Abstract
Background
Hypertension is a major public health challenge affecting more than one billion people worldwide; it disproportionately affects populations in low‐ and middle‐income countries (LMICs), where health systems are generally weak. The increasing prevalence of hypertension is associated with population growth, ageing, genetic factors, and behavioural risk factors, such as excessive salt and fat consumption, physical inactivity, being overweight and obese, harmful alcohol consumption, and poor management of stress. Over the long term, hypertension leads to risk for cardiovascular events, such as heart disease, stroke, kidney failure, disability, and premature mortality.
Cardiovascular events can be preventable when high‐risk populations are targeted, for example, through population‐wide screening strategies. When available resources are limited, taking a total risk approach whereby several risk factors of hypertension are taken into consideration (e.g. age, gender, lifestyle factors, diabetes, blood cholesterol) can enable more accurate targeting of high‐risk groups. Targeting of high‐risk groups can help reduce costs in that resources are not spent on the entire population.
Early detection in the form of screening for hypertension (and associated risk factors) can help identify high‐risk groups, which can result in timely treatment and management of risk factors. Ultimately, early detection can help reduce morbidity and mortality linked to it and can help contain health‐related costs, for example, those associated with hospitalisation due to severe illness and poorly managed risk factors and comorbidities.
Objectives
To assess the effectiveness of different screening strategies for hypertension (mass, targeted, or opportunistic) to reduce morbidity and mortality associated with hypertension.
Search methods
An Information Specialist searched the Cochrane Register of Studies (CRS‐Web), the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, Latin American Caribbean Health Sciences Literature (LILACS) Bireme, ClinicalTrials.gov, and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) without language, publication year, or publication status restrictions. The searches were conducted from inception until 9 April 2020.
Selection criteria
Randomised controlled trials (RCTs) and non‐RCTs (NRCTs), that is, controlled before and after (CBA), interrupted time series (ITS), and prospective analytic cohort studies of healthy adolescents, adults, and elderly people participating in mass, targeted, or opportunistic screening of hypertension.
Data collection and analysis
Screening of all retrieved studies was done in Covidence. A team of reviewers, in pairs, independently assessed titles and abstracts of identified studies and acquired full texts for studies that were potentially eligible. Studies were deemed to be eligible for full‐text screening if two review authors agreed, or if consensus was reached through discussion with a third review author. It was planned that at least two review authors would independently extract data from included studies, assess risk of bias using pre‐specified Cochrane criteria, and conduct a meta‐analysis of sufficiently similar studies or present a narrative synthesis of the results.
Main results
We screened 9335 titles and abstracts. We identified 54 potentially eligible studies for full‐text screening. However, no studies met the eligibility criteria.
Authors' conclusions
There is an implicit assumption that early detection of hypertension through screening can reduce the burden of morbidity and mortality, but this assumption has not been tested in rigorous research studies. High‐quality evidence from RCTs or programmatic evidence from NRCTs on the effectiveness and costs or harms of different screening strategies for hypertension (mass, targeted, or opportunistic) to reduce hypertension‐related morbidity and mortality is lacking.
Plain language summary
Screening strategies for hypertension
Review question
What effects do the different ways of screening for hypertension (mass, targeted, or opportunistic) have in decreasing illness and death?
Background
Hypertension is a long‐term non‐communicable disease (NCD), also known as high, raised, or elevated blood pressure. Blood pressure is expressed by two measurements (systolic (SBP) and diastolic (DBP) pressures), which are the maximum and minimum pressures. High blood pressure is generally diagnosed when resting blood pressure is persistently at SBP ≥ 130/140 millimetres mercury (mmHg) or at DBP ≥ 80/90 mmHg for adults.
Even though blood pressure in the arteries is continuously raised, in many cases, high blood pressure does not cause symptoms. Nonetheless, hypertension can increase the risk for heart failure, stroke, vision loss, and chronic kidney disease, and so on, in the long term. People who have unhealthy diets, consume harmful amounts of alcohol and/or tobacco, and are physically inactive are at higher risk of hypertension.
Early detection, adequate treatment, and good control of high blood pressure can lower the risk of complications associated with hypertension. Although early detection through screening of hypertension has the potential to contain health‐related costs, reducing the burden of hypertension will to some extent involve addressing behavioural and socioeconomic risk factors (such as income, occupation, and level of education). Therefore, it is unclear whether early detection of mild hypertension can positively impact health‐related costs in the long term and improve health outcomes by reducing the need for hospitalisation and management of hypertension‐related complications, which can be severe.
Review methods
We searched various electronic databases on this topic until 9 April 2020. We searched for studies written in any language, whether published or not. We planned to include studies that compared one type of screening strategy for hypertension versus no screening strategy, that is, mass screening versus no screening, targeted screening versus no screening, and opportunistic screening versus no screening. We were interested in studies in which participants were healthy adolescents, adults, and elderly people, and in which researchers measured clinical outcomes, health system outcomes, and adverse events.
Key results
We found no studies that met the criteria described above.
Quality of the evidence
High‐certainty evidence that can tell us whether mass, targeted, or opportunistic screening strategies are effective for reducing illness and death associated with hypertension is lacking.
Background
Description of the condition
Hypertension, also known as high, raised, or elevated blood pressure, is a long‐term non‐communicable medical condition wherein the blood pressure in the arteries is persistently elevated (Guwatudde 2015). Blood pressure can be expressed as two measurements: systolic blood pressure (SBP) and diastolic blood pressure (DBP), which are the maximum and minimum pressures. Table 1 compares previous ‐ WHO 2013 ‐ versus current thresholds for high blood pressure (ACCF 2018; Carey 2018; Whelton 2018).
1. Thresholds for hypertension screening.
| BP category | SBP (mmHg) | DBP (mmHg) |
| Previous guidelines (WHO 2013) | ||
| High | ≥ 140 and | ≥ 90 |
| Current guidelines (ACCF 2018; Carey 2018; Whelton 2018) | ||
| Normal | < 120 and | < 80 |
| Elevated | 120 to 129 and | < 80 |
| Hypertension | Stage 1: 130 to 139 or Stage 2: ≥ 140 or |
80 to 89 ≥ 90 |
| Hypertensive crisis | > 180 and/or | > 120 |
BP: blood pressure.
DBP: diastolic blood pressure.
mmHg: millimetres mercury.
SBP: systolic blood pressure.
Hypertension is a major public health problem and is the most common cardiovascular disorder, affecting approximately one billion people globally. It remains, since the early 2000s, the single leading contributor to the global burden of morbidity and mortality (Guwatudde 2015). In sub‐Saharan Africa, an estimated 10 to 20 million people out of approximately 650 million people may have hypertension (Lloyd‐Sherlock 2014). This high prevalence of hypertension is attributed to population growth (migration from rural to urban areas), changes in dietary habits (resulting in being overweight or obese), ageing of the population, and social stress (Guwatudde 2015; WHO 2013). A large proportion of the population with hypertension remains undiagnosed, untreated, or inadequately treated, with subsequent complications contributing to the rising burden of cardiovascular disease (Ataklte 2015).
Over the long term, hypertension is a major risk factor for cardiovascular events, such as heart disease, stroke, and kidney failure, as well as disability and premature mortality (WHO 2013). Factors that increase the risk of hypertension include genetic and lifestyle factors, such as excessive salt and fat consumption, physical inactivity, harmful alcohol consumption, and poor management of stress (WHO 2013). Growing evidence shows that younger people, such as adolescents, are also at risk of hypertension because of these unhealthy lifestyle factors (Cheung 2017; Kar 2015).
Description of the intervention
Screening programmes for hypertension could help reduce morbidity and mortality linked to it (Legorreta 2015; WHO 2013). Screening is generally defined as the detection of unknown disease among apparently healthy individuals through tests or examinations conducted to identify those at increased risk for the condition (Screening Subcommittee 2008).
Various devices (electronic, mercury, and aneroid) can be used to measure blood pressure (WHO 2013). Two blood pressure measurements should be recorded daily for several days. These measurements should be taken at least a minute apart, ideally in the morning and again in the evening, while the person is seated. For accuracy, measurements taken on the first day are discarded, and an average is taken of all remaining measurements to confirm the diagnosis of hypertension (WHO 2013). It is common practice that diagnosis of hypertension is confirmed if the resting blood pressure is persistently at SBP ≥ 130/140 millimetres mercury (mmHg) or DBP ≥ 80/90 mmHg (ACCF 2018; WHO 2013). This Cochrane Review focused primarily on screening strategies for hypertension and not on the thresholds used for diagnosis.
Key components of screening programmes for hypertension include not only equipment and trained health professionals, but also patient education and informed consent, as well as good relationships between health professions (which are beneficial for referral processes between different healthcare facilities or services) (WHO 2013). These components make screening for hypertension (across an entire population) a costly intervention because of the lengthy time needed to diagnosis and the human and financial resources required.
How the intervention might work
The logic model in Figure 1 outlines how hypertension screening may reduce the burden of disease in terms of participant, intervention, implementation, and contextual factors (Rohwer 2017). Early detection of hypertension through screening could increase awareness for those at risk of hypertension, and thus lead to preventative action or early management, which may ultimately curb the societal and economic burden of the disease (Ataklte 2015).
1.
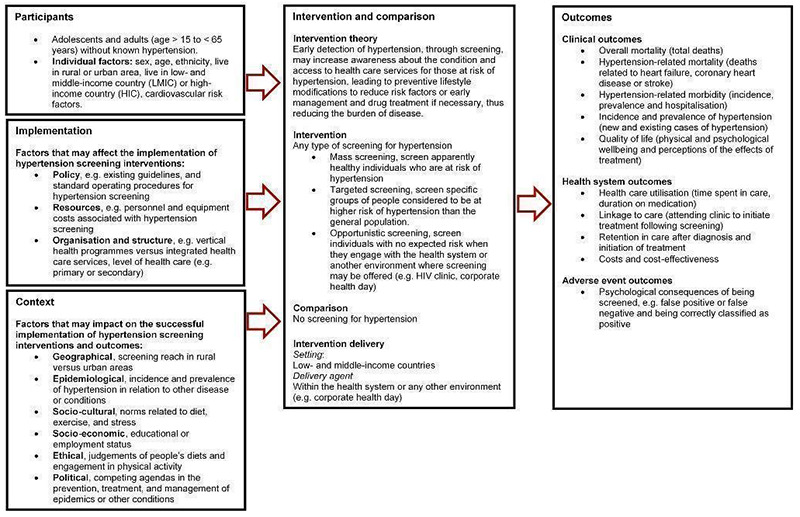
Screening for hypertension
Early detection of hypertension through screening can provide benefits for those identified as hypertensive. Treatment can be especially beneficial for people with moderate to severe hypertension, that is, people with resting blood pressure of greater than 160 over 100 mmHg (Musini 2017; Musini 2019). However, treatment has not proved beneficial for people with mild hypertension (Diao 2012).
On the contrary, screening (including for hypertension) can also lead to harmful consequences for those being screened. For example, patients can sometimes be misdiagnosed as hypertensive when subsequent blood pressure readings are limited over several months. This can result in non‐hypertensive individuals being unnecessarily exposed to treatments and their potential side effects. For the workforce, attending the required number of screening sessions can lead to increased absenteeism from work. Employees may increasingly stay away from work once they are aware that they are potentially hypertensive, and if diagnosed as hypertensive, low adherence to treatment may result in sickness and increased absenteeism (Haynes 1978). Additionally, screening for hypertension can be harmful in settings where the harms of treatment outweigh the benefits.
Why it is important to do this review
Interventions to prevent or manage hypertension should be effective, feasible, affordable, and sustainable. Vertical programmes that focus solely on hypertension are therefore not widely recommended (WHO 2013). Early detection of hypertension may be a feasible and affordable intervention, especially when screening for hypertension is offered as a point‐of‐care or integrated service. However, it is unclear whether or when early detection of hypertension may be an effective intervention, specifically because hypertension is associated with behavioural and socioeconomic risk factors (Bernabé‐Ortiz 2017). Therefore, early detection of mild hypertension may not significantly impact health‐related costs in the long term nor improve health outcomes. Additionally, lifestyle factors associated with hypertension (e.g. changes in diet, physical activity patterns) can generally be attributed to urbanisation in resource‐limited countries. Therefore, preventing hypertension may involve other stakeholders (e.g. policy‐makers) and may extend beyond screening by health professionals (Hunter‐Adams 2017; WHO 2013).
Because it is unclear whether screening for hypertension leads to healthier behaviours and better control of blood pressure levels, it is important to learn from studies that have assessed the impact of screening on hypertension outcomes. A 2014 systematic review informed the US Preventive Services Task Force update of its recommendation on screening for high blood pressure in adults (Piper 2014). The review focused on the role of confirming hypertension diagnoses, re‐screening intervals, ambulatory blood pressure monitoring, and home blood pressure monitoring. Evidence from the systematic review does not provide guidance on different screening strategies.
A recent overview of systematic reviews on diabetes and hypertension screening programmes found that a systematic review is needed to assess the effectiveness and impact of various screening interventions (Durao 2014). Thus, this Cochrane Review aimed to address this gap in the literature, with specific focus on evidence from resource‐limited countries, where behavioural and socioeconomic risk factors for hypertension are similar to the broader problems of urban areas in these countries. It aimed to clarify whether screening for hypertension, in all age groups, helps contain health‐related costs and improve outcomes related to hypertension and associated life‐threatening complications.
Objectives
To assess the effectiveness of different screening strategies for hypertension (mass, targeted, or opportunistic) to reduce morbidity and mortality associated with hypertension.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include randomised trials, non‐randomised trials, controlled before and after (CBA) studies, interrupted time series (ITS), and prospective analytic cohort studies (Cochrane EPOC 2017a). Given the programmatic nature of screening for hypertension, we did not expect to find many randomised controlled trials (RCTs), and so we also planned to include non‐randomised studies (NRSs). RCTs are experimental studies in which people are randomly allocated to one of two or more groups receiving an intervention or control treatment or no treatment. A CBA study is an NRS in which outcomes are measured before and after treatment, both in a group that receives the treatment and in another comparison group. An ITS study is an NRS that measures an outcome at multiple time points before and after an intervention (the ‘interruption'). The design attempts to detect whether the intervention has had an effect greater than any underlying trend over time. To be eligible, ITS studies should be controlled and they must have at least three data points before and after a clearly defined intervention in terms of content and timing (Cochrane EPOC 2017a). Prospective analytic cohort studies were also eligible when participants were already exposed or unexposed to an intervention but had not developed the outcome of interest at the start of the study; participants are followed forward in time, after which outcomes are measured. To be eligible, cohort studies needed to have at least two study arms, so they could provide a comparison of the exposure of interest.
We planned to include studies regardless of their language or publication status.
Types of participants
Healthy adolescents (15 to 24 years old), adults (25 to 64 years old), and elderly people (over 65 years old) without known hypertension. We were also interested in studies in which participants presented with risk factors for hypertension.
Types of interventions
Studies on mass, targeted, or opportunistic hypertension screening compared to no screening with participant follow‐up of at least one year were eligible.
Mass screening involves screening apparently healthy populations regardless of the presence of risk factors (at public places, e.g. markets). Targeted screening involves screening specific groups of people who are considered to be at higher risk of hypertension than the general population. Opportunistic screening involves screening individuals engaging with the health system or in another environment in which screening may be offered (e.g. HIV clinic, corporate health day).
Types of outcome measures
Primary outcomes
Clinical outcomes
1. Overall mortality (total deaths) 2. Hypertension‐related mortality (death related to heart failure, coronary heart disease, stroke, or end‐stage kidney disease) 3. Hypertension‐related morbidity (incidence, prevalence, and hospitalisation due to stroke, coronary heart disease, heart failure, or end‐stage renal disease) 4. Incidence and prevalence of hypertension (ratio of detected hypertension to expected prevalence of hypertension) 5. Quality of life (physical and psychological well‐being and perceptions of the effects of treatment)
Secondary outcomes
Health system outcomes
6. Healthcare utilisation (time spent in care, duration on medication) 7. Linkage to care (attending clinic to initiate treatment following screening) 8. Retention in care after diagnosis and initiation of treatment 9. Costs and cost‐effectiveness (as described in the included studies, or in related sub‐studies)
Adverse events of being screened
10. Psychological consequences of being screened (e.g. false positive or false negative, being correctly classified as positive (new diagnosis))
Search methods for identification of studies
Electronic searches
The Cochrane Hypertension Information Specialist searched the following databases without language, publication year, or publication status restrictions until 9 April 2020.
Cochrane Hypertension Specialised Register via the Cochrane Register of Studies (CRS‐Web).
Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library, via the Cochrane Register of Studies (CRS‐Web).
MEDLINE Ovid (from 1946 onwards), MEDLINE Ovid Epub Ahead of Print, and MEDLINE Ovid In‐Process & Other Non‐Indexed Citations.
Embase Ovid (from 1974 onwards).
Latin American Caribbean Health Sciences Literature (LILACS) Bireme (from 1982 onwards).
ClinicalTrials.gov (www.clinicaltrials.gov).
World Health Organization International Clinical Trials Registry Platform (www.who.it.trialsearch).
The subject strategies for databases were modelled on the search strategy designed for MEDLINE (Appendix 1). When appropriate, these were combined with subject strategy adaptations of the sensitivity‐ and precision‐maximising search strategy designed by Cochrane for identifying RCTs (as described in Box 6.4.c of Higgins 2011). We based the search terms for non‐randomised trials on the EPOC search filter for Ovid MEDLINE (Cochrane EPOC 2017b), and we provide the full search strategies for the listed databases in Appendix 1.
Searching other resources
The Information Specialist searched the Cochrane Hypertension Specialised Register segment (which includes searches of MEDLINE, Embase, the Cochrane Library, and Epistemonikos for systematic reviews) to retrieve published systematic reviews related to this review title, so that we could scan their reference lists to identify additional relevant trials. The Specialised Register also includes searches of the Allied and Complementary Medicine Database (AMED), CAB Abstracts & Global Health, Cumulative Index to Nursing and Allied Health Literature (CINAHL), ProQuest Dissertations & Theses, and Web of Science.
We also contacted experts in this research field to identify relevant studies.
Data collection and analysis
Selection of studies
Pairs of review authors (BS, AH, EN, RM, IT, CB, and TK) independently screened all titles or abstracts, or both, of all records retrieved, to determine their eligibility for full‐text screening. We retrieved the full texts of potentially eligible or unclear studies, which were assessed for inclusion independently by pairs of review authors (BS, AH, EN, RM, TK). We solved disagreements by re‐checking the full‐text article or by consulting a third review author, or both. We present the study selection process in a PRISMA flow diagram (Figure 2), and we list all studies excluded after full‐text assessment along with reasons for their exclusion in the Characteristics of excluded studies table (Liberati 2009).
2.
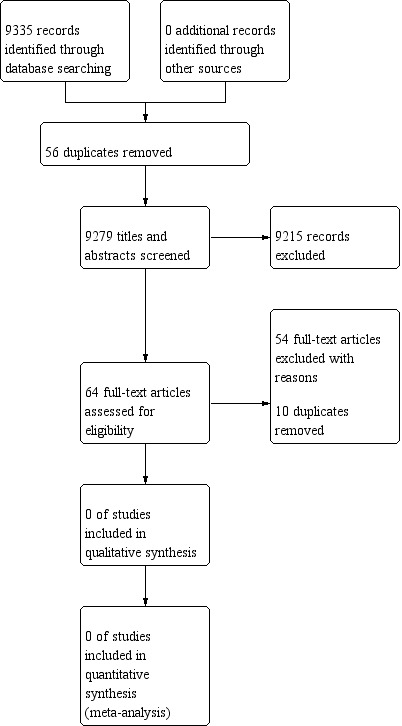
PRISMA diagram of eligible studies
Data extraction and management
We planned to pilot the data extraction form on two included studies to ensure that information is captured in a standardised manner. It was intended that two review authors would independently extract study data related to participant, intervention, comparison, and outcome (PICO) characteristics using the standard data extraction form. Any missing information was going to be noted with a plan to contact the author of the primary study. Any disagreements would have been resolved through discussion or by consultation with a third review author.
Assessment of risk of bias in included studies
We were going to use the ‘Risk of bias' assessment tool modified by the Cochrane Effective Practice and Organisation of Care (EPOC) Group (Cochrane EPOC 2017a). It is widely used and validated for systematic reviews including a wide range of study designs. We planned that two review authors would independently assess the risk of bias in included studies and resolve any disagreements through discussion or by consultation with a third review author. Individual studies would have been classified as having ‘low', ‘unclear', or ‘high' risk of bias. Low risk of bias is plausible bias that is unlikely to alter results, unclear risk of bias is plausible bias that raises some doubt about the results, and high risk of bias is plausible bias that seriously weakens confidence in results. We will follow the recommendations of Cochrane EPOC to score NRCTs as ‘high' risk of bias (Cochrane EPOC 2017a).
We planned to apply the following criteria to the ‘Risk of bias' assessments of RCTs and NRCTs.
Was the allocation sequence adequately generated? (RCTs)
Was the allocation adequately concealed? (RCTs)
Were baseline outcome measurements similar? (all)
Were baseline characteristics similar? (all)
Were incomplete outcome data adequately addressed? (RCTs)
Was knowledge of the allocated intervention adequately prevented during the study? (RCTs)
Was the study adequately protected against contamination? (RCTs)
Was the study free from selective outcome reporting? (RCTs)
Was the study free from other risks of bias? (all)
We planned to apply the following criteria to the ‘Risk of bias' assessments of ITS.
Was the intervention independent of other changes?
Was the shape of the intervention effect pre‐specified?
Was the intervention unlikely to affect data collection?
Was knowledge of the allocated interventions adequately prevented during the study?
Were incomplete outcome data adequately addressed?
Was the study free from selective outcome reporting?
Was the study free from other risks of bias?
Measures of treatment effect
We planned to present dichotomous outcomes as risk ratios, and continuous outcomes as mean differences with standard deviations between the change in intervention and control groups if outcomes were measured in the same way across all studies. In the case that included studies measured continuous outcomes in different ways, we were going to calculate the standardised mean differences between intervention and control groups. We planned to present time‐to‐event outcomes as hazard ratios, and to present 95% confidence intervals (CIs) for all outcomes.
Unit of analysis issues
We planned to consider the level at which randomisation occurred (e.g. in cluster‐randomised trials, groups of individuals may be randomised together to the same intervention). When repeated measurements were taken, there may be multiple observations for the same outcome (Higgins 2011). In the case that more than one comparison was available from the same study, we were going to combine groups into a single pair‐wise comparison. If included cluster‐randomised trials had not appropriately adjusted for the clustering of participants in their analysis, then we were going to re‐analyse them. The design effect (of cluster‐randomised trials) may lead to inflated effect sizes of the intervention, so we had planned to calculate the design effect, which involves an estimation of an intracluster correlation (ICC). We planned to impute estimates of the ICC value using estimates from other included studies that reported ICCs, or using external estimates from empirical research. Also, we planned to examine the impact of clustering by performing sensitivity analyses.
Dealing with missing data
When necessary, we were going to contact the authors of included studies to ask for data related to study methods, attrition rates, and outcomes that were unclear or missing. For example, we were going to request information on the number of participants screened, randomly assigned participants, intention‐to‐treat (ITT), as‐treated or per‐protocol samples, dropouts, losses to follow‐up, or withdrawals. If the study authors did not provide estimates for the entire study sample (e.g. they provide estimates only for each sex group), then we would have calculated these using available information, including imputing data, when appropriate. We would have reported all missing outcome data on the data extraction form and the ‘Risk of bias' table, and we would have assessed the impact of including in the sensitivity analysis studies with missing data.
Assessment of heterogeneity
We planned to assess heterogeneity and variability amongst studies in relation to participant, intervention, comparison, and outcome information, as well as context and type of screening and its implementation. If we had conducted a meta‐analysis, we would have assessed heterogeneity by visually inspecting overlap of CIs and by using statistical methods (i.e. Chi² test and I² statistic values). If the Chi² test had a small P value (P < 0.1) and the I² statistic was 60% or above, we would have concluded that heterogeneity is moderate or substantial (Higgins 2011). We planned to explore reasons for heterogeneity through subgroup analyses.
Assessment of reporting biases
We planned to assess the likelihood of reporting bias for each outcome if a sufficient number of studies (more than 10) were included in a meta‐analysis. We would have used a funnel plot to visually check for asymmetry associated with small‐study effects and publication bias. Through sensitivity analysis, we would have assessed how these factors affect the results and conclusions of the meta‐analysis.
Data synthesis
We planned to conduct a meta‐analysis if the included studies were sufficiently homogenous, and if at least two studies of the same design assess the same intervention, comparison, and outcome. Outcomes should have occurred at clinically relevant time points after hypertension screening, to be analysed (e.g. death within three months of screening may not be clinically relevant). If the characteristics of included studies were excessively heterogeneous, we would have pooled results but would have presented only a narrative synthesis of the results, potentially grouping findings by context measures.
Subgroup analysis and investigation of heterogeneity
We would have considered subgroup analyses according to the following.
Sex: female or male.
Age: adolescents (15 to 24 years old), adults (25 to 64 years old), and elderly people (over 65 years old).
Ethnicity: white, black, Asian, or other.
Setting: rural versus urban; low‐ and middle‐income countries versus high‐income countries (which we would have defined according to the World Bank’s country classifications by income level (World Bank 2018)).
Screening tools: electronic, mercury, or aneroid.
Cardiovascular risk factors: overweight or obesity, physical inactivity, dietary factors (e.g. sodium or salt intake), and comorbid condition.
Sensitivity analysis
We planned to conduct sensitivity analyses to explore the influence of various factors, when applicable, on the effect size. We would have stratified analyses per publication status and level of risk of bias to determine whether studies with high risk of bias skew the results.
Summary of findings and assessment of the certainty of the evidence
We planned to assess the certainty of the overall evidence for each outcome according to the GRADE approach (Guyatt 2008). GRADE is a methodology for rating the certainty of evidence and grading the strength of recommendations in systematic reviews. It includes five criteria for downgrading the certainty of evidence: risk of bias, inconsistency, imprecision, publication bias, and indirectness; and three criteria for upgrading the certainty of evidence: large effect, dose response, and residual confounding opposing the observed effect. We will report the certainty of evidence as either ‘high', ‘moderate', ‘low', or ‘very low'. High certainty means that further research is very unlikely to change our confidence in the estimate of effect; moderate certainty means that further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low certainty means that further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; and very low certainty means that we are very uncertain about the estimate.
We aimed to report GRADE assessments in ‘Summary of findings' table(s). The ‘Summary of findings' table(s) would have included the number of participants and studies included for each outcome, a summary of intervention effect, and a measure of the certainty of evidence against GRADE criteria. We would have presented results for the following outcomes: overall morality, hypertension‐related mortality, hypertension‐related morbidity, incidence and prevalence of hypertension, quality of life, health care utilisation and linkage to care. These are listed as 1 to 7 in the 'Types of outcome measures' section. The seven main outcomes to be presented prioritise clinically important outcomes, followed by adverse effect and health system outcomes. Given the complex nature of the interventions being studied, pre‐specification of the outcomes was challenging (Cochrane EPOC 2017a).
Results
Description of studies
None of the retrieved studies met the inclusion criteria. This ‘empty’ review (no studies were included) will follow the guidelines provided by Cochrane on reporting empty reviews and results from excluded studies. For the discussion, this review will draw on some studies that assessed the effectiveness of screening strategies for hypertension but did not meet the inclusion criteria.
Results of the search
The electronic databases search yielded 9335 records, which were exported into Covidence 2019; 56 duplicates were removed. As is presented in Figure 2, 9279 titles and/or abstracts were screened, from which 64 studies were identified as eligible for full‐text screening. Of these 64, 10 additional duplicates were removed, 54 studies were excluded, no studies are awaiting classification, and no relevant ongoing studies were found. We did not find any studies for inclusion into this review.
Included studies
No studies (RCTs and NRS) met the inclusion criteria.
Excluded studies
Of the 54 excluded studies, 44 did not have a study design eligible for this review (such as cohort studies with no control arm and cross‐sectional studies); two studies included participants already diagnosed with hypertension; and eight studies did not report on our interventions of interest (i.e. mass, targeted, or opportunistic screening). Detailed reasons for exclusion of individual studies are provided in the Characteristics of excluded studies table.
Risk of bias in included studies
No studies met the inclusion criteria for this review.
Allocation
N/A.
Blinding
N/A.
Incomplete outcome data
N/A.
Selective reporting
N/A.
Other potential sources of bias
N/A.
Effects of interventions
No studies met the inclusion criteria for this review.
Discussion
Summary of main results
We did not identify any studies that were eligible for inclusion in the review, nor ongoing studies, making this an ‘empty’ review. About half of the excluded studies (29 of 54) were conducted during the 1970s and 1980s and could potentially have been included in a 2014 systematic review to update the US Preventive Services Task Force recommendation (Whelton 2018). That review, however, focused on the role of confirming hypertension diagnoses, re‐screening intervals, ambulatory blood pressure monitoring, and home blood pressure monitoring instead of different types of screening strategies. We are confident that all relevant literature was identified, given the wide range of appropriate databases that we searched and the double screening of all titles or abstracts and full texts.
The discussion is based on evidence from two studies that did not meet the inclusion criteria of this review but addressed a similar research question related to screening for hypertension. One study conducted in the United States of America (USA) in 2015 aimed to identify the prevalence and characteristics of patients identified with high blood pressure and the proportion of false‐positives after an initial elevated blood pressure reading during non‐primary care visits compared with primary care visits (Handler 2015). This study was excluded because it was a cohort study without a control arm, and there was no analysis comparing screening with no screening of hypertension. The results of 111,996 patients (82.7% in primary care and 17.3% in non‐primary care) suggest that expanding screening for hypertension to non‐primary care settings may improve the detection of hypertension. Although study authors identified non‐primary care as an opportunity to identify individuals with increased blood pressure, they were also concerned about false‐positives. Falsely identifying patients as hypertensive will increase the absolute number of patients who require follow‐up but do not have hypertension. This could lead to wasted time and resources for both patients and the healthcare system in making additional but unnecessary screenings (Handler 2015).
A study conducted in Finland in 1999 assessed a programme for both hypertension screening and hypertension treatment and adherence. We excluded this study from our systematic review because of the absence of a control group (Takala 1983). People aged 40 to 64 years living in a southwestern municipality of Finland were invited to attend screening for hypertension (mass screening). Those identified as hypertensive were divided into two groups: one group received a letter notifying them that they had elevated blood pressure, and the other group received additional written information about hypertension and the importance of receiving treatment. Trial authors report that receiving written information explaining the nature of hypertension and stressing the importance of its treatment, in addition to the notification letter, did not lead to a significant increase in seeking treatment (Handler 2015).
The first study suggests that offering screening across both primary and non‐primary healthcare settings may improve the detection of hypertension, and the second study suggests that there may be no improvement in treatment‐seeking amongst those who are screened, diagnosed, and given encouragement. These studies did not look at different screening strategies for hypertension, but the first study suggests that wider screening of hypertension across healthcare services can improve detection. It is however unclear what the additional costs or harms for screening people in non‐primary care were compared to the benefits of, for example, getting more people on anti‐hypertensive treatment.
Overall completeness and applicability of evidence
N/A.
Quality of the evidence
N/A.
Potential biases in the review process
N/A.
Agreements and disagreements with other studies or reviews
N/A.
Authors' conclusions
Implications for practice.
The latest recommendation related to screening for hypertension was issued by the US Preventive Services Task Force in 2015 (Whelton 2018). The USPSTF recommends screening for high blood pressure in adults aged 18 years or older. However, this recommendation does not provide details on different types of screening strategies for hypertension.
Evidence suggests that screening for hypertension regardless of the type of screening strategy used can reduce morbidity and mortality associated with hypertension. This is based on the notion that early detection can lead hypertensive individuals to seek treatment and positively change lifestyle‐related behaviours. However, falsely diagnosing individuals as hypertensive can have adverse psychological effects on them, and unnecessary screening can lead to wasted resources for patients and the healthcare system.
Currently, evidence to support any specific type of screening strategy for hypertension is lacking. Pragmatically, on the one hand, governments should be cautious in rolling out mass (population‐wide) screening programmes that are costly, without evidence that screening will lead to treatment‐seeking, treatment adherence, and changed lifestyle behaviours. On the other hand, governments may continue to offer screening to those individuals who are at greater risk than the general population, or to individuals who are already engaging with integrated healthcare services.
Implications for research.
Well‐conducted experimental and observational studies are needed to assess the effectiveness of different screening strategies for hypertension (mass, targeted, or opportunistic) to reduce morbidity and mortality associated with hypertension. Knowing which screening strategy is most effective could help decision‐makers, including policy‐makers and practitioners, especially in resource‐limited settings, in prioritising how screening for hypertension should be offered.
What's new
| Date | Event | Description |
|---|---|---|
| 21 May 2020 | Amended | Minor amendment: corrected image type for Figure 1 |
History
Protocol first published: Issue 11, 2018 Review first published: Issue 5, 2020
| Date | Event | Description |
|---|---|---|
| 16 January 2019 | Amended | Amended to include correct citation for the other published version of this protocol in BMJ Open |
Acknowledgements
We would like to thank:
Mrs. Joy Oliver (Cochrane South Africa, South African Medical Research Council), who assisted us with developing the search strategy and scoping the evidence;
Mrs. Rachel Tshibola (Stellenbosch University), who assisted us with screening;
Cochrane Hypertension Group for support provided throughout the review process; and
Professor Naomi Levitt (Division of Diabetic Medicine and Endocrinology, Department of Medicine, University of Cape Town), who contributed to the review question and suggested potential studies for inclusion.
Appendices
Appendix 1. Search strategies
Database: Ovid MEDLINE(R) andEpubAhead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions(R) <1946 to April 06, 2020> Search Date: 7 April 2020 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 mass screening/ 2 early diagnosis/ 3 (screen? or screened or screening?).tw,kf. 4 (early adj3 (detect$ or diagnos$ or intervent$)).tw,kf. 5 detect$.ti. 6 or/1‐5 7 hypertension/di, pc 8 essential hypertension/di, pc 9 (hypertens$ or prehypertens$).ti,kf. 10 ((elevat$ or increas$ or lower or high or rais$ or rising) adj2 (arterial pressure or blood pressure or diastolic pressure or systolic pressure)).tw,kf. 11 ((elevat$ or increas$ or lower or high or rais$ or rising) adj2 (bp or dbp or hbp or sbp)).tw,kf. 12 or/7‐11 13 randomized controlled trial.pt. 14 pragmatic clinical trial.pt. 15 controlled clinical trial.pt. 16 randomi$.ab. 17 placebo.ab. 18 clinical trials as topic/ 19 randomly.ab. 20 trial.ti. 21 multicenter study.pt. 22 non‐randomized controlled trials as topic/ 23 interrupted time series analysis/ 24 controlled before‐after studies/ 25 groups.ab. 26 (multicenter or multi center or multicentre or multi centre).ti. 27 intervention?.ti. 28 (effect? or impact? or controlled or control group? or (before adj5 after) or (pre adj5 post) or ((pretest or pre test) and (posttest or post test)) or quasiexperiment$ or quasi experiment$ or evaluat$ or time series or time point? or repeated measur$).tw. 29 exp cohort studies/ 30 (cohort adj2 (analys$ or design? or stud$)).tw,kf. 31 epidemiologic methods/ 32 limit 31 to yr=1971‐1988 33 or/13‐30,32 34 animals/ not (humans/ and animals/) 35 33 not 34 36 6 and 12 and 35
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: Cochrane Hypertension Specialised Register via Cochrane Register of Studies (CRS‐Web)
Search Date: 8 April 2020 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 MESH DESCRIPTOR Mass Screening AND INSEGMENT #2 MESH DESCRIPTOR Early Diagnosis AND INSEGMENT #3 (screen* OR screened OR screening*) AND INSEGMENT #4 (early NEAR3 (detect* OR diagnos* OR intervent*)) AND INSEGMENT #5 detect*:TI AND INSEGMENT #6 (#1 OR #2 OR #3 OR #4 OR #5) AND INSEGMENT #7 RCT:DE AND INSEGMENT #8 Review:ODE AND INSEGMENT #9 (#7 OR #8) AND INSEGMENT #10 #6 AND #9 AND INSEGMENT #11 * AND INSEGMENT AND 01/11/2018_TO_08/04/2020:CRSCREATED #12 #11 AND #10
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: Cochrane Central Register of Controlled Trials (Issue 3, 2020) via Cochrane Register of Studies (CRS‐Web)
Search Date: 8 April 2020
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 MESH DESCRIPTOR Mass Screening AND CENTRAL:TARGET #2 MESH DESCRIPTOR Early Diagnosis AND CENTRAL:TARGET #3 (screen* OR screened OR screening*) AND CENTRAL:TARGET #4 (early NEAR3 (detect* OR diagnos* OR intervent*)) AND CENTRAL:TARGET #5 detect*:TI AND CENTRAL:TARGET #6 #1 OR #2 OR #3 OR #4 OR #5 AND CENTRAL:TARGET #7 MESH DESCRIPTOR Hypertension WITH QUALIFIER DI PC AND CENTRAL:TARGET #8 MESH DESCRIPTOR Essential Hypertension AND CENTRAL:TARGET #9 (hypertens* OR prehypertens*):TI AND CENTRAL:TARGET #10 ((elevat* OR increas* OR lower OR high OR rais* OR rising) NEAR2 (arterial pressure OR blood pressure OR diastolic pressure OR systolic pressure)) AND CENTRAL:TARGET #11 ((elevat* OR increas* OR lower OR high OR rais* OR rising) NEAR2 (bp OR dbp OR hbp OR sbp)) AND CENTRAL:TARGET #12 #7 OR #8 OR #9 OR #10 OR #11 AND CENTRAL:TARGET #13 #6 AND #12 AND CENTRAL:TARGET #14 * AND 01/11/2018_TO_08/04/2020:CRSINCENTRAL AND CENTRAL:TARGET #15 #14 AND #13
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database:Embase<1974 to 2020 April 03> Search 7 April 2020 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 *mass screening/ 2 *early diagnosis/ 3 (screen? or screened or screening?).ti. 4 (screen? or screened or screening?).ab. /freq=2 5 (early adj3 (detect$ or diagnos$ or intervent$)).tw. 6 or/1‐5 7 hypertension/di, pc 8 (hypertens$ or prehypertens$).ti. 9 ((elevat$ or increas$ or lower or high or rais$ or rising) adj2 (arterial pressure or blood pressure or diastolic pressure or systolic pressure)).tw. 10 ((elevat$ or increas$ or lower or high or rais$ or rising) adj2 (bp or dbp or hbp or sbp)).tw. 11 or/7‐10 12 randomized controlled trial/ 13 crossover procedure/ 14 double‐blind procedure/ 15 (randomi?ed or randomly).tw. 16 (crossover$ or cross‐over$).tw. 17 placebo.ab. 18 (doubl$ adj blind$).tw. 19 assign$.ab. 20 allocat$.ab. 21 time series analysis/ 22 groups.ab. 23 (multicenter or multi center or multicentre or multi centre).ti. 24 intervention?.ti. 25 (effect? or impact? or controlled or control group? or (before adj5 after) or (pre adj5 post) or ((pretest or pre test) and (posttest or post test)) or quasiexperiment$ or quasi experiment$ or evaluat$ or time series or time point? or repeated measur$).tw. 26 exp cohort analysis/ 27 (cohort adj2 (analys$ or design? or stud$)).tw. 28 exp longitudinal study/ 29 exp prospective study/ 30 or/12‐29 31 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 32 30 not 31 33 6 and 11 and 32
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: LILACSBireme Search Date: 9 April 2020
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (tw:("early diagnosis" OR "early detection" OR screening)) AND (tw:("high blood pressure" OR hypertension OR prehypertension)) AND (instance:"regional") AND ( db:("LILACS") AND type_of_study:("clinical_trials" OR "cohort" OR "systematic_reviews") AND limit:("humans"))
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: ClinicalTrials.gov Search Date: 9 April 2020
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Condition or disease: Hypertension Intervention/treatment: "early diagnosis" OR "early detection" OR screening
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: WHO International Clinical Trials Registry Platform (ICTRP) Search Date: 29 November 2018
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Title: high blood pressure OR hypertension OR prehypertension Intervention: "early diagnosis" OR "early detection" OR screening Recruitment status: ALL
OR
Condition: healthy OR hypertension OR prehypertension Intervention: "early diagnosis" OR "early detection" OR screening Recruitment status: ALL
OR
Title: "early diagnosis" OR "early detection" OR screening Condition: healthy OR hypertension OR prehypertension Recruitment status: ALL
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abernethy 1974 | This study reports on hypertension screening for men and women aged 30 to 69 years throughout Australia. Results show the response of the community, the general pattern of blood pressure, and complications in the screened population. There was no control group; therefore this study was excluded from this review |
| Bruneau 2017 | This study aimed to implement and test a cardiovascular assessment screening programme. The intervention described in the study (a cardiovascular screening checklist) is not eligible for inclusion in this review |
| Cretens 1978 | This study reported on a hypertension screening programme that followed up on children who were previously identified with elevated blood pressure. Children are not eligible for this review; therefore this study was excluded from this review |
| Cromwell 2005 | This study evaluated an underlying disorder that is called secondary hypertension, to assess for comorbidities (such as obesity) and to assess for end‐organ damage. It was excluded because of the wrong study design (there was no control group) and the wrong intervention (screening for hypertension was not the intervention of interest) |
| DeSales 2015 | This study aimed to identify the prevalence and characteristics of patients identified with high blood pressure in non‐primary care compared with primary care visits. There was no control group, and the intervention described in this study is not eligible for inclusion in this review |
| Grancio 1979 | This article presents concepts and principles of community control to professional nurses working and living in communities where efforts directed at prevention and control of chronic health problems (such as hypertension) are appropriate. It is not an empirical study; therefore it uses the wrong study design and was excluded from the review |
| Handler 2015 | This study aimed to identify the prevalence and characteristics of patients identified with high blood pressure in non‐primary care compared to primary care visits. It was excluded from this review because of the wrong study design and the wrong intervention |
| Haynes 1978 | This study confirmed that labelling patients as hypertensive can result in increased absenteeism from work. There is no control group; therefore this study was excluded from this review |
| Hoegh 2014 | This study evaluated the cost‐effectiveness of a combined screening programme for the following conditions: abdominal aortic aneurysm, peripheral arterial disease, carotid plaque, hypertension, arrhythmia, and type 2 diabetes. There is no control group, and the intervention is not of interest to this review; therefore this study was excluded from this review |
| Hong 2011 | This study investigated the impact of a national mass screening programme on health service utilisation and medical expenses. There is no control group; therefore this study was excluded from this review |
| Hypertension Detection & Follow‐up Program 1978 | The Hypertension Detection and Follow‐up Program (HDFP) occurred over a 5‐year period and addressed the following questions: (1) Will successful community hypertension intervention programmes have an impact on death and disability rates in the community? (2) Can hypertensives, detected in general populations, be brought under pharmacologic management? (3) Do the benefits of therapy exceed undesirable side effects and costs in the subgroup with mild hypertension? (4) Is antihypertensive therapy effective in young adults and in females, and equally effective in blacks and whites? (5) Can morbidity and mortality from coronary artery disease be decreased by antihypertensive therapy? This study was excluded from this review because it did not have a control group |
| Jastrup 1986 | This study did not have a control group |
| John 2010 | This study was conducted in India to assess whether primary care health workers (Health Aides), similar in training and educational qualifications to the Village Health Nurse, can be sufficiently trained to measure blood pressure without substantially increasing workload. Screening for hypertension was not the intervention of interest; therefore this study was excluded from this review |
| Kaufman 1993 | This study summarised existing data on the epidemiology of hypertension in Africa and outlined important known risk factors. Both the study design and the intervention in this study were not eligible for inclusion into this review |
| Kortge 1976 | The Hypertension Screening and Awareness Project had 3 objectives: (1) to collect data on blood pressure measurements in the community; (2) to provide educational information for the screened population; and (3) to screen the population of south‐central Kansas for undetected hypertension. There was no control group; therefore this study was excluded from this review |
| Kulbertus 1978 | This cross‐sectional study reports on hypertension screening that was conducted in mobile units generally used for detection of tuberculosis and chest diseases. This study was excluded because it used the wrong study design and did not provide follow‐up of at least 1 year |
| Lauridsen 1979 | This cohort study reports on a screening programme for workers in Copenhagen. However, there was no control group; therefore this study was excluded from this review |
| Leshchinskii 1986 | This study assessed early detection of arterial hypertension and ischaemic heart disease in mass screening at the polyclinics of large industrial enterprises. There was no control group; therefore this study was excluded from this review |
| LugoDeOrtellado 2007 | This study evaluated the effectiveness of a programme of Pharmaceutical Care in Paraguay in a controlled prospective study directed at hypertensive patients in community pharmacies. The intervention described in the study was not eligible for inclusion in this review |
| Marin‐Rives 2015 | This study assessed the value of having pharmacists measuring blood pressure to detect hypertension in adults without a previous diagnosis and/or antihypertensive treatment. There was no control group; therefore this study was excluded from this review |
| McAlister 1980 | This study assessed whether an information management system can help family doctors in treating high blood pressure more effectively. The intervention described in the study was not eligible for inclusion in this review |
| Mendy 2014 | The aim of the "Barbers Reaching Out to Help Educate on Routine Screenings" (BROTHERS) initiative was provided for barbers to routinely screen adult black men in the Mississippi Delta region, thereby increasing awareness of high blood pressure, and to refer clients with high blood pressure to a healthcare provider. There was no control group; therefore this study was excluded from this review |
| Miall 1982 | This paper summarised the relevant epidemiological background, evidence from trials concerning the value of treatment for mild hypertension, and technical aspects of mounting screening or case‐finding programmes. This is not an empirical study; therefore it used the wrong study design to be included in this review |
| Miller 1976 | This study evaluated the Franklin County High Blood Pressure Program (FCHHBP), which is a population‐wide network of community blood pressure centres. There was no control group; therefore this study was excluded from this review |
| Mohan 2018 | This paper describes the design and methods of "UDAY" ‐ a comprehensive diabetes and hypertension prevention and management programme in India. Pre‐ and post‐treatment evaluations were conducted, but there was no control group, so this study was excluded from this review |
| Moser 1977 | The Task Force I of the USA National High Blood Pressure Education Program, National Heart, Lung and Blood Institute, was charged with providing practical recommendations for (1) identifying that segment of the total population with high blood pressure, (2) detecting those who could be expected to benefit from antihypertensive therapy, and (3) proposing appropriate therapeutic regimens. The report provides 6 general recommendations for detection, evaluation, and treatment of high blood pressure in adults. This is not an empirical study; therefore it used the wrong study design to be included in this review |
| Musicha 2016 | This study evaluated the uptake of referral for clinical assessment and retention in care following a large rural and urban population screening programme (for hypertension and diabetes) in Malawi. Two cross‐sectional studies were carried out, so this study was excluded from the review for wrong study design |
| Niessen 2014 | The aim of this study was to define and subsequently validate blood pressure cut‐off values to either confirm or reject the diagnosis of hypertension after 1 or 2 duplicate home blood pressure measurements in persons at low and high cardiovascular risk. The intervention described in this study (i.e. home blood pressure measurements) is not eligible for inclusion in this review |
| Nugent 1980 | This study reported on screening for hypertension, referral of hypertensive patients to local physicians for management, and re‐screening of those identified as hypertensive. There was no control group; therefore this study was excluded from this review |
| O'Connell 1985 | This study reported on screening for hypertension and follow‐up in the workplace. There was no control group; therefore this study was excluded from this review |
| O'Sullivan 1979 | This study recorded the blood pressure measurements of non‐pregnant patients between the ages of 20 and 60 years who presented at the Blackburn Clinic. It was excluded from this review because it did not include a control group |
| Petrovitch 1991 | This study reports on the recruitment strategies used in the Systolic Hypertension in the Elderly Program. The intervention described in the study (i.e. recruitment of older individuals) is not eligible for inclusion in this review |
| Prat Gonzalez 2011 | This study evaluated the effectiveness of an intervention to increase youth participation in the Health Prevention and Promotion Activities Programme (PAPPS). The intervention described in the study (letter and phone invitation) is not eligible for inclusion in this review |
| Prins 1980 | This cross‐sectional study reported on people with hypertension and their willingness to have it checked. This study was excluded because it used the wrong study design; there was no control group and follow‐up of at least 1 year was not provided |
| Radice 1984 | The aim of this cross‐sectional study was to assess the efficacy of screening for arterial hypertension in an adult population. The study authors concluded that there was no efficacy in screening for arterial hypertension; however, this study was excluded from this review because it used the wrong study design; there was no control group and follow‐up of at least 1 year was not provided |
| Ramsay 1997 | This paper provided a critique of the Hypertension Detection and Follow‐up Program. It is not an empirical study; therefore it used the wrong study design |
| Risse 2015 | This study evaluated the feasibility and effectiveness of a high blood pressure screening strategy for unknown or insufficiently treated hypertensive patients with Self‐Blood Pressure Measurements (SBPM) in barbershops. There was no control group; therefore this study was excluded from this review |
| Schnohr 1975 | This study reported on a 'heart‐week' intervention whereby people attending supermarkets in Copenhagen were invited to have their blood pressure checked. Data were collected cross‐sectionally; follow‐up of at least 1 year was not provided, and no control group was included; therefore this study was excluded from this review |
| Secrest 1994 | This paper is a commentary by the National Institutes of Health, which is primarily responsible for collecting and disseminating information on detection and treatment of hypertension. This article is not an empirical study; therefore it used the wrong study design to be included in this review |
| Shah 2013 | This cross‐sectional study reported on the prevalence of type 2 diabetes mellitus and hypertension and the influence of possible risk factors. This study was excluded because it used the wrong study design, there was no control group, and follow‐up of at least 1 year was not provided |
| Soricelli 1975 | The aims of this study were to determine the productive results of hypertension detection in private dental offices, to expand screening facilities for hypertension, and to encourage dentists to include blood pressure screening as part of their physical examination of patients. This study was excluded because it included no control group |
| Stamler 1978 | This article is a commentary titled, "Where do we go in hypertension screening?"; it used the wrong study design so was excluded from this review |
| Takala 1983 | This study reported on the Sakyla‐Koylio project, which involved multi‐phasic screening at the health centre level. There was no control group; therefore this study was excluded from this review |
| Taylor 1990 | This cross‐sectional study aimed to identify hypertensive patients engaging with general practice, who were then linked to treatment. It was not eligible for inclusion in this review because it used the wrong study design |
| van der Feen 1980 | This paper reports on a hypertension screening programme that a physician introduced in his practice in The Netherlands, specifically for men and women aged 31 to 60. There was no control group; therefore this study is not eligible for inclusion in this review |
| van der Graaf 2013 | This study aimed to identify predictors of future cardiovascular events. It was excluded from this review because it included the wrong participants (i.e. hypertensive patients) |
| Velez 1983 | This study tested 2 methods of referral for follow‐up evaluation of patients with high blood pressure detected in the walk‐in screening clinic of a Veterans Administration Medical Center (VAMC) during a 6‐week period in June and July 1979. There was no control group, and the intervention being studied is not eligible for this review |
| Verdesca 1974 | This paper is a commentary on hypertension screening and follow‐up. It is not an empirical study; therefore it was excluded from this review |
| Weinberger 1977 | This article explores the approaches used to overcome the hypertension burden in the United States of America. It is a commentary and therefore used the wrong study design |
| Werba 2017 | This study determined the efficacy and cost‐effectiveness (i.e. change in cardiovascular risk factors and lifestyle vs costs) of ProSALUTE, a new organisational model of primary cardiovascular prevention. The intervention described in this study is not eligible for inclusion in this review |
| Whelton 2018 | This paper presents a guideline for prevention, detection, evaluation, and management of high blood pressure in adults in the United States of America. It is not an empirical study; therefore it was excluded from this review |
| Wieliczky 1987 | This cross‐sectional study reported on blood pressure screening for all employees at Mercerville Nursing Center from August 1983 to July 1984. This study was excluded because it used the wrong study design, there was no control group, and follow‐up of at least 1 year was not provided |
| Wilber 1972 | This study evaluated various methods of screening for elevated blood pressure. There was no control group, and the intervention described in this study was not eligible for this review |
| Xi'an Jiaotong University 2016 | This study investigated the effects of interaction between environmental factors and genetic factors on long‐term blood pressure based on 2 established cohorts, including "the cohort of Hanzhong adolescent hypertension study" and "the cohort of Mei county adult salt‐sensitive hypertension study". There was no control group, and the intervention being studied was not eligible for this review |
Differences between protocol and review
There were no differences in the conduct of this review and the protocol.
Contributions of authors
All review authors read and approved the final manuscript.
Sources of support
Internal sources
-
Cochrane South Africa, South African Medical Research Council, South Africa
salary support
External sources
-
Federal Ministry for Education and Research (Bundesministerium für Bildung und Forschung (BMBF)), Germany
This work is part of the Collaboration for Evidence‐Based Healthcare and Public Health in Africa (CEBHA+) (http://cebha-plus.org/Project.html). This is supported through the BMBF funding of Research Networks for Health Innovation in Sub‐Saharan Africa.
Declarations of interest
BS: nothing to declare.
SD: nothing to declare.
IT: nothing to declare.
CMB: nothing to declare.
AH: nothing to declare.
EN: nothing to declare.
JJM: nothing to declare.
TK: nothing to declare.
Edited (no change to conclusions)
References
References to studies excluded from this review
Abernethy 1974 {published data only}
- Abernethy JD. The Australian National Blood Pressure Study. Medical Journal of Australia 1974;1(21):821-4. [PubMed] [Google Scholar]
Bruneau 2017 {published data only}
- Bruneau J. Implementing and Testing a Cardiovascular Assessment Screening Program (CASP). ClinicalTrials.gov 2017.
Cretens 1978 {published data only}
- Cretens ML, Mattson MJ. Hypertension screening program follow-up of previously identified children with elevated blood pressure. Journal of Family Practice 1978;6(4):891-2. [PubMed] [Google Scholar]
Cromwell 2005 {published data only}
- Cromwell PF, Munn N, Zolkowski-Wynne J. Evaluation and management of hypertension in children and adolescents (part two): evaluation and management. Journal of Pediatric Health Care 2005;19(5):309-13. [DOI] [PubMed] [Google Scholar]
DeSales 2015 {published data only}
- De Sales PC. Expanding high blood pressure screening to the nonprimary care setting to improve early recognition. Journal of Clinical Outcomes Management 2015;22(8):350-2. [Google Scholar]
Grancio 1979 {published data only}
- Grancio SD. Nursing role in the detection, evaluation and treatment of high blood pressure. The Massachusetts Nurse 1979. [PubMed]
Handler 2015 {published data only}
- Handler J, Mohan Y, Kanter MH, Reynolds K, Li X, Nguyen M, et al. Screening for high blood pressure in adults during ambulatory nonprimary care visits: opportunities to improve hypertension recognition. Journal of Clinical Hypertension (Greenwich) 2015;17(6):431-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haynes 1978 {published data only}
- Haynes RB, Sackett DL, Taylor DW, Gibson ES, Johnson AL. Increased absenteeism from work after detection and labeling of hypertensive patients. New England Journal of Medicine 1987;299(D):741-4. [DOI] [PubMed] [Google Scholar]
Hoegh 2014 {published data only}
- Hoegh AH. The Intersectional Viborg Screening Program: Cost-(Effectiveness) of Screening for Diabetes and Cardiovascular Diseases (VISP). ClinicalTrials.gov 2014.
Hong 2011 {published data only}
- Hong JM, Lee TJ. The impact of national mass screening program on health service utilizations and medical expenses: in the case of hypertension complications. Value in Health 2011;14(7):A390-A510. [Google Scholar]
Hypertension Detection & Follow‐up Program 1978 {published data only}
- HDFG. Mild hypertensives in the hypertension detection and follow-up program. The Hypertension Detection and Follow-up Cooperative Group. Annals of the New York Academy of Sciences 1978;304(1):254-66. [DOI] [PubMed] [Google Scholar]
Jastrup 1986 {published data only}
- Jastrup B, Faldborg L, Hansen T. Screening for hypertension in men aged 40-64 years. Ugeskrift for Laeger 1986;48(3):119-21. [PubMed] [Google Scholar]
John 2010 {published data only}
- John J, Muliyil J, Balraj V. Screening for hypertension among older adults: a primary care "high risk" approach. Indian Journal of Community Medicine 2010;35(1):67-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaufman 1993 {published data only}
- Kaufman J, Barkey N. Hypertension in Africa: an overview of prevalence rates and causal risk factors. Ethnicity & Disease 1993;3 Suppl:S83-101. [PubMed] [Google Scholar]
Kortge 1976 {published data only}
- Kortge D. Hypertension detection: screening and follow-up. Journal of the Kansas Medical Society 1976;77(9):393-4. [PubMed] [Google Scholar]
Kulbertus 1978 {published data only}
- Kulbertus HE, Leval-Rutten F, Dubois M, Petit JM. Experience with a community screening program for hypertension: results on 24,462 individuals. European Heart Journal 1978;7(5-6):487-97. [PubMed] [Google Scholar]
Lauridsen 1979 {published data only}
- Lauridsen L, Gyntelberg F. A clinical follow-up five years after screening for hypertension in Copenhagen males aged 40-59. International Journal of Epidemiology 1979;8(1):11-7. [Google Scholar]
Leshchinskii 1986 {published data only}
- Leshchinskii LA, Shinkareva IA, Logacheva IV, Zabel'ian OM, Ipatova EN. Early detection of arterial hypertension and ischemic heart disease in mass screening at the polyclinics of large industrial enterprises. Ter Arkh 1986;58(1):20-1. [PubMed] [Google Scholar]
LugoDeOrtellado 2007 {published data only}
- Lugo De Ortellado G, Bittner MR, Chavez H, Perez SEMT. Implementation of a pharmaceutical care program for the detection of hypertension and drug therapy to be followed up in community pharmacies. Acta Farmaceutica Bonaerense 2007;26(4):590-5. [Google Scholar]
Marin‐Rives 2015 {published data only}
- Marin-Rives FR, Sendra-Lillo J, Morales F, Sabater-Hernandez S. Usefulness of blood pressure measurements by community pharmacists in screening for hypertension. The screenBpharm study: a protocol. Latin American Journal of Pharmacy 2015;43(2):237-43. [Google Scholar]
McAlister 1980 {published data only}
- McAlister NH, Covvey HD, Monkman EJ, Wigle ED. Community hypertension management project - design of a randomized, controlled clinical trial to test an automated hypertension management system in primary care. Preventive Medicine 1980;9(3):445. [Google Scholar]
Mendy 2014 {published data only}
- Mendy VL, Perryman B, Hawkins J, Dove C. Planning for the strategic recruitment of barbershops for blood pressure screening and referral in the Mississippi Delta region. Preventing Chronic Disease 2014;11:E126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miall 1982 {published data only}
- Miall WE. Screening for hypertension. British Journal of Hospital Medicine 1982;27(6):592-600. [PubMed] [Google Scholar]
Miller 1976 {published data only}
- Miller FS. Hypertension control in rural Maine. Franklin County High Blood Pressure Program. Journal of the Maine Medical Association 1976;67(9):280-3. [PubMed] [Google Scholar]
Mohan 2018 {published data only}
- Mohan S, Jarhyan P, Ghosh S, Venkateshmurthy NS, et al. UDAY: a comprehensive diabetes and hypertension prevention and management program in India. BMJ Open 2018;8(6):1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moser 1977 {published data only}
- Moser M. Detection, evaluation and treatment of high blood pressure. Hypertension: Current Concepts 1977. [PubMed]
Musicha 2016 {published data only}
- Musicha C, Crampin AC, Kayuni N, Koole O, Amberbir A, Mwagomba B, et al. Accessing clinical services and retention in care following screening for hypertension and diabetes among Malawian adults: an urban/rural comparison. Journal of Hypertension 2016;34(11):2172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Niessen 2014 {published data only}
- Niessen MA, Hoeven NV, den Born BJ, Kalken CK, Kraaijenhagen RA. Home blood pressure measurement as a screening tool for hypertension in a web-based worksite health promotion programme. European Journal of Public Health 2014;24(5):776-81. [DOI] [PubMed] [Google Scholar]
Nugent 1980 {published data only}
- Nugent CA, Gerlach BA. Hypertension control: the role of screening and referral to community physicians. Preventive Medicine 1980;9(4):569-77. [DOI] [PubMed] [Google Scholar]
O'Connell 1985 {published data only}
- O'Connell JK, Price JH, Price JA. Hypertension screening and follow-up in the workplace. Ohio State Medical Journal 1985;81(12):891-2, 896. [PubMed] [Google Scholar]
O'Sullivan 1979 {published data only}
- O'Sullivan J, Carson N, Forsell P. Screening in general practice (2): results of a 12 months screening survey in a group practice, including early observations on therapeutic outcomes. General Practice Research, Australian Family Physician 1979;8(10):1109-12. [PubMed] [Google Scholar]
Petrovitch 1991 {published data only}
- Petrovitch H, Byington R, Bailey G, Borhani P, et al. Systolic Hypertension in the Elderly Program (SHEP). Part 2. Screening and recruitment. Hypertension 1991;17(3 Suppl):16-23. [DOI] [PubMed] [Google Scholar]
Prat Gonzalez 2011 {published data only}
- Prat-Gonzalez I, Juvinya-Canal D, Sanchez-Perez I. Strategy to increase screening for cardiovascular risk factors among young people. Enfermeria Clinica 2011;21(1):3-11. [DOI] [PubMed] [Google Scholar]
Prins 1980 {published data only}
- Prins A. Persons with hypertension and their willingness to have it checked; population screening in Krimpen on the IJssel organized by the local health center. Ned Tijdschr Geneeskd 1980;124(19):723-6. [PubMed] [Google Scholar]
Radice 1984 {published data only}
- Radice M, Alberti D, Alli C, Avanzini F, Di Tullio M, Mariotti G, et al. Efficacy of screening for arterial hypertension in an adult population. Cardiologia 1984;29(11):523-7. [PubMed] [Google Scholar]
Ramsay 1997 {published data only}
- Ramsay LE. The hypertension detection and follow-up program: 17 years on. JAMA 1997;277(2):167-70. [DOI] [PubMed] [Google Scholar]
Risse 2015 {published data only}
- Risse J, Lauriere E, Iraqi M, Fay R, Burnier M, Boivin JM. Screening for hypertension in the barbershop: a Franco-Moroccan feasibility study (the "decoiffa" study). Journal of Hypertension 2015;33(1):e69. [Google Scholar]
Schnohr 1975 {published data only}
- Schnohr P, Hansen AT. Mass screening for hypertension in Copenhagen supermarkets. Lancet 1975;2(7942):969-70. [DOI] [PubMed] [Google Scholar]
Secrest 1994 {published data only}
- Secrest BG. Detecting, evaluating and treating hypertension. Journal of the American Dental Association 1994;125(1):104-6. [DOI] [PubMed] [Google Scholar]
Shah 2013 {published data only}
- Shah A, Afzal M. Prevalence of diabetes and hypertension and association with various risk factors among different Muslim populations of Manipur, India. Journal of Diabetes & Metabolic Disorders 2013;12(52):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Soricelli 1975 {published data only}
- Soricelli SF. Evaluation of a hypertension detection program by dentists. Pennsylvania Dental Journal (Harrisburg) 1975;42(7):15-9. [PubMed] [Google Scholar]
Stamler 1978 {published data only}
- Stamler R, Stamler J. Where do we go in hypertension screening? Urban Health 1978;7(5):8-9. [PubMed] [Google Scholar]
Takala 1983 {published data only}
- Takala J. Screening, treatment and adherence to treatment for hypertension. Scandinavian Journal of Primary Health Care 1983;1(3-4):114-9. [DOI] [PubMed] [Google Scholar]
Taylor 1990 {published data only}
- Taylor FR, Gould SE, Taylor NA. A screening programme for hypertension in general practice. Journal of Human Hypertension 1990;4(4):350-2. [PubMed] [Google Scholar]
van der Feen 1980 {published data only}
- Feen JA. Screening and follow-up. Journal of the Royal College of General Practitioners 1980;1(12):13-4. [Google Scholar]
van der Graaf 2013 {published data only}
- Graaf Y. The SMART-ORACLE Study (SMART-ORACLE). ClinicalTrials.gov 2013.
Velez 1983 {published data only}
- Velez R, Anderson L, Bean S, Magruder-Habib K. A randomized controlled factorial design trial of referral strategies for blood pressure screening. Clinical Research 1983;31(5):840. [Google Scholar]
Verdesca 1974 {published data only}
- Verdesca AS. Hypertension screening and follow-up. Journal of Occupational and Environmental Medicine 1974;16(6):395-401. [PubMed] [Google Scholar]
Weinberger 1977 {published data only}
- Weinberger MH. Hypertension screening, detection, treatment and follow-up: the Indiana experience. Journal of the Indiana State Medical Association 1977;70(8):655-8. [PubMed] [Google Scholar]
Werba 2017 {published data only}
- Werba JP. ProSALUTE: Community Program for Cardiovascular Health (ProSALUTE). ClinicalTrials.gov 2017.
Whelton 2018 {published data only}
- Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. Correction to: 2017 guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018;71(6):1269-324. [DOI] [PubMed] [Google Scholar]
Wieliczky 1987 {published data only}
- Wieliczky l. An annual evaluation study: hypertension screening. Nursing Homes, Senior Citizen Care 1987;36(3):36-7. [PubMed] [Google Scholar]
Wilber 1972 {published data only}
- Wilber JA, Millward D, Baldwin A, Capron B, et al. Atlanta Community High Blood Pressure Program methods of community hypertension screening. Circulation Research 1972;31(Suppl 2):101-9. [PubMed] [Google Scholar]
Xi'an Jiaotong University 2016 {published data only}
- First Affiliated Hospital Xi'an Jiaotong University. Blood Pressure Follow-up Study in Two Chinese Cohorts (C-BPCS). ClinicalTrials.gov 2016.
Additional references
ACCF 2018
- American College of Cardiology Foundation. New ACC/AHA high blood pressure guidelines lower definition of hypertension. www.acc.org/latest-in-cardiology/articles/2017/11/08/11/47/mon-5pm-bp-guideline-aha-2017 (accessed 20 March 2018).
Ataklte 2015
- Ataklte F, Erqou S, Kaptoge S, Taye B, Echouffo-Tcheugui JB, Kengne AP. Burden of undiagnosed hypertension in sub-Saharan Africa: a systematic review and meta-analysis. Hypertension 2015;65(2):291-8. [DOI] [PubMed] [Google Scholar]
Bernabé‐Ortiz 2017
- Bernabe-Ortiz A, Carrillo-Larco RM, Gilman RH, Checkley W, Smeeth L, Miranda JJ. Impact of urbanisation and altitude on the incidence of, and risk factors for, hypertension. BMJ Heart 2017;103(1):827-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carey 2018
- Carey RM, Whelton PK, 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association hypertension guideline. Annals of Internal Medicine 2018;168(5):351-8. [DOI] [PubMed] [Google Scholar]
Cheung 2017
- Cheung EL, Bell CS, Samuel JP, Poffenbarger T, Redwine KM, Samuels JA. Race and obesity in adolescent hypertension. Pediatrics 2017;139(5):e20161433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cochrane EPOC 2017a
- Cochrane Effective Practice and Organisation of Care (EPOC). Resources for review authors. https://epoc.cochrane.org/cochrane-resources-review-authors (accessed 20 March 2018).
Cochrane EPOC 2017b
- Cochrane Effective Practice and Organisation of Care (EPOC) Group. How to develop a search strategy for an intervention review. https://epoc.cochrane.org/sites/epoc.cochrane.org/files/public/uploads/Resources-for-authors2017/how_to_develop_a_search_strategy.pdf (accessed 20 March 2018).
Covidence 2019 [Computer program]
- Covidence systematic review software. Veritas Health Innovation, Melbourne Australia, 2019.Available at www.covidence.org.
Diao 2012
- Diao D, Wright JM, Cundiff DK, Gueyffier F. Pharmacotherapy for mild hypertension. Cochrane Database of Systematic Reviews 2012;8(CD006742):1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Durao 2014
- Durao S, Kredo T. Mapping the evidence on population level interventions to prevent diabetes and hypertension (unpublished). Cochrane South Africa, South African Medical Research Council, 2014.
Guwatudde 2015
- Guwatudde D, Nankya-Mutyoba J, Kalyesubula R, Laurence C, Adebamowo C, Ajayi I, et al. The burden of hypertension in sub-Saharan Africa: a four-country cross sectional study. BMC Public Health 2015;15:1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hunter‐Adams 2017
- Hunter-Adams J, Yongsi BN, Dzasi K, Parnell S, Boufford JI, Pieterse E, et al. How to address non-communicable diseases in urban Africa. Lancet Diabetes & Endocrinology 2017;5(12):932-4. [DOI] [PubMed] [Google Scholar]
Kar 2015
- Kar S, Khandelwal B. Fast foods and physical inactivity are risk factors for obesity and hypertension among adolescent school children in east district of Sikkim, India. Journal of Natural Science, Biology, and Medicine 2015;6(2):356-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Legorreta 2015
- Legorreta AP, Schaff SR, Leibowitz AN, Meijgaard J. Measuring the effects of screening programs in asymptomatic employees: detection of hypertension through worksite screenings. Journal of Occupational and Environmental Medicine 2015;57(6):682-6. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lloyd‐Sherlock 2014
- Lloyd-Sherlock P, Ebrahim S, Grosskurth H. Is hypertension the new HIV epidemic? International Journal of Epidemiology 2014;43(1):8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Musini 2017
- Musini VM, Gueyffier F, Puil L, Salzwedel DM, Wright JM. Pharmacotherapy for hypertension in adults aged 18 to 59 years. Cochrane Database of Systematic Reviews 2017;8(CD008276):1-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Musini 2019
- Musini VM, Tejani AM, Bassett K, Puil L, Wright JM. Pharmacotherapy for hypertension in adults 60 years or older. Cochrane Database of Systematic Reviews 2019;6(CD000028):1-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Piper 2014
- Piper MA, Evans CV, Burda BU, Margolis KL, O’Connor E, Smith N, et al. Screening for high blood pressure in adults: a systematic evidence review for the U.S. Preventive Services Task Force. Evidence Synthesis No. 121 2014:1-296. [PubMed]
Rohwer 2017
- Rohwer A, Pfadenhauer L, Burns J, Brereton L, Gerhardus A, Booth A, et al. Series: Clinical Epidemiology in South Africa. Paper 3: Logic models help make sense of complexity in systematic reviews and health technology assessments. Journal of Clinical Epidemiology 2017;83:37-47. [DOI] [PubMed] [Google Scholar]
Screening Subcommittee 2008
- Screening Subcommittee. Australian Population Health Development Principal Committee. Population Based Screening Framework. Commonwealth of Australia 2008:1-18.
WHO 2013
- World Health Organization. A global brief on hypertension: silent killer, global public health crisis. World Health Day 2013. www.who.int/cardiovascular_diseases/publications/global_brief_hypertension/en/ (accessed 18 June 2017).
World Bank 2018
- The World Bank. New country classifications by income level: 2018-2019. http://blogs.worldbank.org/opendata/allaboutfinance/new-country-classifications-income-level-2018-2019 (accessed 02 October 2018).
References to other published versions of this review
Schmidt 2018
- Schmidt B-M, Durao S, Toews I, Bavuma CM, Meerpohl JJ, Kredo T. Screening strategies for hypertension: a systematic review protocol. BMJ Open 2019;9:e025043. [DOI: 10.1136/bmjopen-2018-025043] [DOI] [PMC free article] [PubMed] [Google Scholar]


