Abstract
INTRODUCTION:
The National Blood Collection and Utilization Survey (NBCUS) has demonstrated declines in blood collection and transfusion in the United States since 2008, including declines of 11.6% in red blood cell (RBC) collections and 13.9% in RBC transfusions during 2013–2015. This study described the 2017 NBCUS results.
METHODS:
The 2017 NBCUS was distributed to all US blood collection centers, all hospitals performing at least 1000 surgeries annually, and a 40% random sample of hospitals performing 100 to 999 surgeries annually. Weighting and imputation were used to generate national estimates for units of blood and components collected, deferred, distributed, transfused, and outdated.
RESULTS:
Response rates for the 2017 NBCUS were 88% for blood collection centers and 86% for transfusing hospitals. Compared with 2015, the number of RBC units collected during 2017 (12,211,000; 95% confidence interval [CI], 11,680,000–12,742,000) declined by 3.0%, and transfused RBC units (10,654,000, 95% CI, 10,314,000–10,995,000) declined by 6.1%. Distributed platelet (PLT) units (2,560,000; 95% CI, 2,391,000–2,730,000 units) increased by 5.1%, and transfused PLT units (1,937,000, 95% CI, 1,794,000–2,079,000) declined by 2.3%. Distributed plasma units (3,209,000; 95% CI, 2,879,000–3,539,000) declined by 13.6%, and transfused plasma units (2,374,000; 95% CI, 2,262,000–2,487,000) declined by 12.9%.
CONCLUSION:
The 2017 NBCUS suggests a continued but slowing decline in demand for RBCs. The decline in blood collection and use will likely continue. Despite decreasing demand and increasing manufacturing costs of blood products, the US blood industry has met the regular and emergent needs of the country.
Blood transfusion is one of the most common hospital procedures in the United States.1 Sufficient availability of safe blood products in the United States relies on the collaboration of numerous entities, including community- and hospital-based blood centers, transfusion facilities, public health and regulatory agencies, and voluntary donors. To collect adequate data about this diverse blood system, national surveys of blood collection centers and hospitals have been conducted since 1971.2–4
Since 2008, the National Blood Collection and Utilization Survey (NBCUS) has documented a decline in red blood cell (RBC) collections and transfusions, including a decline of 11.6% in collections and a 13.9% decline in transfusions between 2013 and 2015.2,3,5 These declines have been attributed to implementation of patient blood management programs, adoption of guidelines encouraging restrictive transfusion thresholds, and advances in technology and surgical techniques.3,6–10 Along with this decline, the prices hospitals pay per unit of blood decreased between 2013 and 2015, and additional safety measures such as infectious disease testing have increased production costs.3,9
To understand the current trends in blood collection and use, we analyzed data from the 2017 NBCUS. Specifically, our objectives were to quantify blood and blood component collection, distribution, and transfusion in the United States in 2017; describe component processing, costs, and donor characteristics; and compare 2017 results with previous years.
METHODS
The 2017 survey methodology and questionnaire design were similar to previous surveys.3 The 2017 NBCUS consisted of 48 questions, including 20 questions applicable to blood collection centers and 28 questions applicable to transfusing hospitals.
The Food and Drug Administration’s Blood Establishment Registration database was used to identify blood collection centers. Sixty-five community-based (eg, non–hospital-based) and 108 hospital-based blood collection centers were identified and surveyed (Fig. 1). The 2015 American Hospital Association annual survey database was used to identify transfusing hospitals. Hospitals were excluded if they performed fewer than 100 inpatient surgeries annually; were located in US territories; were owned by the military or Department of Justice; or were classified as rehabilitation, acute long-term care, or psychiatric facilities. Among the remaining 3783 hospitals on the sampling frame, 40% of hospitals that performed 100 to 999 inpatient surgeries per year were selected at random for participation (n = 633/1569); 100% of hospitals performing 1000 or more inpatient surgeries a year (n = 2214) were sent a survey.
Fig. 1.
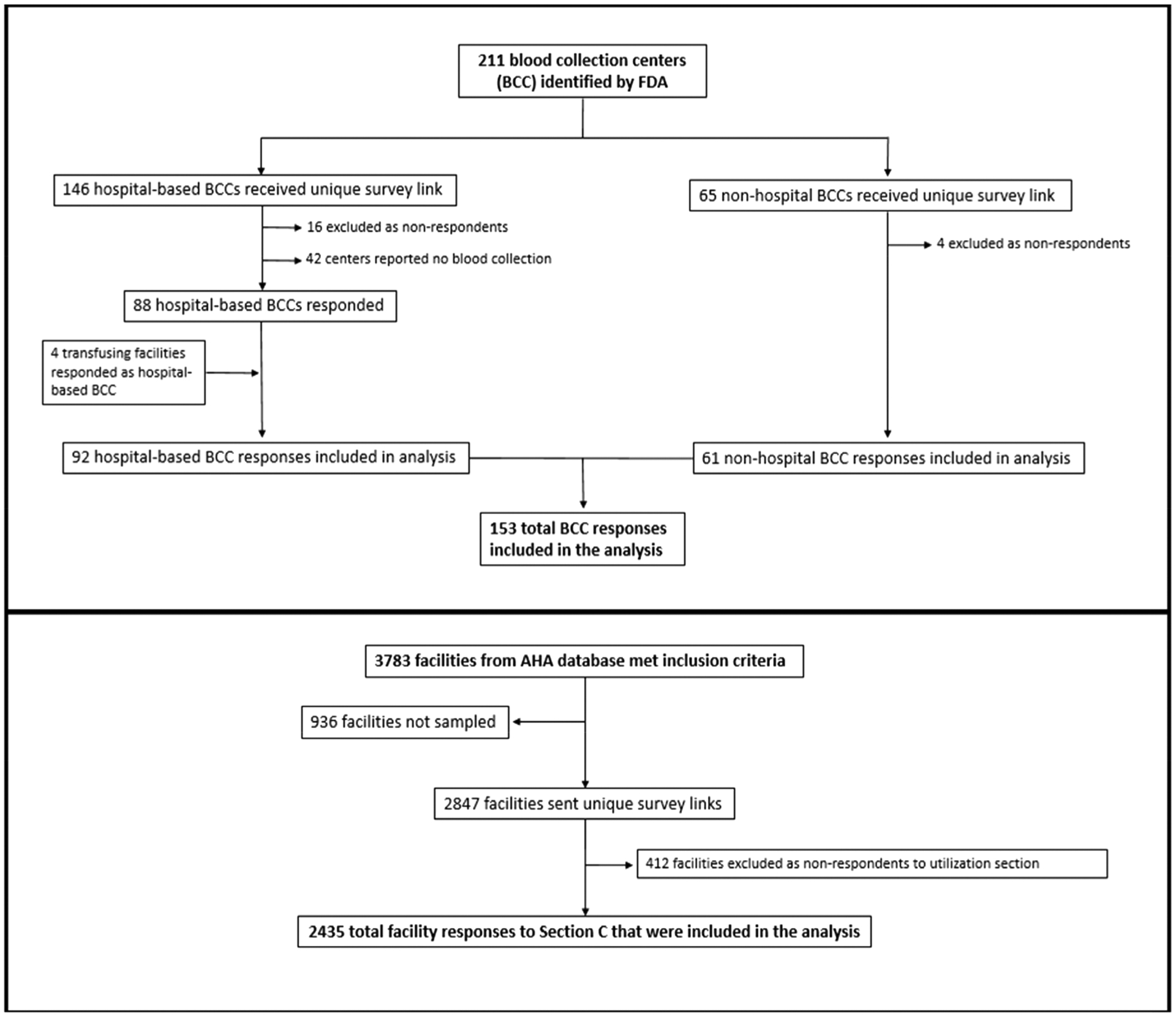
Flow diagram depicting identification, stratification, sampling, exclusion, and recategorization of 2017 National Blood Collection and Use Survey respondents. AHA = American Hospital Association.
The survey was administered using a similar Web-based electronic format as prior years. To reduce the number of nonresponders, facilities were contacted by mail, email, and/or phone to encourage participation.
National estimates for the number of units of blood and blood components collected, distributed, transfused, and outdated were calculated and rounded to the nearest 1000 units. For weighting and imputation purposes, blood collection centers were stratified based on anticipated levels of collection or transfusion in 2017. Community-based blood centers were stratified into 4 categories based on the number of whole blood or RBC units collected in 2015: fewer than 50,000, 50,000 to 199,999, 200,000 to 399,000, and 400,000 or more units. Hospital-based blood centers were stratified into three categories based on the number of inpatient surgical operations performed in 2015: fewer than 1000, 1000 to 7999, and 8000 or more inpatient surgeries. Transfusing hospitals were stratified into six categories based on the number of inpatient surgerical operations performed in 2015: 100 to 999, 1000 to 1399, 1400 to 2399, 2400 to 4999, 5000 to 7999, and 8000 or more surgies.
Responses were weighted to adjust for nonresponse within strata by dividing the total number of eligible participants by the number of actual respondents for each stratum, according to the stratification scheme described above. Blood collection centers with an expected collection volume of more than 400,000 units were assigned a weight of 1.0. Confidence intervals (CIs) for national collection and transfusion estimates were calculated using the Taylor Series method.11
A multiple imputation method was used for missing data. All imputed variables were continuous and non-normally distributed. A two-stage imputation procedure was performed for variables with distributions skewed toward zero.12 According to established multiple imputation logic, imputation factors were considered for each variable to ensure that the variables used for imputation had distributions that were similar to those of the variables requiring imputation.13 Variables that were weighted and imputed included whole blood and apheresis RBCs collected, distributed, rejected, outdated, and transfused; and apheresis platelets (PLTs), plasma, and cryoprecipitate units collected and transfused. Whole blood–derived PLTs were expressed in apheresis equivalents by dividing the number of whole blood–derived PLT units by five.
To project future national blood use, two different approaches were used. The first approach used a previously described method,14 where future RBC use was predicted using trends in transfusions by location in a health care facility because previous NBCUS data suggested the decline in RBC use was driven primarily by decreased RBC use in surgical settings.4 Because the decline in RBC use between 2015 and 2017 appeared to be attributable to decreasing RBC transfusions among smaller hospitals, a second approach was also used, where 2017–2022 RBC use was predicted using 2015–2017 trends in transfusion stratified by annual inpatient surgical volume.
Both mean and median cost per unit for blood and blood components paid by transfusing hospitals were calculated with use of nonweighted data. Medians were preferred over means for comparing differences in unit costs for both 2013 and 2015 due to the presence of outliers, particularly from more remote locations, where blood components are more expensive than in the rest of the United States. To calculate the national rates of whole blood and RBC collection per 1000 population, the total estimated number of units collected before the removal of rejected units was divided by the 2017 US population aged 16 to 64 years. This denominator was used for consistency in comparison with previous NBCUS reports and to reflect the general age range of blood donors (Fig. 2C). To calculate the national rate of whole blood and RBC transfusion per 1000 population, the total estimated number of units transfused was divided by the entire 2017 US population. All population estimates were derived with use of US Census Bureau state-specific and age-specific estimates for 2017.15 To determine whether differences in the collection and usage estimates between the 2015 and 2017 surveys were caused by sampling and response rates, a subset of transfusing hospitals was created including only respondents who had completed both the 2015 and 2017 surveys. This matched subset of NBCUS respondents allowed sensitivity analyses to assess whether differences observed between the two survey years were consistent or disparate when holding the respondents constant. All analyses were conducted with statistical software (SAS version 9.4; SAS Institute).
Fig. 2.
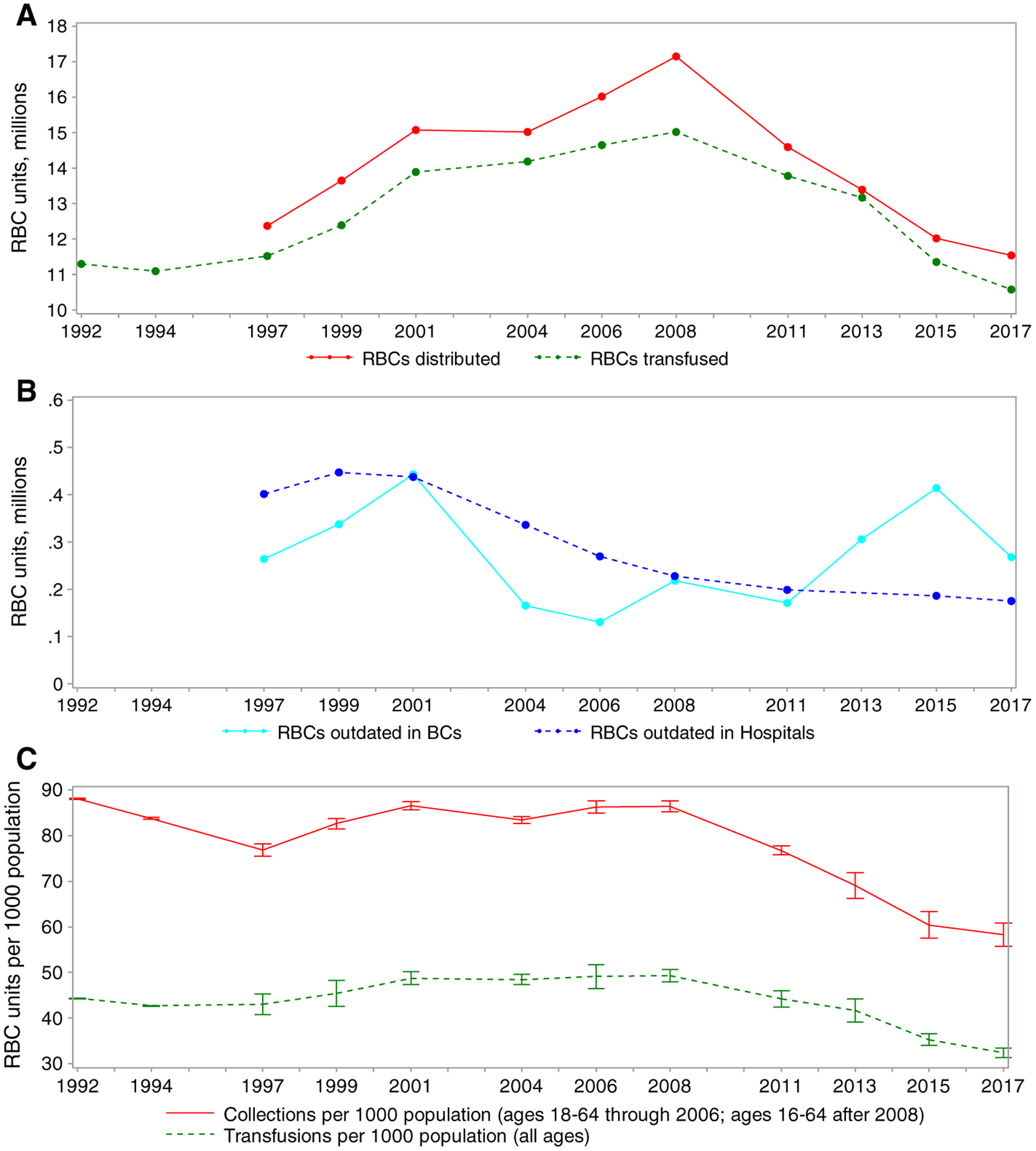
Trends in (A) RBC distributions and transfusions, (B) RBC units outdated in blood centers and hospitals, and (C) RBC collections and transfusion per 1000 population.
RESULTS
Survey participation
The response rates for the 2017 NBCUS were 94% (61/65) for community-based blood centers, 85% (92/108) for hospital-based blood centers, and 86% (2435/2847) transfusing hospitals, which were higher than the response rates for the 2015 survey. The overall number of facilities eligible for inclusion in NBCUS decreased between 2015 and 2017. The total number of hospitals fell slightly from 2892 to 2847, mostly due to smaller facilities dropping below the threshold required for inclusion (≥100 annual inpatient surgical procedures). In comparison to 2015, 15 fewer community-based blood centers and 34 fewer hospital based blood centers were included in the survey. Among blood collection centers collecting <50,000 RBC units, 10 fewer centers were eligible, and among centers collecting 50,000 to 199,000 units, five fewer were eligible. At least seven community-based blood collection centers had been acquired or merged with another center, and three had closed. The reduction in hospital-based blood centers from 2015 to 2017 was almost entirely among medium-volume facilities with hospital-based blood centers performing 1,000 to 7,999 inpatient surgical operations, decreasing from 83 to 45 facilities. Among hospital-based blood centers performing fewer than 1,000 and 8000 or more annual inpatient surgical operations, the number of facilities increased from 12 to 19 and decreased from 47 to 44, respectively, between 2015 and 2017. Fourteen hospital-based blood centers had stopped collecting blood, while 16 centers were registered as blood collection facilities in the FDA Blood Establishment Registry but did not collect blood products.
Whole blood and RBC collections and transfusions
In 2017, 12,211,000 whole blood and apheresis RBC units (95% CI, 11,680,000–12,742,000 units) were collected in the United States (Table 1), reflecting a 3.0% decline in RBC collections since 2015, when 12,591,000 RBC units were collected. Approximately 95.8% of all RBC units collected in 2017 were collected at non–hospital-based collection centers. Of all whole blood units collected, 10,399,000 (95% CI, 9,895,000–10,903,000), or 99.8%, were units collected for allogeneic, nondirected transfusions. An estimated 1,787,000 (95% CI, 1,625,000–1,949,000) apheresis RBC units were collected during 2017, approximately the same as in 2015 (1,797,000 units). The total whole blood and RBC supply available in 2017 after excluding units that were rejected was 11,545,000 units (95% CI, 11,034,000–12,057,000 units), a 4.0% decrease compared with 2015 (12,028,000). Of whole blood and apheresis RBC units rejected after collection in 2017, 11.7% (78,000 units; 95% CI, 70,000–87,000 units) were rejected upon testing for transfusion-transmissible infections, whereas 88.3% (587,000 units; 95% CI, 539,000–635,000 units) were rejected for other reasons, such as insufficient volume or a broken bag.
TABLE 1.
Estimated numbers of whole blood and RBC units collected, transfused, and outdated in 2017 (expressed in thousands)
| Blood centers | Hospitals | Combined totals | 95% CI | 2015 Totals* | % Change 2017–2015 | |
|---|---|---|---|---|---|---|
| Collections | ||||||
| Whole blood units | ||||||
| Allogeneic, nondirected | 9,912 | 487 | 10,399 | (9,895–10,903) | 10,748 | −3.2% |
| Autologous | 7 | 2 | 10 | (7–12) | 25 | −61.8% |
| Directed | 6 | 10 | 16 | (7–24) | 21 | −25.8% |
| Apheresis RBC units† | 1,775 | 12 | 1,787 | (1,625–1,949) | 1,797 | −0.6% |
| Total supply | 11,701 | 510 | 12,211 | (11,680–12,742) | 12,591 | −3.0% |
| Rejected on testing | 74 | 5 | 78 | (70–87) | 53 | 47.6% |
| Rejected for other reasons‡ | 567 | 20 | 587 | (539–635) | 510 | 15.1% |
| Total available supply | 11,059 | 486 | 11,545 | (11,034–12,057) | 12,028 | −4.0% |
| Transfusions | ||||||
| Allogeneic, nondirected | 10,572 | (10,235–10,910) | 11,264 | −6.1% | ||
| Autologous | 27 | (11–42) | 20 | 32.6% | ||
| Directed | 56 | (32–79) | 66 | −15.9% | ||
| Total transfusions | 10,654 | (10,314–10,995) | 11,349 | −6.1% | ||
| Outdated whole blood or RBCs | 269 | 177 | 446 | (419–474) | 600 | −25.6% |
2015 totals were obtained from Ellingson et al.3
Apheresis RBC units include allogeneic, autologous, directed, and concurrent collections.
Units rejected for other reasons does not include outdated units.
An estimated 10,654,000 whole blood–derived and apheresis RBC units (95% CI, 10,314,000–10,995,000 units) were transfused in 2017, a 6.1% decline since 2015 (11,349,000 units) (Table 1). Among these transfused units, 10,572,000 units (95% CI, 10,235,000–10,910,000 units), or 99.2%, were allogeneic (nondirected) transfusions, similar to the proportion reported for 2015 (99.3%). Approximately 446,000 RBC units (95% CI, 419,000–474,000 units) were outdated in 2017, which is a 25.6% decrease from 2015 (600,000 units).
Although the decline in the number of RBC collections and transfusions since 2008 continued, the decline from 2015 to 2017 in collections was smaller than the decline from 2013 to 2015 (Fig. 2A). The population rates of RBC collections and transfusions followed a similar pattern to the trend of total RBC collections and transfusions (Fig. 2C). Declines were observed in the number of RBC units outdated (Fig. 2B).
In subanalyses of 1491 hospitals that provided allogeneic RBC utilization data for both the 2015 and 2017 NBCUS, the median difference between 2015 and 2017 was a 6.5% decline (Table 2). When stratified by annual surgical volume, hospitals performing the fewest inpatient surgical operations had the greatest decline. Hospitals that performed 100 to 999 annual inpatient surgeries transfused 9.1% fewer allogeneic RBC units in 2017 compared with 2015, hospitals that performed 1000 to 1399 annual inpatient surgeries transfused 10.2% fewer RBC units, and hospitals that performed 1400 to 2399 inpatient surgeries transfused 9.1% fewer RBC units. In contrast, the largest hospitals (>8000 annual inpatient surgeries) transfused 1.0% more RBC units during 2017 compared with 2015.
TABLE 2.
Percent difference in allogeneic RBC units transfused in 2015 and 2017 from matched hospitals
| Inpatient surgical volume category | N* | Median (mean) 2017 allogeneic RBCs* | Median % difference† | IQR‡ of % difference† |
|---|---|---|---|---|
| 100–999 surgeries per year | 140 | 400 (564) | −9.1% | 26.5% |
| 1,000–1,399 surgeries per year | 224 | 1,285 (1,493) | −10.2% | 19.7% |
| 1,400–2,399 surgeries per year | 339 | 1,952 (2,209) | −9.1% | 21.8% |
| 2,400–4,999 surgeries per year | 454 | 3,548 (3,928) | −6.5% | 19.1% |
| 5,000–7,999 surgeries per year | 190 | 6,209 (6,884) | −3.6% | 17.2% |
| 8,000 or more surgeries per year | 144 | 13,662 (15,398) | 1.0% | 14.8% |
| Total | 1491 | 2,788 (4,340) | −6.5% | 21.1% |
Based on matched facilities reporting allogeneic RBCs in both 2015 and 2017 NBCUS surveys.
% difference calculated as 100*(2017–2015)/2015.
Interquartile range (75th-25th percentile).
RBC transfusion by location within a health care facility
Among locations within a health care facility, the largest number of RBC units were transfused in inpatient medicine (3,896,000 units; 95% CI, 3,648,000–4,144,000 units), followed by critical care (1,718,000 units; 95% CI, 1,577,000–1,858,000 units), outpatient and nonacute inpatient settings (1,416,000 units; 95% CI, 1,284,000–1,549,000 units), surgery (1,409,000 units; 95% CI, 1,255–1,562,000 units), and the emergency department (1,042,000 units; 95% CI, 962,000–1,254,000 units) (Table 3). The greatest decline in the number of transfused RBC units from 2015 to 2017 was seen in outpatient and nonacute inpatient settings (−15.2%), followed by inpatient medicine (−10.2%), critical care (−5.8%), pediatrics (−4.3%), and surgery (−1.6%). The greatest increase in the number of transfused RBCs from 2015 to 2017 was seen in obstetrics/gynecology (19.4%) and the emergency department (3.3%).
TABLE 3.
RBC units transfused by location in 2017 (expressed in thousands)
| 2017* (95% CI) | 2015 | % diff | Matched median % diff | |
|---|---|---|---|---|
| All surgery (including transplant) | 1,409 (1,255 – 1,562, n = 1,243) | 1,431 | −1.6% | −10.1% (n = 407) |
| Emergency department | 1,042 (962–1,121, n = 1,254) | 1,007 | 3.3% | 15.9% (n = 404) |
| Inpatient medicine (including hematology/oncology) | 3,896 (3,648–4,144, n = 1,285) | 4,293 | −10.2% | −5.9% (n = 440) |
| Obstetrics/Gynecology | 241 (195–287, n = 1,253) | 194 | 19.4% | 5.9% (n = 327) |
| Pediatrics | 143 (101–184, n = 1,310) | 149 | −4.3% | −12.9% (n = 81) |
| Neonates | 106 (84–128, n = 1,338) | 103 | 2.5% | 0.7% (n = 145) |
| Critical care | 1,718 (1,577–1,858, n = 1,175) | 1,817 | −5.8% | −7.7% (n = 318) |
| Outpatient and nonacute inpatient settings† | 1,416 (1,284–1,549, n = 1,274) | 1,631 | −15.2% | −7.0% (n = 359) |
Total number of responses for 2017 was 2138.
Includes outpatient dialysis, rehabilitation, long term care.
Among transfusing hospitals that responded in both 2015 and 2017, the matched median percent difference of transfused RBC units was greatest in pediatrics (−12.9%), followed by surgery (−10.1%), critical care (−7.7%), outpatient and nonacute inpatient settings (−7.0%), and inpatient medicine (−5.9%). The greatest increase in the matched median percent difference of transfused RBCs was in the emergency department (15.9%) and obstetrics/gynecology (5.9%).
Prediction model of RBC transfusion trends
The first RBC use model, assuming that 2015–2017 RBC transfusion trends stratified by location in a health care facility continued, projected a 14.3% decrease by 2022. The second model, assuming that 2015–2017 transfusion trends in transfusions stratified by annual inpatient surgical volume continued, projected a 10.1% decrease by 2022.
PLT, plasma, and cryoprecipitate distribution and transfusion
In 2017, 2,560,000 PLT units were distributed from blood collection centers in the United States (95% CI, 2,391,000–2,730,000 units), 5.1% more units than were distributed in 2015 (2,436,000 units) (Table 4). Of all PLT units distributed, 91.3% (2,2338,000 units; 95% CI, 2,189,000–2,487,000 units) were collected by apheresis and the remaining 8.7%, or 223,000 units (95% CI, 139,000–306,000 units), were whole blood derived.
TABLE 4.
Estimated number of PLTs, plasma, and cryoprecipitate units distributed, transfused, and outdated in 2017 (expressed in thousands)
| Blood centers | Hospitals | Combined Totals | 95% CI | 2015 Totals* | % Change 2017–2015 | |
|---|---|---|---|---|---|---|
| Distributed | ||||||
| Apheresis PLTs | 2,181 | 157 | 2,338 | (2,189–2,487) | 2,234 | 4.6% |
| Whole blood-derived PLTs† | 174 | 49 | 223 | (139–306) | 202 | 10.3% |
| Total PLTs | 2,354 | 206 | 2,560 | (2,391–2,730) | 2,436 | 5.1% |
| Total plasma | 2,982 | 227 | 3,209 | (2,879–3,539) | 3,714 | −13.6% |
| Cryoprecipitate‡ | 1,997 | 171 | 2,168 | (1,908–2,428) | 1,857 | 16.8% |
| Blood center outdates§ | 273 | 47 | 320 | (273–367) | 242 | 32.3% |
| Transfused | ||||||
| Apheresis PLTs | 1,848 | (1,715–1,980) | 1,807 | 2.3% | ||
| Whole blood-derived PLTs† | 82 | (30–133) | 171 | −52.3% | ||
| Total PLTs (includes directed units) | 1,937 | (1,794–2,079) | 1,983 | −2.3% | ||
| Total plasma | 2,374 | (2,262–2,487) | 2,727 | −12.9% | ||
| Cryoprecipitate‡ | 1,064 | (944–1,184) | 1,167 | −8.8% | ||
| Hospital outdates‖ | 446 | (415–477) | 426 | 4.7% |
2015 totals were obtained from Ellingson et al.3
Whole blood-derived PLTs are expressed as apheresis equivalents.
Cryoprecipitates are expressed as individual unit equivalents.
Blood center outdates are units that were outdated at nonhospital and hospital-based blood centers.
Hospital outdates are units that were outdated at transfusing hospitals.
PLTs = platelets.
During 2017, 1,937,000 (95% CI, 1,794,000–2,079,000 units) whole blood–derived (expressed as apheresis equivalents) and apheresis PLT units were transfused, which represents a 2.3% decline compared with 2015 (1,983,000 units). Apheresis PLT units transfused (1,848,000 units; 95% CI, 1,715,000–1,980,000 units) increased by 2.3% since 2015 (1,807,000 units). Whole blood–derived PLT unit transfusions declined by 52.3% between 2015 and 2017, from 171,000 units in 2015 to 82,000 units (95% CI, 30,000–133,000 units) in 2017. Of all PLT units transfused during 2017, 4.2% were whole blood derived, fewer than during 2015, when 8.6% of all transfused PLT units were whole blood derived.
In total, 3,209,000 units of plasma (95% CI, 2,879,000–3,539,000 units) were distributed in 2017, which represents a 13.6% decrease from 2015 (3,714,000 units). This includes fresh-frozen plasma, plasma frozen within 24 hours of collection, cryoprecipitate-reduced plasma, and liquid plasma. A total of 2,374,000 units (95% CI, 2,262,000–2,487,000 units) of plasma were transfused during 2017, a 12.9% decrease since 2015 (2,727,000 units).
A total of 2,168,000 units of cryoprecipitate (95% CI, 1,908,000–2,428,000 units) were distributed during 2015, a 16.8% increase since 2015 (1,857,000 units). Cryoprecipitate transfusions during 2017 (1,064,000 transfusions; 95% CI, 944,000–1,184,000 transfusions) decreased by 8.8% compared with 2015 (1,167,000 transfusions).
Among all PLT, plasma, and cryoprecipitate components produced for distribution during 2017, 320,000 (95% CI, 273,000–367,000) were outdated at blood collection centers, a 32.3% increase compared with 2015. The number and percentage of units outdated at blood centers in 2017 included 165,000 apheresis PLT units (7.1% of apheresis PLT units), 22,800 whole blood-derived PLT apheresis equivalents (10.2%), 33,000 plasma units (1.0%) and 8000 (0.4%) cryoprecipitate units. Approximately 446,000 components were outdated at transfusing hospitals during 2017 (95% CI, 415,000–477,000 outdates), a 4.7% increase compared with 2015. Components outdated at hospitals included 179,000 apheresis PLT units (9.7% of units in hospital inventory), 8000 whole blood–derived PLT apheresis equivalents (9.8%), 154,000 plasma units (6.5%), and 72,000 (6.8%) cryoprecipitate units.
DISCUSSION
The findings of the 2017 NBCUS demonstrate that RBC collection and use has continued to decrease in the United States. From 2015 to 2017, the number of RBC and whole blood collections declined by 3.0%, a slower decline compared with declines from 2008 to 2015. Lower collections are likely a result of lower demand for blood products. Since 2015, transfusions of RBCs and whole blood declined 6.1%, a slower decline compared with declines during 2013–2015 but similar to declines during 2008–2013.
The cause of the slowing decline of RBC and whole blood collections and use is likely continued implementation of technologies and patient blood management policies to reduce blood loss and use, such as restrictive transfusion strategies, a lower dose of transfusions, and avoiding unnecessary blood tests.6,16–20 Patient blood management programs, including adopting restrictive transfusion practices, have demonstrated a substantial reduction in RBC transfusions.21 Many professional organizations have promoted the adoption of patient management programs and policies,16,17,22–29 and an increasing number of hospitals are likely implementing patient blood management programs.4,5 The decline in RBC transfusion was not seen among the largest hospitals (>8000 annual surgeries) responding to both the 2015 and 2017 NBCUS and was more prominent among smaller hospitals. A possible explanation is that larger hospitals have previously adopted transfusion reduction technologies and patient blood management policies, and smaller hospitals are recently adopting similar strategies. Although the greatest proportional reduction in RBC transfusion from 2013 to 2015 was seen in surgical settings,4 the greatest proportional decreases from 2015 to 2017 were seen in inpatient medicine, outpatient and nonacute inpatient settings, and critical care. A possible explanation is that surgical techniques, technologies, and transfusion reduction surgery policies had already been implemented in many hospitals while further implementation of patient blood management policies continued in nonsurgical settings. However, among facilities that responded in both 2015 and 2017, moderate decreases in RBC transfusion in surgery were seen.
The evidence for laboratory thresholds for PLT and plasma transfusion is less established than RBC transfusion threshold evidence.8,10,21 However, many guidelines recommend avoiding plasma transfusions for common indications and the continued reduction in plasma collection and use might be a result of these recommendations.8,10,18,24,30–32 Additionally, vitamin K, prothrombin complex concentrates, and coagulation factor concentrates can be safer and/or more effective than plasma for their specific indications.18,32 In contrast, platelet collection slightly increased, while platelet transfusions slightly decreased from 2015 to 2017. This might reflect the shorter shelf life of platelet products, which requires blood centers to maintain inventory to ensure availability when clinically needed.33 Guidelines for platelet transfusion thresholds have been published8 but reducing plasma and platelet transfusion through blood management programs might be more difficult than reducing RBC transfusions.34 Platelet trends might also be affected by evidence supporting and guidelines recommending early platelet transfusion and a high ratio of platelets to RBCs during resuscitation of severely injured bleeding patients, potentially requiring hospitals to maintain increased numbers of platelets in inventory.35–37
Projections of RBC use in the United States from 2017 to 2022 suggest a continued 10.0% to 14.3% decline. Many industrialized countries have also reported recent declines in RBC use.38–42 In the United States, the rate of whole blood and RBC units collected per 1000 population decreased from 60.4 in 2015 to 58.2 in 2017; the rate of RBC units transfused per 1000 population decreased from 35.3 in 2015 to 32.7 in 2017. During 2017, Hema-Quebec reported a rate of RBC units transfused of 24.2 per 1000 population.39 In 2017, reported rates of RBC units issued per 1000 population (which are slightly higher than transfusion rates) include 25.8 by Canadian Blood Services (for April 2017 through March 2018),38 25.5 in Australia,40 28.1 in New Zealand,42 and 26.1 in the United Kingdom.43 Although blood collection and health care systems vary widely, international trends and recent rates of blood collection and use suggest the United States might further reduce blood use.
As a result of declining blood use, more selective donor criteria, adoption of technologies to enhance blood safety, and the current financial model of blood distribution in the United States, concerns have been raised regarding the sustainability of the blood system and particularly the ability to maintain resilience.9 The findings of the present survey suggest that the cost paid for blood components44 and the demand for blood continues to decrease, but the pace of both is slowing. The blood collection industry might be responding to these financial pressures through mergers.44 Seven percent of blood center respondents to the 2017 NBCUS survey reported financial insolvency. Despite the current trend in collection and use, the US blood supply has been effective at maintaining both safety and availability during recent public health emergencies.9,45–48 However, the ability to continue to maintain resilience or respond to larger or concurrent emergencies is unclear.
These findings are subject to the following limitations. First, imputation and weighting were used to generate national estimates, and comparisons with previous years could be affected by differences in sampling and in response rates. The impact of this limitation is likely mitigated by the higher response rate to the 2015 and 2017 surveys. Second, similar to previous NBCUS surveys, several health care facility types were excluded, including smaller hospitals, military hospitals, and outpatient facilities, which may result in underestimates. Third, outdates of frozen blood products might correspond to collections in 2016 because of the long shelf life. Finally, data are self-reported by responding facilities and were not verified. To reduce the impact of this limitation, follow-up contact was made with certain facilities to clarify responses.
In conclusion, collection and use of RBCs in the United States has continued to decline in 2017, but the decline has slowed since 2015. These declines are largely a result of decreasing use among smaller hospitals. The rate of blood use per capita in the United States continues to be high compared with many other industrialized countries, suggesting that continued decline is possible. We project a slowing decrease as hospitals continue to implement effective blood-saving technologies and patient blood management programs and policies. Although decreasing demand and costs of blood products paid by hospitals combined with increasing manufacturing costs for safer blood products continues to strain blood collection centers, possibly resulting in mergers and closure of blood centers, the US blood industry has become more efficient and continues to provide sufficient blood to meet the regular and emergent needs of the country.
ABBREVIATIONS:
- NBCUS
National Blood Collection and Utilization Survey
Footnotes
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
Publisher's Disclaimer: DISCLAIMER
Publisher's Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Pfuntner A, Wier LM, Stocks C. Most frequent procedures performed in U.S. hospitals, 2010. HCUP statistical brief #149 Rockville (MD): Agency for Healthcare Research and Quality; 2013. [PubMed] [Google Scholar]
- 2.Chung KW, Basavaraju SV, Mu Y, et al. Declining blood collection and utilization in the United States. Transfusion 2016;56: 2184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellingson KD, Sapiano MRP, Haass KA, et al. Continued decline in blood collection and transfusion in the United States-2015. Transfusion 2017;57(Suppl 2):1588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sapiano MRP, Savinkina AA, Ellingson KD, et al. Supplemental findings from the National Blood Collection and Utilization Surveys, 2013 and 2015. Transfusion 2017;57(Suppl 2): 1599–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Department of Health and Human Services. The 2011 national blood collection and utilization survey report. Washington, DC: US Department of Health and Human Services; 2013. [Google Scholar]
- 6.Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB: red blood cell transfusion thresholds and storage. JAMA 2016;316:2025–35. [DOI] [PubMed] [Google Scholar]
- 7.Hebert PC, Carson JL. Transfusion threshold of 7 g per deciliter–the new normal. N Engl J Med 2014;371:1459–61. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2015;162:205–13. [DOI] [PubMed] [Google Scholar]
- 9.Klein HG, Hrouda JC, Epstein JS. Crisis in the sustainability of the U.S. blood system. N Engl J Med 2017;377:1485–8. [DOI] [PubMed] [Google Scholar]
- 10.Roback JD, Caldwell S, Carson J, et al. Evidence-based practice guidelines for plasma transfusion. Transfusion 2010;50:1227–39. [DOI] [PubMed] [Google Scholar]
- 11.Woodruff R. A simple method for approximating the variance of a complicated estimate. J Am Stat Assoc 1971;66:411–4. [Google Scholar]
- 12.He YRT. Tukey’s gh distribution for multiple imputation. Am Stat 2006;60:251–6. [Google Scholar]
- 13.Rubin D. Multiple imputation for nonresponse in surveys. Hoboken (NJ): Wiley; 2004. [Google Scholar]
- 14.Savinkina A, Sapiano MRP, Berger J, et al. Is surgical volume still the most accurate indicator of blood usage in the United States? Transfusion 2019;59:1125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Census Bureau US. Population estimates by state and age. Suitland (MD): US Census Bureau; 2017. [Google Scholar]
- 16.Society for the Advancement of Blood Management. Choosing wisely - five things physicians and patients should question. [cited 2019 Aug 05]. Available from: https://www.sabm.org/wp-content/uploads/2018/08/SABM-Choosing-Wisely-List.pdf.
- 17.American Association of Blood Banks. Choosing wisely: five things physicians and patients should question. [cited 2019 Aug 05]. Available from: http://www.choosingwisely.org/societies/american-association-of-blood-banks/.
- 18.Pavenski K, Stanworth S, Fung M, et al. Quality of evidence-based guidelines for transfusion of red blood cells and plasma: a systematic review. Transfus Med Rev 2018;32:135–43. [DOI] [PubMed] [Google Scholar]
- 19.Retter A, Wyncoll D, Pearse R, et al. Guidelines on the management of anaemia and red cell transfusion in adult critically ill patients. Br J Haematol 2013;160:445–64. [DOI] [PubMed] [Google Scholar]
- 20.Society of Thoracic Surgeons Blood Conservation Guideline Task F, Ferraris VA, Brown JR, et al. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg 2011;91:944–82. [DOI] [PubMed] [Google Scholar]
- 21.Sadana D, Pratzer A, Scher LJ, et al. Promoting high-value practice by reducing unnecessary transfusions with a patient blood management program. JAMA Intern Med 2018;178: 116–22. [DOI] [PubMed] [Google Scholar]
- 22.The Joint Commission. Patient blood management certification. [cited 2019 Aug 05]. Available from: https://www.jointcommission.org/certification/patient_blood_management_certification.aspx.
- 23.AABB. Patient Blood Management Toolkit. [cited 2019 Aug 05]. Available from: http://www.aabb.org/pbm/Pages/pbm-resources.aspx.
- 24.American Association for the Study of Liver Diseases. Choosing wisely. [cited 2019 Aug 05]. Available from: http://www.choosingwisely.org/societies/american-association-for-the-study-of-liver-diseases/
- 25.American Academy of Family Physician. Choosing wisely -twenty things physicians and patients should question. [cited 2019 Aug 05]. Available from: http://www.choosingwisely.org/societies/american-academy-of-family-physicians/.
- 26.Critical Care Societies Collaborative – Critical Care. Chossing wisely - five things physicians and patients should question. [cited 2019 Aug 05]. Available from: http://www.choosingwisely.org/societies/critical-care-societies-collaborative-critical-care/.
- 27.Society of Hospital Medicine – Adult Hospital Medicine. Choosing wisely - five things physicians and patients should question. [cited 2019 Aug 05]. Available from: http://www.choosingwisely.org/societies/society-of-hospital-medicine-adult/.
- 28.American Society of Hematology. Choosing wisely - ten things physicians and patients should question.[cited 2019 Aug 05]. Available from: http://www.choosingwisely.org/societies/american-society-of-hematology/.
- 29.American Hospital Association’s Physician Leadership Forum. Blood management toolkit. Chicago, IL; 2014. Available from: http://www.ahaphysicianforum.org/resources/appropriate-use/blood-management/index.shtml. [Google Scholar]
- 30.Callum JL, Waters JH, Shaz BH, et al. The AABB recommendations for the Choosing Wisely campaign of the American Board of Internal Medicine. Transfusion 2014;54:2344–52. [DOI] [PubMed] [Google Scholar]
- 31.Green L, Bolton-Maggs P, Beattie C, et al. British Society of Haematology Guidelines on the spectrum of fresh frozen plasma and cryoprecipitate products: their handling and use in various patient groups in the absence of major bleeding. Br J Haematol 2018;181:54–67. [DOI] [PubMed] [Google Scholar]
- 32.Yang L, Stanworth S, Hopewell S, et al. Is fresh-frozen plasma clinically effective? An update of a systematic review of randomized controlled trials. Transfusion 2012;52:1673–86 quiz. [DOI] [PubMed] [Google Scholar]
- 33.Dunbar NM, Katus MC, Freeman CM, et al. Easier said than done: ABO compatibility and D matching in apheresis platelet transfusions. Transfusion 2015;55:1882–8. [DOI] [PubMed] [Google Scholar]
- 34.Thakkar RN, Lee KH, Ness PM, et al. Relative impact of a patient blood management program on utilization of all three major blood components. Transfusion 2016;56:2212–20. [DOI] [PubMed] [Google Scholar]
- 35.Cannon JW, Khan MA, Raja AS, et al. Damage control resuscitation in patients with severe traumatic hemorrhage: a practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg 2017;82:605–17. [DOI] [PubMed] [Google Scholar]
- 36.Cardenas JC, Zhang X, Fox EE, et al. Platelet transfusions improve hemostasis and survival in a substudy of the prospective, randomized PROPPR trial. Blood Adv 2018;2:1696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holcomb JB, del Junco DJ, Fox EE, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg 2013;148:127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Canadian Blood Services. Annual report 2017–2018. Ottawa, Canada: Canadian Blood Services; 2018. [Google Scholar]
- 39.Hema-Quebec. 2017–2018 annual report. Quebec City, Canada: Hema-Quebec; 2018. [Google Scholar]
- 40.National Blood Authority Australia. National blood authority annual report 2017–2018. Canberra, Australia: National Blood Authority; 2018. [Google Scholar]
- 41.European Blood Alliance. EBA annual report. The Hague, Netherlands: European Blood Alliance; 2017. p. 2017. [Google Scholar]
- 42.New Zealand Blood Service. Annual report 2017–2018. Auckland, New Zealand: New Zealand Blood Service; 2018. [Google Scholar]
- 43.Bolton-Maggs PH, Poles D, Watt A, et al. The 2017 Annual SHOT Report. Manchester, England: Serious Hazards of Transfusion; 2018. [Google Scholar]
- 44.Sapiano MR. Supplemental findings of the 2017 National Blood Collection and Utilization Survey. Transfusion. 2020;60(Suppl 2):S17–S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.America’s Blood Centers. Statement of America’s Blood Centers hearing on “preparedness, response, and rebuilding: lessons from the 2017 disasters” U.S. House of Representatives Homeland Security Committee March 14, 2018 [cited 2019 Aug 05]. Available from: https://www.americasblood.org/media/81535/abc-statement-to-house-homeland-security-committee-031418.pdf.
- 46.Block B. Crisis in the sustainability of the U.S blood system. N Engl J Med 2018;378:305. [DOI] [PubMed] [Google Scholar]
- 47.Lozada MJ, Cai S, Li M, et al. The Las Vegas mass shooting: an analysis of blood component administration and blood bank donations. J Trauma Acute Care Surg 2019;86:128–33. [DOI] [PubMed] [Google Scholar]
- 48.Vasquez AM, Sapiano MR, Basavaraju SV, et al. Survey of blood collection centers and implementation of guidance for prevention of transfusion-transmitted Zika virus infection–Puerto Rico, 2016. MMWR Morb Mortal Wkly Rep 2016;65:375–8. [DOI] [PubMed] [Google Scholar]


