Abstract
Background:
Evidence about incidence and outcomes of acute coronary syndrome (ACS) after transcatheter aortic valve replacement (TAVR) is scarce.
Methods:
We identified Medicare patients who underwent TAVR from 2012 to 2017 and were admitted with ACS during follow-up. We compared outcomes based on the type of ACS; ST-segment elevation myocardial infarction (STEMI), non-STEMI (NSTEMI), and unstable angina. In patients with non-ST elevation ACS (NSTE-ACS), we compared outcomes based on the treatment strategy (invasive versus conservative) using inverse probability weighting (IPW) analysis.
Results:
Out of 142,845 TAVR patients, 6,741 patients (4.7%) were admitted with ACS after a median time of 297 days (IQR 85–662), with 48% of admissions occurring within 6 months. The most common presentation was NSTEMI. Predictors of ACS were history of coronary artery disease, prior revascularization, diabetes, valve-in-TAVR, and acute kidney injury. STEMI was associated with higher 30-day and 1-year mortality compared to NSTEMI (31.4% versus 15.5% and 51.2% versus 41.3% respectively, p<0.01). Overall, 30.3% of NSTE-ACS patients were treated with invasive approach. On IPW analysis, invasive approach was associated with lower adjusted long-term mortality (aHR 0.69, 95% CI 0.66–0.73, p<0.01) and higher risk of repeat revascularization (aHR 1.29, 95% CI 1.16–1.43, p<0.001).
Conclusion:
After TAVR, ACS is infrequent (<5%), and the most common presentation is NSTEMI. Occurrence of STEMI after TAVR is associated with a high mortality with nearly one-third of patients dying within 30-days. Optimization of care is needed for post-TAVR ACS patients and if feasible, invasive approach should be considered in these high-risk patients.
Keywords: Transcatheter aortic valve replacement, Acute coronary syndrome, Percutaneous coronary intervention
Graphical abstract
CONDENSED ABSTRACT
Among 142,845 Medicare patients who underwent transcatheter aortic valve replacement (TAVR) from 2012 to 2017, 6,741 patients (4.7%) were admitted with acute coronary syndrome after a median time of 297 days (IQR 85–662). The most common presentation was NSTEMI. STEMI was associated with higher 30-day and 1-year mortality compared to NSTEMI (31.4% versus 15.5% and 51.2% versus 41.3% respectively, p<0.01). After propensity score matching and inverse probability weigh analyses, invasive approach was associated with lower 30-day and long-term mortality (aHR 0.69, 95% CI 0.66–0.73, p<0.01), as well as reduction in HF and bleeding admissions.
INTRODUCTION
Transcatheter aortic valve replacement (TAVR) has revolutionized the management of severe aortic stenosis (AS) worldwide. After the recent results from PARTNER 3 and EVOLUT low risk trials, it is expected that the indications for TAVR will expand to include low risk patients(1,2). Furthermore, in light of evidence about potential long-term durability of TAVR valves and improved survival of patients following TAVR (3), it becomes crucial to study long-term cardiac events after TAVR and identify strategies for optimal management.
Aging is a major risk factor for both coronary artery disease (CAD) and AS (4). More than 50% of patients with severe AS who undergo TAVR have concomitant CAD, and majority of these patients have undergone coronary revascularization (5). Evidence about incidence, timing and outcomes of ACS after TAVR at long-term follow up remain scarce (6,7). Furthermore, studies about the optimal management of ACS after TAVR are lacking. The technical considerations involved in performing percutaneous coronary intervention (PCI) in the presence of a TAVR prosthesis raise the question of applicability of prior studies of utility of PCI in management of ACS in TAVR patients (8,9).
The aim of this study is to address this knowledge gap by examining the incidence, timing and predictors of ACS after TAVR in Medicare beneficiaries. We also examined short- and long-term outcomes with different types of ACS and treatment using an invasive approach versus conservative management.
METHODS
Study population
The study cohort included patients who underwent TAVR from January 2012 to December 2017 and were enrolled in Medicare Fee-for-service. These patients were identified using the 100% Medicare Provider and Analysis Review (MEDPAR) Part A files from the Center for Medicare and Medicaid Services (CMS) which include all hospital admissions for Medicare fee-for-service beneficiaries nationwide. TAVR procedures were identified using ICD-9 procedure codes (35.05 and 35.06) for the period until September 2015, and ICD-10 procedure codes (02RF37Z, 02RF38Z, 02RF3JZ, 02RF3KZ, 02RF37H, 02RF38H, 02RF3JH, 02RF3KH, or X2RF332) for the period after September 2015. Patient demographics including age, sex, and race were extracted from Medicare enrollment files, while comorbidities were identified using ICD-9 and ICD-10 codes on inpatient claims during the three years prior to the TAVR admission (Supplemental Table 1). We used comorbidity algorithms originally defined by Elixhauser et al (10), as well as additional conditions that are relevant to our cohort. The dates of beneficiary Medicare enrollment and death were obtained from the 100% Beneficiary Summary file for the study period. Patients were excluded if they had been enrolled in Medicare fee-for-service for less than three years prior to the TAVR.
The Institutional Review Board of the University of Iowa approved this study with waiver for individual informed consent.
Study Outcomes
We identified patients who were admitted after TAVR with a diagnosis of acute coronary syndrome (ACS) including unstable angina (UA), non-ST-segment elevation myocardial infarction (NSTEMI), and ST-segment elevation myocardial infarction (STEMI) using ICD-9 and ICD-10 codes (Supplemental Table 1). We restricted the diagnosis of ACS to the primary diagnosis or the first secondary diagnosis to ensure that the ACS was the main indication for the hospital admission. We excluded ICD-10 code (I21.A1) for type II MI. Patients were followed from the date of TAVR discharge through the end of the follow-up period (December 2017), and were censored due to death, Medicare disenrollment, or end of the study period (December 31, 2017). We also classified ACS admission based on the timing of occurrence after the index TAVR admission into very early (≤7 days), early (>7 to ≤30 days), delayed (>1 to ≤ 6 months) and late (> 6 months) post-TAVR. In the total ACS cohort, we studied 1) the incidence and timing of ACS after TAVR; 2) predictors associated with occurrence of ACS within 6 months after the TAVR procedure; and 3) rate and predictors of invasive approach (coronary angiogram, balloon angioplasty, bare metal stent [BMS], or drug eluting stent [DES]) versus a conservative management in ACS.
We then divided the cohort in 3 groups, patients with UA, NSTEMI and STEMI based on ICD code algorithms that were validated extensively in prior studies with positive predictive value of >90% (11,12). In these 3 groups, we compared 1) differences in in-hospital management (i.e. invasive versus conservative approach, and use of mechanical circulatory support [MCS]); 2) in-hospital outcomes including in-hospital mortality, cardiogenic shock, cardiac arrest, and heart failure; 3) short-term outcomes including 30-day mortality, heart failure (HF), and bleeding admissions; and 3) long-term outcomes including all-cause mortality, readmissions for HF, bleeding, recurrent ACS and need for revascularization. ICD-9 and ICD-10 codes used to define the study outcomes are reported in Supplemental Table 1.
The short- and long-term outcomes with invasive versus conservative approach were further studied among patients with non-ST-segment elevation ACS (NSTE-ACS) patients. Outcomes were reported both pre- and post-propensity score matching that was utilized to balance 41 clinical covariates between the two groups. Dates of deaths occurring through September 1,2018 were available, while data on readmissions was available through December 31,2017.
Statistical analysis
Continuous patient characteristics were described as mean and standard deviation or median and interquartile range as appropriate and were compared between patients with different types of ACS using analysis of variance or Wilcoxon test. Categorical characteristics were described as percentages and were compared using Chi-Square or Fisher Exact test as appropriate.
We used a multivariable logistic regression model to determine factors associated with ACS occurrence within 6 months after TAVR. To determine the predictors of PCI in management of ACS, we created a mixed-effects multivariable logistic regression with hospitals as random-effects to account for clustering of patients within hospitals. Adjusted odds ratios (ORs) are reported with 95% confidence intervals (CI) derived from sandwich estimates of standard errors.
Finally, we compared invasive versus conservative approach in NSTEACS using propensity scores that were created by a logistic regression model with the dependent variable of PCI and a list of 41 covariates as the independent variables (Supplementary Table 3). Propensity score matching with 1 (conservative) to 1 (PCI) was performed with greedy match method and a caliper of 0.01 and robustness of the match algorithm was assessed by comparing the variables between both groups post-matching with a standardized difference lower than 0.1 considered balanced. To account for the matched design, short-term outcomes were compared with conditional logistic regression and asymptotic and exact odds ratio were estimated.(13) For long-term outcomes, we used Cox proportional hazards regression with Breslow method to break ties, which gives identical results to conditional Poisson regression (14), and hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated. Unadjusted Kaplan-Meier (KM) curves with 95% CIs were generated to determine the cumulative proportion of patients with events as a function over time and compared using log-rank or Generalized Wilcoxon statistic, as appropriate. To assess the proportional hazards assumption, Kolmogorov-type supremum test and graphical inspection of Schoenfeld residuals plotted against time were performed. For the long-term outcomes of bleeding and HF admissions, multivariable survival analyses were performed by competing risk regression analysis to account for death using the Fine-Gray proportional sub hazards model, and subdistribution hazard ratios (sHRs) were calculated, along with 95% CIs.(15)
As a sensitivity analysis, we then performed survival time inverse probability weighting to estimate the average treatment effects.(16) First, the unstabilized inverse probability weights (IPWs) for the whole cohort were derived from the propensity scores from the non-parsimonious logistic regression model. Then, survival models to determine the adjusted effect of SAVR/TAVR on the study outcomes were created using the IPWs.(16) The model was generated using a robust sandwich covariance matrix estimate and robust standard error estimates to account for the clustering of patients within hospitals.(17) We also generated IPW-adjusted KM survival curves and adjusted KM estimator compared with log-rank test as proposed previously.(18,19)
A P value of 0.05 was the cutoff for statistical significance. The analysis was done with SAS version 9.4 (SAS Institute, Cary, North Carolina) and R 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Incidence and timing of ACS
Out of 142,845 patients who underwent TAVR during the study period, 6741 patients (4.7%) were admitted for an ACS with a total of 8,862 ACS admissions (single ACS admission n= 4,620, 2 ACS admissions n= 827, 3 ACS admissions n= 405, > 3 ACS admissions n= 242). Table 1 shows baseline characteristics of TAVR patients who experienced ACS during follow up compared to those who did not. Patients who had ACS were younger, more likely to be males and of black race, with a higher burden of comorbidities including hypertension, diabetes mellitus, HF, lung disease, chronic kidney disease, prior CAD, and prior revascularization. Valve-in-TAVR (repeat TAVR) procedure was more common in patients who experienced ACS.
Table 1:
Baseline characteristics of patients with versus without acute coronary syndrome after TAVR
| Variable | Without ACS | With ACS | P value |
|---|---|---|---|
| N | 136,104 | 6,741 | |
| Age | 81.7±8.1 | 80.7±8.8 | <0.0001 |
| Male | 52.7 | 55.3 | <0.001 |
| Other | 3.5 | 3.4 | |
| Hypertension | 93.8 | 95.1 | <0.0001 |
| Diabetes mellitus | 40.9 | 51.5 | <0.0001 |
| Heart failure | 79.3 | 83.9 | <0.001 |
| Deficiency anemia | 40.8 | 50.3 | <0.001 |
| Alcohol abuse | 2.0 | 1.7 | 0.10 |
| Connective tissue disease | 6.7 | 7.7 | <0.001 |
| Chronic lung disease | 38.5 | 43.8 | <0.001 |
| Coagulopathy | 22.4 | 24.1 | 0.001 |
| Depression | 15.2 | 12.7 | 0.002 |
| Drug abuse | 0.7 | 1.0 | 0.02 |
| Hypothyroidism | 25.7 | 26.1 | 0.44 |
| Liver disease | 3.8 | 3.8 | 0.90 |
| Lymphoma | 1.8 | 1.9 | 0.70 |
| Electrolyte abnormality | 43.6 | 51.9 | <0.001 |
| Metastasis | 1.2 | 0.9 | 0.04 |
| Obesity | 24.3 | 27.0 | <0.0001 |
| Paralysis | 4.0 | 4.9 | 0.0004 |
| Peripheral arterial disease | 35.6 | 46.4 | <0.0001 |
| Psychosis | 2.7 | 3.5 | 0.0002 |
| Pulmonary hypertension | 18.2 | 25.5 | <0.0001 |
| Tumor without metastasis | 5.1 | 5.4 | 0.32 |
| Underweight | 1.7 | 1.4 | 0.10 |
| Weight loss | 8.7 | 9.5 | 0.02 |
| Prior bleeding | 34.1 | 41.8 | <0.0001 |
| Cerebral hemorrhage | 0.7 | 0.8 | 0.31 |
| Prior stroke | 16.6 | 22.6 | <0.0001 |
| End stage renal disease on dialysis | 3.5 | 7.0 | <0.0001 |
| Chronic kidney disease | 28.7 | 34.7 | <0.0001 |
| Prior coronary artery disease | 79.8 | 90.8 | <0.0001 |
| Prior smoking | 26.3 | 38.7 | <0.0001 |
| Prior revascularization (within 3 years prior to TAVR) | 35.3 | 55.2 | <0.0001 |
| Prior ACS (within 1 year prior to TAVR) | 7.9 | 21.6 | <0.0001 |
| PCI (within 6 months prior to TAVR) | 7.4 | 14.9 | <0.0001 |
| Prior ICD | 4.2 | 5.1 | 0.0004 |
| Preexisting atrial fibrillation | 43.6 | 40.7 | <0.001 |
| Prior pacemaker | 13.9 | 13.7 | 0.70 |
| Apical TAVR | 7.2 | 12.8 | <0.001 |
| Valve-in-TAVR valve | 0.4 | 1.1 | <0.001 |
| Aortic aneurysm | 1.9 | 1.0 | <0.0001 |
| TAVR in Bicuspid AS | 1.2 | 1.0 | 0.18 |
ACS=Acute coronary syndrome, ICD=intracardiac defibrillator, TAVR=transcatheter aortic valve replacement; PCI= percutaneous coronary intervention
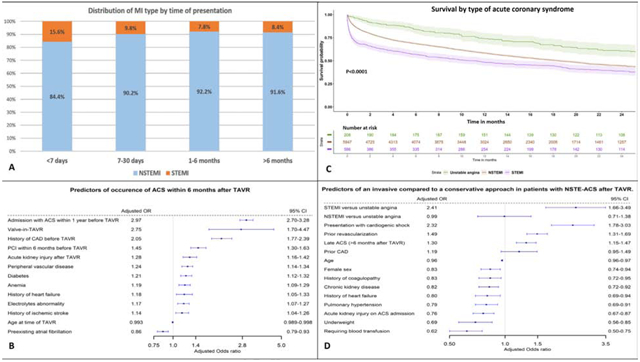
Out of the 6741 first ACS admissions, 586 had STEMI (anterior STEMI n=163, non-anterior STEMI n=423), and 6155 had NSTE-ACS (NSTEMI n= 5,947, UA n= 208) (Supplemental Figure 1). Median time from TAVR to first ACS admission was 297 days (IQ range 85–662) days. Post-TAVR ACS occurred very early (≤7 days) in 562 patients (8.3%), early (7–30 days) in 488 patients (7.2%), delayed (1–6 months) in 1509 patients (22.4%), and late (>6 months) in 4,182 patients (62.0%) (Supplemental Figure 2).
Predictors of ACS within 6 months after TAVR
Important factors associated with occurrence of ACS within 6 months after TAVR included younger age at TAVR (adjusted (aOR) 0.993, 95% CI 0.989–0.998, p=0.008), prior ACS within one year before TAVR (aOR 2.97, 95% CI 2.70–3.28,P<0.0001), history of CAD (aOR 2.05, 95% CI 1.77–2.39, p<0.0001), TAVR-in-TAVR valve (aOR 2.75, 95% CI 1.70–4.47, p<0.0001), prior PCI within 6 months before TAVR (aOR 1.45, 95% CI 1.30–1.63, p=<0.0001), DM (aOR 1.21, 95% 1.12–1.32, p=<0.0001), PAD (aOR 1.24, 95% CI 1.14–1.34, p<0.0001) and post-TAVR acute kidney injury (AKI) (aOR 1.28, 95% CI 1.16–1.42, p<0.0001) (Figure 1).
Figure 1: Predictors of occurence of ACS within 6 months after TAVR.
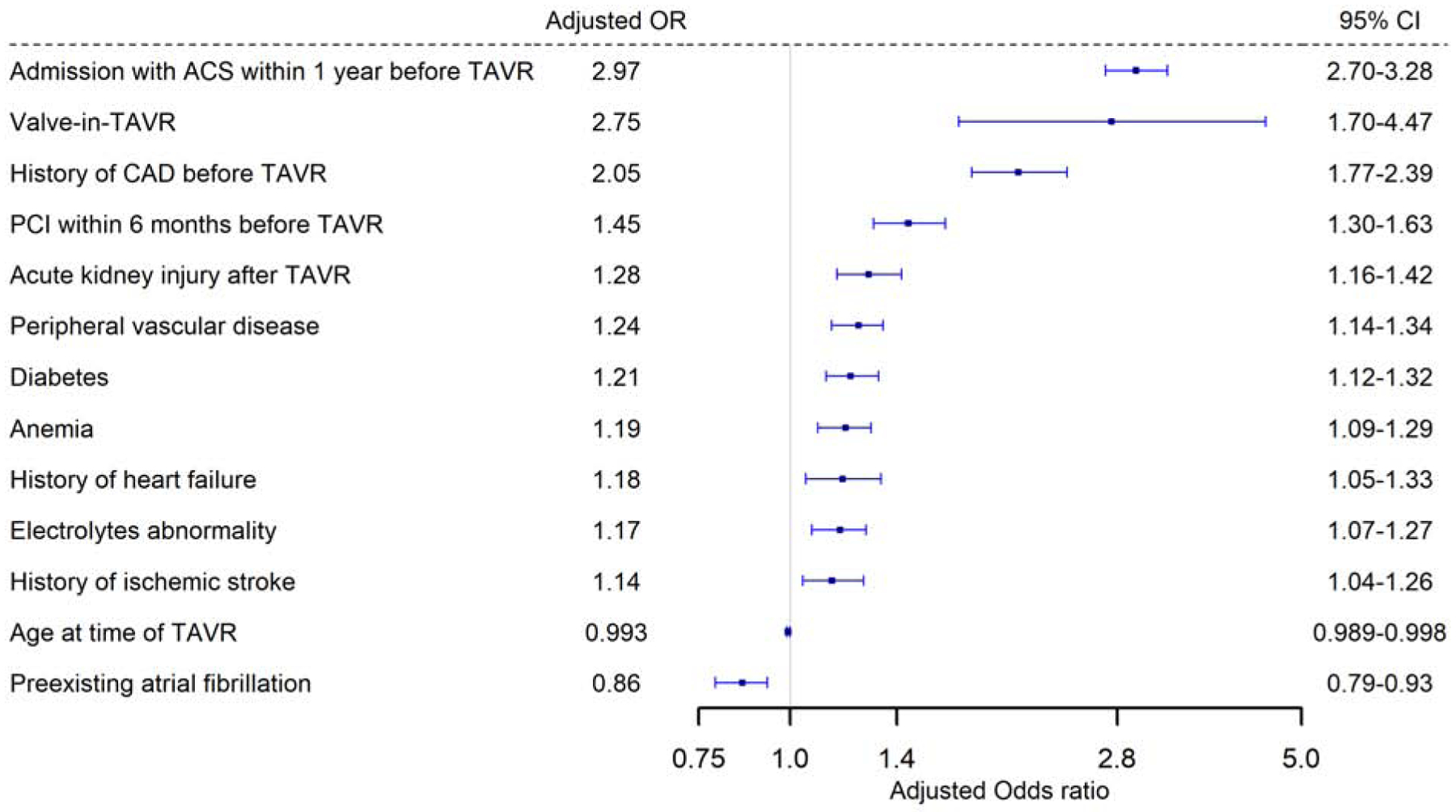
Predictors of occurrence of acute coronary syndrome within 6 months after TAVR on multivariable logistic regression.
ACS= acute coronary syndrome; CAD= coronary artery disease; CI= confidence interval; OR= odds ratio; PCI= percutaneous coronary intervention; TAVR= transcatheter aortic valve replacement
Predictors of invasive management
Overall, 30.3% of the patients were treated with invasive approach (13.9% with coronary angiogram without intervention and 16.6% with PCI). Important predictors of an invasive versus conservative approach included STEMI presentation (aOR 2.41, 95% CI 1.66–3.49, p<0.0001), cardiogenic shock (aOR 2.32, 95% CI 1.78–3.03, p<0.0001), history of prior revascularization (aOR 1.49, 95% CI 1.31–1.69, p<0.0001), and presenting >6 months after TAVR (aOR 1.30, 95% CI 1.15–1.47, p<0.0001) (Figure 2).) In contrary, important predictors that favored conservative approach were older age, female sex, chronic kidney disease, need for blood transfusion, history of coagulopathy or presenting with AKI. Importantly, NSTEMI was not associated with higher rates of invasive approach compared to UA.
Figure 2: Predictors of an invasive compared to a conservative approach in patients with NSTF-ACS after TAVR.
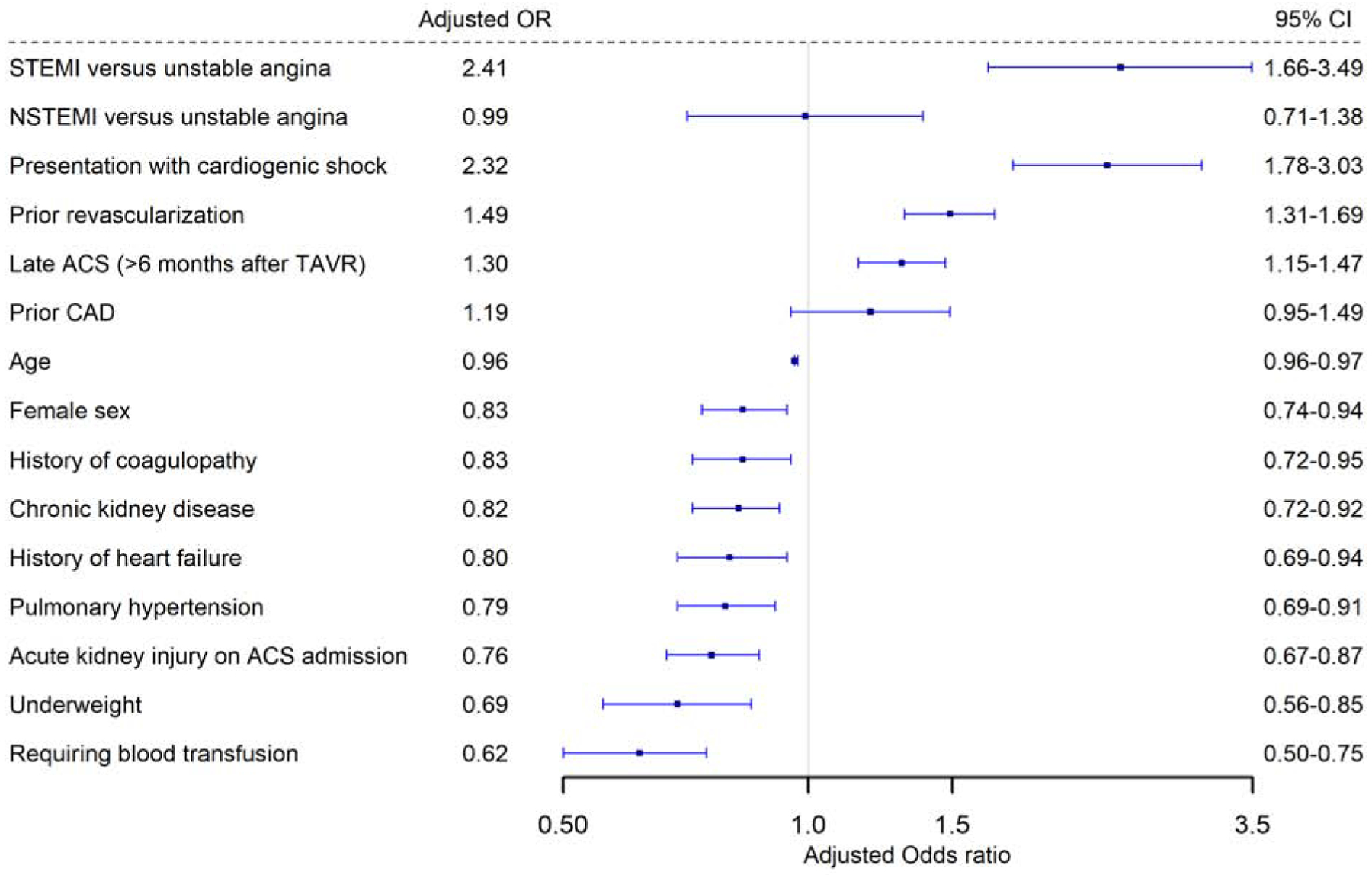
Predictors of an invasive compared to a conservative approach in patients with NSTE-ACS after TAVR.
NSTEMI= Non-ST elevation myocardial infarction; STEMI= ST-elevation myocardial infarction; rest of abbreviations as figure 1.
Outcomes by type of ACS
Table 2 summarizes the baseline characteristics of the three ACS groups. While patients who presented with NSTEMI were relatively younger compared with the other two groups, overall, no significant difference in the prevalence of comorbidities (e.g. DM, hypertension, HF, lung disease, liver disease, or kidney disease) was observed between groups. STEMI patients were also more likely to undergo an invasive approach with half of the patients undergoing coronary angiogram (21.3% had PCI with a drug eluting stent, 6% with bare metal stent, 7% had angioplasty without stenting, 14% had coronary angiogram without intervention and 0.7% had urgent CABG). In patients who underwent intervention, data on number of vessels treated and number of stents placed were available for 99% of the patients. The majority of patients had one stent placed and one vessel treated during the index ACS admission. (Table 3).
Table 2:
Baseline characteristics of patients according to the type of ACS after TAVR
| Unstable angina (n = 208) | NSTEMI (n = 5,947) | STEMI (n = 586) | p value | |
|---|---|---|---|---|
| N | 208 | 5,947 | 586 | |
| Age, yrs | 82.7 ± 7.7 | 80.6 ± 8.8 | 80.9 ± 9.1 | 0.003 |
| Male | 56.7 | 55.7 | 50.5 | 0.05 |
| Other | - | 3.7 | 2.2 | |
| Hypertension | 94.2 | 95.0 | 95.6 | 0.73 |
| Diabetes mellitus | 43.8 | 51.9 | 50.7 | 0.07 |
| Heart failure | 83.2 | 84.1 | 82.9 | 0.74 |
| Deficiency anemia | 46.6 | 50.3 | 51.4 | 0.50 |
| Alcohol abuse | - | 1.7 | 1.9 | 0.37 |
| Connective tissue disease | 5.8 | 7.8 | 8.0 | 0.54 |
| Chronic lung disease | 45.2 | 44.0 | 41.1 | 0.37 |
| Coagulopathy | 20.2 | 24.2 | 23.9 | 0.41 |
| Depression | 21.2 | 17.8 | 15.5 | 0.16 |
| Hypothyroidism | 31.3 | 25.7 | 28.7 | 0.07 |
| Liver disease | - | 3.8 | 5.1 | 0.05 |
| Lymphoma | - | 1.8 | 2.1 | 0.24 |
| Electrolyte abnormality | 47.6 | 52.0 | 52.1 | 0.46 |
| Obesity | 29.8 | 27.0 | 25.9 | 0.56 |
| Paralysis | - | 4.8 | 5.6 | 0.67 |
| Peripheral arterial disease | 47.6 | 46.6 | 44.2 | 0.52 |
| Pulmonary hypertension | 27.4 | 25.7 | 22.0 | 0.12 |
| Tumor without metastasis | 5.8 | 5.3 | 5.5 | 0.96 |
| Weight loss | 6.3 | 9.6 | 9.2 | 0.26 |
| Prior bleeding | 40.4 | 41.7 | 43.3 | 0.68 |
| Prior stroke | 23.6 | 22.5 | 22.9 | 0.92 |
| ESRD | - | 7.2 | 6.5 | 0.10 |
| CKD | 31.7 | 35.0 | 32.6 | 0.32 |
| Prior coronary artery disease | 93.8 | 90.6 | 91.1 | 0.30 |
| Prior smoking | 44.2 | 38.7 | 36.5 | 0.15 |
| Prior revascularization (within 3 yrs before TAVR) | 66.8 | 55.5 | 47.8 | <0.001 |
| Prior ACS (within 1 yr before TAVR) | 18.8 | 22.0 | 18.4 | 0.08 |
| Prior PCI (within 6 months before TAVR) | 14.9 | 14.8 | 15.9 | 0.77 |
| Prior cardiac device | 22.6 | 17.6 | 13.3 | 0.004 |
| Preexisting atrial fibrillation | 42.3 | 40.6 | 41.0 | 0.88 |
| Apical TAVR | 16.8 | 13.0 | 9.6 | 0.01 |
Values are mean ± SD or %.
Cells with N <10 were suppressed with (–).
CKD = chronic kidney disease; ESRD = end-stage renal disease on dialysis; NSTEMI = non–ST-segment elevation myocardial infarction; STEMI = ST-segment elevation myocardial infarction; other abbreviations as in Table 1.
Table 3:
Management and in-hospital outcomes of different types of ACS after TAVR
| Unstable angina (n = 208) | NSTEMI (n = 5,947) | STEMI (n = 586) | p value | |
|---|---|---|---|---|
| N | 208 | 5,947 | 586 | |
| Length of hospital stay,days | 3 (1–4) | 4 (2–7) | 4 (2–8) | <0.0001 |
| Early discharge (≤72 h) | 66.4 | 38.3 | 29.2 | <0.0001 |
| Acute rehabilitation | - | 2.8 | 2.2 | |
| Cardiogenic shock | - | 3.5 | 15.0 | <0.001 |
| Cardiac arrest | - | 2.9 | 13.0 | <0.001 |
| Blood transfusion | 7.7 | 11.2 | 9.9 | 0.19 |
| Acute kidney injury | 8.2 | 33.2 | 32.4 | <0.001 |
| Acute heart failure | - | 34.7 | 25.1 | <0.0001 |
| In-hospital mortality | - | 7.6 | 24.1 | <0.0001 |
| 30-day readmission for recurrent ACS | 4.9 | 12.1 | 28.5 | <0.0001 |
| 30-day need for intervention (after discharge from index ACS admission) | 6.8 | 4.0 | 4.0 | 0.1 |
| 30-day heart failure | - | 4.1 | 5.5 | 0.04 |
| 30-day bleeding | - | 2.5 | - | 0.07 |
| 30-day mortality | - | 15.5 | 31.4 | <0.0001 |
| 1-yr mortality | 26.0 | 41.3 | 51.2 | <0.0001 |
| ACS inpatient management | ||||
| Invasive approach | 30.3 | 28.5 | 48.1 | <0.001 |
| Drug eluting stenting | —13.9 | 11.8 | 21.3 | <0.001 |
| Bare metal stenting | - | 1.4 | 6.0 | <0.001 |
| ≥3 | - | 3.0 | 5.1 | |
| ≥3 | 6.3 | 6.6 | 12.2 | |
| Coronary angiography without intervention | 13.0 | 13.9 | 13.8 | 0.92 |
| Plain old balloon angioplasty alone | - | 1.4 | 7.2 | <0.001 |
| Urgent CABG | 0.0 | 0.5 | - | 0.48 |
| Use of coronary intravascular ultrasound | - | 1.2 | 2.4 | 0.02 |
| Mechanical circulatory support (IABP, ECMO, Impella) | - | 1.4 | 7.2 | <0.001 |
| Nuclear myocardial perfusion imaging testing | 12.5 | 10.4 | 3.8 | <0.001 |
Values are median (interquartile range) or %.
Cells with N <10 were suppressed with (–).
CABG = coronary artery bypass grafting; ECMO = extracorporeal membrane oxygenation; IABP = intra-aortic balloon pump; SNF = skilled nursing facility; other abbreviations as in Tables 1 and 2.
These are proportions out of patients who had PCI with drug-eluting stent or bare-metal stent. Missing data are <1% for both variables.
In-hospital and short-term outcomes
STEMI was associated with significantly higher in-hospital mortality (24.1% compared to 7.6% and 1.4% for NSTEMI and UA, respectively, p<0.001). Also, STEMI patients had higher rates of cardiogenic shock, cardiac arrest, AKI, and need for mechanical circulatory support. NSTEMI patients had the highest incidence of in-patient acute HF (34.7% compared to 25.1% and 4.8% for STEMI and UA, respectively, p<0.001). At 30-days, STEMI was associated with higher mortality (31.4% compared to 15.5% and 4.8% for NSTEMI and UA, respectively, p<0.001). STEMI and NSTEMI both had higher 30-day HF readmission compared to UA (5.5% and 4.4% compared to 1.5%, p=0.04). There were no differences in 30-day bleeding between types of ACS. One-year mortality was highest in STEMI compared to NSTEMI and UA (51.2% versus 41.3% and 26.0% respectively, p <0.01).
Long-term outcomes
Median follow up after ACS was 373 days (IQR 91–676). Long-term mortality remained higher with STEMI compared to NSTE-ACS (58.7 deaths vs 45.8 deaths per 100 person-years in NSTEMI, HR 1.28, 95% CI 1.15–1.43, p<0.0001; and 24.3 deaths per 100 person-years in UA, HR 2.04, 95% CI 1.65–2.51, p<0.0001). NSTEMI also had higher mortality compared to UA (HR 1.59, 95%CI 1.32–1.92, p<0.0001) (Figure 3 and Supplemental Table 2). STEMI was also associated with higher risk of readmission with ACS compared to NSTEMI (HR 1.91, 95% CI 1.63–2.24) and UA (HR 2.89, 95% CI 2.08–4.03) (P<0.001 for both). There was no difference between the three groups in long-term bleeding or HF admissions.
Figure 3: Survival by type of Acute coronary syndrome.
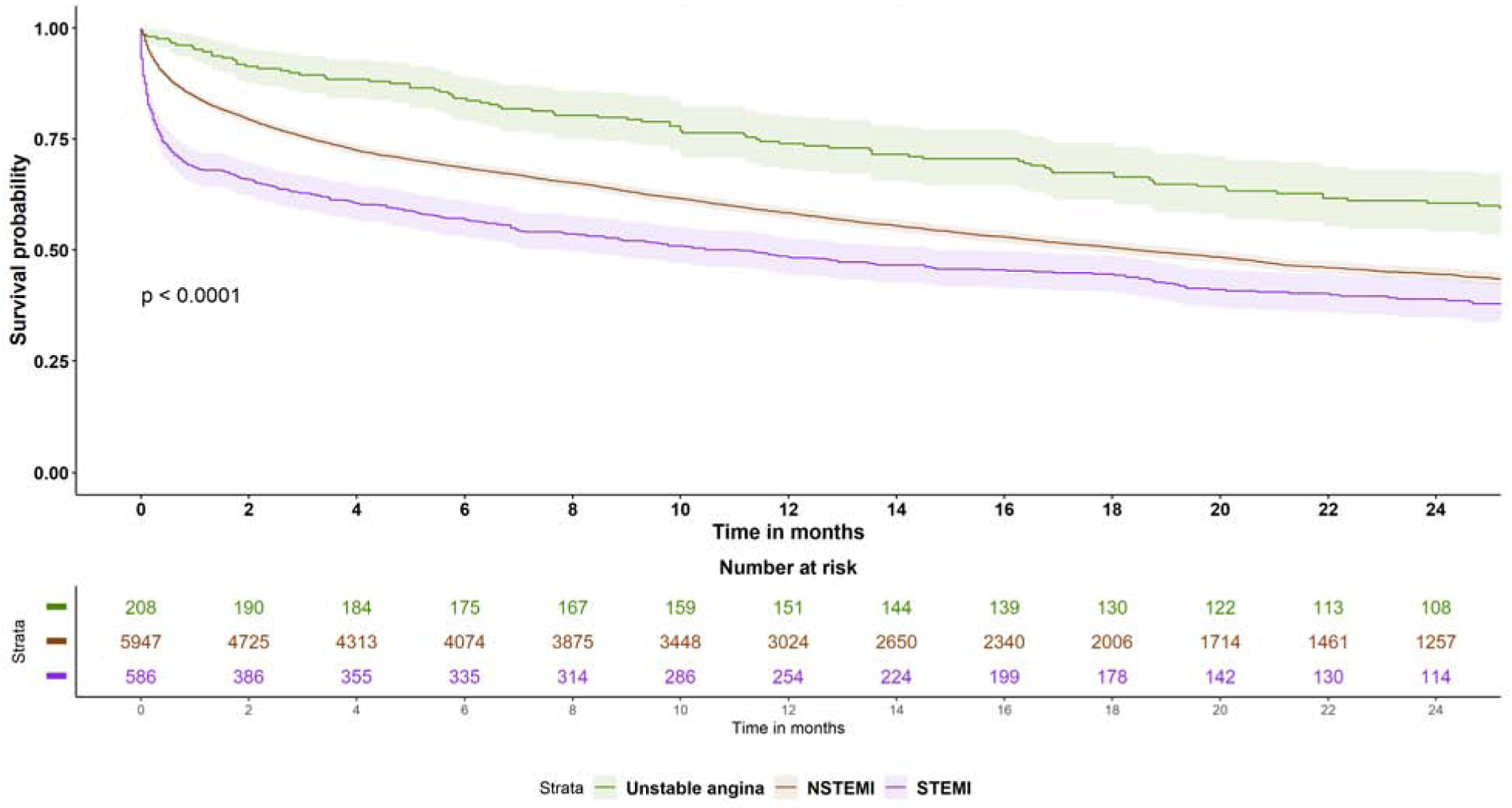
Kaplan Meier curves for survival probability in the three ACS types after TAVR. Abbreviations as figure 2.
Outcomes with invasive versus conservative approach in NSTE-ACS patients
Prior to propensity score matching, invasive approach was associated with lower in-hospital mortality (4.8% versus 8.4%, p<0.0001), 30-day mortality (8.8% versus 17.7%, p<0.0001) and 1-year mortality (51.2% versus 41.3, P<0.001). Invasive approach was also associated with significantly lower long-term mortality, and bleeding readmissions, but a higher risk for repeat revascularization at follow up compared to the conservative approach. There was no difference in the risk of recurrent ACS admissions or heart failure admissions between both management approaches (Supplemental figures 3A–E).
Propensity-matching and Inverse-probability weighting analyses
After propensity-matching, there were 1750 matched pairs managed with invasive versus conservative approach. The two groups were well balanced in all variables (Supplemental Table 3). Invasive approach remained associated with significantly lower in-hospital, 30-days and 1-year mortality (4.8% versus 8.4%, aOR 0.54, 95% CI 0.40–0.72, 8.8% versus 16.9%, aOR 0.47, 95% CI 0.38–0.58, and 29.6% versus 43.8%, aOR 0.52, 95% CI 0.47–0.63, p<0.0001 for all). On IPW-adjusted analysis, Invasive management was also associated with significantly lower long-term mortality (aHR 0.69, 95% CI 0.66–0.73, p<0.0001), lower risk for heart failure (aHR 0.90, 95% CI 0.81–0.99, P=0.03) and bleeding (aHR 0.88, 95% CI 0.78–0.98, P=0.03). There was no difference in long-term risk for recurrent ACS admissions between both groups (aHR 1.00, 95% CI 0.93–1.09, p=0.87. Invasive approach was associated with higher risk of need for revascularization at follow up (aHR 1.29, 95% CI 1.16–1.43, p<0.001). (Figures 4A–D).
Figure 4: IPW Adjusted Kaplan-Meier Curves.
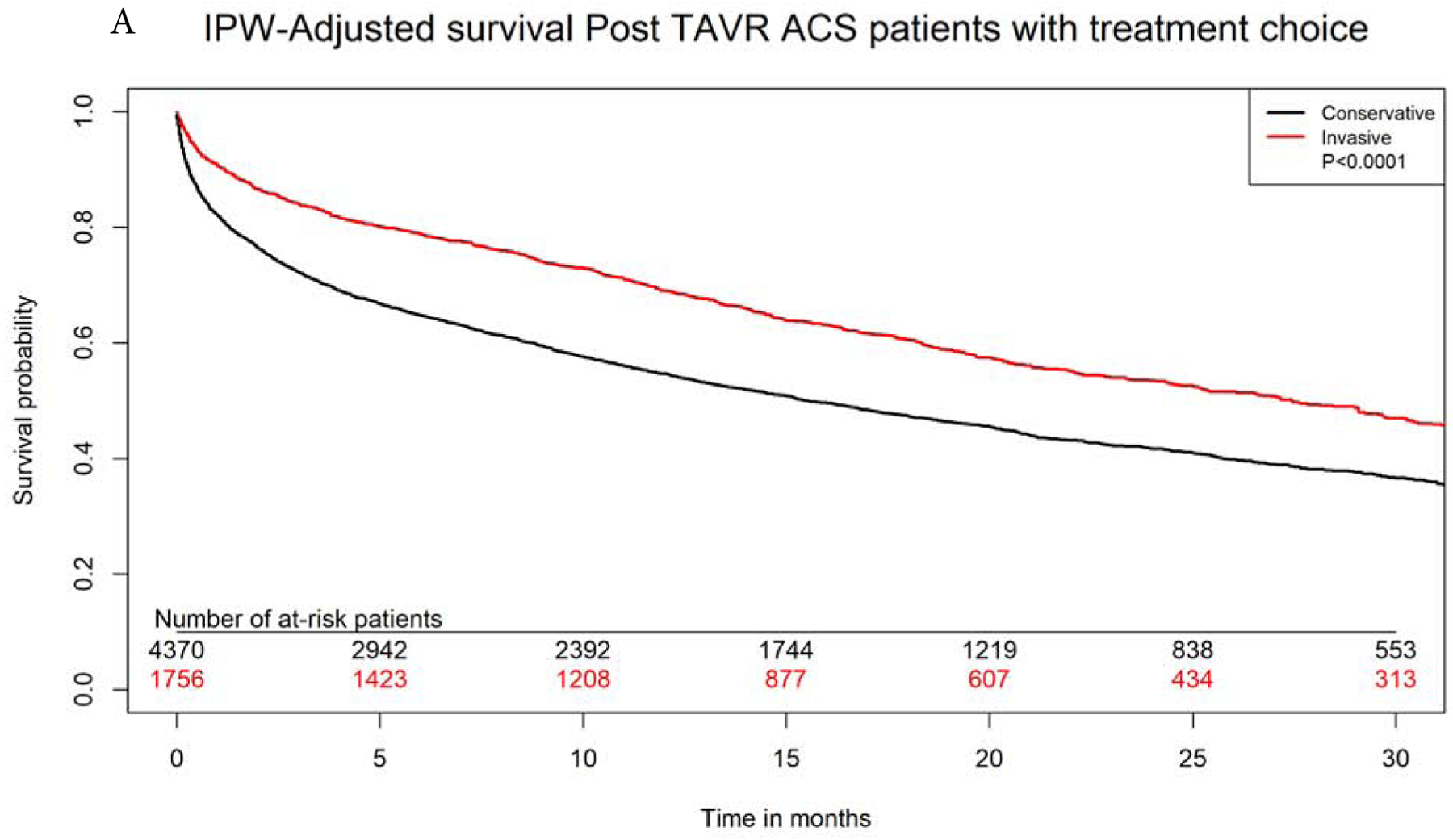
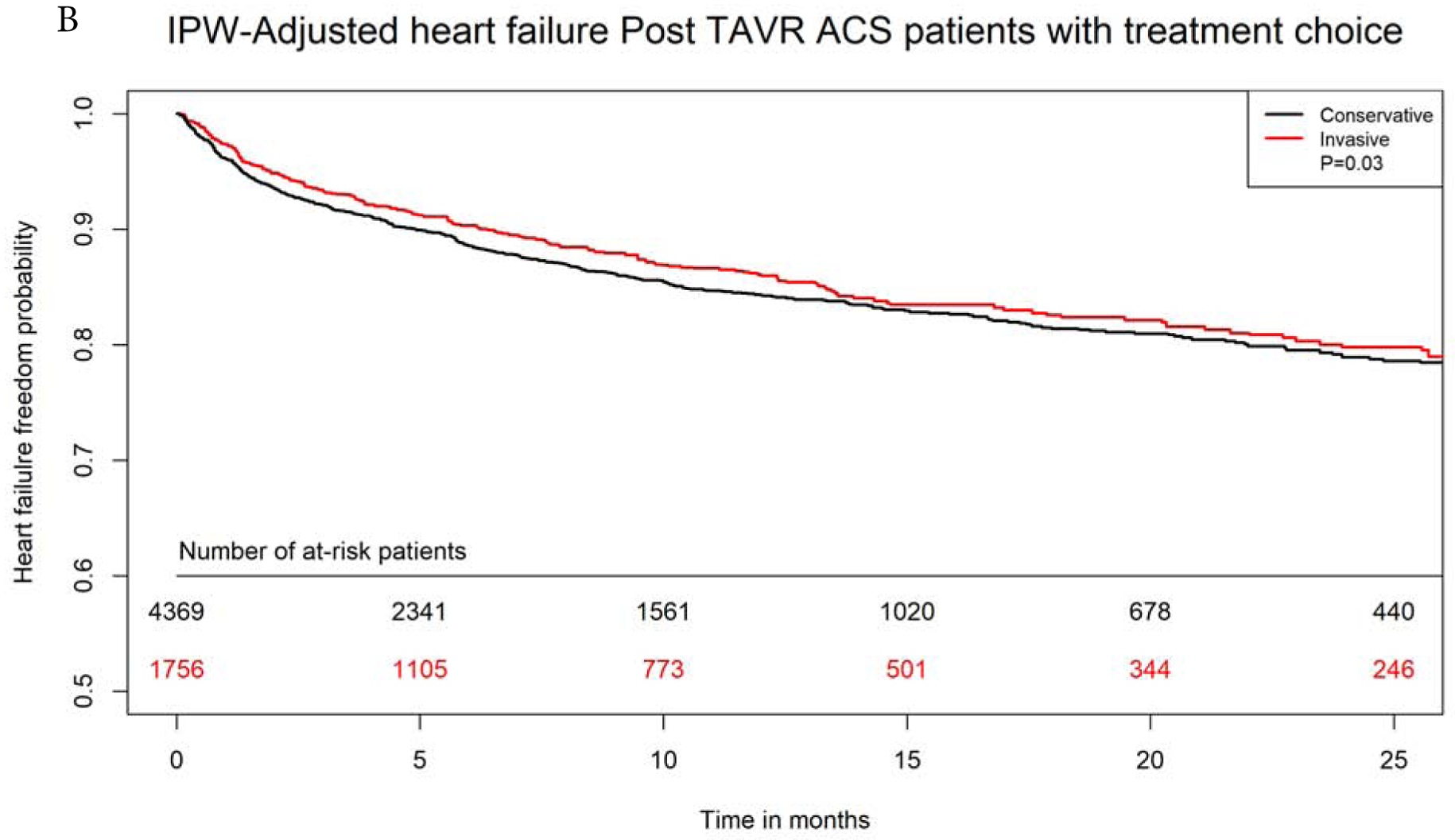
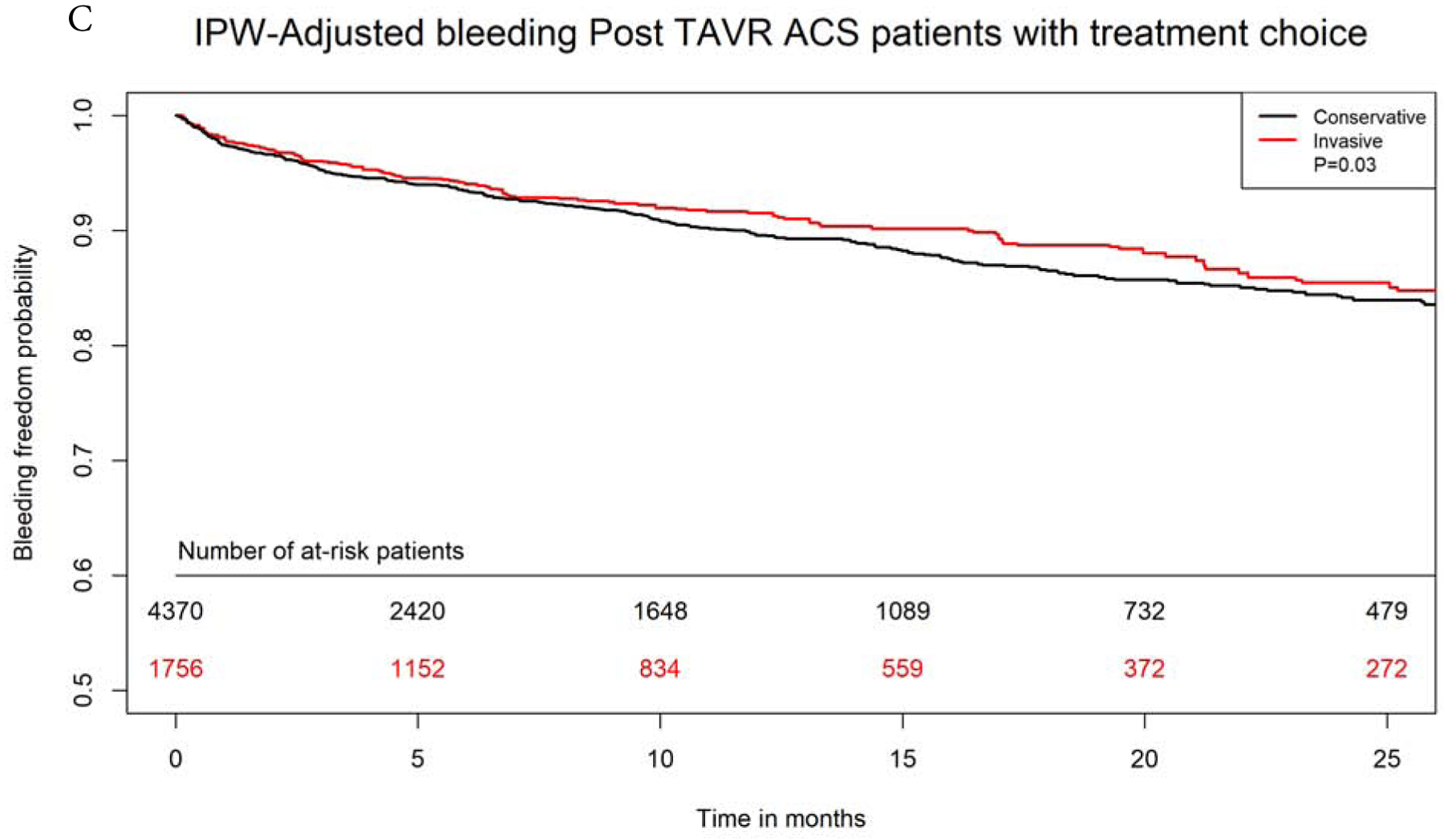
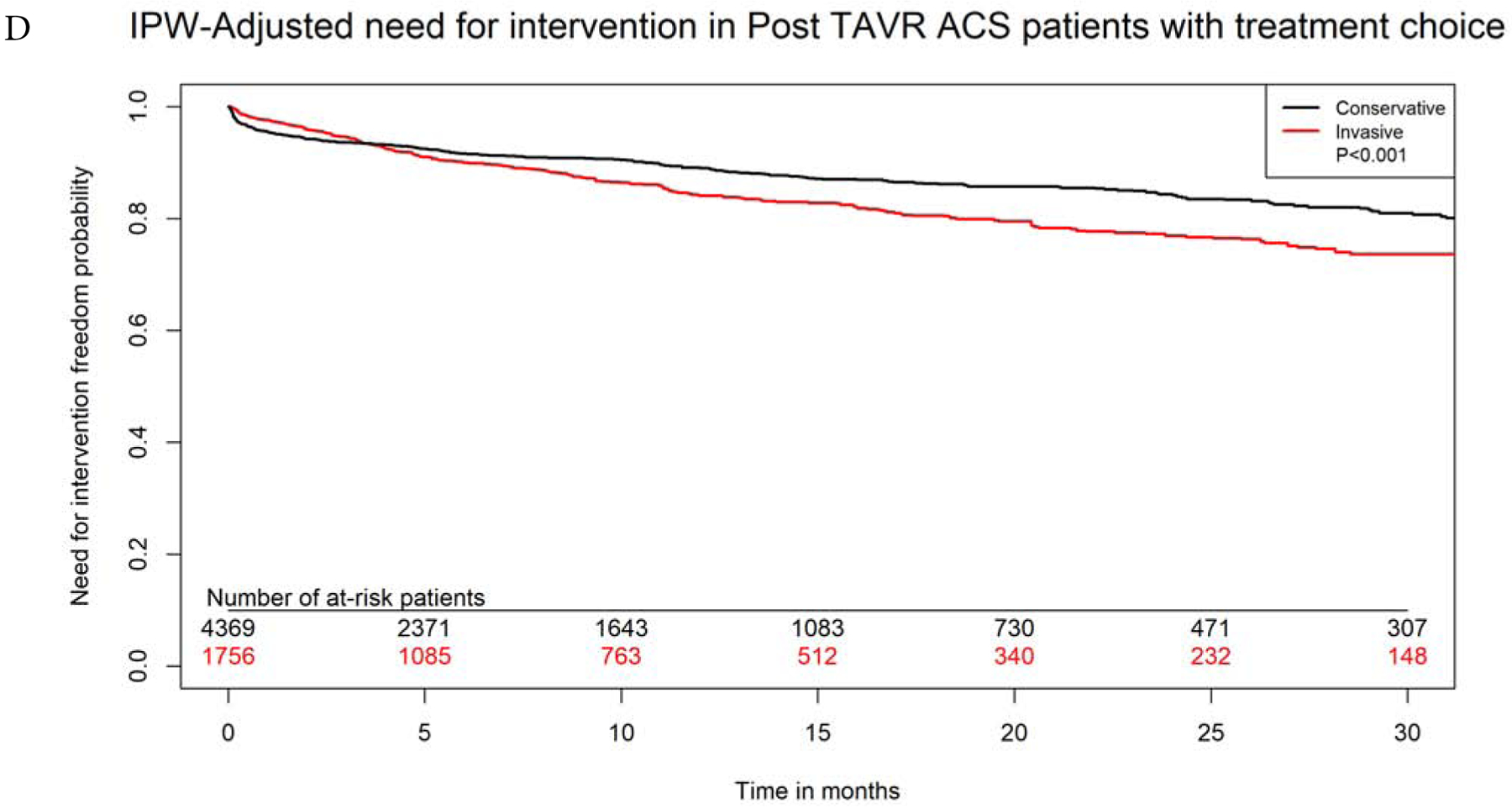
Inverse probability weighting adjusted Kaplan Meier curves of A) survival, B) heart failure, C) bleeding, and D) need for revascularization with invasive versus conservative approach in patients with NSTE-ACS after TAVR
IPW=inverse probability weighting; rest of abbreviations as figure 1.
Central illustration: Panel A: Distribution of types of myocardial infarction by timing of presentation Panel B: Predictors of occurrence of acute coronary syndrome within 6 months after TAVR on multivariable logistic regression. Panel C: Kaplan Meier curves for survival probability in the three ACS types after TAVR. Panel D: Predictors of an invasive compared to a conservative approach in patients with NSTE-ACS after TAVR
DISCUSSION
In this study from Medicare population, we report several important findings. First, the incidence of ACS after TAVR was 4.7%, and the majority of ACS admissions were due to NSTEMI. Second, 48% of ACS admissions occurred within 6 months after TAVR and were associated with prior history of CAD, prior revascularization within 6 months before TAVR, diabetes, post-TAVR AKI and valve-in-TAVR procedures. Third, STEMI was associated with the highest rates of short- and long-term mortality followed by NSTEMI compared to UA with one half of the patients dying within one year. Fourth, about one-third of post-TAVR ACS patients were managed with an invasive approach, especially when presenting with STEMI, cardiogenic shock, late (>6 months) after TAVR, or had prior revascularization. Last, this approach was associated with significantly lower long-term mortality when compared to conservative management (Central Illustration).
There is scarce literature about the true incidence of ACS in long-term follow-up after TAVR in real world. Plausible mechanisms of ACS after TAVR include plaque rupture with intra-coronary thrombus formation, valve thrombosis with embolization into the coronaries, and mechanical obstruction of coronary ostia with the valve structure. In our study, over a median of one year, 4.7% of TAVR patients developed ACS. In a prior single center study, the incidence was higher (10%) (7). This can be explained by its less contemporary cohort, and likely higher risk TAVR population from as early as 2007, its longer follow up compared to our study, and the inclusion of patients with type II MI. In our study, we divided ACS admissions into earlier than or after 6 months post TAVR. ACS occurring earlier than 6 months could be probably related to the TAVR procedure. In one study, delayed coronary obstruction occurring in the first 7 days after the procedure was most probably due to continued expansion of the valve, while obstruction that occurred after 7 days was most likely related to valve thrombosis (6). In that study, 40% of cases of coronary obstruction related to the valve occurred more than two months after the procedure (6). In another study by Vilalta and his colleagues, half of ACS admissions happened in the first year following TAVR. This is consistent with our study where 40% of ACS admissions occurred in the first 6 months. It appears that NSTEMI is the most common type of ACS following TAVR, representing 65% of the cases in the prior study and 85% of the cases in our study (7).
It is crucial to study factors associated with occurrence of ACS after TAVR given the morbidity and mortality associated with ACS. In our study, younger age at TAVR, history of CAD and occurrence of AKI after TAVR were all significantly associated with ACS after multivariable analysis. However, it is interesting that valve-in-TAVR valve was associated with a significantly higher risk of ACS. Valve-in-valve procedures were shown in the past to be associated with delayed coronary obstruction after TAVR in one study (6), and this could be due to high risk of thrombosis or due to crowding of the aortic root with two valve structures. In our study, we were not able to study outcomes with valve-in-surgical valves, because of the limitation of ICD codes in specifying location of prior prosthetic valves (aortic vs mitral) when the surgery was remote.
Some of the factors that were associated with a higher likelihood of an PCI in managing NSTE-ACS in the current study are consistent with the ACC/AHA guidelines in NSTE-ACS in general population, such as hemodynamic instability, ST-segment elevation on presentation, and history of revascularization (20). Furthermore, patients with chronic kidney disease or AKI on presentation were less likely to be treated invasively probably to avoid further kidney injury with exposure to contrast. Similarly, patients with coagulopathy, liver disease and need for transfusion, are likely to be at a higher risk of bleeding with antithrombotic agents, which could explain the lower rates of invasive approach in this population. Interestingly, similar to prior studies in NSTE-ACS, in our study female sex and older age were both associated with less likelihood of PCI (21,22). How prior TAVR affects decision of PCI in ACS patients remains unclear.
Multiple case reports have raised concerns about technical challenges in performing PCI in patients post-TAVR, including challenges in selective cannulation of the right coronary artery (8,23), retrieval of the guiding catheter due to entrapment within the stent frame (9), and the usual difficult location of the culprit lesion in the ostium of the vessel (24). However, procedural success was reported to be around 70–90% in prior studies (6,7). In our study, PCI in patients who presented with NSTE-ACS was associated with lower mortality compared with conservative approach. This indicates that the known benefit of PCI in NSTE-ACS patients may as well extend to include patients with prior TAVR despite the technical challenges (25,26). However, it is worth mentioning that PCI in our study was associated with an increase in the need for revascularization at follow-up. Two plausible explanations include 1) staged PCI for non-culprit lesions found on coronary angiogram; and 2) the risk of in-stent restenosis or thrombosis with PCI. Among the important potential causes of ACS in TAVR population is leaflet thrombosis and subsequent embolization, and the magnitude, prevention as well as the appropriate management of such complication remain under extensive investigation (27).
Limitations
The current study represents the largest and most comprehensive analysis examining the incidence, outcomes and management of ACS after TAVR. However, our study has several limitations. First, we only had inpatient files available for the analysis, so patients who received coronary interventions on an outpatient basis would not be captured. However, our main interest was ACS and coronary intervention in the acute setting. Second, information on serum biomarkers levels such as troponin and EKG tracings were not available for our study cohort. However, we used ICD-codes algorithms that were validated extensively in prior studies (11,12). Third, to avoid including type II MI patients, we excluded ICD codes (I21.A1, 410.9*) and we restricted our cohort to patients with diagnosis of ACS in the first two positions in the inpatient claim. However, there is a possibility that some of NSTEMI patients in our cohort could still represent type II MI, and outcomes could potentially be different in these two etiologies. Fourth, we lacked information on important medications known to affect ACS outcomes including antiplatelets, anticoagulation and statins. Fifth, we lacked information on surgical risk scores such as STS score, coronary anatomy, culprit lesion, success of PCI, and repeat revascularization site. Last, despite propensity score matching analysis to adjust for measured confounders, residual confounding cannot be entirely excluded.
CONCLUSION
In TAVR patients, ACS after TAVR is infrequent (4.7%), and the most common presentation is NSTEMI. Although incidence of STEMI after TAVR is low (<1%), it is associated with a high mortality with nearly one-third of patients dying within 30-days. ACS is most frequently treated with conservative approach and although invasive approach was associated with better outcomes in our study, the role invasive versus conservative approach requires further investigation.
Supplementary Material
Table 4:
Adjusted short outcomes with invasive compared to conservative approach in NST-ACS patients after propensity score matching.
| Conservative | Invasive | Adjusted OR | 95% CI | P value | |
|---|---|---|---|---|---|
| N=1750 | N=1750 | ||||
| In-hospital mortality | 8.4% | 4.8% | 0.54 | 0.40–0.72 | <0.0001 |
| 30-day readmission for ACS | 12.7% | 10.6% | 0.85 | 0.67–1.06 | 0.10 |
| 30-day need for intervention | 6.1% | 3.2% | 0.50 | 0.34–0.73 | 0.0002 |
| 30- day mortality | 16.9% | 8.8% | 0.47 | 0.38–0.58 | <0.0001 |
| 30-day HF | 4.4% | 3.2% | 0.70 | 0.47–1.03 | 0.07 |
| 30-day bleeding | 2.6% | 2.0% | 0.72 | 0.43–1.20 | 0.22 |
| 1 -year mortality | 43.8% | 29.6% | 0.52 | 0.47–0.63 | <0.0001 |
NST-ACS=Non-ST elevation acute coronary syndrome, OR=Odds ratio, CI=Confidence interval, HF=Heart failure, ACS=Acute coronary syndrome.
Table 5:
Cox regression analysis for long-term outcomes for invasive versus conservative approach in NSTE-ACS patients after inverse probability weigh analysis
| Outcome | HR | 95% CI | P value |
|---|---|---|---|
| Mortality | 0.69 | 0.66–0.73 | <0.0001 |
| Heart failure | 0.90 | 0.81–0.99 | 0.03 |
| Bleeding | 0.88 | 0.78–0.98 | 0.03 |
| Need for intervention | 1.29 | 1.16–1.43 | <0.0001 |
| Admission with ACS | 1.00 | 0.93–1.09 | 0.87 |
NST-ACS=Non-ST elevation acute coronary syndrome, HR=Hazard ratio, CI=Confidence interval, ACS=Acute coronary syndrome
PERSPECTIVES.
WHAT IS KNOWN?
Because of the age and comorbidities of patients who undergo transcatheter aortic valve replacement (TAVR), they are at high risk of coronary artery disease and acute coronary syndromes (ACS).
WHAT IS NEW?
Incidence of ACS after TAVR is low (4.7%) and is most commonly non-ST elevation myocardial infarction (NSTEMI). STEMI is associated with higher short- and mid-term mortality than other ACS types. Invasive approach in post-TAVR patients with ACS is potentially associated with better outcomes.
WHAT IS NEXT?
Future studies are encouraged to examine the appropriate preventive and therapeutic approaches to lower the risk and improve the outcomes of post-TAVR ACS in this high-risk population.
Funding:
Dr. Mentias received support from National Institute of Health NRSA institutional grant (T32 HL007121) to the Abboud Cardiovascular Research Center. Dr. Sarrazin is supported by funding from the National Institute of Aging (NIA R01AG055663-01), and by the Health Services Research and Development Service (HSR&D) of the Department of Veterans Affairs.
Disclosures:
Dr. Horwitz receives grant support from Edwards Lifesciences and Boston Scientific.
The remaining authors do not have any conflicts of interest or financial disclosures.
Dr. Sorajja receives grant support from Edwards Lifesciences, Boston Scientific, Medtronic, Abbott Structural; consulting fees from Edwards Lifesciences, Boston Scientific, Medtronic, Abbott Structural, W.L. Gore, Admedus, Cardionomics.
ABBREVIATIONS AND ACRONYMS
- ACS
Acute coronary syndrome
- AKI
Acute kidney injury
- CAD
coronary artery disease
- HF
Heart failure
- NST-ACS
Non-ST elevation acute coronary syndrome
- NSTEMI
Non-ST elevation myocardial infarction
- PCI
percutaneous coronary intervention
- STEMI
ST-elevation myocardial infarction
- TAVR
Transcatheter aortic valve replacement
- UA
unstable angina
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Popma JJ, Deeb GM, Yakubov SJ et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N Engl J Med 2019;380:1706–1715. [DOI] [PubMed] [Google Scholar]
- 2.Mack MJ, Leon MB, Thourani VH et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med 2019;380:1695–1705. [DOI] [PubMed] [Google Scholar]
- 3.Blackman DJ, Saraf S, MacCarthy PA et al. Long-Term Durability of Transcatheter Aortic Valve Prostheses. J Am Coll Cardiol 2019;73:537–545. [DOI] [PubMed] [Google Scholar]
- 4.Montilla Padilla I, Martin-Asenjo R, Bueno H. Management of Acute Coronary Syndromes in Geriatric Patients. Heart Lung Circ 2017;26:107–113. [DOI] [PubMed] [Google Scholar]
- 5.Goel SS, Ige M, Tuzcu EM et al. Severe aortic stenosis and coronary artery disease--implications for management in the transcatheter aortic valve replacement era: a comprehensive review. J Am Coll Cardiol 2013;62:1–10. [DOI] [PubMed] [Google Scholar]
- 6.Jabbour RJ, Tanaka A, Finkelstein A et al. Delayed Coronary Obstruction After Transcatheter Aortic Valve Replacement. J Am Coll Cardiol 2018;71:1513–1524. [DOI] [PubMed] [Google Scholar]
- 7.Vilalta V, Asmarats L, Ferreira-Neto AN et al. Incidence, Clinical Characteristics, and Impact of Acute Coronary Syndrome Following Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv 2018;11:2523–2533. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka A, Jabbour RJ, Testa L et al. Incidence, Technical Safety, and Feasibility of Coronary Angiography and Intervention Following Self-expanding Transcatheter Aortic Valve Replacement. Cardiovasc Revasc Med 2019;20:371–375. [DOI] [PubMed] [Google Scholar]
- 9.Harhash A, Ansari J, Mandel L, Kipperman R. STEMI After TAVR: Procedural Challenge and Catastrophic Outcome. JACC Cardiovasc Interv 2016;9:1412–3. [DOI] [PubMed] [Google Scholar]
- 10.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 11.McCormick N, Lacaille D, Bhole V, Avina-Zubieta JA. Validity of myocardial infarction diagnoses in administrative databases: a systematic review. PLoS One 2014;9:e92286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel AB, Quan H, Welsh RC et al. Validity and utility of ICD-10 administrative health data for identifying ST- and non-ST-elevation myocardial infarction based on physician chart review. CMAJ Open 2015;3:E413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleiss JL, Levin B, Paik MC. Statistical methods for rates and proportions: John Wiley & Sons, 2013. [Google Scholar]
- 14.Cummings P, McKnight B, Greenland S. Matched cohort methods for injury research. Epidemiol Rev 2003;25:43–50. [DOI] [PubMed] [Google Scholar]
- 15.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American statistical association 1999;94:496–509. [Google Scholar]
- 16.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015;34:3661–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee EW, Wei L, Amato DA, Leurgans S. Cox-type regression analysis for large numbers of small groups of correlated failure time observations Survival analysis: state of the art: Springer, 1992:237–247. [Google Scholar]
- 18.Cole SR, Hernan MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed 2004;75:45–9. [DOI] [PubMed] [Google Scholar]
- 19.Xie J, Liu C. Adjusted Kaplan-Meier estimator and log-rank test with inverse probability of treatment weighting for survival data. Stat Med 2005;24:3089–110. [DOI] [PubMed] [Google Scholar]
- 20.Amsterdam EA, Wenger NK, Brindis RG et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;64:e139–e228. [DOI] [PubMed] [Google Scholar]
- 21.Alfredsson J, Stenestrand U, Wallentin L, Swahn E. Gender differences in management and outcome in non-ST-elevation acute coronary syndrome. Heart 2007;93:1357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinclair H, Kunadian V. Coronary revascularisation in older patients with non-ST elevation acute coronary syndromes. Heart 2016;102:416–24. [DOI] [PubMed] [Google Scholar]
- 23.Broyd CJ, Mullen M, Shiu MF. Late Presentation of a Semicomplete Occluded Right Coronary Artery by a Direct Flow Valve Preventing Interventional Therapy for ST-Segment Elevation Myocardial Infarction. JACC Cardiovasc Interv 2016;9:e149–51. [DOI] [PubMed] [Google Scholar]
- 24.Li YJ, Liao YB, Wei X, Feng Y, Chen M. Acute Myocardial Infarction as the Initial Manifestation of Delayed Bioprosthesis Thrombosis After Transcatheter Aortic Valve Replacement. Heart Lung Circ 2018;27:e46–e50. [DOI] [PubMed] [Google Scholar]
- 25.Puymirat E, Taldir G, Aissaoui N et al. Use of invasive strategy in non-ST-segment elevation myocardial infarction is a major determinant of improved long-term survival: FAST-MI (French Registry of Acute Coronary Syndrome). JACC Cardiovasc Interv 2012;5:893–902. [DOI] [PubMed] [Google Scholar]
- 26.Fox KA, Poole-Wilson P, Clayton TC et al. 5-year outcome of an interventional strategy in non-ST-elevation acute coronary syndrome: the British Heart Foundation RITA 3 randomised trial. Lancet 2005;366:914–20. [DOI] [PubMed] [Google Scholar]
- 27.Makkar RR, Fontana G, Jilaihawi H et al. Possible Subclinical Leaflet Thrombosis in Bioprosthetic Aortic Valves. N Engl J Med 2015;373:2015–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


