Background
Acute Kidney Injury (AKI) results in a long-term reduction in renal functional reserve such that affected patients are at an increased risk of progressive chronic kidney disease (CKD)1–4. However, no therapies have been proven to reduce AKI-driven, post-injury CKD progression5. Given the high mortality and morbidity associated with AKI, the lack of effective treatment represents an important and unmet medical need. A main reason there has been a failure to develop therapies for AKI is because patients often present late in the course of AKI, and many of the drugs that been ineffective in AKI clinical trials, were only effective in experimental models of AKI when given before the initiating injury6. One approach to address this limitation is to focus on cellular repair mechanisms that are activated after the initiating injury has occurred7.
Phenotypic vs. target-based drug discovery for AKI
Most post-genomics era drug discovery campaigns are target-based, focusing initially on the identification of compounds that modify the activity of selected molecular targets in vitro. However, many first in class drugs approved by the FDA were discovered in phenotypic, not target-based, screens8. This is relevant for AKI drug discovery where multiple cells types and often poorly defined molecular pathways are involved in regulating injury and repair, such that the selection of a single molecular target for a drug discovery effort may not survive efficacy studies in complex in vivo models. In addition, since access to human AKI kidney biopsies is limited6,9, the clinical significance of target-based therapies, will commonly be unknown until very late in the course of drug development, notably when the results of hypothesis testing Phase 3 clinical trials become available.
With the development of new instrumentation and imaging technologies, there has been increased interest in the use of invertebrate (C. elegans and Drosophila) and vertebrate (zebrafish) models as primary tools for high-content screens (HCS)10–12. While their success is still dependent on choice of a suitable phenotype, these platforms have two major advantages over target-based discovery: a) phenotypic screens are target-agnostic, so do not require prior knowledge of the molecular target, and may identify compounds that simultaneously engage multiple targets. This may be important in the complex milieu of the injured kidney and might be missed in traditional target-based screens; and b) phenotypic screens provide insight into absorption, distribution, metabolism, excretion and toxicity (ADMET) at an early stage in drug development. This has the potential to accelerate drug development by allowing for more rapid transition to animal models with minimal requirement for ADMET optimization.
The use of zebrafish for phenotypic drug discovery in kidney disease
Zebrafish have genetic conservation with humans (including 82% sequence homology with human disease causing proteins), and are genetically malleable so can be used to screen for compounds that correct mutation-associated phenotypes13. Zebrafish are also amenable to HCS: a) screening can be performed using fertilized eggs (~400 per clutch) over the first 72 hours post fertilization (hpf), since larval organs including kidneys, liver and the cardiovascular system, are functional by 24–48 hpf14; b) eggs and larvae are small enough to array in multi-well platforms; can be isolated and dispensed using microfluidic technologies; and can be soaked in robotically dispensed compounds13; c) zebrafish are transparent and genetically malleable. This can be exploited for analysis of internal organs, and for analysis of signaling pathways or cells using fluorescent reporters15. In addition, the zebrafish larval kidney, while simple, has anatomical features of the mammalian kidney that are not conserved in invertebrate HTS models. This includes a glomerular filtration system linked to pulsatile blood flow, and renal segmentation that conserves many of the functions and markers found in the mammalian kidney16. These features make zebrafish larvae an ideal model for phenotypic, kidney-related drug discovery17.
Screening for small molecules that enhance repair after AKI
Our goal was to identify molecules that enhance repair after AKI, so we initially developed proliferation-based assays that were associated with expansion of renal progenitor cells in embryonic and larval zebrafish. Our working hypothesis was that compounds that promote expansion of renal progenitor cells in zebrafish larvae would also enhance proliferative repair after AKI. We screened ~2000 compounds to identify larvae that developed pericardial edema, a sign of abnormal kidney function associated with impaired water excretion. This was followed by a secondary in situ hybridization screen to look for expansion of Lhx1 expressing renal progenitor cells. Using this approach we identified 4-phenylthiobutanoic acid (PTBA), a histone deacetylase inhibitor (HDACi) that caused proliferation-dependent expansion of renal progenitor cells18. To determine whether PTBA also enhanced repair after AKI we initially exploited a model of gentamicin-induced AKI in zebrafish larvae in which intra-cardiac injection of gentamicin causes AKI with renal tubular cell death followed by proliferative repair19. There was increased survival of larvae soaked in the PTBA pro-drug, methyl-4-phenylbutanoate, M4PTB/UPHD25 after gentamicin-induced AKI associated with a marked increase in renal tubular cell proliferation20. We next evaluated the effects of UPHD25 in a mouse model of ischemia reperfusion-induced AKI (IR-AKI)21. UPHD25 treatment initiated 24 hours after injury markedly reduced post-injury renal fibrosis and was associated with increased effective renal tubular cell proliferation20. M4PTB treatment initiated 4 days after injury also reduced post-injury fibrosis and enhanced effective proliferative repair of renal tubular epithelial cells in a mouse model of aristolochic acid induced AKI22.
Automated image-based zebrafish embryonic progenitor cells (EKPC) assays
Encouraged by success of our initial screen we developed an automated zebrafish embryonic kidney progenitor cell (EKPC) assay for further PTBA analog optimization. For this we applied an artificial intelligence-based imaging technique, Cognitive network Technology (CNT), that quantifies fluorescent signals associated with specific fluorescent markers in live zebrafish larvae23. We used two transgenic zebrafish: a) the Lhx1 fluorescent reporter, Tg(lhx1a:EGFP), to quantify renal progenitor cell numbers24; and b) the Cadherin 17 reporter, Tg(cdh17:EGFP), to specifically quantify expansion of renal tubular epithelium25. Using these we developed dose response curves for PTBA analogs (Figure 1)24,25, and found 20/64 analogs were active, with EC50s <3μM25. Of the 6 active compounds (including PTBA) with modifications to acid functionality R2: 3/5 increased survival in zebrafish AKI assays and 2 of these, UPHD25 and UPHD186, reduced fibrosis in a mouse IR-AKI model (Table 1). Furthermore, UPHD186 (but not UPHD25), reduced fibrosis after unilateral ureteric obstruction, and also reduced post-injury renal fibrosis when treatment was delayed until 3 days after IR-AKI25. In addition, treatment with UPHD186 for 7 days at up to 5X the effective therapeutic dose, showed no demonstrable toxicity in mice (unpublished data). These findings indicate that our screening platform can identify non-toxic compounds that enhance effective proliferative repair in multiple mouse models of AKI, including when treatment is delayed days after the initiating injury. This provides the potential for therapeutic intervention across a range of different AKI etiologies that may be given to patients late, after they develop established clinical signs of AKI.
Figure 1.
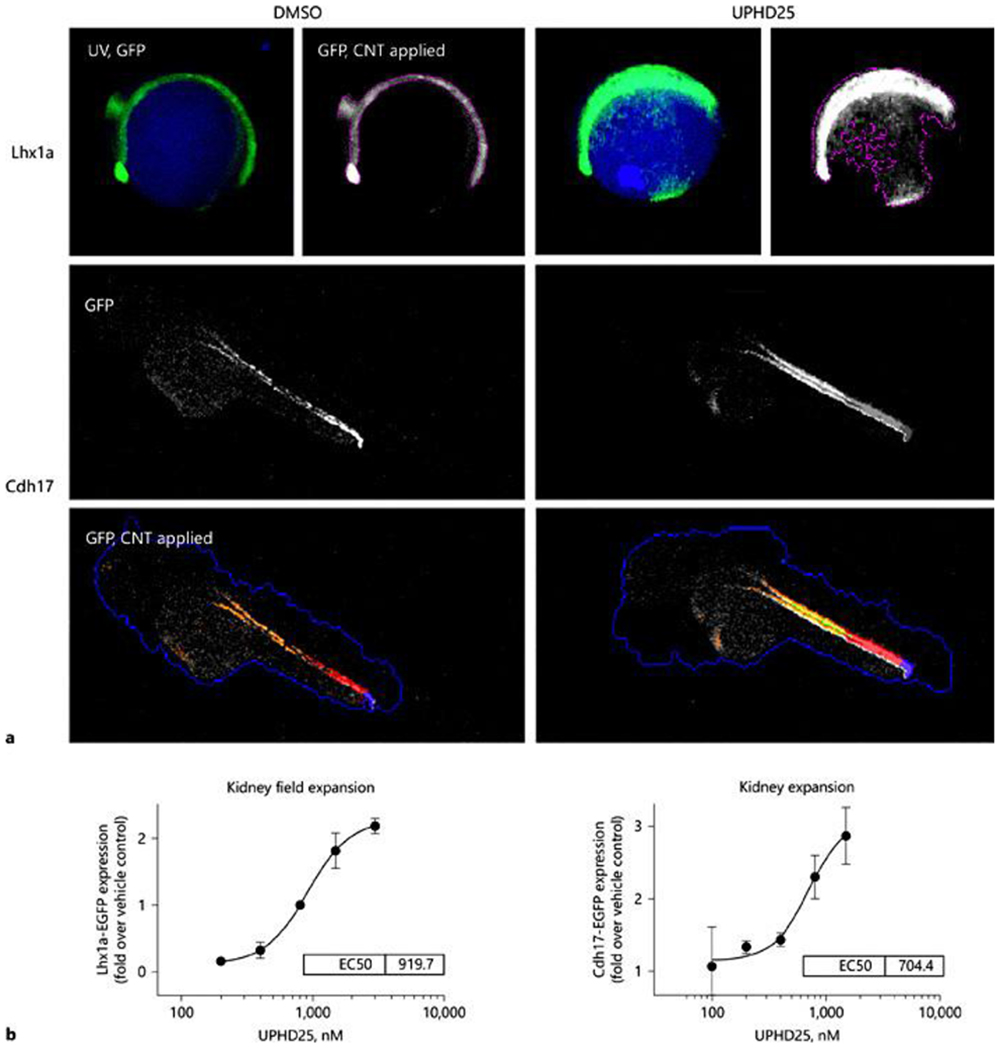
Cognition network technology (CNT) image-based analysis of EGFP-labeled transgenes. a Upper panel: Tg(lhx1a:EGFP)pt303 treated at 3 hpf with vehicle (0.5% DMSO) or UPHD25 (1.5 μM). Images acquired in the UV (DAPI) and green fluorescent protein (GFP) channels after 12 h. A CNT ruleset was applied that detected the whole embryo by auto-fluorescence (UV, GFP) and the transgene as a lower hierarchy sub-object (GFP, CNT applied), permitting transgene-specific measurements of intensity and shape. UPHD25 increases the Lhx1a-EGFP expression and causes characteristic changes in transgene morphology. Lower panel: GFP channel fluorescence micrographs in lateral view of 48 hpf Tg(Cdh17:EGFP)pt305, treated with vehicle or UPHD25 (800 nM). The zebrafish embryonic kidney appears as a pair of tubular structures that converge at the cloaca (triangle). A CNT ruleset was applied that detected the fluorescent kidney (orange). The cloaca was classified based on the brightness and proximity to the zebrafish edge (blue), permitting quantification of Cdh17-EGFP in a defined length tubular segment (red). UPHD25 increases GFP intensity within the embryonic zebrafish kidney. For all panels, rostral is to the left, dorsal pointing up. b Dose-response curves of UPHD25 on kidney progenitor cell expansion using Lhx1a-EGFP and kidney organ size by EGFP-Cdh17 with similar EC50 values for UPHD25 in the 2 models.
Developing an integrated phenotypic and target-based screen for hit to lead optimization
Identification of the mechanism of action (MOA) of a drug is not required for FDA approval for clinical use. However, knowledge of molecular targets helps medicinal chemistry campaigns for lead optimization by guiding structure activity relationship (SAR) studies12. This is of importance to us as UPHD186, while demonstrating favorable toxicity and efficacy profiles, has liabilities that limit its potential for commercial development, such as the requirement to deliver it as a pro-drug. A variety of approaches ranging from serendipity to affinity capture proteomics can be used to identify drug targets26. Forward genetic approaches using new genome editing techniques can also be performed in zebrafish and used for rapid target validation studies27. The molecular targets of PTBA analogs are currently unknown. PTBA, UPHD25 and 186 are all HDIs18,25, and other HDIs such as Trichostatin A are active in EKPC assays18. However, the HDI, MS275, has no effect in EKPC assays, or on zebrafish larval survival after AKI, and does not prevent post-injury fibrosis after IR-AKI (Table 1). These data suggest that while HDACs might be a target of PTBA analogs, their MOA either requires multiple targets or is dependent on a unique HDAC selectivity profile. Our lab is currently using a variety of chemical and genetic approaches to test this hypothesis so that we can develop an iterative target and phenotype driven approach for PTBA analog optimization (Figure 2). By combining this with early and late stage pharmaceutical profiling, and screening through a series of secondary AKI assays, including mouse IR-AKI studies as an initial screen followed by analysis of more complex models reflecting the diversity of injury and co-morbidities seen in clinical practice, as well as the use of human IPS cell-derived kidney organoids to evaluate effects on toxin-induced AKI models that have recently been described28. By completion of this campaign we hope to identify new PTBA-like chemotypes with unique and favorable efficacy profiles (including data on their potential to modify human kidney cell responses to injury), structural and pharmaceutical properties for pre-clinical development.
Acknowledgements
The laboratory of Mark de Caestecker was supported by 1R01DK112688-01A1. The laboratory of Neil Hukriede was supported by the National Institutes of Health Grants R01 DK069403, R01 DK112652, R01 HD053287 and P30 DK079307.
References
- 1.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney international. 2012;81(5):442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lo LJ, Go AS, Chertow GM, et al. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney international. 2009;76(8):893–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wald R, Quinn RR, Luo J, et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA : the journal of the American Medical Association. 2009;302(11):1179–1185. [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. The New England journal of medicine. 2004;351(13):1296–1305. [DOI] [PubMed] [Google Scholar]
- 5.Faubel S, Chawla LS, Chertow GM, Goldstein SL, Jaber BL, Liu KD. Ongoing clinical trials in AKI. Clin J Am Soc Nephrol. 2012;7(5):861–873. [DOI] [PubMed] [Google Scholar]
- 6.Skrypnyk NI, Siskind LJ, Faubel S, de Caestecker MP. Bridging translation for acute kidney injury with better preclinical modeling of human disease. American journal of physiology Renal physiology. 2016;310(10):F972–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Humphreys BD, Cantaluppi V, Portilla D, et al. Targeting Endogenous Repair Pathways after AKI. Journal of the American Society of Nephrology : JASN. 2016;27(4):990–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swinney DC, Anthony J. How were new medicines discovered? Nature reviews Drug discovery. 2011;10(7):507–519. [DOI] [PubMed] [Google Scholar]
- 9.Okusa MD, Rosner MH, Kellum JA, Ronco C, Acute Dialysis Quality Initiative XW. Therapeutic Targets of Human AKI: Harmonizing Human and Animal AKI. Journal of the American Society of Nephrology : JASN. 2016;27(1):44–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strange K Drug Discovery in Fish, Flies, and Worms. ILAR J. 2016;57(2):133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giacomotto J, Segalat L. High-throughput screening and small animal models, where are we? Br J Pharmacol. 2010;160(2):204–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JA, Berg EL. Neoclassic drug discovery: the case for lead generation using phenotypic and functional approaches. Journal of biomolecular screening. 2013;18(10):1143–1155. [DOI] [PubMed] [Google Scholar]
- 13.MacRae CA, Peterson RT. Zebrafish as tools for drug discovery. Nature reviews Drug discovery. 2015;14(10):721–731. [DOI] [PubMed] [Google Scholar]
- 14.Detrich HW 3rd, Westerfield M, Zon LI Overview of the Zebrafish system. Methods Cell Biol. 1999;59:3–10. [DOI] [PubMed] [Google Scholar]
- 15.Williams CH, Hong CC. Zebrafish small molecule screens: Taking the phenotypic plunge. Comput Struct Biotechnol J. 2016;14:350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wingert RA, Davidson AJ. The zebrafish pronephros: a model to study nephron segmentation. Kidney international. 2008;73(10):1120–1127. [DOI] [PubMed] [Google Scholar]
- 17.Swanhart LM, Cosentino CC, Diep CQ, Davidson AJ, de Caestecker M, Hukriede NA. Zebrafish kidney development: basic science to translational research. Birth Defects Res C Embryo Today. 2011;93(2):141–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Groh ED, Swanhart LM, Cosentino CC, et al. Inhibition of histone deacetylase expands the renal progenitor cell population. Journal of the American Society of Nephrology : JASN. 2010;21(5):794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cianciolo Cosentino C, Roman BL, Drummond IA, Hukriede NA. Intravenous microinjections of zebrafish larvae to study acute kidney injury. J Vis Exp. 2010(42). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cianciolo Cosentino C, Skrypnyk NI, Brilli LL, et al. Histone deacetylase inhibitor enhances recovery after AKI. Journal of the American Society of Nephrology : JASN. 2013;24(6):943–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skrypnyk NI, Harris RC, de Caestecker MP. Ischemia-reperfusion model of acute kidney injury and post injury fibrosis in mice. J Vis Exp. 2013(78). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novitskaya T, McDermott L, Zhang KX, et al. A PTBA small molecule enhances recovery and reduces postinjury fibrosis after aristolochic acid-induced kidney injury. American journal of physiology Renal physiology. 2014;306(5):F496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vogt A, Cholewinski A, Shen X, et al. Automated image-based phenotypic analysis in zebrafish embryos. Dev Dyn. 2009;238(3):656–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanker S, Cirio MC, Vollmer LL, et al. Development of High-Content Assays for Kidney Progenitor Cell Expansion in Transgenic Zebrafish. Journal of biomolecular screening. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skrypnyk NI, Sanker S, Skvarca LB, et al. Delayed treatment with PTBA analogs reduces postinjury renal fibrosis after kidney injury. American journal of physiology Renal physiology. 2016;310(8):F705–F716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schenone M, Dancik V, Wagner BK, Clemons PA. Target identification and mechanism of action in chemical biology and drug discovery. Nat Chem Biol. 2013;9(4):232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schenk H, Muller-Deile J, Kinast M, Schiffer M. Disease modeling in genetic kidney diseases: zebrafish. Cell Tissue Res. 2017. [DOI] [PubMed] [Google Scholar]
- 28.Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, Bonventre JV. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol. 2015;33(11):1193–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]


