Abstract
Air pollution has consistently been associated with cardiometabolic outcomes, although associations with obesity have only been recently reported. Studies of air pollution and adiposity have mostly relied on body mass index (BMI) rather than body fat percentage (BF%), and most have not accounted for noise as a possible confounder. Additionally, it is unknown whether genetic predisposition for obesity increases susceptibility to the obesogenic effects of air pollution. To help fill these gaps, we used the UK Biobank, a large, prospective cohort study in the United Kingdom, to explore the relationship between air pollution and adiposity, and modification by a polygenic risk score for BMI. We used 2010 annual averages of air pollution estimates from land use regression (NO2, NOX, PM2.5, PM2.5absorbance, PM2.5–10, PM10), traffic intensity (TI), inverse distance to road (IDTR), along with examiner-measured BMI, waist-hip-ratio (WHR), and impedance measures of BF%, which were collected at enrollment (2006–2010, n=473,026) and at follow-up (2012–2013, n=19,518). We estimated associations of air pollution with BMI, WHR, and BF% at enrollment and follow-up, and with obesity, abdominal obesity, and BF%-obesity at enrollment and follow-up. We used linear and logistic regression and controlled for noise and other covariates. We also assessed interactions of air pollution with a polygenic risk score for BMI. On average, participants at enrollment were 56 years of age, 54% were female, and 32% had completed college or a higher degree. Almost all participants (~95%) were white. All air pollution measures except IDTR were positively associated with at least one continuous measure of adiposity at enrollment. However, NO2 was negatively associated with BMI but positively associated with WHR at enrollment, and IDTR was also negatively associated with BMI. At follow-up (controlling for enrollment adiposity), we observed positive associations for PM2.5–10 with BMI, PM10 with BF%, and TI with BF% and BMI. Associations were similar for binary measures of adiposity, with minor differences for some pollutants. Associations of NOX, NO2, PM2.5absorbance, PM2.5 and PM10, with BMI at enrollment, but not at follow-up, were stronger among individuals with higher BMI polygenic risk scores (interaction p <0.05). In this large, prospective cohort, air pollution was associated with several measures of adiposity at enrollment and follow-up, and associations with adiposity at enrollment were modified by a polygenic risk score for obesity.
Keywords: air pollution, obesity, BMI, gene by environment interactions, epidemiology
Introduction
Worldwide, the prevalence of obesity tripled from 1975 to 2016 (Flegal, Kruszon-Moran et al. 2016, Fryar, Carroll et al. 2016), and in the United States, high BMI has an economic cost in excess of $215 billion per year (Hammond and Levine 2010). Although much attention has been given to the role of personal behaviors and genetics in obesity, the rapid rise of obesity over the past few decades also implies a strong role of environmental factors and gene-environment interactions (Ogden, Yanovski et al. 2007).
Environmental contaminants, and air pollution in particular, may act to alter basal metabolism (Chen and Schwartz 2008), adipose deposition (Heindel and vom Saal 2009), craving and/or satiety mechanisms that are hormonally or neurologically regulated (Bolton, Smith et al. 2012, Chen, Herting et al. 2018), and inflammation and oxidative stress mechanisms (Risom, Møller et al. 2005, Chuang, Chan et al. 2007), which are also closely related to obesity (Keaney Jr, Larson et al. 2003, Fernández-Sánchez, Madrigal-Santillán et al. 2011). Recently, some epidemiological and animal studies suggest that air pollution is not only associated with these biological mechanisms, but also may be associated with clinically relevant obesity outcomes (Jerrett, McConnell et al. 2014, McConnell, Shen et al. 2015, Wei, Zhang et al. 2016, Mao, Nachman et al. 2017), although most studies to date in Western countries have focused on children. In adults, the relationship between air pollution and obesity is relatively unexplored, with existing epidemiological studies reporting mixed results (Li, Dorans et al. 2016, White, Jerrett et al. 2016, James, Kioumourtzoglou et al. 2017, Wallwork, Colicino et al. 2017).
In these early stages of epidemiological research on the relationship between air pollution and obesity, most studies focused on BMI, with only one study that we have identified examining associations with adipose tissue (Li, Dorans et al. 2016). BMI is often used as a proxy for body fat, although BMI cannot distinguish between lean and fat body mass. No studies have examined associations of air pollution with body fat percentage, despite the fact that it is an excellent predictor of subsequent health outcomes, even in the presence of normal BMI (Gómez-Ambrosi, Silva et al. 2011, Shah and Braverman 2012, Shea, King et al. 2012). Additionally, two studies have found that 29%−39% of those classified as normal weight by BMI standards were actually obese by body fat percentage standards (Gómez-Ambrosi, Silva et al. 2012, Phillips, Tierney et al. 2013). However, body fat is a difficult and somewhat expensive metric to capture, while BMI and WHR are easily measured and are currently standard measurements in a wide variety of public health and clinical settings. Thus, evaluating epidemiological associations with multiple measures of obesity may help clarify whether there is any advantage to using body fat over BMI. Additionally, using multiple measures may help reduce outcome misclassification and more fully capture the range of obesity metrics.
The relationship between air pollution and adiposity may also be confounded by noise, which has recently been associated with measures of adiposity (Christensen, Raaschou-Nielsen et al. 2015, Pyko, Eriksson et al. 2015, Pyko, Eriksson et al. 2017, Foraster, Eze et al. 2018). Traffic may be a key generator of both noise and air pollution, which may serve to open a backdoor pathway for noise to confound the air pollution-obesity relationship.
Finally, as genetic factors play a substantial role in susceptibility to obesity, they may also modify the obesogenic effects of environmental factors (Tyrrell, Wood et al. 2017). Since many genes contribute to obesity susceptibility, polygenic risk scores can be used to combine information across multiple single nucleotide polymorphisms (SNPs), and thus capture an individual’s overall level of genetic predisposition towards obesity. By examining whether they modify the effect of an environmental exposure, they can help to identify individuals that are more susceptible to the detrimental effects of that exposure, or help to show that the exposure is similarly detrimental across different individuals.
Here, we use a large, prospective dataset to examine associations of several air pollution measures with measures of obesity, using traditional measures (e.g., BMI and waist-hip ratio (WHR)), as well as body fat percentage, while also controlling for noise and other relevant covariates. We also evaluated a polygenic risk score for obesity as a modifier of these relationships. Since the interpretation of epidemiological investigations of environmental exposures that are measured concurrently with (or after) the outcome may be limited by concerns about temporality, we estimated associations of air pollution measured in 2010 with obesity measured at enrollment (2006–2010), and also with obesity measured at follow-up (2012–2013), controlling for adiposity values at enrollment.
Methods
The UK Biobank is a prospective longitudinal cohort that enrolled approximately 500,000 participants at ages 40 to 69 years across the United Kingdom from 2006–2010. A detailed description of enrollment procedures has been previously published (Sudlow, Gallacher et al. 2015). At enrollment, participants answered questions about demographic and baseline characteristics via a touch-screen questionnaire and a computer-assisted interview. Between 2012 and 2013, approximately 20,000 participants returned for a follow-up visit that included repeat anthropometric measurements. The median time between baseline and follow-up visits was approximately 4.4 years, with minimum and maximum time between visits equaling 2.1 and 6.1 years. Ethical approval of the UK Biobank study was given by the North West Multicentre Research Ethics Committee, the National Information Governance Board for Health & Social Care, and the Community Health Index Advisory Group.
Air pollution values were estimated by the Small Area Health Statistics Unit as part of the BioSHaRE-EU Environmental Determinants of Health project. These values were calculated for the year 2010 using protocols from the European Study of Cohorts for Air Pollution Effects (ESCAPE) (Eeftens, Beelen et al. 2012, Beelen, Hoek et al. 2013). These estimated values for 2010 are a proxy for a measure of chronic, long-term exposure to air pollution. Briefly, annualized estimates of air pollution were derived from land use regression models that were created on a site-specific basis from monitors across Europe. These estimates represent annual averages of air pollution in 2010 for the reported residence at enrollment and include Nitrogen Oxides (NOx), Nitrogen Dioxide (NO2), Particulate Matter≤ 2.5 μm (PM2.5), Particulate Matter 2.5 – 10 μm (PM2.5-10), Particulate Matter ≤ 10 μm (PM10), and PM2.5 Absorbance (a measure of black carbon)(Eeftens, Beelen et al. 2012, Beelen, Hoek et al. 2013). The UK Biobank also provides estimates for other measures of the local environment. For this analysis, we included log inverse distance to nearest major road (1/meters), and log traffic intensity on nearest major road (vehicles per day). We also evaluated an annual average of 24-hour sound level of noise pollution as a potential confounder. Noise estimates were derived from a simplified version of the Common Noise Assessment Methods in European Union (CNOSSOS-EU) framework (Kephalopoulos, Paviotti et al. 2014), which uses land use characteristics including road networks and flows, land cover and meteorology, and the properties of noise propagation from diffraction and refraction, absorption, distance, and angles. These characteristics were used to derive an LDen, or a day-evening-night equivalent level, with a weighted Leq noise level measured over a 24-hour period with a 10 decibel penalty added to the levels between 23:00 and 07:00.
Body weight, anthropometry, and body composition measures were assessed at enrollment and at the first follow-up visit. Height, weight, hip circumference, and waist circumference were measured by an examiner. Body fat percentage was measured with whole-body bio-impedance measures using the Tanita BC418MA body composition analyzer, at enrollment and at follow-up. We used Center for Disease Control (CDC) cutoffs for BMI-based obesity, where a BMI of 30 or above is considered obese, and we used the World Health Organization’s standards for abdominal obesity, where a 0.90 waist-hip-ratio (WHR) and above is abdominally obese for men, and 0.85 and above is abdominally obese for women. We used conventional estimates for body fat percentage cutoffs for obesity, where >25% is obese for men, and >35% is obese for women.
All UK Biobank participants were genotyped as previously described (Bycroft, Freeman et al. 2018). Polygenic risk scores for obesity were calculated based on 96 out of the 97 SNPs identified by Locke et al., that were also available in the UK Biobank (Locke, Kahali et al. 2015). One SNP (rs12016871) was not available in the UK Biobank. All SNPs passed QC criteria of minor allele frequency, Hardy-Weinberg equilibrium, and imputation accuracy. Genotypes at these SNPs were extracted, and for each individual, a polygenic risk score was calculated by taking the sum of BMI-increasing alleles across all 96 SNPs, weighting each one by the estimated effect size from Locke et al., and dividing by the number of non-missing genotypes for that individual.
Statistical Analysis
We first evaluated whether the demographics of the study group at follow-up were different from the group at enrollment with a series of t-tests and chi-squared ANOVA tests. We evaluated them based on their age, average Townsend Deprivation Index score, race/ethnicity, education, annual average air pollution levels for 2010, and body composition measures at enrollment. We also calculated the percentage of people that gained or lost weight between enrollment and follow-up, and calculated the mean change in weight by enrollment weight categories.
We used linear regression to evaluate the relationships between air pollution and body composition at enrollment and at follow-up. Air pollution measures for NOx, NO2, PM2.5, PM2.5absorbance, PM2.5–10, and PM10 were scaled so that a one-unit increase in the measure represented a one inter-quartile-range (IQR) increase. For inverse-distance-to-road and traffic intensity, we used logs of the raw values after examining distributions. Body composition measures included inverse-normalized BMI, inverse-normalized waist-hip-ratio (WHR), and inverse-normalized body fat percentage (BF%), at enrollment and at follow-up. In analyses of associations at follow-up, we additionally controlled for the relevant measure at enrollment (e.g., we controlled for BMI at enrollment when examining associations with BMI at follow-up). In sensitivity analyses, we controlled for BMI in the models of associations between air pollution and WHR. To ease interpretability, we also report effect estimates of 10 unit increases in PM2.5, PM2.5–10, and PM10 with one-unit increases in BMI, WHR, and body fat percentage. Since WHR is expressed as a ratio, we multiplied WHR by 100 in these sensitivity analyses. Since any observed differences between associations at enrollment and associations at follow-up may be due to the differing populations at enrollment and follow-up, we additionally report associations with adiposity at enrollment for the entire population (N=473,020 for NOx and 440,193 for PM), against associations with adiposity at enrollment for only the subset of the population that returned for follow-up (N=19,518).
We used logistic regression to examine associations with BMI-based obesity, WHR-based abdominal obesity, and body-fat based obesity at enrollment and at follow-up. To evaluate associations with a measure that approximates incident obesity, we restricted the follow-up study participants to only those who did not qualify as obese for that particular measure at enrollment (e.g., models of “incident” abdominal obesity were restricted to those who did not qualify as being abdominally obese at enrollment based on their WHR).
To evaluate interactions between air pollution and the polygenic risk score for BMI, we restricted the population to those who identified as British/Irish white. We then evaluated the dose-response relationship between polygenic risk score and the obesity measures using linear regression. We used the chi-squared LRT p-values to assess the interactions between the continuous air pollution variables and the continuous polygenic risk score variable. Since the polygenic risk score variable is a measure of risk for BMI, we evaluated this risk score as a modifier of air pollution only in relation to BMI. We set our alpha for interaction at 0.05. In order to better characterize the scope of interactions, we additionally show the associations between pollutants and BMI by quartile of polygenic risk score. We also calculated average air pollution values by weight change status and genetic polymorphisms for the 5 SNPs with the largest effect size on BMI, based on the original discovery study (Locke, Kahali et al. 2015).
All models were a priori adjusted for sex, white race/ethnicity, age, enrollment centre, education (binary for college or higher), genetic risk score for BMI (Locke, Kahali et al. 2015), and the Townsend Deprivation Index, a community measure of relative deprivation (Townsend 1987). We also controlled for noise, and we present models that did not control for noise separately, since we were partially interested in investigating whether noise is a confounder of the air pollution-obesity relationship.
Results
The complete case participants in this study at enrollment were middle aged (mean age ~57, sd = 8.09), with low Townsend Deprivation Index Scores (mean= −1.35), and moderate education (32% with college degree or higher) (Table 1). Study participants were mostly female (54%) and British or Irish White (95%). Air pollution levels were moderate. For almost all characteristics, the participants that returned for follow-up were significantly different than those that did not.
Table 1.
Characteristics of the Study Participants
| Enrollment N=473,026 | Follow-Up N=19,518 | P for difference | |
|---|---|---|---|
| Age (mean (sd)) | 56.55 (8.09) | 57.17 (7.40) | <0.01 |
| Townsend Deprivation Index (mean (sd)) | −1.35 (3.07) | −2.05 (2.68) | <0.01 |
| Male | 216,154 (45.69%) | 9,521 (48.78%) | <0.01 |
| Race/Ethnicity | |||
| British or Irish White | 448, 320 (94.78)% | 19,096 (97.84%) | <0.01 |
| Education | |||
| College Graduate or Higher | 152,586 (32.26%) | 8,513 (43.62%) | <0.01 |
| Air Pollution in 2010 (mean, IQR) | |||
| NOX | 44.00 (16.35) | 43.22 (16.16) | <0.01 |
| NO2 | 26.63 (9.74) | 26.06 (8.85) | <0.01 |
| PM2.5 | 9.99 (1.28) | 9.97 (1.34) | 0.07 |
| PM2.5absorbance | 1.18 (0.30) | 1.17 (0.24) | <0.01 |
| PM2.5–10 | 6.43 (0.79) | 6.34 (0.60) | <0.01 |
| PM10 | 16.23 (1.76) | 15.94 (1.73) | <0.01 |
| Distance to Road, m (median, sd) | 378.79 (62.94) | 358.42 (70.75) | <0.01 |
| Traffic intensity, VPD (median, sd) | 17,053 (21,049) | 15,763 (21,961) | <0.01 |
| Anthropometric and body composition Measures at Enrollment | |||
| BMI (mean (sd)) | 27.42 (4.78) | 26.89 (4.52) | <0.01 |
| Waist-Hip-Ratio (mean (sd)) | 0.87 (0.09) | 0.86 (0.09) | <0.01 |
| Body Fat % (mean (sd)) | 31.41 (8.53) | 30.54 (8.44) | <0.01 |
| Obesity (BMI) (N %) | 115,041 (24.32%) | 3,929 (20.13%) | <0.01 |
| Abdominal Obesity (WHR) (N %) | 234,240 (49.52%) | 8,851 (45.35%) | <0.01 |
| Body Fat % Obesity (N %) | 262,778 (56.38%) | 10,073 (52.43%) | <0.01 |
| BMI Genetic Risk Score | 0.024 (0.002) | 0.024 (0.002) | <0.01 |
Enrollment numbers include complete cases with values for waist hip ratio, age, NO2 air pollution values, the BMI risk score, education, Townsend Deprivation Index, race/ethnicity, and assessment centre. A smaller number of these participants have available PM values (n=440,193 at enrollment, and 19,514 at follow-up); PM values were not reported for participants in some regions due to unreliability of the air pollution estimates. All air pollution estimates are annual averages from 2010. P for difference indicates the difference between those who returned for follow-up and those who did not return for follow-up (t-tests for continuous variables, chi-square tests for categorical). Traffic intensity in vpd is vehicles per day, and distance to road is meters.
Approximately 45% of participants that returned for follow-up had a lower BMI at follow-up than at enrollment, and 55% of participants had either the same or higher BMIs at follow-up. Across BMI categories (underweight, normal, overweight, obese), participants generally gained weight, although participants in the obese category were more likely to lose weight (Supplementary Table 3).
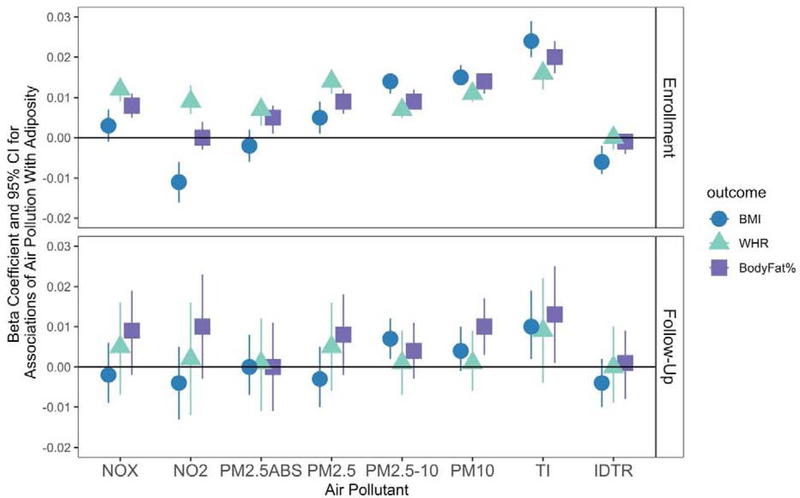
At enrollment, we observed associations between several measures of air pollution at enrollment and the Waist-Hip-Ratio (WHR), BMI, and body fat percentage (BF%) (Figure 1). PM2.5, PM2.5–10, PM10, and traffic intensity on nearest major road were all positively associated with BMI, WHR, and BF% at enrollment. NOX, NO2 and PM2.5 absorbance were positively associated with WHR, but not BMI, at enrollment. In fact, NO2 was unexpectedly negatively associated with BMI at enrollment, as was inverse distance to road. At follow-up, these estimates were generally attenuated towards the null, although PM2.5–10, PM10, and traffic intensity remained associated with BMI, but not WHR or BF%. There was minimal confounding by noise, as estimates were generally negligibly altered (Supplementary Table 1a and Supplementary Table 1b). The only significant associations that changed after controlling for noise were for NOx and BMI at enrollment, and PM10 and BMI at follow-up. The NOx/BMI association was attenuated towards the null after controlling for noise, while the effect estimate for the PM10/ BMI relationship stayed the same, but the confidence intervals widened after accounting for noise. In general, effect sizes were relatively modest. For instance, for a one IQR increase in PM2.5 (1.28 ug/L), inverse-normalized BMI at enrollment increased by 0.005, inverse-normalized WHR at enrollment increased by 0.014, and inverse-normalized body fat percentage at enrollment increased by 0.009. To facilitate interpretation, we also considered 10 unit increases in particulate matter variables with the untransformed outcomes. Thus, these translate such that a 10 unit increase in PM2.5 was associated with a 0.30 unit increase in BMI at enrolment (e.g. from 21 to 21.30), a 0.95 increase in WHR (e.g., an increase from 80.00 to 80.95 for the WHR x 100 variable), and a 0.79 increase in body fat percentage (e.g., an increase from 24.00% to 24.79% body fat) (Supplementary Figure 1). However, the range of PM2.5 in the study participants did not span 10 ppb (the min and max were 5.57 and 12.82); thus the estimates for 10 unit increases extrapolate beyond the observed data.
Figure 1. Associations (ß, 95% CI) of Air Pollution With Continuous Measures of Adiposity at enrollment and at follow-up.
N=473,026 at enrollment for NO and NOx models, N=440,193 for PM models. N=19,518 at follow-up. IDTR = log Inverse distance to road; TI = log Traffic Intensity. Air pollutants are scaled such that a one unit increase represents a one inter-quartile range increase, although inverse distance to road and traffic density are logged. All models controlled for race/ethnicity, age, assessment centre, Townsend Deprivation Index, education, genetic risk score for BMI, noise, and sex. BMI, WHR, and Body Fat percentage are modeled as inverse normalized variables. Associations with measures at follow-up additionally controlled for the relevant body composition measure at enrollment.
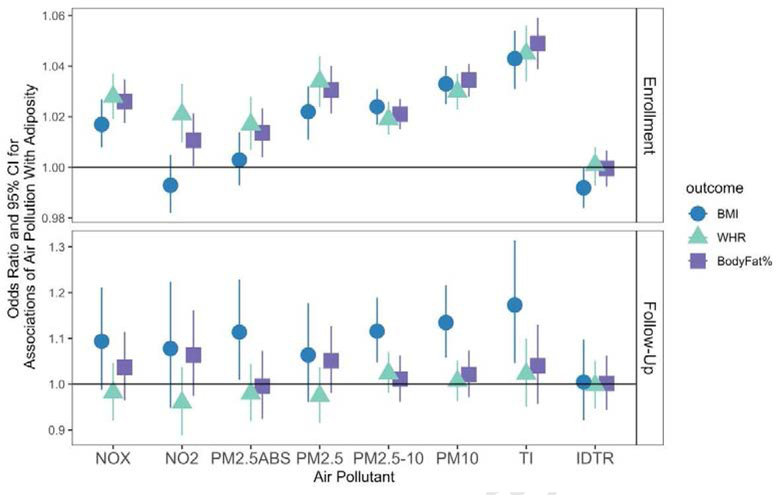
We observed somewhat similar patterns of associations for binary measures of adiposity (Figure 2). For instance, NOX, PM2.5, PM2.5–10, PM10, and traffic intensity were all associated with BMI-based obesity, abdominal obesity, and BF% obesity, at enrollment. NO2 and PM2.5 absorbance were associated with abdominal obesity and body fat obesity, but not BMI obesity, at enrollment. For incident obesity, we observed associations with BMI-obesity for PM2.5 absorbance, PM2.5–10, PM10, and traffic intensity, but not associations with abdominal obesity or BF% obesity. Associations were small to moderate, with significant ORs ranging from 1.011 (NO2 & BF% obesity) to 1.173 (traffic intensity and incident BMI-obesity). Again, associations were not confounded by noise (Supplementary Tables 2a and 2b).
Figure 2. Associations (Odds Ratios & 95% CIs) of Air Pollution With Binary Measures of Adiposity at Enrollment and Follow-Up.
At enrollment, N=473,026 for NOx and NO2 models, and N = 440,193 for PM models. At follow-up, N=19,518 and N= 19,514. BMI indicates BMI-based obesity; WHR indicates WHR-based obesity; and BodyFat% indicates body fat percentage-based obesity. IDTR = log Inverse distance to road; TI = log Traffic Intensity; these were modeled as log variables. Other air pollutants are scaled such that a one unit increase represents a one inter-quartile range increase. Models control for race/ethnicity, age, assessment centre, Townsend Deprivation Index, education, genetic risk score for BMI, noise, and sex. Associations with measures at follow-up are incident associations and exclude participants with the condition at enrollment. Note that the scales are different for enrollment and follow-up, for visualization purposes.
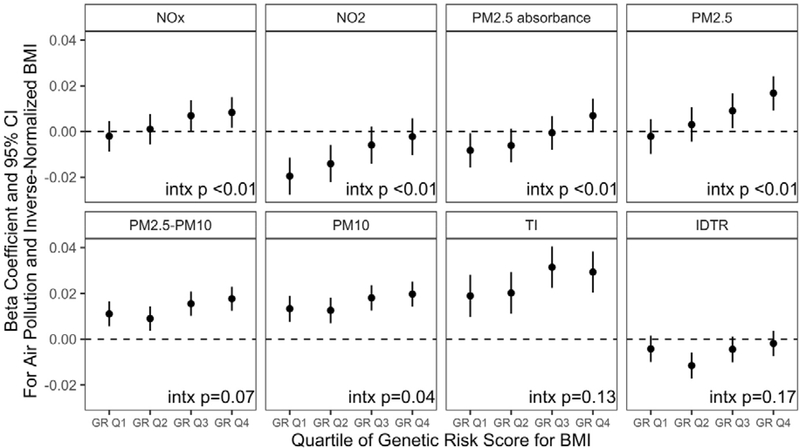
Polygenic risk score for BMI was associated with all measures of body weight in our study participants, at both enrollment and at follow-up (Supplementary Figure 3). For instance, at enrollment, individuals in the fourth quartile of the polygenic risk score had a BMI that was, on average,1.6 units higher than individuals in the first quartile. We observed a dose-response relationship of the polygenic risk score with BMI, at both enrollment and follow-up, such that p-trends were all <0.05. Associations for the polygenic risk score were most precise (e.g., confidence intervals were smallest) for BMI at enrollment, likely because the polygenic risk score is based on BMI. We also observed modification of the air pollution-BMI association at enrollment by GRS for BMI, for NOX, NO2, PM2.5 Absorbance, PM2.5, and PM10 (Figure 3). Associations between air pollution and BMI at enrollment were stronger among individuals with a higher genetic risk score for BMI. For NOx, PM2.5, and PM2.5 absorbance, effect sizes for air pollution on BMI were largest among those in the highest quartiles of genetic risk score for BMI. However, associations were actually negative for NO2 and PM2.5 absorbance among those in the lowest quartiles for genetic risk score for BMI. We did not observe any modification by genetic risk score for associations with adiposity at follow-up.
Figure 3. Associations of Air Pollution with BMI at Enrollment, by Genetic Risk Score for BMI.
At enrollment, N = 445,257 for NOx and NO2 models, N = 440,193 for PM models. Models are restricted to those of British/Irish white race/ethnicity and control for sex, age, enrollment centre, education, noise, and the Townsend Deprivation Index. Interaction p values represent the likelihood ratio test p-value for models of interactions between the air pollutant and the continuous genetic risk score for BMI.
In sensitivity analyses, controlling for BMI in models examining WHR did not materially change the findings, suggesting associations with WHR at enrollment are independent of BMI. When we examined associations of air pollution with obesity measures at enrollment among the full study participant group, compared to associations at enrollment among only those participants who subsequently returned for follow-up, we did not observe materially different associations (Supplementary Figure 2). Generally, the confidence intervals for the associations in the larger sample were narrower but overlapped to a large degree with the confidence intervals for the smaller sample.
Discussion
In this large prospective cohort with low to moderate air pollution levels, we observed associations of several air pollution components with several measures of adiposity. Traffic intensity, PM2.5–10, and PM10 were associated with the most measures of adiposity, including all of the continuous measures at enrollment and follow-up (with the exception of WHR at follow-up), all three binary measures of adiposity at enrollment, and BMI-based obesity at follow-up. We additionally observed modification by genetic susceptibility to BMI, where associations between some air pollution components and adiposity at enrollment were strongest among those with the greatest BMI genetic susceptibility scores.
Reported associations in the prior literature are mixed, with low consistency across pollutants and susceptible populations. Studies of adults in western countries have reported associations between PM2.5 and BMI (although this association in this study appeared to be driven primarily by walkability) (James, Kioumo urtzoglou et al. 2017), and associations with distance to roadway (but not PM2.5) and overall and abdominal adiposity (Li, Dorans et al. 2016). Another study of adults in the United States showed that although PM2.5 was associated with metabolic syndrome, it was not associated with abdominal obesity (Hazard Ratio = 1.00) (Wallwork, Colicino et al. 2017). Interestingly, another study of African American adult women showed no associations of PM2.5 or O3 with BMI change. They also reported an unexpectedly negative association with NO2, with the strongest associations among the leanest participants (White, Jerrett et al. 2016). We also observed an unexpectedly negative association of NO2 with BMI at enrollment, which appeared to be driven by associations among those with lower genetic susceptibility to obesity. However, we also observed a positive association for NO2 and WHR, and positive relationships for NO2 and abdominal obesity and BF%-obesity. These conflicting associations have also been observed with cigarette smoking, where cigarette smoking is routinely associated with lower overall BMI but higher WHR (Barrett-Connor and Khaw 1989, Canoy, Wareham et al. 2005, Chiolero, Faeh et al. 2008). Cigarette smoke contains high levels of nitrogen dioxides (Bokhoven and Niessen 1961, Norman and Keith 1965), which may imply that our findings are in line with prior literature on cigarette smoking and adiposity.
Recent studies in China have reported associations of long-term PM1, PM2.5, PM10, and NO2 with overweight/obesity (Yang, Guo et al. 2019), associations between PM2.5 and gestational weight gain (Liao, Yu et al. 2018), and associations of PM10, NO2, and O3, with obesity, but only among women (Li, Qian et al. 2015). Yang et al 2019 also showed that associations for air pollutants and cardiometabolic risk factors were stronger among those with a family history of CVD, which may support our findings of an interaction with genetic susceptibility to high BMI. Epidemiological studies in children report similar findings. In Southern California children, traffic-related air pollution and NOx has been associated with obesity and/or childhood BMI trajectories (Jerrett, McConnell et al. 2014, Kim, Alderete et al. 2018), while in Barcelona, PM10 was associated with overweight/obese, with some evidence of non-linear relationships reported for NO2, PM2.5, and elemental carbon, with overweight/obese, but generally no consistent linear associations reported for BMI (de Bont, Casas et al. 2019). Another study in the Netherlands reported associations between NO2 and overweight/obesity in children, but no associations for PM2.5 or PM10 (Bloemsma, Wijga et al. 2019), and another in Sweden showed no association between NOx exposures during pregnancy and overweight/obesity at age 4 (Frondelius, Oudin et al. 2018). Virtually no studies, to our knowledge, have been reported on these air pollutants in adults and body fat percentage.
Some of these conflicting findings of significance may be due to sample size. For example, at enrollment, we were exceptionally well-powered with an N of 473,026 to detect very small effect sizes, and at follow-up, we were also well-powered to detect associations with an N of 19,518. In Li et al 2016, they report no association between a 1.5 ug/m3 increase in PM2.5 with BMI (effect size 0.08, 95% CI −0.14, 0.30). In our data, when we estimate a comparably scaled exposure and outcome, we find an effect size of 0.05, 95% CI 0.03, 0.08. The effect size in our data is actually smaller than that reported in Li, although ours reaches significance and theirs does not, possibly due to sample size. Even within our own study, we report no significant associations with BF% obesity at follow-up (when we had approximately 19,000 participants), despite effect sizes for BF% obesity at follow-up being higher than effect sizes for BF% obesity at enrollment (where the n was >450,000). However, these were not significant at follow-up, likely due to the loss of power from the decreased sample size.
In other analyses of cardiometabolic risk factors, a recent meta-analysis reported associations between NO2 and PM10 with triglyceride levels (Gaio, Roquette et al. 2019), while another study showed that near-roadway air pollution exposures were associated with altered fatty acid oxidation in young adults (Chen, Newgard et al. 2019). Air pollution has also been associated with fasting blood glucose (Peng, Bind et al. 2016) and insulin responses (Toledo-Corral, Alderete et al. 2018, Kim, Chen et al. 2019). In mouse models, researchers have shown that in mice fed a high-fat diet, ambient PM2.5 exposure resulted in exaggerated insulin resistance and visceral inflammation and adiposity (Sun, Yue et al. 2009), and the same research group has shown that PM2.5 upregulates mouse genes involved in adipocyte differentiation, lipid-droplet formation, lipogenesis, and lipolysis in white adipose tissue (Mendez, Zheng et al. 2013).
In general, we found that associations were consistent across continuous measures of adiposity, with the exception of NO2, which we discussed earlier. If these findings are generalizable, then BMI is likely a good proxy for WHR and BF% in other studies. However, this may not be true when examining associations with NO2 or cigarette smoking, where WHR may be more appropriate. A few minor differences were observed, which may or may not be clinically meaningful. For instance, at follow-up, continuous measures of BF% seemed to be the most sensitive marker for air pollution-dependent obesity measures. Since these measures controlled for the relevant obesity measure at enrollment, this pattern may reflect a slight tendency for BF% to change in response to air pollution more rapidly than the other metrics. However, the binary measures did not reflect a similar pattern, and at follow-up, only BMI-based obesity was associated with any pollutants. This may be due to the relative utility of the cutoffs for BMI and BF% obesity measures. BMI obesity cutoffs were developed by insurance companies, based on associations with mortality (reviewed in (Komaroff 2016)), and have since withstood the scrutiny of time. However, the BF% cutoffs are a metric borne from less evidence-based research (Ho-Pham, Campbell et al. 2011) and may be less clinically meaningful than BMI. Since the WHR associations were generally null for both the continuous and binary measures at follow-up, this may in part reflect reduced power due to a smaller sample size at enrollment. We did observe strong associations for air pollution and WHR at enrollment, which may be more reflective of a chronic response to air pollution.
Several additional biological mechanisms may underlie the relationship between air pollution and obesity. In recent metabolomic, epigenetic, and toxicological studies of air pollution, leukotriene, valine, fatty acid oxidation, and oxidative stress mechanisms have been implicated (Gruzieva, Xu et al. 2016) (Panni, Mehta et al. 2016, Plusquin, Guida et al. 2017, Liang, Moutinho et al. 2018) (Xu, Yavar et al. 2010). Leukotriene has been associated with obesity and insulin resistance (Martínez-Clemente, Clària et al. 2011, Spite, Hellmann et al. 2011, Bäck, Avignon et al. 2014, Ying, Wollam et al. 2017) and fatty acid oxidation plays an important role in the development of adipose tissue (Kelley, Goodpaster et al. 1999, den Besten, van Eunen et al. 2013, Simopoulos 2016), suggesting that these factors may be mediators in the air pollution-obesity relationship.
Unfortunately, little research has been done on gene-environment interactions for air pollution and obesity. Investigations of genetic modification of air pollution on cardiovascular risk have been reported, with some studies suggesting a role for oxidative stress genes & inflammation (Hee, Adar et al. 2010), metals processing (Bind, Coull et al. 2014), and microRNA processing (Wilker, Preis et al. 2015) (reviewed in (Ward-Caviness 2019)). These studies have predominantly used candidate genes, with a few using GWAS-identified SNPs, to evaluate interactions. Another study of cardiometabolic risk factors reported interactions with an air pollution susceptibility score (Bind, Coull et al. 2014), although in general, genetic susceptibility scores are relatively novel and have yet to be widely adopted,. We used a genetic susceptibility score for obesity, which has also been shown to interact with several lifestyle factors including dietary fat and total energy intake (Celis-Morales, Lyall et al. 2017), and with sedentary behaviors and physical activity (Celis-Morales, Lyall et al. 2019). Many of the SNPs in this BMI genetic risk score may operate through pathways including synaptic plasticity, glutamate receptor activity, insulin action, lipid metabolism and adipogenesis, and may thus partly affect satiety mechanisms and eating behavior (Locke, Kahali et al. 2015). Interestingly, a recent study showed that air pollution increases voluntary intake of highly palatable foods and increases the caloric efficiency of high fat foods in rats (da Silveira, Di Domenico et al. 2018), and a novel epidemiological study showed that even after accounting for access to fast food, higher air pollution levels were associated with higher intake of trans-fats and fast food (Chen, Herting et al. 2018). If the genetic risk score is highly predictive of seeking out a high-fat diet, the observed interaction may be partially capturing this phenomenon. Overall, the prior literature, together with the current findings, suggest genes and air pollution may interact in a complex manner, on multiple different pathways, to affect adiposity.
We did observe some differences by enrollment or follow-up status. For instance, no associations of air pollution with WHR were significant at follow-up, despite being consistently associated with all pollutants and pollutant sources except for distance to road, at enrollment. This may in part reflect a survival effect, since we used a chronic measure of air pollution and controlled for WHR at enrollment. Participants who were susceptible to the effects of air pollution on adiposity may have been already obese/overweight at enrollment, so the associations at follow-up would only be amongst those who were less susceptible to these effects. However, we did still observe associations with BMI and BF. Therefore, it is also possible that air pollution acts more immediately on BMI and body fat, and may take longer to induce changes in abdominal adiposity.
Our study had several strengths and limitations. The strengths include the exceptionally large sample sizes at enrollment and follow-up, the well-validated air pollution metrics, examiner-measured BMI & WHR, impedance-measures of body fat percentage, and a previously validated genetic risk score for BMI. We were able to detect relatively small effect sizes at enrollment and follow-up in our population of nearly half a million, and for the first time, we also report associations with body fat percentage at enrolment & follow-up. Limitations are predominantly centered around the availability of air pollution measures, which were only available from ESCAPE in 2010 for all of the air pollutants included in this study. However, NO2 estimates for 2005, 2006, and 2007 (but not 2008, 2009, or 2010, and not the other air pollutants) were available for participants using a different method, and the correlation coefficients for these years for NO2 ranged from 0.98 to 0.99. This suggests that a given annual average is likely representative of long-term chronic air pollution exposures. Air pollution metrics in 2010 did precede measures at follow-up, which were taken in 2012–2013. Additionally, air pollution and obesity measures may partially reflect residual confounding by other obesogenic factors, such as stress, neighborhood walkability and green space, and residual error in household wealth or income. Finally, this is a cohort of predominantly Caucasian, middle-aged UK participants. The physiologic response to air pollutants may depend on a number of factors, including diet/nutrition, social stress/resiliency, occupation, and other metrics of SES that may be unique to this cohort. Thus, the extent to which our results can be generalized to other populations is not known. Furthermore, as the polygenic that we used was developed based on individuals of European descent, our results may not generalize to individuals of other ancestries.
Conclusions
Air pollution may be associated with markers of adiposity and weight, both at enrollment and follow-up. Associations for some pollutants may be stronger among those who are genetically susceptible to higher BMI.
Supplementary Material
Acknowledgments
Funding
This project was supported by funding from the National Heart, Lung, and Blood Institute R01-HL136528, and by funding from the National Institute of Environmental Health Sciences K99ES028743 (MF).
Footnotes
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bäck M, Avignon A, Stanke-Labesque F, Boegner C, Attalin V, Leprieur E and Sultan A (2014). “Leukotriene production is increased in abdominal obesity.” PloS one 9(12): e104593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett-Connor E and Khaw K-T (1989). “Cigarette smoking and increased central adiposity.” Annals of internal medicine 111(10): 783–787. [DOI] [PubMed] [Google Scholar]
- Beelen R, Hoek G, Vienneau D, Eeftens M, Dimakopoulou K, Pedeli X, Tsai M-Y, Künzli N, Schikowski T and Marcon A (2013). “Development of NO 2 and NO x land use regression models for estimating air pollution exposure in 36 study areas in Europe–the ESCAPE project.” Atmospheric Environment 72: 10–23. [Google Scholar]
- Bind M-A, Coull B, Suh H, Wright R, Baccarelli A, Vokonas P and Schwartz J (2014). “A novel genetic score approach using instruments to investigate interactions between pathways and environment: application to air pollution.” PLoS One 9(4): e96000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemsma LD, Wijga AH, Klompmaker JO, Janssen NA, Smit HA, Koppelman GH, Brunekreef B, Lebret E, Hoek G and Gehring U (2019). “The associations of air pollution, traffic noise and green space with overweight throughout childhood: The PIAMA birth cohort study.” Environmental research 169: 348–356. [DOI] [PubMed] [Google Scholar]
- Bokhoven C and Niessen H (1961). “Amounts of oxides of nitrogen and carbon monoxide in cigarette smoke, with and without inhalation.” Nature 192(4801): 458–459. [DOI] [PubMed] [Google Scholar]
- Bolton JL, Smith SH, Huff NC, Gilmour MI, Foster WM, Auten RL and Bilbo SD (2012). “Prenatal air pollution exposure induces neuroinflammation and predisposes offspring to weight gain in adulthood in a sex-specific manner.” The FASEB Journal 26(11): 4743–4754. [DOI] [PubMed] [Google Scholar]
- Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, Motyer A, Vukcevic D, Delaneau O and O’Connell J (2018). “The UK Biobank resource with deep phenotyping and genomic data.” Nature 562(7726): 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canoy D, Wareham N, Luben R, Welch A, Bingham S, Day N and Khaw KT (2005). “Cigarette Smoking and Fat Distribution in 21, 828 British Men and Women: A Population-based Study.” Obesity research 13(8): 1466–1475. [DOI] [PubMed] [Google Scholar]
- Celis-Morales CA, Lyall DM, Gray SR, Steell L, Anderson J, Iliodromiti S, Welsh P, Guo Y, Petermann F, Mackay DF, Bailey MES, Pell JP, Gill JMR and Sattar N (2017). “Dietary fat and total energy intake modifies the association of genetic profile risk score on obesity: evidence from 48 170 UK Biobank participants.” International Journal Of Obesity 41: 1761. [DOI] [PubMed] [Google Scholar]
- Celis-Morales CA, Lyall DM, Petermann F, Anderson J, Ward J, Iliodromiti S, Mackay DF, Welsh P, Bailey ME and Pell J (2019). “Do physical activity, commuting mode, cardiorespiratory fitness and sedentary behaviours modify the genetic predisposition to higher BMI? Findings from a UK Biobank study.” International Journal of Obesity: 1. [DOI] [PubMed] [Google Scholar]
- Chen J-C and Schwartz J (2008). “Metabolic syndrome and inflammatory responses to long-term particulate air pollutants.” Environmental health perspectives 116(5): 612–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Herting MM, Chatzi L, Belcher BR, Alderete TL, McConnell R and Gilliland FD (2018). “Regional and traffic-related air pollutants are associated with higher consumption of fast food and trans fat among adolescents.” The American journal of clinical nutrition 109(1): 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Newgard CB, Kim JS, IIkayeva O, Alderete TL, Thomas DC, Berhane K, Breton C, Chatzi L and Bastain TM (2019). “Near-roadway air pollution exposure and altered fatty acid oxidation among adolescents and young adults–The interplay with obesity.” Environment international 130: 104935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiolero A, Faeh D, Paccaud F and Cornuz J (2008). “Consequences of smoking for body weight, body fat distribution, and insulin resistance.” The American Journal of Clinical Nutrition 87(4): 801–809. [DOI] [PubMed] [Google Scholar]
- Christensen JS., Raaschou-Nielsen O, Tjønneland A, Nordsborg RB, Jensen SS, Sørensen TI and Sørensen M (2015). “Long-term exposure to residential traffic noise and changes in body weight and waist circumference: a cohort study.” Environmental research 143: 154–161. [DOI] [PubMed] [Google Scholar]
- Chuang K-J, Chan C-C, Su T-C, Lee C-T and Tang C-S (2007). “The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults.” American journal of respiratory and critical care medicine 176(4): 370–376. [DOI] [PubMed] [Google Scholar]
- da Silveira CG, Di Domenico M, Hilário Nascimento Saldiva P and Ramos Rhoden C (2018). “Subchronic air pollution exposure increases highly palatable food intake, modulates caloric efficiency and induces lipoperoxidation.” Inhalation toxicology 30(9–10): 370–380. [DOI] [PubMed] [Google Scholar]
- de Bont J, Casas M, Barrera-Gómez J, Cirach M, Rivas I, Valvi D, Álvarez M, Dadvand P, Sunyer J and Vrijheid M (2019). “Ambient air pollution and overweight and obesity in schoolaged children in Barcelona, Spain.” Environment international 125: 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud D-J and Bakker BM (2013). “The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism.” Journal of lipid research 54(9): 2325–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeftens M, Beelen R, de Hoogh K, Bellander T, Cesaroni G, Cirach M, Declercq C, Dedele A, Dons E and de Nazelle A (2012). “Development of land use regression models for PM2. 5, PM2. 5 absorbance, PM10 and PMcoarse in 20 European study areas; results of the ESCAPE project.” Environmental science & technology 46(20): 11195–11205. [DOI] [PubMed] [Google Scholar]
- Fernández-Sánchez A, Madrigal-Santillán E, Bautista M, Esquivel-Soto J, Morales-González Á, Esquivel-Chirino C, Durante-Montiel I, Sánchez-Rivera G, Valadez-Vega C and Morales-González JA (2011). “Inflammation, oxidative stress, and obesity.” International journal of molecular sciences 12(5): 3117–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD and Ogden CL (2016). “Trends in obesity among adults in the United States, 2005 to 2014.” Jama 315(21): 2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foraster M, Eze IC, Vienneau D, Schaffner E, Jeong A, Héritier H, Rudzik F, Thiesse L, Pieren R and Brink M (2018). “Long-term exposure to transportation noise and its association with adiposity markers and development of obesity.” Environment international 121: 879–889. [DOI] [PubMed] [Google Scholar]
- Frondelius K, Oudin A and Malmqvist E (2018). “Traffic-Related Air Pollution and Child BMI— A Study of Prenatal Exposure to Nitrogen Oxides and Body Mass Index in Children at the Age of Four Years in Malmö, Sweden.” International journal of environmental research and public health 15(10): 2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryar CD, Carroll MD and Ogden CL (2016). “Prevalence of overweight, obesity, and extreme obesity among adults aged 20 and over: United States, 1960–1962 through 2013–2014.” National Center for Health Statistics Data, Health E-Stats 2016. [Google Scholar]
- Gaio V, Roquette R, Dias CM and Nunes B (2019). “Ambient air pollution and lipid profile: Systematic review and meta-analysis.” Environmental Pollution: 113036. [DOI] [PubMed] [Google Scholar]
- Gómez-Ambrosi J, Silva C, Galofré J, Escalada J, Santos S, Millán D, Vila N, Ibañez P, Gil M and Valentí V (2012). “Body mass index classification misses subjects with increased cardiometabolic risk factors related to elevated adiposity.” International journal of obesity 36(2): 286. [DOI] [PubMed] [Google Scholar]
- Gómez-Ambrosi J, Silva C, Galofré JC, Escalada J, Santos S, Gil MJ, Valentí V, Rotellar F, Ramírez B and Salvador J (2011). “Body adiposity and type 2 diabetes: increased risk with a high body fat percentage even having a normal BMI.” Obesity 19(7): 1439–1444. [DOI] [PubMed] [Google Scholar]
- Gruzieva O, Xu C-J, Breton CV, Annesi-Maesano I, Antó JM, Auffray C, Ballereau S, Bellander T, Bousquet J and Bustamante M (2016). “Epigenome-wide meta-analysis of methylation in children related to prenatal NO2 air pollution exposure.” Environmental health perspectives 125(1): 104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond RA and Levine R (2010). “The economic impact of obesity in the United States.” Diabetes, metabolic syndrome and obesity: targets and therapy 3: 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hee VCV, Adar SD, Szpiro AA, Barr RG, Roux AD, Bluemke DA, Sheppard L, Gill EA, Bahrami H, Wassel C, Sale MM, Siscovick DS, Rotter JI, Rich SS and Kaufman JD (2010). “Common Genetic Variation, Residential Proximity to Traffic Exposure, and Left Ventricular Mass: The Multi-Ethnic Study of Atherosclerosis.” Environmental Health Perspectives 118(7): 962–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel JJ and vom Saal FS (2009). “Role of nutrition and environmental endocrine disrupting chemicals during the perinatal period on the aetiology of obesity.” Molecular and cellular endocrinology 304(1–2): 90–96. [DOI] [PubMed] [Google Scholar]
- Ho-Pham LT, Campbell LV and Nguyen TV (2011). More on body fat cutoff points Mayo Clinic Proceedings, Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James P., Kioumourtzoglou M-A, Hart JE, Banay RF, Kloog I and Laden F (2017). “Interrelationships Between Walkability, Air Pollution, Greenness, and Body Mass Index.” Epidemiology (Cambridge, Mass.) 28(6): 780–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrett M, McConnell R, Wolch J, Chang R, Lam C, Dunton G, Gilliland F, Lurmann F, Islam T and Berhane K (2014). “Traffic-related air pollution and obesity formation in children: a longitudinal, multilevel analysis.” Environmental Health 13(1): 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keaney JF Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA and Benjamin EJ (2003). “Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study.” Arteriosclerosis, thrombosis, and vascular biology 23(3): 434–439. [DOI] [PubMed] [Google Scholar]
- Kelley DE, Goodpaster B, Wing RR and Simoneau J-A (1999). “Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss.” American Journal of Physiology-Endocrinology And Metabolism 277(6): E1130–E1141. [DOI] [PubMed] [Google Scholar]
- Kephalopoulos S, Paviotti M, Anfosso-Lédée F, Van Maercke D, Shilton S and Jones N (2014). “Advances in the development of common noise assessment methods in Europe: The CNOSSOS-EU framework for strategic environmental noise mapping.” Science of the Total Environment 482: 400–410. [DOI] [PubMed] [Google Scholar]
- Kim JS, Alderete TL, Chen Z, Lurmann F, Rappaport E, Habre R, Berhane K and Gilliland FD (2018). “Longitudinal associations of in utero and early life near-roadway air pollution with trajectories of childhood body mass index.” Environmental Health 17(1): 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Chen Z, Alderete TL, Toledo-Corral C, Lurmann F, Berhane K and Gilliland FD (2019). “Associations of air pollution, obesity and cardiometabolic health in young adults: The Meta-AIR study.” Environment International 133: 105180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komaroff M (2016). “For researchers on obesity: historical review of extra body weight definitions.” Journal of obesity 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Qian Z, Vaughn M, Boutwell B, Ward P, Lu T, Lin S, Zhao Y, Zeng X-W and Liu R-Q (2015). “Sex-specific difference of the association between ambient air pollution and the prevalence of obesity in Chinese adults from a high pollution range area: 33 communities Chinese health study.” Atmospheric Environment 117: 227–233. [Google Scholar]
- Li W, Dorans KS, Wilker EH, Rice MB, Schwartz J, Coull BA, Koutrakis P, Gold DR, Fox CS and Mittleman MA (2016). “Residential proximity to major roadways, fine particulate matter, and adiposity: The framingham heart study.” Obesity 24(12): 2593–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D, Moutinho JL, Golan R, Yu T, Ladva CN, Niedzwiecki M, Walker DI, Sarnat SE, Chang HH, Greenwald R, Jones DP, Russell AG and Sarnat JA (2018). “Use of high-resolution metabolomics for the identification of metabolic signals associated with traffic-related air pollution.” Environment International 120: 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J, Yu H, Xia W, Zhang B, Lu B, Cao Z, Liang S, Hu K, Xu S and Li Y (2018). “Exposure to ambient fine particulate matter during pregnancy and gestational weight gain.” Environment international 119: 407–412. [DOI] [PubMed] [Google Scholar]
- Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML and Yang J (2015). “Genetic studies of body mass index yield new insights for obesity biology.” Nature 518(7538): 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao G., Nachman RM, Sun Q, Zhang X, Koehler K, Chen Z, Hong X, Wang G, Caruso D, Zong G, Pearson C, Ji H, Biswal S, Zuckerman B, Wills-Karp M and Wang X (2017). “Individual and Joint Effects of Early-Life Ambient PM2.5 Exposure and Maternal Prepregnancy Obesity on Childhood Overweight or Obesity.” Environmental Health Perspectives 125(6): 067005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Clemente M, Clària J and Titos E (2011). “The 5-lipoxygenase/leukotriene pathway in obesity, insulin resistance, and fatty liver disease.” Current Opinion in Clinical Nutrition & Metabolic Care 14(4): 347–353. [DOI] [PubMed] [Google Scholar]
- McConnell R, Shen E, Gilliland FD, Jerrett M, Wolch J, Chang C-C, Lurmann F and Berhane K (2015). “A Longitudinal Cohort Study of Body Mass Index and Childhood Exposure to Secondhand Tobacco Smoke and Air Pollution: The Southern California Children’s Health Study.” Environmental Health Perspectives 123(4): 360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez R, Zheng Z, Fan Z, Rajagopalan S, Sun Q and Zhang K (2013). “Exposure to fine airborne particulate matter induces macrophage infiltration, unfolded protein response, and lipid deposition in white adipose tissue.” American journal of translational research 5(2): 224. [PMC free article] [PubMed] [Google Scholar]
- Norman V and Keith CH (1965). “Nitrogen oxides in tobacco smoke.” Nature 205(4974): 915–916. [Google Scholar]
- Ogden CL, Yanovski SZ, Carroll MD and Flegal KM (2007). “The epidemiology of obesity.” Gastroenterology 132(6): 2087–2102. [DOI] [PubMed] [Google Scholar]
- Panni T, Mehta AJ, Schwartz JD, Baccarelli AA, Just AC, Wolf K, Wahl S, Cyrys J, Kunze S and Strauch K (2016). “Genome-wide analysis of DNA methylation and fine particulate matter air pollution in three study populations: KORA F3, KORA F4, and the normative aging study.” Environmental health perspectives 124(7): 983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C, Bind M-AC, Colicino E, Kloog I, Byun H-M, Cantone L, Trevisi L, Zhong J, Brennan K and Dereix AE (2016). “Particulate air pollution and fasting blood glucose in nondiabetic individuals: Associations and epigenetic mediation in the Normative Aging Study, 2000–2011.” Environmental health perspectives 124(11): 1715–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips CM, Tierney AC, Perez-Martinez P, Defoort C, Blaak EE, Gjelstad IM, Lopez-Miranda J, Kiec-Klimczak M, Malczewska-Malec M and Drevon CA (2013). “Obesity and body fat classification in the metabolic syndrome: impact on cardiometabolic risk metabotype.” Obesity 21(1): E154–E161. [DOI] [PubMed] [Google Scholar]
- Plusquin M, Guida F, Polidoro S, Vermeulen R, Raaschou-Nielsen O, Campanella G, Hoek G, Kyrtopoulos SA, Georgiadis P, Naccarati A, Sacerdote C, Krogh V, Bas Bueno-de-Mesquita H, Monique Verschuren WM, Sayols-Baixeras S, Panni T, Peters A, Hebels DGAJ, Kleinjans J, Vineis P and Chadeau-Hyam M (2017). “DNA methylation and exposure to ambient air pollution in two prospective cohorts.” Environment International 108: 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyko A, Eriksson C, Lind T, Mitkovskaya N, Wallas A, Ögren M, Östenson C-G and Pershagen G (2017). “Long-term exposure to transportation noise in relation to development of obesity—a cohort study.” Environmental health perspectives 125(11): 117005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyko A, Eriksson C, Oftedal B, Hilding A, Östenson C-G, Krog NH, Julin B, Aasvang GM and Pershagen G (2015). “Exposure to traffic noise and markers of obesity.” Occup Environ Med 72(8): 594–601. [DOI] [PubMed] [Google Scholar]
- Risom L., Møller P and Loft S(2005). “Oxidative stress-induced DNA damage by particulate air pollution.” Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 592(1–2): 119–137. [DOI] [PubMed] [Google Scholar]
- Shah NR and Braverman ER (2012). “Measuring adiposity in patients: the utility of body mass index (BMI), percent body fat, and leptin.” PloS one 7(4): e33308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea J, King M, Yi Y, Gulliver W and Sun G (2012). “Body fat percentage is associated with cardiometabolic dysregulation in BMI-defined normal weight subjects.” Nutrition, Metabolism and Cardiovascular Diseases 22(9): 741–747. [DOI] [PubMed] [Google Scholar]
- Simopoulos A (2016). “An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity.” Nutrients 8(3): 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spite M, Hellmann J, Tang Y, Mathis SP, Kosuri M, Bhatnagar A, Jala VR and Haribabu B (2011). “Deficiency of the leukotriene B4 receptor, BLT-1, protects against systemic insulin resistance in diet-induced obesity.” The Journal of Immunology 187(4): 1942–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J and Landray M (2015). “UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age.” PLoS medicine 12(3): e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Yue P, Deiuliis JA, Lumeng CN, Kampfrath T, Mikolaj MB, Cai Y, Ostrowski MC, Lu B and Parthasarathy S (2009). “Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity.” Circulation 119(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Corral C, Alderete T, Habre R, Berhane K, Lurmann F, Weigensberg M, Goran M and Gilliland F (2018). “Effects of air pollution exposure on glucose metabolism in Los Angeles minority children.” Pediatric obesity 13(1): 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend P (1987). “Deprivation.” Journal of Social Policy 16(2): 125–146. [Google Scholar]
- Tyrrell J, Wood AR, Ames RM, Yaghootkar H, Beaumont RN, Jones SE, Tuke MA, Ruth KS, Freathy RM and Davey Smith G (2017). “Gene–obesogenic environment interactions in the UK Biobank study.” International journal of epidemiology 46(2): 559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallwork RS, Colicino E, Zhong J, Kloog I, Coull BA, Vokonas P, Schwartz JD and Baccarelli AA (2017). “Ambient fine particulate matter, outdoor temperature, and risk of metabolic syndrome.” American journal of epidemiology 185(1): 30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward-Caviness CK (2019). “A review of gene-by-air pollution interactions for cardiovascular disease, risk factors, and biomarkers.” Human genetics: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Zhang J, Li Z, Gow A, Chung KF, Hu M, Sun Z, Zeng L, Zhu T, Jia G, Li X, Duarte M and Tang X (2016). “Chronic exposure to air pollution particles increases the risk of obesity and metabolic syndrome: findings from a natural experiment in Beijing.” The FASEB Journal 30(6): 2115–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LF, Jerrett M, Yu J, Marshall JD, Rosenberg L and Coogan PF (2016). “Ambient air pollution and 16-year weight change in African-American women.” American journal of preventive medicine 51(4): e99–e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilker EH, Preis SR, Beiser AS, Wolf PA, Au R, Kloog I, Li W, Schwartz J, Koutrakis P and DeCarli C (2015). “Long-term exposure to fine particulate matter, residential proximity to major roads and measures of brain structure.” Stroke 46(5): 1161–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Yavar Z, Verdin M, Ying Z, Mihai G, Kampfrath T, Wang A, Zhong M, Lippmann M and Chen L-C (2010). “Effect of early particulate air pollution exposure on obesity in mice: role of p47phox.” Arteriosclerosis, thrombosis, and vascular biology 30(12): 2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B-Y., Guo Y, Markevych I, Qian Z, Bloom MS, Heinrich J, Dharmage SC, Rolling CA, Jordan SS, Komppula M, Leskinen A, Bowatte G, Li S, Chen G, Liu K-K, Zeng X-W, Hu L-W and Dong G-H (2019). “Association of Long-term Exposure to Ambient Air Pollutants With Risk Factors for Cardiovascular Disease in ChinaAir Pollutant Exposure and Cardiovascular Disease Risk Factors in ChinaAir Pollutant Exposure and Cardiovascular Disease Risk Factors in China.” JAMA Network Open 2(3): e190318–e190318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying W, Wollam J, Ofrecio JM, Bandyopadhyay G, El Ouarrat D, Lee YS, Li P, Osborn O and Olefsky JM (2017). “Adipose tissue B2 cells promote insulin resistance through leukotriene LTB4/LTB4R1 signaling.” The Journal of clinical investigation 127(3): 1019–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.