Abstract
The nuclear periphery is a hotspot for the accumulation of age-induced damage in eukaryotic cells. The types of damage that occur at the periphery and their phenotypic consequences have begun to be characterized; however, the mechanisms by which cells repair or eliminate nuclear damage remain poorly understood. Using budding yeast meiosis as a natural system to study cellular rejuvenation, we recently discovered a novel nuclear quality control event, in which age-induced damage is sequestered away from dividing chromosomes to a discarded nuclear compartment that we term the GUNC (for “Gametogenesis Uninherited Nuclear Compartment”). Interestingly, extensive nuclear remodeling occurs even in young cells, including a surprising modularity of the nuclear pore complex, suggesting a general contribution to gamete fitness. In this review, we discuss these findings in the context of recent evidence that the nuclear periphery is a highly dynamic region critical for cellular health.
Introduction
The nuclear periphery, which consists of two lipid bilayers – inner and outer nuclear envelope as well as the associated proteins, acts as a guardian to the genome and mediates nucleocytoplasmic transport in all eukaryotic cells. Notably, this region undergoes a number of changes during natural and pathological aging in metazoans. In natural aging, long-lived nuclear pore complexes (NPCs) accumulate damage and display reduced functionality in both Caenorhabditis elegans and mammals (D’Angelo et al., 2009; Savas et al., 2012; Toyama et al., 2013). In pathological aging, the nuclear permeability barrier is disrupted during the course of progressive neurodegenerative diseases, such as amyotrophic lateral sclerosis and Huntington’s disease (Chou et al., 2018; Gasset-Rosa et al., 2017; Grima et al., 2017; Zhang et al., 2015). Changes in nuclear organization may be causal to aging phenotypes, since the premature aging disease Hutchison-Gilford progeria syndrome results from mutations in the nuclear gene lamin A and is associated with enlarged nucleoli (Buchwalter and Hetzer, 2017; Eriksson et al., 2003). Consistent with this, small nucleolar size correlates with increased lifespan in C. elegans, files, mice, and humans (Tiku et al., 2017). The model organism in which aging has been most extensively studied, the budding yeast Saccharomyces cerevisiae, likewise exhibits changes at the nuclear periphery with age including: the appearance of protein aggregates (Cabrera et al., 2017; Saarikangas et al., 2017), the deformation and enlargement of the nucleolus (Morlot et al., 2019; Sinclair et al., 1997; Unal et al., 2011), the clustering and altered stoichiometry of NPCs (Lord et al., 2015; Rempel et al., 2019), and the accumulation of non-chromosomal rDNA circles (Denoth-Lippuner et al., 2014; Sinclair and Guarente, 1997). Given the connection between aging and changes at the nuclear periphery, improved characterization of nuclear envelope organization and dynamics is required.
Cell divisions involve dramatic nuclear envelope remodeling and, therefore, represent an important opportunity for periphery-associated damage to be eliminated. In most metazoan cells, the nuclear envelope undergoes coordinated disassembly and reassembly during cell divisions (reviewed in Ungricht and Kutay, 2017). In addition to facilitating the division of genetic material, these “open” cell divisions allow rejuvenation of the periphery; as a consequence, damage to NPCs and disruption of the nuclear permeability barrier are confined to post-mitotic cells (D’Angelo et al., 2009; Toyama et al., 2019; Toyama et al., 2013). In budding yeast, the nuclear envelope instead remains intact during both mitosis and meiosis (reviewed in Boettcher and Barral, 2013). The nuclear periphery undergoes dramatic morphological changes in these “closed” divisions to accommodate the division of genetic material; however, the elimination of nuclear age-induced damage in offspring requires specialized mechanisms. In mitosis, compartmentalization of the nuclear periphery by the bud neck ensures that age-induced damage is asymmetrically retained in the mother cell (Caudron and Barral, 2009; Clay et al., 2014; Gehlen et al., 2011). In meiosis, age-induced nuclear damage is eliminated as part of gamete rejuvenation (Ünal et al., 2011), but the mechanisms mediating this elimination were unknown until recently.
Principles discovered by studying fungal nuclear divisions are often conserved in metazoan cells (e.g., NIMA-regulated NPC disassembly Laurell et al., 2011 and septin-mediated diffusion barriers Saarikangas and Barral, 2011); as such, we sought to determine how budding yeast meiotic nuclear rejuvenation occurs. We performed in-depth characterization of the nuclear periphery during the meiotic divisions using time-lapse fluorescence microscopy, which allowed us to define a novel remodeling event that facilitates cellular rejuvenation (King and Goodman et al., 2019). In this review, we discuss: (1) the unconventional nuclear reorganization that occurs during budding yeast meiosis; (2) the contribution of nuclear compartmentalization to cellular rejuvenation in budding yeast; and (3) the dynamic structure of NPCs during cell divisions. Further characterization of nuclear behavior during budding yeast meiosis provides a unique opportunity to improve our understanding of nuclear organization and its contributions to cellular health.
Remodeling of the nuclear periphery during budding yeast meiosis
A fundamental property of eukaryotic life is that age-induced damage is not inherited by subsequent generations. Recent work has begun to characterize the specialized mechanisms that facilitate this rejuvenation during sexual reproduction. In metazoans, for example, protein aggregates in the germline are eliminated by activation of the lysosome (Bohnert and Kenyon, 2017; Goudeau and Aguilaniu, 2010). We turned our focus to budding yeast, where meiosis resets lifespan symmetrically such that all resultant gametes are born young (Unal and Amon, 2011; Unal et al., 2011). Coincident with this rejuvenation, various types of age-induced damage associated with the nuclear periphery, including protein aggregates, non-chromosomal rDNA circles, and abnormal nucleolar material, are eliminated (Unal et al., 2011). Given that the nuclear envelope remains continuous during the meiotic divisions (Moens, 1971; Moens and Rapport, 1971), it was unclear how age-associated damage could be separated away from inherited genomic material.
To answer this question, we performed live-cell fluorescence microscopy to observe the behavior of age-induced damage and various other nuclear components during the meiotic divisions (King and Goodman et al., 2019). We found that protein aggregates and abnormal nucleolar material are physically separated away from dividing chromosomes during meiosis II (Figure 1B). Notably, rDNA circles are similarly sequestered, indicating that cells can distinguish them from chromosomal DNA perhaps due to the lack of centromeres in rDNA circles (Figure 1B). The sequestration is coupled to a nuclear envelope remodeling event that takes place in both young and old cells, culminating in the formation of a fifth nuclear envelope-bound compartment outside of the developing gametes (Figure 1A–B). This compartment, which we term the “GUNC” (for “Gametogenesis Un-inherited Nuclear Compartment”), contains select nuclear components – such as the core of the NPC and some nucleolar material – even in young cells (Figure 1A). During gamete maturation, the material in the GUNC is destroyed when the vacuole, the yeast equivalent of the lysosome, undergoes programmed permeabilization and releases its hydrolases (Eastwood et al., 2012; Eastwood and Meneghini, 2015). Notably, the sequestration of nuclear material to this compartment requires nuclear compartmentalization mediated by the formation of de novo gamete plasma membranes. When gamete plasma membrane formation is prevented, NPCs and age-induced protein aggregates become randomly distributed along the nuclear periphery (King and Goodman et al., 2019). The sequestration and elimination of nuclear age-induced damage is likely to be a fundamental aspect of meiotic cellular rejuvenation.
Figure 1. Nuclear compartmentalization in budding yeast meiosis and mitosis.
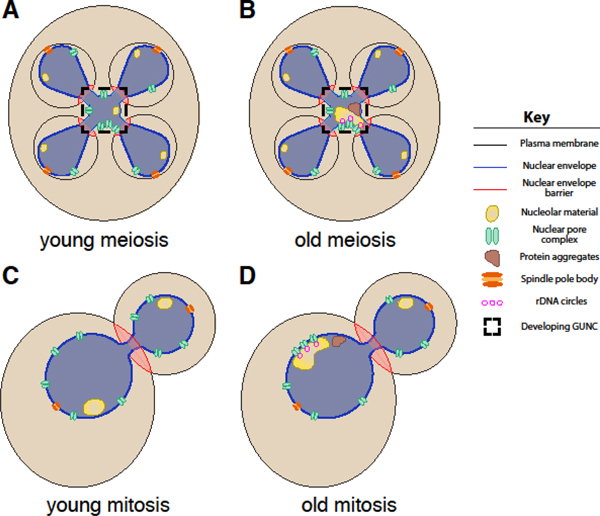
A-B. Gamete plasma membranes result in nuclear compartmentalization during meiosis via an unknown mechanism (King and Goodman et al., 2019). A. In young cells, the gametogenesis un-inherited nuclear compartment (GUNC) contains core NPC subcomplexes and some nucleolar material. B. In old cells, the GUNC also contains non-chromosomal rDNA circles, protein aggregates, and abnormal nucleolar material. C-D. The bud neck organizes a septin-mediated outer nuclear envelope diffusion barrier during mitosis in both C. young and D. old cells (Chao et al., 2014; Clay et al., 2014). In old cells, the diffusion barrier contributes to the asymmetric inheritance of nuclear pore complexes and associated non-chromosomal rDNA circles (Denoth-Lippuner et al., 2014). The narrow constriction of the nucleus at the bud neck also limits diffusion between the mother and daughter nucleoplasm (Boettcher et al., 2012; Gehlen et al., 2011).
Nuclear envelope compartmentalization and its contributions to cellular rejuvenation
Our work provides evidence that compartmentalization of the nucleus is vital to sequestration of age-induced damage away from gametes during budding yeast meiosis (Figure 1A–B). In a similar manner, delineation of mother and daughter nuclear space at the bud neck during mitosis is required for the asymmetric retention of nuclear damage in mother cells (Figure 1C–D; Denoth-Lippuner et al., 2014; Gehlen et al., 2011). In this context, asymmetric partitioning is achieved by compartmentalization of both the nucleoplasm and nuclear envelope during anaphase. Nucleoplasmic partitioning of mother and daughter nuclei results from the nuclear envelope’s geometry, as its narrow constriction at the bud neck and the brief duration of mitotic anaphase limit diffusion of nuclear components like rDNA circles (Boettcher et al., 2012; Gehlen et al., 2011). Nuclear envelope partitioning is the consequence of a sphingolipid diffusion barrier in the outer nuclear membrane, established by a septin-mediated signaling cascade at the bud-neck (Chao et al., 2014; Clay et al., 2014; Singh and Li, 2018). This barrier restricts the movement of large nuclear envelope protein complexes, such as NPCs, and associated molecules, such as rDNA circles tethered to NPCs, into daughter cells (Denoth-Lippuner et al., 2014). However, the barrier between daughter and mother nuclei is not absolute: a cytoplasmic pool of the channel nucleoporin Nsp1 facilitates regulated inheritance of NPCs through the diffusion barrier (Colombi et al., 2013; Makio et al., 2013). Collectively, this work suggests that a dynamic barrier established by at least two parallel mechanisms, namely nucleoplasmic and nuclear envelope partitioning, is responsible for nuclear compartmentalization at the mitotic bud neck.
It is currently unclear what mechanisms achieve nuclear compartmentalization during meiosis. As in mitosis, meiotic compartmentalization relies on a plasma membrane-coupled mechanism, since blocking gamete plasma membrane development prevents sequestration of NPCs and age-induced damage (King and Goodman et al., 2019). The lips of gamete plasma membranes localize to the boundaries of the GUNC, making them attractive candidates to organize a structure that divides the nucleus into distinct domains. However, meiotic septins, known lip-localizing proteins (e.g., the leading edge complex members Don1, Ady3, Irc10), and other constituents of the mitotic diffusion barrier (e.g., Sur2, Epo1, Bud6, Scs2) are not required for sequestration of age-induced protein aggregates or NPCs (unpublished data; King and Goodman et al., 2019), suggesting the existence of a novel mechanism that establishes meiotic nuclear compartmentalization. Intriguingly, the nuclear envelope also exhibits bud neck-independent compartmentalization during mitotic growth, with the nuclear envelope region proximate to the nucleolus preferentially undergoing Cdc5-dependent expansion upon mitotic delay (Walters et al., 2014; Witkin et al., 2012). Together with our study, this implies that multiple mechanisms exist to establish distinct nuclear envelope regions in budding yeast. Given that the nucleoplasm is already known to be organized non-randomly into chromatin domains in both yeast and metazoans (reviewed by Bonev and Cavalli, 2016; Van de Vosse et al., 2011), nuclear envelope compartmentalization seems likely to be an underappreciated layer of nuclear organization that merits future study.
Nuclear pore complex dynamics during cell divisions and differentiation
Given the dynamic behavior of the nuclear periphery during meiosis in young cells, nuclear remodeling is likely to play a role in gamete health beyond facilitating rejuvenation. Dramatic changes to the nuclear periphery occur during “closed” meiosis in other fungal species, including a transient loss of the nuclear permeability barrier in fission yeast (Arai et al., 2010; Asakawa et al., 2010). In this context, virtual nuclear envelope breakdown is required for proper spindle disassembly during meiosis II (Flor-Parra et al., 2018), providing an example of meiosis-specific nuclear behavior that is vital to gamete health. One of the most striking nuclear changes we observe in young cells is the sequestration of NPC cores away from genomic material (King and Goodman et al., 2019). A similar sequestration event occurs during metazoan spermatogenesis coincident with the development of the acrosome, a structure external to the nucleus that may be acting in a manner akin to the yeast gamete plasma membrane (Fawcett and Chemes, 1979; Ho, 2010; Troyer and Schwager, 1982). Moreover, the NPC displays modular behavior during meiosis II, suggesting its dynamics are tightly regulated: the core subcomplexes of the NPC are sequestered and eliminated, while the nuclear basket separates and returns to developing nuclei (Figure 2). Nuclear basket separation in meiosis is a new example of nuclear pore complex plasticity, which is becoming increasingly appreciated as a feature of many types of cell divisions and differentiation (reviewed in Raices and D’Angelo, 2012). As such, determining the mechanism and function underlying this NPC remodeling will improve our understanding of how NPC modularity contributes to cellular health.
Figure 2. Nuclear pore complex modularity during budding yeast meiosis.
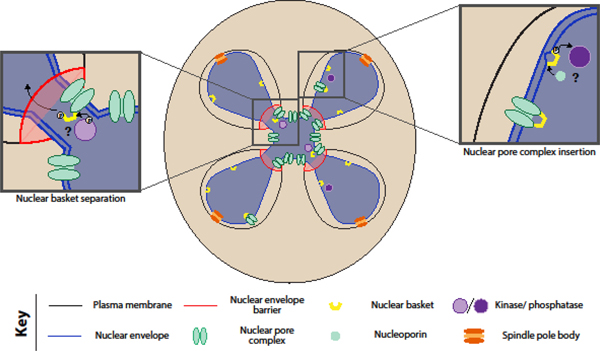
During meiosis II in budding yeast, the core of the nuclear pore complex is sequestered away from dividing chromosomes, while the nuclear basket returns to gamete nuclei (King and Goodman et al., 2019). We postulate that phosphorylation mediates separation of basket from the core, allowing the basket to return to gamete nuclei despite the diffusion barrier created by nascent gamete membranes. Once inside the gamete nuclei, the basket might mediate the insertion of new nuclear pore complexes, via its constituents’ membrane-binding amphipathic helices (Meszaros et al., 2015).
In terms of how this modularity is achieved, we hypothesize that the interaction between the basket and the core is weakened by a post-translational modification such as phosphorylation, similar to regulatory events observed during the cell divisions of other fungal species and metazoans (Figure 2). In Aspergillus nidulans mitosis, the conserved NIMA kinase facilitates partial NPC disassembly, disrupting the nuclear permeability barrier in an otherwise closed cell division (De Souza et al., 2004). In metazoan mitosis and meiosis, nuclear envelope breakdown is initiated by phosphorylation and disassembly of NPCs by a NIMA homolog and other kinases (Laurell et al., 2011; Ungricht and Kutay, 2017). The nuclear basket is also ubiquitinated and sumoylated in budding yeast (Folz et al., 2019; Nino et al., 2016), raising the possibility that these post-translational modifications could instead be involved in nuclear basket separation. Intriguingly, the nuclear basket displays a transient association with the NPC core even during mitotic growth (Denning et al., 2001; Dilworth et al., 2001; Niepel et al., 2013), with a subpopulation of NPCs near the nucleolus lacking nuclear baskets (Galy et al., 2004). Characterization of the proteins required for basket separation during meiosis is thus likely to reveal NPC regulators important in other contexts.
In terms of what this modularity achieves, we hypothesize that nuclear basket separation plays a role in both sequestration of core nucleoporins and insertion of new gamete NPCs (Figure 2). Given that nuclear basket nucleoporins associate with chromatin during cell divisions in many eukaryotes (Dultz et al., 2008; Markossian et al., 2015; Suresh et al., 2017), separation of the nuclear basket from the NPC core may be necessary to disrupt interactions between NPCs and chromosomes that would otherwise prevent NPC core sequestration. Further, basketless pores may become disorganized and clustered, enhancing their sequestration, as basket members have previously been shown to regulate NPC distribution (Niepel et al., 2013). Any association of the nuclear basket with chromatin could then facilitate its return to gamete nuclei, where amphipathic helices in Nup60 and Nup1 would allow it to dock onto and curve the nuclear envelope (Meszaros et al., 2015). Given the increased evidence that nuclear pore insertion takes place via an inside-out mechanism when the nuclear envelope is intact (Otsuka et al., 2016), the sites of nuclear basket binding might initiate insertion of new NPCs in bulk. Meanwhile, core subcomplexes – some of which are extraordinarily long-lived and stable (D’Angelo et al., 2009; Rabut et al., 2004; Toyama et al., 2013) – are eliminated, allowing clearance of any age-induced damage. Consistent with a functional importance for nuclear basket return, deletion of the non-essential nuclear basket member Nup60 causes meiotic defects and gamete inviability (Chu et al., 2017). Determining the consequences of disrupting NPC modularity is therefore likely to provide fundamental insights into nuclear basket function.
Conclusions
In budding yeast, the nuclear periphery is far from a passive participant in closed cell divisions. Its compartmentalization is critical to ensure that offspring are born young in both mitosis and meiosis (Figure 1). Dramatic remodeling of its core constituents, including nuclear pore complexes, occurs during meiosis in young cells with possible consequences to gamete health (Figure 2). Our work contributes to an increasing body of evidence that the nuclear periphery is highly dynamic, with its own dedicated quality control systems (Khmelinskii et al., 2014; Miller et al., 2015; Mochida et al., 2015; Smoyer and Jaspersen, 2019; Smoyer et al., 2019; Webster et al., 2014) and active lipid metabolism (Romanauska and Kohler, 2018). Studying the exceptional changes that take place to the nuclear periphery during budding yeast meiosis will improve our understanding of healthy nuclear organization in yeast and metazoans.
Acknowledgements
We would like to thank Jay Goodman and Tina Sing for their helpful discussions regarding this manuscript. GAK is supported by a National Science Foundation Graduate Research Fellowship (DGE 1752814) and a National Institutes of Health Traineeship (T32 GM007232). EÜ is supported by funds from the Pew Charitable Trusts (00027344), Damon Runyon Cancer Research Foundation (35–15), National Institutes of Health (DP2 AG055946–01), and Glenn Foundation for Medical Research.
References
- 1.Arai K, Sato M, Tanaka K, and Yamamoto M (2010). Nuclear compartmentalization is abolished during fission yeast meiosis. Current biology : CB 20, 1913–1918. [DOI] [PubMed] [Google Scholar]
- 2.Asakawa H, Kojidani T, Mori C, Osakada H, Sato M, Ding DQ, Hiraoka Y, and Haraguchi T (2010). Virtual breakdown of the nuclear envelope in fission yeast meiosis. Current biology : CB 20, 1919–1925. [DOI] [PubMed] [Google Scholar]
- 3.Boettcher B, and Barral Y (2013). The cell biology of open and closed mitosis. Nucleus (Austin, Tex) 4, 160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boettcher B, Marquez-Lago TT, Bayer M, Weiss EL, and Barral Y (2012). Nuclear envelope morphology constrains diffusion and promotes asymmetric protein segregation in closed mitosis. The Journal of cell biology 197, 921–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohnert KA, and Kenyon C (2017). A lysosomal switch triggers proteostasis renewal in the immortal C. elegans germ lineage. Nature 551, 629–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonev B, and Cavalli G (2016). Organization and function of the 3D genome. Nature reviews Genetics 17, 772. [DOI] [PubMed] [Google Scholar]
- 7.Buchwalter A, and Hetzer MW (2017). Nucleolar expansion and elevated protein translation in premature aging. Nature communications 8, 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabrera M, Novarina D, Rempel IL, Veenhoff LM, and Chang M (2017). A simple microfluidic platform to study age-dependent protein abundance and localization changes in Saccharomyces cerevisiae. Microbial cell (Graz, Austria) 4, 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caudron F, and Barral Y (2009). Septins and the lateral compartmentalization of eukaryotic membranes. Developmental cell 16, 493–506. [DOI] [PubMed] [Google Scholar]
- 10.Chao JT, Wong AK, Tavassoli S, Young BP, Chruscicki A, Fang NN, Howe LJ, Mayor T, Foster LJ, and Loewen CJ (2014). Polarization of the endoplasmic reticulum by ER-septin tethering. Cell 158, 620–632. [DOI] [PubMed] [Google Scholar]
- 11.Chou CC, Zhang Y, Umoh ME, Vaughan SW, Lorenzini I, Liu F, Sayegh M, Donlin-Asp PG, Chen YH, Duong DM, et al. (2018). TDP-43 pathology disrupts nuclear pore complexes and nucleocytoplasmic transport in ALS/FTD. Nature neuroscience 21, 228–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu DB, Gromova T, Newman TAC, and Burgess SM (2017). The Nucleoporin Nup2 Contains a Meiotic-Autonomous Region that Promotes the Dynamic Chromosome Events of Meiosis. Genetics 206, 1319–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clay L, Caudron F, Denoth-Lippuner A, Boettcher B, Buvelot Frei S, Snapp EL, and Barral Y (2014). A sphingolipid-dependent diffusion barrier confines ER stress to the yeast mother cell. eLife 3, e01883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colombi P, Webster BM, Frohlich F, and Lusk CP (2013). The transmission of nuclear pore complexes to daughter cells requires a cytoplasmic pool of Nsp1. The Journal of cell biology 203, 215–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Angelo MA, Raices M, Panowski SH, and Hetzer MW (2009). Age-dependent deterioration of nuclear pore complexes causes a loss of nuclear integrity in postmitotic cells. Cell 136, 284–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Souza CP, Osmani AH, Hashmi SB, and Osmani SA (2004). Partial nuclear pore complex disassembly during closed mitosis in Aspergillus nidulans. Current biology : CB 14, 1973–1984. [DOI] [PubMed] [Google Scholar]
- 17.Denning D, Mykytka B, Allen NP, Huang L, Al B, and Rexach M (2001). The nucleoporin Nup60p functions as a Gsp1p-GTP-sensitive tether for Nup2p at the nuclear pore complex. The Journal of cell biology 154, 937–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denoth-Lippuner A, Krzyzanowski MK, Stober C, and Barral Y (2014). Role of SAGA in the asymmetric segregation of DNA circles during yeast ageing. eLife 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dilworth DJ, Suprapto A, Padovan JC, Chait BT, Wozniak RW, Rout MP, and Aitchison JD (2001). Nup2p dynamically associates with the distal regions of the yeast nuclear pore complex. The Journal of cell biology 153, 1465–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dultz E, Zanin E, Wurzenberger C, Braun M, Rabut G, Sironi L, and Ellenberg J (2008). Systematic kinetic analysis of mitotic dis- and reassembly of the nuclear pore in living cells. The Journal of cell biology 180, 857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eastwood MD, Cheung SW, Lee KY, Moffat J, and Meneghini MD (2012). Developmentally programmed nuclear destruction during yeast gametogenesis. Developmental cell 23, 35–44. [DOI] [PubMed] [Google Scholar]
- 22.Eastwood MD, and Meneghini MD (2015). Developmental Coordination of Gamete Differentiation with Programmed Cell Death in Sporulating Yeast. Eukaryot Cell 14, 858–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, Erdos MR, Robbins CM, Moses TY, Berglund P, et al. (2003). Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature 423, 293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fawcett DW, and Chemes HE (1979). Changes in distribution of nuclear pores during differentiation of the male germ cells. Tissue and Cell 11, 147–162. [DOI] [PubMed] [Google Scholar]
- 25.Flor-Parra I, Iglesias-Romero AB, Salas-Pino S, Lucena R, Jimenez J, and Daga RR (2018). Importin alpha and vNEBD Control Meiotic Spindle Disassembly in Fission Yeast. Cell reports 23, 933–941. [DOI] [PubMed] [Google Scholar]
- 26.Folz H, Nino CA, Taranum S, Caesar S, Latta L, Waharte F, Salamero J, Schlenstedt G, and Dargemont C (2019). SUMOylation of the nuclear pore complex basket is involved in sensing cellular stresses. Journal of cell science 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galy V, Gadal O, Fromont-Racine M, Romano A, Jacquier A, and Nehrbass U (2004). Nuclear retention of unspliced mRNAs in yeast is mediated by perinuclear Mlp1. Cell 116, 63–73. [DOI] [PubMed] [Google Scholar]
- 28.Gasset-Rosa F, Chillon-Marinas C, Goginashvili A, Atwal RS, Artates JW, Tabet R, Wheeler VC, Bang AG, Cleveland DW, and Lagier-Tourenne C (2017). Polyglutamine-Expanded Huntingtin Exacerbates Age-Related Disruption of Nuclear Integrity and Nucleocytoplasmic Transport. Neuron 94, 48–57.e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gehlen LR, Nagai S, Shimada K, Meister P, Taddei A, and Gasser SM (2011). Nuclear geometry and rapid mitosis ensure asymmetric episome segregation in yeast. Current biology : CB 21, 25–33. [DOI] [PubMed] [Google Scholar]
- 30.Goudeau J, and Aguilaniu H (2010). Carbonylated proteins are eliminated during reproduction in C. elegans. Aging Cell 9, 991–1003. [DOI] [PubMed] [Google Scholar]
- 31.Grima JC, Daigle JG, Arbez N, Cunningham KC, Zhang K, Ochaba J, Geater C, Morozko E, Stocksdale J, Glatzer JC, et al. (2017). Mutant Huntingtin Disrupts the Nuclear Pore Complex. Neuron 94, 93–107.e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ho HC (2010). Redistribution of nuclear pores during formation of the redundant nuclear envelope in mouse spermatids. J Anat 216, 525–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khmelinskii A, Blaszczak E, Pantazopoulou M, Fischer B, Omnus DJ, Le Dez G, Brossard A, Gunnarsson A, Barry JD, Meurer M, et al. (2014). Protein quality control at the inner nuclear membrane. Nature 516, 410–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.King GA, Goodman JS, Schick JG, Chetlapalli K, Jorgens DM, McDonald KL, and Unal E (2019). Meiotic cellular rejuvenation is coupled to nuclear remodeling in budding yeast. eLife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laurell E, Beck K, Krupina K, Theerthagiri G, Bodenmiller B, Horvath P, Aebersold R, Antonin W, and Kutay U (2011). Phosphorylation of Nup98 by multiple kinases is crucial for NPC disassembly during mitotic entry. Cell 144, 539–550. [DOI] [PubMed] [Google Scholar]
- 36.Lord CL, Timney BL, Rout MP, and Wente SR (2015). Altering nuclear pore complex function impacts longevity and mitochondrial function in S. cerevisiae. The Journal of cell biology 208, 729–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Makio T, Lapetina DL, and Wozniak RW (2013). Inheritance of yeast nuclear pore complexes requires the Nsp1p subcomplex. The Journal of cell biology 203, 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Markossian S, Suresh S, Osmani AH, and Osmani SA (2015). Nup2 requires a highly divergent partner, NupA, to fulfill functions at nuclear pore complexes and the mitotic chromatin region. Molecular biology of the cell 26, 605–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meszaros N, Cibulka J, Mendiburo MJ, Romanauska A, Schneider M, and Kohler A (2015). Nuclear pore basket proteins are tethered to the nuclear envelope and can regulate membrane curvature. Developmental cell 33, 285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller SB, Ho CT, Winkler J, Khokhrina M, Neuner A, Mohamed MY, Guilbride DL, Richter K, Lisby M, Schiebel E, et al. (2015). Compartment-specific aggregases direct distinct nuclear and cytoplasmic aggregate deposition. The EMBO journal 34, 778–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mochida K, Oikawa Y, Kimura Y, Kirisako H, Hirano H, Ohsumi Y, and Nakatogawa H (2015). Receptor-mediated selective autophagy degrades the endoplasmic reticulum and the nucleus. Nature 522, 359–362. [DOI] [PubMed] [Google Scholar]
- 42.Moens PB (1971). Fine structure of ascospore development in the yeast Saccharomyces cerevisiae. Can J Microbiol 17, 507–510. [DOI] [PubMed] [Google Scholar]
- 43.Moens PB, and Rapport E (1971). Spindles, spindle plaques, and meiosis in the yeast Saccharomyces cerevisiae (Hansen). The Journal of cell biology 50, 344–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morlot S, Song J, Leger-Silvestre I, Matifas A, Gadal O, and Charvin G (2019). Excessive rDNA Transcription Drives the Disruption in Nuclear Homeostasis during Entry into Senescence in Budding Yeast. Cell reports 28, 408–422.e404. [DOI] [PubMed] [Google Scholar]
- 45.Niepel M, Molloy KR, Williams R, Farr JC, Meinema AC, Vecchietti N, Cristea IM, Chait BT, Rout MP, and Strambio-De-Castillia C (2013). The nuclear basket proteins Mlp1p and Mlp2p are part of a dynamic interactome including Esc1p and the proteasome. Molecular biology of the cell 24, 3920–3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nino CA, Guet D, Gay A, Brutus S, Jourquin F, Mendiratta S, Salamero J, Geli V, and Dargemont C (2016). Posttranslational marks control architectural and functional plasticity of the nuclear pore complex basket. The Journal of cell biology 212, 167–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Otsuka S, Bui KH, Schorb M, Hossain MJ, Politi AZ, Koch B, Eltsov M, Beck M, and Ellenberg J (2016). Nuclear pore assembly proceeds by an inside-out extrusion of the nuclear envelope. eLife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rabut G, Doye V, and Ellenberg J (2004). Mapping the dynamic organization of the nuclear pore complex inside single living cells. Nature cell biology 6, 1114–1121. [DOI] [PubMed] [Google Scholar]
- 49.Raices M, and D’Angelo MA (2012). Nuclear pore complex composition: a new regulator of tissue-specific and developmental functions. Nature reviews Molecular cell biology 13, 687–699. [DOI] [PubMed] [Google Scholar]
- 50.Rempel IL, Crane MM, Thaller DJ, Mishra A, Jansen DP, Janssens G, Popken P, Aksit A, Kaeberlein M, van der Giessen E, et al. (2019). Age-dependent deterioration of nuclear pore assembly in mitotic cells decreases transport dynamics. eLife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Romanauska A, and Kohler A (2018). The Inner Nuclear Membrane Is a Metabolically Active Territory that Generates Nuclear Lipid Droplets. Cell 174, 700–715.e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saarikangas J, and Barral Y (2011). The emerging functions of septins in metazoans. EMBO reports 12, 1118–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saarikangas J, Caudron F, Prasad R, Moreno DF, Bolognesi A, Aldea M, and Barral Y (2017). Compartmentalization of ER-Bound Chaperone Confines Protein Deposit Formation to the Aging Yeast Cell. Current biology : CB 27, 773–783. [DOI] [PubMed] [Google Scholar]
- 54.Savas JN, Toyama BH, Xu T, Yates JR 3rd, and Hetzer MW (2012). Extremely long-lived nuclear pore proteins in the rat brain. Science (New York, NY) 335, 942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sinclair DA, and Guarente L (1997). Extrachromosomal rDNA circles--a cause of aging in yeast. Cell 91, 1033–1042. [DOI] [PubMed] [Google Scholar]
- 56.Sinclair DA, Mills K, and Guarente L (1997). Accelerated aging and nucleolar fragmentation in yeast sgs1 mutants. Science (New York, NY) 277, 1313–1316. [DOI] [PubMed] [Google Scholar]
- 57.Singh P, and Li R (2018). Emerging roles for sphingolipids in cellular aging. Current genetics 64, 761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smoyer CJ, and Jaspersen SL (2019). Patrolling the nucleus: inner nuclear membrane-associated degradation. Current genetics 65, 1099–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smoyer CJ, Smith SE, Gardner JM, McCroskey S, Unruh JR, and Jaspersen SL (2019). Distribution of Proteins at the Inner Nuclear Membrane Is Regulated by the Asi1 E3 Ligase in Saccharomyces cerevisiae. Genetics 211, 1269–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suresh S, Markossian S, Osmani AH, and Osmani SA (2017). Mitotic nuclear pore complex segregation involves Nup2 in Aspergillus nidulans. The Journal of cell biology 216, 2813–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tiku V, Jain C, Raz Y, Nakamura S, Heestand B, Liu W, Spath M, Suchiman HED, Muller RU, Slagboom PE, et al. (2017). Small nucleoli are a cellular hallmark of longevity. Nature communications 8, 16083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Toyama BH, Arrojo EDR, Lev-Ram V, Ramachandra R, Deerinck TJ, Lechene C, Ellisman MH, and Hetzer MW (2019). Visualization of long-lived proteins reveals age mosaicism within nuclei of postmitotic cells. The Journal of cell biology 218, 433–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Toyama BH, Savas JN, Park SK, Harris MS, Ingolia NT, Yates JR 3rd, and Hetzer MW (2013). Identification of long-lived proteins reveals exceptional stability of essential cellular structures. Cell 154, 971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Troyer D, and Schwager P (1982). Evidence for nuclear membrane fluidity: Proacrosome migration and nuclear pore redistribution during grasshopper spermiogenesis. Cell Motility 2, 355–367. [Google Scholar]
- 65.Unal E, and Amon A (2011). Gamete formation resets the aging clock in yeast. Cold Spring Harbor symposia on quantitative biology 76, 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Unal E, Kinde B, and Amon A (2011). Gametogenesis eliminates age-induced cellular damage and resets life span in yeast. Science (New York, NY) 332, 1554–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ungricht R, and Kutay U (2017). Mechanisms and functions of nuclear envelope remodelling. Nature reviews Molecular cell biology 18, 229–245. [DOI] [PubMed] [Google Scholar]
- 68.Van de Vosse DW, Wan Y, Wozniak RW, and Aitchison JD (2011). Role of the nuclear envelope in genome organization and gene expression. Wiley interdisciplinary reviews Systems biology and medicine 3, 147–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Walters AD, May CK, Dauster ES, Cinquin BP, Smith EA, Robellet X, D’Amours D, Larabell CA, and Cohen-Fix O (2014). The yeast polo kinase Cdc5 regulates the shape of the mitotic nucleus. Current biology : CB 24, 2861–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Webster BM, Colombi P, Jager J, and Lusk CP (2014). Surveillance of nuclear pore complex assembly by ESCRT-III/Vps4. Cell 159, 388–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Witkin KL, Chong Y, Shao S, Webster MT, Lahiri S, Walters AD, Lee B, Koh JL, Prinz WA, Andrews BJ, et al. (2012). The budding yeast nuclear envelope adjacent to the nucleolus serves as a membrane sink during mitotic delay. Current biology : CB 22, 1128–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang K, Donnelly CJ, Haeusler AR, Grima JC, Machamer JB, Steinwald P, Daley EL, Miller SJ, Cunningham KM, Vidensky S, et al. (2015). The C9orf72 repeat expansion disrupts nucleocytoplasmic transport. Nature 525, 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]


