Abstract
The obligate intracellular bacterium Chlamydia trachomatis causes the most prevalent bacterial sexual transmitted infection worldwide. CD4 T cells play a central role in protective immunity against Chlamydia female reproductive tract (FRT) infection, while B cells are thought to be dispensable for resolution of primary Chlamydia infection in mouse models. We recently reported an unexpected requirement of B cells in local Chlamydia-specific CD4 T cell priming and bacterial containment within the FRT. Here, we sought to tackle the precise effector function of B cells during Chlamydia primary infection. Using mixed bone marrow chimeras that lack B cell-dependent Ag presentation (MHCIIB−/−) or devoid of circulating antibodies (AID−/− x μS−/−), we show that Chlamydia-specific CD4 T cell expansion does not rely on Ag presentation by B cells. Importantly, we demonstrate that antibody, but not B cell-dependent antigen presentation, is required for preventing systemic bacterial dissemination following Chlamydia FRT infection.
Keywords: Antibody, antigen presentation, B cells, infection, Chlamydia
Introduction
Chlamydia is a gram-negative obligate intracellular bacterium that causes diseases at various mucosal tissues. Chlamydia trachomatis ocular infection leads to inclusion conjunctivitis and trachoma, the leading cause of infectious blindness worldwide[1]. Sexually transmitted C. trachomatis are tropic for the transitional and columnar epithelial cells in the female reproductive tract (FRT)[2]. While most Chlamydia sexually transmitted infections (STIs) are asymptomatic, pathological immune responses can often times lead to severe tissue damage and result in pelvic inflammatory disease, ectopic pregnancy and infertility [3]–[7]. In addition to the most prevalent STI C. trachomatis strains (serovars D through K), the invasive lymphoma granuloma venereum (LGV) strains (serovars L1-L3) are associated with several bacterial STI outbreaks in recent years[8]–[10]. These strains evade the local draining lymph nodes and disseminate to surrounding tissues[11]. Despite relatively rare clinical presentations, increasing evidence suggests that C. trachomatis infection causes diseases at remote tissues away from the initial mucosal portal of entry, such as arthritis in joints, Fitz-Huge-Curtis syndrome in the peritoneal cavity and chronic colonization in the gastroenteric tract[12]–[17]. Another species, Chlamydia pneumoniae, the etiologic agent for community-acquired pneumonia, is associated with exacerbation of cardiovascular diseases in both mouse models and clinical studies[18]–[20]. These findings suggest that despite mucosal epithelium tropism, Chlamydia infection in otherwise healthy individuals is likely under strict immune surveillance to prevent systemic spread. However, the host immune components that keep Chlamydia contained at mucosal tissues in immunocompetent hosts remain largely undefined.
Chlamydia muridarum infection in female mice closely resembles many features of C. trachomatis infection in women, including protective immune responses, development of immunopathology[21]–[23]. Using the mouse model of C. muridarum infection, research in the past a few decades has established a central role of cell-mediated immunity (CMI) against Chlamydia[24],[25]. In contrast, B cells or antibody-mediated immunity (AMI) are traditionally thought to be dispensable for immune control over the course of Chlamydia primary infection[23],[24]. Depletion of B cells using anti-IgM antibody showed minimum effect on resolution of C. muridarum infection[26]. B cell-deficient mice clear C. muridarum from the FRT with similar kinetics as WT controls[27]. Moreover, passive immune serum transfer into naïve mice fail to protect the hosts from C. muridarum intravaginal challenge[28]. While B cells seem to be dispensable at the FRT mucosa, our previous study reported an unexpected finding that C. muridarum causes disseminated infection in B cell-deficient (μMT) mice after intravaginal inoculation[29]. These mice exhibit delayed Chlamydia-specific CD4 T cell responses and develop ascites during the first a few weeks after C. muridarum FRT infection. We proposed several mutually non-exclusive mechanisms that could account for B cell-dependent control of disseminated bacterial infection: (1) antibody production; (2) B cell dependent antigen-presentation for prompt CD4 T cell responses in the local draining lymph nodes (DLNs); (3) The presence of B cells in the DLNs to maintain a proper architecture for effective CD4 T cell responses. In the current study, we sought to determine the detailed effector function of B cells in control of disseminated Chlamydia. We report that, antibody, but not B cell dependent antigen presentation, is essential for C. muridarum containment in the FRT.
Results
Generating mixed bone marrow chimeric mice
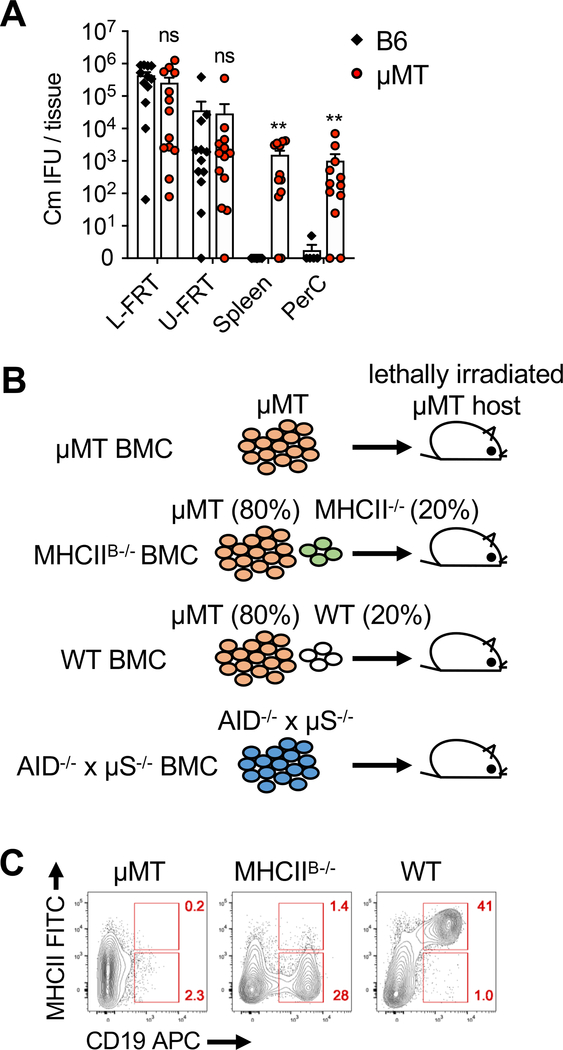
C. muridarum intravaginal inoculation typically induces a contained infection in the female reproductive tract (FRT). Mice deficient in B cells (μMT), however, experience a transient dissemination to distal tissues such as spleen and peritoneal cavity within 2 weeks post infection (Fig. 1A)[29]. This unexpected phenotype prompted us to further examine the mechanism underlying this observation. To disrupt the function of B cells for antigen presentation independent from antibody production, we generated mixed bone marrow chimeras (BMCs) in which MHCII expression was specifically ablated in B cells (MHCIIB−/−) (Fig. 1B) [30],[31]. Loss of MHC class II expression on B cells was confirmed by staining splenocytes from reconstituted BMCs with CD19 and MHCII. As expected, CD19+ B cells in MHCIIB−/− BMCs stained negative for MHCII, whereas B cells in WT BMCs retained high levels of MHCII expression (Fig. 1C). AID−/− x μS−/− BMCs were generated in parallel to address the role of B cells in antibody production (Fig. 1B). Activation-induced deaminase (AID) is essential to initiate antibody class switching, and expression of secretory μ chain (μS) is required for antibody secretion[32],[33]. Thus, AID−/− x μS/− BMCs lack circulating antibodies while retain a polyclonal B cell population expressing functional BCRs and MHCII on their surface [34].
Fig. 1.
Bacterial dissemination in B cell deficient mice and generation of mixed bone marrow chimeras. (A) C57BL/6 (B6) and μMT mice were infected with 1×105 C. muridarum intravaginally. Bacterial burden in the lower (L) and upper (U) female reproductive tract (FRT), spleen and peritoneal cavity (PerC) 13–14 days post infection measured by enumerating IFUs on HeLa229 cells. Data shown are combined results of four independent experiments with a total of 12–13 mice per group. Error bars show mean bacterial counts ± SEM; **p < 0.01; ns, not significant as calculated by Mann-Whitney U test. (B) Cartoon showing μMT bone marrow chimera (BMC) strategy. For MHCIIB−/− BMCs, lethally irradiated μMT mice were reconstituted with μMT and MHCII−/− bone marrow cells mixed at an 80:20 ratio. Therefore, majority (80%) of non-B cells express normal levels of MHCII while none of the B cells express MHCII. (C) Flow cytometry plots showing expression of MHCII on CD19+ B cell. Data shown are representative results of two independent experiments with 3–4 mice per group.
Chlamydia-specific Ab responses were partially diminished in MHCIIB−/− BMCs
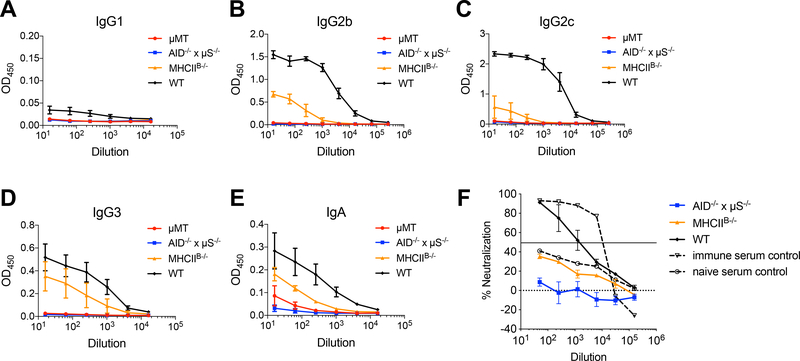
Lack of MHCII expression on B cells could potentially disrupt the cognate interaction between B cells and CD4 T cells, leading to defects in T cell-dependent antibody production. To assess whether Ab responses were affected by lack of MHCII-dependent T-B interactions during Chlamydia FRT infection, we measured antibody levels in MHCIIB−/− BMCs by ELISA. Indeed, MHCIIB−/− BMCs made significantly reduced serum Ig, as demonstrated by the universal reduction of all Ig isotypes measured (Fig. 2A–E). Specifically, high titers of IgG2b and IgG2c were only generated when B cells were expressing MHCII (Fig. 2B and 2C). In contrast, IgG3 responses that were typically induced by T-independent antigens were least affected in MHCIIB−/− BMCs (Fig. 2D) [35]. These results suggest that Chlamydia-specific Ab production only partially relies on MHCII expression on B cells. Of note, although Ab responses were not completely abrogated in MHCIIB−/− BMCs, neutralization activity of these Abs were not detected (Fig. 2F).
Fig. 2.
Serum Ab levels in BMCs 14 days after C. muridarum intravaginal infection.
WT, MHCIIB−/−, AID−/− x μS−/− and μMT BMCs were infected with 1×105 C. muridarum intravaginally. Fourteen days post infection, serum IgG1 (A), IgG2b (B), IgG2c (C), IgG3 (D) and IgA (E) were measured by antibody ELISA; neutralizing Ab levels (F) were measured by Ab neutralization assay. Data shown are representative results of two independent experiments with 3–4 mice per group. Error bars show mean bacterial counts ± SEM.
B-cell dependent antigen presentation is not critical for Chlamydia-specific CD4 T cell responses
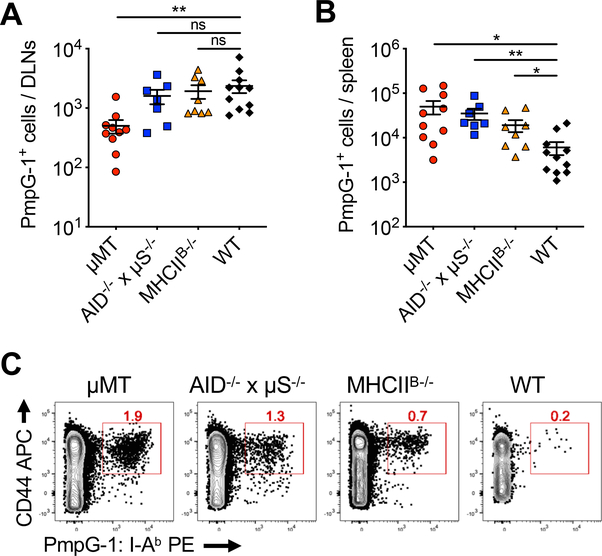
We have previously reasoned that Chlamydia dissemination in B cell deficient mice is, at least in part, due to inefficient CD4 T cell priming in the local draining iliac lymph nodes (DLNs). Consequently, mucosal infections lack effective cell-mediated immune control and dissemination occurs to systemic tissues[29]. The question remains whether the diminished CD4 T cell priming in the DLNs was due to altered lymph node architecture and/or lack of B cell dependent antigen presentation in B cell deficient mice. To dissect the detailed mechanism, we assessed the magnitude of ag-specific CD4 T cell expansion in B cell BMCs using MHC class II tetramers (PmpG-1:I-Ab) specific for an endogenous C. muridarum epitope PmpG-1[29]. As expected, μMT BMCs exhibited reduced Chlamydia-specific CD4 T cell response in the DLNs at day 14 after intravaginal infection. In contrast, Ag-specific CD4 T cell numbers in AID−/− x μS−/− and MHCIIB−/− BMCs were comparable to WT BMCs (Fig. 3A), indicating that both antibody and B cell dependent antigen presentation are dispensable for local CD4 T cell priming. Consistent with our previous observations, bacterial dissemination provoked robust systemic CD4 T cell responses in μMT and AID−/− x μS−/− BMCs, as demonstrated by the large increase of Ag-specific CD4 T cells in the spleens (Fig. 3B and 3C). A mild increase of Chlamydia-specific CD4 T cells was observed in MHCIIB−/− BMCs, while the mechanism remains unclear. Taken together, these results indicate that initial CD4 T cell expansion in response to Chlamydia intravaginal infection does not require antigen presentation by B cells or antibody. Therefore, it is likely that maintaining an intact lymphoid structure by B cells is crucial for efficient CD4 T cell responses.
Fig. 3.
Chlamydia-specific CD4 T cell response is unaltered in the absence of B cell-dependent antigen presentation.
WT, MHCIIB−/−, AID−/− x μS−/− and μMT BMCs were infected with 1×105 C. muridarum intravaginally. Fourteen days post infection, Chlamydia-specific CD4 T cells from spleen and DLNs were analyzed by flow cytometry. (A-B) Total PmpG-1-specific CD4 T cells recovered from the draining lymph nodes (DLNs) (A) and spleens (B). (C) Flow cytometry plots showing representative PmpG-1-specific CD4 T cells from the spleen after tetramer staining and enrichment. All plots were pre-gated on CD11b−F4/80−B220−CD3+CD8−CD4+ cells. Total cell numbers of endogenous PmpG-1-specific CD4 T cells were calculated based on flow cytometry analysis. Data shown are combined results of three independent experiments with a total of 7–11 mice per group. Each data point represents an individual mouse. Error bars show mean ± SEM; *p < 0.05; **p < 0.01; ns, not significant as calculated by unpaired t-test.
Systemic dissemination of Chlamydia in the absence of antibodies
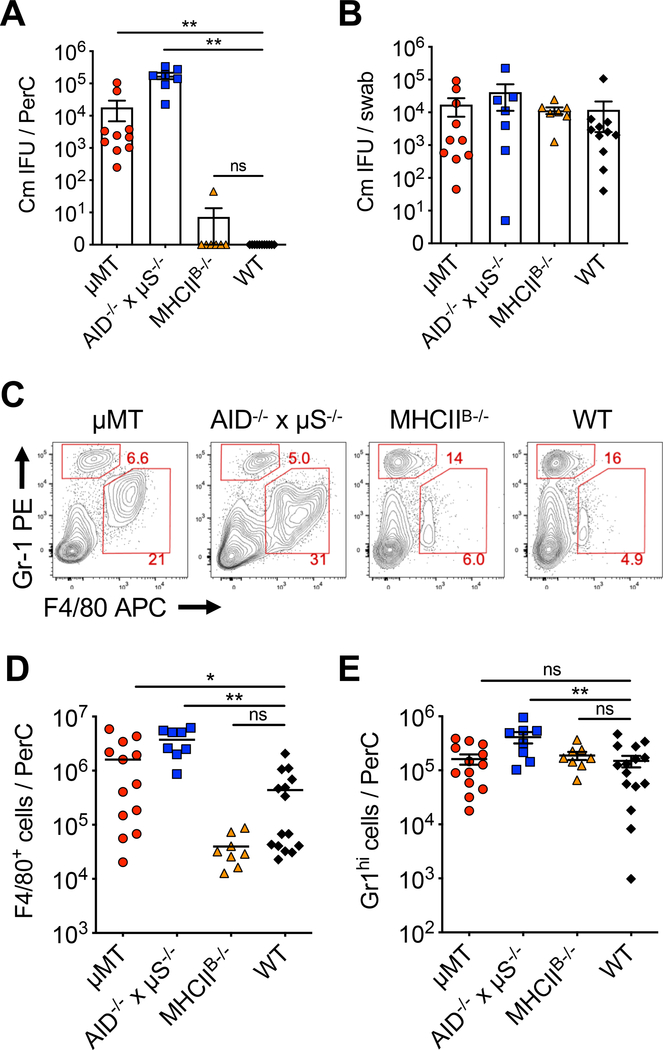
We next examined Chlamydia dissemination of various B cell BMCs. Consistent with our previous findings, high numbers of C. muridarum inclusion forming unites (IFUs) were detected in the peritoneal cavity of μMT BMCs at day 14 after C. muridarum intravaginal infection (Fig. 4A)[29]. Likewise, Chlamydia dissemination were also detected in AID−/− x μS−/− BMCs, as demonstrated by severe ascites and high Chlamydia IFUs in the peritoneal cavity (Fig. 4A). Disseminated infections in μMT and AID−/− x μS−/− BMCs were accompanied by increased F4/80+Gr-1lo macrophage infiltrates into the peritoneal cavity of these mice, as revealed by the analysis of ascites fluid from these mice (Fig. 4C and 4D). A mild increase of F4/80−Gr-1hi neutrophils was also observed in AID−/− x μS−/− BMCs (Fig. 4E). In contrast, no live Chlamydia or elevated macrophage/neutrophil infiltrates were detected in the intraperitoneal lavage of either WT or MHCIIB−/− BMCs (Fig. 4A–E). In addition, bacterial burdens in the FRT as measured by vaginal swabs were similar among all groups (Fig. 4B). These results demonstrate that antibody, but not B cell-dependent antigen presentation, is essential for Chlamydia containment at the FRT mucosa.
Fig. 4.
C. muridarum disseminates to the peritoneal cavity in AID−/− x μS−/− and μMT BMCs.
WT, MHCIIB−/−, AID−/− x μS−/− and μMT BMCs were infected with 1×105 C. muridarum intravaginally. Fourteen days post infection, bacterial burden and cell infiltrates in the peritoneal cavity (PerC) were analyzed. (A-B) Bacteria burden in PerC (A) and the lower FRT (B) measured by enumerating IFUs on HeLa229 cells. Data shown are combined results of three independent experiments with a total of 7–11 mice per group. (C-E) Representative flow cytometry plots (C) and total cell counts of macrophages (F4/80+Gr-1lo) (D) and neutrophils (F4/80−Gr-1hi) (E) isolated from the peritoneal lavage. All plots were pre-gated on FSC/SSC to exclude doublets and dead cells/debris. Total cell numbers were calculated based on flow cytometry analysis. Data shown are combined results of four independent experiments with a total of 8–15 mice per group. Each data point represents an individual mouse. (A-E) Error bars show mean ± SEM; *p < 0.05; **p < 0.01; ns, not significant as calculated by Mann-Whitney U test for bacterial burden and unpaired t-test for cell numbers.
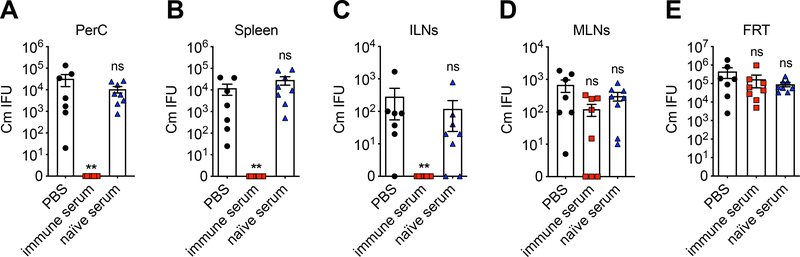
Passive immune serum transfer rescues Chlamydia dissemination in B cell-deficient mice
To functionally complement the loss of antibody in B cell deficient mice, we next conducted passive serum transfer experiments to determine whether antibody is sufficient to rescue the Chlamydia dissemination phenotype in B cell-deficient mice. Immune convalescent serum from WT mice that had resolved a prior C. muridarum intravaginal infection were transferred intraperitoneally into μMT mice before intravaginally challenge with C. muridarum. Bacterial burdens were measured in systemic tissues including spleen, DLNs, mesenteric lymph nodes (MLNs) and peritoneal cavity. As shown in Fig. 5, disseminated bacteria in spleen, ILNs and peritoneal cavity were completely eradicated by immune serum transfer, but not by PBS or naïve serum treatment. Interestingly, live Chlamydia were found in mesenteric lymph nodes in μMT mice, and these bacteria were resistant to passive serum treatment. Consistent with previous reports[28], immune serum transfer had no effect on Chlamydia shedding from the FRT, as C. muridarum IFUs in the lower FRT were similar among all groups at the time of tissue harvest (Fig. 5E).
Fig. 5.
Passive immune serum transfers rescue disseminated C. muridarum from systemic tissues but not the FRT mucosa.
μMT mice were infected with 1×105 C. muridarum intravaginally. Five hundred microliters of immune serum, naïve serum or PBS was injected intraperitoneally into μMT mice on days −1, 0, 3 and 6 after infection. Bacterial burdens in peritoneal cavity (A), spleen (B), draining iliac lymph nodes (C), mesenteric lymph nodes (D) and lower FRT (E) on day 9 post infection as measured by enumerating IFUs on HeLa229 cells. Data shown are combined results of two independent experiments with a total of 7–8 mice per group. Each data point represents an individual mouse. Error bars show mean ± SEM; **p < 0.01; ns, not significant as calculated by Mann-Whitney U test.
Discussion
The multifaceted roles B cells play in host defense against microbial pathogens have been studied extensively in various bacterial and viral infection models, but were rarely being interrogated for Chlamydia, the intracellular bacterium that accounts for the most prevalent bacterial STI worldwide. The lack of focus on B cells is largely due to the facts that both loss-of-function and gain-of-function studies in mouse models failed to establish a protective role for B cells during Chlamydia primary FRT infection[27],[28]. While revisiting the B cell-deficient mice recently, we discovered that B cells are essential for Chlamydia-specific CD4 T cell priming in the DLNs and for preventing Chlamydia systemic dissemination[29]. These findings suggest that B cells play a more important role than previously appreciated, while the detailed mechanisms underlying the protective effect of B cells during Chlamydia primary infection remain to be elucidated.
Antigen sampling by APCs at the site of infection is the critical first step for launching the adaptive immune response. Amongst professional APCs that primes ag-specific CD4 T cells, dendritic cells (DCs) are the most potent APCs that activate naïve CD4 T cells[36]. B cells, in particular marginal zone B cells, has also been shown to participate Ag-presentation at early stage of T cell activation[37]. Our previous observation that Chlamydia-specific CD4 T cell was significantly reduced in the DLNs of B cell deficient mice implies that B cell-dependent Ag presentation may be essential for CD4 T cell priming[29]. Nevertheless, we show here that when MHCII expression is specifically ablated on B cells, clonal expansion of Ag-specific CD4 T cells remains intact. Therefore, the impaired early CD4 T cell responses in μMT mice may primarily attribute to the disrupted lymphoid architecture within the DLNs in the absence of B cells, and consequently inappropriate positioning of DCs and naïve CD4 T cells for effective interaction for CD4 T cell priming[38]. While our data does not exclude the possibility that B cells actively participate in antigen presentation during primary response, and B cells very likely serve as potent APCs during memory response[39], we speculate that submucosa DCs are the major APC population responsible for naïve CD4 T cell activation at the FRT mucosa[40]. These hypotheses will need to be tested experimentally.
The traditional dogma that intracellular bacteria are outside the reach of antibody-mediated immunity (AMI) has been challenged by emerging evidence showing protective antibody responses against intracellular pathogens including Chlamydia[28],[41]–[44]. Our data add to these findings and show definitively that antibody is essential for Chlamydia containment at the FRT mucosa. Antibody-deficient AID−/− x μS−/− BMCs suffer from significant bacteremia, an atypical phenotype following Chlamydia intravaginal infection, but closely mirrors the observation in B cell-deficient μMT mice. Moreover, antibody passive transfer fully complemented the defects of systemically disseminated infection in multiple tissues in μMT mice, including spleen, draining lymph nodes and peritoneal cavity. Of interest, bacterial shedding in the FRT were unaltered by immune serum treatment, indicating that Chlamydia infections in systemic vs mucosal tissues are likely controlled by distinct immune effector mechanisms. Numerous studies have demonstrated direct neutralization of Chlamydia EBs in in vitro tissue culture systems[45]–[47]. However, in vivo findings suggest that antibody does not elicit its effector function simply by neutralization. Consistent with this notion, while in vitro neutralization activity of Abs in MHCIIB−/− BMCs was not evident (Fig. 2F), Ab response in these mice was clearly important for protection. It has been proposed that protective efficacy of antibody depends upon the local context and specific cell types or immune components present at systemic tissues and the pathogen-experienced FRT, but are absent from naïve FRT[48]. While such components for Ab-mediated protection remain elusive, recent studies by Morrison and colleagues shed light on the important roles of IFNγ and neutrophils in Ab-mediated protection against Chlamydia re-infections in the FRT[49],[50].
The immune convalescent serum we used in the serum transfer experiments preclude the possibility of evaluating the early Ab responses for bacterial containment, as high titer, high affinity antibodies are not present during the early stage of an infection. Our results, however, did suggest indirectly that early, T cell-independent Abs are responsible for bacterial containment. In MHCIIB−/− BMCs, cognate interactions between B and T cells were diminished due to the lack of MHCII on B cells to interact with CD4 TCRs. Nevertheless, reduced Ab responses in these mice were sufficient to prevent bacterial systemic spread. This is consistent with other studies documenting early extra-follicular B cell response and its role in protection [48]–[50].
In addition to AMI, CMI plays a central role in host defense against Chlamydia. It is important to note that comparable numbers of Ag-specific CD4 T cells were detected in WT and antibody-deficient AID−/− x μS−/− BMCs, indicating that CMI was not sufficient to rescue the dissemination phenotype in the absence of AMI. Although this conclusion is seemingly contradictory with our previous argument that CD4 T cell response is essential for Chlamydia control in B cell-deficient mice, it is reasonable to speculate that CMI and AMI function synergistically yet independently at early stage of Chlamydia infection. Indeed, a more severe lethal disseminated infection was reported in mice lacking both arms of the adaptive immunity (Rag1−/−), and mice lacking the major Th1 cytokine IFNγ also suffer from disseminated Chlamydia infection[51]–[53]. The non-redundant roles of CMI and AMI in Chlamydia dissemination were further elucidated by a recent study, in which Darville and colleagues showed that adoptive transfer of B cells successfully rescued the lethality of Rag1−/− mice, while bacterial burdens in lung tissues were only partially reduced[54]. Together, these findings contribute to the notion that mucosal surfaces are under stringent immune surveillance by collaborative efforts of CMI and AMI to exclude pathogen from systemic evasion.
In summary, we show in this study that antibody production, but not B cell-dependent antigen presentation to CD4 T cells, is essential for protecting the host from systemic Chlamydia dissemination. Future studies will be implemented to decipher the dynamics and mechanism of antibody-mediated protection in vivo and reveal other possible roles of B cells, such as cytokine production and regulatory functions in protective immunity against Chlamydia[55].
Materials and Methods
Mice
C57BL/6 (B6), μMT (B6.129S2-Ighmtm1Cgn/J), MHCII−/− (B6.129S2-H2dlAb1-Ea/J) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). AID−/− x μS−/− bone marrow cells were kindly provided by Dr. John Harty (University of Iowa). All mice used for experiments were 6–16 weeks old, unless otherwise noted. Mice were maintained under SPF conditions and all mouse experiments were approved by University of Arkansas for Medical Sciences and University of California Davis Institutional Animal Care and Use Committee (IACUC).
Generating bone marrow chimera
Recipient mice were given lethal irradiation at 1000 rad 4–6 h before reconstitution. Bone marrow from donor mice were isolated and five million cells were injected intravenously via the tail vein into the recipient mice. For mixed BMCs, μMT bone marrow was mixed with bone marrow from either MHCII−/− or WT (B6) at an 80:20 ratio. This ratio ensures that the majority (80%) of non-B cells in the BMCs express normal levels of MHCII. Host mice were kept on antibiotic treatment (polymyxin B 150 mg/L, neomycin sulfate 400 mg/L in drinking water) for 4 weeks, and analyzed 8 weeks after reconstitution.
Bacteria
Chlamydia muridarum strain Nigg II was purchased from ATCC (VR-123; Manassas, VA). Elementary bodies (EBs) were propagated in HeLa229 cells, purified by discontinuous density gradient centrifugation and stored at −80°C as previously describe[56],[57]. The stock was tested negative for mycoplasma contamination by both PCR-based mycoplasma detection kit (ATCC, Manassas, VA) and bacteria whole genome sequencing. A fresh aliquot was thawed and used for every infection experiment. The inclusion forming units (IFUs) of EB were determined by infection of HeLa229 cells and enumeration of Chlamydia inclusions stained positive with antiChlamydia MOMP mAb (clone Mo33b, a generous gift from Dr. Harlan Caldwell, NIH).
Chlamydia infection and IFU enumeration
Mice were injected subcutaneously with 2.5 mg Depo-Provera (Greenstone, NJ) 5–7 days prior to intravaginal infection to ensure susceptibly at diestrus phase[58]. For infection, 1×105 C. muridarum EB in SPG buffer were deposited directly into the vaginal vault using a pipet tip. To enumerate bacterial shedding from the FRT, vaginal swabs were collected, disrupted with glass beads suspended in SPG buffer, serial dilutions were plated and IFUs enumerated on HeLa229 cells. To measure bacteria burdens in tissues, spleen, lymph nodes (LNs) were homogenized in SPG buffer, peritoneal cavity (PerC) was lavaged with SPG buffer. Tissue homogenate and lavage were shaked with glass beads. Samples were centrifuged at 500 g for 10 min, supernatants collected and serial dilutions plated on HeLa229 cells for IFU enumeration.
Chlamydia-specific serum Ab ELISA
Mice were bled retro-orbitally and serum was isolated. Heat-killed EBs (HKEBs) were prepared by heating EBs at 56°C for 30 min. High protein binding ELISA plates (Costar) were coated with 1×106 HKEB in 0.1 M carbonate/bicarbonate buffer (pH9.6) overnight at 4°C. Plates were blocked with PBS-T containing 0.1% non-fat milk for 1 hr at room temperature before serial dilutions of serum samples were added to the plates. Chlamydia-specific Abs were detected using HRP-based SBA Clonotyping System (Southern Biotech).
Ab neutralization assay
Antibody neutralization assay were conducted on Syrian hamster kidney (HaK) cells as previously described[59]. Briefly, mouse serum was collected via retro-orbital route and heated at 56°C for 30 min to inactivate complement activity. Serial dilutions of serum samples were mixed with 1500 live EB SPG buffer containing 0.05% BSA at 37°C for 1 hr with shaking (450 rpm). Incubated samples were plated on HaK cells and IFUs enumerated as described above.
Tetramer staining and flow cytometry
Tetramer staining for Chlamydia-specific CD4 T cells was carried out as previously described [29]. Spleen and LNs were harvested from naïve or infected mice and single cell suspensions prepared in FACS buffer (PBS with 2% FCS) containing Chlamydia MHC class-II tetramers (PmpG-1303–311:I-Ab) in Fc block (purified 2.4G2 mAb, 2% mouse serum, 2% rat serum) for 1 hr at 37°C in dark. Cells were washed and tetramer positive cells enriched via magnetic selection using EasySep PE Positive Selection Kit (Stemcell Technologies, Vancouver, BC). The enriched cells were surface stained with a panel of antibodies (listed below) and analyzed on an LSRFortessa flow cytometer (BD Biosciences, San Jose, CA). Antibodies used included B220 (RA3–6B2), CD4 (RM4–5), CD8 (53–6.7), CD11b (M1/70), CD19 (6D5), CD44 (IM7), CD90.2 (53–2.1), F4/80 (BM8), Gr-1 (RB6–8C5), MHCII (M5/114.15.2), Fixable Viability Dye (BioLegend, eBioscience, and BD Biosciences, San Diego, CA). Data were analyzed using FlowJo software (Tree Star, Ashland, OR). Flow cytometry procedures adhere to EJI recently published guidelines[60].
Passive serum transfer
Immune convalescent serum was isolated and pooled from B6 mice at >90 days after C. muridarum intravaginal infection. Five hundred microliters of immune serum, naïve serum or PBS was injected intraperitoneally into recipient mice on days −1, 0, 3 and 6 after infection[28].
Statistical analysis
Statistical analysis was performed by using an unpaired t test for normally distributed continuous variable comparisons and a Mann-Whitney U test for nonparametric comparisons (Prism; GraphPad Software, Inc.).
Supplementary Material
Acknowledgements
We thank Dr. John T. Harty (University of Iowa) for providing us with the AID−/− x μS−/− mouse bone marrow. We thank Dr. Noah S. Butler and Jordan T. Johnson (University of Iowa Carver College of Medicine) for assisting us with the AID−/− x μS−/− mouse blood sample analysis. We thank Dr. Richard Morrison and Sandra Morrison for assisting us with Ab neutralization assay. We acknowledge the UAMS Radiation Core (supported by NIH Institutional Development Award (IDeA) under the grant number P20 GM109005) for assisting with mouse whole body irradiation. This study was supported by grants from the National Institutes of Health to LXL (AI139124 and GM103625) and to SJM (AI103422 and AI117303).
Footnotes
Conflict of interest
The authors declare no financial or commercial conflict of interest.
References
- 1.Taylor HR, Burton MJ, Haddad D, West S, Wright H. Trachoma. Lancet. 2014; 384:2142–2152.DOI: 10.1016/S0140-6736(13)62182-0. [DOI] [PubMed] [Google Scholar]
- 2.Abdelsamed H, Peters J, Byrne GI. Genetic variation in Chlamydia trachomatisand their hosts: impact on disease severity and tissue tropism. Future Microbiology. 2013; 8:1129–1146.DOI: 10.2217/fmb.13.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Sexually Transmitted Infections 2016. –2021 2016:1–64. [PubMed] [Google Scholar]
- 4.Division of STD Prevention CDC. Sexually Transmitted Disease Surveillance 2017. Atlanta; 2018:1–168. [Google Scholar]
- 5.Van Voorhis WC, Barrett LK, Sweeney YT, Kuo CC, Patton DL. Repeated Chlamydia trachomatis infection of Macaca nemestrina fallopian tubes produces a Th1-like cytokine response associated with fibrosis and scarring. Infect. Immun. 1997; 65:2175–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vicetti Miguel RD, Quispe Calla NE, Pavelko SD, Cherpes TL. Intravaginal Chlamydia trachomatis Challenge Infection Elicits TH1 and TH17 Immune Responses in Mice That Promote Pathogen Clearance and Genital Tract Damage Kanellopoulos-Langevin C, ed. PLoS ONE. 2016; 11:e0162445DOI: 10.1371/journal.pone.0162445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lijek RS, Helble JD, Olive AJ, Seiger KW, Starnbach MN. Pathology afterChlamydia trachomatisinfection is driven by nonprotective immune cells that are distinct from protective populations. Proc. Natl. Acad. Sci. U.S.A. 2018; 376:201711356DOI: 10.1073/pnas.1711356115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stary G, Stary A. Lymphogranuloma venereum outbreak in Europe. J Dtsch Dermatol Ges. 2008; 6:935–940.DOI: 10.1111/j.1610-0387.2008.06742.x. [DOI] [PubMed] [Google Scholar]
- 9.de Voux A, Kent JB, Macomber K, Krzanowski K, Jackson D, Starr T, Johnson S, et al. Notes from the Field: Cluster of Lymphogranuloma Venereum Cases Among Men Who Have Sex with Men - Michigan, August 2015-April 2016. MMWR Morb. Mortal. Wkly. Rep. 2016; 65:920–921.DOI: 10.15585/mmwr.mm6534a6. [DOI] [PubMed] [Google Scholar]
- 10.López LS, La Rosa L, Entrocassi AC, Caffarena D, Santos B, Fermepin MR. Rectal Lymphogranuloma Venereum, Buenos Aires, Argentina. Emerging Infect. Dis. 2019; 25:598–599.DOI: 10.3201/eid2503.180600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett JE, Dolin R, Blaser MJ, Mandell GL. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases E-Book. Elsevier Health Sciences; 2009; 180, 2301–2319.e6 [Google Scholar]
- 12.Schumacher HR. Chlamydia-associated reactive arthritis. Isr. Med. Assoc. J 2000; 2:532–535. Available at: https://www.ima.org.il/MedicineIMAJ/viewarticle.aspx?year=2000&month=07&page=532. [PubMed] [Google Scholar]
- 13.Gerard HC, Stanich JA, Whittum-Hudson JA, Schumacher HR, Carter JD, Hudson AP. Patients with Chlamydia-associated arthritis have ocular (trachoma), not genital, serovars of C. trachomatis in synovial tissue. Microb. Pathog. 2010; 48:62–68.DOI: 10.1016/j.micpath.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang SP, Eschenbach DA, Holmes KK, Wager G, Grayston JT. Chlamydia trachomatis infection in Fitz-Hugh-Curtis syndrome. Am. J. Obstet. Gynecol. 1980; 138:1034–1038. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi Y, Takeuchi H, Kitade M, Kikuchi I, Sato Y, Kinoshita K. Pathological study of Fitz-Hugh-Curtis syndrome evaluated from fallopian tube damage. J. Obstet. Gynaecol. Res. 2006; 32:280–285.DOI: 10.1111/j.1447-0756.2006.00399.x. [DOI] [PubMed] [Google Scholar]
- 16.Rank RG, Yeruva L. An alternative scenario to explain rectal positivity in Chlamydia-infected individuals. Clin. Infect. Dis. 2015; 60:1585–1586.DOI: 10.1093/cid/civ079. [DOI] [PubMed] [Google Scholar]
- 17.Chandra NL, Broad C, Folkard K, Town K, Harding-Esch EM, Woodhall SC, Saunders JM, et al. Detection of Chlamydia trachomatis in rectal specimens in women and its association with anal intercourse: a systematic review and meta-analysis. Sex Transm Infect. 2018; 94:320–326.DOI: 10.1136/sextrans-2017-053161. [DOI] [PubMed] [Google Scholar]
- 18.Campbell LA, Kuo C-C. Chlamydia pneumoniae--an infectious risk factor for atherosclerosis? Nat Rev Micro. 2004; 2:23–32.DOI: 10.1038/nrmicro796. [DOI] [PubMed] [Google Scholar]
- 19.Zafiratos MT, Manam S, Henderson KK, Ramsey KH, Murthy AK. CD8+ T cells mediate Chlamydia pneumoniae-induced atherosclerosis in mice. Pathog Dis. 2015; 73:ftv052DOI: 10.1093/femspd/ftv052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xue L, Liang Y-H, Gao Y-Y, Wang X-J. Clinical study of chlamydia pneumoniae infection in patients with coronary heart disease. BMC Cardiovasc Disord. 2019; 19:110DOI: 10.1186/s12872-019-1099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.la Maza de LM, Pal S, Khamesipour A, Peterson EM. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect. Immun. 1994; 62:2094–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Darville T, Hiltke TJ. Pathogenesis of genital tract disease due to Chlamydia trachomatis. J. Infect. Dis. 2010; 201 Suppl 2:S114–25. Available at: https://academic.oup.com/jid/article/201/Supplement_2/S114/805039.DOI: 10.1086/652397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rank RG, Whittum-Hudson JA. Protective immunity to chlamydial genital infection: evidence from animal studies. J. Infect. Dis. 2010; 201 Suppl 2:S168–77.DOI: 10.1086/652399. [DOI] [PubMed] [Google Scholar]
- 24.Brunham RC, Rey-Ladino J. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat. Rev. Immunol. 2005; 5:149–161.DOI: 10.1038/nri1551. [DOI] [PubMed] [Google Scholar]
- 25.Labuda JC, McSorley SJ. Diversity in the T cell response to Chlamydia-sum are better than one. Immunol. Lett. 2018; 202:59–64.DOI: 10.1016/j.imlet.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramsey KH, Soderberg LS, Rank RG. Resolution of chlamydial genital infection in B-celldeficient mice and immunity to reinfection. Infect. Immun. 1988; 56:1320–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrison RP. Chlamydia trachomatis genital tract infection of antibody-deficient gene knockout mice. Infect. Immun. 1997; 65:1993–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrison SG, Morrison RP. A predominant role for antibody in acquired immunity to chlamydial genital tract reinfection. J. Immunol. 2005; 175:7536–7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li L-X, McSorley SJ. B cells enhance antigen-specific CD4 T cell priming and prevent bacteria dissemination following Chlamydia muridarum genital tract infection Wherry EJ, ed. PLoS Pathog. 2013; 9:e1003707DOI: 10.1371/journal.ppat.1003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wojciechowski W, Harris DP, Sprague F, Mousseau B, Makris M, Kusser K, Honjo T, et al. Cytokine-producing effector B cells regulate type 2 immunity to H. polygyrus. Immunity. 2009; 30:421–433.DOI: 10.1016/j.immuni.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Donnell H, Pham OH, Li L-X, Atif SM, Lee S-J, Ravesloot MM, Stolfi JL, et al. Toll-like receptor and inflammasome signals converge to amplify the innate bactericidal capacity of T helper 1 cells. Immunity. 2014; 40:213–224.DOI: 10.1016/j.immuni.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000; 102:553–563. [DOI] [PubMed] [Google Scholar]
- 33.Boes M, Esau C, Fischer MB, Schmidt T, Carroll M, Chen J. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J. Immunol. 1998; 160:4776–4787. Available at: http://www.jimmunol.org/content/160/10/4776.long. [PubMed] [Google Scholar]
- 34.Kumazaki K, Tirosh B, Maehr R, Boes M, Honjo T, Ploegh HL. AID−/−mus−/− mice are agammaglobulinemic and fail to maintain B220-CD138+ plasma cells. J. Immunol. 2007; 178:2192–2203.DOI: 10.4049/jimmunol.178.4.2192. [DOI] [PubMed] [Google Scholar]
- 35.Perlmutter RM, Hansburg D, Briles DE, Nicolotti RA, Davie JM. Subclass restriction of murine anti-carbohydrate antibodies. J. Immunol. 1978; 121:566–572. [PubMed] [Google Scholar]
- 36.Ingulli E, Mondino A, Khoruts A, Jenkins MK. In vivo detection of dendritic cell antigen presentation to CD4(+) T cells. J. Exp. Med. 1997; 185:2133–2141.DOI: 10.1084/jem.185.12.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Attanavanich K, Kearney JF. Marginal zone, but not follicular B cells, are potent activators of naive CD4 T cells. J. Immunol. 2004; 172:803–811.DOI: 10.4049/jimmunol.172.2.803. [DOI] [PubMed] [Google Scholar]
- 38.Willard-Mack CL. Normal structure, function, and histology of lymph nodes. Toxicol Pathol. 2006; 34:409–424.DOI: 10.1080/01926230600867727. [DOI] [PubMed] [Google Scholar]
- 39.Johnson RM, Yu H, Strank NO, Karunakaran K, Zhu Y, Brunham RC. B Cell Presentation of Chlamydia Antigen Selects Out Protective CD4γ13 T Cells: Implications for Genital Tract Tissue-Resident Memory Lymphocyte Clusters. Infect. Immun. 2018; 86:e00614–17.DOI: 10.1128/IAI.00614-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao X, Deak E, Soderberg K, Linehan M, Spezzano D, Zhu J, Knipe DM, et al. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J. Exp. Med. 2003; 197:153–162.DOI: 10.1084/jem.20021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teitelbaum R, Glatman-Freedman A, Chen B, Robbins JB, Unanue E, Casadevall A, Bloom BR. A mAb recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. Proc. Natl. Acad. Sci. U. S. A. 1998; 95:15688–15693.DOI: 10.1073/pnas.95.26.15688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fulop M, Mastroeni P, Green M, Titball RW. Role of antibody to lipopolysaccharide in protection against low- and high-virulence strains of Francisella tularensis. Vaccine. 2001; 19:4465–4472. [DOI] [PubMed] [Google Scholar]
- 43.Nanton MR, Way SS, Shlomchik MJ, McSorley SJ. Cutting edge: B cells are essential for protective immunity against Salmonella independent of antibody secretion. J. Immunol. 2012; 189:5503–5507.DOI: 10.4049/jimmunol.1201413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Casadevall A, Pirofski L-A. A new synthesis for antibody-mediated immunity. Nat. Immunol. 2011; 13:21–28.DOI: 10.1038/ni.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peeling R, Maclean IW, Brunham RC. In vitro neutralization of Chlamydia trachomatis with monoclonal antibody to an epitope on the major outer membrane protein. Infect. Immun. 1984; 46:484–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang YX, Stewart SJ, Caldwell HD. Protective monoclonal antibodies to Chlamydia trachomatis serovar- and serogroup-specific major outer membrane protein determinants. Infect. Immun. 1989; 57:636–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peterson EM, la Maza de LM, Brade L, Brade H. Characterization of a neutralizing monoclonal antibody directed at the lipopolysaccharide of Chlamydia pneumoniae. Infect. Immun. 1998; 66:3848–3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morrison RP, Caldwell HD. Immunity to murine chlamydial genital infection. Infect. Immun. 2002; 70:2741–2751.DOI: 10.1128/IAI.70.6.2741-2751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naglak EK, Morrison SG, Morrison RP. Neutrophils Are Central to Antibody-Mediated Protection against Genital Chlamydia. Infect. Immun. 2017; 85:e00409–17. Available at: https://iai.asm.org/content/85/10/e00409-17.long.DOI: 10.1128/IAI.00409-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naglak EK, Morrison SG, Morrison RP. IFNγ is Required for Optimal Antibody-Mediated Immunity against Genital Chlamydia Infection Palmer GH, ed. Infect. Immun. 2016; 84:3232–3242.DOI: 10.1128/IAI.00749-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sturdevant GL, Caldwell HD. Innate immunity is sufficient for the clearance of Chlamydia trachomatis from the female mouse genital tract. Pathog Dis. 2014; 72:70–73.DOI: 10.1111/2049-632X.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perry LL, Feilzer K, Caldwell HD. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. J. Immunol. 1997; 158:3344–3352. [PubMed] [Google Scholar]
- 53.Cotter TW, Ramsey KH, Miranpuri GS, Poulsen CE, Byrne GI. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infect. Immun. 1997; 65:2145–2152. Available at: https://iai.asm.org/content/65/6/2145.long. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Poston TB, O’Connell CM, Girardi J, Sullivan JE, Nagarajan UM, Marinov A, Scurlock AM, et al. T Cell-Independent Gamma Interferon and B Cells Cooperate To Prevent Mortality Associated with Disseminated Chlamydia muridarum Genital Tract Infection Palmer GH, ed. Infect. Immun. 2018; 86:e00143–18.DOI: 10.1128/IAI.00143-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li L-X, McSorley SJ. A re-evaluation of the role of B cells in protective immunity to Chlamydia infection. Immunol. Lett. 2015; 164:88–93.DOI: 10.1016/j.imlet.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scidmore MA. Cultivation and Laboratory Maintenance of Chlamydia trachomatis. Curr Protoc Microbiol. 2005; Chapter 11:Unit 11A.1–11A.1.25.DOI: 10.1002/9780471729259.mc11a01s00. [DOI] [PubMed] [Google Scholar]
- 57.Li L-X, Labuda JC, Imai DM, Griffey SM, McSorley SJ. CCR7 Deficiency Allows Accelerated Clearance of Chlamydia from the Female Reproductive Tract. J. Immunol. 2017; 199:2547–2554.DOI: 10.4049/jimmunol.1601314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tuffrey M, Taylor-Robinson D. Progesterone as a key factor in the development of a mouse model for genital-tract infection with Chlamydia trachomatis. FEMS Microbiology Letters. 1981; 12:111–115. [Google Scholar]
- 59.Su H, Caldwell HD. In vitro neutralization of Chlamydia trachomatis by monovalent Fab antibody specific to the major outer membrane protein. Infect. Immun. 1991; 59:2843–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cossarizza A, Chang H-D, Radbruch A, Acs A, Adam D, Adam-Klages S, Agace WW, et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition). Eur. J. Immunol. 2019; 49:1457–1973.DOI: 10.1002/eji.201970107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.