Abstract
Background
Over the decades, a variety of psychological interventions for borderline personality disorder (BPD) have been developed. This review updates and replaces an earlier review (Stoffers‐Winterling 2012).
Objectives
To assess the beneficial and harmful effects of psychological therapies for people with BPD.
Search methods
In March 2019, we searched CENTRAL, MEDLINE, Embase, 14 other databases and four trials registers. We contacted researchers working in the field to ask for additional data from published and unpublished trials, and handsearched relevant journals. We did not restrict the search by year of publication, language or type of publication.
Selection criteria
Randomised controlled trials comparing different psychotherapeutic interventions with treatment‐as‐usual (TAU; which included various kinds of psychotherapy), waiting list, no treatment or active treatments in samples of all ages, in any setting, with a formal diagnosis of BPD. The primary outcomes were BPD symptom severity, self‐harm, suicide‐related outcomes, and psychosocial functioning. There were 11 secondary outcomes, including individual BPD symptoms, as well as attrition and adverse effects.
Data collection and analysis
At least two review authors independently selected trials, extracted data, assessed risk of bias using Cochrane's 'Risk of bias' tool and assessed the certainty of the evidence using the GRADE approach. We performed data analysis using Review Manager 5 and quantified the statistical reliability of the data using Trial Sequential Analysis.
Main results
We included 75 randomised controlled trials (4507 participants), predominantly involving females with mean ages ranging from 14.8 to 45.7 years. More than 16 different kinds of psychotherapy were included, mostly dialectical behaviour therapy (DBT) and mentalisation‐based treatment (MBT). The comparator interventions included treatment‐as‐usual (TAU), waiting list, and other active treatments. Treatment duration ranged from one to 36 months.
Psychotherapy versus TAU
Psychotherapy reduced BPD symptom severity, compared to TAU; standardised mean difference (SMD) −0.52, 95% confidence interval (CI) −0.70 to −0.33; 22 trials, 1244 participants; moderate‐quality evidence. This corresponds to a mean difference (MD) of −3.6 (95% CI −4.4 to −2.08) on the Zanarini Rating Scale for BPD (range 0 to 36), a clinically relevant reduction in BPD symptom severity (minimal clinical relevant difference (MIREDIF) on this scale is −3.0 points).
Psychotherapy may be more effective at reducing self‐harm compared to TAU (SMD −0.32, 95% CI −0.49 to −0.14; 13 trials, 616 participants; low‐quality evidence), corresponding to a MD of −0.82 (95% CI −1.25 to 0.35) on the Deliberate Self‐Harm Inventory Scale (range 0 to 34). The MIREDIF of −1.25 points was not reached.
Suicide‐related outcomes improved compared to TAU (SMD −0.34, 95% CI −0.57 to −0.11; 13 trials, 666 participants; low‐quality evidence), corresponding to a MD of −0.11 (95% CI −0.19 to −0.034) on the Suicidal Attempt Self Injury Interview. The MIREDIF of −0.17 points was not reached.
Compared to TAU, psychotherapy may result in an improvement in psychosocial functioning (SMD −0.45, 95% CI −0.68 to −0.22; 22 trials, 1314 participants; low‐quality evidence), corresponding to a MD of −2.8 (95% CI −4.25 to −1.38), on the Global Assessment of Functioning Scale (range 0 to 100). The MIREDIF of −4.0 points was not reached.
Our additional Trial Sequential Analysis on all primary outcomes reaching significance found that the required information size was reached in all cases.
A subgroup analysis comparing the different types of psychotherapy compared to TAU showed no clear evidence of a difference for BPD severity and psychosocial functioning.
Psychotherapy may reduce depressive symptoms compared to TAU but the evidence is very uncertain (SMD −0.39, 95% CI −0.61 to −0.17; 22 trials, 1568 participants; very low‐quality evidence), corresponding to a MD of −2.45 points on the Hamilton Depression Scale (range 0 to 50). The MIREDIF of −3.0 points was not reached.
BPD‐specific psychotherapy did not reduce attrition compared with TAU. Adverse effects were unclear due to too few data.
Psychotherapy versus waiting list or no treatment
Greater improvements in BPD symptom severity (SMD −0.49, 95% CI −0.93 to −0.05; 3 trials, 161 participants), psychosocial functioning (SMD −0.56, 95% CI −1.01 to −0.11; 5 trials, 219 participants), and depression (SMD −1.28, 95% CI −2.21 to −0.34, 6 trials, 239 participants) were observed in participants receiving psychotherapy versus waiting list or no treatment (all low‐quality evidence). No evidence of a difference was found for self‐harm and suicide‐related outcomes.
Individual treatment approaches
DBT and MBT have the highest numbers of primary trials, with DBT as subject of one‐third of all included trials, followed by MBT with seven RCTs.
Compared to TAU, DBT was more effective at reducing BPD severity (SMD −0.60, 95% CI −1.05 to −0.14; 3 trials, 149 participants), self‐harm (SMD −0.28, 95% CI −0.48 to −0.07; 7 trials, 376 participants) and improving psychosocial functioning (SMD −0.36, 95% CI −0.69 to −0.03; 6 trials, 225 participants). MBT appears to be more effective than TAU at reducing self‐harm (RR 0.62, 95% CI 0.49 to 0.80; 3 trials, 252 participants), suicidality (RR 0.10, 95% CI 0.04, 0.30, 3 trials, 218 participants) and depression (SMD −0.58, 95% CI −1.22 to 0.05, 4 trials, 333 participants). All findings are based on low‐quality evidence. For secondary outcomes see review text.
Authors' conclusions
Our assessments showed beneficial effects on all primary outcomes in favour of BPD‐tailored psychotherapy compared with TAU. However, only the outcome of BPD severity reached the MIREDIF‐defined cut‐off for a clinically meaningful improvement. Subgroup analyses found no evidence of a difference in effect estimates between the different types of therapies (compared to TAU) .
The pooled analysis of psychotherapy versus waiting list or no treatment found significant improvement on BPD severity, psychosocial functioning and depression at end of treatment, but these findings were based on low‐quality evidence, and the true magnitude of these effects is uncertain. No clear evidence of difference was found for self‐harm and suicide‐related outcomes.
However, compared to TAU, we observed effects in favour of DBT for BPD severity, self‐harm and psychosocial functioning and, for MBT, on self‐harm and suicidality at end of treatment, but these were all based on low‐quality evidence. Therefore, we are unsure whether these effects would alter with the addition of more data.
Plain language summary
Psychological therapies for people with borderline personality disorder
Background
People affected by borderline personality disorder (BPD) often have difficulties with controlling their impulses and emotions. They may have a poor self‐image, experience rapid changes in mood, harm themselves and find it hard to engage in harmonious interpersonal relationships. Different types of psychological treatments ('talking treatments') have been developed to help people with BPD. The effects of these treatments must be investigated to decide how well they work and if they can be harmful.
Objective
This review summarises what we currently know about the effect of psychotherapy in people with BPD.
Methods
We compared the effects of psychological treatments on people affected by BPD who did not receive treatment or who continued their usual treatment, were on a waiting list or received active treatment.
Findings
We searched for relevant research articles, and found 75 trials (4507 participants, mostly female, mean age ranging from 14.8 to 45.7 years). The trials examined a wide variety of psychological treatments (over 16 different types). They were mostly conducted in outpatient settings, and lasted between one and 36 months. Dialectical behaviour Therapy (DBT) and Mentalisation‐Based Treatment (MBT) were the therapies most studied.
Psychotherapy compared with usual treatment
Psychotherapy reduced the severity of BPD symptoms and suicidality and may reduce self‐harm and depression whilst also improving psychological functioning compared to usual treatment. DBT may be better than usual treatment at reducing BPD severity, self‐harm and improving psychosocial functioning. Similarly, MBT appears to be more effective than usual treatment at reducing self‐harm, suicidality and depression. However, these findings were all based on low‐quality evidence and therefore we are uncertain whether or not these results would change if we added more trials. Most trials did not report adverse effects, and those that did, found no obvious unwanted reactions following psychological treatment. The majority of trials (64 out of 75) were funded by grants from universities, authorities or research foundations. Four trials reported that no funding was received. For the remaining trials (7), funding was not specified.
Psychotherapy versus waiting list or no treatment
Psychotherapy was more effective than waiting list at improving BPD symptoms, psychosocial functioning, and depression, but there was no clear difference between psychotherapy, and waiting list for outcomes of self‐harm, and suicide‐related outcomes.
Conclusions
In general, psychotherapy may be more effective than usual treatment in reducing BPD symptom severity, self‐harm, suicide‐related outcomes and depression, whilst also improving psychosocial functioning. However, only the decrease in BPD symptom severity was found to be at a clinically important level. DBT appears to be better at reducing BPD severity, self‐harm, and improving psychosocial functioning compared to usual treatment and MBT appears more effective than usual treatment at reducing self‐harm and suicidality. However, we are still uncertain about these findings as the quality of the evidence is low.
Summary of findings
Summary of findings 1. Psychotherapy versus treatment‐as‐usual.
| Psychotherapy versus treatment‐as‐usual | ||||||
|
Patient or population: borderline personality disorder Settings: inpatient and outpatient Intervention: psychotherapy Comparison: treatment‐as‐usual (TAU) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect(95% CI) |
Number of participants (RCTs) |
Quality of the evidence (GRADE) |
Comments | |
| Assumed risk | Corresponding risk | |||||
| TAU | Psychotherapy | |||||
|
BPD symptom severity Measured by: clinicians and self‐rated Timing of outcome assessment: end of treatment |
‐ | The mean score in the intervention groups was 0.52 SD lower (0.70 lower to 0.33 lower) | ‐ | 1244 (22 RCTs) |
⊕⊕⊕⊝ Moderatea |
The SMD of −0.52 corresponds to −3.6 on the Zanarini BPD scale. The MIREDIF on this scale is 3.0 points TSA adjusted Cl = −5.49 to −1.90 on the Zanarini BPD scale TSA RIS = 901 |
|
Self‐harm (frequency) Measured by: clinicians and self‐rated Timing of outcome assessment: end of treatment |
‐ | The mean score in the intervention groups was 0.32 SD lower (0.49 lower to 0.14 lower) | ‐ | 616 (13 RCTs) |
⊕⊕⊝⊝ Lowa,b | The SMD of −0. 32 corresponds to −0.82 on the DSHI. The MIREDIF on this scale is −1.25 points (½ SD) TSA adjusted CI = −0.59 to −0.08 on the DSHI TSA RIS = 97 |
|
Suicide‐related outcomes (suicidality) Measured by: clinicians and self‐rated Timing of outcome assessment: end of treatment |
‐ | The mean score in the intervention groups was 0.34 SD lower (0.57 lower to 0.11 lower) | ‐ | 666 (13 RCTs) |
⊕⊕⊝⊝ Lowa,b |
The SMD of −0. 34 corresponds to −0.11 on the SASII. The MIREDIF on this scale is −0.17 points (½ SD) TSA adjusted CI = −0.18 to −0.04 on the SASII TSA RIS = 253 |
|
Psychosocial functioning Measured by: clinicians and self‐rated Timing of outcome assessment: end of treatment |
‐ | The mean score in the intervention groups was 0.45 SD lower (0.68 lower to 0.22 lower) | ‐ | 1314 (22 RCTs) |
⊕⊕⊝⊝ Lowa,c | The SMD of −0.45 corresponds to −2.8 on the GAF. The MIREDIF on this scale is −4.0 points TSA adjusted CI = −3.97 to −1.94 on the GAF TSA RIS = 947 |
|
Depression Measured by: clinicians and self‐rated Timing of outcome assessment: end of treatment |
‐ | The mean score in the intervention groups was 0.39 SD lower (0.61 lower to 0.17 lower) | ‐ | 1568 (22 RCTs) |
⊕⊝⊝⊝ Verylowa,b,c | The SMD of ‐0.45 corresponds to −2.45 on the Hamilton Depression Scale. The MIREDIF on this scale is 3.0 points TSA adjusted CI = −3.34 to −1.72 on the Hamilton Depression Scale TSA RIS = 2274 |
|
Attrition Timing of outcome assessment: end of treatment |
328 per 1000 | 328 per 1000 (95% CI 56 fewer to 66 higher) | RR 1.00 (95% CI 0.83 to 1.20) | 2225 (32 RCTs) |
⊕⊕⊝⊝ Lowa,b | ‐ |
|
Adverse effects Timing of outcome assessment: end of treatment |
74 per 1000 | 6 per 1000 (95% CI 41 fewer to 65 higher) | RR 0.92 (95% CI 0.45 to 1.88) | 381 (2 RCTs) |
⊕⊕⊝⊝ Lowa,b | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; DSHI: Deliberate Self‐Harm Inventory; GAF: Global Assessment of Functioning scale; MIREDIF: Minimum relevant difference; RCTs: Randomised controlled trials; RIS: Required information size; RR: Risk Ratio; SASII: Suicide Attempt Self‐Injury Interview; SD: Standard deviation; SMD: Standardised mean difference; TAU: treatment‐as‐usual; TSA: Trial Sequential Analysis | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: we are very uncertain about the estimate | ||||||
aWe downgraded the quality of this evidence by one level due to risk of bias (other bias). bWe downgraded the quality of this evidence by one level due to imprecision. cWe downgraded the quality of this evidence by one level due to high heterogeneity.
Summary of findings 2. Psychotherapy versus waiting list or no treatment.
| Psychotherapy versus waiting list or no treatment | ||||||
|
Patient or population: borderline personality disorder Settings: inpatient and outpatient Intervention: psychotherapy Comparison: waiting list or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) |
Number of participants (RCTs) |
Quality of the evidence (GRADE) |
Comments | |
| Assumed risk | Corresponding risk | |||||
| Waiting list or no treatment | Psychotherapy | |||||
|
BPD symptom severity Measured by: clinicians and self‐rated Timing of outcome assessment: end of treatment |
‐ | The mean score in the intervention groups was 0.49 SD lower (0.93 lower to 0.05 lower) | ‐ | 161 (3 RCTs) |
⊕⊕⊝⊝ Lowa,b | An SMD of 0.49 represents a moderate effect. |
|
Self‐harm Measured by: clinicians and self‐rated Timing of outcome assessment: end of treatment |
‐ | The mean score in the intervention groups was0.17 SD lower (0.52 lower to 0.18 higher) | ‐ | 128 (2 RCTs) |
⊕⊕⊝⊝ Lowa,b | An SMD of 0.17 represents a small effect. |
|
Suicide‐related outcomes Measured by: self‐rated Timing of outcome assessment: end of treatment |
‐ | The mean score in the intervention groups was 5.62 SD lower (16.39 lower to 5.16 higher) | ‐ | 108 (2 RCTs) |
⊕⊝⊝⊝ Verylowa,b,c |
An SMD of 5.62 represents a large effect. |
|
Psychosocial functioning Measured by: clinicians and self‐rated Timing of outcome assessment: end of treatment |
‐ | The mean score in the intervention groups was 0.56 SD lower (1.01 lower to 0.11 lower) | ‐ | 219 (5 RCTs) |
⊕⊕⊝⊝ Lowa,b | An SMD of 0.56 represents a moderate effect. |
|
Depression Measured by: clinicians and self‐rated Timing of outcome assessment: end of treatment |
‐ | The mean score in the intervention groups was 1.28 SD lower (2.21 lower to 0.34 lower) | ‐ | 239 (6 RCTs) |
⊕⊕⊝⊝ Lowa,b | An effect size of 1.28 represents a large effect. |
|
Attrition Timing of outcome assessment: end of treatment |
81 per 1000 | 147 per 1000 (95% CI 118 fewer to 74 higher) | RR 0.55 (95% CI 0.20 to 1.50) | 144 (3 RCTs) |
⊕⊝⊝⊝ Verylowa,b,c | ‐ |
| Adverse effects (not measured | See comments | See comments | ‐ | ‐ | ‐ | No studies were found that assessed this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RCTs: Randomised controlled trials; RR: Risk ratio; SMD: Standardized mean difference | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: we are very uncertain about the estimate | ||||||
aWe downgraded the quality of this evidence by one level due to risk of bias. bWe downgraded the quality of this evidence by one level due to imprecision (there was a wide CI). cWe downgraded the quality of this evidence by one level due to inconsistency.
Summary of findings 3. Dialectical behavioural therapy or mentalisation‐based therapy versus treatment‐as‐usual.
| Dialectical behavioural therapy or mentalisation‐based therapy versus treatment‐as‐usual | ||||||
|
Patient or population: borderline personality disorder Settings: inpatient and outpatient Intervention: dialectical behavioural therapy (DBT) or mentalisation‐based therapy (MBT) Comparison: treatment‐as‐usual (TAU) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) |
Number of participants (RCTs) |
Quality of the evidence (GRADE) |
Comments | |
| Assumed risk | Corresponding risk | |||||
| TAU | DBT or MBT | |||||
| DBT | ||||||
|
BPD severity Measured by: clinicians Timing of outcome assessment: end of treatment |
‐ | The mean score in the intervention groups was 0.60 SD lower (1.05 lower to 0.14 lower) | ‐ | 149 (3 RCTs) |
⊕⊕⊝⊝ Lowa,b | An SMD of 0.60 represents a moderate effect. |
|
Self‐harm Measured by: clinicians Timing of outcome assessment: end of treatment |
‐ | The mean score in the intervention groups was 0.28 SD lower (0.48 lower to 0.07 lower) | ‐ | 376 (7 RCTs) |
⊕⊕⊝⊝ Lowa,b | An SMD of 0.28 represents a small effect. |
|
Psychosocial functioning Measured by: clinicians and self‐rated Timing of outcome assessment: end of treatment |
‐ | The mean score in the intervention groups was 0.36 SD lower (0.69 lower to 0.03 lower) | ‐ | 225 (6 RCTs) |
⊕⊕⊝⊝ Lowa,b, | An SMD of 0.36 represents a small effect. |
| MBT | ||||||
|
Self‐harm Measured by: clinicians Timing of outcome assessment: end of treatment |
631 per 1000 | 240 per 1000 (95% CI 334 fewer to 126 fewer) | RR 0.62 (95% CI 0.49 to 0.80) | 252 (3 RCTs) |
⊕⊕⊝⊝ Lowa,b | ‐ |
|
Suicide‐related outcomes Measured by: clinicians Timing of outcome assessment: end of treatment |
298 per 1000 | 268 per 1000 (95% CI 286 fewer to 209 fewer) | RR 0.10 (95% CI 0.04 to 0.30) | 218 (3 RCTs) |
⊕⊕⊝⊝ Lowa,b | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; DBT: Dialectical behavioural therapy; MBT: Mentalisation‐based therapy; RCTs: Randomised controlled trials; RR: Risk ratio; SMD: Standardized mean difference; TAU: Treatment‐as‐usual | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: we are very uncertain about the estimate | ||||||
aWe downgraded the quality of this evidence by one level due to risk of bias. bWe downgraded the quality of this evidence by one level due to imprecision (there was a wide CI).
Background
Description of the condition
Borderline personality disorder (BPD) is a condition first formally described in the 20th century (Gunderson 2009). Historically, the term BPD was coined by Adolph Stern to describe a condition in the 'borderland' between psychosis and neurosis (Stern 1938). Subsequent psychoanalytic contributions (especially that of Kernberg 1975) have reaffirmed this distinction, emphasising that the capacity to test reality remains grossly intact but is subject to subtle distortions, especially under stress. The current evidence supports a biopsychological model of the aetiological factors in BPD, all of which may contribute. It is assumed that there is an interaction between the experience of adverse effects during childhood (like neglect, emotional or sexual abuse), and genetic or biological factors. Relevant biological factors include neurobiological structures, such as reduced aymgdala volume, increased volume of the pituitary gland, reduced grey matter volume in the anterior cingulate gyrus, posterior cingulate gyrus or hippocampus, and reduction in size of the right parietal cortex (Leichsenring 2011; Lieb 2004), and neurobiological dysfunctions (especially of the serotonergic system). In combination with psychosocial factors, personality traits (e.g. neuroticisms), personality functioning (self and interpersonal) and proneness to react highly emotionally may contribute to the core components of BPD, like affective and behavioural dysregulation, and disturbed relatedness (Leichsenring 2011; Lieb 2004).
According to current diagnostic criteria, BPD is characterised by a pervasive pattern of instability in affect regulation, impulse control, interpersonal relationships and self‐image (APA 2013; WHO 1993). Clinical hallmarks include, amongst other things, emotional dysregulation, impulsivity, anger, repeated self‐injury and chronic suicidal tendencies, together with inner emptiness and fear of abandonment (Dimaggio 2007; Fonagy 2009; Gunderson 2018; Karterud 2019; Lieb 2004). Despite the difficulties and controversies in defining and delimiting the condition, BPD is being vigorously researched still, not only in adults but also in childhood and adolescence (Chanen 2017), and is the only specific personality disorder to be carried over to the new, eleventh edition of the International Classification of Diseases (ICD‐11) (Bach 2018; WHO 2018). The importance of effective treatments for BPD stems from the considerable psychological suffering of the persons concerned (Stiglmayr 2005; Zanarini 1998), the burden incurred on their families and significant others (Bailey 2014; Bateman 2019a), the significant impact on mental health services (Cailhol 2015; Hörz 2010; Soeteman 2008a; Tyrer 2015; Zanarini 2004; Zanarini 2012), and not least the association of BPD with debilitating functional impairments and premature death (Fok 2012; Gunderson 2011a; Gunderson 2011b; Kjær 2018; Niesten 2016; Skodol 2002; Soeteman 2008b).
The definition of BPD in the Diagnostic and Statistical Manual of Mental Disorders (DSM), Fifth Edition (DSM‐5; APA 2013), Fourth Edition (DMS‐IV; APA 1994) and Fourth Edition Text Revision (DSM‐IV‐TR; APA 2000) comprises nine criteria that cover the features mentioned above. At least five criteria should be met for a definitive, categorical diagnosis of BPD to be made, and four criteria for a probable diagnosis (see Appendix 1). In the alternative diagnostic classification system of the World Health Organization (WHO), the ICD, which is currently in its tenth edition (ICD‐10; WHO 1993), the relating condition is referred to as "Emotionally unstable personality disorder (F60.3)", of which there is an impulsive type (F60.30) and a borderline type (F60.31; see Appendix 2). The latter essentially overlaps with the DSM‐IV definition and DSM‐5 criteria (Ottosen 2002).
In addition to categorical classification systems, the DSM‐5 also includes an alternative model for personality disorders (Section III: "Emerging Measures and Models"). This hybrid model is made up of two dimensions: 1) the severity of impairment in personality (self and interpersonal); and 2) the domains of personality traits (i.e. negative affectivity, detachment, antagonism, disinhibition, psychoticism; APA 2013, Section III). The ICD‐11 (which will be in effect from 2022; WHO 2018) is also moving towards a dimensional approach, where the different types of personality disorders are being replaced with a model that focuses on the severity of core personality functioning instead. However, a specifier relating to a "Borderline pattern" will be retained. Preliminary studies have found that there is a substantial overlap between the current categorical and alternative models found in the DSM‐5 (Bach 2016; Bach 2018; Sellbom 2014), as well as overlap between the dimensional models of the DSM‐5 and ICD‐11 (Bach 2018). Therefore, sufficient continuity between current categorical and upcoming dimensional models is warranted. The findings of this review will be applicable also to populations diagnosed with the DSM‐5 Section III Hybrid BPD type and ICD‐11 Borderline pattern qualifier.
The prevalence of BPD in the general population is estimated to be 1.8 % (95% CI 1.2% to 2.5%) (Winsper 2020). In clinical populations, BPD occurs frequently (Munk‐Jørgensen 2010), with trials reporting a prevalence ranging from 9.3% to 46.3% and a mean point prevalence across studies of 28.5% (Torgersen 2012). BPD usually has its onset in childhood and adolescence, and younger people are affected as much as or even more often than adults (Neacsiu 2017). BPD has been found to peak around 14 to 17 years of age with a linearly decline into adulthood; however, it continues throughout the lifespan and can also be found in older people (Chanen 2007; Newton‐Howes 2015; Sharp 2018; Videler 2019). Though BPD is predominantly diagnosed in women (75%; APA 2000; APA 2013), it is estimated to be almost equally frequent in men in epidemiological studies (Lenzenweger 2007; Ten Have 2016; Torgersen 2001; Torgersen 2012). Moreover, BPD commonly co‐occurs with mood disorders, substance use disorder, eating disorders, post‐traumatic stress disorder (PTSD), attention‐deficit hyperactivity disorder (ADHD), and other specific personality disorders (Coid 2006; Lenzenweger 2007; Stepp 2012; Storebø 2014; Tomko 2014). Suicidal behaviour is reported to occur in up to 84% of people diagnosed with BPD (Goodman 2012; Soloff 2002), and it is estimated that up to 10% of those affected by BPD will die from suicide (Paris 2019). Comorbid mood disorders or substance use disorders are the most common risk factors associated with successful suicide attempts (Black 2004; Doyle 2016; Yen 2004).
Although the short‐ to medium‐term social functioning of people with BPD is poor, diagnostic remission is around 85% within 10 years (Gunderson 2011b; Zanarini 2007). Here, however, remission only means that diagnostic criteria are not fulfilled; it does not indicate the absence of any symptoms. Indeed, whereas acute symptoms — such as self‐mutilation, help‐seeking suicide threats or attempts and impulsivity — decrease with time in most cases, affective symptoms reflecting areas of chronic dysphoria, such as chronic feelings of emptiness, intense anger or profound feelings of abandonment, largely remain (Zanarini 2007). Therefore, the majority of people with BPD still have significant levels of symptoms and experience severe and persistent impairment in social functioning over time (Kongerslev 2015; Ng 2016). Risk factors for poor, long‐term outcomes are comorbid substance use disorders, PTSD, and anxiety cluster disorders (Zanarini 2005; Zanarini 2007), as well as a family history of psychiatric disorder (especially mood disorders and substance use disorders), demographic issues such as older age, longer treatment history, pathological childhood experiences, temperament issues and adult psychosocial functioning (Chanen 2012; De Fruyt 2014; Kongerslev 2015; Zanarini 2007).
People with BPD have severe difficulties in achieving and maintaining vocational and social functioning over time (Hastrup 2019a; Paris 2014; Zanarini 2010). Furthermore, treatment‐seeking people with personality disorders, such as BPD, pose a high economic burden on society (Hastrup 2019b; Van Asselt 2007). Effective treatments could potentially decrease the high costs associated with the condition (Soeteman 2008a). The problem of deliberate self‐harm is also a particular issue within this group (Ayodeji 2015; Kongerslev 2015; Linehan 1997; Rossouw 2012b). In medical settings, people diagnosed with BPD often present after self‐harming behaviour or in suicidal crisis and are treated in emergency settings, often involving repeated psychiatric hospitalisations (Cailhol 2015).
In summary, BPD is a condition that has been studied extensively. It has a major impact on health facilities as those affected often present in crisis. Recovery from symptoms or functional impairment (or both) was previously considered likely for only a small percentage of people diagnosed with BPD. However, the long‐term course, in terms of symptomatic recovery, is favourable (Gunderson 2011b; Zanarini 2007; Zanarini 2012). Nonetheless, people diagnosed with BPD continue to have considerable interpersonal and functional problems, and sustainable recovery appears difficult to attain (Biskin 2015; Kongerslev 2015; Rossouw 2012b).
Description of the intervention
About three‐quarters of people with BPD present to mental healthcare professionals (Tomko 2014), and they are even more likely to do so than people with mood, anxiety, or other personality disorders (Ansell 2007). Most will receive psychological interventions, because drugs are not effective for the BPD core symptoms (Goodman 2010; Tomko 2014), and these psychosocial interventions will often be provided for a relatively long periods of time (e.g. for a period of one year or longer) (Ansell 2007; Zanarini 2015).
A broad range of psychotherapies exist for BPD. The therapy can be delivered in individual or group formats, or a combination of these two treatment modalities. As for most other mental disorders, psychological interventions can be based on the traditional, major psychotherapeutic schools such as psychodynamic psychotherapy, cognitive behaviour therapy (CBT) or client‐centred/humanistic therapy. In addition, several specific treatment approaches have been developed within recent decades to meet the particular challenges of treating BPD. These disorder‐specific approaches are usually precisely structured and manualised (De Groot 2008; Levy 2006; Weinberg 2011). Strategies are provided for addressing interpersonal challenges, such as emotional dysregulation and impulsivity, which are core problems for people diagnosed with BPD and could lead to difficulties in forming a therapeutic alliance. Most BPD‐specific psychological interventions involve multimodal therapy, treatment contracts, actively taking measures to minimise premature non‐completion of treatment, providing a crisis intervention protocol and encouraging the affected one's sense of agency (Bateman 2018; Clarkin 2012; De Groot 2008; Kongerslev 2015; Livesley 2012; Weinberg 2011). They are typically highly focused on affect and the therapeutic relationship, with a relatively active therapist implementing interventions within a supportive and validating atmosphere (Bateman 2018; Clarkin 2012; De Groot 2008; Kongerslev 2015; Livesley 2012; Weinberg 2011). Eclectic therapy is an open, integrative form of psychotherapy, which adapts to the unique needs of each specific client, depending on the problem, the treatment goals and the person’s expectations and motivation (Sansone 2006). Eclectic therapies integrate elements from different forms of psychotherapy.
Among the specific psychological interventions for people diagnosed with BPD, the most commonly used are: transference‐focused therapy (Clarkin 1999; Yeomans 2015); mentalisation‐based treatment (Bateman 2004; Bateman 2006; Bateman 2016); dialectical behaviour therapy (Linehan 1993a; Linehan 2015b); cognitive analytic therapy (Chanen 2014; Ryle 1997); schema‐focused therapy (Arntz 2009; Young 2003); and the systems training for emotional predictability and problem‐solving (STEPPS) (Black 2009). Most of these treatments are designed as outpatient treatments of six to 24 months duration, with once or twice weekly individual sessions. Some also include additional group therapy sessions, inpatient or day‐hospital therapeutic community treatment and psychoeducation. Other potential therapies for BPD include the likes of CBT (Beck 2003), acceptance and commitment therapy (ACT; Gratz 2006), interpersonal psychotherapy (IPT; Markowitz 2006), and psychodynamic psychotherapy (e.g. psychoanalytic‐interactional therapy; Streeck 2009). Broadly speaking, psychodynamic therapies aim to help people understand and reflect on their inner mental processes and make links between their past and current difficulties. Treatments based on CBT place emphasis on self‐directed learning processes: people are encouraged to identify their core beliefs; evaluate and modify their behaviour accordingly; and gain new experiences. Psychotherapy is defined as the "treatment of mental illness or emotional disturbances primarily by verbal or nonverbal communication" (quote; NLM 2009).
Dialectical behavioural therapy (DBT; Linehan 1993a) is a highly structured and complex psychological therapy that was developed using some of the principles of CBT in combination with mindfulness‐based and Zen‐Buddhistic and dialectical thinking strategies. It aims to change behaviour and enhance the ability to tolerate difficult or painful feelings by focusing on improving skills in stress tolerance, emotion regulation, interpersonal behaviour, and mindfulness.
Mentalisation‐based therapy (Bateman 2004; Bateman 2016) is a complex psychodynamic and attachment‐based psychological therapy programme that aims to increase the reflective functioning or mentalising capacity of the individual, helping the person to understand and recognise the feelings they evoke in others and the feelings they experience themselves, as well as improving the capacity for emotion regulation in interpersonal relations.
Schema‐focused therapy (SFT; Young 2003) draws from both cognitive‐behavioural and psychoanalytic theories and helps people with BPD to identify their self‐defeating core themes arising from unmet emotional needs in childhood and presenting as maladaptive coping styles in adulthood. The goal of SFT is to aid people affected by BPD in getting their needs met in adaptive ways.
Transference‐focused psychotherapy (TFP; Clarkin 1999) strives to achieve integrated representations of self and others, modification of primitive defence operations, and resolution of identity diffusion by analysis of the transference within the therapeutic relationship. Primitive object relations, which can be polarised and split, may be transformed to advanced or mature object relations characterised by more integrated object relations. TFP relies on techniques of clarification, confrontation and transference interpretation within the relationship between patient and therapist.
Cognitive analytic therapy (CAT; Ryle 1997) assumes that people with BPD typically experience rapid switching from one self‐state to another in a dissociate manner. The aim is to work with the patient cognitively, to identify procedural sequences, chains of events, emotions, thoughts and motivations, in order to understand how a target problem (like self‐harm) is established and maintained, and to identify reciprocal roles (i.e. how early experiences are replayed later in life).
Systems training for emotional predictability and problem‐solving (STEPPS; Black 2009) combines group‐based psychoeducation with skills training, and targets biased social cognition driven by cognitive filters or schemas.
Dynamic deconstructive psychotherapy (DDP) is a manualised, 12‐ to 18‐month treatment for adults diagnosed with BPD and other complex co‐occurring disorders such as substance use disorders, additional personality disorders or eating disorders (Gregory 2008; Gregory 2010). The DDP model of BPD pathology draws on and combines elements of translational neuroscience, object relations theory, and the philosophy of deconstruction. The aim of DDP is to help people with BPD to connect with and verbalise their experience better, as well as to foster better interpersonal relations and self‐acceptance. DDP was used in a randomised controlled trial (RCT) conducted by its developer (Gregory 2008).
Relaxation techniques and patient education programmes will be considered their own intervention class (i.e. not CBT or psychoanalytically based), as long as they are not explicitly grounded in or taken from a specific treatment approach (such as psychoeducation according to the DBT approach, CBT, or the SFT approach, etc.).
How the intervention might work
Evidence‐based psychological therapies are based on assumptions about causality, core symptoms, and maintenance of the disorder (Kazdin 2004; Livesley 2003; Livesley 2004). The various psychotherapeutic approaches to BPD claim different mechanisms of action according to their respective models of causation (Gunderson 2018; Huprich 2015; Livesley 2004; Livesley 2016). However, they also contain a number of common elements that can account for why a number of seemingly different approaches appear to be effective in ameliorating BPD symptoms (Bateman 2015; Fonagy 2014; Kongerslev 2015; Weinberg 2011), including: a clear and highly structured treatment framework; an explicit model of BPD symptomatology; a consistent focus on the therapeutic relationship, affect regulation, tolerance of emotional states, and biases in social cognition; a high priority given to self‐harm and suicidal behaviour; active therapists who deliver both support and validation as well as explorative and change‐oriented interventions; mix of treatment formats (e.g. includes both individual and group therapy); and therapist support in the form of supervision and regular meetings. The symptoms of BPD are addressed using the following therapeutic approaches. Following Weinberg 2011:
'emotional dysregulation' (e.g. intense anger and affective instability) is addressed through attention to affect, including raising awareness of emotional states, their triggers, and enhancing tolerance and regulative strategies;
'behavioural dysregulation' (e.g. impulsivity, self‐harm and suicidal behaviours) is addressed through change‐oriented interventions, including, for example, challenging negative thoughts, skills training, behavioural experiments, praise, and limit setting; and
'interpersonal dysfunction' (e.g. unstable relationships and stress‐related paranoid ideation) is treated using interventions that enhance the social‐cognitive (or mentalising) capacities of the BPD patient, through making basic and often negatively biased automatic assumptions explicit and more realistic or adaptive, and through paying attention to the establishment and maintenance of a safe and sound working alliance within the therapy sessions.
There is a risk that psychological therapies might not be helpful for all people affected by BPD, either due to the interventions delivered or through factors in the therapeutic relationship (Kongerslev 2015; Lilienfeld 2007; Parry 2016), and very little research has been done on this in people with BPD. The effectiveness of the therapy depends on the skills of the therapist to create the possibility for change with each patient. There is, therefore, the added complexity that the relationship or working alliance between the therapist and the patient itself is an ‘active ingredient’ of the therapy and that the quality of this relationship is an important predictor of outcome (Horvath 2011; Norcross 2011). There is no guarantee that the therapy will deliver what was specified in the manual or what was investigated in a randomised clinical trial (Parry 2016).
Why it is important to do this review
People with BPD and their family and friends experience high levels of psychological suffering. BPD is associated with considerable social costs in terms of service use (e.g. presentation to emergency clinics due to self‐harm or suicidal crises and repeated hospitalisations) and poor psychosocial functioning (e.g. inability to complete education or get/maintain a job). Consequently, identification of effective psychological therapies for BPD is important (Stoffers‐Winterling 2012).
Our review aims to provide a systematic summary of the evidence from randomised controlled trials (RCTs) in order to help people with BPD, their family and friends, mental healthcare workers, and policy and decision managers in general, to make informed decisions about evidence‐based treatment for BPD.
This review is an update of two previous Cochrane Reviews on psychological therapies for BPD (Binks 2006; Stoffers‐Winterling 2012). In addition to updating the two former Cochrane Reviews, our study also seeks to address some of the methodological limitations of both past and current reviews (Bateman 2015; Cristea 2017; Kliem 2010), by using updated methods, including a more comprehensive search strategy. We also had a new protocol published prior to conducting this review (Storebø 2018).
Objectives
To assess the beneficial and harmful effects of psychological therapies for people with BPD.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Persons of all ages, in any setting, with a formal, categorical diagnosis of BPD according to the Diagnostic and Statistical Manual of Mental Disorders (DSM) Third Edition (DSM‐III; APA 1980), Third Edition Revised (DSM‐III‐R; APA 1987), Fourth Edition (DSM‐IV; APA 1994), Fourth Edition Text Revision (DSM‐IV‐TR; APA 2000), and Fifth Edition (DSM‐5; APA 2013), and the International Classification of Diseases and Related Health Problems (ICD) 10th version (WHO 1993), with or without comorbid conditions.
To meet our inclusion criteria, at least 70% of participants of a respective trial had to have a formal diagnosis of BPD. We included trials involving subsamples of people with BPD when data on those with BPD were provided separately (we asked for separate data from trials including less than 70% BPD participants). We did not include trials that focused on people with mental impairment, organic brain disorder, dementia or other severe neurologic/neurodevelopmental diseases.
Types of interventions
Any defined psychological intervention regardless of theoretical orientation (e.g. psychodynamic therapy, CBT, systemic therapy or eclectic therapies designed for BPD treatment), in any kind of treatment setting (e.g. inpatient, outpatient or day clinic), compared to:
control interventions, such as standard care, treatment‐as‐usual (TAU), waiting list or no treatment; and
specific psychotherapeutic interventions (that were well defined and theory driven).
We divided control interventions into two categories: The first category was “waiting list/no treatment”: participants did not receive any treatment or support from the study centre (like, e.g. clinical management, regular medical review, or support/encouragement to find a therapist outside the study centre). The second category, “TAU” included any other kinds of controls: participants were either free to use any treatment except from the respective experiential treatment (optional TAU), or they received usual community treatment, or standardised usual care (obligatory TAU).
We pooled the different types of TAU into one comparison in our main analyses, and compared the effects between the two types of TAU as well as the effect observed by comparison to TAU controls to the effects of comparisons to waiting‐list/no treatment controls (see Subgroup analysis and investigation of heterogeneity).
We allowed concomitant treatments provided they were applied to both treatment conditions.
We accepted trials with active controls, including relaxation techniques such as autogenic training or meditation regimens, or patient education programmes such as self‐management and community‐based education programmes.
Types of outcome measures
Outcomes were either self‐rated by the persons with BPD or observer‐rated by clinicians, with clinician‐rated outcomes being preferred. We included only adequately validated measures (plus spontaneous reporting of adverse effects).
We analysed all outcomes at post‐treatment and at six months follow‐up or longer.
Primary outcomes
BPD symptom severity, assessed by, for example, the Zanarini Rating Scale for Borderline Personality Disorder (Zan‐BPD; Zanarini 2003a); the Borderline Personality Disorder Severity Index, Fourth version (BPDSI‐IV; Arntz 2003) or the Clinical Global Impression Scale for people with Borderline Personality Disorder (CGI‐BPD; Perez 2007).
Self‐harm, in terms of the proportion of participants with self‐harming behaviour, or assessed by, for example, the Deliberate Self‐harm Inventory (DSHI; Gratz 2001) or the Self‐harm behaviour Questionnaire (SHBQ; Guttierez 2001).
Suicide‐related outcomes, assessed by, for example, the Suicidal Behaviours Questionnaire (SBQ; Osman 2001) or the Beck Scale for Suicidal Ideation (BSSI; Beck 1979), or in terms of the proportion of participants with suicidal acts.
Psychosocial functioning, assessed by, for example, the Global Assessment Scale (GAS; Endicott 1976), the Global Assessment of Functioning Scale (GAF; APA 1987) or the Social Functioning Questionnaire (SFQ; Tyrer 2005).
Secondary outcomes
Anger, assessed by, for example, the Hostility subscale of the Symptom Checklist ‐ 90 ‐ Revised (SCL‐90‐R; Derogatis 1994) or the State‐Trait Anger Expression Inventory (STAXI; Spielberger 1988).
Affective instability, assessed by, for example, the relevant item or subscale on the Zan‐BPD (Zanarini 2003a), CGI‐BPD (Perez 2007) or BPDSI‐IV (Arntz 2003).
Chronic feelings of emptiness, assessed by, for example, the relevant item or subscale on the Zan‐BPD (Zanarini 2003a), CGI‐BPD (Perez 2007) or BPDSI‐IV (Arntz 2003).
Impulsivity, assessed by, for example, the Barrett Impulsiveness Scale (BIS; Barrett 1995), or the Anger, Irritability and Assault Questionnaire (AIAQ; Coccaro 1991).
Interpersonal problems, assessed by, for example, the Inventory of Interpersonal Problems (IIP; Horowitz 1988), or the relevant item or subscale on the Zan‐BPD (Zanarini 2003a), CGI‐BPD (Perez 2007), BPDSI‐IV (Arntz 2003), or SCL‐90‐R (Derogatis 1994).
Abandonment, assessed by, for example, the relevant item or subscale on the Zan‐BPD (Zanarini 2003a), CGI‐BPD (Perez 2007) or BPDSI‐IV (Arntz 2003).
Identity disturbance, assessed by, for example, the relevant item or subscale on the Zan‐BPD (Zanarini 2003a), CGI‐BPD (Perez 2007) or BPDSI‐IV (Arntz 2003).
Dissociation and psychotic‐like symptoms, assessed by, for example, the Dissociative Experience Scale (DES; Bernstein 1986), or the Brief Psychiatric Rating Scale (BPRS; Overall 1962).
Depression, assessed by, for example, the Beck Depression Inventory (BDI; Beck 1961) or the Montgomery Åsberg Depression Rating Scale (MADRS; Montgomery 1979).
Attrition, in terms of participants lost after randomisation in each group.
Adverse effects, measured by the use of standardised psychometric rating scales, such as the Systematic Assessment for Treatment Emergent Events (SAFTEE; Levine 1986), or by laboratory values or spontaneous reporting. We defined adverse effects as unfavourable outcomes that occurred during or after psychotherapy but that were not necessarily caused by it (see Chapter 19 in the Cochrane Handbook for Systematic Reviews of Interventions; Peryer 2019. We divided any reported adverse effects into severe and non‐severe, according to the International Committee of Harmonization guidelines (ICH 1996). We defined serious adverse effects as any event that led to death, was life‐threatening, required inpatient hospitalisation or prolongation of existing hospitalisation, resulted in persistent or significant disability, or any important medical event that may have jeopardised the participant’s health or required intervention to prevent one of the aforementioned outcomes occurring. We considered all other adverse effects to be non‐serious.
Search methods for identification of studies
Electronic searches
We searched the electronic databases and trials registers listed below up to March 2019.
Cochrane Central Register of Controlled trials (CENTRAL; 2019, Issue 3), in the Cochrane Library, which includes the Cochrane Developmental, Psychosocial and Learning Problems Specialised Register (searched 20 March 2019).
MEDLINE Ovid (1948 to 20 March 2019).
Embase Ovid (1980 to 20 March 2019).
CINAHL EBSCOhost (Cumulative Index to Nursing and Allied Health Literature; 1980 to 20 March 2019).
PsycINFO Ovid (1806 to 20 March 2019).
ERIC EBSCOhost (Education Resources Information Center; 1966 to 20 March 2019).
BIOSIS Previews Web of Science Clarivate Analytics (1969 to 20 March 2019).
Web of Science Core Collection Clarivate Analytics (1900 to 20 March 2019).
Sociological Abstracts ProQuest (1952 to 20 March 2019).
LILACS (Latin American and Caribbean Health Science Information database; lilacs.bvsalud.org/en; searched 20 August 2019).
OpenGrey (www.opengrey.eu; searched 20 August 2019).
Library Hub Discover,previously COPAC (Library Hub Discover; searched 20 August 2019).
ProQuest Dissertations and Theses A&I (1743 to 20 March 2019).
DART Europe E‐Theses Portal (www.dart-europe.eu/basic-search.php; searched 20 August 2019).
Networked Digital Library of Theses and Dissertations (NDLTD; www.ndltd.org; searched 20 August 2019).
Australian New Zealand Clinical trials Registry (ANZCTR; www.anzctr.org.au/BasicSearch.aspx; searched 20 August 2019).
Clinicaltrials.gov (clinicaltrials.gov; searched 20 March 2019).
EU Clinical trials Register (www.clinicaltrialsregister.eu/ctr-search/search; searched 20 August 2019).
ISRCTN Registry (www.isrctn.com; searched 20 August 2019).
Be Part of research (www.ukctg.nihr.ac.uk/#popoverSearchDivId; searched 20 August 2019).
WHO International Clinical trials Registry Platform (ICTRP; who.int/ictrp/en; searched 20 March 2019).
The search strategies for all databases can be found in Appendix 3. We did not limit our searches by language, year of publication, or type of publication. We sought translation of the relevant sections of non‐English language articles.
Searching other resources
We handsearched relevant journals, including: Journal of Personality Disorders; American Journal of Psychiatry; JAMA Psychiatry; British Journal of Psychiatry; ACTA Psychiatrica Scandinavica; Journal of the American Academy of Child and Adolescent Psychiatry; Personality Disorders: Theory, Research and Treatment; and Journal of Clinical Psychiatry. Additionally, we emailed researchers working in the field, to ask for unpublished data. We also checked abstracts of key conferences for BPD (congresses of the European and the International Society for the Study of Personality Disorders; ESSPD and ISSPD, respectively) and asked for any relevant unpublished data. We traced cross‐references from relevant literature. On 13 December 2019, we ran searches to make sure that none of our included trials had been retracted due to error or fraud. In the next update of this review, we will handsearch additional journal titles for relevant trials, (see Differences between protocol and review).
Data collection and analysis
We conducted this review in accordance with the guidelines set out in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019), and performed analyses using the latest version of Review Manager 5 (RevMan 5), Cochrane's statistical software (Review Manager 2014).
We report only the methods used in successive sections below. Planned but unused methods can be found in the protocol, Storebø 2018, and additional Table 4.
1. Unused methods.
| Section | Protocol (Storebø 2018) | Review |
|
Unit of analysis issues
|
Cross‐over trials We would have included cross‐over trials. We planned to include data up to the point of first cross‐over (first period only; Curtin 2002). We did not intend to use data from subsequent periods due to the likelihood of carry‐over effects from the preceding treatment(s). We planned not to combine repeated participant interventions in one meta‐analysis. |
We did not include any cross‐over trial. |
|
Cluster‐randomised trials Had trials used cluster randomisation, we would have anticipated that investigators would have presented their results after appropriately controlling for clustering effects (robust standard errors or hierarchical linear models). If it had been unclear whether a cluster‐randomised trial had used appropriate controls for clustering, we would have contacted the investigators for further information. We would have requested and re‐analysed individual patient data using multilevel models that controlled for clustering, if appropriate controls had not been used. Following this, we would have analysed effect sizes and standard errors in RevMan 5 (Review Manager 2014), using the generic inverse method (Higgins 2011). If there had been insufficient information to control for clustering, we would have entered outcome data using individuals as the units of analysis, and then conducted a sensitivity analysis to assess the potential biasing effects of inadequately controlled cluster‐randomised trials (Donner 2002). If individual participant data had not been available, we would have looked for information on intra‐class correlation coefficients to adjust for the potential clustering effects. |
We did not include any cluster‐randomised trial. | |
|
Adjustment for multiplicity We planned to adjust the P values and CIs for multiplicity due to the many secondary outcome comparisons following the method described by Jakobsen 2014. |
We only adjusted the primary outcomes and one secondary outcomes for multiplicity, i.e. those outcomes presented in the SoF table. | |
| Dealing with missing data | Had dichotomous data not been presented on the basis of ITT data, we would have added the number of participants lost in each group to the participants with unfavourable results, acting on the assumption that most people with BPD do not get lost at random. | We were unable to perform this analysis due to insufficient information |
| Subgroup analysis and investigation of heterogeneity | We intended to conduct subgroup analyses to make hypotheses about the subgroups mentioned below.
|
We did not conduct these preplanned analyses because of lack of data. |
| Sensitivity analysis | We intended to assess the impact of heterogeneity on the overall pooled effect estimate by removing studies ('outliers') that contributed to heterogeneity. We intended to remove outliers one by one and assess the impact on the overall outcome.
|
We were not able to perform these analyses, due to a lack of sufficient data. |
| TSA | We intended to calculate post hoc, low bias, risk diversity‐adjusted required information size TSA analyses for the primary outcomes. | We were not able to perform these analyses with low risk of bias trials. |
BPD: borderline personality disorder; CI: confidence interval;ITT: intention to treat; TAU: treatment‐as‐usual
Selection of studies
Twelve review authors (OJS, JMSW, BAV, JTM, HEC, AT, CPS, MTK, SSN, MLK, MSJ, EF) worked in six pairs and independently screened titles and abstracts of all records retrieved by the searches. For any record that could have been an eligible RCT, we obtained the full‐text report and assessed it for eligibility against the inclusion criteria (see Criteria for considering studies for this review). During all stages of study selection, we resolved uncertainty or disagreement by consensus. When agreement could not be reached, the review authors discussed disagreements and consulted a third review author (KL, OJS, JMSW or ES).
We list apparently relevant RCTs that did not fulfil the inclusion criteria, along with reasons for their exclusion, in the Characteristics of excluded studies tables. We used Covidence software to keep track of appraised trials and decisions. To ensure transparency of study selection, we provided flow charts according to the QUOROM statement, showing how many records have been excluded for a certain reason (Moher 1999).
Data extraction and management
We developed data extraction forms to facilitate standardisation of data extraction. The form was piloted by OJS, SSN, MTK.
Working in pairs, all review authors extracted data independently using the data collection form to ensure accuracy. We resolved disagreements by discussion or by using an arbiter (ES), if required.
OJS, HEC, AT, EF, and JMSW entered data into RevMan 5 (Review Manager 2014). After all data had been entered, another reviewer (JMSW, OJS) re‐checked the data for completeness and accuracy, to make sure the data were complete, correct and appropriately categorised. Any entered data were verified against the original publication, and we updated the list of outcomes (Appendix 4), if necessary.
Assessment of risk of bias in included studies
Using Cochrane's tool for assessing risk of bias (Higgins 2011), all review authors assessed the risk of bias in each included trial across the following domains: random sequence generation; allocation concealment; blinding of outcome assessment; incomplete outcome data, selective outcome reporting; and other potential sources of bias. Data extractors independently assigned each trial to one of three categories (low risk of bias, unclear (uncertain) risk of bias or high risk of bias), according to guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), using the criteria set out in Appendix 5. We called upon a third review author (ES) to resolve any ongoing disagreements, when needed.
Considering bias due to a lack of blinding is undoubtedly of importance, but it remains unclear how best to deal with this issue in research practice (Boutron 2008). We decided not to judge the likelihood of detection bias due to inadequate blinding of participants and personnel, as it is almost impossible to blind therapists and people receiving treatment in psychological therapy outcome research. However, we assessed the likelihood of detection bias due to inadequate blinding of outcome assessors.
In accordance with Cochrane’s guidelines (Higgins 2011), we also included other potential sources of bias as a final bias component. Here, we included: the likelihood of performance bias due to inadequate treatment adherence; the likelihood of bias due to different amounts of attention given to the treatment groups (attention bias); and other potential sources of bias, such as allegiance bias. We defined allegiance bias as a therapist’s personal belief both in the superiority and the efficacy of a particular treatment. This belief can be based on an education in that particular treatment. This bias is especially strong if the inventor of a treatment is investigating the effects of the particular treatment he/she has invented.
We considered trials with one or more unclear or high risk of bias domains as trials at high risk of bias overall, due to the risk of overestimating beneficial effects and underestimating harmful effects in RCTs with unclear or inadequate methodological quality (Kjaergard 2001; Lundh 2012; Moher 1998; Savović 2012a; Savović 2012b; Schulz 1995; Wood 2008). We defined trials with a low risk of bias in all domains to be at low risk of bias overall.
Measures of treatment effect
Continuous data
For continuous data, we compared the mean score between the two groups to give a mean difference (MD) and presented this with 95% confidence intervals (CIs). We used the overall MD, where possible, to combine the same outcome measures from trials. If two or more different instruments were used to measure the same construct, we reported the effect sizes as standardised mean differences (SMD) in the meta‐analysis. We calculated SMDs on the basis of post‐treatment results and, in separate analyses, follow‐up data. We grouped follow‐up data in six‐month intervals (zero to six months, six to 12 months and 12 months and over). Where the direction of a scale was opposite to most of the other scales, we multiplied the corresponding mean values by −1 to ensure adjusted values. If the trials did not report means and standard deviations (SDs) but reported other values like t‐tests and P values, we tried to transform these into SDs.
To identify the minimum relevant clinical difference (MIREDIF), we transformed the SMD to MD, using the scale with the best validity and reliability for the given outcome. For the analyses of the four primary outcomes in the comparison of psychotherapy versus TAU, we transformed SMDs into MDs on the following scales, to assess whether results exceeded the MIREDIF: ZAN‐BPD Scale, Delibarate Self‐Harm Inventory (DSHI), Suicidal Attempt Self Injury Interview (SASII), Global Assessment of Functioning (GAF), and the Hamilton Depression scale. We identified a MIREDIF of −3.0 points on the ZAN‐BPD, ranging from 0 to 36 points, based on a trial by Crawford 2018a; a MIREDIF of −1.25 points on the DSHI, ranging from 0 to 34 points, which corresponds to ½ SD based on a trial by Farivar 2004; a MIREDIF of −0.17 points on the Suicidal Attempt Self Injury Interview, ranging from 0 to 4 points, which corresponds to ½ SD based on a trial by Farivar 2004; and a MIREDIF of −4.0 on the GAF scale, ranging from 0 to 100, based on a trial by Amri 2014. The MIREDIF of the Hamilton Depression scale is 3.0 points (NICE CG90). For other outcomes, we provided an interpretation of the effect size using Cohen's D, considering 0.2 as a small effect, 0.5 as a medium effect size, and 0.8 as a large effect size (Cohen 1988).
Dichotomous data
We summarised dichotomous data as risk ratios (RRs) with 95% CIs. The RR is the ratio of the risk of an event in the two groups. We decided to use the RR as it may be easier to interpret than odds ratios (ORs).
Unit of analysis issues
Repeated observations
We calculated trial estimates on the basis of post‐treatment group results. We conducted separate analyses for data from different points of measurement (i.e. post‐treatment, follow‐up data of 0‐ to 6‐month, 6‐ to 12‐month, and above 12‐month intervals, where we used the last measurement within these intervals). If a trial reported data at both 7‐month and 11‐month follow‐up periods, we included both; however, we categorised cases like the 11‐month follow‐up as above 12‐month follow‐up. We did not use interim observations (Thalheimer 2002).
Adjustment for multiplicity
Multiplicity reflects the concern that performing multiple comparisons increases the risk of falsely rejecting the null hypothesis. Multiplicity, therefore, may affect the results found within a systematic review and, as a result, needs to be adjusted for. We adjusted the P values and CIs of the primary outcomes and one secondary outcome (depression) for multiplicity using the method described by (Jakobsen 2014).
Dealing with missing data
We tried to obtain any missing data, including incomplete outcome data, by contacting trial authors. We report this information in the 'Risk of bias' tables.
We evaluated the methods used to handle the missing data in the publications and to what extent it was likely that the missing data had influenced the results of outcomes of interest. We calculated effect sizes on the basis of intention‐to‐treat data, if that was possible. If only available case analysis data were reported, we calculated effect sizes on this basis.
We consulted a statistician if data were not reported in an immediately usable way and if data required processing before being analysed. We assessed results derived from statistically processed data in sensitivity analyses. See Sensitivity analysis.
Assessment of heterogeneity
We assessed trials for clinical homogeneity with respect to type of therapy, therapy setting and control group. We evaluated methodological heterogeneity by comparing the designs of trials. For any trials judged as homogeneous and adequate for pooling, we investigated statistical heterogeneity by both visual inspection of the graphs and the I2 statistic (Higgins 2003). We considered I2 values between 0% and 40% as indication of little heterogeneity, between 30% and 60% as indication of moderate heterogeneity, between 50% and 90% as indication of substantial heterogeneity, and between 75% and 100% as indication of considerable heterogeneity (Higgins 2019). Along with the size of the I2 scores, we also took into account the P value, CI and the overall number of included trials in the respective analysis when interpreting the values (Deeks 2019).
Assessment of reporting biases
We drew funnel plots (estimated differences in treatment effects against their standard error) and performed Egger's statistical test for small‐study effects for the primary outcomes; asymmetry in the funnel plot could be due to publication bias or could indicate genuine heterogeneity between small and large trials (Higgins 2019). It is important to assess the funnel axis in the funnel plot as a significant Egger's test could also indicate publication bias or be due to genuine small treatment effects. We did not visually inspect the funnel plot if fewer than 10 trials were included in the meta‐analysis, in accordance with the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019; Egger 1997; Sterne 2017).
Data synthesis
We performed the statistical analyses in accordance with the recommendations in the latest version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019).
In carrying out meta‐analyses, we used the inverse‐variance method, to give more weight to more precise estimates from trials with less variance (mostly larger trials). This minimises the imprecision of the pooled effect estimate, and is a common and simple approach to conducting meta‐analyses (Higgins 2019). We used the random‐effects model for our meta‐analyses, since we expected some degree of clinical heterogeneity to be present in most cases, though not too substantial to prevent pooling in principle. Where only one trial was included in an analysis, we used the fixed‐effect model, and where different models led to different results (Sensitivity analysis), we reported the results of both models.
For trials with a high level of statistical heterogeneity, and where the amount of clinical heterogeneity made it inappropriate to use these trials in meta‐analyses, we provide a narrative description of the trial results. If data pooling seemed feasible, we pooled the primary trials' effects and calculated their 95% CIs.
If a trial reported data for a particular outcome using two or more assessment instruments (e.g. several questionnaires for the assessment of depression), we selected the one used most often in the whole pool of included trials for effect size calculation, in order to minimise heterogeneity of outcomes in form and content. If a trial reported data of two assessment instruments that were equally frequently used, two review authors discussed the issue and chose the one that was, in its content, the most appropriate for the assessment of people affected by BPD.
We divided the doses and the controls into the different comparisons, ensuring that the treatment comparisons were comparable and homogeneous.
We have two main overarching comparisons. In the first comparison, we pooled all of the different types of psychotherapy together and compared them with the different types of TAU pooled together. In the second comparison, we pooled all of the different types of psychotherapy and compared them with waiting list or no treatment. Within in each comparison, we broke down the interventions by therapeutic category compared with different types of TAU, waiting list, no treatment or with another psychotherapy (active control).
Subgroup analysis and investigation of heterogeneity
We conducted the following prespecified subgroup analyses for two primary outcomes (BPD symptom severity and psychosocial functioning), where data were sufficient, in order to make hypotheses.
Therapeutic approaches: specific therapies versus each other (only analyses with two or more trials were included in this subgroup)
Age: mean age of 15 to 18 years versus older than 18 years
Duration: less than six months versus six to 12 months versus over 12 months
Mode of therapy: individual therapy versus group therapy and with the combination of individual and group therapy
Setting: inpatient versus outpatient and the combination of inpatient and outpatient
In addition, we added the following four subgroup analyses post hoc, for the primary outcomes of BPD symptom severity and psychosocial functioning.
Type of raters: self‐rated versus clinician‐rated
Types of TAU: obligatory TAU versus unspecified TAU
Type of comparison group: trials comparing psychotherapy plus TAU versus trials comparing psychotherapy with waiting list or no treatment
Types of scales: different measuring scales versus each other
Trial Sequential Analysis
Trial Sequential Analysis (TSA) is a methodology that combines a required information size (RIS) calculation for a meta‐analysis with the threshold for statistical significance (Brok 2008; Brok 2009; Thorlund 2009; Wetterslev 2008). TSA is a tool for quantifying the statistical reliability of the data in a cumulative meta‐analysis, adjusting P values for sparse data and for repetitive testing on accumulating data (Brok 2008; Brok 2009; Thorlund 2009; Wetterslev 2008).
Comparable to the a priori sample size estimation in a single randomised trial, a meta‐analysis should include an RIS calculation at least as large as the sample size of an adequately powered single trial to reduce the risk of random error. TSA calculates the RIS in a meta‐analysis and provides an alpha‐spending boundary to adjust the significance level for sparse data and repetitive testing on accumulating data (CTU 2011; Wetterslev 2008); hence, the risk of random error can be assessed. Multiple analyses of accumulating data when new trials emerge leads to repeated significant testing and introduces multiplicity. Thus, use of a conventional P value is prone to exacerbate the risk of random error (Berkey 1996; Lau 1995). Meta‐analyses not reaching the RIS are analysed with trial sequential alpha‐spending monitoring boundaries analogous to interim monitoring boundaries in a single trial (Wetterslev 2008). This approach will be crucial in coming updates of the review.
If a TSA does not reveal significant findings (no crossing of the alpha‐spending boundary and no crossing of the conventional boundary of P = 0.05) before the RIS has been reached, then the conclusion should either be that more trials are needed to reject or accept an intervention effect that was used for calculation of the required sample size or — in case the cumulated Z‐curve enters the futility area — the anticipated effect can be rejected.
We used a MIREDIF from studies defining this or, where we could not find this, we used an assumption that the minimal relevant clinical intervention effect was approximately ½ SD on the used scale, which can be used as a MIREDIF (Norman 2003).
We calculated the diversity‐adjusted required information size (DARIS; that is the number of participants required to detect or reject a specific intervention effect in a meta‐analysis), and performed a TSA for the primary outcomes at the end of treatment for the main comparison versus TAU, based on the following a priori assumptions:
the SD of the primary outcomes;
an anticipated MIREDIF defined in a trial reporting on this or we used a ½ SD on the used scale;
a maximum type I error of 2.0% (due to four primary outcomes; Jakobsen 2014);
a maximum type II error of 10% (minimum 90% power; Castellini 2018); and
the diversity observed in the meta‐analysis.
We furthermore performed a TSA for the three secondary outcomes (for the main comparison versus TAU) not closely connected to the BPD core symptoms (depression, attrition and adverse effects) based on the following priori assumptions:
the SD of the primary outcomes;
an anticipated MIREDIF defined in a trial reporting on this or we used a ½ SD on the used scale;
a maximum type I error of 0.8% (due to 11 secondary outcomes; Jakobsen 2014);
a maximum type II error of 10% (minimum 90% power; Castellini 2018); and
the diversity observed in the meta‐analysis.
We only performed a TSA for depression as this was the only significant finding.
Sensitivity analysis
We conducted sensitivity analyses to determine whether findings were sensitive to:
imprecision, as assessed by GRADE, by conducting TSA analyses on all primary outcomes and for the three secondary outcomes (for the main comparison versus TAU) not closely connected to the BPD core symptoms (depression, attrition and adverse effects) with significant findings.
random‐effects or fixed‐effect models.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach to construct 'Summary of findings' tables in which to document the results of review outcomes. Two reviewers (HEC and JSW) , working independently, assessed the quality of the evidence. Any conflicts were resolved by consulting a third author (OJS). The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. Considerations are due to: within‐trial risk of bias; directness of the evidence; heterogeneity of the data; precision of effect estimates; and risk of publication bias (Andrews 2013a; Andrews 2013b; Balshem 2011; Brunetti 2013; Guyatt 2011a; Guyatt 2011b; Guyatt 2011c; Guyatt 2011d; Guyatt 2011e; Guyatt 2011f; Guyatt 2011g; Guyatt 2013a; Guyatt 2013b; Guyatt 2013c; Mustafa 2013). When possible, we reported the MD or the RR, and we used TSA to rate imprecision (Jakobsen 2014). TSA can be used as a secondary analysis in Cochrane Reviews, to provide an additional interpretation of the data from a specific perspective, and can be used for testing imprecision (Thomas 2019).
We report the four primary outcomes (BPD severity, self‐harm, suicide‐related outcomes and psychosocial functioning) and three secondary outcomes not closely connected to the BPD core symptoms (depression, attrition and adverse effects) in 'Summary of findings' tables for our two main comparisons (Atkins 2004): psychotherapy versus treatment‐as‐usual (Table 1); and psychotherapy versus waiting list or no treatment (Table 2). We also created a third 'Summary of findings' table in which we report data from the DBT and MBT treatments with the highest numbers of primary trials, with DBT being the subject of roughly one‐third of all included trials, followed by MBT with seven RCTs. In this table, we report only the primary outcomes (see Table 3).
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies, Characteristics of studies awaiting classification and Characteristics of ongoing studies tables.
Results of the search
All electronic databases and search periods are listed in the Methods section (see Electronic searches). There were no language, date or document format restrictions. This current review is part of a series of reviews on interventions for BPD. Therefore, we used a very comprehensive search strategy, covering all psychotherapeutic or pharmacological treatment (or both) of BPD.
Altogether, the searches generated 22,078 records, of which 3477 were duplicates. Following screening of titles and abstracts, 400 citations merited closer inspection. An assessment of full texts for possible inclusion into this review led to the exclusion of 118 reports. This left 282 reports included. Of these, 50 reports referred to 33 different ongoing trials (see Ongoing studies). Another 12 reports related to 11 different trials that we were unable to classify definitively as included or excluded at this point of time, despite our best efforts to retrieve further information from the trial authors (see Studies awaiting classification). This finally left 220 reports relating to 75 different included trials (see Included studies, and Figure 1).
1.
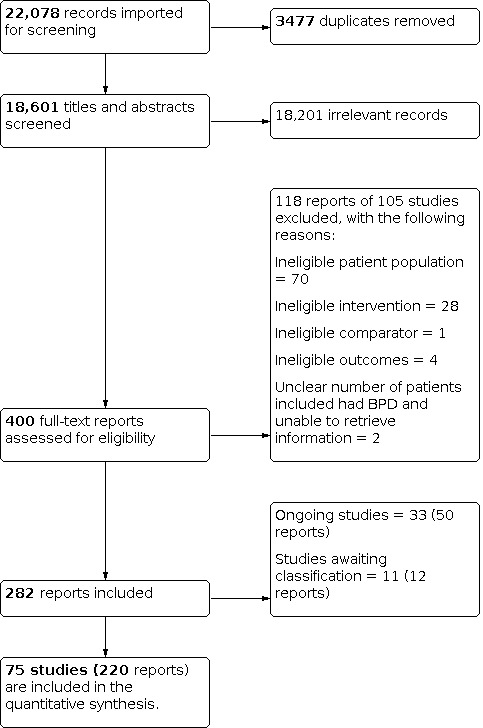
Study flow diagram
Included studies
Below, we summarise the key characteristics of the included trials. For information about which trials correspond to which characteristics for certain categories below, please see Table 5. Further detail can also be found in the Characteristics of included studies tables.
2. Key demographic characteristics of the included studies.
| Category | Study frequency | Study ID |
| Sample size | ||
| Sample size above 100 participants | 5 | Andreoli 2016; Antonsen 2017; Linehan 2006; McMain 2009; McMurran 2016 |
| Setting | ||
| Studies with inpatient settings | 5 | Jahangard 2012; Leichsenring 2016; Mohamadizadeh 2017; Schilling 2018; Stanley 2017 |
| Studies with both inpatient and outpatient settings | 7 | Antonsen 2017; Bateman 1999; Davidson 2014; Gleeson 2012; Kredlow 2017b; Laurenssen 2018; Smith 2012 |
| Gender | ||
| Only females included | 17 | Carter 2010; Doering 2010; Farrell 2009; Gratz 2006; Gratz 2014; Harned 2014; Koons 2001a; Linehan 1991; Linehan 1994; Linehan 2006; Linehan 2015a; Mohamadizadeh 2017; Smith 2012; Van den Bosch 2005; Weinberg 2006; Zanarini 2008; Zanarini 2018 |
| Only males included | 2 | Bianchini 2019; Kamalabadi 2012 |
| Diagnostic classification | ||
| DSM‐III diagnosis | 1 | Linehan 1991 |
| DSM‐III‐R diagnosis | 4 | Bateman 1999; Koons 2001a; Linehan 1994; Turner 2000 |
| DSM‐IV diagnosis | 6 | Borschmann 2013; McMurran 2016; Mohamadizadeh 2017; Priebe 2012; Rossouw 2012b; Schilling 2018 |
| DSM‐IV‐TR diagnosis | 54 | Andreoli 2016; Antonsen 2017; Bateman 2009; Bellino 2006; Bellino 2007; Bellino 2010; Blum 2008; Bos 2010; Carter 2010; Cottraux 2009; Davidson 2006; Davidson 2014; Doering 2010; Elices 2016; Feigenbaum 2012; Feliu-Soler 2017; Giesen-Bloo 2006; Gleeson 2012; Gratz 2006; Gratz 2014; Gregory 2008b; Haeyen 2018; Harned 2014; Jahangard 2012; Jochems 2015; Jørgensen 2013; Kamalabadi 2012; Kramer 2011; Kramer 2014; Kramer 2016: Kredlow 2017a; Kredlow 2017b; Laurenssen 2018; Leppänen 2016; Lin 2019; Linehan 2006; McMain 2009; McMain 2017; Morey 2010; Morton 2012; Nadort 2009; Pascual 2015; Philips 2018; Reneses 2013; Robinson 2016; Santisteban 2015; Schilling 2018; Sinnaeve 2018; Soler 2009; Smith 2012; Van den Bosch 2005; Weinberg 2006; Zanarini 2008; Zanarini 2018 |
| ICD‐10 diagnosis | 1 | Leichsenring 2016 |
| Diagnostic assessment | ||
| BPDSI‐IV (Arntz 2003) | 1 | Kamalabadi 2012 |
| CI‐BPD (Zanarini 2003b) | 1 | Rossouw 2012b |
| DIB (Gunderson 1981) or DIB‐R (Zanarini 1989) | 4 | Cottraux 2009; Feliu-Soler 2017; Linehan 1991; Zanarini 2008 |
| DIB‐R (Zanarini 1989) and the BSI (Conte 1980) | 1 | Farrell 2009 |
| DIB‐R (Zanarini 1989) plus any other DSM‐oriented diagnostic interview SCID‐II (First 1997) | 1 | Bateman 1999 |
| DIPD‐IV (Zanarini 1987) | 3 | Gratz 2006; Gratz 2014; Morey 2010 |
| IPDE (Loranger 1995) | 4 | Andreoli 2016; Harned 2014; McMain 2017; McMurran 2016 |
| IPDE‐self‐rating screening and questionnaire, with the preliminary findings confirmed in clinical interviews by a psychiatrist | 1 | Carter 2010 |
| Millon Clinical Multiaxial Inventory, 3rd Edition (MCMI‐III) | 1 | Jahangard 2012 |
| PDE (Loranger 1988) | 1 | Turner 2000 |
| SCID‐II (First 1997) | 46 | Amianto 2011; Antonsen 2017; Bateman 1999; Bateman 2009; Bellino 2006; Bellino 2007; Bellino 2010; Borschmann 2013; Bos 2010; Davidson 2006; Davidson 2014; Doering 2010; Elices 2016; Feigenbaum 2012; Giesen-Bloo 2006; Gleeson 2012; Gregory 2008b; Haeyen 2018; Jørgensen 2013; Koons 2001a; Kramer 2011; Kramer 2014; Kramer 2016; Kredlow 2017a; Kredlow 2017b; Laurenssen 2018; Leichsenring 2016; Leppänen 2016; Lin 2019; Linehan 1994; Linehan 2006; Linehan 2015a; Koons 2001a; Morton 2012; Nadort 2009; Pascual 2015; Philips 2018; Priebe 2012; Reneses 2013; Robinson 2016; Schilling 2018; Soler 2009; Smith 2012; Van den Bosch 2005; Weinberg 2006; Zanarini 2018 |
| SIDP‐IV (Pfohl 1997) | 1 | Blum 2008 |
| Exclusion criteria in included studies | ||
| Participants with substance abuse or dependence excluded | 43 | Amianto 2011; Andreoli 2016; Antonsen 2017; Bateman 1999; Bateman 2009; Bellino 2006; Bellino 2007; Bellino 2010; Blum 2008; Carmona í Farrés 2019; Cottraux 2009; Davidson 2006; Doering 2010; Feigenbaum 2012; Giesen-Bloo 2006; Gratz 2006; Gratz 2014; Jørgensen 2013; Kamalabadi 2012; Koons 2001a; Kramer 2011; Kredlow 2017a; Laurenssen 2018; Leichsenring 2016; Leppänen 2016; Lin 2019; Linehan 1991; Linehan 1994; McMain 2009; Mohamadizadeh 2017; Morey 2010; Nadort 2009; Pascual 2015; Rossouw 2012b; Schilling 2018; Schuppert 2012; Sinnaeve 2018; Soler 2009; Stanley 2017; Smith 2012; Weinberg 2006; Zanarini 2008; Zanarini 2018 |
| Alcohol or substance abuse and dependence included | 4 | Davidson 2014; Gregory 2008b; Robinson 2016; Santisteban 2015 |
| Antisocial features or full antisocial personality disorders excluded | 9 | Antonsen 2017; Carter 2010; Cottraux 2009; Doering 2010; Giesen-Bloo 2006; Jørgensen 2013; Kamalabadi 2012; Koons 2001a; Nadort 2009 |
| Duration of interventions | ||
| Less than six months | 36 | Andreoli 2016; Antonsen 2017; Blum 2008; Bohus 2013; Borschmann 2013; Bos 2010; Davidson 2014; Elices 2016; Feliu-Soler 2017; Gleeson 2012; Gratz 2006; Gratz 2014; Haeyen 2018; Jahangard 2012; Kamalabadi 2012; Kramer 2011; Kramer 2014; Kramer 2016; Kredlow 2017a; Kredlow 2017b; Leichsenring 2016; Lin 2019; McMain 2017; McMurran 2016; Mehlum 2014; Mohamadizadeh 2017; Morey 2010; Morton 2012; Pascual 2015; Reneses 2013; Schilling 2018; Schuppert 2012; Soler 2009; Weinberg 2006; Zanarini 2008; Zanarini 2018 |
| Between six months and 12 months | 32 | Amianto 2011; Bellino 2006; Bellino 2007; Bellino 2010; Bianchini 2019; Carmona í Farrés 2019; Carter 2010; Cottraux 2009; Davidson 2006; Doering 2010; Farrell 2009; Feigenbaum 2012; Gregory 2008b; Harned 2014; Jochems 2015; Koons 2001a; Leppänen 2016; Linehan 1991; Linehan 1994; Linehan 2006; Linehan 2015a; McMain 2009; Priebe 2012; Robinson 2016; Rossouw 2012b; Salzer 2014; Santisteban 2015; Sinnaeve 2018; Stanley 2017; Smith 2012; Turner 2000; Van den Bosch 2005 |
| Longer than 12 months | 7 | Bateman 1999; Bateman 2009; Giesen-Bloo 2006; Jørgensen 2013; Laurenssen 2018; Nadort 2009; Philips 2018 |
| Formats of interventions | ||
| Individual treatment | 33 | Amianto 2011; Andreoli 2016; Borschmann 2013; Bellino 2006; Bellino 2007; Bellino 2010; Cottraux 2009; Davidson 2006; Davidson 2014; Doering 2010; Giesen-Bloo 2006; Gleeson 2012; Harned 2014; Jahangard 2012; Kramer 2011; Kramer 2014; Kredlow 2017a; Kredlow 2017b; Leichsenring 2016; McMain 2009; McMurran 2016; Mohamadizadeh 2017; Morey 2010; Nadort 2009; Philips 2018; Priebe 2012; Reneses 2013; Salzer 2014; Stanley 2017; Smith 2012; Weinberg 2006; Zanarini 2008; Zanarini 2018 |
| Group treatment | 22 | Antonsen 2017; Blum 2008; Bohus 2013; Bos 2010; Elices 2016; Farrell 2009; Feliu-Soler 2017; Gratz 2006; Gratz 2014; Haeyen 2018; Jochems 2015; Kamalabadi 2012; Kramer 2016; Leppänen 2016; Lin 2019; Linehan 2015a; McMain 2017; Morton 2012; Pascual 2015; Santisteban 2015; Schilling 2018; Soler 2009 |
| Combination of individual and group treatment | 16 | Bateman 1999; Bateman 2009; Bianchini 2019; Carter 2010; Feigenbaum 2012; Gregory 2008b; Jørgensen 2013; Koons 2001a; Linehan 1991; Linehan 1994; Linehan 2006; Laurenssen 2018; Robinson 2016; Rossouw 2012b; Turner 2000; Van den Bosch 2005 |
| Concomitant medication | ||
| Allowed, if needed | 59 | Amianto 2011; Andreoli 2016; Antonsen 2017; Bateman 1999; Bateman 2009; Blum 2008; Bohus 2013; Borschmann 2013; Bos 2010; Carmona í Farrés 2019; Cottraux 2009; Davidson 2006; Doering 2010; Elices 2016; Farrell 2009; Feigenbaum 2012; Feliu‐Soler 2017; Giesen‐Bloo 2006; Gleeson 2012; Gratz 2006; Gratz 2014; Gregory 2008b; Harned 2014; Jochems 2015; Jørgensen 2013; Koons 2001a; Kramer 2011; Kramer 2014; Kramer 2016; Kredlow 2017a; Kredlow 2017b; Laurenssen 2018; Leichsenring 2016; Linehan 1991; Linehan 1994; Linehan 2006; Linehan 2015a; McMain 2009; McMain 2017; McMurran 2016; Mehlum 2014; Morey 2010; Morton 2012; Nadort 2009; Pascual 2015; Priebe 2012; Reneses 2013; Rossouw 2012b; Salzer 2014; Schilling 2018; Schuppert 2012; Sinnaeve 2018; Smith 2012; Soler 2009; Turner 2000; Van den Bosch 2005; Weinberg 2006; Zanarini 2008; Zanarini 2018 |
| All participants of each group received the same kind of concomitant medication | 4 | Bellino 2006; Bellino 2007; Bellino 2010; Jahangard 2012 |
| Partly (50% of each group concomitantly received a specified, concurrent medication, 50% placebo) | 1 | Stanley 2017 |
| Not allowed | 2 | Lin 2019; Mohamadizadeh 2017 |
| Not specified | 9 | Bianchini 2019; Carter 2010; Davidson 2014; Haeyen 2018; Kamalabadi 2012; Leppänen 2016; Philips 2018; Robinson 2016; Santisteban 2015 |
| Control interventions | ||
| Obligatory | 42 | Amianto 2011; Andreoli 2016; Bateman 1999; Bateman 2009; Bellino 2006; Bellino 2010; Bianchini 2019; Borschmann 2013; Bos 2010; Carter 2010; Davidson 2014; Doering 2010; Farrell 2009; Feigenbaum 2012; Gleeson 2012; Gratz 2006; Gratz 2014; Jahangard 2012; Jochems 2015;Jørgensen 2013; Koons 2001a; Kramer 2016; Laurenssen 2018; Leichsenring 2016; Leppänen 2016; Linehan 2006; McMurran 2016; Mehlum 2014; Morton 2012; Mohamadizadeh 2017; Philips 2018; Priebe 2012; Reneses 2013; Robinson 2016; Rossouw 2012b; Schuppert 2012; Soler 2009; Stanley 2017; Smith 2012; Van den Bosch 2005; Weinberg 2006; Zanarini 2018 |
| Optional | 13 | Blum 2008; Bohus 2013; Davidson 2006; Gregory 2008b; Haeyen 2018; Kamalabadi 2012; Kredlow 2017a; Linehan 1991; Linehan 1994; McMain 2017; Mohamadizadeh 2017; Salzer 2014; Zanarini 2008 |
| Funding | ||
| Funded by grants from universities, authorities or research foundations | 62 | Amianto 2011; Antonsen 2017; Bateman 1999; Bateman 2009; Blum 2008; Bohus 2013; Borschmann 2013; Bos 2010; Carmona í Farrés 2019; Cottraux 2009; Davidson 2006; Davidson 2014; Doering 2010; Elices 2016; Farrell 2009; Feigenbaum 2012; Feliu‐Soler 2017; Giesen‐Bloo 2006; Gleeson 2012; Gratz 2006; Gratz 2014; Gregory 2008b; Haeyen 2018; Harned 2014; Jochems 2015; Jørgensen 2013; Kramer 2011; Kramer 2014; Kramer 2016; Kredlow 2017a; Kredlow 2017b; Laurenssen 2018; Leichsenring 2016; Lin 2019; Linehan 1991; Linehan 1994; Linehan 2006; Linehan 2015a; McMain 2009; McMain 2017; McMurran 2016; Mehlum 2014; Morey 2010; Nadort 2009; Pascual 2015; Philips 2018; Priebe 2012; Reneses 2013; Robinson 2016; Rossouw 2012b; Salzer 2014; Santisteban 2015; Schilling 2018; Schuppert 2012; Sinnaeve 2018; Smith 2012; Soler 2009; Stanley 2017; Van den Bosch 2005; Weinberg 2006; Zanarini 2008; Zanarini 2018 |
| No funding received | 4 | Bellino 2006; Bellino 2007; Bellino 2010; Jahangard 2012 |
| Unclear funding | 9 | Andreoli 2016; Bianchini 2019; Carter 2010; Kamalabadi 2012; Koons 2001a; Leppänen 2016; Mohamadizadeh 2017; Morton 2012; Turner 2000 |
| BPDSI‐IV: Borderline Personality Disorder Severity Index; BSI: Borderline Syndrome Index; CI‐BPD: Childhood Interview for DSM‐IV Borderline Personality Disorder; DIB: Diagnostic Interview for Borderline Patients; DIB‐R: Diagnostic Interview for Borderline Patients ‐ revised version; DIPD‐IV: Diagnostic Interview for DSM‐IV Personality Disorders; DSM‐III: Diagnostic and Statistical Manual of Mental Disorders, Third Edition; DSM‐III‐R: Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; DSM‐IV‐TR: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision; ICD‐10: International Classification of Diseases, Tenth Revision; IPDE: International Personality Disorder Examination; PDE: Personality Disorders Examination; SCID‐II: Structured Clinical Interview for DSM DSM‐IV Axis II Personality Disorders (SCID‐II); SIDP‐IV: Structured Interview for DSM‐IV Personality | ||
Design
We included 75 randomised, parallel‐arm trials.
Sample sizes
There was considerable variation in sample sizes between the trials, as the total number of participants with BPD ranged from 16 (Gleeson 2012) to 190 participants (McMain 2009). Five trials had a sample size of more than 100 participants (Table 5).
Setting
Sixty‐three trials were conducted in outpatient settings, five in inpatient settings, and seven in both inpatient and outpatient settings (see Table 5).
Participants
The 75 trials included a total of 4507 participants with BPD; the mean age ranged from 14.8 to 45.7 years. Seventeen trials included only female participants and two trials included only males (see Table 5). All remaining trials included participants of both genders, predominantly females.
Diagnostic criteria
Participants were diagnosed as having BPD according to DSM‐III, DSM‐III‐R, DSM‐IV, DSM‐IV‐TR, or ICD‐10 criteria (see Table 5). The diagnosis was confirmed by standardised means of assessment. The most frequently used assessment instruments were the Structured Clinical Interviews for DSM‐III‐R or DSM‐IV Axis II Personality Disorders (SCID‐II) (First 1997; Spitzer 1989) in 47 trials. Eleven trials applied the Diagnostic Interview for Borderline Personality Disorder patients (DIB; Gunderson 1981) or the Diagnostic Interview for Borderline Personality Disorder Patients ‐ revised (DIB‐R; Zanarini 1989). Six trials made use of the International Personality Disorder Examination (IPDE; Loranger 1995). All assessment instruments used in the included trials are listed in Table 5.
Participants exclusion criteria of primary studies
In 39 trials, people with evident mental impairment, organic brain disorder and neurological conditions were not eligible for participation. In addition, with the exception of Bos 2010 and Kredlow 2017a, schizophrenia, schizoaffective and other psychotic disorders were reasons for exclusion in all trials. Participants with substance abuse or dependence were not eligible for inclusion in 43 trials. Four trials included participants with alcohol or substance abuse/dependence (Davidson 2014; Gregory 2008b; Robinson 2016; Santisteban 2015). Comorbid personality disorders were not a reason for exclusion in 71 trials. Nine trials excluded participants with antisocial features or full antisocial personality disorders. See Table 5 for a list of the trials.
Interventions
Durations of interventions
The duration of the trials ranged from one month (Jahangard 2012) to 36 months (Giesen‐Bloo 2006). Thirty‐six trials had a duration of less than six months. Thirty‐two trials lasted between six months and 12 months. See Table 5.
Formats of interventions
Most of the trials applied individual treatment, with a total of 33 trials investigating the effects of individual therapy. Twenty‐two trials investigated the effects of group therapy whereas 16 trials assessed a combination of individual and group therapy (see Table 5).
Types of interventions
We included any defined, psychological intervention. The following provides a brief overview and description of the interventions investigated in the individual trials. For a more elaborate description, see the relevant references.
Dialectical behaviour therapy (DBT) and modified DBT‐related treatments (DBT; Linehan 1993a; Linehan 1993b). Twenty‐four trials investigated the effect of DBT and DBT‐related treatments (Bohus 2013; Bianchini 2019; Carter 2010; Elices 2016; Feigenbaum 2012; Feliu‐Soler 2017; Gratz 2014; Harned 2014; Kamalabadi 2012; Koons 2001a; Kramer 2016; Lin 2019; Linehan 1991; Linehan 1994; Linehan 2006; Linehan 2015a; McMain 2009; McMain 2017; Mohamadizadeh 2017; Priebe 2012; Soler 2009; Stanley 2017; Turner 2000; Van den Bosch 2005).
Cognitive behavioural therapy (CBT) and CBT‐ related treatments. Eleven trials investigated the effect of CBT and CBT‐related treatments (Bellino 2007; Cottraux 2009; Davidson 2006; Davidson 2014; Jahangard 2012; Kramer 2011; Kramer 2014; Kredlow 2017a; Kredlow 2017b; McMurran 2016; Schilling 2018).
Psychoeducation. One trial investigated a generic form of psychoeducation in which the first step (awareness of BPD) of the STEPPS programme was taught (Pascual 2015), and one trial included a once‐only workshop (Zanarini 2008).
Cognitive analytic therapy (CAT) and CAT‐related treatments. One trial investigated the effect of CAT (Gleeson 2012).
Psychodynamic psychotherapy and related treatments were investigated by three trials: psychic representation‐focused psychotherapy in Reneses 2013; psychoanalytic‐interactional therapy in Leichsenring 2016; and sequential brief Adlerian psychodynamic psychotherapy in Amianto 2011.
Dynamic deconstructive psychotherapy (DDP). One trial investigated the effect of DDP (Gregory 2008b).
Schema‐focused therapy (SFT) and SFT‐related treatments. Four trials investigated the effect of SFT and SFT‐related treatment (Farrell 2009; Giesen‐Bloo 2006; Mohamadizadeh 2017; Nadort 2009).
Acceptance and commitment therapy (ACT) employs mindfulness and acceptance strategies. One trial investigated the effect of ACT (Morton 2012).
Mentalisation‐based treatment (MBT) and MBT‐related treatments. Six trials investigated the effect of MBT and MBT‐related treatments (Bateman 1999; Bateman 2009; Jørgensen 2013; Philips 2018; Robinson 2016; Rossouw 2012b).
Interpersonal psychotherapy (IPT) and IPT‐related treatments. Four trials investigated the effect of IPT (Bellino 2006; Bellino 2007; Bellino 2010; Smith 2012).
Client‐centred therapy (CCT) and related treatments aim to encourage the patient to find solutions to their own problems and to help them decide how they need to change. Two trials investigated the effect of CCT (Cottraux 2009; Turner 2000).
Manual‐assisted cognitive treatment (MACT) is used to treat people who are acutely self‐harming. The treatment is structured around a self‐help book. Two trials investigated the effect of MACT (Morey 2010; Weinberg 2006).
Systems training for emotional predictability and problem‐solving for borderline personality disorder (STEPPS) is a seminar‐like, group treatment programme that combines cognitive‐behavioural elements and skills training. Two trials investigated the effect of STEPPS (Blum 2008; Bos 2010).
Eclectic treatments included art therapy (Haeyen 2018), abandonment psychotherapy and intensive community treatment (Andreoli 2016), a combination of individual SFT and group DBT (Leppänen 2016), combined inpatient and outpatient psychotherapy (Antonsen 2017), integrative BPD‐oriented adolescent family therapy (Santisteban 2015), joint crises plan (Borschmann 2013), web‐based psychoeducation (Zanarini 2018), and motivation feedback (Jochems 2015). Eight trials investigated eclectic treatments.
Concomitant treatment
Medication use was not allowed in three trials (Lin 2019; Mohamadizadeh 2017; Zanarini 2018). The majority of trials allowed participants to continue their respective drug treatments if initiated before the start of the trial (59 trials). Only four out of 75 RCTs gave the same medication to all participants (fluoxetine in the first three trials, selective serotonin reuptake inhibitors not further specified in the latter) (Bellino 2006; Bellino 2007; Bellino 2010; Jahangard 2012). One trial, Stanley 2017, included four treatment groups, which we integrated into two groups of DBT and supportive treatment. Fifty percent of each of these two groups additionally received either fluoxetine or placebo. Concomitant drug use was not specified in nine trials (Bianchini 2019; Carter 2010; Davidson 2014; Haeyen 2018; Kamalabadi 2012; Leppänen 2016; Philips 2018; Robinson 2016; Santisteban 2015). Two trials included medication‐free participants only (Lin 2019; Mohamadizadeh 2017; see Table 5).
Comparisons
The review includes 25 different comparisons.
Control interventions
The kinds of control interventions varied widely and included, for example, treatment‐as‐usual (TAU), waiting list, no treatment or an active treatment. The composition of the control interventions used in the individual trials differed as to whether TAU was obligatory or optional.
The majority of trials (42 trials) included a control intervention with an obligatory TAU. Thirteen trials included a control intervention with optional TAU. See Table 5.
Active treatment
Twenty‐two trials included an active head‐to‐head comparison.
Antonsen 2017: inpatient plus outpatient psychotherapy versus outpatient psychotherapy
Bellino 2007: cognitive therapy versus interpersonal therapy
Carmona í Farrés 2019: dialectical behaviour therapy (DBT) mindfulness versus DBT interpersonal effectiveness
Cottraux 2009: cognitive therapy versus client‐centred therapy (CCT)
Elices 2016: DBT mindfulness versus DBT interpersonal effectiveness
Feliu‐Soler 2017: DBT mindfulness versus loving‐kindness and compassion meditation
Giesen‐Bloo 2006: schema‐focused therapy (SFT) versus transference‐focused therapy
Harned 2014: standard DBT versus DBT prolonged exposure (PE)
Kramer 2011 and Kramer 2014: motive‐oriented therapeutic relationship (MOTR) versus general psychiatric management (GPM)
Kredlow 2017b: cognitive behavioural therapy (CBT) versus trauma‐related plus anxiety‐related psychoeducation
Lin 2019: DBT skills training versus cognitive therapy
Linehan 2015a: standard DBT versus DBT skills plus case management versus DBT individual therapy plus activity
McMain 2009: DBT versus general psychiatric management (GPM)
Mohamadizadeh 2017: DBT versus SFT
Morey 2010: manual‐assisted cognitive therapy (MACT) versus MACT plus therapeutic assessment (TA)
Nadort 2009: SFT versus SFT plus therapist telephone crisis support outside office hours
Pascual 2015: pseudoeducation versus neurocognitive rehabilitation group
Santisteban 2015: BPD‐oriented adolescent family therapy versus individual drug counselling
Schilling 2018: meta‐cognitive training for borderline personality disorder (B‐MCT) versus progressive muscle relaxation training (PMR)
Sinnaeve 2018: standard DBT (outpatient) versus step‐down DBT (combined inpatient and outpatient DBT)
Turner 2000: DBT versus CCT
Kramer 2016: DBT informed and individual treatment versus TAU
Outcomes
When several measures were available for the same outcome, we chose the measure that was used most often, in order to reduce heterogeneity of outcomes in form and content. Outcomes could be either self‐reported by people treated or observer‐rated by clinicians, with clinician‐rated outcomes being preferred. We included outcomes reported at the end of treatment and at ≥ six months follow‐up. trials used a variety of scales to assess both primary and secondary outcomes. The table of outcomes in Appendix 4 presents the list of scales used to assess the outcomes in the individual trials. The list refers to the measurement scales described in the original reports.
Adverse effects
Data on adverse effects were available from five trials (Andreoli 2016; Davidson 2014; McMurran 2016; Robinson 2016; Stanley 2017). Two more trials reported to have recorded adverse effects, but we could not use the data for effect size calculation, either because the final results were not reported (Pascual 2015) or it was unclear in which treatment group the events occurred (Leichsenring 2016). Adverse effects were either recorded as and when people reported them, or the exact method of assessment was not specified.
We categorised adverse effects as serious (life‐threatening, resulting in death or requiring inpatient hospitalisation; available from Andreoli 2016; Davidson 2014; McMurran 2016; Robinson 2016; Stanley 2017) or non‐serious (any other adverse effects; available from McMurran 2016; Stanley 2017).
Funding source
The majority of trials (k = 62) were funded by grants from universities, authorities or research foundations (see Table 5). Four trials reported that no funding was received (Bellino 2006; Bellino 2007; Bellino 2010; Jahangard 2012). The nine remaining trials did not specify funding (Andreoli 2016; Bianchini 2019; Carter 2010; Kamalabadi 2012; Koons 2001a; Leppänen 2016; Mohamadizadeh 2017; Morton 2012; Turner 2000).
Excluded studies
In total, we excluded 105 trials from 118 full‐text reports (see Characteristics of excluded studies tables). Of these, 70 trials included an ineligible patient population, 28 assessed ineligible interventions, 1 included an ineligible comparator, 2 had unclear numbers of participants with BPD included (we were unable to retrieve information), and 4 did not include any relevant outcomes (see Figure 1).
Studies awaiting classification
We included 11 trials as awaiting classification, of which we were unable to retrieve four trials, two of the trials were conference abstracts, two of the trials need to be translated, and three trials did not provide subsample data for people affected by BPD (see Characteristics of studies awaiting classification tables for an elaborated description). All trials investigated different types of psychotherapeutic treatments for people affected by BPD or individuals with PTSD or suicidal behaviour.
Three trials involved CBT treatments for BPD (Akbari 2009; Dorrepaal 2012; Johnson 2017) and five trials involved DBT treatments (Abdelkarim 2016; Cowperthwait 2017; McCauley 2018; Ostermeier 2017; Santamarina 2017). One trial apiece investigated ACT (Ducasse 2018), MBT (Einy 2018) and the STEPPS programme (Isaia 2017).
Three trials involved adolescents (Cowperthwait 2017; McCauley 2018; Santamarina 2017), whereas the rest involved adults.
Ongoing studies
We included 33 ongoing trials assessing different types of psychological interventions for the treatment of BPD, for which the outcome data are not yet available (see Characteristics of ongoing studies tables).
The majority of trials investigated the effect of CBT, DBT or related treatment for individuals with BPD displaying self‐harm or suicidal behaviour. More specifically, nine trials investigated DBT (ACTRN12612001187831; ACTRN12616000236493; NCT02134223; NCT02387736; NCT02517723; NCT02991586; NCT03191565; NCT03297840; NCT03833453), five trials investigated MBT (ACTRN12612000951853; NCT02068326; NCT02771691; NCT03677037; NL2168/NTR2292), three trials investigated cognitive behavioural treatments (DRKS00003605; ISRCTN21802136; NCT01531634), three trials investigated SFT (DRKS00011534; NL1144/NTR1186; NL2266/NTR2392), two trials investigated STEPPS programmes (NCT03092271; NL3856/NTR4016), two trials investigated self‐help or psychoeducation interventions (NCT03185026; NCT03376113), one investigated a meditation‐based treatment (NCT02125942), one investigated internet‐based treatment (NCT03418142), and one an emotional regulation treatment (NCT03011190). Five trials investigated other interventions like early interventions and general care (ACTRN12610000100099; NCT00603421; NCT01823120; NCT02685943; NCT02985047).
One trial investigated adolescents (NCT02771691) whereas the other trials investigated adults.
Risk of bias in included studies
Figure 2 and Figure 3 shows the review authors´ judgements concerning the risk of bias across the included trials and for each individual trial, respectively. Further information for the individual trials can be found in Characteristics of included studies tables.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
There is evidence that poor reporting of randomisation increases the odds of presenting significant outcomes (Chalmers 1983; Schulz 1995). The trials provided further information on how the randomisation sequence had been achieved, or that a stratification was used. We judged 56 trials, where a randomisation method was reported, as having low risk of bias (Amianto 2011; Andreoli 2016; Bateman 2009; Bellino 2006; Bellino 2007; Bellino 2010; Blum 2008; Bohus 2013; Borschmann 2013; Bos 2010; Carmona í Farrés 2019; Carter 2010; Cottraux 2009; Davidson 2006; Davidson 2014; Doering 2010; Elices 2016; Farrell 2009; Feigenbaum 2012; Giesen‐Bloo 2006; Gleeson 2012; Gratz 2006; Gregory 2008b; Harned 2014; Jahangard 2012; Jochems 2015; Kramer 2011; Kramer 2014; Kramer 2016; Kredlow 2017a; Kredlow 2017b; Laurenssen 2018; Leichsenring 2016; Leppänen 2016; Lin 2019; Linehan 1991; Linehan 1994; Linehan 2006; Linehan 2015a; McMain 2009; McMain 2017; McMurran 2016; Mehlum 2014; Morton 2012; Pascual 2015; Philips 2018; Priebe 2012; Reneses 2013; Robinson 2016; Rossouw 2012b; Santisteban 2015; Schuppert 2012; Sinnaeve 2018; Soler 2009; Van den Bosch 2005; Zanarini 2018). The 19 remaining trials did not describe exactly how treatment allocation had been achieved, so we rated these trials at unclear risk of bias (Antonsen 2017; Bateman 1999; Bianchini 2019; Feliu‐Soler 2017; Gratz 2014; Haeyen 2018; Jørgensen 2013; Kamalabadi 2012; Koons 2001a; Mohamadizadeh 2017; Morey 2010; Nadort 2009; Salzer 2014; Schilling 2018; Smith 2012; Stanley 2017; Turner 2000; Weinberg 2006; Zanarini 2008)
Allocation concealment
Thirty‐seven trials, reporting off‐site randomisation or notification of assignment by research coordinators not involved in delivering the therapy, were rated as having a low risk of bias (Amianto 2011; Andreoli 2016; Bateman 1999; Bateman 2009; Bellino 2006; Bellino 2007; Bohus 2013; Borschmann 2013; Bos 2010; Carmona í Farrés 2019; Carter 2010; Cottraux 2009; Davidson 2006; Doering 2010, Feigenbaum 2012; Giesen‐Bloo 2006; Gregory 2008b; Jahangard 2012; Jochems 2015; Kramer 2011; Kramer 2014; Kramer 2016; Kredlow 2017a; Kredlow 2017b; Laurenssen 2018; Leichsenring 2016; Linehan 2006; McMain 2009; McMain 2017; McMurran 2016; Mehlum 2014; Nadort 2009; Philips 2018; Priebe 2012; Rossouw 2012b; Schuppert 2012; Sinnaeve 2018). Thirty‐five trials that did not provide enough information to enable a judgement to be made about adequacy of allocation concealment were rated as having an unclear risk of bias (Antonsen 2017; Bellino 2010; Bianchini 2019; Blum 2008; Davidson 2014; Farrell 2009; Feliu‐Soler 2017; Gratz 2006; Gratz 2014; Haeyen 2018; Harned 2014; Jørgensen 2013; Kamalabadi 2012; Koons 2001a; Leppänen 2016; Lin 2019; Linehan 1991; Linehan 1994; Linehan 2015a; Mohamadizadeh 2017; Morey 2010; Pascual 2015; Reneses 2013; Robinson 2016; Salzer 2014; Santisteban 2015; Schilling 2018; Smith 2012; Soler 2009; Stanley 2017; Turner 2000; Van den Bosch 2005; Weinberg 2006; Zanarini 2008; Zanarini 2018). We rated three trials as being at high risk of bias due to explicit inadequate allocation concealment (Elices 2016; Gleeson 2012; Morton 2012). One example can be found in Gleeson 2012, where the following was stated: "Outcome ratings were made by the trial research assistant who was independent of the treatment, but not blind to treatment allocation because of limited resources" (quote). This could potentially have induced bias.
Blinding
The majority of trials (50 trials) reported that outcome assessors were kept blind to treatment allocation and, for this reason, were considered to be at low risk of bias (Amianto 2011; Andreoli 2016; Bateman 1999; Bateman 2009; Bellino 2006; Bellino 2007; Bellino 2010; Bianchini 2019; Bohus 2013; Borschmann 2013; Carmona í Farrés 2019; Carter 2010; Cottraux 2009; Davidson 2006; Davidson 2014: Doering 2010; Elices 2016; Giesen‐Bloo 2006; Gleeson 2012; Gratz 2006; Gratz 2014; Gregory 2008b; Harned 2014; Jahangard 2012; Jochems 2015; Koons 2001a; Kramer 2011; Kramer 2016; Kredlow 2017a; Kredlow 2017b; Laurenssen 2018; Leichsenring 2016; Leppänen 2016; Linehan 1991; Linehan 1994; Linehan 2006; Linehan 2015a; McMain 2009; McMain 2017; Mehlum 2014; Pascual 2015; Robinson 2016; Rossouw 2012b; Salzer 2014; Schuppert 2012; Soler 2009; Stanley 2017; Turner 2000; Van den Bosch 2005; Weinberg 2006). Fourteen trials did not have an adequate description of the blinding of the outcome assessors and thus were judged to be at unclear risk of bias (Antonsen 2017; Feigenbaum 2012; Feliu‐Soler 2017; Kamalabadi 2012; Kramer 2014; Lin 2019; Mohamadizadeh 2017; Morey 2010; Nadort 2009; Philips 2018; Priebe 2012; Reneses 2013; Schilling 2018; Zanarini 2008). Eleven trials reported inadequate blinding of outcome assessors and thus were considered to be at high risk of bias (Blum 2008; Bos 2010; Farrell 2009; Haeyen 2018; Jørgensen 2013; McMurran 2016; Morton 2012; Santisteban 2015; Sinnaeve 2018; Smith 2012; Zanarini 2018).
Incomplete outcome data
The majority of trials either did not adequately describe the possible reasons for missing data, and therefore were considered to be at unclear risk of bias (30 trials: Bianchini 2019; Blum 2008; Bohus 2013; Davidson 2014; Feliu‐Soler 2017; Jochems 2015; Kamalabadi 2012; Kramer 2016; Leichsenring 2016; Linehan 1991; Linehan 2015a; McMain 2009; Mohamadizadeh 2017; Morey 2010; Morton 2012; Nadort 2009; Pascual 2015; Priebe 2012; Reneses 2013; Robinson 2016; Santisteban 2015; Schilling 2018; Schuppert 2012; Smith 2012; Soler 2009; Stanley 2017; Turner 2000; Van den Bosch 2005; Weinberg 2006; Zanarini 2008), or did not explicitly address obvious missing data and therefore were considered to be at high risk of bias (26 trials: Andreoli 2016; Bateman 1999; Bellino 2006; Bellino 2007; Bellino 2010; Bos 2010; Carmona í Farrés 2019; Carter 2010; Cottraux 2009; Elices 2016; Farrell 2009; Feigenbaum 2012; Gleeson 2012; Gratz 2006; Gregory 2008b; Haeyen 2018; Jørgensen 2013; Koons 2001a; Kramer 2011; Kramer 2014; Laurenssen 2018; Leppänen 2016; Linehan 1994; Philips 2018; Rossouw 2012b; Sinnaeve 2018). Nineteen trials showed no indication of incomplete outcome reporting, and thus were considered to be at low risk of bias (Amianto 2011; Antonsen 2017; Bateman 2009; Borschmann 2013; Davidson 2006; Doering 2010; Giesen‐Bloo 2006; Gratz 2014; Harned 2014; Jahangard 2012; Kredlow 2017a; Kredlow 2017b; Lin 2019; Linehan 2006: McMain 2017; McMurran 2016; Mehlum 2014; Salzer 2014; Zanarini 2018).
Selective reporting
The majority of trials (39 trials) either did not have a published protocol prior to initiating the trials or did not provide sufficient information in the report to judge the extent of reporting bias. We considered these trials to be at unclear risk of bias (Bateman 1999; Bellino 2006; Bellino 2007; Bellino 2010; Bianchini 2019; Bos 2010; Carter 2010; Davidson 2014; Farrell 2009; Feigenbaum 2012; Feliu‐Soler 2017; Giesen‐Bloo 2006; Gratz 2006; Gratz 2014; Jahangard 2012; Jørgensen 2013; Kamalabadi 2012; Koons 2001a; Kramer 2016; Kredlow 2017a; Kredlow 2017b; Leppänen 2016; Linehan 1991; Linehan 1994; Linehan 2006; Mehlum 2014; Mohamadizadeh 2017; Morey 2010; Morton 2012; Pascual 2015; Priebe 2012; Reneses 2013; Salzer 2014; Santisteban 2015; Schilling 2018; Soler 2009; Turner 2000; Van den Bosch 2005; Zanarini 2008). Seventeen trials did not explicitly report data for prespecified outcomes even though they initially had planned to report them. We considered these trials to be at high risk of bias (Andreoli 2016; Antonsen 2017; Blum 2008; Gleeson 2012; Haeyen 2018; Kramer 2011; Kramer 2014; Laurenssen 2018; Leichsenring 2016; Lin 2019; Linehan 2015a; Philips 2018; Rossouw 2012b; Schuppert 2012; Sinnaeve 2018; Smith 2012; Zanarini 2018). We rated 19 trials at low risk of bias due to the fact that they had published a protocol or registered the trial before conducting the trial, and reported all prespecified outcomes (Amianto 2011; Bateman 2009; Bohus 2013; Borschmann 2013; Carmona í Farrés 2019; Cottraux 2009; Davidson 2006; Doering 2010; Elices 2016; Gregory 2008b; Harned 2014; Jochems 2015; McMain 2009; McMain 2017; McMurran 2016; Nadort 2009; Robinson 2016; Stanley 2017; Weinberg 2006).
Other potential sources of bias
Other sources of bias included insufficient treatment adherence, allegiance bias and attention bias. The total score for this outcome was based on the highest score of bias. A detailed description of which domains are critical for the individual trials can be found in the Characteristics of included studies tables.
Most trials (48 trials) exhibited high risk of bias in one or more of the assessed domains (Amianto 2011; Andreoli 2016; Antonsen 2017; Bateman 1999; Bateman 2009; Bellino 2006; Bellino 2010; Bianchini 2019; Blum 2008; Borschmann 2013; Bohus 2013; Bos 2010; Carter 2010; Davidson 2006; Davidson 2014; Farrell 2009; Feliu‐Soler 2017; Gratz 2006; Gratz 2014; Gregory 2008b; Haeyen 2018; Harned 2014; Jørgensen 2013; Kamalabadi 2012; Kramer 2011; Kramer 2014; Kredlow 2017a; Kredlow 2017b; Laurenssen 2018; Leichsenring 2016Leppänen 2016; Linehan 1994; Linehan 2006; Linehan 2015a; McMurran 2016; Mehlum 2014; Morton 2012; Pascual 2015; Philips 2018; Reneses 2013; Robinson 2016; Rossouw 2012b; Salzer 2014; Santisteban 2015; Schilling 2018; Schuppert 2012; Sinnaeve 2018; Smith 2012). Twenty‐two trials had at least one domain that was not adequately described in the trials and therefore were considered to be at unclear risk of bias (Doering 2010; Elices 2016; Feigenbaum 2012; Giesen‐Bloo 2006; Gleeson 2012; Jahangard 2012; Jochems 2015; Kramer 2016; Lin 2019; Linehan 1991; McMain 2009; McMain 2017; Mohamadizadeh 2017; Nadort 2009; Priebe 2012; Soler 2009; Stanley 2017; Turner 2000; Van den Bosch 2005; Weinberg 2006; Zanarini 2008; Zanarini 2018). Only five trials had an overall low risk of bias for all the domains assessed as other potential sources of bias (Bellino 2007; Carmona í Farrés 2019; Cottraux 2009; Koons 2001a; Morey 2010).
Effects of interventions
See: Table 1; Table 2; Table 3
We present the results for each of the primary and secondary outcomes connected to the 25 comparisons below. Where a meta‐analysis involved two or more different instruments to measure the same construct, we reported effect sizes as SMD, otherwise we reported the MD. To identify the MIREDIF, we transformed the SMD to MD for the scale with best validity and reliability for that outcome. For the analyses of the four primary outcomes in the comparison of psychotherapy versus TAU, we transformed the SMD into MD on the following scales, to assess whether results exceeded the minimum clinically important difference: ZAN BPD Scale, DSHI, Suicidal Attempt Self Injury Interview, GAF scale, and the Hamilton Depression scale.
We contacted the authors of 44 trials with unclear or missing data and requested the necessary information. Twenty‐four trial authors replied with answers (Amianto 2011; Bateman 1999; Bellino 2006; Blum 2008; Borschmann 2013; Carmona í Farrés 2019; Carter 2010; Doering 2010; Elices 2016; Farrell 2009; Gleeson 2012; Gratz 2006; Kredlow 2017a; Kredlow 2017b; Leppänen 2016; Mehlum 2014; Morton 2012; Priebe 2012; Robinson 2016; Salzer 2014; Schilling 2018; Sinnaeve 2018; Smith 2012; Soler 2009).
We performed TSA on all primary outcomes and for the secondary outcome depression.at end of treatment for the main comparison versus TSA in our 'Summary of findings' tables, adjusting for multiplicity and sparse data.
We considered all trials as being at high risk of bias overall. However, we used all eligible trials in the meta‐analyses, as the Cochrane Handbook for Systematic Reviews of Interventions recommends doing so when all trials are assigned the same risk of bias. We took account of our 'Risk of bias' assessment when considering the quality of the evidence using the GRADE approach, to ensure that judgements about risk of bias and other factors affecting the quality of the evidence were taken into account when interpreting the results of the review (Higgins 2011; Higgins 2019).
1. Psychotherapy versus treatment‐as‐usual (TAU)
Primary outcomes
1.1 BPD symptom severity (continuous)
Twenty‐three trials reported continuous data on BPD symptom severity (in total for all time points) (Amianto 2011; Blum 2008; Bateman 1999; Bos 2010; Doering 2010; Farrell 2009; Gratz 2006; Gratz 2014; Gregory 2008b; Jørgensen 2013; Koons 2001a; Kredlow 2017a; Laurenssen 2018; Leichsenring 2016; Leppänen 2016; Morton 2012; Philips 2018; Priebe 2012; Reneses 2013; Robinson 2016; Rossouw 2012b; Schuppert 2012; Soler 2009).
Generally, psychotherapy improved BPD symptom severity at end of treatment compared with TAU (SMD −0.52, 95% CI −0.70 to −0.33; 22 trials, 1244 participants; I2 = 57%; P < 0.001; Analysis 1.1.1; moderate‐quality evidence, Table 1). This corresponds to a MD of −3.6 (95% CI −4.4 to ‐2.08) on the ZAN‐BPD scale, which ranges from 0 to 36. This represents a clinical relevant improvement in BPD symptoms. The MIREDIF on this scale is −3.0 points (Crawford 2018a). The TSA analysis showed that the RIS was reached (n = 907), and that there was no risk of type 1 error (TSA adjusted confidence interval −5.49 to −1.90) (see figure 4 in Appendix 6).
1.1. Analysis.

Comparison 1: Psychotherapy vs TAU, Outcome 1: Primary: BPD symptom severity (continuous)
Inspection of the funnel plot (see figure 5 in Appendix 6) suggested potential bias (very small asymmetry), and we found evidence of possible significant publication bias: Egger’s regression intercept (bias) 2.234 (two tailed, P = 0.043). Analysis 1.1.1. might be more difficult to understand as we found asymmetry in the funnel plot and also a significant Egger's test. All effect estimates spread unequivocally at the left‐hand side of the zero line, clearly favouring experimental treatments over controls. However, identifying smaller sample size trials with insignificant results would be unlikely to change our pooled estimate substantially because these smaller trials would only contribute lesser weights to the pooled estimates. Hence, we concluded that our findings were not essentially influenced by publication bias.
Generally, psychotherapy did not reduce BPD symptom severity at zero to six months follow‐up compared with TAU (SMD −0.59, 95% CI −1.23 to 0.05; 2 trials, 41 participants; I2 = 0%; P = 0.17; Analysis 1.1.2).
Generally, psychotherapy did not reduce BPD symptom severity at six to 12 months follow‐up compared with TAU (SMD −0.04, 95% CI −0.36 to 0.27; 2 trials, 157 participants; I2 = 0%; P = 0.79; Analysis 1.1.3).
Generally, psychotherapy did not reduce BPD symptom severity at above 12 months follow‐up compared with TAU (SMD −0.94, 95% CI −2.58 to 0.70; 2 trials, 97 participants; I2 = 92%; P = 0.26; Analysis 1.1.4).
1.2 BPD symptom severity (dichotomous)
One trial reported dichotomous data on BPD symptom severity (Davidson 2006).
Generally, psychotherapy did not reduce BPD symptom severity at above 12 months follow‐up compared with TAU (RR 0.91, 95% CI 0.56 to 1.48; 1 trial, 76 participants; P = 0.71; Analysis 1.2).
1.2. Analysis.

Comparison 1: Psychotherapy vs TAU, Outcome 2: Primary: BPD symptom severity (dichotomous), at above 12 months follow‐up
No data were available for end of treatment, zero to six months and six to 12 months follow‐up.
1.3 Self‐harm (continuous)
Thirteen trials reported continuous data on self‐harm (Amianto 2011; Borschmann 2013; Carter 2010; Feigenbaum 2012; Gratz 2006; Gratz 2014; Koons 2001a; Linehan 1991; Linehan 2006; Philips 2018; Priebe 2012; Van den Bosch 2005; Weinberg 2006).
Generally, psychotherapy improved self‐harm at end of treatment compared with TAU (SMD −0.32, 95% CI −0.49 to −0.14; 13 trials, 616 participants; I2 = 16%; P = 0.0004; low‐quality evidence, Table 1). This corresponds to a MD of −0.82 (95% CI −1.25 to 0.35), on the DSHI, which ranges from 0 to 34. The MIREDIF on this scale is −1.25 points, which corresponds to ½ SD (Farivar 2004). Clinically, this represents no clinically important reduction in self‐harm for people with BPD. The TSA analysis showed that the RIS was reached (n = 97). However, we cannot exclude the potential risk of type 1 error (TSA adjusted confidence interval −0.59 to −0.08) (see figure 6 in Appendix 6).
Inspection of the funnel plot (see figure 7 in Appendix 6) suggested no potential bias (asymmetry), and we found no evidence of possible significant publication bias: Egger’s regression intercept (bias) 1.524 (two tailed, P = 0.237).
Generally, psychotherapy did not reduce self‐harm at zero to six months follow‐up compared with TAU (SMD −0.52, 95% CI −1.28 to 0.23; 1 trial, 28 participants; P = 0.18; Analysis 1.3.2); MD −4.71, 95% CI −11.60 to 2.18; 1 trial, 28 participants; P = 0.18; Analysis 1.3.2).
1.3. Analysis.

Comparison 1: Psychotherapy vs TAU, Outcome 3: Primary: self‐harm (continuous)
Generally, psychotherapy did not reduce self‐harm at six to 12 months follow‐up compared with TAU (SMD −0.18, 95% CI −0.48 to 0.12; 3 trials, 174 participants; I2 = 0%; P = 0.64; Analysis 1.3.3).
No data were available for 12 months and over follow‐up.
1.4 Self‐harm (dichotomous)
Six trials reported dichotomous data on self‐harm (Bateman 1999; Bateman 2009; Bos 2010; Davidson 2006; Doering 2010; Rossouw 2012b).
Generally, psychotherapy did not reduce self‐harm at end of treatment compared with TAU (RR 0.85, 95% CI 0.63 to 1.14; 6 trials, 513 participants; I2 = 73%; P = 0.28; Analysis 1.4.1).
1.4. Analysis.

Comparison 1: Psychotherapy vs TAU, Outcome 4: Primary: self‐harm (dichotomous)
Generally, psychotherapy reduced self‐harm at zero to six months follow‐up compared with TAU (RR 0.14, 95% CI 0.04 to 0.56; 1 trial; P = 0.005; Analysis 1.4.2).
Generally, psychotherapy reduced self‐harm at six to 12 months follow‐up compared with TAU (RR 0.27, 95% CI 0.10 to 0.68; 1 trial; P = 0.006; Analysis 1.4.3).
Generally, psychotherapy reduced self‐harm at above 12 months follow‐up compared with TAU (RR 0.33, 95% CI 0.15 to 0.76; 1 trial, 41 participants; P = 0.009; Analysis 1.4.4).
1.5 Suicide‐related outcomes (continuous)
Thirteen trials reported continuous data on suicide‐related outcomes (Amianto 2011; Andreoli 2016; Davidson 2006; Feigenbaum 2012; Gleeson 2012; Gregory 2008b; Koons 2001a; Leppänen 2016; Linehan 2006; Mehlum 2014; Reneses 2013; Soler 2009; Weinberg 2006).
Generally, psychotherapy reduced suicide‐related outcomes at end of treatment compared with TAU (SMD −0.34, 95% CI −0.57 to −0.11; 13 trials, 666 participants; I2 = 43%; P = 0.004; Analysis 1.5.1; low‐quality evidence, Table 1). The improvement corresponded to a clinical effect MD of −0.11 (95% CI −0.19 to −0.034), on the Suicidal Attempt Self Injury Interview. The MIREDIF on this scale is −0.17 points, which corresponds to ½ SD (Farivar 2004). Clinically, this represents no reduction in suicide‐related outcomes for people with BPD. The TSA analysis showed that the required information size was reached (n = 253). However, we cannot exclude the potential risk of type 1 error, and that there was no risk of type 1 error (TSA adjusted confidence interval −0.18 to −0.04) (see figure 8 in Appendix 6).
1.5. Analysis.

Comparison 1: Psychotherapy vs TAU, Outcome 5: Primary: suicide‐related outcomes (continuous)
Inspection of the funnel plot (see figure 9 in Appendix 6) suggested potential bias (asymmetry), and we found no evidence of possible significant publication bias: Egger’s regression intercept (bias) 0.405 (two tailed, P = 0.735).
Generally, psychotherapy did not reduce suicide‐related outcomes at zero to six months follow‐up compared with TAU (SMD −0.43, 95% CI −1.10 to 0.23; 2 trials, 36 participants; I2 = 0%; P = 0.20; Analysis 1.5.2).
Generally, psychotherapy reduced suicide‐related outcomes at six to 12 months follow‐up compared with TAU (SMD −0.42, 95% CI −0.80 to −0.04; 2 trials, 109 participants; I2 = 0%; P = 0.03; Analysis 1.5.3).
Generally, psychotherapy did not reduce suicide‐related outcomes at above 12 months follow‐up compared with TAU (SMD −0.31, 95% CI −0.77 to 0.14; 1 trial, 76 participants; P = 0.18; MD −1.15, 95% CI −2.86 to 0.56; 1 trial, 76 participants; P = 0.19; Analysis 1.5.4).
1.6 Suicide‐related outcomes (dichotomous)
Five trials reported dichotomous data on suicide‐related outcomes (Bateman 1999; Bateman 2009; Doering 2010; Philips 2018; Stanley 2017).
Generally, psychotherapy reduced suicide‐related outcomes at end of treatment compared with TAU (RR 0.27, 95% CI 0.11 to 0.67; 5 trials, 396 participants; I2 = 45%; P = 0.005; Analysis 1.6.1).
1.6. Analysis.

Comparison 1: Psychotherapy vs TAU, Outcome 6: Primary: suicide‐related outcomes (dichotomous)
Generally, psychotherapy did not reduce suicide‐related outcomes at zero to six months follow‐up compared with TAU (RR 0.25, 95% CI 0.06 to 1.05; 1 trial, 41 participants; P = 0.06; Analysis 1.6.2).
Generally, psychotherapy reduced suicide‐related outcomes at above 12 months follow‐up compared with TAU (RR 0.29, 95% CI 0.11 to 0.74; 1 trial, 41 participants; P = 0.01; Analysis 1.6.3).
No data were available for six to 12 months follow‐up.
1.7 Psychosocial functioning
Twenty‐two trials reported continuous data on psychosocial functioning (Amianto 2011; Andreoli 2016; Bateman 1999; Bateman 2009; Blum 2008; Borschmann 2013; Carter 2010; Davidson 2006; Doering 2010; Farrell 2009; Feigenbaum 2012; Gleeson 2012; Gratz 2014; Gregory 2008b; Jochems 2015; Jørgensen 2013; Kramer 2016; Linehan 1994; Mehlum 2014; Reneses 2013; Salzer 2014; Soler 2009).
Generally, psychotherapy improved psychosocial functioning at end of treatment compared with TAU (SMD −0.45, 95% CI −0.68 to −0.22; 22 trials, 1314 participants; I2 = 72%; P = 0.0001; Analysis 1.7.1; low‐quality evidence, Table 1). This corresponds to a MD of −2.8 (95% CI −4.25 to −1.38), on the GAF scale, which ranges from 0 to 100. The MIREDIF on this scale is −4.0 points (Amri 2014). This finding does not represent a clinically important improvement in psychosocial functioning for people with BPD. The TSA analysis showed that the RIS was reached (n = 947), and that there was no risk of type 1 error (TSA adjusted confidence interval −3.97 to −1.94) (see figure 10 in Appendix 6).
1.7. Analysis.

Comparison 1: Psychotherapy vs TAU, Outcome 7: Primary: psychosocial functioning (continuous)
Inspection of the funnel plot in (see figure 11 in Appendix 6) suggested potential bias (asymmetry), and we found no evidence of possible significant publication bias: Egger’s regression intercept (bias) 1.50 (two tailed, P = 0.241).
Generally, psychotherapy did not improve psychosocial functioning at zero to six months follow‐up compared with TAU (SMD −1.23, 95% CI −2.74 to 0.29; 1 trial, 9 participants; P = 0.11; MD −15.80, 95% CI −2.74 to 0.29; 1 trial, 9 participants; P = 0.11; Analysis 1.7.2).
Generally, psychotherapy did not improve psychosocial functioning at six to 12 months follow‐up compared with TAU (SMD −0.09, 95% CI −0.40 to 0.23; 3 trials, 247 participants; I2 = 30%; P = 0.59; Analysis 1.7.3).
Generally, psychotherapy did not improve psychosocial functioning at above 12 months follow‐up compared with TAU (SMD −0.27, 95% CI −0.60 to 0.05; 6 trials, 499 participants; I2 = 60%; P = 0.10; Analysis 1.7.4).
Secondary outcomes
1.8 Anger
Eight trials reported continuous data on anger (Amianto 2011; Feigenbaum 2012; Gleeson 2012; Koons 2001a; Leppänen 2016; Linehan 1994; Linehan 2006; Soler 2009)
Generally, psychotherapy reduced anger at end of treatment compared with TAU (SMD −0.38, 95% CI −0.64 to −0.12; 8 trials, 323 participants; I2 = 21%; P = 0.005; Analysis 1.8.1).
1.8. Analysis.

Comparison 1: Psychotherapy vs TAU, Outcome 8: Secondary: anger (continuous)
Generally, psychotherapy did not reduce anger at zero to six months follow‐up compared with TAU (SMD −1.20, 95% CI −2.82 to 0.41; 1 trial, 8 participants; P = 0.14; MD −4.50, 95% CI −9.01 to 0.01; 1 trial, 8 participants; P = 0.05; Analysis 1.8.2).
Generally, psychotherapy did not reduce anger at six to 12 months follow‐up compared with TAU (SMD −0.12, 95% CI −0.50 to 0.25; 2 trials, 111 participants; I2 = 0%; P = 0.52; Analysis 1.8.3).
Generally, psychotherapy did not reduce anger at above 12 months follow‐up compared with TAU (SMD 0.02, 95% CI −0.42 to 0.46; 1 trial, 80 participants; P = 0.93; MD 0.01, 95% CI −0.20 to 0.22; 1 trial, 80 participants; P = 0.93; Analysis 1.8.4).
1.9 Affective instability
Twelve trials reported continuous data on affective instability (Amianto 2011; Bianchini 2019; Blum 2008; Farrell 2009; Gratz 2006; Gratz 2014; Leppänen 2016; Morton 2012; Reneses 2013; Salzer 2014; Schuppert 2012; Soler 2009).
Generally, psychotherapy improved affective instability at end of treatment compared with TAU (SMD −0.68, 95% CI −0.98 to −0.39; 12 trials, 620 participants; I2 = 65%; P < 0.001; (Analysis 1.9.1).
1.9. Analysis.

Comparison 1: Psychotherapy vs TAU, Outcome 9: Secondary: affective instability (continuous)
Generally, psychotherapy did not improve affective instability at six to 12 months follow‐up compared with TAU (SMD −0.52, 95% CI −1.21 to 0.18; 1 trial, 33 participants; P = 0.15; MD −0.70, 95% CI −1.59 to 0.19; 1 trial, 33 participants; P = 0.12; Analysis 1.9.2).
No data were available for zero to six months and above 12 months follow‐up.
1.10 Chronic feelings of emptiness
Four trials reported continuous data on chronic feelings of emptiness (Amianto 2011; Leppänen 2016; Reneses 2013; Soler 2009).
Generally, psychotherapy improved chronic feelings of emptiness at end of treatment compared with TAU (SMD −0.39, 95% CI −0.69 to −0.10; 4 trials, 187 participants; I2 = 0%; P = 0.009; Analysis 1.10.1).
1.10. Analysis.

Comparison 1: Psychotherapy vs TAU, Outcome 10: Secondary: chronic feelings of emptiness (continuous)
Generally, psychotherapy did not improve chronic feelings of emptiness at six to 12 months follow‐up compared with TAU (SMD −0.58, 95% CI −1.28 to 0.11; 1 trial, 37 participants; P = 0.10; MD −0.60, 95% CI −1.39 to 0.09; 1 trial, 33 participants; P = 0.09; Analysis 1.10.2).
No data were available for zero to six months and above 12 months follow‐up.
1.11 Impulsivity (continuous)
Ten studiies reported continuous data on impulsivity (Amianto 2011; Bianchini 2019; Blum 2008; Farrell 2009; Gratz 2006; Gratz 2014; Leppänen 2016; Reneses 2013; Soler 2009; Van den Bosch 2005).
Generally, psychotherapy improved impulsivity at end of treatment compared with TAU (SMD −0.54, 95% CI −0.84 to −0.25; 10 trials, 491 participants; I2 = 58%; P = 0.0003; Analysis 1.11.1).
1.11. Analysis.

Comparison 1: Psychotherapy vs TAU, Outcome 11: Secondary: impulsivity (continuous)
Generally, psychotherapy did not improve impulsivity at six to 12 months follow‐up compared with TAU (SMD 0.32, 95% CI −0.13 to 0.77; 2 trials, 77 participants; I2 = 0%; P = 0.16; Analysis 1.11.2).
No data were available for zero to six months and above 12 months follow‐up.
1.12 Impulsivity (dichotomous)
One trial reported dichotomous data on impulsivity (Bos 2010).
Generally, psychotherapy did not improve impulsivity at end of treatment compared with TAU (RR 0.93, 95% CI 0.66 to 1.29; 1 trial, 58 participants; P = 0.65; Analysis 1.12).
1.12. Analysis.

Comparison 1: Psychotherapy vs TAU, Outcome 12: Secondary: impulsivity (dichotomous), at end of treatment
No data were available for zero to six months, six to 12 months, and above 12 months follow‐up.
1.13 Interpersonal problems
Eighteen trials reported continuous data on interpersonal problems (Amianto 2011; Bateman 1999; Bateman 2009; Blum 2008; Bos 2010; Carter 2010; Davidson 2006; Farrell 2009; Gratz 2014; Jørgensen 2013; Kramer 2016; Laurenssen 2018; Leichsenring 2016; Leppänen 2016; Philips 2018; Reneses 2013; Salzer 2014; Soler 2009).
Generally, psychotherapy improved interpersonal problems at end of treatment compared with TAU (SMD −0.42, 95% CI −0.68 to −0.16; 18 trials, 1159 participants; I2 = 77%; P = 0.002; Analysis 1.13.1).
1.13. Analysis.

Comparison 1: Psychotherapy vs TAU, Outcome 13: Secondary: interpersonal problems (continuous)
Generally, psychotherapy did not improve interpersonal problems at zero to six months follow‐up compared with TAU (SMD −0.41, 95% CI −1.01 to 0.20; 1 trial, 53 participants; P = 0.19; MD −0.30, 95% CI −0.76 to 0.16; 1 trial, 53 participants; P = 0.20; Analysis 1.13.2).
Generally, psychotherapy did not improve interpersonal problems at six to 12 months follow‐up compared with TAU (SMD −0.17, 95% CI −0.65 to 0.32; 1 trial, 132 participants; I2 = 39%; P = 0.50; Analysis 1.13.3).
Generally, psychotherapy did not improve interpersonal problems at above 12 months follow‐up compared with TAU (SMD 0.00, 95% CI −0.54 to 0.54; 2 trials, 172 participants; I2 = 65%; P = 1.00; Analysis 1.13.4).
1.14 Abandonment
Two trials reported continuous data on abandonment (Amianto 2011; Leppänen 2016).
Generally, psychotherapy did not improve abandonment at end of treatment compared with TAU (SMD −0.22, 95% CI −0.66 to 0.21; 2 trials, 84 participants; I2 = 0%; P = 0.32; Analysis 1.14.1).
1.14. Analysis.

Comparison 1: Psychotherapy vs TAU, Outcome 14: Secondary: abandonment (continuous)
Generally, psychotherapy did not improve abandonment at six to 12 months follow‐up compared with TAU (SMD −0.39, 95% CI −1.08 to 0.30; 1 trial, 33 participants; P = 0.27; MD ‐−0.39, 95% CI −1.08 to 0.30; 1 trial, 33 participants; P = 0.25; Analysis 1.14.2).
No data were available for zero to six months and above 12 months follow‐up.
1.15 Identity disturbance
Four trials reported continuous data on identity disturbance (Amianto 2011; Leichsenring 2016; Leppänen 2016; Reneses 2013).
Generally, psychotherapy did not improve identity disturbance at end of treatment compared with TAU (SMD −0.37, 95% CI −0.84 to 0.10; 4 trials, 250 participants; I2 = 65%; P = 0.12; Analysis 1.15.1).
1.15. Analysis.

Comparison 1: Psychotherapy vs TAU, Outcome 15: Secondary: identity disturbance (continuous)
Generally, psychotherapy improved identity disturbance at six to 12 months follow‐up compared with TAU (SMD −1.09, 95% CI −1.83 to −0.35; 1 trial, 33 participants; P = 0.004; MD −1.40, 95% CI −2.25 to −0.55; 1 trial, 33 participants; P = 0.001; Analysis 1.15.2).
No data were available for zero to six months and above 12 months follow‐up.
1.16 Dissociation and psychotic‐like symptoms
Six trials reported continuous data on dissociation and psychotic‐like symptoms (Amianto 2011; Blum 2008; Farrell 2009; Gleeson 2012; Gregory 2008b; Kredlow 2017a).
Generally, psychotherapy improved dissociation and psychotic‐like symptoms at end of treatment compared with TAU (SMD −0.47, 95% CI −0.85 to −0.10; 6 trials, 244 participants; I2 = 38%; P = 0.01; Analysis 1.16.1).
1.16. Analysis.

Comparison 1: Psychotherapy vs TAU, Outcome 16: Secondary: dissociation and psychotic‐like symptoms (continuous)
Generally, psychotherapy improved dissociation and psychotic‐like symptoms at zero to six months follow‐up compared with TAU (SMD 0.97, 95% CI −1.69 to −0.26; 2 trials, 35 participants; I2 = 0%; P = 0.008; Analysis 1.16.2).
Generally, psychotherapy did not improve dissociation and psychotic‐like symptoms at six to 12 months follow‐up compared with TAU (SMD −0.59, 95% CI −1.29 to 0.11; 1 trial, 33 participants; P = 0.10; MD −0.70, 95% CI −1.59 to 0.09; 1 trial, 33 participants; P = 0.08; Analysis 1.16.3).
Generally, psychotherapy did not improve dissociation and psychotic‐like symptoms at above 12 months follow‐up compared with TAU (SMD −0.01, 95% CI −0.81 to 0.79; 1 trial, 24 participants; P = 0.98; MD ‐−0.20, 95% CI −20.07 to 19.67; 1 trial, 24 participants; P = 0.98; Analysis 1.16.4).
1.17 Depression (continuous)
Twenty‐two trials reported continuous data on depression (Andreoli 2016; Bateman 1999; Bateman 2009; Blum 2008; Borschmann 2013; Davidson 2006; Davidson 2014; Doering 2010; Gleeson 2012; Gratz 2006; Gratz 2014; Gregory 2008b; Jahangard 2012; Jørgensen 2013; Kredlow 2017a; Laurenssen 2018; Leichsenring 2016; Leppänen 2016; McMurran 2016; Morton 2012; Reneses 2013; Zanarini 2018).
Generally, psychotherapy improved depression at end of treatment compared to TAU (SMD −0.39, 95% CI −0.61 to −0.17; 22 trials, 1568 participants; I2 = 75%; P = 0.0006; Analysis 1.17.1; very low‐quality evidence, Table 1). This corresponds to −2.45 on the Hamilton Depression Scale. The MIREDIF on this scale is 3.0 points (NICE CG90). However, the TSA analysis showed that the RIS was not reached (n = 2274), and that there was a potential risk of type 1 error (TSA adjusted confidence interval −3.34 to −1.72) (see figure 12 in Appendix 6).
1.17. Analysis.

Comparison 1: Psychotherapy vs TAU, Outcome 17: Secondary: depression (continuous)
Generally, psychotherapy improved depression at zero to six months follow‐up compared with TAU (SMD −0.80, 95% CI −1.26 to −0.34; 4 trials, 125 participants; I2 = 23%; P = 0.0006; Analysis 1.17.2).
Generally, psychotherapy did not improve depression at six to 12 months follow‐up compared with TAU (SMD −0.40, 95% CI −0.95 to 0.16; 3 trials, 260 participants; I2 = 77%; P = 0.16; Analysis 1.17.3).
Generally, psychotherapy improved depression at above 12 months follow‐up compared with TAU (SMD −0.40, 95% CI ‐0.74 to −0.06; 5 trials, 311 participants; I2 = 47%; P = 0.02; Analysis 1.17.4).
1.18 Depression (dichotomous)
One trial reported dichotomous data on depression (Rossouw 2012b).
Generally, psychotherapy did not improve depression at end of treatment compared with TAU (RR 0.71, 95% CI 0.49 to 1.03; 1 trial, 80 participants; P = 0.08; Analysis 1.18).
1.18. Analysis.

Comparison 1: Psychotherapy vs TAU, Outcome 18: Secondary: depression (dichotomous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
1.19 Attrition
Thirty‐two trials reported dichotomous data on attrition (Amianto 2011; Andreoli 2016; Bateman 1999; Bateman 2009; Borschmann 2013; Bos 2010; Carter 2010; Davidson 2006; Doering 2010; Farrell 2009; Feigenbaum 2012; Gleeson 2012; Gratz 2006; Gratz 2014; Jørgensen 2013; Koons 2001a; Kramer 2016; Laurenssen 2018; Leichsenring 2016; Leppänen 2016; Linehan 1991; Linehan 1994; Linehan 2006; Morton 2012; Philips 2018; Priebe 2012; Reneses 2013; Robinson 2016; Rossouw 2012b; Stanley 2017; Van den Bosch 2005; Zanarini 2018).
Generally, psychotherapy did not reduce attrition at end of treatment compared with TAU (RR 1.00, 95% CI 0.83 to 1.20; 32 trials, 2225 participants; I2 = 50%; P = 0.98; Analysis 1.19.1; low‐quality evidence, Table 1).
1.19. Analysis.

Comparison 1: Psychotherapy vs TAU, Outcome 19: Secondary: attrition (dichotomous)
Generally, psychotherapy reduced attrition at zero to six months follow‐up compared with TAU (RR 0.58, 95% CI 0.34 to 1.00; 1 trial, 60 participants; P = 0.05; Analysis 19.2.2).
19.2. Analysis.

Comparison 19: Once‐only interventions (individual setting) vs waiting list, Outcome 2: Secondary: interpersonal problems (continuous), at end of treatment
No data were available for six to 12 months and above 12 months follow‐up.
1.20 Non‐serious adverse effects
Two trials reported dichotomous data on non‐adverse effects (McMurran 2016; Stanley 2017).
There was no clear difference in the number of serious adverse effects between psychotherapy and TAU at end of treatment (RR 0.92, 95% CI 0.45 to 1.88; 2 trials, 381 participants, P = 0.81; Analysis 1.20.1; low‐quality evidence, Table 1).
1.20. Analysis.

Comparison 1: Psychotherapy vs TAU, Outcome 20: Secondary: non‐serious adverse effects (dichotomous), at end of treatment
There was no clear difference in the number of adverse effects between psychotherapy and TAU at zero to six months follow‐up (RR 1.40, 95% CI 0.06 to 30.23; 1 trial, 20 participants; P = 0.83; Analysis 1.20.2).
TAU reduced adverse effects compared with psychotherapy at above 12 months follow‐up (RR 1.68, 95% CI 1.34 to 2.10; 1 trial, 183 participants; P < 0.001; Analysis 1.20.3).
No data were available for six to 12 months follow‐up.
1.21 Serious adverse effects
Five trials reported dichotomous data on serious adverse effects (Andreoli 2016; Davidson 2014; McMurran 2016; Robinson 2016; Stanley 2017).
There was no clear difference in the number of serious adverse effects between psychotherapy and TAU at end of treatment ( RR 0.86, 95% CI 0.14 to 5.09; 4 trials, 571 participants, I2 = 46%, P = 0.86; Analysis 1.21).
1.21. Analysis.

Comparison 1: Psychotherapy vs TAU, Outcome 21: Secondary: serious adverse effects (dichotomous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months.
2. Acceptance and commitment therapy (ACT) versus treatment‐as‐usual (TAU)
2.1 Primary outcome: BPD symptom severity
One trial reported continuous data on BPD symptom severity (Morton 2012).
ACT reduced BPD symptom severity at end of treatment compared with TAU (MD −14.66, 95% CI −21.85 to −7.47; 1 trial, 41 participants; P < 0.001; Analysis 2.1).
2.1. Analysis.

Comparison 2: Acceptance and commitment therapy (ACT) vs TAU, Outcome 1: Primary: BPD symptom severity (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available at any time point for self‐harm, suicide‐related outcomes, and psychosocial functioning.
Secondary outcomes
2.2 Affective instability
One trial reported continuous data on affective instability (Morton 2012).
ACT reduced affective instability at end of treatment compared with TAU (MD −27.00, 95% CI −38.86 to −15.14; 1 trial, 41 participants; P < 0.001; Analysis 2.2).
2.2. Analysis.

Comparison 2: Acceptance and commitment therapy (ACT) vs TAU, Outcome 2: Secondary: affective instability (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
2.3 Depression
One trial reported continuous data on depression (Morton 2012).
ACT reduced depression at end of treatment compared with TAU (MD −8.33, 95% CI −15.65 to −1.01; 1 trial, 41 participants; P = 0.03; Analysis 2.3).
2.3. Analysis.

Comparison 2: Acceptance and commitment therapy (ACT) vs TAU, Outcome 3: Secondary: depression (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
2.4 Attrition
One trial reported dichotomous data on attrition (Morton 2012).
ACT did not reduce attrition at end of treatment compared with TAU (RR 1.11, 95% CI 0.45 to 2.74; 1 trial, 41 participants; P = 0.82; Analysis 2.4).
2.4. Analysis.

Comparison 2: Acceptance and commitment therapy (ACT) vs TAU, Outcome 4: Secondary: attrition (dichotomous), at end of treatment
No data were available for zero to months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for anger, chronic feelings of emptiness, impulsivity, interpersonal problems, identity disturbance, dissociation and psychotic‐like symptoms and adverse effects.
3. Dialectical behaviour therapy (DBT) versus treatment‐as‐usual (TAU)
Primary outcomes
3.1 BPD symptom severity
Three trials reported continuous data on BPD symptom severity (Koons 2001a; Priebe 2012; Soler 2009).
DBT reduced BPD symptom severity at end of treatment compared with TAU (SMD −0.60, 95% CI −1.05 to −0.14; 3 trials, 149 participants; I2 = 42%; P = 0.01; Analysis 3.1; low‐quality evidence, Table 3).
3.1. Analysis.

Comparison 3: Dialectical behavior therapy (DBT) vs TAU, Outcome 1: Primary: BPD symptom severity (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
3.2 Self‐harm
Seven trials reported continuous data on self‐harm (Carter 2010; Feigenbaum 2012; Koons 2001a; Linehan 1991; Linehan 2006; Priebe 2012; Van den Bosch 2005).
DBT reduced self‐harm at end of treatment compared with TAU (SMD −0.28, 95% CI −0.48 to −0.07; 7 trials, 376 participants; I2 = 0%; P = 0.008; Analysis 3.2.1; low‐quality evidence, Table 3).
3.2. Analysis.

Comparison 3: Dialectical behavior therapy (DBT) vs TAU, Outcome 2: Primary, self‐harm (continuous)
DBT did not reduce self‐harm at six to 12 months follow‐up compared with TAU (SMD −0.26, 95% CI −0.59 to 0.07; 2 trials, 141 participants; I2 = 0%; P = 0.13; Analysis 3.2.2).
No data were available for zero to six months and above 12 months follow‐up.
3.3 Suicide‐related outcomes
Five trials reported continuous data on suicide‐related outcomes (Feigenbaum 2012; Koons 2001a; Linehan 2006; Mehlum 2014; Soler 2009).
DBT did not reduce suicide‐related outcomes at end of treatment compared with TAU (SMD −0.23, 95% CI −0.68 to 0.23; 5 trials, 231 participants; I2 = 58%; P = 0.33; Analysis 3.3).
3.3. Analysis.

Comparison 3: Dialectical behavior therapy (DBT) vs TAU, Outcome 3: Primary: suicide‐related outcomes (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
3.4 Suicide‐related outcomes, attempts
One trial reported dichotomous data on suicide‐related outcomes (Stanley 2017).
DBT did not reduce suicide‐related outcomes at end of treatment compared with TAU (RR 0.51, 95% CI 0.14 to 1.90; 1 trial, 75 participants; P = 0.32; Analysis 3.4).
3.4. Analysis.

Comparison 3: Dialectical behavior therapy (DBT) vs TAU, Outcome 4: Primary: suicide‐related outcomes, attempts (dichotomous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
3.5 Psychosocial functioning
Six trials reported continuous data on psychosocial functioning (Carter 2010; Feigenbaum 2012; Kramer 2016; Linehan 1994; Mehlum 2014; Soler 2009).
DBT improved psychosocial functioning at end of treatment compared with TAU (SMD −0.36, 95% CI −0.69 to −0.03; 6 trials, 225 participants; I2 = 31%; P = 0.03; Analysis 3.5; low‐quality evidence, Table 3).
3.5. Analysis.

Comparison 3: Dialectical behavior therapy (DBT) vs TAU, Outcome 5: Primary: psychosocial functioning (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
Secondary outcomes
3.6 Anger
Five trials reported continuous data on anger (Feigenbaum 2012; Koons 2001a; Linehan 1994; Linehan 2006; Soler 2009).
DBT reduced anger at end of treatment compared with TAU (SMD −0.47, 95% CI −0.86 to −0.09; 5 trials, 230 participants; I2 = 46%; P = 0.02; Analysis 3.6.1).
3.6. Analysis.

Comparison 3: Dialectical behavior therapy (DBT) vs TAU, Outcome 6: Secondary: anger (continuous)
DBT did not reduce anger at six to 12 months follow‐up compared with TAU (SMD −0.17, 95% CI −0.62 to 0.27; 1 trial, 78 participants; P = 0.44; MD −0.10, 95% CI −0.36 to 0.16; 1 trial, 78 participants; P = 0.45; Analysis 3.6.2).
DBT did not reduce anger at above 12 months follow‐up compared with TAU (SMD 0.02, 95% CI −0.42 to 0.46; 1 trial, 80 participants; P = 0.93; MD 0.01, 95% CI −0.20 to 0.22; 1 trial, 79 participants; P = 0.93; Analysis 3.6.3).
No data were available for zero to six months follow‐up.
3.7 Affective instability
Two trials reported continuous data on affective instability (Bianchini 2019; Soler 2009).
DBT did not reduce affective instability at end of treatment compared with TAU (SMD −0.57, 95% CI −1.64 to 0.51; 2 trials, 80 participants; I2 = 78%; P = 0.30; Analysis 3.7; random‐effects model).
3.7. Analysis.

Comparison 3: Dialectical behavior therapy (DBT) vs TAU, Outcome 7: Secondary: affective instability (continuous), at end of treatment
DBT reduced affective instability at end of treatment compared with TAU (SMD ‐0.75, 95% CI−1.21 to −0.28; 2 trials, 80 participants; I2 = 78%; P = 0.002; analysis not shown; fixed‐effect model).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
3.8 Chronic feelings of emptiness
One trial reported continuous data on chronic feelings of emptiness (Soler 2009).
DBT did not reduce chronic feelings of emptiness at end of treatment compared with TAU (MD −0.67, 95% CI −1.45 to 0.11; 1 trial, 59 participants; P = 0.09; Analysis 3.8).
3.8. Analysis.

Comparison 3: Dialectical behavior therapy (DBT) vs TAU, Outcome 8: Secondary: chronic feelings of emptiness (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
3.9 Impulsivity
Three trials reported continuous data on impulsivity (Bianchini 2019; Soler 2009; Van den Bosch 2005).
DBT reduced impulsivity at end of treatment compared with TAU (SMD −0.35, 95% CI −0.71 to −0.00; 3 trials, 128 participants; I2 = 0%; P = 0.05; Analysis 3.9.1).
3.9. Analysis.

Comparison 3: Dialectical behavior therapy (DBT) vs TAU, Outcome 9: Secondary: impulsivity (continuous)
DBT did not reduce impulsivity at six to 12 months follow‐up compared with TAU (SMD 0.30, 95% CI −0.30 to 0.90; 1 trial, 44 participants; P = 0.33; MD 0.23, 95% CI −0.24 to 0.70; 1 trial, 454 participants; P = 0.33; Van den Bosch 2005; Analysis 3.9.2).
No data were available for zero to six months and above 12 months follow‐up.
3.10 Interpersonal problems
Three trials reported continuous data on interpersonal problems (Carter 2010; Kramer 2016; Soler 2009).
DBT did not reduce interpersonal problems at end of treatment compared with TAU (SMD −0.12, 95% CI −0.45 to 0.20; 3 trials, 148 participants; I2 = 0%; P = 0.45; Analysis 3.10).
3.10. Analysis.

Comparison 3: Dialectical behavior therapy (DBT) vs TAU, Outcome 10: Secondary: interpersonal problems (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
3.11 Dissociation and psychotic‐like symptoms
Four trials reported continuous data on dissociation and psychotic‐like symptoms (Feigenbaum 2012; Koons 2001a; Priebe 2012; Soler 2009).
DBT reduced dissociation and psychotic‐like symptoms at end of treatment compared with TAU (SMD −0.45, 95% CI −0.73 to −0.16; 4 trials, 194 participants; I2 = 0%; P = 0.002; Analysis 3.11).
3.11. Analysis.

Comparison 3: Dialectical behavior therapy (DBT) vs TAU, Outcome 11: Secondary: dissociation and psychotic‐like symptoms (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
3.12 Depression
Five trials reported continuous data on depression (Feigenbaum 2012; Koons 2001a; Linehan 2006; Mehlum 2014; Soler 2009).
DBT did not reduce depression at end of treatment compared with TAU (SMD −0.47, 95% CI −0.98 to 0.03; 5 trials, 219 participants; I2 = 64%; P = 0.07; Analysis 3.12.1; random‐effects model).
3.12. Analysis.

Comparison 3: Dialectical behavior therapy (DBT) vs TAU, Outcome 12: Secondary: depression (continuous)
DBT reduced depression at end of treatment compared with TAU (SMD −0.46, 95% CI −0.73 to −0.18; 5 trials, 219 participants; I2 = 64%; P = 0.001; analysis not shown; fixed‐effect model).
DBT did not reduce depression at six to 12 months follow‐up compared with TAU (SMD −0.23, 95% CI −0.67 to 0.21; 1 trial, 81 participants; P = 0.31; MD −1.80, 95% CI −5.40 to 1.80; 1 trial, 81 participants; P = 0.33; Analysis 3.12.2).
No data were available for zero to six months and above 12 months follow‐up.
3.13 Attrition
Eleven trials reported dichotomous data on attrition (Carter 2010; Feigenbaum 2012; Koons 2001a; Kramer 2016; Linehan 1991; Linehan 1994; Linehan 2006; Priebe 2012; Soler 2009; Stanley 2017; Van den Bosch 2005).
DBT did not reduce attrition at end of treatment compared with TAU (RR 1.27, 95% CI 0.70 to 2.31; 10 trials, 217 participants; I2 = 81%; P = 0.42; Analysis 3.13.1).
3.13. Analysis.

Comparison 3: Dialectical behavior therapy (DBT) vs TAU, Outcome 13: Secondary: attrition (dichotomous)
DBT reduced attrition at zero to six months follow‐up compared with TAU (RR 0.58, 95% CI 0.34 to 1.00; 1 trial, 60 participants; P = 0.05; Analysis 3.13.2).
No data were available for six to 12 months and above 12 months follow‐up.
3.14 Adverse effects
One trial reported dichotomous data on adverse effects (Stanley 2017).
There were no adverse effects at end of treatment in any of the groups (effect not estimable; 1 trial, 75 participants; Analysis 3.14).
3.14. Analysis.

Comparison 3: Dialectical behavior therapy (DBT) vs TAU, Outcome 14: Secondary: adverse effects (dichotomous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
3.15 Serious adverse effects
One trial reported dichotomous data on serious adverse effects (Stanley 2017).
There was no clear difference in the number of serious adverse effects at end of treatment (RR 0.51, 95% CI 0.05 to 5.42; 1 trial, 75 participants; P = 0.58; Analysis 3.15).
3.15. Analysis.

Comparison 3: Dialectical behavior therapy (DBT) vs TAU, Outcome 15: Secondary: serious adverse effects (dichotomous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for abandonment and identity disturbance.
4. Mentalisation‐based therapy (MBT) versus treatment‐as‐usual (TAU)
Primary outcomes
4.1 BPD symptom severity
Six trials reported continuous data on BPD symptom severity (Bateman 1999; Jørgensen 2013; Laurenssen 2018; Philips 2018; Robinson 2016; Rossouw 2012b).
MBT did not reduce BPD symptom severity at end of treatment compared with TAU (SMD −0.13, 95% CI −0.38 to 0.11; 5 trials, 267 participants; I2 = 0%; P = 0.28; Analysis 4.1.1).
4.1. Analysis.

Comparison 4: Mentalisation based therapy (MBT) vs TAU, Outcome 1: Primary: BPD symptom severity (continuous)
MBT did not reduce BPD symptom severity at zero to six months follow‐up compared with TAU (SMD −0.40, 95% CI −1.49 to 0.68; 1 trial, 15 participants; P = 0.47; MD −2.90, 95% CI −10.87 to 5.07; 1 trial, 15 participants; P = 0.48; Analysis 4.1.2).
Above 12 months follow‐up: MBT did not reduce BPD symptom severity at above 12 months follow‐up compared with TAU (SMD −0.94, 95% CI −2.58 to 0.70; 2 trials, 97 participants; I2 = 92%; P = 0.26; Analysis 4.1.3; random‐effects model).
Above 12 months follow‐up: MBT reduced BPD symptom severity at above 12 months follow‐up compared with TAU (SMD −0.76, 95% CI −1.22 to −0.30; 2 trials, 97 participants; I2 = 92%; P = 0.001; analysis not shown; fixed‐effect model).
No data were available for six to 12 months follow‐up.
4.2 Self‐harm (continuous)
One trial reported continuous data on self‐harm (Philips 2018).
MBT did not reduce self‐harm at end of treatment compared with TAU (MD 0.10, 95% CI −4.02 to 4.22; 1 trial, 24 participants; P = 0.96; Analysis 4.2.1).
4.2. Analysis.

Comparison 4: Mentalisation based therapy (MBT) vs TAU, Outcome 2: Primary: self‐harm (continuous), at end of treatment
4.3 Self‐harm (dichotomous)
Three trials reported dichotomous data on self‐harm (Bateman 1999; Bateman 2009; Rossouw 2012b).
MBT reduced self‐harm at end of treatment compared with TAU (RR 0.62, 95% CI 0.49 to 0.80; 3 trials, 252 participants; I2 = 7%; P = 0.0002; Analysis 4.3.1; low‐quality evidence, Table 3).
4.3. Analysis.

Comparison 4: Mentalisation based therapy (MBT) vs TAU, Outcome 3: Primary: self‐harm (dichotomous)
MBT reduced self‐harm at zero to six months follow‐up compared with TAU (RR 0.14, 95% CI 0.04 to 0.56; 1 trial, 41 participants; P = 0.005; Analysis 4.3.2).
MBT reduced self‐harm at six to 12 months follow‐up compared with TAU (RR 0.27, 95% CI 0.10 to 0.68; 1 trial, 41 participants; P = 0.006; Analysis 4.3.3).
MBT reduced self‐harm at above 12 months follow‐up compared with TAU (RR 0.33, 95% CI 0.15 to 0.76; 1 trial, 41 participants; P = 0.009; Analysis 4.3.4).
4.4 Suicide‐related outcomes
Three trials reported dichotomous data on suicide‐related outcomes (Bateman 1999; Bateman 2009; Philips 2018).
MBT reduced suicide‐related outcomes at end of treatment compared with TAU (RR 0.10, 95% CI 0.04 to 0.30; 3 trials, 218 participants; I2 = 0%; P < 0.001; Analysis 4.4.1; low‐quality evidence, Table 3).
4.4. Analysis.

Comparison 4: Mentalisation based therapy (MBT) vs TAU, Outcome 4: Primary: suicide‐related outcomes (dichotomous)
MBT did not reduce suicide‐related outcomes at zero to six months follow‐up compared with TAU (RR 0.25, 95% CI 0.06 to 1.05; 1 trial, 41 participants; P = 0.06; Analysis 4.4.2).
MBT reduced suicide‐related outcomes at above 12 months follow‐up compared with TAU (RR 0.29, 95% CI 0.11 to 0.74; 1 trial, 41 participants; P < 0.001; Analysis 4.4.3).
No data were available for six to 12 months follow‐up.
4.5 Psychosocial functioning
Three trials reported continuous data on psychosocial functioning (Bateman 1999; Bateman 2009; Jørgensen 2013).
MBT did not improve psychosocial functioning at end of treatment compared with TAU (SMD −0.54, 95% CI −1.24 to 0.16; 3 trials, 239 participants; I2 = 83%; P = 0.13; Analysis 4.5.1; random‐effects model).
4.5. Analysis.

Comparison 4: Mentalisation based therapy (MBT) vs TAU, Outcome 5: Primary: psychosocial functioning (continuous)
MBT improved psychosocial functioning at end of treatment compared with TAU (SMD −0.53, 95% CI −0.79 to −0.27; 3 trials, 239 participants; I2 = 83%; P < 0.0001; analysis not shown; fixed‐effect model).
MBT did not improve psychosocial functioning at above 12 months follow‐up compared with TAU (SMD −0.41, 95% CI −0.97 to 0.15; 2 trials, 104 participants; I2 = 47%; P = 0.1507; Analysis 4.5.2).
No data were available for zero to six months and six to 12 months follow‐up.
Secondary outcomes
4.6 Interpersonal problems
Five trials reported continuous data on interpersonal problems (Bateman 1999; Bateman 2009; Jørgensen 2013; Jørgensen 2013; Philips 2018).
MBT reduced interpersonal problems at end of treatment compared with TAU (SMD −0.68, 95% CI −1.33 to −0.02; 5 trials, 357 participants; I2 = 87%; P = 0.04; Analysis 4.6.1).
4.6. Analysis.

Comparison 4: Mentalisation based therapy (MBT) vs TAU, Outcome 6: Secondary: interpersonal problems (continuous)
MBT did not reduce interpersonal problems at zero to six months follow‐up compared with TAU (SMD −0.41, 95% CI −1.01 to 0.20; 1 trial, 53 participants; P = 0.19; MD −0.30, 95% CI −0.76 to 0.16; 1 trial, 53 participants; P = 0.20; Analysis 4.6.2).
MBT did not reduce interpersonal problems at above 12 months follow‐up compared with TAU (SMD −0.28, 95% CI −0.71 to 0.14; 2 trials, 96 participants; I2 = 0%; P = 0.19; Analysis 4.6.3).
No data were available for six to 12 months follow‐up.
4.7 Depression (continuous)
Four trials reported continuous data on depression (Bateman 1999; Bateman 2009; Jørgensen 2013; Laurenssen 2018).
MBT did not reduce depression at end of treatment compared with TAU (SMD −0.58, 95% CI −1.22 to 0.05; 4 trials, 333 participants; I2 = 86%; P = 0.07; Analysis 4.7.1; random‐effects model).
4.7. Analysis.

Comparison 4: Mentalisation based therapy (MBT) vs TAU, Outcome 7: Secondary: depression (continuous)
MBT reduced depression at end of treatment compared with TAU (SMD −0.38, 95% CI − 0.60 to −0.16; 4 trials, 333 participants; I2 = 86%; P = 0.0009; analysis not shown; fixed‐effect model).
MBT did not reduce depression at zero to six months follow‐up compared with TAU (SMD −0.81, 95% CI −1.69 to 0.07; 2 trials, 91 participants; I2 = 72%; P = 0.07; Analysis 4.7.2; random‐effects model).
MBT reduced depression at zero to six months follow‐up compared with TAU (SMD −0.76, 95% CI −1.22 to − 0.30; 2 trials, 91 participants; I2 = 72%; P = 0.001; analysis not shown; fixed‐effect model).
MBT reduced depression at six to 12 months follow‐up compared with TAU (SMD −1.17, 95% CI −1.88 to −0.45; 1 trial, 37 participants; P = 0.001; MD −8.20, 95% CI ‐12.96 to −3.44; 1 trial, 37 participants; P = 0.0007; Analysis 4.7.3).
MBT did not reduce depression at above 12 months follow‐up compared with TAU (SMD −0.72, 95% CI −1.55 to 0.10; 2 trials, 90 participants; I2 = 68%; P = 0.08; Analysis 4.7.4; random‐effects model).
MBT reduced depression at above 12 months follow‐up compared with TAU (SMD −0.68, 95% CI −1.14 to − 0.22; 2 trials, 90 participants; I2 = 68%; P = 0.004; analysis not shown; fixed‐effect model).
4.8 Depression (dichotomous)
One trial reported dichotomous data on depression (Rossouw 2012b).
MBT did not reduce depression at end of treatment compared with TAU (RR 0.71, 95% CI 0.49 to 1.03; 1 trial, 80 participants; P = 0.08; Analysis 4.8.1).
4.8. Analysis.

Comparison 4: Mentalisation based therapy (MBT) vs TAU, Outcome 8: Secondary: depression (dichotomous), at end of treatment
No data were available for zero to six months, six to 12 months, and above 12 months follow‐up.
4.9 Attrition
Seven trials reported dichotomous data on attrition (Bateman 1999; Bateman 2009; Jørgensen 2013; Laurenssen 2018; Philips 2018; Robinson 2016; Rossouw 2012b).
MBT did not reduce attrition at end of treatment compared with TAU (RR 0.99, 95% CI 0.79 to 1.25; 7 trials, 552 participants; I2 = 0%; P = 0.96; Analysis 4.9.1).
4.9. Analysis.

Comparison 4: Mentalisation based therapy (MBT) vs TAU, Outcome 9: Secondary: attrition (dichotomous), at end of treatment
No data were available for zero to six months, six to 12 months, and above 12 months follow‐up.
4.10 Adverse effects
One trial reported dichotomous data on serious adverse effects (Robinson 2016).
There was no clear difference in terms of severe adverse effects at end of treatment (RR 3.00, 95% CI 0.13 to 71.15; 1 trial, P = 0.50; Analysis 4.10).
4.10. Analysis.

Comparison 4: Mentalisation based therapy (MBT) vs TAU, Outcome 10: Secondary: adverse effects (dichotomous), at end of treatment
No data were available for zero to six months, six to 12 months, and above 12 months follow‐up.
* No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, abandonment, identity disturbance, dissociation and psychotic‐like symptoms.
4.11 Mentalisation‐based treatment for eating disorders (MBT‐ED) versus specialist supportive clinical management (SSCM‐ED) (generic inverse variance)
Primary outcome: psychosocial functioning
One trial reported continuous data on psychosocial functioning (Robinson 2016).
MBT‐ED did not improve psychosocial functioning at end of treatment compared with SSCM‐ED (MD −0.07, 95% CI −0.86 to 0.72; 1 trial, 23 participants; P = 0.86; Analysis 4.11.1).
4.11. Analysis.

Comparison 4: Mentalisation based therapy (MBT) vs TAU, Outcome 11: Mentalisation‐based treatment for eating disorders (MBT‐ED) versus specialist supportive clinical management (SSCM‐ED) (generic inverse variance)
MBT‐ED did not improve psychosocial functioning at zero to six months follow‐up compared with SSCM‐ED (MD −0.44, 95% CI −1.52 to 0.64; 1 trial, 15 participants; P = 0.42 Analysis 4.11.2).
No data were available for six to 12 months and above 12 months follow‐up.
No data were available on any time point for BPD symptom severity, self‐harm and suicide‐related outcomes.
Secondary outcomes
Interpersonal problems
One trial reported continuous data on interpersonal problems (Robinson 2016).
MBT‐ED did not reduce interpersonal problems at end of treatment compared with SSCM‐ED (MD −0.10, 95% CI −0.89 to 0.69; 1 trial, 23 participants; P = 0.80; Analysis 4.11.3).
MBT‐ED did not reduce interpersonal problems at zero to six months follow‐up compared with SSCM‐ED (MD −0.06, 95% CI −1.15 to 1.03; 1 trial, 15 participants; P = 0.91; Analysis 4.11.4).
No data were available for six to 12 months and above 12 months follow‐up.
Depression
One trial reported continuous data on depression (Robinson 2016).
MBT‐ED did not reduce depression at end of treatment compared with SSCM‐ED (MD 0.19, 95% CI −0.60 to 0.98; 1 trial, 23 participants; P = 0.64; Analysis 4.11.5).
MBT‐ED did not reduce depression at zero to six months follow‐up compared with SSCM‐ED (MD 0.51, 95% CI −0.62 to 1.64; 1 trial, 15 participants; P = 0.38; Analysis 4.11.6).
No data were available for six to 12 months and above 12 months follow‐up.
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, attrition and adverse effects.
5. Cognitive behaviour therapy (CBT) versus treatment‐as‐usual (TAU)
Primary outcomes
5.1 BPD symptom severity (continuous)
One trial reported continuous data on BPD symptom severity (Kredlow 2017a).
CBT reduced BPD symptom severity at end of treatment compared with TAU (MD −3.08, 95% CI −4.99 to −1.17; 1 trial, 26 participants; P = 0.002; Analysis 5.1.1) (random‐effects model analysis).
5.1. Analysis.

Comparison 5: Cognitive behavioural therapy (CBT) and related treatments vs TAU, Outcome 1: Primary: BPD symptom severity (continuous)
CBT did not reduce BPD symptom severity at zero to six months follow‐up compared with TAU (MD −2.02, 95% CI −4.23 to 0.19; 1 trial, 26 participants; P = 0.07; Analysis 5.1.2) (fixed‐effect model analysis).
No data were available for six to 12 months and above 12 months follow‐up.
5.2 BPD symptom severity (dichotomous)
One trial reported dichotomous data on BPD symptom severity (Davidson 2006).
CBT did not reduce BPD symptom severity at above 12 months follow‐up compared with TAU (RR 0.91, 95% CI 0.56 to 1.48; 1 trial, 76 participants; P = 0.71; Analysis 5.2).
5.2. Analysis.

Comparison 5: Cognitive behavioural therapy (CBT) and related treatments vs TAU, Outcome 2: Primary: BPD symptom severity (dichotomous), at above 12 months follow‐up
No data were available for end of treatment, zero to six months and six to 12 months follow‐up.
5.3 Self‐harm (continuous)
One trial reported continuous data on self‐harm (Weinberg 2006).
CBT reduced self‐harm at end of treatment compared with TAU (MD −3.03, 95% CI −5.68 to −0.38; 1 trial, 28 participants; P = 0.03; Analysis 5.3.1).
5.3. Analysis.

Comparison 5: Cognitive behavioural therapy (CBT) and related treatments vs TAU, Outcome 3: Primary: self‐harm (continuous)
CBT did not reduce self‐harm at zero to six months follow‐up compared with TAU (MD −4.71, 95% CI −11.60 to 2.18; 1 trial, 28 participants; P = 0.18; Analysis 5.3.2).
No data were available for six to 12 months and above 12 months follow‐up.
5.4 Self‐harm (dichotomous)
One trial reported dichotomous data on self‐harm (Davidson 2006).
CBT did not reduce self‐harm at end of treatment compared with TAU (RR 1.17, 95% CI 0.86 to 1.60; 1 trial, 99 participants; P = 0.32; Analysis 5.4).
5.4. Analysis.

Comparison 5: Cognitive behavioural therapy (CBT) and related treatments vs TAU, Outcome 4: Primary: self‐harm (dichotomous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
5.5 Suicide‐related outcomes
Two trials reported continuous data on suicide‐related outcomes (Davidson 2006; Weinberg 2006).
CBT did not reduce suicide‐related outcomes at end of treatment compared with TAU (SMD −0.47, 95% CI −1.02 to 0.08; 2 trials, 104 participants; P = 0.09; Analysis 5.5.1; random‐effects model).
5.5. Analysis.

Comparison 5: Cognitive behavioural therapy (CBT) and related treatments vs TAU, Outcome 5: Primary: suicide‐related outcomes (continuous)
CBT reduced suicide‐related outcomes at end of treatment compared with TAU (SMD −0.42, 95% −0.81, −0.02; 2 trials, 104 participants; P = 0.04; analysis not shown; fixed‐effect model).
CBT did not reduce suicide‐related outcomes at zero to six months follow‐up compared with TAU (SMD −0.45, 95% CI −1.20 to 0.31; 1 trial, 28 participants; P = 0.25; MD −7.73, 95% CI −20.00 to 4.54; 1 trial, 28 participants; P = 0.22; Analysis 5.5.2).
CBT did not reduce suicide‐related outcomes at six to 12 months follow‐up compared with TAU (SMD −0.44, 95% CI −0.89 to 0.02; 1 trial, 76 participants; P = 0.06; MD −1.16, 95% CI −2.47 to 0.15; 1 trial, 76 participants; P = 0.08; Analysis 5.5.3).
CBT did not reduce suicide‐related outcomes at above 12 months follow‐up compared with TAU (SMD −0.31, 95% CI −0.77 to 0.14; 1 trial, 76 participants; P = 0.18; MD −1.15, 95% CI −2.86 to 0.07; 1 trial, 76 participants; P = 0.19; Analysis 5.5.4).
5.6 Psychosocial functioning
Two trials reported continuous data on psychosocial functioning (Davidson 2006; McMurran 2016).
CBT did not improve psychosocial functioning at end of treatment compared with TAU (SMD 0.00, 95% CI −0.39 to 0.39; 1 trial, 99 participants; P = 1.00; MD 0.00, 95% CI −1.78 to 1.78; 1 trial, 99 participants; P = 1.00; Analysis 5.6.1).
5.6. Analysis.

Comparison 5: Cognitive behavioural therapy (CBT) and related treatments vs TAU, Outcome 6: Primary: psychosocial functioning (continuous)
CBT did not improve psychosocial functioning at six to 12 months follow‐up compared with TAU (SMD 0.13, 95% CI −0.28 to 0.55; 1 trial, 90 participants; P = 0.52; MD 0.70, 95% CI −1.43 to 2.83; 1 trial, 90 participants; P = 0.52; Analysis 5.6.2).
CBT did not improve psychosocial functioning at above 12 months follow‐up compared with TAU (SMD 0.04, 95% CI −0.36 to 0.43; 2 trials, 209 participants; I2 = 51%; P = 0.86; Analysis 5.6.3.
No data were available for zero to six months follow‐up.
Secondary outcomes
5.7 Interpersonal problems
One trial reported continuous data on interpersonal problems (Davidson 2006).
CBT did not reduce interpersonal problems at end of treatment compared with TAU (MD 5.40, 95% CI −3.70 to 14.50; 1 trial, 99 participants; P = 0.24; Analysis 5.7.1).
5.7. Analysis.

Comparison 5: Cognitive behavioural therapy (CBT) and related treatments vs TAU, Outcome 7: Secondary: interpersonal problems (continuous)
CBT did not reduce interpersonal problems at six to 12 months follow‐up compared with TAU (MD 0.30, 95% CI −9.17 to 9.77; 1 trial, 99 participants; P = 0.95; Analysis 5.7.2).
TAU reduced interpersonal problems at above 12 months follow‐up compared with CBT (MD 11.70, 95% CI 0.72 to 22.68; 1 trial, 76 participants; P = 0.04; Analysis 5.7.3).
No data were available for zero to six months follow‐up.
5.8 Dissociation and psychotic‐like symptoms
One trial reported continuous data on dissociation and psychotic‐like symptoms (Kredlow 2017a).
CBT did not reduce dissociation and psychotic‐like symptoms at end of treatment compared with TAU (MD −2.30, 95% CI −8.84 to 4.24; 1 trial, 26 participants; P = 0.49; Analysis 5.8.1).
5.8. Analysis.

Comparison 5: Cognitive behavioural therapy (CBT) and related treatments vs TAU, Outcome 8: Secondary: dissociation and psychotic‐like symptoms (continuous)
CBT reduced dissociation and psychotic‐like symptoms at zero to six months follow‐up compared with TAU (MD −13.40, 95% CI −24.49 to −2.31; 1 trial, 26 participants; P = 0.02; Analysis 5.8.2).
No data were available for six to 12 months and above 12 months follow‐up.
5.9 Depression
Five trials reported continuous data on depression (Davidson 2006; Davidson 2014; Jahangard 2012; Kredlow 2017a; McMurran 2016).
CBT did not reduce depression at end of treatment compared with TAU (SMD −0.21, 95% CI −0.77 to 0.35; 5 trials, 314 participants; I2 = 77%; P = 0.47; Analysis 5.9.1).
5.9. Analysis.

Comparison 5: Cognitive behavioural therapy (CBT) and related treatments vs TAU, Outcome 9: Secondary: depression (continuous)
CBT reduced depression at zero to six months follow‐up compared with TAU (SMD −0.96, 95% CI −1.78 to −0.14; 1 trial, 26 participants; P = 0.02; MD −12.70, 95% CI −22.62 to −2.78; 1 trial, 26 participants; P = 0.01; Analysis 5.9.2).
CBT did not reduce depression at six to 12 months follow‐up compared with TAU (SMD −0.29, 95% CI −0.69 to 0.11; 1 trial, 99 participants; P = 0.15; MD −4.60, 95% CI −10.82 to 1.62; 1 trial, 99 participants; P = 0.15; Analysis 5.9.3).
CBT did not reduce depression at above 12 months follow‐up compared with TAU (SMD −0.15, 95% CI −0.43 to 0.13; 2 trials, 197 participants; I2 = 0%; P = 0.30; Analysis 5.9.4).
5.10 Attrition
One trial reported dichotomous data on attrition (Davidson 2006).
CBT did not reduce attrition at end of treatment compared with TAU (RR 0.48, 95% CI 0.09 to 2.52; 1 trial, 106 participants; P = 0.39; Analysis 5.10).
5.10. Analysis.

Comparison 5: Cognitive behavioural therapy (CBT) and related treatments vs TAU, Outcome 10: Secondary: attrition (dichotomous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
5.11 Adverse effects
Two trials reported dichotomous data on non‐serious and serious adverse effects (Davidson 2014McMurran 2016).
There was no clear difference in the number of non‐serious adverse effects at end of treatment (RR 0.92, 95% CI 0.45 to 1.88; 1 trial, 306 participants; P = 0.81; Analysis 5.11.1).
5.11. Analysis.

Comparison 5: Cognitive behavioural therapy (CBT) and related treatments vs TAU, Outcome 11: Secondary: adverse effects (dichotomous), at end of treatment
There was no clear difference in the number of serious adverse effects at end of treatment (RR 2.65, 95% CI 0.31 to 22.93; 2 trials, 326 participants; I2= 0%, P = 0.56; Analysis 5.11.2).
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, abandonment and identity disturbance.
6. Psychodynamic psychotherapy versus treatment‐as‐usual (TAU)
Primary outcomes
6.1 BPD symptom severity
Four trials reported continuous data on BPD symptom severity (Amianto 2011; Gregory 2008b; Leichsenring 2016; Reneses 2013).
Psychodynamic psychotherapy did not reduce BPD symptom severity at end of treatment compared with TAU (SMD −0.29, 95% CI −0.66 to 0.09; 4 trials, 222 participants; I2 = 38%; P = 0.13; Analysis 6.1.1).
6.1. Analysis.

Comparison 6: Psychodynamic psychotherapy vs TAU, Outcome 1: Primary: BPD symptom severity (continuous)
Psychodynamic psychotherapy did not reduce BPD symptom severity at six to 12 months follow‐up compared with TAU (SMD −0.31, 95% CI −1.00 to 0.38; 1 trial, 33 participants; P = 0.38; MD −0.30, 95% CI −0.95 to 0.35; 1 trial, 33 participants; P = 0.37; Analysis 6.1.2).
No data were available for zero to six months and above 12 months follow‐up.
6.2 Self‐harm
Two trials reported continuous data on self‐harm (Amianto 2011; Gregory 2008b).
Psychodynamic psychotherapy did not reduce self‐harm at end of treatment compared with TAU (MD 0.12, 95% CI −0.56 to 0.80; 1 trial, 33 participants; P = 0.73; Analysis 6.2.1).
6.2. Analysis.

Comparison 6: Psychodynamic psychotherapy vs TAU, Outcome 2: Primary: self‐harm (continuous)
Psychodynamic psychotherapy did not reduce self‐harm at six to 12 months follow‐up compared with TAU (MD 0.14, 95% CI −0.55 to 0.82; 1 trial, 33 participants; P = 0.69; Analysis 6.2.2).
No data were available for zero to six months and above 12 months follow‐up.
6.3 Suicide‐related outcomes
Three trials reported continuous data on suicide‐related outcomes (Amianto 2011; Gregory 2008b; Reneses 2013).
Psychodynamic psychotherapy did not reduce suicide‐related outcomes at end of treatment compared with TAU (SMD −0.22, 95% CI −0.62 to 0.17; 3 trials, 101 participants; I2 = 27%; P = 0.27; Analysis 6.3.1).
6.3. Analysis.

Comparison 6: Psychodynamic psychotherapy vs TAU, Outcome 3: Primary: suicide‐related outcomes (continuous)
Psychodynamic psychotherapy did not reduce suicide‐related outcomes at six to 12 months follow‐up compared with TAU (SMD −0.38, 95% CI −1.07 to 0.31; 1 trial, 33 participants; P = 0.28; MD −0.30, 95% CI −0.82 to 0.2; 1 trial, 33 participants; P = 0.26; Analysis 6.3.2).
No data were available for zero to six months and above 12 months follow‐up.
6.4 Psychosocial functioning
Four trials reported continuous data on psychosocial functioning (Amianto 2011; Gregory 2008b; Reneses 2013; Salzer 2014).
Psychodynamic psychotherapy did not improve psychosocial functioning at end of treatment compared with TAU (SMD −0.69, 95% CI −1.98 to 0.59; 4 trials, 140 participants; I2 = 92%; P = 0.29; Analysis 6.4.1; random‐effects model).
6.4. Analysis.

Comparison 6: Psychodynamic psychotherapy vs TAU, Outcome 4: Primary: psychosocial functioning (continuous)
Psychodynamic psychotherapy improved psychosocial functioning at end of treatment compared with TAU (SMD −0.38, 95% CI −0.75 to −0.02; 4 trials, 140 participants; I2 = 92%; P = 0.04; analysis not shown; fixed‐effect model).
Psychodynamic psychotherapy did not improve psychosocial functioning at six to 12 months follow‐up compared with TAU (SMD 0.05, 95% CI −0.64 to 0.73; 1 trial, 33 participants; P = 0.89; MD 0.60, 95% CI −8.07 to 9.27; 1 trial, 33 participants; P = 0.89; Analysis 6.4.2).
Psychodynamic psychotherapy did not improve psychosocial functioning at above 12 months follow‐up compared with TAU (SMD −0.40, 95% CI −1.39 to 0.60; 1 trial, 16 participants; P = 0.44; MD −3.70, 95% CI −12.37 to 4.97; 1 trial, 16 participants; P = 0.40; Analysis 6.4.3).
No data were available for zero to six months.
Secondary outcomes
6.5 Anger
One trial reported continuous data on anger (Amianto 2011).
Psychodynamic psychotherapy did not reduce anger at end of treatment compared with TAU (MD 0.00, 95% CI −0.86 to 0.86; 1 trial, 33 participants; P = 1.00; Analysis 6.5.1).
6.5. Analysis.

Comparison 6: Psychodynamic psychotherapy vs TAU, Outcome 5: Secondary: anger (continuous)
Psychodynamic psychotherapy did not reduce anger at six to 12 months follow‐up compared with TAU (MD 0.00, 95% CI −0.89 to 0.89; 1 trial, 33 participants; P = 1.00; Analysis 6.5.2).
No data were available for zero to six months and above 12 months follow‐up.
6.6 Affective instability
Three trials reported continuous data on affective instability (Amianto 2011; Reneses 2013; Salzer 2014).
Psychodynamic psychotherapy reduced affective instability at end of treatment compared with TAU (SMD −0.50, 95% CI −0.87 to −0.13; 3 trials, 116 participants; I2 = 0%; P = 0.009; Analysis 6.6.1).
6.6. Analysis.

Comparison 6: Psychodynamic psychotherapy vs TAU, Outcome 6: Secondary: affective instability (continuous)
Psychodynamic psychotherapy did not reduce affective instability at six to 12 months follow‐up compared with TAU (SMD −0.52, 95% CI −1.21 to 0.18; 1 trial, 33 participants; P = 0.15; MD −0.70, 95% CI −1.59 to 0.19; 1 trial, 33 participants; P = 0.12; Analysis 6.6.2).
No data were available for zero to six months and above 12 months follow‐up.
6.7 Chronic feelings of emptiness
Two trials reported continuous data on chronic feelings of emptiness (Amianto 2011; Reneses 2013).
Psychodynamic psychotherapy did not reduce chronic feelings of emptiness at end of treatment compared with TAU (SMD −0.49, 95% CI −1.02 to 0.04; 2 trials, 77 participants; I2 = 24%; P = 0.07; Analysis 6.7.1; random‐effects model).
6.7. Analysis.

Comparison 6: Psychodynamic psychotherapy vs TAU, Outcome 7: Secondary: chronic feelings of emptiness (continuous)
Psychodynamic psychotherapy reduced chronic feelings of emptiness at end of treatment compared with TAU (SMD −0.50, 95% CI −0.96 to −0.04; 2 trials, 77 participants; I2 = 24%; P = 0.03; analysis not shown; fixed‐effect model).
Psychodynamic psychotherapy did not reduce chronic feelings of emptiness at six to 12 months follow‐up compared with TAU (SMD −0.58, 95% CI −1.28 to 0.11; 1 trial, 33 participants; P = 0.10; MD −0.60, 95% CI −1.29 to 0.09; 1 trial, 33 participants; P = 0.09; Analysis 6.7.2).
No data were available for six to 12 months and above 12 months follow‐up.
6.8 Impulsivity
Two trials reported continuous data on impulsivity (Amianto 2011; Reneses 2013).
Psychodynamic psychotherapy did not reduce impulsivity at end of treatment compared with TAU (SMD −0.39, 95% CI −0.85 to 0.07; 2 trials, 77 participants; I2 = 0%; P = 0.09; Analysis 6.8.1).
6.8. Analysis.

Comparison 6: Psychodynamic psychotherapy vs TAU, Outcome 8: Secondary: impulsivity (continuous)
Psychodynamic psychotherapy did not reduce impulsivity at six to 12 months follow‐up compared with TAU (SMD 0.35, 95% CI −0.34 to 1.04; 1 trial, 33 participants; P = 0.32; MD 0.20, 95% CI −0.18 to 0.58; 1 trial, 33 participants; P = 0.30; Analysis 6.8.2).
No data were available for zero to six months and above 12 months follow‐up.
6.9 Interpersonal problems
Four trials reported continuous data on interpersonal problems (Amianto 2011; Leichsenring 2016; Reneses 2013; Salzer 2014).
Psychodynamic psychotherapy did not reduce interpersonal problems at end of treatment compared with TAU (SMD −0.21, 95% CI −0.71 to 0.29; 4 trials, 238 participants; I2 = 68%; P = 0.41; Analysis 6.9.1).
6.9. Analysis.

Comparison 6: Psychodynamic psychotherapy vs TAU, Outcome 9: Secondary: interpersonal problems (continuous)
Psychodynamic psychotherapy did not reduce interpersonal problems at six to 12 months follow‐up compared with TAU (SMD −0.51, 95% CI −1.20 to 0.19; 1 trial, 33 participants; P = 0.15; MD −0.60, 95% CI −1.38 to 0.18; 1 trial, 33 participants; P = 0.13; Analysis 6.9.2).
No data were available for zero to six months and above 12 months follow‐up.
6.10 Abandonment
One trial reported continuous data on abandonment (Amianto 2011).
Psychodynamic psychotherapy did not reduce abandonment at end of treatment compared with TAU (MD −0.20, 95% CI −0.95 to 0.55; 1 trial, 33 participants; P = 0.60; Analysis 6.10.1).
6.10. Analysis.

Comparison 6: Psychodynamic psychotherapy vs TAU, Outcome 10: Secondary: abandonment (continuous)
Psychodynamic psychotherapy did not reduce abandonment at six to 12 months follow‐up compared with TAU (MD −0.40, 95% CI −1.08 to 0.28; 1 trial, 33 participants; P = 0.25; Analysis 6.10.2).
No data were available for zero to six months and above 12 months follow‐up.
6.11 Identity disturbance
Three trials reported continuous data on identity disturbance (Amianto 2011; Leichsenring 2016; Reneses 2013).
Psychodynamic psychotherapy did not reduce identity disturbance at end of treatment compared with TAU (SMD −0.38, 95% CI −1.02 to 0.27; 3 trials, 199 participants; I2 = 75%; P = 0.25; Analysis 6.11.1).
6.11. Analysis.

Comparison 6: Psychodynamic psychotherapy vs TAU, Outcome 11: Secondary: identity disturbance (continuous)
Psychodynamic psychotherapy reduced identity disturbance at six to 12 months follow‐up compared with TAU (SMD −1.09, 95% CI −1.83 to −0.35; 1 trial, 33 participants; P = 0.004; MD −1.40, 95% CI −2.25 to −0.55; 1 trial, 33 participants; P = 0.001; Analysis 6.11.2).
No data were available for zero to six months and above 12 months follow‐up.
6.12 Dissociation and psychotic‐like symptoms
Two trials reported continuous data on dissociation and psychotic‐like symptoms (Amianto 2011; Gregory 2008b).
Psychodynamic psychotherapy did not reduce dissociation and psychotic‐like symptoms at end of treatment compared with TAU (SMD −0.18, 95% CI −0.96 to 0.60; 2 trials, 57 participants; I2 = 53%; P = 0.66; Analysis 6.12.1).
6.12. Analysis.

Comparison 6: Psychodynamic psychotherapy vs TAU, Outcome 12: Secondary: dissociation and psychotic‐like symptoms (continuous)
Psychodynamic psychotherapy did not reduce dissociation and psychotic‐like symptoms at six to 12 months follow‐up compared with TAU (SMD −0.59, 95% CI −1.29 to 0.11; 1 trial, 33 participants; P = 0.10; MD −0.70, 95% CI −1.49 to 0.09; 1 trial, 33 participants; P = 0.08; Analysis 6.12.2).
Psychodynamic psychotherapy did not reduce dissociation and psychotic‐like symptoms at above 12 months follow‐up compared with TAU (SMD −0.01, 95% CI −0.81 to 0.79; 1 trial, 24 participants; P = 0.98; MD −0.20, 95% CI −20.07 to 19.67; 1 trial, 24 participants; P = 0.98; Analysis 6.12.3).
No data were available for zero to six months follow‐up.
6.13 Depression
Three trials reported continuous data on depression (Amianto 2011; Leichsenring 2016; Reneses 2013).
Psychodynamic psychotherapy did not reduce depression at end of treatment compared with TAU (SMD −0.17, 95% CI −0.81 to 0.47; 3 trials, 190 participants; I2 = 73%; P = 0.61; Analysis 6.13.1).
6.13. Analysis.

Comparison 6: Psychodynamic psychotherapy vs TAU, Outcome 13: Secondary: depression (continuous)
Psychodynamic psychotherapy did not reduce depression at above 12 months follow‐up compared with TAU (SMD −0.68, 95% CI −1.51 to 0.15; 1 trial, 24 participants; P = 0.11; MD −7.80, 95% CI −16.71 to 1.11; 1 trial, 24 participants; P = 0.09; Analysis 6.13.2).
No data were available for zero to six months and six to 12 months follow‐up.
6.14 Attrition
Three trials reported dichotomous data on attrition (Amianto 2011; Leichsenring 2016; Reneses 2013).
Psychodynamic psychotherapy did not reduce attrition at end of treatment compared with TAU (RR 0.90, 95% CI 0.56 to 1.47; 3 trials, 210 participants; I2 = 0%; P = 0.68; Analysis 6.14).
6.14. Analysis.

Comparison 6: Psychodynamic psychotherapy vs TAU, Outcome 14: Secondary: attrition (dichotomous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for adverse effects.
7. Schema‐focused therapy (SFT) versus treatment‐as‐usual (TAU)
Primary outcomes
7.1 BPD symptom severity
One trial reported continuous data on BPD symptom severity (Farrell 2009).
SFT reduced BPD symptom severity at end of treatment compared with TAU (MD −13.94, 95% CI −19.66 to −8.22; 1 trial, 28 participants; P < 0.001; Analysis 7.1).
7.1. Analysis.

Comparison 7: Schema‐focused therapy (SFT) vs TAU, Outcome 1: Primary: BPD symptom severity (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
7.2 Psychosocial functioning
One trial reported continuous data on psychosocial functioning (Farrell 2009).
SFT improved psychosocial functioning at end of treatment compared with TAU (MD −10.42, 95% CI −16.17 to −4.67; 1 trial, 28 participants; P < 0.001; Analysis 7.2).
7.2. Analysis.

Comparison 7: Schema‐focused therapy (SFT) vs TAU, Outcome 2: Primary: psychosocial functioning (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for self‐harm and suicide‐related outcomes.
Secondary outcomes
7.3 Affective instability
One trial reported continuous data on affective instability (Farrell 2009).
SFT reduced affective instability at end of treatment compared with TAU (MD −3.95, 95% CI −5.75 to −2.15; 1 trial, 28 participants; P < 0.001; Analysis 7.3).
7.3. Analysis.

Comparison 7: Schema‐focused therapy (SFT) vs TAU, Outcome 3: Secondary: affective instability (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
7.4 Impulsivity
One trial reported continuous data on impulsivity (Farrell 2009).
SFT reduced impulsivity at end of treatment compared with TAU (MD −4.02, 95% CI −5.68 to −2.36; 1 trial, 28 participants; P < 0.001; Analysis 7.4).
7.4. Analysis.

Comparison 7: Schema‐focused therapy (SFT) vs TAU, Outcome 4: Secondary: impulsivity (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
7.5 Interpersonal problems
One trial reported continuous data on interpersonal problems (Farrell 2009).
SFT reduced interpersonal problems at end of treatment compared with TAU (MD −7.12, 95% CI −9.65 to −4.59; 1 trial, 28 participants; P < 0.001; Analysis 7.5).
7.5. Analysis.

Comparison 7: Schema‐focused therapy (SFT) vs TAU, Outcome 5: Secondary: interpersonal problems (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
7.6 Dissociation and psychotic‐like symptoms
One trial reported continuous data on dissociation and psychotic‐like symptoms (Farrell 2009).
SFT reduced dissociation and psychotic‐like symptoms at end of treatment compared with TAU (MD −2.56, 95% CI −3.86 to −1.26; 1 trial, 28 participants; P < 0.001; Analysis 7.6).
7.6. Analysis.

Comparison 7: Schema‐focused therapy (SFT) vs TAU, Outcome 6: Secondary: dissociation and psychotic‐like symptoms (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up..
7.7 Attrition
One trial reported dichotomous data on attrition (Farrell 2009).
SFT did not reduce attrition at end of treatment compared with TAU (RR 0.11, 95% CI 0.01 to 1.91; 1 trial, 32 participants; P = 0.13; Analysis 7.7).
7.7. Analysis.

Comparison 7: Schema‐focused therapy (SFT) vs TAU, Outcome 7: Secondary: attrition (dichotomous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
* No data were available on any time point for anger, chronic feelings of emptiness, abandonment, identity disturbance, depression, and adverse effects
8. Systems training for emotional predictability and problem solving (STEPPS) versus treatment‐as‐usual (TAU)
Primary outcomes
8.1 BPD symptom severity
Three trials reported continuous data on BPD symptom severity (Blum 2008; Bos 2010; Schuppert 2012).
STEPPS reduced BPD symptom severity at end of treatment compared with TAU (SMD −0.39, 95% CI −0.63 to −0.15; 3 trials, 273 participants; I2 = 0%; P = 0.001; Analysis 8.1.1).
8.1. Analysis.

Comparison 8: Systems training for emotional predictability and problem solving (STEPPS) vs TAU, Outcome 1: Primary: BPD symptom severity (continuous), at end of treatment
STEPPS did not reduce BPD symptom severity at six to 12 months follow‐up compared with TAU (SMD 0.03, 95% CI −0.33 to 0.38; 1 trial, 124 participants; P = 0.88; MD 0.60, 95% CI −7.45 to 8.65; 1 trial, 124 participants; P = 0.88; Analysis 8.1.2).
No data were available for zero to six months and above 12 months follow‐up.
8.2 Self‐harm
One trial reported dichotomous data on self‐harm (Bos 2010).
STEPPS did not reduce self‐harm at end of treatment compared with TAU (RR 1.32, 95% CI 0.78 to 2.22; 1 trial, 58 participants; P = 0.30; Analysis 8.2).
8.2. Analysis.

Comparison 8: Systems training for emotional predictability and problem solving (STEPPS) vs TAU, Outcome 2: Primary: self‐harm (dichtomous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
8.3 Psychosocial functioning
One trial reported continuous data on psychosocial functioning (Blum 2008).
STEPPS improved psychosocial functioning at end of treatment compared with TAU (MD −7.00, 95% CI −11.43 to −2.57; 1 trial, 124 participants; P = 0.002; Analysis 8.3.1).
8.3. Analysis.

Comparison 8: Systems training for emotional predictability and problem solving (STEPPS) vs TAU, Outcome 3: Primary: psychosocial functioning (continuous)
STEPPS did not improve psychosocial functioning at six to 12 months follow‐up compared with TAU (MD −5.90, 95% CI −12.49 to 0.69; 1 trial, 124 participants; P = 0.08; Analysis 8.3.2).
No data were available for zero to six months and above 12 months follow‐up.
No data were available on any time point for suicide‐related outcomes.
Secondary outcomes
8.4 Affective instability
Two trials reported continuous data on affective instability (Blum 2008; Schuppert 2012).
STEPPS did not reduce affective instability at end of treatment compared with TAU (SMD −0.25, 95% CI −0.52 to 0.02; 2 trials, 221 participants; P = 0.06; Analysis 8.4).
8.4. Analysis.

Comparison 8: Systems training for emotional predictability and problem solving (STEPPS) vs TAU, Outcome 4: Secondary: affective instability (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
8.5 Impulsivity (continuous)
One trial reported continuous data on impulsivity (Blum 2008).
STEPPS did not reduce impulsivity at end of treatment compared with TAU (MD −0.40, 95% CI −1.23 to 0.43; 1 trial, 124 participants; P = 0.35; Analysis 8.5).
8.5. Analysis.

Comparison 8: Systems training for emotional predictability and problem solving (STEPPS) vs TAU, Outcome 5: Secondary: impulsivity (continuous), at end of treatment
8.6 Impulsivity (dichotomous)
One trial reported dichotomous data on impulsivity (Bos 2010).
STEPPS did not reduce impulsivity at end of treatment compared with TAU (RR 0.93, 95% CI 0.66 to 1.29; 1 trial, 58 participants; P = 0.65; Analysis 8.6).
8.6. Analysis.

Comparison 8: Systems training for emotional predictability and problem solving (STEPPS) vs TAU, Outcome 6: Secondary: impulsivity (dichotomous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
8.7 Interpersonal problems
Two trials reported continuous data on interpersonal problems (Blum 2008; Bos 2010).
STEPPS reduced interpersonal problems at end of treatment compared with TAU (SMD −0.38, 95% CI −0.67 to −0.08; 2 trials, 177 participants; I2 = 0%; P = 0.01; Analysis 8.7).
8.7. Analysis.

Comparison 8: Systems training for emotional predictability and problem solving (STEPPS) vs TAU, Outcome 7: Secondary: interpersonal problems (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
8.8 Dissociation and psychotic‐like symptoms
One trial reported continuous data on dissociation and psychotic‐like symptoms (Blum 2008).
STEPPS reduced dissociation and psychotic‐like symptoms at end of treatment compared with TAU (MD −1.00, 95% CI −1.83 to −0.17; 1 trial, 124 participants; P = 0.02; Analysis 8.8).
8.8. Analysis.

Comparison 8: Systems training for emotional predictability and problem solving (STEPPS) vs TAU, Outcome 8: Secondary: dissociation and psychotic‐like symptoms (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
8.9 Depression
One trial reported continuous data on depression (Blum 2008).
STEPPS did not reduce depression at end of treatment compared with TAU (MD −3.80, 95% CI −9.34 to 1.74; 1 trial, 124 participants; P = 0.18; Analysis 8.9.1).
8.9. Analysis.

Comparison 8: Systems training for emotional predictability and problem solving (STEPPS) vs TAU, Outcome 9: Secondary: depression (continuous), at end of treatment
STEPPS did not reduce depression at six to 12 months follow‐up compared with TAU (MD −0.60, 95% CI −8.42 to 9.62; 1 trial, 124 participants; P = 0.90; Analysis 8.9.2).
No data were available for zero to six months and above 12 months follow‐up.
8.10 Attrition
One trial reported dichotomous data on attrition (Bos 2010).
STEPPS did not reduce attrition at end of treatment compared with TAU (RR 1.47, 95% CI 0.59 to 3.65; 1 trial, 79 participants; P = 0.41; Analysis 8.10).
8.10. Analysis.

Comparison 8: Systems training for emotional predictability and problem solving (STEPPS) vs TAU, Outcome 10: Secondary: attrition (dichotomous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for anger, chronic feelings of emptiness, abandonment, identity disturbance, and adverse effects.
9. Cognitive analytic therapy (CAT) versus treatment‐as‐usual (TAU)
Primary outcomes
9.1 Suicide‐related outcomes
One trial reported continuous data on suicide‐related outcomes (Gleeson 2012).
CAT did not reduce suicide‐related outcomes at end of treatment compared with TAU (MD −1.40, 95% CI −4.21 to 1.41; 1 trial, 9 participants; P = 0.33; Analysis 9.1.1).
9.1. Analysis.

Comparison 9: Cognitive analytic therapy (CAT) vs TAU, Outcome 1: Primary: suicide‐related outcomes (continuous)
CAT did not reduce suicide‐related outcomes at zero to six months follow‐up compared with TAU (MD −0.50, 95% CI −2.05 to 1.05; 1 trial, 8 participants; P = 0.53; Analysis 9.1.2).
No data were available for six to 12 months and above 12 months follow‐up.
9.2 Psychosocial functioning
One trial reported continuous data on psychosocial functioning (Gleeson 2012).
CAT improved psychosocial functioning at end of treatment compared with TAU (MD −16.40, 95% CI −31.20 to −1.60; 1 trial, 9 participants; P = 0.03; Analysis 9.2.1).
9.2. Analysis.

Comparison 9: Cognitive analytic therapy (CAT) vs TAU, Outcome 2: Primary: psychosocial functioning (continuous)
CAT improved psychosocial functioning at zero to six months follow‐up compared with TAU (MD −15.80, 95% CI −29.36 to −2.24; 1 trial, 9 participants; P = 0.02; Analysis 9.2.2).
No data were available for six to 12 months and above 12 months follow‐up.
No data were available on any time point for BPD symptom severity, and self‐harm.
Secondary outcomes
9.3 Anger
One trial reported continuous data on anger (Gleeson 2012).
CAT did not reduce anger at end of treatment compared with TAU (MD −1.90, 95% CI −7.97 to 4.17; 1 trial, 9 participants; P = 0.54; Analysis 9.3.1).
9.3. Analysis.

Comparison 9: Cognitive analytic therapy (CAT) vs TAU, Outcome 3: Secondary: anger (continuous)
CAT did not reduce anger at zero to six months follow‐up compared with TAU (MD −4.50, 95% CI −9.01 to 0.01; 1 trial, 8 participants; P = 0.05; Analysis 9.3.2).
No data were available for six to 12 months and above 12 months follow‐up.
9.4 Dissociation and psychotic‐like symptoms
One trial reported continuous data on dissociation and psychotic‐like symptoms (Gleeson 2012).
CAT did not reduce dissociation and psychotic‐like symptoms at end of treatment compared with TAU (MD −6.10, 95% CI −15.01 to 2.81; 1 trial, 9 participants; P = 0.18; Analysis 9.4.1).
9.4. Analysis.

Comparison 9: Cognitive analytic therapy (CAT) vs TAU, Outcome 4: Secondary: dissociation and psychotic‐like symptoms (continuous)
CAT did not reduce dissociation and psychotic‐like symptoms at zero to six months follow‐up compared with TAU (MD −11.70, 95% CI −24.02 to 0.62; 1 trial, 8 participants; P = 0.06; Analysis 9.4.2).
No data were available for six to 12 months and above 12 months follow‐up.
9.5 Depression
One trial reported continuous data on depression (Gleeson 2012).
CAT did not reduce depression at end of treatment compared with TAU (MD −9.70, 95% CI −20.10 to 0.70; 1 trial, 9 participants; P = 0.07; Analysis 9.5.1).
9.5. Analysis.

Comparison 9: Cognitive analytic therapy (CAT) vs TAU, Outcome 5: Secondary: depression (continuous)
CAT did not reduce depression at zero to six months follow‐up compared with TAU (MD −3.70, 95% CI −11.99 to 4.59; 1 trial, 8 participants; P = 0.38; Analysis 9.5.2).
No data were available for six to 12 months and above 12 months follow‐up.
9.6 Attrition
One trial reported dichotomous data on attrition (Gleeson 2012).
CAT did not reduce attrition at end of treatment compared with TAU (RR 1.33, 95% CI 0.43 to 4.13; 1 trial, 16 participants; P = 0.62; Analysis 9.6).
9.6. Analysis.

Comparison 9: Cognitive analytic therapy (CAT) vs TAU, Outcome 6: Secondary: attrition (dichotomous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for affective instability, chronic feelings of emptiness, impulsivity, interpersonal problems, abandonment, identity disturbance, and adverse effects.
10. Motivation feedback (MF) versus treatment‐as‐usual (TAU)
10.1 Primary outcome: psychosocial functioning
One trial reported continuous data on psychosocial functioning (Jochems 2015).
MF did not improve psychosocial functioning at end of treatment compared with TAU (MD −0.42, 95% CI −4.23 to 3.39; 1 trial, 43 participants; P = 0.83; Analysis 10.1).
10.1. Analysis.

Comparison 10: Motivation feedback (MF) vs TAU, Outcome 1: Primary: psychosocial functioning, at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for BPD symptom severity, self‐harm, and suicide‐related outcomes.
Secondary outcomes
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, depression, attrition and adverse effects
11. Psychoeducation versus treatment‐as‐usual (TAU)
Primary outcomes
No data were available on any time point for BPD symptom severity, self‐harm, suicide‐related outcomes, and psychosocial functioning.
Secondary outcomes
11.1 Depression
One trial reported continuous data on depression (Zanarini 2018).
Psychoeducation did not reduce depression at end of treatment compared with TAU (MD −7.03, 95% CI −14.35 to 0.29; 1 trial, 77 participants; P = 0.06; Analysis 11.1).
11.1. Analysis.

Comparison 11: Psychoeducation vs TAU, Outcome 1: Secondary: depression (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
11.2 Attrition
One trial reported continuous data on attrition (Zanarini 2018).
Psychoeducation did not reduce attrition at end of treatment compared with TAU (RR 0.49, 95% CI 0.04 to 5.60; 1 trial, 80 participants; P = 0.56; Analysis 11.2).
11.2. Analysis.

Comparison 11: Psychoeducation vs TAU, Outcome 2: Secondary: attrition (dichotomous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, and adverse effects
12. Transference‐focused psychotherapy (TFP) versus treatment‐as‐usual (TAU)
Primary outcomes
12.1 BPD symptom severity
One trial reported continuous data on BPD symptom severity (Doering 2010).
TFP reduced BPD symptom severity at end of treatment compared with TAU (MD −0.84, 95% CI −1.42 to −0.26; 1 trial, 104 participants; P = 0.004; Analysis 12.1).
12.1. Analysis.

Comparison 12: Transference‐focused psychotherapy (TFP) vs TAU, Outcome 1: Primary: BPD symptom severity (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
12.2 Self‐harm
One trial reported dichotomous data on self‐harm (Doering 2010).
TFP did not reduce self‐harm at end of treatment compared with TAU (RR 1.09, 95% CI 0.84 to 1.40; 1 trial, 104 participants; P = 0.52; Analysis 12.2).
12.2. Analysis.

Comparison 12: Transference‐focused psychotherapy (TFP) vs TAU, Outcome 2: Primary: self‐harm (dichotomous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
12.3 Suicide‐related outcomes
One trial reported dichotomous data on suicide‐related outcomes (Doering 2010).
TFP did not reduce suicide‐related outcomes at end of treatment compared with TAU (RR 0.65, 95% CI 0.27 to 1.54; 1 trial, 104 participants; P = 0.33; Analysis 12.3).
12.3. Analysis.

Comparison 12: Transference‐focused psychotherapy (TFP) vs TAU, Outcome 3: Primary: suicide‐related outcomes (dichotomous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
12.4 Psychosocial functioning
One trial reported continuous data on psychosocial functioning (Doering 2010).
TFP did not improve psychosocial functioning at end of treatment compared with TAU (MD −2.56, 95% CI −5.43 to 0.31; 1 trial, 104 participants; P = 0.08; Analysis 12.4).
12.4. Analysis.

Comparison 12: Transference‐focused psychotherapy (TFP) vs TAU, Outcome 4: Primary: psychosocial functioning (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
Secondary outcomes
12.5 Depression
One trial reported continuous data on depression (Doering 2010).
TFP did not reduce depression at end of treatment compared with TAU (MD 1.65, 95% CI −3.44 to 6.74; 1 trial, 104 participants; P = 0.52; Analysis 12.5).
12.5. Analysis.

Comparison 12: Transference‐focused psychotherapy (TFP) vs TAU, Outcome 5: Secondary: depression (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
12.6 Attrition
One trial reported dichotomous data on attrition (Doering 2010).
TFP reduced attrition at end of treatment compared with TAU (RR 0.57, 95% CI 0.39 to 0.85; 1 trial, 104 participants; P = 0.005; Analysis 12.6).
12.6. Analysis.

Comparison 12: Transference‐focused psychotherapy (TFP) vs TAU, Outcome 6: Secondary: attrition (dichotomous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, and adverse effects.
13. Once‐only interventions (individual setting) versus treatment‐as‐usual (TAU)
Primary outcomes
13.1 Self‐harm
One trial reported continuous data on self‐harm (Borschmann 2013).
Once‐only interventions did not reduce self‐harm at end of treatment compared with TAU (MD 0.60, 95% CI −33.88 to 35.08; 1 trial, 72 participants; P = 0.97; Analysis 13.1).
13.1. Analysis.

Comparison 13: Once‐only interventions (individual setting) vs TAU, Outcome 1: Primary: self‐harm (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
13.2 Psychosocial functioning
One trial reported continuous data on psychosocial functioning (Borschmann 2013).
Once‐only interventions did not improve psychosocial functioning at end of treatment compared with TAU (MD 0.25, 95% CI −3.66 to 4.16; 1 trial, 72 participants; P = 0.90; Analysis 13.2).
13.2. Analysis.

Comparison 13: Once‐only interventions (individual setting) vs TAU, Outcome 2: Primary: psychosocial functioning (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for BPD symptom severity, and suicide‐related outcomes.
Secondary outcomes
13.3 Depression
One trial reported continuous data on depression (Borschmann 2013).
Once‐only interventions did not improve depression at end of treatment compared with TAU (MD 0.27, 95% CI −1.72 to 2.26; 1 trial, 72 participants; P = 0.79; Analysis 13.3).
13.3. Analysis.

Comparison 13: Once‐only interventions (individual setting) vs TAU, Outcome 3: Secondary: depression (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
13.4 Attrition
One trial reported dichotomous data on attrition (Borschmann 2013).
Once‐only interventions did not reduce attrition at end of treatment compared with TAU (RR 1.37, 95% CI 0.53 to 3.52; 1 trial, 88 participants; P = 0.51; Analysis 13.4).
13.4. Analysis.

Comparison 13: Once‐only interventions (individual setting) vs TAU, Outcome 4: Secondary: attrition (dichotomous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, and adverse effects.
14. Eclectic treatments versus treatment‐as‐usual (TAU)
Primary outcomes
14.1 BPD symptom severity
Three trials reported continuous data on BPD symptom severity (Gratz 2006; Gratz 2014; Leppänen 2016).
Eclectic treatments reduced BPD symptom severity at end of treatment compared with TAU (SMD −0.90, 95% CI −1.57 to −0.23; 3 trials, 134 participants; I2 = 67%; P = 0.008; Analysis 14.1).
14.1. Analysis.

Comparison 14: Eclectic treatments vs TAU, Outcome 1: Primary: BPD symptom severity (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
14.2 Self‐harm
Two trials reported continuous data on self‐harm (Gratz 2006; Gratz 2014).
Eclectic treatments reduced self‐harm at end of treatment compared with TAU (SMD −0.84, 95% CI −1.29 to −0.39; 2 trials, 83 participants; I2 = 0%; P = 0.0003; Analysis 14.2).
14.2. Analysis.

Comparison 14: Eclectic treatments vs TAU, Outcome 2: Primary: self‐harm (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
14.3 Suicide‐related outcomes
Two trials reported continuous data on suicide‐related outcomes (Andreoli 2016; Leppänen 2016).
Eclectic treatments did not reduce suicide‐related outcomes at end of treatment compared with TAU (SMD −0.55, 95% CI −1.29 to 0.19; 2 trials, 221 participants; I2 = 78%; P = 0.14; Analysis 14.3; random‐effects model).
14.3. Analysis.

Comparison 14: Eclectic treatments vs TAU, Outcome 3: Primary: suicide‐related outcomes (continuous), at end of treatment
Eclectic treatments reduced suicide‐related outcomes at end of treatment compared with TAU (SMD −0.65, 95% CI −0.98 to − 0.32; 2 trials, 221 participants; I2 = 78%; P = 0.0001; analysis not shown; fixed‐effect model).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
14.4 Psychosocial functioning
Two trials reported continuous data on psychosocial functioning (Andreoli 2016; Gratz 2014).
Eclectic treatments improved psychosocial functioning at end of treatment compared with TAU (SMD −0.57, 95% CI −1.10 to −0.04; 2 trials, 231 participants; I2 = 63%; P = 0.03; Analysis 14.4.1).
14.4. Analysis.

Comparison 14: Eclectic treatments vs TAU, Outcome 4: Primary: psychosocial functioning (continuous)
Eclectic treatments improved psychosocial functioning at above 12 months follow‐up compared with TAU (SMD −0.64, 95% CI −1.04 to −0.24; 1 trial, 170 participants; P = 0.002; MD −7.50, 95% CI −12.30 to −2.70; 1 trial, 170 participants; P = 0.002; Analysis 14.4.2).
No data were available for zero to months and six to 12 months.
Secondary outcomes
14.5 Anger
One trial reported continuous data on anger (Leppänen 2016).
Eclectic treatments did not reduce anger at end of treatment compared with TAU (MD −0.47, 95% CI −1.26 to 0.32; 1 trial, 51 participants; P = 0.24; Analysis 14.5).
14.5. Analysis.

Comparison 14: Eclectic treatments vs TAU, Outcome 5: Secondary: anger (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
14.6 Affective instability
Three trials reported continuous data on affective instability (Gratz 2006; Gratz 2014; Leppänen 2016).
Eclectic treatments reduced affective instability at end of treatment compared with TAU (SMD −0.95, 95% CI −1.74 to −0.15; 3 trials, 134 participants; I2 = 76%; P = 0.02; Analysis 14.6).
14.6. Analysis.

Comparison 14: Eclectic treatments vs TAU, Outcome 6: Secondary: affective instability (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
14.7 Chronic feelings of emptiness
One trial reported continuous data on chronic feelings of emptiness (Leppänen 2016).
Eclectic treatments did not reduce chronic feelings of emptiness at end of treatment compared with TAU (MD −0.59, 95% CI −2.44 to 1.26; 1 trial, 51 participants; P = 0.53; Analysis 14.7).
14.7. Analysis.

Comparison 14: Eclectic treatments vs TAU, Outcome 7: Secondary: chronic feelings of emptiness (continuous), at end of treatment
No data were available for zero to months, six to 12 months and above 12 months follow‐up.
14.8 Impulsivity
Three trials reported continuous data on impulsivity (Gratz 2006; Gratz 2014; Leppänen 2016).
Eclectic treatments reduced impulsivity at end of treatment compared with TAU (SMD −0.76, 95% CI −1.30 to −0.22; 3 trials, 134 participants; I2 = 52%; P = 0.006; Analysis 14.8).
14.8. Analysis.

Comparison 14: Eclectic treatments vs TAU, Outcome 8: Secondary: impulsivity (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
14.9 Interpersonal problems
Two trials reported continuous data on interpersonal problems (Gratz 2014; Leppänen 2016).
Eclectic treatments reduced interpersonal problems at end of treatment compared with TAU (SMD −0.62, 95% CI −1.09 to −0.15; 2 trials, 112 participants; I2 = 32%; P = 0.01; Analysis 14.9).
14.9. Analysis.

Comparison 14: Eclectic treatments vs TAU, Outcome 9: Secondary: interpersonal problems (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
14.10 Abandonment
One trial reported continuous data on abandonment (Leppänen 2016).
Eclectic treatments did not reduce abandonment at end of treatment compared with TAU (MD −0.46, 95% CI −1.39 to 0.47; 1 trial, 51 participants; P = 0.33; Analysis 14.10).
14.10. Analysis.

Comparison 14: Eclectic treatments vs TAU, Outcome 10: Secondary: abandonment (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
14.11 Identity disturbance
One trial reported continuous data on identity disturbance (Leppänen 2016).
Eclectic treatments did not reduce identity disturbance at end of treatment compared with TAU (MD −0.85, 95% CI −1.92 to 0.22; 1 trial, 51 participants; P = 0.12; Analysis 14.11).
14.11. Analysis.

Comparison 14: Eclectic treatments vs TAU, Outcome 11: Secondary: identity disturbance (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
14.14 Depression
Four trials reported continuous data on depression (Andreoli 2016; Gratz 2006; Gratz 2014; Leppänen 2016).
Eclectic treatments reduced depression at end of treatment compared with TAU (SMD −0.82, 95% CI −1.38 to −0.26; 4 trials, 304 participants; I2 = 73%; P = 0.004; Analysis 14.12).
14.12. Analysis.

Comparison 14: Eclectic treatments vs TAU, Outcome 12: Secondary: depression (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
14.13 Attrition
Four trials reported dichotomous data on attrition (Andreoli 2016; Gratz 2006; Gratz 2014; Leppänen 2016).
Eclectic treatments did not reduce attrition at end of treatment compared with TAU (RR 1.10, 95% CI 0.91 to 1.33; 4 trials, 326 participants; I2 = 25%; P = 0.31; Analysis 14.13).
14.13. Analysis.

Comparison 14: Eclectic treatments vs TAU, Outcome 13: Secondary: attrition (dichotomuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
14.14 Adverse effects
One trial reported dichotomous data on adverse effects (Andreoli 2016).
Eclectic treatments did not reduce adverse effects at end of treatment compared with TAU (RR 0.66, 95% CI 0.03 to 15.81; 1 trial, 170 participants; P = 0.80; Analysis 14.14).
14.14. Analysis.

Comparison 14: Eclectic treatments vs TAU, Outcome 14: Secondary: adverse effects (dichotomous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for dissociation and psychotic‐like symptoms.
15. Psychotherapy versus waiting list or no treatment
Primary outcomes
15.1 BPD symptom severity
Three trials reported continuous data on BPD symptom severity (Bellino 2010; Bohus 2013; McMain 2017).
Psychotherapy reduced BPD symptom severity at end of treatment compared with waiting list (SMD −0.49, 95% CI −0.93 to −0.5; 3 trials, 161 participants; I2 = 44%; P = 0.03; Analysis 15.1; low‐quality evidence, Table 2).
15.1. Analysis.

Comparison 15: Psychotherapy vs waiting list or no treatment, Outcome 1: Primary: BPD symptom severity (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
15.2 Self‐harm
Two trials reported continuous data on self‐harm (Bellino 2010; McMain 2017).
Psychotherapy did not reduce self‐harm at end of treatment compared with waiting list (SMD −0.17, 95% CI −0.52 to 0.18; 2 trials, 128 participants; I2 = 0%; P = 0.34; Analysis 15.2; low‐quality evidence, Table 2).
15.2. Analysis.

Comparison 15: Psychotherapy vs waiting list or no treatment, Outcome 2: Primary: self‐harm (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
15.3 Suicide‐related outcomes
Two trials reported continuous data on suicide‐related outcomes (McMain 2017; Mohamadizadeh 2017).
Psychotherapy did not reduce suicide‐related outcomes at end of treatment compared with waiting list or no treatment (SMD −5.62, 95% CI −16.39 to 5.16; 2 trials, 108 participants; I2 = 97%; P = 0.31; Analysis 15.3; very low‐quality evidence, Table 2).
15.3. Analysis.

Comparison 15: Psychotherapy vs waiting list or no treatment, Outcome 3: Primary: suicide‐related outcomes (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
15.4 Psychosocial functioning
Five trials reported continuous data on psychosocial functioning (Bellino 2006; Bellino 2007; Bohus 2013; Haeyen 2018; McMain 2017).
Psychotherapy improved psychosocial functioning at end of treatment compared with waiting list (SMD −0.56, 95% CI −1.01 to −0.11; 5 trials, 219 participants; I2 = 59%; P = 0.01; Analysis 15.4.1; low‐quality evidence, Table 2).
15.4. Analysis.

Comparison 15: Psychotherapy vs waiting list or no treatment, Outcome 4: Primary: psychosocial functioning (continuous)
Psychotherapy improved psychosocial functioning at zero to six months follow‐up compared with waiting list (SMD −0.89, 95% CI −1.65 to −0.13; 1 trial, 30 participants; P = 0.02; MD −12.39, 95% CI −22.00 to −2.78; 1 trial, 30 participants; P = 0.01; Analysis 15.4.2).
Psychotherapy improved psychosocial functioning at six to 12 months follow‐up compared with waiting list (SMD −1.04, 95% CI −1.81 to −0.27; 1 trial, 30 participants; P = 0.008: MD −13.59, 95% CI −22.65 to −4.53; 1 trial, 30 participants; P = 0.003; Analysis 15.4.3).
Psychotherapy did not improve psychosocial functioning at above 12 months follow‐up compared with waiting list (SMD −0.38, 95% CI −1.14 to 0.37; 1 trial, 28 participants; P = 0.32; MD −14.68, 95% CI −46.63 to 17.27; 1 trial, 28 participants; P = 0.37; Analysis 15.4.4).
Secondary outcomes
15.5 Anger
Two trials reported continuous data on anger (Bellino 2010; McMain 2017).
Psychotherapy did not reduce anger at end of treatment compared with waiting list (SMD −0.58, 95% CI −1.70 to 0.55; 2 trials, 128 participants; I2 = 89%; P = 0.32; Analysis 15.5, random‐effects model).
15.5. Analysis.

Comparison 15: Psychotherapy vs waiting list or no treatment, Outcome 5: Secondary: anger (continuous), at end of treatment
Psychotherapy reduced anger at end of treatment compared with waiting list (SMD −0.70, 95% CI −1.06 to −0.33; 2 trials, 128 participants; I2 = 89%; P = 0.0002; analysis not shown; fixed‐effect model).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
15.6 Affective instability
Two trials reported continuous data on affective instability (Bellino 2010; McMain 2017).
Psychotherapy reduced affective instability at end of treatment compared with waiting list (SMD −0.99, 95% CI −1.36 to −0.62; 2 trials, 128 participants; I2 = 0%; P < 0.001; Analysis 15.6.1).
15.6. Analysis.

Comparison 15: Psychotherapy vs waiting list or no treatment, Outcome 6: Secondary: affective instability (continuous)
Psychotherapy did not reduce affective instability at zero to six months follow‐up compared with waiting list (SMD −0.47, 95% CI −1.20 to 0.26; 1 trial, 30 participants; P = 0.21; MD −0.46, 95% CI −1.15 to 0.23; 1 trial, 30 participants; P = 0.19; Analysis 15.6.2).
Psychotherapy did not reduce affective instability at six to 12 months follow‐up compared with waiting list (SMD −0.38, 95% CI −1.10 to 0.34; 1 trial, 30 participants; P = 0.30; MD −0.39, 95% CI −1.12 to 0.34; 1 trial, 30 participants; P = 0.29; Analysis 15.6.3).
Psychotherapy did not reduce affective instability at above 12 months follow‐up compared with waiting list (SMD −0.28, 95% CI −1.01 to 0.44; 1 trial, 30 participants; P = 0.44; MD −0.34, 95% CI −1.17 to 0.49; 1 trial, 30 participants; P = 0.42; Analysis 15.6.4).
15.7 Chronic feelings of emptiness
One trial reported continuous data on chronic feelings of emptiness (Bellino 2010).
Psychotherapy did not reduce chronic feelings of emptiness at end of treatment compared with waiting list (MD 0.04, 95% CI −0.21 to 0.29; 1 trial, 44 participants; P = 0.75; Analysis 15.7).
15.7. Analysis.

Comparison 15: Psychotherapy vs waiting list or no treatment, Outcome 7: Secondary: chronic feelings of emptiness (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
15.8 Impulsivity
Three trials reported continuous data on impulsivity (Bellino 2010; McMain 2017; Zanarini 2008).
Psychotherapy reduced impulsivity at end of treatment compared with waiting list (SMD −0.52, 95% CI −0.82 to ‐0.22; 3 trials, 178 participants; I2 = 0%; P = 0.0007; Analysis 15.8.1).
15.8. Analysis.

Comparison 15: Psychotherapy vs waiting list or no treatment, Outcome 8: Secondary: impulsivity (continuous)
Psychotherapy reduced impulsivity at zero to six months follow‐up compared with waiting list (SMD −0.90, 95% CI −1.66 to −0.14; 1 trial, 30 participants; P = 0.02; MD −1.10, 95% CI −1.95 to −0.25; 1 trial, 30 participants; P = 0.01; Analysis 15.8.2).
Psychotherapy reduced impulsivity at six to 12 months follow‐up compared with waiting list (SMD −0.79, 95% CI −1.54 to −0.04; 1 trial, 30 participants; P = 0.04; MD −1.10, 95% CI −2.06 to −0.14; 1 trial, 30 participants; P = 0.02; Analysis 15.8.3).
Psychotherapy reduced impulsivity at above 12 months follow‐up compared with waiting list (SMD −0.75, 95% CI −1.50 to −0.01; 1 trial, 30 participants; P = 0.05; MD −1.09, 95% CI −2.09 to ‐0.09; 1 trial, 30 participants; P = 0.03; Analysis 15.8.4).
15.9 Interpersonal problems
Three trials reported continuous data on interpersonal problems (Bellino 2010; Haeyen 2018; Zanarini 2008).
Psychotherapy reduced interpersonal problems at end of treatment compared with waiting list (SMD −0.85, 95% CI −1.23 to −0.47; 3 trials, 120 participants; I2 = 0%; P < 0.001; Analysis 15.9.1).
15.9. Analysis.

Comparison 15: Psychotherapy vs waiting list or no treatment, Outcome 9: Secondary: interpersonal problems (continuous)
Psychotherapy reduced interpersonal problems at zero to six months follow‐up compared with waiting list (SMD −1.40, 95% CI −2.21 to −0.59; 1 trial, 30 participants; P = 0.0007; MD −2.00, 95% CI −2.98 to −1.02; 1 trial, 30 participants; P < 0.001; Analysis 15.9.2).
Psychotherapy reduced interpersonal problems at six to 12 months follow‐up compared with waiting list (SMD −0.90, 95% CI −1.66 to −0.14; 1 trial, 30 participants; P = 0.02; MD −1.56, 95% CI −2.74 to −0.38; 1 trial, 30 participants; P = 0.009; Analysis 15.9.3).
Psychotherapy reduced interpersonal problems at above 12 months follow‐up compared with waiting list (SMD −0.86, 95% CI −1.62 to −0.11; 1 trial, 30 participants; P = 0.02; MD −1.48, 95% CI −2.63 to −0.33; 1 trial, 30 participants; P = 0.01; Analysis 15.9.4).
15.10 Abandonment
One trial reported continuous data on abandonment (Bellino 2010).
Psychotherapy did not reduce abandonment at end of treatment compared with waiting list (MD 0.01, 95% CI −0.90 to 0.92; 1 trial, 44 participants; P = 0.98; Analysis 15.10).
15.10. Analysis.

Comparison 15: Psychotherapy vs waiting list or no treatment, Outcome 10: Secondary: abandonment (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
15.11 Identity disturbance
One trial reported continuous data on identity disturbance (Bellino 2010).
Psychotherapy did not reduce identity disturbance at end of treatment compared with waiting list (MD −0.03, 95% CI −0.56 to 0.50; 1 trial, 44 participants; P = 0.91; Analysis 15.11).
15.11. Analysis.

Comparison 15: Psychotherapy vs waiting list or no treatment, Outcome 11: Secondary: identity disturbance (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
15.12 Dissociation and psychotic‐like
Two trials reported continuous data on dissociation and psychotic‐like symptoms (Bellino 2010; Bohus 2013).
Psychotherapy did not reduce dissociation and psychotic‐like symptoms at end of treatment compared with waiting list (SMD −0.13, 95% CI −0.65 to 0.39; 2 trials, 77 participants; I2 = 24%; P = 0.62; Analysis 15.12).
15.12. Analysis.

Comparison 15: Psychotherapy vs waiting list or no treatment, Outcome 12: Secondary: dissociation and psychotic‐like symptoms (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
15.13 Depression
Six trials reported continuous data on depression (Bellino 2006; Bellino 2007; Bohus 2013; McMain 2017; Mohamadizadeh 2017; Smith 2012).
Psychotherapy reduced depression at end of treatment compared with waiting list or no treatment (SMD −1.28, 95% CI −2.21 to −0.34; 6 trials, 239 participants; I2 = 89%; P = 0.007; Analysis 15.13; low‐quality evidence, Table 2).
15.13. Analysis.

Comparison 15: Psychotherapy vs waiting list or no treatment, Outcome 13: Secondary: depression (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
15.14 Attrition
Three trials reported dichotomous data on attrition (Bellino 2006; Bellino 2010; Zanarini 2008).
Psychotherapy did not reduce attrition at end of treatment compared with waiting list (RR 0.55, 95% CI 0.20 to 1.50; 3 trials, 144 participants; I2 = 0%; P = 0.24; Analysis 15.14; very low‐quality evidence, Table 2).
15.14. Analysis.

Comparison 15: Psychotherapy vs waiting list or no treatment, Outcome 14: Secondary: attrition (dichotomous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for adverse effects.
16. Dialectical behaviour therapy (DBT) versus waiting list or no treatment
Primary outcomes
16.1 BPD symptom severity
Two trials reported continuous data on BPD symptom severity (Bohus 2013; McMain 2017).
DBT reduced BPD symptom severity at end of treatment compared with waiting list (SMD −0.71, 95% CI −1.08 to −0.33; 2 trials, 117 participants; I2 = 0%; P = 0.0002; Analysis 16.1).
16.1. Analysis.

Comparison 16: Dialectical behavior therapy (DBT) vs waiting list or no treatment, Outcome 1: Primary: BPD symptom severity (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
16.2 Self‐harm
One trial reported continuous data on self‐harm (McMain 2017).
DBT did not reduce self‐harm at end of treatment compared with waiting list (MD −1.45, 95% CI −3.76 to 0.86; 1 trial, 84 participants; P = 0.22; Analysis 16.2).
16.2. Analysis.

Comparison 16: Dialectical behavior therapy (DBT) vs waiting list or no treatment, Outcome 2: Primary: self‐harm (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
16.3 Suicide‐related outcomes
Two trials reported continuous data on suicide‐related outcomes (McMain 2017; Mohamadizadeh 2017).
Psychotherapy did not reduce suicide‐related outcomes at end of treatment compared with waiting list or no treatment (SMD −5.62, 95% CI −16.39 to 5.16; 2 trials, 108 participants; I2 = 97%; P = 0.30; Analysis 16.3).
16.3. Analysis.

Comparison 16: Dialectical behavior therapy (DBT) vs waiting list or no treatment, Outcome 3: Primary: suicide‐related outcomes (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
16.4 Psychosocial functioning
Two trials reported continuous data on psychosocial functioning (Bohus 2013; McMain 2017).
DBT reduced psychosocial functioning at end of treatment compared with waiting list (SMD −0.73, 95% CI −1.11 to −0.36; 2 trials, 117 participants; I2 = 0%; P = 0.0001; Analysis 16.4).
16.4. Analysis.

Comparison 16: Dialectical behavior therapy (DBT) vs waiting list or no treatment, Outcome 4: Primary: psychosocial functioning (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
Secondary outcomes
16.5 Anger
One trial reported continuous data on anger (McMain 2017).
DBT reduced anger at end of treatment compared with waiting list (MD −11.45, 95% CI −15.73 to −7.17; 1 trial, 84 participants; P < 0.001; Analysis 16.5).
16.5. Analysis.

Comparison 16: Dialectical behavior therapy (DBT) vs waiting list or no treatment, Outcome 5: Secondary: anger (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
16.6 Affective instability
One trial reported continuous data on affective instability (McMain 2017).
DBT reduced affective instability at end of treatment compared with waiting list (MD −20.15, 95% CI −28.49 to −11.81; 1 trial, 84 participants; P < 0.001; Analysis 16.6).
16.6. Analysis.

Comparison 16: Dialectical behavior therapy (DBT) vs waiting list or no treatment, Outcome 6: Secondary: affective instability (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
16.7 Impulsivity
One trial reported continuous data on impulsivity (McMain 2017).
DBT did not reduce impulsivity at end of treatment compared with waiting list (MD −3.41, 95% CI −7.32 to 0.50; 1 trial, 84 participants; P = 0.09; Analysis 16.7).
16.7. Analysis.

Comparison 16: Dialectical behavior therapy (DBT) vs waiting list or no treatment, Outcome 7: Secondary: impulsivity (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
16.8 Dissociation and psychotic‐like symptoms
One trial reported continuous data on dissociation and psychotic‐like symptoms (Bohus 2013).
DBT did not reduce dissociation and psychotic‐like symptoms at end of treatment compared with waiting list (MD −6.45, 95% CI −16.51 to 3.61; 1 trial, 33 participants; P = 0.21; Analysis 16.8).
16.8. Analysis.

Comparison 16: Dialectical behavior therapy (DBT) vs waiting list or no treatment, Outcome 8: Secondary: dissociation and psychotic‐like symptoms (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
16.9 Depression
Three trials reported continuous data on depression (Bohus 2013; McMain 2017; Mohamadizadeh 2017).
DBT reduced depression at end of treatment compared with waiting list or no treatment (SMD −3.20, 95% CI −5.57 to −0.83; 3 trials, 141 participants; P = 0.008; Analysis 16.9).
16.9. Analysis.

Comparison 16: Dialectical behavior therapy (DBT) vs waiting list or no treatment, Outcome 9: Secondary: depression (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for chronic feelings of emptiness, interpersonal problems, abandonment, identity disturbance, attrition and adverse effects.
16.10. DBT couple therapy (CDBT) versus waiting list (generic inverse variance)
Primary outcomes
BPD symptom severity
One trial reported continuous data on BPD symptom severity (Kamalabadi 2012).
End of treatment: CDBT reduced BPD symptom severity at end of treatment compared with waiting list (MD −27.15, 95% CI −31.59 to −22.71; 1 trial, 30 participants; P < 0.001; Analysis 16.10.1).
16.10. Analysis.

Comparison 16: Dialectical behavior therapy (DBT) vs waiting list or no treatment, Outcome 10: DBT‐couple therapy (CDBT) vs waiting list (generic inverse variance)
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
Suicide‐related outcomes
One trial reported continuous data on suicide‐related outcomes (Kamalabadi 2012).
CDBT reduced suicide‐related outcomes at end of treatment compared with waiting list (MD −0.94, 95% CI −1.24 to −0.64; 1 trial, 30 participants; P < 0.001; Analysis 16.10.2).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
Psychosocial functioning
One trial reported continuous data on psychosocial functioning (Kamalabadi 2012).
CDBT improved psychosocial functioning at end of treatment compared with waiting list (MD −10.70, 95% CI −12.31 to −9.09; 1 trial, 30 participants; P < 0.001; Analysis 16.10.3).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for self‐harm.
Secondary outcomes
Anger
One trial reported continuous data on anger (Kamalabadi 2012).
CDBT reduced anger at end of treatment compared with waiting list (MD −1.42, 95% CI −1.72 to −1.12; 1 trial, 30 participants; P < 0.001; Analysis 16.10.4).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
Affective instability
One trial reported continuous data on affective instability (Kamalabadi 2012).
CDBT reduced affective instability at end of treatment compared with waiting list (MD −4.01, 95% CI −5.44 to −2.58; 1 trial, 30 participants; P < 0.001; Analysis 16.10.5).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
Chronic feelings of emptiness
One trial reported continuous data on chronic feelings of emptiness (Kamalabadi 2012).
CDBT reduced chronic feelings of emptiness at end of treatment compared with waiting list (MD −3.54, 95% CI −4.81 to −2.27; 1 trial, 30 participants; P < 0.001; Analysis 16.10.6).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
Impulsivity
One trial reported continuous data on impulsivity (Kamalabadi 2012).
CDBT reduced impulsivity at end of treatment compared with waiting list (MD −0.51, 95% CI −0.72 to −0.30; 1 trial, 30 participants; P < 0.001; Analysis 16.10.7).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
Interpersonal problems
One trial reported continuous data on interpersonal problems (Kamalabadi 2012).
CDBT reduced interpersonal problems at end of treatment compared with waiting list (MD −1.98, 95% CI −2.47 to −1.49; 1 trial, 30 participants;, P < 0.001; Analysis 16.10.8).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
Abandonment
One trial reported continuous data on abandonment (Kamalabadi 2012).
CDBT reduced abandonment at end of treatment compared with waiting list (MD −0.86, 95% CI −1.09 to ‐0.63; 1 trial, 30 participants; P < 0.001; Analysis 16.10.9).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
Identity disturbance
One trial reported continuous data on identity disturbance (Kamalabadi 2012).
CDBT reduced identity disturbance at end of treatment compared with waiting list (MD −2.44, 95% CI −2.77 to −2.11; 1 trial, 30 participants; P < 0.001; Analysis 16.10.10).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
Dissociation and psychotic‐like symptoms
One trial reported continuous data on dissociation and psychotic‐like symptoms (Kamalabadi 2012).
CDBT reduced dissociation or psychotic‐like symptoms at end of treatment compared with waiting list (MD −1.92, 95% CI −2.46 to −1.38; 1 trial, 30 participants; P < 0.001; Analysis 16.10.11).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
Depression
One trial reported continuous data on depression (Kamalabadi 2012).
CDBT reduced depression at end of treatment compared with waiting list (MD −10.43, 95% CI −11.86 to −9.00; 1 trial, number of participants = 30, P < 0.001; Analysis 16.10.12).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for attrition and adverse effects.
17. Schema‐focused therapy (SFT) versus no treatment
17.1 Primary outcome: suicide‐related outcomes
One trial reported continuous data on suicide‐related outcomes (Mohamadizadeh 2017).
SFT reduced suicide‐related outcomes at end of treatment compared with no treatment (MD −16.67, 95% CI −17.70 to −15.64; 1 trial, 24 participants; P < 0.001; Analysis 17.1).
17.1. Analysis.

Comparison 17: Schema‐focused therapy (SFT) vs no treatment, Outcome 1: Primary: suicide‐related outcomes (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for BPD symptom severity, self‐harm, and psychosocial functioning.
17.2 Secondary outcome: depression
One trial reported continuous data on depression (Mohamadizadeh 2017).
SFT reduced depression at end of treatment compared with no treatment (MD −33.92, 95% CI −35.40 to −32.44; 1 trial, 24 participants; P < 0.001; Analysis 17.2).
17.2. Analysis.

Comparison 17: Schema‐focused therapy (SFT) vs no treatment, Outcome 2: Secondary: depression (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, attrition and adverse effects.
18. Interpersonal psychotherapy (IPT) versus waiting list
Primary outcomes
18.1 BPD symptom severity
One trial reported continuous data on BPD symptom severity (Bellino 2010).
IPT did not reduce BPD symptom severity at end of treatment compared with waiting list (MD −0.19, 95% CI −3.71 to 3.33; 1 trial, 44 participants; P = 0.92; Analysis 18.1).
18.1. Analysis.

Comparison 18: Interpersonal psychotherapy (IPT) vs waiting list, Outcome 1: Primary: BPD symptom severity (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
18.2 Self‐harm
One trial reported continuous data on self‐harm (Bellino 2010).
IPT did not reduce self‐harm at end of treatment compared with waiting list (MD 0.03, 95% CI −1.09 to 1.15; 1 trial, 44 participants; P = 0.96; Analysis 18.2).
18.2. Analysis.

Comparison 18: Interpersonal psychotherapy (IPT) vs waiting list, Outcome 2: Primary: self‐harm (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
18.3 Psychosocial functioning
Two trials reported continuous data on psychosocial functioning (Bellino 2006; Bellino 2010).
IPT did not improve psychosocial functioning at end of treatment compared with waiting list (SMD −0.03, 95% CI −0.48 to 0.42; 2 trials, 76 participants; I2 = 0%; P = 0.91; Analysis 18.3.1).
18.3. Analysis.

Comparison 18: Interpersonal psychotherapy (IPT) vs waiting list, Outcome 3: Primary: psychosocial functioning (continuous)
IPT improved psychosocial functioning at zero to six months follow‐up compared with waiting list (SMD −0.89, 95% CI −1.65 to −0.13; 1 trial, 30 participants; P = 0.02; MD −12.39, 95% CI −22.00 to −2.78; 1 trial, 30 participants; P = 0.01; Analysis 18.3.2).
IPT improved psychosocial functioning at six to 12 months follow‐up compared with waiting list (SMD −1.04, 95% CI −1.81 to −0.27; 1 trial, 30 participants; P = 0.008; MD −13.59, 95% CI −22.65 to −4.53; 1 trial, 30 participants; P = 0.003; Analysis 18.3.3).
IPT did not improve psychosocial functioning at above 12 months follow‐up compared with waiting list (SMD −0.38, 95% CI −1.14 to 0.37; 1 trial, 30 participants; P = 0.32; MD −14.68, 95% CI −46.63 to 17.27; 1 trial, 30 participants; P = 0.37; Analysis 18.3.4).
No data were available on any time point for suicide‐related outcomes.
Secondary outcomes
18.4 Anger
One trial reported continuous data on anger (Bellino 2010).
IPT did not reduce anger at end of treatment compared with waiting list (MD 0.01, 95% CI −0.40 to 0.42; 1 trial, 44 participants; P = 0.96; Analysis 18.4).
18.4. Analysis.

Comparison 18: Interpersonal psychotherapy (IPT) vs waiting list, Outcome 4: Secondary: anger (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
18.5 Affective instability
One trial reported continuous data on affective instability (Bellino 2010).
IPT reduced affective instability at end of treatment compared with waiting list (MD −1.02, 95% CI −1.66 to −0.38; 1 trial, 44 participants; P = 0.002; Analysis 18.5.1).
18.5. Analysis.

Comparison 18: Interpersonal psychotherapy (IPT) vs waiting list, Outcome 5: Secondary: affective instability (continuous)
IPT did not reduce affective instability at zero to six months follow‐up compared with waiting list (MD −0.46, 95% CI −1.15 to 0.23; 1 trial, 30 participants; P = 0.19; Analysis 18.5.2).
IPT did not reduce affective instability at six to 12 months follow‐up compared with waiting list (MD −0.39, 95% CI −1.12 to 0.34; 1 trial, 30 participants; P = 0.29; Analysis 18.5.3).
IPT did not reduce affective instability at above 12 months follow‐up compared with waiting list (MD −0.34, 95% CI −1.17 to 0.49; 1 trial, 30 participants; P = 0.42; Analysis 18.5.4).
18.6 Chronic feelings of emptiness
One trial reported continuous data on chronic feelings of emptiness (Bellino 2010).
IPT did not reduce chronic feelings of emptiness at end of treatment compared with waiting list (MD 0.04, 95% CI −0.21 to 0.29; 1 trial, 44 participants; P = 0.75; Analysis 18.6).
18.6. Analysis.

Comparison 18: Interpersonal psychotherapy (IPT) vs waiting list, Outcome 6: Secondary: chronic feelings of emptiness (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
18.7 Impulsivity
One trial reported continuous data on impulsivity (Bellino 2010).
IPT reduced impulsivity at end of treatment compared with waiting list (MD −1.03, 95% CI −1.69 to −0.37; 1 trial, 44 participants; P = 0.002; Analysis 18.7.1).
18.7. Analysis.

Comparison 18: Interpersonal psychotherapy (IPT) vs waiting list, Outcome 7: Secondary: impulsivity (continuous)
IPT reduced impulsivity at zero to six months follow‐up compared with waiting list (MD −1.10, 95% CI −1.95 to −0.25; 1 trial, 30 participants; P = 0.01; Analysis 18.7.2).
IPT reduced impulsivity at six to 12 months compared with waiting list (MD −1.10, 95% CI −2.06 to −0.14; 1 trial, 30 participants; P = 0.02; Analysis 18.7.3).
IPT reduced impulsivity at above 12 months follow‐up compared with waiting list (MD −1.09, 95% CI −2.09 to −0.09; 1 trial, 30 participants; P = 0.03; Analysis 18.7.4).
18.8 Interpersonal problems
One trial reported continuous data on interpersonal problems (Bellino 2010).
IPT reduced interpersonal problems at end of treatment compared with waiting list (MD −1.14, 95% CI −1.94 to −0.34; 1 trial, 44 participants; P = 0.005; Analysis 18.8.1).
18.8. Analysis.

Comparison 18: Interpersonal psychotherapy (IPT) vs waiting list, Outcome 8: Secondary: interpersonal problems (continuous)
IPT reduced interpersonal problems at zero to six months follow‐up compared with waiting list (MD −2.00, 95% CI −2.98 to −1.02; 1 trial, 30 participants; P < 0.001; Analysis 18.8.2).
IPT reduced interpersonal problems at six to 12 months follow‐up compared with waiting list (MD −1.56, 95% CI −2.74 to −0.38; 1 trial, 30 participants; P = 0.009; Analysis 18.8.3).
IPT reduced interpersonal problems above 12 months follow‐up compared with waiting list (MD −1.48, 95% CI −2.63 to −0.33; 1 trial, 30 participants; P = 0.01; Analysis 18.8.4).
18.9 Abandonment
One trial reported continuous data on abandonment (Bellino 2010).
IPT did not reduce abandonment at end of treatment compared with waiting list (MD 0.01, 95% CI −0.90 to 0.92; 1 trial, 44 participants; P = 0.98; Analysis 18.9).
18.9. Analysis.

Comparison 18: Interpersonal psychotherapy (IPT) vs waiting list, Outcome 9: Secondary: abandonment (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
18.10 Identity disturbance
One trial reported continuous data on identity disturbance (Bellino 2010).
IPT did not reduce identity disturbance at end of treatment compared with waiting list (MD −0.03, 95% CI −0.56 to 0.50; 1 trial, 44 participants; P = 0.91; Analysis 18.10).
18.10. Analysis.

Comparison 18: Interpersonal psychotherapy (IPT) vs waiting list, Outcome 10: Secondary: identity disturbance (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
18.11 Dissociation and psychotic‐like symptoms
One trial reported continuous data on dissociation and psychotic‐like symptoms (Bellino 2010).
IPT did not reduce dissociation and psychotic‐like symptoms at end of treatment compared with waiting list (MD 0.23, 95% CI −1.06 to 1.52; 1 trial, 44 participants; P = 0.73; Analysis 18.11).
18.11. Analysis.

Comparison 18: Interpersonal psychotherapy (IPT) vs waiting list, Outcome 11: Secondary: dissociation and psychotic‐like symptoms (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
18.12 Depression
Three trials reported continuous data on depression (Bellino 2006; Bellino 2010; Smith 2012).
IPT did not reduce depression at end of treatment compared with waiting list (SMD −0.52, 95% CI −1.11 to 0.06; 3 trials, 98 participants; P = 0.08; Analysis 18.12; random‐effects model).
18.12. Analysis.

Comparison 18: Interpersonal psychotherapy (IPT) vs waiting list, Outcome 12: Secondary outcome: depression (continuous), at end of treatment
IPT reduced depression at end of treatment compared with waiting list (SMD −0.46, 95% CI − 0.88 to −0.05; 3 trials, 98 participants; P = 0.03; analysis not shown; fixed‐effect model).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
18.13 Attrition
Two trials reported dichotomous data on attrition (Bellino 2006; Bellino 2010).
IPT did not reduce attrition at end of treatment compared with waiting list (RR 0.55, 95% CI 0.20 to 1.50; 2 trials, 94 participants; P = 0.24; Analysis 18.13).
18.13. Analysis.

Comparison 18: Interpersonal psychotherapy (IPT) vs waiting list, Outcome 13: Secondary outcome: attrition (dichotomous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for adverse effects.
19. Once‐only interventions (individual setting) versus waiting list
Primary outcomes
No data were available on any time point for BPD symptom severity, self‐harm, suicide‐related outcomes and psychosocial functioning.
Secondary outcomes
19.1 Impulsivity
One trial reported continuous data on impulsivity (Zanarini 2008).
Once‐only interventions did not reduce impulsivity at end of treatment compared with waiting list (MD −0.48, 95% CI −1.07 to 0.11; 1 trial, 50 participants; P = 0.11; Analysis 19.1).
19.1. Analysis.

Comparison 19: Once‐only interventions (individual setting) vs waiting list, Outcome 1: Secondary: impulsivity (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
19.2 Interpersonal problems
One trial reported continuous data on interpersonal problems (Zanarini 2008).
Once‐only interventions reduced interpersonal problems at end of treatment compared with waiting list (MD −0.88, 95% CI −1.59 to −0.17; 1 trial, 50 participants; P = 0.02; Analysis 19.2).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
19.3 Attrition
One trial reported dichotomous data on attrition (Zanarini 2008).
There was no attrition at end of treatment in any of the groups (effect not estimable; one trial, 50 participants; Analysis 19.3).
19.3. Analysis.

Comparison 19: Once‐only interventions (individual setting) vs waiting list, Outcome 3: Secondary: attrition (dichotomous), at end of treatment
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, depression and adverse effects.
20. Eclectic treatments versus waiting list
20.1 Primary outcome: psychosocial functioning
One trial reported continuous data on psychosocial functioning (Haeyen 2018).
Eclectic treatments improved psychosocial functioning at end of treatment compared with waiting list (MD −18.64, 95% CI −30.06 to −7.22; 1 trial, 16 participants; P = 0.001; Analysis 20.1).
20.1. Analysis.

Comparison 20: Eclectic treatments vs waiting list, Outcome 1: Primary outcome: psychosocial functioning (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for BPD symptom severity, self‐harm, and suicide‐related outcomes.
20.2 Secondary outcome: interpersonal problems
One trial reported continuous data on interpersonal problems (Haeyen 2018).
Eclectic treatments reduced interpersonal problems at end of treatment compared with waiting list (MD −4.89, 95% CI −8.49 to −1.29; 1 trial, 16 participants; P = 0.008; Analysis 20.2).
20.2. Analysis.

Comparison 20: Eclectic treatments vs waiting list, Outcome 2: Secondary outcome: interpersonal problems (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, depression, attrition and adverse effects.
21. Dialectical behaviour therapy (DBT) and related treatments versus active treatment
21.1 Standard DBT (DBT) versus client‐centred therapy (CCT) (continuous)
Primary outcomes
Self‐harm
One trial reported continuous data on self‐harm (Turner 2000).
DBT reduced self‐harm at end of treatment compared with CCT (MD −2.75, 95% CI −4.42 to −1.08; 1 trial, 24 participants; P = 0.001; Analysis 21.1.1).
21.1. Analysis.

Comparison 21: Dialectical behavior therapy (DBT) and related treatments vs active treatment, Outcome 1: Standard DBT (DBT) vs client‐centred therapy (CCT) (continuous)
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
Suicide‐related outcomes
One trial reported continuous data on suicide‐related outcomes (Turner 2000).
DBT reduced suicide‐related outcomes at end of treatment compared with CCT (MD −7.75, 95% CI −14.66 to −0.84; 1 trial, 24 participants; P = 0.03; Analysis 21.1.2).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for BPD symptom severity and psychosocial functioning.
Secondary outcomes
Anger
One trial reported continuous data on anger (Turner 2000).
DBT reduced anger at end of treatment compared with CCT (MD −1.00, 95% CI −1.98 to −0.02; 1 trial, 24 participants; P = 0.05, Analysis 21.1.3).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
Impulsivity
One trial reported continuous data on impulsivity (Turner 2000).
DBT reduced impulsivity at end of treatment compared with CCT (MD −1.50, 95% CI −2.60 to −0.40; 1 trial, 24 participants; P = 0.008; Analysis 21.1.4).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
Dissociation and psychotic‐like symptoms
One trial reported continuous data on dissociation and psychotic‐like symptoms (Turner 2000).
DBT reduced dissociation and psychotic‐like symptoms at end of treatment compared with CCT (MD −7.16, 95% CI −12.15 to −2.17; 1 trial, 24 participants; P = 0.005; Analysis 21.1.5).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
Depression
One trial reported continuous data on depression (Turner 2000).
DBT reduced depression at end of treatment compared with CCT (MD −9.16, 95% CI −14.79 to −3.53; 1 trial, 24 participants; P = 0.001; Analysis 21.1.6).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for affective instability, chronic feelings of emptiness, interpersonal problems, abandonment, identity disturbance, attrition, and adverse effects.
21.2 DBT versus CCT (dichotomous)
Primary outcomes
No data were available on any time point for BPD symptom severity, self‐harm, suicide‐related outcomes, and psychosocial functioning.
Secondary outcome: attrition
One trial reported dichotomous data on attrition (Turner 2000).
DBT did not reduce attrition at end of treatment compared with CCT (RR 0.50, 95% CI 0.16 to 1.55; 1 trial, 24 participants; P = 0.23; Analysis 21.2).
21.2. Analysis.

Comparison 21: Dialectical behavior therapy (DBT) and related treatments vs active treatment, Outcome 2: DBT vs CCT, secondary: attrition (dichotomous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms. depression, and adverse effects.
21.3 Standard DBT (DBT) versus good psychiatric management (GPM) (continuous)
Primary outcomes
BPD symptom severity
One trial reported continuous data on BPD symptom severity (McMain 2009).
DBT did not reduce BPD symptom severity at end of treatment compared with GPM (MD −0.23, 95% CI −1.97 to 1.51; 1 trial, 180 participants; P = 0.80; Analysis 21.3.1).
21.3. Analysis.

Comparison 21: Dialectical behavior therapy (DBT) and related treatments vs active treatment, Outcome 3: Standard DBT (DBT) vs good psychiatric management (GPM) (continuous)
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
Self‐harm
One trial reported continuous data on self‐harm (McMain 2009).
DBT did not reduce self‐harm at end of treatment compared with GPM (MD −8.58, 95% CI −19.38 to 2.22; 1 trial, 180 participants; P = 0.12; Analysis 21.3.2).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for suicide‐related outcomes and psychosocial functioning.
Secondary outcomes
Anger
One trial reported continuous data on anger (McMain 2009).
DBT did not reduce anger at end of treatment compared with GPM (MD −0.15, 95% CI −1.65 to 1.35; 1 trial, 180 participants; P = 0.85; Analysis 21.3.3).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
Interpersonal problems
One trial reported continuous data on interpersonal problems (McMain 2009).
DBT did not reduce interpersonal problems at end of treatment compared with GPM (MD −1.34, 95% CI −15.36 to 12.68; 1 trial, 180 participants; P = 0.85; Analysis 21.3.4).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
Depression
One trial reported continuous data on depression (McMain 2009).
DBT did not reduce depression at end of treatment compared with GPM (MD −2.65, 95% CI −7.18 to 1.88; 1 trial, 180 participants; P = 0.25; Analysis 21.3.5).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for affective instability, chronic feelings of emptiness, impulsivity, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, attrition, and adverse effects.
21.4 DBT versus GPM (dichotomous)
Primary outcomes
No data were available on any time point for BPD symptom severity, self‐harm, suicide‐related outcomes, and psychosocial functioning.
Secondary outcome: attrition
One trial reported dichotomous data on attrition (McMain 2009).
DBT did not reduce attrition at end of treatment compared with GPM (RR 1.03, 95% CI 0.71 to 1.49; 1 trial, 180 participants; P = 0.88; Analysis 21.4).
21.4. Analysis.

Comparison 21: Dialectical behavior therapy (DBT) and related treatments vs active treatment, Outcome 4: DBT vs GPM, secondary: attrition (dichotomous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, depression, and adverse effects.
21.5 Standard DBT (DBT) versus individual DBT therapy + activities group (DBT‐I) (continuous)
Primary outcomes
Self‐harm
One trial reported continuous data on self‐harm (Linehan 2015a).
DBT did not reduce self‐harm at end of treatment compared with DBT‐I (MD −10.40, 95% CI −22.99 to 2.19; 1 trial, 66 participants; P = 0.11; Analysis 21.5.1).
21.5. Analysis.

Comparison 21: Dialectical behavior therapy (DBT) and related treatments vs active treatment, Outcome 5: Standard DBT (DBT) vs individual DBT therapy + activities group (DBT‐I) (continuous)
DBT did not reduce self‐harm at six to 12 months compared with DBT‐I (MD −8.10, 95% CI −19.59 to 3.39; 1 trial, 66 participants; P = 0.17; Analysis 21.5.2).
No data were available for zero to six months and above 12 months follow‐up.
Suicide‐related outcomes
One trial reported continuous data on suicide‐related outcomes (Linehan 2015a).
DBT did not reduce suicide‐related outcomes at end of treatment compared with DBT‐I (MD 0.50, 95% CI −1.37 to 2.37; 1 trial, 66 participants; P = 0.60; Analysis 21.5.3).
DBT reduced suicide‐related outcomes at six to 12 months follow‐up compared with DBT‐I (MD −1.60, 95% CI −2.79 to −0.41; 1 trial, 66 participants; P = 0.009; Analysis 21.5.4).
No data were available for zero to six months and above 12 months follow‐up.
No data were available on any time point for BPD symptom severity and psychosocial functioning.
Secondary outcomes: depression
One trial reported continuous data on depression (Linehan 2015a).
DBT reduced depression at end of treatment compared with DBT‐I (MD −5.90, 95% CI −9.74 to −2.06; 1 trial, 66 participants; P = 0.003, Analysis 21.5.5).
DBT did not reduce depression at six to 12 months follow‐up compared with DBT‐I (MD 1.30, 95% CI −3.10 to 5.70; 1 trial, 66 participants; P = 0.56; Analysis 21.5.6).
No data were available for zero to six months and above 12 months follow‐up.
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, attrition, and adverse effects.
21.6 DBT versus GPM (dichotomous)
Primary outcomes
No data were available on any time point for BPD symptom severity, self‐harm, suicide‐related outcomes, and psychosocial functioning.
Secondary outcome: attrition
One trial reported dichotomous data on attrition (Linehan 2015a).
DBT did not reduce attrition at end of treatment compared with DBT‐I (RR 0.50, 95% CI 0.25 to 1.01; 1 trial, 66 participants; P = 0.05; Analysis 21.6).
21.6. Analysis.

Comparison 21: Dialectical behavior therapy (DBT) and related treatments vs active treatment, Outcome 6: DBT vs DBT‐I, secondary: attrition (dichotomous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, depression, and adverse effects.
21.7 Standard DBT (DBT) versus skills training group + individual case management (DBT‐S) (continuous)
Primary outcomes
Self‐harm
One trial reported continuous data on self‐harm (Linehan 2015a).
DBT did not reduce self‐harm at end of treatment compared with DBT‐S (MD 0.30, 95% CI −8.42 to 9.02; 1 trial, 66 participants, P = 0.95; Analysis 21.7.1).
21.7. Analysis.

Comparison 21: Dialectical behavior therapy (DBT) and related treatments vs active treatment, Outcome 7: Standard DBT (DBT) vs skills training group + individual case management (DBT‐S) (continuous)
DBT did not reduce self‐harm at six to 12 months follow‐up compared with DBT‐S (MD −1.50, 95% CI −9.04 to 6.04, 1 trial, 66 participants; P = 0.70; Analysis 21.7.2).
No data were available for zero to six months and above 12 months follow‐up.
Suicide‐related outcomes
One trial reported continuous data on suicide‐related outcomes (Linehan 2015a).
DBT did not reduce suicide‐related outcomes at end of treatment compared with DBT‐S (MD 0.80, 95% CI −1.06 to 2.66; 1 trial, 66 participants; P = 0.40; Analysis 21.7.3).
DBT reduced suicide‐related outcomes at six to 12 months follow‐up compared with DBT‐S (MD 0.50, 95% CI −0.02 to 1.02; 1 trial, 66 participants; P = 0.06; Analysis 21.7.4).
No data were available for zero to six months and above 12 months follow‐up.
No data were available on any time point for BPD symptom severity and psychosocial functioning.
Secondary outcomes: depression
One trial reported continuous data on depression (Linehan 2015a).
DBT did not reduce depression at end of treatment compared with DBT‐S (MD 1.90, 95% CI −1.60 to 5.40; 1 trial, 66 participants; P = 0.29; Analysis 21.7.5).
DBT did not reduce depression at six to 12 months follow‐up compared with DBT‐S (MD 3.30, 95% CI −0.90 to 7.50; 1 trial, 66 participants; P = 0.12; Analysis 21.7.6).
No data were available for zero to six months, and above 12 months follow‐up.
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, attrition, and adverse effects.
21.8 DBT versus DBT‐S (dichotomous)
Primary outcomes
No data were available on any time point for BPD symptom severity, self‐harm, suicide‐related outcomes and psychosocial functioning.
Secondary outcome: attrition
One trial reported dichotomous data on attrition (Linehan 2015a).
DBT did not reduce attrition at six to 12 months follow‐up compared with DBT‐S (RR 0.62, 95% CI 0.29 to 1.29; 1 trial, 66 participants; P = 0.20; Analysis 21.8).
21.8. Analysis.

Comparison 21: Dialectical behavior therapy (DBT) and related treatments vs active treatment, Outcome 8: DBT vs DBT‐S, secondary: attrition (dichotomous), at 6‐12 months follow‐up
No data were available for end of treatment, zero to six months and above 12 months follow‐up.
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, depression, and adverse effects.
21.9 Standard DBT (DBT) versus step‐down DBT (DBT‐SD) (continuous)
Primary outcomes
BPD symptom severity
One trial reported continuous data on BPD symptom severity (Sinnaeve 2018).
DBT did not reduce BPD severity at end of treatment compared with DBT‐SD (MD −2.83, 95% CI −11.21 to 5.55; 1 trial, 38 participants; P = 0.51; Analysis 21.9.1).
21.9. Analysis.

Comparison 21: Dialectical behavior therapy (DBT) and related treatments vs active treatment, Outcome 9: Standard DBT (DBT) vs step‐down DBT (DBT‐SD) (continuous)
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
Self‐harm
One trial reported continuous data on self‐harm (Sinnaeve 2018).
DBT did not reduce self‐harm at end of treatment compared with DBT‐SD (MD 4.10, 95% CI −4.07 to 12.27; 1 trial, 38 participants; P = 0.33; Analysis 21.9.2).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
Suicide‐related outcomes
One trial reported continuous data on suicide‐related outcomes (Sinnaeve 2018).
DBT did not reduce suicide‐related outcomes at end of treatment compared with DBT‐SD (MD 0.30, 95% CI −0.73 to 1.33; 1 trial, 38 participants; P = 0.57; Analysis 21.9.3).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for psychosocial functioning.
Secondary outcomes
Anger
One trial reported continuous data on anger (Sinnaeve 2018).
DBT did not reduce anger symptoms at end of treatment compared with DBT‐SD (MD −0.53, 95% CI −1.48 to 0.43; 1 trial, 41 participants; P = 0.28; Analysis 21.9.4).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
Affective instability
One trial reported continuous data on affective instability (Sinnaeve 2018).
DBT did not reduce affective instability at end of treatment compared with DBT‐SD (MD −0.71, 95% CI −2.51 to 1.09; 1 trial, 41 participants; P = 0.44; Analysis 21.9.5).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
Chronic feelings of emptiness
One trial reported continuous data on chronic feelings of emptiness (Sinnaeve 2018).
DBT did not reduce chronic feelings of emptiness at end of treatment compared with DBT‐SD (MD 0.18, 95% CI −1.68 to 2.04; 1 trial, 41 participants; P = 0.85; Analysis 21.9.6).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
Impulsivity
One trial reported continuous data on impulsivity (Sinnaeve 2018).
DBT did not reduce impulsivity at end of treatment compared with DBT‐SD (MD 0.24, 95% CI −0.26 to 0.75; 1 trial, 41 participants; P = 0.34; Analysis 21.9.7).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
Interpersonal problems
One trial reported continuous data on interpersonal problems (Sinnaeve 2018).
DBT reduced interpersonal at end of treatment problems compared with DBT‐SD (MD −1.31, 95% CI −2.05 to −0.57; 1 trial, 41 participants; P = 0.005; Analysis 21.9.8).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
Abandonment
One trial reported continuous data on abandonment (Sinnaeve 2018).
DBT reduced abandonment at end of treatment compared with DBT‐SD (MD −1.11, 95% CI −2.14 to −0.08; 1 trial, 41 participants; P = 0.03; Analysis 21.9.9).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
Dissociation and psychotic‐like symptoms
One trial reported continuous data on dissociation and psychotic‐like symptoms (Sinnaeve 2018).
DBT did not reduce dissociation and psychotic‐like symptoms at end of treatment compared with DBT‐SD (MD −0.44, 95% CI −1.56 to 0.68; 1 trial, 41 participants; P = 0.44; Analysis 21.9.10).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for identity disturbance, depression, attrition, and adverse effects.
21.10 DBT versus DBT‐SD (dichotomous)
Primary outcomes
No data were available on any time point for BPD symptom severity, self‐harm, suicide‐related outcomes, and psychosocial functioning.
Secondary outcome: attrition
One trial reported dichotomous data on attrition (Sinnaeve 2018).0.37 [0.17, 0.78]
DBT reduced attrition at end of treatment compared with DBT‐SD (RR 0.37, 95% CI 0.17 to 0.78; 1 trial, 84 participants; P = 0.009; Analysis 21.10).
21.10. Analysis.

Comparison 21: Dialectical behavior therapy (DBT) and related treatments vs active treatment, Outcome 10: DBT vs DBT‐SD, secondary: attrition (dichotomous), at end of treatment
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, depression, and adverse effects.
21.11 Standard DBT (DBT) versus DBT prolonged exposure (DBT‐PE) (continuous)
Primary outcomes
Self‐harm
One trial reported continuous data on self‐harm (Harned 2014).
DBT did not reduce self‐harm at end of treatment compared with DBT‐PE (MD 0.10, 95% CI −1.86 to 2.06; 1 trial, 18 participants; P = 0.92; Analysis 21.11.1).
21.11. Analysis.

Comparison 21: Dialectical behavior therapy (DBT) and related treatments vs active treatment, Outcome 11: Standard DBT (DBT) vs DBT Prolonged Exposure (PE) (continuous)
DBT did not reduce self‐harm at zero to six months follow‐up compared with DBT‐PE (MD −0.30, 95% CI −1.09 to 0.49; 1 trial, 18 participants; P = 0.46; Analysis 21.11.2).
No data were available for six to 12 months and above 12 months follow‐up.
Suicide‐related outcomes
One trial reported continuous data on suicide‐related outcomes (Harned 2014).
There were no suicide attempts in DBT‐PE group at end of treatment (effect not estimable; 1 trial, 18 participants; Analysis 21.11.3).
There were no suicide attempts in DBT group at zero to six months follow‐up (effect not estimable; 1 trial, 18 participants; Analysis 21.11.4).
No data were available for six to 12 months and above 12 months follow‐up.
Psychosocial functioning
One trial reported continuous data on psychosocial functioning (Harned 2014).
DBT did not improve psychosocial functioning at end of treatment compared with DBT‐PE (MD 5.27, 95% CI −2.06 to 12.60; 1 trial, 18 participants; P = 0.16, Analysis 21.11.5).
DBT did not improve psychosocial functioning at zero to six months follow‐up compared with DBT‐PE (MD 2.00, 95% CI −6.27 to 10.27; 1 trial, 18 participants; P = 0.64; Analysis 21.11.6).
No data were available for six to 12 months and above 12 months follow‐up.
No data were available on any time point for BPD symptom severity.
Secondary outcomes
Interpersonal problems
One trial reported continuous data on interpersonal problems (Harned 2014).
DBT did not reduce interpersonal problems at end of treatment compared with DBT‐PE (MD 0.14, 95% CI −0.42 to 0.70; 1 trial, 18 participants; P = 0.62; Analysis 21.11.7).
DBT did not reduce interpersonal problems at zero to six months follow‐up compared with DBT‐PE (MD 0.19, 95% CI −0.48 to 0.86; 1 trial, 18 participants; P = 0.58; Analysis 21.11.8).
No data were available for six to 12 months and above 12 months follow‐up.
Dissociation and psychotic‐like symptoms
One trial reported continuous data on dissociation and psychotic‐like symptoms (Harned 2014).
DBT did not reduce dissociation and psychotic‐like symptoms at end of treatment compared with DBT‐PE (MD 4.60, 95% CI −9.24 to 18.44; 1 trial, 18 participants; P = 0.51; Analysis 21.11.9).
DBT did not reduce dissociation and psychotic‐like symptoms at zero to six months follow‐up compared with DBT‐PE (MD 6.00, 95% CI −9.46 to 21.46; 1 trial, 18 participants; P = 0.45; Analysis 21.11.10).
No data were available for six to 12 months and above 12 months follow‐up.
Depression
One trial reported continuous data on depression (Harned 2014).
DBT did not reduce depression at end of treatment compared with DBT‐PE (MD 3.70, 95% CI −3.19 to 10.59; 1 trial, 18 participants; P = 0.29; Analysis 21.11.11).
DBT did not reduce depression at zero to six months follow‐up compared to DBT‐PE (MD 4.30, 95% CI −1.08 to 9.68; 1 trial, 18 participants; P = 0.12; Analysis 21.11.12).
No data were available for six to 12 months and above 12 months follow‐up.
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, abandonment, identity disturbance, attrition, and adverse effects.
21.12 Standard DBT (DBT) versus DBT prolonged exposure (DBT‐PE) (dichotomous)
Primary outcomes
No data were available on any time point for BPD symptom severity, self‐harm, suicide‐related outcomes, and psychosocial functioning.
Secondary outcome: attrition
One trial reported dichotomous data on attrition Harned 2014).
DBT did not reduce attrition at zero to six months follow‐up compared with DBT‐PE (RR 0.88, 95% CI 0.27 to 2.88; 1 trial, 28 participants; P = 0.84; Analysis 21.12).
21.12. Analysis.

Comparison 21: Dialectical behavior therapy (DBT) and related treatments vs active treatment, Outcome 12: DBT vs DBT‐PE, secondary: attrition (dichotomous), at 0‐6 months follow‐up
No data were available for end of treatment, six to 12 months and above 12 months follow‐up.
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, depression,and adverse effects.
21.13 DBT skills group plus case management (DBT‐S) versus DBT individual therapy plus activity group (DBT‐I) (continuous)
Primary outcomes
Self‐harm
One trial reported continuous data on self‐harm (Linehan 2015a).
DBT‐S did not reduce self harm at end of treatment compared with DBT‐I (MD −10.70, 95% CI −22.47 to 1.07; 1 trial, 66 participants; P = 0.07; Analysis 21.13.1).
21.13. Analysis.

Comparison 21: Dialectical behavior therapy (DBT) and related treatments vs active treatment, Outcome 13: DBT skills group + case management (DBT‐S) vs DBT individual therapy + activity group (DBT‐I) (continuous)
DBT‐S did not reduce self harm at six to 12 months follow‐up compared with DBT‐I (MD −6.60, 95% CI −19.72 to 6.52; 1 trial, 66 participants; P = 0.32; Analysis 21.13.2).
No data were available for six to 12 months and above 12 months follow‐up.
Suicide‐related outcomes
One trial reported continuous data on suicide‐related outcomes (Linehan 2015a).
DBT‐S did not reduce suicide‐related outcomes at end of treatment compared with DBT‐I (MD −0.30, 95% CI −1.72 to 1.12; 1 trial, 66 participants: P = 0.68; Analysis 21.13.3).
DBT‐S reduced suicide‐related outcomes at six to 12 months follow‐up compared with DBT‐I (MD −2.10, 95% CI −3.21 to −0.99; 1 trial, 66 participants; P = 0.0002; Analysis 21.13.4).
No data were available for six to 12 months and above 12 months follow‐up.
No data were available on any time point for BPD symptom severity and psychosocial functioning.
Secondary outcomes: depression
One trial reported continuous data on depression (Linehan 2015a).
DBT‐S reduced depression at end of treatment compared with DBT‐I (MD −7.80, 95% CI −11.27 to −4.33; 1 trial, 66 participants; P < 0.001; Analysis 21.13.5).
DBT‐S did not reduce depression at six to 12 months follow‐up compared with DBT‐I (MD −2.00, 95% CI −6.44 to 2.44; 1 trial, 66 participants; P = 0.38; Analysis 21.13.6).
No data were available for six to 12 months and above 12 months follow‐up.
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, attrition, and adverse effects.
21.14 DBT‐S versus DBT‐I (dichotomous)
Primary outcomes
No data were available on any time point for BPD symptom severity, self‐harm, suicide‐related outcomes, and psychosocial functioning.
Secondary outcome: attrition
One trial reported dichotomous data on attrition (Linehan 2015a).
DBT‐S did not reduce attrition at six to 12 months follow‐up compared with DBT‐I (RR 0.81, 95% CI 0.61 to 1.09; 1 trial, 66 participants; P = 0.17; Analysis 21.14).
21.14. Analysis.

Comparison 21: Dialectical behavior therapy (DBT) and related treatments vs active treatment, Outcome 14: DBT‐S vs DBT‐I, secondary: attrition (dichotomous), at 6‐12 months follow‐up
No data were available for end of treatment, zero to six months and above 12 months follow‐up.
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, depression, and adverse effects.
21.15 DBT skills group (DBT‐S) versus cognitive therapy group (CT‐G) (continuous)
Primary outcomes
BPD symptom severity
One trial reported continuous data on BPD symptom severity (Lin 2019).
DBT‐S did not reduce BPD symptom severity at end of treatment compared with CT‐G (MD 0.26, 95% CI −0.13 to 0.65; 1 trial, 82 participants; P = 0.19; Analysis 21.15.1).
21.15. Analysis.

Comparison 21: Dialectical behavior therapy (DBT) and related treatments vs active treatment, Outcome 15: DBT skills group (DBT‐S) vs cognitive therapy group (CT‐G) (continuous)
DBT‐S reduced BPD symptom severity at zero to six months follow‐up compared with CT‐G (MD −0.96, 95% CI −1.15 to −0.77; 1 trial, 82 participants = 82; P < 0.001; Analysis 21.15.2).
No data were available for six to 12 months and above 12 months follow‐up.
Suicide‐related outcomes
One trial reported continuous data on suicide‐related outcomes (Lin 2019).
DBT‐S did not reduce suicide‐related outcomes at end of treatment compared with CT‐G (MD 0.46, 95% CI −2.47 to 3.39; 1 trial, 82 participants; P = 0.76; Analysis 21.15.3).
DBT‐S reduced suicide‐related outcomes at zero to six months follow‐up compared with CT‐G (MD −2.69, 95% CI −4.89 to −0.49; 1 trial, 82 participants; P = 0.02; Analysis 21.15.4).
No data were available for six to 12 months and above 12 months follow‐up.
No data were available on any time point for self‐harm and psychosocial functioning.
Secondary outcomes: depression
One trial reported continuous data on depression (Lin 2019).
DBT‐S did not reduce depression at end of treatment compared with CT‐G (MD −2.12, 95% CI −5.25 to 1.01; 1 trial, 82 participants; P = 0.18, Analysis 21.15.5).
DBT‐S did not reduce depression at zero to six months follow‐up compared with CT‐G (MD −1.60, 95% CI −5.07 to 1.87; 1 trial, 82 participants, P = 0.37 Analysis 21.15.6).
No data were available for six to 12 months and above 12 months follow‐up.
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, attrition, and adverse effects.
21.16 DBT‐S versus CT‐G (dichotomous)
Primary outcomes
No data were available on any time point for BPD symptom severity, self‐harm, suicide‐related outcomes, and psychosocial functioning.
Secondary outcome: attrition
One trial reported dichotomous data on attrition (Lin 2019).
DBT‐S did not reduce attrition at end of treatment compared with CT‐G (RR 0.71, 95% CI 0.27 to 1.88; 1 trial, 82 participants; P = 0.49; Analysis 21.16).
21.16. Analysis.

Comparison 21: Dialectical behavior therapy (DBT) and related treatments vs active treatment, Outcome 16: DBT‐S vs CT‐G, secondary: attrition (dichotomous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, depression, and adverse effects.
21.17 DBT skills group (DBT‐S) versus schema‐focused therapy group (SFT‐G) (continuous)
Primary outcomes: suicide‐related outcomes
One trial reported continuous data on suicide‐related outcomes (Mohamadizadeh 2017).
DBT‐S did not reduce suicide‐related outcomes at end of treatment compared with SFT‐G (MD 0.92, 95% CI −0.36 to 2.20; 1 trial, 24 participants; P = 0.16; Analysis 21.17.1).
21.17. Analysis.

Comparison 21: Dialectical behavior therapy (DBT) and related treatments vs active treatment, Outcome 17: DBT skills group (DBT‐S) vs schema‐focused therapy group (SFT‐G)
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for BPD symptom severity, self‐harm, and psychosocial functioning.
Secondary outcomes: depression
One trial reported continuous data on depression (Mohamadizadeh 2017).
SFT‐G reduced depression at end of treatment compared with DBT‐S (MD 4.33, 95% CI 2.57 to 6.09; 1 trial, 24 participants; P < 0.001; Analysis 21.17.2).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, attrition and adverse effects.
21.18 DBT mindfulness group (DBT‐M) versus DBT interpersonal effectiveness group (DBT‐IE) (continuous)
Primary outcomes: BPD symptom severity
Two trials reported continuous data on BPD symptom severity (Carmona í Farrés 2019; Elices 2016).
DBT‐M did not reduce BPD symptom severity at end of treatment compared with DBT‐IE (SMD −0.45, 95% CI −1.47 to 0.58, 2 trials, 113 participants; I2 = 86%; P = 0.39; Analysis 21.18.1).
21.18. Analysis.

Comparison 21: Dialectical behavior therapy (DBT) and related treatments vs active treatment, Outcome 18: DBT mindfulness group (DBT‐M) vs DBT interpersonal effectiveness group (DBT‐IE) (continuous)
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for self‐harm, suicide‐related outcomes and psychosocial functioning.
Secondary outcomes: impulsivity
Two trials reported continuous data on impulsivity (Carmona í Farrés 2019; Elices 2016).
DBT‐M did not reduce impulsivity at end of treatment compared with DBT‐IE (SMD −0.36, 95% CI −0.77 to 0.06; 2 trials, 91 participants; I2 = 0%; P = 0.09; Analysis 21.18.2).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, depression, attrition, and adverse effects.
21.19 DBT‐M versus DBT‐IE (dichotomous)
Primary outcomes
No data were available on any time point for BPD symptom severity, self‐harm, suicide‐related outcomes, and psychosocial functioning.
Secondary outcome: attrition
Two trials reported dichotomous data on attrition (Carmona í Farrés 2019; Elices 2016).
DBT‐IE reduced attrition at end of treatment compared with DBT‐M (RR 1.86, 95% CI 1.07 to 3.23; 2 trials, 134 participants; I2 = 0%; P = 0.03; Analysis 21.19).
21.19. Analysis.

Comparison 21: Dialectical behavior therapy (DBT) and related treatments vs active treatment, Outcome 19: DBT‐M vs DBT‐IE, secondary: attrition (dichotomous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, depression, and adverse effects.
21.20 DBT mindfulness group (DBT‐M) versus loving‐kindness and compassion mediation (LKM/CM)
Primary outcomes: BPD symptom severity
One trial reported continuous data on BPD symptom severity (Feliu‐Soler 2017).
DBT‐M did not reduce BPD symptom severity at end of treatment compared with LKM/CM (MD 0.04, 95% CI −0.66 to 0.74; 1 trial, 32 participants; P = 0.91; Analysis 21.20).
21.20. Analysis.

Comparison 21: Dialectical behavior therapy (DBT) and related treatments vs active treatment, Outcome 20: DBT mindfulness group (DBT‐M) vs loving‐kindness and compassion meditation (LK/CM), primary: BPD symptom severity (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for self‐harm, suicide‐related outcomes and psychosocial functioning.
Secondary outcomes
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, depression, attrition and adverse effects.
22. Cognitive behavioural therapy (CBT) and related treatments versus active treatment
22.1 CBT versus brief trauma and anxiety‐related group psychoeducation (continuous)
Primary outcomes
BPD symptom severity
One trial reported continuous data on BPD symptom severity (Kredlow 2017b).
CBT did not reduce BPD symptom severity at end of treatment compared with brief trauma and anxiety‐related group psychoeducation (MD −0.26, 95% CI −1.46 to 0.94; 1 trial, 50 participants; P = 0.67; Analysis 22.1.1).
22.1. Analysis.

Comparison 22: Cognitive behavioural therapy (CBT) and related treatments vs active treatment, Outcome 1: CBT vs trauma‐ and anxiety‐related group psychoeducation (continuous)
CBT did not reduce BPD symptom severity at zero to six months follow‐up compared with brief trauma and anxiety‐related group psychoeducation (MD −0.39, 95% CI −1.60 to 0.82; 1 trial, 50 participants; P = 0.53; Analysis 22.1.2).
CBT did not reduce BPD symptom severity at six to 12 months follow‐up compared to brief trauma and anxiety‐related group psychoeducation (MD 0.57, 95% CI −0.48 to 1.62; 1 trial, number of 50 participants; P = 0.29; Analysis 22.1.3).
No data were available for above 12 months follow‐up.
Psychosocial functioning
One trial reported continuous data on psychosocial functioning (Kredlow 2017b).
CBT did not improve psychosocial functioning at end of treatment compared with brief trauma and anxiety‐related group psychoeducation (MD −0.64, 95% CI −5.76 to 4.48; 1 trial, 50 participants; P = 0.81; Analysis 22.1.4).
CBT did not improve psychosocial functioning at zero to six months follow‐up compared with brief trauma and anxiety‐related group psychoeducation (MD −1.23, 95% CI −6.94 to 4.48; 1 trial, 50 participants; P = 0.67; Analysis 22.1.5).
CBT did not improve psychosocial functioning at six to 12 months follow‐up compared with brief trauma and anxiety‐related group psychoeducation (MD 0.61, 95% CI −5.00 to 6.22; 1 trial, 50 participants; P = 0.83; Analysis 22.1.6).
No data were available for above 12 months follow‐up.
No data were available on any time point for self‐harm and suicide‐related outcomes.
Secondary outcomes: depression
One trial reported continuous data on depression (Kredlow 2017b).
CBT did not reduce depression at end of treatment compared with brief trauma and anxiety‐related group psychoeducation (MD 0.76, 95% CI −8.20 to 9.72, 1 trial; 50 participants; P = 0.87; Analysis 22.1.7).
CBT did not reduce depression at zero to six months follow‐up compared with brief trauma and anxiety‐related group psychoeducation (MD 3.13, 95% CI −4.05 to 10.31; 1 trial, 50 participants; P = 0.39; Analysis 22.1.8).
CBT did not reduce depression at six to 12 months follow‐up compared with brief trauma and anxiety‐related group psychoeducation (MD 0.77, 95% CI −7.08 to 8.62; 1 trial; 50 participants; P = 0.85; Analysis 22.1.9).
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, attrition, and adverse effects.
22.2 CBT versus brief trauma and anxiety‐related group psychoeducation (dichotomous)
Primary outcomes
No data were available on any time point for BPD symptom severity, self‐harm, suicide‐related outcomes, and psychosocial functioning.
Secondary outcome: attrition
One trial reported dichotomous data on attrition (Kredlow 2017b).
CBT did not reduce attrition at end of treatment compared with brief trauma and anxiety‐related group psychoeducation (RR 1.99, 95% CI 0.58 to 6.82, 1 trial, 50 participants, P = 0.27; Analysis 22.2).
22.2. Analysis.

Comparison 22: Cognitive behavioural therapy (CBT) and related treatments vs active treatment, Outcome 2: CBT vs trauma‐ and anxiety‐related group psychoeducation, secondary: attrition (dichotomous), at end of treatment
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, depression, and adverse effects.
22.3 CBT versus interpersonal therapy (IPT) (continuous)
Primary outcome: psychosocial functioning
One trial reported continuous data on psychosocial functioning (Bellino 2007).
CBT did not improve psychosocial functioning at end of treatment compared with IPT (MD −5.30, 95% CI −12.36 to 1.76; 1 trial, 26 participants; P = 0.14; Analysis 22.3.1).
22.3. Analysis.

Comparison 22: Cognitive behavioural therapy (CBT) and related treatments vs active treatment, Outcome 3: CBT vs interpersonal psychotherapy (IPT) (continuous)
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for BPD symptom severity, self‐harm, and suicide‐related outcomes.
Secondary outcomes: depression
One trial reported continuous data on depression (Bellino 2007).
CBT did not reduce depression at end of treatment compared to IPT (MD −0.40, 95% CI −4.72 to 3.92; 1 trial, 26 participants; P = 0.86; Analysis 22.3.2).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, attrition and adverse effects.
22.4 CBT versus interpersonal therapy (IPT) (dichotomous)
Primary outcomes
No data were available on any time point for BPD symptom severity, self‐harm, suicide‐related outcomes, and psychosocial functioning.
Secondary outcome: attrition
One trial reported dichotomous data on attrition (Bellino 2007).
CBT did not reduce attrition at end of treatment compared with IPT (RR 2.00, 95% CI 0.42 to 9.42; 1 trial; 32 participants; P = 0.38; Analysis 22.4).
22.4. Analysis.

Comparison 22: Cognitive behavioural therapy (CBT) and related treatments vs active treatment, Outcome 4: CBT vs IPT, secondary: attrition (dichotomous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, depression, and adverse effects.
22.5 CBT versus Rogerian supportive therapy (continuous)
Primary outcomes
Self‐harm
One trial reported continuous data on self‐harm (Cottraux 2009).
CBT did not reduce self‐harm at end of treatment compared with Rogerian support therapy (MD 0.74, 95% CI −0.03 to 1.51; 1 trial, 38 participants; P = 0.06; Analysis 22.5.1).
22.5. Analysis.

Comparison 22: Cognitive behavioural therapy (CBT) and related treatments vs active treatment, Outcome 5: CBT vs Rogerian supportive therapy (continuous)
CBT did not reduce self‐harm at six to 12 months compared with Rogerian support therapy (MD ‐0.63, 95% CI −1.75 to 0.49; 1 trial, 21 participants; P = 0.27; Analysis 22.5.2).
No data were available for zero to six months and above 12 months follow‐up.
Suicide‐related outcomes
One trial reported continuous data on suicide‐related outcomes (Cottraux 2009).
CBT did not reduce suicide‐related outcomes at end of treatment compared with Rogerian support therapy (MD 0.69, 95% CI −2.60 to 3.9; 1 trial, 38 participants; P = 0.68; Analysis 22.5.3).
CBT did not reduce suicide‐related outcomes at six to 12 months compared with Rogerian support therapy (MD ‐2.43, 95% CI ‐6.14 to 1.28; 1 trial, 21 participants; P = 0.20; Analysis 22.5.4).
No data were available for zero to six months and above 12 months follow‐up.
Psychosocial functioning
One trial reported continuous data on psychosocial functioning (Cottraux 2009).
CBT did not improve psychosocial functioning at end of treatment compared with Rogerian support therapy (MD −0.43, 95% CI −1.31 to 0.45; 1 trial, 38 participants; P = 0.34; Analysis 22.5.5).
CBT did not improve psychosocial functioning at six to 12 months compared with Rogerian support therapy (MD ‐0.98, 95% CI ‐2.02 to 0.06; 1 trial, 21 participants; P = 0.07; Analysis 22.5.6).
No data were available for zero to six months and above 12 months follow‐up.
No data were available on any time point for BPD symptom severity.
Secondary outcomes
Impulsivity
One trial reported continuous data on Impulsivity (Cottraux 2009).
CBT did not reduce impulsivity at end of treatment compared with Rogerian support therapy (MD −1.01, 95% CI −3.85 to 1.83; 1 trial, 38 participants; P = 0.49; Analysis 22.5.7).
CBT did not reduce impulsivity at six to 12 months compared with Rogerian support therapy (MD ‐2.18, 95% CI ‐5.91 to 1.55; 1 trial, 21 participants; P = 0.25; Analysis 22.5.8).
No data were available for zero to six months and above 12 months follow‐up.
Depression
One trial reported continuous data on depression (Cottraux 2009).
CBT did not reduce depression at end of treatment compared with Rogerian support therapy (MD 1.04, 95% CI −5.59 to 7.67; 1 trial, 38 participants; P = 0.76; Analysis 22.5.9).
CBT did reduce depression at six to 12 months compared with Rogerian support therapy (MD ‐5.15, 95% CI ‐9.38 to ‐0.92; 1 trial, 21 participants; P = 0.02; Analysis 22.5.10).
No data were available for zero to six months and above 12 months follow‐up.
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, attrition, and adverse effects.
22.6 CBT versus Rogerian supportive therapy (dichotomous)
Primary outcomes
No data were available on any time point for BPD symptom severity, self‐harm, suicide‐related outcomes, and psychosocial functioning.
Secondary outcome: attrition
One trial reported dichotomous data on attrition (Cottraux 2009).
CBT did not reduce attrition at end of treatment compared with Rogerian support therapy (RR 0.90, 95% CI 0.51 to 1.60; 1 trial, 38 participants; P = 0.72; Analysis 22.6).
22.6. Analysis.

Comparison 22: Cognitive behavioural therapy (CBT) and related treatments vs active treatment, Outcome 6: CBT vs Rogerian supportive therapy, secondary: attrition (dichotomous), end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, depression, and adverse effects.
22.7 MACT (manual‐assisted cognitive therapy) versus MACT + therapeutic assessment (TA) (continuous)
Primary outcomes
BPD symptom severity
One trial reported continuous data on BPD symptom severity (Morey 2010).
MACT did not reduce BPD symptom severity at end of treatment compared with MACT + TA (MD −3.75, 95% CI −14.17 to 6.67; 1 trial, 16 participants; P = 0.48; Analysis 22.7.1).
22.7. Analysis.

Comparison 22: Cognitive behavioural therapy (CBT) and related treatments vs active treatment, Outcome 7: MACT (Manual‐assisted Cognitive Therapy) vs MACT + therapeutic assessment (MACT + TA) (continuous)
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
Self‐harm
One trial reported continuous data on self‐harm (Morey 2010).
MACT did not reduce self‐harm at end of treatment compared with MACT + TA (MD 1.75, 95% CI −18.71 to 22.21; 1 trial, 16 participants; P = 0.87; Analysis 22.7.2).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
Suicide‐related outcomes
One trial reported continuous data on suicide‐related outcomes (Morey 2010).
MACT did not reduce suicide‐related outcomes at end of treatment compared with MACT + TA (MD −0.63, 95% CI −17.71 to 16.45; 1 trial, 16 participants; P = 0.94; Analysis 22.7.3).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for psychosocial functioning.
Secondary outcomes
Affective instability
One trial reported continuous data on affective instability (Morey 2010).
MACT did not reduce affective instability at end of treatment compared with MACT + TA (MD −5.25, 95% CI −12.10 to 1.60; 1 trial, 16 participants; P = 0.13; Analysis 22.7.4).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
Interpersonal problems
One trial reported continuous data on interpersonal problems (Morey 2010).
MACT did not interpersonal problems at end of treatment compared with MACT + TA (MD −0.50, 95% CI −11.24 to 10.24; 1 trial, 16 participants; P = 0.93; Analysis 22.7.5).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
Identity disturbance
One trial reported continuous data on identity disturbance (Morey 2010).
MACT did not reduce identity disturbance at end of treatment compared with MACT + TA (MD −4.88, 95% CI −14.98 to 5.22; 1 trial, 16 participants; P = 0.34; Analysis 22.7.6).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for anger, chronic feelings of emptiness, impulsivity, abandonment, dissociation and psychotic‐like symptoms, depression, attrition, and adverse effects.
22.8 MACT versus MACT + TA (dichotomous)
Primary outcomes
No data were available on any time point for BPD symptom severity, self‐harm, suicide‐related outcomes, and psychosocial functioning.
Secondary outcome: attrition
One trial reported dichotomous data on attrition (Morey 2010).
MACT did not reduce attrition at end of treatment compared with MACT + TA (RR 0.80, 95% CI 0.33 to 1.92; 1 trial, 16 participants; P = 0.62; Analysis 22.8).
22.8. Analysis.

Comparison 22: Cognitive behavioural therapy (CBT) and related treatments vs active treatment, Outcome 8: MACT vs MACT + TA, secondary: attrition (dichotomous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, depression, and adverse effects.
22.9 Meta‐cognitive training for BPD (B‐MCT) versus progressive muscle relaxation (PMR) (continuous)
Primary outcome: BPD symptom severity
One trial reported continuous data on BPD symptom severity (Schilling 2018).
B‐MCT did not reduce BPD symptom severity at end of treatment compared PMR (MD −1.80, 95% CI −4.97 to 1.37; 1 trial, 49 participants; P = 0.27; Analysis 22.9.1).
22.9. Analysis.

Comparison 22: Cognitive behavioural therapy (CBT) and related treatments vs active treatment, Outcome 9: Meta‐Cognitive training for BPD (B‐MCT) vs progressive muscle relaxation training (PMR)
PMR reduced BPD symptom severity at zero to six months follow‐up compared B‐MCT (MD −3.60, 95% CI −7.16 to −0.04; 1 trial, 39 participants; P = 0.05; Analysis 22.9.2).
No data were available for six to 12 months and above 12 months follow‐up.
No data were available on any time point for self‐harm, suicide‐related outcomes and psychosocial functioning.
Secondary outcomes: depression
One trial reported continuous data on depression (Schilling 2018).
B‐MCT did not reduce depression at end of treatment compared with PMR (MD −3.20, 95% CI −9.91 to 3.51; 1 trial, 54 participants; P = 0.35; Analysis 22.9.3).
B‐MCT reduced depression at zero to six months follow‐up compared with PMR (MD 8.50, 95% CI 2.03 to 14.97; 1 trial, 47 participants, P = 0.01 Analysis 22.9.4).
No data were available for six to 12 months and above 12 months follow‐up.
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, attrition and adverse effects.
22.10 B‐MCT versus PMR (dichotomous)
Primary outcomes
No data were available on any time point for BPD symptom severity, self‐harm, suicide‐related outcomes, and psychosocial functioning.
Secondary outcome: attrition
One trial reported dichotomous data on attrition (Schilling 2018).
B‐MCT did not reduce attrition at end of treatment compared with PMR (RR 0.95, 95% CI 0.45 to 2.00; 1 trial, 74 participants; P = 0.89; Analysis 22.10).
22.10. Analysis.

Comparison 22: Cognitive behavioural therapy (CBT) and related treatments vs active treatment, Outcome 10: B‐MCT vs progressive muscle relaxation (PMR) training + TAU (dichotomous). Secondary: attrition (dichotomous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, depression, and adverse effects.
22.11 Motive‐oriented therapeutic relationship (MOTR) versus good psychiatric management (GPM)
Primary outcomes
BPD symptom severity
One trial reported continuous data on BPD symptom severity (Kramer 2014).
MOTR did not reduce BPD symptom severity at end of treatment compared with GPM (MD 0.07, 95% CI −0.38 to 0.52; 1 trial, 74 participants = 74; P = 0.76; Analysis 22.11.1).
22.11. Analysis.

Comparison 22: Cognitive behavioural therapy (CBT) and related treatments vs active treatment, Outcome 11: MOTR (Motive‐Oriented Therapeutic Relationship) vs Good Psychiatric Management (GPM) (continuous)
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
Psychosocial functioning
Two trials reported continuous data on psychosocial functioning (Kramer 2011; Kramer 2014).
MOTR group improved psychosocial functioning at end of treatment compared with GPM (SMD −0.45, 95% CI −0.85 to −0.05: 2 trials, 99 participants; I2 = 0%; P = 0.03; Analysis 22.11.2).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for self‐harm and suicide‐related outcomes.
Secondary outcomes: interpersonal problems
Two trials reported continuous data on interpersonal problems (Kramer 2011; Kramer 2014).
MOTR group improved interpersonal problems at end of treatment compared with GPM (SMD −0.56, 95% CI −0.97 to −0.16: 2 trials, 99 participants; I2 = 0%; P = 0.006; Analysis 22.11.3).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, depression, attrition, and adverse effects.
22.12 MOTR versus GPM (dichotomous)
Primary outcomes
No data were available on any time point for BPD symptom severity, self‐harm, suicide‐related outcomes, and psychosocial functioning.
Secondary outcome: attrition
Two trials reported dichotomous data on attrition (Kramer 2011; Kramer 2014).
MOTR group did not reduce attrition at end of treatment compared with GPM (RR 0.61, 95% CI 0.26 to 1.41: 2 trials, 110 participants; I2 = 34%; P = 0.25; Analysis 22.12).
22.12. Analysis.

Comparison 22: Cognitive behavioural therapy (CBT) and related treatments vs active treatment, Outcome 12: MOTR vs (GPM), secondary: attrition (dichotomous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, depression, and adverse effects.
23. Schema‐focused therapy (SFT) versus active treatment
23.1 SFT versus transference‐focused psychotherapy (TFP) (continuous)
Primary outcome: BPD symptom severity
One trial reported continuous data on BPD symptom severity (Giesen‐Bloo 2006).
SFT reduced BPD symptom severity at end of treatment compared with TFP (MD −4.95, 95% CI −9.59 to −0.31; 1 trial, 86 participants; P = 0.04; Analysis 23.1).
23.1. Analysis.

Comparison 23: Schema‐focused therapy (SFT) vs active treatment, Outcome 1: SFT vs TFP. Primary: BPD symptom severity (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for self‐harm, suicide‐related outcomes, and psychosocial functioning.
Secondary outcomes
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, depression, attrition and adverse effects.
23.2 SFT versus TFP (dichotomous)
Primary outcomes
No data were available on any time point for BPD symptom severity, self‐harm, suicide‐related outcomes, and psychosocial functioning.
Secondary outcome: attrition
One trial reported dichotomous data on attrition (Giesen‐Bloo 2006).
SFT reduced attrition at zero to six months follow‐up compared with TFP (RR 0.52, 95% CI 0.30 to 0.92; 1 trial, 88 participants; P = 0.02; Analysis 23.2).
23.2. Analysis.

Comparison 23: Schema‐focused therapy (SFT) vs active treatment, Outcome 2: SFT vs TFP. Secondary: attrition (dichotomous), at 0‐6 months follow‐up
No data were available for end of treatment, six to 12 months and above 12 months follow‐up.
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, depression, and adverse effects.
23.3 SFT versus SFT + therapist availability (continuous)
Primary outcome: BPD symptom severity
One trial reported continuous data on BPD symptom severity (Nadort 2009).
SFT did not reduce BPD severity at end of treatment compared with TFP (MD −0.30, 95% CI −5.51 to 4.91; 1 trial, 61 participants; P = 0.91; Analysis 23.3).
23.3. Analysis.

Comparison 23: Schema‐focused therapy (SFT) vs active treatment, Outcome 3: SFT vs SFT + therapist availability (TA). Primary: BPD symptom severity (continuous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for self‐harm, suicide‐related outcomes and psychosocial functioning.
Secondary outcomes
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, depression, attrition and adverse effects.
23.4 SFT versus SFT + therapist availability (dichotomous)
Primary outcomes
No data were available on any time point for BPD symptom severity, self‐harm, suicide‐related outcomes, and psychosocial functioning.
Secondary outcome: attrition
One trial reported dichotomous data on attrition (Nadort 2009).
SFT did not reduce attrition at zero to six months follow‐up compared with TFP (RR 0.91, 95% CI 0.35 to 2.41; 1 trial, 62 participants; P = 0.86; Analysis 23.4).
23.4. Analysis.

Comparison 23: Schema‐focused therapy (SFT) vs active treatment, Outcome 4: SFT vs SFT + TA. Secondary: attrition (dichotomous), 0‐6 months follow‐up
No data were available for end of treatment, six to 12 months and above 12 months follow‐up.
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, depression, and adverse effects.
24. Systems training for emotional predictability and problem solving (STEPPS)‐based psychoeducation (STEPPS‐PE) versus cognitive rehabilitation (CR)
24.1 STEPPS‐PE versus CR (continuous)
Primary outcome: BPD symptom severity
One trial reported continuous data on BPD symptom severity (Pascual 2015).
STEPPS‐PE reduced BPD symptom severity at end of treatment compared with cognitive rehabilitation (MD −3.67, 95% CI −6.52 to −0.82; 1 trial, 46 participants; P = 0.01; Analysis 24.1.1).
24.1. Analysis.

Comparison 24: Systems training for emotional predictability and problem solving‐based psychoeducation (STEPPS‐PE) vs cognitive rehabilitation (CR), Outcome 1: STEPPS‐PE vs CR
Cognitive rehabilitation reduced BPD symptom severity at zero to six months follow‐up compared with STEPPS‐PE (MD 4.68, 95% CI 1.42 to 7.94; 1 trial, 42 participants; P = 0.005; Analysis 24.1.2).
No data were available for six to 12 months and above 12 months follow‐up.
No data were available on any time point for self‐harm, suicide‐related outcomes and psychosocial functioning.
Secondary outcomes
Impulsivity
One trial reported continuous data on impulsivity (Pascual 2015).
Cognitive rehabilitation reduced impulsivity at end of treatment compared with STEPPS‐PE (MD 1.99, 95% CI 0.06 to 3.92; 1 trial, 46 participants; P = 0.04; Analysis 24.1.3).
Cognitive rehabilitation reduced impulsivity at zero to six months follow‐up compared with STEPPS‐PE (MD 9.92, 95% CI 7.38 to 12.46; 1 trial, 42 participants; P < 0.001; Analysis 24.1.4).
No data were available for six to 12 months and above 12 months follow‐up.
Depression
One trial reported continuous data on depression (Pascual 2015).
STEPPS‐PE reduced depression at end of treatment compared with cognitive rehabilitation (MD −2.58, 95% CI −3.62 to −1.54; 1 trial, 48 participants; P < 0.001; Analysis 24.1.5).
STEPPS‐PE reduced depression at zero to six months follow‐up compared to cognitive rehabilitation (MD −10.11, 95% CI −11.35 to −8.87; 1 trial, 42 participants; P < 0.001; Analysis 24.1.6).
No data were available for six to 12 months and above 12 months follow‐up.
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, attrition and adverse effects.
24.2 STEPPS‐PE versus CR (dichotomous)
Primary outcomes
No data were available on any time point for BPD symptom severity, self‐harm, suicide‐related outcomes, and psychosocial functioning.
Secondary outcome: attrition
One trial reported dichotomous data on attrition (Pascual 2015).
STEPPS‐PE did not reduce attrition at end of treatment compared with cognitive rehabilitation (RR 0.76, 95% CI 0.39 to 1.47; 1 trial, 70 participants; P = 0.41; Analysis 24.2).
24.2. Analysis.

Comparison 24: Systems training for emotional predictability and problem solving‐based psychoeducation (STEPPS‐PE) vs cognitive rehabilitation (CR), Outcome 2: STEPPS‐PE vs CR. Secondary: attrition (dichotomous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, and adverse effects.
25. Eclectic treatments versus active treatment
25.1 Combined inpatient and outpatient psychotherapy versus outpatient psychotherapy (continuous)
Primary outcome: psychosocial functioning
One trial reported continuous data on psychosocial functioning (Antonsen 2017).
Inpatient and outpatient psychotherapy did not improve psychosocial functioning at end of treatment compared with psychotherapy alone (MD 6.50, 95% CI −0.06 to 13.06; 1 trial, 52 participants; P = 0.05; Analysis 25.1.1).
25.1. Analysis.

Comparison 25: Eclectic treatments vs active treatment, Outcome 1: Combined inpatient and outpatient psychotherapy versus outpatient psychotherapy
Inpatient and outpatient psychotherapy did not improve psychosocial functioning at above 12 months follow‐up compared with psychotherapy alone (MD −5.70, 95% CI −13.33 to 1.93; 1 trial, 52 participants; P = 0.14, Analysis 25.1.2).
No data were available for zero to six months and six to 12 months follow‐up.
No data were available on any time point for BPD symptom severity, self‐harm, and suicide‐related outcomes.
Secondary outcomes
Interpersonal problems
One trial reported continuous data on interpersonal problems (Antonsen 2017).
Inpatient and outpatient psychotherapy did not reduce interpersonal problems at end of treatment (three years) compared with psychotherapy alone (MD 0.05, 95% CI −0.24 to 0.34; 1 trial, 52 participants; P = 0.74; Analysis 25.1.3).
Participants in the inpatient and outpatient psychotherapy reported fewer interpersonal problems at above 12 months follow‐up compared with psychotherapy alone (MD −0.42, 95% CI −0.77 to −0.0; 1 trial, 52 participants; P = 0.02; Analysis 25.1.4).
No data were available for zero to six months and six to 12 months follow‐up.
Identity disturbance
One trial reported continuous data on identity disturbance (Antonsen 2017).
Inpatient and outpatient psychotherapy did not reduce identity disturbance at end of treatment (three years) compared with psychotherapy alone (MD −0.25, 95% CI −0.59 to 0.09; 1 trial, 52 participants; P = 0.15; Analysis 25.1.5).
Inpatient and outpatient psychotherapy reported less identity disturbance at above 12 months follow‐up compared with psychotherapy alone (MD −0.47, 95% CI −0.78 to −0.16; 1 trial, 52 participants; P = 0.003; Analysis 25.1.6).
No data were available for zero to six months and six to 12 months follow‐up.
Depression
One trial reported continuous data on depression (Antonsen 2017).
Inpatient and outpatient psychotherapy did not reduce depression at end of treatment (three years) compared with psychotherapy alone (MD −0.40, 95% CI −6.25 to 5.45; 1 trial, 52 participants; P = 0.8; Analysis 25.1.7).
Inpatient and outpatient psychotherapy did not reduce depression at above 12 months follow‐up compared to psychotherapy alone (MD −4.70, 95% CI −11.02 to 1.62; 1 trial, 52 participants; P = 0.14; Analysis 25.1.8).
No data were available for zero to six months and six to 12 months follow‐up.
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, abandonment, dissociation and psychotic‐like symptoms, attrition and adverse effects.
25.2 Combined inpatient and outpatient psychotherapy versus outpatient psychotherapy (dichotomous)
Primary outcomes
No data were available on any time point for BPD symptom severity, self‐harm, suicide‐related outcomes, and psychosocial functioning.
Secondary outcome: attrition
One trial reported dichotomous data on attrition (Antonsen 2017).
Inpatient and outpatient psychotherapy did not reduce attrition at end of treatment (three years) compared with psychotherapy alone (RR 0.62, 95% CI 0.26 to 1.49; 1 trial, 52 participants; P = 0.28; Analysis 25.2).
25.2. Analysis.

Comparison 25: Eclectic treatments vs active treatment, Outcome 2: Combined inpatient and outpatient psychotherapy versus outpatient psychotherapy. Secondary: attrition (dichotomous), at end of treatment
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, depression, and adverse effects.
25.3 Integrative BPD‐oriented adolescent family therapy (I‐BAFT) versus individual drug counselling (IDC)
Primary outcome: BPD symptom severity
One trial reported dichotomous data on BPD symptom severity (Santisteban 2015).
Integrative BPD did not reduce BPD severity at end of treatment compared with control (RR 0.91, 95% CI 0.50 to 1.64; 1 trial, 40 participants; P = 0.75; Analysis 25.3.1).
25.3. Analysis.

Comparison 25: Eclectic treatments vs active treatment, Outcome 3: integrative BPD‐oriented adolescent family therapy (I‐BAFT) vs individual drug counselling (IDC)
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for self‐harm, suicide‐related outcomes and psychosocial functioning.
Secondary outcome: attrition
One trial reported dichotomous data on attrition (Santisteban 2015).
Integrative BPD did not reduce attrition at end of treatment compared with control (RR 0.50, 95% CI 0.18 to 1.40; 1 trial, 40 participants; P = 0.19; Analysis 25.3.2).
No data were available for zero to six months, six to 12 months and above 12 months follow‐up.
No data were available on any time point for anger, affective instability, chronic feelings of emptiness, impulsivity, interpersonal problems, abandonment, identity disturbance, dissociation and psychotic‐like symptoms, depression, and adverse effects.
Subgroup analyses
Where data permitted, we conducted our planned subgroup analyses, in addition to two post hoc subgroup analyses, for the primary outcomes of BPD symptom severity and psychosocial functioning. For the 'types of comparisons' subgroup, we also conducted analyses for self‐harm and suicide‐related outcomes. Only subgroups with two or more trials were included in the subgroup analyses.
26. Therapeutic approaches
26.1 BPD symptom severity
We compared the effects of DBT versus MBT versus psychodynamic psychotherapy versus STEPPS versus eclectic treatments for the outcome of BPD symptom severity. We found no evidence of significant differences between the subgroups (test for subgroup differences: Chi2 = 6.88, df = 4 (P = 0.14), I2 = 41.8%): DBT SMD = −0.60 (95% CI −1.05 to −0.14; 3 trials, 149 participants; I2 = 42%; P = 0.01; Analysis 26.1.1); MBT SMD = −0.13 (95% CI −0.38 to 0.11; 5 trials, 267 participants; I2 = 0%; P = 0.28; Analysis 26.1.2); psychodynamic psychotherapy SMD = −0.29 (95% CI −0.66 to 0.09; 4 trials, 222 participants; I2 = 38%; P = 0.13; Analysis 26.1.3); STEPPS SMD = −0.39 (95% CI −0.63 to −0.15; 3 trials, 273 participants; I2 = 0%; P = 0.001; Analysis 26.1.4); and eclectic treatments SMD = −0.90 (95% CI −1.57 to −0.23; 2 trials, 134 participants; I2 = 67%; P = 0.008; Analysis 26.1.5). For illustrative purposes, we present the results of other therapeutic approaches from single trials in Analysis 26.1.6 to Analysis 26.1.10.
26.1. Analysis.

Comparison 26: Subgroup analysis: therapeutic approaches, Outcome 1: BPD symptom severity
26.2 Psychosocial functioning
We compared the effects of DBT versus MBT versus psychodynamic psychotherapy versus eclectic treatments for the outcome of psychosocial functioning. We found no evidence of significant differences between the subgroups (test for subgroup differences: Chi2 = 0.67, df = 3 (P = 0.88), I2 = 0%): DBT SMD = −0.36 (95% CI −0.69 to −0.03; 6 trials, 225 participants; I2 = 31%; P = 0.03; Analysis 26.2.1); MBT SMD = −0.54 (95% CI −1.24 to 0.16; 3 trials, 239 participants; I2 = 83%; P = 0.13; Analysis 26.2.2); psychodynamic psychotherapy SMD = −0.69 (95% CI −1.98 to 0.59; 4 trials, 140 participants; I2 = 92%; P = 0.29; Analysis 26.2.3); and eclectic treatments SMD = −0.57 (95% CI −1.10 to −0.04; 2 trials, 231 participants; I2 = 63%; P = 0.03; Analysis 26.2.11). For illustrative purposes, we present the results of other therapeutic approaches from single trials in Analysis 26.2.5 to Analysis 26.2.11.
26.2. Analysis.

Comparison 26: Subgroup analysis: therapeutic approaches, Outcome 2: Psychosocial functioning
27. Age: BPD symptom severity
We compared the effects for the subgroup of 15 to 18 years of age versus above 18 years of age for the outcome of BPD symptom severity. There were significant differences between subgroups (test for subgroup differences: Chi2 = 5.03, df = 1 (P = 0.02), I2 = 80.1%): 15 to 18 years of age SMD = −0.14 (95% CI −0.46 to 0.17; 2 trials, 156 participants; I2 = 0%; P = 0.38; Analysis 27.1.1); and above 18 years of age SMD = −0.57 (95% CI −0.76 to ‐0.37; 20 trials, 1088 participants; I2 = 57%; P < 0.001; Analysis 27.1.2).
27.1. Analysis.

Comparison 27: Subgroup analysis: age, Outcome 1: BPD symptom severity
28. Duration
28.1 BPD symptom severity
We compared the effects of less than six months versus six to 12 months versus above 12 months duration for the outcome of BPD symptom severity. We found no evidence of significant differences between the subgroups (test for subgroup differences: Chi2 = 3.86, df = 2 (P = 0.15), I2 = 48.2%): less than six months SMD = −0.76 (95% CI −1.10 to −0.42; 8 trials, 525 participants; I2 = 69%; P < 0.001; Analysis 28.1.1); six to 12 months SMD = −0.36 (95% CI −0.60 to −0.12; 11 trials, 606 participants; I2 = 50%; P = 0.003; Analysis 28.1.2); and above 12 months SMD = −0.37 (95% CI −0.75 to 0.01; 3 trials, 113 participants; I2 = 0%; P = 0.06; Analysis 28.1.3).
28.1. Analysis.

Comparison 28: Subgroup analysis: duration, Outcome 1: BPD symptom severity
28.2 Psychosocial functioning
We compared the effects of less than six months versus six to 12 months versus above 12 months durations for the outcome of psychosocial functioning. There were no significant differences between subgroups (test for subgroup differences: Chi2 = 2.24, df = 2 (P = 0.33), I2 = 10.9%): less than six months SMD = −0.31 (95% CI −0.73 to 0.11; 6 trials, 468 participants; I2 = 73%; P = 0.14; Analysis 28.2.1); six to 12 months SMD = −0.25 (95% CI −0.49 to −0.01; 10 trials, 535 participants; I2 = 45%; P = 0.04; Analysis 28.2.2); and above 12 months SMD = −0.86 (95% CI −1.62 to −0.10; 4 trials, 263 participants; I2 = 86%; P = 0.03; Analysis 28.2.3).
28.2. Analysis.

Comparison 28: Subgroup analysis: duration, Outcome 2: Psychosocial functioning
29. Mode of therapy
29.1 BPD symptom severity
We compared the effects of individual therapy versus group therapy versus mixed therapy for the outcome of BPD symptom severity. There were significant differences between subgroups (test for subgroup differences: Chi2 = 13.23, df = 2 (P = 0.001), I2 = 84.9%): individual therapy SMD = −0.39 (95% CI −0.63 to −0.16; 8 trials, 520 participants; I2 = 39%; P = 0.001; Analysis 29.1.1); group therapy SMD = −0.89 (95% CI −1.21 to −0.57; 8 trials, 438 participants; I2 = 57%; P < 0.001; Analysis 29.1.2); and mixed therapy SMD = −0.15 (95% CI −0.38 to 0.09; 6 trials, 286 participants; I2 = 0%; P = 0.22; Analysis 29.1.3).
29.1. Analysis.

Comparison 29: Subgroup analysis: mode of therapy, Outcome 1: BPD symptom severity
29.2 Psychosocial functioning
We compared the effects of individual therapy versus group therapy versus mixed therapy for the outcome of psychosocial functioning. There were no significant differences between subgroups (test for subgroup differences: Chi2 = 0.89, df = 2 (P = 0.64), I2 = 0%): individual therapy = SMD −0.31 (95% CI −0.75 to 0.12; 8 trials, 570 participants; I2 = 80%; P = 0.16; Analysis 29.2.1); group therapy SMD = −0.44 (95% CI −0.65 to −0.23; 7 trials, 366 participants; I2 = 0%; P < 0.001; Analysis 29.2.2); and mixed therapy SMD = −0.63 (95% CI −1.14 to −0.13; 7 trials, 378 participants; I2 = 80%; P = 0.01; Analysis 29.2.3).
29.2. Analysis.

Comparison 29: Subgroup analysis: mode of therapy, Outcome 2: Psychosocial functioning
30. Setting
30.1 BPD symptom severity
We compared the effects of inpatient versus outpatient settings for the outcome of BPD symptom severity. There were significant differences between subgroups (test for subgroup differences: Chi2 = 9.18, df = 1 (P = 0.002), I2 = 89.1%): inpatient SMD = −0.07 (95% CI −0.34 to 0.20; 2 trials, 217 participants; I2 = 0%; P = 0.61; Analysis 30.1.1); and outpatient SMD = −0.58 (95% CI −0.77 to −0.39; 20 trials, 1027 participants; I2 = 51%; P < 0.001; Analysis 30.1.2).
30.1. Analysis.

Comparison 30: Subgroup analysis: setting, Outcome 1: BPD symptom severity
30.2 Psychosocial functioning
We compared the effects of inpatient versus outpatient versus combined inpatient and outpatient settings for the outcome of psychosocial functioning. There were significant differences between subgroups (test for subgroup differences: Chi2 = 15.99, df = 2 (P = 0.0003), I2 = 87.5%): inpatient SMD = −0.85 (95% CI −1.24 to −0.46; 2 trials, 179 participants; I2 = 0%; P < 0.001; Analysis 30.2.1); outpatient SMD = −0.31 (95% CI −0.54 to −0.08; 18 trials, 1057 participants; I2 = 67%; P = 0.008; Analysis 30.2.2); and combined inpatient and outpatient SMD = −1.34 (95% CI −1.84 to −0.84; two trials, 78 participants; I2 = 0%; P < 0.001; Analysis 30.2.3).
30.2. Analysis.

Comparison 30: Subgroup analysis: setting, Outcome 2: Psychosocial functioning
31. Type of raters
31.1 BPD symptom severity
We compared the effects of self‐rated versus clinician‐rated for the outcome of BPD symptom severity. There were no significant differences between subgroups (test for subgroup differences: Chi2 = 1.79, df = 1 (P = 0.18), I2 = 44.2%): self‐rated SMD = −0.74 (95% CI −1.19 to −0.29; 8 trials, 408 participants; I2 = 77%; P = 0.001; Analysis 31.1.1); and clinician‐rated SMD = −0.42 (95% CI −0.58 to −0.25; 14 trials, 836 participants; I2 = 24%; P < 0.001; Analysis 31.1.2).
31.1. Analysis.

Comparison 31: Subgroup analysis: types of raters, Outcome 1: BPD symptom severity
31.2 Psychosocial functioning
We compared the effects of self‐rated versus clinician‐rated for the outcome of psychosocial functioning. There were no significant differences between subgroups (test for subgroup differences: Chi2 = 2.86, df = 1 (P = 0.09), I2 = 65.1%): self‐rated SMD = −0.29 (95% CI −0.60 to 0.03; 12 trials, 728 participants; I2 = 76%; P = 0.07; Analysis 31.2.1); and clinician‐rated SMD = −0.66 (95% CI −0.96 to −0.37; 10 trials, 586 participants; I2 = 54%; P < 0.001; Analysis 31.2.2).
31.2. Analysis.

Comparison 31: Subgroup analysis: types of raters, Outcome 2: Psychosocial functioning
32. Types of TAU
32.1 BPD symptom severity
We compared the effects of obligatory TAU versus optional TAU for the outcome of BPD symptom severity. There were no significant differences between subgroups (test for subgroup differences: Chi2 = 0.25, df = 1 (P = 0.61), I2 = 0%): obligatory TAU SMD = −0.50 (95% CI −0.70 to −0.30; 19 trials, 1071 participants; I2 = 60%; P < 0.001; Analysis 32.1.1); and unspecified TAU SMD = −0.63 (95% CI −1.09 to ‐0.17; 3 trials, 173 participants; I2 = 36%; P = 0.007; Analysis 32.1.2).
32.1. Analysis.

Comparison 32: Subgroup analysis: types of TAU, Outcome 1: BPD symptom severity
32.2 Psychosocial functioning
We compared the effects of obligatory TAU versus optional TAU for the outcome of psychosocial functioning. There were no significant differences between subgroups (test for subgroup differences: Chi2 = 3.18, df = 1 (P = 0.07), I2 = 68.6%): obligatory TAU SMD = −0.32 (95% CI −0.56 to −0.09; 17 trials, 1002 participants; I2 = 65%; P = 0.007; Analysis 32.2.1); and unspecified TAU SMD = −0.96 (95% CI −1.62 to −0.30; 5 trials, 312 participants; I2 = 84%; P = 0.004; Analysis 32.2.2).
32.2. Analysis.

Comparison 32: Subgroup analysis: types of TAU, Outcome 2: Psychosocial functioning
33. Types of comparison group
33.1 BPD symptom severity
We compared the effects of trials with TAU versus trials with waiting list for the outcome of BPD symptom severity. There were no significant differences between subgroups (test for subgroup differences: Chi2 = 0.01, df = 1 (P = 0.92), I2 = 0%): trials with TAU SMD = −0.52 (95% CI −0.70 to −0.33; 22 trials, 1244 participants; I2 = 57%; P < 0.001; Analysis 33.1.1); and trials with waiting list (SMD −0.49, 95% CI −0.93 to −0.05; 3 trials, 161 participants; I2 = 44%; P = 0.03; Analysis 33.1.2).
33.1. Analysis.

Comparison 33: Subgroup analysis: type of comparison group, Outcome 1: BPD symptom severity
33.2 Psychosocial functioning
We compared the effects of trials with TAU versus trials with waiting list for the outcome of psychosocial functioning. There were no significant differences between subgroups (test for subgroup differences: Chi2 = 0.18, df = 1 (P = 0.67), I2 = 0%): trials with TAU SMD = −0.45 (95% CI −0.68 to −0.22; 22 trials, 1314 participants; I2 = 72%; P = 0.0001; Analysis 33.2.1); and trials with waiting list SMD = −0.56 (95% CI −1.01 to −0.11; 5 trials, 219 participants; I2 = 59%; P = 0.01; Analysis 33.2.2).
33.2. Analysis.

Comparison 33: Subgroup analysis: type of comparison group, Outcome 2: Psychosocial functioning
34. Types of scales
34.1 BPD symptom severity
We compared the effects of ZAN‐BPD versus SCID versus BEST versus BPDSI on the outcome of BPD symptom severity. There were significant differences between subgroups (test for subgroup differences: Chi2 = 15.40, df = 3 (P = 0.002), I2 = 80.5%): ZAN‐BPD SMD = −0.45 (95% CI −0.69 to −0.20; 4 trials, 261 participants; I2 = 0%; P = 0.0004; Analysis 34.1.1); SCID SMD = −0.58 (95% CI −1.16 to −0.00; 3 trials, 112 participants; I2 = 47%; P = 0.05; Analysis 34.1.2); BEST SMD = −1.10 (95% CI −1.47 to −0.72; 4 trials, 147 participants; I2 = 10%; P < 0.001; Analysis 34.1.3); and BPDSI SMD = −0.21 (95% CI −0.45 to 0.04; 4 trials, 267 participants; I2 = 0%; P = 0.10; Analysis 34.1.4. For illustrative purposes, the results of other scales from single trials are report in Analysis 34.1.5 to Analysis 34.1.11.
34.1. Analysis.

Comparison 34: Subgroup analysis: types of scales, Outcome 1: BPD symptom severity
34.2 Psychosocial functioning
We compared the effects of the GAF versus GAS versus SAS on the outcome of psychosocial functioning. There were no significant differences between subgroups (test for subgroup differences: Chi2 = 1.39, df = 2 (P = 0.50), I2 = 0%): GAF SMD = −0.73 (95% CI −1.41 to −0.05; 4 trials, 204 participants; I2 = 78%; P = 0.04; Analysis 34.2.1); GAS SMD = −0.71 (95% CI −0.96 to −0.46; 4 trials, 330 participants; I2 = 0%; P < 0.001; Analysis 34.2.2); and SAS SMD = −0.23 (95% CI −0.99 to 0.53; 4 trials, 283 participants; I2 = 88%; P = 0.55; Analysis 34.2.3). For illustrative purposes, the results of other scales from single trials are reported in Analysis 34.2.4. to Analysis 34.2.13
34.2. Analysis.

Comparison 34: Subgroup analysis: types of scales, Outcome 2: Psychosocial functioning
Sensitivity analysis
We conducted a TSA as an sensitivity analysis on the significant findings for all primary outcomes and the secondary outcome of depression at end of treatment. The RIS was reached for BPD symptom severity, self‐harm, suicide‐related outcomes and psychosocial functioning. However, due to inconsistency for self‐harm and suicide‐related outcomes, we could only exclude the risk of a false positive finding (type 1 error) for BPD symptom severity and psychosocial functioning. The RIS was not reached for depression.
We used both the fixed‐effect and random‐effects models in all meta‐analyses. However, when there were two or more trials included in an analysis, we chose to report the results of the random‐effects model, which gives greater weight to smaller trials; where only one trial was included in an analysis, we reported the results of the fixed‐effect model. Statistical significance changed in 13 analyses and in those cases we reported the results of both the random‐effects and fixed‐effect models (see Analysis 3.7, Analysis 3.12, Analysis 4.1.3, Analysis 4.5.1, Analysis 4.7.1, Analysis 4.7.2, Analysis 4.7.4, Analysis 5.5.1, Analysis 6.4.1, Analysis 6.7.1, Analysis 14.3, Analysis 15.5, Analysis 18.12).
Discussion
Summary of main results
We considered 400 full‐text reports and, of these, we included 75 trials that randomised a total of 4507 participants. The age ranged from 14.8 to 45.7 mean years, and the majority of included persons were female. The length of the trials ranged from one to 36 months, with most of them performed in outpatient settings. We judged all included trials to be at high risk of bias.
DBT and related treatments were the most intensively studied, with more than one‐third of all included trials comparing DBT to TAU, a waiting list or an alternate active treatment (26 RCTs). Fifteen RCTs investigated the effects of CBT and related treatments, seven of which assessed MBT or eclectic treatments, five evaluated BPD‐specific psychodynamic therapies, four investigated SFT and related treatments, and three assessed STEPPS and IPT, two investigated TFP, PE or MOTR, and one evaluated CAT, ACT, JCP or motivation feedback, respectively.
In the following section, we summarise the main findings for the pooled analyses of psychotherapy versus TAU or waiting list for our primary outcomes (BPD symptom severity, self‐harm, suicide‐related outcomes and psychosocial functioning), and for those secondary outcomes that are not a part of the symptom characteristics of BPD (depression, attrition and adverse effects). We report end‐of‐treatment data. We advise the interested reader to explore the Effects of interventions section for a more elaborated description of the effect of psychotherapy on the remaining secondary outcomes, and follow‐up data. There, they will also find a complete overview of the effects of specific kinds of psychotherapy (such as DBT, MBT, CBT, etc.).
Psychotherapy versus TAU
Comparing the effects of psychotherapy with TAU, we observed significant effects for all primary outcomes at the end of treatment (Table 1).
Psychotherapy significantly reduced BPD symptom severity (SMD −0.52, 95% CI −0.70 to −0.33; 22 trials, 1244 participants) compared with TAU. This corresponds to a MD of −3.6 (95% CI −4.4 to −2.08) on the ZAN‐BPD scale, which ranges from 0 to 36. This represents a clinically relevant improvement in BPD symptoms. The MIREDIF on this scale is −3.0 points (Crawford 2018a).
Psychotherapy also showed a significant reduction in self‐harm when measured as a continuous outcome (SMD −0.32, 95% CI −0.49 to −0.14; 13 trials, 616 participants), but not a dichotomous outcome. The significant effect of continuous outcomes corresponds to a MD of −0.816 (95% CI −1.25 to 0.35) on the DSHI, which ranges from 0 to 34. The MIREDIF on this scale is −1.25 points, which corresponds to ½ SD (Farivar 2004). Clinically, this finding does not represent an important reduction in self‐harm for people with BPD.
Additionally, suicide‐related outcomes were significantly reduced in both continuous (SMD −0.34, 95% CI −0.57 to −0.11; 13 trials, 616 participants) and dichotomous outcomes (RR 0.27, 95% CI 0.11 to 0.67; 5 trials, 396 participants). The improvement corresponded to a MD of −0.11 (95% CI −0.19 to −0.034) on the Suicidal Attempt Self Injury Interview. The MIREDIF on this scale is −0.17 points, which corresponds to ½ SD (Farivar 2004). These findings suggest that suicide‐related outcomes improved following treatment, but these improvements did not quite reach a clinically relevant level. However, when it comes to suicide or suicide attempts, any reduction is of importance.
Psychosocial functioning improved significantly following psychotherapy compared with TAU (SMD −0.45, 95% CI −0.68 to −0.22; 22 trials, 1314 participants). This corresponds to a MD of −2.8 (95% CI −4.25 to −1.38) on the GAF Scale, which ranges from 0 to 100. The MIREDIF on this scale is −4.0 points (Amri 2014); therefore, the improvement did not reach clinical relevance.
Regarding our secondary outcomes, we observed a significant reduction in depression for participants receiving psychotherapy compared to TAU (SMD −0.39, 95% CI −0.61 to −0.17; 22 trials, 1570 participants). This corresponds to −2.45 on the Hamilton Depression Scale, and does not quite reach the MIREDIF on this scale, which is 3.0 points (NICE CG90) .
Sufficient data were not available for effect size calculation for the outcome of adverse effects.
Our additional TSA on all significant findings of the primary outcomes found that the RIS was reached for all primary outcomes but not for the secondary outcome of depression. However, only for BPD symptoms severity and psychosocial functioning could we reject the possibility of type 1 error.
Only 15 trials reported on the effect following end of treatment at zero to six months, six to 12 months and > 12 months (Amianto 2011; Andreoli 2016; Bateman 1999; Blum 2008; Davidson 2006; Gleeson 2012; Gregory 2008b; Jørgensen 2013; Kredlow 2017a; Linehan 2006; McMurran 2016; Robinson 2016; Soler 2009; Van den Bosch 2005; Weinberg 2006). There was no effect on BPD severity at follow‐up when assessed as a continuous measure; however, an improvement was observed at > 12 months follow‐up in the number of people presenting with severe BPD. In addition, the number of persons with self‐harm was reduced at the zero to six months, six to 12 months and > 12 months follow‐up time points. Suicide‐related behaviour was reduced at six to 12 months follow‐up, and a reduction in the number of persons with suicidal behaviour was seen at > 12 months follow‐up. There was an improvement in psychosocial functioning at zero to six months follow‐up; however, this was not sustained at six to 12 months or at > 12 months follow‐up time points.
Regarding the secondary outcomes, there was an improvement in depression at zero to six months and > 12 months follow‐up, as well as a reduction in adverse effects at > 12 months follow‐up only. No improvement in anger, affective instability, impulsivity, emptiness, interpersonal problems or abandonment was observed at follow‐up.
Psychotherapy versus waiting list or no treatment
Comparing the effects of psychotherapy with waiting list, we observed effects in favour of psychotherapy for the primary outcomes of BPD severity (SMD −0.49, 95% CI −0.93 to −0.05; 3 trials, 161 participants) and psychosocial functioning (SMD −0.56, 95% CI −1.01 to −0.11; 5 trials, 219 participants. No clear differences were found for self‐harm and suicide‐related outcomes. See Table 2.
We moreover observed a significant reduction in depression for participants receiving psychotherapy compared to waiting list or no treatment (SMD −1.28, 95% CI −2.21 to −0.34; 6 trials, 239 participants). No reductions were detected in attrition, and once again, sufficient data were not available for effect size calculation for the outcome of adverse effects.
Only one trial, Bellino 2010, reported on the effect following end of treatment (zero to six months, six to 12 months and > 12 months follow‐up periods). Follow‐up data were reported on psychosocial functioning, in which an improvement was observed at zero to six months and six to 12 months post‐treatment. There was no significant improvement observed at > 12 months post‐treatment. Regarding the secondary outcomes, improvements in both impulsivity and interpersonal problems were observed at zero to six months, six to 12 months and > 12 months post‐treatment. No improvement was observed in affective instability at any of the follow‐up time points.
Individual treatment approaches
Among distinct BPD‐specific therapies, most findings were based on single trials. DBT and MBT had the highest numbers of primary trials, with DBT accounting for roughly one‐third of all included trials, followed by MBT with seven RCTs.
Compared to TAU, we observed significant effects of DBT for the primary outcomes of BPD severity (SMD −0.60, 95% CI −1.05 to −0.14; 3 trials, 149 participants), self‐harm (SMD −0.28, 95% CI −0.48 to −0.07; 7 trials, 376 participants) and psychosocial functioning (SMD −0.36, 95% CI −0.69 to −0.03; 6 trials, 225 participants). MBT was found to have significant effects on self‐harm (RR 0.62, 95% CI 0.49 to 0.80; 3 trials, 252 participants) and suicidality (RR 0.10, 95% CI 0.04, 0.30, 3 trials, 218 participants). See Table 3.
Subgroup analysis and investigation of heterogeneity
We had intended to conduct several subgroup analyses, but not all were possible due to a lack of data. See Table 4 for more information.
In one subgroup analysis, we tested for differences between psychotherapeutic approaches appearing in two or more trials. We found no significant subgroup differences between the different therapeutic approaches for BPD symptom severity and psychosocial functioning.
To test the statistical heterogeneity in our analyses of BPD symptom severity and psychosocial functioning, we added a post hoc subgroup analysis exploring the different measurement scales versus each other. We found significant differences between ZAN‐BPD, SCID, BEST and BPDSI, with BEST providing the highest effect estimate. By differentiating between scales, we found that the heterogeneity between trials was I2 = 0% with ZAN‐BPD; I2 = 47% with SCID; I2 = 10% with BEST; and I2 = 0% with BPDSI. The results suggest that much of the statistical heterogeneity found in the analysis of psychotherapy versus TAU for BPD symptom severity was due to the various scales used in trials. We found no significant differences between GAF, GAS and SAS, and no reductions in heterogeneity.
We found significant subgroup differences for BPD symptom severity for age, where the effects of psychotherapy were higher for the subgroup 'older than 18 years of age'; 'mode of therapy', with group therapy being more effective than individual or mixed therapy; and setting, with outpatient groups being more effective than inpatient groups. Additionally, we found significant differences for psychosocial functioning for setting, with the combination of inpatient and outpatient groups and inpatient groups alone showing to be more effective than outpatient groups. We found no subgroup differences for BPD symptom severity for duration and type of raters, or for psychosocial functioning for duration, mode of therapy and types of raters.
trials of psychotherapy versus TAU did not indicate any significant subgroup differences for BPD symptom severity and psychosocial functioning compared with trials of psychotherapy versus waiting list or no treatment. Likewise, we found no subgroup differences for the different types of TAU. Therefore, effect estimates do not seem to differ between comparisons with control groups consisting of any kind of unspecified intervention, such as TAU, standard care or regular appointments, and comparisons with waiting list or no treatment, the participants of which were not paid any special attention at all.
Previous review
In contrast to the previous versions of this review (Binks 2006; Stoffers‐Winterling 2012), we broadened the scope to include the treatment of adolescents also. We were able to include only three RCTs that focused solely on adolescents: the Mehlum 2014 trial on DBT‐A; the Rossouw 2012b trial on MBT‐A; and the Schuppert 2012 trial on ERT, an adaptation of STEPPS for adolescents. Another pilot RCT on HYPE + CAT + SFET included participants from ages 15 to 25 years old, with a mean age of 18.4 years (SD = 2.9) (Gleeson 2012). Subgroup analyses revealed that adolescents benefited less from psychotherapy than those older than 18 years of age. However, the power was much too low to detect any true effects, with sample sizes ranging from 10 participants (subsample of participants with five or more DSM‐IV BPD criteria in the Mehlum 2014 trial) to 97 participants (in Schuppert 2012). Notably, we only included trial samples or subsamples in which at least 70% of participants had full BPD (i.e. fulfilled a minimum of five DSM BPD criteria). Oftentimes, however, adolescents may only fulfil criteria at a subthreshold level while both their personality and BPD are still developing. Nevertheless, they might profit from BPD‐specific treatments in terms of preventing the development of full threshold BPD later in life.
Overall completeness and applicability of evidence
In most cases, we were able to calculate the effect sizes from the data reported in the included trials. If information was missing, we contacted and requested the missing information from the trial authors. Therefore, there is no evident bias within this review as a consequence of inadequate inclusion of existing data.
We rated all trials at high risk of bias, with the most prevalent risk of bias being categorised as 'other risk of bias'; that is insufficient treatment adherence, allegiance bias or attention bias, which might lead to biased data.
Participants
The majority of trials included mainly females, with only two trials solely including males (Bianchini 2019; Kamalabadi 2012). This configuration may reflect the reality of clinical settings, where approximately 75% of all BPD diagnoses are assigned to women (APA 2000). There are, however, contradictory findings pointing towards balanced proportions or an even higher prevalence in men (Sansone 2011). Men with BPD may display a different clinical picture than women, especially with antisocial features being more prevalent in men than in women with BPD (Sansone 2011). Clinically, this may mediate different treatment approaches or special requirements, which may not be reflected in most of the RCT evidence, which stems from either all female or predominantly female samples (see the Characteristics of included studies tables). In addition, in several of the included trials, full antisocial personality disorders or antisocial features were reasons for exclusion. As such, the extent to which the findings in this review are applicable to men with BPD may be limited. Level of functioning at baseline was not available in several of the included trials. However, trials that reported level of functioning using objective measures, such as GAS, GAF, CGI or SFQ, showed that the degree of illness ranged from moderate to severe. Regarding comorbidity, most trials excluded participants with severe mental disorders, organic brain disorders and mental impairments. In sum, the findings of this review may apply mainly to females with a moderate to severe level of illness, and without severe comorbid mental disorders. We do not know how the lack of this information has affected our findings, only that we cannot generalise the findings to men, to females with low levels of illness or to those with severe comorbid mental disorders. On the other hand, several trials concentrated on people with BPD plus distinct comorbidities (i.e. PTSD, depression, eating disorders or substance use disorders). Moreover, subgroups analyses revealed a significant difference, where the effect of psychotherapy on BPD symptom severity and psychosocial functioning seemed lower for adolescents. However, only three trials on adolescents were included in the review, and readers should exercise caution when drawing conclusions.
Some argue that BPD treatment should start before the condition has developed fully, and stress the importance of early assessment and treatment (Chanen 2013; Sharp 2015). Therefore, psychological interventions may also be relevant for patients who have yet to fully fulfil the DSM‐5 criteria. Nevertheless, with respect to the patient sample in this review, we only included adolescent samples if at least 70% of them fulfilled (a minimum five) DSM‐5 criteria for a diagnosis of BPD. We decided to do so in order to assure a minimum level of clinical homogeneity throughout the overall pool of trials.
Interventions
The major psychotherapeutic treatments used in the treatment of BPD (DBT, MBT, SFT, TFP) have been tested in the RCTs included in this review. However, the number of available trials per intervention vary broadly, with the majority of trials investigating DBT; a very limited number of trials assessed SFT and TFP especially, which may not reflect their prevalence in clinical practice. The vast majority of primary trials were conducted in outpatient settings. However, psychotherapy may also be provided on inpatient wards (depending on the respective health system), but only limited evidence was available for this setting. Subgroups analyses showed no evidence of significant differences between psychotherapeutic approaches, indicating that all individual types of psychotherapy are effective in treating people with BPD. Most trials allowed concurrent psychotherapy and psychotropic treatments. As such, it cannot be ruled out that the concurrent treatment may have confounded the corresponding findings. However, we only included trials if possible concurrent treatments were the same for all trial groups.
Some interesting findings revealed that group therapy might be more effective than individual or mixed therapy for reducing BPD symptom severity. However, there was no evidence of subgroup differences for psychosocial functioning. Additionally, outpatient care seemed to be more effective than inpatient care in reducing BPD symptom severity, but not in improving psychosocial functioning, where a combination of inpatient and outpatient treatment was more effective. The duration of treatment does not seem to be a factor in the improvement of BPD symptoms and psychosocial functioning.
Comparisons
The treatments used in the control groups were largely heterogeneous, including TAU, which is a common label for controls. TAU itself may differ between different treatment formats, let alone between different health systems. In some trials, participants receiving TAU were free to use any kind of care, whereas other participants in receipt of TAU were required to receive a minimum standard of care whilst taking part in the trial (see Types of interventions). Consequently, the components of TAU will most certainly vary between countries, as the standard of care is known to differ depending on the national healthcare system.
The use of a control group in randomised trials provides a point of reference from which the effect of a given intervention is measured against. As such, a 'clean' control group is essential when we want to obtain insight into the magnitude of treatment effects and potential adverse effects. In psychiatric research, there is a lack of consensus on what constitutes an appropriate control intervention (Finch 2019). In accordance, we found that control groups across trials were heterogeneous and included, for example, TAU, standard care or waiting list. In 72% of the included trials, either psychotherapeutic treatment was a part of the control intervention or concomitant psychotherapy was allowed while participating in the trial (for more information see Characteristics of included studies tables). As such, a majority of trials compared psychotherapeutic therapy against another intervention that included psychotherapeutic elements. The willingness to let people affected by BPD continue with different variations of psychotherapeutic therapy while included in a control group may indicate a general concern of otherwise withholding them from an important treatment. Yet a drawback of this approach is that it could potentially mask the difference in treatment effects between the control and intervention groups. Concurrently, we found that effects sizes were small, heterogeneous and largely insignificant for the majority of comparisons. There is a substantial need for larger, well‐performed randomised trials that include standardised control groups before we can make firmer conclusions about the effects of psychological therapies in BPD.
Outcomes
The individual trials included a great variety of outcomes, with only minor consensus on what constituted core outcomes for BPD and how these ideally should be assessed. We identified high heterogeneity and significant differences between scales for BPD symptom severity, which might indicate that different scales may give different effect estimates. We found no significant differences between scales for psychosocial functioning, and there were no significant differences for self‐rated and clinician‐rated outcomes. In addition, core symptoms, such as fewer interpersonal conflicts or crises that both caregivers and people with BPD may consider essential when searching for helpful treatments, are sometimes neglected. The outcome of attrition must be interpreted with caution, as the likelihood of leaving a trial may depend on the specific type of control group used. For example, participants assigned to waiting lists may be more likely to leave a trial early than participants assigned to TAU or participants who are provided with regular appointments. Geographical conditions and accessibility of study centres can also play an important role, as rural regions with long distances to study centres may lead to higher dropout rates than urban settings (Blum 2008 trial completer analysis: high dropouts).
Most trials did not consider adverse effects but did consider suicidal or self‐harming behaviour. These outcomes were categorised as primary outcomes in this review (see Appendix 4) and are usually classified as severe adverse effects. Future randomised trials should not only concentrate on these outcomes, but also transparently report non‐serious adverse effects that occur during BPD treatment. Adverse effects of importance would include increased impulsive behaviour or substance use. Such outcomes may occur if psychotherapy induces excessive emotional arousal with which the affected person is not (yet) able to cope (Berk 2009).
For more information, see the Characteristics of included studies tables, where we describe the characteristics of each included trial in greater detail.
Quality of the evidence
We rated the quality of evidence using the GRADE approach, based on the following considerations; risk of bias, inconsistency, indirectness, imprecision and publication bias.
We assessed the risk of bias in all trials in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). The majority of the trials were prone to selection bias, as well as attrition and reporting bias. High risk of bias in randomised clinical trials has been shown to overestimate benefits and underestimate harms (Kjaergard 2001; Lundh 2012; Moher 1998; Savović 2012a; Savović 2012b; Schulz 1995; Wood 2008). The results are based on 75 trials that involved a limited number of people (n = 4507). Most trials included small sample sizes, with the number of participants ranging from 7 to 151 in the individual trials. Heterogeneity in the reporting of outcomes and the interventions investigated in the individual trials hindered the pooling of effect estimates. Except for DBT, ERGT, MBT and MOTR, the review findings are based largely on single trial effects that could not be pooled. The small samples and inability to pool results led to imprecise effect estimates.
The highest risk of bias we observed in the individual trials was for the category of 'other bias', covering attention, affiliation or adherence bias. We rated approximately two‐thirds of trials (49 out of 75) as having a high risk of bias, and another 21 RCTs as having an unclear risk of bias, leaving only five (i.e. 6.7%) RCTs at low risk of bias. We rated two‐thirds of trials as having either a high or an unclear risk of bias in terms of both incomplete outcome data and selective reporting (only 19 trials out of 75 had a low risk of bias in these categories). We rated half or more of the trials as having applied appropriate means of random sequence generation, allocation concealment, and blinding of outcome assessment.
From the analyses regarding psychotherapy and DBT versus waiting list or no treatment for suicide‐related outcomes and depression, we found one trial to be an outlier (Mohamadizadeh 2017). The trial was rated at high risk of bias due to unclear risks of bias in all domains. To ensure transparency, we did not exclude it from the analyses. However, sensitivity analyses showed that the trial's inclusion did not change any conclusions on the aforementioned outcomes.
Notably, there were only two RCTs out of 75 that included medication‐free patients only. The majority of trials allowed participants to continue their respective drug treatments. There were no reports of different medication rates between the groups at baseline from any trial. Apart from those trials that included medication as a compulsory part of their respective treatment regimens for both groups (Bellino 2006; Bellino 2007; Bellino 2010; Jahangard 2012), 24 trials reported on actual medication use during the trial observation period: 17 found no difference in medication use between the groups (Antonsen 2017; Blum 2008; Bohus 2013; Bos 2010; Doering 2010; Elices 2016; Gleeson 2012; Leichsenring 2016; Lin 2019; Linehan 2015a; McMain 2009; McMain 2017; Mohamadizadeh 2017; Reneses 2013; Schuppert 2012; Sinnaeve 2018; Van den Bosch 2005); six reported a higher use of medications in their control groups (Bateman 1999; Bateman 2009; Gregory 2008; Linehan 1994; Linehan 2006; Turner 2000); and only one found a higher rate in the active treatment group (Andreoli 2016). Therefore, medication use as a possible confounding variable can be ruled out whenever beneficial effects for the experimental group were observed.
Potential biases in the review process
This review was designed to minimise the risk of potential biases. We developed a protocol, Storebø 2018, for this review in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). We conducted extensive searches of relevant databases, with no restrictions in language, publication year, publication type or publication status. Two independent review authors selected appropriate trials, extracted data, assessed the risk of bias and graded the quality of the evidence. Disagreements were resolved through discussion. We conducted a TSA, to estimate the RIS needed to either detect or reject a certain intervention effect.
With regard to our inclusion criteria (Criteria for considering studies for this review), we tried to retain a homogeneous pool of primary trials. However, there were some inconsistencies between trials, particularly pertaining to psychiatric comorbidity of trial participants. For example, although the presence of a substance‐related disorder was a common exclusion criterion (see Types of participants), two trials required participants to have such a disorder (Gregory 2008b; Santisteban 2015), and another trial included a mixed sample of participants with and without substance abuse problems (Van den Bosch 2005). In addition, the severity of illness varied between trials, ranging from severe to mild.
We also went to great effort to try and obtain unpublished data pertaining to BPD subsamples where the full sample included fewer than 70% of participants with full BPD. We were able to obtain such subsample data from some trials, but in other cases, trial authors did not respond. We do not know why they did not respond, but it is possible that the subsample data we received are biased in a positive way (i.e. being associated with desired findings).
Agreements and disagreements with other studies or reviews
This updated review has a new conclusion compared to the review published in 2012 (Stoffers‐Winterling 2012). The 2012 review concluded that there were indications of beneficial effects for psychological therapies for BPD core pathology and associated general psychopathology. The overall findings supported a substantial role for psychotherapy in the treatment of people with BPD. However, the authors also reported that the treatments did not have a very robust evidence base, as there were some concerns regarding the quality of individual trials, and therefore indicated a need for replication trials (Stoffers‐Winterling 2012). Following the update of this review, we are now able to state, for the first time, that BPD‐specific psychotherapy seem to be better than usual or control treatments for reducing BPD severity in persons affected by BPD, with effect sizes of clinical relevance. This change in conclusion may result from the following: in 2012, only 28 trials were available for inclusion, and any comparisons were done on treatment approach levels. This means that the review authors did not pool any kind of BPD‐specific treatment, only DBT, MBT and so on. This new review has used updated methods, including a broader search strategy, and thus a new protocol was published in 2018 (Storebø 2018). What is more, this version is the first to include the required information sizes, for at least some comparisons, on the level of BPD‐specific approaches in general (see Table 1), allowing for drawing conclusions on a much more reliable basis.
Our review is in partial agreement with the review by Cristea and colleagues who found that psychotherapy, especially DBT and psychodynamic approaches, are effective for borderline symptoms and related problems (Cristea 2017). Similarly, we found that psychotherapy appears to be more effective than usual treatment at improving BPD symptoms; however, we found no clear differences between the various treatments. We agree that DBT treatments in particular, seem to have a more robust evidence base, due to more clinical trials having been conducted. This discrepancy in conclusions may be due to differences in methodological approach. The study by Cristea and colleagues searched for data in only four databases and included only one publication per trial. They also did not use subsamples of persons with BPD and did not perform an assessment of the overall quality of the evidence. Taken together these limitations suggest that their conclusions may be too optimistic (Faltinsen 2017).
Matthijs Oud and colleagues published a review in 2018 (Oud 2018), which investigated the effects of specialised psychotherapy for BPD. In contrast to our review, they focused on four BPD‐specific treatments (DBT, MBT, SFT, and TFP) and only included adult samples, resulting in the inclusion of 20 trials. In line with the findings of this review, they reported moderate‐quality evidence that psychotherapy is effective in reducing overall BPD severity (SMD of −0.59 compared to our SMD of −0.52). We took a broader approach and included all types of psychotherapy conducted in participants of all ages, which resulted in a more complete picture of the area with a larger evidence base (Oud and colleagues included only 321 participants in their analysis of BPD severity compared to 1244 participants in our analysis of the same outcome).
Authors' conclusions
Implications for practice.
The interventions included in this review represent a broad spectrum of therapies, covering cognitive‐behavioural therapy (CBT), dialectical behavioural therapy (DBT), DBT skills group, eclectic therapy, psychodynamic therapy, systems training for emotional predictability and problem‐solving (STEPPS), schema‐focused therapy (SFT), interpersonal psychotherapy and mentalisation‐based therapy.
Analyses showed beneficial effects of psychotherapy compared to TAU for all primary outcomes. However, only the improvement in BPD severity reached the level of a clinically important improvement compared to the MIREDIF; we considered this to be moderate‐quality evidence, meaning that additional research could impact our confidence in the effect estimate and may change the estimate. All trials had a high risk of bias and, for most outcomes, we considered the data to be of low quality due to imprecision, small sample sizes and inconsistency.
We found no unequivocal, high‐quality evidence to support one BPD‐specific therapy over another in the treatment of BPD; our subgroup analyses showed no differences in effect estimates between the different types of therapies. However, compared to TAU, we observed significant effects in favour of DBT for the primary outcomes of BPD severity, self‐harm and psychosocial functioning, and in favour of MBT for self‐harm, suicidality and depression. We rated the quality of the evidence for these outcomes as low, meaning that the true magnitude of these effects is uncertain.
In relation to implications for practice, low‐certainty evidence shows that that BPD‐tailored therapies (like DBT, MBT, CBT, SFT, TFP and ACT) may be more effective than usual treatments. We must await ongoing and new trials that can compare more validly the different treatment effects for the various types of treatment for BPD, including head‐to‐head comparisons. Current evidence indicates that psychotherapy, especially BPD‐tailored approaches, have beneficial effects on a variety of outcomes. However, only the decrease in BPD symptom severity was found to be clinically important. In order to help people affected by BPD decide on treatment according to their preferences and values, they should be informed about the variety of treatments available, working models, treatment durations and related information.
Though a subgroup analysis indicated that adolescents may benefit less from psychotherapy compared to adults regarding overall BPD severity, this finding is based on low‐quality evidence only and very few observations (only two RCTs of adolescents included; Analysis 27.1.1), so the true effect remains uncertain. In practice, many adolescents often present with BPD symptoms on a subthreshold level, not yet reaching the full diagnostic threshold for BPD. Subthreshold BPD symptoms (even with only one BPD feature present) have been shown to be clinically important and correlated with the presence of mental state disorders, reduced social functioning and suicidal behaviour (Thomson 2019). Therefore, RCTs on early intervention for BPD in adolescence often include participants with subthreshold BPD. We have not included such participants in this review despite accumulating research findings that support the conduct of early interventions (Chanen 2016; Chanen 2018).
Implications for research.
In order to thoroughly investigate the efficacy of the treatments described in this review, there is a need for further high‐quality RCTs with larger patient groups. Here, it is essential that individual researchers are not involved in the delivery or the development of the treatment being investigated. Additionally, to assess the efficacy of psychotherapeutic treatments in BPD, there is a need for a greater consensus on what constitutes the core outcomes of BPD. Here, the selection of outcomes should be based on what people with BPD and their caregivers consider to be of value. Besides the core symptoms of BPD, which are still included in the DSM‐5 (APA 2013) and will be retained in ICD‐11 (WHO 2018), the dimensional outcome domains of personality disorders should also be considered, as this would foster the applicability of BPD research to non‐BPD personality disorders. Furthermore, assessment of outcomes should be based on validated, universal scales that enable a comparison of treatment effects between trials. Fortunately, BPD‐specific measures have been developed and used more often during the past decade. Yet for some important BPD‐specific outcomes (like 'avoidance of abandonment', 'feeling of emptiness'), assessment still relies on single items of questionnaires (like the Zan‐BPD (Zanarini 2003a) or BPDSI‐IV (Arntz 2003)). The development of validated assessments tools would be helpful.
Future trials should include standardised control interventions that are carefully considered and reported fully in order to get a clear picture of what the psychological therapies are being compared to. More research is needed, when it comes to understanding the effects of interventions in certain groups of people, especially adolescents and the elderly, but also in men who are still under‐represented in BPD research. Long‐term trials should be conducted in order to understand which treatment effects are maintained and which diminish over time.
Our findings that different BPD‐specific treatments are helpful is certainly in line with all‐day experiences of clinicians. There are some interventions beyond the prominent paradigms for which we found encouraging results and that deserve further attention. These especially include add‐on therapies such as STEPPS and ERGT. Though the different treatment approaches are theoretically divergent and postulate different mechanisms of change (Gunderson 2018), analyses of the technical aspects reveal many commonalities in terms of structure, focus and interventions, including general factors across therapies such as a structured treatment framework, consistent focus on the therapeutic relationship, active therapists and therapist support (Bateman 2015; De Groot 2008; Kongerslev 2015; Weinberg 2011). However, more research is needed to understand the shared mechanisms of these specialist treatments, and how they contribute to change in BPD symptomatology (Karterud 2020). Future RCTs should consider incorporating measurements of diverse mechanisms of change that are known to be generally effective across therapies and diagnoses (e.g. focusing on the working alliance). This would allow for investigating the role of general mechanisms of change across different treatments in terms of outcome (Chatoor 2001; Kazdin 2009).
Feedback
Feedback, 8 October 2020
Summary
The study ‘Democratic therapeutic community treatment for personality disorder: randomised controlled trial.’ published in the British Journal of Psychiatry in 2017, seems not to have been included in the review, either in the included or excluded studies. Given that it meets the criteria for inclusion, this looks like an oversight. In particular, the study included over 90% of participants suffering from Borderline Personality Disorder; it is an RCT; and it was published in one of the journals which was hand searched. It falls within the time limits of the searches carried out for the review. Authors were not approached to supply data on the subsample of participants with a Borderline Personality Disorder diagnosis. Grateful for your comments on this. Sincerely Dr S Pearce Reference: Pearce S, Scott L, Attwood G, Saunders K, Dean M, De Ridder R, Galea D, Konstandinidou H, Crawford M. Democratic therapeutic community treatment for personality disorder: randomised controlled trial. Br J Psychiatry (2017), Vol. 210, 149‐156. (doi.org/10.1192/bjp.bp.116.184366).
Reply
Thank you for the comment augmenting that we should have included the trial in our Cochrane systematic review: 'Psychological therapies for people with borderline personality disorder'.
We did identify your trial (available at doi.org/10.1192/bjp.bp.116.184366) of democratic therapeutic community (DTC) in our searches, but we excluded it at the title and abstract screening stage, because the intervention is not eligible for our review. In light of your comment, we have discussed this further and have reached the same decision: that it is not eligible for the following reasons.
The intervention that you tested is a socioenvironmental therapy, because it consists of being part of a therapeutic community that is built on democratic principles (consisting of shared decision‐making around group matters, discussion of behavior, feedback on behavior, shared living, creating a community and activities, etc.). Therefore, we regard it as a socioenvironmental intervention rather than psychotherapy.
In our Methods section, we wrote the following about the types of interventions to be included: "Any defined psychological intervention regardless of theoretical orientation (e.g. psychodynamic therapy, CBT, systemic therapy or eclectic therapies designed for BPD treatment), in any kind of treatment setting (e.g. inpatient, outpatient or day clinic)".
Furthermore, in our Background section (Description of the intervention), we noted that although therapeutic community can be used as an add‐on therapy to psychotherapy, we would not regard it as the core therapy in itself and we have excluded trials with similar interventions (e.g. the Carta 2014 trial: 'Sailing can improve quality of life of people with severe mental disorders: results of a cross over randomized controlled trial').
Contributors
Dr S Pearce, Honorary Senior Clinical Lecturer, University of Oxford; Dr Ole Jakob Storebø, Psychiatry of Region Zealand, Denmark; Prof Mike Clarke, DPLP Feedback Editor.
What's new
| Date | Event | Description |
|---|---|---|
| 2 November 2020 | Feedback has been incorporated | Feedback from Dr Pearce submitted via Cochrane Library on 8 October (supplied to DPLP by EMD). No change made. |
History
Protocol first published: Issue 2, 2018 Review first published: Issue 5, 2020
| Date | Event | Description |
|---|---|---|
| 5 May 2020 | Amended | Adding acknowledgement for peer reviewers. |
Notes
This is a new review, which replaces the following, published review: Stoffers JM, Völlm BA, Rücker G, Timmer A, Huband N, Lieb K. Psychological therapies for people with borderline personality disorder. Cochrane Database of Systematic Reviews 2012, Issue 8. Art. No.: CD005652. DOI: 10.1002/14651858.CD005652.pub2.
Acknowledgements
We thank the Cochrane Developmental, Psychosocial and Learning Problems (CDPLP) Editorial Team, especially Joanne Duffield (Managing Editor), Margaret Anderson (Information Specialist), and Geraldine Macdonald (Co‐ordinating Editor).
We are grateful to the German Cochrane Centre for supporting this work, especially Gerd Antes, Director of the German Cochrane Centre, who initially made contact with CDPLP. We thank Maja Kielsholm and Signe Nielsen for their help on the review.
We are grateful to the following peer reviewers for their time and comments: Anees Bahji, Queen’s University, Canada; Fiona Ng, University of Nottingham, UK; and Abhijna Vithal Yergolkar, India. We also thank the following reviewers for their contributions to an earlier version of this review: Gerta Rücker, Antje Timmer and Nick Huband.
The previous version of this review was supported by the Nottinghamshire Healthcare NHS Trust, UK; NHS Cochrane Collaboration Programme Grant Scheme, UK; German Federal Ministry of Education and Research, Germany (grant no. 01KG0609); and a stipend to JSW by the Ministry of Science, Research and Arts of the federal state of Baden‐Württemberg, Germany.
Appendices
Appendix 1. DSM diagnostic criteria for BPD (301.83)
| DSM ‐ Third Edition (DSM‐III;APA 1980) | DSM ‐ Fourth Edition ‐ Text Revision (DSM‐IV‐TR;APA 2000) | DSM ‐ Fifth Edition (DSM‐5;APA 2013) |
| 301.83 BPD | 301.83 BPD | 301.83 BPD |
| Diagnostic criterion A | ||
5 of the following are required
|
A pervasive pattern of instability of interpersonal relationships, self‐image, and affects, and marked impulsivity beginning by early adulthood and present in a variety of contexts, as indicated by 5 (or more) of the following
|
A pervasive pattern of instability of interpersonal relationships, self‐image, and affects and marked impulsivity, beginning by early adulthood and present in a variety of contexts, as indicated by 5 (or more) of the following
|
| Diagnostic criterion B | ||
| If under 18, does not meet the criteria for identity disorder | ‐ | ‐ |
| BPD: Borderline personality disorder; DSM: Diagnostic and Statistical Manual of Mental Disorders | ||
Appendix 2. ICD‐10 research criteria for emotionally unstable personality disorder (F60.3)
| F60.30: Emotionally unstable personality disorder, impulsive type | F60.31: Emotionally unstable personality disorder, borderline type |
| Diagnostic criterion A | |
| The general criteria of personality disorder (F60) must be met | The general criteria of personality disorder (F60) must be met |
| Diagnostic criterion B | |
| At least 3 of the following must be present, 1 of which is 2 1. Marked tendency to act unexpectedly and without consideration of the consequences 2. Marked tendency to quarrelsome behaviour and to conflicts with others, especially when impulsive acts are thwarted or criticised 3. Liability of outbursts of anger or violence, with inability to control the resulting behavioural explosions 4. Difficulty in maintaining any course of action that offers no immediate reward 5. Unstable and capricious mood |
At least 3 of the symptoms mentioned above in criterion B (F60.30) must be present, and, in addition, at least 2 of the following 6. Disturbances in, and uncertainty about, self‐image, aims and internal preferences (including sexual) 7. Liability to become involved in intense and unstable relationships, often leading to emotional crises 8. Excessive efforts to avoid abandonment 9. Recurrent threats or acts of self‐harm 10. Chronic feelings of emptiness |
| ICD‐10: International Classification of Diseases, Tenth Edition | |
Appendix 3. Search strategies
Cochrane Central Register of Controlled Trials, in the Cochrane Library
#1 MeSH descriptor: [Borderline Personality Disorder] explode all trees #2 borderline next state* #3 borderline next personalit* #4 "axis II" or "cluster B" #5 idealization next devaluation #6 (vulnerable or hyperbolic) next temper* #7 (((unstab* or instab* or poor or disturb* or fail* or weak* or dysregulat*) next (self* or impuls* or interperson* or identit* or relation* or emotion* or affect*)) and (person* or character or PD)) #8 impulsiv* near personalit* #9 (self next (injur* or damag* or destruct* or harm* or hurt* or mutilat*)) #10 suicidal next behavio?r #11 (feel* next (empt* or bored*)) #12 (anger next control*) #13 (risk‐taking next (behavior or behaviour)) #14 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13
Medline Ovid
1 Borderline Personality Disorder/ 2 ((borderline or border‐line) adj3 (state* or personalit*)).kf,tw. 3 ("Axis II" or "Cluster B" or flamboyant or "F60.3" or "F60.30" or "F60.31").kf,tw. 4 (idealization adj5 devaluation).kf,tw. 5 ((vulnerable or hyperbolic) adj3 temperament).kf,tw. 6 (((unstab* or instab* or poor or disturb* or fail* or weak or dysregulat*) adj3 (self* or impuls* or interperson* or identit* or relationship* or emotion* or affect*)) and (personality or character or PD)).kf,tw. 7 (impulsiv* adj5 (behavio?r or character or personalit*)).kf,tw. 8 (self adj3 (injur* or damag* or destruct* or harm* or hurt* or mutilat*)).kf,tw. 9 (suicidal adj3 behavio?r).kf,tw. 10 (feel* adj3 (empt* or bored*)).kf,tw. 11 (anger adj5 control*).kf,tw. 12 (risk‐taking adj3 behavio?r).kf,tw. 13 or/1‐12 14 randomised controlled trial.pt. 15 controlled clinical trial.pt. 16 randomi#ed.ab. 17 placebo.ab. 18 randomly.ab. 19 trial.ab. 20 groups.ab. 21 drug therapy.fs. 22 or/14‐21 23 exp Animals/ not Humans/ 24 22 not 23 25 13 and 24
Embase Ovid
1 borderline state/ 2 ((borderline or border‐line) adj3 (personalit* or state*)).kw,tw. 3 ("Axis II" or "Cluster B" or flamboyant or "F60.3" or "F60.30" or "F60.31").kw,tw.) 4 (idealization adj5 devaluation).kw,tw. 5 ((vulnerable or hyberbolic) adj3 temperament).kw,tw. 6 (((unstab* or instab* or poor or disturb* or fail* or weak or dysregulat*) adj3 (self* or impuls* or interperson* or identit* or relationship* or emotion* or affect*)) and (personality or character or PD)).kw,tw. 7 (impulsiv* adj5 (behavio?r or character or personalit*)).kw,tw. 8 (self adj3 (injur* or damag* or destruct* or harm* or hurt* or mutilat*)).kw,tw. 9 (suicidal adj3 (behavior or behaviour)).kw,tw. 10 (feel* adj3 (empt* or bored*)).kw,tw. 11 "anger adj5 control*".kw,tw. 12 (risk‐taking adj3 (behavior or behaviour)).kw,tw. 13 or/1‐12 14 randomised controlled trial/ 15 double blind procedure/ 16 crossover procedure/ 17 single blind procedure/ 18 (random* or factorial* or crossover* or cross‐over* or placebo* or double‐blind* or doubleblind* or single‐blind* or singleblind* or assign* or allocat* or volunteer*).ab,pt,sh,de,ti. 19 or/14‐18 20 13 and 19
CINAHL EBSCOhost (Cumulative Index to Nursing and Allied Health Literature
S1 (MH "Borderline Personality Disorder") S2 TX borderline N3 (state* or personalit*) S3 TX "Axis II" OR "Cluster B" S4 TX idealization N3 devaluation S5 TX ((vulnerable OR hyperbolic) N3 temperament) S6 TX (((unstab* or instab* or poor or disturb* or fail* or weak or dysregulat*) N3 (self or impuls* or interperson* or identit* or relationship* or emotion* or affect*)) AND (person* or character or PD)) S7 TX (impulsiv* N3 (behavio?r OR character or personalit*)) S8 TX (feel* N3 (empt* OR bored*)) S9 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 S10 (MH "randomised Controlled Trials") OR (MH "Random Assignment") OR (MH "Random Sample+") S11 TX random* N4 (trial* OR study OR studies) S12 TX random* N4 (allocat* OR allot* OR assign* OR basis OR divid* OR order) S13 AB placebo* S14 AB trial S15 (MH "Drug Therapy+") S16 S10 OR S11 OR S12 OR S13 OR S14 OR S15 S17 S9 AND S16
PsycINFO Ovid
1 exp Borderline Personality Disorder/ 2 borderline adj3 (personalit* or state*).id,ti,ab. 3 ("Axis II" or "Cluster B").id,ti,ab. 4 (idealization adj5 devaluation).ab,id,ti. 5 ((vulnerable or hyperbolic) adj3 temperament).id,ab,ti. 6 (((unstab* or instab* or poor or disturb* or fail* or weak or dysregulat*) adj3 (self* or impuls* or interperson* or identit* or relationship* or emotion* or affect*)) and (personality or character or PD)).id,ab,ti. 7 (impulsiv* adj5 (behavio?r or character or personalit*)).id,ab,ti. 8 (self adj3 (injur* or damag* or destruct* or harm* or hurt* or mutilat*)).id,ab,ti. 9 (suicidal adj3 behavio?r).id,ab,ti. 10 (feel* adj3 (empt* or bored*)).ab,id,ti. 11 "anger adj5 control*".ab,id,ti. 12 (risk‐taking adj3 behavio?r).id,ab,ti. 13 or/1‐12 14 exp Clinical Trials/ ( 15 (random* adj allocat*).ab. 16 randomi?ed.ab. 17 placebo.ab. 18 randomly.ab. 19 trial.ab. 20 groups.ab. 21 drug therapy.sh. 22 exp Animals/ not Humans/ 23 or/14‐21 24 23 not 22 25 13 and 24
ERIC EBSCOhost (Education Resources Information Center)
S23 S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 S22 TX risk‐taking N5 behaviour S21 TX anger N5 control* S20 TX feel* N3 (empt* or bored*) S19 TX suicidal N3 behavior S18 TX ( (unstab* or instab* or poor or disturb* or fail* or weak or dysregulat*) N3 TX (self or impuls* or interperson* or identit* or relationship* or emotion* or affect*)) AND TX ( personality OR character OR PD ) S17 AB (self AND (injur* or damag* or destruct* or harm or hurt* or mutilat*)) S16 AB impulsivity S15 TI impulsivity S14 TX impulsiv* N3 person* S13 TX "Axis II" OR "Cluster B" S12 TX borderline N3 state S11 TX borderline personality S10 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 S9 AB drug S8 AB trial S7 AB randomly S6 AB placebo S5 AB randomi?ed S4 AB controlled clinical trial* S3 SU controlled clinical trial S2 TX controlled clinical trial S1 DE "randomised Controlled Trials"
BIOSIS Previews Web of Science Clarivate Analytics (1969 to 20 March 2019)
#1 TOPIC: (borderline personality disorder) #2 TOPIC: ((borderline NEAR/3 (state)) #3 TOPIC: ((borderline NEAR/3 personalit*)) #4 TOPIC: (("Axis II" OR "Cluster B")) #5 TOPIC: (idealization NEAR/5 devaluation) #6 TOPIC: ((vulnerable OR hyperbolic) NEAR/3 temperament*) #7 TOPIC: (impulsiv* NEAR/5 personalit*) #8 TOPIC: ((self NEAR/3 (injur* OR damag* OR destruct* OR harm* OR hurt* OR mutilat*))) #9 TOPIC: ((((unstab* OR instab* OR poor OR disturb* OR fail* OR weak OR dysregulat*) NEAR/3 (self* OR impuls* OR interperson* OR identit* OR relationship* OR emotion* OR affect*)) AND (personality OR character OR PD))) #10 TOPIC: (suicidal NEAR/3 behavio?r) #11 TOPIC: (((feel* NEAR/3 (empt* OR bored*)))) #12 TOPIC: ((anger NEAR/5 control*)) #13 TOPIC: (risk‐taking NEAR/3 behavio?r) #14 #13 OR #12 OR #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 #15 TOPIC: (controlled clinical trial) #16 TOPIC: (randomised controlled trial) #17 #16 OR #15 #18 #17 AND #14
Web of Science Core Collection Clarivate Analytics
#18 #17 AND #14 #17 #16 OR #15 #16 TOPIC: (controlled clinical trial) #15 TOPIC: (randomised controlled trial) #14 #13 OR #12 OR #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 #13 TITLE: ((risk‐taking NEAR/3 behavio?r)) #12 TOPIC: ((risk‐taking NEAR/3 behavio?r)) #11 TITLE: ((anger NEAR/5 control*)) #10 TOPIC: ((feel* NEAR/3 (empt* OR bored*))) #9 TITLE: ((feel* NEAR/3 (empt* OR bored*))) #8 TITLE: (suicidal NEAR/3 behavio?r) #7 TITLE: (impulsivity) #6 TOPIC: ((((unstab* OR instab* OR poor OR disturb* OR fail* OR weak OR dysregulat*) NEAR/3 (self* OR impuls* OR interperson* OR identit* OR relationship* OR emotion* OR affect*)) AND (personality OR character OR PD))) #5 TOPIC: ((vulnerable or hyperbolic) NEAR/3 temperament) #4 TOPIC: ((idealization NEAR/5 devaluation)) #3 TOPIC: ("axis II" OR "Cluster B") #2 TOPIC: (borderline NEAR/3 state) #1 TOPIC: (borderline personality disorder)
Sociological Abstracts ProQuest
(((randomised controlled trial) OR (controlled clinical trial) OR SU.exact("CLINICAL TRIALS")) OR AB(randomi?ed) OR AB(randomly) OR AB(placebo) OR AB(trial)) AND ((borderline personality) OR "axis II" OR "Cluster B" OR (idealization AND devaluation) OR ((vulnerable OR hyperbolic) AND temperament) OR (((unstab* OR instab* or poor or disturb* or fail* or weak or dysregulat*) AND (self* or impuls* or interperson* or identit* or relationship* or emotion* or affect*)) AND (personality OR character OR PD)) OR (self AND (injur* OR damag* OR destruct* OR harm OR hurt* OR mutilat*)) OR "suicidal behavio?r" OR "self destructive behavio?r" OR (feel* AND (empt* OR bored*)))
LILACS (Latin American and Caribbean Health Science Information Database)
“Borderline personality disorder”, limits: Controlled clinical study
ProQuest Dissertations A&I
(SU(borderline personality disorder) OR AB("Axis II") OR AB("Cluster B")) AND (("randomised controlled study" OR "controlled clinical study") OR AB(randomi?ed) OR AB(placebo) OR AB(randomly))
OpenGrey:
“Borderline personality disorder”
NDLTD
“Borderline personality disorder”
DART Europe E‐theses portal
“Borderline personality disorder”
ANZCTR
“Borderline personality disorder”
ClinicalTrials.gov
“Borderline personality disorder”
ISRCTN
“Borderline personality disorder”
WHO ICTRP:
“Borderline personality disorder”
UK Clinical Trials Gateway
“Borderline personality disorder”
EU Clinical Trials Register
“Borderline personality disorder”
Library HubDiscover (previously COPAC)
“Borderline personality disorder”
Appendix 4. Table of outcomes
Primary outcomes
1. BPD symptom severity
| Name of scale/means of assessment | Abbreviation | Clinician‐rated (CR)/self‐rated (SR) | Study |
| Borderline Personality Disorder Checklist ‐ 40 | BDP‐40 | SR | Bos 2010 |
| Borderline Evaluation of Severity over Time | BEST | SR | Blum 2008 (12‐month follow‐up data); Gratz 2006; Gratz 2014; Gregory 2008b; Morton 2012 |
| Borderline Personality Disorder Features Scale | BPDFS | SR | Lin 2019 |
| Borderline Personality Features Scale for Children | BPFS‐C | SR | Rossouw 2012b |
| Borderline Personality Inventory | BPI | SR | Leichsenring 2016 |
| Borderline Personality Disorder Severity Index ‐ 4th Edition | BPDSI‐IV | CR | Bellino 2010; Giesen‐Bloo 2006; Kamalabadi 2012; Laurenssen 2018; Leppänen 2016; Nadort 2009; Philips 2018; Sinnaeve 2018 |
| Borderline Personality Disorder Severity Index ‐ 4th Edition for adolescents | BPDSI‐IV‐adol | CR | Schuppert 2012 |
| Borderline Syndrome Index | BSI | SR | Farrell 2009 |
| Borderline Symptom List ‐ 23 items | BSL‐23 | SR | Carmona í Farrés 2019; Elices 2016; Feliu‐Soler 2017; McMain 2017; Kramer 2014; Pascual 2015; Schilling 2018 |
| Clinical Global Impression scale for Borderline Personality Disorder patients | CGI‐BPD | CR | Amianto 2011; Soler 2009 |
| International Personality Disorder Examination ‐ Borderline Personality Disorder criteria | IPDE‐BPD | CR | Bohus 2013 |
| Millon Adolescent Clinical Inventory | MACI | CR | Santisteban 2015 |
| Personality Assessment Inventory ‐ Borderline Scale | PAI‐BOR | SR | Morey 2010 |
| Structured Clinical Interview for DSM‐IV Axis II Personality Disorders, Number of borderline criteria met | SCID‐II, Number of borderline criteria met | CR | Doering 2010; Jørgensen 2013; Koons 2001a; Kredlow 2017a; Kredlow 2017b |
| Structured Clinical Interview for DSM‐IV Axis II Personality Disorders, Still meeting borderline criteria | SCID‐II, Still meeting borderline criteria | CR | Davidson 2006 |
| Zanarini Rating Scale for Borderline Personality Disorder | ZAN‐BPD | CR | Bateman 1999; Blum 2008 (post‐treatment data); Gratz 2014; McMain 2009; Priebe 2012; Reneses 2013; Robinson 2016; Zanarini 2018 |
2. Self‐harm
| Name of scale/means of assessment | Abbreviation | Clinician‐rated (CR)/self‐rated (SR) | Study |
| Borderline Personality Disorder Severity Index ‐ 4th Edition, Parasuicidal Behaviour | BPDSI‐IV, parasuicidal behaviour | CR | Bellino 2010 |
| Cornell Interview for Suicidal and Self‐Harming Behavior‐Self‐Report | CISSB | SR | Doering 2010 |
| Days of self‐harm and type of deliberate self‐harm were recorded in an interview on a structured form | None | None | Priebe 2012 |
| Deliberate Self‐Harm Inventory | DSHI | SR | Gratz 2006; Gratz 2014; McMain 2017 |
| Deliberate Self‐Harm Inventory ‐ Short Form | DSHI‐SF | SR | Philips 2018 |
| Deliberate Self‐Harm Inventory ‐ participants with self‐harm during previous 12 months | DSHI ‐ participants with self‐harm | SR | Davidson 2006 |
| Lifetime Parasuicide Count ‐ Self‐Mutilative Accts | LPC | CR | Gregory 2008b; Sinnaeve 2018; Van den Bosch 2005 |
| Number of self‐harming incidents during previous 12‐month period | Unclear | Unclear | Amianto 2011 |
| Number of self‐harming acts during previous three‐month period | Unclear | Unclear | Carter 2010 |
| Suicide and Self‐Harm Inventory ‐ number of patients with self‐harming behaviour during previous six‐month period | SSHI ‐ patients with self‐harm | CR | Bateman 1999; Bateman 2009 |
| Personality Assessment Inventory, Borderline Features Scale ‐ Self‐Harm | PAI‐BOR‐S | SR | Morey 2010 |
| Parasuicide History Interview ‐ deliberate self‐harm frequency | PHI ‐ deliberate self‐harm frequency | CR | Koons 2001a; Weinberg 2006 |
| Parasuicide History Interview ‐ patients with self‐harming behaviour during previous 12‐month period | PHI ‐ patients with self‐harm | CR | Linehan 1991 |
| Risk‐Taking and Self‐Harm Inventory ‐ participants with self‐harming behaviour | RTSHI ‐ patients with self‐harm | SR | Rossouw 2012b |
| Suicide Attempt and Self‐Injury Interview, Non‐Suicidal Self‐Injury scale | SASII ‐ NSSI/self‐harm | CR | Feigenbaum 2012; Harned 2014; Linehan 2006; Linehan 2015a; |
| Suicide Attempt and Self‐Injury Interview ‐ number of suicidal and self‐injurious episodes | SASII ‐ suicidal and self‐injurious episodes | CR | McMain 2009 |
| Self‐Harming Behaviours Checklist | SHBCL | CR | Cottraux 2009 |
| Self‐Harm Questionnaire ‐ number of suicidal and self‐injurious episodes | SHQ ‐ suicidal and self‐injurious episodes | SR | Borschmann 2013 |
| Target Behaviour Rating ‐ frequency of parasuicide | TBR ‐ frequency of parasuicide | CR | Turner 2000 |
3. Suicide‐related outcomes
| Name of scale/means of assessment | Abbreviation | Clinician‐rated (CR)/self‐rated (SR) | Study |
| The 12‐item Adult Suicidal Ideation Questionnaire— Shortened Version | ASIQ‐S | SR | Lin 2019 |
| Beck Hopelessness Scale | BHS | SR | Cottraux 2009 |
| Beck Scale for Suicidal Ideation | BSS | SR | Davidson 2014; Koons 2001a; Mohamadizadeh 2017; Turner 2000 |
| Clinical Global Impression scale for Borderline Personality Disorder patients, suicidality | CGI‐BPD, Suicidality | CR | Amianto 2011; Soler 2009 |
| Deliberate Self‐Harm Inventory, suicide attempts (cumulative average) | DSHI, Suicide Attempts (cumulative average) | CR | Davidson 2006 |
| Lifetime Suicide Attempt Self‐Injury Interview | LSASI | CR | McMain 2017; Reneses 2013 |
| Number of participants with suicide attempt (recorded via direct contact with patients and health care staff, as well as from reviewing the case records) | None | CR | Philips 2018 |
| Number of suicide attempts | None | None | Stanley 2017 |
| Personality Assessment Inventory ‐ suicidal ideation | PAI‐SI | SR | Morey 2010 |
| Borderline Personality Disorder Severity Index ‐ 4th Edition, parasuicidality, suicide plans and attempts | BPDSI‐IV, parasuicidality, suicide plans and attempts | CR | Leppänen 2016 |
| Suicidal Behaviours Questionnaire | SBQ | SR | Weinberg 2006 |
| Suicidal Ideation Questionnaire ‐ Junior | SIQ‐JR | SR | Mehlum 2014 |
| Suicide attempt and self‐injury interview, suicide attempts | SASII, suicide attempts | CR | Feigenbaum 2012; Harned 2014; Linehan 2006; Linehan 2015a |
| Self‐Harm Inventory (number of participants with life‐threatening suicide attempts in the last 6 months) | SSHI | CR | Bateman 1999; Bateman 2009 |
| Overt Aggression Scale ‐ Modified for outpatients, suicidality | OAS‐M, suicidality | CR | Gleeson 2012 |
| Borderline Personality Disorder Severity Index, parasuicidal | BPDSI, parasuicidal | CR | Kamalabadi 2012 |
4. Psychosocial functioning
| Name of scale/means of assessment | Abbreviation | Clinician‐rated (CR)/self‐rated (SR) | Study |
| Brief Disability Questionnaire, days out of role | BDQ, days out of role | SR | Carter 2010 |
| Children's Global Assessment Scalea | C‐GAS | CR | Mehlum 2014 |
| Clinical Global Impressions scale ‐ severity of illness | CGI‐S | CR | Bellino 2006; Bellino 2010; Cottraux 2009; |
| Clinical Global Impressions scale ‐ improvement ‐ self‐rated | CGI‐I‐SR | SR | Soler 2009 |
| Dutch version of the Health of the Nations Outcome Scales | HoNOS | CR | Jochems 2015 |
| Clinical Outcomes in Routine Evaluation – outcome measure, Functioning subscale | CORE‐OM, Functioning subscale | SR | Feigenbaum 2012 |
| Global Assessment of Functioning scalea | GAF | CR | Amianto 2011; Antonsen 2017; Bohus 2013; Doering 2010; Farrell 2009; Kredlow 2017b; Robinson 2016; Salzer 2014 |
| Global Assessment Scalea | GAS | CR | Andreoli 2016; Blum 2008; Harned 2014; Linehan 1994 |
| General Health Questionnaire, functioning | GHQ, functioning | CR | Kamalabadi 2012 |
| Outcome Questionnaire—45.2, social role | OQ45, social role | SR | Haeyen 2018; Kramer 2011; Kramer 2014; Kramer 2016 |
| Social Functioning Questionnaire | SFQ | SR | Davidson 2006; McMurran 2016 |
| Sheehan Disability Scale | SDS | SR | Gratz 2014; Zanarini 2018 |
| Social Adjustment Scale ‐ self‐rating | SAS‐SR | SR | Bateman 1999; Bateman 2009; Jørgensen 2013; McMain 2017; Reneses 2013 |
| Satisfaction Profile, social functioning | None | SR | Bellino 2010 |
| Social and Occupational Functioning Assessment Scalea | SOFAS | CR | Bellino 2007; Gleeson 2012 |
| Social Provisions Scale ‐ "How many days were you paid for working in the past 30 days?"a | SPS ‐ days paid for working | SR | Gregory 2008b |
| Total Outcome Questionnaire 45 | OQ45 | SR | Haeyen 2018 |
| Work and Social Adjustment Scale | WSAS | SR | Borschmann 2013 |
aFor these scales, higher scores indicate better functioning, as opposed to most other clinical outcome scales (where higher scores indicate higher burden). Scores were multiplied by ( −1) before entering for effect size calculation, to ensure that a negative direction of effect indicates a beneficial effect (like for most other clinical outcomes).
Secondary outcomes
1. Anger
| Name of scale/means of assessment | Abbreviation | Clinician‐rated (CR)/self‐rated (SR) | Study |
| Anger Irritability and Assault Questionnaire, Labile Anger subscale | AIAQ, Labile Anger subscale | SR | Gleeson 2012 |
| Borderline Personality Disorder Severity Index ‐ 4th Edition, anger | BPDSI‐IV, anger | CR | Bellino 2010; Kamalabadi 2012; Leppänen 2016 |
| Clinical Global Impression scale for Borderline Personality Disorder patients, anger | CGI‐BPD, anger | CR | Amianto 2011; Soler 2009 |
| Overt Aggression Scale ‐ Modified, labile anger | OAS‐M, labile anger | CR | Gleeson 2012 |
| Spielberger Anger Expression Scale, anger out | STAXI, anger out | SR | Feigenbaum 2012; Koons 2001a; Linehan 2006; McMain 2009 |
| Spielberger Anger Expression Scale, trait anger | STAXI, trait anger | SR | Linehan 1994; McMain 2017 |
| Target Behaviour Rating, anger | TBR, anger | CR | Turner 2000 |
2. Affective instability
| Name of scale/means of assessment | Abbreviation | Clinician‐rated (CR)/self‐rated (SR) | Study |
| Borderline Personality Disorder Severity Index ‐ 4th Edition, affective instability | BPDSI‐IV, affective instability | CR | Bellino 2010; Kamalabadi 2012; Leppänen 2016 |
| Borderline Personality Disorder Severity Index ‐ 4th Edition for adolescents, affective instability | BPDSI‐IV‐adol, affective instability | CR | Schuppert 2012 |
| Clinical Global Impression scale for Borderline Personality Disorder patients, affective instability | CGI‐BPD, affective instability | CR | Amianto 2011; Soler 2009 |
| Revised Diagnostic Interview for Borderlines, Affect subscale | DIB‐R, Affect subscale | CR | Farrell 2009 |
| Difficulties in Emotion Regulation Scale, Total score | DERS, Total score | SR | Bianchini 2019; Gratz 2006; Gratz 2014; McMain 2017; Morton 2012 |
| Personality Assessment Inventory, affective instability | PAI‐BOR‐A, affective instability | SR | Morey 2010 |
| Strengths and Difficulties Questionnaire, emotional problems | SDQ, emotional problems | SR | Salzer 2014 |
| Zanarini Rating Scale for Borderline Personality Disorder, affective instability | ZAN‐BPD, affective instability | CR | Blum 2008; Reneses 2013; Zanarini 2018 |
3. Chronic feeling of emptiness
| Name of scale/means of assessment | Abbreviation | Clinician‐rated (CR)/self‐rated (SR) | Study |
| Borderline Personality Disorder Severity Index ‐ 4th Edition, emptiness | BPDSI‐IV, emptiness | CR | Bellino 2010; Kamalabadi 2012; Leppänen 2016 |
| Clinical Global Impression scale for Borderline Personality Disorder patients, emptiness | CGI‐BPD, emptiness | CR | Amianto 2011; Soler 2009 |
| Zanarini Rating Scale for Borderline Personality Disorder, feeling of emptiness | Zan‐BPDf, feeling of emptiness | CR | Reneses 2013 |
4. Impulsivity
| Name of scale/means of assessment | Abbreviation | Clinician‐rated (CR)/self‐rated (SR) | Study |
| Barrett Impulsiveness Scale | BIS | SR | Bianchini 2019, McMain 2017; Pascual 2015 |
| Barrett Impulsiveness Scale ‐ 11, non‐planning | BIS‐11, non‐planning | SR | Carmona í Farrés 2019; Elices 2016 |
| Borderline Personality Disorder Severity Index ‐ 4th Edition, impulsivity | BPDSI‐IV, impulsivity | CR | Bellino 2010; Kamalabadi 2012; Leppänen 2016; Van den Bosch 2005 |
| Clinical Global Impression scale for Borderline Personality Disorder patients, impulsivity | CGI‐BPD, impulsivity | CR | Amianto 2011; Soler 2009 |
| Diagnostic Interview for Borderline Personality Disorder ‐ Revised, impulsive | DIB‐R, impulsive | CR | Farrell 2009 |
| Difficulties in Emotion Regulation Scale, impulsive dyscontrol | DERS, impulse dyscontrol | SR | Gratz 2006; Gratz 2014 |
| Eysenck Impulsivity Venturesomeness Empathy Questionnaire, impulsivity | IVE, impulsivity | SR | Cottraux 2009 |
| Target Behaviour Rating, impulsiveness | TBR, impulsiveness | CR | Turner 2000 |
| Zanarini Rating Scale for Borderline Personality Disorder, impulsivity | ZAN‐BPD, impulsivity | CR, SR | Blum 2008; Reneses 2013; Robinson 2016; Zanarini 2018 |
5. Interpersonal problems
| Name of scale/means of assessment | Abbreviation | Clinician‐rated (CR)/self‐rated (SR) | Study |
| Borderline Personality Disorder Severity Index ‐ 4th Edition, interpersonal relationships | BPDSI‐IV, interpersonal relationships | CR | Bellino 2010; Kamalabadi 2012 |
| Borderline Personality Disorder Severity Index ‐ 4th Edition, unstable relationships | BPDSI‐IV, unstable relationships | CR | Leppänen 2016 |
| Clinical Global Impression scale for Borderline Personality Disorder patients, unstable relations | CGI‐BPD, unstable relations | CR | Amianto 2011; Soler 2009 |
| Circumplex of Interpersonal Problems | CIP | SR | Antonsen 2017 |
| Revised Diagnostic Interview for Borderlines, Interpersonal subscale | DIB‐R, Interpersonal subscale | CR | Farrell 2009 |
| EQ‐5D Health‐Related Quality of Life Questionnaire, social relationships | EQ‐5D, social relationships | SR | Robinson 2016 |
| Inventory of Interpersonal Problems ‐ Borderline Personality Disorder, Related composite | IIP‐BPD, Related composite | SR | Gratz 2014 |
| Inventory of Interpersonal Problems ‐ Circumflex version/64‐item version | IIP‐C/IIP‐64 | SR | Bateman 1999; Bateman 2009; Jørgensen 2013; Laurenssen 2018; Leichsenring 2016; McMain 2009; Philips 2018 |
| Inventory of Interpersonal Problems ‐ 25‐item version | IIP‐25 | SR | Harned 2014 |
| Inventory of Interpersonal Problems Short Circumplex ‐ 32‐item version | IIP‐SC/IIP‐32 | SR | Davidson 2006 |
| Outcome Questionnaire 45, interpersonal relations | OQ45, interpersonal relations | SR | Haeyen 2018; Kramer 2011; Kramer 2014; Kramer 2016 |
| Personality Assessment Inventory ‐ Borderline Features Scale ‐ negative relationships | PAI‐BOR‐N | SR | Morey 2010 |
| Strengths and Difficulties Questionnaire, problems in relationships | SDQ, problems in relationships | SR | Salzer 2014 |
| Abbreviated World Health Organization Quality of Life Questionnaire, Social relationships score | WHOQOL‐Bref, Social relationships score | SR | Bos 2010; Carter 2010 |
| Zanarini Rating Scale for Borderline Personality Disorder, disturbed relationships | ZAN‐BPD, disturbed relationships | CR | Blum 2008; Reneses 2013; Zanarini 2018 |
6. Abandonment
| Name of scale/means of assessment | Abbreviation | Clinician‐rated (CR)/self‐rated (SR) | Study |
| Borderline Personality Disorder Severity Index ‐ 4th Edition, abandonment | BPDSI‐IV, abandonment | CR | Bellino 2010; Kamalabadi 2012; Leppänen 2016 |
| Clinical Global Impression scale for Borderline Personality Disorder patients, fear of abandonment | CGI‐BPD, fear of abandonment | CR | Amianto 2011 |
7. Identity disturbance
| Name of scale/means of assessment | Abbreviation | Clinician‐rated (CR)/self‐rated (SR) | Study |
| Borderline Personality Disorder Severity Index ‐ 4th Edition, identity disturbance | BPDSI‐IV, identity disturbance | CR | Bellino 2010; Kamalabadi 2012; Leppänen 2016 |
| Borderline Personality Inventory, identity diffusion | BPI, identity diffusion | SR | Leichsenring 2016 |
| Clinical Global Impression scale for Borderline Personality Disorder patients, identity distortion | CGI‐BPD, identity distortion | CR | Amianto 2011 |
| Personality Assessment Inventory ‐ Borderline Features Scale ‐ identity disturbance | PAI‐BOR‐I | SR | Morey 2010 |
| Severity Indices of Personality Problems | SIPP | SR | Antonsen 2017 |
| Zanarini Rating Scale for Borderline Personality Disorder, Identity subscale | Zan‐BPD, Identity subscale | CR | Reneses 2013 |
8. Dissociation and psychotic‐like symptoms
| Name of scale/means of assessment | Abbreviation | Clinician‐rated (CR)/self‐rated (SR) | Study |
| Borderline Personality Disorder Severity Index ‐ 4th Edition, paranoid ideation | BPDSI‐IV, paranoid ideation | CR | Bellino 2010; Leppänen 2016 |
| Borderline Personality Disorder Severity Index ‐ 4th Edition, dissociation | BPDSI‐IV, dissociation | CR | Kamalabadi 2012 |
| Brief Psychiatric Rating Scale | BPRS | CR | Gleeson 2012; Kredlow 2017a; Soler 2009; Turner 2000 |
| Clinical Global Impression scale for Borderline Personality Disorder patients, dissociative symptoms | CGI‐BPD, dissociative symptoms | CR | Amianto 2011 |
| Revised Diagnostic Interview for Borderlines, Cognitive subscale | DIB‐R, Cognitive subscale | CR | Farrell 2009 |
| Dissociative Experiences Scale | DES | SR | Bohus 2013; Feigenbaum 2012; Gregory 2008b; Koons 2001a |
| Dissociative Experiences Scale ‐ Taxon | DES‐T | SR | Harned 2014 |
| Zanarini Rating Scale for Borderline Personality Disorder, cognitive | Zan‐BPD, cognitive | CR | Blum 2008 |
9. Depression
| Name of scale/means of assessment | Abbreviation | Clinician‐rated (CR)/self‐rated (SR) | Study |
| Beck Depression Inventory | BDI | SR | Antonsen 2017; Bateman 1999; Bateman 2009; Blum 2008; Cottraux 2009; Doering 2010; Gregory 2008b; Koons 2001a; Laurenssen 2018; Leichsenring 2016; McMain 2009; Schilling 2018; Turner 2000 |
| Beck Depression Inventory‐II | BDI‐II | SR | Bohus 2013; Davidson 2006; Feigenbaum 2012; Jørgensen 2013; Kredlow 2017a; Kredlow 2017b; McMain 2017; Mohamadizadeh 2017 |
| Depression Anxiety Stress Scales | DASS | SR | Gratz 2006; Gratz 2014; Morton 2012 |
| Depression Anxiety Stress Scale ‐ 21 | DASS‐21 | SR | Robinson 2016 |
| Diagnostic Interview Schedule for Children – Predictive Scales | DISC ‐ PS | SR | Santisteban 2015 |
| General Health Questionnaire, Depression subscale | GHQ, Depression subscale | SR | Kamalabadi 2012 |
| Hamilton Depression Inventory | Ham‐D | CR | Bellino 2006; Bellino 2007; Bellino 2010; Smith 2012 |
| Hamilton Depression Inventory ‐ 17‐item | Ham‐D‐17 | SR, CR | Linehan 2006; Soler 2009 |
| Hamilton Depression Rating Scale | HDRS | SR | Andreoli 2016; Harned 2014; Jahangard 2012; Linehan 2015a |
| Hamilton Depression and Anxiety Scale | HADS | SR | Borschmann 2013; Davidson 2014; McMurran 2016 |
| Ko’s Depression Inventory | None | SR | Lin 2019 |
| Montgomery Åsberg Depression Rating Scale | MADRS | CR | Gleeson 2012; Mehlum 2014; Pascual 2015; Reneses 2013 |
| Mood and Feelings Questionnaire ‐ participants scoring higher than cut‐off for depression | MFQ ‐ above cut‐off | SR | Rossouw 2012b |
| Clinically Useful Depression Outcome Scale, total score | None | SR | Zanarini 2018 |
10. Adverse effects
| Name of scale/means of assessment | Abbreviation | Clinician‐rated (CR)/self‐rated (SR) | Study |
| Spontaneous reporting | ‐ | ‐ |
Andreoli 2016; Davidson 2014; Haeyen 2018; Leichsenring 2016; McMurran 2016; Pascual 2015; Robinson 2016; Stanley 2017
The remaining trials did not report adverse effects |
Appendix 5. 'Risk of bias' components and criteria for assigning judgements
Selection bias
Random sequence generation
Low risk of bias: The method used was adequate (e.g. computer‐generated random numbers, table of random numbers) or was unlikely to introduce selection bias.
Unclear risk of bias: The information was insufficient for the assessment of whether the method used could introduce selection bias.
High risk of bias: The method used was likely to introduce bias.
Allocation concealment
Low risk of bias: The method used (e.g. central allocation) was unlikely to bias allocation to groups.
Unclear risk of bias: The information was insufficient for assessment of whether the method used could bias allocation to groups.
High risk of bias: The method used (e.g. open random allocation schedule) could bias allocation to groups.
Detection bias: blinding of outcome assessment
Low risk of bias: The method of blinding was described and blinding was conducted in a satisfactory way.
Unclear risk of bias: The information was insufficient for assessment of whether the type of blinding used was likely to bias the estimate of effect.
High risk of bias: There was no blinding or incomplete blinding.
Attrition bias: incomplete outcome data
Low risk of bias: The underlying reasons for missing data probably would not affect outcome measurement, as all missing data can be considered as missing at random or all data were reported.
Unclear risk of bias: The information was insufficient for the assessment of whether the missing data or the method used to handle missing data was likely to bias the estimate of effect.
High risk of bias: The crude estimate of effects could be biased given the reasons for the missing data, or the methods used to handle missing data are unsatisfactory.
Reporting bias: selective outcome reporting
Low risk of bias: The trial protocol was available and all prespecified outcomes of interest were reported.
Unclear risk of bias: The information was insufficient for the assessment of whether selective outcome reporting could have occurred.
High risk of bias: Not all of the primary outcomes specified beforehand were reported or participants were excluded after randomisation.
Other potential sources of bias
Treatment adherence bias
Low risk of bias: Measures were undertaken to assure adequate treatment adherence; for example, by regular supervision or use of adherence ratings of videotaped or audio‐taped therapy sessions.
Unclear risk of bias: There was insufficient information to assess the extent of adequate treatment adherence.
High risk of bias: There was inadequate treatment adherence. Steps/measures were undertaken to assure adequate treatment adherence.
Attention bias
Low risk of bias: The treatment conditions were sufficiently similar in duration and intensity.
Unclear risk of bias: There was insufficient information in regards to treatment duration and intensity.
High risk of bias: One treatment condition was markedly more intense or was of longer duration than (a)nother condition(s).
Affiliation bias
Low risk of bias: The principal investigator was not the developer of the treatment under investigation (if compared to a control condition), or both treatment developers were involved if two treatments were directly compared.
Unclear risk of bias: There was insufficient information to assess affiliation bias.
High risk of bias: The principal investigator was the developer of the treatment under investigation (if compared to a control condition), or only one of the treatment developers was involved if two treatments were directly compared.
Other sources of bias
Low risk of bias: The trial appeared to be free of other sources of bias.
Unclear risk of bias: The information was inadequate for the assessment of other possible sources of bias.
High risk of bias: Other sources of bias were identified.
Appendix 6. Trial Sequential Analysis (TSA) and funnel plots figures
Psychotherapy versus TAU
Primary outcomes
BPD symptom severity
We performed a TSA on the primary outcome of borderline symptom severity at end of treatment. The analysis shows that the required information size was reached. See Figure 237 below.
4.
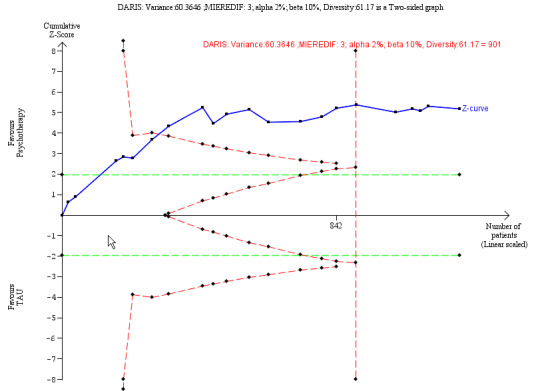
Trial Sequential Analysis on primary outcome: Psychotherapy ‐ borderline symptom severity at end of treatment
We drew a funnel plot for the comparison between psychotherapy and TAU for the primary outcome of BPD symptom severity. The funnel plot shows a small asymmetry. See Figure 238 below.
5.

Funnel plot of comparison 1: Psychotherapy versus TAU, outcome: 1.1 Primary outcome: BPD symptom severity.
Self‐harm
We performed a TSA on the primary outcome of self‐harm at end of treatment. The analysis shows that the required information size was reached. See Figure 239 below.
6.
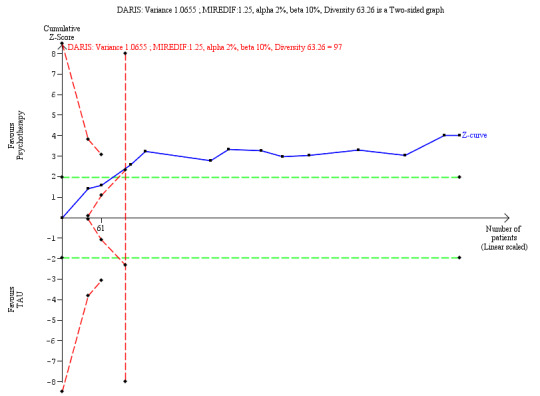
Trial Sequential Analysis on primary outcome: Psychotherapy ‐ self‐harm at end of treatment
We drew a funnel plot for the comparison between psychotherapy and TAU for the outcome of self‐harm. The funnel plot shows symmetry. See Figure 240 below.
7.

Funnel plot of comparison: 1 Psychotherapy versus TAU, outcome: 1.3 Primary outcome: self‐harm.
Suicide‐related outcomes
We performed a TSA on the primary outcome of suicide‐related outcomes at end of treatment. The analysis shows that the required information size was reached. See Figure 241 below.
8.
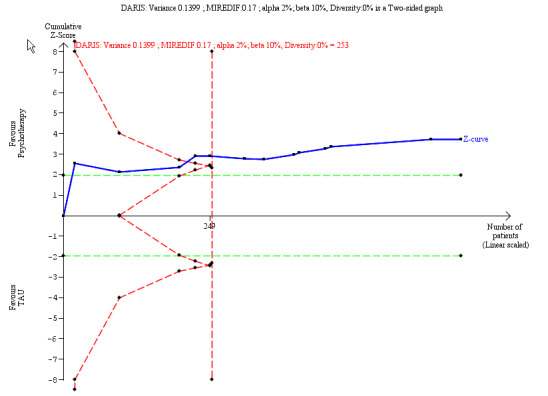
Trial Sequential Analysis on primary outcome: Psychotherapy ‐ suicide‐related outcomes at end of treatment
We drew a funnel plot for the comparison between psychotherapy and TAU for suicide‐related outcomes. The funnel plot shows symmetry. See Figure 242 below.
9.

Funnel plot of comparison: 1 Psychotherapy compared with TAU, outcome: 1.5 Primary outcome: suicide‐related outcomes.
Psychosocial functioning
We performed a TSA on the primary outcome of psychosocial functioning at end of treatment. The analysis shows that the required information size was reached. See Figure 243 below.
10.
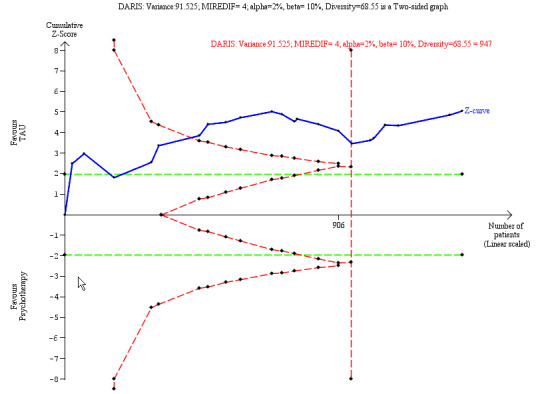
Trial Sequential Analysis on primary outcome: Psychotherapy ‐ psychosocial functioning at end of treatment
We drew a funnel plot for the comparison between psychotherapy and TAU for the outcome of psychosocial functioning. The funnel plot shows symmetry. See Figure 244 below.
11.

Funnel plot of comparison: 1 Psychotherapy compared with TAU, outcome: 1.7 Primary outcome: psychosocial functioning.
Secondary outcome: depression
We performed a TSA on the secondary outcome of depression at end of treatment. The analysis shows that the required information size was not reached. See Figure 245 below.
12.
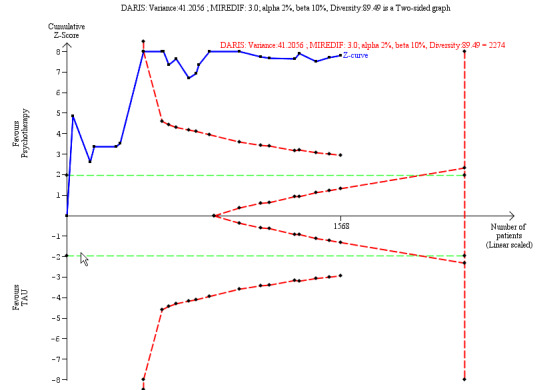
Trial Sequential Analysis on secondary outcome: Psychotherapy ‐ depression at end of treatment
Data and analyses
Comparison 1. Psychotherapy vs TAU.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Primary: BPD symptom severity (continuous) | 23 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1.1 End of treatment | 22 | 1244 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.70, ‐0.33] |
| 1.1.2 0‐6 months follow‐up | 2 | 41 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐1.23, 0.05] |
| 1.1.3 6‐12 months follow‐up | 2 | 157 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.36, 0.27] |
| 1.1.4 12 months and over follow‐up | 2 | 97 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.94 [‐2.58, 0.70] |
| 1.2 Primary: BPD symptom severity (dichotomous), at above 12 months follow‐up | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 1.3 Primary: self‐harm (continuous) | 13 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.3.1 End of treatment | 13 | 616 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.49, ‐0.14] |
| 1.3.2 0‐6 months follow‐up | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐1.28, 0.23] |
| 1.3.3 6‐12 months follow‐up | 3 | 174 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.48, 0.12] |
| 1.4 Primary: self‐harm (dichotomous) | 6 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.4.1 End of treatment | 6 | 513 | Risk Ratio (IV, Random, 95% CI) | 0.85 [0.63, 1.14] |
| 1.4.2 0‐6 months follow‐up | 1 | 41 | Risk Ratio (IV, Random, 95% CI) | 0.14 [0.04, 0.56] |
| 1.4.3 6‐12 months follow‐up | 1 | 41 | Risk Ratio (IV, Random, 95% CI) | 0.27 [0.10, 0.68] |
| 1.4.4 12 months and over follow‐up | 1 | 41 | Risk Ratio (IV, Random, 95% CI) | 0.33 [0.15, 0.76] |
| 1.5 Primary: suicide‐related outcomes (continuous) | 13 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.5.1 End of treatment | 13 | 666 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.57, ‐0.11] |
| 1.5.2 0‐6 months follow‐up | 2 | 36 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐1.10, 0.23] |
| 1.5.3 6‐12 months follow‐up | 2 | 109 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.80, ‐0.04] |
| 1.5.4 Above 12 months follow‐up | 1 | 76 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.77, 0.14] |
| 1.6 Primary: suicide‐related outcomes (dichotomous) | 5 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.6.1 End of treatment | 5 | 396 | Risk Ratio (IV, Random, 95% CI) | 0.27 [0.11, 0.67] |
| 1.6.2 0‐6 months follow‐up | 1 | 41 | Risk Ratio (IV, Random, 95% CI) | 0.25 [0.06, 1.05] |
| 1.6.3 Above 12 months follow‐up | 1 | 41 | Risk Ratio (IV, Random, 95% CI) | 0.29 [0.11, 0.74] |
| 1.7 Primary: psychosocial functioning (continuous) | 23 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.7.1 End of treatment | 22 | 1314 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.68, ‐0.22] |
| 1.7.2 0‐6 months follow‐up | 1 | 9 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.23 [‐2.74, 0.29] |
| 1.7.3 6‐12 months follow‐up | 3 | 247 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.40, 0.23] |
| 1.7.4 Above 12 months follow‐up | 6 | 499 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.60, 0.05] |
| 1.8 Secondary: anger (continuous) | 8 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.8.1 End of treatment | 8 | 323 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.64, ‐0.12] |
| 1.8.2 0‐6 months follow‐up | 1 | 8 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐2.82, 0.41] |
| 1.8.3 6‐12 months follow‐up | 2 | 111 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.50, 0.25] |
| 1.8.4 Above 12 months follow‐up | 1 | 80 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.42, 0.46] |
| 1.9 Secondary: affective instability (continuous) | 12 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.9.1 End of treatment | 12 | 620 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐0.98, ‐0.39] |
| 1.9.2 6‐12 months follow‐up | 1 | 33 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐1.21, 0.18] |
| 1.10 Secondary: chronic feelings of emptiness (continuous) | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.10.1 End of treatment | 4 | 187 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.69, ‐0.10] |
| 1.10.2 6‐12 months follow‐up | 1 | 33 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐1.28, 0.11] |
| 1.11 Secondary: impulsivity (continuous) | 10 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.11.1 End of treatment | 10 | 491 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.84, ‐0.25] |
| 1.11.2 6‐12 months follow‐up | 2 | 77 | Std. Mean Difference (IV, Random, 95% CI) | 0.32 [‐0.13, 0.77] |
| 1.12 Secondary: impulsivity (dichotomous), at end of treatment | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 1.13 Secondary: interpersonal problems (continuous) | 18 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.13.1 End of treatment | 18 | 1159 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.68, ‐0.16] |
| 1.13.2 0‐6 months follow‐up | 1 | 53 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐1.01, 0.20] |
| 1.13.3 6‐12 months follow‐up | 2 | 132 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.65, 0.32] |
| 1.13.4 Above 12 months follow‐up | 3 | 172 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.54, 0.54] |
| 1.14 Secondary: abandonment (continuous) | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.14.1 End of treatment | 2 | 84 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.66, 0.21] |
| 1.14.2 6‐12 months follow‐up | 1 | 33 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐1.08, 0.30] |
| 1.15 Secondary: identity disturbance (continuous) | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.15.1 End of treatment | 4 | 250 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.84, 0.10] |
| 1.15.2 6‐12 months follow‐up | 1 | 33 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐1.83, ‐0.35] |
| 1.16 Secondary: dissociation and psychotic‐like symptoms (continuous) | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.16.1 End of treatment | 6 | 244 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.85, ‐0.10] |
| 1.16.2 0‐6 months follow‐up | 2 | 35 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐1.69, ‐0.26] |
| 1.16.3 6‐12 months follow‐up | 1 | 33 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐1.29, 0.11] |
| 1.16.4 12 months and over follow‐up | 1 | 24 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.81, 0.79] |
| 1.17 Secondary: depression (continuous) | 22 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.17.1 End of treatment | 22 | 1568 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.61, ‐0.17] |
| 1.17.2 0‐6 months follow‐up | 4 | 125 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐1.26, ‐0.34] |
| 1.17.3 6‐12 months follow‐up | 3 | 260 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.95, 0.16] |
| 1.17.4 12 months and over follow‐up | 5 | 311 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.74, ‐0.06] |
| 1.18 Secondary: depression (dichotomous), at end of treatment | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 1.19 Secondary: attrition (dichotomous) | 33 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.19.1 End of treatment | 32 | 2225 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.83, 1.20] |
| 1.19.2 0‐6 months follow‐up | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.34, 1.00] |
| 1.20 Secondary: non‐serious adverse effects (dichotomous), at end of treatment | 2 | 381 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.45, 1.88] |
| 1.21 Secondary: serious adverse effects (dichotomous), at end of treatment | 4 | 571 | Risk Ratio (IV, Fixed, 95% CI) | 0.86 [0.14, 5.09] |
Comparison 2. Acceptance and commitment therapy (ACT) vs TAU.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Primary: BPD symptom severity (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.2 Secondary: affective instability (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.3 Secondary: depression (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.4 Secondary: attrition (dichotomous), at end of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 3. Dialectical behavior therapy (DBT) vs TAU.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Primary: BPD symptom severity (continuous), at end of treatment | 3 | 149 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.05, ‐0.14] |
| 3.2 Primary, self‐harm (continuous) | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.2.1 End of treatment | 7 | 376 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.48, ‐0.07] |
| 3.2.2 6‐12 months follow‐up | 2 | 141 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.59, 0.07] |
| 3.3 Primary: suicide‐related outcomes (continuous), at end of treatment | 5 | 231 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.68, 0.23] |
| 3.4 Primary: suicide‐related outcomes, attempts (dichotomous), at end of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.5 Primary: psychosocial functioning (continuous), at end of treatment | 6 | 225 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.69, ‐0.03] |
| 3.6 Secondary: anger (continuous) | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.6.1 End of treatment | 5 | 230 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.86, ‐0.09] |
| 3.6.2 6‐12 months follow‐up | 1 | 78 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.62, 0.27] |
| 3.6.3 Above 12 months follow‐up | 1 | 80 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.42, 0.46] |
| 3.7 Secondary: affective instability (continuous), at end of treatment | 2 | 80 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐1.64, 0.51] |
| 3.8 Secondary: chronic feelings of emptiness (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.9 Secondary: impulsivity (continuous) | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.9.1 End of treatment | 3 | 128 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.71, ‐0.00] |
| 3.9.2 6‐12 months follow‐up | 1 | 44 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.30, 0.90] |
| 3.10 Secondary: interpersonal problems (continuous), at end of treatment | 3 | 148 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.45, 0.20] |
| 3.11 Secondary: dissociation and psychotic‐like symptoms (continuous), at end of treatment | 4 | 194 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.73, ‐0.16] |
| 3.12 Secondary: depression (continuous) | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.12.1 End of treatment | 5 | 219 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.98, 0.03] |
| 3.12.2 6‐12 months follow‐up | 1 | 81 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.67, 0.21] |
| 3.13 Secondary: attrition (dichotomous) | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.13.1 End of treatment | 10 | 591 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.70, 2.31] |
| 3.13.2 0‐6 months follow‐up | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.34, 1.00] |
| 3.14 Secondary: adverse effects (dichotomous), at end of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.15 Secondary: serious adverse effects (dichotomous), at end of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 4. Mentalisation based therapy (MBT) vs TAU.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Primary: BPD symptom severity (continuous) | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1.1 End of treatment | 5 | 267 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.38, 0.11] |
| 4.1.2 0‐6 months follow‐up | 1 | 15 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐1.49, 0.68] |
| 4.1.3 Above 12 months follow‐up | 2 | 97 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.94 [‐2.58, 0.70] |
| 4.2 Primary: self‐harm (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.3 Primary: self‐harm (dichotomous) | 3 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 4.3.1 End of treatment | 3 | 252 | Risk Ratio (IV, Random, 95% CI) | 0.62 [0.49, 0.80] |
| 4.3.2 0‐6 months follow‐up | 1 | 41 | Risk Ratio (IV, Random, 95% CI) | 0.14 [0.04, 0.56] |
| 4.3.3 6‐12 months follow‐up | 1 | 41 | Risk Ratio (IV, Random, 95% CI) | 0.27 [0.10, 0.68] |
| 4.3.4 Above 12 months follow‐up | 1 | 41 | Risk Ratio (IV, Random, 95% CI) | 0.33 [0.15, 0.76] |
| 4.4 Primary: suicide‐related outcomes (dichotomous) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.4.1 End of treatment | 3 | 218 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.04, 0.30] |
| 4.4.2 0‐6 months follow‐up | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.06, 1.05] |
| 4.4.3 Above 12 months follow‐up | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.11, 0.74] |
| 4.5 Primary: psychosocial functioning (continuous) | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.5.1 End of treatment | 3 | 239 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐1.24, 0.16] |
| 4.5.2 Above 12 months follow‐up | 2 | 104 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.97, 0.15] |
| 4.6 Secondary: interpersonal problems (continuous) | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.6.1 End of treatment | 5 | 357 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐1.33, ‐0.02] |
| 4.6.2 0‐6 months follow‐up | 1 | 53 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐1.01, 0.20] |
| 4.6.3 Above 12 months follow‐up | 2 | 96 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.71, 0.14] |
| 4.7 Secondary: depression (continuous) | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.7.1 End of treatment | 4 | 333 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐1.22, 0.05] |
| 4.7.2 0‐6 months follow‐up | 2 | 91 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.69, 0.07] |
| 4.7.3 6‐12 months follow‐up | 1 | 37 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.17 [‐1.88, ‐0.45] |
| 4.7.4 Above 12 months follow‐up | 2 | 90 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐1.55, 0.10] |
| 4.8 Secondary: depression (dichotomous), at end of treatment | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 4.9 Secondary: attrition (dichotomous), at end of treatment | 7 | 552 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.79, 1.25] |
| 4.10 Secondary: adverse effects (dichotomous), at end of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.11 Mentalisation‐based treatment for eating disorders (MBT‐ED) versus specialist supportive clinical management (SSCM‐ED) (generic inverse variance) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.11.1 Primary: psychosocial functioning (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.86, 0.72] | |
| 4.11.2 Primary: psychosocial functioning (dichotomous), at 0‐6 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | ‐0.44 [‐1.52, 0.64] | |
| 4.11.3 Secondary: interpersonal problems (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.89, 0.69] | |
| 4.11.4 Secondary: interpersonal problems (continuous), at 0‐6 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐1.15, 1.03] | |
| 4.11.5 Secondary: depression (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.19 [‐0.60, 0.98] | |
| 4.11.6 Secondary: depression (continuous), at 0‐6 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.51 [‐0.62, 1.64] |
Comparison 5. Cognitive behavioural therapy (CBT) and related treatments vs TAU.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Primary: BPD symptom severity (continuous) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1.1 End of treatment | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐3.08 [‐4.99, ‐1.17] |
| 5.1.2 0‐6 months follow‐up | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐2.02 [‐4.23, 0.19] |
| 5.2 Primary: BPD symptom severity (dichotomous), at above 12 months follow‐up | 1 | 76 | Risk Ratio (IV, Fixed, 95% CI) | 0.91 [0.56, 1.48] |
| 5.3 Primary: self‐harm (continuous) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.3.1 End of treatment | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐3.03 [‐5.68, ‐0.38] |
| 5.3.2 0‐6 months follow‐up | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐4.71 [‐11.60, 2.18] |
| 5.4 Primary: self‐harm (dichotomous), at end of treatment | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 5.5 Primary: suicide‐related outcomes (continuous) | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.5.1 End of treatment | 2 | 104 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐1.02, 0.08] |
| 5.5.2 0‐6 months follow‐up | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐1.20, 0.31] |
| 5.5.3 6‐12 months follow‐up | 1 | 76 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.89, 0.02] |
| 5.5.4 Above 12 months follow‐up | 1 | 76 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.77, 0.14] |
| 5.6 Primary: psychosocial functioning (continuous) | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.6.1 End of treatment | 1 | 99 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.39, 0.39] |
| 5.6.2 6‐12 months follow‐up | 1 | 90 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.28, 0.55] |
| 5.6.3 Above 12 months follow‐up | 2 | 209 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.36, 0.43] |
| 5.7 Secondary: interpersonal problems (continuous) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.7.1 End of treatment | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | 5.40 [‐3.70, 14.50] |
| 5.7.2 6‐12 months follow‐up | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐9.17, 9.77] |
| 5.7.3 Above 12 months follow‐up | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 11.70 [0.72, 22.68] |
| 5.8 Secondary: dissociation and psychotic‐like symptoms (continuous) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.8.1 End of treatment | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐2.30 [‐8.84, 4.24] |
| 5.8.2 0‐6 months follow‐up | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐13.40 [‐24.49, ‐2.31] |
| 5.9 Secondary: depression (continuous) | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.9.1 End of treatment | 5 | 314 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.77, 0.35] |
| 5.9.2 0‐6 months follow‐up | 1 | 26 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐1.78, ‐0.14] |
| 5.9.3 6‐12 months follow‐up | 1 | 99 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.69, 0.11] |
| 5.9.4 Above 12 months follow‐up | 2 | 197 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.43, 0.13] |
| 5.10 Secondary: attrition (dichotomous), at end of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.11 Secondary: adverse effects (dichotomous), at end of treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.11.1 Non‐serious adverse effects | 1 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.45, 1.88] |
| 5.11.2 Serious adverse effects | 2 | 326 | Risk Ratio (M‐H, Random, 95% CI) | 2.65 [0.31, 22.93] |
Comparison 6. Psychodynamic psychotherapy vs TAU.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Primary: BPD symptom severity (continuous) | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1.1 End of treatment | 4 | 222 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.66, 0.09] |
| 6.1.2 6‐12 months follow‐up | 1 | 33 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐1.00, 0.38] |
| 6.2 Primary: self‐harm (continuous) | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.2.1 End of treatment | 1 | 33 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.56, 0.80] |
| 6.2.2 6‐12 months follow‐up | 1 | 33 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.55, 0.82] |
| 6.3 Primary: suicide‐related outcomes (continuous) | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.3.1 End of treatment | 3 | 101 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.62, 0.17] |
| 6.3.2 6‐12 months follow‐up | 1 | 33 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐1.07, 0.31] |
| 6.4 Primary: psychosocial functioning (continuous) | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.4.1 End of treatment | 4 | 140 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐1.98, 0.59] |
| 6.4.2 6‐12 months follow‐up | 1 | 33 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.64, 0.73] |
| 6.4.3 Above 12 months follow‐up | 1 | 16 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐1.39, 0.60] |
| 6.5 Secondary: anger (continuous) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.5.1 End of treatment | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.86, 0.86] |
| 6.5.2 6‐12 months follow‐up | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.89, 0.89] |
| 6.6 Secondary: affective instability (continuous) | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.6.1 End of treatment | 3 | 116 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.87, ‐0.13] |
| 6.6.2 6‐12 months follow‐up | 1 | 33 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐1.21, 0.18] |
| 6.7 Secondary: chronic feelings of emptiness (continuous) | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.7.1 End of treatment | 2 | 77 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐1.02, 0.04] |
| 6.7.2 6‐12 months follow‐up | 1 | 33 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐1.28, 0.11] |
| 6.8 Secondary: impulsivity (continuous) | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.8.1 End of treatment | 2 | 77 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.85, 0.07] |
| 6.8.2 6‐12 months follow‐up | 1 | 33 | Std. Mean Difference (IV, Random, 95% CI) | 0.35 [‐0.34, 1.04] |
| 6.9 Secondary: interpersonal problems (continuous) | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.9.1 End of treatment | 4 | 238 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.71, 0.29] |
| 6.9.2 6‐12 months follow‐up | 1 | 33 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐1.20, 0.19] |
| 6.10 Secondary: abandonment (continuous) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.10.1 End of treatment | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.95, 0.55] |
| 6.10.2 6‐12 months follow‐up | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.08, 0.28] |
| 6.11 Secondary: identity disturbance (continuous) | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.11.1 End of treatment | 3 | 199 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐1.02, 0.27] |
| 6.11.2 6‐12 months follow‐up | 1 | 33 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐1.83, ‐0.35] |
| 6.12 Secondary: dissociation and psychotic‐like symptoms (continuous) | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.12.1 End of treatment | 2 | 57 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.96, 0.60] |
| 6.12.2 6‐12 months follow‐up | 1 | 33 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐1.29, 0.11] |
| 6.12.3 Above 12 months follow‐up | 1 | 24 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.81, 0.79] |
| 6.13 Secondary: depression (continuous) | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.13.1 End of treatment | 3 | 190 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.81, 0.47] |
| 6.13.2 Above 12 months follow‐up | 1 | 24 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐1.51, 0.15] |
| 6.14 Secondary: attrition (dichotomous), at end of treatment | 3 | 210 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.56, 1.47] |
Comparison 7. Schema‐focused therapy (SFT) vs TAU.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Primary: BPD symptom severity (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.2 Primary: psychosocial functioning (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.3 Secondary: affective instability (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.4 Secondary: impulsivity (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.5 Secondary: interpersonal problems (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.6 Secondary: dissociation and psychotic‐like symptoms (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.7 Secondary: attrition (dichotomous), at end of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 8. Systems training for emotional predictability and problem solving (STEPPS) vs TAU.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Primary: BPD symptom severity (continuous), at end of treatment | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1.1 End of treatment | 3 | 273 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.63, ‐0.15] |
| 8.1.2 6‐12 months follow‐up | 1 | 124 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.33, 0.38] |
| 8.2 Primary: self‐harm (dichtomous), at end of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.3 Primary: psychosocial functioning (continuous) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.3.1 End of treatment | 1 | 124 | Mean Difference (IV, Fixed, 95% CI) | ‐7.00 [‐11.43, ‐2.57] |
| 8.3.2 6‐12 months follow‐up | 1 | 124 | Mean Difference (IV, Fixed, 95% CI) | ‐5.90 [‐12.49, 0.69] |
| 8.4 Secondary: affective instability (continuous), at end of treatment | 2 | 221 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.52, 0.02] |
| 8.5 Secondary: impulsivity (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.6 Secondary: impulsivity (dichotomous), at end of treatment | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 8.7 Secondary: interpersonal problems (continuous), at end of treatment | 2 | 177 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.67, ‐0.08] |
| 8.8 Secondary: dissociation and psychotic‐like symptoms (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.9 Secondary: depression (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.9.1 End of treatment | 1 | 124 | Mean Difference (IV, Fixed, 95% CI) | ‐3.80 [‐9.34, 1.74] |
| 8.9.2 6‐12 months follow‐up | 1 | 124 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐8.42, 9.62] |
| 8.10 Secondary: attrition (dichotomous), at end of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 9. Cognitive analytic therapy (CAT) vs TAU.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Primary: suicide‐related outcomes (continuous) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1.1 End of treatment | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐4.21, 1.41] |
| 9.1.2 0‐6 months follow‐up | 1 | 8 | Mean Difference (IV, Fixed, 95% CI) | ‐0.50 [‐2.05, 1.05] |
| 9.2 Primary: psychosocial functioning (continuous) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.2.1 End of treatment | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | ‐16.40 [‐31.20, ‐1.60] |
| 9.2.2 0‐6 months follow‐up | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | ‐15.80 [‐29.36, ‐2.24] |
| 9.3 Secondary: anger (continuous) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.3.1 End of treatment | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐7.97, 4.17] |
| 9.3.2 0‐6 months follow‐up | 1 | 8 | Mean Difference (IV, Fixed, 95% CI) | ‐4.50 [‐9.01, 0.01] |
| 9.4 Secondary: dissociation and psychotic‐like symptoms (continuous) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.4.1 End of treatment | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | ‐6.10 [‐15.01, 2.81] |
| 9.4.2 0‐6 months follow‐up | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | ‐11.70 [‐24.02, 0.62] |
| 9.5 Secondary: depression (continuous) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.5.1 End of treatment | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | ‐9.70 [‐20.10, 0.70] |
| 9.5.2 0‐6 months follow‐up | 1 | 8 | Mean Difference (IV, Fixed, 95% CI) | ‐3.70 [‐11.99, 4.59] |
| 9.6 Secondary: attrition (dichotomous), at end of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 10. Motivation feedback (MF) vs TAU.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Primary: psychosocial functioning, at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
Comparison 11. Psychoeducation vs TAU.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Secondary: depression (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.2 Secondary: attrition (dichotomous), at end of treatment | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 12. Transference‐focused psychotherapy (TFP) vs TAU.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Primary: BPD symptom severity (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.2 Primary: self‐harm (dichotomous), at end of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.3 Primary: suicide‐related outcomes (dichotomous), at end of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.4 Primary: psychosocial functioning (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.5 Secondary: depression (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.6 Secondary: attrition (dichotomous), at end of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 13. Once‐only interventions (individual setting) vs TAU.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Primary: self‐harm (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 13.2 Primary: psychosocial functioning (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 13.3 Secondary: depression (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 13.4 Secondary: attrition (dichotomous), at end of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 14. Eclectic treatments vs TAU.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 14.1 Primary: BPD symptom severity (continuous), at end of treatment | 3 | 134 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐1.57, ‐0.23] |
| 14.2 Primary: self‐harm (continuous), at end of treatment | 2 | 83 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.29, ‐0.39] |
| 14.3 Primary: suicide‐related outcomes (continuous), at end of treatment | 2 | 221 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐1.29, 0.19] |
| 14.4 Primary: psychosocial functioning (continuous) | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.4.1 End of treatment | 2 | 231 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐1.10, ‐0.04] |
| 14.4.2 Above 12 months follow‐up | 1 | 170 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.04, ‐0.24] |
| 14.5 Secondary: anger (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.6 Secondary: affective instability (continuous), at end of treatment | 3 | 134 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐1.74, ‐0.15] |
| 14.7 Secondary: chronic feelings of emptiness (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.8 Secondary: impulsivity (continuous), at end of treatment | 3 | 134 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐1.30, ‐0.22] |
| 14.9 Secondary: interpersonal problems (continuous), at end of treatment | 2 | 112 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐1.09, ‐0.15] |
| 14.10 Secondary: abandonment (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.11 Secondary: identity disturbance (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.12 Secondary: depression (continuous), at end of treatment | 4 | 304 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.82 [‐1.38, ‐0.26] |
| 14.13 Secondary: attrition (dichotomuous), at end of treatment | 4 | 326 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.85, 1.32] |
| 14.14 Secondary: adverse effects (dichotomous), at end of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 15. Psychotherapy vs waiting list or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 15.1 Primary: BPD symptom severity (continuous), at end of treatment | 3 | 161 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.93, ‐0.05] |
| 15.2 Primary: self‐harm (continuous), at end of treatment | 2 | 128 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.52, 0.18] |
| 15.3 Primary: suicide‐related outcomes (continuous), at end of treatment | 2 | 108 | Std. Mean Difference (IV, Random, 95% CI) | ‐5.62 [‐16.39, 5.16] |
| 15.4 Primary: psychosocial functioning (continuous) | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.4.1 End of treatment | 5 | 219 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐1.01, ‐0.11] |
| 15.4.2 0‐6 months follow‐up | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.65, ‐0.13] |
| 15.4.3 6‐12 months follow‐up | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.04 [‐1.81, ‐0.27] |
| 15.4.4 Above 12 months follow‐up | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐1.14, 0.37] |
| 15.5 Secondary: anger (continuous), at end of treatment | 2 | 128 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐1.70, 0.55] |
| 15.6 Secondary: affective instability (continuous) | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.6.1 End of treatment | 2 | 128 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.99 [‐1.36, ‐0.62] |
| 15.6.2 0‐6 months follow‐up | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐1.20, 0.26] |
| 15.6.3 6‐12 months follow‐up | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐1.10, 0.34] |
| 15.6.4 Above 12 months follow‐up | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐1.01, 0.44] |
| 15.7 Secondary: chronic feelings of emptiness (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 15.8 Secondary: impulsivity (continuous) | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.8.1 End of treatment | 3 | 178 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.82, ‐0.22] |
| 15.8.2 0‐6 months follow‐up | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐1.66, ‐0.14] |
| 15.8.3 6‐12 months follow‐up | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.54, ‐0.04] |
| 15.8.4 Above 12 months follow‐up | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.50, ‐0.01] |
| 15.9 Secondary: interpersonal problems (continuous) | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.9.1 End of treatment | 3 | 120 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.85 [‐1.23, ‐0.47] |
| 15.9.2 0‐6 months follow‐up | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐2.21, ‐0.59] |
| 15.9.3 6‐12 months follow‐up | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐1.66, ‐0.14] |
| 15.9.4 Above 12 months follow‐up | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.62, ‐0.11] |
| 15.10 Secondary: abandonment (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 15.11 Secondary: identity disturbance (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 15.12 Secondary: dissociation and psychotic‐like symptoms (continuous), at end of treatment | 2 | 77 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.65, 0.39] |
| 15.13 Secondary: depression (continuous), at end of treatment | 6 | 239 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.28 [‐2.21, ‐0.34] |
| 15.14 Secondary: attrition (dichotomous), at end of treatment | 3 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.20, 1.50] |
Comparison 16. Dialectical behavior therapy (DBT) vs waiting list or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 16.1 Primary: BPD symptom severity (continuous), at end of treatment | 2 | 117 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐1.08, ‐0.33] |
| 16.2 Primary: self‐harm (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 16.3 Primary: suicide‐related outcomes (continuous), at end of treatment | 2 | 108 | Std. Mean Difference (IV, Random, 95% CI) | ‐5.62 [‐16.39, 5.16] |
| 16.4 Primary: psychosocial functioning (continuous), at end of treatment | 2 | 117 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.73 [‐1.11, ‐0.36] |
| 16.5 Secondary: anger (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 16.6 Secondary: affective instability (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 16.7 Secondary: impulsivity (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 16.8 Secondary: dissociation and psychotic‐like symptoms (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 16.9 Secondary: depression (continuous), at end of treatment | 3 | 141 | Std. Mean Difference (IV, Random, 95% CI) | ‐3.20 [‐5.57, ‐0.83] |
| 16.10 DBT‐couple therapy (CDBT) vs waiting list (generic inverse variance) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 16.10.1 Primary: BPD severity (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | ‐27.15 [‐31.59, ‐22.71] | |
| 16.10.2 Primary: suicide‐related outcomes (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | ‐0.94 [‐1.24, ‐0.64] | |
| 16.10.3 Primary: psychosocial functioning (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | ‐10.70 [‐12.31, ‐9.09] | |
| 16.10.4 Secondary: anger (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | ‐1.42 [‐1.72, ‐1.12] | |
| 16.10.5 Secondary: affective instability (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | ‐4.01 [‐5.44, ‐2.58] | |
| 16.10.6 Secondary: chronic feelings of emptiness (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | ‐3.54 [‐4.81, ‐2.27] | |
| 16.10.7 Secondary: impulsivity (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | ‐0.51 [‐0.72, ‐0.30] | |
| 16.10.8 Secondary: interpersonal problems (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | ‐1.98 [‐2.47, ‐1.49] | |
| 16.10.9 Secondary: abandonment (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | ‐0.86 [‐1.09, ‐0.63] | |
| 16.10.10 Secondary: identity disturbance (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | ‐2.44 [‐2.77, ‐2.11] | |
| 16.10.11 Secondary: dissociation or psychotic‐like symptoms (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | ‐1.92 [‐2.46, ‐1.38] | |
| 16.10.12 Secondary: depression (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | ‐10.43 [‐11.86, ‐9.00] |
Comparison 17. Schema‐focused therapy (SFT) vs no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 17.1 Primary: suicide‐related outcomes (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 17.2 Secondary: depression (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
Comparison 18. Interpersonal psychotherapy (IPT) vs waiting list.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 18.1 Primary: BPD symptom severity (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 18.2 Primary: self‐harm (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 18.3 Primary: psychosocial functioning (continuous) | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 18.3.1 End of treatment | 2 | 76 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.48, 0.42] |
| 18.3.2 0‐6 months follow‐up | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.65, ‐0.13] |
| 18.3.3 6‐12 months follow‐up | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.04 [‐1.81, ‐0.27] |
| 18.3.4 Above 12 months follow‐up | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐1.14, 0.37] |
| 18.4 Secondary: anger (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 18.5 Secondary: affective instability (continuous) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 18.5.1 End of treatment | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐1.02 [‐1.66, ‐0.38] |
| 18.5.2 0‐6 months follow‐up | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐1.15, 0.23] |
| 18.5.3 6‐12 months follow‐up | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐1.12, 0.34] |
| 18.5.4 Above 12 months follow‐up | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐1.17, 0.49] |
| 18.6 Secondary: chronic feelings of emptiness (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 18.7 Secondary: impulsivity (continuous) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 18.7.1 End of treatment | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐1.03 [‐1.69, ‐0.37] |
| 18.7.2 0‐6 months follow‐up | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐1.95, ‐0.25] |
| 18.7.3 6‐12 months follow‐up | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐2.06, ‐0.14] |
| 18.7.4 Above 12 months follow‐up | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐1.09 [‐2.09, ‐0.09] |
| 18.8 Secondary: interpersonal problems (continuous) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 18.8.1 End of treatment | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐1.14 [‐1.94, ‐0.34] |
| 18.8.2 0‐6 months follow‐up | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐2.00 [‐2.98, ‐1.02] |
| 18.8.3 6‐12 months follow‐up | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐1.56 [‐2.74, ‐0.38] |
| 18.8.4 Above 12 months follow‐up | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐1.48 [‐2.63, ‐0.33] |
| 18.9 Secondary: abandonment (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 18.10 Secondary: identity disturbance (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 18.11 Secondary: dissociation and psychotic‐like symptoms (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 18.12 Secondary outcome: depression (continuous), at end of treatment | 3 | 98 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐1.11, 0.06] |
| 18.13 Secondary outcome: attrition (dichotomous), at end of treatment | 2 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.20, 1.50] |
Comparison 19. Once‐only interventions (individual setting) vs waiting list.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 19.1 Secondary: impulsivity (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 19.2 Secondary: interpersonal problems (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 19.3 Secondary: attrition (dichotomous), at end of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 20. Eclectic treatments vs waiting list.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 20.1 Primary outcome: psychosocial functioning (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 20.2 Secondary outcome: interpersonal problems (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
Comparison 21. Dialectical behavior therapy (DBT) and related treatments vs active treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 21.1 Standard DBT (DBT) vs client‐centred therapy (CCT) (continuous) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 21.1.1 Primary: self‐harm (continuous), at end of treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐2.75 [‐4.42, ‐1.08] |
| 21.1.2 Primary: suicide‐related outcomes (continuous), at end of treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐7.75 [‐14.66, ‐0.84] |
| 21.1.3 Secondary: anger (continuous), at end of treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐1.00 [‐1.98, ‐0.02] |
| 21.1.4 Secondary: impulsivity (continuous), at end of treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐1.50 [‐2.60, ‐0.40] |
| 21.1.5 Secondary: dissociation and psychotic‐like symptoms (continuous), at end of treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐7.16 [‐12.15, ‐2.17] |
| 21.1.6 Secondary: depression (continuous), at end of treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐9.16 [‐14.79, ‐3.53] |
| 21.2 DBT vs CCT, secondary: attrition (dichotomous), at end of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21.3 Standard DBT (DBT) vs good psychiatric management (GPM) (continuous) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 21.3.1 Primary: BPD severity (continuous), at end of treatment | 1 | 180 | Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐1.97, 1.51] |
| 21.3.2 Primary: self‐harm (continuous), at end of treatment | 1 | 180 | Mean Difference (IV, Fixed, 95% CI) | ‐8.58 [‐19.38, 2.22] |
| 21.3.3 Secondary: anger (continuous), at end of treatment | 1 | 180 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐1.65, 1.35] |
| 21.3.4 Secondary: interpersonal problems (continuous), at end of treatment | 1 | 180 | Mean Difference (IV, Fixed, 95% CI) | ‐1.34 [‐15.36, 12.68] |
| 21.3.5 Secondary: depression (continuous), at end of treatment | 1 | 180 | Mean Difference (IV, Fixed, 95% CI) | ‐2.65 [‐7.18, 1.88] |
| 21.4 DBT vs GPM, secondary: attrition (dichotomous), at end of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21.5 Standard DBT (DBT) vs individual DBT therapy + activities group (DBT‐I) (continuous) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 21.5.1 Primary: self‐harm (continuous), at end of treatment | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐10.40 [‐22.99, 2.19] |
| 21.5.2 Primary: self‐harm (continuous), at 6‐12 months follow‐up | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐8.10 [‐19.59, 3.39] |
| 21.5.3 Primary: suicide‐related outcomes (continuous), at end of treatment | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | 0.50 [‐1.37, 2.37] |
| 21.5.4 Primary: suicide‐related outcomes (continuous), at 6‐12 months follow‐up | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐2.79, ‐0.41] |
| 21.5.5 Secondary: depression (continuous), at end of treatment | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐5.90 [‐9.74, ‐2.06] |
| 21.5.6 Secondary: depression (continuous), at 6‐12 months follow‐up | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [‐3.10, 5.70] |
| 21.6 DBT vs DBT‐I, secondary: attrition (dichotomous), at end of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21.7 Standard DBT (DBT) vs skills training group + individual case management (DBT‐S) (continuous) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 21.7.1 Primary: self‐harm (continuous), at end of treatment | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐8.42, 9.02] |
| 21.7.2 Primary: self‐harm (continuous), at 6‐12 months follow‐up | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐1.50 [‐9.04, 6.04] |
| 21.7.3 Primary: suicide‐related outcomes (continuous), at end of treatment | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐1.06, 2.66] |
| 21.7.4 Primary: suicide‐related outcomes (continuous), at 6‐12 months follow‐up | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | 0.50 [‐0.02, 1.02] |
| 21.7.5 Secondary: depression (continuous), at end of treatment | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | 1.90 [‐1.60, 5.40] |
| 21.7.6 Secondary: depression (continuous), at 6‐12 months follow‐up | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | 3.30 [‐0.90, 7.50] |
| 21.8 DBT vs DBT‐S, secondary: attrition (dichotomous), at 6‐12 months follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21.9 Standard DBT (DBT) vs step‐down DBT (DBT‐SD) (continuous) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 21.9.1 Primary: BPD symptom severity (continuous), at end of treatment | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐2.83 [‐11.21, 5.55] |
| 21.9.2 Primary: self‐harm (continuous), at end of treatment | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 4.10 [‐4.07, 12.27] |
| 21.9.3 Primary: suicide‐related outcomes (continuous), at end of treatment | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.73, 1.33] |
| 21.9.4 Secondary: anger (continuous), at end of treatment | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐0.53 [‐1.48, 0.43] |
| 21.9.5 Secondary: affective instability (continuous), at end of treatment | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐0.71 [‐2.51, 1.09] |
| 21.9.6 Secondary: chronic feeling of emptiness (continuous), at end of treatment | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐1.68, 2.04] |
| 21.9.7 Secondary: impulsivity (continuous), at end of treatment | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 0.24 [‐0.26, 0.75] |
| 21.9.8 Secondary: interpersonal problems (continuous), at end of treatment | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐1.31 [‐2.05, ‐0.57] |
| 21.9.9 Secondary: abandonment (continuous), at end of treatment | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐1.11 [‐2.14, ‐0.08] |
| 21.9.10 Secondary: dissociation and psychotic‐like symptoms (continuous), at end of treatment | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐0.44 [‐1.56, 0.68] |
| 21.10 DBT vs DBT‐SD, secondary: attrition (dichotomous), at end of treatment | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.17, 0.78] |
| 21.11 Standard DBT (DBT) vs DBT Prolonged Exposure (PE) (continuous) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 21.11.1 Primary: self‐harm (continuous), end of treatment | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐1.86, 2.06] |
| 21.11.2 Primary: self‐harm (continuous), at 0‐6 months follow‐up | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.09, 0.49] |
| 21.11.3 Primary: suicide‐related outcomes (continuous), end of treatment | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 21.11.4 Primary: suicide‐related outcomes (continuous), at 0‐6 months follow‐up | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 21.11.5 Primary: psychosocial functioning (continuous), end of treatment | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 5.27 [‐2.06, 12.60] |
| 21.11.6 Primary: psychosocial functioning (continuous), at 0‐6 months follow‐up | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 2.00 [‐6.27, 10.27] |
| 21.11.7 Secondary: interpersonal problems (continuous), end of treatment | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.42, 0.70] |
| 21.11.8 Secondary: interpersonal problems (continuous), at 0‐6 months follow‐up | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 0.19 [‐0.48, 0.86] |
| 21.11.9 Secondary: dissociation or psychotic‐like symptoms (continuous), end of treatment | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 4.60 [‐9.24, 18.44] |
| 21.11.10 Secondary: dissociation or psychotic‐like symptoms (continuous), at 0‐6 months follow‐up | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 6.00 [‐9.46, 21.46] |
| 21.11.11 Secondary: depression (continuous), end of treatment | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 3.70 [‐3.19, 10.59] |
| 21.11.12 Secondary: depression (continuous), at 0‐6 months follow‐up | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 4.30 [‐1.08, 9.68] |
| 21.12 DBT vs DBT‐PE, secondary: attrition (dichotomous), at 0‐6 months follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21.13 DBT skills group + case management (DBT‐S) vs DBT individual therapy + activity group (DBT‐I) (continuous) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 21.13.1 Primary: self‐harm (continuous), at end of treatment | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐10.70 [‐22.47, 1.07] |
| 21.13.2 Primary: self‐harming behaviour (continuous), at 6‐12 months follow‐up | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐6.60 [‐19.72, 6.52] |
| 21.13.3 Primary: suicide‐related outcomes (continuous), at end of treatment | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.72, 1.12] |
| 21.13.4 Primary: suicide‐related outcomes (continuous), at 6‐12 months follow‐up | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐2.10 [‐3.21, ‐0.99] |
| 21.13.5 Secondary: depression (continuous), at end of treatment | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐7.80 [‐11.27, ‐4.33] |
| 21.13.6 Secondary: depression (continuous), at 6‐12 months follow‐up | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐2.00 [‐6.44, 2.44] |
| 21.14 DBT‐S vs DBT‐I, secondary: attrition (dichotomous), at 6‐12 months follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21.15 DBT skills group (DBT‐S) vs cognitive therapy group (CT‐G) (continuous) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 21.15.1 Primary: BPD symptom severity (continuous), at end of treatment | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | 0.26 [‐0.13, 0.65] |
| 21.15.2 Primary: BPD symptom severity (continuous), at 0‐6 months follow‐up | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐0.96 [‐1.15, ‐0.77] |
| 21.15.3 Primary: suicide‐related outcomes (continuous), at end of treatment | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | 0.46 [‐2.47, 3.39] |
| 21.15.4 Primary: suicide‐related outcomes (continuous), at 0‐6 months follow‐up | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐2.69 [‐4.89, ‐0.49] |
| 21.15.5 Secondary: depression (continuous), at end of treatment | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐2.12 [‐5.25, 1.01] |
| 21.15.6 Secondary: depression (continuous), at 0‐6 months follow‐up | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐5.07, 1.87] |
| 21.16 DBT‐S vs CT‐G, secondary: attrition (dichotomous), at end of treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 21.17 DBT skills group (DBT‐S) vs schema‐focused therapy group (SFT‐G) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 21.17.1 Primary: suicide‐related outcomes (continuous), at end of treatment | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 0.92 [‐0.36, 2.20] |
| 21.17.2 Secondary: depression (continuous), at end of treatment | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 4.33 [2.57, 6.09] |
| 21.18 DBT mindfulness group (DBT‐M) vs DBT interpersonal effectiveness group (DBT‐IE) (continuous) | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 21.18.1 Primary: BPD symptom severity (continuous), at end of treatment | 2 | 113 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐1.47, 0.58] |
| 21.18.2 Secondary: impulsivity (continuous), at end of treatment | 2 | 91 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.77, 0.06] |
| 21.19 DBT‐M vs DBT‐IE, secondary: attrition (dichotomous), at end of treatment | 2 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 1.86 [1.07, 3.23] |
| 21.20 DBT mindfulness group (DBT‐M) vs loving‐kindness and compassion meditation (LK/CM), primary: BPD symptom severity (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
Comparison 22. Cognitive behavioural therapy (CBT) and related treatments vs active treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 22.1 CBT vs trauma‐ and anxiety‐related group psychoeducation (continuous) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 22.1.1 Primary: BPD symptom severity (continuous), at end of treatment | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐1.46, 0.94] |
| 22.1.2 Primary: BPD symptom severity (continuous), at 0‐6 months follow‐up | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐1.60, 0.82] |
| 22.1.3 Primary: BPD symptom severity (continuous), at 6‐12 months follow‐up | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.57 [‐0.48, 1.62] |
| 22.1.4 Primary: psychosocial functioning (continuous), at end of treatment | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.64 [‐5.76, 4.48] |
| 22.1.5 Primary: psychosocial functioning (continuous), at 0‐6 months follow‐up | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐1.23 [‐6.94, 4.48] |
| 22.1.6 Primary: psychosocial functioning (continuous), at 6‐12 months follow‐up | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.61 [‐5.00, 6.22] |
| 22.1.7 Secondary: depression (continuous), at end of treatment | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.76 [‐8.20, 9.72] |
| 22.1.8 Secondary: depression (continuous), at 0‐6 months follow‐up | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 3.13 [‐4.05, 10.31] |
| 22.1.9 Secondary: depression (continuous), at 6‐12 months follow‐up | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.77 [‐7.08, 8.62] |
| 22.2 CBT vs trauma‐ and anxiety‐related group psychoeducation, secondary: attrition (dichotomous), at end of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 22.3 CBT vs interpersonal psychotherapy (IPT) (continuous) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 22.3.1 Primary: psychosocial functioning (continuous), at end of treatment | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐5.30 [‐12.36, 1.76] |
| 22.3.2 Secondary: depression (continuous), at end of treatment | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐4.72, 3.92] |
| 22.4 CBT vs IPT, secondary: attrition (dichotomous), at end of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 22.5 CBT vs Rogerian supportive therapy (continuous) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 22.5.1 Primary: self‐harm (continuous), end of treatment | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 0.74 [‐0.03, 1.51] |
| 22.5.2 Primary: self‐harm (continuous), at 6‐12 months follow‐up | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐0.63 [‐1.75, 0.49] |
| 22.5.3 Primary: suicide‐related outcomes (continuous), at end of treatment | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 0.69 [‐2.60, 3.98] |
| 22.5.4 Primary: suicide‐related outcomes (continuous), at 6‐12 months follow‐up | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐2.43 [‐6.14, 1.28] |
| 22.5.5 Primary: psychosocial functioning (continuous), at end of treatment | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐1.31, 0.45] |
| 22.5.6 Primary: psychosocial functioning (continuous), at 6‐12 months follow‐up | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐0.98 [‐2.02, 0.06] |
| 22.5.7 Secondary: impulsivity (continuous), at end of treatment | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐1.01 [‐3.85, 1.83] |
| 22.5.8 Secondary: impulsivity (continuous), at 6‐12 months follow‐up | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐2.18 [‐5.91, 1.55] |
| 22.5.9 Secondary: depression (continuous), at end of treatment | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 1.04 [‐5.59, 7.67] |
| 22.5.10 Secondary: depression (continuous), at 6‐12 months follow‐up | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐5.15 [‐9.38, ‐0.92] |
| 22.6 CBT vs Rogerian supportive therapy, secondary: attrition (dichotomous), end of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 22.7 MACT (Manual‐assisted Cognitive Therapy) vs MACT + therapeutic assessment (MACT + TA) (continuous) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 22.7.1 Primary: BPD symptom severity (continuous), at end of treatment | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐3.75 [‐14.17, 6.67] |
| 22.7.2 Primary: self‐harm (continuous), at end of treatment | 1 | 16 | Mean Difference (IV, Random, 95% CI) | 1.75 [‐18.71, 22.21] |
| 22.7.3 Primary: suicide‐related outcomes (continuous), at end of treatment | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐17.71, 16.45] |
| 22.7.4 Secondary: affective instability (continuous), at end of treatment | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐5.25 [‐12.10, 1.60] |
| 22.7.5 Secondary: interpersonal problems (continuous), at end of treatment | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐11.24, 10.24] |
| 22.7.6 Secondary: identity disturbance (continuous), at end of treatment | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐4.88 [‐14.98, 5.22] |
| 22.8 MACT vs MACT + TA, secondary: attrition (dichotomous), at end of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 22.9 Meta‐Cognitive training for BPD (B‐MCT) vs progressive muscle relaxation training (PMR) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 22.9.1 Primary: BPD symptom severity (continuous), at end of treatment | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐1.80 [‐4.97, 1.37] |
| 22.9.2 Primary: BPD symptom severity (continuous), at 0‐6 months follow up | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐3.60 [‐7.16, ‐0.04] |
| 22.9.3 Secondary: depression (continuous), at end of treatment | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐3.20 [‐9.91, 3.51] |
| 22.9.4 Secondary: depression (continuous), at 0‐6 months follow‐up | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | 8.50 [2.03, 14.97] |
| 22.10 B‐MCT vs progressive muscle relaxation (PMR) training + TAU (dichotomous). Secondary: attrition (dichotomous), at end of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 22.11 MOTR (Motive‐Oriented Therapeutic Relationship) vs Good Psychiatric Management (GPM) (continuous) | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 22.11.1 Primary: BPD symptom severity (continuous), at end of treatment | 1 | 74 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.39, 0.53] |
| 22.11.2 Primary: psychosocial functioning (continuous), at end of treatment | 2 | 99 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.85, ‐0.05] |
| 22.11.3 Secondary: interpersonal problems (continuous), at end of treatment | 2 | 99 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐0.97, ‐0.16] |
| 22.12 MOTR vs (GPM), secondary: attrition (dichotomous), at end of treatment | 2 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.26, 1.41] |
Comparison 23. Schema‐focused therapy (SFT) vs active treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 23.1 SFT vs TFP. Primary: BPD symptom severity (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 23.2 SFT vs TFP. Secondary: attrition (dichotomous), at 0‐6 months follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 23.3 SFT vs SFT + therapist availability (TA). Primary: BPD symptom severity (continuous), at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 23.4 SFT vs SFT + TA. Secondary: attrition (dichotomous), 0‐6 months follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 24. Systems training for emotional predictability and problem solving‐based psychoeducation (STEPPS‐PE) vs cognitive rehabilitation (CR).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 24.1 STEPPS‐PE vs CR | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 24.1.1 Primary: BPD symptom severity (continuous), at end of treatment | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐3.67 [‐6.52, ‐0.82] |
| 24.1.2 Primary: BPD symptom severity (continuous), at 0‐6 months follow‐up | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 4.68 [1.42, 7.94] |
| 24.1.3 Secondary: impulsivity (continuous), at end of treatment | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 1.99 [0.06, 3.92] |
| 24.1.4 Secondary: impulsivity (continuous), at 0‐6 months follow‐up | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 9.92 [7.38, 12.46] |
| 24.1.5 Secondary: depression (continuous), at end of treatment | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐2.58 [‐3.62, ‐1.54] |
| 24.1.6 Secondary: depression (continuous), at 0‐6 months follow‐up | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐10.11 [‐11.35, ‐8.87] |
| 24.2 STEPPS‐PE vs CR. Secondary: attrition (dichotomous), at end of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 25. Eclectic treatments vs active treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 25.1 Combined inpatient and outpatient psychotherapy versus outpatient psychotherapy | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 25.1.1 Primary: psychosocial functioning (continuous), at end of treatment | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 6.50 [‐0.06, 13.06] |
| 25.1.2 Primary: psychosocial functioning (continuous), at above 12 months follow‐up | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐5.70 [‐13.33, 1.93] |
| 25.1.3 Secondary: interpersonal problems (continuous), at end of treatment | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.24, 0.34] |
| 25.1.4 Secondary: interpersonal problems (continuous), at above 12 months follow‐up | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐0.42 [‐0.77, ‐0.07] |
| 25.1.5 Secondary: identity disturbance (continuous), at end of treatment | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.59, 0.09] |
| 25.1.6 Secondary: identity disturbance (continuous), at above 12 months follow‐up | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐0.47 [‐0.78, ‐0.16] |
| 25.1.7 Secondary: depression (continuous), at end of treatment | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐6.25, 5.45] |
| 25.1.8 Secondary: depression (continuous), at above 12 months follow‐up | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐4.70 [‐11.02, 1.62] |
| 25.2 Combined inpatient and outpatient psychotherapy versus outpatient psychotherapy. Secondary: attrition (dichotomous), at end of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 25.3 integrative BPD‐oriented adolescent family therapy (I‐BAFT) vs individual drug counselling (IDC) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 25.3.1 Primary: BPD symptom severity (dichotomous), at end of treatment | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.50, 1.64] |
| 25.3.2 Secondary: attrition (dichotomous), at 6‐12 months follow‐up | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.18, 1.40] |
Comparison 26. Subgroup analysis: therapeutic approaches.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 26.1 BPD symptom severity | 23 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 26.1.1 DBT | 3 | 149 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.05, ‐0.14] |
| 26.1.2 MBT | 5 | 267 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.38, 0.11] |
| 26.1.3 Psychodynamic psychotherapy | 4 | 222 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.66, 0.09] |
| 26.1.4 STEPPS | 3 | 273 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.63, ‐0.15] |
| 26.1.5 Eclectic treatments | 3 | 134 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐1.57, ‐0.23] |
| 26.1.6 ACT | 1 | 41 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐1.89, ‐0.55] |
| 26.1.7 CBT | 1 | 26 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.28 [‐2.14, ‐0.42] |
| 26.1.8 SFT | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.66 [‐2.54, ‐0.78] |
| 26.1.9 CAT | 1 | 9 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐1.88, 0.83] |
| 26.1.10 Tranference‐focused psychotherapy | 1 | 104 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐0.95, ‐0.16] |
| 26.2 Psychosocial functioning | 22 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 26.2.1 DBT | 6 | 225 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.69, ‐0.03] |
| 26.2.2 MBT | 3 | 239 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐1.24, 0.16] |
| 26.2.3 Psychodynamic psychotherapy | 4 | 140 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐1.98, 0.59] |
| 26.2.4 Eclectic treatments | 2 | 231 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐1.10, ‐0.04] |
| 26.2.5 CBT | 1 | 99 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.39, 0.39] |
| 26.2.6 SFT | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐2.03, ‐0.38] |
| 26.2.7 STEPPS | 1 | 124 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐0.91, ‐0.19] |
| 26.2.8 CAT | 1 | 9 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐2.73, 0.30] |
| 26.2.9 Motivation feedback | 1 | 43 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.69, 0.55] |
| 26.2.10 Tranference‐focused psychotherapy | 1 | 104 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.73, 0.05] |
| 26.2.11 Once‐only intervention | 1 | 72 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.43, 0.49] |
Comparison 27. Subgroup analysis: age.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 27.1 BPD symptom severity | 22 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 27.1.1 15 to 18 years old | 2 | 156 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.46, 0.17] |
| 27.1.2 Above 18 years old | 20 | 1088 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐0.76, ‐0.37] |
Comparison 28. Subgroup analysis: duration.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 28.1 BPD symptom severity | 22 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 28.1.1 Less than 6 months | 8 | 525 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐1.10, ‐0.42] |
| 28.1.2 6 to 12 months | 11 | 606 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.60, ‐0.12] |
| 28.1.3 Above 12 months | 3 | 113 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.75, 0.01] |
| 28.2 Psychosocial functioning | 20 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 28.2.1 Less than 6 months | 6 | 468 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.73, 0.11] |
| 28.2.2 6 to 12 months | 10 | 535 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.49, ‐0.01] |
| 28.2.3 Over 12 months | 4 | 263 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.62, ‐0.10] |
Comparison 29. Subgroup analysis: mode of therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 29.1 BPD symptom severity | 22 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 29.1.1 Individual therapy | 8 | 520 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.63, ‐0.16] |
| 29.1.2 Group therapy | 8 | 438 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.21, ‐0.57] |
| 29.1.3 Mixed therapy | 6 | 286 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.38, 0.09] |
| 29.2 Psychosocial functioning | 22 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 29.2.1 Individual therapy | 8 | 570 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.75, 0.12] |
| 29.2.2 Group therapy | 7 | 366 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.65, ‐0.23] |
| 29.2.3 Mixed therapy | 7 | 378 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐1.14, ‐0.13] |
Comparison 30. Subgroup analysis: setting.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 30.1 BPD symptom severity | 22 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 30.1.1 Inpatient | 2 | 217 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.34, 0.20] |
| 30.1.2 Outpatient | 20 | 1027 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐0.77, ‐0.39] |
| 30.2 Psychosocial functioning | 22 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 30.2.1 Inpatient | 2 | 179 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.85 [‐1.24, ‐0.46] |
| 30.2.2 Outpatient | 18 | 1057 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.54, ‐0.08] |
| 30.2.3 Inpatient and outpatient | 2 | 78 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐1.84, ‐0.84] |
Comparison 31. Subgroup analysis: types of raters.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 31.1 BPD symptom severity | 22 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 31.1.1 Self‐rated | 8 | 408 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐1.19, ‐0.29] |
| 31.1.2 Clinician‐rated | 14 | 836 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.58, ‐0.25] |
| 31.2 Psychosocial functioning | 22 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 31.2.1 Self‐rated | 12 | 728 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.60, 0.03] |
| 31.2.2 Clinician‐rated | 10 | 586 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐0.96, ‐0.37] |
Comparison 32. Subgroup analysis: types of TAU.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 32.1 BPD symptom severity | 22 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 32.1.1 Obligatory TAU | 19 | 1071 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.70, ‐0.30] |
| 32.1.2 Optional TAU | 3 | 173 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐1.09, ‐0.17] |
| 32.2 Psychosocial functioning | 22 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 32.2.1 Obligatory TAU | 17 | 1002 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.56, ‐0.09] |
| 32.2.2 Optional TAU | 5 | 312 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐1.62, ‐0.30] |
Comparison 33. Subgroup analysis: type of comparison group.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 33.1 BPD symptom severity | 25 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 33.1.1 TAU | 22 | 1244 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.70, ‐0.33] |
| 33.1.2 Waiting list | 3 | 161 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.93, ‐0.05] |
| 33.2 Psychosocial functioning | 27 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 33.2.1 TAU | 22 | 1314 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.68, ‐0.22] |
| 33.2.2 Waiting list | 5 | 219 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐1.01, ‐0.11] |
Comparison 34. Subgroup analysis: types of scales.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 34.1 BPD symptom severity | 22 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 34.1.1 ZAN‐BPD | 4 | 261 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.69, ‐0.20] |
| 34.1.2 SCID | 3 | 112 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐1.16, ‐0.00] |
| 34.1.3 BEST | 4 | 147 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐1.47, ‐0.72] |
| 34.1.4 BPDSI | 4 | 267 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.45, 0.04] |
| 34.1.5 BPP‐40 | 1 | 52 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐1.11, 0.00] |
| 34.1.6 CGI‐BPD | 1 | 33 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.68, 0.68] |
| 34.1.7 CCGI‐BPD | 1 | 59 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐1.55, ‐0.47] |
| 34.1.8 BPT | 1 | 122 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.43, 0.28] |
| 34.1.9 BPFS‐C | 1 | 59 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.52, 0.50] |
| 34.1.10 BSI | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.66 [‐2.54, ‐0.78] |
| 34.1.11 Mean number of DSM‐IV symptoms | 1 | 104 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐0.95, ‐0.16] |
| 34.2 Psychosocial functioning | 22 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 34.2.1 GAF | 4 | 204 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.73 [‐1.41, ‐0.05] |
| 34.2.2 GAS | 4 | 330 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐0.96, ‐0.46] |
| 34.2.3 SAS | 4 | 283 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.99, 0.53] |
| 34.2.4 SPS | 1 | 24 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.17 [‐3.22, ‐1.13] |
| 34.2.5 QQ45 | 1 | 41 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐1.09, 0.15] |
| 34.2.6 WSAS | 1 | 72 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.43, 0.49] |
| 34.2.7 CORE‐OM | 1 | 41 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.34, 0.92] |
| 34.2.8 BDQ | 1 | 48 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐1.00, 0.16] |
| 34.2.9 SOFAS | 1 | 9 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐2.73, 0.30] |
| 34.2.10 SFQ | 1 | 99 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.39, 0.39] |
| 34.2.11 SDS | 1 | 61 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.78, 0.23] |
| 34.2.12 CGI | 1 | 59 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.80, 0.22] |
| 34.2.13 HoNOS | 1 | 43 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.69, 0.55] |
Comparison 35. TSA sensitivity analyses: psychotherapy versus TAU.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 35.1 Primary: BPD symptom severity, at end of treatment | 22 | 1244 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.63, ‐0.31] |
| 35.2 Primary: self‐harm | 13 | 616 | Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.69, ‐0.15] |
| 35.3 Primary: suicide‐related outcomes | 13 | 676 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.47, ‐0.14] |
| 35.4 Primary: psychosocial functioning, at end of treatment | 22 | 1314 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.55, ‐0.17] |
| 35.5 Secondary: depression | 22 | 1568 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.54, ‐0.14] |
35.1. Analysis.

Comparison 35: TSA sensitivity analyses: psychotherapy versus TAU, Outcome 1: Primary: BPD symptom severity, at end of treatment
35.2. Analysis.

Comparison 35: TSA sensitivity analyses: psychotherapy versus TAU, Outcome 2: Primary: self‐harm
35.3. Analysis.

Comparison 35: TSA sensitivity analyses: psychotherapy versus TAU, Outcome 3: Primary: suicide‐related outcomes
35.4. Analysis.

Comparison 35: TSA sensitivity analyses: psychotherapy versus TAU, Outcome 4: Primary: psychosocial functioning, at end of treatment
35.5. Analysis.

Comparison 35: TSA sensitivity analyses: psychotherapy versus TAU, Outcome 5: Secondary: depression
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Amianto 2011.
| Study characteristics | ||
| Methods | 12‐month trial with 2 arms
Duration of trial: 10‐12 months intervention, 1 year follow‐up Country: Italy Setting: outpatient, the Mental Health Center of Chivasso, Turin |
|
| Participants |
Method of recruitment of participants: screened through clinical notes of outpatient mental health service. Patients who had been treated and clinically managed at least 1 year were eligible. Overall sample size: 35 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM‐IV‐TR) Means of assessment: Structured Clinical Interview for DSM (SCID) Mean age: 39.5 years (standard deviation = 9.4; range = 24‐57) Sex: not stated Comorbidity: of all the included participants, only 13 participants (37.1%) did not show a comorbid Axis I diagnosis. Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: supervised team management (STM) + sequential brief Adlerian psychodynamic psychotherapy (SB‐APP)
Number randomised to group: 18
Duration: 12 months Control/comparison group Comparison name: STM only Number randomised to group: 17 Duration: 12 months Both groups Concomitant psychotherapy: supervised team management (STM) includes unstructured psychological support focused on socio‐relational impairment as well as rehabilitative interventions and mental health services training. Concomitant pharmacotherapy: supervised team management (STM) includes medications that were administered according to American Psychiatric Association (APA) guidelines for borderline personality disorder. Three classes of drugs were used: antidepressants, mood stabilisers and atypical antipsychotics. Drug treatment was prescribed during the first or second visit and modified if necessary during follow‐up. Proportions of participants taking standing medication during trial observation period: No further information on pharmacotherapy |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: not stated
Ethics approval: yes Comments from review authors:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients, after providing their informed consent, were randomly assigned to SB‐APP in addition to Supervised Team Management (STM; number of participants = 18) or to STM alone (number of participants = 17) groups. The random allocation was generated using a random number table and was carried out by one author (BR), who was not involved in patients’ clinical management". (p 3). |
| Allocation concealment (selection bias) | Low risk | Quote: "[Randomisation] was carried out by one author (BR), who was not involved in patients’ clinical management. Patients were enrolled by a psychiatrist of the MHS who communicated the generalities of the participants who signed the informed consent to BR and received from BR the group assignment. The treatment allocation was assured by both psychiatrist and psychotherapist....Treatments of each branch were conducted simultaneously and both started the week after the enrollment in the study" (p 4). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: The raters were blind with regard to the group assignment of patients. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Intention‐to‐treat (ITT) analysis was used, dropout rates were low and balanced across groups. |
| Selective reporting (reporting bias) | Low risk | Comment: ITT, low dropout that was balanced in each group |
| Other bias | High risk |
Treatment adherence: Adherence to Sequential Brief Adlerian Psychodynamic Psychotherapy (SB‐APP) technique was monitored. However, no results of adherence scores were published. Allegiance bias: SB‐APP was developed by some of the authors. Attention bias: “The number of sessions performed by the two groups in the first year (T0‐T12) was planned to be comparable, reducing the bias about the number of sessions”. Vested interest: No funding was received by any author to perform the present research which was introduced within the ordinary activities of the Chivasso MHS therapeutic team. |
Andreoli 2016.
| Study characteristics | ||
| Methods | 3‐month duration trial with 3 arms
Duration of trial: 3 months Country: Switzerland Setting: community and hospital |
|
| Participants |
Method of recruitment of participants: consecutive patients entering the emergency room of the Geneva (Switzerland) Cantonal University Hospital were screened for deliberate self‐harm by specialised emergency room nurses. Overall sample size: 107 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, (DSM‐IV) Means of assessment: International Personality Disorder Examination (IPDE; Loranger 1995) Mean age: 31.9 years (standard deviation = 10.1) Sex: 84.1% female Comorbidity: major depressive disorder (MDD), substance abuse (10.6%), alcohol dependence (4.1%), alcohol abuse (21.8%) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group 1
Treatment name: abandonment psychotherapy (AP‐P)
Number randomised to group: 70
Duration: 3 months Experimental group 2 Treatment name: abandonment psychotherapy delivered by nurses (AP‐N) Number randomised to group: 70 Duration: 3 months Both experimental groups Concomitant psychotherapy: When therapists were not available, participants could call the 24‐hour emergency room hotline and receive emergency care from the psychiatric staff of the general hospital. Concomitant pharmacotherapy: Abandonment psychotherapy was applied in combination with an antidepressant medication protocol. Antidepressant medication was prescribed in a standard clinical management format by a psychiatrist who was blind to treatment choice. Most patients (n = 125, 89.3%) were prescribed venlafaxine, with an initial 0.5 mg/kg dosage and an optimal 2 to 3 mg/kg dosage. Repeated drug plasma level monitoring was performed at 2 weeks, 1 month, and 2 months to control for compliance. Venlafaxine was chosen because locally it was the medication most frequently prescribed among these patients. Additional mild neuroleptic medication (quetiapine 25 to 75 mg/day) was occasionally prescribed for brief periods, mostly limited to the first weeks of treatment. Not specified which exact proportions of participants received medication in each group Control/comparison group Comparison name: intensive community treatment‐as‐usual Number randomised to group: 30 Duration: 3 months Concomitant psychotherapy: Treatment‐as‐usual included as many nurse visits as required for two weeks and biweekly thereafter, weekly clinical review and medication adjustment from a psychiatrist, group therapy, social worker support, and as much day care, night hospitalisation and family intervention as needed to deal with suicidal relapse, emergency response. Concomitant pharmacotherapy: weekly clinical review and medication adjustment Proportions of participants taking standing medication during trial observation period: "The rate of subjects who were prescribed an antidepressant medication was lower in the TAU group compared to the AP groups (AP‐P: 68, 97.1%; AP‐N: 68, 97.1%, TAU: 23, 76.7%; Fisher’s exact test: p < .003), but the mean number of days spent in antidepressant treatment (AP‐P: 89.6, SD 32.9; AP‐N: 81.8, SD 36.7; TAU: 70.7, SD 55.9) and the number of participants who completed antidepressant treatment (AP‐P: 47, 67.1%; AP‐N: 48, 68.6%; TAU: 17, 56.7%) did not differ in the treatment cells. Among patients assigned to AP, the number of days spent in antidepressant medication and venlafaxine plasma levels did not differ as a function of type of therapist delivering AP" (Andreoli 2016, p. 280). “The analyses were repeated using […] presence of antidepressant medication, number of days spent in antidepressant medication, […] as covariates […]: the results were not materially altered.” (Andreoli 2016, p. 283) "...when we statistically controlled for the presence of additional antidepressant medication, the observed between‐group differences held.” (Andreoli 2016, p.285) |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: yes Ethics approval: yes Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Eligible participants were randomly allocated to treatment by a researcher not involved in the treatment procedures. This was done using a pre‐generated block randomisation scheme developed and held by a statistician, who prepared two series of sealed envelopes. |
| Allocation concealment (selection bias) | Low risk | Comment: Treatment allocation was masked to clinicians through sealed envelopes in charge of the treatments. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: Subjects were assessed at intake and at 3‐month follow‐up by well‐ trained psychologists with clinical experience who were blind to treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Intention‐to‐treat analysis was used and there were relatively low numbers of dropouts. However, the attrition rate was higher in TAU (16.7% who did not come to treatment and 20% who terminated their treatment early) compared to intervention (AP‐P: 5.7% and AP‐N: 2.9% who did not come to treatment and AP‐P: 5.7% and AP‐N: 2.9% who terminated their treatment early). Thus, attrition rates were not balanced between intervention groups and control group. |
| Selective reporting (reporting bias) | High risk | Comment: The authors provided no data on the Hamilton Depression Rating Scale 17 items (HDRS‐17: Hamilton, 1960), even though it was stated as an outcome. |
| Other bias | High risk |
Treatment adherence: No data were provided on the Hamilton Depression Rating Scale 17 items (HDRS‐17: Hamilton, 1960), even though it was stated as an outcome. Allegience bias: It was unclear who developed the manual for abandonment psychotherapy. It was however mentioned that the developers of the manual were involved in supervision p. 275. Attention bias: TAU patients seem to have received more attention given the inpatient treatment. With regards to medication, the intervention groups had received more antidepressants. Vested interest: Unclear who developed manual for abandonment psychotherapy, but there were no clear indications of vested interest. |
Antonsen 2017.
| Study characteristics | ||
| Methods | Approximately 18‐week trial with 2 arms
Duration of trial: 18 weeks Country: Norway Setting: inpatient and outpatient |
|
| Participants |
Method of recruitment of participants: Participants were referred to the respective departments as stated in the individual trials. Overall sample size: 52 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM‐IV) Means of assessment: Structured Clinical Interview for DSM, version 2 (SCID‐II) Mean age: 29 years (standard deviation = 6.7) Sex: 85% female Comorbidity: The distribution of personality disorder (PD) diagnoses was as follows: borderline personality disorder (46%); avoidant personality disorder (40‐41%); personality disorder not otherwise specified (21%); paranoid personality disorder (15%‐14%); obsessive compulsive personality disorder (9%); dependent personality disorder (7%); narcissistic personality disorder (2%); and schizoid personality disorder (1%). The patients had a mean of 3.4 symptom disorders: 74% with major depression; 37% with dysthymia; 8% with bipolar II disorder; 46% with panic disorder; 47% with social phobia; 12% with obsessive compulsive disorder; 48% with general anxiety disorder; 27% with substance misuse disorders; and 14% with eating disorder Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: combination program (CP) of short‐term day hospital psychotherapy (DHP) followed by outpatient combined individual and group psychotherapy
Number randomised to group: 27
Duration: 18 weeks Control/comparison group Comparison name: outpatient individual psychotherapy (OIP) Number randomised to group: 25 Duration: 18 weeks Both groups Concomitant psychotherapy: The outpatient treatment consisted of weekly group therapy (1.5 hours) for a maximum of 4 years, combined with weekly individual therapy for a maximum of 2.5 years. Concomitant pharmacotherapy: All patients received optional psychopharmacological consultations with a psychiatrist as part of the follow‐up evaluations. Proportions of participants taking standing medication during trial observation period: "Patients in the CP tended to use less psychotropic medications over time compared with the OIP, but the difference was not statistically significant (p = .09)." (Antonsen 2017; p. 57) |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: yes (in prior studies) Ethics approval: yes Comments from review authors:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients meeting the entry criteria [...] were consecutively randomly allocated to either day hospital psychotherapy (DHP) [i.e., CP] or outpatient individual psychotherapy (OIP)” (p 72). |
| Allocation concealment (selection bias) | Unclear risk | Comment: no clear details were provided by the authors. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no clear details were provided by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: attrition rates were 14% and 8% attrition rates in the groups. This difference was not statistically significant. ITT analysis was used. The reasons for missing data were similar between groups. |
| Selective reporting (reporting bias) | High risk | Comment: Self‐esteem, self‐destructive behavior, personality pathology, healthcare utilisation, affect consciousness, and reflective functioning included in trial registration and not in Arnevik 2009. |
| Other bias | High risk |
Treatment adherence: day hospital psychotherapy (DHP) “However, there was no formal training for the therapists, nor did the guidelines serve as a standard for treatment adherence.” (p 72) OIP: “The researchers gave no instructions to the OIP therapists regarding the duration and intensity of psychotherapy, nor did they interfere with any treatment decisions in the OIP conditions.” (p 72) Allegiance bias: no obvious risk of bias Attention bias: Similar total length. Frequency of therapy from once a month to three times a week in OIP. Frequency of therapy in groups were different (not reported in Arnevik, 2009). There was an obvious difference in the amount of therapy received. CP patients received 18 weeks’ intensive day hospital treatment followed by conjoint treatment, while in OIP, most patients attended therapy once a week. The mean number of participants who received therapy sessions at 18 months in OIP was 40. (Arnevik 2010, p 199) |
Bateman 1999.
| Study characteristics | ||
| Methods | Randomised controlled trial with 2 arms for 18 months
Duration of trial: up to 18 months Country: UK Setting: partially hospitalised/outpatient |
|
| Participants |
Method of recruitment: Patients referred Overall sample size: 38 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 3rd revision (DSM‐III‐R) Means of assessment: both Structured Clinical Interview for DSM‐IV personality disorders (SCID) and Revised Diagnostic Interview for Borderlines (DIB‐R) Mean age: 31.8 years Sex: 57.9% female Comorbidity: In terms of axis I diagnosis, 70% and 62% had major depression in the intervention and control group, respectively. Inclusion criteria:
Exclusion criteria
|
|
| Interventions |
Experimental group Treatment name: mentalisation‐based treatment (MBT) Number randomised to group: 19 Duration: up to 18 months Control/comparison group Comparison name: standard treatment in the general psychiatric services Number randomised to group: 19 Duration: up to 18 months Both groups Concomitant psychotherapy: none Concomitant pharmacotherapy: antidepressant and antipsychotic drugs prescribed, as appropriate, polypharmacy was discouraged Proportions of participants taking standing medication during trial observation period: "The initial types and doses of medication were the same for both groups." (Bateman 1999, p. 1565) Exact medication use during treatment unclear: higher medication costs in control group (z‐3.9, P < 0.001) (Bateman 2003, Tab. 1, p. 170) indicates more frequent use of medications in the control group. |
|
| Outcomes |
Primary
Both outcomes assessed with the Suicide and Self‐Harm Inventory, a semi‐structured interview. Secondary
|
|
| Notes |
Sample size calculation: not stated Ethics approval: not stated Comments from review authors:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comments: Did not use a minimisation method. This method is used to minimise the imbalance between the number of patients in each treatment group. This method maintains a better balance than traditional blocked randomisation, and its advantage increases with the number of stratification factors. It is reported that random assignment was used but it is unclear how the random assignment was performed. |
| Allocation concealment (selection bias) | Low risk | Comment: Allocation was completed centrally at the university. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: Outcome assessors were blind to intervention group. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Analyses were based on completers only. |
| Selective reporting (reporting bias) | Unclear risk | Comment: There was no clear indication of selective reporting, but there was insufficient information to permit judgement of 'high' or 'low'. |
| Other bias | High risk |
Attention bias: more attention paid to experimental group participants Allegiance bias: there was no indication given for an allegiance effect. However, as both authors are the founders of MBT, the treatment actually used in the experimental group, an allegiance effect seems not improbable. Adherence bias: "All sessions were audiotaped. Adherence to the treatment manuals was determined by randomly selected audiotapes of individual and group sessions drawn from two distinct 6‐months periods of each case using a modified version of the recommended adherence rating scale." (Bateman 2009, online data supplement, p 1) |
Bateman 2009.
| Study characteristics | ||
| Methods | Randomised controlled trial with 2 arms for 18 months
Duration of trial: 18 months Country: UK Setting: outpatient |
|
| Participants |
Methods of recruitment of patients: consecutive referrals Overall sample size: 168 Diagnosis of Borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM‐IV) Means of assessment: Structured and Clinical Interview for DSM axis II disorders Mean age: 31.3 years (standard deviation = 7.6) Sex: 79.9% females Comorbidity: comorbid antisocial personality disorder Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group Treatment name: mentalisation‐based therapy Number randomised to group: 71 Duration: 18 months Control/comparison group Comparison name: structured clinical management Number randomised to group: 63 Duration: 18 months Both groups Concomitant psychotherapy: patients already in long‐term psychotherapeutic treatment were not eligible. Concomitant pharmacotherapy: patients were prescribed medication according to the American Psychological Association (APA) guidelines; all patients were offered medication reviews every 3 months. Proportions of participants taking standing medication during trial observation period: MBT group: 29.6%, control group 57.1%; group effect over time: IRR 0.77, P < 0.001 (less medication use in MBT group) |
|
| Outcomes |
Primary:
Secondary:
|
|
| Notes |
Sample size calculation: yes Ethic approval: The study was approved by Barnet Enfield and Haringey Local Research and Ethics Committee and conducted at the Halliwick Personality Disorder service and in a community outpatient facility. Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization using a stochastic minimization program (MINIM) balancing for age (blocked as 18‐25, 26‐30, > 30 years), gender, and presence of antisocial personality disorder." (Bateman 2009, p 1357) |
| Allocation concealment (selection bias) | Low risk | Quote: "Treatment allocation was made offsite [...] A study psychiatrist informed patients of their assignment." (Bateman 2009, p. 1357) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Assessors were blind to treatment group." (Bateman 2009, p 1358) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: ITT analyses were used by the authors. |
| Selective reporting (reporting bias) | Low risk | Comment: A study protocol is available (ISRCTN27660668); there was no indication of selective reporting. |
| Other bias | High risk |
Adherence bias: "All sessions were audiotaped. Adherence to the MBT‐OP and SCM‐OP manuals was determined by randomly selected audiotapes of individual and group sessions drawn from two distinct 6‐months periods of each case using a modified version of the recommended adherence rating scale." (Bateman 2009, online data supplement, p 1) Allegiance bias: There was no indication given for an allegiance effect. However, as both authors are the founders of MBT, the treatment actually used in the experimental group, an allegiance effect seems not improbable. Attention bias: Equal amounts of attention paid to both groups |
Bellino 2006.
| Study characteristics | ||
| Methods | Randomised controlled trial with 2 treatment arms
Duration of trial: 24 weeks Country: Italy Setting: outpatient |
|
| Participants |
Methods of recruitment of participants: not stated Overall sample size: 39 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text‐Revision (DSM‐IV‐TR) Means of assessment: Structured Clinical Interview for Personality Disorders Mean age: 26.4 years (standard deviation = 3.7) Sex: 60% female Comorbidity: comorbid diagnosis of mild to moderate major depressive episode required for inclusion Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group Treatment name: fluoxetine + interpersonal therapy (IPT) Number randomised to group: 19 Duration: 24 weeks (1 weekly session) Control/comparison group Comparison name: fluoxetine + clinical management (CM) Number randomised to group: 20 Duration: 24 weeks (CM; 6 appointments, first two fortnightly, monthly afterwards) Both groups Concomitant psychotherapy: patients who received psychotherapy during the 2 months prior to the study were not eligible. Concomitant pharmacotherapy: all study participants received 20 to 40 mg fluoxetine daily; patients with psychotropic treatment during the 2 months prior to the study were not eligible for inclusion. Proportions of participants taking standing medication during trial observation period: 100% of each group were taking fluoxetine (see above) |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: not stated Ethics approval: not stated Comments from review authors:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: The authors used a computer random number generator (Bellino 2006) [pers comm]. |
| Allocation concealment (selection bias) | Low risk | Comment: Allocation was by central allocation (Bellino 2010c [pers comm]. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "assessments were performed by an investigator who was blind to the treatment methods" (Bellino 2006, p. 455). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: the authors conducted a completer analysis. Quote: "Owing to noncompliance, 7 patients discontinued treatment during the first 3 weeks,. Of these individuals, 4 were in the medication‐only group, and 3 were in the combined therapy group. We performed analyses on the 32 patients [...] who completed the 24 weeks of treatment." (Bellino 2006, p. 455) |
| Selective reporting (reporting bias) | Unclear risk | Comment: No clear indication for selective reporting, but Insufficient information to permit a judgement of 'high' or 'low' |
| Other bias | High risk |
Adherence bias: "[...] psychotherapist [....] had 5 years of experience practising IPT" (Bellino 2006, p. 455). No specific measures to monitor treatment adherence (Bellino 2010a [pers comm]) Allegiance bias: The authors seemed not to be associated with IPT. Attention bias: More attention paid to EG participants |
Bellino 2007.
| Study characteristics | ||
| Methods | Randomised controlled trial with 2 treatment arms
Duration of trial: 24 weeks Country: Italy Setting: outpatient |
|
| Participants |
Methods of recruitment of participants: not stated Sample size: 32 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM‐IV) Means of assessment: Structured Clinical Interview for Personality Disorders (SCID) Mean age: 30.55 years (standard deviation = 5.75) Sex: 63.2% females Comorbidity: comorbid diagnosis of mild to moderate major depressive episode required for inclusion Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group Treatment name: fluoxetine + interpersonal therapy (IPT) Number randomised to group: 16 Duration: 24 weeks (1 weekly session) Control/comparison group Comparison name: fluoxetine + cognitive therapy Number randomised to group: 16 Duration: 24 weeks (1 weekly session) Both groups Concomitant psychotherapy: patients who received psychotherapy during the 2 months prior to the study were not eligible. Concomitant pharmacotherapy: all study participants received 20 to 40 mg fluoxetine daily, with 7 appointments, the first 2 fortnightly and the last 5 monthly; patients with additional current psychotropic treatment were not eligible for inclusion. Proportions of participants taking standing medication during trial observation period: 100% of each group were taking fluoxetine (see above). |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: not stated Ethics approval: had ethics board approval and followed Declaration of Helsinki guidelines Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients [...] were randomized using the web program Research Randomizer v3.0 (Urbaniak & Plous, Social Psychology Network, 2007)" (Bellino 2007, p. 720). |
| Allocation concealment (selection bias) | Low risk | Comment: Allocation was conducted centrally (Bellino 2010 [pers comm]). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: "A psychiatrist provided pharmacotherapy. He was blind to which type of psychotherapy the patients were receiving [...] The assessments were performed by an investigator who was blind to the treatment methods." (Bellino 2007, p. 720) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: the authors conducted a completer analysis. Quote: "Initially, there were 32 patients enrolled in the study. [...] Owing to noncompliance, 6 patients discontinued treatment during the first 3 weeks. Of these subjects, 2 were in the IPT group, and 4 were in the CT group. We performed statistical analyses of outcome measures on the 26 patients [...] who completed the 24 weeks of treatment." (Bellino 2007, p. 720) |
| Selective reporting (reporting bias) | Unclear risk | Comment: no indication for selective reporting, but Insufficient information to permit judgement of 'Yes' or 'No' |
| Other bias | Low risk |
Adherence bias:"Both psychotherapists received supervision during the treatment to assess their adherence to the psychotherapy manuals." (Bellino 2007, p. 720) Allegiance bias: The authors seemed neither to be associated to IPT nor CT. Attention bias: Equal amounts of attention paid to both groups |
Bellino 2010.
| Study characteristics | ||
| Methods | Parallel‐arm, randomised controlled trial with 2 treatment arms
Duration of trial: 32 weeks Country: Italy Setting: outpatient |
|
| Participants |
Methods of recruitment of participants: participants recruited from patients attending the Service for Personality Disorders of the Unit of Psychiatry 1, Department of Neurosciences, University of Turin, Italy Sample size: 55 Diagnosis of borderline personality disorder diagnosis: Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM‐IV) Means of assessment: Structured Clinical Interview for Personality Disorders (SCID) Mean age: combined therapy + fluoxetine = 26.23 years (standard deviation = 6.4), fluoxetine = 25.86 years (standard deviation = 7.2) Sex: 67.3% female Comorbidity: participants had no comorbid axis‐I or II comorbidities Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group Treatment name: combined therapy of fluoxetine (20 to 40 mg/d) plus weekly individual Interpersonal therapy adapted to borderline personality disorder (IPT‐BPD) Number randomised to group: 27 Duration: 32 weeks Control/comparison group Comparison name: single pharmacotherapy treatment with fluoxetine plus TAU (treatment approach of patients attending the Service for Personality Disorders. A visit lasting 15 to 20 minutes was provided every 2 weeks, dealing with clinical issues) Number randomised to group: 28 Duration: 32 weeks ((20 to 40 mg/d) + clinical management (medical appointments lasting 15 to 20 minutes every 2 weeks, dealing with clinical issues) Both groups Concomitant psychotherapy: eligible patients were not in psychotherapeutic treatment during the last 6 months prior to study entry. Concomitant pharmacotherapy: eligible patients were not receiving psychotropic drugs during the last two months prior to study entry. Proportions of participants taking standing medication during trial observation period: 100% of each group were taking fluoxetine (see above). |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: not stated Ethics approval: followed the Declaration of Helsinki guidelines and received approval from ethics board Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using the web program Research Randomizer version 3.0 (Urbaniak and Plous, [...])." (Bellino 2010, p. 75) |
| Allocation concealment (selection bias) | Unclear risk | Comment: no further details |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Assessments were performed by an investigator who was blind to the treatment methods." (Bellino 2010, p. 76) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: the authors conducted a completer analysis (IPT‐BPD + fluoxetine group: 5 participants lost, CM + fluoxetine group: 6 participants lost). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no clear indication of selective reporting, but Insufficient information to permit judgement of 'high' or 'low' |
| Other bias | High risk |
Performance bias Quote: "Patients in the IPT‐BPD group were treated by a psychotherapist [...] who had at least 5 years of experience practising IPT" (Bellino 2010, p 76). No further information, adherence seems not to have been monitored. Allegiance bias Comment: The working group seems to be experienced in but not associated with IPT (cf. Bellino 2006; Bellino 2007). Attention bias: More attention paid to EG participants |
Bianchini 2019.
| Study characteristics | ||
| Methods | 12‐month trial with 2 arms:
Duration of trial: 12 months Country: Italy Setting: hospital (forensic) |
|
| Participants |
Method of recruitment of participants: participants recruited from men consecutively detained as patients in three high intensity therapeutic facilities Sample size: 21 Diagnosis of borderline personality disorder: measured by the Personality Assessment Inventory (Morey 2007) Means of assessment: diagnosis confirmed in a clinical interview by a psychiatrist Mean age: 41.79 years (standard deviation = 8.14) Sex: 100% males Comorbidity: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group Treatment name: DBT + treatment‐as‐usual Number randomised to group: 10 Duration: 12 months (once‐weekly individual therapy (60 minutes), once‐weekly group sessions (120 minutes)) Concomitant psychotherapy: treatment‐as‐usual included social skills, and cognitive remediation Concomitant pharmacotherapy: treatment‐as‐usual included pharmacotherapy Control/comparison group Comparison name: treatment‐as‐usual (pharmacotherapy, social skills, cognitive remediation) Number randomised to group: 11 Duration: 12 months Concomitant psychotherapy: not stated Concomitant pharmacotherapy: not stated |
|
| Outcomes |
Secondary
|
|
| Notes |
Sample size calculation: no Ethics approval: The study was approved by the Local Ethic Committee. Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: “Each pair was randomised into either a group receiving 12 months of DBT along with other therapies available in the high security hospital (pharmacotherapy, social skills, and cognitive remediation) or a group receiving the other usual therapies alone” (pg. 124). No further details provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: the authors did not specify the process of allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: outcomes were self‐reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk |
Comment: Dropouts were not explicitly specified. However, the authors stated that “All participants completed at least 90% of the DBT sessions offered.” (p.127) Also “Once patients have been admitted […], they are required to complete any treatment programme offered; if a person does not, s/he may be referred back to the magistrate, who must consider if the individual is in breach of his/her order.” (p. 123) No details about the proportion of TAU completers, but, since all participants were convicted inpatients of a secure hospital, “completion” of TAU treatment was very likely. “All participants were reassessed after completion of the DBT programme or, for the control group, after the same time had elapsed”. (p.125) |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. |
| Other bias | High risk |
Adherence bias: therapeutic adherence not assessed Attention bias: DBT (once weekly individual plus group therapy) offered additionally to TAU, hence more attention spent to DBT group Allegiance bias: there was no indication of allegiance bias. |
Blum 2008.
| Study characteristics | ||
| Methods | Randomised controlled trial with 2 treatment arms
Duration of trial: 20 weeks Country: USA Setting: outpatient |
|
| Participants |
Methods of recruitment: participants recruited from the University of Iowa inpatient and outpatient psychiatric service Sample size: 124 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM‐IV) Means of assessment: Structured Interview for DSMIV Personality (SIDP‐IV) Mean age: 31.5 years (standard deviation = 9.5) Sex: 83.1% female Comorbidity: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group Treatment name: systems training for emotional predictability and problem solving (STEPPS) Number randomised to group: 93 Duration: 20 weeks (20 × 2‐hour weekly group therapy sessions + homework assignments + 1 session for family members or significant others; no individual therapy) Control/comparison group Comparison name: treatment‐as‐usual (TAU) Number randomised to group: 72 Duration: 20 weeks Both groups Concomitant psychotherapy: participants were encouraged to continue with ongoing concomitant treatments. 59% of all participants had an additional individual therapy. Concomitant pharmacotherapy: 90% of participants reported at least one psychotropic medication at baseline; on average. Proportions of participants taking standing psychotropic medication during trial observation period: Exact numbers unclear "Psychotropic usage significantly decreased during the 20‐week treatment period for both groups (from 2.9 to 1.3 medications per subject), but there was no group difference in level of change (Mann‐.2 Whitney test: = 0.1, df = 1, p = 0.782). Thus, medication usage did not confound study results.” (Blum 2008; p. 474) |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: not stated Ethics approval: not stated Comments from review authors:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were assigned by coin toss". (Blum 2008, p. 469) |
| Allocation concealment (selection bias) | Unclear risk | Comment: No indication of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "While we intended to conduct blind assessments, we found it nearly impossible to maintain blindness. The convergence of both rater‐ and patient‐administered scales suggests that this may not have been an important deficiency." (Blum 2008, p. 477) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk |
Comment: analysis was conducted on those participants that actually started to receive the allocated intervention, regardless of completion or noncompletion. However, 40 participants that had been randomly allocated did not receive the allocated intervention and were not included in analyses. Quote: "Subjects with at least one post‐baseline assessment were included in the analyses." (Blum 2008, p. 470) |
| Selective reporting (reporting bias) | High risk | Comment: a study protocol was available, but there was no information about primary or secondary outcomes. The authors reported a broad range of outcomes, so there was no indication for selective reporting given. However, there was insufficient information to permit judgment of 'Yes' or 'No'. |
| Other bias | High risk |
Adherence bias: "Adherence to the manual was rated on a 5‐point scale [...] A score of 4 (good) or higher was considered acceptable. Two Ph.D.‐level psychologists who were not involved with the randomized controlled trial but familiar with STEPPS rated 43 randomly selected video‐taped sessions. The mean adherence score was 4.4 (SD = 0.8)." (Blum 2008, p. 470) Allegiance bias: some authors are founders of STEPPS, the treatment actually used in the experimental group, therefore an allegiance effect seemed possible. Attention bias: more attention was paid to STEPPS group participants. |
Bohus 2013.
| Study characteristics | ||
| Methods | Randomised controlled trial with 2 treatment arms
Duration of trial: 3 months of inpatient treatment (i.e. 13 weekly sessions in each condition) + 1 booster session 6 weeks after dismissal Country: Germany Setting: inpatient |
|
| Participants |
Methods of recruitment of patients: referred by local psychiatrist Sample size: 74 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM‐IV) Means of assessment: International Personality Disorder Examination (IPDE) Mean age: 32.9 years (range = 19‐52 years) Sex: 100% female Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group Treatment name: dialectical behavioral therapy for patients with post‐traumatic stress disorder after childhood sexual abuse (DBT‐PTSD); depressive episodes were treated with selective serotonin reuptake inhibitor antidepressive agents (100‐150 mg/d of sertraline); difficulties of sleeping were treated with sleep‐inducing antidepressants (50‐100 mg/d of trimipramine); no benzodiazepines, no neuroleptics Number randomised to group: 36 Duration: 3 months, 13 psychotherapy sessions of 120 minutes each Control/comparison group Comparison name: Waiting list Number randomised to group: 38 Duration: 3 months (continuation of already ongoing treatments for 6 months, inpatient DBT‐PTSD treatment afterwards; points of measurement: baseline, 3 months, 4.5 months and 6 months after study inclusion) Both groups Concomitant psychotherapy: participants in the experimental group did not receive any other individual or group psychotherapy; participants in the waiting‐list condition continued their usual treatments (if any) Concomitant pharmacotherapy: psychiatrists in both treatment arms were free to follow their clinical experience Proportions of participants taking standing psychotropic medication during trial observation period: 82.9% of DBT‐PTSD group, 87.1% of control group (P = 0.74) |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: yes Ethics approval: yes Comments from review authors: Originally, female participants with a diagnosis of post‐traumatic stress disorder (PTSD) and at least 4 criteria of DSM‐IV‐BPD were eligible. We refer to the subsample data of those participants fulfilling 5 or more criteria. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was carried out using the procedure proposed by Efron". (p. 224) (the bootstrapping method) |
| Allocation concealment (selection bias) | Low risk | Quote: "Care was taken that the randomization was concealed to both the patient and to all persons involved in the study until the written informed consent has been given by the patient." (p. 224) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Interviewers were blinded. [...] the diagnostician who was assessing the patient at follow‐up was masked to the assignment." (p. 224) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: No further details provided about attrition rates |
| Selective reporting (reporting bias) | Low risk | Comment: a study protocol was available (NCT00481000 ), no indication for selective reporting |
| Other bias | High risk | Attention bias: more attention paid to active group |
Borschmann 2013.
| Study characteristics | ||
| Methods | 6‐month trial with 2 arms
Duration of trial: 6 months Duration of participation: 6 months Country: UK Setting: outpatient |
|
| Participants |
Method of recruitment of participants: patients under ongoing care of community mental health teams
Sample size: 88 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM‐IV‐TR) Means of assessment: Structured Clinical Interview for Personality Disorders (SCID‐II) Mean age: 35.8 years (standard deviation = 11.6) Sex: 19.3% male Comorbidity: alcohol use disorders (according to AUDIT test score), depression (according to Hamilton Anxiety and Depression Scale ‐ depression subscale score) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: joint crisis plan + treatment‐as‐usual (JCP)
Number randomised to group: 46
Duration: 6 months Control/comparison group Comparison name: treatment‐as‐usual (TAU) Number randomised to group: 42 Duration: 6 months Both groups Concomitant psychotherapy: as provided usually by community mental health team Concomitant pharmacotherapy: yes, as part of standard care, if needed Proportions of participants taking standing psychotropic medication during trial observation period: unclear |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: yes, but with remarks that this was the pilot study
Ethics approval: approved by the South London Research Ethics Committee (reference number: 09/H0803/113) Comments from review authors:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomisation was conducted at the level of the individual and was stratified by alcohol use […] and depression […]. Randomisation was managed electronically by the Clinical Trials Unit at the Kings’s College London Institute of Psychiatry, UK.” (p. 358) |
| Allocation concealment (selection bias) | Low risk | Quote: “Randomisation was managed electronically by the Clinical Trials Unit at the Kings’s College London Institute of Psychiatry, UK.” (p 358) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “Follow‐up data were collected by a research worker who was masked to treatment allocation and all data analyses were conducted by a statistician who was also masked to treatment allocation. The extent to which masking was achieved in the collection of outcome data was assessed at the end of the trial". (p. 358) “…all data analyses were conducted masked to treatment allocation and follow‐up data were collected by a researcher masked to treatment allocation and this masking was maintained in 62 of 73 cases (85%).” (p. 362) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “All analyses were based on the Intention‐to‐treat sample using a statistical analysis plan finalised by the trial statistician (J.M.H.) and approved by the principal investigator (P.M.) in advance of conducting any analyses.“ (p. 359) Dropout rates were similar across both groups. |
| Selective reporting (reporting bias) | Low risk | Comment: Additional use of outcomes not specified in the protocol (esp. HADS), but no indication of bias |
| Other bias | High risk |
Treatment adherence: “Progress of the trial, [and] adherence to protocol […] were overseen by a trial steering committee.” (Borschmann 2013, p 358) “Adherence to the protocol was high, as a total of 41 out of 46 participants in the JCP + TAU group (89.1%) attended their JCP planning meeting and consequently received the active intervention. […] Data gathered at follow‐up indicated that JCPs were used both during and between crises and were viewed favourably by the majority of participants. More than 90 percent of participants were still in possession of their JCP at follow‐up (two participants stated that they had lost their plans) and approximately three‐quarters stated that they had used their JCP during a crisis". (Borschmann 2014, p 170) “All JCP meeting were facilitated by the same person (i.e. me), so fidelity between facilitators was not an issue. We did not record any meetings though – adherence was only measured by the same checklist being completed (by me) during each meeting to ensure uniformity between participants.” (Borschmann 2013 [pers comm] Allegiance bias: Though developers/advocates of JCP were involved (especially Kim Sutherby, George Szmukler), there was no indication of bias, as no significant effects were found. Attention bias: Both groups received TAU, but the JCP group received an additional meeting with carers and healthcare professionals to elaborate their joint crisis plan. Vested interest: no indication of bias |
Bos 2010.
| Study characteristics | ||
| Methods | Multicentre, parallel‐arm trial with 2 treatment arms
Duration of trial: 4.5 months Country: The Netherlands Setting: outpatient |
|
| Participants |
Methods of recruitment of participants: patients recruited from nonacademic outpatient clinics of 2 mental health care institutes in The Netherlands (Lentis, Groningen; Dimence, Deventer) Sample size: 79 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: Structured Clinical Interview for DSM‐IV Axis II Disorders (SCID‐II), Personality Disorders Questionnaire (PDQ‐IV) Mean age: 32.4 years Sex: 86.1% female Comorbidity: not stated Inclusion criteria: none reported Exclusion criteria
|
|
| Interventions |
Experimental group Treatment name: systems training for emotional predictability and problem solving (STEPPS) program + limited individual therapy (STEPPS + LIT; STEPPS) Randomised to group: 42 Duration: 4.5 months (18 weekly sessions) Control/comparison group Comparison name: treatment‐as‐usual (TAU) Randomised to group: 37 Duration: 4.5 months (offered every 1 to 4 weeks) Both groups Concomitant psychotherapy: STEPPS‐related treatments like dialectical behavior therapy (DBT) or family groups or family members of the patients were not allowed; all participants were allowed to have contacts with social worker or another healthcare professional. Concomitant pharmacotherapy: All participants were allowed to have (medication) contacts with a psychiatrist. Proportions of participants taking standing psychotropic medication during trial observation period: Exact proportions unclear. “We further investigated whether the results were confounded by the use of psychotropic medication. Medication use did not differ between the two conditions. Adding medication use at the different time points (yes/no) to the models did not weaken the results; on the contrary, estimated differences became larger and p values smaller. Thus, the results were not likely confounded by medication use.“ (Bos 2010, p. 178) |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: yes Ethics approval: The study protocol was approved by the Medical Ethical Committee for Dutch Mental Health Institutes. Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Allocation was determined by drawing of lots (equal numbers for both groups at each study site) some weeks before start of the STEPPS group after inclusion of all participants. |
| Allocation concealment (selection bias) | Low risk | Comment: Randomisation was carried out by a research assistant. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Quote: "Interviews were conducted by research assistants who were not blind to treatment group assignment." ( p. 300) Comment: Non‐blindness of interviewers may have affected interviewer‐assessed outcomes, i.e. BPDSI‐IV impulsivity and parasuicide scores. All other outcomes were self‐rated by participants. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "All available data of patients who received the intervention according to protocol were used in the analyses. Intention‐to‐treat analyses, in which also patients are included who did not receive the intervention as intended, were performed as well. The per protocol and intention‐to‐treat analyses yielded similar results. We present only the per‐protocol analyses, as we are primarily interested in “method effectiveness” (as opposed to “use effectiveness"). (p. 300) |
| Selective reporting (reporting bias) | Unclear risk | Comment: No indication for selective reporting, but Insufficient information to permit judgement of 'Yes' or 'No' |
| Other bias | High risk |
Adherence bias: "STEPPS therapists met twice a year under the supervision of expert trainers to evaluate the procedure and to preserve uniformity. Individual therapists in the STEPPS condition received a 1‐day training and monthly phone supervision. After each session, individual therapists in both conditions completed a self‐report questionnaire by which the content and frequency of the therapy contacts could be checked." (p. 300) Allegiance bias: "[...] this RCT on STEPPS is the first done by others than its developers." (p. 303) Attention bias: more attention was paid to STEPPS participants. |
Carmona í Farrés 2019.
| Study characteristics | ||
| Methods | Randomised controlled with 2 arms
Duration of trial: 10 weeks Country: Spain Setting: outpatient |
|
| Participants |
Method of recruitment of participants: Patients for this single‐centre randomised trial were recruited from the outpatient facility of the Hospital de la Santa Creu i Sant Pau (Barcelona, Spain). Overall sample size: 70 Diagnosis of borderline personality disorder: Structured clinical interview for DSM IV axis II personality disorders (SCID II) Means of assessment: Diagnostic interview for borderline (DIB‐R) Mean age: 31.9 years Sex: 90% female Comorbidity: no comorbidity Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group Treatment name: dialectical behavioral therapy (DBT) ‐ mindfulness Number randomised to group: 35 Duration: 10 weeks Control/comparison group Comparison name: dialectical behavioral therapy (DBT) ‐ interpersonal effectiveness Number randomised to group: 35 Duration: 10 weeks Both groups Concomitant psychotherapy: not allowed to receive any other type of psychotherapy Concomitant pharmacotherapy: Patients were allowed to continue taking any medications prescribed prior to study inclusion, provided that no modifications of the medication type or dose were made during the intervention period. Proportions of participants taking standing psychotropic medication during trial observation period: unclear |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: no Ethics approval: yes Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An independent statistician randomized the participants using a computer‐generated sequence (blocks of four participants without stratification)." (Carmona í Farrés 2019 [pers comm]) |
| Allocation concealment (selection bias) | Low risk | Quote: "An independent statistician randomized the participants using a computer‐generated sequence (blocks of four participants without stratification), allocation was concealed". (Carmona í Farrés 2019 [pers comm]) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the evaluators of the patients were blinded to the participants' treatment arm throughout the study." (Carmona í Farrés 2019 [pers comm]) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Analyses were conducted on the per‐protocol sample, comprising participants who completed at least 80% of the intervention and for whom all data points (pre‐ and post‐intervention) were available." (Carmona í Farrés 2019, p. 5) "Analyses of primary outcomes [i.e., DERS, BIS‐11] were also conducted in the intention‐to‐treat (ITT) sample, including all enrolled participants, regardless of whether they completed the intervention or not. Missing data were treated with the last observation carried forward method (Little and Rubin 1987). Further analyses [i.e., BSL‐23] were run only for subjects considered completers." Reported data (means, SDs) which were used for effect size calculation in this review were based on completers. "Of the 70 participants who participated in the study, a total of 18 dropped out: 13 in the DBT‐M group (37.14%) and 7 (20%) from the DBT‐IE group. There were no differences between completers and non‐completers in baseline demographic characteristics." (Carmona í Farrés 2019, p. 6) |
| Selective reporting (reporting bias) | Low risk | Comment: Reported outcomes matched study protocol. |
| Other bias | Low risk |
Allegiance bias: No obvious allegiance bias Adherence bias: "In relation to treatment adherence, the group sessions were witnessed by video camera, after the session a feedback to the therapists were provided, but there is no available to Spanish any validated DBT adherence tool measurement." (Soler 2019a [pers comm]). Adequate measures taken to ensure treatment adherence. Attention bias: Equal amounts of attention spent on both groups. Both the DBT‐M and DBT‐IE interventions were delivered in a group format consisting of 9–12 participants. The treatment sessions were 2.5 h in length and held once weekly over a 10‐week period. |
Carter 2010.
| Study characteristics | ||
| Methods | Parallel‐arm, randomised controlled trial with 2 treatment arms
Duration of trial: 6 months (all participants were offered 12 months of DBT, but the comparison between groups was restricted to the first 6 months of DBT vs TAU + WL). Country: Australia Setting: outpatient |
|
| Participants |
Methods of recruitment of participants: Participants were referred from treating general practitioners, treating psychiatrists or public mental health services (any units of Hunter Mental Health Services). Sample size: 73 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM‐IV) Means of assessment: clinical interview, ICD‐10 International Personality Disorder Examination (IPDE) Mean age: 42.5 years (standard deviation = 6.1) Sex: 100% female Comorbidity: participants showed substantial psychopathology with high rates of BPD criteria, International Personality Disorder Examination (IPDE) scores and Axis 1 comorbidity. Inclusion criteria: not stated Exclusion criteria
|
|
| Interventions |
Experimental group Treatment name: DBT Number randomised to group: 38 Duration: 6 months (weekly individual therapy, weekly group‐based skills training, telephone access to an individual therapist, therapist supervision) Control/comparison group Comparison name: TAU + waiting list Number randomised to group: 35 Duration: 6 months (participants were offered DBT treatment after a 6‐month waiting period) Both groups Concomitant psychotherapy: participants were asked to discontinue psychological therapy of any sort for at least the 12‐month duration of dialectical behavior therapy (DBT) Concomitant pharmacotherapy: not specified Proportions of participants taking standing psychotropic medication during trial observation period: unclear |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: yes Ethics approval: not stated Comments from review authors:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: the authors used a computerised random number generator to generate allocations ‐ placed into sealed opaque envelopes (in blocks of 8). Envelope drawn after baseline assessments complete". (Carter 2010 [pers comm]) |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was carried out by the research staff. [...] participants were allocated by selecton of sealed opaque envelopes." (p. 164) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Outcomes were determined [...] by assessors blinded to allocation. [...] All reasonable attempts were made to maintain blindness to allocation status for these raters, but this could not achieve perfect blindness." (pp. 164) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: the authors conducted per protocol analyses (DBT group: 20 completers of treatment and self‐reports out of 38 allocated to this group; TAU group: 31 completers of waiting list and self‐reports out of 35 allocated) |
| Selective reporting (reporting bias) | Unclear risk | Comment: there was no indication for selective reporting, but Insufficient information to permit judgement of 'high' or 'low'. |
| Other bias | High risk |
Performance bias: "The intervention condition was based on the comprehensive DBT model, a team‐based approach including [...] therapist supervision groups." (p. 163). "[...] possible inferiority of training of DBT therapists to that of those in other studies or inferior adherence to the DBT methods despite adequate training" (p. 170). There was no mention of any objective means of assessment. Allegiance bias: no indication of allegiance bias Attention bias: more attention paid to DBT group participants |
Cottraux 2009.
| Study characteristics | ||
| Methods | Randomised controlled trial with 2 treatment arms
Duration of trial: 1 year Country: France Setting: outpatient |
|
| Participants |
Methods of recruitment of participants: borderline personality disorder outpatients recruited and treated at 2 university hospital centres: Lyon (Anxiety Disorder Unit) and Marseille (Behaviour Therapy Unit) Sample size: 65 Diagnosis of borderline personality disorder diagnosis: Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM‐IV) Means of assessment: structured interview screening form, Revised Diagnostic Interview for Borderlines (DIB‐R) Mean age: CT = 34.3 years (standard deviation = 10.2), RST = 32.6 years (standard deviation = 8.3) Sex: 76.9% female Comorbidity: "The MINI investigation found a high DSM‐IV axis‐1 comorbidity throughout the entire sample: 40% of the patients had a social phobia, 26% a panic disorder, 15% agoraphobia, 18% a current PTSD, 15% presented hypomania, 38% bulimia, 23% a somatisation disorder, 26% excessive alcohol consumption, 32% took street drugs irregularly, 53% presented a generalised anxiety disorder, 55% a current major depressive disorder and 46% were at risk of suicide. Frequencies of the diagnoses were comparable in the 2 groups [...]. There was no between‐group difference in psychometric assessment for the 62 patients evaluated before therapy ( table 2 )." (Cottraux 2009, p. 311) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group Treatment name: cognitive therapy Number randomised to group: 33 Duration: 6 months (1‐hour sessions 24 sessions) Control/comparison group Comparison name: Rogerian supportive therapy Number randomised to group: 32 Duration: 6 months (1‐hour sessions 24 sessions) Both groups Concomitant psychotherapy: eligible patients were not to be following psychotherapy at the time of the study Concomitant pharmacotherapy: participants could keep their medication as long as they accepted to have it monitored by the principal investigator Proportions of participants taking standing psychotropic medication during trial observation period: unclear; current psychotropic medication at baseline: CT N = 24 (72.7%), RT N = 26 (81.3%); P = 0.55 |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: not stated Ethics approval: The ethics committee, CCPPRB Lyon B, approved the protocol for the entire country. Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation process used blocks of 4 patients for each centre, and was organised by the Lyon University Hospital's Biostatistics Department." (p. 309) |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation was confidential and delivered via phone call [of the Biostastics Department] to the secretary of each centre." ( p. 309) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Quote: "Psychologists who had not taken part in the treatments performed the assessment. They had no information on either the randomisation or the treatment and did not attend the team meetings about the patients." (p. 310) "[...] evaluators may have received inadvertent or indirect information from the patients about the treatment underway. The evaluators' blindness was not tested." (p. 313) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: the authors per protocol analyses: treatment completers only (20/33 people randomised to CT, 18/32 people randomised to RST) |
| Selective reporting (reporting bias) | Low risk | Comment: Study protocol available (NCT00131781). There was no indication of selective reporting. |
| Other bias | Low risk |
Performance bias: "At the end of each session, the therapists were to complete a checklist of the techniques they used, which was revised and discussed with the prinicipal investigator [...] weekly supervision session." (p. 309) Allegiance bias: there was no indication of an allegiance effect. None of the authors was among the developers of any of the treatments under investigation. Attention bias: equal amounts of attention paid to both groups |
Davidson 2006.
| Study characteristics | ||
| Methods | Multicentre, parallel‐arm RCT, with 2 treatment arms
Duration of trial: 1 year Country: UK Setting: outpatient |
|
| Participants |
Methods of recruitment of participants: not stated Sample size: 106 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DMS‐IV) Means of assessment: Structured Clinical Interview for DSM‐IV Personality Disorders (SCID) Mean age: 31.9 years (standard deviation = 9.1) Sex: 84% female Comorbidity: "We did not rule out comorbid problems such as depression or alcohol and drug abuse that are common in Borderline personality disorder." (quote, p 8) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group Treatment name: cognitive behaviour therapy + treatment‐as‐usual (CBT + TAU) Number randomised to group: 54 Duration: 1 year (mean = 27 sessions) Control/comparison group Comparison name: treatment‐as‐usual (TAU) Number randomised to group: 52 Duration: 1 year Both groups Concomitant psychotherapy: patients currently receiving inpatient treatment for a mental state disorder or a systematic psychological therapy or specialist service were excluded. All other kinds of treatments a patient would have received if the trial had not been in place (e.g. general practitioner care, contact with community mental health teams) were allowed. 90% of participants were in contact with mental health services. Concomitant pharmacotherapy: allowed as part of TAU which allowed "all other kinds of treatments a patients would have received if the trial had not been in place". Proportions of participants taking standing psychotropic medication during trial observation period: There were no details on how many of the study participants actually received psychotropic medical treatment. “Although no information on comorbidity or the use of prescription drugs was collated, there are no major imbalances in resource utilisation to suggest that comorbidity or drug prescribing differed either across the original randomised groups or in the subset of the 76 patients who were followed up.“ (Davidson 2010, p. 461) |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: not stated Ethics approval: not stated Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization schedules were generated by the study center at [...] Glasgow University, and kept securely and confidentially by the trial coordinator at the Study Coordinating Centre." (p. 437) |
| Allocation concealment (selection bias) | Low risk |
Quote: "The randomization schedules were [...] kept securely and confidentially by the trial coordinator [...] The trial coordinator informed the referring agent of the result of randomization immediately and in writing, and then contacted the CBT therapist/s in each area with the patients details so that CBT therapy could be initiated." (p. 437) 106 patients enrolled and randomised |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The research assistants on each site carry out all assessments and are blind to treatment group allocation. In addition, research assistants request that patients do not mention any details of any psychological treatment they may be receiving. [...] The research assistants responsible for the recording of outcomes were unaware of the treatment allocated or received." (p. 439) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The analyses were according to the intention‐to‐treat principle." (p. 454) |
| Selective reporting (reporting bias) | Low risk | Comment: study protocol available (ISRCTN86177428). No indication of selective reporting |
| Other bias | High risk |
Performance bias: "All therapists received training in the protocol at the beginning of the trial and regular meetings of all therapists were held to ensure consistency of approach across the sites. In addition, all therapists received weekly supervision from CBT experts at each site." (p. 452) Allegiance bias: there was no indication of an allegiance effect. However, as one of the authors is the main author of the CT manual according to which the active group was treated, an allegiance effect seems not improbable. Attention bias: more attention paid to CT group participants |
Davidson 2014.
| Study characteristics | ||
| Methods | 6‐session trial with 2 arms
Duration of trial: 6‐session trial, unknown length Country: Scotland Setting: outpatient |
|
| Participants |
Method of recruitment of participants: referral to hospital liaison psychiatry team
Sample size: 20 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM‐IV) Means of assessment: Structured Clinical Interview for DSM‐IV axis II disorders Mean age: not stated Sex: not stated Comorbidity: 4 people had simple personality disorder and 16 had diffuse personality disorder (personality disorder in more than one cluster). The most common diagnoses were borderline personality disorder (n = 17) followed by avoidant (n = 13) and paranoid personality disorder (n = 8). Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: manual‐assisted cognitive therapy (MACT)
Number randomised to group: 14
Duration: 6 sessions Control/comparison group Comparison name: TAU Number randomised to group: 6 Duration: 6 sessions Both groups Concomitant psychotherapy: seems as if both treatments included inpatient care, if required Concomitant pharmacotherapy: not stated Proportions of participants taking standing psychotropic medication during trial observation period: unclear |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: not stated
Ethics approval: yes Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were randomised to MACT or TAU using a random numbers table with an allocation ratio of 2:1 in favour of MACT”. (p.109) |
| Allocation concealment (selection bias) | Unclear risk | Comment: We do not know who conducted the randomisation, or if the participants were informed of allocation prior to baseline assessment. Baseline assessment was conducted by a blinded assessor. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “The research assistant, who assessed patients at baseline and outcome, remained masked to treatment allocation throughout the study.” (p.109) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: the authors used intention‐to‐treat analysis – it did not measure outcome data on all participants (due to attrition), but included all randomised participants in the analysis, evidently. No info on imputation method. 3/14 (21.4%) lost in MACT, 2/6 (33%) in TAU – fairly balanced in numbers. Only one reason given for missing outcome data (suicide). We do not know if reasons differed between the groups. |
| Selective reporting (reporting bias) | Unclear risk | Comment: there was no protocol available for this study. |
| Other bias | High risk |
Adherence bias: "Therapy was delivered to individuals in the community by two therapists, a doctoral‐level clinical psychologist and a psychiatrist, both trained and supervised on a weekly basis in MACT by one of the authors of the manual (K.D.).” (p. 109) Allegiance bias: Main author K.D. is the author of the intervention‐manual. Attention bias: MACT had 6 sessions. There was no mention of how extensive the TAU treatment was, compared to MACT. |
Doering 2010.
| Study characteristics | ||
| Methods | Randomised controlled trial with 2 treatment arms
Duration of trial: 12 months Country: Germany, Austria Setting: outpatient |
|
| Participants |
Methods of recruitment of participants: participants recruited at the outpatient units of the Departments of Psychiatry and Psychotherapy, Technical University of Munich, Germany, and the Psychoanalysis and Psychotherapy Department, Medical University Vienna, Austria Sample size: 104 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th edition, (DSM‐IV) Means of assessment: Structured Clinical Interview for DSM‐IV (SCID) Mean age: 27.3 years Sex: 100% female Comorbidity: "Comorbidity with all other personality disorders and with Axis I disorders except those mentioned above were allowed [...]." (p. 391) "There were no significant differences between the groups with regard to sociodemographic and clinical variables at baseline." (p. 391) Incluson criteria: not stated Exclusion criteria
|
|
| Interventions |
Experimental group Treatment name: transference‐focused psychotherapy (TFP) Number randomised to group: 52 Duration: 12 months (twice‐weekly, individual psychotherapy sessions) Control/comparison group Treatment name: treatment by experienced community psychotherapist (TBE) Number randomised to group: 52 Duration: 12 months Both groups Concomitant psychotherapy: psychotherapy other than the study treatment was not allowed in the experimental group. Concomitant pharmacotherapy: Medication treatment was not standardised, its type and amount were decided on an individual basis by the individuals’ psychiatrists in the community in both groups and registered continuously. “There were no significant differences between the groups with regard to medication at baseline and during the 1‐year treatment period (Fig. 3). The only participant who received amphetamines was in the transference‐focused psychotherapy group. There was no significant influence of psychotropic medication on the outcome variables with the exception of a worse BSI global severity index in medicated participants (F = 43.927, d.f.= 1,101, P = 0.04).“ (p. 392) |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: yes Ethics approval: The study was approved by the ethics commission of the Medical University Innsbruck, Austria, on 24 March 2004 (ID: UN1950). Comments from review authors
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: the authors used random numbers, matching after inclusion of 35th patient according to severity of self‐harming behaviour during the last year and personality organisation (Doering 2010 [pers comm]. |
| Allocation concealment (selection bias) | Low risk | Quote: "The results of the first assessments [screening for inclusion criteria] were sent to a researcher outside the two study centers who performed the randomization." (Doering 2010, p. 5) "After randomization patients were referred to a therapist." (Doering 2010, p. 6) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Research assistants who conducted assessments before randomization and after one year of treatment were blinded for the therapy delivered." (Doering 2010, p. 7) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Last observation carried forward (LOCF) |
| Selective reporting (reporting bias) | Low risk | Comment: Study protocol available (NCT00714311). No indications of selective reporting |
| Other bias | Unclear risk |
Performence bias: "Video recordings of all [EG] sessions were performed and used in the group supervision. [...] Every case was supervised at least every four to six weeks. [...] Experienced community psychotherapists [i.e. CG therapists] attended supervisions according to their usual routine." (Doering 2010, p. 10f.) "For the assessment of adherence and competence of the transference‐focused psychotherapists a German translation of a specific Rating of Adherence and Competence [...] was used. [...] The rating was performed by the supervisor after every video‐guided supervision of a therapy session." (Doering 2010, p. 11) Allegiance bias: Some of the study authors are experienced TFP therapists, but none was personally involved in treatment development. Attention bias: Less attention may have been paid to CG patients depending on the CTBE therapists' main orientation; however, every participant was provided the specifically full amount of necessary attention. |
Elices 2016.
| Study characteristics | ||
| Methods | 10‐week trial with 2 arms
Duration of trial: 10 weeks Country: Spain Setting: outpatient |
|
| Participants |
Method of recruitment of participants: participants recruited from the outpatient BPD Unit at the Department of Psychiatry from the Hospital de la Santa Creu in Sant Pau Sample size: 64 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: Structured Clinical Interview for DSM‐IV axis II personality disorder (SCID‐II) and Diagnostic Interview for Borderlines‐Revised (DIB‐R). Axis I comorbidities were assessed with the Psychiatric Diagnostic Screening Questionnaire (PDSQ) Mean age: MT = 31.56 years (standard deviation = 7.25), IE = 31.72 years (standard deviation = 6.82) Sex: 86% female Comorbidity: All participants in both groups had at least one comorbid Axis I diagnosis, including anxiety disorders, major depressive disorder, and substance abuse. Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: mindfulness training (MT)
Number randomised to group: 32
Duration: 10 weeks Control/comparison group Comparison name: interpersonal effectiveness skills training (IE) Number randomised to group: 32 Duration: 10 weeks Both groups Concomitant psychotherapy: no Concomitant pharmacotherapy: patients under pharmacological treatment were included – provided that no modifications of the medication type or dose were made during the 10‐week intervention period. Proportions of participants taking standing psychotropic medication during trial observation period: no statistically significant difference between proportions taking antidepressants (MT: 83.3%, IE: 65.5%, P = 0.12), benzodiazepines (MT: 50.0%, IE: 53.8%; P = 0.77), antipsychotics (MT: 43.3%, IE: 42.3%, P = 0.93) |
|
| Outcomes |
Primary
Secondary
Some secondary outcomes can be found in a secondary analysis (Soler 2016):
|
|
| Notes |
Sample size calculation: yes
Ethics approval: yes Comments from review authors:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomized allocation was performed with the online Research Randomizer (www.randomizer.org/form.htm), a program that generates 16 sets of 4 numbers each (ranging from 1 to 2 for M and IE, respectively). To obtain the same sample size in each treatment arm, allocation had to be perfectly balanced every four sets. Each group comprised eight individuals corresponding to four consecutive sets of randomization.” (p. 586) |
| Allocation concealment (selection bias) | High risk | Quote: “The research unit coordinator (not blind to treatment condition) was responsible for the randomization process.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “A trained psychiatrist and two psychologists familiar with screening interviews, who were blind to treatment arms, conducted diagnostic interviews.” (p. 586) |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Quote: “(…) analyses were conducted on both per‐protocol (PP) and intention‐to‐treat (ITT) samples. ITT analyses included all enrolled participants (n = 64), regardless of whether they completed the intervention or not. PP analyses comprise only participants who completed at least 80% of the intervention (completers), and for whom, all data points (pre‐ and post‐intervention) are available (M group: number of participants = 19; IE group: number of participants = 25). Missing data were treated with the last observation carried forward (LOCF) method (Little and Rubin 1987)”. (p. 589) “The dropout rate for mindfulness was higher than in the control group (41 vs.19%)” (p. 590) Missing data treated with LOCF, a potentially biased imputation method. There were clear differences in attrition rates between groups which could have affected outcome estimates. The reasons for dropout also varied between groups. The attrition rate was also higher than estimated in the power calculation. |
| Selective reporting (reporting bias) | Low risk | Comment: Matched study protocol |
| Other bias | Unclear risk |
Attention bias: "Participants met once a week in groups of eight for ten consecutive weeks, with each session 150 min in duration. Sessions for both intervention modalities (i.e. M and IE) followed the same structure).” Treatment adherence: “Other team members followed each therapy session using a closed‐circuit television, enabling supervision and feedback.” "Another limitation of the present study is the absence of a treatment adherence measure (TAM), in part because no validated TAM is available in Spanish". Allegiance bias: Last author (JS) is a DBT therapist (http://www.esspd.eu/fileadmin/us er_upload/Board/cvs/Soler_CV.p df). The two arms in this trial were heavily based on DBT. Potential allegiance bias here |
Farrell 2009.
| Study characteristics | ||
| Methods | Randomised controlled trial with 2 treatment arms
Duration of trial: 8 months Country: USA Setting: outpatient |
|
| Participants |
Methods of recruitment of participants: not stated Sample size: 32 Diagnosis of borderline personality disorder: Diagnostic Interview for Borderlines‐Revised (DIB‐R) Means of assessment: Diagnostic Interview for Personality Disorders Revised, Borderline Syndrome Index Mean age: 35.6 years Sex: 100% female Comorbidity: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group Treatment name: group schema focused therapy + individual psychotherapy treatment‐as‐usual (GSFT + PTAU) Number randomised to group: 16 (30 sessions) Duration: 8 months Control/comparison group Comparison name: individual psychotherapy treatment‐as‐usual (PTAU) Number randomised to group: 16 Duration: 8 months Both groups Concomitant psychotherapy: all participants were in individual psychotherapy (eclectic, mainly supportive) throughout the study. Concomitant pharmacotherapy: psychopharmacological treatment was not controlled for. Proportions of participants taking standing psychotropic medication during trial observation period: all participants were stable on at least 1 psychotropic medication at the start of the study, mostly low doses of antipsychotics or selective serotonin reuptake inhibitor, or both. |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: not stated Ethics approval: not stated Comments from review authors:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned using a random number table". (p. 319) |
| Allocation concealment (selection bias) | Unclear risk | Comment: no clear details were reported. After screening for eligibility of 40 patients, N = 8 were excluded. Reasons for exclusion were only given for 3 of them (1 declined participation, 2 did not meet inclusion criteria).Thus, N = 16 were allocated to EG, N = 16 to CG. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Quote: "The DIB‐R structured interviews were conducted by two Ph.D. Clinical Psychologists not involved in treatment delivery. Efforts were made to keep them blind to treatment group membership, but for 10% of the subjects the blind was broken due to patient report." (, p. 319) "Therapists were given a GAFS [Global Assessment of Function Scale] checklist to use so that the anchors for assigning scores were in front of them when they recorded their ratings. They were chosen as raters since they were removed from the hypotheses of the study, although not blind to their patients' group membership and no inter‐rater reliability was possible." ( p. 319) Comment: overall, observer‐rated outcomes were not assessed by blind raters. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: the authors conducted per protocol analyses. |
| Selective reporting (reporting bias) | Unclear risk | Comment: No indication of selective reporting, but Insufficient information to permit judgement of 'high' or 'low' |
| Other bias | High risk |
Adherence bias: "Two of the three groups had the two program developers as therapists and the third had one developer and one clinical psychologist [...] Weekly supervision meetings took place during the course of the study and random videotapes of sessions were reviewed for fidelity by the program developers. The manual developed for the study acted as an additional fidelity check." (p. 322) Allegiance bias: The two program developers were study therapists and have authored this study. Attention bias: more attention paid to SFT‐G participants |
Feigenbaum 2012.
| Study characteristics | ||
| Methods | 12‐month trial with 2 arms
Duration of trial: 1 year Country: UK Setting: outpatient |
|
| Participants |
Method of recruitment of participants: from secondary and tertiary care services Sample size: 42 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM‐IV) Means of assessment: Structured Clinical Interview for DSM‐IV axis II disorders (SCID‐II) Mean age: DBT = 35.4 years (standard deviation = 7.8), treatment‐as‐usual = 34.6 years (standard deviation = 7.4) Sex: 72‐75% female Comorbidity: mood disorders, substance abuse, anxiety disorders, eating disorders Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: dialectical behavioral therapy (DBT)
Number randomised to group: 26
Duration: 1 year
Control/comparison group
Comparison name: treatment‐as‐usual (TAU)
Number randomised to group: 16
Duration: 1 year Both groups Concomitant psychotherapy: no data Concomitant pharmacotherapy: yes, including antidepressants, antipsychotics, and mood stabilisers Proportions of participants taking standing psychotropic medication during trial observation period: "Patients were on a range of medications at time of randomization (predominantly anti‐depressants, anti‐psychotics, and mood stabilizers). Those patients entering DBT were reviewed by a consultant psychiatrist for the appropriateness of medication." (p. 129) Exact proportions of participants in each group unclear |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: yes
Ethics approval: yes Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Treatment allocation was made offsite via telephone randomization using a stochastic minimization programme (MINIM) balancing for sector within the regions to avoid differences in terms of differential referral practices, gender, and presence of BPD. Clients were randomized so that two of three entered DBT and one of three TAU in order to build the caseloads for staff, as this was a new service with no existing clients.” (p. 124) |
| Allocation concealment (selection bias) | Low risk | Quote:"Treatment allocation was made offsite via telephone randomization…” (p. 124) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "While we attempted blinding of assessments, as is often the case with psychosocial treatment trials, those carrying out the research assessments could mostly identify the treatment group of the patient.” (p. 137) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: “All results were analyzed using an intention‐to‐treat analysis based on treatment assignment, 15/26 dropped out in DBT and only 1/16 in TAU. Substantial differences. Unclear [for] the discontinued participants whether they completed the treatment or not.” (p. 127) “Of the 26 assigned to DBT, one withdrew consent for the data to be used at end of treatment and five refused to enter the treatment during the pre‐commitment phase. A further nine patients dropped out of therapy between months 4 and 9 of treatment. (…) Of those assigned to TAU, only one individual dropped out of receiving any form of treatment.” “Those who discontinued treatment continued to contribute data and remained in the trial.” (p. 127) |
| Selective reporting (reporting bias) | Unclear risk | Comment: No information |
| Other bias | Unclear risk |
Treartment adherence: “Adherence to the therapy was not formally measured. However, adherence to the model was monitored by the team through weekly case discussion, verbal reporting of session content, and listening to each other’s audio tapes.” (p. 125) Allegiance bias: First author is Senior international trainer in DBT for British Isles DBT (https://iris.ucl.ac.uk/iris/browse /profile?upi=JFEIG65). Attention bias: “Finally, while information was collected on the types of treatments and services utilized in TAU, the number of hours of TAU intervention was not recorded, thus, it is possible that the differences identified may be due to differing intensities of treatment”. (p. 138) |
Feliu‐Soler 2017.
| Study characteristics | ||
| Methods | 3‐week trial with 2 arms
Allocation: 1:1 Duration of trial: 3 weeks Country: Spain Setting: outpatient |
|
| Participants |
Method of recruitment of participants: not stated
Sample size: 62 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision (DSM‐IV‐TR) Means of assessment: Diagnostic Interview for Borderline Personality Disorders‐Revised (DIB‐R) Mean age: LKM/CM = 35.13 years (standard deviation = 8.25), MCT = 32.5 years (standard deviation = 6.17) Sex: 93.8% female Comorbidity: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: loving‐kindness and compassion meditations (LKM/CM)
Number randomised to group: 16
Duration: 3 weeks Control/comparison group Comparison name: mindfulness continuation training Number randomised to group: 16 Duration: 3 weeks Both groups Concomitant psychotherapy: none Concomitant pharmacotherapy: Psychiatric medication was unaltered during the 3‐week study period. Proportionsof participants taking standing psychotropic medication during trial observation period: exact proportion unclear |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: not stated
Ethics approval: yes Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: “To assess the added value of LKM/CMin this sample, patients were randomly allocated to either 3 weeks of LKM/CM or mindfulness continuation training (MCT)”. (p. 2) Comment: The authors provided insufficient information to make a clear judgement of risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | Comment: the method of concealment was not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk |
Quote: “Missing data were treated with the last‐observation‐carried forward method” (p. 4). Comment: no data on attrition; a more conservative imputation method than last‐observation‐carried‐ forward could have been used. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available to enable a clear judgement of risk of bias. |
| Other bias | High risk |
Allegiance bias: “The interventions were co‐led by two clinical psychologists (JS and AF) with long‐term personal practice in mindfulness meditation and extensive clinical experience with mindfulness‐based programmes and DBT.” (p. 2) Comment: First and last authors carried out the interventions. Adherence bias: no information provided Attention bias: all participants received 10 weeks of mindfulness training. Both groups received 3 weeks of LKM/CM or 3 weeks of MCT. |
Giesen‐Bloo 2006.
| Study characteristics | ||
| Methods | 3‐year trial with 2 treatment arms
Duration of trial: 3 years Country: The Netherlands Setting: outpatient |
|
| Participants |
Methods of recruitment of participants: not stated Sample size: 88 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM‐IV) Means of assessment: Structured Clinical Interview for DSM‐IV (SCID) and Borderline Personality Disorder Severity Index ‐ Version IV (BPDSI‐IV) Mean age: 30.6 years Sex: 93% female Comorbidity: "Comorbid Axis I and Axis II disorders were allowed" (p. 650). Numbers of comorbid Axis I and Axis II disorders were equally distributed across groups. Inclusion criteria: not stated Exclusion criteria
|
|
| Interventions |
Experimental group Treatment name: schema‐focused therapy (SFT) Number randomised to group: 45 Duration: 3 years (50‐minute sessions, twice a week) Control/comparison group Comparison name: transference‐focused psychotherapy (TFP) Number randomised to group: 43 Duration: 3 years (50‐minute sessions, twice a week) Both groups Concomitant psychotherapy: no additional psychotherapeutic treatment allowed Concomitant pharmacotherapy: prescribing according to good clinical practice, similar to American Psychiatric Association guidelines, by psychiatrists from different orientations Proportions of participants taking standing psychotropic medication during trial observation period: Unclear. No difference in psychotropic medication use at baseline: SFT 77.3%, TFP 71.4%; P = 0.87 |
|
| Outcomes |
Primary
|
|
| Notes |
Sample size calculation: yes Ethics approval: The medical ethics committees of the participating centers approved the study. Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization to SFT or TFP was stratified across 4 community mental health centers and was performed [...] after the adaptive biased urn procedure". ( p. 650) |
| Allocation concealment (selection bias) | Low risk |
Quote: "Randomization to SFT or TFP [...] was performed by a study independent person [...] We used this procedure (1) to keep allocation at each site unpredictable until the last patient to avoid unintentionally affecting ongoing screening procedures [...]." ( p. 650) 173 patients were screened for eligibility. 85 of them were excluded, reasons are given (40 declined participation, 24 did not meet inclusion criteria, 19 met exclusion criteria, 2 had insufficient availability); 88 randomised, of 45 allocated to SFT, 44 were included in analyses (1 patient excluded owing to unreliable assessments due to increased patient blindness), of 43 allocated to TFP, 42 were included in analyses (1 patient excluded because untraceable after randomisation; never met or spoke to therapist). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "assessments were made [...] by independent research assistants [...] Study researchers, screeners, research assistant, and SFT/TFP therapists were masked to treatment allocation during the screening procedure and the first assessment". ( p. 650) "most research assistants learned their patients' treatment allocation as the study progressed, as patients talked about their treatment and therapists. However, the results of secondary computer‐assessed self‐report measures [...] concurred with the observer‐rated (interview) findings, making it unlikely that results can be attributed to knowledge of treatment allocation." ( p. 657) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "An intention‐to‐treat approach was applied, using either the last observation during the 3‐year treatment period or the last‐observation‐carried‐forward method [...]." (p. 651) |
| Selective reporting (reporting bias) | Unclear risk | Comment: No indication of selective reporting, but Insufficient information to permit judgement of 'high' or 'low' |
| Other bias | Unclear risk |
Adherence bias: "Weekly local supervision [...], a 1‐day central supervision every 4 months, and a 2‐day central supervision every 9 months. [...] Treatment integrity was monitored by means of supervision. All the raters were independent of the study and masked to treatment outcome. One psychologist, masked to allocation, listened to 1 randomly selected tape of each patient, then stated the treatment administered [...] Other trained therapists for each orientation assessed the TFP Rating of Adherence and Competence Scale or the SFT Therapy Adherence and Competence Scale for BPD." (p. 650‐651). Issues have been raised about treatment integrity of TFP by the study consultant Dr Yeomans (Yeomans 2015). Allegiance bias: experts from both therapies supervised therapists. None of the study authors developed any of the respective therapies. Attention bias: equal amounts of attention spent to both groups |
Gleeson 2012.
| Study characteristics | ||
| Methods | 16 weekly session trial with 2 arms
Duration of trial: 16 weekly sessions Country: Australia Setting: outpatient and inpatient. |
|
| Participants |
Method of recruitment of participants: participants identified by the research assistant in consultation with the outpatient case manager, using a checklist of the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) for borderline personality disorder criteria
Diagnosis of borderline personality disorder: DSM‐IV
Means of assessment: Structured Clinical Interview for the DSM‐IV Axis I Disorders, Patient Edition (SCID‐I/P) and the antisocial and borderline personality disorder modules for Axis II disorders (SCID‐II)
Mean age: 18.4 years (standard deviation = 2.9)
Sex: 82% female Comorbidity: 12 participants had a diagnosis of borderline personality disorder (i.e. greater than or equal to 5 DSM‐IV criteria), and 4 cases had sub‐syndromal borderline personality disorder (4 DSM‐IV criteria) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: HYPE + SFET
Number randomised to group: 8
Duration: 16 weekly sessions
Control/comparison group
Comparison name: SFET
Number randomised to group: 8
Duration: not stated Both groups Concomitant psychotherapy: not stated Concomitant pharmacotherapy: eligible for inclusion in study if had less than 6 months of previous treatment with antipsychotic medication Proportions of participants taking standing psychotropic medication during trial observation period: “patients taking medications. There were seven cases in the HYPE + SFET [87.5%] and five cases in the SFET [62.5%].” (Gleeson 2012, p. 26) Mean adherence did not differ sig. between groups (P = 0.983). (Tab. 2) |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: no
Ethics approval: no Comments from review authors:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization was coordinated by the statistician (SC) and was based on a computer‐generated number list.” (p. 23) |
| Allocation concealment (selection bias) | High risk | Quote: “Outcome ratings were made by the study research assistant (RA) who was independent of the treatment, but not blind to treatment allocation because of limited resources for conducting the pilot study”. (p. 23) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “Outcome ratings were made by the study research assistant (RA) who was independent of the treatment, but not blind to treatment allocation because of limited resources for conducting the pilot study”. (p. 23) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: attrition rates: HYPE + SFET, number of participants = 4/8 and SFET, number of participants = 4/8. No reasons for attrition reported. 50% dropout rate. High risk of bias. No report of imputation method (e.g. ITT, as treated). Potential risk of bias. Summed up: High risk of bias |
| Selective reporting (reporting bias) | High risk | Comment: no report of primary or secondary outcomes in the study (unlike the protocol) |
| Other bias | Unclear risk |
Treatment adherence: Fidelity to CAT was managed by weekly group CAT supervision provided by two trained CAT supervisors (AC and LMc). (p. 23) Allegiance bias: Nothing found. Attention bias: No report of duration of the SFET group. |
Gratz 2006.
| Study characteristics | ||
| Methods | 14‐week trial with 2 arms
Duration of trial: 14 weeks Country: USA Setting: outpatient |
|
| Participants |
Methods of recruitment of participants: not stated Sample size: 25 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: Diagnostic Interview for DSM‐IV Personality Disorders (DIPD‐IV, Zanarini 1996) Mean age: 33.3 years (standard deviation = 9.98) Sex: 100% female Comorbidity: participants excluded if they had comorbid diagnoses Inclusion criteria
Exclusion criteria
Participation in a dialectical behavior therapy (DBT) skills group within the past 6 months |
|
| Interventions |
Experimental group Treatment name: ERG + TAU. 14 weekly, 1.5 hour sessions. Acceptance‐based, behavioural group, combining elements of acceptance and commitment therapy (ACT) and DBT as well as aspects of emotion‐focused psychotherapy and traditional behaviour therapy Number randomised to group: 12 Duration: 14 weeks Control/comparison group Comparison name: TAU + WL Number randomised to group: 10 Duration: 14 weeks Both groups Concomitant psychotherapy: Participants were required to have an individual therapist; average number of individual therapy per week was 1.38 hours. 41% of therapists were clinical psychologists, 27% were psychiatrists, 32% were licensed clinical social workers. Concomitant pharmacotherapy: All study participants continued with their current outpatient treatment over the course of the study. Proportions of participants taking standing psychotropic medication during trial observation period: Unclear. Mean number of psychiatric medications at baseline: ERGT mean = 3.42, SD = 1.39; TAU mean = 3.90, SD = 2.08 (table 1, p. 28), difference NS |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: not stated Ethics approval: not stated Comments from review authors:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants [...] were matched on level of emotion dysregulation and number of lifetime incidents of self‐harm and randomly assigned to either the group treatment plus TAU condition or the TAU waitlist condition." (p. 27) |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Quote: "Research team members were not blind to condition; however, all outcome measures were self‐report, and there was limited interaction between participants and assessors." (p. 30) Outcomes are not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: per protocol (EG: N = 16, CG: N = 12). 24 participants were included and randomised, there were 2 dropouts (one from each condition), with no reasons given. Final analyses referred to N = 22 patients, N = 12 in EG, N = 10 in TAU condition. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no indication of selective reporting, but Insufficient information to permit judgement of 'high' or 'low' |
| Other bias | High risk |
Performance bias: Treatment approach was developed by the first author who was also the therapist; no further information. Allegiance bias: First author developed the treatment approach. Attention bias: more attention paid to ERGT participants |
Gratz 2014.
| Study characteristics | ||
| Methods | 14‐week trial with 2 arms
Duration of trial: 14 weeks. On average, 29 days from randomisation to initial assessment + 14 weeks of treatment + 9 months of follow‐up Country: USA Setting: outpatient |
|
| Participants |
Method of recruitment of participants: referral through clinicians and self‐referral Sample size: N = 61 Number of participants screened: 91 Number of participants included: ERGT + TAU = 31, TAU = 30. 100% female Number of participants followed up: 57 Number of withdrawals: (reason) 5 for ERGT + TAU, 3 for TAU. Reasons in ERGT + TAU: too busy/other responsibilities (n = 3) , moved away (number of participants = 1), not interested (n = 1). Reasons in TAU: could not be reached (n = 2) and not interested (n = 1) Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: Diagnostic Interview for DSM‐IV Personality Disorders (DIPD‐IV; Zanarini 1996) Mean age: ERGT + TAU: 33.3 years, SD = ± 11), TAU: 33.0 years (± 10.9) IQ: NR Ethnicity: ethnic minority ERGT + TAU: 16.1%, TAU: 26.7% Comorbidity: mood disorders, substance use disorder, anxiety disorder, PTSD, eating disorder, Cluster A, B, C PD Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: ERGT + TAU
Number randomised to group: 31
Duration: 90 minutes per week over 14 weeks
Concomitant psychotherapy: yes, ongoing outpatient therapy
Concomitant pharmacotherapy: 1.9 units of psychiatric medication on average per pretreatment. No information regarding patient Control/comparison group Comparison name: TAU Number randomised to group: 30 Duration: average of 15 months of treatment prior to trial. 14 weeks in trial Concomitant psychotherapy: TAU consisted of a variety of different psychotherapies Concomitant pharmacotherapy: allowed (no further details) Proportions of participants taking standing psychotropic medication during trial observation period: Unclear. Mean number of psychiatric medications at baseline: ERGT + TAU 1.9, SD = 1.7; TAU 2.1, SD = 1.2 |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: not stated
Ethics approval: yes Comments from review authors
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “…and randomly assigned by the principal investigator (PI) to either the ERGT + TAU (number of participants = 31) or TAU waitlist (number of participants = 30) condition using a stratified randomization procedure.” (p 2100) |
| Allocation concealment (selection bias) | Unclear risk | Comment: no clear information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “All assessments were conducted by trained assessors masked to participant condition”. (p 2103) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “We adopted a Bayesian approach to growth modeling (Zhang et al. 2007) and fit the models using the Markov chain Monte Carlo routines in Mplus (Muthén & Muthén, 1998–2010) using N(0,1010) priors for the intercepts and paths from Condition to the factors, and G–1 (−1,0) priors for the error variances. This approach implements a multiple imputation strategy to handle missing data (Enders, 2010), enabling an analysis of the intent‐to‐treat (ITT) sample.” (p 2104) |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available to enable a clear judgement to be made. |
| Other bias | High risk |
Treatment adherence: “The PI reviewed all group sessions for adherence and competence (with 25% rated by an independent trained rater with good reliability; κ = 0.90 for adherence ratings and ICC = 0.86 for competence ratings).” (p. 2103). Project therapists were adherent to the protocol, with an average of 8.1 ± 1.1 of the encouraged elements discussed in each group and only one minor non‐protocol event recorded."; (p 2104). Allegiance bias: First author has developed the treatment in the experimental group. Attention bias: Tau participants had received, on average, 15 months of treatment prior to participation, and 54% received < 1 hour a week during treatment, compared to no reports on previous treatment in ERGT group, which received 90 minutes weekly psychotherapy. |
Gregory 2008b.
| Study characteristics | ||
| Methods | 12 months trial with 2 arms
Duration of trial: 12 months Country: USA Setting: outpatient |
|
| Participants |
Methods of recruitment of participants: clinical setting Sample size: 30 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: Structured Clinical Interview for DSM‐IV Axis II Disorders (SCID‐II) Mean age: 28.7 years (standard deviation = 7.7) Sex: 80% female Comorbidity: comorbid diagnosis of active alcohol abuse or dependence (not in full sustained remission) required for inclusion Incluson criteria
Exclusion criteria
|
|
| Interventions |
Experimental group Treatment name: DDP Number randomised to group: 15 Duration: 12 months (post‐treatment), weekly individual Control/comparison group Comparison name: TAU Number randomised to group: 15 Duration: 12 months (post‐treatment) Both groups Concomitant psychotherapy: If not already in treatment, TAU patients were referred to an alcohol rehabilitation centre and given names of clinics and therapists in the community. If they had one, TAU participants were allowed to keep their current psychotherapist. DDP participants were required to end treatment with their present psychotherapist, unless that person served primarily as a case manager or substance use counsellor. 70.0% of participants received individual psychotherapy or alcohol counselling, 30.0% received an additional professional group therapy, 36.7% participated in self‐help groups. Concomitant pharmacotherapy: 63.3% of all participants received separate medication management. The mean number of psychotropic medications was 2.9. Medication management was provided by the DDP therapist for patients in the DDP group according to the American Psychiatric Association guidelines for borderline personality disorder. Medications specifically targeting substance use disorders were not prescribed. [The] “average number of psychotropic medications used during first 12 months of treatment: control group, mean number N = 2.67, SD 1.45; DDP mean number N = 2.34, SD = 1.61" (Gregory 2010, p. 293). 75% of both groups were taking psychotropic medications during the follow‐up period; average number of psychotropic medications used: control group mean number = 1.88 (SD = 1.55), DDP group mean number = 1.63 (SD = 1.30) (Gregory 2010, p. 294). Number of psychotropic medications at end of treatment: DDP 2.0 (SD 1.56), TAU 2.89 (SD 1.69) (Gregory 2008, Tab. 2, p. 33). Proportions of participants taking standing psychotropic medication during trial observation period: n (%) receiving separate medication management: DDP 0%, TAU 56% (Gregory 2008, Tab. 2, p. 33) |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: not stated Ethics approval: not stated Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A minimization method was employed for group assignment [...] ensuring comparability of the two groups on key variables or factors [...] The specific factors that we adjusted for included: age, gender, alcohol abuse versus dependence, current alcohol use, antisocial personality disorder, inpatient utilization, and number of parasuicides." ( p. 31‐32) |
| Allocation concealment (selection bias) | Low risk | Quote: "participants were assigned by the research coordinator to either the investigation treatment or to treatment‐as‐usual (TAU) in the community". (p. 31) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote:"An independent, trained research assistant administered the primary and secondary outcome measures [...] blind to treatment group at the time of interviews, but blindedness was only partial, as she was able to correctly guess group assignment 67% of the time (50% correct guesses were expected by chance alone)." (p. 35) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: the authors conducted per protocol analyses (EG: 10/15 allocated; CG: 9/15 allocated). |
| Selective reporting (reporting bias) | Low risk | Comment: a study protocol is available (NCT00145678) and there were no indications of selective reporting |
| Other bias | High risk |
Performance bias: "Six therapists provided DDP, including the principal investigator [who is one of the two developers of DDP] (PI; N = 6 study participants) and five psychiatry residents (N = 9 participants) who were in their third year of residency training [...] After achieving competency, adherence to technique and treatment integrity for resident therapists was assured through weekly group supervision [...] and individual supervision of videotaped sessions with the PI [principal investigator, developer of DDP] every other week throughout treatment." (Gregory 2008b, p. 34) Allegiance bias: Both developers of the experimental treatment were among the study authors. Attention bias: Though participants of the control group did not receive an alternate, obligatory control treatment, but were free to join alternative treatments, they did not receive less professional attention. "[...] DDP participants received fewer overall treatment contact hours than did participants receiving community care." (p. 39). Also cf. Tab. 2, p. 33. |
Haeyen 2018.
| Study characteristics | ||
| Methods | 3‐month trial with 2 arms
Duration of trial: 3 months Country: The Netherlands Setting: specialised outpatient treatment unit for personality disorders |
|
| Participants |
Method of recruitment of participants: participants recruited from a waiting list of patients targeted for PD treatment in a specialised outpatient treatment unit for personality disorders Sample size: subsample data = 26 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: Structured Clinical Interview for DSM‐IV Axis II Disorders (SCID‐II) Mean age: no data for subsample. Mean age of dropouts from full sample = 40.35 years (standard deviation = 10.03), mean age of completers from full sample = 36.6 years (standard deviation = 10.52) Sex: no data for subsample. Approximately 70% in the full sample Comorbidity: no data on comorbidity in subsample data. Full sample data: paranoid personality disorder (AT = 2.6%, WL = 2.8%); narcisstic personality disorder (AT = 2.6%, WL = 0%); borderline personality disorder (AT = 36.8%, WL = 27.8%); obsessive compulsive personality disorder (AT = 10.5%, WL = 8,3%); dependent personality disorder (AT = 13.2%, WL = 8.3%); avoidant personality disorder (AT = 15.8%, WL = 25%); unspecified personality disorder (AT = 18.4%, WL = 27.8%); cluster B (AT = 36.8%, WL = 27.8%); cluster C (AT = 23.7%, WL = 36.1%); cluster not otherwise specified (AT = 18.4%, WL = 27,6%); 1 personality disorder (AT = 71.1%, WL = 75%); 2 or more personality disorders (AT = 23.7%, WL = 22.2%) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: art therapy
Number randomised to group: 15
Duration: 3 months Control/comparison group Comparison name: waiting list Number randomised to group: 11 Duration: 3 months Both groups Concomitant psychotherapy: not stated Concomitant pharmacotherapy: not stated Proportions of participants taking standing psychotropic medication during trial observation period: unclear |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: yes
Ethics approval: yes. Patients who agreed to participate signed the informed consent form approved by the Medical Ethics Committee of Radboud University. Comments from review authors:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: patients who agreed to participate signed the informed consent form approved by the Medical Ethics Committee of Radboud University and were assigned randomly to either the experimental or the control group, using dice (p 3). Randomisation by simple randomisation (dice). Full sample would be low risk of bias. However, due to the usage of subsample data, we do not know whether the participants are equally distributed between arms. |
| Allocation concealment (selection bias) | Unclear risk | Comment: it was unclear from the reporting who administered the randomisation and how allocation was handled. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: the outcome assessors were not blind to the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: dropout must be analysed to make sure that the results of the GLM repeated measures are not an artifact of a special type of patient quitting the intervention. There were 17 (23%) patients who quit and 57 (77%) who completed the intervention. No imputation method used for the 17 who dropped out. Full sample should be high risk of bias. We compared future dropouts with completers using observations from the pre‐test. Dropouts did not significantly differ from completers on age, dropouts M = 40.35, SD = 10.03; completers M = 36.6, SD = 10.52, t(72) = 1.31; gender, dropouts 58.8% women, completers 73.7% women, X2(1, n = 74) = 1.38; number of PD diagnoses, dropouts 76.5% 1 PD, 17.6% 2 PDs, completers 71.9% 1 PD, 24.6% 2 PDs, X2(2, n =74) = 0.49; the distribution of cluster B/C personality disorders, dropouts 41.2% B, 23.5% C, 17.6% NOS, completers 29.8% B 31.6% C, 24.6% NOS, X2(5, n = 74) = 2.92, P > 0.05; and the OQ‐45 Total score, dropouts M = 84.41, SD = 16.73, completers M = 86.33, SD = 22.46, t(72) = 0.33. These results indicate that dropouts can be considered random and thus will not bias the conclusions. (p 6‐7) |
| Selective reporting (reporting bias) | High risk | Comment: There was no mention of one outcome that would possibly have been of interest (Dutch Mental Health Continuum‐Short Form (MHC‐SF)) in the full report stated as outcomes in trial registry. The authors also failed to follow the primary and secondary outcome distinctions from the protocol in the full report. |
| Other bias | High risk |
Adherence to treatment: There was no mention of adherence check‐up. Attention bias: The intervention was compared to a waiting list control. Allegiance bias: The first author, SH, is the author of an Art Therapy book on which the protocol was based. |
Harned 2014.
| Study characteristics | ||
| Methods | 1‐year trial with 2 arms
Duration of trial: 1‐year intervention Country: USA Setting: outpatient clinic |
|
| Participants |
Method of recruitment of participants: individuals seeking treatment at the clinic, flyers and outreach to area treatment providers
Sample size: 26 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: International Personality Disorder Examination (IPDE, Loranger 1988) Mean age: 32.6 years (range = 19‐55) Sex: 100% female Comorbidity: patients had PTSD, any mood disorder, any anxiety disorder other than PTSD, any eating disorder, any substance use disorder Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: dialectical behavior therapy (DBT) + dialectical behavior therapy prolonged‐exposure (DBT‐PE)
Number randomised to group: 17
Duration: 1 year
Control/comparison group
Comparison name: dialectical behavior therapy (DBT)
Number randomised to group: 9
Duration: 1 year Both groups Concomitant psychotherapy: no Concomitant pharmacotherapy: yes, allowed. A “…minimization randomisation procedure […] was used to match participants on […] current use of SSRI medication.” (p. 10 et seq.) “The standard DBT pharmacotherapy protocol, which makes tapering off psychotropic medications a treatment goal (but not a requirement), was used for all medications except SSRIs. Given that SSRIs are an empirically supported treatment for PTSD, patients on SSRIs were asked to either taper off the medication before starting the DBT‐PE protocol or remain at a constant dose during the DBT‐PE protocol portion of the treatment. Psychotropic medications were prescribed by community (non‐study) providers.” (p 10) No difference in psychotropic medication use at baseline among groups (DBT: 87.5%, DBT + DBT‐PE: 88.2% Proportions of participants taking standing psychotropic medication during trial observation period: unclear |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: no
Ethics approval: yes. Comments from review authors:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: a minimization randomization procedure was used to match participants on five primary prognostic variables: (1) number of suicide attempts in the last year, (2) number of NSSI episodes in the last year, (3) PTSD severity, (4) dissociation and (5) current use of SSRI medication |
| Allocation concealment (selection bias) | Unclear risk | Comment: no clear information was provided on allocation concealment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: all assignments were conducted by independent clinical assessors who were blind to treatment conditions. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: analyses were based on ITT and there seemed not to be any systematic reason influencing dropout rates which were overall similar in both treatment arms. |
| Selective reporting (reporting bias) | Low risk | Comment: matched study protocol NTR3925 |
| Other bias | High risk |
Treatment adherence: the DBT adherence measure was used to code a random selection of 10% of all DBT sessions for adherence. Results indicated that on average therapists in both conditions delivered adherent DBT (M´s = 4.1, SD = 0.2, ICC = 0.99) and adherence ratings did not differ by condition (t(93) = 0.3, P = 0.80)… DBT PE sessions were also delivered with ‘Excellent’ adherence to the protocol. Allegiance bias: Linehan developed DBT and is the last author. Attention bias: DBT‐PE received more attention than DBT alone. |
Jahangard 2012.
| Study characteristics | ||
| Methods | 4‐week trial with 2 arms
Duration of trial: 4 weeks Country: Iran Setting: inpatient |
|
| Participants |
Method of recruitment of participants: inpatients at the Farshchian Psychiatric Center of Hamadan were approached.
Sample size: 30 Diagnosis of borderline personality disorder: evaluated with Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: Millon Clinical Multiaxial Inventory, 3rd edition (MCMI‐III) and a score on the subscale “Personality Disorders” of the MCMI‐III of at least 84. Base rate (cut‐off score) on the Hamilton Depression Rating Scale Mean age: 24.63 years (range = 18‐35) Sex: 53% female Comorbidity: generalised anxiety disorder (GAD), post‐traumatic stress disorder (PTSD), current substance dependence, and other affective disorders Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: emotional intelligence training
Number randomised to group: 15
Duration: 4 weeks, at least 3 sessions (45‐minute/session) per week
Concomitant psychotherapy: not stated
Concomitant pharmacotherapy: at baseline, all had antidepressants (SSRI), and 8/15 had benzodiazepines Control/comparison group Comparison name: control group Number randomised to group: 15 Duration: 4 weeks Concomitant psychotherapy: no psychoeducation, and no instructions in improving emotional intelligence or other interventions, which might be considered as a supportive psychotherapy Concomitant pharmacotherapy: “All patients were pharmacologically treated with SSRIs for depressive disorders, and, if necessary, with benzodiazepines in case of acute sleep difficulties.” (p. 199) At baseline, all had the antidepressants, selective serotonin reuptake Inhibitors (SSRIs). Proportions of participants taking standing psychotropic medication during trial observation period: all took SSRIs, benzodiazepines were taken by 87.5% of the EIT group and 66.7% of the control group (P = 0.44) |
|
| Outcomes |
Secondary
|
|
| Notes |
Sample size calculation: no
Ethics approval: yes Comments from review authors:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We used a simple sample random software: http://www.secutrial.com/blog/2012/05/21/randomisierungen‐in‐ secutrialr/". (Jahangard 2012) [pers comm] |
| Allocation concealment (selection bias) | Low risk | Quote: "patients got codes known only to the study supervisor not otherwise involved in the assessment or intervention". (Jahangard 2012) [pers comm] |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Raters of the outcome variables were unaware of patients' group allocations".(Jahangard 2012) [pers comm] |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All patients sticked on the study conditions, accordingly, we had no missings".(Jahangard 2012) [pers comm] |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcomes were identical in protocol and publication. However, in the protocol primary and secondary outcomes were stated and not in the publication. |
| Other bias | Unclear risk |
Treatment adherence: no information on treatment adherence was provided. Allegiance bias: no indication of bias Attention bias: no indication of bias Vested interest: no indication of bias |
Jochems 2015.
| Study characteristics | ||
| Methods | 12‐month trial with 2 arms
Duration of trial: 12 months Country: The Netherlands Setting: Outpatient |
|
| Participants |
Method of recruitment of participants: “Subsequently, clinicians were asked to provide their caseload to the PI, who randomly selected ten eligible patients for participation (or if fewer than ten eligible patients were available, all the eligible patients were selected). Clinicians explained to the selected patients the contents and procedure of the study and asked for participation. To enhance the likelihood of participation, patients were given an incentive of €15 for participating.“ (quote, p 3053)
Sample size: subsample = 42
Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV)
Means of assessment: “As diagnosed by the psychiatrist of the team using the Diagnostic and Statistical Manual of Mental Disorders‐Text Revision [fourth edition] criteria and obtained from the medical record” (quote, p 3050‐51)
Mean age: no data on subsample. Full sample: motivation feedback = 45.47 years (standard deviation = 10,4), TAU = 42.5 years (standard deviation = 10)
Sex: not stated
Comorbidity: no data on subsample; “Our sample largely represents a broad population of outpatients with diagnoses of psychotic and personality disorders with a variety of comorbid psychiatric disorders”. (quote, p 3061)
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: motivation feedback
Number randomised to group: subsample = 16
Duration: 12 months
Control/comparison group Comparison name: treatment‐as‐usual Number randomised to group: subsample = 26 Duration: 12 months Both groups Concomitant psychotherapy: outpatient care Concomitant pharmacotherapy: no data on subsample Proportions of participants taking standing psychotropic medication during trial observation period: unclear |
|
| Outcomes |
Primary
|
|
| Notes |
Sample size calculation: yes
Ethics approval: “This study was approved by the Medical Ethical Committee for Mental Health Care Institutions (MotivaTe‐IT; trial number NTR2968, Netherlands Trial Register, http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=2968) as well as by the scientific committees of the Western North Brabant Mental Health Center and Breburg Mental Health Center, the specialty mental health institutions where the data were collected.” (quote, p 3050)
Comments from review authors:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a computer‐generated list of random numbers was used to randomly assign each team to a treatment condition, such that all clinicians and patients in the same team were randomized to a similar treatment". (p. 3053). "The randomization sequence was created using software from www.randomization.org with a 1:1 allocation ratio using random block sizes of 1, 2, and 3". (p. 3053) |
| Allocation concealment (selection bias) | Low risk | Quote: "the random allocation sequence was performed by authors ECJ and HJD prior to approaching treatment teams, such that treatment teams and their members were still unknown and were numbered blindly before entering team numbers into the computer program". (p. 3053) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “At baseline, patients were unaware (blind) as to which treatment condition they had been randomized to. Clinicians had to be made aware of treatment condition as those randomized to MF needed to receive the necessary training prior to baseline assessments such that MF could start immediately thereafter. This blinding procedure is common in psychiatric intervention research. At the 12‐month assessment, clinicians and patients were not blind to treatment condition while filling in questionnaires, whereas independent research assistants who looked up information from the medical record and performed interviews with patients were blind to treatment allocation.” (p.3053) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: skewed dropout population of non‐ethnic Dutch participants in full sample. Only observer data used. Unclear handling of data on subsample data |
| Selective reporting (reporting bias) | Low risk | Comment: a protocol was available and the details matched those presented in the report. |
| Other bias | Unclear risk |
Adherence bias: Adherence to treatment: Patients and clinicians in the MF intervention group were asked to fill in a Short Motivation Feedback List (SMFL) every month up to 12 months after baseline assessment. (p. 3051). Clinicians were free to decide for themselves how they would structure this conversation with the patient, such as discussing only one item or several, or discussing differences between patient’s and clinician’s vision, and they were free to decide how long this would take. The duration and frequency of SMFL assessments were monitored by the research team. (p. 3051) During the course of the study, clinicians were regularly contacted by the PI to monitor the MF intervention and to discuss progress and experiences together with other colleagues who also participated in the MF intervention. These evaluation sessions took place four times over the course of the study. (p.3051) We did not seek for uniformity in TAU as such diversity reflects reality. (p.3051); although we performed evaluation sessions with clinicians about MF alongside the trial, we have limited insight into what happened during MF sessions as these were neither recorded nor supervised. (p 3061). Attention bias: There was no control on the amount of treatment received in the TAU group, but that was intended from the authors to test the real life setting of psychotherapy. Allegiance bias: no evidence of allegiance bias found |
Jørgensen 2013.
| Study characteristics | ||
| Methods | 18‐24 month trial with 2 arms
Duration of trial: 18‐24 months Country: Denmark Setting: outpatient |
|
| Participants |
Method of recruitment of participants: referred from outpatient clinics, community psychiatric wards, and psychiatrists in private practice
Sample size: 111
Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV)
Means of assessment: Structured Clinical Interview for DSM‐IV Axis II Disorders (SCID‐III) Mean age: MBT = 29.2 years (standard deviation = 6.1), supportive group = 29 years (standard deviation = 6.4) Sex: 95‐96% female Comorbidity: In the group of patients allocated to mentalisation based therapy (MBT) treatment, 53 (72%) met diagnostic criteria for depression (9 in remission at the time of assessment), 27 (37%) met criteria for anxiety disorder, 14 (19%) met criteria for an obsessive–compulsive disorder and 36 (49%) for a (previous or current) eating disorder. On Axis II, 48 (65%) met criteria for at least 1 personality disorder other than borderline and 16 (22%) for avoidant personality disorder. In the supportive therapy group, 28 (76%) met criteria for depression (11 of these were in remission at assessment), 9 (24%) met criteria for anxiety disorder, 5 (14%) for an obsessive–compulsive disorder and 14 (38%) for a (past or current) eating disorder. 32 (86%) met criteria for at least 1 other personality disorder, 10 (27%) for avoidant personality disorder Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: combined mentalisation‐based therapy (MBT)
Number randomised to group: 74
Duration: 18‐24 months Control/comparison group Comparison name: supportive group treatment Number randomised to group: 37 Duration: 18‐20 months Both groups Concomitant psychotherapy: yes, psychoeducational programme once a month for 6 months Concomitant pharmacotherapy: all participants were offered medical treatment in accordance with American Psychological Association recommendations Proportions of participants taking standing psychotropic medication during trial observation period: “…34% of patients in combined treatment and 48% of patients in supportive therapy had their medication significantly reduced or withdrawn while in treatment (difference NS, P = 0.24). Only 16% in combined and 7% in supportive therapy had their medical treatment intensified during the course of treatment (difference NS, P = 0.49).“ (p. 312) |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: yes
Ethics approval: yes Comments from review authors:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Two‐thirds (n = 74) of the 111 patients included in the study were randomized to combined treatment, while one‐third (n = 37) were offered supportive group therapy”. “The skewed randomization of patients (…), which was dictated partly by a desire on the part of the clinic’s management to offer intensive treatment to as many borderline patients as possible, and partly by available treatment resources, reduced statistical power”. (p. 307) |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Randomization was conducted by individuals outside the clinic.” "Group allocation was not concealed". (Jørgensen 2013) [pers comm] |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: “(…)GAF score was then assessed by team consensus. The team was not blind to treatment group when making these ratings as most patients were known by the team.” (p. 309) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: “Statistical power was further reduced by the relatively high attrition rate (…) owing to some patients’ refusal to complete all assessments at the assigned time points.” “Attrition from the study is relatively high (approximately 43% of included patients with intention to treat, 26% of patients starting treatment)”. “The level of attrition from the two groups was not significantly different (Fisher’s exact test, P = .79).” (p. 315) |
| Selective reporting (reporting bias) | Unclear risk | Comment: there was no study protocol available to compare with the report. |
| Other bias | High risk |
Adherence bias: "the two compared treatments were not based on detailed treatment manuals and our design did not include ongoing systematic monitoring of the two treatment modalities (adherence and competence ratings)”. (p 315) Attention bias: The combined treatment consisted of 45‐min sessions of individual psychotherapy carried out weekly over an 18‐month period and 1½‐h weekly sessions of group psychotherapy over 18–20 months (starting approximately 3 months after the individual therapy). Supportive treatment consisted of one and a half hours of supportive group therapy every fortnight. Allegiance bias: no allegiance found |
Kamalabadi 2012.
| Study characteristics | ||
| Methods | 14‐week trial with 2 arms
Duration of trial: 14‐week intervention Country: Iran Setting: outpatient |
|
| Participants |
Method of recruitment of participants: referred by psychiatrists of Hafez, Ebne Sina and Razy hospitals in Shiraz Sample size: 30 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: Borderline Personality Severity Index ‐ Fourth Version (BPDSI‐IV) Mean age: not stated Sex: 100% male Comorbidity: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: DBT‐C
Number randomised to group: 15
Duration: 14 weekly sessions Control/comparison group Comparison name: waiting list Number randomised to group: 15 Duration: 14 weekly sessions Both groups Concomitant psychotherapy: not stated Concomitant pharmacotherapy: not stated Proportions of participants taking standing psychotropic medication during trial observation period: unclear |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: not stated
Ethics approval: not stated Comments from review authors:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information on random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided on allocation concealment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no clear information provided about blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no information of how many were included and number of dropouts. ANOVA was applied but data analysis was not specified and attrition not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol found, therefore we could not assess the risk of bias due to selective reporting. |
| Other bias | High risk |
Treatment adherence: no clear information provided Allegiance bias: no indication of bias Attention bias: more attention paid to the treatment group since control group was waiting list with no intervention Vested interest: no indication of bias due to vested interests |
Koons 2001a.
| Study characteristics | ||
| Methods | 6‐months trial with 2 arms
Duration of trial: 6 months Country: USA Setting: outpatient |
|
| Participants |
Methods of recruitment of participants: not stated Sample size: 28 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 3rd Edition, Revised (DSM‐III‐R) Means of assessment: Structured Clinical Interview for DSM‐IV Axis II Disorders (SCID‐II) Mean age: 35.0 years Sex: 100% female Comorbidity: excluded patients with several different comorbid diagnoses (see below) Inclusion criteria: 1. women veterans meeting DSM‐III‐R criteria for BPD Exclusion criteria
|
|
| Interventions |
Experimental group Treatment name: DBT Number randomised to group: 14 Duration: 6 months (weekly individual therapy) Control/comparison group Comparison name: TAU Number randomised to group: 14 Duration: 6 months (weekly individual therapy) Both groups Concomitant psychotherapy: all participants received individual psychotherapy (TAU: 4 of the therapists described themselves as cognitive‐behavioural in primary orientation, 2 as psychodynamic, and 2 as eclectic). Group psychotherapy was part of DBT treatment while TAU patients were offered several group therapies at the hospital (4 out of 10 actually attended group therapy). Concomitant pharmacotherapy: all participants were offered pharmacotherapy. Proportions of participants taking standing psychotropic medication during trial observation period: “…all participants, except one in the DBT condition, received pharmacotherapy. In every case, this included an SSRI, and, for some participants, also included a mood stabiliser and/or low‐dose neuroleptic.” (p. 376) "Participants, except for 1 in the DBT condition, received pharmacotherapy, including selective serotonin reuptake inhibitors (SSRIs) in each case and/or an additional mood stabiliser or low‐dose neuroleptic in "some" cases". (quote, p 376) Pharmacotherapy and psychotherapy were provided by separate clinicians in all but 1 TAU case. |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: not stated Ethics approval: not stated Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "28 women were randomized to treatment." (p 374). No further information given |
| Allocation concealment (selection bias) | Unclear risk | Comment: no further details. 28 participants were randomised. 8 were not included in analyses due to not completing treatment (reasons: 2 did not attend the first appointment; 1 dropped out after the first appointment after realising payment was for assessments only, not attending treatment; 2 in TAU and 3 in DBT dropped out after more than one appointment in the first half of treatment citing distance from the medical centre as reason). Analyses referred to N = 10 patients in the DBT and N = 10 patients in the TAU group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Assesment interviews were conducted by two psychology interns who [...] were unaware of subjects' treatment condition." (p 376) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: analyses per protocol (EG: N = 10; CG: N = 10) |
| Selective reporting (reporting bias) | Unclear risk | Comment: no clear indication of selective reporting, but insufficient information to permit judgement of 'high' or 'low' |
| Other bias | Low risk |
Performance bias Quote: EG therapists: "All [DBT therapists] attended the weekly consultation group, and several received additional individual supervision from each other. Two clinicians received supervision briefly from a senior trainer from Linehan's group. [...] All individual and group sessions were videotaped for later coding for adherence using the DBT Expert Rating Scale [...] At the end of treatment, a sample of eight tapes from each therapist‐patient dyad, including the first session and seven others selected randomly, was coded for adherence." (quote, p 377) CG therapists: "Five [out of eight TAU] clinicians [...] received weekly supervision on their cases from attending psychiatrists or staff psychologists." (quote, p 378) Allegiance bias Comment: no indication Attention bias Comment: equal amounts of attention paid to both groups |
Kramer 2011.
| Study characteristics | ||
| Methods | 10‐week trial with 2 arms
Duration of trial: 10 weeks Country: Switzerland Setting: outpatient clinic |
|
| Participants |
Method of recruitment of participants: not stated Sample size: 25 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: Structured Clinical Interview for DSM‐IV Axis II Disorders (SCID‐II) Mean age: MOTR + TAU = 30.29 years (standard deviation = 12.43), TAU = 31.27 years (standard deviation = 8.21) Sex: 77% female Comorbidity: panic disorder, agoraphobia, alcohol abuse, major depression, bulimia, anorexia, somatoform disorder Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: MOTR + TAU
Number randomised to group: 11
Duration: 10 weeks
Concomitant psychotherapy: not stated
Concomitant pharmacotherapy: not stated Control/comparison group Comparison name: TAU Number randomised to group: 14 Duration: 10 weeks Concomitant psychotherapy: not stated Concomitant pharmacotherapy: If necessary, short‐term inpatient treatment was organised, as was adjunct pharmacotherapy Proportions of participants taking standing psychotropic medication during trial observation period: unclear |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: not stated
Ethics approval: The study was approved by the Ethics Committee of the Psychiatric Department involved. All patients gave written consent for the data to be used for research purposes. Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: randomisation was performed by blocks of 10 participants, using a computer‐based program |
| Allocation concealment (selection bias) | Low risk | Comment: The preparation of sealed envelopes containing information on the condition for each participant was done by an independent researcher. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: raters were unaware of the treatment condition. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: in case of missing values, LOCF used. The process analyses were carried out on a restricted sample of 20 patients (TAU = 10; MOTR +TAU = 10), due to missing values (related to early terminations) of 5 individuals having completed too few sessions to be taken into account. |
| Selective reporting (reporting bias) | High risk | Comment: Borderline Symptoms List reported in the protocol did not appear in the publication. |
| Other bias | High risk |
Allegiance bias Comment: Casper involved in the development of analysis plan Attention bias Comment: To counterbalance the increased time investment in condition 2, the therapists in condition 1 filled in a summary form on the patient’s symptoms and problems. |
Kramer 2014.
| Study characteristics | ||
| Methods | 10‐session trial with 2 arms (endpoint data):
Duration of trial: 10 sessions Country: Switzerland Setting: Outpatient |
|
| Participants |
Method of recruitment of participants: not stated
Sample size: 85 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: DSM‐IV diagnoses of borderline personality disorder were established by trained clinicians or clinician researchers for all patients using the Structured Clinical Interview (SCID‐II) for DSM‐IV Mean age: GPM = 30.95 years (standard deviation = 11). MOTR = 34.64 years (standard deviation = 9.97) Sex: 68.2% female Comorbidity: current DSM‐IV diagnoses; depressive disorder = 56/74, anxiety disorder = 13/74, eating disorder = 10/74, substance abuse = 54/74, intelligence limitation = 6/74, sexual disorder = 9/74, attention disorder = 4/74. Axis II cluster A = 11/74, Axis II cluster B = 23/74, Axis II cluster C = 12/74 Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: GPM
Number randomised to group: 43; analysis on 38 only
Duration: 10 sessions; no further information
Concomitant psychotherapy: not stated
Concomitant pharmacotherapy: 23/38 (61%) Control/comparison group Comparison name: MOTR Number randomised to group: 42; analysis on 36 only Duration:10 sessions; no further information Concomitant psychotherapy: not stated Concomitant pharmacotherapy: medication use at baseline: MOTR group 23/38 (61%), TAU group 21/36 (58%); χ2=.04, P = 0.84 Proportions of participants taking standing psychotropic medication during trial observation period: unclear |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: yes Ethics approval: The research protocol was approved by the local ethics board (clearance number 254/08), as well as the research committee of the university department. Comments from review authors:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: randomisation was performed using an internet‐based block randomisation program; sealed envelopes were prepared by an independent researcher and opened when the patient accepted the study (p 179). |
| Allocation concealment (selection bias) | Low risk | Comment: sealed envelopes were prepared by an independent researcher and opened when the patient accepted the study (p 179). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information regarding blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 11 participants dropped out before session two. No reasons provided. No reasons provided either for the 14 who discontinued the treatment. No demographic data on the entire sample (number of participants = 85). Imputation method used = LOCF |
| Selective reporting (reporting bias) | High risk | Comment: Working Alliance Inventory – Short Form, a self‐report questionnaire was presented in the full report but there was no mention of this outcome in protocol. |
| Other bias | High risk |
Treatment adherence Comment: adherence was assessed at the end of each of the 40 treatments (see p 180). Attention bias Comment: nothing found Allegiance bias Ueli Kramer, Franz Caspar & Martin Drapeau are all heavily linked to MOTR. Vested interest Comment: nothing found |
Kramer 2016.
| Study characteristics | ||
| Methods | 1 year trial with 2 arms:
Duration of trial: 1 year Country: Switzerland Setting: outpatient |
|
| Participants |
Method of recruitment of participants: Each recruitment wave was advertised within the psychiatry department where the study took place, in addition to broader information in the community. In order to be included in the treatment, the patients met with the programme‐related researcher for 1 to 2 screening sessions, which explained to them the study and the group treatment programme. Sample size: 41 Diagnosis of Borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: Structured Clinical Interview for DSM‐IV Axis II Disorders (SCID‐II) Mean age: DBT‐informed skills training + TAU = 35.14 years (standard deviations = 9.67), TAU = 33.60 years (standard deviations = 8.57) Sex: 87.8% female Comorbidity: current DSM‐IV diagnoses of depressive disorder, anxiety disorder, eating disorder, substance abuse, intelligence limitation, sexual disorder, attention disorder, axis II cluster A, axis II cluster B, axis II cluster C Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: DBT + TAU
Number randomised to group: 21
Duration: 1 year
Concomitant psychotherapy: Patients in both conditions received TAU, defined as individual treatment (i.e. psychotherapy and psychiatric treatment).
Concomitant pharmacotherapy: medication = 15/21 (71%) Control/comparison group Comparison name: TAU Number randomised to group: 20 Duration: 1 year Concomitant psychotherapy: Patients in both conditions received TAU, defined as individual treatment (i.e. psychotherapy and psychiatric treatment). Concomitant pharmacotherapy: “Psychopharmacological medication was available, if indicated, for all patients as part of the TAU (in both psychiatric and psychotherapeutic models; Table 1). These frequencies were not different between the conditions [at baseline] (using chi‐square statistics).“ (p.194) Medication use at baseline: DBT‐informed skills group 71%, TAU 65% (P = 0.66) Proportions of participants taking standing psychotropic medication during trial observation period: unclear |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: yes
Ethics approval: The research protocol was approved by the university and hospital research ethics board. Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: an internet‐based, block randomisation program was used for each of the 4 waves separately. |
| Allocation concealment (selection bias) | Low risk | Comment: sealed envelopes containing the allocated condition were prepared by an independent researcher and opened when a sufficient number of patients were recruited to form 2 treatment skills groups, together composing 1 recruitment wave. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: assessments and data handling were carried out mainly by 2 research assistants, with the help of a third; all blinded to participants’ treatment condition. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: in all ITT analyses a total of 41 patients were included (DBT ITT n = 21; TAU ITT n = 20); in all completer analyses, 31 patients were included (DBT completers n = 16; TAU completers n = 15); missing data resulted in the strategy of LOCF. 10 participants discontinued treatment (5 from DBT and 5 from TAU). It was not possible to collect research data for these patients at post‐treatment and follow‐up assessment points; discontinuation, if applicable, occurred between sessions 5 and 15. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available |
| Other bias | Unclear risk |
Treatment adherence Comment: treatment attrition concerned 10 (24%) patients in total, 5 (24%) for DBT and 5 (25%) for TAU. These numbers did not differ statistically (χ2(1) = 0.01, P = 0.93) and were below the average reported in the literature for treatments lasting 1 year; moment of discontinuation was not different between the groups. Due to missing questionnaires post‐treatment, the data point at the 3‐month follow‐up involved 33 observations (17 for DBT; 16 for TAU; 2 patients who had dropped out of treatment continued to fill in the questionnaires). Allegiance bias Comment: no apparent source of allegiance bias Attention bias Comment: appeared as if DBT participants were receiving more therapy time and in a more structured way. Vested interest Comment: no apparent conflicts of interest |
Kredlow 2017a.
| Study characteristics | ||
| Methods | 4‐6 month trial with 2 arms
Duration of trial: 4‐6 months Setting: outpatient clinics |
|
| Participants |
Method of recruitment of participants: orientation meetings at outpatient centers, referral from clinicians Sample size: 27 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: Structured Clinical Interview for DSM‐IV Axis II Disorders (SCID‐II) Mean age: 45.7 years (standard deviation = 9.6) Sex: 96% female Comorbidity: major depressive disorder = 67%, bipolar = 33% Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: CBT
Number randomised to group: 15
Duration: 4‐6 months
Control/comparison group
Comparison name: TAU
Number randomised to group: 12
Duration: 4‐6 months Both groups Concomitant psychotherapy: yes, from local community centers Concomitant pharmacotherapy: yes, from local community centers Proportions of participants taking standing psychotropic medication during trial observation period: unclear |
|
| Outcomes |
Primary:
Secondary
|
|
| Notes |
Sample size calculation: yes Ethics approval: yes Comments from review authors:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization was conducted at a central location in the research center by a computer based randomization program with assignments not known in advance by either clinical or research staff.” |
| Allocation concealment (selection bias) | Low risk | Quote: “Randomization was conducted at a central location in the research center by a computer based randomization program with assignments not known in advance by either clinical or research staff. When a client had completed the baseline assessment and his or her eligibility for the study was confirmed, the interviewer called the research center and a member of the research team obtained the randomized assignment from the computer. The client was informed about the assignment by the project coordinator”. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: “All assessments were conducted by Masters or Ph.D. level trained clinical interviewers who were blind to treatment assignment.”; “No specific instances of blind breaking were noted in the study”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “There were no differences between the groups on any demographic, diagnostic, or baseline measures, nor in the rates of follow‐up assessments". |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was published and so a clear judgement could not be made. |
| Other bias | High risk |
Treatment adherence Quote: “No efforts were made to control or modify any of these services provided to study participants.” Allegiance bias Comment: KT Mueser is on the Committee on Research Agenda of the Association for the Advancement of Behavior Therapy, and the Task Force on Empirically Validated Treatments of the American Psychological Association, Division 12 (www.bu.edu/sargent/files/2013/05/MueserCV.pdf). Attention bias Comment: 12‐16 sessions for EG, no information on length of TAU intervention Vested interest Comment: funded by National Institute of Mental Health |
Kredlow 2017b.
| Study characteristics | ||
| Methods | 4‐6 month trial with 2 arms
Duration of trial: 12‐16 weeks Country: USA Setting: partial hospital programmes and outpatient programmes |
|
| Participants |
Method of recruitment of participants: contacted following administration of trauma and PTSD screening at 5 clinic sites Sample size: 55 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: Structured Clinical Interview for DSM‐IV Axis II Disorders (SCID‐II) Mean age: 40.4 years (standard deviation = 9.5) Sex: 78.2% female Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: CBT
Number randomised to group: 29
Duration: 12‐16 weeks
Control/comparison group
Comparison name: brief treatment programme
Number randomised to group: 26
Duration: 3 sessions Both groups Concomitant psychotherapy: all continued to receive case management/usual psychiatric services (see p 502). Concomitant pharmacotherapy: all continued to receive pharmacological treatment. |
|
| Outcomes |
Primary:
Secondary
|
|
| Notes |
Sample size calculation: yes Ethics approval: yes Comments from review authors:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization was conducted at a central location in the research center by a computer based randomization program with assignments not known in advance by either clinical or research staff.” |
| Allocation concealment (selection bias) | Low risk | Quotes: “Randomization was conducted at a central location in the research center by a computer based randomization program with assignments not known in advance by either clinical or research staff". "When a client had completed the baseline assessment and his or her eligibility for the study was confirmed, the interviewer called the research center and a member of the research team obtained the randomized assignment from the computer. The client was informed about the assignment by the project coordinator”. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotea: “All assessments were conducted by Masters or Ph.D. level trained clinical interviewers who were blind to treatment assignment.”. “No specific instances of blind breaking were noted in the study”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “There were no differences between the groups on any demographic, diagnostic, or baseline measures, nor in the rates of follow‐up assessments". |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol published |
| Other bias | High risk |
Treatment adherence Quote: “No efforts were made to control or modify any of these services provided to study participants.” Allegiance bias Comment: KT Mueser is on the Committee on Research Agenda of the Association for the Advancement of Behavior Therapy, and the Task Force on Empirically Validated Treatments of the American Psychological Association, Division 12 (www.bu.edu/sargent/files/2013/05/MueserCV.pdf). Attention bias Comment: 12‐16 sessions for EG, no information on length of TAU intervention Vested interest Comment: funded by National Institute of Mental Health |
Laurenssen 2018.
| Study characteristics | ||
| Methods | 6‐month (on average) trial with 2 arms
Duration of trial: 6 months, on average Country: The Netherlands Setting: inpatient and outpatient |
|
| Participants |
Method of recruitment of participants: 2 mental healthcare centres, both located in Amsterdam, agreed to participate in this study. The City Crisis Service agreed to run the S‐TAU condition. From March 2009 to July 2012, patients were referred to 1 of the 2 mental healthcare centers in Amsterdam.
Sample size: 95 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: Structured Clinical Interview for DSM‐IV Axis I Personality Disorders (SCID‐I), and a total score on the Borderline Personality Disorder Severity Index (BPDSI) of at least 20, reflecting severe borderline personality disorder Mean age: not stated Sex: 79% female Comorbidity: avoidant personality disorder, antisocial personality disorder, paranoid personality disorder, obsessive‐compulsive personality disorder, dependent personality disorder Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: MBT‐DH
Number randomised to group: 54
Duration: The mean number of days was 176 (range = 5–402, median = 149). The mean number of hours in MBT‐DH was 1056 (range = 30–2412, median = 894).
Control/comparison group
Comparison name: S‐TAU
Number randomised to group: 41
Duration: The mean number of hours was 1473 (range = 5–13099, median = 131). Both groups Concomitant psychotherapy: not stated Concomitant pharmacotherapy: Patients could also consult a psychiatrist upon request and medication was prescribed following American Psychiatric Association guidelines (APA 2000). Proportionsof participants taking standing psychotropic medication during trial observation period: unclear |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: yes
Ethics approval: yes, from the Medical Ethics Review Committee of the University of Rotterdam Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: patients agreeing to participate were randomly assigned to either MBT‐DH or S‐TAU using block randomisation taking into account the availability of treatment programs. For this reason, the randomisation was slightly skewed in favor of MBT‐ DH, because the new MBT‐DH groups needed to be filled. (p 2) |
| Allocation concealment (selection bias) | Low risk | Comment: randomisation was done by an independent researcher, away from the site, using a computer algorithm. (p 2) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: research assistants were psychologists with an MSc degree, and were blind for treatment condition. (p 2) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: large attrition rates. Significantly differing rates after baseline. Unclear reasons for attrition. ITT analyses conducted. Multiple imputations conducted, but because imputed and non‐imputed data were similar, imputed data were not reported. |
| Selective reporting (reporting bias) | High risk |
Comment: self‐harm, suicidal behaviour, subjective experiences of symptoms, personality functioning and treatment adherence listed as secondary outcomes in trial registry, but not reported on in study. Quote: "Preliminary screening of the data showed substantial differences in the administration of the SSHI between research assistants, and therefore we decided not to report data using this measure." (p 3, left column) |
| Other bias | High risk |
Adherence to treatment
Comment: adherence was not rated Attention bias Comment: differences in total hourly exposure between groups (mean 1473 versus 1056 hours) Allegiance bias Comment: Patrick Luyten has been involved in the training and dissemination of mentalisation‐based treatments. The other authors declared no competing interests. (see p 7) |
Leichsenring 2016.
| Study characteristics | ||
| Methods | 2‐3 month (approximately) trial with 3 arms
Duration of trial: mean = 77 to 107 days. PIT = 106.7 mean days (standard deviation = 41.71), E‐PDT = and 76.78 mean days (standard deviation = 21.07) Country: Germany Setting: inpatient, Asklepios Clinic, Tiefenbrunn |
|
| Participants |
Method of recruitment of participants: “Patients who applied for treatment and who had been given the presumptive diagnosis of a cluster B personality disorder by the referring clinician were asked to participate in the study.” (quote, p 72)
Sample size: 122 Diagnosis of borderline personality disorder: International Statistical Classification of Diseases and Related Health Problems, 10th version (ICD‐10) Means of assessment: Structured Clinical Interview for DSM‐IV Axis II Disorders (SCID‐II) Mean age: PIT = 28.63 years (standard deviations = 8.71), E‐PDT = 30.43 years (standard deviation = 9.05) Sex: 69% female Comorbidity: avoidant personality disorder in 14 patients (11%), histrionic personality disorder in 2 patients (2%), and narcisstic personality disorder in 28 patients (23%), assessed by SCID‐II. Multiple diagnoses for cluster B personality disorders were possible. Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: PIT
Number randomised to group: 64
Duration: 106.7 mean days (standard deviation = 41.71)
Control/comparison group
Comparison name: E‐PDT
Number randomised to group: 58
Duration: 76.78 mean days (standard deviation = 21.07) Both groups Concomitant psychotherapy: none (inpatient) Concomitant pharmacotherapy: allowed Proportions of participants taking standing psychotropic medication during trial observation period: “In the PIT group, 37.5% received an antidepressive medication (selective serotonin reuptake inhibitors, noradrenalin reuptake inhibitors, or tricyclic antidepressants) as compared with 46.1% in the E‐ PDT group. The difference was not significant (p = 0.311). This was also true for neuroleptics (50 vs. 60.3%, p = 0.251) and anxiolytics (4.7 vs. 5.2%, p = 0.902). In total, 76% of the PIT patients and 79.3% of the E‐ PDT patients received temporarily some form of pharmacotherapy (p = 0.715). Thus, the two treatments did not differ with regard to the applied pharmacotherapy.” (quote, p 76) |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: yes
Ethics approval: yes Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: patients fulfilling the inclusion criteria were randomised to either PIT or E‐PDT by use of a computer‐ generated randomisation list generated by two of the authors (UJ, OM). Randomisation was stratified for sex. (p 74) |
| Allocation concealment (selection bias) | Low risk | Comment: patients were randomised to treatment by use of a random function in Microsoft Excel. This was done by two of the authors (OM and UJ) not involved in any diagnostic assessments pre or post‐therapy (third party assignment). Thus, the diagnosticians assessing and enrolling patients were unaware of the allocation sequence. (email correspondence 28 September 2018) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: 7 specifically trained and independent assessors conducted the interviews. (p 74) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: for ITT analysis, applied multiple imputation by chained equations to account for the uncertainty resulting from missing outcomes. To generate conservative estimates, 50 imputations were created and all available variables were included in the imputation process. (p 74) |
| Selective reporting (reporting bias) | High risk | Comment: Beck Anxiety Inventory was mentioned in protocol, but was not included in full report. |
| Other bias | High risk |
Adherence to treatment Comment: 2 independent masked raters were trained in both PIT and the use of the checklist by one of the developers of PIT (US). They independently rated the 30 videotapes. Having seen the videos, they rated (1) whether PIT or E‐PDT was applied and (2) the overall therapist’s competence in applying principles of PIT. For the latter, a 4‐point Likert rating scale was used comparable to the overall competence rating scale of the Penn Adherence and Competence Scale. (p 75) Attention bias Comment: large difference in duration in the 2 groups. Mean treatment duration was 106.70 days (SD = 41.71) for PIT and 76.78 days (SD = 21.07) for E‐PDT. As this difference was statistically significant (P < 0.0001), treatment duration was included as a covariate in the statistical analysis. (p 75) Allegiance bias Comment: last author, Ulrich Streeck, developed PIT |
Leppänen 2016.
| Study characteristics | ||
| Methods | 1‐year trial with 2 arms
Duration of trial: 1 year Country: Finland Setting: social and health services, including mental services |
|
| Participants |
Method of recruitment of participants: convenience sampling method
Sample size: 71 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: Structured Clinical Interview for DSM‐IV Axis II Disorders (SCID‐II) Mean age: CTBE = 31.9 years (standard deviation = 8.3), TAU = 32.3 years (standard deviation = 8.8) Sex: 85.9% female Comorbidity: not stated Inclusion criteria
Exclusion criteria
Axis I disorders were diagnosed according to Structured Clinical Interview for DSM‐IV Axis I Disorders (SCID‐I) , and the presence of neuropsychiatric disorder and substance abuse was assessed by a clinician. |
|
| Interventions |
Experimental group
Treatment name: CTBE
Number randomised to group: 24
Duration: 1 year (see p 218)
Concomitant psychotherapy: not stated
Concomitant pharmacotherapy: not stated Control/comparison group Comparison name: TAU Number randomised to group: 47 Duration: approximately 1 year Concomitant psychotherapy: not stated Concomitant pharmacotherapy: not stated Proportions of participants taking standing psychotropic medication during trial observation period: unclear |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: not stated
Ethics approval: The Ethics Committee of Oulu University Hospital approved the study (18 June 2009, number: 41/2009). Comments from review authors:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Comment: somewhat unclear, but seems likely that they are referring to a random number table. Quote: “The randomization list was prepared using appropriate statistical methods by a person who had no contact with the patients.” (p 218) |
| Allocation concealment (selection bias) | Unclear risk |
Quote: “The randomization list was prepared using appropriate statistical methods by a person who had no contact with the patients.” (p 218) Comment: The person mentioned (s)he had no contact with patients, but could potentially have had contact with the researchers, enabling them to foresee assignments. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: measure 1: “BPDSI‐IV interviews were blinded and conducted by three interviewers: two psychiatric nurses and a physician.” (p 221); measure 2: “The 15D questionnaires were posted to patients, who were asked to return the completed questionnaire by pre‐paid post.” (p 221) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: higher attrition in TAU than CTBE – imbalance in numbers and reasons for dropout. 31.9% attrition in TAU may have affected effect size estimates in the continuous outcome measures. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol found |
| Other bias | High risk |
Treatment adherence Comment: They did not mention it in the article but did state supervision in the active group (see p 219). Some may have considered this good but we do not think so, especially because it was not videotaped or manualised/rated. Attention bias Comment: Lack of attention to comorbidity and reporting of it. And use of convenience sampling. Potential type 1 and 2 errors Quote: “During the year long intervention, the CTBE patients made, on average, 73 visits to Oulu mental health services compared to an average of only 21 visits in TAU patients. Therefore, the possibility of nonspecific treatment effects may also exist”. (p 228) Other risk of bias Quote: “The likelihood for Type I and Type II errors cannot be excluded, since many statistical comparisons were performed and the subsamples in some statistical analyses may have been too small, thus reducing the statistical power and likelihood of revealing truly significant findings. We considered correcting for multiple comparisons but, due to our limited sample size, such corrections were considered to be rather artificial.” (p 226) |
Lin 2019.
| Study characteristics | ||
| Methods | 8‐week trial with 2 arms:
Duration of trial: 8 weeks Country: Taiwan Setting: outpatient |
|
| Participants |
Method of recruitment of participants: “A total of 256 college students recruited from two university counseling centers (UCC) in southern Taiwan participated in this study.” (quote, p 3)
Sample size: 82 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: Structured Clinical Interview for DSM‐IV Axis II Disorders (SCID‐II) Mean age: DBTSTG = 20.40 years (standard deviation = 0.76), CTG = 20.47 years (standard deviation = 0.71) Sex: 87.8% female Comorbidity: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: DBTSTG
Number randomised to group: 42
Duration: 8 weeks Control/comparison group Comparison name: CTG Number randomised to group: 40 Duration: 8 weeks Concomitant psychotherapy: not stated Concomitant pharmacotherapy: none Proportions of participants taking standing psychotropic medication during trial observation period: "No participants were currently receiving psychotropic medications." (p. 4) |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: yes
Ethics approval: yes Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Following the completion of the intake assessments, eligible participants were referred by counseling centers based on freshman screening during 2009–2014, and were randomly assigned to either the CTG or the DBTSTG for intervention using a computerized randomization procedure with a maximum of 9 cases in one group.” (p 86) |
| Allocation concealment (selection bias) | Unclear risk | Comment: not enough clear information provided in order for a judgement to be made |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: “Blinded assessments were conducted at the baseline and follow‐up; however, blinded assessments during intervention were not possible since the assessment of a suicide attempt involved an evaluation of the circumstances preceding a suicide attempt and the use of mental health services during post‐attempt, which was essential for clinical management.” (p 87) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Quotes: “Generalized linear model analyses were performed to compare participants who were included vs. those who were not included by group (DBTSTG vs. CTG) on the demographic and outcome variables at baseline. Intent‐ to‐treat analyses were conducted to aid in the interpretation of findings by including all those who started treatment but dropped out.” (p 89) "Sixty‐eight participants, including 36 (85.7%) in DBTSTG and 32 (80.0%) in CTG, completed the intervention. There were no intergroup differences in dropout rates (DBTSTG: 14.28%; CTG: 20.00%)....Further comparisons between participants who completed (n = 68) and non‐completed (n = 14) across intervention groups on gender were not significant (χ2 = .75, p = .85; χ2 = .42, p = .47). Moreover, the effects of the sample (included vs. non‐included) x group (DBTSTG vs. CTG) on age, depression and antecedent and response‐focused emotion regulation measures at baseline did not reach significant differences (age: F = .01 p = .69; depression: F = .01 p = .986; cognitive errors: F = .21, p = .74; attentional deployment: F = .02, p = .89, cognitive reappraisal: F = 1.51, p = .219; suppression: F = .75, P = .38 acceptance: F = 3.00, p = .08).” (p 89) |
| Selective reporting (reporting bias) | High risk | Comment: protocol available; data from the Emotion Regulation Scale and Symptom Check List ‐ 90 ‐ Revised not reported in full report |
| Other bias | Unclear risk |
Attention bias Comment: nothing found Treatment adherence Comment: no ratings Quote: "Although each session in both conditions was guided by an intervention manual, and the therapist was required to strictly adhere to the intervention manual and rate the checklists for adherence to the CGT or DSTG manual in an attempt to reduce treatment diffusion, future studies should include independent adherence ratings by objective observers". (p 95) Allegiance bias Comment: no evidence of allegiance bias found |
Linehan 1991.
| Study characteristics | ||
| Methods | 12‐month trial with 2 arms
Duration of trial: 12 months Country: USA Setting: outpatient |
|
| Participants |
Methods of recruitment of participants: not stated Sample size: 62 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 3rd Edition (DSM‐III) Means of assessment: Revised Diagnostic Interview for Borderlines (DIB‐R) Age: not stated Sex: 100% female Comorbidity: not stated Inclusion criteria:
Exclusion criteria
|
|
| Interventions |
Experimental group Treatment name: DBT Number randomised to group: 2 Duration: 12 months (weekly individual therapy, weekly group therapy, telephone contact with the individual therapist between sessions) Control/comparison group Comparison name: TAU Number randomised to group: 22 Duration: 12 months Both groups Concomitant psychotherapy: 13 out of 22 TAU participants were in ongoing individual psychotherapy at pretreatment, 9 out of 22 TAU participants had stable individual therapy for the year. Concomitant pharmacotherapy: "Subjects had to consent to taper off psychotropic medications before entering the study. However, once in the study, failure to terminate or resuming use of medication was not cause for removal from the study." (quote, p 1061) Proportionsof participants taking standing psychotropic medication during trial observation period: unclear |
|
| Outcomes |
Primary
|
|
| Notes |
Sample size calculation: not stated Ethics approval: not stated Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were matched on the number of lifetime parasuicides and psychiatric hospitalization, age, and good vs poor clinical prognosis (with a subthreshold diagnosis on schizophrenia or substance dependence constituting poor prognosis) and randomly assigned to a treatment condition." (p 1061) |
| Allocation concealment (selection bias) | Unclear risk | Comment: no clear details provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Screening and assessment interviews were administered by a team of 13 research assessors. Every effort was made to keep the assessors blind about treatment condition." ( p 1061) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 10 dropped out during pretreatment assessment (EG: N = 5, CG: N = 5). 7 were dropped following pretreatment assessment for refusal or inability to meet study conditions (EG: N = 3, CG: N = 4). 2 EG participants quit the study with 4 or fewer DBT sessions and were dropped from all analyses other than treatment maintenance analyses. Major analyses were conducted for 44 participants, N = 22 in EG and N = 22 in CG treatment. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no indication for selective reporting, but Insufficient information to permit judgement of 'high' or 'low' risk of bias |
| Other bias | Unclear risk | Comment: no further details |
Linehan 1994.
| Study characteristics | ||
| Methods | 12‐months trial with 2 arms
Duration of trial: 12 months Country: USA Setting: outpatient |
|
| Participants |
Methods of recruitment of participants: not stated Sample size: 26 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 3rd Edition (DSM‐III) Means of assessment: Structured Clinical Interview for DSM‐IV (SCID), and Revised Diagnostic Interview for Borderlines (DIB‐R) Mean age: 26.7 years (standard deviation = 7.8) Sex: 100% female Comorbidity: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group Treatment name: DBT Number randomised to group: 13 Duration: 12 months (weekly individual behavioural psychotherapy, weekly psychoeducational skills training groups) Control/comparison group Comparison name: TAU; participants received alternative therapy referrals and were allowed to participate in any type of treatment available in the community Number randomised to group: 13 Duration: 12 months (weekly individual behavioural psychotherapy, weekly psychoeducational skills training groups) Both groups Concomitant psychotherapy: patients assigned to DBT treatment had to terminate other professional mental healthcare. Concomitant pharmacotherapy: no between‐group differences in number of participants using psychotropic medications at pretreatment (use of: antidepressants, anticonvulsants, lithium, anxiolytics) Proportions of participants taking standing psychotropic medication during trial observation period: DBT participants should have tapered off psychotropic medications as 1 goal of therapy, and 8 out of 13 discontinued medication before start of treatment. The remaining 5 DBT participants reported using a mean of 1.80 medications (sedatives, antidepressants, anxiolytics, lithium) over the treatment year, while 9 out of 13 TAU participants reported using a mean of 3.89 different medications (antidepressants, anxiolytics, neuroleptics, sedatives, anticonvulsants). |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: not stated Ethics approval: not stated Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quotes: "assignment of subjects to treatment conditions [...] described in detail in the original outcome study [i.e. Linehan 1991]" (p 1772). "Subjects were matched on the number of lifetime parasuicides and psychiatric hospitalization, age, and good vs poor clinical prognosis (with a subthreshold diagnosis on schizophrenia or substance dependence constituting poor prognosis) and randomly assigned to a treatment condition." (Linehan 1991, p 1061) Comment: no further details |
| Allocation concealment (selection bias) | Unclear risk | Comments: no further details |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Interviews blind to treatment conditions" (p 1772) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: analyses were conducted on a per protocol basis. 26 women included, data set for 26 participants (DBT: N = 13, TAU: N = 13) provided |
| Selective reporting (reporting bias) | Unclear risk | Comment: no indication of selective reporting, but insufficient information to permit judgement of 'high' or 'low' risk of bias |
| Other bias | High risk |
Performance bias Comment: no details provided to indicate if supervision or adherence ratings (or both) had been conducted. However, the same study design was used as for Linehan 1991 ("two cohorts", cf. Linehan 1994, p 1772), where regular supervision was explicitely defined (cf. Characteristics of included studies, Risk of bias table for Linehan 1991). Allegiance bias Comment: "study was conducted at the institution where the treatment was developed." (p 1775) Attention bias Comment: more attention paid to DBT group |
Linehan 2006.
| Study characteristics | ||
| Methods | 12‐month trial with 2 arms
Duration of trial: 12 months Country: USA Setting: outpatient |
|
| Participants |
Method of recruitment of participants: not stated Sample size: 101 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: Structured Clinical Interview for DSM‐IV Axis II Disorders (SCID‐II) and International personality disorder examination (IPDE) Mean age: 29.3 years (standard deviation = 7.5) Sex: 100% female Comorbidity: Current psychiatric diagnoses (DSM‐IV): Major depressive disorder 72.3%, panic disorder 40.6%, PTSD 49.5%, any anxiety disorder 78.2%, any substance use disorder 29.7%, any eating disorder 23.8%, Cluster A PD 1.3%, Cluster B other than BPD 10.9%, Cluster C PD 25.7%. Prevalence rates did not differ significantly between the two treatment groups. Inclusion criteria: not stated Exclusion criteria
|
|
| Interventions |
Experimental group Treatment name: DBT Number randomised to group: 52 Duration: 12 months (weekly individual psychotherapy, group skills training, telephone consultation) Control/comparison group Comparison name: CTBE Number randomised to group: 49 Duration: 12 months Both groups Concomitant psychotherapy: no information given regarding further concomitant psychotherapy Concomitant pharmacotherapy: There were no differences in the types or amounts of psychotropic medication used at pretreatment. Proportions of participants taking standing psychotropic medication during trial observation period: exact proportions unclear. The use of psychotropic medications decreased significantly more in the DBT than the CTBE group during the treatment year. |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: yes Ethics approval: not stated Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quotes: "Using a computerized adaptive minimization randomization procedure, eligible subjects were matched to treatment condition on 5 primary prognostic variables: (1 and 2) the number of lifetime suicide attempts or nonsuicidal self‐injuries combined and psychiatric hospitalizations; (3) a history of only suicide attempts, only nonsuicidal self‐injury, or both; (4) age; and (5) a negative prognostic indicator of a Beck Depression Inventory score higher than 30 or a Global Assessment of Functioning score lower than 45 for a comorbid condition [...] Based on 0.8 power to detect significant differences between conditions (P = .05, 1‐sided), this procedure was used to randomize 101 subjects to DBT (n = 52) or to CTBE (n = 49)." ( p 758)." "The randomization program assigned clients to DBT and CTBE therapists, matching on sex, doctoral vs master's training, and years of clinical experience. Results indicated that therapists' sex and training did not differ in the 2 conditions. The CTBE therapists, however, had more clinical experience, which was expected because they were selected for their expertise." (p 760) |
| Allocation concealment (selection bias) | Low risk |
Quote: "The participant coordinator, who was not blinded to treatment condition, executed the randomization program". (p 758) Comment: improbable that computerised assignment could be foreseen and thus bias be introduced |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Assessments were conducted by blinded independent clinical assessors". (p 758) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: analyses were conducted on an ITT basis. 101 participants randomised, and 60 allocated to the EG and 51 to the CG arms. 8 DBT "training cases" and 2 CBT "pilot cases" excluded from analyses, but the remaining 52 EG and 49 CG participants were analysed regardless of discontinuation or getting lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no clear indication of selective reporting, but there was insufficient information to permit a judgement of 'high' or 'low' risk of bias |
| Other bias | High risk |
Performance bias Quote: "Psychotherapists recommended by colleagues as potentially good DBT therapists were recruited for the study; 8 had no previous DBT exposure and 8 had experience that ranged from workshop attendance to applied practice. [...] Training consisted of a 45‐hour DBT seminar followed by supervised practice. [...] Individual therapists were hired once 6 of 8 consecutive training case sessions were rated as adherent to DBT. During the study, adherence was assessed by coding a random selection of sessions on the DBT Global Rating Scale [...] which codes DBT adherence." (p 759) Allegiance bias Comment: the primary author (MLL) is developer of DBT Attention bias Comment: equal amounts of attention spent to both groups Vested interest Comment: first author is the developer of Dialectical Behavioural Therapy (DBT) – Source: linehaninstitute.org/about/organizations |
Linehan 2015a.
| Study characteristics | ||
| Methods | 49‐week trial with 3 arms
Duration of trial: 49 weeks Country: USA Setting: university clinic and community setting |
|
| Participants |
Method of recruitment of participants: outreach to healthcare practitioners Sample size: 99 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: Structured Clinical Interview for DSM‐IV Axis II Disorders (SCID‐II) and International personality disorder examination (IPDE) Mean age: 30.3 years (range = 18‐60) Sex: 100% female Comorbidity: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: DBT
Number randomised to group: 33
Duration: 52 weeks Control/comparison group 1 Comparison name: DBT‐I Number randomised to group: 33 Duration: 49 weeks Control/comparison group 2 Comparison name: DBT‐S Number randomised to group: 33 Duration: 50 weeks All groups: Concomitant psychotherapy: not stated Concomitant pharmacotherapy: psychotropic medication allowed Proportions of participants taking standing psychotropic medication during trial observation period: exact proportions unclear, but no between‐group differences in use of psychotropic medications |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample calculation: yes
Ethics approval: yes Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “A computerized adaptive randomization procedure (5) matched participants on age, number of suicide attempts, number of NSSI episodes, psychiatric hospitalizations in the past year, and depression severity”. (p 476) |
| Allocation concealment (selection bias) | Unclear risk | Quote: “The participant coordinator, who was not blinded to the treatment condition, executed the randomization and collected treatment‐related data”. (p 476) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “Assessments were conducted before treatment and quarterly during 1 year of treatment and 1 year of follow‐up by blinded independent assessors trained by instrument developers or approved trainers (including K.A.C. and A.M.M.‐G.) and evaluated as reliable for each instrument.” (p 476) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no information on ITT, but all randomised participants were included in analysis, so ITT was likely used. No imputation methods seem to have been used. 26/99 randomised were lost at follow‐up. No differences in rate of dropouts between arms, no evidence that group differences in missing data biased major outcome variables |
| Selective reporting (reporting bias) | High risk | Comment: protocol lists 'coping skills' as a secondary outcome. It was not included in study. 'Reasons for living' and depression and anxiety outcome measures were included in the paper. These were not listed in the protocol. Neither were they mentioned in the paper as post hoc analyses |
| Other bias | High risk |
Treatment adherence Comment: treatment adherence differed significantly between 2 out of 3 groups Adherence bias Comment: first author is the developer of Dialectical Behavioural Therapy (DBT), see http://www.linehaninstitute.org/ab out‐Linehan.php Attention bias Quote: “Participants in standard DBT received significantly more individual sessions than those in DBT‐S owing to weekly sessions in standard DBT and as‐needed sessions in DBT‐S. Participants in standard DBT and DBT‐S received more group therapy sessions than those in DBT‐I owing to the optional nature of group therapy in DBT‐I. Participants in standard DBT attended more groups than those in DBT‐S owing to trend‐level differences in treatment retention.” (p 477) |
McMain 2009.
| Study characteristics | ||
| Methods | 12‐months trial with 2 arms
Duration of trial: 12 months Country: Canada Setting: outpatient |
|
| Participants |
Methods of recruitment of participants: not stated Sample size: 190 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: International Personality Disorder Examination (IPDE) Mean age: 30.4 years (standard deviation = 9.9) Sex: 86.8% female Comorbidity: exclusion of several disorders Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group Treatment name: DBT Number randomised to group: 90 Duration: 12 months (individual sessions 1‐hour weekly; skills group 2 hours weekly; phone coaching 2 hours weekly; consultation team for therapists 2 hours weekly) Control/comparison group Comparison name: general psychiatric management according to American Psychological Association (APA) guideline recommendations Number randomised to group: 90 Duration: 12 months (individual sessions, 1‐hour weekly, including management based on structured drug algorithm, and therapist supervision meeting 90 minutes weekly) Both groups Concomitant psychotherapy: non‐study treatments, such as individual, group, case management, day or inpatient treatment were recorded but participants were not prevented from using them. Concomitant pharmacotherapy: At baseline, 145 patients (81.6%) reported that they were taking psychotropic medications (mean number of medications, 3.08 [SD = 1.64]). Proportions of participants taking standing psychotropic medication during trial observation period: Exact proportions unclear; during treatment, patients in dialectical behavior therapy averaged 1.84 (SD = 1.44) medications, and those in general psychiatric management 2.09 (SD = 1.65), with no significant difference between groups. |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calcuation: yes Ethics approval: The protocol was approved by each centre’s research ethics board, and patients provided written informed consent prior to enrollment. Under the Canadia public healthcare system, participants did not pay for treatment. Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "eligible participants were randomly assigned to treatment arms using a pregenerated block randomization scheme developed and held by the statistician." (p 1366) |
| Allocation concealment (selection bias) | Low risk | Quote: "[...] statistician, who prepared 45 sealed envelopes, each containing the group allocations in random order for four participants." (p 1366) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "[...] assessors who were well trained on study instruments and blind to treatment assignment. [...] Assessors were polled after the treatment phase to ascertain whether they could correctly guess participants' treatment assignment; they did not know treatment assignment for 86% of the cases, suggesting that blinding was largely maintained." (p 1366) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no further details |
| Selective reporting (reporting bias) | Low risk | Comment: study protocol available (NCT00154154). No indication of selective reporting |
| Other bias | Unclear risk | Comment: no further details |
McMain 2017.
| Study characteristics | ||
| Methods | 20‐week trial with 2 arms
Duration of trial: 20 weeks Country: Canada Setting: outpatient |
|
| Participants |
Method of recruitment of participants: not stated Sample size: 84 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: International Personality Disorder examination (IPDE) Mean age: 29.67 years (standard deviation = 8.62) Sex: 78.6% female Comorbidity: current DSM‐IV axis I and axis II diagnoses: major depressive disorder, panic disorder, post‐traumatic stress disorder (PTSD), any anxiety disorders, substance abuse, substance dependence, any eating disorder Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: DBT skills training group
Number randomised to group: 42
Duration: 20 weeks
Control/comparison group
Comparison name: waiting list
Number randomised to group: 42
Duration: 20 weeks Both groups Concomitant psychotherapy: "At baseline, a total of 71 patients (85%) reported that they were receiving some form of psychosocial treatment from a therapist. During their period on wait list, participants could continue with treatment as usual (medication management or other psychosocial treatments)". (quote, p 140) Concomitant pharmacotherapy: "At baseline, a total of 71 patients (86%) were taking psychotropic medications, with a mean of 1.79 ± 1.41 medications per participant. There were no significant between‐group differences in either the number of patients on medication (DBT = 33/42; WL = 38/42; v2(1) = 1.67, P = 0.20) or the mean number of medications taken (DBT = 1.52 ± 0.20; WL = 2.05 ± 0.22; t(80) = 1.73, P > 0.05)." Proportions of participants taking standing psychotropic medication during trial observation period: "At 20 weeks, a total of 57 patients (81%) were taking medication and were averaging 1.62 ± 1.67 medications, with the DBT group reporting both fewer patients on medication (23/32) compared to the WL patients (34/38) and fewer medications (1.52 ± 0.20 vs. 2.05 ± 0.22, t(80) = 2.10, P = 0.04). There was no significant group difference in the average number of medications at the 32‐week follow‐up (t(80) = 0.53, P = 0.60)“. (p. 142) |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: yes
Ethics approval: yes. The study was approved by the CAMH Research Ethics Board. Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: participants were assigned to groups using a standard random block design in block sizes of four. |
| Allocation concealment (selection bias) | Low risk | Comment: statistician prepared 42 envelopes, each containing 2 allocations to each of the conditions in random order. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: participants were assessed by 2 doctoral‐level psychology students and 1 master’s‐level clinician who were well trained on the study instruments and were blinded to treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Comment: all analyses were conducted on the ITT sample (N = 84). Low attrition rate (5 in DBT, 3 on waiting list; reasons for dropout were stated) Quote: “There was no evidence that missing data patterns were biased by group membership”. (p 142) |
| Selective reporting (reporting bias) | Low risk | Comment: protocol published. All outcomes mentioned in the publication except the Borderline Evaluation of Severity over time Scale (secondary outcome), which seems to have been changed to the Borderline Symptom List ‐ 23. Some other outcomes have been added in the publication as well (Beck Depression Inventory ‐ II, the Social Adjustment Scale ‐ Self‐report, the Difficulties in Emotion Regulation Scale, the Distress Tolerance Scale and the Kentucky Inventory of Mindfulness Scale). |
| Other bias | Unclear risk |
Treatment adherence Comment: Treatment adherence ratings were conducted on 10% (n = 22) of sessions. The mean score of 4.44 (SD = 0.11) fell within the ‘adherent’ range. Attention bias Comment: comparator intervention was waiting list. Allegiance bias Comment: authors reported no conflict of interests in relationship to this study. Vested interest Comment: no indication of bias |
McMurran 2016.
| Study characteristics | ||
| Methods | 12‐week trial with 2 arms
Duration of trial: 12 weeks Country: UK Setting: community (outpatient) |
|
| Participants |
Methods of recruitment of participants: referred to PEPS team. Participants were recruited from mental health services in 3 UK NHS trusts.
Sample size: subsample = 183 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: International personality disorder examination (IPDE) Mean age: no data on subsample. Full sample for PEPS therapy = 38.6 years (standard deviation = 10.9), full sample for TAU = 37.8 years (standard deviation = 11) Sex: approximately 75% female Comorbidity: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: PEPS therapy, internet‐based control group with no psychoeducation + TAU
Number randomised to group: 93
Duration: 12 weeks Control/comparison group Comparison name: TAU Number randomised to group: 90 Duration: 12 weeks Both groups Concomitant psychotherapy: not stated Concomitant pharmacotherapy: allowed Proportions of participants taking standing psychotropic medication during trial observation period: unclear |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: yes Ethics approval: yes Comments from review authors:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was based on a computer generated pseudo‐random code using random permuted blocks of randomly varying size created by the Nottingham Clinical Trials Unit [NCTU] in accordance with their standard operating procedure and held on a secure server. Allocation was stratified by recruiting centre and sex." (p xxiv) |
| Allocation concealment (selection bias) | Low risk | Quote: "The sequence of treatment allocations was concealed until recruitment, data collection, and all other trial‐related assessments were complete. The investigator, or an authorised designee, accessed the treatment allocation for each participant by means of a remote, internet‐based randomisation system developed and maintained by the NCTU. Allocation was therefore fully concealed from recruiting staff." (p 14) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "At the start of each follow‐up, participants were reminded of the importance of not disclosing their treatment allocation to the research assistant using a suggested unblinding script (See Appendix 9). If the research assistant was inadvertently unblinded to treatment allocation before completing the final follow‐up, a record of the incident of unblinding was made. Researchers also reported whether or not they were aware of the treatment allocation at the time of completing the primary end‐point assessments. Owing to changes in personnel over the course of the trial, in some cases, end‐point assessments were conducted by researchers who were not unblinded. A record was made of the blinding status of the researcher conducting the final follow‐up data collection." (p 16). "Data analysts remained blinded to allocation during the study by having access to only aggregate data and no access to data that could reveal treatment arm, such as course attendance". "Most of the outcome data were obtained from self‐report questionnaires from participants who were not blind to treatment allocation". (p 16) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Comment: Participants were analysed as randomised, and all were included in the primary analysis by imputation of missing data. Quotes: "We obtained robust variance estimates in all regression models to allow for the potential clustering effect of receiving therapy in groups in the PEPS arm." (p 8) "The primary analysis (ITT) compared the mean SFQ score between PEPS and usual treatment at 72 weeks postrandomisation follow‐up, adjusted for baseline SFQ score and stratification variables (centre and gender), and implemented using maximum likelihood‐based generalised linear modelling." (p 12) "The pattern of missing data was investigated by examining variables recorded at baseline that were associated with ‘missingness’ of SFQ score at the 72‐week follow‐up. Multiple imputation and analysis of multiple imputed data sets were conducted using ‘mi’ procedures in Stata. The imputation model contained site, age, sex,ethnicity, social status, PD category (simple or complex), SFQ at baseline and 24 weeks, baseline EQ‐5D health state score, baseline HADS score, baseline SPSI‐R score and baseline three main problems score, and 20 data sets were imputed." (p 12) |
| Selective reporting (reporting bias) | Low risk | Comment: in terms of outcomes, protocol and full‐text report matched. |
| Other bias | High risk |
Treatment adherence Comment: adherence was self‐rated by the therapist. Attention bias Comment: mean number of treatment weeks: TAU non‐completer = 30 (SD = 25.7), completer = 80.2 (SD = 9,8); PEPS non‐completer = 36.7 (SD = 23,4), completer = 80.3 (SD = 10.4). Seemed that both groups got equal amount of therapy. Allegiance bias Comment: authors (MM, CD) have allegiance to PEPS therapy. Vested interest Comment: The National Institute for Health Research (NIHR) Health Technology Assessment programme funded this study. Hywel Williams is the Deputy Director of this programme but was not involved in the funding decision for this programme. |
Mehlum 2014.
| Study characteristics | ||
| Methods | 19‐week trial with 2 arms
Duration of trial: 19 weeks Country: Norway Setting: outpatient |
|
| Participants |
Method of recruitment of participants: recruited from child and adolescent psychiatric outpatient clinics in Oslo that screened newly referred patients for current self‐harm. Comorbidity in BPD subsample (N = 14, 35.9% of overall sample) unclear Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group Treatment name: DBT‐A Number randomised to group: 39 Duration: 19 weeks Control/comparison group Comparison name: enhanced usual care Number randomised to group: 38 Duration: 19 weeks Both groups: Concomitant psychotherapy: not stated Concomitant pharmacotherapy: Overall sample at baseline (including sub‐threshold BPD): "Three DBT‐A patients (7.7%) used at least 1 psychotropic drug for a mean number of 94.7 days (SD ¼ 64.3), whereas 5 EUC patients (13.2%) used such medication for a mean number of 72.8 days (SD ¼ 16.6), with no significant differences.“ (p. 1087) Medication use in BPD subsample (N = 14, 35.9% of overall sample) unclear Proportions of participants taking standing psychotropic medication during trial observation period: Overall sample (subthreshold including): “Only 7 participants (4 in the DBT‐A group and 3 in the EUC group) had used any psychotropic medication over the follow‐up year (not significant).“ (Mehlum 2016, p. 297) Medication use in BPD subsample unclear (N = 14, 35.9% of overall sample) unclear |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: yes Ethics approval: The study was approved by the Regional Committee for Medical Research Ethics, South‐East Norway, and all patients and parents provided written informed consent. Comments from review authors:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Treatment allocation of participants after baseline assessments was based on a permuted block randomization procedure with an undisclosed and variable blocking factor, and daily management of the randomization procedures was performed by an external group.” (p 1083) |
| Allocation concealment (selection bias) | Low risk | Quote: “…and daily management of the randomization procedures was performed by an external group.” (p 1083) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “Diagnostic assessments were made by experienced clinicians blinded to treatment allocation.” (p 1083) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Quotes: “Data analysis was by intention to treat.”; “Although some patients dropped out of treatment, all patients were followed from baseline to trial completion with no dropouts from the research.” Comment: All participants were assessed for outcome measures at follow‐ups regardless of treatment exposure (see p 1086). Overall, no differences are seen in proportion of withdrawals between groups. The study measured follow‐up outcome data on all participants regardless of amount of treatment exposure. No information was given on how participants with less exposure were included/imputed in the final analyses. |
| Selective reporting (reporting bias) | Unclear risk |
Comment: all outcomes listed in registration were reported on in final paper of the overall sample, including participants with subthreshold BPD. Additionally, hopelessness and BPD symptoms were reported, which had not been listed in the protocol. These were not described as post hoc analyses in the final report. For the sample included here (full‐BPD), only some data were available for depression, suicidality and psychosocial functioning. |
| Other bias | High risk |
Treatment adherence Quote: "adherence to DBT continued to be assessed throughout the trial”. Adherence controlling was conducted for the DBT group. Unclear if it was done for the control group Attention bias Quotes: “Dialectical Behavior Therapy, delivered for 19 weeks, consisted of 1 weekly session of individual therapy (60 minutes), 1 weekly session of multifamily skills training (120 minutes), and family therapy sessions and telephone coaching with individual therapists outside therapy sessions as needed.” “Enhanced usual care was 19 weeks of standard care (enhanced for the purpose of the study by requiring that EUC therapists agree to provide on average no less than 1 weekly treatment session per patient throughout the trial)”. (p 1084) Comment: more attention spent on DBT group Allegiance bias Comment: first author (L Mehlum) is educated in DBT‐therapy (www.med.uio.no/klinmed/personer/vit/lmehlum). Mehlum is one of the most prominent DBT people in Norway, and may have edited a handbook of DBT or received money for educating others in DBT. |
Mohamadizadeh 2017.
| Study characteristics | ||
| Methods | 16 sessions trial with 3 arms
Duration of trial: 16 sessions Country: Iran Setting: inpatient |
|
| Participants |
Method of recruitment of participants: convenience sampling method
Sample size: 36 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: Structured Clinical Interview for DSM‐IV Axis II Disorders (SCID‐II) Mean age: not stated Sex: 100% female Comorbidity: not stated Inclusion criteria: not stated Exclusion criteria
|
|
| Interventions |
Experimental group 1
Treatment name: DBT
Number randomised to group: 12
Duration: 16 sessions for 90 minutes
Experimental group 2
Comparison name: ST
Number randomised to group: 12
Duration: 16 sessions for 90 minutes Control/comparison group Comparison name: no treatment Number randomised to group: 12 Duration: 16 sessions for 90 minutes Both groups Concomitant psychotherapy: none Concomitant pharmacotherapy: none; "The use of any psychiatric medication during training, from the very first session of treatment, and the use of any type of psychological services were abandoned." (quote, p 1027) Proportions of participants taking standing psychotropic medication during trial observation period: equal (medication not allowed) |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: not stated
Ethics approval: not stated Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information. It was unclear if the no treatment control group were randomized or not |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no attrition rates reported; no imputation methods reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol found, so we could not compare planned methods with reported methods |
| Other bias | Unclear risk |
Adherence to treatment Comment: no adherence check stated, but a list over each session was provided Attention bias Comment: no differences between groups Allegiance bias Comment: none |
Morey 2010.
| Study characteristics | ||
| Methods | 1.5 month trial with 2 arms
Duration of trial: 1.5 months Country: USA Setting: outpatient |
|
| Participants |
Methods of recruitment of participants: not stated Samplse size: 16 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: Diagnostic Interview for DSM‐IV Personality Disorders (DIPD‐IV), and Personality Assessment Inventory‐Borderline Scale (PAI‐BOR) Mean age: 31.1 years Sex: 81.3% female Comorbidity: not specified Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group Treatment name: MACT + TA Number randomised to group: 16 Duration: 1.5 months (6 weekly sessions) Control/comparison group Comparison name: MACT Number randomised to group: 16 Duration: 1.5 months (6 weekly sessions) Both groups Concomitant psychotherapy: No other psychosocial interventions were allowed. Concomitant pharmacotherapy: Psychotropic medication was allowed. 56% of participants were taking psychotropic medication at baseline. Proportionsof participants taking standing psychotropic medication during trial observation period: unclear |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: yes Ethics approval: not stated Comments from the review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: study stated that participants were randomised but the specific methods were not clear. |
| Allocation concealment (selection bias) | Unclear risk | Comment: study stated that participnats were randomised but the specific methods were not clear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "[...] assessments [...] were conducted by an independent evaluator". (p 533) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no further details |
| Selective reporting (reporting bias) | Unclear risk | Comment: no indication of selective reporting, but insufficient information to permit judgement of 'high' or 'low' risk of bias |
| Other bias | Low risk |
Adherence bias Quote: "Consenting clients in both conditions were assigned to a project therapist, who worked under the supervision of the primary investigator." (p 532) Allegiance bias Quote: study authors not among treatment developers Attention bias Quote: both groups received comparable amounts of attention. |
Morton 2012.
| Study characteristics | ||
| Methods | 13‐week trial with 2 arms
Duration of trial: 13 weeks of therapy Country: Australia Setting: outpatient, within public‐sector mental health services, Victoria |
|
| Participants |
Method of recruitment of participants: potential participants recruited via referrals from public mental health services to Spectrum (personality disorder service for Victoria) Sample size: 41 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: Structured Clinical Interview for DSM‐IV Axis I Disorders (SCID‐I), and Structured Clinical Interview for DSM‐IV Axis II Disorders (SCID‐II) Mean age: ACT + TAU = 35.6 years (standard deviation = 9.33, range = 19–52), TAU = 34.0 years (standard deviation = 9.02, range = 21–54) Sex: 90.5‐95% female Comorbidity: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: ACT + TAU
Number randomised to group: 21
Duration: 26 weeks
Control/comparison group
Comparison name: TAU
Number randomised to group: 20
Duration: also appears to be 26 weeks Both groups Concomitant psychotherapy: 11 participants (27% of the total sample population of 41) described the service they received as therapy or counselling, rather than case management or medication review. Concomitant pharmacotherapy: TAU offered medications management. Proportions of participants taking standing psychotropic medication during trial observation period: no data provided specifying medication use in the two groups |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: not stated
Ethics approval: not stated Comments from review authors:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quotes: "Available participants at each location were randomized to ACT + TAU or TAU, after stratification of the sample based on the presence or absence of two or more self‐harm episodes in the last year. This stratification of the sample was done in order to ensure equal base rates across the conditions, as recent self‐harm was not a criterion of inclusion in the trial. This resulted in four ACT groups in four locations, each with 4 to 6 participants". The randomization process was planned as follows and was carried out as described: “Randomisation to immediate treatment or waiting list control: When 16 clients with four or more of the criteria for BPD, assessed as suitable, have been recruited for a group at a particular location, a randomisation procedure will be carried out and eight clients will commence treatment as soon as practicable with the remaining eight commencing three months later (waiting list control group). If this procedure would result in an unreasonable delay, a group may commence when 10 clients have been recruited (five will be randomised to an immediate start and five to waiting list). Recruitment will continue for nine months or until 60 clients have been accepted into phase 1 groups whichever occurs sooner. The immediate treatment clients will have a three month period of follow up before phase 2 groups begin. Because change in self‐harm is of interest and yet only about half the sample are expected to be still currently self‐harming, the sample will be stratified based on the presence or absence of two or more self‐harm episodes in the last year. The randomisation procedure will use a randomisation in blocks of four procedure (Pocock, 1983), using resources available at randomization.com. It will be carried out by an independent person who is not part of the research or clinical team.” (personal communication) |
| Allocation concealment (selection bias) | High risk | Quote: "Investigators were not blind to group allocation." (personal communication) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Outcomes were assessed via self‐report questionnaires. Outcome assessors were not blind". (personal communication) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Scores were analyzed using mixed model procedures, which allowed for all available data to be used in the analyses. This approach takes into account the obtained outcomes and missingness for participants with missing data, somewhat reducing the analytic problem presented by missing data. Compound symmetry covariance matrices were used as they were found to provide better model fit with fewer parameters than unstructured matrices as determined by the restricted log likelihood". (p 537) |
| Selective reporting (reporting bias) | Unclear risk | Quote: "The group protocol was developed based on 10 years of experience in providing residential and outpatient treatment for people with a diagnosis of BPD". (p 528) |
| Other bias | High risk |
Treatment adherence Comment: clinicians were instructed not to include any CBT change strategies such as cognitive challenge. Efforts were made to ensure treatment fidelity via review of group materials by ACT trainer Russ Harris and via consultation and supervision sessions, which included discussion framed by the ACT Competencies. Allegiance bias Quote: "An outline of the 12 sessions (of ACT) is provided in Table 2. A copy of the treatment manual, including the handouts and group session outlines) is available from Spectrum." (p 534) Comment: Morton (lead author) developed the treatment manual used in the experimental group .Attention bias Comment: ACT consisted of 12 2‐hour sessions. Unclear how the treatment exposure in TAU compared to this |
Nadort 2009.
| Study characteristics | ||
| Methods | 18‐month trial with two arms
Duration of trial: 18 months Country: The Netherlands Setting: outpatient |
|
| Participants |
Methods of recruitment of participants: Most of the patients were referred by therapists in secondary and tertiary community mental health institutes, some patients were referred by primary care physicians of psychotherapists with private practices. All patients were referred based on a clinical diagnosis of BPD. Patients were then assessed at each study site. Sample size: 62 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: Structured Clinical Interview for DSM‐IV Axis II Disorders (SCID‐II) and Borderline Personality Disorder Severity Index ‐ Version IV (BPDSI‐IV) score > 20 Mean age: 32.0 years Sex: 96.8% female Comorbidity: not specified Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group Treatment name: schema‐focused therapy (SFT) Number randomised to group: N = 30 Duration: 18 months (45‐minute individual sessions twice a week for 12 months, one weekly session in the second year) Control/comparison group Comparison name: schema‐focused therapy + therapist telephone availability outside office hours in case of crisis (SFT + TTA) (45‐minute individual sessions twice a week for 12 months, one weekly session in the second year) Number randomised to group: N = 30 Duration: 18 months Both groups Concomitant psychotherapy: not stated Concomitant pharmacotherapy: medication use allowed. 58% of patients used psychotropic medication at baseline. Proportions of participants taking standing psychotropic medication during trial observation period: unclear |
|
| Outcomes |
Primary
|
|
| Notes |
Sample size calculation: yes Ethics approval: not stated Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no clear information provided on method of randomisation |
| Allocation concealment (selection bias) | Low risk | Quote: "we used a stratified randomization procedure. The stratification procedure was performed by a study‐independent person and concealed for participating therapists, patients and researchers." (p 962) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quotes: "Study researchers, screeners, research assistants and therapists were masked to treatment allocation during the screening period and the first assessment." (p 963) "A limitation of the present study is that the assessments will be performed by research assistants who cannot remain blinded to the treatment condition of the included patients, as is always the case in trials studying the effects of psychotherapy. Nor are the patients blind to treatment condition. In this study, however, added to the main interview‐based outcome measures, self‐report questionnaires will be administered, that will not be influenced by the research assistants." (p 71) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no clear details on attrition provided |
| Selective reporting (reporting bias) | Low risk | Comment: published protocol is available (Nadort 2009), with no indication of selective reporting in the full report. |
| Other bias | Unclear risk | Comment: no clear details provided |
Pascual 2015.
| Study characteristics | ||
| Methods | 16‐week trial with 2 arms
Duration of trial: 16 weeks Country: Spain Setting: outpatient |
|
| Participants |
Method of recruitment of participants: not stated
Sample size: 70 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM‐IV‐TR) Means of assessment: Structured Clinical Interview for DSM‐IV Axis II Disorders (SCID‐II) and Revised Diagnostic Interview for Borderlines (DIB‐R) Mean age: CR = 32.4 years (standard deviation = 6.04), PE = 32.8 years (standard deviation = 8.8) Sex: 74.3% female Comorbidity: No information Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: cognitive rehabilitation (CR)
Number randomised to group: 36
Duration: 16 weeks Control/comparison group Comparison name: psychoeducation (PE) Number randomised to group: 34 Duration: 16 weeks Both groups Concomitant psychotherapy: no Concomitant pharmacotherapy: All patients continued pharmacological treatment if it had been initiated prior to inclusion. At baseline, 75% of the CR group was taking psychotropic medications, and 67.6% of the PE group. The difference was not significant. Proportions of participants taking standing psychotropic medication during trial observation period: unclear |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: yes
Ethics approval: yes Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “All participants were randomized to receive CR or PE in a 1:1 ratio stratified by centre, age, and education level. Generation of random allocation sequence was done with the Research Randomizer (www.randomizer.org)”. (p 2) |
| Allocation concealment (selection bias) | Unclear risk | Comment: no clear information provided about whether or not allocation was concealed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “Study design was a multicenter, randomized, rater‐blind clinical trial”. (p 2) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: reasons for dropout fairly balanced between groups. No differences in overall attrition rates between groups, but the attrition rates exceeded the 30% estimate of the power calculation, which may have increased uncertainty in the overall effect estimates. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no secondary outcomes were included in the trial registration, but they were in the published paper. The paper did not categorise them as post hoc analyses. |
| Other bias | High risk |
Treatment adherence Quote: "To ensure the reliability among centers regarding the evaluation and the treatment fidelity, two meetings were organized before the start of the study to train therapists". (p 2) Allegiance bias: Comment: Google search on first and last author did not reveal any allegiance biases. Attention bias: Comment: CR: group sessions, 120 minutes, twice a week during 16 weeks (32 sessions) PE: 16 weekly group sessions of 120 minutes each (16 sessions) |
Philips 2018.
| Study characteristics | ||
| Methods | 15.5‐18 month trial with 2 arms
Duration of trial: 18 months Country: Sweden Setting: Stockholm Centre for Dependency Disorders |
|
| Participants |
Method of recruitment of participants: “Patients were recruited through outpatient addiction treatment services throughout Stockholm County, through case‐finding among the social service offices in the region and through advertising in newspapers.” (quote, p 3) Sample size: 46 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: Structured Clinical Interview for DSM‐IV Axis II Disorders (SCID‐II) Mean age: 36.7 years (standard devtiation = 9.6, range = 20‐54) Sex: 80.4% female Comorbidity: Current axis I disorders (overall sample, not specified by treatment groups): MDD 28.3%, other depressive disorder including dysthymia 28.3%, bipolar II disorder 6.5%, PTSD 15.2%, any anxiety disorder excluding PTSD 65.2%, any eating disorder 6.5%, somatoform disorder 2.2%, any psychotic disorder 0% Current axis I substance use disorder (overall sample): alcohol 45.7%, amphetamines 13.0%, cannabis 6.5%, opioids 39.1%, sedatives, hypnotics, anxiolytics 21.7% Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: mentalisation‐based therapy (MBT)
Number randomised to group: 24
Duration: mean = 15.5 months (mean = 63.3 sessions)
Concomitant psychotherapy: not stated
Concomitant pharmacotherapy: not stated Control/comparison group Comparison name: treatment‐as‐usual (TAU) Number randomised to group: 22 Duration: 18 months (mean = 10.7 therapy sessions) Concomitant psychotherapy: of the patients randomised to TAU (n = 22), 11 received some sort of psychotherapy Concomitant pharmacotherapy: not stated Proportions of participants taking standing psychotropic medication during trial observation period: unclear |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: yes Ethics approval: Stockholm Regional Ethical Review Board (registration number: 2007/642‐31/1) Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The randomization was conducted by the KTA [Karolinska Trial Alliance] using an urn procedure. The randomization was made in blocks and the researchers were not informed of the block size. KTA prepared sealed randomization envelopes with information about each patient’s treatment assignment.” (p 3) |
| Allocation concealment (selection bias) | Low risk | Quote: “The randomization was conducted by the KTA [Karolinska Trial Alliance] using an urn procedure. The randomization was made in blocks and the researchers were not informed of the block size. KTA prepared sealed randomization envelopes with information about each patient’s treatment assignment.” (p 3) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information on blinding provided by report authors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quotes: “Outcome analyses were made both in the form of completer analyses and ITT analysis with the last observation carried forward. Throughout all measures, completer and ITT analyses gave equivalent results and therefore only the results from completer analyses are described here (Table 2). Outcome analyses of the self‐report and interview measures were seriously challenged by the high attrition rates, as only 24 out of 46 patients came to the measurements at endpoint 18 months (13 out of 24 patients in MBT, and 11 out of 22 patients in the control group).” (p 5) "Due to the recruitment problems in the project, our initial power calculation had to be revised." (p 4) |
| Selective reporting (reporting bias) | High risk | Comment: Beck’s Suicidal Intent Scale, Social Adjustment Scale – Self Report listed as secondary outcomes in trial registration, but not in final report. Outcomes related to health economics and criminality also listed in registration, but not in full report |
| Other bias | High risk |
Adherence bias Quote: "Despite significant efforts to train therapists in the MBT model, most of the them had poor average results on the MDT adherence tests: only 2 therapists passed the threshold for adequate MBT, while the remaining 7 failed to do so." (p 6) Attention bias Quote: "For patients randomized to MBT (n = 24), the treatment duration was in average 15.5 months (SD 4.1, range 3–18) with a mean of 63.3 MBT sessions (SD 26.7, range 10–116). Control group patients received on average 10.7 therapy sessions (SD 14.7, range 0–45)". (p 5) Allegiance bias Comment: none found |
Priebe 2012.
| Study characteristics | ||
| Methods | 12‐month trial with 2 arms
Duration of trial: 12 months Country: UK Setting: outpatient |
|
| Participants |
Method of recruitment of participants: referral
Sample size: 70 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: Structured Clinical Interview for DSM‐IV Axis II Disorders (SCID‐II) Mean age: 32.2 years (standard deviation = 10.8) Sex: 87.5% female Comorbidity: yes, but not specified. Number of axis 1 disorders = 8 Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: dialectical behaviour therapy (DBT)
Number randomised to group: 40
Duration: 12 months
Concomitant psychotherapy: not stated
Concomitant pharmacotherapy: not stated Control/comparison group Comparison name: treatment‐as‐usual Number randomised to group: 40 Duration: 12 months Concomitant psychotherapy: TAU encompasses many different psychotherapies. It is not specified what treatments participants in this group received. Concomitant pharmacotherapy: allowed (utilisation was monitored), no further data Proportions of participants taking standing psychotropic medication during trial observation period: unclear |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: not stated
Ethics approval: yes Comments from review authors:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomisation was computer generated with a 1:1 allocation by an independent statistician, using 6 blocks of 12 randomly permuted treatment allocation sequences, with a final block of 8.” (p 358) |
| Allocation concealment (selection bias) | Low risk | Quote: “Randomisation was computer generated with a 1:1 allocation by an independent statistician, using 6 blocks of 12 randomly permuted treatment allocation sequences, with a final block of 8.” (p 358) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: “Interviewers were therefore not masked to treatment allocation.” (p 358) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: “Of the 40 randomised to receive DBT, 3 people did not start treatment due to various reasons and a further 18 patients did not complete the 1‐year treatment”. (p 359) |
| Selective reporting (reporting bias) | Unclear risk | Comment: The trial registration listed quality of the therapeutic relationship as an outcome measure; however, this measure was not mentioned in the final report. |
| Other bias | Unclear risk |
Treatment adherence Comment: "To establish adherence to the DBT model, individual treatment sessions were audio recorded and 10% of the available recordings were assessed for adherence by a DBT therapist [...] using a 63‐item rating scale”. (p 357) Adherence routines for DBT. No mentioning of adherence routines for TAU Allegiance bias Comment: first author is a licensed psychotherapist. Unclear if he has direct interest in DBT Attention bias Comment: few data on what encompassed TAU – large heterogeneity ‐ and therefore, it may be possible that the participants in the DBT group received more therapy. Vested interest Comment: the authors declared that they have no conflicts of interest. |
Reneses 2013.
| Study characteristics | ||
| Methods | 20‐24 week trial with 3 arms
Duration of trial: 20‐24 weeks Country: Spain Setting: outpatient |
|
| Participants |
Method of recruitment of participants: patients of San Carlos and Hospital Universitario 12 de Octubre of Madrid included in study. The patients were recruited consecutively over a 12‐month period.
Sample size: 53 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM‐IV‐TR) Means of assessment: Structured Clinical Interview for DSM‐IV Axis II Disorders (SCID‐II) Mean age: 33.8 years (standard deviation = 7.5) Sex: 70.5% female Comorbidity: 47.7% with axis 1 Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: psychic representation focused psychotherapy (PRFP) + conventional treatment
Number randomised to group: 25
Duration: 20 weeks
Control/comparison group
Comparison name: conventional treatment (pharmacological treatment and psychological advice)
Number randomised to group: 28
Duration: 24 weeks Both groups Concomitant psychotherapy: optionally, patients could receive nonstandard outpatient psychological advice, but any type of standard psychotherapy excluded Concomitant pharmacotherapy: all participants were receiving drug treatment, 90% of whom were receiving antidepressants, more than 40% mood stabilisers and 30% antipsychotics. |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: not reported
Ethics approval: yes Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The subjects who met the inclusion criteria were assigned to one of the two intervention groups through the generation of simple random sampling through a sequence of randomized numbers generated with EPIDAT 3.1.” (p 141) |
| Allocation concealment (selection bias) | Unclear risk | Comment: no clear information on allocation concealment was provided by the authors |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: the authors did not mention whether or not outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: high attrition rates. Data were imputed with the LOCF, which is potentially inappropriate. Dropout reasons of lack of satisfaction were over‐represented in control group relative to EG |
| Selective reporting (reporting bias) | Unclear risk | Comment: we could not find a published protocol so could not provide a clear judgement of 'high' or 'low' risk of bias |
| Other bias | High risk |
Treatment adherence Comment: adherence routines for EG, no information on CG Quote: “The supervision method consisted in random control by the external supervisor of the project of five sessions of each psychotherapist.” (p 141) Allegiance bias Comment: time‐limited, manualised PDT developed by the research group. Attention bias Comment: EG received 20 weeks of treatment, whereas CG received 24 weeks |
Robinson 2016.
| Study characteristics | ||
| Methods | 1‐year trial with 2 arms
Duration of trial: 1 year Country: UK Setting: outpatient |
|
| Participants |
Method of recruitment of participants: participants recruited from clinical centres by referral from doctors working in the outpatient services of each centre. Referrals received by trial manager, who contacted the potential participant and provided the participant information sheet.
Sample size: 68 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: Structured Clinical Interview for DSM‐IV Axis II Disorders (SCID‐II) Mean age: 31.1 years (standard deviation = 9.9) Sex: 92.7% female Comorbidity: anorexia = 5.9%, bulimia = 63.2%, binge eating disorder = 2.9%, eating disorder not otherwise specified (EDNOS) = 27.9% Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: mentalisation‐based treatment for eating‐disorders (MBT‐ED)
Number randomised to group: 34
Duration: 1 year
Control/comparison group
Comparison name: specialist supportive clinical management for eating disorders (SSCM‐ED)
Number randomised to group: 34
Duration: 1 year Both groups Concomitant psychotherapy: no (excluded if currently in individual or group psychological therapy) Concomitant pharmacotherapy: not stated Proportions of participants taking standing psychotropic medication during trial observation period: unclear |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: yes
Ethics approval: yes Comments from review authors:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The method of randomisation of participants was block randomisation stratified by BMI (15.0–18.5, 18.6–24.9, 25).” (p 552) |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Randomly varying block sizes were implemented in order to maintain pre randomisation allocation concealment. The trial manager used the randomisation result to allocate participants to a treatment.” (p 552) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “single‐blind (researchers and statisticians are blind)”, “the trial statistician and research workers responsible for the collection of the assessments remained blind to treatment allocation during the trial and primary analyses.” (p 550) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk |
Comment: 15/35 in SSCM‐ED and 5/34 in MBT‐ED did not receive interventions – significant differences. Reasons were similar across groups. 41/61 participants were included in the analyses, so this can be said to be a partial ITT analysis. Did not comply with the level of attendance calculated a priori. Quotes: “Participants allocated to SSCM‐ED were significantly more likely to drop out before the start of therapy than those allocated to MBT‐ED.” (p 15) "We set a level of 50% attendance [10] to indicate compliance. That level was achieved by 47.1 % in the MBT‐ ED arm and 37.1% in the SSCM‐ ED arm". (p 556) |
| Selective reporting (reporting bias) | Low risk | Quote: "Not all questionnaires and interviews anticipated in the protocol were included in this analysis. Some were excluded because the small numbers remaining at follow‐up did not justify statistical analysis of outcome over time, and others (the Object Relations Inventory (ORI), treatment adherence, Reading the Mind in the Eyes test and the Reflective Uncelar – they do not use all but it makes sense in statistical terms. Functioning Questionnaire (RFQ)) will be de‐scribed elsewhere". (p 563) |
| Other bias | High risk |
Adherence bias Quotes: “Adherence to the treatment model was tested by the supervisors. After the trial, seven recorded and transcribed sessions each of MBT‐ED (individual therapy, four therapists) and SSCM‐ED (seven therapists) were randomly selected and subjected to adherence rating.” (p 552) "The adherence scores (with number of sessions scoring that level in brackets) were 7 (1), 6 (3), 4 (3). Competence scores were identical to adherence scores". (p 552) Allegiance bias Comment: trial was conducted with support from A Bateman Attention bias Comment: total number of hours in MBT‐ED was 102.7 hours over 12 months; total number of hours in SSCM‐ ED was 20‐26 hours over 12 months. |
Rossouw 2012b.
| Study characteristics | ||
| Methods | 12‐month trial with 2 arms
Duration of trial: 12 months Country: UK Setting: mental health services |
|
| Participants |
Method of recruitment of participants: from consecutive case individuals presenting with self‐harm to community mental health services or acute hospital emergency rooms
Sample size: 80 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: Childhood Interview for DSM‐IV Borderline Personality Disorder (CI‐BPD) Mean age: MBT‐A = 15.4 years (standard deviation = 1.3), TAU = 14.8 years (standard deviation = 1.2) Sex: 85% female Comorbidity: MBT‐A: 30 had borderline personality disorder, TAU = 28 had borderline personality disorder. For the entire sample, 20 had alcohol problems in the MBT‐A group and 15 in TAU, 13 had substance misuse in the MBT‐A group and 9 in TAU, 39 had depression in the MBT‐A group and 38 in TAU Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: mentalisation‐based therapy for adolescents (MBT‐A)
Number randomised to group: 40
Duration: 12 months
Concomitant psychotherapy: not stated
Concomitant pharmacotherapy: Participants who were severely depressed were likely to be offered antidepressants; 16 (40%) received medication during the trial. Control/comparison group Comparison name: treatment‐as‐usual (TAU) Number randomised to group: 40 Duration: 12 months Concomitant psychotherapy: not stated Concomitant pharmacotherapy: 40% of the MBT‐A group and 43% of the TAU group received medication at baseline. Proportions of participants taking standing psychotropic medication during trial observation period: unclear |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: yes
Ethics approval: The study was approved by the North East London NHS Foundation Trust Institutional Review Board, REC 3. Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: eligible consenting participants were randomised by an independent statistician working off‐site using an adaptive minimisation algorithm |
| Allocation concealment (selection bias) | Low risk | Comment: allocations were sent in separate envelopes to an administrator who informed the relevant clinicians |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: assessors and participants were both blinded to treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: ITT analyses were conducted. Only 37 out of 80 randomised participants completed therapy |
| Selective reporting (reporting bias) | High risk | Comment: there were several outcomes in the protocol that were not mentioned in the publication (Millon Adolescent Clinical Inventory, the Reading the Mind in the Eyes Test, trust game and questionnaire, IQ, as well as assessments given by the adolescents’ parent or guardian: Millon Clinical Multiaxial Inventory III, Systemic Inventory of Change, the Reading the Mind in the Eyes Test) |
| Other bias | High risk |
Treatment adherence Comment: supervision sessions included listening to audiotaped sessions and scoring for adherence by consensus using specially developed adherence scales (a copy of the adherence scales is available from the first author on request, see p 1313). However, results of adherence ratings were not presented. Allegiance bias Comment: one of the authors (Fonagy) is one of the developers of MBT and both authors designed and manualised the 12‐month intervention program MBT‐A Attention bias Comment: number of hours of clinical attention received by the two groups did not differ |
Salzer 2014.
| Study characteristics | ||
| Methods | 6‐month trial with 2 arms
Duration of trial: 6 months Country: Germany Setting: inpatient and outpatient |
|
| Participants |
Method of recruitment of participants: during initial interview at a child and adolescent psychiatric outpatient clinic Sample size: 66 Diagnosis of borderline personality disorder: 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD‐10) Means of assessment: Structured Clinical Interview for DSM‐IV Axis II Disorders (SCID‐II) Mean age: 16.49 years (range = 14‐19 years) Sex: 77.7% female Comorbidity: all participants fulfilled ICD‐10 criteria for a diagnosis of F92 (mixed disorder of conduct and emotions) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: PDT Number randomised to group: 32 Duration: 6 months Concomitant psychotherapy: inpatient treatment programme, additional treatment Concomitant pharmacotherapy: tempory pharmacologic treatment to prevent premature discharge in cases of serious impulse control difficulties Control/comparison group Comparison name: WL/TAU Number randomised to group: 34 Duration: 6 months Concomitant psychotherapy: allowed to make use of any alternative psychotherapeutic treatments Concomitant pharmacotherapy: allowed to make use of any psychopharmacological treatments Proportions of participants taking standing psychotropic medication during trial observation period: “In the treatment group, 21 patients (65.6%) received pharmacotherapy during inpatient treatment; neuroleptics (n = 2) or atypical neuroleptics (n = 11), tricyclic antidepressants (n = 5), selective serotonin reuptake inhibitors (n = 3), methylphenidate (n = 3) and an anticonvulsant medication (n = 1) were used.“ (p. 2218); medication use in control group not specified. |
|
| Outcomes |
Secondary
|
|
| Notes |
Sample size calculation: yes Ethics approval: approved by the ethics committee at the University of Goettingen. Adolescent patients and their parents were asked for written consent. Comments from review authors:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "randomly assigned [...] by simple randomization that was conducted by the staff of the out‐patient clinic." (p 2214) Comment: not enough information to make a clear judgement of 'high' or 'low' risk of bias |
| Allocation concealment (selection bias) | Unclear risk |
Quote: "randomly assigned [...] by simple randomization that was conducted by the staff of the out‐patient clinic." (p 2214) Comment: not enough information to make a clear judgement of 'high' or 'low' risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: "Independent assessor" (p 2214). "An independent, trained assessor provided all diagnoses based on a structured interview with the adolescent. This clinical psychologist completed intensive training on the SCID and was not involved in the randomization process, treatment planning or therapy." ( p 2216) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quotes: "For all analyses, the ITT sample was used. In case of missing scores, the last observation carried forward (LOCF) method was applied." (p 2217) "The comparable dropout rates were 15.6% (n = 5) in the treatment group and 17.6% (n = 6) in the WL/TAU condition (p = 0.71). We found no significant differences between the patients who dropped out versus those who remained within the trial regarding number of diagnoses or symptom impairment either within the conditions or in the overall sample (all p > 0.29)." (p 2217) |
| Selective reporting (reporting bias) | Unclear risk | Comment: a study protocol was not available; therefore, there was insufficient information to permit judgement of high or low risk of bias. |
| Other bias | High risk |
Affiliation bias Comment: treatment developer (A Streeck‐Fischer) of the the treatment under investigation is the senior author of the study. Treatment adherence Quote: "All therapists were trained in the PDT and attended an intensive workshop that covered the treatment manual. The therapists were under weekly supervision by the manual’s author. To further ensure treatment fidelity, several videotaped treatment sessions were discussed during the supervision. Treatment adherence was checked in randomly selected videotaped sessions by independent raters based on manual checklists." (p 2214) Attention bias Comment: Participants in the experimental group received inpatient treatment, whereas participants in the control group were put a waiting list and did not receive any treatment by default (though they were allowed to optionally use alternate treatments if necessary). |
Santisteban 2015.
| Study characteristics | ||
| Methods | 7‐month trial with 2 arms
Duration of trial: 7 months Country: USA Setting: university centre |
|
| Participants |
Method of recruitment of participants: About two‐thirds of youths were referred from the juvenile justice system: 38% from the Miami‐Dade Juvenile Services Department and 32% from a juvenile addictions receiving facility. 30% were referred by school counselors or the community in general.
Sample size: 40 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: Revised Diagnostic Interview for Borderlines (RDIB) Mean age: I‐BAFT = 16 years (standard deviations = 0.8), IDC = 15.6 years (standard deviations = 0.8) Sex: 35‐40% female Comorbidity: substance use disorder on the basis of the last 12 months (even if there was no documented use in the past 30 days), depression (I‐BAFT = 35%, IDC = 40%) Inclusion criteria
Exclusion criteria: none stated |
|
| Interventions |
Experimental group
Treatment name: I‐BAFT
Number randomised to group: 20
Duration: 7 months Control/comparison group Comparison name: IDC Number randomised to group: 20 Duration: 7 months Both groups Concomitant psychotherapy: not stated Concomitant pharmacotherapy: not stated Proportions of participants taking standing psychotropic medication during trial observation period: unclear |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: no
Ethics approval: yes Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Permuted block randomization stratified by gender, race/ethnicity and drug use severity in the past 30 days”. (p 57) |
| Allocation concealment (selection bias) | Unclear risk | Comment: not enough information provided to enable a judgement of high or low bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: “Assessors were not blind to the intervention.” (p 57) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no sensitivity analysis was performed after multiple imputations. Relatively high dropout (33% self‐reported substance, 38% urine samples, 33% borderline personality disorder behavior) |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available to enable a clear assessment of selective reporting bias. |
| Other bias | High risk |
Treatment adherence Quote: ”Each therapist was trained by an expert in their respective conditions and met weekly with a supervsor and fellow therapists to view videotaped therapy sessions and to discuss the implementation of the manualised interventions with a high degree of adherence and fidelity”. (p 58) Comment: unclear whether adherence ratings were applied Allegiance bias Comment: I‐BAFT was developed by the authors. Attention bias Comment: matched dosage I‐BAFT Quotes: “I‐BAFT delivered weekly family therapy, individual herapy, and skills‐building intervention in a two‐sessions‐per‐week format over a 7‐month period”. (p 58) “IDC was administered in a two‐sessions‐per‐week format implemented over a 7‐month period”. (p 58) |
Schilling 2018.
| Study characteristics | ||
| Methods | 4‐week trial with 2 arms
Duration of trial: 4 weeks Duration of participation: 4 weeks Country: Germany Setting: not stated |
|
| Participants |
Method of recruitment of participants: recruited from the Deparment of Psychiatry of the Asklepios clinic, Germany
Sample size: 48 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: Structured Clinical Interview for DSM‐IV Axis II Disorders (SCID‐II) Mean age: B‐MACT = 29.36 years (standard deviation = 10.46), PMR = 31.15 years (standard deviation = 9.59) Sex: 91.7% female Comorbidity: 31% = major depressive disorder, 23% = any anxiety disorder (including 12 patients with PTSD), 7% = alcohol or substance abuse, 9% = dependence Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: B‐MACT
Number randomised to group: 22
Duration: 4 weeks
Control/comparison group
Comparison name: PMR
Number randomised to group: 26
Duration: 4 weeks Both groups Concomitant psychotherapy: not stated Concomitant pharmacotherapy: yes; 56 of the 74 treated with psychotropic medication, not specified by group Proportions of participants taking standing psychotropic medication during trial observation period: unclear |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: no sample calculation
Ethics approval: yes Comments from review authors:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information about randomisation method provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information about allocation concealment provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information about the blinding of assessors provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no data on attrition, no mention of ITT or imputation method |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol found preventing us from making a clear assessment of reporting bias |
| Other bias | High risk |
Treatment adherence Comment: no data on treatment adherence Allegiance bias Comment: first author was principal investigator and delivered both interventions. She also developed the manual for B‐ MACT. Attention bias Comment: interventions were similar in length. |
Schuppert 2012.
| Study characteristics | ||
| Methods | 17‐week trial with 2 arms
Duration of trial: 17 weeks Country: The Netherlands Setting: outpatient |
|
| Participants |
Method of recruitment of participants: referral from 1 of 4 mental health centers (for emotion regulation problems or borderline personality disorder features) Sample size: 109 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) (at least 2 borderline personality disorder criteria); 73% of final sample met full criteria for borderline personality disorder. Means of assessment: Structured Clinical Interview for DSM‐IV Axis II Disorders (SCID‐II) Mean age: 15.98 years (standard deviation = 1.22) Sex: not stated Comorbidity: 33 % had excessive use of alcohol, 29 % used addictive drugs and psychotropic medications (however, substance‐dependent patients were excluded). Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group Treatment name: ERT Number randomised to group: 54 Duration: 17 weeks Control/comparison group Comparison name: TAU Number randomised to group: 55 Duration: not specified Both groups Concomitant psychotherapy: yes, free to use mental health care services Concomitant pharmacotherapy: yes, 29.4% on any medication at baseline Proportions of participants taking standing psychotropic medication during trial observation period: no significant differences between groups with regard to medication at baseline or follow‐up |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: not stated Ethics approval: not stated Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Comment: stratified randomisation Quote: “Randomization by drawing lots was performed by an independent, masked research assistant who was not involved in the project.” (p 1316) |
| Allocation concealment (selection bias) | Low risk | Quote: “Randomization by drawing lots was performed by an independent, masked research assistant who was not involved in the project.” (p 1316) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “The assessments were conducted by research psychologists who were blinded to the treatment condition.” (p 1317) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 19% attrition in total. ITT analysis used. Reasons for attrition were not stated – could have been unevenly distributed across groups. |
| Selective reporting (reporting bias) | High risk | Comment: In the protocol, the following outcomes were mentioned: Life Problems Inventory, measuring main symptoms of BPD (however, only the subscale on affective instability reported), Rutgers Alcohol Problems Index, Strengths and Difficulties Questionnaire, raising style, parental stress and parental functioning, consumption of public health services and global functioning. None of these outcomes were reported in the final report. In particular, data from the Children's Depression Inventory (which would have been relevant for this review) were missing. Number of non‐completers (flow‐chart) inconclusive and not reported for the TAU group |
| Other bias | High risk |
Treatment adherence Quotes: “To increase treatment adherence and comparability among the centers, the manuals were highly structured. The treatment integrity of a random sample of 20 audiotaped sessions was checked by an independent rater.” (p 1317); "The competence of all therapists was found to be accurate. On average, the sessions covered 92.4% of the ERT manual." Allegiance bias Quote: "Drs. Schuppert, van Gemert, and Wiersema are authors of the manual on Emotion Regulation Training that is commercially available in the Netherlands." (p 1323) Attention bias Comment: no information about the duration of the treatment‐as‐usual group |
Sinnaeve 2018.
| Study characteristics | ||
| Methods | 9‐12‐month trial with 2 arms
Duration of trial: 9 months for step‐down DBT, and 12 months for standard outpatient DBT Country: The Netherlands Setting: inpatient and outpatient |
|
| Participants |
Method of recruitment of participants: not stated Sample size: 84 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM‐IV‐TR) Means of assessment: Participants were screened with the Vragenlijst Kenmerken Persoonlijkheid. Presence of Axis 1 and Axis 2 disorders assessed with the Mini‐International Neuropsychiatric Interview and the Structured Clinical Interview for DSM Disorders Mean age: step‐down DBT = 26.15 years (standard deviation = 6.18), standard outpatient DBT = 25.63 years (standard deviation = 7.45) Sex: 95% female Comorbidity: “Almost one third of the sample reported a history of sexual abuse (N = 16, 29%) and more than half experienced physical abuse (N = 30, 55%). One out of three participants suffered from post‐traumatic stress disorder (N = 17, 31%), half were diagnosed with major depression (N = 28, 51%), and one out of three participants fulfilled criteria of substance dependence (N = 17, 31%)". (quote, p5) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group Treatment name: step‐down DBT Number randomised to group: 42 Duration: 9 months Control/comparison group Comparison name: standard outpatient DBT Number randomised to group: 42 Duration: 12 months Both groups: Concomitant psychotherapy: not stated Concomitant pharmacotherapy: not stated Proportions of participants taking standing psychotropic medication during trial observation period: unclear |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: yes Ethics approval: The protocol adhered to the principles outlined in the Declaration of Helsinki, approved by the Institutional Review Board and registered in www.clinicaltrials.gov. Comments from review authors:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a computer program, developed by the Amsterdam Medical Centre, generated the sequence. To increase the likelihood of comparable treatment groups, a minimization method was used. Minimization variables were BPDSI score ≥ 40, total lifetime LPC score ≥ 14 and age". (p 14) |
| Allocation concealment (selection bias) | Low risk | Quote: "we ensured that interventions were allocated by means of a concealed randomization procedure." (p 19) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "data collectors were not blind to the assigned intervention." (p 19) |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
QuoteS: "In step‐down DBT, 53% of the participants who started DBT finished the entire 9‐month program. Twelve months of outpatient DBT showed a retention rate of 63%. The results of the Kaplan Meier statistic indicated that there were no significant differences in the time to drop out between conditions, Χ2(1) = .36, p = .55." (p 17) In outpatient DBT, 23 out of 42 participants did not start the allocated treatment. This could be partially due to the fact that waiting times appeared to be longer in outpatient DBT. One participant died by suicide before he received outpatient DBT." (p 16) Comment: high attrition rates in outpatient DBT |
| Selective reporting (reporting bias) | High risk |
Quote: "There are three differences between the study protocol in Trials and this report. First, the name of the residential program was changed from ‘inpatient DBT’ to ‘residential DBT’. Second, our study ended prematurely due to an unexpected close‐down of the Centre for Personality Disorders Jelgersma (CPJ). Third, because of unforeseen waiting list issues, participants who were randomized to outpatient DBT had to wait longer before they met their therapist." (p 14); "...the protocol was published in advance and all analyses were performed by independent experts" (p 19) Comment: The authors addressed changes made from protocol to full report. However, assessing change in level of psychopathological symptoms with The Brief Symptomatology Inventory (BSI)) was excluded as secondary outcome. |
| Other bias | High risk |
Treatment adherence Comment: treatment adherence was evaluated in both conditions (see p 19). Attention bias Comment: differences in duration of treatment (9 months vs 12 months) Allegiance bias Comment: nothing found |
Smith 2012.
| Study characteristics | ||
| Methods | 36‐week trial with 2 arms
Duration of trial: 36 weeks Country: USA Setting: inpatient and outpatient |
|
| Participants |
Method of recruitment of participants: " In total, 1,100 women seeking treatment in the clinic were screened by intake clinicians for study eligibility on the basis of presence of depressive symptoms and self‐ report of sexual abuse before age 18 and absence of exclusion criteria." (quote, p 2)
Sample size: subsample = 70 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: Structured Clinical Interview for DSM‐IV Axis II Disorders (SCID‐II) Mean age: no data for subsample. Full sample = 36.39 years (standard deviation = 9.86) Sex: 100% female Comorbidity: no data for subsample Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: IPT
Number randomised to group: 17 (out of full sample of 37)
Duration: 36 weeks Control/comparison group Comparison name: TAU Number randomised to group: 9 (out of full sample of 33) Duration: 36 Both groups Concomitant psychotherapy: “TAU was individual psychotherapy, TAU therapists described as supportive (53%), cognitive‐behavioral or dialectical‐behavioral (27%), integrated/eclectic (13%), and client‐centered (7%)” (quote, p 4) Concomitant pharmacotherapy: “Women were permitted to enter the study regardless of antidepressant prescription status. A total of 43 women reported having been prescribed antidepressant medications at the baseline assessment (23 of those in the IPT condition, 20 of those in the TAU condition).” (p 125) Proportions of participants taking standing psychotropic medication during trial observation period: unclear |
|
| Outcomes |
Secondary
|
|
| Notes |
Sample size calculation: yes
Ethics approval: yes Comments from review authors:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no clear information on randomisation procedure was available |
| Allocation concealment (selection bias) | Unclear risk | Comment: no clear information on allocation concealment was provided |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "One master’s‐level research assistant, who was aware of patients’ treatment assignments, conducted all assessments." (p 125) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: “In intention‐to‐treat analyses, change over time was assessed with generalized linear models with inference based on generalized estimating equations (GEEs) (32). To test the assumption that data were missing completely at random (MCAR), logistic modeling was performed to determine whether missing assessment data depended on individual patients’ observed responses in previous assessments (Table 2). When the probability of missing values was modeled for each outcome, we found that the shame subscale of the Differential Emotions Scale had informative dropout (P=.04) and violated the MCAR assumption. In that case, weighted GEEs were applied with individual weights estimated from the logistic model for missing data. Women with lower shame scores in earlier assessments were more likely to have missing data in later assessments.” |
| Selective reporting (reporting bias) | High risk |
Comment: extra outcomes included in full report, but not mentioned in the protocol are:
Several outcomes included in full report. Not addressed. No clear differences between primary and secondary outcomes such as in protocol |
| Other bias | High risk |
Attention bias Quote: "Interpersonal psychotherapy participants attended approximately twice as many sessions (12.9±6.5) as those in usual care (6.3±4.2), a significant between‐group difference". (p 127) Adherence bias Comment: all interpersonal psychotherapy sessions were audiotaped or videotaped. The principal investigator reviewed 20% of taped sessions for treatment fidelity and addressed deviations from the model with the therapist. (See p 126) Allegiance bias Comment: none found |
Soler 2009.
| Study characteristics | ||
| Methods | 3‐month trial with 2 arms
Duration of trial: 3 months Country: Spain Setting: outpatient |
|
| Participants |
Methods of recruitment of participants: recruited from the outpatient borderline personality disorder unit at the Department of Psychiatry, Hospital de la Santa Creu i Sant Pau Sample size: 59 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: Structured Clinical Interview for DSM‐IV Axis II Disorders (SCID‐II) and Revised Diagnostic Interview for Borderlines (DIB‐R) Mean age: 29.2 years Sex: 81.3% female Comorbidity: not stated Inclusion criteria:
Exclusion criteria
|
|
| Interventions |
Experimental group Treatment name: DBT‐ST Number randomised to group: 32 Duration: 3 months (13 psychotherapy sessions of 120 minutes each) Control/comparison group Comparison name: SGT Number randomised to group: 32 Duration: 3 months (13 psychotherapy sessions of 120 minutes each) Both groups Concomitant psychotherapy: participants did not receive any other individual or group psychotherapy. Concomitant pharmacotherapy: pharmacological therapy was continued if initiated prior to inclusion, but type and doses could not be modified during the study period. Proportions of participants taking standing psychotropic medication during trial observation period: unclear |
|
| Outcomes |
Primary:
Secondary:
|
|
| Notes |
Sample size calculation: not stated Ethics approval: The study was approved by the ethics committee of the Hospital de la Santa Creu i Sant Pau and carried out in accordance with the Declaration of Helsinki. Comments from review authors:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Blocks of four generated using the SPSS software program served for the randomisation to DBT‐ST or SGT." (p 354) |
| Allocation concealment (selection bias) | Unclear risk | Comment: no further details |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: "... participants were evaluated every two weeks by experienced psychiatrists. Subjects were instructed not to disclose any information about the group (topics, group members or therapists) to maintain blind conditions." (p 354); "Assessment and drug control were carried out by two psychiatrists who were masked to the experimental conditions." (p 355); "We are unable to affirm that all participants refrained from disclosing information about the therapy or the therapists with the psychiatric raters during assessment visits. [...] Indeed, the observer‐rater scales obtained during the interview visits and the results from self‐reported measures filled in by patients during the study showed a good concordance." (p 357) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no further details |
| Selective reporting (reporting bias) | Unclear risk | Comment: no indication of selective reporting from the published paper, but as there was no protocol available, there was insufficient information to permit judgement of 'high' or 'low' risk of bias |
| Other bias | Unclear risk | Comment: no further details |
Stanley 2017.
| Study characteristics | ||
| Methods | 12‐month trial with 4 arms
Duration of trial: 12 months Country: USA Setting: inpatient |
|
| Participants |
Method of recruitment of participants: recruited from emergency departments, clinician referrals and advertisements. Recruitment period ended 6 months prior to study end date.
Sample size: 86 Diagnosis of borderline personality disorder: not stated Means of assessment: not stated Age: not stated Sex: not stated Comorbidity: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: DBT + fluoxetine
Number randomised to group: 18
Duration: 12 months Control/comparison group 1 Comparison name: DBT + placebo Number randomised to group: 19 Duration: 12 months Control/comparison group 2 Comparison name: supportive therapy + fluoxetine Number randomised to group: 20 Duration: 12 months Control/comparison group 3 Comparison name: supportive therapy + placebo Number randomised to group: 18 Duration: 12 months All groups Concomitant psychotherapy: not stated Concomitant pharmacotherapy: all participants were washed out of psychotropic medications and received fluoxetine or placebo, depending on randomisation (50% of each group received fluoxetine or placebo). Benzodiazapines were permitted for sleep. Proportions of participants taking standing psychotropic medication during trial observation period: 50% of each group were taking fluoxetine. |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: not stated
Ethics approval: not stated Comments from review authors:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no clear information on randomisation method was provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no clear information on allocation concealment was provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: outcome assessors were masked to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: dropout rates were even across groups, total rates were around ¼ of participants; no information provided on ITT, but all randomised participants were analysed |
| Selective reporting (reporting bias) | Low risk | Comment: NCT00533117: no apparent bias |
| Other bias | Unclear risk |
Treatment adherence Comment: adherence not stated Attention bias Comment: equal treatment in four groups Allegiance bias Comment: nothing found |
Turner 2000.
| Study characteristics | ||
| Methods | 12‐month trial with two arms
Duration of trial: 12 months Country: USA Setting: outpatient |
|
| Participants |
Methods of recruitment of participants: not stated Sample size: 24 Diagnosis of borderline personality disorders: Diagnostic and Statistical Manual of Mental Disorders, 3rd Edition revised (DSM‐III‐R) Means of assessment: Revised Diagnostic Interview for Borderlines (DIB‐R) Mean age: 22 years Sex: 79.2% female Comorbidity: 23 patients met criteria for a comorbid Axis I disorder. The majority was diagnosed with dysthymia + comorbid generalised anxiety disorder (n = 17). 3 patients met criteria for major depressive disorder, 3 met criteria for dysthymia, 18 met criteria for alcohol abuse, and 20 met criteria for substance abuse. Most patients (n = 18) met criteria for 2 additional personality disorders. Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group Treatment name: DBT Number randomised to group: 12 Duration: 12 months Control/comparison group Comparison name: client‐centered therapy (CCT) Number randomised to group: 12 Duration: 12 months Both groups Concomitant psychotherapy: not stated Concomitant pharmacotherapy: pharmacotherapy was not included in the study treatment regimens; at baseline, 19 patients were out of 24 reported taking prescribed psychotropic medications. Proportions of participants taking standing psychotropic medication during trial observation period: At post‐treatment, 4 DBT patients and 10 CCT patients were taking any medication, difference not significant. |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: not stated Ethics approval: not stated Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Following the initial assessments, patients were randomly assigned to either DBT or CCT." (p 415) Comment: no further details provided |
| Allocation concealment (selection bias) | Unclear risk |
Quote: "Next, patients were sequentially assigned to a mental health clinician." (p 415) Comment: no further details provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The outcome evaluation consisted of independent assessor ratings and patient self‐report. The independent assessor was unaware of the patients' treatment condition but was aware of the purpose of the study." (p 415) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 24 participants were randomly assigned to either DBT (n = 12) or CCT (n = 12). In spite of dropouts from treatment (DBT: n = 4, CCT: n = 6), assessments were available for all 24 participants at all times of assessment. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no indication of selective reporting, but Insufficient information to permit judgement of 'high' or 'low' risk of bias |
| Other bias | Unclear risk | Comment: no further details |
Van den Bosch 2005.
| Study characteristics | ||
| Methods | 12‐month trial with two arms
Duration of trial: 12 months Country: The Netherlands Setting: outpatient |
|
| Participants |
Methods of recruitment of participants: recruited from mental health institutions (n = 39) and addiction treatment services (n = 19) Sample size: 58 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: Structured Clinical Interview for DSM‐IV Axis II Disorders (SCID‐II) Mean age: 34.9 years (standard deviation = 7.7) Sex: 100% female Comorbidity: with or without substance use disorder Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group Treatment name: dialectical behavior therapy (DBT) Number randomised to group: 27 Duration: 12 months Control/comparison group Comparison name: treatment‐as‐usual Number randomised to group: 21 Duration: 12 months Both groups Concomitant psychotherapy: not stated Concomitant pharmacotherapy: no significant differences between the 2 groups with regard to medication use at baseline Proportions of participants taking standing psychotropic medication during trial observation period: "Medication use was monitored [...9. The greater improvement in the dialectical behaviour therapy group could not be explained by greater or other use of psychotropic medications by these patients. In both conditions, three‐quarters of the patients reported use of medication from one or more of the following categories: benzodiazepines, selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants, mood stabilisers and neuroleptics. Use of SSRIs was reported by 14 (52%) of the dialectical behaviour therapy patients and 19 (61%) of treatment‐as‐usual patients (χ2=0.44; P=0.509). These findings eliminate the possibility of confounding by medication use". (Verheul 2003, p 137) |
|
| Outcomes |
Primary:
Secondary:
|
|
| Notes |
Sample size calculation: not stated Ethics approval: not stated Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...patients were randomly assigned to treatment conditions. A minimisation method was used to ensure comparability of the two treatment conditions on age, alcohol problems, drug problems and social problems (as measured by the European version of the Addiction Severity Index [...]". (Verheul 2003, p 135) |
| Allocation concealment (selection bias) | Unclear risk | Comment: no further details |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "although the research assessors were not informed about the treatment condition of their interviewees, it is unlikely that they remained 'masked' throughout the project. Patients might have given them this information, or it could easily have been derived from some of the interviews." (Verheul 2003, p 139) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no further details |
| Selective reporting (reporting bias) | Unclear risk | Comment: no indication of selective reporting, but Insufficient information to permit judgement of 'high' or 'low' risk of bias |
| Other bias | Unclear risk | Comment: no further details |
Weinberg 2006.
| Study characteristics | ||
| Methods | Randomised controlled trial Duration of trial: 6 weekly sessions Country: USA Setting: outpatient |
|
| Participants |
Methods of recruitment of participants: from community through advertising Sample size: 30 Diagnossis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: Structured Clinical Interview for DSM‐IV Axis II Disorders (SCID‐II) and Revised Diagnostic Interview for Borderlines (DIB‐R) Mean age: 28.2 years Sex: 100% female Comorbidity: patients with comorbid disorders were excluded Inclusion criteria:
Exclusion criteria
|
|
| Interventions |
Experimental group Treatment name: manual‐assisted cognitive treatment (MACT) Number randomised to group: 15 Duration: 6 sessions Control/comparison group Comparison name: treatment‐as‐usual (TAU) Number randomised to group: 15 Duration: 6 sessions Both groups Concomitant psychotherapy: both manual‐assisted cognitive treatment (MACT) and treatment‐as‐usual (TAU) participants received treatment as usual; all participants took part in additional treatments that were not further specified. Concomitant pharmacotherapy: both manual‐assisted cognitive treatment (MACT) and treatment‐as‐usual (TAU) participants received treatment‐as‐usual; proportions of participants with medication did not differ at baseline; Fisher = 0.36, df = 1. Proportions of participants taking standing psychotropic medication during trial observation period: unclear |
|
| Outcomes |
Primary:
|
|
| Notes |
Sample size calculation: not stated Ethics approval: not stated Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "randomly assigned" (p 485) Comment: no further details provided regarding randomisation method |
| Allocation concealment (selection bias) | Unclear risk | Comment: no further details |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: "The baseline assessment and administration of the MACT [i.e. the treatment under test] were performed by the primary investigator." (p 486); "Interviewers were randomly assigned for following assessments. The interviewers were blind to baseline ratings and to participants' group allocation". (p 487) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: After screening of 60 referrals by phone, 37 were invited for further assessments. Reasons for exclusion of 7 participants given. 30 participants included in the final sample; n = 15 assigned to the EG and n = 15 to the CG |
| Selective reporting (reporting bias) | Low risk | Comment: no indications for selective reporting |
| Other bias | Unclear risk | Comment: no further details |
Zanarini 2008.
| Study characteristics | ||
| Methods | 12‐week trials with two arms
Duration of trial: 12 weeks Country: USA Setting: outpatient |
|
| Participants |
Methods of recruitment of participants: from community through advertising Sample size: 50 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: Revised Diagnostic Interview for Borderlines (DIB‐R) and Diagnostic Interview for DSM‐IV Personality Disorders (DIPD‐IV) Mean age: 19.3 years (standard deviation = 1.4) Sex: 100% female Comorbidity: lifetime axis I disorders: 39 (78%) met criteria for a mood disorder (mostly major depression), 20 (40%) met criteria for a substance use disorder, 14 (28%) met criteria for an anxiety disorder, and 25 (50%) for an eating disorder. Current prevalence not specified Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group Treatment name: psychoeducation workshop (PEW) Number randomised to group: 30 Duration: 12 weeks Control/comparison group Comparison name: waiting list (WL) Number randomised to group: 20 Duration: 12 weeks Both groups Concomitant psychotherapy: participants that were in any type of current psychiatric treatment were not eligible for study participation. Concomitant pharmacotherapy No significant differences at baseline on treatment histories. 20% had taken medication previously. Proportions of participants taking standing psychotropic medication during trial observation period: unclear |
|
| Outcomes |
Primary:
|
|
| Notes |
Sample size calculation: not stated Ethics approval: not stated Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Using a 3:2 ratio, subjects were either randomized to a workshop that took place within a week of diagnostic disclosure or a waitlist." (p 286) Comment: no further details on method of randomisation provided |
| Allocation concealment (selection bias) | Unclear risk |
Quote: "Fifty subjects were found to meet study criteria for BPD and five who were interviewed did not. These 50 subjects were either randomized to immediate (N = 30) or delayed (N = 20) psychoeducation." (p 286) Comment: no information given about dropouts during the study course |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information given if assessors were blind to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no clear details are provided about attrition rate |
| Selective reporting (reporting bias) | Unclear risk | Comment: no indication of selective reporting, but there was Insufficient information to permit a clear judgement of 'high' or 'low' risk of bias |
| Other bias | Unclear risk | Comment: no further details |
Zanarini 2018.
| Study characteristics | ||
| Methods | 12 month trial with 2 arms
Duration of trial: 12 months Country: USA Setting: outpatient |
|
| Participants |
Method of recruitment of participants: “Recruitment of 80 women between the ages of 18 and 30 years was accomplished through internet‐based advertising in Boston area (primarily on Craiglist)”. (quote, p 53)
Sample size: 80 Diagnosis of borderline personality disorder: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Means of assessment: Structured Clinical Interview for DSM‐IV Axis II Disorders (SCID‐II), Revised Diagnostic Interview for Borderlines (DIB‐R), Background Information Schedule, nonborderline modules of the Diagnostic Interview for DSM‐IV Personality Disorders, and clinician‐administered version of the Zanarini Rating Scale or Borderline Personality Disorder Mean age: internet‐based psychoeducation treatment group = 21.9 years (standard deviation = 3.7), internet‐based control group with no psychoeducation = 20.9 years (standard deviation = 3.1) Sex: 100% female Comorbidity: any mood disorder (58/80), any anxiety disorder (51/80), any substance use disorder (25/80), any eating disorder (16/80). Personality disorders: odd cluster (14/80), anxious cluster (45/80), dramatic cluster (excluding borderline personality disorder) (13/80) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Treatment name: internet‐based psychoeducation treatment
Number randomised to group: 40
Duration: 12 months Control/comparison group Comparison name: internet‐based control group with no psychoeducation Number randomised to group: 40 Duration: 12 months Both groups Concomitant psychotherapy: not stated but patients were excluded if they were in any type of psychiatric treatment Concomitant pharmacotherapy: patients in both groups were taking standing medication as needed at baseline; tendency of more medication use in active group, but not significantly so (treatment group: 32.5% with standing medication at baseline, control group: 15.0%) Proportions of participants taking standing psychotropic medication during trial observation period: unclear |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Sample size calculation: not stated
Ethics approval: yes Comments from review authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Using a computer‐generated list devised by our study statistician”. (p 53) |
| Allocation concealment (selection bias) | Unclear risk | Comment: no clear information was provided about allocation concealment. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: the trial was open‐label meanng there was no blinding. Outcome measurements were based on self‐report completed on the internet, all assesssments were thus unblinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: low attrition rate. 98% completion in EG, and 95% completion in CG. Authors conducted ITT analyses, but did not provide information on imputation method. |
| Selective reporting (reporting bias) | High risk | Comment: Work and Social Adjustment Scale was in the protocol but was excluded from final report. The self‐report version of the ZAN‐BPD was included as an extra outcome in full report relative to the trial registry. No clear indication in trial of which outcome were designated primary and secondary in the registry. 'Borderline evaluation of severity over time' was the only primary outcome. |
| Other bias | Unclear risk |
Attention bias Quote: “Both groups participated in 15 assessment periods that were divided into an acute phase (weeks 1‐12), and a maintenance phase (months 6, 9, 12)”. (p 54) Treatment adherence Comment: no information Allegiance bias Comment: none found |
ACT: acceptance and commitment therapy ADHD: attention deficit hyperactivity disorder AE: adverse events AIAQ: Anger, Irritability and Assault Questionnaire ANOVA: analysis of variance APA: American Psychiatric Association ASIQ‐S: Adult Suicidal Ideation Questionnaire AT: art therapy AUDIT: Alcohol Use Disorders Identification Test BPDFS: Borderline Personality Disorder Feature Scale BPDSI‐IV: Borderline Severity Index, version IV BDI: Beck Depression Inventory BDI‐II: Beck Depression Inventory‐Revision BEST: Borderline Evaluation of Severity over Time BHS: Beck Hopelessness Scale BIS: Barrett Impulsiveness Scale B‐MACT: meta‐cognitive training for borderline patients BMI: body mass index BPD‐40: Borderline Personality Disorder Checklist‐40 BPDSI‐IV(‐A): Borderline Severity Index, Version IV, for Adolescents BPI: Borderline Personality Inventory BPRS: Brief Psychiatric Rating Scale BSI: Borderline Severity Index BSL‐23: Borderline Symptom List‐23 BSS: Beck Scale for Suicidal Ideation CAT: cognitive analytic therapy CCT: client‐centred therapy CG: control group CGI‐BPD: Clinical Global Impression Scale for Borderline Personality Disorder Patients CGI‐S: Clinical Global Impression Scale‐Severity scale CI: confidence interval CM: clinical management CORE‐OM: Clinical Outcomes in Routine Evaluation–Outcome Measure CP: combination psychotherapy CPT II: Conners' Continuous Performance Test II CR: clinician‐rated CT: cognitive therapy CTBE: community treatment by experts DASS: Depression, Anxiety and Stress‐Scale DASS(‐21): Depression, Anxiety and Stress Scale‐21 items DBT: dialectical behavior therapy DBT‐A: DBT adapted for adolescents DB T‐C: DBT couple therapy DBT‐I: DBT individual therapy plus activity group DBT‐IE: DBT interpersonal effectiveness group DBT‐M: DBT mindfulness group DBT‐PE: DBT‐prolonged exposure DBT‐S: DBT skills group plus case management (DBT‐S) DBT‐ST: DBT skills training DDP: dynamic deconstructive psychotherapy DERS: Difficulties in Emotion Regulation Scale DES: Dissociative Experiences Scale DHP: day‐hospital psychotherapy DIB‐R: Revised Diagnostic Interview for Borderlines DIPD‐IV: Diagnostic Interview for DSM‐IV Personality Disorders DPS: Diagnostic Interview Schedule for Children‐Predictive scales DSH: deliberate self‐harm DSHI: Deliberate Self‐Harm Inventory DSM: Diagnostic and Statistical Manual of Mental Disorders DSM‐III‐R: Diagnostic and Statistical Manual of Mental Disorders, 3rd Edition, Revised DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition DSM‐IV‐TR: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision DSM‐5: Diagnostic and Statistical Manual of Mental Disorders, 5th Edition ECR: Experiences in Close Relationships Scale EIT: emotional intelligence training EG: experimental group E‐PDT: psychodynmaic therapy by experts in personality disorders EQ‐5D: EuroQol‐5 Dimensions Questionnaire ERGT: emotion regulation group therapy ERT: emotion regulation training EUC: enhanced usual care FSCRS: Forms of Self‐Criticism/Self‐Attacking and Self‐Reassuring Scale GAD: generalised anxiety disorder GAF(S): Global Assessment of Functioning GAS: Global Assessment Scale GHQ: General Health Questionnaire GPM: general psychiatric management GSFT: group schema‐focused therapy GSI: Global Severity Index of the SCL‐90‐R HADS: Hospital Anxiety and Depression Scale HAM‐D‐17: Hamilton Depression Scale‐17 items HDRS‐17: Hamilton Rating Scale for Depression HoN‐OS: Dutch version of the Health of the Nations Outcome Scales HYPE: helping young people early I‐BAFT: integrative borderline personality disorder‐oriented adolescent family therapy ICD‐10: International Classification of Diseases, 10th Revision IDC: individual drug counselling IIP(‐C)(‐SC): Inventory of Interpersonal Problems ‐ Circumflex version/64‐item version IPDE: Interpersonal Personality Disorder Examination IPT: interpersonal psychotherapy IQ: intelligence quotient IRR: incidence rate ratio ITT: intention to treat JCP: joint crisis plan KDI: Ko's Depression Inventory LIT: limited individual therapy LKM/CM: loving‐kindness and compassion meditation LOCF: last observation carried forward LPC: lifetime parasuicide count LSASI: Lifetime Suicide Attempt Self‐Injury interview MADRS: Montgomery Asberg Depression Rating Scale MCAR: missing completely at random MACI: Millon Adolescent Clinical Inventory MACT: manual‐assisted cognitive psychotherapy MBT: mentalisation‐based treatment MBT‐A: MBT for adolescents MBT‐DH: day hospital MBT MBT‐ED: MBT for eating disorders MBT‐OP: outpatient MBT MCMI‐III: Millon Clinical Multiaxial Inventory, 3rd Edition MCT: meta‐cognitive training MDD: major depressive disorder MF: motivation feedback MFQ: Mood and Feelings Questionnaire MINI: Mini International Neuropsychiatric Interview MOTR: motive‐oriented therapeutic relationship MSc: Master of Science MT: mindfulness training NOS: not otherwise specified NR: not rated ns: not specified NSSI: non‐suicidal self‐injury OAS‐M: Overt Aggression Scale‐Modified for outpatients OIP: outpatient individual psychotherapy OQ‐45: Outcome Questionnaire‐45 ORI: Object Relations Inventory PAI‐BOR: Personality Assessment Inventory ‐ Borderline scale PD: personality disorder PDT: psychodynamic therapy PDQ: Personality Disorders Questionnaire PDSQ: Psychiatric Diagnostic Screening Questionnaire PE: psychoeducation PEPS: psychoeducation and problem solving therapy PEW: psychoeducation workshop PI: principal investigator PIT: psychoanalytic‐interactional therapy PHI: Parasuicide History Interview PP: per protocol PRFP: psychic representation‐focused psychotherapy PTSD: post‐traumatic stress disorder RDIB: Revised Diagnostic Interview for Borderlines SAPAS: Standardised Assessment of Personality: Abbreviated Scale SASS: Social Adaptation Self‐evaluation Scale SASII: Suicide Attempt Self‐Injury Interview SAS‐SR: Social Adaptation Scale‐Self‐Report SB‐APP: sequential brief Adlerian psychodynamic psychotherapy SBQ: Social Behaviour Questionnaire SCID‐I/P: Structured Clinical Interview for DSM‐IV Axis I Disorders Patient Edition SCID‐I: Structured Clinical Interview for DSM‐IV Axis I Disorders SCID‐II: Structured Clinical Interview for DSM‐IV Axis II Personality Disorders SCL‐90‐R: Symptom Checklist‐90‐Revised SCM‐OP: structured clinical management‐outpatient SD: standard deviation SF‐12: 12‐Item Short Form Health Survey SFT(‐G): schema‐focused therapy ‐group SFET: specialist first‐episode psychosis treatment SFQ: Social Functioning Questionnaire SGT: standard group therapy SHBCL: Self‐Harming Behaviours Checklist SIDP‐IV: Structured Interview for DSM‐IV Personality Disorders SMFL: Short Motivation Feedback List SMFQ: Self‐report Mood and Feelings Questionnaire SPS: Social Provisions Scale SSCM‐ED: specialist supportive clinical management for patients with eating disorders SSHI: Suicide and Self‐Harm Inventory ST: schema therapy S‐TAU: specialist treatment as usual STAXI: State‐Trait Anger Expression Inventory STEPPS: systems training for emotional predictability and problem solving STM: supervised team management SUD: substance use disorder TA: therapeutic assessment TAU: treatment‐as‐usual TBR: Target Behaviour Rating TTA: therapist telephone availability UK: United Kingdom WEMBS: Warwick‐Edinburgh Mental Well‐Being Scale WHOQOL‐BREF: World Health Organisation Quality of Life Instruments WL: waiting list WSAS: Work and Social Adjustment Scale ZAN‐BPD: Zanarini Rating Scale for Borderline Personality Disorder
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahmed 2016 | Ineligible patient population |
| Andover 2017 | Ineligible patient population |
| Andreasson 2016 | Proportion of included participants with full BPD unclear. Unable to retreive further information |
| Apsche 2006 | Ineligible patient population |
| Asarnow 2017 | Ineligible patient population |
| Bamelis 2012a | Ineligible patient population |
| Bateman 2019b | Ineligible patient population |
| Bertsch 2013 | Ineligible intervention |
| Bira 2014 | Ineligible patient population |
| Brent 2018 | Ineligible patient population |
| Brune 2012 | Ineligible intervention |
| Buric 2019 | Ineligible patient population |
| Cailhol 2014 | Ineligible intervention |
| Carta 2014 | Ineligible intervention |
| Carter 2013 | Ineligible patient population |
| Chanen 2008 | ineligible population (less than 70% BPD) |
| Charney 2015 | Ineligible patient population |
| Coccaro 1997 | Ineligible patient population |
| Cornelius 1993 | Ineligible intervention |
| Cottrell 2018 | Ineligible patient population |
| Crawford 2018b | Ineligible patient population |
| Cullen 2012a | Ineligible patient population |
| De Beurs 2013 | Ineligible intervention |
| DeSaeger 2014 | Ineligible patient population |
| Dunlop 2012 | Ineligible patient population |
| Ehlers 2013 | Ineligible patient population |
| Ekeblad 2016 | Ineligible patient population |
| EUCTR 2010‐020956‐69 | Ineligible intervention |
| EUCTR 2015‐000749‐21 | Ineligible intervention |
| Fleischer 2015 | Ineligible outcomes |
| Furuno 2018 | Ineligible patient populaiton |
| Ghahramanlou Holloway 2012 | Ineligible patient population |
| Ginty 2016 | Ineligible patient population |
| Gold 2013 | Ineligible intervention |
| Green 2011 | Ineligible patient population |
| Grimholt 2015 | Ineligible patient population |
| Ha 2016 | Ineligible patient population |
| Haddock 2016 | Ineligible patient population |
| Hassanzadeh 2010 | Ineligible patient population |
| Hatcher 2015 | Ineligible patient population |
| Hazell 2009 | Ineligible patient population |
| Hesse 2010 | Ineligible patient population |
| Hewage 2018 | Ineligible patient population |
| Hirayasu 2009 | Ineligible intervention |
| Holder 2017 | Ineligible patient population |
| Hooley 2018 | Ineligible patient population |
| Husain 2011 | Ineligible patient population |
| Husain 2014 | Ineligible patient population |
| Johansson 2010 | Ineligible patient population |
| Kawanishi 2014 | Ineligible patient population |
| Keefe 2016 | Ineligible patient population |
| Keefe 2018 | Ineligible patient population |
| Kellett 2014 | Ineligible patient population |
| Keng 2018 | Ineligible patient population |
| Kennard 2018 | Ineligible patient population |
| Koenigsberg 2003 | Ineligible intervention |
| Krause Utz 2016 | Ineligible intervention |
| Krause Utz 2017 | Ineligible intervention |
| Linehan 1999 | Ineligible outcomes |
| Linehan 2002 | Ineligible outcomes |
| Lorentzen 2013 | Ineligible patient population |
| Maffei 2018 | Ineligible patient population |
| Mahlke 2015 | Ineligible intervention |
| Marasinghe 2012 | Ineligible patient population |
| Marchesi 2006 | Ineligible patient population |
| McAuliffe 2014 | Ineligible patient population |
| Merolla 2017 | Ineligible patient population |
| Moran 2010 | Ineligible intervention |
| Munroe‐Blum 1995 | Ineligible outcomes |
| NCT01311193 | Ineligible intervention |
| NCT02728778 | Ineligible intervention |
| NCT03498937 | Ineligible intervention |
| Nickel 2007a | Ineligible intervention |
| Nickel 2007b | Ineligible intervention |
| Nickel 2008 | Ineligible intervention |
| NL1680/NTR1781 | Ineligible comparator |
| NL5802/NTR5957 | Ineligible intervention |
| O'Connor 2017 | Ineligible patient population |
| Oquendo 2012 | Ineligible patient population |
| Ougrin 2011 | Ineligible patient population |
| Ower 2014 | Ineligible intervention |
| Roberts 2018 | Ineligible patient population |
| Rombold 2014 | Ineligible patient population |
| Rosa 2009 | Ineligible patient population |
| Roussignol 2010 | Ineligible intervention |
| Santamarina Pérez 2017 | Ineligible patient population |
| Schuiringa 2017 | Ineligible patient population |
| Schuppert 2009 | Proportion of participants with full BPD unclear; unable to retreive further information |
| Sharp 2015 | Ineligible intervention |
| Thunnissen 2009 | Ineligible patient population |
| TufekciogIu 2015 | Ineligible patient population |
| Tyrer 2003a | Ineligible patient population |
| Van Beek 2009 | Ineligible patient population |
| Van Dijk 2019 | Ineligible patient population |
| Van Spijker 2010 | Ineligible patient population |
| Vidal 2013 | Ineligible patient population |
| Vijayakumar 2011 | Ineligible patient population |
| Waltz 2009 | Ineligible intervention |
| Watzke 2012 | Ineligible patient population |
| Weertman 2007 | Ineligible patient population |
| Wilks 2018 | Ineligible patient population |
| Wingenfeld 2013 | Ineligible intervention |
| Wingenfeld 2014 | Ineligible intervention |
| Wölwer 2001 | Ineligible patient population |
BPD: borderline personality disorder
Characteristics of studies awaiting classification [ordered by study ID]
Abdelkarim 2016.
| Methods | Not known |
| Participants | People with borderline personality disorder |
| Interventions | Parallel social media in combination with dialectical behaviour therapy (DBT) |
| Outcomes | Compliance to DBT; adverse effects |
| Notes | Conference abstract ‐ no publication yet to enable a definite decision |
Akbari 2009.
| Methods | Randomised controlled trial |
| Participants | People with borderline personality disorder |
| Interventions | Combined cognitive behavioural therapy and pharmacotherapy |
| Outcomes | Depression; anger |
| Notes | Translation required. We will translate it for the next update of this review. |
Cowperthwait 2017.
| Methods | Randomised controlled trial |
| Participants | Adolescents |
| Interventions | Outpatient dialectical behaviour |
| Outcomes | Depression; borderline personality disorder symptoms; emotion dysregulation symptoms; dialectical behaviour therapy skills use |
| Notes | Unable to retrieve paper through contacting the author and help of information retrieval experts |
Dorrepaal 2012.
| Methods | Randomised controlled trial |
| Participants | People with complex post‐traumatic stress disorder (PTSD) and severe comorbidity |
| Interventions | Psychoeducation and cognitive behavioural therapy |
| Outcomes | PTSD symptoms; stress |
| Notes | Subsample data concerning only people with borderline personality disorder is needed. Previous contact with the authors was unsuccessful. We will contact authors again at the next update of this review. |
Ducasse 2018.
| Methods | Randomised controlled trial |
| Participants | Suicidal, adults |
| Interventions | Acceptance and commitment therapy |
| Outcomes | Suicide severity; depressive symptomatology; psychological pain; anxiety; hopelessness; anger; quality of life, and therapeutic processes |
| Notes | Subsample concerning people with borderline personality disorder needed. We will contact authors at next update of this review. |
Einy 2018.
| Methods | Randomised controlled trial |
| Participants | People with borderline personality disorder |
| Interventions | Mentalisation‐based therapy and cognitive‐analytical therapy |
| Outcomes | Insecure attachment; social inefficiency |
| Notes | Translation required. We will translate it for the update of this review. |
Isaia 2017.
| Methods | Not known |
| Participants | People with borderline personality disorder |
| Interventions | Systems training for emotional predictability and problem‐solving (STEPPS) |
| Outcomes | Borderline personality disorder symptomatology; emotion regulation skills |
| Notes | Unable to retrieve paper though contacting the author and help of information retrieval experts |
Johnson 2017.
| Methods | Not known |
| Participants | People with borderline personality disorder |
| Interventions | Cognitive analytic therapy |
| Outcomes | Not known |
| Notes | Unable to retrieve paper though contacting the author and help of information retrieval experts |
McCauley 2018.
| Methods | Randomised controlled trial |
| Participants | Adolescents at high risk of suicide |
| Interventions | Dialectical behaviour therapy |
| Outcomes | Suicide attempts; non‐suicidal self‐injury; self‐harm |
| Notes | Subsample data consisting only of people with borderline personality disorder is needed. We will contact authors again at the next update of this review. |
Ostermeier 2017.
| Methods | Not known |
| Participants | Borderline personality disorder |
| Interventions | Dialectical behaviour therapy |
| Outcomes | Not known |
| Notes | Unable to retrieve paper despite contacting the author twice |
Santamarina 2017.
| Methods | Randomised controlled trial |
| Participants | Adolescents with suicidal behaviour |
| Interventions | Dialectical behaviour therapy |
| Outcomes | Self‐harming behaviour (assessed by number of self‐reported self‐harm episodes); suicidal ideation (assessed with Suicidal Ideation Questionnaire); Clinical Global Impression (CGI) scale for suicide |
| Notes | Conference abstract ‐ no subsequent reports identified for this study |
CGI: Clinical Global Impression scale DBT: dialectical behaviour therapy PTSD: post‐traumatic stress disorder STEPPS: systems training for emotional predictability and problem‐solving
Characteristics of ongoing studies [ordered by study ID]
ACTRN12610000100099.
| Study name |
Public title: A randomised controlled trial of three forms of psychosocial early intervention for borderline personality disorder in youth Scientific title: MOBY: a randomised controlled trial (RCT) of specialised early intervention, with individual cognitive analytic therapy, specialised early intervention without individual psychotherapy, and standard youth mental health care for first‐presentation borderline personality disorder in youth |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria:
|
| Interventions |
|
| Outcomes |
Primary outcomes:
|
| Starting date | 17 March 2011 |
| Contact information |
Name: Dr Andrew Chanen Address: Locked Bag 10, Parkville VIC 3052, Australia E‐mail: achanen@unimelb.edu.au Telephone: +61393422800 |
| Notes | None |
ACTRN12612000951853.
| Study name |
Public title: A clinical trial of mentalization based therapy treatment for borderline personality disorder Scientific title: Outcome effects of a mentalization based treatment service (mindsight) for people with borderline personality disorder in urban Christchurch: a clinical trial and evaluative study |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria:
|
| Interventions | Individual psychotherapy session with a therapist trained in mentalisation‐based treatment method |
| Outcomes |
Primary outcomes:
|
| Starting date | 1 September 2009 (anticipated) |
| Contact information |
Name: Dr David Carlyle Address: Department of Psychological Medicine, University of Otago, PO Box 4345, Christchurch 8011 E‐mail: dave.carlyle@otago.ac.nz Telephone: +64 03 3720400 |
| Notes | None |
ACTRN12612001187831.
| Study name |
Public title: Exploring dialectical behaviour therapy vs conversational model in the treatment of borderline personality disorder: a randomised clinical trial Scientific title: Randomised clinical trial of dialectical behaviour therapy compared to conversational model in the treatment of borderline personality disorder in reducing parasuicidal behaviour and depression |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria:
|
| Interventions | Dialectical behaviour therapy |
| Outcomes | Primary Outcome: Depression scores as measured by Beck Depression Inventory II (BDI‐II) Primary Outcome: Secondary Outcome: Number and severity of BPD symptoms as measured by Borderline Personality Disorder Severity Index (BPDSI) Secondary Outcome: Interpersonal problems as measured by the Inventory of Interpersonal Problems (IIP) Secondary Outcome: Mindfulness skills as measured by the Kentucky Inventory of Mindfulness Scale (KIMS) Secondary Outcome: Dissociation as measured by the Dissociative Experiences Scale (DES) Secondary Outcome: Emotion regulation as measured by the Difficulties in Emotion Regulation Scale (DERS) Secondary Outcome: Sense of self as measured by the Sense of Self Inventory (SSI) |
| Starting date | 12 April 2007 (anticipated) |
| Contact information |
Name: Dr Carla Walton Address: Hunter New England Mental Health Service, PO Box 833, Newcastle NSW 2300, Australia E‐mail: Carla.Walton@hnehealth.nsw.gov.au Telephone: + 61 2 4924 6820 |
| Notes | None |
ACTRN12616000236493.
| Study name |
Public title: Effectiveness of dialectical behaviour therapy (DBT) group skills training for borderline personality disorder (BPD) in community mental health Scientific title: same as public title |
| Methods | Not known |
| Participants |
Inclusion criteria:
|
| Interventions | Dialectical Behaviour Therapy (DBT) Group Skills Training |
| Outcomes |
Primary outcomes:
|
| Starting date | 13 January 2015 |
| Contact information |
Name: Ms Brooke Packham Address: c/o Bordertown Community Health, PO Box 196, Bordertown SA 5268, Australia E‐mail: brooke.packham@sa.gov.au Telephone: + 61887211507 |
| Notes | None |
DRKS00003605.
| Study name | Title: Short‐term psychotherapeutic treatment in adolescents engaging in non‐suicidal self‐injury: a randomised controlled trial |
| Methods | Not known |
| Participants |
Inclusion criteria:
|
| Interventions | The used short‐term treatment programme called “The Cutting Down‐Program” is a manualised short‐term therapy, which is designed for 8 to 12 therapy sessions. It includes elements of the cognitive‐behavioural therapy but also elements of the dialectic‐behavioural therapy. |
| Outcomes |
Primary outcomes:
|
| Starting date | 17 September 2010 |
| Contact information |
Name: Mr Dr Michael Kaess Address: Klinik für Kinder‐ und Jugendpsychiatrie Psychosoziales Zentrum Universitätsklinikum Heidelberg, Blumenstraße 8, 69115 Heidelberg, Germany E‐mail: michael.kaess at med.uni‐heidelberg.de Telephone: 06221‐566914 |
| Notes | None |
DRKS00011534.
| Study name | Title: PRO*BPD: Effectiveness of outpatient treatment PROgrammes for Borderline Personality Disorder: a comparison of schema therapy and dialectical behaviour therapy |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria:
|
| Interventions | Intensive outpatient treatment programme of schema‐focused therapy with one individual and one group session per week for 1.5 years |
| Outcomes |
Primary outcomes:
|
| Starting date | 26 November 2014 |
| Contact information |
Name: Ms Dr Med Eva Faßbinder Address: Klinik für Psychiatrie und Psychotherapie, Universität zu Lübeck, Ratzeburger Allee 160, 23538 Lübeck, Germany E‐mail:eva.fassbinder at uksh.de Telephone: 0451‐50098702 |
| Notes | None |
ISRCTN21802136.
| Study name |
Public title: Coping with Unusual ExperienceS for 12‐18 (CUES+) Scientific title: Coping with Unusual ExperienceS for 12‐18 year olds (CUES+): a transdiagnostic randomised controlled trial of the effectiveness of cognitive therapy in reducing distress associated with unusual experiences in adolescent mental health services: study protocol for a randomised controlled trial |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria:
|
| Interventions | Therapy will consist of up to 16 sessions, given over 16 weeks, including individual cognitive behavioural therapy for psychosis adapted for adolescents, and 3‐4 family support sessions. Family work comprises recognition and understanding of the child’s difficulties, sharing the intervention plan, and troubleshooting any key family difficulties. Individual work focuses on developing a collaborative understanding of UEDs, together with skills in affect regulation, managing negative automatic thoughts, behavioural tests, dealing with social difficulties and adverse life events, recognising and compensating for cognitive biases and a section on taking the work forward and preventing future difficulties. Therapy is tailored to take account of the developmental stage and presenting issues of the child/young person. Therapy materials have been designed to be fun, interactive and engaging. |
| Outcomes |
Primary outcomes:
|
| Starting date | 5 January 2015 |
| Contact information |
Name: Dr Suzanne Jolley Address: King's College London, Institute of Psychiatry, Department of Psychology (PO77), 16 De Crespigny Park, London SE5 8AF, United Kingdom E‐mail: not provided Telephone: not provided |
| Notes | None |
NCT00603421.
| Study name | Title: Effectiveness of a 24 hour phone line on the rate of suicide attempts in people affected by borderline personality disorder |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria:
|
| Interventions | One year of 1. Treatment‐as‐usual (TAU) + access to a 24 hour crisis phone line 2. TAU |
| Outcomes |
Primary outcomes:
|
| Starting date | February 2009 |
| Contact information |
Name: Alexandra Pham‐Scottez; Daniel Guelfi Address: Hôpital St. Anne, Paris, France 75014 E‐mail: a.pham@ch‐sainte‐anne.fr; jd.guelfi@ch‐sainte‐anne.fr Telephone: not provided |
| Notes | None |
NCT01531634.
| Study name | Title: Promoting recovery processes in women with borderline personality disorder using a dynamic cognitive intervention |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria:
|
| Interventions | Behavioural: dynamic cognitive intervention group |
| Outcomes |
Primary outcomes:
|
| Starting date | Not provided. First received 3 February 2012 |
| Contact information |
Name: Orly Tsabar, BOT Address: Tel Aviv University E‐mail: orly.tsabar@gmail.com Telephone: 972‐54‐6852344 |
| Notes | None |
NCT01823120.
| Study name |
Public title: Text message intervention to reduce repeat self‐harm Scientific title: Text message intervention to reduce repeat self‐harm in patients presenting to the emergency department |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria:
|
| Interventions | Supportive and interactive text messages |
| Outcomes | Primary outcomes:
|
| Starting date | March 2015. Specific date not provided |
| Contact information |
Name: Vincent IO Agyapong, MRCPsych MD Address: University of Dublin, Trinity College Dublin E‐mail: not provided Telephone: not provided |
| Notes | None |
NCT02068326.
| Study name |
Public title: MBT in groups for adolescents with BPD or subthreshold BPD versus TAU ‐ the M‐GAB randomized controlled trial (M‐GAB) Scientific title: Mentalization‐based treatment in groups for adolescents with borderline personality disorder (BPD) or subthreshold BPD versus treatment‐as‐usual ‐ the M‐GAB randomised controlled trial (M‐GAB) |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria:
|
| Interventions | Mentalisation‐based treatment |
| Outcomes |
Primary outcomes:
|
| Starting date | September 2015. Specific date not provided |
| Contact information |
Name: Erik Simonsen, PhD Address: Child and Adolescent Psychiatric Department, Region Zealand, Denmark E‐mail: not provided Telephone: not provided |
| Notes | None |
NCT02125942.
| Study name |
Public title: Central meditation and imagery therapy for augmentation of borderline personality disorder treatment Scientific title: Pilot study of central meditation and imagery therapy for borderline personality disorder |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria:
|
| Interventions | Central meditation and imagery therapy |
| Outcomes |
Primary outcomes:
|
| Starting date | April 2014 |
| Contact information |
Name: Felipe Jain, Clinical Instructor of Psychiatry Address: University of California, Los Angeles E‐mail: not provided Telephone: not provided |
| Notes | None |
NCT02126787.
| Study name |
Public title: Short‐term, intensive psychodynamic group therapy versus cognitive‐behavioral group therapy in the day treatment Scientific title: Short‐term, intensive psychodynamic group therapy versus cognitive‐behavioral group therapy in the day treatment of anxiety disorders and comorbid depressive or personality disorders |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria:
|
| Interventions | Intensive group analytic psychotherapy |
| Outcomes | Primary outcomes:
|
| Starting date | September 2014. Specific date not provided |
| Contact information |
Name: Andrzej Kokoszka, MD, PhD Address: Hospital of Wola, Warsaw, Poland, 01‐211 E‐mail:andrzej.kokoszka@wum.edu.pl Telephone: +48603128361 |
| Notes | None |
NCT02134223.
| Study name |
Public title: Methylation status of BDNF gene after dialectical behavior therapy in BPD Scientific title: Changes in methylation status of BDNF gene after receiving dialectical behavior therapy in patients with borderline personality disorder |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria:
|
| Interventions | Dialectical behaviour therapy |
| Outcomes |
Primary outcomes:
|
| Starting date | April 2014. Specific date not provided |
| Contact information |
Name: Shu‐I Wu Address: Mackay Memorial Hospital, Taipei, Taiwan E‐mail:shuiwu624@gmail.com Telephone: +886‐2‐88094661 ext 3055 |
| Notes | None |
NCT02387736.
| Study name |
Public title: DBT for chronically self‐harming individuals with BPD: evaluating the clinical & cost effectiveness of a 6 mo treatment (FASTER‐DBT) Scientific title: Dialectical behaviour therapy for chronically self‐harming individuals with BPD: evaluating the clinical and cost effectiveness of a 6‐month treatment |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria:
|
| Interventions | Dialectical behaviour therapy ‐ 6 months |
| Outcomes |
Primary outcomes:
|
| Starting date | February 2015. Specific date not provided |
| Contact information |
Name: Shelly McMain Address: Centre for Addiction and Mental Health, 60 White Squirrel Way, Toronto, ON M6J 1H4, Canada E‐mail: not provided Telephone: not provided |
| Notes | None |
NCT02517723.
| Study name |
Public title: Narrative exposure therapy in women with borderline personality disorder and post‐traumatic stress disorder Scientific title: A randomized controlled clinical trial (RCCT) to test the effectiveness of narrative exposure therapy (NET) versus dialectical‐behavioral therapy in reducing trauma related symptoms in women suffering from borderline personality disorder (BPD) and post‐traumatic stress disorder (PTSD) |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria:
|
| Interventions |
|
| Outcomes |
Primary outcomes:
|
| Starting date | April 2014. Specific date not provided |
| Contact information |
Name: Professor, Dr Med Martin Driessen; Evangelisches Krankenhaus Bielefeld Address: Clinic of Psychiatry, Evangelisches Krankenhaus Bielefeld, Bielefeld, Germany 33617 E‐mail: not provided Telephone: not provided |
| Notes | None |
NCT02685943.
| Study name |
Public title: A randomised trial for suicidal patients Scientific title: 'Collaborative assessment and management of suicidality' (CAMS) in comparison to 'Treatment‐as‐usual' (TAU) for suicidal patients: a randomized controlled trial |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria:
|
| Interventions | Psychotherapy |
| Outcomes |
Primary outcomes:
|
| Starting date | April 2015. Specific date not provided |
| Contact information |
Name: Dr Roar Fosse Address: Vestre Viken Helseforetak, Drammen, Norway, 3004 E‐mail: not provided Telephone: not provided |
| Notes | None |
NCT02771691.
| Study name |
Public title: Mentalization‐based group therapy for adolescents Scientific title: Efficacy of mentalization‐based group therapy for adolescents: a pilot randomised controlled trial |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria:
|
| Interventions | Mentalisation‐based group therapy for adolescents |
| Outcomes |
Primary outcomes:
|
| Starting date | February 2016. Specific date not provided |
| Contact information |
Name: not provided Address: CAMHS, Edinburgh, Lothian, United Kingdom E‐mail: not provided Telephone: not provided |
| Notes | None |
NCT02985047.
| Study name |
Public title: Brief admission Skåne: replacing general admission for individuals with self‐harm and acute risk of suicide (BAS) Scientific title: Brief admission Skåne: can brief admission replace general admission for individuals with self‐harm and acute risk of suicide |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria:
|
| Interventions | Brief admission |
| Outcomes |
Primary outcomes:
|
| Starting date | 1 September 2015 |
| Contact information |
Name: Sofie Westling, MD, PhD Address: Division of Psychiatry, Region Skåne, Lund, Skåne, Sweden, 22185 E‐mail: not provided Telephone: not provided |
| Notes | None |
NCT02991586.
| Study name |
Public title: Effectiveness study of a dialectical behavioral treatment for families of adolescents with emotional instability (FAL) Scientific title: Effectiveness study of a dialectical behavioral treatment for families of adolescents with emotional instability |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria:
|
| Interventions | Dialectical behaviour therapy |
| Outcomes |
Primary outcomes:
|
| Starting date | May 2016. Specific date not provided |
| Contact information |
Name: María Mayoral, Clinical Psychologist Address: Instituto Provincial de Psiquiatria, Hospital General Universitario Gregorio Maranon. Calle Ibiza 43, Madrid, Spain, 28009 E‐mail: maria.mayoral@iisgm.com Telephone: +34 914265005 |
| Notes | None |
NCT03011190.
| Study name |
Public title: Effectiveness of the iconic therapy for borderline personality disorder symptoms Scientific title: Effectiveness of the iconic therapy in youth with suicidal ideation/self‐injuring behavior and borderline personality traits: study protocol for a randomized controlled trial |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria:
|
| Interventions | Emotional regulation |
| Outcomes |
Primary outcomes:
|
| Starting date | September 2015. Specific date not provided |
| Contact information |
Name: Silvia Elisa Hurtado Santiago, Principal investigator Address: University of Malaga, Malaga, Spain 29190 E‐mail: not provided Telephone: not provided |
| Notes | None |
NCT03092271.
| Study name |
Public title: Randomised trial of stepped care for suicide prevention in teens and young adults (Step2Health) Scientific title: Randomized trial of stepped care for suicide prevention in teens and young adults |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria:
|
| Interventions | Behavioural: stepped care for suicide prevention Behavioural: zero suicide quality improvement |
| Outcomes |
Primary outcomes:
|
| Starting date | 3 April 2017 |
| Contact information |
Name: Dr Joan R Asarnow Address: University of California Los Angeles (UCLA), Los Angeles, California, United States, 90095‐6968 E‐mail:jasarnow@mednet.ucla.edu Telephone: 310 825‐0408 |
| Notes | None |
NCT03185026.
| Study name |
Public title: Psychoeducation for suicidal behavior (PEPSUI) Scientific title: Effectiveness of the first French psychoeducational program for suicidal behavior: a randomized controlled trial |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria:
|
| Interventions | PEPSUI psychoeducational programme |
| Outcomes |
Primary outcomes:
|
| Starting date | 1 July 2017 (estimated) |
| Contact information |
Name: Déborah DUCASSE, MD Address: University Hospital, Montpellier E‐mail:d-ducasse@chu-montpellier.fr Telephone: 00 33 4 67 33 82 89 |
| Notes | None |
NCT03191565.
| Study name |
Public title: Using smartphones for self‐monitoring of skill‐use in dialectical behavior therapy (mDIARY) Scientific title: The mDIARY study: using smartphones for daily self‐monitoring of skill‐use and outcome in dialectical behavior therapy with borderline personality disorder: a combined RCT and time series study |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria:
|
| Interventions | Monsenso dialectical behaviour therapy (DBT)‐app and IT monitoring programme |
| Outcomes |
Primary outcomes:
|
| Starting date | 15 June 2017 |
| Contact information |
Name: Stig Helweg‐Jørgensen PhD Address: Region of Southern Denmark, Denmark E‐mail: not provided Telephone: not provided |
| Notes | None |
NCT03297840.
| Study name |
Public title: Change in mindfulness in borderline personality disorder Scientific title: Change in mindfulness in borderline personality disorder after dialectic‐behavioural therapy (DBT): a pre‐post‐comparison |
| Methods | Registry stated "current observational study" (quote) |
| Participants |
Inclusion criteria:
|
| Interventions | Dialectical behavioural therapy (DBT) |
| Outcomes |
Primary outcomes:
|
| Starting date | 4 May 2017 |
| Contact information |
Name: Katharina Bachmann, Psych Msc Address: Department of Psychiatry and Psychotherapy ‐ University Hospital, University of Oldenburg, Bad Zwischenahn, Germany, 26160 E‐mail:Katharina.Bachmann@uni-oldenburg.de Telephone: 00494119615 1506 |
| Notes | None |
NCT03376113.
| Study name | Title: The effect of a brief psychological intervention on reducing self‐harm repetition: feasibility study |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria:
|
| Interventions | The volitional help sheet |
| Outcomes |
Primary outcomes:
|
| Starting date | 3 November 2017 |
| Contact information |
Name: Shu‐Sen Chang, MD, MSc, PhD Address: National Taiwan University Hospital, Taipei, Taiwan, 100 E‐mail:shusenchang@ntu.edu.tw Telephone: +886 2 33668062 |
| Notes | None |
NCT03418142.
| Study name |
Public title: Evaluating an Internet‐based self‐management intervention for borderline (REVISIT) Scientific title: Research evaluating the effectiVeness of adding an Internet‐based self‐management intervention to usual care in the treatment of borderline personality disorder ‐ REVISIT BPD |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria:
|
| Interventions | Internet‐based self‐management intervention for borderline personality disorder (REVISIT) |
| Outcomes |
Primary outcomes:
|
| Starting date | 29 January 2018 |
| Contact information |
Name: Andrea Hauer Address: Gaia AG Hamburg, Germany, 20144 E‐mail:andrea.hauer@gaia-group.com Telephone: +4940349930382 |
| Notes | None |
NCT03677037.
| Study name |
Public title: The short‐term MBT project (MBT‐RCT) Scientific title: Short‐term versus long‐term mentalization‐based therapy for outpatients with subthreshold or diagnosed borderline personality disorder: a randomized clinical trial |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria:
|
| Interventions |
|
| Outcomes |
Primary outcomes:
|
| Starting date | 24 September 2018 |
| Contact information |
Name: Sophie Juul Msc Address: Stolpegaard Psychotherapy Centre, Mental Health Services, Capital Region of Denmark, Gentofte, Denmark, 2820 E‐mail:sophie.juul@regionh.dk Telephone: 004538645324 |
| Notes | None |
NCT03833453.
| Study name |
Public title: Effectiveness of PTSD‐treatment compared to integrated PTSD‐PD‐treatment in adult patients with comorbid PTSD and BPD (PROSPER‐B) Scientific title: Prediction and outcome study in PTSD and (borderline) personality disorders |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria:
|
| Interventions | behavioural: dialectical behaviour therapy |
| Outcomes |
Primary outcomes:
|
| Starting date | 1 June 2018 |
| Contact information |
Name: Aishah Snoek, MSc Address: Sinai Centrum, Amstelveen, Noord‐Holand, Netherlands, 1180EB E‐mail:aishah.snoek@sinaicentrum.nl Telephone: 0031‐20‐5457200 |
| Notes | None |
NL1144/NTR1186.
| Study name |
Public title: SFT for forensic PD patients Scientific title: Efficacy of schema‐focused therapy (SFT) versus usual treatment in forensic patients with personality disorders: a three‐year randomised clinical trial |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria:
|
| Interventions | Forensic setting, three years of individual SFT |
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
| Starting date | 1 October 2007 |
| Contact information |
Name: Professor David Bernstein Address: University of Maastricht, Departement of Clinical Psychological Science, PO Box 616, 6200 MD, Maastricht, The Netherlands E‐mail: D.Bernstein@dmkep.unimaas.nl Telephone: +31 478 635 200 |
| Notes | None |
NL2168/NTR2292.
| Study name |
Public title: Dosage‐trial mentalisation‐based treatment (MBT): intensive outpatient MBT versus day hospital MBT Scientific title: Intensive outpatient mentalisation‐based treatment versus day hospital mentalisation‐based treatment: a randomised controlled trial |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria:
|
| Interventions | The MBT‐programme offers 18‐month psychotherapy designed specifically for treatment refractory people with complex personality disorders, often complicated by multi‐morbidity, who have typically had a history of unsuccessful treatments. Day hospital MBT (DH‐MBT) consists of daily group psychotherapy, weekly individual psychotherapy, individual crisis planning from a mentalising perspective, art therapy twice a week, and writing therapy. Intensive outpatient MBT (IOP‐MBT) consists of group psychotherapy twice a week, weekly individual psychotherapy, and individual crisis planning from a mentalising perspective. |
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
| Starting date | 8 February 2010 |
| Contact information |
Name: Helene Andrea Address: Viersprong Institute for Studies on Personality Disorders (VISPD), PO Box 7, 4660 AA Halsteren, The Netherlands E‐mail: helene.andrea@deviersprong.nl Telephone: +31 (0)164 632200 |
| Notes | None |
NL2266/NTR2392.
| Study name |
Public title: Group schema therapy for borderline personality disorder Scientific title: Multi‐Site RCT of group schema therapy for borderline personality disorder |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria:
|
| Interventions |
|
| Outcomes |
Primary outcomes:
|
| Starting date | 1 February 2010 |
| Contact information |
Name: Professor Arnoud Arntz Address: University Maastricht (UM), DMKEP, PO Box 616, 6200 MD Maastricht, The Netherlands E‐mail: Arnoud.Arntz@MP.Unimaas.nl Telephone: +31 (0)43 3881228 |
| Notes | None |
NL3856/NTR4016.
| Study name |
Public title: Toetsing van een geïntegreerd behandelprotocol voor patiënten met een bipolaire stoornis en een comorbide borderline persoonlijkheidskenmerken. Een randomized clinical trial Scientific title: The addition of STEPPS in the treatment of people with bipolar disorder and comorbid borderline personality features: a protocol for a randomised controlled trial |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria:
|
| Interventions | Investigational treatment of the study will be the STEPPS training added to individual TAU for bipolar disorder. The STEPPS training is a group treatment developed for people with BPD to improve their emotion regulation. The training consists of 20 weekly sessions of approximately 2.5 hours and consists of 4 parts: psychoeducation, emotion regulation skills, behavioural skills and emotion handling plan. |
| Outcomes |
Primary outcomes:
|
| Starting date | 25 August 2013 |
| Contact information |
Name: G Riemann Address: Saxion Hogescholen Academie Mens en Arbeid Handelskade 75, 7417 DH Deventer, The Netherlands E‐mail: g.riemann@saxion.nl Telephone: not provided |
| Notes | None |
BAS: Brief Admission Skåne BDI(‐II): Beck Depression Inventory (‐Revised) IIBDNF: brain‐derived neurotrophic factor BPD: borderline personality disorder BPDSI: Borderline Personality Severity Index BPFS‐C: Borderline Personality Features Scale for Children BSL‐23: Borderline Symptom List‐23 BSSI: Beck Scale for Suicide Ideation CAMHS: children and adolescents mental health services CAMS: Cognitive and Affective Mindfulness Scale CAPS(‐5): Clinician‐Administered PTSD Scale for DSM‐5 COPE: Coping Orientation to Problems Experienced Inventory C‐SSRS: Columbia Suicide Severity Rating Scale CUES+: Coping with Unusual Experiences for 12–18 year olds DBT: dialectical behavior therapy DERS: Developmental Environmental Rating Scale DES: Dissociative Experiences Scale DH: day hospital DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition DSM‐IV‐TR: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision ED: emergency department ER: emergency room FAL: families of adolescents with emotional instability FASTER‐DBT: the feasibility of a shorter treatment and evaluating responses for dialectical behavior therapy IIP‐C: Inventory of Interpersonal Problems Circumplex Scales IOP: intensive outpatient IPRI: Automatic Anger and Hostility Thoughts Scale IT: information technology KIMS: Kentucky Inventory of Mindfulness Skills MBT: mentalisation‐based treatment mDIARY: using smartphones for self‐monitoring of skill‐use in dialectical behavior therapy M‐GAB: Mentalization‐based treatment in Groups for Adolescence with Borderline personality disorder or subthreshold borderline personality disorder MOBY: Monitoring Outcomes of Borderline personality disorder in Youth NET: narrative exposure therapy NOS: bipolar disorder‐not otherwise specified PD: personality disorder PEPSUI: psychoeducation for suicidal behavior PRO*BPD: PROgrams for Borderline Personality Disorder PROSPER‐B: Prediction and Outcome Study in PTSD and (Borderline) Personality disorders PTSD: post‐traumatic stress disorder RCCT: Rogers' client centred therapy RCT: randomised controlled trial REVISIT: Research Ealuating the effectiVeness of adding an Internet‐based Self‐management Intervention to usual care in the Treatment of Borderline Personality disorder SASII: Suicide Attempt Self‐Injury Interview SAS‐SR: Social Adjustment Scale Self‐Report SCID‐II: Structured Clinical Interview for DSM Axis II disorders SDQ: Strengths and Difficulties Questionnaire SFT: schema focused therapy SITBI‐G: Self‐Injurious Thoughts and Behaviors Interview‐German version SSI: Scale for Suicidal Ideation Step2Health: randomised trial of stepped care for suicide prevention in teens and young adults STEPPS: systems training for emotional predictability and problem solving TAU: treatment‐as‐usual UED: Unusual Experiences associated with Distress ZAN‐BPD: Zanarini Rating Scale for Borderline Personality disorder
Differences between protocol and review
Authorship
The previous version of this review had the following authors.
Jutta M Stoffers
Birgit A Völlm
Gerta Rücker
Antje Timmer
Nick Huband
Klaus Lieb
Gerta Rücker and Antje Timmer were not involved in either preparing the protocol or the updated current version of the review.
The protocol of the current version of the review had the following authors.
Ole Jakob Storebø
Jutta M Stoffers‐Winterling
Birgit A Völlm
Mickey T Kongerslev
Jessica T Mattivi
Maja Lærke Kielsholm
Signe Sofie Nielsen
Mie Sedoc Jørgensen
Erlend Faltinsen
Klaus Lieb
Erik Simonsen
These following authors worked on the final review but not on the protocol stage and received authorship.
Adnan Todorovac
Christian Paul Sales
Henriette E. Callesen
The following authors did not contribute sufficiently to the final review after the protocol stage and did not receive authorship on the current version of the review.
Maja Lærke Kielsholm
Signe Sofie Nielsen
Methods
We were not able to use all of our methods as planned (Storebø 2018). We report the unused methods in Table 4.
Search methods for identification of studies: searching other resources
Whilst not specified in the protocol (Storebø 2018), we intend to handsearch the following journals in future updates of this review, in addition to those already listed under Searching other resources:
Borderline Personality Disorder and Emotion Dysregulation; and
Personality and Mental Health.
Furthermore, we will handsearch any other journal wherein a substantial number of primary trials included in this version of the review have been published, and which may not be included in the list of journals to be handsearched.
Data collection and analysis
Selection of studies
The protocol specified that six review authors (OJS, JMSW, BAV, JTM, MTK, SSN) would work in pairs and independently screen titles and abstracts of all records retrieved by the searches. However, the following additional review authors also selected trials: AT; EF; MSJ and HEC.
The following authors, who worked on the protocol for the review, selected some trials but left the author group early in the development of the review as they no longer wished to be authors: SSN and MTK.
The protocol moreover specified that KL and ES would act as arbiters. However, in the review, OJS and JMSW also functioned as arbiters.
Assessment of risk of bias in included studies
We intend to assess the risk of bias due to unequal rates of medication use in the respective treatment groups in future updates of this review.
Measures of treatment effects: continuous data
For outcomes for which we did not compute an MIREDIF, we specified that we would provide an interpretation of the effect size using Cohen's D (Cohen 1988), considering 0.2 as a small effect, 0.5 as a medium effect size, and 0.8 as a large effect size, in order to help the reader to understand the results.
Unit of analysis issues: adjustment for multiplicity
We planned to adjust the P values and CIs for multiplicity due to the many secondary outcome comparisons following the method described by Jakobsen 2014. However, we only adjusted the primary outcomes and one secondary outcome for multiplicity. We did this because we only wanted to adjust the outcomes presented in the 'Summary of findings' table and felt that adjusting all secondary outcomes was not necessary.
Subgroup analysis and investigation of heterogeneity
Due to a lack of study samples with mean ages above 50 years, we only performed subgroup analyses for the age groups of 15 to under 18 years of age and above 18 years of age.
Moreover, we added the following four, post hoc subgroup analyses.
Types of raters
Types of TAU
Trials comparing psychotherapy with TAU compared with trials comparing psychotherapy with waiting list or no treatment
Types of scales
TSA
When performing the TSA analyses, we did not use a standard deviation (SD) for the primary outcome of 1.0, and we did not always use an anticipated intervention effect of Hedge´s g of 0.5 ( ½ SD) as described in the protocol (Storebø 2018). Instead, we used the SD from the trial as a basis for the transformation of SMD values to MD values. Also, we preferred to use the MIREDIF reported in articles, and only if we could not find it did we use the ½ SD.
Sensitivity analysis
We added one analysis post hoc: imprecision as assessed by GRADE, by conducting TSA analyses on all primary outcomes and the three secondary outcomes (for the main comparison versus TAU) not closely connected to the BPD core symptoms (depression, attrition and adverse effects) with significant findings.
Contributions of authors
Ole Jakob Storebø: protocol development; study selection; data extraction; data entry; assessment of risk of bias and GRADE; data analysis; and writing of final report
Jutta M Stoffers‐Winterling: protocol development; study selection; data extraction; data entry; assessment of risk of bias and GRADE; data analysis; and writing of final report
Birgit A Völlm: protocol development; study selection; data extraction; and writing of final report
Mickey T Kongerslev: protocol development; study selection; data extraction; assessment of risk of bias; and writing of final report
Jessica T Mattivi: protocol development; study selection; data extraction; and writing of final report
Mie Sedoc Jørgensen: study selection; data extraction; assessment of risk of bias; and writing of final report
Erlend Faltinsen: study selection; data extraction; data entry; assessment of risk of bias; data analysis; and writing of final report
Adnan Todorovac: study selection; data extraction; data entry; assessment of risk of bias; data analysis; and writing of final report
Christian Paul Sales: study selection; data extraction; and writing of final report
Henriette E Callesen: study selection, data extraction; assessment of risk of bias and GRADE; data analysis; and writing of final report
Klaus Lieb: protocol development; data extraction; and writing of final report
Erik Simonsen: protocol development; data extraction; and writing of final report
All authors contributed to writing the review. Ole Jakob Storebø is the guarantor for the review.
Sources of support
Internal sources
-
Psychiatric Research Unit, Region Zealand Psychiatry, Roskilde, Denmark
Ole Jakob Storebø, Mickey Kongerslev, Mie Poulsgaard Jørgensen, Henriette Edeman Callesen, Adnan Todorovac, Erlend Faltinsen, and Erik Simonsen worked on the review during office hours.
-
University Medical Center Mainz, Department of Psychiatry and Psychotherapy, Germany
Jutta Stoffers‐Winterling, Jessica Mattivi and Klaus Lieb worked on the review during office hours.
External sources
None, Other
Declarations of interest
Jutta M Stoffers‐Winterling is a board‐certified psychologist, who has worked on a dialectical behaviour therapy (DBT) ward, and attended courses on DBT and schema‐focused therapy (SFT).
Ole Jakob Storebø (OJS) is an Editor with Cochrane Developmental, Psychosocial and Learning Problems (CDPLP). He is involved in a trial investigating group mentalisation‐based treatment (MBT) for adolescents with borderline personality disorder (BPD). This trial is included in the review as an ongoing study. OJS was not involved in the evaluation of this trial. The assessment of eligibility of the trial was done by Erlend Faltinsen and Adnan Todorovac.
Jessica T Mattivi's institution received a grant from the German Federal Ministry of Education and Research for a systematic review on psychosocial interventions for self‐harm in adolescents and a systematic review on pharmacological and non‐pharmacological interventions for post‐operative delirium in older patients.
Birgit A Völlm ‒ none known.
Mickey Kongerslev is a certified specialist in psychotherapy from the Danish Psychological Association. He has received training in group analysis, cognitive behavioural therapy, and MBT. He received money, from private and public agencies, for teaching MBT for BPD, including supervising psychologists under training to becoming licenced 'special psykolog' certified by the Danish National Health Authorities, and has published scientific articles together with the developers of this treatment. He also receives money for teaching and supervision in assessment and management of personality disorder.
Mie Poulsgaard Jørgensen (MPJ) is a trained DBT therapist and currently conducting a trial on group MBT for adolescents with BPD. This trial is included in the review as an ongoing study. MPJ was not involved in the evaluation of this trial. The assessment of eligibility of the trial was done by Erlend Faltinsen and Adnan Todorovac.
Henriette Edemann Callesen ‐ none known.
Adnan Todorovac ‐ none known.
Christian Sales ‐ none known.
Erlend Faltinsen ‒ none known.
Klaus Lieb (KL) is an Editor with CDPLP. He is a board‐certified cognitive behaviour therapist with a special interest in schema therapy. KL has been involved in trials investigating inpatient DBT (Bohus 2004); and inpatient SFT (Reiss 2014). He was not involved in the evaluation of these trials (Bohus 2004: Jutta M Stoffers‐Winterling and Birgit A Völlm did eligibility assessments for the previous version of this review; Reiss 2014: Signe Sofie Nielsen and Mickey Kongerslev did the eligibility assessment for this study for the current review).
Erik Simonsen is a board‐certified therapist in group analysis.
Shared 1st authorship.
Shared 1st authorship.
Shared last authorship.
Shared last authorship.
Edited (no change to conclusions)
References
References to studies included in this review
Amianto 2011 {published data only}
- Amianto F, Ferrero A, Pierò A, Cairo E, Rocca G, Simonelli B, et al. Supervised team management, with or without structured psychotherapy, in heavy users of a mental health service with borderline personality disorder: a two-year follow-up preliminary randomized study. BMC Psychiatry 2011;11:181. [DOI: 10.1186/1471-244X-11-181] [PMC3260092 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01356069. Efficacy of time-limited psychodynamic psychotherapy and informed clinical management in BPD high MHS users (HUMSH) [Sequential brief Adlerian psychodynamic psychotherapy in heavy users of a mental health service with borderline personality disorder: a two years follow-up preliminary randomized study]. clinicaltrials.gov/ct2/show/NCT01356069 (first received 16 May 2011).
Andreoli 2016 {published data only}
- Andreoli A, Burnand Y, Cochennec MF, Marie D, Di Clemente T, Ohlendorf P. Psychoanalytic psychotherapy and venlafaxine among acutely suicidal borderline patients: a randomized clinical study. European Psychiatry 2009;24(Suppl 1):S93. [DOI: 10.1016/S0924-9338(09)70326-8] [S18-03] [DOI] [Google Scholar]
- Andreoli A, Burnand Y, Cochennec MF, Ohlendorf P, Frambati L, Gaudry-Maire D, et al. Disappointed love and suicide: a randomized controlled trial of "abandonment psychotherapy" among borderline patients. Journal of Personality Disorders 2016;30(2):271-87. [DOI: 10.1521/pedi_2015_29_196] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Andreoli A, Burnand Y, Frambati L, Manning D, Frances A. Abandonment psychotherapy and psychosocial functioning among suicidal patients with borderline personality disorder: a 3-year naturalistic follow-up. Journal of Personality Disorders 2019 Feb 20 [Epub ahead of print]. [DOI: 10.1521/pedi_2019_33_423] [PMID: ] [DOI] [PubMed]
- Andreoli A. Re: your study in BPD - Cochrane Collaboration review [personal communication]. Email to: J Stoffers-Winterling 5 June 2018.
Antonsen 2017 {published data only}
- Antonsen BT, Johansen MS, Rø FG, Kvarstein EH, Wilberg T. Is reflective functioning associated with clinical symptoms and long-term course in patients with personality disorders? Comprehensive Psychiatry 2016;64:46-58. [DOI: 10.1016/j.comppsych.2015.05.016] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Antonsen BT, Klungsøyr O, Kamps A, Hummelen B, Johansen MS, Pedersen G, et al. Step-down versus outpatient psychotherapeutic treatment for personality disorders: 6-year follow-up of the Ullevål personality project. BMC Psychiatry 2014;14:119. [DOI: 10.1186/1471-244X-14-119] [PMC4000615] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonsen BT, Kvarstein EH, Urnes Ø, Hummelen B, Karterud S, Wilberg T. Favourable outcome of long-term combined psychotherapy for patients with borderline personality disorder: six-year follow-up of a randomized study. Psychotherapy Research 2017;27(1):51-63. [DOI: 10.1080/10503307.2015.1072283] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Arnevik E, Wilberg T, Urnes Ø, Johansen M, Monsen JT, Karterud S. Psychotherapy for personality disorders: short-term day hospital psychotherapy versus outpatient individual therapy - a randomized controlled study. European Psychiatry 2009;24(2):71-8. [DOI: 10.1016/j.eurpsy.2008.09.004] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Gullestad FS, Johansen MS, Høglend P, Karterud S, Wilberg T. Mentalization as a moderator of treatment effects: findings from a randomized clinical trial for personality disorders. Psychotherapy Research 2013;23(6):674-89. [DOI: 10.1080/10503307.2012.684103] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Gullestad FS. Re: questions regarding your trial on day hospital step-down program [personal communication]. Email to: E Faltinsen 4 December 2017.
- Kvarstein EH, Arnevik E, Halsteinli V, Rø FG, Karterud S, Wilberg T. Health service costs and clinical gains of psychotherapy for personality disorders: a randomized controlled trial of day-hospital-based step-down treatment versus outpatient treatment at a specialist practice. BMC Psychiatry 2013;13:315. [DOI: 10.1186/1471-244X-13-315] [PMC4222503] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bateman 1999 {published data only}
- Bateman A, Fonagy P. 8-year follow-up of patients treated for borderline personality disorder: mentalization-based treatment versus treatment as usual. American Journal of Psychiatry 2008;165(5):631-8. [DOI: 10.1176/appi.ajp.2007.07040636] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Bateman A, Fonagy P. Effectiveness of partial hospitalization in the treatment of borderline personality disorder: a randomized controlled trial. American Journal of Psychiatry 1999;156(10):1563-9. [DOI: 10.1176/ajp.156.10.1563] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Bateman A, Fonagy P. Health care utilization costs for borderline personality disorder patients treated with psychoanalytically oriented partial hospitalization versus general psychiatric care. American Journal of Psychiatry 2003;160(1):169-71. [10.1176/appi.ajp.160.1.169] [12505818] [DOI] [PubMed] [Google Scholar]
- Bateman A, Fonagy P. Treatment of borderline personality disorder with psychoanalytically oriented partial hospitalization: an 18-month follow-up. American Journal of Psychiatry 2001;158(1):36-42. [DOI: 10.1176/appi.ajp.158.1.36] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Bateman A. Important: question regarding your BPD trial for systematic review in Cochrane Collaboration [personal communication]. Email to: K Lieb, J Stoffers 14 December 2010.
- Bateman A. Intensive outpatient and partial hospital care for BPD. In: 157th annual meeting of the American Psychiatric Association. Psychotherapy and pharmacology: dissolving the mind-brain barrier; 2004 May 1-6; New York (NY). Arlington (VA): American Psychiatric Association, 2004:102. [Symposium 100B] [www.psychiatry.org/File%20Library/Psychiatrists/Directories/Library-and-Archive/conference_publications/am_program_2004.pdf]
- Bateman AW, Fonagy P. Drs Bateman and Fonagy reply [to Stern R. Partial hospitalization for borderline personality disorder. American Journal of Psychiatry 2001;158(11):1932-3. DOI: 10.1176/appi.ajp.158.11.1932]. American Journal of Psychiatry 2001;158(11):1932-3. [DOI: 10.1176/appi.ajp.158.11.1932-a] [DOI] [PubMed] [Google Scholar]
- Evans C. Treatment benefits of psychoanalytically oriented partial hospitalisation were maintained over 18 months in borderline personality disorder. Evidence-Based Mental Health 2001;4(3):73. [DOI: 10.1136/ebmh.4.3.73] [DOI] [Google Scholar]
- Fonagy P, Bateman AW. Mentalizing and borderline personality disorder. Journal of Mental Health 2007;16(1):83-101. [DOI: 10.1080/09638230601182045] [DOI] [Google Scholar]
- Stern R. Partial hospitalization for borderline personality disorder. American Journal of Psychiatry 2001;158(11):1932-3. [DOI: 10.1176/appi.ajp.158.11.1932] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bateman 2009 {published data only}ISRCTN27660668
- Bateman A, Fonagy P. Impact of clinical severity on outcomes of mentalisation-based treatment for borderline personality disorder. British Journal of Psychiatry 2013;203(3):221-7. [DOI: 10.1192/bjp.bp.112.121129] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Bateman A, Fonagy P. Randomized controlled trial of outpatient mentalization-based treatment versus structured clinical management for borderline personality disorder. American Journal of Psychiatry 2009;166(12):1355-64. [DOI: 10.1176/appi.ajp.2009.09040539] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Bateman A, Fonagy P. Randomized controlled trial of outpatient mentalization-based treatment versus structured clinical management for borderline personality disorder. Supplementary data. ajp.psychiatryonline.org/doi/suppl/10.1176/appi.ajp.2009.09040539/suppl_file/ajp_166_12_1355_01.pdf (accessed 27 February 2020). [DOI] [PubMed]
- Bateman A, O'Connell J, Lorenzini N, Gardner T, Fonagy P. A randomised controlled trial of mentalization-based treatment versus structured clinical management for patients with comorbid borderline personality disorder and antisocial personality disorder. BMC Psychiatry 2016;16:304. [DOI: 10.1186/s12888-016-1000-9] [PMC5006360] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman A. Important question regarding your BPD trial for systematic review in Cochrane Collaboration [personal communication]. Email to: K Lieb 14 December 2010.
- Bateman AW. Mentalization, borderline personality disorder and what works for whom? Presentation at the 11th International Congress of the International Society for the Study of Personality Disorders; 2009 Aug 21-23. www.borderlinepersonalitydisorder.com/Conferences/ISSPD/isspd_09_Bateman (accessed 21 April 2010).
- ISRCTN27660668. Randomised controlled trial of manualised out-patient individual and group therapy for borderline personality disorder [Effectiveness of intensive out-patient psychotherapy for treatment of borderline personality disorder: a pragmatic randomised single blind controlled study]. www.isrctn.com/ISRCTN27660668 (first received 12 October 2008).
Bellino 2006 {published data only}
- Bellino S, Paradiso E, Zizza M, Di Lorenzo R, Bogetto F. Combined therapy with interpersonal psychotherapy of major depressed patients with borderline personality disorder: a comparison with pharmacotherapy [Terapia combinata con psicoterapia interpersonale di pazienti depressi maggiori con disturbo borderline di personalità: confronto con la farmacoterapia]. Journal of Psychopathology 2005;11(1):34-42. [bit.ly/2rZUh5h] [Google Scholar]
- Bellino S, Zizza M, Rinaldi C, Bogetto F. Combined treatment of major depression in patients with borderline personality disorder: a comparison with pharmacotherapy. Canadian Journal of Psychiatry 2006;51(7):453-60. [DOI: 10.1177/070674370605100707] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bellino 2007 {published data only}
- Bellino S, Zizza M, Rinaldi C, Bogetto F. Combined therapy of major depression with concomitant borderline personality disorder: comparison of interpersonal and cognitive psychotherapy. Canadian Journal of Psychiatry 2007;52(11):718-25. [DOI: 10.1177/070674370705201106] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bellino 2010 {published data only}
- Bellino S, Bozzatello P, Bogetto F. Combined treatment of borderline personality disorder with interpersonal psychotherapy and pharmacotherapy: predictors of response. Psychiatry Research 2015;226(1):284-8. [DOI: 10.1016/j.psychres.2014.12.064] [isiarticles.com/bundles/Article/pre/pdf/34125.pdf] [DOI] [PubMed] [Google Scholar]
- Bellino S, Rinaldi C, Bogetto F. Adaptation of interpersonal psychotherapy to borderline personality disorder: a comparison of combined therapy and single pharmacotherapy. Canadian Journal of Psychiatry 2010;55(2):74-81. [DOI: 10.1177/070674371005500203] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Bellino S. Question regarding your BPD trials for inclusion in Cochrane Collaboration review [personal communication]. Email to: J Stoffers-Winterling 19 December 2010.
- Bellino S. Re: question regarding your BPD trials [personal communication]. Email to: J Stoffers 16 December 2010.
- Bozzatello P, Bellino S. Combined therapy with interpersonal psychotherapy adapted for borderline personality disorder: a two-years follow-up. Psychiatry Research 2016;240:151-6. [DOI: 10.1016/j.psychres.2016.04.014] [www.sciencedirect.com/science/article/pii/S0165178115302389] [DOI] [PubMed] [Google Scholar]
- Zizza M, Fenocchio M, Rinaldi C, Bellino S. Combined treatment of borderline personality disorder: a trial of modified interpersonal therapy. World Psychiatry 2009;8(Suppl 1):234. [Google Scholar]
Bianchini 2019 {published data only}
- Bianchini V, Cofini V, Curto M, Lagrotteria B, Manzi A, Navari S, et al. Dialectical behavior therapy (DBT) for forensic psychiatric patients: an Italian pilot study. Criminal Behavior and Mental Health 2019;29(9):122-30. [DOI: 10.1002/cbm.2102] [PMID: ] [DOI] [PubMed] [Google Scholar]
Blum 2008 {published data only}
- Black D. Question regarding your BPD trial [personal communication]. Email to: J Stoffers 04 January 2011.
- Black DW, Allen J, McCormick B, Blum N. Treatment received by persons with BPD participating in a randomized clinical trial of the systems training for emotional predictability and problem solving programme. Personality and Mental Health 2011;5(3):159-68. [DOI: 10.1002/pmh.167] [DOI] [Google Scholar]
- Black DW, Allen J, St John D, Pfohl B, McCormick B, Blum N. Predictors of response to systems training for emotional predictability and problem solving (STEPPS) for borderline personality disorder: an exploratory study. Acta Psychiatrica Scandinavica 2009;120(1):53-61. [DOI: 10.1111/j.1600-0447.2008.01340.x] [PMC3665337] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black DW, Blum N, Pfohl B, St John D. The STEPPS group treatment program for outpatients with borderline personality disorder. Journal of Contemporary Psychotherapy 2004;34(3):193-210. [DOI: 10.1023/B:JOCP.0000036630.25741.83] [DOI] [Google Scholar]
- Black DW, Pfohl BM. Randomized clinical trial of STEPPS versus treatment as usual. In: 158th annual meeting of the American Psychiatric Association. Psychosomatic medicine: integrating psychiatry & medicine; 2005 May 21-26; Atlanta (GA). Arlington (VA): American Psychiatric Association, 2005:104. [No. 1B] [www.psychiatry.org/File%20Library/Psychiatrists/Directories/Library-and-Archive/syllabus/am_syllabus_2005.pdf]
- Black DW, Simsek-Duran F, Blum N, McCormick B, Allen J. Do people with borderline personality disorder complicated by antisocial personality disorder benefit from the STEPPS treatment program? Personality and Mental Health 2016;10(3):205-15. [DOI: 10.1002/pmh.1326] [PMC4911327] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black DW. RE: [external] questions regarding your trial on the STEPPS program for BPD [personal communication]. Email to: EG Faltinsen 27 November 2017.
- Blum N, Allen J, McCormick B, Black DW. Ms Blum and colleagues reply [to Schulte-Herbrüggen O, Koerting J, Roepke S. Effectiveness of adjunctive STEPPS group treatment in borderline personality disorder patients. American Journal of Psychiatry 2008;165(10):1354-5. DOI: 10.1176/appi.ajp.2008.08030390]. American Journal of Psychiatry 2008;165(10):1354-5. [DOI: 10.1176/appi.ajp.2008.08030390r] [DOI] [PubMed] [Google Scholar]
- Blum N, Franklin J, Hansel R, McCormick B, St John D, Pfohl B, et al. Relationship of age to symptom severity, psychiatric comorbidity and health care utilization in persons with borderline personality disorder. Personality and Mental Health 2008;2(1):25-43. [DOI: 10.1002/pmh.26] [DOI] [PubMed] [Google Scholar]
- Blum N, St John D, Pfohl B, Stuart S, McCormick B, Allen J, et al. Erratum. American Journal of Psychiatry 2008;165(6):777. Erratum for: American Journal of Psychiatry 2008;165(4):468-78. [psycnet.apa.org/record/2008-07854-033] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum N, St John D, Pfohl B, Stuart S, McCormick B, Allen J, et al. Systems training for emotional predictability and problem solving (STEPPS) for outpatients with borderline personality disorder: a randomized controlled trial and 1-year follow-up. American Journal of Psychiatry 2008;165(4):468-78. [DOI: 10.1176/appi.ajp.2007.07071079] [PMC3608469] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson KM. Borderline personality disorder: STEPPS improves symptoms. Evidence-Based Mental Health 2008;11(4):120. [DOI: 10.1136/ebmh.11.4.120] [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT00055315. Treatment for borderline personality disorder [A cognitive group treatment for borderline outpatients]. clinicaltrials.gov/ct2/show/NCT00055315 (first received 25 February 2003).
Bohus 2013 {unpublished data only}
- Bohus M, Dyer AS, Priebe K, Krüger A, Kleindienst N, Schmahl C, et al. Dialectical behaviour therapy for post-traumatic stress disorder after childhood sexual abuse in patients with and without borderline personality disorder: a randomised controlled trial. Psychotherapy and Psychosomatics 2013;82(4):221-33. [DOI: 10.1159/000348451] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Brand S. AW: questions regarding your trial on emotional intelligence training for BPD [personal communication]. Email to: EG Faltinsen 28 November 2017.
- DBT Working Group at the Central Institute for Mental Health, Mannheim, Germany. Requested supplementary data (as supplied 1 October 2010). Data on file.
- NCT00481000. Dialectical cognitive traumatherapy (DCT) on patients with severe PTSD following sexual abuse (PASA) [Dialectical cognitive traumatherapy (DCT) on patients with severe posttraumatic stress disorder following sexual abuse - a randomised controlled trial]. clinicaltrials.gov/ct2/show/NCT00481000 (first received 31 May 2007).
- Steil R, Dyer A, Priebe K, Kleindienst N, Bohus M. Dialectical behavior therapy for posttraumatic stress disorder related to childhood sexual abuse: a pilot study of an intensive treatment program. Journal of Traumatic Stress 2011;24(1):102-6. [DOI: 10.1002/jts.20617] [PMID: ] [DOI] [PubMed] [Google Scholar]
Borschmann 2013 {published data only}ISRCTN12440268
- Borschmann R, Barrett B, Hellier JM, Byford S, Henderson C, Rose D, et al. Joint crisis plans for people with borderline personality disorder: feasibility and outcomes in a randomised controlled trial. British Journal of Psychiatry 2013;202(5):357-64. [DOI: 10.1192/bjp.bp.112.117762] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Borschmann R. Joint crisis plans for BPD - question on publication in British Journal of Psychiatry [pers communication]. Email to: J Stoffers-Winterling 22 August 2017.
- Borschmann R. RE: joint crisis plans for BPD - question on publication in British Journal of Psychiatry [personal communication]. Email to: J Stoffers-Winterling 22 August 2017.
- Borschmann R. The development and testing of joint crisis plans for people with borderline personality disorder: a feasibility study. ethos.bl.uk/OrderDetails.do?uin=uk.bl.ethos.628499 (accessed 27 January 2019).
- Hellier J. RE: regarding your study on on MBT for eating disorders [personal communication]. Email to: E Faltinsen 27 March 2018.
- Hellier J. RE: regarding your study on on MBT for eating disorders [personal communication]. Email to: E Faltinsen 3 January 2018.
- Hellier J. SV: regarding your study on MBT for eating disorders [personal communication]. Email to: EG Faltinsen, P Robinson 27 March 2018.
- ISRCTN12440268. JOSHUA: a pilot randomised controlled trial of joint crisis plans for people who self harm. www.isrctn.com/ISRCTN12440268 (first received 17 September 2009).
Bos 2010 {published data only}
- Bos EH, Van Wel E, Appelo MT, Verbraak MJP. A randomized controlled trial of a Dutch version of systems training for emotional predictability and problem solving for borderline personality disorder. Journal of Nervous and Mental Disease 2010;198(4):299-304. [DOI: 10.1097/NMD.0b013e3181d619cf] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Van Wel EB, Bos EH, Appelo MT, Berendsen EM, Willgeroth FC, Verbraak MJPM. The efficacy of the systems training for emotional predictability and problem solving (STEPPS) in the treatment of borderline personality disorder. A randomized controlled trial [De effectiviteit van de vaardigheidstraining emotieregulatiestoornis (VERS) in de behandeling van de borderlinepersoonlijkheidsstoornis: een gerandomiseerd onderzoek]. Tijdschrift voor Psychiatrie 2009;51(5):291-301. [PMID: ] [PubMed] [Google Scholar]
Carmona í Farrés 2019 {published data only}
- Carmona i Farrés C, Elices M, Soler J, Domínguez-Clavé E, Pomarol-Clotet E, Salvador R, et al. Effects of mindfulness training on borderline personality disorder: impulsivity versus emotional dysregulation. Mindfulness 2019;10:1243-54. [DOI: 10.1007/s12671-018-1071-4] [DOI] [Google Scholar]
- NCT03363230. Effects of mindfulness training on emotion regulation and impulsivity. www.clinicaltrials.gov/ct2/show/NCT03363230 (first received 30 November 2017).
Carter 2010 {published data only}
- Carter G. Question regarding your BPD trial [personal communication]. Email to: J Stoffers 21 December 2010.
- Carter GL, Willcox CH, Lewin TJ, Conrad AM, Bendit N. Hunter DBT project: randomized controlled trial of dialectical behaviour therapy in women with borderline personality disorder. Australian and New Zealand Journal of Psychiatry 2010;44(2):162-73. [DOI: 10.3109/00048670903393621] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cottraux 2009 {published data only}
- Cottraux J, Note ID, Boutitie F, Milliery M, Genouihlac V, Yao SN, et al. Cognitive therapy versus Rogerian supportive therapy in borderline personality disorder. Two-year follow-up of a controlled pilot study. Psychotherapy and Psychosomatics 2009;78(5):307-16. [DOI: 10.1159/000229769] [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT00131781. Cognitive therapy versus supportive therapy in borderline personality disorder. www.clinicaltrials.gov/ct2/show/NCT00131781 (first received 18 August 2005).
Davidson 2006 {published data only}ISRCTN86177428
- Davidson K, Livingstone S, McArthur K, Dickson L, Gumley A. An integrative complexity analysis of cognitive behaviour therapy sessions for borderline personality disorder. Psychology and Psychotherapy 2007;80(Pt 4):513-23. [DOI: 10.1348/147608307X191535] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Davidson K, Norrie J, Tyrer P, Gumley A, Tata P, Murray H, et al. The effectiveness of cognitive behavior therapy for borderline personality disorder: results from the borderline personality disorder study of cognitive therapy (BOSCOT) trial. Journal of Personality Disorders 2006;20(5):450-65. [DOI: 10.1521/pedi.2006.20.5.450] [PMC1852259] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson K, Tyrer P, Gumley A, Tata P, Norrie J, Palmer S, et al. A randomized controlled trial of cognitive behavior therapy for borderline personality disorder: rationale for trial, method, and description of sample. Journal of Personality Disorders 2006;20(5):431-49. [DOI: 10.1521/pedi.2006.20.5.431] [PMC1847748] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson KM, Tyrer P, Norrie J, Palmer SJ, Tyrer H. Cognitive therapy v usual treatment for borderline personality disorder: prospective 6-year follow-up. British Journal of Psychiatry 2010;197(6):456-62. [DOI: 10.1192/bjp.bp.109.074286] [PMID: ] [DOI] [PubMed] [Google Scholar]
- ISRCTN86177428. Borderline personality disorder study of cognitive therapy trial [A randomised controlled trial of cognitive therapy plus treatment as usual versus treatment as usual in the treatment of borderline personality disorder]. www.isrctn.com/ISRCTN86177428 (first received 22 July 2005).
- NCT00538135. BOSCOT: a randomised controlled trial of cognitive behavioural therapy in borderline personality disorder [BOSCOT: a randomised control trial of cognitive behavioural therapy plus treatment as usual versus treatment as usual in the treatment of borderline personality disorder]. www.clinicaltrials.gov/ct2/show/NCT00538135 (first received 29 September 2007).
- Norrie J, Davidson K, Tata P, Gumley A. Influence of therapist competence and quantity of cognitive behavioural therapy on suicidal behaviour and inpatient hospitalisation in a randomised controlled trial in borderline personality disorder: further analyses of treatment effects in the BOSCOT study. Psychology and Psychotherapy 2013;86(3):280-93. [DOI: 10.1111/papt.12004] [PMC4491320] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer S, Davidson K, Tyrer P, Gumley A, Tata P, Norrie J, et al. The cost-effectiveness of cognitive behavior therapy for borderline personality disorder: results from the BOSCOT trial. Journal of Personality Disorders 2006;20(5):466-81. [DOI: 10.1521/pedi.2006.20.5.466] [PMC1852260] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davidson 2014 {published data only}
- Davidson KM, Brown TM, James V, Kirk J, Richardson J. Manual-assisted cognitive therapy for self-harm in personality disorder and substance misuse: a feasibility trial. Psychiatric Bulletin 2014;38(3):108-11. [DOI: 10.1192/pb.bp.113.043109] [PMC4115373] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson KM. Re: question regarding your trial on manual-assisted cognitive therapy [personal communication]. Email to: EG Faltinsen 28 November 2017.
Doering 2010 {published data only}
- Buchheim A, Hörz-Sagstetter S, Doering S, Rentrop M, Schuster P, Buchheim P, et al. Change of unresolved attachment in borderline personality disorders: RCT study of transference-focused psychotherapy. Psychotherapy and Psychosomatics 2017;86(5):314-6. [DOI: 10.1159/000460257] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Diamond D, Levy KN, Clarkin JF, Fischer-Kern M, Cain NM, Doering S, et al. Attachment and mentalization in female patients with comorbid narcissistic and borderline personality disorder. Personality Disorders 2014;5(4):428-33. [DOI: 10.1037/per0000065] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Diamond D, Yeomans FE, Stern B, Levy KN, Hörz S, Doering S, et al. Transference-focused psychotherapy for patients with comorbid narcissistic and borderline personality disorder. Psychoanalytic Inquiry 2013;33(6):527-51. [DOI: 10.1080/07351690.2013.815087] [DOI] [Google Scholar]
- Doering S, Hörz S, Rentrop M, Fischer-Kern M, Schuster P, Benecke C, et al. Transference-focused psychotherapy v treatment by community psychotherapists for borderline personality disorder: randomised controlled trial. British Journal of Psychiatry 2010;196(5):389-95. [DOI: 10.1192/bjp.bp.109.070177] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Doering S. Author's reply [to Kleindienst N, Krumm B, Bohus M. Is transference-focused psychotherapy really efficacious for borderline personality disorder? British Journal of Psychiatry 2011;198(2):156-7. DOI: 10.1192/bjp.198.2.156b]. British Journal of Psychiatry 2011;198(2):156-7. [DOI: 10.1192/bjp.198.2.157] [DOI] [PubMed] [Google Scholar]
- Doering S. Question regarding your BPD trial for systematic review in Cochrane Collaboration [personal communication]. Email to: K Lieb, J Stoffers 10 December 2010.
- Fischer-Kern M, Doering S, Taubner S, Hörz S, Zimmermann J, Rentrop M, et al. Transference-focused psychotherapy for borderline personality disorder: change in reflective function. British Journal of Psychiatry 2015;207(2):173-4. [DOI: 10.1192/bjp.bp.113.143842] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Kleindienst N, Krumm B, Bohus M. Is transference-focused psychotherapy really efficacious for borderline personality disorder? British Journal of Psychiatry 2011;198(2):156-7. [DOI: 10.1192/bjp.198.2.156b] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Levy KN, Meehan KB, Yeomans FE. Transference-focused psychotherapy reduces treatment drop-out and suicide attempters compared with community psychotherapist treatment in borderline personality disorder. Evidence-based Mental Health 2010;13(4):119. [DOI: 10.1136/ebmh.13.4.119] [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT00714311. Efficacy of transference-focused psychotherapy for borderline personality disorder [A randomized-controlled trial of transference-focused psychotherapy vs treatment by experienced community psychotherapists for borderline personality disorder]. www.clinicaltrials.gov/ct2/show/NCT00714311 (first received 9 July 2008).
- Rentrop M, Martius P, Bäuml J, Buchheim P, Döering S, Hörz S. Patients with borderline personality disorder not participating in an RCT: are they different? Psychopathology 2010;43(6):369-72. [DOI: 10.1159/000320351] [PMID: ] [DOI] [PubMed] [Google Scholar]
Elices 2016 {published data only}
- Elices M, Pascual JC, Portella MJ, Feliu-Soler A, Martín-Blanco A, Carmona C, et al. Impact of mindfulness training on borderline personality disorder: a randomized trial. Mindfulness 2016;7:584-95. [DOI: 10.1007/s12671-016-0492-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02397031. Mindfulness and interpersonal effectiveness skills in borderline personality disorder [Randomized, active-controlled, clinical trial comparing effects of mindfulness and interpersonal effectiveness skills in borderline personality disorder]. www.clinicaltrials.gov/ct2/show/NCT02397031 (first received 12 March 2015).
- Soler J, Elices M, Pascual JC, Martín-Blanco A, Feliu-Soler A, Carmona C, et al. Effects of mindfulness training on different components of impulsivity in borderline personality disorder: results from a pilot randomized study. Borderline Personality Disorder and Emotion Dysregulation 2016;3:1. [DOI: 10.1186/s40479-015-0035-8] [PMC4709962] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Farrell 2009 {published data only}
- Farrell JM, Shaw IA, Webber MA. A schema-focused approach to group psychotherapy for outpatients with borderline personality disorder: a randomized controlled trial. Journal of Behavior Therapy and Experimental Psychiatry 2009;40(2):317-28 Corrigendum to: Journal of Behavior Therapy and Experimental Psychiatry 2018; 60: 111. [DOI: 10.1016/j.jbtep.2009.01.002] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Farrell JM. Question regarding your BPD trials [personal communication]. Email to: K Lieb, J Stoffers 9 December 2010.
Feigenbaum 2012 {published data only}
- Feigenbaum JD, Fonagy P, Pilling S, Jones A, Wildgoose A, Bebbington PE. A real-world study of the effectiveness of DBT in the UK National Health Service. British Journal of Clinical Psychology 2012;51(2):121-41. [DOI: 10.1111/j.2044-8260.2011.02017.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Feliu‐Soler 2017 {published data only}
- Feliu-Soler A, Pascual JC, Elices M, Martín-Blanco A, Carmona C, Cebolla A, et al. Fostering self-compassion and loving-kindness in patients with borderline personality disorder: a randomized pilot study. Clinical Psychology & Psychotherapy 2017;24(1):278-86. [DOI: 10.1002/cpp.2000] [PMID: ] [DOI] [PubMed] [Google Scholar]
Giesen‐Bloo 2006 {published and unpublished data}
- Arntz A, Stupar-Rutenfrans S, Bloo J, Van Dyck R, Spinhoven P. Prediction of treatment discontinuation and recovery from borderline personality disorder: results from an RCT comparing schema therapy and transference-focused psychotherapy. Behaviour Research and Therapy 2015;74:60-71. [DOI: 10.1016/j.brat.2015.09.002] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Giesen-Bloo J, Arntz A. Questions concerning the randomized trial of schema-focused therapy vs transference-focused psychotherapy—reply. Archives of General Psychiatry 2007;64(5):610-1. [DOI: 10.1001/archpsyc.64.5.610] [DOI] [PubMed] [Google Scholar]
- Giesen-Bloo J, Van Dyck R, Spinhoven P, Van Tilburg W, Dirksen C, Van Asselt T, et al. Errors in table and figure in: outpatient psychotherapy for borderline personality disorder: randomized trial of schema-focused therapy vs transference-focused psychotherapy. Archives of General Psychiatry 2006;63(9):1008. Erratum for: Archives of General Psychiatry 2006;63(6):649-58. [DOI: 10.1001/archpsyc.63.9.1008] [DOI] [PubMed] [Google Scholar]
- Giesen-Bloo J, Van Dyck R, Spinhoven P, Van Tilburg W, Dirksen C, Van Asselt T, et al. Outpatient psychotherapy for borderline personality disorder: randomized trial of schema-focused therapy vs transference-focused psychotherapy. Archives of General Psychiatry 2006;63(6):649-58. [DOI: 10.1001/archpsyc.63.6.649] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Spinhoven P, Giesen-Bloo J, Van Dyck R, Kooiman K, Arntz A. The therapeutic alliance in schema-focused therapy and transference-focused psychotherapy for borderline personality disorder. Journal of Consulting and Clinical Psychology 2007;75(1):104-15. [DOI: 10.1037/0022-006X.75.1.104] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Van Asselt ADI, Dirksen CD, Arntz A, Giesen-Bloo JH, Van Dyck R, Spinhoven P, et al. Out-patient psychotherapy for borderline personality disorder: cost-effectiveness of schema-focused therapy v transference-focused psychotherapy. British Journal of Psychiatry 2008;192(6):450-7. [DOI: 10.1192/bjp.bp.106.033597] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Van Asselt ADI, Dirksen CD, Arntz A, Severens JL. Difficulties in calculating productivity costs: work disability associated with borderline personality disorder. Value in Health 2008;11(4):637-44. [DOI: 10.1111/j.1524-4733.2007.00288.x] [DOI] [PubMed] [Google Scholar]
- Yeomans F. Questions concerning the randomized trial of schema-focused therapy vs transference-focused psychotherapy. Archives of General Psychiatry 2007;64(5):609-10. [DOI: 10.1001/archpsyc.64.5.609-c] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gleeson 2012 {published data only}
- Gleeson JF, Chanen A, Cotton SM, Pearce T, Newman B, McCutcheon L. Treating co-occurring first-episode psychosis and borderline personality: a pilot randomized controlled trial. Early Intervention in Psychiatry 2012;6(1):21-9. [DOI: 10.1111/j.1751-7893.2011.00306.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Gleeson JFM. RE: separate data [personal communication]. Email to: M Kielsholm 28 October 2016.
Gratz 2006 {published data only}
- Gratz KL. Important: question regarding your BPD trial for systematic review in Cochrane Collaboration [personal communication]. Email to: J Stoffers-Winterling 11 December 2010.
- Gratz KL, Gunderson JG. Preliminary data on an acceptance-based emotion regulation group intervention for deliberate self-harm among women with borderline personality disorder. Behavior Therapy 2006;37(1):25-35. [DOI: 10.1016/j.beth.2005.03.002] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gratz 2014 {published data only}
- Gratz KL, Bardeen JR, Levy R, Dixon-Gordon KL, Tull MT. Mechanisms of change in an emotion regulation group therapy for deliberate self-harm among women with borderline personality disorder. Behaviour Research and Therapy 2015;65:29-35. [DOI: 10.1016/j.brat.2014.12.005] [PMC4306622] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz KL, Dixon-Gordon KL, Tull MT. Predictors of treatment response to an adjunctive emotion regulation group therapy for deliberate self-harm among women with borderline personality disorder. Personality Disorders 2014;5(1):97-107. [DOI: 10.1037/per0000062] [PMC4059504] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz KL, Tull MT, Levy R. Randomized controlled trial and uncontrolled 9-month follow-up of an adjunctive emotion regulation group therapy for deliberate self-harm among women with borderline personality disorder. Psychological Medicine 2014;44(10):2099-112. [DOI: 10.1017/S0033291713002134] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Gratz KL. Question regarding your BPD trials [personal communication]. Email to: J Stoffers 11 December 2010.
Gregory 2008b {published data only}
- Gregory RJ, Chlebowski S, Kang D, Remen A, Soderberg M. Psychodynamic therapy for borderline personality disorder and co-occurring alcohol use disorders: a newly designed ongoing study. Journal of the American Psychoanalytic Association 2006;54(4):1331-4. [DOI: 10.1177/00030651060540040107] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Gregory RJ, Chlebowski S, Kang D, Remen AL, Soderberg MG, Stepkovitch J, et al. A controlled trial of psychodynamic psychotherapy for co-occurring borderline personality disorder and alcohol use disorder. Psychotherapy 2008;45(1):28-41. [DOI: 10.1037/0033-3204.45.1.28] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Gregory RJ, DeLucia-Deranja E, Mogle JA. Dynamic deconstructive psychotherapy versus optimized community care for borderline personality disorder co-occuring with alcohol use disorders: a 30-month follow-up. Journal of Nervous and Mental Disease 2010;198(4):292-8. [DOI: 10.1097/NMD.0b013e3181d6172d] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Gregory RJ, Remen AL, Soderberg M, Ploutz-Snyder RJ. A controlled trial of psychodynamic psychotherapy for co-occurring borderline personality disorder and alcohol use disorder: six-month outcome. Journal of the American Psychoanalytic Association 2009;57(1):199-205. [DOI: 10.1177/00030651090570011006] [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT00145678. Psychodynamic therapy for co-occurring borderline personality disorder and alcohol use disorder [Psychodynamic therapy for patients With borderline personality disorder and alcohol abuse]. www.clinicaltrials.gov/show/NCT00145678 (first received 1 September 2005).
Haeyen 2018 {published data only}
- Haeyen S, Van Hooren S, Hutschemaekers G. Efficacy of art therapy in individuals with personality disorders cluster B/C: a randomised controlled trial. International Journal of Psychology July 2016;51:60. [DOI: 10.1521/pedi_2017_31_312] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Haeyen S, Van Hooren S, Van der Veld W, Hutschemaekers G. Efficacy of art therapy in individuals with personality disorders cluster B/C: a randomised controlled trial. Journal of Personality Disorders 2018;32(4):527-42. [DOI: 10.1521/pedi_2017_31_312] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Haeyen S. Re: Cochrane Collaboration review of psychotherapies for BPD - your RCT of art therapy [personal communication]. Email to: J Stoffers-Winterling 18 August 2018.
Harned 2014 {published data only}
- Harned M. Re: SV: question regarding your trial on brief therapy for attempted suicide [personal communication]. Email to: EG Faltinsen 11 December 2018.
- Harned MS, Korslund KE, Linehan MM. A pilot randomized controlled trial of dialectical behavior therapy with and without the dialectical behavior therapy prolonged exposure protocol for suicidal and self-injuring women with borderline personality disorder and PTSD. Behaviour Research and Therapy 2014;55:7-17. [DOI: 10.1016/j.brat.2014.01.008] [PMC3987949] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harned MS, Wilks CR, Schmidt SC, Coyle TN. Improving functional outcomes in women with borderline personality disorder and PTSD by changing PTSD severity and post-traumatic cognitions. Behaviour Research and Therapy 2018;103:53-61. [DOI: 10.1016/j.brat.2018.02.002] [PMC5837954] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harned MS. RE: questions regarding your trial on brief therapy for attempted suicide [personal communication]. Email to: E Faltinsen 29 November 2017.
Jahangard 2012 {published data only}
- Brand S. AW: questions regarding your trial on emotional intelligence training for BPD [personal communication]. Email to: EG Faltinsen 28 November 2017.
- Brand S. Questions regarding your trial on emotional intelligence training for BPD [personal communication]. Email to: EG Faltinsen 28 November 2017.
- Jahangard L, Haghighi M, Bajoghli H, Ahmadpanah M, Ghaleiha A, Zarrabian MK, et al. Training emotional intelligence improves both emotional intelligence and depressive symptoms in inpatients with borderline personality disorder and depression. International Journal of Psychiatry in Clinical Practice 2012;16(3):197-204. [DOI: 10.3109/13651501.2012.687454] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jochems 2015 {published data only}
- Jochems EC, Van der Feltz-Cornelis CM, Van Dam A, Duivenvoorden HJ, Mulder CL. The effects of motivation feedback in patients with severe mental illness: a cluster randomized controlled trial. Neuropsychiatric Disease and Treatment 2015;11:3049-64. [DOI: 10.2147/NDT.S95190] [PMC4686323] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochems EC. Cochrane Collaboration review on psychotherapies for borderline PD [personal communication]. Email to: EG Faltinsen 2 July 2018.
- Jochems EC. Re: Cochrane Collaboration review on psychotherapies for borderline PD [personal communication]. Email to: J Stoffers-Winterling 02 July 2018.
Jørgensen 2013 {published data only}
- Jørgensen CR, Bøye R, Andersen D, Døssing Blaabjerg AH, Freund C, Jordet H, et al. Eighteen months post-treatment naturalistic follow-up study of mentalization-based therapy and supportive group treatment of borderline personality disorder: clinical outcomes and functioning. Nordic Psychology 2014;66(4):254-73. [DOI: 10.1080/19012276.2014.963649] [DOI] [Google Scholar]
- Jørgensen CR, Freund C, Bøye R, Jordet H, Andersen D, Kjølbye M. Outcome of mentalization-based and supportive psychotherapy in patients with borderline personality disorder: a randomized trial. Acta Psychiatrica Scandinavica 2013;127(4):305-17. [DOI: 10.1111/j.1600-0447.2012.01923.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Jorgensen CR. SV: questions on your trial regarding MBT for BPD patients [personal communication]. Email to: EG Faltinsen 28 November 2017.
Kamalabadi 2012 {published data only}
- Kamalabadi MJ, Ahmadi SA, Etemadi O, Fatehizadeh M, Bahrami F, Firoozabadi A. A study of the effect of couple dialectical behavioral therapy on symptoms and quality of marital relationships and mental health of Iranian borderline personality couples: a controlled trial. Interdisciplinary Journal of Contemporary Research in Business 2012;3(9):1480-7. [psycnet.apa.org/record/2012-22425-047] [Google Scholar]
Koons 2001a {published data only}
- Koons CR, Robins CJ, Tweed JL, Lynch TR, Gonzalez AM, Morse JQ, et al. Efficacy of dialectical behavior therapy in women veterans with borderline personality disorder. Behavior Therapy 2001;32(2):371-90. [DOI: 10.1016/S0005-7894(01)80009-5] [DOI] [Google Scholar]
Kramer 2011 {published data only}
- Kramer U, Berger T, Kolly S, Marquet P, Preisig M, De Roten Y, et al. Effects of motive-oriented therapeutic relationship in early-phase treatment of borderline personality disorder: a pilot study of a randomized trial. Journal of Nervous and Mental Disease 2011;199(4):244-50. [DOI: 10.1097/NMD.0b013e3182125d19] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Kramer U, Caspar F, Drapeau M. Change in biased thinking in a 10-session treatment for borderline personality disorder: further evidence of the motive-oriented therapeutic relationship. Psychotherapy Research 2013;23(6):633-45. [DOI: 10.1080/10503307.2013.791404] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kramer 2014 {published data only}
- Berthoud L, Kramer U, Caspar F, Pascual-Leone A. Emotional processing in a ten-session general psychiatric treatment for borderline personality disorder: a case study. Personality and Mental Health 2015;9(1):73-8. [DOI: 10.1002/pmh.1287] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Berthoud L, Pascual-Leone A, Caspar F, Tissot H, Keller S, Rohde KB, et al. Leaving distress behind: a randomized controlled study on change in emotional processing in borderline personality disorder. Psychiatry 2017;80(2):139-54. [DOI: 10.1080/00332747.2016.1220230] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Keller S, Stelmaszczyk K, Kolly S, De Roten Y, Despland JN, Caspar F, et al. Change in biased thinking in a treatment based on the motive-oriented therapeutic relationship for borderline personality disorder. Journal of Personality Disorders 2018;32(Suppl):75-92. [DOI: 10.1521/pedi.2018.32.supp.75] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Kramer U, Flückiger C, Kolly S, Caspar F, Marquet P, Despland JN, et al. Unpacking the effects of therapist responsiveness in borderline personality disorder: motive-oriented therapeutic relationship, patient in-session experience, and the therapeutic alliance. Psychotherapy and Psychosomatics 2014;83(6):386-7. [DOI: 10.1159/000365400] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Kramer U, Keller S, Caspar F, De Roten Y, Despland JN, Kolly S. Early change in coping strategies in responsive treatments for borderline personality disorder: a mediation analysis. Journal of Consulting and Clinical Psychology 2017;85(5):530-5. [DOI: 10.1037/ccp0000196] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Kramer U, Kolly S, Berthoud L, Keller S, Preisig M, Caspar F, et al. Effects of motive-oriented therapeutic relationship in a ten-session general psychiatric treatment of borderline personality disorder: a randomized controlled trial. Psychotherapy and Psychosomatics 2014;83(3):176-86. [DOI: 10.1159/000358528] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Kramer U, Stulz N, Berthoud L, Caspar F, Marquet P, Kolly S, et al. The shorter the better? A follow-up analysis of 10-session psychiatric treatment including the motive-oriented therapeutic relationship for borderline personality disorder. Psychotherapy Research 2017;27(3):362-70. [DOI: 10.1080/10503307.2015.1110635] [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT01896024. Effects of motive-oriented therapeutic relationship in the early-phase treatment of borderline personality disorder (MOTR). www.clinicaltrials.gov/ct2/show/NCT01896024 (first received 5 July 2013).
Kramer 2016 {published data only}
- Kramer U, Pascual-Leone A, Berthoud L, De Roten Y, Marquet P, Kolly S, et al. Assertive anger mediates effects of dialectical behaviour-informed skills training for borderline personality disorder: a randomized controlled trial. Clinical Psychology & Psychotherapy 2016;23(3):189-202. [DOI: 10.1002/cpp.1956] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Kramer U. The role of coping change in borderline personality disorder: a process-outcome analysis on dialectical-behaviour skills training. Clinical Psychology & Psychotherapy 2017;24(2):302-11. [DOI: 10.1002/cpp.2017] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kredlow 2017a {published data only}
- Kredlow MA, Szuhany KL, Lo S, Xie H, Gottlieb JD, Rosenberg SD, et al. Cognitive behavioral therapy for posttraumatic stress disorder in individuals with severe mental illness and borderline personality disorder. Psychiatry Research 2017;249:86-93. [DOI: 10.1016/j.psychres.2016.12.045] [PMC5325773] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueser K. Re: question regarding your trial on CBT for BPD patients [personal communication]. Email to: EG Faltinsen 28 November 2017.
Kredlow 2017b {published data only}
- Kredlow MA, Szuhany KL, Lo S, Xie H, Gottlieb JD, Rosenberg SD, et al. Cognitive behavioral therapy for posttraumatic stress disorder in individuals with severe mental illness and borderline personality disorder. Psychiatry Research 2017;249:86-93. [DOI: 10.1016/j.psychres.2016.12.045] [PMC5325773] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueser KT, Gottlieb JD, Xie H, Lu W, Yanos PT, Rosenberg SD, et al. Evaluation of cognitive restructuring for post-traumatic stress disorder in people with severe mental illness.. British Journal of Psychiatry 2015;206(6):501-8. [DOI: 10.1192/bjp.bp.114.147926] [PMC4450219] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueser KT, Rosenberg SD, Xie H, Jankowski MK, Bolton EE, Lu W, et al. A randomized controlled trial of cognitive-behavioral treatment for posttraumatic stress disorder in severe mental illness. Journal of Consulting and Clinical Psychology 2008;76(2):259-71. [DOI: 10.1037/0022-006X.76.2.259] [PMC3916092] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueser KT. Re: question regarding your trial on CBT for PTSD participants [personal communication]. Email to: E Faltinsen 28 November 2018.
Laurenssen 2018 {published data only}
- Laurenssen EM, Westra D, Kikkert MJ, Noom MJ, Eeren HV, Van Broekhuyzen AJ, et al. Day hospital mentalization-based treatment (MBT-DH) versus treatment as usual in the treatment of severe borderline personality disorder: protocol of a randomized controlled trial. BMC Psychiatry 2014;14:149. [DOI: 10.1186/1471-244X-14-149] [PMC4045960] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurenssen EMP, Luyten P, Kikkert MJ, Westra D, Peen J, Soons MBJ, et al. Day hospital mentalization-based treatment v specialist treatment as usual in patients with borderline personality disorder: randomized controlled trial. Psychological Medicine 2018;48(15):2522-9. [DOI: 10.1017/S0033291718000132] [PMID: 29478425] [DOI] [PubMed] [Google Scholar]
- NL2058 (NTR2175). Mentalisation-based treatment versus care-as-usual in the treatment of severe borderline personality disorders. www.trialregister.nl/trial/2058 (first received 21 January 2010).
Leichsenring 2016 {published data only}
- Leichsenring F, Masuhr O, Jaeger U, Rabung S, Dally A, Dümpelmann M, et al. Psychoanalytic-interactional therapy versus psychodynamic therapy by experts for personality disorders: a randomized controlled efficacy-effectiveness study in cluster B personality disorders. Psychotherapy and Psychosomatics 2016;85(2):71-80. [DOI: 10.1159/000441731] [PMID: ] [DOI] [PubMed]
Leppänen 2016 {published data only}
- Leppänen V, Hakko H, Sintonen H, Lindeman S. Comparing effectiveness of treatments for borderline personality disorder in communal mental health care: the Oulu BPD study. Community Mental Health Journal 2016;52(2):216-27. [DOI: 10.1007/s10597-015-9866-4] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Leppänen V. Re: questions regarding the Oulu BPD study [personal communication]. Email to: E Faltinsen 20 December 2017.
- Leppänen V. Re: SV: question regarding the Oulu BPD study [personal communication]. Email to: EG Faltinsen 22 December 2017.
Lin 2019 {published data only}
- Lin T-J, Ko H-C, Wu JY-W, Oei TP, Lane H-Y, Chen C-H. The effectiveness of dialectical behavior therapy skills training group vs cognitive therapy group on reducing depression and suicide attempts for borderline personality disorder in Taiwan. Archives of Suicide Research 2019;23(1):82-99. [DOI: 10.1080/13811118.2018.1436104] [PMID: ] [DOI] [PubMed] [Google Scholar]
Linehan 1991 {published data only}
- Hoffman RE. Impact of treatment accessibility on clinical course of parasuicidal patients. Archives of General Psychiatry 1993;50(2):157-8. [DOI: 10.1001/archpsyc.1993.01820140083010] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Lindenboim N. To Know Me is to Keep Me: Self-Verification, Validation, and Therapy Dropout in the Treatment of Borderline Personality Disorder [PhD thesis]. Washington (DC): University of Washington, 2009. [Google Scholar]
- Linehan MM, Armstrong HE, Suarez A, Allmon D, Heard HL. Cognitive-behavioral treatment of chronically parasuicidal borderline patients. Archives of General Psychiatry 1991;48(12):1060-4. [DOI: 10.1001/archpsyc.1991.01810360024003] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Linehan MM, Heard HL. Impact of treatment accessibility on clinical course of parasuicidal patients — reply. Archives of General Psychiatry 1993;50(2):157-8. [DOI: 10.1001/archpsyc.1993.01820140083011] [DOI] [PubMed] [Google Scholar]
Linehan 1994 {published data only}
- Heard HL. Cost-Effectiveness of Dialectical Behavior Therapy in the Treatment of Borderline Personality Disorder [PhD thesis]. Washington (DC): University of Washington, 2000. [Google Scholar]
- Lindenboim N. To Know Me is to Keep Me: Self-Verification, Validation, and Therapy Dropout in the Treatment of Borderline Personality Disorder [PhD thesis]. Washington (DC): University of Washington, 2009. [Google Scholar]
- Linehan MM, Heard HL, Armstrong HE. Naturalistic follow-up of a behavioral treatment for chronically parasuicidal borderline patients. Archives of General Psychiatry 1993;50(12):971-4. [DOI: 10.1001/archpsyc.1993.01820240055007] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Linehan MM, Tutek DA, Heard HL, Armstrong HE. Interpersonal outcome of cognitive behavioral treatment for chronically suicidal borderline patients. American Journal of Psychiatry 1994;151(12):1771-6. [DOI: 10.1176/ajp.151.12.1771] [PMID: ] [DOI] [PubMed] [Google Scholar]
Linehan 2006 {published data only}
- Bedics JD, Atkins DC, Comtois KA, Linehan MM. Treatment differences in the therapeutic relationship and introject during a 2-year randomized controlled trial of dialectical behavior therapy versus nonbehavioral psychotherapy experts for borderline personality disorder. Journal of Consulting and Clinical Psychology 2012;80(1):66-77. [DOI: 10.1037/a0026113] [PMC3265694] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedics JD, Atkins DC, Comtois KA, Linehan MM. Weekly therapist ratings of the therapeutic relationship and patient introject furing the course of dialectical behavioral therapy for the treatment of borderline personality disorder. Psychotherapy 2012;49(2):231-40. [DOI: 10.1037/a0028254] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Bedics JD, Atkins DC, Harned MS, Linehan MM. The therapeutic alliance as a predictor of outcome in dialectical behavior therapy versus nonbehavioral psychotherapy by experts for borderline personality disorder. Psychotherapy 2015;52(1):67-77. [DOI: 10.1037/a0038457] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Brown MZ, Linehan MM, Comtois KA, Murray A, Chapman AL. Shame as a prospective predictor of self-inflicted injury in borderline personality disorder: a multi-modal analysis. Behaviour Research and Therapy 2009;47(10):815-22. [DOI: 10.1016/j.brat.2009.06.008] [PMC2761705] [PMID: 19596223 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle TN, Shaver JA, Linehan MM. On the potential for iatrogenic effects of psychiatric crisis services: the example of dialectical behavior therapy for adult women with borderline personality disorder. Journal of Consulting and Clinical Psychology 2018;86(2):116-24. [DOI: 10.1037/ccp0000275] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Harned MS, Chapman AL, Dexter-Mazza ET, Murray A, Comtois KA, Linehan MM. Treating co-occurring Axis I disorders in recurrently suicidal women with borderline personality disorder: a 2-year randomized trial of dialectical behavior therapy versus community treatment by experts. Journal of Consulting and Clinical Psychology 2008;76(6):1068-75. [DOI: 10.1037/a0014044] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Harned MS, Chapman AL, Dexter-Mazza ET, Murray A, Comtois KA, Linehan MM. Treating co-occurring Axis I disorders in recurrently suicidal women with borderline personality disorder: a 2-year randomized trial of dialectical behavior therapy versus community treatment by experts. Personality Disorders: Theory, Research, and Treatment 2009;S(1):35-45. [DOI: 10.1037/1949-2715.S.1.35] [DOI] [PubMed] [Google Scholar]
- Harned MS, Jackson SC, Comtois KA, Linehan MM. Dialectical behavior therapy as a precursor to PTSD treatment for suicidal and/or self-injuring women with bordreline personality disorder. Journal of Traumatic Stress 2010;23(4):421-9. [DOI: 10.1002/jts.20553] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Lindenboim N, Comtois KA, Linehan MM. Skills practice in dialectical behavior therapy for suicidal women meeting criteria for borderline personality disorder. Cognitive and Behavioral Practice 2007;14(2):147-56. [DOI: 10.1016/j.cbpra.2006.10.004] [DOI] [Google Scholar]
- Linehan MM, Comtois KA, Murray AM, Brown MZ, Gallop RJ, Heard HL, et al. Two-year randomized controlled trial and follow-up of dialectical behavior therapy vs therapy by experts for suicidal behaviors and borderline personality disorder. Archives of General Psychiatry 2006;63(7):757-66. [DOI: 10.1001/archpsyc.63.7.757] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Linehan MM. Error in table in: two-year randomized controlled trial and follow-up of dialectical behavior therapy vs therapy by experts for suicidal behaviors and borderline personality disorder. Archives of General Psychiatry 2007;64(12):1401. Erratum for: Archives of General Psychiatry 2007;64(12):1401. [DOI] [PubMed] [Google Scholar]
- McMain S. Dialectic behaviour therapy reduces suicide attempts compared with non-behavioural psychotherapy in women with borderline personality disorder. Evidence-Based Mental Health 2007;10(1):18. [DOI: 10.1136/ebmh.10.1.18] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Neacsiu AD, Lungu A, Harned MS, Rizvi SL, Linehan MM. Impact of dialectical behavior therapy versus community treatment by experts on emotional experience, expression, and acceptance in borderline personality disorder. Behaviour Research and Therapy 2014;53:47-54. [DOI: 10.1016/j.brat.2013.12.004] [PMC3955205] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neacsiu AD, Rizvi SL, Linehan MM. Dialectical behavior therapy skills use as a mediator and outcome of treatment for borderline personality disorder. Behaviour Research and Therapy 2010;48(9):832-9. [DOI: 10.1016/j.brat.2010.05.017] [PMC2914145] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secrist CD. The Role of Executive Functioning in the Treatment of Borderline Personality Disorder [PhD thesis]. Washington (DC): University of Washington, 2014. [Google Scholar]
Linehan 2015a {published data only}
- Linehan MM, Korslund KE, Harned MS, Gallop RJ, Lungu A, Neacsiu AD, et al. Dialectical behavior therapy for high suicide risk in individuals with borderline personality disorder: a randomized clinical trial and component analysis. JAMA Psychiatry 2015;72(5):475-82 Erratum for: JAMA Psychiatry 2015; 72(9): 951. [DOI: 10.1001/jamapsychiatry.2014.3039] [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT00183651. Treatment of suicidal women with borderline personality disorder [Assessment and treatment of parasuicidal patients]. www.clinicaltrials.gov/ct2/show/NCT00183651 (first received 13 September 2005).
McMain 2009 {published data only}ISRCTN02634417
- Case BG. Letter to the editor on 'McMain SF, Links PS, Gnam WH, Guimond T, Cardish RJ, Korman L, et al. A randomized trial of dialectical behavior therapy versus general psychiatric management for borderline personality disorder. American Journal of Psychiatry 2009;166(12):1365-74. DOI: 10.1176/appi.ajp.2009.09010039'. American Journal of Psychiatry 2010;167(4):475. [DOI: 10.1176/appi.ajp.2009.09101532] [PMID: 20360332 ] [DOI] [PubMed] [Google Scholar]
- ISRCTN02634417. Hope for chronically suicidal patient: evaluating the clinical and health services impact of dialectical behaviour therapy (DBT) in individuals with borderline personality disorder [Evaluating the clinical and health services impact of dialectical behaviour therapy (DBT) in individuals with borderline personality disorder: a randomised controlled trial]. www.isrctn.com/ISRCTN02634417 (first received 16 November 2005). [DOI: 10.1186/ISRCTN02634417] [DOI]
- McMain SF, Fitzpatrick S, Boritz T, Barnhart R, Links P, Streiner DL. Outcome trajectories and prognostic factors for suicide and self-harm behaviors in patients with borderline personaliy disorder following one year of outpatient psychotherapy. Journal of Personality Disorders 2018;32(4):497-512. [DOI: 10.1521/pedi_2017_31_309] [PMID: ] [DOI] [PubMed] [Google Scholar]
- McMain SF, Links PS, Gnam WH, Guimond T, Cardish RJ, Korman L, et al. A randomized trial of dialectical behavior therapy versus general psychiatric management for borderline personality disorder. American Journal of Psychiatry 2009;166(12):1365-74. [DOI: 10.1176/appi.ajp.2009.09010039] [PMID: ] [DOI] [PubMed] [Google Scholar]
- McMain SF. Dr McMain replies to letter to the editor on 'McMain SF, Links PS, Gnam WH, Guimond T, Cardish RJ, Korman L, et al. A randomized trial of dialectical behavior therapy versus general psychiatric management for borderline personality disorder. American Journal of Psychiatry 2009;166(12):1365-74. DOI: 10.1176/appi.ajp.2009.09010039'. American Journal of Psychiatry 2010;167(4):475-6. [DOI: 10.1176/appi.ajp.2009.09101532r)] [DOI] [PubMed] [Google Scholar]
- NCT00154154. Hope for the chronically suicidal patient [Hope for the chronically suicidal patient: evaluating the clinical and health services impact of dialectical behaviour therapy in individuals with borderline personality disorder]. www.clinicaltrials.gov/ct2/show/NCT00154154 (first received 7 September 2005).
McMain 2017 {published data only}
- McMain SF, Guimond T, Barnhart R, Habinski L, Streiner DL. A randomized trial of brief dialectical behaviour therapy skills training in suicidal patients suffering from borderline disorder. Acta Psychiatrica Scandinavica 2017;135(2):138-48. [DOI: 10.1111/acps.12664] [PMID: ] [DOI] [PubMed] [Google Scholar]
McMurran 2016 {published data only}
- McMurran M, Crawford MJ, Reilly J, Delport J, McCrone P, Whitham D, et al. Psychoeducation with problem-solving (PEPS) therapy for adults with personality disorder: a pragmatic randomised controlled trial to determine the clinical effectiveness and cost-effectiveness of a manualised intervention to improve social functioning. Health Technology Assessment 2016;20(52):1-282. [DOI: 10.3310/hta20520] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurran M. RE: Cochrane Collaboration review - PEPS study [personal communication]. Email to: J Stoffers-Winterling 5 July 2018.
- McMurran M. Request for data on BPD subjects in the PEPS rcp-study for use a new Cochrane review [personal communication]. Email to: M Kongerslev 7 March 2017.
Mehlum 2014 {published data only}
- Haga E, Aas E, Grøholt B, Tørmoen AJ, Mehlum L. Cost-effectiveness of dialectical behaviour therapy vs enhanced usual care in the treatment of adolescents with self-harm. Child and Adolescent Psychiatry and Mental Health 2018;12:22. [DOI: 10.1186/s13034-018-0227-2] [PMC5928596] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlum L, Ramberg M, Tørmoen AJ, Haga E, Diep LM, Stanley BH, et al. Dialectical behavior therapy compared with enhanced usual care for adolescents with repeated suicidal and self-harming behavior: outcomes over a one-year follow-up. Journal of the American Academy of Child and Adolescent Psychiatry 2016;55(4):295-300. [DOI: 10.1016/j.jaac.2016.01.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Mehlum L, Tørmoen AJ, Ramberg M, Haga E, Diep LM, Laberg S, et al. Dialectical behavior therapy for adolescents with repeated suicidal and self-harming behavior: a randomized trial. Journal of the American Academy of Child and Adolescent Psychiatry 2014;53(10):1082-91. [DOI: 10.1016/j.jaac.2014.07.003] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Mehlum L. Re: regarding your study of DBT for your with suicidal behavior [personal communication] [Re: angående din studie om DBT for ungdom med selvmordsatferd [personlig kommunikation]]. Email to: E Faltinsen 5 December 2017.
- NCT00675129. Treatment for adolescents with deliberate self harm [A randomized controlled trial for repetitive deliberate self-harm and suicidal behaviours among Norwegian adolescents: a comparison between dialectical behaviour therapy adapted for adolescents (DBT-A) and enhanced usual care (EUC)]. www.clinicaltrials.gov/ct2/show/NCT00675129 (first received 6 May 2008).
- Ramleth RK, Groholt B, Diep LM, Walby FA, Mehlum L. The impact of borderline personality disorder and sub-threshold borderline personality disorder on the course of self-reported and clinician-rated depression in self-harming adolescents. Borderline Personality Disorder and Emotion Dysregulation 2017;4:22. [DOI: 10.1186/s40479-017-0073-5] [PMC5663078] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohamadizadeh 2017 {published data only}
- Mohamadizadeh L, Makvandi B, Pasha R, Bakhtiarpour S, Hafezi F. Comparing of the effect of dialectical behavior therapy (DBT) and schema therapy (ST) on reducing mood activity and suicidal thoughts in patients with borderline personality disorder. Acta Medica Mediterranea 2017;33:1025-31. [DOI: 10.19193/0393-6384_2017_6_162] [www.actamedicamediterranea.com/archive/2017/medica-6/comparing-of-the-effect-of-dialectical-behavior-therapy-dbt-and-schema-therapy-st-on-reducing-mood-activity-and-suicidal-thoughts-in-patients-with-borderline-personality-disorder/pdf] [DOI] [Google Scholar]
Morey 2010 {published data only}
- Morey LC, Lowmaster SE, Hopwood CJ. A pilot study of manual-assisted cognitive therapy with a therapeutic assessment augmentation for borderline personality disorder. Psychiatry Research 2010;178(3):531-5. [DOI: 10.1016/j.psychres.2010.04.055] [PMID: ] [DOI] [PubMed] [Google Scholar]
Morton 2012 {published data only}
- Morton J, Snowdon S, Gopold M, Guymer E. Acceptance and commitment therapy group treatment for symptoms of borderline personality disorder: a public sector pilot study. Cognitive and Behavioral Practice 2012;19(4):527-44. [DOI: 10.1016/j.cbpra.2012.03.005] [DOI] [Google Scholar]
- Morton J. Re: SV: regarding your study on acceptance and commitment therapy for BPD [personal communication]. Email to: EG Faltinsen 22 December 2017.
Nadort 2009 {published data only}
- Nadort M, Arntz A, Smit JH, Giesen-Bloo J, Eikelenboom J, Spinhoven P, et al. Implementation of outpatient schema therapy for borderline personality disorder with versus without crisis support by the therapist outside office hours: a randomized trial. Behaviour Research and Therapy 2009;47(11):961-73. [DOI: 10.1016/j.brat.2009.07.013] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Nadort M, Arntz A, Smit JH, Giesen-Bloo J, Eikelenboom M, Spinhoven P, et al. Implementation of outpatient schema therapy for borderline personality disorder: study design. BMC Psychiatry 2009;9:64. [DOI: 10.1186/1471-244X-9-64] [PMC2762959] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NL1680 (NTR1781). Implementation of out-patient schema-focused therapy for borderline personality disorder in regular mental healthcare. www.trialregister.nl/trial/1680 (first received 29 April 2009).
Pascual 2015 {published data only}
- NCT02033044. Cognitive rehabilitation versus psychoeducation in psychosocial functioning of borderline personality disorder [Randomized controlled trial comparing the effects on psychosocial functioning of cognitive rehabilitation versus psychoeducation in borderline personality disorder]. www.clinicaltrials.gov/ct2/show/NCT02033044 (first received 23 December 2013).
- Pascual JC, Palomares N, Ibáñez Á, Portella MJ, Arza R, Reyes R, et al. Efficacy of cognitive rehabilitation on psychosocial functioning in borderline personality disorder: a randomized controlled trial. BMC Psychiatry 2015;15:255. [DOI: 10.1186/s12888-015-0640-5] [PMC4617906] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Philips 2018 {published data only}
- Philips B, Wenneberg P, Konradsson P, Franck J. Mentalization-based treatment for concurrent borderline personality disorder and substance use disorder: a randomized controlled feasibility study. European Addiction Research 2018;24(1):1-8. [DOI: 10.1159/000485564] [PMC5969093] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Priebe 2012 {published data only}
- Barnicot K. Re: Regarding your study on DBT for self-harming patients [personal communication]. Email to: EG Faltinsen 28 February 2018.
- Priebe S, Bhatti N, Barnicot K, Bremner S, Gaglia A, Katsakou C, et al. Effectiveness and cost-effectiveness of dialectical behaviour therapy for self-harming patients with personality disorder: a pragmatic randomised controlled trial. Psychotherapy and Psychosomatics 2012;81(6):356-65. [DOI: 10.1159/000338897] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Priebe S. Supplemental information [personal communication]. Email to: M Kielsholm 20 May 2016.
Reneses 2013 {published data only}
- Reneses B, Figuera D, Salcedo G, Trujillo M, López-Ibor JJ, Galián M, et al. A controlled randomized study on the efficacy of short-term dinamic psychotherapy in borderline personality disorders (BPD). Preliminary results. European Psychiatry 2011;26(Suppl 1):1040. [DOI: 10.1016/S0924-9338(11)72745-6] [P02-444] [DOI] [Google Scholar]
- Reneses B, Galián M, Serrano R, Figuera D, Fernandez Del Moral A, López-Ibor JJ, et al. A new time limited psychotherapy for BPD: preliminary results of a randomized and controlled trial. Actas Espanolas de Psiquiatria 2013;41(3):139-48. [PMID: ] [PubMed] [Google Scholar]
Robinson 2016 {published data only}ISRCTN51304415
- ISRCTN51304415. Nice OUtcomes for Referrals with Impulsivity, Self Harm and Eating Disorders: the NOURISHED study [A randomised controlled trial of mentalisation based therapy against specialist supportive clinical management in patients with both eating disorders and symptoms of borderline personality disorder]. www.isrctn.com/ISRCTN51304415 (first received 31 January 2011).
- Robinson P, Barrett B, Bateman A, Hakeem A, Hellier J, Lemonsky F, et al. Study protocol for a randomized controlled trial of mentalization based therapy against specialist supportive clinical management in patients with both eating disorders and symptoms of borderline personality disorder. BMC Psychiatry 2014;14:51. [DOI: 10.1186/1471-244X-14-51] [PMC3996076] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson P, Hellier J, Barrett B, Barzdaitiene D, Bateman A, Bogaardt A, et al. The NOURISHED randomised controlled trial comparing mentalisation-based treatment for eating disorders (MBT-ED) with specialist supportive clinical management (SSCM-ED) for patients with eating disorders and symptoms of borderline personality disorder. Trials 2016;17(1):549. [DOI: 10.1186/s13063-016-1606-8] [PMC5114835] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rossouw 2012b {published data only}ISRCTN95266816
- ISRCTN95266816. The emergence of personality disorder traits in adolescents who deliberately self harm and the potential for using a mentalisation based treatment approach as an early intervention for such individuals: a randomised controlled trial. www.isrctn.com/ISRCTN95266816 (first received 29 October 2007).
- Rossouw T. Mentalisation based treatment for adolescents with self harm: an RCT. European Child & Adolescent Psychiatry 2015;24(1 Suppl):S113. [DOI: ] [W1-02-02] [Google Scholar]
- Rossouw T. Self harm in adolescence, is MBT the answer?: an RCT. Adolescent Psychiatry 2012;2(1):102. [DOI: 10.2174/2210676611202010077] [DOI] [Google Scholar]
- Rossouw TI, Fonagy P. Mentalization-based treatment for self-harm in adolescents: a randomized controlled trial. Journal of the American Academy of Child and Adolescent Psychiatry 2012;51(12):1304-13.e3. [DOI: 10.1016/j.jaac.2012.09.018] [PMID: ] [DOI] [PubMed] [Google Scholar]
Salzer 2014 {published data only}
- Salzer S, Cropp C, Jaeger U, Masuhr O, Streeck-Fischer A. Psychodynamic therapy for adolescents suffering from co-morbid disorders of conduct and emotions in an in-patient setting: a randomized controlled trial. Psychological Medicine 2014;44(10):2213-22. [DOI: 10.1017/S003329171300278X] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Salzer S, Streeck-Fischer A. The psychoanalytic-interactional method (PiM) for adolescents with borderline personality disorder [Die psychoanalytisch-interaktionelle methode (PiM) für adoleszente mit borderline-persönlichkeitsstörung]. Personlichkeitsstorungen Theorie und Therapie 2015;19(1):67-76. [elibrary.klett-cotta.de/article/99.120110/ptt-19-1-67] [Google Scholar]
- Salzer S. AW: Re: Cochrane Review on psychotherapies for BPD - RCT testing PiM vs WL [personal communication] [AW:Re: Cochrane Review zur Psychotherapie bei BPS - RCT zu PiM vs WL [persönliche kommunikation]]. Email to: J Stoffers-Winterling 15 July 2018.
Santisteban 2015 {published data only}
- Santisteban DA, Mena MP, Muir J, McCabe BE, Abalo C, Cummings AM. The efficacy of two adolescent substance abuse treatments and the impact of comorbid depression: results of a small randomized controlled trial. Psychiatric Rehabilitation Journal 2015;38(1):55-64. [DOI: 10.1037/prj0000106] [PMC5021542] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schilling 2018 {published data only}
- Schilling L, Moritz S, Köther U, Nagel M. Preliminary results on acceptance, feasibility, and subjective efficacy of the add-on group intervention metacognitive training for borderline patients. Journal of Cognitive Psychotherapy 2015;29(2):153-64. [DOI: 10.1891/0889-8391.29.2.153] [DOI] [PubMed] [Google Scholar]
- Schilling L, Moritz S, Kriston L, Krieger M, Nagel M. Efficacy of metacognitive training for patients with borderline personality disorder: preliminary results. Psychiatry Research 2018;262:459-64. [DOI: 10.1016/j.psychres.2017.09.024] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Schilling L. Re Schilling 2015/2018 - Cochrane Review of psychotherapies for BPD [personal communication] [Re Schilling 2015/2018 - Cochrane Review zur Psychotherapie bei BPS [persönliche Kommunikation]]. Email to: J Stoffers-Winterling 22 January 2019.
Schuppert 2012 {published data only}ISRCTN9758910
- ISRCTN97589104. Evaluation of a group training for adolescents (emotion regulation training) with emotion regulation problems - a randomised controlled clinical trial. www.isrctn.com/ISRCTN97589104 (first received 23 August 2007).
- Schuppert HM, Timmerman ME, Bloo J, Van Gemert TG, Wiersema HM, Minderaa RB, et al. Emotion regulation training for adolescents with borderline personality disorder traits: a randomized controlled trial. Journal of the American Academy of Child & Adolescent Psychiatry 2012;51(12):1314-23. [DOI: 10.1016/j.jaac.2012.09.002] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sinnaeve 2018 {published data only}
- NCT01904227. Intensified, inpatient adaptation of dialectical behavior therapy (DBT) (REDBT) [A randomized controlled study of the efficacy of an intensified, inpatient adaptation of dialectical behavior therapy (DBT) for a population of borderline patients (young adults/adults: 18-40), compared with standard outpatient DBT]. www.clinicaltrials.gov/ct2/show/NCT01904227 (first received 11 July 2013).
- Sinnaeve R, Van den Bosch LM, Van Steenbergen-Weijenburg KM. Change in interpersonal functioning during psychological interventions for borderline personality disorder — a systematic review of measures and efficacy. Personality and Mental Health 2015;9(3):173-94. [DOI: 10.1002/pmh.1296] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Sinnaeve R, Van den Bosch LMC, Hakkaart-van Roijen L, Vansteelandt K. Effectiveness of step-down versus outpatient dialectical behaviour therapy for patients with severe levels of borderline personality disorder: a pragmatic randomized controlled trial. Borderline Personality Disorder and Emotion Dysregulation 2018;5:12. [DOI: 10.1186/s40479-018-0089-5] [PMC6040072] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnaeve R. Cochrane Collaboration review [personal communication]. Email to: J Stoffers-Winterling 11 June 2018.
- Sinnaeve R. Re: BPDSI subscale data Sinnaeve et al (2018) [personal communication]. Email to: A Tadorovac 15 November 2018.
- Sinnaeve R. RE: BPDSI subscale data Sinnaeve et al (2018) [personal communication] [RE: BPDSI subschale data Sinnaeve et al (2018) [personlig kommunikation]]. Email to: A Todorovac 15 November 2018.
- Sinnaeve R. RE: BPDSI subscale data Sinnaeve et al (2018) [personal communication] [RE: BPDSI subschale data Sinnaeve et al (2018) [personlig kommunikation]]. Email to: A Todorovac 9 October 2018.
- Van den Bosch LM, Sinnaeve R, Hakkaart-van Roijen L, Van Furth EF. Efficacy and cost-effectiveness of an experimental short-term inpatient dialectical behavior therapy (DBT) program: study protocol for a randomized controlled trial. Trials 2014;15:152. [DOI: 10.1186/1745-6215-15-152] [PMC4017823] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Roijen LH, Sinnaeve R, Bouwmans C, Van Den Bosch L. Cost-effectiveness and cost-utility of shortterm inpatient dialectical behavior therapy for chronically parasuicidal BPD (young) adults. Journal of Mental Health Policy and Economics 2015;18(Suppl 1):S19-20. [www.icmpe.org/test1/journal/issues/v18s1toc.html] [Google Scholar]
Smith 2012 {published data only}
- Smith PN, Gamble SA, Cort NA, Ward EA, He H, Talbot NL. Attachment and alliance in the treatment of depressed, sexually abused women. Depression and Anxiety 2012;29(2):123-30. [DOI: 10.1002/da.20913] [PMC3325338] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PN. Re: Cochrane Collaboration review of psychotherapies for BPD [personal communication]. Email to: J Stoffers-Winterling 05 July 2018.
Soler 2009 {published data only}
- Soler J, Pascual JC, Tiana T, Cebrià A, Barrachina J, Campins MJ, et al. Dialectical behaviour therapy skills training compared to standard group therapy in borderline personality disorder: a 3-month randomised controlled clinical trial. Behaviour Research and Therapy 2009;47(5):353-8. [DOI: 10.1016/j.brat.2009.01.013] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Soler J. Question regarding your BPD trials [personal communication]. Email to: J Stoffers 15 December 2010.
Stanley 2017 {published data only}
- Fertuck EA, Keilp J, Song I, Morris MC, Wilson ST, Brodsky BS, et al. Higher executive control and visual memory performance predict treatment completion in borderline personality disorder. Psychotherapy and Psychosomatics 2012;81(1):38-43. [DOI: 10.1159/000329700] [PMC3242704] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00533117. Treating suicidal behavior and self-mutilation in people with borderline personality disorder. www.clinicaltrials.gov/ct2/show/NCT00533117 (first received 19 September 2007).
Turner 2000 {published data only}
- Turner RM. Naturalistic evaluation of dialectical behavior therapy-oriented treatment for borderline personality disorder. Cognitive and Behavioral Practice 2000;7(4):413-9. [DOI: 10.1016/S1077-7229(00)80052-8] [DOI] [Google Scholar]
Van den Bosch 2005 {published data only}
- Van den Bosch LM, Koeter MW, Stijnen T, Verheul R, Van den Brink W. Sustained efficacy of dialectical behaviour therapy for borderline personality disorder. Behaviour Research and Therapy 2005;43(9):1231-41. [DOI: 10.1016/j.brat.2004.09.008] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Van den Bosch LM, Verheul R, Schippers GM, Van den Brink W. Dialectical behavior therapy of borderline patients with and without substance use problems. Implementation and long-term effects. Addictive Behaviors 2002;27(6):911-23. [DOI: 10.1016/s0306-4603(02)00293-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Van den Bosch LMC. Efficacy of dialectical behaviour therapy in the treatment of female borderline patients with and without substance abuse problems: results of a Dutch study [Dialectische gedragstherapie bij Nederlandse vrouwen met een borderline persoonlijkheidsstoornis, met en zonder verslavingsproblemen]. Tijdschrift voor Psychiatrie 2005;47(3):127-37. [www.tijdschriftvoorpsychiatrie.nl/assets/articles/articles_1335pdf.pdf] [Google Scholar]
- Verheul R, Van den Bosch LM, Koeter MW, De Ridder MA, Stijnen T, Van den Brink W. Dialectical behaviour therapy for women with borderline personality disorder: 12-month, randomised clinical trial in The Netherlands. British Journal of Psychiatry 2003;182:135-40. [DOI: 10.1192/bjp.182.2.135] [PMID: ] [DOI] [PubMed] [Google Scholar]
Weinberg 2006 {published data only}
- Weinberg I, Gunderson JG, Hennen J, Cutter CJ Jr. Manual assisted cognitive treatment for deliberate self-harm in borderline personality disorder patients. Journal of Personality Disorders 2006;20(5):482-92. [DOI: 10.1521/pedi.2006.20.5.482] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Wilberg T. SV: separate data [personal communication] [SV: separat data [personlig kommunikation]]. Email to: M Kielsholm 25 May 2016.
Zanarini 2008 {published data only}
- Zanarini MC, Frankenburg FR. A preliminary, randomized trial of psychoeducation for women with borderline personality disorder. Journal of Personality Disorders 2008;22(3):284-90. [DOI: 10.1521/pedi.2008.22.3.284] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Zanarini MC, Frankenburg FR. A randomized trial of psychoeducation for patients with BPD. In: 157th annual meeting of the American Psychiatric Association. Psychotherapy and psychopharmacology: dissolving the mind-brain barrier; 2004 May 1-6; New York (NY). Arlington (VA): American Psychiatric Association, 2004:102. [Symposium 100E] [www.psychiatry.org/File%20Library/Psychiatrists/Directories/Library-and-Archive/conference_publications/am_program_2004.pdf]
Zanarini 2018 {published data only}
- Zanarini MC, Conkey LC, Temes CM, Fitzmaurice GM. Randomized controlled trial of web-based psychoeducation for women with borderline personality disorder. Journal of Clinical Psychiatry 2018;79(3):16m11153. [DOI: 10.4088/JCP.16m11153] [PMC5764827] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Ahmed 2016 {published data only}ISRCTN76914248
- Ahmed N, John A, Islam S, Jones R, Anderson P, Davies C, et al. Investigating the feasibility of an enhanced contact intervention in self-harm and suicidal behaviour: a protocol for a randomised controlled trial delivering a Social support and Wellbeing Intervention following Self Harm (SWISH). BMJ Open 2016;6(9):e012043. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Andover 2017 {published data only}
- Andover MS, Schatten HT, Morris BW, Holman CS, Miller IW. An intervention for nonsuicidal self-injury in young adults: a pilot randomized controlled trial. Journal of Consulting and Clinical Psychology 2017;85(6):620-31. [DOI: 10.1037/ccp0000206] [PMC5760269] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Andreasson 2016 {published data only}
- Andreasson K, Krogh J, Wenneberg C, Jessen HKL, Krakauer K, Gluud C, et al. Effectiveness of dialectical behavior therapy versus collaborative assessment and management of suicidality treatment for reduction of self-harm in adults with borderline personality traits and disorder — a randomized observer-blinded clinical trial. Depression & Anxiety 2016;33(6):520-30. [DOI: 10.1002/da.22472] [PMID: ] [DOI] [PubMed] [Google Scholar]
Apsche 2006 {published data only}
- Apsche JA, Bass CK. A treatment study of mode deactivation therapy in an out patient community setting. International Journal of Behavioral Consultation and Therapy 2006;2(1):85-93. [DOI: 10.1037/h0100769] [DOI] [Google Scholar]
Asarnow 2017 {published data only}
- Asarnow JR, Hughes JL, Babeva KN, Sugar CA. Cognitive-behavioral family treatment for suicide attempt prevention: a randomized controlled trial. Journal of the American Academy of Child and Adolescent Psychiatry 2017;56(6):506-14. [DOI: 10.1016/j.jaac.2017.03.015] [PMC5474088] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bamelis 2012a {published data only}
- Bamelis LL, Evers SM, Arntz A. Design of a multicentered randomized controlled trial on the clinical and cost effectiveness of schema therapy for personality disorders. BMC Public Health 2012;12:75. [DOI: 10.1186/1471-2458-12-75] [PMC3305366 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bateman 2019b {published data only}
- Bateman A, Fonagy P. A randomized controlled trial of a mentalization-based intervention (MBT-FACTS) for families of people with borderline personality disorder. Personality Disorders: Theory, Research, & Treatment 2019;10(1):70-9. [DOI: 10.1037/per0000298] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bertsch 2013 {published data only}
- Bertsch K, Gamer M, Schmidt B, Schmidinger I, Walther S, Kästel T, et al. Oxytocin and reduction of social threat hypersensitivity in women with borderline personality disorder. American Journal of Psychiatry 2013;170(10):1169-77. Erratum in: American Journal of Psychiatry 2013;170(10):1218a. [DOI: 10.1176/appi.ajp.2013.13020263] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bira 2014 {published data only}
- Bira LM. Brief Psychological Intervention for Acute Posttraumatic stress: Individual and Trauma Factors Affecting Recovery in Low-SES Minorities [PhD thesis]. Miami (FL): University of Miami, 2014. [Google Scholar]
Brent 2018 {published data only}
- Brent DA, Kennard BD. Impact of a brief inpatient intervention and suicide safety planning app to decrease suicidal behavior after hospital discharge. Journal of the American Academy of Child & Adolescent Psychiatry 2018;57(10 Suppl):S33. [DOI: 10.1016/j.jaac.2018.07.142] [Abstract #22.4] [DOI] [Google Scholar]
Brune 2012 {published data only}
- Brüne M, Ebert A, Kolb M, Tas C, Edel MA, Roser P. Oxytocin influences avoidant reactions to social threat in adults with borderline personality disorder. Human Psychopharmacology 2013;28(6):552-61. [DOI: 10.1002/hup.2343] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Brüne M, Ebert A. Does oxytocin have a role in borderline personality disorder? International Journal of Neuropsychopharmacology 2012;15(Suppl 1):20. [DOI: 10.1017/S1461145712000508] [Abstract # S-15-004] [DOI] [Google Scholar]
Buric 2019 {published data only}
- Buric I, Farias M, Van Mulukom V, Jong J, Kurtev S, Mee C, et al. The neural, genetic and behavioural effects of intensive meditation and yoga on prisoners with personality disorders. Brain, Behavior, and Immunity 2019;76 Suppl:e17. [DOI: 10.1016/j.bbi.2018.11.224] [Abstract #3099] [DOI] [Google Scholar]
Cailhol 2014 {published data only}
- Cailhol L, Roussignol B, Klein R, Bousquet B, Simonetta-Moreau M, Schmitt L, et al. Borderline personality disorder and rTMS: a pilot trial. Psychiatry Research 2014;216(1):155-7. [DOI: 10.1016/j.psychres.2014.01.030] [PMID: ] [DOI] [PubMed] [Google Scholar]
Carta 2014 {published data only}
- Carta MG, Maggiani F, Pilutzu L, Moro MF, Mura G, Sancassiani F, et al. Sailing can improve quality of life of people with severe mental disorders: results of a cross over randomized controlled trial. Clinical Practice and Epidemiology in Mental Health 2014;10:80-6. [DOI: 10.2174/1745017901410010080] [PMC4150378] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carter 2013 {published data only}
- Carter JD, McIntosh VV, Jordan J, Porter RJ, Frampton CM, Joyce PR. Psychotherapy for depression: a randomized clinical trial comparing schema therapy and cognitive behavior therapy. Journal of Affective Disorders 2013;151(2):500-5. [DOI: 10.1016/j.jad.2013.06.034] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chanen 2008 {published data only}
- Chanen AM, Jackson HJ, McCutcheon LK, Jovev M, Dudgeon P, Yuen HP, et al. Early intervention for adolescents with borderline personality disorder using cognitive analytic therapy: randomised controlled trial. British Journal of Psychiatry 2008;193(6):477-84. [PMID: ] [DOI] [PubMed] [Google Scholar]
Charney 2015 {published data only}
- Charney DA, Heath LM, Zikos E, Palacios-Boix J, Gill KJ. Poorer drinking outcomes with citalopram treatment for alcohol dependence: a randomized, double-blind, placebo-controlled trial. Alcoholism: Clinical and Experimental Research 2015;39(9):1756-65. [DOI: 10.1111/acer.12802] [PMID: ] [DOI] [PubMed] [Google Scholar]
Coccaro 1997 {published data only}
- Coccaro EF, Kavoussi RJ. Fluoxetine and impulsive aggressive behavior in personality-disordered subjects. Archives of General Psychiatry 1997;54(12):1081-8. [DOI: 10.1001/archpsyc.1997.01830240035005] [9400343] [DOI] [PubMed] [Google Scholar]
Cornelius 1993 {published data only}
- Cornelius JR, Soloff PH, George A, Ulrich RF, Perel JM. Haloperidol vs phenelzine in continuation therapy of borderline disorder. Psychopharmacology Bulletin 1993;29(2):333-7. [PMID: ] [PubMed] [Google Scholar]
- Cornelius JR, Soloff PH, Perel JM, Ulrich RF. Continuation pharmacotherapy of borderline personality disorder with haloperidol and phenelzine. American Journal of Psychiatry 1993;150(12):1843-8. [DOI: 10.1176/ajp.150.12.1843] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cottrell 2018 {published data only}ISRCTN59793150
- Cottrell DJ, Wright-Hughes A, Collinson M, Boston P, Eisler I, Fortune S, et al. A pragmatic randomised controlled trial and economic evaluation of family therapy versus treatment as usual for young people seen after second or subsequent episodes of self-harm: the self-harm intervention - family therapy (SHIFT) trial. Health Technology Assessment 2018;22(12):1-222. [DOI: 10.3310/hta22120] [PMC5867393 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell DJ, Wright-Hughes A, Collinson M, Boston P, Eisler I, Fortune S, et al. Effectiveness of systemic family therapy versus treatment as usual for young people after self-harm: a pragmatic, phase 3, multicentre, randomised controlled trial. Lancet Psychiatry 2018;5(3):203-16. [DOI: 10.1016/S2215-0366%2818%2930058-0] [PMC5835764 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Crawford 2018b {published data only}ISRCTN14994755
- Crawford MJ, Thana L, Parker J, Turner O, Xing KP, McMurran M, et al. Psychological support for personality (PSP) versus treatment as usual: study protocol for a feasibility randomized controlled trial of a low intensity intervention for people with personality disorder. Trials 2018;19(1):547. [DOI: 10.1186/s13063-018-2920-0] [PMC6180621] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cullen 2012a {published data only}
- Cullen AE, Clarke AY, Kuipers E, Hodgins S, Dean K, Fahy T. A multi-site randomized controlled trial of a cognitive skills programme for male mentally disordered offenders: social-cognitive outcomes. Psychological Medicine 2012;42(3):557-69. [DOI: 10.1017/s0033291711001553] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Cullen AE, Clarke AY, Kuipers E, Hodgins S, Dean K, Fahy T. A multisite randomized trial of a cognitive skills program for male mentally disordered offenders: violence and antisocial behavior outcomes. Journal of Consulting and Clinical Psychology 2012;80(6):1114-20. [DOI: 10.1037/a0030291] [PMID: ] [DOI] [PubMed] [Google Scholar]
De Beurs 2013 {published data only}
- De Beurs DP, De Groot MH, Bosmans JE, De Keijser J, Mokkenstorm J, Verwey B, et al. Reducing patients' suicide ideation through training mental health teams in the application of the Dutch multidisciplinary practice guideline on assessment and treatment of suicidal behavior: study protocol of a randomized controlled trial. Trials 2013;14:372. [DOI: 10.1186/1745-6215-14-372] [PMC3826515] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
DeSaeger 2014 {published data only}
- De Saeger H, Kamphuis JH, Finn SE, Smith JD, Verheul R, Van Busschbach JJ, et al. Therapeutic assessment promotes treatment readiness but does not affect symptom change in patients with personality disorders: findings from a randomized clinical trial. Psychological Assessment 2014;26(2):474-83. Erratum in: Psychological Assessment 2014;26(2):483. [DOI: 10.1037/a0035667] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dunlop 2012 {published data only}
- Dunlop BW, Binder EB, Cubells JF, Goodman MM, Kelley ME, Kinkead B, et al. Predictors of remission in depression to individual and combined treatments (PReDICT): study protocol for a randomized controlled trial. Trials 2012;13:106. [DOI: 106 10.1186/1745-6215-13-106] [PMC3539869 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ehlers 2013 {published data only}
- Ehlers A, Grey N, Wild J, Stott R, Liness S, Deale A, et al. Implementation of cognitive therapy for PTSD in routine clinical care: effectiveness and moderators of outcome in a consecutive sample. Behaviour Research and Therapy 2013;51(11):742-52. [DOI: 10.1016/j.brat.2013.08.006] [PMC3897916] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ekeblad 2016 {published data only}
- Ekeblad A, Falkenström F, Andersson G, Vestberg R, Holmqvist R. Randomized trial of interpersonal psychotherapy and cognitive behavioral therapy for major depressive disorder in a community-based psychiatric outpatient clinic. Depression & Anxiety 2016;33(12):1090-8. [DOI: 10.1002/da.22495] [PMID: ] [DOI] [PubMed] [Google Scholar]
EUCTR 2010‐020956‐69 {published data only}
- EUCTR 2010-020956-69. Effekte von oxytocin bei patientinnen und patienten mit borderline-persönlichkeitsstörung. www.clinicaltrialsregister.eu/ctr-search/trial/2010-020956-69/DE (first received 20 October 2010).
EUCTR 2015‐000749‐21 {published data only}
- EUCTR 2015-000749-21. Botulinumtoxin A for emotional stabilization in borderline personality disorder [Botulinumtoxin A zur emotionalen stabilisierung bei der borderline-persönlichkeitsstörung]. www.clinicaltrialsregister.eu/ctr-search/trial/2015-000749-21/DE (first received 30 December 2015).
Fleischer 2015 {published data only}
- Fleischer J, Wingenfeld K, Kuehl LK, Hinkelmann K, Roepke S, Otte C. Does fludrocortisone influence autobiographical memory retrieval? A study in patients with major depression, patients with borderline personality disorder and healthy controls. Stress 2015;18(6):718-22. [DOI: 10.3109/10253890.2015.1087504] [PMID: ] [DOI] [PubMed] [Google Scholar]
Furuno 2018 {published data only}
- Furuno T, Nakagawa M, Hino K, Yamada T, Kawashima Y, Matsuoka Y, et al. Effectiveness of assertive case management on repeat self-harm in patients admitted for suicide attempt: findings from ACTION-J study. Journal of Affective Disorders 2018;225:460-5. [DOI: 10.1016/j.jad.2017.08.071] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ghahramanlou Holloway 2012 {published data only}
- Ghahramanlou-Holloway M, Bhar SS, Brown GK, Olsen C, Beck AT. Changes in problem-solving appraisal after cognitive therapy for the prevention of suicide. Psychological Medicine 2012;42(6):1185-93. [DOI: 10.1017/S0033291711002169] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ginty 2016 {published data only}
- Ginty AT, Muldoon MF, Kuan DCH, Schirda B, Kamarck TW, Jennings JR, et al. Omega-3 supplementation and the neural correlates of mood and impulsivity: a double-blind, randomized, placebo-controlled trial in midlife adults. Psychosomatic Medicine 2016;78(3):A41. [DOI: 10.1097/PSY.0000000000000343] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gold 2013 {published data only}
- Gold C, Mössler K, Grocke D, Heldal TO, Tjemsland L, Aarre T, et al. Individual music therapy for mental health care clients with low therapy motivation: multicentre randomised controlled trial. Psychotherapy and Psychosomatics 2013;82(5):319-31. [DOI: 10.1159/000348452] [PMID: ] [DOI] [PubMed] [Google Scholar]
Green 2011 {published data only}
- Green JM, Wood AJ, Kerfoot MJ, Trainor G, Roberts C, Rothwell J, et al. Group therapy for adolescents with repeated self harm: randomised controlled trial with economic evaluation. BMJ 2011;342:d682. [DOI: 10.1136/bmj.d682] [PMC3069684] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grimholt 2015 {published data only}
- Grimholt TK, Jacobsen D, Haavet O, Sandvik L, Jorgensen T, Norheim AB, et al. Effect of systematic follow-up by general practitioners after deliberate self-poisoning: a randomised controlled trial. PLOS One 2015;10(12):e0143934. [DOI: 10.1371/journal.pone.0143934] [PMC4667913 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ha 2016 {published data only}
- Ha C. The Effects of Intranasal Oxytocin on Social Cognitive Functioning in Adolescents with Borderline Personality Disorder Compared to a Sample of Non-Clinical Adolescents [PhD thesis]. Houston (TX): University of Houston, 2016. [Google Scholar]
Haddock 2016 {published data only}
- Haddock G, Davies L, Evans E, Emsley R, Gooding P, Heaney L, et al. Investigating the feasibility and acceptability of a cognitive behavioural suicide prevention therapy for people in acute psychiatric wards (the 'INSITE' trial): study protocol for a randomised controlled trial. Trials 2016;17:79. [DOI: 10.1186/s13063-016-1192-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hassanzadeh 2010 {published data only}
- Hassanzadeh M, Khajeddin N, Nojomi M, Fleischmann A, Eshrati T. Brief intervention and contact after deliberate self-harm: an Iranian randomized controlled trial. Iranian Journal of Psychiatry and Behavioral Sciences 2010;4(2):5-12. [ijpsychiatrybs.com/en/articles/2900.html] [Google Scholar]
Hatcher 2015 {published data only}
- Hatcher S, Coupe N, Wikiriwhi K, Durie SM, Pillai A. Te Ira Tangata: a Zelen randomised controlled trial of a culturally informed treatment compared to treatment as usual in Māori who present to hospital after self-harm. Social Psychiatry & Psychiatric Epidemiology 2016;51(6):885-94. [DOI: 10.1007/s00127-016-1194-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Hatcher S, Sharon C, House A, Collings S, Parag V, Collins N. The ACCESS study: a Zelen randomised controlled trial of a treatment package including problem solving therapy compared to treatment as usual in people who present to hospital after self-harm: study protocol for a randomised controlled trial. Trials 2011;12:135. [DOI: 10.1186/1745-6215-12-135] [PMC3117717 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher S, Sharon C, House A, Collins N, Collings S, Pillai A. The ACCESS study: Zelen randomised controlled trial of a package of care for people presenting to hospital after self-harm. British Journal of Psychiatry 2015;206(3):229-36. [DOI: 10.1192/bjp.bp.113.135780] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hazell 2009 {published data only}
- Hazell PL, Martin G, McGill K, Kay T, Wood A, Trainor G, et al. Group therapy for repeated deliberate self-harm in adolescents: failure of replication of a randomized trial. Journal of the American Academy of Child and Adolescent Psychiatry 2009;48(6):662-70. [DOI: 10.1097/CHI.0b013e3181aOacec] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hesse 2010 {published data only}
- Hesse M. Psychoeducation for personality disorders as an add-on to substance abuse treatment versus attention placebo: a controlled trial. Drugs and Alcohol Today 2010;10(1):25-32. [DOI: 10.5042/daat.2010.0125] [DOI] [Google Scholar]
Hewage 2018 {published data only}
- Hewage K, Steel Z, Mohsin M, Tay AK, De Oliveira JC, Da Piedade M, et al. A wait-list controlled study of a trauma-focused cognitive behavioral treatment for intermittent explosive disorder in Timor-Leste. American Journal of Orthopsychiatry 2018;88(3):282-94. [DOI: 10.1037/ort0000280] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hirayasu 2009 {published data only}
- Hirayasu Y, Kawanishi C, Yonemoto N, Ishizuka N, Okubo Y, Sakai A, et al. A randomized controlled multicenter trial of post-suicide attempt case management for the prevention of further attempts in Japan (ACTION-J). BMC Public Health 2009;9:364. [DOI: 10.1186/1471-2458-9-364] [PMC2760885] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Holder 2017 {published data only}
- Holder N, Holliday R, Pai A, Surís A. Role of borderline personality disorder in the treatment of military sexual trauma-related posttraumatic stress disorder with cognitive processing therapy. Behavioral Medicine 2017;43(3):184-90. [DOI: 10.1080/08964289.2016.1276430] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hooley 2018 {published data only}ISRCTN12276176
- Hooley JM, Fox KR, Wang SB, Kwashie AND. Novel online daily diary interventions for nonsuicidal self-injury: a randomized controlled trial. BMC Psychiatry 2018;18:264. [DOI: 10.1186/s12888-018-1840-6] [PMC6106828] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Husain 2011 {published data only}
- Husain N, Fayyaz H, Chaudhry N, Afsar S, Husain M, Rahman R. Culturally adapted manual assisted problem solving training (C-MAPS) for prevention of self harm: an RCT from a low income country. Journal of Psychosomatic Research 2011;70(6):595. [DOI: 10.1016/j.jpsychores.2011.03.006] [DOI] [Google Scholar]
Husain 2014 {published data only}
- Husain N, Afsar S, Ara J, Fayyaz H, Rahman RU, Tomenson B, et al. Brief psychological intervention after self-harm: randomised controlled trial from Pakistan. British Journal of Psychiatry 2014;204(6):462-70. [DOI: 10.1192/bjp.bp.113.138370] [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT01308151. Culturally adapted manual assisted cognitive behavioral therapy for prevention of self harm: a randomized control trial [Culturally adapted cognitive behavioral therapy for prevention of self harm]. www.ichgcp.net/clinical-trials-registry/NCT01308151 (first received 2 March 2011).
Johansson 2010 {published data only}
- Johansson P, Høglend P, Ulberg R, Amlo S, Marble A, Bøgwald K-P, et al. The mediating role of insight for long-term improvements in psychodynamic therapy. Journal of Consulting and Clinical Psychology 2010;78(3):438-48. [DOI: 10.1037/a0019245] [PMID: 20515219] [DOI] [PubMed] [Google Scholar]
Kawanishi 2014 {published data only}
- Kawanishi C, Aruga T, Ishizuka N, Yonemoto N, Otsuka K, Kamijo Y, et al. Assertive case management versus enhanced usual care for people with mental health problems who had attempted suicide and were admitted to hospital emergency departments in Japan (ACTION-J): a multicentre, randomised controlled trial. Lancet Psychiatry 2014;1(3):193-201. [DOI: 10.1016/S2215-0366(14)70259-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
Keefe 2016 {published data only}
- Keefe JR, Webb CA, DeRubeis RJ. In cognitive therapy for depression, early focus on maladaptive beliefs may be especially efficacious for patients with personality disorders. Journal of Consulting and Clinical Psychology 2016;84(4):353-64. [DOI: 10.1037/ccp0000071] [PMC4936187] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Keefe 2018 {published data only}
- Keefe JR, Milrod BL, Gallop R, Barber JP, Chambless DL. What is the effect on comorbid personality disorder of brief panic-focused psychotherapy in patients with panic disorder? Depression & Anxiety 2018;35(3):239-47. [DOI: 10.1002/da.22708] [PMC5842115] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kellett 2014 {published data only}
- Kellett S, Wilbram M, Davis C, Hardy G. Team consultancy using cognitive analytic therapy: a controlled study in assertive outreach. Journal of Psychiatric and Mental Health Nursing 2014;21(8):687-97. [DOI: 10.1111/jpm.12123] [DOI] [PubMed] [Google Scholar]
Keng 2018 {published data only}
- Keng SL, Tan HH. Effects of brief mindfulness and loving-kindness meditation inductions on emotional and behavioral responses to social rejection among individuals with high borderline personality traits. Behaviour Research and Therapy 2018;100:44-53. [DOI: 10.1016/j.brat.2017.11.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kennard 2018 {published data only}
- Kennard BD, Goldstein T, Foxwell AA, McMakin DL, Wolfe K, Biernesser C, et al. As safe as possible (ASAP): a brief app-supported inpatient intervention to prevent postdischarge suicidal behavior in hospitalized, suicidal adolescents. American Journal of Psychiatry 2018;175(9):864-72. [DOI: 10.1176/appi.ajp.2018.17101151] [PMC6169524] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Koenigsberg 2003 {published data only}
- Koenigsberg HW, Reynolds D, Goodman M, New AS, Mitropoulou V, Trestman RL, et al. Risperidone in the treatment of schizotypal personality disorder. Journal of Clinical Psychiatry 2003;64(6):628-34. [DOI: 10.4088/jcp.v64n0602] [PMID: ] [DOI] [PubMed] [Google Scholar]
Krause Utz 2016 {published data only}
- Krause-Utz AD, Walther J-C, Schweizer S, Lis S, Schmahl C, Bohus M. Cognitive control of disturbing social information and heart rate variablility in borderline personality disorder: a randomized-controlled trial on the effects of an emotional working memory training. Biological Psychiatry 2016;79(9 Suppl):415S. [DOI: 10.1016/j.biopsych.2016.03.1410] [Abstract #1187] [DOI] [Google Scholar]
Krause Utz 2017 {published data only}
- Krause-Utz A, Walther J-C, Schweizer S, Lis S, Dalgleish T, Schmahl C, et al. The effectiveness of an emotional working memory training in borderline personality disorder. Biological Psychiatry 2017;81(10 Suppl):S164-5. [DOI: 10.1016/j.biopsych.2017.02.421] [Abstract #404] [DOI] [Google Scholar]
Linehan 1999 {published data only}
- Linehan MM, Schmidt H 3rd, Dimeff LA, Craft JC, Kanter J, Comtois KA. Dialectical behavior therapy for patients with borderline personality disorder and drug-dependence. American Journal on Addictions 1999;8(4):279-92. [DOI: 10.1080/105504999305686] [PMID: ] [DOI] [PubMed] [Google Scholar]
Linehan 2002 {published data only}
- Linehan MM, Dimeff LA, Reynolds SK, Comtois KA, Welch SS, Heagerty P, et al. Dialectical behavior therapy versus comprehensive validation therapy plus 12-step for the treatment of opioid dependent women meeting criteria for borderline personality disorder. Drug and Alcohol Dependence 2002;67(1):13-26. [DOI: 10.1016/s0376-8716(02)00011-x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lorentzen 2013 {published data only}
- Lorentzen S, Ruud T, Fjeldstad A, Høglend P. Comparison of short- and long-term dynamic group psychotherapy: randomised clinical trial. British Journal of Psychiatry 2013;203(3):280-7. [DOI: 10.1192/bjp.bp.112.113688] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Lorentzen S, Ruud T, Fjeldstad A, Høglend PA. Personality disorder moderates outcome in short- and long-term group analytic psychotherapy: a randomized clinical trial. British Journal of Clinical Psychology 2015;54(2):129-46. [DOI: 10.1111/bjc.12065] [PMID: ] [DOI] [PubMed] [Google Scholar]
Maffei 2018 {published data only}
- Maffei C, Cavicchioli M, Movalli M, Cavallaro R, Fossati A. Dialectical behavior therapy skills training in alcohol dependence treatment: findings based on an open trial. Substance Use & Misuse 2018;53(14):2368-85. [DOI: 10.1080/10826084.2018.1480035] [PMID: ] [DOI] [PubMed] [Google Scholar]
Mahlke 2015 {published data only}
- Mahlke CI, Bock T. Peer to peer-support in severe mental disorders: affective disorders, psychosis and personality disorder — a randomized controlled trial. European Psychiatry 2015;30 Suppl 1:28-31. [DOI: 10.1016/S0924-9338(15)30135-8] [Article: 0165] [DOI] [Google Scholar]
Marasinghe 2012 {published data only}
- Marasinghe RB, Edirippulige S, Kavanagh D, Smith A, Jiffry MT. Effect of mobile phone-based psychotherapy in suicide prevention: a randomized controlled trial in Sri Lanka. Journal of Telemedicine and Telecare 2012;18(3):151-5. [DOI: 10.1258/jtt.2012.SFT107] [PMID: ] [DOI] [PubMed] [Google Scholar]
Marchesi 2006 {published data only}
- Marchesi C, De Panfilis C, Cantoni A, Fontò S, Giannelli MR, Maggini C. Personality disorders and response to medication treatment in panic disorder: a 1-year naturalistic study. Progress in Neuro-Psychopharmacology and Biological Psychiatry 2006;30(7):1240-5. [DOI: 10.1016/j.pnpbp.2006.03.010] [PMID: ] [DOI] [PubMed] [Google Scholar]
McAuliffe 2014 {published data only}
- McAuliffe C, McLeavey BC, Fitzgerald T, Corcoran P, Carroll B, Ryan L, at al. Group problem-solving skills training for self-harm: randomised controlled trial. British Journal of Psychiatry 2014;204(5):383-90. [DOI: 10.1192/bjp.bp.111.101816] [PMID: ] [DOI] [PubMed] [Google Scholar]
Merolla 2017 {published data only}
- Merolla AB. A Study of the Relationship between Borderline-Dysregulated Personality and Treatment-Resistant Depression in the Course of the TADS Randomised Controlled Trial [D Clin Psy thesis]. Cholchester (Es): University of Essex, 2017. [Google Scholar]
Moran 2010 {published data only}
- Moran P, Borschmann R, Flach C, Barrett B, Byford S, Hogg J, et al. The effectiveness of joint crisis plans for people with borderline personality disorder: protocol for an exploratory randomised controlled trial. Trials 2010;11:18. [DOI: 10.1186/1745-6215-11-18] [PMC2837648] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Munroe‐Blum 1995 {published data only}
- Munroe-Blum H, Marziali E. A controlled trial of short-term group treatment for borderline personality disorder. Journal of Personality Disorders 1995;9(3):190-8. [DOI: 10.1521/pedi.1995.9.3.190] [DOI] [Google Scholar]
NCT01311193 {published data only}
- NCT01311193. Circadian sleep-wake cycles and light therapy in borderline personality disorder [Circadian sleep-wake cycles, well-being and light therapy in borderline personality disorder]. www.clinicaltrials.gov/ct2/show/NCT01311193 (first received 3 March 2011).
NCT02728778 {published data only}
- NCT02728778. Botulinum toxin A for emotional stabilization in borderline personality disorder (BPD) (BTX-BPD) [Botulinum toxin A for emotional stabilization in borderline personality disorder (BPD)]. clinicaltrials.gov/ct2/show/NCT02728778 (first received 31 March 2016).
NCT03498937 {published data only}
- NCT03498937. Effects of tDCS on impulsiveness among people suffering from borderline personality disorder (TIMBER) [Effet de la tDCS sur i'impulsivité chez les personnes souffrant d'un trouble borderline: TIMBER]. www.clinicaltrials.gov/ct2/show/NCT03498937 (first received 9 April 2018).
Nickel 2007a {published data only}
- Nickel MK. Topiramate treatment of aggression in male borderline patients. Australian and New Zealand Journal of Psychiatry 2007;41(5):461-2. [DOI: 10.1080/00048670701261251] [DOI] [PubMed] [Google Scholar]
Nickel 2007b {published data only}
- Nickel MK. Aripiprazole treatment of patients with borderline personality disorder. Journal of Clinical Psychiatry 2007;68(11):1815-6. [DOI: 10.4088/jcp.v68n1124f] [PMID: ] [DOI] [PubMed] [Google Scholar]
Nickel 2008 {published data only}
- Nickel MK, Loew TH. Treatment of aggression with topiramate in male borderline patients, part II: 18-month follow-up. European Psychiatry 2008;23(2):115-7. [DOI: 10.1016/j.eurpsy.2007.09.004] [DOI] [PubMed] [Google Scholar]
NL1680/NTR1781 {published data only}
- NL1680 (NTR1781). Implementation of out-patient schema-focused therapy for borderline personality disorder in regular mental healthcare. www.trialregister.nl/trial/1680 (first received 29 April 2009).
NL5802/NTR5957 {published data only}
- NL5802 (NTR5957). Mentalization-based treatment with and without competitive memory training for patients with borderline personality disorder: randomized controlled trial. www.trialregister.nl/trial/5802 (first received 3 July 2016).
O'Connor 2017 {published data only}ISRCTN99488269
- O'Connor RC, Ferguson E, Scott F, Smyth R, McDaid D, Park AL, et al. A brief psychological intervention to reduce repetition of self-harm in patients admitted to hospital following a suicide attempt: a randomised controlled trial. Lancet Psychiatry 2017;4(6):451-60. [DOI: 10.1016/S2215-0366(17)30129-3] [PMC5447136] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oquendo 2012 {published data only}
- Oquendo MA, Galfalvy HC, Currier D, Grunebaum MF, Sher L, Sullivan GM, et al. Treatment of suicide attempters with bipolar disorder: a randomized clinical trial comparing lithium and valproate in the prevention of suicidal behavior. American Journal of Psychiatry 2011;168(10):1050-6. Erratum in: American Journal of Psychiatry 2012;169(2):223. [DOI: 10.1176/appi.ajp.2011.11010163] [PMC3767999] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oquendo MA. First RCT comparing lithium and divalproex in the prevention of suicidal behavior in bipolar suicide attempters. Bipolar Disorders 2009;11(Suppl 1):5-6. [DOI: 10.1111/j.1399-5618.2009.00692.x] [DOI] [Google Scholar]
Ougrin 2011 {published data only}ISRCTN81605131
- Ougrin D, Boege I, Stahl D, Banarsee R, Taylor E. Randomised controlled trial of therapeutic assessment versus usual assessment in adolescents with self-harm: 2-year follow-up. Archives of Disease in Childhood 2013;98(10):772-6. [DOI: 10.1136/archdischild-2012-303200] [DOI] [PubMed] [Google Scholar]
- Ougrin D, Zundel T, Ng A, Banarsee R, Bottle A, Taylor E. Trial of therapeutic assessment in London: randomised controlled trial of therapeutic assessment versus standard psychosocial assessment in adolescents presenting with self-harm. Archives of Disease in Childhood 2011;96(2):148-53. [DOI: 10.1136/adc.2010.188755] [DOI] [PubMed] [Google Scholar]
Ower 2014 {published data only}
- Ower N. Effects of Oxytocin on Social Cognition and Empathy in Women with Borderline Personality Disorder/Effekte von Oxytocin auf die Soziale Kognition und Empathie bei Frauen mit Borderline-Persönlichkeitsstörung [PhD thesis]. Freiburg im Breisgau (BW): Albert-Ludwigs-Universität Freiburg, 2014. [Google Scholar]
Roberts 2018 {published data only}
- Roberts ID, Krajbich I, Cheavens JS, Campo JV, Way BM. Acetaminophen reduces distrust in individuals with borderline personality disorder features. Clinical Psychological Science 2018;6(1):145-54. [DOI: 10.1177/2167702617731374] [DOI] [Google Scholar]
Rombold 2014 {published data only}
- Rombold F, Lauterbach E, Felber W, Mueller-Oerlinghausen B, Ahrens B, Bronisch T, et al. Adjunctive lithium treatment in the prevention of suicidal behavior in patients with depression and comorbid personality disorders. International Journal of Psychiatry in Clinical Practice 2014;18(4):300-3. [DOI: 10.3109/13651501.2014.940052] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rosa 2009 {published data only}
- Rosa AR, Cruz N, Comes M, Vieta E. Impulsivity in bipolar disorder: a randomized clinical trial of adjunctive oxcarbazepine. European Neuropsychopharmacology 2009;19 Suppl 1:S75-6. [DOI: 10.1016/S0924-977X(09)70086-0] [Abstract #P.3.16] [DOI] [Google Scholar]
Roussignol 2010 {published data only}
- Roussignol B. Étude de L'effet de la Stimulation Magnétique Transcrânienne Répétitive Chez des Patients Présentant un Trouble de la Personnalité borderline [Thèse d'exercice] / [Effect of Repetitive Transcranial Magnetic Stimulation in Patients with Borderline Personality Disorder] [Exercise thesis]. Toulouse (FR): Médecine Spécialisée Clinique, 2010. [Google Scholar]
Santamarina Pérez 2017 {published data only}
- Santamarina Pérez P, Romero Cela S, Méndez Blanco I, Font Martínez E, Picado Rossi M, Martínez Mallén E, et al. Efficacy of dialectical behavior therapy compared to supportive therapy in adolescents with suicidal behavior. European Neuropsychopharmacology 2017;27 Suppl 4:S853-4. [DOI: 10.1016/S0924-977X(17)31533-X] [Abstract #P.2.f.013] [DOI] [Google Scholar]
Schuiringa 2017 {published data only}
- Schuiringa H, Van Nieuwenhuijzen M, Orobio de Castro B, Lochman JE, Matthys W. Effectiveness of an intervention for children with externalizing behavior and mild to borderline intellectual disabilities: a randomized trial. Cognitive Therapy and Research 2017;41(2):237-51. [DOI: 10.1007/s10608-016-9815-8] [PMC5346153] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schuppert 2009 {published data only}
- Schuppert HM, Giesen-Bloo J, Van Gemert TG, Wiersema HM, Minderaa RD, Emmelkamp PMG, at al. Effectiveness of an emotion regulation group training for adolescents — a randomized controlled pilot study. Clinical Psychology & Psychotherapy 2009;16(6):467-78. [DOI: 10.1002/cpp.637] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sharp 2015 {published data only}
- Sharp C, Venta A, Ha C, Newlin E. Oxytocin and trust in adolescents with borderline personality disorder. Biological Psychiatry 2015;77(9 Suppl):30S. [DOI: 10.1016/j.biopsych.2015.03.006] [Abstract #78] [DOI] [Google Scholar]
Thunnissen 2009 {published data only}
- Thunnissen M, Duivenvoorden H, Van Busschbach J, Hakkaart-van Roijen L, Van Tilburg W, Verheul R, et al. A randomized clinical trial on the effectiveness of a re-integration training after short-term inpatient psychotherapy [De effectiviteit van re-integratietraining versus boostersessies na kortdurende klinische psychotherapie: een gerandomiseerd klinisch onderzoek]. Tijdschrift voor Psychiatrie 2009;51(2):75-86. [PMID: ] [PubMed] [Google Scholar]
TufekciogIu 2015 {published data only}
- TufekciogIu S. Pre-Treatment Axis II Diagnosis and Psychotherapy Process in Two Time-Limited Therapies [PhD thesis]. New York (NY): Adelphi University, The Institute of Advanced Psychological Studies, 2015. [Google Scholar]
- Tufekcioglu S, Muran JC, Safran JD, Winston A. Personality disorder and early therapeutic alliance in two time-limited therapies. Psychotherapy Research 2013;23(6):646-57. [DOI: 10.1080/10503307.2013.843803] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tyrer 2003a {published data only}
- Tyrer P, Jones V, Thompson S, Catalan J, Schmidt U, Davidson K, et al. Service variation in baseline variables and prediction of risk in a randomised controlled trial of psychological treatment in repeated parasuicide: the POPMACT study. International Journal of Social Psychiatry 2003;49(1):58-69. [DOI: 10.1177/0020764003049001148] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Tyrer P, Thompson S, Schmidt U, Jones V, Knapp M, Davidson K, et al. Randomized controlled trial of brief cognitive behaviour therapy versus treatment as usual in recurrent deliberate self-harm: the POPMACT study. Psychological Medicine 2003;33(6):969-76. [DOI: 10.1017/S0033291703008171] [PMID: ] [DOI] [PubMed] [Google Scholar]
Van Beek 2009 {published data only}ISRCTN56421759
- Van Beek W, Kerkhof A, Beekman A. Future oriented group training for suicidal patients: a randomized clinical trial. BMC Psychiatry 2009;9:65. [DOI: 10.1186/1471-244X-9-65] [PMC2767345 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Dijk 2019 {published data only}
- Van Dijk SD, Veenstra MS, Bouman R, Peekel J, Veenstra DH, Van Dalen PJ, et al. Group schema-focused therapy enriched with psychomotor therapy versus treatment as usual for older adults with cluster B and/or C personality disorders: a randomized trial. BMC Psychiatry 2019;19:26. [DOI: 10.1186/s12888-018-2004-4] [PMC6334382 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Spijker 2010 {published data only}
- Van Spijker BA, Van Straten A, Kerkhof AJ. The effectiveness of a web-based self-help intervention to reduce suicidal thoughts: a randomized controlled trial. Trials 2010;11:25. [DOI: 10.1186/1745-6215-11-25] [PMC2841163 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vidal 2013 {published data only}
- Vidal R, Bosch R, Nogueira M, Gómez-Barros N, Valero S, Palomar G, et al. Psychoeducation for adults with attention deficit hyperactivity disorder vs cognitive behavioral group therapy: a randomized controlled pilot study. Journal of Nervous and Mental Disease 2013;201(10):894-900. [DOI: 10.1097/NMD.0b013e3182a5c2c5] [PMID: ] [DOI] [PubMed] [Google Scholar]
Vijayakumar 2011 {published data only}
- Vijayakumar L, Umamaheswari C, Shujaath Ali ZS, Devaraj P, Kesavan K. Intervention for suicide attempters: a randomized controlled study. Indian Journal of Psychiatry 2011;53(3):244-8. [DOI: 10.4103/0019-5545.86817] [PMC3221182] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Waltz 2009 {published data only}
- Waltz J, Dimeff LA, Koerner K, Linehan MM, Taylor L, Miller C. Feasibility of using video to teach a dialectical behavior therapy skill to clients with borderline personality disorder. Cognitive and Behavioral Practice 2009;16(2):214-22. [DOI: 10.1016/j.cbpra.2008.08.004] [DOI] [Google Scholar]
Watzke 2012 {published data only}
- Watzke B, Ruddel H, Jürgensen R, Koch U, Kriston L, Grothgar B, et al. Longer term outcome of cognitive-behavioural and psychodynamic psychotherapy in routine mental health care: randomised controlled trial. Behaviour Research and Therapy 2012;50(9):580-7. [DOI: 10.1016/j.brat.2012.04.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Weertman 2007 {published data only}
- Weertman A, Arntz A. Effectiveness of treatment of childhood memories in cognitive therapy for personality disorders: a controlled study contrasting methods focusing on the present and methods focusing on childhood memories. Behaviour Research and Therapy 2007;45(9):2133-43. [DOI: 10.1016/j.brat.2007.02.013] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wilks 2018 {published data only}
- Wilks CR, Lungu A, Ang SY, Matsumiya B, Yin Q, Linehan MM. A randomized controlled trial of an Internet delivered dialectical behavior therapy skills training for suicidal and heavy episodic drinkers. Journal of Affective Disorders 2018;232:219-28. [DOI: 10.1016/j.jad.2018.02.053] [PMC5859943 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wingenfeld 2013 {published data only}
- Wingenfeld K, Driessen M, Terfehr K, Schlosser N, Fernando S, Otte C, et al. Effects of cortisol on memory in women with borderline personality disorder: role of co-morbid post-traumatic stress disorder and major depression. Psychological Medicine 2013;43(3):495-505. [DOI: 10.1017/S0033291712001961] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wingenfeld 2014 {published data only}
- Wingenfeld K, Kuehl LK, Janke K, Hinkelmann K, Dziobek I, Fleischer J, et al. Enhanced emotional empathy after mineralocorticoid receptor stimulation in women with borderline personality disorder and healthy women. Neuropsychopharmacology 2014;39(8):1799-804. [DOI: 10.1038/npp.2014.36] [PMC4059897] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingenfeld K, Kuehl LK, Janke K, Hinkelmann K, Eckert FC, Roepke S, et al. Effects of mineralocorticoid receptor stimulation via fludrocortisone on memory in women with borderline personality disorder. Neurobiology of Learning and Memory 2015;120:94-100. [DOI: 10.1016/j.nlm.2015.02.013] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wölwer 2001 {published data only}
- Wölwer W, Burtscheidt W, Redner C, Schwarz R, Gaebel W. Out-patient behaviour therapy in alcoholism: impact of personality disorders and cognitive impairments. Acta Psychiatrica Scandinavica 2001;103(1):30-7. [DOI: 10.1034/j.1600-0447.2001.00149.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Abdelkarim 2016 {published data only}
- Abdelkarim A, Nagui Rizk D, Esmaiel M, Helal H. Social media group parallel to dialectical behavior therapy skills training group, the pros and cons. European Psychiatry 2016;33 Suppl:S556. [DOI: 10.1016/j.eurpsy.2016.01.2054] [DOI] [Google Scholar]
Akbari 2009 {published data only}
- Akbari J, Aghamohammadian HR, Ghanbari BA. Effectiveness of CBT, pharmacotherapy and combined approach in improvement of symptoms depression anger in borderline personality disorder. Journal of Psychology 2009;13(3):342-59. [Google Scholar]
Cowperthwait 2017 {published data only}
- Cowperthwait CM. Outpatient Dialectical Behavior Therapy for Adolescents: Treatment Outcomes and Comparison of Treatment Effects in Two Skills Training Group Formats [PhD thesis]. Atlanta (GA): Emroy University, 2016. [EMORY UNIVERSITY: etd.library.emory.edu/concern/etds/0p0967135?locale=es] [Google Scholar]
Dorrepaal 2012 {published data only}
- Dorrepaal E, Thomaes K, Smit JH, Van Balkom AJ, Veltman DJ, Hoogendoorn AW, et al. Stabilizing group treatment for complex posttraumatic stress disorder related to child abuse based on psychoeducation and cognitive behavioural therapy: a multisite randomized controlled trial. Psychotherapy and Psychosomatics 2012;81(4):217-25. [DOI: 10.1159/000335044] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ducasse 2018 {published data only}
- Ducasse D, Jaussent I, Arpon-Brand V, Vienot M, Laglaoui C, Béziat S, et al. Acceptance and commitment therapy for the management of suicidal patients: a randomized controlled trial. Psychotherapy and Psychosomatics 2018;87(4):211-22. [DOI: 10.1159/000488715] [PMID: ] [DOI] [PubMed] [Google Scholar]
Einy 2018 {published data only}
- Einy S, Narimani M, Atadokht A, Basharpoor S, Fariba SM. Effectiveness of mentalization based therapy and cognitive-analytical therapy on improved object relationship of people with borderline personality disorder: a comparison. Payesh 2018;17(3):275-87. [SCIENTIFIC INFORMATION DATABASE: www.sid.ir/en/journal/ViewPaper.aspx?ID=576872] [Google Scholar]
Isaia 2017 {published data only}
- Isaia NC. Effective Therapeutic Components in Systems Training for Emotional Predictability and Problem Solving (STEPPS) for Borderline Personality Disorder [PhD thesis]. Guildford (UK): University of Surrey, 2017. [SURREY RESEARCH INSIGHT: epubs.surrey.ac.uk/842255/] [DOI] [PubMed] [Google Scholar]
Johnson 2017 {published data only}
- Johnson R. Cognitive Analytic Therapy (CAT) for Borderline Personality Disorder : Is CAT effective, and what influences the delivery of this therapy? [DClinPsy thesis]. Sheffield (UK): University of Sheffield, 2017. [WHITE ROSE ETHESES ONLINE: etheses.whiterose.ac.uk/18158/] [Google Scholar]
McCauley 2018 {published data only}
- McCauley E, Berk MS, Asarnow JR, Adrian M, Cohen J, Korslund K, et al. Efficacy of dialectical behavior therapy for adolescents at high risk for suicide: a randomized clinical trial. JAMA Psychiatry 2018;75(8):777-85. [DOI: 10.1001/jamapsychiatry.2018.1109] [PMCID: PMC6584278] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01528020. Collaborative adolescent research on emotions and suicide (CARES) [Treatment of suicidal and self-injurious adolescents with emotional dysregulation]. clinicaltrials.gov/ct2/show/NCT01528020 (first received 2 February 2012).
Ostermeier 2017 {published data only}
- Ostermeier R. Adapting Dialectic Behavioral Therapy Skills Group Based on the Severity Level of Borderline Personality Disorder [PsyD thesis). Eagan (MN): Minnesota School of Professional Psychology, 2014. [Google Scholar]
Santamarina 2017 {published data only}
- Santamarina P, Blanco IM, Picado M, Font E, Moreno E, Martínez E, et al. Dialectical behavior therapy versus supportive therapy for adolescents with suicidal behavior: a randomized controlled trial. Journal of the American Academy of Child and Adolescent Psychiatry 2017;56(10 Suppl):S226-7. [DOI: 10.1016/j.jaac.2017.09.213] [POSTER NUMBER: 3.65] [DOI] [Google Scholar]
References to ongoing studies
ACTRN12610000100099 {published data only}
- ACTRN12610000100099. A randomised controlled trial of three forms of psychosocial early intervention for borderline personality disorder in youth [MOBY: a randomised controlled trial of specialised early intervention with individual Cognitive Analytic Therapy, specialised early intervention without individual psychotherapy, and standard youth mental health care for first-presentation borderline personality disorder in youth]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12610000100099 (first received 21 December 2009).
- Chanen A, Jackson H, Cotton S, Gleeson J, Davey C, Betts J, et al. Comparing three forms of early intervention for youth with borderline personality disorder (the MOBY study). Early Intervention in Psychiatry 2018;12(Supp 1):201. [POSTER NUMBER: C30] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanen A, Jackson H, Cotton SM, Gleeson J, Davey CG, Betts J, et al. Comparing three forms of early intervention for youth with borderline personality disorder (the MOBY study): study protocol for a randomised controlled trial. Trials 2015;16:476. [DOI: 10.1186/s13063-015-1001-x] [PMCID: PMC4618920] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
ACTRN12612000951853 {published data only}
- ACTRN12612000951853. A clinical trial of mentalization based therapy treatment for borderline personality disorder [Outcome effects of a mentalization based treatment service (Mindsight) for people with borderline personality disorder in urban Christchurch: a clinical trial and evaluative study]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=362981 (first received 4 September 2012).
ACTRN12612001187831 {published data only}
- ACTRN12612001187831. Exploring dialectical behaviour therapy vs conversational model in the treatment of borderline personality disorder: a randomised clinical trial [Randomised clinical trial of dialectical behaviour therapy compared to conversational model in the treatment of borderline personality disorder in reducing parasuicidal behaviour and depression]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=363251 (first received 8 November 2012).
ACTRN12616000236493 {published data only}
- ACTRN12616000236493. Effectiveness of dialectical behaviour therapy (DBT) group skills training for borderline personality disorder (BPD) in community mental health. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=370119 (first received 15 February 2016).
DRKS00003605 {published data only}
- DRKS00003605. Short term therapy in adolescents with self-destructive and risk-taking behaviours. www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00003605 (first received 10 July 2012).
- Fischer G, Brunner R, Parzer P, Resch F, Kaess M. Short-term psychotherapeutic treatment in adolescents engaging in non-suicidal self-injury: a randomized controlled trial. Trials 2013;14:294. [DOI: 10.1186/1745-6215-14-294] [PMCID:: PMC3848593] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
DRKS00011534 {published data only}
- DRKS00011534. PRO*BPD: effectiveness of outpatient treatment programmes for borderline personality disorder: a comparison of schema therapy and dialectical behaviour therapy. www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00011534 (first received 11 January 2017).
- Fassbinder E, Assmann N, Schaich A, Heinecke K, Wagner T, Sipos V, et al. PRO*BPD: effectiveness of outpatient treatment programs for borderline personality disorder: a comparison of schema therapy and dialectical behavior therapy: study protocol for a randomized trial. BMC Psychiatry 2018;18(1):341. [DOI: 10.1186/s12888-018-1905-6] [PMCID: PMC6194626] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN21802136 {published data only}ISRCTN21802136
- ISRCTN21802136. Coping with unusual experiences for 12-18 (CUES+) [Coping with unusual experiences for 12-18 year olds (CUES+): a transdiagnostic randomised controlled trial of the effectiveness of cognitive therapy in reducing distress associated with unusual experiences in adolescent mental health services]. www.isrctn.com/ISRCTN21802136 (first received 8 January 2015). [DOI] [PMC free article] [PubMed]
- Jolley S, Browning S, Corrigall R, Laurens KR, Hirsch C, Bracegirdle K, et al. Coping with unusual experiences for 12-18 year olds (CUES+): a transdiagnostic randomised controlled trial of the effectiveness of cognitive therapy in reducing distress associated with unusual experiences in adolescent mental health services: study protocol for a randomised controlled trial. Trials 2017;18(1):586. [DOI: 10.1186/s13063-017-2326-4] [PMCID: PMC5716372] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00603421 {published data only}
- NCT00603421. Effectiveness of a 24 hour phone line on the rate of suicide attempts in borderline patients. clinicaltrials.gov/ct2/show/NCT00603421 (first received 16 January 2008).
NCT01531634 {published data only}
- NCT01531634. Promoting recovery processes in women with borderline personality disorder using a dynamic cognitive intervention. clinicaltrials.gov/ct2/show/NCT01531634 (first received 3 February 2012).
NCT01823120 {published data only}
- Agyapong V, Behre T, Juhas M, Greenshaw A. Preventing self-harm and reducing suicidal ideation through an expedited regular supportive psychotherapy and assertive case management-protocol for a three-arm partial randomised controlled trial. European Psychiatry 2016;33 Suppl:S440. [DOI: 10.1016/j.eurpsy.2016.01.1599] [DOI] [Google Scholar]
- NCT01823120. Text message intervention to reduce repeat self-harm [Text message intervention to reduce repeat self-harm in patients presenting to the emergency department]. clinicaltrials.gov/ct2/show/NCT01823120 (first received 26 March 2013).
NCT02068326 {published data only}
- Beck E, Bo S, Gondan M, Poulsen S, Pedersen L, Pedersen J, et al. Mentalization-based treatment in groups for adolescents with borderline personality disorder (BPD) or subthreshold BPD versus treatment as usual (M-GAB): study protocol for a randomized controlled trial. Trials 2016;17(1):314. [DOI: 10.1186/s13063-016-1431-0] [PMCID: PMC4942923] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02068326. MBT in groups for adolescents with BPD or subthreshold BPD versus TAU - the M-GAB randomized controlled trial (M-GAB) [Mentalization-based treatment in groups for adolescents with borderline personality disorder (BPD) or subthreshold BPD versus treatment as usual - the M-GAB randomized controlled trial]. clinicaltrials.gov/ct2/show/NCT02068326 (first received 19 February 2014).
NCT02125942 {published data only}
- NCT02125942. Central meditation and imagery therapy for augmentation of borderline personality disorder treatment [Pilot study of central meditation and imagery therapy for borderline personality disorder]. clinicaltrials.gov/ct2/show/NCT02125942 (first received 26 April 2014).
NCT02126787 {published data only}
- NCT02126787. Short-term, intensive psychodynamic group therapy versus cognitive-behavioral group therapy in the day treatment [Short-term, intensive psychodynamic group therapy versus cognitive-behavioral group therapy in the day treatment of anxiety disorders and comorbid depressive or personality disorders]. clinicaltrials.gov/ct2/show/NCT02126787 (first received 9 April 2014). [DOI] [PMC free article] [PubMed]
- Suszek H, Holas P, Wyrzykowski T, Lorentzen S, Kokoszka A. Short-term intensive psychodynamic group therapy versus cognitive-behavioral group therapy in day treatment of anxiety disorders and comorbid depressive or personality disorders: study protocol for a randomized controlled trial. Trials 2015;16:319. [DOI: 10.1186/s13063-015-0827-6] [PMCID: PMC4517633] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02134223 {published data only}
- NCT02134223. Methylation status of BDNF gene after dialectical behavior therapy in BPD [Changes in methylation status of BDNF gene after receiving dialectical behavior therapy in patients with borderline personality disorder]. clinicaltrials.gov/ct2/show/NCT02134223 (first received 6 May 2014).
NCT02387736 {published data only}
- McMain SF, Chapman AL, Kuo JR, Guimond T, Streiner DL, Dixon-Gordon KL, et al. The effectiveness of 6 versus 12 months of dialectical behaviour therapy for borderline personality disorder: the feasibility of a shorter treatment and evaluating responses (FASTER) trial protocol. BMC Psychiatry 2018;18(1):230. [DOI: 10.1186/s12888-018-1802-z] [PMCID: PMC6050694] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02387736. DBT for chronically self-harming Individuals with BPD: evaluating the clinical & cost effectiveness of a 6 mo. treatment (FASTER-DBT) [Dialectical behaviour therapy for chronically self-harming individuals with BPD: evaluating the clinical and cost effectiveness of a 6-month treatment]. clinicaltrials.gov/ct2/show/NCT02387736 (first received 20 February 2015).
NCT02517723 {published data only}
- NCT02517723. Narrative exposure therapy in women with borderline personality disorder and posttraumatic stress disorder [A randomized controlled clinical trial (RCCT) to test the effectiveness of narrative exposure therapy (NET) versus dialectical-behavioral therapy in reducing trauma related symptoms in women suffering from borderline personality disorder (BPD) and posttraumatic stress disorder (PTSD)]. clinicaltrials.gov/ct2/show/NCT02517723 (first received 6 January 2014).
- Steuwe C, Rullkötter N, Ertl V, Berg M, Neuner F, Beblo T, et al. Effectiveness and feasibility of narrative exposure therapy (NET) in patients with borderline personality disorder and posttraumatic stress disorder – a pilot study. BMC Psychiatry 2016;16:254. [DOI: 10.1186/s12888-016-0969-4] [PMCID: PMC4955150] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02685943 {published data only}
- NCT02685943. A randomized trial for suicidal patients [Collaborative assessment and management of suicidality (CAMS) in comparison to treatment as usual (TAU) for suicidal patients: a randomized controlled trial]. clinicaltrials.gov/ct2/show/NCT02685943 (first received 8 February 2016).
- Ryberg W, Fossse R, Zahl PH, Borson I, Møller P, Landrø NI, et al. Collaborative assessment and management of suicidality (CAMS) compared to treatment as usual (TAU) for suicidal patients: study protocol for a randomized controlled trial. Trials 2016;17(1):481. [DOI: 10.1186/s13063-016-1602-z] [PMCID: PMC5048411] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02771691 {published data only}
- NCT02771691. Mentalization-based group therapy for adolescents [Efficacy of mentalization-based group therapy for adolescents: a pilot randomised controlled trial]. clinicaltrials.gov/show/nct02771691 (first received 25 April 2016).
NCT02985047 {published data only}
- Liljedahl SI, Helleman M, Daukantaité D, Westrin A, Westling S. A standardized crisis management model for self-harming and suicidal individuals with three or more diagnostic criteria of borderline personality disorder: the Brief Admission Skane randomized controlled trial protocol (BASRCT). BMC Psychiatry 2017;17(1):220. [DOI: 10.1186/s12888-017-1371-6] [PMCID: PMC5472925] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02985047. Brief Admission Skane: replacing general admission for individuals with self-harm and acute risk of suicide (BAS) [Brief Admission Skane: can brief admission replace general admission for individuals with self-harm and acute risk of suicide]. clinicaltrials.gov/ct2/show/NCT02985047 (first received 25 November 2016).
NCT02991586 {published data only}
- NCT02991586. Effectiveness study of a dialectical behavioral treatment for families of adolescents with emotional instability (FAL). clinicaltrials.gov/show/nct02991586 (first received 10 June 2016).
NCT03011190 {published data only}
- Hurtado-Santiago S, Guzmán-Parra J, Bersabé RM, Mayoral F. Effectiveness of iconic therapy for the reduction of borderline personality disorder symptoms among suicidal youth: study protocol for a randomised controlled trial. BMC Psychiatry 2018;18(1):277. [DOI: 10.1186/s12888-018-1857-x] [PMCID: PMC6122595] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03011190. Effectiveness of the iconic therapy for borderline personality disorder symptoms [Effectiveness of the iconic therapy in youth with suicidal ideation/self-injuring behavior and borderline personality traits: study protocol for a randomized controlled trial]. clinicaltrials.gov/ct2/show/NCT03011190 (first received 28 December 2016).
NCT03092271 {published data only}
- NCT03092271. Randomized trial of stepped care for suicide prevention in teens and young adults (Step2Health) [Randomized trial of stepped care for suicide prevention in teens and young adults]. clinicaltrials.gov/ct2/show/NCT03092271 (first received 21 March 2017).
NCT03185026 {published data only}
- NCT03185026. Psychoeducation for suicidal behavior (PEPSUI) [Effectiveness of the first French psychoeducational program for suicidal behavior: a randomized controlled trial]. clinicaltrials.gov/ct2/show/NCT03185026 (first received 11 May 2017).
NCT03191565 {published data only}
- NCT03191565. Using smartphones for self-monitoring of skill-use in dialectical behavior therapy (mDIARY) [The mDIARY study: using smartphones for daily self-monitoring of skill use and outcome in dialectical behavior therapy with borderline personality disorder: a combined RCT and time series study]. clinicaltrials.gov/ct2/show/NCT03191565 (first received 24 March 2017).
NCT03297840 {published data only}
- NCT03297840. Change in mindfulness in borderline personality disorder [Change in mindfulness in borderline personality disorder after dialectic-behavioural therapy (DBT): a pre-post-comparison]. clinicaltrials.gov/ct2/show/NCT03297840 (first received 9 May 2017).
NCT03376113 {published data only}
- NCT03376113. The effect of a brief psychological intervention on reducing self-harm repetition: feasibility study. clinicaltrials.gov/ct2/show/NCT03376113 (first received 2 November 2017).
NCT03418142 {published data only}
- Klein JP, Hauer A, Berger T, Fassbinder E, Schweiger U, Jacob G. Protocol for the REVISIT-BPD trial, a randomized controlled trial testing the effectiveness of an internet-based self-management intervention in the treatment of borderline personality disorder (BPD). Frontiers in Psychiatry 2018;9:439. [DOI: 10.3389/fpsyt.2018.00439] [PMCID: PMC6160537] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03418142. Evaluating an internet-based self-management intervention for borderline (REVISIT) [Research evaluating the effectiveness of adding an internet-based self-management intervention to usual care in the treatment of borderline personality disorder - REVISIT BPD]. clinicaltrials.gov/ct2/show/NCT03418142 (first received 23 January 2018).
NCT03677037 {published data only}
- NCT03677037. The short-term MBT project (MBT-RCT) [Short-term versus long-term mentalization-based therapy for outpatients with subthreshold or diagnosed borderline personality disorder: a randomized clinical trial]. clinicaltrials.gov/ct2/show/NCT03677037 (first received 14 September 2018).
NCT03833453 {published data only}
- NCT03833453. Effectiveness of PTSD-treatment compared to integrated PTSD-PD-treatment in adult patients with comorbid PTSD and BPD [Prediction and outcome study in PTSD and (borderline) personality disorders]. clinicaltrials.gov/ct2/show/NCT03833453 (first received 24 January 2019).
NL1144/NTR1186 {published data only}
- NL1144/NTR1186. SFT for forensic PD patients [Efficacy of schema-focused therapy versus usual treatment in forensic patients with personality disorders: a three-year randomized clinical trial]. www.trialregister.nl/trial/1144 (first received 16 January 2008).
NL2168/NTR2292 {published data only}
- Laurenssen EM, Smits ML, Bales DL, Feenstra DJ, Eeren HV, Noom MJ, et al. Day hospital mentalization-based treatment versus intensive outpatient mentalization-based treatment for patients with severe borderline personality disorder: protocol of a multicentre randomized clinical trial. BMC Psychiatry 2014;14:301. [DOI: 10.1186/s12888-014-0301-0] [PMCID: PMC4240895] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NL2168/NTR2292. Dosage-trial mentalisation-based treatment (MBT): intensive outpatient MBT versus day hospital MBT [Intensive outpatient mentalisation-based treatment versus day hospital mentalisation-based treatment: a randomised controlled trial]. www.trialregister.nl/trial/2168 (first received 16 April 2010).
NL2266/NTR2392 {published data only}
- NL2266/NTR2392. Group schema therapy for borderline personality disorder [Multi-site RCT of group schema therapy for borderline personality disorder]. www.trialregister.nl/trial/2266 (first received 25 June 2010).
- Wetzelaer P, Farrell J, Evers SM, Jacob GA, Lee CW, Brand O, et al. Design of an international multicentre RCT on group schema therapy for borderline personality disorder. BMC Psychiatry 2014;14:319. [DOI: 10.1186/s12888-014-0319-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
NL3856/NTR4016 {published data only}
- NL3856/NTR4016. The addition of STEPPS in the treatment of patients with bipolar disorder and comorbid borderline personality features: a randomized controlled trial [Toetsing van een geïntegreerd behandelprotocol voor patiënten met een bipolaire stoornis en een comorbide borderline persoonlijkheidskenmerken. Een randomized clinical trial]. www.trialregister.nl/trial/3856 (first received 28 May 2013).
- Riemann G, Weisscher N, Goossens PJJ, Draijer N, Apenhorst-Hol M, Kupka RW. The addition of STEPPS in the treatment of patients with bipolar disorder and comorbid borderline personality features: a protocol for a randomized controlled trial. BMC Psychiatry 2014;14:172. [DOI: 10.1186/1471-244X-14-172] [PMCID: PMC4065586] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Amri 2014
- Amri A, Millier M, Toumi M. Minimum clinically important difference in the global assessment functioning in patients with schizophrenia. Value in Health 2014;17(7):A765-6. [DOI: 10.1016/j.jval.2014.08.285] [Abstract #PMH2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Andrews 2013a
- Andrews J, Guyatt G, Oxman AD, Alderson P, Dahm P, Falck-Ytter Y, et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. Journal of Clinical Epidemiology 2013;66(7):719–25. [DOI: 10.1016/j.jclinepi.2012.03.013] [PMID: ] [DOI] [PubMed] [Google Scholar]
Andrews 2013b
- Andrews JC, Schünemann HJ, Oxman AD, Pottie K, Meerpohl JJ, Coello PA, et al. GRADE guidelines: 15. Going from evidence to recommendation - determinants of a recommendation’s direction and strength. Journal of Clinical Epidemiology 2013;66(7):726–35. [DOI: 10.1016/j.jclinepi.2013.02.003] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ansell 2007
- Ansell EB, Sanislow CA, McGlashan TH, Grilo CM. Psychosocial impairment and treatment utilization by patients with borderline personality disorder, other personality disorders, mood and anxiety disorders, and a healthy comparison group. Comprehensive Psychiatry 2007;48(4):329-36. [DOI: 10.1016/j.comppsych.2007.02.001] [PMID: ] [DOI] [PubMed] [Google Scholar]
APA 1980
- American Psychiatric Asssociation. Diagnostic and Statistical Manual of Mental Disorders (DSM-III). 3rd edition. Washington (DC): American Psychiatric Association, 1980. [ISBN 978-0-89042-0416] [Google Scholar]
APA 1987
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-III-R). 3rd edition. Washington (DC): American Psychiatric Association, 1987. [ISBN 978-0-89042-0195] [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). 4th edition. Washington (DC): American Psychiatric Association, 1994. [ISBN 978-0-89042-0610] [Google Scholar]
APA 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR), 4th edition, text revision. Washington (DC): American Psychiatric Association, 2000. [ISBN 978-0-89042-0256] [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th edition. Washington (DC): American Psychiatric Association, 2013. [ISBN 978-0-89042-554-1] [Google Scholar]
Arntz 2003
- Arntz A, Van den Hoorn M, Cornelis J, Verheul R, Van den Bosch W, De Bie AJ. Reliability and validity of the borderline personality disorder severity index. Journal of Personality Disorders 2003;17(1):45-59. [PMID: ] [DOI] [PubMed] [Google Scholar]
Arntz 2009
- Arntz A, Van Genderen H. Schema Therapy for Borderline Personality Disorder. Chichester (UK): Wiley-Blackwell, 2009. [ISBN 978-0-470-51080-3] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490-9. [DOI: ] [PMC428525] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ayodeji 2015
- Ayodeji E, Green J, Roberts C, Trainor G, Rothwell J, Woodham A, et al. The influence of personality disorder on outcome in adolescent self-harm. British Journal of Psychiatry 2015;207(4):313-9. [DOI: 10.1192/bjp.bp.113.138941] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bach 2016
- Bach B, Sellbom M. Continuity between DSM-5 categorial criteria and traits criteria for borderline personality disorder. Canadian Journal of Psychiatry 2016;61(8):489-94. [DOI: 10.1177/0706743716640756] [PMC4959646] [PMID: 27310232] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bach 2018
- Bach B, First MB . Application of the ICD-11 classification of personality disorders. BMC Psychiatry 2018;18:351. [DOI: 10.1186/s12888-018-1908-3] [PMCID: PMC6206910] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bailey 2014
- Bailey RC, Grenyer BF. Supporting a person with personality disorder: a study of carer burden and well-being. Journal of Personality Disorders 2014;28(6):796-809. [DOI: 10.1521/pedi_2014_28_136] [PMID: ] [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401–6. [DOI: 10.1016/j.jclinepi.2010.07.015] [PMID: ] [DOI] [PubMed] [Google Scholar]
Barrett 1995
- Barrett ES, Stanford MS. Impulsiveness. In: Costello CG, editors(s). Personality Characteristics of the Personality Disordered. New York (NY): Wiley, 1995:91-118. [ISBN 978-0-471-01529-1] [Google Scholar]
Bateman 2003
- Bateman A, Fonagy P. Health care utilization costs for borderline personality disorder patients treated with psychoanalytically oriented partial hospitalization versus general psychiatric care. American Journal of Psychiatry 2003;160(1):169-71. [DOI] [PubMed] [Google Scholar]
Bateman 2004
- Bateman A, Fonagy P. Psychotherapy for Borderline Personality Disorder: Mentalisation Based Treatment. Oxford (UK): Oxford University Press, 2004. [ISBN 978-0-19-852766-4] [Google Scholar]
Bateman 2006
- Bateman A, Fonagy P. Mentalization-Based Treatment for Borderline Personality Disorders: A Practical Guide. Oxford (UK): Oxford University Press, 2006. [ISBN 978-0-19-857090-5] [Google Scholar]
Bateman 2015
- Bateman AW, Gunderson J, Mulder R. Treatment of personality disorder. Lancet 2015;385(9969):735-43. [DOI: 10.1016/S0140-6736(14)61394-5] [DOI] [PubMed] [Google Scholar]
Bateman 2016
- Bateman A, Fonagy P. Mentalization-Based Treatment for Personality Disorders: A Practical Guide. Oxford (UK): Oxford University Press, 2016. [ISBN 019968037X] [Google Scholar]
Bateman 2018
- Bateman A, Campbell C, Luyten P, Fonagy P. A mentalization-based approach to common factors in the treatment of borderline personality disorder. Current Opinion in Psychology 2018;21:44-49. [DOI: ] [DOI] [PubMed] [Google Scholar]
Bateman 2019a
- Bateman A, Fonagy P. A randomized controlled trial of a mentalization-based intervention (MBT-FACTS) for families of people with borderline personality disorder. Personality Disorders 2019;10(1):70-9. [DOI: 10.1037/per0000298] [PMID: ] [DOI] [PubMed] [Google Scholar]
Beck 1961
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry 1961;4:561-71. [PMID: ] [DOI] [PubMed] [Google Scholar]
Beck 1979
- Beck AT, Kovacs M, Weissman A. Assessment of suicidal intention: the Scale for Suicide Ideation. Journal of Consulting and Clinical Psychology 1979;47(2):343-52. [PMID: ] [DOI] [PubMed] [Google Scholar]
Beck 2003
- Beck AT, Freeman A. Cognitive Therapy of Personality Disorder. New York (NY): Guilford Press, 2003. [Google Scholar]
Berk 2009
- Berk M, Parker G. The elephant on the couch: side-effects of psychotherapy. Australian & New Zealand Journal of Psychiatry 2009;43(9):787-94. [DOI: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Berkey 1996
- Berkey CS, Mosteller F, Lau J, Antman EM. Uncertainty of the time of first significance in random effects cumulative meta-analysis. Controlled Clinical Trials 1996;17(5):357-71. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bernstein 1986
- Bernstein EM, Putnam FW. Development, reliability, and validity of a dissociation scale. Journal of Nervous and Mental Disease 1986;174(12):727-35. [PMID: ] [DOI] [PubMed] [Google Scholar]
Biskin 2015
- Biskin RS. The lifetime course of borderline personality disorder. Canadian Journal of Psychiatry 2015;60(7):303-8. [DOI: 10.1177/070674371506000702] [PMC4500179] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Black 2004
- Black DW, Blum N, Pfohl B, Hale N. Suicidal behavior in borderline personality disorder: prevalence, risk factors, prediction, and prevention. Journal of Personality Disorders 2004;18(3):226-39. [DOI: 10.1521/pedi.18.3.226.35445] [PMID: ] [DOI] [PubMed] [Google Scholar]
Black 2009
- Black DW, Blum N, St John D. STEPPS: a practical, evidence-based way to treat borderline personality disorder. Current Psychiatry 2009;8:31-7. [Google Scholar]
Bohus 2004
- Bohus M, Haaf B, Simms T, Limberger MF, Schmal C, Unckel C, et al. Effectiveness of inpatient dialectical behavioral therapy for borderline personality disorder: a controlled trial. Behaviour Research and Therapy 2004;42(5):487-99. [DOI: 10.1016/S0005-7967(03)00174-8] [PMID: ] [DOI] [PubMed] [Google Scholar]
Boutron 2008
- Boutron I, Moher D, Altman DG, Schulz K, Ravaud P, CONSORT Group. Extending the CONSORT statement to randomised trials of nonpharmacologic treatment: explanation and elaboration. Annals of Internal Medicine 2008;148(4):295-309. [PMID: ] [DOI] [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. Journal of Clinical Epidemiology 2008;61(8):763-9. [DOI: 10.1016/j.jclinepi.2007.10.007] [PMID: ] [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta-analysis may be inconclusive -- trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. International Journal of Epidemiology 2009;38(1):287-98. [DOI: 10.1093/ije/dyn188] [PMID: ] [DOI] [PubMed] [Google Scholar]
Brunetti 2013
- Brunetti M, Shemilt I, Pregno S, Vale L, Oxman AD, Lord J, et al. GRADE guidelines: 10. Considering resource use and rating the quality of economic evidence. Journal of Clinical Epidemiology 2013;66(2):140–50. [DOI: 10.1016/j.jclinepi.2012.04.012] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cailhol 2015
- Cailhol L, Thalamas C, Garrido C, Birmes P, Lapeyre-Mestre M. Mental health service utilization among borderline personality disorder patients inpatient [Utilisation des services de soin par les patients hospitalisés, présentant un trouble de personnalité borderline en Midi-Pyrénées]. L'Encéphale 2015;41(2):115-22. [DOI: 10.1016/j.encep.2014.10.008] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chalmers 1983
- Chalmers TC, Celano P, Sacks HS, Smith H Jr. Bias in treatment assignment in controlled clinical trials. New England Journal of Medicine 1983;309(22):1358-61. [DOI: 10.1056/NEJM198312013092204] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chanen 2007
- Chanen AM, McCutcheon LK, Jovev M, Jackson HJ, McGorry PD. Prevention and early intervention for borderline personality disorder. Medical Journal of Australia 2007;187(7):S18-21. [DOI: 10.5694/j.1326-5377.2007.tb01330.x] [DOI] [PubMed] [Google Scholar]
Chanen 2012
- Chanen AM, Kaess M. Developmental pathways to borderline personality disorder. Current Psychiatry Reports 2012;14(1):45-53. [DOI: 10.1007/s11920-011-0242-y] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chanen 2013
- Chanen AM, McCutcheon L. Prevention and early intervention for borderline personality disorder: current status and recent evidence. British Journal of Psychiatry 2013;202(54 Suppl):S24-9. [DOI: 10.1192/bjp.bp.112.119180] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chanen 2014
- Chanen AM, McCutcheon L, Kerr IB. HYPE: a cognitive analytic therapy-based prevention and early intervention programme for borderline personality disorder. In: Sharp C, Tackett JL, editors(s). Handbook of Borderline Personality Disorder in Children and Adolescents. New York (NY): Springer, 2014:361-83. [ISBN 978-1-4939-0590-4] [Google Scholar]
Chanen 2016
- Chanen AM, Berk M, Thompson K. Integrating early intervention for borderline personality disorder and mood disorders. Harvard Review of Psychiatry 2016;24(5):330–41. [DOI: ] [DOI] [PubMed] [Google Scholar]
Chanen 2017
- Chanen A, Sharp C, Hoffman P, Global Alliance for the Prevention and Early Intervention for Borderline Personality Disorder. Prevention and early intervention for borderline personality disorder: a novel public health priority. World Psychiatry 2017;16(2):215-6. [DOI: 10.1002/wps.20429] [PMCID: PMC5428197] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chanen 2018
- Chanen AM, Thompson KN. Early intervention for personality disorder. Current Opinion in Psychology 2018;21:132-5. [DOI: ] [DOI] [PubMed] [Google Scholar]
Chatoor 2001
- Chatoor I, Krupnick J. The role of non-speciifc factors in treatment outcome of psychotherapy studies. European Child & Adolescent Psychiatry 2001;10:19-25. [DOI: 10.1007/s007870170004] [DOI] [PubMed] [Google Scholar]
Clarkin 1999
- Clarkin JF, Yeomans FE, Kernberg OF. Psychotherapy for Borderline Personality. New York (NY): John Wiley & Sons, Inc., 1999. [Google Scholar]
Clarkin 2012
- Clarkin JF. An integrated approach to psychotherapy techniques for patients with personality disorder. Journal of Personality Disorders 2012;26(1):43-62. [DOI: 10.1521/pedi.2012.26.1.43] [PMID: ] [DOI] [PubMed] [Google Scholar]
Coccaro 1991
- Coccaro EF, Harvey PD, Kupsaw-Lawrence E, Herbert JL, Bernstein DP. Development of neuropharmacologically-based behavioral assessments of impulsive aggressive behavior. Journal of Neuropsychiatry and Clinical Neurosciences 1991;3(2):S44-S51. [PMID: ] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd edition. Hillsdale (NJ): Lawrence Erlbaum Associates, 1988. [Google Scholar]
Coid 2006
- Coid J, Yang M, Tyrer P, Roberts A, Ullrich S. Prevalence and correlates of personality disorder in Great Britain. British Journal of Psychiatry 2006;188:423-31. [DOI: 10.1192/bjp.188.5.423] [PMID: ] [DOI] [PubMed] [Google Scholar]
Conte 1980
- Conte HR, Plutchik R, Karasu TB, Jerret I. A self-report borderline scale. Discriminative validity and preliminary norms. Journal of Nervous and Mental Disease 1980;168(7):428-35. [DOI: 10.1097/00005053-198007000-00007] [PMID: ] [DOI] [PubMed] [Google Scholar]
Crawford 2018a
- Crawford MJ, Sanatinia R, Barrett B, Cunningham G, Dale O, Ganguli P, et al. The clinical effectiveness and cost-effectiveness of lamotrigine in borderline personality disorder: a randomized placebo-controlled trial. American Journal of Psychiatry 2018;175(8):756-64. [DOI: 10.1176/appi.ajp.2018.17091006] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cristea 2017
- Cristea IA, Gentili C, Cotet CD, Palomba D, Barbui C, Cuijpers P. Efficacy of psychotherapies for borderline personality disorder: a systematic review and meta-analysis. JAMA Psychiatry 2017;74(4):319-28. [DOI: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
CTU 2011
- Copenhagen Trial Unit. TSA - Trial Sequential Analysis. www.ctu.dk/tsa (accessed 12 December 2016).
Curtin 2002
- Curtin F, Elbourne D, Altman DG. Meta-analysis combining parallel and cross-over clinical trials. III: the issue of carry-over. Statistics in Medicine 2002;21(15):2161-73. [DOI: 10.1002/sim.1207] [PMID: ] [DOI] [PubMed] [Google Scholar]
Davidson 2010
- Davidson KM, Tyrer P, Norrie J, Palmer SJ, Tyrer H. Cognitive therapy v usual treatment for borderline personality disorder: prospective 6-year follow-up. British Journal of Psychiatry 2010;197(6):456-62. [DOI: 10.1192/bjp.bp.109.074286] [PMID: ] [DOI] [PubMed] [Google Scholar]
Deeks 2019
- Deeks JJ, Higgins JPT, Altman DG editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane 2019. Available from www.training.cochrane.org/handbook.
De Fruyt 2014
- De Fruyt F, De Clercq B. Antecedents of personality disorder in childhood and adolescence: towards an integrative developmental model. Annual Review of Clinical Psychology 2014;10:449-76. [DOI: 10.1146/annurev-clinpsy-032813-15364] [PMID: ] [DOI] [PubMed] [Google Scholar]
De Groot 2008
- De Groot ER, Verheul R, Trijsburg RW. An integrative perspective on psychotherapeutic treatments for borderline personality disorders. Journal of Personality Disorders 2008;22(4):332-52. [DOI: 10.1521/pedi.2008.22.4.332] [PMID: ] [DOI] [PubMed] [Google Scholar]
Derogatis 1994
- Derogatis LR, Lazarus L. SCL-90-R, Brief Symptom Inventory, and matching clinical rating scales. In: Maruish ME, editors(s). The Use of Psychological Testing for Treatment Planning and Outcomes Assessment. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc., 1994:217-48. [Google Scholar]
Dimaggio 2007
- Dimaggio G, Semerari A, Carcione A, Nicolò G, Procacci M. Psychotherapy of Personality Disorders: Metacognition, States of Mind and Interpersonal Cycles. London (UK): Routledge, 2007. [Google Scholar]
Donner 2002
- Donner A, Klar N. Issues in the meta-analysis of cluster-randomized trials. Statistics in Medicine 2002;21(9):2971-80. [DOI: 10.1002/sim.1301] [PMID: ] [DOI] [PubMed] [Google Scholar]
Doyle 2016
- Doyle M, While D, Mok PL, Windfuhr K, Ashcroft, DM, Kontopantelis E, et al. Suicide risk in primary care patients diagnosed with a personality disorder: a nested case control study. BMC Family Practice 2016;17:106. [DOI: 10.1186/s12875-016-0479-y] [PMC4974738] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Endicott 1976
- Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Archives of General Psychiatry 1976;33(6):766-71. [PMID: ] [DOI] [PubMed] [Google Scholar]
Faltinsen 2017
- Faltinsen EG, Kongerslev M, Storebø OJ. Reviews and meta-analyses of psychotherapy efficacy for borderline personality disorder. JAMA Psychiatry 2017;74(8):853–4. [DOI: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Farivar 2004
- Farivar SS, Liu H, Hays RD. Half standard deviation estimate of the minimally important difference in HRQOL scores? Expert Review of Pharmacoeconomics & Outcomes Research 2004;4(5):515-23. [DOI: 10.1586/14737167.4.5.515] [PMID: ] [DOI] [PubMed] [Google Scholar]
Finch 2019
- Finch EF, Iliakis EA, Masland SR, Choi-Kain LW. A meta-analysis of treatment as usual for borderline personality disorder. Personality Disorders 2019;10(6):491-9. [DOI: 10.1037/per0000353] [PMID: ] [DOI] [PubMed] [Google Scholar]
First 1997
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II). Washington (DC): American Psychiatric Press, 1997. [Google Scholar]
Fok 2012
- Fok ML, Hayes RD, Chang CK, Stewart R, Callard FJ, Moran P. Life expectancy at birth and all-cause mortality among people with personality disorder. Journal of Psychosomatic Research 2012;73(2):104-7. [DOI: 10.1016/j.jpsychores.2012.05.001] [PMID: ] [DOI] [PubMed] [Google Scholar]
Fonagy 2009
- Fonagy P, Luyten P. A developmental, mentalization-based approach to the understanding and treatment of borderline personality disorder. Development and Psychopathology 2009;21(4):1355-81. [DOI: 10.1017/S0954579409990198] [PMID: ] [DOI] [PubMed] [Google Scholar]
Fonagy 2014
- Fonagy P, Allison E. The role of mentalizing and epistemic trust in the therapeutic relationship. Psychotherapy 2014;51(3):372-80. [DOI: 10.1037/a0036505] [PMID: ] [DOI] [PubMed] [Google Scholar]
Goodman 2010
- Goodman M, Patil U, Steffel L, Avedon J, Sasso S, Triebwasser J, et al. Treatment utilization by gender in patients with borderline personality disorder. Journal of Psychiatric Practice 2010;16(3):155-63. [DOI: 10.1097/01.pra.0000375711.47337.27] [PMID: ] [DOI] [PubMed] [Google Scholar]
Goodman 2012
- Goodman M, Roiff T, Oakes AH, Paris J. Suicidal risk and management in borderline personality disorder. Current Psychiatry Reports 2012;14(1):79-85. [DOI: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gratz 2001
- Gratz KL. Measurement of deliberate self-harm: preliminary data on the Deliberate Self-Harm Inventory. Journal of Psychopathology and Behavioral Assessment 2001;23(4):253-63. [DOI: 10.1023/A:1012779403943.pdf] [DOI] [Google Scholar]
Gratz 2004
- Gratz KL, Roemer L. Multidimensional assessment of emotion regulation and dysregulation: development, factor structure, and initial validation of the Difficulties in Emotion Regulation Scale. Journal of Psychopathology and Behavioral Assessment 2004;26:41-54. [DOI: 10.1023/B:JOBA.0000007455.08539.94] [DOI] [Google Scholar]
Gregory 2008
- Gregory RJ, Remen AL. A manual-based psychodynamic therapy for treatment-resistant borderline personality disorder. Psychotherapy: Theory, Research, Practice, Training 2008;45:15-27. [DOI] [PubMed] [Google Scholar]
Gregory 2010
- Gregory RJ, DeLucia-Deranja E, Mogle JA. Dynamic deconstructive psychotherapy versus optimized community care for borderline personality disorder co-occurring with alcohol use disorders: a 30-month follow-up.. Journal of Nervous and Mental Disease 2010;198(4):292-8. [DOI: ] [DOI] [PubMed] [Google Scholar]
Gunderson 1981
- Gunderson JG, Kolb JE, Austin V. The diagnostic interview for borderline patients. American Journal of Psychiatry 1981;138(7):896-903. [DOI: 10.1176/ajp.138.7.896] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gunderson 2009
- Gunderson JG. Borderline personality disorder: ontogeny of a diagnosis. American Journal of Psychiatry 2009;166(5):530-9. [DOI: 10.1176/appi.ajp.2009.08121825] [NIHMS313194] [PMC3145201] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gunderson 2011a
- Gunderson JG. Borderline personality disorder. New England Journal of Medicine 2011;364(21):2037-42. [DOI: ] [DOI] [PubMed] [Google Scholar]
Gunderson 2011b
- Gunderson JG, Stout RL, McGlashan TH, Shea MT, Morey LC, Grilo CM, et al. Ten-year course of borderline personality disorder: psychopathology and function from the Collaborative Longitudinal Personality Disorders study. Archives of General Psychiatry 2011;68(8):827-37. [DOI: ] [PMC3158489] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gunderson 2018
- Gunderson JG, Fruzzetti A, Unruh B, Choi-Kain L. Competing theories of borderline personality disorder. Journal of Personality Disorders 2018;32(2):148-67. [DOI: 10.1521/pedi.2018.32.2.148] [PMID: ] [DOI] [PubMed] [Google Scholar]
Guttierez 2001
- Guttierez PM, Osman A, Barrios FX, Kopper BA. Development and initial validation of the Self-Harm Behavior Questionnaire. Journal of Personality Assessment 2001;77(3):475-90. [DOI: 10.1207/S15327752JPA7703_08] [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction - GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383–94. [DOI: 10.1016/j.jclinepi.2010.04.026] [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. Journal of Clinical Epidemiology 2011;64(4):395–400. [DOI: 10.1016/j.jclinepi.2010.09.012] [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence - study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407–15. [DOI: 10.1016/j.jclinepi.2010.07.017] [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence - publication bias. Journal of Clinical Epidemiology 2011;64(12):1277–82. [DOI: 10.1016/j.jclinepi.2011.01.011] [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2011e
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence - imprecision. Journal of Clinical Epidemiology 2011;64(12):1283–93. [DOI: 10.1016/j.jclinepi.2011.01.012] [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2011f
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence - inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294–302. [DOI: 10.1016/j.jclinepi.2011.03.017] [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2011g
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence - indirectness. Journal of Clinical Epidemiology 2011;64(12):1303–10. [DOI: 10.1016/j.jclinepi.2011.04.014] [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2013a
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso-Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66(2):151–7. [DOI: 10.1016/j.jclinepi.2012.01.006] [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2013b
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables - binary outcomes. Journal of Clinical Epidemiology 2013;66(2):158–72. [DOI: 10.1016/j.jclinepi.2012.01.012] [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2013c
- Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles - continuous outcomes. Journal of Clinical Epidemiology 2013;66(2):173–83. [DOI: 10.1016/j.jclinepi.2012.08.001] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hastrup 2019a
- Hastrup LH, Kongerslev MT, Simonsen E. Low vocational outcome among people diagnosed with borderline personality disorder during first admission to mental health services in Denmark: a nationwide 9-year register-based study. Journal of Personality Disorders 2019;33(3):326-40. [DOI: 10.1521/pedi_2018_32_344] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hastrup 2019b
- Hastrup LH, Jennum P, Ibsen R, Kjellberg J, Simonsen E. Societal costs of borderline personality disorders: a matched-controlled nationwide study of patients and spouses. Acta Psychiatrica Scandinavica 2019;140(5):458-67. [DOI: 10.1111/acps.13094] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI: ] [PMC192859] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Deeks JJ, Altman DG, editor(s), Cochrane Statistical Methods Group. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2019
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019. [Available from: www.training.cochrane.org/handbook] [Google Scholar]
Horowitz 1988
- Horowitz LM, Rosenberg SE, Baer BA, Ureño G, Villaseñor VS. Inventory of interpersonal problems: psychometric properties and clinical applications. Journal of Consulting and Clinical Psychology 1988;56(6):885-92. [PMID: ] [DOI] [PubMed] [Google Scholar]
Horvath 2011
- Horvath AO, Del Re AC, Flückiger C, Symonds D. Alliance in individual psychotherapy. Psychotherapy 2011;48(1):9-16. [DOI: 10.1037/a0022186] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hörz 2010
- Hörz S, Zanarini MC, Frankenburg FR, Reich DB, Fitzmaurice G. Ten-year use of mental health services by patients with borderline personality disorder and with other axis II disorders. Psychiatric Services 2010;61(6):612-6. [DOI: 10.1176/ps.2010.61.6.612] [PMC3889171] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huprich 2015
- Huprich SK. Personality Disorders: Toward Theoretical and Empirical Integration in Diagnosis and Assessment. Washington (DC): American Psychological Association, 2015. [ISBN 978-1-4228-1846-2] [Google Scholar]
ICH 1996
- International Council for Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice E6(R1). www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf (accessed 6 May 2011).
Jakobsen 2014
- Jakobsen JC, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta-analytic methods. BMC Medical Research Methodology 2014;14:120. [DOI: 10.1186/1471-2288-14-120] [PMCID: PMC4251848] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karterud 2019
- Karterud SW, Kongerslev MT. A temperament-attachment-mentalization-based (TAM) theory of personality and its disorders. Frontiers in Psychology 2019;10:518. [DOI: 10.3389/fpsyg.2019.00518] [PMCID: PMC6439347] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karterud 2020
- Karterud S, Kongerslev MT. Psychotherapy of personality disorders needs an integrative theory of personality. Journal of Psychotherapy Integration 2020 Mar 2 [Epub ahead of print]. [DOI: 10.1037/int0000196] [DOI]
Kazdin 2004
- Kazdin AE. Psychotherapy for children and adolescents. In: Lambert MJ, editors(s). Bergin and Garfield's Handbook of Psychotherapy and Behavior Change. 5th edition. New York (NY): John Wiley & Sons, Inc., 2004:543-89. [ISBN 0-471-37755-4] [Google Scholar]
Kazdin 2009
- Kazdin AE. Understanding how and why psychotherapy leads to change. Psychotherapy Research 2009;19(4-5):418-28. [DOI: 10.1080/10503300802448899] [DOI] [PubMed] [Google Scholar]
Kernberg 1975
- Kernberg OF. Borderline Conditions and Pathological Narcissism. New York (NY): Aronson, 1975. [Google Scholar]
Kjær 2018
- Kjær JNR, Biskin R, Vestergaard C, Munk-Jørgensen P. All-cause mortality of hospital-treated borderline personality disorder: a nationwide cohort study. Journal of Personality Disorders 2018;11:1-13. [DOI: 10.1521/pedi_2018_32_403] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Annals of Internal Medicine 2001;135(11):982-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kliem 2010
- Kliem S, Kröger C, Kosfelder J. Dialectical behavior therapy for borderline personality disorder: a meta-analysis using mixed-effects modeling. Journal of Consulting and Clinical Psychology 2010;78(6):936-51. [DOI: 10.1037/a0021015] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kongerslev 2015
- Kongerslev MT, Chanen AM, Simonsen E. Personality disorder in childhood and adolescence comes of age: a review of the current evidence and prospects for future research. Scandinavian Journal of Child and Adolescent Psychiatry and Psychology 2015;3(1):31-48. [DOI: 10.21307/sjcapp-2015-004] [DOI] [Google Scholar]
Lau 1995
- Lau J, Schmid CH, Chalmers TC. Cumulative meta-analysis of clinical trials builds evidence for exemplary medical care. Journal of Clinical Epidemiology 1995;48(1):45-57. [PMID: ] [DOI] [PubMed] [Google Scholar]
Leichsenring 2011
- Leichsenring F, Leibing E, Kruse J, New AS, Leweke F. Borderline personality disorder.. Lancet 2011;377(9759):74-84. [DOI] [PubMed] [Google Scholar]
Lenzenweger 2007
- Lenzenweger MF, Willett JB. Predicting individual change in personality disorder features by simultaneous individual change in personality dimensions linked to neurobehavioral systems: the longitudinal study of personality disorders. Journal of Abnormal Psychology 2007;116(4):684–700. [DOI: 10.1037/0021-843X.116.4.684] [PMID: ] [DOI] [PubMed] [Google Scholar]
Levine 1986
- Levine J, Schooler NR. SAFTEE: a technique for the systematic assessment of side effects in clinical trials. Psychopharmacology Bulletin 1986;22(2):343-81. [PMID: 3774930] [PubMed] [Google Scholar]
Levy 2006
- Levy KN, Clarkin JF, Yeomans FE. The mechanisms of change In the treatment of borderline personality disorder with transference focused psychotherapy. Journal of Clinical Psychology 2006;62(4):481-501. [DOI: ] [DOI] [PubMed] [Google Scholar]
Lieb 2004
- Lieb K, Zanarini MC, Schmahl C, Linehan MM, Bohus M. Borderline personality disorder. Lancet 2004;364(9432):453-61. [DOI: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lilienfeld 2007
- Lilienfeld SO. Psychological treatments that cause harm. Perspectives on Psychology Science 2007;2(1):53-70. [DOI: doi: 10.1111/j.1745-6916.2007.00029.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Linehan 1993a
- Linehan MM. Cognitive-Behavioral Treatment of Borderline Personality Disorder. New York (NY): Guilford Press, 1993. [ISBN 0-89862-183-6] [Google Scholar]
Linehan 1993b
- Linehan MM. Skills Training Manual for Treating Borderline Personality Disorder. New York (NY): Guilford Press, 1993. [Google Scholar]
Linehan 1997
- Linehan MM. Behavioral treatments of suicidal behaviors. Definitional obfuscation and treatment outcomes. Annals of the New York Academy of Sciences 1997;836:302-28. [DOI: 10.1111/j.1749-6632.1997.tb52367.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Linehan 2015b
- Linehan MM. DBT® Skills Training Manual. 2nd edition. New York (NY): Guilford Press, 2015. [ISBN 978-1-4625-1699-5] [Google Scholar]
Links 2015
- Links PS, Ross J, Gunderson JG. Promoting good psychiatric management for patients with borderline personality disorder. Journal of Clinical Psychology 2015;71(8):753-63. [DOI: 10.1002/jclp.22203] [PMID: ] [DOI] [PubMed] [Google Scholar]
Livesley 2003
- Livesley WJ. Practical Management of Personality Disorder. New York (NY): Guilford Press, 2003. [ISBN 1-57230-889-3] [Google Scholar]
Livesley 2004
- Livesley WJ. Changing ideas about the treatment of borderline personality disorder. Journal of Contemporary Psychotherapy 2004;34(3):185-92. [DOI: 10.1023/B:JOCP.0000036697.03467.27] [DOI] [Google Scholar]
Livesley 2012
- Livesley WJ. Moving beyond specialized therapies for borderline personality disorder: the importance of integrated domain-focused treatment. Psychodynamic Psychiatry 2012;40(1):47-74. [DOI: 10.1521/pdps.2012.40.1.4] [PMID: ] [DOI] [PubMed] [Google Scholar]
Livesley 2016
- Livesley WJ, Dimaggio G, Clarkin JF, editor(s). Integrated Treatment for Personality Disorder: A Modular Approach. New York (NY): Guilford Press, 2016. [ISBN 978-1-4625-2288-0] [Google Scholar]
Loranger 1988
- Loranger AW. Personality Disorder Examination (PDE) Manual. Yonkers (NY): DV Communications, 1988. [Google Scholar]
Loranger 1995
- Loranger AW, Janca A, Sartorius N, editor(s). Assessment and Diagnosis of Personality Disorders. The ICD-10 International Personality Disorder Examination (IPDE). Cambridge (Camb): Cambridge University Press, 1995. [WHO IRIS: apps.who.int/iris/handle/10665/41912] [Google Scholar]
Lundh 2012
- Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2012, Issue 12. Art. No: MR000033. [DOI: 10.1002/14651858.MR000033.pub2] [DOI] [PubMed] [Google Scholar]
Markowitz 2006
- Markowitz JC, Skodol AE, Bleiberg K. Interpersonal psychotherapy for borderline personality disorder: possible mechanisms of change. Journal of Clinical Psychology 2006;62(4):431-44. [DOI: 10.1002/jclp.20242] [PMID: ] [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analysis? Lancet 1998;352(9128):609-13. [DOI: 10.1016/S0140-6736(98)01085-X] [PMID: ] [DOI] [PubMed] [Google Scholar]
Moher 1999
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet 1999;354(9193):1896-900. [PMID: ] [DOI] [PubMed] [Google Scholar]
Montgomery 1979
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Morey 2007
- Morey LC. Personality Assessment Inventory (PAI): Professional Manual. 2nd edition. Lutz (FL): Psychological Assessment Resources, 2007. [Google Scholar]
Munk‐Jørgensen 2010
- Munk-Jørgensen P, Najarraq Lund M, Bertelsen A. Use of ICD-10 diagnoses in Danish psychiatric hospital-based services in 2001-2007. World Psychiatry 2010;9(3):183-4. [ PMC2948730] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mustafa 2013
- Mustafa RA, Santesso N, Brozek J, Akl EA, Walter SD, Norman G, et al. The GRADE approach is reproducible in assessing the quality of evidence of quantitative evidence syntheses. Journal of Clinical Epidemiology 2013;66(7):736-42. [DOI: 10.1016/j.jclinepi.2013.02.004] [PMID: ] [DOI] [PubMed] [Google Scholar]
Neacsiu 2017
- Neacsiu AD, Eberle JW, Keng SL, Fang CM, Rosenthal Z. Understanding borderline personality disorder across sociocultural groups: findings, issues, and future directions. Current Psychiatry Reviews 2017;13(3):188-223. [DOI: 10.2174/1573400513666170612122034] [DOI] [Google Scholar]
Newton‐Howes 2015
- Newton-Howes G, Clark LA, Chanen A. Personality disorder across the life course. Lancet 2015;385(9969):727-34. [DOI: 10.1016/S0140-6736(14)61283-6] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ng 2016
- Ng FY, Bourke ME, Grenyer BFS. Recovery from borderline personality disorder: a systematic review of the perspectives of consumers, clinicians, family and carers. PLOS One 2016;11(8):e0160515. [DOI: 10.1371/journal.pone.0160515] [PMC4978398] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE CG90
- National Institute for Health and Care Excellence. Depression in adults: recognition and management. Cinical guideline (CG90). www.nice.org.uk/guidance/cg90 (accessed prior to 16 March 2020).
Niesten 2016
- Niesten IJ, Karan E, Frankenburg FR, Fitzmaurice GM, Zanarini MC. Description and prediction of the income status of borderline patients over 10 years of prospective follow-up. Personality and Mental Health 2016;10(4):285-92. [DOI: 10.1002/pmh.1331] [PMID: ] [DOI] [PubMed] [Google Scholar]
NLM 2009
- US National Library of Medicine. Medical Subject Headings (MeSH Browser). meshb.nlm.nih.gov/search (accessed 31 March 2009).
Norcross 2011
- Norcross JC, editor(s). Psychotherapy Relationships That Work: Evidence-Based Responsiveness. 2nd edition. New York (NY): Oxford University Press, Inc., 2011. [ISBN 978-0-19-973720-8] [Google Scholar]
Osman 2001
- Osman A, Bagge CL, Gutierrez PM, Konick LC, Kopper BA, Barrios FX. The Suicidal Behaviors Questionnaire-Revised (SBQ-R): validation with clinical and nonclinical samples. Assessment 2001;8(4):443-54. [DOI: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ottosen 2002
- Ottosson H, Ekselius L, Grann M, Kullgren G. Cross-system concordance of personality disorder diagnoses of DSM-IV and diagnostic criteria for research of ICD-10. Journal of Personality Disorders 2002;16(3):283-92. [DOI: 10.1521/pedi.16.3.283.22537] [PMID: ] [DOI] [PubMed] [Google Scholar]
Oud 2018
- Oud M, Arntz A, Hermens MLM, Verhoef R, Kendall T. Specialized psychotherapies for adults with borderline personality disorder: a systematic review and meta-analysis. Australian & New Zealand Journal of Psychiatry 2018;52(10):949-61. [DOI: 10.1177/0004867418791257] [PMCID: PMC6151959] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Overall 1962
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports 1962;10(3):799-812. [DOI: 10.2466/pr0.1962.10.3.799] [DOI] [Google Scholar]
Paris 2014
- Paris J. The relevance of social capital for the treatment of personality disorders. Personality and Mental Health 2014;8(1):24-9. [DOI: 10.1002/pmh.1238] [DOI] [PubMed] [Google Scholar]
Paris 2019
- Paris J. Suicidality in borderline personality disorder. Medicina 2019;55(6):E223. [DOI: 10.3390/medicina55060223] [PMCID: PMC6632023] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parry 2016
- Parry GD, Crawford MJ, Duggan C. Iatrogenic harm from psychological therapies - time to move on. British Journal of Psychiatry 2016;208(3):210-2. [DOI: 10.1192/bjp.bp.115.163618] [PMID: ] [DOI] [PubMed] [Google Scholar]
Perez 2007
- Perez V, Barrachina J, Soler J, Pascual JC, Campins MJ, Puigdemont D, et al. The clinical global impression scale for borderline personality disorder patients (CGI-BPD): a scale sensible to detect changes [Impresión clinica global para pacientes con trastorno límite de la personalidad (ICG-TLP): una escala sensible al cambio]. Actas Espanolas de Psiquiatria 2007;35(4):229-35. [PMID: ] [PubMed] [Google Scholar]
Peryer 2019
- Peryer G, Golder S, Junqueira D, Vohra S, Loke YK. Chapter 19: Adverse effects. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019), Cochrane 2019. Available from www.training.cochrane.org/handbook.
Pfohl 1997
- Pfohl B, Blum N, Zimmerman M. Structured Interview for DSM-IV Personality (SIPD-IV). Washington (DC): American Psychiatric Press, 1997. [Google Scholar]
Reiss 2014
- Reiss N, Lieb K, Arntz A, Shaw IA, Farrell J. Responding to the treatment challenge of patients with severe BPD: results of three pilot studies of inpatient schema therapy. Behavioural and Cognitive Psychotherapy 2014;42(3):355-67. [DOI: 10.1017/S1352465813000027] [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ryle 1997
- Ryle A. Cognitive Analytic Therapy and Borderline Personality Disorder: The Model and the Method. Chichester (UK): John Wiley & Sons, Inc., 1997. [ISBN-13: 978-0471976189] [Google Scholar]
Sansone 2006
- Sansone R, Levitt J. Personality Disorders and Eating Disorders. New York: Routledge, 2006. [DOI: ] [Google Scholar]
Sansone 2011
- Sansone RA, Sansone LA. Gender patterns in borderline personality disorder. Innovations in Clinical Neuroscience 2011;8(5):16-20. [PMCID:: PMC3115767] [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Savović 2012a
- Savović J, Jones H, Altman D, Harris R, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta-epidemiological studies. Health Technology Assessment 2012;16(35):1-82. [DOI: 10.3310/hta16350] [PMID: ] [DOI] [PubMed] [Google Scholar]
Savović 2012b
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429-38. [DOI: 10.7326/0003-4819-157-6-201209180-00537] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sellbom 2014
- Sellbom M, Sansone RA, Songer DA, Anderson JL. Convergence between DSM-5 Section II and Section III diagnostic criteria for borderline personality disorder. Australian & New Zealand Journal of Psychiatry 2014;48(4):325-32. [DOI: 10.1177/0004867413511997] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sharp 2018
- Sharp C, Wall K. Personality pathology grows up: adolescence as a sensitive period. Current Opinion in Psychology 2018;21:111-6. [DOI: 10.1016/j.copsyc.2017.11.010] [PMID: ] [DOI] [PubMed] [Google Scholar]
Skodol 2002
- Skodol AE, Gunderson JG, McGlashan TH, Dyck IR, Stout RL, Bender DS, et al. Functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder. American Journal of Psychiatry 2002;159(2):276-83. [DOI: 10.1176/appi.ajp.159.2.276] [PMID: ] [DOI] [PubMed] [Google Scholar]
Soeteman 2008a
- Soeteman DI, Hakkaart-van Roijen L, Verheul R, Busschbach JJV. The economic burden of personality disorders in mental health care. Journal of Clinical Psychiatry 2008;69(2):259–65. [PMID: ] [DOI] [PubMed] [Google Scholar]
Soeteman 2008b
- Soeteman DI, Verheul R, Busschbach JJV. The burden of disease in personality disorders: diagnosis-specific quality of life. Journal of Personality Disorders 2008;22(3):259-68. [DOI: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Soloff 2002
- Soloff PH, Lynch KG, Kelly TM. Childhood abuse as a risk factor for suicidal behavior in borderline personality disorder. Journal of Personality Disorders 2002;16(3):201-14. [PMID: ] [DOI] [PubMed] [Google Scholar]
Spielberger 1988
- Spielberger CD. Manual for the State-Trait Anger Expression Inventory. Odessa (FL): Psychological Assessment Resources, 1988. [Google Scholar]
Spitzer 1989
- Spitzer RL, William JBW, Gibbon M, First MB. Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II). New York (NY): Biometrics Research Department, New York State Psychiatric Institute, 1989. [Google Scholar]
Stepp 2012
- Stepp SD. Development of borderline personality disorder in adolescence and young adulthood: introduction to the special section. Journal of Abnormal Child Psychology 2012;40(1):1-5. [DOI: 10.1007/s10802-011-9594-3] [ PMC3865353] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stern 1938
- Stern A. Psychoanalytic investigation of and therapy in the borderline group of neuroses. Psychoanalytic Quarterly 1938;7:467-89. [Google Scholar]
Sterne 2017
- Sterne JAC, Egger M, Moher D, Boutron I, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Stiglmayr 2005
- Stiglmayr CE, Grathwol T, Linehan MM, Ihorst G, Fahrenberg J, Bohus M. Aversive tension in patients with borderline personality disorder: a computer-based controlled field study. Acta Psychiatrica Scandinavica 2005;111(5):372-9. [DOI: 10.1111/j.1600-0447.2004.00466.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Storebø 2014
- Storebø OJ, Simonsen E. Is ADHD an early stage in the development of borderline personality disorder? Nordic Journal of Psychiatry 2014;68(5):289-95. [DOI: 10.3109/08039488.2013.841992] [PMID: ] [DOI] [PubMed] [Google Scholar]
Streeck 2009
- Streeck U, Leichsenring F. Handbuch Psychoanalytisch-Interaktionelle Therapie. Zur Behandlung von Patienten mit Strukturellen Störungen und Schweren Persönlichkeitsstörungen. Göttingen (NI): Vandenhoeck & Ruprecht, 2009. [Google Scholar]
Ten Have 2016
- Ten Have M, Verheul R, Kaasenbrood A, Van Dorsselaer S, Tuithof M, Kleinjan M, et al. Prevalence rates of borderline personality disorder symptoms: a study based on the Netherlands Mental Health Survey and Incidence Study-2. BMC Psychiatry 2016;16:249. [DOI: ] [PMC4949762] [PMID: 27435813] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thalheimer 2002
- Thalheimer W, Cook S. How to calculate effect sizes from published research: a simplified methodology. www.bwgriffin.com/gsu/courses/edur9131/content/Effect_Sizes_pdf5.pdf (accessed 9 August 2019).
Thomas 2019
- Thomas J, Askie LM, Berlin JA, Elliott JH, Ghersi D, Simmonds M, et al. Chapter 22: Prospective approaches to accumulating evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Thomson 2019
- Thompson KN, Jackson H, Cavelti M, Betts J, McCutcheon L, Jovev M, et al. The clinical significance of subthreshold borderline personality disorder features in outpatient youth. Journal of Personality Disorders 2019;33(1):71-81. [DOI: 10.1521/pedi_2018_32_330] [PMID: ] [DOI] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta-analysis? International Journal of Epidemiology 2009;38(1):276-86. [DOI: 10.1093/ije/dyn179] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tomko 2014
- Tomko RL, Trull TJ, Wood PK, Sher KJ. Characteristics of borderline personality disorder in a community sample: comorbidity, treatment utilization, and general functioning. Journal of Personality Disorders 2014;28(5):734-50. [DOI: ] [PMC3864176] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Torgersen 2001
- Torgersen S, Kringlen E, Cramer V. The prevalence of personality disorders in a community sample. Archives of General Psychiatry 2001;58(6):590–6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Torgersen 2012
- Torgersen S. Epidemiology. In: Widiger TA, editors(s). The Oxford Handbook of Personality Disorders. New York (NY): Oxford University Press, 2012:186-205. [DOI: 10.1093/oxfordhb/9780199735013.013.0009] [ISBN 978-0-19-973501-3] [DOI] [Google Scholar]
Tyrer 2005
- Tyrer P, Nur U, Crawford M, Karlsen S, McLean C, Rao B, et al. The Social Functioning Questionnaire: a rapid and robust measure of perceived functioning. International Journal of Social Psychiatry 2005;51(3):265-75. [PMID: ] [PubMed] [Google Scholar]
Tyrer 2015
- Tyrer P, Reed GM, Crawford MJ. Classification, assessment, prevalence, and effect of personality disorders. Lancet 2015;385(9969):717-26. [DOI: 10.1016/S0140-6736(14)61995-4] [DOI] [PubMed] [Google Scholar]
Van Asselt 2007
- Van Asselt AD, Dirksen CD, Arntz A, Severens JL. The cost of borderline personality disorder: societal cost of illness in BPD-patients. European Psychiatry 2007;22(6):354-61. [DOI: 10.1016/j.eurpsy.2007.04.001] [PMID: ] [DOI] [PubMed] [Google Scholar]
Verheul 2003
- Verheul R, Van den Bosch LM, Koeter MW, De Ridder MA, Stijnen T, Van den Brink W. Dialectical behaviour therapy for women with borderline personality disorder: 12-month, randomised clinical trial in The Netherlands. British Journal of Psychiatry 2003;182:135-40. [DOI: 10.1192/bjp.182.2.135] [PMID: ] [DOI] [PubMed] [Google Scholar]
Videler 2019
- Videler AC, Hutsebaut J, Schulkens JEM, Sobczak S, Van Alphen SPJ. A life span perspective on borderline personality disorder. Current Psychiatry Reports 2019;21(7):51. [10.1007/s11920-019-1040-1] [31161404] [PMC6546651] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vrouva 2010
- Vrouva I, Fonagy P, Fearon PRM, Roussow T. The risk-taking and self-harm inventory for adolescents: development and psychometric evaluation. Psychological Assessment 2010;22(4):852-65. [DOI: 10.1037/a0020583] [PMID: ] [DOI] [PubMed] [Google Scholar]
Weinberg 2011
- Weinberg I, Ronningstam E, Goldblatt MJ, Schechter M, Maltsberger JT. Common factors in empirically supported treatments of borderline personality disorder. Current Psychiatry Reports 2011;13(1):60-8. [DOI: 10.1007/s11920-010-0167-x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. Journal of Clinical Epidemiology 2008;61(1):64-75. [DOI: 10.1016/j.jclinepi.2007.03.013] [PMID: ] [DOI] [PubMed] [Google Scholar]
WHO 1993
- World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research. Geneva (CH): World Health Organization, 1993. [Google Scholar]
WHO 2018
- World Health Organization. ICD-11 for mortality and morbidity statistics. icd.who.int/browse11/l-m/en (accessed 06 December 2019).
Winsper 2020
- Winsper C, Bilgin A, Thompson A, Marwaha S, Chanen AM, Singh S, et al. The prevalence of personality disorders in the community: a global systematic review and meta-analysis.. British Journal of Psychiatry 2020;216(2):69-78. [DOI: 10.1192/bjp.2019.166] [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ 2008;336(7644):601-5. [DOI: 10.1136/bmj.39465.451748.AD] [PMC2267990] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yen 2004
- Yen S, Shea MT, Sanislow CA, Grilo CM, Skodol AE, Gunderson JG, et al. Borderline personality disorder criteria associated with prospectively observed suicidal behavior. American Journal of Psychiatry 2004;161(7):1296-8. [DOI: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Yeomans 2015
- Yeomans FE, Clarkin JF, Kernberg OF. Transference-Focused Psychotherapy for Borderline Personality Disorder: A Clinical Guide. Arlington (VA): American Psychiatric Publishing, 2015. [ISBN 978-1-58562-437-9] [Google Scholar]
Young 2003
- Young JE, Klosko JS, Weishaar ME. Schema Therapy: A Practitioner's Guide. New York (NY): Guilford Press, 2003. [Google Scholar]
Zanarini 1987
- Zanarini MC, Frankenburg FR, Chauncey DL, Gunderson JG. The Diagnostic Interview for Personality Disorders: interrater and test-retest reliability. Comprehensive Psychiatry 1987;28(6):467-80. [DOI: 10.1016/0010-440x(87)90012-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zanarini 1989
- Zanarini MC, Gunderson JG, Frankenburg FR, Chauncey DL. The revised Diagnostic Interview for Borderlines: discriminating BPD from other Axis II disorders. Journal of Personality Disorders 1989;3(1):10-8. [DOI: 10.1521/pedi.1989.3.1.10] [DOI] [Google Scholar]
Zanarini 1996
- Zanarini MC, Frankenburg FR, Sickel AE, Young L. Diagnostic interview for DSM-IV personality disorders (unpublished measure). Boston (MA): McLean Hospital, 1996. [Google Scholar]
Zanarini 1998
- Zanarini MC, Frankenburg FR, DeLuca CJ, Hennen J, Khera GS, Gunderson JG. The pain of being borderline: dysphoric states specific to borderline personality disorder. Harvard Review of Psychiatry 1998;6(4):201-7. [DOI: 10.3109/10673229809000330] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zanarini 2003a
- Zanarini MC, Vujanovic AA, Parachini EA, Boulanger JL, Frankenburg FR, Hennen J. Zanarini Rating Scale for Borderline Personality Disorder (Zan-BPD): a continuous measure of DSM-IV borderline psychopathology. Journal of Personality Disorders 2003;17(3):233-42. [DOI: 10.1521/pedi.17.3.233.22147] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zanarini 2003b
- Zanarini MC. The Childhood Interview for DSM-IV Borderline Personality Disorder (CI-BPD). Belmont (MA): McLean Hospital and Harvard Medical School, 2003. [Google Scholar]
Zanarini 2004
- Zanarini MC, Frankenburg FR, Hennen J, Silk KR. Mental health service utilization by borderline personality disorder patients and Axis II comparison subjects followed prospectively for 6 years. Journal of Clinical Psychiatry 2004;65(1):28-36. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zanarini 2005
- Zanarini MC, Frankenburg FR, Hennen J, Reich DB, Silk KR. The McLean Study of Adult Development (MSAD): overview and implications of the first six years of prospective follow-up. Journal of Personality Disorders 2005;19(5):505-23. [DOI: 10.1521/pedi.2005.19.5.505] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zanarini 2007
- Zanarini MC, Frankenburg FR, Reich DB, Silk KR, Hudson JI, McSweeney LB. The subsyndromal phenomenology of borderline personality disorder: a 10-year follow-up study. American Journal of Psychiatry 2007;164(6):929-35. [DOI: 10.1176/ajp.2007.164.6.929] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zanarini 2010
- Zanarini MC, Frankenburg FR, Reich DB, Fitzmaurice G. The 10-year course of psychosocial functioning among patients with borderline personality disorder and axis II comparison subjects. Acta Psychiatrica Scandinavica 2010;122(2):103-9. [DOI: ] [PMC3876887] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zanarini 2012
- Zanarini MC, Frankenburg FR, Reich B, Fitzmaurice G. Attainment and stability of sustained symptomatic remission and recovery among patients with borderline personality disorder and axis II comparison subjects: a 16-year prospective follow-up study. American Journal of Psychiatry 2012;169(5):476-83. [DOI: 10.1176/appi.ajp.2011.11101550] [PMC3509999] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zanarini 2015
- Zanarini MC, Frankenburg FR, Reich DB, Conkey LC, Fitzmaurice GM. Rates of psychiatric treatment reported by patients with borderline personality disorder and other personality disorders over 16 years of prospective follow-up. Psychiatric Services 2015;66(1):15-20. [DOI: 10.1176/appi.ps.201400055] [NIHMS597508] [PMC4283568] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Binks 2006
- Binks C, Fenton M, McCarthy L, Lee T, Adams CE, Duggan C. Psychological therapies for people with borderline personality disorder. Cochrane Database of Systematic Reviews 2006, Issue 1. Art. No: CD005652. [DOI: 10.1002/14651858.CD005652] [DOI] [PubMed] [Google Scholar]
Stoffers‐Winterling 2012
- Stoffers-Winterling JM, Völlm BA, Rücker G, Timmer A, Huband N, Lieb K. Psychological therapies for people with borderline personality disorder. Cochrane Database of Systematic Reviews 2012, Issue 8. Art. No: CD005652. [DOI: 10.1002/14651858.CD005652.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Storebø 2018
- Storebø OJ, Stoffers-Winterling JM, Völlm BA, Kongerslev MT, Mattivi JT, Kielsholm ML, et al. Psychological therapies for people with borderline personality disorder. Cochrane Database of Systematic Reviews 2018, Issue 2. Art. No: CD012955. [DOI: 10.1002/14651858.CD012955] [DOI] [PMC free article] [PubMed] [Google Scholar]


