Abstract
Background: There is a paucity of data on the dynamics of human papillomavirus (HPV) antibodies in children. We aimed to describe the vertical transmission and clearance of antibodies against HPV6, 11, 16 and 18 in children.
Methods: We used data from pregnant women recruited into the HERITAGE cohort study between 2009 and 2012 who were positive for HPV-DNA at baseline. Dried blood spots were collected during the first trimester in pregnant participants, and at birth, 6, 12, and 24 months of age in children. The level of total immunoglobulin G (IgG) against HPV6, 11, 16 and 18 were measured using Luminex immunoassays. Spearman's coefficients were used to correlate HPV antibody levels between newborns and mothers. Panel and Kaplan-Meier graphics described antibody dynamics in the first 24 months of life.
Findings: Antibodies from newborns and mothers (n = 58 pairs) were moderately to highly correlated with coefficients of 0·81 (95% confidence intervals (CI):0·70–0·88), 0·68 (95% CI:0·5–0·80), 0·90 (95% CI:0·83–0·94) and 0·85 (95% CI:0·76–0·91) against HPV6, 11, 16 and 18, respectively. In newborns seropositive at birth, anti-HPV antibodies were cleared by 80% and 100% at 12 and 24 months, respectively. Only two children presented detectable HPV antibodies at 24 months. The first child had no detectable antibodies at birth and the second presented increasing levels after two undetected measures.
Interpretation: Correlation between mother and newborn IgG antibodies against HPV suggests vertical transfer. Most children cleared anti-HPV antibodies within six to 12 months.
Funding: The Canadian Institutes of Health Research (CIHR)
Keywords: Human papillomavirus, Serology, Mother-newborn correlation, Vertically acquired immunity, Antibody clearance, Antibody dynamics
1. Introduction
Although most commonly sexually transmitted, mucosal human papillomaviruses (HPV) have been detected in newborns and children before their sexual debut [1,2]. Persistent asymptomatic infections have also been described during childhood [3] and despite being rare, clinical manifestations of HPV in children can be catastrophic. For instance, recurrent respiratory papillomatosis is associated with high morbidity and can be life-threatening in severe cases [4].
Maternal antibodies play an essential role in the protection of fetuses and newborns while their immune system develops [5]. Maternal immunoglobulins G (IgG) cross the placental barrier and are gradually cleared after birth [5]. Studies on waning of maternal antibodies against viruses show that most children clear vertically transferred IgG between six and 12 months after birth [6,7]. Maternal vaccination status, coexisting infections and malnutrition, as well as the newborn gestational age can impact the amount of vertically transferred antibodies [6,8]. Clearance time of antibodies is influenced by the concentration of antibodies in the fetal blood at birth, a higher concentration in the newborn allowing the protection to last longer [5,9].
The immune response to HPV infection has several distinguishing features. Because HPV infection involves low levels of exposure to viral proteins and does not induce a phase of viremia and cytolysis [10], the serological response can be delayed and is characterized by low levels of antibodies. In addition, among those infected with HPV, it is thought that only 50–70% will develop specific antibodies [11]. Furthermore, the immune response induced by the HPV vaccine stimulates much higher antibody levels than a natural infection [12], which is contrary to other viral infections that generally stimulate higher antibody levels compared to levels attained after vaccination [6,13]. These features could influence the transmission of mother-child antibodies and the dynamics of these antibodies in children. However, very few studies in the literature have documented the vertical transmission of HPV antibodies and no study has described the dynamics and elimination of HPV antibodies in newborns [14], [15], [16]. Thus, this study sought to describe the natural history of antibodies against HPV6, 11, 16 and 18 in a cohort of children born to mothers recruited before the widespread use of HPV vaccination and followed for up to two years. More specifically, the objectives were: (1) to correlate anti-HPV IgG antibody levels in capillary blood between mothers and newborns at birth; (2) to describe anti-HPV IgG antibodies dynamics in children over the first two years of life; and (3) to estimate median clearance times of vertically transferred anti-HPV IgG antibodies.
2. Methods
HERITAGE is a prospective cohort study which enrolled 1052 pregnant women from three university-affiliated health care centers (Sainte-Justine University Hospital Center, Centre Hospitalier de l'Université de Montréal and Saint-Mary's Hospital Center) in Montreal. HERITAGE was undertaken to study mother-child HPV transmission and adverse birth outcomes associated with HPV. The design of the study was described elsewhere [17]. Participants included in this analysis were recruited in the first phase of HERITAGE and followed between November 2009 and June 2012, for a total of 166 women out of the 207 eligible during this period (participation rate: 80%). Pregnant women aged 18–30 years were eligible to participate if they were between eight and 14 weeks of gestation and planned to give birth at one of the participating hospitals. Women unable to provide informed consent or infected with HIV were excluded. There were 74 children born to mothers who were HPV positive during the first trimester. Of those, there were 58 mother-newborn pairs with valid serological data who were included in the correlation analysis.
At recruitment (first trimester of pregnancy), women provided a self-collected vaginal swab for HPV-testing, and blood samples for HPV serology testing. Blood samples from children born to HPV-DNA positive mothers were collected at birth, six, 12 and 24 months of age for HPV serology testing. Pregnant women provided information on age, ethnicity, vaccination status and gestational age at enrolment. Newborn information on sex, gestational age and birth weight were collected from medical files at delivery.
2.1. Ethics
The study protocol was approved by the institutional ethical and research review boards of each participating site. Written informed consent was obtained from all participating women.
2.2. Swab collection and HPV-DNA testing
Women provided a self-collected vaginal specimen at enrolment using a polyester dry swab. Participants were instructed to insert a swab into the vaginal opening until physically it cannot go any further (at least 2.5 in.) and rotate three times before placing the swab in a dry tube. All samples were individually rinsed with 1.5 mL of PreservCyt in a plastic vial. DNA was extracted using a Master Pure DNA purification kit [18], and stored at −70 °C. Linear Array HPV genotyping assays (LA-HPV; Roche Molecular Systems) were then used to detect the following 36 types of mucosal HPV: 6, 11, 16, 18, 26, 31, 33, 34 (formerly 64), 35, 39, 40, 42, 44 (formerly 55), 45, 51, 52, 53, 54, 56, 58, 59, 61, 62, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 83, 84 and 89. Human β-globin DNA was co-amplified to assess DNA integrity and to screen for the presence of inhibitors [18]. Each amplification run contained a negative, a weak positive and a strong positive control. Samples which were both β-globin and HPV-negative were considered inadequate. HPV52 was detected with a probe that also cross-reacts with types 33, 35 and 58. Samples reactive in LA-HPV were therefore further tested with a validated HPV52-specific real-time polymerase chain reaction (PCR) assay [19].
2.3. Blood collection and HPV serology testing
Dried blood spot (DBS) samples were collected using a Contact-Activated Lancet (BD Microtainer®, USA) in women at enrolment and in babies from HPV-DNA-positive mothers 24–48 h after birth, and at six, 12 and 24 months. After birth, samples from babies were collected at the same time as the routine heel-prick Guthrie test. Expressed blood was placed on Whatman 903™ Protein saver cards (GE Healthcare Ltd., UK) and had to fill two circles of 12.7 mm each, corresponding to a total volume of about 160 μL, to pass quality standards. After drying for a minimum of two to six hours at room temperature, DBS were stored at −80 °C. Serum samples from women at recruitment were also obtained along with each DBS to validate HPV serology testing using DBS. Sera were stored in cryogenic tubes at −80 °C. Both sera and DBS were ultimately transferred from collaborating institutions to the Laboratoire de Santé Publique du Québec (LSPQ), Canada, for testing.
Total IgG antibodies against HPV6, 11, 16 and 18 from sera and DBS specimens were analysed simultaneously using non-competitive Luminex immunoassays (ncLIA). Virus-like particles (VLP) were produced with human embryonic kidney cell line 293TT [20]. HPV-L1 or L1-L2 expression vectors p6SheLLr (~10Kb), p11L1W (~6.2Kb), p16L1-GFP (~5.4Kb) and p18L1L2 (~6.1Kb) were used to produce VLPs for HPV6, 11, 16 and 18, respectively. Their nucleotide sequence can be found at http://home.ccr.cancer.gov/lco/default.asp. IgG antibodies to all four HPV types detected by our in-house assay were then analysed using at least L1 protein as this is the main capsid protein of the virus. DBS samples were classified by percentage of blood cover on filter paper. HPV serology testing on DBS was preceded by a validation phase to determine DBS elution volume and serum dilution, based on the serum-DBS pairs of 20 pregnant women. One and two 6 mm discs of DBS, diluted at 1:4, were compared to sera diluted 1:50 and 1:20 (data not shown). Optimized conditions for DBS elution were obtained with two punches of 6 mm placed in 150 µL of elution buffer (0.05% Tween 20 in phosphate buffered saline (PBS) 0.01 M pH 7.2) and placed in a refrigerator for 18 h with moderated agitation. The elutes were centrifuged at 13,000 x g for four minutes at 20 °C. Fifty µL of DBS elution were then added to 50 µL of serum buffer (1% bovine serum albumin (BSA), 0.1% Tween 20, 0.5% polyvinyl alcohol (PVA), and 0.8% polyvinylpyrrolidone (PVP) in PBS pH 7.2), reaching a final DBS elute dilution of 1:4. Serum samples were diluted 1:50 in serum buffer. Reactions were performed in 96-well plates. DBS elutes, diluted serum samples, in-house HPV-type-specific negative and positive controls, and the HPV16 International Standard of the World Health Organization NIBSC, (cat. No. 05/134) were analysed in each plate. Although the HPV-18 international standard is now available, it was not during our study. HPV6 and HPV11 international standards are still not available. To optimize comparability, maternal serum and DBS elutes as well as offspring DBS elutes were analysed in parallel on the same plate.
VLPs of each HPV type were coupled to distinct fluorescent beads (Luminex Corporation, USA) previously coated with heparin (Sigma-Aldrich, USA). The protocol for heparin coating was adapted from a previous work [21]. Active heparin-coated beads without VLP were used to determine the background signal. A coated beads solution was prepared by pulling all beads together. The Millipore styrene MultiScreen-BV 96-well plate was pre-wetted with 50 μL of PBS buffer pH 7.2 supplemented with 1% BSA and 0.1% Tween 20 (blocking buffer - BB) for ten minutes and washed on a vacuum manifold. Fifty μL/well of coated beads solution and 50 µL/well of diluted serum or eluted DBS reacted in the dark for one hour on a plate shaker at 37 °C. Plates were washed five times with BB and then, incubated in the dark for 90 min at 37 °C with 50 µL/well of a phycoerithrin-conjugated R-donkey anti-human IgG (Jackson ImmunoResearch laboratories Inc., USA) diluted at 1:200 in BB. After five additional washes, the reaction was stopped with 150 µL of BB. Finally, 120 µL from each well were transferred to another 96 well plates for measurement. Fluorescence signals were recorded using LiquiChip 200 Workstation with Luminex 100™ Integrated System, version 2.3 (Luminex Corporation, USA). Fluorescence of individual antigen specific coated beads were expressed as the mean fluorescence intensity (MFI) of at least 100 beads per set per sample. The upper 5% and lower 5% of outliers’ data points were removed before the calculation of the mean. Finally, the sample background was subtracted using the trimmed-mean fluorescent value of beads without antigen.
DBS samples with less than 50% of blood coverage were considered inadequate. Children's DBS samples considered inadequate were excluded. Missing or inadequate DBS data from women were extrapolated from the MFI result of the paired serum sample, whenever available, using linear regression (data not shown). Otherwise, they were also excluded.
To verify reliability of DBS samples, paired serum-DBS results from 154 women were compared (six incompletely paired and six inadequate DBS samplings were excluded from the 166 women recruited). Strong correlation between DBS and serum testing were found with Spearman's coefficients of 0·91 (95% confidence interval (CI): 0·87–0·93), 0·84 (95% CI: 0·79–0·88), 0·88 (95% CI: 0·84–0·91) and 0·72 (95% CI: 0·64–0·79) for HPV6, 11, 16 and 18, respectively. The agreement and the magnitude of the differences between methods were analyzed using Bland-Altman method [22]. The means of difference and the limits of agreement with their 95% CI were calculated from MFI data after natural logarithmic transformation (Supplement Fig. S1). Means of differences by type were influenced by values around and below five (anti-log n: around and below 150 MFI), while for higher levels, the differences between serum and DBS samples were smaller and more constant. The concordance limits were relatively narrow, even considering the influence of lower values. Despite its lower sensitivity compared to serum samples, DBS sample, which is more ethically acceptable in infants, was considered as an effective and satisfactorily reliable alternative method to serum testing.
2.4. Statistical analysis
Correlation between HPV antibody levels in mothers and newborns after birth was individually assessed for anti-HPV6, 11, 16 and 18 antibodies. Type-specific MFI values from DBS samples of newborns and mothers (n = 58 pairs) were used to generate scatter-plots and to calculate Spearman rho (r) coefficients for each type. To describe antibody dynamics in children, MFI values measured at birth, six, 12 and 24 months of age were graphically represented separately by type. Only children with a valid serology testing at birth and at least one second valid measurement during follow-up were included (n = 37) for this analysis. For children with anti-HPV antibody levels at birth ≥ 200 MFI (n = 28, 21, 16 and 9, for types 6, 11, 16 and 18, respectively), median time to antibody clearance was estimated by type using Kaplan-Meier analysis. Entry time was set at birth (time 0) and observations were censored when antibody levels were below 200 MFI, upon loss to follow up or at the last 24-month visit. All statistical analyses were done using Stata/SE statistical software (version 14.0).
3. Role of funding source
The Canadian Institutes of Health Research (CIHR) (funders) did not have any role in the analysis, interpretation of results, drafting of the manuscript or submitting the manuscript for publication.
4. Results
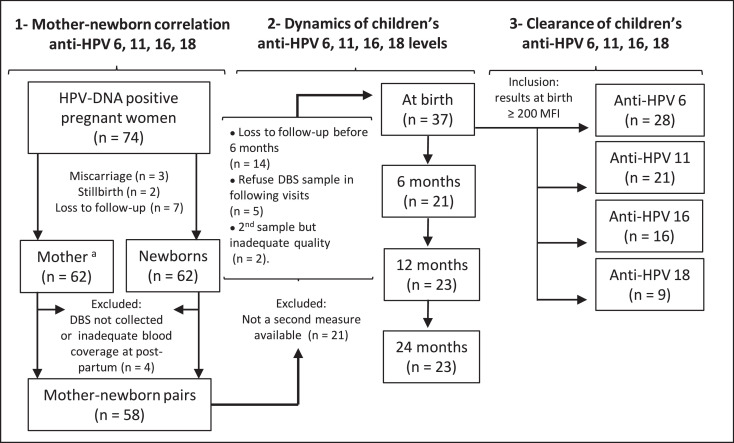
Fig. 1 shows a flow-diagram detailing inclusion and exclusion criteria, and sample size for each specific objective. Out of the 166 women initially followed in the first phase of HERITAGE, 74 women (44.6%) were positive for at least one of the 36 HPV-DNA tested. The 74 HPV-DNA-positive women had 62 children who were born alive and followed (three pregnancies led to miscarriages and two to stillbirths; seven women were lost to follow-up). Finally, 58 women with their children were included in the correlation analysis (n = 58 pairs) as three children did not have a sample collected at birth and one child had inadequate sampling. DBS results of four women were extrapolated from serum sample due to missing or inadequate DBS sampling. Table 1 describes the baseline characteristics and MFI values of pregnant women who participated to the first phase of HERITAGE (n = 166) and their children who were born alive (n = 62) as well as of those who were ultimately included for analysis (n = 58). Women and their child who were included in this analysis were similar to the cohort participants as a whole, except for HPV positivity, as expected. All women included in the pair-analysis were HPV-DNA positive in the first trimester of pregnancy.
Fig. 1.
Number of participants with inclusion/exclusion criteria according to each objective.
Loss to follow-up of the 62 children born alive participating at HERITAGE: at six months (n = 14), at 12 months (n = 7), at 24 months (n = 9).
MFI, mean fluorescence intensity; DBS, dried blood spots
aDue to missing data or inadequate sample, four women had serology results of DBS extrapolated from serum sample (Objective 1).
Table 1.
Baseline characteristics of pregnant women and children.
| Pregnant Women | Recruited (n = 166) | Included for analyses (n = 58) |
| Age, (years) | ||
| Mean (SD) | 26·4 (2·3) | 26·4 (2·6) |
| Median (IQR) | 27·0 (25·0–28·0) | 27·0 (26·0–28·0) |
| Gestational age at enrolment, (weeks) | ||
| Mean (SD) | 11·5 (1·5) | 11·6 (1·7) |
| Median (IQR) | 12·0 (10·4 –12·5) | 12·2 (10·2 – 12·5) |
| Ethnicity, n (%)a | ||
| Caucasian | 130 (77·8) | 46 (79·3) |
| Other | 35 (21·0) | 12 (20·7) |
| Vaginal HPV-DNA status, n (%) | ||
| Negative | 92 (55·4) | – |
| LR-HPV only | 19 (11·5) | 16 (27·6) |
| HR-HPV | 55 (33·1) | 42 (72·4) |
| HPV number of types, n (%) | ||
| None | 92 (55·4) | – |
| 1 type | 37 (22·3) | 31 (53·5) |
| >1 type | 37 (22·3) | 27 (46·5) |
| Vaccinated against HPV, n (%) | ||
| No | 156 (94·0) | 54 (93·1) |
| Yes | 10 (6·0) | 4 (6·9) |
| Anti-HPV6, (MFI)* | ||
| Mean (SD) | 2745·4 (4789·6) | 3199·7 (5296·0) |
| Median (IQR) | 416·5 (105·0–2635·0) | 461·5 (136·0–4323·0) |
| Anti-HPV11, (MFI)* | ||
| Mean (SD) | 1830·2 (4312·1) | 2211·8 (4803·6) |
| Median (IQR) | 356·0 (140·0–1316·0) | 559·0 (207·0–2174·0) |
| Anti-HPV16, (MFI)* | ||
| Mean (SD) | 2979·5 (5639·5) | 4317.1 (6542·4) |
| Median (IQR) | 0·0 (0·0–3258·0) | 333·0 (0·0–7317·0) |
| Anti-HPV18, (MFI)* | ||
| Mean (SD) | 1552·0 (3892·2) | 2900·6 (5125·8) |
| Median (IQR) | 0·0 (0·0–403·0) | 104 (0·0–3690·0) |
| Children born to HPV-positive women | Live births (n = 62) | Included for analyses (n = 58) |
| Gestational age at birth, (weeks) | ||
| Mean (SD) | 39·4 (1·5) | 39·3 (1·5) |
| Median (IQR) | 39·6 (38·9–40·6) | 39·5 (38·7–40·3) |
| Birth weight, (grams) | ||
| Mean (SD) | 3320·0 (462·0) | 3294·0 (457·0) |
| Median (IQR) | 3325·0 (3043·0–3640·0) | 3283·0 (3020·0–3635·0) |
| Sex, n (%) | ||
| Male | 29 (46·8) | 27 (46·5) |
| Female | 33 (53·2) | 31 (53·5) |
| Anti-HPV6, (MFI at birth)* | ||
| Mean (SD) | – | 2753·0 (4926·3) |
| Median (IQR) | – | 365·5 (166·0–2752·0) |
| Anti-HPV11, (MFI at birth)* | ||
| Mean (SD) | – | 1883·3 (4703·0) |
| Median (IQR) | – | 332·0 (73·0–1127·0) |
| Anti-HPV16, (MFI at birth)* | ||
| Mean (SD) | – | 3804·7 (6318·1) |
| Median (IQR) | – | 0·0 (0·0–5117·0) |
| Anti-HPV18, (MFI at birth)* | ||
| Mean (SD) | – | 2188·8 (4421·1) |
| Median (IQR) | – | 0·0 (0·0–1664·0) |
Missing data excluded
Mean fluorescence intensity (MFI) values from dried blood spot (DBS) samples.
n, number; SD, standard deviation; IQR, interquartile range; LR-HPV, low risk human papillomavirus; HR-HPV, high risk human papillomavirus; MFI, mean fluorescence intensity; DBS, dried blood spot.
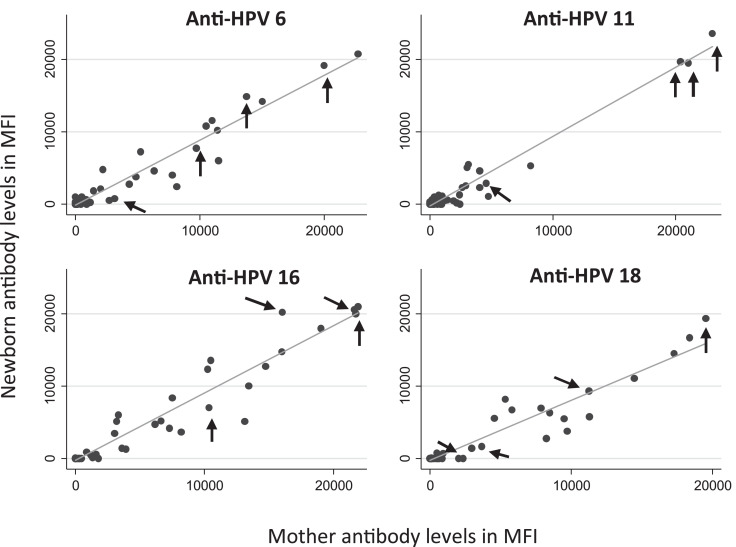
The correlation between MFI antibody levels of newborns at birth and their mothers during pregnancy by HPV type are presented in Fig. 2. Spearman coefficient showed very high correlation for anti-HPV16 (r = 0·90; 95% CI: 0·83–0·94), high correlation for anti-HPV6 (r = 0·81; 95% CI: 0·70–0·88) and anti-HPV18 (r = 0·85; 95% CI: 0·76–0·91), and moderate correlation for anti-HPV11 antibodies (r = 0·68; 95% CI: 0·51–0·80). Interestingly, all the women who self-declared vaccination against HPV had, on average, MFI values greater than 0 (values indicated by arrows in Fig. 2).
Fig. 2.
Mother-newborn antibodies correlation by HPV type (n = 58)
Spearman coefficient: anti-HPV 6 (r = 0·81; 95% CI: 0·70–0·88), anti-HPV 11 (r = 0·68; 95% CI: 0·51–0·80), anti-HPV 16 (r = 0·90; 95% CI: 0·83–0·94), anti-HPV 18 (r = 0·85; 95% CI: 0·76–0·91).
Four women self-reported history of HPV vaccination (arrows).
MFI: Mean fluorescence intensity.
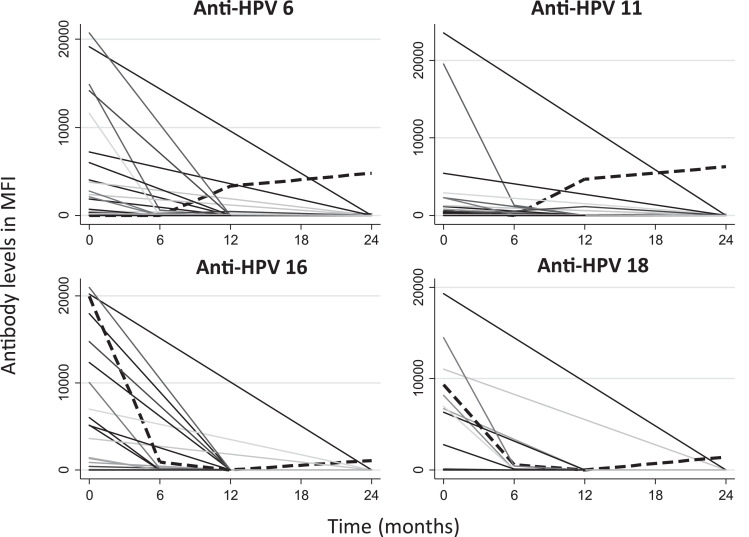
The dynamics of IgG HPV antibodies are presented in Fig. 3. The data of 37 children were available for the analyses, as 14 withdrew from the study or were lost to follow-up before the second measure scheduled at six months. In addition, five mothers refused DBS sampling at each of the following visits and two had inadequate subsequent samplings. In order to evaluate the possible effect of the attrition, we compared the characteristics of the 62 live born children and the subgroup of 37 children included in the analysis, and no significant difference was observed between the groups (data not shown). Anti-HPV antibodies were detected in two children at 24 months (dashed lines). The first, with no prior antibodies detected, presented anti-HPV6 and 11 levels at 12 months (3321 MFI and 4679 MFI, respectively), with persistent levels at 24 months (Anti-HPV6: 4796 MFI; Anti-HPV11: 6280 MFI). In the second child, anti-HPV16 and 18 levels were detectable at birth and at six months (Anti-HPV16: 19,986 MFI and 912 MFI; Anti-HPV 18: 9328 MFI and 570 MFI, respectively). Antibody levels were, however, undetectable at 12 months, and redetected at 24 months (Anti-HPV16: 1101 MFI; Anti-HPV18: 1425 MFI).
Fig. 3.
Antibody levels in children from birth up to 2 years of age (n = 37)
Dashed lines: Children with detectable anti-HPV antibodies at 24 months of follow-up MFI: Mean fluorescence intensity.
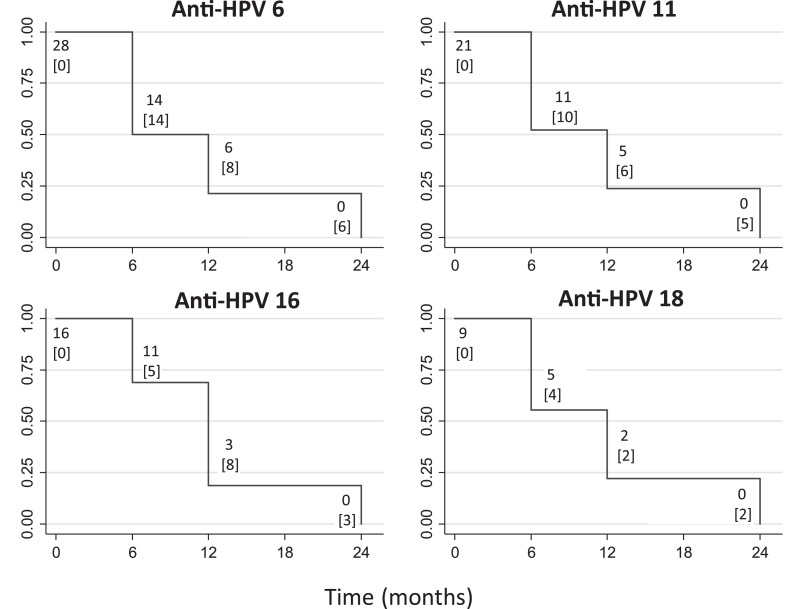
Finally, Kaplan-Meier analyses showed that 80% of children presenting anti-HPV levels of 200 MFI or higher at birth had no anti-HPV detectable before 12 months (Fig. 4). At 24 months, none of the children presented residual antibodies. Median clearance times were six months for anti-HPV6 (95% CI: 6–12, n = 28), and 12 months for anti-HPV11 (95% CI: 6–12, n = 21), anti-HPV16 (95% CI: 6–12, n = 16), and anti-HPV18 (95% CI: 6–12, n = 9).
Fig. 4.
Kaplan-Meier graphics: Clearance of maternal antibodies in children. Kaplan-Meier curves show clearance of antibodies detected at birth (equal or higher to 200 MFI) to levels under 200 MFI between 0 and 24 months, and number of children “at risk”. Number of censored children due to failure (levels under 200 MFI) are represented in brackets under the number at risk. MFI: Mean fluorescence intensity.
5. Discussion
We found that HPV antibodies detected in pregnant women were vertically transferred to infants. Anti-HPV6, 16 and 18 MFI levels from mothers and newborns were highly-to-very-highly correlated, and anti-HPV11 levels, moderately correlated (r = 0·81, 0·90, 0·85, and 0·68, respectively; all p ≤ 0·0001). In addition, anti-HPV IgG antibodies detected at birth against each one of the four types cleared before 24 months of age in all children. Median clearance times varied by type from six to 12 months.
Three other studies previously analysed the correlation between anti-HPV antibody levels of mothers and newborns [14], [15], [16], and our results are consistent with their findings. Heim et al. studied immunoglobulins G, A and M against HPV6, 11, 16, 18 and 31. As expected in vertical immunity, only IgG antibodies were strongly correlated (Spearman r = 0·90, 0·85, 0·74, 0·80 and 0·83 for types 6, 11, 16, 18, and 31, respectively; all p <0·005) [14]. Smith et al. found a strong correlation between IgG serostatus against HPV16, 18, 31 and 33 of mothers and their offspring (kappa 0·78–0·90, all p ≤ 0·001), along with a high global agreement (93%) and low disagreement (McNemar p = 0·30), considering all types together [15]. Matys et al. studied IgG serostatus against HPV6, 11, 16 and 18 from pregnant women who participated in a VLP vaccine efficacy and safety trial and their newborns, and also found strong correlations (Pearson r = 0·8–0·9) [16]. In this study, analyses included women having received a quadrivalent HPV vaccine, or a placebo who presented naturally acquired antibodies against HPV vaccine types. Contrarily to our study that used DBS from finger prick after birth, those three studies used newborn umbilical blood samples [14], [15], [16], which can't completely exclude the risk of maternal blood contamination. It is also important to note that in our study, maternal blood was collected during the first trimester of pregnancy, while newborn blood was collected 24–48 h after birth. The level of the correlation might have been underestimated with this delay. Women may not have detectable antibodies at the time of enrollment but may have seroconverted during pregnancy. On the other hand, women with detectable antibodies at enrolment may have experienced a decrease in antibody levels by the third trimester when the transfer is most likely [5]. In contrast to other studies, we used DBS samples, which are less sensitive for detecting very low levels of antibody and evaluating MFI levels. All this may explain differences with others regarding the correlation of HPV antibodies.
This study is the first to attempt to describe the dynamics of HPV antibodies in children from birth until two years of age. Two children could have seroconverted during the two-year follow-up. The first child was born to an unvaccinated mother and had no reactivity at birth. However, anti-HPV6 and 11 were detectable at 12 and, with increased levels, at 24 months. These findings are difficult to explain because DNA-positivity for each of these types was not detected during the child follow-up, nor in maternal samples at first or third trimester, or placenta. It is possible that HPV infection was not detected because we did not sample in the infected area or because the infection was transient. It is interesting to note however that this child was HPV DNA-positive with a non-phylogenetic related HPV type in the conjunctive (eye) at 18 months (data not shown). We therefore cannot rule out the possibility of cross-reactivity with another type. The second child was born to a vaccinated mother (quadrivalent vaccine) and presented high antibody levels against the four types at birth. Antibody for HPV16 and 18 were detected at six months at lower levels, were undetected at 12 months, and were redetected at 24 months. HPV-DNA-positivity was not observed in this child during follow-up. A false negative result at 12 months and a longer period to clear maternal antibodies could explain this situation, due to the high levels at birth.
Maternal immunoglobulins G (IgG) are the only immunoglobulin group able to cross the placental barrier. Transfer of maternal IgG to fetuses increases gradually during the second and third trimesters, and IgG levels in the fetuses are generally higher than those of their mother at the end of a term pregnancy [5,23]. Maternally transferred antibodies are gradually eliminated, and, in most children, they are cleared between six and 12 months of age [7]. However, the clearance time can vary depending on the immunization status of the mother, and the specificity and levels of the transferred antibodies [6,12]. It is thought that protection after birth is maintained longer if the IgG antibodies received by the fetus are more specific and reach higher levels [6]. For viral infections in general, antibody levels produced after natural infection is generally higher than in vaccine-induced immune responses [6,13]. This is not the case for HPV. Anti-HPV antibodies produced after vaccination usually present levels ten to 100 folds and an avidity three folds higher than after a natural infection [12], which occurs without viremia [7,8,24]. Interestingly, in children born to vaccinated women (n = 4) in our study, anti-HPV antibodies reached higher concentrations at birth and persisted for a longer period at higher levels. This was also found in another study [16]. The small number of vaccinated women in our study did not allow us to estimate the statistical significance of such findings.
Overall, the present study documents HPV-antibody vertical transfer and clearance in infants. Caution should be used when comparing HPV serological studies because of the large methodological variability and because there is no gold standard method for measuring HPV antibodies [25,26]. Despite this important limitation, serological studies are an important tool to study natural immunity, and to monitor HPV vaccination programs. We also used DBS, which is not a standard methodology. However, although it has a lower sensitivity than serum sample, this technique is better accepted, more ethically appropriate and has a major advantage over cord blood sampling at birth as it prevents contamination by maternal blood. Future research should investigate the role of maternal HPV antibodies on child immunity. Efforts should also continue to better define an HPV antibody threshold of protection.
6. Financial support
The HERITAGE study was supported by a Grant from the Canadian Institutes of Health Research (CIHR) [Grant MOP-93564 and MOP-136833] to HT. HT holds a salary award (Research Scholar) from the Fonds de la recherche du Québec en santé (FRQ-S), and from CIHR (New investigator salary award). MHM holds a salary award (Clinical Research Scholar) from the FRQ-S. Funding for quality control of HPV testing was provided in part by the Réseau FRQS SIDA-MI to FC. PM was supported by the Research Institute of the McGill University Health center (Start-up funds).
7. Meeting at which a part of the information has been presented
Part of this manuscript was presented at the 32nd International Papillomavirus Conference (IPVC) October 2018, Sydney, Australia. [M. Zahreddine, M.H. Mayrand, C. Therrien, A. Trevisan, C. Dagenais, P. Monnier, L. Laporte, J. Niyibizi, A.M. Carceller, W.D. Fraser, P. Brassard, J. Lacroix, M.J. Bédard, I. Girard, F. Audibert, F. Coutlée, H. Trottier. Antibodies to human papillomavirus (HPV) types 6, 11, 16 and 18: vertical transfer, clearance, and dynamics in children up to two years of age. Abstract number: IPVC8-0471]
Conflict of interests
FC has received grants through his institution from Becton-Dickson. HT has received occasional lecture from Merck and unrestricted grant form ViiV Healthcare. All other co-authors have no conflict of interests.
Acknowledgments
The authors are grateful to participants. Authors are also grateful to Hasna Meddour, Myra Geoffrion, Kathleen Auclair, Véronique Prévost, Fabiola Correa Botello, Sophie Perreault, Lise-Angela Ouellet (Sainte-Justine Hospital), to Sylvie Daigle, Sophie Leblanc (CHUM Hospital), Siham Aboulfadi (St-Mary's hospital) and to all other contributing research staffs for managing patients and specimens from all sites. Authors are also grateful to Josée Poirier, Audrée Janelle-Montcalm, Isabelle Krauss and Cindy Rousseau for coordinating HERITAGE phase 1 study within the IRNPQEO (3D Project) and to dr. François Beaudoin (in memorian) for their help with the recruitment of patients. Authors are also grateful to Julie Guenoun, Émilie Comète and Pierre Forest for DNA extraction and HPV testing. Authors are also grateful to dr. Michel Couillard (in memorian) from LSPQ for the support to the serology testing. Authors are also grateful to dr. John Schiller from the National Institutes of Health (NIH), USA, who kindly provided the plasmids used to produce VLPs.
Authors' contribution
All authors have directly contributed to the conception and design (HT, MHM, FC, PM, WF, PB) or acquisition of data (MZ, CT, CD, AT, LL, JN, PB, IG, MJB, FA, JL, AMC) or analysis and interpretation (MZ, CT, CD, AT, HT, MHM, FC, PB, AMC) of the study. MZ, HT, MHM and FC wrote the first draft of the manuscript. All authors have subsequently read, revised, and approved the version that is being submitted.
Footnotes
Supplementary data related to this article can be found online at doi:10.1016/j.eclinm.2020.100334.
Supplementary materials
References
- 1.Syrjänen S. Current concepts on human papillomavirus infections in children. APMIS. 2010;118(6–7):494–509. doi: 10.1111/j.1600-0463.2010.02620.x. [DOI] [PubMed] [Google Scholar]
- 2.Merckx M., Liesbeth W.V., Arbyn M. Transmission of carcinogenic human papillomavirus types from mother to child: a meta-analysis of published studies. Eur J Cancer Prev. 2013;22(3):277–285. doi: 10.1097/CEJ.0b013e3283592c46. [DOI] [PubMed] [Google Scholar]
- 3.Cason J., Mant C.A. High risk mucosal human papillomavirus infections during infancy & childhood. J Clin Virol. 2005;32:52–58. doi: 10.1016/j.jcv.2004.12.007. Supplement. [DOI] [PubMed] [Google Scholar]
- 4.Lacey C.J., Lowndes C.M., Shah K.V. Chapter 4: Burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine. 2006;3(24 Suppl 3):S35–S41. doi: 10.1016/j.vaccine.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 5.Simister N.E. Placental transport of immunoglobulin G. Vaccine. 2003;21(24):3365–3369. doi: 10.1016/s0264-410x(03)00334-7. [DOI] [PubMed] [Google Scholar]
- 6.Leuridan E., Van Damme P. Passive transmission and persistence of naturally acquired or vaccine-induced maternal antibodies against measles in newborns. Vaccine. 2007;25(34):6296–6304. doi: 10.1016/j.vaccine.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 7.Nicoara C., Zach K., Trachsel D., Germann D., Matter L. Decay of passively acquired maternal antibodies against measles, mumps, and rubella viruses. Clin Diagn Lab Immunol. 1999;6(6):868–871. doi: 10.1128/cdli.6.6.868-871.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linder N., Tallen-Gozani E., German B., Duvdevani P., Ferber A., Sirota L. Placental transfer of measles antibodies: effect of gestational age and maternal vaccination status. Vaccine. 2004;22(11–12):1509–1514. doi: 10.1016/j.vaccine.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Fu C., Lu L., Wu H. Placental antibody transfer efficiency and maternal levels: specific for measles, coxsackievirus A16, enterovirus 71, poliomyelitis i-iii and HIV-1 antibodies. Sci Rep. 2016;6:38874. doi: 10.1038/srep38874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanley M. Immune responses to human papillomavirus. Vaccine. 2006;1(24 Suppl 1):S16–S22. doi: 10.1016/j.vaccine.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Carter J.J., Koutsky L.A., Hughes J.P. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J Infect Dis. 2000;181(6):1911–1919. doi: 10.1086/315498. [DOI] [PubMed] [Google Scholar]
- 12.Scherpenisse M., Schepp R.M., Mollers M., Meijer C.J., Berbers G.A., van der Klis F.R. Characteristics of HPV-specific antibody responses induced by infection and vaccination: cross-reactivity, neutralizing activity, avidity and IGG subclasses. PLoS ONE. 2013;8(9):e74797. doi: 10.1371/journal.pone.0074797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waaijenborg S., Hahné S.J.M., Mollema L. Waning of maternal antibodies against measles, mumps, rubella, and varicella in communities with contrasting vaccination coverage. J Infect Dis. 2013;208(1):10–16. doi: 10.1093/infdis/jit143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heim K., Hudelist G., Geier A. Type-specific antiviral antibodies to genital human papillomavirus types in mothers and newborns. Reprod Sci. 2007;14(8):806–814. doi: 10.1177/1933719107309546. [DOI] [PubMed] [Google Scholar]
- 15.Smith E.M., Parker M.A., Rubenstein L.M., Haugen T.H., Hamsikova E., Turek L.P. Evidence for vertical transmission of HPV from mothers to infants. Infect Dis Obstet Gynecol. 2010;2010 doi: 10.1155/2010/326369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matys K., Mallary S., Bautista O. Mother-infant transfer of anti-human papillomavirus (HPV) antibodies following vaccination with the quadrivalent HPV (type 6/11/16/18) virus-like particle vaccine. Clin Vaccine Immunol. 2012;19(6):881–885. doi: 10.1128/CVI.00002-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trottier H., Mayrand M.-.H., Coutlée F. Human papillomavirus (HPV) perinatal transmission and risk of HPV persistence among children: design, methods and preliminary results of the HERITAGE study. Papillomavirus Res. 2016;2:145–152. doi: 10.1016/j.pvr.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coutlee F., Rouleau D., Petignat P. Enhanced detection and typing of human papillomavirus (HPV) DNA in anogenital samples with PGMY primers and the linear array HPV genotyping test. J Clin Microbiol. 2006;44(6):1998–2006. doi: 10.1128/JCM.00104-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coutlee F., Rouleau D., Ghattas G. Confirmatory real-time PCR assay for human papillomavirus (HPV) type 52 infection in anogenital specimens screened for HPV infection with the linear array HPV genotyping test. J Clin Microbiol. 2007;45(11):3821–3823. doi: 10.1128/JCM.01145-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buck C.B., Thompson C.D. Production of papillomavirus-based gene transfer vectors. Curr Protoc Cell Biol. 2007 doi: 10.1002/0471143030.cb2601s37. Chapter 26: Unit 26.1. [DOI] [PubMed] [Google Scholar]
- 21.Faust H., Knekt P., Forslund O., Dillner J. Validation of multiplexed human papillomavirus serology using pseudovirions bound to heparin-coated beads. J Gen Virol. 2010;91(Pt 7):1840–1848. doi: 10.1099/vir.0.019349-0. [DOI] [PubMed] [Google Scholar]
- 22.Bland J.M., Altman D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. [PubMed] [Google Scholar]
- 23.Saji F., Samejima Y., Kamiura S., Koyama M. Dynamics of immunoglobulins at the feto-maternal interface. Rev Reprod. 1999;4(2):81–89. doi: 10.1530/ror.0.0040081. [DOI] [PubMed] [Google Scholar]
- 24.Gans H., DeHovitz R., Forghani B., Beeler J., Maldonado Y., Arvin A.M. Measles and mumps vaccination as a model to investigate the developing immune system: passive and active immunity during the first year of life. Vaccine. 2003;21(24):3398–3405. doi: 10.1016/s0264-410x(03)00341-4. [DOI] [PubMed] [Google Scholar]
- 25.Du P., Brendle S., Milici J. Comparisons of VLP-Based ELISA, neutralization assays with native HPV, and neutralization assays with PSV in detecting HPV antibody responses in HIV-Infected women. J AIDS Clin Res. 2015;6(3):1–13. doi: 10.4172/2155-6113.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schiller J.T., Lowy D.R. Immunogenicity testing in human papillomavirus virus-like-particle vaccine trials. J Infect Dis. 2009;200(2):166–171. doi: 10.1086/599988. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.