Abstract
Purpose
Understanding the brain basis of language and cognitive outcomes is a major goal of aphasia research. Prior studies have not often considered the many ways that brain features can relate to behavioral outcomes or the mechanisms underlying these relationships. The purpose of this review article is to provide a new framework for understanding the ways that brain features may relate to language and cognitive outcomes from stroke.
Method
Brain–behavior relationships that may be important for aphasia outcomes are organized into a taxonomy, including features of the lesion and features of brain tissue spared by the lesion. Features of spared brain tissue are categorized into those that change after stroke and those that do not. Features that change are further subdivided, and multiple mechanisms of brain change after stroke are discussed.
Results
Features of the stroke, including size, location, and white matter damage, relate to many behavioral outcomes and likely account for most of the variance in outcomes. Features of the spared brain tissue that are unchanged by stroke, such as prior ischemic disease in the white matter, contribute to outcomes. Many different neurobiological and behavioral mechanisms may drive changes in the brain after stroke in association with behavioral recovery. Changes primarily driven by neurobiology are likely to occur in brain regions with a systematic relationship to the stroke distribution. Changes primarily driven by behavior are likely to occur in brain networks related to the behavior driving the change.
Conclusions
Organizing the various hypothesized brain–behavior relationships according to this framework and considering the mechanisms that drive these relationships may help investigators develop specific experimental designs and more complete statistical models to explain language and cognitive abilities after stroke. Eight main recommendations for future research are provided.
Presentation Video
Long-term aphasia outcomes vary substantially between individual stroke survivors, including differences in the profile of deficits and spared abilities, as well as in the severity of specific deficits (Kertesz & McCabe, 1977). Understanding the basis of these individual differences would help families and clinicians address a person with aphasia's specific challenges earlier, leverage their strengths more fully, and potentially provide new ways in which to intervene in order to improve outcomes. From a purely scientific perspective, this information will also elucidate the brain structures and networks that are important for language and the degree and nature of the plasticity available in the brain. Understanding why people have such different outcomes from stroke is thus a major goal for aphasia research.
Differences in aphasia outcome derive from a variety of sources (Plowman, Hentz, & Ellis, 2012), including the nature of the stroke (Yarnell, Monroe, & Sobel, 1976), psychosocial and societal factors (González-Fernández et al., 2011), brain health (Wright et al., 2018), exposure to speech-language therapy (Brady, Kelly, Godwin, Enderby, & Campbell, 2016), and possibly genetic factors (Cramer, Procaccio, & GAIN Americas and GAIN International Study Investigators, 2012). Understanding how each of these factors contributes to language and cognitive outcomes is important. However, as the brain is the organ of thought and behavior, regardless of the ultimate cause of differences in language and cognitive abilities after stroke, these behavioral differences must be mediated by differences in the brain. As such, the brain is likely to be a key source of information regarding the basis of aphasia outcomes. Furthermore, brain-based neuromodulatory treatments such as medications that alter neurotransmitter availability (Berthier, Pulvermüller, Dávila, Casares, & Gutiérrez, 2011) and noninvasive electrical or magnetic stimulation techniques that affect brain activity (Fregni & Pascual-Leone, 2007) now allow us to directly modulate brain function. Understanding the brain basis of language and cognitive outcomes may help to identify optimal neuromodulatory treatments that make the brain of a person with a poor outcome more like the brain of a person with a good outcome (Hamilton, Chrysikou, & Coslett, 2011; Turkeltaub, 2015).
Neuroscience has made great strides over the past few decades in improving our ability to measure brain structure, function, and connectivity at the level of brain regions and networks in living humans. The measurement of brain regions and networks is an appropriate level of investigation in order to understand stroke outcome because complex behaviors are largely mediated by networks of brain regions working in coordination (Bressler & Menon, 2010). Many studies have now been published associating language or cognitive outcomes from stroke with brain factors such as stroke size and location (e.g., Thye & Mirman, 2018) or activity in brain regions spared by stroke (e.g., Fridriksson, Bonilha, Baker, Moser, & Rorden, 2010). These types of studies often describe how brain features relate to aphasia outcomes without fully considering the biological and behavioral mechanisms underlying these relationships. Furthermore, studies often consider only one or two factors at a time, leading to potentially misleading results given likely interactions between factors (e.g., an inverse relationship between lesion size and perilesional activity). Organizing our thinking about the many ways in which brain features may relate to behavioral outcomes after stroke could help investigators to design studies that examine the brain basis of outcomes more comprehensively. Considering the mechanisms underlying brain–behavior relationships will help researchers to design studies capable of elucidating not only the patterns of brain changes after stroke but also why these changes occur.
In this review article, I will outline a taxonomy of ways in which brain features may relate to stroke outcome, with a focus on aphasia. I developed this framework over the course of several years as I reviewed prior literature on the brain basis of stroke outcome in both humans and in animal models in order to interpret results of my own lab's experiments. My purpose is not to provide a comprehensive review of the brain basis of aphasia outcomes but rather to provide a framework to organize the types of brain–behavior relationships that might occur, giving examples that illustrate major types of relationships suggested by research in animal models and related fields. Other reviews have discussed environmental, behavioral, and genetic variables that relate to aphasia outcomes (Plowman et al., 2012; Watila & Balarabe, 2015). Here, I focus specifically on the ways in which brain features relate to outcomes because, as noted above, all other sources of variation in outcome must be mediated by the brain. I will focus at the level of brain structures and connections that are measurable in living humans, rather than at the cellular or subcellular level that may be of theoretical interest but are not directly measurable in living humans. When relevant, however, I will consider cellular mechanisms that likely determine patterns in large-scale brain features. I hope that the organizational framework and mechanisms for brain–behavior relationships described in this review article will aid researchers to design experiments that consider as many relevant brain variables as possible and to test increasingly specific hypotheses of how and why brain features relate to outcomes after stroke.
Measuring Aphasia Outcome
The choice of outcome measure affects the types of brain–behavior relationships that are observable and the hypotheses one might formulate regarding these relationships. Therefore, before outlining the framework for understanding brain–behavior relationships after stroke, it is worth considering what types of outcome measures are most appropriate to examine in this context. A key to understanding the relationship between the brain and aphasia outcome is the recognition that language is not a singular entity but rather a composite of many interacting processes. Correspondingly, language is performed by widespread brain structures, most importantly a variety of structures in the left cerebral hemisphere (Friederici, 2011; Price, 2012). Although there are ongoing debates about the degree to which cognitive processes are performed by localized brain processors versus distributed networks, it is clear, based on the relationship between lesion location and deficits, that specific structures are important for particular language functions (Mirman et al., 2015). This localization of function leads to behavioral dissociations such as the dramatically different process-level deficits suffered by individuals with classic Broca's and Wernicke's aphasias. Because of these anatomical and behavioral dissociations, two individuals may have mutually exclusive deficits, yet still have the same “overall aphasia severity.” As such, measuring outcomes using global aphasia severity scores is likely to lead researchers to miss brain factors important only for specific language processes and is not likely to yield insight about brain features informative of any individual person's outcome in terms of their strengths and weaknesses. Generally speaking, measuring more specific behavioral outcomes is likely to lead to a finer understanding of brain–behavior relationships after stroke and be more informative for clinicians and stroke survivors.
For some purposes, it may be useful to consider outcomes for specific language processes or representations, such as an orthographic buffer or orthographic representations (Rapp, Purcell, Hillis, Capasso, & Miceli, 2016). Examining this level of specificity is important, for instance, when investigating the impact of specific behavioral treatments that aim to affect specific processes or simply for understanding the mental or neural architecture that underlies behavior. However, because strokes often involve several cubic centimeters of tissue and distributions are determined by relatively stereotyped anatomy of blood vessels, language deficits are typically not restricted to individual processes (Price, Hope, & Seghier, 2017). Furthermore, somewhat broader outcome measures are likely to be clinically important for a wider range of patients and lead to a more generalizable understanding of the brain basis of language outcomes. As such, for many studies, it may be advantageous to consider outcomes at an intermediate level of specificity.
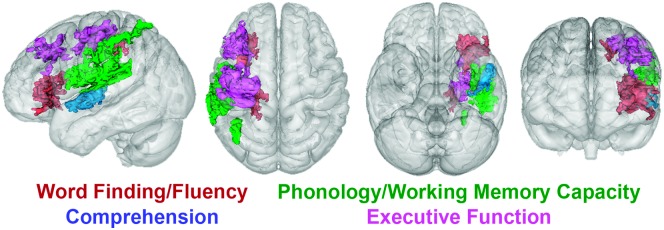
One approach is to conduct a thorough behavioral aphasia assessment battery and then use a statistical technique such as principal components analysis to identify the dissociable functions measured by the battery. Studies using this approach typically identify three to four “core” functions (Butler, Lambon Ralph, & Woollams, 2014; Halai, Woollams, & Lambon Ralph, 2017; Lacey, Skipper-Kallal, Xing, Fama, & Turkeltaub, 2017; Mirman et al., 2015). An example from our own work is provided in Figure 1, showing the results of a principal components factor analysis of scores on a battery of common clinical aphasia assessments in a sample with chronic left hemisphere stroke (Lacey et al., 2017). The analysis identified four factors measured by the assessment battery, and lesion-symptom mapping identified independent lesion locations associated with deficits in each of these behaviors. This approach provides an empirical way to determine outcome measures that are likely to be differently affected by lesions and, for many purposes, serves as a reasonable compromise between global aphasia severity and specific process-level deficits.
Figure 1.
Lesion locations associated with core functions measured by a battery of common aphasia assessments (adapted from Lacey et al., 2017). Thirty-eight individuals with chronic aphasia underwent an extensive aphasia battery consisting of commonly used clinical tests. Principal components factor analysis revealed four underlying language functions measured by the tests, which were labeled by the authors. Support vector regression lesion–symptom mapping was used to identify brain locations where lesions resulted in deficits in each of the language functions. The significant brain areas associated with each language function are shown in color. To provide a full three-dimensional view, the translucent brains (from left to right) show the same results as viewed from the left, from above, from below, and from the front.
A Taxonomy of Brain–Behavior Relationships After Stroke
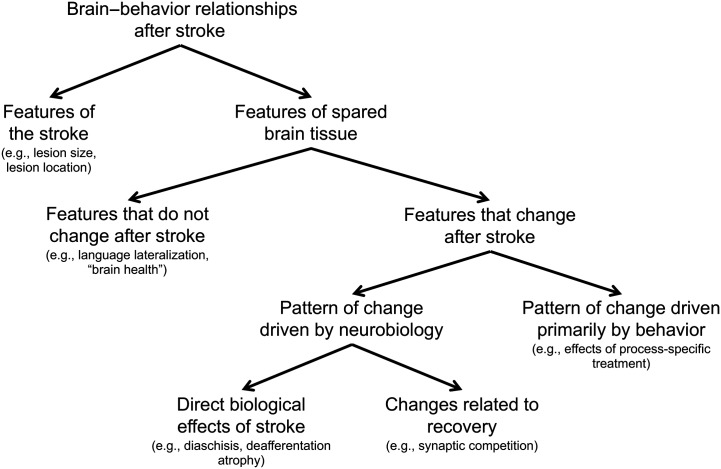
I divide brain factors related to outcome into two groups—features of the stroke itself and features of the brain tissue not directly damaged by the stroke, hereafter referred to as “spared” brain tissue (see Figure 2). When conducting brain imaging studies of stroke survivors, different methods are often used to examine the lesion (e.g., lesion–symptom mapping) and spared brain tissue (e.g., functional magnetic resonance imaging [fMRI]), so this division may help investigators to develop hypotheses depending on the approach of their study. Moreover, this division borrows from other reviews of factors relating to aphasia outcome that have differentiated between lesion-related and patient-related factors (Plowman et al., 2012; Watila & Balarabe, 2015). Instead of “patient-related factors,” this taxonomy includes “features of the spared brain tissue” because, after a stroke, brain differences associated with patient-related factors such as age, education, gender, and handedness can only be measured in brain regions with intact tissue.
Figure 2.
A taxonomy that organizes major ways that brain features can relate to behavioral outcomes from stroke.
Our consideration of brain factors related to poststroke aphasia outcome starts with features of the stroke. The stroke is the reason a person has aphasia, and as such, features of the stroke likely explain most of the variance between individuals in their language and cognitive outcomes (Price et al., 2017). Once the features of the stroke have been considered, the remainder of the explainable variance in aphasia outcomes must be accounted for by features of the spared brain. This is not to say that statistical models based on features of the stroke and the spared brain will ever explain all of the variance in aphasia outcomes across stroke survivors. Technologies for measuring the brain cannot access all relevant features that likely relate to outcomes, and some features will likely be transient and only measured in certain states (Price, Warburton, Moore, Frackowiak, & Friston, 2001), making identifying all sources of variance virtually impossible. Measurement error, along with inadequate mathematical models relating brain measures to behavior, will further preclude this. Nevertheless, any explainable variance not accounted by features of the stroke must be related to features of the spared brain tissue. Features of the spared brain tissue are further divided into those that remain unchanged after stroke and those that change after stroke. Features that change after stroke are subdivided into those for which the pattern of change is driven primarily by neurobiology and those for which the pattern of change is driven primarily by behavior.
A limitation of proposing a taxonomy such as this is that binary divisions oversimplify complex interactions, for instance, between injured and spared brain tissue or between behavioral experience and neurobiology. Notably, dividing the brain into lesioned and spared tissue is an oversimplification as lesions are known to cause anatomical knock-on effects and distant dysfunction in brain structures spared by the stroke (Carrera & Tononi, 2014). One could reasonably argue that no such division is necessary and that most relevant stroke features (e.g., size, location) could equivalently be described in the inverse as features of spared brain tissue (e.g., size of spared brain tissue, location of spared brain tissue). As most researchers and clinicians are in the habit of considering how features of the stroke relate to outcomes and measurement tools are often different for the lesioned and spared tissue, I think the practical benefits of dividing the brain into lesioned versus spared tissue outweigh the ambiguities resulting from this division. Another important caveat is that changes in spared tissue that are related to recovery likely all result from interactions between neurobiology and the environment, most notably the behavioral experience of the stroke survivor (Jones et al., 2009). Nevertheless, making a binary division between brain changes in which the pattern of change is primarily driven by neurobiology versus those primarily driven by behavior facilitates predictions regarding the location and timing of brain changes.
Features of the Stroke That Contribute to Aphasia Outcomes
As noted above, features of the stroke are likely to be the most important brain factors for behavioral outcomes, including aphasia. While lesion-related factors in prior reviews have included both brain measures (e.g., lesion size) and behavioral measures (e.g., initial aphasia severity), I will focus solely on brain measures. Behavioral predictors such as initial severity are themselves the product of a combination of brain features, including both features of the lesion and features of the spared brain related to resilience or vulnerability to deficits, and so considering their relationship to outcomes will not help us to understand brain–behavior relationships after stroke. Certain stroke features relate to broad functional outcomes, including whether the stroke is ischemic or hemorrhagic (Paolucci et al., 2003) and the volume of intraventricular hemorrhage when present (Tuhrim, Horowitz, Sacher, & Godbold, 1999). These and other stroke features may relate to aphasia outcome, but I will focus on three main features of strokes well known to impact aphasia outcomes: lesion size, lesion location, and white matter involvement.
Lesion Size
The first attribute of the stroke to consider is the size of the lesion. Lesion size is an important determiner of outcome for at least two reasons. First, many complex behaviors are performed by large-scale networks in which information flows through pathways that are widely distributed in the brain (Bressler & Menon, 2010). A small lesion may cause only limited disruption of information flow through the network, whereas a large lesion will cause more profound disruption of the overall network architecture, leading to greater behavioral deficits (Thye & Mirman, 2018). Second, even when a behavior relies on only a single small brain region, across the population of stroke survivors, stroke size will often still relate to outcomes in the behavior. This is simply because the larger a stroke, the more likely that any individual region in question is damaged. As a result, across a broad range of behaviors, lesion size is related to outcome. In one study, we examined 20 different scores of widely varied language functions (e.g., auditory sentence comprehension, reading, verbal fluency, picture naming, trails) and found that lesion size significantly related to 15 of them, explaining up to 47% of the variance in individual scores (DeMarco & Turkeltaub, 2018b).
Lesion Location
The second stroke attribute to consider is the location of the lesion (Price et al., 2017). Because many, perhaps all, behaviors rely at least to some degree on specific brain structures, two individuals with lesions of identical size may have mutually exclusive deficits if the lesions affect different brain regions. A canonical example of this is the dissociation between the language deficits typically faced by individuals with anterior versus posterior lesions. One individual may have a large anterior lesion and have Broca's aphasia as a result, whereas another individual may have the same size of the lesion in posterior cortex and have Wernicke's aphasia. Although these two individuals have lesions of the same size, their outcomes, in terms of the specific difficulties they face, are quite different. The relative importance of lesion size versus location to outcome depends largely on the degree to which a function relies on a distributed network of brain structures versus individual structures (Thye & Mirman, 2018). In some cases, lesion location may be more important than size. For example, upper extremity hemiparesis outcomes can be predicted with relatively high accuracy by measuring the degree to which the lesion overlaps with the corticospinal tract, and lesion size does not provide any additional predictive value (Feng et al., 2015). In our work, lesion size has been unrelated to certain deficits, such as depression symptoms or loss of phonotactic knowledge, but lesion–symptom mapping has revealed specific lesion locations associated with these problems, dorsolateral prefrontal cortex in the case of depression (Grajny et al., 2016), and posterior parietal cortex in the case of phonotactic knowledge (Ghaleh et al., 2018). However, even for processes that rely on widely distributed brain networks, some specific gray matter structures may serve as hubs, in that they are connected to many different parts of the network, making them pivotal to the flow of information through the network. Lesions to these structures may cause greater deficits than lesions to other regions (Gleichgerrcht et al., 2016; Warren et al., 2014). For example, we recently demonstrated that virtual lesions using transcranial magnetic stimulation (TMS) cause greater deficits in a working memory task when applied to hub regions as compared with nearby nonhub regions in the same gyrus (Lynch et al., 2018).
White Matter Involvement
A specific consideration regarding lesion location is the degree to which lesions disrupt white matter pathways. White matter damage has long been recognized as a key determinant of outcomes for certain language functions such as severe nonfluency (Naeser & Palumbo, 1994; Naeser, Palumbo, Helm-Estabrooks, Stiassny-Eder, & Albert, 1989). Outcomes for behaviors relying on distributed brain networks may depend on overall white matter lesion burden, whereas outcomes for behaviors that rely on specific white matter tracts relate to the degree of damage to those specific tracts. Recent studies have demonstrated that white matter damage that disrupts global network architecture relates to broad aphasia outcome measures such as overall severity (Marebwa et al., 2017). In our work, we have found that damage in several specific white matter tracts connecting gray matter regions activated during picture naming in control subjects is associated with naming outcomes independent of overall lesion size, whereas damage in the gray matter structures themselves is not (Xing et al., 2018). As predicted by dual stream models, damage in ventral pathways is associated with semantic deficits, whereas damage in dorsal tracts is associated with phonological deficits, both in our work and in others' (Kümmerer et al., 2013; Xing et al., 2018). We have also demonstrated that temporal lobe white matter damage relates to auditory comprehension outcomes, with anterior temporal white matter especially important for word-level comprehension and posterior white matter especially important for sentence-level comprehension (Xing, Lacey, Skipper-Kallal, Zeng, & Turkeltaub, 2017).
Features of Spared Brain Tissue That Contribute to Aphasia Outcomes
As noted above, after accounting for features of the stroke itself, which likely explain most of the variance in outcomes, remaining explainable variance in aphasia outcomes must relate to features of the spared brain tissue. Given the expected outcome based on the severity of an individual's stroke, features of the spared brain tissue determine whether an individual has a better-than-expected or worse-than-expected outcome. I have divided these brain–behavior relationships into two categories, brain features that do not change after the stroke and those that do. The first category encompasses relatively static features that were in place at the time of the stroke that render a person more or less prone to severe deficits from the stroke or more or less able to relearn or compensate after the stroke. The second category, features of spared brain tissue that change after stroke, includes changes that occur as a direct biological effect of the stroke and changes related to recovery.
These two classes of brain features (those that change after stroke and those that do not) can be differentiated using longitudinal measurements or by testing for differences between stroke survivors and matched control groups. A longitudinal change after the stroke or a difference between groups in spared brain tissue suggests that the brain feature changes after the stroke. A relationship between stroke size or location and features of the spared brain tissue also suggests a change in that tissue related to the stroke. The absence of these effects, especially if similar brain–behavior relationships can be demonstrated both in patients and controls, suggests an unchanged feature related to resilience rather than recovery (Pani, Zheng, Wang, Norton, & Schlaug, 2016). While longitudinal measures are needed to confirm that changes have occurred after the stroke and to measure the timing of these changes, the logistical difficulties and extra resources required for longitudinal research often limit the number of participants that can be enrolled, reducing the power available to examine individual differences in the brain that relate to outcomes. Therefore, for many research groups, cross-sectional comparisons between stroke survivors and controls with large sample sizes may be advantageous to address questions about individual differences in spared brain features contributing to outcomes.
Features of Spared Brain Tissue That Do Not Change After Stroke
Several attributes of brains that do not change after stroke make a person resilient or vulnerable to deficits, or more or less able to relearn or compensate after stroke. Prior brain injury from subclinical strokes, microbleeds, and leukoaraiosis (i.e., microvascular white matter injury) portends poor outcomes from further neurologic insults in general (Gardener, Wright, Rundek, & Sacco, 2015) and for poststroke aphasia specifically (Wright et al., 2018; Yarnell et al., 1976). Perhaps not surprisingly, pre-existing dementia at the time of stroke is also associated with poor long-term cognitive outcomes (Hénon et al., 1997).
In addition to so-called brain health factors such as these, features of brain organization prior to stroke may impact the severity of deficits experienced. The most prominent such feature is native lateralization of language processes. Although the vast majority of right-handed people and the majority of left-handed people are left-lateralized for language (Knecht et al., 2000; Szaflarski et al., 2002), there are individual differences in the degree of lateralization even among right-handed people (Szaflarski, Holland, Schmithorst, & Byars, 2006). Virtual lesion studies using TMS demonstrate that the severity of language deficits from left hemisphere disruption depends on the degree of native lateralization (Knecht et al., 2002). Prior studies have not clearly implicated handedness as a factor in aphasia outcome at the population level, but this may be because left-handers represent a small part of the population and most of these individuals are left-lateralized for language, so very large populations and more sensitive language measures than have been used previously might be needed to find effects of handedness on aphasia outcomes (Pedersen, Jørgensen, Nakayama, Raaschou, & Olsen, 1995). Measuring premorbid language lateralization directly would likely be more fruitful if it were possible to obtain lateralization measurements prior to stroke. A more feasible approach is to measure right hemisphere structures shortly after stroke, before any anatomical remodeling takes place. Indeed, prior work has demonstrated that right arcuate fasciculus volume measured shortly after stroke relates to long-term aphasia outcomes (Forkel et al., 2014).
Subtler differences in brain organization may also affect resilience to stroke deficits, although this is less clear at present. For example, there are individual differences in reading strategies employed by children as they go through schooling, for example, relative use of lexical versus sublexical reading strategies (Baron, 1979). Differences in strategies such as these relate to subtle differences in the architecture of networks utilized for reading (Graves et al., 2014; Hoffman, Lambon Ralph, & Woollams, 2015) that may (speculatively) lead to differences in resilience to strokes that damage structures more important for one strategy or another. Similarly, overall higher level of education may lead to richer representations, particularly for orthography, leading to resilience of representational access in the face of brain lesions (González-Fernández et al., 2011). Relatedly, individual differences in brain networks supporting learning and memory could impact one's ability to benefit from behavioral aphasia treatments. These differences could reflect personal traits predating the stroke or could relate to deficits incurred by the stroke (Meinzer et al., 2010), in which case they might best be considered a feature of the stroke itself.
Features of the Spared Brain That Change After Stroke
Many studies, including our own, have provided evidence for changes in the structure and function of spared brain regions after a stroke that relate to behavioral outcomes (Saur et al., 2006; Skipper-Kallal, Lacey, Xing, & Turkeltaub, 2017a, 2017b; Turkeltaub, Messing, Norise, & Hamilton, 2011; Xing et al., 2016; for reviews, see Hartwigsen & Saur, 2019). Often, the nature of such changes are discussed in descriptive terms based on location (e.g., right hemisphere vs. perilesional) or their relationship to behavior (e.g., compensatory, inefficient, maladaptive), sometimes with heuristics for the preferred pattern of activity based on the severity of the stroke (Anglade, Thiel, & Ansaldo, 2014; Hamilton et al., 2011; Heiss & Thiel, 2006). Such descriptions are informative but fall short of a mechanistic understanding of why such patterns arise (Ward, 2017). When specific mechanisms have been proposed, they have often not thoroughly considered other competing hypotheses that might also explain similar patterns of brain change (Heiss & Thiel, 2006). Research in animal models of stroke have demonstrated many different changes that occur in spared brain tissue, including subcellular changes, synaptic plasticity, changes in dendritic arborization, axonal degeneration, axonal sprouting, and others, all of which can contribute to changes in functional maps (Murphy & Corbett, 2009). By considering the specific mechanisms involved in brain changes after stroke, one can develop predictions regarding the expected pattern of changes at the scale measurable by human neuroimaging. Specifically, different mechanisms of change predict different patterns in the type of brain changes (e.g., structure, function, connectivity, properties of network organization), the relationship of brain changes to stroke size and location in the case of biologically driven changes, the relationship to deficits and spared abilities in the case of behaviorally driven changes, the timing of changes in relation to the stroke or to behavioral interventions, and the relationship between observed brain changes and behavioral outcomes. Such mechanistic hypotheses will facilitate the design of experiments capable of providing more specific conclusions regarding the brain basis of aphasia recovery.
Although most brain changes after stroke derive from interactions between biological mechanisms of neural plasticity and the behavioral experience of the stroke survivor (Jones et al., 2009), for the sake of developing specific experimental hypotheses, it is useful to divide these changes into those in which the pattern of change is primarily driven by neurobiology and those in which the pattern of change is primarily driven by behavior.
It is important to note that the specific examples provided below should be viewed as hypotheses regarding how specific mechanisms might result in specific spatiotemporal patterns of brain change measurable in stroke survivors. To the extent possible, I will provide examples from our research or the literature of findings that might correspond to each mechanism of change, but further research will be needed to test whether the exact predicted patterns arise in humans and how important they are for aphasia outcomes. Furthermore, I intend the examples below to illustrate the ways that biological and behavioral mechanisms of change might lead to specific patterns of results, but I do not intend to provide an exhaustive list of all of the possible mechanisms of brain change that contribute to aphasia recovery.
Brain Changes Primarily Driven by Neurobiology
Brain changes primarily driven by neurobiology are those for which the neurobiological mechanism provides the main constraints on the pattern of changes in terms of timing, location, and relationship to behavioral outcome (see Table 1). As such, this type of brain change likely occurs in regions with a systematic relationship to the location of stroke, for example, regions that are directly anatomically connected to the lesioned area, regions that project to the same axonal targets as the lesioned area, or regions that form functional networks with the lesioned area. The exact biological mechanism of change determines the nature of this relationship and also the expected timing of these effects. For example, electrophysiological phenomena such as disinhibition should occur immediately, synaptic plasticity should lead to rapid but not immediate changes, and more substantial anatomical remodeling may result in brain changes over weeks or more. Some changes after stroke occur as a direct biological consequence of the stroke on spared brain tissue. These changes could reasonably be considered features of the lesion itself, but since they are measured in the spared brain tissue, I consider them here instead. The other group of changes I consider here are those associated with recovery, that is, mechanisms of neural plasticity.
Table 1.
Example neurobiological mechanisms of change after stroke and hypothesized spatiotemporal patterns observable in human neuroimaging experiments.
| Mechanism | Location | Timing | Relationship to behavior |
|---|---|---|---|
| Brain changes occurring as a direct biological effect of the lesion | |||
| Maladaptive disinhibition | Region that is directly inhibited by a lesioned tissue, typically the homotopic region of the right hemisphere | Immediate | Impedes performance on behavior performed by a perilesional tissue |
| Deafferentation atrophy | Structure that receives axonal projections from the lesioned structure | Delayed, gradual | Atrophy may correspond with reduced performance for behavior served by the atrophied structure |
| Diaschisis | Regions that form a functional network with a lesioned tissue | Immediate | Dysfunction in the network impedes behavior performed by the network |
| Brain changes related to recovery | |||
| Axonal collateral sprouting | Region that shares axonal targets with the lesioned tissue | Delayed, gradual | Compensates for behavior served by the lesioned tissue, effective to the degree that the new region is similar to the original region in computations and connectivity |
| Engagement of secondary processors due to synaptic plasticity | Network that is already involved in the behavior to some degree or was involved early in development | Early, gradual | Compensates for behavior, likely to be less efficient than the lesioned network was prior to stroke |
Brain Changes Occurring as a Direct Biological Effect of the Lesion
The brain is composed of networks of interconnected and interdependent brain structures, so a stroke to one structure necessarily has effects on other spared structures. Most such indirect effects of the stroke are thought to cause dysfunction of spared brain regions, and so these changes are typically negatively associated with outcomes. Perhaps the most widely discussed example of this category of brain change is the maladaptive release of inter-hemispheric inhibition, a popular theory that guides many brain stimulation treatments for aphasia (Turkeltaub, 2015). In the motor system, transcallosal inhibitory connections between the hemispheres may help to coordinate bimanual movement (Daffertshofer, Peper, & Beek, 2005). After stroke, the interhemispheric inhibitory balance is disrupted and the uninjured motor cortex inhibits the injured side (Rehme, Eickhoff, Wang, Fink, & Grefkes, 2011; Takeuchi & Izumi, 2012), contributing to deficits (Duque et al., 2005; Murase, Duque, Mazzocchio, & Cohen, 2004). If this mechanism also occurs in the language network, one would expect that a stroke to a left hemisphere structure important for language would cause an immediate increase in the activity of the corresponding structure in the right hemisphere and that this increased activity would correspond with poorer performance in a language function performed by the injured left hemisphere tissue. TMS studies have indeed demonstrated that a “virtual lesion” of the left inferior frontal cortex results in immediate increases in activity in the corresponding cortex of the right hemisphere (Thiel et al., 2006), although this increased activity is actually protective against behavioral consequences of the virtual lesion, counter to the theory suggesting such activity should have negative behavioral consequences (Hartwigsen et al., 2013). A prominent longitudinal imaging study demonstrated that right hemisphere language-related brain activity does not arise immediately after a stroke as would be predicted by a direct physiological mechanism such as disinhibition but rather is maximal weeks after the stroke, suggesting a change that occurs more slowly over time (Saur et al., 2006). In a single case, we found that the behavioral benefit of right frontal TMS inhibition for picture naming was unrelated to the level of brain activity at the site of stimulation, that such inhibition did not result in increased activity in the corresponding perilesional left frontal area, and that a subsequent right hemisphere stroke resulted in worsening of aphasia, all findings that fail to support predictions of the theory of maladaptive interhemispheric inhibition (Turkeltaub et al., 2012). Subsequent functional neuroimaging studies from our lab have also failed to find patterns predicted by this theory (Skipper-Kallal et al., 2017a, 2017b). These results raise doubts about whether maladaptive interhemispheric inhibition plays a significant role in aphasia outcomes. The critical point for the purposes of this discussion though is that specific mechanistic theories such as this one lead to specific testable predictions regarding the pattern of expected brain changes in terms of the timing, location, and relationship to behavior. This allows the opportunity to support or disprove these hypotheses, or to alter them to accommodate new scientific evidence. Without such clear hypotheses, the interpretation of results can only be descriptive.
Another example of a change in spared brain tissue that occurs as a direct result of the stroke is atrophy caused by deafferentation of intact brain regions. Atrophy by this mechanism should be limited to structures that receive axonal projections lost due to the lesion, and the behavioral consequences should relate to the role of the atrophied structure in the behavior of interest. For example, structural disconnection of Broca's area is associated with impairment of naming independent of the degree of direct stroke damage to this area (Bonilha, Rorden, & Fridriksson, 2014).
Similarly, a lesion can disrupt information flow through a functional network, causing dysfunction in anatomically intact parts of the network. This phenomenon, referred to as diaschisis, has been recognized for over 100 years (Feeney & Baron, 1986), although the term has sometimes been used for other related phenomena, including structural disconnections (Carrera & Tononi, 2014). Brain changes due to diaschisis are expected in regions functionally connected to the lesion, whether or not they have direct anatomical connections, and decreased functioning should be associated with behavioral deficits. Diaschisis was originally thought to resolve relatively early after stroke, but metabolic imaging studies have demonstrated long-lasting hypometabolism in regions functionally connected to the lesion, even if multiple neurons and synapses intervene between the regions. For instance, years after cerebellar stroke, contralateral cerebral regions that are indirectly connected to the cerebellum via the thalamus demonstrate hypometabolism that relates to functional outcomes (Sobesky et al., 2005). A series of recent studies have demonstrated that poorly localizing behavioral syndromes can be understood by assuming widespread dysfunction in brain regions that form a functional network with the lesioned tissue (Fox, 2018). We have recently developed a new method, termed functional anomaly mapping, that uses resting fMRI data to identify dysfunctional tissue in individual stroke survivors both at the site of the anatomical lesion and in the anatomically spared parts of the brain (DeMarco & Turkeltaub, 2018a). Years after stroke, this technique identifies dysfunction in right hemisphere sites homotopic to the left hemisphere lesion, likely reflecting transcallosal diaschisis (see Figure 3). Furthermore, right cerebellar dysfunction as measured by these maps relates to speech fluency outcomes (DeMarco & Turkeltaub, 2018a), in accordance with prior findings demonstrating right cerebellar hypometabolism in stroke survivors with nonfluent aphasia (Metter et al., 1987).
Figure 3.
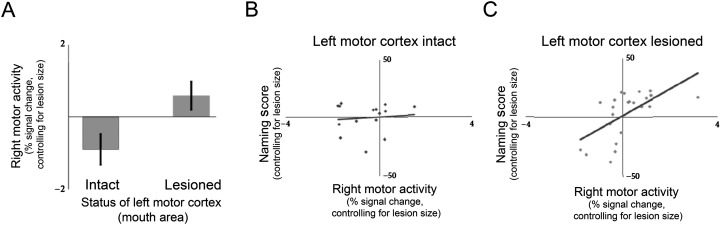
Example of results suggestive of axonal collateral sprouting (adaptedfrom Skipper-Kallal et al., 2017b). Thirty-nine individuals with chronic left hemisphere stroke were tested using the Philadelphia Naming Test and underwent functional magnetic resonance imaging scanning during a delayed response naming task. The group was divided into those with lesions involving the mouth area of motor cortex in the left hemisphere and those with lesions not involving that region. Because the mouth areas of motor cortex in both hemispheres share axonal targets in the brainstem, the principles of axonal collateral sprouting predict the pattern of findings: (A) The mouth area of motor cortex in the right hemisphere was active only when the left mouth area of motor cortex was lesioned, and (B, C) the activity in the right mouth area of motor cortex related to naming ability only when left motor cortex was lesioned.
Brain Changes Associated With Recovery
Brain changes associated with recovery-related neuroplasticity are often constrained by neurobiology, resulting in patterns of changes that are systematically related to the stroke in time and also in location within the brain. One important mechanism of brain change in this category is axonal collateral sprouting due to synaptic competition (Carmichael, 2003). Throughout the central and peripheral nervous systems, neurons that send axons to the same targets compete for synapses. When one of these neurons dies, the others that project to the same target sprout new axonal branches that take over denervated synapses (Edds, 1953). In rat models of stroke, enhancing axonal sprouting in the hemisphere opposite the stroke increases re-innervation of subcortical targets, improving behavioral performance with the impaired limb (Chen, Goldberg, Kolb, Lanser, & Benowitz, 2002; Zai et al., 2009), demonstrating that this mechanism of plasticity is likely to be important for functional outcomes after stroke. In contrast to the immediate changes expected by direct disinhibition described above, brain changes related to axonal collateral sprouting are likely to occur over a longer period of time, given that anatomical remodeling is required (Carmichael, 2003). Whereas direct disinhibition should result in increased activity in regions directly connected to the lesioned area, increased activity or other changes in brain features related to axonal sprouting should occur in regions with cell bodies that share axonal targets with lesioned neurons. These neurons in effect “take over” for the lesioned neurons in the network and are likely to produce positive behavioral outcomes to the degree that their connectivity and computational properties are similar to the lesioned neurons (Ward, 2017). The network can likely never be as effective as it was prior to injury, however, because the postsynaptic neurons at the axonal target are controlled by a smaller number of neurons than they were prior to injury. This results in coarse coordination of activity, a phenomenon that is easily demonstrated in the peripheral nervous system as enlarged and irregular motor unit action potentials on electromyography after peripheral nerve damage and subsequent reinnervation via axonal sprouting (Krarup, Boeckstyns, Ibsen, Moldovan, & Archibald, 2016).
This mechanism of plasticity could theoretically account for well-known patterns observed in aphasia neuroimaging studies. For example, one might expect that nearby cortical regions share axonal targets, so perilesional neurons with similar axonal targets to nearby lesioned neurons are able take over denervated synapses at those targets, resulting in increased activity in perilesional cortex. This mechanistic explanation for perilesional recruitment leads to specific testable hypotheses, for instance, that perilesional regions can effectively compensate for nearby lesioned tissue only to the degree that they share axonal targets. The degree to which two structures share axonal targets could potentially be measurable as shared patterns of structural connectivity. A more generalized version of this hypothesis is that effective behavioral compensation after a lesion to a given cortical processor will occur in regions that have similar structural connectivity patterns to the injured processor.
As in the animal models noted above, contralesional recruitment after stroke may result from competition for shared subcortical axonal targets. For example, we have found that, in a cohort of left hemisphere stroke survivors, only those with damage to the mouth area of left motor cortex activate the corresponding motor area in the right hemisphere during picture naming and only in those individuals does the level of right motor activity relate to naming performance (see Figure 4; Skipper-Kallal et al., 2017b). This pattern is consistent with that expected by axonal collateral sprouting, given the bilateral projections from the mouth area of motor cortex to the brainstem (Triggs, Ghacibeh, Springer, & Bowers, 2005).
Figure 4.
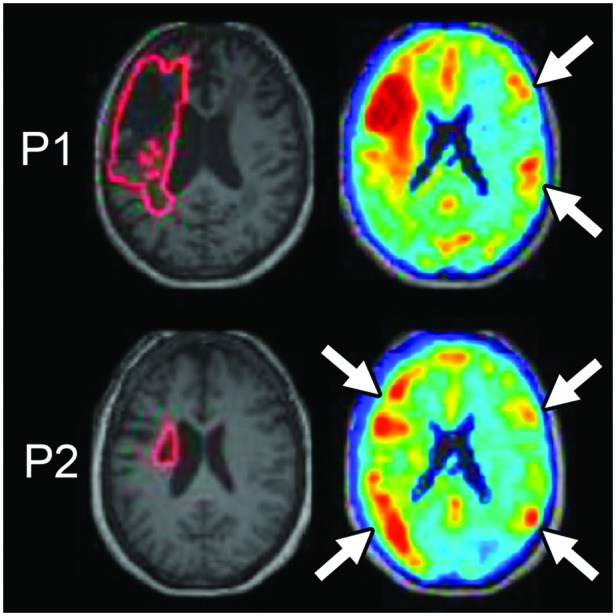
Functional anomaly maps demonstrate abnormal function distant from lesions, consistent with diaschisis (adapted from DeMarco & Turkeltaub, 2018a). Machine learning analysis of resting-state functional magnetic resonance imaging data is used to grade the degree to which spontaneous brain activity differs in individual stroke survivors from a group of controls. Maps are shown for two individuals (P1 and P2). P1 has a large cortical lesion, and dysfunction is identified at the site of the anatomical lesion and also in regions opposite the stroke in the right hemisphere (white arrows). P2 has a subcortical lesion with widespread cortical dysfunction in the lesioned hemisphere and in locations of the right hemisphere opposite areas of prominent left hemisphere dysfunction (white arrows).
More broadly, any mechanism of plasticity that results in synaptic modifications (Abbott & Nelson, 2000), including axonal sprouting, could result in increased activity in spared networks already involved to some degree in a behavior. For example, right hemisphere brain regions homotopic to the left hemisphere language network are often activated to some degree by language tasks (Just, Carpenter, Keller, Eddy, & Thulborn, 1996). These regions may play a minor role in language functions in everyone (Hartwigsen et al., 2010), perhaps as developmental remnants of early bilateral language networks (Newport et al., 2017). When a stroke damages the left hemisphere language network, synaptic plasticity in the spared alternate pathway might result in increased activity, enhanced connectivity, and possibly structural hypertrophy. These changes should occur throughout the network, not just in regions that are directly anatomically connected to the lesioned area or share axonal targets with it. In the case of alternate right hemisphere networks, recruitment is most likely to result in positive outcomes for language processes that are less strongly lateralized, such as auditory comprehension (Hickok & Poeppel, 2007).
These examples demonstrate how considering biological mechanisms of recovery can lead to hypotheses regarding specific patterns of change expected at the region and network level in humans. In general, neurobiologically driven changes should occur in structures or networks that have a systematic relationship to the lesion, with the nature of the relationship determined by the biological mechanism. The nature and timing of these changes is expected to be determined by the mechanism, ranging from immediate electrophysiological effects to slower anatomical remodeling.
Brain Changes Primarily Driven by Behavior
In this category, I include brain changes for which spatiotemporal patterns are primarily determined by the nature of behavioral experiences, rather than by neurobiological constraints. Of course, behaviorally driven effects must ultimately occur through neurobiological mechanisms, and many of the neurobiological mechanisms described above are shaped by behavioral experience, so in a sense, these are not discrete categories. Nevertheless, considering them separately may help to clarify the possible origins of observed brain changes and the experiments and analyses needed to disambiguate different types of changes. For example, a clear hypothesis is that brain changes primarily driven by behavior will occur in brain structures or networks capable of playing a role in the behavior in question. In many cases, this hypothesis will produce different predictions than any of the neurobiologically driven mechanisms described above. Behaviorally driven brain changes may still be constrained by the anatomical distribution of the stroke (Meinzer et al., 2010), although it seems likely that these constraints may, strictly speaking, relate more strongly to the profile of deficits and spared abilities than to the stroke anatomy. The timing of brain changes will correspond to the behavioral experience driving the change, with rapid effects due to priming measurable as early changes in brain activity (Nardo, Holland, Leff, Price, & Crinion, 2017) and slower effects related to anatomical remodeling observable in various brain measures (Jones et al., 2009).
The most obvious example of a behaviorally driven brain change is the effect of behavioral treatment for aphasia on the brain. Here, although treatment-induced changes occur through similar neurobiological mechanisms as described above, the nature of the behavioral training, likely interacting with the individual's capacity to relearn, should principally determine the brain networks that change in response to treatment (see Crinion & Leff, 2015, for a review of recent studies). For example, alongside domain general effects of any behavioral treatment on the brain and with the caveat that behavioral treatments rarely if ever isolate a single language or cognitive process without stimulating others at all, one might expect that treatments targeting phonology should primarily impact brain networks capable of supporting phonology, whereas treatments targeting semantics should primarily impact brain networks capable of supporting semantics. Dissociations such as this have been suggested in small studies (van Hees, McMahon, Angwin, de Zubicaray, & Copland, 2014), but larger studies contrasting different types of behavioral treatment are needed to confirm this hypothesis more broadly. Another straightforward prediction regarding treatment-induced brain changes is that the magnitude of change might be expected to relate to the intensity and duration of treatment.
Other behaviorally driven brain changes may occur as a consequence of behavioral adaptations resulting from living with deficits caused by stroke. For example, difficulties communicating through oral language may provoke use of compensatory strategies with or without formal training to do so. These compensatory strategies, if engaged repeatedly over a period of time, may result in long-lasting changes in the brain networks supporting them. We recently demonstrated that, in a region of the right temporoparietal cortex, gray matter density was greater in left hemisphere stroke survivors with aphasia than in either control participants or left hemisphere survivors without history of aphasia (Xing et al., 2016). The gray matter density in this region related to speech production outcomes as well as verbal working memory after accounting for effects of stroke size and location on these abilities. Although further research would be needed to confirm the mechanism underlying these findings, the pattern might suggest a structural change driven by behavior rather than neurobiology, given that the change occurred across a diverse group irrespective of stroke size and location. In an unselected sample with varied histories of behavioral aphasia therapy, this type of change most likely derived from a compensatory overreliance on a function performed by right temporoparietal cortex. Similarly, attempting to communicate with aphasia may place additional strain on domain general cognitive systems, resulting in changes in these networks (Geranmayeh, Brownsett, & Wise, 2014).
Conversely, diminished use of oral language for communication could result in atrophy of language networks, similar to learned non-use of an impaired limb (Pulvermüller et al., 2001; Taub, Uswatte, Mark, & Morris, 2006). Less discussed in the brain imaging literature on aphasia are the potential consequences of reduced life participation caused by aphasia. Aphasia often reduces social engagement, resulting in fewer opportunities for communication (Dalemans, De Witte, Beurskens, Van Den Heuvel, & Wade, 2010). This deprivation could result in secondary atrophy of both language networks and networks supporting compensatory communication strategies, as well as changes in other networks supporting other social and cognitive functions. If individuals are environmentally deprived but then achieve improvements in functional communication and life participation as a result of either spontaneous recovery or treatment, the potential secondary effects of these successes on brain networks must also be considered when examining brain data. Ultimately, it may be very difficult to tease apart the many potential sources of brain changes in the context of most experiments, but these potential sources of change should be considered alongside more traditional hypotheses regarding the neurobiology of recovery, especially when the pattern of results is unexpected or nonspecific. Studies using detailed measures of language functions as well as daily life behavior in very large numbers of people with aphasia may be needed to tease apart various potential behavioral sources of brain differences after stroke.
A final important type of behaviorally induced effect in people with aphasia is an artifactual change in brain activity related to increased effort during performance of functional neuroimaging tasks. Activity in fMRI and positron emission tomography (PET) studies scales with the effort put toward task performance (Just et al., 1996), and so some changes in brain activity measured in people with aphasia may simply reflect differences in the effort required to perform the task, rather than real changes in brain organization (Wilson, Yen, & Eriksson, 2018). This phenomenon could result in increased activity for people with aphasia compared to control participants or could even result in longitudinal changes in brain activity for individual stroke survivors. For example, in the context of targeted therapies, initial improvement may be achieved effortfully, with associated patterns of activity in brain regions associated with domain general processes, whereas longer term gains or overlearning may be associated with reductions of this activity (DeMarco, Wilson, Rising, Rapcsak, & Beeson, 2018; Kurland et al., 2008). Such reductions in activity after aphasia treatment are typically interpreted as reflecting increased neuronal efficiency (e.g., Nardo et al., 2017) but might instead occur as an epiphenomenon of improved abilities due to correspondingly reduced effort needed to perform the task in the scanner. In our own research, we recently showed that right hemisphere picture-naming activity relates to lesion size in people with left hemisphere strokes, such that people with the largest lesions have the most right hemisphere activity (Skipper-Kallal et al., 2017b). While it is tempting to suggest that this relationship demonstrates the often-suggested increased reliance on the right hemisphere by people with large lesions (Anglade et al., 2014; Heiss & Thiel, 2006), we also identified the same relationship between lesion size and activity in the bilateral visual cortex. This suggests that increased effort for task performance by people with severe aphasia, also marked by longer looking times at the pictures, might explain the relationship between lesion size and activity. Use of tasks that adapt their difficulty to participant performance (Wilson et al., 2018) or use of effort-independent measures such as gray matter structure (Hope et al., 2017; Xing et al., 2016) can address this issue. Task-free measures of brain structure and connectivity have the added benefit of allowing the simultaneous examination of brain–behavior relationships across a variety of specific behavioral outcome measures. In contrast, task-related fMRI requires use of specific tasks, placing practical limits on the number of behaviors one can examine during a typical imaging session. Furthermore, findings from these studies may not generalize beyond the specific tasks used in the scanner.
Examining Both the Stroke and the Spared Brain Tissue
Since both the stroke and the spared brain may contribute to aphasia outcomes, how should these two factors be considered in relation to each other? Given that stroke features are clearly known to play a significant role in outcome, it seems imperative that these factors be included in any attempt to understand outcomes. Considering the features of the stroke alone provides information regarding the degree to which behaviors critically rely on injured regions despite contributions of spared brain regions to resilience and recovery. The variance in outcomes that remains unexplained by these models provides a cap on the degree to which individual differences in the spared brain can explain outcomes. Given that the stroke is the cause of the deficits, measuring features of the spared brain without considering the severity of the stroke is unlikely to lead to clearly interpretable findings.
Some researchers, including ourselves, have combined these factors using hierarchical regression approaches that first enter features of the stroke and subsequently examine features of the spared brain that explain additional variance in behavioral scores (Forkel et al., 2014; Pani et al., 2016; Skipper-Kallal et al., 2017a; Xing et al., 2016). This approach is appealing because it is simple and acknowledges the primacy of the stroke features in causing the behavioral deficits. Given that these statistical models already incorporate information about the stroke before considering features of the spared brain, they are likely biased toward relationships between spared brain features and behavior that are relatively independent of stroke features, in other words, behaviorally driven effects. Other groups have examined features of the stroke and spared brain tissue simultaneously using approaches such as joint independent components analysis (Abel, Weiller, Huber, Willmes, & Specht, 2015; Griffis, Nenert, Allendorfer, & Szaflarski, 2017), which identifies stroke features and spared brain features that co-occur. Each approach thus has value depending on the hypothesized mechanism by which spared brain features contribute to behavior.
An additional consideration is the likelihood that behavioral outcomes may depend on multiple interacting brain features simultaneously, for instance, the presence of a lesion in region w, structural connectivity between regions x and y, and activity in region z. Use of multivariate techniques capable of considering multiple brain regions and imaging modalities simultaneously has already proven useful in understanding the relationship between brain development and cognition (Erus et al., 2015) and may likewise be useful in aphasia research (Pustina et al., 2017).
Recommendations
While describing the taxonomy above, I have implied several recommendations for conducting research on the brain basis of aphasia outcomes. For the sake of clarity, I will restate the main recommendations below:
Examine specific behaviors as outcome measures rather than overall aphasia severity.
Consider the different types of brain–behavior relationships potentially contributing to the outcome and generate specific mechanistic hypotheses to test.
Ensure the study design (e.g., longitudinal vs. cross-sectional, observational vs. treatment) and brain measurements (e.g., gray matter structure, white matter tractography, functional activity) are chosen to test the hypotheses.
The lesion is why a person has aphasia, so always examine the role of the lesion in determining the outcome. Consider features of the lesion commonly associated with outcomes, including lesion size, lesion location, and involvement of key white matter tracts.
Examine the relationship of spared brain features to outcomes in the context of the lesion. Ask whether features of spared brain tissue relate to better- or worse-than-expected outcomes given the severity of the lesion.
Examine the pattern of findings in spared brain tissue to identify the type of brain–behavior relationship. Consider whether the brain feature relates to the same behavior in typical populations, whether there was a change in the feature after the stroke, the timing of changes in longitudinal studies, and the relationship between the changes and the features of the lesion.
Consider how effort might differ between individuals and how this might impact functional neuroimaging results. Use adaptive tasks or non–effort-dependent brain measures of brain structure and connectivity to circumvent this issue.
Given that multiple, different brain features relate to outcomes simultaneously, account for multiple brain features in statistical analyses, using techniques such as hierarchical regression or multivariate analyses.
Conclusions
Understanding the brain factors that result in varied language and cognitive outcomes from stroke is of great clinical and scientific importance. A rapidly growing body of research has contributed to our expanding knowledge of these brain–behavior relationships, but prior studies have generally not tested specific hypotheses regarding the mechanisms by which brain features contribute to behavioral outcomes. The brain basis of aphasia outcomes is complex and multifactorial. It is vital for investigators to consider the many ways that features of the stroke and the spared brain tissue might contribute to behavioral outcomes. Given the number of potential factors contributing to outcomes, organizing the various hypothesized brain–behavior relationships as I have here and considering the mechanisms that drive these relationships may help investigators develop specific experimental designs and more complete statistical models to explain language and cognitive abilities after stroke.
Acknowledgments
This review article stems from the 2018 Research Symposium at ASHA Convention, which was supported by the National Institute on Deafness and Other Communication Disorders under Award R13DC003383. Research reported in this publication was supported by the National Institute on Deafness and Other Communication Disorders under Awards R01DC014960 and R21DC014087 and by the National Center for Clinical and Translational Science via the Georgetown Howard University Center for Clinical and Translational Science under Award KL2TR000102 to Peter Turkeltaub. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding Statement
This review article stems from the 2018 Research Symposium at ASHA Convention, which was supported by the National Institute on Deafness and Other Communication Disorders under Award R13DC003383. Research reported in this publication was supported by the National Institute on Deafness and Other Communication Disorders under Awards R01DC014960 and R21DC014087 and by the National Center for Clinical and Translational Science via the Georgetown Howard University Center for Clinical and Translational Science under Award KL2TR000102 to Peter Turkeltaub. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Abbott L. F., & Nelson S. B. (2000). Synaptic plasticity: Taming the beast. Nature Neuroscience, 3, 1178 https://doi.org/10.1038/81453 [DOI] [PubMed] [Google Scholar]
- Abel S., Weiller C., Huber W., Willmes K., & Specht K. (2015). Therapy-induced brain reorganization patterns in aphasia. Brain, 138(Pt 4), 1097–1112. https://doi.org/10.1093/brain/awv022 [DOI] [PubMed] [Google Scholar]
- Anglade C., Thiel A., & Ansaldo A. I. (2014). The complementary role of the cerebral hemispheres in recovery from aphasia after stroke: A critical review of literature. Brain Injury, 28(2), 138–145. https://doi.org/10.3109/02699052.2013.859734 [DOI] [PubMed] [Google Scholar]
- Baron J. (1979). Orthographic and word-specific mechanisms in children's reading of words. Child Development, 50(1), 60–72. [Google Scholar]
- Berthier M. L., Pulvermüller F., Dávila G., Casares N. G., & Gutiérrez A. (2011). Drug therapy of post-stroke aphasia: A review of current evidence. Neuropsychology Review, 21(3), 302–317. https://doi.org/10.1007/s11065-011-9177-7 [DOI] [PubMed] [Google Scholar]
- Bonilha L., Rorden C., & Fridriksson J. (2014). Assessing the clinical effect of residual cortical disconnection after ischemic strokes. Stroke, 45(4), 988–993. https://doi.org/10.1161/STROKEAHA.113.004137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady M. C., Kelly H., Godwin J., Enderby P., & Campbell P. (2016). Speech and language therapy for aphasia following stroke. Cochrane Database of Systematic Reviews. https://doi.org/10.1002/14651858.CD000425.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler S. L., & Menon V. (2010). Large-scale brain networks in cognition: Emerging methods and principles. Trends in Cognitive Sciences, 14(6), 277–290. https://doi.org/10.1016/j.tics.2010.04.004 [DOI] [PubMed] [Google Scholar]
- Butler R. A., Lambon Ralph M. A., & Woollams A. M. (2014). Capturing multidimensionality in stroke aphasia: Mapping principal behavioural components to neural structures. Brain, 137(12), 3248–3266. https://doi.org/10.1093/brain/awu286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael S. T. (2003). Plasticity of cortical projections after stroke. The Neuroscientist, 9(1), 64–75. https://doi.org/10.1177/1073858402239592 [DOI] [PubMed] [Google Scholar]
- Carrera E., & Tononi G. (2014). Diaschisis: Past, present, future. Brain, 137(9), 2408–2422. https://doi.org/10.1093/brain/awu101 [DOI] [PubMed] [Google Scholar]
- Chen P., Goldberg D. E., Kolb B., Lanser M., & Benowitz L. I. (2002). Inosine induces axonal rewiring and improves behavioral outcome after stroke. Proceedings of the National Academy of Sciences of the United States of America, 99(13), 9031–9036. https://doi.org/10.1073/pnas.132076299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer S. C., Procaccio V., & GAIN Americas and GAIN International Study Investigators. (2012). Correlation between genetic polymorphisms and stroke recovery: Analysis of the GAIN Americas and GAIN International Studies. European Journal of Neurology, 19(5), 718–724. https://doi.org/10.1111/j.1468-1331.2011.03615.x [DOI] [PubMed] [Google Scholar]
- Crinion J. T., & Leff A. (2015). Using functional imaging to understand therapeutic effects in poststroke aphasia. Current Opinion in Neurology, 28(4), 330–337. https://doi.org/10.1097/wco.0000000000000217 [DOI] [PubMed] [Google Scholar]
- Daffertshofer A., Peper C. L., & Beek P. J. (2005). Stabilization of bimanual coordination due to active interhemispheric inhibition: A dynamical account. Biological Cybernetics, 92(2), 101–109. https://doi.org/10.1007/s00422-004-0539-6 [DOI] [PubMed] [Google Scholar]
- Dalemans R. J., De Witte L. P., Beurskens A. J., Van Den Heuvel W. J., & Wade D. T. (2010). An investigation into the social participation of stroke survivors with aphasia. Disability and Rehabilitation, 32, 1678–1685. https://doi.org/10.3109/09638281003649938 [DOI] [PubMed] [Google Scholar]
- DeMarco A. T., & Turkeltaub P. E. (2018a). Functional anomaly mapping reveals local and distant dysfunction caused by brain lesions. bioRxiv. https://doi.org/10.1101/464248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarco A. T., & Turkeltaub P. E. (2018b). A multivariate lesion symptom mapping toolbox and examination of lesion-volume biases and correction methods in lesion-symptom mapping. Human Brain Mapping, 39(11), 4169–4182. https://doi.org/10.1002/hbm.24289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarco A. T., Wilson S. M., Rising K., Rapcsak S. Z., & Beeson P. M. (2018). The neural substrates of improved phonological processing following successful treatment in a case of phonological alexia and agraphia. Neurocase, 24(1), 31–40. https://doi.org/10.1080/13554794.2018.1428352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J., Hummel F., Celnik P., Murase N., Mazzocchio R., & Cohen L. G. (2005). Transcallosal inhibition in chronic subcortical stroke. NeuroImage, 28(4), 940–946. https://doi.org/10.1016/j.neuroimage.2005.06.033 [DOI] [PubMed] [Google Scholar]
- Edds M. V., Jr. (1953). Collateral nerve regeneration. The Quarterly Review of Biology, 28(3), 260–276. [DOI] [PubMed] [Google Scholar]
- Erus G., Battapady H., Satterthwaite T. D., Hakonarson H., Gur R. E., Davatzikos C., & Gur R. C. (2015). Imaging patterns of brain development and their relationship to cognition. Cerebral Cortex, 25(6), 1676–1684. https://doi.org/10.1093/cercor/bht425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney D. M., & Baron J. C. (1986). Diaschisis. Stroke, 17(5), 817–830. https://doi.org/10.1161/01.str.17.5.817 [DOI] [PubMed] [Google Scholar]
- Feng W., Wang J., Chhatbar P. Y., Doughty C., Landsittel D., Lioutas V.-A., … Schlaug G. (2015). Corticospinal tract lesion load: An imaging biomarker for stroke motor outcomes. Annals of Neurology, 78(6), 860–870. https://doi.org/10.1002/ana.24510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forkel S. J., Thiebaut de Schotten M., Dell'Acqua F., Kalra L., Murphy D. G., Williams S. C., & Catani M. (2014). Anatomical predictors of aphasia recovery: A tractography study of bilateral perisylvian language networks. Brain, 137(7), 2027–2039. https://doi.org/10.1093/brain/awu113 [DOI] [PubMed] [Google Scholar]
- Fox M. D. (2018). Mapping symptoms to brain networks with the human connectome. The New England Journal of Medicine, 379(23), 2237–2245. https://doi.org/10.1056/NEJMra1706158 [DOI] [PubMed] [Google Scholar]
- Fregni F., & Pascual-Leone A. (2007). Technology insight: Noninvasive brain stimulation in neurology—Perspectives on the therapeutic potential of rTMS and tDCS. Nature Clinical Practice Neurology, 3(7), 383–393. https://doi.org/10.1038/ncpneuro0530 [DOI] [PubMed] [Google Scholar]
- Fridriksson J., Bonilha L., Baker J. M., Moser D., & Rorden C. (2010). Activity in preserved left hemisphere regions predicts anomia severity in aphasia. Cerebral Cortex, 20(5), 1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici A. D. (2011). The brain basis of language processing: From structure to function. Physiological Reviews, 91(4), 1357–1392. https://doi.org/10.1152/physrev.00006.2011 [DOI] [PubMed] [Google Scholar]
- Gardener H., Wright C. B., Rundek T., & Sacco R. L. (2015). Brain health and shared risk factors for dementia and stroke. Nature Reviews Neurology, 11, 651–657. https://doi.org/10.1038/nrneurol.2015.195 [DOI] [PubMed] [Google Scholar]
- Geranmayeh F., Brownsett S. L., & Wise R. J. (2014). Task-induced brain activity in aphasic stroke patients: What is driving recovery? Brain, 137(Pt. 10), 2632–2648. https://doi.org/10.1093/brain/awu163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaleh M., Skipper-Kallal L. M., Xing S., Lacey E., DeWitt I., DeMarco A., & Turkeltaub P. E. (2018). Phonotactic processing deficit following left-hemisphere stroke. Cortex, 99, 346–357. https://doi.org/10.1016/j.cortex.2017.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleichgerrcht E., Kocher M., Nesland T., Rorden C., Fridriksson J., & Bonilha L. (2016). Preservation of structural brain network hubs is associated with less severe post-stroke aphasia. Restorative Neurology and Neuroscience, 34(1), 19–28. https://doi.org/10.3233/RNN-150511 [DOI] [PubMed] [Google Scholar]
- González-Fernández M., Davis C., Molitoris J. J., Newhart M., Leigh R., & Hillis A. E. (2011). Formal education, socioeconomic status, and the severity of aphasia after stroke. Archives of Physical Medicine and Rehabilitation, 92(11), 1809–1813. https://doi.org/10.1016/j.apmr.2011.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grajny K., Pyata H., Spiegel K., Lacey E. H., Xing S., Brophy C., & Turkeltaub P. E. (2016). Depression symptoms in chronic left hemisphere stroke are related to dorsolateral prefrontal cortex damage. The Journal of Neuropsychiatry and Clinical Neurosciences, 28, 292–298. https://doi.org/10.1176/appi.neuropsych.16010004 [DOI] [PubMed] [Google Scholar]
- Graves W. W., Binder J. R., Desai R. H., Humphries C., Stengel B. C., & Seidenberg M. S. (2014). Anatomy is strategy: Skilled reading differences associated with structural connectivity differences in the reading network. Brain and Language, 133, 1–13. https://doi.org/10.1016/j.bandl.2014.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffis J. C., Nenert R., Allendorfer J. B., & Szaflarski J. P. (2017). Linking left hemispheric tissue preservation to fMRI language task activation in chronic stroke patients. Cortex, 96, 1–18. https://doi.org/10.1016/j.cortex.2017.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halai A. D., Woollams A. M., & Lambon Ralph M. A. (2017). Using principal component analysis to capture individual differences within a unified neuropsychological model of chronic post-stroke aphasia: Revealing the unique neural correlates of speech fluency, phonology and semantics. Cortex, 86, 275–289. https://doi.org/10.1016/j.cortex.2016.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton R. H., Chrysikou E. G., & Coslett B. (2011). Mechanisms of aphasia recovery after stroke and the role of noninvasive brain stimulation. Brain and Language, 118(1–2), 40–50. https://doi.org/10.1016/j.bandl.2011.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwigsen G., Baumgaertner A., Price C. J., Koehnke M., Ulmer S., & Siebner H. R. (2010). Phonological decisions require both the left and right supramarginal gyri. Proceedings of the National Academy of Sciences of the United States of America, 107(38), 16494–16499. https://doi.org/10.1073/pnas.1008121107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwigsen G., & Saur D. (2019). Neuroimaging of stroke recovery from aphasia—Insights into plasticity of the human language network. NeuroImage, 190, 14–31. https://doi.org/10.1016/j.neuroimage.2017.11.056 [DOI] [PubMed] [Google Scholar]
- Hartwigsen G., Saur D., Price C., Ulmer S., Baumgaertner A., & Siebner H. (2013). Perturbation of the left inferior frontal gyrus triggers adaptive plasticity in the right homologous area during speech production. Proceedings of the National Academy of Sciences of the United States of America, 110(41), 16402–16407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss W.-D., & Thiel A. (2006). A proposed regional hierarchy in recovery of post-stroke aphasia. Brain and Language, 98(1), 118–123. https://doi.org/10.1016/j.bandl.2006.02.002 [DOI] [PubMed] [Google Scholar]
- Hénon H., Pasquier F., Durieu M., Godefroy O., Lucas C., Lebert F., & Leys D. (1997). Preexisting dementia in stroke patients. Stroke, 28(12), 2429–2436. https://doi.org/10.1161/01.STR.28.12.2429 [DOI] [PubMed] [Google Scholar]
- Hickok G., & Poeppel D. (2007). The cortical organization of speech processing. Nature Reviews Neuroscience, 8(5), 393–402. [DOI] [PubMed] [Google Scholar]
- Hoffman P., Lambon Ralph M. A., & Woollams A. M. (2015). Triangulation of the neurocomputational architecture underpinning reading aloud. Proceedings of the National Academy of Sciences of the United States of America, 112(28), E3719–E3728. https://doi.org/10.1073/pnas.1502032112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope T. M. H., Leff A. P., Prejawa S., Bruce R., Haigh Z., Lim L., … Price C. J. (2017). Right hemisphere structural adaptation and changing language skills years after left hemisphere stroke. Brain, 140(6), 1718–1728. https://doi.org/10.1093/brain/awx086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. A., Allred R. P., Adkins D. L., Hsu J. E., O'Bryant A., & Maldonado M. A. (2009). Remodeling the brain with behavioral experience after stroke. Stroke, 40, S136–S138. https://doi.org/10.1161/STROKEAHA.108.533653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just A. M., Carpenter P. A., Keller T. A., Eddy W. F., & Thulborn K. R. (1996). Brain activation modulated by sentence comprehension. Science, 274, 114–116. [DOI] [PubMed] [Google Scholar]
- Kertesz A., & McCabe P. (1977). Recovery patterns and prognosis in aphasia. Brain, 100, 1–18. [DOI] [PubMed] [Google Scholar]
- Knecht S., Deppe M., Dräger B., Bobe L., Lohmann H., Ringelstein E.-B., & Henningsen H. (2000). Language lateralization in healthy right-handers. Brain, 123(1), 74–81. [DOI] [PubMed] [Google Scholar]
- Knecht S., Flöel A., Dräger B., Breitenstein C., Sommer J., Henningsen H., … Pascual-Leone A. (2002). Degree of language lateralization determines susceptibility to unilateral brain lesions. Nature Neuroscience, 5(7), 695–699. https://doi.org/10.1038/nn868 [DOI] [PubMed] [Google Scholar]
- Krarup C., Boeckstyns M., Ibsen A., Moldovan M., & Archibald S. (2016). Remodeling of motor units after nerve regeneration studied by quantitative electromyography. Clinical Neurophysiology, 127(2), 1675–1682. https://doi.org/10.1016/j.clinph.2015.08.008 [DOI] [PubMed] [Google Scholar]
- Kurland J., Cortes C. R., Wilke M., Sperling A. J., Lott S. N., Tagamets M. A., … Friedman R. B. (2008). Neural mechanisms underlying learning following semantic mediation treatment in a case of phonologic alexia. Brain Imaging and Behavior, 2(3), 147 https://doi.org/10.1007/s11682-008-9027-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kümmerer D., Hartwigsen G., Kellmeyer P., Glauche V., Mader I., Klöppel S., … Saur D. (2013). Damage to ventral and dorsal language pathways in acute aphasia. Brain, 136(2), 619–629. https://doi.org/10.1093/brain/aws354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey E. H., Skipper-Kallal L. M., Xing S., Fama M. E., & Turkeltaub P. E. (2017). Mapping common aphasia assessments to underlying cognitive processes and their neural substrates. Neurorehabilitation and Neural Repair, 31(5), 442–450. https://doi.org/10.1177/1545968316688797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch C. J., Breeden A. L., Gordon E. M., Cherry J. B. C., Turkeltaub P. E., & Vaidya C. J. (2018). Precision inhibitory stimulation of individual-specific cortical hubs disrupts information processing in humans. Cerebral Cortex, 29(9), 3912–3921. https://doi.org/10.1093/cercor/bhy270 [DOI] [PubMed] [Google Scholar]
- Marebwa B. K., Fridriksson J., Yourganov G., Feenaughty L., Rorden C., & Bonilha L. (2017). Chronic post-stroke aphasia severity is determined by fragmentation of residual white matter networks. Scientific Reports, 7(1), 8188 https://doi.org/10.1038/s41598-017-07607-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer M., Mohammadi S., Kugel H., Schiffbauer H., Flöel A., Albers J., … Deppe M. (2010). Integrity of the hippocampus and surrounding white matter is correlated with language training success in aphasia. NeuroImage, 53(1), 283–290. https://doi.org/10.1016/j.neuroimage.2010.06.004 [DOI] [PubMed] [Google Scholar]
- Metter E. J., Kempler D., Jackson C. A., Hanson W. R., Riege W. H., Camras L. R., … Phelps M. E. (1987). Cerebellar glucose metabolism in chronic aphasia. Neurology, 37(10), 1599 https://doi.org/10.1212/wnl.37.10.1599 [DOI] [PubMed] [Google Scholar]
- Mirman D., Chen Q., Zhang Y., Wang Z., Faseyitan O. K., Coslett H. B., & Schwartz M. F. (2015). Neural organization of spoken language revealed by lesion-symptom mapping. Nature Communications, 6, 6762 https://doi.org/10.1038/ncomms7762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase N., Duque J., Mazzocchio R., & Cohen L. G. (2004). Influence of interhemispheric interactions on motor function in chronic stroke. Annals of Neurology, 55(3), 400–409. https://doi.org/10.1002/ana.10848 [DOI] [PubMed] [Google Scholar]
- Murphy T. H., & Corbett D. (2009). Plasticity during stroke recovery: From synapse to behaviour. Nature Reviews Neuroscience, 10(12), 861–872. https://doi.org/10.1038/nrn2735 [DOI] [PubMed] [Google Scholar]
- Naeser M. A., & Palumbo C. L. (1994). Neuroimaging and language recovery in stroke. Journal of Clinical Neurophysiology, 11(2), 150–174. [DOI] [PubMed] [Google Scholar]
- Naeser M. A., Palumbo C. L., Helm-Estabrooks N., Stiassny-Eder D., & Albert M. L. (1989). Severe nonfluency in aphasia: Role of the medial subcallosal fasciculus and other white matter pathways in recovery of spontaneous speech. Brain, 112(1), 1–38. https://doi.org/10.1093/brain/112.1.1 [DOI] [PubMed] [Google Scholar]
- Nardo D., Holland R., Leff A. P., Price C. J., & Crinion J. T. (2017). Less is more: Neural mechanisms underlying anomia treatment in chronic aphasic patients. Brain, 140(11), 3039–3054. https://doi.org/10.1093/brain/awx234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport E. L., Landau B., Seydell-Greenwald A., Turkeltaub P. E., Chambers C. E., Dromerick A. W., … Gaillard W. D. (2017). Revisiting Lenneberg's hypotheses about early developmental plasticity: Language organization after left-hemisphere perinatal stroke. Biolinguistics, 11, 407–421. [PMC free article] [PubMed] [Google Scholar]
- Pani E., Zheng X., Wang J., Norton A., & Schlaug G. (2016). Right hemisphere structures predict poststroke speech fluency. Neurology, 86(17), 1574–1581. https://doi.org/10.1212/wnl.0000000000002613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolucci S., Antonucci G., Grasso M. G., Bragoni M., Coiro P., De Angelis D., … Pratesi L. (2003). Functional outcome of ischemic and hemorrhagic stroke patients after inpatient rehabilitation: A matched comparison. Stroke, 34(12), 2861–2865. https://doi.org/10.1161/01.STR.0000102902.39759.D3 [DOI] [PubMed] [Google Scholar]
- Pedersen P. M., Jørgensen H. S., Nakayama H., Raaschou H. O., & Olsen T. S. (1995). Aphasia in acute stroke: Incidence, determinants, and recovery. Annals of Neurology, 38(4), 659–666. https://doi.org/10.1002/ana.410380416 [DOI] [PubMed] [Google Scholar]
- Plowman E., Hentz B., & Ellis C. Jr. (2012). Post-stroke aphasia prognosis: A review of patient-related and stroke-related factors. Journal of Evaluation in Clinical Practice, 18(3), 689–694. https://doi.org/10.1111/j.1365-2753.2011.01650.x [DOI] [PubMed] [Google Scholar]
- Price C. J. (2012). A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. NeuroImage, 62(2), 816–847. https://doi.org/10.1016/j.neuroimage.2012.04.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C. J., Hope T. M., & Seghier M. L. (2017). Ten problems and solutions when predicting individual outcome from lesion site after stroke. NeuroImage, 145(Pt B), 200–208. https://doi.org/10.1016/j.neuroimage.2016.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C. J., Warburton E. A., Moore C. J., Frackowiak R. S., & Friston K. J. (2001). Dynamic diaschisis: Anatomically remote and context-sensitive human brain lesions. Journal of Cognitive Neuroscience, 13(4), 419–429. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F., Neininger B., Elbert T., Mohr B., Rockstroh B., Koebbel P., & Taub E. (2001). Constraint-induced therapy of chronic aphasia after stroke. Stroke, 32(7), 1621–1626. https://doi.org/10.1161/01.STR.32.7.1621 [DOI] [PubMed] [Google Scholar]
- Pustina D., Coslett H. B., Ungar L., Faseyitan O. K., Medaglia J. D., Avants B., & Schwartz M. F. (2017). Enhanced estimations of post-stroke aphasia severity using stacked multimodal predictions. Human Brain Mapping, 38(11), 5603–5615. https://doi.org/10.1002/hbm.23752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp B., Purcell J., Hillis A. E., Capasso R., & Miceli G. (2016). Neural bases of orthographic long-term memory and working memory in dysgraphia. Brain, 139(2), 588–604. https://doi.org/10.1093/brain/awv348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehme A. K., Eickhoff S. B., Wang L. E., Fink G. R., & Grefkes C. (2011). Dynamic causal modeling of cortical activity from the acute to the chronic stage after stroke. NeuroImage, 55(3), 1147–1158. https://doi.org/10.1016/j.neuroimage.2011.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur D., Lange R., Baumgaertner A., Schraknepper V., Willmes K., Rijntjes M., & Weiller C. (2006). Dynamics of language reorganization after stroke. Brain, 129(Pt. 6), 1371–1384. https://doi.org/10.1093/brain/awl090 [DOI] [PubMed] [Google Scholar]
- Skipper-Kallal L. M., Lacey E. H., Xing S., & Turkeltaub P. E. (2017a). Functional activation independently contributes to naming ability and relates to lesion site in post-stroke aphasia. Human Brain Mapping, 38(4), 2051–2066. https://doi.org/10.1002/hbm.23504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipper-Kallal L. M., Lacey E. H., Xing S., & Turkeltaub P. E. (2017b). Right Hemisphere remapping of naming functions depends on lesion size and location in poststroke aphasia. Neural Plasticity, 2017, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobesky J., Thiel A., Ghaemi M., Hilker R. H., Rudolf J., Jacobs A. H., … Heiss W.-D. (2005). Crossed cerebellar diaschisis in acute human stroke: A PET study of serial changes and response to supratentorial reperfusion. Journal of Cerebral Blood Flow & Metabolism, 25(12), 1685–1691. https://doi.org/10.1038/sj.jcbfm.9600162 [DOI] [PubMed] [Google Scholar]
- Szaflarski J. P., Binder J. R., Possing E. T., McKiernan K. A., Ward B. D., & Hammeke T. A. (2002). Language lateralization in left-handed and ambidextrous people fMRI data. Neurology, 59(2), 238–244. [DOI] [PubMed] [Google Scholar]
- Szaflarski J. P., Holland S. K., Schmithorst V. J., & Byars A. W. (2006). fMRI study of language lateralization in children and adults. Human Brain Mapping, 27(3), 202–212. https://doi.org/10.1002/hbm.20177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi N., & Izumi S.-I. (2012). Maladaptive plasticity for motor recovery after stroke: Mechanisms and approaches. Neural Plasticity, 2012, 359728 https://doi.org/10.1155/2012/359728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub E., Uswatte G., Mark V. W., & Morris D. M. (2006). The learned nonuse phenomenon: Implications for rehabilitation. Europa Medicophysica, 42(3), 241–256. [PubMed] [Google Scholar]
- Thiel A., Schumacher B., Wienhard K., Gairing S., Kracht L. W., Wagner R., … Heiss W.-D. (2006). Direct demonstration of transcallosal disinhibition in language networks. Journal of Cerebral Blood Flow & Metabolism, 26(9), 1122–1127. https://doi.org/10.1038/sj.jcbfm.9600350 [DOI] [PubMed] [Google Scholar]
- Thye M., & Mirman D. (2018). Relative contributions of lesion location and lesion size to predictions of varied language deficits in post-stroke aphasia. NeuroImage: Clinical, 20, 1129–1138. https://doi.org/10.1016/j.nicl.2018.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triggs W. J., Ghacibeh G., Springer U., & Bowers D. (2005). Lateralized asymmetry of facial motor evoked potentials. Neurology, 65(4), 541–544. https://doi.org/10.1212/01.wnl.0000172916.91302.e7 [DOI] [PubMed] [Google Scholar]
- Tuhrim S., Horowitz D. R., Sacher M., & Godbold J. H. (1999). Volume of ventricular blood is an important determinant of outcome in supratentorial intracerebral hemorrhage. Critical Care Medicine, 27(3), 617–621. [DOI] [PubMed] [Google Scholar]
- Turkeltaub P. E. (2015). Brain stimulation and the role of the right hemisphere in aphasia recovery. Current Neurology and Neuroscience Reports, 15(11), 72 https://doi.org/10.1007/s11910-015-0593-6 [DOI] [PubMed] [Google Scholar]
- Turkeltaub P. E., Coslett H. B., Thomas A. L., Faseyitan O., Benson J., Norise C., & Hamilton R. H. (2012). The right hemisphere is not unitary in its role in aphasia recovery. Cortex, 48(9), 1179–1186. https://doi.org/10.1016/j.cortex.2011.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub P. E., Messing S., Norise C., & Hamilton R. H. (2011). Are networks for residual language function and recovery consistent across aphasic patients? Neurology, 76(20), 1726–1734. https://doi.org/10.1212/WNL.0b013e31821a44c1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hees S., McMahon K., Angwin A., de Zubicaray G., & Copland D. A. (2014). Neural activity associated with semantic versus phonological anomia treatments in aphasia. Brain and Language, 129, 47–57. https://doi.org/10.1016/j.bandl.2013.12.004 [DOI] [PubMed] [Google Scholar]
- Ward N. S. (2017). Restoring brain function after stroke—Bridging the gap between animals and humans. Nature Reviews Neurology, 13(4), 244–255. https://doi.org/10.1038/nrneurol.2017.34 [DOI] [PubMed] [Google Scholar]
- Warren D. E., Power J. D., Bruss J., Denburg N. L., Waldron E. J., Sun H., … Tranel D. (2014). Network measures predict neuropsychological outcome after brain injury. Proceedings of the National Academy of Sciences of the United States of America, 111(39), 14247–14252. https://doi.org/10.1073/pnas.1322173111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watila M. M., & Balarabe S. A. (2015). Factors predicting post-stroke aphasia recovery. Journal of the Neurological Sciences, 352(1–2), 12–18. https://doi.org/10.1016/j.jns.2015.03.020 [DOI] [PubMed] [Google Scholar]
- Wilson S. M., Yen M., & Eriksson D. K. (2018). An adaptive semantic matching paradigm for reliable and valid language mapping in individuals with aphasia. Human Brain Mapping, 39(8), 3285–3307. https://doi.org/10.1002/hbm.24077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A., Tippett D., Saxena S., Sebastian R., Breining B., Faria A., & Hillis A. E. (2018). Leukoaraiosis is independently associated with naming outcome in poststroke aphasia. Neurology, 91(6), e526–e532. https://doi.org/10.1212/WNL.0000000000005945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing S., Lacey E. H., Skipper-Kallal L. M., Jiang X., Harris-Love M. L., Zeng J., & Turkeltaub P. E. (2016). Right hemisphere grey matter structure and language outcomes in chronic left hemisphere stroke. Brain, 139(1), 227–241. https://doi.org/10.1093/brain/awv323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing S., Lacey E. H., Skipper-Kallal L. M., Zeng J., & Turkeltaub P. E. (2017). White matter correlates of auditory comprehension outcomes in chronic post-stroke aphasia [Original research]. Frontiers in Neurology, 8(54) https://doi.org/10.3389/fneur.2017.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing S., Mandal A., Lacey E. H., Skipper-Kallal L. M., Zeng J., & Turkeltaub P. E. (2018). Behavioral effects of chronic gray and white matter stroke lesions in a functionally defined connectome for naming. Neurorehabilitation and Neural Repair, 32(6–7), 613–623. https://doi.org/10.1177/1545968318780351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnell P., Monroe P., & Sobel L. (1976). Aphasia outcome in stroke: A clinical neuroradiological correlation. Stroke, 7(5), 516–522. https://doi.org/10.1161/01.str.7.5.516 [DOI] [PubMed] [Google Scholar]
- Zai L., Ferrari C., Subbaiah S., Havton L. A., Coppola G., Strittmatter S., … Benowitz L. I. (2009). Inosine alters gene expression and axonal projections in neurons contralateral to a cortical infarct and improves skilled use of the impaired limb. The Journal of Neuroscience, 29(25), 8187–8197. https://doi.org/10.1523/JNEUROSCI.0414-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]