Abstract
Exposure to mercury is one of the major global health concerns due to its stability, bioaccumulation and high toxicity. Therefore, the present study was conducted to assess the mean mercury level in hair and breast milk (BM) of Iranian lactating mothers (ILMs) through meta-analysis technique. We conducted a systematic literature search in online electronic databases included main domestic databases (SID, Magiran, Iran medex, Medlib and ISC) and international databases (Embase, Scopus and PubMed) for studies published between 2000 up 2018. Each process of research and evaluation of articles based on inclusion and exclusion criteria is done by two researchers, individually. From10 studies entered to meta-analysis process including 556 ILM, the mean hair mercury level (HML) and mean milk mercury level (MML) was estimated to be 0.15 μg/g (95 CI: 0.11–0.19, I2: 47.6%, P: 0.028) and 0.51 μg/l (95 CI: 0.28–0.74, I2: 1.9%, P: 0.421), respectively. In this meta-analysis, the mean HML and mean MML were estimated to be lower than the standard of World Health Organization (WHO). Although the mean mercury level in hair and BM of ILMs was lower than the WHO standard, but due to toxicity and serious concern of health, management and Periodic monitor are recommended in different cities of the country for evaluate the mercury levels in hair and BM of ILMs and to estimate the infant’s exposure.
Keywords: Mercury; Hair; Breast milk; Mothers, Iran
Introduction
Metals are abundant in everywhere on Earth’s crust, but exposure to some metal pollutants such as arsenic, Lead (Pb), mercury (Hg) and cadmium (Cd) are dangerous and carcinogenic to humans even at very low concentrations [1]. Among the harmful metals to humans, mercury is the as most toxic element known after arsenic and lead, that situated in third rank of pollutants with highest priority by the National Priorities List of the Agency for Toxic Substances and Disease Registry (ATSDR) [2].
Mercury is one of the heavy metals found in water, soil and air that widely enter the environment from through natural and anthropogenic sources [3, 4]. Natural releases of mercury into environment enter through natural sources including the weathering of Hg-containing rocks, geothermal activities, and volcanicity, while the most releases are associated to anthropogenic activities [5, 6].
Also, mercury releases from main sources of anthropogenic is included of the mining, smelting, and production of iron and non-ferrous metals, combustion of coal and other fossil fuels, large-scale gold production, mine production of mercury, cement production, oil refining, contaminated sites, waste of result from consumer products (landfill (mostly) and waste incineration), chlor-alkali industry, Cremation (dental amalgam) and etc. [3, 7].
The other potential sources for human exposure to mercury, is including thermometers, sphygmomanometer, barometers, incandescent lights and batteries and various commercial products including skin creams, germicidal soaps, various medications, teething powders, analgesics, vaccinations and thimerosal (preservative in vaccines) [8].
In recent years, mercury pollution and dangers of it has become a global concern which this topic has led to various conventions such as minamata convention to manage it, the treaty which dedicated purely to mercury. In accordance with article 19 of the Minamata convention, which its main aim is protect the environment and human health, demands from member countries of convention to endeavor and assess the effects of mercury and its compounds on damageable populations such as infants, children, pregnant and lactating women [9].
Mercury is one of the most dangerous environmental pollutants due to environmental sustainability and bioaccumulation in the food chain. Their three main forms are include of elemental mercury (Hg0), inorganic mercury (Hg+2) and organic mercury (MeHg) [10]. All forms of mercury are extremely toxic and harmful to human health. Humans through food, water, air and occupational exposure may be exposed to mercury.
Mercury is the only metal that is liquid in its elemental form. In this state, metal easily evaporates at room temperature and inhalation causes toxicity in humans. The elemental form of mercury is soluble in lipid and readily enters the bloodstream through the alveolus after inhalation, which in this position, the results to bioaccumulation of mercury in the renal cortex, liver, and especially the brain [11].
Inorganic mercury (mercurous and mercuric state) is absorbed by the digestive system and in mercuric salts is more soluble and toxic than elemental form [12]. the main source of exposure whith inorganic mercury is a dental amalgam [13].
The forms of organic mercury include methyl mercury and ethyl mercury. The most dangerous mercury form of organic is dimethyl mercury which is highly toxic. The most usual form of organic mercury is methyl mercury, which is often transformed by microorganisms to other more toxic forms such as methyl mercury in the water, soil and body tissue of creatures [14, 15]. Consuming food such as fish (or other seafoods) and vaccines containing thimerosal are the most important sources of human exposure whith organic mercury, which about 95% of it is absorbed in the digestive system [16–18].
The toxic effects of mercury for humans are related to many factors like the chemical form, dose and exposure rate (quantity, frequency, and duration) [11, 19].
As mercury is potentially toxic, depending on its form, the effects of exposure to mercury and its compounds from natural and anthropogenic sources on humans it can cause irreversible damage to the systems of Neurological (Alzheimer, Erethism, Dementia, Parkinson, Schizophrenia) [20–25], Renal [26–28], respiratory [29–31], immunological [11, 32], Genetic and epigenetic Outcomes [33, 34], Cardiovascular (Arrhythmi, Cardiomyopathy, Irregular pulse, Chest pains) [35–38], and Reproductive Outcomes (Birth defects, Impotency, Impair fertility) [39–41].
Exposures with mercury can be estimated by measuring pollutant levels in various body tissues (such as hair, milk, blood, urine, or nails). Measuring mercury levels in these tissues can be well indicators for different types of mercury exposures [10].
Exposure of women to toxical metals such as mercury during the Pregnancy and Breastfeeding time, even at very low doses, can have effects on fetal and infant growth [42]. During pregnancy, mercury easily crosses the placenta, concentrates in the fetus, and ultimately crosses the fetal blood–brain barrier and it causes brain damage to the developing fetus [43].
It can also be transmitted after birth in the Breastfeeding time to the mammary glands of the Iranian lactating mothers (ILMs) [44].
human breast milk (HBM) of as the best source of nutrition for infants, depending on the mother’s exposure, may contain harmful contaminants such as mercury, so, the exposure to mercury can be injurious effects on infants [45–47]. Once mercury enters the hair, it will no longer returns to the bloodstream and shows a relatively direct relationship with blood mercury levels, so, hair is a good index for evaluating the accumulation of mercury in the body and estimate long- term exposure [48].
In order to perform a risk assessment, exposure results were compared with guidelines of World Health Organization (WHO) [10, 49, 50].
So, the investigation and measurement of this pollutant in hair and Breast Milk (BM) of ILMs is very important to improve the health of mothers and infants.
Various studies have examined the amount of mercury in the hair and milk of ILMs [51, 52]. But, findings are inconsistent in this regard. Specifically, the results of this study can be used to control global mercury pollution to evaluate the effectiveness of the Minamata Convention.
Therefore, the present study was conducted to assess the mean mercury level in hair and BM of ILMs through a systematic review and meta-analysis technique.
Materials and methods
Search strategy
We conducted a systematic search in online electronic databases included main local databases (SID, Magiran, Iran medex, Medlib and ISC) and international databases (Embase, Scopus and PubMed) for studies published (between 1 January 2000 up 31 December 2018) using the following key terms: “heavy metals”, “mercury”, “human milk”, “breast milk”, “Hair”, “mothers” and “Iran” to select related studies. For online electronic databases of national, an equivalent of Persian keywords was used.
Study criteria
Inclusion criteria
The inclusion criteria for studies entered in the meta-analysis process were as follows: 1) the studies of published to Persian and English languages; 2) studies conducted inside Iran; 3) samples containing the mean mercury concentration in hair and BM of ILMs and 4) article of published from 1 January 2000 up 31 December 2018.
Exclusion criteria
The Exclusion criteria were as follows: 1) Articles written in a language other than Persian or English; 2) studies conducted outside Iran; 3) samples containing of non- ILMs or pregnant women; 4) samples containing of mean concentration the other heavy metals (Except of mercury) in hair and BM of ILMs; 5) Studies that did not report mean and standard deviation; and 6) articles that unavailability of information. Also articles that did not have a cross-sectional design or conducted on animals were excluded.
Selecting studies
We reviewed the results of all the studies, and excluded some articles after reviewing based on the titles and abstracts. Two reviewers independently carried out the literature search and evaluation of the searched articles based on the inclusion and exclusion criteria. The search strategy and review processes in present study are in accordance with guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [53]. In the present study, articles qualified for further analyses were collected with initial screening of identified titles or abstracts. Eligible studies were screened against predefined inclusion and exclusion criteria. Then, full text of studies was reviewed to determine of these two referees independently. After accurate review of studies, differences were resolved with consensus or if needed by a third reviewer.
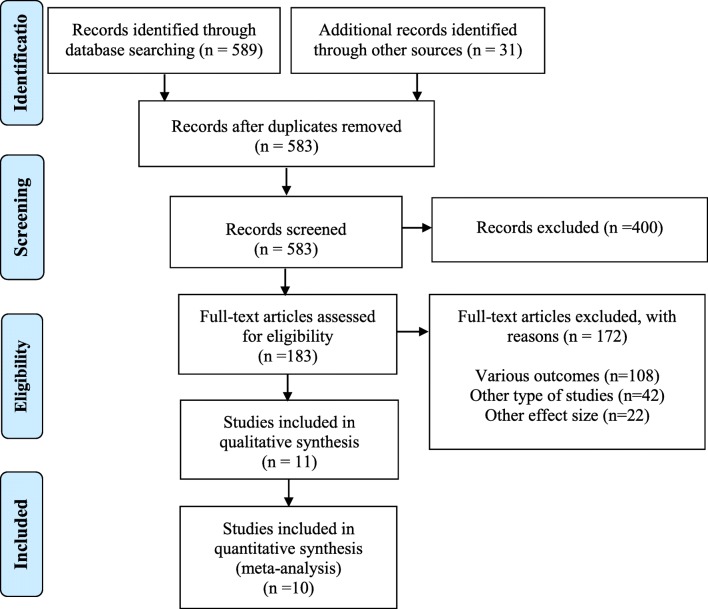
The details and flow diagram of literature review process is given in Fig. 1.
Fig. 1.
PRISMA flow diagram for selecting studies of systematic review
Data extraction and selection
The extracted data were as follows: name of first author, publication year, study place (province and city), sample size, study design, mean of mercury concentration level with standard deviation (SD), analytical technique and age range.
Quality assessment and risk of Bias
The quality of all studies were assessed by Modified Newcastle-Ottawa Scale for cross sectional studies [54]. A score of >7 on the NOS scale for each study, was considered to have a low risk of bias and an excellent methodological domain. Finally, the articles were categorized as low, moderate and high.
Meta-analysis
After data extraction, STATA version 15.0 (Stata Corporation, College Station, TX, USA) was used for meta-analysis. Mean and SD were reported for eligible papers. A Cochran Q test was conducted to assess heterogeneity and an I2 statistic was calculated to estimate the percentage of total variation resulting from between-study variation [55]. Low, moderate or high degrees of heterogeneity were approximated by I2 values of 25%, 50% and 75%, respectively. Heterogeneity was assessed by subgrouping the time of measures, and study population. Publication bias was assessed by Egger and Begg’s test with a significance level of 0.10. In addition, we planned to plot funnel plots if we encountered more than 10 studies for each forest plot; however, the number of studies was not found to be adequate for such plotting.
Results
Search results and studies description
The primary literature searches showed 619 articles, which in databases of Embase, Scopus, PubMed and other databases identified 320, 48, 221 and 31 articles, respectively. After removing duplicates, a total of 605 articles were residual for review. Then, 605 articles were excluded after identification based on titles, abstracts and full-text. Totally, 11 main studies were eligibility for inclusion and exclusion criteria (5 studies dealt with the mercury level in hair of ILMs and six studies dealt with mercury level in BM of mothers). Finally, 10 eligible studies (Hair: Five study, Milk: Five study/ conducted from 2000 to 2018 in 15 of Iran cities) entered the meta-analysis process. The sample size for studies on hair and milk, were 279 and 277, respectively.
One study [56] reported mean mercury level (3.48 μg/L) in BM of ILMs, but do not entered the meta-analysis process due to the lack report of SD (Fig. 1).
The detail data collected from the final included studies and samples specifications are shown in Table 1.
Table 1.
The detail of literature studies in hair and BM of lactating mothers (2000–2018)
| Study/ Year | Place (Province) |
Place (City) |
Sample Size | Study Type | Mean of Mercury Level (Mean ± SD/Rang) |
Analytical Technique | NOS Score |
|---|---|---|---|---|---|---|---|
| Hair (μg/g) | |||||||
| Khammar et al. [57], 2017 | Sistan and Baluchestan | Zahedan | 40 | Cross-sectional |
1.81 ± 0.54 (0.67–3) |
HR-CS-AAS | 7 |
| Okati et al. [58], 2012 | Mazandaran | Nowshahr, Nur and Sari | 93 | Cross-sectional |
3.55 ± 2.52 (0.08–8.97) |
AMA-S-PAAS | 8 |
| Okati et al. [58], 2012 | Mazandaran | Nowshahr | 27 | Cross-sectional |
4.2 ± 2.77 (0.13–8.97) |
AMA-S-PAAS | 8 |
| Okati et al. [58], 2012 | Mazandaran | Nur | 39 | Cross-sectional |
3.3 ± 2.53 (0.08–8.45) |
AMA-S-PAAS | 8 |
| Okati et al. [58], 2012 | Mazandaran | Sari | 27 | Cross-sectional |
3.27 ± 2.19 (0.11–7.42) |
AMA-S-PAAS | 8 |
| Savabieasfahani et al. [59], 2012 | Tehran | Tehran | 6 | Cross-sectional | 0.19 ± 0.12 | ICP-MS | 6 |
| Ghasempouri et al. [60], 2012 | Mazandaran | 5 Regions | 70 | Cross-sectional |
0.19 ± 0.09 (0.06–0.43) |
AMA-S-PAAS | 8 |
| Ghasempouri et al. [60], 2012 | Mazandaran | Nowshahr | 10 | Cross-sectional |
0.29 ± 0.08 (0.18–0.40) |
AMA-S-PAAS | 8 |
| Ghasempouri et al. [60], 2012 | Mazandaran | Nur | 8 | Cross-sectional |
0.24 ± 0.12 (0.12–0.43) |
AMA-S-PAAS | 8 |
| Ghasempouri et al. [60], 2012 | Mazandaran | Chamestan | 17 | Cross-sectional |
0.14 ± 0.09 (0.08–0.42) |
AMA-S-PAAS | 8 |
| Ghasempouri et al. [60], 2012 | Mazandaran | Village of Nur | 13 | Cross-sectional |
0.16 ± 0.04 (0.08–0.24) |
AMA-S-PAAS | 8 |
| Ghasempouri et al. [60], 2012 | Mazandaran | Village of Nowshahr | 22 | Cross-sectional |
0.11 ± 0.03 (0.06–0.38) |
AMA-S-PAAS | 8 |
| Okati et al. [61], 2010 | Mazandaran | Mazandaran | 70 | Cross-sectional |
0.19 ± 0.09 (0.06–0.43) |
AMA-S-PAAS | 7 |
| Milk (μg/L) | |||||||
| Bahmani and Maleki [56], 2018 | Kurdistan | Sanandaj | 100 | Cross-sectional |
3.48 (0.9–3.56) |
ICP-MS | 7 |
| Khammar et al. [57], 2017 | Sistan and Baluchestan | Zahedan | 40 | Cross-sectional |
1.23 ± 0.306 (0.21–1.7) |
HR-CS-AAS | 7 |
| Okati et al. [62], 2013 | Mazandaran | Amol and Sari | 82 | Cross-sectional |
0.43 ± 0.55 (0–2.45) |
AMA-S-PAAS | 8 |
| Okati et al. [62], 2013 | Mazandaran | Amol | 38 | Cross-sectional | 0.37 ± 0.15 | AMA-S-PAAS | 8 |
| Okati et al. [62], 2013 | Mazandaran | Sari | 44 | Cross-sectional | 0.50 ± 0.71 | AMA-S-PAAS | 8 |
| Goudarzi et al. [63], 2013 | Isfahan | Isfahan | 37 | Cross-sectional |
0.92 ± 0.54 (0–2.07) |
CV-AAS | 6 |
| Norouzi et al. [64], 2012 | Isfahan | Lenjan | 38 | Cross-sectional | 7.57 ± 1.08 | AMA-S-PAAS | 8 |
| Dahmardeh Behrooz et al. [65], 2012 | Tehran, Mazandaran and East Azerbaijan | Tehran, Noushahr and Tabriz | 80 | Cross-sectional |
0.39 ± 0.1 (ND* - 5.86) |
AMA-S-PAAS | 7 |
| Dahmardeh Behrooz et al. [65], 2012 | Tehran | Tehran | 34 | Cross-sectional |
0.12 ± 0.06 (ND* - 1.73) |
AMA-S-PAAS | 7 |
| Dahmardeh Behrooz et al. [65], 2012 | Mazandaran | Noushahr | 18 | Cross-sectional |
0.15 ± 0.06 (ND* - 1.21) |
AMA-S-PAAS | 7 |
| Dahmardeh Behrooz et al. [65], 2012 | East Azerbaijan | Tabriz | 28 | Cross-sectional |
0.86 ± 0.26 (0.02–5.86) |
AMA-S-PAAS | 7 |
ND*: Not detectable
Generally, from 10 studies entered to meta-analysis process, mothers age range in done studies on hair and BM of ILMs were 17 to 36 years and 16 to 38 years, respectively.
The lowest and highest number of hair and HBM samples in meta-analysis process, varied from 6 to 93 samples and 37 to 82 samples, respectively. Overall, the risk of bias in primary studies was low (Table 1).
Mean mercury level in hair and BM of ILMs
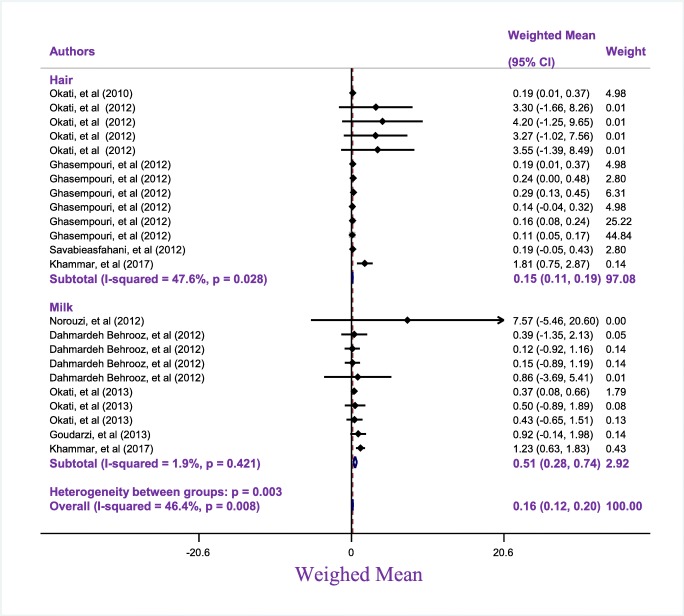
The mean of mercury was obtained to be 0.15 μg/g (95 CI: 0.11–0.19, I2: 47.6%, P: 0.028) and 0.51 μg/l (95 CI: 0.28–0.74, I2: 1.9%, P: 0.421) in hair and BM of ILMs, respectively (Fig. 2).
Fig. 2.
Subgroup analysis Weighed mean of Mercury in Mother based on sample environment (Heir and Milk) in Iran
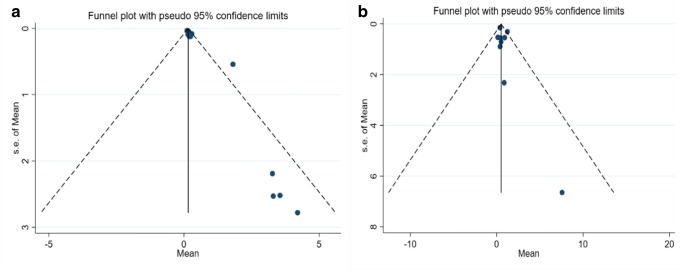
Since the confidence interval (CI) of the test not includes zero (Egger’s Test: t = 1.24, p = 0.0001, CI 95% = 0.73 to 1.75), significant bias occurred in the publication of the results (Fig. 3).
Fig. 3.
Publication bias of weighted Mean of Mercury in mother in Iran (A: Milk, B: Hair)
According to guideline of WHO, for hair and BM of ILMs, four and five studies had lover mercury levels than the limit declared by WHO, respectively.
Based on Table 1, the lowest and highest the mean of mercury level in hair related 0.11 μg/g and 4.2 μg/g, respectively. Also, the lowest and highest mean of mercury in HBM reported 0.12 μg/l 7.57 μg/l, respectively.
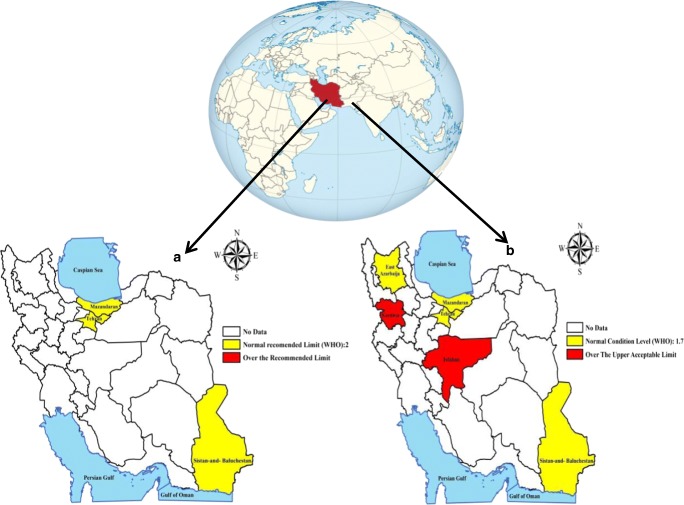
Also, to better highlight of mercury levels in hair and BM of ILMs according to WHO standard, the map of spatial distribution of mean mercury concentrations was generated by using a Geographic Information System (Fig. 4).
Fig. 4.
The geographical distribution of mercury levels in hair (a) and BM (b) of ILMs accord ing to WHO standard
Analytical methods and results reporting
Generally, milk samples of ILMs were collected manually (one study with pump) and then stored in polyethylene (4 study) and polypropylene (one study) containers. Also, the retention temperature of the samples was in range -18 °C to 20 °C.
Also, hair samples in all studies were collected from scalp area of ILMs. The amount of hair needed was for mercury analysis in four study 1 g and in one study about 2–5 g. In all studies, hair samples were cut with stainless steel scissors and then stored in labeled plastic bags.
In the selected studies, mean mercury levels in hair and BM of ILMs were reported in two types of units, μg/L and μg/g, respectively.
The BM mercury content of ILMs was obtained in three studies by Advanced Mercury Analyzer- Single-Purpose Atomic Absorption Spectrometer (AMA-S-PAAS); however, the one study used the Cold Vapor Atomic Absorption Spectroscopy (CV-AAS). Also, two other studies used the mass spectrometry Inductive Coupled Plasma (ICP-MS) and High-Resolution- Continuum Source Atomic Absorption Spectroscopy (HR-CS-AAS).
The hair mercury content of ILMs was obtained in three studies by Advanced Mercury Analyzer- Single-Purpose Atomic Absorption Spectrometer (AMA-S-PAAS). Also, two other studies used the mass spectrometry Inductive Coupled Plasma (ICP-MS) and High-Resolution- Continuum Source Atomic Absorption Spectroscopy (HR-CS-AAS), respectively.
Meta-regression
The Meta regression was used to detect association between independent variables (age and years of publication) and dependent variable (mean of mercury in hair and BM). Results of Meta regression show that the mean of mercy in BM of ILMs did not have association with age (coefficient: 0.035, P: 0.980, 95% CI: −10.61, 10.68) and years of publication (coefficient: -0.025, P: 0.921, 95% CI: −58.73, 58.75). Also results of Meta regression show that the mean of mercury in hair did not have association with age (coefficient: 0.316, P: 0.672, 95% CI: −17.41, 18.04) and years of publication (coefficient: -0.268, P: 0.880, 95% CI: −83.67, 83.00).
Discussion
Successful management and implementation of the Minamata Convention for protect human health and the environment depends on the support of appropriate scientific research and data.
Therefore, to enhance public health research, promote health, population benefit and protection against mercury exposure, we need to have a general understanding of the current situation for planning and formulating appropriate policies in the future.
In the current review study, to the best of our knowledge, for the first time, we determined the mean mercury level in hair and BM among ILMs population.
Hair and HBM are considered as two suitable bio-indicator of mercury contamination for determination of human risk factors in study of toxic metals.
According to Guideline of WHO, the mercury level of 1.4–1.7 μg/l and 2 μg/g were considered as “normal condition levels” in BM of human [49, 50] and “Normal Recommended Limit” in mother’s hair, respectively [10].
Mean of mercury level in milk was estimated to be 0.51 μg/l (95 CI: 0.28–0.74, I2: 1.9%, P: 0.421) for ILMs.
The results of present research indicated a low rate of mean milk mercury level (MML) in ILMs compared with the WHO standard. This is probably due to their low fish consumption and their relatively low mercury levels.
In this meta-analysis, about 37% of the total samples size, the mercury level in milk was reported to be higher than the allowable limit of WHO [56, 64].
The most important risk factor for increasing mercury concentrations in BM is the consumption of mercury-contaminated foods such as fish [10].
In the Bahmani and Maleki study [56] in Kurdistan, since the use of dental amalgam by ILMs was negligible and there was a significant correlation between the MML and fish consumption, so the high MML may be due to high fish consumption.
This is consistent with the results of other studies [48, 62, 66–68]. But in other studies there was no significant relationship found between fish consumption and mercury concentration in BM [69–76].
Also, another risk factor for increasing mercury concentrations in BM is exposure to mercury vapor via amalgam-filled teeth during pregnancy and lactation.
Accordingly, in the study of Norouz et al. [64] in Esfahan, The fish consumption was very low among mothers and there was a significant positive correlation between MML and dental amalgam, which probably the high MML may be due to it. So that the mean milk mercury level in mothers with one to three amalgam-filled teeth and mothers with four to eight amalgam-filled teeth increased from 5.47 μg to 13.33 μg.
Other studies have reported levels above the WHO standard. This rate varies In different countries of the world: Italy (2.6 μg/l) [75], Turkey (3.42 μg/l) [73], Turkey (25.8 μg/l) [72], Brazil (5.7 μg/l) [77], Brazil (5.73 μg/l) [78], Brazil (6.7 μg/l) [71], Brazil (59.41 μg/l) [79], Indonesia, Tanzania and Zimbabwe (1.87 μg/l) [74], and Mexico (2.52 μg/l) [80].
The mean MML in ILMs varies in worldwide, which are within the range of 0.008–59.41 μg/l [79, 81], which in present study was within the range of 0.12–7.57 μg/l.
Various studies have considered different factors to be effective on mercury concentration. These factors include: sampling location, sampling time, sampling method, lactation period, fat content of milk, nutritional status and maternal exposure level [10]. However, factors such as the method of analysis of samples and contaminated samples may also influence the final results.
Although MML are not the same in different countries, but the results of this study are consistent with results from other studies: Saudi Arabia (1.191 μg/l) [45], Korea (0.94 μg/l) [82], Cyprus(0 ± 0.20 μg/l) [83], Saudi Arabia (0.970 μg/l) [84], United Arab Emirates (0.008 μg/l) [81], Slovakia (0.94 μg/l) [85], Austria (1.59 μg/l) [86], Japan (0.81 μg/l) [87], Spain (0.53 μg/l) [68], Brazil (0.36 μg/l) [70], and United Arab Emirates(0.115 μg/l) [88].
Chien et al. [89] In a study estimated that over 99% of mercury exposure in infants was caused by BM. Therefore, BM is a major source of mercury exposure for infants and its consumption can cause serious harm to infants, including nerve damage, immune problems, mental retardation, cerebral palsy, motor disorders, visual impairment, speech and hearing impairment [58, 89, 90].
The results showed that the mean hair mercury level (HML) was estimated to be 0.15 μg/g (95 CI: 0.11–0.19, I2: 47.6%, P: 0.028) for ILMs.
Overall, the mean HML in ILMs was within the range of 0.11–4.2 μg/g. The results of this study showed that the mean HML in ILMs compared with the allowable limit of WHO is lower, which is probably due to their low fish consumptionو their relatively low mercury levels and low amalgams consumption.
In accordance with guideline of WHO, in our study the HML was higher than allowable limit [58].
In this study, there was a significant positive correlation between HMLs and fish consumption as well as amalgam use by ILMs. However, as the concentration of mercury in the mothers without amalgam was also high in this study, this was probably due to the high levels of mercury in the hair and the main exposure of mothers to high fish consumption.
Although fish consumption is an effective factor in increasing the mercury concentration in ILMs, the use of other sources such as cosmetics or chemical shampoos can potentially affect the amount of mercury in hair [91, 92].
Today, mercury-containing cosmetics such as bleach, skin-lightening creams and other beauty products are widely used by women worldwide. Therefore, it is important to provide information on the dangers of this subject, especially for pregnant and ILMs about the care and non-use of these substances [93].
On the other hand, there was no significant relationship between the date of publication and the age of mothers with the mean mercury level in this study, which suggests that there may be other variables that have significant effect on mercury levels in BM and hair.
Thus, although the mean HML of ILMs is not the same in different countries, but the results of this study are consistent with studies in other countries, such as: Spain (1.22 μg/g) [13], Slovakia (0.13 μg/g) [94], Germany (0.109 μg/g) [95], 17 European countries (0.1–1.486 μg/g) [96], and Slovenia (0.377 μg/g) [97].
Limitation:
We observed the heterogeneity of mercury measurement units between different studies in hair and BM of ILMs. Therefore, their analysis was performed separately.
Also, another limitation of the study presented here is that data are not totally representative of a country population.
In addition, exposure with mercury in other studies reporting using biometrics such as blood, urine, nails were not contained in our analysis because of the lack of study in this field.
Therefore, the results of the present study are an overview of the information on mean mercury levels in hair and BM of ILMs, and it doesn’t necessarily show the level of mercury exposure in our country.
Conclusion
Mercury is one of the most dangerous environmental pollutants due to environmental sustainability and bioaccumulation in the food chain.Pregnant and ILMs exposure to mercury and subsequent infant exposure to BM is one of the most important health concerns in the world due to its high toxicity. Exposure to this substance by mothers and infants can cause serious harm to them. In this study, studies conducted on mercury concentrations in hair and BM of ILMs were reviewed. The concentration values of these substances were also compared with WHO standards. While the mean total mercury level in hair and BM of ILMs was lower than the WHO standard, but due to the toxicity and dangers of mercury exposure, management and periodic monitoring of mercury levels in ILMs and newborns in different cities in the country are essential. It is also important to identify all potential risk factors for mercury exposure.
Acknowledgments
The authors want to thank authorities of Iran University of Medical Sciences for their comprehensives support for this study.
Abbreviations
- WHO
World Health Organization
- MML
milk mercury level
- HML
hair mercury level
- SD
standard deviation
- HBM
human Breast Milk
- BM
Breast Milk
- ILMs
Iranian lactating mothers
Compliance with ethical standard
Conflict of interest
The authors of this article declare that they have no conflict of interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Saleh HN, Panahande M, Yousefi M, Asghari FB, Conti GO, Talaee E, et al. Carcinogenic and non-carcinogenic risk assessment of heavy metals in groundwater wells in Neyshabur Plain, Iran. Biological trace element research. 2019;190(1):251–61. [DOI] [PubMed]
- 2.ATSDR. The priority list of hazardous substances that will be the candidates for toxicological profiles. . Agency for Toxic Substances and Disease Registry. 2015.
- 3.Sundseth K, Pacyna JM, Pacyna EG, Pirrone N, Thorne RJ. Global sources and pathways of mercury in the context of human health. International Journal Of Environmental Research And Public Health. 2017;14(1):105. doi: 10.3390/ijerph14010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim K. H, Kabir E, Jahan S. A. A review on the distribution of Hg in the environment and its human health impacts. Journal of Hazardous Materials. 2016 2016/04/05/;306:376–385. [DOI] [PubMed]
- 5.Streets D. G, Horowitz H. M, Jacob D. J, Lu Z, Levin L, ter Schure A. F. H, et al. Total Mercury Released to the Environment by Human Activities. Environmental Science & Technology. 2017 2017/06/06;51(11):5969–5977. [DOI] [PubMed]
- 6.Streets D. G, Horowitz H. M, Lu Z, Levin L, Thackray C. P, Sunderland E. M. Global and regional trends in mercury emissions and concentrations, 2010–2015. Atmospheric Environment. 2019 2019/03/15/;201:417–427.
- 7.UNEP. Global Mercury Assessment 2013: Sources, emissions, releases and environmental transport. UNEP Chemicals Branch, Geneva, Switzerland: United Nations Environment Programme; 2013.
- 8.Guzzi G, La Porta C. A. M. Molecular mechanisms triggered by mercury. Toxicology. 2008 2008/02/03/;244(1):1–12. [DOI] [PubMed]
- 9.Coulter M. A. Minamata Convention on Mercury. International Legal Materials, Cambridge University Press. 2017;55(3):582–616. Epub 01/20.
- 10.UNEP, WHO. Guidance for identifying populations at risk from mercury exposure. Issued by UNEP DTIE Chemicals Branch and WHO Department of Food Safety, Zoonoses and Foodborne Diseases. . Geneva, Switzerland: United Nations Environment Programme / World Health Organization: https://www.who.int/foodsafety/publications/risk-mercury-exposure/en/; 2008.
- 11.Rice KM, Walker EM, Jr, Wu M, Gillette C, Blough ER. Environmental mercury and its toxic effects. J Prev Med Public Health. 2014;47(2):74–83. doi: 10.3961/jpmph.2014.47.2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magos L, Clarkson TW. Overview of the clinical toxicity of mercury. Ann Clin Biochem. 2006;43(4):257–268. doi: 10.1258/000456306777695654. [DOI] [PubMed] [Google Scholar]
- 13.Yusà V, Pérez R, Suelves T, Corpas-Burgos F, Gormáz M, Dualde P, et al. Biomonitoring of mercury in hair of breastfeeding mothers living in the Valencian Region (Spain). Levels and predictors of exposure. Chemosphere. 2017 2017/11/01/;187:106–113. [DOI] [PubMed]
- 14.Akramipour R, Golpayegani M. R, Gheini S, Fattahi N. Speciation of organic/inorganic mercury and total mercury in blood samples using vortex assisted dispersive liquid-liquid microextraction based on the freezing of deep eutectic solvent followed by GFAAS. Talanta. 2018 2018/08/15/;186:17–23. [DOI] [PubMed]
- 15.Clarkson T. W, Magos L. The Toxicology of Mercury and Its Chemical Compounds. Critical Reviews in Toxicology. 2006 2006/01/01;36(8):609–662. [DOI] [PubMed]
- 16.de Souza S. S, Campiglia A. D, Barbosa F. A simple method for methylmercury, inorganic mercury and ethylmercury determination in plasma samples by high performance liquid chromatography–cold-vapor-inductively coupled plasma mass spectrometry. Analytica Chimica Acta. 2013 2013/01/25/;761:11–17. [DOI] [PubMed]
- 17.Marín S, Pardo O, Báguena R, Font G, Yusà V. Dietary exposure to trace elements and health risk assessment in the region of Valencia, Spain: a total diet study. Food Additives & Contaminants: Part A. 2017 2017/02/01;34(2):228–240. [DOI] [PubMed]
- 18.Akerstrom M, Barregard L, Lundh T, Sallsten G. Relationship between mercury in kidney, blood, and urine in environmentally exposed individuals, and implications for biomonitoring. Toxicology and Applied Pharmacology. 2017 2017/04/01/;320:17–25. [DOI] [PubMed]
- 19.Gibb H, O’Leary KG. Mercury exposure and health impacts among individuals in the artisanal and small-scale gold mining community: a comprehensive review. Environ Health Perspect. 2014;122(7):667–672. doi: 10.1289/ehp.1307864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bjørklund G, Tinkov AA, Dadar M, Rahman MM, Chirumbolo S, Skalny AV, Skalnaya MG, Haley BE, Ajsuvakova OP, Aaseth J. Insights into the potential role of mercury in Alzheimer’s disease. J Mol Neurosci. 2019;67(4):511–533. doi: 10.1007/s12031-019-01274-3. [DOI] [PubMed] [Google Scholar]
- 21.Kröger E, Laforce R. Fish consumption, brain mercury, and neuropathology in patients with Alzheimer disease and dementia. Jama. 2016;315(5):465–466. doi: 10.1001/jama.2016.0005. [DOI] [PubMed] [Google Scholar]
- 22.Zellner T, Zellner N, Felgenhauer N, Eyer F., Epilepsy and polyneuropathy in a mercury-exposed patient: investigation, identification of an obscure source and treatment. Case Reports. Dementia. 2016;2016:bcr2016216835. [DOI] [PMC free article] [PubMed]
- 23.Nabi S. Methylmercury and parkinson’s disease. Toxic Effects of Mercury: Springer; 2014. pp. 211–218. [Google Scholar]
- 24.Kim D, Kang YW, Park SW, Lee KH, Lee YS. Relationship of hair copper and mercury contents to personality in chronic schizophrenia. J Prev Med Public Health. 1990;23(3):296–308. [Google Scholar]
- 25.Peplow D, Augustine S. Neurological abnormalities in a mercury exposed population among indigenous Wayana in Southeast Suriname. Environmental Science: Processes & Impacts. 2014;16(10):2415–2422. doi: 10.1039/c4em00268g. [DOI] [PubMed] [Google Scholar]
- 26.Rana MN, Tangpong J, Rahman MM. Toxicodynamics of lead, cadmium, mercury and arsenic-induced kidney toxicity and treatment strategy: a mini review. Toxicol Rep. 2018;5:704–713. doi: 10.1016/j.toxrep.2018.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bridges CC, Zalups RK. The aging kidney and the nephrotoxic effects of mercury. Journal of Toxicology and Environmental Health, Part B. 2017;20(2):55–80. doi: 10.1080/10937404.2016.1243501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duruibe JO, Ogwuegbu M, Egwurugwu J. Heavy metal pollution and human biotoxic effects. International Journal of physical sciences. 2007;2(5):112–118. [Google Scholar]
- 29.Abdel-Rasul GM, Abu-Salem MA, Al-Batanony MA, Al-Dalatony MM, Allam HK. Neurobehavioral, respiratory, and auditory disorders among mercury-exposed fluorescent lamp workers. Menoufia Medical Journal. 2013;26(1):58. [Google Scholar]
- 30.Lim HE, Shim JJ, Lee SY, Lee SH, Kang SY, Jo JY, et al. Mercury inhalation poisoning and acute lung injury. Korean J Intern Med. 1998;13(2):127–130. doi: 10.3904/kjim.1998.13.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smiechowicz J, Skoczynska A, Nieckula-Szwarc A, Kulpa K, Kübler A. Occupational mercury vapour poisoning with a respiratory failure, pneumomediastinum and severe quadriparesis. SAGE Open Med Case Rep. 2017;5:2050313X17695472-2050313X. eng. [DOI] [PMC free article] [PubMed]
- 32.Gardner R. M, Nyland J. F. Immunotoxic effects of mercury. Environmental Influences on the Immune System: Springer; 2016. p. 273–302.
- 33.Rooney J. Further thoughts on mercury, epigenetics, genetics and amyotrophic lateral sclerosis. Neurodegener Dis. 2011;8(6):523–524. doi: 10.1159/000324518. [DOI] [PubMed] [Google Scholar]
- 34.Basu N, Goodrich JM, Head J. Ecogenetics of mercury: from genetic polymorphisms and epigenetics to risk assessment and decision-making. Environ Toxicol Chem. 2014;33(6):1248–1258. doi: 10.1002/etc.2375. [DOI] [PubMed] [Google Scholar]
- 35.Boffetta P, Sällsten G, Garcia-Gómez M, Pompe-Kirn V, Zaridze D, Bulbulyan M, Caballero JD, Ceccarelli F, Kobal AB, Merler E. Mortality from cardiovascular diseases and exposure to inorganic mercury. Occup Environ Med. 2001;58(7):461–466. doi: 10.1136/oem.58.7.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mozaffarian D, Shi P, Morris JS, Spiegelman D, Grandjean P, Siscovick DS, et al. Mercury exposure and risk of cardiovascular disease in two U.S. cohorts. N Engl J Med. 2011;364(12):1116–1125. doi: 10.1056/NEJMoa1006876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Genchi G, Sinicropi MS, Carocci A, Lauria G, Catalano A. Mercury Exposure and Heart Diseases. International journal of environmental research and public health. 2017;14(1):74. doi: 10.3390/ijerph14010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Houston MC. Role of mercury toxicity in hypertension, cardiovascular disease, and stroke. The Journal of Clinical Hypertension. 2011;13(8):621–627. doi: 10.1111/j.1751-7176.2011.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mocevic E, Specht I. O, Marott J. L, Giwercman A, Jönsson B. A. G, Toft G, et al. Environmental mercury exposure, semen quality and reproductive hormones in Greenlandic Inuit and European men: a cross-sectional study. Asian J Androl. 2013;15(1):97–104. Epub 12/10. eng. [DOI] [PMC free article] [PubMed]
- 40.Henriques M. C, Loureiro S, Fardilha M, Herdeiro M. T. Exposure to mercury and human reproductive health: A systematic review. Reproductive Toxicology. 2019 2019/04/01/;85:93–103. [DOI] [PubMed]
- 41.Crump KL, Trudeau VL. Mercury-induced reproductive impairment in fish. Environmental Toxicology and Chemistry. An International Journal. 2009;28(5):895–907. doi: 10.1897/08-151.1. [DOI] [PubMed] [Google Scholar]
- 42.Cerbino MR, Vieira JCS, Braga CP, Oliveira G, Padilha IF, Silva TM, Zara LF, Silva N. J Jr, Padilha PM. Metalloproteomics approach to analyze mercury in breast Milk and hair samples of lactating women in communities of the Amazon Basin. Brazil Biological Trace Element Research. 2018;181(2):216–226. doi: 10.1007/s12011-017-1057-4. [DOI] [PubMed] [Google Scholar]
- 43.Henck J. W. Reproductive Toxicology In: Raymond D. Harbison MMB, and Giffe T. Johnson, editor. Hamilton and Hardy's Industrial Toxicology. 6nd ed Hoboken, New Jersey: Wiley, Inc 2015. p. 1197–1228.
- 44.Jensen AA. Chemical contaminants in human milk. In: GJD GFA, editor. Residue reviews. 89. New York: Springer; 1983. pp. 1–128. [DOI] [PubMed] [Google Scholar]
- 45.Al-Saleh I, Abduljabbar M, Al-Rouqi R, Elkhatib R, Alshabbaheen A, Shinwari N. Mercury (hg) exposure in breast-fed infants and their mothers and the evidence of oxidative stress. Biol Trace Elem Res. 2013;153(1–3):145–154. doi: 10.1007/s12011-013-9687-7. [DOI] [PubMed] [Google Scholar]
- 46.Bose-O'Reilly S, McCarty K. M, Steckling N, Lettmeier B. Mercury exposure and children's health. Curr Probl Pediatr Adolesc Health Care. 2010;40(8):186–215. eng. [DOI] [PMC free article] [PubMed]
- 47.Jedrychowski W, Jankowski J, Flak E, Skarupa A, Mroz E, Sochacka-Tatara E, Lisowska-Miszczyk I, Szpanowska-Wohn A, Rauh V, Skolicki Z, Kaim I, Perera F. Effects of prenatal exposure to mercury on cognitive and psychomotor function in one-year-old infants: epidemiologic cohort study in Poland. Ann Epidemiol. 2006;16(6):439–447. doi: 10.1016/j.annepidem.2005.06.059. [DOI] [PubMed] [Google Scholar]
- 48.Díez S, Montuori P, Pagano A, Sarnacchiaro P, Bayona J. M, Triassi M. Hair mercury levels in an urban population from southern Italy: Fish consumption as a determinant of exposure. Environment International. 2008 2008/02/01/;34(2):162–167. [DOI] [PubMed]
- 49.WHO, IAEA . Minor and trace elements in breast milk : report of a joint WHO/IAEA collaborative study. Geneva: World Health Organization; 1989. [Google Scholar]
- 50.Bansa D. K, Awua A. K, Boatin R, Adom T, Brown-Appiah E. C, Amewosina K. K, et al. Cross-sectional assessment of infants’ exposure to toxic metals through breast milk in a prospective cohort study of mining communities in Ghana. BMC Public Health. 2017 2017/05/25;17(1):505. [DOI] [PMC free article] [PubMed]
- 51.Cherkani-Hassani A, Ghanname I, Mouane N. Total, organic, and inorganic mercury in human breast milk: levels and maternal factors of exposure, systematic literature review, 1976–2017. Critical Reviews in Toxicology. 2019 2019/02/07;49(2):110–121. [DOI] [PubMed]
- 52.Sharma B. M, Sáňka O, Kalina J, Scheringer M. An overview of worldwide and regional time trends in total mercury levels in human blood and breast milk from 1966 to 2015 and their associations with health effects. Environment International. 2019 2019/04/01/;125:300–319. [DOI] [PubMed]
- 53.Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred reporting items for systematic reviews and Meta-analyses: The PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Margulis AV, Pladevall M, Riera-Guardia N, Varas-Lorenzo C, Hazell L, Berkman ND, et al. Quality assessment of observational studies in a drug-safety systematic review, comparison of two tools: the Newcastle–Ottawa scale and the RTI item bank. Clinical epidemiology. 2014;6:359. doi: 10.2147/CLEP.S66677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 56.Bahmani P, Maleki A. Investigation of Mercury, Cadmium and Arsenic Levels in Breast Milk and Their Relationship with the Studied Parameters in Sanandaj, Iran. Zanko Journal of Medical Sciences. 2018;19(62):84–97. eng.
- 57.Khammar S, Pourkhabbaz A, Dahmardeh Behrooz R. Examination of mercury concentration in the hair and Milk mothers and relation to number of dental amalgam filling and mother feeding case study : (city of Zahedan) Journal of Natural Environment. 2017;70(1):77–86. [Google Scholar]
- 58.Okati N, Sari A. E, Ghasempouri S. M. Hair Mercury Concentrations of Lactating Mothers and Breastfed Infants in Iran (Fish Consumption and Mercury Exposure). Biological Trace Element Research. 2012 2012/11/01;149(2):155–162. [DOI] [PubMed]
- 59.Savabieasfahani M, Hoseiny M, Goodarzi S. Toxic and Essential Trace Metals in First Baby Haircuts and Mother Hair from Imam Hossein Hospital Tehran, Iran. Bulletin of Environmental Contamination and Toxicology. 2012 2012/02/01;88(2):140–144. [DOI] [PubMed]
- 60.Ghasempouri SM, Okati N, Esmaili-Sari A. Mercury in Hair of Mothers and Infants: Influencing Factors Assessment in the Southern shores of the Caspian Sea (Iran) Iranian Jornal of Toxicology. 2012;3(3):335–346. [Google Scholar]
- 61.Okati N, Esmaili Sari A, Ghasempouri M. Examination of Mercury Concentration in the Hair of Breast-Feeding Mothers and Relation to Fish Diet, Number of Dental Amalgam Filling, Age and Place of Live. Iranian Journal of Health and Environment. 2010;3(3):327–334. [Google Scholar]
- 62.Okati N, Sari A. E, Ghasempouri S. M. Evaluation of mercury pollution in breast milk and Iranian infants’ hair. International Research Journal of Applied and Basic Sciences. 2013 01/01;4(9):2857–2864.
- 63.Goudarzi M. A, Parsaei P, Nayebpour F, Rahimi E. Determination of mercury, cadmium and lead in human milk in Iran. Toxicology and Industrial Health. 2012 2013/10/01;29(9):820–823. [DOI] [PubMed]
- 64.Norouzi E, Bahramifar N, Ghasempouri S. M. Effect of teeth amalgam on mercury levels in the colostrums human milk in Lenjan. Environmental Monitoring and Assessment. 2012 2012/01/01;184(1):375–380. [DOI] [PubMed]
- 65.Dahmardeh Behrooz R, Esmaili-Sari A, Peer F. E, Amini M. Mercury Concentration in the Breast Milk of Iranian Women. Biological Trace Element Research. 2012 2012/06/01;147(1):36–43. [DOI] [PubMed]
- 66.Grzunov LetiniĿ J, Matek SariĿ M, Piasek M, JurasoviĿ J, Varnai V. M, Sulimanec Grgec A, et al. Use of human milk in the assessment of toxic metal exposure and essential element status in breastfeeding women and their infants in coastal Croatia. Journal of Trace Elements in Medicine and Biology. 2016 2016/12/01/;38:117–125. [DOI] [PubMed]
- 67.Gaxiola-Robles R, Labrada-Martagón V, Acosta-Vargas B, Méndez-Rodríguez LC, Zenteno-Savín T. Interaction between mercury (hg), arsenic (as) and selenium (se) affects the activity of glutathione S-transferase in breast milk; possible relationship with fish and shellfish intake. Nutricion hospitalaria. 2014;30(2):436–446. doi: 10.3305/nh.2014.30.2.7441. [DOI] [PubMed] [Google Scholar]
- 68.García-Esquinas E, Pérez-Gómez B, Fernández M. A, Pérez-Meixeira A. M, Gil E, Paz C. D, et al. Mercury, lead and cadmium in human milk in relation to diet, lifestyle habits and sociodemographic variables in Madrid (Spain). Chemosphere. 2011 2011/09/01/;85(2):268–276. [DOI] [PubMed]
- 69.Dursun A, Yurdakok K, Yalcin S. S, Tekinalp G, Aykut O, Orhan G, et al. Maternal risk factors associated with lead, mercury and cadmium levels in umbilical cord blood, breast milk and newborn hair. The Journal of Maternal-Fetal & Neonatal Medicine. 2016 2016/03/18;29(6):954–961. [DOI] [PubMed]
- 70.Vieira S. M, de Almeida R, Holanda I. B. B, Mussy M. H, Galvão R. C. F, Crispim P. T. B, et al. Total and methyl-mercury in hair and milk of mothers living in the city of Porto Velho and in villages along the Rio Madeira, Amazon, Brazil. International Journal of Hygiene and Environmental Health. 2013 2013/11/01/;216(6):682–689. [DOI] [PubMed]
- 71.Da Cunha LR, da Costa THM, Caldas ED. Mercury concentration in breast milk and infant exposure assessment during the first 90 days of lactation in a midwestern region of Brazil. Biol Trace Elem Res. 2013;151(1):30–37. doi: 10.1007/s12011-012-9542-2. [DOI] [PubMed] [Google Scholar]
- 72.Örün E, Yalçin SS, Aykut O, Orhan G, Koç-Morgil G, Yurdakök K, et al. Mercury exposure via breast-milk in infants from a suburban area of Ankara, Turkey. Turk J Pediatr. 2012;54(2):136–143. [PubMed] [Google Scholar]
- 73.Yalçin SSY, Yurdakök K, Yalçin S, Engür-Karasimav D, Coskun T. Maternal and environmental determinants of breast-milk mercury concentrations. Turk J Pediatr. 2010;52(1):1–9. [PubMed] [Google Scholar]
- 74.Bose-O’Reilly S, Lettmeier B, Roider G, Siebert U, Drasch G. Mercury in breast milk – A health hazard for infants in gold mining areas? International Journal of Hygiene and Environmental Health. 2008 2008/10/01/;211(5):615–623. [DOI] [PubMed]
- 75.Abballe A, Ballard TJ, Dellatte E, A. D D, Ferri F, Fulgenzi AR, et al. Persistent environmental contaminants in human milk: Concentrations and time trends in Italy. Chemosphere. 2008;73(1, Supplement):S220–S2S7. doi: 10.1016/j.chemosphere.2007.12.036. [DOI] [PubMed] [Google Scholar]
- 76.Björnberg KA, Vahter M, Berglund B, Niklasson B, Blennow M, Sandborgh-Englund G. Transport of methylmercury and inorganic mercury to the fetus and breast-fed infant. Environ Health Perspect. 2005;113(10):1381–1385. doi: 10.1289/ehp.7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boischio AAP, Henshel DS. Linear regression models of methyl mercury exposure during prenatal and early postnatal life among Riverside people along the upper Madeira River. Amazon Environmental Research. 2000;83(2):150–161. doi: 10.1006/enrs.2000.4050. [DOI] [PubMed] [Google Scholar]
- 78.Da Costa SL, Malm O, Dórea JG. Breast-milk mercury concentrations and amalgam surface in mothers from Brasilia, Brazil. Biol Trace Elem Res. 2005;106(2):145–151. doi: 10.1385/BTER:106:2:145. [DOI] [PubMed] [Google Scholar]
- 79.dos Santos FA, Cavecci B, Vieira JCS, Franzini VP, Santos A, de Lima LA, et al. A Metalloproteomics study on the Association of Mercury with Breast Milk in samples from lactating women in the Amazon region of Brazil. Arch Environ Contam Toxicol. 2015;69(2):223–229. doi: 10.1007/s00244-015-0161-8. [DOI] [PubMed] [Google Scholar]
- 80.Gaxiola-Robles R, Zenteno-Savín T, Labrada-Martagón V. Celis de la Rosa AdJ, Acosta Vargas B, Méndez-Rodríguez LC. Concentraciones de mercurio en leche de mujeres del noroeste de México: posible asociación a la dieta, tabaco y otros factores maternos. Nutr Hosp. 2013;28(3):934–942. doi: 10.3305/nh.2013.28.3.6447. [DOI] [PubMed] [Google Scholar]
- 81.Abdulrazzaq YM, Osman N, Nagelkerke N, Kosanovic M, Adem A. Trace element composition of plasma and breast milk of well-nourished women. J Environ Sci Health A. 2008;43(3):329–334. doi: 10.1080/10934520701792878. [DOI] [PubMed] [Google Scholar]
- 82.Park Y, Lee A, Choi K, Kim HJ, Lee JJ, Choi G, Kim S, Kim SY, Cho GJ, Suh E, Kim SK, Eun SH, Eom S, Kim S, Kim GH, Moon HB, Kim S, Choi S, Kim YD, Kim J, Park J. Exposure to lead and mercury through breastfeeding during the first month of life: a CHECK cohort study. Sci Total Environ. 2018;612:876–883. doi: 10.1016/j.scitotenv.2017.08.079. [DOI] [PubMed] [Google Scholar]
- 83.Kunter İ, Hürer N, Gülcan HO, Öztürk B, Doğan İ, Şahin G. Assessment of Aflatoxin M1 and heavy metal levels in mothers breast Milk in Famagusta. Cyprus Biological Trace Element Research. 2017;175(1):42–49. doi: 10.1007/s12011-016-0750-z. [DOI] [PubMed] [Google Scholar]
- 84.Al-Saleh I, Abduljabbar M, Al-Rouqi R, Eltabache C, Al-Rajudi T, Elkhatib R, et al. The extent of mercury (hg) exposure among Saudi mothers and their respective infants. Environ Monit Assess. 2015;187(11):678. doi: 10.1007/s10661-015-4858-y. [DOI] [PubMed] [Google Scholar]
- 85.Ursinyova M, Masanova V. Cadmium, lead and mercury in human milk from Slovakia. Food Additives & Contaminants. 2005;22(6):579–589. doi: 10.1080/02652030500135201. [DOI] [PubMed] [Google Scholar]
- 86.Gundacker C, Pietschnig B, Wittmann KJ, Lischka A, Salzer H, Hohenauer L, Schuster E. Lead and mercury in breast Milk. Pediatrics. 2002;110(5):873–878. doi: 10.1542/peds.110.5.873. [DOI] [PubMed] [Google Scholar]
- 87.Iwai-Shimada M, Satoh H, Nakai K, Tatsuta N, Murata K, Akagi H. Methylmercury in the breast milk of Japanese mothers and lactational exposure of their infants. Chemosphere. 2015;126:67–72. doi: 10.1016/j.chemosphere.2014.12.086. [DOI] [PubMed] [Google Scholar]
- 88.Kosanovic M, Adem A, Jokanovic M, Abdulrazzaq YM. Simultaneous determination of cadmium, mercury, Lead, arsenic, copper, and zinc in human breast Milk by ICP-MS/microwave digestion. Anal Lett. 2008;41(3):406–416. doi: 10.1080/00032710701862910. [DOI] [Google Scholar]
- 89.Chien LC, Han BC, Hsu CS, Jiang CB, You HJ, Shieh MJ, Yeh CY. Analysis of the health risk of exposure to breast milk mercury in infants in Taiwan. Chemosphere. 2006;64(1):79–85. doi: 10.1016/j.chemosphere.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 90.Yurdakök K. Lead, mercury, and cadmium in breast milk. Journal of Pediatric and Neonatal Individualized Medicine (JPNIM) 2015;4(2):e040223. [Google Scholar]
- 91.Wilhelm M, Müller F, Idel H. Biological monitoring of mercury vapour exposure by scalp hair analysis in comparison to blood and urine. Toxicol Lett. 1996;88(1):221–226. doi: 10.1016/0378-4274(96)03741-1. [DOI] [PubMed] [Google Scholar]
- 92.Al-Saleh I, Al-Doush I. Mercury content in skin-lightening creams and potential hazards to the health of Saudi women. J Toxicol Environ Health. 1997;51(2):123–130. doi: 10.1080/00984109708984016. [DOI] [PubMed] [Google Scholar]
- 93.Engler DE. Mercury "bleaching" creams. J Am Acad Dermatol. 2005;52(6):1113–1114. doi: 10.1016/j.jaad.2005.01.136. [DOI] [PubMed] [Google Scholar]
- 94.Borošová D, Slotová K, Fabiánová E. Mercury content in hairs of mother-child pairs in Slovakia as a biomarker of environmental exposure. Acta Chimica Slovaca. 2014;7(2):119–122. doi: 10.2478/acs-2014-0020. [DOI] [Google Scholar]
- 95.Schwedler G, Seiwert M, Fiddicke U, Ißleb S, Hölzer J, Nendza J, Wilhelm M, Wittsiepe J, Koch HM, Schindler BK, Göen T, Hildebrand J, Joas R, Joas A, Casteleyn L, Angerer J, Castano A, Esteban M, Schoeters G, den Hond E, Sepai O, Exley K, Bloemen L, Knudsen LE, Kolossa-Gehring M. Human biomonitoring pilot study DEMOCOPHES in Germany: contribution to a harmonized European approach. Int J Hyg Environ Health. 2017;220(4):686–696. doi: 10.1016/j.ijheh.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 96.Den Hond E, Govarts E, Willems H, Smolders R, Casteleyn L, Kolossa-Gehring M, et al. First steps toward harmonized human biomonitoring in Europe: demonstration project to perform human biomonitoring on a European scale. Environ Health Perspect. 2015 Mar;\(3):255–263. Pubmed Central PMCID: PMC4348748. eng. [DOI] [PMC free article] [PubMed]
- 97.Miklavčič A, Cuderman P, Mazej D, Snoj Tratnik J, Krsnik M, Planinšek P, Osredkar J, Horvat M. Biomarkers of low-level mercury exposure through fish consumption in pregnant and lactating Slovenian women. Environ Res. 2011;111(8):1201–1207. doi: 10.1016/j.envres.2011.07.006. [DOI] [PubMed] [Google Scholar]