Abstract
Proteome of seminal plasma provides profound information related to the male reproductive health. This pilot study was conducted to characterize proteomic profile of seminal plasma from men with primary, or secondary infertility and compare it with proven fertile men. Study participants (n = 59) were recruited at the Cleveland Clinic and divided according to their fertility status: proven fertile (n = 39); primary infertility (n = 11) and secondary infertility (n = 9). Proteomic shotgun analysis revealed a total of 515 peptides common to primary infertility and control group; whereas 523 peptides were common to secondary infertility and control group. Bioinformatic analysis revealed dysregulation of biological processes such as cell secretion and vesicle mediated transport in primary infertility, whereas immune system response, regulation of proteolysis and iron homeostasis were dysregulated in secondary infertility. Western blot validation showed overexpression of ANXA2 and CDC42, and underexpression of SEMG2 proteins in primary infertility; and overexpression of ANXA2 and APP proteins in secondary infertility. This study elucidates the potential role of differentially expressed proteins in the seminal plasma as diagnostic biomarker for primary and secondary infertility. Furthermore, our results suggest maturation failure and immune reaction response as the main cause of infertility in men with primary and secondary infertility, respectively. Additional validation of the proteins involved in the above pathways is warranted.
Subject terms: Proteomics, Diagnostic markers
Introduction
Infertility globally affects 15% of couples and is now classified as a disease of the reproductive system by the World Health Organization (WHO)1. Based on the presence or absence of previous successful pregnancies, infertility can be divided as primary and secondary. Couples who were unable to become pregnant after at least 1 year of sexual intercourse without contraceptive methods are referred as primary infertility. On the other hand, couples who were able to get pregnant at least once, but not subsequently are referred as secondary infertility. Prevalence of primary infertility (1.5 to 2.6%) is reported to be lower than secondary infertility (7.2 to 18%)2. Approximately, 50% of all reported couple infertility cases can be attributed to the male factor3,4 though the reasons remain unknown. Basic semen analysis is one of the first steps in the evaluation of male infertility. This analysis provides both macroscopic (volume, pH, color, viscosity) and microscopic characteristics (sperm concentration, total motility, progressive motility, sperm morphology) of semen5. The semen analysis remains the cornerstone of male fertility evaluation. However, it does not provide a systematic explanation for the subcellular changes that occur in the spermatozoa of infertile men, which necessitates a more in-depth analysis and understanding at molecular level6,7.
Spermatozoa acquires fertilizing potential during their epididymal maturation phase before ejaculation8. The ejaculated semen contains both cellular (spermatozoa) and non-cellular (seminal plasma) components. The seminal plasma is composed of secretions from testis, epididymis, prostate, seminal vesicles and bulbo-urethral glands;9,10 it provides nourishment and protection to spermatozoa11,12. It also plays a crucial role in sperm maturation, capacitation, acrosome reaction and fertilization11,12. Composition of the seminal plasma protein and their interaction with sperm surface influence the fertilizing capacity of spermatozoa12.
In recent years, there is an increased number of reports on seminal plasma proteome to identify potential biomarkers for different pathologies and conditions related to infertility. This includes varicocele13–16, oxidative stress mediated male infertility17–20, nonobstructive azoospermia21–23, asthenozoospermia24,25, oligoasthenozoospermia26, secondary hypogonadism27 and prostate cancer19,28,29. Borrachina and collaborators performed a proteomic study in the seminal plasma of infertile patients with normozoospermia, azoospermia, asthenozoospermia and oligoasthenozoospermia and concluded that the current classification of infertile patients based on altered semen parameters resulted in a high heterogeneity in the seminal plasma proteomic profile30. Agarwal and collaborators17 performed proteomic analysis of seminal plasma of infertile men having high levels of seminal reactive oxygen species (ROS) and compared it with proven fertile men with normal ROS in semen. Utilizing proteomic and bioinformatic analysis, it has been suggested that membrane metallo-endopeptidase (MME) and family with sequence similarity 3 (FAM3D) along with ROS levels in the seminal plasma can serve as good markers for diagnosis of male infertility17. Seminal plasma proteomic study in idiopathic oligoasthenozoospermic men revealed differential expression of proteins such as glycosylated epidydimal secretory protein E1(NPC2), galectin-3-binding protein (M2BP) or lipocalin-1 which provides a basis for further investigations of mechanisms underlying oligoasthenozoospermia26. These studies provided important information related to mechanisms associated with male infertility, however did not provide any evidence on the seminal plasma proteomics based on the type of infertility.
The present study was conducted with the following objectives: 1) to profile the seminal plasma proteome of primary and secondary infertile men compared to men with proven fertility, 2) to identify the differentially expressed proteins (DEPs) that could serve as potential biomarkers for primary and secondary infertility.
Materials and Methods
Study subject’s selection
This pilot study (IRB #11–451) was approved by the Institutional Review Board (IRB) of Cleveland Clinic. All the subjects (27–52 years) enrolled in this study signed an informed written consent. Semen samples were obtained from 39 healthy male donors (control group) who had fathered a child in the past 2 years; 11 patients with primary infertility (primary infertility group) and 9 patients with secondary infertility (secondary infertility group). The individuals from the control group had normal semen parameters according to the WHO 2010 guidelines1. All the methods were performed in accordance with the relevant guidelines and regulations according to the declaration of Helsinki (https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/).
Inclusion and exclusion criteria
All subjects enrolled in this study were non-smokers and had never exposed to harmful radiations or environmental stress. Men with azoospermia, oligozoospermia and leukocytospermia were excluded from the study. Furthermore, men under the supportive medication, steroids or drugs were excluded from the study. Additionally, patients with systemic reproductive tract inflammation, genetic defects and sexually transmitted disease were also excluded.
Semen analysis
Semen samples were collected at the Andrology Laboratory by masturbation after sexual abstinence of least 2–7 days. Samples were allowed to liquefy completely for 20 minutes at 37 °C, and semen analysis was performed according to the WHO (2010) guidelines31 using a disposable Leja sperm counting chamber (Spectrum Technologies, Healdsburg, CA) to evaluate sperm count, motility and round cells. After routine semen analysis, the left-over samples were centrifuged for 7 minutes at 1000 × g. The clear seminal plasma was aspirated from the samples and stored at −80 °C for proteomic studies.
Sample preparation for proteomic analysis
The samples used for proteomic analysis were in compliance with the Minimum Information about a Proteomics Experiment (MIAPE) guidelines of the Human Proteome Organization’s Proteomics Standards Initiative (HUPO-PSI) for reporting proteomics studies32. Seminal plasma samples were thawed at room temperature and centrifuged at 3000 × g for 30 minutes to remove any contamination with spermatozoa or other cellular debris. The samples were mixed (1:1 ratio) with the proteinase inhibitor cocktail (Roche, Indianapolis, IN) prepared in phosphate buffer saline (PBS) to prevent proteolysis during sample handling. The protein concentration was determined using a commercial kit, bicinchoninic acid (BCA) kit (Thermo, Rockford, IL), following the manufacturer instructions.
Pooled samples from control (n = 3); primary infertility (n = 3) and secondary infertility (n = 3) were used for proteomic analysis. Equal concentration of proteins from each individual sample was used to normalize the protein concentration in each group. Sample normalization was done by pooling the samples to overcome the biological variation33,34. The samples were mixed with SDS-PAGE buffer and subjected to 1D-PAGE in triplicate runs to overcome the technical variation. After electrophoresis, each gel was cut into 6 pieces, digested with 5 μL trypsin (10 ng/μL), 50 mM ammonium bicarbonate, and incubated overnight at room temperature. Prior to in-gel digestion, the samples (cut lanes) were alkylated with iodoacetamide and reduced with dithiothreitol. The peptides from the digested gel were extracted in two aliquots of 30 μL acetonitrile (10%) with formic acid (5%). The two aliquots were pooled together and evaporated to <10 μL and then diluted with 1% acetic acid to make up a final volume to 30 μL.
Liquid chromatography-tandem mass spectrometry analysis (LC-MS/MS)
Proteomic profiling of seminal plasma was carried out using a Finnigan LTQ linear ion trap mass spectrometer LC-MS/MS system. The peptides were fractionated by injecting 5 μL into a high-performance liquid chromatography (HPLC) column (Phenomenex Jupiter C18 reversed-phase capillary chromatography column). Fractions containing the peptides were eluted in acetonitrile/0.1% formic acid at a flow rate of 0.25 μL/min and were introduced into the source of the mass spectrometer on-line. The micro-electrospray ion source was operated at 2.5 kV. A full spectral scan was performed by utilizing the data dependent multitask ability of the instrument to determine peptide molecular weights and amino acid sequence of the peptides35.
Database searching and protein identification
Tandem mass spectra generated by LC-MS/MS system were retrieved using Proteome Discoverer version 1.4.1.288(https://www.thermofisher.com/us/en/home/industrial/mass-spectrometry/liquid-chromatography-mass-spectrometry-lc-ms/lc-ms-software/multi-omics-data-analysis/proteome-discoverer-software.html). Mascot (Matrix Science, London, UK; version 2.3.02), Sequest (Thermo Fisher Scientific, San Jose, CA, USA; version 1.4.0.288) and X! Tandem (The GPM, thegpm.org; version CYCLONE (2010.12.01.1) search was performed on all the MS/MS raw files. The search was limited to the human reference sequences database (http://www.hprd.org/) assuming the digestion enzyme trypsin. The mass tolerance for parent ion was set to 10 parts per million (ppm) and for fragment ion 1.0 Da. The search results were uploaded into the Scaffold (version 4.0.6.1; Proteome Software Inc., Portland, OR) program as previously described36. Protein probabilities were assigned by the Protein Prophet (Systems Biology, Seattle, WA) algorithm. Annotation of proteins was performed using Gene Ontology (GO) terms from National Center for Biotechnology Information (NCBI).
Quantitative proteomics
The relative quantification of the proteins was performed by comparing the number of spectra, termed spectral counts (SpCs) in control vs primary infertility group and control vs. secondary infertility group. To achieve the false detection rate (FDR) < 1%, protein identification criteria was established at >99% probability as explained in our previous study37. The abundance of the proteins was determined by matching the SpCs and classified as high (H), medium (M), low (L), or very low (VL). To overcome the sample-to-sample variation, normalization of spectral counts was done using the normalized spectral abundance factor (NSAF). In general, longer proteins have more peptide identifications than shorter proteins. NSAF ratio determines the actual expression of the protein in the samples, proteins with ratio <1 and >1 are considered underexpressed and overexpressed, respectively. Different constraints for fold-change cut-offs and significance tests (P value) based on the average SpCs from 3 replicate runs were applied to obtain the DEPs36. Appropriate filters were applied to minimize the errors due to the presence of low abundance proteins. Abundance and the expression of DEPs are based on the following criteria: (i) VL - SpC range, 1.7–7; NSAF ratio (≥2.5 for upregulated and ≤0.4 for downregulated proteins); and P ≤ 0.001, (ii) L - SpC range, 8–19; NSAF ratio (≥2.5 for upregulated and ≤0.4 for downregulated proteins); and P ≤ 0.01, (iii) M - SpC range, between 20 and 79; NSAF ratio (≥2.0 for upregulated and ≤0.5 for downregulated proteins); and P ≤ 0.05, (iv) H - SpC, >80; NSAF ratio (≥1.5 for upregulated and ≤0.67 for downregulated proteins); and P ≤ 0.05.
Bioinformatic analysis
DEPs identified in the control vs primary infertility group and control vs secondary infertility group were subjected to functional annotation and enrichment analysis using both, publicly available bioinformatic annotation tools and databases such as GO Term Finder, GO Term Mapper, UniProt, and Database for Annotation, Visualization and Integrated Discovery (DAVID) (http://david.niaid.nih.gov). Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) analysis was performed using the online tool to identify protein-protein interaction networks (https://string-db.org/). Proprietary software package Metacore (GeneGo Inc.) was also used to identify the upstream regulators involved in the enriched pathways.
Protein validation by western blot and total protein staining
The key proteins involved in reproductive functions and fertilization process were selected for validation. The functions of these proteins are well described in the literature. Based on function and role of proteins related to fertility potential, six DEPs from primary infertility and five DEPs from secondary infertility were selected for validation. The DEPs were validated by western blot (WB) in individual samples from the control group (n = 6) and primary (n = 6) or secondary (n = 6) infertility group. A total of 20 µg of protein per sample was mixed with equal volume of loading buffer (125 mM Tris-HCl, pH 6.8, 2% SDS, 5% glycerol, 0.003% bromophenol blue, and 1% β-mercaptoethanol). The sample mixture was boiled for 10 minutes and kept on the ice for 5 minutes. 30 µL of each sample was loaded into a 4%–15% SDS–polyacrylamide gel and electrophoresed for 2 h at 90 V along with a set of molecular weight marker (Sigma Chemical Co., St. Louis, MO, USA). The resolved protein bands were then transferred onto polyvinylidene difluoride (PVDF) membranes at 20 V for 30 minutes using a transfer buffer (25 mM Tris base, 192 mM glycine, and 20% methanol). PVDF membranes were blocked with Tris-buffered saline-Tween-20 (TBST) with 5% bovine serum albumin (BSA) and used for immunodetection of seminal plasma proteins38. For each protein analysis, specific primary antibodies were incubated overnight at 4 °C overnight (Table 1). Subsequently, the membranes were washed four times with TBST for 10 minutes and incubated with the secondary antibodies at room temperature for 1 h (Table 1). The same membranes were washed four times with TBST (10 minutes) and finally treated with enhanced chemiluminescence (ECL) reagent (GE Healthcare, Marlborough, USA) for 5 minutes. ECL reacted blots were exposed to Chemi-Doc (ChemiDoc MP Imaging System, Bio-Rad, Hercules, USA) to detect the chemiluminescence signals38.
Table 1.
List of the primary and secondary antibodies used in this study.
| Primary | Secondary | |||||
|---|---|---|---|---|---|---|
| Protein | Manufacturer | Source | Dilution | Antibody | Manufacturer | Dilution |
| Annexin A2 | ab54771 | Mouse | 1:1000 | Anti-Mouse Rabbit IgG | ab6728 | 1:10000 |
| CD63 | ab118307 | Rabbit | 1:500 | |||
| CDC42 | ab64533 | 1:1000 | ||||
| PRDX2 | ab71533 | 1:1000 | ||||
| SEMG1 | sc34719 | 1:100 | Anti-Rabbit Goat IgG | ab97051 | ||
| SEMG2 | ab108085 | 1:1000 | ||||
| APP | ab32136 | 1:5000 | ||||
| C4 | ab173577 | 1:1000 | ||||
APP - Amyloid Precursor Protein; PRDX2 - Peroxiredoxin-2; SEMG – Semenogelin.
The total amount of protein present in the membranes were quantified using a Colloidal Gold Total Protein Stain (Bio-Rad, Hercules, USA). The protocol was performed according to manufacturer instructions. Briefly, the membranes were washed twice for 10 minutes in distilled water and stained with total colloidal gold protein stain by gentle shaking for 2 h at room temperature. Stained membranes were washed twice with distilled water for 10 minutes, and the densitometry image was captured using colorimetric mode on Chemi-Doc (ChemiDoc MP Imaging System, Bio-Rad, Hercules, USA)38.
Statistical analysis
MedCalc Statistical Software (V. 17.8; MedCalc Software, Ostend, Belgium) (https://www.medcalc.org/) was used for data analysis. Mann-Whitney test was performed to compare (control vs. primary infertility group and control vs. secondary infertility group) the semen parameters and the expression levels of the proteins validated using WB technique. The results were considered significant with P < 0.05.
Results
Semen parameters of men with primary infertility and men with secondary infertility
Semen analysis showed significant decrease (P < 0.05) in sperm concentration, motility, and total count, and total motile sperm count in both primary infertility and secondary infertility group compared to control group (Table 2).
Table 2.
Semen parameters in control, primary and secondary infertility groups.
| Group | Volume ± SD (ml) | Concentration ± SD (x106 /ml) | Motility ± SD (%) | Total Count ± SD (x106) | Total Motile Sperm ± SD (x106) |
|---|---|---|---|---|---|
| Control | 3.67 ± 1.97 | 76.48 ± 37.34 | 60 ± 10.95 | 273.37 ± 180.20 | 163.39 ± 109.10 |
| Primary Infertility P-value | 3.70 ± 4.06 | 30.48 ± 39.02 | 37 ± 21.47 | 119.52 ± 171.3 | 22.59 ± 70.87 |
| 0.251 | 0.003 | 0.002 | 0.007 | 0.006 | |
| Secondary Infertility P-value | 3.84 ± 1.85 | 39.25 ± 34.52 | 48 ± 13.35 | 166.05 ± 183.80 | 91.49 ± 106.60 |
| 0.711 | 0.009 | 0.021 | 0.056 | 0.029 |
Seminal plasma proteome of primary and secondary infertility
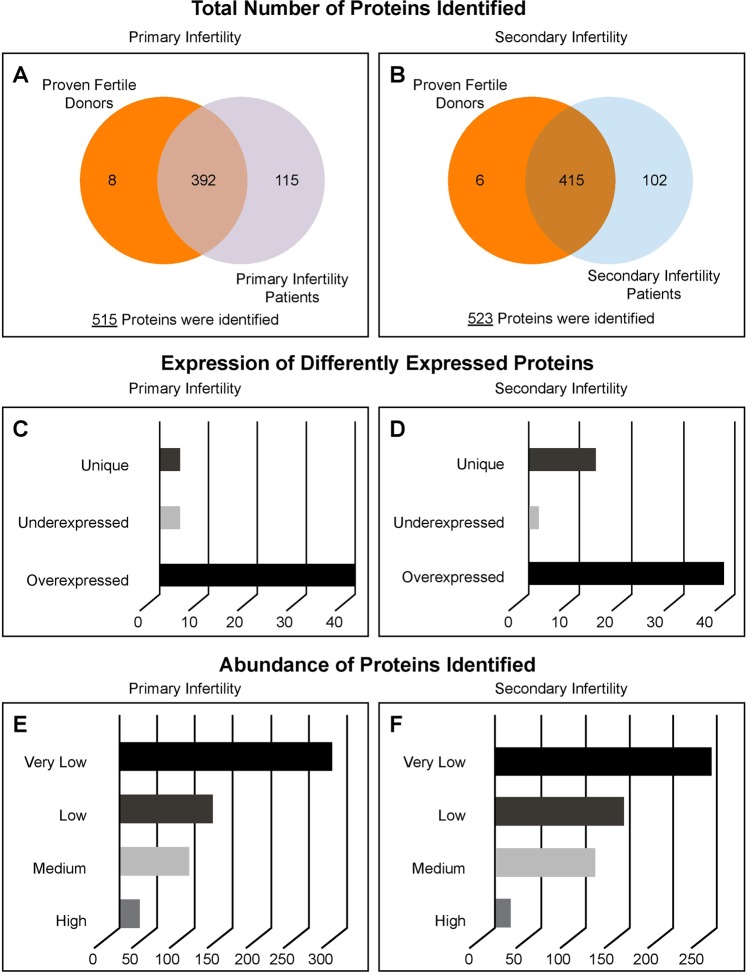
LC-MS/ MS analysis identified a total of 515 different peptides in primary infertility and control group. Out of these, 392 peptides were common to primary infertility and control group, 8 were unique to control group and 115 were unique to primary infertility group (Fig. 1A). Relative abundance of identified peptides revealed 265 peptides with very low, 126 had low, 91 had medium and 25 had high relatively abundance (Fig. 1E). Only 48 seminal plasma proteins were identified as DEPs between primary infertility and control group (Table 3). Of the 48 identified DEPs, 40 were overexpressed, 4 were underexpressed and 4 were unique to primary infertility group compared with control group (Fig. 1C).
Figure 1.
Total number proteins identified in the seminal of proven fertile donors’ group and patients with primary (A) and secondary infertility (B) by LC-MS/MS spectrometry. Differentially expressed proteins of experimental groups (C,D). Distribution of the identified proteins based on their relative abundance (E,F).
Table 3.
Differently expressed proteins identified in seminal plasma from men with primary infertility compared with control men.
| Protein | Accession number | Average SC | Abundance | NSAF ratio | T-Test | Expression | ||
|---|---|---|---|---|---|---|---|---|
| Control | PI | Control | PI | PI/ Control | P-value | |||
| Semenogelin-1 preproprotein | 4506883 | 698.3 | 312.0 | H | H | 0.28 | 0.001 | UE |
| Semenogelin-2 precursor | 4506885 | 1321.3 | 598.0 | H | H | 0.30 | <0.001 | UE |
| Extracellular matrix protein 1 isoform 3 precursor | 322302700 | 66.0 | 80.3 | H | H | 0.63 | 0.001 | UE |
| Prolactin-inducible protein precursor | 4505821 | 258.3 | 277.3 | H | H | 0.66 | 0.002 | UE |
| Actin. Cytoplasmic 2 | 316659409 | 53.7 | 113.3 | M | H | 1.72 | 0.004 | OE |
| Triosephosphate isomerase isoform 2 | 226529917 | 15.0 | 43.7 | L | M | 2.08 | 0.012 | OE |
| Sorbitol dehydrogenase | 156627571 | 19.7 | 47.3 | L | M | 2.10 | 0.008 | OE |
| Transmembrane protease serine 2 isoform 1 | 227499990 | 8.0 | 23.0 | L | M | 2.13 | 0.001 | OE |
| Neprilysin isoform X1 | 578807443 | 15.0 | 63.7 | L | M | 2.18 | 0.008 | OE |
| Phosphoglycerate kinase 1 | 4505763 | 9.3 | 25.0 | L | M | 2.19 | 0.002 | OE |
| Prostaglandin-H2 D-isomerase precursor | 32171249 | 14.0 | 37.0 | L | M | 2.19 | 0.013 | OE |
| Serotransferrin precursor | 4557871 | 63.3 | 282.0 | M | H | 2.24 | <0.001 | OE |
| Peroxiredoxin-2 | 32189392 | 11.3 | 35.7 | L | M | 2.34 | 0.026 | OE |
| Sialate O-acetylesterase isoform 1 precursor | 24850115 | 6.7 | 28.0 | VL | M | 2.38 | 0.034 | OE |
| L-lactate dehydrogenase B chain | 291575128 | 9.0 | 23.7 | L | M | 2.39 | 0.011 | OE |
| Iggfc-binding protein precursor | 154146262 | 16.3 | 54.0 | L | M | 2.41 | 0.004 | OE |
| Serpin B6 isoform a | 41152086 | 8.0 | 23.7 | L | M | 2.41 | 0.004 | OE |
| Chloride intracellular channel protein 1 | 14251209 | 3.3 | 11.7 | VL | L | 2.50 | 0.001 | OE |
| Ras-related protein Rab-3B | 19923750 | 11.0 | 39.0 | L | M | 2.77 | 0.012 | OE |
| Creatine kinase B-type | 21536286 | 15.7 | 57.3 | L | M | 2.80 | 0.005 | OE |
| Di-N-acetylchitobiase precursor | 4758092 | 3.0 | 11.7 | VL | L | 3.28 | 0.003 | OE |
| CD63 antigen isoform A | 383872447 | 3.0 | 10.3 | VL | L | 3.30 | 0.005 | OE |
| Annexin A1 | 4502101 | 7.7 | 34.3 | VL | M | 3.37 | 0.015 | OE |
| L-lactate dehydrogenase A chain isoform 1 | 5031857 | 5.0 | 19.3 | VL | L | 3.37 | 0.010 | OE |
| Cytosolic non-specific dipeptidase isoform X2 | 530414265 | 8.7 | 37.3 | L | M | 3.47 | 0.011 | OE |
| Receptor-type tyrosine-protein phosphatase S isoform X7 | 530425347 | 5.0 | 25.0 | VL | M | 3.66 | 0.003 | OE |
| Annexin A2 isoform 2 | 50845386 | 4.7 | 22.0 | VL | M | 3.86 | 0.016 | OE |
| Ras-related protein Rab-27B isoform X1 | 530414276 | 2.0 | 10.0 | VL | L | 3.87 | 0.009 | OE |
| Protein-glutamine gamma-glutamyltransferase 4 | 156627577 | 31.3 | 255.3 | L | H | 4.10 | <0.001 | OE |
| Collagen alpha-1(XVIII) chain isoform 2 precursor | 110611233 | 1.3 | 9.0 | VL | L | 4.35 | 0.001 | OE |
| Plastin-2 isoform X2 | 530402335 | 10.0 | 62.0 | VL | M | 4.46 | 0.027 | OE |
| Desmocollin-1 isoform Dsc1a preproprotein | 13435361 | 2.0 | 11.3 | VL | L | 5.08 | 0.002 | OE |
| Laminin subunit gamma-1 precursor | 145309326 | 2.0 | 19.7 | VL | L | 5.26 | 0.006 | OE |
| Ras-related protein Rab-27A | 19923264 | 3.0 | 20.7 | VL | M | 5.58 | 0.001 | OE |
| Dipeptidase 3 isoform a precursor | 193211608 | 0.7 | 9.3 | VL | L | 6.84 | 0.009 | OE |
| Desmoplakin isoform I | 58530840 | 9.7 | 56.3 | L | M | 7.52 | 0.048 | OE |
| Agrin precursor | 54873613 | 1.3 | 15.0 | VL | L | 7.72 | 0.005 | OE |
| Amiloride-sensitive amine oxidase [copper-containing] isoform X3 | 578814090 | 2.7 | 40.7 | VL | M | 7.94 | 0.009 | OE |
| Laminin subunit alpha-5 isoform X1 | 578835999 | 1.7 | 27.0 | VL | M | 8.95 | 0.001 | OE |
| Lactoylglutathione lyase | 118402586 | 1.3 | 13.7 | VL | L | 8.96 | 0.001 | OE |
| Golgi apparatus protein 1 isoform 2 precursor | 224586815 | 0.7 | 8.7 | VL | L | 11.35 | 0.002 | OE |
| Choline transporter-like protein 4 isoform 1 | 148612887 | 0.3 | 8.7 | VL | L | 12.95 | 0.001 | OE |
| Junction plakoglobin isoform X1 | 530412116 | 2.0 | 32.7 | VL | M | 21.31 | 0.010 | OE |
| Polymeric immunoglobulin receptor isoform X1 | 530366266 | 0.3 | 24.0 | VL | M | 41.94 | 0.001 | OE |
| Cell division control protein 42 homolog isoform 1 precursor | 4757952 | 0.0 | 3.3 | — | VL | — | <0.001 | Unique |
| Glyoxalase domain-containing protein 4 | 217330598 | 0.0 | 2.3 | — | VL | — | <0.001 | Unique |
| Ferritin heavy chain | 56682959 | 0.0 | 4.0 | — | VL | — | <0.001 | Unique |
| Importin-5 isoform X2 | 530423350 | 0.0 | 5.0 | — | VL | — | 0.001 | Unique |
Abbreviations: SC - Spectral counts; NSAF - Normalized spectral abundance factor; H - High; M - Medium; L - Low; VL - Very Low; UE - Underexpressed; OE – Overexpressed; PI- Primary Infertility.
The analysis identified a total of 523 different peptides in secondary infertility and control group. Of these, 415 peptides were common to secondary infertility and control group, 6 were unique to control group and 102 were unique to secondary infertility group (Fig. 1B). Relative abundance of the identified proteins revealed 245 proteins with very low abundance, 143 with low abundance, 110 with medium abundance and 15 proteins with high relative abundance (Fig. 1F). A total of 53 seminal plasma proteins were found to be differentially expressed between secondary infertility and control group (Table 4). Of these 53 DEPs, 2 were underexpressed, 38 were overexpressed in secondary infertility group compared with control group, and 13 DEPs were unique to secondary infertility group (Fig. 1D).
Table 4.
Differently expressed proteins identified in seminal plasma from men with secondary infertility compared with control men.
| Protein | Accession Number | Average SC | Abundance | NSAF ratio | T-Test | Expression | ||
|---|---|---|---|---|---|---|---|---|
| Control | SI | Control | SI | SI/ Control | P-value | |||
| Semenogelin-2 precursor | 4506885 | 1321.3 | 1310.0 | H | H | 0.54 | 0.001 | UE |
| Semenogelin-1 preproprotein | 4506883 | 698.7 | 742.0 | H | H | 0.59 | 0.010 | UE |
| Serotransferrin precursor | 4557871 | 63.3 | 214.3 | M | H | 1.52 | 0.010 | OE |
| Alpha-1-antichymotrypsin precursor | 50659080 | 40.7 | 144.0 | M | H | 1.90 | 0.001 | OE |
| Elongation factor 1-alpha 1 | 4503471 | 20.3 | 56.7 | M | M | 2.02 | 0.000 | OE |
| 78 glucose-regulated protein precursor | 16507237 | 8.7 | 44.3 | L | M | 2.24 | 0.048 | OE |
| Tubulin beta-4B chain | 5174735 | 13.7 | 44.0 | L | M | 2.27 | 0.011 | OE |
| Creatine kinase B-type | 21536286 | 15.7 | 53.0 | L | M | 2.29 | 0.002 | OE |
| Cytosolic non-specific dipeptidase isoform X2 | 530414265 | 8.7 | 32.0 | L | M | 2.37 | 0.040 | OE |
| Transmembrane protease serine 2 isoform 2 | 205360943 | 8.0 | 34.0 | L | M | 2.46 | 0.004 | OE |
| Annexin A1 | 4502101 | 7.7 | 33.0 | VL | M | 2.75 | 0.013 | OE |
| Kallistatin isoform 2 precursor | 21361302 | 9.0 | 41.0 | L | M | 2.75 | 0.026 | OE |
| Purine nucleoside phosphorylase | 157168362 | 4.7 | 24.0 | VL | M | 2.79 | 0.011 | OE |
| Alpha-2-antiplasmin isoform X1 | 578840157 | 11.0 | 46.0 | L | M | 2.90 | 0.017 | OE |
| Receptor-type tyrosine-protein phosphatase S isoform X1 | 530425335 | 5.0 | 25.3 | VL | M | 2.92 | 0.019 | OE |
| Annexin A2 isoform 1 | 50845388 | 4.7 | 20.0 | VL | M | 3.06 | 0.018 | OE |
| Cullin-associated NEDD8-dissociated protein 1 | 21361794 | 4.3 | 26.3 | VL | M | 3.10 | 0.021 | OE |
| Complement C4-A isoform 1 preproprotein | 67190748 | 7.3 | 49.7 | VL | M | 3.19 | 0.032 | OE |
| L-lactate dehydrogenase A chain isoform 1 | 5031857 | 5.0 | 25.3 | VL | M | 3.25 | 0.001 | OE |
| Ras-related protein Rab-27A isoform X2 | 530406261 | 3.0 | 18.0 | VL | L | 3.28 | 0.005 | OE |
| Beta-hexosaminidase subunit alpha preproprotein | 189181666 | 6.3 | 34.0 | VL | M | 3.50 | 0.002 | OE |
| Ezrin | 21614499 | 2.0 | 9.7 | VL | L | 3.50 | 0.004 | OE |
| Olfactomedin-4 precursor | 32313593 | 3.0 | 25.0 | VL | M | 3.53 | 0.000 | OE |
| Beta-galactosidase isoform b | 119372312 | 3.0 | 22.0 | VL | M | 3.57 | 0.003 | OE |
| Tubulin alpha-1C chain | 14389309 | 9.0 | 40.3 | L | M | 3.60 | 0.001 | OE |
| Maltase-glucoamylase. intestinal isoform X1 | 578814724 | 3.3 | 29.0 | VL | M | 3.69 | 0.010 | OE |
| Heat shock cognate 71 protein isoform X1 | 578822169 | 3.0 | 27.7 | VL | M | 4.02 | 0.003 | OE |
| Plastin-2 isoform X2 | 530402335 | 10.0 | 65.7 | L | M | 4.11 | 0.006 | OE |
| Legumain preproprotein | 56682962 | 3.0 | 21.0 | VL | M | 4.80 | 0.004 | OE |
| Alpha-1-acid glycoprotein 2 precursor | 4505529 | 1.7 | 13.0 | VL | L | 4.87 | 0.006 | OE |
| Lactoylglutathione lyase | 118402586 | 1.3 | 11.0 | VL | L | 5.48 | 0.002 | OE |
| Alpha-1B-glycoprotein precursor | 21071030 | 1.3 | 17.0 | VL | L | 6.11 | 0.003 | OE |
| Laminin subunit alpha-5 isoform X1 | 578835999 | 1.7 | 27.0 | VL | M | 7.06 | 0.022 | OE |
| Lipocalin-15 precursor | 42714611 | 0.7 | 10.0 | VL | L | 8.39 | 0.001 | OE |
| Dipeptidase 3 isoform a precursor | 193211608 | 0.7 | 13.3 | VL | L | 10.10 | 0.002 | OE |
| Complement C3 precursor | 115298678 | 2.7 | 92.0 | VL | H | 15.37 | 0.004 | OE |
| Programmed cell death 6-interacting protein isoform 1 | 22027538 | 0.7 | 21.0 | VL | M | 15.60 | 0.026 | OE |
| Adenylyl cyclase-associated protein 1 | 5453595 | 0.3 | 11.3 | VL | L | 22.01 | 0.002 | OE |
| Polymeric immunoglobulin receptor isoform X1 | 530366266 | 0.3 | 29.0 | VL | M | 45.00 | 0.004 | OE |
| Histone H2A type 2-A | 4504251 | 0.3 | 18.0 | VL | L | 49.22 | 0.005 | OE |
| Ferritin heavy chain | 56682959 | 0.0 | 2.0 | — | VL | — | 0.000 | Unique |
| Fructose-1.6-bisphosphatase 1 | 16579888 | 0.0 | 6.7 | — | VL | — | 0.001 | Unique |
| Nephronectin isoform A precursor | 296011067 | 0.0 | 6.0 | — | VL | — | 0.000 | Unique |
| Transforming protein rhoa precursor | 10835049 | 0.0 | 2.0 | — | VL | — | 0.000 | Unique |
| Ceruloplasmin precursor | 4557485 | 0.0 | 9.3 | — | L | — | 0.000 | Unique |
| Importin-5 isoform X2 | 530423350 | 0.0 | 12.0 | — | L | — | 0.000 | Unique |
| Lysosomal Pro-X carboxypeptidase isoform 1 preproprotein | 4826940 | 0.0 | 10.3 | — | L | — | 0.000 | Unique |
| Heat shock 70 protein 1-like isoform X1 | 530381921 | 0.0 | 8.7 | — | L | — | 0.000 | Unique |
| Carboxylesterase 5 A isoform 1 precursor | 219521907 | 0.0 | 14.3 | — | L | — | 0.001 | Unique |
| Kunitz-type protease inhibitor 1 isoform 1 precursor | 32313599 | 0.0 | 8.0 | — | L | — | 0.004 | Unique |
| Myosin-9 | 12667788 | 0.0 | 17.0 | — | L | — | 0.005 | Unique |
| Amyloid beta A4 protein isoform f precursor | 209915573 | 0.0 | 8.3 | — | L | — | 0.007 | Unique |
| Alpha-crystallin A chain-like isoform X1 | 578836360 | 0.0 | 51.3 | — | M | — | 0.045 | Unique |
Abbreviations: SC - Spectral counts; NSAF - Normalized spectral abundance factor; H - High; M - Medium; L - Low; VL - Very Low; UE - Underexpressed; OE – Overexpressed; SI – Secondary Infertility.
Bioinformatic analysis of seminal plasma DEPs in men with primary and secondary infertility
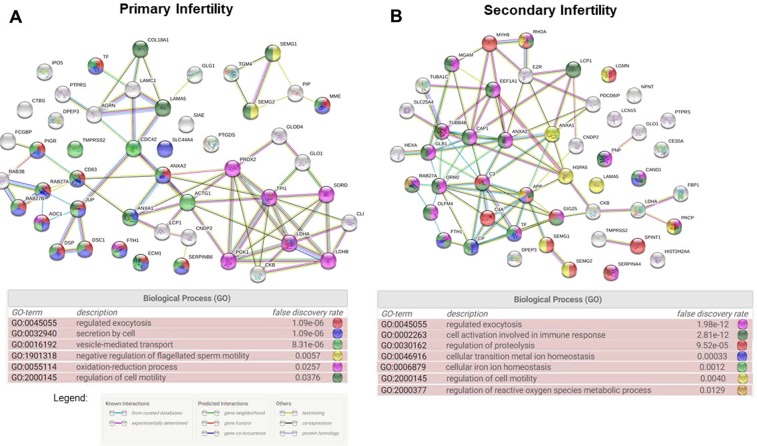
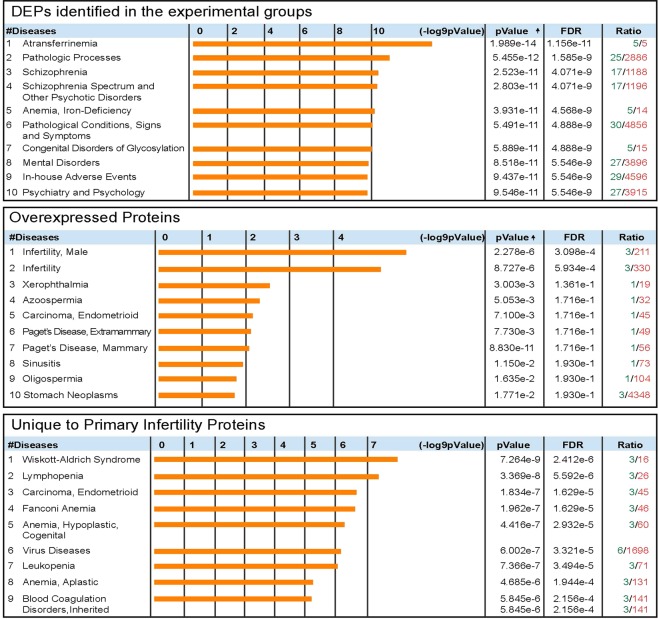
STRING analysis was performed to clarify the protein-protein interaction networks of DEPs identified in the seminal plasma of men with primary infertility (Fig. 2A). This online tool also enabled the identification of biological processes more relevant in the network based on Gene Ontology (GO). In the seminal plasma of men with primary infertility, we identified regulated exocytosis (GO:0045055) (False discovery rate (FDR) = 1.09e−6) and secretion by the cell (GO:0032940) (FDR = 1.09e−6) as the two most important biological processes. Other biological processes identified are listed in Fig. 2A. Using DAVID we were able to identify functional annotations for DEPs identified in seminal plasma of men with primary infertility. Key processes and functions involved in overexpressed, underexpressed and DEPs unique to seminal plasma of men with primary infertility are presented in Table 5. Finally, the diseases related to DEPs from seminal plasma of men with primary infertility were identified using MetaCore enrichment (Fig. 3).
Figure 2.
String network and Biological processes identified in differently expressed proteins of seminal plasma of men with primary infertility (A) and men with secondary infertility (B) compared with proven fertile donors’ group.
Table 5.
DAVID functional annotations for differently expressed proteins identified in seminal plasma.
| Men with primary infertility compared with the control group | |
| Key Processes (# of proteins) | |
| Overexpressed Proteins | Cell migration (5), Oxidation-reduction process (5), Cell adhesion (4), Response to drug (5), Carbohydrate metabolic process (4), ECM organization (4), Angiogenesis (3) |
| Underexpressed Proteins | Coagulation (2), Positive regulation of serine-type endopeptidase activity (2), Negative regulation of sperm motility (2), Protein heterooligomerization (2) |
| Proteins Unique to Primary Infertility | Oxidation-reduction process (1), Immune response (1), Regulation of protein stability (1), Cell-cell adhesion (2) |
| Key Functions (# of proteins) | |
| Overexpressed Proteins | Calcium ion binding (6), Identical protein binding (6), Structural molecule activity (5), Myosin V binding (3), GDP binding (3), Structural constituent of cytoskeleton (3) |
| Underexpressed Proteins | Protease binding (2) |
| Proteins Unique to Primary Infertility | GTPase activity (1), Protein binding (3), Iron-ion binding (1), GTPase inhibitor activity (1) |
| Men with secondary infertility compared with the control group | |
| Key Processes (# of proteins) | |
| Overexpressed Proteins | Platelet degranulation (6), Negative regulation of endopeptidase activity (5), Carbohydrate metabolic process (5), Proteolysis (6), Cell-cell adhesion (4), PKA signaling (2) |
| Underexpressed Proteins | Coagulation (2), Negative regulation of sperm motility (2), Positive regulation of serine-type endopeptidase activity (2), antibacterial humoral response (20, protein heterooligomerization (2) |
| Proteins Unique to Secondary Infertility | ECM organization (3), Protein refolding (2), Cellular iron ion homeostasis (2), Actin cytoskeleton reorganization (2), Negative regulation of endopeptidase activity (2) |
| Key Functions (# of proteins) | |
| Overexpressed Proteins | Cadherin binding involved in cell-cell adhesion (6), Endopeptidase inhibitor activity (3), GTPase activity (4), Unfolded protein binding (3), GTP binding (4), Structural molecule activity (3), Calcium-dependent protein binding (3) |
| Proteins Unique to Secondary Infertility | Ferroxidase activity (2), Serine-type endopeptidase inhibitor activity (2), Unfolded protein binding (2), Identical protein binding (3) |
Figure 3.
Diseases for differently expressed proteins and for overexpressed and unique proteins in seminal plasma of men with primary infertility compared with proven fertile group.
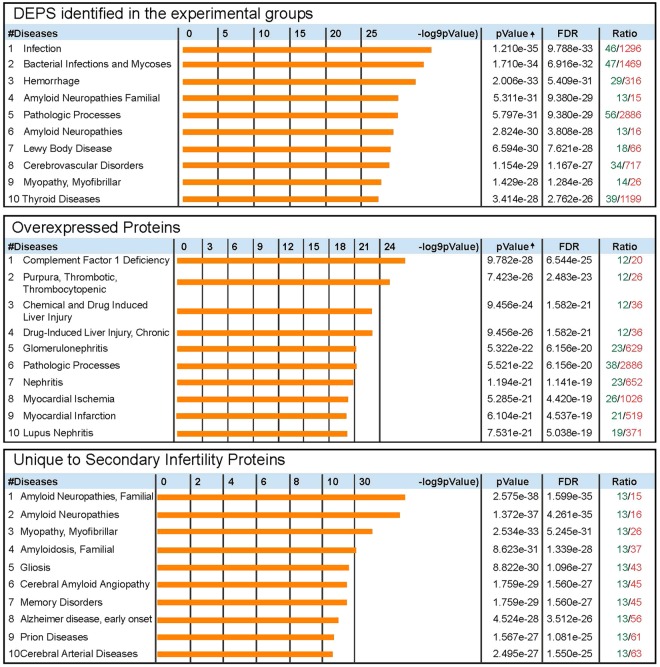
String analysis was performed with the DEPs identified in men with secondary infertility (Fig. 2B). In the seminal plasma of men with secondary infertility, regulated exocytosis (GO:0045055) (FDR = 1.09e−12) and cell activation involved in immune response (GO:0002263) (FDR = 2.81e−12) were the two most important biological processes identified. Other recognized biological processes are presented in Fig. 2B. Using DAVID, we identified key processes and functions involved in overexpressed, underexpressed and DEPs unique to seminal plasma of men with secondary infertility (Table 5). Using MetaCore enrichment, the diseases related with DEPs from seminal plasma of men with secondary infertility were identified and presented in Fig. 4.
Figure 4.
Diseases for differently expressed proteins and for overexpressed and unique proteins in seminal plasma of men with secondary infertility compared with proven fertile group.
Western blot validation of seminal plasma proteins
Based on selection criteria and the biological role, six proteins (ANXA2, PRDX2, CDC42, CD63, SEMG1 and SEMG2) from men with primary infertility and five proteins (ANXA2, C4, APP, SEMG1 and SEMG2) from men with secondary infertility were validated by WB.
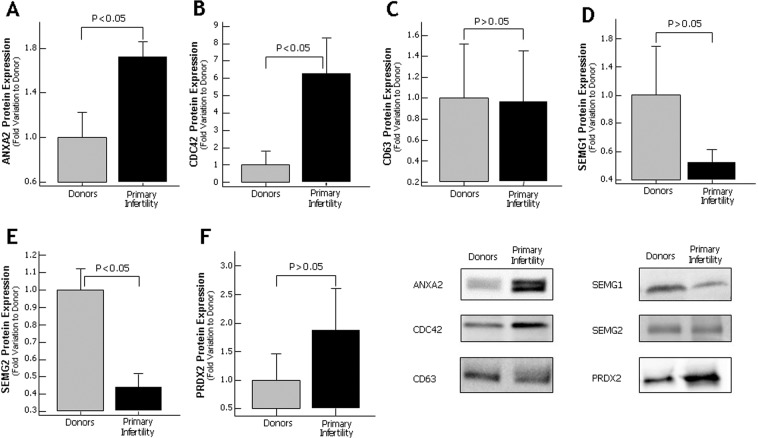
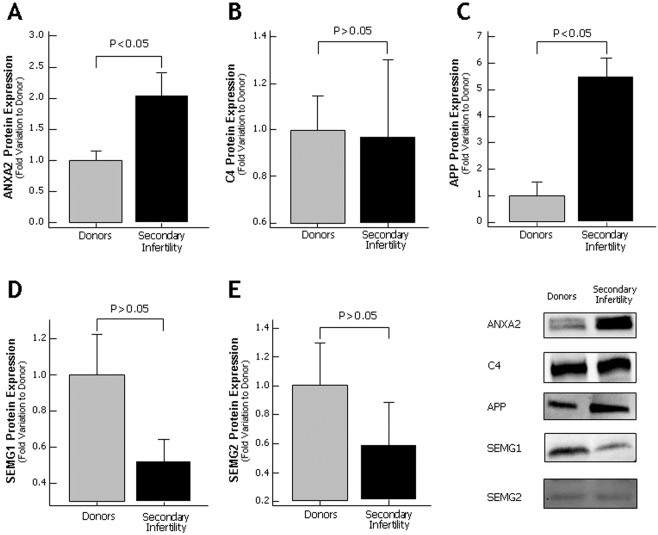
Of the proteins analyzed in men with primary infertility, ANXA2 showed an increased expression when compared to fertile donors (p < 0.05) (Fig. 5A). Other three proteins (PRDX2, CD63 and SEMG1) were detected by WB, but no differences were observed in their expression levels compared to control group (Fig. 5C,D,F). The WB validation of the protein CDC42 showed an increase in protein expression (p < 0.05) (Fig. 5B) that was in concordance with the proteomic results. The other protein SEMG2 selected for validation by western blot was underexpressed in seminal plasma of men with primary infertility when compared to fertile donors (p < 0.05) (Fig. 5E). The protein C4, SEMG1 and SEMG2 selected for validation using western blot in secondary infertility group did not show any change in the expression (Fig. 6B,D,E). Other key proteins such as ANXA2 and APP were overexpressed in secondary infertility group compared with control group (p < 0.05) (Fig. 6A,C).
Figure 5.
Protein expression levels of the differentially expressed proteins selected for validation by Western blot in seminal plasma of proven fertile donors’ group and with primary infertility. (A) Annexin A2; (B) CDC42 protein; (C) CD63; (D) Semenogelin 1 (SEMG1); (E) Semenogelin 2 (SEMG2) and (F) Peroxiredoxin 2; Results are expressed as mean ± SEM and in fold variation to donors’ group. Panel shows a representative image of Western Blot experiments.
Figure 6.
Protein expression levels of the differentially expressed proteins selected for validation by Western blot in seminal plasma of proven fertile donors’ group and with secondary infertility. (A) Annexin A2; (B) C4 protein; (C) Amyloid precursor protein (APP); (D) Semenogelin 1 (SEMG1) and (E) Semenogelin 2 (SEMG2). Results are expressed as mean ± SEM and in fold variation to donors’ group. Panel shows a representative image of Western Blot experiments.
Discussion
The global proteomic approach is currently being used to identify the molecular factors associated with the cause of male infertility. As spermatozoa are transcriptionally and translationally silent they depend on the sperm and the seminal plasma proteins for their normal biological functions. Seminal plasma harbors factors that are essential for protection of spermatozoa during its transit through female reproductive tract and assist in the fertilization process. Semen analysis of men with primary or secondary infertility revealed that all semen parameters were above the WHO 2010 reference values39 (Table 2). Since semen quality seems not to be the cause for infertility in these men, we used proteomic approach to identify the dysregulated seminal plasma proteins. Intasqui et al. carried out proteomic profiling of sperm in men with primary and secondary infertility, and proposed BAG6 and HIST1H2BA as potential biomarker of male infertility40. As a follow up, the current study focused on the proteomic analysis of seminal plasma and its bioinformatic analysis that provides an extensive information about distribution, molecular and functional analysis of the identified proteins9,11,38,41. Furthermore, the proteins selected based on reproductive functions and fertilization process such as regulation of exocytosis42,43, regulation of cell motility44,45 or vesicle mediated-transport46 were validated using WB technique. These biological processes are crucial for fertilization and our analysis revealed dysregulation in both men with primary and secondary infertility. Role of key proteins associated with the pathophysiology of primary and secondary male infertility are discussed in detail.
Annexin 2 (ANXA2) is a Ca2+-dependent phospholipid-binding protein, which is associated with plasma membrane of cells and endosomes47. ANXA2 plays an important role in cellular processes, such as, membrane trafficking events48, lipid reorganization in the membrane and endocytosis49. During abnormal ubiquitination process, an aberrant expression of ANXA2 was observed50,51. With respect to reproductive functions, ANXA2 is involved in maintaining the integrity of blood-testis-barrier and in the release of spermatozoa52. Presence of ANXA2 in sperm is essential for the binding of the sperm in the female tract that is crucial for fertilization53. Earlier studies have reported abnormal expression of ANXA2 in seminal plasma and prostasomes of subfertile or infertile men54–56. In the current study, ANXA2 was found to be involved in several biological function such as exocytosis and secretion by cell, both processes essential in the binding to sperm. Proteomic analysis revealed overexpression of ANXA2 in the seminal plasma of men with primary and secondary infertility. Furthermore, WB findings also confirmed the overexpression of ANXA2 in both primary and secondary infertile men compared to control group. Hence, we suggest that aberrant expression of ANXA2 in the seminal plasma affects the maturation process of the spermatozoa55, resulting in production of the immature sperm in both primary and secondary male infertility conditions.
Peroxiredoxins (PRDXs) are the key proteins that regulate ROS levels and plays an important role in male fertility57. Bioinformatic analysis of DEPs present in the seminal plasma of primary infertility men confirmed that PRDX2 was involved in the oxidation-reduction process. In spermatozoa, the presence of PRDX2 was detected in plasma membrane, acrosome, nucleus, midpiece and flagellum58. PRDX2 reduces the availability of iron which in turn decreases oxidative stress59. Alteration in the expression of this protein has been reported in men with immunological infertility60. Proteomic analysis of seminal plasma of men with primary infertility revealed overexpression PRDX2, whereas WB validation did not reveal difference in the expression levels of PRDX2 compared to the control group. The discrepancies in the results of LC-MS/MS and WB could be mainly due to the differences in the specificity and sensitivity of both techniques. Our seminal plasma proteomic results are concurrent with the findings from earlier reports on male infertility conditions related to varicocele and in men with Hodgkin’s disease61,62. Increased levels of PRDX2 in the seminal plasma of men with primary infertility indicates oxidative stress mediated damage to the spermatozoa leading to impairment of physiological functions related to fertilization process such as hyperactivation, capacitation, acrosome reaction and sperm-oocyte fusion.
The protein CD63 antigen (CD63) is a cellular trafficking molecule63 and is considered an exosomal marker64. Dysregulation in the expression of CD63 affects the exosome-sperm fusion, which is responsible for the production of immature spermatozoa65. In the current study, CD63 was overexpressed in primary infertility group and was involved in vesicle-mediated transport. Alteration in the expression of CD63 protein was reported in spermatozoa of men with testicular cancer66 and seminal plasma of infertile varicocele men55. Furthermore, our bioinformatic analysis revealed that the overexpressed seminal plasma protein CDC42 was also involved in vesicle-mediated transport. CDC42 proteins are expressed in the head of elongated spermatids67 and are involved in the formation of sperm tail and head68. CDC42 are identified as key controllers of capacitation-dependent actin dynamics69 and regulators of the acrosome reaction70. Dube et al. reported decreased levels of CDC42 transcripts in obstructive azoospermia71. Increase levels of CDC42 and CD63 in seminal plasma may play a major role in the pathology associated with vesicle-mediated transport in men with primary infertility. Further, CD63 has a direct impact on the transport of exosomal molecules/ factors to the spermatozoa required for the maturation process whereas CDC42 protein dysfunction may serve as factor for compromised acrosome reaction in men with primary infertility. We were also able to demonstrate the presence of both the proteins (CDC42 and CD63) using WB in the seminal plasma of men with primary infertility.
Immune response and inflammation have negative impact on the male reproductive system and male fertility72. LC-MS/MS analysis and further confirmation by WB, revealed that C4 protein, which is involved in body’s immune response73, was overexpressed in secondary infertile men. Further, bioinformatics analysis also predicted infection as the most relevant dysregulated pathway in secondary infertility. Likewise, the multifunctional protein APP, which was detected in the seminal plasma of secondary infertility men, is involved in many biological processes such as exocytosis, cell activation to response of immune system, proteolysis regulation and regulation of iron homeostasis. Earlier studies demonstrated the localization of APP in tail and head of spermatozoa74,75. In oxidative stress mediated male infertility aberrant expression of APP results in acrosome dysfunction76. In the current study APP was overexpressed and described to have a role in sperm motility and interaction of the sperm with the oocyte75. Moreover, the validation of proteomic results using WB suggests that APP can serve as a seminal plasma marker in diagnosis of secondary infertility. Hence, from the global proteomic and WB results it is clear that proteins associated with immune response are overexpressed in the seminal plasma of men with secondary infertility and thus can a predisposing cause for this condition.
The most abundant proteins in seminal plasma are the semenogelins (1 and 2)77. These proteins are secreted by the seminal vesicles and form the gel-like coagulum with the fibronectin in the ejaculated semen78. The coagulum is liquefied through the action of prostate specific antigen protein, responsible for semenogelins cleavage, inducing motility and releasing spermatozoa. de Lamirande et al. reported that SEMGs are involved in the inhibition of capacitation process of human spermatozoa79. Other global proteomic studies also demonstrated the abnormal expression of SEMGs in seminal plasma of men with abnormal semen parameters80, seminal oxidative stress81 and varicocele82,83. In the present study, proteomic profile of seminal plasma showed both SEMG1 and SEMG2 were underexpressed in primary and secondary infertile men compared to control group. Dysregulation of the semenogelins in both primary and secondary infertility condition may have a severe impact on the capacitation process of the spermatozoa. Furthermore, validation of SEMG2 using WB in the seminal plasma of men with primary infertility suggests that SEMG2 levels can serve as candidate marker to differentiate primary from secondary infertility.
Conclusion
The proteomic data from this pilot study shows an altered seminal plasma proteome in primary and secondary infertility compared with fertile men. Our preliminary results have identified maturation failure and immune reaction response as the causes for infertility in men with primary and secondary infertility. Validation of key proteins suggests that ANXA2 can be a screening biomarker for both primary and secondary infertility, whereas CDC42 and SEMG2 can be useful candidate biomarkers for primary infertility and APP for secondary infertility. This is the first report showing DEPs in seminal plasma of men with primary and secondary infertility, however further studies are warranted to confirm and validate these biomarkers.
Supplementary information
Acknowledgements
The authors thank Rakesh Sharma, PhD, Kristian Leisegang, PhD and Chak-Lam Cho, MD, for their critical reading of the manuscript and helpful suggestions. Belinda Willard, PhD, Director of Proteomic Core Laboratory, Lerner Research Institute, assisted with proteomic analysis while Banu Gopalan, PhD assisted with bioinformatics data analysis. The authors thank Bernastine Buchanan from the Center for Medical Art and Photography for assistance with the figures. Research support was provided by the American Center for Reproductive Medicine at Cleveland Clinic. Ana D Martins funded by “Fundação para a Ciência e Tecnologia” (SFRH/BD/108726/2015) and Fulbright (ID: E0585654).
Author contributions
A.A. conceived the idea and designed the study. A.M. and M.K.P.S. carried out the experiment. Data analysis was carried out by A.M., M.K.P.S. and S.B. The first draft was written by A.M. and revised by A.A., M.K.P.S., M.A. and S.B. All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-64434-1.
References
- 1.WHO. WHO laboratory manual for the examination and processing of human semen. (2010). [PubMed]
- 2.Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reproductive biology and endocrinology: RB&E. 2015;13:37. doi: 10.1186/s12958-015-0032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thoma ME, et al. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertility and Sterility. 2013;99:1324–1331.e1321. doi: 10.1016/j.fertnstert.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medicine PC. o. t. A. S. f. R. Diagnostic evaluation of the infertile male: a committee opinion. Fertility and Sterility. 2015;103:e18–e25. doi: 10.1016/j.fertnstert.2014.12.103. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal, A., Gupta, S. & Sharma, R. in Andrological Evaluation of Male Infertility 39-46 (Springer, 2016).
- 6.Oehninger S, Ombelet W. Limits of current male fertility testing. Fertility and Sterility. 2019;111:835–841. doi: 10.1016/j.fertnstert.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Wang C, Swerdloff RS. Limitations of semen analysis as a test of male fertility and anticipated needs from newer tests. Fertility and Sterility. 2014;102:1502–1507. doi: 10.1016/j.fertnstert.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Jonge C. Biological basis for human capacitation-revisited. Human reproduction update. 2017;23:289–299. doi: 10.1093/humupd/dmw048. [DOI] [PubMed] [Google Scholar]
- 9.Druart X, de Graaf S. Seminal plasma proteomes and sperm fertility. Animal Reproduction Science. 2018;194:33–40. doi: 10.1016/j.anireprosci.2018.04.061. [DOI] [PubMed] [Google Scholar]
- 10.Maxwell W, De Graaf S, Ghaoui R, Evans G. Seminal plasma effects on sperm handling and female fertility. Society of Reproduction and Fertility supplement. 2007;64:13. doi: 10.5661/rdr-vi-13. [DOI] [PubMed] [Google Scholar]
- 11.Samanta L, Parida R, Dias TR, Agarwal A. The enigmatic seminal plasma: a proteomics insight from ejaculation to fertilization. Reproductive biology and endocrinology: RB&E. 2018;16:41. doi: 10.1186/s12958-018-0358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodríguez-Martínez H, Kvist U, Ernerudh J, Sanz L, Calvete JJ. Seminal Plasma Proteins: What Role Do They Play? American Journal of Reproductive Immunology. 2011;66:11–22. doi: 10.1111/j.1600-0897.2011.01033.x. [DOI] [PubMed] [Google Scholar]
- 13.Del Giudice P, et al. Determination of testicular function in adolescents with varicocoele–a proteomics approach. Andrology. 2016;4:447–455. doi: 10.1111/andr.12174. [DOI] [PubMed] [Google Scholar]
- 14.Camargo M, et al. Unbiased label-free quantitative proteomic profiling and enriched proteomic pathways in seminal plasma of adult men before and after varicocelectomy. Human reproduction (Oxford, England) 2013;28:33–46. doi: 10.1093/humrep/des357. [DOI] [PubMed] [Google Scholar]
- 15.Zylbersztejn DS, et al. Proteomic analysis of seminal plasma in adolescents with and without varicocele. Fertil Steril. 2013;99:92–98. doi: 10.1016/j.fertnstert.2012.08.048. [DOI] [PubMed] [Google Scholar]
- 16.Fariello RM, et al. Effect of smoking on the functional aspects of sperm and seminal plasma protein profiles in patients with varicocele. Human reproduction (Oxford, England) 2012;27:3140–3149. doi: 10.1093/humrep/des287. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal A, et al. Comparative proteomic network signatures in seminal plasma of infertile men as a function of reactive oxygen species. Clinical proteomics. 2015;12:23. doi: 10.1186/s12014-015-9094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dias, T. R. et al. Proteomic Signatures Reveal Differences in Stress Response, Antioxidant Defense and Proteasomal Activity in Fertile Men with High Seminal ROS Levels. International journal of molecular sciences20, 10.3390/ijms20010203 (2019). [DOI] [PMC free article] [PubMed]
- 19.Barrio-Munoz M, Abad-Gairin C, Amengual-Guedan JM, Prats-Lopez J. Diagnosis of prostate cancer by analyzing oxidative stress in human seminal plasma: developing unsophisticated tools for noninvasive prostate cancer diagnosis. European journal of cancer prevention: the official journal of the European Cancer Prevention Organisation (ECP) 2016;25:518–523. doi: 10.1097/cej.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 20.Intasqui P, et al. Differences in the seminal plasma proteome are associated with oxidative stress levels in men with normal semen parameters. Fertil Steril. 2015;104:292–301. doi: 10.1016/j.fertnstert.2015.04.037. [DOI] [PubMed] [Google Scholar]
- 21.Cui Z, Agarwal A, da Silva BF, Sharma R, Sabanegh E. Evaluation of seminal plasma proteomics and relevance of FSH in identification of nonobstructive azoospermia: A preliminary study. Andrologia. 2018;50:e12999. doi: 10.1111/and.12999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drabovich AP, et al. Differential diagnosis of azoospermia with proteomic biomarkers ECM1 and TEX101 quantified in seminal plasma. Science Translational Medicine. 2013;5:212ra160. doi: 10.1126/scitranslmed.3006260. [DOI] [PubMed] [Google Scholar]
- 23.Yamakawa K, Yoshida K, Nishikawa H, Kato T, Iwamoto T. Comparative analysis of interindividual variations in the seminal plasma proteome of fertile men with identification of potential markers for azoospermia in infertile patients. Journal of andrology. 2007;28:858–865. doi: 10.2164/jandrol.107.002824. [DOI] [PubMed] [Google Scholar]
- 24.Cao X, et al. Proteomic profile of human spermatozoa in healthy and asthenozoospermic individuals. Reproductive biology and endocrinology: RB&E. 2018;16:16. doi: 10.1186/s12958-018-0334-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J, et al. Proteomic analysis of seminal plasma from asthenozoospermia patients reveals proteins that affect oxidative stress responses and semen quality. Asian journal of andrology. 2009;11:484–491. doi: 10.1038/aja.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giacomini E, et al. Comparative analysis of the seminal plasma proteomes of oligoasthenozoospermic and normozoospermic men. Reproductive biomedicine online. 2015;30:522–531. doi: 10.1016/j.rbmo.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Milardi D, et al. Novel biomarkers of androgen deficiency from seminal plasma profiling using high-resolution mass spectrometry. The Journal of clinical endocrinology and metabolism. 2014;99:2813–2820. doi: 10.1210/jc.2013-4148. [DOI] [PubMed] [Google Scholar]
- 28.Flores-Morales A, Iglesias-Gato D. Quantitative Mass Spectrometry-Based Proteomic Profiling for Precision Medicine in Prostate Cancer. Frontiers in oncology. 2017;7:267. doi: 10.3389/fonc.2017.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hassan MI, et al. Proteomic approach for purification of seminal plasma proteins involved in tumor proliferation. Journal of separation science. 2007;30:1979–1988. doi: 10.1002/jssc.200700028. [DOI] [PubMed] [Google Scholar]
- 30.Barrachina, F. et al. Novel and conventional approaches for the analysis of quantitative proteomic data are complementary towards the identification of seminal plasma alterations in infertile patients. Molecular & Cellular Proteomics, mcp.RA118.001248, 10.1074/mcp.RA118.001248 (2018).
- 31.World Health Organization, D. WHO laboratory manual for the examination and processing of human sperm. World Health Organiz (2010).
- 32.Martínez-Bartolomé S, et al. Guidelines for reporting quantitative mass spectrometry based experiments in proteomics. Journal of proteomics. 2013;95:84–88. doi: 10.1016/j.jprot.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 33.Ayaz, A. et al. Impact of precise modulation of reactive oxygen species levels on spermatozoa proteins in infertile men. Clin Proteomics12, 10.1186/1559-0275-12-4 (2015). [DOI] [PMC free article] [PubMed]
- 34.Bogle O, et al. Identification of protein changes in human spermatozoa throughout the cryopreservation process. Andrology. 2017;5:10–22. doi: 10.1111/andr.12279. [DOI] [PubMed] [Google Scholar]
- 35.Sharma R, et al. Functional proteomic analysis of seminal plasma proteins in men with various semen parameters. Reproductive Biology and Endocrinology. 2013;11:38. doi: 10.1186/1477-7827-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agarwal A, et al. Major protein alterations in spermatozoa from infertile men with unilateral varicocele. Reproductive Biology and Endocrinology. 2015;13:8. doi: 10.1186/s12958-015-0007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Esteves SC, Agarwal A. Afterword to varicocele and male infertility: current concepts and future perspectives. Asian journal of andrology. 2016;18:319. doi: 10.4103/1008-682X.172820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Panner Selvam MK, Agarwal A, Pushparaj PN. A quantitative global proteomics approach to understanding the functional pathways dysregulated in the spermatozoa of asthenozoospermic testicular cancer patients. Andrology. 2019 doi: 10.1111/andr.12620. [DOI] [PubMed] [Google Scholar]
- 39.Organization, W. H. WHO laboratory manual for the examination and processing of human semen. (2010). [PubMed]
- 40.Intasqui, P., Agarwal, A., Sharma, R., Samanta, L. & Bertolla, R. P. Towards the identification of reliable sperm biomarkers for male infertility: A sperm proteomic approach. Andrologia50, 10.1111/and.12919 (2018). [DOI] [PubMed]
- 41.du Plessis SS, Kashou AH, Benjamin DJ, Yadav SP, Agarwal A. Proteomics: a subcellular look at spermatozoa. Reproductive biology and endocrinology: RB&E. 2011;9:36. doi: 10.1186/1477-7827-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akter, Q. S., Rajabi-Toustani, R., Shimizu, K., Kuwahara, Y. & Murase, T. Polymyxin B enhances acrosomal exocytosis triggered by calcium and the calcium ionophore A23187 in ejaculated boar spermatozoa. Animal science journal = Nihon chikusan Gakkaiho, 10.1111/asj.13155 (2019). [DOI] [PMC free article] [PubMed]
- 43.Puga Molina, L. C. et al. Molecular Basis of Human Sperm Capacitation. Frontiers in Cell and Developmental Biology6, 10.3389/fcell.2018.00072 (2018). [DOI] [PMC free article] [PubMed]
- 44.Xu F, Zhu H, Zhu W, Fan L. Human sperm acrosomal status, acrosomal responsiveness, and acrosin are predictive of the outcomes of in vitro fertilization: A prospective cohort study. Reproductive biology. 2018;18:344–354. doi: 10.1016/j.repbio.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 45.Harris AL, et al. Semen parameters on the day of oocyte retrieval predict low fertilization during conventional insemination IVF cycles. Journal of assisted reproduction and genetics. 2019;36:291–298. doi: 10.1007/s10815-018-1336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun X, Kovacs T, Hu YJ, Yang WX. The role of actin and myosin during spermatogenesis. Molecular biology reports. 2011;38:3993–4001. doi: 10.1007/s11033-010-0517-0. [DOI] [PubMed] [Google Scholar]
- 47.Maji S, et al. Exosomal Annexin II Promotes Angiogenesis and Breast Cancer Metastasis. Molecular cancer research: MCR. 2017;15:93–105. doi: 10.1158/1541-7786.Mcr-16-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waisman, D. M. in Signal Transduction Mechanisms 301-322 (Springer, 1995).
- 49.Gerke V, Creutz CE, Moss SE. Annexins: linking Ca 2+ signalling to membrane dynamics. Nature reviews Molecular cell biology. 2005;6:449. doi: 10.1038/nrm1661. [DOI] [PubMed] [Google Scholar]
- 50.He K-L, et al. Endothelial cell annexin A2 regulates polyubiquitination and degradation of its binding partner S100A10/p11. J Biol Chem. 2008;283:19192–19200. doi: 10.1074/jbc.M800100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deng S, Jing B, Xing T, Hou L, Yang Z. Overexpression of Annexin A2 Is Associated with Abnormal Ubiquitination in Breast Cancer. Genomics, Proteomics & Bioinformatics. 2012;10:153–157. doi: 10.1016/j.gpb.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chojnacka K, Bilinska B, Mruk DD. Annexin A2 is critical for blood-testis barrier integrity and spermatid disengagement in the mammalian testis. Biochimica et biophysica acta. Molecular cell research. 2017;1864:527–545. doi: 10.1016/j.bbamcr.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 53.Teijeiro JM, Ignotz GG, Marini PE. Annexin A2 is involved in pig (Sus scrofa)sperm-oviduct interaction. Molecular Reproduction and Development. 2009;76:334–341. doi: 10.1002/mrd.20958. [DOI] [PubMed] [Google Scholar]
- 54.Jodar M, Kalko S, Castillo J, Ballesca JL, Oliva R. Differential RNAs in the sperm cells of asthenozoospermic patients. Human reproduction (Oxford, England) 2012;27:1431–1438. doi: 10.1093/humrep/des021. [DOI] [PubMed] [Google Scholar]
- 55.Panner Selvam, M. K. et al. Protein Fingerprinting of Seminal Plasma Reveals Dysregulation of Exosome-Associated Proteins in Infertile Men with Unilateral Varicocele. The world journal of men’s health, 10.5534/wjmh.180108 (2019). [DOI] [PMC free article] [PubMed]
- 56.Munuce MJ, Marini PE, Teijeiro JM. Expression profile and distribution of Annexin A1, A2 and A5 in human semen. Andrologia. 2019;51:e13224. doi: 10.1111/and.13224. [DOI] [PubMed] [Google Scholar]
- 57.Ryu D-Y, et al. Peroxiredoxin activity is a major landmark of male fertility. Scientific Reports. 2017;7:17174. doi: 10.1038/s41598-017-17488-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O’Flaherty C. Peroxiredoxins: hidden players in the antioxidant defence of human spermatozoa. Basic Clin Androl. 2014;24:4–4. doi: 10.1186/2051-4190-24-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Agarwal, A. & Prabakaran, S. A. Mechanism, measurement, and prevention of oxidative stress in male reproductive physiology. Indian Journal of Experimental Biology43, 963-974 (2005). [PubMed]
- 60.Carlsson L, Ronquist G, Nilsson BO, Larsson A. Dominant Prostasome Immunogens for Sperm-Agglutinating Autoantibodies of Infertile Men. Journal of andrology. 2004;25:699–705. doi: 10.1002/j.1939-4640.2004.tb02844.x. [DOI] [PubMed] [Google Scholar]
- 61.Panner Selvam, M. K. & Agarwal, A. Proteomic Profiling of Seminal Plasma Proteins in Varicocele Patients. The world journal of men’s health, 10.5534/wjmh.180118 (2019). [DOI] [PMC free article] [PubMed]
- 62.Martins, A. D. et al. Alterations of Spermatozoa Proteomic Profile in Men with Hodgkin’s Disease Prior to Cancer Therapy. The world journal of men’s health, 10.5534/wjmh.190012 (2019). [DOI] [PMC free article] [PubMed]
- 63.Pols MS, Klumperman J. Trafficking and function of the tetraspanin CD63. Experimental cell research. 2009;315:1584–1592. doi: 10.1016/j.yexcr.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 64.Vojtech L, et al. Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions. Nucleic acids research. 2014;42:7290–7304. doi: 10.1093/nar/gku347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sullivan R, Saez F, Girouard J, Frenette G. Role of exosomes in sperm maturation during the transit along the male reproductive tract. Blood Cells, Molecules, and Diseases. 2005;35:1–10. doi: 10.1016/j.bcmd.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 66.Panner Selvam, M. K., Agarwal, A. & Pushparaj, P. N. Altered Molecular Pathways in the Proteome of Cryopreserved Sperm in Testicular Cancer Patients before Treatment. International journal of molecular sciences20, 10.3390/ijms20030677 (2019). [DOI] [PMC free article] [PubMed]
- 67.CHAPIN RE, WINE RN, HARRIS MW, BORCHERS CH, HASEMAN JK. Structure and control of a cell‐cell adhesion complex associated with spermiation in rat seminiferous epithelium. Journal of andrology. 2001;22:1030–1052. doi: 10.1002/j.1939-4640.2001.tb03444.x. [DOI] [PubMed] [Google Scholar]
- 68.Huang, C. Y. et al. CDC42 Negatively Regulates Testis-Specific SEPT12 Polymerization. International journal of molecular sciences19, 10.3390/ijms19092627 (2018). [DOI] [PMC free article] [PubMed]
- 69.Bernabo, N. et al. Cyclin-CDK Complexes are Key Controllers of Capacitation-Dependent Actin Dynamics in Mammalian Spermatozoa. International journal of molecular sciences20, 10.3390/ijms20174236 (2019). [DOI] [PMC free article] [PubMed]
- 70.Baltiérrez-Hoyos R, Roa-Espitia AL, Hernandez-Gonzalez EO. The association between CDC42 and caveolin-1 is involved in the regulation of capacitation and acrosome reaction of guinea pig and mouse sperm. Reproduction (Cambridge, England) 2012;144:123–134. doi: 10.1530/REP-11-0433. [DOI] [PubMed] [Google Scholar]
- 71.Dube E, Hermo L, Chan PTK, Cyr DG. Alterations in the Human Blood-Epididymis Barrier in Obstructive Azoospermia and the Development of Novel Epididymal Cell Lines from Infertile Men1. Biology of Reproduction. 2010;83:584–596. doi: 10.1095/biolreprod.110.084459. [DOI] [PubMed] [Google Scholar]
- 72.Agarwal A, et al. Role of oxidative stress, infection and inflammation in male infertility. Andrologia. 2018;50:e13126. doi: 10.1111/and.13126. [DOI] [PubMed] [Google Scholar]
- 73.Wu YL, et al. Three distinct profiles of serum complement C4 proteins in pediatric systemic lupus erythematosus (SLE) patients: tight associations of complement C4 and C3 protein levels in SLE but not in healthy subjects. Advances in experimental medicine and biology. 2006;586:227–247. doi: 10.1007/0-387-34134-x_16. [DOI] [PubMed] [Google Scholar]
- 74.Silva JV, et al. Amyloid precursor protein interaction network in human testis: sentinel proteins for male reproduction. BMC bioinformatics. 2015;16:12–12. doi: 10.1186/s12859-014-0432-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Beeram E, Suman B, Divya B. Proteins as the molecular markers of male fertility. Journal of human reproductive sciences. 2019;12:19. doi: 10.4103/jhrs.JHRS_9_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ayaz A, et al. Proteomic analysis of sperm proteins in infertile men with high levels of reactive oxygen species. Andrologia. 2018;50:e13015. doi: 10.1111/and.13015. [DOI] [PubMed] [Google Scholar]
- 77.Lundwall A, Bjartell A, Olsson AY, Malm J, Semenogelin I., II the predominant human seminal plasma proteins, are also expressed in non-genital tissues. Molecular human reproduction. 2002;8:805–810. doi: 10.1093/molehr/8.9.805. [DOI] [PubMed] [Google Scholar]
- 78.Lilja H, Abrahamsson PA, Lundwall A. Semenogelin, the predominant protein in human semen. Primary structure and identification of closely related proteins in the male accessory sex glands and on the spermatozoa. J Biol Chem. 1989;264:1894–1900. [PubMed] [Google Scholar]
- 79.de Lamirande E, Yoshida K, Yoshiike TM, Iwamoto T, Gagnon C. Semenogelin, the main protein of semen coagulum, inhibits human sperm capacitation by interfering with the superoxide anion generated during this process. Journal of andrology. 2001;22:672–679. [PubMed] [Google Scholar]
- 80.Sharma R, et al. Functional proteomic analysis of seminal plasma proteins in men with various semen parameters. Reproductive biology and endocrinology: RB&E. 2013;11:38. doi: 10.1186/1477-7827-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sharma R, et al. Proteomic analysis of seminal fluid from men exhibiting oxidative stress. Reproductive biology and endocrinology: RB&E. 2013;11:85. doi: 10.1186/1477-7827-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Del Giudice PT, et al. Changes in the seminal plasma proteome of adolescents before and after varicocelectomy. Fertil Steril. 2013;100:667–672. doi: 10.1016/j.fertnstert.2013.04.036. [DOI] [PubMed] [Google Scholar]
- 83.Agarwal A, et al. Spermatozoa protein alterations in infertile men with bilateral varicocele. Asian journal of andrology. 2016;18:43–53. doi: 10.4103/1008-682X.153848. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.