FC (abundance fold-change with log transformation) was used to calculate the gene dependency score for each gene in each experiment. The gene dependency score was calculated by taking the average of Log2FC of all sgRNAs targeting the same gene as previously reported (Wang et al., 2017). The gene dependency score (average Log2FC number) was color-coded to demonstrate the cell viability change upon knockout of the gene. Un-supervised clustering using R package "Pheatmap" was used to cluster genes or experiments with similar phenotypes for visualization.
Culture, infection, and compound treatment of leukemic cells, iMEF cell, 3T3 cell and sarcoma cells
Vitamin B6 depleted medium (VB6−) was prepared by customized pyridoxine (−) RPMI (Gibco) with 10% dialyzed FBS, L-glutamine, and penicillin streptomycin. Pyridoxine, pyridoxamine, pyridoxal or pyridoxal phosphate (Sigma) was added back to VB6(−) medium to make the VB6 (+) medium.
All viral sgRNA infection experiments were performed on Cas9 cell lines. All viral shRNA infection experiments were performed on cell lines carrying rtTA3. The sequences of sgRNA/shRNA are listed in Table S5. Control or gene targeting sgRNAs or shRNAs were delivered by lentivirus. Spin infections were performed at 37°C and 1500 rpm for 60 min. After spin infection, viral supernatants were substituted with leukemic cell culture medium.
For compound treatment, isoniazid, 4'-O-methylpyridoxine or ABT-199 was added in culture medium at indicated concentration. For synergistic effect analysis, Nras(G12D)/MLL-AF9 or MOLM13 cells were plated in 96-well plate at 1,000 cells/well in the presence of isoniazid and ABT-199 titration. Cell number was measured with Guava on day 4. The Combination Index values were calculated with CompuSyn Software (Chou, 2006).
Bone marrow hematopoietic stem and progenitor cells isolation, culture, and compound treatment
For isolation of bone marrow cells, bone marrow from femur and tibia bones was flushed into ice-cold PBS buffer. After red blood cells were lysed with ammonium chloride (STEMCELL 07850), bone marrow cells were resuspended with PBS and filtered into a round bottom tube with cell strainer cap. The hematopoietic cell lineages were stained with biotin mouse lineage panel (BD Biosciences 559971) then isolated by magnetic beads separation (Zhang et al., 2013). For c-Kit positive cells, mouse hematopoietic cells were isolated from bone marrow using MACS CD117 isolation system. Purified bone marrow cells were cultured in RPMI medium supplemented with 10% FBS, L-glutamine, penicillin streptomycin, recombinant mouse SCF, IL-3, and IL-6. Control or Pdxk targeting shRNAs were delivered by lentivirus. Spin infections were performed at 37°C and 1500 rpm for 60 min. After spin infection, viral supernatants were substituted with leukemic cell culture medium. For compound treatment, isoniazid or 4'-O-methylpyridoxine was added in culture medium at indicated concentration.
In vivo competitive proliferation assay
HSPCs isolated from donor (C57BL/6J mice) were infected with retroviruses expressing shRNAs targeting Renilla luciferase, Pdxk, or Rpa3 and mCherry or GFP in the same transcript. Spin infections were performed at 30°C and 1500 rpm for 60 min. After spin infection, viral supernatant was substituted with RPMI medium supplemented with 10% FBS, L-glutamine, penicillin streptomycin, recombinant mouse SCF, IL-3, and IL-6. mCherry positive cells expressing shRNA targeting Renilla luciferase and GFP positive cells expressing shRNA targeting Renilla luciferase, Pdxk, or Rpa3 were mixed at 1 to 1 ratio and transplanted into 7 weeks old lethally irradiated (850 cGy) C57BL/6J mice through tail vein injection. 6 weeks after transplantation, the bone marrow cells were isolated. The relative ratios of GFP positive and mCherry positive Lin−Sca1+c-Kit+ (LSK) bone marrow cells were analyzed.
In vitro competitive proliferation assay
For in vitro competitive proliferation experiments, mouse or human leukemic cells were infected with viruses encoding sgRNAs or shRNAs targeting indicated gene. Spin infection was performed at 37°C and 1500 rpm for 60 min. After spin infection, viral supernatant was substituted with leukemic cell culture medium. Two days after infection, the efficiency was measured by flow cytometry using GFP as an infection marker. GFP+ cells were co-cultured with non-infected cells. The initial percentage of GFP+ cells was ~80%. GFP+ percentage and cell number were monitored during culture using guava flow cytometer. The absolute cell number was measured by using flow cytometry absolute count standard (Bangs Laboratories 580). The experimental results were analyzed by guavasoft software.
Cell differentiation, cell cycle and apoptosis experiments
For differentiation experiment, Nras(G12D)/MLL–AF9 or MOLM13 cells were infected with viruses encoding sgRNAs targeting either Rosa26 or PDXK. On day 9 of infection, cultured cells were stained with c-Kit or Mac-1 antibodies. GFP– and GFP− populations were gated for standard flow cytometry analysis to detect c-Kit and Mac-1 expression levels. Cultured cells were also processed with standard May-Grüwald Giemsa staining.
For cell cycle experiment, Nras(G12D)/MLL–AF9 cells or MOLM13 cells were infected with viruses encoding sgRNAs targeting either Rosa26 or PDXK. On day 9 and 12 of infection, Nras(G12D)/MLL–AF9 cells or MOLM13 cells were stained with EdU Alexa Fluor 647 Flow Cytometry Assay Kit (Invitrogen C10634). Briefly, 10 μM Edu was pulsed in culture for 20 mins. Cells were then washed and fixed with Click-iT fixative for 15 min following by Click-iT reaction. The percentage of S-phase and G1-phase cells in the population were measured by flow cytometry analysis.
For apoptosis experiment, Nras(G12D)/MLL-AF9 cells were infected with viruses encoding sgRNAs targeting either Rosa26 or Pdxk. On day 9 of infection, cultured cells were stained with Annexin-APC and DAPI. GFP+ and GFP− populations were gated for standard flow cytometry analysis.
Leukemia mouse models and Xenogen bioluminescence imaging
The mouse model of human AML was produced as previously described with some modification. For doxycycline-inducible shRNA mouse models, Nras(G12D)/MLL-AF9 leukemic cells infected with viruses encoding shRNAs targeting either Renilla luciferase or Pdxk. Leukemic cells were collected and resuspended in PBS at a concentration of 5 million/ml. 5~6 weeks old NCI B6-Ly5.1/Cr mice (Charles River) were sublethally irradiated (450 cGy) and transplanted with 1 million leukemic cells through tail vein injection. 6 days after transplantation, Xenogen bioluminescence imaging was utilized to monitor luciferase signal intensity. Before imaging, all mice were anesthetized with 2% isoflurane, and anesthesia was also maintained during the image acquisition inside Xenogen dark chamber. Briefly, 300 mg/kg luciferin (Gold Biotechnology) was intraperitoneally injected into mice. 10 min after luciferin injection, Xenogen bioluminescence imaging was applied to monitor luciferase signal intensities of the mice. The bioluminescence was acquired and quantitatively analyzed by the Living Image software. After confirmation of disease establishment, doxycycline was supplemented into mouse diet and water to induce shRNA expression. Xenogen bioluminescence imaging was then utilized on indicated days to monitor luciferase signal intensities and disease progression. For sgRNA mouse models, the procedure was similar to shRNA model except for doxycycline induction.
For the compound injection experiment, Nras(G12D)/MLL-AF9 or AML1/ETO leukemic cells were collected and resuspended in PBS at a concentration of 5 million/ml. 5~6 weeks old NCI B6-Ly5.1/Cr mice (Charles River) were sublethally irradiated (450 cGy) and transplanted with 1 million leukemic cells through tail vein injection. For isoniazid injection, PBS or 90 mg/kg isoniazid was intraperitoneally injected daily. For 4'-O-methylpyridoxine injection, PBS or 7 mg/kg 4'-O-methylpyridoxine was intraperitoneally injected once or twice daily.
For vitamin B6 diet treatment, 5~6 weeks old NCI B6-Ly5.1/Cr mice (Charles River) were fed with a pyridoxine deficient diet (Envigo) for 2 weeks. After transplanted with 1 million Nras(G12D)/MLL-AF9 leukemic cells, the recipient mice were randomly divided into two groups fed with pyridoxine deficient or pyridoxine control diet (Envigo) to endpoint. All animal studies were adherence to the NIH Guide for the Care and Use of Laboratory Animals and conducted following protocols approved by the ethics committee at Cold Spring Harbor Laboratory (566729-10).
Western blot experiment
Cell pellets were lysed with laemmli lysis buffer at room temperature for 4 hr and boiled at 95°C for 5 min. Protein samples were isolated on 4-15% Mini-PROTEAN(r) TGX(tm) Gel and transferred to PVDF membranes (Millipore). After blocking with 5% skimmed milk, membranes were incubated with primary antibodies (1:1000) overnight and HRP-conjugated secondary antibody (1:10000) for 1 hr. Primary antibodies include anti-PDXK antibody (Sigma HPA030196), anti-GFP antibody (Hypromatrix HM2020), and anti-Actin antibody (Sigma A3854). Images were acquired using FluorChem imaging system or X-ray films and quantified by Image J.
RNA-seq library construction and genome-wide transcript counting
To study transcriptomic changes upon PDXK inhibition, total RNA was isolated using the RNeasy minikit (Qiagen) according to standard protocols. Poly-A selection was performed. For sequencing approximately 10 million 76bp single-end reads were acquired per replicate condition. Resulting RNA-Seq data was analyzed by removing adaptor sequences using Trimmomatic (Bolger et al., 2014). RNA-seq reads were then aligned to GRCh37 (hg19), GRCh38 (hg38), or GRCm38 (mm10) with STAR (Dobin et al., 2013) accordingly. Genome-wide transcript counting was performed by subreads (Liao et al., 2013) to generate a matrix of reads per kilobase of exon per million fragments mapped (RPKM). Differentially expressed genes were called by the DESeq2 algorithm using the read counts table (Love et al., 2014).
Measurement of plasma PLP level
Briefly, mice were anesthetized with 2% isoflurane. Then, approximate 100 μL blood was taken from orbital sinus. The blood sample was temporally stored in BD Microtainer with K2EDTA on ice. The plasma was isolated by centrifuge at 2,000 xg and 4°C for 10 min. Then, the supernatant was transferred into a new tube and stored under −80°C. The plasma PLP level was measured with A/C Enzymatic Vitamin B6 Assay (A/C Diagnostics, 510k #0111260) or LC-MS as described below.
Measurement of intracellular Vitamin B6 species
LC-MS grade solvents were purchased from Fisher Scientific, metabolite standards were purchased from Sigma unless stated otherwise. Nras(G12D)/MLL–AF9 cells infected with viruses encoding control or Pdxk shRNAs were harvested after 5 days of doxycycline induction. Human MOLM13 cells infected with viruses encoding control or PDXK sgRNAs were harvested at day 11 after infection. Human THP1 cells and sarcoma cells infected with viruses encoding control or PDXK sgRNAs were harvested at day 8 after infection. Cells treated with isoniazid or 4'-O-methylpyridoxine were harvested after 4 days (for human pluripotent stem cell derived HSPC cells) or 5 days of treatment.
In each case, 1-2 million cells were collected by centrifugation. Media was aspirated and 100 μL of ice-cold 10 % trichloroacetic acid containing 100 ng/mL [3D]-pyridoxal ([3D]-PL), [3D]-pyridoxine ([3D]-PN), [3D]-pyridoxamine ([3D]-PM), [3D]-pyridoxal phosphate ([3D]-PLP) (IsoSciences) was added for metabolite extraction. The intracellular vitamin B6 species were compared to the respective isotope labeled internal controls. For experiments containing isoniazid treatment, cells were washed once with PBS to remove residual drug and minimize post-lysis reactions between isoniazid and PLP, which was confirmed by monitoring the [3D]-PLP internal standard. After overnight incubation at −80°C, samples were vortexed well, transferred to 1.7 mL conical Eppendorf tubes and centrifuged at 21,000 x g for 20 min at 4°C. The supernatant was transferred to autosampler vials for LC-MS analysis. Vitamin B species separation was performed using an Agilent 1290 infinity pump and XSelect HSS T3 column (150 x 2.1 mm, 3.5 μm; Waters Corporation). Mobile phase A was 0.1 % heptaflurobutyric acid (HFBA, Thermo Fisher) and 0.1% formic acid (FA, Thermo Fisher) in water, mobile phase B was 0.1 % HFBA and 0.1 % FA in acetonitrile. The injection volume was 10 μL and LC gradient conditions were: 0 min: 0% B; 1 min: 0% B; 7 min: 25% B; 8 min: 25% B; 9 min: 100 % B; 10.5 min: 100 % B, with 4.5 min of re-equilibration time. MS detection was performed using an Agilent 6230 TOF accurate mass spectrometer with Dual JetStream source operating in positive ionization mode. MS parameters were: gas temp 250°C; gas flow: 13 L/min; nebulizer pressure: 45 psig; sheath gas temp: 225°C; sheath gas flow: 12 L/min; VCap: 3,500 V; Fragmentor: 175 V; Skimmer: 65 V; Octopole RF: 750 V. Active mass axis correction was done using a second nebulizer and pyridine (80.049480) and roxithromycin (837.53185) as reference ions. Data were acquired from m/z 50 - 1700 at 1.5 spectra/sec. Accurate mass (± 20 ppm) and retention time were confirmed relative to the pure standards. Data was analyzed using MassHunter software (Agilent).
Stable isotope labeling assay
Nras(G12D)/MLL-AF9 or iMEF cells were infected with viruses encoding sgRNAs targeting Cr8 or Pdxk and harvested on day 8 or day 12 respectively. 8 hr before harvesting the cells, the culture media was substituted with RPMI media containing dialyzed FBS, and the respective tracer (U-[13C]-glucose, U-[13C]-glutamine, [15N]-alpha-glutamine or [15N]-amide-glutamine). Media was removed and ice-cold extraction solvent (80:20 methanol:water without internal standards) was immediately added to the cells without prior PBS wash.
Metabolite profiling
Approximately 1-2 million cells were washed once with PBS for metabolite detection and isoniazid treatment. The supernatant was dried under vacuum (Genevac EZ-2 Elite). Cells were harvested as described above, and metabolites were extracted using 80:20 methanol:water containing 1.5 μM [13C,15N]-labeled amino acid mix (Cambridge Isotope Laboratories) and 50 ng/mL vitamin B6 internal standards. After overnight incubation at −80°C, samples were vortexed well, transferred to 1.7 mL conical Eppendorf tubes and centrifuged at 21,000 x g for 20 mins at 4°C. Samples were re-suspended in 70 μL of water and divided between HILIC and HFBA methods. For HFBA positive mode profiling (described in the previous section) 20 μL MPA was added to 30 μL of re-suspended and analyzed as described for B6 species. For HILIC negative mode profiling 40 μL of re-suspended extract was diluted with 60 μL of acetonitrile. Chromatographic separation by HILIC was performed using an Agilent 1290 Infinity pump and a ZIC-pHILIC polymeric column (PEEK 150 x 2.1 mm, 5 μm; Merck Sequant). Mobile phase A was 90:10 water: acetonitrile containing 10 mM ammonium bicarbonate (pH 9.4), mobile phase B was 90:10 acetonitrile: water containing 10 mM ammonium bicarbonate (pH 9.4). The injection volume was 5 μL and LC gradient conditions were as follows: 0 min: 95% B; 1 min: 95% B; 10 min: 50% B; 13 min: 50% B; 14 min: 30% B; 16 min 30% B, with 7 min of re-equilibration time. MS detection was done using an Agilent 6545 Q-TOF mass spectrometer with Dual JetStream source operating in negative ionization mode. MS parameters were: gas temp: 200 °C; gas flow: 10 L/min; nebulizer pressure: 40 psig; sheath gas temp: 300 °C; sheath gas flow: 12 L/min; VCap: 3,000 V; Fragmentor: 125 V; Skimmer: 45 V; Octopole RF: 750 V. Active reference mass correction was performed through a second nebulizer using masses with m/z: 119.03632 and 980.016375. Data was acquired from m/z 50 - 1700 at 1 spectra/sec. Accurate mass (±20 ppm), reference metabolite standards and MSMS spectra collected from pooled QC samples were used to confirm the identity of the metabolites. Data analysis was performed within MassHunter software (Agilent) and metabolites reported have <30% CV in pooled QC samples injected regularly throughout the analytical batch. The metabolite profiling data are represented with heatmap. For metabolism profiling analysis, the row Z-Score was used to represented the effects of genetic depletion on each metabolite in leukemic or MEF cells. The row Z-Score was calculated by measuring the standard deviations of each data point away from the mean of each row.
Plasmid construction
The pLKO5.sgRNA.EFS.GFP vector (Addgene 57822) was used for sgRNA experiments. RT3GEN vector (Fellmann et al., 2013) was used for shRNA experiments. miR-E based MLS retroviral vector was used for c-kit positive bone marrow cells viral shRNA infection experiment. shRNA sequences and sgRNA sequences are listed in Table S5. The full length of mouse or human PDXK cDNA was cloned into MSCV Hygro-PGK-Hygro vector (Clontech 634401). The deletion and point mutant of PDXK were made by QuikChange Lightning Multi Site-Directed Mutagenesis Kit (Agilent Technologies).
QUANTIFICATION AND STATISTICAL ANALYSIS
All statistical analyses were performed by GraphPad PRISM 7. The t-test and the Wilcox test was used for the comparisons of the means of two groups. The one-way ANOVA test was used for the comparisons of the means of more than two groups. The Log-rank (Mantel-Cox) test was used for the survival curves analyses. N represents biological replicates in cell culture experiments and the number of mice in animal studies.
DATA AND SOFTWARE AVAILABILITY
All deep sequencing data are available from the Gene Expression Omnibus database with accession numbers GSE139908, GSE139892 and GSE139905.
Supplementary Material
Table S1. List of metabolic genes that are enriched in AML cells. Related to Figure 1.
Table S2. CRISPR/Cas9 functional genomics screen identified metabolic vulnerabilities in AML. Related to Figure 1.
Table S3. RNA-Seq gene expression profiling identified genes differentially expressed upon genetic depletion of Pdxk in mouse AML cells. Related to Figure 1.
Table S4. RNA-Seq gene expression profiling identified genes differentially expressed upon genetic depletion of PDXK in human AML cells. Related to Figure 1.
Table S5. List of sgRNA and shRNAs sequences. Related to STAR Methods.
Significance.
Dysregulated metabolism contributes to cancer cell proliferation. However, beyond mutated metabolic enzymes, metabolic dependencies cannot be inferred from genomic analysis. We performed a CRISPR/Cas9 functional genomic screen and identified PDXK and the vitamin B6 pathway as selective metabolic dependencies in acute myeloid leukemia. PDXK kinase activity and vitamin B6 are required for leukemic cell proliferation and the disruption of PLP-dependent metabolism results in metabolic changes including reduced nucleotide and polyamine levels. Pharmacological blockade of the vitamin B6 pathway, at either the PDXK or PLP level, exhibited anti-leukemic activity. Our results suggest that vitamin B6-dependent metabolism supports multiple metabolic pathways critical for leukemia maintenance. We suggest that vitamin B6 pathway inhibitors, by simultaneously attenuating these pathways, could be promising anti-leukemia agents.
Highlights.
CRISPR/Cas9 screen identifies PDXK as an AML selective metabolic dependency
PDXK kinase activity and PLP are selectively required for leukemic cell proliferation
PLP-dependent enzymes ODC1 and GOT2 selectively support leukemic cell proliferation
The vitamin B6 pathway is a therapeutically actionable dependency in leukemia
ACKNOWLEDGMENTS
We thank Y. Hao of bioinformatics shared resource at CSHL for helping with gene expression analysis and K. Rivera of mass spectrometry shared resource at CSHL for helping with LC-MS experiments. We thank E. Manchado, A. Banito, and K. Tsanov at MSKCC for sharing reagents, technical assistance and advice. We thank Dr. B. Stillman, members of the Stillman laboratory, members of the Lowe laboratory, and members of the Zhang laboratory for discussions and suggestions. We thank R. Ramos, A. Pickard and M. Berisa for suggestions and assistance with metabolomics data analysis. This work is supported by CSHL President’s Council, NIH/NCI grant (P30 CA045508), Northwell Cancer Translational Research grant, NIH/NHLBI grant (U01 HL127522), Edward P. Evans Foundation EvansMDS Young Investigator Award to L. Zhang and NIH/NCI grant (R01 CA190261) and Agilent Thought Leader Award to S.W. Lowe, and the MSKCC cancer center support grant (P30 CA008748). S.W. Lowe is an investigator in the Howard Hughes Medical Institute.
Footnotes
DECLARATION OF INTERESTS
S.W.L. is a founder and scientific advisory board member of Blueprint Medicines, ORIC Pharmaceuticals, and Mirimus, Inc., and is a scientific advisory board member of PMV Pharmaceuticals, Petra Pharmaceuticals, and Constellation Pharmaceuticals. A patent on treating AML with PDXK inhibitors was filed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agathocleous M, Meacham CE, Burgess RJ, Piskounova E, Zhao Z, Crane GM, Cowin BL, Bruner E, Murphy MM, Chen W, et al. (2017). Ascorbate regulates haematopoietic stem cell function and leukaemogenesis. Nature 549, 476–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banito A, Li X, Laporte AN, Roe JS, Sanchez-Vega F, Huang CH, Dancsok AR, Hatzi K, Chen CC, Tschaharganeh DF, et al. (2018). The SS18-SSX Oncoprotein Hijacks KDM2B-PRC1.1 to Drive Synovial Sarcoma. Cancer Cell 33, 527–541.e528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birsoy K, Wang T, Chen WW, Freinkman E, Abu-Remaileh M, and Sabatini DM (2015). An Essential Role of the Mitochondrial Electron Transport Chain in Cell Proliferation Is to Enable Aspartate Synthesis. Cell 162, 540–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, and Usadel B (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasky TM, White E, and Chen CL (2017). Long-Term, Supplemental, One-Carbon Metabolism-Related Vitamin B Use in Relation to Lung Cancer Risk in the Vitamins and Lifestyle (VITAL) Cohort. J Clin Oncol, JCO2017727735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns RA, Harris IS, and Mak TW (2011). Regulation of cancer cell metabolism. Nat Rev Cancer 11, 85–95. [DOI] [PubMed] [Google Scholar]
- Cantor JR, and Sabatini DM (2012). Cancer cell metabolism: one hallmark, many faces. Cancer Discov 2, 881–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Liu Y, Lu C, Cross JR, Morris JP, Shroff AS, Ward PS, Bradner JE, Thompson C, and Lowe SW (2013). Cancer-associated IDH2 mutants drive an acute myeloid leukemia that is susceptible to Brd4 inhibition. Genes Dev 27, 1974–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TC (2006). Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev 58, 621–681. [DOI] [PubMed] [Google Scholar]
- Cimmino L, Dolgalev I, Wang Y, Yoshimi A, Martin GH, Wang J, Ng V, Xia B, Witkowski MT, Mitchell-Flack M, et al. (2017). Restoration of TET2 Function Blocks Aberrant Self-Renewal and Leukemia Progression. Cell 170, 1079–1095.e1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, and Gingeras TR (2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doulatov S, Vo LT, Chou SS, Kim PG, Arora N, Li H, Hadland BK, Bernstein ID, Collins JJ, Zon LI, and Daley GQ (2013). Induction of multipotential hematopoietic progenitors from human pluripotent stem cells via respecification of lineage-restricted precursors. Cell Stem Cell 13, 459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovey OM, Cooper JL, Mupo A, Grove CS, Lynn C, Conte N, Andrews RM, Pacharne S, Tzelepis K, Vijayabaskar MS, et al. (2017). Molecular synergy underlies the co-occurrence patterns and phenotype of. Blood 130, 1911–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliot AC, and Kirsch JF (2004). Pyridoxal phosphate enzymes: mechanistic, structural, and evolutionary considerations. Annu Rev Biochem 73, 383–415. [DOI] [PubMed] [Google Scholar]
- Fellmann C, Hoffmann T, Sridhar V, Hopfgartner B, Muhar M, Roth M, Lai DY, Barbosa IA, Kwon JS, Guan Y, et al. (2013). An optimized microRNA backbone for effective single-copy RNAi. Cell Rep 5, 1704–1713. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Vacchelli E, Michels J, Garcia P, Kepp O, Senovilla L, Vitale I, and Kroemer G (2013). Effects of vitamin B6 metabolism on oncogenesis, tumor progression and therapeutic responses. Oncogene 32, 4995–5004. [DOI] [PubMed] [Google Scholar]
- Gandhi AK, Desai JV, Ghatge MS, di Salvo ML, Di Biase S, Danso-Danquah R, Musayev FN, Contestabile R, Schirch V, and Safo MK (2012). Crystal structures of human pyridoxal kinase in complex with the neurotoxins, ginkgotoxin and theophylline: insights into pyridoxal kinase inhibition. PLoS One 7, e40954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi AK, Ghatge MS, Musayev FN, Sease A, Aboagye SO, di Salvo ML, Schirch V, and Safo MK (2009). Kinetic and structural studies of the role of the active site residue Asp235 of human pyridoxal kinase. Biochem Biophys Res Commun 381, 12–15. [DOI] [PubMed] [Google Scholar]
- Heckl D, Kowalczyk MS, Yudovich D, Belizaire R, Puram RV, McConkey ME, Thielke A, Aster JC, Regev A, and Ebert BL (2014). Generation of mouse models of myeloid malignancy with combinatorial genetic lesions using CRISPR-Cas9 genome editing. Nat Biotechnol 32, 941–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarty MD, Norris MD, Davis K, Liu X, Evageliou NF, Hayes CS, Pawel B, Guo R, Zhao H, Sekyere E, et al. (2008). ODC1 is a critical determinant of MYCN oncogenesis and a therapeutic target in neuroblastoma. Cancer Res 68, 9735–9745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CH, Lujambio A, Zuber J, Tschaharganeh DF, Doran MG, Evans MJ, Kitzing T, Zhu N, de Stanchina E, Sawyers CL et al. (2014). CDK9-mediated transcription elongation is required for MYC addiction in hepatocellular carcinoma. Genes Dev 28, 1800–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi D, Yoshimura T, Johno A, Ishikawa M, Sasaki K, and Wada K (2015). Decrease in pyridoxal-5'-phosphate concentration and increase in pyridoxal concentration in rat plasma by 4'-O-methylpyridoxine administration. Nutr Res 35, 637–642. [DOI] [PubMed] [Google Scholar]
- Koike-Yusa H, Li Y, Tan EP, Velasco-Herrera M. e. C., and Yusa K (2014). Genome-wide recessive genetic screening in mammalian cells with a lentiviral CRISPR-guide RNA library. Nat Biotechnol 32, 267–273. [DOI] [PubMed] [Google Scholar]
- Lainé-Cessac P, Cailleux A, and Allain P (1997). Mechanisms of the inhibition of human erythrocyte pyridoxal kinase by drugs. Biochem Pharmacol 54, 863–870. [DOI] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, and Shi W (2013). The Subread aligner: fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Res 41, e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, and Shi W (2014). featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930. [DOI] [PubMed] [Google Scholar]
- Love MI, Huber W, and Anders S (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DB, and Chen H (1999). Update on interconversions of vitamin B-6 with its coenzyme. J Nutr 129, 325–327. [DOI] [PubMed] [Google Scholar]
- Pais RC, Vanous E, Hollins B, Faraj BA, Davis R, Camp VM, and Ragab AH (1990). Abnormal vitamin B6 status in childhood leukemia. Cancer 66, 2421–2428. [DOI] [PubMed] [Google Scholar]
- Pavlova NN, and Thompson CB (2016). The Emerging Hallmarks of Cancer Metabolism. Cell Metab 23, 27–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock CF, Malone M, Booth M, Evans M, Morgan G, Greil J, and Cotter FE (1995). BCL-2 expression by leukaemic blasts in a SCID mouse model of biphenotypic leukaemia associated with the t(4;11)(q21;q23) translocation. Br J Haematol 90, 855–867. [DOI] [PubMed] [Google Scholar]
- Rauscher B, Heigwer F, Breinig M, Winter J, and Boutros M (2017). GenomeCRISPR - a database for high-throughput CRISPR/Cas9 screens. Nucleic Acids Res 45, D679–D686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjana NE, Shalem O, and Zhang F (2014). Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods 11, 783–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnellbaecher A, Binder D, Bellmaine S, and Zimmer A (2019). Vitamins in cell culture media: Stability and stabilization strategies. Biotechnol Bioeng 116, 1537–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman MH, Yu RT, Engle DD, Ding N, Atkins AR, Tiriac H, Collisson EA, Connor F, Van Dyke T, Kozlov S, et al. (2014). Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 159, 80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Wang E, Milazzo JP, Wang Z, Kinney JB, and Vakoc CR (2015). Discovery of cancer drug targets by CRISPR-Cas9 screening of protein domains. Nat Biotechnol 33, 661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M, Perera RM, Ferrone CR, Mullarky E, Shyh-Chang N, et al. (2013). Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 496, 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueland PM, Ulvik A, Rios-Avila L, Midttun Ø, and Gregory JF (2015). Direct and Functional Biomarkers of Vitamin B6 Status. Annu Rev Nutr 35, 33–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unissa AN, Subbian S, Hanna LE, and Selvakumar N (2016). Overview on mechanisms of isoniazid action and resistance in Mycobacterium tuberculosis. Infect Genet Evol 45, 474–492. [DOI] [PubMed] [Google Scholar]
- Valk PJ, Verhaak RG, Beijen MA, Erpelinck CA, Barjesteh van Waalwijk van Doorn-Khosrovani S, Boer JM, Beverloo HB, Moorhouse MJ, van der Spek PJ, Löwenberg B, and Delwel R (2004). Prognostically useful gene-expression profiles in acute myeloid leukemia. N Engl J Med 350, 1617–1628. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG (2011). Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov 10, 671–684. [DOI] [PubMed] [Google Scholar]
- Vilter RW, Mueller JF, Glazer HS, Jarrold T, Abraham J, Thompson C, and Hawkins VR (1953). The effect of vitamin B6 deficiency induced by desoxypyridoxine in human beings. J Lab Clin Med 42, 335–357. [PubMed] [Google Scholar]
- Visser ME, Texeira-Swiegelaar C, and Maartens G (2004). The short-term effects of anti-tuberculosis therapy on plasma pyridoxine levels in patients with pulmonary tuberculosis. Int J Tuberc Lung Dis 8, 260–262. [PubMed] [Google Scholar]
- Wang T, Yu H, Hughes NW, Liu B, Kendirli A, Klein K, Chen WW, Lander ES, and Sabatini DM (2017). Gene Essentiality Profiling Reveals Gene Networks and Synthetic Lethal Interactions with Oncogenic Ras. Cell 168, 890–903.e815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir DR, and Morningstar WA (1954). The effect of pyridoxine deficiency induced by desoxypyridoxine on acute lymphatic leukemia of adults. Blood 9, 173–182. [PubMed] [Google Scholar]
- Yogarajah M, and Stone RM (2018a). A concise review of BCL-2 inhibition in acute myeloid leukemia. Expert Rev Hematol 11, 145–154. [DOI] [PubMed] [Google Scholar]
- Yogarajah M, and Stone RM (2018b). A concise review of BCL-2 inhibition in acute myeloid leukemia. Expert Rev Hematol, 1–10. [DOI] [PubMed] [Google Scholar]
- Yun J, Mullarky E, Lu C, Bosch KN, Kavalier A, Rivera K, Roper J, Chio II, Giannopoulou EG, Rago C, et al. (2015). Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science 350, 1391–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Prak L, Rayon-Estrada V, Thiru P, Flygare J, Lim B, and Lodish HF (2013). ZFP36L2 is required for self-renewal of early burst-forming unit erythroid progenitors. Nature 499, 92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Chen CC, Rillahan CD, Shen R, Kitzing T, McNerney ME, Diaz-Flores E, Zuber J, Shannon K, Le Beau MM, et al. (2015). Cooperative loss of RAS feedback regulation drives myeloid leukemogenesis. Nat Genet 47, 539–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber J, McJunkin K, Fellmann C, Dow LE, Taylor MJ, Hannon GJ, and Lowe SW (2011a). Toolkit for evaluating genes required for proliferation and survival using tetracycline-regulated RNAi. Nat Biotechnol 29, 79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber J, Radtke I, Pardee TS, Zhao Z, Rappaport AR, Luo W, McCurrach ME, Yang MM, Dolan ME, Kogan SC, et al. (2009). Mouse models of human AML accurately predict chemotherapy response. Genes Dev 23, 877–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber J, Rappaport AR, Luo W, Wang E, Chen C, Vaseva AV, Shi J, Weissmueller S, Fellmann C, Fellman C, et al. (2011b). An integrated approach to dissecting oncogene addiction implicates a Myb-coordinated self-renewal program as essential for leukemia maintenance. Genes Dev 25, 1628–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. List of metabolic genes that are enriched in AML cells. Related to Figure 1.
Table S2. CRISPR/Cas9 functional genomics screen identified metabolic vulnerabilities in AML. Related to Figure 1.
Table S3. RNA-Seq gene expression profiling identified genes differentially expressed upon genetic depletion of Pdxk in mouse AML cells. Related to Figure 1.
Table S4. RNA-Seq gene expression profiling identified genes differentially expressed upon genetic depletion of PDXK in human AML cells. Related to Figure 1.
Table S5. List of sgRNA and shRNAs sequences. Related to STAR Methods.
Data Availability Statement
All deep sequencing data are available from the Gene Expression Omnibus database with accession numbers GSE139908, GSE139892 and GSE139905.