Abstract
Trichomonas vaginalis is responsible for the most common non-viral sexually-transmitted disease worldwide. Standard treatment is with oral nitro-heterocyclic compounds, metronidazole or tinidazole, but resistance to these drugs is emerging and adverse effects can be problematic. Topical treatment offers potential benefits for increasing local drug concentrations and efficacy, while reducing systemic drug exposure, but no topical strategies are currently approved for trichomoniasis. The anti-rheumatic drug, auranofin (AF), was recently discovered to have significant trichomonacidal activity, but has a long plasma half-life and significant adverse effects. Here, we used this drug as a model to develop a novel topical formulation composed of AF-loaded nanoparticles (NP) embedded in a thermoresponsive hydrogel for intravaginal administration. The AF-NP composite gel showed sustained drug release for at least 12 h, and underwent sol-gel transition with increased viscoelasticity within a minute. Intravaginal administration in mice showed excellent NP retention for >6 h and markedly increased local AF levels, but reduced plasma and liver levels compared to oral treatment with a much higher dose. Furthermore, intravaginal AF-NP gel greatly outperformed oral AF in eliminating vaginal trichomonad infection in mice, while causing no systemic or local toxicity. These results show the potential of the AF-NP hydrogel formulation for effective topical therapy of vaginal infections.
Keywords: Trichomoniasis, antimicrobial, drug delivery, nanoparticle, hydrogel
Table of content entry:
The anti-rheumatic drug, auranofin (AF) is encapsulated into polymeric nanoparticles, which are embedded into a thermosensitive hydrogel for intravaginal administration. The composite hydrogel gel (denoted ‘AF-NP’ gel) facilitates tissue adhesion and sustained AF release, Intravaginal administration of AF-NP gel significantly outperforms oral AF in eliminating vaginal trichomonad infection in mice with minimum systemic or local toxicity, demonstrating the potential for effective topical therapy of vaginal infections.
Graphical Abstract
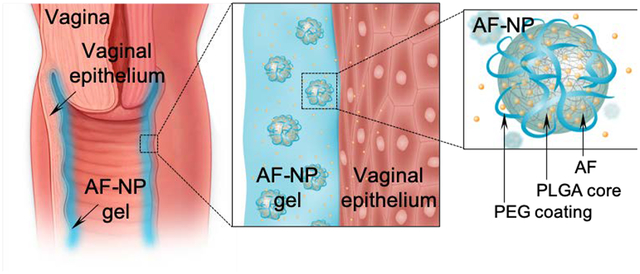
1. Introduction
Trichomonas vaginalis is a human protozoan pathogen responsible for the most common non-viral sexually-transmitted disease worldwide. In the United States, 3 to 5 million new cases of trichomoniasis occur annually, with overall prevalence rates of 0.5–3% [1]. Disease symptoms include purulent discharge accompanied by local irritation in women, and urethritis, epididymitis or prostatitis in men [2]. In addition to urogenital infections, trichomoniasis increases the risk of adverse pregnancy outcomes and HIV transmission, and the incidence and severity of cervical and possibly prostate cancers [3, 4]. Given its prevalence and association with multiple diseases, development of new treatment strategies against trichomoniasis remains an urgent need, particularly in women where infection can persist for months or even years compared to generally less than ten days in men [5].
Only two drugs are FDA-approved for the treatment of trichomoniasis, the nitro-heterocyclic compounds metronidazole and tinidazole. Oral administration of either drug leads to clinical and microbiological cure in the majority of cases, although treatment failures occur in 1–17% of patients [6, 7]. A United States survey found that 4.3% of 538 T. vaginalis isolates showed metronidazole resistance [8]. Higher oral metronidazole doses can sometimes lead to cure of refractory infections, but tend to be poorly tolerated [9]. While generally safe and effective, the current nitro-heterocyclic drugs have significant liabilities, with important adverse effects, including a host of neurologic maladies (peripheral neuropathy, cerebellar syndrome, encephalopathy, and meningitis), and intolerable nausea and gastric cramping [10], when given by the only approved oral route for trichomoniasis. Effects on the intestinal microbiota are also of increasing concern, given the broad-spectrum activity of nitro-heterocyclic drugs on multiple Gram-negative and anaerobic microbes.
In efforts to identify new trichomonacidal agents, numerous compounds have been tested in vitro against T. vaginalis. For example, a screen of 1,040 compounds in the U.S. Drug Collection Library revealed several drugs with significant trichomonacidal activity, although no drug was as effective as nitro-heterocyclic antimicrobials, and therefore none were tested in animal models or humans [11]. In another study, the anti-rheumatic drug, auranofin (AF), was shown to be active against T. vaginalis at low μM levels in vitro and have in vivo efficacy in a murine infection model [12]. AF, an Au(I) complex of tetraacetyl-thio-glucopyranoside thiolate and triethyl phosphine (Figure 1), is a potent inhibitor of thioredoxin reductase (TrxR), an enzyme involved in the defense against oxidative damage in eukaryotic cells [13]. Monovalent gold, Au(I), is thought to be released from AF, bind to critical thiol or selenosulfide residues in TrxR, which inactivates the enzyme. TrxR uses NADPH to reduce thioredoxin, which reduces catalytically active cysteines of thioredoxin peroxidase, thiol peroxidase, and other important targets, thereby maintaining redox homeostasis. The mechanism of the trichomonacidal action of AF has not been fully defined, but TrxR inhibition is likely to be involved. The enzyme is a key component of antioxidant defense in trichomonads, which lack the mammalian antioxidant defense systems of glutathione reductase and catalase but protect themselves against damage from toxic reactive oxygen species using NADPH oxidase (which reduces oxygen to hydrogen peroxide) and thioredoxin -dependent peroxidases. Consequently, T. vaginalis treated with AF are more susceptible to oxidative damage [12]. Given its putative action mechanism, the targets of AF are predictably separate from those of nitro-heterocyclic drugs and thus has promise as a new class of trichomonacidal agents. Although approved by the FDA for the treatment of rheumatic diseases, AF has significant adverse effects after oral administration that have contributed to its diminished use in recent years. Part of the reason for the toxicity may be the long plasma half-life (35 days) of AF [14], resulting in sustained systemic exposure even after short-term therapeutic objectives may have been achieved.
Figure 1.
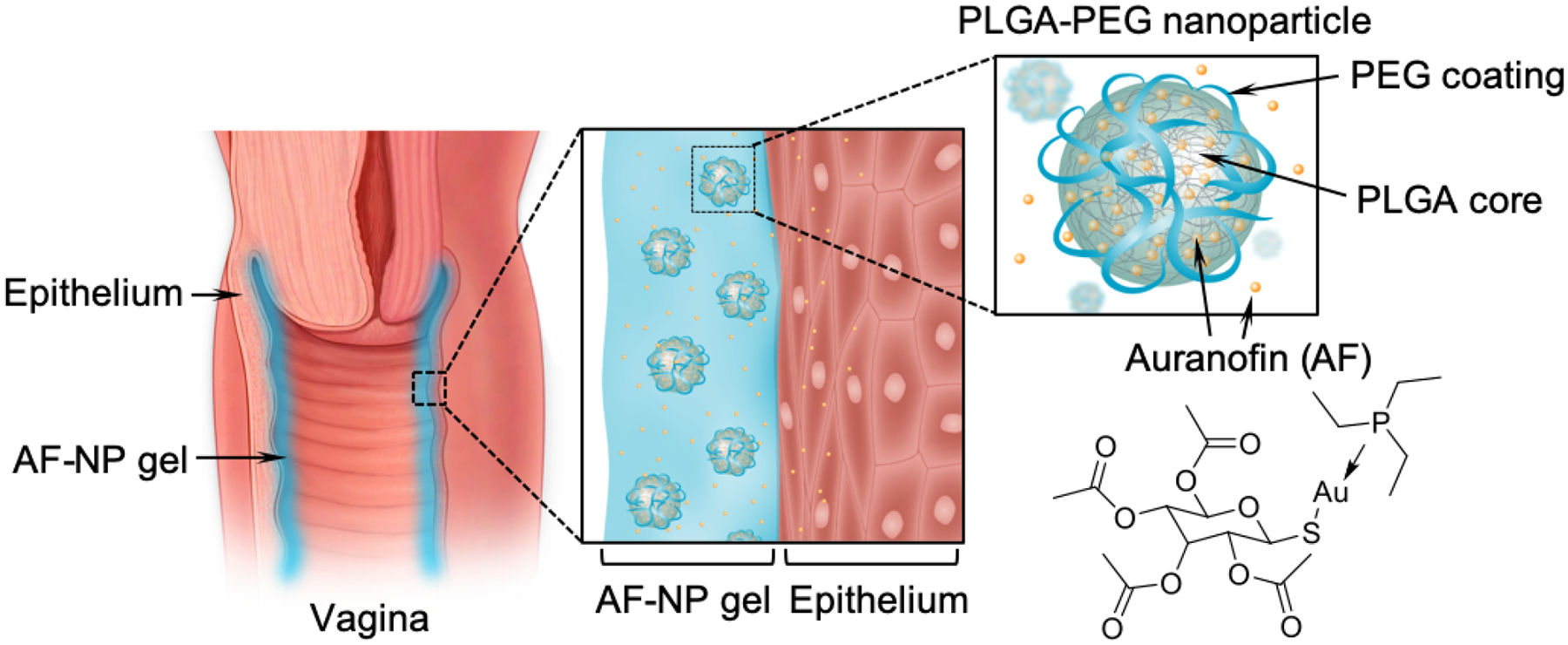
Scheme of AF-NP composite hydrogel. The composite gel consists of auranofin (AF)-loaded nanoparticles (AF-NP) embedded into a thermoresponsive chitosan-based hydrogel. Upon intravaginal instillation of the liquid gel, the shift to body temperature causes a sol-gel transition that helps to anchor the gel on the surface of the vaginal epithelium. AF is topically released from the NPs in a sustained fashion. NPs are made from poly(lactic-co-glycolic) acid (PLGA) and are functionalized with polyethylene glycol (PEG) to increase stability. The structure of AF is shown at the bottom right.
As a localized infection of the urogenital tract, trichomoniasis provides an attractive target for topical therapies that might limit systemic drug exposure. Although possible in principle for vaginal infections, as shown for mycoses [15], topical treatments have so far proven to be less effective than systemic strategies for trichomoniasis [16, 17]. However, improved strategies for effective topical drug delivery would hold great promise for changing the prevalent systemic treatment paradigm for T. vaginalis infection in women, as it would be expected to combine excellent local efficacy with diminished risk of systemic adverse effects.
Several advanced formulations have been proposed for topical treatment in the urogenital tract and at other mucosal sites [18–21]. Among those, hybrid systems that combine multiple constituents and functionalities are particularly attractive, because they can combine advantages intrinsic to each constituent for synergy. In this perspective, nanoparticle (NP)-hydrogel hybrids are particularly attractive. For the NPs, a range of different materials and fabrication methods have been reported, including liposomes [22, 23], polymeric NPs [24], graphene quantum dots [25], metal NPs [26, 27], silica NPs [28], and carbon nanotubes [29]. NPs allow for encapsulation of various drugs for controlled delivery, but their utility may be limited by uneven distribution and insufficient retention in the target tissues due to their small size. This limitation may be overcome by embedding NPs into a hydrogel matrix for maximizing tissue retention. NP embedding can be achieved by various means, including simple mixing of NPs and matrix, or controlled NP growth inside a matrix [30–32]. The utility of such formulations has been demonstrated in a range of applications from drug delivery [25, 33] to detoxification [34], and biosensing [35]. NP-hydrogel composites have been explored for topical applications in various organs [36]. The vaginal tissues poses unique challenges that must be overcome for effective drug delivery, because it is a fibromuscular, collapsible tube lined with a thick and dynamic layer of mucin for clearing external particles [37]. In this study, we set out to address these challenges with a novel thermoresponsive NP-hydrogel composite for the effective topical treatment of trichomoniasis.
2. Results
2.1. Fabrication and physico-chemical characterization of nanoparticle composite hydrogel
To construct a NP composite hydrogel for topical vaginal administration (Figure 1), we employed a two-step fabrication process. First, AF-loaded NPs (denoted “AF-NPs’) were synthesized by nanoprecipitation [38]. For this, AF and poly(lactic-co-glycolic) acid (PLGA) were co-dissolved in acetone, added to an aqueous solution of the nonionic surfactant pluronic, F-127, and stirred overnight to allow acetone to evaporate. The self-assembled AF-loaded NPs displayed a hydrodynamic diameter of ~130 nm similar to that of AF-free control NPs (Figure 2A,B). AF-NPs and control NPs also had similar negative surface charges with zeta-potentials of about −25 mV. These findings indicated that AF loading did not interfere with NP self-assembly or altered the physical characteristics of the NPs. For higher AF loading, we prepared three different formulations with increasing AF input. After removing the free AF, encapsulated AF was quantified with HPLC. AF loading yield increased with AF input, reaching 3.1% (w/w) at an input of 30% (Figure 2C). At this AF input, the drug loading efficiency of AF into the NPs was 10.3%. This formulation was selected for all subsequent studies. Additionally, both free and encapsulated AF showed similar elution times and peak shape (Figure 2D), suggesting that the drug was structurally intact after loading into the AF-NPs.
Figure 2.
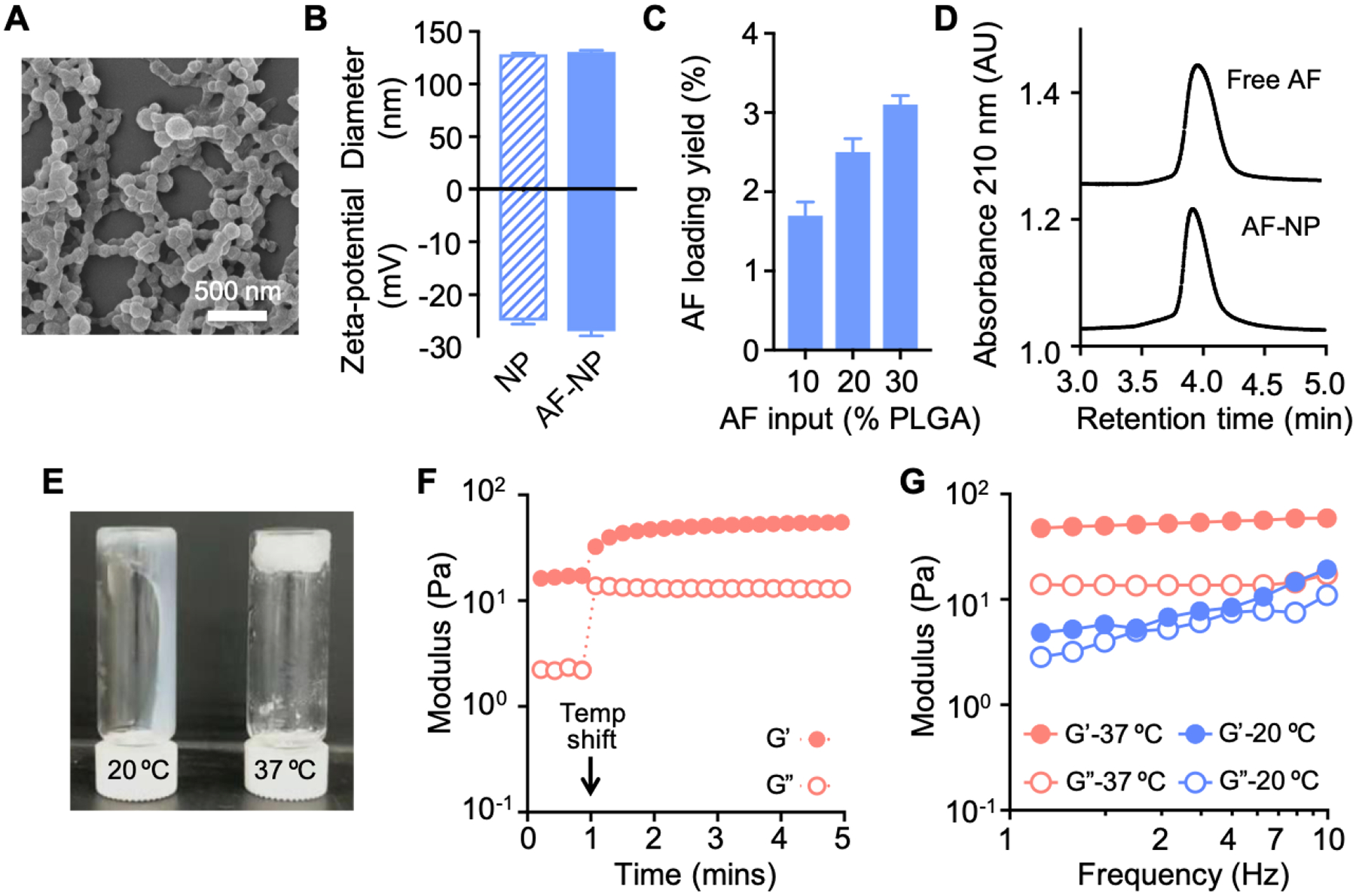
Physico-chemical characteristics of AF-NP gel. (A) The image shows the morphology of AF-NPs examined by scanning electron microscopy. (B) Measurement of the diameter and zeta-potential of AF-NPs (mean ± SEM, n = 3). (C) Quantification of AF loading yield in AF-NPs at different AF inputs (mean ± SEM, n=3). (D) Characteristic HPLC chromatograms of AF before and after loading into NPs. (E) Qualitative demonstration of thermoresponsive nature of chitosan hydrogel. The gel was loaded at ambient temperature (20 °C) into two tubes, one of which was warmed to 37°C in a water bath for 5 min. Both tubes were then inverted and photographed after 1 min. The left tube (20°C) shows the gel running down the side, while the gel remained a coherent plug in the right tube (37°C). (F) Rheological measurement of gelation time after temperature shift from 20 to 37℃ at the 1 min mark (arrow). (G) Frequency sweep of the rheological properties of the AF-NP gel at 20 and 37℃.
Following fabrication, AF-NPs were embedded into a thermoresponsive hydrogel that allows temperature-dependent gelation upon a shift to body temperature after topical administration in vivo. To prepare AF-NP gel, AF-NPs were added to a mixture of β-glycerophosphate and chitosan precursor solution with constant stirring under ice-cold conditions. In the final formulation, the AF-NP concentration was 64 mg/ml (as determined by PLGA content). Together, with the AF loading yield of the NPs, we calculated that the final AF concentration in the AF-NP gel was 64 mg/ml × 3.1% = 1.98 mg/mL. To investigate the thermoresponsive behavior of the AF-NP gel, we monitored the storage modulus (G’) and loss modulus (G”) with a rheometer in an oscillatory time sweep mode. Upon temperature shifting from 20 to 37℃, both G’ and G” increased within 1 min and remained stable thereafter, indicating a rapid temperature-induce gelation process (Figure 2E, F). Moreover, both moduli became less sensitive to low frequencies in the sweeps at 37℃ compared to 20℃ (Figure 2G), suggesting the formation of a cross-linked gel structure at the higher temperature. Together, these results demonstrate the thermoresponsive nature of the AF-NP gel.
To determine the storage stability of the drug within the AF-NP gel formulation, we synthesized AF-NP gel and stored the samples under 4, 25, or 37℃ for 7 days. Samples were then allowed to release the encapsulated AF and assayed by HPLC. AF from samples under different storage conditions showed similar elution times and peak shapes, which also comparable to those of freshly dissolved AF. Quantitative analysis of the areas under the curve revealed comparable drug concentrations in all samples (100.3 ± 0.6%, 99.3 ± 0.8%, and 99.3 ± 0.3% after storage at 4, 25, and 37 °C storage for 7 days, respectively, relative to the loaded AF; mean ± SD, n=3). These results validate the storage stability of AF within the AF-NP gel at different temperatures.
2.2. In vitro activity of AF-NP gel
To determine the drug release properties, AF-NP gel and free AF-NPs as controls were dialyzed against water at 37°C, and the unreleased AF was quantified by inductively coupled plasma-optical emission spectrometry (ICP-OES) for the central gold atom. AF was released from both the AF-NP gel and the free AF-NPs in a time-dependent, delayed fashion with 70–80% release after 12 h of incubation (Figure 3A). The similar release kinetics of AF-NP gel and free AF-NPs indicates that release is primarily determined by the NPs, while the hydrogel matrix has minimal impact on release. Furthermore, AF release from the NPs showed weak pH dependence as similar release kinetics were observed at pH 7.0 and pH 4.5, the pH range in the vaginal lumen under different physiological conditions (Figure 3B).
Figure 3.
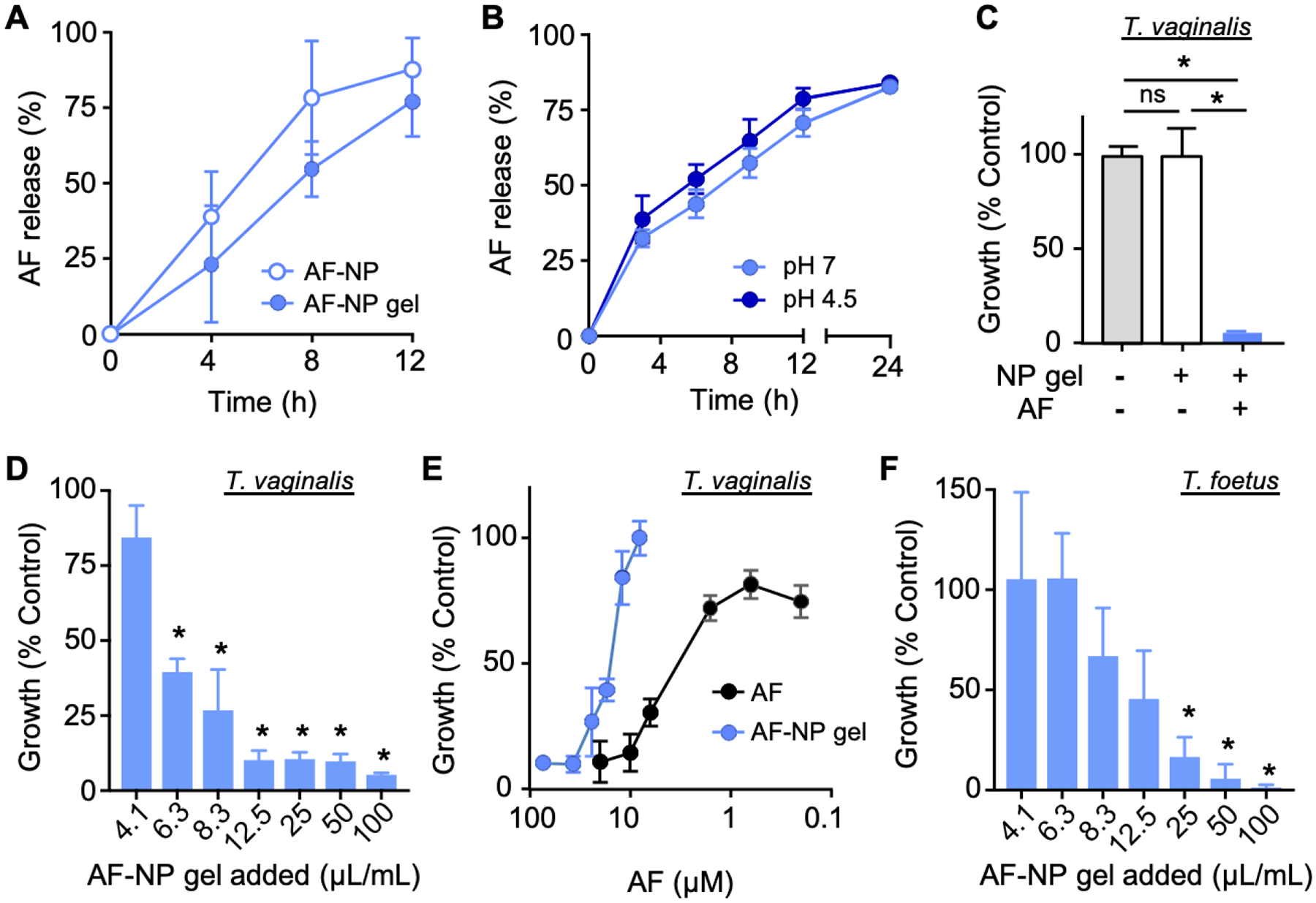
In vitro activity of AF-NP composite gel against trichomonads. (A, B) Cumulative drug release from AF-NP gel and AP-NPs without gel at pH 7 (A), or of AP-NPs at the indicated pH (B), over 12–24 h were determined by dialysis followed by ICP-AES or HPLC analysis (mean ± SEM, n=3). (C-F) AF-NP gel or AF-free NP control gel were diluted with growth media to 100 μL/ml (C) or the indicated dilutions (v/v; D, F), or plain AF was diluted in growth media to the indicated concentrations (E). T. vaginalis or T. foetus trophozoites were added, and cultures were incubated for 24 h under anaerobic conditions. Cell growth and viability were determined with a luminescent ATP assay. Data are mean ± SEM (n=3) relative to growth media alone (C, E) or AF-free NP gel at the same dilution (D, F). AF concentrations in the AF-NP gel treated cultures were calculated from the AF content of the NPs, the NP content in the hybrid gel, and the dilution of AF-NP gel in the final culture volumes. *p<0.05 vs growth media alone (C) or AF-free NP-gel at the same dilution (D, F), as determined by Kruskal-Wallis with Dunn’s post-hoc test or Mann-Whitney tests; ns, not significant.
Next, we evaluated the trichomonacidal activity of the AP-NP gel in vitro. Equal volumes of AF-NP gel or AF-free NP gel were added to varying volumes of growth media, and trophozoites of two trichomonad species, T. vaginalis and T. foetus, were added. Cultures were incubated for 24 h, after which parasite growth and survival were determined. The AF-NP gel completely inhibited parasite growth, whereas the control NP gel without AF had no impact on growth (Figure 3C). Furthermore, the activity of the AF-NP gel against T. vaginalis was dose-dependent, with significant growth inhibition seen at dilutions as low as 0.63% (v/v) (Figure 3D). Comparison of the dose-response curves measured from AF-NP gel and plain AF based on the AF concentrations showed that the NP gel formulation was ~8-fold less potent than the plain drug, with EC50 values of 22 and 2.7 μM, respectively (Figure 3E). Similar results were obtained for T. foetus, though 4-fold more of the AF-NP gel was required to achieve complete growth inhibition (Figure 3F). These results demonstrate that the AF-NP gel was highly effective even at a small volume fraction (≤2.5%), although the AF-NP gel formulation was not as potent in vitro as plain AF.
2.3. Local vaginal retention of AF-NP composite hydrogel
The principal constituent of the AF-NP gel, chitosan, is a mucoadhesive polymer by virtue of its extensive interactions with mucins through electrostatic attraction, hydrogen bonding, and hydrophobic effects [39]. Consequently, we hypothesized that the chitosan-based gel facilitates the topical retention of the AF-NPs, thereby allowing for sustained local drug delivery while minimizing systemic exposure. To test this hypothesis, we fabricated NPs loaded with a fluorescent dye, DiD (denoted ‘DiD-NPs’), embedded them into a chitosan-based hydrogel, and administered the resulting DiD-NP gel intravaginally to adult female mice. A thick layer of DiD fluorescence was observed overlying the vaginal epithelium at 24 h after the administration (Figure 4A), suggesting that the DiD-NP gel was well retained and evenly distributed in the target tissue. A time course analysis of fluorescence confirmed that the DiD-NP gel was retained significantly better in the vaginal tissue than free DiD-NPs (Figure 4B). Nearly 50% of the initially administered dose (as determined by total red fluorescence) was still present in the vaginal tissue after 6 h, while free DiD-NPs without gel were eliminated by that time. These results demonstrate that the topically applied hydrogel-embedded NPs were well retained in the vaginal tissue with minimal systemic absorption.
Figure 4.
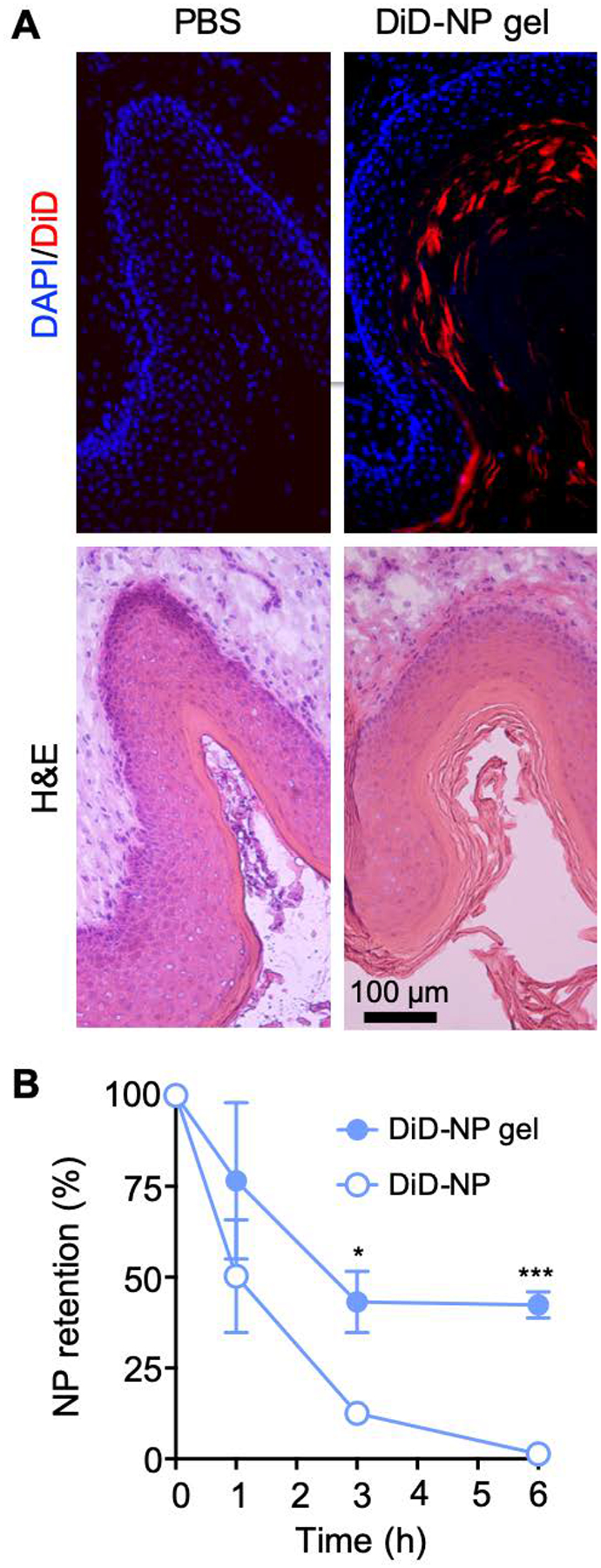
Biodistribution of NP composite gel. (A) Female BALB/c mice were injected intravaginally with a DiD-NP gel or PBS as a control. After 24 h, frozen sections of the vaginal tissue were stained with the nuclear stain, DAPI, and examined by fluorescence microscopy for DiD (red, top row) and DAPI (blue, top row). Parallel sections were stained with H&E (bottom row). (B) Retention of DiD-NPs after intravaginal administration of DiD-NP gel or plain DiD-NPs without gel, as determined by fluorescence spectroscopy of vaginal extracts at different times after NP administration (mean ± SEM, n=3; ***p<0.001 and *p<0.05 by unpaired two-tailed t-test vs. DiD-NPs at the same time).
2.4. Topical drug delivery and in vivo efficacy of AF-NP gel
To determine drug release from the topically applied AF-NP gel in vivo, we administered five doses (10 μg AF in 5 μL per dose; 50 μg total) of the composite gel intravaginally to adult female mice over three days, and assayed drug levels in tissue extracts on day 4 by ICP-OES. Vaginal AF levels ranged from 0.5–1 μg/g tissue, whereas untreated control mice had no detectable drug (Figure 5A). In the same mice, AF levels in the liver and the vaginal were comparable, while AF levels in plasma were modestly (2-fold) higher. By comparison, mice given five oral AF doses over three days at the highest tolerable dose (30 mg AF/kg; 3,000 μg total) had much lower vaginal AF levels (<0.25 μg/g tissue) on day 4, but slightly higher plasma AF levels and markedly higher liver AF levels (Figure 5B). Importantly, both liver and plasma levels were significantly higher than vaginal AF levels after oral AF administration, which contrasted sharply from the findings in the topically treated mice. Thus, despite the lower overall administered dose of AF, the local drug levels in the vaginal tissue were higher with topical administration of the AF-NP gel compared to oral treatment with free AF.
Figure 5.
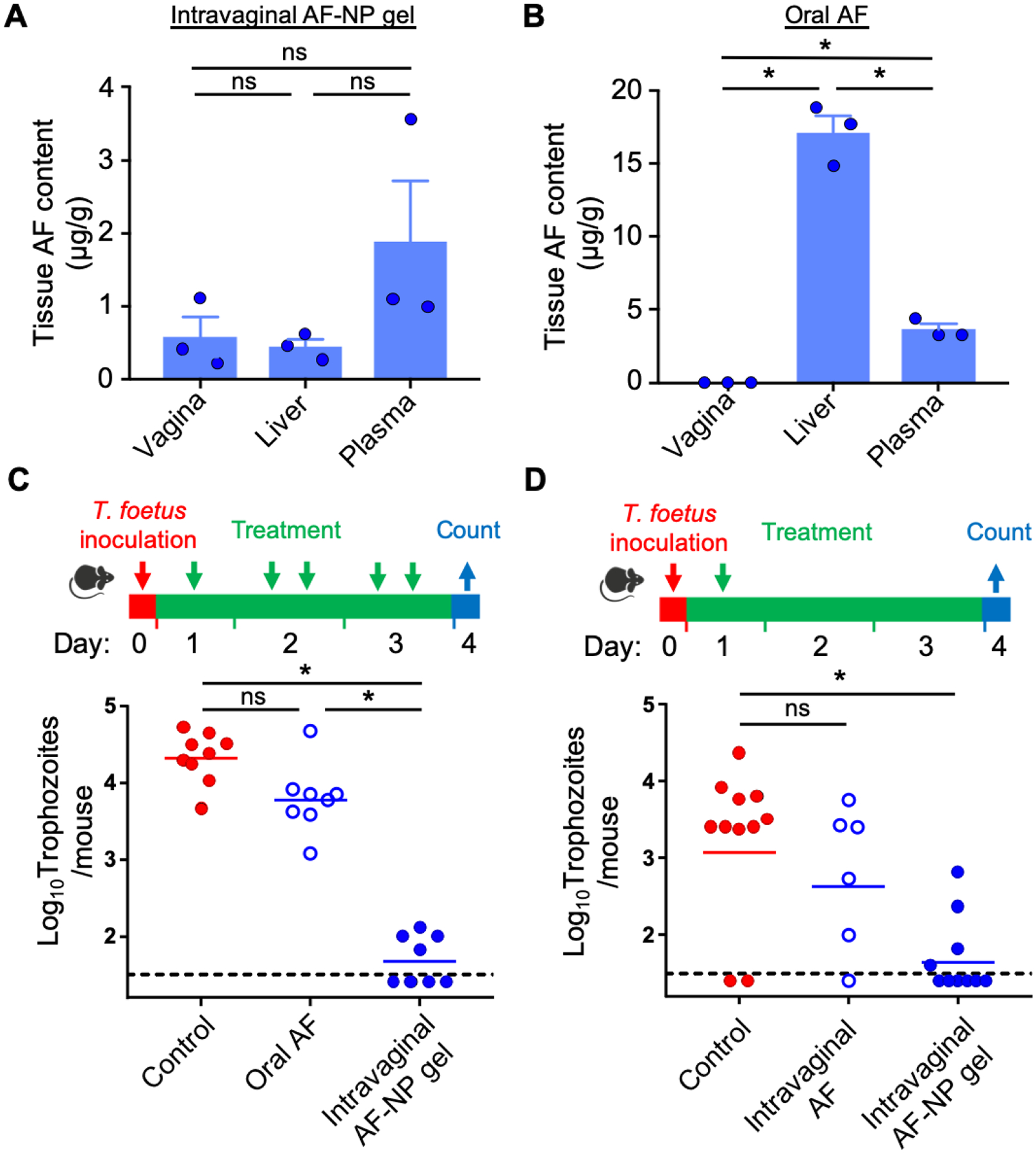
In vivo drug release and efficacy of AF-NP gel. (A, B) Tissue levels of AF after intravaginal administration of AF-NP gel (5 x doses of 10 μg AF in 5 μL per dose over 3 days; 50 μg total AF) or oral administration of plain AF (5 x doses at 30 mg/kg over 3 days; 3,000 μg total AF dose) to female BALB/c mice (bars are mean + SEM, n=3; circles show individual mice; *p<0.05 by Kruskal-Wallis with Dunn’s post-hoc test or Mann-Whitney test; ns, not significant). AF levels were determined by ICP-AES in tissue extracts. (C, D) In vivo efficacy of AF-NP gel. Weanling BALB/c mice were inoculated intravaginally with T. foetus trophozoites. After one day, mice were treated five times (C) or one time (D) at the indicated times by oral administration of plain AF (30 mg/kg per dose), or by intravaginal administration of AF-NP gel (10 μg AF/dose in 5 μL) or plain AF (10 μg/dose in 5 μL) in PBS. Infected mice given PBS served as the control. Live trophozoites were enumerated in vaginal washes on day 4 (bars are mean ± SEM, circles show individual mice; n=6–10 mice/group; *p<0.05 by Kruskal-Wallis with Dunn’s post-hoc test or Mann-Whitney test; ns, not significant). The dashed lines depict the detection limit of the assay.
We then sought to determine the efficacy of the AF-NP composite gel in a vaginal trichomonad infection model. Mice were inoculated intravaginally with T. foetus trophozoites and treated, beginning after one day, with five intravaginal doses (total dose, 50 μg AF/mouse) of the AF-NP gel over three days, or were left untreated. Topical treatment markedly reduced infection in all mice and led to eradication in half of the mice, whereas oral administration of free AF at the highest tolerable dose (30 mg/kg; total AF dose, 3,000 μg/mouse) had only modest effects on the parasite load that did not reach significance (Figure 5C). Furthermore, even a single dose of the AF-NP gel (10 μg AF/mouse) led to marked parasite clearance (Figure 5D). By comparison, intravaginal administration of an equivalent single dose (10 μg) of free AF in PBS had no significant therapeutic effect (Figure 5D). Together, these results show that the topical AF-NP gel greatly outperformed oral AF in treating trichomonad infection despite a much lower total drug dosage, and that the NP gel formulation was critical for topical AF efficacy.
2.5. Toxicological evaluation of topically administered AF-NP gel
AF has significant acute systemic toxicity [40], which had limited our oral drug administration regimen to doses that were only marginally effective. We hypothesized that AP-NP gel, when topically administered, would reduce the adverse effects otherwise caused by AF. To evaluate systemic toxicity, we assayed the activity of TrxR in the liver, since this enzyme is a well-characterized target of the drug [41] and liver exposure to the drug was particularly high after oral AF administration (Figure 5B). Topical drug treatment with five doses had no significant effect on hepatic TrxR activity, whereas oral AF treatment caused the expected inhibition of TrxR (Figure 6A, B). These data provide important functional evidence for the observation that the liver AF levels were >30-fold lower after topical compared to oral dosing (Figure 5A, B).
Figure 6.
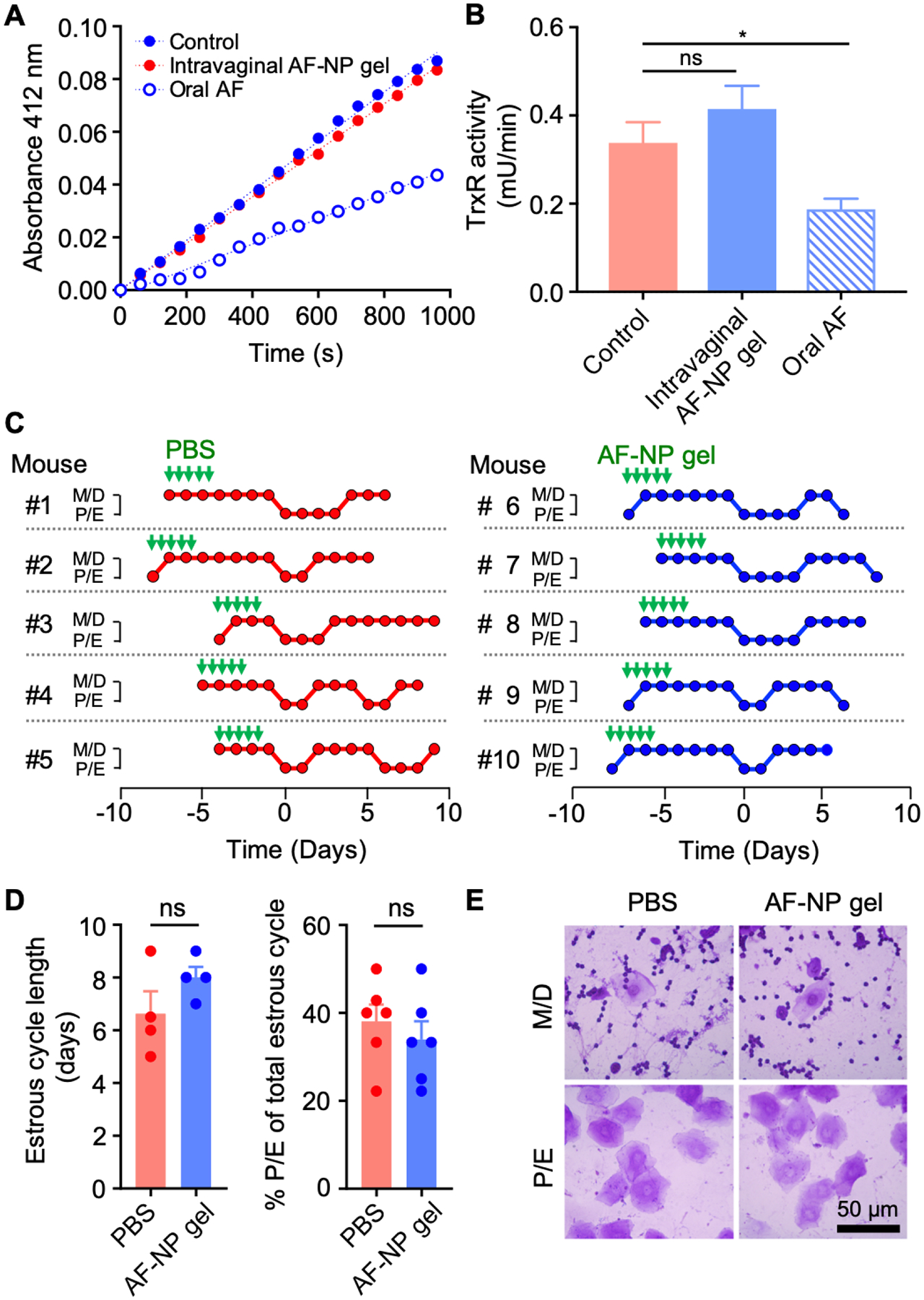
Toxicity evaluation of AF-NP gel. (A, B) Adult female BALB/c mice were given fives doses over three days of either intravaginal AF-NP gel (10 μg AF/dose) or oral unformulated AF (30 mg/kg per dose), with untreated mice as controls. Liver extracts were prepared and assayed for TrxR activity by DTNB reduction. Representative reaction kinetics are shown in panel A, and enzyme activities are shown in panel B (mean ± SEM, n=18; *p<0.05 by Kruskal-Wallis test with Dunn’s post-hoc test; ns, not significant). (C-E) Estrus cycle analysis. Adult female mice were given intravaginal AF-NP gel (10 μg/dose) for five doses over three days, or PBS as controls, as indicated by the green arrows. Estrus cycle phases were determined by daily cytological analysis of crystal violet-stained vaginal washes in individual mice (C). Metestrus and diestrus (M/D) are cytologically characterized by predominant leukocytes mixed with occasional epithelial cells, while proestrus and estrus (P/E) are characterized by >90% nucleated and/or cornified epithelial cells. Phases are lined up in the graphs for the first detected M/D to P/E transition (day 0). Cycle length was determined by the time between M/D to P/E transitions or P/E to M/D transitions, or the average of both in any given mouse. The percentage of the P/E phase within the total cycle was calculated by days in the P/E phase divided by the total cycle length (D; mean ± SEM, n=5/group; ns, not significant by Mann-Whitney test). (E) Representative photographs of stained vaginal smears for mice treated with 5 x doses of AF-NP gel or PBS as a control. No morphological abnormalities were observed after AP-NP gel administration.
To examine potential local toxicity of the AF-NP gel, we evaluated the impact of the treatment on the estrus cycle in female mice, since this complex physiological function depends on multiple hormonal, molecular and cellular processes, and can thus serve as a comprehensive measure of normal vaginal physiology [42]. Treatment did not significantly alter the estrus cycle, as determined by cycle length, duration of proestrus and estrus phases relative to the total cycle, and the characteristic cellular composition and morphology during the different cycle phases (Figure 6C–E). Together, our findings show that the AF-NP gel has no apparent adverse systemic or local effects upon repeated topical vaginal application of efficacious doses.
3. Discussion
Our study demonstrates that AF-NP gel is an effective and safe topical treatment for vaginal trichomonad infection. Compared to oral AF administration, the topically applied AF-NP gel significantly increased the drug concentration in the vaginal tissue leading to greater efficacy, but with lower systemic drug exposure and no apparent liver or topical toxicity. The superior in vivo performance of the AF-NP gel is likely related to its modular structure with two major elements, i.e., the AF-loaded NPs and the chitosan-based hydrogel. Our data show that the NPs effectively controlled the drug release, which was sustained for at least 12 h. By comparison, in a different AF-loaded NP system for a dermal indication, drug release was complete within 8 h [43]. The difference in release kinetics may be explained by the larger diameter of our NPs and the additional hydrophilic barrier of the hydrogel matrix. Importantly, for the NPs to achieve full drug release in our system, it was critical that they were retained in the vaginal tissue for sufficiently long periods (>12 h). This could not be achieved by direct topical NP administration, which led to complete NP loss within 6 h, but required formulation in a mucoadhesive gel for sustained retention. Together, these synergistic interactions between sustained AF release from the NPs and prolonged NP retention in the vaginal tissue through NP embedding in a hydrogel facilitated maximal topical drug efficacy.
Topical treatment of vaginal infections is an alternative to oral formulations for a number of infectious agents [15], but efficacy against vaginal trichomoniasis has so far been inconsistent [16, 17]. The reasons are not well understood, but limitations in drug potency or pharmacokinetics are presumably important. The vaginal tissue poses unique challenges for topical formulations, since abundant moisture, mucins, and motility promote ready clearance of luminally applied formulations [37]. Gels made from carbomer [44], cellulose [18], hyaluronic acid [19], or alginate [20] have been developed as drug carriers, but the hydrophilic nature of these polymers limits drug loading yields for many hydrophobic pharmaceutical compounds such as AF, which has a logP value of 1.6 and aqueous solubility of only 0.015% (w/w) [45]. In contrast, drug loading into PLGA-based NPs allowed for a 13-fold higher loading yield of 0.2% (w/w) in the final AF-NP gel, thus permitting a much higher local drug dose in a smaller volume. The AF loading into NPs comes at the expense of reduced acute potency compared to free AF, an observation also made for other drugs, such as the anti-cancer compound doxorubicin [46]. However, this limitation did not appear to impact in vivo efficacy, because the AF-NP gel was markedly more efficacious than much higher oral AF doses. Importantly, polymeric NPs or microspheres with similar properties could be made with starting materials other than PLGA to achieve optimal loading yields for drugs with different hydrophobicity [47, 48], suggesting that NP composite hydrogels can provide broadly permissive topical formulation platforms with high loading yields for the treatment of other topical diseases with a wide range of hydrophilic and hydrophobic drugs.
Another challenge facing topical formulations in vaginal drug delivery is the possibility of uneven distribution of the dosage forms along the vaginal epithelium, which can limit the drug availability in infected areas. We found that a thermoresponsive hydrogel offers an effective approach to this challenge. The thermoresponsive materials, such as poloxamer [49], guar-based natural polymers [50,] dextran/poly(N-isopropylacrylamide) copolymers [25], or, in our case, a mixture of β-glycerophosphate and chitosan [51], in such gels are liquid at room temperature but crosslink into a highly viscous network at body temperature. The low viscosity of the liquid application form allows efficient spreading along the vagina and ectocervix, while the gelation after the temperature shift promotes mucoadhesion by entanglement between the polymer chains and mucin and anchoring in tissue folds [52]. Another advantage of the thermoresponsive chitosan-based hydrogel is its ability to reduce transmural absorption across the vaginal epithelium [53], which predictably contributed to achieving highly effective local drug levels with minimal systemic exposure [54]. Minimal systemic absorption is particularly important for a drug such as AF, which has a very long plasma half-life of 35 days [14].
New drugs and formulations must be safe to be considered for clinical trials, so comprehensive safety studies in different in vitro and in vivo models are required for both drug and drug carrier. For a drug such as AF, which is already FDA-approved for another indication [40], but has the potential to be repurposed for trichomoniasis [12], the focus of safety evaluations can be on potential topical impact. For example, localized exposure to potentially high concentrations of AF released from a topically applied formulation was found to be safe in a bacterial skin infection model [55], suggesting that toxicity associated with topical AF should not be a significant impediment for vaginal drug application. Furthermore, our initial analysis of selected systemic and topical tissue responses also suggest that the NP-hydrogel will prove to be safe in clinical applications. The two primary components of the formulation, PLGA and chitosan, are well known to be biocompatible [56–58]. PLGA is an FDA-approved natural biodegradable material that is used in surgical, drug delivery applications, and tissue engineering [59–61]. NPs made from PLGA have proven to be biocompatible and safe in a number of topical formulations including for skin and eyes [55, 62, 63], so similar characteristics would be expected in vaginal applications [64]. We note that it may be possible to make precipitates from hydrophohic drugs such as AF with nano-scale dimensions, thereby obviating the need for PLGA as a NP scaffold, but the size and shape of such particles would be difficult to control in fabrication. In contrast, PLGA provides a consistent “backbone” for the reliable construction of nanoparticles with defined physical properties, so drug loading into PLGA-based nanoparticles can avoid fabrication uncertainties and provide a broadly applicable drug delivery platform.
Our studies provide new impetus for the concept that topical treatment can be effective against vaginal trichomoniasis. Conflicting findings on topical effectiveness of trichomonacidal agents have been reported. In one pilot study, topical metronidazole had lower cure rates than oral formulation at equal dosage (44% vs. 100%) [16]. In a case report of metronidazole-resistant trichomoniasis, the patient failed to respond to a high dose of oral and topical metronidazole, but was successfully treated with an intravaginal paromomycin formulation [65]. Notably, the topical formulations used in these studies were not optimized for prolonged vaginal retention, which might explain some of the treatment failures. In another pilot study, patients with T. vaginalis infection treated with vaginal suppositories consisting of a combination of metronidazole and miconazole had cure rates that were not significantly different from those after oral metronidazole [17]. Differences in dosing and administration frequencies are likely to account for some of these discrepant outcomes, which underlines that effective topical delivery of trichomonacidal drugs is presumably critical for high and reliable efficacy. Our studies suggest that such efficacy can be achieved for vaginal trichomonad infection with a composite AF- NP gel formulation.
4. Conclusion
In summary, we synthesized a thermoresponsive NP-hydrogel composite system by embedding AF-NPs into a chitosan hydrogel matrix. The resulting topical drug delivery platform, namely AF-NP gel, was proven to be a safe and effective treatment against trichomonad infection, outperforming oral treatment with better efficacy at a markedly reduced total AF dosage. The findings demonstrate that topical treatment can be highly effective in eliminating vaginal trichomonad infection. Our formulation strategy has the potential to be generalized for combatting other localized infections in women, such as bacterial vaginitis and vaginal yeast infections.
5. Experimental Section
Materials:
High molecular weight chitosan, β-glycerophosphate disodium salt hydrate, glacial acetic acid, and pluronic F-127 were purchased from Sigma Aldrich. Acetone, nitric acid (trace metal grade), hydrochloric acid (trace metal grade) were purchased from Fisher Scientific. AF was purchased from Enzo Life Sciences. 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine, 4-chlorobenzenesulfonate salt (DiD; excitation/emission, 644/665 nm) were purchased from ThermoFisher Scientific. PLGA (50:50, 0.67 dL/g) was purchased from LACTEL Absorbable Polymers.
Construction of AF-NP composite gel:
AF-loaded NPs were produced with a nanoprecipitation method by combining 20 mL of 5 mg/mL PLGA in acetone with variable amounts of AF, and adding the mixture to 40 mL of a 5 mg/mL aqueous solution of the nonionic surfactant pluronic, F-127 (which provides a hydrophilic coating of polyethylene glycol to the hydrophobic PLGA core). The mixture was stirred overnight to evaporate the acetone. To prepare fluorescently labeled NPs, PLGA was mixed with DiD at a polymer-to-dye weight ratio of 1,000:1 and added to the solution of pluronic F127. The AP-NPs or DiD-NPs were collected and washed by centrifugation (25,000 g for 20 mins), and samples were resuspended in double-distilled water. After synthesis, AF-NPs or DiD-NPs were embedded into the chitosan-based hydrogel. Specifically, a chitosan hydrogel precursor solution was prepared by dissolving high molecular weight chitosan powder in 0.5% (v/v) acetic acid with continuous stirring to reach a final concentration of 13 mg/mL. β-glycerophosphate was added to the chitosan solution in an ice bath. Concentrated AF-NP suspension was then slowly added to β-glycerophosphate pre-mixed with chitosan under constant stirring. The resulting composite AF-NP or DiD-NP gels were stored at 4℃ until use. Final concentrations of AF, PLGA, chitosan and β-glycerophosphate were 2 mg/mL, 64 mg/mL, 10 mg/mL, and 308 mg/mL, respectively.
Physico-chemical characterization of AP-NP gel:
NP morphology was analyzed by scanning electron microscope (FEI XL30 SFEG instrument). Size and zeta-potential of the NPs were determined by dynamic light scattering (DLS, Malvern ZEN 3600 Zetasizer). Drug loading yield in the AF-NP was assayed by high performance liquid chromatography (HPLC, PerkinElmer Flexar series 200) equipped with a Brownlee SPP C18 column (100 mm length ×4.6 mm diameter column filled with 2.7 μm diameter beads). Briefly, acetonitrile was used to dissolve the NPs and release the encapsulated AF. An equal volume of water was added to precipitate the PLGA polymer. The supernatant was analyzed for AF by HPLC using a mobile phase of 50% acetonitrile, 50% water (v/v) at 1 mL/min at ambient temperature, and absorbance measurements at 210 nm. The drug loading yield was calculated as the weight percentage of AF relative to the total PLGA mass. For stability test, AF-NP gel was stored at 4, 25, or 37℃ for 7 days. Afterwards, AF-NPs were collected by centrifugation (20,000 ×g, 20 min) and dissolved in acetonitrile for 3 h to completely release AF. Drug content was analyzed by HPLC.
Rheological tests:
The rheological characteristics and gelation behavior of the AF-NP gel were tested on a strain-controlled AR-G2 rheometer with a 20 mm diameter parallel-plate geometry (TA Instruments Inc., New Castle, DE). To perform the test, 200 μL AF-NP gel was extruded onto the sample holder before the gap between the holder and probe was set to 500 μm. The gelation time was determined by monitoring changes in the mechanical gel properties after temperature shift from 20℃ (ambient temperature) to 37℃ (body temperature) at 2% strain and 1 rad/s. For frequency sweeping, the mechanical gel properties were monitored at 20 and 37℃ in an oscillatory time sweep mode at 2% strain with frequencies ranging from 1 to 10 Hz.
AF release assays:
To evaluate AF release, AF-NP gel or free AF-NPs were loaded into dialysis cups (10 kDa cutoff; Slide-A-Lyzer™ MINI Dialysis Devices) and dialyzed at 37℃ against 2 L of phosphate-buffered water (pH of 7.0 or 4.5). The buffer was changed every 2 h for the first 12 h. At various time points, the amount of NP-associated AF remaining in the cups was determined by ICP-OES (Perkin Elmer Optima 3000) or HPLC. For ICP-OES, NPs were dissolved for 2 h in aqua regia (hydrochloride acid:nitric acid, 3:1; v/v) to release the gold ion, followed by acid removal through overnight heating at 80℃. The crystallized gold salt was dissolved in 5 mL of 2% nitric acid, and the gold content was analyzed by ICP-OES based on the gold emission line at 242.8 nm. For HPLC, NPs were dissolved in acetonitrile for 3 h. For both assays, AF amounts were derived from standard curves.
Trichomonad cultures and in vitro activity assays:
T. vaginalis strain BRIS/92/STDL/F1623 (F1623) [66] and T. foetus strain D1 [67] were grown at 37 °C in TYM Diamond’s medium supplemented with 180 μM ferrous ammonium sulphate (Modified TYM media) under anaerobic conditions (AnaeroPackTM-Anaero System; Remel). To test the in vitro activity, a fixed amount (5 μL) AF-NP composite hydrogel was diluted with modified TYM media, and trophozoites (1 × 104/ well in 24- or 48-well plates) were added. After 24 h culture at 37 °C, growth and viability of the trophozoites were determined with a luminescent ATP assay using BacTiter-GloTM Microbial Cell Viability Assay reagent (Promega) [66].
Animal studies:
Breeding pairs of BALB/c mice (originally obtained from Jackson Labs) were maintained in our animal facility, and freshly weaned (21–24 days) or adult females were used for the studies. Animals were housed at a constant temperature room (22 ± 1 °C) with a 12 h light/12 h dark cycle, and provided access to food and water ad libitum. All animal studies were reviewed and approved under protocol number S00205 by the Institutional Animal Care and Use Committee of the University of California, San Diego.
To determine tissue retention, 5 μL of fluorescent DiD-NP gel were administered intravaginally to weanling mice using a positive displacement micropipette (Wiretrol, Drummond). After 24 h, the entire vagina was collected and frozen in Tissue-Teck O.C.T. compound (Sakura Finetek). Frozen sections were prepared and cover-slipped directly with ProLong Gold Antifade Mountant (Thermo Fisher Scientific). Parallel sections were fixed and stained with hematoxylin and eosin. Fluorescence and bright-field images were captured with a fluorescence microscope with an attached 12 Mp camera (Eclipse 50i, Nikon).
For drug distribution studies, female weanling mice were either treated with five doses over three days of either plain AF in a suspension of 1% hypromellose by oral gavage (200 μL, 30 mg/kg per dose) or topically by intravaginal injection of AF-NP gel (10 μg AF/dose in 5 μL). Organs were collected one day after the last dose, and AF content was determined by ICP-OES as described above. For efficacy testing, T. foetus D1 trophozoites were grown to mid-logarithmic phase, and intravaginally inoculated (106 in a 5 μL volume in growth medium) into weanling mice. After one day, AF-NP gel or solvent was applied intravaginally (10 μg AF/dose in 5 μL) with a positive displacement micropipette or given orally (30 mg/kg) for five doses over three days (1 x dose on day 1; 2 x doses each on days 2 and 3). In other experiments, mice were given a single topical dose of the AF-NP gel (10 μg AF/dose in 5 μL) or plain AF in PBS (10 μg AF/dose in 5 μL) one day after infection. For all experiments, live trophozoites were enumerated in a counting chamber on day 4 in washes of 30 μL of PBS [68].
Toxicity evaluation:
Adult female BALB/c mice were treated five times over three days with AF, either orally (30 mg/kg) or topically with the AF-NP gel (10 μg AF/dose in 5 μL), or were left untreated. One day after the last dose, the liver was collected and snap-frozen for later analysis of the systemic impact of AF. Organ extracts were made in 100 mM potassium phosphate buffer (pH 7.0) with a protease inhibitor cocktail (Halt; Thermo Fisher Scientific) by homogenization with zirconium beads in a Mini Beadbeater-16. Samples were cleared by centrifugation and protein concentrations were determined with a DC protein assay kit (Bio-rad). Activity of the AF-sensitive enzyme, TrxR, was assayed in liver extracts adjusted to 50 μg/ml protein in 100 mM potassium phosphate buffer (pH 7.0), 0.5 mM 5,5′-dithio-bis-(2-nitrobenzoic acid) (DTNB), and 0.2 mM NADPH. Conversion of DTNB to 2-nitro-5-thiobenzoic acid was assayed by absorbance at 412 nm [12]. To control for TrxR-independent DTNB reduction, the TrxR-specific inhibitor, aurothiomalate (20 μM), was added to parallel reactions. The difference in activity with and without aurothiomalate was taken as TrxR-dependent DTNB reduction [13].
For analysis of the local impact of topical AF, the estrus cycle was determined in adult female BALB/c mice. Mice were treated or not with five topical doses of the AF-NP gel as described above, and the phase of the estrus cycle was determined by analysis of vaginal smears made from 5–10 μL vaginal lavages. Air-dried smears were stained with 0.1% crystal violet solution and examined microscopically. The four phases of the mouse estrus cycle were divided into two groups for the purpose of this study: proestrus/estrus (P/E) with >90% nucleated and/or cornified epithelial cells, and metestrus/diestrus (M/D) with predominantly leukocytes mixed with occasional epithelial cells [69].
Statistical analysis:
Graphpad Prism 6 was used for statistical analysis. Differences with p<0.05 were considered to be statistically significant. Single comparison was done by Mann-Whitney test for non-parametric data. Multiple comparisons were performed with one-way ANOVA or Kruskal-Wallis test, followed by Dunn’s post-hoc test, for parametric and non‐parametric data, respectively.
Acknowledgements
We thank Elaine Hanson and Christine Le for technical support. The work was supported by NIH grants AI146387, DK120515 and CA200574, and a Takeda Science Foundation Grant to S. Ihara.
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
Contributor Information
Yue Zhang, Department of NanoEngineering and Moores Cancer Center, University of California San Diego, La Jolla, California 92093, USA.
Yukiko Miyamoto, Department of Medicine, University of California San Diego, La Jolla, California 92093, USA.
Sozaburo Ihara, Department of Medicine, University of California San Diego, La Jolla, California 92093, USA; Division of Gastroenterology, The Institute for Adult Diseases, Asahi Life Foundation, Tokyo, Japan.
Justin Z. Yang, Department of Medicine, University of California San Diego, La Jolla, California 92093, USA
Douglas E. Zuill, Department of Medicine, University of California San Diego, La Jolla, California 92093, USA
Pavimol Angsantikul, Department of NanoEngineering and Moores Cancer Center, University of California San Diego, La Jolla, California 92093, USA.
Qiangzhe Zhang, Department of NanoEngineering and Moores Cancer Center, University of California San Diego, La Jolla, California 92093, USA.
Weiwei Gao, Department of NanoEngineering and Moores Cancer Center, University of California San Diego, La Jolla, California 92093, USA.
Liangfang Zhang, Department of NanoEngineering and Moores Cancer Center, University of California San Diego, La Jolla, California 92093, USA.
Lars Eckmann, Department of Medicine, University of California San Diego, La Jolla, California 92093, USA.
References
- [1].Patel EU, Gaydos CA, Packman ZR, Quinn TC, Tobian AAR, Clin Infect Dis, 2018, 67, 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Edwards T, Burke P, Smalley H, Hobbs G, Crit Rev Microbiol, 2016, 42, 406. [DOI] [PubMed] [Google Scholar]
- [3].Marous M, Huang WY, Rabkin CS, Hayes RB, Alderete JF, Rosner B, Grubb RL 3rd, Winter AC, Sutcliffe S, Cancer Causes Control, 2017, 28, 889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yang S, Zhao W, Wang H, Wang Y, Li J, Wu X, Eur J Obstet Gynecol Reprod Biol, 2018, 228, 166. [DOI] [PubMed] [Google Scholar]
- [5].Kissinger P, Curr Infect Dis Rep, 2015, 17, 484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Das S, Huengsberg M, Shahmanesh M, Int J STD AIDS, 2005, 16, 284. [DOI] [PubMed] [Google Scholar]
- [7].Upcroft JA, Dunn LA, Wal T, Tabrizi S, Delgadillo-Correa MG, Johnson PJ, Garland S, Siba P, Upcroft P, Sex Health, 2009, 6, 334. [DOI] [PubMed] [Google Scholar]
- [8].Kirkcaldy RD, Augostini P, Asbel LE, Bernstein KT, Kerani RP, Mettenbrink CJ, Pathela P, Schwebke JR, Secor WE, Workowski KA, Davis D, Braxton J, Weinstock HS, Emerg Infect Dis, 2012, 18, 939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sobel JD, Nyirjesy P, Brown W, Clin Infect Dis, 2001, 33, 1341. [DOI] [PubMed] [Google Scholar]
- [10].Andersson KE, Scand J Infect Dis Suppl, 1981, 26, 60. [PubMed] [Google Scholar]
- [11].Goodhew EB, Secor WE, Sex Transm Infect, 2013, 89, 479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hopper M, Yun JF, Zhou B, Le C, Kehoe K, Le R, Hill R, Jongeward G, Debnath A, Zhang L, Miyamoto Y, Eckmann L, Land KM, Wrischnik LA, Int J Antimicrob Agents, 2016, 48, 690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Arner ES, Zhong L, Holmgren A, Methods Enzymol, 1999, 300, 226. [DOI] [PubMed] [Google Scholar]
- [14].Capparelli EV, Bricker-Ford R, Rogers MJ, McKerrow JH, Reed SL, Antimicrob Agents Chemother, 2017, 61, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fan S, Liu X, Liang Y, Gynecol Obstet Invest, 2015, 80, 113. [DOI] [PubMed] [Google Scholar]
- [16].duBouchet L, McGregor JA, Ismail M, McCormack WM, Sex Transm Dis, 1998, 25, 176. [DOI] [PubMed] [Google Scholar]
- [17].Schwebke JR, Lensing SY, Sobel J, Sex Transm Dis, 2013, 40, 710. [DOI] [PubMed] [Google Scholar]
- [18].Tugcu-Demiroz F, Chem Pharm Bull (Tokyo), 2017, 65, 660. [DOI] [PubMed] [Google Scholar]
- [19].Nowak J, Laffleur F, Bernkop-Schnurch A, Int J Pharm, 2015, 478, 383. [DOI] [PubMed] [Google Scholar]
- [20].Wu SY, Chang HI, Burgess M, McMillan NA, J Control Release, 2011, 155, 418. [DOI] [PubMed] [Google Scholar]
- [21].Goyal AK, Singh R, Chauhan G, Rath G, Artif Cells Nanomed Biotechnol, 2018, 46, 539. [DOI] [PubMed] [Google Scholar]
- [22].Liang Y, Kiick KL, Biomacromolecules, 2016, 17, 601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gao W, Vecchio D, Li J, Zhu J, Zhang Q, Fu V, Thamphiwatana S, Lu D, Zhang L, ACS Nano, 2014, 8, 2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kang CE, Baumann MD, Tator CH, Shoichet MS, Cells Tissues Organs, 2013, 197, 55. [DOI] [PubMed] [Google Scholar]
- [25].Yue J, He L, Tang Y, Yang L, Wu B, Ni J, J Photochem Photobiol B, 2019, 197, 111530. [DOI] [PubMed] [Google Scholar]
- [26].Wu Y, Wang H, Gao F, Xu Z, Dai F, Liu W, Advanced Functional Materials, 2018, 28, 1801000. [Google Scholar]
- [27].Wang L, Chen S, Zhou J, Yang J, X. C, Ji Y, Liu X, Zha L, Macromolecular Materials and Engineering, 2017, 302, 1700181. [Google Scholar]
- [28].Dong L, Peng H, Wang S, Zhang Z, Li J, Ai F, Zhao Q, Luo M, Xiong H, Chen L, Journal Applied Polymer Science, 2014, 131, 40477. [Google Scholar]
- [29].Zhang X, Pint CL, Lee MH, Schubert BE, Jamshidi A, Takei K, Ko H, Gillies A, Bardhan R, Urban JJ, Wu M, Fearing R, Javey A, Nano Lett, 2011, 11, 3239. [DOI] [PubMed] [Google Scholar]
- [30].Kim JH, Lee TR, Langmuir, 2007, 23, 6504. [DOI] [PubMed] [Google Scholar]
- [31].Fang W, Liu C, Yu F, Liu Y, Li Z, Chen L, Bao X, Tu T, ACS Appl Mater Interfaces, 2016, 8, 20583. [DOI] [PubMed] [Google Scholar]
- [32].Mei R, Wang Y, Liu W, Chen L, ACS Appl Mater Interfaces, 2018, 10, 23605. [DOI] [PubMed] [Google Scholar]
- [33].Hao J, Wang X, Bi Y, Teng Y, Wang J, Li F, Li Q, Zhang J, Guo F, Liu J, Colloids Surf B Biointerfaces, 2014, 114, 111. [DOI] [PubMed] [Google Scholar]
- [34].Wang F, Gao W, Thamphiwatana S, Luk BT, Angsantikul P, Zhang Q, Hu CM, Fang RH, Copp JA, Pornpattananangkul D, Lu W, Zhang L, Adv Mater, 2015, 27, 3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Endo T, Ikeda R, Yanagida Y, Hatsuzawa T, Anal Chim Acta, 2008, 611, 205. [DOI] [PubMed] [Google Scholar]
- [36].Thoniyot P, Tan MJ, Karim AA, Young DJ, Loh XJ, Adv Sci (Weinh), 2015, 2, 1400010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bouchemal K, Bories C, Loiseau PM, Clin Microbiol Rev, 2017, 30, 811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bilati U, Allemann E, Doelker E, Eur J Pharm Sci, 2005, 24, 67. [DOI] [PubMed] [Google Scholar]
- [39].Ensign LM, Tang BC, Wang YY, Tse TA, Hoen T, Cone R, Hanes J, Sci Transl Med, 2012, 4, 138ra79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Davis P, Clin Rheum Dis, 1984, 10, 369. [PubMed] [Google Scholar]
- [41].Rackham O, Shearwood AM, Thyer R, McNamara E, Davies SM, Callus BA, Miranda-Vizuete A, Berners-Price SJ, Cheng Q, Arner ES, Filipovska A, Free Radic Biol Med, 2011, 50, 689. [DOI] [PubMed] [Google Scholar]
- [42].Cho WS, Han BS, Lee H, Kim C, Nam KT, Park K, Choi M, Kim SJ, Kim SH, Jeong J, Jang DD, Food Chem Toxicol, 2008, 46, 1666. [DOI] [PubMed] [Google Scholar]
- [43].Diez-Martinez R, Garcia-Fernandez E, Manzano M, Martinez A, Domenech M, Vallet-Regi M, Garcia P, Sci Rep, 2016, 6, 19525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Pavelic Z, Skalko-Basnet N, Schubert R, Int J Pharm, 2001, 219, 139. [DOI] [PubMed] [Google Scholar]
- [45].Marzo T, Cirri D, Gabbiani C, Gamberi T, Magherini F, Pratesi A, Guerri A, Biver T, Binacchi F, Stefanini M, Arcangeli A, Messori L, ACS Med Chem Lett, 2017, 8, 997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Talelli M, Iman M, Varkouhi AK, Rijcken CJ, Schiffelers RM, Etrych T, Ulbrich K, van Nostrum CF, Lammers T, Storm G, Hennink WE, Biomaterials, 2010, 31, 7797. [DOI] [PubMed] [Google Scholar]
- [47].Reis CP, Neufeld RJ, Ribeiro AJ, Veiga F, Nanomedicine, 2006, 2, 8. [DOI] [PubMed] [Google Scholar]
- [48].Peng H, Xiong H, Li J, Xie M, Liu Y, Bai C, Chen L, Food Chemistry, 2010, 121, 23. [Google Scholar]
- [49].Liu Y, Yang F, Feng L, Yang L, Chen L, Wei G, Lu W, Acta Pharm Sin B, 2017, 7, 502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Parameswaran-Thankam A, Parnell CM, Watanabe F, RanguMagar AB, Chhetri BP, Szwedo PK, Biris AS, Ghosh A, ACS Omega, 2018, 3, 15158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Yang TT, Cheng YZ, Qin M, Wang YH, Yu HL, Wang AL, Zhang WF, Biomed Res Int, 2017, 2017, 3564060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Caramella CM, Rossi S, Ferrari F, Bonferoni MC, Sandri G, Adv Drug Deliv Rev, 2015, 92, 39. [DOI] [PubMed] [Google Scholar]
- [53].Malli S, Bories C, Pradines B, Loiseau PM, Ponchel G, Bouchemal K, Eur J Pharm Biopharm, 2017, 112, 143. [DOI] [PubMed] [Google Scholar]
- [54].Powers BL, Wing DA, Carr D, Ewert K, Di Spirito M, J Clin Pharmacol, 2008, 48, 26. [DOI] [PubMed] [Google Scholar]
- [55].Thangamani S, Mohammad H, Abushahba MF, Sobreira TJ, Seleem MN, Int J Antimicrob Agents, 2016, 47, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Cappellano G, Comi C, Chiocchetti A, Dianzani U, Int J Mol Sci, 2019, 20, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Yin W, Li W, Rubenstein DA, Meng Y, Biointerphases, 2016, 11, 04B301. [DOI] [PubMed] [Google Scholar]
- [58].Almomen A, Cho S, Yang CH, Li Z, Jarboe EA, Peterson CM, Huh KM, Janat-Amsbury MM, Pharm Res, 2015, 32, 2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Makadia HK, Siegel SJ, Polymers (Basel), 2011, 3, 1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Li J, Cai C, Sun T, Wang L, Wu H, Yu G, Molecules, 2018, 23, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Di Martino A, Sittinger M, Risbud MV, Biomaterials, 2005, 26, 5983. [DOI] [PubMed] [Google Scholar]
- [62].Kalam MA, Alshamsan A, Biomed Pharmacother, 2017, 94, 402. [DOI] [PubMed] [Google Scholar]
- [63].Rao SB, Sharma CP, J Biomed Mater Res, 1997, 34, 21. [DOI] [PubMed] [Google Scholar]
- [64].Machado A, Cunha-Reis C, Araujo F, Nunes R, Seabra V, Ferreira D, das Neves J, Sarmento B, Acta Biomater, 2016, 44, 332. [DOI] [PubMed] [Google Scholar]
- [65].Coelho DD, Genitourin Med, 1997, 73, 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Miyamoto Y, Kalisiak J, Korthals K, Lauwaet T, Cheung DY, Lozano R, Cobo ER, Upcroft P, Upcroft JA, Berg DE, Gillin FD, Fokin VV, Sharpless KB, Eckmann L, Proc Natl Acad Sci U S A, 2013, 110, 17564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Ikeda JS, BonDurant RH, Campero CM, Corbeil LB, J Clin Microbiol, 1993, 31, 3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Cobo ER, Eckmann L, Corbeil LB, Am J Trop Med Hyg, 2011, 85, 667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].McLean AC, Valenzuela N, Fai S, Bennett SA, J Vis Exp, 2012, e4389. [DOI] [PMC free article] [PubMed] [Google Scholar]


