Abstract
At the core of human thought, for the majority of individuals in the developed nations at least, there is the tacit assumption that as a species we are unfettered by the demands imposed by our biology and that we can do what we want, at whatever time we choose, whereas in reality every aspect of our physiology and behaviour is constrained by a 24 h beat arising from deep within our evolution. Our daily circadian rhythms and sleep/wake cycle allow us to function optimally in a dynamic world, adjusting our biology to the demands imposed by the day/night cycle. The themes developed in this review focus upon the growing realization that we ignore the circadian and sleep systems at our peril, and this paper considers the mechanisms that generate and regulate circadian and sleep systems; what happens mechanistically when these systems collapse as a result of societal pressures and disease; how sleep disruption and stress are linked; why sleep disruption and mental illness invariably occur together; and how individuals and employers can attempt to mitigate some of the problems associated with working against our internal temporal biology. While some of the health costs of sleep disruption can be reduced, in the short-term at least, there will always be significant negative consequences associated with shift work and sleep loss. With this in mind, society needs to address this issue and decide when the consequences of sleep disruption are justified in the workplace.
Keywords: CBTi, circadian, health, insomnia, sleep, stress
Thus passes the day for the virtuous man. And when night comes, I take care not to summon sleep! He, the lord of the virtues, does not care to be summoned!
—Friedrich Nietzsche, Thus Spoke Zarathustra
1. Introduction and mammalian circadian rhythms
Almost all life on earth uses an internal biological clock to anticipate the profound changes that result from the Earth's rotation upon its axis. In organisms as varied as photosynthetic bacteria and humans, physiology and behaviour are ‘fine-tuned’ to the varied, yet predictable, demands of the day/night cycle. Creatures effectively ‘know’ the time of day, and these internally generated daily cycles are called ‘circadian rhythms’, which comes from the Latin circa (about) and dies (day) [1]. In addition to the alignment of the internal and external day, a circadian clock also ensures that biological processes occur in the appropriate temporal sequence. For cells to function properly they need the right materials in the right place at the right time. Thousands of genes have to be switched on and off in a specific order. Proteins, enzymes, fats, carbohydrates, hormones, nucleic acids and other compounds have to be absorbed, broken down, metabolized and produced in a precise time window. Energy has to be obtained, and then partitioned across the cellular economy and allocated to growth, reproduction, metabolism, locomotion and cellular repair. Without this internal temporal compartmentalization, our biology would be profoundly compromised [1].
Circadian rhythms must also be synchronized or entrained to the external environment using signals that provide time of day information (zeitgebers), and the patterns of light produced by the Earth's 24 h rotation provide the dominant entrainment cue. However, in many species, other environmental zeitgebers such as temperature, food availability, rainfall and even predation can contribute to entrainment. The key point is that circadian rhythms are not driven by an external cycle but are generated internally, and then synchronized to the external 24 h world [2].
Relating this to our own species, human physiology is organized around the daily cycle of activity and sleep. In the active phase, when energy expenditure is higher and food and water are consumed, organs need to be prepared for the intake, processing and uptake of nutrients. The activity of organs such as the stomach, liver, small intestine, pancreas and the blood supply to these organs need internal synchronization, which a clock can provide. During sleep, although energy expenditure and digestive processes decrease, many essential activities occur including cellular repair, toxin clearance, and memory consolidation and information processing by the brain. Disrupting this pattern, as happens with jet lag or shift work (see below), leads to internal desynchrony and the failure to do the right thing at the right time [3].
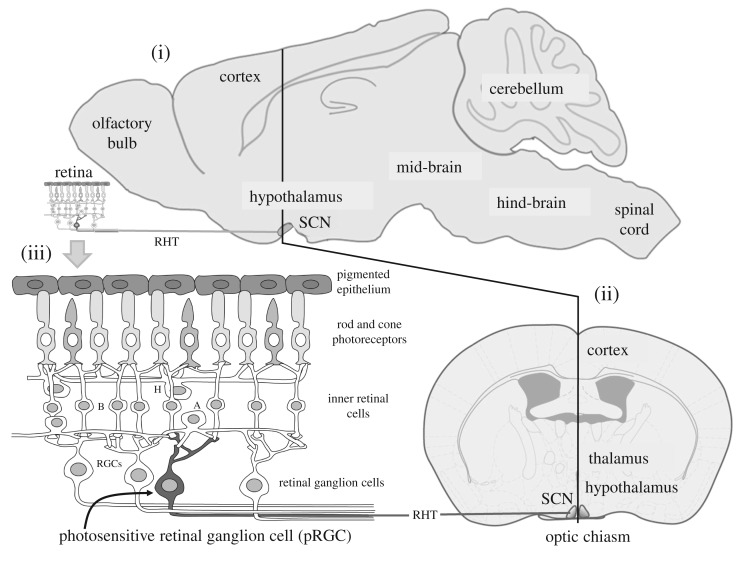
At the heart of the circadian system of mammals is a structure located deep within the brains hypothalamus called the ‘suprachiasmatic nuclei’ or SCN (figure 1). The discovery of this structure has a fascinating history. Experiments in the 1950s and 1960s lesioned/destroyed small parts of the rat brain in the hunt for ‘the clock’, and narrowed it down to somewhere deep in the brain, probably the hypothalamus. Then in a series of experiments in the early 1970s, and based upon the logic that circadian rhythms are entrained by the light/dark cycle, structures within the hypothalamus were identified that received a direct projection from the eye. The SCN receives a major projection from the retina, and when the SCN was lesioned circadian rhythms were abolished [4,5]. Almost 20 years later the critical role of the SCN was confirmed by transplanting small neural grafts from the SCN region of a mutant hamster with a short circadian period of 20 h into non-mutant hamsters whose own SCN had been destroyed and 24 h rhythms were abolished. The transplant not only restored circadian rhythms, but critically, the restored rhythms were 20 h, showing that an essential component of the clock—its period, had been transplanted with the SCN [6].
Figure 1.
The mammalian suprachiasmatic nuclei (SCN) and retina. (i) The mouse brain from the side showing the suprachiasmatic nuclei (SCN) which contains the master circadian pacemaker of mammals. The SCN receive a dedicated projection from the retina called the retino-hypothalamic tract (RHT); (ii) the frontal view of the brain shows the small-paired SCN which are located either side of the third ventricle and sit on top of the optic chiasm (where the optic nerves combine). In mice, the SCN comprises approximately 20 000 neurons, and in humans 50 000 neurons. See text for details. (iii) Retinal rods and cones convey visual information to the retinal ganglion cells (RGCs) via the second order neurons of the inner retina—the bipolar (B), horizontal (H) and amacrine (A) neurons. The optic nerve is formed from the axons of all the ganglion cells and this large nerve takes light information to the brain. A subset of photosensitive retinal ganglion cells (pRGC—shown in dark grey) can also detect light directly. The pRGCs use the blue light-sensitive photopigment, melanopsin or OPN4. Thus photodetection in the retina occurs in three types of cell: the rods, cones and pRGCs. The pRGCs also receive signals from the rods and cones, and, although not required, they can help drive light responses by the pRGCs.
The SCN of humans comprises about 50 000 cellular circadian oscillators sufficiently stable to generate circadian rhythms of neuronal firing for at least six weeks in vitro. This was first shown in dispersed SCN neurons from neonatal rats, placed into the culture on a grid of microelectrodes. Individual neurons displayed robust circadian rhythms in electrical firing, but the phases of these individual rhythms were all different, showing that SCN neurons act as individual clocks and that the basic oscillation lay within individual cells, and was not the emergent property of a network of individual neurons [7].
The subcellular molecular generation of a circadian oscillation arises from a complex interaction between key clock genes and their protein products. At its core, the molecular clockwork comprises a transcriptional/translational feedback loop (TTFL), whereby genes and their protein products interact and feedback to inhibit their own transcription, generating a 24 h cycle of protein production and degradation. The key elements of this molecular clockwork are illustrated in figure 2. For further details, see [10].
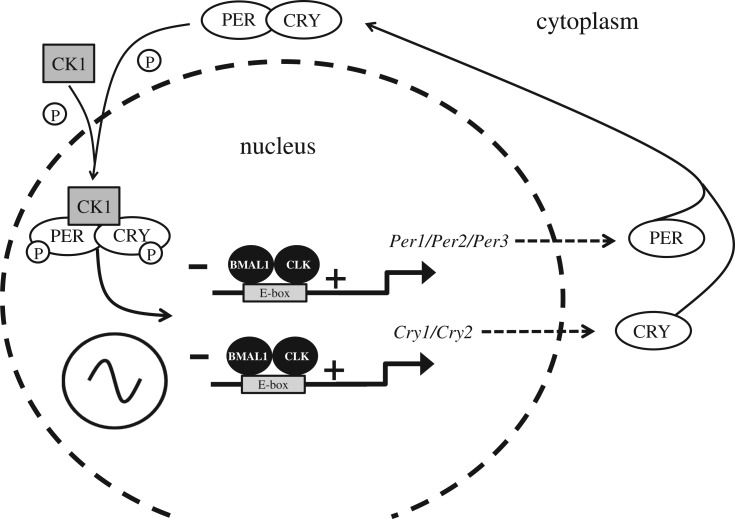
Figure 2.
The mammalian molecular clock. The driving force of the mammalian molecular clockwork is transcriptional/translational feedback loop (TTFL) the transcriptional drive is provided by two proteins named ‘Circadian Locomotor Output Cycles Kaput’, or less torturously CLOCK (CLK), which links with ‘Brain muscle arnt-like 1’ or BMAL1. The CLK–BMAL1 complex binds to E-box promoters driving transcription of five core clock genes, three Period genes (Per) giving rise to the proteins PER1, PER2 and PER3, and two Cryptochrome genes (Cry) which encode the CRY1 and CRY2 proteins. The PER proteins combine with the kinase CK1 (Casein kinase 1) and are phosphorylated. The PER–CK1 complex then binds to the CRYs to form a CRY–PER–CK1 complex. Within the complex of CRY–PER–CK1, CRY and PER are phosphorylated by other kinases which then allows the CRY–PER–CK1 complex to move into the nucleus and inhibit CLK–BMAL1 transcription of the Per and Cry genes forming the negative limb of the TTFL. The CRY–PER–CK1 protein complex levels rise throughout the day, peak at dusk, are then degraded and decline to their lowest level the following dawn. The net result is a TTFL, whereby the Per and Cry genes and their protein products interact and feedback to inhibit their own transcription, generating a 24 h cycle of protein production and degradation. Note, multiple other genes and their proteins, generate additional feedback loops to provide further stability to the circadian oscillation [8]. Significantly, polymorphisms in several of these clock genes have been associated with human ‘morning types’ (larks) and ‘evening types’ (owls) [9].
The SCN projects directly to approximately 35 brain regions, mostly located within the hypothalamus, and particularly those regions of the hypothalamus that regulate hormone release. Indeed, many hormones under pituitary control, like cortisol, are under tight circadian regulation [11,12]. Furthermore, the SCN regulates the activity of the autonomic nervous system, which acts to time-stamp many aspects of physiology, including the sensitivity of target tissues to hormonal signals [13]. In addition to these direct neuronal connections, the SCN communicates to the rest of the body using diffusible chemical signals [14]. This was first shown by transplanting SCN contained within tiny semi-permeable capsules into SCN-lesioned animals. The capsule prevented neural connections being re-established but allowed chemical communication from the transplanted SCN to diffuse out. Even without a neural connection, some circadian rhythms were restored [15]. In more recent years the identity of these chemical signals has begun to emerge [16].
Although the SCN is the ‘master clock’ in mammals, it is not the only clock [17]. There are cellular clocks, using essentially the same subcellular mechanisms (figure 2), within the liver, muscles, pancreas, adipose tissue and probably in every organ and tissue of the body [18]. Destruction of the SCN abolishes multiple rhythms, such as locomotor activity, and this was the reason that the SCN was considered to ‘drive’ 24 h rhythmicity. However, it is now appreciated that the loss of overt rhythmicity occurs because (i) some of the individual peripheral clock cells dampen, and lose rhythmicity after several cycles; but more commonly, because (ii) the individual cellular clocks become uncoupled from each other. The cells continue to tick, but at different phases so that an overt 24 h rhythm within the tissue or organ is lost [19]. This discovery led to the appreciation that the SCN acts as a pacemaker to coordinate, but not drive, the circadian activity of billions of individual peripheral circadian oscillators throughout the tissues and organs of the body. The signalling pathways used by the SCN to entrain these peripheral clocks are still uncertain, but we know that the SCN does not send out countless separate signals around the body targeted at specific individual clocks. Rather, there seems to be a limited number of neuronal and humoral signals. The SCN also receives feedback signals from the periphery that allows the whole body to function in synchrony with the varying demands of the 24 h light/dark cycle [19]. The result is a complex circadian network that coordinates rhythmic physiology and behaviour.
2. Shedding light on the clock
Eye loss in all groups of mammals abolishes the capacity to entrain circadian rhythms to the light/dark cycle [20]. However, astonishingly, the visual cells of the retina, the rods and cones (figure 1), are not required for the detection of the dawn/dusk signal. There exists a third class of photoreceptor within the eye [21,22]. Our studies in the 1990s showed that mice lacking all rod and cone photoreceptors (rdta/cl and rd/rd cl) could still regulate their circadian rhythms to light perfectly normally. But when the eyes were removed the ability to entrain was lost [21,22]. These experiments showed that there had to be another photoreceptor within the eye. The rodless/coneless mouse models provided a powerful approach to characterize this third photoreceptor [23], and along with studies in the rat [24] and monkey [25], the retina was shown to contain a small population (around 1–2%) of photosensitive retinal ganglion cells (pRGCs) that use a blue light-sensitive photopigment called ‘melanopsin’ or OPN4. The OPN4 gene was originally isolated from the light-sensitive pigment cells or ‘melanophores’ found in the skin of amphibians, including frogs and toads [26]. The name ‘melanopsin’ has stuck, and is often confused with ‘melatonin’ but the two molecules are entirely unrelated. Genetic ablation of the rods, cones and melanopsin-pRGCs eliminates circadian responses to light, demonstrating that there are no additional photoreceptors that contribute to circadian entrainment either in the eye or elsewhere. However, although the rods and cones are not required for circadian entrainment, they are now known to contribute to the light responses of the melanopsin pRGCs under certain circumstances. Genetic silencing of melanopsin in the pRGCs does not block photoentrainment in mice. Mice can still entrain but with reduced sensitivities [27–30]. Rods and cones send indirect projections to the pRGCs, and it seems that in the absence of endogenous OPN4, the rods and cones can partially compensate for the loss of OPN4. A complex pattern is emerging of how the different photoreceptor populations interact to bring about entrainment [31–33].
In collaboration with colleagues at Harvard University, we studied humans who had lost all of their rods and cones as a result of genetic disease. Just like the rodless/coneless mice, human circadian entrainment was found to be intact, mediated by pRGCs using the photopigment melanopsin maximally sensitive to blue light around 480 nm [34]. This finding is having a significant impact in the clinic [35,36]. For example, genetic diseases that result in the loss of the rods and cones and cause visual blindness, often spare the pRGCs. Under these circumstances, individuals who have their eyes but are visually blind, yet possess pRGCS, should be advised to expose their eyes to sufficient morning and evening light to entrain their circadian system. The realization that the eye provides us with both our sense of space and our sense of time, via entrainment of the SCN, is redefining the definition and treatment of human blindness.
3. Biology of sleep
The regular cycle of sleep and wakefulness is the most obvious 24 h pattern in our behaviour, and the sleep/wake cycle involves a highly complex set of interactions involving multiple neural circuits, neurotransmitters and hormones, none of which are exclusive to the generation of sleep [37,38]. The major brain structures and neurotransmitter systems involved in the sleep/wake cycle are summarized in figure 3.
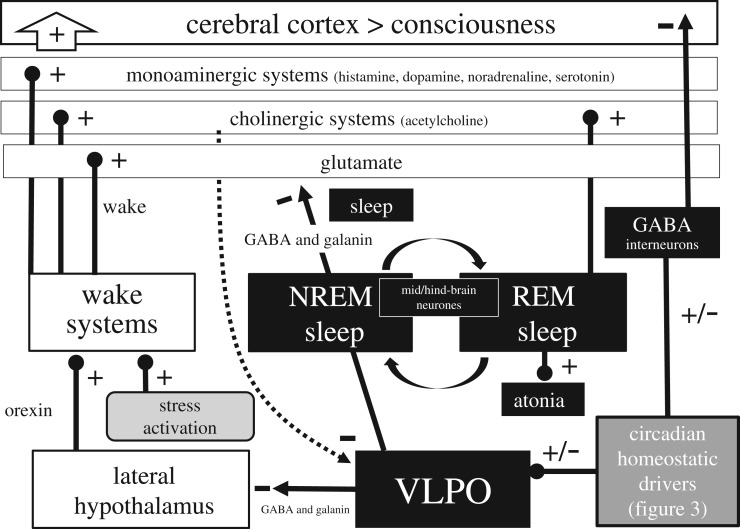
Figure 3.
Sleep/wake states arise from mutually excitatory and inhibitory circuits that result in two distinct behavioural states of wake (consciousness) and sleep. The diagram illustrated here represents a greatly simplified version of the interactions associated with the wake/sleep switch. During wake orexin (also known as hypocretin) neurons in the lateral hypothalamus project to and excite (+) different populations of wake-promoting neurons within the hind- and mid-brain including monoaminergic neurons which release histamine, dopamine, noradrenaline and serotonin; cholinergic neurons in the hind-brain which release acetylcholine; and an important group of broadly distributed neurons that release glutamate. These neurotransmitters drive wakefulness and consciousness within the cortex. In addition, acute activation of the stress axis (figure 5) will also contribute to sleep/wake regulation, acting to promote wake and inhibit sleep. During wake, the monoaminergic neurons project to (dotted line) and inhibit (−) the ventrolateral preoptic nuclei (VLPO). During sleep, circadian and homeostatic sleep drivers (figure 4) activate the VLPO which releases the neurotransmitters gamma-aminobutyric acid (GABA) and galanin to inhibit the orexin neurons in the lateral hypothalamus, and the monoamninergic, cholinergic and glutamatergic neuronal populations (−) directly. Further, a subpopulation of interneurons in the cortex project long distances to the cerebral cortex and release the inhibitory neurotransmitter GABA during sleep. These neurons are activated during sleep in a manner proportional to the homeostatic sleep drive for sleep (figure 4). The primary measures used to define sleep in mammals is the electroencephalogram (EEG) which characterizes sleep as either rapid eye movement (REM) or non-rapid eye movement (NREM) states. The NREM–REM switch occurs every approximately 60–90 min and is driven by a network of neurons within the mid- and hind-brain. During REM sleep monoaminergic neurons remain inhibited, but cholinergic neurons are activated (+). REM-on neurons project to the spinal cord and drive muscle paralysis (atonia) [38]. If the atonia pathway fails to activate, conditions termed REM sleep behaviour disorder (RBD) can arise. Further, the level of loss of atonia can predict the development of Parkinson's disease [39]. It is worth emphasizing that we have only a rudimentary understanding of the real function of REM versus NREM sleep [40].
The complex interactions associated with sleep/wake generation are regulated, under normal circumstances, by two endogenous drivers, termed the homeostatic process (Process S), which increases as a function of wakefulness and a circadian process (Process C). This has been termed the ‘Two-Process’ model of sleep which broadly explains how the sleep/wake cycle is aligned to the night/day cycle [41] (figure 4).
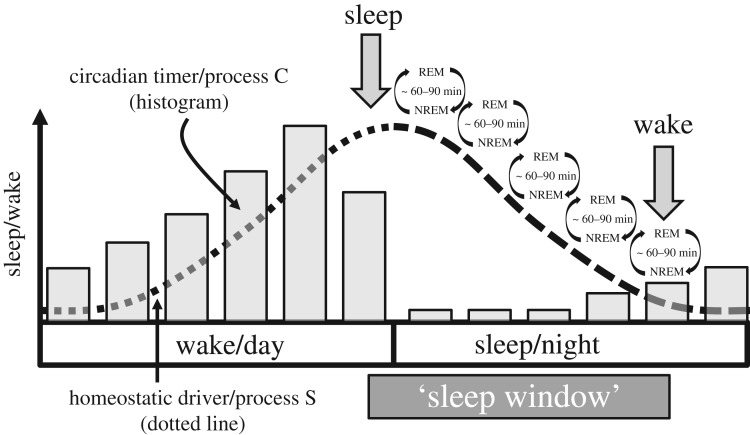
Figure 4.
A depiction of the two-process model of sleep regulation [41]. A 24 h circadian timer (process C) and a homeostatic driver (process S, dotted line) interact to determine the timing, duration and structure of sleep. A circadian driven rhythm of sleep promotion during the night and wake during the day is opposed by a homeostatic driver which increasingly promotes sleep (S) during the day, and then during sleep, homeostatic sleep pressure is dissipated towards the end of the sleep episode. The time of day most suitable for sleep—the ‘sleep window’ occurs as a result of the combined effects of the circadian and homeostatic drivers. Sleep pressure within the sleep window will be highest during the first part of the night but increasingly reduced as the homeostatic drive for sleep dissipates towards the end of the night. During sleep most humans experience 4–5 cycles of NREM/REM sleep and, without the influence of an alarm clock, we wake naturally from REM sleep [42].
In humans, several agents have been implicated in driving sleep homeostasis, and adenosine has emerged as a strong candidate [43]. Adenosine increases in the brain during wake and after enforced sleep deprivation. Furthermore, perfusion of adenosine into the brain of freely moving rodents reduces wakefulness and activates neurons associated with sleep promotion. Caffeine is a potent stimulant and alerting agent, and seems to function by blocking adenosine receptors. Other sleep-promoting factors include prostaglandin [44]. The circadian process seems to drive both wake- and sleep-promoting behaviour, promoting the maintenance of wakefulness during the day, opposing the increasing homeostatic drive for sleep, while at night Process C promotes sleep (figure 4). The model depicted in figure 4 has been very powerful in understanding the basic interactions between the circadian system and homeostatic drivers regulating sleep, but in reality, the regulation of sleep is likely to be much more complicated. Not least, that sleep in humans and other animals is often not a single consolidated block of sleep but can be ‘biphasic’ or even ‘polyphasic’ with two or more periods of sleep separated by short periods of wake [45,46]. How such fragmentary sleep is generated is uncertain and will require additional inputs to the model depicted in figure 4.
Melatonin is often confusingly termed the ‘sleep hormone’, and this is misleading. Melatonin is synthesized mainly in the pineal gland, although the retina and other regions of the body can also produce small amounts. The pineal is regulated by the SCN to produce a circadian pattern of melatonin release, with levels rising at dusk, peaking in the blood around 02.00–03.00 and then declining before dawn. Light, detected by the pRGCs, also acts to inhibit melatonin production acutely [47]. As a result, melatonin acts as a biological marker of the dark. In relation to sleep, melatonin receptors located on SCN neurons are thought to detect nocturnal melatonin to provide an additional zeitgeber for clock entrainment, reinforcing light entrainment signals from the eye [48,49]. However, although some studies suggest that taking melatonin may shorten sleep latency (the time taken to fall asleep) and increase total sleep time, the effects of melatonin [50], and agonists of melatonin, on sleep are modest [51]. While melatonin production occurs at night during sleep in diurnal animals such as humans, nocturnal animals like mice and rats also produce melatonin at night when they are active. Certainly, sleep propensity in humans is closely correlated with the melatonin profile but this may be correlation and not causation. Indeed, individuals who do not produce melatonin (e.g. tetraplegic individuals, people on beta-blockers or pinealectomized patients) still exhibit circadian sleep/wake rhythms with only very minor changes in sleep [52].
4. Sleep loss and harmful stress
Harmful stress, defined here as ‘a physical, mental, or emotional stimulus that results in impaired health or performance’, can arise from disrupted or shortened sleep, and is a common feature across many sectors of society, from teenagers [53], the business and public sectors, such as night shift workers [54], to the elderly [55]. Inadequate sleep usually means a sleep duration shorter than 7–8 h every 24 h. However, there is considerable individual variation, and self-assessments of sleep-need are very important. The main symptom of sleep loss is excessive daytime sleepiness, but a combination of the criteria in table 1 are additionally helpful for self-assessments. For additional background see [56].
Table 1.
Self-assessments of sleep-need. There is considerable individual variation in sleep duration and timing. As a result, it is important for individuals to define their own sleep needs using some or all of the criteria listed here. Once sleep-need is established, then sleep timing and duration should be defended by altered behaviours.
| inadequate sleep is suggested if some/all of the following are experienced |
|---|
| are dependent upon an alarm clock, or another person, to get you out of bed |
| over sleep extensively (get up late) on free days |
| take a long time to wake up and feel alert |
| feel sleepy and irritable during the day |
| feel you need a mid-afternoon nap to function adequately |
| are unable to concentrate and exhibit overly impulsive behaviours |
| crave caffeinated and sugar-rich drinks |
| receive advice from family, friends, work colleagues that your behaviour has changed, specifically you are more irritable, lack empathy in social situations, and are less reflective (disinhibited) |
| experience increased worry, anxiety, mood swings and depression |
The sleep loss experienced by night shift workers can be profound. Shift workers try and sleep during the day and invariably experience shorter (less than 5–6 h in every 24 h) and more disrupted sleep. In effect, shift workers are at work when their biology is in the sleep state and then try to sleep when their biology is prepared for wake. Irrespective of the years spent on a permanent night shift, nearly all (approx. 97%) of night shift workers do not adjust to the nocturnal regime but remain synchronized to daytime [57]. This is directly related to light exposure. Artificial light in the office or factory is dim compared to environmental light. Shortly after dawn, natural light is some 50–100 times brighter than the 300–400 lux experienced in the workplace, and by noon natural light is 500–1000 times brighter [58]. After leaving the night shift, an individual will usually experience bright natural light during the day and the circadian system will always lock onto the brighter light signal as daytime and align internal biology to the diurnal state. In one study, night shift workers were exposed to 2000 lux in the workplace and then completely shielded from natural light during the day. Under these circumstances, they became nocturnal. However, this is not a practical solution for most night shift workers [59].
Many individuals, and night shift workers, in particular, experience chronic sleep deprivation and circadian rhythm disruption [60], and there is increasing evidence that such problems act together to alter the release of corticosteroids (cortisol) regulated by the hypothalamic-pituitary-adrenal (HPA) axis. Cortisol release starts with the stimulation of the pituitary gland to release adrenocorticotropin (ACTH) into the blood. ACTH reaches the adrenal gland and stimulates the adrenal cortex to release glucocorticoids (corticosteroids). ACTH release is under circadian control, resulting in high levels of cortisol being secreted just prior and during the active part of the day, with lower levels of release towards evening and sleep. In addition to this 24 h variation, there is an ultradian rhythm of ACTH release that drives pulses of cortisol secretion from the adrenal cortex. Under normal circumstances, the circadian and pulsatile release of cortisol helps regulate and ‘fine-tune’ metabolic and immune responses to the varied demands of activity and sleep [61]. Under conditions of sleep disruption (and other stressors), the HPA is activated acutely resulting in elevated levels of ACTH which then drives high levels of cortisol.
In addition to the elevation of cortisol, sleep deprivation activates the sympatho-adreno-medullary (SAM) drive, which, via the sympathetic nervous system, stimulates the release of catecholamines (primarily epinephrine/adrenaline) from the adrenal medulla. Chronically elevated cortisol and adrenaline together drive a wide-spread stress response that if sustained will mobilize and release glucose into the bloodstream while reducing insulin release; increase heart rate and blood pressure; suppress the immune response; slow digestion; limit tissue repair, and can reduce memory consolidation and cognitive function [62]. If sustained, such physiological changes promote poor health and an increased difficulty coping with life (figure 5). The abnormal HPA secretory activity seen in shift workers and chronic insomniacs can be replicated in laboratory studies. For example, in one study, healthy young males were only allowed to sleep 4 h over six consecutive nights. This resulted in increased levels of cortisol in the afternoon and early evening and the rate of decrease of free cortisol in saliva was approximately six times slower in sleep-restricted individuals compared to rested controls [63,64]. Furthermore, chronic short sleepers have higher levels of cortisol compared to normal sleepers [65].
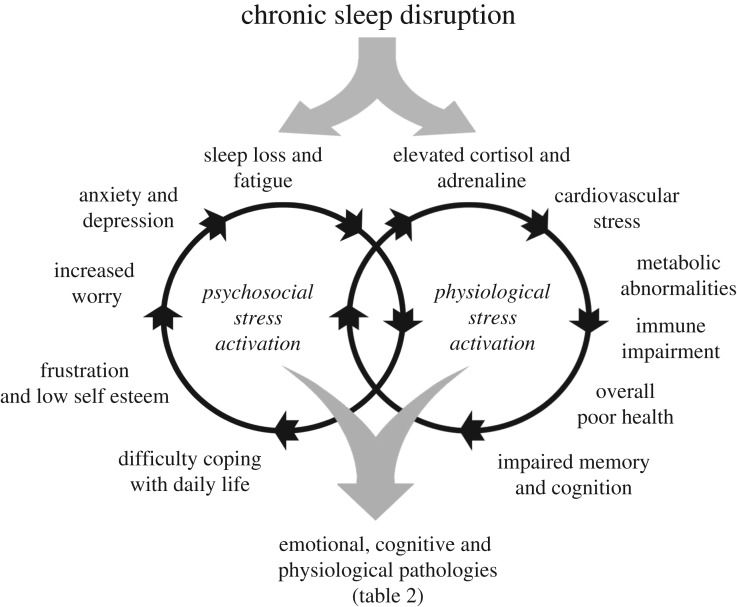
Figure 5.
The impact of chronic sleep disruption and reduced sleep on the promotion and interaction of physiological stress via the hypothalamic-pituitary-adrenal (HPA) and sympatho-adreno-medullary (SAM) axes and psychosocial stress whereby sleep loss and fatigue result in an imbalance between the demands placed upon an individual and an inability of the individual to manage these demands. Ultimately, the combined and interlocking effects of physiological and psychosocial stress lead to emotional, cognitive and physiological pathologies (table 2).
In addition to the activation of the HPA and SAM arms of the stress response, chronic sleep disruption gives rise to sleepiness and fatigue that can precipitate psychosocial stress [66]. Under these circumstances, an individual will experience an imbalance between the demands placed upon them and their perceived inability to manage these demands. This impaired ability to cope with the demands of life acts as an additional stressor to augment the activation of the HPA and SAM stress responses, and can lead directly to behavioural changes including frustration and low self-esteem, increased worry, anxiety and depression. Such behaviours promote further sleep loss and fatigue [67]. The relationships and consequences associated with sleep disruption, the chronic release of cortisol and adrenaline and psychosocial stress are summarized in figure 5.
5. The varied impacts and consequences of sleep disruption
The complexity of sleep generation and regulation (figures 3 and 4) renders this behavioural state very vulnerable to sleep and circadian rhythm disruption (SCRD) from multiple causes. For clarification, the term ‘SCRD’ is used as an ‘umbrella term’ in this review to refer to any form of sleep or circadian disruption, and does not distinguish between cause and effect. Thus, insufficient sleep arising from lack of opportunity and sleep loss due to disease would both be examples of SCRD. The term SCRD encompasses problems with the quality, timing and amount of sleep, and includes the 83 types of disorder included in the International Classification of Sleep Disorders (ICSD) 3rd edition [68]. The ICSD divides sleep disorders into seven main categories: (i) Insomnia (difficulty falling asleep or staying asleep); (ii) Sleep-related breathing disorders (e.g. obstructive sleep apnoea); (iii) Central disorders of hypersomnolence (e.g. Narcolepsy); (iv) Circadian rhythm sleep–wake disorders, illustrated in figure 6); (v) Parasomnias (e.g. sleep walking and night terrors); (vi) Sleep-related movement disorders (e.g. restless legs syndrome); (vii) Other sleep disorders, which do not fulfil the criteria of the other six classifications. Some of the consequences of these seven categories are illustrated in figure 6.
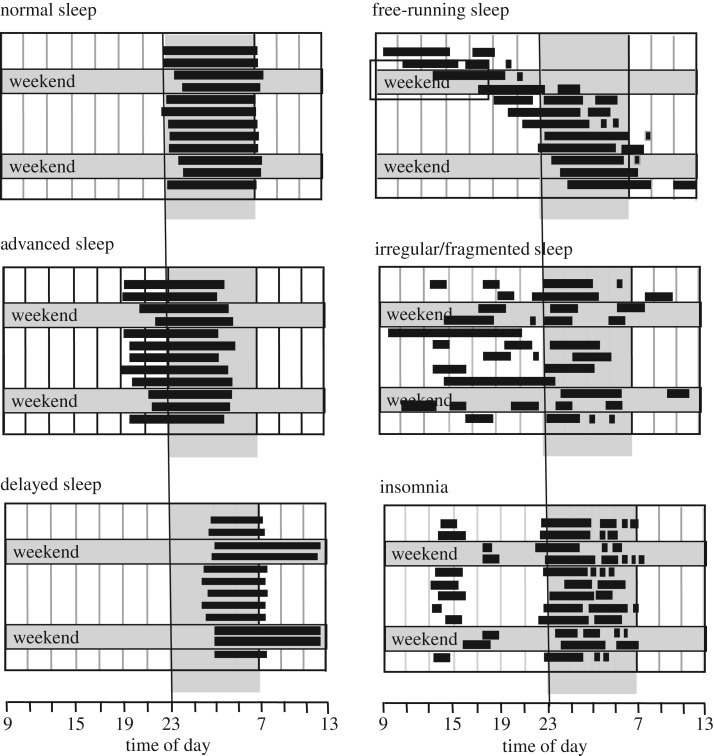
Figure 6.
Illustration of altered sleep patterns arising from multiple causes. Filled horizontal bars represent periods of sleep on consecutive work days and at the weekend. Advanced sleep phase disorder (ASPD) is characterized by difficulty staying awake in the evening and difficulty staying asleep in the early morning. Typically, individuals go to bed and rise about 3 or more hours earlier than the societal norm; delayed sleep phase disorder (DSPD) is characterized by 3 h delay or more in sleep onset and offset. This often leads to greatly reduced sleep duration during the working week and extended sleep on free days. ASPD and DSPD can be considered as pathological extremes of morning (lark) or evening (owl) preferences. It is important to stress that ASPD and DSPD are not merely shifted sleep/wake patterns, but conditions that cause distress or impairment because they conflict with the schedules demanded by societal pressures or personal preferences; Free-running or non-24-h sleep/wake disorder describes a condition where an individual's sleep occurs later and later each day. This has been observed in individuals with complete eye loss or other conditions such as schizophrenia; irregular or completely fragmented sleep is typically observed in individuals who lack a circadian clock. Note: ASPD, DSPS, free-running and irregular sleep/wake patterns are most often, but not exclusively, linked to circadian rhythm abnormalities. Insomnia can be used to describe both a symptom or a disorder, and if a disorder describes a condition that leads to difficulty falling asleep or staying asleep, even when a person has the chance to do so. Insomnia is frequently associated with reduced sleep (hyposomnia) and can arise from multiple causes [68].
As illustrated in figure 5, a major driver of SCRD arises from chronic stress resulting from physiological and/or psychosocial factors. However, there are multiple additional and interlinked drivers of SCRD. These relationships are illustrated in figure 7. In brief, multiple illnesses, and illness resulting in pain or other discomfort are a major driver for SCRD [69]. In addition, the varied problems that arise from the 24/7 society (extended working hours, reduced sleep, shift work, jet lag—essentially working against the biological drivers of sleep) also predispose individuals to SCRD. As illustrated in figure 7, the net result is circadian rhythm disruption, insomnia and sleep deprivation and disturbed social behaviours. These initial impacts can then lead to fatigue (a feeling of lack of energy and motivation that can be physical, mental or both); daytime sleepiness (persistent and overwhelming sleepiness during the day); and psychosocial anxiety (imbalance between the demands placed upon an individual and their perceived failure to manage these demands) [70,71]. Fatigue, daytime sleepiness and psychosocial disruption can precipitate a global disruption in physiology, important behavioural changes and the chronic activation of the physiological stress axis. The short- and long-term consequences in emotional, cognitive and physiological health are summarized in table 2. It is important to note that many of these interactions are bi-directional resulting in a matrix of positive feedback loops that can reinforce each other and precipitate a major breakdown in health and overall wellbeing.
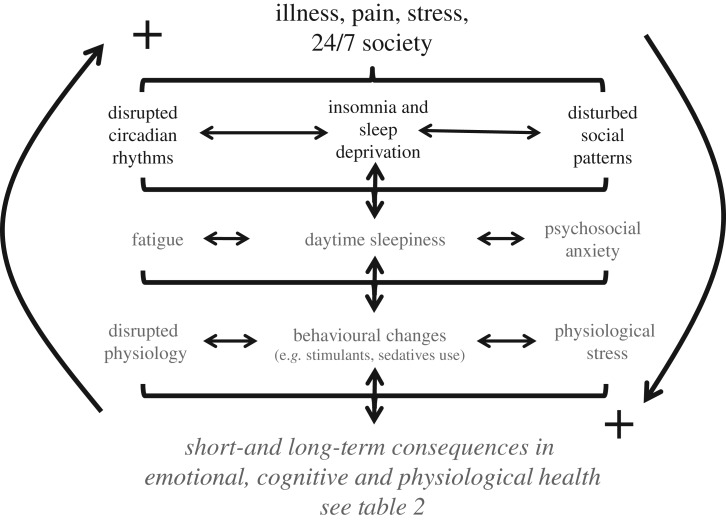
Figure 7.
The drivers of emotional, cognitive and physiological poor health. Factors such as Illness, illness resulting in pain, stressful situations and/or the impact of shift work and the 24/7 society can all lead to disrupted circadian rhythms, insomnia, sleep deprivation and abnormal patterns in social behaviour. Collectively these problems can give rise to fatigue, daytime sleepiness and psychosocial anxiety arising from altered patterns of social interaction. These altered behaviours will, in turn, disrupt physiology (e.g. metabolic abnormalities), drive abnormal patterns of behaviour (promote the use of stimulants and sedatives) and stimulate physiological stress (chronic release of cortisol and adrenaline). Collectively, this cascade of events underpins short- and long-term somatic and mental illness (table 2). Furthermore, it is important to appreciate that many of these interactions are bi-directional, acting to reinforce each other via multiple positive feedback loops.
Table 2.
The impact of chronic sleep and circadian rhythm disruption (SCRD) upon human emotional responses, cognition, physiology and health. Such associations have long been a concern for shift workers, who suffer from extreme forms of SCRD. Citations: fluctuations in mood [72–75], depression and psychosis [76–79], anxiety, irritability, loss of empathy, frustration [80–82], risk-taking and impulsivity [83–86], negative salience [87], stimulant, sedative and alcohol abuse [88–92], illegal drug use [93]; impaired cognitive performance and the ability to multi-task [94–96], memory, attention and concentration [97–100], communication and decision-making [90,101–104], creativity and productivity [105–108], motor performance [96,109], dissociation/detachment [110,111]; day time sleepiness, micro-sleeps, unintended sleep [112–115], altered stress response [116,117], altered sensory thresholds [118–120], impaired immunity and infection [121,122], cancer [123–125], metabolic abnormalities and diabetes II [63,126–129], cardiovascular disease [129–131].
| emotional |
cognitive |
physiology and health |
|---|---|---|
| increased | impaired | increased risk |
| fluctuations in mood | cognitive performance | day time sleepiness |
| irritability | ability to multi-task | micro-sleeps |
| anxiety | memory | cardiovascular disease |
| loss of empathy | attention | altered stress response |
| frustration | concentration | altered sensory thresholds |
| risk-taking and impulsivity | communication | infection, lowered immunity |
| negative salience | decision-making | cancer |
| stimulant use (caffeine) | creativity and productivity | metabolic abnormalities |
| sedative use (alcohol) | motor performance | diabetes II |
| illegal drug use | dissociation/detachment | depression and psychosis |
As summarized in table 2, chronic SCRD, of the sort experienced by shift workers or other groups experiencing SCRD, can lead to an increased risk in serious health conditions. For example, nurses are one of the best-studied groups of night shift workers and many years of shift work has been associated with a broad range of health problems including type II diabetes, gastrointestinal disorders and even breast and colorectal cancers. Cancer risk increases with the number of years of shift work, the frequency of rotating work schedules, and the number of hours per week working at night [132]. The correlations are so strong that shift work is now officially classified as ‘probably carcinogenic [Group 2A]’ by the World Health Organization. Other studies of shift workers show increased heart and stroke problems, obesity and depression (table 2). A study of over 3000 people in southern France found that those who had worked some type of extended night shift work for 10 or more years had much lower overall cognitive and memory scores than those who had never worked on the night shift [133]. Similar findings have been shown in long-haul airline pilots and aircrew [134,135].
SCRD also impairs glucose regulation and metabolism. Under laboratory conditions, sleep restriction in healthy young men led to signs of insulin resistance, which can ultimately lead to type II diabetes. Two gut hormones, leptin and ghrelin, seem to play a key role in this process. Leptin is produced by fat cells and is a signal of satiety; ghrelin is produced by the stomach and signals hunger, particularly for sugars. Together, these hormones regulate hunger and appetite. Restricting the sleep time of healthy young men under laboratory conditions for 7 days caused their leptin levels to fall (approx. 17%) and their ghrelin levels to rise (approx. 28%), and increased their appetite, especially for fatty and sugary foods (increased by 35–40%) [63]. Such a SCRD-induced distortion of appetite may be the explanation why shift workers have a higher risk of weight gain, obesity and type II diabetes. Significantly, night shift workers have elevated levels of the stress hormone cortisol, which has also been shown to suppress the action of insulin and raise blood glucose [64]. At another level, there is also a striking association between SCRD and smoking. For example, independent of social background and region, the number of smokers in the population increases with higher levels of SCRD [136]. Further, the consumption of alcohol and caffeine increases with SCRD [136]. Finally, and based upon the scores from the Beck Depression Inventory [137], the tendency towards depression increases when work times are not compatible with circadian sleep times [138], which leads to the next topic.
6. The special case of sleep disruption in mental illness
SCRD is a common co-morbidity in numerous psychiatric disorders [139]. Most studies have focused upon mood disorders, especially unipolar depression and seasonal affective disorder, yet SCRD is also prominent in the more severe, psychotic disorders such as schizophrenia [140,141]. Interestingly, such links between schizophrenia and abnormal sleep pre-date observations in mood disorders, and were first described in the late nineteenth century by the German psychiatrist Emil Kraepelin [142]. Today, clinical levels of insomnia are reported in more than 80% of patients with schizophrenia, and SCRD is increasingly recognized as one of the most common features of the disorder [143]. SCRD in schizophrenia is very variable, and the abnormal sleep/wake patterns illustrated in figure 6 have all been noted [142–147]. Importantly, schizophrenia patients with SCRD score badly on many quality-of-life clinical subscales, highlighting the human cost of SCRD [143,148,149], and, significantly, schizophrenia patients often comment that an improvement in sleep is one of their highest priorities during treatment [150]. It is also becoming clear that SCRD impacts upon the onset, outcome and relapse of mental illness [151–153]. These findings suggested that there are causal relationships between SCRD and psychoses, perhaps mediated via common (or overlapping) mechanisms [154].
The association of mental illness in general and SCRD has until recently been considered to arise from exogenous factors including social isolation, antipsychotic medication and/or activation of the stress axis [139]. Such a linear explanation between psychosis and SCRD now appears to be overly simplistic. For example, our studies have addressed this association by examining SCRD in patients with schizophrenia and comparing these individuals with unemployed control subjects [141]. The results demonstrated that severe SCRD exists in schizophrenia and persists independently of antipsychotic medication. Further, sleep disruption cannot be explained on the basis of lack of employment as unemployed individuals show remarkably stable sleep/wake patterns [141]. These results are consistent with an alternative hypothesis, which suggests that psychoses and SCRD may share common and overlapping mechanistic pathways [154]. As discussed above, the sleep and circadian timing system is the product of a complex interaction between multiple genes, brain regions, neurotransmitters and modulatory hormones (figures 2 and 3). As a consequence, abnormalities in any of the underlying neurotransmitter systems that predispose individuals to mental illness would almost certainly impinge upon the sleep/circadian timing systems at some level. Similarly, psychosis involves several distributed brain circuits, affecting a range of neurotransmitter systems, many of which overlap with those underlying sleep and circadian rhythm generation [154]. Viewed in this context, it is no surprise that SCRD is common in psychoses, or that SCRD will, in turn, have wide-spread effects, ranging across many aspects of the neural and neuroendocrine function as outlined in table 2. Significantly, many of the pathologies caused by SCRD (table 2) are reported routinely as co-morbid with neuropsychiatric illness but are rarely linked to the disruption of sleep. Furthermore, the consequences of SCRD result in abnormal (reduced) light exposure and atypical patterns of social behaviour (figure 7), closing a vicious cycle to further destabilize sleep/circadian physiology [155,156]. The common and overlapping mechanisms of psychosis and SCRD are illustrated in figure 8. Critically, these relationships explain how relatively small changes in either the impact of SCRD or mental illness will be amplified by physiological feedbacks to increase an individual's vulnerability to neuropsychiatric illness and co-morbid health problems.
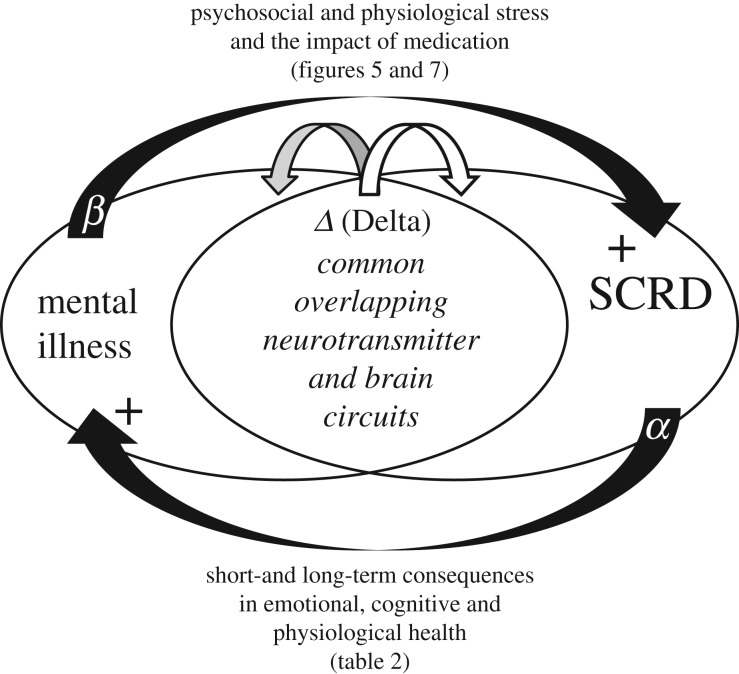
Figure 8.
Diagram illustrating the possible relationship between mental illness and sleep and circadian rhythm disruption (SCRD). The diagram illustrates the hypothesis that mental illness and SCRD share common and overlapping pathways within the brain. As a result, an altered pattern of neurotransmitter release (shown as Δ Delta) that predispose an individual to mental illness will result in a parallel impact upon the sleep/circadian systems. Disruption of sleep (shown as α) will, likewise, impact upon multiple aspects of brain function with both short- and long-term consequences in emotional, cognitive and physiological health (figures 5 and 7; and table 2), and in the young may even have developmental consequences. The consequences of mental illness (shown as β), giving rise to psychosocial (e.g. social isolation) and physiological stress (figures 5 and 7), along with the impact of medication, will impinge upon the sleep and circadian systems. A positive feedback loop could rapidly be established whereby a small change in neurotransmitter release could be amplified via positive feedback loops into more pronounced SCRD and poorer mental health.
The conceptual framework outlined in figure 8 allows four explicit predictions, all of which can be strongly supported by recent findings. Specifically, (i) genes linked to mental illness will play a role in sleep and circadian rhythm generation and regulation [154,157]; (ii) genes that generate and regulate sleep and circadian rhythms will play a role in mental health and illness [154]; (iii) that SCRD will precede mental illness under some circumstances [157]; (iv) that SCRD amelioration will have a positive impact upon mental illness. For this prediction, it is worth mentioning one recent publication. In this study, the aim was to determine whether treating insomnia would reduce levels of paranoia and hallucinations in university students with insomnia. The trial involved a randomized controlled trial at 26 UK universities, and students with insomnia were randomly assigned to receive either digital cognitive behavioural therapy for insomnia (CBTi) (n = 1891) or no intervention (n = 1864). The primary outcome measures were for insomnia, paranoia and hallucinatory experiences. The results showed that a reduction in insomnia, by using CBTi, was correlated with a highly significant reduction in paranoia and hallucinations over the study period. The study concluded that insomnia is a causal factor in the occurrence of psychotic experiences and other mental health problems [158]. These findings are highly significant as they show that treatments for SCRD represent a potentially new and powerful therapeutic target for the reduction of symptoms in mental illness (figure 8).
7. Potential actions as an individual and as an employer
Based upon the findings summarized above, we can legitimately ask the question: ‘what evidence-based approaches can be used to mitigate the causes and consequences of SCRD, both as individuals and as employers?’ Some possible actions are summarized in tables 3 and 4, and discussed below.
Table 3.
Individual actions to achieve better sleep. Actions that can be undertaken during the day, before going to bed, in the bedroom (sleeping space) and in bed to help improve sleep. Such approaches, either alone or in parallel with clinically directed cognitive behavioural therapy for insomnia (CBTi), can lead to a marked improvement in sleep.
| during the day | before bed | the bedroom | in bed |
|---|---|---|---|
| for most individuals, get as much natural morning light as possible; the timed use of light boxes can also help regulate sleep | reduce light levels approximately 30 min before bedtime | not too warm (18–22°C) | try to keep a routine—go to bed and get up at the same time each day |
| if you nap ensure it is no longer than 20 min and not within 6 h of bedtime | stop using electronic devices approximately 30 min before bedtime | keep it quiet, or use ‘white noise’ or a relaxing sound such as the sea | ensure the bed is large enough with a good mattress and pillows |
| exercise—but not too close to bedtime | ideally avoid prescription sedatives | keep it dark; use black-out curtains | keep bedside lights low |
| concentrate food intake to the first and middle parts of the day | do not use alcohol, antihistamines or other peoples' sedatives | remove TV, computers/tablets, smartphones | consider using relaxing oils (e.g. lavender) |
| avoid excessive consumption of caffeine-rich drinks especially in the afternoon | avoid the discussion or consideration of stressful topics immediately before bed | do not ‘clock watch’—consider removing an illuminated clock | ear plugs—if your partner snores; ensure snoring is not due to sleep apnoea |
| make time to step back from stressful situations—do not let stress accumulate | adopt behaviours that relax you, listening to music, reading, mindfulness or a relaxing bath can be useful | do not obsess about sleep apps | if you wake stay calm: consider leaving the bed, keep the lights low and find a relaxing activity, then return |
| above all—define what works best for you and stick to your routine | |||
Table 4.
SCRD-induced employee issue and potential employer responses. On the basis of existing information, employers should establish ‘best practice’ approaches to mitigate some of the inevitable consequences of SRCD in the workplace (table 2). However, it must be emphasized that as a society we have to acknowledge that shift work and patterns of employment that disrupt sleep are detrimental to health, and that currently there is no way of eliminating completely the health problems listed in table 2.
| SCRD-induced employee issue | employer response |
|---|---|
| after extended periods of work or night shifts, loss of vigilance and micro-sleeps at work and/or the drive home | provide vigilance devices in the workplace and for the drive home which alert an individual that they are falling asleep, provide transport (e.g. Taxis) following the night shift for the commute home |
| loss of vigilance and tiredness in the workplace | provide sufficiently bright light to increase alertness |
| poor physical and mental health | higher frequency health-checks to detect problems early and institute interventions to prevent chronic conditions |
| obesity, diabetes II, metabolic abnormalities | appropriate food/nutrition; reduce the high-fat, high-sugar diet available and replace with small protein-rich and easy to digest snacks |
| failure to appreciate the consequences of shortened sleep and night shift work by the employee and family | develop educational materials for the employee and family explaining the impact and consequences of sleep loss |
| anxiety over sleep apps, screen and computer use, and confused recommendations regarding sleep education | develop educational materials and advice regarding best sleep practice |
| varied ability to cope with morning versus evening versus night shift schedules | chronotype individuals in an attempt to accommodate preferred sleep/wake patterns for the allocation of timed patterns of work |
7.1. Actions as an individual
As outlined in table 3, there are a range of relatively simple actions that individuals can undertake to help improve insomnia. Such interventions fall under the general term of ‘cognitive behavioural therapy for insomnia’ or CBTi, which aims to control the environment and the behaviours that precede sleep. Key aspects of CBTi are considered below and are targeted at four different times:
(i) During the day. Individuals should get as much morning natural light as possible, as this has been shown to advance the circadian clock [159]. An earlier bedtime results in extended sleep. Note that a minority of individuals who are very early chronotypes, who go to bed and get up very early, might benefit from late afternoon/evening light exposure which would delay the clock and align them more closely to the rest of the population; in the absence of natural light, timed light exposure using a light box has also been shown to be helpful for some sleep/wake timing problems [160]; if there is a need to nap, the nap should be approximately 20 min as recovery from longer naps can lead to sleep inertia (impaired cognitive and sensory-motor performance) [161]. In addition, naps close to bedtime (within approx. 6 h) will act to reduce the homeostatic drive for sleep (figure 4), and this will delay sleep onset [161]; exercising close to bedtime will elevate core body temperature and this may delay sleep onset in some people, particularly if the exercise is very vigorous [162]. The delayed sleep may be linked to the fact that sleep initiation seems to involve/require a small reduction in core temperature [163], and exercise may override this circadian driven change in body temperature; feeding during the latter part of the day has been shown to predispose individuals to weight gain and increased susceptibility to metabolic abnormalities such as diabetes II [164]. Weight gain can predispose to obstructive sleep apnoea where the walls of the throat relax and narrow during sleep, interrupting normal breathing [165]. If untreated this can lead to multiple health issues. For example, obesity, diabetes and other sleep difficulties, such as restless legs syndrome [166]. In addition, digestive processes (e.g. gut mobility, release of digestive enzymes) are reduced towards the evening. If the major meal of the day is prior to bedtime, this can predispose individuals to digestive health problems such as excessive stomach acid production and a greater risk of stomach ulcers [167]; caffeine can have a major alerting effect on the brain as it blocks the receptors in the brain that respond to adenosine which provides one of the homeostatic drivers for sleep (figure 4). There is considerable individual variability in responses to caffeine, depending on body weight, pregnancy status, medication, liver health and caffeine exposure, but in healthy adults, the half-life is approximately 5–6 h (the time required to reduce caffeine to half of its initial value). As a result, a strong coffee or tea in the afternoon could delay sleep onset [168]; short-term emotional stress is also a very powerful agent for the disruption of sleep (figure 7) [169]. As a result, try to resolve stressful situations during the day.
(ii) Before bed. Light can have an alerting effect upon consciousness and lead to delayed sleep onset [170]. As a result, reducing light exposure 30 min prior to bed may be useful physiologically (reducing alertness) and perhaps psychologically as part of a routine of ‘sleep preparation’. There has also been extensive and a somewhat confusing discussion regarding the impact of light intensity and wavelength prior to bedtime on shifting the circadian system. The light around dusk and in the evening will delay the circadian system; while light in the morning will advance the circadian system. This fact has been used to support the argument that computer or smartphone use prior to bedtime will disrupt sleep/wake timing, thus promoting later sleep times. Further, the software has been developed to shift the colour spectrum of computer screens, lowering the blue light content and so reducing the activation of the ‘blue light sensitive’ pRGCs (figure 1). While this is logical, the impact of different colours of light on alertness is complex [171], and the extent to which the light from screens before bedtime represents a significant problem remains unresolved. For example, one recent study compared the impact of reading a light-emitting ebook (LE-eBook) versus reading from a printed book for 4 h prior to bedtime. The light intensity of the LE-eBook was on the maximum setting (approx. 31 lux), while the light reflected off the page of the printed book was approximately 0.9 lux. The results showed that LE-eBook use delayed sleep onset by less than 10 min compared to reading the print book. Although the results were statistically significant, a delay of 10 min is not particularly noteworthy [172]. In summary, while it is probably sensible to minimize light exposure prior to bedtime to reduce levels of alertness, and to prepare psychologically for bed, the impact of light from digital devices needs further investigation. Although the physiological impact of light exposure from screen use remains unresolved, technology-related behaviours (games, computer or phone use) does impact negatively on sleep and daytime function, and is a particular problem for teenagers [173]. The use of prescription sedatives to aid sleep can be useful in the short-term to adjust sleep, but long-term use, particularly in night shift workers, can cause problems because of side-effects. For example, the chronic use of benzodiazepines (e.g. Xanax, Valium, Ativan and Librium), which are anti-anxiety medications and increase drowsiness, are potentially addictive and can lead to impaired memory consolidation and reduced attention during the day [174]. Non-prescription sedatives such as alcohol and antihistamines (e.g. diphenhydramine and doxylamine) should be avoided, as the side-effects can severely impact upon health and daytime functioning [175]. It is important to avoid the discussion or consideration of stressful topics immediately before bed. The acute elevation of cortisol and adrenaline will increase alertness and delay sleep (figure 5); a relaxing behaviour, such as a bath or shower, or warming the hands and feet [176], can be useful as it may promote peripheral vasodilation, and the lowering of core body temperature which can help sleep initiation. In addition, a bath can be part of a bedtime routine that psychologically prepares you for sleep.
(iii) The bedroom. Making the bedroom or sleeping space suitable for sleep is a much overlooked yet critical part of getting satisfactory sleep. If the bedroom is too warm, this will affect the ability to lower core body temperature, and hence delay sleep onset. Ideally, the bedroom should promote sleep by minimizing distractions and stimuli that alert the individual. The sleeping space should be quiet and dark, and devices such as televisions, computers, smartphones should be removed. Smartphones are now used routinely as alarm clocks, and so removing them from the sleeping space can be problematic. However, if the phone is a distraction, then it should be replaced by an alarm clock; but this is also not straightforward. Many individuals ‘clock watch’ and get anxious about the amount of time left available for sleep and constantly check and re-check the alarm clock generating more anxiety [177]. Under these circumstances, the alarm setting can be used, but the face of the clock should be covered. Sleep apps can be useful in providing a quantitative measure for sleep duration, and in this regard, most are reasonably accurate. By contrast, measures of REM versus non-REM or even ‘deep sleep’ are more difficult to assess from the currently available devices, and may even be profoundly misleading. In theory, such monitoring systems could be useful in recording that a change in behaviour (table 3) has translated into improved sleep. But because most of the commercial apps available fail to provide an accurate measure of overall sleep, individuals can become anxious if their device inaccurately reports ‘insufficient restful sleep’ or ‘low levels of REM sleep’. The lack of validation or FDA approval of the currently available devices is an additional concern, and it is worth noting that very few sleep apps have been endorsed by the sleep academies or sleep specialists [178]. As a result, it would be wise not to take sleep apps too seriously.
(iv) In bed. Keeping a good bedtime routine of getting-up and going to bed at the same time, and importantly, at a time that is optimal for sleep-need, has been shown to be important for maintaining good sleep [179]. Such a schedule reinforces the exposure to environmental zeitgebers, especially light and food, which act to entrain the circadian system and stabilize the sleep/wake cycle. Individuals who are ‘natural long sleepers', needing 9 h or more of sleep each night, may not be able to achieve sufficient sleep during the working week, and it remains unclear whether these individuals might benefit from oversleeping on free days. A good mattress, pillows and bedding make intuitive sense for good sleep, but surprisingly, strong empirical evidence for mattress quality is lacking [180]. Bedside lights should be bright enough for reading, but kept as low as possible to reduce alertness. Relaxing oils are often proposed to help improve sleep. However, the evidence-base is largely absent [181], and any effects could well be placebo. Further research is needed, but for some individuals relaxing oils do anecdotally improve sleep. Perhaps because the association of a distinctive ‘conditioning’ smell, such as lavender, can be part of a bedtime routine that psychologically prepares individuals for sleep. Ear plugs can help if a sleeping partner snores, or if there is external noise [182]. If a partner's snoring becomes too disruptive, then an alternative sleeping space should be identified [183]. Waking at night can occur for multiple reasons, and need not mean the end of sleep. Under such circumstances, it is important not to activate stress responses by remaining in bed and becoming increasingly frustrated by the failure to sleep. Some individuals find it useful to leave the bed, keep the lights low and engage in a relaxing activity such as reading or listening to music. Significantly, and as mentioned above, a single period of consolidated sleep (monophasic sleep) may not be the ‘universal state of sleep’, and could represent an artefact of a shortened night, and greatly compressed sleep. Biphasic sleep (sleeping during two periods interrupted by wake) or polyphasic sleep (multiple sleep/wake episodes) is the normal situation for most animals, and may have been for humans before the Industrial Revolution [184–186]. Although there is no universal agreement [187], the original concept that the natural state of human sleep is polyphasic was partly developed based upon human historical research [188,189], and therefore provides a good example of how historical studies can inform contemporary science. Indeed, laboratory-based studies subsequently supported the idea that human sleep is polyphasic [45,190]. This raises the important point, that if the natural state of human sleep is indeed polyphasic, then we need to re-think our interpretation of ‘disrupted sleep’ at night. Collectively, the data emerging suggest that if an individual wakes at night, then sleep is likely to return, if sleep is not sacrificed to social media and/or other alerting behaviours.
In table 3, different forms of CBTi are listed that have been used to help improve sleep. Nevertheless, it is important to stress that there is remarkable variation in sleep duration, timing and structure, not only between individuals but also within the same individual across the lifespan. This means that the individual has to identify what works best for them, and then defend those behaviours that promote optimal sleep.
7.2. Actions as an employer
In parallel with individual actions and CBTi, employers could implement measures in the workplace to help address some problems arising from SCRD. Potential employer responses are summarized in table 4.
Outlined in table 4 are some actions that employers could undertake to improve employee safety, health and welfare arising from work-related SCRD. For example, night shift work and extended working is associated with the loss of vigilance and a high frequency of micro-sleeps, and this can be dangerous both in the working environment and on the commute home (table 2). Driver fatigue has long been recognized as a major cause of road accidents [191]. A recent study showed that 57% of junior doctors had either had a motor vehicle crash or near miss after working on the night shift [192]. For this reason, some hospitals provide taxis for staff to get them home after the night shift. For many years, the rail industry has used some form of ‘Dead Man's Switch’ or Driver Safety Device to alert the driver that they have lost vigilance or fallen asleep, but such preventative measures have not been widely adopted in either domestic or commercial motor vehicles until recently. Part of the problem has been the lack of availability of non-invasive Driver Drowsiness Detection technology. However, in recent years a range of devices including, Steering pattern monitoring; Vehicle position in lane monitoring; and Driver eye/face monitoring to detect drowsiness have been developed and commercialized [193]. Employers could make such devices available to employees who are at risk of fatigue or who undertake shiftwork and then commute home in their own vehicle. Perhaps the reason why some employers have not supplied such devices is that this might be seen as an admission of liability. However, not to do so could also constitute a failure of ‘duty of care’ by an employer, with serious legal consequences.
Loss of vigilance in the workplace could be improved by illuminating the working environment with light sufficiently bright to promote alertness. Although increasing light levels to the 1000 lux range seems to be useful, more studies are needed to define precisely when and how much light is needed in different settings [194]; as long-term sleep loss and night shift work are associated with a range of physical and mental health problems (table 2), employers could offer higher frequency health-checks for those at-risk individuals to detect problems early so that the appropriate interventions can be implemented to prevent chronic health conditions developing. In the same way, knowing that metabolic abnormalities and cardiovascular diseases have a much higher prevalence in shift workers and the chronically tired (table 2), appropriate nutrition could be made available to help reduce the development of these conditions in the workplace, combined with educational advice regarding diet when not at work. Indeed, the development of educational materials by an independent body, such as the Department of Health in the UK or equivalent bodies in other countries, for employees, employee partners and family, explaining the impact and consequences of sleep loss could be of immense value in terms of developing coping strategies. Multiple studies have shown that the divorce rate is higher and that social interactions are more negative when one partner is involved in shiftwork [194]. Part of the problem may be the failure of the partner to understand some of the negative impacts upon behaviour as a consequence of shift work or sleep loss. Again, the state, working with employers, could provide educational materials to support employees and those with whom they share their lives. Finally, there is considerable variation across the population in terms of chronotype. Studies have shown that the greater the mismatch between an individual's endogenous sleep/wake timing versus the time when an individual is required to work (often called social jet lag), the greater the risk of developing health problems (table 2) [195]. Employers could attempt to match an individual's chronotype to specific work schedules. Put simply, the ‘larks’ would be better suited to the morning shifts and the ‘owls’ to the night shifts. Clearly, this is not the complete solution to shift work, but it could mitigate some of the significant problems of working against internal time.
8. Conclusion
This review has considered the biology of sleep and circadian rhythms, some of the consequences of disrupting these rhythms as a result of disease or societal pressures, and has outlined approaches to help mitigate a few of the problems associated with SCRD. It is disconcerting that although there has been a major increase in our understanding of the importance of sleep and of the consequences of sleep disruption, and a realization that SCRD dominates the lives of millions of individuals in both the developed and developing nations, there has not been commensurate action to deal with the problems. At an organizational level, we are not training our healthcare professionals in this critical area of bioscience; governments have failed to address the broad issue of sleep health with appropriate legislation or the development of clear evidence-based guidelines; and there remains a dearth of research information to allow government and employers to provide evidence-based and specific advice to the workforce regarding how to cope with work-related SCRD. At a broader level, and at least in the short-term, there is no ‘magic bullet’ for the impact of shift work or work-related sleep loss. Employers and employees have to accept that there will always be significant health consequences associated with sleep loss, and that currently, the best we can hope to achieve is a reduction in the severity of symptoms associated with SCRD. As a result, society needs to consider very seriously the circumstances where the consequences of SCRD are justified in the workplace. Such decisions must emerge from an evidence-based discussion involving academia, government, industry and above all the workforce, and hopefully, these discussions will take place before litigation distorts and derails the debate.
Acknowledgements
The author would like to thank Sally Shuttleworth, Elizabeth Foster and Suzanne Ftouni for their constructive advice and feedback on this manuscript. I would also like to thank the Wellcome Trust for their support of our research.
Data accessibility
This article has no additional data.
Competing interests
I declare I have no competing interests.
Funding
I received no funding for this study.
References
- 1.Foster RG, Kreitzman L. 2014. The rhythms of life: what your body clock means to you! Exp. Physiol. 99, 599–606. ( 10.1113/expphysiol.2012.071118) [DOI] [PubMed] [Google Scholar]
- 2.Foster RG, Kreitzman L. 2004. Rhythms of life: the biological clocks that control the daily lives of every living thing, xii, 276 pp London, UK: Profile Books. [Google Scholar]
- 3.Lockley SW, Foster RG. 2012. Sleep: a very short introduction, xvi, 146 pp Oxford, NY: Oxford University Press. [Google Scholar]
- 4.Moore RY, Lenn NJ. 1972. A retinohypothalamic projection in the rat. J. Comp. Neurol. 146, 1–14. ( 10.1002/cne.901460102) [DOI] [PubMed] [Google Scholar]
- 5.Stephan FK, Zucker I. 1972. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc. Natl Acad. Sci. USA 69, 1583–1586. ( 10.1073/pnas.69.6.1583) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ralph MR, Foster RG, Davis FC, Menaker M. 1990. Transplanted suprachiasmatic nucleus determines circadian period. Science 247, 975–978. ( 10.1126/science.2305266) [DOI] [PubMed] [Google Scholar]
- 7.Welsh DK, Logothetis DE, Meister M, Reppert SM. 1995. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron 14, 697–706. ( 10.1016/0896-6273(95)90214-7) [DOI] [PubMed] [Google Scholar]
- 8.Takahashi JS. 2017. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 18, 164–179. ( 10.1038/nrg.2016.150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalmbach DA, Schneider LD, Cheung J, Bertrand SJ, Kariharan T, Pack AI, Gehrman PR. 2017. Genetic basis of chronotype in humans: insights from three landmark GWAS. Sleep 40, zsw048 ( 10.1093/sleep/zsw048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster RG, Kreitzmank L. 2017. Circadian rhythms: a very short introduction, xx, 143 pp., 1st edn Oxford, UK: Oxford University Press. [Google Scholar]
- 11.Kalsbeek A, Palm IF, La Fleur SE, Scheer FA, Perreau-Lenz S, Ruiter M, Kreier F, Cailotto C, Buijs RM. 2006. SCN outputs and the hypothalamic balance of life. J. Biol. Rhythms 21, 458–469. ( 10.1177/0748730406293854) [DOI] [PubMed] [Google Scholar]
- 12.Dickmeis T. 2009. Glucocorticoids and the circadian clock. J. Endocrinol. 200, 3–22. ( 10.1677/JOE-08-0415) [DOI] [PubMed] [Google Scholar]
- 13.Kalsbeek A, Yi CX, Cailotto C, la Fleur SE, Fliers E, Buijs RM. 2011. Mammalian clock output mechanisms. Essays Biochem. 49, 137–151. ( 10.1042/bse0490137) [DOI] [PubMed] [Google Scholar]
- 14.Kalsbeek A, Fliers E, Hofman MA, Swaab DF, Buijs RM. 2010. Vasopressin and the output of the hypothalamic biological clock. J. Neuroendocrinol. 22, 362–372. ( 10.1111/j.1365-2826.2010.01956.x) [DOI] [PubMed] [Google Scholar]
- 15.Silver R, LeSauter J, Tresco PA, Lehman MN. 1996. A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature 382, 810–813. ( 10.1038/382810a0) [DOI] [PubMed] [Google Scholar]
- 16.Freeman GM, Herzog ED. 2011. Neuropeptides go the distance for circadian synchrony. Proc. Natl Acad. Sci. USA 108, 13 883–13 884. ( 10.1073/pnas.1110844108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagoshi E, Saini C, Bauer C, Laroche T, Naef F, Schibler U. 2004. Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell 119, 693–705. ( 10.1016/j.cell.2004.11.015) [DOI] [PubMed] [Google Scholar]
- 18.Richards J, Gumz ML. 2012. Advances in understanding the peripheral circadian clocks. FASEB J. 26, 3602–3613. ( 10.1096/fj.12-203554) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albrecht U. 2012. Timing to perfection: the biology of central and peripheral circadian clocks. Neuron 74, 246–260. ( 10.1016/j.neuron.2012.04.006) [DOI] [PubMed] [Google Scholar]
- 20.Foster RG. 1998. Shedding light on the biological clock. Neuron 20, 829–832. ( 10.1016/S0896-6273(00)80464-X) [DOI] [PubMed] [Google Scholar]
- 21.Freedman MS, Lucas RJ, Soni B, von Schantz M, Munoz M, David-Gray Z, Foster R. 1999. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science 284, 502–504. ( 10.1126/science.284.5413.502) [DOI] [PubMed] [Google Scholar]
- 22.Lucas RJ, Freedman MS, Munoz M, Garcia-Fernandez JM, Foster RG. 1999. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science 284, 505–507. ( 10.1126/science.284.5413.505) [DOI] [PubMed] [Google Scholar]
- 23.Sekaran S, Foster RG, Lucas RJ, Hankins MW. 2003. Calcium imaging reveals a network of intrinsically light-sensitive inner-retinal neurons. Curr. Biol. 13, 1290–1298. ( 10.1016/S0960-9822(03)00510-4) [DOI] [PubMed] [Google Scholar]
- 24.Berson DM, Dunn FA, Takao M. 2002. Phototransduction by retinal ganglion cells that set the circadian clock. Science 295, 1070–1073. ( 10.1126/science.1067262) [DOI] [PubMed] [Google Scholar]
- 25.Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau KW, Gamlin PD. 2005. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature 433, 749–754. ( 10.1038/nature03387) [DOI] [PubMed] [Google Scholar]
- 26.Provencio I, Jiang G, De Grip WJ, Hayes WP, Rollag MD. 1998. Melanopsin: an opsin in melanophores, brain, and eye. Proc. Natl Acad. Sci. USA 95, 340–345. ( 10.1073/pnas.95.1.340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW. 2003. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science 299, 245–247. ( 10.1126/science.1077293) [DOI] [PubMed] [Google Scholar]
- 28.Hattar S, et al. 2003. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature 424, 76–81. ( 10.1038/nature01761) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruby NF, Brennan TJ, Xie X, Cao V, Franken P, Heller HC, O'Hara BF. 2002. Role of melanopsin in circadian responses to light. Science 298, 2211–2213. ( 10.1126/science.1076701) [DOI] [PubMed] [Google Scholar]
- 30.Panda S, et al. 2003. Melanopsin is required for non-image-forming photic responses in blind mice. Science 301, 525–527. ( 10.1126/science.1086179) [DOI] [PubMed] [Google Scholar]
- 31.van Diepen HC, Ramkisoensing A, Peirson SN, Foster RG, Meijer JH. 2013. Irradiance encoding in the suprachiasmatic nuclei by rod and cone photoreceptors. FASEB J. 27, 4204–4212. ( 10.1096/fj.13-233098) [DOI] [PubMed] [Google Scholar]
- 32.van Oosterhout, et al. 2012. Ultraviolet light provides a major input to non-image-forming light detection in mice. Curr. Biol. 22, 1397–1402. ( 10.1016/j.cub.2012.05.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Diepen HC, Foster RG, Meijer JH. 2015. A colourful clock. PLoS Biol. 13, e1002160 ( 10.1371/journal.pbio.1002160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaidi FH, et al. 2007. Short-wavelength light sensitivity of circadian, pupillary, and visual awareness in humans lacking an outer retina. Curr. Biol. 17, 2122–2128. ( 10.1016/j.cub.2007.11.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morjaria R, Alexander I, Purbrick RMJ, Safa R, Chong NV, Wulff K, Foster RG, Downes SM. 2019. Impact of diabetic retinopathy on sleep, mood, and quality of life. Invest. Ophthalmol. Vis. Sci. 60, 2304–2310. ( 10.1167/iovs.18-26108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alexander I, Cuthbertson FM, Ratnarajan G, Safa R, Mellington FE, Foster RG, Downes SM, Wulff K. 2014. Impact of cataract surgery on sleep in patients receiving either ultraviolet-blocking or blue-filtering intraocular lens implants. Invest. Ophthalmol. Vis. Sci. 55, 4999–5004. ( 10.1167/iovs.14-14054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scammell TE, Arrigoni E, Lipton JO. 2017. Neural circuitry of wakefulness and sleep. Neuron 93, 747–765. ( 10.1016/j.neuron.2017.01.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saper CB, Fuller PM. 2017. Wake-sleep circuitry: an overview. Curr. Opin. Neurobiol. 44, 186–192. ( 10.1016/j.conb.2017.03.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Postuma RB, Gagnon JF, Rompre S, Montplaisir JY. 2010. Severity of REM atonia loss in idiopathic REM sleep behavior disorder predicts Parkinson disease. Neurology 74, 239–244. ( 10.1212/WNL.0b013e3181ca0166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vyazovskiy VV, Delogu A. 2014. NREM and REM sleep: complementary roles in recovery after wakefulness. Neuroscientist 20, 203–219. ( 10.1177/1073858413518152) [DOI] [PubMed] [Google Scholar]
- 41.Borbely AA. 1982. A two process model of sleep regulation. Hum. Neurobiol. 1, 195–204. [PubMed] [Google Scholar]
- 42.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. 2010. Sleep state switching. Neuron 68, 1023–1042. ( 10.1016/j.neuron.2010.11.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Donlea JM, Alam MN, Szymusiak R. 2017. Neuronal substrates of sleep homeostasis; lessons from flies, rats and mice. Curr. Opin. Neurobiol. 44, 228–235. ( 10.1016/j.conb.2017.05.003) [DOI] [PubMed] [Google Scholar]
- 44.Greene RW, Bjorness TE, Suzuki A. 2017. The adenosine-mediated, neuronal-glial, homeostatic sleep response. Curr. Opin. Neurobiol. 44, 236–242. ( 10.1016/j.conb.2017.05.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wehr TA. 1992. In short photoperiods, human sleep is biphasic. J. Sleep Res. 1, 103–107. ( 10.1111/j.1365-2869.1992.tb00019.x) [DOI] [PubMed] [Google Scholar]
- 46.Nunn CL, Samson DR, Krystal AD. 2016. Shining evolutionary light on human sleep and sleep disorders. Evol. Med. Public Health 2016, 227–243. ( 10.1093/emph/eow018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lucas RJ, Foster RG. 1999. Neither functional rod photoreceptors nor rod or cone outer segments are required for the photic inhibition of pineal melatonin. Endocrinology 140, 1520–1524. ( 10.1210/endo.140.4.6672) [DOI] [PubMed] [Google Scholar]
- 48.Arendt J. 2005. Melatonin in humans: it's about time. J. Neuroendocrinol. 17, 537–538. ( 10.1111/j.1365-2826.2005.01333.x) [DOI] [PubMed] [Google Scholar]
- 49.Arendt J, Skene DJ. 2005. Melatonin as a chronobiotic. Sleep Med. Rev. 9, 25–39. ( 10.1016/j.smrv.2004.05.002) [DOI] [PubMed] [Google Scholar]
- 50.Ferracioli-Oda E, Qawasmi A, Bloch MH. 2013. Meta-analysis: melatonin for the treatment of primary sleep disorders. PLoS ONE 8, e63773 ( 10.1371/journal.pone.0063773) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lockley SW, et al. 2015. Tasimelteon for non-24-hour sleep-wake disorder in totally blind people (SET and RESET): two multicentre, randomised, double-masked, placebo-controlled phase 3 trials. Lancet 386, 1754–1764. ( 10.1016/S0140-6736(15)60031-9) [DOI] [PubMed] [Google Scholar]
- 52.Whelan A, Halpine M, Christie SD, McVeigh SA. In press. Systematic review of melatonin levels in individuals with complete cervical spinal cord injury. J. Spinal Cord Med. ( 10.1080/10790268.2018.1505312) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carskadon MA. 2011. Sleep in adolescents: the perfect storm. Pediatr. Clin. North Am. 58, 637–647. ( 10.1016/j.pcl.2011.03.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.AUSTRALIA C. See https://www.sleephealthfoundation.org.au/files/Asleep_on_the_job/Asleep_on_the_Job_SHF_report-WEB_small.pdf. August 2017.
- 55.Ancoli-Israel S, Ayalon L, Salzman C. 2008. Sleep in the elderly: normal variations and common sleep disorders. Harv. Rev. Psychiatry 16, 279–286. ( 10.1080/10673220802432210) [DOI] [PubMed] [Google Scholar]
- 56.Buysse DJ. 2014. Sleep health: can we define it? Does it matter? Sleep 37, 9–17. ( 10.5665/sleep.3298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Folkard S. 2008. Do permanent night workers show circadian adjustment? A review based on the endogenous melatonin rhythm. Chronobiol. Int. 25, 215–224. ( 10.1080/07420520802106835) [DOI] [PubMed] [Google Scholar]
- 58.Dilaura DL, Houser KW, Mistrick RG, Steffy GR (eds). 2019. The lighting handbook: reference and application (Illuminating Engineering Society of North America//Lighting Handbook), 10th edn New York, NY: Illuminating Engineering. [Google Scholar]
- 59.Czeisler CA, Dijk DJ. 1995. Use of bright light to treat maladaptation to night shift work and circadian rhythm sleep disorders. J. Sleep Res. 4, 70–73. ( 10.1111/j.1365-2869.1995.tb00231.x) [DOI] [PubMed] [Google Scholar]
- 60.Hirotsu C, Tufik S, Andersen ML. 2015. Interactions between sleep, stress, and metabolism: from physiological to pathological conditions. Sleep Sci. 8, 143–152. ( 10.1016/j.slsci.2015.09.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kalafatakis K, Russell GM, Lightman SL. 2019. MECHANISMS IN ENDOCRINOLOGY: does circadian and ultradian glucocorticoid exposure affect the brain? Eur. J. Endocrinol. 180, R73–R89. ( 10.1530/EJE-18-0853) [DOI] [PubMed] [Google Scholar]
- 62.Buckley TM, Schatzberg AF. 2005. On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J. Clin. Endocrinol. Metab. 90, 3106–3114. ( 10.1210/jc.2004-1056) [DOI] [PubMed] [Google Scholar]
- 63.Van Cauter E, et al. 2007. Impact of sleep and sleep loss on neuroendocrine and metabolic function. Horm. Res. 67(Suppl. 1), 2–9. [DOI] [PubMed] [Google Scholar]
- 64.Van Cauter E, Spiegel K, Tasali E, Leproult R. 2008. Metabolic consequences of sleep and sleep loss. Sleep Med. 9(Suppl. 1), S23–S28. ( 10.1016/S1389-9457(08)70013-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abell JG, Shipley MJ, Ferrie JE, Kivimaki M, Kumari M. 2016. Recurrent short sleep, chronic insomnia symptoms and salivary cortisol: a 10-year follow-up in the Whitehall II study. Psychoneuroendocrinology 68, 91–99. ( 10.1016/j.psyneuen.2016.02.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Akerstedt T. 2006. Psychosocial stress and impaired sleep. Scand. J. Work Environ. Health. 32, 493–501. ( 10.5271/sjweh.1054) [DOI] [PubMed] [Google Scholar]
- 67.Schwarz J, Gerhardsson A, van Leeuwen W, Lekander M, Ericson M, Fischer H, Kecklund G, Åkerstedt T. 2018. Does sleep deprivation increase the vulnerability to acute psychosocial stress in young and older adults? Psychoneuroendocrinology 96, 155–165. ( 10.1016/j.psyneuen.2018.06.003) [DOI] [PubMed] [Google Scholar]
- 68.Medicine AAoS. 2014. International classification of sleep disorders, 3rd edn Darien, IL: American Academy of Sleep Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stocks J, Tang NK, Walsh DA, Warner SC, Harvey HL, Jenkins W, Abhishek A, Doherty M, Valdes A. 2018. Bidirectional association between disturbed sleep and neuropathic pain symptoms: a prospective cohort study in post-total joint replacement participants. J. Pain Res. 11, 1087–1093. ( 10.2147/JPR.S149830) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leyva-Vela B, Jesus Llorente-Cantarero F, Henarejos-Alarcon S, Martinez-Rodriguez A. 2018. Psychosocial and physiological risks of shift work in nurses: a cross-sectional study. Cent. Eur. J. Public Health 26, 183–189. ( 10.21101/cejph.a4817) [DOI] [PubMed] [Google Scholar]
- 71.Nea FM, Pourshahidi LK, Kearney JM, Livingstone MBE, Bassul C, Corish CA. 2018. A qualitative exploration of the shift work experience: the perceived effect on eating habits, lifestyle behaviours and psychosocial wellbeing. J. Public Health (Oxf) 40, e482–ee92. ( 10.1093/pubmed/fdy047) [DOI] [PubMed] [Google Scholar]
- 72.Banks S, Dinges DF. 2007. Behavioral and physiological consequences of sleep restriction. J. Clin. Sleep Med. 3, 519–528. ( 10.5664/jcsm.26918) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oginska H, Pokorski J. 2006. Fatigue and mood correlates of sleep length in three age-social groups: school children, students, and employees. Chronobiol. Int. 23, 1317–1328. ( 10.1080/07420520601089349) [DOI] [PubMed] [Google Scholar]
- 74.Scott JP, McNaughton LR, Polman RC. 2006. Effects of sleep deprivation and exercise on cognitive, motor performance and mood. Physiol. Behav. 87, 396–408. ( 10.1016/j.physbeh.2005.11.009) [DOI] [PubMed] [Google Scholar]
- 75.Selvi Y, Gulec M, Agargun MY, Besiroglu L. 2007. Mood changes after sleep deprivation in morningness-eveningness chronotypes in healthy individuals. J. Sleep Res. 16, 241–244. ( 10.1111/j.1365-2869.2007.00596.x) [DOI] [PubMed] [Google Scholar]
- 76.Johnson EO, Roth T, Breslau N. 2006. The association of insomnia with anxiety disorders and depression: exploration of the direction of risk. J. Psychiatr. Res. 40, 700–708. ( 10.1016/j.jpsychires.2006.07.008) [DOI] [PubMed] [Google Scholar]
- 77.Kahn-Greene ET, Killgore DB, Kamimori GH, Balkin TJ, Killgore WD. 2007. The effects of sleep deprivation on symptoms of psychopathology in healthy adults. Sleep Med. 8, 215–221. ( 10.1016/j.sleep.2006.08.007) [DOI] [PubMed] [Google Scholar]
- 78.Riemann D, Voderholzer U. 2003. Primary insomnia: a risk factor to develop depression? J. Affect. Disord. 76, 255–259. ( 10.1016/S0165-0327(02)00072-1) [DOI] [PubMed] [Google Scholar]
- 79.Sharma V, Mazmanian D. 2003. Sleep loss and postpartum psychosis. Bipolar Disord. 5, 98–105. ( 10.1034/j.1399-5618.2003.00015.x) [DOI] [PubMed] [Google Scholar]
- 80.Dahl RE, Lewin DS. 2002. Pathways to adolescent health sleep regulation and behavior. J. Adolesc. Health 31(6 Suppl), 175–184. ( 10.1016/S1054-139X(02)00506-2) [DOI] [PubMed] [Google Scholar]
- 81.Kelman BB. 1999. The sleep needs of adolescents. J. Sch. Nurs. 15, 14–19. ( 10.1177/105984059901500205) [DOI] [PubMed] [Google Scholar]
- 82.Muecke S. 2005. Effects of rotating night shifts: literature review. J. Adv. Nurs. 50, 433–439. ( 10.1111/j.1365-2648.2005.03409.x) [DOI] [PubMed] [Google Scholar]
- 83.Acheson A, Richards JB, de Wit H. 2007. Effects of sleep deprivation on impulsive behaviors in men and women. Physiol. Behav. 91, 579–587. ( 10.1016/j.physbeh.2007.03.020) [DOI] [PubMed] [Google Scholar]
- 84.McKenna BS, Dickinson DL, Orff HJ, Drummond SP. 2007. The effects of one night of sleep deprivation on known-risk and ambiguous-risk decisions. J. Sleep Res. 16, 245–252. ( 10.1111/j.1365-2869.2007.00591.x) [DOI] [PubMed] [Google Scholar]
- 85.O'Brien EM, Mindell JA. 2005. Sleep and risk-taking behavior in adolescents. Behav. Sleep Med. 3, 113–133. ( 10.1207/s15402010bsm0303_1) [DOI] [PubMed] [Google Scholar]
- 86.Venkatraman V, Chuah YM, Huettel SA, Chee MW. 2007. Sleep deprivation elevates expectation of gains and attenuates response to losses following risky decisions. Sleep 30, 603–609. ( 10.1093/sleep/30.5.603) [DOI] [PubMed] [Google Scholar]
- 87.Walker MP, Stickgold R. 2006. Sleep, memory, and plasticity. Annu. Rev. Psychol. 57, 139–166. ( 10.1146/annurev.psych.56.091103.070307) [DOI] [PubMed] [Google Scholar]
- 88.Baranski JV, Pigeau RA. 1997. Self-monitoring cognitive performance during sleep deprivation: effects of modafinil, d-amphetamine and placebo. J. Sleep Res. 6, 84–91. ( 10.1111/j.1365-2869.1997.00032.x) [DOI] [PubMed] [Google Scholar]
- 89.Boivin DB, Tremblay GM, James FO. 2007. Working on atypical schedules. Sleep Med. 8, 578–589. ( 10.1016/j.sleep.2007.03.015) [DOI] [PubMed] [Google Scholar]
- 90.Killgore WD, Balkin TJ, Wesensten NJ. 2006. Impaired decision making following 49 h of sleep deprivation. J. Sleep Res. 15, 7–13. ( 10.1111/j.1365-2869.2006.00487.x) [DOI] [PubMed] [Google Scholar]
- 91.Roehrs T, Roth T. 2001. Sleep, sleepiness, sleep disorders and alcohol use and abuse. Sleep Med. Rev. 5, 287–297. ( 10.1053/smrv.2001.0162) [DOI] [PubMed] [Google Scholar]
- 92.Roehrs T, Roth T. 2001. Sleep, sleepiness, and alcohol use. Alcohol Res. Health. 25, 101–109. [PMC free article] [PubMed] [Google Scholar]
- 93.Mednick SC, Christakis NA, Fowler JH. 2010. The spread of sleep loss influences drug use in adolescent social networks. PLoS ONE 5, e9775 ( 10.1371/journal.pone.0009775) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dinges DF, Pack F, Williams K, Gillen KA, Powell JW, Ott GE, Aptowicz C, Pack AI. 1997. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep 20, 267–277. ( 10.1093/sleep/20.4.267) [DOI] [PubMed] [Google Scholar]
- 95.Lamond N, Jay SM, Dorrian J, Ferguson SA, Jones C, Dawson D. 2007. The dynamics of neurobehavioural recovery following sleep loss. J. Sleep Res. 16, 33–41. ( 10.1111/j.1365-2869.2007.00574.x) [DOI] [PubMed] [Google Scholar]
- 96.Pilcher JJ, Huffcutt AI. 1996. Effects of sleep deprivation on performance: a meta-analysis. Sleep 19, 318–326. ( 10.1093/sleep/19.4.318) [DOI] [PubMed] [Google Scholar]
- 97.Chee MW, Chuah LY. 2008. Functional neuroimaging insights into how sleep and sleep deprivation affect memory and cognition. Curr. Opin. Neurol. 21, 417–423. ( 10.1097/WCO.0b013e3283052cf7) [DOI] [PubMed] [Google Scholar]
- 98.Dworak M, Schierl T, Bruns T, Struder HK. 2007. Impact of singular excessive computer game and television exposure on sleep patterns and memory performance of school-aged children. Pediatrics 120, 978–985. ( 10.1542/peds.2007-0476) [DOI] [PubMed] [Google Scholar]
- 99.Goder R, Scharffetter F, Aldenhoff JB, Fritzer G. 2007. Visual declarative memory is associated with non-rapid eye movement sleep and sleep cycles in patients with chronic non-restorative sleep. Sleep Med. 8, 503–508. ( 10.1016/j.sleep.2006.11.014) [DOI] [PubMed] [Google Scholar]
- 100.Oken BS, Salinsky MC, Elsas SM. 2006. Vigilance, alertness, or sustained attention: physiological basis and measurement. Clin. Neurophysiol. 117, 1885–1901. ( 10.1016/j.clinph.2006.01.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Baranski JV, Thompson MM, Lichacz FM, McCann C, Gil V, Pasto L, Pigeau RA. 2007. Effects of sleep loss on team decision making: motivational loss or motivational gain? Hum. Factors 49, 646–660. ( 10.1518/001872007X215728) [DOI] [PubMed] [Google Scholar]
- 102.Harrison Y, Horne JA. 2000. The impact of sleep deprivation on decision making: a review. J. Exp. Psychol. Appl. 6, 236–249. ( 10.1037/1076-898X.6.3.236) [DOI] [PubMed] [Google Scholar]
- 103.Killgore WD, Killgore DB, Day LM, Li C, Kamimori GH, Balkin TJ. 2007. The effects of 53 hours of sleep deprivation on moral judgment. Sleep 30, 345–352. ( 10.1093/sleep/30.3.345) [DOI] [PubMed] [Google Scholar]
- 104.Lucidi F, Russo PM, Mallia L, Devoto A, Lauriola M, Violani C. 2006. Sleep-related car crashes: risk perception and decision-making processes in young drivers. Accid. Anal. Prev. 38, 302–309. ( 10.1016/j.aap.2005.09.013) [DOI] [PubMed] [Google Scholar]
- 105.Horne JA. 1988. Sleep loss and “divergent” thinking ability. Sleep 11, 528–536. ( 10.1093/sleep/11.6.528) [DOI] [PubMed] [Google Scholar]
- 106.Jones K, Harrison Y. 2001. Frontal lobe function, sleep loss and fragmented sleep. Sleep Med. Rev. 5, 463–475. ( 10.1053/smrv.2001.0203) [DOI] [PubMed] [Google Scholar]
- 107.Killgore WD, Kahn-Greene ET, Lipizzi EL, Newman RA, Kamimori GH, Balkin TJ. 2008. Sleep deprivation reduces perceived emotional intelligence and constructive thinking skills. Sleep Med. 9, 517–526. ( 10.1016/j.sleep.2007.07.003) [DOI] [PubMed] [Google Scholar]
- 108.Randazzo AC, Muehlbach MJ, Schweitzer PK, Walsh JK. 1998. Cognitive function following acute sleep restriction in children ages 10–14. Sleep 21, 861–868. ( 10.1093/sleep/21.8.861) [DOI] [PubMed] [Google Scholar]
- 109.Kahol K, Leyba MJ, Deka M, Deka V, Mayes S, Smith M, Ferrara JJ, Panchanathan S. 2008. Effect of fatigue on psychomotor and cognitive skills. Am. J. Surg. 195, 195–204. ( 10.1016/j.amjsurg.2007.10.004) [DOI] [PubMed] [Google Scholar]
- 110.Tucker AM, Whitney P, Belenky G, Hinson JM, Van Dongen HP. 2010. Effects of sleep deprivation on dissociated components of executive functioning. Sleep 33, 47–57. ( 10.1093/sleep/33.1.47) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Giesbrecht T, Smeets T, Leppink J, Jelicic M, Merckelbach H. 2007. Acute dissociation after 1 night of sleep loss. J. Abnorm. Psychol. 116, 599–606. ( 10.1037/0021-843X.116.3.599) [DOI] [PubMed] [Google Scholar]
- 112.Basner M, Glatz C, Griefahn B, Penzel T, Samel A. 2008. Aircraft noise: effects on macro- and microstructure of sleep. Sleep Med. 9, 382–387. ( 10.1016/j.sleep.2007.07.002) [DOI] [PubMed] [Google Scholar]
- 113.Philip P, Akerstedt T. 2006. Transport and industrial safety, how are they affected by sleepiness and sleep restriction? Sleep Med. Rev. 10, 347–356. ( 10.1016/j.smrv.2006.04.002) [DOI] [PubMed] [Google Scholar]
- 114.Pilcher JJ, Lambert BJ, Huffcutt AI. 2000. Differential effects of permanent and rotating shifts on self-report sleep length: a meta-analytic review. Sleep 23, 155–163. ( 10.1093/sleep/23.2.1b) [DOI] [PubMed] [Google Scholar]
- 115.Scott LD, Hwang WT, Rogers AE, Nysse T, Dean GE, Dinges DF. 2007. The relationship between nurse work schedules, sleep duration, and drowsy driving. Sleep 30, 1801–1807. ( 10.1093/sleep/30.12.1801) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Meerlo P, Sgoifo A, Suchecki D. 2008. Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med. Rev. 12, 197–210. ( 10.1016/j.smrv.2007.07.007) [DOI] [PubMed] [Google Scholar]
- 117.Phan TX, Malkani RG. 2019. Sleep and circadian rhythm disruption and stress intersect in Alzheimer's disease. Neurobiol Stress 10, 100133 ( 10.1016/j.ynstr.2018.10.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kundermann B, Krieg JC, Schreiber W, Lautenbacher S. 2004. The effect of sleep deprivation on pain. Pain Res. Manag. 9, 25–32. ( 10.1155/2004/949187) [DOI] [PubMed] [Google Scholar]
- 119.Landis CA, Savage MV, Lentz MJ, Brengelmann GL. 1998. Sleep deprivation alters body temperature dynamics to mild cooling and heating not sweating threshold in women. Sleep 21, 101–108. ( 10.1093/sleep/21.1.101) [DOI] [PubMed] [Google Scholar]
- 120.Roehrs T, Hyde M, Blaisdell B, Greenwald M, Roth T. 2006. Sleep loss and REM sleep loss are hyperalgesic. Sleep 29, 145–151. ( 10.1093/sleep/29.2.145) [DOI] [PubMed] [Google Scholar]
- 121.Irwin M. 2002. Effects of sleep and sleep loss on immunity and cytokines. Brain Behav. Immun. 16, 503–512. ( 10.1016/S0889-1591(02)00003-X) [DOI] [PubMed] [Google Scholar]
- 122.Lorton D, Lubahn CL, Estus C, Millar BA, Carter JL, Wood CA, Bellinger DL. 2006. Bidirectional communication between the brain and the immune system: implications for physiological sleep and disorders with disrupted sleep. Neuroimmunomodulation 13, 357–374. ( 10.1159/000104864) [DOI] [PubMed] [Google Scholar]
- 123.Davis S, Mirick DK. 2006. Circadian disruption, shift work and the risk of cancer: a summary of the evidence and studies in Seattle. Cancer Causes Control. 17, 539–545. ( 10.1007/s10552-005-9010-9) [DOI] [PubMed] [Google Scholar]
- 124.Hansen J. 2006. Risk of breast cancer after night- and shift work: current evidence and ongoing studies in Denmark. Cancer Causes Control. 17, 531–537. ( 10.1007/s10552-005-9006-5) [DOI] [PubMed] [Google Scholar]
- 125.Kakizaki M, Kuriyama S, Sone T, Ohmori-Matsuda K, Hozawa A, Nakaya N, Tsuji I. 2008. Sleep duration and the risk of breast cancer: the Ohsaki Cohort Study. Br. J. Cancer 99, 1502–1505. ( 10.1038/sj.bjc.6604684) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. 2005. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. 28, 1289–1296. ( 10.1093/sleep/28.10.1289) [DOI] [PubMed] [Google Scholar]
- 127.Knutson KL, Spiegel K, Penev P, Van Cauter E. 2007. The metabolic consequences of sleep deprivation. Sleep Med. Rev. 11, 163–178. ( 10.1016/j.smrv.2007.01.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Laposky AD, Bass J, Kohsaka A, Turek FW. 2008. Sleep and circadian rhythms: key components in the regulation of energy metabolism. FEBS Lett. 582, 142–151. ( 10.1016/j.febslet.2007.06.079) [DOI] [PubMed] [Google Scholar]
- 129.Luyster FS, Strollo PJ Jr, Zee PC, Walsh JK. 2012. Boards of Directors of the American Academy of Sleep Medicine and the Sleep Research Society. Sleep: a health imperative. Sleep 35, 727-734. ( 10.5665/sleep.1846) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Maemura K, Takeda N, Nagai R. 2007. Circadian rhythms in the CNS and peripheral clock disorders: role of the biological clock in cardiovascular diseases. J. Pharmacol. Sci. 103, 134–138. ( 10.1254/jphs.FMJ06003X2) [DOI] [PubMed] [Google Scholar]
- 131.Young ME, Bray MS. 2007. Potential role for peripheral circadian clock dyssynchrony in the pathogenesis of cardiovascular dysfunction. Sleep Med. 8, 656–667. ( 10.1016/j.sleep.2006.12.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hansen J. 2017. Night shift work and risk of breast cancer. Curr. Environ. Health Rep. 4, 325–339. ( 10.1007/s40572-017-0155-y) [DOI] [PubMed] [Google Scholar]
- 133.Marquie JC, Tucker P, Folkard S, Gentil C, Ansiau D. 2015. Chronic effects of shift work on cognition: findings from the VISAT longitudinal study. Occup. Environ. Med. 72, 258–264. ( 10.1136/oemed-2013-101993) [DOI] [PubMed] [Google Scholar]
- 134.Cho K. 2001. Chronic 'jet lag' produces temporal lobe atrophy and spatial cognitive deficits. Nat. Neurosci. 4, 567–568. ( 10.1038/88384) [DOI] [PubMed] [Google Scholar]
- 135.Cho K, Ennaceur A, Cole JC, Suh CK. 2000. Chronic jet lag produces cognitive deficits. J. Neurosci. 20, RC66 ( 10.1523/JNEUROSCI.20-06-j0005.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wittmann M, Dinich J, Merrow M, Roenneberg T. 2006. Social jetlag: misalignment of biological and social time. Chronobiol. Int. 23, 497–509. ( 10.1080/07420520500545979) [DOI] [PubMed] [Google Scholar]
- 137.Beck AT, Steer RA, Ball R, Ranieri W. 1996. Comparison of Beck depression inventories -IA and -II in psychiatric outpatients. J. Pers. Assess. 67, 588–597. ( 10.1207/s15327752jpa6703_13) [DOI] [PubMed] [Google Scholar]
- 138.Levandovski R, Dantas G, Fernandes LC, Caumo W, Torres I, Roenneberg T, Hidalgo MPL, Allebrandt KV. 2011. Depression scores associate with chronotype and social jetlag in a rural population. Chronobiol. Int. 28, 771–778. ( 10.3109/07420528.2011.602445) [DOI] [PubMed] [Google Scholar]
- 139.Wulff K, Gatti S, Wettstein JG, Foster RG. 2010. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat. Rev. Neurosci. 11, 589–599. ( 10.1038/nrn2868) [DOI] [PubMed] [Google Scholar]
- 140.Benca RM, Obermeyer WH, Thisted RA, Gillin JC. 1992. Sleep and psychiatric disorders. A meta-analysis. Arch. Gen. Psychiatry 49, 651–668; discussion 69–70 ( 10.1001/archpsyc.1992.01820080059010) [DOI] [PubMed] [Google Scholar]
- 141.Wulff K, Dijk DJ, Middleton B, Foster RG, Joyce EM. 2012. Sleep and circadian rhythm disruption in schizophrenia. Br. J. Psychiatry 200, 308–316. ( 10.1192/bjp.bp.111.096321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Manoach DS, Stickgold R. 2009. Does abnormal sleep impair memory consolidation in schizophrenia? Front. Hum. Neurosci. 3, 21 ( 10.3389/neuro.09.021.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cohrs S. 2008. Sleep disturbances in patients with schizophrenia: impact and effect of antipsychotics. CNS Drugs 22, 939–962. ( 10.2165/00023210-200822110-00004) [DOI] [PubMed] [Google Scholar]
- 144.Martin J, Jeste DV, Caliguiri MP, Patterson T, Heaton R, Ancoli-Israel S. 2001. Actigraphic estimates of circadian rhythms and sleep/wake in older schizophrenia patients. Schizophr. Res. 47, 77–86. ( 10.1016/S0920-9964(00)00029-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Martin JL, Jeste DV, Ancoli-Israel S. 2005. Older schizophrenia patients have more disrupted sleep and circadian rhythms than age-matched comparison subjects. J. Psychiatr. Res. 39, 251–259. ( 10.1016/j.jpsychires.2004.08.011) [DOI] [PubMed] [Google Scholar]
- 146.Wulff K, Joyce E, Middleton B, Dijk DJ, Foster RG. 2006. The suitability of actigraphy, diary data, and urinary melatonin profiles for quantitative assessment of sleep disturbances in schizophrenia: a case report. Chronobiol. Int. 23, 485–495. ( 10.1080/07420520500545987) [DOI] [PubMed] [Google Scholar]
- 147.Wulff K, Porcheret K, Cussans E, Foster RG. 2009. Sleep and circadian rhythm disturbances: multiple genes and multiple phenotypes. Curr. Opin. Genet. Dev. 19, 237–246. ( 10.1016/j.gde.2009.03.007) [DOI] [PubMed] [Google Scholar]
- 148.Goldman M, Tandon R, DeQuardo JR, Taylor SF, Goodson J, McGrath M. 1996. Biological predictors of 1-year outcome in schizophrenia in males and females. Schizophr. Res. 21, 65–73. ( 10.1016/0920-9964(96)00021-7) [DOI] [PubMed] [Google Scholar]
- 149.Hofstetter JR, Lysaker PH, Mayeda AR. 2005. Quality of sleep in patients with schizophrenia is associated with quality of life and coping. BMC Psychiatry 5, 13 ( 10.1186/1471-244X-5-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Auslander LA, Jeste DV. 2002. Perceptions of problems and needs for service among middle-aged and elderly outpatients with schizophrenia and related psychotic disorders. Community Ment. Health J. 38, 391–402. ( 10.1023/A:1019808412017) [DOI] [PubMed] [Google Scholar]
- 151.Ehlers CL, Frank E, Kupfer DJ. 1988. Social zeitgebers and biological rhythms. A unified approach to understanding the etiology of depression. Arch. Gen. Psychiatry 45, 948–952. ( 10.1001/archpsyc.1988.01800340076012) [DOI] [PubMed] [Google Scholar]
- 152.Chemerinski E, Ho BC, Flaum M, Arndt S, Fleming F, Andreasen NC. 2002. Insomnia as a predictor for symptom worsening following antipsychotic withdrawal in schizophrenia. Compr. Psychiatry 43, 393–396. ( 10.1053/comp.2002.34627) [DOI] [PubMed] [Google Scholar]
- 153.Gruber J, Miklowitz DJ, Harvey AG, Frank E, Kupfer D, Thase ME, Sachs GS, Ketter TA. 2011. Sleep matters: sleep functioning and course of illness in bipolar disorder. J. Affect. Disord. 134, 416–420. ( 10.1016/j.jad.2011.05.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pritchett D, Wulff K, Oliver PL, Bannerman DM, Davies KE, Harrison PJ, Peirson SN, Foster RG. 2012. Evaluating the links between schizophrenia and sleep and circadian rhythm disruption. J. Neural Transm. (Vienna) 119, 1061–1075. ( 10.1007/s00702-012-0817-8) [DOI] [PubMed] [Google Scholar]
- 155.Uhlhaas PJ, Singer W. 2006. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron 52, 155–168. ( 10.1016/j.neuron.2006.09.020) [DOI] [PubMed] [Google Scholar]
- 156.Richardson G, Wang-Weigand S. 2009. Effects of long-term exposure to ramelteon, a melatonin receptor agonist, on endocrine function in adults with chronic insomnia. Hum. Psychopharmacol. 24, 103–111. ( 10.1002/hup.993) [DOI] [PubMed] [Google Scholar]
- 157.Jagannath A, Peirson SN, Foster RG. 2013. Sleep and circadian rhythm disruption in neuropsychiatric illness. Curr. Opin. Neurobiol. 23, 888–894. ( 10.1016/j.conb.2013.03.008) [DOI] [PubMed] [Google Scholar]
- 158.Freeman D, et al. 2017. The effects of improving sleep on mental health (OASIS): a randomised controlled trial with mediation analysis. Lancet Psychiatry 4, 749–758. ( 10.1016/S2215-0366(17)30328-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wright KP Jr, McHill AW, Birks BR, Griffin BR, Rusterholz T, Chinoy ED. 2013. Entrainment of the human circadian clock to the natural light-dark cycle. Curr. Biol. 23, 1554-1558. ( 10.1016/j.cub.2013.06.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.van Maanen A, Meijer AM, van der Heijden KB, Oort FJ. 2016. The effects of light therapy on sleep problems: a systematic review and meta-analysis. Sleep Med. Rev. 29, 52–62. ( 10.1016/j.smrv.2015.08.009) [DOI] [PubMed] [Google Scholar]
- 161.Milner CE, Cote KA. 2009. Benefits of napping in healthy adults: impact of nap length, time of day, age, and experience with napping. J. Sleep Res. 18, 272–281. ( 10.1111/j.1365-2869.2008.00718.x) [DOI] [PubMed] [Google Scholar]
- 162.Stutz J, Eiholzer R, Spengler CM. 2019. Effects of evening exercise on sleep in healthy participants: a systematic review and meta-analysis. Sports Med. 49, 269–287. ( 10.1007/s40279-018-1015-0) [DOI] [PubMed] [Google Scholar]
- 163.Murphy PJ, Campbell SS. 1997. Nighttime drop in body temperature: a physiological trigger for sleep onset? Sleep 20, 505–511. ( 10.1093/sleep/20.7.505) [DOI] [PubMed] [Google Scholar]
- 164.Beccuti G, Monagheddu C, Evangelista A, Ciccone G, Broglio F, Soldati L, Bo S. 2017. Timing of food intake: sounding the alarm about metabolic impairments? A systematic review. Pharmacol. Res. 125:132-141. ( 10.1016/j.phrs.2017.09.005) [DOI] [PubMed] [Google Scholar]
- 165.Stradling J. 2016. Obstructive sleep apnoea: is it moving into primary care? Br. J. Gen. Pract. 66, e149–e151. ( 10.3399/bjgp16X683785) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jehan S, Myers AK, Zizi F, Pandi-Perumal SR, Jean-Louis G, McFarlane SI. 2018. Obesity, obstructive sleep apnea and type 2 diabetes mellitus: epidemiology and pathophysiologic insights. Sleep Med. Disord. 2, 52–58. ( 10.15406/smdij.2018.02.00045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ruddick-Collins LC, Johnston JD, Morgan PJ, Johnstone AM. 2018. The big breakfast study: chrono-nutrition influence on energy expenditure and bodyweight. Nutr. Bull. 43, 174–183. ( 10.1111/nbu.12323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.O'Callaghan F, Muurlink O, Reid N. 2018. Effects of caffeine on sleep quality and daytime functioning. Risk Manag. Healthc. Policy 11, 263–271. ( 10.2147/RMHP.S156404) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Verlander LA, Benedict JO, Hanson DP. 1999. Stress and sleep patterns of college students. Percept. Mot. Skills 88, 893–898. ( 10.2466/pms.1999.88.3.893) [DOI] [PubMed] [Google Scholar]
- 170.Cajochen C, Munch M, Kobialka S, Krauchi K, Steiner R, Oelhafen P, Orgül S, Wirz-Justice A. 2005. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J. Clin. Endocrinol. Metab. 90, 1311–1316. ( 10.1210/jc.2004-0957) [DOI] [PubMed] [Google Scholar]
- 171.Mehta R, Zhu RJ. 2009. Blue or red? Exploring the effect of color on cognitive task performances. Science 323, 1226–1229. ( 10.1126/science.1169144) [DOI] [PubMed] [Google Scholar]
- 172.Chang AM, Aeschbach D, Duffy JF, Czeisler CA. 2015. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc. Natl Acad. Sci. USA 112, 1232–1237. ( 10.1073/pnas.1418490112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Harbard E, Allen NB, Trinder J, Bei B. 2016. What's keeping teenagers up? Prebedtime behaviors and actigraphy-assessed sleep over school and vacation. J. Adolesc. Health 58, 426–432. ( 10.1016/j.jadohealth.2015.12.011) [DOI] [PubMed] [Google Scholar]
- 174.Lemmer B. 2007. The sleep-wake cycle and sleeping pills. Physiol. Behav. 90, 285–293. ( 10.1016/j.physbeh.2006.09.006) [DOI] [PubMed] [Google Scholar]
- 175.Singleton RA, Wolfson AR. 2009. Alcohol consumption, sleep, and academic performance among college students. J. Stud. Alcohol Drugs 70, 355–363. ( 10.15288/jsad.2009.70.355) [DOI] [PubMed] [Google Scholar]
- 176.Raymann RJ, Swaab DF, Van Someren EJ. 2007. Skin temperature and sleep-onset latency: changes with age and insomnia. Physiol. Behav. 90, 257–266. ( 10.1016/j.physbeh.2006.09.008) [DOI] [PubMed] [Google Scholar]
- 177.Berk M. 2009. Sleep and depression - theory and practice. Aust. Fam. Physician 38, 302–304. [PubMed] [Google Scholar]
- 178.Ko PR, Kientz JA, Choe EK, Kay M, Landis CA, Watson NF. 2015. Consumer sleep technologies: a review of the landscape. J. Clin. Sleep Med. 11, 1455–1461. ( 10.5664/jcsm.5288) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.LeBourgeois MK, Giannotti F, Cortesi F, Wolfson AR, Harsh J. 2005. The relationship between reported sleep quality and sleep hygiene in Italian and American adolescents. Pediatrics 115(Suppl. 1), 257–265. ( 10.1542/peds.2004-0815H) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bader GG, Engdal S. 2000. The influence of bed firmness on sleep quality. Appl. Ergon. 31, 487–497. ( 10.1016/S0003-6870(00)00013-2) [DOI] [PubMed] [Google Scholar]
- 181.Lytle J, Mwatha C, Davis KK. 2014. Effect of lavender aromatherapy on vital signs and perceived quality of sleep in the intermediate care unit: a pilot study. Am. J. Crit. Care 23, 24–29. ( 10.4037/ajcc2014958) [DOI] [PubMed] [Google Scholar]
- 182.Robertson S, Loughran S, MacKenzie K. 2006. Ear protection as a treatment for disruptive snoring: do ear plugs really work? J. Laryngol. Otol. 120, 381–384. ( 10.1017/S0022215106000363) [DOI] [PubMed] [Google Scholar]
- 183.Blumen M, Quera Salva MA, d'Ortho MP, Leroux K, Audibert P, Fermanian C , Chabolle F, Lofaso F. 2009. Effect of sleeping alone on sleep quality in female bed partners of snorers. Eur. Respir. J. 34, 1127–1131. ( 10.1183/09031936.00012209) [DOI] [PubMed] [Google Scholar]
- 184.Ekirch AR. 2001. Sleep we have lost: pre-industrial slumber in the British Isles. Am. Hist. Rev. 106, 343–386. ( 10.2307/2651611) [DOI] [PubMed] [Google Scholar]
- 185.Ekirch AR. 2016. Segmented sleep in preindustrial societies. Sleep 39, 715–716. ( 10.5665/sleep.5558) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ekirch AR. 2018. What sleep research can learn from history. Sleep Health. 4, 515–518. ( 10.1016/j.sleh.2018.10.004) [DOI] [PubMed] [Google Scholar]
- 187.Yetish G, Kaplan H, Gurven M, Wood B, Pontzer H, Manger PR, Wilson C, Mcgregor R, Siegel JM. 2015. Natural sleep and its seasonal variations in three pre-industrial societies. Curr. Biol. 25, 2862–2868. ( 10.1016/j.cub.2015.09.046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ekirch AR. 2005. At day's close: A history of nighttime. London, UK: W. W. Norton & Company. [Google Scholar]
- 189.Handley S. 2016. Sleep in early modern England, xii, 280 pp New Haven, CT: Yale University Press. [Google Scholar]
- 190.Duncan WC, Barbato G, Fagioli I, Garcia-Borreguero D, Wehr TA. 2009. A biphasic daily pattern of slow wave activity during a two-day 90-minute sleep wake schedule. Arch. Ital. Biol. 147, 117–130. [PubMed] [Google Scholar]
- 191.Lal SK, Craig A. 2001. A critical review of the psychophysiology of driver fatigue. Biol. Psychol. 55, 173–194. ( 10.1016/S0301-0511(00)00085-5) [DOI] [PubMed] [Google Scholar]
- 192.McClelland L, Holland J, Lomas JP, Redfern N, Plunkett E. 2017. A national survey of the effects of fatigue on trainees in anaesthesia in the UK. Anaesthesia 72, 1069–1077. ( 10.1111/anae.13965) [DOI] [PubMed] [Google Scholar]
- 193.Cai Q, Gao ZK, Yang YX, Dang WD, Grebogi C. 2019. Multiplex limited penetrable horizontal visibility graph from EEG signals for driver fatigue detection. Int. J. Neural Syst. 29, 1850057 ( 10.1142/S0129065718500570) [DOI] [PubMed] [Google Scholar]
- 194.Lok R, Smolders K, Beersma DGM, de Kort YAW. 2018. Light, alertness, and alerting effects of white light: a literature overview. J. Biol. Rhythms 33, 589–601. ( 10.1177/0748730418796443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Parsons MJ, Moffitt TE, Gregory AM, Goldman-Mellor S, Nolan PM, Poulton R, Caspi A. 2015. Social jetlag, obesity and metabolic disorder: investigation in a cohort study. Int. J. Obes. (Lond.) 39, 842–848. ( 10.1038/ijo.2014.201) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.