Abstract
Melatonin is a pleiotropic, indole secreted, and synthesized by the human pineal gland. Melatonin has biological effects including anti-apoptosis, protecting mitochondria, anti-oxidation, anti-inflammation, and stimulating target cells to secrete cytokines. Its protective effect on cardiomyocytes in acute myocardial infarction (AMI) has caused widespread interest in the actions of this molecule. The effects of melatonin against oxidative stress, promoting autophagic repair of cells, regulating immune and inflammatory responses, enhancing mitochondrial function, and relieving endoplasmic reticulum stress, play crucial roles in protecting cardiomyocytes from infarction. Mitochondrial apoptosis and dysfunction are common occurrence in cardiomyocyte injury after myocardial infarction. This review focuses on the targets of melatonin in protecting cardiomyocytes in AMI, the main molecular signaling pathways that melatonin influences in its endogenous protective role in myocardial infarction, and the developmental prospect of melatonin in myocardial infarction treatment.
Keywords: melatonin, cardioprotective, cardiomyocyte, myocardial infarction, mitochondrion
Introduction
With the general improvement of the human living standard, the change of living habits and the prolongation of life span, the prevalence rate of cardiovascular diseases has risen sharply. According to China Cardiovascular Disease Report in 2018, the population with cardiovascular diseases in China has reached up to 290 million, and the number of patients with acute myocardial infarction (AMI) is about 2.5 million annually. AMI has become a disease seriously affecting people’s life span and quality. Despite great progress of modern medicine, science and technology, iterative new anticoagulants and antiplatelet drug, improvements in the management of AMI patients (Amanakis et al., 2019; Heusch, 2019), including the more frequent coronary reperfusion using fibrinolysis, primary percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG), the prognosis of AMI has improved, yet, there are frequent occurrences of malignant arrhythmia events, a decline of cardiac function following AMI leading to the development of heart failure and a poor prognosis (Botker et al., 2018; Davidson et al., 2018; Joshi et al., 2019). Due to ischemia and hypoxia of the infarct area, a large number of inflammatory cells infiltrate the lesion, cardiomyocyte apoptosis is frequent and scar repair is common (Li et al., 2010; Galluzzi et al., 2018). Due to the non-regenerative feature of cardiomyocytes, irreversible cardiomyocyte apoptosis and infarction caused by acute ischemia are main factors for poor prognosis of AMI (Abukar et al., 2018; Beckendorf et al., 2018). After myocardial infarction, the myocardial tissue in the infarcted area and its adjacent non-infarcted areas undergoes apoptosis and necrosis due to ischemia, hypoxia and inflammatory response (Zhou et al., 2018c; Hu et al., 2019). Thus, the hemodynamic parameters and electrical nerve conduction of the myocardium are changed, and bring about cardiac systolic, diastolic dysfunction and dysrhythmia, and result in ventricular remodeling and electrocardiogram (ECG) changes (Zhang et al., 2014; Joshi et al., 2019). Mitochondria are organelles that generate ATP (Kowaltowski, 2019; Lee B.W.L. et al., 2019). They play a significant role in myocardial injury after myocardial infarction (Zhou et al., 2018h; Zhou et al., 2020). Stabilizing the structure and function of mitochondria effectively inhibits cardiomyocyte injury and necrosis (Audia et al., 2018; Lu J. et al., 2019). Melatonin, an indoleamine secreted by pineal gland, is a highly effective antioxidant and beneficial to many diseases including diabetes, infectious diseases, metabolic syndrome, depression, and neurodegenerative diseases (Zaouali et al., 2011; de Oliveira Junior et al., 2019). The value of melatonin in the treatment of myocardial infarction has drawn wide-spread attention in recent years. Highly compatible with mitochondrial membrane receptors, melatonin effectively reduces mitochondrial dysfunction, thus inhibiting post-myocardial infarction damage of cardiomyocytes. This review summarizes the effect of melatonin in protecting cardiomyocytes and improving prognosis after myocardial infarction by enhancing the adaptability of cardiomyocytes to ischemia and hypoxia via stabilizing mitochondrial function. We hope this introduction will be helpful to the research of melatonin in the treatment of myocardial infarction.
Pathophysiological Changes of Heart During Myocardial Infarction
Lipid Deposition and Atherosclerotic Plaque Formation
The direct cause of AMI is myocardial necrosis caused by prolonged ischemia and hypoxia resulting from coronary artery occlusion or spasm. The key factor leading to acute myocardial ischemia is the rupture of an atherosclerotic plaque in the coronary artery and the gradual formation of a thrombus (Zhou et al., 2018e; Bacmeister et al., 2019). The pathogenesis of atherosclerosis includes lipid deposition, inflammation, thrombosis, endothelial dysfunction, smooth muscle cell cloning and other processes (Nofe et al., 2010; Zhou et al., 2018b; Zhao J. et al., 2019). The most important risk factors are lipid metabolism disorder and endothelial cell injury. Under long-term lipid metabolism abnormality, low density lipoprotein cholesterol (LDL-C) enters intima through damaged arterial endothelium where it is converted to oxidized LDL cholesterol (Ox LDL-C), causing further damage to the intima of the coronary artery (Liu et al., 2019; Aimo et al., 2020). Ox LDL-C is engulfed by macrophages, gradually forming foam cells; there after, atherosclerotic plaques gradually develop along with the aggregation of foam cells and lipids (Abdelnaseer et al., 2016; Wu et al., 2019). If the intima of a coronary artery ruptures, the atheromatous plaque substance enters the lumen and becomes an embolus which completely occludes the blood vessel (Yuqi et al., 2015; Tian et al., 2018; Xiong et al., 2019). Thus, regulating the lipid metabolism balance, delaying the formation of atherosclerotic plaques and inhibiting plaque rupture are key measures in preventing myocardial infarction.
Platelet Activation Aggregation and Thrombosis
When atherosclerotic plaques are formed and protrude into the lumen, local arterial stenosis causes changes in blood flow turbulence and shear force, leading to an interruption of arterial intima continuity, contraction of endothelial cells, exposure of tissues such as subendothelial collagen, and activation of platelets; this causes further adhesion and aggregation of platelets on the arterial intima, and finally formation of atherosclerotic thrombotic disease (Cohen et al., 2019; Trindade et al., 2019). Reports show that the mural thrombosis plays a vital role in pathophysiological changes of myocardial infarction progression (Hu S.Y. et al., 2017; Zhou et al., 2017b). At present, there are three types of antiplatelet drugs used in the clinic: (1) cyclooxygenase-1 (COX-1) inhibitor: aspirin. (2) ADP receptor antagonists: clopidogrel, prasugrel, ticagrelor. (3) platelet glycoprotein IIb/IIIa (GP IIb/IIIa) receptor antagonists: tirofiban, etibadin, abciximab. Among these, aspirin is a first-line antiplatelet drug and can irreversibly inactivate COX-1, an enzyme expressed in platelets, which moderates the synthesis of thromboxane; thromboxane is a potent platelet aggregation agent (Davidson et al., 2018; Merz et al., 2018).
Inflammatory Cell Infiltration and Inflammatory Response
The microenvironment of the infarcted cardiomyocyte is a dynamic and complex area. Inflammation and immune responses play important roles in the initiation and progression of myocardial infarction. After myocardial infarction, inflammatory cells such as neutrophils, monocytes, and macrophages are activated, causing their number to increase sharply (Basalay et al., 2018; Wang Z. et al., 2019; Faghfouri et al., 2020). A large amount of inflammatory cytokines are released and move toward the injured area, and the permeability of endothelial cells increases, which results in the infiltration of inflammatory cells (Chen et al., 2016; Thieltges et al., 2018). Cytokine release and the inflammatory response are important conditions for tissue healing after myocardial infarction, but an excessive inflammatory response leads to myocardial tissue remodeling, activating apoptosis signals in cardiomyocytes with destruction of the integrity of extracellular matrix, which is not conducive to the survival of cardiomyocytes and the recovery of cardiac function (Nahrendorf et al., 2010; Krause et al., 2018). Apoptosis and the inflammatory response are initiated by myocardial tissue injury in the ischemic area. Specific cytokines and mediators including interleukin-1 (IL-1), IL-6, nuclear transcription factor kB (NF-κB) p65, NF-κB p50, Toll-like receptor 4(TLR4), Tenascin-C (TNC) and other inflammatory response regulatory proteins, as well as apoptosis regulatory proteins caspase-3, Bcl-2, etc. are produced. Excessive inflammatory response and fiber proliferation lead to ventricular remodeling (Nokik et al., 2017; Crist et al., 2018; Bocci et al., 2019; Chen M. et al., 2019). TLRs are a major reaction pathway of the inflammatory response after myocardial infarction. Recognition receptors are expressed by inflammatory cells and recognize the danger signal released by injured cells (Jager and Hoefer, 2019; Sircana et al., 2019; Yang M.Y. et al., 2019). Studies have shown that adjusting the activity of TLRs enhances the positive effect of the inflammatory response on myocardial tissue healing and limits damage to tissue, thus providing a new therapeutic process for promoting myocardial tissue recovery after myocardial infarction (Dominguez-Rodriguez et al., 2010b; Cao et al., 2019). Protein kinase D1 (PKD1) mediates the processes of myocardial remodeling, angiogenesis and myocardial contraction (Ren, 2016; Serocki et al., 2018), but the mechanism of PKD1 in the processes of the inflammatory response and cell injury in the microenvironment after myocardial infarction has not been determined.
Cardiomyocyte Death
Cardiomyocyte injury following AMI is a very complex with multi-linked pathophysiological processes, including not only an inflammatory response, immune response, and cell signal transduction, but also complex processes such as apoptosis, necrosis, autophagy, and mitochondrial dysfunction (Westman et al., 2016; Hockings et al., 2018; Heckmann et al., 2019). Immunological studies show that cardiomyocyte in the myocardial infarction region is seriously damaged, apoptosis is severe, the expressions of Bax and caspase-3 are significantly increased, while the expression of Bcl-2 is reduced (Jin et al., 2018). It is reported that, caspase-8 is involved in fatty acid synthase (Fas)/FasL-related death receptor pathway, caspase-9 is depended on mitochondrial damage, and caspase-12 is related to endoplasmic reticulum stress (Fuhrmann and Brune, 2017; Karwi et al., 2018; Jiang et al., 2019). These are associated with cardiomyocyte apoptosis following myocardial infarction. After AMI, the focal area mainly consists of a peripheral ischemic penumbra and inner infarct area. The ischemic penumbra is mainly composed of apoptotic cardiomyocytes, and the inner infarcted area contains numerous dead cardiomyocytes (Niccoli et al., 2016; Hadebe et al., 2018). Studies reveal that the number of necrotic cells is seven times that of apoptotic cells; thus apoptosis plays only a minor role in the progression of myocardial infarction (Adameova et al., 2016; Zhou et al., 2018a; Zhu P. et al., 2018). Mitochondrial reactive oxygen species (ROS) and xanthine oxidase activity in ischemic areas lead to oxidative stress, and the calcium-induced calcium release (CICR) leads to intracellular calcium overload (Zollbrecht et al., 2016; Espinosa-Diez et al., 2018; Graczyk-Jarzynka et al., 2019; Guidarelli et al., 2019). Oxidative stress and inflammatory response activate receptor interaction protein kinase 3 (Ripk3) via interaction with cell death receptors; the common receptors are the Fas receptor, TRAIL receptor and TNFR1 (Zhou et al., 2017e; Zhou and Toan, 2020). Ripk3 destroys cell membrane integrity by mediating cell membrane-related chromosome translocation, resulting in cell necrosis (Kohlstedt et al., 2018; Zhong et al., 2019).
Mitochondrial Dysfunction and Mitophagy
As organelles with important functions in eukaryotic cells, mitochondria mainly generate ATP, moderating apoptosis and oxidative stress reactions and exhibit dynamic changes. Their development and degradation, fusion and fission, and their quantity and morphology are precisely regulated and controlled based on changes in the environment where the cells are located (Zhou et al., 2017a,f; Chen S. et al., 2019; Mayorov et al., 2019; Simula et al., 2019). Mitochondrial dynamics include mitochondrial fission, fusion and mitophagy. Mitochondrial fission aggravates the damage to mitochondrial structure and function by inducing mitochondrial fragmentation. The fission process is considered as an initiation event for mitochondrial apoptosis (Zhou et al., 2017c; Xu Z. et al., 2019; Zhou et al., 2019). Mitochondrial fusion reduces mitochondrial damage and maintains function of these organelles by promoting the integration of mitochondrial fragments, enhancing the stability of mitochondrial genes, and promoting the exchange of mitochondrial contents (Filadi et al., 2018; Kanaan et al., 2018; Yao et al., 2019). As a process of organelle autophagy, mitophagy removes and digests damaged mitochondria fragment via mitochondrial degradation moderated by lysosomes (Zhou et al., 2018d, e; Breda et al., 2019; Mei et al., 2019). Mitophagy is specific and a main pathway of mitochondrial metabolism, and plays a key role in maintaining cell function stability (Liu et al., 2012; Bhandari et al., 2014; Kang et al., 2016).
When cellular damage causes mitochondrial structural dysfunction, cells firstly maintain their original structure through antioxidant factors, DNA repair, protein folding, and so on. If this first line of defense is breached, autophagy, fusion, and biogenesis of the mitochondria are activated, which is a more effective and extensive quality control system (Yang M. et al., 2019). The process of mitophagy is closely related to ubiquitin/proteasome system (UPS). First, the damaged mitochondria are ubiquitinated through modification and then recognized by ubiquitin receptors. The substrates are induced to mitophagy degradation by binding to LC3 on phages, and finally the components after degradation are released to the cytoplasm for recycling, providing nutrients and energy for cell survival (Ji and Kwon, 2017). Recent studies reveal that mitophagy participates in mitochondrial protection induced by mitochondrial fusion (Kornicka et al., 2019). Mitochondrial fusion initially promotes the integration of fragmented mitochondria, and then “cleans” mitochondria by proteolysis in lysosomes to moderately degrade damaged mitochondrial fragments, thus maintaining the stability of mitochondrial quality and quantity (Tahrir et al., 2019; Wang et al., 2020). In most cases, mitophagy can remove defective mitochondria from AMI injuries, playing a protective and adaptive role. The experimental results of Siddall et al. (2013) show that the enhancement of mitophagy activity protects cardiomyocytes by reducing the production of mitochondrial ROS and inhibiting calcium overload (Reddy et al., 2018). The role of mitophagy in myocardial infarction cell injury, however, is still controversial. However, experimental data of Yang et al. (2018) indicates that the rise of mitophagy reduces the energy supply of cells, thus aggravating cell injury (Koentges et al., 2018; Zhou et al., 2018g).
The heart has a very high demand for energy. There are a large number of mitochondria in cardiomyocytes. Their total volume of mitochondria in a cardiomyocyte accounts for approximately 22–37%. The energy is used to maintain the normal blood pumping function of the heart. The distribution and supply of energy are related closely to the functional state of mitochondria (Battogtokh et al., 2018; Joshi and Mochly-Rosen, 2018; Zhong et al., 2019). The mitochondrial membrane is a bilayer structure, with a non-specific ion channel in the inner membrane. This channel is known as the mitochondrial permeability transition pore (MPTP), which plays a critical role in maintaining Ca2+ dynamic balance and apoptosis (Meyer and Leuschner, 2018; Venugopal et al., 2018). The opening of MPTP leads to an increase of mitochondrial intimal permeability, resulting in imbalance of electrochemical driving force of ions inside and outside the membrane, a decrease of Na+-K+-ATP enzyme activity, and transmembrane transport barrier of sodium and potassium ions, which further leads to mitochondrial dysfunction, a drop of the mitochondrial inner membrane potential with the release of cytochrome c (Cyt c), and activation of the apoptosis program (Xiao et al., 2018; Xu T. et al., 2019). Under normal circumstances, MPTP is closed state and is activated when the concentration of oxygen-derived free radicals and Ca2+ is elevated (Yang et al., 2016; Xu S.F. et al., 2019). During AMI, cardiomyocytes are in a microenvironment of ischemia and hypoxia, with an extensive infiltration of inflammatory cells and oxygen-derived free radicals. Mitochondria then release apoptosis-inducing factors, Cyt c, pro-interleukin 1, and other mediators that induce apoptosis in this harsh microenvironment; this mediates the release of IL-1 through an inflammatory cascade reaction resulting in cardiomyocyte injury and necrosis (Teixeira et al., 2018; Lee E. et al., 2019). In addition, a large number of free radicals and inflammatory agents destroy the structure and function of mitochondria, causing inactivation of various enzymes, destruction of double membrane barrier and opening of the MPTP, causing mitochondrial edema, disintegration, and dysfunction of energy generation, and eventually leading to cardiomyocyte death and cardiac function damage. During the process of AMI, opening of MPTP plays an important role (Boengler et al., 2018; Schreiber et al., 2019). The cardiomyocyte injury in ischemia is reduced by inhibiting opening of the MPTP, lowering the release of Cyt c and limiting oxygen-derived free radicals and Ca2+ overload (Seidlmayer et al., 2015; Morell et al., 2018).
Target the Melatonin Protects Cardiomyocytes in Acute Myocardial Infarction
Melatonin Plays Anti-oxidative Stress Effect and Inhibits Inflammatory Response
Melatonin was originally discovered in the bovine pineal gland and was named after its ability to change pigmentation (melanin) in amphibian skin. Controlled by sympathetic nervous system, the synthesis and secretion of melatonin are in phase with the fluctuations in the light:dark cycle with little secretion during the day and high amounts of secretion at night (Perez-Gonzalez et al., 2019). Some of the biological effects of melatonin are related to its ability to effectively scavenge free radicals and enhance the activity of antioxidant enzymes; melatonin’s metabolites also exhibit high radical scavenging activity (Galano et al., 2013). Melatonin is highly potent free radical scavenger due to multiple mechanisms (Galano and Reiter, 2018). Free radicals that are eliminated include nitric oxide (NO⋅), superoxide anion radical (O2–⋅) and hydroxyl radical (OH⋅), etc. Its hydrophilicity and high lipophilicity, allows melatonin to pass through the cell membrane and the nuclear membrane easily, thus exerting strong antioxidant effects in cytoplasm and nucleus (Garcia et al., 2015). In addition to directly scavenging free radicals, melatonin also induces the expression of antioxidant enzymes to achieve indirect antioxidant effects (Kleszczynski et al., 2016). Nrf2 (NF-E2-related factor2), a transcription factor, plays a key role in cell oxidative stress response, and controls the expression of various antioxidant response genes after linking to the DNA antioxidant response element (ARE), while melatonin mainly influences the pathway through a nuclear retinoid-related orphan receptor (RZR/RORα) (Giudice et al., 2010).
Melatonin also plays an antioxidant role in coordination with reduced glutathione, nicotinamide adenine dinucleotide phosphate (NADPH), vitamin C, vitamin E, etc. (Yang C.H. et al., 2019). Its synergistic anti-inflammatory effects are mainly realized by up-regulating the activity of enzymes synthesizing such antioxidants so as to increase total content of antioxidants in the organism (Yoon et al., 2019). The anti-inflammatory mechanisms of melatonin include inhibiting the aggregation of inflammatory cells and the release of inflammatory cytokines including TNF-α, IL-1β, and IL-6 all of which are important inflammatory mediators of the inflammatory response. These inflammatory factors directly cause tissue injury and also stimulate other inflammatory cells to release inflammatory mediators, causing a chain reaction (Amin et al., 2019). Experiments show that melatonin increases the release of anti-inflammatory mediators such as IL-10 and while inhibiting the release of inflammatory mediators such as TNF-α, IL-1β, and IL-6, thus achieving an anti-inflammatory effect. In addition, animal experiments show that anti-inflammatory actives of melatonin are related to its inhibition of adhesion molecule related gene expression (Hu C. et al., 2017).
The TLRs mentioned above are the main response pathway of the inflammatory response after myocardial infarction. In a rat myocardial infarction model (Zhao Y. et al., 2019), the expression of the TLR4 signaling pathway is inhibited when melatonin is injected into the heart before ischemic injury. In addition, melatonin also blocks upstream signals (e.g., lipopolysaccharide binding protein CD14) of TLR4. This process can significantly reduce the release of inflammatory factors such as Granulocyte-Monocyte Colony-Stimulating Factor (GM-CSF), TNF-α, C-C Motif Chemokine Ligand 2 (CCL 2), IL-1β, IL-6, C-reactive protein (CRP), serum amyloid A, α-1 antitrypsin, while the content of Nrf2, IL-1α, heme oxygenase-1, and other anti-inflammatory cytokines rise significantly (Ter Horst et al., 2018; Ostjen et al., 2019; Tang et al., 2019).
Dyslipidemia is an independent risk factor for attack of coronary heart disease (CHD). It is reported that (Tengattini et al., 2008) melatonin regulates blood lipid, and reduces the Ox LDL-C, both of which are helpful for reducing the overall incidence of myocardial infarction injury. In terms of protecting vascular endothelial cells, melatonin reduces the degree of injury of endothelial cells in the process of myocardial infarction by inhibiting the activity of myosin light streptokinase, thus delaying the progression of atherosclerosis (Rezzani et al., 2013). In addition, several studies demonstrate that melatonin induces calcium overload and ROS generation in platelets, and activates caspase pathway and depolarizes mitochondrial membrane to mediate platelet inactivation, and this process also involves peroxisome proliferator-activated receptor γ(PPARγ)/ FUN14 domain containing 1 (FUNDC1) /mitophagy pathways (Zhou et al., 2017b; NaveenKumar et al., 2019). In addition to directly mediating platelet dysfunction, melatonin also down-regulates adhesion molecules and delays NO metabolism, thus indirectly inhibiting platelet aggregation (Girish et al., 2013). This antithrombotic effect reduces cardiomyocyte injury after myocardial infarction, and plays a role in protecting the myocardium (Dominguez-Rodriguez et al., 2010a). The circadian rhythm of melatonin release significantly reduces the activity of platelets at night, while the early morning with low melatonin levels is often the time for the occurrence of cardiovascular events. Therefore, supplementation of melatonin through external sources may effectively prevent cardiovascular events (Arushanian, 2013; Lansink et al., 2016).
Melatonin Mediates Myocardial Protection Through Receptor and Non-receptor Pathways
In rat models of myocardial infarction, melatonin concentrations in plasma and left ventricle tissue increase sharply within 1 day, and mRNA levels of the MT1, a member of melatonin receptor, rise significantly after 2 weeks, indicating that melatonin may play an endogenous protective role in myocardial infarction (Sallinen et al., 2007). Melatonin has a biological role mainly by being bound to receptors including both membrane and nuclear binding sites. Among them, membrane receptors include melatonin receptor, TNF receptor and Notch receptor. Melatonin nuclear receptors are members of the retinoic acid related orphan nuclear receptor/retinoic acid Z receptor (ROR/RZR) family, including three subtypes: α, β, γ. The subtype α is referred to as novel endogenous myocardial infarction injury defense agent in new development progress. In rats that lack RORα receptors, the size of the myocardial infarct and degree of cardiac dysfunction after cell injury increases significantly (He et al., 2016). The related pathways by which melatonin executes its cardiac protective role via the receptor pathway includes the reperfusion injury salvage kinase (RISK) pathway, SAFE pathway and Notch pathway, with a complex association among the downstream signaling molecules (Botker et al., 2018; Coverstone et al., 2018; Shanmugam et al., 2019; Yarana et al., 2019).
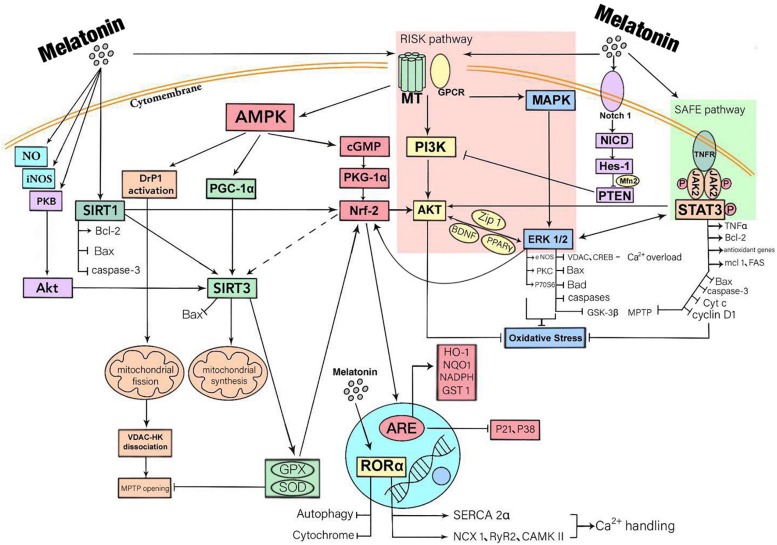
The RISK pathway has an intracellular biological role primarily through the best known melatonin receptors including MT1, MT2, and MT3, all of which belong to G-protein coupled receptor family (GPCR). MT1 and MT2 have a high affinity with melatonin, while MT3 has a low affinity (Cho et al., 2019). Researchers have discovered a large number of MT1 and MT2 in the heart of rats, ducks, and coronary arteries of chicken and human beings, indicating that the cardiovascular system is a major target organ of melatonin (Hukic et al., 2017). A non-specific melatonin receptor antagonist Luzindole, eliminates the protective action of melatonin on cardiomyocytes, thus confirming their role in mediating the protective effect of melatonin on the heart (Pan et al., 2015). There are three downstream signal pathways of MT1/2, namely MAPK-ERK signal pathway, AMP-dependent protein kinase (AMPK) signal pathway and PI3K-Akt signal pathway. The three routes transmit MT1/2 activation signals from extracellular to intracellular level and mediate intracellular second messenger transmission. The downstream signaling molecules of the three pathways are crossed and connected. (a) The MAPK-ERK signaling pathway is mediated by MT1/2, the activation of MAPK-ERK up-regulates of the level of antioxidant factor Nrf2, and Nrf2 couples with DNA antioxidant reaction elements (ARE) to up-regulate the expressions of HO-1, NADPH, quinone oxidoreductase 1 (NQO1), and glutathione s-transferase 1 (GST1), and reduces the expressions of apoptotic proteins, p21 and p38 (Audia et al., 2018; Canugovi et al., 2019; Kim C.Y. et al., 2019; Liu et al., 2019). The activity of the voltage dependent anion channel (VDAC) and the transcription factor of IP3R-cAMP response element binding protein (CREB) are inhibited by activated extracellular signal regulating kinase (ERK), while excessive activation of VDAC and CREB leads to intracellular calcium overload and then causes mitochondrial dysfunction, eventually bringing about cardiomyocyte necrosis (Li et al., 2018; Zhu H. et al., 2018). Activation of this pathway also leads to inactivation of glycogen synthase kinase-3β (GSK-3β) (Nduhirabandi et al., 2012). The downstream effects also involve the activation of endothelial nitric oxide synthase (eNOS), PKC, and p70 ribosomal protein S6 (p70S6), and down-regulation of the expression of apoptosis related factors such as Bax, Bad, and phosphorylation of caspases (Paradies et al., 2015; Eid et al., 2018; Wang S. et al., 2019; Xu N. et al., 2019). In addition, the activation of MAPK-ERK signaling pathway directly inhibits the opening of MPTP. (b) In the AMPK signaling pathway, Nrf2 is also a downstream signaling molecule of AMPK-PKG1α pathway (Wu et al., 2018b; Lu M. et al., 2019). The AMPK pathway and MAPK-ERK pathways are interrelated through Nrf2 and have a synergistic role in antioxidative stress processes and reducing apoptosis (Yu et al., 2018). In addition, the activation of AMPK inhibits the activity of mitochondrial motility related protein Drp1, which promotes mitochondrial fission, thereby activating VDAC-HK opening and ultimately promoting the MPTP opening (Singhanat et al., 2018). SIRT1 and SIRT3 are both important downstream signaling molecules that aid melatonin in its cardioprotective role. SIRT3 is the downstream target of peroxisome proliferator-activated receptor γ co-activator 1α (PGC-1α) which is stimulated by AMPK. The effect of this pathway is to reduce the transfer of Bax to mitochondria, promote the deacetylation of mitochondrial antioxidant enzyme GPX, boost the biosynthesis of mitochondria, and enhance the activity of superoxide dismutase (SOD) (Lombard and Zwaans, 2014; Lochner et al., 2018). (c) The primary downstream molecular effect of the PI3K-Akt signaling pathway is the reduction of cellular oxidative stress. The activation of Akt promotes phosphorylation of signal transducer and activator of transcription 3 (STAT3), thereby elevating TNFα release for cardiac protection (Yu et al., 2016; Kim E.H. et al., 2019). The enhanced activity of GPX and SOD raises the level of Nrf2, thus promoting the Akt signaling pathway (Song et al., 2017; Zhang H. et al., 2019). Melatonin regulates the activity of ERK through the Akt pathway. Signaling molecules in this pathway include Zrt/Irt-like protein 1 (Zip1), brain-derived neurotrophic factor (BDNF) and PPARγ. In the nuclear receptor signaling pathway, melatonin regulates autophagy and Cyt c release through ROR α, and also enhances the expression of the myocardial sarcoplasmic reticulum Ca2+-ATPase (SERCA)2α, sodium-calcium exchange 1 (NCX1), Ryanodine receptor 2 (RyR2), Ca2+-calmodulin-dependent kinase II (CAMKII) and other protein-related genes, thus enhancing the ability of cells to process calcium ions and reduce the stress injury to and apoptosis of the cardiomyocytes (Gebhard et al., 2018; Na et al., 2019).
In addition to the conventional melatonin receptor pathway, melatonin also binds to other receptors on the cell membrane for signal transduction, including the SAFE pathway and the Notch pathway. In the SAFE pathway, melatonin plays a role in phosphorylation of JAK2-STAT3 through TNF receptor on the cell membrane. Downstream molecular effects include the promotion of expression of BCL-2, antioxidant genes, TNFα, mcl 1, FAS and the inhibition of Bax, caspase-3, Cyt c, cyclin-dependent kinase (cyclin D1), P21 and GSK-3β (Yang et al., 2013). Melatonin also directly inhibits MPTP opening through this pathway. Phosphorylation of STAT3 activates the ERK and Akt pathways, which also promote phosphorylation of STAT3. In the Notch pathway, melatonin promotes the expression of Hairy and enhancer of split 1 (Hes 1) through Notch 1-Notch Intracellular area (NICD), while Hes1 inhibits the negative regulatory effect of chromosome 10 (PTEN) on phosphatidylinositol 3-kinase (PI3K). Notch pathway also reduces the effects of cardiomyocyte apoptosis by regulating mitophagy with mitochondrial fusion related protein (Mfn2) (Pei et al., 2016).
In addition to binding to receptors, melatonin also enters cells where it has direct biological effects (Mauriz et al., 2013). Melatonin enters the cytosol to promote the release of NO, enhances the activity of nitric oxide synthase (iNOS) and boosts the expression of SIRT3 via the activation of PKB-Akt. Activated by melatonin, SIRT1 regulates oxidative stress in cardiomyocytes, mitophagy and apoptosis by enhancing the expression of Bcl-2 and weakening Bax and caspase-3. Studies have shown that SIRT1 is an important upstream molecule of Nrf2 and can also be bound to the SIRT3 promoter to enhance the expression of SIRT3. However, whether melatonin regulates the expression of SIRT3 through the SIRT1-Nrf2 pathway requires verification (Figure 1).
FIGURE 1.
A summary of the mechanisms that myocardial protection mediated by melatonin through receptor and non-receptor pathways. Melatonin has a biological role mainly by being bound to receptors. There are three downstream signal pathways of MT1/2, namely MAPK-ERK signal pathway, AMP-dependent protein kinase (AMPK) signal pathway and PI3K-Akt signal pathway. Melatonin also activates STAT3, a signal transducer and activator of transcription factor for antioxidant enzymes, by activating SAFE pathway. Besides, melatonin activates the Notch1 pathway, thus inhibiting PIK3 function. These downstream signaling molecules are crossed and connected within the cell. In addition, melatonin also enters cells where it has direct biological effects. It promotes the release of NO, enhances the activity of iNOS and boosts the expression of SIRT3 via the activation of PKB-Akt. The ultimate effects of these responses are to reduce oxidative stress, inflammatory responses, and to protect mitochondrial function, thereby reducing cardiomyocyte apoptosis.
Melatonin Stabilizes the Structure and Function of Mitochondria After AMI
Mitochondria are the site of ATP and oxygen-derived free radical production, and the target of attack by various free radicals (Connolly et al., 2018; Boengler et al., 2019; Yuan et al., 2019). Melatonin protects cardiomyocytes by stabilizing the structure and function of mitochondria and regulates mitochondrial oxidative stress, raises mitochondrial antioxidant enzyme levels, restores mitochondrial energy metabolism (Soto-Heras et al., 2019), maintains mitochondrial membrane potential stability, reduces mitochondrial injury, and inhibits mitochondrial apoptosis (Yan et al., 2018) through receptors including MT1/2. In addition, mitochondrial biosynthesis, DNA homeostasis and regulation of SIRT3 system are closely related to the function of melatonin (Reiter et al., 2017; Zhou et al., 2017d). After myocardial infarction, injured tissue releases a large quantity of oxygen-derived free radicals, inflammatory mediators and other harmful substances, which cause direct injury to mitochondria (Kalkavan and Green, 2018; Riehle and Bauersachs, 2018; Nanadikar et al., 2019). Mitochondrial dysfunction aggravates cell injury, which then becomes a vicious cycle. Melatonin breaks this vicious cycle by virtue of its potent free radical scavenging ability and antioxidant effects, thus playing a protective role in myocardium such as in ischemia-reperfusion injury (Reina and Martínez, 2018). Studies show that melatonin, as a potent free radical scavenger, is abundant in mitochondria (Venegas et al., 2012). This shows that melatonin likely prevents mitochondrial injury during the oxidative stress response (Ma et al., 2016). Melatonin also up-regulates the activity of the four respiratory complexes, thus reducing electron leakage and the generation of oxygen-derived free radicals (Nair et al., 2011). Melatonin inhibits mitochondrion fission, prevents disintegration of VDAC1 and hexokinase 2 (HK2), inhibits MTPT opening, limits endothelial cell injury, improves endothelial barrier function, reduces inflammatory cell infiltration, restores eNOS content and blood flow, lowers infarct size, preserves myocardial microvasculature, and improve prognosis (Zhang and Zhang, 2014; Li et al., 2019). Based on published reports (Wang et al., 2016), activation of MT1/2 receptor strengthens AMPK signaling pathway, up-regulates optic atrophy 1 (OPA1) level and then modulates mitophagy during myocardial infarction. Mitophagy degrades injured mitochondria so as to maintain the structure and function of normal mitochondria, reduces cardiomyocyte apoptosis and lowers the degree of myocardial injury during infarction (Dominguez-Rodriguez et al., 2017a; Zhang Y. et al., 2019).
Clinical Application and Prospect of Melatonin Use in Acute Myocardial Infarction
Due to the non-renewability of cardiomyocytes, it is essential to reduce cardiac damage and improve long-term prognosis of patients with myocardial infarction by clarifying the mechanisms of injury and necrosis of cardiomyocytes after a cardiovascular episode (Gaspar et al., 2018; Wu et al., 2018a; Heusch, 2019). In addition to the mechanisms of inflammatory cell infiltration and oxidative stress injury which have been reported many times, mitochondrial injury and apoptosis are the key initiating processes of myocardial injury following myocardial infarction (Hofmann, 2018; Villalobos et al., 2018; Wang Y. et al., 2018; Boengler et al., 2019; Phan et al., 2019). There is an urgent need to find a method which can effectively reduce mitochondrial injury and to improve cardiomyocyte function following myocardial infarction and provide a new target for clinical treatment.
As an indole and age-related molecule secreted by the human pineal gland, melatonin has remarkable functions including antioxidant, anti-apoptosis, anti-fibrosis, direct free radical scavenging, and mitochondrial protection (Jumnongprakhon et al., 2016). Melatonin receptors also play a role in reducing cardiac damage (Liu et al., 2016). In models of myocardial reperfusion injury, melatonin promotes mitochondrial fusion through AMPK/OPA1 signaling pathway and OPA1 binds with lysine 70 residues of mitophagy receptor FUNDC1 to mediate mitophagy (Del Dotto et al., 2018; Wang B. et al., 2018). Recent studies reveal that mitochondria exhibit biological rhythms, which may be regulated by the melatonin cycle. However, studies have not been conducted to clarify the role of melatonin in the mitochondrial biological clock and the myocardial protective effects that involve these rhythms. As mentioned above, melatonin is secreted in large quantities at night according to its circadian rhythm. Studies illustrates that the nocturnal secretion of melatonin in patients with CHD decreases significantly compared with healthy people and it may be associated with various disease risk factors in these patients which can explain why AMI always happen (at a peak) in early morning when melatonin levels are adequate (Dominguez-Rodriguez et al., 2006, 2007a).
In terms of clinical disease treatment, most studies on melatonin-induced cardiovascular effect are in phase 2a of clinical trials, and according to literatures, melatonin has a beneficial therapeutic effect on hypertension, atherosclerosis, CHD, and other chronic cardiovascular diseases (Yang et al., 2014; Simko et al., 2016; Baltatu et al., 2019) (Table 1). Since it is an age-related molecule, it declines with age. Because of its high safety profile, it has important potential clinical applications. Clinical trials demonstrate that melatonin significantly reduce the area of myocardial infarction when used in the treatment of STEMI (ST-elevation myocardial infarction) patients after PCI (Dominguez-Rodriguez et al., 2017a). In addition, the use of melatonin before surgery significantly reduces CABG related oxidative stress and cardiac injury, and on the other hand increase the activity of Nrf2 in patients (Haghjooy Javanmard et al., 2013; Shafiei et al., 2018). Melatonin plays a role in reducing nocturnal hypertension in patients by affecting circadian cardiovascular rhythms of blood pressure (Grossman et al., 2011). The experimental results of Ma et al. (2018) show that melatonin eliminates ROS by regulating the mitophagy in macrophages, thus inhibiting the activation of NLRP3 (nucleotide-binding domain and leucine-rich repeat pyrin domain containing 3) inflammasome and ultimately inhibiting the progression of atherosclerosis, which is mediated at least in part through the Sirt3 signaling pathway.
TABLE 1.
A summary of the results of some clinical trials (there are many more), which illustrate the beneficial effects of melatonin in clinical acute myocardial infarction.
| Type of study | Study population | Administration route | Results | Possible mechanism |
| Unicenter, randomized, double-blind, parallel-group, placebo-controlled study (Dominguez-Rodriguez et al., 2007a) | 272 patients with AMI and be expected to undergo primary angioplasty (PA); melatonin group (n = 136), placebo group (n = 136). | Patients received a total intravenous melatonin dose of 11.61 mg (approximately 166 μg/kg) or placebo. The temporal distribution of perfusion was: 30 min previous to percutaneous revascularization and remainder doses in a subsequent 120 min (1 h during the angioplasty + 60 min post-intervention). | The infarction size of melatonin group and placebo group was 9.0% and 19.5%, respectively (P < 0.05). | The cardiac-protection effect of melatonin was most likely through its direct free radical scavenging activities, indirect antioxidant activity and its ability to increase mitochondrial bioenergetics. |
| Case-control study (Dominguez-Rodriguez et al., 2008) | 90 patients with STEMI and 70 healthy humans. | No melatonin was administered. | Melatonin value kept adiurnal variation but with a significantly lower dose in STEMI patients (P < 0.001). The mean nocturnal melatonin levels in these patients was lower than in the control group (P < 0.001). | The lower melatonin production rate in AMI patients was correlated with the stage of the disease, and some immunological factors, such as CRP and cytokines, could play an important role in the pathogenesis. |
| Prospective cohort study (Dominguez-Rodriguez et al., 2010a) | 180 patients with first STEMI who underwent PCI within 6 h from onset of symptoms. 63 patients (35%) were angiographic no-reflow after PCI. | No melatonin was administered. | Patients with angiographic no-reflow had lower intraplatelet melatonin levels compared to patients without no-reflow (12.32 ± 3.64 vs. 18.62 ± 3.88 ng/100,000 platelets, P < 0.0001) | Platelets have Melatonin inhibits platelet cyclooxygenase and decreases arachidonic acid-induced aggregation and thromboxane B2 production and thus inhibits platelet aggregation. |
| Prospective cohort study (Dominguez-Rodriguez et al., 2012) | 161 patients with AMI. | No melatonin was administered. | Melatonin levels (OR = 2.10, CI 95% 1.547–2.870, P < 0.001) were an independent predictor of LV remodeling. | The anti-fibrotic and antioxidant effect of melatonin. |
| Nested case-control study (McMullan et al., 2017) | 209 women with incident cases of fatal and non-fatal MI and were matched to 209 controls. | No melatonin was administered. | Lower melatonin secretion was significantly associated with a higher risk of MI. Women in the highest concentration had an estimated absolute risk of MI of 84 cases per 100,000 person-years compared with 197 cases per 100,000 person-years in the lowest concentration, and the association was strongly modified by body mass index (BMI) (p = 0.02). | Melatonin reduces platelet aggregation, against plaque rupture, and regulates the immune system and inflammation. |
| Prospective, multicenter, randomized, double blind, placebo-controlled study (Dominguez-Rodriguez et al., 2017b) | 146 patients with STEMI; melatonin group (n = 73), placebo group (n = 73). | The experimental drug was a formulation of melatonin in polyethylene glycol solution. Patients in the melatonin group received a dose of 51.7 μmol intravenously given by a time period of 60 min starting immediately before PCI and a bolus of 8.6 μmol of intracoronary melatonin given through the PCI-guiding catheter after restoring the blood flow to the infarct related artery. The placebo group received a matching placebo formulation. | No significant differences in the myocardial infarct size between the two group. Both left ventricular end-diastolic and end-systolic volumes were lower in the placebo group (P = 0.01). No significant differences in the incidence of adverse events at 1 year in both groups (P = 0.150). | The median pain-to-balloon time (200 min) was so long that it likely negated the benefits of melatonin in reducing lethal IRI. |
| Unicenter, randomized, double-blinded, placebo controlled (Ekeloef et al., 2017) | 48 patients with STEIMI; melatonin group (n = 24), placebo group (n = 24). | Patients were randomized to receive either intracoronary or intravenous melatonin (total 50 mg) or placebo (isotonic saline) | Melatonin did not exert a significant effect on myocardial salvage index after PCI. The myocardial salvage index at day 4 (±1 day) after PCI was similar in the melatonin group (n = 22) at 55.3% (95% CI 47.0–63.6) and the placebo group (n = 19) at 61.5% (95% CI 57.5–65.5), p = 0.21. | The cardioprotective effects of melatonin might be largely dependent on a clinically effective distribution of the drug in the myocardial area at risk, prior to ischemia and definitely prior to reperfusion |
| Prospective, multicenter, randomized, double blind, placebo-controlled study (Dominguez-Rodriguez et al., 2017a) | 146 patients with STEMI; melatonin group (n = 73), placebo group (n = 73). Randomized patients were divided into tertiles according to symptoms onset to balloon time: first tertile (136 ± 23 min), second tertile (196 ± 19 min), and third tertile (249 ± 41 min). | The experimental drug was a formulation of melatonin in polyethylene glycol solution. Patients in the melatonin group received a dose of 51.7 μmol intravenously given by a time period of 60 min starting immediately before PCI and a bolus of 8.6 μmol of intracoronary melatonin given through the PCI-guiding catheter after restoring the blood flow to the infarct related artery. The placebo group received a matching placebo formulation. | In the first tertile, the infarct size was significantly smaller in the melatonin-treated subjects compared with placebo (14.6 ± 14.2 vs. 24.9 ± 9.0%; P = 0.003). Treatment with melatonin was associated with a larger infarct size in the group of patients included in the third tertile (20.5 ± 8.7% vs. 11.2 ± 5.2%; P = 0.001), resulting in a significant interaction (P = 0.001). | Melatonin administered earlier may result in a greater cardioprotective effect compared with delayed administration. Treatments that are able to reduce mitochondrial dysfunction appear to be more effective after shorter ischemic periods. |
AMI, acute myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
Studies reveal that (Dominguez-Rodriguez et al., 2017a), from the onset of myocardial infarction symptoms to the beginning of interventional therapy, the application of melatonin in a short time window can effectively reduce the size of a myocardial infarct. Repair after a myocardial infarction is a complex process involved with multiple factors, but whether melatonin is involved with these repair processes have not been investigated. The combination of melatonin with other myocardial protective drugs (e.g., antithrombotic drugs and new-type myocardial metabolic regulation drug GLP1) has not yet been reported, and further research is required to confirm the myocardial protective actions of melatonin treatment.
Author Contributions
ZF conceived and designed the review. ZF, YJ, JW, YZ, and MS collected the literatures. YJ and ZF wrote the manuscript. ZF, RR, and YC reviewed and edited the manuscript. QX revised the manuscript and the language. All authors read and approved the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study was supported by National Natural Science Foundation of China (fund numbers 81500269, 81500202, and 81570383).
References
- Abdelnaseer M., Elfayomi N., Esmail E. H., Kamal M. M., Hamdy A., Samie R. M. A., et al. (2016). Relationship between matrix metalloproteinase-9 and common carotid artery intima media thickness. Neurol. Sci. 37 117–122. 10.1007/s10072-015-2358-z [DOI] [PubMed] [Google Scholar]
- Abukar Y., Ramchandra R., Hood S. G., McKinley M. J., Booth L. C., Yao S. T., et al. (2018). Increased cardiac sympathetic nerve activity in ovine heart failure is reduced by lesion of the area postrema, but not lamina terminalis. Basic Res. Cardiol. 113:35. [DOI] [PubMed] [Google Scholar]
- Adameova A., Goncalvesova E., Szobi A., Dhalla N. S. (2016). Necroptotic cell death in failing heart: relevance and proposed mechanisms. Heart Fail. Rev. 21 213–221. 10.1007/s10741-016-9537-8 [DOI] [PubMed] [Google Scholar]
- Aimo A., Cerbai E., Bartolucci G., Adamo L., Barison A., Lo Surdo G., et al. (2020). Pirfenidone is a cardioprotective drug: mechanisms of action and preclinical evidence. Pharmacol. Res. 155:104694 10.1016/j.phrs.2020.104694 [DOI] [PubMed] [Google Scholar]
- Amanakis G., Kleinbongard P., Heusch G., Skyschally A. (2019). Attenuation of ST-segment elevation after ischemic conditioning maneuvers reflects cardioprotection online. Basic Res. Cardiol. 114:22 10.1007/s00395-019-0732-3 [DOI] [PubMed] [Google Scholar]
- Amin N., Shafabakhsh R., Reiter R. J., Asemi Z. (2019). Melatonin is an appropriate candidate for breast cancer treatment: based on known molecular mechanisms. J. Cell. Biochem. 120 12208–12215. 10.1002/jcb.28832 [DOI] [PubMed] [Google Scholar]
- Arushanian E. B. (2013). Effect of melatonin on the thrombocyte hemostasis and its circadian organization. Eksp. Klin. Farmakol. 76 32–36. [PubMed] [Google Scholar]
- Audia J. P., Yang X. M., Crockett E. S., Housley N., Haq E. U., O’Donnell K., et al. (2018). Caspase-1 inhibition by VX-765 administered at reperfusion in P2Y12 receptor antagonist-treated rats provides long-term reduction in myocardial infarct size and preservation of ventricular function. Basic Res. Cardiol. 113:32 10.1007/s00395-018-0692-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacmeister L., Schwarzl M., Warnke S., Stoffers B., Blankenberg S., Westermann D., et al. (2019). Inflammation and fibrosis in murine models of heart failure. Basic Res. Cardiol. 114:19. [DOI] [PubMed] [Google Scholar]
- Baltatu O. C., Senar S., Campos L. A., Cipolla-Neto J. (2019). Cardioprotective melatonin: translating from proof-of-concept studies to therapeutic use. Int. J. Mol. Sci. 20:4342 10.3390/ijms20184342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basalay M. V., Davidson S. M., Gourine A. V., Yellon D. M. (2018). Neural mechanisms in remote ischaemic conditioning in the heart and brain: mechanistic and translational aspects. Basic Res. Cardiol. 113:25 10.1007/s00395-018-0684-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battogtokh G., Choi Y. S., Kang D. S., Park S. J., Shim M. S., Huh K. M., et al. (2018). Mitochondria-targeting drug conjugates for cytotoxic, anti-oxidizing and sensing purposes: current strategies and future perspectives. Acta Pharm. Sin. B 8 862–880. 10.1016/j.apsb.2018.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckendorf J., van den Hoogenhof M. M. G., Backs J. (2018). Physiological and unappreciated roles of CaMKII in the heart. Basic Res. Cardiol. 113:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari P., Song M., Chen Y., Burelle Y., Dorn G. W. (2014). Mitochondrial contagion induced by Parkin deficiency in Drosophila hearts and its containment by suppressing mitofusin. Circ. Res. 114 257–265. 10.1161/CIRCRESAHA.114.302734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocci M., Sjolund J., Kurzejamska E., Lindgren D., Marzouka N. A., Bartoschek M., et al. (2019). Activin receptor-like kinase 1 is associated with immune cell infiltration and regulates CLEC14A transcription in cancer. Angiogenesis 22 117–131. 10.1007/s10456-018-9642-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boengler K., Bornbaum J., Schluter K. D., Schulz R. (2019). P66shc and its role in ischemic cardiovascular diseases. Basic Res. Cardiol. 114:29 10.1007/s00395-019-0738-x [DOI] [PubMed] [Google Scholar]
- Boengler K., Lochnit G., Schulz R. (2018). Mitochondria “THE” target of myocardial conditioning. Am. J. Physiol. Heart Circ. Physiol. 315 H1215–H1231. 10.1152/ajpheart.00124.2018 [DOI] [PubMed] [Google Scholar]
- Botker H. E., Hausenloy D., Andreadou I., Antonucci S., Boengler K., Davidson S. M., et al. (2018). Practical guidelines for rigor and reproducibility in preclinical and clinical studies on cardioprotection. Basic Res. Cardiol. 113:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breda C. N. S., Davanzo G. G., Basso P. J., Saraiva Camara N. O., Moraes-Vieira P. M. M. (2019). Mitochondria as central hub of the immune system. Redox Biol. 26:101255 10.1016/j.redox.2019.101255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canugovi C., Stevenson M. D., Vendrov A. E., Hayami T., Robidoux J., Xiao H., et al. (2019). Increased mitochondrial NADPH oxidase 4 (NOX4) expression in aging is a causative factor in aortic stiffening. Redox Biol. 26:101288 10.1016/j.redox.2019.101288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao T., Fan S., Zheng D., Wang G., Yu Y., Chen R., et al. (2019). Increased calpain-1 in mitochondria induces dilated heart failure in mice: role of mitochondrial superoxide anion. Basic Res. Cardiol. 114:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Ye K., Zhang B., Xin Q., Li P., Kong A. N., et al. (2019). Paris Saponin II inhibits colorectal carcinogenesis by regulating mitochondrial fission and NF-kappaB pathway. Pharmacol. Res. 139 273–285. 10.1016/j.phrs.2018.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Dong G., Wu S., Liu N., Zhang W., Sheng C. (2019). Novel fluorescent probes of 10-hydroxyevodiamine: autophagy and apoptosis-inducing anticancer mechanisms. Acta Pharm. Sin. B 9 144–156. 10.1016/j.apsb.2018.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Spitzl A., Mathes D., Nikolaev V. O., Werner F., Weirather J., et al. (2016). Endothelial actions of ANP enhance myocardial inflammatory infiltration in the early phase after acute infarction. Circ. Res. 119 237–248. 10.1161/CIRCRESAHA.115.307196 [DOI] [PubMed] [Google Scholar]
- Cho J. H., Tae H. J., Kim I. S., Song M., Kim H., Lee T. K., et al. (2019). Melatonin alleviates asphyxial cardiac arrest-induced cerebellar Purkinje cell death by attenuation of oxidative stress. Exp. Neurol. 320:112983 10.1016/j.expneurol.2019.112983 [DOI] [PubMed] [Google Scholar]
- Cohen A. T., Berger S. E., Milenkovic D., Hill N. R., Lister S. (2019). Anticoagulant selection for patients with VTE-Evidence from a systematic literature review of network meta-analyses. Pharmacol. Res. 143 166–177. 10.1016/j.phrs.2019.03.017 [DOI] [PubMed] [Google Scholar]
- Connolly N. M. C., Theurey P., Adam-Vizi V., Bazan N. G., Bernardi P., Bolanos J. P., et al. (2018). Guidelines on experimental methods to assess mitochondrial dysfunction in cellular models of neurodegenerative diseases. Cell Death Differ. 25 542–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coverstone E. D., Bach R. G., Chen L., Bierut L. J., Li A. Y., Lenzini P. A., et al. (2018). A novel genetic marker of decreased inflammation and improved survival after acute myocardial infarction. Basic Res. Cardiol. 113:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist A. M., Lee A. R., Patel N. R., Westhoff D. E., Meadows S. M. (2018). Vascular deficiency of Smad4 causes arteriovenous malformations: a mouse model of Hereditary Hemorrhagic Telangiectasia. Angiogenesis 21 363–380. 10.1007/s10456-018-9602-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S. M., Arjun S., Basalay M. V., Bell R. M., Bromage D. I., Botker H. E., et al. (2018). The 10th biennial hatter cardiovascular institute workshop: cellular protection-evaluating new directions in the setting of myocardial infarction, ischaemic stroke, and cardio-oncology. Basic Res. Cardiol. 113:43 10.1007/s00395-018-0704-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira Junior E. R., Nascimento T. L., Salomao M. A., da Silva A. C. G., Valadares M. C., Lima E. M. (2019). Increased nose-to-brain delivery of melatonin mediated by polycaprolactone nanoparticles for the treatment of glioblastoma. Pharm. Res. 36:131 10.1007/s11095-019-2662-z [DOI] [PubMed] [Google Scholar]
- Del Dotto V., Fogazza M., Lenaers G., Rugolo M., Carelli V., Zanna C. (2018). OPA1: how much do we know to approach therapy? Pharmacol. Res. 131 199–210. 10.1016/j.phrs.2018.02.018 [DOI] [PubMed] [Google Scholar]
- Dominguez-Rodriguez A., Abreu-Gonzalez P., Arroyo-Ucar E., Reiter R. J. (2012). Decreased level of melatonin in serum predicts left ventricular remodelling after acute myocardial infarction. J. Pineal Res. 53 319–323. 10.1111/j.1600-079X.2012.01001.x [DOI] [PubMed] [Google Scholar]
- Dominguez-Rodriguez A., Abreu-Gonzalez P., de la Torre-Hernandez J. M., Consuegra-Sanchez L., Piccolo R., Gonzalez-Gonzalez J., et al. (2017a). Usefulness of early treatment with melatonin to reduce infarct size in patients with ST-segment elevation myocardial infarction receiving percutaneous coronary intervention (from the melatonin adjunct in the acute myocardial infarction treated with angioplasty trial). Am. J. Cardiol. 120 522–526. 10.1016/j.amjcard.2017.05.018 [DOI] [PubMed] [Google Scholar]
- Dominguez-Rodriguez A., Abreu-Gonzalez P., de la Torre-Hernandez J. M., Gonzalez-Gonzalez J., Garcia-Camarero T., Consuegra-Sanchez L., et al. (2017b). Effect of intravenous and intracoronary melatonin as an adjunct to primary percutaneous coronary intervention for acute ST-elevation myocardial infarction: results of the Melatonin Adjunct in the acute myocaRdial Infarction treated with Angioplasty trial. J. Pineal Res. 62:e12374 10.1111/jpi.12374 [DOI] [PubMed] [Google Scholar]
- Dominguez-Rodriguez A., Abreu-Gonzalez P., Garcia-Gonzalez M., Reiter R. J. (2006). Prognostic value of nocturnal melatonin levels as a novel marker in patients with ST-segment elevation myocardial infarction. Am. J. Cardiol. 97 1162–1164. 10.1016/j.amjcard.2005.11.033 [DOI] [PubMed] [Google Scholar]
- Dominguez-Rodriguez A., Abreu-Gonzalez P., Garcia-Gonzalez M. J., Kaski J. C., Reiter R. J., Jimenez-Sosa A. (2007a). A unicenter, randomized, double-blind, parallel-group, placebo-controlled study of melatonin as an adjunct in patients with acute myocardial infarction undergoing primary angioplasty the melatonin adjunct in the acute myocardial infarction treated with angioplasty (MARIA) trial: study design and rationale. Contemp. Clin. Trials 28 532–539. 10.1016/j.cct.2006.10.007 [DOI] [PubMed] [Google Scholar]
- Dominguez-Rodriguez A., Abreu-Gonzalez P., Garcia-Gonzalez M. J., Reiter R. J. (2007b). Relation of nocturnal melatonin levels to serum matrix metalloproteinase-9 concentrations in patients with myocardial infarction. Thromb. Res. 120 361–366. 10.1016/j.thromres.2006.10.010 [DOI] [PubMed] [Google Scholar]
- Dominguez-Rodriguez A., Abreu-Gonzalez P., Garcia-Gonzalez M. J., Samimi-Fard S., Kaski J. C., Reiter R. J. (2008). Light/dark patterns of soluble vascular cell adhesion molecule-1 in relation to melatonin in patients with ST-segment elevation myocardial infarction. J. Pineal Res. 44 65–69. 10.1111/j.1600-079X.2007.00529.x [DOI] [PubMed] [Google Scholar]
- Dominguez-Rodriguez A., Abreu-Gonzalez P., Jimenez-Sosa A., Avanzas P., Bosa-Ojeda F., Kaski J. C. (2010a). Usefulness of intraplatelet melatonin levels to predict angiographic no-reflow after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. Am. J. Cardiol. 106 1540–1544. 10.1016/j.amjcard.2010.07.030 [DOI] [PubMed] [Google Scholar]
- Dominguez-Rodriguez A., Abreu-Gonzalez P., Sanchez-Sanchez J. J., Kaski J. C., et al. (2010b). Melatonin and circadian biology in human cardiovascular disease. J. Pineal Res. 49 14–22. 10.1111/j.1600-079X.2010.00773.x [DOI] [PubMed] [Google Scholar]
- Eid R. A., Alkhateeb M. A., Eleawa S., Al-Hashem F. H., Al-Shraim M., El-Kott A. F., et al. (2018). Cardioprotective effect of ghrelin against myocardial infarction-induced left ventricular injury via inhibition of SOCS3 and activation of JAK2/STAT3 signaling. Basic Res. Cardiol. 113:13. [DOI] [PubMed] [Google Scholar]
- Ekeloef S., Halladin N., Fonnes S., Jensen S. E., Zaremba T., Rosenberg J., et al. (2017). Effect of intracoronary and intravenous melatonin on myocardial salvage index in patients with ST-elevation myocardial infarction: a randomized placebo controlled trial. J. Cardiovasc. Transl. Res. 10 470–479. 10.1007/s12265-017-9768-7 [DOI] [PubMed] [Google Scholar]
- Espinosa-Diez C., Miguel V., Vallejo S., Sanchez F. J., Sandoval E., Blanco E., et al. (2018). Role of glutathione biosynthesis in endothelial dysfunction and fibrosis. Redox Biol. 14 88–99. 10.1016/j.redox.2017.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faghfouri A. H., Zarrin R., Maleki V., Payahoo L., Khajebishak Y. (2020). A comprehensive mechanistic review insight into the effects of micronutrients on toll-like receptors functions. Pharmacol. Res. 152:104619 10.1016/j.phrs.2019.104619 [DOI] [PubMed] [Google Scholar]
- Filadi R., Greotti E., Pizzo P. (2018). Highlighting the endoplasmic reticulum-mitochondria connection: focus on mitofusin 2. Pharmacol. Res. 128 42–51. 10.1016/j.phrs.2018.01.003 [DOI] [PubMed] [Google Scholar]
- Fuhrmann D. C., Brune B. (2017). Mitochondrial composition and function under the control of hypoxia. Redox Biol. 12 208–215. 10.1016/j.redox.2017.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galano A., Reiter R. J. (2018). Melatonin and its metabolites vs oxidative stress: from individual actions to collective protection. J. Pineal Res. 65:e12514 10.1111/jpi.12514 [DOI] [PubMed] [Google Scholar]
- Galano A., Tan D. X., Reiter R. J. (2013). On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J. Pineal Res. 54 245–257. 10.1111/jpi.12010 [DOI] [PubMed] [Google Scholar]
- Galluzzi L., Vitale I., Aaronson S. A., Abrams J. M., Adam D., Agostinis P., et al. (2018). Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 25 486–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J. A., Volt H., Venegas C., Doerrier C., Escames G., Lopez L. C., et al. (2015). Disruption of the NF-kappaB/NLRP3 connection by melatonin requires retinoid-related orphan receptor-alpha and blocks the septic response in mice. FASEB J. 29 3863–3875. 10.1096/fj.15-273656 [DOI] [PubMed] [Google Scholar]
- Gaspar A., Lourenco A. P., Pereira M. A., Azevedo P., Roncon-Albuquerque R., Jr., et al. (2018). Randomized controlled trial of remote ischaemic conditioning in ST-elevation myocardial infarction as adjuvant to primary angioplasty (RIC-STEMI). Basic Res. Cardiol. 113:14. [DOI] [PubMed] [Google Scholar]
- Gebhard C., Maafi F., Stahli B. E., Dang J., Nachar W., de Oliveira Moraes A. B., et al. (2018). Apolipoprotein A-I proteolysis in aortic valve stenosis: role of cathepsin S. Basic Res. Cardiol. 113:30. 10.1007/s00395-018-0689-7 [DOI] [PubMed] [Google Scholar]
- Girish K. S., Paul M., Thushara R. M., Hemshekhar M., Shanmuga Sundaram M., Rangappa K. S., et al. (2013). Melatonin elevates apoptosis in human platelets via ROS mediated mitochondrial damage. Biochem. Biophys. Res. Commun. 438 198–204. 10.1016/j.bbrc.2013.07.053 [DOI] [PubMed] [Google Scholar]
- Giudice A., Arra C., Turco M. C. (2010). Review of molecular mechanisms involved in the activation of the Nrf2-ARE signaling pathway by chemopreventive agents. Methods Mol. Biol. 647 37–74. 10.1007/978-1-60761-738-9_3 [DOI] [PubMed] [Google Scholar]
- Graczyk-Jarzynka A., Goral A., Muchowicz A., Zagozdzon R., Winiarska M., Bajor M., et al. (2019). Inhibition of thioredoxin-dependent H2O2 removal sensitizes malignant B-cells to pharmacological ascorbate. Redox Biol. 21:101062. 10.1016/j.redox.2018.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman E., Laudon M., Zisapel N. (2011). Effect of melatonin on nocturnal blood pressure: meta-analysis of randomized controlled trials. Vasc. Health Risk Manag. 7 577–584. 10.2147/VHRM.S24603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidarelli A., Fiorani M., Cerioni L., Cantoni O. (2019). Calcium signals between the ryanodine receptor- and mitochondria critically regulate the effects of arsenite on mitochondrial superoxide formation and on the ensuing survival vs apoptotic signaling. Redox Biol. 20 285–295. 10.1016/j.redox.2018.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadebe N., Cour M., Lecour S. (2018). The SAFE pathway for cardioprotection: is this a promising target? Basic Res. Cardiol. 113:9. 10.1007/s00395-018-0670-5 [DOI] [PubMed] [Google Scholar]
- Haghjooy Javanmard S., Ziaei A., Ziaei S., Ziaei E., Mirmohammad-Sadeghi M. (2013). The effect of preoperative melatonin on nuclear erythroid 2-related factor 2 activation in patients undergoing coronary artery bypass grafting surgery. Oxid. Med. Cell. Longev. 2013:676829. 10.1155/2013/676829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B., Zhao Y. C., Xu L. W., Gao L. C., Su Y. Y., Lin N., et al. (2016). The nuclear melatonin receptor RORα is a novel endogenous defender against myocardial ischemia/reperfusion injury. J. Pineal Res. 60 313–326. 10.1111/jpi.12312 [DOI] [PubMed] [Google Scholar]
- Heckmann B. L., Tummers B., Green D. R. (2019). Crashing the computer: apoptosis vs. necroptosis in neuroinflammation. Cell Death Differ. 26 41–52. 10.1038/s41418-018-0195-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heusch G. (2019). Coronary microvascular obstruction: the new frontier in cardioprotection. Basic Res. Cardiol. 114:45. 10.1007/s00395-019-0756-8 [DOI] [PubMed] [Google Scholar]
- Hockings C., Alsop A. E., Fennell S. C., Lee E. F., Fairlie W. D., Dewson G., et al. (2018). Mcl-1 and Bcl-xL sequestration of Bak confers differential resistance to BH3-only proteins. Cell Death Differ. 25 719–732. 10.1038/s41418-017-0010-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann F. (2018). A concise discussion of the regulatory role of cGMP kinase I in cardiac physiology and pathology. Basic Res. Cardiol. 113:31. 10.1007/s00395-018-0690-1 [DOI] [PubMed] [Google Scholar]
- Hu C., Wang P., Zhang S., Ren L., Lv Y., Yin R., et al. (2017). Neuroprotective effect of melatonin on soluble Abeta1-42-induced cortical neurodegeneration via Reelin-Dab1 signaling pathway. Neurol. Res. 39 621–631. 10.1080/01616412.2017.1312805 [DOI] [PubMed] [Google Scholar]
- Hu S. Y., Zhang Y., Zhu P. J., Zhou H., Chen Y. D. (2017). Liraglutide directly protects cardiomyocytes against reperfusion injury possibly via modulation of intracellular calcium homeostasis. Geriatr. Cardiol. 14 57–66. 10.11909/j.issn.1671-5411.2017.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C., Zhang X., Wei W., Zhang N., Wu H., Ma Z., et al. (2019). Matrine attenuates oxidative stress and cardiomyocyte apoptosis in doxorubicin-induced cardiotoxicity via maintaining AMPKalpha/UCP2 pathway. Acta Pharm. Sin. B 9 690–701. 10.1016/j.apsb.2019.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hukic D. S., Lavebratt C., Olsson E., Ostenson C. G., Eriksson S. V., Erlinge D., et al. (2017). Troponin T levels associated with genetic variants in NOTCH2 and MTNR1B in women with psychosis. Psychiatry Res. 250 217–220. 10.1016/j.psychres.2017.01.030 [DOI] [PubMed] [Google Scholar]
- Jager S. C. A. D., Hoefer I. E. (2019). Local inflammatory responses take their toll on the heart. Int. J. Cardiol. 293 254–255. 10.1016/j.ijcard.2019.07.055 [DOI] [PubMed] [Google Scholar]
- Ji C. H., Kwon Y. T. (2017). Crosstalk and interplay between the ubiquitin-proteasome system and autophagy. Mol. Cells 40 441–449. 10.14348/molcells.2017.0115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q., Xiang B., Wang H., Huang K., Kong H., Hu S. (2019). Remote ischaemic preconditioning ameliorates sinus rhythm restoration rate through Cox maze radiofrequency procedure associated with inflammation reaction reduction. Basic Res. Cardiol. 114:14. 10.1007/s00395-019-0723-4 [DOI] [PubMed] [Google Scholar]
- Jin Q., Li R., Hu N., Xin T., Zhu P., Hu S., et al. (2018). DUSP1 alleviates cardiac ischemia/reperfusion injury by suppressing the Mff-required mitochondrial fission and Bnip3-related mitophagy via the JNK pathways. Redox Biol. 14 576–587. 10.1016/j.redox.2017.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi A. U., Mochly-Rosen D. (2018). Mortal engines: mitochondrial bioenergetics and dysfunction in neurodegenerative diseases. Pharmacol. Res. 138 2–15. 10.1016/j.phrs.2018.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi C., Bapat R., Anderson W., Dawson D., Hijazi K., Cherukara G. (2019). Detection of periodontal microorganisms in coronary atheromatous plaque specimens of myocardial infarction patients: a systematic review and meta-analysis. Trends Cardiovasc. Med. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Jumnongprakhon P., Govitrapong P., Tocharus C., Tocharus J. (2016). Inhibitory effect of melatonin on cerebral endothelial cells dysfunction induced by methamphetamine via NADPH oxidase-2. Brain Res. 1650 84–92. 10.1016/j.brainres.2016.08.045 [DOI] [PubMed] [Google Scholar]
- Kalkavan H., Green D. R. (2018). MOMP, cell suicide as a BCL-2 family business. Cell Death Differ. 25 46–55. 10.1038/cdd.2017.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan G. N., Ichim B., Gharibeh L., Maharsy W., Patten D. A., Xuan J. Y., et al. (2018). Glutaredoxin-2 controls cardiac mitochondrial dynamics and energetics in mice, and protects against human cardiac pathologies. Redox Biol. 14 509–521. 10.1016/j.redox.2017.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J. W., Hong J. M., Lee S. M. (2016). Melatonin enhances mitophagy and mitochondrial biogenesis in rats with carbon tetrachloride-induced liver fibrosis. J. Pineal Res. 60 383–393. 10.1111/jpi.12319 [DOI] [PubMed] [Google Scholar]
- Karwi Q. G., Bice J. S., Baxter G. F. (2018). Pre- and postconditioning the heart with hydrogen sulfide (H2S) against ischemia/reperfusion injury in vivo: a systematic review and meta-analysis. Basic Res. Cardiol. 113:6. 10.1007/s00395-017-0664-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C. Y., Kang B., Suh H. J., Choi H. S. (2019). Parthenolide, a feverfew-derived phytochemical, ameliorates obesity and obesity-induced inflammatory responses via the Nrf2/Keap1 pathway. Pharmacol. Res. 145:104259. 10.1016/j.phrs.2019.104259 [DOI] [PubMed] [Google Scholar]
- Kim E. H., Wong S. W., Martinez J. (2019). Programmed necrosis and disease:we interrupt your regular programming to bring you necroinflammation. Cell Death Differ. 26 25–40. 10.1038/s41418-018-0179-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleszczynski K., Zillikens D., Fischer T. W. (2016). Melatonin enhances mitochondrial ATP synthesis, reduces reactive oxygen species formation, and mediates translocation of the nuclear erythroid 2-related factor 2 resulting in activation of phase-2 antioxidant enzymes (gamma-GCS, HO-1, NQO1) in ultraviolet radiation-treated normal human epidermal keratinocytes (NHEK). J. Pineal Res. 61 187–197. 10.1111/jpi.12338 [DOI] [PubMed] [Google Scholar]
- Koentges C., Pepin M. E., Musse C., Pfeil K., Alvarez S. V. V., Hoppe N., et al. (2018). Gene expression analysis to identify mechanisms underlying heart failure susceptibility in mice and humans. Basic Res. Cardiol. 113:8. 10.1007/s00395-017-0666-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlstedt K., Trouvain C., Fromel T., Mudersbach T., Henschler R., Fleming I. (2018). Role of the angiotensin-converting enzyme in the G-CSF-induced mobilization of progenitor cells. Basic Res. Cardiol. 113:18. 10.1007/s00395-018-0677-y [DOI] [PubMed] [Google Scholar]
- Kornicka K., Szlapka-Kosarzewska J., Smieszek A., Marycz K. (2019). 5-Azacytydine and resveratrol reverse senescence and ageing of adipose stem cells via modulation of mitochondrial dynamics and autophagy. J. Cell. Mol. Med. 23 237–259. 10.1111/jcmm.13914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowaltowski A. J. (2019). Strategies to detect mitochondrial oxidants. Redox Biol. 21:101065. 10.1016/j.redox.2018.101065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause J., Loser A., Lemoine M. D., Christ T., Scherschel K., Meyer C., et al. (2018). Rat atrial engineered heart tissue: a new in vitro model to study atrial biology. Basic Res. Cardiol. 113:41. 10.1007/s00395-018-0701-2 [DOI] [PubMed] [Google Scholar]
- Lansink M. O., Görlinger K., Hartmann M., de Groot H., Effenberger-Neidnicht K. (2016). Melatonin does not affect disseminated intravascular coagulation but diminishes decreases in platelet count during subacute endotoxaemia in rats. Thromb. Res. 139 38–43. 10.1016/j.thromres.2015.10.025 [DOI] [PubMed] [Google Scholar]
- Lee B. W. L., Ghode P., Ong D. S. T. (2019). Redox regulation of cell state and fate. Redox Biol. 25:101056. 10.1016/j.redox.2018.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E., Hwang I., Park S., Hong S., Hwang B., Cho Y., et al. (2019). MPTP-driven NLRP3 inflammasome activation in microglia plays a central role in dopaminergic neurodegeneration. Cell Death Differ. 26 213–228. 10.1038/s41418-018-0124-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. Y., Zheng X. Y., Ma X. Y., Xu X. Y., Du Y., Lv Q. J., et al. (2019). Melatonin protects against chromium(VI)-induced cardiac injury via activating the AMPK/Nrf2 pathway. J. Inorg. Biochem. 197:110698. 10.1016/j.jinorgbio.2019.110698 [DOI] [PubMed] [Google Scholar]
- Li Q., Turdi S., Thomas D. P., Zhou T., Ren J. (2010). Intra-myocardial delivery of mesenchymal stem cells ameliorates left ventricular and cardiomyocyte contractile dysfunction following myocardial infarction. Toxicol. Lett. 195 119–126. 10.1016/j.toxlet.2010.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Xin T., Li D., Wang C., Zhu H., Zhou H. (2018). Therapeutic effect of Sirtuin 3 on ameliorating nonalcoholic fatty liver disease: the role of the ERK-CREB pathway and Bnip3-mediated mitophagy. Redox Biol. 18 229–243. 10.1016/j.redox.2018.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. B., Clough S. J., Hutchinson A. J., Adamah-Biassi E. B., Popovska-Gorevski M., Dubocovich M. L. (2016). MT1 and MT2 melatonin receptors: a therapeutic perspective. Annu. Rev. Pharmacol. Toxicol. 56 361–383. 10.1146/annurev-pharmtox-010814-124742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Feng D., Chen G., Chen M., Zheng Q., Song P., et al. (2012). Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat. Cell Biol. 14 177–185. 10.1038/ncb2422 [DOI] [PubMed] [Google Scholar]
- Liu Y., Xu W., Zhai T., You J., Chen Y. (2019). Silibinin ameliorates hepatic lipid accumulation and oxidative stress in mice with non-alcoholic steatohepatitis by regulating CFLAR-JNK pathway. Acta Pharm. Sin. B 9 745–757. 10.1016/j.apsb.2019.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochner A., Marais E., Huisamen B. (2018). Melatonin and cardioprotection against ischaemia/reperfusion injury: what’s new? A review. J. Pineal Res. 65:e12490. 10.1111/jpi.12490 [DOI] [PubMed] [Google Scholar]
- Lombard D. B., Zwaans B. M. M. (2014). SIRT3: as simple as it seems? Gerontology 60 56–64. 10.1159/000354382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Li J., Hu Y., Guo Z., Sun D., Wang P., et al. (2019). Chrysophanol protects against doxorubicin-induced cardiotoxicity by suppressing cellular PARylation. Acta Pharm. Sin. B 9 782–793. 10.1016/j.apsb.2018.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M., Wang P., Qiao Y., Jiang C., Ge Y., Flickinger B., et al. (2019). GSK3beta-mediated Keap1-independent regulation of Nrf2 antioxidant response: a molecular rheostat of acute kidney injury to chronic kidney disease transition. Redox Biol. 26:101275. 10.1016/j.redox.2019.101275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S., Chen J., Feng J., Zhang R., Fan M., Han D., et al. (2018). Melatonin ameliorates the progression of atherosclerosis via mitophagy activation and NLRP3 inflammasome inhibition. Oxid. Med. Cell. Longev. 2018:9286458. 10.1155/2018/9286458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z. Q., Yang Y., Fan C. X., Han J., Wang D. J., Di S. Y., et al. (2016). Melatonin as a potential anticarcinogen for non-small-cell lung cancer. Oncotarget 7 46768–46784. 10.18632/oncotarget.8776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauriz J. L., Collado P. S., Veneroso C., Reiter R. J., Gonzalez-Gallego J. (2013). A review of the molecular aspects of melatonin’s anti-inflammatory actions: recent insights and new perspectives. J. Pineal Res. 54 1–14. 10.1111/j.1600-079X.2012.01014.x [DOI] [PubMed] [Google Scholar]
- Mayorov V., Uchakin P., Amarnath V., Panov A. V., Bridges C. C., Uzhachenko R., et al. (2019). Targeting of reactive isolevuglandins in mitochondrial dysfunction and inflammation. Redox Biol. 26:101300. 10.1016/j.redox.2019.101300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullan C. J., Rimm E. B., Schernhammer E. S., Forman J. P. (2017). A nested case-control study of the association between melatonin secretion and incident myocardial infarction. Heart 103 694–701. 10.1136/heartjnl-2016-310098 [DOI] [PubMed] [Google Scholar]
- Mei D., Chen B., He B., Liu H., Lin Z., Lin J., et al. (2019). Actively priming autophagic cell death with novel transferrin receptor-targeted nanomedicine for synergistic chemotherapy against breast cancer. Acta Pharm. Sin. B 9 1061–1077. 10.1016/j.apsb.2019.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz J., Albrecht P., von Garlen S., Ahmed I., Dimanski D., Wolf D., et al. (2018). Purinergic receptor Y2 (P2Y2)- dependent VCAM-1 expression promotes immune cell infiltration in metabolic syndrome. Basic Res. Cardiol. 113:45. 10.1007/s00395-018-0702-1 [DOI] [PubMed] [Google Scholar]
- Meyer I. S., Leuschner F. (2018). The role of Wnt signaling in the healing myocardium: a focus on cell specificity. Basic Res. Cardiol. 113:44. 10.1007/s00395-018-0705-y [DOI] [PubMed] [Google Scholar]
- Morell M., Burgos J. I., Gonano L. A., Vila Petroff M. (2018). AMPK-dependent nitric oxide release provides contractile support during hyperosmotic stress. Basic Res. Cardiol. 113:7. 10.1007/s00395-017-0665-7 [DOI] [PubMed] [Google Scholar]
- Na H. J., Yeum C. E., Kim H. S., Lee J., Kim J. Y., Cho Y. S. (2019). TSPYL5-mediated inhibition of p53 promotes human endothelial cell function. Angiogenesis 22 281–293. 10.1007/s10456-018-9656-z [DOI] [PubMed] [Google Scholar]
- Nahrendorf M., Pittet M. J., Swirski F. K. (2010). Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation 121 2437–2445. 10.1161/CIRCULATIONAHA.109.916346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S. M., Rahman R. M., Clarkson A. N., Sutherland B. A., Taurin S., Sammut I. A., et al. (2011). Melatonin treatment following stroke induction modulates L-arginine metabolism. J. Pineal Res. 51 313–323. 10.1111/j.1600-079X.2011.00891.x [DOI] [PubMed] [Google Scholar]
- Nanadikar M. S., Vergel Leon A. M., Borowik S., Hillemann A., Zieseniss A., Belousov V. V., et al. (2019). O2 affects mitochondrial functionality ex vivo. Redox Biol. 22:101152. 10.1016/j.redox.2019.101152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NaveenKumar S. K., Hemshekhar M., Kemparaju K., Girish K. S. (2019). Hemin-induced platelet activation and ferroptosis is mediated through ROS-driven proteasomal activity and inflammasome activation: protection by melatonin. Biochim. Biophys. Acta 1865 2303–2316. 10.1016/j.bbadis.2019.05.009 [DOI] [PubMed] [Google Scholar]
- Nduhirabandi F., du Toit E. F., Lochner A. (2012). Melatonin and the metabolic syndrome: a tool for effective therapy in obesity-associated abnormalities? Acta Physiol. 205 209–223. 10.1111/j.1748-1716.2012.02410.x [DOI] [PubMed] [Google Scholar]
- Niccoli G., Scalone G., Lerman A., Crea F. (2016). Coronary microvascular obstruction in acute myocardial infarction. Eur. Heart J. 37 1024–1033. 10.1093/eurheartj/ehv484 [DOI] [PubMed] [Google Scholar]
- Nofe J. R., Brodde M. F., Kehrel B. E. (2010). High-density lipoproteins, platelets and the pathogenesis of atherosclerosis. Clin. Exp. Pharmacol. Physiol. 37 726–735. 10.1111/j.1440-1681.2010.05377.x [DOI] [PubMed] [Google Scholar]
- Nokik S. E., Nobuyuki M., Kazuko T., Dongzhu X., Taizo K., Rujie Q., et al. (2017). Rev-erb agonist improves adverse cardiac remodeling and survival in myocardial infarction through an anti-inflammatory mechanism. PLoS One 12:e0189330. 10.1371/journal.pone.0189330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostjen C. A., Rosa C. G. S., Hartmann R. M., Schemitt E. G., Colares J. R., Marroni N. P. (2019). Anti-inflammatory and antioxidant effect of melatonin on recovery from muscular trauma induced in rats. Exp. Mol. Pathol. 106 52–59. 10.1016/j.yexmp.2018.12.001 [DOI] [PubMed] [Google Scholar]
- Pan S., Wever-Pinzon O., Rao S., Levin A., Garan A., Takeda K., et al. (2015). Myocardial recovery from short- and long-term cardiac support devices: results from the UNOS registry. J. Heart Lung Transplant. 34:S41. [DOI] [PubMed] [Google Scholar]
- Paradies G., Paradies V., Ruggiero F. M., Petrosillo G. (2015). Protective role of melatonin in mitochondrial dysfunction and related disorders. Arch. Toxicol. 89 923–939. 10.1007/s00204-015-1475-z [DOI] [PubMed] [Google Scholar]
- Pei H. F., Du J., Song X. F., He L., Zhang Y. F., Li X. C., et al. (2016). Melatonin prevents adverse myocardial infarction remodeling via Notch1/Mfn2 pathway. Free Radic. Biol. Med. 97 408–417. 10.1016/j.freeradbiomed.2016.06.015 [DOI] [PubMed] [Google Scholar]
- Perez-Gonzalez A., Castaneda-Arriaga R., Alvarez-Idaboy J. R., Reiter R. J., Galano A. (2019). Melatonin and its metabolites as chemical agents capable of directly repairing oxidized DNA. J. Pineal Res. 66:e12539. 10.1111/jpi.12539 [DOI] [PubMed] [Google Scholar]
- Phan T. K., Williams S. A., Bindra G. K., Lay F. T., Poon I. K. H., Hulett M. D. (2019). Phosphoinositides: multipurpose cellular lipids with emerging roles in cell death. Cell Death Differ. 26 781–793. 10.1038/s41418-018-0269-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy K. R. K., Dasari C., Duscharla D., Supriya B., Ram N. S., Surekha M. V., et al. (2018). Dimethylarginine dimethylaminohydrolase-1 (DDAH1) is frequently upregulated in prostate cancer, and its overexpression conveys tumor growth and angiogenesis by metabolizing asymmetric dimethylarginine (ADMA). Angiogenesis 21 79–94. 10.1007/s10456-017-9587-0 [DOI] [PubMed] [Google Scholar]
- Reina M., Martínez A. (2018). A new free radical scavenging cascade involving melatonin and three of its metabolites (3OHM, AFMK and AMK). Comput. Theor. Chem. 1123 111–118. 10.1016/j.comptc.2017.11.017 [DOI] [Google Scholar]
- Reiter R. J., Rosales-Corral S., Tan D. X., Jou M. J., Galano A., Xu B. (2017). Melatonin as a mitochondria-targeted antioxidant: one of evolution’s best ideas. Cell. Mol. Life Sci. 74 3863–3881. 10.1007/s00018-017-2609-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren B. (2016). Protein kinase D1 signaling in angiogenic gene expression and VEGF-mediated angiogenesis. Front. Cell Dev. Biol. 4:37. 10.3389/fcell.2016.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezzani R., Favero G., Stacchiotti A., Rodella L. F. (2013). Endothelial and vascular smooth muscle cell dysfunction mediated by cyclophylin A and the atheroprotective effects of melatonin. Life Sci. 92 875–882. 10.1016/j.lfs.2012.11.011 [DOI] [PubMed] [Google Scholar]
- Riehle C., Bauersachs J. (2018). Of mice and men: models and mechanisms of diabetic cardiomyopathy. Basic Res. Cardiol. 114:2. 10.1007/s00395-018-0711-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallinen P., Manttari S., Leskinen H., Ilves M., Vakkuri O., Ruskoaho H., et al. (2007). The effect of myocardial infarction on the synthesis, concentration and receptor expression of endogenous melatonin. J. Pineal Res. 42 254–260. 10.1111/j.1600-079X.2006.00413.x [DOI] [PubMed] [Google Scholar]
- Schreiber T., Salhofer L., Quinting T., Fandrey J. (2019). Things get broken: the hypoxia-inducible factor prolyl hydroxylases in ischemic heart disease. Basic Res. Cardiol. 114:16. 10.1007/s00395-019-0725-2 [DOI] [PubMed] [Google Scholar]
- Seidlmayer L. K., Juettner V. V., Kettlewell S., Pavlov E. V., Blatter L. A., Dedkova E. N. (2015). Distinct mPTP activation mechanisms in ischaemia-reperfusion: contributions of Ca2+, ROS, pH, and inorganic polyphosphate. Cardiovasc. Res. 106 237–248. 10.1093/cvr/cvv097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serocki M., Bartoszewska S., Janaszak-Jasiecka A., Ochocka R. J., Collawn J. F., Bartoszewski R. (2018). miRNAs regulate the HIF switch during hypoxia: a novel therapeutic target. Angiogenesis 21 183–202. 10.1007/s10456-018-9600-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiei E., Bahtoei M., Raj P., Ostovar A., Iranpour D., Akbarzadeh S., et al. (2018). Effects of N-acetyl cysteine and melatonin on early reperfusion injury in patients undergoing coronary artery bypass grafting: a randomized, open-labeled, placebo-controlled trial. Medicine 97:e11383. 10.1097/MD.0000000000011383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam G., Challa A. K., Litovsky S. H., Devarajan A., Wang D., Jones D. P., et al. (2019). Enhanced Keap1-Nrf2 signaling protects the myocardium from isoproterenol-induced pathological remodeling in mice. Redox Biol. 27:101212. 10.1016/j.redox.2019.101212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddall H. K., Yellon D. M., Ong S. B., Mukherjee U. A., Burke N., Hall A. R., et al. (2013). Loss of PINK1 increases the heart’s vulnerability to ischemia-reperfusion injury. PLoS One 8:e62400. 10.1371/journal.pone.0062400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simko F., Baka T., Paulis L., Reiter R. J. (2016). Elevated heart rate and nondipping heart rate as potential targets for melatonin: a review. J. Pineal Res. 61 127–137. 10.1111/jpi.12348 [DOI] [PubMed] [Google Scholar]
- Simula L., Campanella M., Campello S. (2019). Targeting Drp1 and mitochondrial fission for therapeutic immune modulation. Pharmacol. Res. 146:104317. 10.1016/j.phrs.2019.104317 [DOI] [PubMed] [Google Scholar]
- Singhanat K., Apaijai N., Chattipakorn S. C., Chattipakorn N. (2018). Roles of melatonin and its receptors in cardiac ischemia-reperfusion injury. Cell. Mol. Life Sci. 75 4125–4149. 10.1007/s00018-018-2905-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sircana A., De Michieli F., Parente R., Framarin L., Leone N., Berrutti M., et al. (2019). Gut microbiota, hypertension and chronic kidney disease: recent advances. Pharmacol. Res. 144 390–408. 10.1016/j.phrs.2018.01.013 [DOI] [PubMed] [Google Scholar]
- Song C., Zhao J., Fu B., Li D., Mao T., Peng W., et al. (2017). Melatonin-mediated upregulation of Sirt3 attenuates sodium fluoride-induced hepatotoxicity by activating the MT1-PI3K/AKT-PGC-1alpha signaling pathway. Free Radic. Biol. Med. 112 616–630. 10.1016/j.freeradbiomed.2017.09.005 [DOI] [PubMed] [Google Scholar]
- Soto-Heras S., Catala M. G., Roura M., Menendez-Blanco I., Piras A. R., Izquierdo D., et al. (2019). Effects of melatonin on oocyte developmental competence and the role of melatonin receptor 1 in juvenile goats. Reprod. Domest. Anim. 54 381–390. 10.1111/rda.13378 [DOI] [PubMed] [Google Scholar]
- Tahrir F. G., Langford D., Amini S., Mohseni Ahooyi T., Khalili K. (2019). Mitochondrial quality control in cardiac cells: mechanisms and role in cardiac cell injury and disease. J. Cell. Physiol. 234 8122–8133. 10.1002/jcp.27597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang V., Fu S., Rayner B. S., Hawkins C. L. (2019). 8-Chloroadenosine induces apoptosis in human coronary artery endothelial cells through the activation of the unfolded protein response. Redox Biol. 26:101274. 10.1016/j.redox.2019.101274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira J., Basit F., Swarts H. G., Forkink M., Oliveira P. J., Willems P., et al. (2018). Extracellular acidification induces ROS- and mPTP-mediated death in HEK293 cells. Redox Biol. 15 394–404. 10.1016/j.redox.2017.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tengattini S., Reiter R. J., Tan D. X., Terron M. P., Rodella L. F., Rezzani R. (2008). Cardiovascular diseases: protective effects of melatonin. J. Pineal Res. 44 16–25. 10.1111/j.1600-079X.2007.00518.x [DOI] [PubMed] [Google Scholar]
- Ter Horst E. N., Krijnen P. A. J., Hakimzadeh N., Robbers L., Hirsch A., Nijveldt R., et al. (2018). Elevated monocyte-specific type I interferon signalling correlates positively with cardiac healing in myocardial infarct patients but interferon alpha application deteriorates myocardial healing in rats. Basic Res. Cardiol. 114:1. 10.1007/s00395-018-0709-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thieltges K. M., Avramovic D., Piscitelli C. L., Markovic-Mueller S., Binz H. K., Ballmer-Hofer K. (2018). Characterization of a drug-targetable allosteric site regulating vascular endothelial growth factor signaling. Angiogenesis 21 533–543. 10.1007/s10456-018-9606-9 [DOI] [PubMed] [Google Scholar]
- Tian L., Lu L., Feng J., Melancon M. P. (2018). Radiopaque nano and polymeric materials for atherosclerosis imaging, embolization and other catheterization procedures. Acta Pharm. Sin. B 8 360–370. 10.1016/j.apsb.2018.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trindade F., Vitorino R., Leite-Moreira A., Falcao-Pires I. (2019). Pericardial fluid: an underrated molecular library of heart conditions and a potential vehicle for cardiac therapy. Basic Res. Cardiol. 114:10. 10.1007/s00395-019-0716-3 [DOI] [PubMed] [Google Scholar]
- Venegas C., Garcia J. A., Escames G., Ortiz F., Lopez A., Doerrier C., et al. (2012). Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. J. Pineal Res. 52 217–227. 10.1111/j.1600-079X.2011.00931.x [DOI] [PubMed] [Google Scholar]
- Venugopal S., Kao C., Chandna R., Sulochana K. N., Subramanian V., Chen M., et al. (2018). Angio-3, a 10-residue peptide derived from human plasminogen kringle 3, suppresses tumor growth in mice via impeding both angiogenesis and vascular permeability. Angiogenesis 21 653–665. 10.1007/s10456-018-9616-7 [DOI] [PubMed] [Google Scholar]
- Villalobos C., Gutierrez L. G., Hernandez-Morales M., Del Bosque D., Nunez L. (2018). Mitochondrial control of store-operated Ca(2+) channels in cancer: pharmacological implications. Pharmacol. Res. 135 136–143. 10.1016/j.phrs.2018.08.001 [DOI] [PubMed] [Google Scholar]
- Wang B., Nie J. L., Wu L. J., Hu Y. Y., Wen Z., Dong L. L., et al. (2018). AMPKα2 protects against the development of heart failure by enhancing mitophagy via PINK1 phosphorylation. Circ. Res. 122 712–729. 10.1161/circresaha.117.312317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zhu P., Li R., Ren J., Zhou H. (2020). Fundc1-dependent mitophagy is obligatory to ischemic preconditioning-conferred renoprotection in ischemic AKI via suppression of Drp1-mediated mitochondrial fission. Redox Biol. 30:101415. 10.1016/j.redox.2019.101415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Dong G., Sheng C. (2019). Structural simplification: an efficient strategy in lead optimization. Acta Pharm. Sin. B 9 880–901. 10.1016/j.apsb.2019.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. N., Shu H., Wang M. W., Sun Y. Y., Liu C. F., Liu W. L., et al. (2016). Melatonin alleviates lipopolysaccharide-compromised integrity of blood brain barrier by activation of AMP-activated protein kinase in old mice. Chin. J. Pharmacol. Toxicol. 30 1021–1022. 10.1111/acel.12572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Lei T., Yuan J., Wu Y., Shen X., Gao J., et al. (2018). GCN2 deficiency ameliorates doxorubicin-induced cardiotoxicity by decreasing cardiomyocyte apoptosis and myocardial oxidative stress. Redox Biol. 17 25–34. 10.1016/j.redox.2018.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Xu G., Gao Y., Zhan X., Qin N., Fu S., et al. (2019). Cardamonin from a medicinal herb protects against LPS-induced septic shock by suppressing NLRP3 inflammasome. Acta Pharm. Sin. B 9 734–744. 10.1016/j.apsb.2019.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westman P. C., Lipinski M. J., Luger D., Waksman R., Bonow R. O., Wu E., et al. (2016). Inflammation as a driver of adverse left ventricular remodeling after acute myocardial infarction. J. Am. Coll. Cardiol. 67 2050–2060. 10.1016/j.jacc.2016.01.073 [DOI] [PubMed] [Google Scholar]
- Wu C., Xi C., Tong J., Zhao J., Jiang H., Wang J., et al. (2019). Design, synthesis, and biological evaluation of novel tetrahydroprotoberberine derivatives (THPBs) as proprotein convertase subtilisin/kexin type 9 (PCSK9) modulators for the treatment of hyperlipidemia. Acta Pharm. Sin. B 9 1216–1230. 10.1016/j.apsb.2019.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Hu Q., Tan B., Rose P., Zhu D., Zhu Y. Z. (2018a). Amelioration of mitochondrial dysfunction in heart failure through S-sulfhydration of Ca(2+)/calmodulin-dependent protein kinase II. Redox Biol. 19 250–262. 10.1016/j.redox.2018.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Wang H., Teng T., Duan S., Ji A., Li Y. (2018b). Hydrogen sulfide and autophagy: a double edged sword. Pharmacol. Res. 131 120–127. 10.1016/j.phrs.2018.03.002 [DOI] [PubMed] [Google Scholar]
- Xiao Y., Karam C., Yi J., Zhang L., Li X., Yoon D., et al. (2018). ROS-related mitochondrial dysfunction in skeletal muscle of an ALS mouse model during the disease progression. Pharmacol. Res. 138 25–36. 10.1016/j.phrs.2018.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Q., Wang Z., Yu Y., Wen Y., Suguro R., Mao Y., et al. (2019). Hydrogen sulfide stabilizes atherosclerotic plaques in apolipoprotein E knockout mice. Pharmacol. Res. 144 90–98. 10.1016/j.phrs.2019.04.006 [DOI] [PubMed] [Google Scholar]
- Xu N., Wang Q., Jiang S., Wang Q., Hu W., Zhou S., et al. (2019). Fenofibrate improves vascular endothelial function and contractility in diabetic mice. Redox Biol. 20 87–97. 10.1016/j.redox.2018.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S. F., Zhang Y. H., Wang S., Pang Z. Q., Fan Y. G., Li J. Y., et al. (2019). Lactoferrin ameliorates dopaminergic neurodegeneration and motor deficits in MPTP-treated mice. Redox Biol. 21:101090. 10.1016/j.redox.2018.101090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T., Ding W., Ao X., Chu X., Wan Q., Wang Y., et al. (2019). ARC regulates programmed necrosis and myocardial ischemia/reperfusion injury through the inhibition of mPTP opening. Redox Biol. 20 414–426. 10.1016/j.redox.2018.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Fu T., Guo Q., Sun W., Gan Z. (2019). Mitochondrial quality orchestrates muscle-adipose dialog to alleviate dietary obesity. Pharmacol. Res. 141 176–180. 10.1016/j.phrs.2018.12.020 [DOI] [PubMed] [Google Scholar]
- Yan G. G., Yu L. Z., Jiang S. M., Zhu J. F. (2018). Melatonin antagonizes oxidative stress-induced mitochondrial dysfunction in retinal pigmented epithelium cells via melatonin receptor 1 (MT1). J. Toxicol. Sci. 43 659–669. 10.2131/jts.43.659 [DOI] [PubMed] [Google Scholar]
- Yang C. H., Xu J. H., Ren Q. C., Duan T., Mo F., Zhang W. (2019). Melatonin promotes secondary hair follicle development of early postnatal cashmere goat and improves cashmere quantity and quality by enhancing antioxidant capacity and suppressing apoptosis. J. Pineal Res. 67:e12569 10.1111/jpi.12569 [DOI] [PubMed] [Google Scholar]
- Yang M., Linn B. S., Zhang Y., Ren J. (2019). Mitophagy and mitochondrial integrity in cardiac ischemia-reperfusion injury. Biochim. Biophys. Acta 1865 2293–2302. 10.1016/j.bbadis.2019.05.007 [DOI] [PubMed] [Google Scholar]
- Yang M. Y., Yu Q. L., Huang Y. S., Yang G. (2019). Neuroprotective effects of andrographolide derivative CX-10 in transient focal ischemia in rat: involvement of Nrf2/AE and TLR/NF-kappaB signaling. Pharmacol. Res. 144 227–234. 10.1016/j.phrs.2019.04.023 [DOI] [PubMed] [Google Scholar]
- Yang Q., He G. W., Underwood M. J., Yu C. M. (2016). Cellular and molecular mechanisms of endothelial ischemia/reperfusion injury: perspectives and implications for postischemic myocardial protection. Am. J. Transl. Res. 8 765–777. [PMC free article] [PubMed] [Google Scholar]
- Yang X., Wang H., Ni H.-M., Xiong A., Wang Z., Sesaki H., et al. (2017). Inhibition of Drp1 protects against senecionine-induced mitochondria-mediated apoptosis in primary hepatocytes and in mice. Redox Biol. 12, 264–273. 10.1016/j.redox.2017.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Duan W., Jin Z., Yi W., Yan J., Zhang S., et al. (2013). JAK2/STAT3 activation by melatonin attenuates the mitochondrial oxidative damage induced by myocardial ischemia/reperfusion injury. J. Pineal Res. 55 275–286. 10.1111/jpi.12070 [DOI] [PubMed] [Google Scholar]
- Yang Y., Sun Y., Yi W., Li Y., Fan C. X., Xin Z. L., et al. (2014). A review of melatonin as a suitable antioxidant against myocardial ischemia–reperfusion injury and clinical heart diseases. J. Pineal Res. 57 357–366. 10.1111/jpi.12175 [DOI] [PubMed] [Google Scholar]
- Yao X., Zhang J., Jing X., Ye Y., Guo J., Sun K., et al. (2019). Fibroblast growth factor 18 exerts anti-osteoarthritic effects through PI3K-AKT signaling and mitochondrial fusion and fission. Pharmacol. Res. 139 314–324. 10.1016/j.phrs.2018.09.026 [DOI] [PubMed] [Google Scholar]
- Yarana C., Thompson H., Chaiswing L., Butterfield D. A., Weiss H., Bondada S., et al. (2019). Extracellular vesicle-mediated macrophage activation: an insight into the mechanism of thioredoxin-mediated immune activation. Redox Biol. 26:101237. 10.1016/j.redox.2019.101237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon Y. M., Kim H. J., Lee J. H., Lee S. H. (2019). Melatonin enhances mitophagy by upregulating expression of heat shock 70 kDa protein 1L in human mesenchymal stem cells under oxidative stress. Int. J. Mol. Sci. 20:4545 10.3390/ijms20184545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L. M., Di W. C., Dong X., Li Z., Zhang Y., Xue X. D., et al. (2018). Melatonin protects diabetic heart against ischemia-reperfusion injury, role of membrane receptor-dependent cGMP-PKG activation. Biochim. Biophys. Acta 1864 563–578. 10.1016/j.bbadis.2017.11.023 [DOI] [PubMed] [Google Scholar]
- Yu L. M., Li B. Y., Zhang M., Jin Z. X., Duan W. X., Zhao G. L., et al. (2016). Melatonin reduces PERK-eIF2α-ATF4-mediated endoplasmic reticulum stress during myocardial ischemia-reperfusion injury: role of RISK and SAFE pathways interaction. Apoptosis 21 809–824. 10.1007/s10495-016-1246-1 [DOI] [PubMed] [Google Scholar]
- Yuan T., Yang T., Chen H., Fu D., Hu Y., Wang J., et al. (2019). New insights into oxidative stress and inflammation during diabetes mellitus-accelerated atherosclerosis. Redox Biol. 20 247–260. 10.1016/j.redox.2018.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuqi L., Lei G., Yanqiu S., Qiao X., Yu W., Yundai C. (2015). Efficacy and safety of limus-eluting versus paclitaxel-eluting coronary artery stents in patients with diabetes mellitus: a meta-analysis. Int. J. Cardiol. 64 680–691. 10.1016/j.ijcard.2015.02.002 [DOI] [PubMed] [Google Scholar]
- Zaouali M. A., Reiter R. J., Padrissa-Altes S., Boncompagni E., Garcia J. J., Ben Abnennebi H., et al. (2011). Melatonin protects steatotic and nonsteatotic liver grafts against cold ischemia and reperfusion injury. J. Pineal Res. 50 213–221. 10.1111/j.1600-079X.2010.00831.x [DOI] [PubMed] [Google Scholar]
- Zhang H., Jin B., Faber J. E. (2019). Mouse models of Alzheimer’s disease cause rarefaction of pial collaterals and increased severity of ischemic stroke. Angiogenesis 22 263–279. 10.1007/s10456-018-9655-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. M., Zhang Y. (2014). Melatonin: a well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 57 131–146. 10.1111/jpi.12162 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Mi S. L., Hu N., Doser T. A., Sun A., Ge J., et al. (2014). Mitochondrial aldehyde dehydrogenase 2 accentuates aging-induced cardiac remodeling and contractile dysfunction: role of AMPK, Sirt1, and mitochondrial function. Free Radic. Biol. Med. 71 208–220. 10.1016/j.freeradbiomed.2014.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Wang Y., Xu J. N., Tian F., Hu S. Y., Chen Y. D., et al. (2019). Melatonin attenuates myocardial ischemia-reperfusion injury via improving mitochondrial fusion/mitophagy and activating the AMPK-OPA1 signaling pathways. J. Pineal Res. 66:e12542. 10.1111/jpi.12542 [DOI] [PubMed] [Google Scholar]
- Zhao J., Gao J. L., Zhu J. X., Zhu H. B., Peng X., Jiang M., et al. (2019). The different response of cardiomyocytes and cardiac fibroblasts to mitochondria inhibition and the underlying role of STAT3. Basic Res. Cardiol. 114:12. 10.1007/s00395-019-0721-6 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Wang H. Y., Chen W., Chen L. F., Liu D. M., Wang X., et al. (2019). Melatonin attenuates white matter damage after focal brain ischemia in rats by regulating the TLR4/NF-kappaB pathway. Brain Res. Bull. 150 168–178. 10.1016/j.brainresbull.2019.05.019 [DOI] [PubMed] [Google Scholar]
- Zhong J., Tan Y., Lu J., Liu J., Xiao X., Zhu P., et al. (2019). Therapeutic contribution of melatonin to the treatment of septic cardiomyopathy: a novel mechanism linking Ripk3-modified mitochondrial performance and endoplasmic reticulum function. Redox Biol. 26:101287. 10.1016/j.redox.2019.101287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Hu S., Jin Q., Shi C., Zhang Y., Zhu P., et al. (2017a). Mff-dependent mitochondrial fission contributes to the pathogenesis of cardiac microvasculature ischemia/reperfusion injury via induction of mROS-mediated cardiolipin oxidation and HK2/VDAC1 disassociation-involved mPTP opening. J. Am. Heart Assoc. 6:e005328 10.1161/JAHA.116.005328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Li D., Zhu P., Hu S., Hu N., Ma S., et al. (2017b). Melatonin suppresses platelet activation and function against cardiac ischemia/reperfusion injury via PPARgamma/FUNDC1/mitophagy pathways. J. Pineal Res. 63:e12438. 10.1111/jpi.12438 [DOI] [PubMed] [Google Scholar]
- Zhou H., Zhang Y., Hu S., Shi C., Zhu P., Ma Q., et al. (2017c). Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission-VDAC1-HK2-mPTP-mitophagy axis. J. Pineal Res. 63:e12413 10.1111/jpi.12413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Zhang Y., Hu S. Y., Shi C., Zhu P. J., Ma Q., et al. (2017d). Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission-VDAC1-HK2-mPTP-mitophagy axis. J. Pineal Res. 63;e12413. 10.1111/jpi.12413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Zhu P., Guo J., Hu N., Wang S., Li D., et al. (2017e). Ripk3 induces mitochondrial apoptosis via inhibition of FUNDC1 mitophagy in cardiac IR injury. Redox Biol. 13 498–507. 10.1016/j.redox.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Li D., Zhu P., Ma Q., Toan S., Wang J., et al. (2018a). Inhibitory effect of melatonin on necroptosis via repressing the Ripk3-PGAM5-CypD-mPTP pathway attenuates cardiac microvascular ischemia-reperfusion injury. J. Pineal Res. 65:e12503. 10.1111/jpi.12503 [DOI] [PubMed] [Google Scholar]
- Zhou H., Li N., Yuan Y., Jin Y. G., Guo H., Deng W., et al. (2018b). Activating transcription factor 3 in cardiovascular diseases: a potential therapeutic target. Basic Res. Cardiol. 113:37. [DOI] [PubMed] [Google Scholar]
- Zhou H., Ma Q., Zhu P., Ren J., Reiter R. J., Chen Y. (2018c). Protective role of melatonin in cardiac ischemia-reperfusion injury: from pathogenesis to targeted therapy. J. Pineal Res. 64:e12471 10.1111/jpi.12471 [DOI] [PubMed] [Google Scholar]
- Zhou H., Shi C., Hu S., Zhu H., Ren J., Chen Y. (2018d). BI1 is associated with microvascular protection in cardiac ischemia reperfusion injury via repressing Syk-Nox2-Drp1-mitochondrial fission pathways. Angiogenesis 21 599–615. 10.1007/s10456-018-9611-z [DOI] [PubMed] [Google Scholar]
- Zhou H., Wang J., Zhu P., Zhu H., Toan S., Hu S., et al. (2018e). NR4A1 aggravates the cardiac microvascular ischemia reperfusion injury through suppressing FUNDC1-mediated mitophagy and promoting Mff-required mitochondrial fission by CK2alpha. Basic Res. Cardiol. 113:23. 10.1007/s00395-018-0682-1 [DOI] [PubMed] [Google Scholar]
- Zhou H., Wang S., Hu S., Chen Y., Ren J. (2018f). ER-mitochondria microdomains in cardiac ischemia-reperfusion injury: a fresh perspective. Front. Physiol. 9:755. 10.3389/fphys.2018.00755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Wang S., Zhu P., Hu S., Chen Y., Ren J. (2018g). Empagliflozin rescues diabetic myocardial microvascular injury via AMPK-mediated inhibition of mitochondrial fission. Redox Biol. 15 335–346. 10.1016/j.redox.2017.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Zhu P., Wang J., Zhu H., Ren J., Chen Y. (2018h). Pathogenesis of cardiac ischemia reperfusion injury is associated with CK2alpha-disturbed mitochondrial homeostasis via suppression of FUNDC1-related mitophagy. Cell Death Differ. 25 1080–1093. 10.1038/s41418-018-0086-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Toan S. (2020). Pathological roles of mitochondrial oxidative stress and mitochondrial dynamics in cardiac microvascular ischemia/reperfusion injury. Biomolecules 10:85 10.3390/biom10010085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Toan S., Zhu P., Wang J., Ren J., Zhang Y. (2020). DNA-PKcs promotes cardiac ischemia reperfusion injury through mitigating BI-1-governed mitochondrial homeostasis. Basic Res. Cardiol. 115:11. 10.1007/s00395-019-0773-7 [DOI] [PubMed] [Google Scholar]
- Zhou H., Wang J., Hu S., Zhu H., Toanc S., Ren J. (2019). BI1 alleviates cardiac microvascular ischemia-reperfusion injury via modifying mitochondrial fission and inhibiting XO/ROS/F-actin pathways. J. Cell Physiol. 234 5056–5069. 10.1002/jcp.27308 [DOI] [PubMed] [Google Scholar]
- Zhu H., Jin Q., Li Y., Ma Q., Wang J., Li D., et al. (2018). Melatonin protected cardiac microvascular endothelial cells against oxidative stress injury via suppression of IP3R-[Ca(2+)]c/VDAC-[Ca(2+)]m axis by activation of MAPK/ERK signaling pathway. Cell Stress Chaperones 23 101–113. 10.1007/s12192-017-0827-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P., Hu S., Jin Q., Li D., Tian F., Toan S., et al. (2018). Ripk3 promotes ER stress-induced necroptosis in cardiac IR injury: a mechanism involving calcium overload/XO/ROS/mPTP pathway. Redox Biol. 16 157–168. 10.1016/j.redox.2018.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollbrecht C., Persson A. E. G., Lundberg J. O., Weitzberg E., Carlström M. (2016). Nitrite-mediated reduction of macrophage NADPH oxidase activity is dependent on xanthine oxidoreductase-derived nitric oxide but independent of S-nitrosation. Redox Biol. 10 119–127. 10.1016/j.redox.2016.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]