Abstract
Introduction:
Glioblastoma (GBM) is a highly aggressive brain tumor and is one of the most lethal human cancers. Chimeric antigen receptor (CAR) T cell therapy has markedly improved survival in previously incurable disease; however, this vanguard treatment still faces challenges in GBM. Likewise, checkpoint blockade therapies have not enjoyed the same victories against GBM. As it becomes increasingly evident that a mono-therapeutic approach is unlikely to provide anti-tumor efficacy, there evolves a critical need for combined treatment strategies.
Areas covered:
This review highlights the clinical successes observed with CAR T cell therapy as well the current efforts to overcome its perceived limitations. The review also explores employed combinations of CAR T cell approaches with immune checkpoint blockade strategies, which aim to potentiate immunotherapeutic benefits while restricting the impact of tumor heterogeneity and T cell exhaustion.
Expert Opinion:
Barriers such as tumor heterogeneity and T cell exhaustion have exposed the weaknesses of various mono-immunotherapeutic approaches to GBM, including CAR T cell and checkpoint blockade strategies. Combining these potentially complementary strategies, however, may proffer a rational means of mitigating these barriers and advancing therapeutic successes against GBM and other solid tumors.
Keywords: CAR T cells, Exhaustion, solid malignancy, immunotherapy, inhibitory immune checkpoint blockade, Program Death-1 (PD-1), glioblastoma
Introduction
Immunotherapy has become an attractive therapeutic strategy against cancer, but the success against glioblastoma (GBM) has been limited. There is a crucial need to improve the efficacy of immunotherapeutic regimens, as the current standard of care does little to alter the poor prognosis.
In its simplest and earliest forms, immunotherapy aimed to boost the body’s endogenous cancer defenses by activating immune cells and improving immunosurveillance functions. The field of cancer immunotherapy has vastly expanded, however, and now includes complex treatment approaches such as chimeric antigen receptor (CAR) T cell therapy and immune checkpoint blockade. Despite their complexity and promise, the success for both therapeutic strategies has been limited in GBM. Therapeutic shortcomings and future directions are discussed.
1.1. GBM Epidemiology
GBM is the most common primary malignant brain tumor found in adults. It is the most aggressive form of astrocytoma and is classified as a grade IV glioma by the World Health Organization. GBM accounts for 54% of all gliomas and 16% of all primary brain tumors [1, 2]. On average, the annual age-adjusted incidence rate of GBM varies from 0.59 to 3.69 per 100,000 persons [1, 2]. Patients diagnosed with GBM have a median age of 64 [1, 2]. Primary GBM occurs more in males than in females, and rates in European Americans are 2.5 times higher than in African Americans [2]. Non-Hispanics also suffer a higher incidence of GBM than Hispanics [2]. Accounting for more deaths than kidney cancer or melanoma, GBM is uniformly lethal. It is likewise the most frequent cause of cancer death in children and young adults. Overall survival in patients remains dismal 15–20 months after standard-of-care treatment [3].
1.2. GBM Standard of Care
The current standard of care for GBM involves maximal safe surgical resection, external beam radiotherapy, concomitant and adjuvant chemotherapy, and tumor treating fields (TTFields) as an optional therapy [4]. Despite treatment, nearly all patients experience tumor recurrence, and many suffer incapacitating neurological damage as a result of the tumor or even the standard-of-care regimens.
Following surgical resection, patients with newly diagnosed GBM are typically treated with radiation therapy, temozolomide (TMZ) and optional TTFields. The standard radiation dose is 60 Gy divided over 30 fractions, while concomitant TMZ is given at 75 mg/m2/day for the same 6 weeks [5]. Following this regimen, another six routine maintenance cycles of TMZ are given. Recent meta-analyses have shown that combinatorial radiation and TMZ support improves overall survival (OS) and progression-free survival (PFS). When tumors recur, patients have treatment options that include supportive care, bevacizumab, reoperation, reirradiation, systemic therapies, and various combinatorial treatments [6]. Clinical trials are also commonly offered to this population.
1.3. CAR T Cells
First described in 1988 by Steven Rosenberg, adoptive cellular therapy (ACT) proved to be an effective treatment for metastatic melanoma [7]. This immunotherapy leveraged lymphocytes extracted from patient melanomas, expanded in vitro, and reintroduced to the patient. In the presence of IL-2, these lymphocytes, mostly T cells, were stimulated to target and kill tumor cells. Further studies characterized a unique subpopulation of T cells arriving at the site of the tumor in mouse studies. These tumor-infiltrating lymphocytes (TILs) were harvested from tumors and, once activated and expanded ex vivo, reinfused into the patient for therapeutic benefit. Such ACTs were the early predecessors of the CAR T cell platform [7].
Often cited as the workhorses of the immune system, T cells are the backbone of CAR T cell therapy. CARs, in turn, are recombinant receptors that recognize cell surface antigens, much like an antibody. While T cell receptors (TCRs) can only engage human leukocyte antigen (HLA)-peptide complexes, CARs recognize and engage antigen independently of HLA expression or antigen processing [8, 9]. Thus, T cells genetically engineered to express CARs are granted antibody-type specificity for cell surface antigens, allowing CAR T cells to attach and kill tumor cells that express the relevant specific proteins [9, 10]. Likewise, CARs are not limited to peptide antigens, as they can target glycolipids and carbohydrates.
First-generation CARs include an scFv from a monoclonal antibody paired with the zeta-chain of the CD3 complex (CD3ζ), which facilitates TCR signaling and T cell activation. While T cells engineered with first-generation CARs have more efficient recognition of tumor targets, they lack costimulatory signals. This limits the CAR T cell proliferation potential upon repeated antigen engagement in the host. Overall, first-generation CAR T cells have demonstrated limited persistence and efficacy [11].
In comparison, second- and third-generation CARs were developed by combining the activation domain of first-generation CARs with one (second-generation CARs) or more (third-generation CARs) costimulatory domains. For example, second-generation CARs may exhibit a costimulatory signaling domain, and third generations exhibit more than one (i.e., CD28 and 4-1BB) [12–14]. The addition of other costimulatory molecules, such as the OX40, ICOS, CD27 and IL-15 cytokine signaling domains, is currently being evaluated in second- and third-generation CARs with the expectation of providing increased persistence, proliferation, and cytokine production upon CAR-antigen binding [15, 16]. Two recent studies on third-generation CARs have demonstrated the potential benefit of including 4-1BB, CD28, and CD3ζ within third-generation CARs [17]. Likewise, the addition of cytokine signaling domains enhanced third-generation CAR T cell survival when compared to a second-generation CAR containing CD28 and CD3ζ [15, 16].
The targeting flexibility inherent to CAR T cells has led to preclinical and clinical investigation with CARs aimed at a broad range of tumor antigens. Table I contains a select list of glioblastoma-associated antigens that are currently being explored in clinical trials. An ideal target antigen for CAR T cells is tumor-specific and expressed on nearly all tumor cells and not expressed on normal cells. Antigens that meet these characteristics are scarce [18]; however, the continued discovery of such antigens may lead to breakthrough cancer treatments.
Table I.
Targetable CAR antigens currently under active clinical investigation
| ClinicalTrials.gov Code | Target Antigen | Function | CAR T Construct |
|---|---|---|---|
|
NCT03500991 NCT02792114 NCT03423992 |
HER2 | Intracellular signaling pathway activator in response to extracellular signals | 4-1BB/CD3ζ |
|
NCT02208362 NCT04003649 NCT03423992 |
IL13-Rα2 | Internalization of IL13 | 4-1BB/CD3ζ |
|
NCT03283631 NCT03423992 NCT03726515 |
EGFRvIII | Cell differentiation and proliferation |
CD28/4-1BB/CD3ζ |
| NCT03423992 | EphA2 | Bidirectional signaling pathway |
N/A |
|
NCT03473457 NCT03423992 |
CD133 | Potential organizer of cell membrane topology | 4-1BB/CD3ζ |
2. CAR T Cell Therapy and GBM
CAR T cell therapy offers a potentially transformative immunotherapeutic strategy for GBM. In 2017, the FDA approved the CAR T cell therapies KYMRIAH® (tisagenlecleucel) for B cell acute lymphoblastic leukemia (ALL) [19] and YESCARTA® (axicabtagene ciloleucel) for large B cell lymphoma [20]. Trials with both drugs demonstrated the curative potential of the CAR T cell platform in hematological cancers. While there have been great successes with such hematological tumors, targeting solid malignancies such as GBM has proven more challenging.
Several preclinical and clinical studies have been conducted in the context of GBM, each revolving around a distinct antigenic target. These proof-of-principle studies were largely focused on feasibility and safety. Recent clinical trials in GBM have evaluated four specific antigenic targets: IL-13 receptor a2 (IL-13Rα2); human epidermal growth factor receptor 2 (HER2); erythropoietin-producing hepatocellular carcinoma A2 (EphA2) receptor; and epidermal growth factor receptor variant III (EGFRvIII). Most of these trials provided early clinical evidence for the safety and utility of CAR T cell therapy for GBM. While we will focus our discussion on these four antigens, other antigens such as EGFR [21, 22], CD133 [23], and chondroitin sulfate proteoglycan 4 (CSPG4) [24] have also been shown to be highly expressed in GBM and could serve as potential targets in future clinical studies.
2.1. IL-13Rα2
One of the most studied glioma-associated targets for CAR T cell therapy is IL-13Rα2. This cell surface receptor is overexpressed in GBM but not expressed at significant levels by normal brain tissue. Kahlon et al. developed a second-generation CAR that recognizes IL-13Rα2 via a membrane-tethered IL-13 ligand [25], known as an IL-13-zetakine. This CAR contains a point mutation in the IL-13 ligand domain (E13Y) for preferential binding to IL-13Rα2 [25, 26]. The preferred binding reduces the affinity to the more ubiquitously expressed IL-13Rα1 to offset potential on-target toxicity. IL-13-zetakine CAR T cells were capable of eliminating gliomas in orthotopic mouse tumor models when injected intracranially into immunocompromised mice [25]. More recently, the Gottschalk group utilized a scFv against IL-13Rα2 in a new CAR design and experimented different combinations of costimulatory moieties to generate first-, second-, and third-generation CARs against IL-13Rα2 [27]. Gottschalk’s CARs demonstrated tumor cytotoxicity and efficacy both in vitro and in vivo, with the second- and third-generation CARs performing better than the first-generation CARs [27].
Several studies have assessed the clinical bioactivity, safety, localization, and efficacy of IL-13Rα2 CARs [28–30]. Bioactivity and safety were assessed in patients receiving first-generation IL-13Rα2 CAR T cells delivered intracranially [28]. These studies demonstrated that multiple intracranial infusions of IL-13Rα2 CAR T cells were safe and were capable of reducing the density of IL-13Rα2 expression in tumors, which is an indication of antigen-specific tumor killing [28].
In a recent study, Brown et al. reported a case of a 50-year-old patient with recurrent multifocal IDH1-wild-type, O6-methylguanine DNA methyltransferase (MGMT)-nonmethylated GBM [30]. Second-generation IL-13Rα2 CAR T cells were administered intracranially six times weekly and were then switched to intraventricular administration weekly after progressive disease was observed distant from the injection site. During the treatment, all lesions continued to resolve and were not measurable by MRI or PET. This clinical response was sustained for 7.5 months after the initiation of therapy; however, the patient’s disease eventually recurred at four new locations 228 days after the initial treatment [30].
2.2. HER2
HER2 (also commonly referred to as HER2/Neu or ERBB2) is an orphan receptor member of the EGFR family. While commonly found in many tissues during adulthood, HER2 expression is absent in both neuronal and glial tissue. HER2 is a proto-oncogene that is activated and expressed at high levels in primary malignant brain tumors such as GBM. The expression of HER2 can be found in 20–80% of GBMs, with a modest degree of heterogeneity; however, due to its absence in normal brain tissue, it has become an attractive target for CAR T cell therapy [31, 32]. Developed by Ahmed et al., second-generation HER2-specific CAR T cells aim to target primary GBM stem cells. When cultured in vitro or infused intracranially into mice with primary GBM xenografts, HER2 CAR T cells were able to eradicate autologous GBM stem cells.[33]. GBM cells that were HER2 negative, however, were not killed [33].
Ahmed’s group advanced their second-generation HER2-specific CAR T cell therapy into a phase I clinical trial by enrolling patients with progressive, recurrent HER2-positive GBM [34]. A total of 16 evaluable patients received one or more systemic intravenous infusions of HER2 CAR T cells. Patients completed standard-of-care cytotoxic therapy at least 4 weeks prior to their CAR T cell infusion. No patient suffered complications due to treatment. Of 16 patients, one had a partial response (PR) for more than 9 months, and three had stable disease after 24 months [34]. The dose-escalation study established the safety of treatment, as well as the potential clinical benefit, with a median overall survival (OS) of 11.1 months after CAR T cell infusion.
2.3. EphA2
EphA2 is a cell surface tyrosine kinase receptor whose ligands belong to the Ephrin family. EphA2 expression is generally very low and is found in proliferating epithelium and other organs [35]; however, EphA2 is overexpressed in 60–90% of anaplastic astrocytomas and primary and recurrent GBMs [36]. Its overexpression leads to enhanced tumorigenesis, tumor cell invasion, angiogenesis and metastasis, making it an attractive target for GBM CARs. Chow et al. designed a second-generation EphA2-specific CAR based on a humanized EphA2 monoclonal antibody [37]. They found that their EphA2 CAR T cells could recognize and kill EphA2-positive glioma cell lines, such as the U373 and U87 cell lines. Furthermore, EphA2 CAR T cells killed neurospheres generated from the U87 cell line. In vivo efficacy was also studied in immunocompromised mice using the U373 cell line. Their group found that intracranially infused EphA2 CAR T cells could induce GBM regression [37].
2.4. EGFRvIII
EGFRvIII, the result of a mutation in the wild-type receptor, is exclusively expressed on the surface of GBM and other tumors [38]. EGFRvIII expression has been detected in approximately ~30% of GBM patients [39] and has become a potentially ideal target for CAR T cell therapy, as its unique extracellular epitope is easily recognizable by monoclonal antibodies, and the mutation is absent from all normal tissues. Preclinically, several investigators have developed human EGFRvIII CARs containing different scFv and costimulatory domains capable of eliminating tumor deposits in immunocompromised mice [40–43]. Notably, Sampson and colleagues developed a third-generation, EGFRvIII-specific murine CAR T cell that was tested in a fully immune-competent mouse model of malignant glioma [44, 45]. Their research illustrated that following lymphodepleting conditioning and elevated doses of CAR T cells, therapy led to cures in all mice with brain tumors [44, 45].
In the clinical realm, O’Rourke et al. utilized third-generation EGFRvIII CAR T cells in a phase I trial enrolling ten patients with refractory, multifocal, MGMT-unmethylated recurrent GBM [46]. Notably, this study did not employ a lymphodepleting regimen prior to treatment. All intravenously infused subjects had detectable engraftment of EGFRvIII CAR T cells in their peripheral blood. Tumor resections posttreatment demonstrated that CAR T cells successfully trafficked into the tumor and reduced the density of EGFRvIII expression, indicative of antigen-specific tumor killing [46].
In a separate study, Goff et al. developed a third-generation EGFRvIII CAR T cell for a phase I pilot trial. Eighteen patients were treated intravenously with an escalating dose of EGFRvIII CAR T cells following lymphodepleting host conditioning [47]. Two patients survived more than one year, and a third patient was alive at 59 months. Persistence of CAR cells was observed and correlated with cell dose. Unfortunately, no objective responses were obtained [47] despite the lymphodepleting regimen.
2.5. Antigenic Heterogeneity
Since trials of novel CAR T cell therapies for solid malignancies have not shown similar efficacy to those in hematologic malignancies, studies have shifted focus to understand the factors limiting success in solid tumors. Recent studies have demonstrated that the antigenic and molecular profiles in GBM can vary drastically between patients [48, 49], as well as within an individual patient’s tumor, suggesting a high degree of intertumoral and intratumoral heterogeneity in GBM [50–52]. To create an effective CAR T cell therapy, appropriate tumor antigenic targets need to be identified that meet certain criteria: 1) potential antigens should be concentrated on tumor cells and less/not expressed on normal tissue; and 2) the antigen should be broadly and homogenously expressed on the majority of tumor cells. Even with several studied antigenic targets in GBM, success has remained elusive. In the absence of an antigen that sufficiently satisfies the above criteria, a newer focus must be on strategies that can accommodate or bypass the substantial intratumoral heterogeneity.
Investigators are indeed examining several ways to overcome or sidestep tumor antigenic heterogeneity by varying CAR designs. Tandem CAR T cells have been designed specifically to tackle antigen escape by expressing two antigen binding domains. These CARs are engineered to be activated in the presence of either of the two antigens they have been designed to target. In GBM, the most notable example of tandem CARs was developed by Hegde et al. which joined a HER2 scFv and an IL-13Rα2-binding IL-13 mutant and used CD28 as the costimulatory factor [53]. Their tandem CAR was capable of lysing autologous GBM cells by binding to either HER2 or IL-13Rα2. If both antigens were encountered simultaneously, the tandem CAR promoted “super-additive” T cell activation. Additionally, tandem CAR T cell activity was more sustained while being less exhaustible. Tandem CAR T cells were also injected intratumorally into immunocompromised mice with xenografted GBM. The tandem CARs improved antitumor efficacy and mitigated antigen escape. The same group expanded on this approach by designing a trivalent CAR T cell targeting HER2, IL-13Rα2, and EphA2. The trivalent CAR captured nearly 100% of tumor cells in autologous GBM patient-derived xenograft (PDX) models and improved survival in treated animals [54].
Significant barriers to effective immune-based platforms for GBM are found in its characteristic molecular and histological heterogeneity [48–52], as well as its highly immunosuppressive tumor microenvironment (TME) [55, 56]. Poor T cell infiltration [57, 58], regulatory T cell (Treg)-mediated suppression [59–61], and rampant T cell exhaustion all contribute to crippled immune responses [62, 63], which are exacerbated by a dramatic influx of monocytes and macrophages that likely further serve to restrict antitumor immunity [64–66]. Of interest, recent demonstrations of CAR T cell exhaustion within tumor environments make exhaustion a focal mechanism of limiting CAR T cell antitumor efficacy that must be addressed [46].
This review will aim to provide a deeper analysis of the role that T cell exhaustion in the efficacy of CAR T cell treatment and presents current strategies aimed at overcoming CAR T cell exhaustion.
3. Exhaustion
3.1. T Cell Exhaustion
T cell exhaustion was initially described in the setting of chronic lymphocytic choriomeningitis virus (LCMV) infection [67, 68]. Seminal work by Wherry et al. demonstrated that LCMV-specific CD8+ T cells were unable to produce IFNγ, TNFα, and IL-2 when chronically exposed to viral antigen [69]. These exhausted CD8+ T cells demonstrated a unique gene expression signature distinct from that of memory T cells or activated effector T cells [67]. Exhausted CD8+ T cells were characterized by increased expression of immune checkpoint receptors such as programmed-death 1 (PD-1). In addition to PD-1, expression of alternative immune checkpoint receptors, including lymphocyte activation gene-3 (LAG-3), T cell immunoglobulin and mucin domain-3 (Tim-3), and B- and T-lymphocyte attenuator (BTLA) [67, 70], was closely tied to a progressive loss of function. Importantly, T cells may express PD-1 or CTLA-4 without being functionally impaired or exhibiting the transcriptional signatures of T cell exhaustion. Indeed, transient expression of PD-1 is characteristic of normal T cell activation, while persistent antigen exposure induces the sustained expression of PD-1 characteristic of T cell exhaustion [71]. T cell exhaustion, therefore, is characterized not only by the expression of immune checkpoints such as PD-1 but also by co-expression of immune checkpoints receptors and the loss of effector function. The loss of function in T cell exhaustion has been shown to be highly regulated and hierarchical, with IL-2 secretion lost early, TNFα secretion lost at the intermediate stage, and IFNγ and granzyme B production lost last [69]. Ultimately, chronic viral antigen recognition and immune checkpoint engagement, are responsible for the loss of T cell function and the exhaustion phenotype [67].
New studies have also demonstrated the presence of T cell exhaustion in preclinical tumor models [72–74] and cancer [75–77]. Blockade of immune checkpoint receptor signaling, either by blocking the receptor or ligand, has been shown to ameliorate early stages of T cell exhaustion [78]. Conversely, terminally exhausted T cells are less likely to respond to immune checkpoint blockade [71]. Recent studies have shown that while PD-1 single-positive exhausted T cells can be rescued by PD-1 blockade, T cells co-expressing PD-1+Tim-3+LAG-3+ cannot. Required instead is inhibition of multiple immune checkpoint receptors, which can be accomplished via mechanisms such as the addition of anti-Tim-3 or anti-LAG-3 to anti-PD-1 therapy [73, 74].
Currently, there are several monoclonal blocking antibodies utilized in the clinical setting aimed at blocking different immune checkpoint molecules: anti-PD-1 antibodies such as nivolumab and pembrolizumab are among the most widely utilized and are FDA-approved in solid tumors; the anti-CTLA-4 antibodies ipilimumab and tremelimumab are both used as effective blocking antibodies, often in melanoma; the anti-PD-L1 antibodies (targeting the PD-1 ligand PD-L1) avelumab, atezolizumab, and durvalumab are all under current clinical investigation; and the anti-Tim-3 antibody MBG453 and the anti-LAG-3 BMS-986016 are recently developed antibodies targeting alternative immune checkpoint molecules [79].
3.2. T Cell Exhaustion in Glioblastoma
T cell exhaustion in human GBM has recently begun to be characterized [62, 63, 80, 81]. Multiple studies have demonstrated that T cells infiltrating human GBM tumors are more likely to demonstrate T cell exhaustion, as measured through the expression of immune checkpoints and decreased functional capacity, than T cells isolated from peripheral blood of patients with GBM [62, 63, 81]. One study showed that human GBM TILs, whether isolated from newly diagnosed or recurrent tumors, expressed significantly higher levels of PD-1, CD39, and Tim-3 than T cells isolated from patient peripheral blood [62]. Woroniecka et al. found similar increased expression of immune checkpoints on GBM TILs. Additionally, the functional capacity of GBM TILs was closely tied to the expression of immune checkpoints, with mounting expression of immune checkpoints associated with decreased T cell functional capacity [63]. A separate study isolated TILs from 98 patients with newly diagnosed GBM, examined the expression of immune checkpoint molecules and transcription factors by flow cytometry, and examined T cell capacity for proliferation via stimulation with anti-CD3 [82]. TILs isolated from patients with high percentages of exhausted T cells did not proliferate in response to anti-PD-1 blockade in vitro [82].
Murine studies have expanded upon these initial findings in humans. TILs infiltrating murine GBM are likewise susceptible to T cell exhaustion [63]. Importantly, TILs isolated from murine models of GBM demonstrate greater degrees of T cell exhaustion than TILs isolated from murine models of other brain tumors, including models of breast, lung, and melanoma metastasis [63]. Woroniecka et al. demonstrated that T cell exhaustion signatures varied with tumor type and not with intracranial or peripheral location [63]. GBM elicits a severe T cell exhaustion signature regardless of the location in which it is implanted, suggesting tumor-specific mechanisms for eliciting exhaustion.
Preclinical studies utilizing immune checkpoint blockade strategies in murine models of GBM have shown mixed results [83–86]. A recent publication has reconciled these observations by demonstrating that the immune profile of the tumor microenvironment established by the orthotopic murine tumor cell line determines the impact of the immune checkpoint blockade strategy [85, 86]. Those cell lines whose tumor microenvironment resembles that of clinical GBM (such as CT2A) were not responsive to immune checkpoint blockade strategies, while a particular cell line, GL261, whose tumor microenvironment did not resemble that of GBM, was responsive to checkpoint blockade [86]. These results raised the question of whether immune checkpoint blockade strategies will be effective in patients with GBM.
Clinical evaluation of immune checkpoint blockade in recurrent GBM has indeed been disappointing. A recent large randomized study evaluating the impact of nivolumab on survival failed to demonstrate any significant benefit [87]. Recent studies have highlighted the possibility that low mutational burden is a key contributor to checkpoint blockade failure in GBM, since GBM patients with defects in DNA mismatch repair (MMR) pathway and exhibiting a high mutational burden responded to checkpoint blockade treatment [88–90]. Mutations in the MMR pathway are relatively common in the GBM patient population, particularly in patients with recurrent GBM (~25%) [91]. MMR mutations may result in increased T cell infiltration in these patients, which could potentiate responses to immune checkpoint blockade [91]. A recent retrospective study by Zhao et al. supports the concept that certain GBM patients with particular genetic mutations, specific immune profiles, and high levels of immune infiltration could benefit from checkpoint blockade therapies [92]. However, these observations have yet to be evaluated in a prospective phase III study.
Despite the promising observations in GBM patients with mutations in the MMR pathway, novel strategies to harness the potential benefit of immune checkpoint blockade strategies are still needed. Recent studies focused on the administration of nivolumab or pembrolizumab in the neoadjuvant setting [93, 94]. The results obtained were quite promising, albeit all studies had relatively small patient numbers. Cloughesy et al. conducted an elegant study involving 35 patients with recurrent resectable GBM. Patients were randomized to receive either neoadjuvant pembrolizumab with continued adjuvant therapy after surgery or adjuvant pembrolizumab after surgery only [93]. Patients who received neoadjuvant pembrolizumab had an OS of 13.5 months, while those in the adjuvant-only arm had a dismal 7.5-month median survival [93]. Further support for this approach comes from a study conducted by Schalper et al. with 30 GBM patients, 27 of which had recurrent disease and 3 of which were newly diagnosed [94]. In this single arm study, administration of nivolumab in the neoadjuvant setting was associated with increased T cell infiltration and alterations in the immune profile of the tumor microenvironment. However, the OS in this population was not drastically enhanced, as had been seen in the Cloughesy study. Further strategies employing chemotherapy and radiation in combination with other immunomodulatory agents are under current evaluation [95] (Table II).
Table II.
Clinical trials investigating checkpoint blockade in Glioblastoma
| ClinicalTrials.gov Code | Phase study | Checkpoint inhibition strategy | GBM disease status | Sample size |
|---|---|---|---|---|
| NCT03636477 | Phase I | Ad-RTS-hIL-12 + Nivolumab | Recurrent or Progressive | 18 |
| NCT02311920 | Phase I | Ipilimumab and/or Nivolumab with Temozolomide | Newly Diagnosed | 32 |
| NCT03493932 | Phase I | Nivolumab + Anti-LAG-3 BMS-986016 | Recurrent | 20 |
| NCT03707457 | Phase I | Nivolumab + anti-GITR or IDO1 inhibitor or Ipilimumab | Recurrent | 30 |
| NCT03422094 | Phase I | Neoantigen vaccine with Nivolumab +/−Ipilimumab | Newly Diagnosed | 30 |
| NCT04015700 | Phase I | Neoantigen vaccine with Nivolumab +/−Ipilimumab | Newly Diagnosed, MGMT-Unmethylated | 30 |
| NCT03233152 | Phase I | Intra-tumoral Ipilimumab + Intravenous Nivolumab |
Recurrent | 6 |
| NCT02529072 | Phase I | Nivolumab With DC Vaccines | Recurrent | 7 |
| NCT02658981 | Phase I | Anti-LAG-3 Alone & in Combination w/ Nivolumab | Recurrent | 100 |
| NCT03961971 | Phase I | Anti-Tim-3 in Combination With Anti-PD-1 and SRS |
Recurrent | 15 |
| NCT03722342 | Phase I | TTAC-0001 and Pembrolizumab | Recurrent | 20 |
| NCT02530502 | Phase I | Radiation Therapy With Temozolomide and Pembrolizumab |
Newly Diagnosed | 4 |
| NCT03426891 | Phase I | Pembrolizumab and Vorinostat Combined With Temozolomide | Newly Diagnosed | 32 |
| NCT02287428 | Phase I | Personalized NeoAntigen Cancer Vaccine w RT Plus Pembrolizumab for Patients |
MGMT Unmethylated, Newly Diagnosed | 46 |
| NCT03341806 | Phase I | Avelumab With Laser Interstitial Therapy | Recurrent | 30 |
| NCT03430791 | Phase II | TTF with Nivolumab +/− Ipilimumab | Recurrent | 60 |
| NCT03743662 | Phase II | Nivolumab with Radiation therapy | Recurrent | 94 |
| NCT02550249 | Phase II | Neoadjuvant Nivolumab | Recurrent | 29 |
| NCT03452579 | Phase II | Nivolumab + Bevacizumab | Recurrent | 90 |
| NCT03367715 | Phase II | Nivolumab + Ipilimumab and radiotherapy | MGMT-Unmethylated Newly Diagnosed | 24 |
| NCT03014804 | Phase II | Vaccine with Nivolumab | Recurrent | 30 |
| NCT03890952 | Phase II | Nivolumab + Bevacizumab | Recurrent | 40 |
| NCT03718767 | Phase II | Nivolumab |
IDH-Mutant Gliomas With and Without Hypermutator Phenotype | 95 |
| NCT02794883 | Phase II | Tremelimumab and Durvalumab in Combination or Alone | Recurrent | 36 |
| NCT02336165 | Phase II | Anti-PD-L1 | Newly Diagnosed Unmethylated MGMT And Recurrent |
159 |
| NCT03661723 | Phase II | Pembrolizumab and Reirradiation with Bevacizumab |
Recurrent | 60 |
| NCT03018288 | Phase II | Radiation Therapy Plus Temozolomide and Pembrolizumab With and Without HSPPC-96 |
Newly Diagnosed | 108 |
| NCT02337491 | Phase II | Pembrolizumab +/− Bevacizumab |
Recurrent | 80 |
| NCT03899857 | Phase II | Pembrolizumab | Newly Diagnosed | 56 |
| NCT03405792 | Phase II | Adjuvant Temozolomide Plus TTFields Plus Pembrolizumab |
Newly Diagnosed | 29 |
| NCT04013672 | Phase II | Pembrolizumab Plus SurVaxM for Glioblastoma |
Recurrent | 51 |
| NCT02337686 | Phase II | Pembrolizumab |
Recurrent | 20 |
| NCT03197506 | Phase II | Pembrolizumab and Standard Therapy | Newly Diagnosed | 90 |
| NCT02798406 | Phase II | Combination Adenovirus + Pembrolizumab to Trigger Immune Virus Effects |
Recurrent | 49 |
| NCT03797326 | Phase II | Efficacy and Safety of Pembrolizumab (MK-3475) Plus Lenvatinib |
Glioblastoma and other cancers | 180 |
| NCT01174121 | Phase II | Imunotherapy Using Tumor Infiltrating Lymphocytes | Glioblastoma and other cancers | 332 |
| NCT02968940 | Phase II | Avelumab With Radiation Therapy | IDH1 Mutant | 43 |
| NCT03047473 | Phase II | Avelumab |
Newly Diagnosed | 30 |
| NCT03291314 | Phase II | Avelumab and Axitinib | Recurrent | 52 |
| NCT02327078 | Phase I/II | Epacadostat IDO inhibitor administered with Nivolumab | Glioblastoma Plus Other Advanced Cancers | 307 |
| NCT02335918 | Phase I/II | Anti-CD27 Varlilumab with Nivolumab | Glioblastoma Plus Other Advanced Cancers | 175 |
| NCT03576612 | Phase I/II | AdV-TK + Nivolumab and Radiation in | Newly Diagnosed High Grade Gliomas | 36 |
| NCT03684811 | Phase I/II | FT-2102 + Nivolumab and Chemotherapy | IDH Glioblastoma Plus Other Advanced Cancers | 200 |
| NCT03665545 | Phase I/II | Pembrolizumab With the IMA950/Poly-ICLC Vaccine |
Relapsing | 24 |
| NCT03532295 | Phase I/II | Epacadostat With Radiation Therapy and Avelumab | Recurrent | 55 |
| NCT03174197 | Phase I/II | Atezolizumab in Combination With Temozolomide and Radiation |
Newly Diagnosed | 60 |
| NCT03673787 | Phase I/II | Ipatasertib in Combination With Atezolizumab | Recurrent | 51 |
| NCT03973879 | Phase I/II | PVSRIPO and Atezolizumab |
Recurrent | 31 |
| NCT03750071 | Phase I/II | VXM01 Plus Avelumab |
Progressive | 30 |
| NCT03277638 | Phase I/II | Laser Interstitial Thermotherapy (LITT) Combined With Pembrolizumab |
Recurrent | 34 |
| NCT02866747 | Phase I/II | Hypofractionated SRS and Durvalumab |
Recurrent | 62 |
| NCT02667587 | Phase III | Temozolomide + Radiation Therapy With Nivolumab |
Newly Diagnosed | 693 |
| NCT02617589 | Phase III | Nivolumab Compared to Temozolomide, Each Given With Radiation Therapy |
Newly Diagnosed | 550 |
| NCT02017717 | Phase III | Nivolumab Compared to Bevacizumab and to Nivolumab With or WithoutIpilimumab |
Recurrent | 626 |
A novel application of immune checkpoint blockade is combination therapy with vaccines or CAR T cell therapy. This strategy is of particular relevance as it aims to overcome checkpoint inhibitor resistant tumors. Activation of the immune system via vaccines or CAR T cell therapy can potentially convert “cold” tumors like GBM, into “hot” tumors and improve the efficacy of immune checkpoint blockade therapy. Here, two distinct types of immunotherapy form a mutualistic relationship to generate a more robust therapy.
3.3. CAR T Cell Exhaustion in GBM
Utilization of immune checkpoint blockade strategies in combination with CAR T cell therapy could overcome CAR T cell exhaustion existing in patients with solid malignancies, including GBM. This combinatorial therapy could markedly improve CAR T cell efficacy. [63]. For instance, CAR T cell hypofunctionality was associated with the expression of several surface inhibitory receptors, including PD-1, LAG-3, and Tim-3, as well as with upregulation of the intrinsic T cell inhibitory enzyme SHP-1, in a preclinical study evaluating the efficacy of CAR T cells targeting mesothelin (MSLN) in mesothelioma [96]. In the clinical setting, third generation CARs targeting GD2 in melanoma gained surface expression of several immune checkpoint molecules, including PD-1 and PD-L1 [97]. This expression was associated with decreased CAR T cell viability and persistence, although the cells retained functionality, as assessed by CD107 expression [97].
In a phase I clinical trial of a second-generation CAR targeting EGFRvIII in GBM, CAR T cells were shown to traffic to the brain tumor, proliferate, and exert some direct anti-EGFRvIII activity, but the clinical response was incomplete and associated with tumor microenvironment upregulation of PD-L1 [46]. Although not directly evident of T cell exhaustion, immunosuppressive changes, such as the upregulation of PD-L1, suggest that human CAR T cells may be limited by ligand signaling through immune checkpoint receptors. These findings also suggest that strategies to add checkpoint blockade to CAR T cell therapies may be justified.
Recent preclinical animal studies in both murine and canine models of GBM provide further rationale for combining immune checkpoint blockade strategies with CAR T cell platforms in these tumors. Numerous studies have demonstrated that CAR T cells targeting IL-13Rα2 can become exhausted in GBM-bearing hosts and that such exhaustion may be overcome with the addition of checkpoint blockade [98–100]. Sengupta et al. characterized CAR T cell exhaustion by highlighting their upregulation of PD-1 [98], while Wang et al. denoted the additional upregulation of Tim-3, 2B4 and LAG-3 [100]. Studies performed by Yin et al. showed that increases in PD-1, Tim-3 and CTLA-4 levels on CAR T cells were associated with decreased functionality [99]. In all of these studies, however, blockade of checkpoint receptor signaling resulted in increased CAR T cell antitumor efficacy and prolonged animal survival.
4. Overcoming Exhaustion in CAR T Cell Therapy for GBM
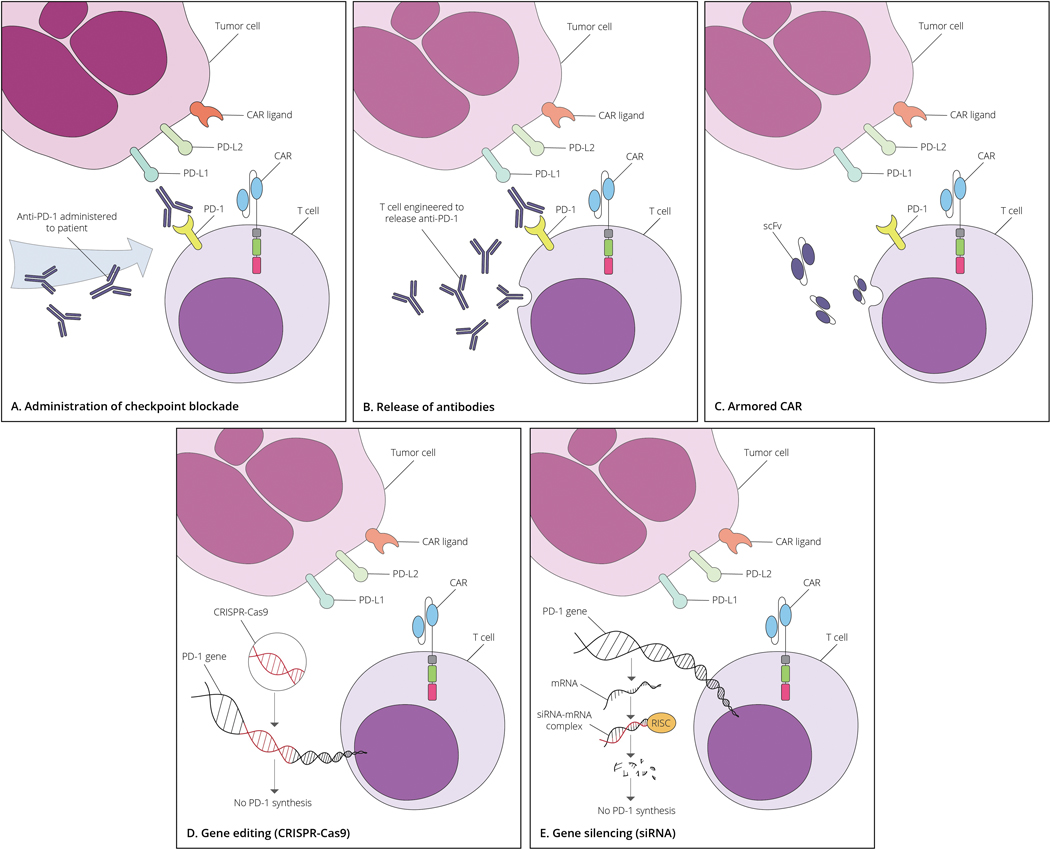
As illustrated in Figure 1, several strategies have emerged for combining CAR T cell activity with checkpoint blockade to overcome CAR T cell exhaustion. Strategies range from simply co-delivering the platforms to endowing CAR T cells themselves with the capacity to inhibit the interaction between checkpoint receptors and ligands. The immune checkpoint blockade component may be furnished by 1) co-administering a monoclonal checkpoint blockade antibody; 2) engineering CAR T cells to secrete a monoclonal checkpoint blockade antibody or scFv fragment; 3) gene editing; or 4) gene silencing of the immune checkpoint receptor.
Figure 1.
Strategies combining monoclonal antibody therapy, gene transduction, and gene editing to examine the role of checkpoint blockade on CAR T cells
A) checkpoint blockade through the administration of monoclonal antibody therapy; B&C) genetic manipulation of CAR T cells to force secretion of a full monoclonal blocking antibody or scFv against the inhibitory immune checkpoint receptor, respectively; D) genetic editing of the inhibitory immune checkpoint receptor gene by CRISPR/Cas9; and E) siRNA gene silencing of the inhibitory immune checkpoint receptor.
4.1. Antibody-based Strategies
An emerging strategy to overcome tumor-induced exhaustion of CAR T cells is to simply co-administer immune checkpoint blocking antibody-based agents with CAR T cells. Preclinical studies in HER2-expressing breast cancer models [101] and in IL-13Rα2-expressing GBM [99] suggest that codelivery of immune checkpoint blockade antibodies increases the functionality of CAR T cells. Similar results were obtained in a pleural mesothelioma model using an MSLN CAR [102].
The combination of immune checkpoint blockade and CAR T cells is just beginning to be explored in the clinical setting in GBM (Table III). Two recently initiated phase I studies have focused their efforts on GBMs expressing EGFRvIII or IL-13Rα2. In the first instance, a single-arm study in newly diagnosed EGFRvIII-expressing MGMT-unmethylated GBM, 7 patients will receive EGFRvIII CAR T cells in combination with the PD-1 monoclonal antibody pembrolizumab to determine the safety and tolerability of the combined therapy ( NCT03726515). In the second study, 60 IL-13Rα2-expressing recurrent GBM patients will be randomized to two arms: arm 1 will receive nivolumab and ipilimumab intravenously 14 days prior to IL-13Rα2 CAR T cell infusion; and arm 2 will receive IL-13Rα2 CAR T cells only. IL-13Rα2 CAR T cells will be delivered intracranially every week, and after each infusion, both arms will receive nivolumab intravenously every other week ( NCT04003649).
Table III.
Clinical trials investigating CAR T cell therapy with checkpoint blockade in Glioblastoma
| ClinicalTrials.gov Code | Phase study | Checkpoint inhibition strategy | CAR T cell | Malignancy | Sample size |
|---|---|---|---|---|---|
| NCT03726515 | Phase I | CAR T cell plus αPD-1 mAb Pembrolizumab | EGFRvIII CAR |
EGFRvIII+ Glioblastoma | 7 |
| NCT04003649 | Phase I | CAR T cell plus αPD-1 mAb Nivolumab and αCTLA-4 Ipilimumab | IL-13Rα2 CAR |
IL-13Rα2+ Glioblastoma | 60 |
| NCT02873390 | Phase I/II | CAR T cell expressing αPD-1 mAb | EGFR CAR |
EGFR+ cancers | 20 |
| NCT03182816 | Phase I/II | CAR T cell expressing αPD-1 mAb and αCTLA-4 | EGFR CAR |
EGFR+ cancers | 40 |
While co-infusion of clinically approved checkpoint blockade antibodies with CAR T cells has been the preferred mechanism for combining these platforms to date due to the availability of these agents, genetic engineering approaches aimed at endowing CAR T cells with the capacity to secrete such checkpoint-blocking agents could prove to be a more potent and even safer strategy. Using CAR T cells to increase the relative local concentration of the checkpoint-blocking agent at the tumor site may help overcome limits to tumor penetrance, a theoretical downside to systemic antibody delivery. For example, CAR T cells may be engineered to wield either membrane-bound or secreted antibodies or to secrete a smaller blocking scFv [103, 104].
This strategy recently reached the clinical setting for the treatment of solid malignancies (Table III). Two registered studies have continued evaluating the safety and efficacy of PD-1-antibody-expressing CAR T cells in EGFR-expressing advanced solid malignancies, with potential inclusion of GBM patients ( NCT02873390). A similar approach is being tried with CTLA-4 blockade ( NCT03182816). It is expected that more of these studies will open for recruitment, with some instead incorporating a strategy for secreted scFv. CAR-based antibody secretion has the potential to avoid many of the toxicities associated with systemically administered checkpoint blockade.
Both direct administration and CAR-based secretion of monoclonal antibodies are promising means for overcoming exhaustion in CAR T cell therapy. Likewise, these approaches may allow the host to overcome or prevent exhaustion among tumor-infiltrating endogenous T cells. This may facilitate the development of much needed heterogeneous antigenic memory for tumor cells, countering the therapeutic restrictions imposed by both loss of antigen variants and tumor heterogeneity.
4.2. Gene editing and silencing strategies
The development of gene editing methods, including clustered regularly interspaced short palindromic repeats-Cas9 ribonucleoprotein (CRISPR-Cas9), transcription activator-like effector nucleases, meganucleases, and zinc-finger nucleases, has allowed for the targeted interruption of selected genes [105]. Of these approaches, CRISPR-Cas9 has gained the most attention due to its high efficiency and ease of use [106]. CRISPR-Cas9 has been used as a gene editing tool to selectively excise the PD-1 [107, 108] and LAG-3 [109] checkpoint receptors. Deletion of these receptors on CAR T cells eliminates their signaling and minimizes their contribution to exhaustion. This approach also obviates monoclonal checkpoint blockade antibodies and their systemic toxicities. However, the impact of such strategies on CAR T cell therapy for GBM has yet to be explored in the clinical setting.
An alternative modality to gene editing is gene silencing. Gene silencing takes advantage of an evolutionarily conserved pathway existing in plants and all mammals known as RNA interference (siRNA) [110]. In RNA interference, the target gene (in this case, an immune checkpoint receptor gene) is effectively silenced since no protein will be translated in the absence of the target gene’s mRNA. Like gene editing, this approach has been used to selectively silence the PD-1 gene to improve CAR T cell function and antitumor efficacy.
PD-1 gene silencing with siRNA was first examined in the preclinical setting against MSLN in a pleural mesothelioma model [102]. In this study, administration of CAR T cells with siRNA-silenced PD-1 resulted in enhanced CAR T cell function and efficacy equivalent to that of CAR T cells plus a monoclonal anti-PD-1 antibody. The approach has been applied to other checkpoint molecules, such as CTLA-4, to enhance in vitro cytotoxic CAR T cell function [111]. Gene silencing with siRNA has become an attractive technique in preclinical studies and has been applied clinically; however, such strategies in GBM have yet to be evaluated.
5. Toxicity
5.1. Toxicities Associated with CAR T Cell Therapy for GBM
CAR T cell therapies mediate potent tumor regression in hematological malignancies, but these successes are often accompanied by manageable toxicities, such as cytokine release syndrome (CRS) and neurotoxicity [112]. While potentially life threatening, an understanding of the contributions made by the tumor burden and IL-6 and IFNγ serum levels to CRS has led to proper management [113]. Addressing neurotoxicity has been more challenging, as the origins remain largely unclear. Recent studies have suggested that CAR-induced neurotoxicity is associated with vascular permeability and increased concentrations of inflammatory cytokines in the cerebrospinal fluid (CSF) [114]. Interestingly, however, these toxicities have been observed at a much lower frequency (or not at all) in patients with solid tumors, including GBM.
Experience with CAR T cell therapy in GBM has been relatively limited by comparison. Most of the CAR T cell trials in GBM patients have been phase I studies with a small number of patients. In one study with 3 patients, intracranial administration of IL-13Rα2 CARs was deemed safe and well tolerated, with only manageable temporary brain inflammation and neurotoxicity at the highest dose of CAR T cells [28]. These toxicities were attributed to the CAR T cell treatment and faded away once steroids were introduced. In a case report of a 50-year-old patient undergoing intracranial administration of CAR T cells via two different routes, intracavitary and intraventricular, the two routes yielded similar toxicity profiles [30]. No adverse events were observed. Nonetheless, it remains unclear whether a particular route (intracavitary, intratumoral or intraventricular) will provide better safety or efficacy within the CNS.
In studies employing intracranial HER2 CAR T cells, no adverse events or dose-limiting toxicities were observed, even when pediatric cases were treated [34]. Such safety is important in light of the death of a colorectal cancer patient receiving high-dose HER2 CAR T cells in a separate study [42]. Similar safety has been observed in two trials utilizing EGFRvIII CAR T cells. In these studies, nonlymphodepleted patients experienced no dose-limiting toxicities after receiving 1–5×108 CAR T cells [46]. In lymphodepleted patients, however, dose-limiting toxicities were observed [47]. Two out of 18 patients experienced severe hypoxia with higher doses, and one death was observed at the highest dose of 6×1010 cells [47]. Therefore, careful dose escalation processes should be followed in the setting of intravenous CAR T cell administration, particularly in those protocols incorporating host myeloablative or lymphodepleting regimens.
5.2. Toxicities Associated with Checkpoint Blockade in GBM
Checkpoint blockade strategies for GBM patients are in their relative infancy. The safety and efficacy were evaluated in 45 patients with recurrent GBM. Recent results obtained from an exploratory phase I cohort of the phase III trial Checkmate-143 ( NCT02017717) in patients with recurrent GBM detailed the adverse events and toxicities seen with nivolumab and ipilimumab in this patient population [87]. The toxicities did not differ significantly from those observed in other cancer populations [115, 116].
In Checkmate-143, 45 patients were randomized to three arms that received nivolumab alone at 3 mg/kg; nivolumab 1 mg/kg + ipilimumab 3 mg/kg; or nivolumab 3 mg/kg + ipilimumab 1 mg/kg [87]. The most common adverse events observed in all study arms were fatigue, increased lipase, nausea, and diarrhea. Headache was the most common neurotoxic event. While no grade 3 or 4 adverse events were reported in the nivolumab alone arm, severe grade 3 and 4 toxicities were reported in the other arms in which patients received ipilimumab [87]. Grade 3 or 4 events were reported in 90% and 30% of patients in the second and third arms above, respectively [87]. The most common events were increased liver enzymes, colitis, diarrhea, fatigue, and increased lipase [87]. Other less common serious adverse events were hypothyroidism and pneumonitis. The combination arms were thus discontinued. Despite a manageable toxicity profile for nivolumab alone, no increase in OS was observed over historical controls [87].
As new checkpoint blockade studies in patients with GBM continue to open and enroll (Table II), more information regarding the safety profile of these agents will become available. In the meantime, the Checkmate-143 trial suggests that these immune checkpoint inhibitors will produce similar toxicity profiles in GBM patients as those seen with other cancers. Furthermore, combinatorial treatment targeting immune checkpoint inhibitors may elicit increased toxicity.
5.3. Potential Toxicities: CAR T Cells Plus Checkpoint Blockade for GBM
Considering the toxicities observed with CAR T cell therapy and immune checkpoint blockade, it is plausible to expect that the combination of these two immunotherapeutic modalities could increase the number of serious adverse effects.
Increasing CAR T cell function by deleting, editing, or silencing immune checkpoint genes could result in increased and sustained cytokine secretion and unwelcome inflammation within the brain. In addition, potential off-target toxicities might be unmasked by inhibiting immune checkpoint genes. Off-target toxicity may occur when CAR T cell populations inadvertently bind antigens other than the intended one or activate themselves indiscriminately [117]. Inhibition of immune checkpoint genes such as PD-1 could reduce the threshold for T cell activation [118]. Off-target toxicity could be mediated by the endogenous TCR of the CAR T cells binding their antigens or by CARs binding their own targets with low affinity. Potentially life-threatening autoimmunity could ensue if tolerance is broken to antigens known to be T cell epitopes in multiple sclerosis or other immune-mediated neurodegenerative diseases. In contrast, a recent study suggested that gene editing of PD-1 in CAR T cells could be detrimental to CAR T cell proliferation [118]. Nonetheless, strategies aimed at eliminating potential CAR on-target and off-target toxicities continue to be investigated. Examples include incorporating an inducible Caspase 9 (icasp9) suicide gene [119], furnishing a CAR T cell surface target that can be recognized by a depleting monoclonal antibody [120], and building in biological switches [121].
While the strategies above specifically address CAR toxicity, additional measures may be necessary to limit the scope of immune responses once checkpoint blockade strategies are superimposed. One approach geared at avoiding the systemic complications of dual therapy has been the forced expression of secreted scFv against PD-1 and other checkpoints by CAR T cells. A recent study showed that MUC1 CAR T cells engineered to secrete a soluble blocking scFv against PD-1 demonstrated superior safety and antitumor activity when compared to co-administered CAR T cells and an anti-PD-1 antibody [104]. This was attributed by the authors to the preferred tumor localization of the secreted scFv.
The need for limiting the toxicity of superimposed CAR/checkpoint blockade strategies ultimately still needs to be determined. The results of the ongoing phase I and II clinical studies employing CAR T cells in combination with checkpoint blockade (Table III) will provide pivotal information regarding the potential efficacy, pitfalls, and toxicities of these combined approaches.
The cost of combinatorial immunotherapy puts an additional burden on cancer patients. Financial toxicity from the cost of treatment presents itself in the form of monetary and psychological stress. A recent report evaluated 74 observational studies of financial toxicity in 598,751 patients with cancer [122]. Overall, 49% of patients reported some form of physical or psychological financial burdens. The burden was not influenced by the site of disease but was associated with early disease and poor quality of life. Patients with GBM experience poor quality of life due to the nature of their highly aggressive disease [2]. Due to the combination of standard of care with costly CAR T cell therapy and immune checkpoint blockade therapy, patients will likely endure some form of financial toxicity.
6. Expert Opinion
CAR T cells are an emerging immunotherapy with the potential to effectively treat a variety of malignancies. The platform is predicated upon removing host T cells and endowing them with the capacity to target tumor surface antigens in an HLA-independent fashion. Whereas tumor heterogeneity and CAR T cell exhaustion have provided barriers to success in GBM, strategies for including checkpoint blockade in the overall approach may provide clever ways for overcoming these restrictions to efficacy.
While tumor heterogeneity is not as prominent among hematological cancers, solid malignancies are characterized by both intertumoral and intratumoral heterogeneity. The heterogeneous expression of tumor antigens severely limits the impact of strategies targeting one or a few antigens in solid tumors and has been shown to mitigate tumor escape following CAR T cell therapy. Approaches aimed at enhancing the endogenous T cell activity directed at the tumor, such as checkpoint blockade, may help to scale the barriers erected by tumor heterogeneity. Meanwhile, in the case of GBM, the concomitant presence of a highly immunosuppressive tumor microenvironment provides significant challenges even if a ubiquitously expressed strong tumor antigen is afforded. In particular, tumor-imposed T cell exhaustion has proved to be a challenge for CAR T cells specifically and likewise limits the efficacy of checkpoint blockade therapies. Therefore, strategies to counter exhaustion may license efficacy for both treatment platforms.
Because of the flexibility afforded by the ability to genetically modify CAR T cells, creative solutions to the above problems are increasingly emerging. While simply co-administering CAR T cells and monoclonal checkpoint blockade antibodies may strengthen immune responses and provide complementary strategies, more interesting approaches can help focus responses at the tumor and limit on-target and off-target toxicities. CAR T cells can be engineered to wield antibodies to immune checkpoints and may have their own expression of checkpoints modified or silenced. These novel approaches have begun making their way into the clinic and provide a glimpse at the next generation of much needed combinatorial and rational immunotherapeutic platforms.
Article highlights.
The current standard-of-care regimen for glioblastoma using resection, radiotherapy, and chemotherapy only modestly improves survival in patients due to its inability to effectively eradicate all malignant cells
Chimeric antigen receptor T cell therapy is highly effective at treating cancer in hematologic malignancies but has shown limited efficacy in solid tumors like glioblastoma
Checkpoint blockade therapy, a mainstay of cancer immunotherapy also fails in glioblastoma due to a lack of functional, non-exhausted endogenous T cells at the tumor
Given the complexity of the disease, it is becoming increasingly evident that mono-therapeutic approaches are unlikely to provide anti-tumor efficacy, thus prompting the use of a combined therapeutic approach
By combining both chimeric antigen T cell therapy and checkpoint blockade, glioblastoma can be treated in two specific approaches with each treatment also mitigating the other’s limitations
Acknowledgments
Funding
This work was supported by grants from the National Institutes of Health (NIH) National Cancer Institute [P50-CA190991 (JH Sampson)] and the NIH National Institute of Neurological Disorders and Stroke [R01-NS099463 (JH Sampson), R01-NS085412 (JH Sampson), and U01-NS090284 (JH Sampson)]. Additional support was provided by a National Science Foundation Graduate Research Fellowship (KAR).
Footnotes
Declaration of Interests
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
References
- 1.Tamimi AF and Juweid M, Epidemiology and Outcome of Glioblastoma, in Glioblastoma S. De Vleeschouwer, Editor. 2017: Brisbane (AU). [PubMed] [Google Scholar]
- 2.Hanif F, et al. , Glioblastoma Multiforme: A Review of its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac J Cancer Prev, 2017. 18(1): p. 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ostrom QT, et al. , CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011–2015. Neuro Oncol, 2018. 20(suppl_4): p. iv1-iv86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fabian D, et al. , Treatment of Glioblastoma (GBM) with the Addition of Tumor-Treating Fields (TTF): A Review. Cancers (Basel), 2019. 11(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim M, et al. , Current state of immunotherapy for glioblastoma. Nat Rev Clin Oncol, 2018. 15(7): p. 422–442. [DOI] [PubMed] [Google Scholar]
- 6.Gallego O, Nonsurgical treatment of recurrent glioblastoma. Curr Oncol, 2015. 22(4): p. e273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg SA, et al. , Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med, 1988. 319(25): p. 1676–80. [DOI] [PubMed] [Google Scholar]
- 8.Geldres C, Savoldo B, and Dotti G, Chimeric antigen receptor-redirected T cells return to the bench. Semin Immunol, 2016. 28(1): p. 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kershaw MH, et al. , Supernatural T cells: genetic modification of T cells for cancer therapy. Nat Rev Immunol, 2005. 5(12): p. 928–40. [DOI] [PubMed] [Google Scholar]
- 10.Fesnak AD, June CH, and Levine BL, Engineered T cells: the promise and challenges of cancer immunotherapy. Nat Rev Cancer, 2016. 16(9): p. 566–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brocker T. and Karjalainen K, Signals through T cell receptor-zeta chain alone are insufficient to prime resting T lymphocytes. J Exp Med, 1995. 181(5): p. 1653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abate-Daga D. and Davila ML, CAR models: next-generation CAR modifications for enhanced T-cell function. Mol Ther Oncolytics, 2016. 3: p. 16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawalekar OU, et al. , Distinct Signaling of Coreceptors Regulates Specific Metabolism Pathways and Impacts Memory Development in CAR T Cells. Immunity, 2016. 44(2): p. 380–90. [DOI] [PubMed] [Google Scholar]
- 14.Savoldo B, et al. , CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest, 2011. 121(5): p. 1822–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nair S, et al. , Functional Improvement of Chimeric Antigen Receptor Through Intrinsic Interleukin-15Ralpha Signaling. Curr Gene Ther, 2019. 19(1): p. 40–53. [DOI] [PubMed] [Google Scholar]
- 16.Kagoya Y, et al. , A novel chimeric antigen receptor containing a JAK-STAT signaling domain mediates superior antitumor effects. Nat Med, 2018. 24(3): p. 352–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramos CA, et al. , In Vivo Fate and Activity of Second- versus Third-Generation CD19-Specific CAR-T Cells in B Cell Non-Hodgkin’s Lymphomas. Mol Ther, 2018. 26(12): p. 2727–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, et al. , New Chimeric Antigen Receptor Design for Solid Tumors. Front Immunol, 2017. 8: p. 1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maude SL, et al. , Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med, 2018. 378(5): p. 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neelapu SS, et al. , Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med, 2017. 377(26): p. 2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caruso HG, et al. , Redirecting T-Cell Specificity to EGFR Using mRNA to Self-limit Expression of Chimeric Antigen Receptor. J Immunother, 2016. 39(5): p. 205–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caruso HG, et al. , Tuning Sensitivity of CAR to EGFR Density Limits Recognition of Normal Tissue While Maintaining Potent Antitumor Activity. Cancer Res, 2015. 75(17): p. 3505–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu X, et al. , Patient-derived glioblastoma stem cells are killed by CD133-specific CAR T cells but induce the T cell aging marker CD57. Oncotarget, 2015. 6(1): p. 171–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pellegatta S, et al. , Constitutive and TNFalpha-inducible expression of chondroitin sulfate proteoglycan 4 in glioblastoma and neurospheres: Implications for CAR-T cell therapy. Sci Transl Med, 2018. 10(430). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kahlon KS, et al. , Specific recognition and killing of glioblastoma multiforme by interleukin 13-zetakine redirected cytolytic T cells. Cancer Res, 2004. 64(24): p. 9160–6. [DOI] [PubMed] [Google Scholar]
- 26.Brown MP, Ebert LM, and Gargett T, Clinical chimeric antigen receptor-T cell therapy: a new and promising treatment modality for glioblastoma. Clin Transl Immunology, 2019. 8(5): p. e1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krenciute G, et al. , Characterization and Functional Analysis of scFv-based Chimeric Antigen Receptors to Redirect T Cells to IL13Ralpha2-positive Glioma. Mol Ther, 2016. 24(2): p. 354–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown CE, et al. , Bioactivity and Safety of IL13Ralpha2-Redirected Chimeric Antigen Receptor CD8+ T Cells in Patients with Recurrent Glioblastoma. Clin Cancer Res, 2015. 21(18): p. 4062–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keu KV, et al. , Reporter gene imaging of targeted T cell immunotherapy in recurrent glioma. Sci Transl Med, 2017. 9(373). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown CE, et al. , Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. N Engl J Med, 2016. 375(26): p. 2561–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hynes NE and Lane HA, ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer, 2005. 5(5): p. 341–54. [DOI] [PubMed] [Google Scholar]
- 32.Citri A. and Yarden Y, EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol, 2006. 7(7): p. 505–16. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed N, et al. , HER2-specific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors. Clin Cancer Res, 2010. 16(2): p. 474–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmed N, et al. , HER2-Specific Chimeric Antigen Receptor-Modified Virus-Specific T Cells for Progressive Glioblastoma: A Phase 1 Dose-Escalation Trial. JAMA Oncol, 2017. 3(8): p. 1094–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walker-Daniels J, et al. , Differential regulation of EphA2 in normal and malignant cells. Am J Pathol, 2003. 162(4): p. 1037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wykosky J, et al. , Interleukin-13 receptor alpha 2, EphA2, and Fos-related antigen 1 as molecular denominators of high-grade astrocytomas and specific targets for combinatorial therapy. Clin Cancer Res, 2008. 14(1): p. 199–208. [DOI] [PubMed] [Google Scholar]
- 37.Chow KK, et al. , T cells redirected to EphA2 for the immunotherapy of glioblastoma. Mol Ther, 2013. 21(3): p. 629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sampson JH, et al. , Tumor-specific immunotherapy targeting the EGFRvIII mutation in patients with malignant glioma. Semin Immunol, 2008. 20(5): p. 267–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Humphrey PA, et al. , Anti-synthetic peptide antibody reacting at the fusion junction of deletion-mutant epidermal growth factor receptors in human glioblastoma. Proc Natl Acad Sci U S A, 1990. 87(11): p. 4207–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bullain SS, et al. , Genetically engineered T cells to target EGFRvIII expressing glioblastoma. J Neurooncol, 2009. 94(3): p. 373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohno M, et al. , Retrovirally engineered T-cell-based immunotherapy targeting type III variant epidermal growth factor receptor, a glioma-associated antigen. Cancer Sci, 2010. 101(12): p. 2518–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson LA, et al. , Rational development and characterization of humanized anti-EGFR variant III chimeric antigen receptor T cells for glioblastoma. Sci Transl Med, 2015. 7(275): p. 275ra22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miao H, et al. , EGFRvIII-specific chimeric antigen receptor T cells migrate to and kill tumor deposits infiltrating the brain parenchyma in an invasive xenograft model of glioblastoma. PLoS One, 2014. 9(4): p. e94281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sampson JH, et al. , EGFRvIII mCAR-modified T-cell therapy cures mice with established intracerebral glioma and generates host immunity against tumor-antigen loss. Clin Cancer Res, 2014. 20(4): p. 972–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suryadevara CM, et al. , Temozolomide lymphodepletion enhances CAR abundance and correlates with antitumor efficacy against established glioblastoma. Oncoimmunology, 2018. 7(6): p. e1434464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Rourke DM, et al. , A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med, 2017. 9(399). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goff SL, et al. , Pilot Trial of Adoptive Transfer of Chimeric Antigen Receptor-transduced T Cells Targeting EGFRvIII in Patients With Glioblastoma. J Immunother, 2019. 42(4): p. 126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cancer Genome Atlas Research, N., Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature, 2008. 455(7216): p. 1061–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eckel-Passow JE, et al. , Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. N Engl J Med, 2015. 372(26): p. 2499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patel AP, et al. , Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science, 2014. 344(6190): p. 1396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meyer M, et al. , Single cell-derived clonal analysis of human glioblastoma links functional and genomic heterogeneity. Proc Natl Acad Sci U S A, 2015. 112(3): p. 851–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dagogo-Jack I. and Shaw AT, Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol, 2018. 15(2): p. 81–94. [DOI] [PubMed] [Google Scholar]
- 53.Hegde M, et al. , Tandem CAR T cells targeting HER2 and IL13Ralpha2 mitigate tumor antigen escape. J Clin Invest, 2016. 126(8): p. 3036–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bielamowicz K, et al. , Trivalent CAR T cells overcome interpatient antigenic variability in glioblastoma. Neuro Oncol, 2018. 20(4): p. 506–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Quail DF and Joyce JA, The Microenvironmental Landscape of Brain Tumors. Cancer Cell, 2017. 31(3): p. 326–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tomaszewski W, et al. , Brain Tumor Microenvironment and Host State: Implications for Immunotherapy. Clin Cancer Res, 2019. 25(14): p. 4202–4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lohr J, et al. , Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-beta. Clin Cancer Res, 2011. 17(13): p. 4296–308. [DOI] [PubMed] [Google Scholar]
- 58.Han S, et al. , Rescuing defective tumor-infiltrating T-cell proliferation in glioblastoma patients. Oncol Lett, 2016. 12(4): p. 2924–2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fecci PE, et al. , Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res, 2006. 66(6): p. 3294–302. [DOI] [PubMed] [Google Scholar]
- 60.Sayour EJ, et al. , Increased proportion of FoxP3+ regulatory T cells in tumor infiltrating lymphocytes is associated with tumor recurrence and reduced survival in patients with glioblastoma. Cancer Immunol Immunother, 2015. 64(4): p. 419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.El Andaloussi A. and Lesniak MS, An increase in CD4+CD25+FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme. Neuro Oncol, 2006. 8(3): p. 234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mohme M, et al. , Immunophenotyping of Newly Diagnosed and Recurrent Glioblastoma Defines Distinct Immune Exhaustion Profiles in Peripheral and Tumor-infiltrating Lymphocytes. Clin Cancer Res, 2018. 24(17): p. 4187–4200. [DOI] [PubMed] [Google Scholar]
- 63.Woroniecka K, et al. , T-Cell Exhaustion Signatures Vary with Tumor Type and Are Severe in Glioblastoma. Clin Cancer Res, 2018. 24(17): p. 4175–4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Strik HM, Stoll M, and Meyermann R, Immune cell infiltration of intrinsic and metastatic intracranial tumours. Anticancer Res, 2004. 24(1): p. 37–42. [PubMed] [Google Scholar]
- 65.Charles NA, et al. , The brain tumor microenvironment. Glia, 2012. 60(3): p. 502–14. [DOI] [PubMed] [Google Scholar]
- 66.Bowman RL, et al. , Macrophage Ontogeny Underlies Differences in Tumor-Specific Education in Brain Malignancies. Cell Rep, 2016. 17(9): p. 2445–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wherry EJ, et al. , Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity, 2007. 27(4): p. 670–84. [DOI] [PubMed] [Google Scholar]
- 68.Zajac AJ, et al. , Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med, 1998. 188(12): p. 2205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wherry EJ, et al. , Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol, 2003. 77(8): p. 4911–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blackburn SD, et al. , Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol, 2009. 10(1): p. 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wherry EJ and Kurachi M, Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol, 2015. 15(8): p. 486–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schietinger A, et al. , Tumor-Specific T Cell Dysfunction Is a Dynamic Antigen-Driven Differentiation Program Initiated Early during Tumorigenesis. Immunity, 2016. 45(2): p. 389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Woo SR, et al. , Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res, 2012. 72(4): p. 917–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sakuishi K, et al. , Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med, 2010. 207(10): p. 2187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thommen DS and Schumacher TN, T Cell Dysfunction in Cancer. Cancer Cell, 2018. 33(4): p. 547–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Akbay EA, et al. , Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov, 2013. 3(12): p. 1355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koyama S, et al. , STK11/LKB1 Deficiency Promotes Neutrophil Recruitment and Proinflammatory Cytokine Production to Suppress T-cell Activity in the Lung Tumor Microenvironment. Cancer Res, 2016. 76(5): p. 999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hirano F, et al. , Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res, 2005. 65(3): p. 1089–96. [PubMed] [Google Scholar]
- 79.Li Y, et al. , Targeting the tumor microenvironment to overcome immune checkpoint blockade therapy resistance. Immunol Lett, 2019. [DOI] [PubMed] [Google Scholar]
- 80.Woroniecka KI, et al. , T-cell Dysfunction in Glioblastoma: Applying a New Framework. Clin Cancer Res, 2018. 24(16): p. 3792–3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Davidson TB, et al. , Expression of PD-1 by T Cells in Malignant Glioma Patients Reflects Exhaustion and Activation. Clin Cancer Res, 2019. 25(6): p. 1913–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Park J, et al. , Immune Checkpoint Inhibitor-induced Reinvigoration of Tumor-infiltrating CD8(+) T Cells is Determined by Their Differentiation Status in Glioblastoma. Clin Cancer Res, 2019. 25(8): p. 2549–2559. [DOI] [PubMed] [Google Scholar]
- 83.Reardon DA, et al. , Glioblastoma Eradication Following Immune Checkpoint Blockade in an Orthotopic, Immunocompetent Model. Cancer Immunol Res, 2016. 4(2): p. 124–35. [DOI] [PubMed] [Google Scholar]
- 84.Hung AL, et al. , TIGIT and PD-1 dual checkpoint blockade enhances antitumor immunity and survival in GBM. Oncoimmunology, 2018. 7(8): p. e1466769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Genoud V, et al. , Responsiveness to anti-PD-1 and anti-CTLA-4 immune checkpoint blockade in SB28 and GL261 mouse glioma models. Oncoimmunology, 2018. 7(12): p. e1501137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Garg AD, et al. , Preclinical efficacy of immune-checkpoint monotherapy does not recapitulate corresponding biomarkers-based clinical predictions in glioblastoma. Oncoimmunology, 2017. 6(4): p. e1295903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Omuro A, et al. , Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: results from exploratory phase I cohorts of CheckMate 143. Neuro Oncol, 2018. 20(5): p. 674–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bouffet E, et al. , Immune Checkpoint Inhibition for Hypermutant Glioblastoma Multiforme Resulting From Germline Biallelic Mismatch Repair Deficiency. J Clin Oncol, 2016. 34(19): p. 2206–11. [DOI] [PubMed] [Google Scholar]
- 89.AlHarbi M, et al. , Durable Response to Nivolumab in a Pediatric Patient with Refractory Glioblastoma and Constitutional Biallelic Mismatch Repair Deficiency. Oncologist, 2018. 23(12): p. 1401–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hodges TR, et al. , Mutational burden, immune checkpoint expression, and mismatch repair in glioma: implications for immune checkpoint immunotherapy. Neuro Oncol, 2017. 19(8): p. 1047–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Indraccolo S, et al. , Genetic, Epigenetic, and Immunologic Profiling of MMR-Deficient Relapsed Glioblastoma. Clin Cancer Res, 2019. 25(6): p. 1828–1837. [DOI] [PubMed] [Google Scholar]
- 92.Zhao J, et al. , Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nat Med, 2019. 25(3): p. 462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cloughesy TF, et al. , Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med, 2019. 25(3): p. 477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schalper KA, et al. , Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat Med, 2019. 25(3): p. 470–476. [DOI] [PubMed] [Google Scholar]
- 95.Maxwell R, Jackson CM, and Lim M, Clinical Trials Investigating Immune Checkpoint Blockade in Glioblastoma. Curr Treat Options Oncol, 2017. 18(8): p. 51. [DOI] [PubMed] [Google Scholar]
- 96.Moon EK, et al. , Multifactorial T-cell hypofunction that is reversible can limit the efficacy of chimeric antigen receptor-transduced human T cells in solid tumors. Clin Cancer Res, 2014. 20(16): p. 4262–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gargett T, et al. , BRAF and MEK inhibition variably affect GD2-specific chimeric antigen receptor (CAR) T-cell function in vitro. J Immunother, 2015. 38(1): p. 12–23. [DOI] [PubMed] [Google Scholar]
- 98.Sengupta S, et al. , Glycogen synthase kinase 3 inhibition lowers PD-1 expression, promotes long-term survival and memory generation in antigen-specific CAR-T cells. Cancer Lett, 2018. 433: p. 131–139. [DOI] [PubMed] [Google Scholar]
- 99.Yin Y, et al. , Checkpoint Blockade Reverses Anergy in IL-13Ralpha2 Humanized scFv-Based CAR T Cells to Treat Murine and Canine Gliomas. Mol Ther Oncolytics, 2018. 11: p. 20–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang D, et al. , Glioblastoma-targeted CD4+ CAR T cells mediate superior antitumor activity. JCI Insight, 2018. 3(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.John LB, et al. , Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res, 2013. 19(20): p. 5636–46. [DOI] [PubMed] [Google Scholar]
- 102.Cherkassky L, et al. , Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest, 2016. 126(8): p. 3130–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yoon DH, et al. , Incorporation of Immune Checkpoint Blockade into Chimeric Antigen Receptor T Cells (CAR-Ts): Combination or Built-In CAR-T. Int J Mol Sci, 2018. 19(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rafiq S, et al. , Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat Biotechnol, 2018. 36(9): p. 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Osborn MJ, et al. , Gene editing and its application for hematological diseases. Int J Hematol, 2016. 104(1): p. 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Singh N, et al. , Genome-Editing Technologies in Adoptive T Cell Immunotherapy for Cancer. Curr Hematol Malig Rep, 2017. 12(6): p. 522–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rupp LJ, et al. , CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci Rep, 2017. 7(1): p. 737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guo X, et al. , Disruption of PD-1 Enhanced the Anti-tumor Activity of Chimeric Antigen Receptor T Cells Against Hepatocellular Carcinoma. Front Pharmacol, 2018. 9: p. 1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang Y, et al. , CRISPR-Cas9 mediated LAG-3 disruption in CAR-T cells. Front Med, 2017. 11(4): p. 554–562. [DOI] [PubMed] [Google Scholar]
- 110.Sontheimer EJ, Assembly and function of RNA silencing complexes. Nat Rev Mol Cell Biol, 2005. 6(2): p. 127–38. [DOI] [PubMed] [Google Scholar]
- 111.Simon B, et al. , The siRNA-mediated downregulation of PD-1 alone or simultaneously with CTLA-4 shows enhanced in vitro CAR-T-cell functionality for further clinical development towards the potential use in immunotherapy of melanoma. Exp Dermatol, 2018. 27(7): p. 769–778. [DOI] [PubMed] [Google Scholar]
- 112.Gauthier J. and Turtle CJ, Insights into cytokine release syndrome and neurotoxicity after CD19-specific CAR-T cell therapy. Curr Res Transl Med, 2018. 66(2): p. 50–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hay KA, et al. , Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood, 2017. 130(21): p. 2295–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gust J, et al. , Endothelial Activation and Blood-Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T Cells. Cancer Discov, 2017. 7(12): p. 1404–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Motzer RJ, et al. , Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med, 2015. 373(19): p. 1803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Larkin J, et al. , Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med, 2015. 373(1): p. 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sun S, et al. , Immunotherapy with CAR-Modified T Cells: Toxicities and Overcoming Strategies. J Immunol Res, 2018. 2018: p. 2386187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wei J, et al. , PD-1 silencing impairs the anti-tumor function of chimeric antigen receptor modified T cells by inhibiting proliferation activity. J Immunother Cancer, 2019. 7(1): p. 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gargett T. and Brown MP, The inducible caspase-9 suicide gene system as a “safety switch” to limit on-target, off-tumor toxicities of chimeric antigen receptor T cells. Front Pharmacol, 2014. 5: p. 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kao RL, et al. , A Cetuximab-Mediated Suicide System in Chimeric Antigen Receptor-Modified Hematopoietic Stem Cells for Cancer Therapy. Hum Gene Ther, 2019. 30(4): p. 413–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lienert F, et al. , Synthetic biology in mammalian cells: next generation research tools and therapeutics. Nat Rev Mol Cell Biol, 2014. 15(2): p. 95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Smith GL, et al. , Financial Burdens of Cancer Treatment: A Systematic Review of Risk Factors and Outcomes. J Natl Compr Canc Netw, 2019. 17(10): p. 1184–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]