Abstract
Background:
High trait impulsive sensation seeking (ISS), the tendency to engage in behavior without forethought and to seek out new/extreme experiences, is a transdiagnostic risk factor for externalizing and mood disorders, particularly bipolar disorder (BD). We published a positive association between trait ISS and reward expectancy-related activity in the left ventrolateral prefrontal cortex (L vlPFC) and ventral striatum (VS). We aimed to replicate this finding and extend it by testing for mediation effects of ISS on relationships between reward expectancy-related activity and measures denoting hypomania.
Methods:
A transdiagnostic sample of 127 adults aged 18-25 completed a card-based guessing fMRI task as well as measures of ISS (inattention, motor impulsivity, fun seeking, positive and negative urgency) and the Moods Spectrum (MOODS) as a measure of hypomania. A sample of 98 from the original Chase et al. (2017) study was included for confirmatory and mediation analyses.
Results:
We replicated a positive relationship between reward expectancy-related L vlPFC activity and negative urgency, an ISS component (β=0.28,t=2.44,p=0.0169). We combined these data with the original paper sample, confirming this finding (β=0.27,t=2.41,p=0.0184). Negative urgency statistically mediated the relationship between reward expectancy-related L vlPFC activity and MOODS factors associated with hypomania. No other associations between ISS measures and reward-expectancy related activity were replicated.
Conclusions:
We replicated findings showing that reward expectancy-related L vlPFC activity is a biomarker for negative urgency, the tendency to react with frustration during distressing conditions. Negative urgency also statistically mediated the relationship between L vlPFC activity and measures indicative of hypomanic symptoms.
Keywords: fMRI, bipolar disorder, replication, reward expectancy, impulsive sensation seeking, ventrolateral prefrontal cortex
Introduction
Impulsive sensation seeking (ISS) is a complex trait comprising impulsivity, i.e., behavior without advance thought, and sensation seeking, the tendency to seek new or extreme experiences.(1) High trait ISS is associated with alcohol and substance abuse,(2, 3) suicide attempt,(4) and attention deficit hyperactivity disorder (ADHD)(5). Furthermore, in prospective studies, impulsive behavior has shown utility in predicting progression to bipolar disorder (BD) versus other psychiatric diagnoses, leading some to suggest that high impulsivity may represent a BD prodrome.(6) Indeed, individuals with BD show high ISS, as do at-risk individuals who develop BD.(7-9) High ISS also differentiates a subgroup of individuals diagnosed with major depressive disorder who experience cyclothymic and other subthreshold hypomanic symptoms associated with risk for BD from those who do not experience these symptoms.(10)
High ISS is related to greater valuation and motivational salience of potential reward.(11) One context relevant to ISS is reward expectancy, a state of motivated approach following a cue denoting potential reward. During reward expectancy, high ISS individuals are more likely to make impulsive decisions to obtain reward. These findings are consistent with a conceptual model of risk for externalizing disorders that posits heightened activation of a biobehavioral system in response to reward cues.(12, 13) Elucidating the relationship between reward expectancy-related activity and ISS can thus help identify objective, transdiagnostic biological risk markers for psychiatric illness.
In a previous study using a card-based reward task,(14, 15) we identified ISS components associated with reward expectancy-related reward network activity in a transdiagnostic sample of young adults. We observed a positive association between scales measuring inattention, motor impulsivity, fun seeking, and positive/negative urgency and reward expectancy-related activity in the left ventrolateral prefrontal cortex (L vlPFC), and bilateral ventral striatum (VS).(16) These findings parallel other studies showing that the L vlPFC and the VS are associated with reward processing, and show abnormal reward processing-related (especially reward expectancy-related) activity in individuals with or at risk for BD and alcohol and substance use disorders.(17-24)
The L vlPFC is associated with evaluating and linking stimuli to possible outcomes.(25, 26) Heightened reward expectancy-related L vlPFC activity may thus represent enhanced encoding of potential reward, although there is some literature that would suggest enhanced vlPFC activity might also be related to effortful emotional regulation or cognitive control/reappraisal.(27-29) More specifically, there is evidence for greater L vlPFC activity during encoding of high versus low reward stimuli.(30) Furthermore, differences in L vlPFC responsiveness on the basis of reward valuation are greater for individuals with high reward sensitivity.(31) There is also much evidence demonstrating the role of the VS in reward processing, especially motivational salience and reward learning,(32, 33) and VS activity during reward expectancy is positively correlated with novelty seeking and impulsivity.(22) Our previous findings of a positive association between ISS and reward expectancy-related L vlPFC and bilateral VS activity thus highlight these regions as potentially important biomarkers for psychiatric disorders associated with heightened reward sensitivity, including BD.(34)
Although there is evidence for the role of a distributed reward network in risk for psychopathology, this finding must be replicated. There have been few successful replications in psychiatric neuroimaging. This has led some to describe a “replication crisis” in brain-behavior research.(35, 36) There are likely high false positive rates due to small sample sizes, a lack of consistent standards for statistical reporting, and problems with variation in methodological approaches and standards in fMRI.(36) For example, a recent series of confirmatory replication analyses failed to replicate any findings in 17 MRI studies.(37) Lack of replications hinders progress, and will result in misdirected efforts. Thus, there is a need for psychiatric neuroimaging replication studies.
In this study, our goal was twofold. First, we aimed to replicate our findings of an association between ISS and reward expectancy-related activity in an independent study with an identical experimental design as our prior work.(16) As such, we hypothesized that reward expectancy-related L vlPFC and VS activity would be positively associated with ISS. Secondly, in order to refine and extend our understanding of the relationships between ISS and reward expectancy-related activity, we examined the relationship between L vlPFC and VS activity and each ISS component separately, in contrast to the original study. Given previous findings of a positive relationship between reward expectancy-related activity in these regions and ISS, greater reward expectancy-related L vlPFC and VS activity might not only represent enhanced reward encoding, but may also serve as an important transdiagnostic biomarker. As high ISS has been associated with BD risk and differentiates BD from unipolar depression, we hypothesized associations among reward expectancy-related activity, ISS, and predisposition to BD. To measure predisposition to BD in this sample of young adults, we used specific measures of subthreshold to syndromal-level affective symptoms that have been shown to denote the presence of and/or predisposition to BD, and that, in prior work, have differentiated BD from unipolar depression: the Moods Spectrum (MOODS) factors of Suicidality, Mixed Instability, and Psychomotor Activation.(38) Given associations between L vlPFC and VS reward expectancy-related activity and risk for psychopathology, as well as high trait ISS and risk for BD, we hypothesized that the relationship between reward expectancy-related activity in reward-associated regions (L vlPFC, VS) and the three MOODS factors would be explained in part by high ISS.
Methods and Materials
Participants
Sixty-five adults (47 female) ages 18-25 who were seeking treatment for psychological distress, regardless of diagnosis, were recruited from the Pittsburgh community. Only two had a present BD diagnosis (Supplemental). Sixty-eight healthy age- and sex-matched adults were recruited via community advertisement. All participants were right-handed and all healthy participants had no family/personal history of psychiatric illness (Supplemental). The University of Pittsburgh Institutional Review Board approved this study. Participants gave written informed consent following a complete description of the study. Six were excluded due to excessive motion (as in the original sample; Supplemental). The final sample included 61 distressed and 66 healthy participants (Table 1). We conducted additional analyses combining this sample with the sample from the original study (Supplemental).
Table 1:
Replication Sample Participant Demographics and Symptom Measures
| Healthy | Distressed | Statistical Comparison | |
|---|---|---|---|
| Gender | 53 F, 13 M | 44 F, 17 M | X2=1.17, p=0.28 |
| Age | 22.01 (2.04) | 21.45 (2.20) | T=1.48(122.03), p=0.14 |
| Scanner | 15 TRIO, 51 PRISMA | 2 TRIO, 59 PRISMA | X2=10.34, p=0.001 |
| Educational Attainment (no college/some college or more) | 8/58 | 13/48 | X2=1.93, p=0.16 |
| NART IQ | 107.62 (6.33) | 108.94 (7.18) | T=−1.089(119.97), p=0.28 |
| Framewise displacement | 0.21 (0.07) | 0.24 (0.07) | T=−2.30(122.18), p=0.02 |
| BIS-11 Attention | 8.58 (2.54) | 11.05 (2.72) | T=−5.28(122.33), p<0.0001 |
| BIS-11 Motor | 13.21 (2.38) | 13.93 (3.83) | T=−1.27(98.76), p=0.21 |
| BIS/BAS Fun Seeking | 11.85 (2.15) | 11.70 (2.20) | T=0.37(123.69), p=0.71 |
| UPPS-P Positive Urgency | 20.85 (5.44) | 25.64 (9.42) | T=−3.48(94.43), p=0.001 |
| UPPS-P Negative Urgency | 21.59 (5.68) | 29.36 (7.01) | T=−6.83(114.57), p<0.0001 |
| YMRS Total Score | 0.32 (1.24) | 2.49 (1.74) | X2=87.25, p<0.0001 |
| HAMD Total Score | 1.27 (2.09) | 14.67 (6.33) | T=−15.75(72.06), p<0.0001 |
| MOODS Mixed Instability Factor | 0.52 (0.86) | 1.87 (1.81) | X2=23.81, p<0.0001 |
| MOODS Psychomotor Activation Factor | 0.95 (1.62) | 5.41 (3.77) | X2=59.65, p<0.0001 |
| MOODS Suicidality Factor | 0.05 (0.21) | 2.30 (1.94) | Χ2=41.34, p<0.0001 |
Assessments
The Structured Clinical Interview for DSM-V was administered to assess for exclusion criteria (Supplemental).(39) All participants were administered the Hamilton Rating Scale for Depression (HAMD)(40) and the Young Mania Rating Scale (YMRS).(41) Participants completed the Mood Spectrum Scale (MOODS), a 15-factor measure designed to assess lifetime incidence of hypo/manic and depressive symptoms/traits that characterize subsyndromal or prodromal states of affective disturbance, as well as threshold symptomatic criteria. Previous studies identified three specific MOODS factors associated with the presence of and/or predisposition to BD, which can be used to differentiate BD from unipolar depression: Psychomotor Activation, Mixed Instability, and Suicidality factors.(42) Participants completed the specific measures of ISS in which components were shown, in our original study, to be associated with reward expectancy-related L vlPFC and VS activity: the Barrett Impulsiveness Scale (BIS),(43) Behavioral Inhibition/Activation Scale (BIS/BAS)(44) and the Urgency, Premeditation (lack of), Perseverance (lack of), Sensation Seeking, Positive Urgency, (UPPS-P) Impulsive Behavior Scale.(45)
MRI data acquisition
Data were collected using a 3.0 Tesla Siemens Trio 2 (n=17) or a 3.0 Tesla PRISMA MRI (n=110) scanner at the University of Pittsburgh Magnetic Resonance Research Center (Supplemental).
FMRI task
Participants completed a 16-minute event-related task to assess Blood Oxygen Level Dependent (BOLD) signal during anticipation and receipt of reward that was identical to our original study.(15) For each trial, participants were asked to indicate via button press whether they guessed that a subsequently-presented card would have a value higher or lower than five. A reward expectancy cue was then presented for 2-6 seconds denoting one of four trial types (24 trials for each trial type overall), followed by a 1 second outcome and a 0.5-1.5 second jittered ISI (Supplemental). The four trial types included: win (win or no change); loss (loss or no change); mixed win and loss trials with win or loss outcomes; neutral trials with no change.
Neuroimaging data preprocessing
FMRI data were preprocessed using Nipype (Supplemental).(46)
First-level modeling
First-level general linear models were created for each participant in SPM12 (Supplemental). The reward expectancy condition was our parametric regressor of interest, corresponding to the 2-6 second window following choice but before outcome. We weighted reward expectancy to reflect the expected value for trial conditions: +.5 for win trials (50% chance of winning $1), −.375 for loss trials (50% chance of losing $.75), 0 for neutral trials, and +.125 for mixed trials (50/50 chance of winning $1 or losing $.75).
For completeness and to determine specificity of findings, we computed parametric regressors for prediction error and outcome expectancy (Supplemental).
Second-level modeling
A total of three regression analyses (one for each region of interest), and three mediation analyses (one for each of three MOODS factors), were performed in this study.
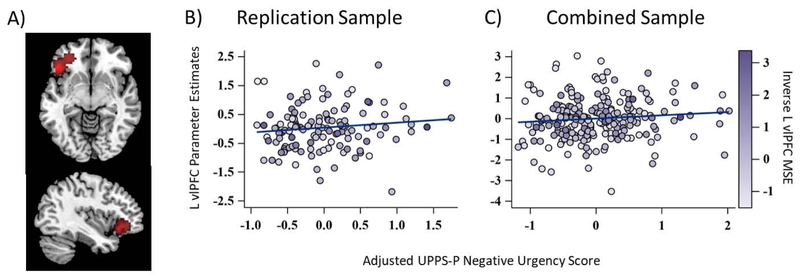
Signal was extracted from a priori regions of interest (L vlPFC, right and left VS) in SPM12. The striatal regions of interest were created from activation maps from an independent study.(19) The L vlPFC region of interest, used in the original study(16), was derived from an activation likelihood estimation meta-analysis of studies showing increased reward-related activity in this region (Figure 1a).(19, 22-24, 47)
Figure 1.
Weighted regression results for extracted parameter estimates during reward expectancy for the A) left ventrolateral prefrontal cortex (L vlPFC) region of interest, UPPS-P Negative Urgency scores in the B) independent replication sample (t=2.44, p=0.02), and C) combined sample (t=2.41, p=0.02). The points on the scatter plots represent raw mean reward expectancy-related L vlPFC values, and the color bar represents the relative weighting of each point by inverse of L vlPFC mean squared error (MSE). The lines represent the association between reward expectancy-related L vlPFC activity as a function of adjusted UPPS-P Negative Urgency Scores. These values have been adjusted by first regressing out the remaining independent variables from the primary statistical models. The lines thus represent the semi-partial correlations (i.e. the unique contributions) of negative urgency to reward expectancy-related L vlPFC activity accounting for all of the other covariates. The squared correlation coefficients from these relationship are akin to the change in R2 obtained by adding negative urgency to the weighted regression models that have already accounted for the variance that could be explained by the other covariates.
Three parallel linear regressions were performed with extracted reward expectancy-related signal in each region of interest as the dependent variables and ISS components identified in our previous study as independent variables (BIS Attention, BIS Motor, BIS/BAS Fun Seeking, and UPPS-P Positive and Negative Urgency). Covariates included age, sex, education, scanner, and framewise displacement (FWD). FWD was not significantly correlated with any experimental variables of interest (Supplemental). After first-level model fitting, we observed a substantial scanner difference in the mean squared error (MSE) maps generated for each subject, representing differences in residual variance by scanner. We therefore extracted MSE for each participant from each region of interest and weighted all regressions using the standardized inverse of MSE per participant for each respective region of interest. This approach offers a sophisticated control for signal to noise ratio differences.(48) The original Chase and colleagues study employed elastic net regression to identify ISS component measures; these measures were then transformed to create a composite ISS variable for additional analyses. In contrast, we performed standard linear regression models with each of the five ISS components identified in the original study, to better assess the relationship of each of these components with reward expectancy-related activity. These regression analyses were also performed for a combined sample that included 98 additional participants (two with BD) from the original study (n=225). Results for the independent and combined samples were considered significant at p<0.02727, FDR corrected for three models.(49) Analyses were repeated for outcome expectancy and prediction error (Supplemental).
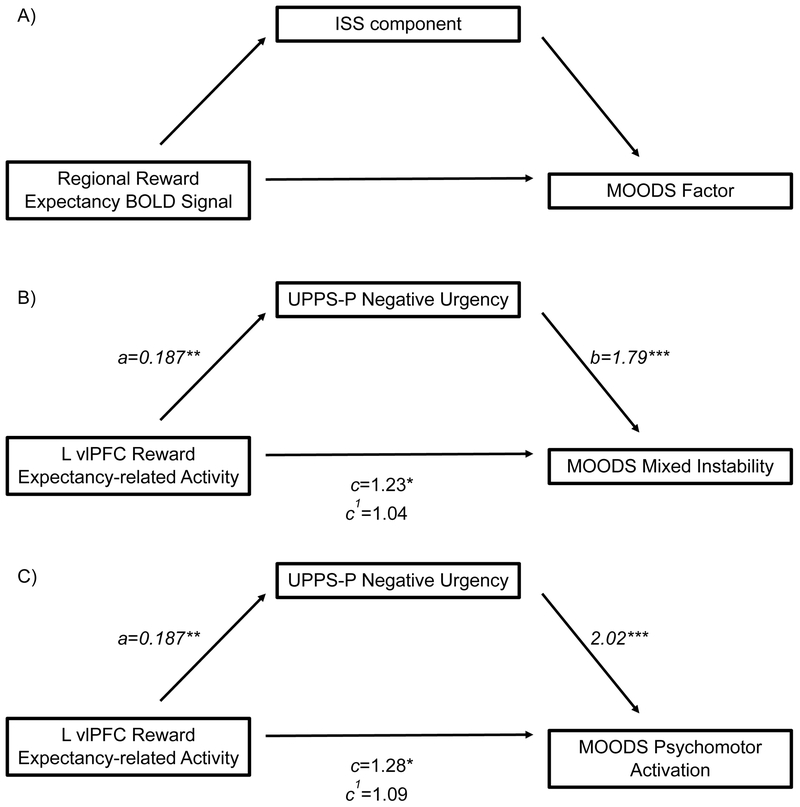
To extend findings of the original study, we used Baron and Kenny’s framework(50) to examine whether significant ISS components identified in the primary regression models statistically mediated the relationship between reward expectancy-related activity and MOODS factors that differentiate BD from unipolar depression (MOODS Mixed Instability, Psychomotor Activation, and Suicidality; Figure 2a).(38) Since MOODS scores were distributed as count variables, associations were estimated using negative binomial regressions. To confirm finding specificity, we tested for mediation effects of ISS on the relationship between reward expectancy-related activity and YMRS and HAMD (Supplemental).
Figure 2.
A) Hypothesized mediation model B) Model for the relationship between reward expectancy-related L vlPFC activity and MOODS Mixed Instability Factor, as mediated by UPPS-P Negative Urgency. The indirect model resulted in an 81.67% reduction in the contribution of reward expectancy-related L vlPFC activity to MOODS factor score versus the direct model. C) Model for the relationship between reward expectancy-related L vlPFC activity and MOODS Psychomotor Activation factor, as mediated by UPPS-P Negative Urgency. The indirect model resulted in a 67.98% reduction versus the direct model. Values represent standardized β for linear regression (a) and IRR for negative binomial regressions (b, c, c1). Percent reductions for IRR are the ratio of change in IRR between the direct and indirect models over the direct model IRR.(79) *p<0.05, **p<0.005,***p<0.0001
Exploratory whole brain analyses were conducted to assess for associations between ISS and brain activity during reward expectancy, outcome expectancy, and prediction error (p<0.05,FWE corrected, Supplemental).
Results
Region of Interest Analyses and ISS Components
We observed a positive association between UPPS-P Negative Urgency and reward expectancy-related L vlPFC activity (β=0.28, t=2.44, p=0.0169 Figure 1b). No other ISS component (BIS Attention, BIS Motor, BIS/BAS Fun Seeking, or UPPS-P Positive Urgency) was significantly associated with reward expectancy-related L vlPFC activity. There were no findings for the VS (ps>0.05, Table 2). The omnibus tests were nonsignificant for all three primary regression analyses (L vlPFC, left and right VS, ps>0.05).
Table 2a:
Description of region of interest statistics for three parallel regression models, independent sample
| Dependent Variable | Independent Variables | ||||
|---|---|---|---|---|---|
| BIS-11 Attention |
BIS-11 Motor |
BIS/BAS Fun Seeking |
UPPS-P Negative Urgency |
UPPS-P Positive Urgency |
|
| L vlPFC Reward Expectancy |
β =0.07 (−0.12,0.26) |
β =−0.06 (−0.24,0.12) |
β =−0.01 (−0.21,0.19) |
β =0.28 (0.05,0.51)* |
β =−0.20 (−0.45,0.04) |
| L VS Reward Expectancy |
β =0.17 (−0.13,0.47) |
β =−0.04 (−0.36,0.27) |
β =−0.09 (−0.36,0.19) |
β =0.25 (−0.14,0.64) |
β =−0.45 (−0.93,0.03) |
| R VS Reward Expectancy |
β =0.27 (−0.05,0.59) |
β =−0.04 (−0.35,0.27) |
β =−0.16 (−0.49,0.16) |
β =0.03 (−0.39,0.45) |
β =−0.14 (−0.66,0.39) |
Standardized β and 95% Confidence Intervals for linear regression models in the,
p<0.02727 (FDR correction threshold).
In the combined sample, UPPS-P Negative Urgency was associated with reward expectancy-related L vlPFC activity (β=0.27, t=2.41, p=0.0184, Figure 1c). No other ISS component was associated with reward expectancy-related L vlPFC activity. There were no findings in the VS (ps>0.05, Table 3).
Table 3:
Description of region of interest statistics for three parallel regression models, combined sample
|
Dependent Variable |
Independent Variables | ||||
|---|---|---|---|---|---|
| BIS-11 Attention |
BIS-11 Motor |
BIS/BAS Fun Seeking |
UPPS-P Negative Urgency |
UPPS-P Positive Urgency |
|
| L vlPFC Reward Expectancy |
β =0.07 (−0.11,0.26) |
β =−0.08 (−0.25,0.09) |
β =0.01 (−0.18,0.20) |
β =0.27 (0.05,0.49)* |
β =−0.18 (−0.41,0.05) |
| L VS Reward Expectancy |
β =0.04 (−0.15,0.23) |
β =0.02 (−0.19,0.24) |
β =0.02 (−0.20,0.24) |
β=0.13 (−0.16,0.41) |
β =−0.12 (−0.34,0.11) |
| R VS Reward Expectancy |
β =0.15 (−0.07,0.36) |
β =−0.03 (−0.25,0.18) |
β =0.04 (−0.23,0.31) |
β=−0.12 (−0.44,0.21) |
β =0.10 (−0.16,0.37) |
Standardized β and 95% Confidence Intervals for linear regression models in the,
p<0.02727 (FDR correction threshold).
Statistical Mediation (combined sample)
We modeled reward expectancy-related L vlPFC activity and MOODS factors (Mixed Instability, Psychomotor Activation, Suicidality). Results were considered significant at p<0.02727, FDR corrected for these three factors.(49) There was a significant positive relationship between reward expectancy-related L vlPFC activity and MOODS Mixed Instability (Incidence Rate Ratio (IRR)=1.23, χ2=5.39, p=0.02) and Psychomotor Activation (IRR=1.28, χ2=6.52, p=0.01), but not Suicidality (IRR=1.15, χ2=1.00, p=0.32). As only the relationship between reward expectancy-related L vlPFC activity and negative urgency was significant, we next examined whether negative urgency statistically mediated the relationship between reward expectancy-related L vlPFC activity and MOODS Mixed Instability and Psychomotor Activation.
In the mediation model, reward expectancy-related L vlPFC activity was associated with UPPS-P Negative Urgency (β=0.187, t=2.88, p=0.004). There was an association between negative urgency and MOODS Mixed Instability (IRR=1.79, χ2=63.22, p<0.0001). We calculated the percent reduction in IRR by dividing the difference in IRR between the two models by the IRR for the direct model, as there is currently no available inferential test for mediation models including both linear and count variables. The indirect model resulted in an 81.67% reduction in the contribution of reward expectancy-related L vlPFC activity to MOODS Mixed Instability (L vlPFC IRR=1.04, χ2=0.29, p=0.59, Figure 2b). Negative urgency was also associated with MOODS Psychomotor Activation (IRR=2.02, χ2=80.29, p<0.0001). Inclusion of negative urgency in the model resulted in a 67.98% reduction in the contribution of reward expectancy-related L vlPFC activity to the MOODS factor outcome (IRR=1.09, χ2=1.06, p=0.30. Figure 2c). Reward expectancy-related L vlPFC activity did not mediate between negative urgency and MOODS scores (Supplemental).
Whole Brain Analyses
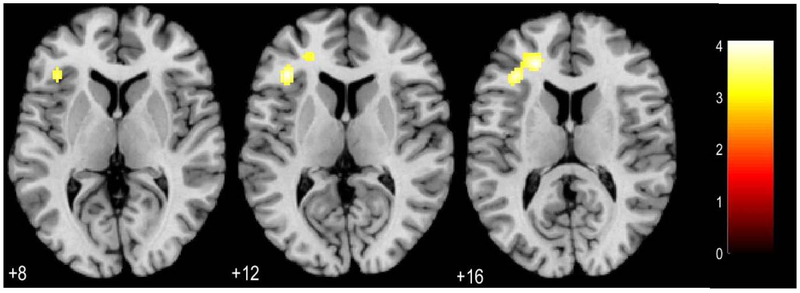
There were no findings at p<0.05, FWE corrected. There was a positive relationship between reward expectancy-related L vlPFC activity and negative urgency, p<0.001 uncorrected, k=20 (Figure 3, Supplemental).
Figure 3.
Whole brain associations between reward expectancy and UPPS-P Negative Urgency in the left ventrolateral prefrontal cortex. Maps thresholded at p<0.001 uncorrected, k=20. Color bar reflects t values, numbers below axial slices represent z coordinates.
Post hoc Analyses
There were no changes when medicated (n=3) participants were excluded and no diagnosis effects (Supplemental), as in the original sample.
Discussion
We replicated a positive association between negative urgency and reward expectancy-related L vlPFC activity in an independent, transdiagnostic sample of young adults, and confirmed this result after combining the replication and original samples. No other findings from the Chase et al., 2017 publication were replicated (i.e., associations with the bilateral VS or other components of ISS). We further showed that negative urgency statistically mediated, or accounted for, positive relationships between reward expectancy-related L vlPFC activity and the MOODS Psychomotor Activation and with the Mixed Instability factors. Importantly, both of these factors have been shown to differentiate BD from unipolar depression.(38) Given these results, the fact that high ISS is a risk factor for BD, and that high scores on the above MOODS factors denote presence of and/or predisposition to BD versus unipolar depression, our findings point to reward expectancy-related L vlPFC activity as an important neural biomarker of negative urgency, a transdiagnostic risk factor for psychiatric disorders, particularly BD.
Negative urgency is the tendency to act without forethought during emotional distress. People with high negative urgency respond with impulsivity, impatience, frustration, or irritation to negative events or to relatively benign contexts such as reward anticipation.(51-53) In contrast, positive urgency is the tendency to act impulsively while experiencing positive emotions.(54) Most studies of BD and impulsivity focus on positive urgency, demonstrating high positive urgency in rewarding contexts.(55, 56) These findings are framed as a failure of response inhibition caused by heightened reward sensitivity/pursuit in BD.(12) The relationship between impulsivity and negative urgency has been less well-characterized in the BD literature, although high negative urgency is evident among remitted patients with BD and among those with co-occurring substance use, anxiety disorders, and suicidal ideation.(57) Furthermore, factor analyses of hypo/manic symptoms demonstrated a prominent irritable or negative affective component to hypo/mania that is distinct from impulsivity, concluding that hypo/mania can be characterized by both positive and negative affect.(58, 59) Irritability, a closely related construct to negative urgency, is more prevalent in depressive episodes in BD than in unipolar depression.(60, 61) Irritability predicts longer episode duration, greater symptom severity, and higher levels of suicidal behavior, (62, 63) as well as first major affective episode in BD.(64) It is the most reported mood state before a manic episode.(65, 66) Moreover, negative affective components of mania likely result in some of the more harmful consequences associated with manic episodes, such as legal problems and interpersonal violence. Negative urgency is also a transdiagnostic risk factor, and our findings contribute to the emerging literature highlighting negative affective arousal in people experiencing psychiatric distress who may be at risk for developing externalizing disorders. These results indicate a need for longitudinal study of the neural correlates of negative urgency in at-risk cohorts.
The neural correlates of negative urgency have been best characterized in the context of addiction and obesity. Negative urgency has been associated with dorsal striatal functional and structural alterations (67) and correlated with ventromedial prefrontal activity during reward cues.(68) Other studies have shown volume reductions in the left inferior frontal gyrus in cocaine-dependent individuals,(69) as well as a positive relationship between negative urgency and L vlPFC activation during inhibitory trials in a Go/No-Go task.(70) We observed a relationship between negative urgency and L vlPFC activity during reward expectancy, but not outcome expectancy or prediction error. This is consistent with our previous findings, as well as the literature associating reward expectancy-related L vlPFC activity with impulsivity and reward sensitivity.(71, 72) The vlPFC encodes potential outcome value, and left-lateralized reward expectancy-related vlPFC activity may be specifically related to approach behavior.(73) We speculate that reward expectancy-related L vlPFC activity may thus represent a state of frustration or impatience during anticipation of reward. This is consistent with the clinical presentation of hypo/manic states, which are often characterized by elevated or irritable mood in addition to heightened impulsivity and behavioral activation.(59) Indeed, the YMRS assesses for reactive aggression and annoyance in response to benign frustrations, as well as increases in interpersonal conflicts, when assessing the negative affective components of hypo/mania.(41)
We showed a statistical mediation effect of negative urgency on the relationship between reward expectancy-related L vlPFC activity and Psychomotor Activation and Mixed Instability factors, an incremental extension of the original Chase et al. study.(38) Heightened behavioral activation is an important factor in the differential diagnosis of BD,(74) and enquiry about episodes of psychomotor hyperactivity more reliably differentiates BD than enquiries about euphoric episodes.(75, 76) Interestingly, we did not observe a mediation effect of brain activity on the relationship between negative urgency and MOODS factors, indicating specificity of the relationship between reward expectancy-related activity in the L vlPFC and proximal transdiagnostic risk.
Importantly, there was no significant relationship between reward expectancy-related L vlPFC activity and depression symptoms, or the MOODS Suicidality factor, suggesting that reward expectancy-related L vlPFC activity may have utility for assessing specific risk for hypo/mania via its relationships with negative urgency. There was no significant relationship between reward expectancy-related L vlPFC activity and YMRS score, but this may reflect the low range of scores on this measure, given that only two of the participants in the combined sample of 225 had BD. To our knowledge, there have been no prospective longitudinal studies that have specifically examined the MOODS as a predictor of conversion to BD. A recent study found that, in young adults with remitted unipolar depression, the MOODS reliably identified a subgroup with high trait impulsivity and subthreshold manic symptoms, as well as altered VS-inferior frontal gyrus connectivity.(77) These findings indicate that the MOODS shows promise as a more proximal measure of biomarkers for hypo/mania.
Limitations in this study include the use of two scanners, for which we adjusted by including scanner as a covariate and by weighting all regressions by extracted MSE. While we were underpowered to test for moderation effects of gender, the overrepresentation of women in our sample is consistent with the demographics of individuals seeking treatment. The overrepresentation of women in the present study may limit generalizability of our findings, however. Similarly, the age range was restricted to 18-25 years, given our interest in assessing risk for psychopathology during this critical developmental period. Our findings may not generalize to other age groups. While we only partially replicated our previous findings, we demonstrated important relationships between ISS components and brain activity. For example, we did not observe a relationship between positive urgency and reward-expectancy related activity, even though positive urgency is typically associated with reward processing. One interpretation of these findings is that participants experienced frustration and negative affect during periods of uncertain reward, resulting in more prominent negative urgency findings. This study was cross-sectional, and demonstrates a statistical mediation between brain, negative urgency, and MOODS factors. Longitudinal studies can help determine if negative urgency mediates the relationship between L vlPFC activity and changes in hypo/manic symptoms or diagnosis. We recruited a transdiagnostic sample of young adults experiencing psychiatric distress. Although a proportion of these young adults will likely convert to BD, given that BD onset commonly occurs up until late twenties-early thirties(78), the sample was not recruited based on a specific risk factor for BD per se, i.e., a genetic risk factor or first degree relative. Future studies with longitudinal designs that oversample for BD risk specifically are needed to clarify the specificity of negative urgency as a behavioral construct associated with BD risk versus other disorders associated with high ISS.
This study makes an important contribution – first by partially replicating our previous findings, identifying a single ISS component, negative urgency, associated with reward expectancy-related L vlPFC activity, and second by observing that the relationship between reward expectancy-related L vlPFC activity and factors known to differentiate BD from unipolar depression is largely explained by heightened negative urgency. Our findings thereby improve understanding of the neural mechanisms underlying risk for psychiatric disorders, and highlight the importance of the L vlPFC as a potential biomarker and target for interventions to reduce negative affective symptoms.
Supplementary Material
Acknowledgements
Funding sources for this study include NIMH R01 MH100041 04 and T32 MH16804 (Phillips). Portions of this study were presented as a poster at the Society for Biological Psychiatry Annual Meeting, 2019.
Footnotes
Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Zuckerman M (2007): Sensation seeking and risky behavior. Washington, DC: American Psychological Association. [Google Scholar]
- 2.Schepis TS, Desai RA, Smith AE, Cavallo DA, Liss TB, McFetridge A, et al. (2008): Impulsive sensation seeking, parental history of alcohol problems, and current alcohol and tobacco use in adolescents. Journal of addiction medicine. 2:185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doumas DM, Miller R, Esp S (2017): Impulsive sensation seeking, binge drinking, and alcohol-related consequences: Do protective behavioral strategies help high risk adolescents? Addictive behaviors. 64:6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ortin A, Lake AM, Kleinman M, Gould MS (2012): Sensation seeking as risk factor for suicidal ideation and suicide attempts in adolescence. Journal of affective disorders. 143:214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopez R, Dauvilliers Y, Jaussent I, Billieux J, Bayard S (2015): A multidimensional approach of impulsivity in adult attention deficit hyperactivity disorder. Psychiatry research. 227:290–295. [DOI] [PubMed] [Google Scholar]
- 6.Conus P, Ward J, Hallam KT, Lucas N, Macneil C, McGorry PD, et al. (2008): The proximal prodrome to first episode mania--a new target for early intervention. Bipolar disorders. 10:555–565. [DOI] [PubMed] [Google Scholar]
- 7.Alloy LB, Abramson LY, Walshaw PD, Cogswell A, Grandin LD, Hughes ME, et al. (2008): Behavioral Approach System and Behavioral Inhibition System sensitivities and bipolar spectrum disorders: prospective prediction of bipolar mood episodes. Bipolar disorders. 10:310–322. [DOI] [PubMed] [Google Scholar]
- 8.Urosevic S, Abramson LY, Harmon-Jones E, Alloy LB (2008): Dysregulation of the behavioral approach system (BAS) in bipolar spectrum disorders: review of theory and evidence. Clinical psychology review. 28:1188–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saddichha S, Schuetz C (2014): Is impulsivity in remitted bipolar disorder a stable trait? A meta-analytic review. Comprehensive psychiatry. 55:1479–1484. [DOI] [PubMed] [Google Scholar]
- 10.Fornaro M, Ventriglio A, De Pasquale C, Pistorio ML, De Berardis D, Cattaneo CI, et al. (2013): Sensation seeking in major depressive patients: relationship to sub-threshold bipolarity and cyclothymic temperament. Journal of affective disorders. 148:375–383. [DOI] [PubMed] [Google Scholar]
- 11.Smillie LD, Pickering AD, Jackson CJ (2006): The new reinforcement sensitivity theory: implications for personality measurement. Personality and social psychology review : an official journal of the Society for Personality and Social Psychology, Inc. 10:320–335. [DOI] [PubMed] [Google Scholar]
- 12.Johnson SL (2005): Mania and dysregulation in goal pursuit: a review. Clinical psychology review. 25:241–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray J (1994): Three fundamental emotion systems In: Eckman PD, RJ, editor. The nature of emotion: fundamental questions. New York: Oxford University Press, pp 243–247. [Google Scholar]
- 14.Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA (2000): Tracking the hemodynamic responses to reward and punishment in the striatum. Journal of neurophysiology. 84:3072–3077. [DOI] [PubMed] [Google Scholar]
- 15.Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, et al. (2009): Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. The American journal of psychiatry. 166:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chase HW, Fournier JC, Bertocci MA, Greenberg T, Aslam H, Stiffler R, et al. (2017): A pathway linking reward circuitry, impulsive sensation-seeking and risky decision-making in young adults: identifying neural markers for new interventions. Translational psychiatry. 7:e1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cattarinussi G, Di Giorgio A, Wolf RC, Balestrieri M, Sambataro F (2019): Neural signatures of the risk for bipolar disorder: A meta-analysis of structural and functional neuroimaging studies. Bipolar disorders. 21:215–227. [DOI] [PubMed] [Google Scholar]
- 18.Manelis A, Ladouceur CD, Graur S, Monk K, Bonar LK, Hickey MB, et al. (2016): Altered functioning of reward circuitry in youth offspring of parents with bipolar disorder. Psychological medicine. 46:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chase HW, Nusslock R, Almeida JR, Forbes EE, LaBarbara EJ, Phillips ML (2013): Dissociable patterns of abnormal frontal cortical activation during anticipation of an uncertain reward or loss in bipolar versus major depression. Bipolar disorders. 15:839–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirschner M, Cathomas F, Manoliu A, Habermeyer B, Simon JJ, Seifritz E, et al. (2019): Shared and dissociable features of apathy and reward system dysfunction in bipolar I disorder and schizophrenia. Psychological medicine.1–12. [DOI] [PubMed] [Google Scholar]
- 21.Lee MS, Anumagalla P, Talluri P, Pavuluri MN (2014): Meta-analyses of developing brain function in high-risk and emerged bipolar disorder. Frontiers in psychiatry. 5:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh MK, Kelley RG, Howe ME, Reiss AL, Gotlib IH, Chang KD (2014): Reward processing in healthy offspring of parents with bipolar disorder. JAMA psychiatry. 71:1148–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nusslock R, Almeida JR, Forbes EE, Versace A, Frank E, Labarbara EJ, et al. (2012): Waiting to win: elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar disorder adults. Bipolar Disord. 14:249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caseras X, Lawrence NS, Murphy K, Wise RG, Phillips ML (2013): Ventral striatum activity in response to reward: differences between bipolar I and II disorders. The American journal of psychiatry. 170:533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SW, O'Doherty JP, Shimojo S (2015): Neural computations mediating one-shot learning in the human brain. PLoS biology. 13:e1002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boorman ED, Rajendran VG, O'Reilly JX, Behrens TE (2016): Two Anatomically and Computationally Distinct Learning Signals Predict Changes to Stimulus-Outcome Associations in Hippocampus. Neuron. 89:1343–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snyder HR, Banich MT, Munakata Y (2011): Choosing our words: retrieval and selection processes recruit shared neural substrates in left ventrolateral prefrontal cortex. Journal of cognitive neuroscience. 23:3470–3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silvers JA, Weber J, Wager TD, Ochsner KN (2015): Bad and worse: neural systems underlying reappraisal of high- and low-intensity negative emotions. Social cognitive and affective neuroscience. 10:172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Badre D, Poldrack RA, Pare-Blagoev EJ, Insler RZ, Wagner AD (2005): Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 47:907–918. [DOI] [PubMed] [Google Scholar]
- 30.Cohen MS, Rissman J, Suthana NA, Castel AD, Knowlton BJ (2014): Value-based modulation of memory encoding involves strategic engagement of fronto-temporal semantic processing regions. Cognitive, affective & behavioral neuroscience. 14:578–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen MS, Rissman J, Suthana NA, Castel AD, Knowlton BJ (2016): Effects of aging on value-directed modulation of semantic network activity during verbal learning. NeuroImage. 125:1046–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diekhof EK, Kaps L, Falkai P, Gruber O (2012): The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude - an activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia. 50:1252–1266. [DOI] [PubMed] [Google Scholar]
- 33.Cox J, Witten IB (2019): Striatal circuits for reward learning and decision-making. Nature reviews Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alloy LB, Olino T, Freed RD, Nusslock R (2016): Role of Reward Sensitivity and Processing in Major Depressive and Bipolar Spectrum Disorders. Behavior therapy. 47:600–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ioannidis JP (2012): Why Science Is Not Necessarily Self-Correcting. Perspectives on psychological science : a journal of the Association for Psychological Science. 7:645–654. [DOI] [PubMed] [Google Scholar]
- 36.Maxwell SE, Lau MY, Howard GS (2015): Is psychology suffering from a replication crisis? What does "failure to replicate" really mean? The American psychologist. 70:487–498. [DOI] [PubMed] [Google Scholar]
- 37.Boekel W, Wagenmakers EJ, Belay L, Verhagen J, Brown S, Forstmann BU (2015): A purely confirmatory replication study of structural brain-behavior correlations. Cortex; a journal devoted to the study of the nervous system and behavior. 66:115–133. [DOI] [PubMed] [Google Scholar]
- 38.Cassano GB, Rucci P, Benvenuti A, Miniati M, Calugi S, Maggi L, et al. (2012): The role of psychomotor activation in discriminating unipolar from bipolar disorders: a classification-tree analysis. The Journal of clinical psychiatry. 73:22–28. [DOI] [PubMed] [Google Scholar]
- 39.First MB, Williams JBW, Karg RS, Spitzer RL (2015): Structured Clinical Interview for DSM-5, Research Version. Arlington, VA: American Psychiatric Association. [Google Scholar]
- 40.Hamilton M (1960): A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry. 23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Young RC, Biggs JT, Ziegler VE, Meyer DA (1978): A rating scale for mania: reliability, validity and sensitivity. The British journal of psychiatry : the journal of mental science. 133:429–435. [DOI] [PubMed] [Google Scholar]
- 42.Dell'Osso L, Armani A, Rucci P, Frank E, Fagiolini A, Corretti G, et al. (2002): Measuring mood spectrum: comparison of interview (SCI-MOODS) and self-report (MOODS-SR) instruments. Comprehensive psychiatry. 43:69–73. [DOI] [PubMed] [Google Scholar]
- 43.Patton JH, Stanford MS, Barratt ES (1995): Factor structure of the Barratt impulsiveness scale. Journal of clinical psychology. 51:768–774. [DOI] [PubMed] [Google Scholar]
- 44.Carver CS, White TL (1994): Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. Journal of personality and social psychology. 67:319–333. [Google Scholar]
- 45.Whiteside SP, Lynam DR (2003): Understanding the role of impulsivity and externalizing psychopathology in alcohol abuse: application of the UPPS impulsive behavior scale. Experimental and clinical psychopharmacology. 11:210–217. [DOI] [PubMed] [Google Scholar]
- 46.Gorgolewski K, Burns CD, Madison C, Clark D, Halchenko YO, Waskom ML, et al. (2011): Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Frontiers in neuroinformatics. 5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bermpohl F, Kahnt T, Dalanay U, Hagele C, Sajonz B, Wegner T, et al. (2010): Altered representation of expected value in the orbitofrontal cortex in mania. Human brain mapping. 31:958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loh JM, Lindquist MA, Wager TD (2008): Residual analysis for detecting mis-modeling in fMRI. Statistica Sinica. 18:1421–1448. [Google Scholar]
- 49.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I (2001): Controlling the false discovery rate in behavior genetics research. Behavioural brain research. 125:279–284. [DOI] [PubMed] [Google Scholar]
- 50.Baron RM, Kenny DA (1986): The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. Journal of personality and social psychology. 51:1173–1182. [DOI] [PubMed] [Google Scholar]
- 51.Brown RC, Overstreet C, Sheerin C, Berenz E, Hawn S, Pickett T, et al. (2018): The Nomological Network of a Behavioral Distress Tolerance Task in Veterans. Journal of traumatic stress. 31:876–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zorrilla EP, Koob GF (2019): Impulsivity Derived From the Dark Side: Neurocircuits That Contribute to Negative Urgency. Frontiers in behavioral neuroscience. 13:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gipson CD, Beckmann JS, Adams ZW, Marusich JA, Nesland TO, Yates JR, et al. (2012): A translational behavioral model of mood-based impulsivity: Implications for substance abuse. Drug and alcohol dependence. 122:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cyders MA, Smith GT (2008): Emotion-based dispositions to rash action: positive and negative urgency. Psychological bulletin. 134:807–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giovanelli A, Hoerger M, Johnson SL, Gruber J (2013): Impulsive responses to positive mood and reward are related to mania risk. Cognition & emotion. 27:1091–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muhtadie L, Johnson SL, Carver CS, Gotlib IH, Ketter TA (2014): A profile approach to impulsivity in bipolar disorder: the key role of strong emotions. Acta psychiatrica Scandinavica. 129:100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson SL, Carver CS, Tharp JA (2017): Suicidality in Bipolar Disorder: The Role of Emotion-Triggered Impulsivity. Suicide & life-threatening behavior. 47:177–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gonzalez-Pinto A, Ballesteros J, Aldama A, Perez de Heredia JL, Gutierrez M, Mosquera F, et al. (2003): Principal components of mania. Journal of affective disorders. 76:95–102. [DOI] [PubMed] [Google Scholar]
- 59.Akiskal HS, Azorin JM, Hantouche EG (2003): Proposed multidimensional structure of mania: beyond the euphoric-dysphoric dichotomy. Journal of affective disorders. 73:7–18. [DOI] [PubMed] [Google Scholar]
- 60.Schaffer A, Cairney J, Veldhuizen S, Kurdyak P, Cheung A, Levitt A (2010): A population-based analysis of distinguishers of bipolar disorder from major depressive disorder. Journal of affective disorders. 125:103–110. [DOI] [PubMed] [Google Scholar]
- 61.Fletcher K, Parker G, Barrett M, Synnott H, McCraw S (2012): Temperament and personality in bipolar II disorder. Journal of affective disorders. 136:304–309. [DOI] [PubMed] [Google Scholar]
- 62.Berk L, Hallam KT, Venugopal K, Lewis AJ, Austin DW, Kulkarni J, et al. (2017): Impact of irritability: a 2-year observational study of outpatients with bipolar I or schizoaffective disorder. Bipolar disorders. 19:184–197. [DOI] [PubMed] [Google Scholar]
- 63.Parmentier C, Etain B, Yon L, Misson H, Mathieu F, Lajnef M, et al. (2012): Clinical and dimensional characteristics of euthymic bipolar patients with or without suicidal behavior. European psychiatry : the journal of the Association of European Psychiatrists. 27:570–576. [DOI] [PubMed] [Google Scholar]
- 64.Skjelstad DV, Holte A, Malt UF (2011): Genuine clinical predictors of bipolar II disorder: an exploration of temporal and contextual characteristics. Journal of affective disorders. 135:419–423. [DOI] [PubMed] [Google Scholar]
- 65.Noto MN, Noto C, Caribe AC, Miranda-Scippa A, Nunes SO, Chaves AC, et al. (2015): Clinical characteristics and influence of childhood trauma on the prodrome of bipolar disorder. Revista brasileira de psiquiatria. 37:280–288. [DOI] [PubMed] [Google Scholar]
- 66.Judd LL, Schettler PJ, Akiskal H, Coryell W, Fawcett J, Fiedorowicz JG, et al. (2012): Prevalence and clinical significance of subsyndromal manic symptoms, including irritability and psychomotor agitation, during bipolar major depressive episodes. Journal of affective disorders. 138:440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cardenas D, Madinabeitia I, Vera J, Perales JC, Garcia-Ramos A, Ortega E, et al. (2018): Strength, Affect Regulation, and Subcortical Morphology in Military Pilots. Medicine and science in sports and exercise. 50:722–728. [DOI] [PubMed] [Google Scholar]
- 68.Chester DS, Lynam DR, Milich R, DeWall CN (2016): Craving versus control: Negative urgency and neural correlates of alcohol cue reactivity. Drug and alcohol dependence. 163 Suppl 1:S25–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moreno-Lopez L, Catena A, Fernandez-Serrano MJ, Delgado-Rico E, Stamatakis EA, Perez-Garcia M, et al. (2012): Trait impulsivity and prefrontal gray matter reductions in cocaine dependent individuals. Drug and alcohol dependence. 125:208–214. [DOI] [PubMed] [Google Scholar]
- 70.Chester DS, Lynam DR, Milich R, Powell DK, Andersen AH, DeWall CN (2016): How do negative emotions impair self-control? A neural model of negative urgency. NeuroImage. 132:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Joseph JE, Liu X, Jiang Y, Lynam D, Kelly TH (2009): Neural correlates of emotional reactivity in sensation seeking. Psychological science. 20:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Krebs RM, Schott BH, Duzel E (2009): Personality traits are differentially associated with patterns of reward and novelty processing in the human substantia nigra/ventral tegmental area. Biological psychiatry. 65:103–110. [DOI] [PubMed] [Google Scholar]
- 73.Davidson RJ, Shackman AJ, Maxwell JS (2004): Asymmetries in face and brain related to emotion. Trends in cognitive sciences. 8:389–391. [DOI] [PubMed] [Google Scholar]
- 74.Hantouche EG, Akiskal HS (2005): Bipolar II vs. unipolar depression: psychopathologic differentiation by dimensional measures. Journal of affective disorders. 84:127–132. [DOI] [PubMed] [Google Scholar]
- 75.Benazzi F, Akiskal HS (2003): Refining the evaluation of bipolar II: beyond the strict SCID-CV guidelines for hypomania. Journal of affective disorders. 73:33–38. [DOI] [PubMed] [Google Scholar]
- 76.Akiskal HS (2005): Searching for behavioral indicators of bipolar II in patients presenting with major depressive episodes: the "red sign," the "rule of three" and other biographic signs of temperamental extravagance, activation and hypomania. Journal of affective disorders. 84:279–290. [DOI] [PubMed] [Google Scholar]
- 77.Kling LR, Bessette KL, DelDonno SR, Ryan KA, Drevets WC, McInnis MG, et al. (2018): Cluster analysis with MOODS-SR illustrates a potential bipolar disorder risk phenotype in young adults with remitted major depressive disorder. Bipolar disorders. 20:697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU (2012): Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. International journal of methods in psychiatric research. 21:169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Geldhof GJA, Katherine P; Selig James P.; Mendez-Luck Carolyn A. (2018): Accommodating binary and count variables in mediation: A case for conditional indirect effects. International Journal of Behavioral Development. 42:300–308. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.