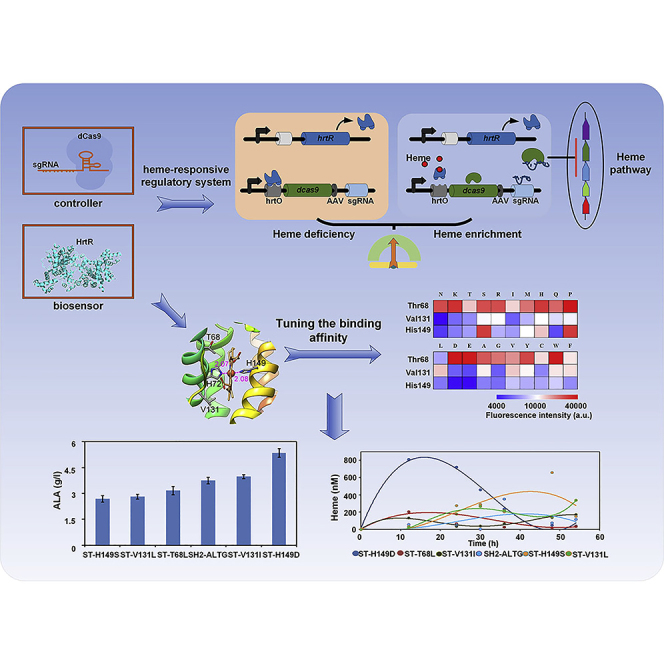
Summary
Current challenge for dynamic pathway control in metabolic engineering is enabling the components of the artificial regulatory system to be tunable. Here, we designed and built a heme-responsive regulatory system containing a heme biosensor HrtR and CRISPRi to regulate chemicals production while maintaining the intracellular heme homeostasis. A series of engineered biosensors with varied sensitivity and threshold were obtained by semi-rational design with site saturated mutation of HrtR. The modified metabolite-binding affinity of HrtR was confirmed by heme titration and molecular dynamic simulation. Dynamic regulation pattern of the system was validated by the fluctuation of gene expression and intracellular heme concentration. The efficiency of this regulatory system was proved by improving the 5-aminolevulinic acid (ALA) production to 5.35g/L, the highest yield in batch fermentation of Escherichia coli. This system was also successfully used in improving porphobilinogen (PBG) and porphyrins biosynthesis and can be applied in many other biological processes.
Subject Areas: Bioengineering, Metabolic Engineering, Biotechnology
Graphical Abstract
Highlights
-
•
Designed and built a heme-responsive regulatory system employing HrtR and CRISPRi
-
•
Turning the binding affinity of HrtR by site saturation mutations
-
•
Optimizing the system to achieve dynamic regulation of target genes
-
•
The system was applied to the ALA, PBG, and porphyrins production
Bioengineering; Metabolic Engineering; Biotechnology
Introduction
Traditional microbial production of valuable chemicals mainly involves constitutive or inducible expression of pathway enzymes under static control, which imposes burden and even generates suboptimal growth caused by imbalanced cofactors or toxic intermediates accumulation (Glick, 1995, Martin et al., 2003). In comparison, natural cells maintain robust growth and withstand environmental fluctuations by dynamically adjusting cellular metabolism through complex regulatory networks (Shen-Orr et al., 2002). Thus, “dynamic control” of metabolic pathway would reinforce hosts robustness and high production yield.
A synthetic dynamic control system/circuit typically consists of a biosensor and a genetic controller. Biosensor is a key component of dynamic regulatory system, which is metabolites responsive and should provide desired input-output relationships. Therefore, the tunability of the biosensor is of great importance; it must respond to a certain range of metabolites concentration with the appropriate sensitivity and threshold to ensure the precise regulation of host metabolism. The application of biosensors and genetic control circuits in metabolic engineering has been extensively reviewed (Brophy and Voigt, 2014, Liu et al., 2015a, Mahr and Frunzke, 2016). So far, many biosensor-based regulatory circuits have been built, whereas a few pioneering studies (Liu et al., 2015b, Xu et al., 2014, Zhang et al., 2012) can actually realize the detected dynamic regulation and can prove the existence of metabolite fluctuation. Several reports have demonstrated that tuning the biosensor performance can increase production (Liu et al., 2015b, Xu et al., 2014). However, these studies mainly focus on TF expression level, such as plasmid copy number, number of TF binding sites (Trabelsi et al., 2018) and promoter engineering (Mannan et al., 2017) (Blazeck and Alper, 2013, Feng et al., 2018). Few studies reported the alteration of the sensitivity and threshold of the biosensor through the modification of the ligand-binding affinity (Taylor et al., 2016).
Heme is a critical biological macromolecule that serves as a redox active prosthetic group required for many cellular processes, such as respiration, cellular differentiation, signal transduction, circadian rhythm pathways, and gas sensing (Bonyhady et al., 1982, Chen and London, 1981, Shelver et al., 1997). Therefore, heme is necessary for cells to maintain normal physiological functions (Tsiftsoglou et al., 2006). However, excessive free heme (>1 μM) is toxic to cells (Ryter and Tyrrell, 2000). In Escherichia coli, the biosynthesis of heme involves the formation of 5-aminolevulinic acid (ALA) as a precursor (Layer et al., 2010) and subsequent condensation of two ALA molecules into porphobilinogen (PBG), which finally generates heme via porphyrins (Choby and Skaar, 2016). The indispensability and toxicity of heme raises the difficulties in engineering the metabolic pathway related to heme biosynthesis, such as ALA, vitamin B12, siroheme, and chlorophyll. Therefore, it is essential to develop a regulatory system to enhance metabolic flux while still maintaining in vivo heme homeostasis.
Here, we designed and constructed a heme-responsive regulatory system to control the metabolic pathway dynamically and precisely. HrtR, a heme-sensing transporter regulator from Lactococcus lactis (Sawai et al., 2012), was used as the biosensor and CRISPR interference (CRISPRi) (Fontana et al., 2018, Qi et al., 2013) as a controller. This synthetic regulatory system was optimized with regard to its sensing and controlling components and was applied to pathway engineering.
Results
Design and Characterization of a Heme-Responsive Biosensor
Organism has evolved sophisticated heme regulatory system to maintain in vivo heme at a reasonable level via heme-sensing proteins (Frunzke et al., 2011). These proteins bind heme reversibly (Baureder and Hederstedt, 2013) and are usually not conserved in prokaryotic (Qi et al., 1999) and eukaryotic cells (Ding et al., 1994, Han et al., 2007). HrtR acts as a heme-sensing repressor for the regulation of heme-efflux system through hrtRBA operon in Lactococcus lactis (Sawai et al., 2012), binds to a 15-nt special DNA sequence (hrtO) located in the promoter region, and controls heme homeostasis by sensing intracellular heme (Lechardeur et al., 2012). Therefore, HrtR and hrtO were selected for a heme-responsive biosensor in this study.
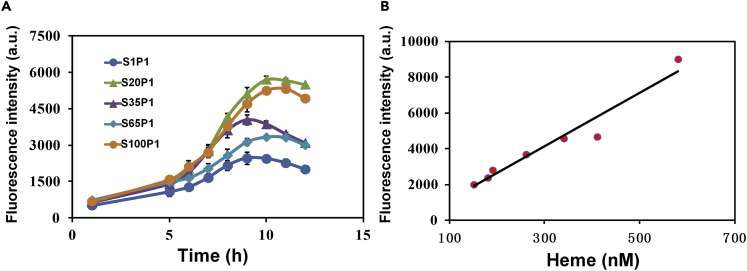
To evaluate the heme-responsive biosensor, the hrtO-hybrid trc promoter was placed upstream of gfp under the control of HrtR, resulting in plasmid P1. A recombinant E. coli strain containing different copy numbers of glutamyl-tRNA reductase gene (hemA) and glutamate-1-semialdehyde aminotransferase gene (hemL) on the genome was employed to achieve different intracellular heme accumulation (Cui et al., 2019). Strains S1, S20, S35, S65, and S100 represented 1, 20, 35, 65, and 100 copies of hemA/hemL integrated on the genome. The above five strains were obtained through chemically inducible chromosomal evolution (CIChE) method previously in our laboratory (Tyo et al., 2009). Then plasmid P1 was transformed into recombinant E. coli strains. The fluorescence intensity in these strains was gradually enhanced with the increased hemA/hemL copy numbers (Figure 1A). To investigate the correlation between heme and fluorescence intensity, the intracellular heme concentration and green fluorescence intensity were also measured and analyzed. As shown in Figure 1B, the fluorescence intensity was positively correlated with intracellular heme concentration and proved that HrtR can be used as a heme-responsive biosensor.
Figure 1.
The Evaluation the Relationship of Fluorescence Intensity and Intracellular Free Heme Concentration of the Heme Responsive Biosensor
(A) The GFP expression intensity of E. coli strains S1P1, S20P1, S35P1, S65P1, and S100P1.
(B) Linear relationship between green fluorescence intensity and intercellular heme concentration. Error bars represent ±1 SD from the mean of three replicate cultures.
Semi-Rational Design of Heme-Responsive Biosensor with Varied Binding Affinity
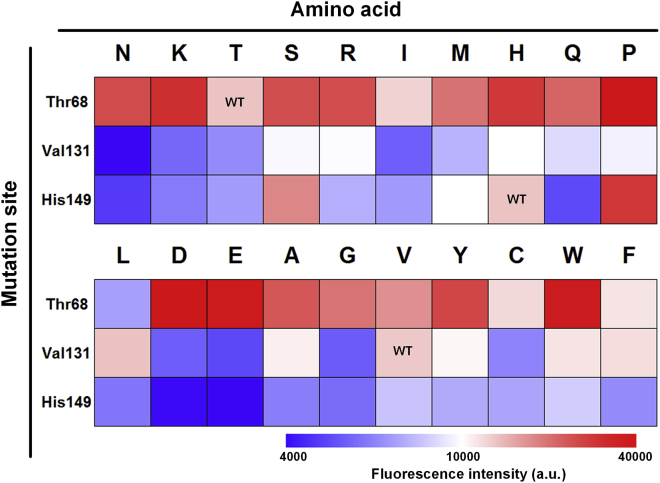
Biosensor with tailor-made ligand-binding affinity is the prerequisite for precise regulation. Heme interacts with HrtR through two histidines, His-72 and His-149, coordinates to the heme iron. The coordination between Histidines 72 and 149 with heme was supposed to form a strong affinity between HrtR and heme (Sawai et al., 2012). In vitro experiments showed that the addition of 1 μM heme was sufficient to fully dissociate HrtR from DNA. Based on the crystal structure of holo HrtR (PDB : 3VP5), three residues including H149 that form coordinate bond with Fe atom, V131 that locates at the entrance loop (P125-G135) of heme binding cavity, and the polar residue T68 that locates close to the nonpolar part of heme porphyrin were selected and were performed saturated mutation. The mutants were found to affect the binding affinity dramatically, which was reflected in the variation of fluorescence from 32.4% up to 280.5% of the wild-type (Figure 2, Table S1). Among the 19 mutants at the T68 site, most had higher fluorescence intensity than the wild-type except mutant T68L. On the contrary, mutations at the V131 site caused an obvious decrease in fluorescence intensity except V131L. Remarkably, the alteration of the coordination bond had a significant effect on the fluorescence intensity. Compared with the wild-type, the H149D and H149E reduced the fluorescence intensity by approximately 67%, whereas the H149S and H149P increased the fluorescence intensity by approximately 122%. Since protein engineering at key sites of heme binding changed biosensor's output effectively (Figure 3B), a precise calibration of the biosensor was carried out.
Figure 2.
The GFP Expression Intensity Heatmaps of Promoters Regulated by HrtR Saturation Mutant Library
Experimental data are available in Table S1.
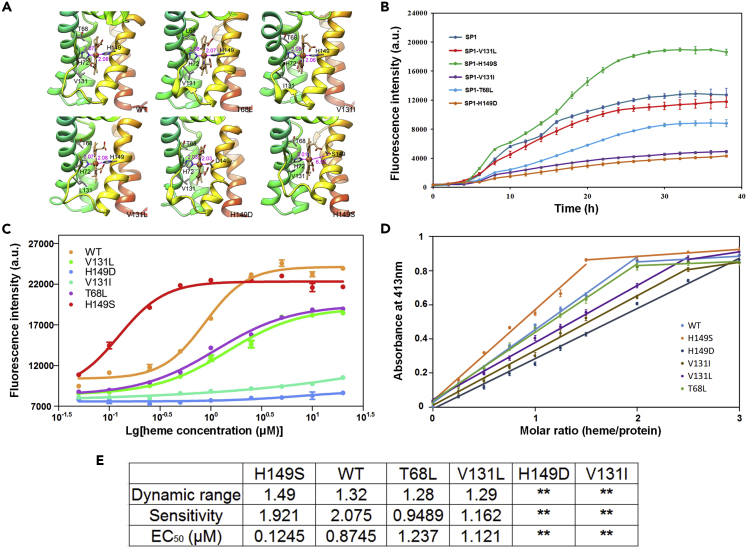
Figure 3.
Characterization of Heme Biosensors with Different HrtR Variations
(A) The equilibrated conformations of the heme-binding regions of the wild-type and mutated HrtR (V131I, V131L, T68L, H149D, H149S) derived from molecular dynamics simulations.
(B) Time-dependent GFP expression intensity under the regulation of HrtR mutants H149D, H149S, V131L, V131I, T68L.
(C) Fluorescence of mutants changes after 8 h cultivation with different concentrations of heme.
(D) Changes in absorbance at 413 nm after titration of mutants with heme at different concentrations. Proteins final concentration was 5 μM.
(E) The Dynamic range, Sensitivity, and EC50 of mutants. Dynamic range is the ratio of rising fluorescence to background fluorescence, Sensitivity is the slope at EC50. Error bars represent ±1 SD from the mean of three replicate cultures.
To characterize the function of constructed heme-responsive biosensors, the dose-response curves of the wild-type and five chosen mutants, H149D, H149S, V131L, V131I, and T68L, were determined. Since E. coli K-12 strains have no natural heme uptake system, heme transporters from three different origins were selected to express in E. coli DH5α, such as HasA/R from Serratia marcescens and HutA from Bartonella. Only ChuA from E. coli O157:H7 EDL933 was effective. We established a dose-response relationship of heme and the GFP output expression of the biosensors in the ChuA-expressing strain. The fluorescence intensity was measured after cultivation for 8 h with different heme concentrations (0.01–20 μM). Since the addition of heme will affect the measurement of fluorescence, the maximum concentration added is set as 20 μM. The results are shown in Figure 3C. HrtR and its mutants H149S, T68L, and V131L showed standard dose-response curves. But 20 μM heme is not enough to support complete dissociation of mutants H149D and V131I. In addition to H149D and V131I, the dynamic range, sensitivity, and EC50 of the other biosensors were all calculated (Figure 3E). Compared with the wild-type, the dynamic ranges of the mutants were slightly reduced and the sensitivities of T68L and V131L to heme was significantly reduced. The EC50 of the different mutants in ascending order was H149S, WT, T68L, V131L.
To verify the heme responses of H149D and V131I, the in vitro heme affinity of HrtR and the five mutants was detected by titrating heme into apo-HrtR and measuring the change in absorbance at 413 nm (Figure 3D) (Sawai et al., 2012). When the molar ratio of heme to protein reached 1.5, H149S first reached saturation. As the ratio increased, other mutants became saturated in turn. This indicates that the heme affinity of H149D and V131I was less than that of other mutants. Figures 3D and 3E together showed that the descending order of affinity to heme is H149S, WT, T68L, V131L, V131I, H149D.
Molecular docking and molecular dynamics simulation revealed the binding affinity change of HrtR and its mutants at the molecular level. Molecular dynamics (Mazumder and Case, 2007) simulations demonstrated that replacing the original T68 or V131 with bulkier leucine or isoleucine residues showed marginal effect on the coordinate bonding between heme and HrtR histidines (H72 and H149) (Figure 3A). However, the binding affinity between heme and HrtR became more energetically unfavorable caused by increased steric repulsions (Table S2). The H149D mutation led to an apparent decrease of the coordinate bond lengths (Figure 3A) and consequently a dramatic increase of steric repulsion with most unfavorable binding free energy (Table S2). In contrast, the H149S mutation resulted in a five-coordinate heme-binding complex in which heme formed most energetically favorable binding with the H149S mutant by locating at a much more relaxed hydrophobic pocket (Table S2 and Figure 3A). The binding free energy result showed that the ligand-binding affinity of the mutants followed the order of H149S > WT > V131L > T68L > V131I > H149D. The trend is consistent with the results of our in vitro and in vivo experiments.
Design and Construction of a Heme-Responsive Regulatory System
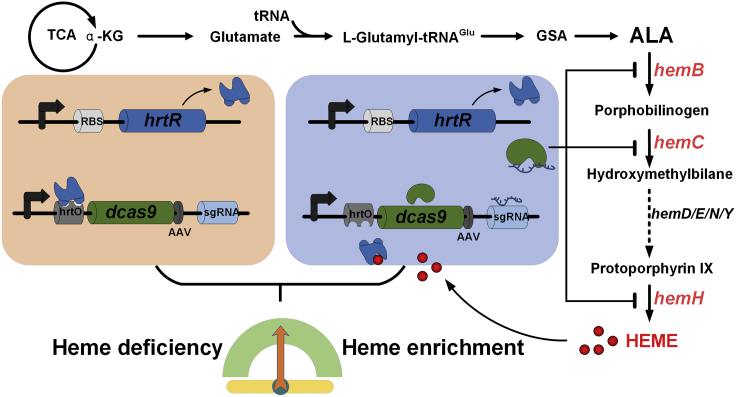
The heme-responsive regulatory system was designed by incorporation of heme biosensor with CRISPRi regulation. We synthesized a hybrid promoter containing the DNA-binding site (hrtO) of HrtR, whereas HrtR expression was driven by a constitutive promoter (Figure 4). The expression of dCas9 and sgRNA is driven by hrtO-hybrid promoters with the cis-regulatory hrtO-operator sequence located within or adjacent to the promoter. The general mechanism of the constructed system is depicted in Figure 4. In the early growth period, heme is absent/low, the constitutively expressed HrtR binds to the hrtO, which hinders the expression of CRISPRi. When heme is synthesized and accumulated, it interacts with HrtR and allows it dissociate from hrtO of dcas9 promoter, resulting in dCas9 expression that inhibits target genes guiding by sgRNA. This further leads to the reduced heme synthesis. Again, low levels of heme results in more HrtR binding to hrtO and turns off CRISPRi gradually, which leads to the increased expression of target gene and the constant increase of intracellular heme. The spontaneous cycle of the system ensures the feasibility of dynamic regulation of heme biosynthesis. To increase the system turnover rate, a degradation tag AAV was added to the C terminus of the dcas9 protein.
Figure 4.
Process of the Heme-Responsive Dynamic Regulation System: HrtR Sensed Excess Heme and Dissociated from hrtO, Protein dcas9 Inhibited Target Gene Expression and Reduced the Intracellular Heme Concentration
Low level of heme results in HrtR turning off the expression of CRISPRi by recombining to hrtO.
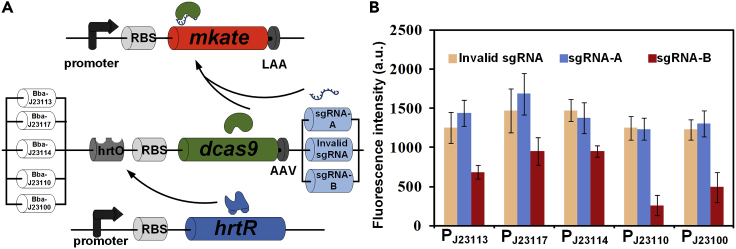
Red fluorescent protein mKATE2 was used as the second marker protein to characterize the inhibition effect of CRISPRi. Similar to the design above, HrtR controlled the expression of CRISPRi and sgRNA; sgRNA was artificially designed with a complementary region to mkate2. Degradation tags LAA were added to the C terminus of mKATE2 to facilitate the turnover. Thus, red fluorescence intensity (i.e., the inhibitory effect of the system) can reflect the intracellular heme concentration change (Figure 5A). Five promoters with different strengths from the iGEM promoter library were selected to initiate expression of dcas9, and two different sgRNA targeting different positions of mkate2 were selected to guide dcas9 (Figure S1). Compared with strains containing sgRNA-A and control strains, the strains containing sgRNA-B located between RBS and promoter of mkate2 showed lower red fluorescence intensity. Among them, the strain containing the promoter BBa-J23110 showed the lowest fluorescence intensity, which means that it had the best inhibition of mKATE2 (Figure 5B). So, the promoter BBa-J23110 and sgRNA-B were selected for next experiments.
Figure 5.
Optimization of CRISPRi Action Site and Expression Intensity in Heme Regulatory System
(A) Schematic representation of the dynamic regulation system represented by the reporter mKATE2.
(B) Red fluorescence in strains containing different promoters of dcas9 and different sgRNA. Error bars represent ±1 SD from the mean of three replicate cultures.
Dynamic Pathway Regulation Using the Heme-Responsive Regulation System
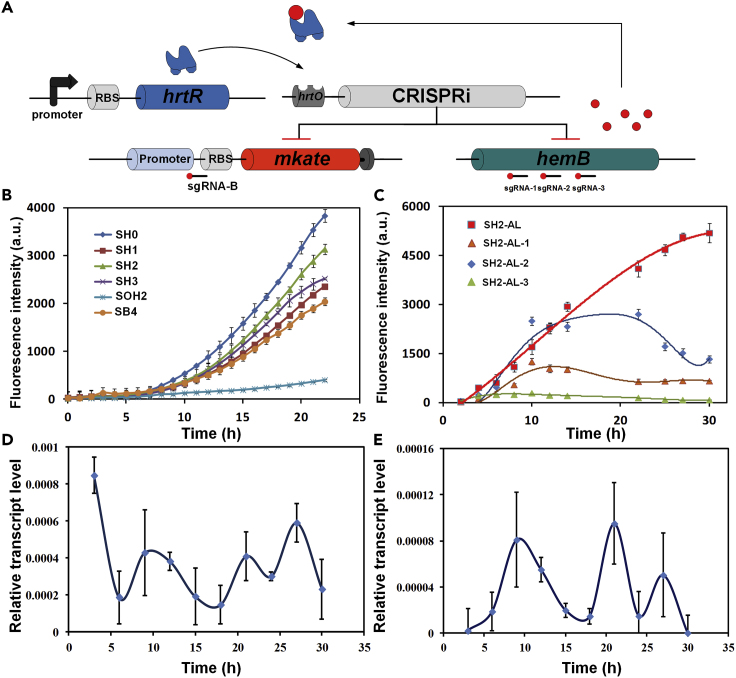
To investigate the dynamic regulation pattern of this system, an sgRNA targeting the hemB was designed (Figure S2). When hemB is inhibited, the intracellular heme concentration is reduced and CRISPRi expression is turned off, thereby canceling hemB inhibition. mKATE2 can be the visualization of the expression of hemB via the change of fluorescence. Similarly, in order to find the most suitable inhibition site for hemB, three various sgRNAs targeting different positions of hemB were used for further screening (Figure 6A). Three constructions were used as blank controls: SH0 (only contained sgRNA targeting hemB rather than mkate2); SB4 (only contained sgRNA targeting mkate2 rather than hemB); SOH2 (removed off the hrtO site within the promoter of CRISPRi system, resulting the constitutive expression of dcas9 and sgRNA) (Figures S1 and S2). The red fluorescence intensity of SH0 was the highest and that of SOH2 was the lowest. The strains in which hemB was inhibited had a higher fluorescence intensity than SB4 (Figure 6B). This indicated that the CRISPRi had a significant inhibitory effect on mKATE2 in normal heme accumulating E. coli. When hemB was inhibited, the intracellular heme concentration decreased, less dcas9 and sgRNA were expressed, resulting in increase in the red fluorescence intensity. This was in line with our design and proved the effectiveness of the synthetic regulatory system.
Figure 6.
Verification of the Function of the Heme Dynamic Regulation System
(A) Schematic diagram of dynamic control system acting on both hemB and mkate2.
(B) Red fluorescence intensity of strains containing both sgRNA targeting mkate2 and hemB. SH0 (mkate2 is not inhibited by CRISPRi), SH1 (sgRNA-A and sgRNA-1), SH2 (sgRNA-A and sgRNA-2), SH3 (sgRNA-A and sgRNA-3), SOH2 (removed hrtO based on SH2, making CRISPRi constitutive expression), SB4 (only contains sgRNA-A, no sgRNA targeting hemB).
(C) Red fluorescence intensity change after replacing the mkate2 promoter and RBS, SH2-AL: Ptac and RBSB0034; SH2-AL-1: PJ23110 and RBSB0032; SH2-AL-2: PJ23101 and RBSB0032; SH2-AL-3: PJ23106 and RBSB0032.
(D) The relative expression level of dcas9 in SH2-AL changes with time.
(E) The relative expression level of hemB in SH2-AL changes with time. Error bars represent ±1 SD from the mean of three replicate cultures.
To visualize the in vivo fluctuation of the gene expression, two sgRNAs simultaneously affected by in vivo heme concentration were designed to target mkate2 and hemB, respectively. According to our design, dynamic regulation of hemB made the heme accumulation exhibit an oscillatory changing pattern and so does the expression of hemB and mkate2. However, results showed that the strain in which hemB was inhibited (SH2) had no dynamic fluctuations of red fluorescence. This may be due to the low intracellular heme concentration. To improve intracellular heme concentration, hemA from Salmonella arizona and hemL from E. coli were added to SH2 to obtain SH2-AL. No significant fluorescence fluctuations were observed again in SH2-AL. To solve this problem, we changed the promoter and RBS of mKATE2 to reduce the expression of mkate2. Finally, obvious fluctuations of fluorescence intensity were observed (Figure 6C). Among them, SH2-AL-2 (promoter: BBa-J23101, RBS: B0032) showed a significant fluorescence intensity change. These results indicated that the synthetic regulatory system can dynamically regulate metabolic pathway by perturbing the genes expression.
To further verify the dynamic regulation in different aspects, real-time fluorescence quantitative PCR was used to analyze the expression of hemB and dcas9 at the transcriptional level. SH2-AL-2 was cultivated in a shake flask with 50 mL of LB medium and sampled for mRNA analysis every 3 h. Fluctuations were observed at different time points in the strains SH2-AL-2 (Figures 6D and 6E). The RT-PCR result proved that the synthetic regulatory system was capable of dynamically regulating dcas9 and hemB expression.
Increased ALA Production Using Heme-Responsive Regulatory System
Previous studies in ALA showed that direct overexpression of the key genes in heme synthesis pathway accumulated large quantity of downstream products including heme and porphyrins, which are toxic and affected cell growth (Kang et al., 2011).
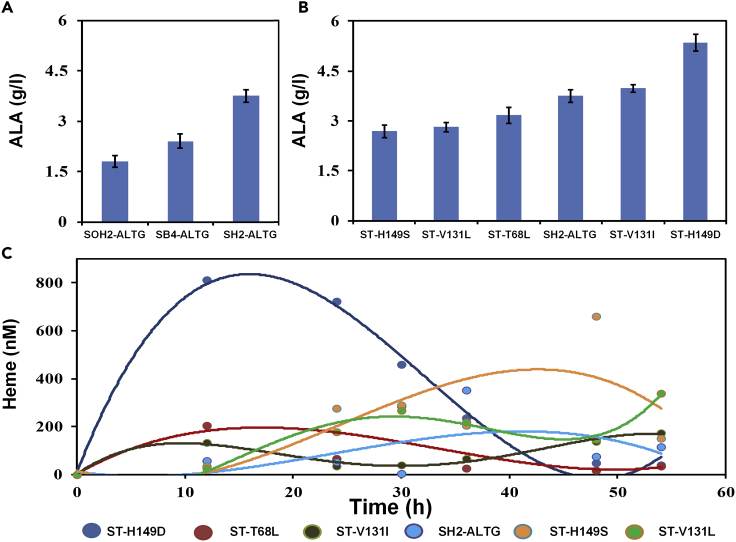
Based on SH2-AL, an ALA production strain was constructed by overexpression of gltW (tRNAGlu, the tRNA responsible for charging glutamic acid), rhtA (inner membrane transporter), and gdhA (glutamate dehydrogenase) to obtain the strain SH2-ALTG; strains SB4-ALTG (expression of hemAL, gltW, gdhA, rhtA on the basis of SB4) and SOH2-ALTG (expression of hemAL, gltW, gdhA, rhtA on the basis of SOH2) were set as control. After cultivation, strains SH2-ALTG, SB4-ALTG, SOH2-ALTG have no significant difference in cell growth and glucose consumption. SH2-ALTG accumulated 3.75g/L ALA, which was 1.54-fold of SB4-ALTG (2.42g/L) and 2.09-fold of SOH2-AL (1.8 g/L) (Figure 7A). The results showed that the dynamic regulation of hemB contributes to the increased ALA production.
Figure 7.
The Application of Heme Dynamic Regulation System in ALA Production
(A) Improvement of ALA production through regulation of hemB by heme dynamic regulation system. SOH2-ALTG: Mssing hrtO, constitutive expression of CRISPRi, hemB was continuously inhibited; SB4-ALTG: without sgRNA targeting hemB, hemB was not inhibited; SH2-ALTG: hemB was regulated by dynamic regulation system.
(B) The ALA production of the engineered strains in which hemB was, respectively regulated by wild-type and five HrtR mutants. Strains ST-H149S, ST-V131L, ST-T68L, ST-V131I, and ST-H149D have amino acid mutations that occurred on the basis of SH2-ALTG.
(C) Intracellular free heme concentration curve of fermentation strain. Error bars represent ±1 SD from the mean of three replicate cultures.
Mutant biosensors with different binding affinity were applied to investigate the effect of ligand-binding affinity on regulation efficiency. As expected, the binding affinity of biosensor had a significant effect on ALA production (Figure 7B). During ALA fermentation, intracellular free heme of these six strains showed a pattern of rise-decrease-rise (Figure 7B). The intracellular heme concentration of the mutant H149D was the highest. At the same time, H149D accumulates the most ALA, 5.35 g/L, which indicates that a high in vivo heme level at the early stage of growth is beneficial for cell growth and the ALA production.
Application of Synthetic Regulatory System in Porphyrin and Porphobilinogen Synthesis
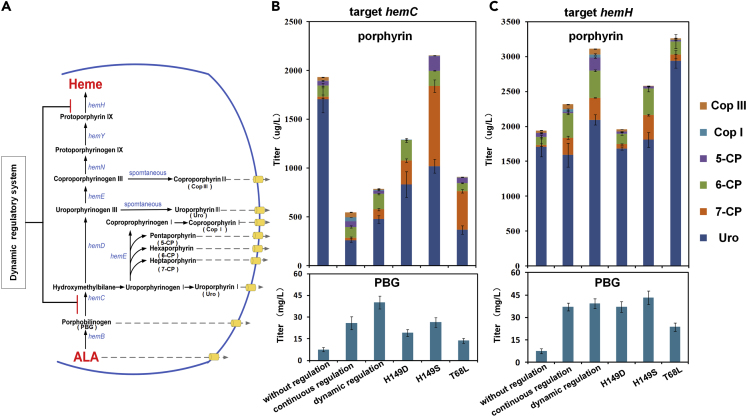
Porphyrins play an important role in the fields of medical and materials chemistry (Birnbaum et al., 1995, Karpishin et al., 1994). First, dynamic regulation using heme-responsive biosensor was tried to synthesis PBG by designing sgRNA targeting hemC (encoding hydroxymethylbilane synthase). PBG accumulation of the strain under the dynamic regulation is 40.25 mg/L, 428.4% higher than that of the un-regulated strain and 80% higher than that of the constitutive-regulated strain (Figure 8B). Meanwhile, the six porphyrin compounds found in the fermentation broth were uroporphyrin (Uro), heptaporphyrin (7-CP), hexaporphyrin (6-CP), pentaporphyrin (5-CP), coproporphyrin I (CopI), and coproporphyrin III (CopIII) (among them, uroporphyrin is a mixture of uroporphyrin I and uroporphyrin III) (Figure 8A). The downstream porphyrin compounds reduced by 55.6% after dynamic regulation of hemC, whereas the un-regulated strains accumulated large amounts of porphyrins. Mutants (H149D, H149S, T69L) were used to investigate the regulation role of different biosensors. Among these HrtR and its mutants, wild-type HrtR was the most effective biosensor on the accumulation of PBG. The intracellular free heme of all dynamic strains showed a fluctuation trend with time. The intracellular free heme concentration of H149D was the highest within 30 h (Figures S6B and S6D).
Figure 8.
The Application of Heme Dynamic Regulation System in ALA Production
(A) Heme biosynthesis pathway in Escherichia coli. hemA: glutamyl-tRNA reductase; hemL: glutamate-1-semialdehyde aminotransferase; hemB: 5-ALA dehydratase; hemC: PBG deaminase; hemD: uroporphyrinogen III synthase; hemE: uroporphyrinogen decarboxylase; hemN: coproporphyrinogen III oxidase; hemY: protoporphyrinogen oxidase; hemH: protoporphyrin ferrochelatase.
(B) PBG and porphyrin productions in dynamic regulation hemC strains.
(C) PBG and porphyrin productions in dynamic regulation hemH strains. Error bars represent ±1 SD from the mean of three replicate cultures.
We also designed sgRNA targeting hemH (encoding coproporphyrin ferrochelatase). The strain that dynamically regulates hemH (SAL-H), strain constitutively inhibiting hemH (SAL-HO) and SB4-AL were used for fermentation. The strain with dynamic regulatory system accumulated 65% higher porphyrin compounds than the control strains. The mutants T68L resulted in the highest porphyrin production and reached 3,263.29 μg/L (Figure 8C).
Discussion
Biosensors with suitable sensitivity and threshold are the key for regulating the pathway dynamically and precisely. Since heme may act as an allosteric molecule that binds to regulatory proteins and regulate the heme biosynthesis, degradation, and transportation, a universal heme-responsive biosensor can be designed and constructed. Initially, two prokaryotic heme-responsive proteins were selected: iron response regulator (Irr) from Rhizobium leguminosarum (Hamza et al., 1998) and HrtR from Lactococcus lactis. However, Irr has more than two Heme Regulatory Motifs (Lohrmann et al., 2019) and was also affected by iron level (Singleton et al., 2010), which resulted in complexity and uncertainty. Thus, it was finally discarded after several trials.
To characterize the function of heme-responsive biosensor, a dose-response curve dependent on heme concentration should be determined. However, E. coli K-12 strains have no natural heme uptake system. To address this issue, we employed multi-copy integrated hemA/hemL strains that can provide different in vivo heme concentration (Cui et al., 2019). The positive correlation between the green fluorescence intensity and the in vivo heme concentration was observed and guaranteed the characterization of the regulatory system (Figure 1B). And we tried to express the heme transporter HasA/R from Serratia marcescens and the transporter HutA from Bartonella in E. coli, but this did not work (data not shown); finally, this goal was achieved by using ChuA from E. coli O157:H7 EDL933. To tune the binding affinity of heme biosensor, three key sites of HrtR that were supposed to be involved in heme binding were selected for saturated mutation based on the protein structure (Sawai et al., 2012). Evaluating biosensor characteristics is necessary before its application. Based on this, a series of heme-responsive biosensors with different sensitivity and threshold were obtained. Our results proved that modifying the metabolite-binding affinity generated a very clear horizontal shift of the dose-response curve with more than 8-fold EC50 change and over 2-fold sensitivity change. Detailed biophysical analysis such as heme titration and molecular dynamics simulation can contribute to revealing strategies for biosensor precise control and operational direction. In a sense, some analysis like molecular dynamics simulation can be effective auxiliary methods.
The fine-tuned biosensors can be applied in many different pathways. For example, insensitive biosensor needs high heme concentration for dissociation of HrtR from the promoter regulatory site and may be suitable for heme tolerant process. When biosensor H149D was applied in ALA production, 5.35 g/L ALA was obtained. This is the highest ALA production in batch cultivation of E. coli.
Compared with antisense RNA (asRNA), RNA interference (RNAi), and protein degradation systems (Cameron and Collins, 2014, Na et al., 2013, Yang et al., 2015), CRISPRi is a robust RNA-guided modulation system that acts at the transcriptional level other than post-transcriptional level and post-translational level. It has many advantages, such as reversibility (Qi et al., 2013). However, it was reported that dCas9 may continuously bind to DNA and thus CRISPRi has a relatively slower turnover rate, which may affect the dynamic regulation efficiency (Qi et al., 2013). Therefore, we designed a degradation tag to the C terminus of dcas9 to speed up the turnover rate. The oscillatory expression of hemB and dcas9 was observed after this modification (Figures 6D and 6E). The oscillation results under dynamic regulation were also verified by the results of red fluorescence and intracellular heme fluctuation (Figures 6C and 7C).
Since the heme biosynthesis pathway possesses many important compounds, such as B12 (Fang et al., 2018), siroheme, and chlorophyll (Chen et al., 2018), and heme is a small molecule necessary for cell respiration, the heme-responsive regulatory system can be applied for many processes, including basic metabolism (Zhang et al., 2017). In a broader scientific context, biosensor with tailor-made sensitivity provides the possibility of regulating the biological process more dynamically and precisely.
Limitations of the Study
Owing to the transport efficiency of heme transporter and the fact that excessive addition of heme will affect the fluorescence measurement, we could not determine the EC50 of the mutants H149D and V131I and only analyzed it by heme titration.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (31730003, 31670047, and 31770095), the National Key R&D Program of China (2019YFA0904900), and Young Scholars Program of Shandong University.
Author Contributions
J.Z. completed all experiments and the writing of the original manuscript; Z.W. was responsible for the work of molecular dynamics simulation; T.S. participated in the design of the regulatory system and some preliminary work; H.S. and Y.Z. participated in the construction of some plasmids; Q.Q. and Q.W. designed and supervised the entire work and completed the writing of the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: May 22, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101067.
Contributor Information
Qingsheng Qi, Email: qiqingsheng@sdu.edu.cn.
Qian Wang, Email: qiqi20011983@gmail.com.
Supplemental Information
References
- Baureder M., Hederstedt L. Heme proteins in lactic acid bacteria. Adv. Microb. Physiol. 2013;62:1–43. doi: 10.1016/B978-0-12-410515-7.00001-9. [DOI] [PubMed] [Google Scholar]
- Birnbaum E.R., Grinstaff M.W., Labinger J.A., Bercaw J.E., Gray H.B. On the mechanism of catalytic alkene oxidation by molecular oxygen and halogenated iron porphyrins. J. Mol. Catal. A Chem. 1995;104:L119–L122. [Google Scholar]
- Blazeck J., Alper H.S. Promoter engineering: recent advances in controlling transcription at the most fundamental level. Biotechnol. J. 2013;8:46–58. doi: 10.1002/biot.201200120. [DOI] [PubMed] [Google Scholar]
- Bonyhady R.E., Hendry I.A., Hill C.E., McLennan I.S. Effects of haemin on neurones derived from the neural crest. Dev. Neurosci. 1982;5:125–129. doi: 10.1159/000112669. [DOI] [PubMed] [Google Scholar]
- Brophy J.A., Voigt C.A. Principles of genetic circuit design. Nat. Methods. 2014;11:508–520. doi: 10.1038/nmeth.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron D.E., Collins J.J. Tunable protein degradation in bacteria. Nat. Biotechnol. 2014;32:1276–U1149. doi: 10.1038/nbt.3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G.E., Canniffe D.P., Barnett S.F.H., Hollingshead S., Brindley A.A., Vasilev C., Bryant D.A., Hunter C.N. Complete enzyme set for chlorophyll biosynthesis in Escherichia coli. Sci. Adv. 2018;4:eaaq1407. doi: 10.1126/sciadv.aaq1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.J., London I.M. Hemin enhances the differentiation of mouse 3t3-cells to adipocytes. Cell. 1981;26:117–122. doi: 10.1016/0092-8674(81)90039-8. [DOI] [PubMed] [Google Scholar]
- Choby J.E., Skaar E.P. Heme synthesis and acquisition in bacterial pathogens. J. Mol. Biol. 2016;428:3408–3428. doi: 10.1016/j.jmb.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z.Y., Jiang Z.N., Zhang J.H., Zheng H.H., Jiang X., Gong K., Liang Q.F., Wang Q., Qi Q.S. Stable and efficient biosynthesis of 5-aminolevulinic acid using plasmid-free Escherichia coli. J. Agric. Food Chem. 2019;67:1478–1483. doi: 10.1021/acs.jafc.8b06496. [DOI] [PubMed] [Google Scholar]
- Ding J.M., Chen D., Weber E.T., Faiman L.E., Rea M.A., Gillette M.U. Resetting the biological clock: mediation of nocturnal circadian shifts by glutamate and NO. Science. 1994;266:1713–1717. doi: 10.1126/science.7527589. [DOI] [PubMed] [Google Scholar]
- Fang H., Li D., Kang J., Jiang P., Sun J., Zhang D. Metabolic engineering of Escherichia coli for de novo biosynthesis of vitamin B12. Nat. Commun. 2018;9:4917. doi: 10.1038/s41467-018-07412-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Xie Z., Jiang X., Li Z., Shen Y., Wang B., Liu J. The applications of promoter-gene-engineered biosensors. Sensors. 2018;18 doi: 10.3390/s18092823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana J., Dong C., Ham J.Y., Zalatan J.G., Carothers J.M. Regulated expression of sgRNAs tunes CRISPRi in E. coli. Biotechnol. J. 2018;13:e1800069. doi: 10.1002/biot.201800069. [DOI] [PubMed] [Google Scholar]
- Frunzke J., Gatgens C., Brocker M., Bott M. Control of heme homeostasis in corynebacterium glutamicum by the two-component system HrrSA. J. Bacteriol. 2011;193:1212–1221. doi: 10.1128/JB.01130-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick B.R. Metabolic load and heterologous gene expression. Biotechnol. Adv. 1995;13:247–261. doi: 10.1016/0734-9750(95)00004-a. [DOI] [PubMed] [Google Scholar]
- Hamza I., Chauhan S., Hassett R., O'Brian M.R. The bacterial irr protein is required for coordination of heme biosynthesis with iron availability. J. Biol. Chem. 1998;273:21669–21674. doi: 10.1074/jbc.273.34.21669. [DOI] [PubMed] [Google Scholar]
- Han Y., Meyer M.H., Keusgen M., Klug G. A haem cofactor is required for redox and light signalling by the AppA protein of Rhodobacter sphaeroides. Mol. Microbiol. 2007;64:1090–1104. doi: 10.1111/j.1365-2958.2007.05724.x. [DOI] [PubMed] [Google Scholar]
- Kang Z., Wang Y., Gu P.F., Wang Q., Qi Q.S. Engineering Escherichia coli for efficient production of 5-aminolevulinic acid from glucose. Metab. Eng. 2011;13:492–498. doi: 10.1016/j.ymben.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Karpishin T.B., Grinstaff M.W., Komarpanicucci S., Mclendon G., Gray H.B. Electron-transfer in cytochrome-C depends upon the structure of the intervening medium. Structure. 1994;2:415–422. doi: 10.1016/s0969-2126(00)00043-5. [DOI] [PubMed] [Google Scholar]
- Layer G., Reichelt J., Jahn D., Heinz D.W. Structure and function of enzymes in heme biosynthesis. Protein Sci. 2010;19:1137–1161. doi: 10.1002/pro.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechardeur D., Cesselin B., Liebl U., Vos M.H., Fernandez A., Brun C., Gruss A., Gaudu P. Discovery of intracellular heme-binding protein HrtR, which controls heme efflux by the conserved HrtB-HrtA transporter in Lactococcus lactis. J. Biol. Chem. 2012;287:4752–4758. doi: 10.1074/jbc.M111.297531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Evans T., Zhang F. Applications and advances of metabolite biosensors for metabolic engineering. Metab. Eng. 2015;31:35–43. doi: 10.1016/j.ymben.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Liu D., Xiao Y., Evans B.S., Zhang F. Negative feedback regulation of fatty acid production based on a malonyl-CoA sensor-actuator. ACS Synth. Biol. 2015;4:132–140. doi: 10.1021/sb400158w. [DOI] [PubMed] [Google Scholar]
- Lohrmann V., Ohl M., Michalik P., Pitts J.P., Jeanneau L., Perrichot V. Notes on rhopalosomatid wasps of Dominican and Mexican amber (Hymenoptera: Rhopalosomatidae) with a description of the first fossil species of Rhopalosoma Cresson, 1865. Foss Rec. 2019;22:31–44. [Google Scholar]
- Mahr R., Frunzke J. Transcription factor-based biosensors in biotechnology: current state and future prospects. Appl. Microbiol. Biotechnol. 2016;100:79–90. doi: 10.1007/s00253-015-7090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannan A.A., Liu D., Zhang F., Oyarzun D.A. Fundamental design principles for transcription-factor-based metabolite biosensors. ACS Synth. Biol. 2017;6:1851–1859. doi: 10.1021/acssynbio.7b00172. [DOI] [PubMed] [Google Scholar]
- Martin V.J., Pitera D.J., Withers S.T., Newman J.D., Keasling J.D. Engineering a mevalonate pathway in Escherichia coli for production of terpenoids. Nat. Biotechnol. 2003;21:796–802. doi: 10.1038/nbt833. [DOI] [PubMed] [Google Scholar]
- Mazumder D., Case D.A. AMBER Score in DOCK6: application of molecular dynamics simulations and implicit solvent model (GB/SA) in protein-ligand docking. Abstr. Pap. Am. Chem. S. 2007;233:20. [Google Scholar]
- Na D., Yoo S.M., Chung H., Park H., Park J.H., Lee S.Y. Metabolic engineering of Escherichia coli using synthetic small regulatory RNAs. Nat. Biotechnol. 2013;31:170–174. doi: 10.1038/nbt.2461. [DOI] [PubMed] [Google Scholar]
- Qi L.S., Larson M.H., Gilbert L.A., Doudna J.A., Weissman J.S., Arkin A.P., Lim W.A. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell. 2013;152:1173–1183. doi: 10.1016/j.cell.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z., Hamza I., O'Brian M.R. Heme is an effector molecule for iron-dependent degradation of the bacterial iron response regulator (Irr) protein. Proc. Natl. Acad. Sci. U S A. 1999;96:13056–13061. doi: 10.1073/pnas.96.23.13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter S.W., Tyrrell R.M. The Heme synthesis and degradation pathways: role in oxidant sensitivity - heme oxygenase has both pro- and antioxidant properties. Free Radic. Bio. Med. 2000;28:289–309. doi: 10.1016/s0891-5849(99)00223-3. [DOI] [PubMed] [Google Scholar]
- Sawai H., Yamanaka M., Sugimoto H., Shiro Y., Aono S. Structural basis for the transcriptional regulation of heme homeostasis in Lactococcus lactis. J. Biol. Chem. 2012;287:30755–30768. doi: 10.1074/jbc.M112.370916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelver D., Kerby R.L., He Y.P., Roberts G.P. CooA, a CO-sensing transcription factor from Rhodospirillum rubrum, is a CO-binding heme protein. Proc. Natl. Acad. Sci. U S A. 1997;94:11216–11220. doi: 10.1073/pnas.94.21.11216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Orr S.S., Milo R., Mangan S., Alon U. Network motifs in the transcriptional regulation network of Escherichia coli. Nat. Genet. 2002;31:64–68. doi: 10.1038/ng881. [DOI] [PubMed] [Google Scholar]
- Singleton C., White G.F., Todd J.D., Marritt S.J., Cheesman M.R., Johnston A.W., Le Brun N.E. Heme-responsive DNA binding by the global iron regulator Irr from Rhizobium leguminosarum. J. Biol. Chem. 2010;285:16023–16031. doi: 10.1074/jbc.M109.067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor N.D., Garruss A.S., Moretti R., Chan S., Arbing M.A., Cascio D., Rogers J.K., Isaacs F.J., Kosuri S., Baker D. Engineering an allosteric transcription factor to respond to new ligands. Nat. Methods. 2016;13:177–183. doi: 10.1038/nmeth.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabelsi H., Koch M., Faulon J.L. Building a minimal and generalizable model of transcription factor-based biosensors: showcasing flavonoids. Biotechnol. Bioeng. 2018;115:2292–2304. doi: 10.1002/bit.26726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiftsoglou A.S., Tsamadou A.I., Papadopoulou L.C. Heme as key regulator of major mammalian cellular functions: molecular, cellular, and pharmacological aspects. Pharmacol. Therapeut. 2006;111:327–345. doi: 10.1016/j.pharmthera.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Tyo K.E., Ajikumar P.K., Stephanopoulos G. Stabilized gene duplication enables long-term selection-free heterologous pathway expression. Nat. Biotechnol. 2009;27:760–765. doi: 10.1038/nbt.1555. [DOI] [PubMed] [Google Scholar]
- Xu P., Li L., Zhang F., Stephanopoulos G., Koffas M. Improving fatty acids production by engineering dynamic pathway regulation and metabolic control. Proc. Natl. Acad. Sci. U S A. 2014;111:11299–11304. doi: 10.1073/pnas.1406401111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.P., Lin Y.H., Li L.Y., Linhardt R.J., Yan Y.J. Regulating malonyl-CoA metabolism via synthetic antisense RNAs for enhanced biosynthesis of natural products. Metab. Eng. 2015;29:217–226. doi: 10.1016/j.ymben.2015.03.018. [DOI] [PubMed] [Google Scholar]
- Zhang F., Carothers J.M., Keasling J.D. Design of a dynamic sensor-regulator system for production of chemicals and fuels derived from fatty acids. Nat. Biotechnol. 2012;30:354–359. doi: 10.1038/nbt.2149. [DOI] [PubMed] [Google Scholar]
- Zhang T.T., Bu P.L., Zeng J., Vancura A. Increased heme synthesis in yeast induces a metabolic switch from fermentation to respiration even under conditions of glucose repression. J. Biol. Chem. 2017;292:16942–16954. doi: 10.1074/jbc.M117.790923. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.