ABSTRACT
Membrane trafficking pathways are essential for the viability and growth of cells, and play a major role in the interaction of cells with their environment. In this At a Glance article and accompanying poster, we outline the major cellular trafficking pathways and discuss how defects in the function of the molecular machinery that mediates this transport lead to various diseases in humans. We also briefly discuss possible therapeutic approaches that may be used in the future treatment of trafficking-based disorders.
KEY WORDS: Disease, Endocytic pathway, Genetic disorder, Membrane traffic, Secretory pathway, Vesicle
Summary: This At a Glance article and poster summarise the major intracellular membrane trafficking pathways and associated molecular machineries, and describe how defects in these give rise to disease in humans.
Introduction
Membrane trafficking pathways are essential for cells to maintain critical functions, to grow, and to accommodate to their chemical and physical environment. Membrane flux through these pathways is high, and in specialised cells in some tissues can be enormous. For example, pancreatic acinar cells synthesise and secrete amylase, one of the many enzymes they produce, at a rate of approximately 0.5% of cellular protein mass per hour (Allfrey et al., 1953), while in Schwann cells, the rate of membrane protein export must correlate with the several thousand-fold expansion of the cell surface that occurs during myelination (Pereira et al., 2012). The population of cell surface proteins is constantly monitored and modified via the endocytic pathway. In some cells, endocytosis accounts for the complete turnover of surface membrane over a period of an hour or so (Steinman et al., 1976). Given such rates of trafficking, it is not surprising that even subtle alterations in transport caused by mutation or insufficiency of the trafficking machinery can impair cell function and lead to disease over the course of a lifetime.
This At a Glance article describes the essential features of membrane trafficking pathways, including the crucial molecular events that drive transport. We identify instances where the mutation or loss of trafficking machinery components is associated with disease, and attempt to rationalise these effects. Several topics are not covered or are mentioned only briefly due to space limitations, including the folding and quality control of soluble or membrane-bound cargo, as exemplified by cystic fibrosis transmembrane conductance regulator (CFTR) in cystic fibrosis; motor proteins and their adaptors, which move vesicle-bound cargo around the cell; the biogenesis of mitochondria, peroxisomes, or non-membranous organelles; compartment-specific proteins that define essential organelle functions; non-vesicular lipid transport pathways; and exosome trafficking. Similarly, we only briefly discuss autophagy, which relies on membrane input from both the secretory and endocytic pathways and fusion of autophagosomes with lysosomes (Søreng et al., 2018).
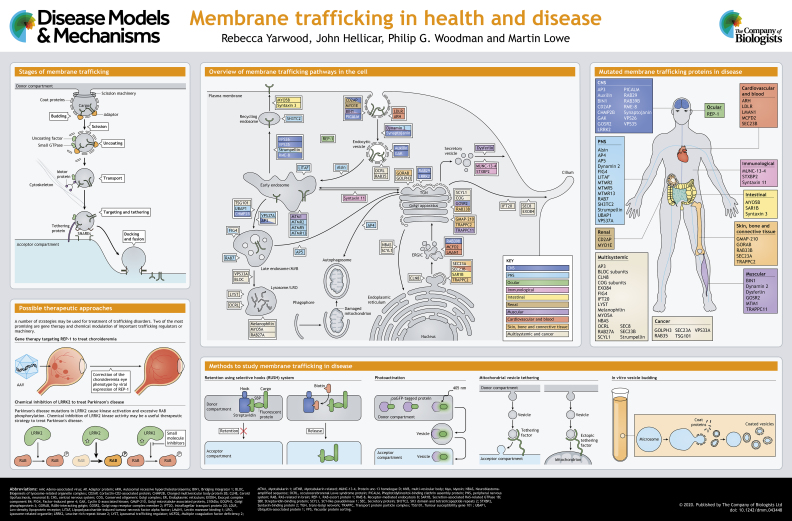
General principles of membrane trafficking
Transport of proteins between compartments is initiated by (1) selection of cargo and its segregation from resident proteins of the donor compartment by the action of ‘adaptors’; (2) encapsulation of cargo-bound adaptors within a protein scaffold or ‘coat’, which drives membrane deformation and ultimately scission to form a transport vesicle, or in some cases a tubular transport intermediate; (3) movement of the vesicle to the target compartment; (4) membrane tethering, in which the vesicle is drawn towards the target membrane by extended proteins/protein complexes that work in conjunction with RAB (RAS-related in brain) GTPases; and (5) docking and membrane fusion, in which the vesicle is first tightly attached to the target membrane, followed by merging of the lipid bilayers, both processes being mediated by soluble N-ethylmaleimide-sensitive-factor attachment protein receptor (SNARE) protein complexes and supported by accessory factors (see poster). While these steps are generic to transport reactions, the compartmental specificity of the components within protein families ensures transport fidelity. Much of our understanding of these processes stems from important experimental methodologies, which we describe in Box 1.
Box 1. Experimental approaches used to study trafficking.
Many molecular cell biological approaches have been used to study membrane traffic. Historically, cell-free assays that reconstitute transport reactions (Balch et al., 1984) and yeast genetics (Novick and Schekman, 1979) provided great advances in identifying the crucial molecular components, and these approaches are still relevant today for dissecting transport mechanisms (see poster, ‘In vitro vesicle budding’). Cell culture models remain a powerful tool, with recent advances including growing cells in 3D to better mimic the tissue environment (Torras et al., 2018), the use of induced pluripotent stem cells that can be isolated from human patients and differentiated into any relevant cell type (Avior et al., 2016), and the use of stem cell-generated organoids, which provide a close approximation of tissue organisation in an in vitro setting (Lancaster and Huch, 2019; Rossi et al., 2018). Animal models also remain a valuable tool to study disease mechanisms attributable to trafficking defects, and have been used very successfully in this regard (see, for example, Smits et al., 2010). Analysis of human patients is also a powerful way to assess the functional relevance of gene products in a physiological setting, and provides a direct indication of the importance of trafficking factors for human health (FitzGerald et al., 2018).
A number of more recent or specialised approaches can be applied to the study of membrane traffic, some of which are highlighted in the poster. Various methods have been developed to allow synchronous transport along the secretory pathway (Kreis and Lodish, 1986; Chen et al., 2013; Kuismanen and Saraste, 1989; Rivera et al., 2000). One of the most commonly used is the retention using selective hooks (RUSH) system, in which synchronous transport is triggered by the addition of exogenous biotin, which triggers release of cargo from an organelle-resident ‘hook’ (Boncompain et al., 2012). The use of split-fluorescent protein technology allows researchers to assess delivery into secretory compartments (Feng et al., 2017). Here, cargo and organelle-resident proteins are separately tagged with two units of a fluorescent protein that, when combined, emit fluorescence, allowing for visualisation of cargo delivery to the organelle of interest. Photo-activation or photo-switching of fluorescently tagged cargo proteins or machinery can also be used to visualise transport dynamics (Sengupta and Lippincott-Schwartz, 2013).
Mitochondrial relocation is a useful tool for assessing protein-protein interactions, but more recently has been adapted to allow visualisation of vesicle tethering in intact cells. Here, tethering factors were artificially localised to mitochondria to allow direct visualisation of tethering by light and electron microscopy (Wong and Munro, 2014). Proximity biotinylation is a recently developed and widely used technology to identify closely associated proteins within cells. There are several variations of the method, which all rely on the promiscuous activity of a biotin ligase attached to any protein of interest, allowing for biotinylation of nearby proteins and their isolation and identification by mass spectrometry (Branon et al., 2018; Hung et al., 2016; Roux et al., 2012). The approach can be used in the context of membrane traffic to identify the machinery involved in particular trafficking reactions, cargo components of transport vesicles, or the protein complements of organelles within the endomembrane system. Quantitative proteomics can also be used to identify entire complements of secreted or plasma membrane proteins (Eichelbaum et al., 2012; Steinberg et al., 2013), allowing for unbiased and comprehensive analysis of how these protein complements may change in response to perturbation of various trafficking pathways.
The secretory pathway
The biogenesis of most integral membrane proteins, secreted proteins and organelle content markers occurs at the endoplasmic reticulum (ER) (see poster). Correctly folded and post-translationally modified membrane-bound or lumenal cargoes are then selected for export by adaptor proteins that engage the coat protein complex (COP) II vesicle machinery, or by binding COPII directly (Jensen and Schekman, 2011; McCaughey and Stephens, 2018). COPII vesicle production is initiated when the ER-associated guanine nucleotide exchange factor (GEF) secretion protein (SEC) 12 (also known as PREB), activates secretion-associated RAS-related GTPase 1 (SAR1) and SAR1-GTP subsequently anchors to the membrane. The COPII coat is formed as SAR1 sequentially recruits multiple SEC23/SEC24 dimers, followed by SEC13/SEC31, to sequester adaptors and drive membrane deformation to produce COPII vesicles. These vesicles tether at and fuse with the ER-Golgi intermediate compartment (ERGIC), from which they are delivered to the cis-side of the Golgi apparatus, processes mediated by tethering factors and complexes of ERGIC- and Golgi-associated SNARE proteins (Brandizzi and Barlowe, 2013).
Cargo subsequently moves through the Golgi complex, where it can undergo post-translational modification and processing, most notably at the level of glycosylation, by enzymes that each localise within a narrow range of Golgi cisternae. How cargo moves forward is controversial, but the current consensus is that a Golgi cisterna moves ‘en bloc’, with cargo encountering Golgi-resident enzymes, as these are distilled backwards via selective incorporation into COPI vesicles (Pantazopoulou and Glick, 2019). COPI works analogously to COPII, with vesicle production initiated by the activation and membrane anchoring of ADP-ribosylation factor (ARF) 1 GTPase (Beck et al., 2009). The COPI coat is recruited en masse, and includes moieties that bind cargo and cargo adaptors, and those that scaffold the assembly and induce membrane curvature. Meanwhile, ARF GTPase activating proteins (ARF-GAPs) sense completion of COPI budding, and facilitate coat disassembly. Conserved oligomeric Golgi complex (COG) is a crucial membrane-tethering complex for COPI vesicles, working in conjunction with RAB GTPases and golgin coiled-coil proteins, while membrane fusion involves Golgi-specific SNAREs (Fisher and Ungar, 2016). In addition to intra-Golgi transport, COPI also recycles proteins from the ERGIC and Golgi apparatus back to the ER (Brandizzi and Barlowe, 2013).
Proteins exit the Golgi at the trans-Golgi network en route to the cell surface or towards the endosomal system (discussed below) (De Matteis and Luini, 2008). In the case of secretory/surface cargo, where export is constitutive, carriers appear to be tubular. In contrast, cargoes subject to regulated secretion are concentrated into specialised granules, which fuse with the surface in a Ca2+-regulated manner (Anantharam and Kreutzberger, 2019). SNARE-mediated fusion of these granules with the cell surface is facilitated by a range of specialised accessory proteins, including members of the synaptotagmin family of Ca2+ sensors.
The endocytic pathway
Surface membrane proteins define the interface between cells and their environment, and cells constantly refine the population of proteins at the surface via rounds of endocytosis and subsequent endosomal sorting (see poster). Endocytosis also brings in soluble proteins, either as ligands to surface receptors or as bulk-flow constituents. The best-characterised uptake pathway is clathrin-mediated endocytosis (McMahon and Boucrot, 2011). Here, clathrin provides the membrane-deforming scaffold, the assembly of which onto the plasma membrane is mediated by cargo-binding adaptor complexes. The best known of these is adaptor protein complex (AP) 2, which is part of a wider family of hetero-tetrameric adaptor complexes, AP1-5. AP2 binds to peptide motifs within the cytoplasmic domains of a range of membrane proteins, while also binding clathrin. Other clathrin adaptors engage client cargoes more selectively. Meanwhile, numerous accessory proteins promote key steps towards vesicle formation, leading ultimately to the recruitment of the scission GTPase, dynamin. Clathrin-coated vesicle formation also relies on local actin dynamics, and on the local generation of phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2] which aids both actin and coat protein recruitment. PtdIns(4,5)P2 phosphatases, notably synaptojanin, complete the vesicle cycle.
Other endocytic mechanisms employ membrane-deforming proteins that selectively engage client membrane cargo while often utilising actin to provide a driving force (Sandvig et al., 2018). Examples include those mediated by flotilin, endophilin and cell division control protein 42 homologue (CDC42). Caveolae, comprised of the membrane protein caveolin and the structural protein cavin, provide a prominent and clinically important example of plasma membrane invagination (Parton, 2018). They primarily appear to function as a reservoir for surface membrane that forms or is dissipated according to alterations in membrane tension, and they are particularly enriched in elastic tissues such as the lung and muscle. Their role may also extend to the sequestration of some signalling pathway components. Caveolae can also undergo endocytosis, although the mechanisms remain poorly defined (Parton, 2018).
Endocytic vesicles fuse to form early endosomes, which are the major sorting stations within the endocytic pathway. The early (or sorting) endosomes are defined by the presence of RAB5 and phosphatidylinositol 3-phosphate (PtdIns3P), which promote the recruitment of numerous effector proteins to the endosomal membrane (Wandinger-Ness and Zerial, 2014). Eventually, endosomes mature as RAB5 is replaced with RAB7 and PtdIns3P is converted to phosphatidylinositol 3,5-bisphosphate [PtdIns(3,5)P2] to generate late endosomes. These fuse with and discharge into lysosomes, leading to the digestion of lumenal content (Wartosch et al., 2015)
To allow the degradation of integral membrane proteins, these must move from the endosomal limiting membrane into the lysosomal lumenal space. Hence, these membrane proteins are incorporated into intralumenal vesicles (ILVs), giving rise to the multivesicular body (MVB). The signal for ILV sorting, K63-linked polyubiquitin, is recognised by a series of endosomal sorting complexes required for transport (ESCRT) complexes and accessory factors (Christ et al., 2017), of which ESCRT-0 and ESCRT-I form the principal ubiquitin receptors. Cargo is passed onwards to ESCRT-III, a membrane-deforming polymer that combines with the AAA ATPase vacuolar protein sorting (VPS) 4 to mediate membrane fission and ILV completion.
Endocytic cargo can escape from the MVB-lysosome pathway by recycling to the cell surface or diverting to the Golgi complex (Cullen and Steinberg, 2018). These pathways involve the formation of tubular or vesicular intermediates that bud away from the endosome. The retriever and retromer complexes are important players in recycling from the sorting endosome that interact with sorting nexin proteins. Recycling to the plasma membrane can occur via a ‘fast’ direct route, or a ‘slow’ indirect route by which cargo is first delivered to the recycling endosome, marked by RAB11, and utilises a distinct set of molecular machineries such as EH domain-containing protein 1 (EHD1) and molecule interacting with CasL protein-like 1 (MICAL-L1), which remain less well characterised than those at the sorting endosome (Goldenring, 2015).
Synaptic vesicles are the mediators of neurotransmitter release at neuronal synapses. Synaptic vesicle biogenesis within the nerve terminal can occur directly from the plasma membrane via endocytosis, or from pre-existing endosomes through selective budding from this compartment (Saheki and De Camilli, 2012), and is therefore highly dependent upon the endocytic trafficking machinery. Fusion of synaptic vesicles with the plasma membrane for neurotransmitter release is tightly regulated, and occurs in a similar way to the regulated exocytosis of secretory granules described above, being mediated by SNAREs and controlled by Ca2+ sensors (Südhof, 2013).
Diseases that are caused by defective membrane traffic
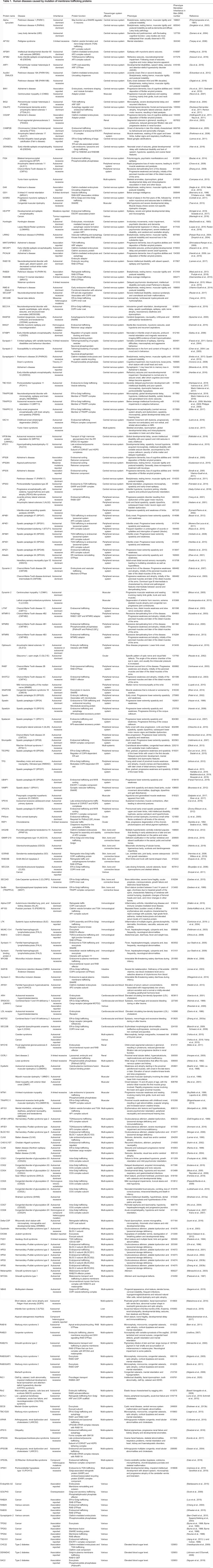
Diseases associated with defective membrane traffic collectively manifest in practically all tissues and organ systems, with some affecting multiple systems and others restricted to one tissue type or organ. Diseases most often arise from mutations that cause loss of expression or function of transport machinery components, but some are caused by toxic gain-of-function mutations. Diseases attributable to defective trafficking machinery can be developmental in nature, or can arise during the lifespan, often manifesting during ageing. Here, we categorise membrane trafficking-related diseases based upon their tissue and organ system involvement (also see poster). The discussion is not exhaustive; for a more comprehensive list of diseases associated with defective trafficking please consult Table 1.
Table 1.
Human diseases caused by mutation of membrane trafficking proteins
Neurological disease
Major neurodegenerative diseases are strongly associated with defects in membrane traffic, particularly within the endosomal system (Schreij et al., 2016). Genetic association studies link variants or altered expression levels of the clathrin-mediated endocytosis components phosphatidylinositol-binding clathrin assembly protein (PICALM) (Harold et al., 2009; Jun et al., 2010), bridging integrator 1 (BIN1)/amphiphysin 2 (Hu et al., 2011; Seshadri et al., 2010), cortactin-CD2-associated protein (CD2AP) (Hollingworth et al., 2011; Naj et al., 2011) and synaptojanin (McMahon and Boucrot, 2011; Miranda et al., 2018), with the risk of acquiring Alzheimer's disease (AD). Additionally, deficiency in VPS26 and VPS35, two subunits of the retromer complex for endosomal recycling, has been observed in AD (Small et al., 2005). In AD, differences in endocytic trafficking and processing of amyloid precursor protein (APP) to its cytotoxic product Aβ can explain the involvement of endocytic traffic in AD pathogenesis (Toh and Gleeson, 2016). Endocytic traffic may also affect AD pathogenesis in other ways; for example, by influencing the susceptibility of neurons to Aβ (which itself can disrupt endocytic traffic), the uptake of toxic Aβ aggregates from the cell exterior, the production of synaptic vesicles or abundance of post-synaptic receptors, or by altering lysosome homeostasis and autophagy pathways that are important for cell viability (Nixon, 2017). Increased processing of APP to Aβ may also arise from altered trafficking at the Golgi apparatus, although the molecular details are less clear (Joshi and Wang, 2015).
Parkinson's disease (PD) is also strongly associated with defective endocytic traffic, including the mutation or altered expression of various endocytic components (Abeliovich and Gitler, 2016). These include cyclin G-associated kinase (GAK) (Nagle et al., 2016; Nalls et al., 2014), auxilin (Edvardson et al., 2012; Olgiati et al., 2016) and synaptojanin (Krebs et al., 2013; Quadri et al., 2013) [which function in clathrin-mediated endocytosis (McMahon and Boucrot, 2011)], the retromer subunit VPS35 (Vilarino-Guell et al., 2011; Zimprich et al., 2011) and the retromer-associated protein receptor-mediated endocytosis 8 (RME-8) (Vilarino-Guell et al., 2014). As in AD, endocytic traffic may lead to PD pathology in several ways; for example, by influencing the uptake of toxic α-synuclein aggregates, by altering synaptic vesicle or neurotransmitter receptor traffic, or by affecting lysosome homeostasis and autophagy (Abeliovich and Gitler, 2016). Of note, mutations of several lysosomal proteins are strongly associated with PD (Dehay et al., 2013), as is defective autophagic clearance of mitochondria (Ryan et al., 2015).
Defective traffic in the early secretory pathway is also relevant for PD pathogenesis. RAB39B, a mutation of which is associated with early-onset PD as well as X-linked intellectual disability (Giannandrea et al., 2010; Mata et al., 2015; Wilson et al., 2014), is required for ER-to-Golgi transport of the synaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic (AMPA) receptor subunit GluA2 (Mignogna et al., 2015). Interestingly, excess α-synuclein can also disrupt ER-to-Golgi traffic, most likely at the level of COPII vesicle tethering or fusion (Cooper et al., 2006; Thayanidhi et al., 2010). α-Synuclein appears to normally function in synaptic vesicle fusion (Burre et al., 2010; Chandra et al., 2005), hence its aggregation or loss of function likely also directly affect neurotransmitter release (Carstea et al., 1997; Polymeropoulos et al., 1997; Singleton et al., 2003). As for AD, PD pathology may arise from defects in other trafficking pathways. A protein of much current interest is leucine-rich repeat kinase 2 (LRRK2), which is mutated in ∼1% of sporadic and ∼5% of familial PD (Paisan-Ruiz et al., 2004; Zimprich et al., 2004). LRRK2 phosphorylates several RAB GTPases (Steger et al., 2016), functioning in diverse trafficking steps, and the most common PD mutations cause LRRK2 activation (West et al., 2005). Excessive LRRK2-mediated phosphorylation alters the ability of these RABs to engage with regulatory factors and effector proteins, thereby disrupting traffic (Steger et al., 2016). Of interest, RAB29 stimulates LRRK2 activation at cellular membranes (Gomez et al., 2019; Purlyte et al., 2018), and is also independently linked to PD, indicating that these proteins (co)operate in a common disease pathway (MacLeod et al., 2013).
Frontotemporal dementia (FTD) is a neurodegenerative disease that is commonly associated with early onset of symptoms (Warren et al., 2013). Mutation of C9orf72, which encodes a RAB GEF, is strongly associated with familial FTD (DeJesus-Hernandez et al., 2011; Renton et al., 2011). Expansion of nucleotide repeats may cause a toxic gain of function at the RNA level, whereas a loss of protein function may also contribute to FTD pathology by altering trafficking to the lysosome, with likely downstream effects upon autophagy (Balendra and Isaacs, 2018). Consistent with this, FTD can be caused by mutation of the ESCRT-III subunit charged multivesicular body protein 2B (CHMP2B), which is involved in MVB sorting (Skibinski et al., 2005).
Amyotrophic lateral sclerosis (ALS), or motor neuron disease, results in progressive degeneration of motor neurons (Hardiman et al., 2017). ALS and FTD represent two extremes of a phenotypic spectrum, and share common pathogenic mechanisms (Ferrari et al., 2011). Thus, C9orf72 mutation causes ALS as well as FTD (DeJesus-Hernandez et al., 2011; Renton et al., 2011). Other endocytic proteins are mutated in ALS, including the RAB5 GEF Alsin (also known as ALS2) and the inositol phosphatase factor-induced gene 4 (FIG4) (Chow et al., 2009; Yang et al., 2001). Dysregulation of endocytic transport, in turn affecting lysosome function and autophagy, is therefore associated with ALS. Of note, mutation of several ALS-associated proteins, including superoxide dismutase 1 (SOD1), RNA-binding protein fused in sarcoma (FUS) and TAR DNA-binding protein 43 (TDP43; also known as TARDBP), have been reported to disrupt the secretory pathway, suggesting additional mechanisms linking defective traffic to ALS (Soo et al., 2015).
Charcot Marie-Tooth (CMT) disease is a genetically and clinically diverse group of peripheral neuropathies (Rossor et al., 2013). Most CMT forms result from altered expression or mutation of myelin components, but mutation of several endocytic proteins is also a cause. For example, recessive demyelinating forms of CMT (CMT4) result from mutation of the endocytic recycling protein SH3 domain and tetratricopeptide repeats 2 (SH3TC2) (Senderek et al., 2003), as well as the myotubularins and FIG4, which influence traffic by acting upon endosomal phosphoinositides (Azzedine et al., 2003; Bolino et al., 2000; Nakhro et al., 2013; Zhang et al., 2008). Lipopolysaccharide-induced tumour necrosis factor alpha factor (LITAF), a protein involved in endocytic protein sorting, causes the autosomal dominant demyelinating CMT1 (also known as SIMPLE) (Street et al., 2003). Meanwhile, mutation of RAB7 causes a dominant axonal form of CMT (Verhoeven et al., 2003).
Similarly, mutation of endocytic factors is associated with hereditary spastic paraplegia (HSP), a genetically diverse disorder that manifests as progressive loss of lower limb movement control (Blackstone et al., 2011). Notable HSP-involved proteins are spastin (SPG4; also known as SPAST), which couples membrane remodelling with microtubule dynamics, including during endocytic traffic (Hazan et al., 1999), spartin (SPG20; also known as SPART), an endosomal protein that also associates with microtubules (Patel et al., 2002), and strumpellin (SPG8; also known as WASHC5), which is part of the WASH WASP and SCAR (WASH) homologue complex that operates in retromer-mediated endocytic recycling (Valdmanis et al., 2007). Interestingly, spastin also functions at the ER, and mutations in Atlastin (also known as ATL1), another ER membrane remodelling protein, also cause HSP (Zhao et al., 2001). It is currently unclear how changes in ER morphology lead to HSP. Another group of HSPs is caused by mutations within subunits of the AP4 and AP5 adaptor complexes that function in post-Golgi trafficking (Bauer et al., 2012; Hardies et al., 2015; Moreno-De-Luca et al., 2011; Slabicki et al., 2010; Verkerk et al., 2009), which likely affect endolysosomal function (Sanger et al., 2019). Interestingly, AP4 is important for trafficking of the autophagy-initiating factor ATG9A, suggesting a link between HSP and dysregulated autophagy (Mattera et al., 2017; Davies et al., 2018). HSP also results from mutations in ubiquitin-associated protein 1 (UBAP1) and VPS37A (Farazi Fard et al., 2019; Zivony-Elboum et al., 2012), components of ESCRT-I required for MVB sorting (Schmidt and Teis, 2012). Mutations in Trk-fused gene (TFG) and tectonin beta-propeller repeat-containing protein 2 (TECPR2), proteins that associate with COPII and help mediate ER-to-Golgi transport, also cause HSP, indicating that defective trafficking in the early secretory pathway can also cause this type of disorder (Beetz et al., 2013; Stadel et al., 2015).
Mutations in the endocytic machinery are prevalent in other rare neurological disorders (Table 1). The consequent defects in endocytosis and endosomal recycling may alter presynaptic vesicle biogenesis or postsynaptic neurotransmitter receptor availability. Meanwhile, defects in the later stages of the endocytic pathway can affect lysosome homeostasis and autophagy, which, if impaired, result in cytotoxic stress. Defective traffic in the secretory pathway is also associated with several neurological diseases. Here, defective transport may alter axon and dendrite morphogenesis, affect the surface levels of neurotransmitter receptors, or induce cytotoxic ER stress due to cargo accumulation in this compartment.
Ocular disease
Eye pathology has been reported in several trafficking-related multi-systemic disorders, including the ciliopathies and the X-linked Lowe syndrome, which are described below. Choroideremia, which is an eye-specific disorder, manifests as degeneration of rod photoreceptors and retinal pigment epithelial cells (Moosajee et al., 2014). It is caused by mutations in RAB escort protein 1 (REP-1), a chaperone required for the prenylation of all RABs, grossly disrupting membrane traffic in the affected cells (Alory and Balch, 2001; Sankila et al., 1992; Seabra et al., 1993). The retinal tissue-restricted nature of choroideremia is likely because a second RAB escort protein, REP-2, compensates for the loss of REP-1 in other cell types, but is not expressed in the retina (Cremers et al., 1994).
Skin, bone and connective tissue disorders
The extracellular matrix, which surrounds cells in our skin, bone and connective tissues, is a major secreted product in the human body. Consequently, matrix-rich tissues appear particularly susceptible to mutations affecting the secretory pathway that disrupt matrix deposition. Mutations in SEC23A, a component of the COPII coat, cause the skeletal disorder cranio-lentico-sutural dysplasia (CLSD) (Boyadjiev et al., 2006). Although COPII is essential for secretion, CLSD is tissue restricted, because most cells also express the functionally analogous SEC23B, sustaining COPII functionality (Khoriaty et al., 2018). Mutations in Sedlin (also known as TRAPPC2), a component of the transport protein particle (TRAPP) complex operating between the ER and Golgi, a RAB GEF and possible vesicle-tethering factor (Barrowman et al., 2010), cause X-linked spondyloepiphyseal dysplasia tarda (SEDT) (Gedeon et al., 1999). Sedlin also regulates SAR1, and both CLSD and SEDT mutations give rise to defective procollagen export from the ER, causing matrix defects and skeletal dysplasia (Boyadjiev et al., 2011; Venditti et al., 2012). Null and hypomorphic mutations in the Golgi vesicle-tethering factor Golgi microtubule-associated protein of 210 kDa [GMAP-210; also known as thyroid hormone receptor interactor 11 (TRIP11)] are responsible for the lethal skeletal dysplasia achondrogenesis type 1A (ACG1A) and the milder odontochondrodysplasia (ODCD), respectively (Smits et al., 2010; Wehrle et al., 2019). In both cases, the major pathogenic mechanism is defective traffic and improper glycosylation of matrix proteins within the Golgi (Smits et al., 2010; Wehrle et al., 2019). GMAP-210 is also important for cargo traffic to the primary cilium (Follit et al., 2008), and the phenotype may therefore partly arise from defective ciliary signalling that is required to maintain chondrocyte differentiation (Wang et al., 2013). Similarly, mutations in the trans-Golgi protein RAB6-interacting golgin (GORAB), which functions in COPI-mediated traffic, cause the skin and bone disorder gerodermia osteodysplastica, likely as a consequence of disrupted matrix protein glycosylation (Hennies et al., 2008; Witkos et al., 2019). This not only affects matrix assembly, but is also important for controlling TGFβ (also known as TGFB1) signalling to prevent cell senescence (Chan et al., 2018). Mutations in Golgi RAB33B cause Smith-McCort syndrome (Dupuis et al., 2013), an osteochondrodysplasia. This is likely due to defects in Golgi traffic and autophagosome formation, both RAB33B-dependent processes (Morgan et al., 2019).
Immunological disease
Membrane traffic is vital for innate and adaptive immunity; for example, in mediating phagocytosis of invading microorganisms, supporting the biosynthesis and signalling of the many receptors found on immune cells, and facilitating the secretion of antibodies, cytokines and other immunomodulatory factors. Consequently, several immunological diseases, including immunodeficiencies and autoimmune disorders, can be attributed to defective membrane trafficking. These include familial haemophagocytic lymphohistiocytosis, an immune disorder caused by mutations in protein unc-13 homologue D (MUNC-13-4; also known as UNC13D) (Feldmann et al., 2003), syntaxin 11 (zur Stadt et al., 2005) or syntaxin binding protein 2 (zur Stadt et al., 2009). These proteins control lytic granule release at the T-cell and natural killer (NK) cell immune synapse and platelet granule exocytosis. As a result, cells with these mutations have a compromised ability to mediate cell killing, leading to hyperactivation of the immune system (Gholam et al., 2011). Another interesting example is leukocyte tyrosine kinase receptor (LTK), an ER-associated tyrosine kinase that controls COPII assembly and ER-to-Golgi traffic (Centonze et al., 2019). Gain-of-function mutations in LTK are associated with the autoimmune disorder systemic lupus erythematosus, and it has been proposed that increased LTK activity, and therefore increased COPII-mediated ER export, allows plasma cells to cope better with the increased production and secretion of autoantibodies, thereby contributing to the autoimmune phenotype seen in lupus (Centonze et al., 2019; Li et al., 2004).
Intestinal disorders
Defective traffic within both the secretory and endocytic pathways can affect enterocyte function and cause intestinal disease. Enterocytes absorb fats from the intestine and package them into chylomicron particles, which form at the ER and are secreted into the bloodstream. Chylomicron retention disease is a rare disorder caused by mutation in SAR1B (Jones et al., 2003), which impairs chylomicron particle export from the ER, reducing their secretion and the availability of fats and fat-soluble vitamins throughout the body (Roy et al., 1987). The disease is restricted to enterocytes, most likely because the paralogue SAR1A fulfils SAR1 function in other cell types. Microvillus inclusion disease also affects enterocytes, with a loss of microvilli from the apical surface and impaired nutrient absorption (Davidson et al., 1978). It is caused by mutations in the actin motor myosin (MYO) 5B, which is required for endosomal recycling to the apical membrane (Muller et al., 2008), or in syntaxin 3, which is required for vesicle fusion at the apical membrane (Wiegerinck et al., 2014).
Liver disease
Membrane trafficking is important in hepatocytes, which secrete a multitude of proteins into the bloodstream. Mutation of SCY1-like pseudokinase 1 (SCYL1) or neuroblastoma-amplified sequence (NBAS), which function in COPI vesicle traffic, can manifest in the liver, but typically also affect other tissues, and are discussed further in the ‘Multi-systemic disorders’ section below.
Cardiovascular disease and blood disorders
Cholesterol is transported in the blood as low-density lipoprotein (LDL) particles. These are internalised, particularly into hepatocytes, by receptor-mediated endocytosis. Defective LDL uptake causes hypercholesterolaemia, which can manifest as atherosclerosis and premature coronary heart disease (Brown and Goldstein, 1986). Mutations in the LDL receptor (LDLR) that abolish LDL binding have been reported, but of more relevance to this article are LDLR mutations that disrupt binding to disabled homologue 2 (DAB2) and autosomal recessive hypercholesterolaemia (ARH; also known as LDLRAP1), adaptor proteins that mediate LDLR uptake by clathrin-dependent endocytosis (Davis et al., 1986; He et al., 2002; Maurer and Cooper, 2006). Similarly, mutation of ARH itself can also cause hypercholesterolaemia (Garcia et al., 2001).
Defects within the secretory pathway can affect red blood cell production and the production of clotting factors. In the former, mutations within the COPII subunit SEC23B cause congenital dyserythropoietic anaemia type II (Bianchi et al., 2009; Schwarz et al., 2009), likely due to perturbation of ER-to-Golgi traffic in erythroblasts that impairs the delivery and glycosylation of proteins required for red blood cell formation (Denecke and Marquardt, 2009). The widespread expression of the paralogue SEC23A likely accounts for the restricted phenotype of SEC23B mutation. The blood clotting disorder combined factor V and VIII deficiency results from mutations in multiple coagulation factor deficiency 2 (MCFD2) and lectin mannose binding 1 (LMAN1) (Nichols et al., 1998; Zhang et al., 2003a). MCFD2 and LMAN1 combine to form a cargo receptor for the ER-to-Golgi transport of blood clotting factors V and VIII and thus are essential for their secretion (Zhang et al., 2005).
Renal disorders
Renal dysfunction occurs in several multi-systemic trafficking disorders, most notably the ciliopathies. Mutations in the actin-associated proteins CD2AP and MYO1E cause focal segmental glomerulosclerosis, which progressively reduces the ability of the glomerulus to filter the blood, ending in renal failure (Kim et al., 2003; Mele et al., 2011). Both proteins participate in endocytosis, which is required to maintain podocyte foot processes and thus effective filtration, but whether defective traffic constitutes a disease mechanism is unclear (Inoue and Ishibe, 2015). The proteins may directly act upon actin within the foot processes (Inoue and Ishibe, 2015), while CD2AP is also a component of the slit diaphragm (Shih et al., 2001). Dent disease and cystinosis are proximal tubulopathies in which the ability of the proximal tubule to re-absorb proteins by endocytosis is disrupted (Ivanova et al., 2015; Piwon et al., 2000; Wang et al., 2000). However, in both diseases, the mutations are not in the trafficking machinery; Dent disease is caused by mutation in the endosomal chloride channel chloride channel protein 5 (ClC-5; also known as CLCN5) (Lloyd et al., 1996), and cystinosis by mutation of a lysosomal cystine transporter (Town et al., 1998).
Muscular disorders
Centronuclear myopathies (CNMs) are a group of muscle disorders that derive their name from centrally located muscle cell nuclei. Mutations in the membrane fission protein dynamin 2 or in BIN1, a BAR domain protein able to sculpt membrane shape, cause congenital CNM (Bitoun et al., 2005; Nicot et al., 2007). These two proteins, which physically interact, participate in endocytosis in most cells (Takei et al., 1999). However, in muscle, they are critical for the formation and maintenance of T-tubules, membrane invaginations that penetrate into muscle cells (Chin et al., 2015; Lee et al., 2002). Thus, disruption of T-tubule morphogenesis and function is a major disease mechanism in CNMs. Mutation of myotubularin 1 (MTM1), a member of the myotubularin family of endosomal inositol phosphatases, causes an X-linked CNM (Buj-Bello et al., 1999; Laporte et al., 2000). MTM1 binds to BIN1 (Royer et al., 2013), and, as seen in congenital CNMs, MTM1 mutation disrupts T-tubules, indicating a likely common disease mechanism (Al-Qusairi et al., 2009; Dowling et al., 2009). Mutation of dysferlin, a Ca2+-binding protein with homology to the membrane fusion regulator synaptotagmin, causes muscular dystrophy (Bashir et al., 1998; Illa et al., 2001; Liu et al., 1998). The likely pathological mechanism is disruption of muscle cell integrity due to a defect in vesicle fusion and repair of the plasma membrane (Bansal et al., 2003; Lek et al., 2012). Mutations in two proteins required for ER-to-Golgi transport, the TRAPP complex subunit TRAPPC11, and the SNARE Golgi SNAP receptor complex member 2 (GOSR2), are also linked to muscular dystrophy (Bögershausen et al., 2013; Tsai et al., 2013). Hypoglycosylation of α-dystroglycan occurs in both cases (Larson et al., 2018). Because α-dystroglycan glycosylation is important for linking the muscle sarcolemma to the extracellular matrix (Barresi and Campbell, 2006), these glycosylation defects can explain the destabilisation of muscle fibres seen in patients.
Multi-systemic disorders
There are numerous multi-systemic disorders associated with mutations in the membrane trafficking machinery (Table 1). Several belong to larger disease classes such as congenital disorders of glycosylation (CDGs), ciliopathies and lysosomal storage disorders (LSDs). Many CDGs are caused by loss of Golgi glycosylation enzyme or ion or sugar transporter activity, but mutations within the COG vesicle-tethering complex account for several (Ng and Freeze, 2018). Here, impaired COPI-dependent recycling of glycosylation enzymes in the Golgi stack leads to their inefficient retention, affecting the glycosylation of proteins and lipids (Fisher and Ungar, 2016).
The ciliopathies are a large disease class associated with loss of cilia or defective ciliary signalling (Reiter and Leroux, 2017). The commonly affected tissues include the brain, retina and kidney. Several ciliopathies are associated with defective transport of proteins to or within the cilium, although the latter is not vesicle mediated (Reiter and Leroux, 2017). Vesicle-mediated transport from the Golgi apparatus to the cilium is important for the generation and maintenance of cilia, with RAB8 and its effector, the exocyst vesicle-tethering complex, constituting the key machinery of this trafficking step (Hsiao et al., 2012). Indeed, mutations in two exocyst subunits, exocyst complex component 84 (EXO84; also known as EXOC8) and SEC8 (also known as EXOC4), have been found in the ciliopathies Joubert syndrome and Meckel–Gruber syndrome (Dixon-Salazar et al., 2012; Shaheen et al., 2013a,b). Intraflagellar transport protein 20 (IFT20) is an important player in Golgi-to-cilium transport of certain membrane proteins (Follit et al., 2008; Monis et al., 2017), and mutation of VPS15, which also causes a ciliopathy, impairs this transport pathway (Stoetzel et al., 2016). Interestingly, IFT20 is anchored to the Golgi by GMAP-210 (Follit et al., 2008), suggesting that the two skeletal dysplasias caused by GMAP-210 mutation (ACG1A and ODCD, discussed above) may have a ciliary component (Smits et al., 2010; Wehrle et al., 2019).
LSDs are a third broad class of disease, defined by impaired lysosome-mediated degradation (Platt et al., 2018). Many LSDs result from the loss of hydrolase expression, but some involve defective hydrolase trafficking. For example, ceroid-lipofuscinosis, neuronal 8 (CLN8) is a cargo receptor for trafficking of newly synthesised hydrolases from the ER to the Golgi (di Ronza et al., 2018), and mutations in CLN8 cause the LSD Batten disease (Ranta et al., 1999). Mutation of VPS33A, a common component of the class C core vacuole/endosome tethering (CORVET) and homotypic fusion and protein sorting (HOPS) multi-subunit vesicle-tethering complexes that operate at the early and late endosome/lysosome, respectively, causes the LSD mucopolysaccharidosis (Kondo et al., 2017).
Lysosome-related organelles (LROs) are found in specific cell types and carry out specialised functions (Marks et al., 2013). Examples include melanosomes in skin melanocytes and retinal pigment epithelial cells, which are important for pigmentation, lytic granules of NK and T-cells that mediate target cell killing, and Weibel–Palade bodies in endothelial cells that contribute to blood clotting. Chediak–Higashi, Griscelli and Hermansky–Pudlak syndromes are all associated with defective LRO biogenesis, and in many cases are due to defects in the relevant LRO trafficking machinery (Huizing et al., 2008). For example, Griscelli syndrome, characterised by hypopigmentation and immunodeficiency, can be caused by mutations in RAB27A, its effector melanophilin, or the actin motor MYO5A, which together facilitate melanosome movement to the cell periphery for delivery of pigment to neighbouring keratinocytes (Ménasché et al., 2003; Ménasché et al., 2000; Pastural et al., 1997). Hermansky–Pudlak syndrome, which presents as hypopigmentation, bleeding and additional symptoms depending on the subtype, is caused by mutations in subunits of the biogenesis of lysosome-related organelle complex (BLOC)-1 (Li et al., 2003; Morgan et al., 2006), BLOC-2 (Anikster et al., 2001; Zhang et al., 2003b), BLOC-3 (Oh et al., 1996; Suzuki et al., 2002) or AP3 (Ammann et al., 2016; Dell'Angelica et al., 1999) complexes that are involved in transport of cargo proteins from endosomes to LROs. Chediak–Higashi syndrome, which manifests as albinism, excessive bleeding and immunodeficiency, is caused by mutations in lysosomal trafficking regulator (LYST) (Karim et al., 2002), which appears to function in endolysosomal trafficking (Gil-Krzewska et al., 2016).
Lysosome dysfunction has also been reported in the rare X-linked disorder Lowe syndrome, which affects the brain, eyes and kidneys, and is caused by mutation of the inositol phosphatase occulocerebrorenal Lowe syndrome protein (OCRL) (Attree et al., 1992). The aetiology of Lowe is complex, since build-up of the OCRL substrate PtdIns(4,5)P2 disrupts not only lysosomal function, which results in an additional autophagy defect, but also affects endocytosis, endocytic recycling and trafficking to the cilium (De Matteis et al., 2017). Hence, disruption of several trafficking steps is likely to cause the Lowe syndrome phenotypes seen in patients. Interestingly, mutations in OCRL also cause Dent-2 disease, for which the symptoms are largely restricted to the kidney (Hoopes et al., 2005). The reasons for this dual pathophenotype remain unclear.
Defective COPI-dependent recycling from the Golgi apparatus to the ER is associated with two multi-systemic genetic disorders, both affecting the liver. Mutation of the COPI accessory protein SCYL1 causes low γ-glutamyl-transferase cholestasis, acute liver failure, and neurodegeneration (CALFAN) syndrome, manifesting as hepatocyte death and liver failure, as well as ataxia resulting from cerebellar neurodegeneration (Lenz et al., 2018; Schmidt et al., 2015). Mutation of NBAS, a component of the NBAS/RINT1/ZW10 (NRZ) ER-localised COPI vesicle-tethering complex results in a nearly identical liver phenotype, and also causes bone, connective tissue, retina and immune system defects (Balasubramanian et al., 2017; Haack et al., 2015; Maksimova et al., 2010; Segarra et al., 2015). These findings suggest a high requirement for the secretory pathway in the affected cell types, including hepatocytes, consistent with them secreting large amounts of material into the bloodstream.
Cancer
Membrane trafficking is intimately linked with cancer, with trafficking in both the secretory and endocytic pathways playing an important role in many types of cancer. Endocytic trafficking is responsible for the abundance and signalling capacity of mitogenic receptors, adhesion molecules and immune modulators that determine the ability of the immune system to detect cancer cells (Mellman and Yarden, 2013). Hence, changes in the expression levels or degree of phosphorylation of endocytic trafficking machinery can correlate with cancer susceptibility or prognosis. In addition, cancer-causing mutations within the components of the endocytic machinery have been described. A recent example is RAB35, which mediates various endocytic trafficking steps (Klinkert and Echard, 2016). Oncogenic mutations in RAB35, although extremely rare, have been shown to cause its constitutive activation and promiscuous growth factor signalling from endosomal compartments (Wheeler et al., 2015). Altered expression and splicing of tumour susceptibility gene 101 (TSG101), has been found in cancer (Jiang et al., 2013), whereby impaired growth factor receptor downregulation at the endosome may contribute to tumourigenesis (Lu et al., 2003). Interestingly, toxic gain-of-function mutation of p53 can also promote the recycling of integrins and growth factor receptors, which is responsible for increased cell migration and metastatic potential of tumour cells (Muller et al., 2009).
The secretory pathway can influence cancer susceptibility and disease progression in a number of ways (Dejeans et al., 2014). We know that cell surface glycans, which are generated within the secretory pathway, are important for processes contributing to cancer development and metastasis, including signalling, adhesion and migration (Pinho and Reis, 2015). A particularly interesting example of an oncogenic trafficking protein is Golgi phosphoprotein 3 (GOLPH3), which is highly expressed in several cancers (Scott et al., 2009). GOLPH3 appears to participate in intra-Golgi transport, which is required for Golgi enzyme retention and correct protein glycosylation (Ali et al., 2012; Chang et al., 2013; Isaji et al., 2014; Pereira et al., 2014), as well as export of cargo from the trans-Golgi (Rahajeng et al., 2019). GOLPH3 overexpression stimulates a number of mitogenic signalling pathways, which may be a consequence of altering the cell surface glycan profile and thus the signalling capacity of surface receptors (Rizzo et al., 2017). In addition, GOLPH3 has been implicated in a DNA stress response pathway, linking DNA damage to the Golgi apparatus (Farber-Katz et al., 2014). In this context, GOLPH3 overexpression can promote cell survival upon DNA damage, which may be relevant to the cancer phenotype. Another interesting example is mutation of the ER-to-Golgi trafficking protein LMAN1 in colorectal cancers, which causes reduced secretion of the LMAN1 client protein α-1-antitrypsin (A1AT; also known as SERPINA1), an angiogenesis inhibitor, thereby contributing to tumour blood supply and growth (Roeckel et al., 2009).
Diabetes
Exocytosis of insulin from pancreatic beta cells, and the endocytic and secretory trafficking of insulin receptors and glucose transporters in target cells, may all directly affect diabetes susceptibility or progression. For example, the inositol phosphatase suppressor of actin 2 (SAC2; also known as INPP5F) functions in insulin granule exocytosis from pancreatic beta cells, and its levels are reduced in type II diabetic patients, suggesting that SAC2 insufficiency might contribute to impaired insulin release in these patients (Nguyen et al., 2019). Another protein of interest is clathrin heavy chain 22 (CHC22), which is involved in the trafficking of glucose transporter type 4 (GLUT4; also known as SLC2A4) in muscle and fat cells, where it mediates glucose uptake in response to insulin signalling (Vassilopoulos et al., 2009). Two CHC22 variants exist in the human population, which differ in their ability to traffic GLUT4 and thus remove glucose from the bloodstream (Fumagalli et al., 2019). The ‘new’ variant, which appeared later in evolution, increases cell surface levels of GLUT4 and glucose removal from the bloodstream, whereas the ‘older’ variant has a lower capacity to traffic GLUT4 to the cell surface and therefore to clear blood glucose. However, it remains to be seen whether people carrying the ‘older’ variant have a greater diabetes risk.
Summary and conclusions
Membrane trafficking is a ubiquitous process and fundamentally important to all tissues. However, defects in components of the trafficking machinery often manifest as a tissue-specific phenotype. The nature of the observed defect depends upon the tissue expression of the trafficking component in question and its degree of functional redundancy, the rate-limiting trafficking steps within different cell types, and the abundance and types of cargo proteins expressed in different cells. The nature of the mutation itself is also important, as it can result in either a complete loss of expression or function of the trafficking component, a partial loss of expression or function, or, in some cases, a toxic overexpression or gain of function. This is expected to cause corresponding changes in the associated trafficking pathway(s), resulting in the observed phenotype. With regard to the tissue-specific nature of the diseases, it is interesting that the nervous system is particularly sensitive to disruption of the endolysosomal system, possibly due to the importance of endocytic traffic to maintain neurotransmission as well as the sensitivity of neurons to disrupted lysosome function and autophagy. Skin, bone and connective tissues are more sensitive to defective secretory traffic, reflecting the high secretory load in these tissues. Despite these generalisations, it is often hard to predict the phenotype one might expect upon mutation of a particular trafficking component, and understanding the disease mechanisms underlying most trafficking-related disorders is not trivial.
Defective traffic can manifest in a particular phenotype for several reasons. In some cases, it may be the failure to deliver a cargo protein to the correct destination compartment, causing dysfunction of that organelle, or the impaired ability of cells to secrete or internalise cargo effectively, resulting in systemic effects. In other cases, the inability to traffic proteins from their donor compartments may be problematic, as in the case of ER stress induction when proteins fail to exit this compartment. Similarly, the inability to degrade substrates by autophagy is cytotoxic. It is also worth noting that although impaired traffic can cause disease, in some contexts, trafficking might be required to sustain a disease phenotype. This appears to be true in cancer, where endocytic traffic is required to sustain proliferative signalling and cell migration, important for tumour growth and metastasis. Thus, in terms of developing therapeutics for trafficking disorders, a range of strategies is possible (Box 2). Gene therapy is one possible route, but drugs can potentially rescue defective organelle function. Therapeutic strategies could alleviate the cell stress that occurs downstream from organelle dysfunction, restore the disrupted trafficking step, or, in some cases, inhibit a transport step that is driving the disease phenotype. As we identify more rare diseases attributable to defects in membrane traffic, and better understand the mechanisms that underlie these and other more common disorders, we will undoubtedly be able to deliver better treatments and long-term therapies in the future.
Box 2. Therapeutic approaches to rescue traffic-dependent phenotypes.
We lack effective therapies to treat most of the diseases associated with defective membrane trafficking. In principle, diseases caused by genetic mutation could be treated with gene therapy, but using this approach to successfully treat human disease remains in its infancy (Dunbar et al., 2018). A promising example is gene therapy for retinal dystrophy choroideremia, which is caused by loss of the RAB escort protein REP-1 (Sankila et al., 1992; Seabra et al., 1993). Here, CHM, the gene that encodes REP-1, is administered to the eye via a viral delivery vector. The therapy is currently undergoing phase 3 clinical trials following promising results in earlier stages of clinical testing (Xue et al., 2018). Many trafficking regulators, which are enzymes, are potentially amenable to treatment with small-molecule drugs. This approach remains to be explored more fully, but there is significant interest in targeting the protein kinase LRRK2 for treatment of Parkinson's disease (Zhao and Dzamko, 2019). Pathogenic LRRK2 mutations lead to overactive kinase activity and so chemically inhibiting this activity could protect against Parkinson's disease. As such, clinical trials are underway to test the safety and efficacy of LRRK2 inhibitors in human patients.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
R.Y. is supported by a Wellcome Trust PhD studentship (203995/Z/16/Z) and J.H. by a joint University of Manchester and Agency for Science, Technology and Research (Singapore A*STAR) PhD studentship. Research in the P.G.W. laboratory is supported by grants from the Wellcome Trust (212246/Z/18/Z), the Biotechnology and Biological Sciences Research Council (BBSRC) (BB/R015864/1) and the Leverhulme Trust (RPG-2018-091). Research in the M.L. laboratory is supported by grants from the BBSRC (BB/S014799/1, BB/T000945/1), the Lowe Syndrome Trust (ML/MU/LST NOV/18) and the Leverhulme Trust (RPG-2019-134).
At a glance
A high-resolution version of the poster is available for downloading in the online version of this article at http://dmm.biologists.org/content/13/4/dmm043448/F1.poster.jpg.
References
- Abeliovich A. and Gitler A. D. (2016). Defects in trafficking bridge Parkinson's disease pathology and genetics. Nature 539, 207-216. 10.1038/nature20414 [DOI] [PubMed] [Google Scholar]
- Alazami A. M., Hijazi H., Kentab A. Y. and Alkuraya F. S (2014). NECAP1 loss of function leads to a severe infantile epileptic encephalopathy. J. Med. Genet. 51, 224-228. 10.1136/jmedgenet-2013-102030 [DOI] [PubMed] [Google Scholar]
- Ali M. F., Chachadi V. B., Petrosyan A. and Cheng P. W. (2012). Golgi phosphoprotein 3 determines cell binding properties under dynamic flow by controlling Golgi localization of core 2 N-acetylglucosaminyltransferase 1. J. Biol. Chem. 287, 39564-39577. 10.1074/jbc.M112.346528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aligianis I. A., Johnson C. A., Gissen P., Chen D. R., Hampshire D., Hoffmann K., Maina E. N., Morgan N. V., Tee L., Morton J. et al. (2005). Mutations of the catalytic subunit of RAB3GAP cause Warburg Micro syndrome. Nat. Genet. 37, 221-223. 10.1038/ng1517 [DOI] [PubMed] [Google Scholar]
- Aligianis I. A., Morgan N. V., Mione M., Johnson C. A., Rosser E., Hennekam R. C., Adams G., Trembath R. C., Pilz D. T., Stoodley N. et al. (2006). Mutation in Rab3 GTPase-activating protein (RAB3GAP) noncatalytic subunit in a kindred with Martsolf syndrome. Am. J. Hum. Genet. 78, 702-707. 10.1086/502681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allfrey V., Daly M. M. and Mirsky A. E. (1953). Synthesis of protein in the pancreas. II. The role of ribonucleoprotein in protein synthesis. J. Gen. Physiol. 37, 157-175. 10.1085/jgp.37.2.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alory C. and Balch W. E. (2001). Organization of the Rab-GDI/CHM superfamily: the functional basis for choroideremia disease. Traffic 2, 532-543. 10.1034/j.1600-0854.2001.20803.x [DOI] [PubMed] [Google Scholar]
- Al-Qusairi L., Weiss N., Toussaint A., Berbey C., Messaddeq N., Kretz C., Sanoudou D., Beggs A. H., Allard B., Mandel J.-L. et al. (2009). T-tubule disorganization and defective excitation-contraction coupling in muscle fibers lacking myotubularin lipid phosphatase. Proc. Natl. Acad. Sci. USA 106, 18763-18768. 10.1073/pnas.0900705106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammann S., Schulz A., Krageloh-Mann I., Dieckmann N. M., Niethammer K., Fuchs S., Eckl K. M., Plank R., Werner R., Altmuller J. et al. (2016). Mutations in AP3D1 associated with immunodeficiency and seizures define a new type of Hermansky-Pudlak syndrome. Blood 127, 997-1006. 10.1182/blood-2015-09-671636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantharam A. and Kreutzberger A. J. B. (2019). Unraveling the mechanisms of calcium-dependent secretion. J. Gen. Physiol. 151, 417-434. 10.1085/jgp.201812298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anikster Y., Huizing M., White J., Shevchenko Y. O., Fitzpatrick D. L., Touchman J. W., Compton J. G., Bale S. J., Swank R. T., Gahl W. A. et al. (2001). Mutation of a new gene causes a unique form of Hermansky-Pudlak syndrome in a genetic isolate of central Puerto Rico. Nat. Genet. 28, 376-380. 10.1038/ng576 [DOI] [PubMed] [Google Scholar]
- Assoum M., Philippe C., Isidor B., Perrin L., Makrythanasis P., Sondheimer N., Paris C., Douglas J., Lesca G., Antonarakis S. et al. (2016). Autosomal-recessive mutations in AP3B2, adaptor-related protein complex 3 Beta 2 subunit, cause an early-onset epileptic encephalopathy with optic atrophy. Am. J. Hum. Genet. 99, 1368-1376. 10.1016/j.ajhg.2016.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attree O., Olivos I. M., Okabe I., Bailey L. C., Nelson D. L., Lewis R. A., McInnes R. R. and Nussbaum R. L. (1992). The Lowe's oculocerebrorenal syndrome gene encodes a protein highly homologous to inositol polyphosphate-5-phosphatase. Nature 358, 239-242. 10.1038/358239a0 [DOI] [PubMed] [Google Scholar]
- Avior Y., Sagi I. and Benvenisty N. (2016). Pluripotent stem cells in disease modelling and drug discovery. Nat. Rev. Mol. Cell Biol. 17, 170-182. 10.1038/nrm.2015.27 [DOI] [PubMed] [Google Scholar]
- Azzedine H., Bolino A., Taieb T., Birouk N., Di Duca M., Bouhouche A., Benamou S., Mrabet A., Hammadouche T., Chkili T. et al. (2003). Mutations in MTMR13, a new pseudophosphatase homologue of MTMR2 and Sbf1, in two families with an autosomal recessive demyelinating form of Charcot-Marie-Tooth disease associated with early-onset glaucoma. Am. J. Hum. Genet. 72, 1141-1153. 10.1086/375034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian M., Hurst J., Brown S., Bishop N. J., Arundel P., DeVile C., Pollitt R. C., Crooks L., Longman D., Caceres J. F. et al. (2017). Compound heterozygous variants in NBAS as a cause of atypical osteogenesis imperfecta. Bone 94, 65-74. 10.1016/j.bone.2016.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E., Dunphy W. G., Braell W. A. and Rothman J. E. (1984). Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Cell 39, 405-416. 10.1016/0092-8674(84)90019-9 [DOI] [PubMed] [Google Scholar]
- Baldassarre T., Watt K., Truesdell P., Meens J., Schneider M. M., Sengupta S. K. and Craig A. W (2015). Endophilin A2 promotes TNBC cell invasion and tumor metastasis. Mol. Cancer Res. 13, 1044-1055. 10.1158/1541-7786.MCR-14-0573 [DOI] [PubMed] [Google Scholar]
- Balendra R. and Isaacs A. M. (2018). C9orf72-mediated ALS and FTD: multiple pathways to disease. Nat. Rev. Neurol. 14, 544-558. 10.1038/s41582-018-0047-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal D., Miyake K., Vogel S. S., Groh S., Chen C. C., Williamson R., McNeil P. L. and Campbell K. P. (2003). Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature 423, 168-172. 10.1038/nature01573 [DOI] [PubMed] [Google Scholar]
- Barresi R. and Campbell K. P. (2006). Dystroglycan: from biosynthesis to pathogenesis of human disease. J. Cell Sci. 119, 199-207. 10.1242/jcs.02814 [DOI] [PubMed] [Google Scholar]
- Barrowman J., Bhandari D., Reinisch K. and Ferro-Novick S. (2010). TRAPP complexes in membrane traffic: convergence through a common Rab. Nat. Rev. Mol. Cell Biol. 11, 759-763. 10.1038/nrm2999 [DOI] [PubMed] [Google Scholar]
- Basel-Vanagaite L., Sarig O., Hershkovitz D., Fuchs-Telem D., Rapaport D., Gat A., Isman G., Shirazi I., Shohat M., Enk C. D. et al. (2009). RIN2 deficiency results in macrocephaly, alopecia, cutis laxa, and scoliosis: MACS syndrome. Am. J. Hum. Genet. 85, 254-263. 10.1016/j.ajhg.2009.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir R., Britton S., Strachan T., Keers S., Vafiadaki E., Lako M., Richard I., Marchand S., Bourg N., Argov Z. et al. (1998). A gene related to Caenorhabditis elegans spermatogenesis factor fer-1 is mutated in limb-girdle muscular dystrophy type 2B. Nat. Genet. 20, 37-42. 10.1038/1689 [DOI] [PubMed] [Google Scholar]
- Bauer P., Leshinsky-Silver E., Blumkin L., Schlipf N., Schröder C., Schicks J., Lev D., Riess O., Lerman-Sagie T. and Schöls L. (2012). Mutation in the AP4B1 gene cause hereditary spastic paraplegia type 47 (SPG47). Neurogenetics 13, 73-76. 10.1007/s10048-012-0314-0 [DOI] [PubMed] [Google Scholar]
- Baulac S., Lenk G. M., Dufresnois B., Ouled Amar Bencheikh B., Couarch P., Renard J., Larson P. A., Ferguson C. J., Noe E., Poirier K. et al. (2014). Role of the phosphoinositide phosphatase FIG4 gene in familial epilepsy with polymicrogyria. Neurology 82, 1068-1075. 10.1212/WNL.0000000000000241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck R., Rawet M., Wieland F. T. and Cassel D. (2009). The COPI system: molecular mechanisms and function. FEBS Lett. 583, 2701-2709. 10.1016/j.febslet.2009.07.032 [DOI] [PubMed] [Google Scholar]
- Beetz C., Johnson A., Schuh A. L., Thakur S., Varga R. E., Fothergill T., Hertel N., Bomba-Warczak E., Thiele H., Nurnberg G. et al. (2013). Inhibition of TFG function causes hereditary axon degeneration by impairing endoplasmic reticulum structure. Proc. Natl. Acad. Sci. USA 110, 5091-5096. 10.1073/pnas.1217197110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bem D., Yoshimura S.-I., Nunes-Bastos R., Bond F. F., Kurian M. A., Rahman F., Handley M. T. W., Hadzhiev Y., Masood I., Straatman-Iwanowska A. A. et al. (2011). Loss-of-function mutations in RAB18 cause Warburg Micro syndrome. Am. J. Hum. Genet. 88, 499-507. 10.1016/j.ajhg.2011.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Chetrit N., Chetrit D., Russell R., Körner C., Mancini M., Abdul-Hai A., Itkin T., Carvalho S., Cohen-Dvashi H., Koestler W. J. et al. (2015). Synaptojanin 2 is a druggable mediator of metastasis and the gene is overexpressed and amplified in breast cancer. Sci. Signal. 8, ra7 10.1126/scisignal.2005537 [DOI] [PubMed] [Google Scholar]
- Bianchi P., Fermo E., Vercellati C., Boschetti C., Barcellini W., Iurlo A., Marcello A. P., Righetti P. G. and Zanella A. (2009). Congenital dyserythropoietic anemia type II (CDAII) is caused by mutations in the SEC23B gene. Hum. Mutat. 30, 1292-1298. 10.1002/humu.21077 [DOI] [PubMed] [Google Scholar]
- Bienvenu T., des Portes V., Saint Martin A., McDonell N., Billuart P., Carrie A., Vinet M. C., Couvert P., Toniolo D., Ropers H. H. et al. (1998). Non-specific X-linked semidominant mental retardation by mutations in a Rab GDP-dissociation inhibitor. Hum. Mol. Genet. 7, 1311-1315. 10.1093/hmg/7.8.1311 [DOI] [PubMed] [Google Scholar]
- Bitoun M., Maugenre S., Jeannet P. Y., Lacene E., Ferrer X., Laforet P., Martin J. J., Laporte J., Lochmuller H., Beggs A. H. et al. (2005). Mutations in dynamin 2 cause dominant centronuclear myopathy. Nat. Genet. 37, 1207-1209. 10.1038/ng1657 [DOI] [PubMed] [Google Scholar]
- Blackstone C., O'Kane C. J. and Reid E. (2011). Hereditary spastic paraplegias: membrane traffic and the motor pathway. Nat. Rev. Neurosci. 12, 31-42. 10.1038/nrn2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolino A., Muglia M., Conforti F. L., LeGuern E., Salih M. A., Georgiou D. M., Christodoulou K., Hausmanowa-Petrusewicz I., Mandich P., Schenone A. et al. (2000). Charcot-Marie-Tooth type 4B is caused by mutations in the gene encoding myotubularin-related protein-2. Nat. Genet. 25, 17-19. 10.1038/75542 [DOI] [PubMed] [Google Scholar]
- Boncompain G., Divoux S., Gareil N., de Forges H., Lescure A., Latreche L., Mercanti V., Jollivet F., Raposo G. and Perez F. (2012). Synchronization of secretory protein traffic in populations of cells. Nat. Methods 9, 493-498. 10.1038/nmeth.1928 [DOI] [PubMed] [Google Scholar]
- Bögershausen N., Shahrzad N., Chong J. X., von Kleist-Retzow J.-C., Stanga D., Li Y., Bernier F. P., Loucks C. M., Wirth R., Puffenberger E. G. et al. (2013). Recessive TRAPPC11 mutations cause a disease spectrum of limb girdle muscular dystrophy and myopathy with movement disorder and intellectual disability. Am. J. Hum. Genet. 93, 181-190. 10.1016/j.ajhg.2013.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borck G., Wunram H., Steiert A., Volk A. E., Korber F., Roters S., Herkenrath P., Wollnik B., Morris-Rosendahl D. J. and Kubisch C (2011). A homozygous RAB3GAP2 mutation causes Warburg Micro syndrome. Hum. Genet. 129, 45-50. 10.1007/s00439-010-0896-2 [DOI] [PubMed] [Google Scholar]
- Bourassa C. V., Meijer I. A., Merner N. D., Grewal K. K., Stefanelli M. G., Hodgkinson K., Ives E. J., Pryse-Phillips W., Jog M., Boycott K. et al. (2012). VAMP1 mutation causes dominant hereditary spastic ataxia in Newfoundland families. Am. J. Hum. Genet. 91, 548-552. 10.1016/j.ajhg.2012.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyadjiev S. A., Fromme J. C., Ben J., Chong S. S., Nauta C., Hur D. J., Zhang G., Hamamoto S., Schekman R., Ravazzola M. et al. (2006). Cranio-lenticulo-sutural dysplasia is caused by a SEC23A mutation leading to abnormal endoplasmic-reticulum-to-Golgi trafficking. Nat. Genet. 38, 1192-1197. 10.1038/ng1876 [DOI] [PubMed] [Google Scholar]
- Boyadjiev S. A., Kim S. D., Hata A., Haldeman-Englert C., Zackai E. H., Naydenov C., Hamamoto S., Schekman R. W. and Kim J. (2011). Cranio-lenticulo-sutural dysplasia associated with defects in collagen secretion. Clin. Genet. 80, 169-176. 10.1111/j.1399-0004.2010.01550.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandizzi F. and Barlowe C. (2013). Organization of the ER-Golgi interface for membrane traffic control. Nat. Rev. Mol. Cell Biol. 14, 382-392. 10.1038/nrm3588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branon T. C., Bosch J. A., Sanchez A. D., Udeshi N. D., Svinkina T., Carr S. A., Feldman J. L., Perrimon N. and Ting A. Y. (2018). Efficient proximity labeling in living cells and organisms with TurboID. Nat. Biotechnol. 36, 880-887. 10.1038/nbt.4201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S. and Goldstein J. L. (1986). A receptor-mediated pathway for cholesterol homeostasis. Science 232, 34-47. 10.1126/science.3513311 [DOI] [PubMed] [Google Scholar]
- Buj-Bello A., Biancalana V., Moutou C., Laporte J. and Mandel J. L. (1999). Identification of novel mutations in the MTM1 gene causing severe and mild forms of X-linked myotubular myopathy. Hum. Mutat. 14, 320-325. [DOI] [PubMed] [Google Scholar]
- Burre J., Sharma M., Tsetsenis T., Buchman V., Etherton M. R. and Südhof T. C. (2010). Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 329, 1663-1667. 10.1126/science.1195227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne J. A., Tomasetto C., Garnier J. M., Rouyer N., Mattei M. G., Bellocq J. P., Rio M. C. and Basset P (1995). A screening method to identify genes commonly overexpressed in carcinomas and the identification of a novel complementary DNA sequence. Cancer Res. 55, 2896-2903. [PubMed] [Google Scholar]
- Byrne J. A., Mattei M.-G. and Basset P (1996). Definition of the tumor protein D52 (TPD52) gene family through cloning of D52 homologues in human (hD53) and mouse (mD52). Genomics 35, 523-532. 10.1006/geno.1996.0393 [DOI] [PubMed] [Google Scholar]
- Byrne J. A., Nourse C. R., Basset P. and Gunning P (1998). Identification of homo- and heteromeric interactions between members of the breast carcinoma-associated D52 protein family using the yeast two-hybrid system. Oncogene 16, 873-881. 10.1038/sj.onc.1201604 [DOI] [PubMed] [Google Scholar]
- Cai X., Chen X., Wu S., Liu W., Zhang X., Zhang D., He S., Wang B., Zhang M., Zhang Y. et al. (2016). Homozygous mutation of VPS16 gene is responsible for an autosomal recessive adolescent-onset primary dystonia. Sci. Rep. 6, 25834 10.1038/srep25834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau P. M., Lenk G. M., Lu J. T., Bae Y., Burrage L., Turnpenny P., Roman Corona-Rivera J., Morandi L., Mora M., Reutter H. et al. (2013). Yunis-Varon syndrome is caused by mutations in FIG4, encoding a phosphoinositide phosphatase. Am. J. Hum. Genet. 92, 781-791. 10.1016/j.ajhg.2013.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstea E. D., Morris J. A., Coleman K. G., Loftus S. K., Zhang D., Cummings C., Gu J., Rosenfeld M. A., Pavan W. J., Krizman D. B. et al. (1997). Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science 277, 228-231. 10.1126/science.277.5323.228 [DOI] [PubMed] [Google Scholar]
- Centonze F. G., Reiterer V., Nalbach K., Saito K., Pawlowski K., Behrends C. and Farhan H. (2019). LTK is an ER-resident receptor tyrosine kinase that regulates secretion. J. Cell Biol. 218, 2470-2480. 10.1083/jcb.201903068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan W. L., Steiner M., Witkos T., Egerer J., Busse B., Mizumoto S., Pestka J. M., Zhang H., Hausser I., Khayal L. A. et al. (2018). Impaired proteoglycan glycosylation, elevated TGF-β signaling, and abnormal osteoblast differentiation as the basis for bone fragility in a mouse model for gerodermia osteodysplastica. PLoS Genet. 14, e1007242 10.1371/journal.pgen.1007242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S., Gallardo G., Fernández-Chacón R., Schlüter O. M. and Südhof T. C. (2005). Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell 123, 383-396. 10.1016/j.cell.2005.09.028 [DOI] [PubMed] [Google Scholar]
- Chang W.-L., Chang C.-W., Chang Y.-Y., Sung H.-H., Lin M.-D., Chang S.-C., Chen C.-H., Huang C.-W., Tung K.-S. and Chou T.-B. (2013). The Drosophila GOLPH3 homolog regulates the biosynthesis of heparan sulfate proteoglycans by modulating the retrograde trafficking of exostosins. Development 140, 2798-2807. 10.1242/dev.087171 [DOI] [PubMed] [Google Scholar]
- Chen Q., He G., Qin W., Chen Q.-Y., Zhao X.-Z., Duan S.-W., Liu X.-M., Feng G.-Y., Xu Y.-F., St Clair D. et al. (2004). Family-based association study of synapsin II and schizophrenia. Am. J. Hum. Genet. 75, 873-877. 10.1086/425588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Gibson E. S. and Kennedy M. J. (2013). A light-triggered protein secretion system. J. Cell Biol. 201, 631-640. 10.1083/jcb.201210119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. W., Lahad J. P., Kuo W.-L., Lapuk A., Yamada K., Auersperg N., Liu J., Smith-McCune K., Lu K. H., Fishman D. et al. (2004). The RAB25 small GTPase determines aggressiveness of ovarian and breast cancers. Nat. Med. 10, 1251-1256. 10.1038/nm1125 [DOI] [PubMed] [Google Scholar]
- Chin Y. H., Lee A., Kan H. W., Laiman J., Chuang M. C., Hsieh S. T. and Liu Y. W. (2015). Dynamin-2 mutations associated with centronuclear myopathy are hypermorphic and lead to T-tubule fragmentation. Hum. Mol. Genet. 24, 5542-5554. 10.1093/hmg/ddv285 [DOI] [PubMed] [Google Scholar]
- Chow C. Y., Landers J. E., Bergren S. K., Sapp P. C., Grant A. E., Jones J. M., Everett L., Lenk G. M., McKenna-Yasek D. M., Weisman L. S. et al. (2009). Deleterious variants of FIG4, a phosphoinositide phosphatase, in patients with ALS. Am. J. Hum. Genet. 84, 85-88. 10.1016/j.ajhg.2008.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ L., Raiborg C., Wenzel E. M., Campsteijn C. and Stenmark H. (2017). Cellular functions and molecular mechanisms of the ESCRT membrane-scission machinery. Trends Biochem. Sci. 42, 42-56. 10.1016/j.tibs.2016.08.016 [DOI] [PubMed] [Google Scholar]
- Cooper A. A., Gitler A. D., Cashikar A., Haynes C. M., Hill K. J., Bhullar B., Liu K., Xu K., Strathearn K. E., Liu F. et al. (2006). Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science 313, 324-328. 10.1126/science.1129462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett M. A., Schwake M., Bahlo M., Dibbens L. M., Lin M., Gandolfo L. C., Vears D. F., O'Sullivan J. D., Robertson T., Bayly M. A. et al. (2011). A mutation in the Golgi Qb-SNARE gene GOSR2 causes progressive myoclonus epilepsy with early ataxia. Am. J. Hum. Genet. 88, 657-663. 10.1016/j.ajhg.2011.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremers F. P., Armstrong S. A., Seabra M. C., Brown M. S. and Goldstein J. L. (1994). REP-2, a Rab escort protein encoded by the choroideremia-like gene. J. Biol. Chem. 269, 2111-2117. [PubMed] [Google Scholar]
- Cullen P. J. and Steinberg F. (2018). To degrade or not to degrade: mechanisms and significance of endocytic recycling. Nat. Rev. Mol. Cell Biol. 19, 679-696. 10.1038/s41580-018-0053-7 [DOI] [PubMed] [Google Scholar]
- Cullinane A. R., Straatman-Iwanowska A., Zaucker A., Wakabayashi Y., Bruce C. K., Luo G., Rahman F., Gürakan F., Utine E., Özkan T. B. et al. (2010). Mutations in VIPAR cause an arthrogryposis, renal dysfunction and cholestasis syndrome phenotype with defects in epithelial polarization. Nat. Genet. 42, 303-312. 10.1038/ng.538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Adamo P., Menegon A., Lo Nigro C., Grasso M., Gulisano M., Tamanini F., Bienvenu T., Gedeon A. K., Oostra B., Wu S. K. et al. (1998). Mutations in GDI1 are responsible for X-linked non-specific mental retardation. Nat. Genet. 19, 134-139. 10.1038/487 [DOI] [PubMed] [Google Scholar]
- Damseh N., Danson C. M., Al-Ashhab M., Abu-Libdeh B., Gallon M., Sharma K., Yaacov B., Coulthard E., Caldwell M. A., Edvardson S. et al. (2015). A defect in the retromer accessory protein, SNX27, manifests by infantile myoclonic epilepsy and neurodegeneration. Neurogenetics 16, 215-221. 10.1007/s10048-015-0446-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson G. P., Cutz E., Hamilton J. R. and Gall D. G. (1978). Familial enteropathy: a syndrome of protracted diarrhea from birth, failure to thrive, and hypoplastic villus atrophy. Gastroenterology 75, 783-790. 10.1016/0016-5085(78)90458-4 [DOI] [PubMed] [Google Scholar]
- Davies A. K., Itzhak D. N., Edgar J. R., Archuleta T. L., Hirst J., Jackson L. P., Robinson M. S. and Borner G. H. H. (2018). AP-4 vesicles contribute to spatial control of autophagy via RUSC-dependent peripheral delivery of ATG9A. Nat. Commun. 9, 3958 10.1038/s41467-018-06172-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. G., Lehrman M. A., Russell D. W., Anderson R. G., Brown M. S. and Goldstein J. L. (1986). The J.D. mutation in familial hypercholesterolemia: amino acid substitution in cytoplasmic domain impedes internalization of LDL receptors. Cell 45, 15-24. 10.1016/0092-8674(86)90533-7 [DOI] [PubMed] [Google Scholar]
- De Matteis M. A. and Luini A. (2008). Exiting the Golgi complex. Nat. Rev. Mol. Cell Biol. 9, 273-284. 10.1038/nrm2378 [DOI] [PubMed] [Google Scholar]
- De Matteis M. A., Staiano L., Emma F. and Devuyst O. (2017). The 5-phosphatase OCRL in Lowe syndrome and Dent disease 2. Nat. Rev. Nephrol. 13, 455-470. 10.1038/nrneph.2017.83 [DOI] [PubMed] [Google Scholar]
- Dehay B., Martinez-Vicente M., Caldwell G. A., Caldwell K. A., Yue Z., Cookson M. R., Klein C., Vila M. and Bezard E. (2013). Lysosomal impairment in Parkinson's disease. Mov. Disord. 28, 725-732. 10.1002/mds.25462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejeans N., Manié S., Hetz C., Bard F., Hupp T., Agostinis P., Samali A. and Chevet E. (2014). Addicted to secrete - novel concepts and targets in cancer therapy. Trends Mol. Med. 20, 242-250. 10.1016/j.molmed.2013.12.003 [DOI] [PubMed] [Google Scholar]
- DeJesus-Hernandez M., Mackenzie I. R., Boeve B. F., Boxer A. L., Baker M., Rutherford N. J., Nicholson A. M., Finch N. A., Flynn H., Adamson J. et al. (2011). Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72, 245-256. 10.1016/j.neuron.2011.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Angelica E. C., Shotelersuk V., Aguilar R. C., Gahl W. A. and Bonifacino J. S. (1999). Altered trafficking of lysosomal proteins in Hermansky-Pudlak syndrome due to mutations in the β3A subunit of the AP-3 adaptor. Mol. Cell 3, 11-21. 10.1016/S1097-2765(00)80170-7 [DOI] [PubMed] [Google Scholar]
- Denecke J. and Marquardt T. (2009). Congenital dyserythropoietic anemia type II (CDAII/HEMPAS): where are we now? Biochim. Biophys. Acta 1792, 915-920. 10.1016/j.bbadis.2008.12.005 [DOI] [PubMed] [Google Scholar]
- di Ronza A., Bajaj L., Sharma J., Sanagasetti D., Lotfi P., Adamski C. J., Collette J., Palmieri M., Amawi A., Popp L. et al. (2018). CLN8 is an endoplasmic reticulum cargo receptor that regulates lysosome biogenesis. Nat. Cell Biol. 20, 1370-1377. 10.1038/s41556-018-0228-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon-Salazar T. J., Silhavy J. L., Udpa N., Schroth J., Bielas S., Schaffer A. E., Olvera J., Bafna V., Zaki M. S., Abdel-Salam G. H. et al. (2012). Exome sequencing can improve diagnosis and alter patient management. Sci. Transl. Med. 4, 138ra78 10.1126/scitranslmed.3003544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. J., Vreede A. P., Low S. E., Gibbs E. M., Kuwada J. Y., Bonnemann C. G. and Feldman E. L. (2009). Loss of myotubularin function results in T-tubule disorganization in zebrafish and human myotubular myopathy. PLoS Genet. 5, e1000372 10.1371/journal.pgen.1000372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar C. E., High K. A., Joung J. K., Kohn D. B., Ozawa K. and Sadelain M. (2018). Gene therapy comes of age. Science 359, eaan4672 10.1126/science.aan4672 [DOI] [PubMed] [Google Scholar]
- Dupuis N., Lebon S., Kumar M., Drunat S., Graul-Neumann L. M., Gressens P. and El Ghouzzi V. (2013). A novel RAB33B mutation in smith–McCort dysplasia. Hum. Mutat. 34, 283-286. 10.1002/humu.22235 [DOI] [PubMed] [Google Scholar]
- Edvardson S., Cinnamon Y., Ta-Shma A., Shaag A., Yim Y.-I., Zenvirt S., Jalas C., Lesage S., Brice A., Taraboulos A. et al. (2012). A deleterious mutation in DNAJC6 encoding the neuronal-specific clathrin-uncoating co-chaperone auxilin, is associated with juvenile parkinsonism. PLoS ONE 7, e36458 10.1371/journal.pone.0036458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvardson S., Gerhard F., Jalas C., Lachmann J., Golan D., Saada A., Shaag A., Ungermann C. and Elpeleg O (2015). Hypomyelination and developmental delay associated with VPS11 mutation in Ashkenazi-Jewish patients. J. Med. Genet. 52, 749-753. 10.1136/jmedgenet-2015-103239 [DOI] [PubMed] [Google Scholar]
- Eichelbaum K., Winter M., Berriel Diaz M., Herzig S. and Krijgsveld J. (2012). Selective enrichment of newly synthesized proteins for quantitative secretome analysis. Nat. Biotechnol. 30, 984-990. 10.1038/nbt.2356 [DOI] [PubMed] [Google Scholar]
- Elliott A. M., Simard L. R., Coghlan G., Chudley A. E., Chodirker B. N., Greenberg C. R., Burch T., Ly V., Hatch G. M. and Zelinski T (2013). A novel mutation in KIAA0196: identification of a gene involved in Ritscher-Schinzel/3C syndrome in a First Nations cohort. J. Med. Genet. 50, 819-822. 10.1136/jmedgenet-2013-101715 [DOI] [PubMed] [Google Scholar]
- Eymard-Pierre E., Lesca G., Dollet S., Santorelli F. M., di Capua M., Bertini E. and Boespflug-Tanguy O (2002). Infantile-onset ascending hereditary spastic paralysis is associated with mutations in the alsin gene. Am. J. Hum. Genet. 71, 518-527. 10.1086/342359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizi G. M., Ferrarini M., Cavallaro T., Cabrini I., Cerini R., Bertolasi L. and Rizzuto N (2007). Two novel mutations in dynamin-2 cause axonal Charcot–Marie–Tooth disease. Neurology 69, 291-295. 10.1212/01.wnl.0000265820.51075.61 [DOI] [PubMed] [Google Scholar]
- Farazi Fard M. A., Rebelo A. P., Buglo E., Nemati H., Dastsooz H., Gehweiler I., Reich S., Reichbauer J., Quintáns B., Ordóñez-Ugalde A. et al. (2019). Truncating mutations in UBAP1 cause hereditary spastic paraplegia. Am. J. Hum. Genet. 104, 767-773. 10.1016/j.ajhg.2019.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber-Katz S. E., Dippold H. C., Buschman M. D., Peterman M. C., Xing M., Noakes C. J., Tat J., Ng M. M., Rahajeng J., Cowan D. M. et al. (2014). DNA damage triggers Golgi dispersal via DNA-PK and GOLPH3. Cell 156, 413-427. 10.1016/j.cell.2013.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassio A., Patry L., Congia S., Onofri F., Piton A., Gauthier J., Pozzi D., Messa M., Defranchi E., Fadda M. et al. (2011). SYN1 loss-of-function mutations in autism and partial epilepsy cause impaired synaptic function. Hum. Mol. Genet. 20, 2297-2307. 10.1093/hmg/ddr122 [DOI] [PubMed] [Google Scholar]
- Feinstein M., Flusser H., Lerman-Sagie T., Ben-Zeev B., Lev D., Agamy O., Cohen I., Kadir R., Sivan S., Leshinsky-Silver E. et al. (2014). VPS53 mutations cause progressive cerebello-cerebral atrophy type 2 (PCCA2). J. Med. Genet. 51, 303-308. 10.1136/jmedgenet-2013-101823 [DOI] [PubMed] [Google Scholar]
- Feldmann J., Callebaut I., Raposo G., Certain S., Bacq D., Dumont C., Lambert N., Ouachée-Chardin M., Chedeville G., Tamary H. et al. (2003). Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3). Cell 115, 461-473. 10.1016/S0092-8674(03)00855-9 [DOI] [PubMed] [Google Scholar]
- Feng S., Sekine S., Pessino V., Li H., Leonetti M. D. and Huang B. (2017). Improved split fluorescent proteins for endogenous protein labeling. Nat. Commun. 8, 370 10.1038/s41467-017-00494-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari R., Kapogiannis D., Huey E. D. and Momeni P. (2011). FTD and ALS: a tale of two diseases. Curr. Alzheimer Res. 8, 273-294. 10.2174/156720511795563700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira C. R., Xia Z.-J., Clément A., Parry D. A., Davids M., Taylan F., Sharma P., Turgeon C. T., Blanco-Sánchez B., Ng B. G. et al. (2018). A recurrent de novo heterozygous COG4 substitution leads to saul-wilson syndrome, disrupted vesicular trafficking, and altered proteoglycan glycosylation. Am. J. Hum. Genet. 103, 553-567. 10.1016/j.ajhg.2018.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher P. and Ungar D. (2016). Bridging the gap between glycosylation and vesicle traffic. Front. Cell Dev. Biol. 4, 15 10.3389/fcell.2016.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald G., Botstein D., Califf R., Collins R., Peters K., Van Bruggen N. and Rader D. (2018). The future of humans as model organisms. Science 361, 552-553. 10.1126/science.aau7779 [DOI] [PubMed] [Google Scholar]
- Follit J. A., San Agustin J. T., Xu F., Jonassen J. A., Samtani R., Lo C. W. and Pazour G. J. (2008). The Golgin GMAP210/TRIP11 anchors IFT20 to the Golgi complex. PLoS Genet. 4, e1000315 10.1371/journal.pgen.1000315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulquier F., Vasile E., Schollen E., Callewaert N., Raemaekers T., Quelhas D., Jaeken J., Mills P., Winchester B., Krieger M. et al. (2006). Conserved oligomeric Golgi complex subunit 1 deficiency reveals a previously uncharacterized congenital disorder of glycosylation type II. Proc. Natl. Acad. Sci. USA 103, 3764-3769. 10.1073/pnas.0507685103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulquier F., Ungar D., Reynders E., Zeevaert R., Mills P., Garcia-Silva M. T., Briones P., Winchester B., Morelle W., Krieger M. et al. (2007). A new inborn error of glycosylation due to a Cog8 deficiency reveals a critical role for the Cog1-Cog8 interaction in COG complex formation. Hum. Mol. Genet. 16, 717-730. 10.1093/hmg/ddl476 [DOI] [PubMed] [Google Scholar]
- Fumagalli M., Camus S. M., Diekmann Y., Burke A., Camus M. D., Norman P. J., Joseph A., Abi-Rached L., Benazzo A., Rasteiro R. et al. (2019). Genetic diversity of CHC22 clathrin impacts its function in glucose metabolism. Elife 8, e41517 10.7554/eLife.41517.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbes L., Kim K., Riess A., Hoyer-Kuhn H., Beleggia F., Bevot A., Kim M. J., Huh Y. H., Kweon H. S., Savarirayan R. et al. (2015). Mutations in SEC24D, encoding a component of the COPII machinery, cause a syndromic form of osteogenesis imperfecta. Am. J. Hum. Genet. 96, 432-439. 10.1016/j.ajhg.2015.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia C. K., Wilund K., Arca M., Zuliani G., Fellin R., Maioli M., Calandra S., Bertolini S., Cossu F., Grishin N. et al. (2001). Autosomal recessive hypercholesterolemia caused by mutations in a putative LDL receptor adaptor protein. Science 292, 1394-1398. 10.1126/science.1060458 [DOI] [PubMed] [Google Scholar]
- Garcia C. C., Blair H. J., Seager M., Coulthard A., Tennant S., Buddles M., Curtis A. and Goodship J. A (2004). Identification of a mutation in synapsin I, a synaptic vesicle protein, in a family with epilepsy. J. Med. Genet. 41, 183-186. 10.1136/jmg.2003.013680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X., Gong H., Dumas K., Litwin J., Phillips J. J., Waisfisz Q., Weiss M. M., Hendriks Y., Stuurman K. E., Nelson S. F. et al. (2016). Missense-depleted regions in population exomes implicate ras superfamily nucleotide-binding protein alteration in patients with brain malformation. NPJ Genom. Med. 1, 16036 10.1038/npjgenmed.2016.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gedeon A. K., Colley A., Jamieson R., Thompson E. M., Rogers J., Sillence D., Tiller G. E., Mulley J. C. and Gécz J. (1999). Identification of the gene (SEDL) causing X-linked spondyloepiphyseal dysplasia tarda. Nat. Genet. 22, 400-404. 10.1038/11976 [DOI] [PubMed] [Google Scholar]
- Gershlick D. C., Ishida M., Jones J. R., Bellomo A., Bonifacino J. S. and Everman D. B (2019). A neurodevelopmental disorder caused by mutations in the VPS51 subunit of the GARP and EARP complexes. Hum. Mol. Genet. 28, 1548-1560. 10.1093/hmg/ddy423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholam C., Grigoriadou S., Gilmour K. C. and Gaspar H. B. (2011). Familial haemophagocytic lymphohistiocytosis: advances in the genetic basis, diagnosis and management. Clin. Exp. Immunol. 163, 271-283. 10.1111/j.1365-2249.2010.04302.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannandrea M., Bianchi V., Mignogna M. L., Sirri A., Carrabino S., D'Elia E., Vecellio M., Russo S., Cogliati F., Larizza L. et al. (2010). Mutations in the small GTPase gene RAB39B are responsible for X-linked mental retardation associated with autism, epilepsy, and macrocephaly. Am. J. Hum. Genet. 86, 185-195. 10.1016/j.ajhg.2010.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Krzewska A., Wood S. M., Murakami Y., Nguyen V., Chiang S. C. C., Cullinane A. R., Peruzzi G., Gahl W. A., Coligan J. E., Introne W. J. et al. (2016). Chediak-Higashi syndrome: Lysosomal trafficking regulator domains regulate exocytosis of lytic granules but not cytokine secretion by natural killer cells. J. Allergy Clin. Immunol. 137, 1165-1177. 10.1016/j.jaci.2015.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gissen P., Johnson C. A., Morgan N. V., Stapelbroek J. M., Forshew T., Cooper W. N., McKiernan P. J., Klomp L. W. J., Morris A. A. M., Wraith J. E. et al. (2004). Mutations in VPS33B, encoding a regulator of SNARE-dependent membrane fusion, cause arthrogryposis-renal dysfunction-cholestasis (ARC) syndrome. Nat. Genet. 36, 400-404. 10.1038/ng1325 [DOI] [PubMed] [Google Scholar]
- Goldenring J. R. (2015). Recycling endosomes. Curr. Opin. Cell Biol. 35, 117-122. 10.1016/j.ceb.2015.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez R. C., Wawro P., Lis P., Alessi D. R. and Pfeffer S. R. (2019). Membrane association but not identity is required for LRRK2 activation and phosphorylation of Rab GTPases. J. Cell Biol.. 218, 4157-4170. 10.1083/jcb.201902184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guelly C., Zhu P.-P., Leonardis L., Papić L., Zidar J., Schabhüttl M., Strohmaier H., Weis J., Strom T. M., Baets J. et al. (2011). Targeted high-throughput sequencing identifies mutations in atlastin-1 as a cause of hereditary sensory neuropathy type I. Am. J. Hum. Genet. 88, 99-105. 10.1016/j.ajhg.2010.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavsson E. K., Guella I., Trinh J., Szu-Tu C., Rajput A., Rajput A. H., Steele J. C., McKeown M., Jeon B. S., Aasly J. O. et al. (2015). Genetic variability of the retromer cargo recognition complex in parkinsonism. Mov. Disord. 30, 580-584. 10.1002/mds.26104 [DOI] [PubMed] [Google Scholar]
- Haack T. B., Staufner C., Köpke M. G., Straub B. K., Kölker S., Thiel C., Freisinger P., Baric I., McKiernan P. J., Dikow N. et al. (2015). Biallelic mutations in NBAS cause recurrent acute liver failure with onset in infancy. Am. J. Hum. Genet. 97, 163-169. 10.1016/j.ajhg.2015.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadano S., Hand C. K., Osuga H., Yanagisawa Y., Otomo A., Devon R. S., Miyamoto N., Showguchi-Miyata J., Okada Y., Singaraja R. et al. (2001). A gene encoding a putative GTPase regulator is mutated in familial amyotrophic lateral sclerosis 2. Nat. Genet. 29, 166-173. 10.1038/ng1001-166 [DOI] [PubMed] [Google Scholar]
- Hady-Cohen R., Ben-Pazi H., Adir V., Yosovich K., Blumkin L., Lerman-Sagie T. and Lev D (2018). Progressive cerebello-cerebral atrophy and progressive encephalopathy with edema, hypsarrhythmia and optic atrophy may be allelic syndromes. Eur. J. Paediatr. Neurol. 22, 1133-1138. 10.1016/j.ejpn.2018.07.003 [DOI] [PubMed] [Google Scholar]
- Halperin D., Kadir R., Perez Y., Drabkin M., Yogev Y., Wormser O., Berman E. M., Eremenko E., Rotblat B., Shorer Z. et al. (2019). SEC31A mutation affects ER homeostasis, causing a neurological syndrome. J. Med. Genet. 56, 139-148. 10.1136/jmedgenet-2018-105503 [DOI] [PubMed] [Google Scholar]
- Han C., Alkhater R., Froukh T., Minassian A. G., Galati M., Liu R. H., Fotouhi M., Sommerfeld J., Alfrook A. J., Marshall C. et al. (2016). Epileptic encephalopathy caused by mutations in the guanine nucleotide exchange factor DENND5A. Am. J. Hum. Genet. 99, 1359-1367. 10.1016/j.ajhg.2016.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanein S., Martin E., Boukhris A., Byrne P., Goizet C., Hamri A., Benomar A., Lossos A., Denora P., Fernandez J. et al. (2008). Identification of the SPG15 gene, encoding spastizin, as a frequent cause of complicated autosomal-recessive spastic paraplegia, including Kjellin syndrome. Am. J. Hum. Genet. 82, 992-1002. 10.1016/j.ajhg.2008.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardies K., May P., Djemie T., Tarta-Arsene O., Deconinck T., Craiu D., AR working group of the EuroEPINOMICS RES Consortium, Helbig I., Suls A., Balling R. et al. (2015). Recessive loss-of-function mutations in AP4S1 cause mild fever-sensitive seizures, developmental delay and spastic paraplegia through loss of AP-4 complex assembly. Hum. Mol. Genet. 24, 2218-2227. 10.1093/hmg/ddu740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardies K., Cai Y., Jardel C., Jansen A. C., Cao M., May P., Djemie T., Hachon Le Camus C., Keymolen K., Deconinck T., (2016). Loss of SYNJ1 dual phosphatase activity leads to early onset refractory seizures and progressive neurological decline. Brain 139, 2420-2430. 10.1093/brain/aww180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardiman O., Al-Chalabi A., Chio A., Corr E. M., Logroscino G., Robberecht W., Shaw P. J., Simmons Z. and van den Berg L. H. (2017). Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers 3, 17071 10.1038/nrdp.2017.71 [DOI] [PubMed] [Google Scholar]
- Harlalka G. V., McEntagart M. E., Gupta N., Skrzypiec A. E., Mucha M. W., Chioza B. A., Simpson M. A., Sreekantan-Nair A., Pereira A., Günther S. et al. (2016). Novel genetic, clinical, and pathomechanistic insights into TFG-associated hereditary spastic paraplegia. Hum. Mutat. 37, 1157-1161. 10.1002/humu.23060 [DOI] [PubMed] [Google Scholar]
- Harold D., Abraham R., Hollingworth P., Sims R., Gerrish A., Hamshere M. L., Pahwa J. S., Moskvina V., Dowzell K., Williams A. et al. (2009). Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nat. Genet. 41, 1088-1093. 10.1038/ng.440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harripaul R., Vasli N., Mikhailov A., Rafiq M. A., Mittal K., Windpassinger C., Sheikh T. I., Noor A., Mahmood H., Downey S. et al. (2018). Mapping autosomal recessive intellectual disability: combined microarray and exome sequencing identifies 26 novel candidate genes in 192 consanguineous families. Mol. Psychiatry 23, 973-984. 10.1038/mp.2017.60 [DOI] [PubMed] [Google Scholar]
- Hazan J., Fonknechten N., Mavel D., Paternotte C., Samson D., Artiguenave F., Davoine C. S., Cruaud C., Durr A., Wincker P. et al. (1999). Spastin, a new AAA protein, is altered in the most frequent form of autosomal dominant spastic paraplegia. Nat. Genet. 23, 296-303. 10.1038/15472 [DOI] [PubMed] [Google Scholar]
- He G., Gupta S., Yi M., Michaely P., Hobbs H. H. and Cohen J. C. (2002). ARH is a modular adaptor protein that interacts with the LDL receptor, clathrin, and AP-2. J. Biol. Chem. 277, 44044-44049. 10.1074/jbc.M208539200 [DOI] [PubMed] [Google Scholar]
- Helbig I., Lopez-Hernandez T., Shor O., Galer P., Ganesan S., Pendziwiat M., Rademacher A., Ellis C. A., Humpfer N., Schwarz N. et al. (2019). A recurrent missense variant in AP2M1 impairs clathrin-mediated endocytosis and causes developmental and epileptic encephalopathy. Am. J. Hum. Genet. 104, 1060-1072. 10.1016/j.ajhg.2019.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennies H. C., Kornak U., Zhang H., Egerer J., Zhang X., Seifert W., Kühnisch J., Budde B., Nätebus M., Brancati F. et al. (2008). Gerodermia osteodysplastica is caused by mutations in SCYL1BP1, a Rab-6 interacting golgin. Nat. Genet. 40, 1410-1412. 10.1038/ng.252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth P., Harold D., Sims R., Gerrish A., Lambert J. C., Carrasquillo M. M., Abraham R., Hamshere M. L., Pahwa J. S., Moskvina V. et al. (2011). Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer's disease. Nat. Genet. 43, 429-435. 10.1038/ng.803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoopes R. R. Jr, Shrimpton A. E., Knohl S. J., Hueber P., Hoppe B., Matyus J., Simckes A., Tasic V., Toenshoff B., Suchy S. F. et al. (2005). Dent Disease with mutations in OCRL1. Am. J. Hum. Genet. 76, 260-267. 10.1086/427887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao Y.-C., Tuz K. and Ferland R. J. (2012). Trafficking in and to the primary cilium. Cilia 1, 4 10.1186/2046-2530-1-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Pickering E., Liu Y. C., Hall S., Fournier H., Katz E., Dechairo B., John S., Van Eerdewegh P., Soares H. et al. (2011). Meta-analysis for genome-wide association study identifies multiple variants at the BIN1 locus associated with late-onset Alzheimer's disease. PLoS ONE 6, e16616 10.1371/journal.pone.0016616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizing M., Helip-Wooley A., Westbroek W., Gunay-Aygun M. and Gahl W. A. (2008). Disorders of lysosome-related organelle biogenesis: clinical and molecular genetics. Annu. Rev. Genomics Hum. Genet. 9, 359-386. 10.1146/annurev.genom.9.081307.164303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung V., Udeshi N. D., Lam S. S., Loh K. H., Cox K. J., Pedram K., Carr S. A. and Ting A. Y. (2016). Spatially resolved proteomic mapping in living cells with the engineered peroxidase APEX2. Nat. Protoc. 11, 456-475. 10.1038/nprot.2016.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illa I., Serrano-Munuera C., Gallardo E., Lasa A., Rojas-Garcia R., Palmer J., Gallano P., Baiget M., Matsuda C. and Brown R. H. (2001). Distal anterior compartment myopathy: a dysferlin mutation causing a new muscular dystrophy phenotype. Ann. Neurol. 49, 130-134. <130::AID-ANA22>3.0.CO;2-0 [PubMed] [Google Scholar]
- Inoue K. and Ishibe S. (2015). Podocyte endocytosis in the regulation of the glomerular filtration barrier. Am. J. Physiol. Renal. Physiol. 309, F398-F405. 10.1152/ajprenal.00136.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaji T., Im S., Gu W., Wang Y., Hang Q., Lu J., Fukuda T., Hashii N., Takakura D., Kawasaki N. et al. (2014). An oncogenic protein Golgi phosphoprotein 3 up-regulates cell migration via sialylation. J. Biol. Chem. 289, 20694-20705. 10.1074/jbc.M113.542688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiura H., Sako W., Yoshida M., Kawarai T., Tanabe O., Goto J., Takahashi Y., Date H., Mitsui J., Ahsan B. et al. (2012). The TRK-fused gene is mutated in hereditary motor and sensory neuropathy with proximal dominant involvement. Am. J. Hum. Genet. 91, 320-329. 10.1016/j.ajhg.2012.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova E. A., De Leo M. G., Van Den Heuvel L., Pastore A., Dijkman H., De Matteis M. A. and Levtchenko E. N. (2015). Endo-lysosomal dysfunction in human proximal tubular epithelial cells deficient for lysosomal cystine transporter cystinosin. PLoS ONE 10, e0120998 10.1371/journal.pone.0120998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova E. L., Mau-Them F. T., Riazuddin S., Kahrizi K., Laugel V., Schaefer E., de Saint Martin A., Runge K., Iqbal Z., Spitz M.-A. et al. (2017). Homozygous truncating variants in TBC1D23 cause pontocerebellar hypoplasia and alter cortical development. Am. J. Hum. Genet. 101, 428-440. 10.1016/j.ajhg.2017.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi K., Brett M., Nishi E., Drunat S., Tan E.-S., Fujiki K., Lebon S., Cham B., Masuda K., Arakawa M. et al. (2016). ARCN1 mutations cause a recognizable craniofacial syndrome due to COPI-mediated transport defects. Am. J. Hum. Genet. 99, 451-459. 10.1016/j.ajhg.2016.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen D. and Schekman R. (2011). COPII-mediated vesicle formation at a glance. J. Cell Sci. 124, 1-4. 10.1242/jcs.069773 [DOI] [PubMed] [Google Scholar]
- Jiang Y., Ou Y. and Cheng X. (2013). Role of TSG101 in cancer. Front. Biosci. (Landmark Ed) 18, 279-288. 10.2741/4176 [DOI] [PubMed] [Google Scholar]
- Johnson A. D. and O'Donnell C. J (2009). An open access database of genome-wide association results. BMC Med. Genet. 10, 6 10.1186/1471-2350-10-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B., Jones E. L., Bonney S. A., Patel H. N., Mensenkamp A. R., Eichenbaum-Voline S., Rudling M., Myrdal U., Annesi G., Naik S. et al. (2003). Mutations in a Sar1 GTPase of COPII vesicles are associated with lipid absorption disorders. Nat. Genet. 34, 29-31. 10.1038/ng1145 [DOI] [PubMed] [Google Scholar]
- Joshi G. and Wang Y. (2015). Golgi defects enhance APP amyloidogenic processing in Alzheimer's disease. BioEssays 37, 240-247. 10.1002/bies.201400116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josifova D. (2007). RAB23 mutations in carpenter syndrome imply an unexpected role for hedgehog signaling in cranial-suture development and obesity (vol 80, pg 1162, 2007). Am. J. Hum. Genet. 81, 1114-1114. 10.1086/522891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun G., Naj A. C., Beecham G. W., Wang L. S., Buros J., Gallins P. J., Buxbaum J. D., Ertekin-Taner N., Fallin M. D., Friedland R. et al. (2010). Meta-analysis confirms CR1, CLU, and PICALM as alzheimer disease risk loci and reveals interactions with APOE genotypes. Arch. Neurol. 67, 1473-1484. 10.1001/archneurol.2010.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim M. A., Suzuki K., Fukai K., Oh J., Nagle D. L., Moore K. J., Barbosa E., Falik-Borenstein T., Filipovich A., Ishida Y. et al. (2002). Apparent genotype-phenotype correlation in childhood, adolescent, and adult Chediak-Higashi syndrome. Am. J. Med. Genet. 108, 16-22. 10.1002/ajmg.10184 [DOI] [PubMed] [Google Scholar]
- Kato K., Oka Y., Muramatsu H., Vasilev F. F., Otomo T., Oishi H., Kawano Y., Kidokoro H., Nakazawa Y., Ogi T. et al. (2019). Biallelic VPS35L pathogenic variants cause 3C/Ritscher-Schinzel-like syndrome through dysfunction of retriever complex. J. Med. Genet. 57, 245-253. 10.1136/jmedgenet-2019-106213 [DOI] [PubMed] [Google Scholar]
- Khoriaty R., Hesketh G. G., Bernard A., Weyand A. C., Mellacheruvu D., Zhu G., Hoenerhoff M. J., McGee B., Everett L., Adams E. J. et al. (2018). Functions of the COPII gene paralogs SEC23A and SEC23B are interchangeable in vivo. Proc. Natl. Acad. Sci. USA 115, E7748-E7757. 10.1073/pnas.1805784115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. M., Wu H., Green G., Winkler C. A., Kopp J. B., Miner J. H., Unanue E. R. and Shaw A. S. (2003). CD2-associated protein haploinsufficiency is linked to glomerular disease susceptibility. Science 300, 1298-1300. 10.1126/science.1081068 [DOI] [PubMed] [Google Scholar]
- Klinkert K. and Echard A. (2016). Rab35 GTPase: a central regulator of phosphoinositides and F-actin in endocytic recycling and beyond. Traffic 17, 1063-1077. 10.1111/tra.12422 [DOI] [PubMed] [Google Scholar]
- Kodera H., Ando N., Yuasa I., Wada Y., Tsurusaki Y., Nakashima M., Miyake N., Saitoh S., Matsumoto N. and Saitsu H (2015). Mutations in COG2 encoding a subunit of the conserved oligomeric golgi complex cause a congenital disorder of glycosylation. Clin. Genet. 87, 455-460. 10.1111/cge.12417 [DOI] [PubMed] [Google Scholar]
- Kondo H., Maksimova N., Otomo T., Kato H., Imai A., Asano Y., Kobayashi K., Nojima S., Nakaya A., Hamada Y. et al. (2017). Mutation in VPS33A affects metabolism of glycosaminoglycans: a new type of mucopolysaccharidosis with severe systemic symptoms. Hum. Mol. Genet. 26, 173-183. 10.1093/hmg/ddw377 [DOI] [PubMed] [Google Scholar]
- Korpal M., Ell B. J., Buffa F. M., Ibrahim T., Blanco M. A., Celià-Terrassa T., Mercatali L., Khan Z., Goodarzi H., Hua Y. et al. (2011). Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nat. Med. 17, 1101-1108. 10.1038/nm.2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsopoulos O. S., Kretz C., Weller C. M., Roux A., Mojzisova H., Böhm J., Koch C., Toussaint A., Heckel E., Stemkens D. et al. (2013). Dynamin 2 homozygous mutation in humans with a lethal congenital syndrome. Eur. J. Hum. Genet. 21, 637-642. 10.1038/ejhg.2012.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz C., Ng B. G., Sun L., Sharma V., Eklund E. A., Miura Y., Ungar D., Lupashin V., Winkel R. D., Cipollo J. F. et al. (2007). COG8 deficiency causes new congenital disorder of glycosylation type IIh. Hum. Mol. Genet. 16, 731-741. 10.1093/hmg/ddm028 [DOI] [PubMed] [Google Scholar]
- Krebs C. E., Karkheiran S., Powell J. C., Cao M., Makarov V., Darvish H., Di Paolo G., Walker R. H., Shahidi G. A., Buxbaum J. D. et al. (2013). The Sac1 domain of SYNJ1 identified mutated in a family with early-onset progressive Parkinsonism with generalized seizures. Hum. Mutat. 34, 1200-1207. 10.1002/humu.22372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis T. E. and Lodish H. F. (1986). Oligomerization is essential for transport of vesicular stomatitis viral glycoprotein to the cell surface. Cell 46, 929-937. 10.1016/0092-8674(86)90075-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuismanen E. and Saraste J. (1989). Low temperature-induced transport blocks as tools to manipulate membrane traffic. Methods Cell Biol. 32, 257-274. 10.1016/S0091-679X(08)61174-7 [DOI] [PubMed] [Google Scholar]
- Lamers I. J. C., Reijnders M. R. F., Venselaar H., Kraus A., Jansen S., de Vries B. B. A., Houge G., Gradek G. A., Seo J., Choi M. et al. (2017). Recurrent De Novo mutations disturbing the GTP/GDP binding pocket of RAB11B cause intellectual disability and a distinctive brain phenotype. Am. J. Hum. Genet. 101, 824-832. 10.1016/j.ajhg.2017.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster M. A. and Huch M. (2019). Disease modelling in human organoids. Dis. Model. Mech. 12, dmm039347 10.1242/dmm.039347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laporte J., Biancalana V., Tanner S. M., Kress W., Schneider V., Wallgren-Pettersson C., Herger F., Buj-Bello A., Blondeau F., Liechti-Gallati S. et al. (2000). MTM1 mutations in X-linked myotubular myopathy. Hum. Mutat. 15, 393-409. [DOI] [PubMed] [Google Scholar]
- Larson A. A., Baker P. R. II, Milev M. P., Press C. A., Sokol R. J., Cox M. O., Lekostaj J. K., Stence A. A., Bossler A. D., Mueller J. M. et al. (2018). TRAPPC11 and GOSR2 mutations associate with hypoglycosylation of alpha-dystroglycan and muscular dystrophy. Skelet Muscle 8, 17 10.1186/s13395-018-0163-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E., Marcucci M., Daniell L., Pypaert M., Weisz O. A., Ochoa G. C., Farsad K., Wenk M. R. and De Camilli P. (2002). Amphiphysin 2 (Bin1) and T-tubule biogenesis in muscle. Science 297, 1193-1196. 10.1126/science.1071362 [DOI] [PubMed] [Google Scholar]
- Lee H. J., Song J. Y., Kim J. W., Jin S.-Y., Hong M. S., Park J. K., Chung J.-H., Shibata H. and Fukumaki Y (2005). Association study of polymorphisms in synaptic vesicle-associated genes, SYN2 and CPLX2, with schizophrenia. Behav. Brain Funct. 1, 15 10.1186/1744-9081-1-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lek A., Evesson F. J., Sutton R. B., North K. N. and Cooper S. T. (2012). Ferlins: regulators of vesicle fusion for auditory neurotransmission, receptor trafficking and membrane repair. Traffic 13, 185-194. 10.1111/j.1600-0854.2011.01267.x [DOI] [PubMed] [Google Scholar]
- Lemos R. R., Oliveira D. F., Zatz M. and Oliveira J. R. M (2011). Population and computational analysis of the MGEA6 P521A variation as a risk factor for familial idiopathic basal ganglia calcification (Fahr's Disease). J. Mol. Neurosci. 43, 333-336. 10.1007/s12031-010-9445-7 [DOI] [PubMed] [Google Scholar]
- Lenk G. M., Szymanska K., Debska-Vielhaber G., Rydzanicz M., Walczak A., Bekiesinska-Figatowska M., Vielhaber S., Hallmann K., Stawinski P., Buehring S. et al. (2016). Biallelic mutations of VAC14 in pediatric-onset neurological disease. Am. J. Hum. Genet. 99, 188-194. 10.1016/j.ajhg.2016.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz D., McClean P., Kansu A., Bonnen P. E., Ranucci G., Thiel C., Straub B. K., Harting I., Alhaddad B., Dimitrov B. et al. (2018). SCYL1 variants cause a syndrome with low γ-glutamyl-transferase cholestasis, acute liver failure, and neurodegeneration (CALFAN). Genet. Med. 20, 1255-1265. 10.1038/gim.2017.260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner T. J., Boustany R. M. N., Anderson J. W., Darigo K. L., Schlumpf K., Buckler A. J., Gusella J. F., Haines J. L., Kremmidiotis G., Lensink I. L. et al. (1995). Isolation of a novel gene underlying batten-disease, CLN3. Cell 82, 949-957. 10.1016/0092-8674(95)90274-0 [DOI] [PubMed] [Google Scholar]
- Li L. and Cohen S. N (1996). Tsg101: a novel tumor susceptibility gene isolated by controlled homozygous functional knockout of allelic loci in mammalian cells. Cell 85, 319-329. 10.1016/S0092-8674(00)81111-3 [DOI] [PubMed] [Google Scholar]
- Li W., Zhang Q., Oiso N., Novak E. K., Gautam R., O'Brien E. P., Tinsley C. L., Blake D. J., Spritz R. A., Copeland N. G. et al. (2003). Hermansky-Pudlak syndrome type 7 (HPS-7) results from mutant dysbindin, a member of the biogenesis of lysosome-related organelles complex 1 (BLOC-1). Nat. Genet. 35, 84-89. 10.1038/ng1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Nakamura K., Jiang Y., Tsurui H., Matsuoka S., Abe M., Ohtsuji M., Nishimura H., Kato K., Kawai T. et al. (2004). Gain-of-function polymorphism in mouse and human Ltk: implications for the pathogenesis of systemic lupus erythematosus. Hum. Mol. Genet. 13, 171-179. 10.1093/hmg/ddh020 [DOI] [PubMed] [Google Scholar]
- Li S., Tiab L., Jiao X., Munier F. L., Zografos L., Frueh B. E., Sergeev Y., Smith J., Rubin B., Meallet M. A. et al. (2005). Mutations in PIP5K3 are associated with Francois-Neetens mouchetee fleck corneal dystrophy. Am. J. Hum. Genet. 77, 54-63. 10.1086/431346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liegel R. P., Handley M. T., Ronchetti A., Brown S., Langemeyer L., Linford A., Chang B., Morris-Rosendahl D. J., Carpanini S., Posmyk R. et al. (2013). Loss-of-function mutations in TBC1 D20 cause cataracts and male infertility in blind sterile mice and warburg micro syndrome in humans. Am. J. Hum. Genet. 93, 1001-1014. 10.1016/j.ajhg.2013.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lines M. A., Ito Y., Kernohan K. D., Mears W., Hurteau-Miller J., Venkateswaran S., Ward L., Khatchadourian K., McClintock J., Bhola P. et al. (2017). Yunis-Varon syndrome caused by biallelic VAC14 mutations. Eur. J. Hum. Genet. 25, 1049-1054. 10.1038/ejhg.2017.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Aoki M., Illa I., Wu C., Fardeau M., Angelini C., Serrano C., Urtizberea J. A., Hentati F., Hamida M. B. et al. (1998). Dysferlin, a novel skeletal muscle gene, is mutated in Miyoshi myopathy and limb girdle muscular dystrophy. Nat. Genet. 20, 31-36. 10.1038/1682 [DOI] [PubMed] [Google Scholar]
- Lloyd S. E., Pearce S. H., Fisher S. E., Steinmeyer K., Schwappach B., Scheinman S. J., Harding B., Bolino A., Devoto M., Goodyer P. et al. (1996). A common molecular basis for three inherited kidney stone diseases. Nature 379, 445-449. 10.1038/379445a0 [DOI] [PubMed] [Google Scholar]
- Lopes F., Barbosa M., Ameur A., Soares G., de Sá J., Dias A. I., Oliveira G., Cabral P., Temudo T., Calado E. et al. (2016). Identification of novel genetic causes of Rett syndrome-like phenotypes. J. Med. Genet. 53, 190-199. 10.1136/jmedgenet-2015-103568 [DOI] [PubMed] [Google Scholar]
- Lu Q., Hope L. W., Brasch M., Reinhard C. and Cohen S. N. (2003). TSG101 interaction with HRS mediates endosomal trafficking and receptor down-regulation. Proc. Natl. Acad. Sci. USA 100, 7626-7631. 10.1073/pnas.0932599100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubbehusen J., Thiel C., Rind N., Ungar D., Prinsen B. H. C. M. T., de Koning T. J., van Hasselt P. M. and Korner C (2010). Fatal outcome due to deficiency of subunit 6 of the conserved oligomeric Golgi complex leading to a new type of congenital disorders of glycosylation. Hum. Mol. Genet. 19, 3623-3633. 10.1093/hmg/ddq278 [DOI] [PubMed] [Google Scholar]
- Luo M. L., Gong C., Chen C. H., Hu H., Huang P., Zheng M., Yao Y., Wei S., Wulf G., Lieberman J. et al. (2015). The Rab2A GTPase promotes breast cancer stem cells and tumorigenesis via Erk signaling activation. Cell Rep 11, 111-124. 10.1016/j.celrep.2015.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupski J. R., Reid J. G., Gonzaga-Jauregui C., Rio Deiros D., Chen D. C., Nazareth L., Bainbridge M., Dinh H., Jing C., Wheeler D. A. et al. (2010). Whole-genome sequencing in a patient with Charcot-Marie-Tooth neuropathy. N. Engl. J. Med. 362, 1181-1191. 10.1056/NEJMoa0908094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald M. E., Ambrose C. M., Duyao M. P., Myers R. H., Lin C., Srinidhi L., Barnes G., Taylor S. A., James M., Groot N. et al. (1993). A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell 72, 971-983. 10.1016/0092-8674(93)90585-E [DOI] [PubMed] [Google Scholar]
- MacLeod D. A., Rhinn H., Kuwahara T., Zolin A., Di Paolo G., McCabe B. D., Marder K. S., Honig L. S., Clark L. N., Small S. A. et al. (2013). RAB7L1 interacts with LRRK2 to modify intraneuronal protein sorting and Parkinson's disease risk. Neuron 77, 425-439. 10.1016/j.neuron.2012.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddirevula S., Alzahrani F., Al-Owain M., Al Muhaizea M. A., Kayyali H. R., AlHashem A., Rahbeeni Z., Al-Otaibi M., Alzaidan H. I., Balobaid A. et al. (2019). Autozygome and high throughput confirmation of disease genes candidacy. Genet. Med. 21, 736-742. 10.1038/s41436-018-0138-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksimova N., Hara K., Nikolaeva I., Chun-Feng T., Usui T., Takagi M., Nishihira Y., Miyashita A., Fujiwara H., Oyama T. et al. (2010). Neuroblastoma amplified sequence gene is associated with a novel short stature syndrome characterised by optic nerve atrophy and Pelger-Huet anomaly. J. Med. Genet. 47, 538-548. 10.1136/jmg.2009.074815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manteghi S., Gingras M. C., Kharitidi D., Galarneau L., Marques M., Yan M., Cencic R., Robert F., Paquet M., Witcher M. et al. (2016). Haploinsufficiency of the ESCRT component HD-PTP predisposes to cancer. Cell Rep 15, 1893-1900. 10.1016/j.celrep.2016.04.076 [DOI] [PubMed] [Google Scholar]
- Marin-Valencia I., Gerondopoulos A., Zaki M. S., Ben-Omran T., Almureikhi M., Demir E., Guemez-Gamboa A., Gregor A., Issa M. Y., Appelhof B. et al. (2017). Homozygous mutations in TBC1D23 lead to a non-degenerative form of pontocerebellar hypoplasia. Am. J. Hum. Genet. 101, 441-450. 10.1016/j.ajhg.2017.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Valencia I., Novarino G., Johansen A., Rosti B., Issa M. Y., Musaev D., Bhat G., Scott E., Silhavy J. L., Stanley V. et al. (2018). A homozygous founder mutation in TRAPPC6B associates with a neurodevelopmental disorder characterised by microcephaly, epilepsy and autistic features. J. Med. Genet. 55, 48-54. 10.1136/jmedgenet-2017-104627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks M. S., Heijnen H. F. and Raposo G. (2013). Lysosome-related organelles: unusual compartments become mainstream. Curr. Opin. Cell Biol. 25, 495-505. 10.1016/j.ceb.2013.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama H., Morino H., Ito H., Izumi Y., Kato H., Watanabe Y., Kinoshita Y., Kamada M., Nodera H., Suzuki H. et al. (2010). Mutations of optineurin in amyotrophic lateral sclerosis. Nature 465, 223-226. 10.1038/nature08971 [DOI] [PubMed] [Google Scholar]
- Mata I. F., Jang Y., Kim C. H., Hanna D. S., Dorschner M. O., Samii A., Agarwal P., Roberts J. W., Klepitskaya O., Shprecher D. R. et al. (2015). The RAB39B p.G192R mutation causes X-linked dominant Parkinson's disease. Mol Neurodegener 10, 50 10.1186/s13024-015-0045-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattera R., Park S. Y., De Pace R., Guardia C. M. and Bonifacino J. S. (2017). AP-4 mediates export of ATG9A from the trans-Golgi network to promote autophagosome formation. Proc. Natl. Acad. Sci. USA 114, E10697-E10706. 10.1073/pnas.1717327114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer M. E. and Cooper J. A. (2006). The adaptor protein Dab2 sorts LDL receptors into coated pits independently of AP-2 and ARH. J. Cell Sci. 119, 4235-4246. 10.1242/jcs.03217 [DOI] [PubMed] [Google Scholar]
- McCaughey J. and Stephens D. J. (2018). COPII-dependent ER export in animal cells: adaptation and control for diverse cargo. Histochem. Cell Biol. 150, 119-131. 10.1007/s00418-018-1689-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon H. T. and Boucrot E. (2011). Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 12, 517-533. 10.1038/nrm3151 [DOI] [PubMed] [Google Scholar]
- Ménasché G., Pastural E., Feldmann J., Certain S., Ersoy F., Dupuis S., Wulffraat N., Bianchi D., Fischer A., Le Deist F. et al. (2000). Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat. Genet. 25, 173-176. 10.1038/76024 [DOI] [PubMed] [Google Scholar]
- Mele C., Iatropoulos P., Donadelli R., Calabria A., Maranta R., Cassis P., Buelli S., Tomasoni S., Piras R., Krendel M. et al. (2011). MYO1E mutations and childhood familial focal segmental glomerulosclerosis. N. Engl. J. Med. 365, 295-306. 10.1056/NEJMoa1101273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I. and Yarden Y. (2013). Endocytosis and cancer. Cold Spring Harb. Perspect Biol. 5, a016949 10.1101/cshperspect.a016949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménasché G., Ho C. H., Sanal O., Feldmann J., Tezcan I., Ersoy F., Houdusse A., Fischer A. and de saint Basile G. (2003). Griscelli syndrome restricted to hypopigmentation results from a melanophilin defect (GS3) or a MYO5A F-exon deletion (GS1). J. Clin. Investig. 112, 450-456. 10.1172/JCI200318264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignogna M. L., Giannandrea M., Gurgone A., Fanelli F., Raimondi F., Mapelli L., Bassani S., Fang H., Van Anken E., Alessio M. et al. (2015). The intellectual disability protein RAB39B selectively regulates GluA2 trafficking to determine synaptic AMPAR composition. Nat. Commun. 6, 6504 10.1038/ncomms7504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milev M. P., Grout M. E., Saint-Dic D., Cheng Y. H., Glass I. A., Hale C. J., Hanna D. S., Dorschner M. O., Prematilake K., Shaag A. et al. (2017). Mutations in TRAPPC12 manifest in progressive childhood encephalopathy and golgi dysfunction. Am. J. Hum. Genet. 101, 291-299. 10.1016/j.ajhg.2017.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir A., Kaufman L., Noor A., Motazacker M. M., Jamil T., Azam M., Kahrizi K., Rafiq M. A., Weksberg R., Nasr T. et al. (2009). Identification of mutations in TRAPPC9, which encodes the NIK- and IKK-β-binding protein, in nonsyndromic autosomal-recessive mental retardation. Am. J. Hum. Genet. 85, 909-915. 10.1016/j.ajhg.2009.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda A. M., Herman M., Cheng R., Nahmani E., Barrett G., Micevska E., Fontaine G., Potier M. C., Head E., Schmitt F. A. et al. (2018). Excess synaptojanin 1 contributes to place cell dysfunction and memory deficits in the aging hippocampus in three types of Alzheimer's disease. Cell Rep 23, 2967-2975. 10.1016/j.celrep.2018.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura Y., Tay S. K., Aw M. M., Eklund E. A. and Freeze H. H (2005). Clinical and biochemical characterization of a patient with congenital disorder of glycosylation (CDG) IIx. J. Pediatr. 147, 851-853. 10.1016/j.jpeds.2005.07.038 [DOI] [PubMed] [Google Scholar]
- Mochida G. H., Mahajnah M., Hill A. D., Basel-Vanagaite L., Gleason D., Hill R. S., Bodell A., Crosier M., Straussberg R. and Walsh C. A (2009). A truncating mutation of TRAPPC9 is associated with autosomal-recessive intellectual disability and postnatal microcephaly. Am. J. Hum. Genet. 85, 897-902. 10.1016/j.ajhg.2009.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monis W. J., Faundez V. and Pazour G. J. (2017). BLOC-1 is required for selective membrane protein trafficking from endosomes to primary cilia. J. Cell Biol. 216, 2131-2150. 10.1083/jcb.201611138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecchiani C., Pedace L., Lo Giudice T., Casella A., Mearini M., Gaudiello F., Pedroso J. L., Terracciano C., Caltagirone C., Massa R. et al. (2016). ALS5/SPG11/KIAA1840 mutations cause autosomal recessive axonal Charcot-Marie-Tooth disease. Brain 139, 73-85. 10.1093/brain/awv320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montpetit A., Côté S., Brustein E., Drouin C. A., Lapointe L., Boudreau M., Meloche C., Drouin R., Hudson T. J., Drapeau P. et al. (2008). Disruption of AP1S1, causing a novel neurocutaneous syndrome, perturbs development of the skin and spinal cord. PLoS Genet. 4, e1000296 10.1371/journal.pgen.1000296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosajee M., Ramsden S. C., Black G. C., Seabra M. C. and Webster A. R. (2014). Clinical utility gene card for: choroideremia. Eur. J. Hum. Genet. 22, 572 10.1038/ejhg.2013.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-De-Luca A., Helmers S. L., Mao H., Burns T. G., Melton A. M. A., Schmidt K. R., Fernhoff P. M., Ledbetter D. H. and Martin C. L. (2011). Adaptor protein complex-4 (AP-4) deficiency causes a novel autosomal recessive cerebral palsy syndrome with microcephaly and intellectual disability. J. Med. Genet. 48, 141-144. 10.1136/jmg.2010.082263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan N. V., Pasha S., Johnson C. A., Ainsworth J. R., Eady R. A., Dawood B., McKeown C., Trembath R. C., Wilde J., Watson S. P. et al. (2006). A germline mutation in BLOC1S3/reduced pigmentation causes a novel variant of Hermansky-Pudlak syndrome (HPS8). Am. J. Hum. Genet. 78, 160-166. 10.1086/499338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan N. E., Cutrona M. B. and Simpson J. C. (2019). Multitasking Rab proteins in autophagy and membrane trafficking: a focus on Rab33b. Int. J. Mol. Sci. 20, 3916 10.3390/ijms20163916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller T., Hess M. W., Schiefermeier N., Pfaller K., Ebner H. L., Heinz-Erian P., Ponstingl H., Partsch J., Rollinghoff B., Kohler H. et al. (2008). MYO5B mutations cause microvillus inclusion disease and disrupt epithelial cell polarity. Nat. Genet. 40, 1163-1165. 10.1038/ng.225 [DOI] [PubMed] [Google Scholar]
- Muller P. A., Caswell P. T., Doyle B., Iwanicki M. P., Tan E. H., Karim S., Lukashchuk N., Gillespie D. A., Ludwig R. L., Gosselin P. et al. (2009). Mutant p53 drives invasion by promoting integrin recycling. Cell 139, 1327-1341. 10.1016/j.cell.2009.11.026 [DOI] [PubMed] [Google Scholar]
- Nagle M. W., Latourelle J. C., Labadorf A., Dumitriu A., Hadzi T. C., Beach T. G. and Myers R. H. (2016). The 4p16.3 Parkinson disease risk locus is associated with GAK expression and genes involved with the synaptic vesicle membrane. PLoS ONE 11, e0160925 10.1371/journal.pone.0160925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naj A. C., Jun G., Beecham G. W., Wang L. S., Vardarajan B. N., Buros J., Gallins P. J., Buxbaum J. D., Jarvik G. P., Crane P. K. et al. (2011). Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer's disease. Nat. Genet. 43, 436-441. 10.1038/ng.801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhro K., Park J.-M., Hong Y. B., Park J. H., Nam S. H., Yoon B. R., Yoo J. H., Koo H., Jung S.-C., Kim H.-L. et al. (2013). SET binding factor 1 (SBF1) mutation causes Charcot-Marie-Tooth disease type 4B3. Neurology 81, 165-173. 10.1212/WNL.0b013e31829a3421 [DOI] [PubMed] [Google Scholar]
- Nalls M. A., Pankratz N., Lill C. M., Do C. B., Hernandez D. G., Saad M., DeStefano A. L., Kara E., Bras J., Sharma M. et al. (2014). Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson's disease. Nat. Genet. 46, 989-993. 10.1038/ng.3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbit M. A., Hannan F. M., Howles S. A., Reed A. A., Cranston T., Thakker C. E., Gregory L., Rimmer A. J., Rust N., Graham U. et al. (2013). Mutations in AP2S1 cause familial hypocalciuric hypercalcemia type 3. Nat. Genet. 45, 93-97. 10.1038/ng.2492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng B. G. and Freeze H. H. (2018). Perspectives on glycosylation and its congenital Disorders. Trends Genet. 34, 466-476. 10.1016/j.tig.2018.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen P. M., Gandasi N. R., Xie B., Sugahara S., Xu Y. and Idevall-Hagren O. (2019). The PI(4)P phosphatase Sac2 controls insulin granule docking and release. J. Cell Biol. 218, 3714-3729. 10.1083/jcb.201903121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols W. C., Seligsohn U., Zivelin A., Terry V. H., Hertel C. E., Wheatley M. A., Moussalli M. J., Hauri H.-P., Ciavarella N., Kaufman R. J. et al. (1998). Mutations in the ER-Golgi intermediate compartment protein ERGIC-53 cause combined deficiency of coagulation factors V and VIII. Cell 93, 61-70. 10.1016/S0092-8674(00)81146-0 [DOI] [PubMed] [Google Scholar]
- Nichols W. C., Terry V. H., Wheatley M. A., Yang A., Zivelin A., Ciavarella N., Stefanile C., Matsushita T., Saito H., de Bosch N. B. et al. (1999). ERGIC-53 gene structure and mutation analysis in 19 combined factors V and VIII deficiency families. Blood 93, 2261-2266. [PubMed] [Google Scholar]
- Nicot A.-S., Toussaint A., Tosch V., Kretz C., Wallgren-Pettersson C., Iwarsson E., Kingston H., Garnier J.-M., Biancalana V., Oldfors A. et al. (2007). Mutations in amphiphysin 2 (BIN1) disrupt interaction with dynamin 2 and cause autosomal recessive centronuclear myopathy. Nat. Genet. 39, 1134-1139. 10.1038/ng2086 [DOI] [PubMed] [Google Scholar]
- Nixon R. A. (2017). Amyloid precursor protein and endosomal-lysosomal dysfunction in Alzheimer's disease: inseparable partners in a multifactorial disease. FASEB J. 31, 2729-2743. 10.1096/fj.201700359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourse C. R., Mattei M.-G., Gunning P. and Byrne J. A (1998). Cloning of a third member of the D52 gene family indicates alternative coding sequence usage in D52-like transcripts. Biochim. Biophys. Acta 1443, 155-168. 10.1016/S0167-4781(98)00211-5 [DOI] [PubMed] [Google Scholar]
- Novick P. and Schekman R. (1979). Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 76, 1858-1862. 10.1073/pnas.76.4.1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J., Bailin T., Fukai K., Feng G. H., Ho L., Mao J. I., Frenk E., Tamura N. and Spritz R. A. (1996). Positional cloning of a gene for Hermansky-Pudlak syndrome, a disorder of cytoplasmic organelles. Nat. Genet. 14, 300-306. 10.1038/ng1196-300 [DOI] [PubMed] [Google Scholar]
- Ohira M., Oshitani N., Hosomi S., Watanabe K., Yamagami H., Tominaga K., Watanabe T., Fujiwara Y., Maeda K., Hirakawa K. et al. (2009). Dislocation of Rab13 and vasodilator-stimulated phosphoprotein in inactive colon epithelium in patients with Crohn's disease. Int. J. Mol. Med. 24, 829-835. 10.3892/ijmm_00000300 [DOI] [PubMed] [Google Scholar]
- Olgiati S., Quadri M., Fang M., Rood J. P., Saute J. A., Chien H. F., Bouwkamp C. G., Graafland J., Minneboo M., Breedveld G. J. et al. (2016). DNAJC6 mutations associated with early-onset Parkinson's disease. Ann. Neurol. 79, 244-256. 10.1002/ana.24553 [DOI] [PubMed] [Google Scholar]
- Orlacchio A., Babalini C., Borreca A., Patrono C., Massa R., Basaran S., Munhoz R. P., Rogaeva E. A., St George-Hyslop P. H., Bernardi G. et al. (2010). SPATACSIN mutations cause autosomal recessive juvenile amyotrophic lateral sclerosis. Brain 133, 591-598. 10.1093/brain/awp325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz-Levi D., Ben-Zeev B., Ruzzo E. K., Hitomi Y., Gelman A., Pelak K., Anikster Y., Reznik-Wolf H., Bar-Joseph I., Olender T. et al. (2012). Mutation in TECPR2 reveals a role for autophagy in hereditary spastic paraparesis. Am. J. Hum. Genet. 91, 1065-1072. 10.1016/j.ajhg.2012.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paesold-Burda P., Maag C., Troxler H., Foulquier F., Kleinert P., Schnabel S., Baumgartner M. and Hennet T (2009). Deficiency in COG5 causes a moderate form of congenital disorders of glycosylation. Hum. Mol. Genet. 18, 4350-4356. 10.1093/hmg/ddp389 [DOI] [PubMed] [Google Scholar]
- Paisan-Ruiz C., Jain S., Evans E. W., Gilks W. P., Simon J., van der Brug M., Lopez de Munain A., Aparicio S., Gil A. M., Khan N. et al. (2004). Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron 44, 595-600. 10.1016/j.neuron.2004.10.023 [DOI] [PubMed] [Google Scholar]
- Pantazopoulou A. and Glick B. S. (2019). A kinetic view of membrane traffic pathways can transcend the classical view of golgi compartments. Front. Cell Dev. Biol. 7, 153 10.3389/fcell.2019.00153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson N., Ince P. G., Smith M. O., Highley R., Skibinski G., Andersen P. M., Morrison K. E., Pall H. S., Hardiman O., Collinge J. et al. (2006). ALS phenotypes with mutations in CHMP2B (charged multivesicular body protein 2B). Neurology 67, 1074-1077. 10.1212/01.wnl.0000231510.89311.8b [DOI] [PubMed] [Google Scholar]
- Parton R. G. (2018). Caveolae: structure, function, and relationship to disease. Annu. Rev. Cell Dev. Biol. 34, 111-136. 10.1146/annurev-cellbio-100617-062737 [DOI] [PubMed] [Google Scholar]
- Pasteris N. G., Cadle A., Logie L. J., Porteous M. E., Schwartz C. E., Stevenson R. E., Glover T. W., Wilroy R. S. and Gorski J. L (1994). Isolation and characterization of the faciogenital dysplasia (Aarskog-Scott syndrome) gene: a putative Rho/Rac guanine nucleotide exchange factor. Cell 79, 669-678. 10.1016/0092-8674(94)90552-5 [DOI] [PubMed] [Google Scholar]
- Pastural E., Barrat F. J., Dufourcq-Lagelouse R., Certain S., Sanal O., Jabado N., Seger R., Griscelli C., Fischer A. and de Saint Basile G. (1997). Griscelli disease maps to chromosome 15q21 and is associated with mutations in the myosin-Va gene. Nat. Genet. 16, 289-292. 10.1038/ng0797-289 [DOI] [PubMed] [Google Scholar]
- Patel H., Cross H., Proukakis C., Hershberger R., Bork P., Ciccarelli F. D., Patton M. A., McKusick V. A. and Crosby A. H. (2002). SPG20 is mutated in Troyer syndrome, an hereditary spastic paraplegia. Nat. Genet. 31, 347-348. 10.1038/ng937 [DOI] [PubMed] [Google Scholar]
- Pereira J. A., Lebrun-Julien F. and Suter U. (2012). Molecular mechanisms regulating myelination in the peripheral nervous system. Trends Neurosci. 35, 123-134. 10.1016/j.tins.2011.11.006 [DOI] [PubMed] [Google Scholar]
- Pereira N. A., Pu H. X., Goh H. and Song Z. (2014). Golgi phosphoprotein 3 mediates the Golgi localization and function of protein O-linked mannose beta-1,2-N-acetlyglucosaminyltransferase 1. J. Biol. Chem. 289, 14762-14770. 10.1074/jbc.M114.548305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe O., Rio M., Carioux A., Plaza J. M., Guigue P., Molinari F., Boddaert N., Bole-Feysot C., Nitschke P., Smahi A. et al. (2009). Combination of linkage mapping and microarray-expression analysis Identifies NF-κB signaling defect as a cause of autosomal-recessive mental retardation. Am. J. Hum. Genet. 85, 903-908. 10.1016/j.ajhg.2009.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho S. S. and Reis C. A. (2015). Glycosylation in cancer: mechanisms and clinical implications. Nat. Rev. Cancer 15, 540-555. 10.1038/nrc3982 [DOI] [PubMed] [Google Scholar]
- Piwon N., Gunther W., Schwake M., Bosl M. R. and Jentsch T. J. (2000). ClC-5 Cl- -channel disruption impairs endocytosis in a mouse model for Dent's disease. Nature 408, 369-373. 10.1038/35042597 [DOI] [PubMed] [Google Scholar]
- Platt F. M., d'Azzo A., Davidson B. L., Neufeld E. F. and Tifft C. J. (2018). Lysosomal storage diseases. Nat. Rev. Dis. Primers 4, 27 10.1038/s41572-018-0025-4 [DOI] [PubMed] [Google Scholar]
- Pohler E., Mamai O., Hirst J., Zamiri M., Horn H., Nomura T., Irvine A. D., Moran B., Wilson N. J., Smith F. J. et al. (2012). Haploinsufficiency for AAGAB causes clinically heterogeneous forms of punctate palmoplantar keratoderma. Nat. Genet. 44, 1272-1276. 10.1038/ng.2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos M. H., Lavedan C., Leroy E., Ide S. E., Dehejia A., Dutra A., Pike B., Root H., Rubenstein J., Boyer R. et al. (1997). Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science 276, 2045-2047. 10.1126/science.276.5321.2045 [DOI] [PubMed] [Google Scholar]
- Purlyte E., Dhekne H. S., Sarhan A. R., Gomez R., Lis P., Wightman M., Martinez T. N., Tonelli F., Pfeffer S. R. and Alessi D. R. (2018). Rab29 activation of the Parkinson's disease-associated LRRK2 kinase. EMBO J. 37, 1-18. 10.15252/embj.201798099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadri M., Fang M., Picillo M., Olgiati S., Breedveld G. J., Graafland J., Wu B., Xu F., Erro R., Amboni M. et al. (2013). Mutation in the SYNJ1 gene associated with autosomal recessive, early-onset Parkinsonism. Hum. Mutat. 34, 1208-1215. 10.1002/humu.22373 [DOI] [PubMed] [Google Scholar]
- Rafiullah R., Aslamkhan M., Paramasivam N., Thiel C., Mustafa G., Wiemann S., Schlesner M., Wade R. C., Rappold G. A. and Berkel S (2016). Homozygous missense mutation in the LMAN2L gene segregates with intellectual disability in a large consanguineous Pakistani family. J. Med. Genet. 53, 138-144. 10.1136/jmedgenet-2015-103179 [DOI] [PubMed] [Google Scholar]
- Rahajeng J., Kuna R. S., Makowski S. L., Tran T. T. T., Buschman M. D., Li S., Cheng N., Ng M. M. and Field S. J. (2019). Efficient golgi forward trafficking requires GOLPH3-driven, PI4P-dependent membrane curvature. Dev. Cell 50, 573-585.e5. 10.1016/j.devcel.2019.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranta S., Zhang Y., Ross B., Lonka L., Takkunen E., Messer A., Sharp J., Wheeler R., Kusumi K., Mole S. et al. (1999). The neuronal ceroid lipofuscinoses in human EPMR and mnd mutant mice are associated with mutations in CLN8. Nat. Genet. 23, 233-236. 10.1038/13868 [DOI] [PubMed] [Google Scholar]
- Ranta S., Topcu M., Tegelberg S., Tan H., Ustubutun A., Saatci I., Dufke A., Enders H., Pohl K., Alembik Y. et al. (2004). Variant late infantile neuronal ceroid lipofuscinosis in a subset of Turkish patients is allelic to Northern epilepsy. Hum. Mutat. 23, 300-305. 10.1002/humu.20018 [DOI] [PubMed] [Google Scholar]
- Raza M. H., Mattera R., Morell R., Sainz E., Rahn R., Gutierrez J., Paris E., Root J., Solomon B., Brewer C. et al. (2015). Association between rare variants in AP4E1, a component of intracellular trafficking, and persistent stuttering. Am. J. Hum. Genet. 97, 715-725. 10.1016/j.ajhg.2015.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter J. F. and Leroux M. R. (2017). Genes and molecular pathways underpinning ciliopathies. Nat. Rev. Mol. Cell Biol. 18, 533-547. 10.1038/nrm.2017.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton A. E., Majounie E., Waite A., Simon-Sanchez J., Rollinson S., Gibbs J. R., Schymick J. C., Laaksovirta H., van Swieten J. C., Myllykangas L. et al. (2011). A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72, 257-268. 10.1016/j.neuron.2011.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M. S., Tawamie H., Buchert R., Hosny Gebril O., Froukh T., Thiel C., Uebe S., Ekici A. B., Krumbiegel M., Zweier C. et al. (2017). Diagnostic yield and novel candidate genes by exome sequencing in 152 consanguineous families with neurodevelopmental disorders. JAMA Psychiatry 74, 293-299. 10.1001/jamapsychiatry.2016.3798 [DOI] [PubMed] [Google Scholar]
- Reynders E., Foulquier F., Leão Teles E., Quelhas D., Morelle W., Rabouille C., Annaert W. and Matthijs G (2009). Golgi function and dysfunction in the first COG4-deficient CDG type II patient. Hum. Mol. Genet. 18, 3244-3256. 10.1093/hmg/ddp262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaie T., Child A., Hitchings R., Brice G., Miller L., Coca-Prados M., Heon E., Krupin T., Ritch R., Kreutzer D. et al. (2002). Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science 295, 1077-1079. 10.1126/science.1066901 [DOI] [PubMed] [Google Scholar]
- Rivera V. M., Wang X., Wardwell S., Courage N. L., Volchuk A., Keenan T., Holt D. A., Gilman M., Orci L., Cerasoli F. Jr. et al. (2000). Regulation of protein secretion through controlled aggregation in the endoplasmic reticulum. Science 287, 826-830. 10.1126/science.287.5454.826 [DOI] [PubMed] [Google Scholar]
- Rizzo R., Parashuraman S., D'Angelo G. and Luini A. (2017). GOLPH3 and oncogenesis: What is the molecular link? Tissue Cell 49, 170-174. 10.1016/j.tice.2016.06.008 [DOI] [PubMed] [Google Scholar]
- Rodan L. H., Cohen J., Fatemi A., Gillis T., Lucente D., Gusella J. and Picker J. D (2016). A novel neurodevelopmental disorder associated with compound heterozygous variants in the huntingtin gene. Eur. J. Hum. Genet. 24, 1826-1827. 10.1038/ejhg.2016.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeckel N., Woerner S. M., Kloor M., Yuan Y.-P., Patsos G., Gromes R., Kopitz J. and Gebert J. (2009). High frequency of LMAN1 abnormalities in colorectal tumors with microsatellite instability. Cancer Res. 69, 292-299. 10.1158/0008-5472.CAN-08-3314 [DOI] [PubMed] [Google Scholar]
- Rossi G., Manfrin A. and Lutolf M. P. (2018). Progress and potential in organoid research. Nat. Rev. Genet. 19, 671-687. 10.1038/s41576-018-0051-9 [DOI] [PubMed] [Google Scholar]
- Rossor A. M., Polke J. M., Houlden H. and Reilly M. M. (2013). Clinical implications of genetic advances in Charcot-Marie-Tooth disease. Nat. Rev. Neurol. 9, 562-571. 10.1038/nrneurol.2013.179 [DOI] [PubMed] [Google Scholar]
- Roux K. J., Kim D. I., Raida M. and Burke B. (2012). A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J. Cell Biol. 196, 801-810. 10.1083/jcb.201112098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy C. C., Levy E., Green P. H., Sniderman A., Letarte J., Buts J. P., Orquin J., Brochu P., Weber A. M., Morin C. L. et al. (1987). Malabsorption, hypocholesterolemia, and fat-filled enterocytes with increased intestinal apoprotein B. Chylomicron retention disease. Gastroenterology 92, 390-399. 10.1016/0016-5085(87)90133-8 [DOI] [PubMed] [Google Scholar]
- Royer B., Hnia K., Gavriilidis C., Tronchère H., Tosch V. and Laporte J. (2013). The myotubularin-amphiphysin 2 complex in membrane tubulation and centronuclear myopathies. EMBO Rep. 14, 907-915. 10.1038/embor.2013.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan B. J., Hoek S., Fon E. A. and Wade-Martins R. (2015). Mitochondrial dysfunction and mitophagy in Parkinson's: from familial to sporadic disease. Trends Biochem. Sci. 40, 200-210. 10.1016/j.tibs.2015.02.003 [DOI] [PubMed] [Google Scholar]
- Saheki Y. and De Camilli P. (2012). Synaptic vesicle endocytosis. Cold Spring Harb. Perspect Biol. 4, a005645 10.1101/cshperspect.a005645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitsu H., Kato M., Mizuguchi T., Hamada K., Osaka H., Tohyama J., Uruno K., Kumada S., Nishiyama K., Nishimura A. et al. (2008). De novo mutations in the gene encoding STXBP1 (MUNC18-1) cause early infantile epileptic encephalopathy. Nat. Genet. 40, 782-788. 10.1038/ng.150 [DOI] [PubMed] [Google Scholar]
- Salpietro V., Lin W., Delle Vedove A., Storbeck M., Liu Y., Efthymiou S., Manole A., Wiethoff S., Ye Q., Saggar A. et al. (2017). Homozygous mutations in VAMP1 cause a presynaptic congenital myasthenic syndrome. Ann. Neurol. 81, 597-603. 10.1002/ana.24905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Kavaliauskiene S. and Skotland T. (2018). Clathrin-independent endocytosis: an increasing degree of complexity. Histochem. Cell Biol. 150, 107-118. 10.1007/s00418-018-1678-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger A., Hirst J., Davies A. K. and Robinson M. S. (2019). Adaptor protein complexes and disease at a glance. J. Cell Sci. 132, jcs222992 10.1242/jcs.222992 [DOI] [PubMed] [Google Scholar]
- Sankila E.-M., Tolvanen R., van den Hurk J. A. M., Cremers F. P. and de la Chapelle A. (1992). Aberrant splicing of the CHM gene is a significant cause of choroideremia. Nat. Genet. 1, 109-113. 10.1038/ng0592-109 [DOI] [PubMed] [Google Scholar]
- Schmidt O. and Teis D. (2012). The ESCRT machinery. Curr. Biol. 22, R116-R120. 10.1016/j.cub.2012.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W. M., Rutledge S. L., Schule R., Mayerhofer B., Zuchner S., Boltshauser E. and Bittner R. E. (2015). Disruptive SCYL1 mutations underlie a syndrome characterized by recurrent episodes of liver failure, peripheral neuropathy, cerebellar atrophy, and ataxia. Am. J. Hum. Genet. 97, 855-861. 10.1016/j.ajhg.2015.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreij A. M., Fon E. A. and McPherson P. S. (2016). Endocytic membrane trafficking and neurodegenerative disease. Cell. Mol. Life Sci. 73, 1529-1545. 10.1007/s00018-015-2105-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz K., Iolascon A., Verissimo F., Trede N. S., Horsley W., Chen W., Paw B. H., Hopfner K. P., Holzmann K., Russo R. et al. (2009). Mutations affecting the secretory COPII coat component SEC23B cause congenital dyserythropoietic anemia type II. Nat. Genet. 41, 936-940. 10.1038/ng.405 [DOI] [PubMed] [Google Scholar]
- Scott K. L., Kabbarah O., Liang M. C., Ivanova E., Anagnostou V., Wu J., Dhakal S., Wu M., Chen S., Feinberg T. et al. (2009). GOLPH3 modulates mTOR signalling and rapamycin sensitivity in cancer. Nature 459, 1085-1090. 10.1038/nature08109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabra M. C., Brown M. S. and Goldstein J. L. (1993). Retinal degeneration in choroideremia: deficiency of rab geranylgeranyl transferase. Science 259, 377-381. 10.1126/science.8380507 [DOI] [PubMed] [Google Scholar]
- Segarra N. G., Ballhausen D., Crawford H., Perreau M., Campos-Xavier B., van Spaendonck-Zwarts K., Vermeer C., Russo M., Zambelli P.-Y., Stevenson B. et al. (2015). NBAS mutations cause a multisystem disorder involving bone, connective tissue, liver, immune system, and retina. Am. J. Med. Genet. A 167, 2902-2912. 10.1002/ajmg.a.37338 [DOI] [PubMed] [Google Scholar]
- Senderek J., Bergmann C., Stendel C., Kirfel J., Verpoorten N., De Jonghe P., Timmerman V., Chrast R., Verheijen M. H., Lemke G. et al. (2003). Mutations in a gene encoding a novel SH3/TPR domain protein cause autosomal recessive Charcot-Marie-Tooth type 4C neuropathy. Am. J. Hum. Genet. 73, 1106-1119. 10.1086/379525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta P. and Lippincott-Schwartz J. (2013). Photohighlighting approaches to access membrane dynamics of the Golgi apparatus. Methods Cell Biol. 118, 217-234. 10.1016/B978-0-12-417164-0.00013-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri S., Fitzpatrick A. L., Ikram M. A., DeStefano A. L., Gudnason V., Boada M., Bis J. C., Smith A. V., Carassquillo M. M., Lambert J. C. et al. (2010). Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA 303, 1832-1840. 10.1001/jama.2010.574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setta-Kaffetzi N., Simpson M. A., Navarini A. A., Patel V. M., Lu H.-C., Allen M. H., Duckworth M., Bachelez H., Burden A. D., Choon S.-E. et al. (2014). AP1S3 mutations are associated with pustular psoriasis and impaired Toll-like receptor 3 trafficking. Am. J. Hum. Genet. 94, 790-797. 10.1016/j.ajhg.2014.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen R., Ansari S., Alshammari M. J., Alkhalidi H., Alrukban H., Eyaid W. and Alkuraya F. S (2013a). A novel syndrome of hypohidrosis and intellectual disability is linked to COG6 deficiency. J. Med. Genet. 50, 431-436. 10.1136/jmedgenet-2013-101527 [DOI] [PubMed] [Google Scholar]
- Shaheen R., Faqeih E., Alshammari M. J., Swaid A., Al-Gazali L., Mardawi E., Ansari S., Sogaty S., Seidahmed M. Z., AlMotairi M. I. et al. (2013b). Genomic analysis of Meckel-Gruber syndrome in Arabs reveals marked genetic heterogeneity and novel candidate genes. Eur. J. Hum. Genet. 21, 762-768. 10.1038/ejhg.2012.254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen V. L., Ganesh V. S., Topcu M., Sebire G., Bodell A., Hill R. S., Grant P. E., Shugart Y. Y., Imitola J., Khoury S. J. et al. (2004). Mutations in ARFGEF2 implicate vesicle trafficking in neural progenitor proliferation and migration in the human cerebral cortex. Nat. Genet. 36, 69-76. 10.1038/ng1276 [DOI] [PubMed] [Google Scholar]
- Shen X.-M., Selcen D., Brengman J. and Engel A. G (2014). Mutant SNAP25B causes myasthenia, cortical hyperexcitability, ataxia, and intellectual disability. Neurology 83, 2247-2255. 10.1212/WNL.0000000000001079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X.-M., Scola R. H., Lorenzoni P. J., Kay C. S., Werneck L. C., Brengman J., Selcen D. and Engel A. G (2017). Novel synaptobrevin-1 mutation causes fatal congenital myasthenic syndrome. Ann. Clin. Transl. Neurol. 4, 130-138. 10.1002/acn3.387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih N.-Y., Li J., Cotran R., Mundel P., Miner J. H. and Shaw A. S. (2001). CD2AP localizes to the slit diaphragm and binds to nephrin via a novel C-terminal domain. Am. J. Pathol. 159, 2303-2308. 10.1016/S0002-9440(10)63080-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava-Ranjan P., Faundez V., Fang G., Rees H., Lah J. J., Levey A. I. and Kahn R. A (2008). Mint3/X11γ is an ADP-ribosylation factor-dependent adaptor that regulates the traffic of the Alzheimer's Precursor protein from the trans-Golgi network. Mol. Biol. Cell 19, 51-64. 10.1091/mbc.e07-05-0465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton A. B., Farrer M., Johnson J., Singleton A., Hague S., Kachergus J., Hulihan M., Peuralinna T., Dutra A., Nussbaum R. et al. (2003). alpha-Synuclein locus triplication causes Parkinson's disease. Science 302, 841 10.1126/science.1090278 [DOI] [PubMed] [Google Scholar]
- Skibinski G., Parkinson N. J., Brown J. M., Chakrabarti L., Lloyd S. L., Hummerich H., Nielsen J. E., Hodges J. R., Spillantini M. G., Thusgaard T. et al. (2005). Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat. Genet. 37, 806-808. 10.1038/ng1609 [DOI] [PubMed] [Google Scholar]
- Slabicki M., Theis M., Krastev D. B., Samsonov S., Mundwiller E., Junqueira M., Paszkowski-Rogacz M., Teyra J., Heninger A. K., Poser I. et al. (2010). A genome-scale DNA repair RNAi screen identifies SPG48 as a novel gene associated with hereditary spastic paraplegia. PLoS Biol. 8, e1000408 10.1371/journal.pbio.1000408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slosarek E. L., Schuh A. L., Pustova I., Johnson A., Bird J., Johnson M., Frankel E. B., Bhattacharya N., Hanna M. G., Burke J. E. et al. (2018). Pathogenic TFG mutations underlying hereditary spastic paraplegia impair secretory protein trafficking and axon fasciculation. Cell Rep. 24, 2248-2260. 10.1016/j.celrep.2018.07.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small S. A., Kent K., Pierce A., Leung C., Kang M. S., Okada H., Honig L., Vonsattel J. P. and Kim T. W. (2005). Model-guided microarray implicates the retromer complex in Alzheimer's disease. Ann. Neurol. 58, 909-919. 10.1002/ana.20667 [DOI] [PubMed] [Google Scholar]
- Smigiel R., Landsberg G., Schilling M., Rydzanicz M., Pollak A., Walczak A., Stodolak A., Stawinski P., Mierzewska H., Sasiadek M. M. et al. (2018). Developmental epileptic encephalopathy with hypomyelination and brain atrophy associated with PTPN23 variants affecting the assembly of UsnRNPs. Eur. J. Hum. Genet. 26, 1502-1511. 10.1038/s41431-018-0179-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits P., Bolton A. D., Funari V., Hong M., Boyden E. D., Lu L., Manning D. K., Dwyer N. D., Moran J. L., Prysak M. et al. (2010). Lethal skeletal dysplasia in mice and humans lacking the golgin GMAP-210. N. Engl. J. Med. 362, 206-216. 10.1056/NEJMoa0900158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soo K. Y., Halloran M., Sundaramoorthy V., Parakh S., Toth R. P., Southam K. A., McLean C. A., Lock P., King A., Farg M. A. et al. (2015). Rab1-dependent ER-Golgi transport dysfunction is a common pathogenic mechanism in SOD1, TDP-43 and FUS-associated ALS. Acta Neuropathol. 130, 679-697. 10.1007/s00401-015-1468-2 [DOI] [PubMed] [Google Scholar]
- Søreng K., Neufeld T. P. and Simonsen A. (2018). Membrane trafficking in autophagy. Int. Rev. Cell Mol. Biol. 336, 1-92. 10.1016/bs.ircmb.2017.07.001 [DOI] [PubMed] [Google Scholar]
- Sowada N., Hashem M. O., Yilmaz R., Hamad M., Kakar N., Thiele H., Arold S. T., Bode H., Alkuraya F. S. and Borck G (2017). Mutations of PTPN23 in developmental and epileptic encephalopathy. Hum. Genet. 136, 1455-1461. 10.1007/s00439-017-1850-3 [DOI] [PubMed] [Google Scholar]
- Spaenij-Dekking E. H., Van Delft J., Van Der Meijden E., Hiemstra H. S., Falkenburg J. H., Koning F., Drijfhout J. W. and Kluin-Nelemans J. C (2003). Synaptojanin 2 is recognized by HLA class II-restricted hairy cell leukemia-specific T cells. Leukemia 17, 2467-2473. 10.1038/sj.leu.2403174 [DOI] [PubMed] [Google Scholar]
- Sprecher E., Ishida-Yamamoto A., Mizrahi-Koren M., Rapaport D., Goldsher D., Indelman M., Topaz O., Chefetz I., Keren H., O'Brien T J. et al. (2005). A mutation in SNAP29, coding for a SNARE protein involved in intracellular trafficking, causes a novel neurocutaneous syndrome characterized by cerebral dysgenesis, neuropathy, ichthyosis, and palmoplantar keratoderma. Am. J. Hum. Genet. 77, 242-251. 10.1086/432556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadel D., Millarte V., Tillmann K. D., Huber J., Tamin-Yecheskel B. C., Akutsu M., Demishtein A., Ben-Zeev B., Anikster Y., Perez F. et al. (2015). TECPR2 cooperates with LC3C to regulate COPII-dependent ER export. Mol. Cell 60, 89-104. 10.1016/j.molcel.2015.09.010 [DOI] [PubMed] [Google Scholar]
- Steger M., Tonelli F., Ito G., Davies P., Trost M., Vetter M., Wachter S., Lorentzen E., Duddy G., Wilson S. et al. (2016). Phosphoproteomics reveals that Parkinson's disease kinase LRRK2 regulates a subset of Rab GTPases. Elife 5, e12813 10.7554/eLife.12813.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg F., Gallon M., Winfield M., Thomas E. C., Bell A. J., Heesom K. J., Tavaré J. M. and Cullen P. J. (2013). A global analysis of SNX27-retromer assembly and cargo specificity reveals a function in glucose and metal ion transport. Nat. Cell Biol. 15, 461-471. 10.1038/ncb2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Brodie S. E. and Cohn Z. A. (1976). Membrane flow during pinocytosis. A stereologic analysis. J. Cell Biol. 68, 665 10.1083/jcb.68.3.665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevanin G., Santorelli F. M., Azzedine H., Coutinho P., Chomilier J., Denora P. S., Martin E., Ouvrard-Hernandez A. M., Tessa A., Bouslam N. et al. (2007). Mutations in SPG11, encoding spatacsin, are a major cause of spastic paraplegia with thin corpus callosum. Nat. Genet. 39, 366-372. 10.1038/ng1980 [DOI] [PubMed] [Google Scholar]
- Stoetzel C., Bär S., De Craene J.-O., Scheidecker S., Etard C., Chicher J., Reck J. R., Perrault I., Geoffroy V., Chennen K. et al. (2016). A mutation in VPS15 (PIK3R4) causes a ciliopathy and affects IFT20 release from the cis-Golgi. Nat. Commun. 7, 13586 10.1038/ncomms13586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street V. A., Bennett C. L., Goldy J. D., Shirk A. J., Kleopa K. A., Tempel B. L., Lipe H. P., Scherer S. S., Bird T. D. and Chance P. F. (2003). Mutation of a putative protein degradation gene LITAF/SIMPLE in Charcot-Marie-Tooth disease 1C. Neurology 60, 22-26. 10.1212/WNL.60.1.22 [DOI] [PubMed] [Google Scholar]
- Südhof T. C. (2013). Neurotransmitter release: the last millisecond in the life of a synaptic vesicle. Neuron 80, 675-690. 10.1016/j.neuron.2013.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Li W., Zhang Q., Karim A., Novak E. K., Sviderskaya E. V., Hill S. P., Bennett D. C., Levin A. V., Nieuwenhuis H. K. et al. (2002). Hermansky-Pudlak syndrome is caused by mutations in HPS4, the human homolog of the mouse light-ear gene. Nat. Genet. 30, 321-324. 10.1038/ng835 [DOI] [PubMed] [Google Scholar]
- Takei K., Slepnev V. I., Haucke V. and De Camilli P. (1999). Functional partnership between amphiphysin and dynamin in clathrin-mediated endocytosis. Nat. Cell Biol. 1, 33-39. 10.1038/9004 [DOI] [PubMed] [Google Scholar]
- Tarpey P. S., Stevens C., Teague J., Edkins S., O'Meara S., Avis T., Barthorpe S., Buck G., Butler A., Cole J. et al. (2006). Mutations in the gene encoding the Sigma 2 subunit of the adaptor protein 1 complex, AP1S2, cause X-linked mental retardation. Am. J. Hum. Genet. 79, 1119-1124. 10.1086/510137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayanidhi N., Helm J. R., Nycz D. C., Bentley M., Liang Y. and Hay J. C. (2010). Alpha-synuclein delays endoplasmic reticulum (ER)-to-Golgi transport in mammalian cells by antagonizing ER/Golgi SNAREs. Mol. Biol. Cell 21, 1850-1863. 10.1091/mbc.e09-09-0801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh W. H. and Gleeson P. A. (2016). Dysregulation of intracellular trafficking and endosomal sorting in Alzheimer's disease: controversies and unanswered questions. Biochem. J. 473, 1977-1993. 10.1042/BCJ20160147 [DOI] [PubMed] [Google Scholar]
- Torras N., García-Díaz M., Fernández-Majada V. and Martínez E. (2018). Mimicking epithelial tissues in three-dimensional cell culture models. Front Bioeng Biotechnol 6, 197 10.3389/fbioe.2018.00197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Town M., Jean G., Cherqui S., Attard M., Forestier L., Whitmore S. A., Callen D. F., Gribouval O., Broyer M., Bates G. P. et al. (1998). A novel gene encoding an integral membrane protein is mutated in nephropathic cystinosis. Nat. Genet. 18, 319-324. 10.1038/ng0498-319 [DOI] [PubMed] [Google Scholar]
- Tsai L., Schwake M., Corbett M. A., Gecz J., Berkovic S. and Shieh P. B. (2013). P.1.20 GOSR2: a novel form of congenital muscular dystrophy. Neuromuscul. Disord. 23, 748 10.1016/j.nmd.2013.06.404 [DOI] [Google Scholar]
- Unlu G., Qi X., Gamazon E. R., Melville D. B., Patel N., Rushing A. R., Hashem M., Al-Faifi A., Chen R., Li B. et al. (2020). Phenome-based approach identifies RIC1-linked Mendelian syndrome through zebrafish models, biobank associations and clinical studies. Nat. Med. 26, 98-109. 10.1038/s41591-019-0705-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uwineza A., Caberg J.-H., Hitayezu J., Wenric S., Mutesa L., Vial Y., Drunat S., Passemard S., Verloes A., El Ghouzzi V. et al. (2019). VPS51 biallelic variants cause microcephaly with brain malformations: a confirmatory report. Eur. J. Med. Genet. 62, 103704 10.1016/j.ejmg.2019.103704 [DOI] [PubMed] [Google Scholar]
- Valdmanis P. N., Meijer I. A., Reynolds A., Lei A., MacLeod P., Schlesinger D., Zatz M., Reid E., Dion P. A., Drapeau P. et al. (2007). Mutations in the KIAA0196 gene at the SPG8 locus cause hereditary spastic paraplegia. Am. J. Hum. Genet. 80, 152-161. 10.1086/510782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilopoulos S., Esk C., Hoshino S., Funke B. H., Chen C. Y., Plocik A. M., Wright W. E., Kucherlapati R. and Brodsky F. M. (2009). A role for the CHC22 clathrin heavy-chain isoform in human glucose metabolism. Science 324, 1192-1196. 10.1126/science.1171529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venditti R., Scanu T., Santoro M., Di Tullio G., Spaar A., Gaibisso R., Beznoussenko G. V., Mironov A. A., Mironov A. Jr, Zelante L. et al. (2012). Sedlin controls the ER export of procollagen by regulating the Sar1 cycle. Science 337, 1668-1672. 10.1126/science.1224947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven K., De Jonghe P., Coen K., Verpoorten N., Auer-Grumbach M., Kwon J. M., FitzPatrick D., Schmedding E., De Vriendt E., Jacobs A. et al. (2003). Mutations in the small GTP-ase late endosomal protein RAB7 cause Charcot-Marie-Tooth type 2B neuropathy. Am. J. Hum. Genet. 72, 722-727. 10.1086/367847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkerk A. J., Schot R., Dumee B., Schellekens K., Swagemakers S., Bertoli-Avella A. M., Lequin M. H., Dudink J., Govaert P., van Zwol A. L. et al. (2009). Mutation in the AP4M1 gene provides a model for neuroaxonal injury in cerebral palsy. Am. J. Hum. Genet. 85, 40-52. 10.1016/j.ajhg.2009.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilarino-Guell C., Wider C., Ross O. A., Dachsel J. C., Kachergus J. M., Lincoln S. J., Soto-Ortolaza A. I., Cobb S. A., Wilhoite G. J., Bacon J. A. et al. (2011). VPS35 mutations in Parkinson disease. Am. J. Hum. Genet. 89, 162-167. 10.1016/j.ajhg.2011.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilarino-Guell C., Rajput A., Milnerwood A. J., Shah B., Szu-Tu C., Trinh J., Yu I., Encarnacion M., Munsie L. N., Tapia L. et al. (2014). DNAJC13 mutations in Parkinson disease. Hum. Mol. Genet. 23, 1794-1801. 10.1093/hmg/ddt570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronov S. V., Frere S. G., Giovedi S., Pollina E. A., Borel C., Zhang H., Schmidt C., Akeson E. C., Wenk M. R., Cimasoni L. et al. (2008). Synaptojanin 1-linked phosphoinositide dyshomeostasis and cognitive deficits in mouse models of Down's syndrome. Proc. Natl. Acad. Sci. USA 105, 9415-9420. 10.1073/pnas.0803756105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandinger-Ness A. and Zerial M. (2014). Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb. Perspect Biol. 6, a022616 10.1101/cshperspect.a022616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. S., Devuyst O., Courtoy P. J., Wang X. T., Wang H., Wang Y., Thakker R. V., Guggino S. and Guggino W. B. (2000). Mice lacking renal chloride channel, CLC-5, are a model for Dent's disease, a nephrolithiasis disorder associated with defective receptor-mediated endocytosis. Hum. Mol. Genet. 9, 2937-2945. 10.1093/hmg/9.20.2937 [DOI] [PubMed] [Google Scholar]
- Wang C., Yuan X. and Yang S. (2013). IFT80 is essential for chondrocyte differentiation by regulating Hedgehog and Wnt signaling pathways. Exp. Cell Res. 319, 623-632. 10.1016/j.yexcr.2012.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren J. D., Rohrer J. D. and Rossor M. N. (2013). Clinical review. Frontotemporal dementia. BMJ 347, f4827 10.1136/bmj.f4827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartosch L., Bright N. A. and Luzio J. P. (2015). Lysosomes. Curr. Biol. 25, R315-R316. 10.1016/j.cub.2015.02.027 [DOI] [PubMed] [Google Scholar]
- Watkin L. B., Jessen B., Wiszniewski W., Vece T. J., Jan M., Sha Y., Thamsen M., Santos-Cortez R. L., Lee K., Gambin T. et al. (2015). COPA mutations impair ER-Golgi transport and cause hereditary autoimmune-mediated lung disease and arthritis. Nat. Genet. 47, 654-660. 10.1038/ng.3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrle A., Witkos T. M., Unger S., Schneider J., Follit J. A., Hermann J., Welting T., Fano V., Hietala M., Vatanavicharn N. et al. (2019). Hypomorphic mutations of TRIP11 cause odontochondrodysplasia. JCI Insight 4, e124701 10.1172/jci.insight.124701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West A. B., Moore D. J., Biskup S., Bugayenko A., Smith W. W., Ross C. A., Dawson V. L. and Dawson T. M. (2005). Parkinson's disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc. Natl. Acad. Sci. USA 102, 16842-16847. 10.1073/pnas.0507360102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler D. B., Zoncu R., Root D. E., Sabatini D. M. and Sawyers C. L. (2015). Identification of an oncogenic RAB protein. Science 350, 211-217. 10.1126/science.aaa4903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegerinck C. L., Janecke A. R., Schneeberger K., Vogel G. F., van Haaften-Visser D. Y., Escher J. C., Adam R., Thoni C. E., Pfaller K., Jordan A. J. et al. (2014). Loss of syntaxin 3 causes variant microvillus inclusion disease. Gastroenterology 147, 65-68.e10. 10.1053/j.gastro.2014.04.002 [DOI] [PubMed] [Google Scholar]
- Wilson G. R., Sim J. C., McLean C., Giannandrea M., Galea C. A., Riseley J. R., Stephenson S. E., Fitzpatrick E., Haas S. A., Pope K. et al. (2014). Mutations in RAB39B cause X-linked intellectual disability and early-onset Parkinson disease with alpha-synuclein pathology. Am. J. Hum. Genet. 95, 729-735. 10.1016/j.ajhg.2014.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkos T. M., Chan W. L., Joensuu M., Rhiel M., Pallister E., Thomas-Oates J., Mould A. P., Mironov A. A., Biot C., Guerardel Y. et al. (2019). GORAB scaffolds COPI at the trans-Golgi for efficient enzyme recycling and correct protein glycosylation. Nat. Commun. 10, 127 10.1038/s41467-018-08044-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M. and Munro S. (2014). Membrane trafficking. The specificity of vesicle traffic to the Golgi is encoded in the golgin coiled-coil proteins. Science 346, 1256898 10.1126/science.1256898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Steet R. A., Bohorov O., Bakker J., Newell J., Krieger M., Spaapen L., Kornfeld S. and Freeze H. H (2004). Mutation of the COG complex subunit gene COG7 causes a lethal congenital disorder. Nat. Med. 10, 518-523. 10.1038/nm1041 [DOI] [PubMed] [Google Scholar]
- Xue K., Jolly J. K., Barnard A. R., Rudenko A., Salvetti A. P., Patrício M. I., Edwards T. L., Groppe M., Orlans H. O., Tolmachova T. et al. (2018). Beneficial effects on vision in patients undergoing retinal gene therapy for choroideremia. Nat. Med. 24, 1507-1512. 10.1038/s41591-018-0185-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Hentati A., Deng H.-X., Dabbagh O., Sasaki T., Hirano M., Hung W.-Y., Ouahchi K., Yan J., Azim A. C. et al. (2001). The gene encoding alsin, a protein with three guanine-nucleotide exchange factor domains, is mutated in a form of recessive amyotrophic lateral sclerosis. Nat. Genet. 29, 160-165. 10.1038/ng1001-160 [DOI] [PubMed] [Google Scholar]
- Yang X.-Y., Zhou X.-Y., Wang Q. Q., Li H., Chen Y., Lei Y.-P., Ma X.-H., Kong P., Shi Y., Jin L. et al. (2013). Mutations in the COPII vesicle component gene SEC24B are associated with human neural tube defects. Hum. Mutat. 34, 1094-1101. 10.1002/humu.22338 [DOI] [PubMed] [Google Scholar]
- Yehia L., Niazi F., Ni Y., Ngeow J., Sankunny M., Liu Z., Wei W., Mester J. L., Keri R. A., Zhang B. et al. (2015). Germline heterozygous variants in SEC23B are associated with cowden syndrome and enriched in apparently sporadic thyroid cancer. Am. J. Hum. Genet. 97, 661-676. 10.1016/j.ajhg.2015.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehia L., Jindal S., Komar A. A. and Eng C (2018). Non-canonical role of cancer-associated mutant SEC23B in the ribosome biogenesis pathway. Hum. Mol. Genet. 27, 3154-3164. 10.1093/hmg/ddy226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarranz J. J., Alegre J., Gómez-Esteban J. C., Lezcano E., Ros R., Ampuero I., Vidal L., Hoenicka J., Rodriguez O., Atarés B. et al. (2004). The new mutation, E46K, of α-synuclein causes parkinson and Lewy body dementia. Ann. Neurol. 55, 164-173. 10.1002/ana.10795 [DOI] [PubMed] [Google Scholar]
- Zhang B., Cunningham M. A., Nichols W. C., Bernat J. A., Seligsohn U., Pipe S. W., McVey J. H., Schulte-Overberg U., de Bosch N. B., Ruiz-Saez A. et al. (2003a). Bleeding due to disruption of a cargo-specific ER-to-Golgi transport complex. Nat. Genet. 34, 220-225. 10.1038/ng1153 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Zhao B., Li W., Oiso N., Novak E. K., Rusiniak M. E., Gautam R., Chintala S., O'Brien E. P., Zhang Y. et al. (2003b). Ru2 and Ru encode mouse orthologs of the genes mutated in human Hermansky-Pudlak syndrome types 5 and 6. Nat. Genet. 33, 145-153. 10.1038/ng1087 [DOI] [PubMed] [Google Scholar]
- Zhang B., Kaufman R. J. and Ginsburg D. (2005). LMAN1 and MCFD2 form a cargo receptor complex and interact with coagulation factor VIII in the early secretory pathway. J. Biol. Chem. 280, 25881-25886. 10.1074/jbc.M502160200 [DOI] [PubMed] [Google Scholar]
- Zhang X., Chow C. Y., Sahenk Z., Shy M. E., Meisler M. H. and Li J. (2008). Mutation of FIG4 causes a rapidly progressive, asymmetric neuronal degeneration. Brain 131, 1990-2001. 10.1093/brain/awn114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Alvarado D., Rainier S., Lemons R., Hedera P., Weber C. H., Tukel T., Apak M., Heiman-Patterson T., Ming L. et al. (2001). Mutations in a newly identified GTPase gene cause autosomal dominant hereditary spastic paraplegia. Nat. Genet. 29, 326-331. 10.1038/ng758 [DOI] [PubMed] [Google Scholar]
- Zhao Y. and Dzamko N. (2019). Recent developments in LRRK2-targeted therapy for Parkinson's disease. Drugs 79, 1037-1051. 10.1007/s40265-019-01139-4 [DOI] [PubMed] [Google Scholar]
- Zimprich A., Biskup S., Leitner P., Lichtner P., Farrer M., Lincoln S., Kachergus J., Hulihan M., Uitti R. J., Calne D. B. et al. (2004). Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 44, 601-607. 10.1016/j.neuron.2004.11.005 [DOI] [PubMed] [Google Scholar]
- Zimprich A., Benet-Pagès A., Struhal W., Graf E., Eck S. H., Offman M. N., Haubenberger D., Spielberger S., Schulte E. C., Lichtner P. et al. (2011). A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am. J. Hum. Genet. 89, 168-175. 10.1016/j.ajhg.2011.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivony-Elboum Y., Westbroek W., Kfir N., Savitzki D., Shoval Y., Bloom A., Rod R., Khayat M., Gross B., Samri W. et al. (2012). A founder mutation in Vps37A causes autosomal recessive complex hereditary spastic paraparesis. J. Med. Genet. 49, 462-472. 10.1136/jmedgenet-2012-100742 [DOI] [PubMed] [Google Scholar]
- Zuchner S., Noureddine M., Kennerson M., Verhoeven K., Claeys K., De Jonghe P., Merory J., Oliveira S. A., Speer M. C., Stenger J. E. et al. (2005). Mutations in the pleckstrin homology domain of dynamin 2 cause dominant intermediate Charcot-Marie-Tooth disease. Nat. Genet. 37, 289-294. [DOI] [PubMed] [Google Scholar]
- zur Stadt U., Schmidt S., Kasper B., Beutel K., Diler A. S., Henter J. I., Kabisch H., Schneppenheim R., Nurnberg P., Janka G. et al. (2005). Linkage of familial hemophagocytic lymphohistiocytosis (FHL) type-4 to chromosome 6q24 and identification of mutations in syntaxin 11. Hum. Mol. Genet. 14, 827-834. 10.1093/hmg/ddi076 [DOI] [PubMed] [Google Scholar]
- zur Stadt U., Rohr J., Seifert W., Koch F., Grieve S., Pagel J., Strauss J., Kasper B., Nurnberg G., Becker C. et al. (2009). Familial hemophagocytic lymphohistiocytosis type 5 (FHL-5) is caused by mutations in Munc18-2 and impaired binding to syntaxin 11. Am. J. Hum. Genet. 85, 482-492. 10.1016/j.ajhg.2009.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]